Exhibit 99.1

ZIOPHARM ONCOLOGY
35th Annual J.P. Morgan
Healthcare Conference
January 11, 2017

2
Forward-looking Statements
This presentation contains certain forward-looking information about ZIOPHARM Oncology, Inc. that is intended to be covered by the safe harbor for “forward-looking statements” provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” and “believes.” These statements include, but are not limited to, statements regarding the progress, timing and results of preclinical and clinical trials involving the Company’s drug candidates, and the progress of the Company’s research and development programs. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not limited to: whether chimeric antigen receptor T cell (CAR T) approaches,Ad-RTS-IL-12, TCR and NK cell-based therapies, or any of our other therapeutic candidates will advance further in thepre-clinical or clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether chimeric antigen receptor T cell (CAR T) approaches,Ad-RTS-IL-12, TCR and NK cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and enforceability of our intellectual property rights; competition from other pharmaceutical and biotechnology companies; and the other risk factors contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form10-K for the fiscal year ended December 31, 2015, and our Quarterly Report on Form10-Q for the quarter ended September 30, 2016. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of ornon-occurrence of any events.
ZIOPHARM Oncology

3
Cost and Control
The next generation ofDNA-based immunotherapies will need to master cost and control
Bloomberg Markets Tech Pursuits Politics Opinion Businessweek
Deaths in Cancer Trials Remain Mystery for Promising Therapy
Opinion: Balancing Risks and Rewards
of CART-Cell Therapy
New approaches to treating cancer have shown great promise, but they also come with serious risks that give us cause for concern.
By David Harris | December 15, 2016
Analys Questions High Cost of T Cell Therapies as Potential Frontline Treatment for Blood Cancers
Experimental cancer therapy holds great promise- but at great cost
FiercePharma
What do theCAR-T Patient Deaths Mean for the Future of the Field?
others face higher manufacturing costs withCAR-T cell treatments
ZIOPHARM Oncology
4
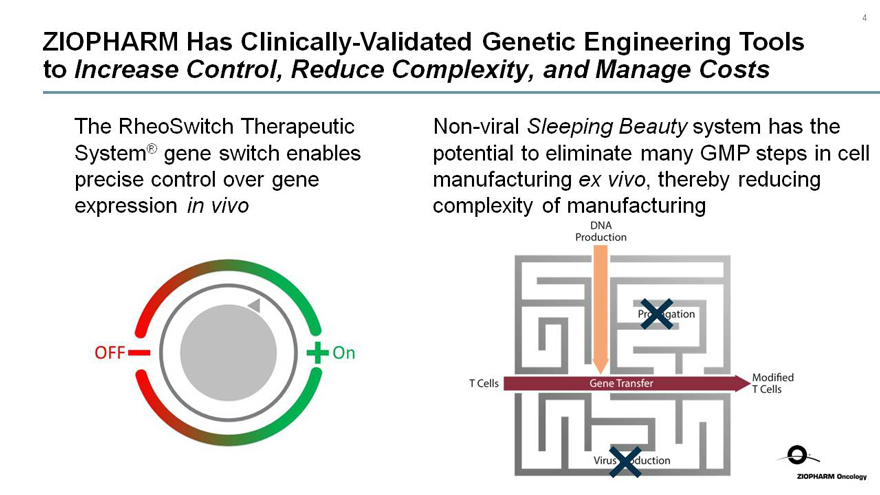
ZIOPHARM Has Clinically-Validated Genetic Engineering Tools to Increase Control, Reduce Complexity, and Manage Costs
The RheoSwitch Therapeutic System® gene switch enables precise control over gene expression in vivo
OFF
ON
Non-viral Sleeping Beauty system has the potential to eliminate many GMP steps in cell manufacturing ex vivo, thereby reducing complexity of manufacturing
DNA Production
T Cells
Gene Transfer
Modified T Cells
Virus
ZIOPHARM Oncology
5
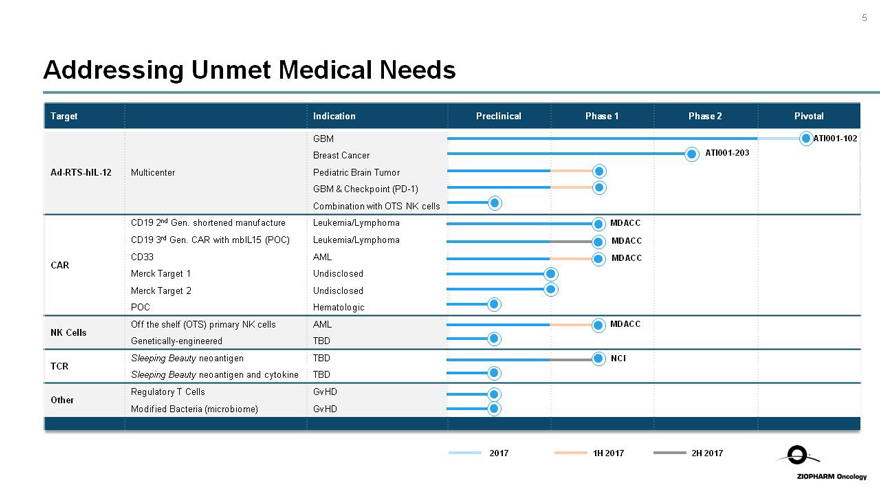
Addressing Unmet Medical Needs
Target
Indication
Preclinical
Phase 1
Phase 2
Pivotal
Ad-RTS-hIL-12
Multicenter
GBM
Breast Cancer
Pediatric Brain Tumor
GBM & Checkpoint(PD-1)
Combination with OTS NK cells
ATI001-102
ATI001-203
CAR
CD19 2nd Gen. shortened manufacture
CD19 3rd Gen. CAR with mbIL15 (POC)
CD33
Merck Target 1
Merck Target 2
POC
Leukemia/Lymphoma
Leukemia/Lymphoma
AML
Undisclosed
Undisclosed
Hematologic
MDACC
MDACC
MDACC
NK Cells
Off the shelf (OTS) primary NK cells
Genetically-engineered
AML
TBD
MDACC
TCR
Sleeping Beauty neoantigen
Sleeping Beauty neoantigen and cytokine
TBD
TBD
NCI
Other
Regulatory T Cells
Modified Bacteria (microbiome)
GvHD
GvHD
2017
1H 2017
2H 2017
ZIOPHARM Oncology

Control
Ad-RTS-hIL-12 + Veledimex
ZIOPHARM Oncology
7
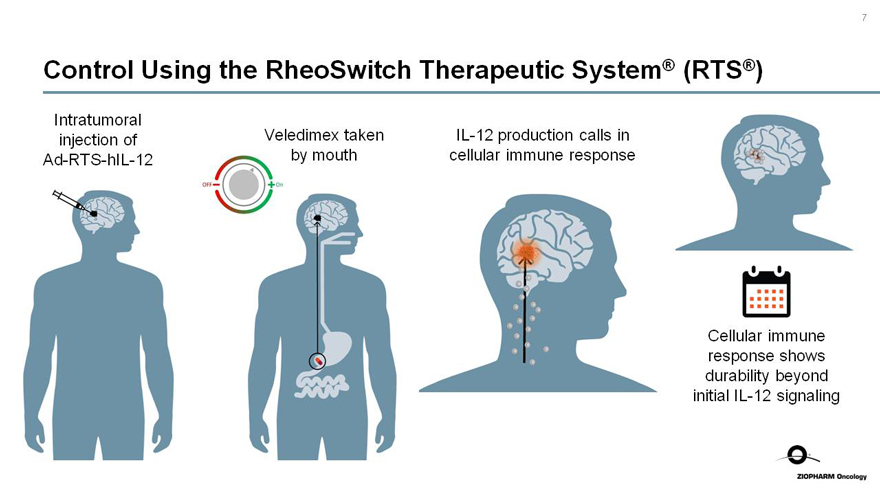
Control Using the RheoSwitch Therapeutic System® (RTS®)
Intratumoral injection ofAd-RTS-hIL-12
Veledimex taken by mouth
OFF On
IL-12 production calls in cellular immune response
Cellular immune response shows durability beyond initialIL-12 signaling
ZIOPHARM Oncology
8
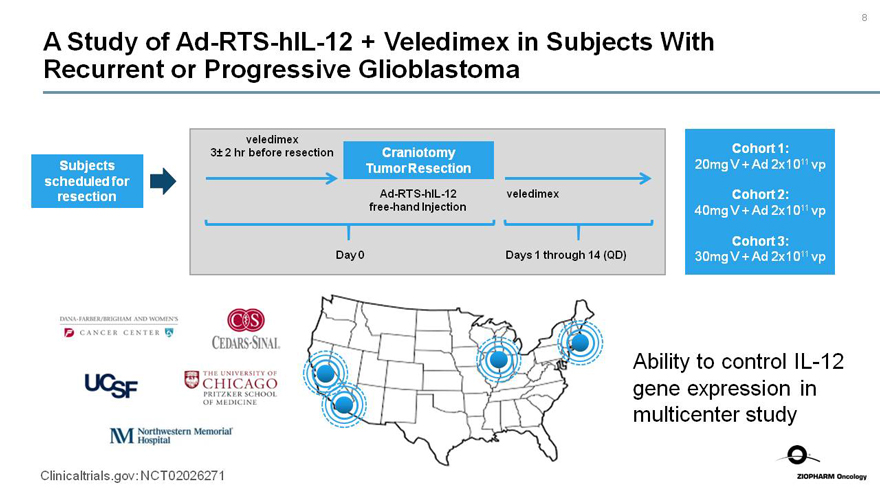
A Study ofAd-RTS-hIL-12 + Veledimex in Subjects With
Recurrent or Progressive Glioblastoma
Subjects scheduled for resection
veledimex 3± 2 hr before resection
Craniotomy Tumor Resection
Ad-RTS-hIL-12
free-hand Injection
veledimex
Day 0
Days 1 through 14 (QD)
Cohort 1:
20mg V + Ad 2x1011 vp
Cohort 2:
40mg V + Ad 2x1011 vp
Cohort 3:
30mg V + Ad 2x1011 vp
DANA-FARBER/BRIGHAM AND WOMEN’S
CANCER CENTER
CEDARS-SINAI
UCSF
THE UNIVERSITY OF
CHICAGO
PRITZKER SCHOOL OF MEDICINE
Northwestern Memorial Hospital Ability to controlIL-12 gene expression in multicenter study
Clinicaltrials.gov: NCT02026271
ZIOPHARM Oncology
9
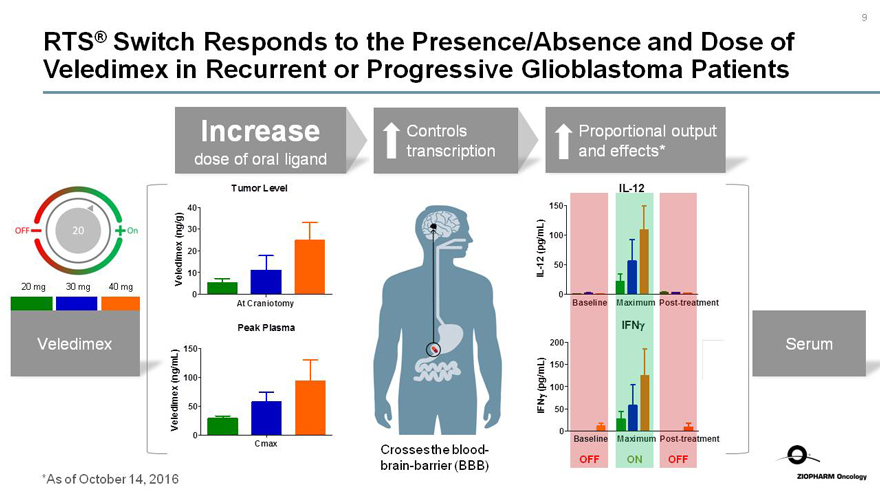
RTS® Switch Responds to the Presence/Absence and Dose of Veledimex in Recurrent or Progressive Glioblastoma Patients
OFF 20 On
20 mg 30 mg 40 mg
Veledimex
Increase
dose of oral ligand
Tumor Level
Veledime (ng/g)
40 30 20 10 0
At Craniotomy
Peak Plasma
Veledime (ng/mL)
150 100 50 0
Cmax
Controls
transcription
Crosses the blood-
brain-barrier (BBB)
Proportional output and effects*
IL-12(pg/mL)
IL-12
150 100 50 0
Baseline
Maximum
Post-treatment
IFNy
IFNy (pg/ML)
200 150 100 50 0
Baseline
Maximum
Post-treatment
OFF
ON
OFF
Serum
*As of October 14, 2016
ZIOPHARM Oncology
10
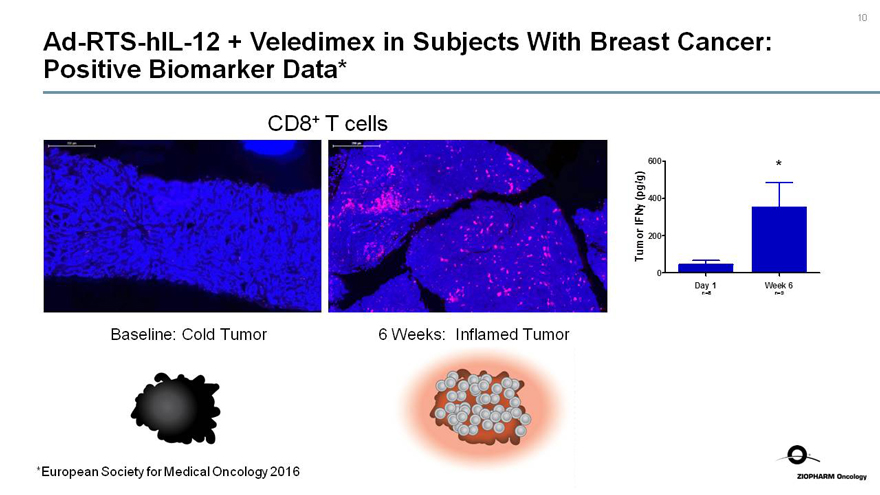
Ad-RTS-hIL-12 + Veledimex in Subjects With Breast Cancer: Positive Biomarker Data*
CD8+ T cells
Baseline: Cold Tumor
6 Weeks: Inflamed Tumor
Tumor IFNy (pg/g)
600
400
200
0
*
Day 1
Week 6
n=8
n=9
*European Society for Medical Oncology 2016
ZIOPHARM Oncology
11
Interim Study Results
Based on tolerability and survival benefit (median OS=12.7 months, n=15), 20 mg was selected for an expansion cohort and we are following patients’ overall survival data*
Ad-RTS-hIL-12 + veledimex is well tolerated and suggests a survival benefit over historical controls at 6, 9, and 12 months (median OS=9.6 months, n=25)
Toxicities were tolerable, predictable and reversible upon discontinuing veledimex
There is a strong correlation between veledimex dose, BBB penetration, andIL-12 production
These data demonstrate that the RTS® gene switch works in humans toggling not only as a switch to turn on and off the production ofIL-12, but also as a rheostat to control the level ofIL-12
* As of January 6, 2017
ZIOPHARM Oncology
12
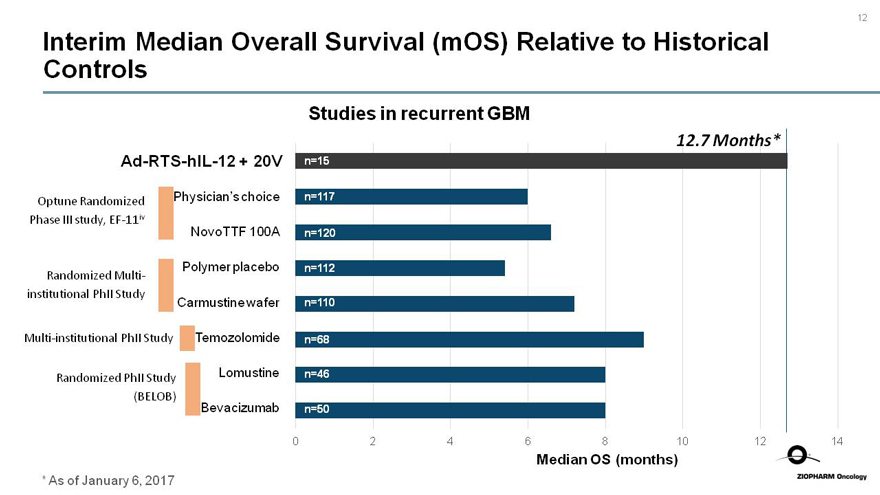
Interim Median Overall Survival (mOS) Relative to Historical Controls
Studies in recurrent GBM
12.7 Months*
Ad-RTS-hIL-12 + 20V n=15
Optune Randomized Phase III study,EF-11iv Physician’s choice n=117
NovoTTF 100A n=120
Polymer placebo n=112
Randomized Multi-institutional PhII Study
Carmustine wafer n=110
Multi-institutional PhII Study Temozolomide n=68
Randomized PhII Study (BELOB) Lomustine n=46
Bevacizumab n=50
0 2 4 6 8 10 12 14
Median OS (months)
* As of January 6, 2017
ZIOPHARM Oncology
13
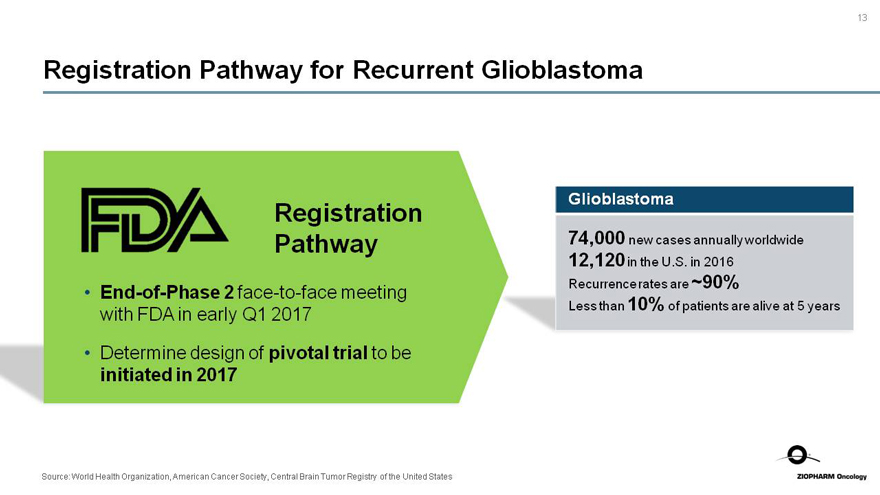
Registration Pathway for Recurrent Glioblastoma
FDA Registration
Pathway
End-of-Phase 2face-to-face meeting with FDA in early Q1 2017
Determine design of pivotal trial to be initiated in 2017
Glioblastoma
74,000 new cases annually worldwide
12,120 in the U.S. in 2016
Recurrence rates are ~90%
Less than 10% of patients are alive at 5 years
Source: World Health Organization, American Cancer Society, Central Brain Tumor Registry of the United States
ZIOPHARM Oncology
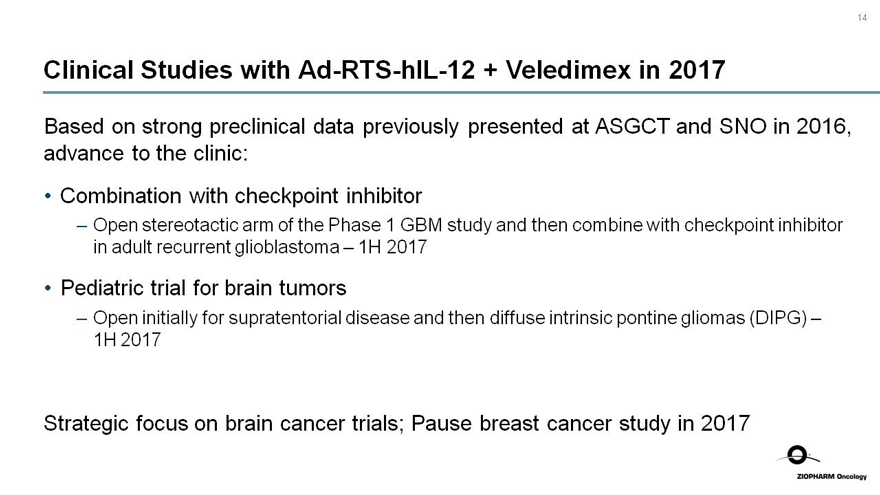
14
Clinical Studies withAd-RTS-hIL-12 + Veledimex in 2017
Based on strong preclinical data previously presented at ASGCT and SNO in 2016, advance to the clinic:
Combination with checkpoint inhibitor
- Open stereotactic arm of the Phase 1 GBM study and then combine with checkpoint inhibitor in adult recurrent glioblastoma – 1H 2017
Pediatric trial for brain tumors
- Open initially for supratentorial disease and then diffuse intrinsic pontine gliomas (DIPG) – 1H 2017
Strategic focus on brain cancer trials; Pause breast cancer study in 2017
ZIOPHARM Oncology
15
Cost and Control
Competitive Edge in Cell Therapy
ZIOPHARM Oncology
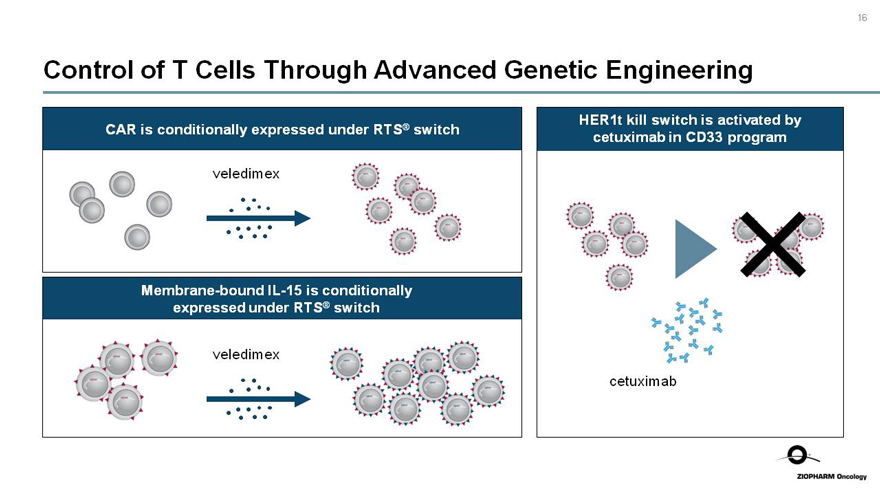
16
Control of T Cells Through Advanced Genetic Engineering
CAR is conditionally expressed under RTS® switch
veledimex
Membrane-boundIL-15 is conditionally expressed under RTS® switch
veledimex
HER1t kill switch is activated by
cetuximab in CD33 program
cetuximab
ZIOPHARM Oncology
17
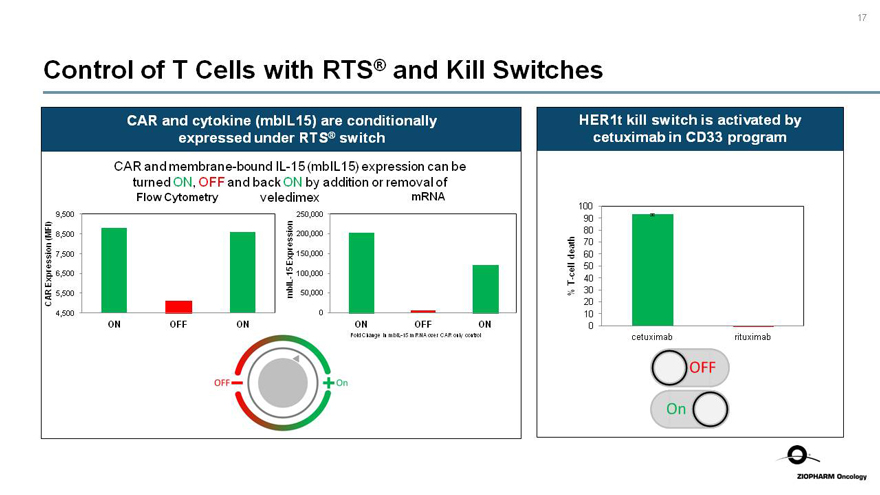
Control of T Cells with RTS® and Kill Switches
CAR and cytokine (mbIL15) are conditionally expressed under RTS® switch
CAR and membrane-boundIL-15 (mbIL15) expression can be turned ON, OFF and back ON by addition or removal of
Flow Cytometry veledimex mRNA
CAR Expression (MFI)
mbIL-15 Expression
9,500 250,000
8,500 200,000
7,500 150,000
6,500 100,000
5,500 50,000
4,500 0
ON OFF ON ON OFF ON
OFF
On
Fold Change inmbIL-15 mRNA over CAR only control
HER1t kill switch is activated by cetuximab in CD33 program
%T-cell death
H
100
90
80
70
60
50
40
30
20
10
0
cetuximab rituximab
OFF
On
ZIOPHARM Oncology
18
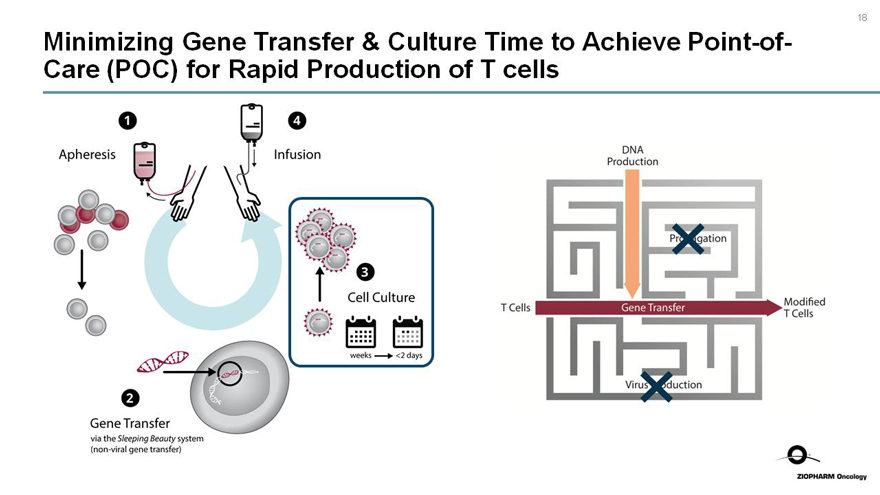
Minimizing Gene Transfer & Culture Time to AchievePoint-of-Care (POC) for Rapid Production of T cells
1 Apheresis
4 Infusion
2 Gene Transfer
via the Sleeping Beauty system
(non-viral gene transfer)
3 Cell Culture
weeks <2 days
DNA Production
X
Propagation
T Cells
Gene Transfer
Modified T Cells
X
Virus Production
ZIOPHARM Oncology

Cost and Control
CD19 CAR+ T: Improving Autologous Cell Therapies
ZIOPHARM Oncology
20
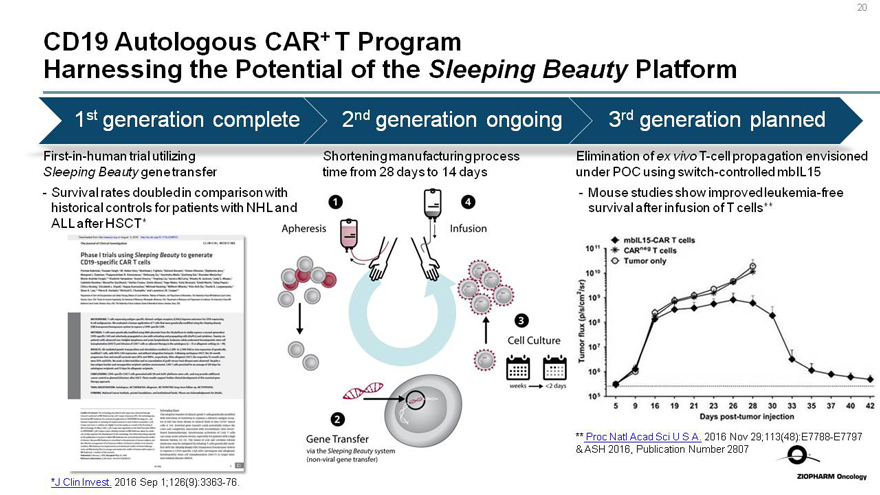
CD19 Autologous CAR+ T Program
Harnessing the Potential of the Sleeping Beauty Platform
1st generation complete
First-in-human trial utilizing
Sleeping Beauty gene transfer
- Survival rates doubled in comparison with historical controls for patients with NHL and ALL after HSCT*
*J Clin Invest. 2016 Sep1;126(9):3363-76.
2nd generation ongoing
Shortening manufacturing process time from 28 days to 14 days
Apheresis
Infusion
Cell Structure
weeks <2 days 1 2 3 4
Gene Transfer
Via the Sleeping Beauty system
(non-viral gene transfer)
3rd generation planned
Elimination of ex vivoT-cell propagation envisioned under POC using switch-controlled mbIL15
- Mouse studies show improved leukemia-free survival after infusion of T cells**
Tumor flux (p/s/cm2/sr)
1011
1010
109
108
107
106
105
mbIL15-CAR T cells
CARneg T cells
Tumor only
5 9 16 19 21 23 26 28 30 33 35 37 40 42
Days post-tumor injection
** Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7788-E7797
& ASH 2016, Publication Number 2807
ZIOPHARM Oncology
21
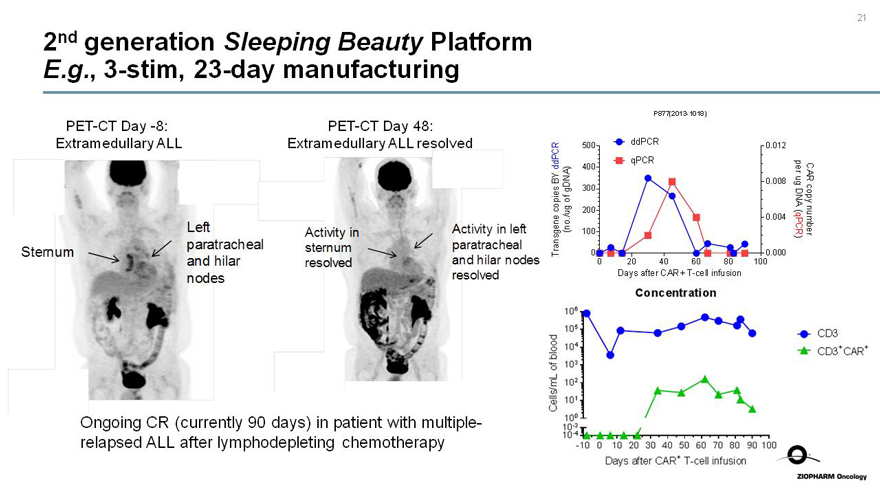
2nd generation Sleeping Beauty Platform E.g.,3-stim,23-day manufacturing
PET-CT Day-8:
Extramedullary ALL
Left
Sternum paratracheal
and hilar
nodes
PET-CT Day 48:
Extramedullary ALL resolved
Activity in Activity in left
sternum paratracheal
resolved and hilar nodes
resolved
P877(2013-1018)
Transgene copies BY ddPCR (no./ug of gDNA)
ddPCR
500 0.012
qPCR
400
0.008
300
200
0.004
100
0 0.000
0 20 40 60 80 100
Days after CAR+T-cell infusion
Concentration
Cells/mL of blood
106
105
104
103
102
101
100
10-3
10-4
-10
0
10
20
30
40
50
60
70
80
90
100
Days after CAR+T-cell infusion
CD3+CAR+
Ongoing CR (currently 90 days) in patient with multiple-relapsed ALL after lymphodepleting chemotherapy
ZIOPHARM Oncology

Technologies Extend to Other Immunologic Therapies
INTREXON
ZIOPHARM Oncology
23
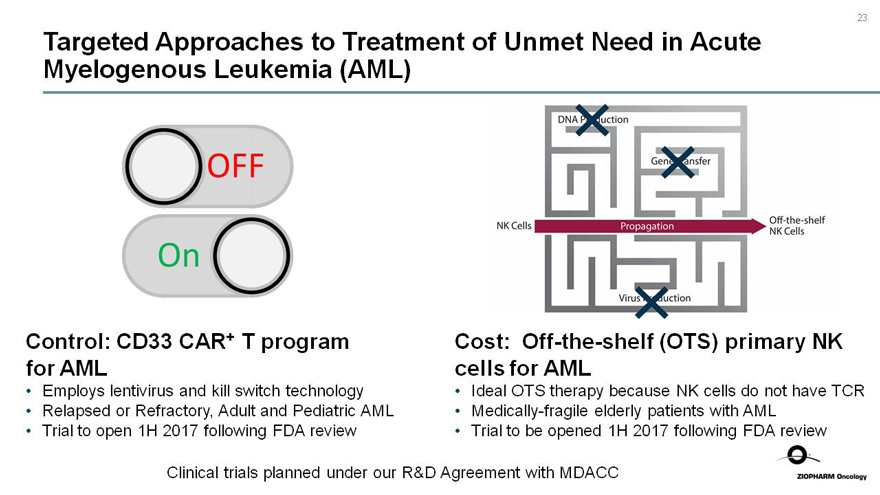
Targeted Approaches to Treatment of Unmet Need in Acute Myelogenous Leukemia (AML)
OFF
On
Control: CD33 CAR+ T program for AML
Employs lentivirus and kill switch technology
Relapsed or Refractory, Adult and Pediatric AML
Trial to open 1H 2017 following FDA review
NK Cells Propagationoff-the-shelf
NK Cells
Cost:Off-the-shelf (OTS) primary NK cells for AML
Ideal OTS therapy because NK cells do not have TCR
Medically-fragile elderly patients with AML
Trial to be opened 1H 2017 following FDA review
Clinical trials planned under our R&D Agreement with MDACC
ZIOPHARM Oncology
24
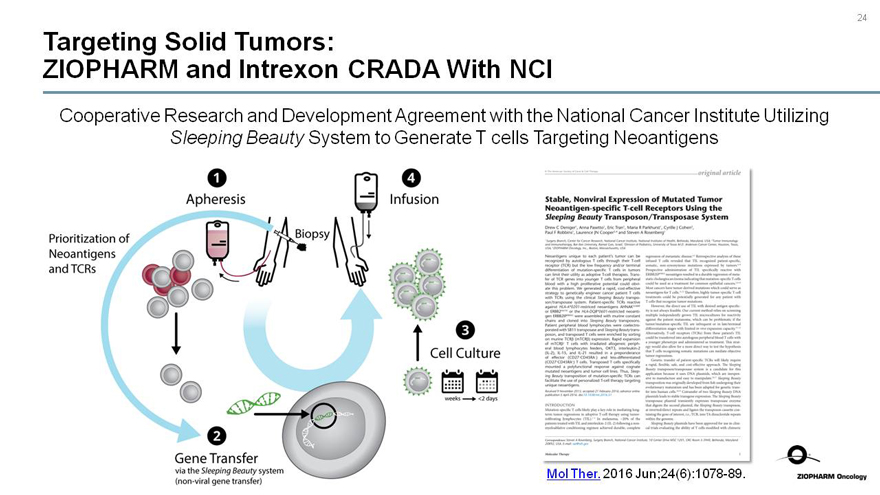
Targeting Solid Tumors:
ZIOPHARM and Intrexon CRADA With NCI
Cooperative Research and Development Agreement with the National Cancer Institute Utilizing Sleeping Beauty System to Generate T cells Targeting Neoantigens
Apheresis
Prioritization of Neoantigens and TCRs
Biopsy
Gene Transfer via the Sleeping Beauty system(non-viral gene transfer)
Infusion
Cell Culture
weeks <2 days
Mol Ther. 2016Jun;24(6):1078-89.
ZIOPHARM Oncology
25
Undertaking the Pipeline Through Partnerships and Collaborations
Research Translation Collaboration
INTREXON UCSF THE UNIVERSITY OF TEXAS THE UNIVERSITY OF CALIFORNIA UCLA EMO SERONO
UNIVERSITY OF TEXAS
MD Anderson
Cancer Center Memorial Sloan Kettering CancerCenter Northwestern Memorial Hospital UNIVERSITY OF TEXAS MD Anderson Cancer Center THE UNIVERSITY OF CHICAGO PRITZKER SCHOOL OF MEDICINE NATIONAL CANCER INSTITUTE
INTREXON
ZIOPHARM Oncology

26
Corporate and Financial Highlights
Amended Exclusive Channel Collaborations with Intrexon
All unpartnered programs divided 80:20 in favor of ZIOPHARM
Provides greater reward for investing in development
Increases attractiveness to potential strategic partners
Sublicensed programs continue to be divided 50:50*
Consideration for new terms is dilutive only under certain developments
Well Capitalized
Approximately $94.7M in cash as of 3Q16
Cash runway into 1Q18
Shares outstanding as of November 3, 2016: 131.7M
Highly Efficient Operations with a Headcount of 35
Partnerships support preclinical, clinical and commercial development
*Includes exclusive agreement with Intrexon for the development and commercialization ofCAR-T products with Merck KGaA, Darmstadt, Germany
ZIOPHARM Oncology
27
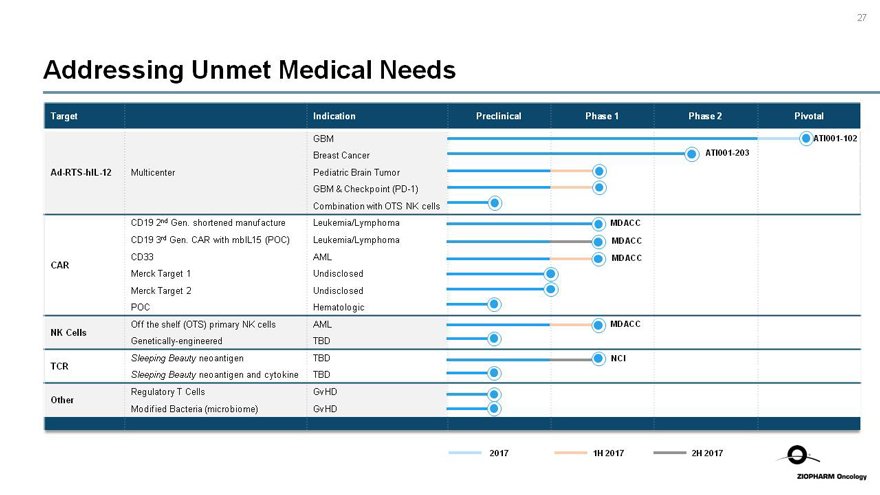
Addressing Unmet Medical Needs
Target Indication Preclinical Phase 1 Phase 2 Pivotal
GBMATI001-102
Breast CancerATI001-203
Ad-RTS-hIL-12 Multicenter Pediatric Brain Tumor
GBM & Checkpoint(PD-1)
Combination with OTS NK cells
CD19 2nd Gen. shortened manufacture Leukemia/Lymphoma MDACC
CD19 3rd Gen. CAR with mbIL15 (POC) Leukemia/Lymphoma MDACC
CD33 AML MDACC
CAR
Merck Target 1 Undisclosed
Merck Target 2 Undisclosed
POC Hematologic
Off the shelf (OTS) primary NK cells AML MDACC
NK Cells
Genetically-engineered TBD
Sleeping Beauty neoantigen TBD NCI
TCR
Sleeping Beauty neoantigen and cytokine TBD
Regulatory T Cells GvHD
Other
Modified Bacteria (microbiome) GvHD
2017 1H 2017 2H 2017
ZIOPHARM Oncology

ZIOPHARM ONCOLOGY
35th Annual J.P. Morgan
Healthcare Conference
January 11, 2017



























