drives us… What …is our passionate pursuit of improving patients’ lives. ENDO PHARMACEUTICALS Pacific Growth Equities Life Sciences Conference June 13, 2006 Exhibit 99.1 |
1 © 2006 Endo Pharmaceuticals What drives us… Forward-Looking Statements This presentation contains forward-looking statements, within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on management’s beliefs and assumptions, current expectations, estimates and projections. These statements are subject to risks and uncertainties and, therefore, actual results may differ materially from those expressed or implied by these forward-looking statements. Forward-looking statements are not historical facts and include information regarding the Company’s possible or assumed results of operations. Also, statements or expressions that are preceded by, followed by, or that include, the words “believes,” “anticipates,” “plans,” “expects,” “intends,” “estimates” or similar expressions are forward-looking statements. Endo’s estimated or anticipated future results, product performance or other non-historical facts are forward-looking and reflect Endo’s current perspective on existing trends and information. Many of the factors that will determine the Company’s future results are beyond the ability of the Company to control or predict. The reader should not rely on any forward-looking statement. The Company undertakes no obligations to update any forward-looking statements whether as a result of new information, future events or otherwise. None of the development products in the Company’s pipeline have been established as safe and effective by the FDA or approved by the FDA. Several important factors, in addition to the specific factors discussed in connection with these forward-looking statements individually, could affect the future results of Endo and could cause those results to differ materially from those expressed in the forward-looking statements contained herein. Important factors that may affect future results include, but are not limited to: the Company’s ability to successfully develop, commercialize and market new products; results of clinical trials on new products; competition for the business of the Company’s branded and generic products, and in connection with the Company’s acquisition of rights to intellectual property assets; market acceptance of the Company’s future products; government regulation of the pharmaceutical industry; the Company’s dependence on a small number of products; the Company’s dependence on outside manufacturers for the manufacture of its products; the Company’s dependence on third parties to supply raw materials and to provide services for the core aspects of its business; new regulatory action or lawsuits relating to the Company’s use of narcotics in most of its core products; the Company’s exposure to product liability claims and product recalls and the possibility that the Company may not be able to adequately insure itself; the Company’s ability to protect its proprietary technology; the Company’s ability to successfully implement its acquisition strategy; the availability of controlled substances that constitute the active ingredients of some of the Company’s products and products in development; the availability of third-party reimbursement for the Company’s products; the Company’s dependence on sales to a limited number of large pharmacy chains and wholesale drug distributors for a large portion of its total net sales; and other risks and uncertainties detailed in Endo’s filings with the Securities and Exchange Commission, including its Registration Statement on Form S-3 filed with the SEC on March 21, 2006. Readers should evaluate any statement in light of these important factors. |
2 © 2006 Endo Pharmaceuticals What drives us… Endo Pharmaceuticals Profile Fully integrated specialty pharma company with market leadership in pain management; expanding into complementary therapeutic areas Well-developed commercial capability: Portfolio of branded prescription products including Lidoderm ® , Frova ® and Percocet ® , amongst others Promising pipeline, with seven mid- to late-stage products Oxymorphone ER and IR (brand names upon FDA approval) are the nearest-term opportunities Strong cash flow and no debt |
3 © 2006 Endo Pharmaceuticals What drives us… Lidoderm ® Product Profile Topical patch launched in 1999 Covered by five Orange Book- listed patents through 2015 First FDA-approved drug for the treatment of the pain of post- herpetic neuralgia, a form of neuropathic pain Provides analgesia (without anesthesia) directly to the affected nerves |
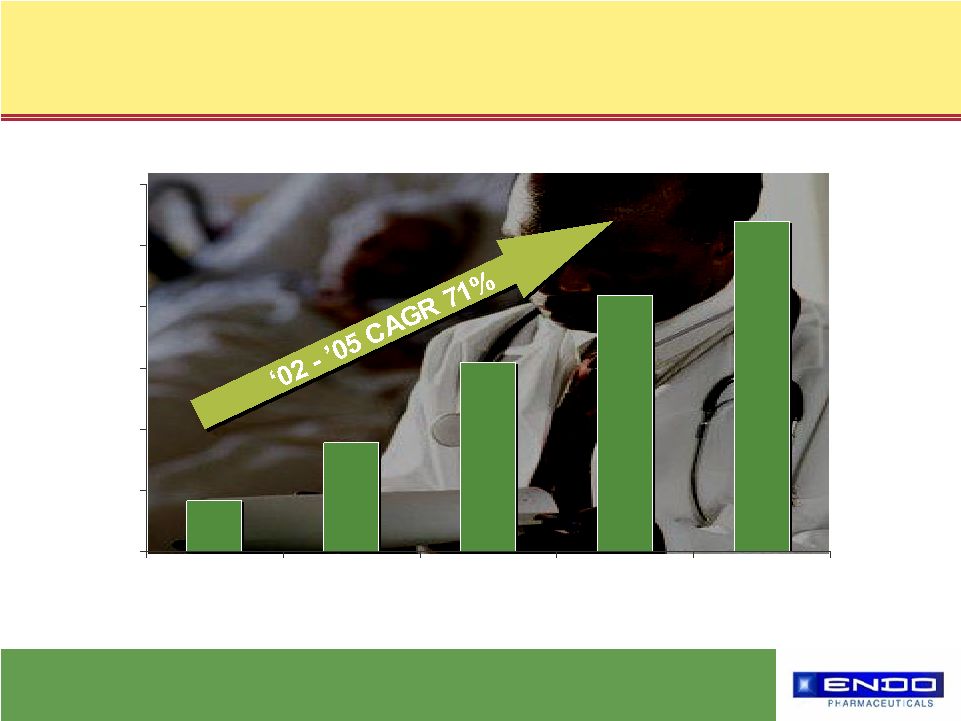
4 © 2006 Endo Pharmaceuticals What drives us… $83 $178 $309 $419 $540 $0 $100 $200 $300 $400 $500 $600 2002 2003 2004 2005 2006* *Represents high end of company guidance Lidoderm ® Net Sales ($ in millions) |
5 © 2006 Endo Pharmaceuticals What drives us… Lidoderm ® Future Growth Drivers Other Neuropathies: Peripheral Diabetic AIDS Cancer Trauma Goal is to explore possible utility in other neuropathic and chronic pain segments Neuropathic Pain Market PHN |
6 © 2006 Endo Pharmaceuticals What drives us… Frova ® Profile Triptan indicated for acute treatment of migraine headaches in adults Commercial strategy to implement marketing, education and clinical plan to differentiate Frova ® in the marketplace Low recurrence rate Long half-life Target specialty physician audience Focus on neurologists, pain management specialists Leverage existing coverage of high prescribers Create advocacy base among thought leaders Phase III development (two pivotal trials) completed for an indication of prophylaxis of Menstrual Migraine |
7 © 2006 Endo Pharmaceuticals What drives us… Selective focus on: Generic Product Strategy Niche therapeutic areas Difficult-to-develop generics Internally developed proprietary sustained-release technology AB-rated MS Contin, OxyContin generics Current marketed portfolio substantially consists of: Oxycodone ER Endocet ® Morphine Sulfate ER |
8 © 2006 Endo Pharmaceuticals What drives us… Substantial Pipeline Opportunities FDA-Approved - Launch shortly Synera™ (2) Transdermal sufentanil patch (3) Frova ® (Menstrual Migraine) (2) Topical ketoprofen patch (3) Rapinyl™ (3) Selected Endo Development Projects PDUFA date June 22, 2006 Oxymorphone ER (1) and IR Status Filed (1) Co-developed (2) Licensed marketing rights (3) Licensed marketing and development rights |
9 © 2006 Endo Pharmaceuticals What drives us… Topical, local anesthetic patch indicated for use in children and adults to numb the skin before various medical procedures; e.g., blood draws or IV cannulation Thin layer of local anesthetic formulation integrated with an oxygen-activated heating element Exclusive N. American rights licensed from ZARS Pharma Market Need Addressed: Hospitalized pediatric patients Anticipated benefits include: • Fast onset of action • Ease of administration Status: FDA approval in June 2005 • Safety and efficacy demonstrated in more than 660 pediatric (aged 3-17 years) and adult patients undergoing superficial dermatological procedures Anticipated launch in second half of 2006 Pediatric immunization studies planned Description / Indication: Synera TM |
10 © 2006 Endo Pharmaceuticals What drives us… Oral oxymorphone hydrochloride in extended (ER) and immediate (IR) release formulations Intended to treat moderate-to-severe pain IR a complementary treatment to ER for breakthrough pain Market Need Addressed: Will compete in strong opioid market We believe that oxymorphone ER will provide equivalent analgesia with only half the milligram dosage of OxyContin Convenience of twice-daily dosing Status: FDA “Approvable Letters” received 10/03 Positive results in Phase III trials for ER and IR conducted with FDA agreed-upon endpoints Filed complete responses with FDA on 12/22/06 PDUFA date 6/22/06 Description / Indications: Oxymorphone ER / IR Profile |
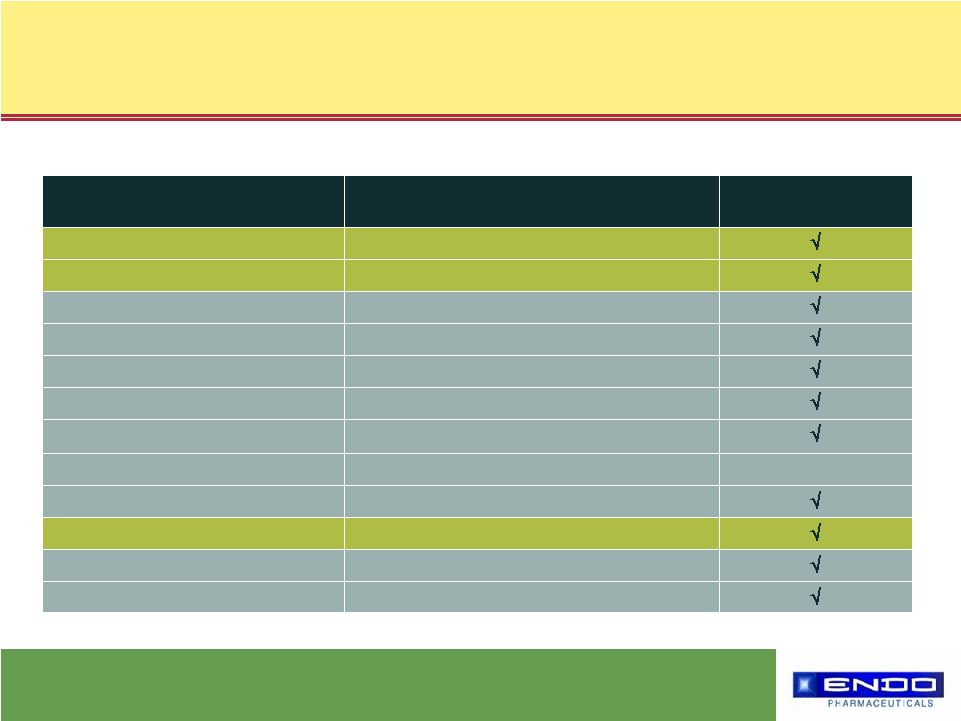
11 © 2006 Endo Pharmaceuticals What drives us… Oxymorphone ER / IR Clinical Trials Oxycodone IR and Placebo Post-Surgical Pain (SPA) Placebo Chronic Low Back Pain (SPA) Placebo Chronic Low Back Pain Oxycodone IR and Placebo Post-Surgical Pain Oxycodone IR and Placebo Post-Surgical Pain Oxycodone ER Cancer Pain --- Morphine Sulfate ER Cancer Pain Morphine Sulfate ER, Oxycodone ER Cancer Pain Placebo Osteoarthritis Pain Oxycodone ER and Placebo Chronic Low Back Pain Oxycodone ER and Placebo Osteoarthritis Pain Placebo Post-Surgical Pain Primary Outcome p<0.05 Comparator Indication Extensive program in more than 3,000 subjects, including 15 Phase II/III studies |
12 © 2006 Endo Pharmaceuticals What drives us… Oxymorphone ER Development Program FDA has accepted one clinical trial as pivotal proof of efficacy Two additional clinical trials conducted to file at least one additional pivotal trial First positive 12-week double-blind, placebo-controlled trials ever conducted with an opioid in chronic low back pain patients – One trial (under SPA) in opioid-naïve patients – One trial in opioid-experienced patients Efficacy data from these additional trials presented at APS Annual Meeting, May 3-6, in San Antonio, Texas |
13 © 2006 Endo Pharmaceuticals What drives us… Oxymorphone ER Data from APS 2006 Conclusions from ER studies: Patients titrated to effective, well-tolerated dose had pain level maintained effectively at same dose level for three months Statistically significant reduction in average pain intensity compared to placebo (p<0.0001) Both trials met all of their secondary endpoints Low drop-out rate due to AEs in treatment periods in both trials: Opioid-naïve (8.6% treated group vs. 8.0% placebo) Opioid-experienced (10% treated group vs. 11% placebo) |
14 © 2006 Endo Pharmaceuticals What drives us… Frova ® Clinical Development in MM Two double-blind, placebo-controlled studies in Menstrual Migraine (“MM”) prophylaxis; long-term open-label safety & tolerance study Frova ® taken for six days, starting two days prior to onset of expected MM headache Primary efficacy endpoint: reduction in the incidence of MM headache (statistical and clinical significance achieved in both trials) p<0.0001 to p < 0.01 (vs. placebo) Secondary endpoints also achieved - reduction in severity and duration of MM headache Results of first Phase III efficacy trial published in July 2004 Neurology Positive confirmatory Phase III efficacy study announced May 8, 2006 Expect to file sNDA in coming weeks |
15 © 2006 Endo Pharmaceuticals What drives us… Oral, fast-dissolving sublingual fentanyl tablet Intended for the treatment of breakthrough pain • Expected to compete with Actiq and FEBT Exclusive North American marketing and development rights licensed from Orexo AB Market Need Addressed: Intended to compete in the market for treatments of breakthrough pain Anticipated benefits include: • Fast onset of action • Enhanced absorption characteristics • Added convenience Status: Initiated Phase III clinical trials in Dec. 2005 Expect to file NDA in 2H 2007 Description / Indication: Rapinyl TM |
16 © 2006 Endo Pharmaceuticals What drives us… Topical patch intended for localized treatment of acute pain associated with soft-tissue injuries: • Tendonitis • Joint Sprains and Strains Licensed marketing and development rights from ProEthic in March 2005 Market Need Addressed: Ketoprofen (NSAID) currently only available in the U.S. in oral form Will compete in the ~$2.5 billion soft-tissue injury market primarily consisting of NSAIDs and COX-IIs Anticipated benefits include: • Bypassing the bloodstream • Local / targeted pain control • Once-daily dosing Status: Expect to enter Phase III trials in U.S. shortly Description / Indications: Topical Ketoprofen Patch |
17 © 2006 Endo Pharmaceuticals What drives us… Recent Accomplishments Completed clinical development program and filed responses to approvable letters on oxymorphone ER and IR Strengthened pipeline by licensing rights to two new development products Topical ketoprofen patch Transdermal sufentanil patch Initiated Phase III trials for Rapinyl TM Licensed Synera™, an FDA-approved topical local anesthetic patch from ZARS Pharma |
18 © 2006 Endo Pharmaceuticals What drives us… Key Milestones for 2006 PDUFA date for oxymorphone ER and IR 6/22/06 File sNDA for Frova ® in MM prophylaxis Coming weeks Initiate Phase III trials for topical ketoprofen patch 1 st Half Launch oxymorphone ER and IR 2 nd Half Launch Synera TM 2 nd Half Advance pipeline development Ongoing Acquire / in-license opportunities in pain and complementary areas such as neurology, perioperative care and supportive care oncology Ongoing Building a solid platform for sustainable growth: |
19 © 2006 Endo Pharmaceuticals What drives us… Summary Fully integrated specialty pharma company with market leadership in pain management; expanding into complementary therapeutic areas Well-developed commercial capability Broadest and deepest pipeline in the pain management arena Strong financial condition |
drives us… What …is our passionate pursuit of improving patients’ lives. ENDO PHARMACEUTICALS Nasdaq: ENDP |