|
Exhibit 99.1 
|
Exhibit 99.1
ZIOPHARM
Jefferies Immuno-Oncology Summit
April 2016
Forward-looking statements
This presentation contains certain forward-looking information about ZIOPHARM Oncology, Inc. that is intended to be covered by the safe harbor for “forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” and “believes.” These statements include, but are not limited to, statements regarding the progress, timing and results of preclinical and clinical trials involving the Company’s drug candidates, and the progress of the Company’s research and development programs. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not limited to: whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, or any of our other therapeutic candidates will advance further in the pre-clinical or clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and enforceability of our intellectual property rights; competition from other pharmaceutical and biotechnology companies; and the other risk factors contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2015. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events.
2
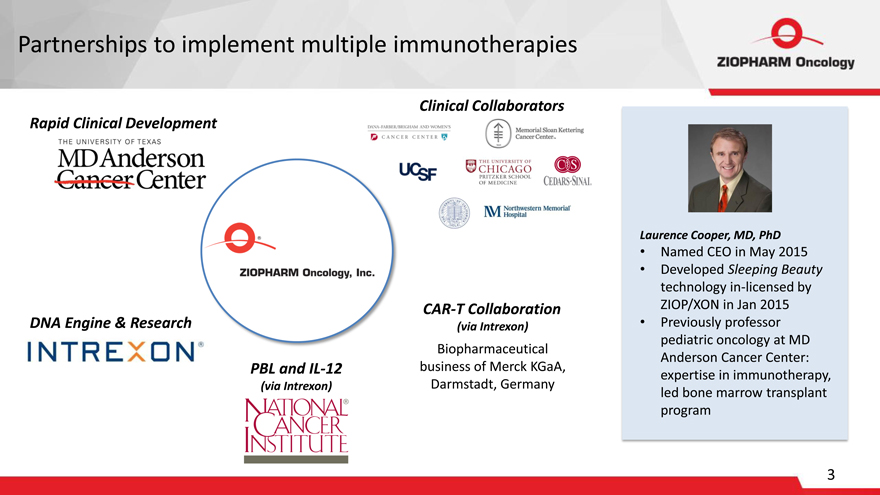
Partnerships to implement multiple immunotherapies
Clinical Collaborators
Rapid Clinical Development
CAR-T Collaboration DNA Engine & Research (via Intrexon)
Biopharmaceutical PBL and IL-12 business of Merck KGaA, (via Intrexon) Darmstadt, Germany
Laurence Cooper, MD, PhD
Named CEO in May 2015
Developed Sleeping Beauty
technology in-licensed by
ZIOP/XON in Jan 2015
Previously professor
pediatric oncology at MD
Anderson Cancer Center:
expertise in immunotherapy,
led bone marrow transplant
program
3
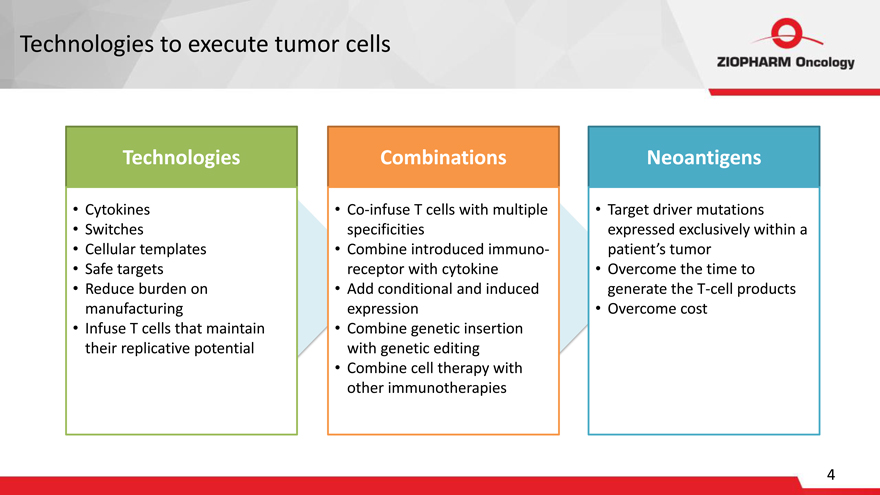
Technologies to execute tumor cells
Technologies
Cytokines Switches
Cellular templates
Safe targets Reduce burden on manufacturing Infuse T cells that maintain their replicative potential
Combinations
Co-infuse T cells with multiple specificities
Combine introduced immuno-receptor with cytokine Add conditional and induced expression Combine genetic insertion with genetic editing
Combine cell therapy with other immunotherapies
Neoantigens
Target driver mutations expressed exclusively within a patient’s tumor
Overcome the time to generate the T-cell products Overcome cost
4
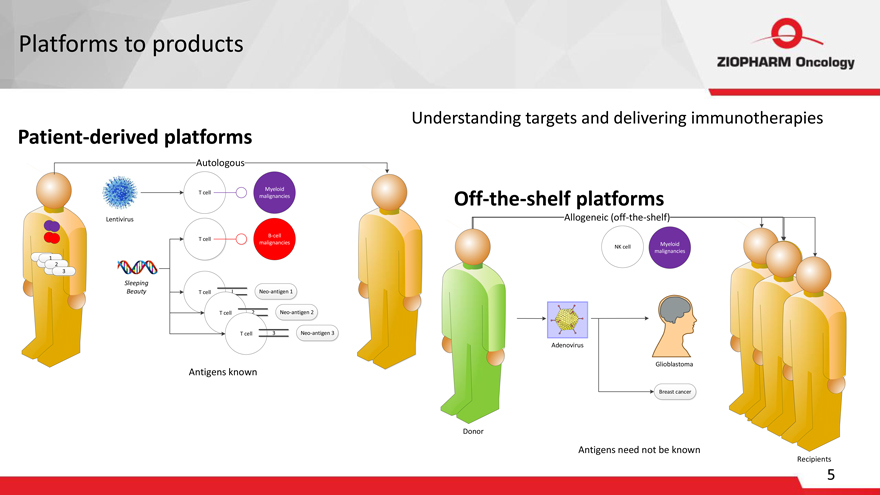
Platforms to products
Patient-derived platforms
Autologous
Myeloid T cell malignancies
Lentivirus
B-cell malignancies
Sleeping
Beauty T cell I Neo-antigen 1 T cell 2 Neo-antigen 2 T cell 3 Neo-antigen 3
Antigens known
Understanding targets and delivering immunotherapies
Off-the-shelf platforms Allogeneic (off-the-shelf)
Myeloid malignancies
Glioblastoma
Breast cancer
Donor
not be known
Recipients
5
NK cell Adenovirus Antigens need
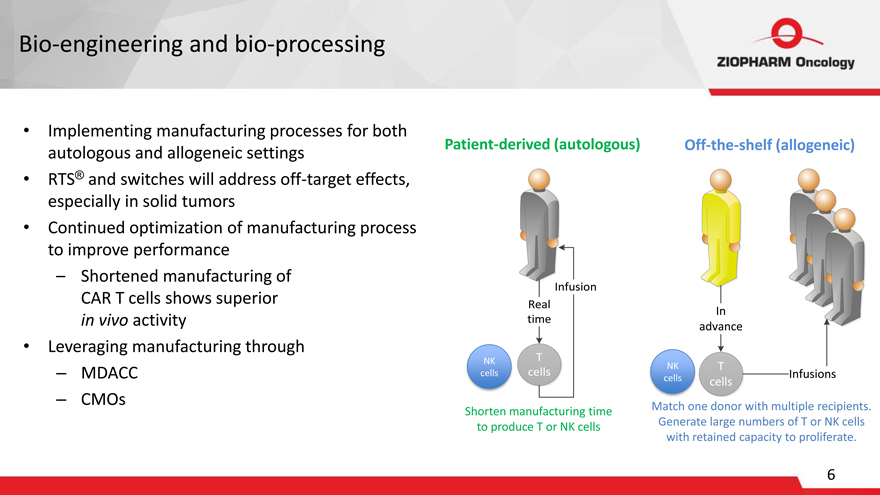
Bio-engineering and bio-processing
Implementing manufacturing processes for both autologous and allogeneic settings RTS® and switches will address off-target effects, especially in solid tumors Continued optimization of manufacturing process to improve performance
– Shortened manufacturing of CAR T cells shows superior in vivo activity
Leveraging manufacturing through
– MDACC
– CMOs
Patient-derived (autologous)
Infusion Real time
NK T cells cells
Shorten manufacturing time to produce T or NK cells
Off-the-shelf (allogeneic)
In advance
NK T
cells Infusions cells
Match one donor with multiple recipients. Generate large numbers of T or NK cells with retained capacity to proliferate.
6
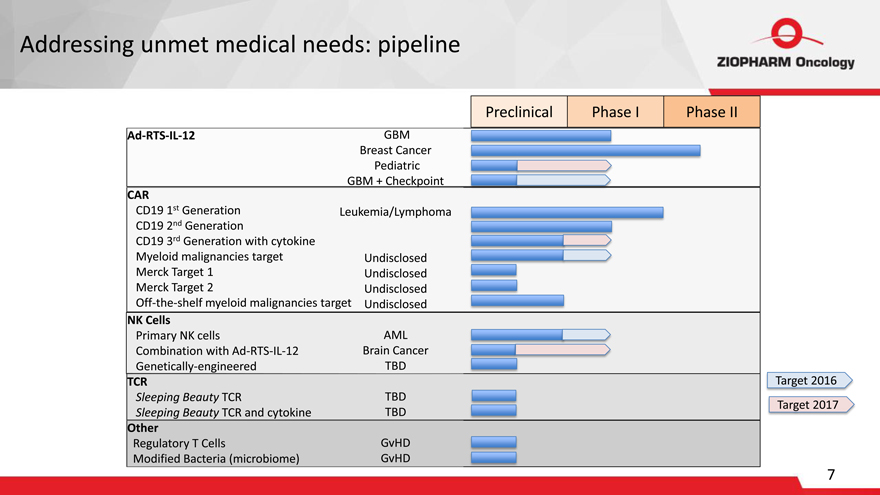
Addressing unmet medical needs: pipeline
Preclinical Phase I Phase II
Ad-RTS-IL-12 GBM
Breast Cancer
Pediatric
GBM + Checkpoint
CAR
CD19 1st Generation Leukemia/Lymphoma
CD19 2nd Generation
CD19 3rd Generation with cytokine
Myeloid malignancies target Undisclosed
Merck Target 1 Undisclosed
Merck Target 2 Undisclosed
Off-the-shelf myeloid malignancies target Undisclosed
NK Cells
Primary NK cells AML
Combination with Ad-RTS-IL-12 Brain Cancer
Genetically-engineered TBD
TCR Target 2016
Sleeping Beauty TCR TBD
Target 2017
Sleeping Beauty TCR and cytokine TBD
Other
Regulatory T Cells GvHD
Modified Bacteria (microbiome) GvHD
7
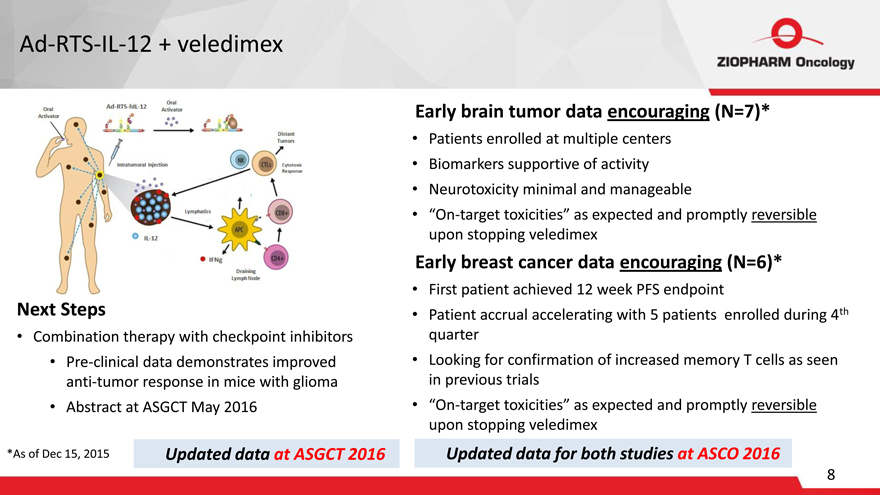
Ad-RTS-IL-12 + veledimex
Oral Activator Ad-RTS-hiL-12 Oral Activator Distant Tumors Cytotoxic Response Lymphatics IL-12 IFNg APC CD8+ CD4+
Next Steps
Combination therapy with checkpoint inhibitors
Pre-clinical data demonstrates improved
anti-tumor response in mice with glioma
Abstract at ASGCT May 2016
*As of Dec 15, 2015 Updated data at ASGCT 2016
Early brain tumor data encouraging (N=7)*
Patients enrolled at multiple centers Biomarkers supportive of activity Neurotoxicity minimal and manageable
“On-target toxicities” as expected and promptly reversible upon stopping veledimex
Early breast cancer data encouraging (N=6)*
First patient achieved 12 week PFS endpoint
Patient accrual accelerating with 5 patients enrolled during 4th quarter Looking for confirmation of increased memory T cells as seen in previous trials
“On-target toxicities” as expected and promptly reversible upon stopping veledimex
Updated data for both studies at ASCO 2016
8
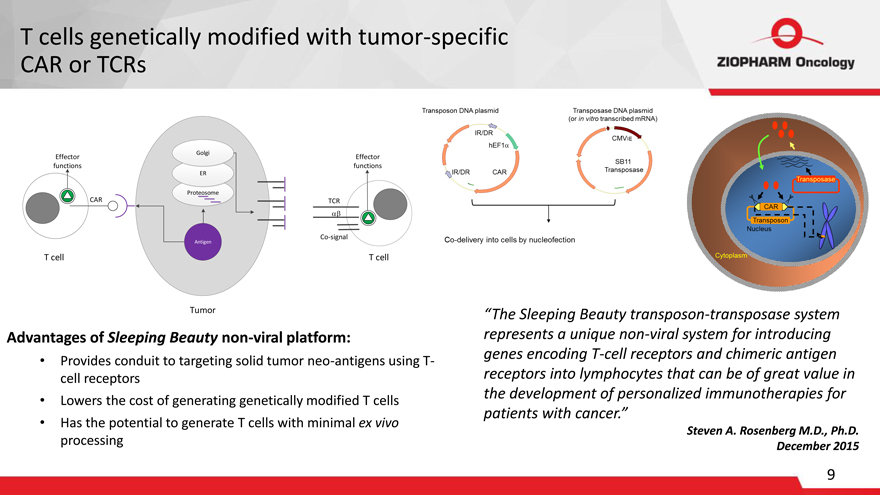
T cells genetically modified with tumor-specific CAR or TCRs
Golgi
Effector Effector functions functions
ER
Proteosome
CAR TCR
Antigen Co-signal
T cell T cell
Tumor
Transposon DNA plasmid IR/DR IR/DR CAR hEF1
Transposase DNA plasmid (or in vitro transcribed mRNA) CMVIE SB11 Transposase Co-delivery into cells by nucleofection
Transposase Transposon CAR Nucleus Cytoplasm “The Sleeping Beauty transposon-transposase system represents a unique non-viral system for introducing genes encoding T-cell receptors and chimeric antigen receptors into lymphocytes that can be of great value in the development of personalized immunotherapies for patients with cancer.”
Steven A. Rosenberg M.D., Ph.D. December 2015
Advantages of Sleeping Beauty non-viral platform:
Provides conduit to targeting solid tumor neo-antigens using T-
cell receptors
Lowers the cost of generating genetically modified T cells
Has the potential to generate T cells with minimal ex vivo
processing
9
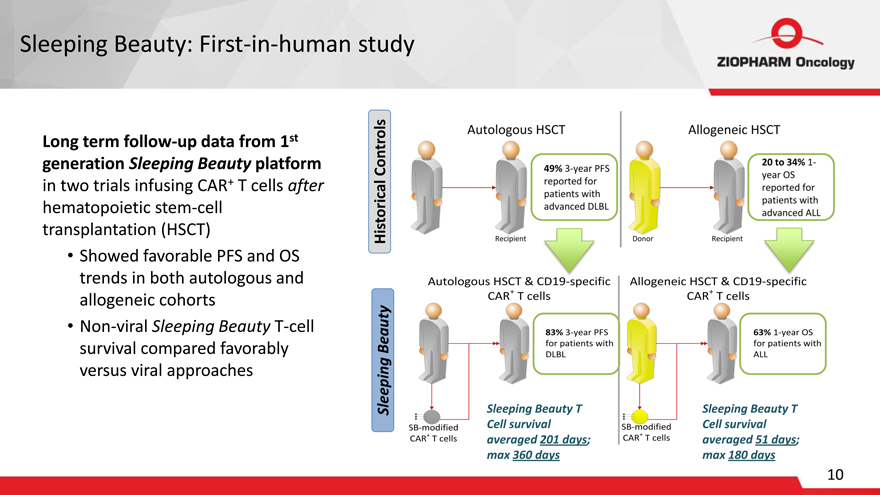
Sleeping Beauty: First-in-human study
Long term follow-up data from 1st generation Sleeping Beauty platform in two trials infusing CAR+ T cells after hematopoietic stem-cell transplantation (HSCT)
Showed favorable PFS and OS trends in both autologous and allogeneic cohorts
Non-viral Sleeping Beauty T-cell survival compared favorably versus viral approaches
Historical Controls
Sleeping Beauty
Autologous HSCT
Recipient 49% 3-year PFS reported for patients with advanced DLBL
Recipient Donor Allogeneic HSCT 20 to 34% 1-year Os reported for patients with advanced ALL
Autologous HSCT & CD19-specific CAR+T cells 83% 3-year PFS for patients with DLBL SB-modified CAR+T cells Allogeneic HSCT & CD19-specific CAR+T cells 63% 1-year Os for patients with ALL
SB-modified CAR+T cells
Sleeping Beauty T Cell survival averaged 201 days; max 360 days
Sleeping Beauty T Cell survival averaged 51 days; max 180 days
10
Intrexon/Merck KGaA, Darmstadt, Germany in CAR-T
Exclusive agreement to develop and commercialize CAR-T cancer therapies 2 novel CAR T targets nominated
Merck KGaA, Darmstadt, Germany to lead IND filing and pre-IND interactions, clinical development and commercialization Intrexon and ZIOPHARM retain ability to explore targets independently, granting Merck KGaA opt-in rights during clinical development Economics divided evenly between ZIOPHARM and Intrexon
$413 million per product in milestones
Tiered royalties up to lower-double digits on net sales
11
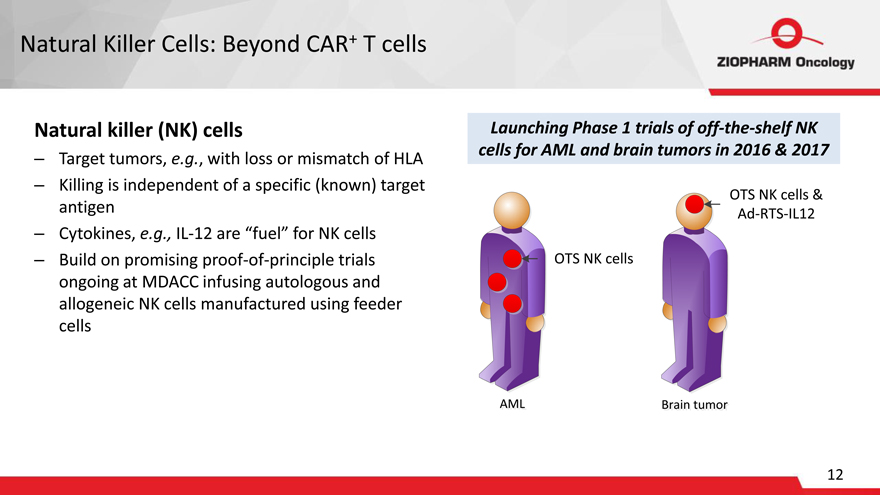
Natural Killer Cells: Beyond CAR+ T cells
Natural killer (NK) cells
– Target tumors, e.g., with loss or mismatch of HLA
– Killing is independent of a specific (known) target antigen
– Cytokines, e.g., IL-12 are “fuel” for NK cells
– Build on promising proof-of-principle trials ongoing at MDACC infusing autologous and allogeneic NK cells manufactured using feeder cells
Launching Phase 1 trials of off-the-shelf NK cells for AML and brain tumors in 2016 & 2017
OTS NK cells & Ad-RTS-IL12
OTS NK cells
AML L Brain Brain tumor tu or
12
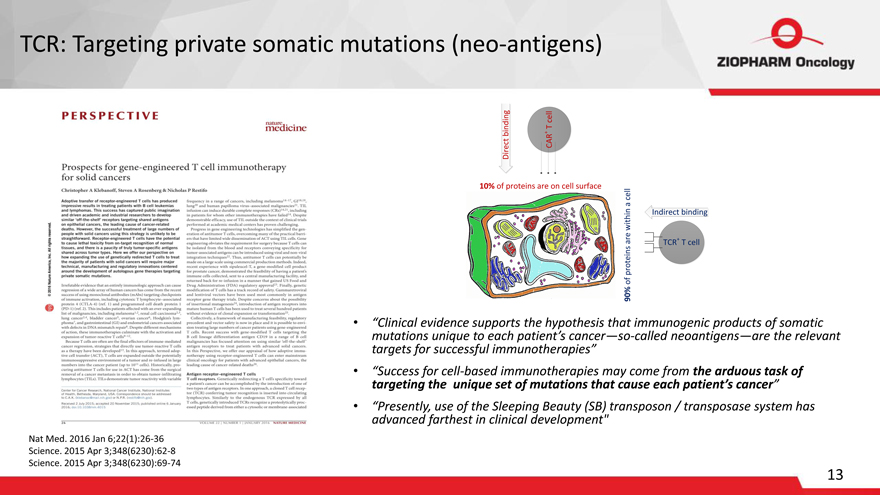
TCR: Targeting private somatic mutations (neo-antigens)
PERSPECTIVE nature medicine Prospects for gene-engineered T cell immunotherapy for solid cancers
Nat Med. 2016 Jan 6;22(1):26-36
Science. 2015 Apr 3;348(6230):62-8
Science. 2015 Apr 3;348(6230):69-74
Direct binding
CAR+ T cell
10% of proteins are on cell surface
Indirect binding
TCR+ T cell
90% of proteins are within a cell
“Clinical evidence supports the hypothesis that immunogenic products of somatic mutations unique to each patient’s cancer—so-called neoantigens—are the relevant targets for successful immunotherapies”
“Success for cell-based immunotherapies may come from the arduous task of targeting the unique set of mutations that cause each patient’s cancer”
“Presently, use of the Sleeping Beauty (SB) transposon / transposase system has advanced farthest in clinical development”
13
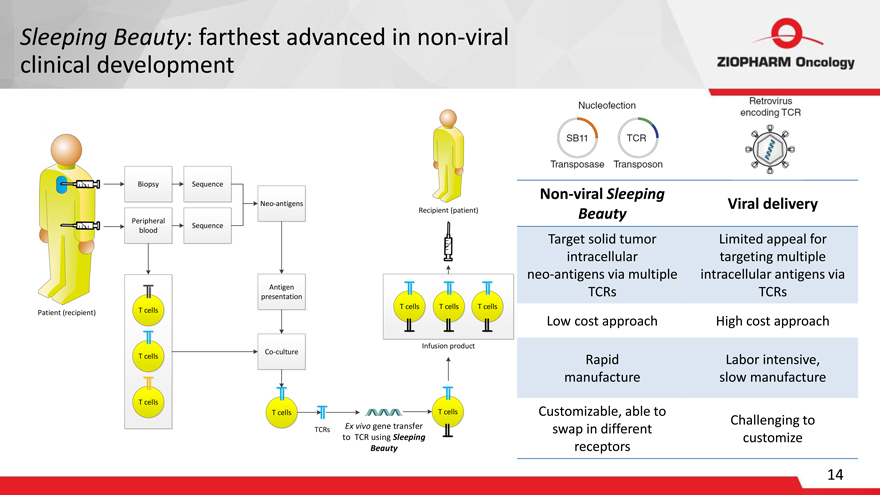
Sleeping Beauty: farthest advanced in non-viral clinical development
Patient (recipient) Biopsy Sequence Peripheral blood Sequence Neo-antigens T cells T cells
T cells
Antigen presentation Co-culture T cells
T cells
T cells
T cells
T cells
TCRs Ex vivo gene transfer to TCR using Sleeping Beauty
Nucleofection SB11 TCR Transposase Transposon Retrovirus encoding TCR
Non-viral Sleeping
Viral delivery
Beauty
Target solid tumor Limited appeal for intracellular targeting multiple neo-antigens via multiple intracellular antigens via TCRs TCRs
Low cost approach High cost approach
Rapid Labor intensive, manufacture slow manufacture
Customizable, able to
Challenging to swap in different customize receptors
14
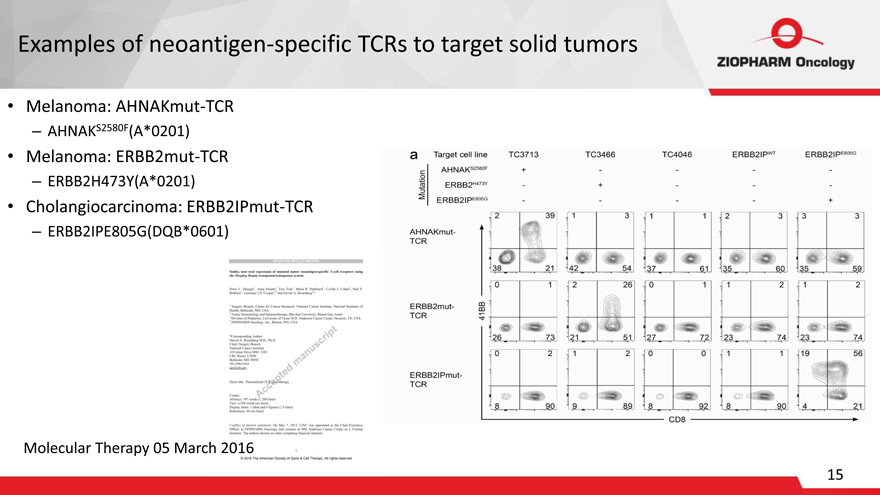
Examples of neoantigen-specific TCRs to target solid tumors
Melanoma: AHNAKmut-TCR
AHNAKS2580F(A*0201)
Melanoma: ERBB2mut-TCR
ERBB2H473Y(A*0201)
Cholangiocarcinoma: ERBB2IPmut-TCR
ERBB2IPE805G(DQB*0601)
Molecular Therapy 05 March 2016
15
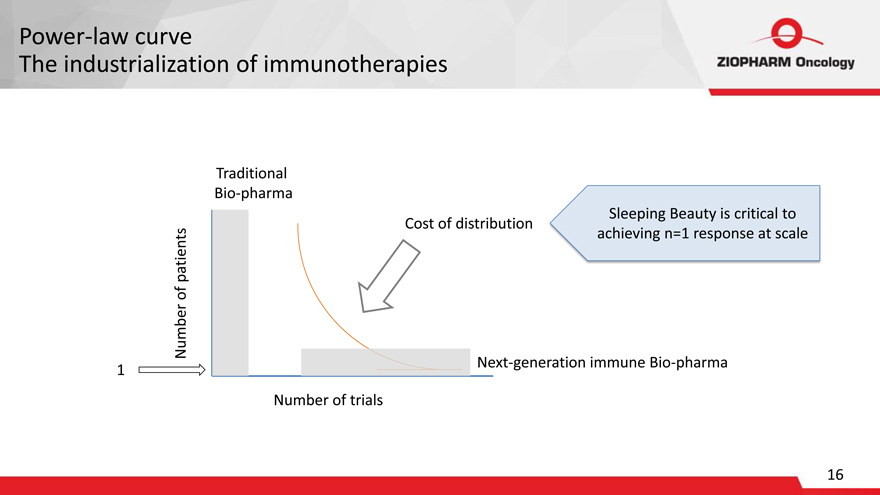
Power-law curve
The industrialization of immunotherapies
Traditional Bio-pharma
Sleeping Beauty is critical to patients Cost of distribution achieving n=1 response at scale of Number Next-generation immune Bio-pharma 1
Number of trials
16
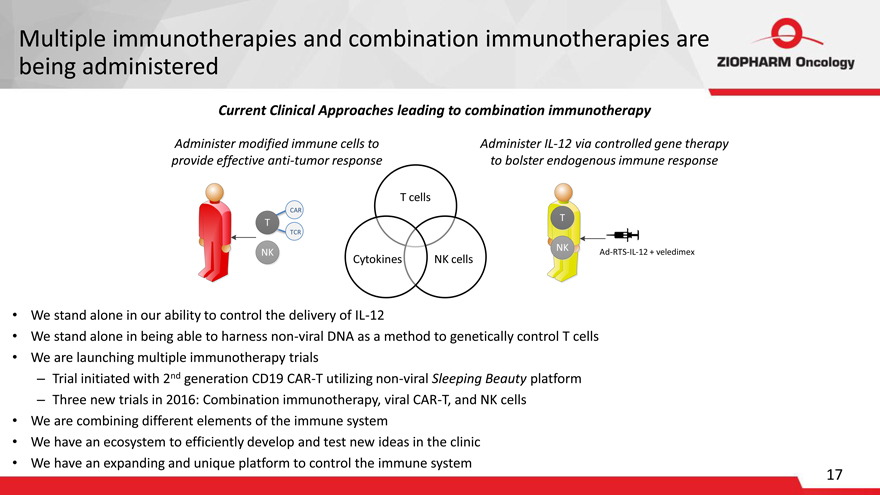
Multiple immunotherapies and combination immunotherapies are being administered
Current Clinical Approaches leading to combination immunotherapy
Administer modified immune cells to Administer IL-12 via controlled gene therapy provide effective anti-tumor response to bolster endogenous immune response
T cells
CAR
T T
TCR
NK
NK Ad-RTS-IL-12 + veledimex
Cytokines NK cells
We stand alone in our ability to control the delivery of IL-12
We stand alone in being able to harness non-viral DNA as a method to genetically control T cells
We are launching multiple immunotherapy trials
Trial initiated with 2nd generation CD19 CAR-T utilizing non-viral Sleeping Beauty platform
Three new trials in 2016: Combination immunotherapy, viral CAR-T, and NK cells
We are combining different elements of the immune system
We have an ecosystem to efficiently develop and test new ideas in the clinic
We have an expanding and unique platform to control the immune system
17
ZIOPHARM
Jefferies Immuno-Oncology Summit
April 2016