Exhibit 10.3
CO-EXCLUSIVE LICENSE AGREEMENT
This LICENSE AGREEMENT (“Agreement”), dated as of June 2, 2005 (the “Effective Date”), is by and between Edwards Lifesciences PVT, Inc., a Delaware corporation (“Edwards”), on the one hand, and 3F Therapeutics, Inc., a Delaware corporation (“3F”), on the other hand. Each of Edwards and 3F may be referred to herein individually as a “Party” or collectively as the “Parties.”
RECITALS
WHEREAS Edwards is the owner or has the right to sublicense certain patents and patent applications relating to catheter-delivered heart valves and venous valves;
WHEREAS 3F desires to obtain a co-exclusive license to such patents and patent applications in the Fields of Use defined below; and
WHEREAS Edwards is willing to grant such license to 3F under the following terms and conditions.
AGREEMENT
NOW, THEREFORE, in consideration of the covenants and agreements set forth herein, which constitutes good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
1. Definitions. The following definitions shall apply to the following terms:
1.1 “3F” shall have the meaning set forth in the preamble.
1.2 “Additional License” shall mean either a Compelled License or a Granted License.
1.3 “Affiliate” shall mean, with respect to any specified Person, a Person that directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, the Person specified.
1.4 “Agreement” shall have the meaning set forth in the preamble.
1.5 “Calendar Quarter” shall mean each three-month-period beginning January 1, April 1, July 1, and October 1 of each calendar year.
1.6 “Combination Price” shall be calculated by multiplying the gross invoice price charged and the value of any other consideration owed for such combined Licensed Product or Unauthorized Product and other product(s) that are not Licensed Products or Unauthorized Products by the fraction 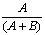 where A is the gross selling price of the Licensed Product or Unauthorized Product sold separately and B is the gross selling price of the other product(s) sold separately. Notwithstanding any of the foregoing, in no event shall the fraction
where A is the gross selling price of the Licensed Product or Unauthorized Product sold separately and B is the gross selling price of the other product(s) sold separately. Notwithstanding any of the foregoing, in no event shall the fraction 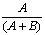 used in the calculation of the Combination Price be less than one-half (1/2).
used in the calculation of the Combination Price be less than one-half (1/2).
1
1.7 “Compelled License” shall have the meaning set forth in Section 7.5.
1.8 “Edwards” shall have the meaning set forth in the preamble.
1.9 “Effective Date” shall have the meaning set forth in the preamble.
1.10 “Field-Restricted Royalty” shall have the meaning set forth in Section 4.1.
1.11 “Fields of Use” shall mean the Surgical Field of Use and the Venous Field of Use.
1.12 “Granted License” shall have the meaning set forth in Section 7.5.
1.13 “Heartport License” shall mean the license agreement, dated December 21, 2000, by and between Heartport, Inc., together with its predecessor Stanford Surgical Technologies, and Edwards’ predecessor-in-interest Percutaneous Valve Technologies, Inc., attached hereto as Exhibit D.
1.14 “Licensed Patents” shall mean the Surgical Licensed Patents and the Venous Licensed Patents.
1.15 “Licensed Products” shall mean the Surgical Licensed Products and the Venous Licensed Products.
1.16 “Litigation” shall have the meaning set forth in Section 7.2.
1.17 “Litigation Expenses” shall mean all attorneys’ fees and costs (including reasonable fees and costs attributable for work performed by in-house counsel), all expert fees and costs, all court and/or arbitration expenses, and any other fees and costs properly incurred, including any fees and costs incurred in bringing and prosecuting the Litigation and/or enforcing any order, judgment, ruling, or award granted as part of such Litigation.
1.18 “Net Invoice Price” means (a) the gross invoice price charged and the value of any other consideration owed for a Licensed Product or Unauthorized Product, or (b) in those instances where the Licensed Product or Unauthorized Product is sold in combination with one or more other products that are not Licensed Products or Unauthorized Products, the Combination Price, less the following items, but only to the extent that they actually pertain to the disposition of such Licensed Product or Unauthorized Product and are identified separately on a bill or invoice:
i) allowances actually granted to customers for rejections, returns, or prompt payment and volume discounts;
ii) freight, transport packing, and insurance charges associated with transportation; and
iii) rebates or discounts paid or credited pursuant to applicable law.
2
Notwithstanding the foregoing, for purposes of this Section and this Agreement, in no event shall the Combination Price be used if such other product(s) are essential for the use or delivery of the Licensed Product or Unauthorized Product. By way of example, the Net Invoice Price of a stented, tissue heart valve combined with a catheter delivery system shall be the gross invoice price charged and the value of any other consideration owed for both the stented, tissue heart valve and catheter delivery system, and not the Combination Price.
1.19 “Net Sales” shall mean the Net Invoice Price charged by 3F or its Affiliates to a Third Party.
1.20 “Outside-Field Royalty” shall have the meaning set forth in Section 4.2.
1.21 “Party” shall have the meaning set forth in the preamble.
1.22 “Parties” shall have the meaning set forth in the preamble.
1.23 “Person” shall mean any individual, partnership, firm, corporation, association, trust, unincorporated organization or other entity, as well as any syndicate or group that would be deemed to be a person under Section 13(d)(3) of the Exchange Act.
1.24 “Royalty” or “Royalties” shall mean the Field-Restricted Royalty and/or Outside-Field Royalty, as appropriate.
1.25 “Royalty Report” shall have the meaning set forth in Section 5.1.
1.26 “Sublicensed Patents” shall mean (i) the patents and patent applications listed on Exhibit A attached hereto and (ii) any continuations, divisionals, reexaminations, reissues, extensions and foreign counterparts thereof, if and only to the extent that such rights are possessed by Edwards.
1.27 “Surgical Field of Use” shall mean the surgical insertion through the chest cavity of a stented, tissue heart valve using a catheter delivery system of no greater than 50 centimeters in usable length (i.e., the length that can be inserted into the human body); provided, however, that such Surgical Field of Use does not include the delivery of a stented, tissue heart valve to the heart by way of any vessel other than solely through the thoracic aorta.
1.28 “Surgical Licensed Patents” shall mean the patents and patent applications listed on Exhibit B attached hereto, and any continuations, divisionals, reexaminations, reissues, extensions and foreign counterparts thereof.
1.29 “Surgical Licensed Product” shall mean a product in the Surgical Field of Use covered by any Valid Claim of the Surgical Licensed Patents or Sublicensed Patents.
3
1.30 “Third Party” shall mean any Person other than Edwards, 3F, or an Affiliate of either.
1.31 “Unauthorized Product” shall mean a product sold by 3F that would be a Licensed Product under this Agreement if such product was used in the Surgical Field of Use or Venous Field of Use, as applicable, but which is actually used, with or without the direct involvement or prior knowledge of 3F, outside such applicable Field of Use.
1.32 “Valid Claim” shall mean a claim of an issued and unexpired patent included in the Licensed Patents or Sublicensed Patents which has not been revoked or held unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and which has not been disclaimed, denied, or admitted to be invalid or otherwise unenforceable through reissue, reexamination, disclaimer, or otherwise.
1.33 “Venous Field of Use” shall mean the treatment and repair outside of the heart of venous valves with venous insufficiency; provided, however, that such Venous Field of Use shall also include venous valves placed in the superior or inferior vena cava for treatment of the tricuspid valve.
1.34 “Venous Licensed Patents” shall mean the patents and patent applications listed on Exhibit C attached hereto, and any continuations, divisionals, reexaminations, reissues, extensions and foreign counterparts thereof.
1.35 “Venous Licensed Product” shall mean a product in the Venous Field of Use covered by any Valid Claim of the Venous Licensed Patents or Sublicensed Patents.
2. License Grants.
2.1 Edwards hereby grants to 3F a worldwide, non-transferable (except as set forth in Section 13.2.1 below), royalty-bearing, co-exclusive license under the Surgical Licensed Patents, without the right to sublicense, to manufacture, have manufactured, use, import, offer for sale, and sell Surgical Licensed Products solely in the Surgical Field of Use.
2.2 Edwards hereby further grants to 3F a worldwide, non-transferable (except as set forth in Section 13.2.1 below), royalty-bearing, co-exclusive license under the Venous Licensed Patents, without the right to sublicense, to manufacture, have manufactured, use, import, offer for sale, and sell Venous Licensed Products solely in the Venous Field of Use.
2.3 Edwards also hereby further grants to 3F a worldwide, non-transferable (except as set forth in Section 13.2.1 below), royalty-bearing co-exclusive sublicense under the Sublicensed Patents, without the right to further sublicense, to manufacture, have manufactured, use, import, offer for sale, and sell Licensed Products solely in the Fields of Use.
2.4 3F agrees and acknowledges that the limited scope of the Fields of Use set forth in this Agreement are a material and essential part of this Agreement. As such, the licenses and sublicenses set forth in this Section 2 include a covenant by 3F to refrain from taking any actions that would permit, encourage, promote, market, fund, sponsor, or facilitate, directly or indirectly,
4
any off label use or any other use of any Licensed Product outside the Surgical Field of Use or Venous Field of Use, as applicable, and to use its best efforts to prevent any such off label use or any other use outside such applicable Field of Use. Any breach by 3F of this covenant shall be deemed a material breach of this Agreement.
2.5 The co-exclusive licenses and sublicense set forth in this Section 2 shall be co-exclusive with Edwards, its Affiliates, successors, and/or assigns. During the term of this Agreement, Edwards shall have no right to grant to any third party any license under the Surgical Licensed Patents in the Surgical Field of Use or Venous Licensed Patents in the Venous Field of Use or sublicense under the Sublicensed Patents in the Surgical Field of Use or the Venous Field of Use, except as provided in Section 7.5 hereof.
2.6 No implied patent or other intellectual property rights or licenses are granted by Edwards hereunder or in connection herewith other than those licenses and the sublicense expressly granted in this Agreement.
2.7 3F, as a sublicensee to the Sublicensed Patents, hereby agrees to comply with all terms and conditions applicable to a sublicensee under the Heartport License.
3. Product Labeling.
3.1 Upon written notice from Edwards of a specific Licensed Patent that applies to a specific Licensed Product, each Licensed Product sold by 3F shall be marked in compliance with 35 U.S.C. § 287, or the relevant corresponding statute under the law of the country in which the Licensed Product is sold.
3.2 Each Licensed Product manufactured by or for 3F shall be labeled for use solely in the Surgical Field of Use or Venous Field of Use, as applicable.
4. Royalties.
4.1 In consideration of the grant of rights under the Licensed Patents and Sublicensed Patents in this Agreement, and except as provided by Section 4.2 below, 3F shall pay Edwards a royalty (the “Field-Restricted Royalty”) of four percent (4%) of the Net Sales of any Licensed Product sold by 3F. The Field-Restricted Royalty accrues and shall be deemed payable at the time that each Licensed Product is sold.
4.2 In light of the essential nature of the limited scope of the Fields of Use, 3F shall pay Edwards a royalty (the “Outside-Field Royalty”) of twenty-five percent (25%) of the Net Sales of all Unauthorized Products above the first fifty (50) Unauthorized Products sold by 3F in any year during the term hereof. 3F agrees and acknowledges that the Outside-Field Royalty represents a fair and reasonable Royalty for such Unauthorized Products and shall not be considered a penalty or fine.
5. Payments.
5.1 Within thirty (30) days of the last day of each Calendar Quarter, 3F shall pay Edwards all Royalties accrued during such Calendar Quarter, along with a written report (the
5
“Royalty Report”) detailing for each country, for the Calendar Quarter covered, the calculation of any Royalties along with (i) the number of Surgical and Venous Licensed Products sold by 3F; (ii) the Net Sales charged for each such Licensed Product; (iii) the number of any Unauthorized Products; and (iv) the Net Sales charged for each such Unauthorized Product.
5.2 No Royalties accrued under this Agreement shall be reduced by any taxes, fees, or other charges imposed by the government of such country on the remittance of such Royalties. 3F shall further be responsible for all bank charges associated with remittance of any Royalties.
5.3 All Royalties accrued pursuant to this Agreement shall be paid in United States dollars. When Licensed or Unauthorized Products are sold for monies other than United States dollars, the Royalties will be first determined in such foreign currency and then converted into equivalent United States dollars based on the exchange rate quoted in The Wall Street Journal on the last business day of the applicable Calendar Quarter.
5.4 Any amount payable to Edwards pursuant to this Agreement and which is not paid when due shall bear interest at the lesser of (i) one percent (1.0%) per month or (ii) the maximum amount allowable by law.
5.5 Within five (5) days of becoming insolvent, as defined in Section 9.3, 3F shall give written notice to Edwards of such insolvency.
6. Right to Audit.
6.1 3F shall keep, and maintain, complete and accurate records concerning the sale of Licensed or Unauthorized Products by 3F at its principal executive offices in California or such other locations as the Parties shall agree. On fourteen (14) days prior written notice by Edwards, 3F shall permit said records to be inspected at Edward’s expense, at any time, by an independent auditor appointed by Edwards and reasonably accepted by 3F for this purpose. In the event of an underpayment by 3F, 3F shall immediately pay Edwards for any such underpayment plus applicable interest. In the event that the audit reveals an underpayment of Royalties by 3F of more than five percent (5%), 3F shall reimburse Edwards for all such reasonable costs of such audit.
7. Infringement and Enforcement.
7.1 If any time during the term of this Agreement, 3F learns or believes that any Third Party is infringing any of the Licensed Patents or Sublicensed Patents hereunder in the Fields of Use, 3F shall notify Edwards in writing of the existence of such alleged infringement.
7.2 Edwards may, but has no obligation to, take any and all actions to enforce the Licensed Patents or Sublicensed Patents (including without limitation instituting litigation) against any infringement in the Fields of Use (“Litigation”); provided, however, that Edwards agrees that, upon notice from 3F pursuant to Section 7.1, Edwards will make a good faith evaluation as to whether and when to institute any such Litigation, in light of the factors that Edwards believes are relevant, including without limitation the potential costs and risks associated with such Litigation. Edwards shall bear all the expenses and costs with respect to
6
any such Litigation and, except as expressly set forth herein, Edwards shall be entitled to all damages recovered in such Litigation.
7.3 At Edwards’ request, 3F agrees to cooperate in any such Litigation, including but not limited to participating in such Litigation as a named party. Edwards agrees to pay 3F’s reasonable costs and expenses in connection with such Litigation. In the event that 3F desires to retain separate counsel in connection with such Litigation, however, 3F shall bear its own costs and expenses concerning the Litigation, including without limitation the costs and expenses of such separate counsel. Notwithstanding 3F’s participation in such Litigation, Edwards shall retain the full right to control such Litigation, including without limitation any settlement of such Litigation. Edwards shall have the right, in its sole and absolute discretion, to settle any Litigation on such terms and conditions that Edwards deems to be appropriate.
7.4 With respect to any monetary award received by Edwards in the Litigation, or amounts received by Edwards in settlement of the Litigation:
(i) Attributable to infringement of the Sublicensed Patents in the Fields of Use, and after deducting all Litigation Expenses, 3F shall be entitled to receive twenty-four percent (24%) of such award or settlement; or
(ii) Attributable to infringement of the Licensed Patents in the Fields of Use, and after deducting all Litigation Expenses, 3F shall be entitled to receive forty-eight percent (48%) of such award or settlement.
7.5 To the extent not governed by Section 7.4 above, in the event that, in connection with any settlement of the Litigation, Edwards grants a license within the Fields of Use and receives on-going royalty payments for such license in the Fields of Use (“Granted License”) or, in the event that an injunction against future infringement is not granted and a party is permitted to continue to practice either the Licensed Patents or the Sublicensed Patents within the Fields of Use and Edwards receives on-going royalty payments for such practice in the Fields of Use (“Compelled License”), then:
(i) With respect to such royalty payments received for such Additional License for the Sublicensed Patents, and after deducting all Litigation Expenses to the extent such Litigation Expenses have not been reimbursed pursuant to Section 7.4, 3F shall be entitled to receive twenty-four percent (24%) of such payments; or
(ii) With respect to such royalty payments received for such Additional License for the Licensed Patents, and after deducting all Litigation Expenses to the extent such Litigation Expenses have not been reimbursed pursuant to Section 7.4, 3F shall be entitled to receive forty-eight percent (48%) of such payments.
7.6 Nothing in this Agreement shall require Edwards to provide 3F with any compensation or portion of any recovery in connection with any litigation initiated by Heartport, Inc., or its successor or assignee, in accordance with Section 5.4 of the Heartport License.
7
7.7 In no event shall any amounts pursuant to Sections 7.4 and/or 7.5 be payable by Edwards to 3F before thirty (30) days after Edwards actually receives payment of such amounts from the Third Party.
8. Representations and Warranties.
8.1 Edwards hereby represents and warrants to 3F that
(i) it has all of the requisite power and authority to enter into this Agreement and to perform its obligations hereunder and that this Agreement has been duly and validly authorized, executed, and delivered by Edwards; and
(ii) it is the owner of, or has the exclusive license to, the patents and patent applications subject to the Agreement and that it has the entire right to enter into these licenses.
8.2 EXCEPT AS EXPRESSLY SET FORTH IN SECTION 8.1 AND TO THE EXTENT THAT EDWARDS PROVIDES NOTICE IN ACCORDANCE WITH SECTION 3.1, EDWARDS DOES NOT MAKE, AND THERE ARE NO, WARRANTIES, REPRESENTATIONS, OR CONDITIONS, EXPRESS OR IMPLIED, STATUTORY OR OTHERWISE RELATING TO THE LICENSED PATENTS OR SUBLICENSED PATENTS, INCLUDING WITHOUT LIMITATION ANY REPRESENTATIONS OR WARRANTIES OF VALIDITY, ENFORCEABILITY, NON-INFRINGEMENT, OR FITNESS FOR ANY PARTICULAR PURPOSE.
8.3 3F hereby represents and warrants to Edwards that it has all of the requisite power and authority to enter into this Agreement and that this Agreement has been duly and validly authorized, executed, and delivered by 3F.
9. Term and Termination.
9.1 The term of this Agreement shall commence on the Effective Date and, subject to earlier termination as provided herein, shall continue to the date of expiration of the last to expire of any Licensed Patent or Sublicensed Patent covering any Licensed Product.
9.2 Either Party may terminate this Agreement for a material breach by the other Party that remains uncured for ninety (90) days following notice to the breaching Party except for non-payment of Royalties for which 3F shall have forty-five (45) days to cure such nonpayment breach.
9.3 This Agreement, including all licenses and sublicenses herein, shall automatically and immediately terminate, without any further action or notice by Edwards, in the event that 3F is or becomes insolvent. For purposes of this Agreement, the term “insolvent” shall mean 3F shall be deemed “insolvent” if it is unable to pay its debts and obligations as and when they come due..
8
10. Notices.
Any notice which is required or permitted to be given to a Party pursuant to this Agreement shall be deemed to have been given only if such notice is reduced to writing and (i) delivered personally, (ii) sent by reputable overnight courier service for next business day delivery to the person in question a the address given below, or (iii) sent by facsimile machine (with proof transmission capability) to the fax number set forth below, with a hard copy to be sent by first class mail to the person at the address given below:
If to Edwards: |
| If to 3F: |
|
|
|
Edwards Lifesciences PVT, Inc. |
| 3F Therapeutics, Inc. |
|
|
|
with a copy to (which copy shall not constitute |
| with a copy to (which copy shall not |
|
|
|
Gibson, Dunn & Crutcher LLP |
| Reed Smith, LLP |
or to such other address or facsimile number as either Party shall have specified by notice in writing to the other Party.
If delivered personally or by facsimile during normal business hours on a business day, a notice shall be deemed delivered when actually received at the address specified above. In any other case, notice shall be deemed delivered on the next business day following the date on which it was sent.
11. Indemnification.
11.1 3F shall indemnify, defend, and hold Edwards harmless against any and all losses arising out of a Third Party claim to the extent such claim arises from the negligence, willful misconduct, breach of contract, or violations of law by 3F, its employees, agents, subcontractors, or assigns in the performance of this Agreement. The losses covered by this Section 11 include, but are not limited to, settlements, judgments (court costs, attorneys’ fees, expert fees, and other litigation expenses), fines and penalties arising out of actual or alleged (i) injury to or death of any person; (ii) loss of or damage to tangible or intangible property; (iii) patent mismarking; and (iv) breach of contract.
11.2 Edwards shall indemnify, defend, and hold 3F harmless against any and all losses arising out of a Third Party claim to the extent such claim arises from the negligence, willful misconduct, breach of contract, or violations of law by Edwards, its employees, agents,
9
subcontractors, or assigns in the performance of this Agreement. The losses covered by this Section 11 include, but are not limited to, settlements, judgments (court costs, attorneys’ fees, expert fees, and other litigation expenses), fines and penalties arising out of actual or alleged (i) loss of or damage to intangible property; (ii) patent mismarking to the extent that Edwards provides notice pursuant to Section 3.1; and (iii) breach of contract.
12. Limitation of Liability or Damages.
12.1 EXCEPT WITH RESPECT TO EDWARDS’ INDEMNITY OBLIGATIONS PURSUANT TO SECTION 11, IN NO EVENT SHALL EDWARDS, ITS AGENTS OR EMPLOYEES, BE LIABLE FOR ANY INDIRECT, SPECIAL, INCIDENTAL, OR CONSEQUENTIAL DAMAGES, OR ANY PUNITIVE DAMAGES, INCLUDING WITHOUT LIMITATION LOST PROFITS, IN CONNECTION WITH THIS AGREEMENT, THE LICENSED PATENTS, OR THE SUBLICENSED PATENTS, HOWEVER SO CAUSED, WHETHER ARISING IN CONTRACT (INCLUDING BREACH), TORT (INCLUDING NEGLIGENCE), OR OTHERWISE. The Parties acknowledge and agree that the exclusion of liability herein is reasonable and appropriate in the circumstances and was a material factor in determining the terms of this Agreement.
12.2 Without limiting the generality of Section 12.1, nothing contained in this Agreement shall be construed as:
(i) requiring the enforcement of any patent or patent application, including any obligation by Edwards to institute any suit or action for infringement of any of the Licensed Patents or Sublicensed Patents;
(ii) reflecting a determination by Edwards of the applicability of any of the Licensed Patents or Sublicensed Patents to the Fields of Use or any Licensed Products of 3F;
(iii) a warranty or representation by Edwards as to the validity or scope of any Licensed Patents or Sublicensed Patents;
(iv) any promise, obligation, or duty by or imposed on Edwards to obtain any clarification, declaration, or other determination, listing, or identification of any patents, patent applications, or rights licensed or possessed by Edwards under the Heartport License or, correspondingly, sublicensed to 3F as part of the Sublicensed Patents; or
(v) any obligation by Edwards to furnish any assistance under this Agreement.
13. Miscellaneous.
13.1 Relationship of the Parties. Nothing in this Agreement shall be construed to create a partnership, joint venture, employment or agency relationship, or any other form of legal association between Edwards and 3F. Except as expressly set forth in this Agreement, each Party shall conduct business in its own name and shall be solely responsible for the acts and conduct of its employees and agents.
10
13.2 Assignability.
13.2.1 This Agreement is not assignable or transferable by 3F, in whole or in part, except (i) with the prior written consent of Edwards; (ii) an assignment in connection with the sale of all or substantially all of 3F’s business; or (iii) the licenses and sublicense set forth in Section 2 can be separately assigned by 3F to a purchaser of all or substantially all of the assets of the business relating to the Surgical Field of Use or Venous Field of Use, as applicable; provided, however, that in the event of any assignment pursuant to this Section, such assignee shall agree to be bound and comply with all of the applicable obligations of this Agreement. By way of example, in connection with subsection (iii) of this Section, if 3F sells all or substantially all of its business relating to the Surgical Field of Use, the license set forth in Section 2.1 and the sublicense relating to the Surgical Field of Use set forth in Section 2.3 may be assigned to the purchaser of such business, provided that such assignee agrees to be bound and comply with all of the obligations of this Agreement related to such assigned license and sublicense, including without limitation, for example, Sections 2.4, 2.7, 3, 4, 5, 6, 7, 11.1, 12, and 13.
13.2.2 Edwards may assign or transfer this Agreement, in whole or in part; provided, however, that Edwards provide written notice to 3F and that such assignee agrees to be bound and comply with all of the applicable obligations of this Agreement.
13.3 Governing Law. This Agreement shall be governed by and construed in accordance with the internal laws of the State of California, without giving effect to the choice of law rules thereof. Each of the Parties hereto irrevocably consents to the jurisdiction of the state and federal courts located within Orange County, State of California, and irrevocably agrees that all actions or proceedings relating to this Agreement, the Licensed Patents, or the Sublicensed Patents shall be litigated in such courts, and each of the Parties expressly waive any objection or defenses that it may have based on lack of personal jurisdiction, improper venue, or forum non conveniens with respect to such courts. The Parties’ consent and waiver set forth in this Section 13.3 expressly shall apply in the event that any such action is transferred by the United States District Court sitting in Orange County, on its own motion or upon filing, to any other division of the United States District Court for the Central District of California.
13.4 Attorneys’ Fees. If any Party to this Agreement shall bring any action, suit, arbitration, mediation, counterclaim or appeal for any relief against any other Party, declaratory or otherwise, to enforce the terms hereof or to declare rights hereunder, the Prevailing Party in such action shall be entitled to recover as recoverable costs in any such action its attorneys’ fees and costs (including reasonable fees and costs for in-house counsel), all expert fees and costs, all court and/or arbitration expenses, and any other costs reasonably and properly incurred, including any fees and costs incurred in bringing and prosecuting such action and/or enforcing any order, judgment, ruling, or award granted as part of such action. As used in this Section, “Prevailing Party” shall include, without limitation, a party who agrees to dismiss an action or who obtains substantially the relief sought by it.
13.5 Counterparts. This Agreement may be executed in two or more counterparts (including by means of facsimile), each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
11
13.6 Severability. Should any part or provision of this Agreement be rendered or declared invalid by reason of any law or by decree of a court of competent jurisdiction, the validity of any other term, clause, or provision shall not be affected provided that such invalid or unenforceable provision is and can be replaced with an enforceable clause which most closely achieves the result intended by such invalid clause.
13.7 Survivability. The provisions of Sections 1, 4, 5, 6, 7, 8, 11, 12, and 13 shall survive any termination or expiration of this Agreement.
13.8 Headings. The headings used in this Agreement are for purpose of reference only and shall not affect the meaning or interpretation of any provision of this Agreement.
13.9 Drafting. Each Party has had the opportunity to consult with competent, independent counsel in connection with this Agreement and has participated in the drafting of this Agreement. Accordingly, this Agreement shall not be construed against either Party as the drafter.
13.10 Waiver. No waiver or delay by either Party of any breach of the covenants contained herein to be performed by the other Party shall be construed as a waiver of any succeeding breach of the same or any other covenants or conditions hereof.
13.11 Entirety of Agreement. This Agreement supersedes any prior understandings or agreements, whether written or oral, and any contemporaneous oral agreements, between the Parties hereto in regard to the subject matter hereof and, together with the Master Agreement and other Related Agreements (as defined in the Master Agreement), contain the entire agreement between the Parties in regard to the subject matter hereof. This Agreement may not be changed or modified orally, but only by an agreement, in writing, signed by all parties hereto.
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed and delivered by their duly authorized representations, effective as of the Effective Date set forth above.
EDWARDS LIFESCIENCES PVT, INC. |
| 3F THERAPEUTICS, INC. | |||||
|
|
| |||||
|
|
| |||||
By: | /s/ Jay P. Wertheim |
|
| By: | /s/ Walter Cuevas |
| |
Name: Jay P. Wertheim |
|
| Name: | Walter Cuevas |
| ||
Title: Vice President, Associate General |
| Title: | President and Chief Executive Officer |
| |||
Counsel and Secretary |
|
|
|
| |||
12