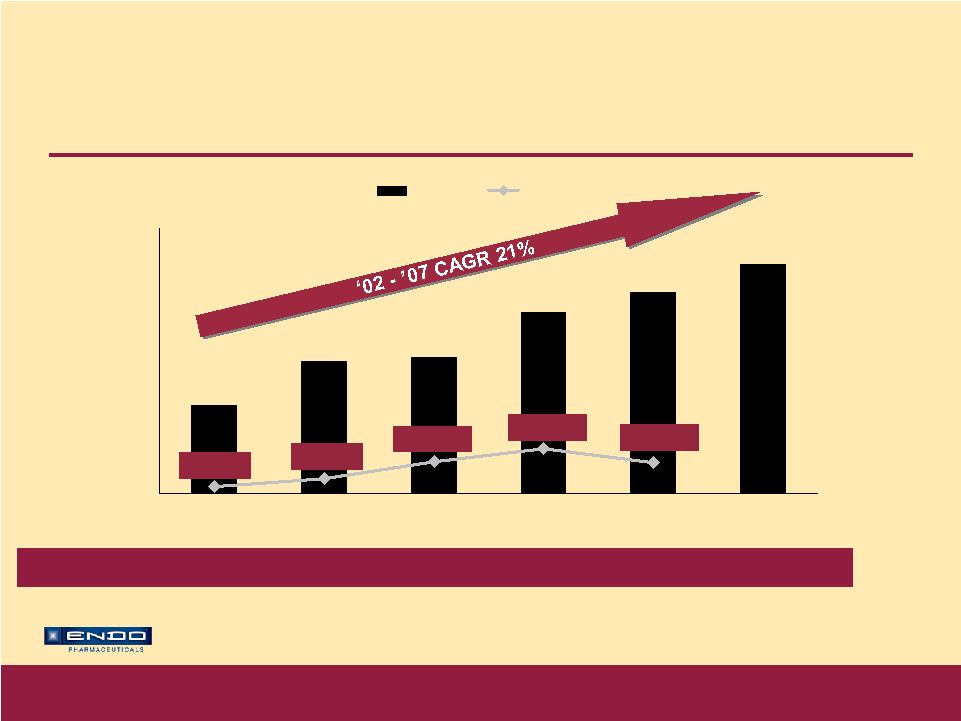
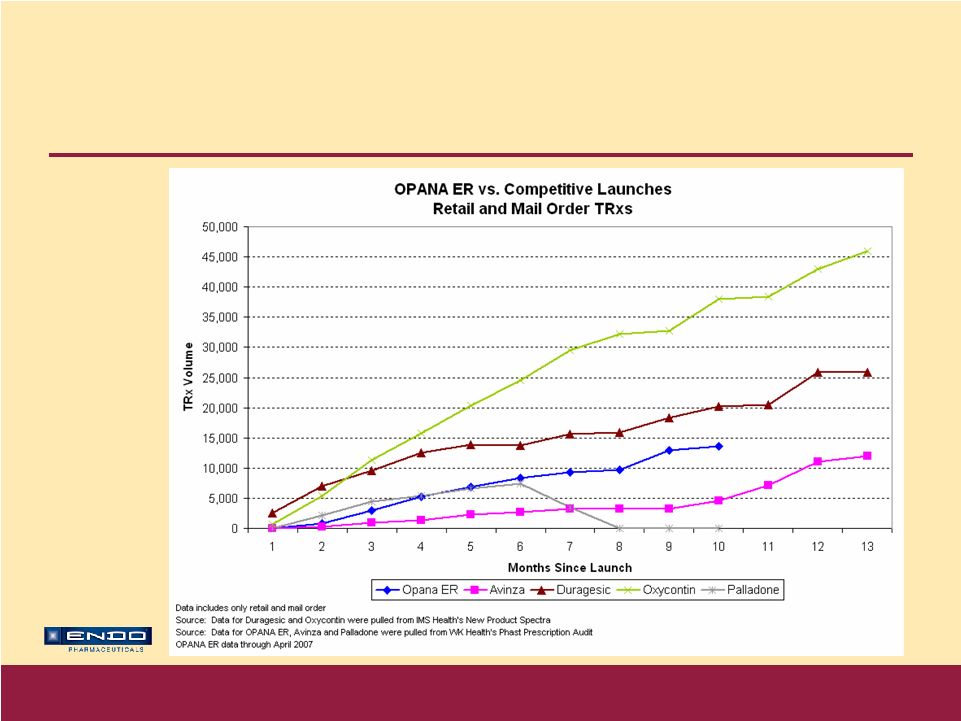
Accelerating Our Growth © 2007 Endo Pharmaceuticals Opana ® ER – Broad Label Broad indication: For the relief of moderate-to-severe pain in patients requiring continuous, around-the-clock opioid treatment for an extended period of time First time oxymorphone available in oral ER formulation Proven efficacy in broad range of appropriate pain patients Key messages of clinical differentiation • Twice-daily dosing • Effective pain control shown at stable dose for three months in clinical trials underscores durability of Opana ® ER’s analgesic effect • Generally well tolerated when titrated effectively* * Most common adverse events (> 10%) in trials were nausea, constipation, dizziness, vomiting, pruritus, somnolence, headache, increased sweating, and sedation |