
Exhibit 99.2 Third Quarter 2022 Results November 14, 2022

Forward Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” “believes” or other words or terms of similar meaning. These statements include, but are not limited to, statements regarding Alaunos Therapeutics, Inc.'s (”Alaunos or the Company ) business and strategic plans, the anticipated outcome of preclinical and clinical studies by the Company or its third-party collaborators, the Company’s manufacturing capacity, the Company's ability to raise capital, and the timing of the Company's research and development programs, including the anticipated dates for enrolling and dosing patients in the Company’s clinical trials. Although the management team of Alaunos believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Alaunos, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, among other things, changes in the Company’s operating plans that may impact its cash expenditures; the uncertainties inherent in research and development, future clinical data and analysis, including whether any of Alaunos’ product candidates will advance further in the preclinical research or clinical trial process, including receiving clearance from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies to conduct clinical trials and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indication; the strength and enforceability of Alaunos’ intellectual property rights; and competition from other pharmaceutical and biotechnology companies as well as risk factors discussed or identified in the public filings with the Securities and Exchange Commission made by Alaunos, including those risks and uncertainties listed in the most recent period report filed by Alaunos with the Securities and Exchange Commission. We are providing this information as of the date of this presentation, and Alaunos does not undertake any obligation to update or revise the information contained in this presentation whether as a result of new information, future events, or any other reason. 2

Speakers on Today’s Call Drew Deniger, Ph.D. Kevin S. Boyle, Sr. VP, Research & Development Chief Executive Officer Mike Wong Abhishek Srivastava, Ph.D. VP, Technical Operations VP, Finance Melinda Lackey 3
Confirmed Partial Response Following Treatment with Sleeping Beauty TCR-T Cells at First Dose Level TCR-T Library Phase 1/2 Trial Enrolling; Confirmed Partial Response in First Patient (NSCLC); Now Treating at Dose Level 2 1 Increasing cGMP Manufacturing Facility Capacity; Consistently Producing High Quality TCR-T Cells at Clinical Scale 2 Growing Clinical Library of TCRs (KRAS, TP53, EGFR) Increasing the Addressable Market 3 Proprietary TCR Discovery Platform,hunTR™ is Expanding and Advancing the Pipeline 4 60% Fewer Employees and 50%+ Reduction in Operating Cash Burn Year-over-Year 4
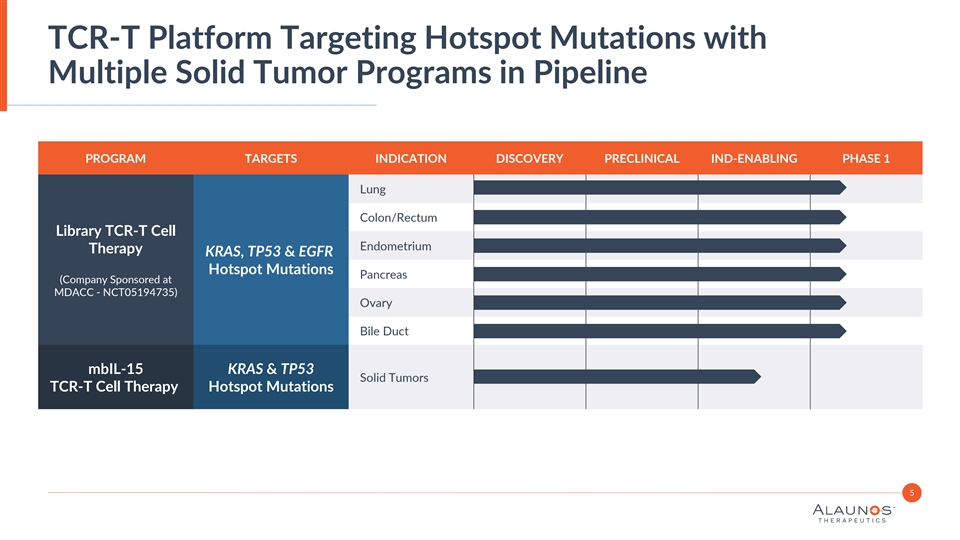
TCR-T Platform Targeting Hotspot Mutations with Multiple Solid Tumor Programs in Pipeline PROGRAM TARGETS INDICATION DISCOVERY PRECLINICAL IND-ENABLING PHASE 1 Lung Colon/Rectum Library TCR-T Cell Endometrium Therapy KRAS, TP53 & EGFR Hotspot Mutations Pancreas (Company Sponsored at MDACC - NCT05194735) Ovary Bile Duct mbIL-15 KRAS & TP53 Solid Tumors TCR-T Cell Therapy Hotspot Mutations 5

Clinical Update 6
Encouraging Early Clinical Data for Non-viral TCR-T Therapy: Safety, Persistence and Efficacy with 1 Confirmed PR • First in human confirmed response in solid tumor by TCR-T cell therapy using Sleeping Beauty • Patients have been treated with KRAS and TP53 mutation specific cells • Two patients treated; now treating at dose level 2 • Manageable safety profile with no neurotoxicity • Persistence of TCR-T cells observed at six months • Confirmed Partial Response in Patient 1 with Non-Small Cell Lung Cancer (NSCLC) • Early clinical validation highlights potential of TCR-T cell therapy in high value indications with significant unmet medical need 7

Patient 1: PR in NSCLC Six-month imaging
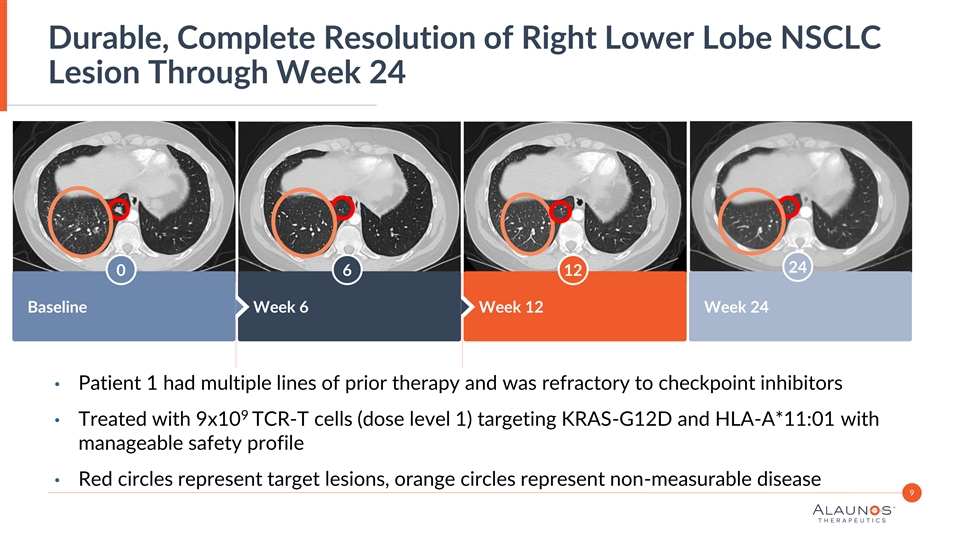
Durable, Complete Resolution of Right Lower Lobe NSCLC Lesion Through Week 24 Insert Image Here Insert Image Here Insert Image Here 24 0 6 12 Baseline Week 6 Week 12 Week 24 • Patient 1 had multiple lines of prior therapy and was refractory to checkpoint inhibitors 9 • Treated with 9x10 TCR-T cells (dose level 1) targeting KRAS-G12D and HLA-A*11:01 with manageable safety profile • Red circles represent target lesions, orange circles represent non-measurable disease 9
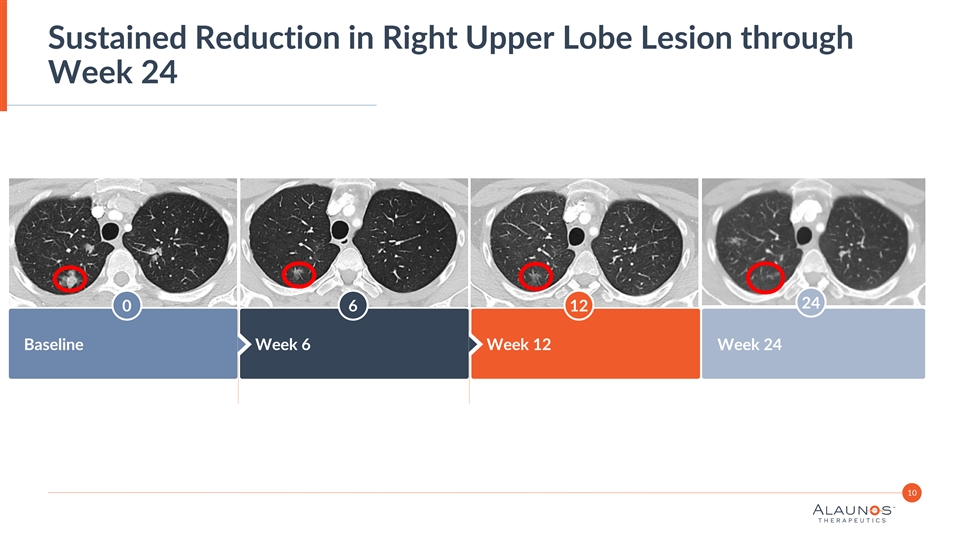
Sustained Reduction in Right Upper Lobe Lesion through Week 24 24 0 6 12 Baseline Week 6 Week 12 Week 24 10
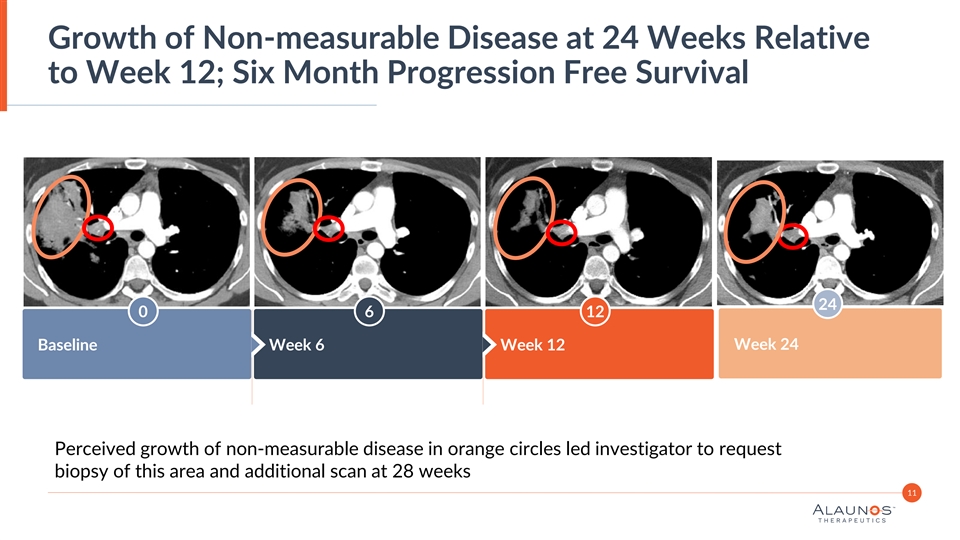
Growth of Non-measurable Disease at 24 Weeks Relative to Week 12; Six Month Progression Free Survival 24 0 6 12 Baseline Week 6 Week 12 Week 24 Perceived growth of non-measurable disease in orange circles led investigator to request biopsy of this area and additional scan at 28 weeks 11
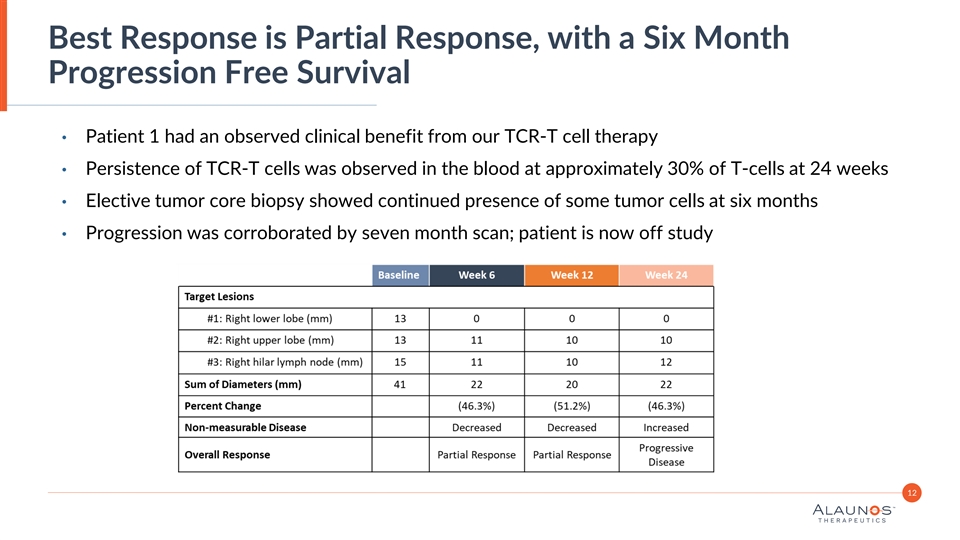
Best Response is Partial Response, with a Six Month Progression Free Survival • Patient 1 had an observed clinical benefit from our TCR-T cell therapy • Persistence of TCR-T cells was observed in the blood at approximately 30% of T-cells at 24 weeks • Elective tumor core biopsy showed continued presence of some tumor cells at six months • Progression was corroborated by seven month scan; patient is now off study 12

Patient 2: Colorectal Cancer

Patient 2: Previously Treated Advanced Colorectal Cancer Achieved Best Overall Response of Stable Disease 9 • Patient 2 received one prior line of therapy and was treated with 64x10 TCR-T cells (dose level 2) targeting TP53- R175H and HLA-A*02:01 • Treatment was well tolerated with manageable safety events; patient received one dose of tocilizumab with no neurotoxicity • Some evidence of efficacy at six weeks with reduction of pelvic mass and overall decrease in target lesions combined • Persistence of TCR-T cells was observed in the blood at approximately 20% of T-cells at 12 weeks • Patient off study due to disease progression at Week 12 due to new lesions in the liver and lung 14

Clinical Summary to Date

Encouraging Clinical Activity from Sleeping Beauty TCR-T Therapy Demonstrating Safety, Persistence and Efficacy • Treatment was well tolerated with no DLTs or ICANS in either patient • Persistence of TCR-T cells was noted in both patients to last follow-up • Indications of efficacy observed in both patients • Six-month progression free survival in KRAS NSCLC patient, comparable to other therapeutics • Infiltration of TCR-T cells into the tumor was observed at six months suggesting the cells could home to the tumor microenvironment • Progressing tumor had both mutation and HLA expressed indicating that the target was intact and still required for the cancer 16

Manufacturing Update
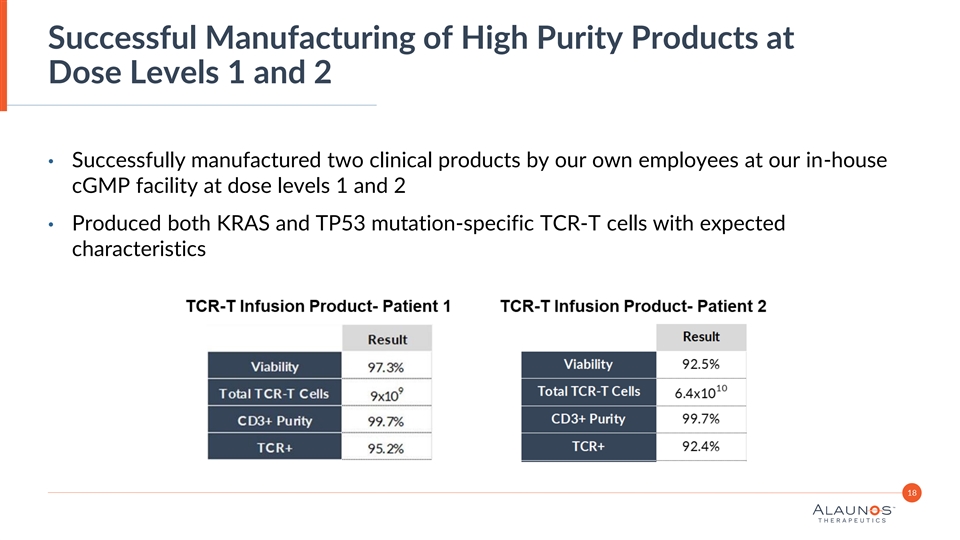
Successful Manufacturing of High Purity Products at Dose Levels 1 and 2 • Successfully manufactured two clinical products by our own employees at our in-house cGMP facility at dose levels 1 and 2 • Produced both KRAS and TP53 mutation-specific TCR-T cells with expected characteristics 18

Execution Against Multipronged Expansion Strategy Has Doubled Manufacturing Capacity • Changes in Process & Procedures • Updated SOPs to allow for simultaneous manufacture of multiple products in the cGMP suite • Expect to file IND amendment to move from fresh to cryopreserved product in 4Q 2022 and implement change in 1H 2023 • Reduced Manufacturing Time by 13% • Implementation of cryopreservation anticipated to shorten manufacturing time from 30 to 26 days, increasing cGMP suite throughput potential • Expanded Manufacturing Team • Hired and trained additional staff to enable manufacture of simultaneous products 19

R&D Update

TM hunTR Is Differentiated from Competing TCR Discovery Platforms Differentiator Alaunos Competition Blood from healthy donors Starting material TILs from patient with target without target Virally transduced donor T cells TCR screening Reporter cell line (fast, universal) (donor-to-donor variability, labor intensive and time consuming) Limited and commonly HLA and mutation All mutations and HLAs dependent on peptide prediction screening algorithms 21
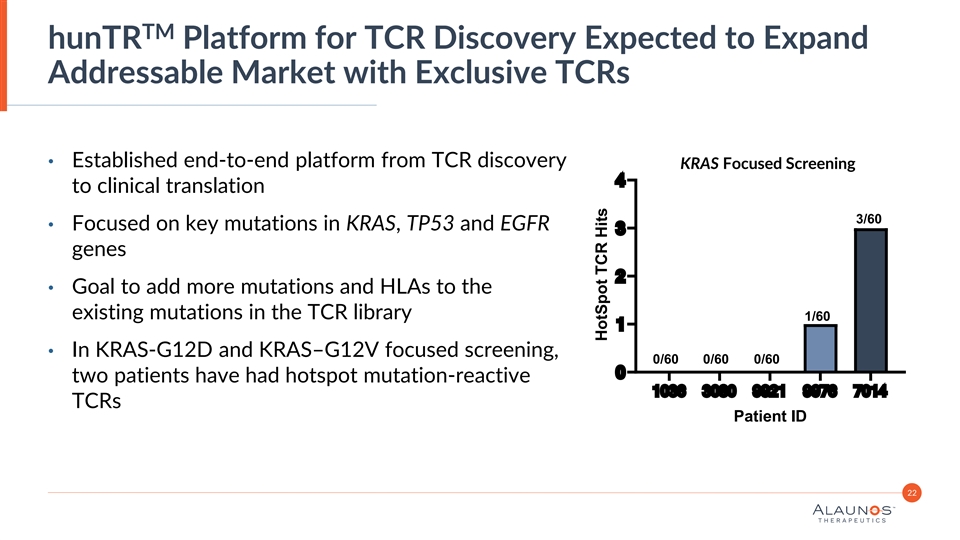
TM hunTR Platform for TCR Discovery Expected to Expand Addressable Market with Exclusive TCRs • Established end-to-end platform from TCR discovery KRAS Focused Screening 4 to clinical translation 3/60 • Focused on key mutations in KRAS, TP53 and EGFR 3 genes 2 • Goal to add more mutations and HLAs to the existing mutations in the TCR library 1/60 1 • In KRAS-G12D and KRAS–G12V focused screening, 0/60 0/60 0/60 0 two patients have had hotspot mutation-reactive 1036 3080 9921 9976 7014 TCRs Patient ID 22 HotSpot TCR Hits
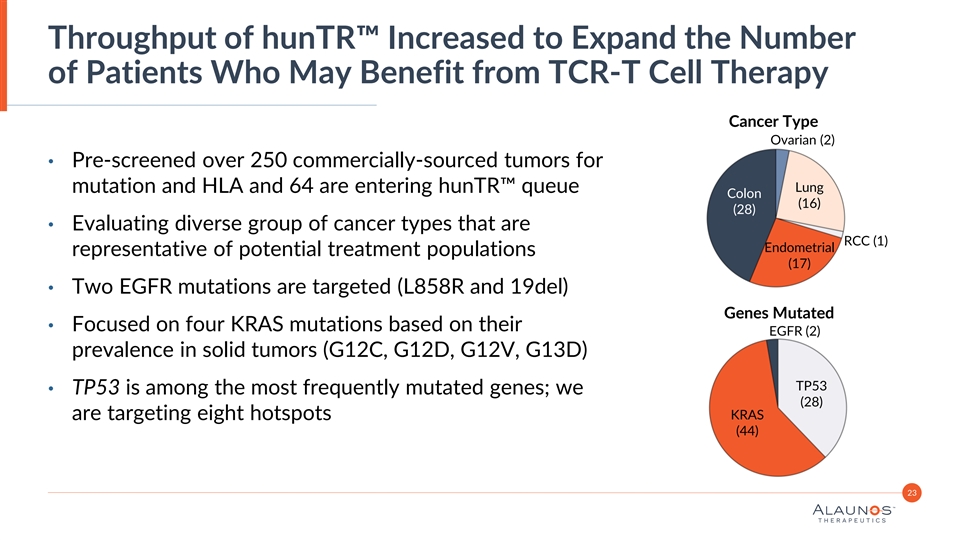
Throughput of hunTR™ Increased to Expand the Number of Patients Who May Benefit from TCR-T Cell Therapy Cancer Type Ovarian (2) • Pre-screened over 250 commercially-sourced tumors for Lung mutation and HLA and 64 are entering hunTR™ queue Colon (16) (28) • Evaluating diverse group of cancer types that are RCC (1) Endometrial representative of potential treatment populations (17) • Two EGFR mutations are targeted (L858R and 19del) Genes Mutated • Focused on four KRAS mutations based on their EGFR (2) prevalence in solid tumors (G12C, G12D, G12V, G13D) TP53 • TP53 is among the most frequently mutated genes; we (28) are targeting eight hotspots KRAS (44) 23

Financial Update

Selected Financial Data for the Third Quarter; Over a 50% Reduction in Operating Cash Burn Year-Over-Year Revenue Operating Expense Cash & Net Cash • Collaboration revenue of $2.9 • R&D costs decreased 46%: as • Cash: $37.8 million million: primarily related to a result of focused • Restricted Cash: $13.9 million the first commercial sale of efforts on our TCR-T platform darinaparsin by Solasia Pharma and lower employee related • Debt Outstanding: $22.7 million K.K. costs; includes one-time milestone expense of $2.5 • Operating Cash Burn: $6.1 million associated with the million in third quarter of 2022, Solasia collaboration revenue. compared to $9.6 million in third quarter of 2021 a decrease of • G&A decreased 60%: a result 36%; Year-to-date of $22.1 of reduced headcount and million in 2022, compared to implemented efficiencies $46.3 in 2021, a decrease of 52% 25

Upcoming Milestones

Continue to Grow Platform to Expand Number of Patients Who May Benefit from Sleeping Beauty TCR-T Poised for Continued Progress Generating Additional Clinical Data and Building Pipeline 1 Treating Additional Patients on Library TCR-T Cell Clinical Trial 2 Amending IND to Include Two New TCRs and Reducing Manufacturing Process Time with Cryopreserved Product 3 Planning to File IND for mbIL-15 TCR-T Cell Therapy in 2H 2023 4 Collaboration with NCI for Personalized Sleeping Beauty TCR-T Approach 27


























