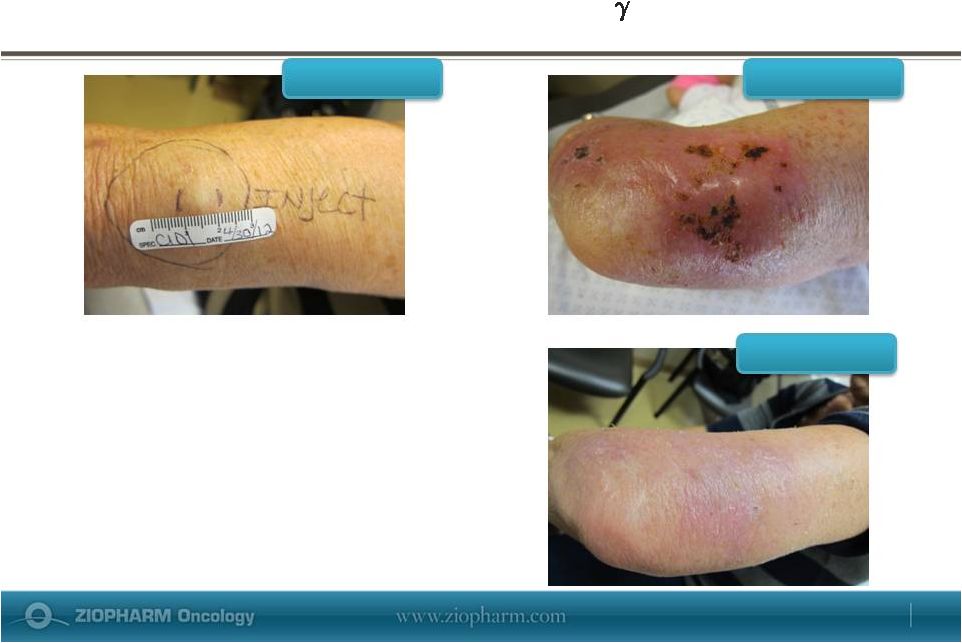
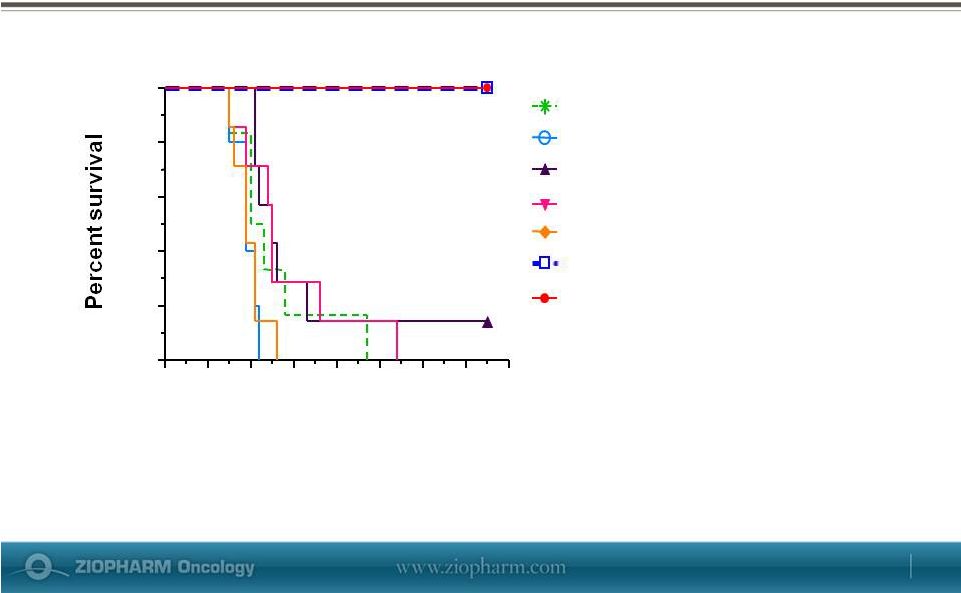
Forward-Looking Statements This presentation contains certain forward-looking information about ZIOPHARM Oncology that is intended to be covered by the safe harbor for “forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts. Words such as “expect(s),” “feel(s),” “believe(s),” “will,” “may,” “anticipate(s)” and similar expressions are intended to identify forward-looking statements. These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products; our ability to expand our long-term business opportunities; financial projections and estimates and their underlying assumptions; and future performance. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, but are not limited to: whether any of our therapeutic candidates will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether any of our therapeutic candidates will be successfully marketed if approved; whether our DNA-based biotherapeutics discovery and development efforts will be successful; our ability to achieve the results contemplated by our collaboration agreements; the strength and enforceability of our intellectual property rights; competition from pharmaceutical and biotechnology companies; the development of and our ability to take advantage of the market for DNA-based biotherapeutics; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; and the other risk factors contained in our periodic and interim reports filed with the SEC including, but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2012, and our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2013. Our audience is cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. May 29, 2013 |