UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________
FORM 10-K
|
| |
| (Mark One) | |
| ý | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| FOR THE FISCAL YEAR ENDED DECEMBER 31, 2016 |
| OR |
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| FOR THE TRANSITION PERIOD FROM TO |
Commission File No. 001-15943
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
(Exact Name of Registrant as Specified in Its Charter)
|
| | |
| Delaware | | 06-1397316 |
(State or Other Jurisdiction of Incorporation or Organization) | | (I.R.S. Employer Identification No.) |
251 Ballardvale Street Wilmington, Massachusetts | | 01887 |
| (Address of Principal Executive Offices) | | (Zip Code) |
____________________________________________________________________________
(Registrant’s telephone number, including area code): (781) 222-6000
Securities registered pursuant to Section 12(b) of the Act: |
| | |
| Title of each class | | Name of each exchange on which registered |
| Common Stock, $0.01 par value | | New York Stock Exchange |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark whether the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ý No o
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No ý
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files.) Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
| | | | | | |
Large accelerated filer ý | | Accelerated filer o | | Non-accelerated filer o (Do not check if smaller reporting company) | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
On June 25, 2016, the aggregate market value of the Registrant’s voting common stock held by non-affiliates of the Registrant was approximately $3,735,593,230. As of January 27, 2017, there were 47,372,995 shares of the Registrant’s common stock outstanding, $0.01 par value per share.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement for its 2017 Annual Meeting of Shareholders scheduled to be held on May 9, 2017, which will be filed with the Securities and Exchange Commission (SEC) not later than 120 days after December 31, 2016, are incorporated by reference into Part III of this Annual Report on Form 10-K. With the exception of the portions of the 2017 Proxy Statement expressly incorporated into this Annual Report on Form 10-K by reference, such document shall not be deemed filed as part of this Form 10-K.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
ANNUAL REPORT ON FORM 10-K
FOR FISCAL YEAR 2016
TABLE OF CONTENTS
|
| | |
| Item | | Page |
| | PART I | |
| 1 | | |
| 1A | | |
| 1B | | |
| 2 | | |
| 3 | | |
| 4 | Mine Safety Disclosures | |
| | PART II | |
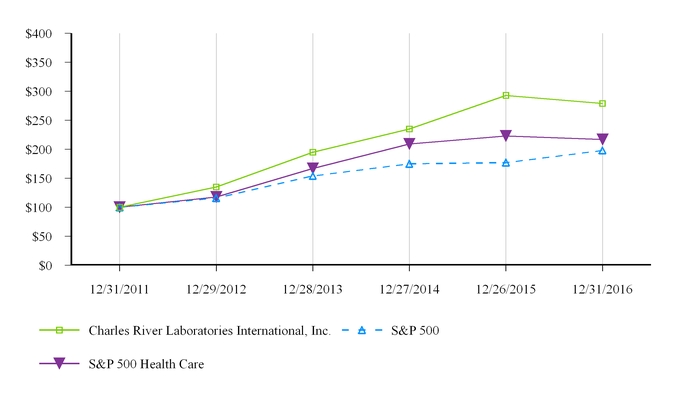
| 5 | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | |
| 6 | | |
| 7 | | |
| 7A | | |
| 8 | | |
| 9 | | |
| 9A | | |
| 9B | | |
| | PART III | |
| 10 | | |
| 11 | | |
| 12 | | |
| 13 | | |
| 14 | | |
| | PART IV | |
| 15 | | |
| 16 | Form 10-K Summary | |
| | | |
| Signatures | |
| Exhibit Index | |
PART I
Item 1. Business
General
This Annual Report on Form 10-K contains forward-looking statements regarding future events and the future results of Charles River Laboratories International, Inc. that are based on our current expectations, estimates, forecasts and projections about the industries in which we operate and the beliefs and assumptions of our management. Words such as “expect,” “anticipate,” “target,” “goal,” “project,” “intend,” “plan,” “believe,” “seek,” “estimate,” “will,” “likely,” “may,” “designed,” “would,” “future,” “can,” “could” and other similar expressions that are predictions, indicate future events and trends or which do not relate to historical matters are intended to identify such forward-looking statements. These statements are based on our current expectations and beliefs and involve a number of risks, uncertainties and assumptions that are difficult to predict. For example, we may use forward-looking statements when addressing topics such as: goodwill and asset impairments still under review; future demand for drug discovery and development products and services, including the outsourcing of these services; our expectations regarding stock repurchases, including the number of shares to be repurchased, expected timing and duration, the amount of capital that may be expended and the treatment of repurchased shares; present spending trends and other cost reduction activities by our clients; future actions by our management; the outcome of contingencies; changes in our business strategy, business practices and methods of generating revenue; the development and performance of our services and products; market and industry conditions, including competitive and pricing trends; our strategic relationships with leading pharmaceutical companies and venture capital limited partnerships, and opportunities for future similar arrangements; our cost structure; the impact of completed and in-process acquisitions (including Argenta, BioFocus, VivoPath, ChanTest, Sunrise, Celsis, Oncotest, WIL Research, Blue Stream, and Agilux) and the timing of closing of in-process acquisitions; our expectations with respect to revenue growth and operating synergies (including the impact of specific actions intended to cause related improvements); the impact of specific actions intended to improve overall operating efficiencies and profitability (and our ability to accommodate future demand with our infrastructure), including gains and losses attributable to businesses we plan to close, consolidate or divest; changes in our expectations regarding future stock option, restricted stock, performance share units and other equity grants to employees and directors; expectations with respect to foreign currency exchange; assessing (or changing our assessment of) our tax positions for financial statement purposes; and our liquidity. In addition, these statements include the impact of economic and market conditions on us and our clients; the effects of our cost-saving actions and the steps to optimize returns to shareholders on an effective and timely basis.
You should not rely on forward-looking statements because they are predictions and are subject to risks, uncertainties and assumptions that are difficult to predict. Therefore, actual results may differ materially and adversely from those expressed in any forward-looking statements. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this document or in the case of statements incorporated by reference, on the date of the document incorporated by reference. Factors that might cause or contribute to such differences include, but are not limited to, those discussed in this Form 10-K under the sections entitled “Our Strategy,” “Risk Factors,” "Management's Discussion and Analysis of Financial Condition and Results of Operations,” in our press releases and other financial filings with the SEC. We have no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or risks. New information, future events or risks may cause the forward-looking events we discuss in this report not to occur.
Corporate History
We began operating in 1947 and since then, we have undergone several changes to our business structure. Charles River Laboratories International, Inc. was incorporated in 1994 and in 2000 we completed our initial public offering. Our stock is traded on the New York Stock Exchange under the symbol “CRL” and is included in the Standard & Poor's MidCap 400 and Composite 1500 indices, the Dow Jones U.S. Biotechnology Index, the NYSE Arca Biotechnology Index, the NYSE Composite and Healthcare Sector indices, and many of the Russell indices, among others. We are headquartered in Wilmington, Massachusetts. Our headquarters mailing address is 251 Ballardvale Street, Wilmington, MA, 01887, and the telephone number at that location is (781) 222-6000. Our Internet site is www.criver.com. Material contained on our Internet site is not incorporated by reference into this Form 10-K. Unless the context otherwise requires, references in this Form 10-K to “Charles River,” “we,” “us” “the Company” or “our” refer to Charles River Laboratories International, Inc. and its subsidiaries.
This Form 10-K, as well as all other reports filed with the SEC, is available free of charge through the Investor Relations section of our Internet site as soon as practicable after we electronically file such material with, or furnish it to, the SEC. You may read and copy any materials we file with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington,
DC 20549. In addition, you may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains an Internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
Overview
We are a full service, early-stage contract research organization (CRO). We have built upon our core competency of laboratory animal medicine and science (research model technologies) to develop a diverse portfolio of discovery and safety assessment services, both Good Laboratory Practice (GLP) and non-GLP, which is able to support our clients from target identification through non-clinical development. We also provide a suite of products and services to support our clients’ manufacturing activities. Utilizing our broad portfolio of products and services enables our clients to create a more flexible drug development model, which reduces their costs, enhances their productivity and effectiveness, and increases speed to market.
Discovery represents the earliest stages of research in the life sciences, directed at the identification, screening, and selection of a lead molecule for future drug development. Discovery activities typically extend anywhere from 4 to 6 years in conventional pharmaceutical research and development timelines.
Development activities, which follow, and which can take up to 7 to10 years, are directed at demonstrating the safety, tolerability, and clinical efficacy of the selected drug candidates. During the non-clinical stage of the development process, a drug candidate is tested in vitro (non-animal, typically on a cellular or sub-cellular level in a test tube or multi-well petri plate) and in vivo (in research models) to support planned or on-going human clinical trials.
The development of new drugs requires the steadily increasing investment of time and money. Various studies and reports estimate that it takes between 10 to 15 years, up to $2.0 billion excluding time costs, and exploration of between 10,000 and 15,000 drug molecules to produce a single Food and Drug Administration (FDA)-approved drug. We are positioned to leverage our leading portfolio in early-stage drug research in an efficient and cost-effective way to aid our clients in bringing their drugs to market faster. Our clients reduce their costs, increase their speed, and improve their productivity and effectiveness in early-stage discovery and development by using our broad portfolio of products and services.
For 70 years, we have been in the business of providing the research models required in research and development of new drugs, devices, and therapies. Over this time, we have built upon our core competency of in vivo biology to develop a diverse and expanding portfolio of products and services, which now encompasses the broader early-stage drug research process. Our client base includes global pharmaceutical companies, biotechnology companies, government agencies, and hospitals and academic institutions around the world. We currently operate 75 facilities in 23 countries worldwide, which numbers exclude our Insourcing Solutions (IS) sites. Our products and services, supported by our global infrastructure and deep scientific expertise, enable our clients to overcome many of the challenges of early-stage life sciences research. In 2016, our total revenue was $1.7 billion and our operating income from continuing operations, before income taxes, was $222.9 million.
We have three reporting segments: Research Models and Services (RMS), Discovery and Safety Assessment (DSA), and Manufacturing Support (Manufacturing).
In April 2016, we acquired WRH, Inc. (WIL Research). WIL Research’s safety assessment business is reported in our DSA reportable segment and its contract development and manufacturing (CDMO) services business created a new operating segment, Contract Manufacturing, which is reported as part of our Manufacturing reportable segment. We divested the CDMO business on February 10, 2017.
The revised reportable segments are as follows:
|
| | |
| Research Models and Services | Discovery and Safety Assessment | Manufacturing Support |
| Research Models | Discovery Services | Microbial Solutions |
| Research Model Services | Safety Assessment | Avian |
| | | Biologics |
| | | Contract Manufacturing |
Through our RMS segment, we have been supplying research models to the drug development industry since 1947. With over 150 different strains, we continue to maintain our position as the global leader in the production and sale of the most widely used rodent research model strains, principally genetically and microbiologically defined purpose-bred rats and mice. We also provide a variety of related services that are designed to assist our clients in supporting the use of research models in drug discovery and development. We maintain multiple production centers, including barrier rooms and/or isolator facilities, on three
continents (North America, Europe, and Asia). In 2016, RMS accounted for 29.4% of our total revenue and approximately 3,200 of our employees, including approximately 110 science professionals with advanced degrees.
Our DSA business segment provides services that enable our clients to outsource their innovative drug discovery research, their related drug development activities, and regulatory-required safety assessment of potential new drugs, industrial chemicals, and agrochemicals to us. The demand for these services has historically been driven by the needs of large global pharmaceutical companies that have exceeded their internal capacity and by the needs of biotechnology companies and non-governmental organizations (NGOs) who traditionally outsourced most of their discovery, development and safety testing programs. Global pharmaceutical, biotechnology, and chemical companies choose to outsource their discovery, development, and safety activities because outsourcing reduces the significant investment in personnel and facilities and capital resources necessary to efficiently and effectively conduct required scientific studies. Additionally, outsourcing to Charles River provides companies access to scientific expertise that they may not have internally or available to them.
We are the largest provider of drug discovery, non-clinical development, and safety testing services worldwide and offer a comprehensive portfolio of services required for regulatory submission of pharmaceuticals, chemicals, and agrochemicals. We have extensive expertise in the discovery of small molecule clinical candidates and in the design, execution, and reporting of safety assessment studies for both small and large molecules and argochemicals. We currently provide discovery and safety assessment services at multiple facilities located in the United States (U.S.), Canada, and Europe. Our DSA segment represented 49.8% of our total revenue in 2016 and employed approximately 5,900 of our employees including approximately 960 science professionals with advanced degrees.
Through our Manufacturing segment, we help ensure the safe production and release of products manufactured by our clients. Our Microbial Solutions business provides in vitro methods for conventional and rapid quality control testing of sterile and non-sterile pharmaceuticals and consumer products. Our Biologics Testing Solutions business provides specialized testing of biologics and devices frequently outsourced by global pharmaceutical and biotechnology companies. Our Avian Vaccine Services business provides specific-pathogen-free (SPF) fertile chicken eggs and chickens used in the manufacture of live viruses.
In 2016, Manufacturing accounted for 20.9% of our total revenue from continuing operations and approximately 1,400 of our employees, including approximately 140 science professionals with advanced degrees.
In recent years, we have focused our efforts on unifying our businesses and improving the efficiency of our global operations to enhance our ability to support our key clients. Our key pharmaceutical and biotechnology clients are increasingly seeking full service, “one-stop” global partners to whom they can outsource more of their drug discovery and development efforts. It is estimated that the market for regulated safety assessment services is at least 50% outsourced, while emerging growth areas such as early and in vivo discovery and certain research model services are currently believed to be less outsourced.
Research Models and Services (RMS). Our RMS segment is comprised of (1) Research Models and (2) Research Model Services.
Research Models. Our Research Models business is comprised of the production and sale of research models.
Research Models. A significant portion of this business is comprised of the commercial production and sale of research models, principally purpose-bred rats and mice for use by researchers. We provide our rodent models to numerous clients around the world, including most pharmaceutical companies, a broad range of biotechnology companies, and many government agencies, hospitals, and academic institutions. We have a global footprint with production facilities strategically located in 8 countries, in close proximity to our clients. Our research models include standard stocks and strains and disease models such as those with compromised immune systems, which are in demand as early-stage research tools. The FDA and foreign regulatory agencies typically require that the safety and efficacy of new drug candidates be tested on research models like ours prior to testing in humans. As a result, our research models are an essential part of the drug discovery and development process.
Our rodent species have been, and continue to be, some of the most extensively used research models in the world, largely as a result of our geographic footprint and continuous commitment to innovation and quality. Our research models are bred and maintained in a variety of controlled environments, which are designed to ensure that the models are free of specific viral and bacterial agents and other contaminants that can disrupt research operations and distort research results. With our production capabilities, we are able to deliver consistently high-quality research models worldwide.
Our research models include:
| |
| • | outbred, which are purposefully bred for heterogeneity; |
| |
| • | inbred, which are bred to be homogeneous; |
| |
| • | spontaneous mutant, whose genotype results in a naturally occurring genetic mutation (such as immune deficiency); |
| |
| • | hybrid, which are the offspring of two different inbred parents; and |
| |
| • | other genetically modified research models, such as knock-out models with one or more disabled genes and transgenic models. |
Certain of our research models are proprietary, disease-specific rodent models used to research treatments for diseases such as diabetes, obesity, cardiovascular and kidney disease.
We are also a premier provider of high quality, purpose bred, SPF large research models to the biomedical research community.
Research Model Services. RMS also offers a variety of services designed to support our clients' use of research models in basic research and screening non-clinical drug candidates. These services address the need among pharmaceutical and biotechnology companies to outsource the non-core aspects of their drug discovery activities. Our services include those which are related to the maintenance and monitoring of research models, and managing research operations for government entities, academic organizations, and commercial clients. We currently have three service offerings in research models services: Genetically Engineered Models and Services, Insourcing Solutions, and Research Animal Diagnostic Services.
Genetically Engineered Models and Services (GEMS). We breed and maintain research models purchased or purposefully created by our clients for biomedical research activities. The creation of a genetically engineered model (GEM) is a critical scientific event, but it is only the first step in the discovery process. Productive utilization of GEMs requires significant additional technical expertise in order to properly support basic and early discovery research. We provide breeding expertise and colony development, quarantine, health and genetic testing and monitoring, germplasm cryopreservation, and rederivation including assisted reproduction and model creation. Our team of project managers is supported by a technologically advanced Internet Colony Management (ICM™) system that allows for real-time data exchange. We provide these services to clients around the world, including pharmaceutical and biotechnology companies, hospitals, universities, and government agencies.
Insourcing Solutions (IS). We manage research operations (including recruitment, training, staffing, and management services) for government entities, academic organizations, and commercial clients. Some research institutions prefer to retain certain elements of their research in-house, while outsourcing staffing and management, thus driving demand for our services. We believe that our expertise in early-stage drug research, and in particular research model care, scientific and technical support, facility operations, and discovery and development services, enhances the productivity and quality of our clients' research programs.
Research Animal Diagnostic Services (RADS). We monitor and analyze the health profiles of research models and cell lines used by our clients. We developed this capability internally in order to address the diagnostic needs of our own research model business. We are able to serve as their sole-source testing laboratory, or as an alternative source supporting our clients’ internal laboratory capabilities. We believe we are the reference laboratory of choice for health testing of laboratory research models and an industry leader in the field of animal diagnostics.
Discovery and Safety Assessment (DSA)
We currently offer discovery and safety assessment services, both regulated and non-regulated, in which we include both in vitro and in vivo studies, supporting laboratory services, and strategic non-clinical consulting and program management to support product development.
Discovery Services. We offer a full spectrum of discovery services from identification of a novel druggable target, followed by high-throughput screening and medical chemistry, through delivery of non-clinical drug and therapeutic candidates ready for safety assessment. Our Early Discovery and In Vivo Discovery businesses are integrated into a single business line - Discovery Services - as evidence of our efforts to streamline and enhance the support we can provide for clients’ integrated drug discovery programs. One seamless discovery organization allows us to better engage with clients at the earliest stages of drug discovery and support their complex scientific needs. We support a variety of therapeutic areas including oncology, central nervous system, bone and musculoskeletal, inflammation, metabolic diseases, respiratory and fibrotic diseases, cardiovascular, gastrointestinal, genito-urinary, anti-infectives, and ophthalmology. We also provide expertise in the growing area of rare and orphan diseases, which are typically diseases of high unmet medical need in smaller patient populations, such as myotonic dystrophy, cystic fibrosis, and Huntington’s Disease. We believe there are emerging opportunities to assist our clients in a variety of drug discovery applications and platforms from target discovery to candidate selection.
Early Discovery. We are a global leader in integrated drug discovery services, with a predominant focus on in vitro biology capabilities and medicinal chemistry. Our knowledge and expertise allow us to support our clients as they drive their molecules forward through design and implementation of clear program plans. Our full suite of service offerings allows us to support our clients at the earliest stages of their research, and to stay with them through the entire early-stage process. Our Early Discovery service capabilities include: target discovery and validation, hit identification, medicinal chemistry, and testing how a drug is absorbed, distributed in the body, metabolized, and excreted (ADME). We also offer ion channel testing and in vitro cardiac safety assessment services, for both discovery and non-clinical purposes. In addition, we offer custom in vivo and in vitro genome editing. With this technology, we are able to develop more translational research models designed to improve the efficiency and effectiveness of the drug discovery process. These services extend from the early discovery screening process through to in vitro GLP safety assessment testing.
In Vivo Discovery Services. In Vivo Discovery Services are essential in early stage, non-clinical discovery, directed at the identification, screening, and selection of a lead compound for drug development. In vivo activities typically extend anywhere from 4 to 6 years in conventional pharmaceutical research and development timelines. We offer research and development expertise, capabilities, and services globally to accelerate our clients' drug discovery pipelines from lead generation to candidate selection and on occasion, complete in vivo studies in support of clinical efforts or post-marketing work. We complement and extend clients' capabilities and expertise to improve their decision-making, increase their flexibility, and reduce their internal costs and product development timelines. In addition, we provide in vitro and in vivo assays in support of lead optimization to candidate selection activities. Examples of this include early pharmacokinetic and pharmacodynamic studies and in vitro and in vivo assays to assess mechanism, bioavailability, metabolism, efficacy, and safety pharmacology.
In September 2016, we acquired Agilux Laboratories, Inc. (Agilux), a CRO that provides a suite of integrated discovery small and large molecule bioanalytical services, drug metabolism and pharmacokinetic services, and pharmacology services. This acquisition supports our strategy to offer clients a broader, integrated portfolio that provides services continuously from the earliest stages of drug research through the non-clinical development process.
Safety Assessment. We offer a full range of safety assessment studies required for regulatory submission on a global basis.
Bioanalysis, Drug Metabolism and Pharmacokinetics. In support of non-clinical drug safety testing, our clients are required to demonstrate appropriate exposure, stability in the collected sample, pharmacokinetics of their drug or compound in circulation, the presence of metabolites, and, with biologics, the presence or absence of anti-drug antibodies. We have scientific depth in the sophisticated bioanalytical techniques required to satisfy these requirements for a number of drug classes. After performing sample analysis in support of non-clinical studies, we have the opportunity to capture the benefits of bridging the non-clinical bioanalysis with subsequent clinical development. Once the analysis is complete, our scientists evaluate the data to provide information on the pharmacokinetics and/or toxicokinetics of the drug, and complete an evaluation of the biologic disposition of the drug and its potential metabolites. Pharmacokinetics refers to understanding what the body does to a drug or compound once administered, including the process by which the drug is absorbed, distributed in the body, metabolized and excreted (ADME); toxicokinetics refers to the same understanding as applied at higher doses that may result in adverse effects. These studies are required for the full non-clinical assessment of the disposition of the drug and the results are used in the final non-clinical safety evaluation of the compound to support the start of clinical trials.
Safety Pharmacology. In support of non-clinical drug safety testing, our clients are required to demonstrate that the test article as formulated does not have the potential to prolong the cardiac QT interval. We have the assays (both in vitro and in vivo) and can perform the screening for this demonstration that is required for an investigational new drug submission.
Toxicology. We have expertise in the design and execution of development programs in support of both chemically-derived (small molecule) and biotechnology-derived (large molecule) pharmaceuticals. Once a lead molecule is selected, toxicology studies are required to support clinical trials in humans and new drug registrations. These toxicology studies focus on assessing the safety of the molecule to determine if administration of the molecules to humans might cause any unintended harmful effects. These studies are typically performed in research models to identify any potential adverse effects that a compound has on an organism over a variety of doses and over various time periods.
Our toxicology services feature:
| |
| • | a broad offering of in vitro and in vivo capabilities and study types designed to identify possible safety risks for potential therapeutics, industrial chemicals, and agrochemicals as they transition from discovery into regulated drug development, toxicology, and human clinical testing, or as they are submitted for regulatory registration; |
| |
| • | all the standard in vitro and in vivo studies in support of general toxicology (acute, sub-acute, and chronic studies), genetic toxicology, safety pharmacology, and carcinogenicity bioassays that are required for either regulatory submissions supporting “first-in-human” to “first-to-the-market” strategies, or for national chemical registration; |
| |
| • | all the standard in vitro and in vivo studies in support of general toxicology (acute, sub-acute, and chronic studies), genetic toxicology, reproductive and developmental toxicology, environmental toxicology, and carcinogenicity bioassays that are required for regulatory submissions supporting the registration of industrial chemicals, food additives, agrochemicals, and biocides; |
| |
| • | expertise in standard and specialty routes of administration (e.g., infusion, intravitreal, intrathecal, and inhalation) that are important not only for the testing of potential pharmaceuticals and biopharmaceuticals, but also for the safety testing of medical devices, nutraceuticals, animal health products, and other materials; |
| |
| • | expertise in the conduct and assessment of reproductive, developmental, and juvenile toxicology studies (in support of larger-scale and later-stage human clinical trials or chemical registration); |
| |
| • | expertise in environmental toxicology (aquatic and terrestrial) and regulatory submissions required for chemical registration; |
| |
| • | services in important specialty areas such as ocular, bone, juvenile/neonatal, immune-toxicology, photobiology, inhalation, and dermal testing; |
| |
| • | expertise in all major therapeutic areas; |
| |
| • | study design and strategic advice to our clients based on our wealth of experience and scientific expertise in support of drug development and chemical registration; and |
| |
| • | a strong history of assisting our clients in achieving their regulatory and/or internal milestones for the safety testing of numerous therapy types including stem cells, vaccines, proteins, antibodies, drug conjugates, oligonucleotide biotherapeutics, small molecules, medical devices, chemicals, and agrochemicals. |
Our safety assessment facilities comply with GLP to the extent required by the FDA, Environmental Protection Agency, USDA, European Medicines Agency, European Chemicals Agency, Organization for Economic Co-operation and Development (OECD), as well as other international regulatory agencies. Furthermore, our early-stage discovery work, which is not subject to GLP standards, is typically carried out under a quality management system such as ISO 9100 or similarly constructed internally developed quality systems. Our facilities are regularly inspected by U.S. and other regulatory compliance monitoring authorities, our clients' quality assurance departments, and our own internal quality assessment program.
Pathology Services. The ability to identify and characterize clinical and anatomic pathologic changes is critical in determining the safety and efficacy of potential new therapeutics and agrochemicals. Key “go/no-go” decisions regarding continued product development are typically dependent on the identification, characterization and evaluation of fluid, tissue, and cellular changes that our experts identify and interpret for our clients. We employ a large number of highly trained veterinary anatomic and clinical pathologists and other scientists who use state-of-the-art techniques to identify potential test compound-related changes within tissues, fluids, and cells. In addition to all standard anatomic and clinical pathology techniques, we provide specialized evaluations such as cytology, platelet function, assay development, immunohistochemistry, in situ blood hybridization electron microscopy, tissue morphometry, and stereology services.
Manufacturing Support (Manufacturing)
Microbial Solutions. Our Microbial Solutions business provides in vitro methods for conventional and rapid quality control testing of sterile and non-sterile biopharmaceutical and consumer products. Our legacy business provided lot release testing of medical devices and injectable drugs for endotoxin contamination. Our Celsis business provides rapid microbial detection systems for quality control testing in the pharmaceutical and consumer products industries. Our Accugenix business provides state-of-the-art microbial identification and genetic sequencing services for manufacturing in the biopharmaceutical, medical device, nutraceutical, and consumer care industries.
Endotoxin testing is an in vitro process which uses a processed extract from the raw materials of the horseshoe crab, known as limulus amebocyte lysate (LAL). The LAL test is the first and most successful FDA-validated alternative to an in vivo test to date. The extraction of the raw materials for LAL does not harm the crabs, which are subsequently returned to their natural ocean environment. Our Microbial Solutions business produces and distributes a comprehensive portfolio of endotoxin testing, microbial detection and identification kits, reagents, software, accessories, instruments, and associated microbial quality control
laboratory services to a broad range of companies manufacturing and releasing products from the pharmaceutical, biotechnology, consumer products, and dairy industries worldwide. We are a market leader in endotoxin testing products and services, which are used for FDA-required quality control testing of injectable drugs and medical devices, their components, and the processes by which they are manufactured.
The growth in our Microbial Solutions business is driven by our FDA-approved line of next-generation endotoxin testing products. This line is based on the Endosafe Portable Testing System (Endosafe®-PTS™) technology, which allows rapid endotoxin testing in the central laboratory or manufacturing environment. In recent years, we expanded the PTS product portfolio to include a multiple sample testing system known as the Endosafe®-MCS™ (multi-cartridge system) to satisfy the demand of our clients who require higher sample throughput. We anticipate our clients' demand for rapid testing methods will continue to increase as they respond to the FDA's Process Analytical Technology (PAT) Initiative, as well as move to faster, simpler testing methods for their technicians. In 2013, we launched the first fully automated robotic system developed specifically for high-volume endotoxin testing: Endosafe®-Nexus™. We expect to see expanded use of this rapid endotoxin testing technology in non-traditional areas such as renal dialysis, nuclear and compounding pharmacies, and cellular therapy.
Celsis’ systems are principally used for product-release testing to help ensure the safe manufacture of pharmaceutical, pharmaceutical, and consumer products. The Advance II™, Accel™ and Innovate™ systems for non-sterile applications complement our PTS-Micro™, a rapid bacterial (bioburden) detection system for sterile biopharmaceutical applications. We expect our comprehensive portfolio to drive increased adoption of our quality control testing solutions across both sterile and non-sterile applications.
Our Accugenix subsidiary is the premier global provider of current ISO 17025 and Good Manufacturing Practice (cGMP)-compliant contract microbial identification and genetic sequencing testing. Accugenix is an acknowledged industry leader in species-level identification and strain typing of bacteria and fungi that are recovered from manufacturing facilities. Utilizing state-of-the-art and proprietary in vitro technologies, coupled with scientific expertise and analysis, Accugenix excels in providing accurate, timely, and cost-effective microbial identification services required to meet internal quality standards and government regulations.
Biologics Testing Solutions. We perform specialized testing of biologics frequently outsourced by global pharmaceutical and biotechnology companies. Our laboratories in the U.S., Germany, Scotland, Ireland, and France provide timely and regulatory-compliant molecular biology, virology, bioanalysis, immunochemistry, microbiology, and related services. We confirm that biological processes and the drug candidates and drugs produced are consistent, correctly defined, stable, and essentially contaminant free. This testing is required by the FDA and other international regulatory authorities for our clients to obtain new drug approvals, to maintain government licensed manufacturing facilities, and to release approved therapeutic products for patient treatment.
Our manufacturing services group grows and stores well-characterized early-stage client cell lines for later development or manufacture of therapeutic proteins and vaccines for clinical trials. We further design and provide viral clearance projects for Phase I, II, and III studies in our German and U.S. facilities.
In June 2016, we acquired Blue Stream Laboratories, Inc. (Blue Stream), an analytical CRO supporting the development of complex biologics and biosimilars.
Avian Vaccine Services. We are the global leader for the supply of SPF fertile chicken eggs and chickens. SPF chicken embryos are used by animal health companies as self-contained “bioreactors” for the manufacture of live viruses. These viruses are used as a raw material primarily in poultry as well as human and veterinary vaccine applications. The production of SPF eggs is performed under biosecure conditions, similar in many ways to our research model production. We have a worldwide presence, with several SPF egg production facilities in the U.S., and contracted production capabilities in Hungary. We also operate a specialized avian laboratory in the U.S., which provides in-house quality control testing of the SPF flocks, offers testing services to vaccine companies and commercial poultry operations, and manufactures poultry diagnostics and bulk antigens for poultry vaccines.
Contract Manufacturing. Through our acquisition of WIL Research in April 2016, we acquired its QS Pharma subsidiary. This business specializes in contract formulation development and manufacturing (including analytical services and stability testing) with a focus on high potency compounds, oral solid and liquid dose formulations, and manufacturing. On February 10, 2017, we divested this business. For additional information, see Note 17, “Subsequent Events” included in Item 8, “Financial Statements and Other Supplementary Data” in this Annual Report on Form 10-K.
Our Strategy
Our objective is to be the preferred strategic global partner for our clients. Our strategy is to deliver a comprehensive and integrated portfolio of drug discovery and non-clinical development products, services, and solutions to support our clients' discovery and early-stage drug research, process development, scale up, and manufacturing efforts, and enable them to bring new and improved therapies to market faster and more cost effectively. In addition, we believe we can improve and augment drug discovery and early-stage development effectiveness by coordinating the dialog between large pharmaceutical, biotechnology, academic and non-governmental organizations, and venture capitalists. Separately, through our various Manufacturing segment businesses, we aim to be the premier provider of products and services that ensure our clients produce and release their products safely. As these groups increasingly rely on and interact with one another in this field, we assist them in working together by developing deeper strategic relationships with each of these constituencies.
We believe we have certain competitive advantages in executing this strategy, as a result of our continuing focus on the following:
Integrated Early-Stage Portfolio. We are the only large, global CRO with a portfolio of products, services, and solutions that focuses on drug discovery and early-stage development. We provide research models and associated services, discovery research studies and services, and comprehensive safety assessment studies in both regulated and non-regulated environments. As such, we are able to collaborate with clients from target discovery through candidate selection. When critical decisions are made regarding which therapeutics will progress from discovery to development, we continue to work alongside our clients as the drug candidates move downstream. Our recognized expertise in early-stage drug research and pharmacology provides us with a competitive advantage. We understand our clients' therapies and the challenges they face during the discovery and development process, including mechanism of action, efficacy, drug metabolism, safety assessment, and toxicological testing critical for making “go/no-go” decisions.
Pharmaceutical Manufacturing Support Portfolio. We also offer a portfolio of products, services, and solutions that supports the process development, scale up, and quality control efforts of the biopharmaceutical industry. We provide products and services that support the development and release of commercialized biologics products. In particular, we are an industry leader in the areas of microbial detection and microbial identification to support process development and ongoing commercial production. Our portfolio spans a broad range of traditional and rapid methods, which provide the highest testing quality, enhance productivity, and reduce cycle time.
Deep Scientific Expertise. We provide a breadth and depth of scientific expertise across a broad range of therapeutic areas which may be too costly for our clients to build and/or maintain in-house. We provide essential capabilities, including biomarkers, biologics, medicinal chemistry, in vitro screening, in vivo pharmacology, immunology, pathology, biologics process development testing, microbial detection and identification, and other specialty service areas that have high infrastructure costs or are cost-prohibitive for clients to maintain in-house. We continue to expand our portfolio in key therapeutic and pharmacology areas to align with our clients' internal drug discovery and development areas of focus. These areas of disease focus and expertise include oncology, metabolism and obesity, immunology, respiratory, bone and musculoskeletal, diabetes, cardiovascular, infectious disease, and central nervous system. In the areas of functional expertise, it includes synthetic and medicinal chemistry, library design, cell line development, in vitro and in vivo assay development screening, non-clinical imaging, structural biology, process chemistry, toxicology, veterinary pathology, bioanalysis, scale up, and formulation development. We also continue to enhance our small molecule and biologics manufacturing portfolio in areas of greatest industry need, where outsourcing provides major benefits for our clients and where we could provide significant benefits given our unique early development portfolio and global footprint.
Commitment to Animal Welfare. We are committed to being the worldwide leader in the humane care of laboratory animals and implementation of the “3Rs” (Replacement, Reduction, and Refinement). As researchers, we are responsible to our clients and the public for the health and well-being of the animals in our care. We work hand-in-hand with the scientific community to understand how living conditions, handling procedures, and reduction of stress play an important role in the quality and efficiency of research.
Superior Quality and Client Support. We maintain scientific rigor and high quality standards through management of key performance indicators and an intense focus on biosecurity. These standards allow clients to access our global portfolio of products and services with the confidence that they will obtain consistent results no matter where they choose to obtain their products or conduct their research.
Flexible and Customized Environment to Provide the Right Solutions. Each of our clients is different, with unique needs and specific requirements. We understand the importance of flexibility, and leverage the expertise embedded in our integrated early-stage portfolio to provide customized solutions tailored to the specific need or therapeutic area for a particular client. By utilizing our streamlined and efficient facilities, we help clients create a flexible infrastructure in order to improve their workload and staffing requirements. This allows our clients to reduce internal capacity and/or staff. We provide enhanced value to clients who use us as a full-service integrated partner over a longer period of time.
Large, Global Partner. We believe there is a particular advantage in being a full service, high-quality provider of research models and associated services, discovery and non-clinical in vivo and in vitro services, and manufacturing support on a global scale. Many of our clients, especially large biopharmaceutical companies, have decided to limit the number of suppliers with which they work. Their preference is to partner with large Tier 1 CROs like Charles River, who can offer clients support across the early-stage drug research process as a result of broader portfolios and experience in project management. This includes extensive scientific, technical, and therapeutic area expertise, real-time access to data through secure portals, a global footprint, and streamlined and simplified processes and communications including professional project and relationship management. We are focused on leveraging our competitive advantages to ensure we are recognized as the premier preferred provider, thereby enabling us to build broader and deeper long-term strategic relationships with our clients.
Global biopharmaceutical companies are continuing to make the decision to outsource more significant tranches of their drug discovery, development, and manufacturing processes. Over the past few years we have entered into strategic relationships with leading global biopharmaceutical companies and expanded existing preferred provider agreements with other leading global biopharmaceutical companies. For example, in 2016, we extended the term of our longstanding integrated drug discovery alliance with Genentech, a member of the Roche Group. Through this alliance, we provide Genentech early discovery services, including medicinal chemistry, in vitro and in vivo biology, structural biology, and computer-aided drug design to help identify promising candidates for non-clinical development. And, in 2015, we extended the term of our collaboration with AstraZeneca for outsourced regulated safety assessment, and development drug metabolism and pharmacokinetics until 2020. For some of our partners, we provide a broad suite of research models and discovery and safety assessment services and for others we provide a customized and select array of discovery and safety assessment services and/or research models. Offering flexibility enables our clients to utilize our products and services to deliver innovative health solutions in a manner which best suits their individual needs.
There have been fundamental changes in our clients' research and development needs, particularly with regard to the large pharmaceutical industry. First, these clients are increasingly emphasizing studies that have greater translation to the clinic so that they can make appropriate decisions regarding the progression of potential therapeutic entities earlier in the development process. The result is a greater focus on discovery services, including in vivo pharmacology studies consisting of efficacy and non-GLP DMPK (drug metabolism and pharmacokinetics) studies. Second, these clients are choosing to outsource additional discovery and safety assessment services in order to increase the efficiency and effectiveness of their drug selection processes.
We believe that this changing environment will provide enhanced outsourcing opportunities for us in the future. We remain optimistic that our clients are increasingly receptive to partnering with CROs as a means of meeting their discovery and non-clinical support needs. We believe that the successful development of new therapies and outsourcing by the pharmaceutical industry will continue to be positive drivers of demand for our products and services.
We also believe that larger biopharmaceutical companies will increasingly focus on efficiencies and execution. They will continue to reassess what are core differentiators from research and development to commercialization. We expect they will also continue to be conservative in re-building infrastructure and expertise. This should lead to more opportunities for strategic outsourcing as clients choose to utilize external resources rather than invest in internal infrastructure. In the aggregate, we believe that the evolving large biopharmaceutical research and development business model will make our essential products and services even more relevant to our clients, and allow them to leverage our integrated offerings and expertise to drive their research, non-clinical development, and manufacturing efficiency and cost effectiveness.
We believe it is critical to participate in the strategic partnering process because these relationships are likely to extend for lengthy periods of time - three to five years. Furthermore, both the client and the CRO invest heavily in the initial phases of the relationship to successfully transfer work streams and establish governance processes. Given this investment, clients are less likely to change CROs at the conclusion of the initial relationship. Our goal is to prevail in the majority of these opportunities.
We also believe that our portfolio provides flexible solutions that meet the customized needs for virtual and small biotechnology companies, which have limited or no infrastructure. These clients also value our ability to provide a broad range
of services and integrated services where we work hand in hand with our customers to design, plan, and manage integrated projects and programs. This includes classically outsourced services, “insourced” services, and hybrid offerings blending resources from both our clients and our staff. Our clients have utilized this capability, which blends resources both inside and outside their walls.
We maintain an intense focus on initiatives designed to allow us to drive profitable growth and maximize value for shareholders, and better position ourselves to operate successfully in the current and future business environment. As a result, we believe that we are well positioned to exploit both existing and new outsourcing opportunities.
We intend to continue to broaden the scope of the products and services we provide across the drug discovery and early-stage development continuum primarily through internal development, and, as needed, through focused acquisitions and alliances. Acquisitions are an integral part of our growth strategy, both to expand our portfolio and broaden our geographic footprint. We are committed to a disciplined approach that seeks to target businesses that are a sound strategic fit and that offer the prospect of enhancing shareholder value, typically including the achievement of a hurdle rate for return on invested capital above our weighted average cost of capital. For example, in each of 2015 and 2016, we completed significant strategic acquisitions. In 2015, we acquired Celsis Group Limited., a leading provider of rapid bacterial detection systems for sterile and non-sterile quality control testing in the biopharmaceutical and consumer products industries. In 2016, we made three acquisitions. In April, we acquired WIL Research, a premier provider of safety assessment and contract development and manufacturing services to biopharmaceutical and agricultural and industrial chemical companies worldwide. In June, we acquired Blue Stream, an analytical CRO supporting the development of complex biologics and biosimilars. In September, we acquired Agilux, a CRO that provides a suite of integrated discovery small and large molecule bioanalytical services, drug metabolism and pharmacokinetic services, and pharmacology services.
Our acquisition strategy also takes into account geographic as well as strategic expansion of existing core services. For example, in 2015, we acquired Oncotest, a Germany-based CRO providing discovery services for oncology, which complements our existing In Vivo Discovery businesses in the U.S. and Finland, and Sunrise, a producer of SPF fertile chicken eggs and chickens used in the manufacture of live viruses.
We are also partnering with a number of venture capital firms primarily investing in life sciences, healthcare, and technology companies with an emphasis on early-stage emerging growth companies. Through these partnerships we are able to promote contract research services for discovery and safety assessment to these companies. For example, in 2016, we committed to invest $10 million in BioMotiv, LLC, the therapeutic accelerator company associated with The Harrington Project for Discovery and Development. Through this agreement, we will be the preferred drug discovery and non-clinical development partner for BioMotiv’s portfolio of technologies and companies. This offers us the opportunity to establish ourselves as a provider of choice for a unique client group which has emerged as biopharmaceutical companies rationalize and prioritize their development pipelines.
Customers
We maintain a three-pronged sales organization with a focus on:
| |
| • | global biopharmaceutical companies; |
| |
| • | small and mid-sized pharmaceutical, biotechnology, agrochemical, industrial chemical, and veterinary medicine companies, as well as contract research organizations; and |
| |
| • | academic and government institutions. |
We also maintain several sales specialists which either have specific technical expertise (often degreed scientists) or cover unique markets.
Our clients continue to consist primarily of all of the major biopharmaceutical companies; many biotechnology, agricultural and chemical, life science, veterinary medicine, medical device, diagnostic, and consumer product companies; contract research and contract manufacturing organizations; and other commercial entities, as well as leading hospitals, academic institutions, and government agencies. We have stable, long-term relationships with many of our clients. During 2016, no single commercial client accounted for more than 3% of our total revenue and no single customer accounted for more than 10% of the revenue of any of our three business segments.
We continue to pursue a goal of expanding our relationships with our large biopharmaceutical clients, and with many of our larger mid-market clients. These relationships take different forms, from preferred provider arrangements to strategic partnerships. The structure of these relationships incentivizes clients to purchase more products and services across our early-
stage portfolio. Because of the strength of these relationships, we have better insight into our clients' planning processes, and therefore, better visibility than in the past. For information regarding revenue attributable to each of our business segments for the last three fiscal years, please see Note 15, “Segment and Geographic Information” included in the Notes to Consolidated Financial Statements included elsewhere in this Form 10-K. For information regarding revenue and long-lived assets attributable to operations in the United States, Europe, Canada, Japan, and other countries for each of the last three fiscal years, please review Note 15, “Segment and Geographic Information” included in the Notes to Consolidated Financial Statements included elsewhere in this Form 10-K.
Sales, Marketing and Customer Support
We have designated dedicated sales people for each of our three client segments (global biopharmaceutical, small and mid-sized pharmaceutical and biotechnology companies, and academic and government institutions). This enhances our ability to meet client needs by offering customized, tailored solutions across our entire portfolio. In addition, our mid-market pharmaceutical and biotechnology clients benefit by additional support from a combination of account managers with broad portfolio knowledge and specialists with specific scientific expertise. This allows us to provide comprehensive coverage of all of the market segments among our diverse client population. We also apply the use of dedicated sales specialists for certain technical product lines, such as in our Manufacturing business.
We sell our products and services principally through our direct sales force and account management teams who work in North America, Europe, and the Asia-Pacific countries. In addition to interactions with our direct sales force, our primary promotional activities include organizing scientific symposia, publishing scientific papers and newsletters, hosting webinars and making presentations at, and participating in, scientific conferences and trade shows in North America, Europe, and Asia. We supplement these scientifically based marketing activities with internet-based marketing, advertising, and direct mail. In certain areas, our direct sales force is supplemented by international distributors and agents.
Our internal marketing/product management teams support the field sales staff and account management teams while developing and implementing programs to create close working relationships with our clients in the biomedical research industry. We maintain customer service, technical assistance, and consulting service departments (in addition to project managers for our service businesses), which address both our clients' routine and more specialized needs and generally serve as a scientific resource for them. We frequently assist our clients in solving problems related to animal husbandry, health and genetics, biosecurity, non-clinical study design, regulatory consulting, protocol development, and other areas in which our expertise is widely recognized as a valuable resource by our clients.
Our marketing efforts are focused on stimulating demand for further outsourcing across our entire services portfolio. We believe that our ability to provide solutions that address all aspects of early-stage drug research are increasingly attractive to our clients, and we continue to design and market our commercial activities to deliver flexible, customized programs designed by segment to meet our clients' global and site-specific needs.
Competition
Our goal is to be a leader in each of the markets in which we participate. We compete in the marketplace on the basis of our therapeutic and scientific expertise in early-stage drug research, quality, reputation, flexibility, responsiveness, pricing, innovation, and global capabilities. We are able to offer a unique portfolio of early-stage products and services to support drug discovery and development.
We encounter a broad range of competitors of different sizes and capabilities in each of our three businesses segments, although the largest competitors within any segment vary. We also face competition from the internal discovery and development resources of our clients.
| |
| • | For RMS, we have five main competitors of which one is a government funded, not-for-profit entity; one is part of a large public company; two are privately held in Europe and one is privately held in the U.S. We believe that none of these competitors compares to us in global reach, financial strength, breadth of product and services offerings, technical expertise, or pharmaceutical and biotechnology industry relationships. |
| |
| • | For DSA, both our Discovery Services and Safety Assessment businesses have numerous competitors. Discovery has hundreds of competitors, as it is a highly competitive and fragmented market. Safety Assessment has seven main competitors; one is part of a large public company in the U.S.; one is a privately held company in the U.K.; one is a public company in China; two are privately held companies in the U.S.; one is privately held in Canada; and one is privately held in France. Our DSA segment also competes with in-house departments of pharmaceutical and biotechnology companies, universities, and teaching hospitals. |
| |
| • | For Manufacturing, each of our underlying businesses has several competitors. In addition to many smaller competitors, Biologics has five main competitors, of which two are public companies in Europe, one is a private company in the U.S., one is a public company in China, and one is a public company in the U.S. Avian has one main competitor to its SPF eggs business, which is privately held in Europe, and numerous competitors for services provided through our specialized avian laboratory. Microbial Solutions has five main competitors, of which three are public companies in Europe and two are privately held in the U.S. Contract Manufacturing has five main competitors, of which two are public companies in the U.S. and three are privately held in Europe. |
Industry Support and Animal Welfare
One of our core values is a concern for, and commitment to, animal welfare. We have been in the forefront of animal welfare improvements in our industry, and continue to show our commitment with special recognition programs for employees who demonstrate an extraordinary commitment in this critical aspect of our business. We created our own Humane Care Initiative, which is directed by our Animal Welfare and Training Group. The goal of the initiative is to assure that we continue as a worldwide leader in the humane care of laboratory animals and implementation of the 3Rs (Replacement, Reduction and Refinement). Laboratory animals are an important resource that further our knowledge of living systems and contribute to the discovery of life-saving drugs and procedures. We work hand-in-hand with the scientific community to understand how living conditions, handling procedures and stress play a role in the quality and efficiency of research. As researchers, we are responsible to our clients and the public for the health and well-being of the animals in our care.
We are firmly committed to the 3Rs and to reducing the number of animals used by emphasizing health and genetic integrity to decrease study data variability. Whenever possible, we use technological advances such as new diagnostic tests for screening pathogens in laboratory rodents, microsampling and in vitro assays. We also partner with customers to develop study designs decreasing the number of animals needed and suggesting pilot studies where appropriate. We also maintain a quarterly award recognizing our employees’ efforts to continually implement the 3Rs at our sites globally.
We support a wide variety of organizations and individuals working to further animal welfare as well as the interests of the biomedical research community. We fund scholarships to laboratory animal training programs, provide financial support to non-profit institutions that educate the public about the benefits of animal research and provide awards and prizes to outstanding leaders in the laboratory animal medicine field and the supporters of 3Rs.
Employees
As of December 31, 2016, we had approximately 11,000 employees (including approximately 1,200 science professionals with advanced degrees, including Ph.D.s, D.V.M.s and M.D.s). Our employees are not unionized in the U.S. Employees at some of our European facilities are represented by works councils and/or unions, which is consistent with local customs for our industry. We believe we have good relationships with our employees, based on a number of factors including employee retention and survey results.
Backlog
Our backlog for our RMS, DSA and Manufacturing reportable segments was $88.0 million, $551.8 million and $39.5 million, respectively, as of December 31, 2016, as compared to $106.6 million, $327.8 million and $36.2 million, respectively, as of December 26, 2015. Related services are performed over varying durations, from short to extended periods of time, which may be as long as several years. We maintain an order backlog to track anticipated revenue from studies and projects that either have not started, but are anticipated to begin in the near future, or are in process and have not been completed. We only recognize a study or project in backlog after we have received written evidence of a client's intention to proceed. Canceled studies or projects are removed from backlog.
We believe our aggregate backlog as of any date is not necessarily a meaningful indicator of our future results for a variety of reasons. First, studies vary in duration (i.e., some studies or projects that are included in December 31, 2016 backlog may be completed in 2017, while others may be completed in later years). Second, the scope of studies or projects may change, which may either increase or decrease their value. Third, studies or projects included in backlog may be subject to bonus or penalty payments. Fourth, studies or projects may be terminated or delayed at any time by the client or regulatory authorities for a number of reasons, including the failure of a drug to satisfy safety and efficacy requirements, or a sponsor making a strategic decision that a study or service is no longer necessary. Delayed contracts remain in our backlog until a determination of whether to continue, modify, or cancel the study has been made. We cannot provide any assurance that we will be able to realize all or most of the net revenues included in backlog or estimate the portion to be filled in the current year.
Regulatory Matters
As our business operates in a number of distinct operating environments and in a variety of locations worldwide, we are subject to numerous, and sometimes overlapping, regulatory environments.
The Animal Welfare Act (AWA) governs the care and use of certain species of animals used for research in the U.S. other than laboratory rats, mice and chickens. As a result, most of our U.S. small animal research models activities and our avian vaccine services operations are not subject to regulation under the AWA. For regulated species, the AWA and the associated Animal Care regulations require producers and users of regulated species to provide veterinary care and to utilize specific husbandry practices such as cage size, shipping conditions, sanitation and environmental enrichment to assure the welfare of these animals. Separately, facilities using live vertebrate animals in research funded by the U.S. Public Health Service (PHS) must also adhere to the PHS Policy on Humane Care and Use of Laboratory Animals and follow the Guide for the Care and Use of Laboratory Animals produced by the Institute for Laboratory Animal Research.
We comply with licensing and registration requirement standards set by the United States Department of Agriculture (USDA) and similar agencies in other countries such as the European Union, China, Japan, and Canada for the care and use of regulated species. Our animal production facilities in the U.S., our DSA facilities in the U.S. and Canada, and most of our DSA and RMS sites in the Europe are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International, a private, nonprofit, international organization that promotes the humane treatment of animals in science through voluntary accreditation and assessment programs.
Our import and export of animals and our operations in foreign countries are subject to international agreements and conventions, as well as a variety of national, regional, and local laws and regulations, which establish the standards for the humane treatment, care, handling, and transport of animals by dealers and research facilities.
We conduct non-clinical safety assessment studies to support the submissions for approval or licensing of our clients' products throughout the world. Many of these studies must comply with national statutory or regulatory requirements for Good Laboratory Practice (GLP). GLP regulations describe a quality system for the organizational process and the conditions under which non-clinical studies are planned, performed, monitored, recorded, reported and archived. GLP compliance is required by such regulatory agencies as the FDA, United States Environmental Protection Agency, European Medicines Agency, Medicines and Healthcare Products Regulatory Agency in the United Kingdom (U.K.), Health Products Regulatory Authority in Ireland, Health Canada and other similar monitoring authorities in the countries where we operate. GLP requirements are significantly harmonized throughout the world and our laboratories are capable of conducting studies in compliance with all necessary requirements.
Our Manufacturing businesses produce endotoxin test kits, reagents, cell banks used in research and biopharmaceutical production, clinical trial vaccines, vaccine support products and provided GMP contract manufacturing of clinical and marketed products. Additionally, several of our laboratories conduct analytical testing such as identity, stability, sterility and potency testing in support of our clients' manufacturing programs working with our clients to fulfill their validation requirements as applicable. These activities are subject to regulation and consequently require these businesses to be inspected by the FDA and other national regulatory agencies under their respective current Good Manufacturing Practice (cGMP) regulations. These regulations require that we manufacture our products or perform testing in a prescribed manner with respect to cGMP compliance, and maintain records of our manufacturing, testing and control activities. In addition, the specific activities of some of our businesses require us to hold specialized licenses for the manufacture, distribution and/or marketing of particular products.
All of our sites are subject to licensing and regulation under international treaties and conventions, including national, regional and local laws relating to:
| |
| • | the surface and air transportation of chemicals, biological reagents and laboratory specimens; |
| |
| • | the handling, use, storage, and disposal of chemicals (including narcotics and psychotropic drugs), biological reagents, laboratory specimens, hazardous waste, and radioactive materials; |
| |
| • | the procurement, handling, use, storage, and disposal of human cells, tissues, and cellular and tissue based products for research purposes; |
| |
| • | the safety and health of employees and visitors to our facilities; and |
| |
| • | protection of the environment and general public. |
Global compliance programs are centralized under a single group responsible for global quality programs and systems to ensure that all business sectors comply with applicable statutory and regulatory requirements and satisfy our clients’ expectations for
quality and regulatory compliance. To assure these compliance obligations, we established quality assurance units (QAUs) in each of our regulated businesses that require independent oversight. The QAUs operate independently from those individuals that direct and conduct studies, manufacturing or analytical testing that studies that supports manufacturing.
Intellectual Property
We develop and implement computer software and technically derived procedures and products intended to maximize the quality and effectiveness of our services. Although our intellectual property rights are valuable to our success, we believe that such factors as the technical expertise, proprietary know-how, ability, and experience of our professionals are more important, and that, overall, these technological capabilities provide significant benefits to our clients. Where we consider it appropriate, steps are taken to protect our know-how through confidentiality agreements and registrations. In addition, we in-license technology and products from other companies when it enhances our product and services businesses. In the future, in-licensing may become a larger initiative to enhance our offerings, particularly as we focus on therapeutic area expertise. With the exception of technology related to our Microbial Solutions testing business, we have no patents, trademarks, licenses, franchises, or concessions which are material and upon which any of our products or services are dependent.
Corporate Governance
We are committed to operating our business with integrity and accountability. We strive to meet or exceed all of the corporate governance standards established by the New York Stock Exchange, the SEC, and the Federal government as implemented by the Sarbanes-Oxley Act of 2002 and the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010. Eight of the nine members of our Board of Directors are independent and have no significant financial, business, or personal ties to us or management and all of our board committees (with the exception of our Executive Committee and our Strategic Planning and Capital Allocation Committee) are composed entirely of independent directors. The Board adheres to our Corporate Governance Guidelines and a Code of Business Conduct and Ethics which has been communicated to employees and posted on our website. We are diligent in complying with established accounting principles and are committed to providing financial information that is transparent, timely, and accurate. We have a Related Person Transactions Policy designed to promote the timely identification of such transactions and to ensure we give appropriate consideration to any real or perceived conflicts in our commercial arrangements. We have a global process through which employees, either directly or anonymously, can notify management (and the Audit Committee of the Board of Directors) of alleged accounting and auditing concerns or violations including fraud. Our internal Disclosure Committee meets regularly and operates pursuant to formal disclosure procedures and guidelines which help to ensure that our public disclosures are accurate and timely. Copies of our Corporate Governance Guidelines, Code of Business Conduct and Ethics, and Related Person Transactions Policy are available on our website at www.ir.criver.com.
Executive Officers of the Registrant (pursuant to Instruction 3 to Item 401(b) of Regulation S-K)
Below are the names, ages and principal occupations of each of our current executive officers. All such persons have been elected to serve until their successors are elected and qualified or until their earlier resignation or removal.
James C. Foster, age 66, joined us in 1976 as General Counsel. During his tenure, Mr. Foster has held various staff and managerial positions, and was named our President in 1991, Chief Executive Officer in 1992 and our Chairman in 2000.
William D. Barbo, age 56, joined us in 1982 as a laboratory technician. Between 1982 and 2005, Mr. Barbo served in a variety of positions of increasing responsibilities. He was named Corporate Vice President of Research Models and Services in 2005, Corporate Senior Vice President of Global Sales and Marketing in 2010, and Corporate Executive Vice President and Chief Commercial Officer in October 2016.
David P. Johst, age 55, joined us in 1991 as Corporate Counsel and was named Vice President, Human Resources in 1995. He became Vice President, Human Resources and Administration in 1996, a Senior Vice President in 1999, and a Corporate Executive Vice President in 2005. He currently serves as our General Counsel and Chief Administrative Officer and is responsible for overseeing our corporate legal function, Human Resources department, and several other corporate staff departments. Prior to joining us, Mr. Johst was in private practice at the law firm of Hale and Dorr (now WilmerHale). Mr. Johst currently serves as a trustee of Mt. Ida College.
Davide Molho, age 47, joined our Italian operations in 1999 and was promoted to Director of Operations for RMS Italy in 2002. In 2005, his role was expanded to include French RMS operations and in 2007, he became Corporate Vice President, European Research Models and Services with responsibility for all European RMS operations. In July 2009, Dr. Molho was promoted to Corporate Senior Vice President, North American and European Research Models and Services. He was subsequently promoted to Corporate Executive Vice President and President, Global Research Models and Services in
December 2010. In 2011, Dr. Molho was named Corporate Executive Vice President, North America Operations and in December 2013, he was named Corporate Executive Vice President and President, Global RMS and DSA Operations.
David R. Smith, age 51, has served as our Corporate Executive Vice President and Chief Financial Officer since August 2015. He joined us as Corporate Vice President, Discovery Services through our acquisition of Argenta and BioFocus from Galapagos NV in March 2014 and was promoted to Corporate Senior Vice President, Global Discovery Services, in October 2014. At Galapagos, he served in various capacities, including as Chief Executive Officer of its Galapagos Services division and as Chief Financial Officer. Mr. Smith served as Chief Financial Officer for Cambridge University Hospitals from 2007 to 2013. Mr. Smith spent eight years at PricewaterhouseCoopers prior to joining AstraZeneca in 1997, where he spent the next nine years in various finance and business roles of increasingly greater responsibility.
Item 1A. Risk Factors
Set forth below, elsewhere in this Form 10-K and in other documents we file with the SEC are risks and uncertainties that could cause actual results to differ materially from the results contemplated by the forward-looking statements contained in this Form 10-K. We note that factors set forth below, individually or in the aggregate, may cause our actual results to differ materially from expected and historical results. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties.
The outsourcing trend in non-clinical (discovery and safety assessment) stages of drug discovery and development may decrease, which could impair our growth.
Over the past decade, pharmaceutical and biotechnology companies have generally increased their outsourcing of non-clinical research support activities, such as discovery and safety assessment. While many industry analysts expect the outsourcing trend to continue to increase for the next several years (although with different growth rates for different phases of drug discovery and development), decreases in such outsourcing may result in a diminished growth rate in the sales of any one or more of our service lines and may adversely affect our financial condition and results of operations. For additional discussion of the factors that we believe have recently been influencing outsourcing demand from our clients, please see the section entitled “Our Strategy” included elsewhere in this Form 10-K.
A reduction in research and development budgets at pharmaceutical and biotechnology companies may adversely affect our business.
Our clients include researchers at pharmaceutical and biotechnology companies. Our ability to continue to grow and win new business is dependent in large part upon the ability and willingness of the pharmaceutical and biotechnology industries to continue to spend on molecules in the non-clinical phases of research and development (and in particular discovery and safety assessment) and to outsource the products and services we provide. Fluctuations in the expenditure amounts in each phase of the research and development budgets of these researchers and their organizations could have a significant effect on the demand for our products and services. Research and development budgets fluctuate due to changes in available resources, mergers of pharmaceutical and biotechnology companies, spending priorities (including available resources of our biotechnology clients, particularly those that are cash-negative, who may be highly focused on rationing their liquid assets in a challenging funding environment), general economic conditions, and institutional budgetary policies. Available funding for biotechnology clients in particular may be affected by the capital markets, investment objectives of venture capital investors, and priorities of biopharmaceutical industry sponsors.
Our business could be adversely affected by any significant decrease in drug research and development expenditures by pharmaceutical and biotechnology companies, as well as by academic institutions, government laboratories, or private foundations. Similarly, economic factors and industry trends that affect our clients in these industries also affect their research and development budgets and, consequentially, our business as well. Furthermore, our clients (particularly larger biopharmaceutical companies) continue to search for ways to maximize the return on their investments with a focus on leaner research and development costs per drug candidate. For additional discussion of the factors that we believe have recently been influencing research and development budgets at our clients, please see the sections entitled “Our Strategy” and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this Form 10-K.
A reduction or delay in government funding of research and development may adversely affect our business.
A portion of revenue in our RMS segment is derived from clients at academic institutions and research laboratories whose funding is partially dependent on both the level and timing of funding from government sources such as the U.S. National Institutes of Health (NIH) and similar domestic and international agencies, which can be difficult to forecast. Government
funding of research and development is subject to the political process, which is inherently fluid and unpredictable. Our revenue may be adversely affected if our clients delay purchases as a result of uncertainties surrounding the approval of government budget proposals. Also, government proposals to reduce or eliminate budgetary deficits have sometimes included reduced allocations to the NIH and other government agencies that fund research and development activities. Other programs, such as homeland security or defense, or general efforts to reduce the federal budget deficit could be viewed by the U.S. government as a higher priority. These budgetary pressures may result in reduced allocations in the future to government agencies that fund research and development activities. A reduction in government funding for the NIH or other government research agencies could adversely affect our business and our financial results. Also, there is no guarantee that NIH funding will be directed towards projects and studies that require use of our products and services.
Changes in government regulation or in practices relating to the pharmaceutical or biotechnology industries, including potential healthcare reform, could decrease the need for the services we provide.
Governmental agencies throughout the world, but particularly in the U.S., strictly regulate the drug development process. Our business involves helping pharmaceutical and biotechnology companies, among others, navigate the regulatory drug approval process. Accordingly, many regulations, and often new regulations, are expected to result in higher regulatory standards and often additional revenues for companies that service these industries. However, some changes in regulations, such as a relaxation in regulatory requirements or the introduction of streamlined or expedited drug approval procedures, or an increase in regulatory requirements that we have difficulty satisfying or that make our services less competitive, could eliminate or substantially reduce the demand for our services.
Although we believe we are currently in compliance in all material respects with national, regional, and local laws, as well as other accepted guidance used by oversight bodies (which include the USDA, the standards set by the International Air Transport Association, the Convention on International Trade in Endangered Species of Wild Fauna and Flora, U.S. Fish and Wildlife Service, The Centers for Disease Control, the Department of Transportation, the Department of State, the office of Laboratory Animal Welfare of NIH, the Drug Enforcement Agency, as well as numerous other oversight agencies in Canada, Europe, and Asia), failure to comply could subject us to denial of the right to conduct business, fines, criminal penalties, and other enforcement actions. In addition, if regulatory authorities were to mandate a significant reduction in safety assessment procedures which utilize laboratory animals (as has been advocated by certain groups), certain segments of our business could be materially adversely affected.
In March 2010, the U.S. Congress enacted healthcare reform legislation intended over time to expand health insurance coverage and impose health industry cost containment measures. In June 2012, the U.S. Supreme Court upheld the constitutionality of this legislation. The Court’s decision allows implementation of key provisions impacting drug manufacturers going forward, including, but not limited to, (1) expansion of access to health insurance coverage, (2) expansion of the Medicaid program, (3) enactment of an industry fee on pharmaceutical companies, and (4) imposition of an excise tax on the sale of medical devices. Since the law and its implementation continue to face challenges in Congress and federal courts, and from certain state governments, opposition advocacy groups, and some small business organizations, as well as from the incoming president and his administration, we are uncertain as to the ultimate effects of this legislation on our business and are unable to predict what legislative proposals will be adopted in the future.
Implementation of healthcare reform legislation may have certain benefits, but also may contain costs that could limit the profits that can be made from the development of new drugs. This could adversely affect research and development expenditures by pharmaceutical and biotechnology companies, which could in turn decrease the business opportunities available to us both in the U.S. and abroad. In addition, new laws or regulations may create a risk of liability, increase our costs, or limit our service offerings. Furthermore, if health insurers were to change their practices with respect to reimbursements for pharmaceutical products, our clients may spend less, or reduce their growth in spending on research and development.
In addition, the recent presidential and congressional elections in the U.S. may result in significant changes in, and uncertainty with respect to, legislation, regulation and government policy. While it is not possible to predict whether and when any such changes will occur, changes at the local, state or federal level may significantly impact our domestic and foreign businesses and/or those of our clients. Specific legislative and regulatory proposals discussed during and after the election that may have a material impact on us or our clients include, but are not limited to, appeal or reform of the Health Care Reform Act; and modifications to international trade policy, public company reporting requirements, environmental regulation and antitrust enforcement.
Contaminations in our animal populations can damage our inventory, harm our reputation for contaminant-free production, result in decreased sales and cause us to incur additional costs.
Our research models and fertile chicken eggs must be free of certain infectious agents such as certain viruses and bacteria because the presence of these contaminants can distort or compromise the quality of research results and could adversely impact human or animal health. The presence of these infectious agents in our animal production facilities and certain service operations could disrupt our contaminant-free research model and fertile egg production as well as our animal services businesses including GEMS, harm our reputation for contaminant-free production, and result in decreased sales.
If they occur, contaminations typically require cleaning up, renovating, disinfecting, retesting, and restarting production or services. Such clean-ups result in inventory loss, clean-up and start-up costs, and reduced sales as a result of lost client orders and potentially credits for prior shipments. In addition to microbiological contaminations, the potential for genetic mix-ups or mis-matings also exists and may require the restarting of the applicable colonies. While this does not require the complete clean-up, renovation, and disinfection of the barrier room, it would likely result in inventory loss, additional start-up costs and possibly reduced sales. Contaminations also expose us to risks that clients will request compensation for damages in excess of our contractual indemnification requirements. There also exists a risk that contaminations from models that we produce may affect our client's facilities, with similar impact to them for which we could be liable for damages. In some cases, we may produce or import animals carrying infectious agents capable of causing disease in humans; and in the case of such a contamination or undiagnosed infection, there could be a possible risk of human exposure and infection.
We are also subject to similar contamination risks with respect to our large research models. While often we own these models, they may be maintained on our behalf at a site operated by the original provider. Accordingly, risk of contamination may be outside of our control, and we depend on the practices and protocols of third parties to ensure a contamination-free environment. A contamination may require extended CDC quarantine with subsequent reduced sales as a result of lost client orders as well as the potential for complete inventory loss and disinfection of the affected quarantine rooms. Furthermore, while we often negotiate for contractual risk indemnification, we may be exposed in the event of such contaminations if the third party does not fulfill its indemnification obligation or is unable to as a result of insolvency or other impediments.
All such contaminations described above are unanticipated and difficult to predict and could adversely impact our financial results. Many of our operations are comprised of complex mechanical systems which are subject to periodic failure, including aging fatigue. Such failures are unpredictable, and while we have made significant capital expenditures designed to create redundancy within these mechanical systems, strengthen our biosecurity, improve our operating procedures to protect against such contaminations, and replace impaired systems and equipment in advance of such events, failures and/or contaminations may still occur.
Any failure by us to comply with applicable regulations and related guidance could harm our reputation and operating results, and compliance with new regulations and guidance may result in additional costs.
Any failure on our part to comply with applicable regulations could result in the termination of ongoing research or the disqualification of data for submission to regulatory authorities. This could harm our reputation, our prospects for future work and our operating results. For example, the issuance of a notice of objectionable observations or a warning from the FDA based on a finding of a material violation by us for GLP or cGMP requirements could materially and adversely affect us. If our operations are found to violate any applicable law or other governmental regulations, we might be subject to civil and criminal penalties, damages and fines. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management's attention from the operation of our business and damage our reputation.
In addition, regulations and guidance worldwide concerning the production and use of laboratory animals for research purposes continue to be updated. Notably, the European Directive 2010/63/EU requires new standards for animal housing and accommodations that require implementation by 2017. Some of these new standards require additional operating and capital expenses that will impact not only us and our industry competitors, but clients in the biomedical research community through both changes in the pricing of goods and services and changes in their own operations.
Similarly, guidance has been and continues to be developed for other areas that impact the biomedical research community on both a national and international basis including transportation, mandated contingency planning, euthanasia guidance, import and export requirements of biological materials, health monitoring requirements and the use of disinfectants.
We could experience a breach of the confidentiality of the information we hold or of the security of our computer systems.
We operate large and complex computer systems that contain significant amounts of client data. As a routine element of our business, we collect, analyze, and retain substantial amounts of data pertaining to the non-clinical studies we conduct for our clients. Unauthorized third parties could attempt to gain entry to such computer systems for the purpose of stealing data or disrupting the systems. We believe that we have taken appropriate measures to protect them from intrusion, and we continue to improve and enhance our systems in this regard, but in the event that our efforts are unsuccessful, we could suffer significant harm. Our contracts with our clients typically contain provisions that require us to keep confidential the information generated from these studies. In the event the confidentiality of such information was compromised, we could suffer significant harm.
Our revenue generating agreements contain termination and service reduction provisions or may otherwise terminate according to their term, which may result in less contract revenue than we anticipate.
Many of our agreements with both large and small clients, including those which underlie our strategic relationships with some of our more significant customers, provide for termination or reduction in scope with little or no notice. In addition, we sell our products and services to our competitors, and similarly they sell products and services to us. For instance, we have historically entered into, and currently are party to, contracts with certain of our competitors to distribute specialty research models in locations where our competitors may not have distribution capabilities.
Clients and/or competitors may elect to terminate their agreements with us for various reasons including:
| |
| • | the products being tested fail to satisfy safety requirements; |
| |
| • | unexpected or undesired study results; |
| |
| • | production problems resulting in shortages of the drug being tested; |
| |
| • | a client's decision to forego or terminate a particular study; |
| |
| • | establishment of alternative distribution channels by our competitors; |
| |
| • | the loss of funding for the particular research study; or |
| |
| • | general convenience/counterparty preference. |
If a client or competitor terminates a contract with us, we are typically entitled under the terms of the contract to receive revenue earned to date as well as certain other costs and, in some cases, termination fees. Cancellation of a large contract or proximate delay, cancellation or conclusion of multiple contracts could materially adversely affect our business and, therefore, may adversely affect our operating results.
Many of our contracts are fixed price and may be delayed or terminated or reduced in scope for reasons beyond our control, or we may under‑price or overrun cost estimates with these contracts, potentially resulting in financial losses.
Many of our contracts provide for services on a fixed price or fee-for-service with a cap basis and, accordingly, we bear the financial risk if we initially under-price our contracts or otherwise overrun our cost estimates. In addition, these contracts may be terminated or reduced in scope either immediately or upon notice. Cancellations may occur for a variety of reasons, and often at the discretion of the client. The loss, reduction in scope or delay of a large contract or the loss or delay of multiple contracts could materially adversely affect our business, although our contracts frequently entitle us to receive the costs of winding down the terminated projects, as well as all fees earned by us up to the time of termination. Some contracts also entitle us to a predetermined termination fee and irrevocably committed costs/expenses.
Several of our product and service offerings are dependent on a limited source of supply, which if interrupted could adversely affect our business.
We depend on a limited international source of supply for certain products, such as large research models. Disruptions to their continued supply may arise from health problems, export or import laws/restrictions or embargoes, international trade regulations, foreign government or economic instability, severe weather conditions, increased competition among suppliers for models, disruptions to the air travel system, activist campaigns, commercial disputes, supplier insolvency, or other normal-course or unanticipated events. Any disruption of supply could harm our business if we cannot remove the disruption or are unable to secure an alternative or secondary supply source on comparable commercial terms.
If we are not successful in selecting and integrating the businesses and technologies we acquire, or in managing our current and future divestitures, our business may suffer.
During the past fifteen years, we have steadily expanded our business through numerous acquisitions. We plan to continue to acquire businesses and technologies and form strategic alliances. However, businesses and technologies may not be available on terms and conditions we find acceptable. We risk spending time and money investigating and negotiating with potential acquisition or alliance partners, but not completing transactions.
In April 2016, we acquired WIL Research, a premier provider of safety assessment and contract development manufacturing services to biopharmaceutical, agricultural, and industrial chemical companies worldwide. This transaction was our largest acquisition in over ten years.
Acquisitions and alliances involve numerous risks which may include:
| |
| • | difficulties in achieving business and financial success; |
| |
| • | difficulties and expenses incurred in assimilating and integrating operations, services, products, technologies, or pre-existing relationships with our customers, distributors, and suppliers; |
| |
| • | challenges with developing and operating new businesses, including those which are materially different from our existing businesses and which may require the development or acquisition of new internal capabilities and expertise; |
| |
| • | potential losses resulting from undiscovered liabilities of acquired companies that are not covered by the indemnification we may obtain from the seller or the insurance we acquire in connection with the transaction; |
| |
| • | the presence or absence of adequate internal controls and/or significant fraud in the financial systems of acquired companies; |
| |
| • | diversion of management's attention from other business concerns; |
| |
| • | becoming subject to a more expansive regulatory environment; |
| |
| • | acquisitions could be dilutive to earnings, or in the event of acquisitions made through the issuance of our common stock to the shareholders of the acquired company, dilutive to the percentage of ownership of our existing shareholders; |
| |
| • | risks of not being able to overcome differences in foreign business practices, customs, and importation regulations, language, and other cultural barriers in connection with the acquisition of foreign companies; |
| |
| • | new technologies and products may be developed which cause businesses or assets we acquire to become less valuable; and |
| |
| • | risks that disagreements or disputes with prior owners of an acquired business, technology, service, or product may result in litigation expenses and diversion of our management's attention. |
In the event that an acquired business, technology, or an alliance does not meet our expectations, our results of operations may be adversely affected.
Some of the same risks exist when we decide to sell a business, site, or product line. In addition, divestitures could involve additional risks, including the following:
| |
| • | difficulties in the separation of operations, services, products, and personnel; |
| |
| • | diversion of management's attention from other business concerns; and |
| |
| • | the need to agree to retain or assume certain current or future liabilities in order to complete the divestiture. |
We continually evaluate the performance and strategic fit of our businesses. These and any divestitures may result in significant write-offs, including those related to goodwill and other intangible assets, which could have an adverse effect on our results of operations and financial condition. In addition, we may encounter difficulty in finding buyers, or, alternative exit strategies at acceptable prices and terms, and in a timely manner. We may not be successful in managing these or any other significant risks that we encounter in divesting a business, site, or product line, and as a result, we may not achieve some or all of the expected benefits of the divestiture.
Impairment of goodwill or other intangible assets may adversely impact future results of operations.
We have intangible assets, including goodwill, on our balance sheet due to our acquisitions of businesses. The initial identification and valuation of these intangible assets and the determination of the estimated useful lives at the time of acquisition involve use of management judgments and estimates. These estimates are based on, among other factors, projections of cash flows that arise from identifiable intangible assets of acquired businesses and discount rates based on an analysis of our weighted average cost of capital, adjusted for specific risks associated with the assets. Disruptions in global financial markets and deterioration of economic conditions could, among other things, impact the discount rate and other assumptions used in the valuations and actual cash flows arising from a particular intangible asset could vary from projected cash flows, which could imply different carrying values from those established at the dates of acquisition and which could result in impairment of such assets.
If the future growth and operating results of our business are not as strong as anticipated, overall macroeconomic or industry conditions deteriorate and/or our market capitalization declines, this could impact the assumptions used in establishing the carrying value of goodwill or other intangible assets. To the extent goodwill or other intangible assets are impaired, their carrying value will be written down to their implied fair values and a charge will be made to our income from continuing operations. Such an impairment charge could materially and adversely affect our operating results. As of December 31, 2016, the carrying amount of goodwill and other intangibles on our consolidated balance sheet was $1,182.0 million.
Our business is subject to risks relating to operating internationally.
A significant part of our revenue is derived from operations outside the U.S. Our international revenue represented approximately one-half of our total revenue in recent years. We expect that international revenue will continue to account for a significant percentage of our total revenue for the foreseeable future. There are a number of risks associated with our international business including:
| |
| • | foreign currencies we receive for sales and in which we record expenses outside the U.S. could be subject to unfavorable exchange rates with the U.S. dollar and reduce the amount of revenue and cash flow (and increase the amount of expenses) that we recognize and cause fluctuations in reported financial results; |
| |
| • | certain contracts, particularly in Canada, are frequently denominated in currencies other than the currency in which we incur expenses related to those contracts, and where expenses are incurred in currencies other than those in which contracts are priced, fluctuations in the relative value of those currencies could have a material adverse effect on our results of operations; |
| |
| • | general economic and political conditions in the markets in which we operate; |
| |
| • | potential international conflicts, including terrorist acts; |
| |
| • | potential trade restrictions, exchange controls, adverse tax consequences, and legal restrictions on the repatriation of funds into the U.S.; |
| |
| • | difficulties and costs associated with staffing and managing foreign operations, including risks of work stoppages and/or strikes, as well as violations of local laws or anti-bribery laws such as the U.S. Foreign Corrupt Practices Act, the UK Bribery Act, and the OECD Convention on Combating Bribery of Foreign Public Officials in International Business Transactions; |
| |
| • | unexpected changes in regulatory requirements; |
| |
| • | the difficulties of compliance with a wide variety of foreign laws and regulations; |
| |
| • | unfavorable labor regulations in foreign jurisdictions; |
| |
| • | potentially negative consequences from changes in or interpretations of U.S. and foreign tax laws; |
| |
| • | exposure to business disruption or property damage due to geographically unique natural disasters; |
| |
| • | longer accounts receivable cycles in certain foreign countries; and |
| |
| • | import and export licensing requirements. |
These risks, individually or in the aggregate, could have an adverse effect on our results of operations and financial condition. For example, as mentioned above, we are subject to compliance with the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws, which generally prohibit companies and their intermediaries from making improper payments to foreign
government officials for the purpose of obtaining or retaining business. While our employees, distributors and agents are required to comply with these laws, we cannot be sure that our internal policies and procedures will always protect us from violations of these laws despite our commitment to legal compliance and corporate ethics. The occurrence or allegation of these types of risks may adversely affect our business, performance, prospects, value, financial condition, and results of operations.
New technologies may be developed, validated, and increasingly used in biomedical research that could reduce demand for some of our products and services.
The scientific and research communities continue to explore methods to develop improved models and systems that would improve the translation of cellular and animal models to human studies and vice-versa and possibly replace or supplement the use of traditional living animals as test platforms in biomedical research. Some companies have developed techniques in these areas that may have scientific merit to improve translation between species. In addition, technological improvements to existing or new processes, such as imaging and other translational biomarker technologies, could result in the refinement and utility for the number of animal research models necessary to improve the translation from non-clinical to clinical studies. There is an increasing push to focus on in vitro technologies such that employ human materials, stem cell technology, and other model creation technology. However, the increasing availability and utility of these in vitro models is partially offset by these technologies facilitating the creation of humanized, highly specialized and specific disease-mimicking models we can produce.
It is our strategy to explore these in vitro technologies to refine and potentially reduce the utilization of animal models as these new methods become validated. For example, ChanTest Corporation has a well-developed program to evaluate the cardiac properties of induced pluripotent stem cell-derived cardiomyocytes. We may not be successful in commercializing these methods, and, furthermore, revenues from these new models and approaches if successfully developed may not offset reduced sales or profits from research models. In addition, alternative research methods could decrease the need for future research models, and we may not be able to develop new products effectively or in a timely manner to replace any lost sales. Lastly, other companies or entities may develop research models with characteristics different than the ones that we produce, and which may be viewed as more desirable by some of our clients.
Negative attention from special interest groups may impair our business.
The products and services which we provide our clients are essential to the drug discovery, development and manufacturing processes, and are almost universally mandated by law. Notwithstanding, certain special interest groups categorically object to the use of animals for valid research purposes. Historically, our core research model activities with rats, mice and other rodents have not been the subject of significant animal rights media attention. However, research activities with animals have been the subject of adverse attention, including shareholder proposals and attempts to disrupt air carriers from transporting research models, impacting the industry. This has included periodic demonstrations near facilities operated by us and at our annual meetings, as well as shareholder proposals we received for some of our past Annual Meetings of Shareholders. Any negative attention, threats or acts of vandalism directed against either our animal research activities or our third party service providers such as our airline carriers in the future could impair our ability to operate our business efficiently.
Our debt level could adversely affect our business and growth prospects.
As of December 31, 2016, we had $1.2 billion of debt. Our debt could have significant adverse effects on our business, including making it more difficult for us to obtain additional financing on favorable terms; requiring us to dedicate a substantial portion of our cash flows from operations to the repayment of debt and the interest on this debt; limiting our ability to capitalize on significant business opportunities; and making us more vulnerable to rising interest rates. For additional information regarding our debt, please see Note 7, “Long-Term Debt and Capital Lease Obligations”, included in the Notes to Consolidated Financial Statements included elsewhere in this Form 10-K.
The drug discovery, development services and manufacturing support industries are highly competitive.
The drug discovery, non-clinical development, and manufacturing support services industries are highly competitive. We often compete for business not only with other CROs, but also with internal discovery and development departments within our larger clients, who may have greater resources than ours. We also compete with universities and teaching hospitals for outsourced services. We compete on a variety of factors, including:
| |
| • | reputation for on-time quality performance; |
| |
| • | reputation for regulatory compliance; |
| |
| • | expertise and experience in multiple specialized areas; |
| |
| • | scope and breadth of service and product offerings across the drug discovery and development spectrum; |
| |
| • | scope and breadth of service and product offerings across the manufacturing support spectrum; |
| |
| • | ability to provide flexible and customized solutions to support our clients' drug discovery, non-clinical development, and manufacturing support needs; |
| |
| • | broad geographic availability (with consistent quality); |
| |
| • | technological expertise and efficient drug development processes; |
| |
| • | ability to acquire, process, analyze, and report data in an accurate manner; and |
| |
| • | accessibility of client data through secure portals. |
If we do not compete successfully, our business will suffer. Increased competition might lead to price and other concessions that could adversely affect our operating results. The drug discovery and development services industry has continued to see a trend towards consolidation, particularly among the biotechnology companies, who are targets for each other and for larger pharmaceutical companies. If this trend continues, it is likely to produce more competition among the larger companies and CROs generally, with respect to both clients and acquisition candidates. In addition, small, specialized entities considering entering the CRO industries will continue to find lower barriers to entry, and private equity firms may determine that there are opportunities to acquire and consolidate these companies, thus further increasing possible competition. More generally, our competitors or others might develop technologies, services or products that are more effective or commercially attractive than our current or future technologies, services, or products, or that render our technologies, services, or products less competitive or obsolete. If competitors introduce superior technologies, services, or products and we cannot make enhancements to ours to remain competitive, our competitive position, and in turn our business, revenue, and financial condition, would be materially and adversely affected. In the aggregate, these competitive pressures may affect the attractiveness of our technologies, services, or products and could adversely affect our financial results.
Potential Changes in U.S. and International Tax Law.
In the U.S., there are several proposals to reform corporate tax law that are currently under consideration. These proposals include reducing the corporate statutory tax rate, broadening the corporate tax base through the elimination or reduction of deductions, exclusions, and credits, implementing a territorial regime of taxation, limiting the ability of U.S. corporations to deduct interest expense, modifying the foreign tax credit rules, and reducing the ability to defer U.S. tax on offshore earnings. These or other changes in the U.S. tax laws could increase our effective tax rate which would affect our profitability.
We have substantial operations in Canada and the United Kingdom which currently benefit from favorable corporate tax arrangements. We receive substantial tax credits in Canada, from both the Canadian federal and Quebec governments, and the U.K. Any reduction in the availability or amount of these tax credits due to tax law changes or outcomes of tax controversies could have a material adverse effect on our profits, cash flow, and effective tax rate.
Currently, the OECD has developed an action plan to address concerns regarding base erosion and profit shifting (BEPS). This initiative has resulted in proposed and enacted changes to tax laws in various countries including France, Germany, and the U.K. Future changes to tax laws or interpretation of tax laws resulting from the BEPS project could increase our effective tax rate, which would affect our profitability.
Contract research services create a risk of liability.
As a CRO, we face a range of potential liabilities which may include:
| |
| • | errors or omissions in reporting of study detail in non-clinical studies that may lead to inaccurate reports, which may undermine the usefulness of a study or data from the study, or which may potentially advance studies absent the necessary support or inhibit studies from proceeding to the next level of testing; |
| |
| • | risks associated with our possible failure to properly care for our clients' property, such as research models and samples, study compounds, records, work in progress, other archived materials, or goods and materials in transit, while in our possession; |
| |
| • | risks that models in our breeding facilities or in facilities that we manage may be infected with diseases that may be harmful and even lethal to them or humans, despite preventive measures contained in our policies for the quarantine and handling of imported animals; and |
| |
| • | risks that we may have errors and omissions and/or product liabilities related to our products designed to conduct lot release testing of medical devices, injectable drugs, food, beverages, and home and beauty products (primarily through our Microbial Solutions business), or in the testing of biologics and other services performed by our Biologics business, which could result in us or our clients failing to identify unsafe or contaminated materials. |
While we attempt to mitigate these risks through a variety of methods, it is impossible to completely eradicate such risks. In our RMS business, we mitigate these risks to the best of our abilities through our regimen of animal testing, quarantine procedures, and veterinary staff vigilance, through which we seek to control the exposure of animal related disease or infections. In our DSA and Manufacturing businesses, we attempt to reduce these risks by contractual risk transfer provisions entitling us to be indemnified subject to a limitation of liability, by insurance maintained by our clients and/or by us, and by various regulatory requirements we must follow in connection with our business.
Contractual risk transfer indemnifications generally do not protect us against liability arising from certain of our own actions, such as negligence or misconduct. We could be materially and adversely affected if we are required to pay damages or bear the costs of defending any claim that is outside any contractual indemnification provision, or if a party does not fulfill its indemnification obligations, or the damage is beyond the scope or level of insurance coverage. We also often contractually indemnify our clients (subject to a limitation of liability), similar to the way they indemnify us, and we may be materially adversely affected if we have to fulfill our indemnity obligations. Furthermore, there can be no assurance that neither we nor a party required to indemnify us will be able to maintain such insurance coverage (either at all or on terms acceptable to us).
Upgrading and integrating our business systems could result in implementation issues and business disruptions.
In recent years we implemented a project to replace many of our numerous legacy business systems at certain sites worldwide with an enterprise wide, integrated enterprise resource planning (ERP) system. The expansion of the ERP system to other international locations may occur at a future date based on value to the business. In general, the process of planning and preparing for these types of integrated, wide-scale implementations is extremely complex and we are required to address a number of challenges including data conversion, system cutover, and user training. Problems in any of these areas could cause operational problems during implementation including delayed shipments, missed sales, billing and accounting errors, and other operational issues. There have been numerous, well-publicized instances of companies experiencing difficulties with the implementation of ERP systems, which resulted in negative business consequences.
The drug discovery and development industry has a history of patent and other intellectual property litigation, and we might be involved in costly intellectual property lawsuits.
The drug discovery and development industry has a history of patent and other intellectual property litigation and these lawsuits will likely continue.
In July 2015, IDEXX Laboratories, Inc. and IDEXX Distribution, Inc. (collectively, IDEXX) filed a complaint in the United States District Court for the District of Delaware alleging we have infringed three (3) recently issued patents related to a blood spot sample collection method used in determining the presence or absence of an infectious disease in a population of rodents. We filed our answer to the complaint on July 21, 2016. In addition, on July 29, 2016, we initiated an inter partes review (IPR) procedure with the United States Patent and Trademark Office challenging the validity of the IDEXX patents. On February 6, 2017, we entered into a settlement agreement with IDEXX, which involved the withdrawal by IDEXX of their complaint and withdrawal by us of the IPR.
Legal proceedings relating to intellectual property are expensive, take significant time, and divert management's attention from other business concerns, whether we win or lose. If we do not prevail in an infringement lawsuit brought against us, we might have to pay substantial damages, including treble damages, and we could be required to stop the infringing activity or obtain a license to use technology on unfavorable terms.
We may not be able to successfully develop and market new services and products.
We may seek to develop and market new services and products that complement or expand our existing business or service offerings. We believe our ability to in-license new technologies from third parties will be critical to our ability to offer new products and services to our customers. Our ability to gain access to technologies that we need for new products and services depends, in part, on our ability to convince inventors and their agents or assignees that we can successfully commercialize their inventions. We cannot guarantee that we will be able to identify new technologies of interest to our customers. Even if we are able to identify new technologies of interest, we may not be able to negotiate license agreements on acceptable terms, or at all. If we are unable to develop new services and products and/or create demand for those newly developed services and products, our future business, results of operations, financial condition, and cash flows could be adversely affected.
We depend on key personnel and may not be able to retain these employees or recruit additional qualified personnel, which would harm our business.
Our success depends to a significant extent on the continued services of our senior management and other members of management. James C. Foster, our Chief Executive Officer since 1992 and Chairman since 2000, has held various positions with us for four decades. We have no employment agreement with Mr. Foster or other members of our non-European based senior management. If Mr. Foster or other members of senior management do not continue in their present positions, our business may suffer.
Because of the specialized scientific nature of our business, we are highly dependent upon attracting and retaining qualified scientific, technical, and managerial personnel. While we have a strong record of employee retention, there is still significant competition for qualified personnel in the veterinary, pharmaceutical, and biotechnology fields. Therefore, we may not be able to attract and retain the qualified personnel necessary for the development of our business. The loss of the services of existing personnel, as well as the failure to recruit additional key scientific, technical, and managerial personnel in a timely manner, could harm our business.
Our quarterly operating results may vary, which could negatively affect the market price of our common stock.
Our results of operations in any quarter may vary from quarter to quarter and are influenced by such factors as:
| |
| • | changes in the general global economy; |
| |
| • | the number and scope of ongoing client engagements; |
| |
| • | the commencement, postponement, delay, progress, completion, or cancellation of client contracts in the quarter; |
| |
| • | changes in the mix of our products and services; |
| |
| • | competitive pricing pressures; |
| |
| • | the extent of cost overruns; |
| |
| • | holiday buying patterns of our clients; |
| |
| • | budget cycles of our clients; |
| |
| • | changes in tax laws, rules, regulations, and tax rates in the locations in which we operate; |
| |
| • | the timing and charges associated with completed acquisitions and other events; |
| |
| • | the financial performance of our venture capital investments; |
| |
| • | the occasional extra week (“53rd week”) that we recognize in a fiscal year (and fourth fiscal quarter thereof) due to our fiscal year ending on the last Saturday in December; and |
| |
| • | exchange rate fluctuations. |
We believe that operating results for any particular quarter are not necessarily a meaningful indication of future results. Nonetheless, fluctuations in our quarterly operating results could negatively affect the market price of our common stock.
Referendum on the United Kingdom’s membership in the European Union (“Brexit”) may adversely affect our business.
On June 23, 2016, the U.K. held a referendum in which voters approved an exit from the European Union (E.U.), referred to as “Brexit.” As a result of the referendum, it is expected that the British government will begin negotiating the terms of the U.K.’s future relationship with the E.U. The decision by referendum to withdraw the U.K. from the E.U. has caused significant
volatility in global stock markets and currency exchange rate fluctuations, including the strengthening of the U.S. dollar against foreign currencies. The execution of Brexit also may create global economic uncertainty, which may cause our customers and potential customers to monitor their costs and reduce their budgets for our products and services. In addition, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the U.K. determines which E.U. laws to replace or replicate. Given that we conduct a substantial portion of our business in the E.U. and the U.K., these effects of Brexit, among others, could adversely affect our business, business opportunities, results of operations, financial condition, and cash flows.
Our industry has a history of patent and other intellectual property litigation, which can be costly.
Our industry has a history of intellectual property litigation. On July 31, 2015, IDEXX Laboratories, Inc. and IDEXX Distribution, Inc. filed a complaint in the United States District Court for the District of Delaware alleging we infringed three recently issued patents related to a dried blood spot sample collection method used in determining the presence or absence of an infectious disease in a population of rodents. Legal proceedings relating to intellectual property can be expensive, take significant time, and divert management’s attention from other business concerns, regardless of the outcome of the litigation. On February 6, 2017, we entered into a settlement agreement with IDEXX, which involved the withdrawal by IDEXX of their complaint and withdrawal by us of the IPR.
Since we do not expect to pay any cash dividends for the foreseeable future, our shareholders will benefit from an investment in our common stock only if it appreciates in value.
We have not declared or paid any cash dividends on our common stock, and do not anticipate that we will pay any dividends to holders of our common stock for the foreseeable future. Any payment of cash dividends will be at the discretion of our Board of Directors and will depend on our financial condition, capital requirements, legal requirements, earnings and other factors. Consequently, our shareholders should not rely on dividends to receive a return on their investment.
Item 1B. Unresolved Staff Comments
There are no unresolved comments to be reported in response to Item 1B.
Item 2. Properties
We own or lease the land and buildings where we have facilities. We own large facilities (facilities over 50,000 square feet) for our DSA businesses in Canada, France, Ireland, Netherlands, Scotland, and the U.S. and lease large facilities in England and the U.S. We own large RMS facilities in Canada, China, France, Germany, Italy, Japan, England, and the U.S. We own large Manufacturing segment facilities in the U.S. and China. None of our leases is individually material to our business operations. Many of our leases have an option to renew, and we believe that we will be able to successfully renew expiring leases on terms satisfactory to us. We believe that our facilities in each of our reportable segments are adequate for our operations and that suitable additional space will be available when needed. For additional information, see Note 7, “Long-Term Debt and Capital Lease Obligations” and Note 13, “Commitments and Contingencies” included in Item 8, “Financial Statements and Other Supplementary Data” in this Annual Report on Form 10-K.
Capacity at our Safety Assessment businesses within our DSA segment is primarily based on physical room infrastructure designed towards meeting specific scientific and regulatory requirements. We track room utilization on an ongoing basis and depending on the needs of our clients at given times, we may need to execute on contingent plans for expansion, which average between six and fifteen months to complete.
We may also expand at specific sites in order to accommodate needs resulting from any consolidation strategy. We continue to employ a master site planning strategy to proactively evaluate our real estate needs. In certain circumstances, we dispose of or consolidate operations, which could result in impairment charges. In situations where the associated real estate is leased, and depending on the resolution of these situations, we may be encumbered with the remaining real estate lease obligations.
Item 3. Legal Proceedings
We are not party to any material legal proceedings, other than ordinary routine litigation incidental to our business that is not material to our business or financial condition.
In May 2013, with the assistance of the law firm of Davis Polk & Wardwell LLP, we commenced an investigation into inaccurate billing with respect to certain government contracts. This issue had been reported to our senior management by a Charles River employee. We promptly reported these matters to the relevant government contracting officers, the Department of Health and Human Services’ Office of the Inspector General, and the Department of Justice, and we are cooperating with these agencies to ensure the proper repayment and resolution of this matter. The investigation confirmed that our RMS business
segment billed the Department of Health and Human Services for certain work that had not been performed with respect to a small subset of our government contracts. It has been determined that when employees regularly assigned to work in research model barrier rooms associated with these contracts were absent, other employees' names would be substituted on time-keeping records associated with the relevant contracts. We billed the government for the hours associated with these substitute employees, despite the fact that, in many cases, these employees did not perform any services in connection with the relevant government contracts. Based on the findings of the investigation to date, we believe that this conduct was limited to our research model facilities in Raleigh, North Carolina, and Kingston, New York. We previously identified approximately $1.5 million of excess amounts billed on these contracts, and recorded a liability for such amount. Based on our ongoing discussions with the government, we have recorded an additional charge of $0.3 million during the fiscal year 2016. Our best estimate, which totals $1.8 million, may be subject to change based on the terms of any final settlement with the Department of Justice and the Department of Health and Human Services’ Office of the Inspector General. We have already taken appropriate steps to prevent this conduct from recurring, and will consider additional remedial measures following the conclusion of the matter.
In July 2015, IDEXX filed a complaint in the United States District Court for the District of Delaware alleging we have infringed three (3) recently issued patents related to a blood spot sample collection method used in determining the presence or absence of an infectious disease in a population of rodents. On September 21, 2015, we timely filed a motion to dismiss the complaint on the grounds that all of the claims are directed to unpatentable subject matter and therefore are invalid. On October 7, 2015, IDEXX filed an amended complaint, which substantially asserted the same patents and infringement allegations as asserted in the original complaint, and on October 26, 2015, we timely filed a motion to dismiss this amended complaint. The hearing on the motion to dismiss was held on January 12, 2016. On July 1, 2016, the Court issued an opinion denying the motion to dismiss. We filed our answer to the complaint on July 21, 2016. In addition, on July 29, 2016, we initiated an inter partes review (IPR) procedure with the United States Patent and Trademark Office, challenging the validity of the IDEXX patents. On February 6, 2017, we entered into a settlement agreement with IDEXX, which involved the withdrawal by IDEXX of their complaint and withdrawal by us of the IPR.
Item 4. Mine Safety Disclosures
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock began trading on the New York Stock Exchange on June 23, 2000 under the symbol “CRL.” The following table shows the high and low sales prices for our common stock:
|
| | | | | | | |
| Fiscal 2017 | High | | Low |
| First quarter (through January 27, 2017) | $ | 82.89 |
| | $ | 75.25 |
|
| Fiscal 2016 | High | | Low |
| First quarter | $ | 81.61 |
| | $ | 65.70 |
|
| Second quarter | 87.95 |
| | 73.42 |
|
| Third quarter | 89.18 |
| | 75.54 |
|
| Fourth quarter | 84.53 |
| | 67.20 |
|
| Fiscal 2015 | High | | Low |
| First quarter | $ | 84.69 |
| | $ | 63.22 |
|
| Second quarter | 80.30 |
| | 68.59 |
|
| Third quarter | 78.50 |
| | 63.75 |
|
| Fourth quarter | 80.44 |
| | 59.99 |
|
There were no equity securities that were not registered under the Securities Act of 1933, as amended, sold during fiscal year 2016.
Shareholders
As of January 27, 2017, there were approximately 380 registered shareholders of the outstanding shares of common stock.
Dividends
We have not declared or paid any cash dividends on shares of our common stock in the past two years and we do not intend to pay cash dividends in the foreseeable future. We currently intend to retain any earnings to finance future operations and expansion.
Issuer Purchases of Equity Securities
The following table provides information relating to our purchases of shares of our common stock during the fourth quarter of 2016: |
| | | | | | | | | | | | | |
| Total Number
of Shares
Purchased |
| Average
Price Paid
per Share |
| Total Number of
Shares Purchased
as Part of Publicly
Announced Plans
or Programs |
| Approximate Dollar
Value of Shares
That May Yet Be
Purchased Under the
Plans or Programs |
| For the period | | | | | | | (in thousands) |
| September 25, 2016 to October 22, 2016 | 482 |
| | $ | 83.34 |
| | — |
| | $ | 69,694 |
|
| October 23, 2016 to November 19, 2016 | — |
| | — |
| | — |
| | 69,694 |
|
| November 20, 2016 to December 31, 2016 | 20 |
| | 71.10 |
| | — |
| | 69,694 |
|
| Total | 502 |
| | | | — |
| | |
|
In July 2010, our Board of Directors authorized a $500.0 million stock repurchase program, and subsequently approved increases to the program of $250.0 million in fiscal year 2010, $250.0 million in fiscal year 2013 and $150.0 million in fiscal year 2014, for an aggregate authorization of $1,150.0 million. During the fourth quarter of fiscal year 2016, we did not repurchase any shares of common stock under our Rule 10b5-1 Purchase Plan or in open market trading. Additionally, our stock-based compensation plans permit the netting of common stock upon vesting of restricted stock, performance share units, and restricted stock units in order to satisfy individual minimum statutory tax withholding requirements.
Comparison of 5-Year Cumulative Total Return
The following stock performance graph compares the annual percentage change in the Company’s cumulative total shareholder return on its Common Stock during a period commencing on December 31, 2011 and ending on December 31, 2016 (as measured by dividing (1) the sum of (A) the cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and (B) the difference between the Company’s share price at the end and the beginning of the measurement period; by (2) the share price at the beginning of the measurement period) with the cumulative total return of the S&P 500 Index and the S&P 500 Health Care Index during such period. The Company has not paid any dividends on the Common Stock, and no dividends are included in the representation of the Company’s performance. The stock price performance on the graph below is not necessarily indicative of future price performance. The graph is not “soliciting material,” is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference in any filing of the Company under the Securities Act of 1933 or the Securities Exchange Act of 1934 whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. Information used in the graph was obtained from Standards & Poor’s Institutional Market Services, a source believed to be reliable, but the Company is not responsible for any errors or omissions in such information
COMPARISON OF 5-YEAR CUMULATIVE TOTAL RETURN
Among Charles River Laboratories International, Inc., The S&P 500 Index And
The S&P 500 Health Care Index
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| | 2011 | | 2012 | | 2013 | | 2014 | | 2015 | | 2016 |
| Charles River Laboratories International, Inc. | $ | 100 |
| | $ | 135 |
| | $ | 195 |
| | $ | 235 |
| | $ | 293 |
| | $ | 279 |
|
| S&P 500 | 100 |
| | 116 |
| | 154 |
| | 175 |
| | 177 |
| | 198 |
|
| S&P 500 Health Care | 100 |
| | 118 |
| | 167 |
| | 209 |
| | 223 |
| | 217 |
|
Item 6. Selected Consolidated Financial Data
The selected financial data presented below is derived from our audited consolidated financial statements and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” contained in Item 7 and “Financial Statements and Supplementary Data” contained in Item 8 of this Annual Report on Form 10-K. Our fiscal year is typically based on 52-weeks, with each quarter composed of 13 weeks ending on the last Saturday on, or closest to, March 31, June 30, September 30, and December 31. A 53rd week was included in fiscal year 2016, which is occasionally necessary to align with a December 31 calendar year-end. The additional week was included in the fourth quarter.
|
| | | | | | | | | | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 | | 2013 | | 2012 |
| | | | | | | | | | |
| | (in thousands, except per share amounts) |
| Statement of Income Data | | | | | | | | | |
| Total revenue | $ | 1,681,432 |
| | $ | 1,363,302 |
| | $ | 1,297,662 |
| | $ | 1,165,528 |
| | $ | 1,129,530 |
|
| Income from continuing operations, net of income taxes | 156,086 |
| | 152,037 |
| | 129,924 |
| | 105,416 |
| | 102,118 |
|
| Income (loss) from discontinued operations, net of income taxes | 280 |
| | (950 | ) | | (1,726 | ) | | (1,265 | ) | | (4,252 | ) |
| Common Share Data | | | | | | | | | |
| Earnings per common share from continuing operations: | | | | | | | | | |
| Basic | $ | 3.28 |
| | $ | 3.23 |
| | $ | 2.76 |
| | $ | 2.18 |
| | $ | 2.12 |
|
| Diluted | $ | 3.22 |
| | $ | 3.15 |
| | $ | 2.70 |
| | $ | 2.15 |
| | $ | 2.10 |
|
| Other Data | | | | | | | | | |
| Depreciation and amortization | $ | 126,658 |
| | $ | 94,881 |
| | $ | 96,445 |
| | $ | 96,636 |
| | $ | 81,275 |
|
| Capital expenditures | 55,288 |
| | 63,252 |
| | 56,925 |
| | 39,154 |
| | 47,534 |
|
| Balance Sheet Data (as of period end) | | | | | | | | | |
| Cash and cash equivalents | $ | 117,626 |
| | $ | 117,947 |
| | $ | 160,023 |
| | $ | 155,927 |
| | $ | 109,685 |
|
Total assets(1) | 2,711,800 |
| | 2,068,497 |
| | 1,870,578 |
| | 1,623,438 |
| | 1,577,111 |
|
Long-term debt, net and capital leases(1) | 1,207,696 |
| | 845,997 |
| | 740,557 |
| | 635,226 |
| | 520,712 |
|
| Redeemable noncontrolling interest | 14,659 |
| | 28,008 |
| | 28,419 |
| | 20,581 |
| | — |
|
| | | | | | | | | | |
(1) During the second quarter of 2015, we elected early adoption of Accounting Standards Update (ASU) 2015-03, “Simplifying the Presentation of Debt Issuance Costs,” and applied the changes retrospectively to all prior periods. During the fourth quarter of 2015, we elected early adoption of ASU 2015-17, “Balance Sheet Classification of Deferred Taxes,” and applied the changes retrospectively to all prior periods. Prior years’ amounts have been updated to conform to current presentation. |
Refer to Note 2, “Business Acquisitions” included in Item 8, “Financial Statements and Other Supplementary Data” in this Annual Report on Form 10-K for additional information concerning the impact of our recent acquisitions.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read in conjunction with our consolidated financial statements and related notes appearing in Item 8, “Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. The following discussion contains forward-looking statements. Actual results may differ significantly from those projected in the forward-looking statements. Factors that might cause future results to differ materially from those projected in the forward-looking statements include, but are not limited to, those discussed in Item 1A, “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Certain percentage changes from period over period may not recalculate due to rounding.
Overview
We are a full service, early-stage contract research organization (CRO). For 70 years, we have been in the business of providing the research models required in research and development of new drugs, devices, and therapies. Over this time, we have built upon our original core competency of laboratory animal medicine and science (research model technologies) to develop a diverse portfolio of discovery and safety assessment services, both Good Laboratory Practice (GLP) and non-GLP, which are able to support our clients from target identification through non-clinical development. We also provide a suite of products and services to support our clients’ manufacturing activities. Utilizing our broad portfolio of products and services enables our clients to create a more flexible drug development model, which reduces their costs, enhances their productivity and effectiveness, and increases speed to market.
Our client base includes all of the major global biopharmaceutical companies, many biotechnology companies, CROs, agricultural and industrial chemical companies, life science companies, veterinary medicine companies, contract manufacturing organizations, medical device companies, and diagnostic and other commercial entities, as well as leading hospitals, academic institutions, and government agencies around the world. We currently operate approximately 75 facilities in 23 countries worldwide, which numbers exclude our Insourcing Solutions (IS) sites.
Business Trends
The demand for our products and services increased in fiscal year 2016. Our pharmaceutical and biotechnology clients continued to intensify their use of strategic outsourcing to improve their operating efficiency and to access capabilities that they do not maintain internally. Many of our large biopharmaceutical clients have refocused on their drug discovery and early-stage development efforts, after a period of greater emphasis on late-stage programs to bring new drugs to market. In addition, small and mid-size biopharmaceutical clients benefited from the continued strength in the biotechnology funding environment in fiscal year 2016, from capital markets, partnering with large biopharmaceutical companies, and investment by venture capital. Academia has also benefited from partnering activities, as large biopharmaceutical companies have increasingly utilized academic research capabilities to broaden the scope of their research activities. Our full service, early-stage portfolio continued to lead to additional client discussions in fiscal year 2016 regarding strategic relationships, where clients seek to outsource larger portions of their early-stage drug research programs to us.
The primary result of these trends was improved demand for our safety assessment services in fiscal year 2016, particularly from biotechnology clients. This improvement led to increased capacity utilization in our safety assessment facilities, with utilization approaching optimal levels. Price also improved moderately in fiscal year 2016, as industry capacity utilization continued to increase. In view of client demand, we expanded our global footprint and reinforced our scientific leadership in safety assessment services by acquiring WRH, Inc. (WIL Research) in April 2016. We also opened small amounts of new capacity in fiscal year 2016, including the re-opening of our Charles River Massachusetts facility. We believe our scientific expertise, quality, and responsiveness remain key criteria when our clients make the decision to outsource to us.
Demand for our products and services that support our clients’ manufacturing activities was also robust in fiscal year 2016. Demand for our Microbial Solutions business remained strong as manufacturers continued to increase their use of our rapid microbial testing solutions. Our Biologics Testing Solutions (Biologics) business continued to benefit from increased demand for services associated with the growing proportion of biologic drugs in the pipeline and on the market. To enhance our ability to support biologic and biosimilar development, we acquired Blue Stream Laboratories, Inc. (Blue Stream) in June 2016.
As our clients continue to pursue their goal of more efficient and effective drug research, they are evaluating outsourcing new areas of their research programs, such as discovery services. We have enhanced our Discovery Services capabilities over the past three years to enable us to work with clients at the earliest stages of the discovery process. In fiscal year 2016, demand from biotechnology clients was strong for discovery services, but demand from larger biopharmaceutical clients fluctuated, particularly for our early discovery capabilities. We believe this is due to the fact that large biopharmaceutical companies have significant internal discovery capabilities, on which they can choose to rely. In order for large biopharmaceutical clients to
increasingly outsource more work to us, we must continue to demonstrate that our services can augment and accelerate our clients’ drug discovery process. We implemented business changes, including a small site consolidation and realignment of sales strategies, in fiscal year 2016 in our early discovery business to expedite this process. Demand for our in vivo discovery services continued to increase in fiscal year 2016, and we acquired Agilux Laboratories, Inc. (Agilux) in September 2016 to strengthen our bioanalytical services offering, and reinforce the linkage between our discovery and safety assessment capabilities.
Demand for research models and services improved modestly in fiscal year 2016. We remain confident in the long-term drivers of this business because research models and services remain essential tools for our clients’ drug discovery and early-stage development efforts.
Acquisitions
We continued to make strategic acquisitions designed to expand our portfolio of services to support the drug discovery and early-stage development continuum and position us as a market leader in the outsourced discovery services market. Fiscal year 2016 acquisitions included:
| |
| • | On April 4, 2016, we acquired WIL Research, a provider of safety assessment and contract development and manufacturing (CDMO) services to biopharmaceutical and agricultural and industrial chemical companies worldwide. The acquisition enhanced our position as a leading global early-stage CRO by strengthening our ability to partner with clients across the drug discovery and development continuum. The purchase price for WIL Research was $604.8 million, including assumed liabilities of $0.4 million, and was funded by cash on hand and borrowings on our amended credit facility. |
| |
| • | On June 27, 2016, we acquired Blue Stream, an analytical CRO supporting the development of complex biologics and biosimilars. Combining Blue Stream with our existing discovery, safety assessment, and biologics capabilities creates a leading provider with the ability to support biologic and biosimilar development from characterization through clinical testing and commercialization. The purchase price for Blue Stream was $11.7 million, including $3.0 million in contingent consideration, and was subject to certain customary adjustments. The acquisition was funded by borrowings on our revolving credit facility. |
| |
| • | On September 28, 2016, we acquired Agilux, a CRO that provides a suite of integrated discovery small and large molecule bioanalytical services, drug metabolism and pharmacokinetic (DMPK) services, and pharmacology services. The acquisition supports our strategy to offer clients a broader, integrated portfolio that provides services continuously from the earliest stages of drug research through the nonclinical development process. The purchase price for Agilux was $64.9 million in cash and was funded by borrowings on our revolving credit facility. |
Segment Reporting
We report our performance in three reportable segments: Research Models and Services (RMS), Discovery and Safety Assessment (DSA), and Manufacturing Support (Manufacturing). We aggregate our operating segments into a reportable segment if (a) they have similar economic characteristics; (b) they are similar in the in the nature of the products or services, nature of the production process, type or class of customer for their products and services, methods used to distribute their products and services and nature of the regulatory environment; and (c) the aggregation helps users better understand our performance.
In the second quarter of 2016, we acquired WIL Research. WIL Research’s safety assessment business is reported in our DSA reportable segment and its CDMO business created a new operating segment, Contract Manufacturing, that is reported as part of our Manufacturing reportable segment. On February 10, 2017, we divested the CDMO business. In addition, amounts due to changes in our market strategy for certain services and resulting information provided to the Chief Operating Decision Maker were reclassified from our RMS reportable segment to our Manufacturing reportable segment, including revenue of $2.8 million and $3.7 million for fiscal years 2015 and 2014, respectively, and operating income of $0.5 million and $0.6 million for fiscal years 2015 and 2014, respectively.
We reported segment results on this basis for all periods presented in this Annual Report on Form 10-K.
The revised reportable segments are as follows: |
| | |
| Research Models and Services | Discovery and Safety Assessment | Manufacturing Support |
| Research Models | Discovery Services | Microbial Solutions |
| Research Model Services | Safety Assessment | Avian |
| | | Biologics |
| | | Contract Manufacturing |
Our RMS segment includes the Research Models and Research Model Services businesses. Research Models includes the commercial production and sale of small research models, as well as the supply of large research models. Research Model Services includes three business units: Genetically Engineered Models and Services (GEMS), which performs contract breeding and other services associated with genetically engineered research models; Research Animal Diagnostic Services (RADS), which provides health monitoring and diagnostics services related to research models; and IS, which provides management of our clients’ research operations (including recruitment, training, staffing, and management services). Our DSA segment includes services required to take a drug through the early development process including discovery services, which are non-regulated services to assist clients with the identification, screening, and selection of a lead compound for drug development, and regulated and non-regulated safety assessment services. Our Manufacturing segment includes Microbial Solutions, which includes in vitro (non-animal) lot-release testing products and microbial detection, conventional and rapid quality control testing of sterile and non-sterile biopharmaceutical and consumer products, and species identification services; Biologics, which performs specialized testing of biologics; Avian Vaccine Services (Avian), which supplies specific-pathogen-free fertile chicken eggs and chickens; and Contract Manufacturing, which, until we divested this business on February 10, 2017, specialized in formulation design and development, manufacturing, and analytical and stability testing for small molecules.
Fiscal Quarters
Our fiscal year is typically based on 52-weeks, with each quarter composed of 13 weeks ending on the last Saturday on, or closest to, March 31, June 30, September 30, and December 31. A 53rd week was included in fiscal year 2016, which is occasionally necessary to align with a December 31 calendar year-end. The additional week was included in the fourth quarter.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements prepared in accordance with generally accepted accounting principles in the United States (U.S.). The preparation of these financial statements requires us to make certain estimates and assumptions that may affect the reported amounts of assets and liabilities, the reported amounts of revenues and expenses during the reported periods and related disclosures. These estimates and assumptions are monitored and analyzed by us for changes in facts and circumstances, and material changes in these estimates could occur in the future. We base our estimates on our historical experience, trends in the industry, and various other factors that are believed to be reasonable under the circumstances. Actual results may differ from our estimates under different assumptions or conditions.
We believe that our application of the following accounting policies, each of which require significant judgments and estimates on the part of management, are the most critical to aid in fully understanding and evaluating our reported financial results. Our significant accounting policies are more fully described in Note 1, “Description of Business and Summary of Significant Accounting Policies”, to our consolidated financial statements contained in Item 8, “Financial Statements and Other Supplementary Data” in this Annual Report on Form 10-K.
We believe the following represent our critical accounting policies and estimates used in the preparation of our financial statements:
Revenue Recognition
We recognize revenue when all of the following conditions are satisfied: persuasive evidence of an arrangement exists, delivery has occurred or services have been provided, our price to the customer is fixed or determinable, and collectibility is reasonably assured.
Service revenue is generally evidenced by client contracts, which range in duration from a few weeks to a few years and typically take the form of an agreed upon rate per unit or fixed fee arrangements. Such contracts typically do not contain acceptance provisions based upon the achievement of certain study or laboratory testing results. Revenue of agreed upon rate per unit contracts is recognized as services are performed, based upon rates specified in the contract. In cases where
performance spans reporting periods, revenue of fixed fee contracts is recognized as services are performed, measured on the ratio of outputs or performance obligations completed to the total contractual outputs or performance obligations to be provided. Changes in estimated effort to complete the fixed fee contract are reflected in the period in which the change becomes known. Changes in scope of work are common, especially under long-term contracts, and generally result in a change in contract value. Once the parties have agreed to the changes in scope and renegotiated pricing terms, the contract value is amended and revenue is typically recognized as described above.
Most contracts are terminable by the client, either immediately or upon notice. These contracts often require payment to us of expenses to wind down the project, fees earned to date or, in some cases, a termination fee. Such payments are included in revenues when earned.
We recognize product revenue, net of allowances for estimated returns, rebates and discounts, when title and risk of loss pass to customers. When we sell equipment with specified acceptance criteria, we assess our ability to meet the acceptance criteria in order to determine the timing of revenue recognition. We would defer revenue until completion of customer acceptance testing if we are not able to demonstrate the ability to meet such acceptance criteria.
A portion of our revenue is from multiple-element arrangements that include multiple products and/or services as deliverables in a single arrangement, with each deliverable, or a combination of the deliverables, representing a separate unit of accounting. We allocate revenues to each element in a multiple-element arrangement based upon the relative selling price of each deliverable. Revenue allocated to each deliverable is then recognized when all revenue recognition criteria are met. Judgments as to the identification of deliverables, units of accounting, the allocation of consideration to the deliverable, and the appropriate timing of revenue recognition are critical with respect to these arrangements.
At the inception of each arrangement that includes milestone payments, we evaluate whether each milestone is substantive. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) our performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from our performance to achieve the milestone; (b) the consideration relates solely to past performance; and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, clinical, regulatory, and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required, and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment. If a substantive milestone is achieved and collection of the related receivable is reasonably assured, we recognize revenue related to the milestone in its entirety in the period in which the milestone is achieved. If we were to achieve milestones that we consider substantive under any of our revenue arrangements, we may experience significant fluctuations in our revenue from quarter to quarter and year to year depending on the timing of achieving such substantive milestones. In those circumstances where a milestone is not substantive, we recognize as revenue, on the date the milestone is achieved, an amount equal to the applicable percentage of the performance period that had elapsed as of the date the milestone was achieved, with the balance being deferred and recognized over the remaining period of performance. As of December 31, 2016, we had no significant milestones that were deemed substantive.
The Company records shipping charges billed to customers in total revenue and records shipping costs in cost of revenue (excluding amortization of intangible assets) for all periods presented.
Income Taxes
We prepare and file income tax returns based on our interpretation of each jurisdiction’s tax laws and regulations. In preparing our consolidated financial statements, we estimate our income tax liability in each of the jurisdictions in which we operate by estimating our actual current tax expense together with assessing temporary differences resulting from differing treatment of items for tax and financial reporting purposes. These differences result in deferred tax assets and liabilities, which are included in our consolidated balance sheets. Significant management judgment is required in assessing the realizability of our deferred tax assets. In performing this assessment, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. In making this determination, under the applicable financial accounting standards, we are allowed to consider the scheduled reversal of deferred tax liabilities, projected future taxable income, and the effects of tax planning strategies. In the event that actual results differ from our estimates, we adjust our estimates in future periods and we may need to establish a valuation allowance, which could materially impact our financial position and results of operations.
We account for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. We evaluate uncertain tax positions on a quarterly basis and consider various factors, that include, but are not limited
to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, information obtained during in process audit activities and changes in facts or circumstances related to a tax position. We adjust the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. Our liabilities for uncertain tax positions can be relieved only if the contingency becomes legally extinguished through either payment to the taxing authority or the expiration of the statute of limitations, the recognition of the benefits associated with the position meet the “more-likely-than-not” threshold or the liability becomes effectively settled through the controversy process. We consider matters to be effectively settled once the taxing authority has completed all of its required or expected examination procedures, including all appeals and administrative reviews; we have no plans to appeal or litigate any aspect of the tax position; and we believe that it is highly unlikely that the taxing authority would re-examine the related tax position. We also accrue for potential interest and penalties related to unrecognized tax benefits in income tax expense.
As of December 31, 2016, our non-U.S. subsidiaries’ undistributed foreign earnings included in consolidated retained earnings were $704.6 million. As of the end of fiscal year 2016, our policy with respect to the undistributed earnings of our non-U.S. subsidiaries is to maintain an indefinite reinvestment assertion as they are required to fund needs outside of the U.S. and cannot be repatriated in a manner that is substantially tax-free. This assertion is made on a jurisdiction by jurisdiction basis and takes into account the liquidity requirements in both the U.S. and our foreign subsidiaries. If we decide to repatriate funds to the U.S. in the future to execute our growth initiatives or to fund any other liquidity needs, the resulting tax consequences could negatively impact our results of operations through a higher effective tax rate and dilution of our earnings. On December 18, 2015, the U.S. enacted the Consolidated Appropriations Act, which reinstated and extended the controlled foreign corporation look-through rules through the fiscal year 2019. This rule allows us to access Chinese and Canadian cash in a more tax-efficient manner and utilize the cash outside of the U.S. without triggering residual U.S. tax. As such, we are accruing foreign withholding taxes to reflect this change for the years in which the rules are reinstated.
Goodwill and Intangible Assets
We use assumptions and estimates in determining the fair value of assets acquired and liabilities assumed in a business combination. The determination of the fair value of intangible assets, which represent a significant portion of the purchase price in many of our acquisitions, requires the use of significant judgment with regard to (i) the fair value; and (ii) whether such intangibles are amortizable or non-amortizable and, if the former, the period and the method by which the intangible asset will be amortized. We utilize commonly accepted valuation techniques, such as the income approach and the cost approach, as appropriate, in establishing the fair value of intangible assets. Typically, key assumptions include projections of cash flows that arise from identifiable intangible assets of acquired businesses as well as discount rates based on an analysis of our weighted average cost of capital, adjusted for specific risks associated with the assets.
We review definite-lived intangible assets for impairment when indication of potential impairment exists, such as a significant reduction in cash flows associated with the assets. Actual cash flows arising from a particular intangible asset could vary from projected cash flows which could imply different carrying values from those established at the dates of acquisition and which could result in impairment of such asset.
During fiscal year 2016, we determined that the carrying values of certain DSA intangible assets were not recoverable and recorded an impairment charge of $1.9 million, which was included in costs of services provided (excluding amortization of intangible assets).
We evaluate goodwill for impairment annually, during the fourth quarter, and when events occur or circumstances change that may reduce the fair value of the asset below its carrying amount. Events or circumstances that might require an interim evaluation include unexpected adverse business conditions, economic factors, unanticipated technological changes or competitive activities, loss of key personnel and acts by governments and courts. Estimates of future cash flows require assumptions related to revenue and operating income growth, asset-related expenditures, working capital levels and other factors. Different assumptions from those made in our analysis could materially affect projected cash flows and our evaluation of goodwill for impairment.
We have the option to first assess qualitative factors to determine whether it is necessary to perform the two-step goodwill impairment test. If we elect this option and believe, as a result of the qualitative assessment, that it is more-likely-than-not that the carrying value of goodwill is not recoverable, the quantitative two-step impairment test is required; otherwise, no further testing is required. Alternatively, we may elect to not first assess qualitative factors and immediately perform the quantitative two-step impairment test. In the first step, we compare the fair value of our reporting units to their carrying values. If the carrying values of the net assets assigned to the reporting units exceed the fair values of the reporting units, then the second step
of the impairment test is performed in order to determine the implied fair value of our goodwill. If the carrying value of the reporting unit’s goodwill exceeds its implied fair value, then we would record an impairment loss equal to the difference.
In fiscal years 2016, 2015 and 2014, we performed the first step of the two-step goodwill impairment test for our reporting units. Fair value was determined by using a weighted combination of a market-based approach and an income approach, as this combination was deemed to be the most indicative of our fair value in an orderly transaction between market participants. Under the market-based approach, we utilized information about our Company as well as publicly available industry information to determine earnings multiples and sales multiples that are used to value our reporting units. Under the income approach, we determined fair value based on the estimated future cash flows of each reporting unit, discounted by an estimated weighted-average cost of capital, which reflects the overall level of inherent risk of the reporting unit and the rate of return an outside investor would expect to earn.
Our 2016, 2015 and 2014 impairment tests indicated that goodwill was not impaired.
In the second quarter of 2016, we revised the composition of our reportable segments to align with the view of the business following our acquisition of WIL Research. See Note 1, "Description of Business and Summary of Significant Accounting Policies." As a result, goodwill was allocated from our RMS reportable segment to our Manufacturing reportable segment based on the fair value of each business group within its original reporting unit relative to the fair value of that reporting unit. In addition, we completed an assessment of any potential goodwill impairment for all reporting units immediately prior to the reallocation and determined that no impairment existed.
Valuation and Impairment of Long-Lived Assets
Long-lived assets to be held and used, including property, plant, and equipment, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets or asset group may not be recoverable. Factors we consider important which could trigger an impairment review include, but are not limited to, the following:
| |
| • | significant underperformance relative to expected historical or projected future operating results; |
| |
| • | significant negative industry or economic trends; or |
| |
| • | significant changes or developments in strategy or operations that negatively affect the utilization of our long-lived assets. |
Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset, net of any sublease income, if applicable, and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written-down to their fair values. We measure any impairment based on a projected discounted cash flow method using a discount rate determined by management to be commensurate with the risk inherent in our current business model. Significant judgments are required to estimate future cash flows, including the selection of appropriate discount rates and other assumptions. We may also estimate fair value based on market prices for similar assets, as appropriate. Changes in these estimates and assumptions could materially affect the determination of fair value for these assets.
Pension and Other Post-Retirement Benefit Plans
Several of our U.S. and non-U.S. subsidiaries sponsor defined benefit pension and other post-retirement benefit plans. We recognize the funded status of our defined benefit pension and other postretirement benefit plans as an asset or liability. This amount is defined as the difference between the fair value of plan assets and the benefit obligation. We measure plan assets and benefit obligations as of the date of our fiscal year end.
The cost and obligations of these arrangements are calculated using many assumptions to estimate the benefits that the employee earns while working, the amount of which cannot be completely determined until the benefit payments cease. Major assumptions used in the accounting for these employee benefit plans include the expected return on plan assets, withdrawal and mortality rates, discount rate, and rate of increase in employee compensation levels. Assumptions are determined based on our data and appropriate market indicators, and are evaluated each year as of the plans’ measurement date. Should any of these assumptions change, they would have an effect on net periodic pension costs and the unfunded benefit obligation.
The expected long-term rate of return on plan assets reflects the average rate of earnings expected on the funds invested, or to be invested, to provide for the benefits included in the projected benefit obligations. In determining the expected long-term rate of return on plan assets, we consider the relative weighting of plan assets, the historical performance of total plan assets and individual asset classes and economic and other indicators of future performance.
The discount rate reflects the rate we would have to pay to purchase high-quality investments that would provide cash sufficient to settle our current pension obligations.
The rate of compensation increase reflects the expected annual salary increases for the plan participants based on historical experience and the current employee compensation strategy.
In fiscal year 2016, new mortality improvement scales were issued in the U.S. reflecting a decline in longevity projection from the 2015 releases that we adopted, which decreased our benefit obligations by $1.3 million as of December 31, 2016. In fiscal year 2015, new mortality improvement scales were issued in the U.S. and the United Kingdom (U.K.) reflecting a decline in longevity projection from the 2014 releases that we adopted, which decreased our benefit obligations by $3.3 million as of December 26, 2015.
Stock-Based Compensation
We grant stock options, restricted stock, restricted stock units, and performance share units (PSUs) to employees, and stock options, restricted stock, and restricted stock units to non-employee directors under stock-based compensation plans. We make certain assumptions in order to value and record expense associated with awards made under our stock-based compensation arrangements. Changes in these assumptions may lead to variability with respect to the timing and amount of expense we recognize in connection with share-based payments.
Determining the appropriate valuation model and related assumptions requires judgment. The fair value of stock options granted is calculated using the Black-Scholes model and the fair value of PSUs is calculated using a lattice model with a Monte Carlo simulation, both of which require the use of subjective assumptions including volatility and expected term, among others.
Determining the appropriate amount to expense based on the anticipated achievement of PSU’s performance targets requires judgment, including forecasting the achievement of future financial targets. The estimate of expense is revised periodically based on the probability of achieving the required performance targets. The cumulative impact of any changes to our estimates is reflected in the period of change.
We also estimate forfeitures over the requisite service period when recognizing share-based compensation expense based on historical rates and forward looking factors; these estimates are adjusted to the extent that actual forfeitures differ, or are expected to materially differ, from our estimates.
New Accounting Pronouncements
For a discussion of new accounting pronouncements, refer to Note 1, “Description of Business and Summary of Significant Accounting Policies” to our consolidated financial statements contained in Item 8, “Financial Statements and Other Supplementary Data,” in this Annual Report on Form 10-K.
Results of Operations
Fiscal Year 2016 Compared to Fiscal Year 2015
Revenue
The following table presents consolidated revenue by reportable segment:
|
| | | | | | | | | | | | | | | | | |
| | Fiscal Year | | | | | | |
| | 2016 | | 2015 | | $ change | | % change | | Impact of FX |
| | | | | | | | | | |
| | (in millions, except percentages) |
| RMS | $ | 494.0 |
| | $ | 470.4 |
| | $ | 23.6 |
| | 5.0 | % | | (0.2 | )% |
| DSA | 836.6 |
| | 612.2 |
| | 224.4 |
| | 36.7 | % | | (2.7 | )% |
| Manufacturing | 350.8 |
| | 280.7 |
| | 70.1 |
| | 25.0 | % | | (0.8 | )% |
| Total revenue | $ | 1,681.4 |
| | $ | 1,363.3 |
| | $ | 318.1 |
| | 23.3 | % | | (1.5 | )% |
Revenue for fiscal year 2016 increased $318.1 million, or 23.3%, compared with fiscal year 2015. The negative effect of changes in foreign currency exchange rates decreased revenue by $20.0 million, or 1.5%, when compared to the prior year.
RMS revenue increased $23.6 million due to higher research model services revenue in North America, Europe, and Japan and higher research model revenue in North America, Europe, and Asia; partially offset by the negative effect of changes in foreign currency exchange rates.
DSA revenue increased $224.4 million due to higher revenue in the Safety Assessment business, primarily as a result of the WIL Research acquisition that contributed $163.5 million to revenue growth, and increased study volume, mix of services, and pricing in our legacy business; and higher revenue in Discovery Services’ In Vivo business, which includes the acquisitions of Oncotest and Agilux that contributed $14.6 million to revenue growth; partially offset by lower Early Discovery revenue due primarily to softer demand from global clients; and the negative effect of changes in foreign currency exchange rates.
Manufacturing revenue increased $70.1 million due to higher revenue in the Microbial Solutions business, which includes the acquisition of the Celsis business that contributed $17.9 million to revenue growth; higher revenue in the Biologics business, which includes the Blue Stream acquisition that contributed $4.1 million to revenue growth; higher revenue in the Avian business, primarily due to the acquisition of the Sunrise business that contributed $4.9 million to revenue growth; and Contract Manufacturing revenue related to the CDMO services of WIL Research acquired in April 2016 that contributed $12.6 million to revenue growth; partially offset by the negative effect of changes in foreign currency exchange rates.
The following table presents consolidated revenue by type:
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2016 | | 2015 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| Service revenue | $ | 1,130.7 |
| | $ | 858.2 |
| | $ | 272.5 |
| | 31.7 | % |
| Product revenue | 550.7 |
| | 505.1 |
| | 45.6 |
| | 9.0 | % |
| Total revenue | $ | 1,681.4 |
| | $ | 1,363.3 |
| | $ | 318.1 |
| | 23.3 | % |
Service revenue increased $272.5 million due to higher revenue in the Safety Assessment business, primarily as a result of the WIL Research acquisition that contributed $163.5 million to service revenue growth, and increased study volume, mix of services, and pricing in our legacy business; and higher revenue in Discovery Services’ In Vivo business, which includes the acquisitions of Oncotest and Agilux that contributed $14.6 million to revenue growth; Contract Manufacturing revenue related to the CDMO services of WIL Research acquired in April 2016 that contributed $12.6 million to revenue growth; higher revenue in the Biologics business, which includes the Blue Stream acquisition that contributed $4.1 million to revenue growth; and higher research model services revenue in North America, Europe, and Japan; partially offset by lower Early Discovery revenue due primarily to softer demand from global clients; and the negative effect of changes in foreign currency exchange rates.
Product revenue increased $45.6 million due to higher revenue in Microbial Solutions and Avian, which included the acquisitions of the Celsis and Sunrise businesses, respectively, and in total contributed $22.1 million to product revenue growth; and higher research model revenue in North America, Europe, and Asia; partially offset by the negative effect of changes in foreign currency exchange rates.
Cost of Services Provided and Products Sold (Excluding Amortization of Intangible Assets)
The following table presents consolidated cost of services provided and products sold (excluding amortization of intangible assets) by reportable segment:
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2016 | | 2015 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| RMS | $ | 292.8 |
| | $ | 284.2 |
| | $ | 8.6 |
| | 3.0 | % |
| DSA | 572.4 |
| | 407.0 |
| | 165.4 |
| | 40.6 | % |
| Manufacturing | 169.5 |
| | 141.0 |
| | 28.5 |
| | 20.3 | % |
| Total cost of services provided and products sold (excluding amortization of intangible assets) | $ | 1,034.7 |
| | $ | 832.2 |
| | $ | 202.5 |
| | 24.3 | % |
Cost of services provided and products sold (excluding amortization of intangible assets) (Costs) for fiscal year 2016 increased $202.5 million, or 24.3%, compared with fiscal year 2015. Costs as a percentage of revenue for fiscal year 2016 were 61.5%, an increase of 0.5%, from 61.0% for fiscal year 2015.
RMS Costs increased $8.6 million due primarily to the growth of the business, partially offset by cost savings achieved as a result of our efficiency initiatives. RMS Costs as a percentage of revenue for fiscal year 2016 were 59.3%, a decrease of 1.1%, from 60.4% for fiscal year 2015.
DSA Costs increased $165.4 million due primarily to an increase in Safety Assessment Costs, which included a higher cost base due to the acquisition of WIL Research, the growth of the legacy business; an increase in Discovery Services Costs, which included a higher cost base due to the acquisitions of Oncotest and Agilux; a charge of $1.9 million related to an impairment of certain intangibles; and a restructuring charge of $9.4 million related to the consolidation of small DSA facilities in the U.S., Ireland, and the U.K.; partially offset by the favorable effect of changes in foreign currency exchange rates. DSA Costs as a percentage of revenue for fiscal year 2016 were 68.4%, an increase of 1.9%, from 66.5% for fiscal year 2015, primarily due to the acquisition of WIL Research.
Manufacturing Costs increased $28.5 million due primarily to an increase in Biologics Costs resulting from the growth of the business and the acquisition of Blue Stream; an increase in Contract Manufacturing Costs related to the CDMO services of WIL Research acquired in April 2016; an increase in Microbial Solutions Costs resulting from the acquisition of Celsis and the growth of the legacy business; and an increase in Avian Costs, primarily due to the acquisition of the Sunrise business; partially offset by $4.1 million due to lower amortization of inventory fair value adjustments related to the Celsis acquisition. Manufacturing Costs as a percentage of revenue for fiscal year 2016 were 48.3%, a decrease of 1.9%, from 50.2% for fiscal year 2015.
The following table presents consolidated cost of services provided and products sold (excluding amortization of intangible assets) by type:
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2016 | | 2015 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| Cost of services provided | $ | 757.7 |
| | $ | 568.2 |
| | $ | 189.5 |
| | 33.4 | % |
| Cost of products sold | 277.0 |
| | 264.0 |
| | 13.0 |
| | 4.9 | % |
| Total cost of services provided and products sold (excluding amortization of intangible assets) | $ | 1,034.7 |
| | $ | 832.2 |
| | $ | 202.5 |
| | 24.3 | % |
Cost of services provided increased $189.5 million due to an increase in Safety Assessment Costs, which included a higher cost base due to the acquisition of WIL Research, the growth of the legacy business; an increase in Discovery Services Costs, which included a higher cost base due to the acquisitions of Oncotest and Agilux; a charge of $1.9 million related to an impairment of certain intangibles; a restructuring charge of $9.4 million related to the consolidation of small DSA facilities in the U.S., Ireland, and the U.K.; higher Biologics Costs resulting from the growth of the business and the acquisition of Blue Stream; an increase in Contract Manufacturing Costs related to the CDMO services of WIL Research acquired in April 2016; and increased research model services costs due to growth in the business; partially offset by the favorable effect of changes in foreign currency exchange rates primarily related to the Safety Assessment and Discovery Services businesses.
Cost of products sold increased $13.0 million due primarily to higher Microbial Solutions Costs as a result of the acquisition of Celsis and the growth of the legacy business; higher Avian Costs, primarily due to the acquisition of the Sunrise business; and
higher research model costs due to growth in the business; partially offset by $4.1 million due to lower amortization of inventory fair value adjustments related to the Celsis acquisition and savings associated with global efficiency initiatives in the research models business.
Selling, General and Administrative Expenses
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2016 | | 2015 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| RMS | $ | 62.5 |
| | $ | 62.1 |
| | $ | 0.4 |
| | 0.5 | % |
| DSA | 98.3 |
| | 69.2 |
| | 29.1 |
| | 42.0 | % |
| Manufacturing | 65.1 |
| | 57.9 |
| | 7.2 |
| | 12.5 | % |
| Unallocated corporate | 141.6 |
| | 111.2 |
| | 30.4 |
| | 27.4 | % |
| Total selling, general and administrative | $ | 367.5 |
| | $ | 300.4 |
| | $ | 67.1 |
| | 22.3 | % |
Selling, general and administrative expenses (SG&A) for fiscal year 2016 increased $67.1 million, or 22.3%, compared with fiscal year 2015. SG&A as a percentage of revenue for fiscal year 2016 was 21.9%, a decrease of 0.1%, from 22.0% for fiscal year 2015.
The increase in RMS SG&A of $0.4 million was related to an increase of $1.3 million in external consulting and other service expenses; an increase of $0.5 million in operating expenses, including information technology infrastructure and facility expenses; an increase of $0.3 million in compensation, benefits, and other employee-related expenses; and an increase of $0.2 million in stock-based compensation expense; partially offset by a decrease of $0.8 million in severance expense; a decrease of $0.3 million in costs associated with the evaluation and integration of acquisitions; a decrease of $0.2 million in bad debt expense; and a decrease of $0.6 million in other expenses. RMS SG&A as a percentage of revenue for fiscal year 2016 was 12.6%, a decrease of 0.6%, from 13.2% for fiscal year 2015.
The increase in DSA SG&A of $29.1 million was related to an increase of $12.5 million in compensation, benefits, and other employee-related expenses; an increase of $5.9 million in operating expenses, including information technology infrastructure and facility expenses; an increase of $5.7 million in costs associated with the evaluation and integration of acquisitions; an increase of $2.9 million in severance expense; an increase of $1.5 million in external consulting and other service expenses; an increase of $1.3 million in depreciation expense; an increase of $1.2 million in stock-based compensation expense; and an increase of $0.3 million in other expenses; partially offset by a decrease of $2.2 million in bad debt expense. DSA SG&A as a percentage of revenue for fiscal year 2016 was 11.8%, an increase of 0.5%, from 11.3% for fiscal year 2015.
The increase in Manufacturing SG&A of $7.2 million was related to an increase of $6.7 million in compensation, benefits, and other employee-related expenses; an increase of $1.2 million in external consulting and other service expenses; an increase of $1.0 million in operating expenses, including information technology infrastructure and facility expenses; an increase of $0.7 million in stock-based compensation; and an increase of $0.6 million in other expenses; partially offset by a decrease of $1.8 million in severance expense; a decrease of $1.0 million in costs associated with the evaluation and integration of acquisitions; and a decrease of $0.2 million in depreciation expense. Manufacturing SG&A as a percentage of revenue for fiscal year 2016 was 18.6%, a decrease of 2.0%, from 20.6% for fiscal year 2015.
The increase in unallocated corporate SG&A of $30.4 million was related to an increase of $8.0 million in external consulting and other service expenses; an increase of $6.2 million in compensation, benefits, and other employee-related expenses; an increase of $4.8 million in information technology expenses; an increase of $4.0 million in costs associated with the evaluation and integration of acquisitions; an increase of $1.5 million in stock-based compensation; an increase of $1.0 million in depreciation expense; and an increase of $4.9 million in other expenses.
Amortization of Intangible Assets Amortization of intangibles for fiscal year 2016 was $41.7 million, an increase of $17.5 million, or 72.1%, from $24.2 million for fiscal year 2015, due primarily to certain intangibles acquired in connection with the Agilux, Blue Stream, WIL Research, Oncotest, Celsis, and Sunrise acquisitions.
Interest Income Interest income, which represents earnings on held cash, cash equivalents, and time deposits was $1.3 million for fiscal year 2016, an increase of $0.3 million, or 26.0%, compared to $1.0 million for fiscal year 2015.
Interest Expense Interest expense for fiscal year 2016 was $27.7 million, an increase of $12.6 million, or 83.8%, compared to $15.1 million for fiscal year 2015. The increase was primarily due to the write-off of a portion of debt issuance costs in connection with the modification of our $1.3B Credit Facility, a higher average debt balance outstanding as a result of business
acquisitions, a higher average interest rate as a result of a higher leverage ratio, and an increased interest expense related to capital leases.
Other Income (Expense), Net Other income (expense), net, was a net other income of $11.9 million for fiscal year 2016, an increase of $8.9 million, or 295.5%, compared to a net other income of $3.0 million for fiscal year 2015. The increase in other income (expense), net was driven by the absence of an expense of $10.4 million due to a reversal of the indemnification asset associated with a previous acquisition in the corresponding period in 2015; an increase of $6.5 million in gains on our venture capital investments accounted for under the equity method; a higher net gain of $2.1 million on life insurance policy investments; a $0.7 million gain on remeasurement of previously held equity interest in an entity acquired in a step acquisition; and an increase of $0.6 million in other activity; partially offset by the absence of a bargain purchase gain of $9.9 million associated with the acquisition of Sunrise in May 2015; and a $1.5 million charge recorded in connection with the modification of the option to purchase the remaining 13% equity interest in Vital River.
Income Taxes Income tax expense was $66.8 million for fiscal year 2016, an increase of $23.4 million, compared to $43.4 million for fiscal year 2015. Our effective tax rate was 30.0% in the fiscal year 2016, compared to 22.2% in the fiscal year 2015. The increase was primarily driven by non-deductible expenses associated with acquisitions and restructurings. In addition, we recognized a reduction in unrecognized tax benefits and related interest of $10.4 million due to the expiration of the statute of limitations associated with pre-acquisition tax positions on the forgiveness of debt and a non-taxable bargain purchase gain of $9.9 million associated with the acquisition of Sunrise in the fiscal year 2015.
Fiscal Year 2015 Compared to Fiscal Year 2014
Revenue
The following table presents consolidated revenue by reportable segment:
|
| | | | | | | | | | | | | | | | | |
| | Fiscal Year | | | | | | |
| | 2015 | | 2014 | | $ change | | % change | | Impact of FX |
| | | | | | | | | | |
| | (in millions, except percentages) |
| RMS | $ | 470.4 |
| | $ | 503.7 |
| | $ | (33.3 | ) | | (6.6 | )% | | (6.3 | )% |
| DSA | 612.2 |
| | 538.2 |
| | 74.0 |
| | 13.7 | % | | (3.4 | )% |
| Manufacturing | 280.7 |
| | 255.8 |
| | 24.9 |
| | 9.7 | % | | (7.6 | )% |
| Total revenue | $ | 1,363.3 |
| | $ | 1,297.7 |
| | $ | 65.6 |
| | 5.1 | % | | (5.3 | )% |
Revenue for fiscal year 2015 increased $65.6 million, or 5.1%, compared with fiscal year 2014. The negative effect of changes in foreign currency exchange rates decreased revenue by $69.4 million, or 5.3%, when compared to the prior period.
RMS revenue decreased $33.3 million due primarily to the negative effect of changes in foreign currency exchange rates. Excluding the impact of foreign exchange rates, RMS revenue decreased slightly due to lower research model services revenue and lower research models revenue in Japan; partially offset by higher research models revenue in North America, China, and Europe.
DSA revenue increased $74.0 million due to higher revenue in the Safety Assessment business, as a result of increased study volume; higher revenue in the Discovery Services business, primarily as a result of the Argenta, BioFocus, ChanTest, and Oncotest acquisitions that contributed $27.0 million to revenue growth; partially offset by the negative effect of changes in foreign currency exchange rates.
Manufacturing revenue increased $24.9 million, as higher revenue for Microbial Solutions and Avian, which include the Celsis and Sunrise acquisitions, respectively, was partially offset by the negative effect of changes in foreign currency exchange rates.
The following table presents consolidated revenue by type:
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2015 | | 2014 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| Service revenue | $ | 858.2 |
| | $ | 797.8 |
| | $ | 60.4 |
| | 7.6 | % |
| Product revenue | 505.1 |
| | 499.9 |
| | 5.2 |
| | 1.0 | % |
| Total revenue | $ | 1,363.3 |
| | $ | 1,297.7 |
| | $ | 65.6 |
| | 5.1 | % |
Service revenue increased $60.4 million due to higher revenue in the Safety Assessment business, as a result of increased study volume; and higher revenue in the Discovery Services business, which included the acquisitions of Argenta, BioFocus,
ChanTest, and Oncotest that contributed $27.0 million to service revenue growth; partially offset by lower revenue in our research model services and the negative effect of changes in foreign currency exchange rates.
Product revenue increased $5.2 million due to higher revenue for Microbial Solutions and Avian, which include the acquisitions of Celsis and Sunrise, respectively, that contributed $16.7 million to product revenue growth; higher research models revenue in North America, China, and Europe; partially offset by lower revenue in our research models and the negative effect of changes in foreign currency exchange rates.
Cost of Services Provided and Products Sold (Excluding Amortization of Intangible Assets)
The following table presents consolidated cost of services provided and products sold (excluding amortization of intangible assets) by reportable segment:
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2015 |
| 2014 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| RMS | $ | 284.2 |
| | $ | 314.7 |
| | $ | (30.5 | ) | | (9.7 | )% |
| DSA | 407.0 |
| | 387.3 |
| | 19.7 |
| | 5.1 | % |
| Manufacturing | 141.0 |
| | 123.0 |
| | 18.0 |
| | 14.6 | % |
| Total cost of services provided and products sold (excluding amortization of intangible assets) | $ | 832.2 |
| | $ | 825.0 |
| | $ | 7.2 |
| | 0.9 | % |
Costs for fiscal year 2015 increased $7.2 million, or 0.9%, compared with fiscal year 2014. Costs as a percentage of revenue for fiscal year 2015 were 61.0%, a decrease of 2.6%, from 63.6% for fiscal year 2014.
RMS costs decreased $30.5 million due primarily to favorable effect of changes in foreign currency exchange rates, cost savings achieved as a result of our efficiency initiatives, and reduced restructuring costs. RMS costs as a percentage of revenue for fiscal year 2015 were 60.4%, a decrease of 2.1%, from 62.5% for fiscal year 2014.
DSA costs increased $19.7 million due primarily to an increase in Discovery Services costs, which included a higher cost base due to the acquisitions of Argenta, BioFocus, ChanTest, and Oncotest; partially offset by the favorable effect of changes in foreign currency exchange rates. Safety Assessment costs increased due to higher costs resulting from the growth of the business, partially offset by the favorable effect of changes in foreign currency exchanges rates. DSA costs as a percentage of revenue for fiscal year 2015 were 66.5%, a decrease of 5.5%, from 72.0% for fiscal year 2014, primarily due to improved operating leverage as a result of increased study volume in our Safety Assessment business.
Manufacturing costs increased $18.0 million due primarily to the Celsis and Sunrise acquisitions, partially offset by the favorable effect of changes in foreign currency exchange rates. Manufacturing costs as a percentage of revenue for fiscal year 2015 were 50.2%, an increase of 2.1%, from 48.1% for fiscal year 2014.
The following table presents consolidated cost of services provided and products sold (excluding amortization of intangible assets) by type:
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2015 | | 2014 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| Cost of services provided | $ | 568.2 |
| | $ | 558.6 |
| | $ | 9.6 |
| | 1.7 | % |
| Cost of products sold | 264.0 |
| | 266.4 |
| | (2.4 | ) | | (0.9 | )% |
| Total cost of services provided and products sold (excluding amortization of intangible assets) | $ | 832.2 |
| | $ | 825.0 |
| | $ | 7.2 |
| | 0.9 | % |
Cost of services provided increased $9.6 million due to a higher cost base, as a result of the acquisitions of Argenta, BioFocus, ChanTest, and Oncotest as well as increased Safety Assessment revenues; partially offset by the favorable effect of changes in foreign currency exchange rates and lower costs for our research model services as a result of lower revenue.
Cost of products sold decreased $2.4 million due to savings associated with global efficiency initiatives, reduced restructuring costs and the favorable effect of changes in foreign currency exchange rates; partially offset by increased costs as a result of the acquisitions of Sunrise and Celsis.
Selling, General and Administrative Expenses
|
| | | | | | | | | | | | | | |
| | Fiscal Year | | | | |
| | 2015 | | 2014 | | $ change | | % change |
| | | | | | | | |
| | (in millions, except percentages) |
| RMS | $ | 62.1 |
| | $ | 65.7 |
| | $ | (3.6 | ) | | (5.5 | )% |
| DSA | 69.2 |
| | 63.1 |
| | 6.1 |
| | 9.7 | % |
| Manufacturing | 57.9 |
| | 48.1 |
| | 9.8 |
| | 20.4 | % |
| Unallocated corporate | 111.2 |
| | 92.1 |
| | 19.1 |
| | 20.7 | % |
| Total selling, general and administrative | $ | 300.4 |
| | $ | 269.0 |
| | $ | 31.4 |
| | 11.7 | % |
SG&A for fiscal year 2015 increased $31.4 million, or 11.7%, compared with fiscal year 2014. SG&A as a percentage of revenue for fiscal year 2015 was 22.0%, an increase of 1.3%, from 20.7% for fiscal year 2014.
The decrease in RMS SG&A of $3.6 million was related to a decrease of $1.4 million in external consulting and other service expenses; a decrease of $1.2 million in depreciation expense; a decrease of $1.1 million in compensation, benefits and other employee related expenses; and a decrease of $0.4 million in other expenses; partially offset by an increase of $0.5 million in stock-based compensation, primarily related to our annual stock-based grants made in the first quarter of 2015, which included a new retirement vesting provision. RMS SG&A as a percentage of revenue for fiscal year 2015 was 13.2%, an increase of 0.1%, from 13.1% for fiscal year 2014.
The increase in DSA SG&A of $6.1 million was related to an increase of $5.9 million in compensation, benefits and other employee related expenses; an increase of $1.4 million in external consulting and other service expenses; an increase of $0.4 million in operating expenses, including information technology infrastructure and facility expenses; an increase of $0.4 million in bad debt expense; and an increase of $0.3 million in depreciation expense; partially offset by a decrease of $1.8 million in severance expense and a decrease of $0.5 million in other expenses. DSA SG&A as a percentage of revenue for fiscal year 2015 was 11.3%, a decrease of 0.4%, from 11.7% for fiscal year 2014.
The increase in Manufacturing SG&A of $9.8 million was related to an increase of $4.8 million in compensation, benefits and other employee related expenses; an increase of $1.7 million in external consulting and other service expenses; an increase of $1.6 million in severance expense; an increase of $1.0 million in operating expenses, including information technology infrastructure and facility expenses; an increase of $0.9 million in depreciation expense; and an increase of $0.5 million in stock-based compensation, primarily related to our annual stock-based grants made in the first quarter of 2015, which included a new retirement vesting provision; partially offset by a decrease of $0.7 million in other expenses. Manufacturing SG&A as a percentage of revenue for fiscal year 2015 was 20.6%, an increase of 1.8% from 18.8% for fiscal year 2014.
The increase in unallocated corporate SG&A of $19.1 million was related to an increase of $7.3 million in stock-based compensation, primarily related to our annual stock-based grants made in the first quarter of 2015, which included a new retirement vesting provision and the modification of certain stock-based awards as part of executive retirement transitions; an increase of $7.3 million in costs associated with the evaluation and integration of acquisitions and compensation costs related to business acquisitions; an increase of $2.2 million in compensation, benefits and other employee-related expenses; an increase of $2.0 million in external consulting and other service expenses; an increase of $1.9 million in information technology related expenses; and an increase of $0.4 million in other expenses; partially offset by a decrease of $2.0 million in contingent consideration related to business acquisitions.
Amortization of Intangible Assets Amortization of intangibles for fiscal year 2015 was $24.2 million, a decrease of $1.8 million, or 6.7%, from $26.0 million for fiscal year 2014, due primarily to certain intangibles acquired in connection with several Discovery Services and Safety Assessment businesses becoming fully amortized and the effect of changes in foreign currency exchange rates, partially offset by an increase due to recent acquisitions, primarily Argenta, BioFocus, ChanTest, Sunrise, Celsis and Oncotest.
Interest Income Interest income, which represents earnings on held cash, cash equivalents, and time deposits, was $1.0 million for fiscal year 2015, a decrease of $0.2 million, or 9.4%, compared to $1.2 million for fiscal year 2014.
Interest Expense Interest expense for fiscal year 2015 was $15.1 million, an increase of $3.1 million, or 26.1%, compared to $12.0 million for fiscal year 2014. The increase was due primarily to the write-off of a portion of debt issuance costs in connection with the modification of our $970M Credit Facility in April 2015, interest expense related to new capital leases, and overall higher average debt due to additional borrowings related to business acquisitions.
Other Income (Expense), Net Other income (expense), net was net other income of $3.0 million for fiscal year 2015, a decrease of $7.7 million, or 71.9%, compared to net other income of $10.7 million for fiscal year 2014. The decrease in other
income (expense), net was driven by a decrease of $10.4 million due to a reversal of the indemnification asset associated with a pre-acquisition tax position and corresponding unrecognized tax benefit; a decrease of $5.5 million in income from our venture capital investments accounted for under the equity method; and the absence of a noncash gain of $2.1 million related to assets assumed at our Frederick, Maryland, facility following the termination of a customer contract, which was recorded in fiscal year 2014; partially offset by a bargain purchase gain of $9.8 million associated with the acquisition of Sunrise and an increase of $0.5 million from other activity.
Income Taxes Income tax expense was $43.4 million in fiscal year 2015, a decrease of $4.3 million compared to $47.7 million for fiscal year 2014. Our effective tax rate was 22.2% in fiscal year 2015, compared to 26.8% in fiscal year 2014. The decrease was primarily attributable to a $10.4 million reduction in unrecognized tax benefits and related interest due to the expiration of the statute of limitations associated with pre-acquisition tax positions on the forgiveness of debt and a non-taxable bargain purchase gain of $9.8 million associated with the acquisition of Sunrise. These benefits were offset by a tax accrual of $6.6 million of withholding taxes in order to access cash from our Canadian and Chinese operations for use outside of the U.S.
Liquidity and Capital Resources
We currently require cash to fund our working capital needs, pension obligations, capital expansion, acquisitions, and to pay our debt obligations. Our principal sources of liquidity have been our cash flows from operations, supplemented by long-term borrowings. Based on our current business plan, we believe that our existing funds, when combined with cash generated from operations and our access to financing resources, are sufficient to fund our operations for the foreseeable future.
The following table presents our cash, cash equivalents and investments:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in millions) |
| Cash and cash equivalents: | | | |
| Held in the U.S. entities | $ | 10.6 |
| | $ | 3.6 |
|
| Held in non-U.S. entities | 107.0 |
| | 114.3 |
|
| Total cash and cash equivalents | 117.6 |
| | 117.9 |
|
| Investments: | | | |
| Held in the U.S. entities | — |
| | 4.5 |
|
| Held in non-U.S. entities | 3.8 |
| | 16.0 |
|
| Total cash, cash equivalents and investments | $ | 121.4 |
| | $ | 138.4 |
|
Borrowings
In April 2015, we amended and restated our $970M Credit Facility, creating a $1.3 billion facility ($1.3B Credit Facility) that provides for a $400.0 million term loan facility and a $900.0 million multi-currency revolving facility. The interest rates applicable to term loans and revolving loans under the Company’s $1.3B Credit Facility were, at our option, equal to either the alternate base rate (which is the higher of (1) the prime rate, (2) the federal funds rate plus 0.5% or (3) the one-month adjusted LIBOR rate plus 1%) or the adjusted LIBOR rate, plus an interest rate margin based upon our leverage ratio.
On March 30, 2016, we amended and restated our $1.3B Credit Facility, creating a $1.65 billion credit facility ($1.65B Credit Facility) which (1) extends the maturity date for the credit facility and (2) makes certain other amendments in connection with our acquisition of WIL Research. The $1.65B Credit Facility provides for up to approximately $1.65 billion in financing, including a $650.0 million term loan facility and a $1.0 billion multi-currency revolving facility. The term loan facility matures in 19 quarterly installments, with the last installment due March 30, 2021. The revolving facility matures on March 30, 2021, and requires no scheduled payment before that date. Under specified circumstances, we have the ability to increase the term loans and/or revolving line of credit by up to $500.0 million in the aggregate.
Amounts outstanding under the $1.65B Credit Facility were as follows as of December 31, 2016 and December 26, 2015:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in millions) |
| Term loans | $ | 633.8 |
| | $ | 390.0 |
|
| Revolving credit facility | 578.8 |
| | 446.0 |
|
| Total | $ | 1,212.6 |
| | $ | 836.0 |
|
The interest rates applicable to term loan and revolving loans under the $1.65B Credit Facility are, at our option, equal to either the base rate (which is the higher of (1) the prime rate, (2) the federal funds rate plus 0.50%, or (3) the one-month adjusted LIBOR rate plus 1%) or the adjusted LIBOR rate, plus an interest rate margin based upon our leverage ratio.
Repurchases of Common Stock
In July 2010, our Board of Directors authorized a $500.0 million stock repurchase program, and subsequently approved increases for an aggregate authorization of $1,150.0 million. During fiscal year 2016, we did not repurchase any shares under our authorized stock repurchase program. As of December 31, 2016, we had $69.7 million remaining on the authorized stock repurchase program. Our stock-based compensation plans permit the netting of common stock upon vesting of restricted stock, PSUs, and restricted stock units in order to satisfy individual minimum statutory tax withholding requirements. During fiscal year 2016, we acquired approximately 0.2 million shares for $12.3 million.
Cash Flows
The following table presents our net cash provided by operating activities:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in millions) |
| Income from continuing operations | $ | 156.1 |
| | $ | 152.0 |
| | $ | 129.9 |
|
| Adjustments to reconcile net income from continuing operations to net cash provided by operating activities | 174.3 |
| | 126.6 |
| | 126.0 |
|
| Changes in assets and liabilities | (30.0 | ) | | 9.6 |
| | (3.8 | ) |
| Net cash provided by operating activities | $ | 300.4 |
| | $ | 288.2 |
| | $ | 252.1 |
|
Cash flows from operating activities represent the cash receipts and disbursements related to all of our activities other than investing and financing activities. Operating cash flow is derived by adjusting our income from continuing operations for (1) non-cash operating items such as depreciation and amortization, stock-based compensation, gains on venture capital investments, and gains on bargain purchases, as well as (2) changes in operating assets and liabilities, which reflect timing differences between the receipt and payment of cash associated with transactions and when they are recognized in our results of operations. The increase in cash provided by operating activities from fiscal year 2015 to 2016 was primarily driven by higher income from continuing operations; and an increase in non-cash adjustments, primarily an increase in depreciation and amortization as well as stock-based compensation; partially offset by a negative change in operating assets and liabilities. The increase in cash provided by operating activities from fiscal year 2014 and 2015 was primarily driven by higher income from continuing operations and a positive change in operating assets and liabilities. Our days sales outstanding, which includes deferred revenue as an offset to accounts receivable in the calculation, was 52 days as of December 31, 2016, compared to 51 days as of December 26, 2015, and 52 days as of December 27, 2014.
The following table presents our net cash used in investing activities:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in millions) |
| Acquisition of businesses and assets, net of cash acquired | $ | (648.5 | ) | | $ | (247.7 | ) | | $ | (234.3 | ) |
| Capital expenditures | (55.3 | ) | | (63.3 | ) | | (56.9 | ) |
| Investments, net | 13.7 |
| | (7.1 | ) | | (5.6 | ) |
| Other, net | 3.7 |
| | (2.2 | ) | | (1.2 | ) |
| Net cash used in investing activities | $ | (686.4 | ) | | $ | (320.3 | ) | | $ | (298.0 | ) |
The principal use of cash in investing activities in fiscal year 2016 was related to our acquisitions of WIL Research for $577.4 million, net of cash acquired; Agilux for $62.0 million, net of cash acquired; and Blue Stream for $8.7 million, net of cash acquired; as well as our capital expenditures; partially offset by proceeds from the sale of investments and distributions from venture capital investments, net of purchases. The principal use of cash in fiscal year 2015 was related to our acquisitions of Celsis for $202.0 million, net of cash acquired; Oncotest for $35.2 million, net of cash acquired; and Sunrise for $9.6 million, net of cash acquired; as well as our capital expenditures. The principal use of cash in fiscal year 2014 was primarily related to our acquisitions of Argenta and BioFocus for $182.5 million, net of cash acquired; and ChanTest for $51.1 million, net of cash acquired; as well as our capital expenditures.
The following table presents our net cash provided by financing activities:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in millions) |
| Proceeds from long-term debt and revolving credit facility | $ | 1,044.7 |
| | $ | 492.5 |
| | $ | 298.9 |
|
| Proceeds from exercises of stock options | 23.2 |
| | 39.3 |
| | 73.7 |
|
| Payments on long-term debt, revolving credit facility, and capital lease obligations | (656.6 | ) | | (417.3 | ) | | (194.5 | ) |
| Purchase of treasury stock | (12.3 | ) | | (117.5 | ) | | (122.0 | ) |
| Other, net | (8.2 | ) | | 7.5 |
| | 5.3 |
|
| Net cash provided by financing activities | $ | 390.8 |
| | $ | 4.5 |
| | $ | 61.4 |
|
For fiscal year 2016, cash provided by financing activities reflected net borrowings of $388.0 million and proceeds from exercises of employee stock options of $23.2 million; partially offset by treasury stock purchases of $12.3 million due to the netting of common stock upon vesting of stock-based awards in order to satisfy individual minimum statutory tax withholding requirements and other activity. For fiscal year 2015, cash provided by financing activities reflected net borrowings of $75.2 million; proceeds from exercises of employee stock options of $39.3 million, and other activity; partially offset by treasury stock purchases of $117.5 million made pursuant to our authorized stock repurchase program. For fiscal year 2014, cash provided by financing activities reflected net borrowings of $104.4 million; proceeds from exercises of employee stock options of $73.7 million, and other activity; partially offset by treasury stock purchases of $122.0 million made pursuant to our authorized stock repurchase program.
Contractual Commitments and Obligations
Minimum future payments of our contractual obligations as of December 31, 2016 are as follows: |
| | | | | | | | | | | | | | | | | | | |
| | Payments Due by Period |
| | Total | | Less than
1 Year | | 1 - 3 Years | | 3 - 5 Years | | More Than
5 Years |
| | | | | | | | | | |
| | (in millions) |
Notes payable (1) | $ | 1,212.7 |
| | $ | 24.6 |
| | $ | 89.4 |
| | $ | 1,098.7 |
| | $ | — |
|
Operating leases (2) | 101.3 |
| | 23.4 |
| | 37.4 |
| | 21.4 |
| | 19.1 |
|
| Capital leases | 43.2 |
| | 4.1 |
| | 6.5 |
| | 4.6 |
| | 28.0 |
|
Redeemable noncontrolling interest (3) | 14.1 |
| | — |
| | 14.1 |
| | — |
| | — |
|
Venture capital investment commitments (4) | 46.6 |
| | 29.9 |
| | 15.7 |
| | 1.0 |
| | — |
|
Contingent consideration (5) | 3.8 |
| | 3.8 |
| | — |
| | — |
| | — |
|
Unconditional purchase obligations (6) | 86.2 |
| | 79.1 |
| | 7.1 |
| | — |
| | — |
|
| Total contractual cash obligations | $ | 1,507.9 |
| | $ | 164.9 |
| | $ | 170.2 |
| | $ | 1,125.7 |
| | $ | 47.1 |
|
| |
(1) | Notes payable includes the principal payments on our debt. |
| |
(2) | We lease properties and equipment for use in our operations. In addition to rent, the leases may require us to pay additional amounts for taxes, insurance, maintenance, and other operating expenses. Amounts reflected within the table detail future minimum rental commitments under non-cancellable operating leases for each of the periods presented. |
| |
(3) | The estimated cash obligation for redeemable noncontrolling interest is based on the amount that would be paid if settlement occurred as of the balance sheet date based on the contractually defined redemption value as of December 31, 2016. |
| |
(4) | The timing of the remaining capital commitment payments to venture capital funds is subject to the procedures of the limited liability partnerships and limited liability companies; the above table reflects the earliest possible date the payment can be required under the relevant agreements. |
| |
(5) | In connection with business acquisitions, we agreed to make additional payments of up to $3.8 million based upon the achievement of certain financial targets. The contingent consideration obligation included in the table above has not been probability adjusted or discounted. |
| |
(6) | Unconditional purchase obligations include agreements to purchase goods or services that are enforceable and legally binding and that specify all significant terms, including fixed or minimum quantities to be purchased, fixed, minimum or variable price provisions, and the approximate timing of the transaction. Purchase obligations exclude agreements that are cancellable at any time without penalty. |
The above table excludes obligations related to our pension and other post-retirement benefit plans. Refer to Item 8, “Financial Statements and Other Supplementary Data,” in this Annual Report on Form 10-K for more details.
Tax Related Obligations
We excluded liabilities pertaining to uncertain tax positions from our summary of contractual obligations presented above, as we cannot make a reliable estimate of the period of cash settlement with the respective taxing authorities. As of December 31, 2016, we had $24.2 million of liabilities associated with uncertain tax positions.
Off-Balance Sheet Arrangements
As of December 31, 2016, we did not have any significant off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K promulgated under the Exchange Act, except as disclosed below.
Venture Capital Investments
We invest in several venture capital funds that invest in start-up companies, primarily in the life sciences industry. Our total commitment to these entities as of December 31, 2016 was $84.8 million, of which we had funded $38.2 million. Refer to Note 4, “Venture Capital Investments and Marketable Securities,” to our consolidated financial statements contained in Item 8, “Financial Statements and Other Supplementary Data,” in this Annual Report on Form 10-K for further details.
Letters of Credit
Our off-balance sheet commitments related to our outstanding letters of credit as of December 31, 2016 were $4.9 million.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risk from changes in interest rates and currency exchange rates, which could affect our future results of operations and financial condition. We manage our exposure to these risks through our regular operating and financing activities.
Interest Rate Risk
We are exposed to changes in interest rates while conducting normal business operations as a result of ongoing financing activities. As of December 31, 2016, our debt portfolio was comprised primarily of floating interest rate borrowings. A 100-basis point increase in interest rates would increase our annual pre-tax interest expense by approximately $12.1 million.
Foreign Currency Exchange Rate Risk
We operate on a global basis and have exposure to some foreign currency exchange rate fluctuations for our financial position, results of operations, and cash flows.
While the financial results of our global activities are reported in U.S. dollars, our foreign subsidiaries typically conduct their operations in their respective local currency. The principal functional currencies of the Company’s foreign subsidiaries are the Euro, British Pound, and Canadian Dollar. During fiscal year 2016, the most significant drivers of foreign currency translation adjustment that the Company recorded as part of other comprehensive income (loss) were the Euro, British Pound, Canadian Dollar, and to a lesser extent, the Chinese Yuan Renminbi and Japanese Yen.
Fluctuations in the foreign currency exchange rates of the countries in which we do business will affect our financial position, results of operations, and cash flows. As the U.S. dollar strengthens against other currencies, particularly as a result of Brexit and other recent developments, the value of our non-U.S. revenue, expenses, assets, liabilities, and cash flows will generally decline when reported in U.S. dollars. The impact to net income as a result of a U.S. dollar strengthening will be partially mitigated by the value of non-U.S. expense, which will also decline when reported in U.S. dollars. As the U.S. dollar weakens versus other currencies, the value of the non-U.S. revenue and expenses, assets, liabilities, and cash flows will generally increase when reported in U.S. dollars. For fiscal year 2016, our revenue would have decreased by approximately $65.9 million and our operating income would have decreased by approximately $2.8 million, respectively, if the U.S. dollar exchange rate would have strengthened by 10% with all other variables held constant.
We attempt to minimize this exposure by using certain financial instruments in accordance with our overall risk management and our hedge policy. We do not enter into speculative derivative agreements.
During fiscal year 2016, we utilized foreign exchange contracts, principally to hedge certain balance sheet exposures resulting from currency fluctuations.
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
| |
| |
| Consolidated Statements of Income for fiscal years 2016, 2015 and 2014 | |
| Consolidated Statements of Comprehensive Income for fiscal years 2016, 2015 and 2014 | |
| Consolidated Balance Sheets as of December 31, 2016 and December 26, 2015 | |
| Consolidated Statements of Cash Flows for fiscal years 2016, 2015 and 2014 | |
| Consolidated Statements of Changes in Equity for fiscal years 2016, 2015 and 2014 | |
| |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Charles River Laboratories International, Inc.:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of income, comprehensive income, changes in equity and cash flows present fairly, in all material respects, the financial position of Charles River Laboratories International, Inc. and its subsidiaries at December 31, 2016 and December 26, 2015, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2016 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2016, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As described in Management's Report on Internal Control over Financial Reporting, management has excluded WRH, Inc., Blue Stream Laboratories, Inc., and Agilux Laboratories, Inc. from its assessment of internal control over financial reporting as of December 31, 2016 because they were acquired by the Company during 2016. We have also excluded WRH, Inc., Blue Stream Laboratories, Inc., and Agilux Laboratories, Inc. from our audit of internal control over financial reporting. WRH, Inc., Blue Stream Laboratories, Inc., and Agilux Laboratories, Inc. are wholly-owned subsidiaries whose total assets and total revenues represent 7.8 percent and 11.1 percent, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2016.
/s/ PricewaterhouseCoopers LLP
Boston, Massachusetts
February 14, 2017
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF INCOME
(in thousands, except per share amounts)
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| Service revenue | $ | 1,130,733 |
| | $ | 858,244 |
| | $ | 797,765 |
|
| Product revenue | 550,699 |
| | 505,058 |
| | 499,897 |
|
| Total revenue | 1,681,432 |
| | 1,363,302 |
| | 1,297,662 |
|
| Costs and expenses: | | | | | |
| Cost of services provided (excluding amortization of intangible assets) | 757,732 |
| | 568,227 |
| | 558,578 |
|
| Cost of products sold (excluding amortization of intangible assets) | 277,034 |
| | 263,983 |
| | 266,424 |
|
| Selling, general and administrative | 367,548 |
| | 300,414 |
| | 269,033 |
|
| Amortization of intangible assets | 41,699 |
| | 24,229 |
| | 25,957 |
|
| Operating income | 237,419 |
| | 206,449 |
| | 177,670 |
|
| Other income (expense): | | | | | |
| Interest income | 1,314 |
| | 1,043 |
| | 1,154 |
|
| Interest expense | (27,709 | ) | | (15,072 | ) | | (11,950 | ) |
| Other income (expense), net | 11,897 |
| | 3,008 |
| | 10,721 |
|
| Income from continuing operations, before income taxes | 222,921 |
| | 195,428 |
| | 177,595 |
|
| Provision for income taxes | 66,835 |
| | 43,391 |
| | 47,671 |
|
| Income from continuing operations, net of income taxes | 156,086 |
| | 152,037 |
| | 129,924 |
|
| Income (loss) from discontinued operations, net of income taxes | 280 |
| | (950 | ) | | (1,726 | ) |
| Net income | 156,366 |
| | 151,087 |
| | 128,198 |
|
| Less: Net income attributable to noncontrolling interests | 1,601 |
| | 1,774 |
| | 1,500 |
|
| Net income attributable to common shareholders | $ | 154,765 |
| | $ | 149,313 |
| | $ | 126,698 |
|
| Earnings (loss) per common share | | | | | |
| Basic: | | | | | |
| Continuing operations attributable to common shareholders | $ | 3.28 |
| | $ | 3.23 |
| | $ | 2.76 |
|
| Discontinued operations | $ | 0.01 |
| | $ | (0.02 | ) | | $ | (0.04 | ) |
| Net income attributable to common shareholders | $ | 3.29 |
| | $ | 3.21 |
| | $ | 2.72 |
|
| Diluted: | | | | | |
| Continuing operations attributable to common shareholders | $ | 3.22 |
| | $ | 3.15 |
| | $ | 2.70 |
|
| Discontinued operations | $ | 0.01 |
| | $ | (0.02 | ) | | $ | (0.04 | ) |
| Net income attributable to common shareholders | $ | 3.23 |
| | $ | 3.13 |
| | $ | 2.66 |
|
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| See Notes to Consolidated Financial Statements. |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(in thousands)
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| Net income | $ | 156,366 |
| | $ | 151,087 |
| | $ | 128,198 |
|
| Other comprehensive income (loss): | | | | | |
| Foreign currency translation adjustment and other | (73,243 | ) | | (61,982 | ) | | (48,955 | ) |
| Cumulative translation adjustment related to intercompany loan forgiveness | — |
| | (2,341 | ) | | — |
|
| Pension and other post-retirement benefit plans (Note 10): | | | | | |
| Prior service cost and gains (losses) arising during the period | (60,678 | ) | | (302 | ) | | (42,236 | ) |
| Amortization of net gains (losses) and prior service benefit included in net periodic pension cost | 1,711 |
| | 2,617 |
| | 1,234 |
|
| Comprehensive income, before income taxes | 24,156 |
| | 89,079 |
| | 38,241 |
|
Income tax expense (benefit) related to items of other comprehensive income
(Note 8) | (12,369 | ) | | 530 |
| | (9,897 | ) |
| Comprehensive income, net of income taxes | 36,525 |
| | 88,549 |
| | 48,138 |
|
| Less: Comprehensive income (loss) related to noncontrolling interests, net of income taxes | (24 | ) | | 537 |
| | 1,044 |
|
| Comprehensive income attributable to common shareholders, net of income taxes | $ | 36,549 |
| | $ | 88,012 |
| | $ | 47,094 |
|
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| See Notes to Consolidated Financial Statements. |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
CONSOLIDATED BALANCE SHEETS
(in thousands, except per share amounts)
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 117,626 |
| | $ | 117,947 |
|
| Trade receivables, net | 364,050 |
| | 270,068 |
|
| Inventories | 95,833 |
| | 93,735 |
|
| Prepaid assets | 34,315 |
| | 30,198 |
|
| Other current assets | 45,008 |
| | 47,286 |
|
| Total current assets | 656,832 |
| | 559,234 |
|
| Property, plant and equipment, net | 755,827 |
| | 677,959 |
|
| Goodwill | 787,517 |
| | 438,829 |
|
| Client relationships, net | 320,157 |
| | 213,374 |
|
| Other intangible assets, net | 74,291 |
| | 67,430 |
|
| Deferred tax assets | 28,746 |
| | 40,028 |
|
| Other assets | 88,430 |
| | 71,643 |
|
| Total assets | $ | 2,711,800 |
| | $ | 2,068,497 |
|
| Liabilities, Redeemable Noncontrolling Interest and Equity | | | |
| Current liabilities: | | | |
| Current portion of long-term debt and capital leases | $ | 27,313 |
| | $ | 17,033 |
|
| Accounts payable | 68,485 |
| | 36,675 |
|
| Accrued compensation | 93,471 |
| | 72,832 |
|
| Deferred revenue | 127,731 |
| | 81,343 |
|
| Accrued liabilities | 84,470 |
| | 89,494 |
|
| Other current liabilities | 26,500 |
| | 12,544 |
|
| Current liabilities of discontinued operations | 1,623 |
| | 1,840 |
|
| Total current liabilities | 429,593 |
| | 311,761 |
|
| Long-term debt, net and capital leases | 1,207,696 |
| | 845,997 |
|
| Deferred tax liabilities | 55,717 |
| | 48,223 |
|
| Other long-term liabilities | 159,239 |
| | 89,062 |
|
| Long-term liabilities of discontinued operations | 5,771 |
| | 7,890 |
|
| Total liabilities | 1,858,016 |
| | 1,302,933 |
|
| Commitments and contingencies (Notes 2, 7, 9, 10, 13, and 17) |
| |
|
| Redeemable noncontrolling interest | 14,659 |
| | 28,008 |
|
| Equity: | | | |
| Preferred stock, $0.01 par value; 20,000 shares authorized; no shares issued and outstanding | — |
| | — |
|
| Common stock, $0.01 par value; 120,000 shares authorized; 86,301 shares issued and 47,363 shares outstanding as of December 31, 2016 and 85,464 shares issued and 46,698 shares outstanding as of December 26, 2015 | 863 |
| | 855 |
|
| Additional paid-in capital | 2,477,371 |
| | 2,397,960 |
|
| Retained earnings | 165,303 |
| | 10,538 |
|
| Treasury stock, at cost, 38,938 shares and 38,766 shares as of December 31, 2016 and December 26, 2015, respectively | (1,553,005 | ) | | (1,540,738 | ) |
| Accumulated other comprehensive loss | (253,764 | ) | | (135,548 | ) |
| Total equity attributable to common shareholders | 836,768 |
| | 733,067 |
|
| Noncontrolling interests | 2,357 |
| | 4,489 |
|
| Total equity | 839,125 |
| | 737,556 |
|
| Total liabilities, redeemable noncontrolling interest and equity | $ | 2,711,800 |
| | $ | 2,068,497 |
|
| See Notes to Consolidated Financial Statements. |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| Cash flows relating to operating activities | | | | | |
| Net income | $ | 156,366 |
| | $ | 151,087 |
| | $ | 128,198 |
|
| Less: Income (loss) from discontinued operations, net of income taxes | 280 |
| | (950 | ) | | (1,726 | ) |
| Income from continuing operations, net of income taxes | 156,086 |
| | 152,037 |
| | 129,924 |
|
| Adjustments to reconcile net income from continuing operations to net cash provided by operating activities: |
| Depreciation and amortization | 126,658 |
| | 94,881 |
| | 96,445 |
|
| Amortization of debt issuance costs and discounts | 2,831 |
| | 2,380 |
| | 1,725 |
|
| Stock-based compensation | 43,642 |
| | 40,122 |
| | 31,035 |
|
| Deferred income taxes | 1,945 |
| | 2,689 |
| | 7,060 |
|
| Gain on venture capital investments | (10,284 | ) | | (3,823 | ) | | (9,301 | ) |
| Gain on bargain purchase | 16 |
| | (9,837 | ) | | — |
|
| Other, net | 9,499 |
| | 168 |
| | (982 | ) |
| Changes in assets and liabilities: | | | | | |
| Trade receivables, net | (52,780 | ) | | (16,963 | ) | | (28,088 | ) |
| Inventories | (4,021 | ) | | 3,364 |
| | (2,956 | ) |
| Other assets | (6,215 | ) | | 850 |
| | (5,145 | ) |
| Accounts payable | 22,076 |
| | 1,174 |
| | 4,599 |
|
| Accrued compensation | 9,298 |
| | 8,414 |
| | 13,631 |
|
| Deferred revenue | 14,580 |
| | 6,274 |
| | 22,244 |
|
| Accrued liabilities | (11,487 | ) | | 14,069 |
| | 8,284 |
|
| Taxes payable and prepaid taxes | (1,800 | ) | | (3,906 | ) | | (7,090 | ) |
| Other liabilities | 331 |
| | (3,659 | ) | | (9,253 | ) |
| Net cash provided by operating activities | 300,375 |
| | 288,234 |
| | 252,132 |
|
| Cash flows relating to investing activities | | | | | |
| Acquisition of businesses and assets, net of cash acquired | (648,482 | ) | | (247,651 | ) | | (234,267 | ) |
| Capital expenditures | (55,288 | ) | | (63,252 | ) | | (56,925 | ) |
| Purchases of investments | (40,248 | ) | | (34,235 | ) | | (26,648 | ) |
| Proceeds from sale of investments and distributions from venture capital investments | 53,954 |
| | 27,072 |
| | 21,000 |
|
| Other, net | 3,694 |
| | (2,221 | ) | | (1,150 | ) |
| Net cash used in investing activities | (686,370 | ) | | (320,287 | ) | | (297,990 | ) |
| Cash flows relating to financing activities | | | | | |
| Proceeds from long-term debt and revolving credit facility | 1,044,666 |
| | 492,514 |
| | 298,920 |
|
| Proceeds from exercises of stock options | 23,197 |
| | 39,367 |
| | 73,688 |
|
| Payments on long-term debt, revolving credit facility, and capital lease obligations | (656,636 | ) | | (417,331 | ) | | (194,536 | ) |
| Purchase of treasury stock | (12,267 | ) | | (117,478 | ) | | (122,018 | ) |
| Other, net | (8,234 | ) | | 7,476 |
| | 5,360 |
|
| Net cash provided by financing activities | 390,726 |
| | 4,548 |
| | 61,414 |
|
| Discontinued operations | | | | | |
| Net cash used in operating activities from discontinued operations | (2,056 | ) | | (1,876 | ) | | (1,081 | ) |
| Effect of exchange rate changes on cash and cash equivalents | (2,996 | ) | | (12,695 | ) | | (10,379 | ) |
| Net change in cash and cash equivalents | (321 | ) | | (42,076 | ) | | 4,096 |
|
| Cash and cash equivalents, beginning of period | 117,947 |
| | 160,023 |
| | 155,927 |
|
| Cash and cash equivalents, end of period | $ | 117,626 |
| | $ | 117,947 |
| | $ | 160,023 |
|
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| See Notes to Consolidated Financial Statements. |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS (continued)
(in thousands)
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| Supplemental cash flow information: | | | | | |
| Cash paid for income taxes | $ | 42,868 |
| | $ | 24,436 |
| | $ | 29,704 |
|
| Cash paid for interest | $ | 22,756 |
| | $ | 11,101 |
| | $ | 10,199 |
|
| Non-cash investing and financing activities: | | | | | |
| Capitalized interest | $ | 4 |
| | $ | 424 |
| | $ | 1,032 |
|
| Additions to property, plant and equipment, net | $ | 5,333 |
| | $ | 6,720 |
| | $ | 4,355 |
|
| Assets acquired under capital lease | $ | 1,335 |
| | $ | 10,281 |
|
| $ | 18,690 |
|
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| | | | | | |
| See Notes to Consolidated Financial Statements. |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
(in thousands)
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common stock | | Additional Paid-In Capital | | Retained Earnings (Accumulated Deficit) | | Accumulated Other Comprehensive Income (Loss) | | Treasury Stock | | Total Equity Attributable to Common Shareholders | | Noncontrolling Interests | | Total Equity |
| Shares | | Amount | | | | | Shares | | Amount | | | |
| December 28, 2013 | 82,523 |
| | $ | 825 |
| | $ | 2,206,155 |
| | $ | (265,473 | ) | | $ | 5,357 |
| | 34,969 |
| | $ | (1,305,880 | ) | | $ | 640,984 |
| | $ | 3,093 |
| | $ | 644,077 |
|
| Net income | — |
| | — |
| | — |
| | 126,698 |
| | — |
| | — |
| | — |
| | 126,698 |
| | 645 |
| | 127,343 |
|
| Other comprehensive loss | — |
| | — |
| | — |
| | — |
| | (79,604 | ) | | — |
| | — |
| | (79,604 | ) | | (14 | ) | | (79,618 | ) |
| Adjustment of redeemable noncontrolling interest to fair value | — |
| | — |
| | (7,425 | ) | | — |
| | — |
| | — |
| | — |
| | (7,425 | ) | | — |
| | (7,425 | ) |
| Tax benefit associated with stock issued under employee compensation plans | — |
| | — |
| | 4,301 |
| | — |
| | — |
| | — |
| | — |
| | 4,301 |
| | — |
| | 4,301 |
|
| Issuance of stock under employee compensation plans | 1,980 |
| | 20 |
| | 73,574 |
| | — |
| | — |
| | — |
| | — |
| | 73,594 |
| | — |
| | 73,594 |
|
| Acquisition of treasury shares | — |
| | — |
| | — |
| | — |
| | — |
| | 2,207 |
| | (117,380 | ) | | (117,380 | ) | | — |
| | (117,380 | ) |
| Stock-based compensation | — |
| | — |
| | 31,035 |
| | — |
| | — |
| | — |
| | — |
| | 31,035 |
| | — |
| | 31,035 |
|
| December 27, 2014 | 84,503 |
| | 845 |
| | 2,307,640 |
| | (138,775 | ) | | (74,247 | ) | | 37,176 |
| | (1,423,260 | ) | | 672,203 |
| | 3,724 |
| | 675,927 |
|
| Net income | — |
| | — |
| | — |
| | 149,313 |
| | — |
| | — |
| | — |
| | 149,313 |
| | 936 |
| | 150,249 |
|
| Other comprehensive loss | — |
| | — |
| | — |
| | — |
| | (61,301 | ) | | — |
| | — |
| | (61,301 | ) | | (171 | ) | | (61,472 | ) |
| Adjustment of redeemable noncontrolling interest to fair value | — |
| | — |
| | 183 |
| | — |
| | — |
| | — |
| | — |
| | 183 |
| | — |
| | 183 |
|
| Tax benefit associated with stock issued under employee compensation plans | — |
| | — |
| | 10,608 |
| | — |
| | — |
| | — |
| | — |
| | 10,608 |
| | — |
| | 10,608 |
|
| Issuance of stock under employee compensation plans | 961 |
| | 10 |
| | 39,407 |
| | — |
| | — |
| | — |
| | — |
| | 39,417 |
| | — |
| | 39,417 |
|
| Acquisition of treasury shares | — |
| | — |
| | — |
| | — |
| | — |
| | 1,590 |
| | (117,478 | ) | | (117,478 | ) | | — |
| | (117,478 | ) |
| Stock-based compensation | — |
| | — |
| | 40,122 |
| | — |
| | — |
| | — |
| | — |
| | 40,122 |
| | — |
| | 40,122 |
|
| December 26, 2015 | 85,464 |
| | 855 |
| | 2,397,960 |
| | 10,538 |
| | (135,548 | ) | | 38,766 |
| | (1,540,738 | ) | | 733,067 |
| | 4,489 |
| | 737,556 |
|
| Net income | — |
| | — |
| | — |
| | 154,765 |
| | — |
| | — |
| | — |
| | 154,765 |
| | 924 |
| | 155,689 |
|
| Other comprehensive loss | — |
| | — |
| | — |
| | — |
| | (118,216 | ) | | — |
| | — |
| | (118,216 | ) | | (154 | ) | | (118,370 | ) |
| Dividends declared to noncontrolling interests | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | — |
| | (2,902 | ) | | (2,902 | ) |
| Adjustment of redeemable noncontrolling interest to fair value | — |
| | — |
| | 1,690 |
| | — |
| | — |
| | — |
| | — |
| | 1,690 |
| | — |
| | 1,690 |
|
Purchase of additional equity in redeemable noncontrolling interest
| — |
| | — |
| | 1,593 |
| | — |
| | — |
| | — |
| | — |
| | 1,593 |
| | — |
| | 1,593 |
|
| Tax benefit associated with stock issued under employee compensation plans | — |
| | — |
| | 9,274 |
| | — |
| | — |
| | — |
| | — |
| | 9,274 |
| | — |
| | 9,274 |
|
| Issuance of stock under employee compensation plans | 837 |
| | 8 |
| | 23,212 |
| | — |
| | — |
| | — |
| | — |
| | 23,220 |
| | — |
| | 23,220 |
|
| Acquisition of treasury shares | — |
| | — |
| | — |
| | — |
| | — |
| | 172 |
| | (12,267 | ) | | (12,267 | ) | | — |
| | (12,267 | ) |
| Stock-based compensation | — |
| | — |
| | 43,642 |
| | — |
| | — |
| | — |
| | — |
| | 43,642 |
| | — |
| | 43,642 |
|
| December 31, 2016 | 86,301 |
| | $ | 863 |
| | $ | 2,477,371 |
| | $ | 165,303 |
| | $ | (253,764 | ) | | 38,938 |
| | $ | (1,553,005 | ) | | $ | 836,768 |
| | $ | 2,357 |
| | $ | 839,125 |
|
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| See Notes to Consolidated Financial Statements. |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Description of Business
Charles River Laboratories International, Inc. (the Company), together with its subsidiaries, is a full service, early-stage contract research organization (CRO). The Company has built upon its core competency of laboratory animal medicine and science (research model technologies) to develop a diverse portfolio of discovery and safety assessment services, both Good Laboratory Practice (GLP) and non-GLP, which are able to support its clients from target identification through non-clinical development. The Company also provides a suite of products and services to support its clients’ manufacturing activities.
Principles of Consolidation
The Company’s consolidated financial statements reflect its financial statements and those of its subsidiaries in which the Company holds a controlling financial interest. For consolidated entities in which the Company owns or is exposed to less than 100% of the economics, the Company records net income (loss) attributable to noncontrolling interests in its consolidated statements of income equal to the percentage of the economic or ownership interest retained in such entities by the respective noncontrolling parties. Intercompany balances and transactions are eliminated in consolidation.
The Company’s fiscal year is typically based on 52-weeks, with each quarter composed of 13 weeks ending on the last Saturday on, or closest to, March 31, June 30, September 30, and December 31. A 53rd week was included in fiscal year 2016, which is occasionally necessary to align with a December 31 calendar year-end. The additional week was included in the fourth quarter.
Reclassifications
Certain reclassifications have been made to prior year statements to conform to the current year presentation. These reclassifications have no impact on period reported net income or cash flow.
Segment Reporting
The Company reports its results in three reportable segments: Research Models and Services (RMS), Discovery and Safety Assessment (DSA), and Manufacturing Support (Manufacturing). The Company aggregates its operating segments into a reportable segment if (a) they have similar economic characteristics; (b) they are similar in the in the nature of the products or services, nature of the production process, type or class of customer for their products and services, methods used to distribute their products and services and nature of the regulatory environment; and (c) the aggregation helps users better understand the Company’s performance.
During the second quarter of 2016, the Company acquired WRH, Inc. (WIL Research), a provider of safety assessment and contract development and manufacturing (CDMO) services. WIL Research’s safety assessment business is reported in the Company’s DSA reportable segment and its CDMO business created a new operating segment, Contract Manufacturing, that is reported as part of the Company’s Manufacturing reportable segment. On February 10, 2017, the Company divested the CDMO business. In addition, amounts due to changes in the Company’s market strategy for certain services and resulting information provided to the Chief Operating Decision Maker were reclassified from the Company’s RMS reportable segment to its Manufacturing reportable segment, including revenue of $2.8 million and $3.7 million for fiscal years 2015 and 2014, respectively, and operating income of $0.5 million and $0.6 million for fiscal years 2015 and 2014, respectively. The Company reported segment results on this basis for all periods presented.
The revised reportable segments are as follows: |
| | |
| Research Models and Services | Discovery and Safety Assessment | Manufacturing Support |
| Research Models | Discovery Services | Microbial Solutions |
| Research Model Services | Safety Assessment | Avian |
| | | Biologics |
| | | Contract Manufacturing |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Use of Estimates
The preparation of consolidated financial statements in accordance with generally accepted accounting principles in the United States (U.S. GAAP) requires that the Company makes estimates and judgments that may affect the reported amounts of assets, liabilities, revenues, expenses, and related disclosure of contingent assets and liabilities. On an on-going basis, the Company evaluates its estimates, judgments and methodologies. The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results may differ from these estimates under different assumptions or conditions. Changes in estimates are reflected in reported results in the period in which they become known.
Cash and Cash Equivalents
Cash equivalents include money market funds, time deposits and other investments with remaining maturities at the purchase date of three months or less.
Investments
Marketable securities are reported at fair value. Realized gains and losses on marketable securities are included in other income (expense), net and are determined using the specific identification method. Unrealized gains and losses on available-for-sale marketable securities are included in accumulated other comprehensive income (loss). Time deposits with original maturities of greater than three months are reported as investments.
Trade Receivables, Net
The Company records trade receivables net of an allowance for doubtful accounts. An allowance for doubtful accounts is established based on historical collection information, a review of major client accounts receivable balances and current economic conditions in the geographies in which it operates. Amounts determined to be uncollectible are charged or written off against the allowance.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents, investments and trade receivables. The Company places cash and cash equivalents and investments in various financial institutions with high credit rating and limits the amount of credit exposure to any one financial institution. Trade receivables are primarily from clients in the pharmaceutical and biotechnology industries, as well as academic and government institutions. Concentrations of credit risk with respect to trade receivables, which are typically unsecured, are limited due to the wide variety of customers using the Company’s products and services as well as their dispersion across many geographic areas. No single client accounted for more than 5% of revenue or trade receivables for the periods ended December 31, 2016 and December 26, 2015.
Fair Value Measurements
The accounting standard for fair value measurements defines fair value, establishes a framework for measuring fair value in accordance with U.S. GAAP, and requires certain disclosures about fair value measurements. Under this standard, fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Company has certain financial assets and liabilities recorded at fair value which have been classified as Level 1, 2 or 3 within the fair value hierarchy:
| |
| • | Level 1 - Fair values are determined utilizing prices (unadjusted) in active markets for identical assets or liabilities that the Company has the ability to access; |
| |
| • | Level 2 - Fair values are determined by utilizing quoted prices for identical or similar assets and liabilities in active markets or other market observable inputs such as interest rates, yield curves and foreign currency spot rates; |
| |
| • | Level 3 - Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable. |
The fair value hierarchy level is determined by asset, liability and redeemable noncontrolling interest class based on the lowest level of significant input. The observability of inputs may change for certain assets or liabilities. This condition could cause an asset or liability to be reclassified between levels. The Company recognizes transfers between levels within the fair value hierarchy, if any, at the end of each quarter.
Valuation methodologies used for assets and liabilities measured or disclosed at fair value are as follows:
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
| |
| • | Cash equivalents - Valued at market prices determined through third-party pricing services; |
| |
| • | Mutual funds - Valued at the unadjusted quoted net asset value of shares held by the Company; |
| |
| • | Foreign currency forward contracts - Valued using readily observable market inputs, such as forward foreign exchange points and foreign exchanges rates; |
| |
| • | Life insurance policies - Valued at cash surrender value based on the fair value of underlying investments; |
| |
| • | Contingent consideration - Valued based on a probability weighting of the future cash flows associated with the potential outcomes; |
| |
| • | Redeemable noncontrolling interest - Valued using the income approach based on estimated future cash flows of the underlying business discounted by a weighted average cost of capital. |
Inventories
Inventories are stated at the lower of cost or net realizable value. Cost is determined on the average cost method for the small model business and first-in-first-out for the Company’s large model and Microbial Solutions businesses. For the small model business, cost includes direct materials such as feed and bedding, costs of personnel directly involved in the care of the models, and an allocation of facility overhead. For the large model business, cost is primarily the external cost paid to acquire the model. Certain businesses value inventory based on standard costs, which are periodically compared to and adjusted to actual costs. Inventory costs are charged to cost of revenue in the period the products are sold to an external party. The Company analyzes its inventory levels on a quarterly basis and writes down inventory that is determined to be damaged, obsolete or otherwise unmarketable, with a corresponding charge to cost of products sold.
Property, Plant and Equipment, Net
Property, plant and equipment, including improvements that significantly add to productive capacity or extend useful life, are carried at cost and are subject to review for impairment whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. The cost of normal, recurring, or periodic repairs and maintenance activities related to property, plant and equipment is expensed as incurred. In addition, the Company capitalizes certain internal use computer software development costs. Costs incurred during the preliminary project stage are expensed as incurred, while costs incurred during the application development stage are capitalized and amortized over the estimated useful life of the software. The Company also capitalizes costs related to specific upgrades and enhancements when it is probable the expenditures will result in additional functionality. Maintenance and training costs related to software obtained for internal use are expensed as incurred.
Interest costs incurred during the construction of major capital projects are capitalized until the underlying asset is ready for its intended use, at which point the interest costs are amortized as depreciation expense over the life of the underlying asset.
The Company generally depreciates the cost of its property, plant and equipment using the straight-line method over the estimated useful lives of the respective assets as follow:
|
| |
| | Estimated
Useful Lives |
| | (in years) |
| Land | Indefinite |
| Buildings | 20 - 40 |
| Machinery and equipment | 3 - 20 |
| Furniture and fixtures | 5 - 10 |
| Computer hardware and software | 3 - 8 |
| Vehicles | 3 - 5 |
Leasehold improvements are amortized over the shorter of the estimated useful life of the asset or the lease term. Capital lease assets are amortized over the lease term, however, if ownership is transferred by the end of the capital lease, or there is a bargain purchase option, such capital lease assets are amortized over the useful life that would be assigned if such assets were owned.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
When the Company disposes of property, plant and equipment, it removes the associated cost and accumulated depreciation from the related accounts on its consolidated balance sheet and includes any resulting gain or loss in its consolidated statement of income.
Business Acquisitions
The Company accounts for acquisitions as business combinations under the acquisition method of accounting. The Company allocates the amounts that it pays for each acquisition to the assets it acquires and liabilities it assumes based on their fair values at the dates of acquisition, including identifiable intangible assets. The Company bases the fair value of identifiable intangible assets acquired in a business combination on valuations that use information and assumptions determined by management and which consider management’s best estimates of inputs and assumptions that a market participant would use.
Contingent Consideration
The consideration for the Company’s acquisitions often includes future payments that are contingent upon the occurrence of a particular event. The Company records an obligation for such contingent payments at fair value on the acquisition date. The Company estimates the fair value of contingent consideration obligations through valuation models that incorporate probability adjusted assumptions related to the achievement of the milestones and thus likelihood of making related payments. The Company revalues these contingent consideration obligations each reporting period. Changes in the fair value of the contingent consideration obligations are recognized in the Company’s consolidated statements of income as a component of selling, general and administrative expenses. Changes in the fair value of the contingent consideration obligations can result from changes to one or multiple inputs, including adjustments to the discount rates and changes in the assumed probabilities of successful achievement of certain financial targets.
Discount rates in the Company’s valuation models represent a measure of the credit risk associated with settling the liability. The period over which the Company discounts its contingent obligations is typically based on when the contingent payments would be triggered. These fair value measurements are based on significant inputs not observable in the market. See Note 5, “Fair Value.”
Goodwill and Intangible Assets
Goodwill represents the difference between the purchase price and the fair value of the identifiable tangible and intangible net assets when accounted for using the purchase method of accounting. Goodwill is not amortized, but reviewed for impairment on an annual basis, during the fourth quarter, or more frequently if an event occurs or circumstances change that would more-likely-than-not reduce the fair value of the Company's reporting units below their carrying amounts.
The Company has the option to first assess qualitative factors to determine whether it is necessary to perform the two-step impairment test. If the Company elects this option and believes, as a result of the qualitative assessment, that it is more-likely-than-not that the carrying value of goodwill is not recoverable, the quantitative two-step impairment test is required; otherwise, no further testing is required. Alternatively, the Company may elect to not first assess qualitative factors and immediately perform the quantitative two-step impairment test. In the first step, the Company compares the fair value of its reporting units to their carrying values. If the carrying values of the net assets assigned to the reporting units exceed the fair values of the reporting units, then the second step of the impairment test is performed in order to determine the implied fair value of the Company’s goodwill. If the carrying value of the reporting unit’s goodwill exceeds its implied fair value, then the Company would record an impairment loss equal to the difference.
Definite-lived intangible assets, including client relationships, are amortized over the pattern in which the economic benefits of the intangible assets are utilized and reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets or asset group may not be recoverable. Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset, which requires the use of customer attribution rates and other assumptions. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the definite-lived intangible assets, the definite-lived intangible assets are written-down to their fair values.
Valuation and Impairment of Long-Lived Assets
Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets or asset group may not be recoverable.
Determination of recoverability is based on an estimate of undiscounted future cash flows resulting from the use of the asset and its eventual disposition. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the assets, the assets are written-down to their fair values.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Long-lived assets to be disposed of are carried at fair value less costs to sell.
Venture Capital Investments
The Company invests in several venture capital funds that invest in start-up companies primarily in the life sciences industry. The Company’s ownership interest in these funds ranges from 0.7% to 12.0%. The Company accounts for such investments in limited liability partnerships (LLP), which are variable interest entities, under the equity or cost method of accounting. The Company is not the primary beneficiary because it has no power to direct the activities that most significantly affect the LLPs’ economic performance. The Company accounts for the investments in limited liability companies, which are not variable interest entities, under the equity method of accounting.
Under the equity method of accounting, the Company’s portion of the investment gains and losses, as reported in the fund’s financial statements on a quarterly lag each reporting period, is recorded in other income (expense), net. In addition, the Company adjusts the carrying value of these investments to reflect its estimate of changes to fair value since the fund’s financial statements based on information from the fund’s management team, market prices of known public holdings of the fund and other information.
Under the cost method of accounting, the Company’s investment is initially measured at cost, with distributions recognized in other income (expense), net. Distributions received in excess of earnings subsequent to the date of investment are considered a return of investment and are recorded as reductions of cost of the investment. The Company reviews its cost method investments to determine whether a decline in fair value below the cost basis is other-than-temporary. If the decline in fair value is determined to be other-than-temporary, the cost basis of the investment is written down to fair value.
Life Insurance Contracts
Investments in life insurance contracts are recorded at cash surrender value. The initial investment at the transaction price is recognized and remeasured based on fair value of underlying investments or contractual value each reporting period. Investments in and redemptions of these life insurance contracts are reported as cash flows from investing activities in the consolidated statement of cash flows. As of December 31, 2016 and December 26, 2015, the Company held 43 and 42 contracts, respectively, with a face value of $61.4 million and $60.5 million, respectively.
Stock-Based Compensation
The Company grants stock options, restricted stock, restricted stock units, and performance share units (PSUs) to employees and stock options, restricted stock, and restricted stock units to non-employee directors under stock-based compensation plans. Stock-based compensation is recognized as an expense in the consolidated financial statements based on the grant date fair value, adjusted for estimated forfeitures, over the requisite service period.
For stock options, restricted stock and restricted stock units that vest based on service conditions, the Company uses the straight-line method to allocate compensation expense to reporting periods. Where awards are made with non-substantive vesting periods, where a portion of the award continues to vests after the employee’s retirement, the Company recognizes expense based on the period from the grant date to the date on which the employee is retirement eligible. The Company records the expense for PSU grants subject to performance and/or market conditions using the accelerated attribution method over the remaining service period when management determines that achievement of the performance-based milestone is probable.
The fair value of stock options granted is calculated using the Black-Scholes option-pricing model and the fair value of PSUs is estimated using a lattice model with a Monte Carlo simulation, both of which require the use of subjective assumptions including volatility and expected term, among others. The expected volatility assumption is typically determined using the historical volatility of the Company’s common stock over the expected life of the stock-based award. The expected term is determined using historical option exercise activity. The fair value of restricted stock and restricted stock units is based on the market value of the Company’s common stock on the date of grant.
Revenue Recognition
The Company recognizes revenue when all of the following conditions are satisfied: persuasive evidence of an arrangement exists, delivery has occurred or services have been provided, the price to the customer is fixed or determinable, and collectibility is reasonably assured.
Service revenue is generally evidenced by client contracts, which range in duration from a few weeks to a few years and typically take the form of an agreed upon rate per unit or fixed fee arrangements. Such contracts typically do not contain acceptance provisions based upon the achievement of certain study or laboratory testing results. Revenue of agreed upon rate per unit contracts is recognized as services are performed, based upon rates specified in the contract. In cases where
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
performance spans reporting periods, revenue of fixed fee contracts is recognized as services are performed, measured on the ratio of outputs or performance obligations completed to the total contractual outputs or performance obligations to be provided. Changes in estimated effort to complete the fixed fee contract are reflected in the period in which the change becomes known. Changes in scope of work are common, especially under long-term contracts, and generally result in a change in contract value. Once the client has agreed to the changes in scope and renegotiated pricing terms, the contract value is amended and revenue is typically recognized as described above.
Billing schedules and payment terms are generally negotiated on a contract-by-contract basis. Payments received in excess of revenue recognized are recorded as deferred revenue. As the contracted services are subsequently performed and the associated revenue is recognized, the deferred revenue balance is reduced by the amount of revenue recognized during the period. In other cases, services may be provided and revenue is recognized before the client is invoiced. In these cases, revenue recognized will exceed amounts billed and the difference, representing amounts which are currently unbillable to the customer pursuant to contractual terms, is recorded as an unbilled receivable. Once the client is invoiced, the unbilled receivable is reduced for the amount billed, and a corresponding trade receivable is recorded.
Most contracts are terminable by the client, either immediately or upon notice. These contracts often require payment to the Company of expenses to wind down the project, fees earned to date or, in some cases, a termination fee. Such payments are included in revenues when earned.
The Company recognizes product revenue net of allowances for estimated returns, rebates and discounts when title and risk of loss pass to customers. When the Company sells equipment with specified acceptance criteria, it assesses its ability to meet the acceptance criteria in order to determine the timing of revenue recognition. The Company would defer revenue until completion of customer acceptance testing if it is not able to demonstrate the ability to meet such acceptance criteria.
A portion of the Company’s revenue is from multiple-element arrangements that include multiple products and/or services as deliverables in a single arrangement with each deliverable, or a combination of the deliverables, representing a separate unit of accounting. The Company allocates revenues to each element in a multiple-element arrangement based upon the relative selling price of each deliverable. Revenue allocated to each deliverable is then recognized when all revenue recognition criteria are met. Judgments as to the identification of deliverables, units of accounting, the allocation of consideration to the deliverable, and the appropriate timing of revenue recognition are critical with respect to these arrangements.
At the inception of each arrangement that includes milestone payments, the Company evaluates whether each milestone is substantive. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the Company’s performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the Company’s performance to achieve the milestone; (b) the consideration relates solely to past performance; and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. If a substantive milestone is achieved and collection of the related receivable is reasonably assured, the Company recognizes revenue related to the milestone in its entirety in the period in which the milestone is achieved. In those circumstances where a milestone is not substantive, the Company recognizes as revenue, on the date the milestone is achieved, an amount equal to the applicable percentage of the performance period that had elapsed as of the date the milestone was achieved, with the balance being deferred and recognized over the remaining period of performance. As of December 31, 2016, the Company had no significant milestones that were deemed substantive.
The Company records shipping charges billed to customers in total revenue and records shipping costs in cost of revenue (excluding amortization of intangible assets) for all periods presented.
Advertising Costs
Advertising costs are expensed as incurred. For fiscal years 2016, 2015 and 2014, advertising costs totaled $1.4 million, $1.2 million and $1.3 million, respectively.
Income Taxes
The provision for income taxes includes federal, state, local and foreign taxes. Income taxes are accounted for under the liability method. Deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial statements carrying amounts and their respective tax basis. The Company measures deferred tax assets and liabilities using the enacted tax rates in effect when the temporary differences are expected to be settled. The Company evaluates the realizability of its deferred tax assets and establishes a valuation allowance when it is more likely than not that all or a portion of deferred tax assets will not be realized.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The Company accounts for uncertain tax positions using a “more-likely-than-not” threshold for recognizing and resolving uncertain tax positions. The Company evaluates uncertain tax positions on a quarterly basis and considers various factors, including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, information obtained during in process audit activities and changes in facts or circumstances related to a tax position. The Company also accrues for potential interest and penalties related to unrecognized tax benefits in income tax expense.
Foreign Currency Contracts
Foreign currency contracts are recorded at fair value in the Company’s consolidated balance sheet and are not designated as hedging instruments. Any gains or losses on such contracts are immediately recognized in other income (expense), net.
Translation of Foreign Currencies
For the Company’s subsidiaries that transact in a functional currency other than the U.S. dollar, assets and liabilities are translated at current rates of exchange as of the balance sheet date. Income and expense items are translated at the average foreign exchange rates for the period. Adjustments resulting from the translation of the financial statements of the Company’s foreign operations into U.S. dollars are excluded from the determination of net income and are recorded in accumulated other comprehensive income (loss), a separate component of equity.
Pension and Other Post-Retirement Benefit Plans
The Company recognizes the funded status of its defined benefit pension and other post-retirement benefit plans as an asset or liability. This amount is defined as the difference between the fair value of plan assets and the benefit obligation. The Company measures plan assets and benefit obligations as of its fiscal year end.
The key assumptions used to calculate benefit obligations and related pension costs include expected long-term rate of return on plan assets, withdrawal and mortality rates, expected rate of increase in employee compensation levels and discount rate. Assumptions are determined based on the Company’s data and appropriate market indicators, and evaluated each year as of the plan’s measurement date.
The expected long-term rate of return on plan assets reflects the average rate of earnings expected on the funds invested, or to be invested, to provide for the benefits included in the projected benefit obligations. In determining the expected long-term rate of return on plan assets, the Company considers the relative weighting of plan assets, the historical performance of total plan assets and individual asset classes and economic and other indicators of future performance.
In fiscal year 2016, new mortality improvement scales were issued in the U.S. reflecting a decline in longevity projection from the 2015 releases that the Company adopted, which decreased the Company’s benefit obligations by $1.3 million as of December 31, 2016. In fiscal year 2015, new mortality improvement scales were issued in the U.S. and the United Kingdom (U.K.) reflecting a decline in longevity projection from the 2014 releases that the Company adopted, which decreased the Company’s benefit obligations by $3.3 million as of December 26, 2015.
The discount rate reflects the rate the Company would have to pay to purchase high-quality investments that would provide cash sufficient to settle its current pension obligations.
The rate of compensation increase reflects the expected annual salary increases for the plan participants based on historical experience and the current employee compensation strategy.
The Company is required to recognize as a component of other comprehensive income, net of tax, the actuarial gains or losses and prior service costs or credits that arise but were not previously required to be recognized as components of net periodic benefit cost. Other comprehensive income is adjusted as these amounts are later recognized in income as components of net periodic benefit cost.
Earnings Per Share
Basic earnings per share are calculated by dividing net income attributable to common shareholders by the weighted average number of common shares outstanding during the period. Except where the result would be anti-dilutive to income from continuing operations, diluted earnings per share is computed using the treasury stock method, assuming the exercise of stock options and the vesting of restricted stock awards, restricted stock units, or PSUs, as well as their related income tax effects.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Newly Adopted Accounting Pronouncements
In July 2015, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2015-11, “Simplifying the Measurement of Inventory,” that simplifies the subsequent measurement of inventories by replacing the current lower of cost or market test with a lower of cost or net realizable value test. The ASU is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted. During the fourth quarter of 2016, the Company adopted this standard, which had no impact on inventories as of December 31, 2016.
In August 2014, the FASB issued ASU 2014-15, “Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern.” The standard requires management to assess if there is substantial doubt about an entity’s ability to continue as a going concern within one year after the issuance date and, as applicable, provide additional disclosures on management’s plan to alleviate the substantial doubt. The ASU is effective for fiscal years ending after December 15, 2016, and interim periods within annual periods beginning after December 15, 2016. Early adoption is permitted. During the fourth quarter of 2016, the Company adopted this standard, which had no impact on the Company’s consolidated financial statements and related disclosures.
Newly Issued Accounting Pronouncements
In January 2017, the FASB issued ASU 2017-04, “Simplifying the Test for Goodwill Impairment.” The standard simplifies the accounting for goodwill impairment by removing Step 2 of the goodwill impairment test, which requires a hypothetical purchase price allocation. The ASU is effective for annual or interim goodwill impairment tests in fiscal years beginning after December 15, 2019, and should be applied on a prospective basis. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The adoption of this standard is not expected to have a material impact on the consolidated financial statements and related disclosures.
In January 2017, the FASB issued ASU 2017-01, “Clarifying the Definition of a Business.” The standard clarifies the definition of a business by adding guidance to assist entities in evaluating whether transactions should be accounted for as acquisitions of assets or businesses. The ASU is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted for certain transactions. The Company is still evaluating the impact this standard will have on its consolidated financial statements and related disclosures.
In November 2016, the FASB issued ASU 2016-18, “Restricted Cash.” The standard addresses the classification and presentation of restricted cash and restricted cash equivalents within the statement of cash flows. The ASU is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted. The Company is still evaluating the impact this standard will have on its consolidated financial statements and related disclosures.
In October 2016, the FASB issued ASU 2016-16, “Intra-Entity Transfers of Assets Other Than Inventory.” The standard requires the immediate recognition of tax effects for an intra-entity asset transfer other than inventory. The ASU is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted. The adoption of this standard is not expected to have a material impact on the consolidated financial statements and related disclosures.
In August 2016, the FASB issued ASU 2016-15, “Classification of Certain Cash Receipts and Cash Payments.” The standard addresses the classification of certain transactions within the statement of cash flows, including cash payments for debt prepayment or debt extinguishment costs, contingent consideration payments made after a business combination, and distributions received from equity method investments. The ASU is effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. Early adoption is permitted. The Company is still evaluating the impact this standard will have on its consolidated financial statements and related disclosures.
In March 2016, the FASB issued ASU 2016-09, “Improvements to Employee Share-Based Payment Accounting.” The standard reduces complexity in several aspects of the accounting for employee share-based compensation, including the income tax consequences, classification of awards as either equity or liabilities and classification on the statement of cash flows. The ASU is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. Early adoption is permitted. The Company is still evaluating the impact this standard will have on its consolidated financial statements and related disclosures.
In February 2016, the FASB issued ASU 2016-02, “Leases.” The standard established the principles that lessees and lessors will apply to report useful information to users of financial statements about the amount, timing and uncertainty of cash flows arising from a lease. The ASU is effective for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years. Early adoption is permitted. The Company is still evaluating the full impact this standard will have on its
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
consolidated financial statements and related disclosures but expects to recognize substantially all of its leases on the balance sheet, by recording a right-to-use asset and a corresponding lease liability.
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers.” The standard, including subsequently issued amendments, will replace most existing revenue recognition guidance in U.S. GAAP when it becomes effective and permits the use of either the full retrospective or modified retrospective transition method. The standard will require an entity to recognize the amount of revenue to which it expects to be entitled for the transfer of promised goods or services to customers. The standard will be effective for annual and interim periods beginning after December 15, 2017. The Company has selected the modified retrospective transition method and is still evaluating the impact the adoption will have on its consolidated financial statements and related disclosures.
2. BUSINESS ACQUISITIONS
Agilux
On September 28, 2016, the Company acquired Agilux Laboratories, Inc. (Agilux), a CRO that provides a suite of integrated discovery small and large molecule bioanalytical services, drug metabolism and pharmacokinetic services, and pharmacology services. The acquisition supports the Company’s strategy to offer clients a broader, integrated portfolio that provides services continuously from the earliest stages of drug research through the non-clinical development process. The purchase price for Agilux was $64.9 million in cash and was funded by borrowings on the Company’s revolving credit facility. The business is reported as part of the Company’s DSA reportable segment.
The purchase price allocation of $62.0 million, net of $2.9 million of cash acquired, was as follows:
|
| | | |
| | September 28, 2016 |
| | (in thousands) |
| Trade receivables (contractual amount of $4,799) | $ | 4,799 |
|
| Other current assets (excluding cash) | 1,509 |
|
| Property, plant and equipment | 3,907 |
|
| Other long-term assets | 11 |
|
| Definite-lived intangible assets | 21,900 |
|
| Goodwill | 43,899 |
|
| Current liabilities | (3,987 | ) |
| Long-term liabilities | (10,013 | ) |
| Total purchase price allocation | $ | 62,025 |
|
The purchase price allocations are subject to change as additional information becomes available concerning the fair value and tax basis of the assets acquired and liabilities assumed. Any additional adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the date of acquisition.
The breakout of definite-lived intangible assets acquired was as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 16,700 |
| | 17 |
| Other intangible assets | 5,200 |
| | 4 |
| Total definite-lived intangible assets | $ | 21,900 |
| | 14 |
The goodwill resulting from the transaction is primarily attributable to the potential growth of the Company’s DSA businesses from customers and technology introduced through Agilux, and the assembled workforce of the acquired business. The goodwill attributable to Agilux is not deductible for tax purposes.
The Company incurred transaction and integration costs of $1.7 million in connection with the acquisition during fiscal year 2016, which were included in selling, general and administrative expenses.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Pro forma financial information as well as actual revenue and operating income (loss) have not been included because Agilux’s financial results are non-significant when compared with the Company’s consolidated financial results.
Blue Stream
On June 27, 2016, the Company acquired Blue Stream Laboratories, Inc. (Blue Stream), an analytical CRO supporting the development of complex biologics and biosimilars. Combining Blue Stream with the Company’s existing discovery, safety assessment, and biologics capabilities creates a leading CRO that has the ability to support biologic and biosimilar development from characterization through clinical testing and commercialization. The purchase price for Blue Stream was $11.7 million, including $3.0 million in contingent consideration, and was subject to certain customary adjustments. The acquisition was funded by borrowings on the Company’s revolving credit facility. The business is reported in the Company’s Manufacturing reportable segment.
The contingent consideration is a one-time payment that could become payable based on the achievement of a revenue target. If achieved, the payment will become due in the third quarter of fiscal year 2017. The aggregate, undiscounted amount of contingent consideration that the Company may pay is $3.0 million. The Company estimated the fair value of this contingent consideration based on a probability-weighted set of outcomes.
The purchase price allocation of $11.7 million, net of a non-significant amount of cash acquired, was as follows:
|
| | | |
| | June 27, 2016 |
| | (in thousands) |
| Trade receivables (contractual amount of $1,104) | $ | 1,104 |
|
| Other current assets (excluding cash) | 15 |
|
| Property, plant and equipment | 912 |
|
| Other long-term assets | 187 |
|
| Definite-lived intangible assets | 1,230 |
|
| Goodwill | 10,477 |
|
| Current liabilities | (1,132 | ) |
| Long-term liabilities | (1,044 | ) |
| Total purchase price allocation | $ | 11,749 |
|
The purchase price allocations are subject to change as additional information becomes available concerning the fair value and tax basis of the assets acquired and liabilities assumed. From the date of the acquisition through December 31, 2016, the Company recorded a measurement-period adjustment related to the acquisition that resulted in an immaterial change to the purchase price allocation. Any additional adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the date of acquisition.
The breakout of definite-lived intangible assets acquired was as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 650 |
| | 10 |
| Other intangible assets | 580 |
| | 5 |
| Total definite-lived intangible assets | $ | 1,230 |
| | 7 |
The goodwill resulting from the transaction is primarily attributable to the potential growth of the Company’s Manufacturing business from customers and technology introduced through Blue Stream, the assembled workforce of the acquired business, expected synergies, and the development of future proprietary processes. The goodwill attributable to Blue Stream is not deductible for tax purposes.
The Company incurred $0.6 million of transaction and integration costs in connection with the acquisition during fiscal year 2016, which were included in selling, general and administrative expenses.
Pro forma financial information as well as actual revenue and operating income (loss) have not been included because Blue Stream’s financial results are non-significant when compared with the Company’s consolidated financial results.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
WIL Research
On April 4, 2016, the Company acquired WIL Research, a provider of safety assessment and CDMO services to biopharmaceutical and agricultural and industrial chemical companies worldwide. The acquisition enhanced the Company’s position as a leading global early-stage CRO by strengthening its ability to partner with clients across the drug discovery and development continuum. The purchase price for WIL Research was $604.8 million, including assumed liabilities of $0.4 million. The purchase price includes payment for estimated working capital, which was subject to final adjustment based on the actual working capital of the acquired business. The acquisition was funded by cash on hand and borrowings on the Company’s amended credit facility. See Note 7, “Long-Term Debt and Capital Lease Obligations.” WIL Research’s safety assessment and CDMO businesses are reported in the Company’s DSA and Manufacturing reportable segments, respectively.
The purchase price allocation of $577.4 million, net of $27.4 million of cash acquired, was as follows:
|
| | | |
| | April 4, 2016 |
| | (in thousands) |
| Trade receivables (contractual amount of $48,625) | $ | 48,157 |
|
| Inventories | 2,296 |
|
| Other current assets (excluding cash) | 3,814 |
|
| Property, plant and equipment | 129,066 |
|
| Other long-term assets | 1,060 |
|
| Definite-lived intangible assets | 164,800 |
|
| Goodwill | 330,602 |
|
| Deferred revenue | (39,103 | ) |
| Other current liabilities | (27,386 | ) |
| Long-term liabilities | (35,915 | ) |
| Total purchase price allocation | $ | 577,391 |
|
The purchase price allocations are subject to change as additional information becomes available concerning the fair value and tax basis of the assets acquired and liabilities assumed. From the date of the acquisition through December 31, 2016, the Company recorded measurement-period adjustments related to the acquisition that resulted in an immaterial change to the purchase price allocation on a consolidated basis. Any additional adjustments to the purchase price allocation will be made as soon as practicable but no later than one year from the date of acquisition.
The breakout of definite-lived intangible assets acquired was as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 137,500 |
| | 15 |
| Developed technology | 20,700 |
| | 3 |
| Backlog | 6,600 |
| | 1 |
| Total definite-lived intangible assets | $ | 164,800 |
| | 13 |
The goodwill resulting from the transaction, $19.0 million of which is deductible for tax purposes due to a prior asset acquisition, is primarily attributed to the potential growth of the Company’s DSA and Manufacturing businesses from clients introduced through WIL Research, the assembled workforce of the acquired business, and expected cost synergies.
The Company incurred transaction and integration costs in connection with the acquisition of $15.5 million and $3.2 million during fiscal years 2016 and 2015, respectively, which were included in selling, general and administrative expenses.
WIL Research revenue and operating income from April 4, 2016 through December 31, 2016 was $176.1 million and $12.5 million, respectively. Beginning on April 4, 2016, WIL Research has been included in the operating results of the Company.
The following selected unaudited pro forma consolidated results of operations are presented as if the WIL Research acquisition had occurred as of the beginning of the period immediately preceding the period of acquisition after giving effect to certain adjustments. For fiscal year 2016, these adjustments included additional amortization of intangible assets and depreciation of
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
fixed assets of $0.4 million, reversal of interest expense on borrowings of $2.6 million, elimination of intercompany activity and other one-time costs, and the tax impacts of these adjustments. For fiscal year 2015, these adjustments included additional amortization of intangible assets and depreciation of fixed assets of $13.6 million, reversal of interest expense on borrowings of $10.5 million, inclusion of acquisition-related transaction costs of $11.5 million, elimination of intercompany activity and other one-time costs, and the tax impacts of these adjustments.
|
| | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 |
| | | | |
| | (in thousands, except per share amounts) |
| | (unaudited) |
| Revenue | $ | 1,741,964 |
| | $ | 1,578,133 |
|
| Net income attributable to common shareholders | 175,779 |
| | 153,974 |
|
| Earnings per common share: | | | |
| Basic | $ | 3.74 |
| | $ | 3.31 |
|
| Diluted | $ | 3.67 |
| | $ | 3.23 |
|
These unaudited pro forma results of operations have been prepared for comparative purposes only, and they do not purport to be indicative of the results of operations that actually would have resulted had the acquisition occurred on the date indicated or that may result in the future. No effect has been given for synergies, if any, that may have been realized through the acquisition.
Oncotest
On November 18, 2015, the Company acquired Oncotest GmbH (Oncotest), a German CRO providing discovery services for oncology, one of the largest therapeutic areas for biopharmaceutical research and development spending. With this acquisition, the Company has expanded its oncology services capabilities, enabling it to provide clients with access to a more comprehensive portfolio of technologies, including patient-derived xenograft (PDX) and syngeneic models. The purchase price for Oncotest was $36.0 million, including $0.3 million in contingent consideration. The acquisition was funded by borrowings on the Company's revolving credit facility. The business is reported in the Company’s DSA reportable segment.
The contingent consideration earn-out period ended in the fourth quarter of 2016. As a result, the related contingent consideration liability was reversed and a gain of $0.3 million was recorded in selling, general and administrative expenses, as no payments were expected to be made. The contingent consideration was a one-time payment that could have become payable based on the achievement of a revenue target for fiscal year 2016. If achieved, the payment would have become due in the first quarter of fiscal year 2017. The aggregate, undiscounted amount of contingent consideration that the Company could have paid was €2.0 million ($2.1 million as of December 31, 2016). The Company estimated the fair value of this contingent consideration based on a probability-weighted set of outcomes.
The purchase price allocation of $35.4 million, net of $0.6 million of cash acquired, was as follows:
|
| | | |
| | November 18, 2015 |
| | (in thousands) |
| Trade receivables (contractual amount of $3,546) | $ | 3,520 |
|
| Inventories | 129 |
|
| Other current assets (excluding cash) | 706 |
|
| Property, plant and equipment | 2,528 |
|
| Definite-lived intangible assets | 13,330 |
|
| Goodwill | 22,894 |
|
| Other long-term assets | 250 |
|
| Current liabilities | (3,456 | ) |
| Long-term liabilities | (4,470 | ) |
| Total purchase price allocation | $ | 35,431 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The breakout of definite-lived intangible assets acquired was as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 7,146 |
| | 19 |
| Developed technology | 5,960 |
| | 19 |
| Other intangible assets | 224 |
| | 3 |
| Total definite-lived intangible assets | $ | 13,330 |
| | 19 |
The goodwill resulting from the transaction is primarily attributed to the potential growth in the Company's DSA businesses from customers and technology introduced through Oncotest, the assembled workforce of the acquired business and expected cost synergies. The goodwill attributable to Oncotest is not deductible for tax purposes.
The Company incurred non-significant transaction and integration costs in connection with the acquisition during fiscal year 2016 and costs of $2.1 million during fiscal year 2015, which were included in selling, general and administrative expenses.
Pro forma financial information as well as actual revenue and operating income (loss) have not been included because Oncotest’s financial results are non-significant when compared with the Company’s consolidated financial results.
Celsis
On July 24, 2015, the Company acquired Celsis Group Limited (Celsis), a leading provider of rapid testing systems for non-sterile bacterial contamination for the biopharmaceutical and consumer products industries. The purpose of this acquisition was to enhance the Company’s portfolio of rapid microbial detection products and services with the addition of a rapid bioburden testing product. The purchase price for Celsis was $214.5 million, including assumed debt and certain liabilities of $10.3 million. The acquisition was funded by cash on hand and borrowings on the Company’s revolving credit facility. The business is reported in the Company’s Manufacturing reportable segment.
The purchase price allocation of $212.2 million, net of $2.3 million of cash acquired, was as follows:
|
| | | |
| | July 24, 2015 |
| | (in thousands) |
| Trade receivables (contractual amount of $5,410) | $ | 5,288 |
|
| Inventories | 10,103 |
|
| Other current assets (excluding cash) | 13,269 |
|
| Property, plant and equipment | 4,639 |
|
| Definite-lived intangible assets | 118,140 |
|
| Goodwill | 105,550 |
|
| Other long-term assets | 537 |
|
| Current debt | (9,766 | ) |
| Other current liabilities | (7,136 | ) |
| Long-term liabilities | (28,388 | ) |
| Total purchase price allocation | $ | 212,236 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The breakout of definite-lived intangible assets acquired was as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 71,000 |
| | 16 |
| Developed technology | 39,140 |
| | 14 |
| Trademark and trade names | 5,200 |
| | 14 |
| Non-compete | 2,800 |
| | 5 |
| Total definite-lived intangible assets | $ | 118,140 |
| | 15 |
The goodwill resulting from the transaction is primarily attributed to the potential growth of the Company’s Manufacturing business from clients introduced through Celsis, the assembled workforce of the acquired business and expected cost synergies. The goodwill attributable to Celsis is not deductible for tax purposes.
The Company incurred transaction and integration costs in connection with the acquisition of $1.0 million and $8.8 million during fiscal years 2016 and 2015, which were included in selling, general and administrative expenses.
Celsis revenue and operating loss from July 24, 2015 through December 26, 2015 was $11.1 million and $6.1 million, respectively. Beginning on July 24, 2015, Celsis has been included in the operating results of the Company.
The following selected unaudited pro forma consolidated results of operations are presented as if the Celsis acquisition had occurred as of the beginning of the period immediately preceding the period of acquisition after giving effect to certain nonrecurring costs and other adjustments, resulting in a reversal of $0.6 million and additional expenses of $13.1 million for fiscal years 2015 and 2014, respectively, related to depreciation and amortization of property, plant and equipment, inventory fair value adjustments and intangible assets.
|
| | | | | | | |
| | Fiscal Year |
| | 2015 | | 2014 |
| | | | |
| | (in thousands, except per share amounts) |
| | (unaudited) |
| Revenue | $ | 1,380,493 |
| | $ | 1,329,025 |
|
| Net income attributable to common shareholders | 162,672 |
| | 110,387 |
|
| Earnings per common share: | | | |
| Basic | $ | 3.50 |
| | $ | 2.37 |
|
| Diluted | $ | 3.42 |
| | $ | 2.32 |
|
These unaudited pro forma results of operations have been prepared for comparative purposes only, and they do not purport to be indicative of the results of operations that actually would have resulted had the acquisition occurred on the date indicated or that may result in the future. No effect has been given for synergies, if any, that may have been realized through the acquisition.
Sunrise
On May 5, 2015, the Company acquired Sunrise Farms, Inc. (Sunrise), a producer of specific-pathogen-free fertile chicken eggs and chickens used in the manufacture of live viruses. The purpose of this business acquisition was to expand the capabilities of the Company’s existing Avian Vaccine Services business. The purchase price of the acquisition was $9.6 million and was funded by cash on hand and borrowings on the Company’s revolving credit facility. The business is reported in the Company’s Manufacturing reportable segment.
The Company recorded a bargain purchase gain of $9.8 million, which represents the excess of the estimated fair value of the net assets acquired over the purchase price. The bargain purchase gain was recorded in other income (expense), net in the Company’s consolidated statement of income and was not recognized for tax purposes. The Company believes there were several factors that contributed to this transaction resulting in a bargain purchase gain, including the highly specialized nature of Sunrise’s business falling outside of the seller’s core activities and a limited pool of potential buyers.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Before recognizing the gain from the bargain purchase, the Company reassessed its initial identification and valuation of assets acquired and liabilities assumed to validate that all assets and liabilities that the Company was able to identify at the acquisition date were properly recognized.
The purchase price allocation of $9.6 million, net of less than $0.1 million of cash acquired, was as follows:
|
| | | |
| | May 5, 2015 |
| | (in thousands) |
| Trade receivables (contractual amount of $995) | $ | 965 |
|
| Inventories | 1,518 |
|
| Other current assets (excluding cash) | 973 |
|
| Property, plant and equipment | 13,698 |
|
| Definite-lived intangible assets | 3,400 |
|
| Current liabilities | (925 | ) |
| Long-term liabilities | (250 | ) |
| Fair value of net assets acquired | 19,379 |
|
| Bargain purchase gain | (9,821 | ) |
| Total purchase price allocation | $ | 9,558 |
|
The identifiable definite-lived intangible assets acquired represent the client relationships intangible, which is being amortized over the weighted average estimated useful life of approximately 15 years.
The Company incurred non-significant transaction and integration costs in connection with the acquisition during fiscal year 2016 and costs of $1.5 million during fiscal year 2015, which were included in selling, general and administrative expenses.
Pro forma financial information as well as actual revenue and operating income (loss) have not been included because Sunrise’s financial results are non-significant when compared with the Company’s consolidated financial results.
ChanTest
On October 29, 2014, the Company acquired ChanTest Corporation (ChanTest), a leading provider of ion channel testing services to the biopharmaceutical industry. The acquisition augments the Company’s early discovery capabilities and enhances the Company’s ability to support clients’ target discovery and lead optimization efforts. The purchase price of the acquisition was $59.2 million, including $0.3 million in contingent consideration, and was funded by borrowings on the Company’s revolving credit facility and cash on hand. The business is reported in the Company’s DSA reportable segment.
The contingent consideration earn-out period ended in the fourth quarter of 2015. As a result, the related contingent consideration liability was reversed and a gain of $0.3 million was recorded in selling, general and administrative expenses, as no payments were expected to be made. The aggregate, undiscounted amount of contingent consideration that could have become payable was $2.0 million. The Company estimated the fair value of this contingent consideration based on a probability-weighted set of outcomes.
The purchase price allocation of $52.0 million, net of $7.2 million in cash acquired, is as follows:
|
| | | |
| | October 29, 2014 |
| | (in thousands) |
| Current assets (excluding cash) | $ | 4,669 |
|
| Property, plant and equipment | 1,637 |
|
| Definite-lived intangible assets | 23,920 |
|
| Goodwill | 34,775 |
|
| Current liabilities | (3,486 | ) |
| Long-term liabilities | (9,486 | ) |
| Total purchase price allocation | $ | 52,029 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The breakout of definite-lived intangible assets acquired is as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 19,000 |
| | 13 |
| Other intangible assets | 4,920 |
| | 9 |
| Total definite-lived intangible assets | $ | 23,920 |
| | 12 |
The definite-lived intangibles are largely attributed to the expected cash flows related to client relationships existing at the acquisition closing date. The goodwill resulting from the transaction is primarily attributed to the potential growth of the business and is not deductible for tax purposes.
The Company incurred non-significant transaction and integration costs in connection with the acquisition during both fiscal years 2016 and 2015, and costs of $1.1 million during fiscal year 2014, which were included in selling, general and administrative expenses.
Pro forma financial information as well as actual revenue and operating income (loss) have not been included because ChanTest’s financial results are non-significant when compared with the Company’s consolidated financial results.
VivoPath
On June 16, 2014, the Company acquired substantially all of the assets of VivoPath LLC (VivoPath), a discovery services company specializing in the rapid, in vivo compound evaluation of molecules in the therapeutic areas of metabolism, inflammation and oncology. The purchase price was $2.3 million, including $1.6 million in contingent consideration, and was allocated primarily to the intangible assets acquired. The Company estimated the fair value of this contingent consideration based on a probability-weighted set of outcomes. The undiscounted total amount of contingent consideration was a maximum of $2.4 million, payable over three years based on the achievement of revenue growth targets and other contractual requirements. During fiscal year 2016, the Company paid the second year tranche of the contingent consideration of $0.2 million. During fiscal year 2015, the Company paid the first year tranche of the contingent consideration of $0.6 million and recorded a gain of $0.8 million, primarily due to a decrease in the expected future contingent consideration payments. As of December 31, 2016, the remaining contingent consideration payable is a maximum of $0.2 million. The business is reported in the Company’s DSA reportable segment.
Argenta and BioFocus
On April 1, 2014, the Company acquired (1) 100% of the shares of the U.K. based entities Argenta and BioFocus, and (2) certain Dutch assets. These businesses have formed the core of the Company’s Early Discovery business. With this acquisition, the Company has enhanced its position as a full service, early-stage CRO, with integrated in vitro and in vivo capabilities from target discovery through non-clinical development. The purchase price of the acquisition was $191.8 million, including $0.9 million in contingent consideration. The acquisition was funded by cash on hand and borrowings on the Company’s revolving credit facility. The businesses are reported in the Company’s DSA reportable segment.
The contingent consideration earn-out period ended on April 1, 2015. As a result, the related contingent consideration liability, as adjusted for subsequent changes in fair value, was reversed and a gain of $0.8 million was recorded in selling, general and administrative expenses during fiscal year 2015, as no payments were expected to be made. The contingent consideration was a one-time payment that could have become payable in the second quarter of 2015 based on the achievement of a certain revenue target for the twelve-month period following the acquisition. The aggregate, undiscounted amount of contingent consideration that the Company could have paid was €5.0 million ($5.3 million as of December 31, 2016). The Company estimated the fair value of this contingent consideration based on a probability-weighted set of outcomes.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The purchase price allocation of $183.6 million, net of $8.2 million of cash acquired, was as follows:
|
| | | |
| | April 1, 2014 |
| | (in thousands) |
| Current assets (excluding cash) | $ | 31,682 |
|
| Property, plant and equipment | 21,008 |
|
| Other long-term assets | 11,140 |
|
| Definite-lived intangible assets | 104,470 |
|
| Goodwill | 65,235 |
|
| Current liabilities | (13,139 | ) |
| Long-term liabilities | (36,802 | ) |
| Total purchase price allocation | $ | 183,594 |
|
The breakout of definite-lived intangible assets acquired was as follows:
|
| | | | | |
| | Definite-Lived Intangible Assets | | Weighted Average Amortization Life |
| | (in thousands) | | (in years) |
| Client relationships | $ | 94,000 |
| | 18 |
| Backlog | 5,900 |
| | 1 |
| Trademark and trade names | 1,170 |
| | 3 |
| Leasehold interests | 1,000 |
| | 13 |
| Other intangible assets | 2,400 |
| | 19 |
| Total definite-lived intangible assets | $ | 104,470 |
| | 17 |
The goodwill resulting from the transaction is primarily attributed to the potential growth of the Company’s DSA businesses from clients introduced through Argenta and BioFocus, the assembled workforce of the acquired businesses and expected cost synergies. The goodwill attributable to Argenta and BioFocus is not deductible for tax purposes.
The Company incurred non-significant transaction and integration costs in connection with the acquisition during both fiscal years 2016 and 2015, and costs of $5.3 million during fiscal year 2014, which were included in selling, general and administrative expenses.
Argenta and BioFocus revenue and operating income for fiscal year 2014 were $71.4 million and $1.8 million, respectively. Beginning on April 1, 2014, Argenta and BioFocus have been included in the operating results of the Company.
The following selected unaudited pro forma consolidated results of operations are presented as if the Argenta and BioFocus acquisition had occurred as of the beginning of the period immediately preceding the period of acquisition after giving effect to certain adjustments, including amortization of intangible assets and depreciation of fixed assets of $3.7 million and other nonrecurring costs.
|
| | | |
| | Fiscal Year |
| | 2014 |
| | (in thousands, except per share amounts) |
| | (unaudited) |
| Revenue | $ | 1,322,771 |
|
| Net income attributable to common shareholders | 128,195 |
|
| Earnings per common share: | |
| Basic | $ | 2.75 |
|
| Diluted | $ | 2.70 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
These unaudited pro forma results of operations have been prepared for comparative purposes only, and they do not purport to be indicative of the results of operations that actually would have resulted had the acquisition occurred on the date indicated or that may result in the future. No effect has been given for synergies, if any, that may have been realized through the acquisition.
3. SUPPLEMENTAL BALANCE SHEET INFORMATION
The composition of trade receivables, net is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Client receivables | $ | 283,997 |
| | $ | 230,010 |
|
| Unbilled revenue | 82,203 |
| | 45,996 |
|
| Total | 366,200 |
| | 276,006 |
|
| Less: Allowance for doubtful accounts | (2,150 | ) | | (5,938 | ) |
| Trade receivables, net | $ | 364,050 |
| | $ | 270,068 |
|
Recoveries to the allowance for doubtful accounts in fiscal year 2016 were $0.5 million. Provisions to the allowance for doubtful accounts in fiscal years 2015 and 2014 were $1.8 million and $0.5 million, respectively.
The composition of inventories is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Raw materials and supplies | $ | 18,893 |
| | $ | 15,998 |
|
| Work in process | 13,681 |
| | 12,101 |
|
| Finished products | 63,259 |
| | 65,636 |
|
| Inventories | $ | 95,833 |
| | $ | 93,735 |
|
The composition of other current assets is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Investments | $ | 3,771 |
| | $ | 20,516 |
|
| Prepaid income tax | 40,705 |
| | 26,350 |
|
| Restricted cash | 532 |
| | 271 |
|
| Other | — |
| | 149 |
|
| Other current assets | $ | 45,008 |
| | $ | 47,286 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The composition of property, plant and equipment, net is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Land | $ | 47,392 |
| | $ | 39,846 |
|
Buildings (1) | 784,129 |
| | 713,841 |
|
| Machinery and equipment | 403,123 |
| | 362,695 |
|
| Leasehold improvements | 47,071 |
| | 41,477 |
|
| Furniture and fixtures | 24,148 |
| | 21,783 |
|
| Computer hardware and software | 127,283 |
| | 113,466 |
|
| Vehicles | 4,118 |
| | 3,819 |
|
| Construction in progress | 24,703 |
| | 25,845 |
|
| Total | 1,461,967 |
| | 1,322,772 |
|
| Less: Accumulated depreciation | (706,140 | ) | | (644,813 | ) |
| Property, plant and equipment, net | $ | 755,827 |
| | $ | 677,959 |
|
(1) The balances as of December 31, 2016 and December 26, 2015 include capital lease buildings. See Note 7, “Long-Term Debt and Capital Lease Obligations.”
Depreciation expense in fiscal years 2016, 2015 and 2014 was $85.0 million, $70.7 million and $70.5 million, respectively.
The composition of other assets is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Life insurance policies | $ | 29,456 |
| | $ | 27,554 |
|
| Venture capital investments | 45,331 |
| | 32,730 |
|
| Restricted cash | 1,736 |
| | 1,745 |
|
| Other | 11,907 |
| | 9,614 |
|
| Other assets | $ | 88,430 |
| | $ | 71,643 |
|
The composition of other current liabilities is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Accrued income taxes | $ | 25,621 |
| | $ | 12,168 |
|
| Other | 879 |
| | 376 |
|
| Other current liabilities | $ | 26,500 |
| | $ | 12,544 |
|
The composition of other long-term liabilities is as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Long-term pension liability | $ | 89,984 |
| | $ | 34,604 |
|
| Accrued executive supplemental life insurance retirement plan and deferred compensation plan | 32,880 |
| | 30,188 |
|
| Other | 36,375 |
| | 24,270 |
|
| Other long-term liabilities | $ | 159,239 |
| | $ | 89,062 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
4. VENTURE CAPITAL INVESTMENTS AND MARKETABLE SECURITIES
Venture Capital Investments
During fiscal years 2016, 2015, and 2014, the Company recognized gains related to the venture capital investments of $10.3 million, $3.8 million and $9.3 million, respectively. The Company’s total commitment to these entities as of December 31, 2016 was $84.8 million, of which the Company had funded $38.2 million as of December 31, 2016. During fiscal years 2016, 2015, and 2014, the Company received dividends totaling $7.1 million, $7.3 million, and $7.4 million, respectively. As of December 31, 2016 and December 26, 2015, the Company’s consolidated retained earnings (accumulated deficit) included $4.4 million and $2.4 million, respectively, of the undistributed earnings related to these entities.
Marketable Securities
The Company held no marketable securities as of December 31, 2016.
The following is a summary of the Company’s marketable securities, all of which are classified as available-for-sale, as of December 26, 2015:
|
| | | | | | | | | | | | | | | |
| | Amortized Cost | | Gross Unrealized Gains | | Gross Unrealized Losses | | Fair Value |
| | | | | | | | |
| | (in thousands) |
| Mutual fund | $ | 4,650 |
| | $ | — |
| | $ | (141 | ) | | $ | 4,509 |
|
| Total | $ | 4,650 |
| | $ | — |
| | $ | (141 | ) | | $ | 4,509 |
|
During fiscal year 2016, the Company realized non-significant losses and received proceeds of $4.6 million from the sale of its available-for-sale securities. There were no sales of available-for-sale securities during fiscal year 2015.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
5. FAIR VALUE
Assets, liabilities, and redeemable noncontrolling interest measured at fair value on a recurring basis are summarized below:
|
| | | | | | | | | | | | | | | |
| | December 31, 2016 |
| | Level 1 | | Level 2 | | Level 3 | | Total |
| | | | | | | | |
| | (in thousands) |
| Cash equivalents | $ | — |
| | $ | 21 |
| | $ | — |
| | $ | 21 |
|
| Other assets: | | | | | | | |
| Life insurance policies | — |
| | 22,121 |
| | — |
| | 22,121 |
|
| Total assets measured at fair value | $ | — |
| | $ | 22,142 |
| | $ | — |
| | $ | 22,142 |
|
| | | | | | | | |
| Other current liabilities: | | | | | | | |
| Contingent consideration | $ | — |
| | $ | — |
| | $ | 3,621 |
| | $ | 3,621 |
|
| Total liabilities measured at fair value | $ | — |
| | $ | — |
| | $ | 3,621 |
| | $ | 3,621 |
|
|
| | | | | | | | | | | | | | | |
| | December 26, 2015 |
| | Level 1 | | Level 2 | | Level 3 | | Total |
| | | | | | | | |
| | (in thousands) |
| Cash equivalents | $ | — |
| | $ | 190 |
| | $ | — |
| | $ | 190 |
|
| Other current assets: | | | | | | | |
| Marketable securities | 4,509 |
| | — |
| | — |
| | 4,509 |
|
| Foreign currency forward contracts | — |
| | 15 |
| | — |
| | 15 |
|
| Other assets: | | | | | | | |
| Life insurance policies | — |
| | 20,364 |
| | — |
| | 20,364 |
|
| Total assets measured at fair value | $ | 4,509 |
| | $ | 20,569 |
| | $ | — |
| | $ | 25,078 |
|
| | | | | | | | |
| Other current liabilities: | | | | | | | |
| Contingent consideration | $ | — |
| | $ | — |
| | $ | 1,172 |
| | $ | 1,172 |
|
| Other long-term liabilities: | | | | | | | |
| Contingent consideration | — |
| | — |
| | 198 |
| | 198 |
|
| Redeemable noncontrolling interest | — |
| | — |
| | 28,008 |
| | 28,008 |
|
| Total liabilities and redeemable noncontrolling interest measured at fair value | $ | — |
| | $ | — |
| | $ | 29,378 |
| | $ | 29,378 |
|
During fiscal years 2016 and 2015, there were no transfers between fair value levels.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Contingent Consideration
The following table provides a rollforward of the contingent consideration related to previous business acquisitions. See Note 2, “Business Acquisitions.”
|
| | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 |
| | | | |
| | (in thousands) |
| Beginning balance | $ | 1,370 |
| | $ | 2,828 |
|
| Additions | 3,600 |
| | 973 |
|
| Payments | (872 | ) | | (600 | ) |
| Total gains or losses (realized/unrealized): | | | |
| Reversal of previously recorded contingent liability and change in fair value | (477 | ) | | (1,831 | ) |
| Ending balance | $ | 3,621 |
| | $ | 1,370 |
|
The unobservable inputs used in the fair value measurement of the Company’s contingent consideration are the probabilities of successful achievement of certain financial targets and a discount rate. Increases or decreases in any of the probabilities of success would result in a higher or lower fair value measurement, respectively. Increases or decreases in the discount rate would result in a lower or higher fair value measurement, respectively.
Debt Instruments
The book value of the Company’s term and revolving loans, which are variable rate loans carried at amortized cost, approximates their fair value based on current market pricing of similar debt. As the fair value is based on significant other observable inputs, including current interest and foreign currency exchange rates, it is deemed to be Level 2 within the fair value hierarchy.
Redeemable Noncontrolling Interest
The Company’s redeemable noncontrolling interest resulted from the acquisition of an interest in Vital River in January 2013 and July 2016.
The following table provides a rollforward of the fair value of the Company’s redeemable noncontrolling interest for fiscal year 2015:
|
| | | |
| | Redeemable Noncontrolling Interest |
| | (in thousands) |
| December 27, 2014 | $ | 28,419 |
|
| Total gains or losses (realized/unrealized): | |
| Net income attributable to noncontrolling interest | 838 |
|
| Foreign currency translation | (1,066 | ) |
| Change in fair value, included in additional paid-in capital | (183 | ) |
| December 26, 2015 | 28,008 |
|
| Total gains or losses (realized/unrealized): | |
| Net income attributable to noncontrolling interest | 320 |
|
| Foreign currency translation | (653 | ) |
| Change in fair value, included in additional paid-in capital | (1,690 | ) |
| July 7, 2016 | $ | 25,985 |
|
Since July 7, 2016, the redeemable noncontrolling interest is no longer reported at fair value. See Note 8, “Equity and Redeemable Noncontrolling Interest.”
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
6. GOODWILL AND INTANGIBLE ASSETS
Goodwill
The following table provides a rollforward of the Company’s goodwill:
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Adjustments to Goodwill | | | | Adjustments to Goodwill | | |
| | December 27, 2014 | | Acquisitions | | Foreign Exchange | | December 26, 2015 | | Acquisitions | | Transfers | | Foreign Exchange | | December 31, 2016 |
| | | | | | | | | | | | | | | | |
| | (in thousands) |
| RMS | $ | 59,196 |
| | $ | — |
| | $ | (1,029 | ) | | $ | 58,167 |
| | $ | — |
| | $ | (342 | ) | | $ | (1,428 | ) | | $ | 56,397 |
|
| DSA | 1,234,302 |
| | 22,146 |
| | (4,398 | ) | | 1,252,050 |
| | 337,872 |
| | — |
| | (21,446 | ) | | 1,568,476 |
|
| Manufacturing | 32,579 |
| | 105,567 |
| | (4,534 | ) | | 133,612 |
| | 46,859 |
| | 342 |
| | (13,169 | ) | | 167,644 |
|
| Gross carrying amount | 1,326,077 |
| |
|
| |
|
| | 1,443,829 |
| |
|
| |
|
| |
|
| | 1,792,517 |
|
| Accumulated impairment loss - DSA | (1,005,000 | ) | | — |
| | — |
| | (1,005,000 | ) | | — |
| | — |
| | — |
| | (1,005,000 | ) |
| Goodwill | $ | 321,077 |
| |
|
| |
|
| | $ | 438,829 |
| |
|
| | | |
|
| | $ | 787,517 |
|
During the second quarter of 2016, the Company revised the composition of its reportable segments to align with the view of the business following its acquisition of WIL Research. See Note 1, "Description of Business and Summary of Significant Accounting Policies." As a result, goodwill was allocated from the Company's RMS reportable segment to its Manufacturing reportable segment, as shown in the preceding table within "transfers." The allocation was based on the fair value of each business group within its original reporting unit relative to the fair value of that reporting unit. In addition, the Company completed an assessment of any potential goodwill impairment for all reporting units immediately prior to the reallocation and determined that no impairment existed.
Based on the Company’s step one goodwill impairment test for fiscal years 2016, 2015 and 2014, the fair value of each reporting unit exceeded the reporting unit’s book value and, therefore, goodwill was not impaired.
Intangible Assets, Net
The following table displays intangible assets, net by major class:
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | Gross | | Accumulated
Amortization | | Net | | Gross | | Accumulated
Amortization | | Net |
| | | | | | | | | | | | |
| | (in thousands) |
| Backlog | $ | 8,370 |
| | $ | (6,390 | ) | | $ | 1,980 |
| | $ | 50,568 |
| | $ | (50,554 | ) | | $ | 14 |
|
| Technology | 71,425 |
| | (14,314 | ) | | 57,111 |
| | 60,350 |
| | (5,911 | ) | | 54,439 |
|
| Trademarks and trade names | 8,177 |
| | (4,124 | ) | | 4,053 |
| | 11,495 |
| | (5,944 | ) | | 5,551 |
|
| Other | 16,775 |
| | (5,628 | ) | | 11,147 |
| | 14,711 |
| | (7,285 | ) | | 7,426 |
|
| Other intangible assets | 104,747 |
| | (30,456 | ) | | 74,291 |
| | 137,124 |
| | (69,694 | ) | | 67,430 |
|
| Client relationships | 519,123 |
| | (198,966 | ) | | 320,157 |
| | 396,537 |
| | (183,163 | ) | | 213,374 |
|
| Intangible assets | $ | 623,870 |
| | $ | (229,422 | ) | | $ | 394,448 |
| | $ | 533,661 |
| | $ | (252,857 | ) | | $ | 280,804 |
|
During fiscal year 2016, the Company determined that the carrying values of certain DSA intangible assets were not recoverable and recorded an impairment charge of $1.9 million, which was included in costs of services provided (excluding amortization of intangible assets).
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Amortization expense of definite-lived intangible assets, including client relationships, for fiscal years 2016, 2015 and 2014 was $41.7 million, $24.2 million and $26.0 million, respectively. Estimated amortization expense for intangible assets for each of the next five fiscal years is expected to be as follows:
|
| | | | |
| Fiscal Year | | Amortization Expense |
| | | (in thousands) |
| 2017 | | $ | 42,525 |
|
| 2018 | | 40,731 |
|
| 2019 | | 34,995 |
|
| 2020 | | 34,382 |
|
| 2021 | | 32,994 |
|
7. LONG-TERM DEBT AND CAPITAL LEASE OBLIGATIONS
Long-Term Debt
Long-term debt, net consists of the following:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Term loans | $ | 633,750 |
| | $ | 390,000 |
|
| Revolving credit facility | 578,759 |
| | 446,041 |
|
| Other long-term debt | 185 |
| | 193 |
|
| Total debt | 1,212,694 |
| | 836,234 |
|
| Less: Current portion of long-term debt | (24,560 | ) | | (15,193 | ) |
| Long-term debt | 1,188,134 |
| | 821,041 |
|
| Debt discount and debt issuance costs | (7,633 | ) | | (6,805 | ) |
| Long-term debt, net | $ | 1,180,501 |
| | $ | 814,236 |
|
As of December 31, 2016 and December 26, 2015, the weighted average interest rate on the Company’s debt was 1.89% and 1.33%, respectively.
In April 2015, the Company amended and restated the $970M Credit Facility, creating a $1.3 billion facility ($1.3B Credit Facility) that provides for a $400.0 million term loan facility and a $900.0 million multi-currency revolving facility. The interest rates applicable to term loans and revolving loans under the Company’s $1.3B Credit Facility were, at the Company’s option, equal to either the alternate base rate (which is the higher of (1) the prime rate, (2) the federal funds rate plus 0.5% or (3) the one-month adjusted LIBOR rate plus 1%) or the adjusted LIBOR rate, plus an interest rate margin based upon the Company’s leverage ratio.
On March 30, 2016, the Company amended and restated its $1.3B credit facility creating a $1.65 billion credit facility ($1.65B Credit Facility) which (1) extends the maturity date for the credit facility and (2) makes certain other amendments in connection with the Company’s acquisition of WIL Research. The amendment was accounted for as a debt modification with a partial extinguishment of debt. In connection with the transaction, the Company capitalized approximately $3.3 million and expensed approximately $1.4 million of debt issuance costs.
The $1.65B Credit Facility provides for a $650.0 million term loan and a $1.0 billion multi-currency revolving facility. The term loan facility matures in 19 quarterly installments with the last installment due March 30, 2021. The revolving facility matures on March 30, 2021, and requires no scheduled payment before that date. Under specified circumstances, the Company has the ability to increase the term loan and/or revolving line of credit by up to $500 million in the aggregate.
The interest rates applicable to term loan and revolving loans under the $1.65B Credit Facility are, at the Company’s option, equal to either the base rate (which is the higher of (1) the prime rate, (2) the federal funds rate plus 0.50%, or (3) the one-month adjusted LIBOR rate plus 1%) or the adjusted LIBOR rate, plus an interest rate margin based upon the Company’s leverage ratio.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The $1.65B Credit Facility includes certain customary representations and warranties, events of default, notices of material adverse changes to the Company’s business and negative and affirmative covenants. These covenants include (1) maintenance of a ratio of consolidated earnings before interest, taxes, depreciation and amortization (EBITDA) less capital expenditures to consolidated cash interest expense, for any period of four consecutive fiscal quarters, of no less than 3.50 to 1.0 as well as (2) maintenance of a ratio of consolidated indebtedness to consolidated EBITDA for any period of four consecutive fiscal quarters, of no more than 4.25 to 1.0 with step downs to 3.50 to 1.0 by the last day of the fourth quarter of 2017. As of December 31, 2016, the Company was compliant with all covenants.
The obligations of the Company under the $1.65B Credit Facility are collateralized by substantially all of the assets of the Company.
Principal maturities of existing debt for the periods set forth in the table below, are as follows:
|
| | | | |
| | | Principal |
| | | (in thousands) |
| 2017 | | $ | 24,560 |
|
| 2018 | | 36,563 |
|
| 2019 | | 52,813 |
|
| 2020 | | 81,250 |
|
| 2021 | | 1,017,508 |
|
| Total | | $ | 1,212,694 |
|
Letters of Credit
As of both December 31, 2016 and December 26, 2015, the Company had $4.9 million outstanding under letters of credit.
Capital Lease Obligations
The Company acquired a build-to-suit lease as part of its acquisition of Argenta and BioFocus. In accordance with accounting guidance applicable to entities involved with the construction of an asset that will be leased when the construction is completed, the Company was considered the owner, for accounting purposes, of this property during the construction period. Accordingly, the Company recorded an asset and a corresponding financing obligation on its consolidated balance sheet for the amount of total project costs incurred related to the construction in progress for this property through completion of the construction period. Upon completion of the construction during the second quarter of fiscal year 2015, the Company determined that it was no longer considered the owner of the property because it did not have continuing involvement. Consequently, the Company recorded a successful sale leaseback and derecognized the property and the associated financing obligation from the Company’s consolidated balance sheet and recorded a capital lease asset and a corresponding liability of $35.8 million.
As of December 31, 2016, the minimum lease payments under capital leases for each of the next five years and total thereafter were as follows:
|
| | | | |
| | | Minimum Lease Payments |
| | | (in thousands) |
| 2017 | | $ | 4,097 |
|
| 2018 | | 3,503 |
|
| 2019 | | 3,005 |
|
| 2020 | | 2,385 |
|
| 2021 | | 2,250 |
|
| Thereafter | | 27,974 |
|
| Total | | $ | 43,214 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
8. EQUITY AND REDEEMABLE NONCONTROLLING INTEREST
Earnings Per Share
The following table reconciles the numerator and denominator in the computations of basic and diluted earnings per share:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Numerator: | | | | | |
| Income from continuing operations, net of income taxes | $ | 156,086 |
| | $ | 152,037 |
| | $ | 129,924 |
|
| Income (loss) from discontinued operations, net of income taxes | 280 |
| | (950 | ) | | (1,726 | ) |
| Less: Net income attributable to noncontrolling interests | 1,601 |
| | 1,774 |
| | 1,500 |
|
| Net income attributable to common shareholders | $ | 154,765 |
| | $ | 149,313 |
| | $ | 126,698 |
|
| | | | | | |
| Denominator: | | | | | |
| Weighted-average shares outstanding—Basic | 47,014 |
| | 46,496 |
| | 46,627 |
|
| Effect of dilutive securities: | | | | | |
| Stock options, restricted stock units, performance share units and restricted stock | 944 |
| | 1,138 |
| | 931 |
|
| Weighted-average shares outstanding—Diluted | 47,958 |
| | 47,634 |
| | 47,558 |
|
Options to purchase approximately 0.8 million shares, 0.5 million shares, and 0.6 million shares for fiscal years 2016, 2015 and 2014, respectively, as well as a non-significant number of restricted stock, restricted stock units (RSUs), and performance share units (PSUs), were not included in computing diluted earnings per share because their inclusion would have been anti-dilutive. Basic weighted average shares outstanding for fiscal years 2016, 2015 and 2014 excluded the impact of approximately 1.1 million shares, 1.1 million shares, and 1.2 million shares, respectively, of non-vested restricted stock, restricted stock units, and PSUs.
Treasury Shares
In July 2010, the Company’s Board of Directors authorized a $500.0 million stock repurchase program, and subsequently approved increases to the stock repurchase program of $250.0 million in 2010, $250.0 million in 2013 and $150.0 million in 2014, for an aggregate authorization of $1,150.0 million. Under its authorized stock repurchase program, the Company did not repurchase any shares in fiscal year 2016, and repurchased approximately 1.5 million shares for $108.8 million and approximately 2.1 million shares for $110.6 million in fiscal years 2015 and 2014, respectively. As of December 31, 2016, the Company had $69.7 million remaining on the authorized stock repurchase program. In addition, the Company’s stock-based compensation plans permit the netting of common stock upon vesting of restricted stock, restricted stock units and performance share units in order to satisfy individual minimum statutory tax withholding requirements. The Company acquired approximately 0.2 million shares for $12.3 million, approximately 0.1 million shares for $8.7 million, and approximately 0.1 million shares for $6.8 million in fiscal years 2016, 2015 and 2014, respectively, to satisfy individual minimum statutory tax withholding requirements.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Accumulated Other Comprehensive Income (Loss)
Changes to each component of accumulated other comprehensive income (loss), net of income taxes, are as follows:
|
| | | | | | | | | | | |
| | Foreign Currency Translation and Other(3) | | Pension and Other Post-Retirement Benefit Plans | | Total |
| | | | | | |
| | (in thousands) |
| December 27, 2014 | $ | (19,891 | ) | | $ | (54,356 | ) | | $ | (74,247 | ) |
Other comprehensive loss before reclassifications (1) | (60,745 | ) | | (302 | ) | | (61,047 | ) |
| Amounts reclassified from accumulated other comprehensive income (loss) | (2,341 | ) | | 2,617 |
| | 276 |
|
| Net current period other comprehensive income (loss) | (63,086 | ) | | 2,315 |
| | (60,771 | ) |
| Income tax expense | — |
| | 530 |
| | 530 |
|
| December 26, 2015 | (82,977 | ) | | (52,571 | ) | | (135,548 | ) |
Other comprehensive loss before reclassifications (2) | (71,618 | ) | | (60,678 | ) | | (132,296 | ) |
| Amounts reclassified from accumulated other comprehensive income (loss) | — |
| | 1,711 |
| | 1,711 |
|
| Net current period other comprehensive income (loss) | (71,618 | ) | | (58,967 | ) | | (130,585 | ) |
| Income tax expense (benefit) | — |
| | (12,369 | ) | | (12,369 | ) |
| December 31, 2016 | $ | (154,595 | ) | | $ | (99,169 | ) | | $ | (253,764 | ) |
(1) The impact of the foreign currency translation adjustment to other comprehensive income (loss) before reclassifications for fiscal year 2015 was primarily due to the effect of changes in foreign currency exchange rates of the Euro and Canadian Dollar and to a lesser extent due to the impact of changes in the British Pound. |
(2) The impact of the foreign currency translation adjustment to other comprehensive income (loss) before reclassifications for fiscal year 2016 was primarily due to the effect of changes in foreign currency exchange rates of the Euro, British Pound, and Canadian Dollar and to a lesser extent due to the impact of changes in the Chinese Yuan Renminbi and Japanese Yen. |
(3) Foreign currency translation and other includes a non-significant amount of unrealized gains (losses) on available-for-sale marketable securities. |
Nonredeemable Noncontrolling Interests
The Company has investments in several entities, whose financial results are consolidated in the Company’s financial statements, as it has the ability to exercise control over these entities. The interests of the respective noncontrolling parties in these entities have been recorded as nonredeemable noncontrolling interests.
Redeemable Noncontrolling Interest
In January 2013, the Company acquired a 75% ownership interest of Vital River, a commercial provider of research models and related services in China, for $24.2 million, net of $2.7 million of cash acquired. Concurrent with the acquisition, the Company entered into an agreement with the noncontrolling interest holders that provided the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining 25% of the entity for cash at its fair value beginning in January 2016.
On July 7, 2016, the Company purchased an additional 12% equity interest in Vital River for $10.8 million, resulting in total ownership of 87%. The Company recorded a $1.6 million gain in equity equal to the excess fair value of the 12% equity interest over the purchase price. Concurrent with the transaction, the original agreement was amended providing the Company with the right to purchase, and the noncontrolling interest holders with the right to sell, the remaining 13% equity interest at a contractually defined redemption value, subject to a redemption floor (embedded derivative). These rights are exercisable beginning in 2019 and are accelerated in certain events. The Company recorded a charge of $1.5 million in other income (expense), net, equal to the excess fair value of the hybrid instrument (equity interest with an embedded derivative) over the fair value of the 13% equity interest. The redeemable noncontrolling interest is measured at the greater of the amount that would be paid if settlement occurred as of the balance sheet date based on the contractually defined redemption value ($14.1 million as of December 31, 2016) and its carrying amount adjusted for net income (loss) attributable to the noncontrolling interest. As the
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
noncontrolling interest holders have the ability to require the Company to purchase the remaining 13% interest, the noncontrolling interest is classified in the mezzanine section of the consolidated balance sheet, which is presented above the equity section and below liabilities. The agreement does not limit the amount that the Company could be required to pay to purchase the remaining 13% equity interest.
The following table provides a rollforward of the Company’s redeemable noncontrolling interest subsequent to the acquisition of the additional 12% equity interest on July 7, 2016:
|
| | | |
| | Redeemable Noncontrolling Interest |
| | (in thousands) |
| July 7, 2016 | $ | 25,985 |
|
| Purchase of 12% equity interest | (12,360 | ) |
| Total gains or losses (realized/unrealized): | |
| Net income attributable to noncontrolling interest | 357 |
|
| Foreign currency translation | (818 | ) |
| Modification of 13% purchase option | 1,495 |
|
| December 31, 2016 | $ | 14,659 |
|
See Note 5, “Fair Value,” for the activity within the redeemable noncontrolling interest prior to July 7, 2016.
9. INCOME TAXES
The components of income from continuing operations before income taxes and the related provision for income taxes are presented below:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Income from continuing operations before income taxes: | |
| | | | |
| U.S. | $ | 59,255 |
| | $ | 76,157 |
| | $ | 71,002 |
|
| Non-U.S. | 163,666 |
| | 119,271 |
| | 106,593 |
|
| | $ | 222,921 |
| | $ | 195,428 |
| | $ | 177,595 |
|
| Income tax provision: | |
| | | | |
| Current: | |
| | | | |
| Federal | $ | 18,592 |
| | $ | 23,687 |
| | $ | 13,733 |
|
| Foreign | 39,829 |
| | 8,572 |
| | 20,364 |
|
| State | 5,263 |
| | 6,819 |
| | 4,746 |
|
| Total current | 63,684 |
| | 39,078 |
| | 38,843 |
|
| Deferred: | |
| | | | |
| Federal | 7,206 |
| | 1,790 |
| | 12,982 |
|
| Foreign | (4,024 | ) | | 3,064 |
| | (4,672 | ) |
| State | (31 | ) | | (541 | ) | | 518 |
|
| Total deferred | 3,151 |
| | 4,313 |
| | 8,828 |
|
| | $ | 66,835 |
| | $ | 43,391 |
| | $ | 47,671 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The components of deferred tax assets and liabilities are as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Deferred tax assets: | | | |
| Compensation | $ | 70,863 |
| | $ | 55,259 |
|
| Accruals and reserves | 8,103 |
| | 8,941 |
|
| Inventory reserves and valuations | 3,447 |
| | 2,022 |
|
| Financing related | — |
| | 902 |
|
| Net operating loss and credit carryforwards | 58,081 |
| | 35,233 |
|
| Other | 2,921 |
| | 2,593 |
|
| Valuation allowance | (10,101 | ) | | (6,112 | ) |
| Total deferred tax assets | 133,314 |
| | 98,838 |
|
| Deferred tax liabilities: | | | |
| Goodwill and other intangibles | (121,256 | ) | | (73,208 | ) |
| Financing related | (854 | ) | | — |
|
| Depreciation related | (32,271 | ) | | (23,664 | ) |
| Venture capital investments | (5,084 | ) | | (3,570 | ) |
| Foreign withholding taxes | (821 | ) | | (6,590 | ) |
| Total deferred tax liabilities | (160,286 | ) | | (107,032 | ) |
| Net deferred taxes | $ | (26,972 | ) | | $ | (8,194 | ) |
Reconciliations of the statutory U.S. Federal income tax rate to effective tax rates are as follows:
|
| | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| U.S. statutory income tax rate | 35.0 | % | | 35.0 | % | | 35.0 | % |
| Foreign tax rate differences | (10.3 | )% | | (8.6 | )% | | (9.4 | )% |
| State income taxes, net of Federal tax benefit | 1.6 | % | | 1.9 | % | | 1.9 | % |
| Research tax credits and enhanced deductions | (3.5 | )% | | (2.6 | )% | | (4.1 | )% |
| Enacted tax rate changes | (0.8 | )% | | (1.5 | )% | | — | % |
| Impact of tax uncertainties | 0.2 | % | | (5.2 | )% | | (0.7 | )% |
| Foreign withholding taxes | 2.0 | % | | 3.4 | % | | — | % |
| Impact of acquisitions and restructuring | 1.8 | % | | (2.0 | )% | | 1.6 | % |
| Other | 4.0 | % | | 1.8 | % | | 2.5 | % |
| Effective income tax rate | 30.0 | % | | 22.2 | % | | 26.8 | % |
The tax rate benefit for enacted tax rate changes is primarily associated with a reduction in the U.K.’s statutory tax rates.
As of December 31, 2016, the Company had foreign net operating loss and tax credit carryforwards of $58.5 million, as compared to $34.6 million as of December 26, 2015. Of this amount, $5.2 million will expire beginning after 2016, $40.5 million will begin to expire in 2028 and beyond, and the remainder of $12.8 million can be carried forward indefinitely. In accordance with Canadian Federal tax law, the Company claims Scientific Research and Experimental Development (SR&ED) credits on qualified research and development costs incurred in its Safety Assessment facility in Montreal, and currently maintains $16.8 million in credit carryforwards, which will begin to expire in 2033. Additionally, the Company records a benefit to operating income for research and development credits in both Quebec and the U.K. related to its Safety Assessment and Early Discovery facilities.
The Company has fully recognized its deferred tax assets on the belief that it is more likely than not that they will be realized. The only exceptions relate to deferred tax assets primarily for net operating losses in France, Hong Kong, Luxembourg, the
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Netherlands and Germany, capital losses in the U.S., and fixed assets in the U.K. The valuation allowance increased by $4.0 million from $6.1 million as of December 26, 2015 to $10.1 million as of December 31, 2016.
A reconciliation of the Company’s beginning and ending unrecognized income tax benefits is as follows:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Beginning balance | $ | 23,338 |
| | $ | 34,627 |
| | $ | 18,475 |
|
| Additions to tax positions for current year | 2,194 |
| | 2,362 |
| | 1,700 |
|
| Additions to tax positions for prior years | 2,035 |
| | 3,028 |
| | 18,502 |
|
| Reductions to tax positions for current year | — |
| | — |
| | — |
|
| Reductions to tax positions for prior years | (1,866 | ) | | (3,991 | ) | | (3,722 | ) |
| Settlements | (918 | ) | | (1,946 | ) | | (308 | ) |
| Expiration of statute of limitations | (597 | ) | | (10,742 | ) | | (20 | ) |
| Ending balance | $ | 24,186 |
| | $ | 23,338 |
| | $ | 34,627 |
|
The $0.8 million increase in unrecognized income tax benefits during fiscal year 2016 is primarily attributable to pre-acquisition tax positions taken by WIL Research, as well as an additional year of Canadian SR&ED credit, offset by a settlement related to the tax year ended 2014 for Canadian SR&ED credits and favorable foreign exchange movement.
The amount of unrecognized income tax benefits that, if recognized, would favorably impact the effective tax rate was $21.4 million as of December 31, 2016 and $20.1 million as of December 26, 2015. The $1.3 million increase is primarily pre-acquisition tax positions taken by WIL Research, as well as an additional year of Canadian SR&ED credit, offset by favorable foreign exchange movement. It is reasonably possible as of December 31, 2016 that the liability for unrecognized tax benefits for the uncertain tax position will decrease by $4.6 million over the next twelve month period, primarily as a result of the outcome of a pending tax ruling and competent authority proceedings. The Company continues to recognize interest and penalties related to unrecognized income tax benefits in income tax expense. The total amount of accrued interest related to unrecognized income tax benefits as of December 31, 2016 and December 26, 2015 was $1.7 million and $1.0 million, respectively. The total amount of accrued penalties related to unrecognized income tax benefits as of December 31, 2016 was $0.2 million.
The Company conducts business in a number of tax jurisdictions. As a result, it is subject to tax audits on a regular basis including, but not limited to, such major jurisdictions as the U.S., the U.K., China, Japan, France, Germany, and Canada. With few exceptions, the Company is no longer subject to U.S. and international income tax examinations for years before 2013.
The Company and certain of its subsidiaries have ongoing tax controversies in the U.S., Canada, Germany, and France. The Company does not anticipate resolution of these audits will have a material impact on its financial statements.
During 2015, the Company applied with the Internal Revenue Service (IRS) and Canadian Revenue Authority (CRA) for relief pursuant to the competent authority procedure provided in the tax treaty between the U.S. and Canada for transfer pricing tax assessments related to the tax years 2008 through 2012. The Company believes that the controversy will likely be ultimately settled via the competent authority process and accordingly have recorded both a Canadian liability and a U.S. receivable. The actual amounts of the liability for Canadian taxes and the asset for the correlative relief in the U.S. could be different based upon the agreement reached between the IRS and the CRA.
In accordance with the Company’s policy, the undistributed earnings of the Company’s non-U.S. subsidiaries remain indefinitely reinvested outside of the U.S. as of the end of 2016 as they are required to fund needs outside the U.S. and cannot be repatriated in a manner that is substantially tax free. As of December 31, 2016, the earnings of non-U.S. subsidiaries considered to be indefinitely reinvested totaled $704.6 million. No provision for U.S. income taxes has been provided herein. Determination of the amount of unrecognized deferred income tax liabilities on these earnings is not practicable because of the complexities with the hypothetical calculation. Additionally, the amount of liability is dependent on circumstances existing if and when remittance occurs. On December 18, 2015, the U.S. enacted the Consolidated Appropriations Act, which provides for a reinstatement and extension of the controlled foreign corporation look-through rules. This rule allows the Company to access Chinese and Canadian cash in a more tax-efficient manner and utilize the cash outside of the U.S. without triggering residual U.S. tax. As such, in 2016 the Company accrued $4.5 million of foreign withholding taxes.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
10. EMPLOYEE BENEFIT PLANS
Pension Plans
The Charles River Laboratories, Inc. Pension Plan (CRL Pension Plan) is a qualified, non-contributory defined benefit plan covering certain U.S. employees. Effective 2002, the plan was amended to exclude new participants from joining and in 2008 the accrual of benefits was frozen.
The Charles River Pension Plan is a defined contribution and defined benefit pension plan covering certain U.K. employees. Benefits are based on participants’ final pensionable salary and years of service. Participants’ rights vest immediately. Effective December 31, 2002, the plan was amended to exclude new participants from joining the defined benefit section of the plan and a defined contribution section was established for new entrants. Contributions under the defined contribution plan are determined as a percentage of gross salary. In the fourth quarter of 2015, the Charles River Pension Plan was amended such that the members of the defined benefit section of the plan ceased to accrue additional benefits; however, their benefits continue to be adjusted for changes in their final pensionable salary or a specified inflation index, as applicable.
In addition, the Company has several defined benefit plans in certain other countries in which it maintains an operating presence, including Japan, Canada, France and the Netherlands.
Charles River Laboratories Deferred Compensation Plan and Executive Supplemental Life Insurance Retirement Plan
The Company maintains a non-qualified deferred compensation plan, known as the Charles River Laboratories Deferred Compensation Plan (DCP), which allows a select group of eligible employees to defer a portion of their compensation. At the present time, no contributions are credited to the DCP, except as set forth below. Participants must specify the distribution date for deferred amounts at the time of deferral, in accordance with applicable IRS regulations. Generally, amounts may be paid in lump sum or installments upon retirement or termination of employment, or later if the employee terminates employment after age 55 and before age 65. Amounts may also be distributed during employment, subject to a minimum deferral requirement of three years.
The Company provides certain active employees an annual contribution into their DCP account of 10% of the employee’s base salary plus the lesser of their target annual bonus or actual annual bonus.
In addition to the DCP, certain officers and key employees also participate, or in the past participated, in the Company’s Executive Supplemental Life Insurance Retirement Plan (ESLIRP), which is a non-funded, non-qualified arrangement. Annual benefits under this plan will equal a percentage of the highest five consecutive years of compensation, offset by amounts payable under the CRL Pension Plan and Social Security. In connection with the establishment of the DCP, certain active ESLIRP participants, who agreed to convert their accrued ESLIRP benefit to a comparable deferred compensation benefit, discontinued their direct participation in the ESLIRP. Instead, the present values of the accrued benefits of ESLIRP participants were credited to their DCP accounts, and future accruals are converted to present values and credited to their DCP accounts annually.
The costs associated with these plans, including the ESLIRP, for fiscal years 2016, 2015 and 2014 totaled $2.2 million, $2.6 million and $3.3 million, respectively.
The Company has invested in several corporate-owned key-person life insurance policies with the intention of using these investments to fund the ESLIRP and the DCP. Participants have no interest in any such investments. As of December 31, 2016 and December 26, 2015, the cash surrender value of these life insurance policies were $29.5 million and $27.6 million, respectively.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The following table provides a reconciliation of benefit obligations and plan assets of the Company’s pension, DCP and ESLIRP plans:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Change in projected benefit obligations: | |
| | |
|
| Benefit obligation at beginning of year | $ | 345,220 |
| | $ | 359,130 |
|
| Service cost | 2,453 |
| | 4,293 |
|
| Interest cost | 12,046 |
| | 12,974 |
|
| Benefit payments | (13,383 | ) | | (8,191 | ) |
| Curtailment | (279 | ) | | — |
|
| Settlements | (5,499 | ) | | — |
|
| Plan amendments | 188 |
| | — |
|
| Transfer in from acquisition | 5,271 |
| | — |
|
| Actuarial loss (gain) | 71,006 |
| | (10,362 | ) |
| Administrative expenses paid | (605 | ) | | (411 | ) |
| Effect of foreign exchange | (36,476 | ) | | (12,213 | ) |
| Benefit obligation at end of year | $ | 379,942 |
| | $ | 345,220 |
|
| Change in fair value of plan assets: | | | |
| Fair value of plan assets at beginning of year | $ | 275,480 |
| | $ | 281,290 |
|
| Actual return on plan assets | 23,388 |
| | 6,263 |
|
| Employer contributions | 10,551 |
| | 6,762 |
|
| Settlements | (5,499 | ) | | — |
|
| Transfer in from acquisition | 508 |
| | — |
|
| Benefit payments | (13,383 | ) | | (8,191 | ) |
| Administrative expenses paid | (605 | ) | | (411 | ) |
| Effect of foreign exchange | (33,537 | ) | | (10,233 | ) |
| Fair value of plan assets at end of year | $ | 256,903 |
| | $ | 275,480 |
|
| | | | |
| Net balance sheet liability | $ | 123,039 |
| | $ | 69,740 |
|
| | | | |
| Amounts recognized in balance sheet: | | | |
| Noncurrent assets | $ | — |
| | $ | 261 |
|
| Current liabilities | 1,120 |
| | 6,133 |
|
| Noncurrent liabilities | 121,919 |
| | 63,868 |
|
Amounts recognized in accumulated other comprehensive loss related to the Company’s pension, DCP and ESLIRP plans are as follows:
|
| | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 |
| | | | |
| | (in thousands) |
| Net actuarial loss | $ | 123,743 |
| | $ | 73,412 |
|
| Net prior service cost (credit) | (3,300 | ) | | (4,584 | ) |
| Net amount recognized | $ | 120,443 |
| | $ | 68,828 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The accumulated benefit obligation and fair value of plan assets for the Company’s pension, DCP and ESLIRP plans with accumulated benefit obligations in excess of plan assets are as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Accumulated benefit obligation | $ | 346,122 |
| | $ | 306,433 |
|
| Fair value of plan assets | 242,172 |
| | 253,225 |
|
The projected benefit obligation and fair value of plan assets for the Company’s pension, DCP and ESLIRP plans with projected benefit obligations in excess of plan assets are as follows:
|
| | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | | | |
| | (in thousands) |
| Projected benefit obligation | $ | 379,942 |
| | $ | 336,155 |
|
| Fair value of plan assets | 256,903 |
| | 266,154 |
|
The amounts in accumulated other comprehensive income expected to be recognized as components of net periodic benefit cost over the next fiscal year are as follows:
|
| | | |
| | December 31, 2016 |
| | (in thousands) |
| Amortization of net actuarial loss | $ | 3,867 |
|
| Amortization of net prior service credit | (475 | ) |
Components of net periodic benefit cost for the Company’s pension, DCP and ESLIRP plans are as follows:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Service cost | $ | 2,453 |
| | $ | 4,293 |
| | $ | 4,155 |
|
| Interest cost | 12,046 |
| | 12,974 |
| | 13,831 |
|
| Expected return on plan assets | (14,164 | ) | | (16,987 | ) | | (17,444 | ) |
| Amortization of prior service cost (credit) | (292 | ) | | (581 | ) | | 1,211 |
|
| Amortization of net loss (gain) | 2,003 |
| | 3,198 |
| | 23 |
|
| Curtailment | (279 | ) | | — |
| | — |
|
| Settlements | 788 |
| | — |
| | — |
|
| Net periodic cost (benefit) | $ | 2,555 |
| | $ | 2,897 |
| | $ | 1,776 |
|
Assumptions
Weighted-average assumptions used to determine projected benefit obligations are as follows:
|
| | | | | |
| | December 31, 2016 | | December 26, 2015 |
| Discount rate | 3.01 | % | | 3.89 | % |
| Rate of compensation increase | 3.25 | % | | 3.17 | % |
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Weighted-average assumptions used to determine net periodic benefit cost are as follows:
|
| | | | | | | | |
| | December 31, 2016 | | December 26, 2015 | | December 27, 2014 |
| Discount rate | 3.89 | % | | 3.75 | % | | 4.44 | % |
| Expected long-term return on plan assets | 5.83 | % | | 6.24 | % | | 6.41 | % |
| Rate of compensation increase | 3.17 | % | | 3.18 | % | | 3.36 | % |
A 0.5% decrease in the expected rate of return would increase annual pension expense by $1.3 million.
Plan assets
The Company invests its pension assets with the objective of achieving a total long-term rate of return sufficient to fund future pension obligations and to minimize future pension contributions. The Company is willing to tolerate a commensurate level of risk to achieve this objective. The Company controls its risk by maintaining a diversified portfolio of assets classes. Plan assets did not include any of the Company’s common stock as of December 31, 2016 or December 26, 2015. The weighted-average target asset allocations are approximately 45.0% to equity securities, approximately 31.5% to fixed income securities and approximately 23.5% to other securities.
The fair value of the Company’s pension plan assets by asset category are as follows:
|
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, 2016 | | December 26, 2015 |
| | Level 1 | | Level 2 | | Level 3 | | Total | | Level 1 | | Level 2 | | Level 3 | | Total |
| | | | | | | | | | | | | | | | |
| | (in thousands) |
| Cash | $ | 108 |
| | $ | — |
| | $ | — |
| | $ | 108 |
| | $ | 92 |
| | $ | — |
| | $ | — |
| | $ | 92 |
|
Equity securities(1) | 63,348 |
| | 6,252 |
| | — |
| | 69,600 |
| | 65,890 |
| | 5,941 |
| | — |
| | 71,831 |
|
Debt securities(2) | 59,294 |
| | 3,269 |
| | — |
| | 62,563 |
| | 68,489 |
| | 2,822 |
| | — |
| | 71,311 |
|
Mutual funds(3) | 64,698 |
| | 56,596 |
| | — |
| | 121,294 |
| | 63,689 |
| | 65,725 |
| | — |
| | 129,414 |
|
| Other | 1,318 |
| | 586 |
| | 1,434 |
| | 3,338 |
| | 1,021 |
| | 49 |
| | 1,762 |
| | 2,832 |
|
| Total | $ | 188,766 |
| | $ | 66,703 |
| | $ | 1,434 |
| | $ | 256,903 |
| | $ | 199,181 |
| | $ | 74,537 |
| | $ | 1,762 |
| | $ | 275,480 |
|
(1) This category comprises equity securities held by non-U.S. pension plans valued at the quoted closing price, and translated into U.S. dollars using a foreign currency exchange rate at year end. |
(2) This category comprises debt securities held by non-U.S. pension plans valued at the quoted closing price, and translated into U.S. dollars using a foreign currency exchange rate at year end. |
(3) This category comprises mutual funds valued at the net asset value of shares held at year end. |
The activity within the Level 3 pension plan assets was non-significant during the periods presented.
During fiscal year 2016, the Company contributed $4.0 million to the pension plans and expects to contribute $4.0 million to its pension plans in fiscal year 2017.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Expected benefit payments are estimated using the same assumptions used in determining the Company’s benefit obligation as of December 31, 2016. Benefit payments will depend on future employment and compensation levels, among other factors, and changes in any of these factors could significantly affect these estimated future benefit payments. Estimated future benefit payments during the next five years and in the aggregate for fiscal years thereafter, are as follows:
|
| | | | |
| Fiscal Year | | Pension Plans |
| | | (in thousands) |
| 2017 | | $ | 8,610 |
|
| 2018 | | 8,818 |
|
| 2019 | | 9,682 |
|
| 2020 | | 10,014 |
|
| 2021 | | 30,717 |
|
| Thereafter | | 57,705 |
|
Post-Retirement Health and Life Insurance Plans
The Company’s Canadian location offers post-retirement life insurance benefits to its employees and post-retirement medical and dental insurance coverage to certain executives. The plan is non-contributory and unfunded. As of December 31, 2016 and December 26, 2015, the accumulated benefit obligation related to the plan was $1.2 million and $0.9 million, respectively. The amounts included in other accumulated comprehensive income as well as expenses related to the plan were non-significant for fiscal years 2016, 2015, and 2014.
Charles River Laboratories Employee Savings Plan
The Charles River Laboratories Employee Savings Plan is a defined contribution plan in the form of a qualified 401(k) plan in which substantially all U.S. employees are eligible to participate upon employment. The plan contains a provision whereby the Company matches a percentage of employee contributions. During fiscal years 2016, 2015 and 2014, the costs associated with this defined contribution plan totaled $6.2 million, $5.3 million and $4.9 million, respectively.
11. STOCK-BASED COMPENSATION
The Company has stock-based compensation plans under which employees and non-employee directors may be granted stock-based awards such as stock options, restricted stock, restricted stock units and PSUs.
During fiscal years 2016, 2015 and 2014, the primary share-based awards and their general terms and conditions are as follows:
| |
| • | Stock options, which entitle the holder to purchase a specified number of shares of common stock at an exercise price equal to the closing market price of common stock on the date of grant; typically vest over 4 years; and typically expire 5 to 7 years from date of grant. |
| |
| • | Restricted stock units, which represent an unsecured promise to grant at no cost a set number of shares of common stock upon the completion of the vesting schedule, and typically vest over 2 to 4 years. With respect to restricted stock units, recipients are not entitled to cash dividends and have no voting rights on the stock during the vesting period. |
| |
| • | Restricted stock, which is an award of common stock issued on the grant date and subject to vesting, typically over 2 to 4 years. Recipients cannot sell or transfer the shares until the restriction period has lapsed, but are entitled to forfeitable cash dividends and to vote their respective shares upon grant. |
| |
| • | PSUs, which entitle the holder to receive at no cost, a specified number of shares of common stock within a range of shares from zero to a specified maximum and typically vest over 3 years. Payout of this award is contingent upon achievement of certain performance and market conditions. |
In May 2007, the Company’s shareholders approved the 2007 Incentive Plan, which was amended in 2009, 2011, 2013 and 2015 (2007 Plan). The 2007 Plan provided no further awards to be granted under preexisting stock option and incentive plans; provided, however, that any shares that have been forfeited or canceled in accordance with the terms of the applicable award under a preexisting plan may be subsequently awarded in accordance with the terms of the preexisting plan. The 2007 Plan allows a maximum of 18.7 million shares to be awarded, of which restricted stock grants, restricted stock units, and
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
performance based stock awards count as 2.3 shares and stock options count as 1.0 shares. Any stock options and other share-based awards that were granted under prior plans and were outstanding in May 2007 continue in accordance with the terms of the respective plans.
In May 2016, the Company’s shareholders approved the 2016 Incentive Plan (2016 Plan). The 2016 Plan provided no further awards to be granted under preexisting stock option and incentive plans; provided, however, that any shares that have been forfeited or canceled in accordance with the terms of the applicable award under a preexisting plan may be subsequently awarded in accordance with the terms of the preexisting plan. The 2016 Plan allows a maximum of 6.1 million shares to be awarded, of which restricted stock grants, restricted stock units and performance based stock awards count as 2.3 shares and stock options count as 1.0 shares. Any stock options and other share-based awards that were granted under prior plans and were outstanding in May 2016 continue in accordance with the terms of the respective plans.
As of December 31, 2016, approximately 7.3 million shares were authorized for future grants under the Company’s share-based compensation plans. The Company settles employee share-based compensation awards with newly issued shares. The following table provides the financial statement line items in which stock-based compensation is reflected:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Cost of revenue (excluding amortization of intangible assets) | $ | 6,508 |
| | $ | 6,511 |
| | $ | 5,382 |
|
| Selling, general and administrative | 37,134 |
| | 33,611 |
| | 25,653 |
|
| Stock-based compensation expense, before income taxes | 43,642 |
| | 40,122 |
| | 31,035 |
|
| Provision for income taxes | (15,548 | ) | | (14,225 | ) | | (11,006 | ) |
| Stock-based compensation, net of income taxes | $ | 28,094 |
| | $ | 25,897 |
| | $ | 20,029 |
|
During fiscal year 2015, the Company modified certain stock-based awards granted in previous years as part of executive retirement transitions. For the stock-based awards granted to employees during and subsequent to fiscal year 2015, the Company introduced a new retirement provision, which allows for continued vesting of such awards after the employee’s retirement if certain eligibility conditions are met. The introduction of the new retirement provision and stock-based award modifications increased the Company’s stock-based compensation expense for 2015 by $4.5 million.
The Company capitalized no stock-based compensation related costs for fiscal years 2016, 2015 and 2014.
The Company’s pool of excess tax benefits, which is computed in accordance with the long form method, was $32.2 million as of December 31, 2016, $22.3 million as of December 26, 2015, and $10.8 million as of December 27, 2014. During fiscal year 2016, the Company recorded a tax benefit of $9.3 million to additional paid-in capital related to the exercise of stock options and vesting of restricted shares and restricted stock units, compared to a tax benefit of $10.6 million in fiscal year 2015.
Stock Options
The following table summarizes stock option activities under the Company’s stock-based compensation plans:
|
| | | | | | | | | | | | |
| | Number of shares | | Weighted Average
Exercise Price | | Weighted Average
Remaining
Contractual Life
(in years) | | Aggregate
Intrinsic
Value |
| | | | | | | | |
| | (in thousands, except per share amounts) |
| Options outstanding as of December 26, 2015 | 2,066 |
| | $ | 50.62 |
| | | | |
|
| Options granted | 588 |
| | $ | 74.13 |
| | | | |
|
| Options exercised | (601 | ) | | $ | 38.52 |
| | | | |
|
| Options canceled | (83 | ) | | $ | 62.66 |
| | | | |
|
| Options outstanding as of December 31, 2016 | 1,970 |
| | $ | 60.82 |
| | 3.4 | | $ | 30,638 |
|
| Options exercisable as of December 31, 2016 | 746 |
| | $ | 48.34 |
| | 2.6 | | $ | 20,817 |
|
| Options expected to vest as of December 31, 2016 | 1,202 |
| | $ | 68.40 |
| | 3.8 | | $ | 9,690 |
|
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
The fair value of stock options granted was estimated using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
| | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| Expected life (in years) | 3.6 |
| | 3.6 |
| | 4.2 |
|
| Expected volatility | 25 | % | | 28 | % | | 30 | % |
| Risk-free interest rate | 1.2 | % | | 1.1 | % | | 1.5 | % |
| Expected dividend yield | 0 | % | | 0 | % | | 0 | % |
The weighted-average grant date fair value of stock options granted was $15.12, $17.24 and $15.19 for fiscal years 2016, 2015 and 2014, respectively.
As of December 31, 2016, the unrecognized compensation cost related to unvested stock options expected to vest was $10.8 million. This unrecognized compensation will be recognized over an estimated weighted-average amortization period of 2.2 years.
The total intrinsic value of options exercised during fiscal years 2016, 2015 and 2014 was $23.0 million, $28.3 million and $30.5 million, respectively, with intrinsic value defined as the difference between the market price on the date of exercise and the exercise price.
Restricted Stock and Restricted Stock Units
The following table summarizes the restricted stock and restricted stock units activity for fiscal year 2016:
|
| | | | | | |
| | Restricted Stock and Restricted Stock Units | | Weighted
Average
Grant Date
Fair Value |
| | (in thousands) | | |
| December 26, 2015 | 607 |
| | $ | 55.52 |
|
| Granted | 236 |
| | $ | 75.10 |
|
| Vested | (296 | ) | | $ | 49.29 |
|
| Canceled | (32 | ) | | $ | 63.36 |
|
| December 31, 2016 | 515 |
| | $ | 67.62 |
|
As of December 31, 2016, the unrecognized compensation cost related to shares of unvested restricted stock and restricted stock units expected to vest was $19.2 million, which is expected to be recognized over an estimated weighted-average amortization period of 1.2 years. The total fair value of restricted stock and restricted stock unit grants that vested during fiscal years 2016, 2015 and 2014 was $14.6 million, $15.7 million and $13.9 million, respectively.
Performance Based Stock Award Program
The Company issues PSUs to certain corporate officers. The number of shares of common stock issued for each PSU is adjusted based on a performance condition linked to the Company’s financial performance. Certain awards are further adjusted based on a market condition, which is calculated based on the Company’s stock performance relative to a peer group over the three-year vesting period. The fair value of the market condition is reflected in the fair value of the award at grant date.
The Company utilizes a Monte Carlo simulation valuation model to value these awards. Information pertaining to the Company’s PSUs and the related estimated weighted-average assumptions used to calculate their fair value were as follows:
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| PSUs granted | 190,628 |
| | 148,900 |
| | 214,823 |
|
| Weighted average per share fair value | $ | 80.38 |
| | $ | 88.62 |
| | $ | 67.82 |
|
| Key Assumptions: | | | | | |
| Expected volatility | 24 | % | | 23 | % | | 29 | % |
| Risk-free interest rate | 0.91 | % | | 0.96 | % | | 0.63 | % |
| Expected dividend yield | — | % | | — | % | | — | % |
| 20 trading day average stock price on grant date | (4.8 | )% | | 20.6 | % | | 13.1 | % |
The maximum amount of common shares to be issued upon vesting of PSUs is approximately 0.3 million. For fiscal years 2016, 2015 and 2014, the Company recognized stock-based compensation related to PSUs of $19.7 million, $14.7 million and $8.5 million, respectively. The total fair value of PSUs that vested during fiscal years 2016 and 2015 was $18.0 million and $6.6 million, respectively.
In fiscal year 2016, the Company also issued approximately 18,000 PSUs using a weighted average fair value per share of $73.70. These PSUs vest upon the achievement of financial targets and other performance measures.
12. FOREIGN CURRENCY CONTRACTS
The Company enters into foreign exchange forward contracts to limit its foreign currency exposure related to intercompany loans that are not of a long-term investment nature. These contracts are recorded at fair value in the Company’s consolidated balance sheet and are not designated as hedging instruments. Any gains or losses on such contracts are immediately recognized in other income (expense), net, and are largely offset by the remeasurement of the underlying intercompany loan balances.
The Company did not have any foreign currency contracts open as of December 31, 2016. The notional amount and fair value of the Company’s foreign currency forward contracts as of December 26, 2015 were as follows:
|
| | | | | | | | |
| Notional Amount | | Fair Value | | Balance Sheet Location |
| | | | | |
| (in thousands) |
| $ | 88,483 |
| | $ | 15 |
| | Other current assets |
The following table summarizes gains (losses) recognized on foreign exchange forward contracts related to intercompany loans denominated in British Pounds and Euros on the Company’s consolidated statement of income:
|
| | | | | | | | |
| | | Fiscal Year |
| Location of Gain (Loss) | | 2016 | | 2015 |
| | | | | |
| | | (in thousands) |
| Other income (expense), net | | $ | 3,373 |
| | $ | (4,917 | ) |
The forward contracts outstanding as of December 26, 2015 had durations of approximately 3 months.
13. COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company rents laboratory and office space, land, vehicles and certain equipment under non-cancellable operating leases. These lease agreements contain various clauses for renewal at the Company’s option and, in certain cases, rent escalation clauses. Rental expense under these leases amounted to $21.8 million, $23.4 million and $14.2 million in fiscal years 2016, 2015 and 2014, respectively. In addition to rent, the leases may require the Company to pay additional amounts for taxes, insurance, maintenance and other operating expenses.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
As of December 31, 2016, minimum rental commitments under non-cancellable leases, net of income from subleases, for each of the next five years and total thereafter were as follows:
|
| | | | |
| | | Minimum Lease Payments |
| | | (in thousands) |
| 2017 | | $ | 23,410 |
|
| 2018 | | 20,273 |
|
| 2019 | | 17,119 |
|
| 2020 | | 13,254 |
|
| 2021 | | 8,104 |
|
| Thereafter | | 19,116 |
|
| Total | | $ | 101,276 |
|
Insurance
The Company maintains certain insurance policies that maintain large deductibles up to approximately $5.0 million, some with or without stop-loss limits, depending on market availability. Insurance policies at certain locations are based on a percentage of the insured assets, for which deductibles for certain property may exceed $5.0 million in the event of a catastrophic event.
Litigation
Various lawsuits, claims and proceedings of a nature considered normal to its business are pending against the Company. While the outcome of any of these proceedings cannot be accurately predicted, the Company does not believe the ultimate resolution of any of these existing matters would have a material adverse effect on the Company’s business or financial condition.
In July 2015, IDEXX Laboratories, Inc. and IDEXX Distribution, Inc. (collectively, IDEXX) filed a complaint in the United States District Court for the District of Delaware alleging the Company has infringed three recently issued patents related to a blood spot sample collection method used in determining the presence or absence of an infectious disease in a population of rodents. On September 21, 2015, the Company timely filed a motion to dismiss the complaint on the grounds that all of the claims are directed to unpatentable subject matter and therefore are invalid. On October 7, 2015, IDEXX filed an amended complaint, which substantially asserted the same patents and infringement allegations as asserted in the original complaint, and on October 26, 2015, the Company timely filed a motion to dismiss this amended complaint. The hearing on the motion to dismiss was held on January 12, 2016. On July 1, 2016, the Court issued an opinion denying the motion to dismiss. The Company filed its answer to the complaint on July 21, 2016. In addition, on July 29, 2016, the Company initiated an inter partes review (IPR) procedure with the United States Patent and Trademark Office challenging the validity of the IDEXX patents. On February 6, 2017, we entered into a settlement agreement with IDEXX, which involved the withdrawal by IDEXX of their complaint and withdrawal by us of the IPR.
In May 2013, the Company commenced an investigation into inaccurate billing with respect to certain government contracts. The Company promptly reported these matters to the relevant government contracting officers, the Department of Health and Human Services’ Office of the Inspector General, and the Department of Justice, and the Company is cooperating with these agencies to ensure the proper repayment and resolution of this matter. The Company previously identified approximately $1.5 million of excess amounts billed on these contracts since January 1, 2007, and recorded a liability for such amount. Based on its ongoing discussions with the government, the Company has recorded an additional charge of $0.3 million during the fiscal year 2016. The Company’s best estimate, which totals $1.8 million, may be subject to change based on the terms of any final settlement with the Department of Justice and the Department of Health and Human Services’ Office of the Inspector General.
Guarantees
The Company enters into certain agreements with other parties in the ordinary course of business that contain indemnification provisions. These typically include agreements with directors and officers, business partners, contractors, landlords, and customers. Under these provisions, the Company generally indemnifies and holds harmless the indemnified party for losses suffered or incurred by the indemnified party as a result of the Company’s activities. These indemnification provisions generally survive termination of the underlying agreement. The maximum potential amount of future payments the Company could be required to make under these indemnification provisions is unlimited. However, to date the Company has not incurred
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
material costs to defend lawsuits or settle claims related to these indemnification provisions. As a result, the estimated fair value of these obligations is minimal.
Purchase Obligations
The Company enters into unconditional purchase obligations, in the ordinary course of business, that include agreements to purchase goods or services that are enforceable and legally binding and that specify all significant terms including: fixed or minimum quantities to be purchased; fixed, minimum or variable price provisions; and the approximate timing of the transaction. Purchase obligations exclude agreements that are cancellable at any time without penalty. The aggregate amount of the Company’s unconditional purchase obligations totaled $86.2 million as of December 31, 2016.
14. RESTRUCTURING AND ASSET IMPAIRMENTS
Workforce Reductions
The Company periodically implements workforce reductions to improve operating efficiency at various sites. The following table provides a rollforward of the Company’s severance and retention costs liability:
|
| | | | | | | | | | | |
| | December 31, 2016 | | December 26, 2015 | | December 27, 2014 |
| | | | | | |
| | (in thousands) |
| Balance, beginning of period | $ | 2,969 |
| | $ | 2,666 |
| | $ | 2,782 |
|
| Expense | 8,454 |
| | 6,173 |
| | 7,792 |
|
| Payments / utilization | (7,473 | ) | | (5,820 | ) | | (7,900 | ) |
| Foreign currency adjustments | (270 | ) | | (50 | ) | | (8 | ) |
| Balance, end of period | $ | 3,680 |
| | $ | 2,969 |
| | $ | 2,666 |
|
As of December 31, 2016 and December 26, 2015, $3.6 million and $2.6 million of severance and retention costs liability, respectively, was included in accrued compensation and $0.1 million and $0.3 million, respectively, was included in other long-term liabilities on the Company's consolidated balance sheets.
The following table presents severance and retention costs by classification on the consolidated statements of income:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Cost of services provided and products sold (excluding amortization of intangible assets) | $ | 4,717 |
| | $ | 735 |
| | $ | 3,342 |
|
| Selling, general and administrative | 3,737 |
| | 5,438 |
| | 4,450 |
|
| Total severance and retention costs | $ | 8,454 |
| | $ | 6,173 |
| | $ | 7,792 |
|
The following presents severance and retention costs by reportable segment:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| RMS | $ | 759 |
| | $ | 1,338 |
| | $ | 4,593 |
|
| DSA | 7,689 |
| | 1,068 |
| | 2,912 |
|
| Manufacturing | 6 |
| | 1,639 |
| | 166 |
|
| Unallocated corporate | — |
| | 2,128 |
| | 121 |
|
| Total severance and retention costs | $ | 8,454 |
| | $ | 6,173 |
| | $ | 7,792 |
|
Facilities
In fiscal year 2016, the Company commenced a consolidation of small DSA facilities in the U.S., Ireland, and the United Kingdom. As a result, an asset impairment charge of $9.4 million was recorded related to the consolidation plans.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
In fiscal year 2015, the Company commenced a consolidation of certain RMS facilities in the U.S., Europe, and Japan. As a result, an asset impairment charge of $1.8 million was recorded related to the consolidation plans.
In fiscal year 2014, the Company committed to plans to consolidate certain research model operations in the U.S., Japan, and Europe. As a result, the Company recorded $2.2 million of asset impairments and other charges and $4.3 million of accelerated depreciation related to certain facilities impacted by the consolidation plans. Also, in fiscal year 2014, the Company recorded a gain of $1.0 million on the sale of a European facility.
15. SEGMENT AND GEOGRAPHIC INFORMATION
The Company revised its reportable segments during fiscal year 2016 to align with its view of the business following its acquisition of WIL Research. See Note 1, “Description of Business and Summary of Significant Accounting Policies.” The Company reported segment results on this basis retrospectively for all comparable prior periods. Asset information on a reportable segment basis is not disclosed as this information is not separately identified and internally reported to the Company’s Chief Operating Decision Maker.
The following table presents revenue and other financial information by reportable segment:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| RMS | | | | | |
| Revenue | $ | 494,037 |
| | $ | 470,411 |
| | $ | 503,656 |
|
| Operating income | 136,365 |
| | 120,973 |
| | 120,736 |
|
| Depreciation and amortization | 20,853 |
| | 22,526 |
| | 27,309 |
|
| Capital expenditures | 11,642 |
| | 17,398 |
| | 18,669 |
|
| DSA | | | |
| | |
|
| Revenue | $ | 836,593 |
| | $ | 612,173 |
| | $ | 538,218 |
|
| Operating income | 138,157 |
| | 121,981 |
| | 69,749 |
|
| Depreciation and amortization | 71,816 |
| | 46,812 |
| | 47,138 |
|
| Capital expenditures | 27,493 |
| | 30,333 |
| | 19,759 |
|
| Manufacturing | | | | | |
| Revenue | $ | 350,802 |
| | $ | 280,718 |
| | $ | 255,788 |
|
| Operating income | 104,543 |
| | 74,675 |
| | 79,260 |
|
| Depreciation and amortization | 25,566 |
| | 18,129 |
| | 14,295 |
|
| Capital expenditures | 12,247 |
| | 9,814 |
| | 15,621 |
|
For fiscal years ended 2016, 2015 and 2014, reconciliations of segment operating income and capital expenditures to the respective consolidated amounts are as follows:
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | Operating Income | | Capital Expenditures |
| | Fiscal Year | | Fiscal Year |
| | 2016 | | 2015 | | 2014 | | 2016 | | 2015 | | 2014 |
| | | | | | | | | | | | |
| | (in thousands) |
| Total reportable segments | $ | 379,065 |
| | $ | 317,629 |
| | $ | 269,745 |
| | $ | 51,382 |
| | $ | 57,545 |
| | $ | 54,049 |
|
| Unallocated corporate | (141,646 | ) | | (111,180 | ) | | (92,075 | ) | | 3,906 |
| | 5,707 |
| | 2,876 |
|
| Total consolidated | $ | 237,419 |
| | $ | 206,449 |
| | $ | 177,670 |
| | $ | 55,288 |
| | $ | 63,252 |
| | $ | 56,925 |
|
Revenue for each significant product or service offering is as follows:
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| RMS | $ | 494,037 |
| | $ | 470,411 |
| | $ | 503,656 |
|
| DSA | 836,593 |
| | 612,173 |
| | 538,218 |
|
| Manufacturing | 350,802 |
| | 280,718 |
| | 255,788 |
|
| Total revenue | $ | 1,681,432 |
| | $ | 1,363,302 |
| | $ | 1,297,662 |
|
A summary of unallocated corporate overhead consists of the following:
|
| | | | | | | | | | | |
| | Fiscal Year |
| | 2016 | | 2015 | | 2014 |
| | | | | | |
| | (in thousands) |
| Stock-based compensation expense | $ | 27,272 |
| | $ | 25,751 |
| | $ | 18,474 |
|
| Salary, bonus, and fringe | 39,189 |
| | 33,026 |
| | 30,838 |
|
| Consulting, audit, and professional services | 23,421 |
| | 15,418 |
| | 13,431 |
|
| IT related expenses | 13,233 |
| | 8,400 |
| | 6,528 |
|
| Depreciation expense | 8,423 |
| | 7,414 |
| | 7,703 |
|
| Acquisition related adjustments | 15,608 |
| | 11,644 |
| | 6,285 |
|
| Other general unallocated corporate expenses | 14,500 |
| | 9,527 |
| | 8,816 |
|
| Total unallocated corporate overhead costs | $ | 141,646 |
| | $ | 111,180 |
| | $ | 92,075 |
|
Other general unallocated corporate expenses consist of various departmental costs including those associated with departments such as senior executives, corporate accounting, legal, tax, human resources, treasury, and investor relations.
Revenue and long-lived assets by geographic area are as follows:
|
| | | | | | | | | | | | | | | | | | | | | | | |
| | U.S. | | Europe | | Canada | | Japan | | Other Non-U.S. | | Consolidated |
| | | | | | | | | | | | |
| | (in thousands) |
| 2016 | | | | | | | | | | | |
| Revenue | $ | 850,422 |
| | $ | 520,937 |
| | $ | 194,210 |
| | $ | 46,829 |
| | $ | 69,034 |
| | $ | 1,681,432 |
|
| Long-lived assets | 462,330 |
| | 177,423 |
| | 78,866 |
| | 20,941 |
| | 16,267 |
| | 755,827 |
|
| 2015 | | | | | | | | | | | |
| Revenue | $ | 659,466 |
| | $ | 435,491 |
| | $ | 172,349 |
| | $ | 40,520 |
| | $ | 55,476 |
| | $ | 1,363,302 |
|
| Long-lived assets | 402,238 |
| | 159,445 |
| | 77,535 |
| | 22,348 |
| | 16,393 |
| | 677,959 |
|
| 2014 | | | | | | | | | | |
|
|
| Revenue | $ | 588,531 |
| | $ | 446,263 |
| | $ | 163,490 |
| | $ | 49,921 |
| | $ | 49,457 |
| | $ | 1,297,662 |
|
| Long-lived assets | 386,624 |
| | 153,203 |
| | 95,272 |
| | 23,896 |
| | 17,802 |
| | 676,797 |
|
Included in the other non-U.S. category above are operations located in China, Korea, Australia, Singapore, and India. Revenues represent sales originating in entities physically located in the identified geographic area. Long-lived assets include property, plant, and equipment and other long-lived assets.
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
16. SELECTED QUARTERLY FINANCIAL DATA (unaudited)
The following table contains quarterly financial information for fiscal years 2016 and 2015. The operating results for any quarter are not necessarily indicative of future period results.
|
| | | | | | | | | | | | | | | |
| | First Quarter | | Second Quarter | | Third Quarter | | Fourth Quarter |
| | | | | | | | |
| Fiscal Year 2016 | (in thousands, except per share amounts) |
| Total revenue | $ | 354,868 |
| | $ | 434,055 |
| | $ | 425,720 |
| | $ | 466,789 |
|
Gross profit(1) | 140,768 |
| | 169,747 |
| | 156,270 |
| | 179,881 |
|
| Operating income | 51,472 |
| | 58,061 |
| | 58,795 |
| | 69,091 |
|
| Net income attributable to common shareholders | 37,143 |
| | 35,207 |
| | 37,735 |
| | 44,680 |
|
| Earnings (loss) per common share | | | | | | | |
| Basic: | | | | | | | |
| Continuing operations attributable to common shareholders | $ | 0.80 |
| | $ | 0.75 |
| | $ | 0.79 |
| | $ | 0.95 |
|
| Discontinued operations | $ | — |
| | $ | — |
| | $ | 0.01 |
| | $ | — |
|
| Net income attributable to common shareholders | $ | 0.80 |
| | $ | 0.75 |
| | $ | 0.80 |
| | $ | 0.95 |
|
| Diluted: | | | | | | | |
| Continuing operations attributable to common shareholders | $ | 0.78 |
| | $ | 0.73 |
| | $ | 0.78 |
| | $ | 0.93 |
|
| Discontinued operations | $ | — |
| | $ | — |
| | $ | 0.01 |
| | $ | — |
|
| Net income attributable to common shareholders | $ | 0.78 |
| | $ | 0.73 |
| | $ | 0.79 |
| | $ | 0.93 |
|
| Fiscal Year 2015 | | | | | | | |
| Total revenue | $ | 320,414 |
| | $ | 339,573 |
| | $ | 349,465 |
| | $ | 353,850 |
|
Gross profit(1) | 119,660 |
| | 132,783 |
| | 138,075 |
| | 140,574 |
|
| Operating income | 43,005 |
| | 55,735 |
| | 55,440 |
| | 52,269 |
|
| Net income attributable to common shareholders | 31,541 |
| | 48,509 |
| | 37,379 |
| | 31,884 |
|
| Earnings (loss) per common share | | | | | | | |
| Basic: | | | | | | | |
| Continuing operations attributable to common shareholders | $ | 0.67 |
| | $ | 1.04 |
| | $ | 0.81 |
| | $ | 0.71 |
|
| Discontinued operations | $ | — |
| | $ | — |
| | $ | — |
| | $ | (0.02 | ) |
| Net income attributable to common shareholders | $ | 0.67 |
| | $ | 1.04 |
| | $ | 0.81 |
| | $ | 0.69 |
|
| Diluted: | | | | | | | |
| Continuing operations attributable to common shareholders | $ | 0.66 |
| | $ | 1.02 |
| | $ | 0.79 |
| | $ | 0.69 |
|
| Discontinued operations | $ | — |
| | $ | — |
| | $ | — |
| | $ | (0.02 | ) |
| Net income attributable to common shareholders | $ | 0.66 |
| | $ | 1.02 |
| | $ | 0.79 |
| | $ | 0.67 |
|
(1) Gross profit is calculated as total revenues minus cost of revenue (excluding amortization of intangible assets). |
Full-year amounts may not sum due to rounding.
17. SUBSEQUENT EVENTS
On February 10, 2017, the Company completed the divestiture of its CDMO business to Quotient Clinical Ltd., based in London, England, for $75.0 million in cash, subject to certain post-closing adjustments.
The CDMO business, which represented approximately 1% of the Company’s 2016 consolidated revenue, was acquired in April 2016 as part of the acquisition of WIL Research. See Note 1, “Description of Business and Summary of Significant Accounting Policies” and Note 2, “Business Acquisitions.” Following a strategic review, the Company determined that the CDMO business was not optimized within the Company’s portfolio at its current scale, and that the capital could be better deployed in other long-term growth opportunities.
The Company is in the process of evaluating the transaction and its impact on the financial statements, including evaluating the resulting gain (loss) that will be recognized. As of December 31, 2016, the carrying amounts of the major classes of assets and liabilities associated with the CDMO business were as follows:
CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
|
| | | |
| | December 31, 2016 |
| | (in thousands) |
| Assets | |
| Current assets | $ | 4,270 |
|
| Property, plant and equipment, net | 11,313 |
|
| Goodwill | 35,857 |
|
| Long-term assets | 17,332 |
|
| Total assets | $ | 68,772 |
|
| Liabilities | |
| Current liabilities | $ | 5,840 |
|
| Long-term liabilities | 7,856 |
|
| Total liabilities | $ | 13,696 |
|
As of December 31, 2016, the assets and liabilities of the CDMO business were not classified as held-for-sale as management had not committed to a formal plan to sell the business and the sale was not deemed probable.
Item 9. Changes in and Disagreement with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures
Based on their evaluation, required by paragraph (b) of Rules 13a-15 or 15d-15, promulgated by the Securities Exchange Act of 1934, as amended (Exchange Act), the Company’s principal executive officer and principal financial officer have concluded that the Company’s disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act are effective, at a reasonable assurance level, as of December 31, 2016, to ensure that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in Securities and Exchange Commission rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the Company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurances of achieving the desired control objectives, and management necessarily was required to apply its judgment in designing and evaluating the controls and procedures.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our CEO and CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our assessment and those criteria, management concluded that the Company maintained effective internal control over financial reporting as of December 31, 2016.
We have excluded the business acquisitions completed during fiscal year 2016, including WRH, Inc., Blue Stream Laboratories, Inc., and Agilux Laboratories, Inc., from the assessment of the effectiveness of internal control over financial reporting as of December 31, 2016. The acquired businesses are wholly-owned subsidiaries whose total assets and total revenues collectively represent 7.8% and 11.1%, respectively, of the related consolidated financial statement amounts as of and for fiscal year ended December 31, 2016.
The effectiveness of our internal control over financial reporting as of December 31, 2016, has been audited by PricewaterhouseCoopers LLP, an Independent Registered Public Accounting Firm, as stated in their report which is included in Item 8, “Financial Statements and Other Supplementary Data” in this Annual Report on Form 10-K.
(b) Changes in Internal Controls
During the fourth quarter of 2016, the Company continued to execute a plan to centralize certain accounting transaction processing functions to internal shared service centers. This planned effort is expected to continue in subsequent quarters. There were no other changes in the Company’s internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of the Exchange Act Rules 13a-15 or 15d-15 that occurred during the fourth quarter of 2016 that materially affected, or were reasonably likely to materially affect, the Company’s internal control over financial reporting.
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
A. Directors and Compliance with Section 16(a) of the Exchange Act
The information required by this Item regarding our directors and compliance with Section 16(a) of the Exchange Act by our officers and directors will be included in the 2017 Proxy Statement under the sections captioned “Nominees for Directors” and “Section 16(a) Beneficial Ownership Reporting Compliance” and is incorporated herein by reference thereto. The information required by this Item regarding our corporate governance will be included in the 2017 Proxy Statement under the section captioned “Corporate Governance” and is incorporated herein by reference thereto.
B. Our Executive Officers
The information required by this Item regarding our executive officers is reported in Part I of this Form 10-K under the heading “Item 1. Business”
C. Audit Committee Financial Expert
The information required by this Item regarding the audit committee of the Board of Directors and financial experts will be included in the 2017 Proxy Statement under the section captioned “The Board of Directors and its Committees-Audit Committee and Financial Experts” and is incorporated herein by reference thereto.
D. Code of Ethics
We have adopted a Code of Business Conduct and Ethics that applies to all of our employees and directors, including our principal executive officer, principal financial officer, principal accounting officer, controller, or persons performing similar functions. Our Code of Business Conduct and Ethics is posted on our website and can be accessed by selecting the “Corporate Governance” link at http://ir.criver.com. We will provide to any person, without charge, a copy of our Code of Business Conduct and Ethics. To obtain a copy, please mail a request to the Secretary, Charles River Laboratories, Inc., 251 Ballardvale Street, Wilmington, MA 01887. Information on our website is not incorporated by reference in this annual report.
E. Changes to Board Nomination Procedures
Since December 2008, there have been no material changes to the procedures by which security holders may recommend nominees to our Board of Directors.
Item 11. Executive Compensation
The information required by this Item will be included in the 2017 Proxy Statement under the sections captioned “2016 Director Compensation,” “Compensation Discussion and Analysis,” “Executive Compensation and Related Information,” “Compensation Committee Interlocks and Insider Participation” and “Report of Compensation Committee,” and is incorporated herein by reference thereto.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this Item will be included in the 2017 Proxy Statement under the sections captioned “Beneficial Ownership of Securities” and “Equity Compensation Plan Information” and is incorporated herein by reference thereto.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item will be included in the 2017 Proxy Statement under the sections captioned “Related Person Transaction Policy” and “Corporate Governance-Director Qualification Standards; Director Independence” and is incorporated herein by reference thereto.
Item 14. Principal Accountant Fees and Services
The information required by this Item will be included in the 2017 Proxy Statement under the section captioned “Statement of Fees Paid to Independent Registered Public Accounting Firm” and is incorporated herein by reference thereto.
PART IV
Item 15. Exhibits and Financial Statement Schedules
Item 15(a)(1) and (2) Financial Statements and Schedules
See "Index to Consolidated Financial Statements and Financial Statements Schedules" at Item 8 to this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
Item 15(a)(3) and Item 15(b) Exhibits
The exhibits filed as part of this Annual Report on Form 10-K are listed in the Exhibit Index immediately preceding the exhibits. We have identified in the Exhibit Index each management contract and compensation plan filed as an exhibit to this Annual Report on Form 10-K in response to Item 15(c) of Form 10-K.
Item 16. Form 10-K Summary
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
| | | |
| | | CHARLES RIVER LABORATORIES INTERNATIONAL, INC. |
| | | By: | /s/ DAVID R. SMITH |
| | | David R. Smith |
| Date: | February 14, 2017 | Corporate Executive Vice President, Chief Financial Officer and Chief Accounting Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
|
| | | |
| Signatures | Title | Date |
| By: | /s/ JAMES C. FOSTER | President, Chief Executive Officer and Chairman | February 14, 2017 |
| | James C. Foster | | |
| | | | |
| By: | /s/ DAVID R. SMITH | Corporate Executive Vice President, Chief | February 14, 2017 |
| | David R. Smith | Financial Officer and Chief Accounting Officer | |
| | | | |
| By: | /s/ ROBERT J. BERTOLINI | Director | February 14, 2017 |
| | Robert J. Bertolini | | |
| | | | |
| By: | /s/ STEPHEN D. CHUBB | Director | February 14, 2017 |
| | Stephen D. Chubb | | |
| | | | |
| By: | /s/ GEORGE E. MASSARO | Director | February 14, 2017 |
| | George E. Massaro | | |
| | | | |
| By: | /s/ DEBORAH KOCHEVAR | Director | February 14, 2017 |
| | Deborah Kochevar | | |
| | | | |
| By: | /s/ GEORGE M. MILNE, JR. | Director | February 14, 2017 |
| | George M. Milne, Jr. | | |
| | | | |
| By: | /s/ C. RICHARD REESE | Director | February 14, 2017 |
| | C. Richard Reese | | |
| | | | |
| By: | /s/ CRAIG B. THOMPSON | Director | February 14, 2017 |
| | Craig B. Thompson | | |
| | | | |
| By: | /s/ RICHARD F. WALLMAN | Director | February 14, 2017 |
| | Richard F. Wallman | | |
EXHIBIT INDEX
|
| | | | | |
| Exhibit No. | Description | Filed with this Form 10-K | Incorporation by Reference |
| Form | Filing Date | Exhibit No. |
| 3.1 | Second Amended and Restated Certificate of Incorporation of Charles River Laboratories International, Inc. dated June 5, 2000 | | S-1/A | June 23, 2000 | 3.1 |
| 3.2 | Fifth Amended and Restated By-Laws of Charles River Laboratories International, Inc. | | 8-K | May 16, 2016 | 3.2 |
| 4.1 | Form of Common Stock certificate, $0.01 par value, of Charles River Laboratories International, Inc. | | S-1 | June 23, 2000 | 4.1 |
| 4.2 | Charles River Laboratories International, Inc. Form of Performance Share Unit granted under the 2007 Incentive Plan | | 10-K | February 27, 2013 | 4.4 |
| 4.3 | Charles River Laboratories International, Inc. Form of Performance Share Unit granted under the 2016 Incentive Plan | X | | | |
| 10.1* | Charles River Laboratories International, Inc. 2007 Incentive Plan, as amended | | 10-K | February 17, 2015 | 10.13 |
| 10.2* | Charles River Laboratories International, Inc. 2016 Incentive Plan | | 10-Q | August 3, 2016 | 10.1 |
| 10.3* | Charles River Laboratories International, Inc. Form of Stock Option granted under the 2007 Incentive Plan, as amended | | 10-K | February 20, 2008 | 10.17 |
| 10.4* | Charles River Laboratories International, Inc. Form of Stock Option granted under the 2016 Incentive Plan | X | | | |
| 10.5* | Charles River Laboratories International, Inc. Form of Restricted Stock Award granted under the 2007 Incentive Plan, as amended | | 10-K | February 20, 2008 | 10.18 |
| 10.6* | Charles River Laboratories International, Inc. Form of Restricted Stock Unit granted under the 2007 Incentive Plan | X | | | |
| 10.7* | Charles River Laboratories International, Inc. Form of Restricted Stock Unit granted under the 2016 Incentive Plan | X | | | |
| 10.8* | Charles River Corporate Officer Separation Plan dated April 30, 2010 | | 10-Q | August 3, 2010 | 10.1 |
| 10.9* | Form of Change in Control Agreement | | 10-K | February 23, 2009 | 10.7 |
| 10.10* | Executive Incentive Compensation Plan dated January 1, 2016 | | 10-K | February 12, 2016 | 10.4 |
| 10.11* | Charles River Laboratories International, Inc. Non-Employee Directors Deferral Plan dated April 5, 2016 | | 10-Q | May 4, 2016 | 10.1 |
| 10.12* | Charles River Laboratories, Inc. Executive Life Insurance/Supplemental Retirement Income Plan | | 10-K | March 9, 2005 | 10.23 |
| 10.13* | Charles River Laboratories amended and restated Deferred Compensation Plan, as amended | | 10-K | February 27, 2012 | 10.11 |
| 10.14* | Amended and Restated Deferred Compensation Plan Document dated July 17, 2012 | | 10-Q | August 7, 2012 | 10.1 |
| 10.15* | Letter Agreements with Davide Molho dated May 22, 2009 | | 10-K | February 23, 2011 | 10.17 |
| 10.16* | Agreement between Thomas Ackerman and Charles River Laboratories, Inc. dated February 25, 2015 | | 8-K | February 27, 2015 | 99.10 |
| 10.17* | Agreement between David Smith and Charles River Laboratories, Inc. dated March 3, 2015 | | 10-K | February 12, 2016 | 10.16 |
| 10.18 | Charles River Laboratories International, Inc. Seventh Amended and Restated Credit Agreement dated March 30, 2016 | | 8-K | April 5, 2016 | 10.1 |
| 21.1 | Subsidiaries of Charles River Laboratories International, Inc. | X | | | |
| 23.1 | Consent of PricewaterhouseCoopers LLP | X | | | |
| 31.1 | Rule 13a-14(a)/15d-14(a) Certification of Chief Executive Officer | X | | | |
| 31.2 | Rule 13a-14(a)/15d-14(a) Certification of Chief Financial Officer | X | | | |
| 32.1 | Section 1350 Certification of the Chief Executive Officer and Chief Financial Officer | X | | | |
| 101.INS | eXtensible Business Reporting Language (XBRL) Instance Document | X | | | |
| 101.SCH | XBRL Taxonomy Extension Schema | X | | | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase | X | | | |
|
| | | | | |
| Exhibit No. | Description | Filed with this Form 10-K | Incorporation by Reference |
| Form | Filing Date | Exhibit No. |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase | X | | | |
| 101.LAB | XBRL Taxonomy Extension Labels Linkbase | X | | | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase | X | | | |
* Management contract or compensatory plan, contract or arrangement. |