Exhibit 99.2

September, 2014

Protalex , Inc. Forward - Looking Statement Statements made in this presentation stating the Company ’ s beliefs, intentions, and expectations are forward - looking statements. The company ’ s actual results could differ materially from those projected. Additional information is contained in the company ’ s SEC filings such as our Form 10 - K and Form 10 - Qs filed at www.sec.gov . 2 Protalex , Inc .
Protalex , Inc . (Ticker: PRTX) Biotechnology company developing a new class of drugs with the potential to revolutionize the treatment of autoimmune diseases Lead product: PRTX - 100 ▪ Highly purified formulation of Staphylococcal protein A, a natural protein isolated from cultured bacteria ▪ Safe and well - tolerated in five human clinical studies ▪ Efficient and inexpensive proprietary production process 3 Protalex , Inc.
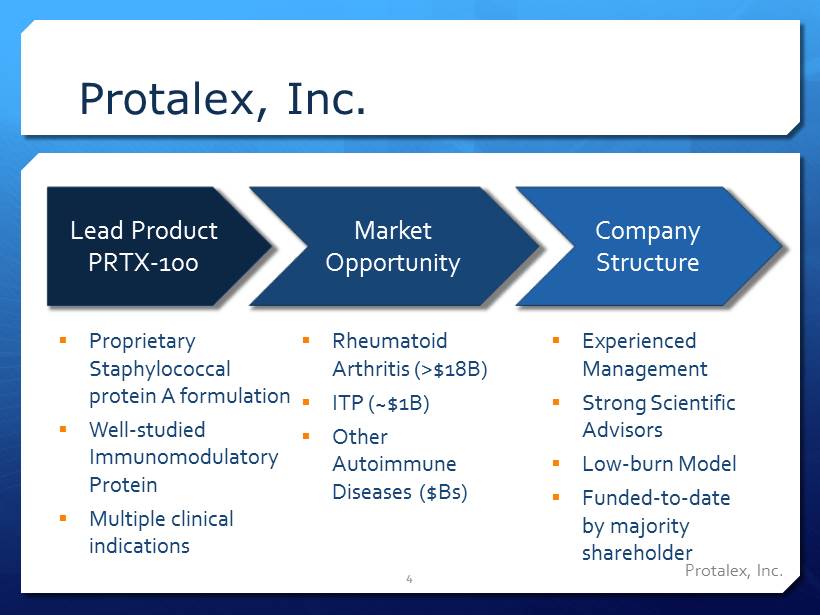
Protalex , Inc. Protalex , Inc. 4 Lead Product PRTX - 100 Market Opportunity Company Structure ▪ Rheumatoid Arthritis (>$18B) ▪ ITP (~$1B) ▪ Other Autoimmune Diseases ($Bs) ▪ Experienced Management ▪ Strong Scientific Advisors ▪ Low - burn Model ▪ Funded - to - date by majority shareholder ▪ Proprietary Staphylococcal protein A formulation ▪ Well - studied Immunomodulatory Protein ▪ Multiple clinical indications

Protalex , Inc. Protalex Investment Thesis PRTX - 100 is a novel immunomodulatory biological with potential to be a blockbuster drug in various autoimmune diseases ▪ ITP — an orphan disease ▪ Rheumatoid arthritis — the largest autoimmune market To date, 5 clinical studies conducted demonstrate that PRTX - 100 is safe and well - tolerated in humans Positive therapeutic effects seen in RA patients and in ITP preclinical models Potential efficacy in a number of orphan disease indications Validated manufacturing process; significantly lower cost of goods relative to other biologics Strong and growing IP position 5 Protalex , Inc.

Protalex , Inc. Animal Studies of PRTX - 100 PRTX - 100 reduces footpad swelling in the murine CIA model of arthritis PRTX - 100 inhibits platelet phagocytosis by human macrophages in vitro — model for ITP, and orphan disease PRTX - 100 is not immunosuppressive like anti - TNFs ▪ Pretreat w drug ▪ Challenge w Candida albicans ▪ Anti - TNF potentiates infection 6 0 20 40 60 80 100 -1 1 2 3 4 5 6 7 8 9 PERCENT SURVIVAL STUDY DAY Vehicle Etanercept PRTX 50 PRTX 250 anti-TNF

Protalex , Inc. Efficacy trends from two phase I clinical studies in RA patients — US and So Africa PRTX - 100 - treated patients showed greater response than placebo - treated patients in all common measures of disease activity, including: ▪ ACR20/50/70 (American College of Rheumatology “patient only” index) ▪ CDAI (Clinical Disease Activity Index, only clinical parameters) ▪ DAS28 - CRP (Disease Activity Score, clinical and blood parameters) In most recent trial, 43 % of PRTX - 100 treated patients achieved DAS28 - CRP < 3.2 (mean pretreatment DAS28 - CRP was 4.93 ) Other promising trends with categorical analyses Furthermore , the magnitude of the benefit compares favorably to published efficacy data for in - market RA biologicals with “black box” safety warnings 7 Protalex , Inc.
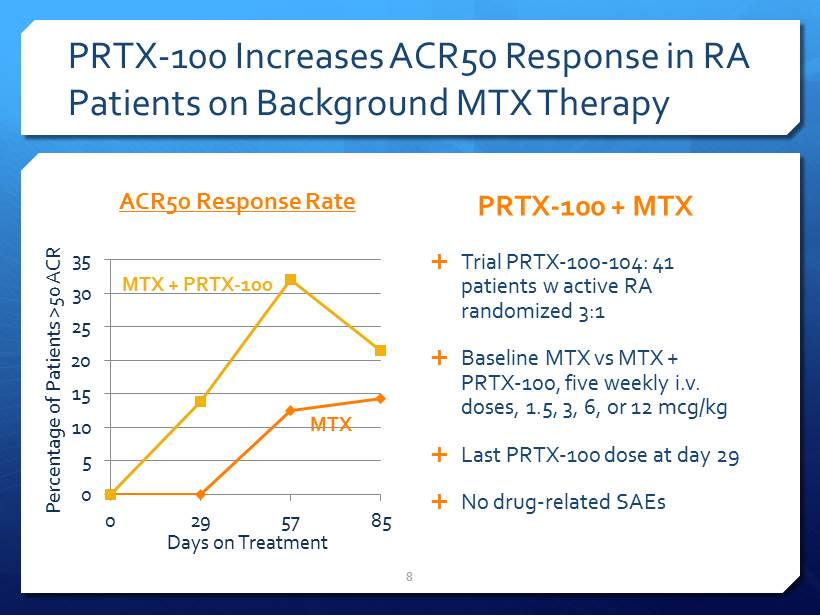
PRTX - 100 Increases ACR50 Response in RA Patients on Background MTX Therapy ACR50 Response Rate 0 5 10 15 20 25 30 35 0 29 57 85 PRTX - 100 + MTX Trial PRTX - 100 - 104: 41 patients w active RA randomized 3:1 Baseline MTX vs MTX + PRTX - 100, five weekly i.v . doses, 1.5, 3, 6, or 12 mcg/kg Last PRTX - 100 dose at day 29 No drug - related SAEs 8 Percentage of Patients >50 ACR Days on Treatment MTX MTX + PRTX - 100
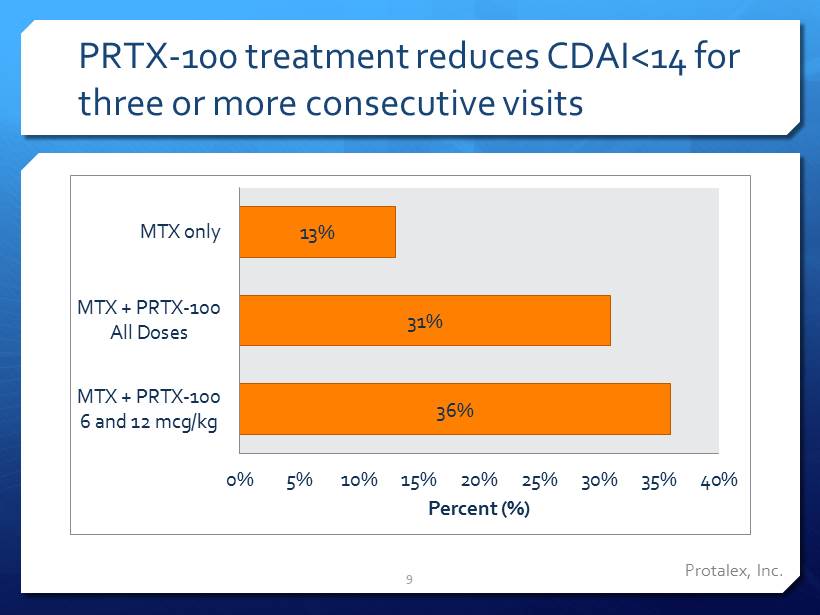
Protalex , Inc. PRTX - 100 treatment reduces CDAI<14 for three or more consecutive visits 36% 31% 13% 0% 5% 10% 15% 20% 25% 30% 35% 40% MTX + PRTX-100 6 and 12 mcg/kg MTX + PRTX-100 All Doses MTX only Percent (%) 9
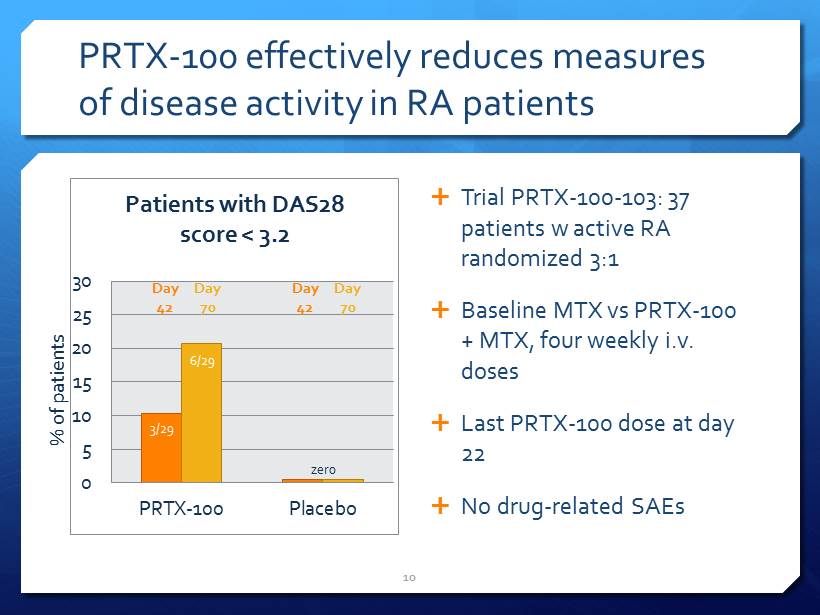
PRTX - 100 effectively reduces measures of disease activity in RA patients 0 5 10 15 20 25 30 PRTX-100 Placebo Patients with DAS28 score < 3.2 3/29 6/29 Trial PRTX - 100 - 103: 37 patients w active RA randomized 3:1 Baseline MTX vs PRTX - 100 + MTX, four weekly i.v . doses Last PRTX - 100 dose at day 22 No drug - related SAEs 10 % of patients Day 42 Day 70 Day 42 Day 70 zero
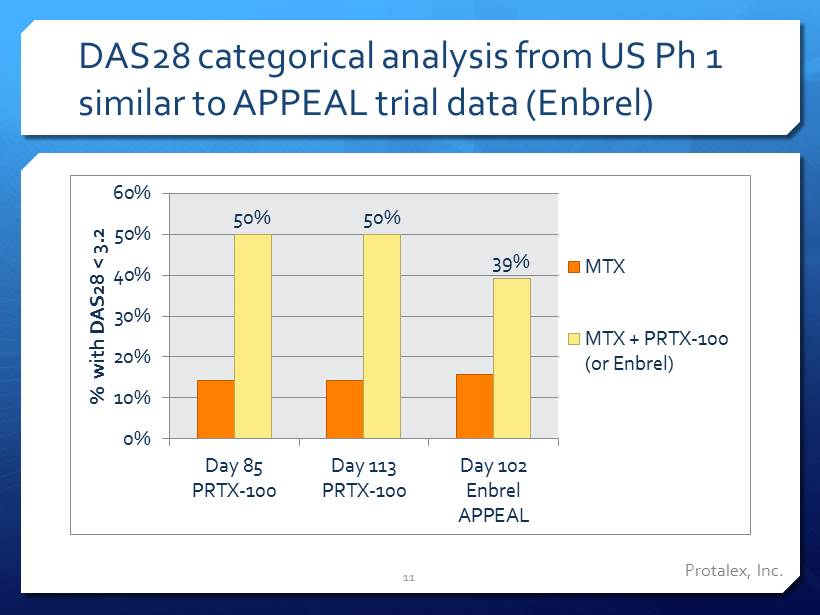
Protalex , Inc. DAS28 categorical analysis from US Ph 1 similar to APPEAL trial data ( Enbrel ) 50% 50% 39% 0% 10% 20% 30% 40% 50% 60% Day 85 PRTX-100 Day 113 PRTX-100 Day 102 Enbrel APPEAL % with DAS28 < 3.2 MTX MTX + PRTX-100 (or Enbrel) 11

PRTX - 100 Competitive Advantage Considerably lower cost production No immunosuppression / no “Black Box” Attractive safety profile allows for potential use in combination therapy Potentially applicable across a broad set of autoimmune diseases PRTX - 100 vs. current Biologicals 12 Protalex , Inc.

PRTX - 100 Development Plans 13 2012 2013 2014 2015 2016 2017 PRTX - 100 Rheumatoid Arthritis PRTX - 100 Immune Thrombocytopenia Phase 1 So. Africa PRTX - 100 - 103 Phase 1 in USA PRTX - 100 - 104 Phase 2 Phase 3 Explore Partnership Preclinical Phase 1/2 Phase 3 Protalex , Inc.
Protalex , Inc. Patents and Intellectual Property Patents (five issued in US and one in Japan) ▪ Initial US patent 7,211,258, “Protein A compositions and methods of use” filed 2002 and issued 2007 for RA, juvenile RA, and systemic lupus erythematosus ▪ Continuation patents expanding use were issued for: o ITP or autoimmune TP in 2008 o Acute inflammatory response or inflammation in 2012 o Psoriasis and scleroderma in 2012 o MS in 2013 ▪ Japanese patent issued with 2023 expiration date o April 2014 notice of allowance for psoriasis, scleroderma, Crohn’s Disease ▪ Additional patent applications pending in Europe, Canada, Japan, and US Other Intellectual Property ▪ Considerable know - how in the manufacture and QA of highly purified SPA expected to remain trade secret 14 Protalex , Inc.

Protalex , Inc. Protalex Key Team Members Arnold P Kling – President, Director; Principal of Niobe Ventures, LLC, experienced investor in and manager of early stage technology companies James W Dowe III – Vice - Chair of SAB; active investor in biotechnology, computer software and investment management companies William E. Gannon, MD – Chief Medical Officer; more than 20 years experience in clinical development and regulatory affairs at Quintiles, PPD, and other companies Bruce McClain, MD – Medical Director; more than 20 years experience in clinical development and product safety; senior roles at Aeras Global and MedImmune Richard Francovitch , Ph.D . -- VP of ITP Programs; 27 years pharma experience, former Head of Hematology Franchise and Global Commercial Leader for Promacta / Revolade at GSK Benjamin R Bowen, Ph.D . – Senior Advisor; background in pharma and biotech R&D at Genentech, Ciba - Geigy, and Novartis; ten years in investment banking Michelle Catalina, Ph.D . – Director of Preclinical Studies; academic research background in immunology, former instructor at U Mass Medical Center 15
Protalex , Inc. Protalex Milestones x 2Q13 Safety data from first three cohorts of PRTX - 100 - 104 x 3Q13 Initiation of cohort 5 extension study, to investigate monthly maintenance doses x 2Q14 Top - line results of PRTX - 100 - 104 trial □ 3Q14 Filing IND for PRTX - 100 in ITP □ 4Q14 Top - line results from cohort 5 extension study □ 4Q14 First dose in Phase 1/2 study of PRTX - 100 in ITP □ 1Q15 Submit end of study report from PRTX - 100 - 104 trial 16

Protalex , Inc. Protalex Investment Thesis Summary PRTX - 100 – potentially a blockbuster drug ▪ Multiple clinical indications in both large and niche markets (RA, ITP) ▪ Potentially applicable across a broad set of autoimmune diseases ▪ Considerable cost advantage over competitors ▪ Preliminary results demonstrate safety and proof - of - principle Market Opportunity ▪ RA = $18 Billion annual market size ▪ ITP = $1 B market ▪ Future expansion into other disease areas Company Structure ▪ Experienced Management and Advisory Teams with “skin in the game” ▪ Committed support from expert Scientific Advisory Board ▪ Established IP protection and trade secrets 17

September, 2014

















