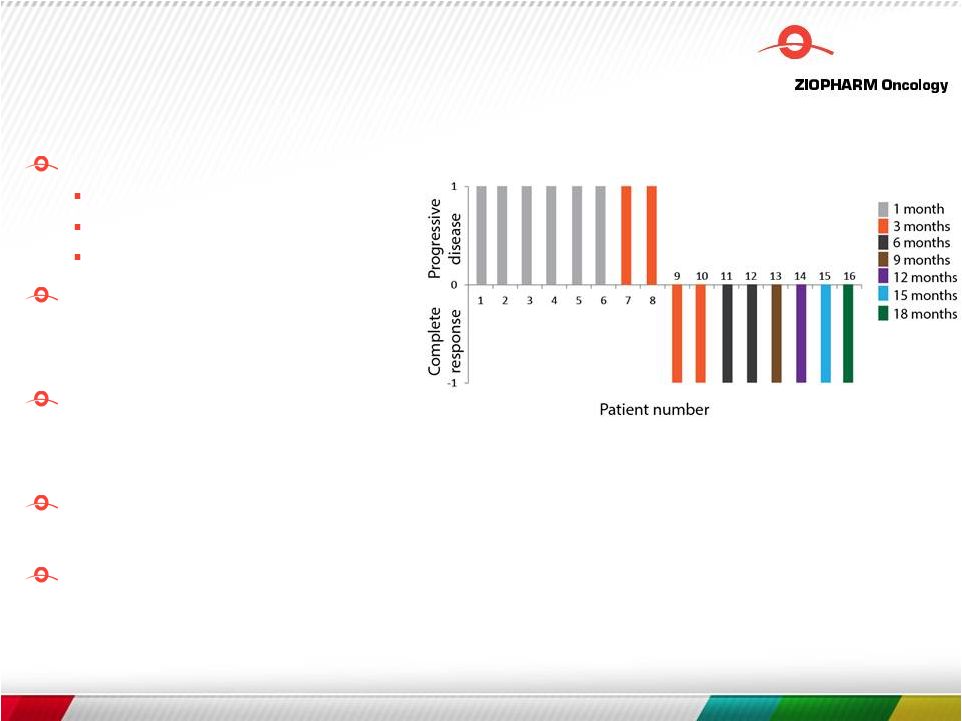
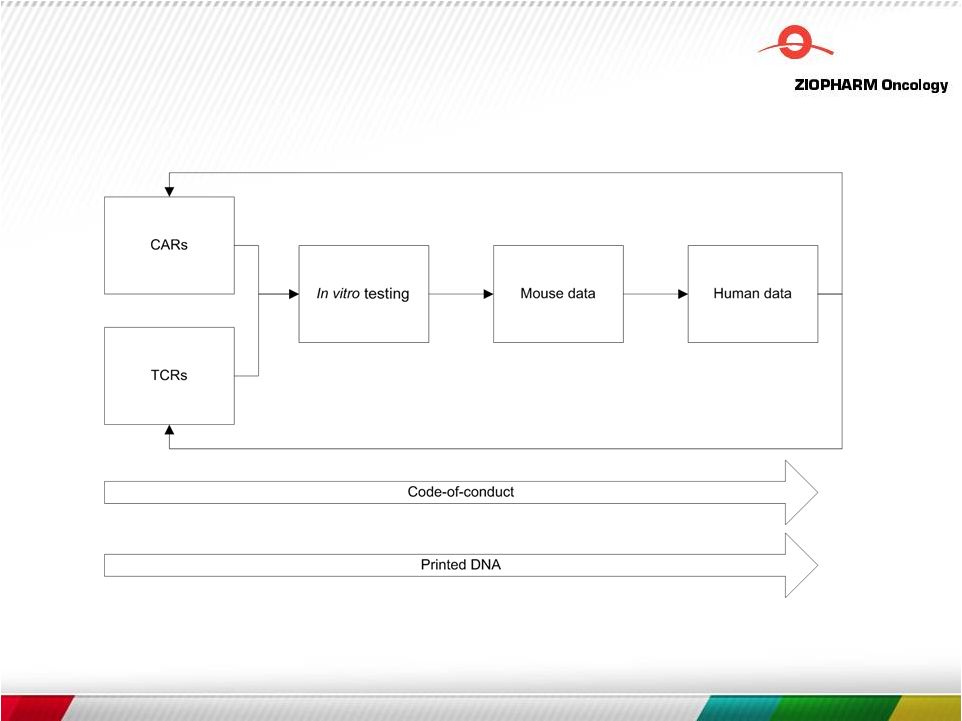
Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
TCRT similar filings
- 21 Aug 15 Entry into a Material Definitive Agreement
- 10 Aug 15 ZIOPHARM Reports Second-Quarter 2015 Financial Results and Recent Activities
- 19 Jun 15 Submission of Matters to a Vote of Security Holders
- 18 Jun 15 Regulation FD Disclosure
- 8 Jun 15 Entry into a Material Definitive Agreement
- 2 Jun 15 Appointed Chief Operating Officer
- 15 May 15 Departure of Directors or Certain Officers
Filing view
External links