Exhibit 99.1

Exhibit 99.1
ENDO PHARMACEUTICALS
Credit Suisse Healthcare Conference
What drives us…
November 16, 2006
…is our passionate pursuit of improving patients’ lives.

Forward-Looking Statements
This presentation contains forward-looking statements, within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, as amended, that are based on management’s beliefs and assumptions, current expectations, estimates and projections. These statements are subject to risks and uncertainties and, therefore, actual results may differ materially from those expressed or implied by these forward-looking statements. Forward-looking statements are not historical facts and include information regarding the Company’s possible or assumed results of operations. Also, statements or expressions that are preceded by, followed by, or that include, the words “believes,” “anticipates,” “plans,” “expects,” “intends,” “estimates” or similar expressions are forward-looking statements. Endo’s estimated or anticipated future results, product performance or other non-historical facts are forward-looking and reflect Endo’s current perspective on existing trends and information. Many of the factors that will determine the Company’s future results are beyond the ability of the Company to control or predict. The reader should not rely on any forward-looking statement. The Company undertakes no obligations to update any forward-looking statements whether as a result of new information, future events or otherwise. None of the development products in the Company’s pipeline have been established as safe and effective by the FDA or approved by the FDA. Several important factors, in addition to the specific factors discussed in connection with these forward-looking statements individually, could affect the future results of Endo and could cause those results to differ materially from those expressed in the forward-looking statements contained herein. Important factors that may affect future results include, but are not limited to: the Company’s ability to successfully develop, commercialize and market new products; results of clinical trials on new products; competition for the business of the Company’s branded and generic products, and in connection with the Company’s acquisition of rights to intellectual property assets; market acceptance of the Company’s future products; government regulation of the pharmaceutical industry; the Company’s dependence on a small number of products; the Company’s dependence on outside manufacturers for the manufacture of its products; the Company’s dependence on third parties to supply raw materials and to provide services for the core aspects of its business; new regulatory action or lawsuits relating to the Company’s use of narcotics in most of its core products; the Company’s exposure to product liability claims and product recalls and the possibility that the Company may not be able to adequately insure itself; the Company’s ability to protect its proprietary technology; the Company’s ability to successfully implement its acquisition strategy; the availability of controlled substances that constitute the active ingredients of some of the Company’s products and products in development; the availability of third-party reimbursement for the Company’s products; the Company’s dependence on sales to a limited number of large pharmacy chains and wholesale drug distributors for a large portion of its total net sales; and other risks and uncertainties detailed in Endo’s filings with the Securities and Exchange Commission, including its Registration Statement on Form S-3 filed with the SEC on March 21, 2006. Readers should evaluate any statement in light of these important factors.
What drives us…
© 2006 Endo Pharmaceuticals

Endo – A Leader in Pain Management
Fully integrated specialty pharma company
Market leadership in pain management; expanding into complementary therapeutic areas Established commercial capability Pipeline focused on delivering and executing
Five products in mid- to late-stage development
Strong cash flow and no debt
What drives us…
© 2006 Endo Pharmaceuticals

Growth Strategy
Capitalize on established brand names and brand awareness Develop/acquire proprietary products in our therapeutic areas and generic products with significant barriers to market entry Build balanced, sustainable pipeline across development phases Strengthen position in Pain Management and drive expansion into complementary therapeutic areas such as:
Neurology Perioperative Care Supportive Care Oncology
What drives us…
© 2006 Endo Pharmaceuticals

Expanding Commercial Portfolio
Patent-protected, branded products including:
Lidoderm® Frova®
Opana® ER/Opana® Synera
And more to come…
What drives us…
© 2006 Endo Pharmaceuticals
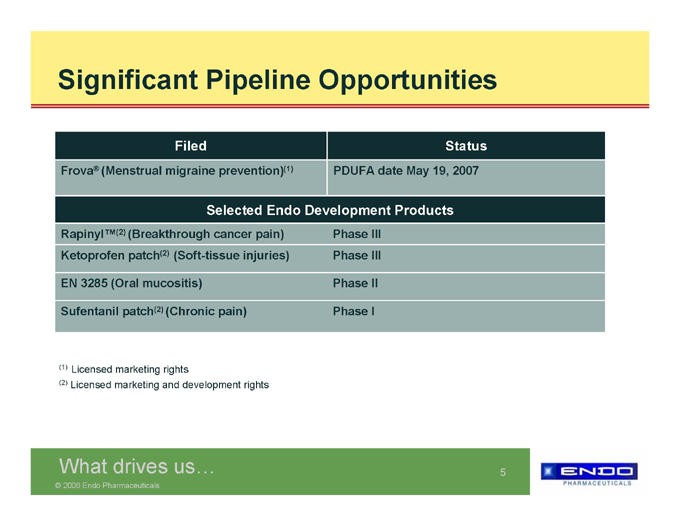
Significant Pipeline Opportunities
Filed Status
Frova® (Menstrual migraine prevention)(1) PDUFA date May 19, 2007
Selected Endo Development Products
Rapinyl™(2) (Breakthrough cancer pain) Phase III
Ketoprofen patch(2) (Soft-tissue injuries) Phase III
EN 3285 (Oral mucositis) Phase II
Sufentanil patch(2) (Chronic pain) Phase I
(1) | | Licensed marketing rights |
(2) | | Licensed marketing and development rights |
What drives us…
© 2006 Endo Pharmaceuticals

Key Milestones for 2006-2007
Building a solid platform for sustainable growth:
FDA action letter on Frova® sNDA in MM 5/19/07
Launch Opana® ER and Opana® Ongoing
Launch SyneraTM Ongoing
Advance pipeline development Ongoing
Acquire / in-license opportunities in pain and
complementary areas Ongoing
What drives us…
© 2006 Endo Pharmaceuticals

ENDO PHARMACEUTICALS
Nasdaq: ENDP
What drives us…
…is our passionate pursuit of improving patients’ lives.







