Exhibit 99.1
Poster #109
A Multi-Center Phase 1, Dose —Escalation Trial to Determine the Safety and Pharmacokinetics/Pharmacodynamics
of BAY 86-9766 (RDEA119), a MEK Inhibitor, in Advanced Cancer Patients
C. D. Weekes1, D. D. Von Hoff2, A. A. Adjei3, L. Yeh4, D. Leffingwell4, B. Sheedy4, C. Iverson4, P. Rajagopalan5, R. Dubowy5, N. Clendeninn4
1University of Colorado Cancer Center, Aurora, CO;2TGen, Scottsdale, AZ,3Roswell Park Cancer Institute, Buffalo, NY,4Ardea Biosciences, Inc. San Diego, CA,5Bayer
| | | | |
| | | HealthCare Pharmaceuticals, Montville, NJ | | |
| |
| | | | | Thank you to all the patients who participated in this study. |
Introduction
The RAS-RAF-MEK-ERK pathway has emerged as a significant focus for molecularly targeted oncology therapeutics. MEK inhibitors may have broad utility in the treatment of human cancers driven by activation of this pathway as a result of phosphorylation of ERK by MEK. BAY 86-9766 is an oral, potent, non-ATP competitive, highly selective inhibitor of MEK1/2 with favorable pharmacokinetics (PK). It is highly protein bound, 98.0%. BAY 86-9766 is active as monotherapy and in combination with other anti-cancer agents in xenograft models of colon, pancreatic, and hepatocellular cancer and other tumor types. Following multiple once daily (qd) dosing of BAY 86-9766 in healthy adult male volunteers at 10 mg qd or 20 mg qd, BAY 86-9766 was readily absorbed with mean Tmax ranging between 1.75 to 2.25 hours and half lives between 13-15 hours. It is currently in Phase 1/2 development as a single agent and in combination targeting solid tumors. The results of the monotherapy dose escalation study RDEA119-101 in advanced cancer patients are presented here.
Methods
| | – | | Determine the safety and the maximum tolerated dose (MTD) of BAY 86-9766. |
| | – | | Evaluate the plasma PK profile of BAY 86-9766. |
| |
| | – | | Assess pharmacodynamic (PD) markers. |
| |
| | – | | Evaluate the safety and tolerability of the recommended phase 2 dose. |
| Ø | | Key Eligibility Criteria: |
| | – | | Advanced metastatic or locally recurrent solid tumor: |
| | • | | No proven effective therapy exists. |
| |
| | • | | No central nervous system (CNS) metastases requiring treatment. |
| | – | | Males and females, age > 18 years. |
| |
| | – | | ECOG Performance Status of 0-1. |
| |
| | – | | Adequate hematologic, renal, and hepatic function without significant cardiovascular risk. |
| | – | | Patients were given a single dose on Day 1 to determine PK, were off drug for 7 days, and then began a 28-day course of treatment with steady-state PK obtained on Days 15 and 28. |
| |
| | – | | Safety was assessed by adverse events (AEs), clinical laboratory tests, vital signs, ECGs, ECHO/MUGA scans and physical exams. |
| |
| | – | | Patients were evaluated every 2 courses for tumor response by RECIST criteria. |
| |
| | – | | PD assessments included phosphorylated ERK (pERK) and cytokine levels from peripheral blood mononuclear cells (PBMCs) and pERK from hair follicles. |
| |
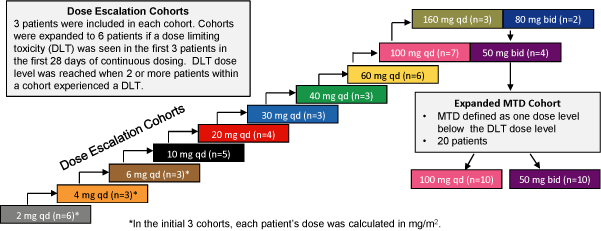
| | – | | Dose escalation scheme followed a modified Fibonacci series as shown in Figure 1. |
Figure 1: Study Design Scheme
Results
Patient Demographics and Baseline Characteristics
| Ø | | Of the 69 patients enrolled in the study, the mean age was 61 years (range: 19 — 82 years) across all dose levels. |
| |
| Ø | | A majority of patients were male (39 [57%]) and of white, non-Hispanic ethnic origin (63 [91%]). |
| |
| Ø | | The ECOG Performance Status score at screening was 1 in a majority of patients (49 [71%]). |
| |
| Ø | | Mean time since first cancer diagnosis was 5 years (range: 0.2 — 18.6 years). |
| |
| Ø | | Most frequently reported tumor type was colorectal; data for all tumor types are summarized in Table 1. |
| |
| Ø | | Most common prior cancer therapies were surgery in 66 (97%) patients, chemotherapy in 65 (96%) patients, and radiation in 39 (57%) patients. |
Results (cont’d)
Table 1: Primary Tumor Type by Total Daily Dose
| | | | | | | | | | | |
| | | Lower Dose Levels | | Higher Dose Levels | | Overall |
| | | 2 mg - 40 mg | | 60 mg | | 100 mg** | | 160 mg | | All dose levels |
| Primary Tumor Type | | N = 27 | | N = 6 | | N = 31 | | N = 5 | | N = 69 |
| | | |
| Colorectal | | 4 | | 3 | | 11 | | 4 | | 22 |
| Melanoma | | 4 | | 0 | | 4 | | 0 | | 8 |
| NSCLC | | 2 | | 0 | | 2 | | 1 | | 5 |
| Prostate | | 3 | | 0 | | 2 | | 0 | | 5 |
| Pancreatic | | 1 | | 0 | | 4 | | 0 | | 5 |
| Other* | | 13 | | 3 | | 8 | | 0 | | 24 |
| |
| Totals | | 27 | | 6 | | 31 | | 5 | | 69 |
| |
| | |
| |
| * | | Other: 2 mg - 40 mg:Adrenal (3), breast (2), GIST, Ewing’s sarcoma, ovarian, mesothelioma, vaginal, papillary thyroid, perivascular epithelioid sarcoma, chondrosarcoma;60 mg:GE junction adenocarcinoma, biliary, retroperitoneal sarcoma;100 mg: Cholangiocarcinoma (3), hepatocellular, basal cell, chondrosarcoma, liposarcoma, appendiceal, |
| |
| ** | | Includes all patients treated at the MTD dose level of 100 mg daily in the dose escalation and MTD cohorts. |
Patient Disposition
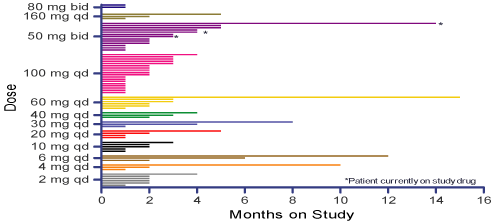
| Ø | | Of the 69 patients enrolled, 57 (83%) patients completed the study (28 days of continuous dosing in Course 1). |
| |
| Ø | | A majority of patients (53 [77%]) discontinued from the study due to disease progression categorized as either objective evidence of disease progression (48 [70%]) or symptomatic deterioration (5 [7%]). |
| |
| Ø | | The remaining patients discontinued from the study due to adverse events (12 [17%]) and withdrawn consent (1 [1%]). |
Figure 2: Exposure to RDEA119 In Months By Treatment Group
Safety
| Ø | | RDEA119 was generally well tolerated with AEs reported that are commonly associated with MEK inhibitors. |
| |
| Ø | | A total of 56 SAEs were reported at any dose level of which 12 (21%) were considered possibly related to treatment. |
| |
| Ø | | The DLT dose level was determined to be 160 mg per day (Table 3). |
| |
| Ø | | At the MTD dose level (100 mg per day), 6 (19%) patients discontinued from the study due to an adverse event of which 4 (13%) were considered to be possibly related to treatment with study drug. AE data are presented in Table 2. |
| |
| Ø | | Of the 31 patients treated at the MTD dose level (100 mg per day), 17 (55%) patients had a total of 22 dose interruptions; 4 (13%) of these patients required their dose of study drug to be reduced due to an adverse event of rash (2 patients), peripheral edema (1 patient), or gait imbalance (1 patient). |
| | |
| Table 2: | | Overall, Most Frequently Reported (≤ 15% of patients) Treatment Related AEs, all Grades, by Total Daily Dose |
| | | | | | | | | | | |
| | | Lower Dose Levels | | Higher Dose Levels | | Overall |
| | | 2 mg - 40 mg | | 60 mg | | 100 mg* | | 160 mg | | All dose levels |
| Preferred Term | | N (%) | | N (%) | | N (%) | | N (%) | | N (%) |
| Rash** | | 13 (48) | | 6 (100) | | 26 (84) | | 4 (80) | | 49 (71) |
| Diarrhoea | | 6 (22) | | 1 (17) | | 13 (42) | | 2 (33) | | 22 (32) |
| Nausea | | 5 (19) | | 1 (17) | | 12 (39) | | 0 | | 18 (26) |
| Vomiting | | 7 (26) | | 0 | | 9 (29) | | 1 (17) | | 17 (25) |
| Fatigue | | 4 (15) | | 2 (33) | | 9 (29) | | 2 (33) | | 17 (25) |
| Oedema peripheral | | 0 | | 0 | | 9 (29) | | 1 (17) | | 10 (15) |
| | |
| * | | Includes all patients treated at the MTD dose level of 100 mg daily in the dose escalation and MTD cohorts. |
| |
| ** | | The term rash includes the Preferred Terms rash, rash macular, rash maculo papular, rash papular, and dermatitis acneiform. |
Results (cont’d)
Safety (cont’d)
| Ø | | Rash was the most common AE . A total of 90 events of rash were reported by 49 (71%) patients; 97% (87) were grade 1/2 in severity and 4% (3) were grade 3. |
| | • | | Treatment guidelines were provided to the sites to minimize rash as follows: Patients were instructed to moisturize the skin twice daily with a thick alcohol-free emollient and minimize exposure to sunlight. Treatment with a topical hydrocortisone 2.5% and/or clindamycin 1% gel for grade 1; same as for mild rash plus doxycycline 100 mg bid or Minocycline 100 mg bid for grade 2 rash; treatment for moderate rash plus Medrol dose pack for a grade 3 rash. |
Table 3 : Summary of DLT Events (possibly related CTCAE* grade 3 or higher)
| | | | | |
|
| Dose (mg) | | Dose Limiting Toxicity | | Action Taken with BAY 86-9766 |
| 2 mg qd | | Lipase increased | | Treatment held for 1 week, dosing resumed, at same dose. |
| 60 mg qd | | Hypophosphataemia | | No action, continued on study. |
| 100 mg qd | | Pain of skin and rash | | Treatment held ~ 2 weeks, dosing resumed at a reduced dose of 60 mg qd. |
| 160 mg qd | | Diarrhoea | | Patient withdrew consent. |
| | | Presyncope | | Treatment held for 1 week, dosing resumed at 100 mg qd. |
| 80 mg bid | | Somnolence and rash | | Patient discontinued study due to DLTs. |
| | |
| * | | CTCAE (Common Terminology Criteria for Adverse Events) V 3.0 |
CNS and ocular toxicities have been reported with the use of other MEK inhibitors. Those seen in this study and considered possibly related to study drug include:
ØAt the DLT dose (total daily dose of 160 mg), 1 case each of the CNS toxicities of presyncope and somnolence, grade 3, were reported and considered DLTs.
ØAt the MTD dose level (100 mg per day), a total of 10 (32%) patients reported CNS toxicities that were considered possibly related to study drug.
| | • | | Grade 3/4 AEs of hallucinations, somnolence, confusion, and neurotoxicity were reported in 3 (10%) patients. |
| |
| | • | | Grade 1/2 AEs reported in both dose groups were similar and included confusion, dizziness, and abnormal dreams. |
Ø At the MTD dose level (100 mg per day), 1 patient reported grade 1 chorioretinopathy during course 1 that recovered following dose reduction, and 1 patient with ocular melanoma developed grade 2 hemi-retinal vein occlusion after 5 courses of treatment, that resolved with intravitreal bevacizumab.
Pharmacokinetics
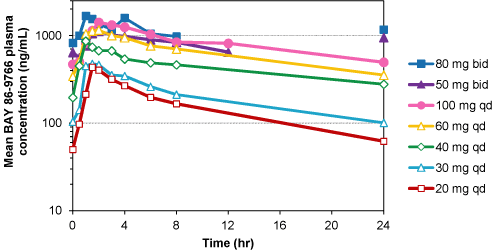
| Ø | | Cmax and AUC0-24 values increased with dose in a dose linear manner following single or multiple doses up to 100 mg. |
| |
| Ø | | Accumulation of BAY 86-9766 was modest (median accumulation ratio of Cmax: 1.4, (range 0.4-3.8); AUC0-24: 1.6
(range 0.9-2.5) in all patients) following multiple doses. |
| |
| Ø | | Half life of BAY 86-9766 was approximately 10 to 20 hours following single dose on Day 1. |
Figure 3: Mean Plasma Concentration Profiles of BAY 86-9766 at Steady State
Results (cont’d)
Pharmacodynamics
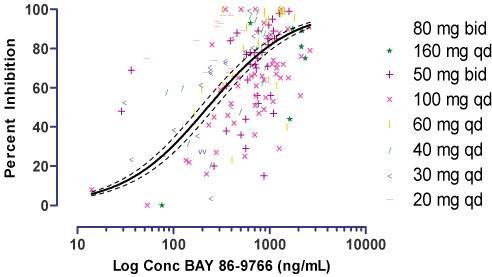
| Ø | | BAY 86-9766 shows a concentration-dependent increase in inhibition of pERK (Figure 4). |
| |
| Ø | | Emax of 100% pERK inhibition was achieved at multiple doses. |
| |
| Ø | | Estimated leukocyte pERK ED50 corresponds to mean 24 hour steady state plasma levels achieved by the 30 mg to 40 mg qd dose range. |
| |
| Ø | | pERK was also decreased in tumor biopsies (data not shown). |
| |
| Ø | | Stable disease was confirmed in 10 patients across multiple dose groups and is summarized in Table 4. |
| | |
| Figure 4: | | Isolated Patient Leukocytes Show a Concentration-Dependent pERK Inhibition with resulting ED50 of 227 ng/mL |
Methods:Leukocytes were isolated from whole blood after dosing with BAY 86-9766 and stimulating phosphorylation of ERKex vivowith TPA. Data graphed above are from PD and PK samples taken at 2 hours, 3 hours, and 24 hours post dosing. Curve fitting was done using normalized response parameters, n = 169.
| | |
| Table 4: | | Confirmed Stable Disease Achieved in Ten Patients Across Multiple Tumor Types and Dose Groups |
| | | | | |
| Dose | | Tumor Type | | Number of Courses |
| 4 mg qd | | Ovarian | | 10 |
| 6 mg qd | | Adrenal | | 6 |
| | | Colorectal | | 12 |
| 20 mg qd | | Breast | | 5 |
| 30 mg qd | | Colorectal | | 8 |
| 60 mg qd | | Liposarcoma | | 15 |
| 50 mg bid | | Colorectal* | | 14 (ongoing) |
| | | Ocular melanoma | | 5 |
| | | Ocular melanoma | | 5 (ongoing) |
| 160 mg qd | | Colorectal** | | 5 |
| | |
| * | | Began dosing at 50 mg bid. Drug held from C4D28-C5D21 due to CNS AEs. Re-started drug at 30 mg bid. |
| |
| ** | | Began dosing at 160 mg qd. Drug held from C1D15-C1D28 due to CNS AEs. Re-started drug at 100 mg qd. Note: One additional patient in the 50 mg bid group is ongoing in Course 4 with stable disease yet to be confirmed. |
Conclusions
| Ø | | BAY 86-9766 was generally well tolerated at doses£ 100 mg daily with rash being the most common treatment-related adverse event. |
| |
| Ø | | Exposure of BAY 86-9766 increased linearly with increasing doses. |
| |
| Ø | | At the MTD, significant inhibition of leukocyte pERK and associated cytokines was observed. |
| |
| Ø | | Stable disease was achieved in 10 patients for an average duration of 8 months. |
| |
| Ø | | Based on the results of this study, Phase 2 development is indicated and being pursued. |
22ndEORTC-NCI-AACR Symposium on “Molecular Targets and Cancer Therapeutics”, Berlin, November 16-19, 2010