ZIOPHARM / Intrexon
Graft-Versus-Host Disease
Exclusive Channel Collaboration
SEPTEMBER 28, 2015
Forward-looking Statements
This presentation contains certain forward-looking information about ZIOPHARM Oncology, Inc. that is intended to be covered by the safe harbor for “forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” and “believes.” These statements include, but are not limited to, statements regarding the progress, timing and results of preclinical and clinical trials involving the Company’s drug candidates, and the progress of the Company’s research and development programs. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not limited to: whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, or any of our other therapeutic candidates will advance further in the pre-clinical or clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and enforceability of our intellectual property rights; competition from other pharmaceutical and biotechnology companies; and the other risk factors contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2014, and our Quarterly Report on Form 10Q for the quarter ended June 30, 2015. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events.
2
Allogeneic Hematopoietic Stem-Cell
Transplantation (HSCT)
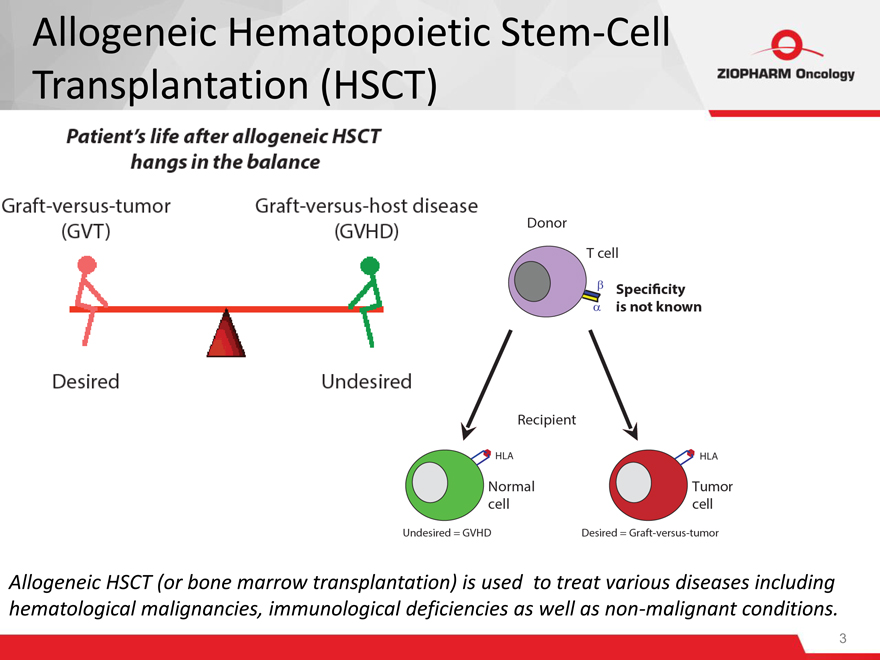
Allogeneic HSCT (or bone marrow transplantation) is used to treat various diseases including hematological malignancies, immunological deficiencies as well as non-malignant conditions.
3
Role of T cells in Recipients of Allogeneic HSCT
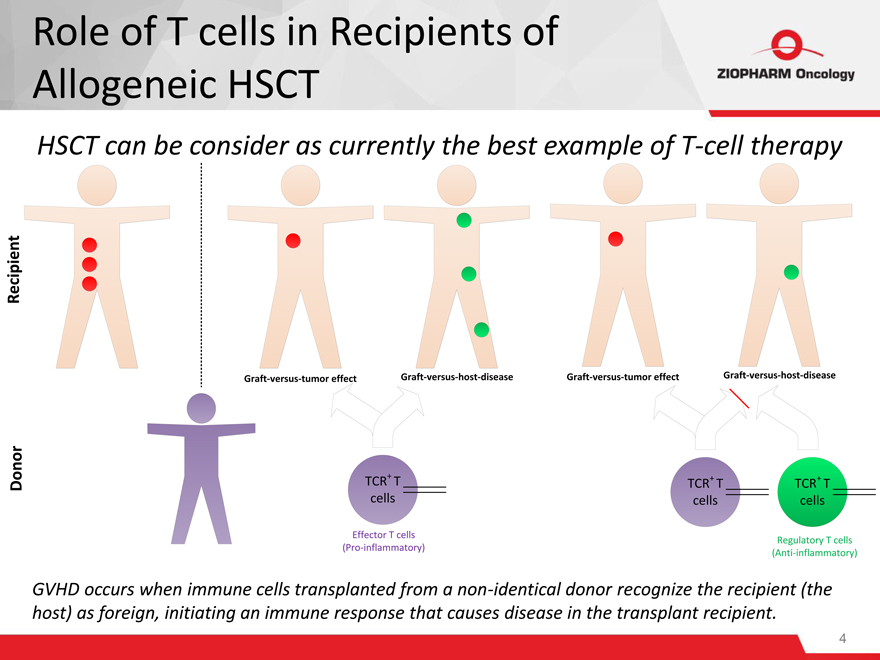
HSCT can be consider as currently the best example of T-cell therapy
Recipient
Graft-versus-tumor effect Graft-versus-host-disease Graft-versus-tumor effect Graft-versus-host-disease
TCR+ T + + Donor TCR T TCR T cells cells cells
Effector T cells
(Pro-inflammatory) Regulatory T cells (Anti-inflammatory)
GVHD occurs when immune cells transplanted from a non-identical donor recognize the recipient (the host) as foreign, initiating an immune response that causes disease in the transplant recipient.
4
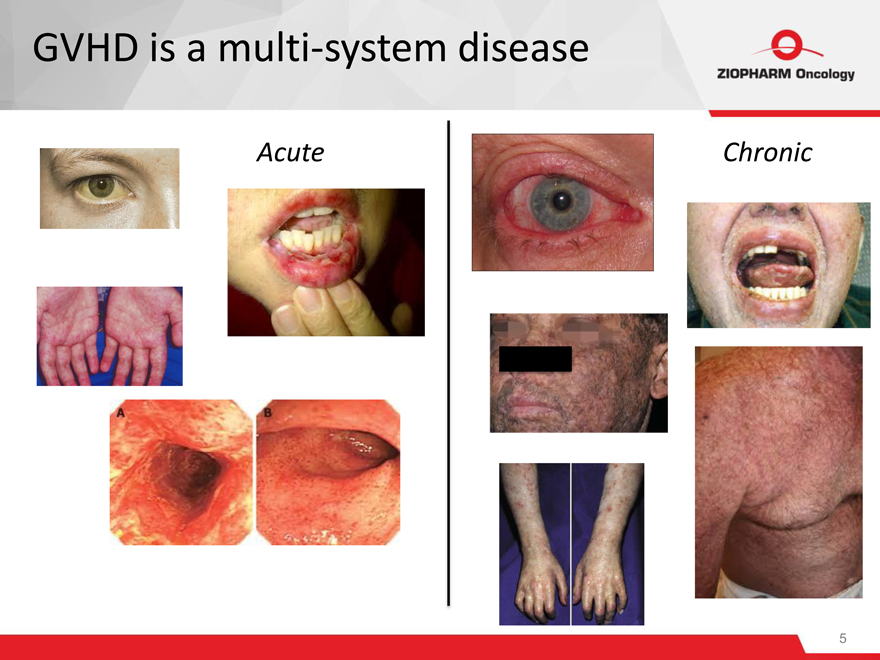
GVHD is a multi-system disease
Acute Chronic
5
Graft-versus-Host Disease (GVHD)
Acute and chronic GVHD are multisystem disorders that are common complications of allogeneic hematopoietic stem-cell transplantation (HSCT) Principal risk factors for non-relapse deaths after allogeneic HSCT are older age and GVHD
– J Clin Oncol. 2011 Jun 1;29(16):2230-9
Patients with steroid-resistant acute GVHD have a dismal prognosis, with mortality rates in excess of 90%.
– Adv Hematol. 2011;2011:601953
Despite aggressive treatment, chronic GVHD affects up to 50% of long-term allogeneic HSCT survivors and is lethal in 40% of severely affected recipients
– Br J Haematol. 2015 Jul 26. [Epub ahead of print]
– Blood. 2014 Jul 17;124(3):374-84.
Chronic GVHD is the single major factor determining long-term quality of life following allogeneic HSCT
Chronic GVHD is the leading cause of non-relapse mortality in patients surviving more than 2 years
– N Engl J Med. 1999 Jul 1;341(1):14-21
6
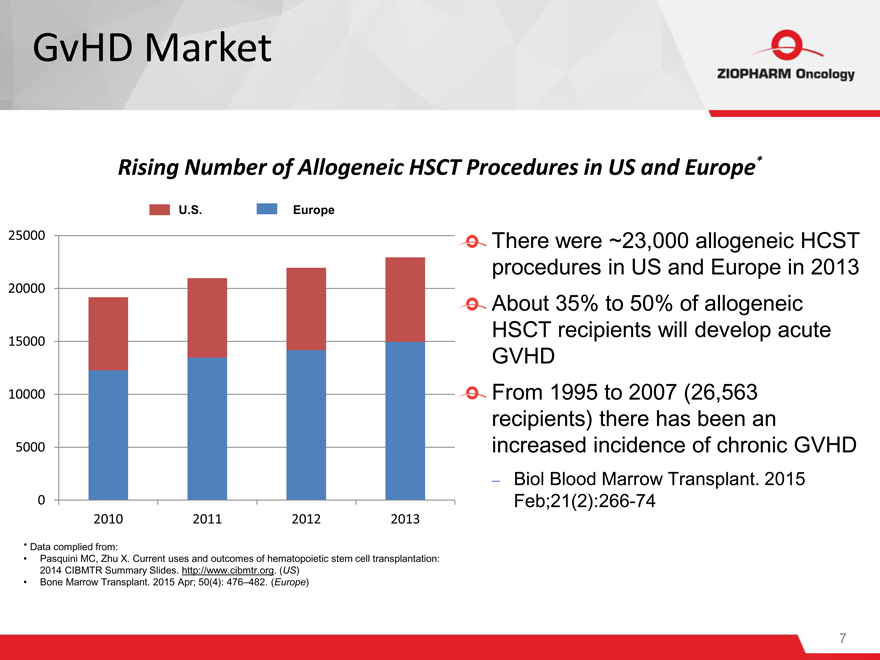
GvHD Market
Rising Number of Allogeneic HSCT Procedures in US and Europe*
U.S. Europe
25000
20000
15000
10000
5000
0
2010 2011 2012 2013
There were ~23,000 allogeneic HCST procedures in US and Europe in 2013 About 35% to 50% of allogeneic HSCT recipients will develop acute GVHD
From 1995 to 2007 (26,563 recipients) there has been an increased incidence of chronic GVHD
– Biol Blood Marrow Transplant. 2015 Feb;21(2):266-74
* Data complied from:
Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. http://www.cibmtr.org. (US)
Bone Marrow Transplant. 2015 Apr; 50(4): 476–482. (Europe)
7
Overcoming Limitations; Expanding
Potential Patient Population
Immunosuppressive agents and systemic steroids routinely used to prevent and treat GvHD have limited efficacy and toxicity, defining the need for safer, more effective therapies.
The ECC intends to overcome these limitations through the application of IL-2 immunotherapy under Intrexon’s control technologies and/or the ActoBiotics® platform’s ability for targeted delivery to the digestive tract which plays a significant role in the body’s immune system.
These new ways of treating and preventing GvHD have the potential to increase the market opportunity through:
1. Broadening of patient eligibility to receive allogeneic HCST
2. Increasing number of effective donor/recipient combinations
8
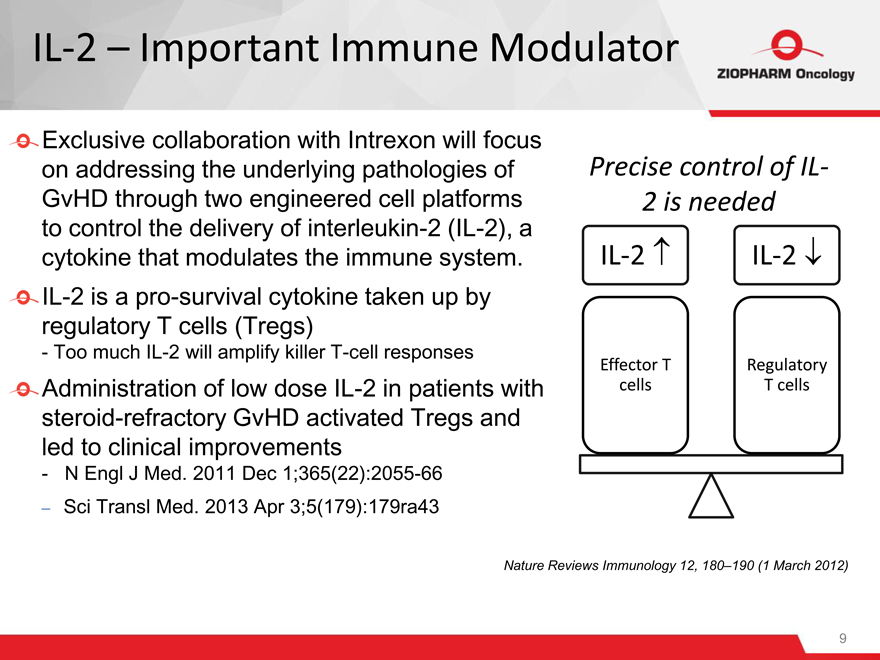
IL-2 – Important Immune Modulator
Exclusive collaboration with Intrexon will focus on addressing the underlying pathologies of GvHD through two engineered cell platforms to control the delivery of interleukin-2 (IL-2), a cytokine that modulates the immune system. IL-2 is a pro-survival cytokine taken up by regulatory T cells (Tregs)
- Too much IL-2 will amplify killer T-cell responses
Administration of low dose IL-2 in patients with steroid-refractory GvHD activated Tregs and led to clinical improvements
- N Engl J Med. 2011 Dec 1;365(22):2055-66
– Sci Transl Med. 2013 Apr 3;5(179):179ra43
Precise control of IL-
2 is needed
IL-2 IL-2
Effector T Regulatory
cells T cells
Nature Reviews Immunology 12, 180–190 (1 March 2012)
9
Current Human Application of
Tregs and IL-2
Clin Cancer Res. 2014 Apr 15;20(8):2215-25.
Blood. 2013 Apr 11;121(15):2864-74.
Best Pract Res Clin Haematol. 2011 Sep;24(3):459-66.
Sci Transl Med. 2011 May 18;3(83):83ra41.
Semin Immunol. 2006 Apr;18(2):78-88.
Blood. 2011 Jan 20;117(3):1061-70.
10
Clinical Application of Gene
Control Technologies
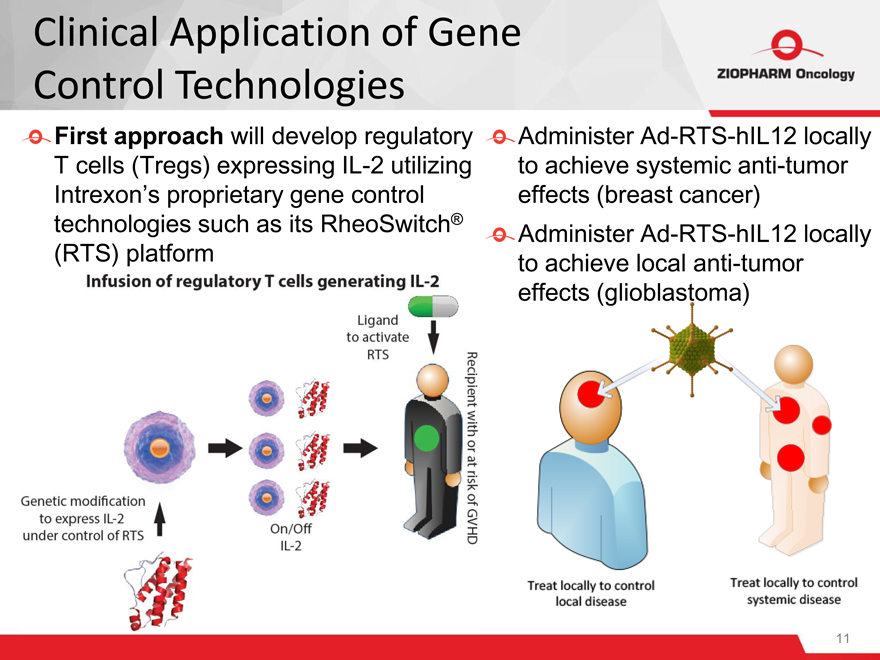
First approach will develop regulatory Administer Ad-RTS-hIL12 locally
T cells (Tregs) expressing IL-2 utilizing to achieve systemic anti-tumor
Intrexon’s proprietary gene control effects (breast cancer)
technologies such as its RheoSwitch® Administer Ad-RTS-hIL12 locally
(RTS) platform to achieve local anti-tumor
effects (glioblastoma)
11
Expression of IL-2 through
ActoBiotics® microbes
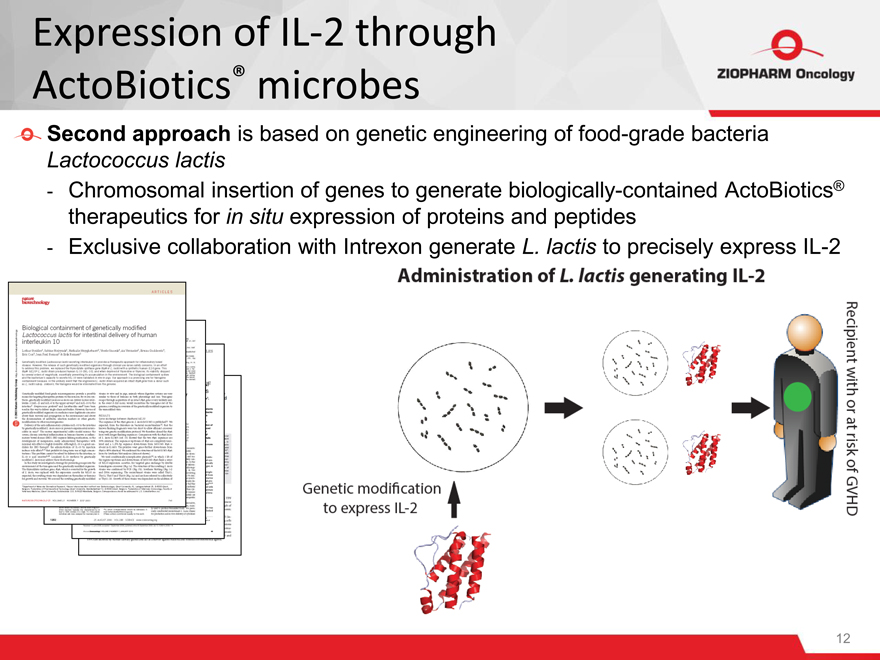
Second approach is based on genetic engineering of food-grade bacteria
Lactococcus lactis
- Chromosomal insertion of genes to generate biologically-contained ActoBiotics® therapeutics for in situ expression of proteins and peptides
- Exclusive collaboration with Intrexon generate L. lactis to precisely express IL-2
12
Targeted ActoBiotics® Delivery to GI Tract
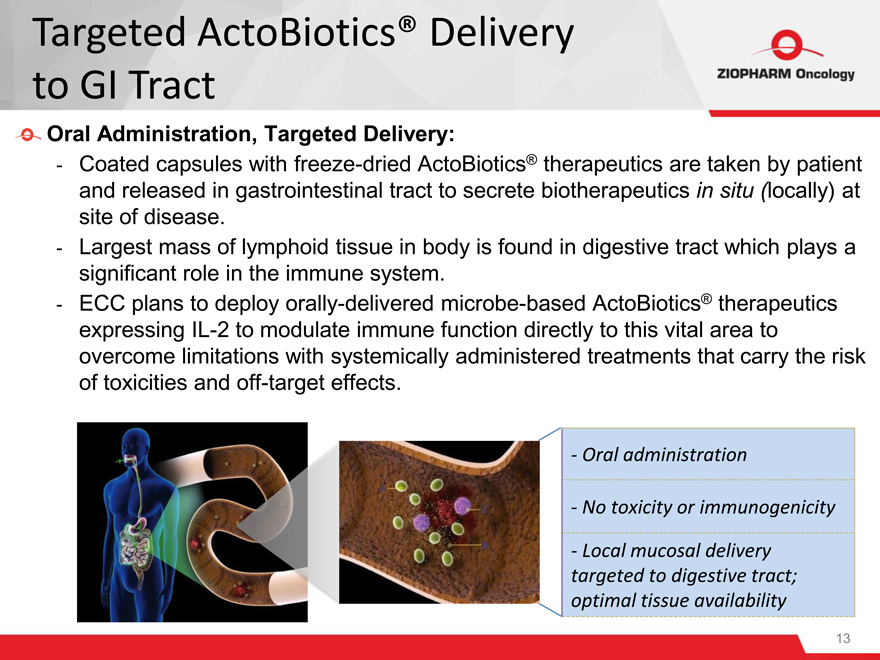
Oral Administration, Targeted Delivery:
- Coated capsules with freeze-dried ActoBiotics® therapeutics are taken by patient and released in gastrointestinal tract to secrete biotherapeutics in situ (locally) at site of disease.
- Largest mass of lymphoid tissue in body is found in digestive tract which plays a significant role in the immune system.
- ECC plans to deploy orally-delivered microbe-based ActoBiotics® therapeutics expressing IL-2 to modulate immune function directly to this vital area to overcome limitations with systemically administered treatments that carry the risk of toxicities and off-target effects.
- Oral administration
- No toxicity or immunogenicity
- Local mucosal delivery targeted to digestive tract; optimal tissue availability
13
Synergies with Existing Programs
Virotherapy T-cell therapy
Genetic modification
Off-the-shelf therapy NK-cell therapy
14
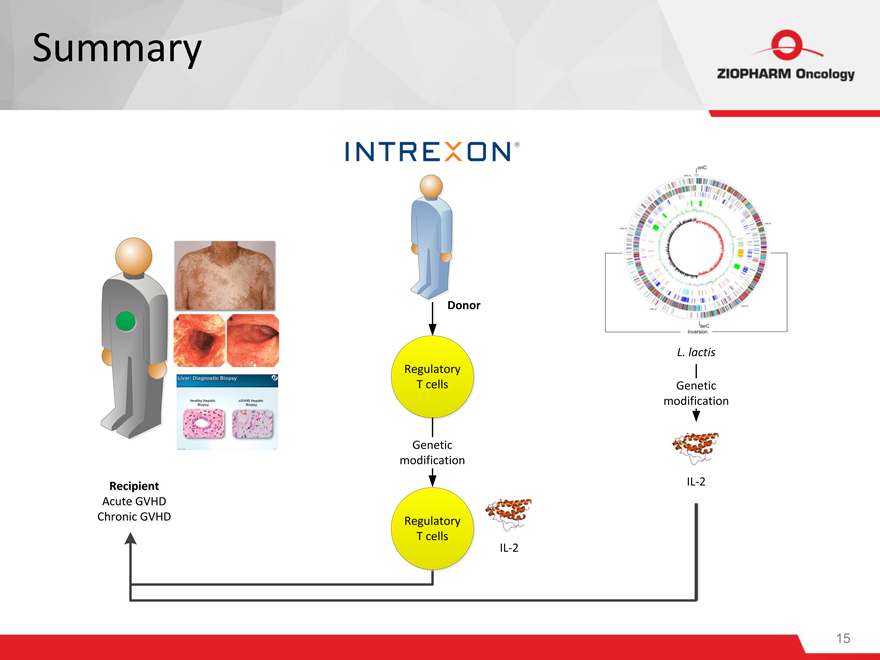
Summary
Donor
L. lactis
Regulatory
T cells Genetic
modification
Genetic
modification
Recipientecipient IL-2
Acutecute GVHD
ChronicChronic GVHD Regulatory
T ce
IL-2
15
Conclusions
Allogeneic HSCT is a widely accepted approach to T-cell therapy that does not rely on a prior knowing the tumor antigens ZIOPHARM is delivering biologics based on genetic modification of the immune system to treat cancer
– This is based on existing ECC with Intrexon
ZIOPHARM will leverage its expertise to deliver biologics to improve the outcome of patients receiving T-cell therapy in the context of allogeneic HSCT
– This is based on a new ECC with Intrexon
The number of recipients that can benefit from allogeneic HSCT can be increased if GVHD can be prevented or treated
– This will impact treatment of oncology as well as non-malignant diseases
16
ZIOPHARM / Intrexon
Graft-Versus-Host Disease
Exclusive Channel Collaboration
SEPTEMBER 28, 2015