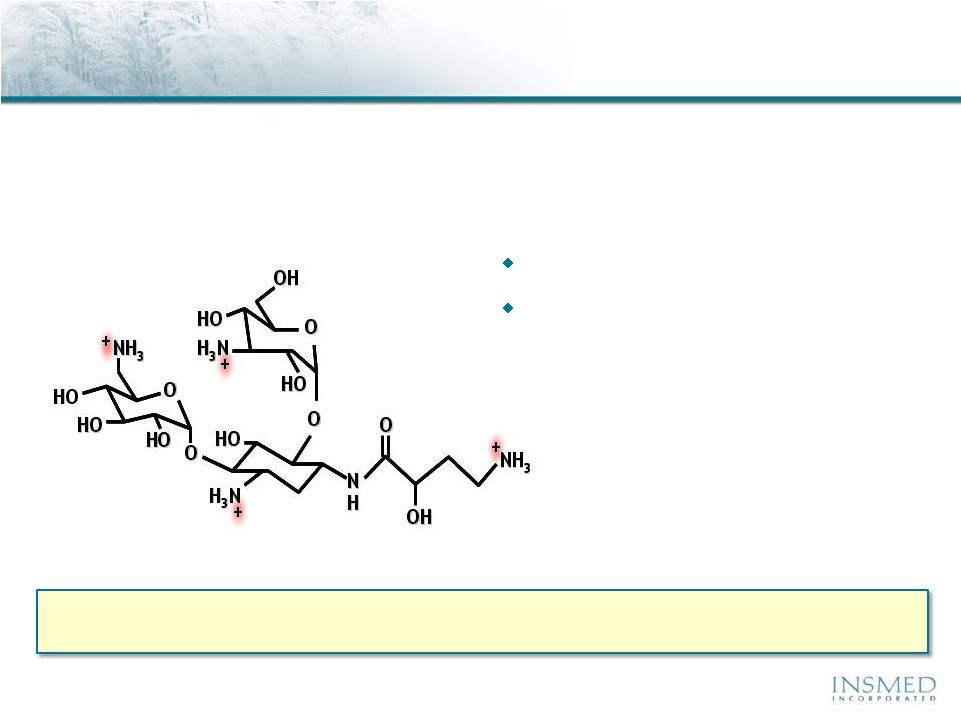
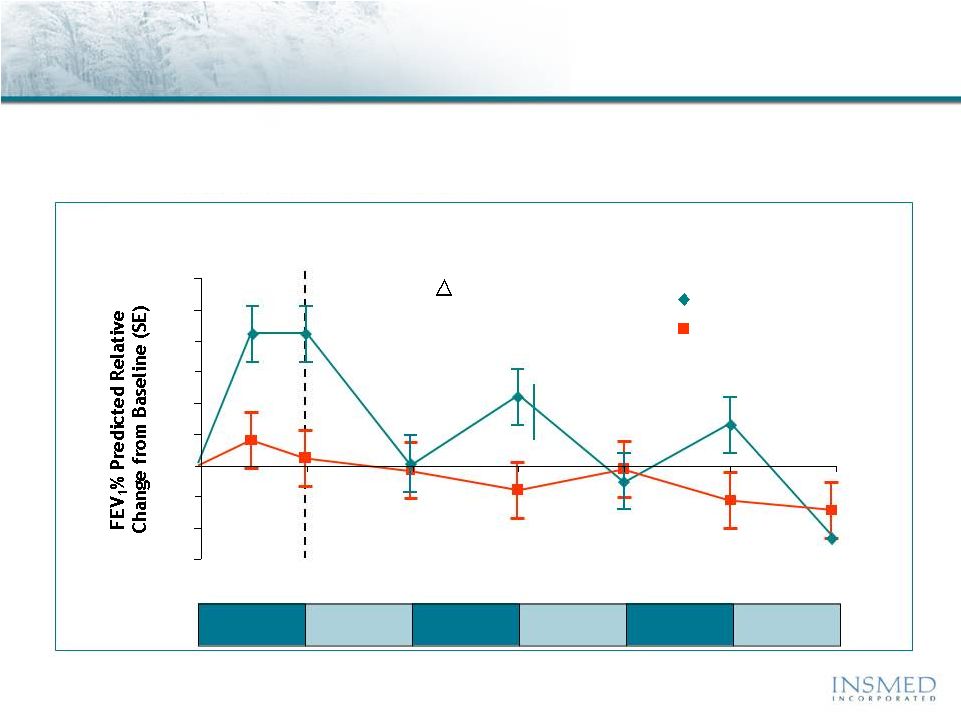
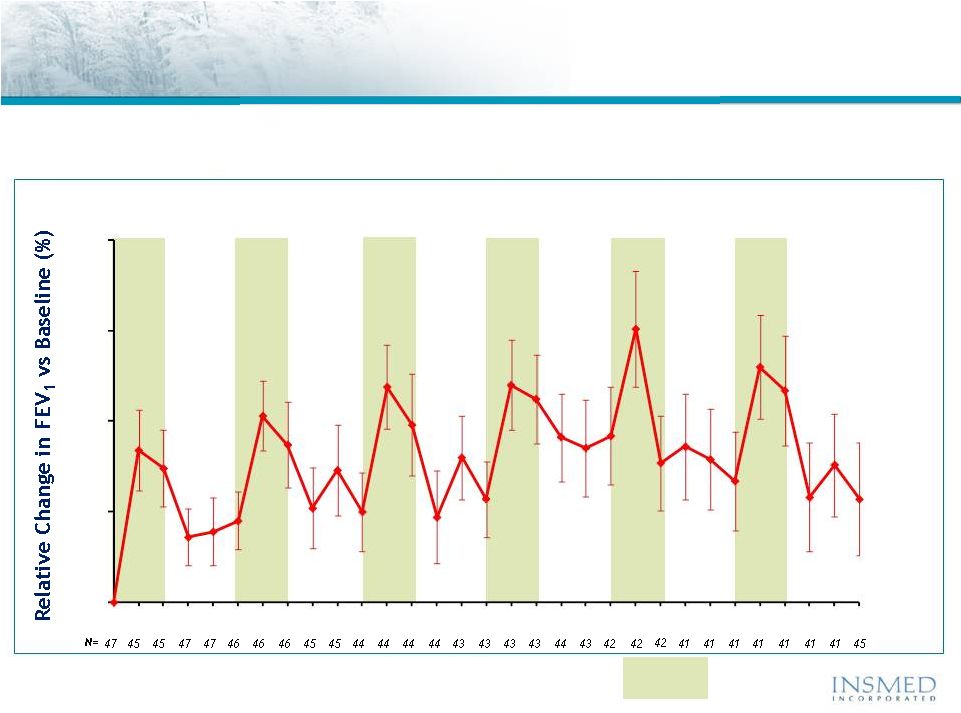
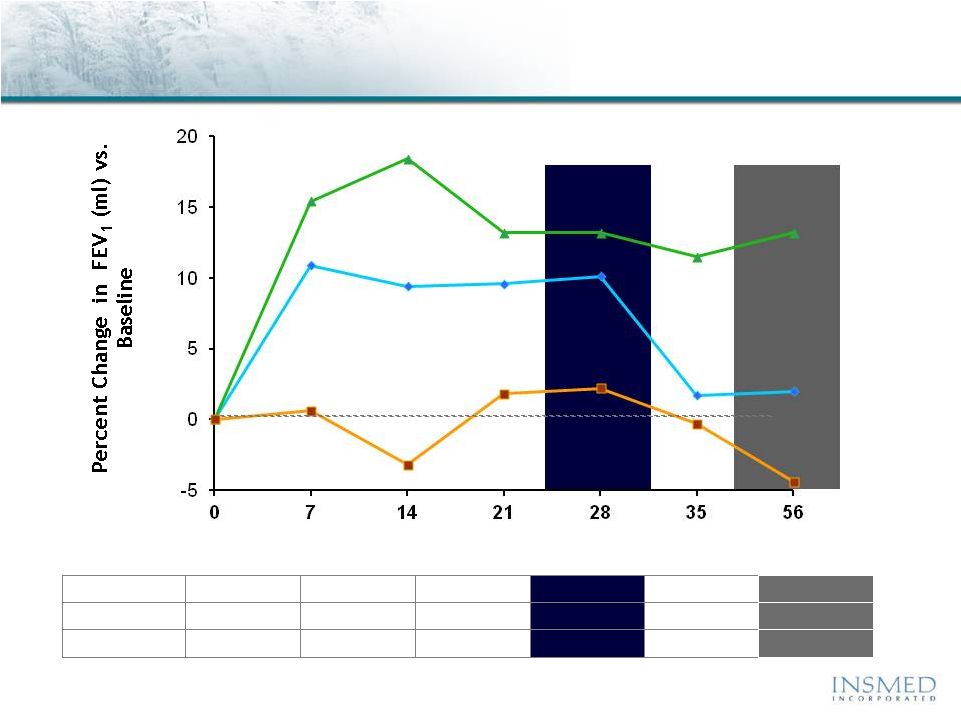
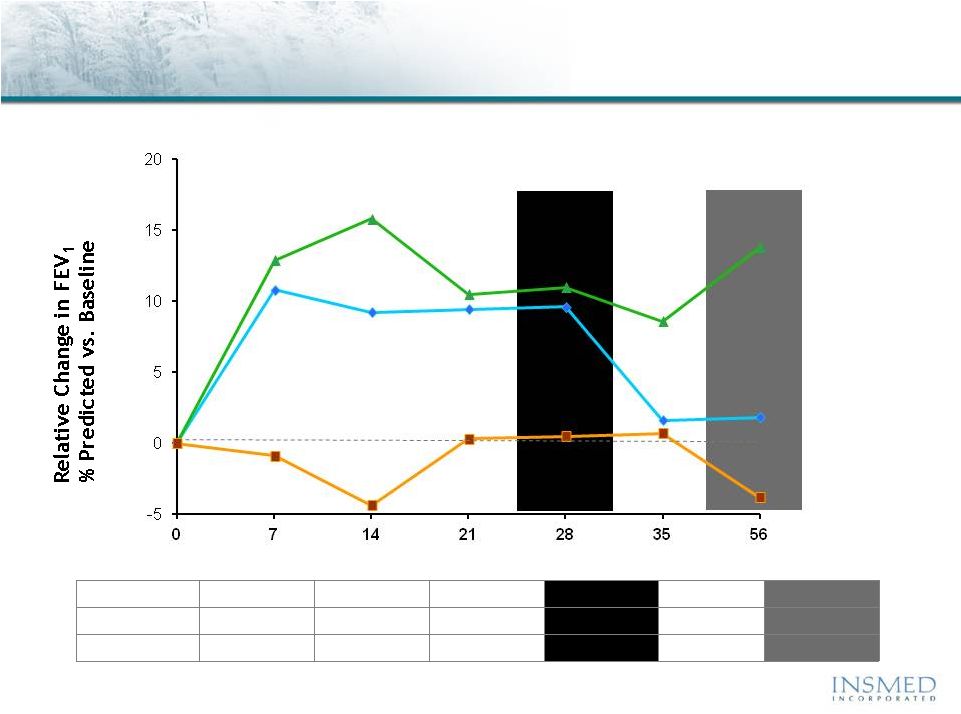
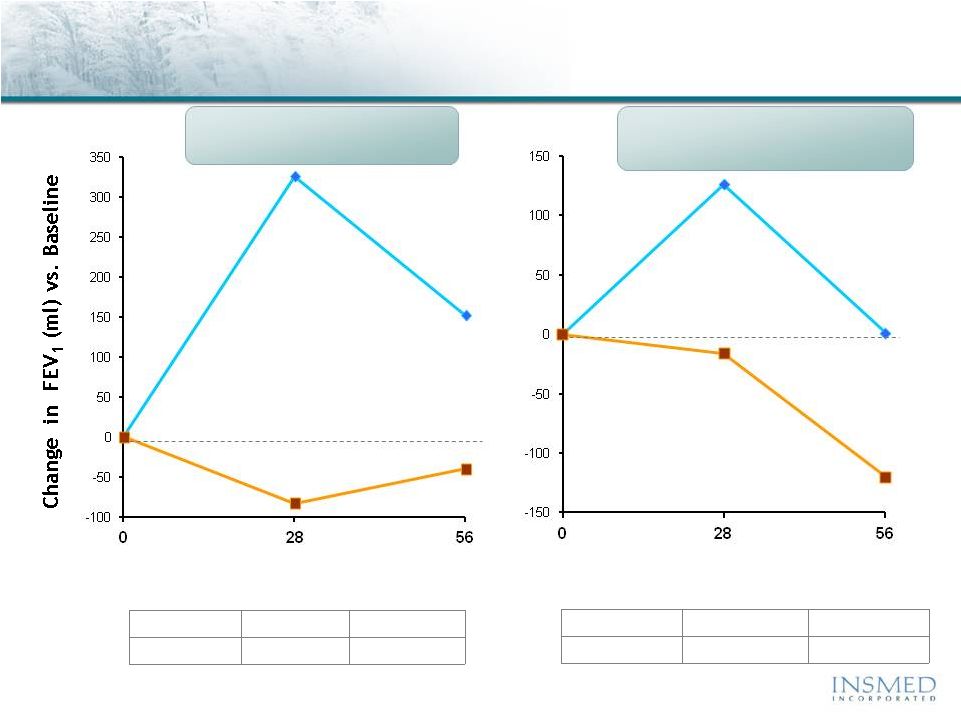
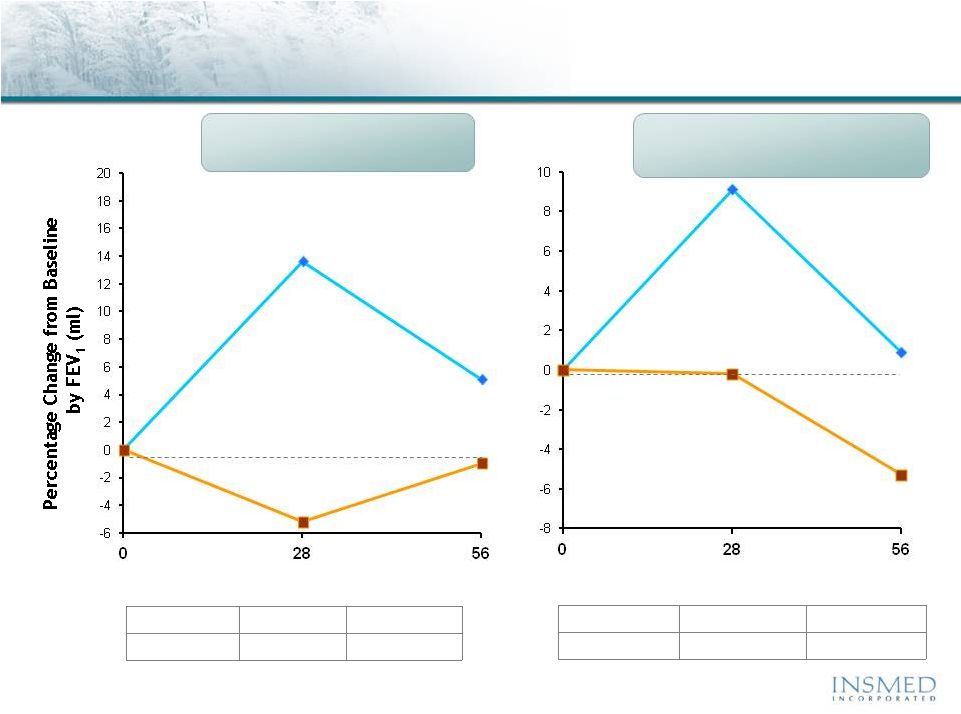
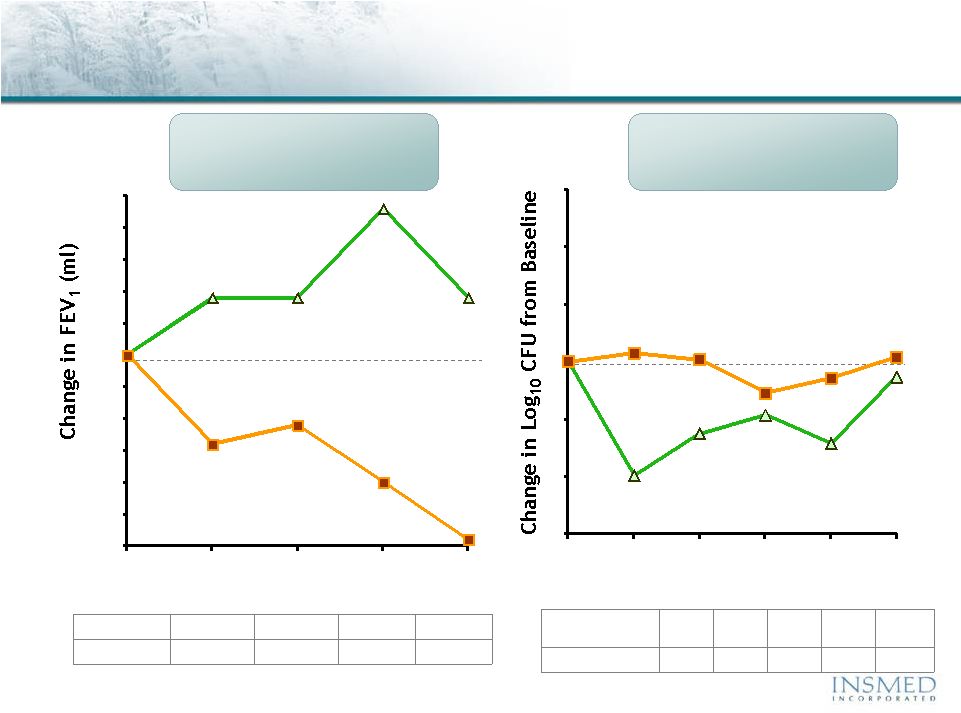
19 Summary: Addressing Potential for Cross-Resistance in ARIKACE While resistance to TOBI (tobramycin) has been documented, we believe there is no cross-resistance in ARIKACE (amikacin) for the following reasons. Well-characterized clinical isolates of Pseudomonas aeruginosa (Pa) from Dr. Burns’ collection have been tested against amikacin and ARIKACE. ARIKACE has shown activity against aminoglycoside-resistant and multi-drug resistant isolates. Dr. Burns felt ARIKACE performed a bit better than free amikacin. (Report on file.) Overall, amikacin has lower potential for inducing resistance as compared to tobramycin (literature). Additionally, aminoglycoside-inactivating enzymes elaborated by Pa are different for these two aminoglycosides. Thus, we there is no complete cross resistance. The issue of emerging tobramycin resistance secondary to TOBI (inhaled antibiotic) use is not completely quantified. However, it is primarily due to poor compliance with the prescribed regimen of TOBI. Patients do not take the drug twice a day consistently. This leads to drug levels much below the MICs of most phenotypes of Pa for prolonged periods, and thus increased potential for emergence of resistance. Additionally, there is non-specific binding of cationic tobramycin to sputum and further low levels available to microbes. Typically, levels >10x of the MICs are needed for entire dosing interval. Thus, compliance with dosing regimen is critical as is penetration of antibiotics into biofilms. Features of ARIKACE that overcome some of the issues responsible for resistance include: charge neutral liposomes shield amikacin, providing penetration into biofilm, and high Cmax and AUC, enabling once a day dosing and improved compliance. The unique features of ARIKACE will reduce potential for emergence of amikacin resistance vs. free aminoglycoside for inhalation. Most importantly, the sustained clinical benefit of Arikace in the “off month” and convenience of once a day will shape the use of inhalation antibiotics in CF patients. Use of ciprofloxacin is known to contribute to emergence of Pa isolates with antimicrobial resistance. Tobramycin is also used as IV for treatment of exacerbations and for tune-ups. This may also be contributing to emergence of resistance as low levels of drug reach the lung after IV use. Our phase 2 data have shown that 65% of isolates were resistant to aminoglycosides and ~90% were mucoid variant. However, we were able to demonstrate reduction in bacterial density and improvement in lung function and pros. Thus, we expect to have significant treatment effect in phase 3 studies even if isolates are resistant. We have also done in vitro work against mdr isolates and shown ARIKACE to be effective. |