FORWARD-LOOKING STATEMENTS
Some of the statements made in this presentation are
forward-looking statements. These forward-looking
statements are based upon our current expectations and
projections about future events and generally relate to our
plans, objectives and expectations for the development of
and commercialization of in-licensed cancer drugs. Although
management believes that the plans and objectives reflected
in or suggested by these forward-looking statements are
reasonable, all forward-looking statements involve risks and
uncertainties and actual future results may be materially
different from the plans, objectives and expectations
expressed in this presentation.
ZIOPHARM Mission and Strategy
Better cancer medicine.
Low cost small molecules.
Oral and global.
Improved quality of life.
Portfolio of Mid-Stage Development Candidates
Focus of current resources:
ZymafosTM (palifosfamide), novel DNA-alkylating molecule in
randomized phase II expected to define registration trial 1H 2010;
oral form at IND stage
To follow:
ZybulinTM (indibulin), novel oral tubulin-binding molecule in phase
I trials expected to enter phase I/II breast cancer trial 2H 2009 with
mathematically-derived dosing schedule (Dr. Norton); nanoparticle
formulation (for oral, IV) in preclinical evaluation
ZinaparTM (darinaparsin), novel IV mitochondrial-targeted
molecule in phase II trials with the potential for registration trial in
1H 2010; oral form in phase I
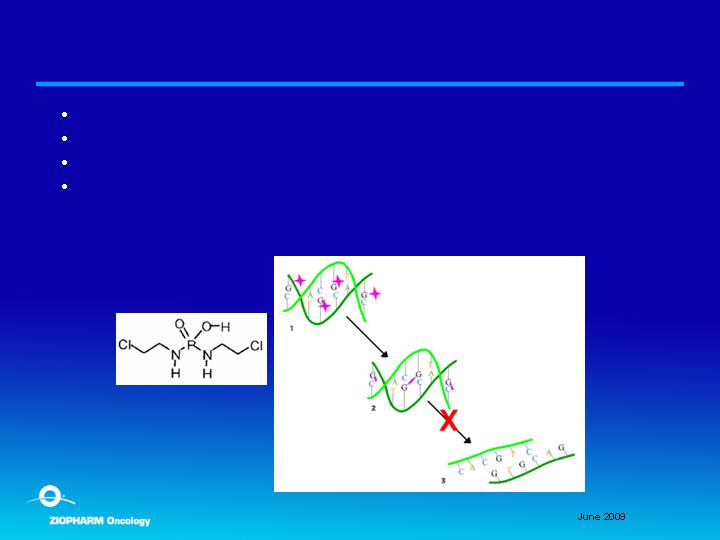
Palifosfamide
Novel alkylating molecule; patent applications U.S. and internationally
Anticipated low cost to manufacture
IV form in randomized phase II and oral developed for IND
Target indication of soft-tissue sarcoma (front/second-line setting)
Orphan Drug Designation (soft-tissue sarcoma) in U.S. and Europe
Palifosfamide Opportunity
Palifosfamide, novel DNA alkylating agent, related to ifosfamide
and cyclophosphamide family
Active preclinically in diverse cancers including ifosfamide and
cyclophosphamide resistant tumors
Development premise: less toxic, more efficacious, enhanced
quality of life, easier to administer than related drugs, cost-effective
Niche market development for soft-tissue sarcoma with estimated
sales potential in the front- and second-line setting of $250 MM
Replacing ifosfamide in lymphoma $400 MM, and with use in other
solid tumors (for ifosfamide/cyclophosphamide) including breast,
ovarian and prostate, significant further potential
Palifosfamide Development Leading to ASCO
Active in Phase I with anticipated toxicity profile
Active in Phase II advanced sarcoma
Synergistic with doxorubicin in preclinical study
US and EU experts recommend randomized phase II (front-/second-
line setting, palifosfamide +/- doxorubicin) in soft-tissue sarcoma
(PICASSO); trial actively enrolling with initial drug study safety
monitoring committee meeting concluding completing trial as planned,
possibly enrolling less patients.
Phase I palifosfamide/doxorubicin combination data presented at
ASCO 2009
Select ASCO data:
A Phase I Study of Palifosfamide
in Combination with Doxorubicin:
Safety and Preliminary Efficacy
Luis H. Camacho1, Sant Chawla2, Victoria Chua2, Giovanni Abbadessa3,
Philip Komarnitsky 3, Barbara Wallner3, Jan Stevens3, Jonathan Lewis3
1Oncology Consultants; 2Sarcoma Oncology Center, Santa Monica, CA;
3ZIOPHARM Oncology, Inc., Boston, MA
Preliminary Exposure and
Efficacy
Exposure
13 treated; 0 ongoing
Best Response
SD or better in 42% of 12 evaluated patients
3 PR: STS (2) and SCLC (1)
SD or better in 75% of STS patients
Patients age =65 (n=4): 2 SD (50%), 1 PR (25%)
Soft-Tissue Sarcomas:
Best Response (N=8)
0
2
0
LMS (2)
0
1
0
MPNS Tumor
25
1
0
1
0
PD
0
0
Angiosarcoma
0
2
Rhabdomyosarcoma (2)
50
25
% of STS patients:
0
0
Pleomorphic Sarcoma
1
0
Endometrial Stromal Cell Sarcoma
SD
PR
75 % SD or better
Adverse events primarily mild to moderate in severity
No encephalopathy, no hemorrhagic cystitis, no renal
toxicity
Most common adverse events include:
Neutropenia 6 (46%)
Thrombocytopenia 6 (46%)
Micro Hematuria 5 (38%)
Anemia 5 (38%)
Nausea 4 (31%)
Vomiting 4 (31%)
Adverse Events
Conclusions
Palifosfamide 150 mg/m2 , 3 times per week combined with
doxorubicin 75 mg/m2 once every 3 weeks is a very well tolerated
outpatient regimen.
There has been no encephalopathy, no hemorrhagic cystitis, no
renal toxicity. Adverse events are primarily hematologic and easy to
manage.
Preliminary efficacy:
3/12 PRs
2/8 PRs in STS 4/8 SD in STS
Ease of administration, favorable toxicity profile, and preliminary
efficacy support further evaluation of this agent in sarcoma. A
randomized, controlled Phase II study in STS comparing
palifosfamide 150 mg/m2 plus doxorubicin 75 mg/m2 vs. doxorubicin
75 mg/m2 alone is in full progress.
Palifosfamide Registration Strategy
Palifosfamide / doxorubicin vs. doxorubicin in front- and
second-line patients with unresectable or metastatic soft-
tissue sarcoma:
Phase II randomized trial helps shape registration trial
Single registration trial powered for PFS and survival
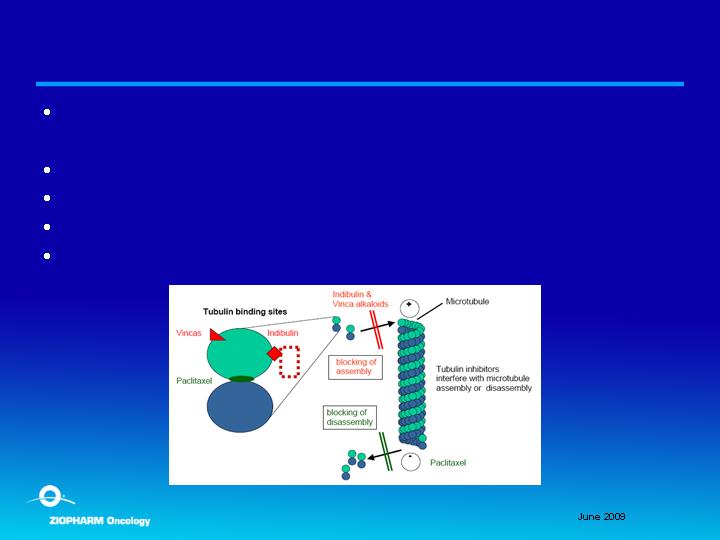
Indibulin
Novel oral tubulin binding agent; issued patents and
applications
Anticipated low cost to manufacture
Targets cell mitosis and movement
Expected low toxicity (no neurotoxicity)
Target indication of subset of breast cancer
Indibulin Opportunity
Taxanes widely used and a more efficacious/less toxic
oral treatment expected to have billion dollar sales
potential
Distinct mechanism and
Oral dosing
Lack of neurotoxicity
Potential efficacy in tumors with MDR
Treatment approaches, initially in breast cancer:
Norton dose density
Combination therapy
Indibulin Development Leading to ASCO
Single agent activity in phase I in multiple tumor types;
confirmatory activity in phase I PET data; DLT not
reached, minimal toxicity and no neurotoxicity
Highly synergistic in preclinical study
Phase I oral capecitabine with oral indibulin presented at
ASCO 2009
Preclinical evaluation (Dr. Norton) evaluating dose
density schedule presented at ASCO 2009
Select ASCO presented data:
Indibulin, a Novel Tubulin
Targeting-agent, in
Combination with
Capecitabine, is Suitable for
Mathematically-Optimized
Dose-Scheduling
Jonathan J. Lewis 1, Matthew D. Galsky 2,6, Luis H. Camacho 3, David
M. Loesch 4,6, Philip B. Komarnitsky 1, Barbara Wallner 1, Jan Stevens
Larry Norton 5
1 ZIOPHARM Oncology, New York, NY; 2 Comprehensive Cancer Centers of
Nevada, Las Vegas, NV; 3 Oncology Consultants P.A., Houston, TX; 4 Central
Indiana Cancer Centers, Indianapolis, IN; 5 Harmon Hill, New York, NY, 6 US
Oncology, Translational Oncology Program, Houston, TX
Preliminary Clinical Activity
Median SD 6 Cycles
Breast and colon cancer SD for 6 Cycles
Bladder cancer SD for 9 Cycles
Prostate cancer SD for 9 Cycles
Breast and Colon Cancer 6 Cycles
Bladder and Prostate Cancer 9 Cycles
Preliminary Safety
AEs that are Related and occurred in
2
or more pts
(
=
29
%)
Frequency, %
Grade 1/2 AEs that were related
•
Fatigue
4 (57)
•
Anorexia
2 (29)
•
Dyspnea
2 (29)
•
Hand Foot Syndrome
2 (29)
•
Mucositis
2 (29)
•
Vomiting
2 (29)
Grade 3/4 AEs that were related
•
Hypophosphatemia
1 (14)
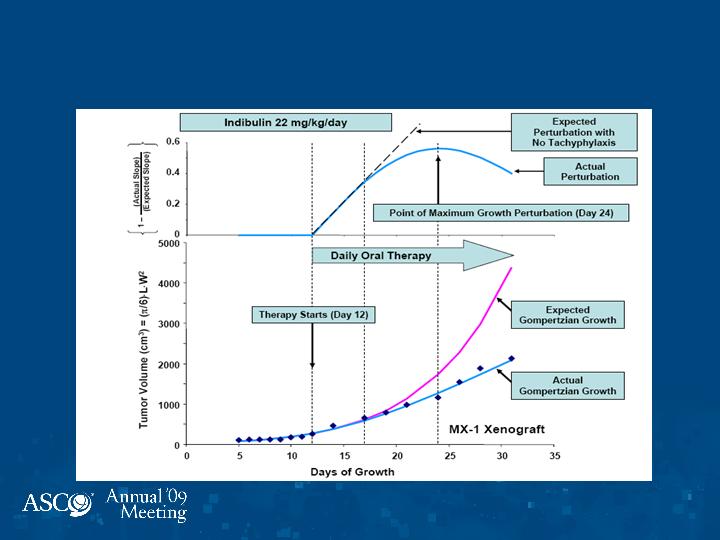
Indibulin: Optimization of
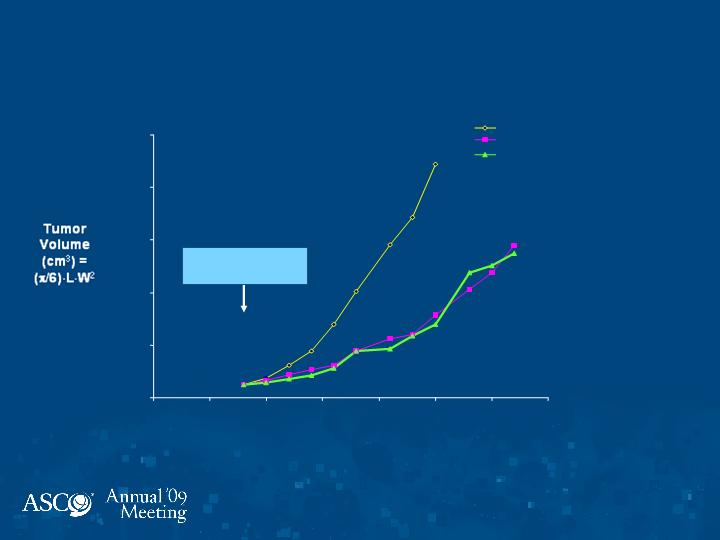
Dosing Schedules
Days of Growth
Therapy Starts
(Day 8)
MX-1 Xenograft
0
500
1000
1500
2000
2500
0
5
10
15
20
25
30
35
Vehicle Control
daily
Indibulin 22 mg/kg
daily
Indibulin 29 mg/kg 5 days on, 5
days off
Indibulin: Optimization of
Dosing Schedules
Conclusions
Oral indibulin in combination with capecitabine is very
well tolerated with no neurotoxicity. Early activity in
breast, colon, bladder, and prostate cancers.
Formal analyses of preclinical data utilizing Norton-
Simon Modeling reveals that the major effect of therapy
occurs in five days of exposure, which is not
manifest on gross inspection until one week thereafter.
Hence, an intermittent schedule based on five days of
drug administration preserves full activity while
minimizing toxicity. This may also minimize acquired
resistance.
A Phase I-II study in breast cancer using this novel
scheduling strategy is in development.
Darinaparsin
Novel mitochondrial-targeted agent (organic arsenic);
first in a new class of molecules
IV formulation in multiple phase I/II studies with
confirmed activity in lymphoma
Oral form ongoing in Phase I
Families of compounds covered by issued patents with
further applications pending in U.S. and internationally
Anticipated low cost to manufacture
Target indication of refractory peripheral T-cell
lymphoma / lymphoma, potential sales of $250 million,
with other lymphoma use, $400 million; with oral form,
significant additional potential
Darinaparsin Opportunity
Inorganic arsenic use limited by cardiotoxcity (“Black
Box” warning)
IV darinaparsin (organic arsenic) in several phase I/II
studies with no cardiotoxicity, well tolerated; MTD not
yet reached with oral form
IV phase II data in hematological malignancies
presented at ASCO 2009 -- advisors believe data can
support potential registration trial
Select ASCO Symposium Data:
Novel Organic Arsenic
Molecule Darinaparsin:
Development of IV and Oral
Forms
I. S. Lossos1, M. D. Craig2, M. S. Tallman3, R. V. Boccia4,
P. R. Conkling5, C. Becerra6, P. B. Komarnitsky7, B. Wallner7,
J.J.Lewis7, W. H. Miller8
1 Miller School Of Medicine, University of Miami, Miami, FL; 2 West Virginia University Hospitals, Morgantown,
WV; 3 Northwestern University Medical School, Chicago, IL; 4 Associates In Oncology/Hematology,
Bethesda, MD; 5 Virginia Oncology Associates, Norfolk, VA; 6 Baylor University Medical Center,
Dallas, TX; 7 ZIOPHARM Oncology, Inc., Boston, MA; 8 McGill University Jewish General Hospital,
Montreal, QC
IV Darinaparsin Efficacy
Complete Responses: 3
66 year old female, PTCL (3 + months*)
3 prior treatment regimens: CHOP x 6, ICE and
EPOCH x 2
Patient taken off study for autologous BMT
73 year old female, PTCL + senile EBV-associated, B-cell
lymphoproliferative disorder (5 + months)
6 prior regimens: ABVD x 3, ICE, autologous bone
marrow transplant, gemzar and radiation
65 year old male, DLBCL (6 months)
4 prior regimens: RCHOP, EPOCH, transplant, gemzar
IV Darinaparsin Efficacy
1 Marginal Zone Non-Hodgkin Lymphoma transformed to DLBCL (14 +
months)*
5 prior treatment regimens: chlorambucil, RCHOPx5, RICEx3, RT
and BMT
1 Marginal Zone Non-Hodgkin Lymphoma (3 months)
Rituximab x 8, RCVP x 1, and gemcitabine x 1
1 PTCL (4 months)
EPOCH x 2, dox, cytoxan
1 Hodgkin’s Nodular Sclerosis (8 months)
ICE x 1, CBV x 1, gemcitabine + MDX x 6
1 PTCL, 2 Hodgkin's, 1 B-cell (3 – 9+ months)
Prolonged Stable Disease: 4
Partial Responses: 4
IV Darinaparsin Related Adverse
Events
Events that were
grade 3
N
%
Fatigue
1
3
Alk. Phos Increased
1
3
Events that were considered SAEs
N
%
Fall
1
3
Neutropenic Fever
1
3
No
QT prolongation
Oral Efficacy
MTD not reached
1 PR (H&N) duration 5+ months
15 prolonged SD (H&N, lymphoma,
colon, pancreas)
Duration 3 – 6+ months
Summary
IV Lymphoma
7 / 19 objective responses (3 CRs, 4 PRs)
3 / 5 objective responses PTCL (2 CRs, 1 PR)
4 prolonged SD
PO All comers
not yet at MTD
1 PR, 15 prolonged SD
Portfolio Highlights
Palifosfamide pivotal trial design expected from IV
phase II randomized study to initiate as early as 1H 2010
and focus of current resources
Indibulin phase I and dose scheduling oral data
highlight potential benefit over widely used tubulin
targeted agents with phase I/II novel dose schedule in
breast expected 2H 2009
Darinaparsin phase II lymphoma data highly
encouraging for possible registration trial 1H 2010