
Third Quarter 2020 Financial Results and Update 05 November 2020 Exhibit 99.2

This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as "may," "will," "could," "expects," "plans," "anticipates," and "believes." These statements include, but are not limited to, statements regarding the Company's business and strategic plans, the availability of cash resources, the Company’s hiring expectations and expected additions to its Board of Directors or Scientific Advisory Board, the progress, design and timing of the Company's research and development programs, including the anticipated dates for the initiation, completion and readouts of its clinical trials, the Company’s expectations regarding the number of patients or the timelines for the commencement of patient dosing in its clinical trials, the Company’s expectations regarding the timing of IND filings, the buildout and expansion of the Company’s facilities, and the Company’s expectations regarding the impact of the ongoing COVID-19 pandemic, including the expected duration of disruption and immediate and long-term impact and effect on its business and operations. Although Ziopharm’s management team believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Ziopharm, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include among other things, changes in our operating plans that may impact our cash expenditures, the uncertainties inherent in research and development, future clinical data and analysis, including whether any of Ziopharm’s product candidates will advance further in the preclinical research or clinical trial process, including receiving clearance from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies to conduct clinical trials and whether and when, if at all, they will receive final approval from the U.S. FDA or equivalent foreign regulatory agencies and for which indication; the strength and enforceability of Ziopharm’s intellectual property rights; competition from other pharmaceutical and biotechnology companies as well as risk factors discussed or identified in the public filings with the Securities and Exchange Commission made by Ziopharm, including those risks and uncertainties listed in Ziopharm’s Quarterly Report on Form 10-Q filed by Ziopharm with the Securities and Exchange Commission. In addition, the extent to which the COVID-19 pandemic impacts the Company’s business, clinical development and regulatory efforts and the value of its common stock, will depend on future developments that are highly uncertain and cannot be predicted with confidence at this time, such as the ultimate duration of the pandemic, travel restrictions, quarantines, social distancing and business closure requirements, and the effectiveness of actions taken globally to contain and treat the disease. The global economic slowdown, the overall disruption of global healthcare systems and the other risks and uncertainties associated with the pandemic could have a material adverse effect on the Company’s business, financial condition, results of operations and growth prospects. We are providing this information as of the date of this press release, and Ziopharm does not undertake any obligation to update or revise the information contained in this press release whether as a result of new information, future events or any other reason. Forward Looking Statements
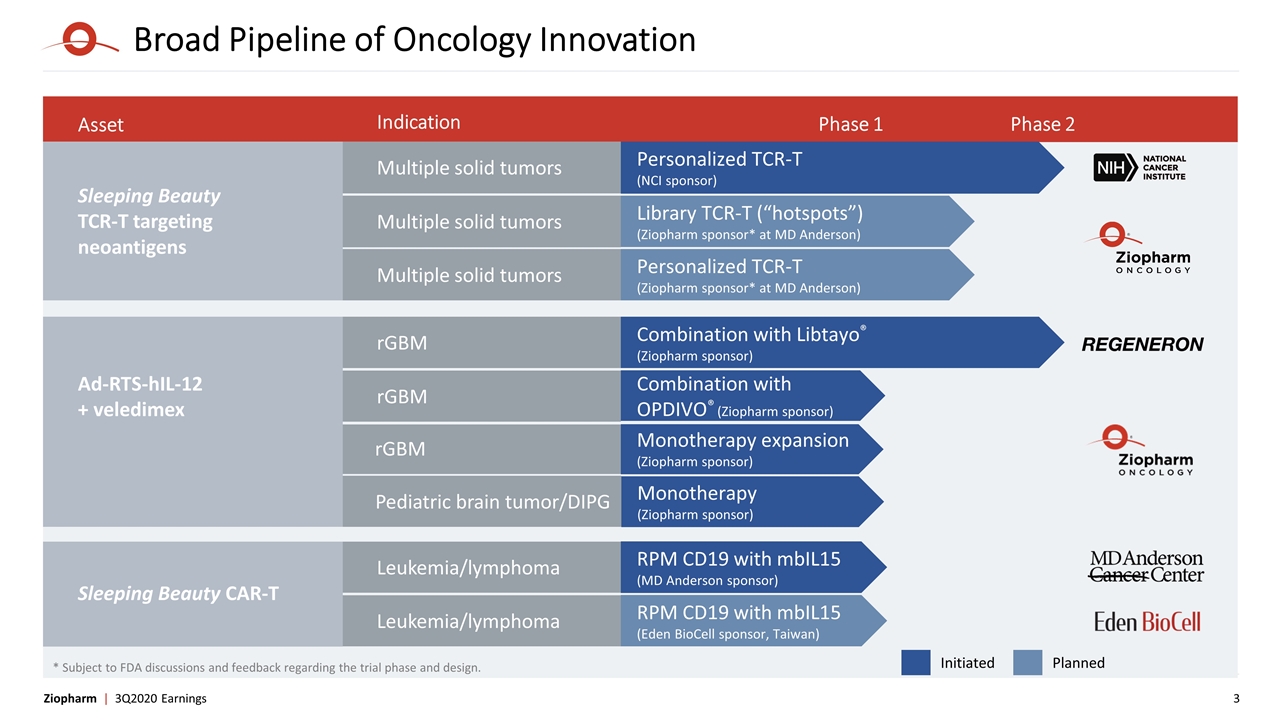
Asset Indication Phase 1 Phase 2 Multiple solid tumors rGBM Combination with Libtayo® (Ziopharm sponsor) Combination with OPDIVO® (Ziopharm sponsor) rGBM rGBM Monotherapy expansion (Ziopharm sponsor) Ad-RTS-hIL-12 + veledimex Broad Pipeline of Oncology Innovation Sleeping Beauty TCR-T targeting neoantigens Multiple solid tumors Personalized TCR-T (NCI sponsor) Library TCR-T (“hotspots”) (Ziopharm sponsor* at MD Anderson) Multiple solid tumors Personalized TCR-T (Ziopharm sponsor* at MD Anderson) Multiple solid tumors Initiated Planned * Subject to FDA discussions and feedback regarding the trial phase and design. Pediatric brain tumor/DIPG Monotherapy (Ziopharm sponsor) Sleeping Beauty CAR-T Leukemia/lymphoma RPM CD19 with mbIL15 (MD Anderson sponsor) Leukemia/lymphoma RPM CD19 with mbIL15 (Eden BioCell sponsor, Taiwan)

Third Quarter Results and Update Response to COVID-19: Ziopharm employees primarily work remotely with exceptions such as in our laboratory; safely and advancing programs; adapting to pandemic Sleeping Beauty TCR-T Program: Ziopharm on track to file IND for library hotspot trial in Q1 2021 through build out of team and infrastructure in Houston Further expanded library of TCRs targeting neoantigen hotspots through additional license of IP from NCI Completed technology transfer of Ziopharm's engineering runs to the NCI; NCI authenticated methodology and now validating in GMP facility Queue of eligible patients with solid tumors at NCI depleted during shutdown due to COVID-19; NCI is screening patients for neoantigens and TCRs to expand patient pool for TCR-T trial; Pace of adding patients is slower than before pandemic NCI guides that enrollment to Personalized TCR-T trial to begin next year Controlled IL-12 Program: Three abstracts accepted at Society for Neuro-Oncology Annual Meeting November 19-21, 2020 Initial observations from phase 1/2 pediatric trial for diffuse intrinsic pontine gliomas (DIPG) Update from phase 1 combination trial of Controlled IL-12 with Opdivo® First presentation of data from phase 2 trial of Controlled IL-12 in combination with Libtayo® All three clinical sites active and screening for potential DIPG patients Sleeping Beauty CD19-specific CAR-T Program: MD Anderson screening patients for phase 1 Sleeping Beauty allogeneic CAR-T trial using Rapid Personalized Manufacturing (RPM) Eden BioCell commenced filing IND for clinical trial in Taiwan to assess autologous CAR-T produced using RPM Eden BioCell and partners report dosing of several patients with relapsed CD19+ malignancies under compassionate use, infusing autologous CAR-T the day after gene transfer per RPM Board Refreshment and Operational: Named Kevin Buchi and James Huang to Ziopharm Board of Directors Populated Scientific Advisory Board under Carl June, M.D., Chairman Recruited Dr. Adam Levy from Gilead as EVP, IR and Corporate Communications

Ziopharm’s TCR-T Clinical Program – Library “Hotspot” Trial First Clinical Program – Evaluating Ziopharm’s Existing TCR Library in Multiple Cancers Ziopharm’s TCR Library Clinical Trial Timing: On track to file IND in Q1 2021; begin dosing patients in mid-2021 Indications: gynecologic, colorectal, pancreatic, non-small cell lung cancer and cholangiocarcinoma Casting a wide net to bolster enrollment TCRs: Initiate with TCRs from existing library targeting prevalent mutations and HLA; Additional TCRs from library will be added to trial as it progresses Ongoing activities: Several workstreams with MD Anderson, including identifying potential populations, intended to streamline program Unique relationship with MD Anderson facilitates approach Attributes of Ziopharm’s Existing Library Existing library includes 30+ TCRs, capable of treating 19 unique mutation/HLA combinations Initial TCRs target subsets of common mutations in KRAS and TP53 hotspot genes Mutation targets are pan-cancer and considered drivers of tumor biology KRAS Dominant oncogenic mutations of KRAS are single amino acid substitutions present in multiple cancers Small molecules address only one KRAS target (G12C); We are building a library that targets multiple genetic changes to KRAS Data confirm targeting KRAS can result in anti-cancer responses; Bodes well for our TCR-T library that includes TCRs against multiple KRAS mutations TP53 Considered among the most mutated gene in human cancers Functions as a transcription factor to regulate cell division and stabilize the genome TCRs in library have broad HLA representation

Ziopharm’s TCR-T Clinical Program – Personalized TCR-T Trial Second Clinical Program – Evaluating Personalized TCR Approach in Multiple Cancers Overview: Personalized approach evaluates therapies using patient-derived TCRs targeting neoantigens specific to each patient “TCRs from the patient, for the patient” Treatment: Each patient may receive TCRs with multiple specificities per infusion Takes advantage of scalable Sleeping Beauty manufacturing approach to create commercial solution Timing: Commence after the initiation of TCR-T Library trial Indications: Multiple cancers Process improvements: Building on methodologies from the NCI’s Personalized TCR-T trial; Commercializing the approach by reducing overall time to treatment Library expansion: Personalized TCR-T trial may serve to feed expansion of TCRs in library
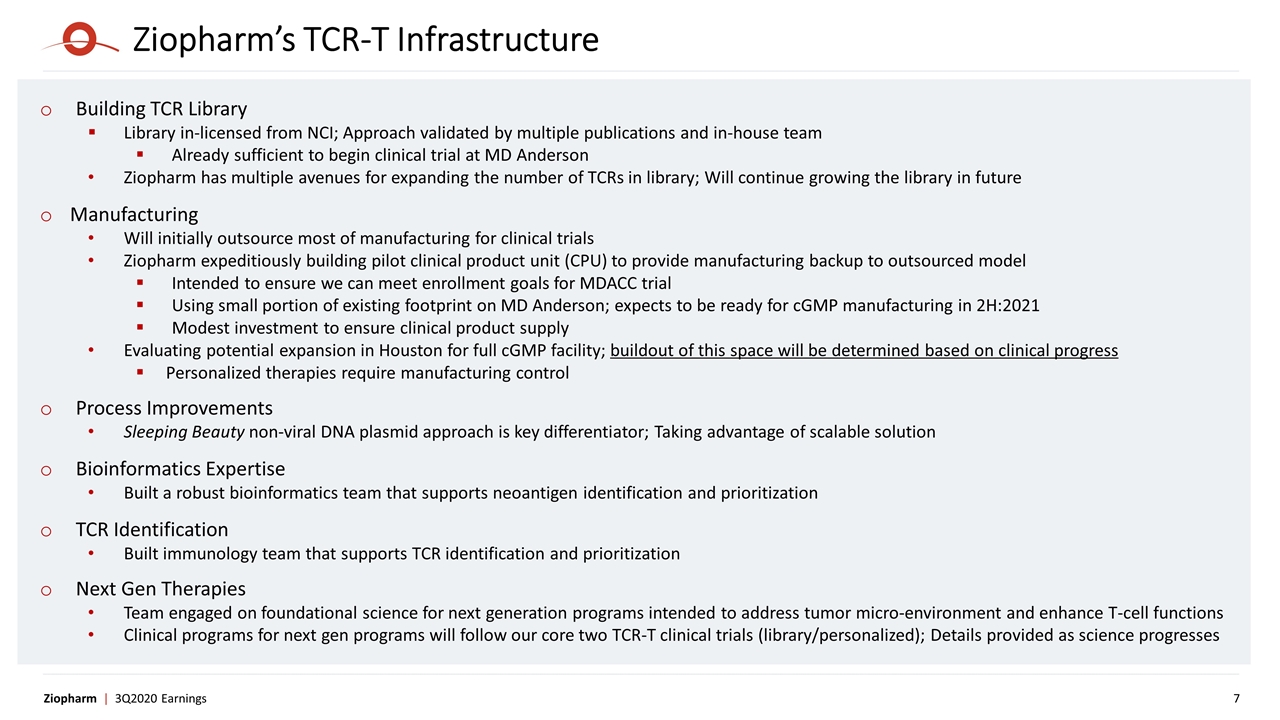
Ziopharm’s TCR-T Infrastructure Building TCR Library Library in-licensed from NCI; Approach validated by multiple publications and in-house team Already sufficient to begin clinical trial at MD Anderson Ziopharm has multiple avenues for expanding the number of TCRs in library; Will continue growing the library in future Manufacturing Will initially outsource most of manufacturing for clinical trials Ziopharm expeditiously building pilot clinical product unit (CPU) to provide manufacturing backup to outsourced model Intended to ensure we can meet enrollment goals for MDACC trial Using small portion of existing footprint on MD Anderson; expects to be ready for cGMP manufacturing in 2H:2021 Modest investment to ensure clinical product supply Evaluating potential expansion in Houston for full cGMP facility; buildout of this space will be determined based on clinical progress Personalized therapies require manufacturing control Process Improvements Sleeping Beauty non-viral DNA plasmid approach is key differentiator; Taking advantage of scalable solution Bioinformatics Expertise Built a robust bioinformatics team that supports neoantigen identification and prioritization TCR Identification Built immunology team that supports TCR identification and prioritization Next Gen Therapies Team engaged on foundational science for next generation programs intended to address tumor micro-environment and enhance T-cell functions Clinical programs for next gen programs will follow our core two TCR-T clinical trials (library/personalized); Details provided as science progresses
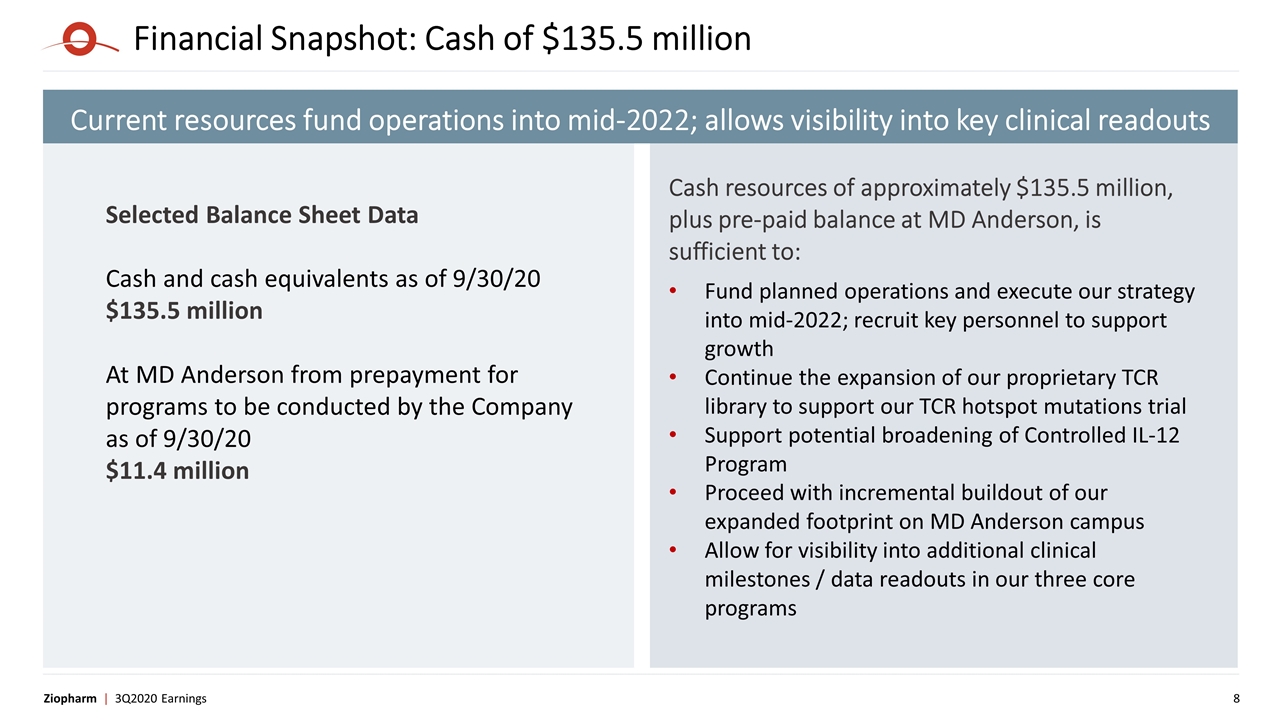
Financial Snapshot: Cash of $135.5 million Selected Balance Sheet Data Cash and cash equivalents as of 9/30/20 $135.5 million At MD Anderson from prepayment for programs to be conducted by the Company as of 9/30/20 $11.4 million Cash resources of approximately $135.5 million, plus pre-paid balance at MD Anderson, is sufficient to: Fund planned operations and execute our strategy into mid-2022; recruit key personnel to support growth Continue the expansion of our proprietary TCR library to support our TCR hotspot mutations trial Support potential broadening of Controlled IL-12 Program Proceed with incremental buildout of our expanded footprint on MD Anderson campus Allow for visibility into additional clinical milestones / data readouts in our three core programs Current resources fund operations into mid-2022; allows visibility into key clinical readouts

Anticipated Milestones Present additional clinical data for Controlled IL-12 program at SNO 2020 Advance enrollment and dosing of DIPG patients in pediatric trial for Controlled IL-12 at multiple sites Commence patient dosing in allogeneic RPM CD19-specific CAR-T study; Initiated at MD Anderson Complete IND filing for autologous RPM CD19-specific CAR-T trial in Taiwan with Eden BioCell this year IND in early 2021 for Ziopharm's TCR-T Library trial to be undertaken at MD Anderson; Treat patients mid-2021 Dose first patient in NCI-led Sleeping Beauty TCR-T phase 2 trial targeting solid tumors Host virtual R&D Day with team and guest speakers in Q1 2021 Continue review of potential additions to Board of Directors; Recruitment of key executive leadership

Thank you… 05 November 2020









