As filed with the Securities and Exchange Commission on October 29, 2007
Registration Statement No. 333-145356
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 3
TO
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
NANOSPHERE, INC.
(Exact name of registrant as specified in its charter)
| | | | | |
| | | | | |
Delaware
(State or other jurisdiction of
incorporation or organization) | | 2835
(Primary Standard Industrial
Classification Code Number) | | 36-4339870
(I.R.S. Employer
Identification Number) |
4088 Commercial Avenue
Northbrook, Illinois 60062
(847) 400-9000
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
William P. Moffitt III
President and Chief Executive Officer
Nanosphere, Inc.
4088 Commercial Avenue
Northbrook, Illinois 60062
(847) 400-9000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| | | |
Esteban A. Ferrer, Esq.
Ann Lawrence, Esq.
Paul, Hastings, Janofsky & Walker LLP
1055 Washington Boulevard
Stamford, CT 06901
Telephone: (203) 961-7400
Facsimile: (203) 674-7716 | | William J. Whelan III, Esq.
Cravath, Swaine & Moore LLP
Worldwide Plaza
825 Eighth Avenue
New York, NY 10019
Telephone: (212) 474-1000
Facsimile: (212) 474-3700 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
CALCULATION OF REGISTRATION FEE
| | | | | | | | | | | | | |
| | | | | | | Proposed Maximum
| | | Proposed Maximum
| | | |
Title of Each Class of
| | | Amount to
| | | Offering Price
| | | Aggregate Offering
| | | Amount of
|
| Securities to be Registered | | | be Registered(1) | | | Per Share | | | Price | | | Registration Fee(2) |
| Common Stock, $0.01 par value per share | | | 8,050,000 | | | $16.00 | | | $128,800,000 | | | $3,955 |
| | | | | | | | | | | | | |
| | |
| (1) | | Includes 1,050,000 shares that the underwriters have the option to purchase to cover over-allotments, if any. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
|
SUBJECT TO COMPLETION, DATED OCTOBER 29, 2007
7,000,000 Shares
Common Stock
Prior to this offering, there has been no public market for our common stock. The initial public offering price of the common stock is expected to be between $14.00 and $16.00 per share. We have applied to list our common stock on the NASDAQ Global Market under the symbol “NSPH”.
We are offering 7,000,000 shares of our common stock. The underwriters have an option to purchase a maximum of 1,050,000 additional shares of common stock to cover over-allotments of shares.
Investing in our common stock involves risks. See “Risk Factors” beginning on page 9.
Brookside Capital Partners Fund, L.P., one of our principal stockholders, has indicated an interest in purchasing $12.5 million of our common stock in this offering at the initial offering price. Brookside Capital Partners Fund, L.P. is not under any obligation to purchase any shares in this offering, and their interest in purchasing shares in this offering is not a commitment to do so.
| | | | | | | | | | | | | |
| | | | | | Underwriting
| | | | |
| | | Price to
| | | Discounts and
| | | Proceeds to
| |
| | | Public | | | Commissions | | | Nanosphere, Inc. | |
| |
| Per Share to Public | | $ | | | | $ | | | | $ | | |
| Per Share to Brookside Capital Partners Fund, L.P. | | $ | | | | $ | | | | $ | | |
| Total | | $ | | | | $ | | | | $ | | |
Delivery of the shares of common stock in book-entry form will be made on or about , 2007.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Credit Suisse
Piper Jaffray
Leerink Swann
Allen & Company LLC
The date of this prospectus is , 2007.
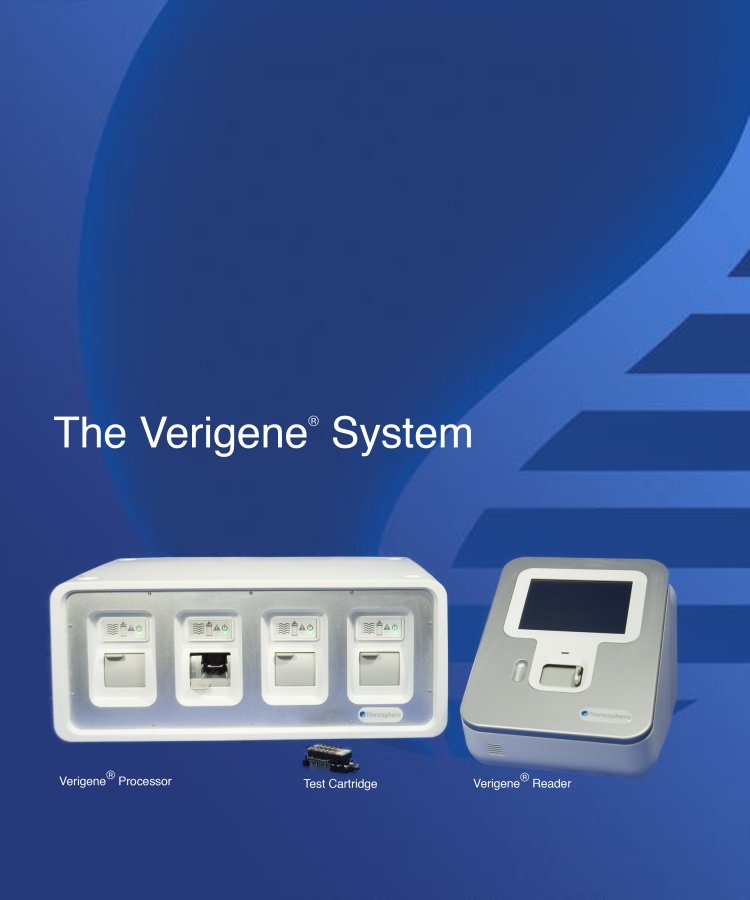
| The Verigene® System Verigene® Processor Test Cartridge Verigene® Reader |
TABLE OF CONTENTS
You should rely only on the information contained in this document or to which we have referred you. We have not authorized anyone to provide you with information that is different. This document may only be used where it is legal to sell these securities. The information contained in this document is accurate only on the date of this document. Our business, financial condition, results of operations and prospectus may have changed since that date.
Dealer Prospectus Delivery Obligation
Until , 2007 (25 days after the commencement of the offering), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
This summary highlights selected information contained elsewhere in this prospectus. This summary does not contain all of the information you should consider before investing in our common stock. You should read this entire prospectus carefully, especially the risks of investing in our common stock discussed under “Risk Factors” beginning on page 9, and the financial statements and notes to those financial statements, before making an investment decision. Unless the context indicates otherwise, the references in this prospectus to “Nanosphere,” “we,” “us” and “our” refer to Nanosphere, Inc. Nanosphere, Inc. does not have any subsidiaries.
Our Company
Nanosphere develops, manufactures and markets an advanced molecular diagnostics platform, the Verigene System, that enables simple, low cost and highly sensitive genomic and protein testing on a single platform. Our proprietary nanoparticle technology simplifies molecular diagnostic testing, achieves ultra-sensitive protein detection at limits beyond current diagnostic technologies, provides the ability to multiplex, or run multiple tests at the same time on the same sample, and enables the development of a broad menu of test assays to be performed on a single platform. We received 510(k) clearance from the United States Food and Drug Administration, or FDA, for the Verigene System and our warfarin metabolism assay on September 17, 2007 and for our hyper-coagulation assay on October 12, 2007. Upon receipt of FDA clearance, we commenced sales to hospital-based laboratories and academic research institutions in the United States, which we believe is the primary market for our products.
We are currently developing diagnostic tests for a variety of medical conditions including cancer, neurodegenerative, cardiovascular and infectious diseases, as well as pharmaco-genomics, or tests for personalized medicine. We anticipate that we will submit applications to the FDA for clearance of tests for cystic fibrosis, herpes, cervical cancer, respiratory illness, recurrent prostate cancer and cardiovascular disease during the next 36 months, and we anticipate we will submit two such tests within the next 12 months. Since our inception, we have had minimal revenues which have been derived from the sale of the Verigene System, including cartridges and related products, to research laboratories pursuant to government contracts.
Our Market Opportunity
According to Boston Biomedical Consultants, the global in vitro diagnostics market was estimated to be $34 billion in 2006. One of the fastest growing segments of the in vitro diagnostics market is the $2.3 billion molecular diagnostics market which, together with our estimated market for our initial protein assays, comprises a more than $3.0 billion current market opportunity. Growth in our market will be driven by the continued conversion of traditional testing methods to molecular methods, an acceleration in the discovery of genomic biomarkers resulting in opportunities for novel tests, the emergence of tests for pharmaco-genomics, the availability of technology for more sensitive protein detection resulting in novel protein tests and the growing understanding of the inter-relationship between genetics and proteins in disease states.
The most widely used method for genomic testing is polymerase chain reaction, or PCR, which involves amplifying, or generating billions of copies of, the DNA sequence in question and then detecting the DNA with the use of fluorescent dyes. Due to the complexity, susceptibility to contamination and significant costs related to PCR and other amplification technologies, the molecular diagnostics market remains limited to reference laboratories, research facilities and laboratories associated with major hospitals, typically at academic teaching institutions, of which there are 200 to 300 in the United States. Moreover, due to the limited capability of many existing technologies, numerous testing platforms are required to perform even a limited menu of tests. PCR and other target amplification technologies also lack the capacity to multiplex in a cost effective manner.
Advances in molecular diagnostics have led to a broad array of new tests to detect genomic markers, however, many diseases are manifested at the protein, rather than the genetic, level. The most widely used method for protein testing is enzyme-linked immunosorbent assay, or ELISA. However, ELISA is often not sufficiently sensitive to detect protein biomarkers until the disease has progressed to an advanced stage and
1
biomarkers for several diseases have not been validated or commercialized because they exist in concentrations too low to be detected by current technologies. While mass spectrometry has emerged as an alternative approach to ELISA for protein detection due to its greater sensitivity, it is extremely costly, requires significant time and effort by highly trained personnel and is unable to detect long peptide chain proteins or misfolded proteins which are biomarkers for diseases such as transmissible spongiform encephalopathy, or mad cow, and Alzheimer’s.
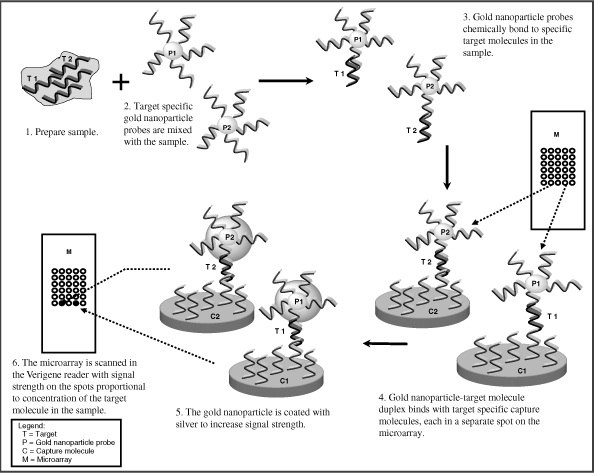
The Verigene System
The Verigene System, recently cleared by the FDA, is a compact, bench-top molecular diagnostics workstation. It allows multiple tests to be performed on a single platform, including both genomic and protein assays, unlike most existing systems, which provide a diagnostic result for one test or specific niche. The Verigene System is comprised of a microfluidics processor, a reader, and disposable test cartridges. With a prepared sample, the Verigene System completes tests in 45 to 90 minutes and requires less than 20 minutes of technician time. The system incorporates several key features which we believe will make it attractive to a wide range of laboratories, including:
| | |
| | • | Low Cost and Complexity. The Verigene System is a low cost platform without the need for sophisticated instrumentation or complex reagent kits. |
| |
| | • | On Demand Testing. The Verigene System allows laboratories to economically run tests at the time they are ordered, unlike other systems where laboratories must wait to process patient samples in batches to control reagent and labor costs. |
| |
| | • | Multiplexing. The Verigene System enables high count multiplexing, or the ability to identify a large number of target molecules on the same sample in a single assay. |
| |
| | • | Direct Genomic Detection. The Verigene System utilizes a proprietary method to detect nucleic acids with greater specificity and without the complexity and risk of contamination inherent in the use of amplification techniques such as PCR, thereby increasing the reliability of test results. |
| |
| | • | Ultra-Sensitive Protein Detection. The Verigene System allows ultra-sensitive detection of proteins with at least 100 times greater sensitivity than current technologies such as ELISA. This may enable earlier detection of disease and the development of completely novel tests. |
We believe that the Verigene System’s ease of use, rapid turnaround times, relatively low cost and ability to support a broad test menu will simplify work flow and reduce costs for laboratories already performing molecular diagnostic testing and allow a broader range of laboratories, including those operated by local hospitals, to perform molecular diagnostic testing.
Our Initial Test Menu
We have received 510(k) clearance from the FDA for two initial assays: the warfarin metabolism test for which clearance was received in September 2007, and the hyper-coagulation test for which clearance was received in October 2007.
| | |
| | • | Hyper-coagulation. This assay is for the detection of genetic mutations associated with an increased risk for the development of blood clots. Hyper-coagulation tests for mutations associated with a pre-disposition to blood clots are currently among the most frequently conducted human genetic tests. Our hyper-coagulation panel consists of a multiplex of three genetic markers associated with a pre-disposition to blood clots. We believe that our assay enables the detection of these markers on a much simpler platform than current alternatives. |
| |
| | • | Warfarin Metabolism. This assay is a pharmaco-genomic test for the detection of genetic mutations that determine an individual’s ability to metabolize the oral anticoagulant warfarin, including Coumadin. Warfarin decreases blood clotting and is the most widely prescribed oral anticoagulant in North America and Europe. Because individuals metabolize warfarin differently, if administration of the drug is not managed carefully, it can lead to serious bleeding complications. Our assay is the first, and |
2
| | |
| | | currently the only, FDA cleared genetic diagnostic test to assess warfarin metabolism. Our test panel detects three genetic markers that play a critical role in metabolizing warfarin. Through detection of these genetic markers, doctors are able to determine the appropriate dosage level in a safer and more efficient manner than current methods. |
We are developing additional genomic assays, including tests for cystic fibrosis and for a range of infectious diseases including herpes simplex virus, human papillomavirus, and respiratory viruses, which we anticipate we will submit to the FDA for 510(k) clearance during the next 36 months.
In addition, we have an active program to develop tests based on established protein biomarkers and to validate new biomarkers where our ultra-sensitive technology may enable earlier detection of a broad range of diseases. We are conducting clinical studies to demonstrate the diagnostic value of our first two ultra-sensitive protein assays, which we anticipate we will submit to the FDA for 510(k) clearance during the next 36 months:
| | |
| | • | Prostate Specific Antigen — Recurrent Prostate Cancer. This assay is being developed to enable the early detection of recurrent prostate cancer in men following prostate removal. Prostate specific antigen, or PSA, is a protein produced by the cells of the prostate gland and may be found in an increased amount in those with prostate cancer. However, current technologies have limited detection capabilities and on average can only diagnose recurrence three and a half years later. We expect that our ultra-sensitive PSA detection assay will detect recurrence within a few months after surgery enabling earlier intervention and treatment. |
| |
| | • | Cardiac Troponin I — Cardiac Risk Stratification. This assay is being developed for the detection of cardiac troponin I in patients suspected of having cardiovascular disease. Troponin I is a protein that is found in cardiac muscle and is released when the heart is injured, for instance during a myocardial infarction. Cardiac troponin tests are used to diagnose a heart attack and evaluate mild to severe heart injury in patients experiencing heart/chest discomfort. However, limitations of current detection levels of cardiac troponin I often result in the failure to accurately diagnose all cases of cardiovascular disease. Our initial clinical tests have demonstrated the ability to reliably identify a rise in cardiac troponin I well below the current limits of detection. |
Through our biomarker validation program, we are also working to validate novel protein targets for Alzheimer’s disease, stroke, sepsis, and kidney disease, which we believe will create new protein-based diagnostic tests.
Other Applications of Our Technology
Our technology is broadly applicable beyond the clinical diagnostics market in both research and industrial applications and we expect to continue to seek opportunities, either directly or through outlicensing arrangements, to commercialize our technology in these markets. For example, for over two years the Verigene System has been in use in research laboratories supporting collaborations and independent research in areas including ovarian cancer, mad cow disease, and HIV. In addition, we are currently working with the FDA on a joint research program to develop an H5N1 avian flu assay. In the industrial market, we have developed and delivered a biosecurity platform for the detection of various bioterrorism agents to the Technical Support Working Group, an agency affiliated with the U.S. Department of Defense.
Our Intellectual Property Portfolio
Our patent portfolio is comprised, on a worldwide basis, of 80 issued patents and 150 pending patent applications which, in either case, we own directly or for which we are the exclusive licensee. We licensed our initial core technology from the International Institute for Nanotechnology at Northwestern University in May 2000. This formed the basis for a sustained relationship with Northwestern whereby we have rights to future developments in the field of biodiagnostics. This relationship provides us with access to ongoing research and innovation which we utilize in our research and development of new applications and products.
3
Our Strategy
Our goal is to establish a new standard in molecular diagnostics characterized by our low cost, easy to use platform for genomic and protein testing and to develop new diagnostic tests where none exist today. To achieve this objective, we intend to:
| | |
| | • | Target Key Customer Segments. We will focus our sales efforts on hospital laboratories, where there is significant demand for molecular diagnostic testing, but where cost, complexity and resource needs of existing technologies have limited their ability to process tests in-house. |
| |
| | • | Employ a Direct Sales Force Model. We are currently marketing and selling the Verigene System through our own sales and marketing organization, which is currently comprised of 19 people, including sales representatives, clinical support staff and product managers. |
| |
| | • | Market FDA Cleared Products. We will seek FDA clearance for all our products. We believe that there is strong market demand for FDA cleared tests versus tests developed in-house by individual laboratories known as “home-brew” tests. FDA cleared tests require less skilled laboratory technician time and do not subject the laboratory to the additional regulatory requirements imposed on laboratories using “home-brew” tests. |
| |
| | • | Establish a Broad Test Menu. We are developing a broad test menu to maximize the value of the Verigene System, generate cartridge sales and support placements of systems in those laboratories that demand a broad testing menu before implementing a new testing platform. |
| |
| | • | Validate New Biomarkers and Commercialize New Tests Using Our Ultra-Sensitive Protein Detection Methods. We are applying our ultra-sensitive protein detection methods to the development of established protein biomarkers and the validation of novel protein targets that may lead to earlier detection of medical conditions in the area of cancer, neurodegenerative disorders including Alzheimer’s disease, sepsis and mad cow disease. |
| |
| | • | Capitalize on Strong Intellectual Property and Development Capabilities. We will continue to develop assays based on both our in-house and in-licensed intellectual property through our ongoing relationship with the International Institute for Nanotechnology at Northwestern University and other third parties to expand the utility of our Verigene System. |
Risk Factors
Our ability to execute our strategy and capitalize on our advantages is subject to a number of risks discussed more fully in the “Risk Factors” section and elsewhere in this prospectus. The principal risks facing our business include, among others:
History of Losses. We have a history of losses, our losses are likely to increase significantly and we may never achieve or maintain profitability.
Acceptance of Verigene System. Our financial results depend on commercial acceptance of the Verigene System, its array of tests, and the development and regulatory clearance of additional tests. If we do not achieve significant product revenue, we may not be able to meet our cash requirements without obtaining additional capital from external sources, and if we are unable to do so, we may have to curtail or cease operations.
Intellectual Property. Third parties may claim we are infringing their intellectual property rights, which could prevent us from selling or commercializing our products unless we obtain a license from such third party. Our products could infringe patent rights of others, which may require costly litigation and, if we are not successful, could cause us to pay substantial damages or limit our ability to commercialize our products.
Competition. We face increasing competition from current and potential competitors, some of which have greater name recognition, more substantial intellectual property portfolios and longer operating histories.
4
Government Regulation. Our products are subject to regulation by the FDA and numerous other federal and state governmental authorities. We may incur significant expenses to comply with, and experience delays in our product commercialization as a result of, these regulations.
Corporate Information
We were founded in September 1998 as Nanosphere LLC, an Illinois limited liability company, by Dr. Robert L. Letsinger and Dr. Chad A. Mirkin, two Professors of Chemistry at Northwestern University. We established Nanosphere, Inc. in December 1999 as a Delaware corporation, into which we merged Nanosphere LLC in January 2000. Our executive offices are located at 4088 Commercial Avenue, Northbrook, IL 60062. Our telephone number at that address is(847) 400-9000 and our website is www.nanosphere.us. The information contained on, or that can be accessed through, our website is not incorporated by reference into this prospectus and should not be considered to be part of this prospectus.
| | | | | |
| Clearread, Verigene, and the | |  | | logo are our registered trademarks. |
Ruggid, Valid and Biobarcode are the subject of pending trademark applications owned by us.
We also have registrations or pending applications for registration of some of our trademarks in other jurisdictions, including Europe, People’s Republic of China, Hong Kong, Japan, Republic of Korea, Taiwan, Canada, Malaysia, and Singapore. All other trademarks, trade names, and service marks appearing in this prospectus are the trademarks of their respective owners.
5
The Offering
| | |
| Issuer | | Nanosphere, Inc. |
| |
| Common stock offered | | 7,000,000 shares |
| | |
| Underwriters’ option to purchase additional shares | | 1,050,000 shares |
| | |
| Common stock to be outstanding after this offering | |
21,064,198 shares, or 22,114,198 shares if the underwriters exercise their over-allotment option in full. |
| | |
| Use of Proceeds | | We estimate that the net proceeds to us in the offering will be approximately $95 million, assuming an initial public offering price of $15.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. We expect to use approximately $50 million of the net proceeds of this offering to finance ongoing research and development and the continued investments in and development of our product manufacturing infrastructure and approximately $40 million to fund additional sales, marketing and service personnel and initiatives. We expect to use the remainder of the net proceeds for additional working capital and general corporate purposes. |
| |
| Dividend Policy | | We have never declared or paid any cash dividends on our capital stock and do not expect to pay any dividends for the foreseeable future. |
| |
| NASDAQ Global Market symbol | | NSPH |
The number of shares of common stock to be outstanding upon completion of this offering is based on the following (all as of October 29, 2007): 932,646 shares of common stock, 17,007 shares of Series B Convertible Preferred Stock, 10,050,007 shares of and 16,666 warrants to purchase Series C Convertible Preferred Stock, 128,825,044 shares of and 3,438,690 warrants to purchaseSeries C-2 Convertible Preferred Stock, and 168,392,966 shares of Series D Convertible Preferred Stock, and excludes as of that date:
| | |
| | • | 3,251,548 shares of common stock issuable upon exercise of options outstanding at a weighted average exercise price of $4.98 per share; |
| |
| | • | 1,625,321 shares of common stock reserved for future issuance under our long-term incentive plan in effect immediately prior to the closing of this offering; and |
| |
| | • | 1,307,773 shares of common stock issuable upon exercise of warrants to purchase Series D Convertible Preferred Stock, which will become exercisable for common stock immediately prior to the closing of this offering. |
Except as otherwise indicated, all of the information in this prospectus assumes:
| | |
| | • | no exercise of the underwriters’ option to purchase additional shares; |
| |
| | • | adoption of our amended and restated certificate of incorporation and amended and restated by-laws to be effective upon the consummation of this offering; |
| |
| | • | the conversion on a 10.2-for-one basis of our Series B Convertible Preferred Stock into common stock to be effected immediately prior to the closing of this offering; |
| | |
| | • | the conversion on a 25-for-one basis of our Series C, Series C-2 and Series D Convertible Preferred Stock into common stock including common stock issued in connection with accrued and unpaid dividends which were an aggregate of 700,986 shares as of October 29, 2007 (at an assumed initial public offering price of $15.00 per share which is the midpoint of the range listed on the cover page of this prospectus) to be effected immediately prior to the closing of this offering; |
| | |
| | • | the exercise of all outstanding Series C andSeries C-2 Convertible Preferred Stock warrants, which expire immediately prior to the closing of this offering unless exercised, for an aggregate of 138,205 shares of common stock for an aggregate purchase price of $1,213,542; |
| |
| | • | the conversion of the outstanding warrants to purchase 32,694,562 shares of Series D Convertible Preferred Stock into warrants to purchase 1,307,773 shares of common stock to be effected immediately prior to the closing of this offering; and |
| |
| | • | a 25-for-one reverse stock split of our common stock effected on October 16, 2007. |
6
Summary Financial Data
The following statements of operations data for each of the years ended December 31, 2004, 2005 and 2006 and the six month period ended June 30, 2007 and the balance sheet data at December 31, 2005, December 31, 2006 and June 30, 2007 have been derived from our audited financial statements and related notes which are included elsewhere in this prospectus. The statements of operations data for the six month period ended June 30, 2006 have been derived from our unaudited financial statements and related notes which are included elsewhere in this prospectus. In the opinion of management, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments necessary for the fair presentation of our financial position and results of operations for these periods. The summary financial data set forth below should be read in conjunction with our financial statements, the related notes, “Risk Factors,” “Use of Proceeds,” “Capitalization,” “Selected Financial Data,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in the document. The historical results are not necessarily indicative of the results to be expected for any future period. The accompanying financial statements for the years ended December 31, 2004, 2005 and 2006 have been restated. See Note 12 to the financial statements.
| | | | | | | | | | | | | | | | | | | | | |
| | | | | | Six Month Period
| |
| | | Years Ended December 31, | | | Ended June 30, | |
Statements of Operations Data: | | 2004 | | | 2005 | | | 2006 | | | 2006 | | | 2007 | |
| | | (As restated) | | | (As restated) | | | (As restated) | | | | | | | |
| |
| Revenue: | | | | | | | | | | | | | | | | | | | | |
| Grant and contract revenue | | $ | 2,768,125 | | | $ | 1,777,667 | | | $ | 1,006,351 | | | $ | 438,512 | | | $ | 726,503 | |
| Product sales | | | — | | | | 136,850 | | | | 131,660 | | | | 27,630 | | | | 53,670 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | | 2,768,125 | | | | 1,914,517 | | | | 1,138,011 | | | | 466,142 | | | | 780,173 | |
| | | | | | | | | | | | | | | | | | | | | |
| Costs and expenses: | | | | | | | | | | | | | | | | | | | | |
| Cost of product sales | | | — | | | | 125,118 | | | | 31,049 | | | | — | | | | 18,367 | |
| Research and development | | | 10,366,473 | | | | 13,244,872 | | | | 17,447,227 | | | | 7,874,596 | | | | 10,219,047 | |
| Sales, general and administrative | | | 3,131,390 | | | | 4,502,970 | | | | 5,415,525 | | | | 2,663,931 | | | | 5,256,583 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total costs and expenses | | | 13,497,863 | | | | 17,872,960 | | | | 22,893,801 | | | | 10,538,527 | | | | 15,493,997 | |
| | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (10,729,738 | ) | | | (15,958,443 | ) | | | (21,755,790 | ) | | | (10,072,385 | ) | | | (14,713,824 | ) |
| Change in fair value of convertible derivative liability | | | — | | | | — | | | | (2,916,822 | ) | | | (2,916,822 | ) | | | — | |
| Change in fair value of preferred stock warrants | | | (8,801 | ) | | | (8,314 | ) | | | (119,914 | ) | | | (244,104 | ) | | | (276,612 | ) |
| Foreign exchange loss | | | — | | | | — | | | | — | | | | — | | | | (13,770 | ) |
| Interest expense - related party | | | (204,335 | ) | | | (37,919 | ) | | | (146,550 | ) | | | (146,550 | ) | | | — | |
| Interest expense | | | — | | | | — | | | | (7,506 | ) | | | (1,585 | ) | | | (823,748 | ) |
| Interest income | | | 40,963 | | | | 69,376 | | | | 1,415,001 | | | | 483,755 | | | | 758,433 | |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | (10,901,911 | ) | | | (15,935,300 | ) | | | (23,531,581 | ) | | | (12,897,691 | ) | | | (15,069,521 | ) |
| Accumulated convertible preferred stock dividends | | | — | | | | — | | | | (4,413,591 | ) | | | (1,350,933 | ) | | | (3,180,329 | ) |
| Convertible preferred stock redemption value adjustment | | | (10,156,393 | ) | | | (2,898,787 | ) | | | (17,737,544 | ) | | | (17,737,544 | ) | | | (608,940 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss attributable to common stock | | $ | (21,058,304 | ) | | $ | (18,834,087 | ) | | $ | (45,682,716 | ) | | $ | (31,986,168 | ) | | $ | (18,858,790 | ) |
| | | | | | | | | | | | | | | | | | | | | |
| Net loss per common share: | | | | | | | | | | | | | | | | | | | | |
| basic and diluted | | $ | (34.44 | ) | | $ | (30.80 | ) | | $ | (52.78 | ) | | $ | (40.15 | ) | | $ | (20.22 | ) |
| Weighted average number of common shares: | | | | | | | | | | | | | | | | | | | | |
| basic and diluted | | | 611,466 | | | | 611,496 | | | | 865,559 | | | | 796,729 | | | | 932,646 | |
7
| | | | | | | | | | | | | | | | | |
| | | As of December 31, | | | As of June 30, 2007 | |
Balance Sheet Data: | | 2005 | | | 2006 | | | Actual | | | As Adjusted(1) | |
| | | (As restated) | | | (As restated) | | | | | | | |
| |
| Cash and cash equivalents | | $ | 3,641,338 | | | $ | 29,112,429 | | | $ | 26,970,383 | | | $ | 122,833,925 | |
| Working capital | | $ | (2,642,582 | ) | | $ | 27,332,463 | | | $ | 23,894,749 | | | $ | 119,758,291 | |
| Total assets | | $ | 11,346,514 | | | $ | 41,037,834 | | | $ | 40,435,013 | | | $ | 136,298,555 | |
| Long-term debt (net of discount of $0, $0, $1,670,405) | | | — | | | $ | 58,802 | | | $ | 9,302,976 | | | $ | 9,302,976 | |
| Convertible preferred stock | | $ | 51,143,984 | | | $ | 108,868,040 | | | $ | 113,985,907 | | | | — | |
| Stockholders’ equity (deficit)(2) | | $ | (59,961,290 | ) | | $ | (105,238,071 | ) | | $ | (123,241,672 | ) | | $ | 121,339,904 | |
| |
| (1) | On an as adjusted basis giving effect to: (1) the conversion of all outstanding shares of our Series B, Series C, Series C-2 and Series D Convertible Preferred Stock into common stock; (2) the exercise of all outstanding warrants to purchase Series C and Series C-2 Convertible Preferred Stock which expire immediately prior to the closing of this offering unless exercised for an aggregate of 138,205 shares of common stock for an aggregate purchase price of $1,213,542; and (3) to reflect the sale of 7,000,000 shares of our common stock in this offering at an assumed initial public offering price of $15.00 per share, the midpoint of the range on the cover page of this prospectus. Each $1.00 increase or decrease in the assumed initial public offering price of $15.00 per share would increase or decrease cash and cash equivalents, total stockholders’ equity (deficit) by approximately $6.5 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. |
| |
| (2) | Since our incorporation, we have been principally financed through convertible preferred stock equity investments. In accordance with Securities and Exchange Commission, or SEC, rules and regulations, our convertible preferred stock is recorded outside of stockholders’ equity (deficit), while our accumulated deficit, representing our accumulated losses to-date, allocation of convertible preferred stock dividends and convertible preferred stock redemption value adjustments are recorded as reductions to stockholders’ deficit. See Note 8 to the financial statements for more information on our convertible preferred stock and related accounting. |
8
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below together with all of the other information contained in this prospectus, including the financial statements and the related notes appearing at the end of this prospectus, before making an investment decision. If any of the following risks or uncertainties actually occurs, our business, financial condition or operating results could materially suffer. In that event, the trading price of our common stock could decline and you may lose all or part of your investment.
Risks Related to Our Business
We have a history of losses, our losses are likely to increase significantly, and we may never achieve or maintain profitability.
We are a development-stage company with limited operating history. We have incurred significant losses in each fiscal year since our inception, including net losses attributable to common stock of $18.8 million, $45.7 million, and $18.9 million in the years ended December 31, 2005 and 2006, and the six month period ended June 30, 2007, respectively. As of June 30, 2007, we had an accumulated deficit during the development stage of $124.8 million. These losses resulted principally from costs incurred in our research and development programs and from our general and administrative expenses. In recent years, we have incurred significant costs in connection with the development of the Verigene System and its range of tests. We expect our research and development expense levels to remain high for the foreseeable future as we seek to enhance our existing product and develop new products. After we begin selling our products, we expect our losses to continue to increase as a result of ongoing research and development expenses, as well as increased manufacturing, sales and marketing expenses. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. If we fail to achieve profitability in the future, the market price of our common stock could decline.
Our financial results depend on commercial acceptance of the Verigene System, its array of tests, and the development of additional tests.
Our future depends on the success of the Verigene System, which depends primarily on its acceptance by hospitals, research institutions, and independent diagnostic laboratories as a reliable, accurate and cost-effective replacement for traditional molecular diagnostic measurement methods. Many hospitals and laboratories already use expensive molecular diagnostic testing instruments in their laboratories and may be reluctant to change their current procedures for performing such analyses.
The Verigene System currently does not process a sufficiently broad menu of tests for some hospitals and laboratories to consider adopting it. Although we continue to develop additional tests to respond to hospitals’ and laboratories’ needs, we cannot guarantee that we will be able to develop enough additional tests quickly enough or in a manner that is cost-effective or at all. The development of new or enhanced products is a complex and uncertain process requiring the accurate anticipation of technological and market trends, as well as precise technological execution. We are currently not able to estimate when or if we will be able to develop, commercialize or sell additional tests or enhance existing products. If we are unable to increase sales of the Verigene System and its tests or to successfully develop and commercialize other products or tests, our revenues and our ability to achieve profitability would be impaired.
The regulatory approval process is expensive, time consuming and uncertain and the failure to obtain such approvals will prevent us from commercializing our future products.
Our products will be subject to approval or clearance by the FDA or foreign governmental entities prior to their marketing for commercial use. The 510(k) clearance and pre-market approval processes as well as the foreign approvals required to initiate sales outside the United States can be expensive, time consuming and uncertain. It generally takes from four to twelve months from submission to obtain 510(k) clearance, and from
9
one to three years from submission to obtain pre-market approval; however, it may take longer, and 510(k) clearance or pre-market approval may never be obtained. Delays in receipt of, or failure to obtain, clearances or approvals for future products, including tests that are currently in development, would result in delayed, or no, realization of revenues from such products and in substantial additional costs which could decrease our profitability. We have limited experience in filing FDA applications for 510(k) clearance and pre-market approval. There are no assurances that we will obtain any required clearance or approval. Any such failure, or any material delay in obtaining the clearance or approval, could harm our business, financial condition and results of operations.
We and our customers are subject to various governmental regulations, and we may incur significant expenses to comply with, and experience delays in our product commercialization as a result of, these regulations.
The products we develop, manufacture and market are subject to regulation by the FDA and numerous other federal, state and foreign governmental authorities. We generally are prohibited from marketing our products in the United States unless we obtain either 510(k) clearance or pre-market approval from the FDA.
In addition, we are required to continue to comply with applicable FDA and other regulatory requirements once we have obtained clearance or approval for a product. These requirements include the Quality System Regulation, labeling requirements, the FDA’s general prohibition against promoting products for unapproved or “off-label” uses and adverse event reporting regulations. Failure to comply with applicable FDA product regulatory requirements could result in warning letters, fines, injunctions, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspension of production, the FDA’s refusal to grant future pre-market clearances or approvals, withdrawals or suspensions of current product applications and criminal prosecution. Any of these actions, in combination or alone, could prevent us from selling our products and would likely harm our business.
Our manufacturing facilities are subject to periodic regulatory inspections by the FDA and other federal and state regulatory agencies. The use of our diagnostic products by our customers is also affected by the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and related federal and state regulations that provide for regulation of laboratory testing. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, participation in proficiency testing, patient test management, quality and inspections. Current or future CLIA requirements or the promulgation of additional regulations affecting laboratory testing may prevent some laboratories from using some or all of our diagnostic products.
The FDA and foreign governmental regulators have made, and may continue to make, changes in approval requirements and processes. We cannot predict what these changes will be, how or when they will occur or what effect they will have on the regulation of our products. Any new regulations, including regulations specifically related to nanotechnology, may impose additional costs or lengthen review times of our products. Delays in receipt of or failure to receive regulatory approvals or clearances for our new products would have a material adverse effect on our business, financial condition and results of operations.
If third-party payors do not reimburse our customers for the use of our clinical diagnostic products or if they reduce reimbursement levels, our ability to sell our products will be harmed.
We intend to sell our products primarily to hospital-based laboratories and academic research institutions, substantially all of which receive reimbursement for the health care services they provide to their patients from third-party payors, such as Medicare, Medicaid and other domestic and international government programs, private insurance plans and managed care programs. Most of these third-party payors may deny reimbursement if they determine that a medical product was not used in accordance with cost-effective treatment methods, as determined by the third-party payor, or was used for an unapproved indication. Third-party payors also may refuse to reimburse for procedures and devices deemed to be experimental.
In the United States, the American Medical Association assigns specific Current Procedural Terminology, or CPT, codes, which are necessary for reimbursement of diagnostic tests. Once the CPT code is established,
10
the Centers for Medicare and Medicaid Services establish reimbursement payment levels and coverage rules under Medicaid and Medicare, and private payors establish rates and coverage rules independently. Although the tests performed by our assays in development have previously assigned CPT Codes, we cannot guarantee that our assays are covered by such CPT codes and are therefore approved for reimbursement by Medicare and Medicaid as well as most third-party payors. Additionally, certain of our future products may not be approved for reimbursement. Third-party payors may choose to reimburse our customers on a per test basis, rather than on the basis of the number of results given by the test. This may result in reference laboratories, public health institutions and hospitals electing to use separate tests to screen for each disease so that they can receive reimbursement for each test they conduct. In that event, these entities likely would purchase separate tests for each disease, rather than products that multiplex.
Third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement for medical products and services. Increasingly, Medicare, Medicaid and other third-party payors are challenging the prices charged for medical services, including clinical diagnostic tests. Levels of reimbursement may decrease in the future, and future legislation, regulation or reimbursement policies of third-party payors may adversely affect the demand for and price levels of our products. If our customers are not reimbursed for our products, they may reduce or discontinue purchases of our products, which would cause our revenues to decline.
We may fail to receive positive clinical results from the diagnostic tests currently in development that require clinical trials, and even if we receive positive clinical results, we may still fail to receive the necessary clearances or approvals to market our products.
We are investing in the research and development of new products to expand the menu of testing options for the Verigene System. In order to commercialize our products, we are required to undertake time consuming and costly development activities, sometimes including clinical trials for which the outcome is uncertain. Products that appear promising during early development and preclinical studies may, nonetheless, fail to demonstrate the results needed to support regulatory approval. Even if we receive positive clinical results, we may still fail to obtain the necessary FDA clearance and approvals.
Our operating results may be variable and unpredictable.
The sales cycles for our products may be lengthy, which will make it difficult for us to accurately forecast revenues in a given period, and may cause revenues and operating results to vary significantly from period to period. In addition to its length, the sales cycle associated with our products is subject to a number of significant risks, including the budgetary constraints of our customers, their inventory management practices and possibly internal acceptance reviews, all of which are beyond our control. Sales of our products will also involve the purchasing decisions of large, medium and small hospitals and laboratories which can require many levels of pre-approvals, further lengthening sales time. As a result, we may expend considerable resources on unsuccessful sales efforts or we may not be able to complete transactions on the scheduled anticipated.
If we do not achieve significant product revenue, we may not be able to meet our cash requirements without obtaining additional capital from external sources, and if we are unable to do so, we may have to curtail or cease operations.
We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure, commercialization, manufacturing, and research and development activities. We anticipate that our current cash and cash equivalents, together with the net proceeds of this offering, will be sufficient to meet our currently estimated needs for at least the next three years. However, we operate in a market that makes our prospects difficult to evaluate, and we may need additional financing to execute on our current or future business strategies. The amount of additional capital we may need to raise depends on many factors, including:
| | |
| | • | the level of research and development investment required to maintain and improve our technology; |
| |
| | • | the amount and growth rate, if any, of our revenues; |
11
| | |
| | • | changes in product development plans needed to address any difficulties in manufacturing or commercializing the Verigene System and enhancements to our system; |
| |
| | • | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| |
| | • | competing technological and market developments; |
| |
| | • | our need or decision to acquire or license complementary technologies or acquire complementary businesses; |
| |
| | • | the expansion of our sales force; and |
| |
| | • | changes in regulatory policies or laws that affect our operations. |
We cannot be certain that additional capital will be available when and as needed or that our actual cash requirements will not be greater than anticipated. If we require additional capital at a time when investment in diagnostics companies or in the marketplace in general is limited due to the then prevailing market or other conditions, we may not be able to raise such funds at the time that we desire or any time thereafter. In addition, if we raise additional funds through the issuance of common stock or convertible securities, the percentage ownership of our stockholders could be significantly diluted, and these newly issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain additional debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us.
If our products do not perform as expected or the reliability of the technology on which our products are based is questioned, we could experience lost revenue, delayed or reduced market acceptance of our products, increased costs and damage to our reputation.
Our success depends on the market’s confidence that we can provide reliable, high-quality diagnostics systems. We believe that customers in our target markets are likely to be particularly sensitive to product defects and errors.
Our reputation and the public image of our products or technologies may be impaired if our products fail to perform as expected or our products are perceived as difficult to use. Our products are complex and may develop or contain undetected defects or errors. Any defects or errors could lead to the filing of product liability claims, which could be costly and time-consuming to defend and result in substantial damages. If we experience a sustained material defect or error, this could result in loss or delay of revenues, delayed market acceptance, damaged reputation, diversion of development resources, legal claims, increased insurance costs or increased service and warranty costs, any of which could materially harm our business. We cannot assure you that our product liability insurance would protect our assets from the financial impact of defending a product liability claim. A product liability claim could have a serious adverse effect on our business, financial condition and results of operations.
We rely on third-party license agreements for patents and other technology related to our products, and the termination of these agreements could delay or prevent us from being able to commercialize our products.
We depend on an exclusive license to certain patents and patent applications owned by Northwestern University that are related to nanotechnology and biobarcode technology in the biodiagnostics field. Although this license is irrevocable, we have an obligation to use commercially reasonable efforts to commercialize the subject inventions of the licensed patents, and if we fail to meet this obligation, we could potentially lose exclusivity in the licensed patents. If, in such an event, Northwestern were to provide a license to these patents to one or more of our competitors thereafter, our ability to compete in the market may be diminished.
12
We also depend on non-exclusive patent license agreements. If we fail to comply with our material obligations under these non-exclusive patent license agreements, such licenses may be terminated.
The exclusive and non-exclusive licenses expire at various times, corresponding to the subject patents’ expirations, which currently range from 2009 to 2023. We may also need to license other technology or patents to commercialize future products, but such licenses may not be available to us on commercially reasonable terms or at all.
If we are unable to obtain, maintain and enforce intellectual property protection covering our products, others may be able to make, use, or sell our products, which could adversely affect our ability to compete in the market.
Our success is dependent in part on obtaining, maintaining and enforcing intellectual property rights, including patents. If we are unable to obtain, maintain and enforce intellectual property legal protection covering our products, others may be able to make, use or sell products that are substantially identical to ours without incurring the sizeable discovery, development and licensing costs that we have incurred, which would adversely affect our ability to compete in the market.
We seek to obtain and maintain patents and other intellectual property rights to restrict the ability of others to market products that compete with our products. Currently, our patent portfolio is comprised, on a worldwide basis, of 80 issued patents and 150 pending patent applications which, in either case, we own directly or for which we are the exclusive licensee. However, patents may not issue from any pending or future patent applications owned by or licensed to us, and moreover, issued patents owned or licensed to us now or in the future may be found by a court to be invalid or otherwise unenforceable. Also, even if our patents are determined by a court to be valid and enforceable, they may not be sufficiently broad to prevent others from marketing products similar to ours or designing around our patents, despite our patent rights, nor provide us with freedom to operate unimpeded by the patent rights of others.
Furthermore, we cannot be certain that we were the first to make the invention claimed in our United States issued patents or pending patent applications, or that we were the first to file for protection of the inventions claimed in our foreign issued patents or pending patent applications. We may become subject to interference proceedings conducted in the patent and trademark offices of various countries to determine our entitlement to patents, and these proceedings may conclude that other patents or patent applications have priority over our patents or patent applications. It is also possible that a competitor may successfully challenge our patents through various proceedings and those challenges may result in the elimination or narrowing of our patents, and therefore reduce our patent protection. Accordingly, rights under any of our issued patents, patent applications or future patents may not provide us with commercially meaningful protection for our products or afford us a commercial advantage against our competitors or their competitive products or processes.
We have a number of foreign patents and applications. However, the laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as laws in the United States, and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties or we are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
We may initiate litigation to enforce our patent rights, which may prompt our adversaries in such litigation to challenge the validity, scope or enforceability of our patents. Patent litigation is complex and often difficult and expensive, and would consume the time of our management and other significant resources. In addition, the outcome of patent litigation is uncertain. If a court decides that our patents are not valid, not enforceable or of a limited scope, we may not have the right to stop others from using the subject matter covered by those patents.
We also rely on trade secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult to obtain or enforce. We may not be able to protect our trade secrets adequately. In addition, we rely on non-disclosure and confidentiality agreements with our employees, consultants and
13
other parties to protect, in part, our trade secrets and other proprietary technology. These agreements may be breached and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential data into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us.
Our products could infringe patent rights of others, which may require costly litigation and, if we are not successful, could cause us to pay substantial damages or limit our ability to commercialize our products.
Our commercial success depends on our ability to operate without infringing the patents and other proprietary rights of third parties. We are aware of third party patents that may relate to our products and technology. There may also be other patents that relate to our products and technology of which we are not aware. We may unintentionally infringe upon valid patent rights of third parties. Although we are currently not involved in any litigation involving patents, a third party patent holder could assert a claim of patent infringement against us in the future. Alternatively, we may initiate litigation against the third party patent holder to request that a court declare that we are not infringing the third party’s patentand/or that the third party’s patent is invalid or unenforceable. If a claim of infringement is asserted against us and is successful, and therefore we are found to infringe, we could be required to pay damages for infringement, including treble damages if it is determined that we knew or became aware of such a patent and we failed to exercise due care in determining whether or not we infringed the patent. If we have supplied infringing products to third parties or have licensed third parties to manufacture, use or market infringing products, we may be obligated to indemnify these third parties for damages they may be required to pay to the patent holder and for any losses they may sustain. We can also be prevented from selling or commercializing any of our products that use the infringing technology in the future, unless we obtain a license from such third party. A license may not be available from such third party on commercially reasonable terms, or may not be available at all. Any modification to include a non-infringing technology may not be possible or if possible may be difficult or time-consuming to develop, and require revalidation, which could delay our ability to commercialize our products.
Any infringement action asserted against us, even if we are ultimately successful in defending against such action, would likely delay the regulatory approval process of our products, harm our competitive position, be expensive and require the time and attention of our key management and technical personnel.
We have limited experience in sales and marketing and may be unable to successfully commercialize our Verigene System, or it may be difficult to build brand loyalty.
We have limited marketing, sales and distribution experience and capabilities. We have only recently established a sales force. Our ability to achieve profitability depends on attracting customers for the Verigene System and building brand loyalty. To successfully perform sales, marketing, distribution and customer support functions ourselves, we will face a number of risks, including:
| | |
| | • | our ability to attract and retain the skilled support team, marketing staff and sales force necessary to commercialize and gain market acceptance for our technology and our products; |
| |
| | • | the ability of our sales and marketing team to identify and penetrate the potential customer base including hospitals, research institutions, and independent diagnostic laboratories; |
| |
| | • | the time and cost of establishing a support team, marketing staff and sales force; and |
| |
| | • | the difficulty of establishing brand recognition and loyalty for our products. |
In addition, we may seek to enlist one or more third parties to assist with sales, distribution and customer support globally or in certain regions of the world. If we do seek to enter into such arrangements, we may not be successful in attracting desirable sales and distribution partners, or we may not be able to enter into such arrangements on favorable terms. If our sales and marketing efforts, or those of any third-party sales and distribution partners, are not successful, our technologies and products may not gain market acceptance, which would materially impact our business operations.
14
We may be unsuccessful in our long-term goal of expanding our product offerings outside the United States.
To the extent we begin to offer our products broadly outside the United States, we expect that we will be dependent on third-party distribution relationships. Distributors may not commit the necessary resources to market and sell our products to the level of our expectations. If distributors do not perform adequately, or we are unable to locate distributors in particular geographic areas, our ability to realize long-term international revenue growth would be materially adversely affected.
Additionally, our products may require regulatory clearances and approvals from jurisdictions outside the United States. These products may not be sold in these jurisdictions until the required clearances and approvals are obtained. We cannot assure you that we will be able to obtain these clearances or approvals on a timely basis, or at all.
Manufacturing risks and inefficiencies may adversely affect our ability to produce products.
We must manufacture or engage third parties to manufacture components of our products in sufficient quantities and on a timely basis, while maintaining product quality and acceptable manufacturing costs and complying with regulatory requirements. In determining the required quantities of our products and the manufacturing schedule, we must make significant judgments and estimates based on historical experience, inventory levels, current market trends and other related factors. Because of the inherent nature of estimates, there could be significant differences between our estimates and the actual amounts of products we require. Additionally, some of the components of the Verigene System are custom-made by only a few outside vendors. We may not be able to meet the demand for our products if one or more of these vendors are not able to supply us with the needed components or components that meet our specifications. We have not arranged for alternate suppliers, and it may be difficult to find alternate suppliers in a timely manner and on terms acceptable to us.
We may experience unforeseen technical complications in the processes we use to develop, manufacture, customize or receive orders for our products. These complications could materially delay or limit the use of products we attempt to commercialize, substantially increase the anticipated cost of our products or prevent us from implementing our processes at appropriate quality and scale levels, thereby causing our business to suffer. In addition, our manufacturing operations use highly technical processes involving unique, proprietary techniques that our manufacturing personnel must continuously monitor and update, especially as we develop more products. In order to be profitable, we must manufacture greater quantities of products than we have to date and we must do this more efficiently than we have in the past. We may not be able to do so.
We will need to develop manufacturing capacity by ourselves or with third parties.
We will need to either continue to build internal manufacturing capacity or contract with one or more manufacturing partners, or both. We currently use a combination of outsourced and internal manufacturing activities. We have not commercially manufactured any instruments, products or supplies. We may encounter difficulties in manufacturing our products and, due to the complexity of our technology and our manufacturing process, we cannot be sure we fully understand all of the factors that affect our manufacturing processes or product performance. We may not be able to build manufacturing capacity internally or find one or more suitable manufacturing partners, or both, to meet the volume and quality requirements necessary to be successful in the market. If our products do not consistently meet our customers’ performance expectations, we may be unable to generate sufficient revenues to become profitable. Significant additional resources, implementation of additional manufacturing equipment and changes in our manufacturing processes and organization may be required for thescale-up of each new product prior to commercialization or to meet increasing customer demand once commercialization begins, and this work may not be successfully or efficiently completed. Any delay in establishing or inability to expand our manufacturing capacity could delay our ability to develop or sell our products, which would result in lost revenue and seriously harm our business, financial condition and results of operations.
15
Our business and future operating results may be adversely affected by events outside of our control.
We develop and manufacture the Verigene System and assays in our facility located in Northbrook, Illinois. This facility and the manufacturing equipment we use would be costly to replace and could require substantial lead time to repair or replace. Our business and operating results may be harmed due to interruption of our manufacturing by events outside of our control, including earthquakes, tornadoes and fires. Other possible disruptions may include power loss and telecommunications failures. In the event of a disruption, we may lose customers and we may be unable to regain those customers thereafter. Our insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, or at all.
We face intense competition from established and new companies in the molecular diagnostics field.
We compete with companies that design, manufacture and market already existing and new molecular diagnostics systems. We anticipate that we will face increased competition in the future as new companies enter the market with new technologies and our competitors improve their current products. One or more of our competitors may offer technology superior to ours and render our technology obsolete or uneconomical. Most of our current competitors, as well as many of our potential competitors, have greater name recognition, more substantial intellectual property portfolios, longer operating histories, significantly greater resources to invest in new technologies and more substantial experience in new product development, regulatory expertise, manufacturing capabilities and the distribution channels to deliver products to customers. If we are not able to compete successfully, we may not generate sufficient revenue to become profitable.
Our success may depend upon how we and our competitors anticipate and adapt to market conditions.
The markets for our products are characterized by rapidly changing technology, evolving industry standards, changes in customer needs, emerging competition and new product introductions. The success of our products will depend on our ability to continue to increase their performance and decrease their price. New technologies, techniques or products could emerge with similar or better price-performance than our system and could exert pricing pressures on our products. It is critical to our success for us to anticipate changes in technology and customer requirements and to successfully introduce enhanced and competitive technology to meet our customers’ and prospective customers’ needs on a timely basis. We may not be able to maintain our technological advantages over emerging technologies in the future and we will need to respond to technological innovation in a rapidly changing industry. If we fail to keep pace with emerging technologies our system will become uncompetitive, our market share will decline and our business, revenue, financial condition and operating results could suffer materially.
We may not be able to manage our anticipated growth, and we may experience constraints or inefficiencies caused by unanticipated acceleration and deceleration of customer demand.
Unanticipated acceleration and deceleration of customer demand for our products may result in constraints or inefficiencies related to our manufacturing, sales force, implementation resources and administrative infrastructure. Such constraints or inefficiencies may adversely affect us as a result of delays, lost potential product sales or loss of current or potential customers due to their dissatisfaction. Similarly, over-expansion or investments in anticipation of growth that does not materialize, or develops more slowly than we expect, we could harm our financial results and result in overcapacity.
To manage our anticipated future growth effectively, we must enhance our manufacturing capabilities and operations, information technology infrastructure, and financial and accounting systems and controls. Organizational growth andscale-up of operations could strain our existing managerial, operational, financial and other resources. Our growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of new products or enhancements of existing products. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our revenue could grow more slowly than expected and we may not be able to achieve our research and
16
development and commercialization goals. Our failure to manage our anticipated growth effectively could have a material adverse effect on our business, operating results or financial condition.
We use hazardous chemicals, biological materials, and infectious diseases in our business. Any claims relating to improper handling, storage or disposal of these materials could be time consuming and costly.
Our research and development and manufacturing processes involve the controlled use of hazardous materials, including chemicals, biological materials and infectious diseases. Our operations produce hazardous waste products. We cannot eliminate the risk of accidental contamination or discharge and any resultant injury from these materials. We may be sued for any injury or contamination that results from our use or the use by third parties of these materials, and our liability may exceed our insurance coverage and our total assets. Federal, state and local laws and regulations govern the use, manufacture, storage, handling and disposal of these hazardous materials and specified waste products, as well as the discharge of pollutants into the environment and human health and safety matters. Compliance with environmental laws and regulations may be expensive, and may impair our research, development and production efforts. If we fail to comply with these requirements, we could incur substantial costs, including civil or criminal fines and penalties,clean-up costs, or capital expenditures for control equipment or operational changes necessary to achieve and maintain compliance. In addition, we cannot predict the impact on our business of new or amended environmental laws or regulations, or any changes in the way existing and future laws and regulations are interpreted and enforced.
If we are unable to recruit and retain key executives and scientists, we may be unable to achieve our goals.
Our performance is substantially dependent on the performance of our senior management and key scientific and technical personnel. The loss of the services of any member of our senior management or our scientific or technical staff could divert management’s attention to transition matters and identification of suitable replacements, if any, and have a material adverse effect on our business, operating results and financial condition. Each of our executive officers and other key employees could terminate his or her relationship with us at any time. We do not maintain key man life insurance on any of our employees.
In addition, our product development and marketing efforts could be delayed or curtailed if we are unable to attract, train and retain highly skilled employees and scientific advisors, particularly our management team, senior scientists and engineers and sales and marketing personnel. To expand our research, product development and sales efforts we need additional people skilled in areas such as protein science, information services, manufacturing, sales, marketing and technical support. Because of the complex and technical nature of our system and the dynamic market in which we compete, any failure to attract and retain a sufficient number of qualified employees could materially harm our ability to develop and commercialize our technology. We may not be successful in hiring or retaining qualified personnel and our failure to do so could have a material adverse effect on our business, financial condition and results of operations.
Healthcare reform and restrictions on reimbursement may adversely affect our profitability.
In the United States, healthcare providers that purchase our products and other diagnostic products generally rely on third-party payors to reimburse all or part of the cost of the procedure. In international markets, reimbursement and healthcare payment systems vary significantly by country, and include both government-sponsored healthcare and private insurance. Third-party payors can affect the pricing or the relative attractiveness of our products by regulating the maximum amount of reimbursement provided by such payors for laboratory testing services. Lower-than-expected or decreases in reimbursement amounts for tests performed using our products may decrease amounts physicians and other practitioners are able to charge patients, which in turn may adversely affect the willingness of physicians and other practitioners to purchase our products at prices we target, or at all. If we were not able to sell our products at target prices, then we will suffer a decrease in expected profitability that would likely adversely affect our business, financial condition and results of operations.
17
Risks Related to This Offering and Ownership of Our Common Stock
There has been no prior market for our common stock, and an active trading market for our common stock may not develop.
Prior to this offering, there has been no public market for our common stock. Although we have applied to have our common stock listed on the NASDAQ Global Market, an active trading market for shares of our common stock may never develop or be sustained following this offering. If no trading market develops, securities analysts may not initiate or maintain research coverage of our company, which could further depress the market for our common stock. As a result, investors may not be able to sell their shares of our common stock at or above the initial public offering price or at the time that they would like to sell. An inactive market may also impair our ability to raise capital by selling additional shares and may impair our ability to acquire other companies or technologies by using our shares as consideration.
The market price of our common stock may be volatile and fluctuate significantly, which could result in substantial losses for investors purchasing shares in this offering and subject us to securities class action litigation.
The initial public offering price for shares of our common stock sold in this offering will be determined by negotiations between the underwriters and us. This initial public offering price may vary from the market price of our common stock after the offering. If an active market for our stock develops and continues, our stock price nevertheless may be volatile. Market prices of diagnostics companies have been volatile. Among the factors that may cause the market price of our common stock to fluctuate are the risks described in this “Risk Factors” section and other factors, including:
| | |
| | • | fluctuations in our quarterly operating results or the operating results of our competitors; |
| |
| | • | changes in estimates of our financial results or recommendations by securities analysts; |
| |
| | • | variance in our financial performance from the expectations of securities analysts; |
| |
| | • | changes in the estimation of the future size and growth rate of our markets; |
| |
| | • | changes in accounting principles or changes in interpretations of existing principles, which could affect our financial results; |
| |
| | • | failure of our products to achieve or maintain market acceptance or commercial success; |
| |
| | • | conditions and trends in the markets we serve; |
| |
| | • | changes in general economic, industry and market conditions; |
| |
| | • | success of competitive products and services; |
| |
| | • | changes in market valuations or earnings of our competitors; |
| |
| | • | changes in our pricing policies or the pricing policies of our competitors; |
| |
| | • | announcements of significant new products, contracts, acquisitions or strategic alliances by us or our competitors; |
| |
| | • | changes in legislation or regulatory policies, practices, or actions; |
| |
| | • | the commencement or outcome of litigation involving our company, our general industry or both; |
| |
| | • | recruitment or departure of key personnel; |
| |
| | • | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| |
| | • | actual or expected sales of our common stock by our stockholders; and |
| |
| | • | the trading volume of our common stock. |
In addition, the stock market in general, the NASDAQ and the market for diagnostics companies in particular, may experience a loss of investor confidence. Such loss of investor confidence may result in extreme price and volume fluctuations in our common stock that are unrelated or disproportionate to the operating performance of our business, financial condition or results of operations. These broad market and
18
industry factors may materially harm the market price of our common stock and expose us to securities class action litigation. Such litigation, even if unsuccessful, could be costly to defend and divert management’s attention and resources, which could further materially harm our financial condition and results of operations.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our common stock, the price of our common stock could decline.
The liquidity of the trading market for our common stock may be affected in part by the research and reports that equity research analysts publish about us and our business. We do not control the opinions of these analysts. The price of our stock could decline if one or more equity analysts downgrade our stock or if those analysts issue other unfavorable commentary or cease publishing reports about us or our business.
Certain provisions of our corporate governing documents could make an acquisition of our company more difficult.
Certain provisions of our organizational documents could discourage potential acquisition proposals, delay or prevent a change in control of us or limit the price that investors may be willing to pay in the future for shares of our common stock. For example, our amended and restated certificate of incorporation and amended and restated by-laws will:
| | |
| | • | authorize the issuance of preferred stock that can be created and issued by our board of directors without prior stockholder approval, commonly referred to as “blank check” preferred stock, with rights senior to those of our common stock; |
| |
| | • | limit the persons who can call special stockholder meetings; |
| |
| | • | provide that a majority vote of our stockholders is required to amend our amended and restated certificate of incorporation and amended and restated by-laws; |
| |
| | • | establish advance notice requirements to nominate persons for election to our board of directors or to propose matters that can be acted on by stockholders at stockholder meetings; |
| |
| | • | not provide for cumulative voting in the election of directors; and |
| |
| | • | provide for the filling of vacancies on our board of directors by action of a majority of the directors and not by the stockholders. |
These and other provisions in our organizational documents could allow our board of directors to affect your rights as a stockholder in a number of ways, including making it more difficult for stockholders to replace members of the board of directors. Because our board of directors is responsible for approving the appointment of members of our management team, these provisions could in turn affect any attempt to replace the current management team. These provisions could also limit the price that investors would be willing to pay in the future for shares of our common stock.
Our amended and restated articles of incorporation provide that Section 203 of the Delaware General Corporation Law, an anti-takeover law, will not apply to us. Section 203 generally prohibits an interested stockholder from engaging in certain types of business combinations with a Delaware corporation for three years after becoming an interested stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns 15% or more of the corporation. See “Description of Capital Stock — Anti-Takeover Effects of Delaware Law and Provisions of Our Amended and Restated Certificate of Incorporation and Our Amended and Restated By-Laws.”
Investors purchasing common stock in this offering will experience immediate and substantial dilution.
The assumed initial public offering price of our common stock is substantially higher than the net tangible book value per share of our outstanding common stock immediately after this offering. As a result, you will pay a price per share that substantially exceeds the book value of our tangible assets after subtracting our liabilities. Purchasers of our common stock in this offering will incur immediate and substantial dilution of $9.44 per share, representing the difference between the assumed initial public offering price of $15.00 per share and our adjusted pro forma net tangible book value per share after giving effect to this offering. In addition, investors purchasing shares of common stock in this offering will have contributed 47% of the total amount invested to fund our company, but will own only 33% of the common stock outstanding after this offering.
19
Moreover, we have issued options and warrants in the past to acquire common stock at prices significantly below the initial public offering price. Upon completion of this offering, after giving effect to the reverse stock split and the conversion of all series of convertible preferred stock into common stock, there will be 1,307,773 shares of common stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $10.66 per share and 3,251,548 shares subject to outstanding options at a weighted average exercise price of $4.98 per share. To the extent that these warrants or outstanding options are ultimately exercised, you will incur further dilution of your investment in our common stock. See “Dilution” for a further description of the dilution that you will experience immediately after this offering.
Future sales of our common stock may depress our share price.
After this offering, we will have 21,064,198 shares of common stock outstanding. Sales of substantial shares of our common stock in the public market following this offering, or the perception that these sales may occur, could cause the market price of our common stock to decline. After thelock-up agreements pertaining to this offering expire, additional stockholders will be able to sell their shares in the public market, subject to legal restrictions on transfer. As soon as practicable upon completion of this offering, we also intend to file a registration statement covering shares of our common stock issued or reserved for issuance under our stock option plan. In addition, under our stockholders’ agreement, some of our stockholders are entitled to registration rights. Following the expiration of thelock-up agreements, registration of the sale of these shares of our common stock would generally permit their sale into the market immediately after registration. These registration rights of our stockholders could impair our ability to raise capital by depressing the price of our common stock. We may also sell additional shares of common stock in subsequent public offerings, which may adversely affect market prices for our common stock. See “Shares Eligible for Future Sale” for more information.
We have broad discretion in the use of the net proceeds from this offering, and our investment of these proceeds may not yield a favorable return.
We cannot specify with certainty the particular uses of the net proceeds we will receive from this offering, and these uses may vary substantially from our current plans. Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in “Use of Proceeds.” Accordingly, you will have to rely upon the judgment of our management with respect to the use of the proceeds. Our management may spend a portion or all of the net proceeds from this offering in ways that our stockholders may not desire or that may not yield a significant return or any return at all. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may also invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We do not currently intend to pay dividends on our capital stock and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our common stock.
We have never declared or paid any cash dividends on our capital stock, and we currently intend to invest our future earnings, if any, to fund the development and growth of our business. Therefore, we do not anticipate declaring or paying cash dividends on our common stock in the foreseeable future. The payment of dividends will be at the discretion of our board of directors and will depend on our results of operations, capital requirements, financial condition, future prospects, contractual arrangements, restrictions imposed by applicable law, any limitations on payments of dividends present in our current and future debt agreements, and other factors our board of directors may deem relevant. If we do not pay dividends, your ability to achieve a return on your investment in our company will depend on any future appreciation in the market price of our common stock. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
We and our independent registered public accounting firm have identified material weaknesses in our internal control over financial reporting. Failure to achieve and maintain effective internal control over financial reporting could result in our failure to accurately report our financial results.
Management and our independent registered public accounting firm reported to the audit committee of our board of directors two material weaknesses and a significant deficiency in the design and operation of our internal control over financial reporting identified during the audit of the six month period ended June 30, 2007, which did not include an audit of our internal control over financial reporting.
20
Our independent registered public accounting firm was not engaged to perform an audit of our internal control over financial reporting, however they and management identified material weaknesses related to the lack of segregation of duties of finance personnel and our internal controls related to the accounting for expenses incurred and assets received in the proper period. Specifically, we did not have:
| | |
| | • | sufficient personnel with appropriate financial accounting and reporting expertise; |
| |
| | • | sufficient segregation of duties due to our limited number of finance and accounting personnel; |
| |
| | • | sufficient internal controls for addressing accounting for complex transactions, such as those related to our equity transactions; |
| |
| | • | a formal process and related controls to identify and appropriately record and disclose certain contractual obligations, such as licenses and other agreements; and |
| |
| | • | internal controls related to cutoff, impacting the accounting and reporting of inventory, prepaid expenses, accounts payable and accrued expenses. |
The identified significant deficiency related to the perpetual inventory recording. Specifically, we have not maintained a perpetual record of our inventory of purchased components that formally tracked inventory during interim financial periods.
While we are taking steps to address the identified material weaknesses and significant deficiency, there is no guarantee that these remediation steps will be sufficient to remediate the identified material weaknesses and significant deficiency or prevent additional material weaknesses and significant deficiencies from occurring. If we fail to remediate the material weaknesses or significant deficiency, or if additional material weaknesses or significant deficiencies are discovered in the future, we may fail to meet our future reporting obligations and our financial statements may contain material misstatements. Any such failure could also adversely affect the results of the periodic management evaluations and annual auditor attestation reports regarding the effectiveness of our internal control over financial reporting that will be required when the rules under Section 404 of the Sarbanes-Oxley Act of 2002 become applicable to us beginning with the required filing of our Annual Report onForm 10-K for the fiscal year ended December 31, 2008.
We will incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which may adversely affect our operating results and failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act could cause investors to lose confidence in our operating results and in the accuracy of our financial reports and could have a material adverse effect on our business and on the price of our common stock.
As a public company, we will be required, pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting for the first full fiscal year beginning after the effective date of this offering. The controls and other procedures are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is disclosed accurately and is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. We are in the early stages of developing our disclosure controls and procedures and we may not be able to complete our evaluation, testing and any required remediation needed to comply with Section 404 in a timely fashion. Our independent registered public accounting firm was not engaged to perform an audit of our internal control over financial reporting. Our independent registered public accounting firm’s audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of our internal control over financial reporting. Accordingly, no such opinion was expressed; however, we and our independent registered public accounting firm have identified two material weaknesses and a significant deficiency in the design and operation of our internal control over financial reporting as of June 30, 2007, which if not properly remediated, could result in material misstatements in our financial statements in future periods. Even if we develop effective controls, these new controls may become inadequate because of changes in conditions or the degree of compliance with these policies or procedures may deteriorate. Even after we develop these new procedures additional weaknesses in our internal controls may be discovered. In the event that we are not able to demonstrate compliance with Section 404 of the Sarbanes-Oxley Act in a timely manner, or are unable to produce timely
21
or accurate financial statements, we may be subject to sanctions or investigations by regulatory authorities such as the SEC or NASDAQ and investors may lose confidence in our operating results and our stock price could decline. Furthermore, if we or our auditors are unable to certify that our internal control is effective and in compliance with Section 404 we may be subject to sanctions or investigations by regulatory authorities such as the SEC or NASDAQ and we could lose investor confidence in the accuracy and completeness of our financial reports, which would have a material adverse effect on our business and on the price of our common stock.
Furthermore, as a public company, we will incur significant additional legal, accounting and other expenses that we did not incur as a private company. In addition, changing laws, regulations and standards relating to corporate governance and public disclosure, including regulations implemented by the Securities and Exchange Commission and the NASDAQ may increase legal and financial compliance costs and make some activities more time consuming. These laws, regulations and standards are subject to varying interpretations and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue-generating activities to compliance activities. If notwithstanding our efforts to comply with new laws, regulations and standards, we fail to comply, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Failure to comply with these rules might also make it more difficult for us to obtain certain types of insurance, including director and officer liability insurance, and we might be forced to accept reduced policy limits and coverageand/or incur substantially higher costs to obtain the same or similar coverage. The impact of these events could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on committees of our board of directors, or as executive officers.
Concentration of ownership among some of our stockholders, including directors and management may limit your ability to influence corporate matters.
Upon completion of this offering, approximately 65% of our common stock including the conversion of all outstanding series of convertible preferred stock into common stock and the exercise of all outstanding warrants and options to purchase our common stock exercisable within 60 days of October 29, 2007 will be beneficially held by our directors, our executive officers, and greater than five percent stockholders and their respective affiliates. Lurie Investment Fund, L.L.C., Lurie Investments, Inc., AOQ Trust, Alfa-Tech, L.L.C., and their respective affiliates, collectively the Lurie Entities, will own 29% of our common stock, and Bain Capital Venture Fund 2005, L.P. and Brookside Capital Partners Fund, L.P., and their respective affiliates, collectively the Bain Entities, will own 30% of our common stock upon completion of this offering, or 34% of our common stock if Brookside Capital Partners Fund, L.P. purchases $12.5 million of our common stock in this offering. Consequently, a small number of our stockholders may be able to substantially influence our management and affairs. If they choose to act together, they would be able to influence most matters requiring the approval by our stockholders, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets and any other significant corporate transaction. The concentration of ownership may also delay or prevent a change in control of us even if such changes might otherwise be beneficial to our stockholders. In addition, the significant concentration of share ownership may adversely affect the trading price of our common stock because investors often perceive disadvantages in owning shares in companies with controlling stockholders.
22
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements about us and our industry that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this prospectus regarding our strategy, future operations, future financial position, future net sales, projected expenses, prospects and plans and objectives of management are forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievement to be materially different from those expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “might,” “plan,” “project,” “will,” “would,” “should,” “could,” “can,” “predict,” “potential,” “continue,” “objective,” or the negative of these terms, and similar expressions intended to identify forward-looking statements. However, not all forward-looking statements contain these identifying words. These forward-looking statements reflect our current views about future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Actual events or results could differ materially from those expressed or implied by these forward-looking statements as a result of various factors, including the risk factors described in greater detail in the section entitled “Risk Factors.”
These forward-looking statements represent our estimates and assumptions only as of the date of this prospectus. Unless required by U.S. federal securities laws, we do not intend to update any of these forward-looking statements to reflect circumstances or events that occur after the statement is made or to conform these statements to actual results.
23
We estimate that the net proceeds to us from the sale of common stock that we are offering will be approximately $95 million, assuming an initial public offering price of $15.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. A $1.00 increase or decrease in the assumed initial public offering price of $15.00 per share would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $6.5 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We expect to use the net proceeds from this offering as follows:
| | |
| | • | approximately $50 million to finance ongoing research and development in connection with the development of additional genomic and protein tests and the next generation Verigene System and the continued investments in and development of our product manufacturing infrastructure, including purchasing manufacturing equipment, tooling and facilities leasehold improvements, as well as increased manufacturing personnel; |
| |
| | • | approximately $40 million to fund additional sales, marketing and service personnel and marketing initiatives in connection with future test and system product launches; and |
| |
| | • | the remainder for additional working capital and other general corporate purposes, such as business development, financial and administrative support services, the employment of additional personnel and the costs of operating as a public company. |
Although we currently have no agreements or commitments for any specific acquisitions, we may use a portion of the net proceeds to expand our current business through strategic alliances with, or acquisitions of, other businesses, products or technologies. This expected use of the net proceeds of this offering represents our current intentions based upon our present plans and business condition. The amounts and timing of our actual expenditures may vary significantly and will depend upon numerous factors, including cash flows from operations and the anticipated growth of our business. We will retain broad discretion in the allocation and use of our net proceeds. Pending the allocation of the net proceeds of this offering, we intend to invest the net proceeds of this offering in short-term, interest-bearing obligations, investment grade instruments, certificates of deposit or guaranteed obligations of the U.S. government.
We have never declared or paid any cash dividends on our capital stock and do not expect to pay any dividends for the foreseeable future. We currently intend to retain any future earnings to fund the operation, development and expansion of our business. Any future determination to pay dividends will be at the sole discretion of our board of directors and will depend upon a number of factors, including our results of operations, capital requirements, financial condition, future prospects, contractual arrangements, restrictions imposed by applicable law, any limitations on payments of dividends present in our current and future debt arrangements, and other factors our board of directors may deem relevant.
24
The following table sets forth our capitalization as of June 30, 2007 on an actual basis, and on an as adjusted basis to reflect (i) the conversion of all outstanding shares of Series B, Series C, Series C-2 and Series D Convertible Preferred Stock into common stock; (ii) the exercise of all outstanding warrants to purchase Series C andSeries C-2 Convertible Preferred Stock, which expire immediately prior to closing unless exercised for an aggregate of 138,205 shares of common stock for an aggregate purchase price of $1,213,542; (iii) the conversion of all outstanding warrants to purchase Series D Convertible Preferred Stock into warrants to purchase shares of common stock; and (iv) the receipt and use by us of the estimated net proceeds from this offering as described in “Use of Proceeds,” as if they had occurred on June 30, 2007.
The table should be read in conjunction with the more detailed information contained elsewhere in this prospectus, including “Use of Proceeds,” “Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus.
| | | | | | | | | |
| | | As of June 30, 2007 | |
| | | | | | As
| |
| | | Actual | | | Adjusted(1)(2) | |
| Long-term debt | | $ | 9,302,976 | | | $ | 9,302,976 | |
| Convertible preferred stock | | | 113,985,907 | | | | — | |
| | | | | | | | | |
| Stockholders’ equity (deficit) | | | | | | | | |
| Common stock: par value $0.01 per share; 450,000,000 shares authorized, 932,646 shares issued and outstanding on an actual basis and 100,000,000 shares authorized, 20,907,558 shares issued and outstanding on an as adjusted basis | | | 92,580 | | | | 214,496 | |
| Additional paid-in capital(3) | | | 2,906,315 | | | | 245,660,104 | |
| Note receivable from chief executive officer(4) | | | (1,440,000 | ) | | | (1,440,000 | ) |
| Common stock warrants(3) | | | — | | | | 1,705,871 | |
| Deficit accumulated during the development stage | | | (124,800,567 | ) | | | (124,800,567 | ) |
| | | | | | | | | |
| Total stockholders’ equity (deficit) | | | (123,241,672 | ) | | | 121,339,904 | |
| | | | | | | | | |
| Total capitalization | | $ | 47,211 | | | | 130,642,880 | |
| | | | | | | | | |
| | |
| (1) | | Each $1.00 increase or decrease in the assumed initial public offering price of $15.00 per share would increase or decrease, as applicable, total stockholders’ equity (deficit) and total capitalization by approximately $6.5 million, assuming the number of shares offered by us, as set forth on the cover of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. |
| | |
| (2) | | Assumes (i) the conversion of all outstanding shares of Series B, Series C,Series C-2 and Series D Convertible Preferred Stock for shares of common stock on a 25-for-one basis including common stock issued in connection with accrued and unpaid dividends which were 544,346 as of June 30, 2007 (at an assumed initial public offering price of $15.00 per share which is the midpoint of the range listed on the cover page of this prospectus); (ii) the exercise of all outstanding warrants to purchase Series C andSeries C-2 Convertible Preferred Stock, which expire immediately prior to closing unless exercised for an aggregate of 138,205 shares of common stock for an aggregate purchase price of $1,213,542; and (iii) the conversion of all outstanding warrants to purchase Series D Convertible Preferred Stock into warrants to purchase 1,307,773 shares of common stock at a weighted average exercise price of $10.66 immediately prior to the closing of this offering. |
| | |
| (3) | | As adjusted reflects the conversion of all convertible preferred stock to common stock resulting in the reclassification of $32.7 million convertible derivative liability into additional paid-in capital, and the conversion of all outstanding warrants to purchase Series D Convertible Preferred Stock into warrants to purchase 1,307,773 shares of common stock which are re-classified to common stock warrants. |
| |
| (4) | | This note was repaid on August 8, 2007. |
25
As of June 30, 2007, we had a net tangible book deficit of our common stock of $128.4 million, or approximately $137.70 per share of common stock, not taking into account (i) the conversion of all outstanding series of convertible preferred stock into common stock, and (ii) the exercise of all outstanding warrants to purchase Series C andSeries C-2 Convertible Preferred Stock which expire immediately prior to the closing of this offering unless exercised for common stock. Net tangible book deficit per share represents the amount of our total tangible assets less total liabilities and convertible preferred stock, divided by the number of common shares outstanding.
Our pro forma net tangible book value after giving effect to (i) the conversion of all outstanding series of convertible preferred stock into common stock and (ii) the exercise of our outstanding warrants to purchase Series C andSeries C-2 Convertible Preferred Stock for common stock as of June 30, 2007 was $21.5 million, or $1.55 per share of common stock. Pro forma net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the number of shares of common stock outstanding as of June 30, 2007 after giving effect to the conversion of all outstanding series of convertible preferred stock into shares of common stock which will occur immediately prior to the completion of this offering and assumes the exercise of all outstanding warrants to purchase Series C andSeries C-2 Convertible Preferred Stock for common stock which expire immediately prior to the closing of this offering unless exercised.
After giving effect to the sale by us of shares of common stock in this offering at an assumed initial public offering price of $15.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our adjusted pro forma net tangible book value as of June 30, 2007 would have been approximately $116.2 million, or approximately $5.56 per share. This amount represents an immediate increase in adjusted pro forma net tangible book value of $4.01 per share to our existing stockholders and an immediate dilution in adjusted pro forma net tangible book value of approximately $9.44 per share to new investors purchasing shares of common stock in this offering at the assumed initial public offering price.
A $1.00 increase or decrease in the assumed initial public offering price of $15.00 per share would increase or decrease, as applicable, the net proceeds to us from this offering by approximately $6.5 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We determine dilution by subtracting the adjusted pro forma net tangible book value per share after this offering from the amount of cash that a new investor paid for a share of common stock.
The following table illustrates this dilution on a per share basis:
| | | | | | | | | |
| Assumed initial public offering price | | | | | | $ | 15.00 | |
| | | | | | | | | |
| Pro forma net tangible book value as of June 30, 2007 | | $ | 1.55 | | | | | |
| | | | | | | | | |
| Increase attributable to new stockholders | | $ | 4.01 | | | | | |
| | | | | | | | | |
| Adjusted pro forma net tangible book value after this offering | | $ | 5.56 | | | | | |
| | | | | | | | | |
| Dilution in pro forma net tangible book value to new stockholders | | | | | | $ | 9.44 | |
| | | | | | | | | |
The following table summarizes, as of June 30, 2007, the differences for our existing stockholders and new investors in this offering, with respect to the number of shares of common stock purchased from us, the total consideration paid and the average price per share paid before deducting fees and expenses at an assumed initial public offering price of $15.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The following table assumes (i) the conversion of all series of convertible preferred stock into common stock which will be effected immediately prior to the completion of this offering and (ii) the exercise
26
of all outstanding warrants to purchase Series C and Series C-2 Convertible Preferred Stock for common stock which expire immediately prior to the closing of this offering unless exercised.
| | | | | | | | | | | | | | | | | | | | | |
| | | Total Shares | | | Total Consideration | | | Average Price
| |
| | | Number | | | Percentage | | | Amount | | | Percentage | | | per Share | |
| |
| Existing stockholders | | | 13,907,558 | | | | 67 | % | | $ | 118,517,036 | | | | 53 | % | | $ | 8.52 | |
| New stockholders in this offering | | | 7,000,000 | | | | 33 | % | | | 105,000,000 | | | | 47 | % | | | 15.00 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | | 20,907,558 | | | | 100.0 | % | | $ | 223,517,036 | | | | 100.0 | % | | | | |
| | | | | | | | | | | | | | | | | | | | | |
A $1.00 increase or decrease in the assumed initial public offering price of $15.00 per share would increase or decrease, as applicable, total consideration paid by investors in this offering and total consideration paid by all stockholders by $6.5 million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same.
The discussion and tables above assume no exercise of the underwriters’ over-allotment option. If the underwriters’ over-allotment option is exercised in full:
| | |
| | • | the number of shares of common stock held by existing stockholders will represent 63% of the total number of shares of common stock to be outstanding after this offering; the number of shares of common stock held by investors participating in this offering will represent 37% of the total number of shares of common stock to be outstanding after this offering; and |
| | |
| | • | our adjusted pro forma net tangible book value at June 30, 2007 will be $130.8 million, or $5.96 per share of common stock, representing an immediate increase in pro forma net tangible book value of $4.41 per share of common stock to our existing stockholders and an immediate dilution of $9.04 per share to investors purchasing shares in this offering. |
In addition, the above discussion and tables assume no exercise of stock options or the outstanding warrants to purchase Series D Convertible Preferred Stock which will become exercisable to purchase 1,307,773 shares of common stock at an exercise price of $10.66 per share immediately prior to the completion of this offering.
As of June 30, 2007, we had outstanding options to purchase a total of 3,173,548 shares of common stock at a weighted average exercise price of $4.99 per share and outstanding warrants to purchase a total of 32,694,562 shares of Series D Convertible Preferred Stock, which will be converted into warrants to purchase 1,307,773 shares of common stock immediately prior to the completion of this offering.
If all of these outstanding options and warrants had been exercised as of June 30, 2007, our pro forma net tangible book value would have been $2.79 per share of common stock, adjusted pro forma net tangible book value after this offering would be $5.75 per share of common stock and dilution in adjusted pro forma net tangible book value to investors in this offering would be $9.25 per share of common stock.
In addition, if all of these outstanding options and warrants as of June 30, 2007 were exercised, on an as adjusted basis before deducting underwriting discounts and estimated offering expenses payable by us, (i) existing stockholders would have purchased 18,388,879 shares representing 72% of the total shares for $148,301,724, or approximately 59% of the total consideration paid, with an average price per share of $8.06 and (ii) 7,000,000 shares purchased by new stockholders in this offering would represent approximately 28% of total shares for approximately $105,000,000, or approximately 41% of the total consideration paid.
27
The statements of operations data for the years ended December 31, 2004, 2005 and 2006 and for the six month period ended June 30, 2007, and the balance sheet data at December 31, 2005, December 31, 2006 and June 30, 2007, are derived from the financial statements that have been audited by our independent registered public accounting firm, which are included elsewhere in this prospectus. The statement of operations for the years ended December 31, 2002 and 2003 and the balance sheet data at December 31, 2004 have been derived from our audited financial statements and notes that do not appear in this prospectus. The statements of operations data for the six month period ended June 30, 2006 have been derived from our unaudited financial statements and related notes which are included elsewhere in this prospectus. The historical results presented below are not necessarily indicative of future results. The following selected financial data should be read in conjunction with, and is qualified by reference to, our financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this prospectus. The accompanying selected financial data for the years ended December 31, 2004, 2005 and 2006 have been restated. See Note 12 to the financial statements.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | Six Month Period
| |
| | | Years Ended December 31, | | | Ended June 30, | |
| Statements of Operations Data: | | 2002 | | | 2003 | | | 2004 | | | 2005 | | | 2006 | | | 2006 | | | 2007 | |
| | | | | | | | | (As restated) | | | (As restated) | | | (As restated) | | | | | | | |
| Revenue: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Grant and contract revenue | | $ | 567,731 | | | $ | 1,440,880 | | | $ | 2,768,125 | | | $ | 1,777,667 | | | $ | 1,006,351 | | | $ | 438,512 | | | $ | 726,503 | |
| Product sales | | | — | | | | 30,020 | | | | — | | | | 136,850 | | | | 131,660 | | | | 27,630 | | | | 53,670 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | | 567,731 | | | | 1,470,900 | | | | 2,768,125 | | | | 1,914,517 | | | | 1,138,011 | | | | 466,142 | | | | 780,173 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Costs and expenses: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of product sales | | | — | | | | 16,452 | | | | — | | | | 125,118 | | | | 31,049 | | | | — | | | | 18,367 | |
| Research and development | | | 4,854,757 | | | | 8,358,249 | | | | 10,366,473 | | | | 13,244,872 | | | | 17,447,227 | | | | 7,874,596 | | | | 10,219,047 | |
| Sales, general and administrative | | | 1,886,910 | | | | 3,086,823 | | | | 3,131,390 | | | | 4,502,970 | | | | 5,415,525 | | | | 2,663,931 | | | | 5,256,583 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Total costs and expenses | | | 6,741,667 | | | | 11,461,524 | | | | 13,497,863 | | | | 17,872,960 | | | | 22,893,801 | | | | 10,538,527 | | | | 15,493,997 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (6,173,936 | ) | | | (9,990,624 | ) | | | (10,729,738 | ) | | | (15,958,443 | ) | | | (21,755,790 | ) | | | (10,072,385 | ) | | | (14,713,824 | ) |
| Change in fair value of convertible derivative liability | | | — | | | | — | | | | — | | | | — | | | | (2,916,822 | ) | | | (2,916,822 | ) | | | — | |
| Change in fair value of preferred stock warrants | | | — | | | | — | | | | (8,801 | ) | | | (8,314 | ) | | | (119,914 | ) | | | (244,104 | ) | | | (276,612 | ) |
| Foreign exchange loss | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | (13,770 | ) |
| Interest expense-related party | | | — | | | | — | | | | (204,335 | ) | | | (37,919 | ) | | | (146,550 | ) | | | (146,550 | ) | | | — | |
| Interest expense | | | — | | | | — | | | | — | | | | — | | | | (7,506 | ) | | | (1,585 | ) | | | (823,748 | ) |
| Interest income | | | 45,110 | | | | 70,765 | | | | 40,963 | | | | 69,376 | | | | 1,415,001 | | | | 483,755 | | | | 758,433 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | (6,128,826 | ) | | | (9,919,859 | ) | | | (10,901,911 | ) | | | (15,935,300 | ) | | | (23,531,581 | ) | | | (12,897,691 | ) | | | (15,069,521 | ) |
| Accumulated convertible preferred stock dividends | | | — | | | | — | | | | — | | | | — | | | | (4,413,591 | ) | | | (1,350,933 | ) | | | (3,180,329 | ) |
| Convertible preferred stock redemption value adjustment | | | — | | | | — | | | | (10,156,393 | ) | | | (2,898,787 | ) | | | (17,737,544 | ) | | | (17,737,544 | ) | | | (608,940 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss attributable to common stock | | $ | (6,128,826 | ) | | $ | (9,919,859 | ) | | $ | (21,058,304 | ) | | $ | (18,834,087 | ) | | $ | (45,682,716 | ) | | $ | (31,986,168 | ) | | $ | (18,858,790 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss per common share: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| basic and diluted | | $ | (10.04 | ) | | $ | (16.24 | ) | | $ | (34.44 | ) | | $ | (30.80 | ) | | $ | (52.78 | ) | | $ | (40.15 | ) | | $ | (20.22 | ) |
| Weighted average number of common shares: | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| basic and diluted | | | 610,533 | | | | 610,972 | | | | 611,466 | | | | 611,496 | | | | 865,559 | | | | 796,729 | | | | 932,646 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | As of December 31, | | | | | | As of June 30, | |
| Balance Sheet Data: | | 2002 | | | 2003 | | | 2004 | | | 2005 | | | 2006 | | | | | | 2007 | |
| | | | | | | | | (As restated) | | | (As restated) | | | (As restated) | | | | | | | |
| |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 12,453,311 | | | $ | 1,150,108 | | | $ | 6,314,008 | | | $ | 3,641,338 | | | $ | 29,112,429 | | | | | | | $ | 26,970,383 | |
| Working capital | | | 11,893,231 | | | | 125,034 | | | | 4,542,299 | | | | (2,642,582 | ) | | | 27,332,463 | | | | | | | | 23,894,749 | |
| Total assets | | | 14,233,017 | | | | 4,674,142 | | | | 12,198,575 | | | | 11,346,514 | | | | 41,037,834 | | | | | | | | 40,435,013 | |
| Long-term debt | | | — | | | | — | | | | — | | | | — | | | | 58,802 | | | | | | | | 9,302,976 | |
| Convertible preferred stock | | | 23,793,897 | | | | 23,793,897 | | | | 40,998,231 | | | | 51,143,984 | | | | 108,868,040 | | | | | | | | 113,985,907 | |
| Stockholders’ deficit | | | (10,306,050 | ) | | | (20,328,435 | ) | | | (41,348,431 | ) | | | (59,961,290 | ) | | | (105,238,071 | ) | | | | | | | (123,241,672 | ) |
28
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read together with our financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements based upon current expectations that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements” and elsewhere in this prospectus.
Restatement
Subsequent to the issuance of our 2006 restated financial statements, we determined that warrants to purchase shares of various series of our convertible preferred stock were not accounted for as liabilities consistent with the provisions of Financial Accounting Standards Board’s Staff PositionNo. 150-5, Issuer’s Accounting under FASB Statement No. 150 for Freestanding Warrants and Other Similar Instruments on Shares that are Redeemable. The warrants had been recorded at no value upon their issuance and were included in mezzanine equity in the financial statements combined with the amounts for the related series of preferred stock. The warrants should have been accounted for as a liability due to the redemption feature included within the convertible preferred stock to be acquired upon exercise of the warrants. The warrants, when classified as a liability, are required to be initially recorded at fair value and to be marked to fair value at the end of each reporting period, with changes in the fair value being recorded as a component of other income and expense in our statements of operations.
In addition, we have identified an error in the Black-Scholes option pricing model that we have historically used to value our common stock options and the warrants. The error arose due to an incorrect formula embedded in the model we use to calculate fair value for these financial instruments.
The accompanying financial statements for the years ended December 31, 2004, 2005 and 2006 have been restated. See Note 12 to the financial statements. The accompanying management’s discussion and analysis of financial condition and results of operations reflects the restatement described above.
Business Overview
Nanosphere develops, manufactures and markets an advanced molecular diagnostics platform, the Verigene System, that enables simple, low cost and highly sensitive genomic and protein testing on a single platform. Our proprietary nanoparticle technology simplifies the ability to perform molecular diagnostic tests, achieves ultra-sensitive protein detection at limits beyond current diagnostic technologies, provides the ability to multiplex, or run multiple tests at the same time on the same sample, and enables the development of a broad menu of test assays to be performed on a single platform. We are currently developing diagnostic tests for a variety of medical conditions including cancer, neurodegenerative, cardiovascular and infectious diseases, as well as pharmaco-genomics, or tests for personalized medicine. There is a growing demand among laboratories to implement molecular diagnostic capabilities, but the cost and complexity of existing technologies and the need for specialized personnel and facilities have limited the number of laboratories with these capabilities. We believe that the Verigene System’s ease of use, rapid turnaround times, relatively low cost and ability to support a broad test menu will simplify work flow and reduce costs for laboratories already performing molecular diagnostic testing and allow a broader range of laboratories, including those operated by local hospitals, to perform molecular diagnostic testing. Our ability to detect proteins, which is at least 100 times more sensitive than current technologies, may enable earlier detection of and intervention in diseases associated with known biomarkers and the introduction of tests for new biomarkers that exist in concentrations too low to be detected by current technologies. We are focusing our efforts on the clinical diagnostics market and may seek opportunities either directly or through outlicensing arrangements to commercialize our technology in the industrial, biosecurity and other markets.
We received 510(k) clearance from the FDA for the Verigene System and our warfarin metabolism assay on September 17, 2007 and for our hyper-coagulation assay on October 12, 2007. We are currently developing
29
diagnostic tests for a variety of medical conditions including cancer, neurodegenerative, cardiovascular and infectious diseases, as well as pharmaco-genomics, or tests for personalized medicine. We anticipate that we will submit applications to the FDA for clearance of tests for cystic fibrosis, herpes, cervical cancer, respiratory illness, recurrent prostate cancer and cardiovascular disease during the next 36 months, and we anticipate that we will submit two of such tests within the next 12 months.
Since our inception we have incurred net losses each year, and we expect to continue to incur losses for the foreseeable future. Our net losses attributable to common stock for fiscal 2004, 2005 and 2006 were $21.1 million, $18.8 million and $45.7 million, respectively, and $18.9 million for the six month period ended June 30, 2007. As of June 30, 2007, we had an accumulated deficit during the development stage of $124.8 million. Our operations to date have been funded principally through capital contributions from investors in our convertible preferred stock and our debt borrowings. We expect to incur increasing expenses over the next several years, principally to further develop our products and to develop additional diagnostic tests, as well as to further increase our spending to manufacture, sell and market our products.
Financial Operations Overview
Revenue
Revenue received to date is limited to funds received under contracts and government grants, including funds for the reimbursement of certain research and development expenses, and minimal product revenues derived from the sale of the Verigene System, including cartridges and related products to research laboratories.
Cost of Product Sales
Cost of product sales represents the cost of materials, direct labor, other overhead costs associated with manufacturing, delivering and selling the Verigene System, including cartridges and related products, including royalties on product sales.
Research and Development Expenses
Research and development expenses primarily include all expenses related to the development of the Verigene System and assays, and the research and development costs associated with fulfilling our obligations to the United States government and under other contracts and grants. Such expenses include salaries and benefits for research and development personnel, contract services and other expenses. We expense all research and development costs in the periods in which they are incurred. We expect research and development expenses to increase as we work to develop future generations of the Verigene System, and additional genomic and protein tests.
Sales, General and Administrative Expenses
Sales, general and administrative expenses principally include compensation for employees in our sales, customer service, marketing, management and administrative functions. We also include professional services, facilities, communications and administrative expenses in sales, general and administrative. The professional services costs primarily consist of legal, intellectual property and accounting costs. We expect sales and marketing expenses to continue to increase in the future as a result of anticipated growth in our sales and customer support functions to support growth in our product sales. We expect general and administrative expenses to increase after this offering as a result of the need to hire additional administrative personnel and due to higher legal, accounting and related expenses associated with being a public company.
Interest Income
Interest income principally includes interest earned on our excess cash balances. Such balances are primarily invested in money market and bank checking accounts at major financial institutions.
30
Interest Expense
Interest expense includes the interest charges incurred on bridge financing arrangements entered into prior to 2007 as part of our convertible preferred stock issuances, and in 2007, includes interest expense related to our debt borrowings, including non-cash interest expense relating to the amortization of debt discount and issue costs.
Results of Operations
Six Month Period Ended June 30, 2007 Compared to Six Month Period Ended June 30, 2006
Revenues
Revenues were $780,173 for the six month period ended June 30, 2007, as compared to $466,142 for the comparable period in 2006. These revenues were primarily derived from contracts and government grants and secondarily derived from a small level of product sales to research customers. Revenues from contracts and government grants were $726,503 in 2007 and $438,512 in 2006. The increase resulted from revenue associated with contracts with the Technical Support Working group within the U.S. Department of Defense and Northwestern University. As product revenue grows, we do not expect revenue from contracts and government grants to be a material percentage of our business.
Cost of Product Sales
For the six month period ended June 30, 2007, cost of product sales was $18,367 consisting primarily of product manufacturing costs. No cost of product sales was recorded for the six month period ended June 30, 2006.
Research and Development Expenses
Research and development expenses were $10.2 million for the six month period ended June 30, 2007, an increase from the $7.9 million of research and development expense for the comparable period in 2006. This expense growth was a result of increased research and development personnel and contract services to prepare for the commercial launch of the Verigene System and to develop protein assays in parallel with genomic tests.
The $2.3 million increase in research and development expenses for the six month period ended June 30, 2007 versus 2006 consists primarily of $1.0 million in staffing and $1.3 million in cartridge development.
Sales, General and Administrative Expenses
Sales, general and administrative expenses increased to $5.3 million for the six month period ended June 30, 2007, from $2.7 million for the comparable period in 2006. The increase was due primarily to $1.1 million related to the hiring of a direct sales force in preparation for the anticipated product launch of our Verigene System, $0.6 million related to stock option equity compensation, $0.2 million increase in legal fees, $0.2 million increase in building maintenance expense and $0.2 million increase in travel expense.
Change in Fair Value of Convertible Derivative Liability
OurSeries C-2 and Series D Convertible Preferred Stock contain conversion features which are embedded derivatives and therefore require bifurcation and accounting at fair value separate and distinct from the convertible preferred stock. Changes in the fair value of the conversion liability are recognized in earnings. During 2006 the fair value of the conversion liability increased, resulting in a $2.9 million charge to earnings.
Change in the Fair Value of the Preferred Stock Warrants
The change in the fair value of the preferred stock warrants reduced earnings by $244,104 and $276,612 for the six month periods ended June 30, 2006 and 2007, respectively.
31
Interest Income
Interest income was $758,433 for the six month period ended June 30, 2007, as compared to $483,755 in the comparable period in 2006. This $274,678 increase was due to higher average cash balances and higher interest rates during the six month period ended June 30, 2007.
Interest Expense
Interest expense was $823,748 for the six month period ended June 30, 2007, as compared to $148,135 in the comparable period in 2006. Our interest expense in 2007 was due to our debt borrowings, and our 2006 interest expense resulted primarily from bridge loans related to our convertible preferred stock issuance in 2006.
Fiscal 2006 Compared to 2005
Revenues
Revenues were $1.1 million for fiscal 2006, as compared to $1.9 million for fiscal 2005. Product revenues to the research market associated with sales of the Verigene System, including cartridges and related products, for fiscal 2006 were $131,660, as compared to $136,850 in fiscal 2005. Grant and contract revenues were $1.0 million for fiscal 2006, down from $1.8 million in fiscal 2005. Grant and contract revenues in both periods were for technology feasibility evaluations and biosecurity product development.
Cost of Product Sales
For fiscal 2006, cost of product sales was $31,049, as compared to $125,118 for fiscal 2005. All costs recorded for these periods consist primarily of product manufacturing costs. Cost of product sales in 2005 included our normal product cost as well as the cost of customized components associated with a government contract. These costs were included in the contract revenues with no mark up in 2005 and such costs did not exist in 2006.
Research and Development Expenses
Research and development expenses increased to $17.4 million for fiscal 2006, from $13.2 million for fiscal 2005. The increase in research and development expenses is attributable to increased spending on Verigene System platform development activities, development of genomic assays and development of protein assays. The increase in research and development spending from 2005 to 2006 includes $1.1 million in staffing, $1.0 million in lab supplies, $0.9 million in system design support and $0.5 million in cartridge development. The staffing expense increase resulted from hiring approximately 15 people in research, development and manufacturing. Most of the personnel increase was to support our expanded number of tests in development. Our increase in design support, lab supplies and cartridge development relates to purchases of design services, materials and components required to commercialize our initial genomic tests and the Verigene System.
Sales, General and Administrative Expenses
Sales, general and administrative expenses increased to $5.4 million for fiscal 2006, from $4.5 million for fiscal 2005. The $0.9 million increase is primarily driven by the $0.6 million increase in sales and marketing personnel, $0.2 million in stock option compensation expense and $0.1 million increase in legal fees.
Change in Fair Value of Convertible Derivative Liability
OurSeries C-2 and Series D Convertible Preferred Stock contain conversion features which are embedded derivatives and therefore require bifurcation and accounting at fair value separate and distinct from the Convertible Preferred Stock. Changes in the fair value of the conversion liability are recognized in earnings. During 2006 the fair value of the conversion liability increased, resulting in a $2.9 million charge to earnings.
32
Change in the Fair Value of the Preferred Stock Warrants
The change in the fair value of the preferred stock warrants reduced earnings by $8,314 and $119,914 in 2005 and 2006, respectively.
Interest Income
Interest income was $1.4 million for fiscal 2006, compared with $69,376 in fiscal 2005. This increase was due to higher average cash balances and higher interest rates during fiscal 2006.
Interest Expense
Interest expense increased to $154,056 for fiscal 2006, from $37,919 in 2005 due to bridge loans from existing investors prior to the closing of our Series D Convertible Preferred Stock financing in April 2006. The bridge loan was initially made by Lurie Investment Fund, L.L.C., on December 9, 2005 in the principal amount of $5.0 million at a fixed annual interest rate of 10.0%. Bridge loans were also made on January 6, 2006 by the following existing investors R. Capital II, Ltd., Adam N. Mirkin, Rhoderic Peter Mirkin, Richard Segal, and Steven E. Mather in the aggregate principal amount of $0.1 million at a fixed annual interest rate of 10%, and on March 15, 2006, by Lurie Investment Fund, L.L.C. in the principal amount of $1.3 million at a fixed annual interest rate of 4.58%. The promissory notes issued as consideration for such bridge loans were cancelled on April 12, 2006 in exchange for shares of Series D Convertible Preferred Stock. See “Certain Relationships and Related Party Transactions.”
Fiscal 2005 Compared to 2004
Revenues
Revenues for fiscal 2005 were $1.9 million, of which $1.8 million was from grants and contracts. Of this grant and contract revenue, $1.0 million was related to a biosecurity contract with the U.S. Department of Defense and the remaining revenue consisted of several grants and a contract with Northwestern University. We recorded $2.8 million in grant and contract revenues for fiscal 2004 associated with several contracts and government grants. The largest contract was $1.4 million with the U.S. Department of Defense to develop a biotoxin application on a modified Verigene System platform. There was no product revenue recorded for fiscal 2004.
Cost of Product Sales
For fiscal 2005, cost of product sales was $125,118 which consist primarily of product manufacturing costs. No cost of product sales was recorded for fiscal 2004.
Research and Development Expenses
Research and development expenses were $13.2 million for fiscal 2005, up from $10.4 million for fiscal 2004. The increase of $2.8 million was driven by a $1.4 million increase in research and development personnel expenses and an increase of $0.9 million in depreciation on equipment and lab supplies for developing system and cartridge prototypes.
Sales, General and Administrative Expenses
Sales, general and administrative expenses were $4.5 million for fiscal 2005, up from $3.1 million for fiscal 2004. The increase of $1.4 million relates primarily to a $1.0 million increase in marketing and corporate staffing and recruiting expense. This increase was also driven by a $0.4 million rise in demonstration supplies and materials.
33
Change in the Fair Value of the Preferred Stock Warrants
The change in the fair value of the preferred stock warrants reduced earnings by $8,801 and $8,314 in 2004 and 2005, respectively.
Interest Income
Interest income increased to $69,376 for fiscal 2005, from $40,963 for fiscal 2004. This increase was due to higher average cash and investment balances in 2005 as compared to 2004.
Interest Expense
Interest expense was $37,919 for fiscal 2005, as compared to $204,335 for fiscal 2004. The interest expense in 2005 was due to the $5.0 million bridge loan from Lurie Investment Fund, L.L.C. prior to the closing of our Series D Convertible Preferred Stock financing which was outstanding for one month in 2005, and in 2004, related to bridge loans issued in advance of ourSeries C-2 Convertible Preferred Stock financing. All promissory notes issued as consideration for such bridge loans in 2004 and 2005 were cancelled in exchange for our convertible preferred stock. See “Certain Relationships and Related Party Transactions.”
Liquidity and Capital Resources
From our inception in December 1999 through June 30, 2007, we had received net proceeds of $103.9 million from the sale of convertible preferred stock and issuance of notes payable that were exchanged for convertible preferred stock, $12.5 million from our debt borrowings and $8.5 million from grant and contract revenue. We have devoted substantially all of these funds to research and development and general and administrative expenses. Since our inception, we have had minimal revenues which have been derived from the sale of the Verigene System, including cartridges and related products, to research laboratories and pursuant to government contracts. Since inception, we have incurred significant losses and, as of June 30, 2007, we had an accumulated deficit during the development stage of approximately $124.8 million. As of December 31, 2005 and 2006, the accumulated deficit was $60.3 million and $105.9 million, respectively. We have not yet achieved profitability and anticipate that we will continue to incur net losses for the foreseeable future. We expect that our research and development, sales, general and administrative expenses will continue to increase and, as a result, we will need to generate significant revenues to achieve profitability.
Our historical product sales have been minimal because our products, including the Verigene System, did not receive FDA clearance until September 17, 2007. On September 17, 2007, we received 510(k) clearance from the FDA for the Verigene System and our warfarin metabolism assay, and on October 12, 2007 we received FDA clearance for our hyper-coagulation assay. We have now commenced commercial sales of the Verigene System and this initial assay. Our expenses are likely to exceed our product sales for the next few years as we begin to market our FDA cleared products and continue to expand our FDA cleared tests. During this period we expect to spend approximately $50 million to finance ongoing research and development in connection with the development of additional tests and the next generation Verigene System and continued investments in and development of our product manufacturing infrastructure, including purchasing manufacturing equipment, tooling and facilities leasehold improvements, as well as increased manufacturing personnel. We also expect to spend approximately $40 million to fund additional sales, marketing and service personnel and marketing initiatives in connection with future test and system product launches. Positive cash flow from operations will depend upon revenue resulting from adoption of our initial products and expansion of our FDA cleared tests.
As of June 30, 2007, we had $27.0 million in cash and cash equivalents, compared to $29.1 million at December 31, 2006. The decrease of $2.1 million was principally due to our operating and investing activities of $14.4 million, offset by our new debt borrowings of $12.5 million. Our working capital as of June 30, 2007 was $23.9 million.
In February 2007, we entered into two loan and security agreements, with commitments for an aggregate of $25.0 million in debt financing with Venture Lending & Leasing IV, Inc., and Venture Lending & Leasing V,
34
Inc. Pursuant to these loan agreements, we borrowed $12.5 million in February 2007. We are required to pay interest and a minimal amount of principal, for the initial twelve month period followed by a thirty month period within which the note principal will be amortized. Interest is paid during the initial twelve month period at a fixed annual interest rate of 12.5%, and during the following thirty month period at a fixed annual interest rate of 10.0%.
The second tranche of notes under this agreement can be drawn down at our option during December 2007. The terms of that borrowing, should it occur, would require us to pay interest and a minimal amount of principal for the initial nine month period followed by a twenty-four month period within which the note principal would be amortized. Interest will be paid during the initial nine month period at a fixed annual interest rate equivalent to the greater of the prime rate at the time of the borrowing plus 4.25%, or 12.5% at June 30, 2007, and during the followingtwenty-four month period at a fixed annual interest rate equivalent to the higher of the prime rate at the time of the borrowing plus 1.75%, or 10.0% at June 30, 2007. The loans are secured by a first priority lien on all of our assets including intellectual property. In connection with the execution of the commitment, we issued to the lenders 3,928,650 shares of our Series D Convertible Preferred Stock. Also in February 2007, in connection with the initial note issuance of $12.5 million, we issued an additional 1,607,174 shares of Series D Convertible Preferred Stock to the lenders. Under the current terms of the agreement, if we elect to draw down the additional $12.5 million of commitment, we would be required to issue an additional 1,607,176 shares of Series D Convertible Preferred Stock to the lenders. Assuming successful completion of this offering, we do not intend to draw-down the second tranche of notes.
The $12.5 million of proceeds received were allocated to debt and the Series D Convertible Preferred Stock based on their fair values at the borrowing date with $1.9 million allocated to Series D Convertible Preferred Stock and the remaining $10.6 million allocated to debt. The discount on the debt of $1.9 million results in an effective interest rate on the debt of 21% and the discount will be amortized to interest expense over the term of the debt following the interest method.
Net cash used in operating activities was $12.7 million for the six month period ended June 30, 2007, compared to $9.3 million for the six month period ended June 30, 2006. The increase in cash used of $3.4 million was primarily due to an increase in research and development expenses of $2.3 million and an increase of sales, general and administrative expenses of $2.6 million. Net cash used in investing activities was $1.7 million for the six month period ended June 30, 2007, compared to $1.2 million for the six month period ended June 30, 2006. Cash from financing activities in the six month period ended June 30, 2007, contributed $12.3 million primarily from the debt borrowings described above. For the six month period ended June 30, 2006, cash from financing activities consisted of $48.1 million related to the closing of our Series D Convertible Preferred Stock financing. As part of the financing, bridge loans were converted into shares of our Series D Convertible Preferred Stock upon the closing of the Series D Convertible Preferred Stock financing. Such bridge loans were made by the following existing stockholders, R. Capital II, Ltd., Adam N. Mirkin, Rhoderic Peter Mirkin, Richard Segal, and Steven E. Mather in the aggregate principal amount of $0.1 million at a fixed annual interest rate of 10%, and on March 15, 2006, by Lurie Investment Fund, L.L.C. in the principal amount of $1.3 million at a fixed annual interest rate of 4.58% per year.
Net cash used in operating activities was $10.0 million, $15.0 million and $18.7 million for fiscal 2004, 2005 and 2006, respectively. Net cash used in operating activities for these periods consisted primarily of cash expended in connection with our research and development activities as well as general operating activities, partially offset by $5.8 million in revenues generated principally through government grants and contracts. Net cash used in investing activities was $2.1 million, $2.8 million and $3.9 million for fiscal 2004, 2005 and 2006, respectively. Our investing activities for these periods consisted primarily of purchases of property and equipment and investments in intellectual property, principally patent-related costs. Financing activities consisted primarily of the sale of ourSeries C-2 and Series D Convertible Preferred Stock, including the issuance of bridge notes in connection therewith, for fiscal 2004, 2005 and 2006.Series C-2 and Series D Convertible Preferred Stock issuances in those years raised $80.5 million.
We anticipate that our current cash and cash equivalents, together with the net proceeds of this offering, will be sufficient to meet our currently estimated needs for at least the next three years. However, we may need additional financing to execute on our current or future business strategies. We expect capital outlays and operating expenditures to increase over the next several years as we expand our infrastructure,
35
commercialization, manufacturing, and research and development activities. The amount of additional capital we may need to raise depends on many factors, including:
| | |
| | • | the level of research and development investment required to maintain and improve our technology; |
| |
| | • | the amount and growth rate, if any, of our revenues; |
| |
| | • | changes in product development plans needed to address any difficulties in manufacturing or commercializing the Verigene System and enhancements to our system; |
| |
| | • | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; |
| |
| | • | competing technological and market developments; |
| |
| | • | our need or decision to acquire or license complementary technologies or acquire complementary businesses; and |
| |
| | • | changes in regulatory policies or laws that affect our operations. |
We cannot be certain that additional capital will be available when and as needed or that our actual cash requirements will not be greater than anticipated. If we require additional capital at a time when investment in diagnostics companies or in the marketplace in general is limited due to the then prevailing market or other conditions, we may not be able to raise such funds at the time that we desire or any time thereafter. In addition, if we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly-issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain additional debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we raise additional funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products, or grant licenses on terms that are not favorable to us.
Income Taxes
Since inception, we have incurred operating losses and, accordingly, have not recorded a provision for income taxes for any of the periods presented. As of June 30, 2007, we had net operating loss carryforwards for federal and state income tax purposes of $76.8 million. We also had federal research and development tax credit carryforwards of $4.7 million. If not utilized, the federal net operating loss and tax credit carryforwards will expire beginning in 2020. Utilization of net operating loss and credit carryforwards may be subject to a substantial annual limitation due to restrictions contained in the Internal Revenue Code that are applicable if we experience an ownership change which may occur, for example, as a result of this offering being aggregated with certain other sales of our stock before or after this offering. The annual limitation may result in the expiration of our net operating loss and tax credit carryforwards before they can be used.
Contractual Obligations
As of December 31, 2006, the annual amounts of future minimum payments under certain of our contractual obligations were:
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period | |
Contractual Obligations | | Total | | | Less than 1 Year | | | 1-3 Years | | | 3-5 Years | | | More than 5 Years | |
| |
| Long-term debt obligations(1) | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Capital lease | | | 91,246 | | | | 32,444 | | | | 58,802 | | | | — | | | | — | |
| Operating lease | | | 1,743,735 | | | | 495,128 | | | | 1,027,708 | | | | 220,899 | | | | — | |
| Obligations under license agreements | | | 1,539,500 | | | | 197,500 | | | | 372,000 | | | | 245,000 | | | | 725,000 | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 3,374,481 | | | $ | 725,072 | | | $ | 1,458,510 | | | $ | 465,899 | | | $ | 725,000 | |
| | | | | | | | | | | | | | | | | | | | | |
36
| | |
| (1) | | Since December 31, 2006 we entered into a debt agreement, under which we borrowed $12.5 million. As part of this agreement we are obligated to pay principal and interest totaling $0.2 million, $3.6 million, $4.8 million and $3.9 million in 2007, 2008, 2009 and 2010, respectively. |
Our long-term commitment under our operating lease agreement shown above, which expires in 2010, consists of payments for our office and laboratory space. We have also entered into a capital lease agreement expiring in 2009 for a piece of laboratory equipment.
License Agreements
Through June 30, 2007, we have paid aggregate initial license fees of $1.8 million for these licenses, and have agreed to pay a percentage of net sales as royalties, in percentage amounts ranging from 1% to 12.0%. Certain of the license agreements have minimum annual royalty payments, and such minimum payments are as shown above. These licenses expire at various times, corresponding to the subject patents expirations, which currently range from 2009 to 2023.
In 2006, we entered into a new license agreement with Northwestern University, that replaced our prior agreement, and provides us with an exclusive license to certain patents and patent applications related to the application of nanotechnology to biodiagnostics and to biobarcode technology, which involves the analysis of oligonucleotides as reporter molecules, through January 1, 2013, after which date we have the right of first negotiation for an exclusive license on future inventions. Our research team utilizes the research and patents developed at Northwestern to develop diagnostic applications including additional genomic and protein testing assays for use in the Verigene System. See “Certain Relationships and Related Party Transactions.”
Off-Balance Sheet Arrangements
We do not have any off-balance sheet financing or unconsolidated special-purpose entities.
Critical Accounting Policies and Significant Judgments and Estimates
This discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in conformity with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as revenues and expenses during the reporting periods. We evaluate our estimates and judgments on an ongoing basis. We base our estimates on historical experience and on various other factors we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could therefore differ materially from those estimates under different assumptions or conditions.
Our significant accounting policies are described in Note 2 to our consolidated financial statements included elsewhere in this prospectus. We believe the following critical accounting policies reflect our more significant estimates and assumptions used in the preparation of our financial statements.
Revenue Recognition
We are in the development stage and have generated limited revenues since our inception. We recognize revenue under grants and contracts and for reimbursement of related research and development expenses at the time the relevant expenses are incurred. For product sales, revenue is recognized when persuasive evidence of an arrangement exists, title and risk of loss is transferred to customers, the price to the buyer is fixed or determinable, and collectibility is reasonably assured.
Verigene System instrument units are sold outright to customers or via sales-type lease arrangement. Verigene System units will also be leased to customers pursuant to operating leases. We recognize revenue
37
from sales of the Verigene System, including cartridges and related products generally upon shipment or, in certain transactions, upon customer acceptance. Revenue and related costs for Verigene System instrument units sold via sales-type leases are generally recognized at the time of shipment based on the present value of the minimum lease payments with interest income recognized over the life of the lease using the interest method. Under operating lease arrangements, revenue is recognized on an installment basis over the life of the lease while the cost of the leased equipment is carried on the Company’s balance sheet and amortized over its estimated useful life. To date, we have not sold any products via sales-type leases.
Stock-Based Compensation Expense
Effective January 1, 2005, we adopted, on a prospective basis, the provisions of SFAS No. 123 (Revised), Share-Based Payment, or SFAS No. 123(R), which provides for recognition of compensation expense based on the fair value of the stock-based compensation utilizing various assumptions regarding the underlying attributes of the options and stock. The estimated fair value of options granted, net of expected forfeitures expected to occur during the vesting period, is amortized as compensation expense on a straight-line basis over the vesting period of the options. Previously, we applied the provisions of SFAS No. 123 to our stock-based compensation and adoption of SFAS No. 123(R) did not have a material impact on our financial position, results of operations, or cash flows.
We have granted share-based compensation consisting of common stock options issued to employees, consultants and founders. Compensation expense is recognized based on the fair value of the stock-based awards granted utilizing various assumptions regarding the underlying attributes of the options and our common stock. The estimated fair value of options granted, net of forfeitures expected to occur during the vesting period, is determined using the Black-Scholes option-pricing model and then amortized as compensation expense on a straight-line basis over the vesting period of the options. All of the stock options granted have exercise prices at or above the estimated fair value of the common stock on the date of grant, as determined by our board of directors, who use their knowledge of us and our affairs along with third-party valuation assessments, to determine the fair value of our common stock. In addition to the grant date fair value of our common stock, the Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Since we do not have a history of traded common stock activity, expected volatility of the options is based on historical data from various peer public companies with product portfolios similar to ours. The expected life of options granted is derived from the average of the vesting period and the term of the option following the guidance in SEC Staff Accounting Bulletin No. 107. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant.
Convertible Derivative Liability
Our Series C-2 and Series D Convertible Preferred Stock contains conversion features which result in an embedded derivative that requires bifurcation and accounting as an embedded derivative pursuant to paragraph 12 of Statement of Financial Accounting Standards No. 133, “Accounting for Derivative Instruments and Hedging Activities” separate and distinct from the convertible preferred stock. The fair value of the convertible derivative liability is determined, at each reporting date, using the option pricing method detailed in the AICPA Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation.”
Key inputs for the option pricing model include the current price of our convertible preferred stock (based on an estimate of our enterprise value or the measurement date), the dividend yield on the common stock into which the preferred stock can convert, the risk-free interest rate, volatility, the time to maturity, (which reflects the expected time to liquidity), and a discount for lack of marketability. The determination of the value of the stock, the dividend yield, the risk-free interest rate and volatility are all determined in a manner consistent with the approval used in computing our stock-based compensation expense. The term of the redemption feature embedded in the convertible preferred stock was deemed as the best-expected term. An at-the-money put option analysis was used to determine the lack of marketability discount.
38
Recent Accounting Pronouncements
In June 2006, the FASB issued FASB Interpretation No. 48, Accounting for Uncertainty in Income Taxes-an interpretation of FASB Statement No. 109, or FIN No. 48, which clarifies the accounting for uncertainty in tax positions. FIN No. 48 requires that we recognize in our financial statements the impact of a tax position if that position is more likely than not of being sustained upon examination, based on the technical merits of the position. The provisions of FIN No. 48 were effective for us as of January 1, 2007, with the cumulative effect of the change in accounting principle, if any, recorded as an adjustment to opening retained earnings. The adoption of FIN No. 48 did not have an impact on our financial position, results of operations or cash flows.
In September 2006, the FASB issued SFAS No. 157, Fair Value Measurements. SFAS No. 157 defines fair value, establishes a framework for measuring fair value and expands disclosure of fair value measurements. SFAS No. 157 applies under other accounting pronouncements that require or permit fair value measurements and accordingly, does not require any new fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007. We are currently evaluating the impact of this pronouncement on our financial statements.
In February 2007, the FASB issued SFAS No. 159, The Fair Value Option for Financial Assets and Financial Liabilities. SFAS No. 159 permits entities to choose, at specified election dates, to measure eligible items at fair value (“fair value option”) and to report in earnings unrealized gains and losses on those items for which the fair value option has been elected. SFAS No. 159 also requires entities to display the fair value of those assets and liabilities on the face of the balance sheet. SFAS No. 159 establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar types of assets and liabilities. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year beginning after November 15, 2007. Early adoption is permitted as of the beginning of the previous fiscal year provided that the entity makes that choice in the first 120 days of that fiscal year and also elects to apply the provisions of Statement 157. We are currently evaluating the impact of this pronouncement on our financial statements.
Qualitative and Quantitative Disclosures about Market Risk
Our exposure to market risk is currently confined to our cash and cash equivalents. We have not used derivative financial instruments for speculation or trading purposes. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while at the same time maximizing the income we receive from our investments without significantly increasing risk. To achieve these objectives, our investment policy allows us to maintain a portfolio of cash equivalents and short-term investments through a variety of securities, including commercial paper, money market funds and corporate debt securities. Our cash and cash equivalents through December 31, 2006 included amounts in bank checking and liquid money market accounts. As a result, we believe we have minimal interest rate risk; however, a one percentage point decrease in the average interest rate on our portfolio would have reduced interest income for 2006 by $282,442.
Controls and Procedures
Our independent registered public accounting firm was not engaged to perform an audit of our internal control over financial reporting. Our independent registered public accounting firm’s audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of our internal control over financial reporting. Accordingly, no such opinion was expressed. However, in connection with the audit of our consolidated financial statements for the six month period ended June 30, 2007, we and our independent registered public accounting firm noted two material weaknesses and a significant deficiency in our internal controls over financial reporting.
The Public Company Accounting Oversight Board, or PCAOB, defines material weakness as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The PCAOB defines a significant deficiency as a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material
39
weakness, yet important enough to merit attention by those responsible for oversight of the company’s financial reporting.
In its report to our audit committee, dated September 26, 2007, our independent registered public accounting firm noted material weaknesses relating to internal controls over financial reporting, specifically, that we did not have:
| | |
| | • | sufficient personnel with appropriate financial accounting and reporting expertise; |
| |
| | • | sufficient segregation of duties due to our limited number of finance and accounting personnel; |
| |
| | • | sufficient internal controls for addressing accounting for complex transactions, such as those related to our equity transactions; |
| |
| | • | a formal process and related controls to identify and appropriately record and disclose certain contractual obligations, such as licenses and other agreements; and |
| |
| | • | internal controls related to cutoff, impacting the accounting and reporting of inventory, prepaid expenses, accounts payable and accrued expenses. |
In addition, our independent registered public accounting firm identified a significant deficiency in our internal controls over financial reporting relating to our failure to maintain a perpetual record of our inventory of purchased components that formally tracks inventory during interim financial periods. Since the date of our independent registered public accounting firm’s report on our financial statements and through the date of this prospectus, we have taken steps intended to remediate the material weaknesses and significant deficiency, primarily through the implementation of additional controls and procedures and the hiring of additional accounting personnel. Commencing with our fiscal year ending December 31, 2008, we must perform system and process evaluations and testing of our internal control over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal control over financial reporting, as required under Section 404 of the Sarbanes-Oxley Act.
The actions we plan to take are subject to continued management review supported by confirmation and testing, as well as audit committee oversight. While we expect to remediate the material weaknesses and significant deficiency, we cannot assure you that we will be able to do so in a timely manner, which could impair our ability to accurately and timely report our financial position, results of operations or cash flows. See “Risk Factors — Risk Relating to Our Business — we and our independent registered public accounting firm have identified material weaknesses in our internal control over financial reporting. Failure to achieve and maintain effective internal control over financial reporting could result in our failure to accurately report our financial results.”
40
Overview
Nanosphere develops, manufactures and markets an advanced molecular diagnostics platform, the Verigene System, that enables simple, low cost and highly sensitive genomic and protein testing on a single platform. Our proprietary nanoparticle technology simplifies the ability to perform molecular diagnostic tests, achieves ultra-sensitive protein detection at limits beyond current diagnostic technologies, provides the ability to multiplex, or run multiple tests at the same time on the same sample, and enables the development of a broad menu of test assays to be performed on a single platform. We are currently developing diagnostic tests for a variety of medical conditions including cancer, neurodegenerative, cardiovascular and infectious diseases, as well as pharmaco-genomics, or tests for personalized medicine. There is a growing demand among laboratories to implement molecular diagnostic capabilities, but the cost and complexity of existing technologies and the need for specialized personnel and facilities have limited the number of laboratories with these capabilities. We believe that the Verigene System’s ease of use, rapid turnaround times, relatively low cost and ability to support a broad test menu will simplify work flow and reduce costs for laboratories already performing molecular diagnostic testing and allow a broader range of laboratories, including those operated by local hospitals, to perform molecular diagnostic testing. Our ability to detect proteins, which is at least 100 times more sensitive than current technologies, may enable earlier detection of and intervention in diseases associated with known biomarkers and the introduction of tests for new biomarkers that exist in concentrations too low to be detected by current technologies.
We received 510(k) clearance from the United States Food and Drug Administration, or FDA, for commercial sale of the Verigene System in September 2007. We also received clearance for two diagnostic tests: our warfarin metabolism assay, which is a pharmaco-genomic test to determine how an individual metabolizes the drug warfarin, including Coumadin, the most-prescribed oral anticoagulant in North America and Europe, and our hyper-coagulation test, one of the highest volume genetic tests currently performed, to determine an individual’s risk for the development of blood clots in September 2007 and October 2007, respectively. Upon receipt of FDA clearance, we commenced sales to hospital-based laboratories and academic research institutions in the United States, which we believe is the primary market for our products. We have established a direct sales organization within the United States and are focusing our initial commercial efforts on the hospital-based laboratory market. Our minimal revenues to date have been derived from the sale of the Verigene System, including cartridges and related products, within the United States to research laboratories and pursuant to government contracts.
We are currently developing diagnostic tests for a variety of medical conditions including cancer, neurodegenerative, cardiovascular and infectious diseases, as well as pharmaco-genomics, or tests for personalized medicine. We anticipate that we will submit applications to the FDA for clearance of tests for cystic fibrosis, herpes, cervical cancer, respiratory illness, recurrent prostate cancer and cardiovascular disease during the next 36 months, and we anticipate that we will submit two of such tests within the next 12 months. In addition, we have an active program to develop protein tests based on established biomarkers and to validate new biomarkers where our ultra-sensitive protein detection technology may enable earlier detection of a broad range of diseases. Through our biomarker validation program, we are working to validate novel protein targets for Alzheimer’s disease, stroke, sepsis and kidney disease, which we believe will lead to new protein-based diagnostic tests.
Our technology is broadly applicable beyond the clinical diagnostic market in both research and industrial applications. For over two years the Verigene System has been in use in research laboratories supporting collaborations and independent research in areas including ovarian cancer, mad cow disease and HIV. We are currently working with the FDA on a joint research program to develop an H5N1 avian flu assay. We have developed and delivered a biosecurity platform for the detection of various bioterrorism agents to the Technical Support Working Group, an agency affiliated with the U.S. Department of Defense.
Currently, our patent portfolio is comprised, on a worldwide basis, of 80 issued patents and 150 pending patent applications which, in either case, we own directly or for which we are the exclusive licensee. We
41
exclusively licensed our initial core technology from the International Institute for Nanotechnology at Northwestern University in May 2000. This formed the basis for a sustained relationship with Northwestern whereby we have rights to future developments in the field of biodiagnostics. This relationship provides us with access to ongoing research and innovation which we utilize in our research and development of new applications and products.
Limitations of Existing Technologies
Industry Background
In vitro diagnostic tests are used to detect the presence and quantity of certain substances in biological samples to diagnose disease, monitor and guide treatment, and assist in managing chronic conditions. The global market for in vitro diagnostic products was estimated to be $34 billion in 2006 according to Boston Biomedical Consultants. Molecular diagnostics is a new and expanding part of the in vitro diagnostics market that emerged in response to a need for more rapid, sensitive and specific diagnostic tests than were previously available using traditional techniques, such as immunoassays. Our current market opportunity is more than $3.0 billion, including the $2.3 billion molecular diagnostics market and our estimated market for our initial protein assays. According to Boston Biomedical Consultants, the genomic market grew 11% in 2006. This market is comprised primarily of infectious disease and human genetic tests, and excludes protein tests. Market growth has been driven primarily by conversion of traditional testing methods to molecular methods. In addition to the continuation of this conversion, we believe that four primary trends will expand the market for molecular and other advanced testing:
| | |
| | • | Expanded Human Genetic Testing. Advances in the understanding of the role genes play in disease have led to the emergence of human genetic tests that today are primarily used in prenatal screening for inherited diseases such as Down Syndrome or in perinatal testing for diseases such as cystic fibrosis. As technology advances, we expect a growing number of genetic tests will emerge that will be able to establish an individual’s predisposition to a wide range of diseases such as cancer. |
| |
| | • | Pharmaco-genomics. There is growing recognition of the role that genetics play in how well individuals tolerate and respond to many drugs. As a result, we expect growing use of genetic testing to guide personalized medicine. |
| |
| | • | Protein Testing. As in the field of molecular diagnostics, the introduction of more sensitive testing technologies is expected to revolutionize protein testing by enabling physicians to detect target proteins at lower concentrations than can be detected using traditional technologies. This is expected to expand the market for tests associated with existing biomarkers and drive the emergence of new tests for biomarkers that cannot be detected using current technologies. |
| |
| | • | Convergence of Genomic and Protein Testing. The growing medical understanding of the inter-relationship between genetics and proteins in disease states will drive a convergence of genomic and protein testing. |
The Genomic Testing Market
Background on Genomic Testing
Nucleic acids, DNA and RNA, are molecules found inside cells and viruses that contain genes, the unique blueprint of each living creature. The diversity of living organisms results from variability in genetic content which is determined by the sequence of four nucleotide bases that form the chemical building blocks of DNA and encode an organism’s genetic instructions. Variability can also be the result of differences in gene expression, the process by which a gene’s DNA sequence is converted into proteins that in turn regulate or perform most of the physiological functions of the body. Variations or mutations in the sequences of genes may be introduced by environmental or other factors, such as errors in the replication of genes. Mutations in individual genes have been associated with diseases such as cystic fibrosis, and mutations in multiple genes have been associated with diseases such as cancer, cardiovascular disease and drug metabolism. Most molecular diagnostic tests target only one or a few genes.
42
The Limitations of Current Genomic Testing Methods
Over the past 20 years, scientists have developed a variety of genomic analysis methods, including DNA sequencing, gene expression analysis and genotyping, to measure an ever-increasing number of genomic biomarkers and to more effectively detect diseases. The most widely used method for genetic testing is polymerase chain reaction, or PCR, which involves amplifying, or generating billions of copies of, the DNA sequence in question and then detecting the DNA with the use of fluorescent dyes.
Due to the complexity, susceptibility to contamination and significant costs related to PCR and other existing technologies, the genomic testing market remains limited to reference laboratories, research facilities and laboratories associated with the top 200 to 300 hospitals, primarily at academic teaching institutions.
A typical molecular diagnostics laboratory in a hospital or research laboratory setting is a dedicated facility that employs highly skilled technologists and is supervised by a technician with a Ph.D. or M.D./Ph.D. To guard against contamination, which is a common result of target amplification, a typical laboratory will require at least three separate rooms, or isolation areas, to perform PCR-based assay methods for genomic testing. Moreover, due to the limited capability of many existing technologies, numerous testing platforms are required to perform even a limited menu of tests. In addition, due to the complexity of test procedures and the cost of reagents and supplies, tests are typically batch processed, often only on a weekly basis. These issues require molecular diagnostics laboratories to maintain multiple pieces of equipment, each with a very limited testing menu, creating a laboratory model that is complex, costly and one that demands a significant commitment of highly specialized and skilled laboratory technologists due to the training required to utilize the numerous techniques and pieces of equipment and the significant amount of technologist time required by current test systems. Moreover, these target amplification technologies continue to lack the capacity to run multiple tests at the same time on the same sample, or multiplex, in a cost effective manner.
The Protein Testing Market
Background on Protein Testing
Proteins are one of the primary structural and functional components of the human body. While genes are typically associated with presence or absence of disease states, proteins often reflect the activity of a disease state. Therefore, many diseases are both diagnosed as well as monitored at the protein level, rather than at the genetic level. Proteins often serve as the primary biomarkers for detecting disease states such as post-surgical recurrent prostate cancer or cardiovascular disease, and monitoring the progress of these diseases during treatment. As a result, the simultaneous detection of both protein biomarkers and genomic biomarkers is emerging as a way to more comprehensively diagnose and monitor the entire disease process.
The Limitations of Current Protein Testing Methods
Protein detection methods have been evolving over the last 40 years. The most widely used method for protein testing is enzyme-linked immunosorbent assay, or ELISA, which was the first method that allowed for widespread use of protein detection for discovery and diagnostic applications. ELISA and similar predecessor technologies have proven effective in detecting many of the common protein markers associated with diseases including some forms of cancer, cardiovascular disease and various infectious diseases. However, they are often not sufficiently sensitive to detect the protein biomarkers until the disease has progressed to an advanced stage and the need for greater sensitivity has become apparent in protein detection for biomarkers, drug discovery and diagnostics. Moreover, biomarkers for medical conditions such as stroke, various forms of cancer and neurodegenerative diseases such as Alzheimer’s have not been validated or commercialized because they exist in concentrations too low to be detected by current technologies.
As a result of this limitation, an alternative approach to protein detection called mass spectrometry has been used in research laboratories to detect protein biomarkers undetectable with ELISA technology. Mass spectrometry systems first purify the sample and then break the proteins down into pieces. Each protein constituent is then injected into the mass spectrometer, where it is ionized and information regarding mass and charge data is then extracted from the system. Although mass spectrometry is highly sensitive, it is extremely
43
costly and requires significant time and effort by highly trained personnel. Additionally, mass spectrometry is not able to detect long peptide chain proteins or misfolded proteins, which are biomarkers for diseases such as mad cow and Alzheimer’s, and therefore is not practical for commercial molecular diagnostics.
Our Solution
We are commercializing a platform based on our proprietary nanotechnology that we believe will significantly advance and expand the market for molecular diagnostic testing. Our technology makes possible the combination of direct genomic detection and ultra-sensitive protein detection on one simple to use, low cost platform. Our technology will enable faster and simpler genomic detection than other currently used genomic testing technologies. In addition, our ultra-sensitive protein detection technology, which is at least 100 times more sensitive than ELISA, will enable earlier detection of disease and the validation and commercialization of new biomarkers where no test exists today.
The Verigene System
The Verigene System is a bench-top workstation, which is comprised of a microfluidics processor, a touch screen reader, and disposable test cartridges. With a prepared sample, the Verigene System completes tests in 45 to 90 minutes and requires less than 20 minutes of technician time. Specifically, the system incorporates several key features which we believe will make it attractive to a wide range of laboratories, including:
| | |
| | • | Low Cost and Complexity. The Verigene System is a low cost platform without the need for sophisticated instrumentation or complex reagent kits. The versatility of the cartridge-based design eliminates the need for multiple testing platforms. Automation of key process steps eliminates manual intervention and algorithms provide test results without the need for operator interpretation or data manipulation. By eliminating the need for skilled technicians and multiple complex platforms, costs are reduced, work flow is simplified and molecular diagnostic testing can be decentralized to a greater number of laboratories around the world. |
| |
| | • | On Demand Testing. The Verigene System allows laboratories to economically run tests at the time they are ordered, unlike other systems where laboratories must process patient samples in batches to control reagent and labor costs. This results in faster turn-around times and potentially improved patient care. |
| |
| | • | Multiplexing. The Verigene System enables high count multiplexing, or the ability to identify a large number of target molecules on the same sample in a single assay. Potential applications include combination genomic and protein test panels and cartridges that allow for the simultaneous detection of multiple genetic mutations associated with complex diseases such as cystic fibrosis. |
| |
| | • | Direct Genomic Detection. The Verigene System utilizes a proprietary method to detect nucleic acids with greater specificity and without the complexity and risk of contamination inherent in the use of amplification techniques such as PCR, thereby increasing the reliability of test results. |
| |
| | • | Ultra-Sensitive Protein Detection. The Verigene System allows ultra-sensitive detection of proteins with at least 100 times greater sensitivity than current technologies such as ELISA. This may enable earlier detection of disease, potentially improving clinical outcomes and enabling the development of completely novel tests. |
The combination of ease of use, improved laboratory work flow, high sensitivity and a versatile test menu, including genomic and protein testing, makes our system a significant improvement for laboratories and enables broad market decentralization of molecular diagnostic testing.
44
Strategy
Our goal is to establish a new standard in molecular diagnostics characterized by our low cost, easy to use platform for genomic and protein testing and to develop new diagnostic tests where none exists today. To achieve this objective, we intend to:
| | |
| | • | Target Key Customer Segments. We will focus our sales efforts on hospital laboratories, where there is large and growing demand for molecular diagnostic testing, but where cost, complexity and resource requirements of existing technologies have limited their ability to process tests in-house. We will emphasize the ease of use, bench-top convenience and high quality and consistent results of our system, as well as the flexibility afforded by a broad test menu on a single platform. |
| |
| | • | Employ a Direct Sales Force Model. We are currently marketing and selling the Verigene System through our own sales and marketing organization, which is currently comprised of 19 people, including sales representatives, clinical support staff and product managers. In our experience, technical sales of new technologies such as ours are best accomplished directly through an in-house organization, which allows us to control the pace of our commercialization. |
| |
| | • | Market FDA Cleared Products. We will seek FDA clearance for all of our products. Many tests currently on the market are sold as analyte specific reagents, or ASR, assays, which laboratories use to create “home-brew” tests. We believe that there is strong market demand for FDA cleared tests because FDA cleared tests require less skilled laboratory technician time and do not subject the laboratory to the additional regulatory requirements imposed on laboratories using “home-brew” tests. Marketing only FDA cleared products will expand diagnostic testing into smaller hospitals and other laboratories that lack the capability to develop their own “home-brew” tests or do not have laboratories that meet the higher quality standards imposed on laboratories performing “home-brew” tests. |
| |
| | • | Establish a Broad Test Menu. We are developing a broad genomic and protein test menu for the Verigene System, focusing on assays that are either already in high demand or projected to experience rapid growth. This will maximize the value of the Verigene System and support placements of systems in those laboratories that demand a broad testing menu before implementing a new testing platform. Likewise, for those laboratories that implement our system with a narrower initial test menu, an expanding range of tests will drive incremental cartridge sales. |
| |
| | • | Utilize Our Ultra-Sensitive Protein Detection Methods to Validate New Biomarkers and Commercialize New Diagnostic Tests. We are applying our ultra-sensitive protein detection methods to the development of established protein biomarkers and the validation of novel protein targets that may lead to earlier detection of medical conditions including cancer, neurodegenerative disorders including Alzheimer’s disease, sepsis and mad cow disease, for blood screening and veterinary applications. |
| | |
| | • | Capitalize on Strong Intellectual Property and Development Capabilities. We will continue to develop our product capabilities based on our strong in-house and licensed intellectual property to expand the utility of established biomarkers and enable the creation of entirely new tests by validating new biomarkers. Our ongoing relationship with the International Institute for Nanotechnology at Northwestern University and our internal expertise will allow us to develop new genomic and protein assays for the Verigene System. Currently we have six tests in development beyond those recently cleared by the FDA. |
We are focusing our efforts on the clinical diagnostics market, and may seek opportunities, either directly or through outlicensing arrangements, to commercialize our technology in the industrial, biosecurity and other markets.
Our Products
The Verigene System is a bench-top molecular diagnostics workstation that is a universal platform for genomic and protein testing. While many systems currently available on the market provide a diagnostic result
45
for one test or specific niche, the Verigene System provides for multiple tests to be performed on a single platform including genomic and protein assays.
The Verigene System is comprised of a microfluidics processor, a touchscreen reader and disposable test cartridges. The microfluidics processor interacts with and manipulates various functional components of the test cartridge, including target binding to the nucleic acid or protein array, nanoparticle probe hybridization, intermediate washes and signal amplification steps. The reader houses the optical detection module that illuminates the test slide and automated spot recognition software analyzes the resulting signal intensities and provides the test results. The reader also serves as the control station and has a simple and intuitive touchscreen interface that allows users to track samples and test cartridges, initiate and monitor test processing, analyze results and generate reports. The reader is web-enabled to allow remote access to results and reports.
To perform a test, the operator adds a prepared sample to a designated port in the test cartridge, enters sample identification and test cartridge information into the reader using the touchscreen keyboard or via the barcode wand, and inserts the test cartridge into the processor. The processor assimilates information received from the reader and matches it to the inserted test cartridge and initiates the specified test protocol. Once the assay process is complete the test array is introduced into the reader for image analysis and result reporting.
Our Applications
We are commercializing or have in development several genomic and protein assays including the following:
| | | | | |
| |
| Assay | | Status | | Condition |
| |
| |
Genomic Tests | | | | |
| Hyper-coagulation | | FDA clearance received October 2007 | | Increased risk of blood clots, stroke and pulmonary embolism |
| Warfarin Metabolism | | FDA clearance received September 2007 | | Initial dosing of leading anticoagulant |
| Cystic Fibrosis | | In development | | Cystic fibrosis gene mutation; prenatal carrier screening |
| Herpes Simplex Virus | | In development | | Herpes viral infection |
| Human Papillomavirus | | In development | | Cervical cancer |
| Respiratory Virus Panel | | In development | | Respiratory illness |
| | | | | |
Protein Tests | | | | |
| Prostate Specific Antigen | | In development | | Post-surgical recurrent prostate cancer |
| Cardiac Troponin I | | In development | | Cardiovascular disease; risk stratification; diagnosis of heart attack |
| |
| |
We anticipate that we will submit applications to the FDA for clearance of two additional tests within the next 12 months and all of the tests listed above that are in development during the next 36 months. We are also continuing to research the expansion of our test menu in areas including, cancer, neurodegenerative, cardiovascular and infectious diseases and pharmaco-genomics. Specifically, we are researching assays for the detection of sexually transmitted diseases associated with chlamydia, gonorrhea and HIV.
Genomic Assays
Hyper-coagulation. This assay is for the detection of genetic mutations associated with an increased risk for the development of blood clots. Blood clots can travel to the lungs, resulting in a life threatening pulmonary embolism, and according to the National Hemophilia Foundation, more than 600,000 Americans die from abnormal blood clots each year. Patients that test positively for an increased risk of blood clots can be managed with anticoagulant therapy such as warfarin. Hyper-coagulation tests for mutations associated with a pre-disposition to blood clots are currently among the most frequently conducted human genetic tests.
46
Currently available technologies for this test are limited by contamination issues associated with PCR, and by complex and costly work flow including test time, lab technician time and the number of process steps. Our hyper-coagulation panel consists of a multiplex of three genetic markers. This test enables the direct detection of mutations associated with a pre-disposition to blood clots on a much simpler platform than current alternatives. Our hyper-coagulation assay received 510(k) clearance from the FDA in October 2007.
Warfarin Metabolism. This assay is a pharmaco-genomic test for the detection of genetic mutations that determine an individual’s ability to metabolize the oral anticoagulant warfarin, including Coumadin. Warfarin decreases the blood’s clotting ability and is the most widely prescribed oral anticoagulant in North America and Europe, with more than 12 million new prescriptions from July 2006 to June 2007 in the United States alone according to IMS Health Incorporated. Individuals metabolize warfarin differently, and if its administration is not managed carefully, life threatening side effects may occur. For example, patients that metabolize warfarin slowly will experience dangerously high levels of warfarin in the body which can lead to serious bleeding complications. The FDA has indicated that warfarin is among the top 10 drugs with the largest number of serious adverse event reports during this decade and it is recommended that regular monitoring be performed on all patients who receive warfarin. According to the FDA, warfarin is the second most common drug, after insulin, implicated in emergency room visits for adverse drug events.
Our warfarin metabolism assay received 510(k) clearance from the FDA in September 2007. Our assay is the first, and currently the only, FDA cleared genetic diagnostic test to assess warfarin metabolism. The only other assays available today to detect mutations associated with warfarin metabolism are those developed in-house by individual laboratories, known as “home-brew” tests, which are more complex than traditional diagnostic tests. They are based on analyte specific reagent kits, which are not cleared for marketing by the FDA.
Our warfarin metabolism panel detects three genetic markers that play a critical role in metabolizing warfarin. Through detection of these genetic markers, doctors are able to determine the appropriate initial warfarin dosage level in a safer and more efficient manner than current methods.
Cystic Fibrosis. This assay is currently in development. We anticipate that it will provide a multiplex panel for the detection of mutations in the CFTR gene that contribute to a higher risk of cystic fibrosis. Cystic fibrosis is an inherited chronic disease that affects the lungs and digestive system of about 30,000 children and adults in the United States and 70,000 worldwide, according to the Cystic Fibrosis Foundation. More than 10 million Americans are symptomless carriers of the defective cystic fibrosis gene. The American College of Medical Genetics and The American College of Obstetricians and Gynecologists recommend testing for 23 mutations associated with this risk as part of prenatal carrier screening. According to the United States Center for Disease Control, or CDC, there are over 4 million pregnancies annually in the United States.
Currently available technologies for this test are limited by complex and costly work flows including test time, lab technician time and the number of process steps. Our direct detection method enables each of the 23 mutations to be detected in a single test cartridge. Through detection of these genetic markers, physicians will be able to reliably diagnose, and determine the predisposition for this disease without the need for additional testing.
Herpes Simplex Virus. This assay is currently in development. We anticipate that it will provide a multiplex panel for the detection of this common viral infection that targets the oral, genital and central nervous system. According to the CDC, over 45 million Americans, or one out of five adolescents and adults, are infected by the genital herpes simplex virus.
Human Papillomavirus, or HPV. This assay is currently under development and we anticipate that it will provide a multiplex panel for the detection of 14 specific viral strains that are sexually transmitted and are the cause of 95% of cervical cancer cases worldwide. Any sexually active woman is at risk of infection and, according to the CDC, more than 50% of such women become infected with HPV, which exhibits no symptoms. In the United States, more than 20 million people are infected with HPV. We are working to develop a strain specific diagnostic test that will detect all of the high risk viral types in a cartridge based assay, allowing for better clinical diagnosis in a decentralized setting.
47
Respiratory Virus Panel. This assay is currently under development. We anticipate that it will provide a multiplex panel for the detection of both the most common and variant forms of respiratory virus infections and provide a definitive diagnosis in less than two hours. According to the CDC, in the United States, 5% to 20% of the population gets the flu, more than 200,000 people are hospitalized from flu complications, and about 36,000 people die annually. Despite this need, the market is currently underserved because rapid tests lack the necessary specificity to provide a definitive diagnosis and traditional microbiology is extremely time-consuming.
Protein Assays
Our initial assay development efforts are focused on the earlier detection of disease through application of our ultra-sensitive protein detection technology to existing biomarkers. The following assays are in development.
Prostate Specific Antigen — Recurrent Prostate Cancer. This assay is currently in development. We anticipate that this assay will enable the early detection of recurrent prostate cancer in men following prostate removal, a standard treatment for prostate cancer. According to the Prostate Cancer Foundation, prostate cancer is the most common non-skin cancer in America, affecting one in six men. In the United States in 2007, more than 218,000 men will be diagnosed with prostate cancer, and more than 27,000 men will die from the disease. The American Cancer Society recommends that all men begin annual prostate specific antigen testing by age 50.
Prostate specific antigen, or PSA, is a protein produced by the cells of the prostate gland and may be found in an increased amount in those with prostate cancer. However, despite regular PSA testing post-surgery, current technologies on average can only diagnose recurrence three and a half years later, even though forty percent of patients suffer recurrence. Because early stage prostate cancer recurrence may not cause PSA to increase to currently detectable levels. We expect that our ultra-sensitive PSA detection assay will diagnose recurrence within a few months, rather than years, after surgery. We also believe that our test will have the ability to determine which patients will not have a recurrence of the disease, relieving some of the burden on caregivers and patients. We are in the process of transferring this assay to the Verigene System format.
Cardiac Troponin I. This assay is currently in development. This assay is being developed for the detection of cardiac troponin I in patients suspected of having cardiovascular disease. According to the American Heart Association, coronary disease is the leading cause of death in the United States, and in adults, heart attacks, or myocardial infarctions, cause one out of every five deaths. The National Institutes of Health estimate that more than 1.2 million cases of myocardial infarction occur each year in the United States and about 460,000 of these are fatal. Although there are various diagnostic tests used to detect acute myocardial infarction, both the American College of Cardiology and the American Heart Association issued guidelines asserting that testing for cardiac troponin is the new, definitive laboratory standard for the diagnosis of myocardial infarction. Troponin assays are not only more sensitive but also more specific than tests for other existing biomarkers.
Troponin I is a protein that is found in cardiac muscle and is released when the heart is injured, for instance during a myocardial infarction. Cardiac troponin blood diagnostic tests are used to diagnose a heart attack and evaluate mild to severe heart injury in patients experiencing heart/chest discomfort. However, limitations of current detection levels often result in the failure to accurately diagnose all cases of cardiovascular disease. Each year, millions of patients with chest pain are discharged from hospital emergency departments after current technologies fail to diagnose acute myocardial infarctions. Tens of thousands of these patients are subsequently readmitted to the hospital or die from cardiac arrest within a few weeks or months after the initial visit.
Current methods of troponin testing are only sensitive to concentrations at least 100 times higher than the true normal, which is zero level in healthy patients. As a result, no approved test exists that can identify a patient who may be experiencing transient ischemic events, which are precursors to a cardiac event. Our initial clinical studies have demonstrated our ability to reliably identify a rise in cardiac troponin well below the current limits of detection, at levels where the assay can diagnose acute myocardial infarctions earlier and detect precursor cardiac events including unstable angina, cardiac necrosis and ischemia. Furthermore, this
48
level of sensitivity could also lead to more accurate risk stratification associated with angina. We believe that a new definition of “normal” will be defined at a lower troponin concentration level that the current market cannot detect. We are also in the process of performing additional research to determine new diagnostic attributes of the assay, including its predictive value.
Biomarker Validation. We are also applying our ultra-sensitive protein detection methods to the development of established protein biomarkers and the validation of novel protein targets that may lead to earlier detection of medical conditions including in the areas of cancer, neurodegenerative disorders including Alzheimer’s disease, sepsis and transmissible spongiform encephalopathy, or mad cow disease, for blood screening and veterinary applications.
Our Technology
We believe our technology will drive the use of ultra-sensitive and multiplexed protein and genomic diagnostics in routine clinical laboratories, much like ELISA accelerated the use of protein testing in the 1970s and 1980s and PCR catalyzed the emergence of nucleic acid diagnostics in the 1990s.
Our Gold Nanoparticle Molecular Probes
At the core of our technology are gold nanoparticles which offer a unique set of physical properties that can be exploited in the detection of biological molecules. In 1998, Dr. Mirkin and Dr. Letsinger at Northwestern University developed a novel process to prepare stable probes by covalently attaching oligonucleotides to gold nanoparticles. This method, protected by patents, is exclusively assigned to or owned by us. We have refined the synthesis methods to enable highly reproducible production of nanoparticle probes with diameters in the13-50 nanometer range required for highly sensitive biomedical analysis. Subsequently, we have also developed methods for attaching antibodies to gold nanoparticles, thereby producing highly stable probes for ultra-sensitive detection of proteins.
The properties of nanoparticle probes can be tailored by controlling the size of the particles, the density of recognition-oligomers or antibodies on the nanoparticles, the use of diluent oligonucleotides, the use of spacer oligonucleotides and the salt concentration. Combined, the optimization of these properties enables us to deliver superior analytical performance characteristics versus other methods, for example:
| | |
| | • | High Signal-to-Noise Ratio. Our nanoparticle probes deliver significantly stronger signals than the fluorescent probes, or fluorophores, used in diagnostic platforms today. Nanoparticles are typically10-100 nm in diameter and therefore significantly larger than conventional fluorophores. This size difference enables nanoparticles to produce up to 10,000 times more signal via light scattering than a fluorophore. A single nanoparticle can be detected with simple optical instrumentation with very high sensitivity, thus eliminating the need to employ our amplification techniques. |
| |
| | • | Orders of Magnitude Greater Sensitivity and Lower Detection Limits. The sensitivity and limits of detection of our technology are further enhanced by a silver-staining step, which effectively amplifies the signal from each nanoparticle bound to a target molecule. In this process, silver is coated onto the gold nanoparticle surface, producing larger particles with enhanced optical properties. Whereas the leading technologies today can detect molecules at the picomolar range (10-12), our technology is capable of up to a million times higher sensitivity at the femtomolar (10-15) and attomolar (10-18) range, enabling the unprecedented analysis of rarely expressed genes or low abundance proteins for early disease detection and diagnosis. |
| |
| | • | High Count Multiplexing. Our core technology enables high count multiplexing, or simultaneous multiple target identification in a single sample using a simple low-density microarray. A sample and probe mixture is introduced simultaneously into a single self-contained reaction chamber pre-printed with multiple reaction spots, each containing capture strand oligonucleotides or proteins that are complementary to a specific target molecule of interest. By utilizing the sharp melt transition of the nanoparticle probes, multiple targets can be discretely identified in a single sample. This methodology eliminates the need for complex and costly means of physically isolating individual target molecules. |
49
| | |
| | • | Detection of Genomic and Protein Molecules Simultaneously. We are able to synthesize our gold nanoparticle probes for the simultaneous multiplexed detection of both protein and genomic targets in the same assay. |
| |
| | • | Superior Reaction Kinetics. The sharp melt transition curves in our gold nanoparticles increase binding affinity thereby leading to improved assay kinetics and efficiency. |
| |
| | • | Long-Term Stability. The high density of oligonucleotides per nanoparticle, which serve both as a protective and recognition layer on the nanoparticle surface, ensures the long-term stability of our nanoparticles. We have patented approaches using localized salt and buffer concentrations that deliver long-shelf life for our technology and reagent set. |
| |
| | • | Unparalleled Specificity. A key property of the oligonucleotide-linked gold nanoparticle is an extremely sharp melting curve. Our nanoparticles exhibit dissociation transitions of less than one degree, whereas PCR transitions are typically20-30 degrees in range or more. These qualities eliminate errors caused by mismatched nucleotide pairs, thereby allowing genomic targets differing by a single nucleotide (base pair) to be distinguished with unprecedented selectivity. |
Assay Format
Our silver-enhanced gold nanoparticles and related optical detection technology are used for diagnostic assays which detect genomic and proteomic targets captured onto microarrays as shown below. The microarray format enables high count multiplexing of assay targets, facilitating the development of a broad menu of tests, including for complex diseases where multiple targets must be evaluated to provide a diagnosis, in a simple, scalable format.
Two probes can be used in an assay. Oligonucleotide probes are used for genomic assays and antibodies for protein assays. One probe, complementary to a specific site on the target molecule, is attached to a surface such as a glass slide and the other probe, complementary to a different site on the target molecule, is attached to the surface of gold nanoparticles. In the presence of the target molecule of interest, the probes and target form a three dimensional, cross-linked aggregate. After silver coating the gold nanoparticles, light scatter is measured on the surface of the microarray slide. The silver-enhanced gold nanoparticle probes located on the slide surface scatter light in proportion to the concentration of the target in the sample, which is detected through optical imaging and translated into clinical results via our proprietary software algorithms.
50
Schematic of Microarray Based Detection Using Nanoparticle Probes
Research and Development
Our research and development efforts are focused on:
| | |
| | • | Expanding and Enhancing the Capabilities of Our Instrument Platform. Design elements and components of our current instrument platform, the Verigene System, will serve as the foundation for future generation development. We plan to enhance the microfluidics processor and touchscreen reader in the existing Verigene System in a second generation instrument which will also add sample preparation for infectious diseases. This feature will further simplify the processing of clinical samples from swab, cerebrospinal fluid and serum. We are also developing a fully automated instrument with increased throughput and sample preparation for both infectious disease and human genetic tests. By basing future generations of our instrument platform on existing design elements, each new generation of development will process assays developed for previous generations. |
| |
| | • | Developing Additional Genomic and Protein Assays. We are in various phases of developing and commercializing new assays for detecting protein biomarkers, infectious diseases and human genetic markers. Currently we are developing additional genomic assays including a cystic fibrosis test, a human papillomavirus test, a respiratory virus panel and a herpes simplex viral test. In addition, we are working to integrate ultra-sensitive assays for prostate specific antigen and cardiac troponin I into the current Verigene System instrument platform. |
51
| | |
| | • | Validating and Commercializing New Biomarkers. We have a dedicated team of protein scientists and assay developers who conduct assay development to support feasibility testing and new protein biomarker validation. This team is collaborating with clinical researchers in academic and private settings to apply our ultra-sensitive protein detection technology to the researchers’ efforts to create diagnostic methods with greater clinical sensitivity and specificity. We are also applying our ultra-sensitivity methods to the development of established protein biomarkers that may lead to earlier detection of medical conditions including cancer, neurodegenerative disorders including Alzheimer’s disease, sepsis and mad cow disease, for blood screening and veterinary applications. |
| |
| | • | Enhancing Performance of Established Product Systems and Developing New Applications. We have entered into a license agreement with Northwestern University which provides us with an exclusive license to certain patents and patent applications related to the application of nanotechnology to biodiagnostics and to biobarcode technology, which involves the analysis of oligonucleotides as reporter molecules, through January 1, 2013, after which date we have the right of first negotiation for an exclusive license on future inventions. Our research team utilizes the research and patents developed at Northwestern to develop diagnostic applications including additional genomic and protein testing assays for use in the Verigene System. See “Certain Relationships and Related Party Transactions.” |
Government Grants and Contracts
We have received grants over the last five years that have allowed for the evaluation and development of new technologies and also allowed for development of market specific diagnostic products.
We have benefited from Small Business Innovation Research grants to prove feasibility of gold nanoparticle based detection technology as well as evaluate potential new technologies and medical diagnostic applications.
We have received government contracts for the development of automated biological agent detection systems using nanoparticle probes that are capable of rapidly detecting biological warfare agents and biological toxins. These products have potential application for both government contractors and civilian first responders. Through June 30, 2007, we have recorded revenue of approximately $3.2 million and $5.3 million under these grants and contracts respectively.
Manufacturing
We assemble and package all our finished products at our corporate headquarters in Northbrook, Illinois. There, we manufacture our proprietary nanoparticle probes and assay reagents and test cartridges and Verigene System instrumentation. We outsource much of the disposable component molding and component assembly. Reagent manufacturing and cartridge filling is performed under the current Good Manufacturing Practice — Quality System Regulation which is required by the FDA for the manufacture of in vitro diagnostic products.
We have implemented a quality system which complies with FDA regulations governing in vitro diagnostic products. These regulations carefully control the manufacture, testing and release of diagnostics products as well as raw material receipt and control. To ensure that products are manufactured consistently to meet quality requirements, we have built and validated a quality system that we believe complies with FDA guidelines and regulations, including the FDA’s Quality System Regulation. We are required to register with the FDA as an owner and operator of an establishment that manufacturers a device intended for human use, which includes in vitro diagnostics products, within 30 days of the shipment of the first commercial sale of our product. Our manufacturing facility occupies approximately 12,000 square feet of the 40,945 square feet which we lease at our Northbrook, Illinois facility. Class 10,000 clean room facilities are available for the assembly of sub-assembled disposable plastic components in a semi-automated fashion.
We have controlled methods for the consistent manufacturing of our proprietary nanoparticles and production oligonucleotides at very high purity (greater than 95%). We also manufacture at our Northbrook facility a proprietary linker to ensure stable bonding of the oligonucleotide to the gold nanoparticle.
52
All quality control tests are validated to ensure product quality measurements are accurate. Manufacturing of the Verigene System including test cartridges is tightly controlled with the use of manufacturing batch records. These records control which product is produced and ensure that each batch of product is manufactured consistently and according to the intended design.
We plan to continue to manufacture components that we determine are highly proprietary or highly difficult to produce consistently while outsourcing commodity components. We are likely to establish additional outsourcing partnerships as we manufacture additional products. While we believe our current facilities and expansion rights are adequate to meet our manufacturing needs for at least the next three years, we may need to lease additional space.
Marketing
As a part of our business strategy, we have established a direct sales and marketing organization to support the launch of the Verigene System and the initial menu of tests in the United States. This organization comprises geographically dispersed sales representatives and clinical support specialists as well as a centralized staff of market and product managers. We believe that the primary market for our diagnostic applications will be hospital-based laboratories and academic research institutions in the United States. At the customer’s option we expect to sell the Verigene System or enter into a leasing arrangement. Our lease arrangements take the form of what are known as “reagent rentals” where an instrument is placed at a customer location and the customer commits to purchase a certain minimum volume of cartridges annually.
Our sales and marketing team will provide customer service for order fulfillment, technical service and product support, and distribution logistics. Upon our receipt of 510(k) clearance from the FDA in September 2007, we have commenced sales and are proceeding with customer evaluation, validations and preparations for installations following efforts by the sales representatives to pre-qualify prospective customers.
We believe that the primary international customers for our diagnostic applications will be hospital-based laboratories and academic research institutions. We have commenced the process of obtaining CE Mark approval for sale of the Verigene System in European Union countries. In Europe and most other international markets we may enter in the future, we anticipate using marketing partners and distributors. A distribution strategy is being developed for each relevant international market. We expect to supplement marketing partnerships with specialists who will train our partners’ sales forces and provide technical support.
Competition
We primarily face competition in the nucleic acid based testing market from companies that provide PCR-based technologies. These competitors include Abbott Diagnostics, Applera Corporation, Gen-Probe, Inc., Luminex Corporation, Roche Diagnostics, Siemens Medical Solutions Diagnostics and Third Wave Technologies, Inc. We believe that the Verigene System will compete with these companies primarily on the following factors: (1) cost effectiveness; (2) ease of use; (3) multiplex capability; (4) range of tests offered; (5) immediacy of results; and (6) reliability.
We also face competition in the protein detection market from companies that provide mass spectrometry systems, including Applera Corporation, Cepheid, Ciphergen Biosystems, Inc. and PerkinElmer, Inc. Although mass spectrometry systems offer high sensitivity, they are extremely costly, require significant time and effort by sophisticated staff and cannot detect many complex, disease-causing proteins. These significant limitations have rendered mass spectrometry systems impractical for commercial protein diagnostics laboratories.
The protein detection market also includes companies that provide ELISA-based testing systems, including Abbott Diagnostics, Beckman Coulter, Inc., Dade Behring, the Ortho-Clinical Diagnostics division of Johnson & Johnson, Roche Diagnostics and Siemens Medical Solutions Diagnostics. However, we believe that our technology, which is at least 100 times more sensitive than current diagnostic technologies, provides a significant advantage because it can detect proteins at lower concentrations equating to earlier detection of disease. This sensitivity will create new value for existing biomarkers and allow the discovery of novel biomarkers for the treatment and monitoring of disease where none exist today.
53
Many of our competitors have substantially greater financial, technical, research and other resources and larger, more established marketing, sales and distribution services organizations than we do. Many of our competitors also offer broader product lines and have greater brand recognition than we do. Moreover, our competitors may make rapid technological developments that may result in our technologies and products becoming obsolete before we recover the expenses incurred to develop them or before they generate significant revenue. Our success will depend on our ability to establish competitive marketing, sales and distribution services.
Government Regulation
We anticipate that the testing, manufacture and sale of our diagnostic products will be subject to regulation by numerous governmental authorities, principally the FDA and corresponding state and foreign regulatory agencies.
Regulation by the United States Food and Drug Administration
In the United States, the FDA regulates the sale and distribution, in interstate commerce, of medical devices, including in vitro diagnostic test kits. Pursuant to the federal Food, Drug, and Cosmetic Act, the FDA regulates the preclinical and clinical design, testing, manufacture, labeling, distribution and promotion of medical devices. We will not be able to commence marketing or commercial sales in the United States of new medical devices under development that fall within the FDA’s jurisdiction until we receive clearance from the FDA.
In the United States, medical devices are classified into one of three classes (i.e., Class I, II or III) on the basis of the controls deemed necessary by the FDA to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls (e.g., establishment registration, medical device listing, labeling regulations, possible premarket notification, possible adherence to current Good Manufacturing Practice). However, most Class I devices are exempt from premarket notification (510(k) clearance) and adherence to current Good Manufacturing Practice. Class II devices are subject to general and special controls (e.g., special labeling requirements, mandatory performance standards, premarket notification (510(k) clearance) often with guidance from an FDA special control guideline, adherence to current Good Manufacturing Practice, possible post-market surveillance). Generally, Class III devices are subject to general and special controls and must receive premarket approval, or PMA, by the FDA to ensure their safety and effectiveness (e.g., new devices for which insufficient information exists to assure safety and effectiveness through general and special controls; often such devices are life-sustaining, life-supporting and implantable). Many devices that have been approved by way of premarket approval are required to perform post-market surveillance.
510(k) Clearance
The FDA will grant 510(k) clearance if the submitted information establishes that the proposed device is “substantially equivalent” to a legally marketed Class I or Class II medical device or apre-amendment Class III medical device for which the FDA has not sought PMAs. The FDA has recently been requiring more rigorous demonstration of substantial equivalence than in the past, including in some cases requiring submission of clinical data. It generally takes from four to twelve months from submission to obtain 510(k) premarket clearance, but it may take longer. The FDA may determine that a proposed device is not substantially equivalent to a legally marketed device or that additional information is needed before a substantial equivalence determination can be made. A “not substantially equivalent” determination, or a request for additional information, could prevent or delay the market introduction of new products that fall into this category. For any devices that are cleared through the 510(k) process, modifications or enhancements that could significantly affect safety or effectiveness, or constitute a major change in the intended use of the device, require new 510(k) submissions and clearances.
54
PMA Approval
A PMA application must be filed if a proposed device is a new device not substantially equivalent to a legally marketed Class I or Class II device, or if it is apre-amendment Class III device for which the FDA has sought PMAs. A PMA application must be supported by valid scientific evidence to demonstrate the safety and effectiveness of the device, typically including the results of clinical investigations, bench tests, and laboratory and animal studies. The PMA application must also contain a complete description of the device and its components and a detailed description of the method, facilities and controls used to manufacture the device. In addition, the submission must include the proposed labeling, advertising literature and any training materials. The PMA approval process can be expensive, uncertain and lengthy, and a number of devices for which FDA approval has been sought by other companies have never been approved for marketing.
Upon receipt of a PMA application, the FDA makes a threshold determination as to whether the application is sufficiently complete to permit a substantive review. If the FDA determines that the PMA application is complete, the FDA will accept the application for filing. Once the submission is accepted, the FDA begins an in-depth review of the PMA. The FDA’s review of a PMA application generally takes one to three years from the date the application is accepted, but may take significantly longer. The review time is often extended by the FDA asking for more information or clarification of information already provided in the submission. During the review period, an advisory committee, typically a panel of clinicians, will likely be convened to review and evaluate the application and provide recommendations to the FDA as to whether the device should be approved. The FDA is not bound by the recommendation of the advisory panel. Toward the end of the PMA review process, the FDA generally will conduct an inspection of the manufacturer’s facilities to ensure that the facilities are in compliance with applicable current Good Manufacturing Practices requirements.
If FDA evaluations of both the PMA application and the manufacturing facilities are favorable, the FDA may issue either an approval letter or an approvable letter, which usually contains a number of conditions that must be met in order to secure final approval of the PMA. When and if those conditions have been fulfilled to the satisfaction of the FDA, the agency will issue a PMA approval letter, authorizing commercial marketing of the device for certain indications. If the FDA’s evaluation of the PMA application or manufacturing facilities is not favorable, the FDA will deny approval of the PMA application or issue a non-approvable letter. The FDA may determine that additional clinical investigations are necessary, in which case the PMA may be delayed for one or more years while additional clinical investigations are conducted and submitted in an amendment to the PMA.
Modifications to a device that is the subject of an approved PMA, including its labeling or manufacturing process, may require approval by the FDA of PMA supplements or new PMAs. Supplements to an approved PMA often require the submission of the same type of information required for an initial PMA, except that the supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA.
Clinical Investigations
Before we can submit a medical device for 510(k) clearance, we may have to perform a short (i.e., months) method comparison study at clinical sites to ensure that end-users can perform the test successfully. This is a study in a clinical environment, but is not usually considered a clinical trial. Alternatively, when we submit a PMA, we generally must conduct a longer (i.e., years) clinical trial of the device which supports the clinical utility of the device and how the device will be used.
Although clinical investigations of most devices are subject to the investigational device exemption, or IDE requirements, clinical investigations of in vitro diagnostic tests, including our products and products under development, are exempt from the IDE requirements. Thus, our tests do not require the FDA’s prior approval, provided the testing is non-invasive, does not require an invasive sampling procedure that presents a significant risk, does not intentionally introduce energy into the subject, and is not used as a diagnostic procedure without confirmation by another medically established test or procedure. In addition, our tests must be labeled “for
55
research use only” or “for investigational use only,” and distribution controls must be established to assure that our tests distributed for research, method comparisons or clinical trials are used only for those purposes.
Obtaining FDA Clearance for Our Products
In March 2007, we submitted 510(k) premarket notifications with respect to the Verigene System instrumentation platform and two independent application cartridges: (1) the hyper-coagulation test panel, which detects three genetic single nucleotide polymorphisms, or SNPs, that correlate to a person’s propensity to form blood clots, and (2) the warfarin metabolism test panel, which detects three other SNPs that define a person’s ability to metabolize warfarin. On September 17, 2007, we received 510(k) clearance from the FDA for the Verigene System and our warfarin test panel and on October 12, 2007, we received 510(k) clearance from the FDA for our hyper-coagulation test panel. The Verigene System and the initial assays are considered Class II medical devices since there are predicate devices already in the market. Most of our tests have special control guidances for 510(k) clearance. Some of our future tests may be Class III devices. We also plan to conduct method comparison studies or clinical trials of our products currently under development, which we intend to distribute in the United States. Our future developments may not be exempt from IDE requirements and may require us to obtain approval from the FDA through the PMA process rather than 510(k) clearance. In addition, any failure to maintain compliance with the IDE exemption requirements could result in, among other things, the loss of the IDE exemption or the imposition of other restrictions on the distribution of our products.
Regulation After FDA Approval or Clearance
Any devices we manufacture or distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. We are required to adhere to applicable regulations setting forth detailed current Good Manufacturing Practices requirements, which include testing, control and documentation requirements. Non-compliance with these standards can result in, among other things, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, failure of the government to grant 510(k) clearance or premarket approval for devices, withdrawal of marketing approvals and criminal prosecutions. We have designed and implemented our manufacturing facilities under the current Good Manufacturing Practices requirements.
Because we are a manufacturer of medical devices, we must also comply with medical device reporting requirements by reporting to the FDA any incident in which our product may have caused or contributed to a death or serious injury. We must also report any incident in which our product malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur. Labeling and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Current FDA enforcement policy prohibits the marketing of approved medical devices for unapproved uses.
We are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. We have numerous policies and procedures in place to ensure compliance with these laws and to minimize the risk of occupational exposure to hazardous materials. In addition, we do not expect the operations of our products to produce significant quantities of hazardous or toxic waste that would require extraordinary disposal practices. Although the costs to comply with these applicable laws and regulations have not been material, we cannot predict the impact on our business of new or amended laws or regulations, or any changes in the way existing and future laws and regulations are interpreted or enforced. Moreover, as we develop toxin and pathogen detection products for the food and agriculture markets, we may be subject to the regulations of various food safety organizations, including the United States Department of Agriculture.
56
Export of Our Products
Export of products subject to the 510(k) notification requirements, but not yet cleared to market, are permitted with FDA authorization provided certain requirements are met. Unapproved products subject to the PMA requirements must be approved by the FDA for export. To obtain FDA export approval, we must meet certain requirements, including, with some exceptions, documentation demonstrating that the product is approved for import into the country to which it is to be exported and, in some instances, safety data for the devices.
Third-Party Payor Reimbursements
Successful sales of our products in the United States and other countries will depend on the availability of reimbursement from third-party payors such as private insurance plans, managed care organizations, and Medicare and Medicaid. In the United States, the American Medical Association assigns specific Current Procedural Terminology, or CPT, codes, which are necessary for reimbursement of diagnostic tests. Once the CPT code is established, the Centers for Medicare and Medicaid Services establish reimbursement payment levels and coverage rules under Medicaid and Medicare, and private payors establish rates and coverage rules independently. We believe that each of the tests performed by our assays are covered by established CPT codes and are therefore approved for reimbursement by Medicare and Medicaid as well as most third-party payors. However, we have just initiated commercial sales of the Verigene System and our warfarin assay and we have no experience obtaining reimbursement for our tests. Also, outside of the United States, health care reimbursement systems vary from country to country, and to the extent we begin to sell our products outside the United States, we may not be able to obtain adequate reimbursement coverage, if any, for our products.
In addition, we may develop tests in the future that do not relate to previously established CPT codes and we may need to obtain new CPT codes in order to obtain reimbursement. Reimbursement by a third-party payor depends on a number of factors, including the level of demand by health care providers and the payor’s determination that the use of the product represents a clinical advance. In addition, third-party payors are routinely limiting reimbursement coverage for diagnostic tests.
Clinical Laboratory Improvement Amendments of 1988
The use of our products is also affected by the Clinical Laboratory Improvement Amendments of 1988, or CLIA, and related federal and state regulations, which provide for regulation of laboratory testing. These regulations mandate that clinical laboratories must be certified by the federal government, by a federally-approved accreditation agency or by a state that has been deemed exempt from the regulation’s requirements. Moreover, these laboratories must meet quality assurance, quality control and personnel standards, and they must undergo proficiency testing and inspections. The CLIA standards applicable to clinical laboratories are based on the complexity of the method of testing performed by the laboratory, which range from “waived” to “moderately complex” to “highly complex.” We expect that most of our products will be categorized as either “moderately complex” or “highly complex.”
Foreign Government Regulation
We anticipate that our products will be introduced in foreign markets in the future. We have commenced the process of obtaining CE Mark approval for sale of the Verigene System in European Union countries. The regulatory review process varies from country to country, and many countries also impose product standards, packaging requirements, labeling requirements and import restrictions on devices. Each country has its own tariff regulations, duties and tax requirements.
Facilities
Our executive, research and development and manufacturing functions are all located at a 40,945 square foot leased facility in Northbrook, Illinois. The lease for our Northbrook facility expires in May 2010.
57
We do not own any real property. We believe that our leased facilities are adequate to meet our needs for the foreseeable future.
Employees
As of September 30, 2007, we had 102 full-time employees. Of these employees, 51 were in research and development, 23 were in manufacturing, 19 were in sales and marketing and 9 were in general and administrative functions. We have never had a work stoppage and none of our employees are covered by collective bargaining agreements or represented by a labor union. We believe our employee relations are good.
Legal Proceedings
We are from time to time subject to various claims and legal actions during the ordinary course of our business. We believe that there are currently no claims or legal actions that would in management’s judgment based on information currently available, have a material adverse effect on our results of operations or financial condition.
58
Executive Officers and Directors
The following table sets forth information regarding our directors and executive officers, including their ages and positions as of September 30, 2007:
| | | | | | | |
Name | | Age | | Position |
| |
| William P. Moffitt III | | | 60 | | | President, Chief Executive Officer, Director |
| J. Roger Moody, Jr. | | | 40 | | | Chief Financial Officer, Vice President of Finance & Administration, Treasurer, Secretary |
| William H. Cork | | | 47 | | | Chief Technology Officer, Vice President, Research & Development |
| Michael K. McGarrity | | | 44 | | | Chief Marketing Officer |
| Gregory W. Shipp, M.D. | | | 50 | | | Chief Medical Officer, Vice President, Medical and Regulatory Affairs and Quality Assurance |
| Winton G. Gibbons | | | 45 | | | Senior Vice President, Business Development |
| Mark Slezak(2)(3) | | | 48 | | | Chairman of the Board |
| Jeffrey R. Crisan(1) | | | 34 | | | Director |
| André de Bruin(1)(2) | | | 60 | | | Director |
| Chad A. Mirkin, Ph.D. | | | 43 | | | Director |
| James J. Nahirny(2)(3) | | | 41 | | | Director |
| Sheli Z. Rosenberg(1)(3) | | | 65 | | | Director |
| | |
| (1) | | Member of audit committee. |
| |
| (2) | | Member of compensation committee. |
| |
| (3) | | Member of corporate governance and nominating committee. |
William P. Moffitt III. Mr. Moffitt became President and Chief Executive Officer of Nanosphere in July 2004, and serves on the board of directors. Mr. Moffitt has over 30 years of experience in the diagnostics and medical device industry, and has spent the last 20 years developing novel technologies into products and solutions that have helped shape the industry. Prior to joining Nanosphere, he served as president and chief executive officer of i-STAT Corporation, a developer, manufacturer and marketer of diagnostic products in the point-of-care blood analysis market. Mr. Moffitt led i-STAT from its early stage to commercialization and its initial public offering in 1992 to its acquisition by Abbott Laboratories in 2003. Prior to i-STAT, Mr. Moffitt held increasingly responsible executive positions from 1973 through 1989 with Baxter Healthcare Corporation, a manufacturer and distributor of healthcare products, and American Hospital Supply Corporation, a diversified manufacturer and distributor of healthcare products, which Baxter acquired in 1985. Mr. Moffitt earned a B.S. in zoology from Duke University.
J. Roger Moody, Jr. Mr. Moody joined Nanosphere in May 2007 as Chief Financial Officer and Vice President of Finance & Administration. Mr. Moody has more than 17 years of experience in leading finance, corporate development and operations for high growth healthcare and technology companies. Previously, Mr. Moody spent six years at Medsn, a medical education company where he began as chief financial officer and was promoted to chief operating officer where he led Medsn’s United States and off-shore operations. Mr. Moody also served as chief financial officer and led corporate development for two private venture backed companies sold to strategic partners. Additionally, Mr. Moody provided mergers and acquisition and strategic advisory services to technology and healthcare companies for Volpe Brown Whelan & Company. Mr. Moody began his career at IBM. Mr. Moody received his B.S. from Syracuse University and his M.B.A. from the University of Chicago, Graduate School of Business.
William H. Cork. Mr. Cork joined Nanosphere in March 2001 as Chief Technology Officer and Vice President, Research & Development. Prior to joining Nanosphere, Mr. Cork held the position of Vice President, Research & Development, at Baxter International’s Fenwal division, where he managed development and global launch of various platelet and cell collection therapy product lines. During his more than 10 years
59
at Baxter, responsibilities included product launches in Europe, Japan and the United States amid significant regulatory environments, both global and domestic, including FDA reviews. Mr. Cork led multifunctional teams in regulatory, software and electrical engineering, clinical research, quality, manufacturing and marketing. Prior to Baxter, Mr. Cork led the product development team in software system and instrument design at Hollister, Inc. He also founded and operated Prism Technologies, and has invented numerous diagnostic devices with 10 successful patents. Mr. Cork received a B.S. degree in electrical engineering/computer science at Northwestern University, with a minor in biomedical engineering.
Michael K. McGarrity. Mr. McGarrity joined Nanosphere in September 2005 as Chief Marketing Officer. Mr. McGarrity, who has more than 17 years of sales and marketing experience in the medical device industry, joined Nanosphere after 13 years with Stryker Corporation. At Stryker, he served in leadership roles in marketing and strategic development, most recently as vice president of marketing for Stryker Instruments, where he had executive general management responsibility for a newly created business focused on interventional pain management. Mr. McGarrity is a graduate of the University of Notre Dame and began his career in commercial banking in Chicago.
Gregory W. Shipp, M.D. Dr. Shipp joined Nanosphere in June 2005, and serves as our Chief Medical Officer and Vice President, Medical and Regulatory Affairs and Quality Assurance. He was previously medical director and chief medical executive at i-STAT Corporation, an Abbott Point-of-Care business. Prior to i-STAT, he was director of clinical affairs for Boston Scientific Medi-tech, where he managed the process of obtaining FDA and ethical committee approvals to begin clinical evaluation of a stent-graft for endovascular exclusion of infra-renal abdominal aortic aneurysms. Dr. Shipp was senior medical officer at Bio-Reg Associates, where he led clinical studies of Class III medical devices in the areas of ophthalmology, plastic surgery, urology and bone physiology. He holds an M.D. from the University of North Carolina School of Medicine and M.S. and B.S. degrees in biomedical engineering from Northwestern University.
Winton G. Gibbons. Mr. Gibbons joined us in July 2007 as Senior Vice President, Business Development. From 2005 to 2007, he was senior vice president for strategic and global product marketing at Biosite (now Inverness Medical). For the period of 1997 through 2005, he was a sell-side equity analyst for the investment firm of William Blair & Company, L.L.C., covering diagnostic, life science and biotechnology companies, and during which he became a principal, as well as group head for healthcare. Prior to that position, from 1994 to 1997, Mr. Gibbons was vice president of strategy and business development for the Patient Care Division of Boehringer Mannheim Diagnostics (now Roche Diagnostics). He has also been a director of management services at Merck & Co., a consultant and manager at McKinsey & Company, and held marketing and sales positions at Conoco Chemicals, where he began his career. Mr. Gibbons holds an M.B.A. in Finance and Business Policy from the University of Chicago, Graduate School of Business and a B.S. degree in Chemistry from Duke University.
Mark Slezak. Mr. Slezak has served as Chairman of our board of directors since 2000. Mr. Slezak is the chief executive officer and member of the board of directors of Lurie Investments, Inc. As such, Mr. Slezak oversees financial activities as (i) a trustee of AOQ Trust, (ii) a managing member of Eagle Capital Management, LLC, which is the managing member of Alfa-Tech, LLC, (iii) the investment manager of LFT Partnership, (iv) vice president and a director of the Ann and Robert H. Lurie Foundation, and (v) the managing member of WASK Investments, LLC. Lurie Investments, Inc. and Eagle Capital Management, LLC are both managing members of Lurie Investment Fund, L.L.C. These entities are our affiliates or related to our affiliates. He is chairman of the board at NanoInk and a member of the board of directors at Ardesta, Kionix and numerous other private companies and foundations. Since 1979, Mr. Slezak has held various accounting and financial positions with Arthur Rubloff & Company and Equity Group Investments, Inc. From 1989 to 1996, Mr. Slezak held the position of senior vice president and treasurer of Equity Group Investments, Inc., for which he has been a member of the board of directors since 1997. Mr. Slezak is also a managing member of several investment funds investing in venture and public markets. He holds a B.S./B.A. degree from Roosevelt University in Chicago, Illinois.
Jeffrey R. Crisan. Mr. Crisan has served as a member of our board of directors since April 2006. Mr. Crisan is a director at Bain Capital Venture Partners, LLC, which is related to an affiliate of ours. Prior to joining Bain Capital Ventures, Mr. Crisan worked at Bain Capital Private Equity, focusing primarily on
60
technology and healthcare investments, including software, semiconductors, telecommunications, business services and healthcare services. Prior to Bain Capital, Mr. Crisan was a consultant with Bain & Company. Mr. Crisan is also a general partner of BCIP Associates III and BCIP Associates III-B, which are the managers and sole members of BCIP Associates III, LLC and BCIP Associates III-B, LLC, respectively. These entities are our affiliates or related to our affiliates. Mr. Crisan also serves as a member of the board of directors of Eyetel Imaging, Inc. and the Princeton Review, Inc. Mr. Crisan received an M.B.A. with Distinction from Harvard Business School and a B.A., magna cum laude, Phi Beta Kappa in Mathematics and Government from Dartmouth College.
André de Bruin. Mr. de Bruin has served as member of our board of directors since June 2005. Mr. de Bruin has more than 35 years of global healthcare industry experience spanning the bio-pharmaceutical, medical device and diagnostics markets. Mr. de Bruin is the founder and chief executive officer of Duraparts, Inc., a manufacturer of steel and fabric structures. Prior to his retirement in 2004 as executive chairman of Quidel Corporation’s board of directors, Mr. de Bruin served as the company’s chief executive officer from 1998 until 2001. He was president and chief executive officer of Somatogen and was elected chairman in 1996. Prior to joining Somatogen, de Bruin was chairman, president and chief executive officer of Boehringer Mannheim Corporation, a global healthcare concern subsequently acquired by Hoffman-La Roche. Past experience includes advisory services for Ferrer, Freeman and Company LLC and various boards of directors. Mr. De Bruin graduated from the University of Potchefstroom in South Africa, where he earned a B.S. in finance, economics and business.
Chad A. Mirkin, Ph.D. Dr. Mirkin, one of our co-founders, has served as a member of our board of directors since 2000. Dr. Mirkin is a scientist and pioneer in the development of ultra-high sensitivity and selectivity assays based upon nanostructures. He is currently the director of the Northwestern University International Institute for Nanotechnology and the George B. Rathmann Professor of Chemistry, Professor of Medicine, and Professor of Materials Science and Engineering. Dr. Mirkin received his undergraduate training at Dickinson College (B.S., 1986) and his graduate training at the Pennsylvania State University, where he completed his Ph.D. in chemistry in 1989. That same year, he moved to MIT as a National Science Foundation Postdoctoral Fellow. Dr. Mirkin joined the faculty at Northwestern University in 1991. He has won over 50 national and international awards for his research, including the ACS Nobel Signature Award, the NIH Director’s Pioneer Award, the Feynman Prize, the Leo Hendrik Baekeland Award, the ACS Award in Pure Chemistry, the Sackler Prize, the Materials Research Society’s Outstanding Young Investigator Award, the E. Bright Wilson Prize, and an A. P. Sloan Foundation Fellowship. He is the author or co-author of more than 290 publications and 320 patents (61 issued), has served on the editorial advisory board of over 20 scientific journals, and has been a consultant with several major chemical companies.
James J. Nahirny. Mr. Nahirny has served as a member of our board since April 2006. Mr. Nahirny is a managing director of Bain Capital Venture Partners, LLC and Bain Capital Venture Investors, LLC, which is a general partner of Bain Capital Venture Partners 2005, L.P., which is the general partner of Bain Capital Venture Fund 2005, L.P. Mr. Nahirny is a general partner of BCIP Associates III, which is the manager and sole member of BCIP Associates III, LLC, where he leads investment advisory services. These entities are our affiliates or related to our affiliates. Prior to Bain Capital Ventures, Mr. Nahirny was a partner at McKinsey & Company, the global management consulting firm, where he worked with the senior management and boards of his clients on a broad range of strategic and operational issues. Before joining McKinsey, Mr. Nahirny was in the mergers and acquisitions group at the investment bank First Boston. Mr. Nahirny received an M.B.A. with High Distinction from Harvard Business School, where he was named a Baker Scholar, and a B.A. magna cum laude from Yale University.
Sheli Z. Rosenberg. Ms. Rosenberg has served as a member of our board of directors since October 2002. Ms. Rosenberg is the retired chief executive officer, president and vice chairwoman of Equity Group Investments, Inc. She joined Equity in 1980 as General Counsel. She sits on the boards of five New York Stock Exchange corporations: CVS Corporation, Avis Budget Group, Inc., Equity LifeStyle Properties, Inc., Equity Residential Properties Trust and Ventas, Inc.
61
Board of Directors
We currently have seven directors. Each of these directors was elected pursuant to a stockholders agreement among us, Mr. Moffitt, Mr. Slezak, Dr. Mirkin, Ms. Rosenberg and other investors. Under the terms of our amended and restated certificate of incorporation in effect prior to the closing of this offering, the holders of outstanding shares of ourSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock, each respectively voting as a class, are each entitled to elect two members of our board of directors. Pursuant to the second amended and restated stockholders agreement in effect prior to the closing of this offering, holders of theSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock agreed that Mr. Slezak, Ms. Rosenberg, Mr. Nahirny and Mr. Crisan would be elected as members of the board and would serve as the directors entitled to be elected by the holders ofSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock. In addition, the second amended and restated stockholders agreement provided that Mr. Moffitt and Dr. Mirkin would be elected as directors and two additional members would be designated by a majority of the other directors including an affirmative vote from either Mr. Slezak or Ms. Rosenberg and an affirmative vote from either Mr. Nahirny or Mr. Crisan. The two additional members may not be an officer or employee of us or any stockholder or an affiliate of a stockholder. Mr. de Bruin currently serves in such capacity and there is a vacancy for the second member. The Second Amended and Restated Stockholders Agreement, as amended, will be terminated upon the closing of this offering. Upon the termination of this agreement and the amendment and restatement of our existing certificate of incorporation, there will be no further contractual obligations regarding the election of our directors. Our directors hold office until their successors have been elected and qualified or until the earlier of their resignation or removal.
Our board of directors has considered the relationships of all directors and, where applicable, the transactions involving them are described under “Certain Relationships and Related Party Transactions.” After such consideration, our board determined that none of the directors, with the exception of Mr. Moffitt, our President and Chief Executive Officer, and Dr. Mirkin, have any relationship which would interfere with the exercise of independent judgment in carrying out his or her responsibility as a director and that each director, other than Mr. Moffitt and Dr. Mirkin, qualifies as an “independent director” under the applicable rules of the NASDAQ Global Market and the SEC.
Board Committees
Our board of directors has established an audit committee, a compensation committee and a corporate governance and nominating committee. The composition and function of each of our committees complies with the applicable rules of the SEC and the NASDAQ Global Market, and we intend to comply with additional requirements to the extent that they become applicable to us.
Audit Committee. Jeffrey R. Crisan, André de Bruin and Sheli Z. Rosenberg currently serve on our audit committee. Mr. Crisan is the chairman of our audit committee. The audit committee’s responsibilities include, but are not limited to:
| | |
| | • | appointing, approving the compensation of, and assessing the independence of our independent registered public accounting firm; |
| |
| | • | pre-approving auditing and permissible non-audit services, and the terms of such services, to be provided by our independent registered public accounting firm; |
| |
| | • | reviewing and discussing with management and the independent registered public accounting firm our annual and quarterly financial statements and related disclosures; |
| |
| | • | coordinating the oversight and reviewing the adequacy of our internal control over financial reporting; |
| |
| | • | establishing policies and procedures for the receipt and retention of accounting related complaints and concerns; and |
| |
| | • | preparing the audit committee report required by SEC rules to be included in our annual proxy statement. |
Our board of directors has determined that Ms. Rosenberg qualifies as an “audit committee financial expert” as defined under the Securities Exchange Act of 1934, or the Exchange Act, and the applicable rules of the NASDAQ Global Market. The board has determined that each of Ms. Rosenberg and Mr. de Bruin is independent and that Mr. Crisan is not independent pursuant toRule 10A-3 of the Exchange Act. We believe
62
that the composition of our audit committee meets the requirements for independence and financial sophistication under the current requirements of the NASDAQ Global Market and SEC rules and regulations.
Compensation Committee. Mark Slezak, André de Bruin and James J. Nahirny currently serve on the compensation committee. Mr. Slezak is the chairman of our compensation committee. We believe that the composition of our compensation committee meets the requirements for independence under the current requirements of the NASDAQ Global Market and SEC rules and regulations. The compensation committee’s responsibilities include, but are not limited to:
| | |
| | • | annually reviewing and approving corporate goals and objectives relevant to compensation of our chief executive officer; |
| |
| | • | evaluating the performance of our chief executive officer in light of such corporate goals and objectives and reviewing and approving the compensation of our chief executive officer and recommending such compensation to the board; |
| |
| | • | reviewing and approving the compensation of our other executive officers and recommending such compensation to the board; |
| |
| | • | overseeing and administering our compensation, welfare, benefit and pension plans and similar plans; and |
| |
| | • | reviewing and making recommendations to the board with respect to director compensation. |
Corporate Governance and Nominating Committee. Sheli Z. Rosenberg, Mark Slezak and James J. Nahirny will serve on the corporate governance and nominating committee. Ms. Rosenberg is the chairman of our corporate governance and nominating committee. We believe that the composition of our corporate governance and nominating committee meets the requirements for independence under the current requirements of the NASDAQ Global Market. The corporate governance and nominating committee’s responsibilities include, but are not limited to:
| | |
| | • | developing and recommending to the board criteria for board and committee membership; |
| |
| | • | establishing procedures for identifying and evaluating director candidates including nominees recommended by stockholders; |
| |
| | • | identifying individuals qualified to become board members; |
| |
| | • | recommending to the board the persons to be nominated for election as directors and to each of the board’s committees; |
| |
| | • | developing and recommending to the board a code of business conduct and ethics and a set of corporate governance guidelines; and |
| |
| | • | overseeing the evaluation of the board and management. |
Compensation Committee Interlocks and Insider Participation
None of the members of the compensation committee has been or is an officer or employee of ours. None of our executive officers serves on the board of directors or compensation committee of a company that has an executive officer that serves on our board or compensation committee. No member of our board is an executive officer of a company in which one of our executive officers serves as a member of the board of directors or compensation committee of that company. Lurie Investment Fund, L.L.C. and Bain Capital Venture Fund 2005, L.P. directly hold more than 5% of our capital stock. Mr. Slezak, chairman of our board of directors, is related to the Lurie Entities. Mr. Nahirny, a member of our board of directors, is related to the Bain Entities. These ownership interests are described under the caption “Principal Stockholders.”
Scientific Advisory Board
We have assembled a group of scientific, clinical and health policy advisors who are leaders in fields related to nanobiotechnology and proteomic and genomic biomarkers in cardiovascular disease, cancer, infectious disease, neurodegenerative disease and reproductive health. The Scientific Advisory Board meets
63
periodically and independently with our scientific managers and the senior management team to discuss present and long-term research and development activities. The Scientific Advisory Board members include:
Marvin Caruthers, Ph.D. —Professor of Chemistry, University of Colorado — Dr. Caruthers’ work led to the development of automated DNA synthesis which made oligonucleotides of any desired sequence available.
Anthony K. Cheetham, Ph.D. —Director of the Materials Research Laboratory and Professor at the University of California-Santa Barbara (UCSB) — Dr. Cheetham’s research interests include the area of zeolites and metal oxides and span the synthesis, characterization and applications of such materials.
M. Reza Ghadiri, Ph.D. —Professor, Departments of Chemistry and Molecular Biology, and Member of the Skaggs Institute for Chemical Biology at the Scripps Research Institute — Dr. Ghadiri’s research interests include de novo design of synthetic proteins and enzymes, self-assembling peptide nanotubes and biomaterials, transmembrane ion channels and antimicrobial agents, design of novel biosensors, self-replicating molecular systems and self-organized chemical networks, and molecular computation.
Robert Letsinger, Ph.D. (co-founder) —Professor Emeritus, Northwestern University— Dr. Letsinger is a pioneer in creating the solid support concept for organic synthesis and in developing the rapid and efficient method for chemical synthesis of DNA used throughout the world today. In collaboration with Dr. Chad A. Mirkin, he invented the methods for attaching a reproducible number of oligonucleotides to gold nanoparticles.
Chad A. Mirkin, Ph.D. (co-founder) — Director, Northwestern University International Institute for Nanotechnology — Dr. Mirkin is a pioneer in the development of ultra-high sensitivity and selectivity assays based upon nanostructures. He is one of the most influential people in the field of nanotechnology. In collaboration with Dr. Robert Letsinger, he invented the methods for attaching a reproducible number of oligonucleotides to gold nanoparticles.
David Nash, M.D., M.B.A. —Professor of Health Policy and Medicine at Jefferson Medical College of Thomas Jefferson University — Dr. Nash directs the Office of Health Policy and Clinical Outcomes at Jefferson University Hospital. In 1996, he was named the first Associate Dean for Health Policy at Jefferson Medical College.
Steven T. Rosen, M.D. — Professor of Medicine at Northwestern University Medical School and Director of the Robert H. Lurie Comprehensive Cancer Center at Northwestern University — Dr. Rosen is internationally known for his leadership roles in cancer research. Dr. Rosen’s laboratory research focuses on experimental therapeutics and hematologic malignancies.
Alan Wu, Ph.D. —Chief of the Clinical Chemistry and Toxicology Laboratories in the Department of Laboratory Medicine at San Francisco General Hospital, University of California at San Francisco — Dr. Wu’s research interests include protein and nucleic acid biomarkers for diagnostics and therapeutics. His areas of expertise include cardiac biomarkers (e.g., cardiac troponin, B-type natriuretic peptide), stroke markers, toxicology and personalized therapeutics (i.e., the use of biomarkers to determine proper selection and dosing of drugs to maximize efficacy and minimize adverse drug reactions).
Corporate Governance
We expect to adopt a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics will be available on our internet site at www.nanosphere.us. We expect that any amendments to the code will be disclosed on our website.
Limitation of Liability and Indemnification
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law, or DGCL, provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer, director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid
64
in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses which such officer or director has actually and reasonably incurred. Our amended and restated certificate of incorporation and amended and restated bylaws provide for the indemnification of our directors and officers to the fullest extent permitted under the DGCL.
Section 102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability:
| | |
| | • | for any transaction from which the director derives an improper personal benefit; |
| |
| | • | for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| |
| | • | for improper payment of dividends or redemptions of shares; or |
| |
| | • | for any breach of a director’s duty of loyalty to the corporation or its stockholders. |
Our amended and restated certificate of incorporation and amended and restated bylaws include such a provision. Expenses incurred by any officer or director in defending any such action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director or officer is not entitled to be indemnified by us.
Prior to the completion of this offering, we will execute indemnification agreements which will be in effect upon the closing of this offering, with each of our directors and executive officers that require us to indemnify such persons against any and all expenses including attorneys’ fees, witness fees, damages, judgments, fines, settlements and other amounts incurred in connection with any action, suit or proceeding, whether actual or threatened, to which any such person may be made a party by reason of the fact that such person is or was a director, an officer or an employee of our or any of our affiliated enterprises, provided that such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to our best interests and, with respect to any criminal proceeding, had no reasonable cause to believe his or her conduct was unlawful. The indemnification agreements also set forth procedures that will apply in the event of a claim for indemnification thereunder.
At present, there is no pending litigation or proceeding involving a director or executive officer as to which indemnification is being sought and we are not aware of any threatened litigation that may result in claims for indemnification by any of our directors or executive officers.
We maintain an insurance policy covering our directors and executive officers with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
65
Compensation Discussion and Analysis for Named Executive Officers
Overview of Compensation Program
The compensation committee of the board of directors is responsible for establishing and implementing our compensation philosophy, as detailed below. The compensation committee reviews and approves all of our compensation policies, including executive officer salaries, bonuses and equity incentive compensation. The committee ensures that the total compensation paid to the executive management is fair, reasonable, competitive, and includes incentives that are designed to appropriately drive corporate performance.
The compensation committee has the authority to review and approve, the annual compensation for our executive officers, other than our chief executive officer, for which the compensation committee has the authority to review and recommend the annual compensation for the approval of the board of directors. In 2006, the compensation committee did not engage the services of a compensation consultant in connection with its review of executive compensation.
Overview of Compensation Philosophy and Objectives
The compensation of our executive officers is based in part on the terms of the employment agreements that we entered into with each of our named executive officers. In addition, our “pay-for-performance” philosophy on both an individual and corporate level is among the fundamental tenants of our executive compensation program. We have adopted an approach to compensation comprised of a mix of short-term and long-term components that are designed to provide proper incentives and reward our senior management team.
Our intent regarding the compensation of our executive officers is to provide salary levels and compensation incentives that:
| | |
| | • | are competitive within the life sciences industry; |
| |
| | • | attract and retain talented and experienced executives; |
| |
| | • | motivate our executives to manage our business to meet our short-term and long-term business objectives; |
| |
| | • | align the interests of our executives and stockholders by motivating the executives to increase stockholder value; and |
| |
| | • | tie executive compensation to the achievement of certain short-term and long-term individual and corporate objectives. |
Role of Our Compensation Committee
Our compensation committee is responsible for administering our compensation practices. Our compensation committee was appointed by our board of directors, and consists entirely of directors who are “outside directors” for purposes of Section 162(m) of the Internal Revenue Code, as amended, or the Code, and non-employee directors for purposes ofRule 16b-3 under the Exchange Act. Our compensation committee is comprised of Mark Slezak, André de Bruin and James J. Nahirny, with Mr. Slezak as our compensation committee chairperson. Our compensation committee holds meetings as required, but meets not less than six times throughout the year.
Within the context of the overall objectives of our executive compensation program, the compensation committee determines the specific amounts of compensation to be paid to each of our named executive officers based on a number of factors including:
| | |
| | • | the roles and responsibilities of our executives; |
| |
| | • | the individual experience and skills of, and expected contributions from, our executives; and |
66
| | |
| | • | our executives’ historical compensation at our company. |
When discussing performance evaluations and setting new compensation levels, the compensation committee considers recommendations from Mr. Moffitt, our chief executive officer, regarding the compensation for executive officers and the senior management team, other than for himself. In its sole discretion, the compensation committee may accept or deny, in whole or in part, the recommendations of Mr. Moffitt. For 2006, the compensation committee reviewed and accepted the recommendations of Mr. Moffitt without adjustment. Mr. Moffitt does not participate in determining the amount of his own compensation. With the exception of our chief executive officer, the compensation committee has the final authority regarding the overall compensation structure for the executive officers and the senior management team. In the case of Mr. Moffitt, the compensation committee evaluates Mr. Moffitt’s performance and recommends compensation levels to the board of directors. In its sole discretion, the board of directors may accept or deny, in whole or in part, the recommendations of the compensation committee with respect to Mr. Moffitt’s overall compensation. For 2006, however, the board of directors reviewed and accepted the recommendations of the compensation committee without adjustment.
Elements of Compensation
The compensation of our named executive officers consists primarily of five components:
| | |
| | • | base salary; |
| |
| | • | annual incentive cash bonuses; |
| |
| | • | equity-based incentives; |
| |
| | • | other benefits; and |
| |
| | • | severance and termination protection, in the case of some, but not all of our executive officers. |
In general, total compensation is geared to be sufficient to attract and retain the best possible human resource talent. In determining the adjustments to the compensation of our executive officers for the fiscal year ended December 31, 2006 and prior periods, we relied on the experience of the members of our compensation committee who serve on the boards of directors and compensation committees for other companies, and we annually take into account the performance evaluations of each executive officer, their contributions toward our success, and our growth and stage of development. For fiscal year ended December 31, 2006 and prior periods, we did not conduct a peer group study or perform a benchmarking survey. In August 2007, we engaged the services of Radford Surveys + Consulting to perform a formal review of our executive compensation structure, as discussed in the “Compensation Discussion and Analysis — Post-2006 Actions” section.
We use a mix of short-term compensation (base salaries and cash incentive bonuses) and long-term compensation (equity incentive compensation) to provide a total compensation structure that is designed to achieve our pay-for-performance philosophy and our compensation objectives. We discuss each of the principal elements of our executive compensation in detail below.
Annual Cash Compensation
Base Salary
In general the base salaries are designed to provide a consistent base of income and to attract the appropriate level of talent. Our executives’ base salaries reflect (1) the initial base salaries that we negotiated with each of them at the time of their initial employment or promotion, and (2) our subsequent adjustments to these amounts, which are primarily attributable to annual performance, any changes in our executives’ roles and responsibilities and a cost of living adjustment, generally between 3% and 5% each year.
67
In 2006, our increases in the annual rate of the base salaries for our named executive officers was limited to a cost of living adjustment as follows:
| | | | | | | | | |
| | | 2006 | | | 2005 | |
| |
William P. Moffitt III, | | | | | | | | |
President and Chief Executive Officer | | $ | 395,000 | | | $ | 370,000 | |
Stephen G. Wasko, | | | | | | | | |
Chief Financial Officer | | $ | 204,000 | | | $ | 196,100 | |
William H. Cork, | | | | | | | | |
Chief Technology Officer | | $ | 263,000 | | | $ | 253,300 | |
Michael K. McGarrity, | | | | | | | | |
Chief Marketing Officer | | $ | 250,000 | | | $ | 235,000 | |
Gregory W. Shipp, | | | | | | | | |
Chief Medical Officer | | $ | 236,000 | | | $ | 227,000 | |
The base salaries of our executive officers are reviewed and adjusted annually. We may also increase the base salary of an executive officer at other times if a change in the scope of the officer’s responsibilities justifies such consideration or in order to maintain salary equity among our executive officers.
Annual Cash Incentive Compensation
Annual cash incentive awards are designed to reward near-term operating performance and the achievement of milestones critical to our success in both the near and the long-term. For years prior to 2003, our compensation committee has, on occasion, granted discretionary cash bonuses to our executive officers. Consistent with our emphasis on pay-for-performance, we have adopted a management incentive bonus program. Executive officers will have an opportunity to earn cash bonuses based on the attainment of specified individual goals. The target bonuses and our establishment of business goals for each executive officer reinforces three of our compensation goals — namely, to motivate our executives toward even higher achievement and business results, to tie our executives’ goals and interests to ours and our stockholders’ and to enable us to attract and retain highly qualified individuals.
Since inception of our management incentive bonus program in 2003, our employment offer letters to the executive officers provide for participation in this incentive program and establish the target bonus amounts. Accordingly, the annual target bonus amounts reflect the amount that we negotiated with each executive officer at the time of their initial employment or promotion (or determined in our discretion upon the commencement of the management incentive bonus program, as in the case of Mr. Cork). The target bonuses merely reflect an opportunity to receive the specified award, conditioned upon complete satisfaction of the performance targets, but are not guarantees for their payout. Under our management incentive bonus program, we may pay less than the target bonus in the event the performance goals are only partially achieved. The ultimate payout approximates the targeted amount multiplied by the percentage of the targets achieved, as demonstrated in the table below.
The board of directors may increase the target bonus amount for our chief executive officer, and the compensation committee may increase the target bonus amounts for other executive officers, as further incentives to motivate our executives to meet our business objectives. For 2006, the board of directors increased Mr. Moffitt’s target bonus opportunity to $200,000 from the prior year’s target bonus of $150,000. Similarly, the compensation committee increased Dr. Shipp’s target bonus opportunity to $50,000 from the prior year’s target bonus of $45,000. Increases in the target bonuses are reflective of our increasing emphasis on performance-based cash compensation, rather than significant increases in base salaries. This approach to compensation is consistent with our overall pay-for-performance philosophy.
With respect to the performance targets, the goals typically are a mix between company milestones and individual performance targets. Our chief executive officer, in consultation with the other executive officers, develops corporate and individual level performance targets for the executive officers, and submits the recommended goals for the approval of the compensation committee. In its sole discretion, the compensation committee may accept or deny, in whole or in part, the performance targets recommended by our chief
68
executive officer. For 2006, the compensation committee reviewed and accepted without adjustment the performance targets that Mr. Moffitt recommended for the executive officers.
In the case of Mr. Moffitt, the compensation committee, in consultation with Mr. Moffitt, develops corporate and individual level performance targets and submits the recommended targets for the approval of the board of directors. In its sole discretion, the board of directors may accept or deny, in whole or in part, the recommendations of the compensation committee with respect to the performance targets for Mr. Moffitt. For 2006, the board of directors reviewed and accepted the recommended goals without adjustment.
The performance targets are objectively determinable and measurable and their outcomes are substantially uncertain at the time established, evident by only partial completion of several targets and payouts less than the target bonus amounts, as set forth in the table below. The compensation committee authorizes bonuses to the executive officers, other than the chief executive officer, in amounts that are commensurate with each executive officer’s target bonus and the result achieved by the end of the year. At the close of the performance period, our chief executive officer assesses the achievement of the objectively determinable targets of the executive officers, reports his findings to the compensation committee, and submits recommendations for bonus payouts for the approval of the compensation committee. The compensation committee reviews our chief executive officer’s analysis and in its sole discretion may accept or deny, in whole or in part, the recommendations of Mr. Moffitt. For 2006, the compensation committee reviewed and accepted Mr. Moffitt’s bonus recommendations for all executive officers except Mr. Cork, our chief technology officer. The compensation committee made an upward adjustment in the form of a discretionary bonus for Mr. Cork, as discussed below.
For our chief executive officer, the compensation committee assesses the achievement of the objectively determinable performance targets and reports its findings and bonus recommendations to the board of directors. In its sole discretion, the board of directors may accept or deny, in whole or in part, the bonus recommendations of the compensation committee. For 2006, the board of directors reviewed, and accepted without adjustment, the compensation committee’s bonus recommendations with respect to Mr. Moffitt.
In 2006, the business objectives, potential awards, results and actual payouts (which were paid in 2007) were as follows:
| | | | | | | | | | | | | | | |
| | | | | | | | Percentage
| | | | |
| | | Performance Targets | | Target | | | Achieved | | | Payout | |
| |
William P. Moffitt III
President and
Chief Executive Officer | | Develop a comprehensive launch plan for product; develop a strategic plan for approval of board of directors; complete feasibility studies for product; team building. | | $ | 200,000 | | | | 75 | % | | $ | 150,000 | |
| | | | | | | | | | | | | | | |
William H. Cork
Chief Technology
Officer | | Product launch; assure successful field evaluation of specified product; conclude the development of assays; develop two additional assays for clinical evaluations; assess the commercialization of the specified technology. | | $ | 75,000 | | | | 64 | % | | $ | 47,813 | |
| | | | | | | | | | | | | | | |
Michael K. McGarrity
Chief Marketing
Officer | | Establish direct U.S. distribution channel; commercial launch and U.S. placement of product; develop international strategy for 2007 launch; develop a government business strategy for launch; business development. | | $ | 90,000 | | | | 68 | % | | $ | 61,000 | |
| | | | | | | | | | | | | | | |
Gregory W. Shipp
Chief Medical
Officer | | Complete clinical studies leading to FDA clearance and commercial launch of product; perform initial validation studies for several assays; perform initial validation studies for at least one of two product applications; form advisory board supporting commercialization and clinical validation of specified product. | | $ | 50,000 | | | | 86 | % | | $ | 43,125 | |
69
In the absence of pre-established goals, Mr. Stephen G. Wasko, our chief financial officer, did not participate in the management incentive bonus program for fiscal year ended December 31, 2006.
The board of directors applied a comprehensive assessment of Mr. Moffitt’s goals and determined that each goal was substantially completed with the exception of the launch of the product. Although Mr. Cork did not achieve the target related to the product launch and only partially achieved the additional goal of developing two additional assays, the compensation committee determined that the remainder of his goals were substantially completed. The compensation committee determined that Mr. McGarrity fully completed his goal related to the establishment of a U.S. distribution channel, and that significant progress had been made with respect to the remainder of his goals. The compensation committee determined that Dr. Shipp fully completed his goals with respect to the completion of clinical studies leading to FDA clearance and the commercial launch of our product, as well as the performance of validation studies for several assays, while significant progress had been made with respect to the remainder of his goals.
The compensation committee may also, in its discretion, award bonuses from time to time to executive officers based upon such other terms and conditions as the compensation committee may determine.
The compensation committee (and the board of directors with respect to Mr. Moffitt) determined the performance targets, business objectives and potential awards under the management incentive bonus program for fiscal year 2007 are as follows:
| | | | | | | |
| | | | | Potential
| |
| | | | | Award
| |
| | | Performance Targets | | Amount | |
| |
William P. Moffitt III
President and Chief
Executive Officer | | Receive FDA clearance of the Verigene System and assays; sales of the Verigene System and assays to customers; manage expenses; team building. | | $ | 225,000 | |
J. Roger Moody, Jr.
Chief Financial Officer | | Lead initial public offering; develop and implement performance management system; design reporting system; team building. | | $ | 90,000 | |
William Cork
Chief Technology Officer | | Develop infectious disease assay; integrate new technologies into the Verigene System; develop cost reduction programs; team building. | | $ | 90,000 | (1) |
Michael McGarrity
Chief Marketing Officer | | Commercial launch and U.S. placement of product; develop international business plan; develop and implement marketing strategy for protein assays. | | $ | 90,000 | |
Gregory Shipp
Chief Medical Officer | | Complete clinical studies leading to FDA clearance and commercial launch of product; perform validation studies for several assays; form advisory board supporting commercialization and clinical validation of specified product; establish complaint handling system; define regulatory strategy for international market development. | | $ | 50,000 | |
| | |
| (1) | | On April 3, 2007, the compensation committee increased Mr. Cork’s target bonus opportunity to $90,000 from the prior year’s target bonus of $75,000. |
In addition to the $47,813 we paid to Mr. Cork, our chief technology officer, pursuant to our management incentive bonus program, the compensation committee, in its discretion, awarded Mr. Cork an additional $7,187 for a combined payment of $55,000. We believe the incentive bonus, supplemented with the discretionary bonus, is more reflective of Mr. Cork’s progress and accomplishments of the majority of his performance goals.
On March 16, 2006, we entered into a bonus arrangement with Mr. Moffitt to retain him as our chief executive officer. Under the agreement, Mr. Moffitt is eligible to receive a cash bonus in the amount of $2.3 million, subject to his continuous employment with us until March 16, 2011, or if earlier, upon (1) termination by Mr. Moffitt for good reason, (2) termination by us without cause, (3) termination by us due to non-renewal of his initial employment term, expiring July 19, 2008, or (4) his death or permanent disability. In addition, our obligation to pay the bonus to Mr. Moffitt will become immediately payable upon our filing for bankruptcy, immediately prior to our filing a registration statement in connection with an initial public offering of our securities, or immediately prior to a change in control. The bonus was paid in full in August
70
2007 for the achievement of the strategic milestone of the filing of our initial registration statement, which has provided significant value to our stockholders. See “Post-2006 Actions.”
Equity Incentive Compensation
We grant equity incentive awards in the form of stock options and restricted stock purchase awards to align the interests of our executive officers with our stockholders, by providing our executive officers with strong incentives to increase stockholder value. Our decisions regarding the amount and type of equity incentive compensation and relative weighting of these awards among total executive compensation have been initially based on our negotiations with our executives in connection with their initial employment or promotion by our company.
We typically make grants of equity incentive awards to our executive officers on a periodic basis, approximately every two to three years. All such grants are approved by the compensation committee at regularly scheduled committee meetings throughout the year. Awards to our chief executive officer are approved by the compensation committee and are subject to approval by the board of directors. The date of grant and the fair market value of the awards are established on the date of the committee meeting, or board of directors meeting, in the case of an award to our chief executive officer.
Following the completion of this offering, all equity awards will be granted with an exercise price equal to or above the fair market value of our common stock on the date of grant. Such fair market value will be defined as the closing market price of a share of our common stock on the date of grant. We do not have any program, plan or practice of setting the exercise price at a price less than fair market value of our common stock on the grant date. We do not have any program, plan or obligation that requires us to grant equity compensation on specified dates to our named executive officers.
Stock Option Awards
Stock option awards provide our executive officers with the right to purchase shares of our common stock at a fixed exercise price typically for a period of up to ten years, subject to continued employment with our company. In general, we provide our executives and all of our employees, with service-based stock options that have both gradual and cliff vesting schedules. The gradually-vesting stock options are earned on the basis of continued service to us and generally vest over four years, 25% on each of the four anniversaries of the date of grant. The cliff-vested stock options vest in full on the seventh anniversary of the date of grant. However, the vesting of these stock options is subject to acceleration based on the achievement of distinct corporate milestones relating to product launch, revenues and profit margins, which are identical for all executive officers and for all employees generally. In practice, half of the total grants of stock options are typically subject to the service-based vesting, and the other half are granted with a seven year cliff-vesting schedule, subject to acceleration based on the achievement of distinct corporate milestones.
With respect to the acceleration of cliff-vested awards, if there are five milestones associated with the grant of cliff-vested stock options, then 20% of the options granted shall immediately vest and become exercisable upon the achievement of each performance milestone. For our 2005 grants of equity award, the five milestones include (i) the commercial release of the Verigene System, (ii) attaining $20 million in cumulative revenue from our biosecurity system, (iii) the commercial release of the AutoLab System, (iv) corporate collaborations for joint product development, and (v) the development of our ultra-sensitive assay capabilities.
Historically, our compensation committee has granted stock options pursuant to our 2000 Equity Incentive Plan, or the 2000 Plan. The exercise price of each stock option is not less than the fair market value of our common stock on the date of grant. The fair market value of our common stock for purposes of determining the exercise price of stock options has been determined by our board of directors based on a number of factors applicable to common stock of privately-held companies including, among others, the total company valuation implied by the most recent venture capital round of financing, the market value of similarly situated public companies, our anticipated future risks and opportunities, the rights and preferences of our convertible preferred stock existing at the time, the lack of a liquid market and an independent analysis of the value of our common stock.
71
We have granted stock options as incentive stock options in accordance with Section 422 of the Code, subject to the volume limitations contained in the Code, as well as non-qualified stock options. Generally, for stock options that do not qualify as incentive stock options, we are entitled to a tax deduction in the year in which the stock options are exercised equal to the spread between the exercise price and the fair market value of the stock for which the stock option was exercised. The holders of the non-qualified stock options are generally taxed on this same amount in the year of exercise. For stock options that qualify as incentive stock options, we do not receive a tax deduction, and the holder of the stock option may receive more favorable tax treatment than he or she would for a non-qualified stock option. Historically, we have primarily granted incentive stock options to provide these potential tax benefits to our executives and because of the limited expected benefits to our company of the potential tax deductions as a result of our historical net losses.
In Mr. McGarrity’s offer letter, we committed to evaluate his performance in 2006 and to consider adjusting his cash compensation and granting him options based on his performance and position with respect to comparables in the industry. In March 2006, Mr. McGarrity’s performance was evaluated by our chief executive officer, and submitted to the compensation committee for review. Our chief executive officer’s evaluation was based upon two primary objectives (i) completing an analysis of commercialization and distribution methods and (ii) determination of an implementation strategy of such methods. The compensation committee concluded that Mr. McGarrity fully met both objectives and established the course for commercialization of the company’s first products.
Consistent with our commitment made within Mr. McGarrity’s offer letter, our chief executive officer recommended an award of additional stock options, which was approved by the compensation committee. On March 14, 2006, we granted to Mr. McGarrity, additional stock options, on apost-split basis, to purchase 30,000 shares for a price of $4.50 per share, an amount determined by the board of directors to be greater than the fair market value of $3.25 for the common stock on the date of grant. The stock options have a ten-year term and cliff-vest on the seventh anniversary of the date of grant, with accelerated vesting in the event we achieve certain performance-based milestones as described above. We granted the stock options to Mr. McGarrity based on his outstanding performance over the initial months that ensued since the commencement of his employment with us in September 2005.
As discussed further in the “Post-2006 Actions” section of this Compensation Discussion and Analysis, we adopted the 2007 Long-Term Incentive Plan, or the 2007 Plan, in March 2007. The 2007 Plan replaced our existing 2000 Plan, and no future grants will be made under the 2000 Plan.
Restricted Stock Purchase Awards
We may grant restricted stock purchase awards from time to time to provide our executive officers with the ability to purchase shares of our common stock at a fixed purchase price at the time of grant pursuant to a restricted stock purchase agreement. To date, restricted stock purchase awards have been granted only to our Chief Executive Officer in connection with the commencement of his employment with us. The shares of restricted stock may have a vesting period and may be subject to mandatory repurchase by us in connection with termination of employment.
In connection with the commencement of Mr. Moffitt’s services as our Chief Executive Officer in July 2004, we initially granted stock options to Mr. Moffitt. In lieu of effecting those stock options, we replaced the award in March 2006 with the right to purchase 320,000 shares of restricted common stock at $4.50, after giving effect to the reverse stock split, an amount determined by the board of directors to be in excess of the fair market value of $3.25 on the date of grant. Mr. Moffitt executed a full recourse promissory note in the principal amount of $1,440,000 in our favor under which an aggregate principal amount of $1,440,000 remained outstanding at December 31, 2006. The proceeds of the note were used to purchase 320,000 shares of our restricted common stock. The note carried interest at a rate of 4.51% per annum and was repaid in full in August 2007. Pursuant to the restricted stock purchase agreement, prior to vesting, the stock is subject to purchase by us for the price Mr. Moffitt paid in the event Mr. Moffitt is terminated by us for cause or resigns without good reason. Of the 320,000 shares, 160,000 shares vested immediately upon purchase, the restrictions lapsed for 80,000 more shares on July 19, 2006 (the anniversary of Mr. Moffitt’s employment), and for the remaining 80,000 shares on July 19, 2007.
72
Other Compensation
All of our executive officers are eligible for benefits offered to employees generally, including life, health, disability and dental insurance and participation in our 401(k) plan. We intend to continue to maintain our current benefits for our executive officers. The compensation committee in its discretion may revise, amend or add to the officer’s executive benefits and perquisites if it deems it advisable. We do not believe it is necessary for the attraction or retention of management talent to provide the officers with a substantial amount of compensation in the form of perquisites. In 2006, the only perquisites we provided were certain relocation expenses in connection with the hiring of certain new employees.
Post-Employment Severance and Change in Control Benefits
Chief Executive Officer
We entered into an employment agreement with Mr. Moffitt, which provides for severance pay should Mr. Moffitt incur a loss of employment or a significant change in employment. Mr. Moffitt’s employment may be terminated at any time during the term of employment (a) by us with or without cause upon 60 days’ prior written notice; (b) by the executive upon 60 days’ prior written notice; (c) upon death of the executive; and (d) by us at any time after 180 consecutive days or two or more periods of 90 consecutive days in each 360 day period of Mr. Moffitt’s disability.
Termination Other than in Connection with a Change in Control. If Mr. Moffitt’s employment is terminated by voluntary resignation for good reason, by us without cause, or by our non-renewal of his employment agreement, Mr. Moffitt will be entitled to (a) severance compensation in the amount equal to 18 months of his base salary (payable in accordance with customary payroll practices), plus payment of the full target amount of his performance bonus, plus the accelerated amount of his bonus agreement entered into in March 2006; and (b) an immediate and full vesting, on the date of termination, of all outstanding options and restricted stock awards, in which case the options shall remain exercisable for a period of one year following the date of termination.
Termination in Connection with a Change in Control. In the event Mr. Moffitt’s employment is terminated within one year after a change in control by voluntary resignation for good reason or by us without cause, Mr. Moffitt shall be entitled to the same benefits enumerated above, plus an additional twelve months of base salary. In the aggregate, the thirty months of base salary is payable in accordance with customary payroll practices. In addition, Mr. Moffitt is entitled to be reimbursed for any tax imposed by Section 4999 of the Code on any portion of the compensation or benefits payable by us.
Death; Disability. In the event Mr. Moffitt’s employment is terminated due to death or disability during the course of employment, he (or his estate or designated beneficiary) will be entitled to (1) an acceleration of the bonus agreement award entered into in March 2006, and (2) immediate and full vesting, on the date of termination, of all outstanding options and restricted stock awards, in which case the options shall remain exercisable for a period of one year following the date of termination.
Mr. McGarrity
In the event Mr. McGarrity is terminated for reasons other than for cause, including as a result of death or disability, he will be entitled to a lump sum severance payment equivalent to six months’ base salary plus a prorated annual bonus.
Other Named Executive Officers
The employment agreements for our other named executive officers do not contain severance arrangements. Accordingly, upon a termination for cause, without cause, in connection with a change in control or any other reason, Mr. Wasko, Mr. Cork and Dr. Shipp shall receive their accrued salary, earned bonus, unreimbursed expenses and other entitlements to the date of termination, unless we decide at that time to provide additional severance payments.
The post-employment severance benefits for our executive officers are quantified in the “Estimate of Post-Employment Payments” table.
73
Accounting and Tax Considerations
Effective January 1, 2005, we adopted, on a prospective basis, the fair value provisions of SFAS 123(R), “Share-Based Payment,” or SFAS 123(R). Under SFAS 123(R), the estimated fair value of options granted, net of forfeitures expected to occur during the vesting period is amortized as compensation expense on a straight line basis over the vesting period of the options.
We generally intend for our executive compensation program to comply with Section 162(m) of the Code once we are a public company subject to these rules, as well as Code Section 409A. The compensation committee intends for all compensation paid to the named executive officers to be tax deductible to us pursuant to Section 162(m) of the Code. Under Section 162(m) of the Code, compensation paid to the named executive officers in excess of $1,000,000 cannot be deducted by us for federal income tax purposes, unless such amounts satisfy the performance-based exception to the deduction disallowance.
Section 409A of the Code addresses certain non-qualified deferred compensation benefits payable to our executives and provides that if such benefits do not comply with Section 409A, they will be taxable in the first year they are not subject to a substantial risk of forfeiture. In such case, our executives would be subject to regular federal income tax, interest and an additional federal income tax of 20% of the benefit includible in income.
We have granted stock options as incentive stock options in accordance with Section 422 of the Code subject to the volume limitations contained in the Code. Generally, the exercise of an incentive stock option does not trigger any recognition of income or gain to the holder. If the stock is held until at least one year after the date of exercise (or two years from the date the option is granted, whichever is later), all of the gain on the sale of the stock, when recognized for income tax purposes will be capital gain, rather than ordinary income to the recipient. Consequently, we do not receive a tax deduction. For stock options that do not qualify as incentive stock options, we are entitled to a tax deduction in the year in which the stock options are exercised equal to the spread between the exercise price and the fair market value of the stock for which the stock option was exercised. The holders of the non-qualified stock options are generally taxed on this same amount in the year of exercise.
Post-2006 Actions
We engaged the compensation advisory services of Radford Surveys + Consulting to provide a competitive analysis of our compensation program, and review our plan designs, salaries, incentive compensation, employment agreements and overall executive compensation structure.
Effective March 27, 2007, we adopted, as approved by our shareholders, the 2007 Plan that will afford more flexibility to our compensation committee by allowing grants of a wide variety of equity awards to our key employees, directors and consultants, including non-qualified stock options, shares of restricted stock and other awards that are valued by reference to the fair market value of our common stock. This plan is designed to assist us in attracting, retaining, motivating and rewarding key employees, directors and consultants and providing long-term value for our stockholders by closely aligning the interests of these individuals with those of our stockholders. The 2007 Plan replaced our existing 2000 Plan, and no future grants will be made under the 2000 Plan. See “Equity Compensation Plans.”
On April 3, 2007, the following named executive officers received incentive stock options to purchase the following number of shares under the 2007 Plan at the price of $4.50 per share (a price determined by the board of directors to be equal to the fair market value on that date: 600,000 shares to Mr. Moffitt; 160,000 shares to Mr. Cork; 184,000 shares to Mr. McGarrity; and 40,000 shares to Dr. Shipp. For each of these option grants, one-half of the shares vest ratably over a four year period and one-half of the shares cliff-vest on the seventh anniversary of date of grant, subject to immediate vesting upon the achievement of corporate performance milestones related to our 2007 grants of equity awards as discussed below.
On April 25, 2007, we entered into an employment agreement with Mr. J. Roger Moody, Jr. in connection with his employment as our Chief Financial Officer. The employment agreement provides an initial base salary of $235,000, with a performance bonus opportunity of $90,000 per year. In connection with the commencement of his employment as our Chief Financial Officer, Mr. Moody received a $15,000 sign-on bonus and
74
incentive stock options to purchase 180,000 shares. On August 3, 2007, our chief executive officer recommended and the compensation committee approved an award of 20,000 additional options to Mr. Moody in connection with the commencement of his employment. For each of these option grants: (1) the exercise price is $4.50 per share (a price determined by the board of directors to be equal to the fair market value on that date), and (2) one-half of the shares vest ratably over a four-year period and one-half of the shares cliff-vest on the seventh anniversary of the date of grant, subject to immediate vesting upon the achievement of corporate performance milestones related to our 2007 grants of equity awards, as discussed below.
With respect to the acceleration of all cliff-vested stock options granted in 2007, there are four performance milestones associated with such options. Accordingly, 25% of such options granted shall immediately vest and become exercisable upon the achievement of each performance milestone. The four milestones are: (i) the achievement of a minimum annualized revenue and minimum gross profit margin from product sales for two consecutive quarters; (ii) the achievement of an increased minimum gross profit margin from product sales for two consecutive quarters; (iii) the commercial launch of the first infectious disease assay; and (iv) the commercial launch of the first ultra-sensitive protein assay. The achievement of these performance milestones shall be determined in the sole discretion of the compensation committee. We believe that each of the milestones is likely to be achieved and are commensurate with our long-term growth objectives.
Effective June 4, 2007, Mr. Stephen Wasko resigned as our Chief Financial Officer, Vice President of Finance and Treasurer, and Mr. J. Roger Moody, Jr. became our Chief Financial Officer, Vice President, Finance & Administration and Treasurer, effective the same date. In the discretion of the compensation committee, Mr. Wasko is receiving severance payments in the amount equal to five months of his base salary (payable in accordance with customary payroll practices). The severance payments may be extended for up to an additional three months or shortened, depending on the level of Mr. Wasko’s income replacement.
On August 8, 2007, Mr. Moffitt received $2.3 million pursuant to the terms of the bonus agreement, as amended. Under the bonus agreement, we paid Mr. Moffitt the bonus prior to the filing of our first registration statement on Form S-1 with the SEC.
Summary Compensation Table
The following summary compensation table sets forth certain information with respect to compensation for the year ended December 31, 2006 earned by or paid to our Chief Executive Officer, Chief Financial Officer and our three other most highly compensated executive officers, who are referred to as the named executive officers.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | Change in
| | | | | | | |
| | | | | | | | | | | | | | | | | | Non-Equity
| | | Pension Value
| | | | | | | |
| | | Fiscal
| | | | | | | | | Stock
| | | Option
| | | Incentive Plan
| | | and NQDC
| | | All Other
| | | | |
Name and Principal Position | | Year | | | Salary | | | Bonus | | | Awards | | | Awards(4) | | | Compensation(5) | | | Earnings | | | Compensation | | | Total | |
| |
William P. Moffitt III,
President and Chief Executive Officer(1) | | | 2006 | | | $ | 395,000 | | | | — | | | | — | (3) | | | — | | | $ | 150,000 | | | | — | | | | — | | | $ | 545,000 | |
Stephen G. Wasko,
Chief Financial Officer(2) | | | 2006 | | | $ | 204,000 | | | | — | | | | — | | | $ | 11,445 | | | | — | | | | — | | | | — | | | $ | 215,445 | |
William H. Cork,
Chief Technology Officer | | | 2006 | | | $ | 263,000 | | | $ | 7,187(6 | ) | | | — | | | $ | 34,732 | | | $ | 47,813 | | | | — | | | | — | | | $ | 352,732 | |
Michael K. McGarrity,
Chief Marketing Officer | | | 2006 | | | $ | 244,807 | | | | — | | | | — | | | $ | 28,756 | | | $ | 61,000 | | | | — | | | | — | | | $ | 334,563 | |
Gregory W. Shipp,
Chief Medical Officer | | | 2006 | | | $ | 232,088 | | | | — | | | | — | | | $ | 13,097 | | | $ | 43,125 | | | | — | | | $ | 1,875 | (7) | | $ | 290,185 | |
| | |
| (1) | | Mr. Moffitt also served as a director. A director who is an employee does not receive payment for service as a director. |
| |
| (2) | | Effective June 4, 2007, Mr. Stephen Wasko resigned as our Chief Financial Officer. |
75
| | |
| (3) | | On March 16, 2006, we offered to Mr. Moffitt (in lieu of an option grant he would have received under his 2004 employment agreement, but for cancellation of that grant) an ability to purchase 320,000 shares of restricted common stock for a price fixed at an amount that was determined by the board of directors to be greater than the fair market value of the shares on the date of the offer. Since the purchase price of the restricted stock awards is above fair market value, the fair value of the award was de-minimus and resulted in no compensation expense associated with this award. The terms of the restricted stock purchase awards are described in “Compensation Discussion and Analysis for Named Executive Officers — Restricted Stock Purchase Awards” and the “Grants of Plan-Based Awards” table. |
| |
| (4) | | The fair values of our option awards were estimated at the dates of grant using the Black-Scholes option pricing model with the following assumptions: |
| | | | | | | | | | | | | | | | | |
| | | 2004 | | | 2005 | | | 2006 | | | 2007 | |
| |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
| Expected volatility | | | 75 | % | | | 80 | % | | | 85 | % | | | 77 | % |
| Risk free interest rate | | | 3.94 | | | | 4.13 | | | | 4.82 | | | | 4.66 | |
| Weighted-average expected option life | | | 6.3 years | | | | 7.5 years | | | | 8.4 years | | | | 7.0 years | |
| Estimated weighted average fair value on the date of grant based on the above assumptions | | $ | 1.24 | | | $ | 2.28 | | | $ | 2.56 | | | $ | 3.31 | |
| Estimated forfeiture rate for unvested options | | | N/A | | | | 12.5 | % | | | 12.5 | % | | | 4.6 | % |
| | |
| | Expected volatility is based on calculated stock volatilities for publicly traded companies in the same industry and general stage of development as us. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of the grants for periods consistent with the expected life of the option. The expected life of options granted is derived from the average of the vesting period and the term of the option as defined in the respective incentive plans, following the guidance in SEC Staff Accounting Bulletin No. 107,Share-Based Payment. |
| |
| (5) | | Amounts shown in the “Non-Equity Incentive Plan Compensation” column reflect the annual incentive award granted and earned during fiscal year 2006, and paid in fiscal year 2007. These annual awards are described in further detail under “Compensation Discussion and Analysis for Named Executive Officers — Annual Cash Incentive Compensation” and are also reflected in the table “Grants of Plan-Based Awards” under the column “Estimated Future Payouts Under Non-Equity Incentive Plan Awards.” |
| |
| (6) | | The compensation committee supplemented Mr. Cork’s cash award under the management incentive bonus program with a discretionary bonus for a combined payout of $55,000. The award is described further in the “Compensation Discussion and Analysis for Named Executive Officers — Annual Cash Incentive Compensation.” |
| |
| (7) | | The amount reflects the relocation expenses provided to Dr. Shipp. |
Grants of Plan-Based Awards
Pursuant to our management incentive bonus plan and the 2000 Plan, we granted both cash and equity awards during fiscal year 2006. The cash incentives awards were granted, subject to a target performance threshold. The equity incentive awards were granted in the form of incentive stock options and restricted stock purchase awards.
76
The following table shows information with respect to awards granted to the named executive officers during the fiscal year 2006 under the management incentive bonus plan and the 2000 Plan.
Estimated Future Payouts Under Non-Equity Incentive Plan Awards:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | Exercise or
| | | | |
| | | | | | | | | | | | | | | Base Price
| | | | |
| | | | | | | | | | | | All Other
| | | of
| | | | |
| | | | | | Estimated Future
| | | All Other
| | | Options
| | | Restricted
| | | | |
| | | | | | Payouts Under
| | | Stock
| | | Awards:
| | | Stock
| | | | |
| | | | | | Non-Equity
| | | Awards:
| | | Number of
| | | Purchase
| | | Grant Date
| |
| | | | | | Incentive Plan
| | | Number of
| | | Securities
| | | Awards and
| | | Fair Value of
| |
| | | | | | Awards
| | | Shares of
| | | Underlying
| | | Option
| | | Option
| |
Name | | Grant Date | | | Target(1) | | | Stock or Units | | | Options | | | Awards(5) | | | Awards | |
| |
| William P. Moffitt III | | | — | | | $ | 200,000 | | | | — | | | | — | | | | — | | | | — | |
| | | | 3/16/2006 | | | | — | | | | 320,000 | (3) | | | — | | | $ | 4.50 | | | | — | |
| Stephen G. Wasko | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| William H. Cork | | | — | | | $ | 75,000 | | | | — | | | | — | | | | — | | | | — | |
| | | | — | | | $ | 50,000 | (2) | | | — | | | | — | | | | — | | | | — | |
| Michael K. McGarrity | | | 3/14/2006 | | | | — | | | | — | | | | 30,000 | (4) | | $ | 4.50 | | | $ | 76,917 | (6) |
| | | | | | | $ | 90,000 | | | | — | | | | — | | | | — | | | | — | |
| Gregory W. Shipp | | | — | | | $ | 50,000 | | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | Unless otherwise provided, amounts shown in the “Estimated Future Payouts Under Non-Equity Incentive Plan Awards Target” column reflect the cash incentive awards payable under the management incentive bonus plan to the named executive officers, provided the executive officer achieves certain performance-based milestones. |
| |
| (2) | | Mr. Cork was granted a potential cash incentive opportunity of $50,000 conditioned upon the full achievement of the product launch timeline, as set forth in our 2007 board of directors-approved operating budget. |
| |
| (3) | | This amount reflects the number of shares acquired by Mr. Moffitt pursuant to a restricted stock purchase award at $4.50 per share, a price the board of directors determined to be greater than the $3.25 per share fair market value of the shares on the date of the offer, for an aggregate purchase price of $1,440,000. Mr. Moffitt purchased the shares by delivering an executed promissory note to us secured by the underlying shares and repaid the loan in August 2007. |
| |
| (4) | | On March 14, 2006, Mr. McGarrity received options to purchase 30,000 shares of common stock at the price of $4.50 per share, a price the board of directors determined to be greater than the $3.25 per share fair market value on the date of grant. The shares have a term of ten years and vest on the seventh anniversary of the date of grant, subject to immediate vesting upon the achievement of corporate performance milestones. See “Compensation Discussion and Analysis for Named Executive Officers — Stock Option Awards.” |
| |
| (5) | | The exercise price of the stock options and the price paid for the purchase of restricted stock awards reflect an amount that the board of directors determined to be in excess of the fair market value of $3.25 per share. The determination of the exercise price of the stock options is described in further detail in the “Compensation Discussion and Analysis for Named Executive Officers — Stock Option Awards.” |
| | |
| (6) | | We determined the total grant-date fair value of Mr. McGarrity’s options using the Black-Scholes option pricing model with the assumptions set forth in footnote 4 of the “Summary Compensation” table. |
The nonequity incentive plan compensation varies between the targets reported on the “Grants of Plan-Based Awards” table and the “Summary Compensation” table. The compensation committee established the management incentive bonus plan, in which the compensation committee establishes business goals for the named executive officers for the year, the results of which are substantially uncertain at the time they are established. The performance goals generally relate to the acceleration of product launches, development of additional assays, the creation of a strategic plan for a particular business unit, and submission of FDA applications. The potential incentive compensation is set forth within the employment agreement for the executive officers, which may be adjusted from time to time in the discretion of the compensation committee. The compensation committee has the discretion to pay a reduced amount of incentive compensation in the event that the targeted goals are only partially achieved.
77
Mr. McGarrity’s grant of stock option awards is subject to a seven year cliff-vesting schedule which may be accelerated in 20% increments upon the achievement of each of five performance milestones. See “Compensation Discussion and Analysis of Named Executive Officers — Stock Options Awards” for details regarding the milestones.
Outstanding Equity Awards at December 31, 2006
The following table shows information with respect to the unexercised options and other equity-based awards held by the named executive officers as of December 31, 2006.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Stock Awards | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | Equity
| |
| | | Option Award | | | | | | | | | | | | Incentive Plan
| |
| | | | | | | | | Equity
| | | | | | | | | | | | | | | Equity
| | | Awards:
| |
| | | | | | | | | Incentive Plan
| | | | | | | | | | | | | | | Incentive Plan
| | | Market or
| |
| | | | | | | | | Awards:
| | | | | | | | | | | | Market
| | | Awards:
| | | Payout Value
| |
| | | | | | Number of
| | | Number of
| | | | | | | | | | | | Value of
| | | Number of
| | | of Unearned
| |
| | | | | | Securities
| | | Securities
| | | | | | | | | Number of
| | | Shares or
| | | Unearned
| | | Shares, Units
| |
| | | | | | Underlying
| | | Underlying
| | | | | | | | | Shares or
| | | Units of
| | | Shares, Units or
| | | or Other
| |
| | | | | | Unexercised
| | | Unexercised
| | | Option
| | | Option
| | | Units of Stock
| | | Stock that
| | | Other Rights
| | | Rights That
| |
| | | | | | Options
| | | Unearned
| | | Exercise
| | | Expiration
| | | that Have Not
| | | Have Not
| | | that Have Not
| | | Have Not
| |
Name | | Exercisable | | | Unexercisable | | | Options | | | Price | | | Date(1) | | | Vested(4) | | | Vested | | | Vested | | | Vested | |
| |
| William P. Moffitt III | | | — | | | | — | | | | — | | | | — | | | | — | | | | 80,000 | | | $ | 360,000 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | �� | | |
| Stephen G. Wasko | | | 10,500 | (2) | | | 3,500 | (2) | | | — | | | $ | 7.50 | | | | 10/22/2013 | | | | — | | | | — | | | | — | | | | — | |
| | | | 1,500 | (2) | | | 1,500 | (2) | | | | | | $ | 7.50 | | | | 1/5/2014 | | | | | | | | | | | | | | | | | |
| | | | — | (3) | | | 22,800 | (3) | | | | | | $ | 4.50 | | | | 5/12/2015 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| William H. Cork | | | 9,600 | (2) | | | — | (2) | | | — | | | $ | 11.50 | | | | 3/19/2011 | | | | — | | | | — | | | | — | | | | — | |
| | | | 3,200 | (2) | | | — | (2) | | | | | | $ | 36.75 | | | | 1/1/2012 | | | | | | | | | | | | | | | | | |
| | | | 9,000 | (2) | | | 3,000 | (2) | | | | | | $ | 7.50 | | | | 1/15/2013 | | | | | | | | | | | | | | | | | |
| | | | 10,000 | (2) | | | 10,000 | (2) | | | | | | $ | 7.50 | | | | 1/5/2014 | | | | | | | | | | | | | | | | | |
| | | | — | (3) | | | 80,000 | (3) | | | | | | $ | 4.50 | | | | 5/12/2015 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Michael K. McGarrity | | | 5,000 | (2) | | | 15,000 | (2) | | | — | | | $ | 4.50 | | | | 9/29/2015 | | | | — | | | | — | | | | — | | | | — | |
| | | | — | (3) | | | 30,000 | (3) | | | | | | $ | 4.50 | | | | 9/29/2015 | | | | | | | | | | | | | | | | | |
| | | | — | (3) | | | 30,000 | (3) | | | | | | $ | 4.50 | | | | 3/14/2016 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Gregory W. Shipp | | | — | (3) | | | 40,000 | (3) | | | — | | | $ | 4.50 | | | | 6/13/2015 | | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | The expiration date of each incentive stock option occurs ten years after the date of grant. |
| |
| (2) | | The incentive stock options vest in 25% increments beginning on the first anniversary of the date of grant and on each anniversary thereafter. |
| |
| (3) | | The incentive stock options cliff vest on the seventh anniversary of the date of grant. Upon our achievement of certain performance-based milestones, vesting may be accelerated. See “Compensation Discussion and Analysis for Named Executive Officers — Stock Options” for details regarding the milestones. |
| |
| (4) | | In the event of Mr. Moffitt’s voluntary resignation without good reason or our termination of his employment for cause prior to July 19, 2007, one-fourth of the shares covered by Mr. Moffitt’s restricted stock purchase agreement (i.e., the 80,000 restricted shares included in this table) was subject to mandatory repurchase by us prior to July 19, 2007. |
78
Option Exercises and Stock Vested
The following table shows information regarding options exercised and vesting of restricted stock during fiscal 2006. No options were exercised in fiscal year 2006.
| | | | | | | | | | | | | | | | | |
| | | Option Awards | | | Stock Awards | |
| | | Number of Shares Acquired
| | | Value Realized on
| | | Number of Shares
| | | Value Realized on
| |
| | | on Exercise | | | Exercise | | | Acquired on Vesting(1) | | | Vesting | |
| |
| William P. Moffitt III | | | — | | | | — | | | | 240,000 | | | | — | |
| Stephen G. Wasko | | | — | | | | — | | | | — | | | | — | |
| William H. Cork | | | — | | | | — | | | | — | | | | — | |
| Michael K. McGarrity | | | — | | | | — | | | | — | | | | — | |
| Gregory W. Shipp | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | On March 16, 2006, Mr. Moffitt purchased 320,000 shares of restricted common stock. The shares were subject to our ability to repurchase the shares for $4.50, the price paid by Mr. Moffitt, in the event his termination of employment by us for cause or his resignation without good reason. The repurchase restrictions lapsed with respect to 160,000 shares immediately on the date of purchase, and an additional 80,000 shares on July 19, 2006, the anniversary of his employment. The repurchase restrictions on the remaining 80,000 shares lapsed on July 19, 2007. The terms of the restricted stock purchase awards are described in “Compensation Discussion and Analysis of Named Executive Officers — Restricted Stock Purchase Awards” as well as the “Grants of Plan-Based Awards” table. |
Equity Compensation Plans
2007 Long-Term Incentive Plan
Our board of directors adopted, and our shareholders approved, the Nanosphere 2007 Long-Term Incentive Plan on March 27, 2007 (as amended as of June 4, 2007), in order to provide incentives and awards to employees, directors, consultants and advisors of ours and our affiliates, referred to herein as eligible persons. Prior to completion of this offering, we intend to amend the 2007 Plan to increase the number of authorized shares under the 2007 Plan and to include an “evergreen” provision, which provides for an annual increase in the number of shares available for issuance under the plan on the first day of each fiscal year beginning 2009 to 2012. The maximum aggregate number of shares issuable under the 2007 Plan will be 4,016,141 shares, plus up to an additional 1,181,128 shares of common stock that will become available in the event that awards made under the 2000 Plan expire, are forfeited or cancelled, plus an annual increase in the number of shares equal to the least of:
| | |
| | • | 900,000 shares of common stock; |
| |
| | • | 4.0% of our outstanding shares of common stock as of such date; and |
| |
| | • | an amount determined by our board of directors. |
The 2007 Plan permits awards of options, shares appreciation rights, restricted shares, restricted share units, unrestricted shares, deferred share units and performance shares.
Shares Subject to the 2007 Plan. Currently, the 2007 Plan provides that no more than 2,616,141 shares of common stock, plus up to an additional 1,181,128 shares of common stock that will become available in the event that awards made under the 2000 Plan expire, are forfeited or cancelled, may be issued pursuant to amounts under the 2007 Plan. The number of shares available for awards, as well as the terms of outstanding awards, are subject to adjustment for stock splits, stock dividends, recapitalizations and other similar events. Shares of common stock that are subject to any award that expires, or is forfeited, cancelled or becomes unexercisable will again be available for subsequent awards, except as prohibited by law.
Administration. Either the board of directors or a committee appointed by the board of directors will administer the 2007 Plan. The compensation committee of the board of directors is currently acting as the committee for purposes of the 2007 Plan.
Subject to the terms of the 2007 Plan, the committee has express authority to determine the eligible persons who will receive awards, the number of shares of common stock, units or dollars to be covered by
79
each award, and the terms and conditions of awards. The committee has broad discretion to prescribe, amend and rescind rules relating to the 2007 Plan and its administration, to interpret and construe the 2007 Plan and the terms of all award agreements and to take all actions necessary or advisable to administer the 2007 Plan. Within the limits of the 2007 Plan, the committee may accelerate the vesting of any award, allow the exercise of unvested awards and may modify, replace, cancel or renew them.
Eligibility. The committee may grant options that are intended to qualify as incentive stock options, or ISOs, only to employees, and may grant all other awards to eligible persons. The 2007 Plan provides that no participant may receive options and share appreciation rights, or SARs, that relate to more than 1,000,000 shares of common stock during any twelve month period. As of September 30, 2007, all of our 102 employees (including officers) and all five of our non-employee directors would have been eligible to participate in the 2007 Plan.
Options. Options granted under the 2007 Plan provide participants with the right to purchase shares of common stock at a predetermined exercise price. The committee may grant options that are intended to qualify as ISOs or options that are not intended to so qualify, or Non-ISOs. The 2007 Plan also provides that ISO treatment may not be available for options that become first exercisable in any calendar year to the extent the value of the underlying shares that are the subject of the option exceed $100,000 (based upon the fair market value of the shares of common stock on the option grant date).
Share Appreciation Rights. A share appreciation right generally permits a participant who receives it to receive, upon exercise, cashand/or shares of common stock equal in value to an amount determined by multiplying (1) the excess of the fair market value, on the date of exercise, of the shares of common stock with respect to which the SAR is being exercised, over the exercise price of the SAR for such shares by (2) the number of shares with respect to which the SARs are being exercised. The committee may grant SARs in tandem with options or independently of them. SARs that are independent of options may limit the value payable on its exercise to a percentage, not exceeding 100%, of the excess value.
Exercise Price for Options and SARs. The exercise price of ISOs, Non-ISOs and SARs may not be less than 100% of the fair market value on the grant date of the shares of common stock subject to the award (110% of fair market value for ISOs granted to employees who, at the time of grant, own more than 10% of our outstanding shares of common stock).
Exercise of Options and SARs. To the extent exercisable in accordance with the agreement granting them, an option or SAR may be exercised in whole or in part, and from time to time during its term; subject to earlier termination relating to a holder’s termination of employment or service. With respect to options, the committee has the discretion to accept payment of the exercise price in any of the following forms (or combination of them): cash or check in U.S. dollars, certain shares of common stock and cashless exercise under a program the committee approves. The term over which participants may exercise options and SARs may not exceed ten years from the date of grant (five years in the case of ISOs granted to employees who, at the time of grant, own more than 10% of our outstanding shares of common stock).
Subject to the terms of the agreement evidencing an option grant, the option may be exercised during the three month period after the optionee retires, during the one year period after the optionee’s termination of service due to death or permanent disability and during the 90 day period after the optionee’s termination of employment without cause (but in no case later than the termination date of the option). The agreements evidencing the grant of an option may, in the discretion of the committee, set forth additional or different terms and conditions applicable to such option upon a termination or change in status of the employment or service of the option holder. All SARs are to be settled in shares of our stock and shall be counted in full against the number of shares available for award under the 2007 Plan, regardless of the number of exercise gain shares issued upon settlement of the SARs.
Restricted Shares, Restricted Share Units, Unrestricted Shares and Deferred Share Units. Under the 2007 Plan, the committee may grant restricted shares that are forfeitable until certain vesting requirements are met, may grant restricted share units which represent the right to receive shares of common stock after certain vesting requirements are met, and may grant unrestricted shares as to which the participant’s interest is immediately vested. For restricted awards, the 2007 Plan provides the committee with discretion to determine the terms and conditions under which a participant’s interests in such awards becomes vested. The 2007 Plan
80
provides for deferred share units in order to permit certain directors, consultants, agents, or select members of management to defer their receipt of compensation payable in cash or shares of common stock (including shares that would otherwise be issued upon the vesting of restricted shares and restricted share units).
Whenever shares of common stock are delivered pursuant to these awards, the participant will be entitled to receive additional shares of common stock equal to the sum of (1) any stock dividends that our stockholders received between the date of the award and issuance or release of the shares of common stock and (2) a number of additional shares of common stock equal to the shares of common stock that the participant could have purchased at fair market value on the payment date of any cash dividends for shares of common stock if the Participant had received such cash dividends between its grant date and its settlement date.
Performance Awards. The 2007 Plan authorizes the committee to grant performance-based awards in the form of performance units that the committee may or may not designate as “performance compensation awards” that are intended to be exempt from Section 162(m) of the Code limitations. In either case, performance compensation awards vest and become payable based upon the achievement, within the specified period of time, of performance objectives applicable to the individual or us. Performance compensation awards are payable in shares of common stock, cash or some combination of the two and shall not exceed 1,000,000 shares of common stock and $1,000,000 in cash. The committee decides the length of performance periods, but the periods may not be less than one of our fiscal years.
With respect to performance compensation awards, the 2007 Plan requires that the committee specify in writing the performance period to which the award relates, and an objective formula by which to measure whether and the extent to which the award is earned on the basis of the level of performance achieved with respect to one or more performance measures. Once established for a performance period, the performance measures and performance formula applicable to the award may not be amended or modified in a manner that would cause the compensation payable under the award to fail to constitute performance-based compensation under Section 162(m) of the Code.
Buyout Provision. The committee may at any time offer to buy out an option, in exchange for a payment in cash or shares of common stock, based on such terms and conditions as the committee shall establish and communicate to the participant at the time that such offer is made. In addition, if the fair market value for shares of common stock subject to an option is more than 33% below their exercise price for more than 30 consecutive business days, the committee may unilaterally terminate and cancel the option either (1) by paying the participant, in cash or shares of common stock, an amount not less than the Black-Scholes or other value of the vested portion of the option, or (2) if approved by shareholders, by irrevocably committing to grant a new option, on a designated date more than six months after such termination and cancellation of such option (but only if the participant’s continuous service has not terminated prior to such designated date), pursuant to terms and conditions determined by the committee.
Certain Corporate Transactions. The committee shall equitably adjust the number of shares covered by each outstanding award, and the number of shares that have been authorized for issuance under the 2007 Plan, as well as the price per share covered by each such outstanding award, to reflect any increase or decrease in the number of issued shares resulting from a stock split, reverse stock split, stock dividend, combination, recapitalization or reclassification of the shares, or any other increase or decrease in the number of issued shares effected without receipt of consideration by us. In the event of any such transaction or event, the committee may provide in substitution for any or all outstanding options under the 2007 Plan such alternative consideration (including securities of any surviving entity) as it may in good faith determine to be equitable under the circumstances and may require in connection therewith the surrender of all options so replaced. In any case, such substitution of securities will not require the consent of any person who is granted options pursuant to the 2007 Plan.
In addition, in the event or in anticipation of a change in control, as defined in the 2007 Plan, the committee may at any time in its sole and absolute discretion and authority, without obtaining the approval or consent of our stockholders or any participant with respect to his or her outstanding awards (except to the extent an award provides otherwise), take one or more of the following actions: (1) arrange for or otherwise provide that each outstanding award will be assumed or substituted with a substantially equivalent award by a successor corporation or a parent or subsidiary of such successor corporation; (2) accelerate the vesting of
81
awards for any period (and may provide for termination of unexercised options and SARs at the end of that period) so that awards shall vest (and, to the extent applicable, become exercisable) as to the shares of common stock that otherwise would have been unvested and provide that our repurchase rights with respect to shares of common stock issued upon exercise of an award shall lapse as to the shares of common stock subject to such repurchase right; (3) arrange or otherwise provide for payment of cash or other consideration to participants in exchange for the satisfaction and cancellation of outstanding awards; or (4) terminate awards upon the consummation of the transaction, provided that the committee may in its sole and absolute discretion provide for vesting of all or some outstanding awards in full as of a date immediately prior to consummation of the change in control. To the extent that an award is not exercised prior to consummation of a transaction in which the award is not being assumed or substituted, such award shall terminate upon such consummation.
In the event of any distribution to our stockholders of securities of any other entity or other assets (other than dividends payable in cash or our stock) without receipt of consideration by us, the committee may, in its discretion, appropriately adjust the price per share covered by each outstanding award to reflect the effect of such distribution. Finally, if we dissolve or liquidate, all awards will terminate immediately prior to such dissolution or liquidation, subject to the ability of the board of directors to exercise any discretion that it may exercise in the case of a change in control.
2000 Equity Incentive Plan
Our board of directors adopted our 2000 Equity Incentive Plan on September 8, 2000 (as amended as of February 2, 2006), in order to provide incentives and awards to eligible persons. The 2000 Plan permits awards of options, shares appreciate rights and restricted shares. In connection with the approval of the 2007 Plan, we terminated the 2000 Plan and therefore, we may not make any further awards of options, share appreciation rights or restricted shares under the 2000 Plan.
Shares Subject to the 2000 Plan. The 2000 Plan provides that no more than 1,600,000 shares of common stock may be issued pursuant to awards under the 2000 Plan. The number of shares available for awards, as well as the terms of outstanding awards, are subject to adjustment as provided in the 2000 Plan for stock splits, stock dividends, recapitalizations and other similar events. Shares of common stock that remain unissued at the termination of the 2000 Plan shall cease to be subject to the 2000 Plan.
Administration. Either the board of directors or a committee which shall be comprised of at least two directors of the board will administer the 2000 Plan. The board of directors and any committee exercising discretion under the 2000 Plan from time to time are referred to in this section as the committee.
Subject to the terms of the 2000 Plan, the committee has express authority to determine the eligible persons who will receive awards, the number of shares of common stock to be covered by each award, and the terms and conditions of awards. The committee has broad discretion to prescribe, amend, and rescind rules relating to the 2000 Plan and its administration, to interpret and construe the 2000 Plan and the terms of all award agreements, and to take all actions necessary or advisable to administer the 2000 Plan.
Eligibility. The committee may grant options that are intended to qualify as ISOs only to employees, and may grant all other awards to eligible persons. As of September 30, 2007, all of our 102 employees (including officers) and all five of our non-employee directors would have been eligible to participate in the 2000 Plan.
Options. Options granted under the 2000 Plan provide participants with the right to purchase shares of common stock at a predetermined exercise price. The committee may grant options that are intended to qualify as ISOs or Non-ISOs. The 2000 Plan also provides that ISO treatment may not be available for options that become first exercisable in any calendar year to the extent the value of the underlying shares that are the subject of the option exceed $100,000.
Stock Appreciation Rights. The committee may grant SARs in tandem with options or independently of them.
Exercise Price for Options and SARs. The exercise price of ISOs and Non-ISOs may not be less than 100% of the fair market value on the grant date of the shares of common stock subject to the award (110% of
82
fair market value for ISOs granted to employees who, at the time of grant, own more than 10% of our outstanding shares of common stock).
Exercise of Options and SARs. To the extent exercisable in accordance with the agreement granting them, an option or SAR may be exercised in whole or in part, and from time to time during its term; subject to earlier termination relating to a holder’s termination of employment or service. With respect to options, the option price may be paid in any one or a combination of cash, personal check, personal note, certain shares of common stock or a broker exercise notice. The term over which participants may exercise options and SARs may not exceed ten years from the date of grant (five years in the case of ISOs granted to employees who, at the time of grant, own more than 10% of our outstanding shares of common stock).
Subject to the terms of the agreement evidencing an option grant, the option may be exercised during the three month period after the optionee terminates employment with us and during the one year period after the optionee’s termination of service due to death or permanent disability. The agreements evidencing the grant of an option may, in the discretion of the committee, set forth additional or different terms and conditions applicable to such option upon a termination or change in status of the employment or service of the option holder.
Restricted Shares. Under the 2000 Plan, the committee may grant restricted shares that are forfeitable until certain vesting requirements are met. For restricted awards, the 2000 Plan provides the committee with discretion to determine the terms and conditions under which a participant’s interests in such awards becomes vested.
Repurchase Right in Case of Termination. Following the termination of a participant’s employment for any reason prior to a the shares being registered under the Securities Act of 1933, we have the right, but not the obligation, to purchase any shares of common stock acquired by the participant pursuant to an option. The purchase price is determined in accordance with the provisions of the 2000 Plan.
Repurchase Right in Case of Change in Control. In the event of a change in control, as that term is defined in the 2000 Plan, transaction pursuant to which shares of common stock are entitled to receive consideration, we have the right to cause the participant to sell all or any portion of any unexpired or unexercised option or right, whether vested or unvested. The purchase price is determined in accordance with the provisions of the 2000 Plan and can paid in either cash or in the form of consideration that is being paid to our shareholders as determined by us in our sole discretion.
Arrangements with Named Executive Officers
Mr. William P. Moffitt III. We entered into an employment agreement dated July 19, 2004 with Mr. Moffitt, in connection with his employment as our President and Chief Executive Officer. The employment agreement provides an initial base salary of $350,000 per year, which shall be reviewed annually and may be increased, but not decreased below $350,000, by our board of directors. The agreement also provides Mr. Moffitt with a target bonus opportunity of $75,000 in 2004 and $150,000 in 2005, subject to the achievement of agreed goals and milestones. For calendar years ended after 2005, Mr. Moffitt’s target bonus amount may be increased, but not reduced below $150,000, by our board of directors. In addition, Mr. Moffitt’s employment agreement provided for the grant of options to purchase shares of our common stock representing 5% of our fully diluted capital stock. The options would vest in substantially equal annual installments during his initial employment term, commencing July 19, 2004 and ending July 18, 2008. This option grant was ultimately cancelled without any awards being granted to Mr. Moffitt.
In 2006, Mr. Moffitt’s annual rate of salary was increased to $395,000 and the target amount of his performance bonus opportunity was increased to $200,000. On March 16, 2006, we entered into an amended employment agreement with Mr. Moffitt, in which a restricted stock purchase award was granted in lieu of effecting the option grant under the initial agreement. Accordingly, Mr. Moffitt purchased 320,000 shares of our common stock at a price of $4.50 per share, a price the board of directors determined to be greater than the fair market value on the date of grant, for an aggregate purchase price of $1,440,000. The shares were issued to Mr. Moffitt upon his execution and delivery of a promissory note, secured by such shares. The restricted stock contains a repurchase right, which effectively requires Mr. Moffitt to resell to us any “unvested shares,” purchased at the price paid by Mr. Moffitt, in the event of his voluntary resignation without good
83
reason or our termination of his employment for cause. Of the 320,000 shares, 160,000 shares vested immediately upon purchase; 80,000 shares vested on July 19, 2006; and 80,000 shares vested on July 19, 2007. Also on March 16, 2006, we entered into a bonus arrangement with Mr. Moffitt to retain him as our Chief Executive Officer for the next several years in order to assist us with possible strategic transactions. Under the agreement, Mr. Moffitt is eligible to receive a cash bonus in the amount of $2,300,000, subject to his continuous employment with us until March 16, 2011, or if earlier, upon (1) termination by Mr. Moffitt for good reason, (2) termination by us without cause, (3) termination by us due to non-renewal of his initial employment term, expiring July 19, 2008, or (4) his death or permanent disability. In addition, our obligation to pay the bonus to Mr. Moffitt will become immediately payable upon bankruptcy of the company, immediately prior to our filing a registration statement in connection with an initial public offering of our securities, or immediately prior to a change in control. The bonus was paid in full in August 2007. See“Post-2006 Actions.”
In the event we terminate Mr. Moffitt’s employment without cause or we terminate Mr. Moffitt’s employment by a non-renewal of the employment agreement, or Mr. Moffitt resigns for good reason, we must pay to Mr. Moffitt: (1) his base salary for a period of 18 months (30 months if the termination was within one year after on a change in control), (2) his full target bonus for the year of termination, and (3) immediate and full vesting of all outstanding options and restricted stock awards, which, in the case of options, shall remain exercisable for a period of one year following the date of termination. In the event Mr. Moffitt’s employment is terminated due to his permanent disability or death, Mr. Moffitt (or his estate) shall be entitled to immediate and full vesting of all outstanding options and restricted stock awards, and to exercise such options within one year of the date of termination of employment. In the event we terminate Mr. Moffitt for cause, any unvested options shall be forfeited and any vested options shall expire and shall no longer be exercisable as of the date of termination. Mr. Moffitt’s employment agreement also provides for an excise tax gross-up payment if payments received under the agreement and other payments received under other agreements or employee benefit plans in connection with a change in our control result in the imposition of a golden parachute excise tax under Section 4999 of the Code.
Michael K. McGarrity. We entered into an employment agreement dated September 8, 2005 with Mr. McGarrity, in connection with his employment as our Chief Marketing Officer. The employment agreement provides an initial base salary of $235,000 per year, or such greater amount as our board of directors may from time to time establish. The agreement also provides Mr. McGarrity with a performance bonus opportunity of $90,000 per year. In the event we terminate Mr. McGarrity’s employment for reasons other than cause, including as a result of death or disability, Mr. McGarrity will be entitled to a severance payment equal to six months’ base salary plus a prorated calculation of his annual bonus.
William H. Cork. We entered into an employment agreement dated January 2, 2001 with Mr. Cork, in connection with his employment as our Vice President of Product Development. The employment agreement provides an initial base salary of $176,000 per year, or such greater amount as our board of directors may from time to time establish. Mr. Cork’s employment agreement establishes an at-will employee relationship and does not provide for any severance arrangements. Accordingly, upon a termination for cause, without cause, change in control or any other reason, Mr. Cork shall receive his accrued salary, earned bonus, unreimbursed expenses and other entitlements to the date of termination, unless we decide at that time to provide additional severance payments. We have not entered into a new employment agreement with Mr. Cork in connection with his current position as our Chief Technology Officer.
Gregory W. Shipp. We entered into an employment agreement dated May 13, 2005 with Dr. Shipp, in connection with his employment as our Vice President of Medical Affairs. The employment agreement provides an initial base salary of $227,000 per year, or such greater amount as our board of directors may from time to time establish. The agreement also provides Dr. Shipp with an initial performance bonus opportunity of $45,000 per year, in which our committee increased the performance bonus opportunity to $50,000 per year as of 2006. Dr. Shipp’s employment agreement establishes an at-will employee relationship and does not provide for any severance arrangements. Accordingly, upon a termination for cause, without cause, change in control or any other reason, Dr. Shipp shall receive his accrued salary, earned bonus, unreimbursed expenses and other entitlements to the date of termination, unless we decide at that time to provide additional severance payments. We have not entered into a new employment agreement with Dr. Shipp
84
in connection with his current position as Chief Medical Officer, Vice President, Medical and Regulatory Affairs and Quality Assurance.
Each of our executive officers has entered into our standard employment agreement, which contains customary provisions relating to the handling of proprietary and confidential information, as well as restrictions on competition and solicitation during the period of employment and for one year after termination.
Estimate of Post-Employment Payments
(Assumes a December 31, 2006 Employment Termination Event)
The following table sets forth the additional amounts that could have been realized by each named executive officer if termination of his employment were to occur as of December 31, 2006 under the following circumstances.
| | | | | | | | | | | | | |
| | | Cash Severance
| | | Excise Tax &
| | | Total Termination
| |
Name and Termination Event | | Payment(2) | | | Gross-Up | | | Benefits | |
| |
William P. Moffitt III | | | | | | | | | | | | |
| Without cause, good reason, or non-renewal of agreement by us | | $ | 3,092,500 | (3) | | $ | — | | | $ | 3,092,500 | |
| Disability | | $ | 2,300,000 | (4) | | $ | — | | | $ | 2,300,000 | |
| Death | | $ | 2,300,000 | (4) | | $ | — | | | $ | 2,300,000 | |
| Involuntary or good reason after change in control | | $ | 3,487,500 | (5) | | $ | 1,016,733 | (7) | | $ | 4,504,233 | |
Michael K. McGarrity | | | | | | | | | | | | |
| Without cause or good reason | | $ | 215,000 | (6) | | $ | — | | | $ | 215,000 | |
| Disability | | $ | 215,000 | (6) | | $ | — | | | $ | 215,000 | |
| Death | | $ | 215,000 | (6) | | $ | — | | | $ | 215,000 | |
| Involuntary or good reason after change in control | | $ | 215,000 | (6) | | $ | — | | | $ | 215,000 | |
Stephen G. Wasko(1), William H. Cork and Gregory W. Shipp | | | | | | | | | | | | |
| Without cause or good reason | | $ | — | | | $ | — | | | $ | — | |
| Disability | | $ | — | | | $ | — | | | $ | — | |
| Death | | $ | — | | | $ | — | | | $ | — | |
| Involuntary or good reason after change in control | | $ | — | | | $ | — | | | $ | — | |
| | |
| (1) | | Effective June 4, 2007, Mr. Wasko resigned as our Chief Financial Officer. In the absence of a severance agreement with us, Mr. Wasko was not entitled to any severance payments, unless the compensation committee decides otherwise at the time of his termination of employment. The compensation committee granted Mr. Wasko five months of continued salary (payable in accordance with customary payroll practices). The severance payments may be extended up to three additional months or shortened, depending on the level of Mr. Wasko’s income replacement. |
| |
| (2) | | Accrued salary, unreimbursed expenses and other entitlements to the date of termination, or Entitlements. |
| |
| (3) | | Eighteen months’ salary ($592,500), plus target performance bonus ($200,000), plus 2006 bonus agreement ($2,300,000), plus entitlements. |
| |
| (4) | | The 2006 bonus agreement ($2,300,000), plus Entitlements. |
| |
| (5) | | Thirty months’ salary ($987,500), plus target performance bonus ($200,000), plus 2006 bonus agreement ($2,300,000), plus Entitlements. |
| |
| (6) | | Six months’ salary ($125,000), plus a prorated calculation of annual bonus ($90,000), plus Entitlements. |
| |
| (7) | | The excise tax gross-up is calculated assuming the excise tax rate of 20% of the excess of the value of the change in control payments over Mr. Moffitt’s average W-2 earnings for calendar year 2005 and annualized earnings for 2004. The excise tax gross-up is based on a combined marginal federal and state tax rate of 48% for the executive. The estimated tax gross-up payment has been calculated assuming no value is assigned to the non-compete and other restrictive covenants that may apply to the executive. The estimated |
85
| | |
| | tax gross-up payment has been calculated assuming no value is assigned to the non-compete and other restrictive covenants that apply to Mr. Moffitt. |
Non-Employee Director Compensation Table
During fiscal year ended December 31, 2006, our directors did not receive any cash fees for their services on the board of directors, but were entitled to reimbursement of all reasonableout-of-pocket expenses incurred in connection with their attendance at board of directors and board committee meetings. Our non-employee directors were eligible to receive stock options under the 2000 Plan.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Change in
| | | | | | | |
| | | | | | | | | | | | | | | | | | Pension
| | | | | | | |
| | | | | | | | | | | | | | | Non-equity
| | | Value and
| | | | | | | |
Name and Principal
| | | | | Fees Earned
| | | Stock
| | | Option
| | | Incentive Plan
| | | NQDC
| | | All Other
| | | | |
Position | | Year | | | or Paid(1) | | | Awards | | | Awards(3) | | | Compensation | | | Earnings | | | Compensation | | | Total | |
| |
| Chad A. Mirkin, Ph.D. | | | 2006 | | | | — | | | | — | | | $ | 151,715 | (4) | | | — | | | | — | | | $ | 99,996 | (2) | | $ | 251,711 | |
| André de Bruin | | | 2006 | | | | — | | | | — | | | $ | 16,039 | (5) | | | — | | | | — | | | | — | | | $ | 16,039 | |
| Sheli Z. Rosenberg | | | 2006 | | | | — | | | | — | | | $ | 16,201 | (6) | | | — | | | | — | | | | — | | | $ | 16,201 | |
| Mark Slezak | | | 2006 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| James J. Nahirny | | | 2006 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| Jeffrey R. Crisan | | | 2006 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | | | | — | |
| | |
| (1) | | The directors did not receive any compensation from us for fiscal year 2006. |
| |
| (2) | | Dr. Mirkin received fees in his capacity as a consultant. See “Certain Relationships and Related Party Transactions.” |
| |
| (3) | | The fair value for awards is calculated for option awards, by using the Black-Scholes option pricing model. This value does not reflect estimated forfeitures or awards actually forfeited during the year. The actual value, if any, that will be realized upon the exercise of an option will depend upon the difference between the exercise price of the option and the market price of the common stock on the date the option is exercised. |
| |
| (4) | | On July 14, 2005, Dr. Mirkin received options to purchase 120,000 shares of common stock at the price of $4.50 per share, an amount determined by the board of directors to be greater than the fair market value on that date of $3.25 per share, which vest ratably every six months commencing on the six month anniversary of the date of grant. |
| |
| (5) | | On June 2, 2005, Mr. de Bruin received options to purchase 32,000 shares of common stock at the price of $4.50 per share, an amount determined by the board of directors to be greater than the fair market value on that date of $3.25 per share, which vest ratably over a four year period commencing on the anniversary of the date of grant. |
| |
| (6) | | On March 14, 2005, Ms. Rosenberg received options to purchase 32,000 shares of common stock at the price of $4.50 per share, an amount determined by the board of directors to be greater than the fair market value on that date of $3.25 per share, which vest ratably over a four year period commencing on the anniversary of the date of grant. |
On April 3, 2007, the board of directors granted options to three of the directors. Dr. Mirkin received options to purchase 460,000 shares of common stock at the price of $4.50 per share, a price the board of directors determined to be equal to the fair market value of the shares on the date of grant, 115,000 of which vested immediately and the remaining 345,000 vest ratably on an annual basis over a three year period commencing on the anniversary of the date of grant. Mr. de Bruin and Ms. Rosenberg each received options to purchase 32,000 shares of common stock at the price of $4.50 per share, a price the board of directors determined to be equal to the fair market value of the shares on the date of grant, which vest ratably on a monthly basis over a four year period commencing one month after the date of grant.
86
The following table sets forth information with respect to the beneficial ownership of our common stock as of October 29, 2007, and as adjusted to reflect the sale of common stock offered by us in this offering, for:
| | |
| | • | each person or group of affiliated persons known by us to own beneficially of more than 5% of our outstanding common stock; |
| |
| | • | each of our directors; |
| |
| | • | each of our named executive officers; and |
| |
| | • | all of our executive officers and directors as a group. |
We have determined beneficial ownership in accordance with the rules of the SEC. Except as otherwise indicated in the footnotes below, all of the shares reflected in the table are shares of common stock and all persons listed below have sole voting and investment power with respect to the shares beneficially owned by them, subject to applicable community property laws.
Percentage ownership calculations are based on 14,064,198 shares outstanding as of October 29, 2007, which assumes the conversion of all outstanding series of convertible preferred stock into common stock including common stock issued in connection with accrued and unpaid dividends which were an aggregate of 700,986 shares as of October 29, 2007 (at an assumed initial public offering price of $15.00 per share which is the midpoint of the range listed on the cover page of this prospectus) and the exercise of all outstanding warrants for the purchase of Series C andSeries C-2 Preferred Stock into an aggregate of 13,131,552 shares of common stock. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we have deemed outstanding shares of common stock subject to options held by that person that are exercisable within 60 days of October 29, 2007. We have not deemed these shares outstanding for the purpose of computing the percentage ownership of any other person.
Unless otherwise indicated below, the address for each of the beneficial owners in the table below isc/o Nanosphere, Inc., 4088 Commercial Avenue, Northbrook, Illinois 60062.
| | | | | | | | | | | | | | | | | |
| | | Shares Beneficially
| | | Shares Beneficially
| |
| | | Owned
| | | Owned
| |
| | | Prior to the Offering | | | After Offering | |
Name and Address of Beneficial Owner | | Number | | | Percentage | | | Number | | | Percent | |
| |
5% Stockholders | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| AOQ Trust(1)(2) | | | 1,156,325 | | | | 8.22 | % | | | 1,156,325 | | | | 5.49 | % |
| | | | | | | | | | | | | | | | | |
| Alfa-Tech, L.L.C.(1)(2) | | | 1,261,642 | | | | 8.97 | % | | | 1,261,642 | | | | 5.99 | % |
| | | | | | | | | | | | | | | | | |
| Bain Capital Venture Fund 2005, L.P.(3)(4) | | | 2,079,130 | | | | 14.48 | % | | | 2,079,130 | | | | 9.73 | % |
| | | | | | | | | | | | | | | | | |
| Brookside Capital Partners Fund, L.P.(5)(6) | | | 4,154,754 | | | | 28.35 | % | | | 4,988,087 | | | | 23.03 | %(21) |
| | | | | | | | | | | | | | | | | |
| Lurie Investment Fund, L.L.C.(2)(7)(8) | | | 3,347,798 | | | | 23.58 | % | | | 3,347,798 | | | | 15.79 | % |
| | | | | | | | | | | | | | | | | |
| Lurie Investments, Inc.(2)(7)(8) | | | 3,372,016 | | | | 23.75 | % | | | 3,372,016 | | | | 15.91 | % |
| | | | | | | | | | | | | | | | | |
| William White(2)(7)(9) | | | 3,406,299 | | | | 23.98 | % | | | 3,406,299 | | | | 16.06 | % |
| | | | | | | | | | | | | | | | | |
Directors and Named Executive Officers | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| William P. Moffitt III | | | 320,000 | | | | 2.28 | % | | | 320,000 | | | | 1.52 | % |
| | | | | | | | | | | | | | | | | |
| J. Roger Moody, Jr. | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| William H. Cork(10) | | | 39,800 | | | | | * | | | 39,800 | | | | | * |
| | | | | | | | | | | | | | | | | |
| Michael K. McGarrity(11) | | | 10,000 | | | | | * | | | 10,000 | | | | | * |
| | | | | | | | | | | | | | | | | |
| Gregory W. Shipp, M.D. | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | |
| Mark Slezak(1)(7)(12) | | | 6,447,707 | | | | 45.33 | % | | | 6,447,707 | | | | 30.38 | % |
| | | | | | | | | | | | | | | | | |
| Jeffrey R. Crisan(13)(14)(15) | | | 302,196 | | | | 2.14 | % | | | 302,196 | | | | 1.43 | % |
| | | | | | | | | | | | | | | | | |
| André de Bruin(16) | | | 25,333 | | | | | * | | | 25,333 | | | | | * |
| | | | | | | | | | | | | | | | | |
| Chad A. Mirkin, Ph.D.(17) | | | 534,400 | | | | 3.74 | % | | | 534,400 | | | | 2.51 | % |
| | | | | | | | | | | | | | | | | |
| James J. Nahirny(3)(14)(18) | | | 2,374,840 | | | | 16.49 | % | | | 2,374,840 | | | | 11.10 | % |
| | | | | | | | | | | | | | | | | |
| Sheli Z. Rosenberg(19) | | | 95,294 | | | | | * | | | 95,294 | | | | | * |
| | | | | | | | | | | | | | | | | |
| All executive officers and directors as a group (12 persons)(20) | | | 9,853,860 | | | | 60.82 | % | | | 9,853,860 | | | | 42.47 | %(21) |
| | |
| * | | Represents less than 1% of the outstanding shares of common stock. |
| |
| (1) | | Mark Slezak is (i) a trustee of AOQ Trust, (ii) managing member of Eagle Capital Management, LLC, which is the managing member of Alfa-Tech, LLC, (iii) investment manager of LFT Partnership, (iv) vice president and a director of the Ann and Robert H. Lurie Foundation, and (v) managing member of WASK |
87
| | |
| | Investments, LLC. Mr. Slezak, may be deemed to have beneficial ownership of the shares and warrants held by each of AOQ Trust, Alfa-Tech, LLC, LFT Partnership, Ann and Robert H. Lurie Foundation and WASK Investments, LLC. |
| |
| (2) | | The address of AOQ Trust, Alfa-Tech, LLC, Lurie Investment Fund, L.L.C., Lurie Investments, Inc. and William White is c/o Lurie Investments, Inc., 440 W. Ontario Street, Chicago, Illinios 60610. |
| |
| (3) | | Mr. Nahirny is a managing director of Bain Capital Venture Partners, LLC and Bain Capital Venture Investors, LLC. Bain Capital Venture Investors, LLC is the general partner of Bain Capital Venture Partners 2005, L.P., which is the general partner of Bain Capital Venture Fund 2005, L.P. Mr. Nahirny may be deemed to have beneficial ownership of shares and warrants held by Bain Capital Venture Fund 2005, L.P. |
| |
| (4) | | Includes warrants convertible into 295,348 shares of common stock. |
| |
| (5) | | Brookside Capital Investors, L.P. is the sole general partner of Brookside Capital Partners Fund, L.P. Brookside Capital Management, LLC is the sole general partner of Brookside Capital Investors, L.P. Mr. Domenic J. Ferrante is the sole managing member of Brookside Capital Management, LLC. Brookside Capital Investors, L.P., Brookside Capital Management, LLC, and Mr. Ferrante may be deemed to have beneficial ownership of shares and warrants held by Brookside Capital Partners Fund, L.P. and they each disclaim beneficial ownership of all such shares and warrants except to the extent of their pecuniary interest therein. |
| |
| (6) | | Includes warrants convertible into 590,199 shares of common stock. |
| |
| (7) | | Mark Slezak is the chief executive officer of Lurie Investments, Inc. Mr. Slezak is the managing member of Eagle Capital Management, LLC. Lurie Investments, Inc. and Eagle Capital Management, LLC are both managing members of Lurie Investment Fund, L.L.C. William White is also a managing member of Lurie Investment Fund, L.L.C. Mr. Slezak, Eagle Capital Management, LLC, Lurie Investments, Inc. and Mr. White may be deemed to have beneficial ownership of the shares and warrants held by Lurie Investment Fund, L.L.C. Mr. Slezak may be deemed to have beneficial ownership of shares and warrants held by Lurie Investments, Inc. and Eagle Capital Management, LLC. |
| |
| (8) | | Includes warrants convertible into 136,340 shares of common stock. |
| |
| (9) | | Includes warrants convertible into 140,350 shares of common stock. |
| |
| (10) | | Includes options to purchase 39,800 shares of common stock that are exercisable within 60 days. |
| |
| (11) | | Includes options to purchase 10,000 shares of common stock that are exercisable within 60 days. |
| |
| (12) | | Includes warrants convertible into 160,965 shares of common stock. |
| |
| (13) | | Mr. Crisan is a general partner of BCIP Associates III-B which is the manager and sole member of BCIP Associates III-B, LLC. Mr. Crisan may be deemed to have beneficial ownership of shares and warrants held by BCIP Associates III-B, LLC. |
| |
| (14) | | Mr. Nahirny and Mr. Crisan are each general partners of BCIP Associates III which is the manager and sole member of BCIP Associates III, LLC. Mr. Nahirny and Mr. Crisan may be deemed to have beneficial ownership of shares and warrants held by BCIP Associates III, LLC. |
| |
| (15) | | Includes warrants convertible into 42,928 shares of common stock. |
| |
| (16) | | Includes options to purchase 25,333 shares of common stock that are exercisable within 60 days. |
| |
| (17) | | Includes options to purchase 235,000 shares of common stock that are exercisable within 60 days. |
| |
| (18) | | Includes warrants convertible into 337,355 shares of common stock. |
| |
| (19) | | Includes options to purchase 29,833 shares of common stock that are exercisable within 60 days. |
| | |
| (20) | | Includes warrants convertible into 541,248 shares of common stock and options to purchase 339,966 shares of common stock exercisable within 60 days. |
| | |
| (21) | | Percentage ownership calculations give effect to the $12.5 million of our common stock, which represents 833,333 shares of our common stock assuming a purchase price per share equal to $15.00, the midpoint of the price range indicated on the cover page of this prospectus, that Brookside Capital Partners Fund, L.P. has indicated an interest in purchasing. |
88
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Northwestern License Agreement
We entered into a license agreement with Northwestern University dated May 10, 2000, or the Original License Agreement, pursuant to which we received an exclusive license to all technology developed in the laboratories of Dr. Chad A. Mirkin or Dr. Robert Letsinger of Northwestern University, to the extent that such technology relates to biological diagnostics involving nanoparticles. Dr. Mirkin has been a member of our board of directors since 2000.
We entered into a new license agreement with Northwestern University dated January 1, 2006, or the New License Agreement, which supersedes the Original License Agreement. Under the New License Agreement, we have an exclusive license to certain patents and patent applications owned by Northwestern that are related to (1) nanotechnology, which technology involves a particle where no single dimension is greater than 100 nanometers, or Nanotechnology, and (2) biobarcode technology, which is analysis where oligonucleotides act as surrogate targets or reporter molecules, or Biobarcode Technology. The license is limited to the “Biodiagnostics Field” defined as qualitative or quantitative in vitro analysis, testing, measurement, or detection of various biodiagnostics field subjects and target combinations.
The New License Agreement includes licenses to patents and patent applications based on existing inventions and future inventions developed in the laboratory of Dr. Mirkin or Dr. Letsinger, by or under their direct supervision, and conceived prior to January 1, 2013 that are Nanotechnology or Biobarcode Technology referred to herein as Licensed Patents. We have an obligation to use commercially reasonable efforts to bring the subject inventions of the Licensed Patents to market. If the parties disagree as to whether we are meeting this diligence requirement, an arbitrator may require us to comply with a timeline for cure or convert our exclusive license to a non-exclusive license; Northwestern does not have the right to revoke any license to the Licensed Patents already granted to us.
We also have the first right to negotiate an exclusive license to inventions developed in the laboratory of Dr. Mirkin or Dr. Letsinger, by or under their direct supervision, and (1) conceived after January 1, 2013 that are Nanotechnology or Biobarcode Technology and (2) that are not Nanotechnology or Biobarcode Technology, but otherwise within the Biodiagnostics Field, conceived prior to January 1, 2013. Both (1) and (2) are herein referred to as Future Inventions. If the parties cannot agree on the terms of the license for the Future Inventions, the parties shall submit to arbitration to determine reasonable terms. For inventions conceived after January 1, 2013 that are not Nanotechnology or Biobarcode Technology, but otherwise within the Biodiagnostics Field, we have the right to negotiate a license if Northwestern offers such inventions to third parties. If we have a license based on Future Inventions, Northwestern has the right to terminate the license upon any material breach that we do not cure or upon our bankruptcy.
We have an obligation to pay Northwestern a royalty at a rate that is a percentage of the gross profits of licensed products, subject to certain adjustments. We paid Northwestern $5,000, $1,000 and $31,000 for the years ended December 31, 2004, 2005 and 2006, respectively in connection with the Original License Agreement, and $30,000 for the six month period ended June 30, 2007 in connection with the New License Agreement.
We have entered into various research subcontracting agreements with Northwestern, pursuant to which we collaborate with it on focused research projects. We have received $260,000 and $204,000 for the years ended December 31, 2005 and 2006, and $46,000 for the six month period ended June 30, 2007, from Northwestern in connection with these agreements and products sales.
Mirkin Consulting Agreement
We entered into a Consulting and Non-Competition Agreement with Dr. Mirkin dated as of October 31, 2002, as amended as of February 23, 2004. Pursuant to the terms of this agreement, we have engaged Dr. Mirkin (1) to provide scientific advice and counsel to us with regard to our technology, (2) to represent and promote us and our technology at scientific meetings and other public forums, (3) to participate, either individually or with one of our representatives, at meetings and presentations on our behalf, and (4) to participate in capital-raising activities on our behalf. The term of the agreement extends through October 31,
89
2012 and is automatically renewed for successive one year periods unless either party gives the other party 60 days’ prior written notice of non-renewal. We pay Dr. Mirkin $100,000 per annum as compensation for his services. We paid Dr. Mirkin $69,999, $99,996 and $99,996 in the years ended December 31, 2004, 2005 and 2006, and have paid him $49,998 for the six month period ended June 30, 2007. If the consulting agreement is terminated for any reason before October 31, 2012, Dr. Mirkin shall continue to provide patent prosecution support and similar services as we shall reasonably request or as shall be required under any other agreement directly or indirectly applicable to Dr. Mirkin and as compensation therefor, Dr. Mirkin shall be paid at such hourly market rate as we and Dr. Mirkin shall agree to in good faith and absent such agreement, at the rate of $300.00 per hour. The consulting agreement may be terminated by mutual agreement of the parties. Dr. Mirkin has also agreed not to engage in a competing business (other than NanoInk, Inc.) in the continental United States during the term of the consulting agreement and for a period of two years after termination for any reason.
Second Amended and Restated Stockholders’ Agreement
In connection with the issuance of our Series D Convertible Preferred Stock, we entered into a Second Amended and Restated Stockholders’ Agreement dated as of April 12, 2006 with Mr. William P. Moffitt III, our chief executive officer and a member of our board of directors, Mr. Mark Slezak, Dr. Chad Mirkin and Ms. Sheli Rosenberg, who are members of our board of directors, AOQ Trust, Alfa-Tech, L.L.C., Lurie Investment Fund, L.L.C., Lurie Investments, Inc., William White and their respective affiliates, and Bain Capital Venture Fund 2005, L.P., Brookside Capital Partners Fund, L.P., and their respective affiliates and other stockholders.
We intend to terminate the Second Amended and Restated Stockholders’ Agreement upon completion of this offering.
Registration Rights Agreement
Pursuant to our Amended and Restated Registration Rights Agreement dated as of April 12, 2006, we have granted the following demand registration rights to Mr. Mark Slezak and Ms. Sheli Rosenberg, who are members of our board of directors, AOQ Trust, Alfa-Tech, L.L.C., Lurie Investment Fund, L.L.C., Lurie Investments, Inc., William White and their respective affiliates, and Bain Capital Venture Fund 2005, L.P., Brookside Capital Partners Fund, L.P., and their respective affiliates and other stockholders. Mr. William P. Moffitt III, our chief executive officer and a member of our board of directors, and Dr. Chad Mirkin, a member of our board of directors, are parties to the Amended and Restated Registration Rights Agreement, but do not have the right to demand registration. At any time after the earlier to occur of (1) 120 days after the completion of this offering, or (2) April 1, 2010:
| | |
| | • | Long-Form Registrations. Stockholders holding at least 20% of the then outstanding shares of our common stock that are subject to the registration rights agreement, which we refer to as registrable securities, have the right to demand that we file a registration statement under the Securities Act onForm S-1 or any similar long-form registration covering their registrable securities. However, we are not obligated to file a long-form registration statement on more than three occasions upon the request of our stockholders. |
| |
| | • | Short-Form Registrations. Stockholders holding at least 10% of the then outstanding registrable securities have the right to demand that we file a registration statement onForm S-3 or any similar short-form registration covering their registrable securities, provided that such short-form registration is then available to us under applicable law. Such stockholders are entitled to request an unlimited number of short-form registrations, but we are not obligated to effect more than 2 short-form registrations during any twelve month period. |
If our board of directors believes in its reasonable good faith that any demand registration would have a material adverse effect on a proposal or plan that we intend to undertake, we may delay the registration once in any twelve month period for up to 90 days. Moreover, if the demand registration is an underwritten offering, we may reduce the number of shares of our registrable securities to be registered upon the advice of the underwriters that such offering exceeds the number of securities that can be sold in an orderly manner within an acceptable price range. If shares of our stock requested to be included in a registration must be
90
excluded pursuant to the underwriters’ advice, we will generally register a pro rata portion of the shares requested to be registered.
Under the piggyback registration provisions, if we propose to register any securities under the Securities Act, other than pursuant to a demand registration, and the registration form to be used may be used for the registration of registrable securities, stockholders holding such registrable securities have the right to include their shares in the registration statement. However, if the registration is an underwritten offering, we may reduce the number of shares to be registered under the piggyback registration provisions upon the advice of the underwriters that such offering exceeds the number of securities that can be sold in an orderly manner within an acceptable price range. If shares of our stock requested to be included in a registration must be excluded pursuant to the underwriters’ advice, we will generally register a pro rata portion of the shares requested to be registered under the piggyback registration provisions. The piggyback registration rights granted under the registration rights agreement have no expiration date.
Expenses of Registration. We will generally pay all registration expenses in connection with the demand and piggyback registrations described above, including all registration and filing fees, expenses and fees of compliance with securities laws, and fees and disbursements of all counsel, independent certified public accountants, underwriters (excluding discounts and commissions) and other persons retained by us. We will also pay the reasonable fees and disbursements of one counsel chosen by the selling stockholders in each demand or piggyback registration.
Transferability. The demand and piggyback registration rights described above are generally transferable to any subsequent holder of registrable securities.
Loans from Lurie Investment Fund, L.L.C.
Prior to ourSeries C-2 financing in September 2004, we entered into three note and warrant purchase agreements with Lurie Investment Fund, L.L.C., or the Fund, pursuant to which the Fund made us loans, and we issued to it convertible promissory notes in the principal amounts of the loans and issued warrants to purchase our convertible preferred stock. Specifically, on February 10, 2004, the Fund loaned us $4,000,000, and we issued to it a convertible promissory note in the principal amount of $4,000,000 at an annual interest rate of 7.5%, and warrants to purchase 666,667 shares of Series C Convertible Preferred Stock at an exercise price of $0.60 per share. On June 8, 2004, the Fund loaned us $1,000,000, and we issued to it a convertible promissory note in the principal amount of $1,000,000 at annual interest rate of 7.5%, and warrants to purchase 41,667 shares of Series C Convertible Preferred Stock at an exercise price of $0.60 per share (or convertible into the equity securities available in our next financing). On July 8, 2004, the Fund loaned to us $1,250,000, and we issued to it a convertible promissory note in the principal amount of $1,250,000 at an annual interest rate of 7.5%, and warrants to purchase 52,083 shares of Series C Convertible Preferred Stock at an exercise price of $0.60 per share (or convertible into the equity securities available in our next financing).
We also entered into a note purchase agreement with the Fund on August 13, 2004, pursuant to which the Fund loaned to us $650,668 and we issued a short-term convertible promissory note in the principal amount of $650,668 at an annual interest rate of 7.5%. On September 2, 2004, we issued 20,293,345 shares ofSeries C-2 Convertible Preferred Stock to the Fund in exchange for the cancellation of the convertible promissory notes issued on February 10, June 8, July 8, and August 13, 2004, including all principal amounts and accrued interest, which totaled $7,102,671.
Prior to our Series D financing in April 2006, we entered into one note and warrant purchase agreement with the Fund on December 9, 2005, pursuant to which the Fund loaned us $5,000,000, and we issued to it an unsecured promissory note in the principal amount of $5,000,000 at an annual interest rate of 10.0%, and a warrant to purchase $500,000 of shares in our next qualified offering. We also entered into a note purchase agreement with the Fund on March 15, 2006, pursuant to which the Fund loaned us $1,250,000 and we issued an unsecured promissory note in the principal amount of $1,250,000 at an annual interest rate of 4.58%. On April 12, 2006, we separately issued 15,714,286 shares and 3,571,429 shares of our Series D Convertible Preferred Stock, and warrants to purchase 2,756,892 shares and 626,566 shares of Series D Convertible Preferred Stock at a minimum price of $0.35 per share in exchange for the cancellation of the promissory
91
notes issued on December 9, 2005 and March 15, 2006, and the exercise of the warrant issued on December 9, 2005.
Loan and Security Agreement
In March 2006, in connection with his purchase of 320,000 shares of our common stock for an aggregate price of $1.44 million, William P. Moffitt III, our chief executive officer and a director, executed a loan and security agreement payable to us for $1.44 million which bore interest at 4.51% per annum. The note was secured by a pledge of the 320,000 shares of our common stock purchased by Mr. Moffitt. For the year ended December 31, 2006 and for the six month periods ended June 30, 2006 and 2007, $51,414, $18,942 and $32,472 respectively of interest had accrued on the note. The entire principal and accrued interest was repaid in full in August 2007.
Purchase of Shares in this Offering
Brookside Capital Partners Fund, L.P., one of our principal stockholders and an affiliate of the Bain Entities, has indicated an interest in purchasing $12.5 million of our common stock in this offering, which represents 833,333 shares of our common stock assuming a purchase price per share equal to $15.00, the midpoint of the price range indicated on the cover page of this prospectus. Brookside Capital Partners Fund, L.P. is not under any obligation to purchase any shares in this offering, and their interest in purchasing shares in this offering is not a commitment to do so. These shares, if purchased, will be subject to the 180-day lock-up agreement that Brookside Capital Partners Fund, L.P. executed in connection with this offering. See “Shares Eligible for Future Sale — Lock-up Agreements.” Brookside Capital Partners Fund, L.P. beneficially owned 3,564,555 shares of our common stock and warrants to purchase an additional 590,199 shares or an aggregate of 28.35% of our common stock immediately prior to this offering. Assuming an initial public offering price of $15.00 per share, which is the midpoint of the range listed on the cover page of this prospectus, upon completion of this offering, the Bain Entities collectively will own approximately 30% of our common stock, or 33% of our common stock if Brookside Capital Partners Fund, L.P. purchases $12.5 million of our common stock in this offering, which includes warrants to purchase 928,475 shares of our common stock.
Private Placements — Preferred Stock, Warrant and Option Issuances
The following is a description of transactions from January 1, 2004 to the date of the prospectus in which we were or are a party, in which the amount involved in the transaction exceeded or exceeds $120,000, and in which any of our directors, executive officers or holders of more than five percent of our capital stock had or will have a direct or indirect material interest. We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or amounts that would have been paid or received, as applicable, in arm’s-length transactions.
| | | | | | | | | |
| | | Relationship
| | | | | | |
| | | to the
| | Date of
| | Number and Type of
| | |
Name | | Company | | Issuance | | Shares Issued | | Consideration |
| |
| AOQ Trust | | 5% Stockholder | | September 2, 2004 | | 571,428 shares of common stock (14,285,715 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $5,000,000 |
| | | | | September 30, 2004 | | 519,881 shares of common stock (12,997,036 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $4,548,963 |
| Alfa-Tech, L.L.C. | | 5% Stockholder | | September 30, 2004 | | 390,706 shares of common stock (9,767,665 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 7,378,983 shares of Series A Convertible Preferred Stock |
| | | | | June 17, 2005 | | 228,571 shares of common stock (5,714,286 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $7,000,000 |
| | | | | August 31, 2005 | | 571,428 shares of common stock 14,285,714 shares of Series C-2 Convertible (Preferred Stock prior to conversion) | | |
92
| | | | | | | | | |
| | | Relationship
| | | | | | |
| | | to the
| | Date of
| | Number and Type of
| | |
Name | | Company | | Issuance | | Shares Issued | | Consideration |
| |
| Lurie Investment Fund, L.L.C. | | 5% Stockholder | | February 10, 2004 | | 45,714 shares of common stock (Warrants to purchase 666,667 shares of Series C Preferred Stock at an exercise price of $0.60 per share prior to conversion) | | $4,000,000(1) |
| | | | | June 8, 2004 | | 14,285 shares of common stock (Warrants to purchase 41,667 shares of Series C Preferred Stock and warrants to purchase 166,667 shares of Series C Preferred Stock (or convertible into our equity securities available in the next financing) at an exercise price of $0.60 per share prior to conversion) | | $1,000,000(1) |
| | | | | July 8, 2004 | | 17,856 shares of common stock (Warrants to purchase 52,083 shares of Series C Preferred Stock and warrants to purchase 208,334 shares of Series C Preferred Stock (or convertible into our equity securities available in the next financing) at an exercise price of $0.60 per share prior to conversion) | | $1,250,000 bridge loan(1) |
| | | | | September 2, 2004 | | 811,733 shares of common stock (20,293,345 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $7,102,671(2) |
| | | | | September 30, 2004 | | 1,287,949 shares of common stock (32,198,737 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 3,027,211 shares of Series B Convertible Preferred Stock and 11,365,930 shares of Series C Convertible Preferred Stock |
| | | | | June 17, 2005 | | 45,714 shares of common stock (1,142,857 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $400,000 |
| | | | | December 9, 2005 | | Warrants to purchase $500,000 of shares our next qualified financing (greater than $25,000,000) | | $5,000,000(3) |
| | | | | April 12, 2006 | | 771,428 shares of common stock and warrants to purchase 135,337 shares of common stock at an exercise price of $10.9375 per share (15,714,286 shares and 3,571,429 shares of Series D Convertible Preferred Stock (issued separately) and warrants to purchase 2,756,892 shares and 626,566 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 per share prior to conversion) | | $6,750,000(4) |
| Lurie Investments, Inc. | | 5% Stockholder | | June 17, 2005 | | 22,857 shares of common stock (571,428 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $200,000 |
| WASK Investments, L.L.C. | | Affiliate of Director Mark Slezak | | April 12, 2006 | | 34,285 shares of common stock and warrants to purchase 6,015 shares of common stock at an exercise price of $10.9375 per share (857,143 shares of Series D Convertible Preferred Stock and warrants to purchase 150,376 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 per share prior to conversion) | | $300,000 |
| Ann and Robert H. Lurie Foundation | | Affiliate of Director Mark Slezak | | April 12, 2006 | | 106,079 shares of common stock and warrants to purchase 18,610 shares of common stock at an exercise price of $10.9375 per share (2,651,987 shares of Series D Convertible Preferred Stock and warrants to purchase 465,261 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 per share prior to conversion) | | $928,195 |
| Eagle Capital Management, LLC | | Affiliate of Director Mark Slezak | | September 30, 2004 | | 22,857 shares of common stock (571,429 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 333,333 shares of Series C Convertible Preferred Stock |
93
| | | | | | | | | |
| | | Relationship
| | | | | | |
| | | to the
| | Date of
| | Number and Type of
| | |
Name | | Company | | Issuance | | Shares Issued | | Consideration |
| |
| LFT Partnership | | Affiliate of Director Mark Slezak | | September 2, 2004 | | 137,142 shares of common stock (3,428,571 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 2,000,000 shares of Series C Convertible Preferred Stock |
| | | | | June 17, 2005 | | 274,285 shares of common stock (6,857,143 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $2,400,000 |
| William White | | 5% Stockholder | | September 2, 2004 | | 28,571 shares of common stock (714,286 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 102,041 shares of Series B Convertible Preferred Stock and 166,667 shares of Series C Convertible Preferred Stock |
| | | | | April 12, 2006 | | 22,857 shares of common stock and warrants to purchase 4,010 shares of common stock at an exercise price of $10.9375 (571,429 shares of Series D Convertible Preferred Stock and warrants to purchase 100,251 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $200,000 |
| Laura Mondrowski (Bermont) | | Affiliate of 5% Stockholder Lurie Investments, Inc. | | April 12, 2006 | | 5,714 shares of common stock and warrants to purchase 1,002 shares of common stock at an exercise price of $10.9375 (142,857 shares of Series D Convertible Preferred Stock and warrants to purchase 25,063 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $50,000 |
| Bain Capital Venture Fund 2005, L.P. | | 5% Stockholder | | April 12, 2006 | | 1,683,488 shares of common stock and warrants to purchase 295,348 shares of common stock at an exercise price of $10.9375 (42,087,201 shares of Series D Convertible Preferred Stock and warrants to purchase 7,383,719 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $14,730,520(5) |
| Brookside Capital Partners Fund, L.P. | | 5% Stockholder | | April 12, 2006 | | 3,364,136 shares of common stock and warrants to purchase 590,199 shares of common stock at an exercise price of $10.9375 (84,103,401 shares of Series D Convertible Preferred Stock and warrants to purchase 14,754,983 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $29,436,190(5) |
| BCIP Associates III, LLC | | Affiliate of Directors Jeffrey Crisan and James Nahirny | | April 12, 2006 | | 239,439 shares of common stock and warrants to purchase 42,007 shares of common stock at an exercise price of $10.9375 (5,985,996 shares of Series D Convertible Preferred Stock and warrants to purchase 1,050,175 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $2,095,099 |
| BCIP Associates III-B, LLC | | Affiliate of Directors Jeffrey Crisan and James Nahirny | | April 12, 2006 | | 5,253 shares of common stock and warrants to purchase 921 shares of common stock at an exercise price of $10.9375 (131,331 shares of Series D Convertible Preferred and warrants to purchase 23,041 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $45,966 |
| William P. Moffitt III | | Executive Officer and Director | | March 16, 2006 | | 320,000 shares of common stock | | $1,440,000 |
| | | | | April 3, 2007 | | 600,000 incentive stock options at an exercise price of $4.50 per share(6) | | |
| J. Roger Moody, Jr. | | Executive Officer | | May 16, 2007 | | 180,000 incentive stock options at an exercise price of $4.50 per share(6) | | |
| | | | | August 3, 2007 | | 20,000 incentive stock options at an exercise price of $4.50(6) | | |
94
| | | | | | | | | |
| | | Relationship
| | | | | | |
| | | to the
| | Date of
| | Number and Type of
| | |
Name | | Company | | Issuance | | Shares Issued | | Consideration |
| |
| William Cork | | Executive Officer | | January 5, 2004 | | 20,000 incentive stock options at an exercise price of $7.50(6) | | |
| | | | | May 12, 2005 | | 80,000 incentive stock options at an exercise price of $4.50(6) | | |
| | | | | April 3, 2007 | | 160,000 incentive stock options at an exercise price of $4.50 per share(6) | | |
| Michael K. McGarrity | | Executive Officer | | September 29, 2005 | | 50,000 incentive stock options at an exercise price of $4.50 per share(6) | | |
| | | | | March 14, 2006 | | 30,000 incentive stock options at an exercise price of $4.50(6) | | |
| | | | | April 3, 2007 | | 184,000 incentive stock options at an exercise price of $4.50 per share(6) | | |
| Gregory W. Shipp | | Executive Officer | | June 13, 2005 | | 40,000 incentive stock options at an exercise price of $4.50(6) | | |
| | | | | April 3, 2007 | | 40,000 incentive stock options at an exercise price of $4.50(6) | | |
| Mark Slezak | | Director | | September 2, 2004 | | 22,857 shares of common stock (571,427 shares ofSeries C-2 Convertible Preferred Stock prior to conversion) | | 136,054 shares of Series B Convertible Preferred Stock |
| André de Bruin | | Director | | June 2, 2005 | | 32,000 non-qualified stock options at an exercise price of $4.50 per share(6) | | |
| | | | | April 3, 2007 | | 32,000 non-qualified stock options at an exercise price of $4.50 per share(6) | | |
| Dr. Chad A. Mirkin | | Director | | July 14, 2005 | | 120,000 non-qualified stock options at an exercise price of $4.50 per share(6) | | |
| | | | | April 3, 2007 | | 460,000 non-qualified stock options at an exercise price of $4.50 per share(6) | | |
| Sheli Z. Rosenberg | | Director | | September 2, 2004 | | 40,004 shares of common stock (1,000,117 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 136,054 shares of Series B Convertible Preferred Stock and 250,070 shares of Series C Convertible Preferred Stock |
| | | | | March 14, 2005 | | 32,000 non-qualified stock options at an exercise price of $4.50 per share | | |
| | | | | April 12, 2006 | | 17,142 shares of common stock and warrants to purchase 3,007 shares of common stock at an exercise price of $10.9375 per share (428,571 shares of Series D Convertible Preferred Stock and warrants to purchase 75,188 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 per share prior to conversion) | | $150,000 |
| | | | | April 3, 2007 | | 32,000 non-qualified stock options at an exercise price of $4.50 per share | | |
| Allen & Company LLC | | Underwriter | | April 12, 2006 | | 182,857 shares of common stock and warrants to purchase 32,080 shares of common stock at an exercise price of $10.9375 per share (4,571,429 shares of Series D Convertible Preferred Stock and warrants to purchase 802,005 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $1,600,000 |
| | | | | April 12, 2006 | | Warrants to purchase 164,925 shares of common stock at an exercise price of $8.75 (Warrants to purchase 4,123,131 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | (7) |
95
| | | | | | | | | |
| | | Relationship
| | | | | | |
| | | to the
| | Date of
| | Number and Type of
| | |
Name | | Company | | Issuance | | Shares Issued | | Consideration |
| |
| Adam N. Mirkin(12) | | Affiliate of Director Chad A. Mirkin | | July 23, 2004 | | Warrants to purchase 9 shares of common stock at a purchase price of $0.60 per share (Warrants to purchase 134 shares of Series C Convertible Preferred Stock at an exercise price of $0.60 per share prior to conversion) | | $3,222(1) |
| | | | | September 2, 2004 | | 371 shares of common stock (9,281 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $3,222(8) |
| | | | | September 30, 2004 | | 2,859 shares of common stock (71,488 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 41,701 shares of Series C Convertible Preferred Stock |
| | | | | January 6, 2006 | | Warrants to purchase $200 of shares our next qualified financing (greater than $25,000,000) | | $2,000(3) |
| | | | | April 12, 2006 | | 531 shares of common stock and warrants to purchase 93 shares of common stock at an exercise price of $10.9375 per share (13,286 shares of Series D Convertible Preferred Stock and warrants to purchase 2,331 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $4,650(9) |
| Rhoderic Peter Mirkin(12) | | Affiliate of Director Chad A. Mirkin | | July 23, 2004 | | Warrants to purchase 55 shares of common stock at an exercise price of $0.60 per share (Warrants to purchase 806 shares of Series C Convertible Preferred Stock at an exercise price of $0.60 per share prior to conversion) | | $19,335(1) |
| | | | | September 2, 2004 | | 2,227 shares of common stock (55,692 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $19,335(10) |
| | | | | September 30, 2004 | | 17,157 shares of common stock (428,929 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | 250,209 shares of Series C Convertible Preferred Stock |
| | | | | September 30, 2004 | | 10,263 shares of common stock (256,594 shares of Series C-2 Convertible Preferred Stock prior to conversion) | | $89,808 |
| | | | | January 6, 2006 | | Warrants to purchase $3,493.02 of shares our next qualified financing (greater than $25,000,000) | | $34,930(3) |
| | | | | April 12, 2006 | | 15,819 shares of common stock and warrants to purchase 2,775 shares of common stock at an exercise price of $10.9375 per share (395,495 shares of Series D Convertible Preferred Stock and warrants to purchase 69,385 shares of Series D Convertible Preferred Stock at a minimum exercise price of $0.35 prior to conversion) | | $138,423(11) |
| | |
| (1) | | We issued such warrants as partial consideration for a bridge loan made to us on the same date therewith. We also issued a convertible promissory note to the lender in the principal amount of the bridge loan, which was convertible into Series C Convertible Preferred Stock. |
| |
| (2) | | The total consideration for the issuance of 20,293,345 shares ofSeries C-2 Convertible Preferred Stock was $7,102,671, which was the total principal amount and accrued interest on convertible promissory notes issued on February 10, June 8, July 8, and August 13, 2004. We issued the August 13, 2004 convertible promissory note in the amount of $650,668 as the sole consideration for a $650,668 bridge loan to us. |
| |
| (3) | | We issued such warrants as partial consideration for a bridge loan made to us on the same date therewith. We also issued a promissory note to the lender in the principal amount of the bridge loan. The promissory note and warrants were cancelled pursuant to a Note and Warrant Cancellation Agreement executed on April 12, 2006 in conjunction with the Series D financing. |
| |
| (4) | | This is comprised of the cancellation of the $5,000,000 bridge loan of December 9, 2005, the cancellation of a $1,250,000 bridge loan made on March 15, 2006, and the conversion of $500,000 in warrants received on December 9, 2005. |
96
| | |
| (5) | | This purchase of our Series D Convertible Preferred Stock resulted in the purchaser becoming a 5% or greater stockholder in our capital stock. |
| |
| (6) | | See “Executive Compensation” for further details. |
| |
| (7) | | We issued warrants to Allen & Company LLC pursuant to the terms of the Allen & Company LLC engagement letter dated November 21, 2005. |
| |
| (8) | | The consideration for the issuance of 9,281 shares ofSeries C-2 Convertible Preferred Stock was the cancellation of the total principal amount of $3,222 and accrued interest on a convertible promissory note issued July 23, 2004. |
| |
| (9) | | This is comprised of $2,650 cash consideration, the cancellation of the $2,000 convertible promissory note dated January 6, 2006, and the conversion of $200 in warrants received on January 6, 2006. |
| |
| (10) | | The consideration for the issuance of 55,692 shares ofSeries C-2 Convertible Preferred Stock was the cancellation of the total principal amount of $19,335 and accrued interest on a convertible promissory note issued July 23, 2004. |
| |
| (11) | | This is comprised of $100,000 cash consideration, the cancellation of the $34,930 convertible promissory note dated January 6, 2006, and the conversion of $3,493 in warrants received on January 6, 2006. |
| |
| (12) | | On July 23, 2004, Adam Mirkin and Rhoderic Peter Mirkin elected to exercise their preemptive rights as stockholders and paid to us $3,222 and $19,335, respectively, for which they received convertible promissory notes and warrants. In conjunction with ourSeries C-2 financing, we issued to them shares ofSeries C-2 Convertible Preferred Stock and cancelled the related outstanding promissory notes. Similarly, on January 6, 2006, they elected to exercise their preemptive rights and paid to us $2,000 and $34,930, respectively, for which they received unsecured promissory notes and warrants. In conjunction with our Series D financing, we issued to them shares of Series D Convertible Preferred Stock in exchange for the cancellation of the promissory notes and the exercise of the warrants issued on January 6, 2006. |
Policies and Procedures for Related Party Transactions
Upon completion of this offering, our audit committee charter will provide that our audit committee must review and approve in advance any related party transaction. All of our directors, officers and employees are required to report to our audit committee any such related party transaction for approval prior to its completion. In approving or rejecting a proposed related party transaction, our audit committee shall consider the relevant facts and circumstances available and deemed relevant to the audit committee, including, but not limited to, the risks, costs and benefits to us, the terms of the transaction and the impact on a director’s independence. Our audit committee shall approve only those related party transactions that, in the light of known circumstances, are in, or are not inconsistent with, our best interests, as our audit committee determines in the good faith exercise of its discretion. A related party transaction includes any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, the amount involved exceeds $120,000, and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness, and employment by us of a related person.
In addition, any related person transaction previously approved by the audit committee or otherwise already existing that is ongoing in nature will be reviewed by the audit committee on an ongoing basis to ensure that such related person transaction has been conducted in accordance with the previous approval granted by the audit committee, if any, and that all required disclosures regarding the related person transaction are made.
97
DESCRIPTION OF CAPITAL STOCK
General
Upon completion of this offering, our authorized capital stock will consist of 100,000,000 shares of common stock, par value $0.01 per share, and 10,000,000 shares of undesignated preferred stock, par value $0.01 per share. The following description of our capital stock is intended as a summary only and is qualified in its entirety by reference to our amended and restated certificate of incorporation and amended and restated by-laws to be in effect at the closing of this offering, copies of which are available as set forth under “Where You Can Find More Information.”
Common Stock
As of October 29, 2007, there were 932,646 shares of our common stock outstanding that were held of record by 18 stockholders. Upon completion of this offering, there will be 21,064,198 shares of our common stock outstanding.
After giving effect to our amended and restated certificate of incorporation and our amended and restated by-laws, the holders of our common stock will be entitled to the following rights.
Holders of our common stock are entitled to one vote for each share of common stock held of record for the election of directors and on all matters submitted to a vote of stockholders. Holders of our common stock are entitled to receive dividends ratably, if any, as may be declared by our board of directors out of legally available funds, subject to any preferential dividend rights of any preferred stock then outstanding. In the event of our dissolution, liquidation or winding up, holders of our common stock are entitled to share ratably in our net assets legally available after the payment of all of our debts and other liabilities, subject to the liquidation preferences of any preferred stock then outstanding. Holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future. All outstanding shares of our common stock are, and all shares of common stock to be issued in connection with this offering will be, fully paid and nonassessable. Except as described below in “Anti-Takeover Effects of Delaware Law Provisions of Our Amended and Restated Certificate of Incorporation and Our Amended and Restated By-Laws,” a majority vote of common stockholders is generally required to take action under our amended and restated certificate of incorporation and amended and restated by-laws.
Preferred Stock
Upon completion of this offering, our board of directors will be authorized, without action by the stockholders, to designate and issue up to 10,000,000 shares of preferred stock in one or more series. Our board of directors can fix or alter the rights, preferences and privileges of the shares of each series and any of its qualifications, limitations or restrictions, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences and the number of shares constituting a class or series. The issuance of preferred stock could, under certain circumstances, result in one or more of the following adverse effects:
| | |
| | • | decreasing the market price of our common stock; |
| |
| | • | restricting dividends on our common stock; |
| |
| | • | diluting the voting power of our common stock; |
| |
| | • | impairing the liquidation rights of our common stock; or |
| |
| | • | delaying or preventing a change in control of us without further action by our stockholders. |
Our board of directors will make any determination to issue such shares based on its judgment as to our best interests and the best interests of our stockholders. We have no current plans to issue any shares of preferred stock.
98
Warrants
Upon completion of this offering, all outstanding warrants to purchase Series C andSeries C-2 Convertible Preferred Stock must be exercised or they will be forfeited in accordance with their terms. The 32,694,562 outstanding warrants to purchase Series D Convertible Preferred Stock will be converted into warrants to purchase 1,307,773 shares of common stock at a currentweighted-average exercise price of $10.66.
Anti-Takeover Effects of Delaware Law and Provisions of Our Amended and Restated Certificate of Incorporation and Our Amended and Restated By-Laws
Section 203 of the Delaware General Corporation Law
We have elected not to be governed by the provisions of Section 203 of the Delaware General Corporation Law. In general, Section��203 regulates acquisitions of Delaware corporations by prohibiting a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three year period following the time date this stockholder became an interested stockholder.
In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owing 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Section 203 defines an “interested stockholder” as:
| | |
| | • | any person who owns 15% or more of the corporation’s outstanding voting stock. |
| |
| | • | any person associated or affiliated with the corporation who owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s outstanding voting stock; or |
| |
| | • | affiliates and amounts of any such person. |
Amended and Restated Certificate of Incorporation and Amended and Restated By-Laws
Certain provisions of our amended and restated certificate of incorporation and amended and restated by-laws summarized below may be deemed to have an anti-takeover effect and may delay, deter or prevent a tender offer or takeover attempt that a stockholder might consider to be in its best interests, including attempts that might result in a premium being paid over the market price for the shares held by stockholders.
Our amended and restated certificate of incorporation and amended and restated by-laws contain provisions that could have the effect of discouraging potential acquisition proposals or tender offers or delaying or preventing a change of control of our company. In particular, our amended and restated certificate of incorporation and amended and restated by-laws, as applicable, among other things:
| | |
| | • | provide that special meetings of the stockholders may be called only by our Chairman of the Board, Chief Executive Officer, notice by at least two members of the board of directors or a written request of holders of at least a majority of our outstanding capital stock; |
| |
| | • | establish procedures with respect to stockholder proposals and stockholder nominations, including requiring that advance written notice of a stockholder proposal or director nomination generally must be received at our principal executive offices not less than 90 nor more than 120 days prior to the first anniversary date of mailing of our proxy statement released to stockholders in connection with the previous year’s annual meeting of stockholders; |
| |
| | • | do not include a provision for cumulative voting in the election of directors. Under cumulative voting, a minority stockholder holding a sufficient number of shares may be able to ensure the election of one or more directors. The absence of cumulative voting may have the effect of limiting the ability of minority stockholders to effect changes in the board of directors and, as a result, may have the effect of deterring a hostile takeover or delaying or preventing changes in control or management of our company; |
99
| | |
| | • | provide that vacancies on our board of directors may be filled by a majority of directors in office, although less than a quorum, and not by the stockholders; |
| |
| | • | require that the vote of holders of a majority of the voting power of the outstanding shares entitled to vote generally in the election of directors is required to amend various provisions of our amended and restated certificate of incorporation and amended and restated by-laws, including provisions relating to: |
| | |
| | • | the number of directors on our board of directors; |
| |
| | • | the election, qualification and term of office of our directors; |
| |
| | • | removal of members of our board of directors; and |
| |
| | • | certain amendments to our amended and restated certificate of incorporation and amended and restated by-laws; and |
| | |
| | • | provide that the board of directors has the power to alter, amend or repeal the amended and restated by-laws without stockholder approval. |
Following the completion of this offering, our amended and restated certificate of incorporation will authorize our board of directors, without further vote or action by the stockholders, to issue up to 10,000,000 shares of preferred stock, par value $0.01 per share, in one or more classes or series, and to fix or alter:
| | |
| | • | the number of shares constituting any class or series; |
| |
| | • | the designations, powers and preferences of each class or series; |
| |
| | • | the relative, participating, optional and other special rights of each class or series; and |
| |
| | • | any qualifications, limitations or restrictions on each class or series. |
The above provisions are intended to promote continuity and stability in the composition of our board of directors and in the policies formulated by the board, and to discourage certain types of transactions that may involve an actual or threatened change of control. These provisions are expected to reduce our vulnerability to unsolicited acquisition attempts as well as discourage certain tactics that may be used in proxy fights. Such provisions, however, could discourage others from making tender offers for our shares and, as a consequence, may also inhibit fluctuations in the market price of our common stock that could result from actual or rumored takeover attempts. These provisions could also operate to prevent changes in our management.
NASDAQ Global Market Listing
We have applied to have our common stock listed on the NASDAQ Global Market under the trading symbol “NSPH”.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock will be American Stock Transfer & Trust Company.
100
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and a significant public market for our common stock may not develop or be sustained after this offering. Actual or anticipated sales of significant amounts of our common stock in the future, including shares of our outstanding common stock and shares issued upon exercise of outstanding options or convertible preferred stock warrants, could adversely affect the market price of our common stock. Such sales could also impair our future ability to raise capital by selling additional shares.
Sale of Restricted Shares
Upon the closing of this offering, we will have 21,064,198 shares of common stock outstanding based on the number of shares outstanding as of October 29, 2007. This assumes no exercise of the underwriters’ over-allotment option and no exercise of outstanding options to purchase common stock or warrants to purchase Series D Convertible Preferred Stock which will be exercisable for common stock upon the closing of this offering.
Of these shares, 7,000,000 (or 6,166,667 if Brookside Capital Partners Fund, L.P. acquires 833,333 shares in this offering) of the shares of common stock sold in this offering, plus any additional shares sold upon exercise of the underwriters over-allotment option, will be freely tradable without restriction under the Securities Act. However, shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, including up to 833,333 shares that may be sold to Brookside Capital Partners Fund, L.P. in this offering (assuming a public offering price in this offering of $15.00 per share, the midpoint of the price range indicated on the cover page of this prospectus), will not be freely tradeable and will be subject to volume limitations and other restrictions that are described below. Shares purchased by affiliates may generally only be sold pursuant to an effective registration statement or in compliance with Rule 144 under the Securities Act.
The remaining 14,064,198 (or 14,897,531 if Brookside Capital Partners Fund, L.P. acquires 833,333 shares this offering) shares of common stock held by existing stockholders are “restricted securities” within the meaning of Rule 144 under the Securities Act. These shares are only eligible for public sale if registered under the Securities Act or sold in accordance with Rules 144, 144(k) or 701 of the Securities Act, which are summarized below. As a result oflock-up agreements described below and the provisions of Rules 144, 144(k) and 701, these restricted securities will be available for sale in the public market as follows:
| | | | | | | | | |
Days after date of
| | Shares Eligible
| | | | | |
this Prospectus | | for Sale | | | | | Comment |
| |
| Upon effectiveness | | | 6,166,667 | | | (1) | | Shares sold in the offering |
| Upon effectiveness | | | 125,764 | | | | | Freely tradable shares saleable under Rule 144(k) that are not subject to the lock-up |
| 180 days | | | 14,771,767 | | | (1) | | Lock-up released; shares saleable under Rules 144 and 701 |
| | |
| (1) | | Assumes the purchase by Brookside Capital Partners Fund, L.P. of 833,333 shares of our common stock in this offering. |
Lock-up Agreements
We have agreed that, subject to certain exceptions, we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of Credit Suisse Securities (USA) LLC for a period of 180 days after the date of this prospectus. However, in the event that either (1) during the last 17 days of the“lock-up” period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the“lock-up” period, we announce that we will release earnings results during the 16 day period beginning on the last day of the“lock-up” period, then in either case the expiration of the
101
“lock-up” will be extended until the expiration of the 18 day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless Credit Suisse Securities (USA) LLC waives, in writing, such an extension.
Our officers and directors and certain existing stockholders have agreed that, subject to certain exceptions, they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of Credit Suisse Securities (USA) LLC for a period of 180 days after the date of this prospectus. However, in the event that either (1) during the last 17 days of the“lock-up” period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the“lock-up” period, we announce that we will release earnings results during the 16 day period beginning on the last day of the“lock-up” period, then in either case the expiration of the“lock-up” will be extended until the expiration of the 18 day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless Credit Suisse Securities (USA) LLC waives, in writing, such an extension.
We have agreed to indemnify the underwriters and their controlling persons against certain liabilities, including liabilities under the Securities Act. If we are unable to provide this indemnification, we have agreed to contribute to payments the underwriters and their controlling persons may be required to make in respect of those liabilities.
Employee Benefit Plans
In addition to the 21,064,198 shares of common stock outstanding immediately after this offering, options to purchase 3,251,548 shares of our common stock were outstanding as of October 29, 2007. As soon as practicable upon completion of this offering, we intend to file a registration statement onForm S-8 under the Securities Act to register 4,876,869 shares of our common stock subject to outstanding stock options or reserved for issuance under our 2007 Plan. Accordingly, shares of our common stock registered under such registration statement will be available for sale in the open market upon exercise by the holders, subject to vesting restrictions, contractuallock-up restrictionsand/or market stand-off provisions applicable to each option agreement that prohibit the sale or other disposition of the shares of common stock underlying the options for a period of 180 days after the date of this prospectus without the prior written consent from Credit Suisse Securities (USA) LLC.
Registration Rights
Upon completion of this offering, the holders of at least 14,439,322 shares of common stock and warrants to purchase common stock will have certain rights with respect to the registration of such shares under the Securities Act. See “Certain Relationships and Related Party Transactions — Registration Rights Agreement.” Upon the effectiveness of a registration statement, these shares, other than shares purchased by our affiliates, would become freely tradable without restriction under the Securities Act.
Rule 144
In general, Rule 144 allows a stockholder (or stockholders whose shares are aggregated) who has beneficially owned shares of our common stock for at least one year to sell within any three month period commencing 90 days after the date of this prospectus a number of those shares that does not exceed the greater of:
| | |
| | • | 1% of the number of shares of our common stock then outstanding, which will equal approximately 210,642 shares immediately after this offering; or |
| | |
| | • | the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of the Form 144 with respect to such sale. |
102
Sales under Rule 144, however, are subject to specific manner of sale provisions, notice requirements, and the availability of current public information about us. We cannot estimate the number of shares of common stock our existing stockholders will sell under Rule 144 since this will depend on the market price for our common stock, the personal circumstances of the stockholders, and other factors.
Rule 144(k)
In general, under Rule 144(k), a stockholder (or stockholders whose shares are aggregated) who has beneficially owned the shares proposed to be sold for at least two years and who is not deemed to have been our affiliate at any time during the immediately preceding three months may sell such shares without complying with the manner of sale provisions, notice requirements, public information requirements, or volume limitations of Rule 144. Affiliates, however, must always sell pursuant to Rule 144, even after the otherwise applicable Rule 144 holding periods have been satisfied.
Rule 701
Rule 701 generally allows any of our employees, officers, directors or consultants who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been our affiliate during the immediately preceding 90 days to sell those shares under Rule 144 without complying with the public information, holding period, volume limitation, or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, may not sell their Rule 701 shares until 90 days after the date of this prospectus.
In addition, Rule 701 will apply to stock options granted by us before this offering, along with the shares acquired upon exercise of such options, including exercises after the date of this prospectus.
103
CERTAIN MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX
CONSIDERATIONS TONON-U.S. HOLDERS
The following is a discussion of the material U.S. federal income and estate tax considerations with respect to the ownership and disposition of our common stock that may be relevant to anon-U.S. holder that acquires our common stock pursuant to this offering. The discussion is based on provisions of the Internal Revenue Code of 1986, as amended, or the Code, applicable U.S. Treasury regulations promulgated thereunder and U.S. Internal Revenue Service, or IRS, rulings and pronouncements and judicial decisions, all as in effect on the date of this prospectus and all of which are subject to change (possibly on a retroactive basis) or to differing interpretations so as to result in tax considerations different from those summarized below. We can not assure you that a change in law will not alter significantly the tax considerations that we describe in this summary.
The discussion is limited tonon-U.S. holders that hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). As used in this discussion, the term“non-U.S. holder” means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
| | |
| | • | an individual who is a citizen or resident of the United States; |
| |
| | • | a corporation (including any entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any political subdivision thereof; |
| |
| | • | a partnership (including any entity treated as a partnership for U.S. federal income tax purposes); |
| |
| | • | an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
| |
| | • | a trust (1) if a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) that has made a valid election to be treated as a U.S. person for such purposes. |
This discussion does not address the U.S. federal income and estate tax rules applicable to any person who holds our common stock through entities treated as partnerships for U.S. federal income tax purposes or to such entities themselves. If a partnership (including any entity or arrangement treated as a partnership for U.S. federal income tax purposes) owns our common stock, the tax treatment of a partner in that partnership will depend upon the status of the partner and the activities of the partnership. A holder that is a partnership or a holder of interests in a partnership should consult such holder’s tax advisor regarding the tax consequences of the purchase, ownership and disposition of our common stock.
This discussion does not consider:
| | |
| | • | any state, local or foreign tax consequences; |
| |
| | • | any tax consequences or computation of the alternative minimum tax; |
| |
| | • | any U.S. federal gift tax consequences; or |
| |
| | • | any U.S. federal tax considerations that may be relevant to anon-U.S. holder in light of its particular circumstances or tonon-U.S. holders that may be subject to special treatment under U.S. federal tax laws, including without limitation, banks or other financial institutions, insurance companies, tax-exempt organizations, certain trusts, hybrid entities, “controlled foreign corporations,” “passive foreign investment companies,” certain former citizens or residents of the U.S., holders subject to U.S. federal alternative minimum tax, broker-dealers, dealers or traders in securities or currencies and holders that hold our common stock as part of a “straddle,” “hedge,” “conversion transaction,” “synthetic security” or other integrated investment. |
Prospective investors are urged to consult their tax advisors regarding the application of the U.S. federal income and estate tax laws to their particular situations and the consequences under U.S. federal gift tax laws, as well as foreign, state and local laws and tax treaties.
104
Dividends
As previously discussed, we do not anticipate paying dividends on our common stock in the foreseeable future. If we pay dividends on our common stock, however, those payments will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed our current and accumulated earnings and profits, the distributions will constitute a return of capital and first reduce thenon-U.S. holder’s adjusted tax basis, but not below zero, and then will be treated as gain from the sale of stock, as described in the section of this prospectus entitled “Gain on Disposition of Common Stock.”
A dividend paid to anon-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate, or a lower rate under an applicable income tax treaty, unless the dividend is effectively connected with the conduct of a trade or business of thenon-U.S. holder within the U.S. (and, if an applicable income tax treaty so requires, is attributable to a permanent establishment of thenon-U.S. holder within the U.S.).Non-U.S. holders (generally on a properly executed IRSForm W-8 BEN) will be required to satisfy certain certification and disclosure requirements in order to claim a reduced rate of withholding pursuant to an applicable income tax treaty. These forms must be periodically updated.Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty. Special rules apply in the case of common stock held by certainnon-U.S. holders that are entities rather than individuals.
Dividends that are effectively connected with anon-U.S. holder’s conduct of a trade or business in the United States and, if an applicable income tax treaty so requires, attributable to a permanent establishment in the United States will be taxed on a net income basis at the regular graduated U.S. federal income tax rates in the same manner as if thenon-U.S. holder were a resident of the United States. In such cases, we will not have to withhold U.S. federal income tax if thenon-U.S. holder complies with applicable certification and disclosure requirements. In addition, a “branch profits tax” may be imposed at a 30% rate, or a lower rate under an applicable income tax treaty, on dividends received by a foreign corporation that are effectively connected with the conduct of a trade or business in the United States.
Anon-U.S. holder may obtain a refund or credit of any excess amounts withheld by filing an appropriate claim for a refund together with the required information with the IRS.
Gain on Disposition of Common Stock
Anon-U.S. holder generally will not be subject to U.S. federal income tax with respect to gain realized on a sale or other disposition of our common stock unless one of the following applies:
| | |
| | • | the gain is effectively connected with thenon-U.S. holder’s conduct of a trade or business in the U.S. or, if an applicable income tax treaty so requires, is attributable to a permanent establishment maintained by the non-U.S. holder in the United States; in these cases, thenon-U.S. holder generally will be taxed on its net gain derived from the disposition at the regular graduated rates; |
| |
| | • | and in the manner applicable to United States persons and, if thenon-U.S. holder is a foreign corporation, the “branch profits tax” described above may also apply; |
| |
| | • | thenon-U.S. holder is an individual present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met; in this case, thenon-U.S. holder will be subject to a 30% tax on the amount by which the gain derived from the sale or other disposition of our common stock and any otherU.S.-source capital gains realized by thenon-U.S. holder in the same taxable year exceed theU.S.-source capital losses realized by thenon-U.S. holder in that taxable year unless an applicable income tax treaty provides an exemption or a lower rate; or |
| |
| | • | we are or have been a “U.S. real property holding corporation” for U.S. federal income tax purposes at any time within the shorter of the five year period ending on the date of disposition or the period that thenon-U.S. holder held our common stock. We do not believe that we have been, are, or will become, a U.S. real property holding corporation, although there can be no assurance in this regard. If we are, or were to become, a U.S. real property holding corporation at any time during the applicable period, however, any gain recognized on a disposition of our common stock by a non-U.S. holder that did not own (directly, indirectly or constructively) more than 5% of our common stock during the applicable period generally |
105
| | |
| | | would not be subject to U.S. federal income tax, provided that our common stock is “regularly traded on an established securities market” (within the meaning of Section 897(c)(3) of the Code). |
Federal Estate Tax
Common stock owned or treated as owned by an individual who is anon-U.S. holder at the time of death will be included in the individual’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax or other treaty provides otherwise, and, therefore, such individual may be subject to U.S. federal estate tax.
Information Reporting and Backup Withholding Tax
Dividends and proceeds from the sale or other taxable disposition of our common stock are potentially subject to backup withholding. In general, backup withholding will not apply to dividends on our common stock paid by us or our paying agents, in their capacities as such, to anon-U.S. holder if the holder has provided the required certification that it is anon-U.S. holder.
Generally, we must report to the IRS the amount of dividends paid, the name and address of the recipient, and the amount, if any, of tax withheld. Pursuant to income tax treaties or some other agreements, the IRS may make its reports available to tax authorities in the recipient’s country of residence.
In general, backup withholding and information reporting will not apply to proceeds from the disposition of our common stock paid to anon-U.S. holder within the United States or conducted through certainU.S.-related financial intermediaries the holder has provided the required certification that it is anon-U.S. holder.
Backup withholding is not an additional tax. Any amount withheld may be refunded or credited against the holder’s U.S. federal income tax liability, if any, provided that the required information is furnished to the IRS in a timely manner.
Non-U.S. holders should consult their tax advisors regarding the application of the information reporting and backup withholding rules to them.
Prospectivenon-U.S. holders of our common stock should consult their tax advisors with respect to the particular tax consequences to them of owning and disposing of our common stock, including the consequences under the laws of any state, local or foreign jurisdiction or under any applicable tax treaty.
106
Under the terms and subject to the conditions contained in an underwriting agreement dated , 2007, we have agreed to sell to the underwriters named below, for whom Credit Suisse Securities (USA) LLC is acting as representative, the following respective numbers of shares of common stock:
| | | | | |
| | | Number
| |
Underwriter | | of Shares | |
| |
| Credit Suisse Securities (USA) LLC | | | | |
| Piper Jaffray & Co. | | | | |
| Leerink Swann LLC | | | | |
| Allen & Company LLC | | | | |
| | | | | |
| | | | | |
| Total | | | 7,000,000 | |
| | | | | |
The underwriting agreement provides that the underwriters are obligated to purchase all the shares of common stock in the offering if any are purchased, other than those shares covered by the over-allotment option described below. The underwriting agreement also provides that if an underwriter defaults the purchase commitments of non-defaulting underwriters may be increased or the offering may be terminated.
We have granted to the underwriters a 30 day option to purchase on a pro rata basis up to 1,050,000 additional shares at the initial public offering price less the underwriting discounts and commissions. The option may be exercised only to cover any over-allotments of common stock.
The underwriters propose to offer the shares of common stock initially at the public offering price on the cover page of this prospectus and to selling group members at that price less a selling concession of $ per share. After the initial public offering the underwriters may change the public offering price and concession.
The following table summarizes the compensation and estimated expenses we will pay:
| | | | | | | | | | | | | | | | | |
| | | Per Share | | | Total | |
| | | Without
| | | With
| | | Without
| | | With
| |
| | | Over-
| | | Over-
| | | Over-
| | | Over-
| |
| | | Allotment | | | Allotment | | | Allotment | | | Allotment | |
| |
| Underwriting Discounts and Commissions paid by us | | $ | | | | $ | | | | $ | | | | $ | | |
| Expenses payable by us | | $ | | | | $ | | | | $ | | | | $ | | |
As described under “Certain Relationships and Related Party Transactions — Purchase of Shares in this Offering,” Brookside Capital Partners Fund, L.P. has indicated an interest in purchasing $12.5 million of our common stock in this offering, which represents 833,333 shares of our common stock assuming a purchase price per share equal to $15.00, the midpoint of the price range indicated on the cover page of this prospectus. These shares offered to Brookside Capital Partners Fund, L.P. will be subject to an underwriting discount of 3.33%. Brookside Capital Partners Fund, L.P. is not under any obligation to purchase any shares in this offering, and their interest in purchasing shares in this offering is not a commitment to do so. These shares, if purchased, will be subject to the 180-day lock-up agreement that Brookside Capital Partners Fund, L.P. executed in connection with this offering. See “Shares Eligible for Future Sale — Lock-up Agreements.” Brookside Capital Partners Fund, L.P. beneficially owned 3,564,555 shares of our common stock and warrants to purchase an additional 590,199 shares or an aggregate of 28.35% of our common stock immediately prior to this offering.
The underwriters have informed us that they do not expect to sell to accounts over which the underwriters have discretionary authority.
We have agreed that, subject to certain exceptions, we will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, or file with the SEC a registration statement under the Securities Act relating to, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing, without the prior written consent of Credit Suisse Securities (USA) LLC for a period of 180 days after the date of this prospectus. However, in the event that either (1) during the last 17 days of the
107
“lock-up” period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the“lock-up” period, we announce that we will release earnings results during the 16 day period beginning on the last day of the“lock-up” period, then in either case the expiration of the“lock-up” will be extended until the expiration of the 18 day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless Credit Suisse Securities (USA) LLC waives, in writing, such an extension.
Our officers and directors and certain existing stockholders have agreed that, subject to certain exceptions, they will not offer, sell, contract to sell, pledge or otherwise dispose of, directly or indirectly, any shares of our common stock or securities convertible into or exchangeable or exercisable for any shares of our common stock, enter into a transaction that would have the same effect, or enter into any swap, hedge or other arrangement that transfers, in whole or in part, any of the economic consequences of ownership of our common stock, whether any of these transactions are to be settled by delivery of our common stock or other securities, in cash or otherwise, or publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement, without, in each case, the prior written consent of Credit Suisse Securities (USA) LLC for a period of 180 days after the date of this prospectus. However, in the event that either (1) during the last 17 days of the“lock-up” period, we release earnings results or material news or a material event relating to us occurs or (2) prior to the expiration of the“lock-up” period, we announce that we will release earnings results during the16-day period beginning on the last day of the“lock-up” period, then in either case the expiration of the“lock-up” will be extended until the expiration of the18-day period beginning on the date of the release of the earnings results or the occurrence of the material news or event, as applicable, unless Credit Suisse Securities (USA) LLC waives, in writing, such an extension.
Credit Suisse Securities (USA) LLC does not currently anticipate waiving any of thelock-up agreements restricting the sale of shares and does not have any pre-established conditions for such waivers.
The underwriters have reserved for sale at the initial public offering price up to 350,000 shares of the common stock for employees, directors and other persons associated with us who have expressed an interest in purchasing common stock in the offering. The number of shares available for sale to the general public in the offering will be reduced to the extent these persons purchase the reserved shares. Any reserved shares not so purchased will be offered by the underwriters to the general public on the same terms as the other shares.
We have agreed to indemnify the underwriters against liabilities under the Securities Act, or contribute to payments that the underwriters may be required to make in that respect.
We have applied to list the shares of common stock on the NASDAQ Global Market under the symbol “NSPH”.
Prior to this offering, there has been no market for our common stock. The initial public offering price will be determined by negotiation between us and the underwriters and will not necessarily reflect the market price of the common stock following this offering. The principal factors that will be considered in determining the initial public offering price will include:
| | |
| | • | the information presented in this prospectus and otherwise available to the underwriters; |
| |
| | • | the history of and the prospects for the industry in which we will compete; |
| |
| | • | the ability of our management; |
| |
| | • | the prospects for our future earnings; |
| |
| | • | the present state of our development and our current financial condition; |
| |
| | • | the recent market prices of, and the demand for, publicly-traded common stock of generally comparable companies; and |
| |
| | • | the general condition of the securities markets at the time of this offering. |
We cannot assure you that the initial public offering price will correspond to the price at which the common stock will trade in the public market subsequent to this offering or that an active trading market for the common stock will develop and continue after this offering.
108
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions, penalty bids and passive market making in accordance with Regulation M under the Exchange Act.
| | |
| | • | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. |
| |
| | • | Over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment optionand/or purchasing shares in the open market. |
| |
| | • | Syndicate covering transactions involve purchases of the common stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| |
| | • | Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| |
| | • | In passive market making, market makers in the common stock who are underwriters or prospective underwriters may, subject to limitations, make bids for or purchases of our common stock until the time, if any, at which a stabilizing bid is made. |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of the common stock. As a result the price of our common stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the NASDAQ Global Market or otherwise and, if commenced, may be discontinued at any time.
Certain of the underwriters and their respective affiliates have from time to time performed, and may in the future perform, various financial advisory, commercial banking and investment banking services for us and our affiliates in the ordinary course of business, for fees and expenses.
In August 2007, we entered into an engagement letter with Allen & Company LLC, pursuant to which they will provide us financial advisory services for which we expect to pay an aggregate of $250,000 in fees and a maximum of $20,000 in out-of-pocket expenses.
A prospectus in electronic format may be made available on the web sites maintained by one or more of the underwriters, or selling group members, if any, participating in this offering and one or more of the underwriters participating in this offering may distribute prospectuses electronically. The representative may agree to allocate a number of shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations.
The shares of common stock are offered for sale in those jurisdictions in the United States, Europe, Asia and elsewhere where it is lawful to make such offers.
Each of the underwriters has represented and agreed that it has not offered, sold or delivered and will not offer, sell or deliver any of the shares of common stock directly or indirectly, or distribute this prospectus or any accompanying prospectus or any other offering material relating to the shares of common stock, in or
109
from any jurisdiction except under circumstances that will result in compliance with the applicable laws and regulations thereof and that will not impose any obligations on us except as set forth in the underwriting agreement.
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a Relevant Member State), each underwriter represents and agrees that with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State (the Relevant Implementation Date) it has not made and will not make an offer of shares of common stock to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares of common stock which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the competent authority in that Relevant Member State, all in accordance with the Prospectus Directive, except that it may, with effect from and including the Relevant Implementation Date, make an offer of shares of common stock to the public in that Relevant Member State at any time,
| | |
| | • | to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| |
| | • | to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| |
| | • | to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive) subject to obtaining the prior consent of the manager for any such offer; or |
| |
| | • | in any other circumstances which do not require the publication by the Issuer of a prospectus pursuant to Article 3 of the Prospectus Directive. |
For the purposes of this provision, the expression an “offer of Shares to the public” in relation to any shares of the common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the shares of common stock to be offered so as to enable an investor to decide to purchase or subscribe the shares of common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression Prospectus Directive means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Notice to Investors in the United Kingdom
Each of the underwriters severally represents, warrants and agrees as follows:
| | |
| | • | it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) to persons who have professional experience in matters relating to investments falling with Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005 or in circumstances in which section 21 of FSMA does not apply to the company; and |
| |
| | • | it has complied with, and will comply with all applicable provisions of FSMA with respect to anything done by it in relation to the shares of common stock in, from or otherwise involving the United Kingdom. |
Notice to Residents of Hong Kong
The underwriters and each of their affiliates have not (i) offered or sold, and will not offer or sell, in Hong Kong, by means of any document, our shares of common stock other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32 of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance or (ii) issued or had in its possession for the purposes of issue, and will not issue or have in its possession for the purposes of issue, whether in Hong Kong or elsewhere any
110
advertisement, invitation or document relating to our shares of common stock which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to our securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance any rules made under that Ordinance. The contents of this document have not been reviewed by any regulatory authority in Hong Kong. You are advised to exercise caution in relation to the offer. If you are in any doubt about any of the contents of this document, you should obtain independent professional advice.
Notice to Residents of Japan
The underwriters will not offer or sell any of our shares of common stock directly or indirectly in Japan or to, or for the benefit of any Japanese person or to others, for re-offering or re-sale directly or indirectly in Japan or to any Japanese person, except in each case pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Securities and Exchange Law of Japan and any other applicable laws and regulations of Japan. For purposes of this paragraph, “Japanese person” means any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Residents of Singapore
This prospectus or any other offering material relating to our shares of common stock has not been and will not be registered as a prospectus with the Monetary Authority of Singapore, and the shares of common stock will be offered in Singapore pursuant to exemptions under Section 274 and Section 275 of the Securities and Futures Act, Chapter 289 of Singapore (the “Securities and Futures Act”). Accordingly our shares of common stock may not be offered or sold, or be the subject of an invitation for subscription or purchase, nor may this prospectus or any other offering material relating to our shares of common stock be circulated or distributed, whether directly or indirectly, to the public or any member of the public in Singapore other than (a) to an institutional investor or other person specified in Section 274 of the Securities and Futures Act, (b) to a sophisticated investor, and in accordance with the conditions specified in Section 275 of the Securities and Futures Act or (c) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the Securities and Futures Act.
111
NOTICE TO CANADIAN RESIDENTS
Resale Restrictions
The distribution of the shares of common stock in Canada is being made only on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of shares of common stock are made. Any resale of the shares of common stock in Canada must be made under applicable securities laws which will vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the shares of common stock.
Representations of Purchasers
By purchasing shares of common stock in Canada and accepting a purchase confirmation a purchaser is representing to us and the dealer from whom the purchase confirmation is received that:
| | |
| | • | the purchaser is entitled under applicable provincial securities laws to purchase the shares of common stock without the benefit of a prospectus qualified under those securities laws; |
| |
| | • | where required by law, that the purchaser is purchasing as principal and not as agent; |
| |
| | • | the purchaser has reviewed the text above under Resale Restrictions; and |
| |
| | • | the purchaser acknowledges and consents to the provision of specified information concerning its purchase of the shares of common stock to the regulatory authority that by law is entitled to collect the information. |
Further details concerning the legal authority for this information is available on request.
Rights of Action — Ontario Purchasers Only
Under Ontario securities legislation, certain purchasers who purchase a security offered by this prospectus during the period of distribution will have a statutory right of action for damages, or while still the owner of the shares of common stock, for rescission against us in the event that this prospectus contains a misrepresentation without regard to whether the purchaser relied on the misrepresentation. The right of action for damages is exercisable not later than the earlier of 180 days from the date the purchaser first had knowledge of the facts giving rise to the cause of action and three years from the date on which payment is made for the shares of common stock. The right of action for rescission is exercisable not later than 180 days from the date on which payment is made for the shares of common stock. If a purchaser elects to exercise the right of action for rescission, the purchaser will have no right of action for damages against us. In no case will the amount recoverable in any action exceed the price at which the shares of common stock were offered to the purchaser and if the purchaser is shown to have purchased the securities with knowledge of the misrepresentation, we will have no liability. In the case of an action for damages, we will not be liable for all or any portion of the damages that are proven to not represent the depreciation in value of the shares of common stock as a result of the misrepresentation relied upon. These rights are in addition to, and without derogation from, any other rights or remedies available at law to an Ontario purchaser. The foregoing is a summary of the rights available to an Ontario purchaser. Ontario purchasers should refer to the complete text of the relevant statutory provisions.
112
Enforcement of Legal Rights
All of our directors and officers as well as the experts named herein may be located outside of Canada and, as a result, it may not be possible for Canadian purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
Taxation and Eligibility for Investment
Canadian purchasers of shares of common stock should consult their own legal and tax advisors with respect to the tax consequences of an investment in the shares of common stock in their particular circumstances and about the eligibility of the shares of common stock for investment by the purchaser under relevant Canadian legislation.
113
The validity of the shares of common stock offered hereby will be passed upon for us by Paul, Hastings, Janofsky & Walker LLP. Cravath, Swaine & Moore LLP is representing the underwriters in this offering.
The financial statements as of December 31, 2005 and 2006, and June 30, 2007, and for each of the three years in the period ended December 31, 2006 and for the six month period ended June 30, 2007, included in this prospectus, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in the registration statement (which report expresses an unqualified opinion and includes explanatory paragraphs that indicate the accompanying 2004, 2005 and 2006 financial statements have been restated and Nanosphere, Inc. is in the development stage). Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement onForm S-1 under the Securities Act of 1933 with respect to the shares of common stock offered by this prospectus. This prospectus does not contain all of the information included in the registration statement. For further information pertaining to us and our common stock, you should refer to the registration statement and to its exhibits. Whenever we make reference in this prospectus to any of our contracts, agreements or other documents, the references are not necessarily complete, and you should refer to the exhibits attached to the registration statement for copies of the actual contract, agreement or other document.
Upon completion of this offering, we will be subject to the information and periodic reporting requirements of the Securities Exchange Act of 1934 and will file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facility at 100 F Street, N.E., Room 1580 Washington, D.C., 20549. Please call the SEC at1-800-SEC-0330 for further information on the operation of the public reference facility.
114
INDEX TO FINANCIAL STATEMENTS
| | | | | |
| | | Page |
| |
| | | F-2 | |
| | | F-3 | |
| | | F-4 | |
| | | F-5 | |
| | | F-6 | |
| | | F-7 | |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Nanosphere, Inc.
Northbrook, Illinois
We have audited the accompanying balance sheets of Nanosphere, Inc. (a development stage company) (the “Company”) as of December 31, 2005, December 31, 2006, and June 30, 2007 and the related statements of operations, stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2006, for the six month period ended June 30, 2007 and for the cumulative period from December 30, 1999 (date of incorporation) through June 30, 2007. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2005, December 31, 2006, and June 30, 2007 and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2006, for the six month period ended June 30, 2007 and for the cumulative period from December 30, 1999 (date of incorporation) through June 30, 2007, in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 1, the Company is in the development stage.
As discussed in Note 12, the accompanying 2004, 2005 and 2006 financial statements have been restated.
/s/ DELOITTE & TOUCHE LLP
Chicago, Illinois
September 26, 2007
(October 16, 2007 as to the effects of the stock split described in Note 1, and to the last four paragraphs of Note 11)
F-2
Nanosphere, Inc.
(A Development Stage Company)
Balance Sheets
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | June 30,
| |
| | | As of December 31, | | | June 30,
| | | 2007
| |
| | | 2005 | | | 2006 | | | 2007 | | | Pro Forma | |
| | | (As restated
| | | (As restated
| | | | | | (Unaudited
| |
| | | see note 12) | | | see note 12) | | | | | | see note 13) | |
| ASSETS |
| CURRENT ASSETS: | | | | | | | | | | | | | | | | |
| Cash and cash equivalents | | $ | 3,641,338 | | | $ | 29,112,429 | | | $ | 26,970,383 | | | $ | 28,183,925 | |
| Accounts receivable | | | 247,621 | | | | 44,816 | | | | 99,800 | | | | 99,800 | |
| Inventories | | | 123,765 | | | | 884,849 | | | | 1,188,909 | | | | 1,188,909 | |
| Other current assets | | | 436,219 | | | | 462,858 | | | | 954,447 | | | | 954,447 | |
| | | | | | | | | | | | | | | | | |
| Total current assets | | | 4,448,943 | | | | 30,504,952 | | | | 29,213,539 | | | | 30,427,081 | |
| | | | | | | | | | | | | | | | | |
| PROPERTY AND EQUIPMENT — At cost: | | | | | | | | | | | | | | | | |
| Computer equipment and software | | | 558,258 | | | | 730,144 | | | | 808,655 | | | | 808,655 | |
| Laboratory equipment | | | 1,599,395 | | | | 2,788,164 | | | | 2,895,947 | | | | 2,895,947 | |
| Furniture and fixtures | | | 49,357 | | | | 143,134 | | | | 213,638 | | | | 213,638 | |
| Leasehold improvements | | | 2,086,977 | | | | 2,086,977 | | | | 2,120,329 | | | | 2,120,329 | |
| Manufacturing equipment | | | 1,407,603 | | | | 2,056,830 | | | | 2,586,474 | | | | 2,586,474 | |
| Office equipment | | | 58,925 | | | | 66,007 | | | | 66,007 | | | | 66,007 | |
| Tooling | | | 435,733 | | | | 707,018 | | | | 943,316 | | | | 943,316 | |
| | | | | | | | | | | | | | | | | |
| Total property and equipment — at cost | | | 6,196,248 | | | | 8,578,274 | | | | 9,634,366 | | | | 9,634,366 | |
| Less accumulated depreciation | | | (1,808,391 | ) | | | (2,999,802 | ) | | | (3,773,146 | ) | | | (3,773,146 | ) |
| | | | | | | | | | | | | | | | | |
| Net property and equipment — at cost | | | 4,387,857 | | | | 5,578,472 | | | | 5,861,220 | | | | 5,861,220 | |
| | | | | | | | | | | | | | | | | |
| INTANGIBLE ASSETS — Net of accumulated amortization | | | 2,431,254 | | | | 4,865,950 | | | | 5,180,336 | | | | 5,180,336 | |
| | | | | | | | | | | | | | | | | |
| OTHER ASSETS | | | 78,460 | | | | 88,460 | | | | 179,918 | | | | 179,918 | |
| | | | | | | | | | | | | | | | | |
| TOTAL | | $ | 11,346,514 | | | $ | 41,037,834 | | | $ | 40,435,013 | | | $ | 41,648,555 | |
| | | | | | | | | | | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) |
| CURRENT LIABILITIES: | | | | | | | | | | | | | | | | |
| Accounts payable | | $ | 1,210,996 | | | $ | 1,499,514 | | | $ | 1,068,804 | | | $ | 1,068,804 | |
| Accrued compensation | | | 378,571 | | | | 872,508 | | | | 1,337,271 | | | | 1,337,271 | |
| Accrued license fees | | | | | | | 360,000 | | | | 402,109 | | | | 402,109 | |
| Accrued legal expenses | | | 96,797 | | | | 137,088 | | | | 457,031 | | | | 457,031 | |
| Other current liabilities | | | 386,099 | | | | 270,935 | | | | 594,334 | | | | 594,334 | |
| Note payable — related party | | | 5,019,062 | | | | | | | | | | | | | |
| Long term debt — current portion | | | | | | | | | | | 1,424,400 | | | | 1,424,400 | |
| Lease payable — current portion | | | | | | | 32,444 | | | | 34,841 | | | | 34,841 | |
| | | | | | | | | | | | | | | | | |
| Total current liabilities | | | 7,091,525 | | | | 3,172,489 | | | | 5,318,790 | | | | 5,318,790 | |
| | | | | | | | | | | | | | | | | |
| LONG-TERM LIABILITIES: | | | | | | | | | | | | | | | | |
| Accrued license fees — noncurrent | | | | | | | 330,000 | | | | 336,885 | | | | 336,885 | |
| Lease payable — noncurrent portion | | | | | | | 58,802 | | | | 40,518 | | | | 40,518 | |
| Long term debt — noncurrent portion | | | | | | | | | | | 9,262,458 | | | | 9,262,458 | |
| Preferred stock warrants | | | 189,790 | | | | 1,761,533 | | | | 2,038,146 | | | | | |
| Convertible derivative liability | | | 12,882,505 | | | | 32,085,041 | | | | 32,693,981 | | | | | |
| | | | | | | | | | | | | | | | | |
| Total liabilities | | | 20,163,820 | | | | 37,407,865 | | | | 49,690,778 | | | | 14,958,651 | |
| | | | | | | | | | | | | | | | | |
| CONVERTIBLE PREFERRED STOCK: | | | | | | | | | | | | | | | | |
Series B Convertible Preferred Stock, $0.01 par value;
Liquidation preference of $25,396 at June 30, 2007. | | | 25,396 | | | | 25,396 | | | | 25,396 | | | | | |
Series C Convertible Preferred Stock, $0.01 par value;
Liquidation preference of $6,030,003 at June 30, 2007. | | | 6,030,003 | | | | 6,030,003 | | | | 6,030,003 | | | | | |
Series C-2 Convertible Preferred Stock, $0.01 par value;
Liquidation preference of $47,794,091 at June 30, 2007. | | | 45,088,585 | | | | 47,037,902 | | | | 48,423,199 | | | | | |
Series D Convertible Preferred Stock, $0.01 par value;
Liquidation preference of $60,439,375 at June 30, 2007. | | | | | | | 55,774,739 | | | | 59,507,309 | | | | | |
| | | | | | | | | | | | | | | | | |
| Total Convertible Preferred Stock | | | 51,143,984 | | | | 108,868,040 | | | | 113,985,907 | | | | | |
| | | | | | | | | | | | | | | | | |
| STOCKHOLDERS’ EQUITY (DEFICIT): | | | | | | | | | | | | | | | | |
| Common stock, $0.01 par value; 250,000,000, 450,000,000 and 450,000,000, shares authorized at December 31, 2005, December 31, 2006 and June 30, 2007, respectively | | | 12,347 | | | | 92,580 | | | | 92,580 | | | | 144,496 | |
| Additional paid-in capital | | | 285,424 | | | | 2,051,126 | | | | 2,906,315 | | | | 151,080,104 | |
| Note receivable from chief executive officer | | | | | | | (1,440,000 | ) | | | (1,440,000 | ) | | | (1,440,000 | ) |
| Common stock warrants | | | | | | | | | | | | | | | 1,705,871 | |
| Deficit accumulated during the development stage | | | (60,259,061 | ) | | | (105,941,777 | ) | | | (124,800,567 | ) | | | (124,800,567 | ) |
| | | | | | | | | | | | | | | | | |
| Total stockholders’ equity (deficit) | | | (59,961,290 | ) | | | (105,238,071 | ) | | | (123,241,672 | ) | | | 26,689,904 | |
| | | | | | | | | | | | | | | | | |
| TOTAL | | $ | 11,346,514 | | | $ | 41,037,834 | | | $ | 40,435,013 | | | $ | 41,648,555 | |
| | | | | | | | | | | | | | | | | |
See notes to financial statements.
F-3
Nanosphere, Inc.
(A Development Stage Company)
Statements of Operations
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Period From
| |
| | | | | | | | | | | | Six Month
| | | Six Month
| | | December 30, 1999
| |
| | | | | | | | | | | | Period Ended
| | | Period Ended
| | | (Date of Incorporation)
| |
| | | Years Ended December 31, | | | June 30,
| | | June 30,
| | | Through
| |
| | | 2004 | | | 2005 | | | 2006 | | | 2006 | | | 2007 | | | June 30, 2007 | |
| | | (As restated
| | | (As restated
| | | (As restated
| | | (Unaudited)
| | | | | | | |
| | | See note 12) | | | See note 12) | | | See note 12) | | | | | | | | | | |
| |
| REVENUE: | | | | | | | | | | | | | | | | | | | | | | | | |
| Grant and contract revenue | | $ | 2,768,125 | | | $ | 1,777,667 | | | $ | 1,006,351 | | | $ | 438,512 | | | $ | 726,503 | | | $ | 8,487,257 | |
| Product sales | | | | | | | 136,850 | | | | 131,660 | | | | 27,630 | | | | 53,670 | | | | 352,200 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total revenue | | | 2,768,125 | | | | 1,914,517 | | | | 1,138,011 | | | | 466,142 | | | | 780,173 | | | | 8,839,457 | |
| COSTS AND EXPENSES: | | | | | | | | | | | | | | | | | | | | | | | | |
| Cost of product sales | | | | | | | 125,118 | | | | 31,049 | | | | | | | | 18,367 | | | | 190,986 | |
| Research and development | | | 10,366,473 | | | | 13,244,872 | | | | 17,447,227 | | | | 7,874,596 | | | | 10,219,047 | | | | 67,589,471 | |
| Sales, general, and administrative | | | 3,131,390 | | | | 4,502,970 | | | | 5,415,525 | | | | 2,663,931 | | | | 5,256,583 | | | | 24,555,735 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total costs and expenses | | | 13,497,863 | | | | 17,872,960 | | | | 22,893,801 | | | | 10,538,527 | | | | 15,493,997 | | | | 92,336,192 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Loss from operations | | | (10,729,738 | ) | | | (15,958,443 | ) | | | (21,755,790 | ) | | | (10,072,385 | ) | | | (14,713,824 | ) | | | (83,496,735 | ) |
| OTHER INCOME (EXPENSE): | | | | | | | | | | | | | | | | | | | | | | | | |
| Change in fair value of convertible derivative liability | | | | | | | | | | | (2,916,822 | ) | | | (2,916,822 | ) | | | | | | | (2,916,822 | ) |
| Change in fair value of preferred stock warrants | | | (8,801 | ) | | | (8,314 | ) | | | (119,914 | ) | | | (244,104 | ) | | | (276,612 | ) | | | (413,641 | ) |
| Foreign exchange loss | | | | | | | | | | | | | | | | | | | (13,770 | ) | | | (13,770 | ) |
| Interest expense — related party | | | (204,335 | ) | | | (37,919 | ) | | | (146,550 | ) | | | (146,550 | ) | | | | | | | (388,804 | ) |
| Interest expense | | | | | | | | | | | (7,506 | ) | | | (1,585 | ) | | | (823,748 | ) | | | (831,254 | ) |
| Interest income | | | 40,963 | | | | 69,376 | | | | 1,415,001 | | | | 483,755 | �� | | | 758,433 | | | | 2,666,582 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total other income (expense) | | | (172,173 | ) | | | 23,143 | | | | (1,775,791 | ) | | | (2,825,306 | ) | | | (355,697 | ) | | | (1,897,709 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| NET LOSS | | | (10,901,911 | ) | | | (15,935,300 | ) | | | (23,531,581 | ) | | | (12,897,691 | ) | | | (15,069,521 | ) | | | (85,394,444 | ) |
| Accumulated convertible preferred stock dividends | | | | | | | | | | | (4,413,591 | ) | | | (1,350,933 | ) | | | (3,180,329 | ) | | | (8,004,459 | ) |
| Convertible preferred stock redemption value adjustment | | | (10,156,393 | ) | | | (2,898,787 | ) | | | (17,737,544 | ) | | | (17,737,544 | ) | | | (608,940 | ) | | | (31,401,664 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| NET LOSS ATTRIBUTABLE TO COMMON STOCK | | $ | (21,058,304 | ) | | $ | (18,834,087 | ) | | $ | (45,682,716 | ) | | $ | (31,986,168 | ) | | $ | (18,858,790 | ) | | $ | (124,800,567 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss per common share — basic and diluted | | $ | (34.44 | ) | | $ | (30.80 | ) | | $ | (52.78 | ) | | $ | (40.15 | ) | | $ | (20.22 | ) | | | | |
| Weighted average number of common shares outstanding — basic and diluted | | | 611,466 | | | | 611,496 | | | | 865,559 | | | | 796,729 | | | | 932,646 | | | | | |
See notes to financial statements.
F-4
Nanosphere, Inc.
(A Development Stage Company)
Statements of Stockholders’ Deficit
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | Note
| | | Deficit
| | | | |
| | | | | | | | | | | | Receivable
| | | Accumulated
| | | | |
| | | | | | | | | Additional
| | | From Chief
| | | During the
| | | | |
| | | Common Stock | | | Paid-In
| | | Executive
| | | Development
| | | | |
| | | Shares | | | Par Value | | | Capital | | | Officer | | | Stage | | | Total | |
| |
| BALANCE — December 30, 1999 | | | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| Issuance of common stock upon incorporation | | | 606,000 | | | | 12,036 | | | | | | | | | | | | | | | | 12,036 | |
| Issuance of common stock in exchange for a license | | | 3,915 | | | | | | | | | | | | | | | | | | | | — | |
Exercise of stock options on common stock
(since incorporation) | | | 1,551 | | | | 248 | | | | 25,951 | | | | | | | | | | | | 26,199 | |
| Issuance of dividends on Series A Convertible Preferred Stock (since incorporation) | | | | | | | | | | | | | | | | | | | (410,539 | ) | | | (410,539 | ) |
| Net loss (since incorporation) | | | | | | | | | | | | | | | | | | | (19,956,131 | ) | | | (19,956,131 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — December 31, 2003 | | | 611,466 | | | | 12,284 | | | | 25,951 | | | | | | | | (20,366,670 | ) | | | (20,328,435 | ) |
| Share-based compensation related to stock options (As restated, see note 12) | | | | | | | | | | | 38,308 | | | | | | | | | | | | 38,308 | |
| Convertible preferred stock redemption value adjustment (As restated, see note 12) | | | | | | | | | | | | | | | | | | | (10,156,393 | ) | | | (10,156,393 | ) |
| Net loss (As restated, see note 12) | | | | | | | | | | | | | | | | | | | (10,901,911 | ) | | | (10,901,911 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — December 31, 2004 (As restated, see note 12) | | | 611,466 | | | | 12,284 | | | | 64,259 | | | | | | | | (41,424,974 | ) | | | (41,348,431 | ) |
| Exercise of stock options on common stock | | | 250 | | | | 63 | | | | 1,812 | | | | | | | | | | | | 1,875 | |
| Share-based compensation related to stock options (As restated, see note 12) | | | | | | | | | | | 219,353 | | | | | | | | | | | | 219,353 | |
| Convertible preferred stock redemption value adjustment | | | | | | | | | | | | | | | | | | | (2,898,787 | ) | | | (2,898,787 | ) |
| Net loss (As restated, see note 12) | | | | | | | | | | | | | | | | | | | (15,935,300 | ) | | | (15,935,300 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — December 31, 2005 (As restated, see note 12) | | | 611,716 | | | | 12,347 | | | | 285,424 | | | | | | | | (60,259,061 | ) | | | (59,961,290 | ) |
| Issuance of common stock on March 16, 2006 at $4.50 per share in exchange for a note receivable | | | 320,000 | | | | 80,000 | | | | 1,360,000 | | | | (1,440,000 | ) | | | | | | | | |
| Exercise of stock options on common stock | | | 930 | | | | 233 | | | | 6,742 | | | | | | | | | | | | 6,975 | |
| Share-based compensation related to stock options (As restated, see note 12) | | | | | | | | | | | 398,960 | | | | | | | | | | | | 398,960 | |
Undeclared and unpaid 6% dividends, earned in 2006 onSeries C-2 and Series D Convertible Preferred Stock | | | | | | | | | | | | | | | | | | | (4,413,591 | ) | | | (4,413,591 | ) |
| Convertible preferred stock redemption value adjustment (As restated, see note 12) | | | | | | | | | | | | | | | | | | | (17,737,544 | ) | | | (17,737,544 | ) |
| Net loss (As restated, see note 12) | | | | | | | | | | | | | | | | | | | (23,531,581 | ) | | | (23,531,581 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — December 31, 2006 (As restated, see note 12) | | | 932,646 | | | | 92,580 | | | | 2,051,126 | | | | (1,440,000 | ) | | | (105,941,777 | ) | | | (105,238,071 | ) |
| Share-based compensation related to stock options | | | | | | | | | | | 790,247 | | | | | | | | | | | | 790,247 | |
| Interest received on note receivable from chief executive officer | | | | | | | | | | | 64,942 | | | | | | | | | | | | 64,942 | |
Undeclared and unpaid 6% dividends, earned onSeries C-2 and Series D Convertible Preferred Stock | | | | | | | | | | | | | | | | | | | (3,180,329 | ) | | | (3,180,329 | ) |
| Convertible preferred stock redemption value adjustment | | | | | | | | | | | | | | | | | | | (608,940 | ) | | | (608,940 | ) |
| Net loss | | | | | | | | | | | | | | | | | | | (15,069,521 | ) | | | (15,069,521 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| BALANCE — June 30, 2007 | | | 932,646 | | | $ | 92,580 | | | $ | 2,906,315 | | | $ | (1,440,000 | ) | | $ | (124,800,567 | ) | | $ | (123,241,672 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
See notes to financial statements.
F-5
Nanosphere, Inc.
(A Development Stage Company)
Statements of Cash Flows
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Period From
| |
| | | | | | | | | | | | Six Month
| | | Six Month
| | | December 30, 1999
| |
| | | | | | | | | | | | Period Ended
| | | Period Ended
| | | (Date of Incorporation)
| |
| | | Years Ended December 31, | | | June 30,
| | | June 30,
| | | Through
| |
| | | 2004 | | | 2005 | | | 2006 | | | 2006 | | | 2007 | | | June 30, 2007 | |
| | | (As restated
| | | (As restated
| | | (As restated
| | | (unaudited)
| | | | | | | |
| | | See note 12)
| | | See note 12)
| | | See note 12)
| | | | | | | | | | |
| |
| CASH FLOWS FROM OPERATING ACTIVITIES: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (10,901,911 | ) | | $ | (15,935,300 | ) | | $ | (23,531,581 | ) | | $ | (12,897,691 | ) | | | (15,069,521 | ) | | $ | (85,394,444 | ) |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Depreciation and amortization | | | 481,926 | | | | 854,556 | | | | 1,239,855 | | | | 585,393 | | | | 837,162 | | | | 3,940,282 | |
| Amortization of financing costs and accretion of debt discount | | | | | | | | | | | | | | | | | | | 279,409 | | | | 279,409 | |
| Loss from write-off of intangible assets | | | 167,730 | | | | 89,534 | | | | 41,659 | | | | | | | | 206,683 | | | | 505,606 | |
| Share-based compensation | | | 38,308 | | | | 219,353 | | | | 398,960 | | | | 196,315 | | | | 790,247 | | | | 1,446,868 | |
| Change in fair value of preferred stock warrants | | | 8,801 | | | | 8,314 | | | | 119,914 | | | | 244,104 | | | | 276,612 | | | | 413,641 | |
| Change in fair value of convertible derivative liability | | | | | | | | | | | 2,916,822 | | | | 2,916,822 | | | | | | | | 2,916,822 | |
| Changes in operating assets and liabilities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Accounts receivable | | | (122,468 | ) | | | (93,161 | ) | | | 202,805 | | | | (39,588 | ) | | | (54,984 | ) | | | (99,800 | ) |
| Inventories | | | (18,438 | ) | | | (23,067 | ) | | | (761,084 | ) | | | (736,511 | ) | | | (304,060 | ) | | | (1,188,909 | ) |
| Other current assets | | | (300,323 | ) | | | (66,542 | ) | | | (80,533 | ) | | | 2,786 | | | | (220,015 | ) | | | (736,767 | ) |
| Other assets | | | | | | | 9,158 | | | | (10,000 | ) | | | (10,000 | ) | | | 10,830 | | | | (77,630 | ) |
| Accounts payable | | | (189,694 | ) | | | 185,929 | | | | 157,868 | | | | 201,776 | | | | (368,282 | ) | | | 764,821 | |
| Accrued and other current liabilities | | | 846,645 | | | | (250,290 | ) | | | 564,563 | | | | 191,331 | | | | 876,704 | | | | 2,267,302 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash used in operating activities | | | (9,989,424 | ) | | | (15,001,516 | ) | | | (18,740,752 | ) | | | (9,345,263 | ) | | | (12,739,215 | ) | | | (74,962,799 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES: | | | | | | | | | | | | | | | | | | | | | | | | |
| Proceeds from investment maturities | | | | | | | | | | | 53,894 | | | | 320 | | | | | | | | 53,894 | |
| Purchases of property and equipment | | | (1,657,526 | ) | | | (2,219,353 | ) | | | (2,184,616 | ) | | | (752,952 | ) | | | (1,122,347 | ) | | | (9,374,869 | ) |
| Investments in intangible assets | | | (393,484 | ) | | | (618,491 | ) | | | (1,752,016 | ) | | | (471,248 | ) | | | (572,238 | ) | | | (4,924,795 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash used in investing activities | | | (2,051,010 | ) | | | (2,837,844 | ) | | | (3,882,738 | ) | | | (1,223,880 | ) | | | (1,694,585 | ) | | | (14,245,770 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES: | | | | | | | | | | | | | | | | | | | | | | | | |
| Proceeds from related party notes payable | | | | | | | 5,019,062 | | | | 1,320,148 | | | | 1,320,148 | | | | | | | | 6,339,210 | |
| Proceeds from issuance of debt | | | | | | | | | | | | | | | | | | | 12,500,000 | | | | 12,500,000 | |
| Repayment of long term debt | | | | | | | | | | | | | | | | | | | (142,736 | ) | | | (142,736 | ) |
| Payments on capital lease obligation | | | | | | | | | | | (26,439 | ) | | | (5,619 | ) | | | (15,887 | ) | | | (42,326 | ) |
| Proceeds from interest on CEO note receivable | | | | | | | | | | | | | | | | | | | 64,942 | | | | 64,942 | |
| Deferred financing fees | | | | | | | | | | | | | | | | | | | (114,565 | ) | | | (114,565 | ) |
| Proceeds from the issuance of common stock | | | | | | | 1,875 | | | | 6,975 | | | | 2,700 | | | | | | | | 47,085 | |
| Proceeds from the issuance of Series A Convertible Preferred Stock | | | | | | | | | | | | | | | | | | | | | | | 3,008,357 | |
| Proceeds from the issuance of Series B Convertible Preferred Stock | | | | | | | | | | | | | | | | | | | | | | | 5,375,001 | |
| Proceeds from the issuance of Series C Convertible Preferred Stock | | | | | | | | | | | | | | | | | | | | | | | 15,000,000 | |
Proceeds from the issuance ofSeries C-2 Convertible Preferred Stock | | | 17,204,334 | | | | 10,145,753 | | | | | | | | | | | | | | | | 27,350,087 | |
| Proceeds from the issuance of Series D Convertible Preferred Stock | | | | | | | | | | | 46,793,897 | | | | 46,793,897 | | | | | | | | 46,793,897 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net cash provided by financing activities | | | 17,204,334 | | | | 15,166,690 | | | | 48,094,581 | | | | 48,111,126 | | | | 12,291,754 | | | | 116,178,952 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | | | 5,163,900 | | | | (2,672,670 | ) | | | 25,471,091 | | | | 37,541,983 | | | | (2,142,046 | ) | | | 26,970,383 | |
| CASH AND CASH EQUIVALENTS — Beginning of period | | | 1,150,108 | | | | 6,314,008 | | | | 3,641,338 | | | | 3,641,338 | | | | 29,112,429 | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| CASH AND CASH EQUIVALENTS — End of period | | $ | 6,314,008 | | | $ | 3,641,338 | | | $ | 29,112,429 | | | $ | 41,183,321 | | | $ | 26,970,383 | | | $ | 26,970,383 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| NONCASH INVESTING AND FINANCING ACTIVITIES: | | | | | | | | | | | | | | | | | | | | | | | | |
| Equipment acquired under capital lease | | $ | — | | | $ | — | | | $ | 117,685 | | | $ | 117,685 | | | $ | | | | $ | 117,685 | |
| Capital expenditures included in accounts payable | | | 348,973 | | | | 128,341 | | | | 208,066 | | | | 82,286 | | | | 141,811 | | | | 208,066 | |
| Patent costs capitalized and included in accounts payable | | | 143,545 | | | | 107,420 | | | | 158,344 | | | | 113,821 | | | | 162,171 | | | | 158,344 | |
| Patent and license costs capitalized and included in accrued liabilities | | | 25,432 | | | | 35,430 | | | | 757,290 | | | | 50,000 | | | | 766,112 | | | | 757,290 | |
| Common stock issued for note payable — related party | | | | | | | | | | | 1,440,000 | | | | 1,440,000 | | | | | | | | 1,440,000 | |
| Payment of Series A Convertible Preferred Stock dividends in additional shares of Series A Convertible Preferred Stock | | | | | | | | | | | | | | | | | | | | | | | 410,539 | |
Accumulated dividends onSeries C-2 and Series D Convertible Preferred Stock — undeclared and unpaid | | | | | | | | | | $ | 4,413,591 | | | | 1,350,933 | | | | 3,180,329 | | | | 7,593,920 | |
Conversion of related party notes payable and accrued interest of $204,335, intoSeries C-2 Convertible Preferred Stock | | | 7,212,156 | | | | | | | | | | | | | | | | | | | | 7,212,156 | |
| Conversion of related party notes payable and accrued interest of $177,358 into Series D Convertible Preferred Stock | | | | | | | | | | | 6,516,568 | | | | 6,516,568 | | | | | | | | 6,516,568 | |
| Equity offering transaction costs included in other current assets and accrued liabilities | | | | | | | | | | | | | | | | | | | 271,573 | | | | 271,573 | |
See notes to financial statements.
F-6
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements
As of December 31, 2005 and 2006 and June 30, 2007, and
For The Years Ended December 31, 2004, 2005 and 2006,
for the six month periods ended June 30, 2006 (Unaudited) and 2007 and
for the Cumulative Period From December 30, 1999
(Date of Incorporation) Through June 30, 2007
| |
| 1. | Description of Business |
Nanosphere, Inc. (the “Company”) was incorporated in Delaware on December 30, 1999. On January 3, 2000, Nanosphere, Inc. merged with Nanosphere LLC, an entity owned by the founders of the Company. The Company began operations upon incorporation on December 30, 1999. The Company develops, manufactures and markets an advanced molecular diagnostics platform, the Verigene System, that enables simple, low cost, and highly sensitive genomic and protein testing on a single platform. The Company has been, since its inception, in the development stage, as defined by the Statement of Financial Accounting Standards (“SFAS”) No. 7,Accounting and Reporting by Development Stage Enterprises.
Basis of Presentation — The accompanying financial statements have been prepared on a going-concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred net losses attributable to common stock of $124.8 million since inception, and has funded those losses primarily through the sale and issuance of equity securities and secondarily through research and development contracts.
As discussed in Note 11, all common stock share and per share data (except par value), for all periods presented, have been adjusted to reflect the effect of theone-for-25 stock split, effected on October 16, 2007. In addition, the number of shares of common stock issuable upon exercise of stock options and convertible preferred stock warrants, the ratio by which the number of shares of convertible preferred stock is multiplied by to determine the number of shares of common stock into which such preferred stock converts, as well as the number of shares of common stock reserved for issuance under our equity incentive plans, were proportionately decreased in accordance with the terms of those respective agreements and plans and the Company’s Amended and Restated Certificate of Incorporation, as amended.
| |
| 2. | Summary of Significant Accounting Policies |
Cash and Cash Equivalents — The Company considers all highly liquid investments with a maturity of three months or less, at date of purchase, to be cash equivalents. The majority of these funds are held in interest-bearing money market and bank checking accounts. Interest income is recorded on an accrual basis as earned.
Receivables — Accounts receivable consist of amounts due to the Company under various contracts and government grants. An allowance for doubtful accounts is not recorded because the Company believes that all receivables are fully collectible.
Inventories — Inventories are carried at the lower of cost or market, using thefirst-in, first-out method. Inventory on hand at December 31, 2005, December 31, 2006, and June 30, 2007 consisted of $123,765, $27,209 and $447,928 of finished goods, respectively, and $0, $857,640 and $740,981 ofwork-in-process component parts related to the Company’s diagnostic instrument product lines, respectively.
F-7
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
Property and Equipment — Property and equipment are recorded at cost and depreciated using the straight-line method over the assets’ estimated useful lives, which are:
| | | | | |
| Computers and office equipment | | | 3 years | |
| Engineering and laboratory equipment, including tooling | | | 5 years | |
| Furniture and fixtures | | | 7 years | |
| Manufacturing equipment | | | 7 years | |
| Leasehold improvements | | | 7 years | |
Assets classified as leasehold improvements are amortized over the shorter of their estimated useful lives or the lease term using the straight-line method. Capitalized leased assets are amortized using the straight-line method over the estimated useful life. Maintenance and repair costs are expensed as incurred.
Intangible Assets — Intangible assets are stated at cost less accumulated amortization and consist of patent costs and purchased intellectual property. For patent costs, amortization begins upon the patent grant date and is calculated using the straight-line method over the remaining lives of the granted patents, which range from 10.5 to 16.25 years. Purchased intellectual property represents licenses and is associated with patents owned by third-parties for technologies which are embedded in the Company’s diagnostic instruments and diagnostic test products that the Company licensed in anticipation of sales of such products. Amortization of purchased intellectual property will begin upon the Company obtaining FDA clearance to sell products containing the licensed technology and will be calculated using the straight-line method over the remaining expected life of the licensed technology, which range from 2 to 17 years.
Impairment of Long-Lived Assets — The Company assesses the recoverability of long-lived assets, including intangible assets, by periodically evaluating the carrying value of such assets whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. If impairment is indicated, the Company will value the asset at its estimated fair value.
Use of Estimates — The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements. Estimates also affect the reported amounts of expenses during the reporting period. The Company’s more significant estimates and assumptions include its accounting for stock-based compensation. Actual results could differ from those estimates.
Revenue Recognition — The Company is in the development stage and has generated limited revenues since its inception. The Company recognizes revenue under grants and contracts and for reimbursement of related research and development expenses at the time the relevant expenses are incurred. For product sales, revenue is recognized when persuasive evidence of an arrangement exists, title and risk of loss is transferred to customers, the price to the buyer is fixed or determinable, and collectibility is reasonably assured.
Verigene System instrument units are sold outright to customers or via sales-type lease arrangements. Verigene System units are also leased to customers pursuant to operating leases. The Company recognizes revenue from sales of the Verigene System, including cartridges and related products generally upon shipment or, in certain transactions, upon customer acceptance. Revenue and related cost for Verigene System instrument units sold via sales-type leases are generally recognized at the time of shipment based on the present value of the minimum lease payments with interest income recognized over the life of the lease using the interest method. Under operating lease arrangements revenue is recognized on an installment basis over the life of the lease while the cost of the leased equipment is carried on the Company’s balance sheet and amortized over its estimated useful life. To date, the Company has not sold any products via sales-type leases.
Research and Development Costs — Research and development costs are expensed as incurred.
F-8
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
Income Taxes — The Company accounts for income taxes under the provisions of SFAS No. 109,Accounting for Income Taxes. SFAS No. 109 requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. An allowance is provided to reduce net deferred tax assets to the amount management believes will, more likely than not, be recovered.
In June 2006, Financial Accounting Standards Board (“FASB”) issued FASB Interpretation No. FIN 48,Accounting for Uncertainty in Income Taxes. This interpretation clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with SFAS No. 109. This interpretation prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. Under this interpretation, the evaluation of a tax position is a two-step process. First, the enterprise determines whether it is more likely than not that a tax position will be sustained upon examination, based on the technical merits of the position. The second step is measuring the benefit to be recorded from tax positions that meet the more-likely-than-not recognition threshold, whereby the enterprise determines the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement, and recognizes that benefit in its financial statements. The Company adopted FIN 48 on January 1, 2007. Adoption of FIN 48 did not have an impact on the Company’s financial position, results of operations, or cash flows.
Share-Based Compensation — The Company issues share-based compensation consisting of common stock options issued to employees, consultants, and founders. Effective January 1, 2005, the Company adopted, on a prospective basis, the provisions of SFAS No. 123 (Revised),Share-Based Payment(“SFAS No. 123(R)”) which provides for recognition of compensation expense based on the fair value of the stock-based compensation utilizing various assumptions regarding the underlying attributes of the options and stock. The estimated fair value of options granted, net of forfeitures expected to occur during the vesting period, is amortized as compensation expense on a straight-line basis over the vesting period of the options. Previously, the Company applied the provisions of SFAS No. 123 to its stock-based compensation and adoption of SFAS No. 123(R) did not have a material impact on the Company’s financial position, results of operations, or cash flows.
Fair Value of Financial Instruments — The carrying amount of the Company’s financial instruments, including cash and cash equivalents, accounts receivable and accounts payable approximate their fair values.
Deferred Financing Costs — Deferred financing costs incurred in connection with the Company’s issuance of debt are amortized over the life of the debt using the effective interest rate method with amortization of such costs being charged to interest expense.
Preferred Stock — The Company recognizes changes in the redemption value of its preferred stock immediately as they occur and adjusts the carrying value of the preferred stock to be equal to the redemption value of the preferred stock at the end of each reporting period.
New Accounting Standards Issued not yet Adopted — In September 2006 the FASB issued SFAS No. 157, “Fair Value Measurement” and in February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities.” SFAS No. 157 was issued to eliminate the diversity in practice that exists due to the different definitions of fair value and the limited guidance in applying these definitions. SFAS No. 157 encourages entities to combine fair value information disclosed under SFAS No. 157 with other accounting pronouncements, including SFAS No. 107, “Disclosures about Fair Value of Financial Instruments,” where applicable. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007. SFAS No. 159 permits entities to choose to measure many financial instruments and certain other items at fair value. The objective is to improve financial reporting by providing entities with the
F-9
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
opportunity to mitigate volatility in reported earnings caused by measuring related assets and liabilities differently without having to apply complex hedge accounting provisions. SFAS No. 159 is effective as of the beginning of an entity’s first fiscal year that begins after November 15, 2007. The Company has not yet determined the impact that adoption of these statements will have on its financial position, results of operations, or cash flows.
Net Loss Per Common Share —Basic and diluted net loss per common share have been calculated in accordance with SFAS No. 128,Earnings Per Share,for the years ended December 31, 2004, 2005 and 2006 and for the six month periods ended June 30, 2006 and 2007. As the Company had a net loss in each of the periods presented, basic and diluted net loss per common share are the same.
The computations of diluted net loss per common share for the years ended December 31, 2004, 2005 and 2006 and the six month periods ended June 30, 2006 and 2007, did not include the effects of the following options, convertible preferred stock and convertible preferred stock warrants as the inclusion of these securities would have been antidilutive.
| | | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | | | Six month period ended June 30, | |
| | | 2004 | | | 2005 | | | 2006 | | | 2006 | | | 2007 | |
| | | | | | | | | | | | (unaudited)
| | | | |
| Stock options | | | 209,296 | | | | 850,136 | | | | 861,128 | | | | 874,116 | | | | 3,173,548 | |
| Convertible preferred stock | | | 4,396,168 | | | | 5,555,683 | | | | 12,069,968 | | | | 12,069,968 | | | | 12,291,401 | |
| Convertible preferred stock warrants | | | 138,214 | | | | 138,214 | | | | 1,445,997 | | | | 1,445,997 | | | | 1,445,997 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 4,743,678 | | | | 6,544,033 | | | | 14,377,093 | | | | 14,390,081 | | | | 16,910,946 | |
| | | | | | | | | | | | | | | | | | | | | |
Intangible assets, consisting of purchased intellectual property and capitalized patents costs, as of December 31, 2005 and 2006, and June 30, 2007 comprise the following:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2005 | | | 2006 | | | June 30, 2007 | |
| | | Carrying
| | | Accumulated
| | | Carrying
| | | Accumulated
| | | Carrying
| | | Accumulated
| |
| | | Value | | | Amortization | | | Value | | | Amortization | | | Value | | | Amortization | |
| |
| Intellectual property | | | | | | | | | | | | | | | | | | | | | | | | |
| — licenses | | $ | 100,000 | | | $ | — | | | $ | 1,796,359 | | | $ | — | | | $ | 1,821,682 | | | $ | — | |
| Patents | | | 2,385,554 | | | | (54,300 | ) | | | 3,172,335 | | | | (102,744 | ) | | | 3,525,216 | | | | (166,562 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 2,485,554 | | | $ | (54,300 | ) | | $ | 4,968,694 | | | $ | (102,744 | ) | | $ | 5,346,898 | | | $ | (166,562 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Amortization expense for intangible assets for the years ended December 31, 2004, 2005 and 2006 and the six month periods ended June 30, 2006 (unaudited) and 2007, amounted to $16,353, $29,475, $48,444, $18,955 and $63,818, respectively. Estimated future amortization expense is as follows:
| | | | | |
Years Ending December 31 | | | |
| |
| 2007 (Period from July 1 to December 31) | | $ | 105,083 | |
| 2008 | | | 325,270 | |
| 2009 | | | 182,279 | |
| 2010 | | | 109,886 | |
| 2011 | | | 109,886 | |
Patent amortization commences on the grant date of the underlying patent and continues over the remaining life of the granted patent. Licenses are amortized from the date of FDA clearance of products
F-10
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
associated with the licensed technology and continues over the remaining life of the license. The future amortization expense reflected above is based on patents granted as of June 30, 2007 and licenses related to FDA cleared products. The amortization period related to $1.3 million of licenses is not known as the diagnostic test products associated with the licensed technology have not been cleared by the FDA and, accordingly, amortization expense associated with the licenses is not included in the table above.
Costs deferred related to pending patent applications for which amortization has not commenced totaled $1.9 million, $2.3 million and $2.3 million as of December 31, 2005, December 31, 2006 and June 30, 2007, respectively. Costs deferred are written off if, and when, patent applications are abandoned or allowed to expire. Deferred patent costs written off in 2004, 2005 and 2006 and the six month periods ended June 30, 2006 (unaudited) and 2007 were $167,730, $89,534, $41,659, $0 and $206,683, respectively.
| |
| 4. | Related Party Transactions |
Robert Letsinger and Chad Mirkin, co-founders of the Company, provide contracted research and development services to the Company, which are reimbursed based upon negotiated contract rates. The Company incurred expenses of $150,000 for these services in each of the years 2004, 2005 and 2006, and $75,000 for the six month periods ended June 30, 2006 (unaudited) and 2007, which are recorded as research and development costs in the accompanying statements of operations.
Upon the merger of Nanosphere LLC into the Company in January 2000, Robert Letsinger and Chad Mirkin each received 299,400 shares of common stock in the Company as a conversion of their Class A and Class B units of Nanosphere LLC.
During May 2005, the Company issued a short term note to Lurie Investment Fund, L.L.C. for total proceeds of $1,000,000. This note accrued interest at an annual rate of 8.0% through its maturity. This note was paid in full, along with $7,111 in interest, on June 27, 2005.
In 2006, the Company issued short term notes for total proceeds of $1,320,148 to the following existing stockholders, R. Capital II, Ltd., Adam N. Mirkin, Rhoderic Peter Mirkin, Richard Segal, Steven E. Mather and Lurie Investment Fund, L.L.C. Such notes, along with $5,019,062 of similar notes issued to existing stockholders Lurie Investment Fund, L.L.C. and Steven E. Mather in 2005, were converted into Series D Convertible Preferred Stock, along with unpaid interest of $177,358 accrued thereon (see Note 8).
In March 2006, the Company issued to William Moffitt, the Company’s chief executive officer and a director, 320,000 shares of restricted common stock at a price of $4.50 per share, for an aggregate price of $1,440,000. As of December 31, 2006, the Company would be required to repurchase for a price of $4.50 a share 80,000 shares of this restricted stock in the event of certain occurrences, all of which would involve the termination of William Moffitt’s employment with the Company. The restrictions on the common stock lapsed in July 2007. In connection with the sale, the Company received a full recourse, long-term promissory note from William Moffitt for a total of $1,440,000, which note is secured by the shares of common stock purchased. Interest on the promissory note accrues and is paid annually in cash at an interest rate of 4.51%. For the year ended December 31, 2006, $51,414 of interest had accrued on the note. During the six month period ended June 30, 2007, $64,942 of interest was paid to the Company on the note. Interest on the note is recorded as additional paid in capital when received. Interest income on this note was $18,942 and $32,472 for the six month periods ended June 30, 2006 (unaudited) and 2007 respectively. In August 2007, the note receivable, along with the accrued and unpaid interest, due from the Company’s chief executive officer was repaid.
In August 2007, in accordance with terms of the Amended Bonus Agreement between the Company and the chief executive officer, the Company paid a $2.3 million bonus payment to the chief executive officer.
F-11
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
The Company’s 2000 Equity Incentive Plan, as amended (the “Plan”), permits the grant of options to employees, founders, and consultants for up to 1,600,000 shares of common stock. Option awards are generally granted with an exercise price equal to or above the fair value of the Company’s common stock at the date of grant; those option awards have various vesting structures and have 10 year contractual terms. In March 2007 the Company’s board of directors adopted and the shareholders approved the Nanosphere 2007 Long-Term Incentive Plan (the “2007 Plan”). The 2007 Plan authorizes the compensation committee to grant stock options, share appreciation rights, restricted shares, restricted share units, unrestricted shares incentive stock options, deferred share units and performance awards. The total awards to be granted under this plan cannot exceed 2,616,141 shares. Option awards under the 2007 Plan are generally similar to those under the 2000 Plan. Certain employee options vest ratably over four years of service, while other employee options vest after seven years of service but provide for accelerated vesting contingent upon the achievement of various company-wide performance goals, such as decreasing time to market for new products and entering into corporate collaborations (as defined in the option grant agreements). For these “accelerated vesting” options,20-25% of the granted option shares will vest upon the achievement of each of four or five milestones as defined in the option grant agreements, with any remaining unvested options vesting on the seven year anniversary of the option grant dates. Approximately 31% of the options granted and outstanding contain “accelerated vesting” provisions.
The fair values of the Company’s option awards were estimated at the dates of grant using the Black Scholes option pricing model with the following assumptions:
| | | | | | | | | | | | | | | | | |
| | | 2004 | | | 2005 | | | 2006 | | | 2007 | |
| |
| Expected dividend yield | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
| Expected volatility | | | 75 | % | | | 80 | % | | | 85 | % | | | 77 | % |
| Risk free interest rate | | | 3.94 | | | | 4.13 | | | | 4.82 | | | | 4.66 | |
| Weighted-average expected option life | | | 6.3 years | | | | 7.5 years | | | | 8.4 years | | | | 7.0 years | |
| Estimated weighted-average fair value | | | | | | | | | | | | | | | | |
| on the date of grant based on the above assumptions | | $ | 1.24 | | | $ | 2.28 | | | $ | 2.56 | | | $ | 3.31 | |
| Estimated forfeiture rate for unvested options | | | N/A | | | | 12.5 | % | | | 12.5 | % | | | 4.6 | % |
Expected volatility is based on calculated stock volatilities for publicly traded companies in the same industry and general stage of development as the Company. The risk-free rate is based on the U.S. Treasury yield curve in effect at the time of the grants for periods consistent with the expected life of the option. The expected life of options granted is derived from the average of the vesting period and the term of the option as defined in the Plans, following the guidance in SEC Staff Accounting Bulletin No. 107. Total compensation cost recognized in 2004, 2005 and 2006 and for the six month periods ended June 30, 2006 (unaudited) and 2007 was $38,308, $219,353, $398,960, $196,315 and $790,247, respectively.
As of June 30, 2007, the total compensation cost not yet recognized related to the nonvested awards is approximately $7,556,337, which amount is expected to be recognized over the next five years, which is a weighted average term, without taking into account any potential acceleration of vesting that might occur as discussed above because the milestone events that would trigger acceleration are not yet deemed probable. While the Company does not have a formally established policy, as a practice the Company has delivered newly issued shares of its common stock upon the exercise of stock options.
F-12
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
A summary of option activity under the Plan as of June 30, 2007, and for the six month period then ended is presented below:
| | | | | | | | | | | | | | | | | |
| | | | | | | | | Weighted
| | | | |
| | | | | | Weighted
| | | Average
| | | | |
| | | | | | Average
| | | Remaining
| | | Aggregate
| |
| | | Number of
| | | Exercise
| | | Contractual
| | | Intrinsic
| |
Options | | Shares | | | Price | | | Term | | | Value of Options | |
| |
| Outstanding — January 1, 2007 | | | 860,728 | | | $ | 6.25 | | | | | | | | | |
| Granted | | | 2,312,820 | | | $ | 4.50 | | | | | | | | | |
| Exercised | | | | | | | | | | | | | | | | |
| Forfeited | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding — June 30, 2007 | | | 3,173,548 | | | $ | 4.99 | | | | 9.17 | | | $ | 18,288 | |
| | | | | | | | | | | | | | | | | |
| Exercisable — June 30, 2007 | | | 427,115 | | | $ | 8.04 | | | | 6.94 | | | $ | 18,288 | |
| | | | | | | | | | | | | | | | | |
| Vested and Expected to Vest — June 30, 2007 | | | 3,034,990 | | | $ | 5.01 | | | | 9.16 | | | $ | 18,288 | |
| | | | | | | | | | | | | | | | | |
There was no intrinsic value associated with any options exercised during 2005, 2006 or 2007, or the six month periods ended June 30, 2006 (unaudited) and 2007. No options were exercised during 2004.
Included in the number of options outstanding at June 30, 2007, are 1,384,310 options with an exercise price of $4.50 per share and accelerated vesting provisions based on the criteria mentioned above. None of the milestones which would accelerate vesting have occurred, therefore the related options are not exercisable as of June 30, 2007. The total fair value of shares vested during 2004, 2005 and 2006 and for the six month periods ended June 30, 2006 (unaudited) and 2007 was $11,672, $26,636, $241,387, $142,261 and $482,888, respectively.
| | | | | | | | | |
| | | | | | Weighted Average
| |
| | | Number of
| | | Grant Date
| |
Nonvested Options | | Options | | | Fair Value | |
| |
| Nonvested at January 1, 2007 | | | 618,952 | | | $ | 2.25 | |
| Granted | | | 2,312,820 | | | | 3.25 | |
| Vested | | | (185,339 | ) | | | 2.25 | |
| Forfeited | | | | | | | | |
| | | | | | | | | |
| Nonvested at June 30, 2007 | | | 2,746,433 | | | $ | 3.25 | |
| | | | | | | | | |
Net deferred tax assets consist primarily of net operating loss (“NOL”) carryforwards related to U.S. federal and state taxes. Realization of future tax benefits related to deferred tax assets is dependent on many factors, including the Company’s ability to generate future taxable income. Due to the uncertainty of future earnings, management is unable to predict whether these net deferred tax assets will be realized, and accordingly, has recorded a full valuation allowance against these assets.
NOL carryforwards of approximately $76.8 million for income tax purposes are available to offset future taxable income. If not used, these carryforwards will expire in varying amounts beginning in 2020. The Company also has federal research and development tax credit carryforwards of $4.7 million which will begin to expire in 2020.
F-13
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
The following is a summary of the significant components of the Company’s deferred tax assets and liabilities as of December 31, 2005, December 31, 2006 and June 30, 2007:
| | | | | | | | | | | | | |
| | | 2005 | | | 2006 | | | 2007 | |
| |
| Deferred tax assets/(liabilities): | | | | | | | | | | | | |
| Net operating losses | | $ | 17,647,760 | | | $ | 25,120,442 | | | $ | 30,556,271 | |
| Research and development credits | | | 2,808,513 | | | | 4,263,902 | | | | 4,736,523 | |
| Depreciation on property and equipment | | | (381,656 | ) | | | (391,640 | ) | | | (334,679 | ) |
| Amortization of intangible assets | | | (1,231 | ) | | | (1,288 | ) | | | 11,550 | |
| | | | | | | | | | | | | |
| | | | 20,073,386 | | | | 28,991,416 | | | | 34,969,665 | |
| Less valuation allowance | | | (20,073,386 | ) | | | (28,991,416 | ) | | | (34,969,665 | ) |
| | | | | | | | | | | | | |
| Deferred tax asset — net | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | |
The reconciliation of the federal statutory rate to the Company’s effective tax rate of zero percent for the years ended December 31, 2005 and 2006 is as follows:
| | | | | | | | | | | | | | | | | | | | | |
| | | Years Ended
| | | | |
| | | December 31, | | | Six Month Periods Ended June 30, | |
| | | 2004 | | | 2005 | | | 2006 | | | 2006 | | | 2007 | |
| | | | | | | | | | | | (unaudited) | | | | |
| |
| Tax provision at the statutory federal rate | | | 34.0 | | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % | | | 34.0 | % |
| State income taxes, net of federal income tax benefit | | | 4.0 | % | | | 4.0 | % | | | 4.0 | % | | | 4.0 | % | | | 4.0 | % |
| Valuation allowance | | | (38.0 | )% | | | (38.0 | )% | | | (38.0 | )% | | | (38.0 | )% | | | (38.0 | )% |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % | | | 0 | % |
| | | | | | | | | | | | | | | | | | | | | |
The Company entered into a license agreement with Northwestern University (“Northwestern”) in May 2000 (the “Original License Agreement”). Pursuant to the Original License Agreement and the previous related license issued to Nanosphere LLC at no cost, the Company had been granted an exclusive, world-wide, royalty free, perpetual license, with right to sublicense, to all technology developed by, or under the supervision of, Chad Mirkin at Northwestern, to the extent that such technology relates to biological diagnostics involving nanoparticles. In exchange, the Company issued to Northwestern 3,915 shares of the Company’s common stock. As the fair value of the Company’s common stock at the time the Original License Agreement was entered into was deemed to be de minimus, this license was recorded at a zero value in the Company’s balance sheet. As part of the Original License Agreement, the Company agreed, at its own expense, to bear all of the costs for the prosecution of any and all patents, domestic and international, that arise from the licensed technology. Under the Original License Agreement, the Company had the right, but not the obligation, at its own expense, to prosecute any infringements or defend any claims of invalidity or unenforceability of any of its licensed technology rights.
In 2006, the Company entered into a new license agreement with Northwestern, which replaced the prior agreement and provides the Company with an exclusive license to certain patents and patent applications owned by Northwestern that are related to (1) nanotechnology, which technology involves a particle where no single dimension is greater than 100 nanometers, or Nanotechnology, and (2) biobarcode technology, which is analysis where oligonucleotides act as surrogate targets or reporter molecules, or Biobarcode Technology. The license is limited to the “Biodiagnostics Field” defined as qualitative or quantitative in vitro analysis, testing, measurement, or detection of various biodiagnostics field subjects and target combinations.
F-14
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
The New License Agreement includes licenses to patents and patent applications based on existing inventions and future inventions developed in the laboratory of Dr. Mirkin or Dr. Letsinger, by or under their direct supervision, and conceived prior to January 1, 2013 that are Nanotechnology or Biobarcode Technology referred to herein as Licensed Patents. The Company has an obligation to use commercially reasonable efforts to bring the subject inventions of the Licensed Patents to market. If the parties disagree as to whether they are meeting this diligence requirement, an arbitrator may require us to comply with a timeline for cure or convert our exclusive license to a non-exclusive license; Northwestern does not have the right to revoke any license to the Licensed Patents already granted to the Company.
The Company also has the first right to negotiate an exclusive license to inventions developed in the laboratory of Dr. Mirkin or Dr. Letsinger, by or under their direct supervision, and (1) conceived after January 1, 2013 that are Nanotechnology or Biobarcode Technology and (2) that are not Nanotechnology or Biobarcode Technology, but otherwise within the Biodiagnostics Field, conceived prior to January 1, 2013. Both (1) and (2) are herein referred to as Future Inventions. If the parties cannot agree on the terms of the license for the Future Inventions, the parties shall submit to arbitration to determine reasonable terms. For inventions conceived after January 1, 2013 that are not Nanotechnology or Biobarcode Technology, but otherwise within the Biodiagnostics Field, the Company has the right to negotiate a license if Northwestern offers such inventions to third parties. If the Company has a license based on Future Inventions, Northwestern has the right to terminate the license upon any material breach that the Company does not cure or upon our bankruptcy.
The Company has an obligation to pay Northwestern a royalty at a rate that is a percentage of the gross profits of licensed products, subject to certain adjustments. The Company’s obligation for payments to Northwestern pursuant to this agreement began on January 1, 2007. To date, the Company has paid Northwestern $5,000, $1,000, $31,000, for the years ended December 31, 2004, 2005 and 2006 respectively in connection with the Original License Agreement, and $30,000 for the six month period ended June 30, 2007 in connection with the New License Agreement.
The Company has entered into several nonexclusive license agreements with various companies covering certain technologies which are embedded in the Company’s diagnostic instruments and diagnostic test products. As of June 30, 2007, the Company has paid aggregate initial license fees of $1,821,682 for these licenses, and has agreed to pay a percentage of net sales as royalties, in percentage amounts ranging from 1% to 12.0%. Certain of the license agreements have minimum annual royalty payments, and such minimum payments are $197,500 in 2007, $183,000 in 2008, $189,000 in 2009, $120,000 in 2010, $125,000 in 2011 and are $40,000 to $160,000 annually thereafter through the dates the respective licenses terminate. These licenses expire at various times, corresponding to the subject patents expirations, which currently range from 2009 to 2023.
| |
| 8. | Convertible Preferred Stock |
Series A Convertible Preferred Stock — In 2000, the Company sold to a single stockholder 4,328,571 shares of its Series A Convertible Preferred Stock, for $0.4633 per share, for an aggregate consideration of $2,005,571. Series A Convertible Preferred Stock carried a dividend rate of 6.5%, which was paid in shares of Series A Convertible Preferred Stock at the Company’s option. Dividends for this class of convertible preferred stock in the amounts of 886,125 shares of Series A Convertible Preferred Stock were declared and paid in2000-2002. A warrant to purchase 2,164,287 shares of Series A Convertible Preferred Stock at $0.4633 per share was also provided to the stockholder at the time of the original purchase and was exercised in 2001 for total consideration of $1,002,786. All outstanding shares of the Series A Convertible Preferred Stock were converted into shares ofSeries C-2 Convertible Preferred Stock in 2004. Upon conversion, $976,766 of the Series A Convertible Preferred Stock book value was reclassified to convertible derivative liability. The Series A Convertible Preferred Stock is no longer authorized under the Company’s Amended and Restated Certificate of Incorporation.
F-15
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
Series B Convertible Preferred Stock — In 2001, the Company sold 3,486,395 shares of its Series B Convertible Preferred Stock for $1.47 per share and, in 2002, sold 113,122 shares of its Series B Convertible Preferred Stock for $2.21 per share, for an aggregate consideration of $5,375,001. In 2004, 3,582,510 shares of the Series B Convertible Preferred Stock were converted into shares ofSeries C-2 Convertible Preferred Stock. Upon conversion, $1,528,571 of the Series B Preferred Stock book value was reclassified to convertible derivative liability. The number of shares of Series B Convertible Preferred Stock authorized at December 31, 2005, December 31, 2006 and June 30, 2007 was 21,520, 17,007 and 17,007, respectively; and 17,007 shares were issued and outstanding at December 31, 2005, December 31, 2006 and June 30, 2007.
Series C Convertible Preferred Stock — In 2002, the Company sold 25,000,000 shares of its Series C Convertible Preferred Stock for $0.60 per share, for an aggregate consideration of $15,000,000. In 2004, 14,949,993 shares of the Series C Convertible Preferred Stock were converted into shares ofSeries C-2 Convertible Preferred Stock. Upon conversion, $2,562,856 of the Series C Convertible Preferred Stock book value was reclassified to convertible derivative liability. The number of shares of Series C Convertible Preferred Stock authorized at December 31, 2005, December 31, 2006 and June 30, 2007 was 10,820,000, 10,066,673 and 10,066,673, respectively; and 10,050,007 shares were issued and outstanding at December 31, 2005, December 31, 2006 and June 30, 2007.
Series C-2 Convertible Preferred Stock — In 2004, the Company sold 49,155,241 shares of itsSeries C-2 Convertible Preferred Stock for $0.35 per share, for an aggregate consideration of $17,204,334. In 2005, the Company sold to existing stockholders 28,987,866 shares of itsSeries C-2 Convertible Preferred Stock for $0.35 per share, for an aggregate consideration of $10,145,753. Of the total consideration received, $7,814,312 was allocated to the convertible derivative liability. The number of shares ofSeries C-2 Convertible Preferred Stock authorized at December 31, 2005, December 31, 2006 and June 30, 2007 was 180,000,000, 132,263,734 and 132,263,734, respectively; and 128,825,044 shares were issued and outstanding at December 31, 2005, December 31, 2006 and June 30, 2007.
Series D Convertible Preferred Stock — On April 12, 2006, the Company sold to new and existing investors 162,857,142 shares of its Series D Convertible Preferred Stock for $0.35 per share, for an aggregate consideration of $53,310,465. Of the total consideration received, $16,285,714 was allocated to the convertible derivative liability. The Company issued 5,535,824 shares of Series D Convertible Preferred Stock in February 2007 in connection with its debt financing as described in Note 10. The number of shares of Series D Convertible Preferred Stock authorized at December 31, 2006 and June 30, 2007 was 196,980,276 and 204,123,276, respectively; and 162,857,142 and 168,392,966 shares were issued and outstanding at December 31, 2006 and June 30, 2007, respectively.
Prior to the closing of certain of the convertible preferred stock issuances described above, the Company entered into bridge financing with existing investors through the issuance of bridge notes payable. The notes, and the interest accrued thereon, were ultimately converted into shares of convertible preferred stock in the next following issued series of convertible preferred stock on the same terms as the other shares issued in that series of convertible preferred stock. Interest expense accrued on such notes totaled $204,335, forSeries C-2 Convertible Preferred Stock in 2004 and $177,358 for Series D Convertible Preferred Stock in 2006.
Rights and Privileges on Convertible Preferred Stock
At June 30, 2007, the Company had four series of convertible preferred stock subject to certain rights and privileges under the Company’s Amended and Restated Certificate of Incorporation.
At any time subsequent to April 12, 2013, the holders of a majority of the then outstandingSeries C-2 Convertible Preferred Stock or Series D Convertible Preferred Stock may require the Company to redeem all of the respective series of preferred shares. The price paid by the Company for the preferred shares would be the greater of a) the liquidation value of the preferred share series being redeemed, plus any declared and unpaid dividends not previously added to the liquidation value, or b) the fair market value per share, on the date the redemption request was made, of the series of preferred stock being redeemed. Upon such request by
F-16
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
a majority of the holders of one series of the convertible preferred stock, holders of the other series of convertible preferred stock may request redemption of their series of convertible preferred stock, and such series will be redeemed if a majority of the holders of such series make a redemption request. The redemption price for the Series B and Series C Convertible Preferred Stock, if redeemed, is the greater of the liquidation value or the fair value of the convertible preferred shares.
The Company classifies the convertible preferred stock as mezzanine equity on the balance sheet. The Company recognizes changes in the redemption value immediately as they occur via direct charges to Accumulated Deficit and adjusts the carrying value of the Preferred Stock to equal its redemption value at the end of each reporting period.
Series B, C, C-2 and D Convertible Preferred Stock — Series B Convertible Preferred Stockholders, Series C Convertible Preferred Stockholders,Series C-2 Convertible Preferred Stockholders and Series D Convertible Preferred Stockholders have the following rights and privileges:
Voting — Holders of each Series B, C, C-2 and D Convertible Preferred Stock shall have voting rights on an as if converted (to common shares) basis. The holders of theSeries C-2 Convertible Preferred Stock and the Series D Convertible Preferred Stock, respectively as a single class, shall each have the right to elect two directors to the Company’s Board of Directors. All remaining members of the Board of Directors are elected by the holders of the Series B, C, C-2 and D Convertible Preferred Stock, along with the common stockholders, voting as a single class.
Conversion — The holder of any shares of Series B, C, C-2 and D Convertible Preferred Stock have the right at the holder’s option, at any time, to convert any of such shares into such number of fully paid and nonassessable shares of common stock as is determined (i) in the case of the Series B Convertible Preferred Stock, by multiplying 0.098 by the number of Series B Convertible Preferred Stock shares being converted into Common Stock at the time of conversion, (ii) in the case of the Series C Convertible Preferred Stock,Series C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock, by multiplying 0.04 by the number of Series C Preferred,Series C-2 Preferred or Series D Preferred shares, respectively, being converted into common stock at the time of conversion. The conversion rate for all series of convertible preferred stock is adjustable under certain circumstances (primarily if the Company sells equity for a price or at a conversion rate which is less than the original sales price of the respective convertible preferred stock series). Further, certain events, such as a Qualified Public Offering (as defined) would trigger an automatic conversion of the Convertible Preferred Stock to Common Stock.
The convertible feature in the Company’sSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock is accounted for separately from the convertible preferred stock, and has been accounted for as a derivative liability in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 133, “Accounting for Derivative Instruments and Hedging Activities.” The conversion feature of the convertible preferred stock meets the criteria of an embedded derivative as defined by paragraph 12 of SFAS 133, and accordingly, is bifurcated from the convertible preferred stock and accounted for separately as a liability, the convertible derivative liability. The conversion feature, when classified as a derivative liability, is required to be initially recorded at fair value and to be marked to fair value at the end of each reporting period, which results in a non-cash charge to other income or expense in the Statement of Operations. In 2004, 2005, 2006 and for the six month periods ended June 30, 2006 (unaudited) and 2007, such charges were zero, zero, $2.9 million, $2.9 million and zero, respectively. The fair value of the convertible derivative liability is determined, at each reporting date, using the option pricing method detailed in the AICPA Practice Aid, “Valuation of Privately-Held-Company Equity Securities Issued as Compensation.”
Dividends — The holders of shares ofSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock are entitled to receive, when and if declared by the Board of Directors, out of assets of the Company which are by law available therefore under the Delaware General Corporation Law and other applicable law, prior and in preference to any declaration or payment on the common stock, Series B Convertible Preferred
F-17
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
Stock or Series C Convertible Preferred Stock, cumulative dividends at an annual rate of six percent (6%) of the Liquidation Value (as defined), beginning effective April 12, 2006, payable in cash. At June 30, 2007, there were cumulative undeclared and unpaid dividends of $7,593,920 on the Company’sSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock.
If the Company declares or pays any dividends upon the common stock, other than dividends payable solely in shares of common stock, the Company will also declare and pay dividends to the holders of theSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock, on an as-if converted to common basis. Holders of Series B Convertible Preferred Stock and Series C Convertible Preferred Stock are not entitled to receive dividends.
Liquidation — Upon any liquidation, dissolution or winding up of the Company, whether voluntary or involuntary, each holder ofSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock are entitled to receive (in cash or other consideration) from the assets of the Company available for distribution to stockholders prior and in preference to the holders of all other classes and series of stock, including holders of Series B Convertible Preferred Stock, Series C Convertible Preferred Stock or common stock, an amount equal to the greater of (a) the sum of the aggregate Liquidation Value of allSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock, as applicable, plus an amount equal to all accrued and unpaid dividends on such shares of Convertible preferred stock, which have not previously been added to the Liquidation Value thereof or (b) the aggregate amount such holder would receive in a liquidation, dissolution or winding up of the Company with respect to all of theSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock, as applicable, held by such holder if such convertible preferred stock was converted into common stock. Liquidation Value as toSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock is defined as the sum (as proportionately adjusted for stock splits, stock dividends, stock combinations and other recapitalizations affecting theSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock, as applicable) of $0.35 per share plus an amount equal to any dividends on such series of convertible preferred stock that have accrued on any Dividend Payment Date that have not been paid. The Series C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock shall not be entitled to any further payment.
After payment to theSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock holders, each holder of the Series B Convertible Preferred Stock and Series C Convertible Preferred Stock shall be paid, on a pari passu basis, before distribution to holders of the common stock, an amount (in cash or other consideration), if any, equal to the greater of (a) the sum of the aggregate Liquidation Value of all Series B Convertible Preferred Stock ($1.9305 per share) and Series C Convertible Preferred Stock ($0.6934 per share), as applicable (as proportionately adjusted for stock splits, stock dividends, stock combinations and other recapitalizations affecting the Series B Convertible Preferred Stock and Series C Convertible Preferred Stock, as applicable), or (b) the aggregate amount such holder would receive in a liquidation, dissolution or winding up of the Company with respect to all of the Series B Convertible Preferred Stock and Series C Convertible Preferred Stock, as applicable, held by such holder if such convertible preferred stock was converted into common stock.
If, upon the occurrence of such event, the assets and funds to be distributed among the holders of shares of the Company’s convertible preferred and common stockholders are insufficient to permit payment to such holders of the aggregate amount to which they are entitled as described above, then any such distributions shall be first to theSeries C-2 Convertible Preferred Stock and Series D Convertible Preferred Stock holders proportionately based upon the amount to which such holders are entitled; second to the Series C Convertible Preferred Stock and Series B Convertible Preferred Stock proportionately based upon the amount to which such holders are entitled; with any remaining assets and funds available distributed to the holders of the Company’s common stock on a proportionate basis.
F-18
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
The Company’s convertible preferred stock transactions from December 30, 1999 (date of incorporation) through June 30, 2007, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Total
| |
| | | | | | | | | | | | | | | | | | Convertible Preferred
| |
| | | Series A | | | Series B | | | Series C | | | Series C-2 | | | Series D | | | Stock | |
| |
| Issuance of 4,328,571 shares of Series A Convertible Preferred Stock on January 18, 2000 | | $ | 2,005,571 | | | | | | | | | | | | | | | | | | | $ | 2,005,571 | |
| Exercise of warrant for 2,164,287 shares of Series A Convertible Preferred Stock on March 13, 2001 | | | 1,002,786 | | | | | | | | | | | | | | | | | | | | 1,002,786 | |
| Issuance of 886,125 shares of Series A Convertible Preferred Stock as dividends on Series A Convertible Preferred Stock | | | 410,539 | | | | | | | | | | | | | | | | | | | | 410,539 | |
| Issuance of 3,486,395 shares of Series B Convertible Preferred Stock on March 13, 2001 | | | | | | $ | 5,125,001 | | | | | | | | | | | | | | | | 5,125,001 | |
| Issuance of 113,122 shares of Series B Convertible Preferred Stock on February 13, 2002 | | | | | | | 250,000 | | | | | | | | | | | | | | | | 250,000 | |
| Issuance of 25,000,000 shares of Series C Convertible Preferred Stock on November 6, 2002 | | | | | | | | | | $ | 15,000,000 | | | | | | | | | | | | 15,000,000 | |
Issuance of 34,891,877 shares ofSeries C-2 Convertible Preferred Stock on September 2, 2004 | | | | | | | | | | | | | | $ | 12,212,156 | | | | | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | (3,489,188 | ) | | | | | | | 8,722,968 | |
Issuance of 14,263,364 shares ofSeries C-2 Convertible Preferred Stock on September 30, 2004 | | | | | | | | | | | | | | | 4,992,178 | | | | | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | (1,426,337 | ) | | | | | | | 3,565,841 | |
Conversion of 7,378,983 shares of Series A Convertible Preferred Stock into 9,767,665 shares ofSeries C-2 Convertible Preferred Stock in September 2004 | | | (3,418,896 | ) | | | | | | | | | | | 3,418,896 | | | | | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | (976,766 | ) | | | | | | | (976,766 | ) |
Conversion of 3,582,510 shares of Series B Convertible Preferred Stock into 15,285,714 shares ofSeries C-2 Convertible Preferred Stock in September 2004 | | | | | | | (5,349,605 | ) | | | | | | | 5,349,605 | | | | | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | (1,528,571 | ) | | | | | | | (1,528,571 | ) |
Conversion of 14,949,993 shares of Series C Convertible Preferred Stock into 25,628,558 shares ofSeries C-2 Convertible Preferred Stock in September 2004 | | | | | | | | | | | (8,969,997 | ) | | | 8,969,997 | | | | | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | (2,562,856 | ) | | | | | | | (2,562,856 | ) |
| Redemption value adjustment, September 2004 | | | | | | | | | | | | | | | 9,983,718 | | | | | | | | 9,983,718 | |
| Allocation to preferred stock warrant liability | | | | | | | | | | | | | | | (172,675 | ) | | | | | | | | |
| Redemption value adjustment, December 2004 | | | | | | | | | | | | | | | 172,675 | | | | | | | | | |
Issuance of 28,987,866 shares ofSeries C-2 Convertible Preferred Stock from June to September 2005 | | | | | | | | | | | | | | | 10,145,753 | | | | | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | (2,898,787 | ) | | | | | | | 7,246,966 | |
| Redemption value adjustment, June — September 2005 | | | | | | | | | | | | | | | 2,898,787 | | | | | | | | 2,898,787 | |
| Allocation to preferred stock warrant liability | | | | | | | | | | | | | | | (2,166 | ) | | | | | | | | |
| Redemption value adjustment, March 2006 | | | | | | | | | | | | | | | 2,166 | | | | | | | | | |
F-19
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | Total
| |
| | | | | | | | | | | | | | | | | | Convertible Preferred
| |
| | | Series A | | | Series B | | | Series C | | | Series C-2 | | | Series D | | | Stock | |
| |
| Issuance of 162,857,142 shares of Series D Convertible Preferred Stock on April 12, 2006 | | | | | | | | | | | | | | | | | | | 53,310,465 | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | | | | | (16,285,714 | ) | | | 37,024,751 | |
| Allocation to preferred stock warrant liability | | | | | | | | | | | | | | | | | | | (1,449,664 | ) | | | (1,449,664 | ) |
| Redemption value adjustment, June 2006 | | | | | | | | | | | | | | | | | | | 17,735,378 | | | | 17,735,378 | |
| Issuance of 5,535,824 shares of Series D Convertible Preferred Stock in connection with debt borrowings in February 2007 | | | | | | | | | | | | | | | | | | | 1,937,538 | | | | | |
| less allocation to convertible derivative liability | | | | | | | | | | | | | | | | | | | (608,940 | ) | | | 1,328,598 | |
| Redemption value adjustment, February 2007 | | | | | | | | | | | | | | | | | | | 608,940 | | | | 608,940 | |
| Undeclared and unpaid dividends on | | | | | | | | | | | | | | | | | | | | | | | | |
Series C-2 and Series D Convertible Preferred Stock earned | | | | | | | | | | | | | | | 3,334,614 | | | | 4,259,306 | | | | 7,593,920 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Convertible preferred stock outstanding at June 30, 2007 | | $ | — | | | $ | 25,396 | | | $ | 6,030,003 | | | $ | 48,423,199 | | | $ | 59,507,309 | | | $ | 113,985,907 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Warrants — The Company has also issued, in connection with certain of the convertible preferred stock financings, warrants to purchase shares of the related series of the Company’s convertible preferred stock. The exercise price of the warrants was the same as the purchase price of the related series of convertible preferred stock issued at the same time the warrant was issued. In 2004, certain warrants to acquire shares of Series B Convertible Preferred Stock and Series C Convertible Preferred Stock were converted into warrants to acquire shares ofSeries C-2 Convertible Preferred Stock, in connection with the corresponding conversion of Series B Convertible Preferred Stock and Series C Convertible Preferred Stock into shares ofSeries C-2 Convertible Preferred Stock. At December 31, 2006, there were outstanding warrants to acquire 16,666 shares of Series C Convertible Preferred Stock at an exercise price of $0.60 per share, 3,438,687 shares ofSeries C-2 Convertible Preferred Stock at an exercise price of $0.35 per share, and 32,694,562 shares of Series D Convertible Preferred Stock at an exercise price of $0.35 or $0.4375 per share. The exercise price on 28,571,431 of the Series D Convertible Preferred Stock warrants increases annually, in increments of $0.0875 per year to $0.70 in the final year of the warrant term. These warrants are accounted for as liabilities initially at fair value and are marked to fair value at the end of each reporting period, which results in a non-cash charge to other income or expense in the Statement of Operations. 32,737,419 warrants to acquire preferred stock have terms that change the warrant to be a warrant to acquire common stock if and when all outstanding shares of the series of preferred stock to which the warrant is associated are converted to common stock. Upon any such conversion, the warrant would be reclassified to Stockholders’ Deficit and would no longer require adjustment to fair value at each reporting date. Substantially all of the warrants allow the warrant to be exercised using a net exercise provision by which the warrant holder can exercise the warrant without cash payment for a reduced number of shares of preferred stock with such reduction being based on the fair market value of the preferred stock and the exercise price of the warrant on the date of exercise.
F-20
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
The expiration dates of the warrants vary and are as follows:
| | | | | | | | | |
Series of Preferred Stock to
| | Number of
| | | Expiration
| |
| which the Warrant is Exercisable | | Warrants | | | Date | |
| |
| Series C | | | 16,666 | | | | October 2012 | |
| Series C-2 | | | 361,759 | | | | June 2009 | |
| Series C-2 | | | 453,526 | | | | July 2009 | |
| Series C-2 | | | 1,423,806 | | | | October 2012 | |
| Series C-2 | | | 1,155,197 | | | | February 2014 | |
| Series C-2 | | | 1,542 | | | | March 2014 | |
| Series C-2 | | | 42,857 | | | | January 2016 | |
| Series D | | | 28,571,431 | | | | April 2011 | |
| Series D | | | 4,123,131 | | | | April 2013 | |
The warrants to acquire Series D Convertible Preferred Stock have terms that trigger an earlier expiration, generally upon a liquidation of the Company or a Qualified Public Offering, as defined in the Company’s Amended and Restated Certificate of Incorporation, as amended. The warrants to acquire Series C andSeries C-2 Convertible Preferred Stock also have terms that trigger an earlier expiration, including a) upon the closing of Company’s first underwritten initial public offering of the Company’s Common Stock pursuant to an effective registration statement under the Securities Act of 1933, b) the sale or transfer of all or substantially all of the Company’s assets, or c) a change of control of the Company via acquisition, merger or other similar transaction.
The fair values of the Company’s convertible preferred stock warrants were estimated as of December 31, 2005, December 31, 2006 and June 30, 2007 using either a Black-Scholes or a binominal pricing model, as appropriate given the terms of the warrant, with the following assumptions:
| | | | | | | | | | | | | |
| | | December 31,
| | | December 31,
| | | June 30,
| |
| | | 2005 | | | 2006 | | | 2007 | |
| |
| Expected dividend yield | | | 8 | % | | | 6 | % | | | 6 | % |
| Expected volatility | | | 35 | % | | | 40 | % | | | 47 | % |
| Risk free interest rate | | | 4.36 | % | | | 4.70 | % | | | 4.90 | % |
| Weighted-average warrant term | | | 6.5 years | | | | 4.7 years | | | | 4.2 years | |
| Estimated weighted-average fair value | | $ | 0.06 | | | $ | 0.05 | | | $ | 0.06 | |
The Company has an operating lease agreement expiring in 2010 for its office and laboratory space. The Company has also entered into a capital lease agreement expiring in 2009 for a piece of laboratory equipment. Rent expense was $730,134, $721,635, $703,418, $345,096 and $339,267 in 2004, 2005 and 2006, and for the six month periods ended June 30, 2006 (unaudited) and 2007 respectively. The Company has a bargain purchase option of $11,563 on equipment under capital lease at the end of the lease term in 2009. The gross and net book values of the equipment acquired under the capital lease is $117,685 and $96,670, respectively at June 30, 2007.
F-21
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
Annual future minimum obligations for operating and capital leases as of June 30, 2007, are as follows:
| | | | | | | | | |
| | | Operating
| | | Capital
| |
Years Ending December 31 | | Leases | | | Leases | |
| |
| 2007 (Period from July 1 to December 31) | | $ | 248,576 | | | $ | 21,090 | |
| 2008 | | | 506,473 | | | | 42,180 | |
| 2009 | | | 519,135 | | | | 22,108 | |
| 2010 | | | 264,425 | | | | | |
| | | | | | | | | |
| Total minimum lease payments | | $ | 1,538,609 | | | | 85,378 | |
| | | | | | | | | |
| Less amounts representing interest | | | | | | | (10,019 | ) |
| | | | | | | | | |
| Net present value | | | | | | | 75,359 | |
| Less current portion of net minimum lease payments | | | | | | | (34,841 | ) |
| | | | | | | | | |
| Long-term portion of net minimum lease payments | | | | | | $ | 40,518 | |
| | | | | | | | | |
In February 2007, the Company entered into two loan and security agreements, with commitments for an aggregate of $25.0 million in debt financing with Venture Lending & Leasing IV, Inc., and Venture Lending & Leasing V, Inc. These agreements give the Company the right to issue secured notes during two time periods during 2007. The Company borrowed $12.5 million under these agreements in February 2007. The Company will pay interest, and a minimal amount of principal, for the initial twelve month period followed by a thirty month period within which the note principal would be amortized. Interest will be paid during the initial twelve month period at a fixed annual interest rate of 12.5%, and during the following thirty month period at a fixed annual interest rate of 10.0%. The second tranche of notes under this agreement can be drawn down during December 2007. The terms of that borrowing, should it occur, would require the Company to pay interest and a minimal amount of principal for the initial nine month period followed by a twenty-four month period within which the note principal would be amortized. Interest will be paid during the initial nine month period at a fixed annual interest rate equal to the higher of the Prime Rate at the time of the borrowing plus 4.25%, or 12.5% at June 30, 2007, and during the following twenty-four month period at a fixed annual interest rate equal to the higher of the Prime Rate at the time of the borrowing plus 1.75%, or 10.0% at June 30, 2007. Notes issued pursuant to this commitment are secured by a first security lien on all of the Company’s assets including intellectual property. In connection with the execution of the commitment, the Company issued to the lenders 3,928,650 shares of the Company’s Series D Convertible Preferred Stock. Also in February 2007, in connection with the initial note issuance of $12.5 million, the Company issued an additional 1,607,174 shares of Series D Convertible Preferred Stock to the lenders. Under the current terms of the agreement, if the Company elects to draw down the additional $12.5 million of commitment, the Company would be required to issue an additional 1,607,176 shares of Series D Convertible Preferred Stock to the lenders.
The $12.5 million of proceeds received were allocated to debt and the Series D Convertible Preferred Stock based on their fair values at the borrowing date with $1.9 million allocated to Series D Convertible Preferred Stock and the remaining $10.6 million allocated to debt. The discount on the debt of $1.9 million results in an effective interest rate on the debt of 21% and the discount will be amortized to interest expense over the term of the debt following the interest method. Interest expense on this debt for the six month period ended June 30, 2007 was $806,271, which includes $267,133 of discount amortization.
F-22
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
Aggregate annual principal payments on long-term debt are as follows:
| | | | | |
| 2007 (July 1 to December 31) | | $ | 23,937 | |
| 2008 | | | 3,597,823 | |
| 2009 | | | 4,818,211 | |
| 2010 | | | 3,917,293 | |
| | | | | |
| | | $ | 12,357,264 | |
| | | | | |
On August 13, 2007, the Company filed a registration statement onForm S-1 to register shares for an initial public offering of its common stock. The registration statement has not been declared effective and there can be no assurance that the planned offering will actually close. As of June 30, 2007, $271,573 of transaction costs associated with the Company’s filing of a registration statement for an initial public offering of its common stock are included in other current assets and will ultimately offset the proceeds received from the offering if the offering is successfully closed, or will be expensed if the offering is abandoned.
On September 17, 2007, the Company received 510(k) clearance from the U.S. Food and Drug Administration (the “FDA”) for the Verigene System and warfarin metabolism assay.
On October 12, 2007, the Company received 510(k) clearance from the FDA for its hyper-coagulation assay.
As of October 16, 2007, the Company received written notices of exercise from the holders of at least 84% of the Company’s outstanding warrants to purchase Series C-2 Convertible Preferred Stock. Such exercise is conditional upon the closing and effectiveness of the Company’s contemplated initial public offering.
On October 16, 2007 (the “Effective Date”), each share of common stock, par value $0.01 per share (the “Old Common Stock”), issued and outstanding immediately prior to the Effective Date, was automatically reclassified as and converted into 1/25 of a share of common stock, par value $0.01 per share, of the Company. Any stock certificate that, immediately prior to the Effective Date, represented shares of the Old Common Stock will, from and after the Effective Date, automatically and without the necessity of presenting the same for exchange, represent the number of shares of common stock, as equals the product obtained by multiplying the number of shares of Old Common Stock represented by such certificate immediately prior to the Effective Date by 1/25. This stock split is referred to herein as the “one-for-25 stock split” and all common stock share and per share amounts (except par value) for all periods presented have been adjusted to reflect the one-for-25 stock split. Additionally, in accordance with the Company’s Amended and Restated Certificate of Incorporation, as amended, the conversion ratio of the Company’s convertible preferred stock has also been proportionately decreased, and such conversion ratios are also restated herein.
On October 16, 2007, the majority of the shareholders of the Company’s Series C-2 and Series D Convertible Preferred Stock, each series voting as a separate class, in accordance with the Company’s Amended and Restated Certificate of Incorporation, as amended, approved the conversion of all of the Company’s convertible preferred stock into shares of the Company’s common stock, contingent upon the closing and effectiveness of the Company’s contemplated initial public offering and the listing of the Company’s common stock on the NASDAQ Global Market.
12. Restatement of Financial Statements
Subsequent to the issuance of the 2006 financial statements included in the Company’s registration statement onForm S-1, filed on August 13, 2007 with the United States Securities and Exchange Commission,
F-23
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
the Company determined that warrants to purchase shares of various series of the Company’s Convertible Preferred Stock (collectively, the “Warrants”) were not accounted for as liabilities consistent with the provisions of Financial Accounting Standards Board’s Staff PositionNo. 150-5, “Issuer’s Accounting under FASB Statement No. 150 for Freestanding Warrants and Other Similar Instruments on Shares that are Redeemable.” The Warrants had been recorded at no value upon their issuance and were included in mezzanine equity in the financial statements combined with the amounts for the related series of preferred stock. The Warrants should have been accounted for as a liability due to the redemption feature included within the Convertible Preferred Stock to be acquired upon exercise of the Warrants. The Warrants, when classified as a liability, are required to be initially recorded at fair value and to be marked to fair value at the end of each reporting period, with changes in the fair value being recorded as a component of other income and expense in the Statements of Operations.
In addition, the Company identified an error in the Black-Scholes option pricing model that the Company has historically used to value its Common Stock options and the Warrants. The error arose due to an incorrect formula embedded in the model the Company’s uses to calculate fair value for these financial instruments.
The following tables summarize the impact of the restatements discussed above on the previously issued balance sheets as of December 31, 2005 and 2006 and the previously issued statements of operations and cash flows for the years ended December 31, 2004, 2005 and 2006. Financial information for the three month periods ended March 31, 2006 and 2007 was unaudited and was included in the Company’s previously issued financial statements are not presented herein and are affected by this restatement. Net loss for the three month periods ended March 31, 2006 and 2007 was $5,072,522 and $6,710,076, respectively, as previously presented. When corrected by the restatement, such net loss for the three month periods ended March 31, 2006 and 2007 was $5,092,189 and $6,636,367, respectively.
| | | | | | | | | | | | | | | | | |
| | | December 31, 2005 | | | December 31, 2006 | |
| | | As Previously
| | | | | | As Previously
| | | | |
| | | Reported | | | As Restated | | | Reported | | | As Restated | |
| |
Balance Sheets | | | | | | | | | | | | | | | | |
| Preferred stock warrants | | | | | | $ | 189,790 | | | | | | | $ | 1,761,533 | |
| Total liabilities | | $ | 19,974,030 | | | $ | 20,163,820 | | | $ | 35,646,332 | | | $ | 37,407,865 | |
Additionalpaid-in-capital | | $ | 111,306 | | | $ | 285,424 | | | $ | 1,628,076 | | | $ | 2,051,126 | |
| Deficit accumulated during the development stage | | $ | (59,895,153 | ) | | $ | (60,259,061 | ) | | $ | (103,757,194 | ) | | $ | (105,941,777 | ) |
| Total stockholders’ deficit | | $ | (59,771,500 | ) | | $ | (59,961,290 | ) | | $ | (103,476,538 | ) | | $ | (105,238,071 | ) |
F-24
Nanosphere, Inc.
(A Development Stage Company)
Notes to Financial Statements — (Continued)
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, 2004 | | | Year Ended December 31, 2005 | | | Year Ended December 31, 2006 | |
| | | As Previously
| | | | | | As Previously
| | | | | | As Previously
| | | | |
| | | Reported | | | As Restated | | | Reported | | | As Restated | | | Reported | | | As Restated | |
| |
Statements of Operations | | | | | | | | | | | | | | | | | | | | | | | | |
| Sales, general, and administrative | | $ | 3,093,082 | | | $ | 3,131,390 | | | $ | 4,367,160 | | | $ | 4,502,970 | | | $ | 5,166,594 | | | $ | 5,415,525 | |
| Total costs and expenses | | $ | 13,459,555 | | | $ | 13,497,863 | | | $ | 17,737,150 | | | $ | 17,872,960 | | | $ | 22,644,870 | | | $ | 22,893,801 | |
| Loss from operations | | $ | (10,691,430 | ) | | $ | (10,729,738 | ) | | $ | (15,822,633 | ) | | $ | (15,958,443 | ) | | $ | (21,506,859 | ) | | $ | (21,755,790 | ) |
| Change in fair value of preferred stock warrants | | | | | | $ | (8,801 | ) | | | | | | $ | (8,314 | ) | | | | | | $ | (119,914 | ) |
| Total other income (expense) | | $ | (163,372 | ) | | $ | (172,173 | ) | | $ | 31,457 | | | $ | 23,143 | | | $ | (1,655,877 | ) | | $ | (1,775,791 | ) |
| Net loss | | $ | (10,854,802 | ) | | $ | (10,901,911 | ) | | $ | (15,791,176 | ) | | $ | (15,935,300 | ) | | $ | (23,162,736 | ) | | $ | (23,531,581 | ) |
| Convertible preferred stock redemption value adjustment | | $ | (9,983,718 | ) | | $ | (10,156,393 | ) | | | | | | | | | | $ | (16,285,714 | ) | | $ | (17,737,544 | ) |
| Net loss attributed to common stock | | $ | (20,838,520 | ) | | $ | (21,058,304 | ) | | $ | (18,689,963 | ) | | $ | (18,834,087 | ) | | $ | (43,862,041 | ) | | $ | (45,682,716 | ) |
| Net loss per common share — basic and diluted | | $ | (34.08 | ) | | $ | (34.44 | ) | | $ | (30.56 | ) | | $ | (30.80 | ) | | $ | (50.67 | ) | | $ | (52.78 | ) |
Statements of Cash Flows | | | | | | | | | | | | | | | | | | | | | | | | |
Cash Flows From Operating Activities: | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | $ | (10,854,802 | ) | | $ | (10,901,911 | ) | | $ | (15,791,176 | ) | | $ | (15,935,300 | ) | | $ | (23,162,736 | ) | | $ | (23,531,581 | ) |
| Share-based compensation | | | | | | $ | 38,308 | | | $ | 83,543 | | | $ | 219,353 | | | $ | 150,028 | | | $ | 398,960 | |
| Change in fair value of preferred stock warrants | | | | | | $ | 8,801 | | | | | | | $ | 8,314 | | | | | | | $ | (119,914 | ) |
13. Pro Forma Financial Information (Unaudited)
The accompanying financial statements include pro forma information to present the Company’s balance sheet as of June 30, 2007 as if a) the Company’s one-for-25 stock split of its common stock, which was effective on October 16, 2007, had occurred on June 30, 2007, b) all of the Company’s warrants to purchase Series C and Series C-2 Convertible Preferred Stock, which expire upon the closing of an initial public offering, were exercised for proceeds of $1,213,542 on June 30, 2007, and c) all of the Company’s preferred stock had been converted into common stock on June 30, 2007, including the conversion into common stock of $7,593,920 accrued and unpaid dividends on theSeries C-2 and Series D convertible preferred stock. The conversion of the Company’s convertible preferred stock into common stock will be automatic upon the successful closing of an initial public offering of the Company’s common stock. The conversion of the Company’s convertible preferred stock to common stock also results in the reclassification of the Company’s $32.7 million convertible derivative liability into additional paid-in capital, and the Company’s outstanding warrants to acquire Series D Convertible Preferred Stock become warrants to acquire 1,307,773 shares of common stock and are re-classified to stockholders’ deficit. On a pro forma basis, as of June 30, 2007, the Company would have issued and outstanding 14,453,521 shares of its common stock and warrants to acquire 1,307,773 shares of its common stock. The pro forma financial information has not been audited. The unaudited pro forma financial information is presented for informational purposes only. The pro forma information is not necessarily indicative of what the financial position and shares of common stock and warrants to acquire common stock outstanding actually would have been had the one-for-25 stock split, exercise of the Series C andSeries C-2 preferred stock warrants, and the conversion of preferred stock into common stock been completed as June 30, 2007.
F-25
Part II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
| |
| Item 13. | Other Expenses of Issuance And Distribution |
The following table sets forth the various expenses, other than the underwriting discounts and commissions, payable by us in connection with the sale and distribution of the common stock being registered. All amounts shown are estimates, except the Securities and Exchange Commission registration fee, the National Association of Securities Dealers, Inc. filing fee and the NASDAQ listing fee.
| | | | | |
| SEC registration fee | | $ | 3,955 | |
| NASD filing fee | | | 13,380 | |
| NASDAQ listing fee | | | 100,000 | |
| Accounting fees and expenses | | | 1,150,000 | |
| Legal fees and expenses | | | 1,600,000 | |
| Printing and engraving expenses | | | 350,000 | |
| Transfer agent fees and expenses | | | 3,500 | |
| Miscellaneous fees and expenses | | | 237,540 | |
| | | | | |
| Total | | $ | 3,458,375 | |
| | | | | |
| |
| Item 14. | Indemnification of Directors and Officers |
Section 145 of the Delaware General Corporation Law, or DGCL, provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent to the corporation. The DGCL provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise.
Section 102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for any breach of the director’s duty of loyalty to the corporation or its stockholders, for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, or for any transaction from which the director derived an improper personal benefit.
Our amended and restated certificate of incorporation and amended and restated by-laws, in each case, that will be adopted upon consummation of this offering, will include provisions to (i) eliminate the personal liability of our directors for monetary damages resulting from breaches of their fiduciary duty to the extent permitted by Section 102(b)(7) of the DGCL and (ii) require the registrant to indemnify its directors and officers to the fullest extent permitted by Section 145 of the DGCL, including circumstances in which indemnification is otherwise discretionary. Pursuant to Section 145 of the DGCL, a corporation generally has the power to indemnify its present and former directors, officers, employees and agents against expenses incurred by them in connection with any suit to which they are, or are threatened to be made, a party by reason of their serving in such positions so long as they acted in good faith and in a manner they reasonably believed to be in or not opposed to, the best interests of the corporation and with respect to any criminal action, they had no reasonable cause to believe their conduct was unlawful. We believe that these provisions are necessary to attract and retain qualified persons as directors and officers. These provisions do not eliminate the directors’ duty of care, and, in appropriate circumstances, equitable remedies such as injunctive or other forms of non-monetary relief will remain available under DGCL. In addition,
II-1
each director will continue to be subject to liability for breach of the director’s duty of loyalty to the registrant, for acts or omissions not in good faith or involving intentional misconduct, for knowing violations of law, for acts or omissions that the director believes to be contrary to the best interests of the registrant or its stockholders, for any transaction from which the director derived an improper personal benefit, for acts or omissions involving a reckless disregard for the director’s duty to the registrant or its stockholders when the director was aware or should have been aware of a risk of serious injury to the registrant or its stockholders, for acts or omissions that constitute an unexcused pattern of inattention that amounts to an abdication of the director’s duty to the registrant or its stockholders, for improper transactions between the director and the registrant and for improper distributions to stockholders and loans to directors and officers. The provision also does not affect a director’s responsibilities under any other law, such as the federal securities law or state or federal environmental laws.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing, we have been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Prior to the completion of this offering, we intend to enter into indemnification agreements with our directors and officers. The indemnification agreements will provide indemnification to our directors and officers under certain circumstances for acts or omissions which may not be covered by directors’ and officers’ liability insurance, and may, in some cases, be broader than the specific indemnification provisions contained under Delaware law.
At present, there is no pending litigation or proceeding involving any of our directors or officers as to which indemnification is being sought nor are we aware of any threatened litigation that may result in claims for indemnification by any officer or director.
We have an insurance policy covering our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act or otherwise.
| |
| Item 15. | Recent Sales of Unregistered Securities |
Since March 31, 2004, the registrant has sold the following securities which were not registered under the Securities Act of 1933:
(a) Stock Option Grants
(1) From April 2004 through November 2006, we granted stock options to our employees and directors under our 2000 Equity Incentive Plan pursuant to which the optionees may purchase up to an aggregate of 774,000 shares of our common stock at the current weighted average exercise price of $4.50 per share. Of the options we granted during this period, options to purchase a total of 71,420 shares of our common stock have been forfeited, and 1,180 have been exercised. The sale and issuance of these securities were exempt from registration under Rule 701 under the Securities Act.
(2) From April 2007 to October 29, 2007, we granted stock options to our employees, directors and consultants under our 2007 Long-Term Incentive Plan pursuant to which the optionees may purchase up to an aggregate of 2,390,820 shares of our common stock at the current weighted average exercise price of $4.50 per share. Of the options we granted during this period, none have been forfeited and none have been exercised. The sale and issuance of these securities were exempt from registration under Rule 701 under the Securities Act.
| |
| (b) | Issuances of Common and Preferred Stock and Warrants |
(1) In September 2004, we exchanged 7,378,983 existing shares of Series A Convertible Preferred Stock into 9,767,665 shares ofSeries C-2 Convertible Preferred Stock.
II-2
(2) In September 2004, 339,001 warrants to acquire shares of Series B Convertible Preferred Stock and 1,150,347 shares of Series C Convertible Preferred Stock were converted into an aggregate of 3,395,830 warrants to acquire shares ofSeries C-2 Convertible Preferred Stock.
(3) In September 2004, we exchanged 3,582,510 shares of Series B Convertible Preferred Stock into 15,285,714 shares ofSeries C-2 Convertible Preferred Stock.
(4) In September 2004, we exchanged 14,949,993 shares of Series C Convertible Preferred Stock into 25,628,558 shares ofSeries C-2 Convertible Preferred Stock.
(5) In September 2004, we issued and sold 49,155,241 shares ofSeries C-2 Convertible Preferred Stock at a price per share of $0.35 to various accredited investors, for an aggregate consideration of $17,204,334.
(6) In June through September 2005 we issued and sold 28,987,866 shares ofSeries C-2 Convertible Preferred Stock at a price per share of $0.35 to existing stockholders who were accredited investors, for an aggregate consideration of $10,145,753.
(7) In March 2006, we issued and sold 320,000 shares of common stock at a price per share of $4.50 to William Moffitt pursuant to a restricted stock purchase agreement.
(8) In April 2006, we issued and sold 162,857,142 shares of Series D Convertible Preferred Stock at a price per share of $0.35 to new and existing accredited investors, for an aggregate consideration of $53,310,465.
(9) In February 2007, we issued 1,964,325 shares of Series D Convertible Preferred Stock to Venture Lending & Leasing IV, Inc. and 1,964,325 shares of Series D Convertible Preferred Stock to Venture Lending & Leasing V, Inc. as consideration for their execution of a commitment to provide loans in an aggregate amount of $25 million. In February 2007, we borrowed $12.5 million pursuant to those loan agreement and we issued an additional aggregate amount of 1,607,174 shares of Series D Convertible Preferred Stock to Venture Landing & Leasing IV, Inc. and Venture Landing & Leasing V, Inc. in connection with the Company’s drawdown of $12.5 million.
No underwriters were involved in the foregoing sales of securities. The securities described in this paragraph (b) of Item 15 were sold and issued to accredited investors in reliance upon the exemption from the registration requirements of the Securities Act, as set forth in Section 4(2) under the Securities Act and Rule 506 of Regulation D under the Securities Act, in each case, to the extent an exemption from such registration was required. The purchasers of shares and warrants described above represented to us in connection with their purchase that they were accredited investors and were acquiring the shares for investment and not distribution, that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration or an available exemption from such registration. The sales of these securities were made without general solicitation or advertising.
| |
| Item 16. | Exhibits and Financial Statement Schedules. |
(a) Exhibits
| | | | | |
Exhibit
| | |
No. | | Description of Document |
| |
| | 1 | .1 | | Form of Underwriting Agreement |
| | 3 | .1 | | Form of Second Amended and Restated Certificate of Incorporation of Nanosphere, Inc., to be effective upon the closing of the offering to which this Registration Statement relates (3) |
| | 3 | .2 | | Form of Amended and Restated Bylaws of Nanosphere, Inc., to be effective upon the closing of the offering to which this Registration Statement relates (3) |
| | 3 | .3 | | Amended and Restated Certificate of Incorporation of Nanosphere, Inc., dated as of April 12, 2006, as amended (3) |
| | 4 | .1 | | Nanosphere, Inc. Second Amended and Restated Stockholders Agreement, dated as of April 12, 2006, as amended (3) |
II-3
| | | | | |
Exhibit
| | |
No. | | Description of Document |
| |
| | 4 | .2 | | Nanosphere, Inc. Amended and Restated Registration Rights Agreement, dated as of April 12, 2006 (1) |
| | 4 | .3 | | Specimen of stock certificate for common stock |
| | 5 | .1 | | Opinion of Paul, Hastings, Janofsky & Walker LLP, related to the shares of common stock being sold in the initial public offering |
| | 10 | .1 | | Nanosphere, Inc. 2000 Equity Incentive Plan, as amended (1) |
| | 10 | .2 | | Form of Nanosphere, Inc. 2000 Equity Incentive Plan Non-Qualified Stock Option Award Agreement, as amended (1) |
| | 10 | .3 | | Form of Nanosphere, Inc. 2000 Equity Incentive Plan Option Award Agreement (1) |
| | 10 | .4 | | Nanosphere, Inc. 2007 Long-Term Incentive Plan, as amended and restated |
| | 10 | .5 | | Form of Nanosphere, Inc. 2007 Long-Term Incentive Plan Incentive Stock Option Award Agreement (Time Vested) (1) |
| | 10 | .6 | | Form of Nanosphere, Inc. 2007 Long-Term Incentive Plan Non-Qualified Stock Option Award Agreement (Time Vested) (1) |
| | 10 | .7 | | Form of Nanosphere, Inc. 2007 Long-Term Incentive Plan Option Award Agreement (Cliff-vested, performance-accelerated) (1) |
| | 10 | .8 | | Employment Agreement, dated as of July 19, 2004, by and between Nanosphere, Inc. and William P. Moffitt III, as amended (1) |
| | 10 | .9 | | Restricted Stock Purchase Agreement, dated as of March 16, 2006, by and between Nanosphere, Inc. and William P. Moffitt III (3) |
| | 10 | .10 | | Employment Agreement, dated January 2, 2001, by and between Nanosphere, Inc. and William Cork |
| | 10 | .11 | | Employment Agreement, dated May 13, 2005, by and between Nanosphere, Inc. and Gregory Shipp (1) |
| | 10 | .12 | | Employment Agreement, dated September 5, 2005, by and between Nanosphere, Inc. and Michael McGarrity (1) |
| | 10 | .13 | | Employment Agreement, dated April 25, 2007, by and between Nanosphere, Inc. and J. Roger Moody, Jr. (1) |
| | 10 | .14 | | Employment Agreement, dated May 31, 2007, by and between Nanosphere, Inc. and Winton Gibbons (1) |
| | 10 | .15 | | Severance Agreement, dated as of June 4, 2007, by and between Nanosphere, Inc. and Stephen Wasko (1) |
| | 10 | .16 | | License Agreement, dated as of January 1, 2006, by and between Northwestern University and Nanosphere, Inc. (2)# |
| | 10 | .17 | | Non-Exclusive License Agreement, dated as of December 20, 2002, by and between Nanosphere, Inc. and Abbott Laboratories (2)# |
| | 10 | .18 | | Lease with Motorola, Inc., dated as of March 24, 2003, as amended (1) |
| | 10 | .19 | | Loan and Security Agreement, dated as of February 7, 2007, by and between Nanosphere, Inc. and Venture Lending & Leasing IV, Inc. (1) |
| | 10 | .20 | | Loan and Security Agreement, dated as of February 21, 2007, by and between Nanosphere, Inc. and Venture Lending & Leasing V, Inc. (1) |
| | 10 | .21 | | Consulting and Non-Competition Agreement, dated as of October 31, 2002, by and between Nanosphere, Inc. and Chad A. Mirkin, as amended (1) |
| | 10 | .22 | | Bonus Agreement, dated as of March 16, 2006, by and between Nanosphere, Inc. and William P. Moffitt III, as amended (2) |
| | 10 | .23 | | Series C-2 Preferred Stock Purchase Agreement, dated as of September 2, 2004 (2) |
| | 10 | .24 | | Series D Preferred Stock and Warrant Purchase Agreement, dated as of April 12, 2006 (2) |
| | 10 | .25 | | Note and Warrant Purchase Agreement, dated as of February 10, 2004, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C. (2) |
| | 10 | .26 | | Form of Note and Warrant Purchase Agreement, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C., dated as of June 8, 2004 and July 8, 2004 (2) |
II-4
| | | | | |
Exhibit
| | |
No. | | Description of Document |
| |
| | 10 | .27 | | Note and Warrant Purchase Agreement, dated as of December 9, 2005, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C. (2) |
| | 10 | .28 | | Note Purchase Agreement, dated as of March 15, 2006, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C. (2) |
| | 10 | .29 | | Form of Indemnification Agreement (3) |
| | 10 | .30 | | Non-Exclusive Financial Advisory Services Engagement Letter, dated as of August 8, 2007, by and between Nanosphere, Inc. and Allen & Company LLC |
| | 23 | .1 | | Consent of Deloitte & Touche LLP |
| | 23 | .3 | | Consent of Paul, Hastings, Janofsky & Walker LLP (included in Exhibit 5.1) |
| | 24 | .1 | | Power of Attorney (seepage II-6 of this Registration Statement) (1) |
| | |
| * | | To be filed by amendment. |
| |
| # | | Confidential treatment has been requested with respect to certain provisions of this agreement. Omitted portions have been filed separately with the SEC. |
| |
| (1) | | Previously filed with theForm S-1 filed by the Registrant on August 13, 2007. |
| |
| (2) | | Previously filed with Amendment No. 1 to Form S-1 filed by the Registrant on September 27, 2007. |
| | |
| (3) | | Previously filed with Amendment No. 2 toForm S-1 filed by the Registrant on October 17, 2007. |
(b) Financial Statement Schedules
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
(a) The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
(b) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(c) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For purposes of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initialbona fideoffering thereof.
(3) If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other
II-5
than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(4) For the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser
II-6
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Northbrook, State of Illinois, on October 29, 2007.
NANOSPHERE, INC. (Registrant)
| | |
| | By: | /s/ William P. Moffitt III |
William P. Moffitt III
President and Chief Executive Officer
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed below by the following persons in the capacities and on the dates indicated below.
| | | | | | | |
Signature | | Title(s) | | Date |
| |
| | | | | |
/s/ William P. Moffitt III
William P. Moffitt III | | President, Chief Executive Officer, Director
(principal executive officer) | | October 29, 2007 |
| | | | | |
/s/ J. Roger Moody, Jr.
J. Roger Moody, Jr. | | Chief Financial Officer, Vice President of Finance & Administration, Treasurer, Secretary
(principal financial and accounting officer) | | October 29, 2007 |
| | | | | |
*
Mark Slezak | | Chairman of the board of directors | | October 29, 2007 |
| | | | | |
*
Jeffrey R. Crisan | | Director | | October 29, 2007 |
| | | | | |
*
André de Bruin | | Director | | October 29, 2007 |
| | | | | |
*
Chad A. Mirkin | | Director | | October 29, 2007 |
| | | | | |
*
James J. Nahirny | | Director | | October 29, 2007 |
| | | | | |
*
Sheli Z. Rosenberg | | Director | | October 29, 2007 |
| | | | | | | |
| By: | | /s/ William P. Moffitt III
William P. Moffitt III
President and Chief Executive Officer | | | | |
II-7
INDEX TO EXHIBITS
| | | | | |
Exhibit
| | |
No. | | Description of Document |
| |
| | 1 | .1 | | Form of Underwriting Agreement |
| | 3 | .1 | | Form of Second Amended and Restated Certificate of Incorporation of Nanosphere, Inc., to be effective upon the closing of the offering to which this Registration Statement relates (3) |
| | 3 | .2 | | Form of Amended and Restated Bylaws of Nanosphere, Inc., to be effective upon the closing of the offering to which this Registration Statement relates (3) |
| | 3 | .3 | | Amended and Restated Certificate of Incorporation of Nanosphere, Inc., dated as of April 12, 2006, as amended (3) |
| | 4 | .1 | | Nanosphere, Inc. Second Amended and Restated Stockholders Agreement, dated as of April 12, 2006, as amended (3) |
| | 4 | .2 | | Nanosphere, Inc. Amended and Restated Registration Rights Agreement, dated as of April 12, 2006 (1) |
| | 4 | .3 | | Specimen of stock certificate for common stock |
| | 5 | .1 | | Opinion of Paul, Hastings, Janofsky & Walker LLP, related to the shares of common stock being sold in the initial public offering |
| | 10 | .1 | | Nanosphere, Inc. 2000 Equity Incentive Plan, as amended (1) |
| | 10 | .2 | | Form of Nanosphere, Inc. 2000 Equity Incentive Plan Non-Qualified Stock Option Award Agreement, as amended (1) |
| | 10 | .3 | | Form of Nanosphere, Inc. 2000 Equity Incentive Plan Option Award Agreement (1) |
| | 10 | .4 | | Nanosphere, Inc. 2007 Long-Term Incentive Plan, as amended and restated |
| | 10 | .5 | | Form of Nanosphere, Inc. 2007 Long-Term Incentive Plan Incentive Stock Option Award Agreement (Time Vested) (1) |
| | 10 | .6 | | Form of Nanosphere, Inc. 2007 Long-Term Incentive Plan Non-Qualified Stock Option Award Agreement (Time Vested) (1) |
| | 10 | .7 | | Form of Nanosphere, Inc. 2007 Long-Term Incentive Plan Option Award Agreement (Cliff-vested, performance-accelerated) (1) |
| | 10 | .8 | | Employment Agreement, dated as of July 19, 2004, by and between Nanosphere, Inc. and William P. Moffitt III, as amended (1) |
| | 10 | .9 | | Restricted Stock Purchase Agreement, dated as of March 16, 2006, by and between Nanosphere, Inc. and William P. Moffitt III (3) |
| | 10 | .10 | | Employment Agreement, dated January 2, 2001, by and between Nanosphere, Inc. and William Cork |
| | 10 | .11 | | Employment Agreement, dated May 13, 2005, by and between Nanosphere, Inc. and Gregory Shipp (1) |
| | 10 | .12 | | Employment Agreement, dated September 5, 2005, by and between Nanosphere, Inc. and Michael McGarrity (1) |
| | 10 | .13 | | Employment Agreement, dated April 25, 2007, by and between Nanosphere, Inc. and J. Roger Moody, Jr. (1) |
| | 10 | .14 | | Employment Agreement, dated May 31, 2007, by and between Nanosphere, Inc. and Winton Gibbons (1) |
| | 10 | .15 | | Severance Agreement, dated as of June 4, 2007, by and between Nanosphere, Inc. and Stephen Wasko (1) |
| | 10 | .16 | | License Agreement, dated as of January 1, 2006, by and between Northwestern University and Nanosphere, Inc. (2)# |
| | 10 | .17 | | Non-Exclusive License Agreement, dated as of December 20, 2002, by and between Nanosphere, Inc. and Abbott Laboratories (2)# |
| | 10 | .18 | | Lease with Motorola, Inc., dated as of March 24, 2003, as amended (1) |
| | 10 | .19 | | Loan and Security Agreement, dated as of February 7, 2007, by and between Nanosphere, Inc. and Venture Lending & Leasing IV, Inc. (1) |
| | | | | |
Exhibit
| | |
No. | | Description of Document |
| |
| | 10 | .20 | | Loan and Security Agreement, dated as of February 21, 2007, by and between Nanosphere, Inc. and Venture Lending & Leasing V, Inc. (1) |
| | 10 | .21 | | Consulting and Non-Competition Agreement, dated as of October 31, 2002, by and between Nanosphere, Inc. and Chad A. Mirkin, as amended (1) |
| | 10 | .22 | | Bonus Agreement, dated as of March 16, 2006 by and between Nanosphere, Inc. and William P. Moffitt III, as amended (2) |
| | 10 | .23 | | Series C-2 Preferred Stock Purchase Agreement, dated as of September 2, 2004 (2) |
| | 10 | .24 | | Series D Preferred Stock and Warrant Purchase Agreement, dated as of April 12, 2006 (2) |
| | 10 | .25 | | Note and Warrant Purchase Agreement, dated as of February 10, 2004, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C. (2) |
| | 10 | .26 | | Form of Note and Warrant Purchase Agreement, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C., dated as of June 8, 2004 and July 8, 2004 (2) |
| | 10 | .27 | | Note and Warrant Purchase Agreement, dated as of December 9, 2005, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C. (2) |
| | 10 | .28 | | Note Purchase Agreement, dated as of March 15, 2006, by and between Nanosphere, Inc. and Lurie Investment Fund, L.L.C. (2) |
| | 10 | .29 | | Form of Indemnification Agreement (3) |
| | 10 | .30 | | Non-Exclusive Financial Advisory Services Engagement Letter, dated as of August 8, 2007, by and between Nanosphere, Inc. and Allen & Company LLC |
| | 23 | .1 | | Consent of Deloitte & Touche LLP |
| | 23 | .3 | | Consent of Paul, Hastings, Janofsky & Walker LLP (included in Exhibit 5.1) |
| | 24 | .1 | | Power of Attorney (seepage II-6 of this Registration Statement) (1) |
| | |
| * | | To be filed by amendment. |
| |
| # | | Confidential treatment has been requested with respect to certain provisions of this agreement. Omitted portions have been filed separately with the SEC. |
| |
| (1) | | Previously filed with theForm S-1 filed by the Registrant on August 13, 2007. |
| |
| (2) | | Previously filed with Amendment No. 1 toForm S-1 filed by the Registrant on September 27, 2007. |
| | |
| (3) | | Previously filed with Amendment No. 2 toForm S-1 filed by the Registrant on October 17, 2007. |