UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-Q
(Mark One)
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the Quarterly Period Ended June 30, 2023
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||||
For the transition period from to
Commission file number 1-15525
EDWARDS LIFESCIENCES CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 36-4316614 | |||||||||||||
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |||||||||||||
One Edwards Way
Irvine, California 92614
(Address of principal executive offices and zip code)
(949) 250-2500
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
| Common Stock, par value $1.00 per share | EW | New York Stock Exchange | ||||||
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company," and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☒ | Accelerated filer | ☐ | Non-accelerated filer | ☐ | Smaller reporting company | ☐ | Emerging growth company | ☐ | ||||||||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The number of shares outstanding of the registrant's common stock, $1.00 par value, as of July 25, 2023 was 607,916,300.
EDWARDS LIFESCIENCES CORPORATION
FORM 10-Q
For the quarterly period ended June 30, 2023
TABLE OF CONTENTS
Page Number | ||||||||
| Item 6. | ||||||||
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. We intend the forward-looking statements contained in this report to be covered by the safe harbor provisions of such Acts. Statements other than statements of historical or current fact in this report or referred to or incorporated by reference into this report are "forward-looking statements" for purposes of these sections. These statements include, among other things, the expected impact of COVID-19 on our business, the expected impact of macroeconomic conditions on our business, any predictions, opinions, expectations, plans, strategies, objectives and any statements of assumptions underlying any of the foregoing relating to our current and future business and operations, including, but not limited to, financial matters, development activities, clinical trials and regulatory matters, manufacturing and supply operations, and product sales and demand. These statements can sometimes be identified by the use of the forward-looking words such as "may," "believe," "will," "expect," "project," "estimate," "should," "anticipate," "plan," "goal," "continue," "seek," "pro forma," "forecast," "intend," "guidance," "optimistic," "aspire," "confident," other forms of these words or similar words or expressions or the negative thereof. Statements regarding past performance, efforts, or results about which inferences or assumptions may be made can also be forward-looking statements and are not indicative of future performance or results; these statements can be identified by the use of words such as "preliminary," "initial," diligence," "industry-leading," "compliant," "indications," or "early feedback" or other forms of these words or similar words or expressions or the negative thereof. These forward-looking statements are subject to substantial risks and uncertainties that could cause our results or future business, financial condition, results of operations or performance to differ materially from our historical results or experiences or those expressed or implied in any forward-looking statements contained in this report. These risks and uncertainties include, but are not limited to: our success in developing new products and avoiding manufacturing and quality issues; clinical trial or commercial results or new product approvals and therapy adoption; the impact of public health crises, including the COVID-19 pandemic; the impact of domestic and global conditions; competitive dynamics in the markets in which we operate; our reliance on vendors, suppliers, and other third parties; damage, failure or interruption of our information technology systems; consolidation in the healthcare industry; our ability to protect our intellectual property; our compliance with applicable regulations; our exposure to product liability claims; use of our products in unapproved circumstances; changes to reimbursement for our products; the impact of currency exchange rates; unanticipated actions by the United States Food and Drug Administration and other regulatory agencies; changes to tax laws; unexpected impacts or expenses of litigation or internal or government investigations; and other risks detailed under “Risk Factors” in our annual report on Form 10-K for the year ended December 31, 2022, as such risks and uncertainties may be amended, supplemented or superseded from time to time by our subsequent reports on Forms 10-Q and 8-K we file with the United States Securities and Exchange Commission. These forward-looking statements speak only as of the date on which they are made and we do not undertake any obligation to update any forward-looking statement to reflect events or circumstances after the date of the statement. If we do update or correct one or more of these statements, investors and others should not conclude that we will make additional updates or corrections.
Unless otherwise indicated or otherwise required by the context, the terms "we," "our," "it," "its," "Company," "Edwards," and "Edwards Lifesciences" refer to Edwards Lifesciences Corporation and its subsidiaries.
Part I. Financial Information
Item 1. Financial Statements
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED CONDENSED BALANCE SHEETS
(in millions, except par value; unaudited)
| June 30, 2023 | December 31, 2022 | ||||||||||
| ASSETS | |||||||||||
| Current assets | |||||||||||
| Cash and cash equivalents | $ | 1,042.6 | $ | 769.0 | |||||||
| Short-term investments (Note 4) | 466.7 | 446.3 | |||||||||
| Accounts receivable, net of allowances of $8.1 and $7.9, respectively | 754.4 | 643.0 | |||||||||
| Other receivables | 62.5 | 56.1 | |||||||||
| Inventories (Note 2) | 980.2 | 875.5 | |||||||||
| Prepaid expenses | 123.2 | 110.0 | |||||||||
| Other current assets | 217.4 | 195.9 | |||||||||
| Total current assets | 3,647.0 | 3,095.8 | |||||||||
| Long-term investments (Note 4) | 856.2 | 1,239.0 | |||||||||
| Property, plant, and equipment, net | 1,662.2 | 1,632.8 | |||||||||
| Operating lease right-of-use assets | 84.9 | 92.3 | |||||||||
| Goodwill | 1,299.5 | 1,164.3 | |||||||||
| Other intangible assets, net (Note 6) | 431.4 | 285.2 | |||||||||
| Deferred income taxes | 610.7 | 484.0 | |||||||||
| Other assets (Note 5) | 412.1 | 299.1 | |||||||||
| Total assets | $ | 9,004.0 | $ | 8,292.5 | |||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||||||
| Current liabilities | |||||||||||
| Accounts payable | $ | 193.0 | $ | 201.9 | |||||||
| Accrued and other liabilities (Note 2) | 939.9 | 795.0 | |||||||||
| Operating lease liabilities | 24.3 | 25.5 | |||||||||
| Total current liabilities | 1,157.2 | 1,022.4 | |||||||||
| Long-term debt | 596.7 | 596.3 | |||||||||
| Contingent consideration liabilities (Note 7) | — | 26.2 | |||||||||
| Taxes payable | 81.2 | 143.4 | |||||||||
| Operating lease liabilities | 63.5 | 69.5 | |||||||||
| Uncertain tax positions | 303.7 | 267.5 | |||||||||
| Litigation settlement accrual (Note 2) | 112.5 | 143.0 | |||||||||
| Other liabilities | 242.7 | 217.5 | |||||||||
| Total liabilities | 2,557.5 | 2,485.8 | |||||||||
| Commitments and contingencies (Note 11) | |||||||||||
| Stockholders' equity | |||||||||||
| Preferred stock, $0.01 par value, authorized 50.0 shares, no shares outstanding | — | — | |||||||||
| Common stock, $1.00 par value, 1,050.0 shares authorized, 649.1 and 646.3 shares issued, and 607.9 and 608.3 shares outstanding, respectively | 649.1 | 646.3 | |||||||||
| Additional paid-in capital | 2,145.5 | 1,969.3 | |||||||||
| Retained earnings | 8,237.6 | 7,590.0 | |||||||||
| Accumulated other comprehensive loss (Note 12) | (255.5) | (254.9) | |||||||||
| Treasury stock, at cost, 41.2 and 38.0 shares, respectively | (4,401.0) | (4,144.0) | |||||||||
| Total Edwards Lifesciences Corporation stockholders' equity | 6,375.7 | 5,806.7 | |||||||||
| Noncontrolling interest (Note 6) | 70.8 | — | |||||||||
| Total stockholders' equity | 6,446.5 | 5,806.7 | |||||||||
| Total liabilities and equity | $ | 9,004.0 | $ | 8,292.5 | |||||||
The accompanying notes are an integral part of these
consolidated condensed financial statements.
1
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED CONDENSED STATEMENTS OF OPERATIONS
(in millions, except per share information; unaudited)
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Net sales | $ | 1,530.2 | $ | 1,373.9 | $ | 2,989.8 | $ | 2,715.1 | |||||||||||||||
| Cost of sales | 343.0 | 269.4 | 672.5 | 568.7 | |||||||||||||||||||
| Gross profit | 1,187.2 | 1,104.5 | 2,317.3 | 2,146.4 | |||||||||||||||||||
| Selling, general, and administrative expenses | 468.7 | 409.0 | 905.0 | 779.3 | |||||||||||||||||||
| Research and development expenses | 270.3 | 250.8 | 531.5 | 479.4 | |||||||||||||||||||
| Intellectual property agreement and litigation expense (Note 3) | 147.9 | 6.1 | 191.4 | 13.2 | |||||||||||||||||||
| Change in fair value of contingent consideration liabilities (Note 7) | (26.9) | (20.9) | (26.2) | (23.8) | |||||||||||||||||||
| Operating income, net | 327.2 | 459.5 | 715.6 | 898.3 | |||||||||||||||||||
| Interest income, net | (9.1) | (0.9) | (17.7) | (1.5) | |||||||||||||||||||
| Other income, net | (2.2) | (4.3) | (3.8) | (1.0) | |||||||||||||||||||
| Income before provision for income taxes | 338.5 | 464.7 | 737.1 | 900.8 | |||||||||||||||||||
| Provision for income taxes | 33.0 | 58.3 | 91.1 | 120.8 | |||||||||||||||||||
| Net income | 305.5 | 406.4 | 646.0 | 780.0 | |||||||||||||||||||
| Net loss attributable to noncontrolling interest (Note 6) | (1.6) | — | (1.6) | — | |||||||||||||||||||
| Net income attributable to Edwards Lifesciences Corporation | $ | 307.1 | $ | 406.4 | $ | 647.6 | $ | 780.0 | |||||||||||||||
Share information (Note 13) | |||||||||||||||||||||||
| Earnings per share: | |||||||||||||||||||||||
| Basic | $ | 0.51 | $ | 0.65 | $ | 1.07 | $ | 1.26 | |||||||||||||||
| Diluted | $ | 0.50 | $ | 0.65 | $ | 1.06 | $ | 1.24 | |||||||||||||||
| Weighted-average number of common shares outstanding: | |||||||||||||||||||||||
| Basic | 606.9 | 620.9 | 607.2 | 621.5 | |||||||||||||||||||
| Diluted | 610.3 | 626.7 | 610.6 | 628.1 | |||||||||||||||||||
The accompanying notes are an integral part of these
consolidated condensed financial statements.
2
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED CONDENSED STATEMENTS OF COMPREHENSIVE INCOME
(in millions; unaudited)
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Net income | $ | 305.5 | $ | 406.4 | $ | 646.0 | $ | 780.0 | |||||||||||||||
| Other comprehensive loss, net of tax (Note 12): | |||||||||||||||||||||||
| Foreign currency translation adjustments | (11.5) | (43.2) | (7.7) | (53.2) | |||||||||||||||||||
| Unrealized gain (loss) on hedges | 5.3 | 31.3 | (11.9) | 44.7 | |||||||||||||||||||
| Unrealized pension credits (costs) | 0.2 | (0.1) | 0.1 | (0.1) | |||||||||||||||||||
| Unrealized gain (loss) on available-for-sale investments | 2.1 | (30.9) | 11.1 | (68.0) | |||||||||||||||||||
| Reclassification of realized investment losses to earnings | 3.8 | 5.1 | 7.8 | 9.9 | |||||||||||||||||||
| Other comprehensive loss | (0.1) | (37.8) | (0.6) | (66.7) | |||||||||||||||||||
| Comprehensive income | 305.4 | 368.6 | 645.4 | 713.3 | |||||||||||||||||||
| Comprehensive loss attributable to noncontrolling interest | (1.6) | — | (1.6) | — | |||||||||||||||||||
| Comprehensive income attributable to Edwards Lifesciences Corporation | $ | 307.0 | $ | 368.6 | $ | 647.0 | $ | 713.3 | |||||||||||||||
The accompanying notes are an integral part of these
consolidated condensed financial statements.
3
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED CONDENSED STATEMENTS OF CASH FLOWS
(in millions; unaudited)
| Six Months Ended June 30, | |||||||||||
| 2023 | 2022 | ||||||||||
| Cash flows from operating activities | |||||||||||
| Net income | $ | 646.0 | $ | 780.0 | |||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | |||||||||||
| Depreciation and amortization | 71.3 | 68.8 | |||||||||
| Non-cash operating lease cost | 13.7 | 13.9 | |||||||||
| Stock-based compensation (Note 9) | 76.3 | 68.6 | |||||||||
| Change in fair value of contingent consideration liabilities (Note 7) | (26.2) | (23.8) | |||||||||
| Loss (gain) on investments, net | (3.5) | 38.2 | |||||||||
| Deferred income taxes | (134.7) | (102.4) | |||||||||
| Other | 2.8 | 4.2 | |||||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts and other receivables, net | (124.3) | (77.7) | |||||||||
| Inventories | (113.7) | (85.8) | |||||||||
| Accounts payable and accrued liabilities | 76.2 | (81.7) | |||||||||
| Income taxes | 31.0 | 49.7 | |||||||||
| Prepaid expenses and other current assets | (43.4) | 25.7 | |||||||||
| Intellectual property agreement accrual | (14.5) | (19.7) | |||||||||
| Long-term prepaid royalties (Note 3) | (114.0) | — | |||||||||
| Other | 4.7 | (32.5) | |||||||||
| Net cash provided by operating activities | 347.7 | 625.5 | |||||||||
| Cash flows from investing activities | |||||||||||
| Capital expenditures | (109.4) | (115.8) | |||||||||
| Purchases of held-to-maturity investments (Note 4) | (15.5) | (180.7) | |||||||||
| Proceeds from held-to-maturity investments (Note 4) | 83.5 | 277.5 | |||||||||
| Purchases of available-for-sale investments (Note 4) | (6.8) | (114.3) | |||||||||
| Proceeds from available-for-sale investments (Note 4) | 314.5 | 584.1 | |||||||||
| Business combination, net of cash (Note 6) | (141.2) | — | |||||||||
| Payment for acquisition options (Note 5) | (15.0) | (55.5) | |||||||||
| Issuances of notes receivable | (22.5) | (45.5) | |||||||||
| Collections of notes receivable | — | 18.0 | |||||||||
| Other | (16.9) | (8.7) | |||||||||
| Net cash provided by investing activities | 70.7 | 359.1 | |||||||||
| Cash flows from financing activities | |||||||||||
| Purchases of treasury stock | (256.8) | (760.7) | |||||||||
| Proceeds from stock plans | 102.7 | 86.5 | |||||||||
| Other | (0.4) | (2.0) | |||||||||
| Net cash used in financing activities | (154.5) | (676.2) | |||||||||
| Effect of currency exchange rate changes on cash, cash equivalents, and restricted cash | 11.0 | 27.1 | |||||||||
| Net increase in cash, cash equivalents, and restricted cash | 274.9 | 335.5 | |||||||||
| Cash, cash equivalents, and restricted cash at beginning of period | 772.6 | 867.4 | |||||||||
| Cash, cash equivalents, and restricted cash at end of period | $ | 1,047.5 | $ | 1,202.9 | |||||||
The accompanying notes are an integral part of these
consolidated condensed financial statements.
4
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED CONDENSED STATEMENTS OF STOCKHOLDERS' EQUITY
(in millions; unaudited)
| Common Stock | Treasury Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Par Value | Shares | Amount | Additional Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Loss | Total Edwards Lifesciences Corporation Stockholders' Equity | Noncontrolling Interest | Total Stockholders' Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2022 | 646.3 | $ | 646.3 | 38.0 | $ | (4,144.0) | $ | 1,969.3 | $ | 7,590.0 | $ | (254.9) | $ | 5,806.7 | $ | — | $ | 5,806.7 | |||||||||||||||||||||||||||||||||||||||||
| Net income | 340.5 | 340.5 | — | 340.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | (0.5) | (0.5) | (0.5) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock issued under stock plans | 0.8 | 0.8 | 41.1 | 41.9 | 41.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 38.9 | 38.9 | 38.9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purchases of treasury stock | 3.1 | (249.5) | (249.5) | (249.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Changes to noncontrolling interest | — | 84.0 | 84.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at March 31, 2023 | 647.1 | 647.1 | 41.1 | (4,393.5) | 2,049.3 | 7,930.5 | (255.4) | 5,978.0 | 84.0 | 6,062.0 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Net income | 307.1 | 307.1 | (1.6) | 305.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | (0.1) | (0.1) | (0.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock issued under equity plans | 2.0 | 2.0 | 58.8 | 60.8 | 60.8 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 37.4 | 37.4 | 37.4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purchases of treasury stock | 0.1 | (7.5) | (7.5) | (7.5) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Changes to noncontrolling interest | — | (11.6) | (11.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at June 30, 2023 | 649.1 | $ | 649.1 | 41.2 | $ | (4,401.0) | $ | 2,145.5 | $ | 8,237.6 | $ | (255.5) | $ | 6,375.7 | $ | 70.8 | $ | 6,446.5 | |||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these
consolidated condensed financial statements.
5
EDWARDS LIFESCIENCES CORPORATION
CONSOLIDATED CONDENSED STATEMENTS OF STOCKHOLDERS' EQUITY
(in millions; unaudited)
| Common Stock | Treasury Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Par Value | Shares | Amount | Additional Paid-in Capital | Retained Earnings | Accumulated Other Comprehensive Loss | Total Stockholders' Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | 642.0 | $ | 642.0 | 17.9 | $ | (2,416.9) | $ | 1,700.4 | $ | 6,068.1 | $ | (157.7) | $ | 5,835.9 | |||||||||||||||||||||||||||||||||||||||||||||
| Net income | 373.6 | 373.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | (28.9) | (28.9) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock issued under stock plans | 0.9 | 0.9 | 36.6 | 37.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 32.4 | 32.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purchases of treasury stock | 3.6 | (405.6) | (405.6) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at March 31, 2022 | 642.9 | 642.9 | 21.5 | (2,822.5) | 1,769.4 | 6,441.7 | (186.6) | 5,844.9 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Net income | 406.4 | 406.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other comprehensive income, net of tax | (37.8) | (37.8) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Common stock issued under equity plans | 2.1 | 2.1 | 46.9 | 49.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation expense | 36.2 | 36.2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Purchases of treasury stock | 3.7 | (355.1) | (355.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Balance at June 30, 2022 | 645.0 | $ | 645.0 | 25.2 | $ | (3,177.6) | $ | 1,852.5 | $ | 6,848.1 | $ | (224.4) | $ | 5,943.6 | |||||||||||||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these
consolidated condensed financial statements.
6
1. BASIS OF PRESENTATION
The accompanying interim consolidated condensed financial statements and related disclosures have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission ("SEC") and should be read in conjunction with the consolidated financial statements and notes included in Edwards Lifesciences' Annual Report on Form 10-K for the year ended December 31, 2022. Certain information and footnote disclosures normally included in financial statements prepared in accordance with generally accepted accounting principles in the United States of America ("GAAP") have been condensed or omitted.
The consolidated condensed financial statements include the accounts of all wholly-owned subsidiaries and variable interest entities for which the Company is the primary beneficiary. The Company attributes the net income or losses of its consolidated variable interest entities to controlling and noncontrolling interests using the hypothetical liquidation at book value method. All intercompany accounts and transactions have been eliminated in consolidation.
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements. Actual results could differ from those estimates.
In the opinion of management, the interim consolidated condensed financial statements reflect all adjustments necessary for a fair statement of the results for the interim periods presented. All such adjustments, unless otherwise noted herein, are of a normal, recurring nature. The results of operations for the interim periods are not necessarily indicative of the results of operations to be expected for the full year.
There have been no material changes to the Company's significant accounting policies from those described in the Company's Annual Report on Form 10-K for the year ended December 31, 2022.
New Accounting Standards Not Yet Adopted
In March 2023, the Financial Accounting Standards Board ("FASB") issued an amendment to the accounting guidance on investments in tax credit structures to allow entities to elect to account for their tax equity investments, regardless of the tax credit program from which the income tax credits are received, using the proportional amortization method if certain conditions are met. The guidance is effective for fiscal years beginning after December 15, 2023, including interim periods within those fiscal years. The Company does not expect that the adoption of this guidance will have a material impact on its consolidated financial results.
2. OTHER CONSOLIDATED FINANCIAL STATEMENT DETAILS
Composition of Certain Financial Statement Captions
(in millions)
Components of selected captions in the consolidated condensed balance sheets consisted of the following:
| June 30, 2023 | December 31, 2022 | ||||||||||
| Inventories | |||||||||||
| Raw materials | $ | 181.2 | $ | 156.4 | |||||||
| Work in process | 223.0 | 177.4 | |||||||||
| Finished products | 576.0 | 541.7 | |||||||||
| $ | 980.2 | $ | 875.5 | ||||||||
At June 30, 2023 and December 31, 2022, $149.7 million and $128.6 million, respectively, of the Company's finished products inventories were held on consignment.
7
| June 30, 2023 | December 31, 2022 | ||||||||||
| Accrued and other liabilities | |||||||||||
| Employee compensation and withholdings | $ | 312.7 | $ | 268.7 | |||||||
| Taxes payable | 106.6 | 50.6 | |||||||||
| Property, payroll, and other taxes | 56.1 | 45.6 | |||||||||
| Research and development accruals | 70.7 | 66.9 | |||||||||
| Accrued rebates | 130.1 | 116.1 | |||||||||
| Fair value of derivatives | 17.8 | 20.7 | |||||||||
| Accrued marketing expenses | 16.9 | 17.0 | |||||||||
| Legal and insurance | 30.4 | 28.1 | |||||||||
Litigation settlement (a) | 69.2 | 53.3 | |||||||||
| Accrued relocation costs | 23.3 | 25.2 | |||||||||
| Accrued professional services | 10.2 | 6.6 | |||||||||
| Accrued realignment reserves | 14.4 | 15.6 | |||||||||
| Other accrued liabilities | 81.5 | 80.6 | |||||||||
| $ | 939.9 | $ | 795.0 | ||||||||
_______________________________________
(a) As of June 30, 2023, $69.2 million was accrued in "Accrued and Other Liabilities" and $112.5 million was accrued in "Litigation Settlement Accrual" on the consolidated condensed balance sheet related to a settlement agreement with Abbott Laboratories and its direct and indirect subsidiaries.
Supplemental Cash Flow Information
(in millions)
| Six Months Ended June 30, | |||||||||||
| 2023 | 2022 | ||||||||||
| Cash paid during the year for: | |||||||||||
| Income taxes | $ | 193.5 | $ | 173.0 | |||||||
| Amounts included in the measurement of lease liabilities: | |||||||||||
| Operating cash flows from operating leases | $ | 14.3 | $ | 14.5 | |||||||
| Non-cash investing and financing transactions: | |||||||||||
| Right-of-use assets obtained in exchange for new lease liabilities | $ | 6.0 | $ | 10.6 | |||||||
| Capital expenditures accruals | $ | 26.9 | $ | 24.6 | |||||||
Cash, Cash Equivalents, and Restricted Cash
(in millions)
| June 30, 2023 | December 31, 2022 | |||||||||||||
| Cash and cash equivalents | $ | 1,042.6 | $ | 769.0 | ||||||||||
| Restricted cash included in other current assets | 1.4 | 0.5 | ||||||||||||
| Restricted cash included in other assets | 3.5 | 3.1 | ||||||||||||
| Total cash, cash equivalents, and restricted cash | $ | 1,047.5 | $ | 772.6 | ||||||||||
Amounts included in restricted cash primarily represent funds placed in escrow related to litigation.
3. INTELLECTUAL PROPERTY AGREEMENT AND LITIGATION EXPENSE
On April 12, 2023, Edwards entered into an Intellectual Property Agreement (the "Intellectual Property Agreement") with Medtronic, Inc. ("Medtronic") pursuant to which the parties agreed to a 15-year global covenant not to sue ("CNS") for infringement of certain patents in the structural heart space owned or controlled by each other. In consideration for the global CNS and related mutual access to certain intellectual property rights, Edwards paid to Medtronic a one-time, lump sum payment of $300.0 million and will pay annual royalty payments that are tied to net sales of certain Edwards products. Based upon the terms of the Intellectual Property Agreement, the Company identified the relevant elements for accounting purposes and allocated the $300.0 million upfront payment based on their respective fair values. The Company recorded a $37.0 million
8
pre-tax charge in "Intellectual Property Agreement and Litigation Expense" in March 2023 related primarily to prior commercial sales incurred through March 31, 2023. The Company recorded a prepaid royalty asset of $124.0 million in April 2023 related to future commercial sales, which will be amortized to expense during the term of the Intellectual Property Agreement. Separately, the Company recorded a $139.0 million pre-tax charge in "Intellectual Property Agreement and Litigation Expense" in April 2023 related to products currently in development.
4. INVESTMENTS
Debt Securities
Investments in debt securities at the end of each period were as follows (in millions):
| June 30, 2023 | December 31, 2022 | ||||||||||||||||||||||||||||||||||||||||||||||
| Held-to-maturity | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | |||||||||||||||||||||||||||||||||||||||
| Bank time deposits | $ | 28.1 | $ | — | $ | — | $ | 28.1 | $ | 96.0 | $ | — | $ | — | $ | 96.0 | |||||||||||||||||||||||||||||||
| Available-for-sale | |||||||||||||||||||||||||||||||||||||||||||||||
| U.S. government and agency securities | 103.4 | — | (5.0) | 98.4 | 137.7 | — | (6.1) | 131.6 | |||||||||||||||||||||||||||||||||||||||
| Asset-backed securities | 262.5 | — | (10.0) | 252.5 | 380.6 | — | (14.0) | 366.6 | |||||||||||||||||||||||||||||||||||||||
| Corporate debt securities | 864.9 | — | (34.0) | 830.9 | 1,028.1 | — | (47.8) | 980.3 | |||||||||||||||||||||||||||||||||||||||
| Municipal securities | 2.8 | — | (0.2) | 2.6 | 2.7 | — | (0.2) | 2.5 | |||||||||||||||||||||||||||||||||||||||
| Total | $ | 1,233.6 | $ | — | $ | (49.2) | $ | 1,184.4 | $ | 1,549.1 | $ | — | $ | (68.1) | $ | 1,481.0 | |||||||||||||||||||||||||||||||
The cost and fair value of investments in debt securities, by contractual maturity, as of June 30, 2023, were as follows:
| Held-to-Maturity | Available-for-Sale | ||||||||||||||||||||||
| Amortized Cost | Fair Value | Amortized Cost | Fair Value | ||||||||||||||||||||
| (in millions) | |||||||||||||||||||||||
| Due in 1 year or less | $ | 26.3 | $ | 26.3 | $ | 450.9 | $ | 440.4 | |||||||||||||||
| Due after 1 year through 5 years | 1.8 | 1.8 | 472.0 | 446.7 | |||||||||||||||||||
| Due after 5 years through 10 years | — | — | 4.6 | 4.4 | |||||||||||||||||||
Instruments not due at a single maturity date (a) | — | — | 306.1 | 292.9 | |||||||||||||||||||
| $ | 28.1 | $ | 28.1 | $ | 1,233.6 | $ | 1,184.4 | ||||||||||||||||
_______________________________________
(a) Consists of mortgage- and asset-backed securities.
Actual maturities may differ from the contractual maturities due to call or prepayment rights.
The following tables present gross unrealized losses and fair values for those investments that were in an unrealized loss position as of June 30, 2023 and December 31, 2022, aggregated by investment category and the length of time that individual securities have been in a continuous loss position (in millions):
| June 30, 2023 | |||||||||||||||||||||||||||||||||||
| Less than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||||||||||||||
| Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | ||||||||||||||||||||||||||||||
| U.S. government and agency securities | $ | 10.9 | $ | (0.1) | $ | 86.2 | $ | (4.9) | $ | 97.1 | $ | (5.0) | |||||||||||||||||||||||
| Asset-backed securities | 50.9 | (0.8) | 199.9 | (9.2) | 250.8 | (10.0) | |||||||||||||||||||||||||||||
| Corporate debt securities | 23.3 | (0.1) | 793.3 | (33.9) | 816.6 | (34.0) | |||||||||||||||||||||||||||||
| Municipal securities | — | — | 2.6 | (0.2) | 2.6 | (0.2) | |||||||||||||||||||||||||||||
| $ | 85.1 | $ | (1.0) | $ | 1,082.0 | $ | (48.2) | $ | 1,167.1 | $ | (49.2) | ||||||||||||||||||||||||
9
| December 31, 2022 | |||||||||||||||||||||||||||||||||||
| Less than 12 Months | 12 Months or Greater | Total | |||||||||||||||||||||||||||||||||
| Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | Fair Value | Gross Unrealized Losses | ||||||||||||||||||||||||||||||
| U.S. government and agency securities | $ | 61.6 | $ | (1.5) | $ | 69.5 | $ | (4.6) | $ | 131.1 | $ | (6.1) | |||||||||||||||||||||||
| Asset-backed securities | 103.3 | (1.3) | 254.6 | (12.7) | 357.9 | (14.0) | |||||||||||||||||||||||||||||
| Corporate debt securities | 189.0 | (5.3) | 784.8 | (42.5) | 973.8 | (47.8) | |||||||||||||||||||||||||||||
| Municipal securities | — | — | 2.5 | (0.2) | 2.5 | (0.2) | |||||||||||||||||||||||||||||
| $ | 353.9 | $ | (8.1) | $ | 1,111.4 | $ | (60.0) | $ | 1,465.3 | $ | (68.1) | ||||||||||||||||||||||||
The Company reviews its investments in debt securities to determine if there has been an other-than-temporary decline in fair value. Consideration is given to 1) the financial condition and near-term prospects of the issuer, including the credit quality of the security's issuer, 2) the Company's intent to sell the security, and 3) whether it is more likely than not the Company will have to sell the security before recovery of its amortized cost. The decline in fair value of the debt securities was largely due to changes in interest rates, not credit quality, and as of June 30, 2023, the Company did not intend to sell the securities, and it was not more likely than not that it will be required to sell the securities before recovery of the unrealized losses, and, therefore, the unrealized losses are considered temporary.
Investments in Unconsolidated Entities
The Company has a number of equity investments in unconsolidated entities. These investments are recorded in "Long-term Investments" on the consolidated condensed balance sheets, and are as follows:
| June 30, 2023 | December 31, 2022 | ||||||||||
| (in millions) | |||||||||||
| Equity method investments | |||||||||||
| Carrying value of equity method investments | $ | 23.5 | $ | 21.4 | |||||||
| Equity securities | |||||||||||
| Carrying value of non-marketable equity securities | 86.9 | 86.9 | |||||||||
| Total investments in unconsolidated entities | $ | 110.4 | $ | 108.3 | |||||||
During the six months ended June 30, 2023, the Company made $1.7 million of equity investments in limited liability companies that invest in qualified community development entities ("CDEs") through the New Markets Tax Credit ("NMTC") program. The NMTC program provides federal tax incentives to investors to make investments in distressed communities and promotes economic improvements through the development of successful businesses in these communities. The NMTC is equal to 39% of the qualified investment and is taken over seven years. These limited liability companies are variable interest entities ("VIEs"). The Company determined that it is not the primary beneficiary of the VIEs because it does not have the power to direct the activities that most significantly impact the economic performance of the VIEs and, therefore, the Company does not consolidate these entities. Instead, the NMTC investments are accounted for as equity method investments.
Non-marketable equity securities consist of investments in privately held companies without readily determinable fair values, and are reported at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or similar investment of the same issuer. As of June 30, 2023, the Company had recorded cumulative upward adjustments of $8.8 million based on observable price changes, and cumulative downward adjustments of $3.1 million due to impairments and observable price changes.
During the three and six months ended June 30, 2023, the gross realized gains or losses from sales of available-for-sale investments were not material.
5. INVESTMENTS IN VARIABLE INTEREST ENTITIES
The Company reviews its investments in other entities to determine whether the Company is the primary beneficiary of a variable interest entity ("VIE"). The Company would be the primary beneficiary of the VIE, and would be required to
10
consolidate the VIE, if it has the power to direct the significant activities of the entity and the obligation to absorb losses or receive benefits from the entity that may be significant to the VIE. The Company's maximum loss exposure to variable interest entities, prior to the exercise of options to acquire the entities, is limited to its investment in the variable interest entities, which include equity investments, options to acquire, and promissory notes.
Consolidated VIEs
In February 2023, the Company acquired a majority equity interest in a medical technology company pursuant to a preferred stock purchase agreement, and amended and restated a previous option agreement to acquire the remaining equity interest. Edwards concluded that it is the primary beneficiary and consolidated the VIE. See Note 6 for additional information.
Unconsolidated VIEs
Edwards has relationships with various VIEs that it does not consolidate as Edwards lacks the power to direct the activities that significantly impact the economic success of these entities.
In March 2023, the Company agreed to pay a medical device company up to $45.0 million as consideration for an option to acquire the medical device company, of which $15.0 million has been paid as of June 30, 2023. Also, in March 2023, Edwards advanced $5.0 million to the medical device company under a convertible promissory note. The option and the note are included in "Other Assets" on the consolidated condensed balance sheet.
In January 2023, the Company loaned a privately-held medical device company (the "Investee") $10.0 million under a $45.0 million secured promissory note agreement. In April 2023, the Investee drew an additional $7.5 million under the promissory note. Previously, in 2021, the Company invested $39.3 million, included in "Long-term Investments," in the Investee's preferred equity securities and paid $13.1 million, included in "Other Assets," for an option to acquire the Investee. Per the agreement, the Company may be required to invest up to an additional $6.5 million in the Investee's preferred equity securities and up to an additional $14.4 million for the option to acquire the Investee.
In August 2022, the Company entered into an option agreement with a medical device company. Under the option agreement, Edwards paid $47.1 million for an option to acquire the medical device company. The $47.1 million option is included in "Other Assets" on the consolidated condensed balance sheet.
In June 2022, the Company entered into a convertible promissory note and amended its existing warrant agreement with a medical device company. Under the convertible promissory note agreement, the Company agreed to loan the medical device company up to $47.5 million, of which $32.5 million has been advanced as of June 30, 2023. In addition, in 2019 the Company paid $35.0 million for an option to acquire the medical device company. The $35.0 million option and the $32.5 million note receivable are included in "Other Assets" on the consolidated condensed balance sheet.
In May 2022, the Company entered into an option agreement with a medical technology company. Under the option agreement, Edwards paid $60.0 million for an option to acquire the medical technology company, of which $10.0 million was paid in 2021. The $60.0 million option is included in "Other Assets" on the consolidated condensed balance sheet.
In addition, Edwards has made equity investments through the NMTC program in limited liability companies that are considered VIEs. For more information, see Note 4.
6. BUSINESS COMBINATION
On February 28, 2023, the Company acquired 61% of the then outstanding shares of a medical technology company in an all cash transaction. The Company determined it was the primary beneficiary of this VIE, and the VIE has been consolidated in the Company's consolidated condensed financial statements. In addition, the Company amended and restated its previous option agreement with the medical technology company. The option agreement gives Edwards the option to acquire the remaining equity interest in the medical technology company.
11
The medical technology company is dedicated to developing technologies for detecting and managing patients with cardiovascular disease. The transaction was accounted for as a business combination. Tangible and intangible assets and liabilities acquired were recorded based on their estimated fair values at the acquisition date. The excess of the purchase price over the fair value of net assets acquired was recorded to goodwill. The following table summarizes the fair values of the assets acquired and liabilities assumed (in millions):
| Assets | $ | 8.1 | |||||||||
| Goodwill | 133.2 | ||||||||||
| In-process research and development | 136.6 | ||||||||||
| Liabilities assumed | (1.7) | ||||||||||
| Deferred tax liabilities | (28.0) | ||||||||||
| Fair value of net assets acquired | 248.2 | ||||||||||
Less: Noncontrolling interest (a) | (72.4) | ||||||||||
| Total purchase price | 175.8 | ||||||||||
| Less: cash acquired | (6.8) | ||||||||||
Total purchase price, net of cash acquired (b) | $ | 169.0 | |||||||||
_______________________________________
(a) Includes the fair value of the noncontrolling interest of $94.4 million, offset by the purchase consideration allocated to the option of $22.0 million, which was ascribed to the noncontrolling interest.
(b) Includes $22.5 million paid in a previous year under option agreements and $5.3 million for the settlement of a pre-existing note.
Goodwill includes expected synergies and other benefits the Company believes will result from the acquisition. Goodwill was assigned to the Company’s Rest of World segment and is not deductible for tax purposes.
Pro forma results have not been presented as the results of the medical technology company are not material in relation to the consolidated financial statements of Edwards Lifesciences.
7. FAIR VALUE MEASUREMENTS
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. The Company prioritizes the inputs used to determine fair values in one of the following three categories:
Level 1—Quoted market prices in active markets for identical assets or liabilities.
Level 2—Inputs, other than quoted prices in active markets, that are observable, either directly or indirectly.
Level 3—Unobservable inputs that are not corroborated by market data.
In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the level in the fair value hierarchy within which the fair value measurement in its entirety falls has been determined based on the lowest level input that is significant to the fair value measurement in its entirety.
The consolidated condensed financial statements include financial instruments for which the fair market value of such instruments may differ from amounts reflected on a historical cost basis. Financial instruments of the Company consist of cash deposits, accounts and other receivables, investments, accounts payable, certain accrued liabilities, and borrowings under a revolving credit agreement. The carrying value of these financial instruments generally approximates fair value due to their short-term nature. Financial instruments also include notes payable. As of June 30, 2023, the fair value of the notes payable, based on Level 2 inputs, was $580.5 million.
12
Assets and Liabilities Measured at Fair Value on a Recurring Basis
The following table summarizes the Company's financial instruments which are measured at fair value on a recurring basis (in millions):
| June 30, 2023 | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||
| Assets | |||||||||||||||||||||||
| Cash equivalents | $ | 635.0 | $ | — | $ | — | $ | 635.0 | |||||||||||||||
| Available-for-sale investments: | |||||||||||||||||||||||
| Corporate debt securities | — | 830.9 | — | 830.9 | |||||||||||||||||||
| Asset-backed securities | — | 252.5 | — | 252.5 | |||||||||||||||||||
| United States government and agency securities | — | 98.4 | — | 98.4 | |||||||||||||||||||
| Municipal securities | — | 2.6 | — | 2.6 | |||||||||||||||||||
| Investments held for deferred compensation plans | 125.3 | — | — | 125.3 | |||||||||||||||||||
| Derivatives | — | 63.9 | — | 63.9 | |||||||||||||||||||
| $ | 760.3 | $ | 1,248.3 | $ | — | $ | 2,008.6 | ||||||||||||||||
| Liabilities | |||||||||||||||||||||||
| Derivatives | $ | — | $ | 17.8 | $ | — | $ | 17.8 | |||||||||||||||
| Other liability | — | — | 14.0 | 14.0 | |||||||||||||||||||
| $ | — | $ | 17.8 | $ | 14.0 | $ | 31.8 | ||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||
| Assets | |||||||||||||||||||||||
| Cash equivalents | $ | 280.4 | $ | — | $ | — | $ | 280.4 | |||||||||||||||
| Available-for-sale investments: | |||||||||||||||||||||||
| Corporate debt securities | — | 980.3 | — | 980.3 | |||||||||||||||||||
| Asset-backed securities | — | 366.6 | — | 366.6 | |||||||||||||||||||
| United States government and agency securities | 37.1 | 94.5 | — | 131.6 | |||||||||||||||||||
| Municipal securities | — | 2.5 | — | 2.5 | |||||||||||||||||||
| Investments held for deferred compensation plans | 112.1 | — | — | 112.1 | |||||||||||||||||||
| Derivatives | — | 65.5 | — | 65.5 | |||||||||||||||||||
| $ | 429.6 | $ | 1,509.4 | $ | — | $ | 1,939.0 | ||||||||||||||||
| Liabilities | |||||||||||||||||||||||
| Derivatives | $ | — | $ | 27.2 | $ | — | $ | 27.2 | |||||||||||||||
| Contingent consideration liabilities | — | — | 26.2 | 26.2 | |||||||||||||||||||
| Other liability | — | — | 14.0 | 14.0 | |||||||||||||||||||
| $ | — | $ | 27.2 | $ | 40.2 | $ | 67.4 | ||||||||||||||||
Cash Equivalents and Available-for-sale Investments
Cash equivalents included money market funds for the periods presented above. The Company estimates the fair values of its money market funds based on quoted prices in active markets for identical assets. The Company estimates the fair values of its corporate debt securities, asset-backed securities, United States and foreign government and agency securities, and municipal securities by taking into consideration valuations obtained from third-party pricing services. The pricing services use industry standard valuation models, including both income and market-based approaches, for which all significant inputs are observable, either directly or indirectly, to estimate fair value. These inputs include reported trades and broker-dealer quotes on the same or similar securities, benchmark yields, credit spreads, prepayment and default projections based on historical data, and other observable inputs. The Company independently reviews and validates the pricing received from the third-party pricing service by comparing the prices to prices reported by a secondary pricing source. The Company’s validation procedures have not resulted in an adjustment to the pricing received from the pricing service.
Deferred Compensation Plans
The Company holds investments in trading securities related to its deferred compensation plans. The investments are in a variety of stock, bond and money market mutual funds. The fair values of these investments are based on quoted market prices.
13
Derivative Instruments
The Company uses derivative financial instruments in the form of foreign currency forward exchange contracts and cross currency swap contracts to manage foreign currency exposures. All derivatives contracts are recognized on the balance sheet at their fair value. The fair value of the derivative financial instruments was estimated based on quoted market foreign exchange rates, cross currency swap basis rates, and market discount rates. Judgment was employed in interpreting market data to develop estimates of fair value; accordingly, the estimates presented herein are not necessarily indicative of the amounts that the Company could realize in a current market exchange. The use of different market assumptions or valuation methodologies could have a material effect on the estimated fair value amounts.
Contingent Consideration Liabilities
Certain of the Company's acquisitions involve contingent consideration arrangements. Payment of additional consideration is contingent upon the acquired company reaching certain performance milestones, such as attaining specified sales levels or obtaining regulatory approvals. These contingent consideration liabilities are measured at estimated fair value using either a probability weighted discounted cash flow analysis or a Monte Carlo simulation model, both of which consider significant unobservable inputs. As of June 30, 2023 the probability of milestone achievement was determined to be 0% and, accordingly, the contingent consideration liability was reduced to zero.
The following tables summarize the changes in fair value of the contingent consideration and the other liability (in millions):
| Contingent Consideration | Other liability | Total | |||||||||||||||
| Balance at December 31, 2022 | $ | 26.2 | $ | 14.0 | $ | 40.2 | |||||||||||
| Changes in fair value | (26.2) | — | (26.2) | ||||||||||||||
| Balance at June 30, 2023 | $ | — | $ | 14.0 | $ | 14.0 | |||||||||||
| Contingent Consideration | Other liability | Total | |||||||||||||||
| Balance at December 31, 2021 | $ | 62.0 | $ | 14.0 | $ | 76.0 | |||||||||||
| Changes in fair value | (23.8) | — | (23.8) | ||||||||||||||
| Balance at June 30, 2022 | $ | 38.2 | $ | 14.0 | $ | 52.2 | |||||||||||
8. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
The Company uses derivative financial instruments to manage its currency exchange rate risk and its interest rate risk as summarized below. Notional amounts are stated in United States dollar equivalents at spot exchange rates at the respective dates. The Company does not enter into these arrangements for trading or speculation purposes.
| Notional Amount | |||||||||||
| June 30, 2023 | December 31, 2022 | ||||||||||
| (in millions) | |||||||||||
| Foreign currency forward exchange contracts | $ | 1,763.7 | $ | 1,678.4 | |||||||
| Cross currency swap contracts | 300.0 | 300.0 | |||||||||
Derivative financial instruments involve credit risk in the event the counterparty should default. It is the Company's policy to execute such instruments with global financial institutions that the Company believes to be creditworthy. The Company diversifies its derivative financial instruments among counterparties to minimize exposure to any one of these entities. The Company also uses International Swap Dealers Association master-netting agreements. The master-netting agreements provide for the net settlement of all contracts through a single payment in a single currency in the event of default, as defined by the agreements.
The Company uses foreign currency forward exchange contracts and cross currency swap contracts to manage its exposure to changes in currency exchange rates from (a) future cash flows associated with intercompany transactions and certain local currency expenses expected to occur within the next 13 months (designated as cash flow hedges), (b) its net
14
investment in certain foreign subsidiaries (designated as net investment hedges) and (c) foreign currency denominated assets or liabilities (designated as fair value hedges). The Company also uses foreign currency forward exchange contracts that are not designated as hedging instruments to offset the transaction gains and losses associated with revaluation of certain assets and liabilities denominated in currencies other than their functional currencies (resulting principally from intercompany and local currency transactions).
All derivative financial instruments are recognized at fair value in the consolidated condensed balance sheets. For each derivative instrument that is designated as a fair value hedge, the gain or loss on the derivative included in the assessment of hedge effectiveness is recognized immediately to earnings, and offsets the loss or gain on the underlying hedged item. The Company reports in "Accumulated Other Comprehensive Loss" the gain or loss on derivative financial instruments that are designated, and that qualify, as cash flow hedges. The Company reclassifies these gains and losses into earnings in the same line item and in the same period in which the underlying hedged transactions affect earnings. Changes in the fair value of net investment hedges are reported in "Accumulated Other Comprehensive Loss" as a part of the cumulative translation adjustment and would be reclassified into earnings if the underlying net investment is sold or substantially liquidated. The portion of the change in fair value related to components excluded from the hedge effectiveness assessment are amortized into earnings over the life of the derivative. The gains and losses on derivative financial instruments for which the Company does not elect hedge accounting treatment are recognized in the consolidated statements of operations in each period based upon the change in the fair value of the derivative financial instrument. Cash flows from net investment hedges are reported as investing activities in the consolidated statements of cash flows, and cash flows from all other derivative financial instruments are reported as operating activities.
The following table presents the location and fair value amounts of derivative instruments reported in the consolidated condensed balance sheets (in millions):
| Fair Value | ||||||||||||||||||||
| Derivatives designated as hedging instruments | Balance Sheet Location | June 30, 2023 | December 31, 2022 | |||||||||||||||||
| Assets | ||||||||||||||||||||
| Foreign currency contracts | Other current assets | $ | 32.2 | $ | 24.9 | |||||||||||||||
| Cross currency swap contracts | Other assets | $ | 31.7 | $ | 40.6 | |||||||||||||||
| Liabilities | ||||||||||||||||||||
| Foreign currency contracts | Accrued and other liabilities | $ | 17.8 | $ | 20.7 | |||||||||||||||
| Foreign currency contracts | Other liabilities | $ | — | $ | 6.5 | |||||||||||||||
The following table presents the effect of master-netting agreements and rights of offset on the consolidated condensed balance sheets (in millions):
| Gross Amounts Not Offset in the Consolidated Balance Sheet | |||||||||||||||||||||||||||||||||||
| Gross Amounts Offset in the Consolidated Balance Sheet | |||||||||||||||||||||||||||||||||||
| Net Amounts Presented in the Consolidated Balance Sheet | |||||||||||||||||||||||||||||||||||
| June 30, 2023 | Gross Amounts | Financial Instruments | Cash Collateral Received | Net Amount | |||||||||||||||||||||||||||||||
| Derivative assets | |||||||||||||||||||||||||||||||||||
| Foreign currency contracts | $ | 32.2 | $ | — | $ | 32.2 | $ | (8.6) | $ | — | $ | 23.6 | |||||||||||||||||||||||
| Cross currency swap contracts | $ | 31.7 | $ | — | $ | 31.7 | $ | — | $ | — | $ | 31.7 | |||||||||||||||||||||||
| Derivative liabilities | |||||||||||||||||||||||||||||||||||
| Foreign currency contracts | $ | 17.8 | $ | — | $ | 17.8 | $ | (8.6) | $ | — | $ | 9.2 | |||||||||||||||||||||||
| December 31, 2022 | |||||||||||||||||||||||||||||||||||
| Derivative assets | |||||||||||||||||||||||||||||||||||
| Foreign currency contracts | $ | 24.9 | $ | — | $ | 24.9 | $ | (12.0) | $ | — | $ | 12.9 | |||||||||||||||||||||||
| Cross currency swap contracts | $ | 40.6 | $ | — | $ | 40.6 | $ | — | $ | — | $ | 40.6 | |||||||||||||||||||||||
| Derivative liabilities | |||||||||||||||||||||||||||||||||||
| Foreign currency contracts | $ | 27.2 | $ | — | $ | 27.2 | $ | (12.0) | $ | — | $ | 15.2 | |||||||||||||||||||||||
The following tables present the effect of derivative and non-derivative hedging instruments on the consolidated condensed statements of operations and consolidated condensed statements of comprehensive income (in millions):
15
| Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion) | Amount of Gain or (Loss) Reclassified from Accumulated OCI into Income (Effective Portion) | |||||||||||||||||||||||||||||||
| Three Months Ended June 30, | Location of Gain or (Loss) Reclassified from Accumulated OCI into Income | Three Months Ended June 30, | ||||||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||||||
| Cash flow hedges | ||||||||||||||||||||||||||||||||
| Foreign currency contracts | $ | 23.2 | $ | 57.9 | Cost of sales | $ | 13.9 | $ | 16.3 | |||||||||||||||||||||||
| Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion) | Amount of Gain or (Loss) Reclassified from Accumulated OCI into Income (Effective Portion) | |||||||||||||||||||||||||||||||
| Six Months Ended June 30, | Location of Gain or (Loss) Reclassified from Accumulated OCI into Income | Six Months Ended June 30, | ||||||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||||||
| Cash flow hedges | ||||||||||||||||||||||||||||||||
| Foreign currency contracts | $ | 26.9 | $ | 82.6 | Cost of sales | $ | 43.7 | $ | 23.6 | |||||||||||||||||||||||
| Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion) | Amount of Gain or (Loss) Recognized in Income on Derivative (Amount Excluded from Effectiveness Testing) | |||||||||||||||||||||||||||||||
| Three Months Ended June 30, | Location of Gain or (Loss) Recognized in Income on Derivative (Amount Excluded from Effectiveness Testing) | Three Months Ended June 30, | ||||||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||||||
| Net investment hedges | ||||||||||||||||||||||||||||||||
| Cross currency swap contracts | $ | (6.5) | $ | 19.4 | Interest income, net | $ | 1.8 | $ | 2.0 | |||||||||||||||||||||||
| Amount of Gain or (Loss) Recognized in OCI on Derivative (Effective Portion) | Amount of Gain or (Loss) Recognized in Income on Derivative (Amount Excluded from Effectiveness Testing) | |||||||||||||||||||||||||||||||
| Six Months Ended June 30, | Location of Gain or (Loss) Reclassified from Accumulated OCI into Income | Six Months Ended June 30, | ||||||||||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||||||
| Net investment hedges | ||||||||||||||||||||||||||||||||
| Cross currency swap contracts | $ | (9.0) | $ | 23.3 | Interest income, net | $ | 3.5 | $ | 3.6 | |||||||||||||||||||||||
The cross currency swap contracts have an expiration date of June 15, 2028. At maturity of the cross currency swap contracts, the Company will deliver the notional amount of €257.2 million and will receive $300.0 million from the counterparties. The Company will receive semi-annual interest payments from the counterparties based on a fixed interest rate until maturity of the agreements.
| Amount of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| Three Months Ended June 30, | ||||||||||||||||||||
| Location of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| 2023 | 2022 | |||||||||||||||||||
| Fair value hedges | ||||||||||||||||||||
| Foreign currency contracts | Other income, net | $ | 11.3 | $ | — | |||||||||||||||
16
| Amount of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| Six Months Ended June 30, | ||||||||||||||||||||
| Location of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| 2023 | 2022 | |||||||||||||||||||
| Fair value hedges | ||||||||||||||||||||
| Foreign currency contracts | Other income, net | $ | 11.3 | $ | — | |||||||||||||||
| Amount of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| Three Months Ended June 30, | ||||||||||||||||||||
| Location of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| 2023 | 2022 | |||||||||||||||||||
| Derivatives not designated as hedging instruments | ||||||||||||||||||||
| Foreign currency contracts | Other income, net | $ | 9.9 | $ | 26.6 | |||||||||||||||
| Amount of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| Six Months Ended June 30, | ||||||||||||||||||||
| Location of Gain or (Loss) Recognized in Income on Derivative | ||||||||||||||||||||
| 2023 | 2022 | |||||||||||||||||||
| Derivatives not designated as hedging instruments | ||||||||||||||||||||
| Foreign currency contracts | Other income, net | $ | 4.5 | $ | 42.1 | |||||||||||||||
The following tables present the effect of fair value and cash flow hedge accounting on the consolidated condensed statements of operations (in millions):
| Location and Amount of Gain or (Loss) Recognized in Income on Fair Value and Cash Flow Hedging Relationships | |||||||||||||||||||||||||||||||||||||||||||||||
| Three Months Ended June 30, 2023 | Six Months Ended June 30, 2023 | ||||||||||||||||||||||||||||||||||||||||||||||
| Cost of sales | Other income, net | Cost of sales | Other income, net | ||||||||||||||||||||||||||||||||||||||||||||
| Total amounts of income and expense line items presented in the consolidated condensed statements of operations in which the effects of fair value or cash flow hedges are recorded | $ | (343.0) | $ | 2.2 | $ | (672.5) | $ | 3.8 | |||||||||||||||||||||||||||||||||||||||
| The effects of fair value and cash flow hedging: | |||||||||||||||||||||||||||||||||||||||||||||||
| Gain (loss) on fair value hedging relationships: | |||||||||||||||||||||||||||||||||||||||||||||||
| Foreign currency contracts: | |||||||||||||||||||||||||||||||||||||||||||||||
| Hedged items | $ | — | $ | (10.1) | $ | — | $ | (8.9) | |||||||||||||||||||||||||||||||||||||||
| Derivatives designated as hedging instruments | $ | — | $ | 10.1 | $ | — | $ | 8.9 | |||||||||||||||||||||||||||||||||||||||
| Amount excluded from effectiveness testing recognized in earnings based on an amortization approach | $ | — | $ | 1.2 | $ | — | $ | 2.4 | |||||||||||||||||||||||||||||||||||||||
| Gain (loss) on cash flow hedging relationships: | |||||||||||||||||||||||||||||||||||||||||||||||
| Foreign currency contracts: | |||||||||||||||||||||||||||||||||||||||||||||||
| Amount of gain (loss) reclassified from accumulated OCI into income | $ | 13.9 | $ | — | $ | 43.7 | $ | — | |||||||||||||||||||||||||||||||||||||||
17
| Location and Amount of Gain or (Loss) Recognized in Income on Fair Value and Cash Flow Hedging Relationships | |||||||||||||||||||||||||||||||||||||||||||||||
| Three Months Ended June 30, 2022 | Six Months Ended June 30, 2022 | ||||||||||||||||||||||||||||||||||||||||||||||
| Cost of sales | Cost of sales | ||||||||||||||||||||||||||||||||||||||||||||||
| Total amounts of income and expense line items presented in the consolidated condensed statements of operations in which the effects of fair value or cash flow hedges are recorded | $ | (269.4) | $ | (568.7) | |||||||||||||||||||||||||||||||||||||||||||
| The effects of fair value and cash flow hedging: | |||||||||||||||||||||||||||||||||||||||||||||||
| Gain (loss) on cash flow hedging relationships: | |||||||||||||||||||||||||||||||||||||||||||||||
| Foreign currency contracts: | |||||||||||||||||||||||||||||||||||||||||||||||
| Amount of gain (loss) reclassified from accumulated OCI into income | $ | 16.3 | $ | 23.6 | |||||||||||||||||||||||||||||||||||||||||||
The Company expects that during the next twelve months it will reclassify to earnings a $9.0 million gain currently recorded in "Accumulated Other Comprehensive Loss."
9. STOCK-BASED COMPENSATION
Stock-based compensation expense related to awards issued under the Company's incentive compensation plans for the three and six months ended June 30, 2023 and 2022 was as follows (in millions):
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Cost of sales | $ | 6.2 | $ | 6.9 | $ | 13.2 | $ | 13.1 | |||||||||||||||
| Selling, general, and administrative expenses | 22.5 | 21.1 | 44.8 | 39.8 | |||||||||||||||||||
| Research and development expenses | 8.7 | 8.2 | 18.3 | 15.7 | |||||||||||||||||||
| Total stock-based compensation expense | 37.4 | 36.2 | 76.3 | 68.6 | |||||||||||||||||||
| Income tax benefit | (6.8) | (5.9) | (11.5) | (10.3) | |||||||||||||||||||
| Total stock-based compensation expense, net of tax | $ | 30.6 | $ | 30.3 | $ | 64.8 | $ | 58.3 | |||||||||||||||
At June 30, 2023, the total remaining compensation cost related to nonvested stock options, restricted stock units, market-based restricted stock units, and employee stock purchase plan ("ESPP") subscription awards amounted to $275.0 million, which will be amortized on a straight-line basis over each award's requisite service period. The weighted-average remaining requisite service period is 34 months.
During the six months ended June 30, 2023, the Company granted 1.9 million stock options at a weighted-average
exercise price per share of $88.72, and 0.8 million restricted stock units at a weighted-average grant-date fair value per share of $88.06. During the six months ended June 30, 2023, the Company also granted 0.1 million market-based restricted stock units at a weighted-average grant-date fair value per share of $110.10. The market-based restricted stock units granted during the six months ended June 30, 2023 vest based on a combination of certain service and market conditions. The actual number of shares issued will be determined based on the Company's total shareholder return relative to a selected industry peer group over a three-year performance period and may range from 0% to 175% of the target number of shares granted.
18
Fair Value Disclosures
The fair value of market-based restricted stock units was determined using a Monte Carlo simulation model, which uses multiple input variables to determine the probability of satisfying the market condition requirements. The weighted-average assumptions used to determine the fair value of the market-based restricted stock units granted during the six months ended June 30, 2023 and 2022 included a risk-free interest rate of 3.6% and 2.8%, respectively, and an expected volatility rate of 32.6% and 33.8%, respectively.
The following table includes the weighted-average grant-date fair values of stock options granted during the periods indicated and the related weighted-average assumptions used in the Black-Scholes option pricing model:
Option Awards | Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Risk-free interest rate | 3.4 | % | 3.0 | % | 3.4 | % | 3.0 | % | |||||||||||||||
| Expected dividend yield | None | None | None | None | |||||||||||||||||||
| Expected volatility | 32.8 | % | 31.4 | % | 32.8 | % | 31.4 | % | |||||||||||||||
| Expected term (years) | 5.1 | 5.0 | 5.1 | 5.0 | |||||||||||||||||||
| Fair value, per option | $ | 31.03 | $ | 35.07 | $ | 31.01 | $ | 35.08 | |||||||||||||||
The following table includes the weighted-average grant-date fair values for ESPP subscriptions granted during the periods indicated and the related weighted-average assumptions used in the Black-Scholes option pricing model:
ESPP | Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Risk-free interest rate | 4.7 | % | 1.1 | % | 4.6 | % | 0.3 | % | |||||||||||||||
| Expected dividend yield | None | None | None | None | |||||||||||||||||||
| Expected volatility | 34.8 | % | 31.5 | % | 31.5 | % | 32.1 | % | |||||||||||||||
| Expected term (years) | 0.6 | 0.6 | 0.6 | 0.6 | |||||||||||||||||||
| Fair value, per share | $ | 22.02 | $ | 23.63 | $ | 19.03 | $ | 28.63 | |||||||||||||||
10. ACCELERATED SHARE REPURCHASE
During 2023 and 2022, the Company entered into accelerated share repurchase ("ASR") agreements providing for the repurchase of the Company's common stock based on the volume-weighted average price ("VWAP") of the Company's common stock during the term of the applicable agreement, less a discount. The following table summarizes the terms of the ASR agreements (dollars and shares in millions, except per share data):
| Initial Delivery | Final Settlement | |||||||||||||||||||||||||||||||||||||||||||
| Agreement Date | Amount Paid | Shares Received | Price per Share | Value of Shares as % of Contract Value | Settlement Date | Total Shares Received | Average Price per Share | |||||||||||||||||||||||||||||||||||||
| January 2022 | $ | 250.0 | 1.9 | $ | 104.87 | 80 | % | February 2022 | 2.3 | $ | 110.31 | |||||||||||||||||||||||||||||||||
| February 2023 | $ | 200.0 | 2.0 | $ | 80.44 | 80 | % | March 2023 | 2.5 | $ | 79.28 | |||||||||||||||||||||||||||||||||
The ASR agreements were each accounted for as two separate transactions: (1) the value of the initial delivery of shares was recorded as shares of common stock acquired in a treasury stock transaction on the acquisition date, and (2) the remaining amount of the purchase price paid was recorded as a forward contract indexed to the Company's own common stock and was initially recorded in "Additional Paid-in Capital" and subsequently, upon settlement, was transferred to "Treasury Stock" on the consolidated condensed balance sheets. The initial delivery of shares resulted in an immediate reduction of the outstanding shares used to calculate the weighted-average common shares outstanding for basic and diluted earnings per share. The Company determined that the forward contracts indexed to the Company's common stock met all the applicable criteria for equity classification and, therefore, were not accounted for as a derivative instrument.
19
11. COMMITMENTS AND CONTINGENCIES
The Company is reviewing and investigating whether business activities in Japan and other markets violate certain provisions of the Foreign Corrupt Practices Act ("FCPA"). The Company voluntarily notified the SEC and the United States Department of Justice ("DOJ") during 2021 that it has engaged outside counsel to conduct this review and investigation. The Company has provided status updates to the SEC and DOJ since that time. Any determination that the Company’s operations or activities are not in compliance with existing laws, including the FCPA, could result in the imposition of fines, penalties, and equitable remedies. The Company cannot currently predict the outcome of the review and investigation or the potential impact on its financial statements.
On September 28, 2021, Aortic Innovations LLC, a non-practicing entity, filed a lawsuit against Edwards Lifesciences Corporation and certain of its subsidiaries ("Edwards") in the United States District Court for the District of Delaware alleging that Edwards’ SAPIEN 3 Ultra product infringes certain of its patents. The Company is unable to predict the ultimate outcome of this matter or estimate a range of possible exposure; therefore, no amounts have been accrued. The Company intends to vigorously defend itself in this litigation.
The Company is or may be a party to, or may otherwise be responsible for, pending or threatened lawsuits including those related to products and services currently or formerly manufactured or performed, as applicable, by the Company, workplace and employment matters, matters involving real estate, Company operations or health care regulations, or governmental investigations (the "Lawsuits"). The Lawsuits raise difficult and complex factual and legal issues and are subject to many uncertainties, including, but not limited to, the facts and circumstances of each particular case or claim, the jurisdiction in which each suit is brought, and differences in applicable law. Management does not believe that any loss relating to the Lawsuits would have a material adverse effect on the Company's overall financial condition, results of operations or cash flows. However, the resolution of one or more of the Lawsuits in any reporting period, could have a material adverse impact on the Company's financial results for that period. The Company is not able to estimate the amount or range of any loss for legal contingencies related to the Lawsuits for which there is no reserve or additional loss for matters already reserved.
20
12. ACCUMULATED OTHER COMPREHENSIVE LOSS
The following tables summarize the activity for each component of "Accumulated Other Comprehensive Loss" (in millions):
| Foreign Currency Translation Adjustments | Unrealized Gain on Hedges | Unrealized Loss on Available-for-sale Investments | Unrealized Pension Credits | Total Accumulated Other Comprehensive Loss | |||||||||||||||||||||||||
| December 31, 2022 | $ | (218.8) | $ | 23.8 | $ | (65.6) | $ | 5.7 | $ | (254.9) | |||||||||||||||||||
| Other comprehensive gain (loss) before reclassifications | 4.9 | 6.7 | 9.0 | (0.1) | 20.5 | ||||||||||||||||||||||||
| Amounts reclassified from accumulated other comprehensive loss | (1.7) | (29.8) | 4.0 | — | (27.5) | ||||||||||||||||||||||||
| Deferred income tax benefit | 0.6 | 5.9 | — | — | 6.5 | ||||||||||||||||||||||||
| March 31, 2023 | $ | (215.0) | $ | 6.6 | $ | (52.6) | $ | 5.6 | $ | (255.4) | |||||||||||||||||||
| Other comprehensive (loss) gain before reclassifications | (11.3) | 33.5 | 2.1 | 0.2 | 24.5 | ||||||||||||||||||||||||
| Amounts reclassified from accumulated other comprehensive loss | (1.8) | (25.2) | 3.8 | — | (23.2) | ||||||||||||||||||||||||
| Deferred income tax benefit (expense) | 1.6 | (3.0) | — | — | (1.4) | ||||||||||||||||||||||||
| June 30, 2023 | $ | (226.5) | $ | 11.9 | $ | (46.7) | $ | 5.8 | $ | (255.5) | |||||||||||||||||||
| Foreign Currency Translation Adjustments | Unrealized Gain on Hedges | Unrealized Loss on Available-for-sale Investments | Unrealized Pension Costs | Total Accumulated Other Comprehensive Loss | |||||||||||||||||||||||||
| December 31, 2021 | $ | (172.5) | $ | 29.7 | $ | (6.9) | $ | (8.0) | $ | (157.7) | |||||||||||||||||||
| Other comprehensive (loss) gain before reclassifications | (7.4) | 24.7 | (47.6) | — | (30.3) | ||||||||||||||||||||||||
| Amounts reclassified from accumulated other comprehensive loss | (1.6) | (7.3) | 4.8 | — | (4.1) | ||||||||||||||||||||||||
| Deferred income tax (expense) benefit | (1.0) | (4.0) | 10.5 | — | 5.5 | ||||||||||||||||||||||||
| March 31, 2022 | $ | (182.5) | $ | 43.1 | $ | (39.2) | $ | (8.0) | $ | (186.6) | |||||||||||||||||||
| Other comprehensive (loss) gain before reclassifications | (36.5) | 57.9 | (20.9) | (0.1) | 0.4 | ||||||||||||||||||||||||
| Amounts reclassified from accumulated other comprehensive loss | (2.0) | (16.3) | 5.1 | — | (13.2) | ||||||||||||||||||||||||
| Deferred income tax expense | (4.7) | (10.3) | (10.0) | — | (25.0) | ||||||||||||||||||||||||
| June 30, 2022 | $ | (225.7) | $ | 74.4 | $ | (65.0) | $ | (8.1) | $ | (224.4) | |||||||||||||||||||
21
The following table provides information about amounts reclassified from "Accumulated Other Comprehensive Loss" (in millions):
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||||||||
| Affected Line on Consolidated Condensed Statements of Operations | |||||||||||||||||||||||||||||
Details about Accumulated Other Comprehensive Loss Components | 2023 | 2022 | 2023 | 2022 | |||||||||||||||||||||||||
| Foreign currency translation adjustments | $ | 1.8 | $ | 2.0 | $ | 3.5 | $ | 3.6 | Other income, net | ||||||||||||||||||||
| (0.4) | (0.5) | (0.8) | (0.9) | Provision for income taxes | |||||||||||||||||||||||||
| $ | 1.4 | $ | 1.5 | $ | 2.7 | $ | 2.7 | Net of tax | |||||||||||||||||||||
| Gain on hedges | $ | 13.9 | $ | 16.3 | $ | 43.7 | $ | 23.6 | Cost of sales | ||||||||||||||||||||
| 11.3 | — | 11.3 | — | Other income, net | |||||||||||||||||||||||||
| 25.2 | 16.3 | 55.0 | 23.6 | Total before tax | |||||||||||||||||||||||||
| (4.8) | (4.3) | (11.9) | (6.4) | Provision for income taxes | |||||||||||||||||||||||||
| $ | 20.4 | $ | 12.0 | $ | 43.1 | $ | 17.2 | Net of tax | |||||||||||||||||||||
| Loss on available-for-sale investments | $ | (3.8) | $ | (5.1) | $ | (7.8) | $ | (9.9) | Interest income, net | ||||||||||||||||||||
| 0.9 | 1.2 | 1.9 | 2.4 | Provision for income taxes | |||||||||||||||||||||||||
| $ | (2.9) | $ | (3.9) | $ | (5.9) | $ | (7.5) | Net of tax | |||||||||||||||||||||
13. EARNINGS PER SHARE
Basic earnings per share is computed by dividing net income by the weighted-average common shares outstanding during the period. Diluted earnings per share is computed based on the weighted-average common shares outstanding plus the effect of dilutive potential common shares outstanding during the period calculated using the treasury stock method. Dilutive potential common shares include employee equity share options, nonvested shares, and similar equity instruments granted by the Company. Potential common share equivalents have been excluded where their inclusion would be anti-dilutive.
The table below presents the computation of basic and diluted earnings per share (in millions, except for per share information):
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Basic: | |||||||||||||||||||||||
| Net income attributable to Edwards Lifesciences Corporation | $ | 307.1 | $ | 406.4 | $ | 647.6 | $ | 780.0 | |||||||||||||||
| Weighted-average shares outstanding | 606.9 | 620.9 | 607.2 | 621.5 | |||||||||||||||||||
| Basic earnings per share | $ | 0.51 | $ | 0.65 | $ | 1.07 | $ | 1.26 | |||||||||||||||
| Diluted: | |||||||||||||||||||||||
| Net income attributable to Edwards Lifesciences Corporation | $ | 307.1 | $ | 406.4 | $ | 647.6 | $ | 780.0 | |||||||||||||||
| Weighted-average shares outstanding | 606.9 | 620.9 | 607.2 | 621.5 | |||||||||||||||||||
| Dilutive effect of stock plans | 3.4 | 5.8 | 3.4 | 6.6 | |||||||||||||||||||
| Dilutive weighted-average shares outstanding | 610.3 | 626.7 | 610.6 | 628.1 | |||||||||||||||||||
| Diluted earnings per share | $ | 0.50 | $ | 0.65 | $ | 1.06 | $ | 1.24 | |||||||||||||||
Stock options, restricted stock units, and market-based restricted stock units to purchase an aggregate of 6.7 million and 3.6 million common shares for the three months ended June 30, 2023 and 2022, respectively, and 5.9 million and 2.6 million shares for the six months ended June 30, 2023 and 2022, respectively, were outstanding, but were not included in the computation of diluted earnings per share for such periods because the effect would have been anti-dilutive.
22
14. INCOME TAXES
The Company's effective income tax rates were 9.7% and 12.5% for the three months ended June 30, 2023 and 2022, respectively, and 12.4% and 13.4% for the six months ended June 30, 2023 and 2022, respectively. The decrease in the effective rate between the six months ended June 30, 2023 and 2022 is primarily due to an increase in the benefit from the federal research and development credit and the Intellectual Property Agreement with Medtronic (see Note 3), partially offset by a reduced tax benefit from employee share-based compensation. In addition, the effective rates for the six months ended June 30, 2023 and 2022 were lower than the federal statutory rate of 21% primarily due to (1) foreign earnings taxed at lower rates, (2) Federal and California research and development credits, and (3) the tax benefit from employee share-based compensation. The effective rates include a tax benefit from employee share-based compensation of $9.3 million and $19.8 million for the three months ended June 30, 2023 and 2022, respectively, and $12.3 million and $33.2 million for the six months ended June 30, 2023 and 2022, respectively.
In the normal course of business, the Internal Revenue Service (“IRS”) and other taxing authorities are in different stages of examining various years of the Company's tax filings. During these audits the Company may receive proposed audit adjustments that could be material. Therefore, there is a possibility that an adverse outcome in these audits could have a material effect on the Company's results of operations and financial condition. The Company strives to resolve open matters with each tax authority at the examination level and could reach agreement with a tax authority at any time. While the Company has accrued for matters it believes are more likely than not to require settlement, the final outcome with a tax authority may result in a tax liability that is more or less than that reflected in the consolidated financial statements. Furthermore, the Company may later decide to challenge any assessments, if made, and may exercise its right to appeal. The uncertain tax positions are reviewed quarterly and adjusted as events occur that affect potential liabilities for additional taxes, such as lapsing of applicable statutes of limitations, proposed assessments by tax authorities, negotiations between tax authorities, identification of new issues, and issuance of new legislation, regulations, or case law.
As of June 30, 2023 and December 31, 2022, the gross liability recorded for income taxes associated with uncertain tax positions was $520.0 million and $475.3 million, respectively. The Company estimates that these liabilities would be reduced by $205.1 million and $182.1 million, respectively, from offsetting tax benefits associated with the correlative effects of potential transfer pricing adjustments, state income taxes, and timing adjustments. The net amounts of $314.9 million and $293.2 million, respectively, if not required, would favorably affect the Company's effective tax rate.
The Company executed an Advance Pricing Agreement ("APA") in 2018 between the United States and Switzerland governments for tax years 2009 through 2020 covering various, but not all, transfer pricing matters. The unagreed transfer pricing matters, namely Surgical Structural Heart and Transcatheter Aortic Valve Replacement (collectively “Surgical/TAVR”) intercompany royalty transactions, then reverted to IRS Examination for further consideration as part of the respective years' regular tax audits. In addition, the Company executed other bilateral APAs as follows: during 2017, an APA between the United States and Japan covering tax years 2015 through 2019; and during 2018, APAs between Singapore and Japan and between Switzerland and Japan covering tax years 2015 through 2019. The Company has filed to renew all of the APAs which cover transactions with Japan for the years 2020 and forward. An APA between Switzerland and Japan covering tax years 2020 through 2024 was executed in 2021. The execution of some or all of these APA renewals depends on many variables outside of the Company's control.
The audits of the Company’s United States federal income tax returns through 2014 have been closed. The IRS audit field work for the 2015 through 2017 tax years was completed during the second quarter of 2021, except for certain transfer pricing and related matters. The IRS began its examination of the 2018 through 2020 tax years during the first quarter of 2022.
The audits of the Company's material state, local, and foreign income tax matters have been concluded for years through 2015. While not material, the Company continues to address matters in India for years from 2010 and on.
During 2021, the Company received a Notice of Proposed Adjustment (“NOPA”) from the IRS for the 2015-2017 tax years relating to transfer pricing involving Surgical/TAVR intercompany royalty transactions between the Company's United States and Switzerland subsidiaries. The NOPA proposed an increase to the Company's United States taxable income, which could result in additional tax expense for this period of approximately $220 million and reflects a significant departure from the transfer pricing methods the Company had previously agreed upon with the IRS for these types of transactions. The Company disagreed with the NOPA and submitted a formal protest on the matter during the fourth quarter of 2021. During the second quarter of 2022, the Company received the IRS's rebuttal to the Company's protest and was notified that the case had been transferred to the IRS Independent Office of Appeals ("Appeals"). The opening conference was held with Appeals during March 2023 and discussions with Appeals continued throughout the second quarter of 2023. While Appeals still maintains
23
jurisdiction over the matter as of June 30, 2023, the Company and Appeals have now concluded that a satisfactory resolution of this matter at the administrative level is not possible.
The Company now plans to vigorously contest the proposed adjustments through the judicial process. Final resolution of this matter is not likely within the next 12 months. The Company believes the amounts previously accrued related to this uncertain tax position are appropriate based on prior experience and interpretation and application of relevant tax law and accounting standards to the Company's facts and, accordingly, has not accrued any additional amount based on the NOPA received or as a result of the Appeals proceedings to date. Nonetheless, the outcome of the judicial process cannot be predicted with certainty, and it is possible that the outcome of that process could have a material impact on the Company's consolidated financial statements. As noted below, similar material tax disputes may arise for the 2018-2023 tax years. While no payment of any amount related to the NOPA has yet been required, the Company made a partial deposit in November 2022 to prevent the further accrual of interest on that portion of any potential deficiency that may be ultimately sustained.
Surgical/TAVR intercompany royalty transactions covering tax years 2018-2023 that were not resolved under the APA program remain subject to IRS examination, and those transactions and related tax positions remain uncertain as of June 30, 2023. The Company has considered this information, as well as information regarding the NOPA, the rebuttal and Appeals discussions described above, in its evaluation of its uncertain tax positions. The impact of these unresolved transfer pricing matters, net of any correlative tax adjustments, may be significant to the Company’s consolidated financial statements. Based on the information currently available and numerous possible outcomes, the Company cannot reasonably estimate what, if any, changes in its existing uncertain tax positions may occur in the next 12 months and, therefore, has continued to record the uncertain tax positions as a long-term liability.
15. SEGMENT INFORMATION
Edwards Lifesciences conducts operations worldwide and is managed in the following geographical regions: United States, Europe, Japan, and Rest of World. All regions sell products that are used to treat advanced cardiovascular disease.
The Company's geographic segments are reported based on the financial information provided to the Chief Operating Decision Maker (the Chief Executive Officer). The Company evaluates the performance of its geographic segments based on net sales and operating income. Segment net sales and segment operating income are based on internally derived foreign exchange rates and do not include inter-segment profits. Because of the interdependence of the reportable segments, the operating profit as presented may not be representative of the geographical distribution that would occur if the segments were not interdependent. Net sales by geographic area are based on the location of the customer. There were no customers that represented 10% or more of the Company's total net sales.
Certain items are maintained at the corporate level and are not allocated to the segments. The non-allocated items include corporate research and development expenses, manufacturing variances, corporate headquarters costs, net interest income, global marketing expenses, special gains and charges, stock-based compensation, foreign currency hedging activities, certain litigation costs, changes in the fair value of contingent consideration liabilities, and most of the Company's amortization expense. Although most of the Company's depreciation expense is included in segment operating income, due to the Company's methodology for cost build-up, it is impractical to determine the amount of depreciation expense included in each segment and, therefore, a portion is maintained at the corporate level. The Company neither discretely allocates assets to its operating segments, nor evaluates the operating segments using discrete asset information.
24
The table below presents information about Edwards Lifesciences' reportable segments (in millions):
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Segment Net Sales | |||||||||||||||||||||||
| United States | $ | 895.3 | $ | 800.8 | $ | 1,744.4 | $ | 1,550.3 | |||||||||||||||
| Europe | 336.0 | 309.8 | 671.4 | 609.9 | |||||||||||||||||||
| Japan | 109.7 | 145.1 | 214.0 | 286.0 | |||||||||||||||||||
| Rest of World | 182.2 | 145.9 | 347.8 | 288.2 | |||||||||||||||||||
| Total segment net sales | $ | 1,523.2 | $ | 1,401.6 | $ | 2,977.6 | $ | 2,734.4 | |||||||||||||||
| Segment Operating Income | |||||||||||||||||||||||
| United States | $ | 592.4 | $ | 549.5 | $ | 1,155.8 | $ | 1,061.0 | |||||||||||||||
| Europe | 177.7 | 164.5 | 359.4 | 331.1 | |||||||||||||||||||
| Japan | 63.4 | 95.9 | 126.8 | 195.4 | |||||||||||||||||||
| Rest of World | 80.1 | 54.6 | 150.6 | 114.3 | |||||||||||||||||||
| Total segment operating income | $ | 913.6 | $ | 864.5 | $ | 1,792.6 | $ | 1,701.8 | |||||||||||||||
The table below presents reconciliations of segment net sales to consolidated net sales and segment operating income to consolidated pre-tax income (in millions):
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Net Sales Reconciliation | |||||||||||||||||||||||
| Segment net sales | $ | 1,523.2 | $ | 1,401.6 | $ | 2,977.6 | $ | 2,734.4 | |||||||||||||||
| Foreign currency | 7.0 | (27.7) | 12.2 | (19.3) | |||||||||||||||||||
| Consolidated net sales | $ | 1,530.2 | $ | 1,373.9 | $ | 2,989.8 | $ | 2,715.1 | |||||||||||||||
| Pre-tax Income Reconciliation | |||||||||||||||||||||||
| Segment operating income | $ | 913.6 | $ | 864.5 | $ | 1,792.6 | $ | 1,701.8 | |||||||||||||||
| Unallocated amounts: | |||||||||||||||||||||||
| Corporate items | (492.4) | (450.4) | (961.3) | (869.8) | |||||||||||||||||||
| Intellectual property agreement and litigation expense | (147.9) | (6.1) | (191.4) | (13.2) | |||||||||||||||||||
| Change in fair value of contingent consideration liabilities | 26.9 | 20.9 | 26.2 | 23.8 | |||||||||||||||||||
| Foreign currency | 27.0 | 30.6 | 49.5 | 55.7 | |||||||||||||||||||
| Consolidated operating income | 327.2 | 459.5 | 715.6 | 898.3 | |||||||||||||||||||
| Non-operating income | 11.3 | 5.2 | 21.5 | 2.5 | |||||||||||||||||||
| Consolidated pre-tax income | $ | 338.5 | $ | 464.7 | $ | 737.1 | $ | 900.8 | |||||||||||||||
25
Enterprise-wide Information
(in millions)
The following enterprise-wide information is based on actual foreign exchange rates used in the Company's consolidated condensed financial statements.
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Net Sales by Geographic Region | |||||||||||||||||||||||
| United States | $ | 895.3 | $ | 800.8 | $ | 1,744.4 | $ | 1,550.3 | |||||||||||||||
| Europe | 336.2 | 302.8 | 667.3 | 613.9 | |||||||||||||||||||
| Japan | 117.9 | 122.9 | 232.0 | 258.4 | |||||||||||||||||||
| Rest of World | 180.8 | 147.4 | 346.1 | 292.5 | |||||||||||||||||||
| $ | 1,530.2 | $ | 1,373.9 | $ | 2,989.8 | $ | 2,715.1 | ||||||||||||||||
| Net Sales by Major Product Group | |||||||||||||||||||||||
| Transcatheter Aortic Valve Replacement | $ | 991.6 | $ | 906.9 | $ | 1,939.5 | $ | 1,788.2 | |||||||||||||||
| Transcatheter Mitral and Tricuspid Therapies | 47.6 | 27.9 | 89.2 | 54.9 | |||||||||||||||||||
| Surgical Structural Heart | 256.3 | 228.5 | 504.5 | 449.3 | |||||||||||||||||||
| Critical Care | 234.7 | 210.6 | 456.6 | 422.7 | |||||||||||||||||||
| $ | 1,530.2 | $ | 1,373.9 | $ | 2,989.8 | $ | 2,715.1 | ||||||||||||||||
26
Item 2. Management's Discussion and Analysis of Financial Condition and Results of Operations
Overview
The following discussion and analysis contains forward-looking statements within the meaning of the federal securities laws, and should be read in conjunction with the disclosures we make concerning risks and other factors that may affect our business and operating results. See “Note Regarding Forward-Looking Statements” preceding Part I, Item 1 in this Quarterly Report on Form 10-Q.
We are the global leader in patient-focused medical innovations for structural heart disease and critical care monitoring. Driven by a passion to help patients, we partner with the world's leading clinicians and researchers and invest in research and development to transform care for those impacted by structural heart disease or who require hemodynamic monitoring during surgery or in intensive care. We conduct operations worldwide and are managed in the following geographical regions: United States, Europe, Japan, and Rest of World. Our products are categorized into the following groups: Transcatheter Aortic Valve Replacement ("TAVR"), Transcatheter Mitral and Tricuspid Therapies ("TMTT"), Surgical Structural Heart ("Surgical"), and Critical Care.
Financial Highlights and Market Update
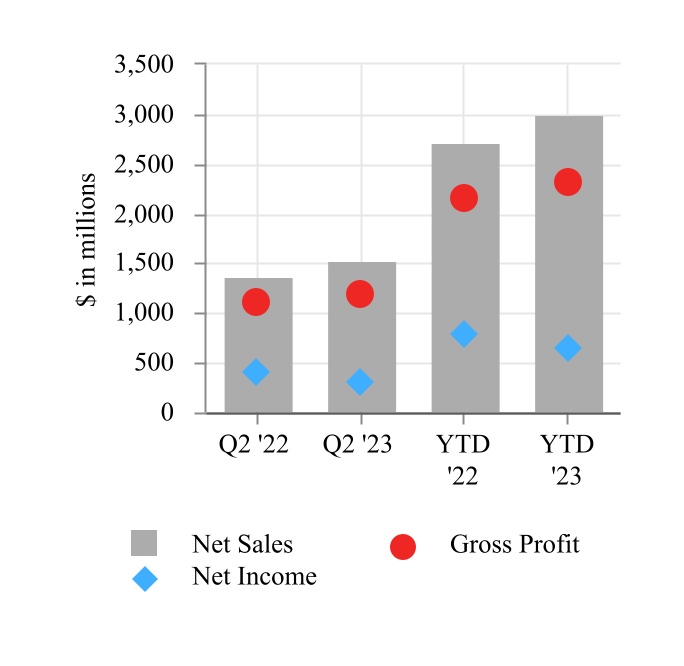
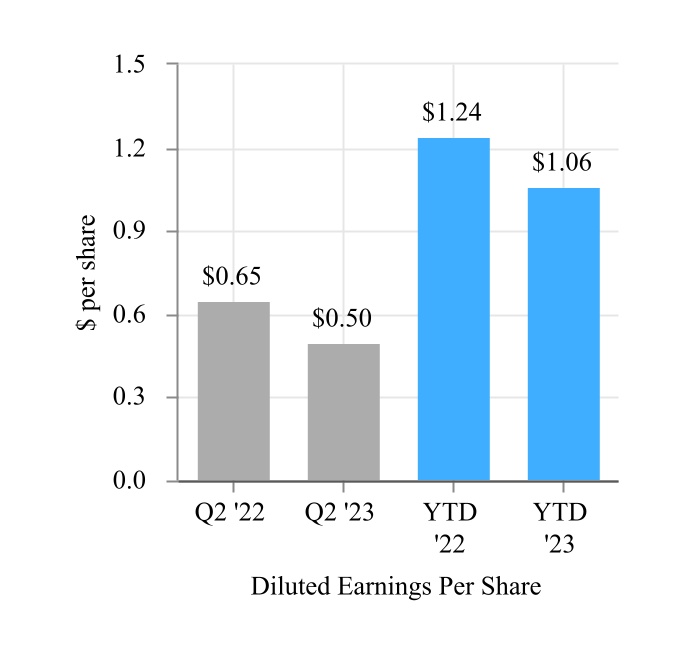
COVID-19 and Macroeconomic Uncertainties
While conditions related to the COVID-19 pandemic have improved compared to 2022, we have continued to experience the impacts of the COVID-19 pandemic in 2023, particularly relating to lingering headwinds in Japan and disruptions related to staffing shortages in the United States and Europe. The COVID-19 pandemic adversely impacted, and may further adversely impact, aspects of our business and markets, including our workforce and the operations of our customers, suppliers, and business partners. Our priority has been to maintain access for patients to our life-saving technologies while providing continuous front-line support to our clinician partners, and protecting the well-being of our employees. Our manufacturing operations have continued to respond to impacts related to COVID-19, and we have been able to supply our technologies around the world. Across our organization, we are proactively managing inventory, assessing alternative logistics options, and closely monitoring the supply of components to address potential supply constraints.
In addition to the impacts described above, the global economy, including the financial and credit markets, continues to experience extreme volatility and disruptions, including inflationary conditions, rising interest rates, declines in consumer confidence, and uncertainty about economic growth and stability. The severity and duration of the impact of these conditions on our business cannot be predicted. See Item 1A, "Risk Factors," of our Annual Report on Form 10-K for the year ended December 31, 2022 for additional information.
27
Financial Highlights
Despite the challenges to our business due to COVID-19 and macroeconomic factors, our net sales for the first six months of 2023 were $3.0 billion, representing an increase of $274.7 million over the first six months of 2022, driven primarily by sales of our TAVR products.
Our gross profit increased in the six months ended June 30, 2023, driven by our sales growth. Gross profit as a percentage of sales decreased primarily due to the impact of foreign currency exchange rate fluctuations. The decrease in our diluted earnings per share in the six months ended June 30, 2023 was driven by an after-tax charge of $142.2 million related to an intellectual property agreement.
Healthcare Environment, Opportunities, and Challenges
The medical technology industry is highly competitive and continues to evolve. Our success is measured both by the development of innovative products and the value we bring to our stakeholders. We are committed to developing new technologies and providing innovative patient care, and we are committed to defending our intellectual property in support of those developments. In the first six months of 2023, we invested 17.8% of our net sales in research and development.
We are dedicated to generating robust clinical, economic, and quality-of-life evidence increasingly expected by patients, clinicians, and payors in the current healthcare environment, with the goal of encouraging the adoption of innovative new medical therapies that demonstrate superior outcomes.
New Accounting Standards
Information on new accounting standards is included in Note 1 to the "Consolidated Condensed Financial Statements."
Results of Operations
Net Sales by Region
(dollars in millions)
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||||||||||||||||||||||||||
| Percent Change | Percent Change | ||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | 2022 | Change | 2023 | 2022 | Change | ||||||||||||||||||||||||||||||||||||||||||
| United States | $ | 895.3 | $ | 800.8 | $ | 94.5 | 11.8 | % | $ | 1,744.4 | $ | 1,550.3 | $ | 194.1 | 12.5 | % | |||||||||||||||||||||||||||||||
| Europe | 336.2 | 302.8 | 33.4 | 11.0 | % | 667.3 | 613.9 | 53.4 | 8.7 | % | |||||||||||||||||||||||||||||||||||||
| Japan | 117.9 | 122.9 | (5.0) | (4.1) | % | 232.0 | 258.4 | (26.4) | (10.2) | % | |||||||||||||||||||||||||||||||||||||
| Rest of World | 180.8 | 147.4 | 33.4 | 22.8 | % | 346.1 | 292.5 | 53.6 | 18.3 | % | |||||||||||||||||||||||||||||||||||||
| Outside of the United States | 634.9 | 573.1 | 61.8 | 10.8 | % | 1,245.4 | 1,164.8 | 80.6 | 6.9 | % | |||||||||||||||||||||||||||||||||||||
| Total net sales | $ | 1,530.2 | $ | 1,373.9 | $ | 156.3 | 11.4 | % | $ | 2,989.8 | $ | 2,715.1 | $ | 274.7 | 10.1 | % | |||||||||||||||||||||||||||||||
Net sales outside of the United States include the impact of foreign currency exchange rate fluctuations. The impact of foreign currency exchange rate fluctuations on net sales is not necessarily indicative of the impact on net income due to the corresponding effect of foreign currency exchange rate fluctuations on international manufacturing and operating costs, and our hedging activities.
Net Sales by Product Group
(dollars in millions)
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||||||||||||||||||||||||||
| Percent Change | Percent Change | ||||||||||||||||||||||||||||||||||||||||||||||
| 2023 | 2022 | Change | 2023 | 2022 | Change | ||||||||||||||||||||||||||||||||||||||||||
| Transcatheter Aortic Valve Replacement | $ | 991.6 | $ | 906.9 | $ | 84.7 | 9.3 | % | $ | 1,939.5 | $ | 1,788.2 | $ | 151.3 | 8.5 | % | |||||||||||||||||||||||||||||||
| Transcatheter Mitral and Tricuspid Therapies | 47.6 | 27.9 | 19.7 | 71.4 | % | 89.2 | 54.9 | 34.3 | 62.6 | % | |||||||||||||||||||||||||||||||||||||
| Surgical Structural Heart | 256.3 | 228.5 | 27.8 | 12.2 | % | 504.5 | 449.3 | 55.2 | 12.3 | % | |||||||||||||||||||||||||||||||||||||
| Critical Care | 234.7 | 210.6 | 24.1 | 11.4 | % | 456.6 | 422.7 | 33.9 | 8.0 | % | |||||||||||||||||||||||||||||||||||||
| Total net sales | $ | 1,530.2 | $ | 1,373.9 | $ | 156.3 | 11.4 | % | $ | 2,989.8 | $ | 2,715.1 | $ | 274.7 | 10.1 | % | |||||||||||||||||||||||||||||||
28
Transcatheter Aortic Valve Replacement Sales
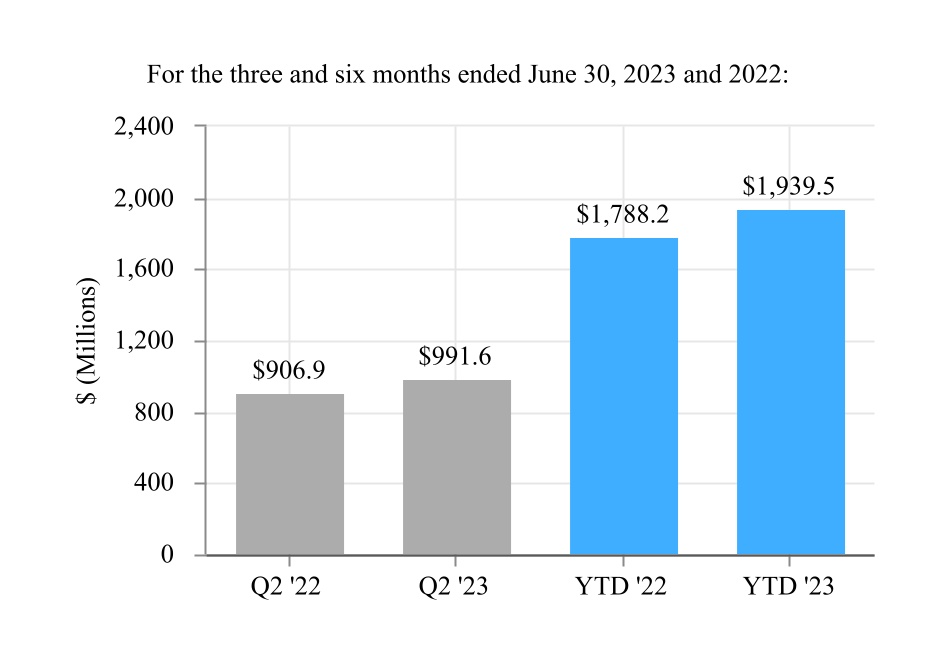
Net sales of TAVR products increased for the three and six months ended June 30, 2023 driven by:
•higher sales of the Edwards SAPIEN platform in 2023, primarily the Edwards SAPIEN 3 Ultra valve in the United States;
partially offset by:
•foreign currency exchange rate fluctuations, which decreased net sales outside of the United States by $3.3 million for the three months ended June 30, 2023, primarily due to the weakening of the Japanese yen against the United States dollar, and $28.5 million for the six months ended June 30, 2023, primarily due the weakening of the Japanese yen and the Euro against the United States dollar.
During the first half of 2023, we continued to enroll our PROGRESS pivotal trial, studying moderate aortic stenosis patients. In March 2023, we launched the Edwards SAPIEN 3 Ultra RESILIA valve in Japan. In July 2023, we announced the restart of enrollment in our pivotal trial, ALLIANCE, designed to study our next generation TAVR technology, SAPIEN X4.
29
Transcatheter Mitral and Tricuspid Therapies Sales
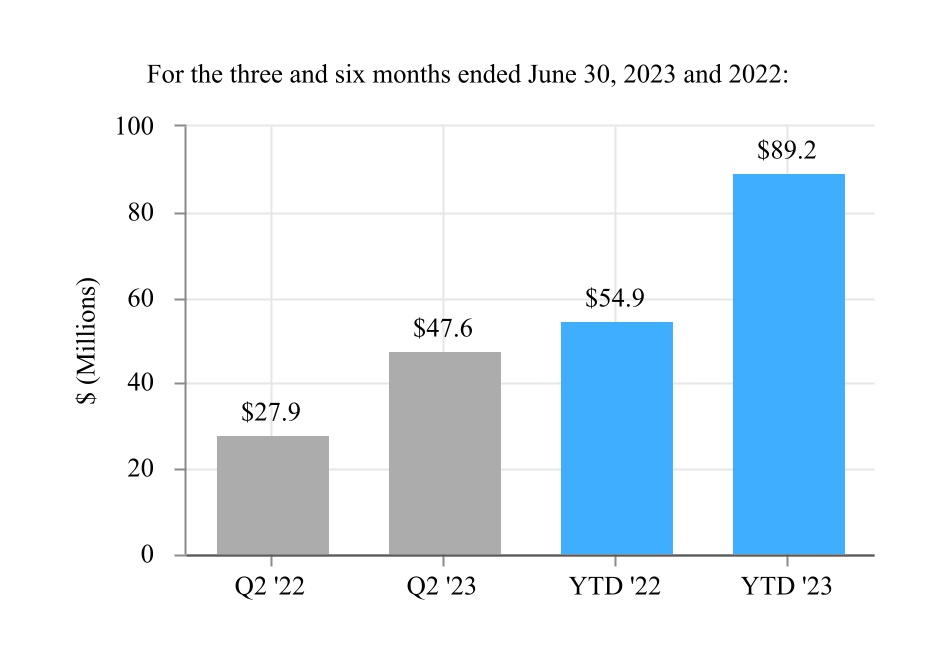
Net sales of TMTT products increased for the three and six months ended June 30, 2023 primarily due to the continued adoption of our PASCAL system in Europe and its launch in the United States.
During the first six months of 2023, we continued to enroll the CLASP IIF pivotal trial with PASCAL for patients with functional mitral regurgitation. In mitral replacement, enrollment continued in the ENCIRCLE pivotal trial for SAPIEN M3. In tricuspid, we completed the enrollment of the full cohort of the TRISCEND II pivotal trial of the EVOQUE replacement system. In the United States, in December 2022, the Food and Drug Administration approved continued access allowing hospitals that were involved in the clinical trial to continue to have EVOQUE as a therapy option. In addition, enrollment continued in the CLASP IITR pivotal trial with the PASCAL repair system in patients with symptomatic, severe tricuspid regurgitation.
30
Surgical Structural Heart Sales
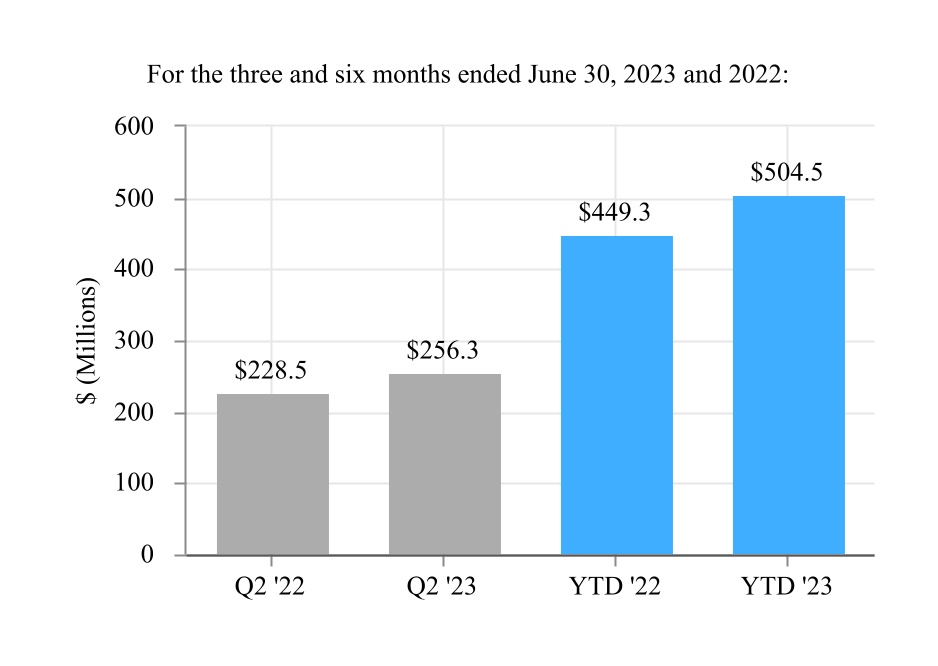
Net sales of Surgical products increased for the three and six months ended June 30, 2023 primarily due to increased sales of the INSPIRIS RESILIA aortic valve, primarily in the United States and Europe, and the MITRIS RESILIA valve, primarily in the United States. These increases were partially offset by the impact of foreign currency exchange rate fluctuations, which decreased net sales outside of the United States by $2.1 million for the three months ended June 30, 2023, primarily due to the weakening of the Japanese yen against the United States dollar, and $10.5 million for the six months ended June 30, 2023, primarily due to the weakening of the Japanese yen and the Euro against the United States dollar.
We are continuing to enroll patients in our MOMENTIS clinical study to demonstrate the durability of RESILIA tissue in the mitral position.
31
Critical Care Sales
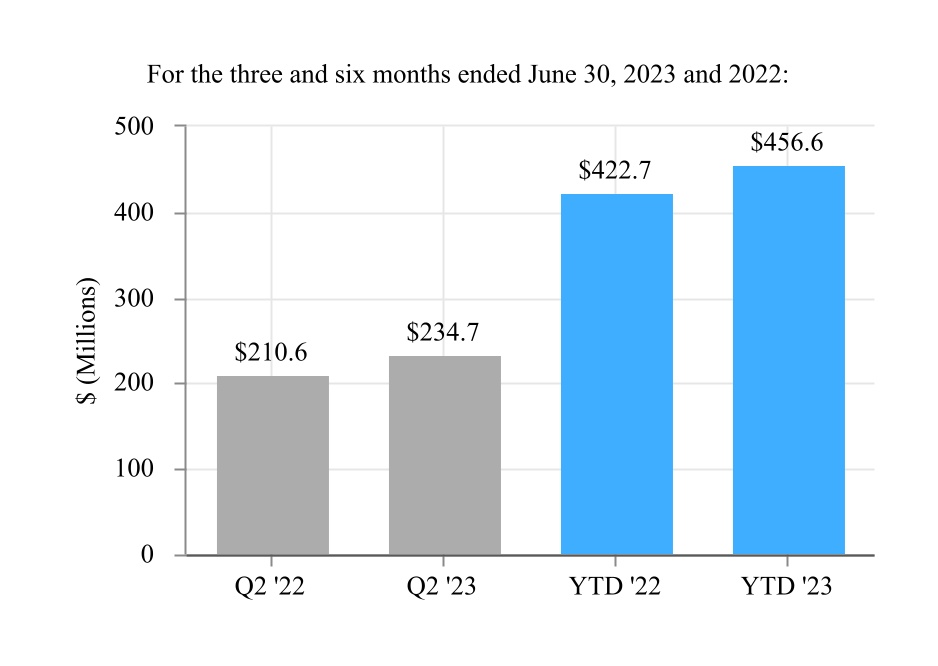
Net sales of Critical Care products increased for the three and six months ended June 30, 2023 primarily due to:
•increased demand for our enhanced surgical recovery products and pressure monitoring products, primarily in the United States;
partially offset by:
•foreign currency exchange rate fluctuations, which decreased net sales outside of the United States by $2.5 million and $11.3 million for the three and six months ended June 30, 2023, respectively, primarily due to the weakening of the Japanese yen against the United States dollar.
32
Gross Profit
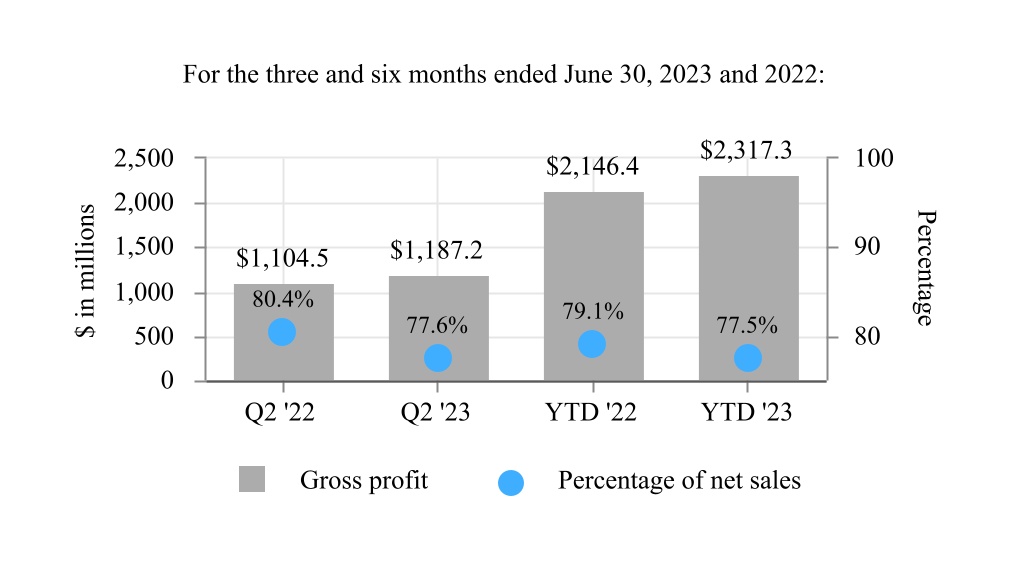
The decrease in gross profit as a percentage of net sales for the three and six months ended June 30, 2023 was driven by a 2.2 percentage point and 1.5 percentage point decrease, respectively, from the impact of foreign currency exchange rate fluctuations, primarily the strengthening of the United States dollar against the Euro.
Selling, General, and Administrative ("SG&A") Expenses
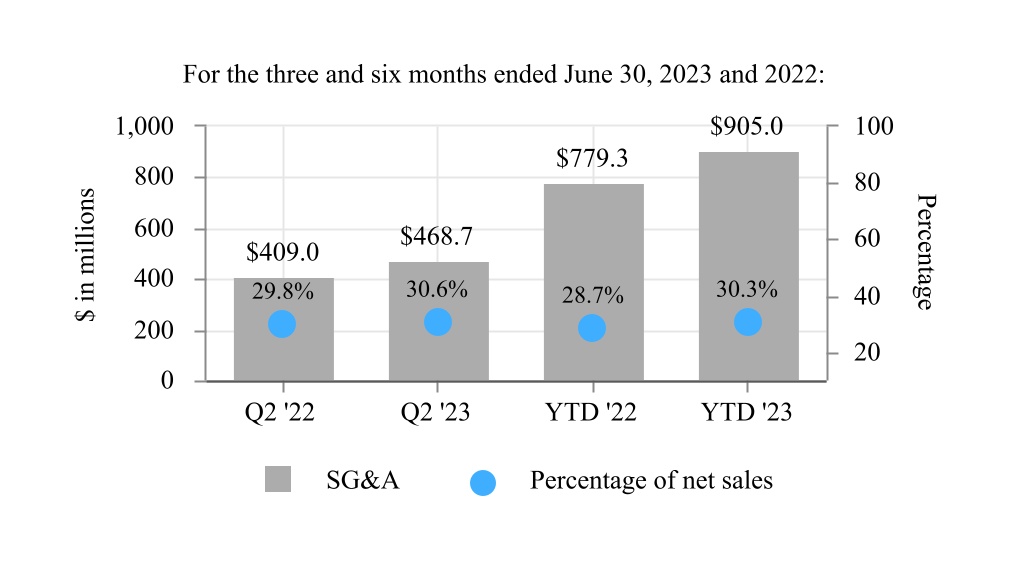
SG&A expenses increased for the three and six months ended June 30, 2023 primarily due to higher field-based personnel-related costs, primarily TAVR and TMTT in the United States, and higher performance-based compensation. Foreign currency exchange rate fluctuations decreased expenses by $1.4 million and $9.9 million for the three and six months ended June 30, 2023, respectively, due to the strengthening of the United States dollar against multiple foreign currencies.
33
Research and Development ("R&D") Expenses
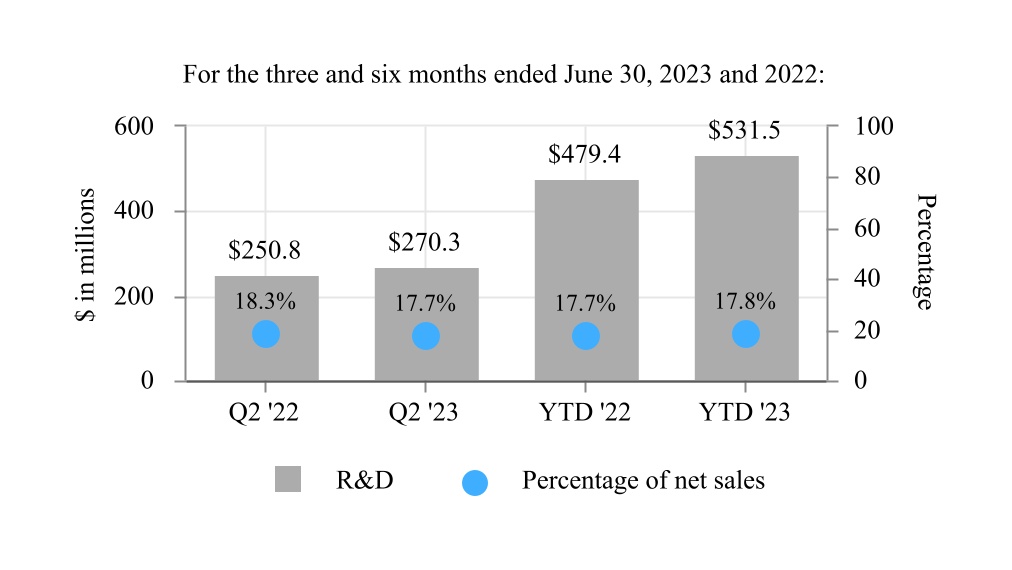
R&D expenses increased for the three and six months ended June 30, 2023 primarily due to continued investments in our transcatheter aortic valve innovations, including increased clinical trial activity.
Intellectual Property Agreement and Litigation Expense
We incurred intellectual property agreement and litigation expenses of $147.9 million and $6.1 million during the three months ended June 30, 2023 and 2022, respectively, and $191.4 million and $13.2 million during the six months ended June 30, 2023 and 2022, respectively. On April 12, 2023, we entered into an Intellectual Property Agreement (the "Intellectual Property Agreement") with Medtronic, Inc. ("Medtronic") and recorded a $37.0 million charge in March 2023 and a $139.0 million charge in April 2023. For more information, see Note 3 to the "Consolidated Condensed Financial Statements."
Change in Fair Value of Contingent Consideration Liabilities
The change in fair value of contingent consideration liabilities resulted in a gain of $26.9 million and $26.2 million for the three and six months ended June 30, 2023, respectively, and a gain of $20.9 million and $23.8 million for the three and six months ended June 30, 2022, respectively. The gains in 2023 and 2022 were each due to changes in projected probabilities and timing of milestone achievement and the projected timing of cash inflows. For further information, see Note 7 to the "Consolidated Condensed Financial Statements."
Other Income, net
(in millions)
| Three Months Ended June 30, | Six Months Ended June 30, | ||||||||||||||||||||||
| 2023 | 2022 | 2023 | 2022 | ||||||||||||||||||||
| Foreign exchange (gains) losses, net | $ | (2.5) | $ | 1.8 | $ | (3.8) | $ | 4.3 | |||||||||||||||
| Loss (gain) on investments | 0.3 | (0.8) | 0.2 | 0.1 | |||||||||||||||||||
| Gain on insurance settlement | — | (3.8) | — | (3.8) | |||||||||||||||||||
| Other | — | (1.5) | (0.2) | (1.6) | |||||||||||||||||||
| Other income, net | $ | (2.2) | $ | (4.3) | $ | (3.8) | $ | (1.0) | |||||||||||||||
The net foreign exchange (gains) losses relate to the foreign currency fluctuations primarily in our global trade and intercompany receivable and payable balances, partially offset by the gains and losses on foreign currency derivative instruments.
The loss (gain) on investments primarily represents our net share of gains and losses in investments accounted for under the equity method, and realized gains and losses on investments in equity securities.
34
The gain on insurance settlement in the three and six months ended June 30, 2022 relates to an insurance recovery for
damaged cargo shipments of heart valves.
Provision for Income Taxes
The provision for income taxes consists of provisions for federal, state, and foreign income taxes. We operate in an international environment with significant operations in various locations outside the United States which have statutory tax rates typically lower than the United States tax rate. Accordingly, the consolidated income tax rate is a composite rate reflecting the earnings in the various locations and the applicable rates.
Our effective income tax rate was 9.7% and 12.5% for the three months ended June 30, 2023 and 2022, respectively, and 12.4% and 13.4% for the six months ended June 30, 2023 and 2022, respectively. The decrease in the effective rate between the six months ended June 30, 2023 and 2022 is primarily due to an increase in the benefit from the Federal research and development credit and the Intellectual Property Agreement with Medtronic (see Note 3 to the "Consolidated Condensed Financial Statements"), partially offset by a reduced tax benefit from employee share-based compensation. In addition, the effective rates for the six months ended June 30, 2023 and 2022 were lower than the federal statutory rate of 21% primarily due to (1) foreign earnings taxed at lower rates, (2) Federal and California research and development credits, and (3) the tax benefit from employee share-based compensation.
In the normal course of business, the Internal Revenue Service (“IRS”) and other taxing authorities are in different stages of examining various years of our tax filings. During these audits we may receive proposed audit adjustments that could be material. Therefore, there is a possibility that an adverse outcome in these audits could have a material effect on our results of operations and financial condition. We strive to resolve open matters with each tax authority at the examination level and could reach agreement with a tax authority at any time. While we have accrued for matters we believe are more likely than not to require settlement, the eventual outcome with a tax authority may result in a tax liability that is more or less than that reflected in the consolidated financial statements. Furthermore, we may later decide to challenge any assessments, if made, and may exercise our right to appeal. The uncertain tax positions are reviewed quarterly and adjusted as events occur that affect potential liabilities for additional taxes, such as lapsing of applicable statutes of limitations, proposed assessments by tax authorities, negotiations between tax authorities, identification of new issues, and issuance of new legislation, regulations, or case law.
We executed an Advance Pricing Agreement ("APA") in 2018 between the United States and Switzerland governments for tax years 2009 through 2020 covering various, but not all, transfer pricing matters. The unagreed transfer pricing matters, namely Surgical Structural Heart and Transcatheter Aortic Valve Replacement (collectively "Surgical/TAVR") intercompany royalty transactions, then reverted to IRS Examination for further consideration as part of the respective years' regular tax audits. In addition, we executed other bilateral APAs as follows: during 2017, an APA between the United States and Japan covering tax years 2015 through 2019; and during 2018, APAs between Singapore and Japan and between Switzerland and Japan covering tax years 2015 through 2019. We have filed to renew all the APAs which cover transactions with Japan for the years 2020 and forward. An APA between Switzerland and Japan covering tax years 2020 through 2024 was executed in 2021. The execution of some or all these APA renewals depends on many variables outside of our control.
The audits of our United States federal income tax returns through 2014 have been closed. The IRS audit field work for the 2015 through 2017 tax years was completed during the second quarter of 2021, except for certain transfer pricing and related matters. The IRS began its examination of the 2018 through 2020 tax years during the first quarter of 2022.
The audits of our material state, local, and foreign income tax matters have been concluded for years through 2015. While not material, we continue to address matters in India for years from 2010 and on.
During 2021, we received a Notice of Proposed Adjustment (“NOPA”) from the IRS for the 2015-2017 tax years relating to transfer pricing involving Surgical/TAVR intercompany royalty transactions between our United States and Switzerland subsidiaries. The NOPA proposed an increase to our United States taxable income, which could result in additional tax expense for this period of approximately $220 million and reflects a significant departure from the transfer pricing methods we had previously agreed upon with the IRS for these types of transactions. We have disagreed with the NOPA and submitted a formal protest on the matter during the fourth quarter of 2021. During the second quarter of 2022, we received the IRS's rebuttal to our protest and were notified that the case had been transferred to the IRS Independent Office of Appeals ("Appeals"). The opening conference was held with Appeals during March 2023 and discussions with Appeals continued throughout the second quarter of 2023. While Appeals still maintains jurisdiction over the matter as of June 30, 2023, we and Appeals have now concluded that a satisfactory resolution of this matter at the administrative level is not possible.
35
We now plan to vigorously contest the proposed adjustments through the judicial process. Final resolution of this matter is not likely within the next 12 months. We believe the amounts previously accrued related to this uncertain tax position are appropriate based on prior experience and interpretation and application of relevant tax law and accounting standards to our facts and, accordingly, have not accrued any additional amount based on the NOPA received or as a result of the Appeals proceedings to date. Nonetheless, the outcome of the judicial process cannot be predicted with certainty, and it is possible that the outcome of that process could have a material impact on our consolidated financial statements. As noted below, similar material tax disputes may arise for the 2018-2023 tax years. While no payment of any amount related to the NOPA has yet been required, we made a partial deposit in November 2022 to prevent the further accrual of interest on that portion of any potential deficiency that may ultimately be sustained.
Surgical/TAVR intercompany royalty transactions covering tax years 2018-2023 that were not resolved under the APA program remain subject to IRS examination, and those transactions and related tax positions remain uncertain as of June 30, 2023. We have considered this information, as well as information regarding the NOPA, the rebuttal and Appeals discussions described above, in our evaluation of our uncertain tax positions. The impact of these unresolved transfer pricing matters, net of any correlative tax adjustments, may be significant to our consolidated financial statements. Based on the information currently available and numerous possible outcomes, we cannot reasonably estimate what, if any, changes in our existing uncertain tax positions may occur in the next 12 months and, therefore, have continued to record the uncertain tax positions as a long-term liability.
On August 16, 2022, the Inflation Reduction Act of 2022 (“IRA”) was signed into law. The IRA includes, among other provisions, changes to the U.S. corporate income tax system, including a 15% minimum tax based on “adjusted financial statement income,” which is effective for tax years beginning after December 31, 2022, and a one percent excise tax on net repurchases of stock after December 31, 2022. Based upon our analysis to date, we do not expect the IRA will have a material impact on our consolidated financial statements.
Liquidity and Capital Resources
Our sources of cash liquidity include cash and cash equivalents, short-term investments, cash from operations, and amounts available under credit facilities. We believe that these sources are sufficient to fund the current and long-term requirements of working capital, capital expenditures, and other financial commitments. However, we periodically consider various financing alternatives and may, from time to time, seek to take advantage of favorable interest rate environments or other market conditions.
As of June 30, 2023, cash and cash equivalents and short-term investments held in the United States and outside of the United States were $1,177.3 million and $332.0 million, respectively.
We have a Five-year Credit Agreement (the "Credit Agreement") which provides for a $750.0 million multi-currency unsecured revolving credit facility and matures on July 15, 2027. We may increase the amount available under the Credit Agreement by up to an additional $250.0 million in the aggregate and extend the maturity date for an additional year, subject to agreement of the lenders. As of June 30, 2023, no amounts were outstanding under the Credit Agreement.
In June 2018, we issued $600.0 million of 4.3% fixed-rate unsecured senior notes (the "2018 Notes") due June 15, 2028. As of June 30, 2023, the carrying value of the 2018 Notes was $596.7 million.
From time to time, we repurchase shares of our common stock under share repurchase programs authorized by the Board of Directors. We consider several factors in determining when to execute share repurchases, including, among other things, expected dilution from stock plans, cash capacity, and the market price of our common stock. During the six months ended June 30, 2023, under the Board authorized repurchase program, we repurchased a total of 3.2 million shares at an aggregate cost of $249.1 million. See Part II, Item 2, “Unregistered Sales of Equity Securities and Use of Proceeds,” for additional information about our share repurchase program. As of June 30, 2023, we had remaining authority to purchase $666.5 million of our common stock under the share repurchase program.
On April 12, 2023, we entered into the Intellectual Property Agreement with Medtronic pursuant to which the parties agreed to a 15-year global covenant not to sue ("CNS") for infringement of certain patents in the structural heart space owned or controlled by each other. In consideration for the global CNS, we paid Medtronic a one-time, lump sum payment of $300.0 million and will pay annual royalty payments that are tied to net sales of certain Edwards products. For more information, see Note 3 to the "Consolidated Condensed Financial Statements."
36
On February 28, 2023, we acquired a majority equity interest in a medical technology company. In addition, we amended and restated our previous option agreement with the medical technology company. The option agreement gives us the option to acquire the remaining equity interest in the medical technology company. For more information, see Note 6 to the "Consolidated Condensed Financial Statements."
At June 30, 2023, there had been no material changes in our cash requirements from known contractual and other obligations, including commitments for capital expenditures, as disclosed in Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations," of our Annual Report on Form 10-K for the year ended December 31, 2022.
Consolidated Cash Flows - For the six months ended June 30, 2023 and 2022:
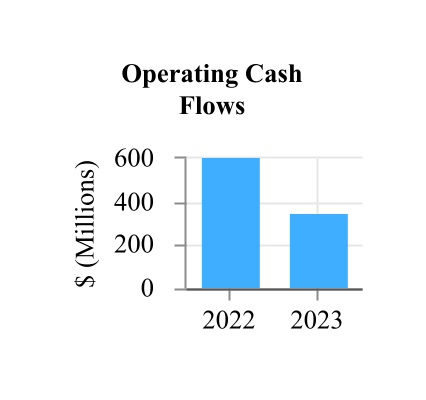
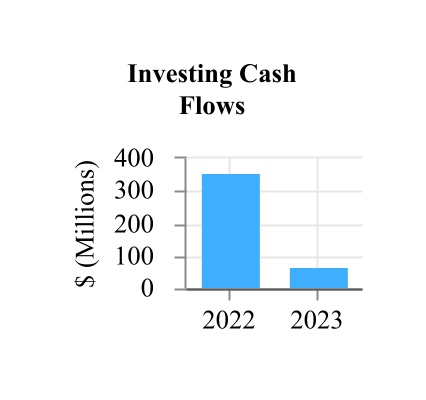
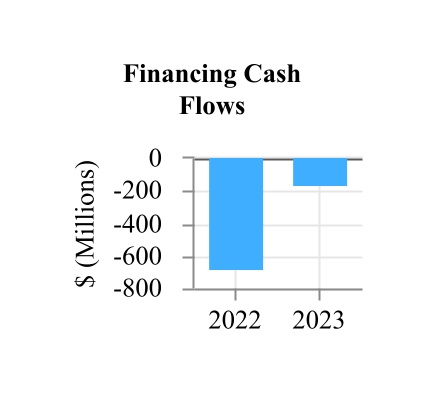
Net cash flows provided by operating activities of $347.7 million for the six months ended June 30, 2023 decreased $277.8 million over the same period last year primarily due to a $300.0 million payment in 2023 under the Intellectual Property Agreement, partially offset by a higher bonus payout in 2022 associated with 2021 performance.
Net cash provided by investing activities of $70.7 million for the six months ended June 30, 2023 consisted primarily of net proceeds from investments of $371.8 million, partially offset by a payment of $141.2 million to acquire a majority interest in another company (see Note 6 to the "Consolidated Condensed Financial Statements") and capital expenditures of $109.4 million.
Net cash provided by investing activities of $359.1 million for the six months ended June 30, 2022 consisted primarily of net proceeds from investments of $558.4 million, partially offset by capital expenditures of $115.8 million.
Net cash used in financing activities of $154.5 million for the six months ended June 30, 2023 consisted primarily of purchases of treasury stock of $256.8 million, partially offset by proceeds from stock plans of $102.7 million.
Net cash used in financing activities of $676.2 million for the six months ended June 30, 2022 consisted primarily of purchases of treasury stock of $760.7 million, partially offset by proceeds from stock plans of $86.5 million.
Critical Accounting Policies and Estimates
The consolidated condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States which require us to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated condensed financial statements and sales and expenses during the periods reported. Actual results could differ from those estimates. Information with respect to our critical accounting policies and estimates which we believe could have the most significant effect on our reported results and require subjective or complex judgments by management is contained on pages 34-36 in Item 7, "Management's Discussion and Analysis of Financial Condition and Results of Operations," of our Annual Report on Form 10-K for the year ended December 31, 2022. There have been no significant changes from the information discussed therein.
37
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk, Foreign Currency Risk, Credit Risk, and Concentrations of Risk
For a complete discussion of our exposure to interest rate risk, foreign currency risk, credit risk, and concentrations of risk, refer to Item 7A "Quantitative and Qualitative Disclosures About Market Risk" in our Annual Report on Form 10-K for the year ended December 31, 2022. There have been no material changes from the information discussed therein.
Investment Risk
We are exposed to investment risks related to changes in the underlying financial condition and credit capacity of certain of our investments. As of June 30, 2023, we had $1.2 billion of investments in debt securities, of which $0.7 billion were long-term. In addition, we had $110.4 million of investments in equity instruments of public and private companies. Should these companies experience a decline in financial performance, financial condition or credit capacity, or fail to meet certain development milestones, a decline in the investments' value may occur, resulting in unrealized or realized losses. See Note 4 to the "Consolidated Condensed Financial Statements" for additional information.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures. Our management, including the Chief Executive Officer and the Chief Financial Officer, performed an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended) as of June 30, 2023. Based on their evaluation, the Chief Executive Officer and Chief Financial Officer have concluded as of June 30, 2023 that our disclosure controls and procedures are designed at a reasonable assurance level and effective in providing reasonable assurance that the information we are required to disclose in the reports we file or submit under the Securities Exchange Act of 1934, as amended, is recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission's rules and forms, and that such information is accumulated and communicated to our management, including the Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Changes in Internal Control Over Financial Reporting. There have been no changes in our internal control over financial reporting during the quarter ended June 30, 2023 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
38
Part II. Other Information
Item 1. Legal Proceedings
We are reviewing and investigating whether business activities in Japan and other markets violate certain provisions of the Foreign Corrupt Practices Act ("FCPA"). We voluntarily notified the United States Securities and Exchange Commission ("SEC") and the United States Department of Justice ("DOJ") during 2021 that we have engaged outside counsel to conduct this review and investigation. We have provided status updates to the SEC and DOJ since that time. Any determination that our operations or activities are not in compliance with existing laws, including the FCPA, could result in the imposition of fines, penalties, and equitable remedies. We cannot currently predict the outcome of the review and investigation or the potential impact on our financial statements.
On September 28, 2021, Aortic Innovations LLC, a non-practicing entity, filed a lawsuit against Edwards Lifesciences Corporation and certain of its subsidiaries (“Edwards”) in the United States District Court for the District of Delaware alleging that Edwards’ SAPIEN 3 Ultra product infringes certain of its patents. We are unable to predict the ultimate outcome of this matter or estimate a range of possible exposure; therefore, no amount has been accrued. We intend to vigorously defend ourselves in this litigation.
We are subject to various environmental laws and regulations both within and outside of the United States. Our operations, like those of other medical device companies, involve the use of substances regulated under environmental laws, primarily in manufacturing and sterilization processes. While it is difficult to quantify the potential impact of continuing compliance with environmental protection laws, management believes that such compliance will not have a material impact on our financial results. Our threshold for disclosing material environmental legal proceedings involving a governmental authority where potential monetary sanctions are involved is $1 million.
Item 1A. Risk Factors
A description of the risk factors associated with our business is contained in the “Risk Factors” section of our Annual Report on Form 10-K for our fiscal year ended December 31, 2022. There have been no material changes to our risk factors as previously reported.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
Issuer Purchases of Equity Securities
| Period | Total Number of Shares (or Units) Purchased (a) | Average Price Paid per Share (or Unit) | Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs | Approximate Dollar Value of Shares that May Yet Be Purchased Under the Plans or Programs (in millions) (b) | |||||||||||||||||||||||||||||||
| April 1, 2023 through April 30, 2023 | 258 | $ | 82.77 | — | $ | 666.5 | |||||||||||||||||||||||||||||
| May 1, 2023 through May 31, 2023 | 83,511 | 88.49 | — | 666.5 | |||||||||||||||||||||||||||||||
| June 1, 2023 through June 30, 2023 | — | — | — | 666.5 | |||||||||||||||||||||||||||||||
| Total | 83,769 | 88.48 | — | ||||||||||||||||||||||||||||||||
(a) The difference between the total number of shares (or units) purchased and the total number of shares (or units) purchased as part of publicly announced plans or programs is due to shares withheld by us to satisfy tax withholding obligations in connection with the vesting of restricted stock units issued to employees.
(b) In July 2022, the Board of Directors approved a stock repurchase program providing for up to $1.5 billion of repurchases of our common stock, effective July 28, 2022. Repurchases under the program may be made on the open market, including pursuant to a Rule 10b5-1 plan, and in privately negotiated transactions. The repurchase program does not have an expiration date.
39
Item 5. Other Information
Rule 10b5-1 Trading Plans
On May 30, 2023, Daveen Chopra, Corporate Vice President of TMTT, entered into a 10b5-1 trading plan (the “Plan”) intended to satisfy the affirmative defense of Rule 10b5-1(c) under the Exchange Act. The Plan provides for the potential sale of 20,500 shares of the Company’s stock commencing September 6, 2023. The Plan terminates on the earlier of March 6, 2024 or the date all shares are sold.
Item 6. Exhibits
The exhibits listed in the Exhibit Index below are filed, furnished, or incorporated by reference as part of this report on Form 10-Q.
| Exhibit No. | Description | ||||||||||
| 3.1 | |||||||||||
| 3.2 | |||||||||||
| 3.3 | |||||||||||
| 3.4 | |||||||||||
| 31.1 | |||||||||||
| 31.2 | |||||||||||
| 32 | |||||||||||
| 101.INS | XBRL Inline Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | ||||||||||
| 101.SCH | XBRL Taxonomy Extension Schema Document | ||||||||||
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | ||||||||||
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document | ||||||||||
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document | ||||||||||
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | ||||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | ||||||||||
40
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| EDWARDS LIFESCIENCES CORPORATION | |||||||||||
| (Registrant) | |||||||||||
| Date: | July 28, 2023 | By: | /s/ SCOTT B. ULLEM | ||||||||
Scott B. Ullem Chief Financial Officer (Principal Financial Officer; Duly Authorized Officer) | |||||||||||
| Date: | July 28, 2023 | By: | /s/ ROBERT W.A. SELLERS | ||||||||
Robert W.A. Sellers Corporate Controller (Principal Accounting Officer) | |||||||||||
41