Exhibit 99.1
2012 Investor Day
Endo Health Solutions
October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved
1
Forward Looking Statements
This presentation contains forward-looking statements within the meaning of the Private Securities
Litigation Reform Act of 1995. Statements including words such as “believes,” “expects,”
“anticipates,” “intends,” “estimates,” “plan,” “will,” “may,” “look forward,” “intend,” “guidance,” “future” or similar expressions are forward-looking statements. Because these statements reflect our current views, expectations and beliefs concerning future events, these forward-looking statements involve risks and uncertainties. Investors should note that many factors, as more fully described under the caption “Risk Factors” in our Form 10-K, Form 10-Q and Form 8-K filings with the Securities and Exchange Commission and as otherwise enumerated herein or therein, could affect our future financial results and could cause our actual results to differ materially from those expressed in forward-looking statements contained in our Annual Report on Form 10-K. The forward-looking statements in this presentation are qualified by these risk factors. These are factors that, individually or in the aggregate, could cause our actual results to differ materially from expected and historical results. We assume no obligation to publicly update any forward-looking statements, whether as a result of new information, future developments or otherwise.
© 2012 Endo Pharmaceuticals Inc. All rights reserved
2
Non-GAAP Financial Measures; Trademarks
This presentation may refer to non-GAAP financial measures that are not prepared in accordance with accounting principles generally accepted in the United States and that may be different from non-GAAP financial measures used by other companies. Investors are encouraged to review Endo’s current report on Form 8-K filed with the SEC for Endo’s reasons for including those non-GAAP financial measures in this presentation. Reconciliations of certain non-GAAP financial measures to the most directly comparable GAAP financial measures are provided at the end of this presentation.
All product and service names appearing in a typeface different from that of the surrounding text or with a trademark symbol, including the Endo and product logos, are trademarks and/or service marks owned by or licensed to Endo, its subsidiaries or its affiliates other than Voltaren®, which is a registered trademark of Novartis Corporation, BEMA®, which is a registered trademark of BioDelivery Sciences International, Inc., Lidoderm®, which is a registered trademark of Hind Health Care, Inc., Frova®, which is a registered trademark of Vernalis Development Limited and Urocidin®, which is a trademark of Bioniche Life Sciences Inc.
© 2012 Endo Pharmaceuticals Inc. All rights reserved
3
2012 Investor Day
Endo Health Solutions Vision & Strategy
David Holveck
President and Chief Executive Officer
October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved
4
2012 Investor Day Agenda
Welcome
• Blaine Davis, SVP Corporate Affairs Endo Strategy and Vision
• Dave Holveck, President & CEO
Qualitest
• Julie McHugh, Chief Operating Officer AMS
• Camille Farhat, President, AMS
HealthTronics
• Kelly Huang, President, HealthTronics Endo Pharmaceuticals
• Julie McHugh, Chief Operating Officer R&D
• Ivan Gergel, EVP, Research & Development Finance
• Alan Levin, EVP & Chief Financial Officer Closing Comments
• Dave Holveck, President & CEO
Q&A
• All Speakers
© 2012 Endo Pharmaceuticals Inc. All rights reserved
5
Endo Health Solutions—Transformation
2008 – 2011 Diversifying our Business Created Optionality
2012 – 2013 Transformation to Offset Pharmaceutical Loss
of Exclusivity
2014 – Beyond Sustaining Growth, Durability & Long Term
Expansion
© 2012 Endo Pharmaceuticals Inc. All rights reserved
6
Endo Health Solutions Today Has
Track record of revenue growth and profitability
Durable cash flow
Organic and long-term growth across business segments
Disciplined capital allocation
International capabilities
Diversified pipeline
© 2012 Endo Pharmaceuticals Inc. All rights reserved
7
Diversifying Our Business
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
8
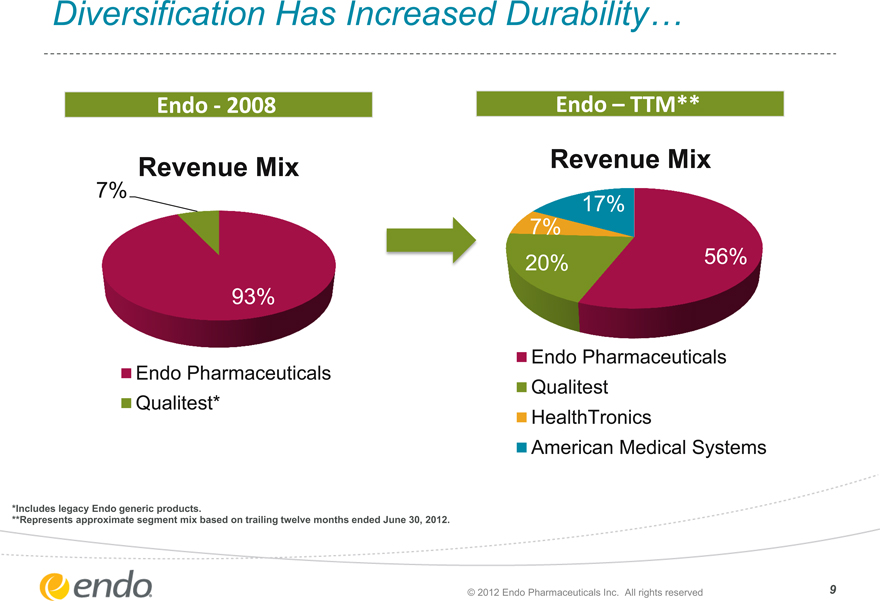
Diversification Has Increased Durability…
Endo - 2008
Revenue Mix
7%
93%
Endo Pharmaceuticals Qualitest*
Endo – TTM**
Revenue Mix
17%
7% 20%
Endo Pharmaceuticals Qualitest HealthTronics American Medical Systems
*Includes legacy Endo generic products.
**Represents approximate segment mix based on trailing twelve months ended June 30, 2012.
© 2012 Endo Pharmaceuticals Inc. All rights reserved
9
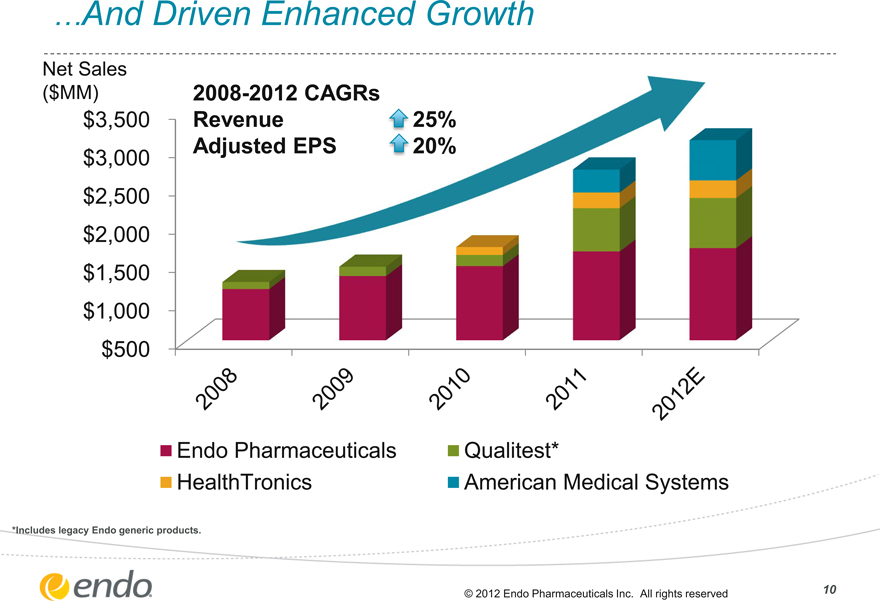
…And Driven Enhanced Growth
Net Sales
($MM)
$3,500 $3,000 $2,500 $2,000 $1,500 $1,000 $500
2008-2012 CAGRs
Revenue 25%
Adjusted EPS 20%
2008 2009 2010 2011 2012E
Endo Pharmaceuticals HealthTronics
Qualitest*
American Medical Systems
*Includes legacy Endo generic products.
© 2012 Endo Pharmaceuticals Inc. All rights reserved
10
Transforming Our Enterprise
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
11
Vision & Mission
VISION
To enable better care by redefining healthcare value
MISSION
To meet the needs of today’s healthcare customers by providing high quality and effective solutions that improve treatment outcomes, access to care, and economic value
© 2012 Endo Pharmaceuticals Inc. All rights reserved
12
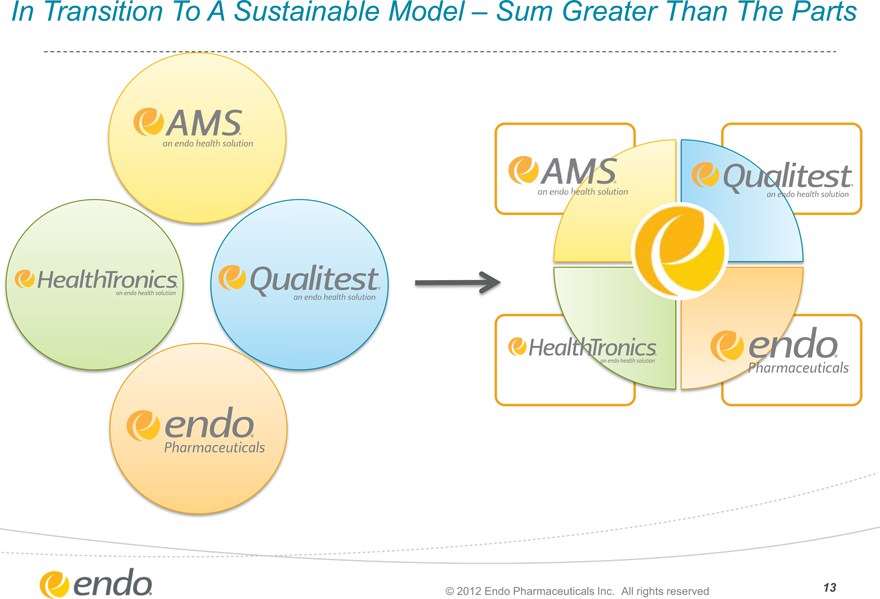
In Transition To A Sustainable Model – Sum Greater Than The Parts
AMS an endo health solution
HealthTronics an endo health solution
Qualitest an endo health solution
endo Pharmaceuticals
AMS an endo health solution
Qualitest an endo health solution
HealthTronics an endo health solution
endo Pharmaceuticals
© 2012 Endo Pharmaceuticals Inc. All rights reserved
13
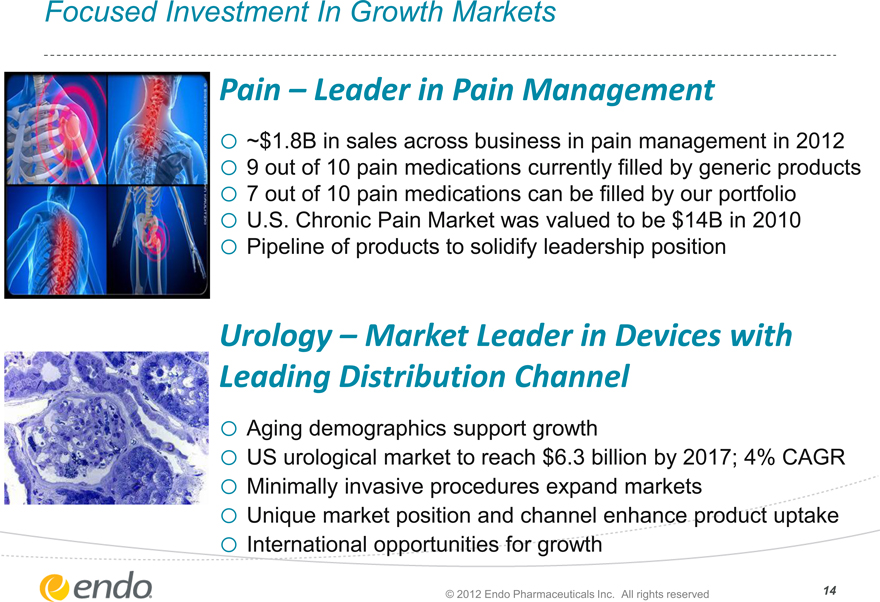
Focused Investment In Growth Markets
Pain – Leader in Pain Management
o ~$1.8B in sales across business in pain management in 2012 o 9 out of 10 pain medications currently filled by generic products o 7 out of 10 pain medications can be filled by our portfolio o U.S. Chronic Pain Market was valued to be $14B in 2010 o Pipeline of products to solidify leadership position
Urology – Market Leader in Devices with Leading Distribution Channel
o Aging demographics support growth o US urological market to reach $6.3 billion by 2017; 4% CAGR o Minimally invasive procedures expand markets o Unique market position and channel enhance product uptake o International opportunities for growth
© 2012 Endo Pharmaceuticals Inc. All rights reserved
14
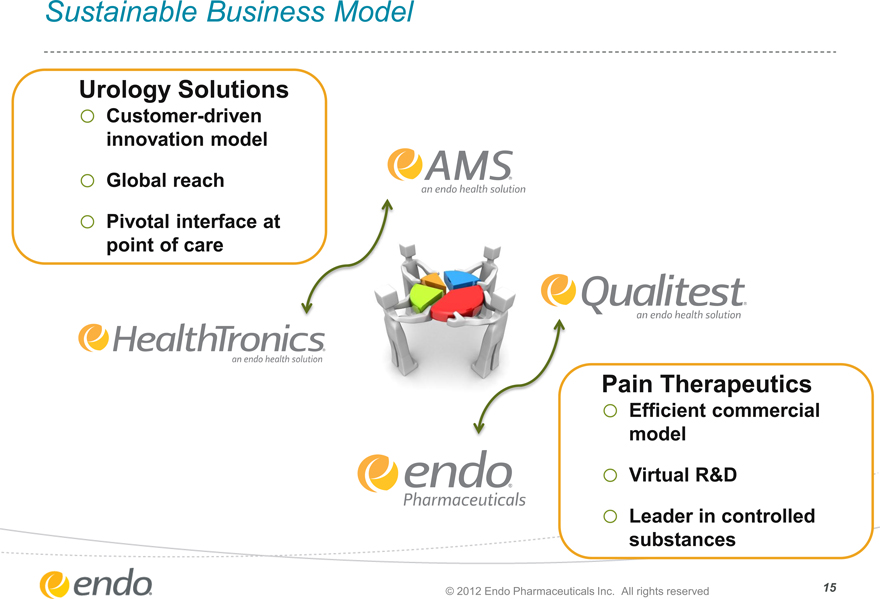
Sustainable Business Model
Urology Solutions
o Customer-driven
innovation model
o Global reach
o Pivotal interface at
point of care
AMS an endo health solution
Qualitest an endo health solution
HealthTronics an endo health solution
endo Pharmaceuticals
Pain Therapeutics
o Efficient commercial
model
o Virtual R&D
o Leader in controlled
substances
© 2012 Endo Pharmaceuticals Inc. All rights reserved
15
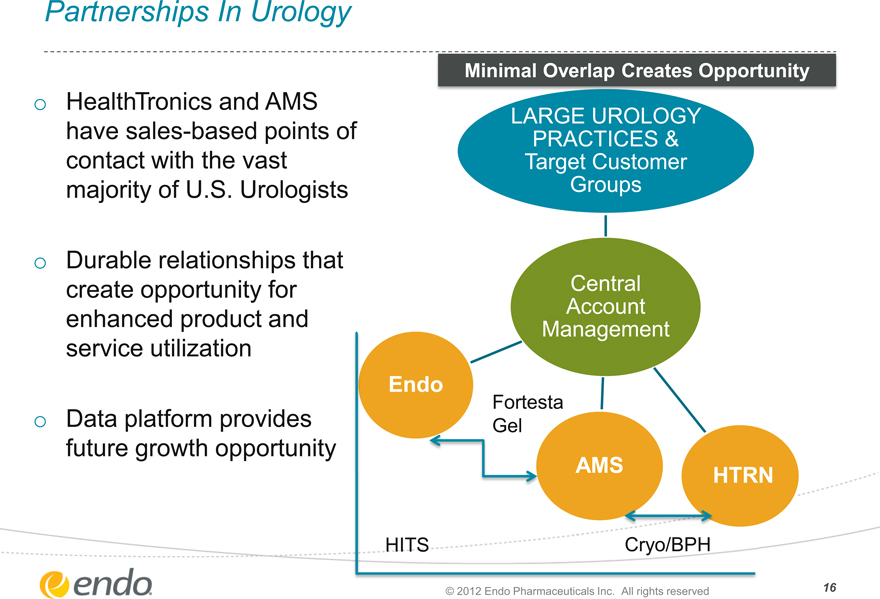
Partnerships In Urology
HealthTronics and AMS have sales-based points of contact with the vast majority of U.S. Urologists
Durable relationships that create opportunity for enhanced product and service utilization
Data platform provides future growth opportunity
Minimal Overlap Creates Opportunity
LARGE UROLOGY PRACTICES & Target Customer
Groups
Central Account Management
Endo
Fortesta Gel
AMS
HTRN
HITS
Cryo/BPH
© 2012 Endo Pharmaceuticals Inc. All rights reserved
16
Sustaining Growth & Profitability
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
17
Qualitest Has Outperformed Expectations
Durable business with sustained double digit revenue growth and improving margins through 2015
Brought us enhanced supply chain flexibility o Manufacture of Percocet® and Endocet® transferred to Huntsville facility in early 2012 o Preserving nearly $200 million of annual sales that previously would have been at-risk to the Novartis facility shutdown
Accelerated synergy capture of $50 million in 2012
o Manufacturing cost reductions versus third party o API savings created by qualifying a second source of supply for more products
© 2012 Endo Pharmaceuticals Inc. All rights reserved
18
Investment In Innovation – R&D
Research and Development diverse growth opportunities:
o Branded Pharmaceuticals - Pain and Urology
o Generics - higher value lines of business
o Devices that enhance current technologies
© 2012 Endo Pharmaceuticals Inc. All rights reserved
19
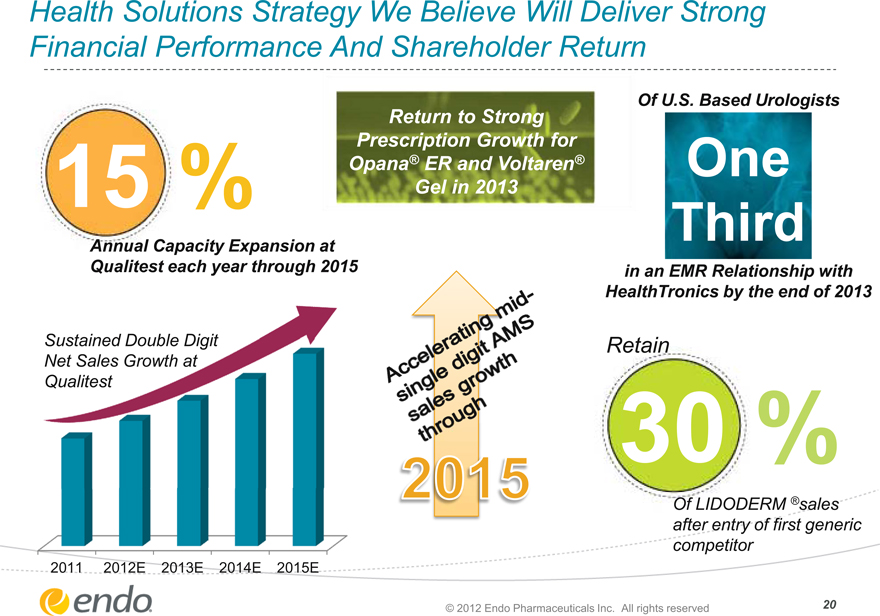
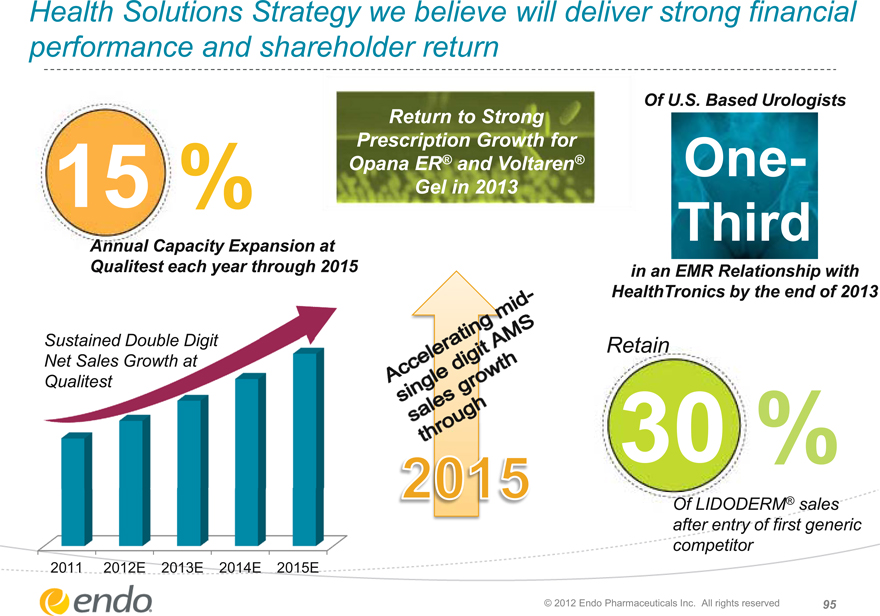
Health Solutions Strategy We Believe Will Deliver Strong Financial Performance And Shareholder Return
15%
Annual Capacity Expansion at
Qualitest each year through 2015
Sustained Double Digit
Net Sales Growth at Qualitest
2011 2012E 2013E 2014E 2015E
Return to Strong Prescription Growth for Opana® ER and Voltaren® Gel in 2013
Accelerating mid-single digit AMS
Sales growth through
2015
Of U.S. Based Urologists
One Third
in an EMR Relationship with HealthTronics by the end of 2013
Retain
30%
Of LIDODERM ®sales after entry of first generic competitor
© 2012 Endo Pharmaceuticals Inc. All rights reserved
20
Endo Health Solutions Will Continue To Have
Track record of revenue growth and profitability
Durable cash flow
Organic and long-term growth across business segments Disciplined capital allocation International capabilities Diversified pipeline
© 2012 Endo Pharmaceuticals Inc. All rights reserved
21
2012 Investor Day
Qualitest
Julie McHugh
Chief Operating Officer
October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved
22
Qualitest Key Takeaways
Double-digit net sales growth expected through 2015 Sustained gross margins expected through 2015 Capacity expansion and pricing flexibility drive topline growth
15% annual increases in capacity planned through 2015 Pricing flexibility driven by portfolio optimization
Leader and expert in controlled substances and liquids supports future growth Sizable ANDA portfolio in higher value lines of business
© 2012 Endo Pharmaceuticals Inc. All rights reserved
23
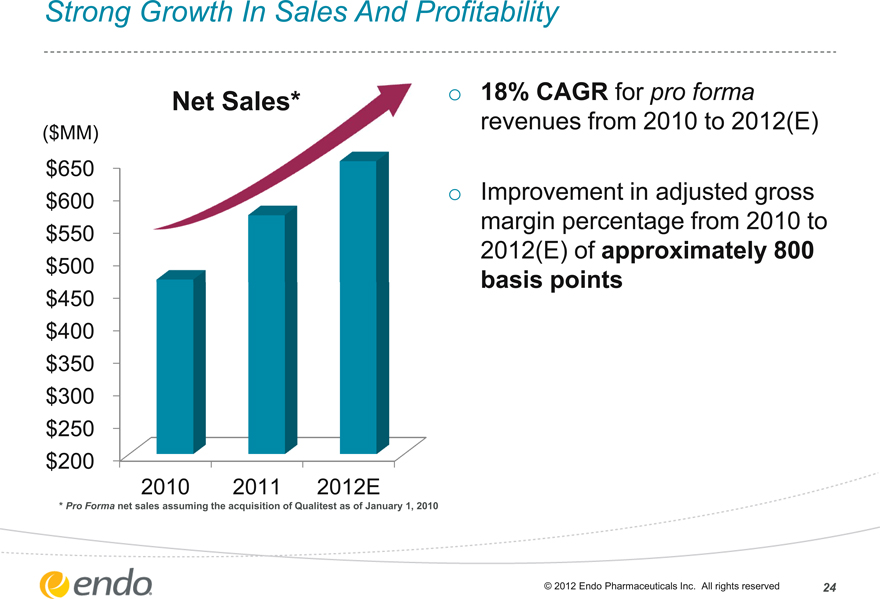
Strong Growth In Sales And Profitability
Net Sales*
($MM)
$650 $600 $550 $500 $450 $400 $350 $300 $250 $200
2010 2011 2012E
* Pro Forma net sales assuming the acquisition of Qualitest as of January 1, 2010
18% CAGR for pro forma revenues from 2010 to 2012(E)
Improvement in adjusted gross margin percentage from 2010 to 2012(E) of approximately 800 basis points
© 2012 Endo Pharmaceuticals Inc. All rights reserved
24
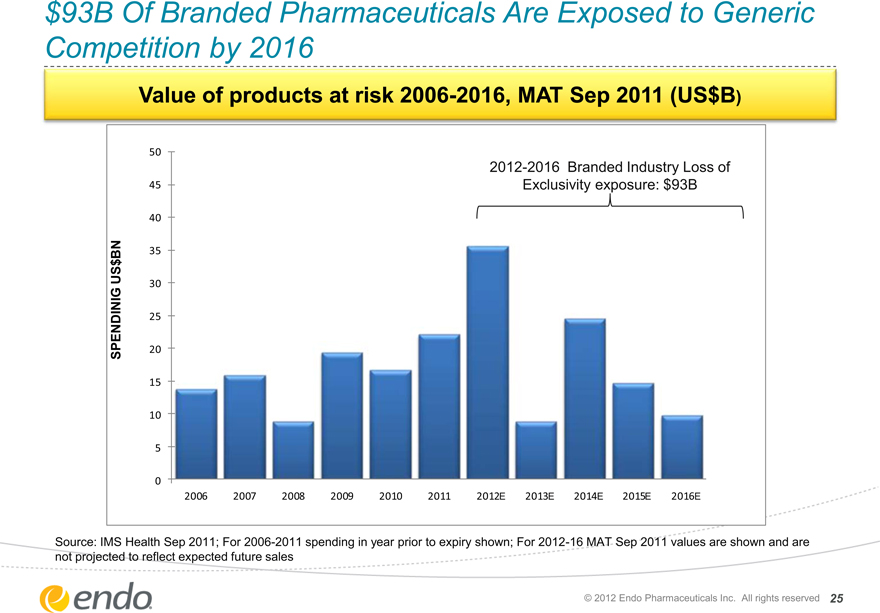
$93B Of Branded Pharmaceuticals Are Exposed to Generic Competition by 2016
Value of products at risk 2006-2016, MAT Sep 2011 (US$B)
2012-2016 Branded Industry Loss of
Exclusivity exposure: $93B
50
45
40
BN 35
$
US 30
SPENDING US$BN
25
20
15
10
5
0
2006 2007 2008 2009 2010 2011 2012E 2013E 2014E 2015E 2016E
Source: IMS Health Sep 2011; For 2006-2011 spending in year prior to expiry shown; For 2012-16 MAT Sep 2011 values are shown and are not projected to reflect expected future sales
© 2012 Endo Pharmaceuticals Inc. All rights reserved
25
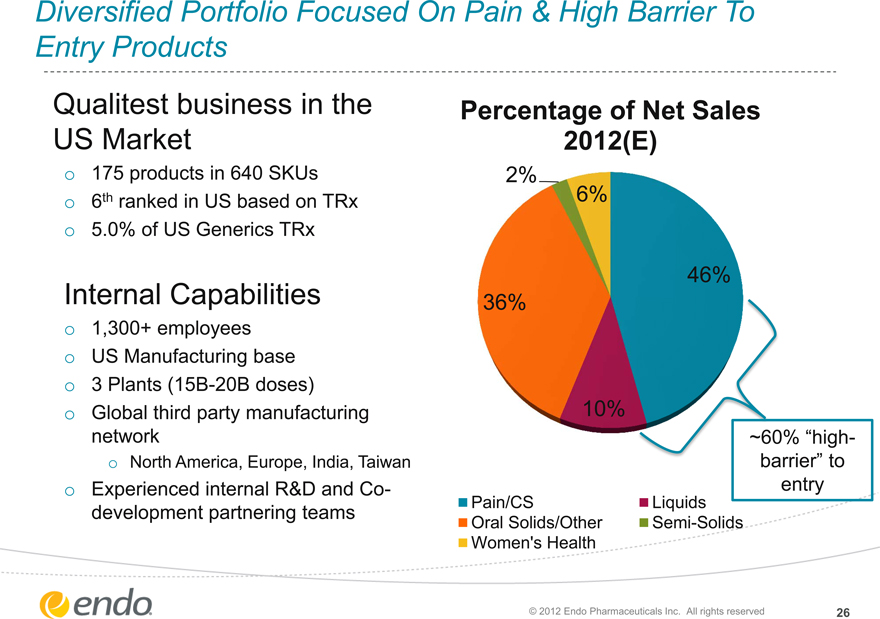
Diversified Portfolio Focused On Pain & High Barrier To Entry Products
Qualitest business in the US Market
175 products in 640 SKUs 6th ranked in US based on TRx 5.0% of US Generics TRx
Internal Capabilities
1,300+ employees US Manufacturing base
3 Plants (15B-20B doses) Global third party manufacturing network
North America, Europe, India, Taiwan
Experienced internal R&D and Co-development partnering teams
Percentage of Net Sales 2012(E)
2% 6%
46% 36%
10%
~60% “high-barrier” to entry
Pain/CS Liquids Oral Solids/Other Semi-Solids Women’s Health
© 2012 Endo Pharmaceuticals Inc. All rights reserved
26
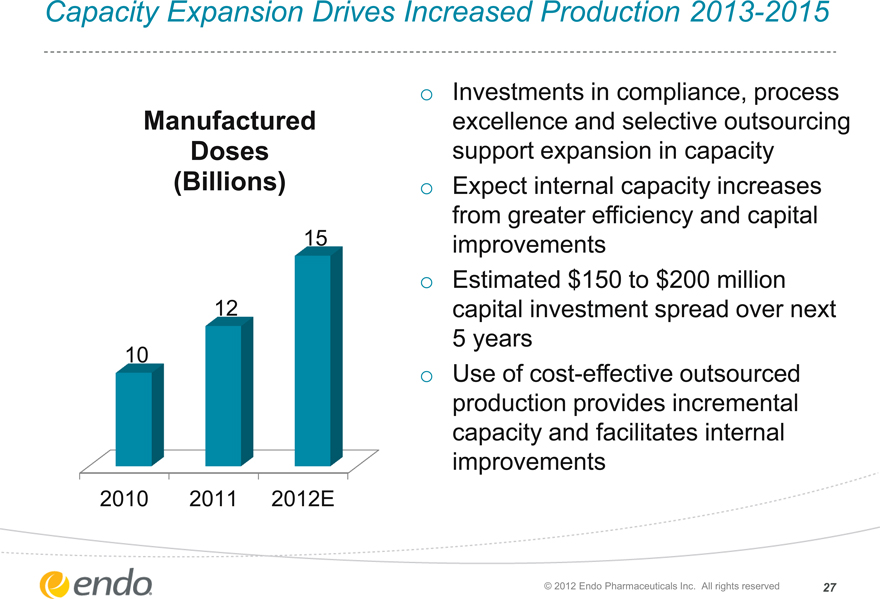
Capacity Expansion Drives Increased Production 2013-2015
Manufactured Doses (Billions)
15
12
10
2010 2011 2012E
Investments in compliance, process excellence and selective outsourcing support expansion in capacity Expect internal capacity increases from greater efficiency and capital improvements Estimated $150 to $200 million capital investment spread over next 5 years Use of cost-effective outsourced production provides incremental capacity and facilitates internal improvements
© 2012 Endo Pharmaceuticals Inc. All rights reserved
27
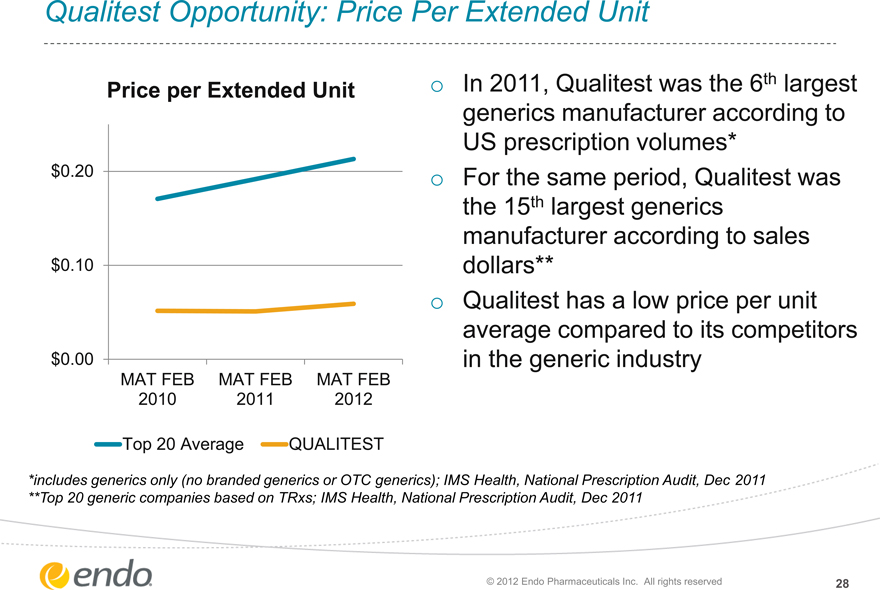
Qualitest Opportunity: Price Per Extended Unit
Price per Extended Unit
$0.20
$0.10
$0.00
MAT FEB MAT FEB MAT FEB 2010 2011 2012
Top 20 Average QUALITEST
In 2011, Qualitest was the 6th largest generics manufacturer according to US prescription volumes* For the same period, Qualitest was the 15th largest generics manufacturer according to sales dollars** Qualitest has a low price per unit average compared to its competitors in the generic industry
*includes generics only (no branded generics or OTC generics); IMS Health, National Prescription Audit, Dec 2011 **Top 20 generic companies based on TRxs; IMS Health, National Prescription Audit, Dec 2011
© 2012 Endo Pharmaceuticals Inc. All rights reserved
28
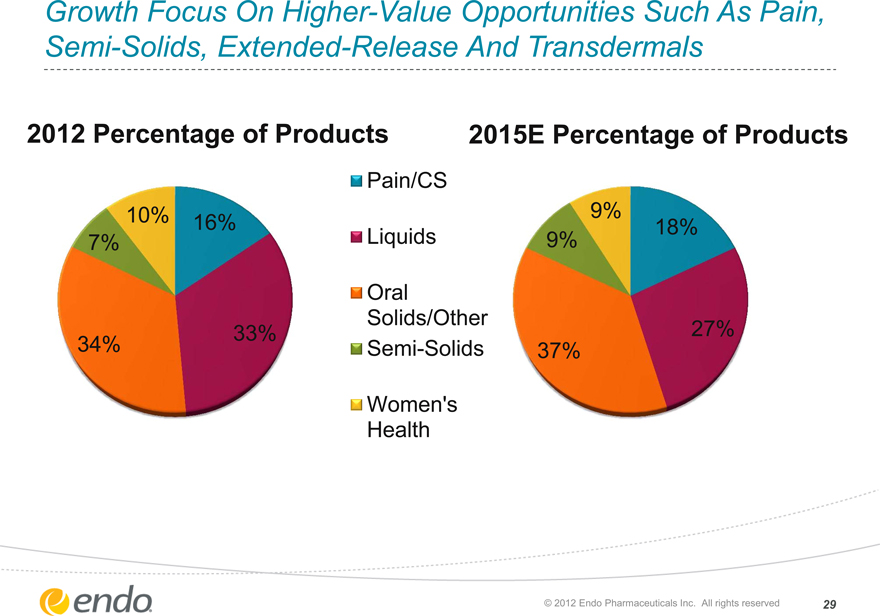
Growth Focus On Higher-Value Opportunities Such As Pain, Semi-Solids, Extended-Release And Transdermals
2012 Percentage of Products 2015E Percentage of Products
10% 16% 7%
33% 34%
9%
18% 9%
27% 37%
Pain/CS
Liquids
Oral Solids/Other
Semi-Solids
Women’s Health
© 2012 Endo Pharmaceuticals Inc. All rights reserved
29
R&D—Balance Of Organic Development And Strategic Acquisition
Pipeline investments differentiated based on portfolio needs
Capitalizing on current infrastructure scale and expertise in core technologies
Accelerating pipeline and commercial products through external partnerships, licensing and asset acquisitions
© 2012 Endo Pharmaceuticals Inc. All rights reserved
30
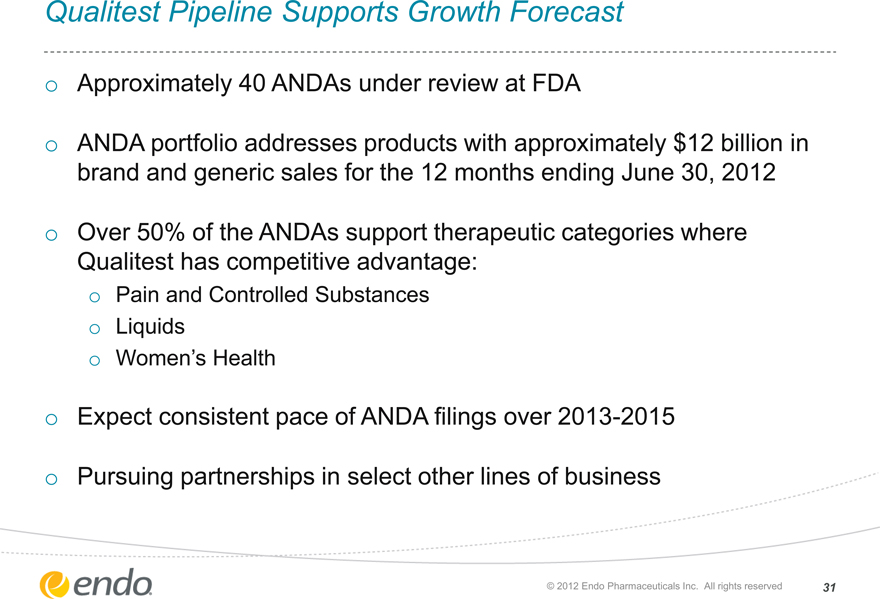
Qualitest Pipeline Supports Growth Forecast
Approximately 40 ANDAs under review at FDA
ANDA portfolio addresses products with approximately $12 billion in brand and generic sales for the 12 months ending June 30, 2012
Over 50% of the ANDAs support therapeutic categories where Qualitest has competitive advantage:
Pain and Controlled Substances Liquids
Women’s Health
Expect consistent pace of ANDA filings over 2013-2015
Pursuing partnerships in select other lines of business
© 2012 Endo Pharmaceuticals Inc. All rights reserved
31
Qualitest Key Takeaways
Double-digit net sales growth expected through 2015 Sustained or improving margin expected through 2015 Capacity expansion and pricing flexibility drive topline growth
15% annual increases in capacity planned through 2015 Pricing flexibility driven by portfolio optimization
Leader and expert in controlled substances and liquids supports future growth Sizable ANDA portfolio in higher value lines of business
© 2012 Endo Pharmaceuticals Inc. All rights reserved
32
2012 Investor Day
AMS
Camille Farhat President, AMS October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved
33
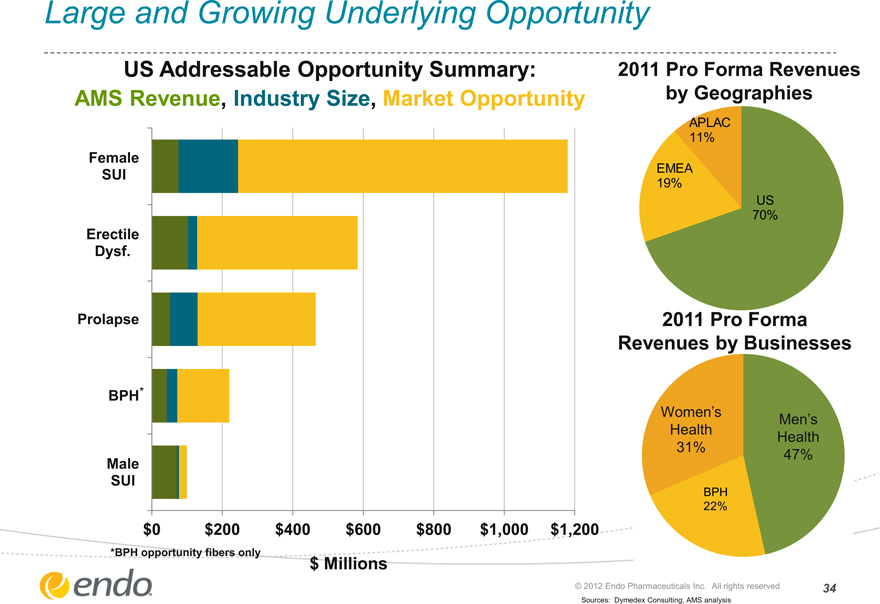
Large and Growing Underlying Opportunity
US Addressable Opportunity Summary: AMS Revenue, Industry Size, Market Opportunity
Female
SUI
Erectile
Dysf.
Prolapse
BPH*
Male
SUI
$0 $200 $400 $600 $800 $1,000 $1,200
*BPH opportunity fibers only
$ Millions
2011 Pro Forma Revenues by Geographies
APLAC 11%
EMEA 19%
US 70%
2011 Pro Forma Revenues by Businesses
Women’s Men’s
Health Health
31% 47%
BPH
22%
© 2012 Endo Pharmaceuticals Inc. All rights reserved
Sources: Dymedex Consulting, AMS analysis
34
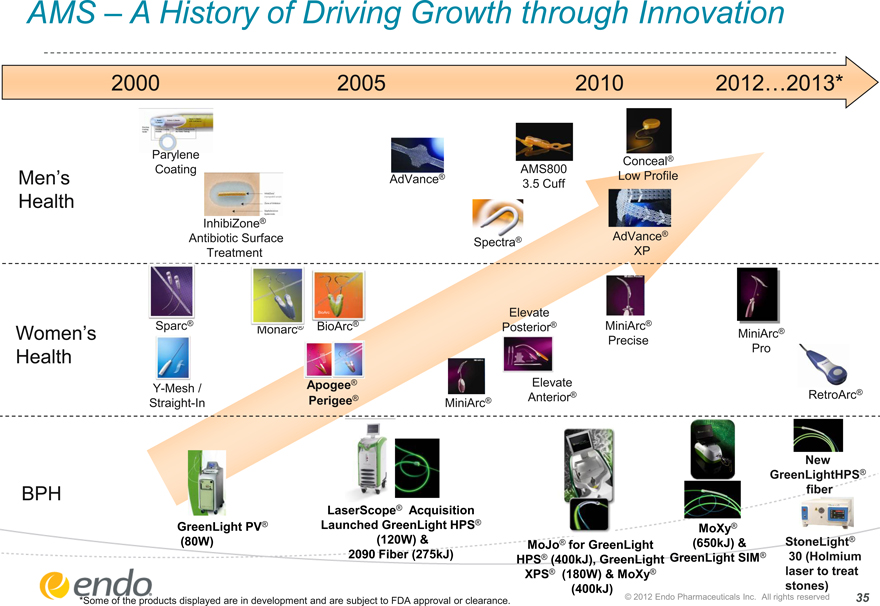
AMS – A History of Driving Growth through Innovation 2000 2005 2010 2012…2013* Men’s Health Women’s Health BPH GreenLight PV® (80W) LaserScope® Acquisition Launched GreenLight HPS® (120W) & 2090 Fiber (275kJ) MoXy® (650kJ) & GreenLight SIM®
MoJo® for GreenLight HPS® (400kJ), GreenLight XPS® (180W) & MoXy®
(400kJ) Sparc® Y-Mesh / Straight-In Elevate Posterior® Monarc® BioArc® MiniArc® Elevate Anterior®
Apogee® Perigee® BioArcBioArcMiniArc® Precise MiniArc® Pro RetroArc® AMS800 3.5 Cuff Conceal® Low Profile AdVance®
AdVance®
XP InhibiZone® Antibiotic Surface Treatment Parylene Coating StoneLight® 30 (Holmium laser to treat stones) New GreenLightHPS® fiber Spectra® *Some of the products displayed are in development and are subject to FDA approval or clearance. © 2012 Endo Pharmaceuticals Inc. All rights reserved
35
AMS Uniquely Positioned to Capitalize on Relationships and Reputation
© 2012 Endo Pharmaceuticals Inc. All rights reserved
36
AMS Growth Projections 2012—2015
Key Business Drivers
Men’s Health Steady profitable growth
Women’s Health Data, therapy awareness and education to return to growth, with support from physician training
BPH Increase utilization through clinical evidence, training, and access
International Therapy Awareness
2012 2015E
Mid-to-High Single Digit Growth
$515M-$535M
Men’s Health Women’s Health BPH
AMS = solid, balanced growth from all businesses
© 2012 Endo Pharmaceuticals Inc. All rights reserved
37
Men’s Health Business Overview
\Men’s Health: A Foundational Business for AMS
Erectile Restoration
$584M US opportunity / $1.7B globally
92% patient satisfaction rate1 96% partner satisfaction rate1 >70% physician interested in increasing implant surgeries2 Market Share Leader
Male Continence
$200M global opportunity / 60% penetration
92% of AMS 800® patients would have the device placed again3 94% of AdVance® patients recommend procedure to a friend4 Market Share Leader
Sources: 1Montorsi F, et al. AMS three-piece inflatable implants for erectile dysfunction: a long-term multi-institution study in 200 consecutive patients. Eur Urol. 2000;37:50-55 (n=200). 2AMS/KJT Group. Physician implanter research quantitative results, June 2010, page 77 (n=200). 3Litweller SE, et al. Post-prostatectomy incontinence and the artificial urinary sphincter: a long-term study of patient satisfaction and criteria for success. J Urol. 1996 Dec;156(6):1975-1980 (n=50). 4Suskind AM, et al. Patient-perceived outcomes of the AdVance sling up to months post procedure. Neurourol Urodyn. 2011 Sept;30(7):1267-70 (n=36).
© 2012 Endo Pharmaceuticals Inc. All rights reserved
38
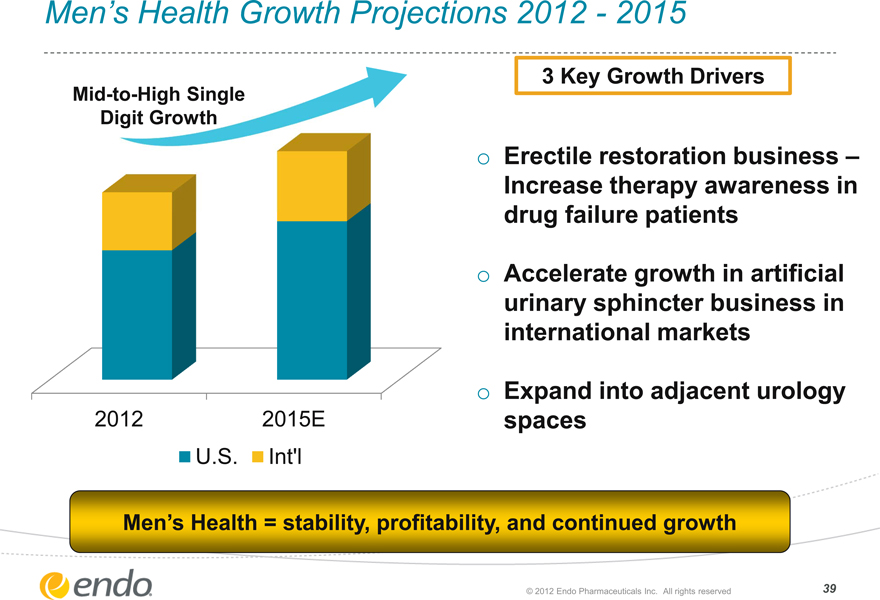
Men’s Health Growth Projections 2012—2015
Mid-to-High Single Digit Growth
2012 2015E
U.S. Int’l
3 Key Growth Drivers
Erectile restoration business – Increase therapy awareness in drug failure patients
Accelerate growth in artificial urinary sphincter business in international markets
Expand into adjacent urology spaces
Men’s Health = stability, profitability, and continued growth
© 2012 Endo Pharmaceuticals Inc. All rights reserved
39
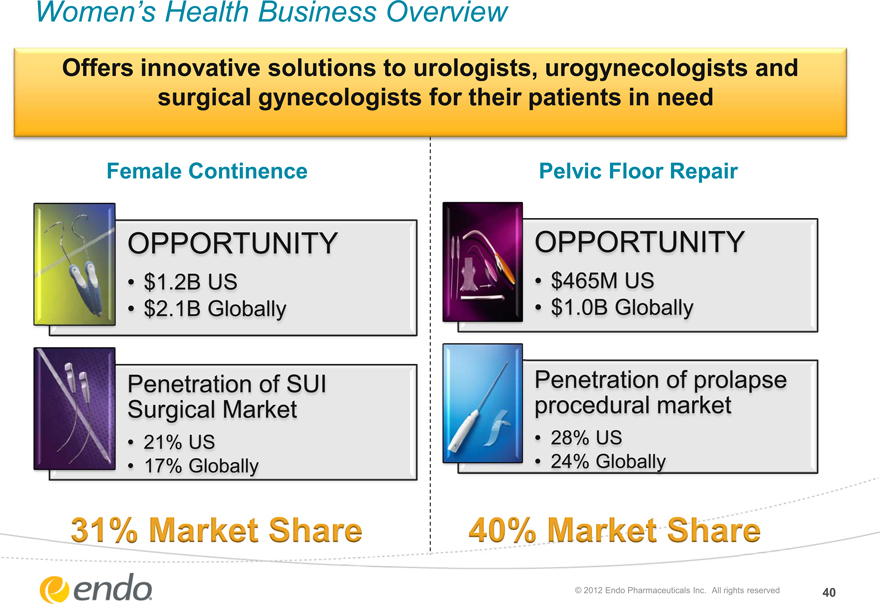
Women’s Health Business Overview
Offers innovative solutions to urologists, urogynecologists and surgical gynecologists for their patients in need
Female Continence
Pelvic Floor Repair
OPPORTUNITY
$1.2B US
$2.1B Globally
$465M US
$1.0B Globally
Penetration of SUI Surgical Market
21% US 17% Globally
Penetration of prolapse procedural market
28% US 24% Globally
31% Market Share 40% Market Share
© 2012 Endo Pharmaceuticals Inc. All rights reserved
40
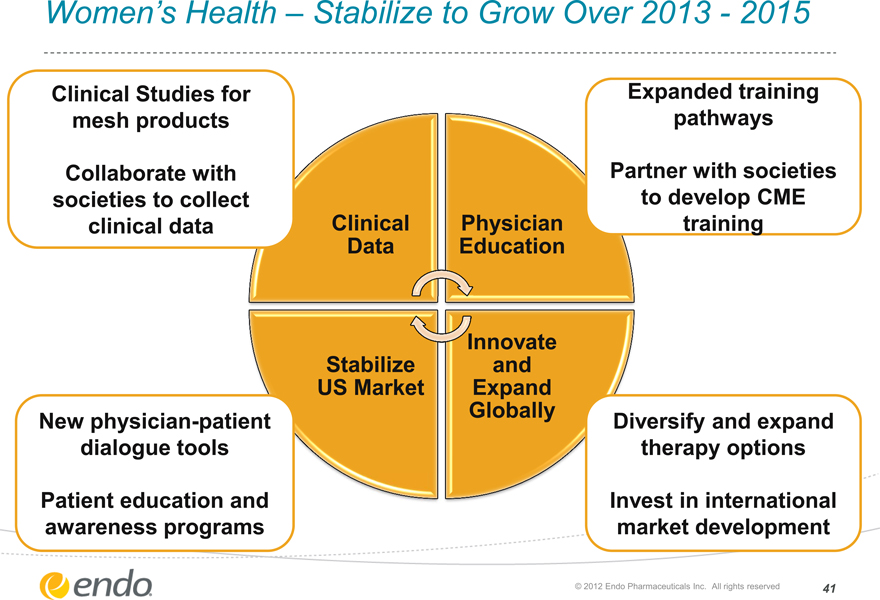
Women’s Health – Stabilize to Grow Over 2013—2015
Clinical Studies for mesh products
Collaborate with societies to collect clinical data
Clinical
Data
Physician Education
Expanded training pathways
Partner with societies to develop CME training
New physician-patient dialogue tools
Patient education and awareness programs Stabilize
US Market
Innovate and Expand Globally
Diversify and expand therapy options
Invest in international market development
© 2012 Endo Pharmaceuticals Inc. All rights reserved
41
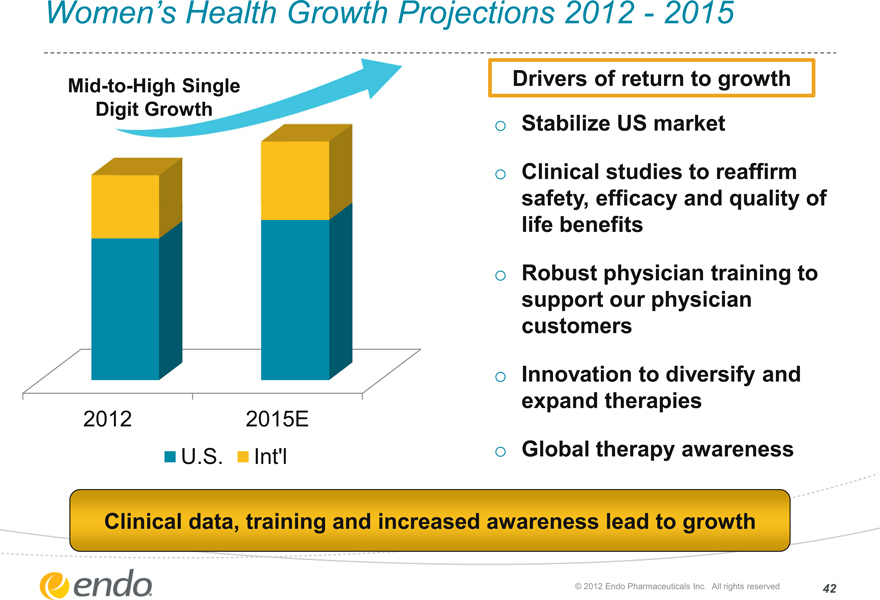
Women’s Health Growth Projections 2012—2015
Mid-to-High Single Digit Growth
2012
2015E
U.S.
Int’l
Drivers of return to growth
Stabilize US market
Clinical studies to reaffirm safety, efficacy and quality of life benefits
Robust physician training to support our physician customers
Innovation to diversify and expand therapies
Global therapy awareness
Clinical data, training and increased awareness lead to growth
© 2012 Endo Pharmaceuticals Inc. All rights reserved
42
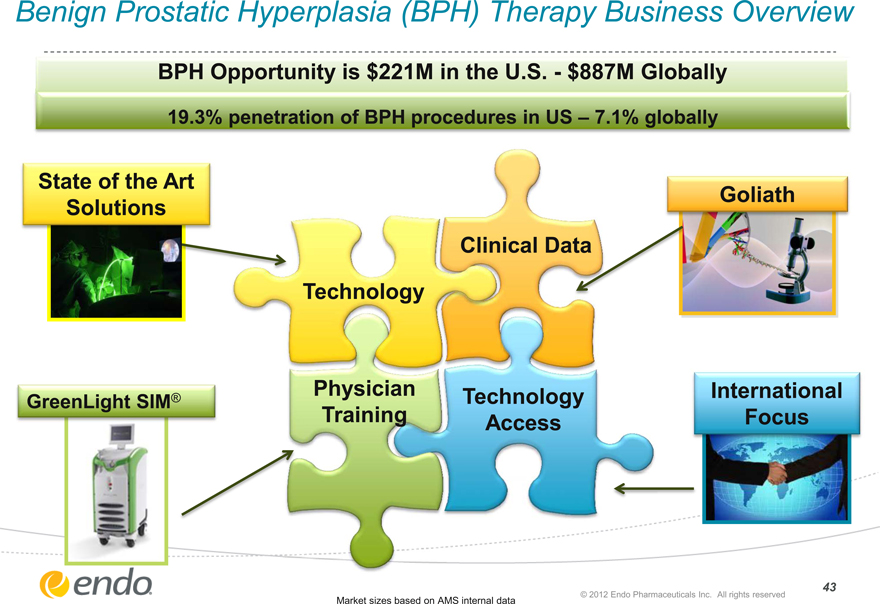
Benign Prostatic Hyperplasia (BPH) Therapy Business Overview
BPH Opportunity is $221M in the U.S.—$887M Globally
19.3% penetration of BPH procedures in US – 7.1% globally
State of the Art Solutions
GreenLight SIM®
Technology
Physician Training
Clinical Data
Technology Access
Goliath
International Focus
Market sizes based on AMS internal data
© 2012 Endo Pharmaceuticals Inc. All rights reserved
43
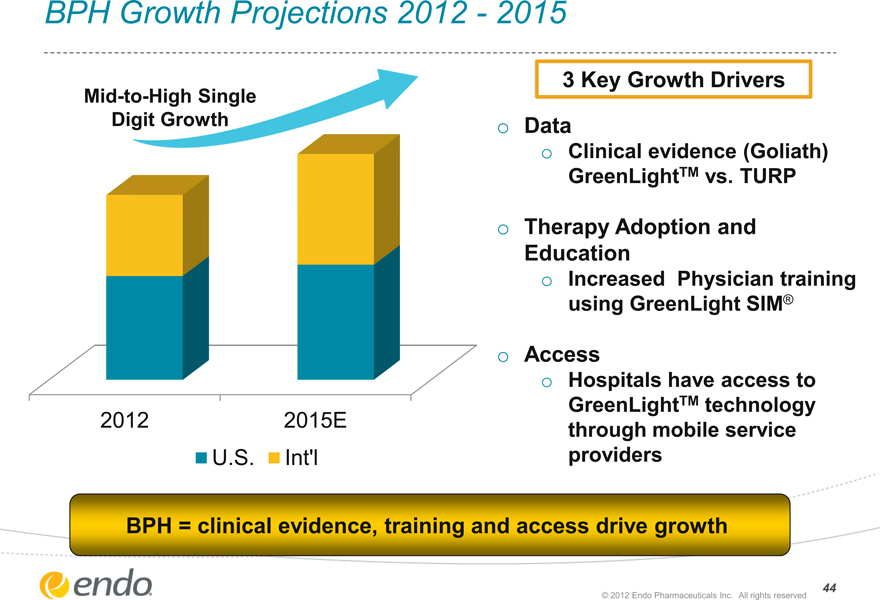
BPH Growth Projections 2012—2015
Mid-to-High Single Digit Growth
2012
2015E
U.S.
Int’l
3 Key Growth Drivers
Data
Clinical evidence (Goliath) GreenLightTM vs. TURP
Therapy Adoption and Education
Increased Physician training using GreenLight SIM®
Access
Hospitals have access to GreenLightTM technology through mobile service providers
BPH = clinical evidence, training and access drive growth
© 2012 Endo Pharmaceuticals Inc. All rights reserved
44
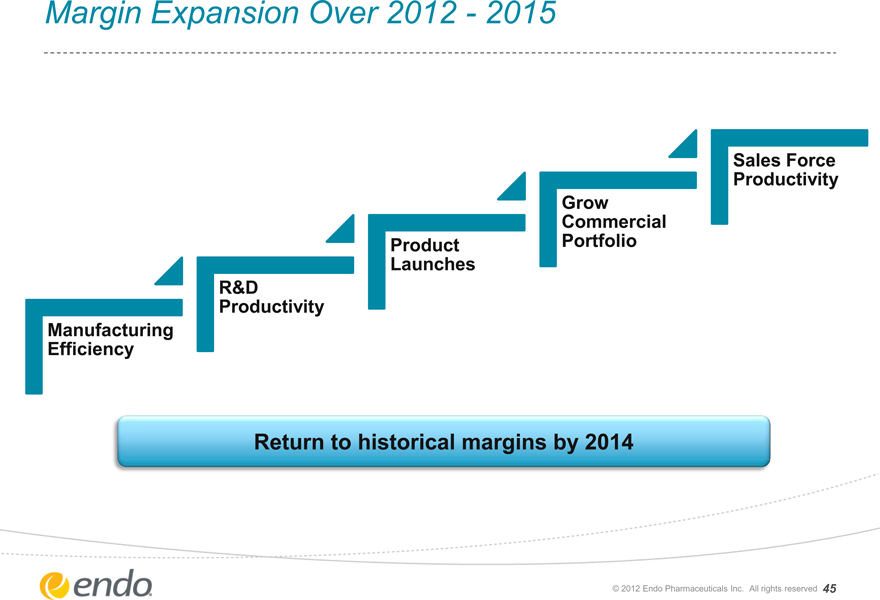
Margin Expansion Over 2012—2015
Manufacturing Efficiency
R&D Productivity
Product Launches
Grow Commercial Portfolio
Sales Force Productivity
Return to historical margins by 2014
© 2012 Endo Pharmaceuticals Inc. All rights reserved
45
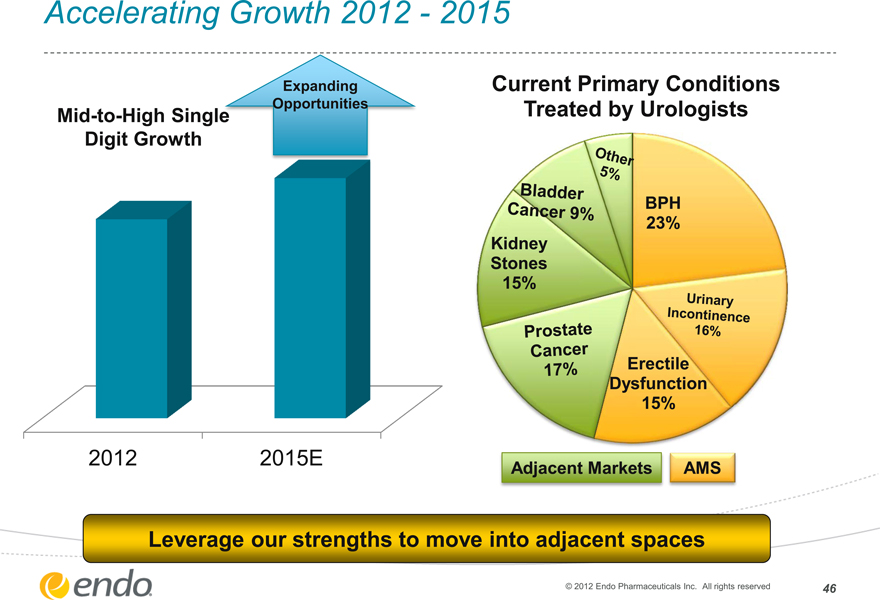
Accelerating Growth 2012—2015
Mid-to-High Single Digit Growth
Expanding Opportunities
2012
2015E
Current Primary Conditions Treated by Urologists
Other 5%
Bladder Cancer 9%
Kidney Stones 15%
Prostate Cancer 17%
BPH 23%
Urinary Incontinence 16%
Erectile Dysfunction 15%
Adjacent Markets
AMS
Leverage our strengths to move into adjacent spaces
© 2012 Endo Pharmaceuticals Inc. All rights reserved
46
AMS Key Takeaways
Strong organic growth opportunities remain within AMS core business lines – mid-to-high single digit CAGR 2012-2015
AMS is uniquely positioned in urology
Leverage Endo partnership to enhance leadership in urology
Expect to return operating margin to historical levels (high 20 percent to low 30 percent by 2014)
Opportunities in adjacent spaces with existing customers
© 2012 Endo Pharmaceuticals Inc. All rights reserved
47
2012 Investor Day
HealthTronics
Kelly Huang
President, HealthTronics October 4, 2012
©2012 HealthTronics, Inc. All rights reserved.
48
HealthTronics Key Takeaways
HealthTronics has deep, durable relationships with the US-based Urology community
HealthTronics has a stable, core business that is profitable and cash flow positive
HealthTronics has several platforms for growth including Cryotherapy, Electronic Medical Records and Data-Enabled Services & Analytics
HealthTronics, through its channel presence in Urology, has strategic value for the Endo enterprise
©2012 HealthTronics, Inc. All rights reserved.
49
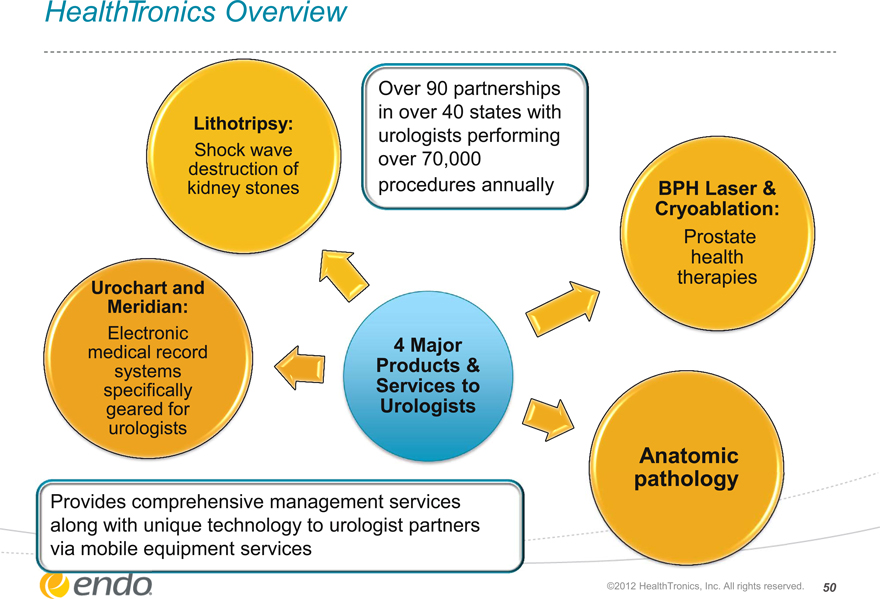
HealthTronics Overview
Lithotripsy:
Shock wave destruction of kidney stones
Over 90 partnerships in over 40 states with urologists performing over 70,000 procedures annually
BPH Laser & Cryoablation:
Prostate health therapies
Urochart and Meridian:
Electronic medical record systems specifically geared for urologists
4 Major Products & Services to Urologists
Anatomic pathology
Provides comprehensive management services along with unique technology to urologist partners via mobile equipment services
©2012 HealthTronics, Inc. All rights reserved.
50
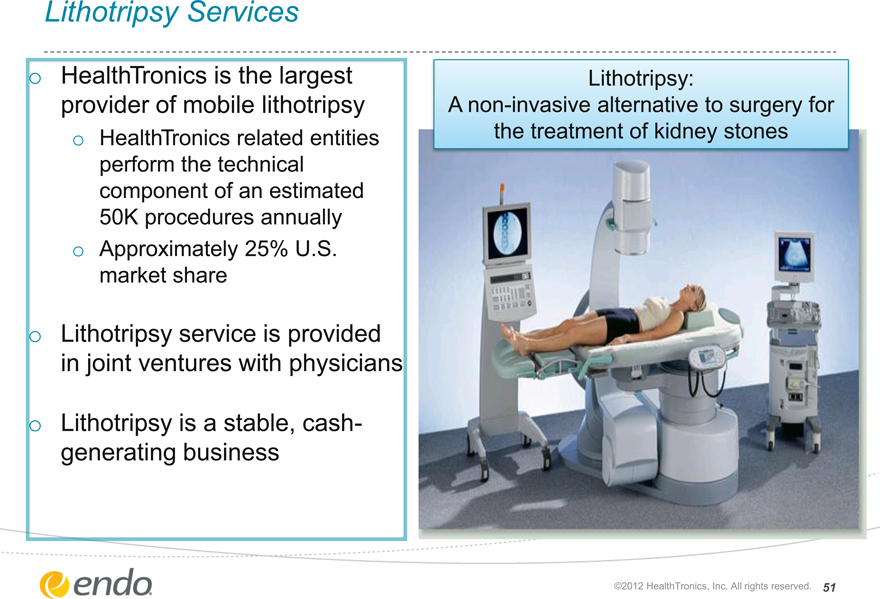
Lithotripsy Services
HealthTronics is the largest provider of mobile lithotripsy
HealthTronics related entities perform the technical component of an estimated 50K procedures annually
Approximately 25% U.S. market share
Lithotripsy service is provided in joint ventures with physicians
Lithotripsy is a stable, cash- generating business
Lithotripsy:
A non-invasive alternative to surgery for the treatment of kidney stones
©2012 HealthTronics, Inc. All rights reserved.
51
Cryotherapy Services
Cryotherapy:
A minimally invasive alternative to surgery for the treatment of prostate cancer
HealthTronics is leading mobile provider of Cryotherapy equipment with 65% market share
Clinical research is driving momentum
Expect to submit FDA application for new Partial Gland Treatment indication in 4Q12 Technology has significant potential for growth as we look to expand its indicated uses to other areas
Next generation Cryotherapy System in development
Reduction in procedure time and data connectivity with EMR systems
©2012 HealthTronics, Inc. All rights reserved.
52
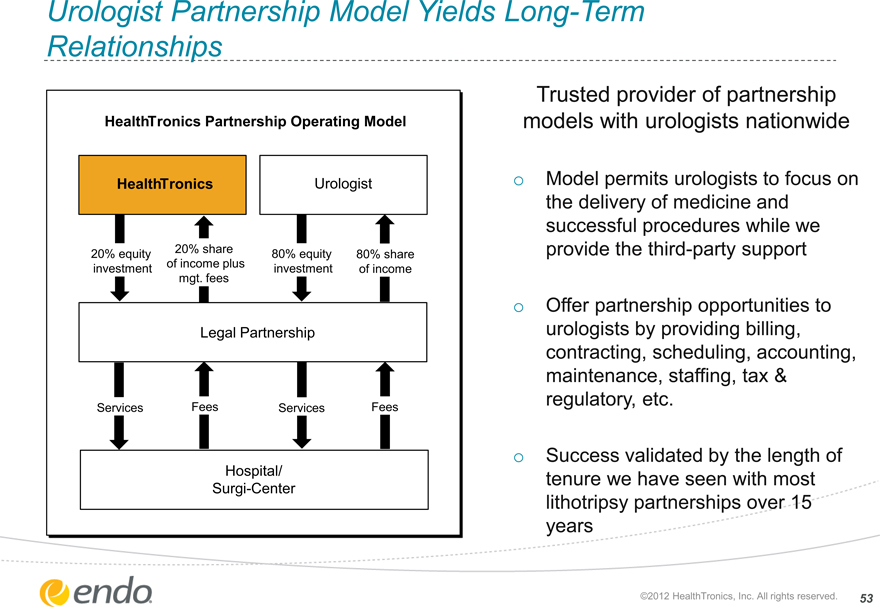
Urologist Partnership Model Yields Long-Term Relationships
HealthTronics Partnership Operating Model
HealthTronics
Urologist
20% equity investment
20% share of income plus mgt. fees
80% equity investment
80% share of income
Legal Partnership
Services
Fees
Services
Fees
Hospital/ Surgi-Center
Trusted provider of partnership models with urologists nationwide
Model permits urologists to focus on the delivery of medicine and successful procedures while we provide the third-party support
Offer partnership opportunities to urologists by providing billing, contracting, scheduling, accounting, maintenance, staffing, tax & regulatory, etc.
Success validated by the length of tenure we have seen with most lithotripsy partnerships over 15 years
©2012 HealthTronics, Inc. All rights reserved.
53
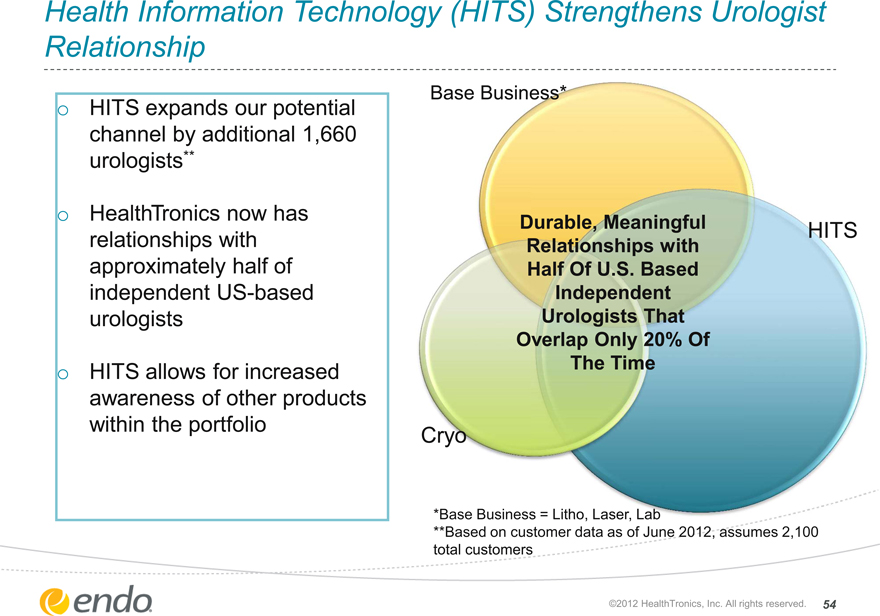
Health Information Technology (HITS) Strengthens Urologist Relationship
HITS expands our potential channel by additional 1,660 urologists**
HealthTronics now has relationships with approximately half of independent US-based urologists
HITS allows for increased awareness of other products within the portfolio
Base Business*
Durable, Meaningful Relationships with Half Of U.S. Based Independent Urologists That Overlap Only 20% Of The Time
HITS
Cryo
*Base Business = Litho, Laser, Lab
**Based on customer data as of June 2012, assumes 2,100 total customers
©2012 HealthTronics, Inc. All rights reserved.
54
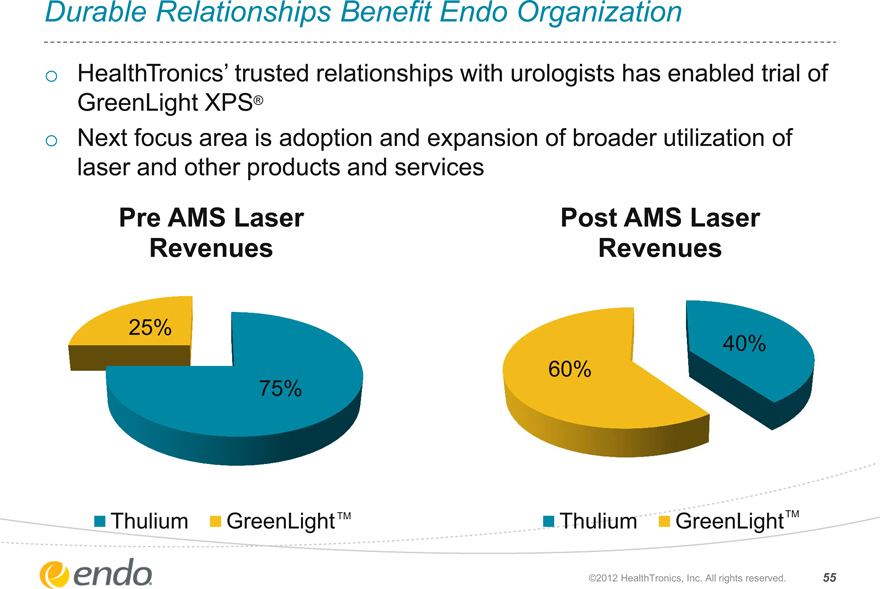
Durable Relationships Benefit Endo Organization
HealthTronics’ trusted relationships with urologists has enabled trial of
GreenLight XPS®
Next focus area is adoption and expansion of broader utilization of laser and other products and services
Pre AMS Laser Revenues
Post AMS Laser Revenues
25%
75%
60%
40%
Thulium
GreenLightTM
Thulium
GreenLightTM
©2012 HealthTronics, Inc. All rights reserved.
55
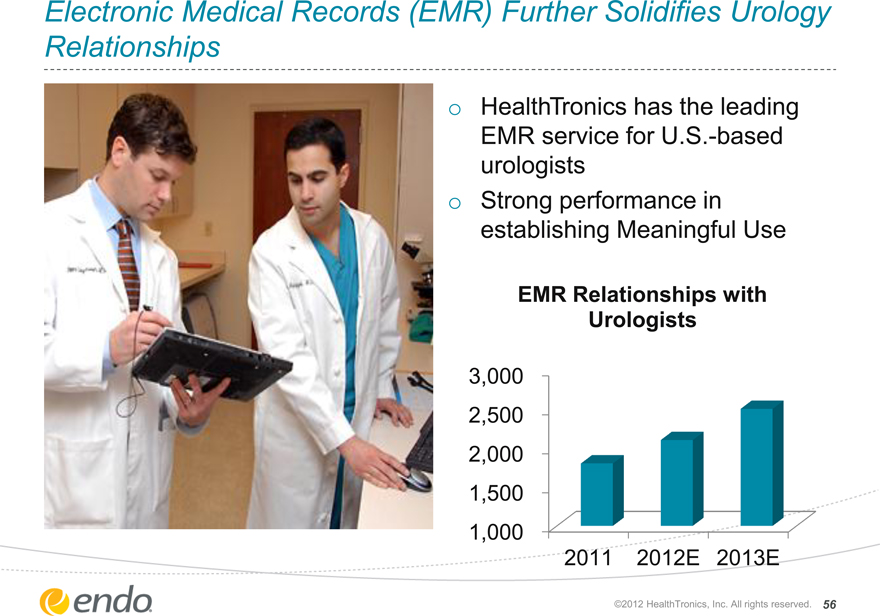
Electronic Medical Records (EMR) Further Solidifies Urology Relationships
HealthTronics has the leading EMR service for U.S.-based urologists
Strong performance in establishing Meaningful Use
EMR Relationships with Urologists
3,000
2,500
2,000
1,500
1,000
2011 2012E 2013E
©2012 HealthTronics, Inc. All rights reserved.
56
Electronic Medical Records (EMR) In The Future
EMR supports how doctors practice medicine in seamless manner driving urologist adoption
EMR generated data will grow in the future as there is increased emphasis on:
Patient-Centric Solutions
Outcomes Based Reimbursement
Reduced Readmission & Revision to Lower Costs
EMR Will Be Crucial to a Physician’s Practice
Inter-op Clinical Decision Support
Patient-Specific Outcome Correlations
Practice Economic Performance
Revenue Opportunities, e.g. Clinical Enrollment
©2012 HealthTronics, Inc. All rights reserved.
57
HealthTronics Key Takeaways
HealthTronics has deep, durable relationships with the US-based Urology community
HealthTronics has a stable, core business that is profitable and is cash flow positive
HealthTronics has several platforms for growth including Cryotherapy, Electronic Medical Records and Data-Enabled Services & Analytics
HealthTronics, through its channel presence in Urology, has strategic value for the Endo enterprise
©2012 HealthTronics, Inc. All rights reserved.
58
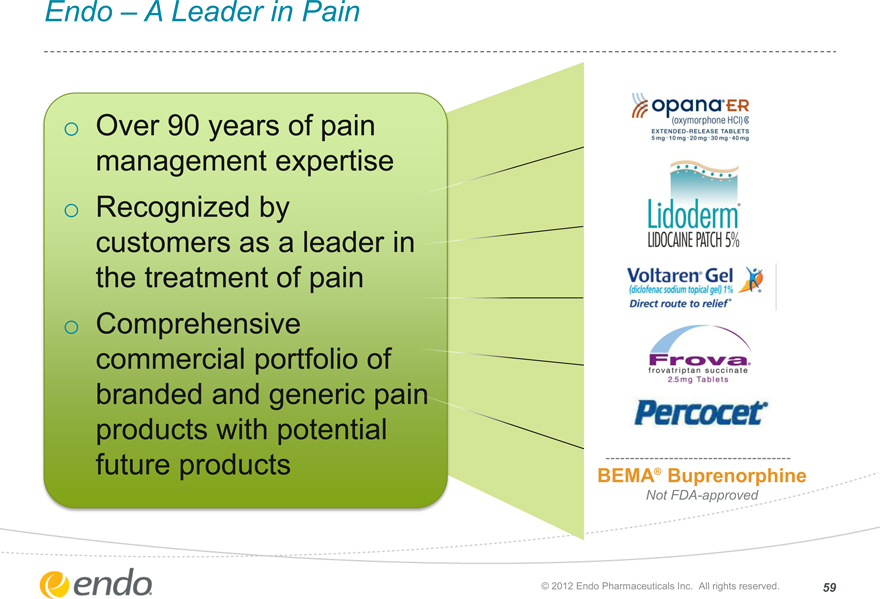
Endo – A Leader in Pain
Over 90 years of pain management expertise
Recognized by customers as a leader in the treatment of pain
Comprehensive commercial portfolio of branded and generic pain products with potential future products
BEMA® Buprenorphine
Not FDA-approved
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
59
2012 Investor Day
Endo Pharmaceuticals
Julie McHugh
Chief Operating Officer
October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved
60
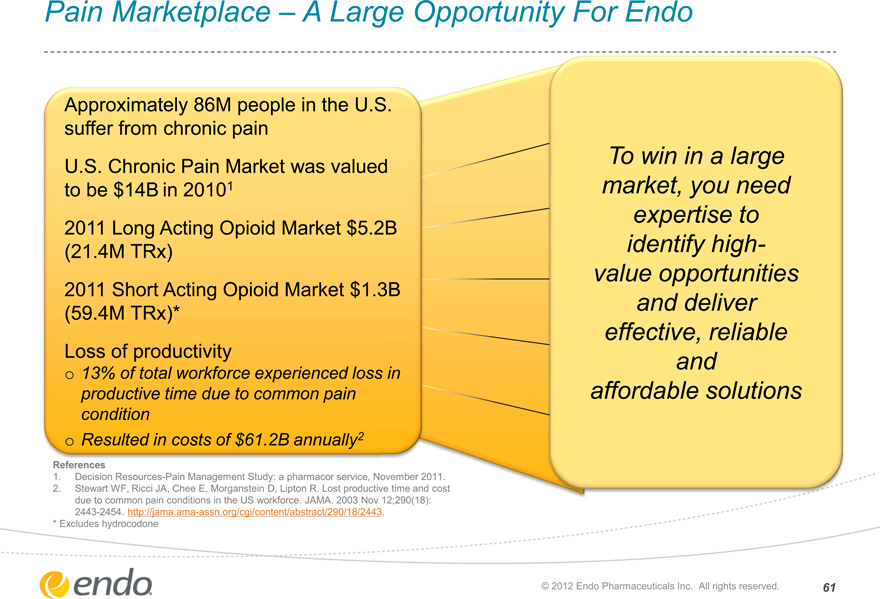
Pain Marketplace – A Large Opportunity For Endo
Approximately 86M people in the U.S. suffer from chronic pain U.S. Chronic Pain Market was valued to be $14B in 20101 2011 Long Acting Opioid Market $5.2B (21.4M TRx) 2011 Short Acting Opioid Market $1.3B (59.4M TRx)* Loss of productivity
13% of total workforce experienced loss in productive time due to common pain condition Resulted in costs of $61.2B annually2
To win in a large market, you need expertise to identify high- value opportunities and deliver effective, reliable and affordable solutions
References
1. Decision Resources-Pain Management Study: a pharmacor service, November 2011.
2. Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003 Nov 12;290(18): 2443-2454. http://jama.ama-assn.org/cgi/content/abstract/290/18/2443.
* Excludes hydrocodone
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
61
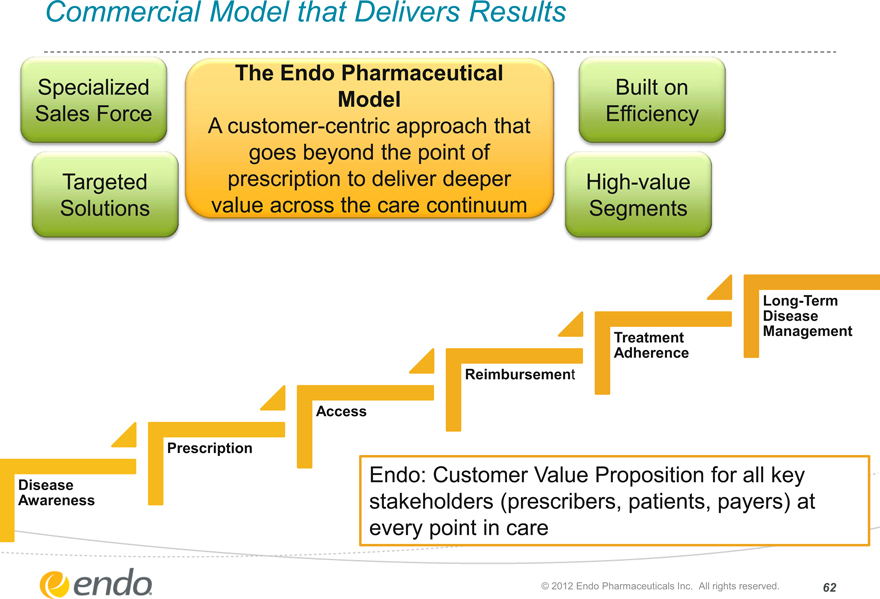
Commercial Model that Delivers Results
Specialized
Sales Force
Targeted Solutions
The Endo Pharmaceutical Model
A customer-centric approach that goes beyond the point of prescription to deliver deeper value across the care continuum
Built on
Efficiency
High-value Segments
Disease Awareness
Prescription
Access
Reimbursement
Treatment Adherence
Long-Term Disease Management
Endo: Customer Value Proposition for all key stakeholders (prescribers, patients, payers) at every point in care
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
62
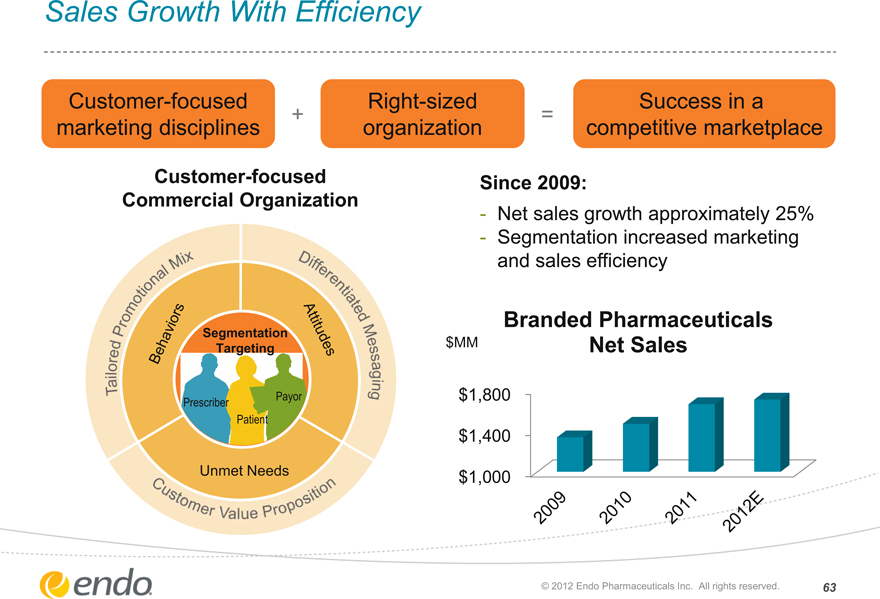
Sales Growth With Efficiency
Customer-focused marketing disciplines
+
Right-sized organization
=
Success in a competitive marketplace
Customer-focused Commercial Organization
Tailored Promotional Mix
Differentiated Messaging
Customer Value Proposition
Behaviors Attitudes
Segmentation Targeting
Payor
Prescriber
Patient
Unmet Needs
Since 2009:
Net sales growth approximately 25% Segmentation increased marketing and sales efficiency
Branded Pharmaceuticals Net Sales
$MM
$ 1,800
$ 1,400
$ 1,000
2009
2010
2011
2012E
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
63
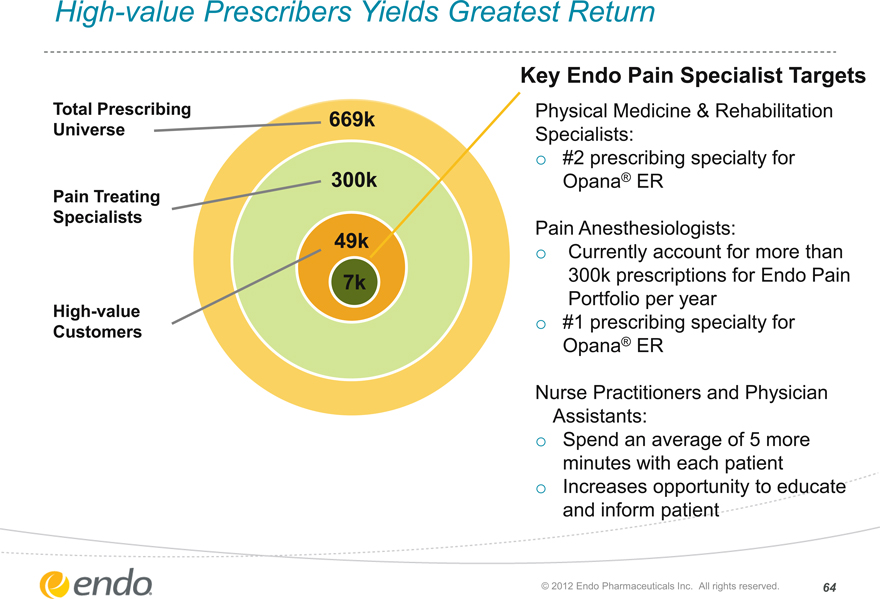
High-value Prescribers Yields Greatest Return
Total Prescribing Universe
Pain Treating Specialists
High-value Customers
669k
300k
49k
7k
Key Endo Pain Specialist Targets
Physical Medicine & Rehabilitation Specialists:
#2 prescribing specialty for Opana® ER
Pain Anesthesiologists:
Currently account for more than 300k prescriptions for Endo Pain Portfolio per year
#1 prescribing specialty for Opana® ER
Nurse Practitioners and Physician Assistants:
Spend an average of 5 more minutes with each patient Increases opportunity to educate and inform patient
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
64
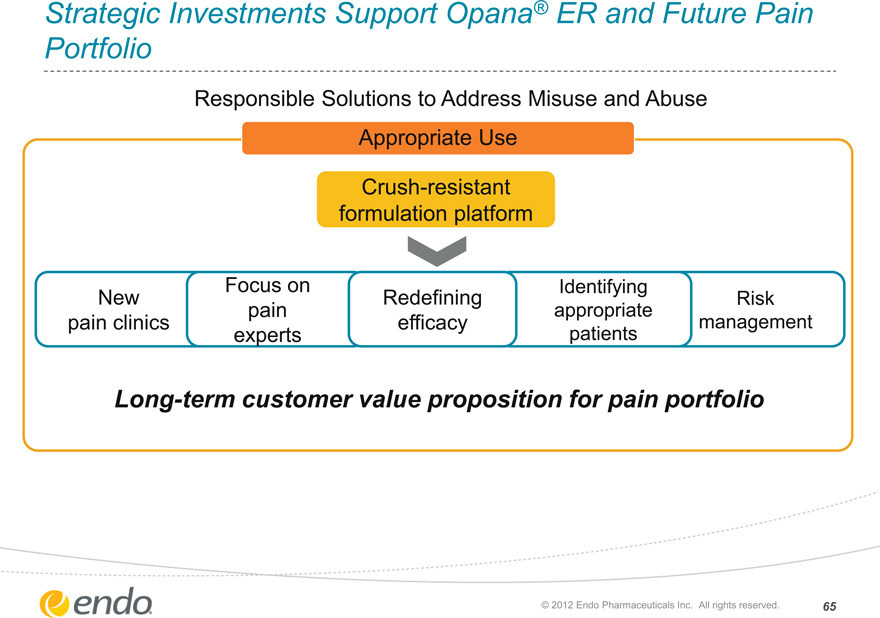
Strategic Investments Support Opana® ER and Future Pain Portfolio
Responsible Solutions to Address Misuse and Abuse
Appropriate Use
Crush-resistant formulation platform
New pain clinics
Focus on pain experts
Redefining efficacy
Identifying appropriate patients
Risk management
Long-term customer value proposition for pain portfolio
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
65
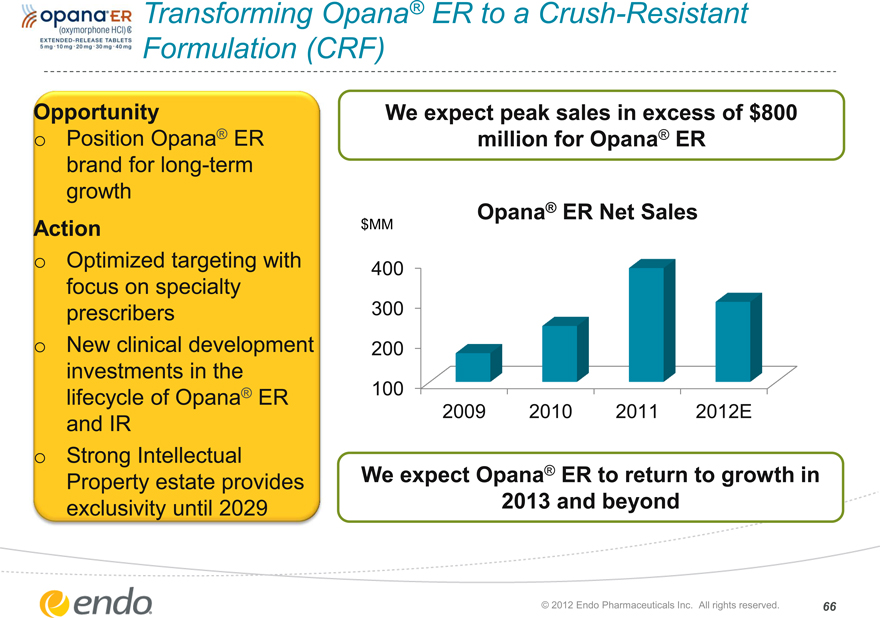
Transforming Opana® ER to a Crush-Resistant Formulation (CRF)
Opportunity
Position Opana® ER brand for long-term growth
Action
Optimized targeting with focus on specialty prescribers New clinical development investments in the lifecycle of Opana® ER and IR
Strong Intellectual
Property estate provides exclusivity until 2029
We expect peak sales in excess of $800 million for Opana® ER
Opana® ER Net Sales
$MM
400
300
200
100
2009
2010
2011
2012E
We expect Opana® ER to return to growth in 2013 and beyond
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
66
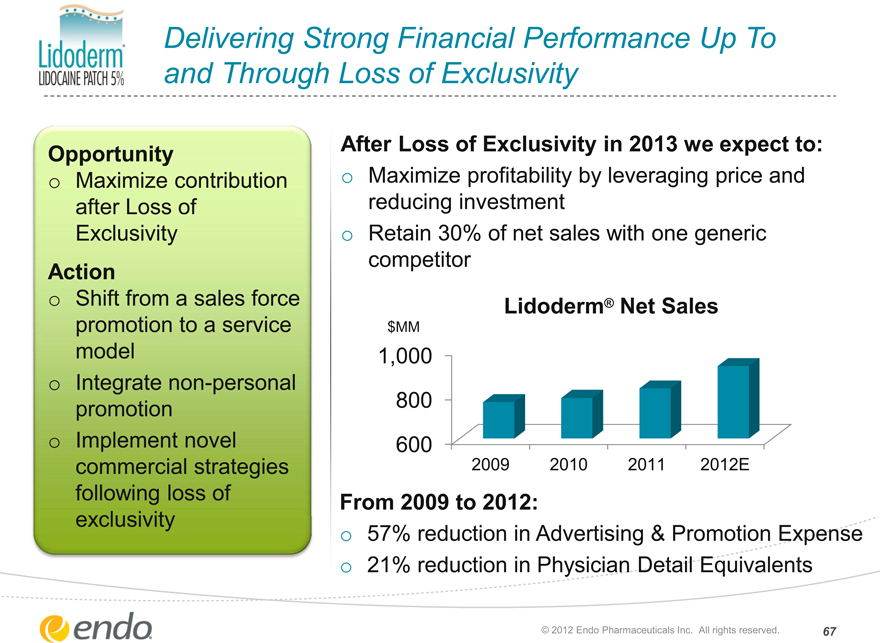
Delivering Strong Financial Performance Up To and Through Loss of Exclusivity
Opportunity
Maximize contribution after Loss of Exclusivity
Action
Shift from a sales force promotion to a service model
Integrate non-personal promotion Implement novel commercial strategies following loss of exclusivity
After Loss of Exclusivity in 2013 we expect to:
Maximize profitability by leveraging price and reducing investment Retain 30% of net sales with one generic competitor
Lidoderm® Net Sales
$MM
1,000
800,
600
2009
2010
2011
2012E
From 2009 to 2012:
57% reduction in Advertising & Promotion Expense 21% reduction in Physician Detail Equivalents
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
67
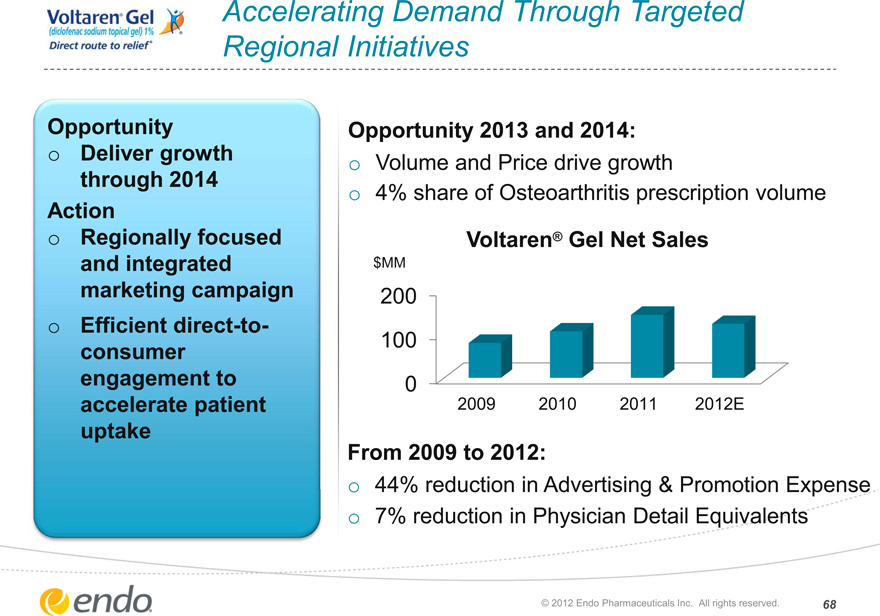
Accelerating Demand Through Targeted Regional Initiatives
Opportunity
Deliver growth through 2014
Action
Regionally focused and integrated marketing campaign Efficient direct-to-consumer engagement to accelerate patient uptake
Opportunity 2013 and 2014:
Volume and Price drive growth
4% share of Osteoarthritis prescription volume
Voltaren® Gel Net Sales
$MM
200
100
0
2009
2010
2011
2012E
From 2009 to 2012:
44% reduction in Advertising & Promotion Expense 7% reduction in Physician Detail Equivalents
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
68
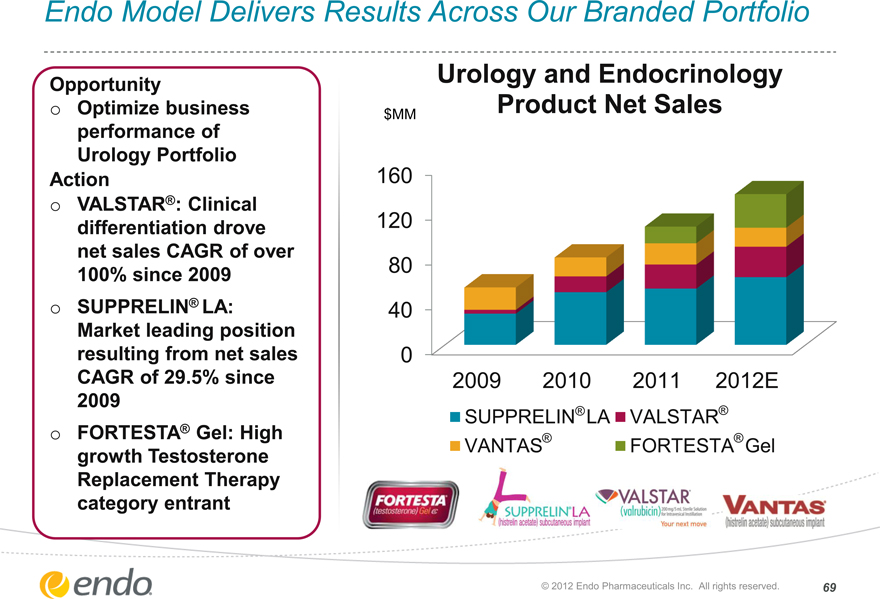
Endo Model Delivers Results Across Our Branded Portfolio
Opportunity
Optimize business performance of Urology Portfolio
Action
VALSTAR®: Clinical differentiation drove net sales CAGR of over 100% since 2009 SUPPRELIN® LA: Market leading position resulting from net sales CAGR of 29.5% since 2009 FORTESTA® Gel: High growth Testosterone Replacement Therapy category entrant
Urology and Endocrinology Product Net Sales
$MM
160
120
80
40
0
2009
2010
2011
2012E
SUPPRELIN® LA
VANTAS®
VALSTAR®
FORTESTA® Gel
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
69
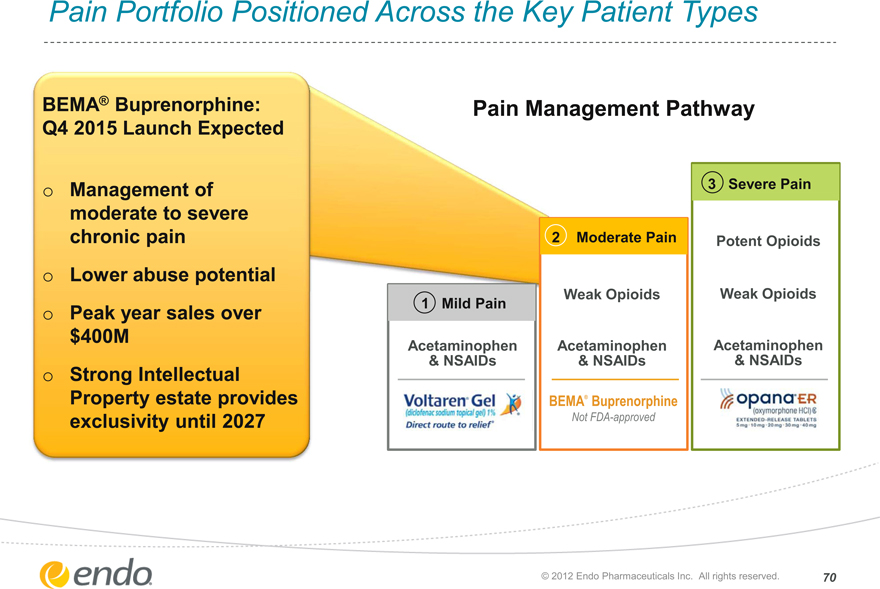
Pain Portfolio Positioned Across the Key Patient Types
BEMA® Buprenorphine: Q4 2015 Launch Expected
Management of moderate to severe chronic pain Lower abuse potential Peak year sales over $400M
Strong Intellectual Property estate provides exclusivity until 2027
Pain Management Pathway
1 Mild Pain
Acetaminophen
& NSAIDs
2 Moderate Pain
Weak Opioids
Acetaminophen
& NSAIDs
BEMA® Buprenorphine
Not FDA-approved
3 Severe Pain
Potent Opioids Potent Opioids
Weak Opioids
Acetaminophen
& NSAIDs
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
70
Pharmaceutical Business —Strong Growth Potential
Commercial model allows Endo to manage through the loss of exclusivity for Lidoderm® and support our competitive position BD Opportunities
Long-term patent exclusivity on portfolio growth drivers:
Opana® ER Intellectual Property through 2029
C
BEMA® Buprenorphine Intellectual Property through 2027
BEMA® Buprenorphine launch expected late 2015
Expect multiple Branded Pharmaceuticals products to exceed $100M in sales by 2017
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
71
2012 Investor Day
Research & Development
Ivan Gergel
Chief Scientific Officer
October 4, 2012
©2012 Endo Pharmaceuticals Inc. All rights reserved.
72
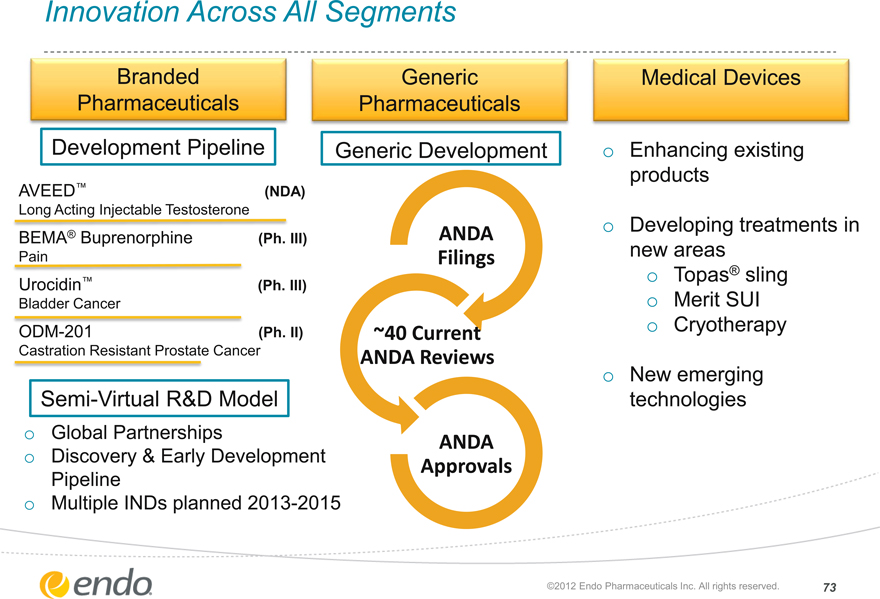
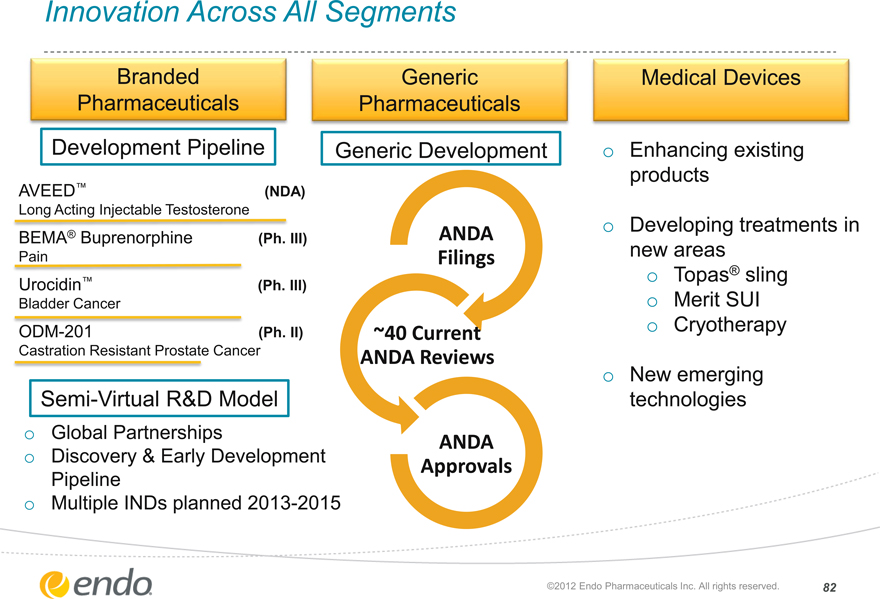
Innovation Across All Segments
Branded Pharmaceuticals
Generic
Pharmaceuticals
Medical Devices
Development Pipeline
Generic Development
AVEED™ (NDA)
Long Acting Injectable Testosterone
BEMA® Buprenorphine (Ph. III)
Pain
Urocidin™ (Ph. III)
Bladder Cancer
ODM-201 (Ph. II)
Castration Resistant Prostate Cancer
Semi-Virtual R&D Model
Global Partnerships
Discovery & Early Development Pipeline Multiple INDs planned 2013-2015
ANDA Filings
~40 Current ANDA Reviews
ANDA Approvals
Enhancing existing
products
Developing treatments in
new areas
Topas® sling
Merit SUI Cryotherapy
New emerging technologies
©2012 Endo Pharmaceuticals Inc. All rights reserved.
73
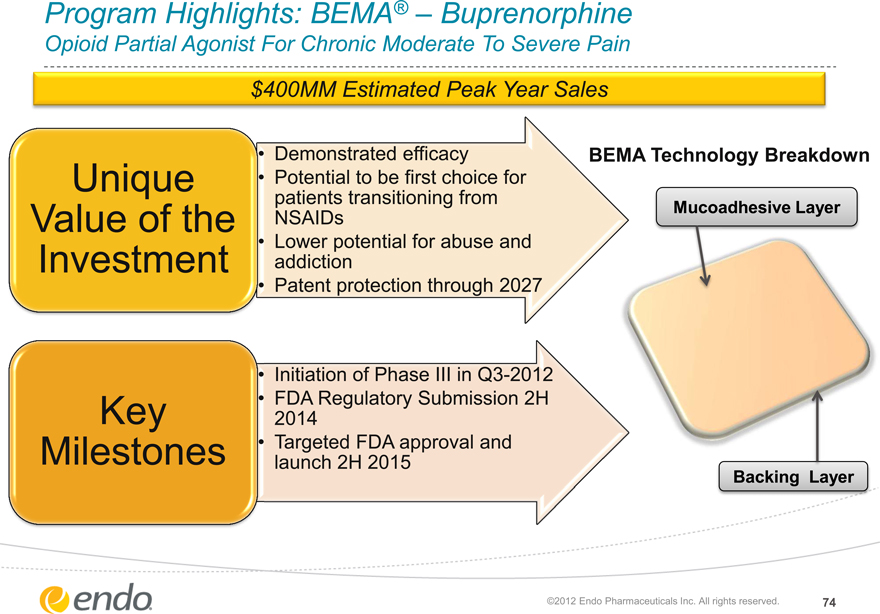
Program Highlights: BEMA® – Buprenorphine
Opioid Partial Agonist For Chronic Moderate To Severe Pain
$400MM Estimated Peak Year Sales
Unique Value of the Investment
Demonstrated efficacy
Potential to be first choice for patients transitioning from NSAIDs
Lower potential for abuse and addiction
Patent protection through 2027
BEMA Technology Breakdown
Mucoadhesive Layer
Key Milestones
Initiation of Phase III in Q3-2012 FDA Regulatory Submission 2H 2014 Targeted FDA approval and launch 2H 2015
Backing Layer
©2012 Endo Pharmaceuticals Inc. All rights reserved.
74
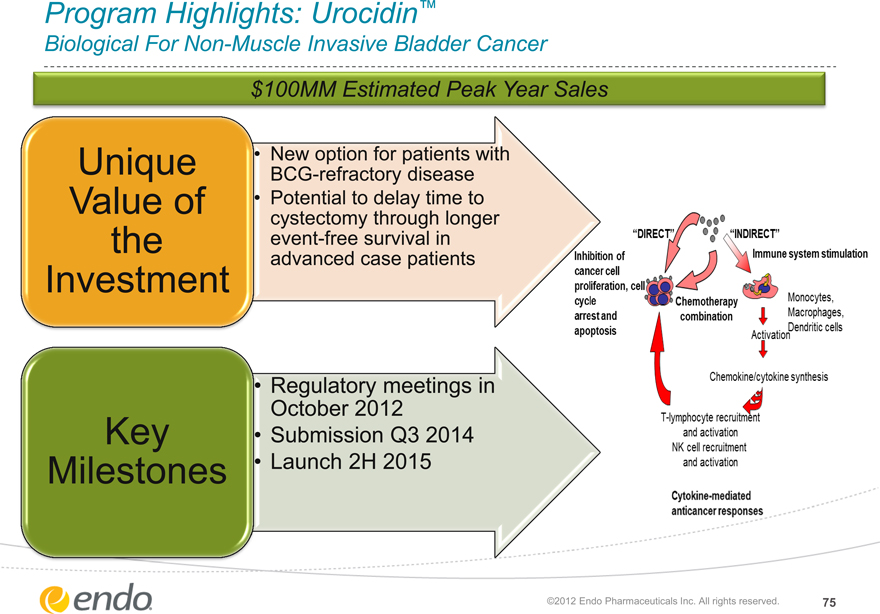
Program Highlights: Urocidin™
Biological For Non-Muscle Invasive Bladder Cancer
$100MM Estimated Peak Year Sales
Unique Value of the Investment
New option for patients with BCG-refractory disease Potential to delay time to cystectomy through longer event-free survival in advanced case patients
“DIRECT” “INDERECT”
Inhibition of cancer cell proliferation, cell cycle arrest and apoptosis
Immune system stimulation
Chemotherapy combination
Monocytes, Macrophages, Dendritic cells
Activation
Chemokine/cytokines synthesis
T-lymphocyte recruitment and activation NK cell recruitment and activation
Cytokine-mediated anticancer responses
Key Milestones
Regulatory meetings in
October 2012
Submission Q3 2014 Launch 2H 2015
©2012 Endo Pharmaceuticals Inc. All rights reserved.
75
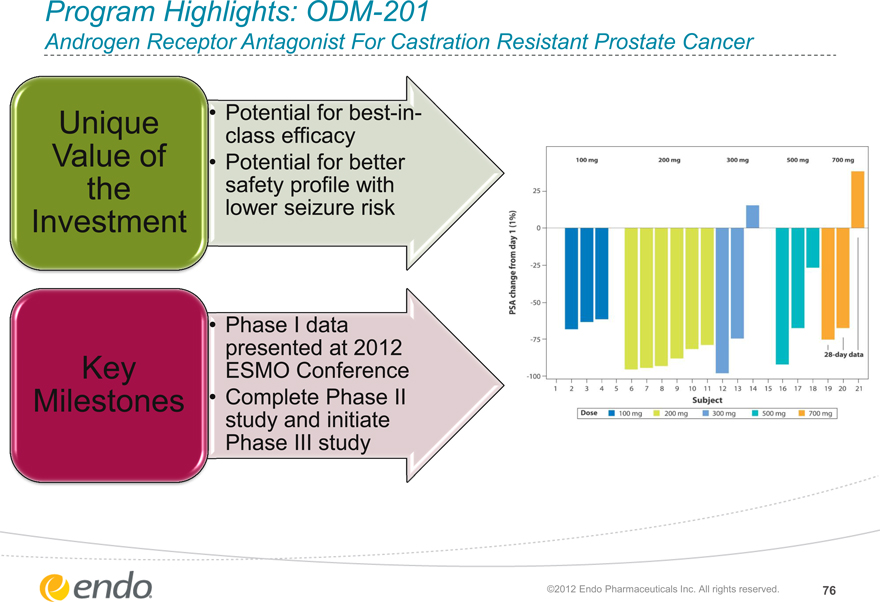
Program Highlights: ODM-201
Androgen Receptor Antagonist For Castration Resistant Prostate Cancer
Unique Value of the Investment
Potential forbest-in-class efficacy
Potential for better safety profile with lower seizure risk
Key Milestones
Phase I data presented at 2012 ESMO Conference Complete Phase II study and initiate
Phase III study
©2012 Endo Pharmaceuticals Inc. All rights reserved.
76
Endo’s Semi-Virtual Drug Discovery Strategy
Fast-follower/ validated targets
Clinical POC Competitors in Phase I/II
Maximize opportunity by leveraging partnerships
No infrastructure build up Shared risk
Differentiation from competition
Efficacy and /or safety advantage
©2012 Endo Pharmaceuticals Inc. All rights reserved.
77
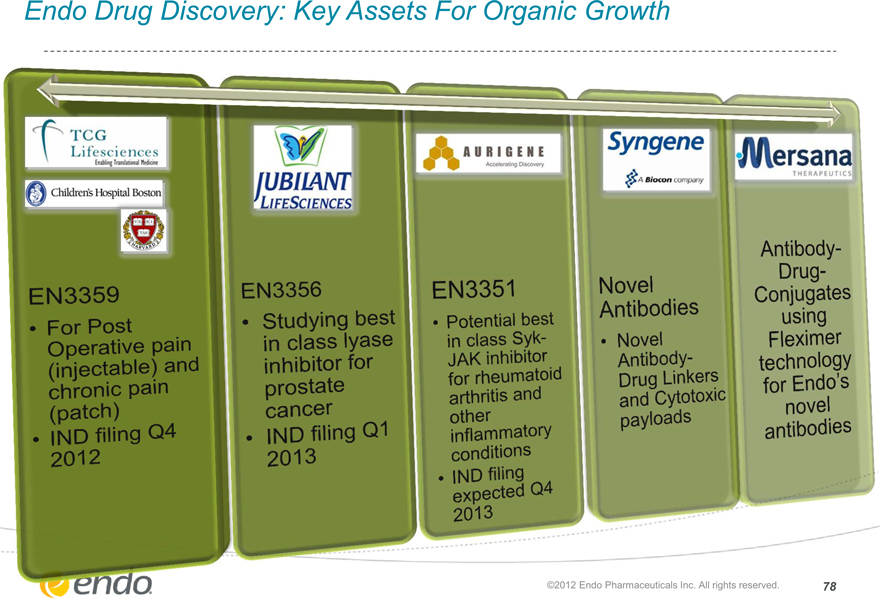
Endo Drug Discovery: Key Assets For Organic Growth
EN3359
For Post Operative pain (injectable and chronic pain (patch)
IND filing Q4 2012
EN3356
Studying best in class lyase inhibitor for prostate cancer
IND filing Q1 2013
EN3351
Potential best in class Syk-JAK inhibitor for rheumatoid arthritis and other inflammatory conditions
IND filing expected Q4 2013
Novel Antibodies
Novel Antibody-Drug Linkers and Cytotoxic payloads
Antibody-Drug-Conjugates using Fleximer technology for Endo’s novel antibodies
©2012 Endo Pharmaceuticals Inc. All rights reserved.
78
Qualitest Pipeline Approach – Organic Development And Strategic Acquisition Of Products And Target Technologies
Differentiate pipeline investments based on portfolio
Strengthen current infrastructure scale and expertise in core technologies e.g. controlled substances, extended release, liquids
Accelerate pipeline and commercial products through external partnerships and licensing
©2012 Endo Pharmaceuticals Inc. All rights reserved.
79
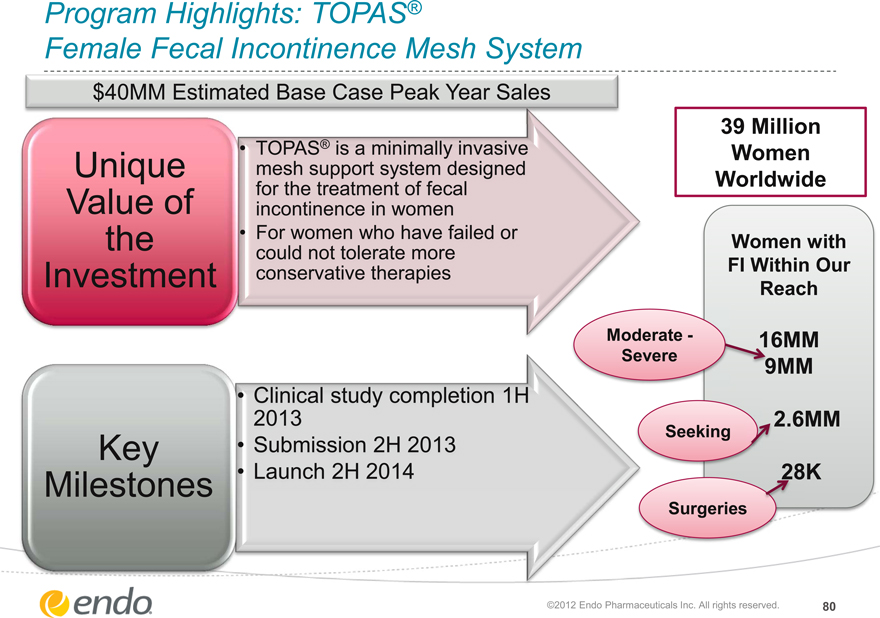
Program Highlights: TOPAS®
Female Fecal Incontinence Mesh System
$40MM Estimated Base Case Peak Year Sales
Unique Value of the Investment
TOPAS® is a minimally invasive mesh support system designed for the treatment of fecal incontinence in women For women who have failed or could not tolerate more conservative therapies
Key Milestones
Clinical study completion 1H 2013
Submission 2H 2013
Launch 2H 2014
39 Million Women Worldwide
Women with FI Within Our Reach
Moderate—Severe
16MM 9MM
Seeking
2.6MM
Surgeries
28K
©2012 Endo Pharmaceuticals Inc. All rights reserved.
80
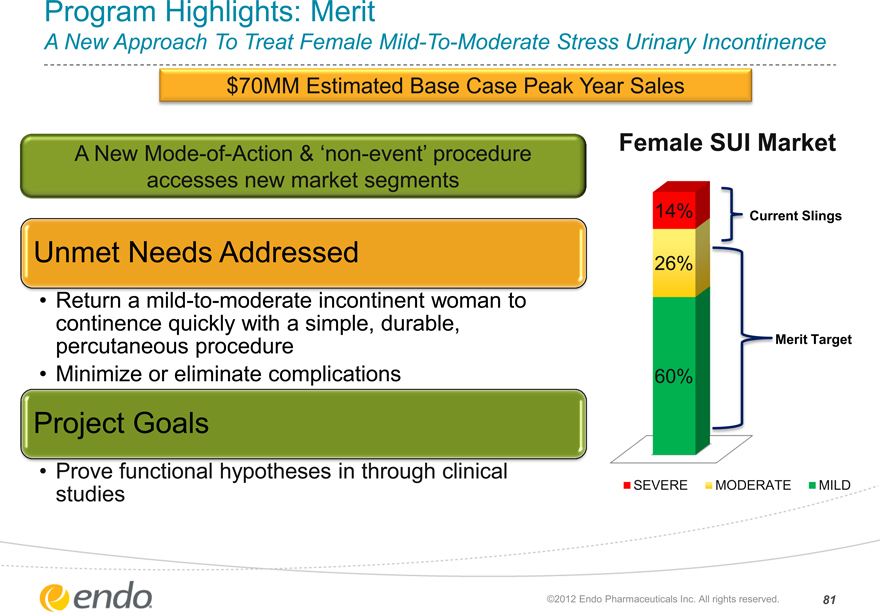
Program Highlights: Merit
A New Approach To Treat Female Mild-To-Moderate Stress Urinary Incontinence
$70MM Estimated Base Case Peak Year Sales
A New Mode-of-Action & ‘non-event’ procedure accesses new market segments
Unmet Needs Addressed
• Return a mild-to-moderate incontinent woman to continence quickly with a simple, durable, percutaneous procedure
• Minimize or eliminate complications
Project Goals
• Prove functional hypotheses in through clinical studies
Female SUI Market
14%
26%
60%
Current Slings
Merit Target
SEVERE MODERATE MILD
©2012 Endo Pharmaceuticals Inc. All rights reserved. 81
Innovation Across All Segments
Branded Pharmaceuticals
Development Pipeline
Generic
Pharmaceuticals
Generic Development
Medical Devices
AVEED™ (NDA)
Long Acting Injectable Testosterone
BEMA® Buprenorphine (Ph. III)
Pain
Urocidin™ (Ph. III)
Bladder Cancer
ODM-201 (Ph. II)
Castration Resistant Prostate Cancer
Semi-Virtual R&D Model
o Global Partnerships o Discovery & Early Development Pipeline o Multiple INDs planned 2013-2015
ANDA Filings
~40 Current ANDA Reviews
ANDA Approvals
Enhancing existing products
Developing treatments in new areas o Topas® sling o Merit SUI o Cryotherapy
New emerging technologies
©2012 Endo Pharmaceuticals Inc. All rights reserved. 82
2012 Investor Day
Finance
Alan Levin
Chief Financial Officer
October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved 83
Key Takeaways
Stable Base Business Assuming 2013 Lidoderm® Loss of Exclusivity
Revenue CAGR in the low single digit range from 2012 to 2015 Adjusted Diluted EPS CAGR in the low-single digit range from 2012 to
2015
Base case assumes one generic competitor for Lidoderm® through 2015 Excluding Lidoderm®, expect double-digit growth for company revenues
Capital Allocation
Balance of share repurchase, debt paydown and business development to support additional growth Financial flexibility and significant cash flow from operations support growth in 2014 and beyond Explore business development opportunities to support the organic growth potential in our business
© 2012 Endo Pharmaceuticals Inc. All rights reserved 84
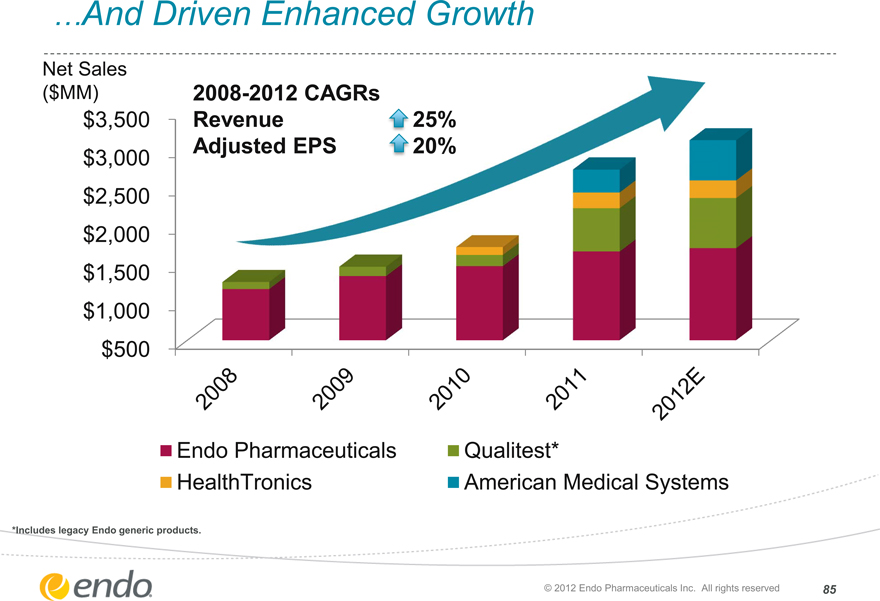
…And Driven Enhanced Growth
Net Sales
($MM)
$3,500 $3,000 $2,500 $2,000 $1,500 $1,000 $500
2008-2012 CAGRs Revenue
Adjusted EPS
25%
20%
2008 2009 2010 2011 2012E
Endo Pharmaceuticals HealthTronics
Qualitest*
American Medical Systems
*Includes legacy Endo generic products.
© 2012 Endo Pharmaceuticals Inc. All rights reserved 85
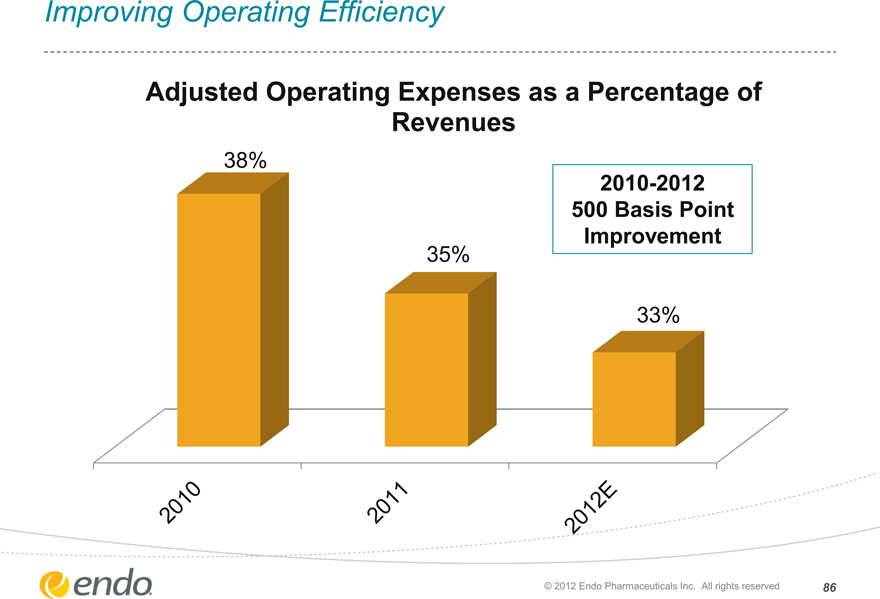
Improving Operating Efficiency
Adjusted Operating Expenses as a Percentage of Revenues
38%
35%
33%
2010-2012 500 Basis Point
Improvement
2010 2011 2012E
© 2012 Endo Pharmaceuticals Inc. All rights reserved 86
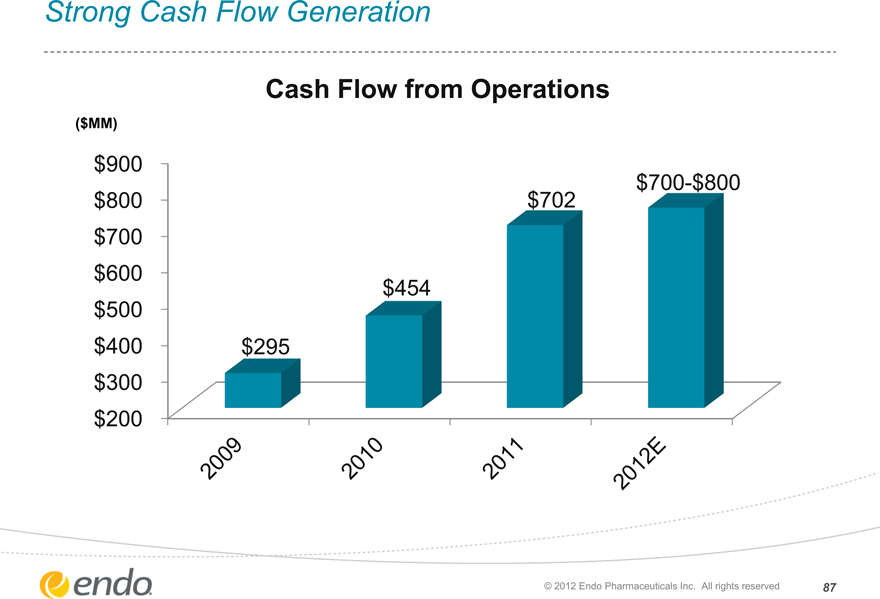
Strong Cash Flow Generation
Cash Flow from Operations
($MM)
$900 $800 $700 $600 $500 $400 $300 $200
$700-$800 $702
$454
$295
2009 2010 2011 2012E
© 2012 Endo Pharmaceuticals Inc. All rights reserved 87
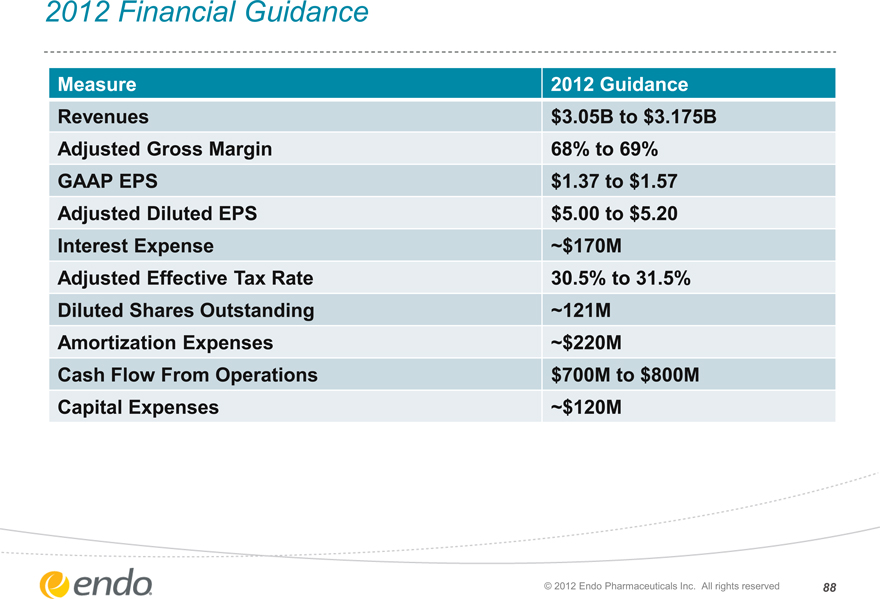
2012 Financial Guidance
Measure Revenues
Adjusted Gross Margin GAAP EPS
Adjusted Diluted EPS Interest Expense Adjusted Effective Tax Rate Diluted Shares Outstanding Amortization Expenses Cash Flow From Operations Capital Expenses
2012 Guidance $3.05B to $3.175B 68% to 69% $1.37 to $1.57 $5.00 to $5.20
~$170M
30.5% to 31.5% ~121M
~$220M $700M to $800M
~$120M
© 2012 Endo Pharmaceuticals Inc. All rights reserved 88
2012 Financial Guidance—Q3/Q4 Update
Expect Q3 2012 Adjusted Diluted EPS of approximately $1.25
Average selling price per prescription of Opana® ER is lower than expected due to dosage strength mix
Greater proportion of sales from lower strength dosage forms in Q3 than projected
Previously announced Operating Expense reductions have limited effect on Q3 but in full effect by Q4
Reaffirm full year 2012 adjusted diluted EPS guidance of $5.00—$5.20
© 2012 Endo Pharmaceuticals Inc. All rights reserved 89
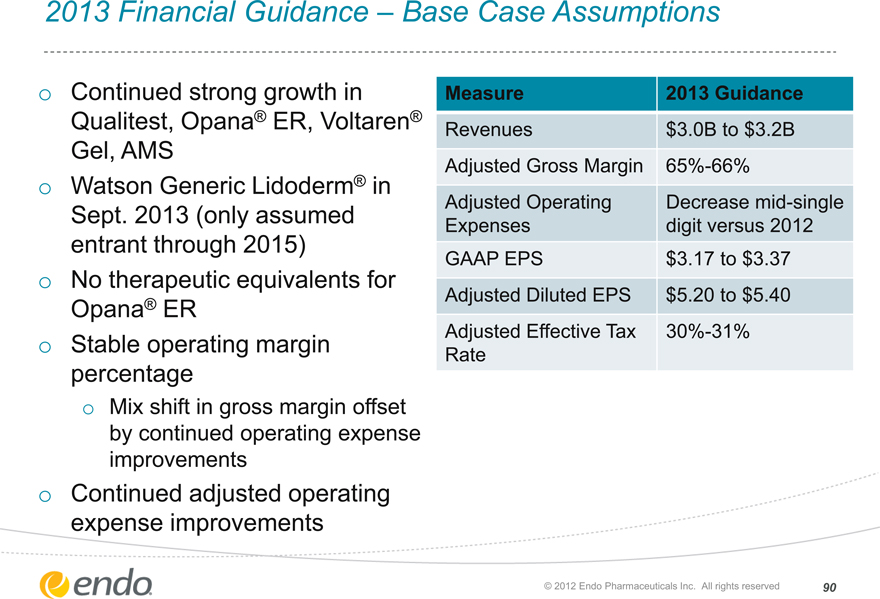
2013 Financial Guidance – Base Case Assumptions
Continued strong growth in Qualitest, Opana® ER, Voltaren® Gel, AMS
Watson Generic Lidoderm® in Sept. 2013 (only assumed entrant through 2015) No therapeutic equivalents for Opana® ER
Stable operating margin percentage
Mix shift in gross margin offset by continued operating expense improvements
Continued adjusted operating expense improvements
Measure
Revenues
Adjusted Gross Margin Adjusted Operating Expenses GAAP EPS
Adjusted Diluted EPS Adjusted Effective Tax Rate
2013 Guidance $3.0B to $3.2B 65%-66% Decrease mid-single digit versus 2012 $3.17 to $3.37 $5.20 to $5.40 30%-31%
© 2012 Endo Pharmaceuticals Inc. All rights reserved 90
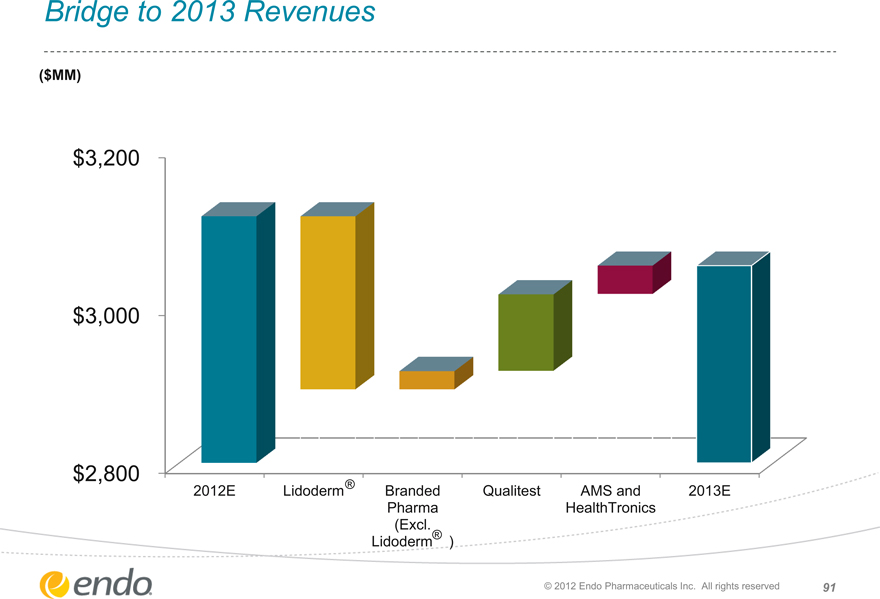
Bridge to 2013 Revenues
($MM)
$3,200 $3,000 $2,800
2012E Lidoderm®
Branded Pharma
(Excl.
Lidoderm®)
Qualitest AMS and HealthTronics
2013E
© 2012 Endo Pharmaceuticals Inc. All rights reserved 91
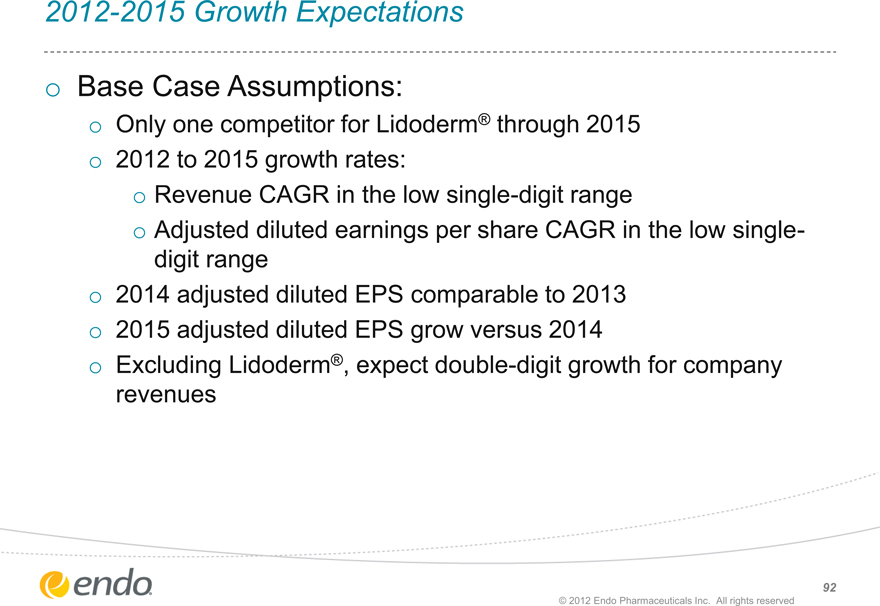
2012-2015 Growth Expectations
Base Case Assumptions:
Only one competitor for Lidoderm® through 2015
2012 to 2015 growth rates:
Revenue CAGR in the low single-digit range
Adjusted diluted earnings per share CAGR in the low single-digit range
2014 adjusted diluted EPS comparable to 2013
2015 adjusted diluted EPS grow versus 2014
Excluding Lidoderm®, expect double-digit growth for company revenues
92
© 2012 Endo Pharmaceuticals Inc. All rights reserved
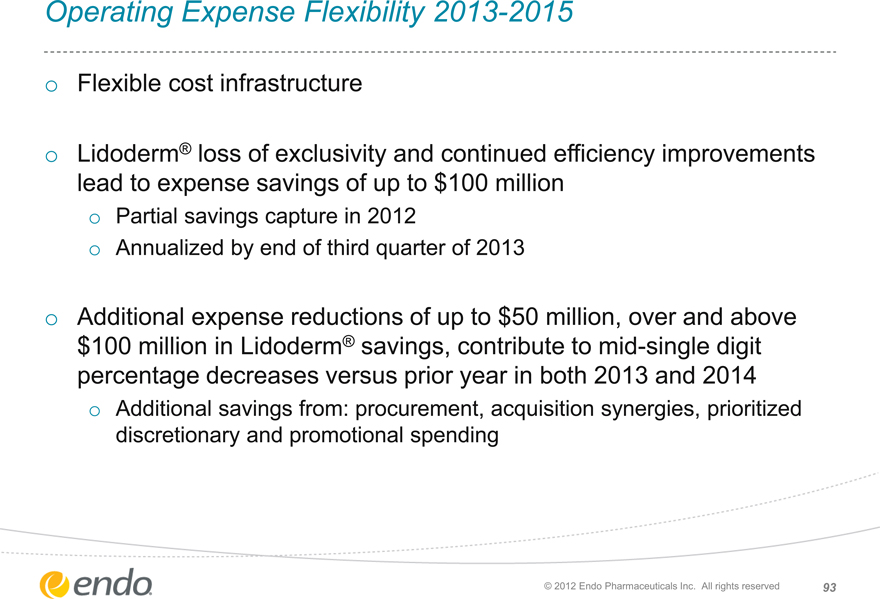
Operating Expense Flexibility 2013-2015
Flexible cost infrastructure
Lidoderm® loss of exclusivity and continued efficiency improvements lead to expense savings of up to $100 million
Partial savings capture in 2012
Annualized by end of third quarter of 2013
Additional expense reductions of up to $50 million, over and above $100 million in Lidoderm® savings, contribute to mid-single digit percentage decreases versus prior year in both 2013 and 2014
Additional savings from: procurement, acquisition synergies, prioritized discretionary and promotional spending
© 2012 Endo Pharmaceuticals Inc. All rights reserved 93
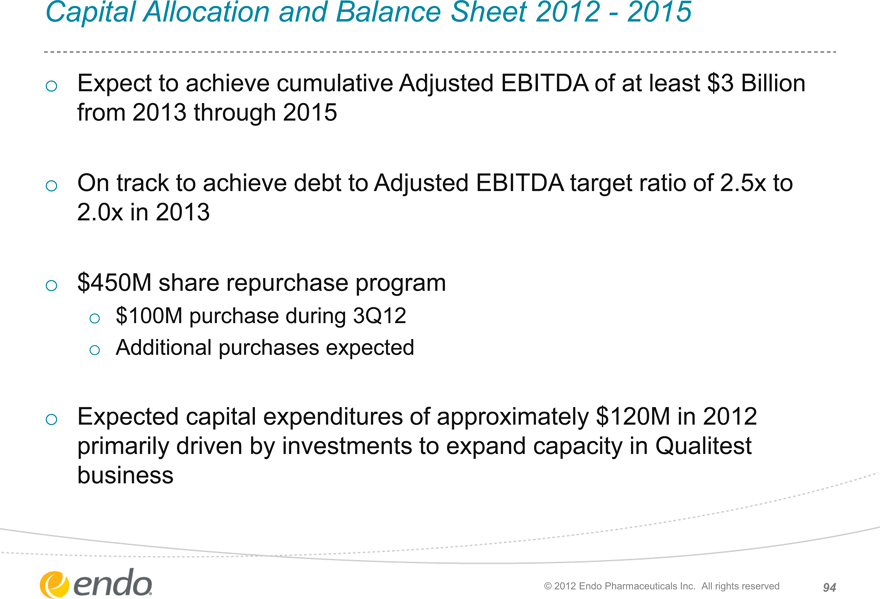
Capital Allocation and Balance Sheet 2012—2015
Expect to achieve cumulative Adjusted EBITDA of at least $3 Billion from 2013 through 2015
On track to achieve debt to Adjusted EBITDA target ratio of 2.5x to 2.0x in 2013
$450M share repurchase program
$100M purchase during 3Q12 Additional purchases expected
Expected capital expenditures of approximately $120M in 2012 primarily driven by investments to expand capacity in Qualitest business
© 2012 Endo Pharmaceuticals Inc. All rights reserved 94
Health Solutions Strategy we believe will deliver strong financial performance and shareholder return
15%
Return to Strong Prescription Growth for Opana ER® and Voltaren® Gel in 2013
Annual Capacity Expansion at Qualitest each year through 2015
Of U.S. Based Urologists
One- Third
in an EMR Relationship with HealthTronics by the end of 2013
Sustained Double Digit Net Sales Growth at Qualitest
Accelerating mid-single digit AMS sales growth through 2015
Retain
30%
Of LIDODERM® sales after entry of first generic competitor
2011 2012E 2013E 2014E 2015E
© 2012 Endo Pharmaceuticals Inc. All rights reserved 95
Key Takeaways
Stable Base Business Assuming 2013 Lidoderm® Loss of Exclusivity
Revenue CAGR in the low single digit range from 2012 to 2015
Adjusted Diluted EPS CAGR in the low-single digit range from 2012 to
2015
Base case assumes one generic competitor for Lidoderm® through 2015
Excluding Lidoderm®, expect double-digit growth for company revenues
Capital Allocation
Balance of debt paydown, business development and share repurchase to support additional growth
Financial flexibility and significant cash flow from operations support growth in 2014 and beyond
Explore business development opportunities to support the organic growth potential in our business
© 2012 Endo Pharmaceuticals Inc. All rights reserved 96
endo® a vision for healthcare™
endo AMS Endo Pharmaceuticals HealthTronics Qualitest
97
Supplementary Financial Data
© 2012 Endo Pharmaceuticals Inc. All rights reserved 98
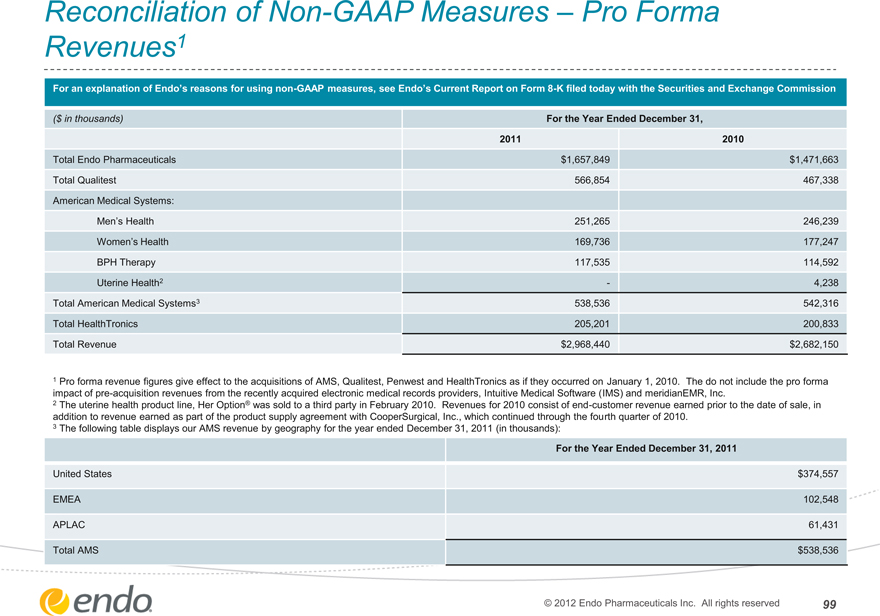
Reconciliation of Non-GAAP Measures – Pro Forma Revenues1
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with the Securities and Exchange Commission
($ in thousands)
Total Endo Pharmaceuticals Total Qualitest American Medical Systems:
Men’s Health
Women’s Health
BPH Therapy
Uterine Health2
Total American Medical Systems3 Total HealthTronics Total Revenue
For the Year Ended December 31,
2011 2010
$1,657,849 $1,471,663
566,854 467,338
251,265 246,239
169,736 177,247
117,535 114,592
— 4,238
538,536 542,316
205,201 200,833
$2,968,440 $2,682,150
1 Pro forma revenue figures give effect to the acquisitions of AMS, Qualitest, Penwest and HealthTronics as if they occurred on January 1, 2010. The do not include the pro forma impact of pre-acquisition revenues from the recently acquired electronic medical records providers, Intuitive Medical Software (IMS) and meridianEMR, Inc.
2 The uterine health product line, Her Option® was sold to a third party in February 2010. Revenues for 2010 consist of end-customer revenue earned prior to the date of sale, in addition to revenue earned as part of the product supply agreement with CooperSurgical, Inc., which continued through the fourth quarter of 2010.
3 The following table displays our AMS revenue by geography for the year ended December 31, 2011 (in thousands):
For the Year Ended December 31, 2011
United States
EMEA
APLAC
Total AMS
$374,557 102,548 61,431 $538,536
© 2012 Endo Pharmaceuticals Inc. All rights reserved 99
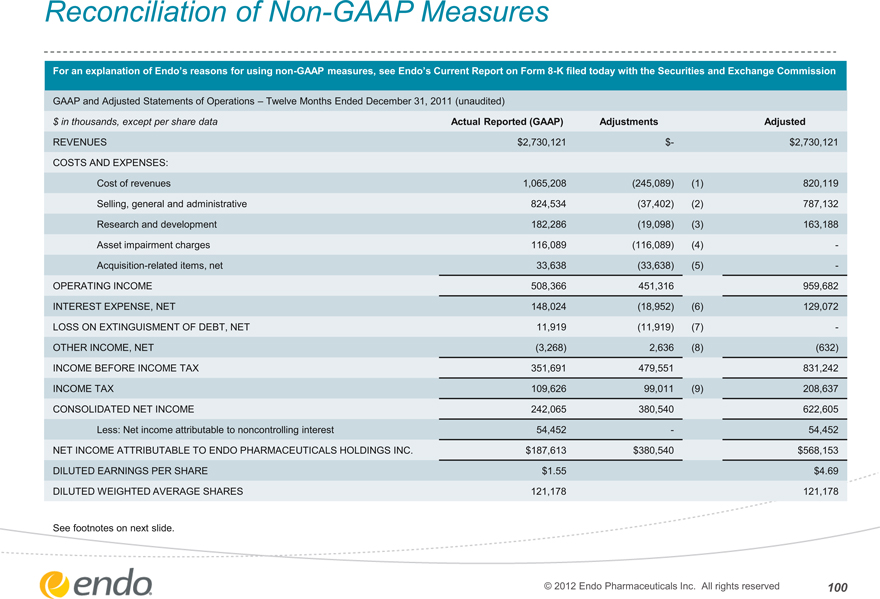
Reconciliation of Non-GAAP Measures
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with the Securities and Exchange Commission
GAAP and Adjusted Statements of Operations – Twelve Months Ended December 31, 2011 (unaudited)
$ in thousands, except per share data Actual Reported (GAAP) Adjustments Adjusted
REVENUES $2,730,121 $- $2,730,121
COSTS AND EXPENSES:
Cost of revenues 1,065,208 (245,089) (1) 820,119
Selling, general and administrative 824,534 (37,402) (2) 787,132
Research and development 182,286 (19,098) (3) 163,188
Asset impairment charges 116,089 (116,089) (4) -
Acquisition-related items, net 33,638 (33,638) (5) -
OPERATING INCOME 508,366 451,316 959,682
INTEREST EXPENSE, NET 148,024 (18,952) (6) 129,072
LOSS ON EXTINGUISMENT OF DEBT, NET 11,919 (11,919) (7) -
OTHER INCOME, NET (3,268) 2,636 (8) (632)
INCOME BEFORE INCOME TAX 351,691 479,551 831,242
INCOME TAX 109,626 99,011 (9) 208,637
CONSOLIDATED NET INCOME 242,065 380,540 622,605
Less: Net income attributable to noncontrolling interest 54,452 — 54,452
NET INCOME ATTRIBUTABLE TO ENDO PHARMACEUTICALS HOLDINGS INC. $187,613 $380,540 $568,153
DILUTED EARNINGS PER SHARE $1.55 $4.69
DILUTED WEIGHTED AVERAGE SHARES 121,178 121,178
See footnotes on next slide.
© 2012 Endo Pharmaceuticals Inc. All rights reserved 100
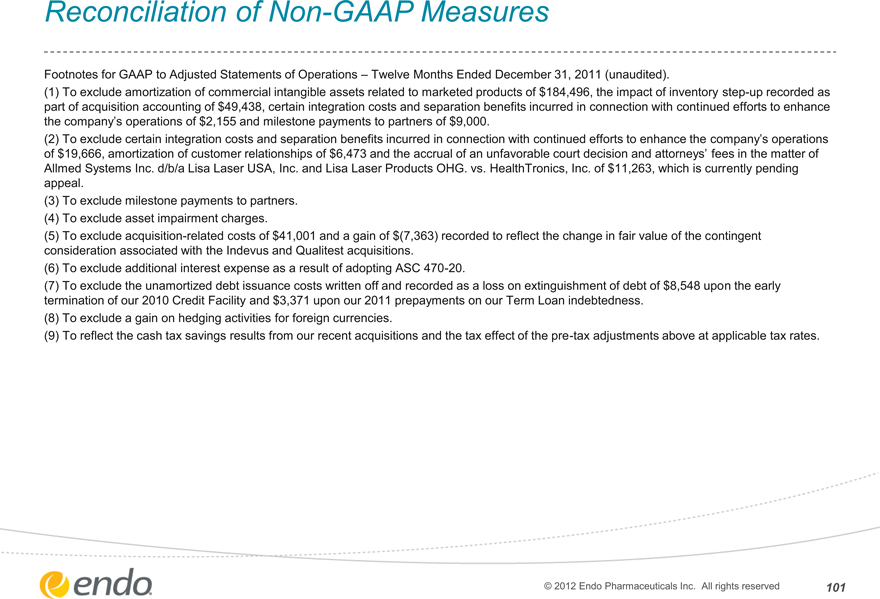
Reconciliation of Non-GAAP Measures
Footnotes for GAAP to Adjusted Statements of Operations – Twelve Months Ended December 31, 2011 (unaudited).
(1) To exclude amortization of commercial intangible assets related to marketed products of $184,496, the impact of inventory step-up recorded as part of acquisition accounting of $49,438, certain integration costs and separation benefits incurred in connection with continued efforts to enhance the company’s operations of $2,155 and milestone payments to partners of $9,000.
(2) To exclude certain integration costs and separation benefits incurred in connection with continued efforts to enhance the company’s operations of $19,666, amortization of customer relationships of $6,473 and the accrual of an unfavorable court decision and attorneys’ fees in the matter of
Allmed Systems Inc. d/b/a Lisa Laser USA, Inc. and Lisa Laser Products OHG. vs. HealthTronics, Inc. of $11,263, which is currently pending appeal.
(3) To exclude milestone payments to partners. (4) To exclude asset impairment charges.
(5) To exclude acquisition-related costs of $41,001 and a gain of $(7,363) recorded to reflect the change in fair value of the contingent consideration associated with the Indevus and Qualitest acquisitions.
(6) To exclude additional interest expense as a result of adopting ASC 470-20.
(7) To exclude the unamortized debt issuance costs written off and recorded as a loss on extinguishment of debt of $8,548 upon the early termination of our 2010 Credit Facility and $3,371 upon our 2011 prepayments on our Term Loan indebtedness.
(8) To exclude a gain on hedging activities for foreign currencies.
(9) To reflect the cash tax savings results from our recent acquisitions and the tax effect of the pre-tax adjustments above at applicable tax rates.
© 2012 Endo Pharmaceuticals Inc. All rights reserved
101
Reconciliation of Non-GAAP Measures
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with the Securities and Exchange Commission
GAAP and Adjusted Statements of Operations – Twelve Months Ended December 31, 2010 (unaudited)
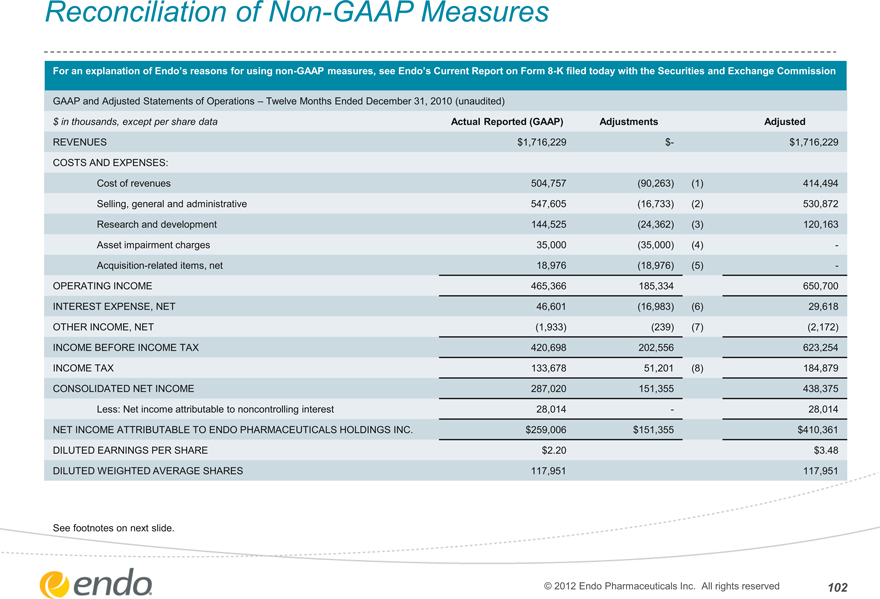
$ in thousands, except per share data Actual Reported (GAAP) Adjustments Adjusted
REVENUES $1,716,229 $- $1,716,229
COSTS AND EXPENSES:
Cost of revenues 504,757 (90,263) (1) 414,494
Selling, general and administrative 547,605 (16,733) (2) 530,872
Research and development 144,525 (24,362) (3) 120,163
Asset impairment charges 35,000 (35,000) (4) -
Acquisition-related items, net 18,976 (18,976) (5) -
OPERATING INCOME 465,366 185,334 650,700
INTEREST EXPENSE, NET 46,601 (16,983) (6) 29,618
OTHER INCOME, NET (1,933) (239) (7) (2,172)
INCOME BEFORE INCOME TAX 420,698 202,556 623,254
INCOME TAX 133,678 51,201 (8) 184,879
CONSOLIDATED NET INCOME 287,020 151,355 438,375
Less: Net income attributable to noncontrolling interest 28,014 — 28,014
NET INCOME ATTRIBUTABLE TO ENDO PHARMACEUTICALS HOLDINGS INC. $259,006 $151,355 $410,361
DILUTED EARNINGS PER SHARE $2.20 $3.48
DILUTED WEIGHTED AVERAGE SHARES 117,951 117,951
See footnotes on next slide.
© 2012 Endo Pharmaceuticals Inc. All rights reserved
102
Reconciliation of Non-GAAP Measures
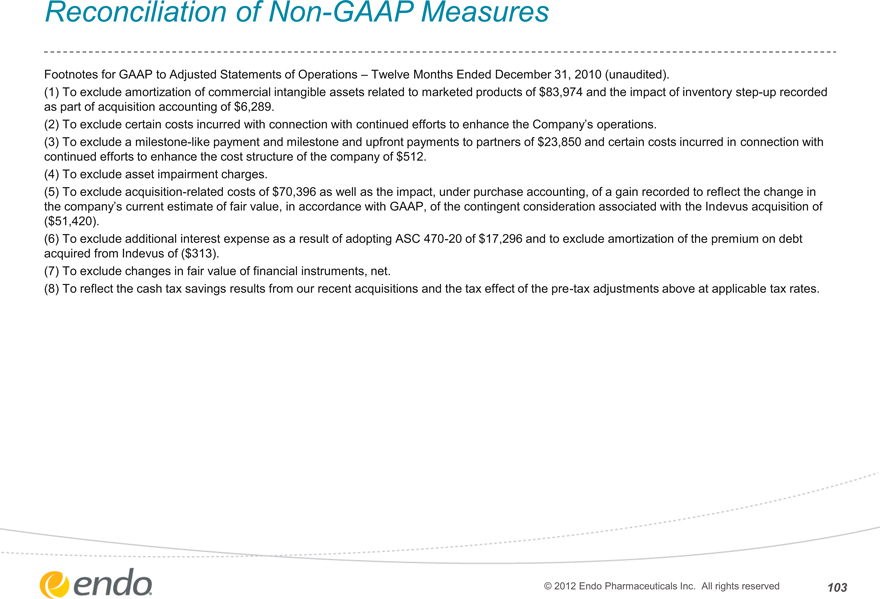
Footnotes for GAAP to Adjusted Statements of Operations – Twelve Months Ended December 31, 2010 (unaudited).
(1) To exclude amortization of commercial intangible assets related to marketed products of $83,974 and the impact of inventory step-up recorded as part of acquisition accounting of $6,289.
(2) To exclude certain costs incurred with connection with continued efforts to enhance the Company’s operations.
(3) To exclude a milestone-like payment and milestone and upfront payments to partners of $23,850 and certain costs incurred in connection with continued efforts to enhance the cost structure of the company of $512.
(4) To exclude asset impairment charges.
(5) To exclude acquisition-related costs of $70,396 as well as the impact, under purchase accounting, of a gain recorded to reflect the change in the company’s current estimate of fair value, in accordance with GAAP, of the contingent consideration associated with the Indevus acquisition of
($51,420).
(6) To exclude additional interest expense as a result of adopting ASC 470-20 of $17,296 and to exclude amortization of the premium on debt acquired from Indevus of ($313).
(7) To exclude changes in fair value of financial instruments, net.
(8) To reflect the cash tax savings results from our recent acquisitions and the tax effect of the pre-tax adjustments above at applicable tax rates.
© 2012 Endo Pharmaceuticals Inc. All rights reserved 103
Reconciliation of Non-GAAP Measures – Q3 2012
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with
the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per Share Guidance for the Quarter Ending
September 30, 2012
Projected GAAP diluted income per common share $1.04
Upfront and milestone-related payments to partners $0.04
Amortization of commercial intangible assets and inventory step-up $0.48
Acquisition and integration costs related to recent acquisitions $0.06
Watson litigation settlement ($0.36)
Interest expense adjustment for ASC 470-20 and other treasury items $0.04
Tax effect of pre-tax adjustments at the applicable tax rates and certain other expected cash ($0.05)
tax savings as a result of recent acquisitions
Diluted adjusted income per common share guidance $1.25
The company’s guidance is being issued based on certain assumptions including:
•Certain of the above amounts are based on estimates and there can be no assurance that Endo will achieve these results
•Includes all completed business development transactions as of September 30, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved 104
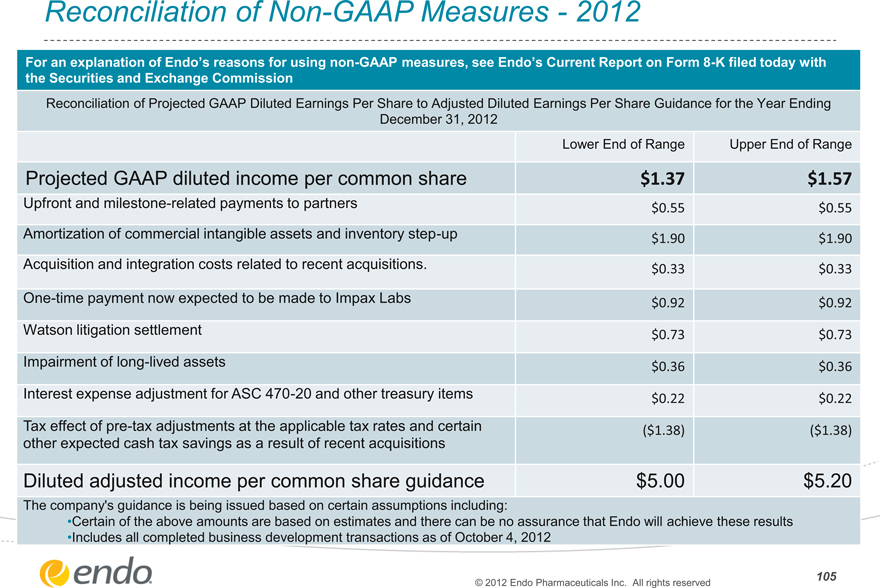
Reconciliation of Non-GAAP Measures—2012
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with
the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per Share Guidance for the Year Ending
December 31, 2012
Lower End of Range Upper End of Range
Projected GAAP diluted income per common share $1.37 $1.57
Upfront and milestone-related payments to partners $0.55 $0.55
Amortization of commercial intangible assets and inventory step-up $1.90 $1.90
Acquisition and integration costs related to recent acquisitions. $0.33 $0.33
One-time payment now expected to be made to Impax Labs $0.92 $0.92
Watson litigation settlement $0.73 $0.73
Impairment of long-lived assets $0.36 $0.36
Interest expense adjustment for ASC 470-20 and other treasury items $0.22 $0.22
Tax effect of pre-tax adjustments at the applicable tax rates and certain ($1.38) ($1.38)
other expected cash tax savings as a result of recent acquisitions
Diluted adjusted income per common share guidance $5.00 $5.20
The company’s guidance is being issued based on certain assumptions including:
•Certain of the above amounts are based on estimates and there can be no assurance that Endo will achieve these results
•Includes all completed business development transactions as of October 4, 2012
105
© 2012 Endo Pharmaceuticals Inc. All rights reserved
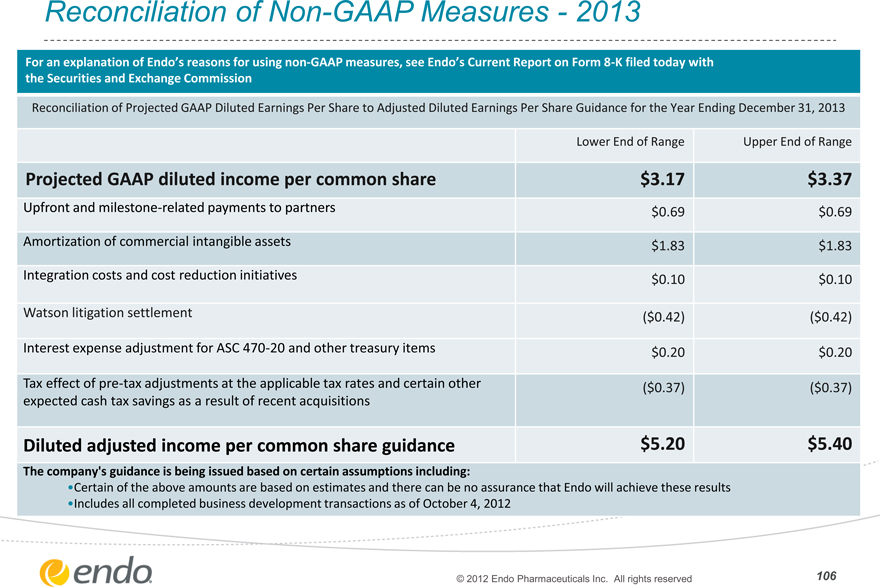
Reconciliation of Non-GAAP Measures—2013
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s Current Report on Form 8-K filed today with
the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per Share Guidance for the Year Ending December 31, 2013
Lower End of Range Upper End of Range
Projected GAAP diluted income per common share $3.17 $3.37
Upfront and milestone-related payments to partners $0.69 $0.69
Amortization of commercial intangible assets $1.83 $1.83
Integration costs and cost reduction initiatives $0.10 $0.10
Watson litigation settlement ($0.42) ($0.42)
Interest expense adjustment for ASC 470-20 and other treasury items $0.20 $0.20
Tax effect of pre-tax adjustments at the applicable tax rates and certain other ($0.37) ($0.37)
expected cash tax savings as a result of recent acquisitions
Diluted adjusted income per common share guidance $5.20 $5.40
The company’s guidance is being issued based on certain assumptions including:
•Certain of the above amounts are based on estimates and there can be no assurance that Endo will achieve these results
•Includes all completed business development transactions as of October 4, 2012
© 2012 Endo Pharmaceuticals Inc. All rights reserved 106
Supplementary Information
107
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
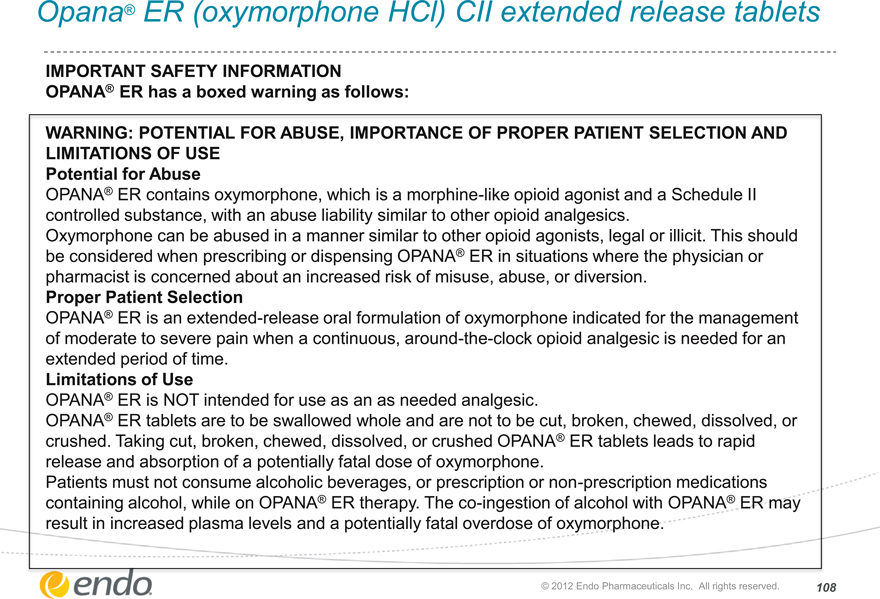
Opana® ER (oxymorphone HCl) CII extended release tablets
IMPORTANT SAFETY INFORMATION
OPANA® ER has a boxed warning as follows:
WARNING: POTENTIAL FOR ABUSE, IMPORTANCE OF PROPER PATIENT SELECTION AND LIMITATIONS OF USE
Potential for Abuse
OPANA®ER contains oxymorphone, which is a morphine-like opioid agonist and a Schedule II controlled substance, with an abuse liability similar to other opioid analgesics.
Oxymorphone can be abused in a manNer similar to other opioid agonisits, legal or illicit. This should be considered when prescribing or dispensing OPANA® ER in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
Proper Patient Selection
OPANA® ER is an extended-release oral formulation of oxymorphone indicated for the management of moderate to severe pain when a continuous, around-the-clock opioid analgesic is needed for an extended period of time.
Limitations of Use
OPANA® ER is NOT intended for use as an needed analgesic.
OPANA® ER tablets are to be swallowed whole and are not to be cut, broken, chewed, dissolved, or crushed. Taking cut, broken, chewed, dissolved, or crushed OPANA® ER tablets leads to rapid release and absorption of a potentially fatal dose of oxymorphone.
Patients must not consume alcoholic beverages, or prescription or non-prescription medications containing alcohol, while on OPANA® ER therapy. The co-ingestion of alcohol with OPANA® ER may result in increased plasma levels and a potentially fatal overdose of oxymorphone.
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 108
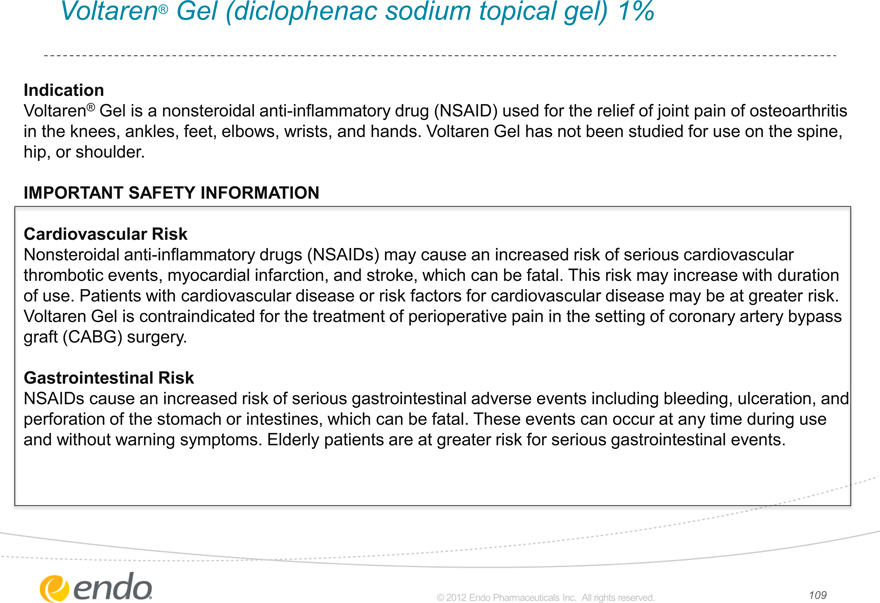
Voltaren® Gel (diclophenac sodium topical gel) 1%
Indication
Voltaren® Gel is a nonsteroidal anti-inflammatory drug (NSAID) used for the relief of joint pain of osteoarthritis in the knees, ankles, feet, elbows, wrists, and hands. Voltaren Gel has not been studied for use on the spine, hip, or shoulder.
IMPORTANT SAFETY INFORMATION
Cardiovascular Risk
Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk. Voltaren Gel is contraindicated for the treatment of perioperative pain in the setting of coronary artery bypass graft (CABG) surgery.
Gastrointestinal Risk
NSAIDs cause in increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events.
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 109
Lidoderm® (lidocaine patch 5%)
Indication
LIDODERM® (lidocaine patch 5%) is indicated for relief of pain associated with post-herpetic neuralgia. Apply only to intact skin.
Important Safety Information
LIDODERM is contraindicated in patients with a history of sensitivity to local anesthetics (amide type) or any product component.
Even a used LIDODERM patch contains a large amount of lidocaine (at least 665 mg). The potential exists for a small child or pet to suffer serious adverse effects from chewing or ingesting a new or used LIDODERM patch, although the risk with this formulation has not been evaluated. It is important for patients to store and dispose of LIDODERM out of reach of children, pets, and others.
Excessive dosing, such as applying LIDODERM to larger areas or for longer than the recommended wearing time, could result in increased absorption of lidocaine and high blood concentrations leading to serious adverse effects.
Avoid contact of LIDODERM with the eye. If contact occurs, immediately wash the eye with water or saline and protect it until sensation returns. Avoid the use of external heat sources as this has not been evaluated and may increase plasma lidocaine levels.
Patients with severe hepatic disease are at greater risk of developing toxic blood concentrations of lidocaine, because of their inability to metabolize lidocaine normally. LIDODERM should be used with caution in patients receiving Class I antiarrhythmic drugs (such as tocainide and mexiletine) since the toxic effects are additive and potentially synergistic. LIDODERM should also be used with caution in pregnant (including labor and delivery) or nursing mothers.
Allergic reactions, although rare, can occur.
During or immediately after LIDODERM treatment, the skin at the site of application may develop blisters, bruising, burning sensation, depigmentation, dermatitis, discoloration, edema, erythema, exfoliation, irritation, papules, petechia, pruritus, vesicles, or may be the locus of abnormal sensation. These reactions are generally mild and transient, resolving spontaneously within a few minutes to hours. Other reactions may include dizziness, headache and nausea.
When LIDODERM is used concomitantly with local anesthetic products, the amount absorbed from all formulations must be considered.
Immediately discard used patches or remaining unused portions of cut patches in household trash in a manner that prevents accidental application or ingestion by children, pets, or others.
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 110
Endocare® Cryocare Systems
The Endocare® Cryocare Systems consists of a compact, easy-to-operate console and associated accessories that include Endocare
CryoProbe™ devices to deliver cold temperatures to the therapeutic tissue and Endocare TempProbe to monitor temperatures in the surrounding tissue. The Cryocare Systems are intended for use in open, minimally invasive or endoscopic procedures in the areas in general surgery, urology, gynecology, oncology, neurology, dermatology, ENT, proctology, pulmonary surgery and thoracic surgery. The systems are designed to freeze/ablate tissue by the application of extreme cold temperatures including prostate and kidney tissue, liver metastases, tumors, skin lesions, and warts.
Prior to using these devices, please review the Operator’s Manual and directions for use for a complete listing of indications, warnings, precautions and safety information. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 111
AMS 700® and Inhibizone®
The AMS 700® Series Inflatable Penile Prosthesis is intended for use in the treatment of chronic, organic, male erectile dysfunction (impotence). These devices are contraindicated in patients who have active urogenital infections or active skin infections in the region of surgery or (for the AMS 700 with InhibiZone® have a known sensitivity or allergy to rifampin, minocycline, or other tetracyclines). Implantation will make latent natural or spontaneous erections, as well as other interventional treatment options, impossible. Men with diabetes, spinal cord injuries or open sores may have an increased risk of infection. Failure to evaluate and treat device erosion may result in infection and loss of tissue. Implantation may result in penile shortening, curvature, or scarring. Possible adverse events include, but are not limited to, urogenital pain (usually associated with healing), urogenital edema, urogenital ecchymosis, urogenital erythema, reservoir encapsulation, patient dissatisfaction, auto-inflation, mechanical malfunction, and impaired urination.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 112
AMS Spectra®
The AMS Spectra® Concealable Penile Prosthesis is a sterile, non-pyrogenic, single-use implant that is intended for use in the treatment of chronic, organic, erectile dysfunction (impotence) in men who are determined to be suitable candidates for implantations surgery. These devices are contraindicated in patients who: have active urogenital infections or active skin infections in the region of surgery; patients whose proximal corporal length measurements is less than the proximal rigid section of the Spectra cylinders, or whose total intracorporal length is not within the range of 12cm to 27.5cm; patients who require repeated endoscopic procedures; or patients who have compromised tissue and as a result cannot withstand constant pressure. Implantation will make latent natural or spontaneous erections, as well as other interventional treatment options, impossible. Men with diabetes, spinal cord injuries or open sores may have an increased risk of infection. Failure to evaluate and treat device erosion may result in infection and loss of tissue. Implantation may result in penile shortening, curvature, or scarring. Possible adverse events include, but are not limited to: infection, erosion, migration, extrusion, mechanical malfunction, patient dissatisfaction, adverse tissue reaction, allergic reaction, pain, urinary obstruction, and silicone particle migration. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 113
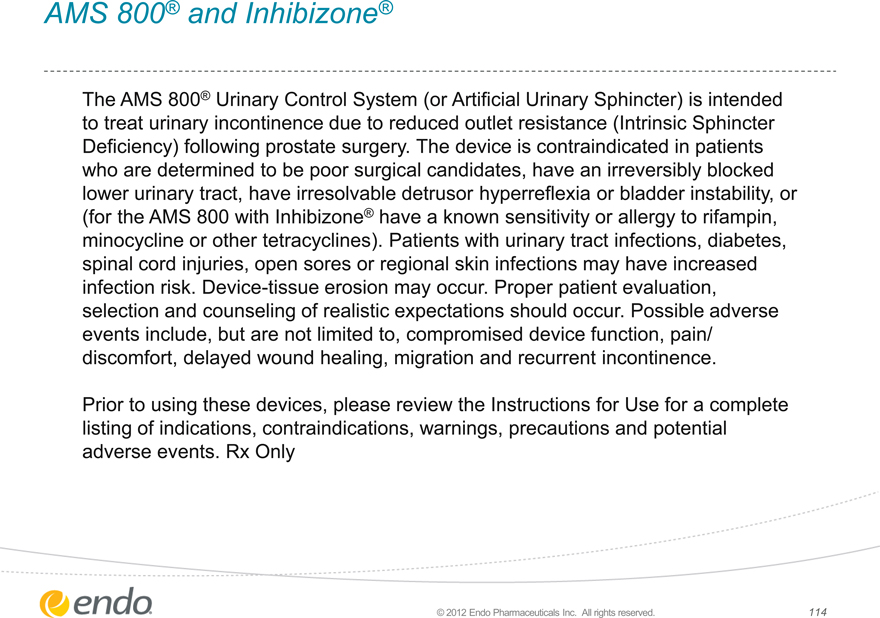
AMS 800® and Inhibizone®
The AMS 800® Urinary Control System (or Artificial Urinary Sphincter) is intended to treat urinary incontinence due to reduced outlet resistance (Intrinsic Sphincter Deficiency) following prostate surgery. The device is contraindicated in patients who are determined to be poor surgical candidates, have an irreversibly blocked lower urinary tract, have irresolvable detrusor hyperreflexia or bladder instability, or (for the AMS 800 with Inhibizone® have a known sensitivity or allergy to rifampin, minocycline or other tetracyclines). Patients with urinary tract infections, diabetes, spinal cord injuries, open sores or regional skin infections may have increased infection risk. Device-tissue erosion may occur. Proper patient evaluation, selection and counseling of realistic expectations should occur. Possible adverse events include, but are not limited to, compromised device function, pain/ discomfort, delayed wound healing, migration and recurrent incontinence.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 114
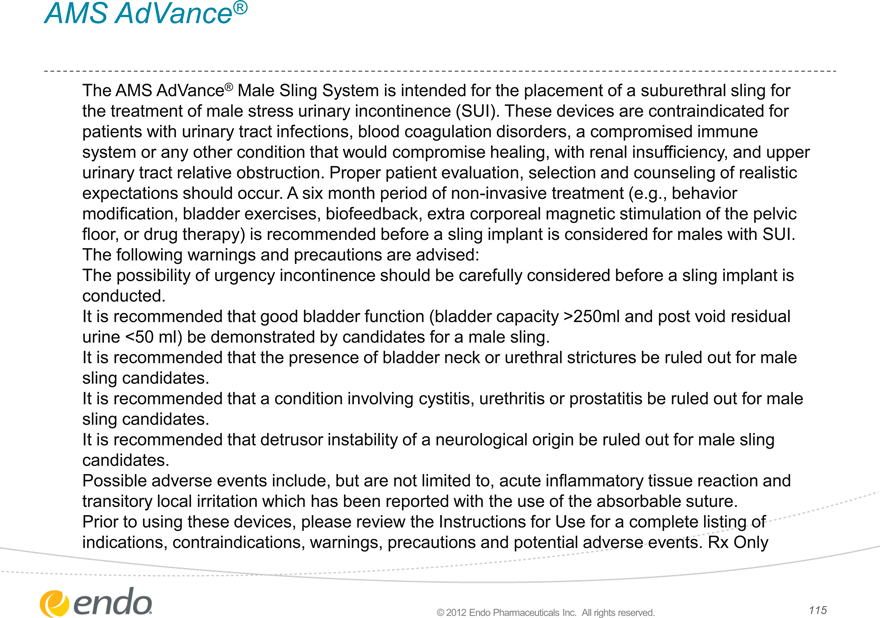
AMS AdVance®
The AMS AdVance® Male Sling System is intended for the placement of a suburethral sling for the treatment of male stress urinary incontinence (SUI). These devices are contraindicated for patients with urinary tract infections, blood coagulation disorders, a compromised immune system or any other condition that would compromise healing, with renal insufficiency, and upper urinary tract relative obstruction. Proper patient evaluation, selection and counseling of realistic expectations should occur. A six month period of non-invasive treatment (e.g., behavior modification, bladder exercises, biofeedback, extra corporeal magnetic stimulation of the pelvic floor, or drug therapy) is recommended before a sling implant is considered for males with SUI. The following warnings and precautions are advised: The possibility of urgency incontinence should be carefully considered before a sling implant is conducted.
It is recommended that good bladder function (bladder capacity >250ml and post void residual urine <50 ml) be demonstrated by candidates for a male sling.
It is recommended that the presence of bladder neck or urethral strictures be ruled out for male sling candidates.
It is recommended that a condition involving cystitis, urethritis or prostatitis be ruled out for male sling candidates.
It is recommended that detrusor instability of a neurological origin be ruled out for male sling candidates.
Possible adverse events include, but are not limited to, acute inflammatory tissue reaction and transitory local irritation which has been reported with the use of the absorbable suture. Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 115
Elevate®, Apogee®, Perigee®
As with any surgical procedure, inherent risks are present. Although rare, some of the most severe risks associated with prolapse procedures include bleeding (hematoma), perforation of vessels, nerves, bladder, ureter, urethra, or bowel; erosion of the implant through neighboring tissue, and infection. Some of the most common risks include; vaginal extrusion, De Novo/ worsening incontinence, dyspareunia, and pain.
If infection or erosion occur, the entire mesh may have to be removed or revised. Prolapse repair may unmask pre-existing incontinence. Do not perform this procedure on pregnant patients.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, and potential adverse events. This document is written for professional medical audiences. Contact AMS for lay publications. AMS periodically updates product literature. If you have questions regarding the currency of this information please contact AMS. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 116
BioArc®, MiniArc®, MiniArc Precise™, MiniArc® Pro, Monarc®, RetroArc®, Sparc®
As with any surgical procedure, inherent risks are present. Although rare, some of the most severe risks with sling procedures include infection, erosion and vessel or urethra perforation. Some of the most common risks include urinary tract infections, urge symptoms and urinary retention. Do not perform this procedure on pregnant patients.
Prior to using these devices, please review the Instructions for Use for a complete listing of indications, contraindications, warnings, precautions, and potential adverse events. This document is written for professional medical audiences. Contact AMS for lay publications. AMS periodically updates product literature. If you have questions regarding the currency of this information please contact AMS. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 117
GreenLight™
The GreenLight™ laser system is intended for incision/excision, vaporization, ablation, hemostasis and coagulation of soft tissue, including photoselective vaporization of the prostate for benign prostatic hyperplasia (BPH). The laser system is contraindicated for patients who: are contraindicated for surgery, have calcified tissue, require hemostasis in >2mm vessels, have uncontrolled bleeding disorders, have prostate cancer, have acute urinary tract infection (UTI) or severe urethral stricture. Possible adverse events include, but are not limited to, irritative symptoms (dysuria, urgency, frequency), retrograde ejaculation, hematuria, urinary incontinence, erectile dysfunction, hematuria—gross, UTI, bladder neck contracture/outlet obstruct, urinary retention, perforation—prostate, urethral stricture.
Prior to using these devices, please review the Operator’s Manual and any accompanying instructions for use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. Rx Only
118
© 2012 Endo Pharmaceuticals Inc. All rights reserved.
StoneLight®
The StoneLight® laser system is intended for endoscopic, laparoscopic and open surgical procedures involving incision/excision, vaporization, ablation, hemostasis and coagulation of soft tissue and lithotripsy and percutaneous urinary lithotripsy of urinary, urethral and kidney calculi. The laser system is contraindicated when the patient is unsuitable for surgery, and where appropriate anesthesia is contraindicated by patient history. Possible complications include, but are not limited to, local and /or systemic infection, pain, chills, edema, hemorrhage, tissue perforation or air embolism.
Prior to using these devices, please review the Operator’s Manual and any accompanying instructions for use for a complete listing of indications, contraindications, warnings, precautions and potential adverse events. Rx Only
© 2012 Endo Pharmaceuticals Inc. All rights reserved. 119