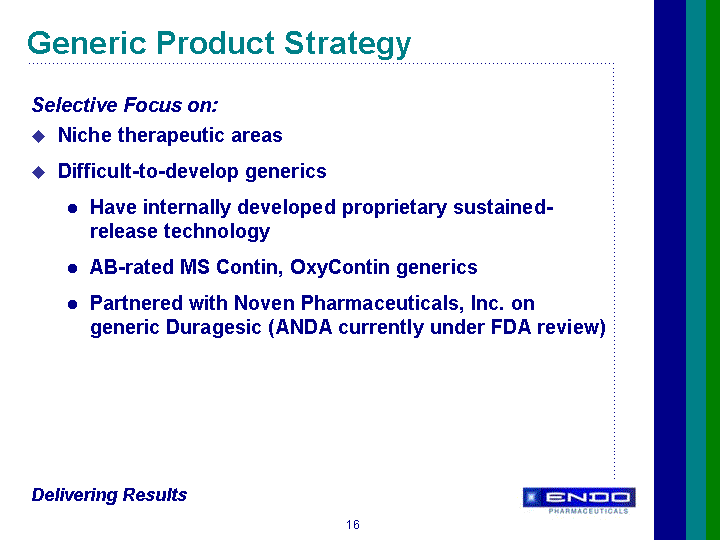
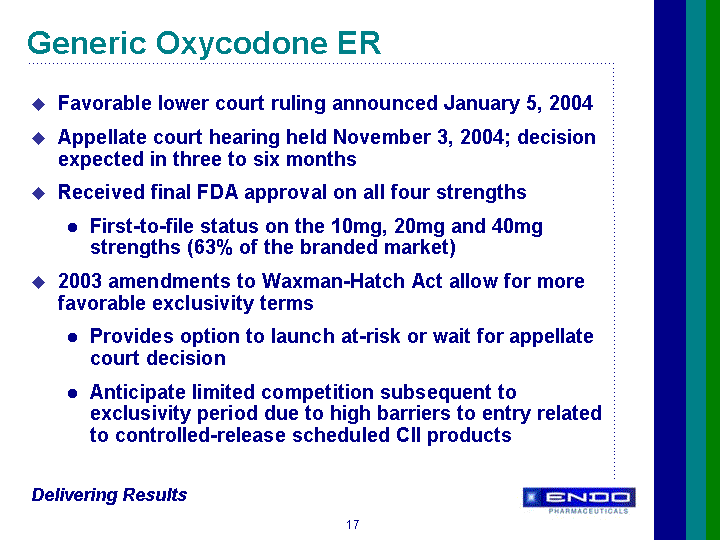
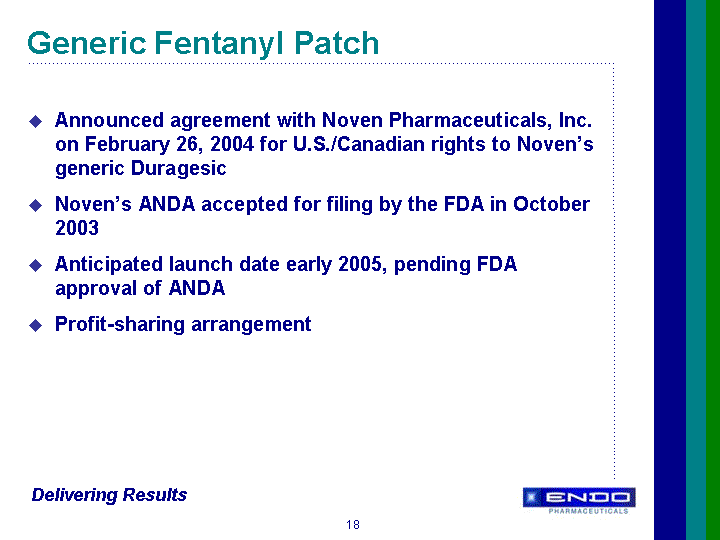
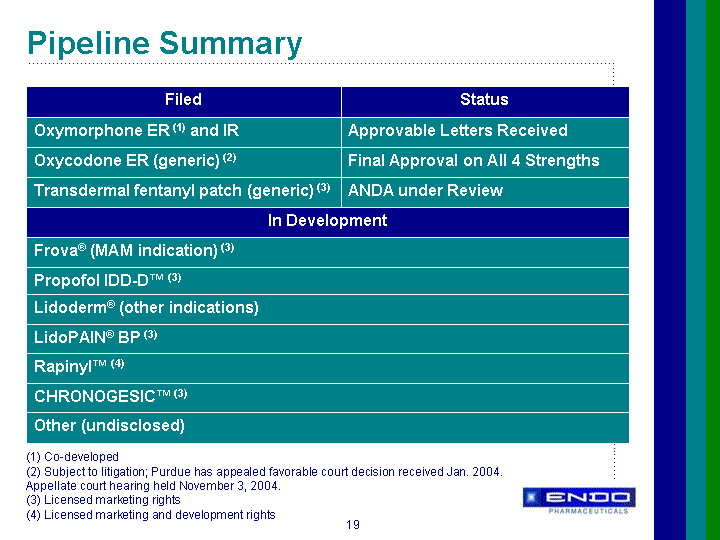
| Forward-Looking Statements This presentation contains forward-looking statements, within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on management's beliefs and assumptions, current expectations, estimates and projections. These statements are subject to risks and uncertainties and, therefore, actual results may differ materially from those expressed or implied by these forward-looking statements. Forward-looking statements are not historical facts and include information regarding the Company's possible or assumed results of operations. Also, statements or expressions that are preceded by, followed by, or that include, the words "believes," "anticipates," "plans," "expects," "intends," "estimates" or similar expressions are forward-looking statements. Endo's estimated or anticipated future results, product performance or other non-historical facts are forward-looking and reflect Endo's current perspective on existing trends and information. Many of the factors that will determine the Company's future results are beyond the ability of the Company to control or predict. The reader should not rely on any forward-looking statement. The Company undertakes no obligations to update any forward-looking statements whether as a result of new information, future events or otherwise. None of the development products in the Company's pipeline have been established as safe and effective by the FDA or approved by the FDA. Several important factors, in addition to the specific factors discussed in connection with these forward-looking statements individually, could affect the future results of Endo and could cause those results to differ materially from those expressed in the forward-looking statements contained herein. Important factors that may affect future results include, but are not limited to: the Company's ability to successfully develop, commercialize and market new products; results of clinical trials on new products; competition for the business of the Company's branded and generic products, and in connection with the Company's acquisition of rights to intellectual property assets; market acceptance of the Company's future products; government regulation of the pharmaceutical industry; the Company's dependence on a small number of products; the Company's dependence on outside manufacturers for the manufacture of its products; the Company's dependence on third parties to supply raw materials and to provide services for the core aspects of its business; new regulatory action or lawsuits relating to the Company's use of narcotics in most of its core products; the Company's exposure to product liability claims and product recalls and the possibility that the Company may not be able to adequately insure itself; the Company's ability to protect its proprietary technology; the Company's ability to successfully implement its acquisition strategy; the availability of controlled substances that constitute the active ingredients of some of the Company's products and products in development; the availability of third-party reimbursement for the Company's products; the Company's dependence on sales to a limited number of large pharmacy chains and wholesale drug distributors for a large portion of its total net sales; and other risks and uncertainties detailed in Endo's Registration Statement on Form S-4 filed with the Securities and Exchange Commission on June 9, 2000, as amended, and in Endo's Registration Statement on Form S-3 dated October 17, 2001, in Endo's Registration Statement on Form S-3 filed with the SEC on July 1, 2003, and in Endo's Registration Statement on Form S-3 filed with the SEC on April 30, 2004, as amended. Readers should evaluate any statement in light of these important factors. |