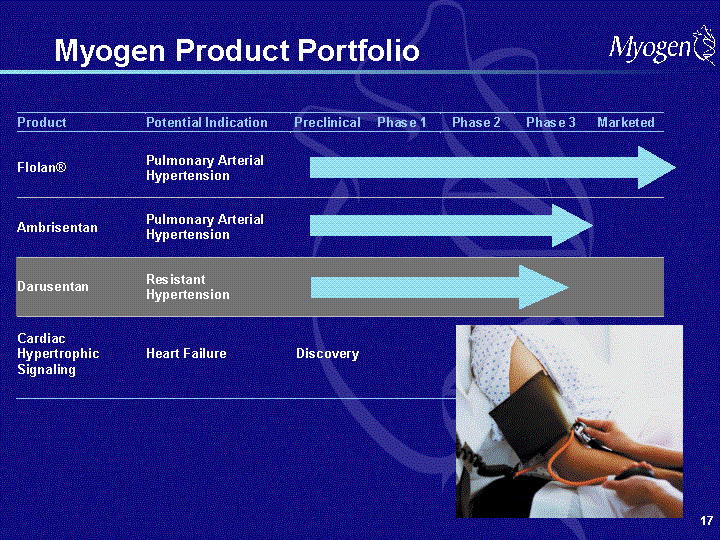
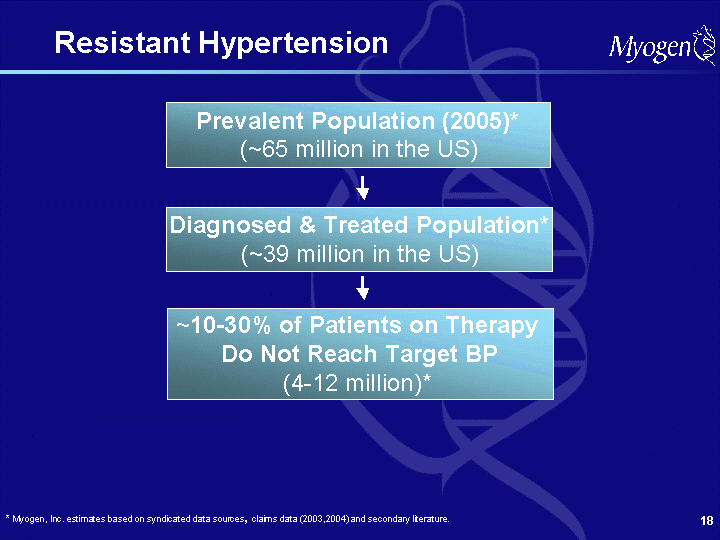
| Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including statements relating to the completion and reporting of results from the Company's clinical trials and the potential advantages of Myogen's product candidates. Actual results could differ materially from those projected and the Company cautions investors not to place undue reliance on the forward-looking statements contained in, or made in connection with, the presentation. Among other things, the projected commencement and completion of any of the Company's clinical trials and the dissemination of the results of the clinical trials may be affected by difficulties or delays, including difficulties or delays caused by regulatory issues, patient enrollment, patient treatment, data collection or data analysis. In addition, the Company's results may be affected by its effectiveness at managing its financial resources, its ability to successfully develop and market its current products, its ability to obtain and enforce patent protection for its products, difficulties or delays in manufacturing its products, and regulatory developments involving current and future products. Delays in clinical programs, whether caused by competition, adverse events, patient enrollment rates, regulatory issues or other factors, could adversely affect the Company's financial position and prospects. Prior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. Preliminary clinical trial results may not be confirmed upon full analysis of the detailed results of a trial. If the Company's product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Even if the Company's product candidates meet safety and efficacy endpoints, regulatory authorities may not approve them, or the Company may face post- approval problems that require the withdrawal of its product from the market. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing of and outcomes of clinical trials, competitive developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale back or discontinue one or more of its drug development or discovery research programs. Myogen may not ever have any products that generate significant revenue. It is Myogen's policy to update or reconfirm its public guidance only by issuing a press release or filing updated guidance in a publicly accessible document with the SEC. Clinical guidance is as of September 26, 2006 and financial guidance relating to the Company's current cash, cash equivalents and investments is as of August 7, 2006. Myogen undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in the Company's expectations. |