Exhibit 99.1 Attacking Solid Tumors with Novel TCR-T Cell Therapies January 2023

Forward Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. Forward- looking statements are statements that are not historical facts, and in some cases can be identified by terms such as “may,” “will,” “could,” “expects,” “plans,” “anticipates,” “believes” or other words or terms of similar meaning. These statements include, but are not limited to, statements regarding the Alaunos Therapeutics, Inc.'s (”Alaunos or the Company ) business and strategic plans, the Company's ability to raise capital, and the timing of the Company's research and development programs, including the anticipated dates for enrolling and dosing patients in the Company’s clinical trials. Although the management team of Alaunos believes that the expectations reflected in such forward-looking statements are reasonable, investors are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Alaunos, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include, among other things, changes in the Company’s operating plans that may impact its cash expenditures; the uncertainties inherent in research and development, future clinical data and analysis, including whether any of Alaunos’ product candidates will advance further in the preclinical research or clinical trial process, including receiving clearance from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies to conduct clinical trials and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indication; the strength and enforceability of Alaunos’ intellectual property rights; and competition from other pharmaceutical and biotechnology companies as well as risk factors discussed or identified in the public filings with the Securities and Exchange Commission made by Alaunos, including those risks and uncertainties listed in the most recent Form 10-Q and Form 10-K filed by Alaunos with the Securities and Exchange Commission. We are providing this information as of the date of this presentation, and Alaunos does not undertake any obligation to update or revise the information contained in this presentation whether as a result of new information, future events, or any other reason. 2
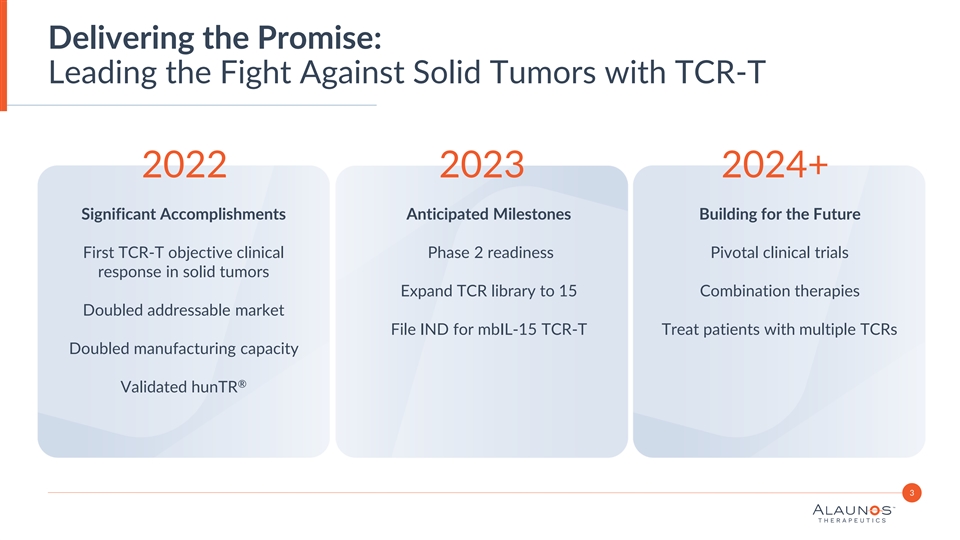
Delivering the Promise: Leading the Fight Against Solid Tumors with TCR-T 2022 2023 2024+ Significant Accomplishments Anticipated Milestones Building for the Future First TCR-T objective clinical Phase 2 readiness Pivotal clinical trials response in solid tumors Expand TCR library to 15 Combination therapies Doubled addressable market File IND for mbIL-15 TCR-T Treat patients with multiple TCRs Doubled manufacturing capacity ® Validated hunTR 3
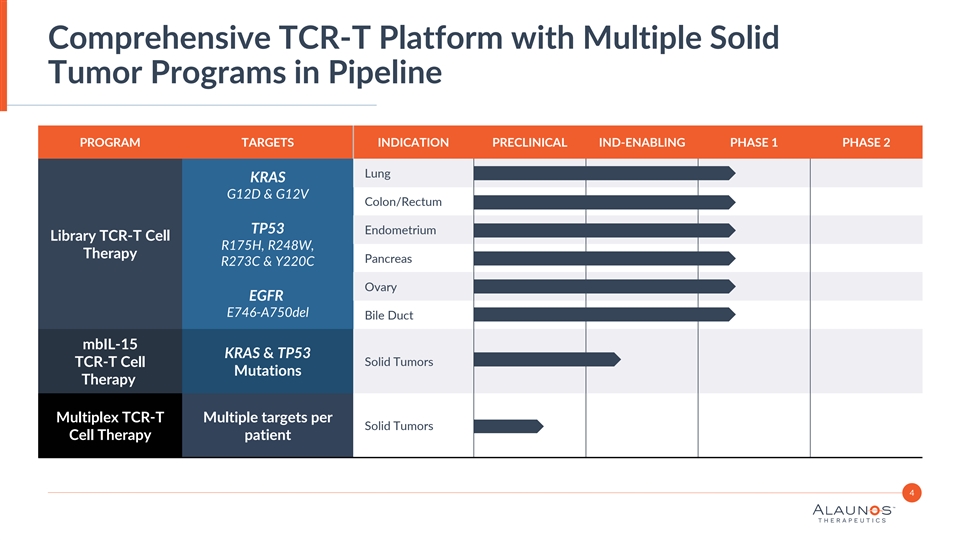
Comprehensive TCR-T Platform with Multiple Solid Tumor Programs in Pipeline PROGRAM TARGETS INDICATION PRECLINICAL IND-ENABLING PHASE 1 PHASE 2 Lung KRAS G12D & G12V Colon/Rectum TP53 Endometrium Library TCR-T Cell R175H, R248W, Therapy Pancreas R273C & Y220C Ovary EGFR E746-A750del Bile Duct mbIL-15 KRAS & TP53 TCR-T Cell Solid Tumors Mutations Therapy Multiplex TCR-T Multiple targets per Solid Tumors Cell Therapy patient 4
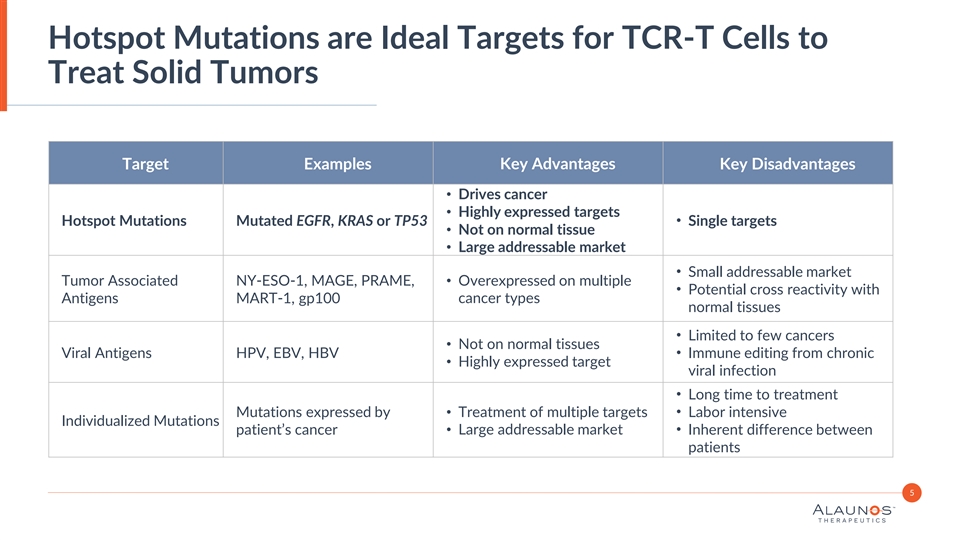
Hotspot Mutations are Ideal Targets for TCR-T Cells to Treat Solid Tumors Target Examples Key Advantages Key Disadvantages • Drives cancer • Highly expressed targets • Hotspot Mutations Mutated EGFR, KRAS or TP53 Single targets • Not on normal tissue • Large addressable market • Small addressable market Tumor Associated NY-ESO-1, MAGE, PRAME, • Overexpressed on multiple • Potential cross reactivity with Antigens MART-1, gp100 cancer types normal tissues • Limited to few cancers • Not on normal tissues • Viral Antigens HPV, EBV, HBV Immune editing from chronic • Highly expressed target viral infection • Long time to treatment Mutations expressed by • Treatment of multiple targets• Labor intensive Individualized Mutations patient’s cancer• Large addressable market• Inherent difference between patients 5
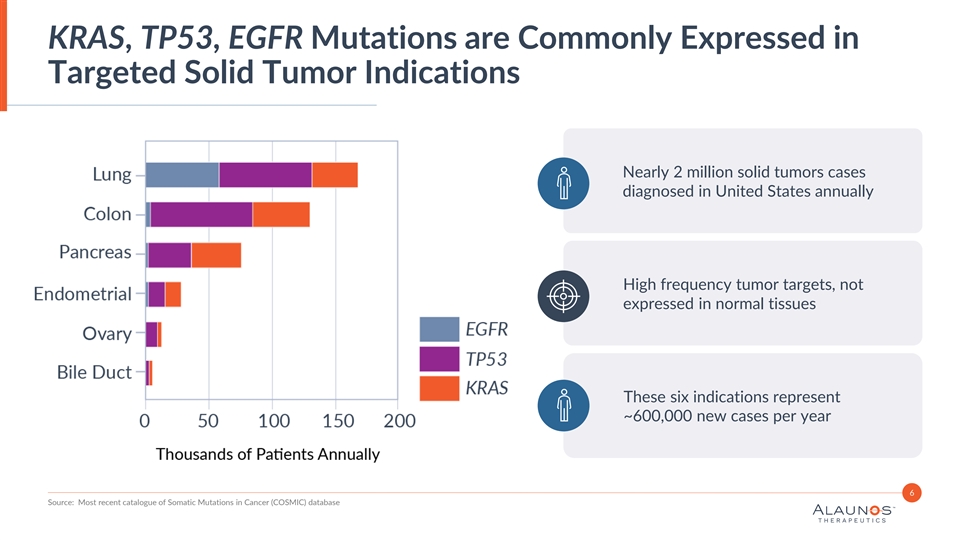
KRAS, TP53, EGFR Mutations are Commonly Expressed in Targeted Solid Tumor Indications Nearly 2 million solid tumors cases diagnosed in United States annually High frequency tumor targets, not expressed in normal tissues These six indications represent ~600,000 new cases per year 6 Source: Most recent catalogue of Somatic Mutations in Cancer (COSMIC) database
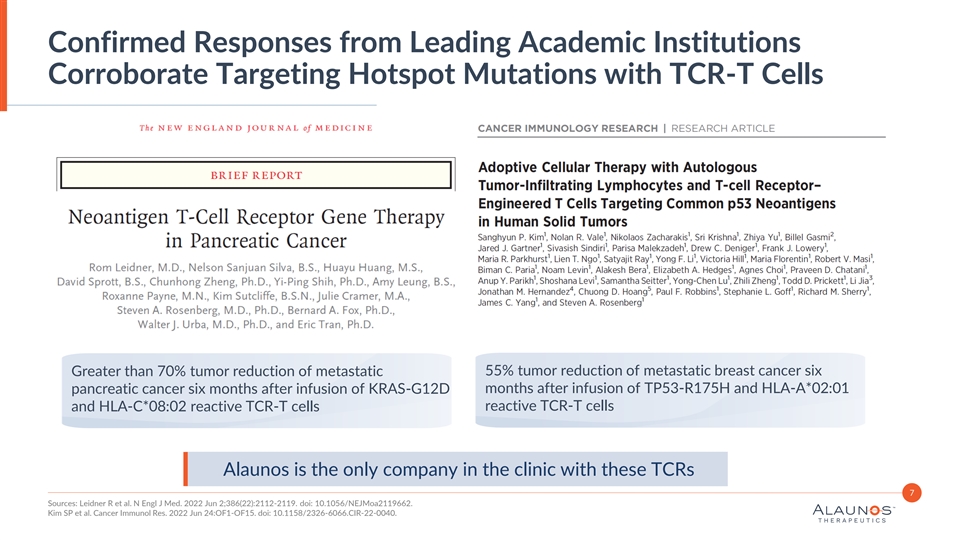
Confirmed Responses from Leading Academic Institutions Corroborate Targeting Hotspot Mutations with TCR-T Cells Greater than 70% tumor reduction of metastatic 55% tumor reduction of metastatic breast cancer six months after infusion of TP53-R175H and HLA-A*02:01 pancreatic cancer six months after infusion of KRAS-G12D reactive TCR-T cells and HLA-C*08:02 reactive TCR-T cells Alaunos is the only company in the clinic with these TCRs 7 Sources: Leidner R et al. N Engl J Med. 2022 Jun 2;386(22):2112-2119. doi: 10.1056/NEJMoa2119662. Kim SP et al. Cancer Immunol Res. 2022 Jun 24:OF1-OF15. doi: 10.1158/2326-6066.CIR-22-0040.
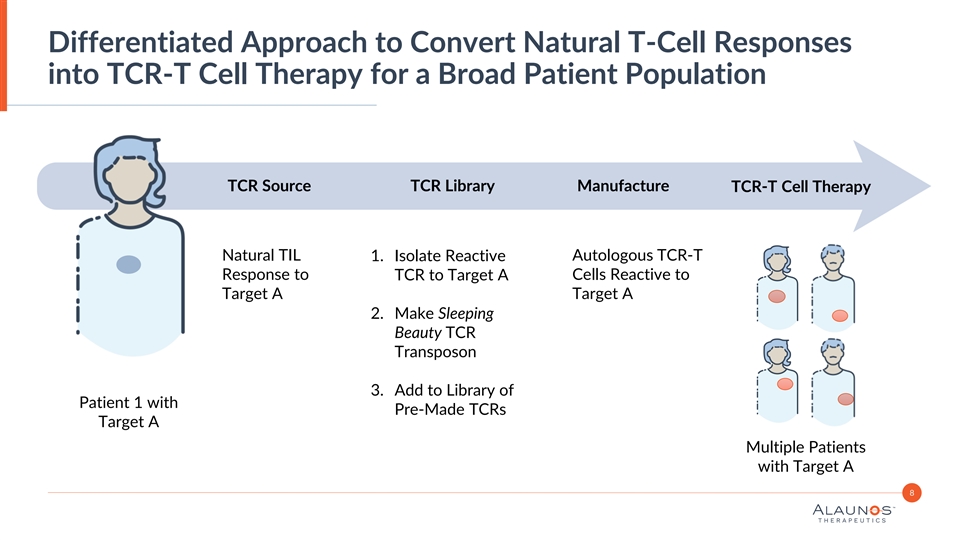
Differentiated Approach to Convert Natural T-Cell Responses into TCR-T Cell Therapy for a Broad Patient Population TCR Source TCR Library Manufacture TCR-T Cell Therapy Natural TIL 1. Isolate Reactive Autologous TCR-T Response to TCR to Target A Cells Reactive to Target A Target A 2. Make Sleeping Beauty TCR Transposon 3. Add to Library of Patient 1 with Pre-Made TCRs Target A Multiple Patients with Target A 8

Unparalleled TCR Library Captures Both High Frequency Mutations and HLA Types Recently added TCRs via IND amendment: KRAS-G12V and DRB1*07:01 TP53-R273C and DPB1*04:02 Our TCR library contains mutations from genes that are known to drive cancer and are highly expressed by tumors Mutations in our library are among the most frequent and most mutated genes in solid tumors HLAs that present our mutations are prevalent in the United States 9

Non-viral Sleeping Beauty Platform Well Suited for Manufacturing TCR-T Cells without Complexity of Gene Editing • Efficient, essentially random integration without complexity of gene editing or viral approaches • Rapid, cost-effective manufacturing • Flexible approach to add TCRs; attractive choice for library • Platform can accommodate large transgene size • Expected to be scalable for commercialization 10 10

TCR-T Cells Recognize KRAS, TP53, EGFR Mutations and Kill Solid Tumor Cells Powerful TCRs: Naturally-occurring, high avidity TCRs recognize low levels of neoantigens No off-target toxicity observed: Specificity for the mutation with negligible recognition of wild type sequences Tumor killing: Recognition of tumor cells that express mutation and HLA Note: Refers to TP53 mutant reactive TCRs 11
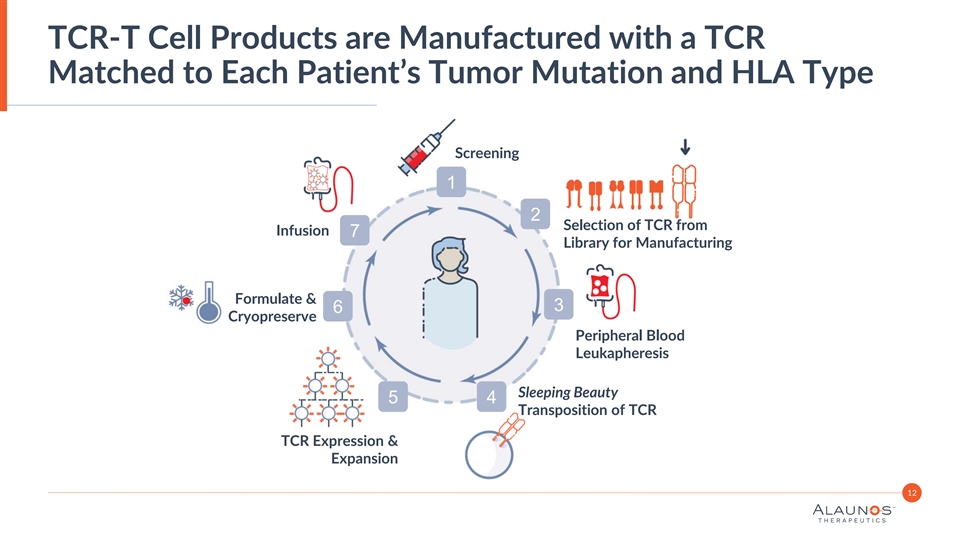
TCR-T Cell Products are Manufactured with a TCR Matched to Each Patient’s Tumor Mutation and HLA Type Screening 1 2 Selection of TCR from Infusion 7 Library for Manufacturing Formulate & 3 6 Cryopreserve Peripheral Blood Leukapheresis Sleeping Beauty 5 4 Transposition of TCR TCR Expression & Expansion 12
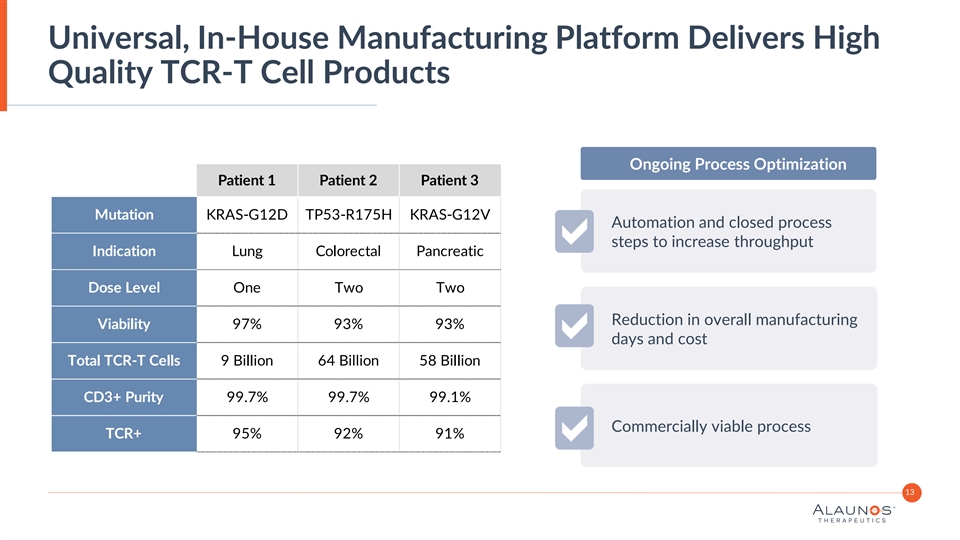
Universal, In-House Manufacturing Platform Delivers High Quality TCR-T Cell Products Ongoing Process Optimization Patient 1 Patient 2 Patient 3 Mutation KRAS-G12D TP53-R175H KRAS-G12V Automation and closed process steps to increase throughput Indication Lung Colorectal Pancreatic Dose Level One Two Two Reduction in overall manufacturing Viability 97% 93% 93% days and cost Total TCR-T Cells 9 Billion 64 Billion 58 Billion CD3+ Purity 99.7% 99.7% 99.1% Commercially viable process TCR+ 95% 92% 91% 13

First-in-Human TCR-T Clinical Trial Actively Enrolling with Innovative Library Approach • Defines safety and dose with any TCR and any indication Phase 1 • Enrolling in one of three dose cohorts: 5, 40 and 100 billion TCR-T cells • Defines efficacy at recommended dose with any TCR in library Phase 2 • Each cancer type will be a distinct cohort • Selected indications and selected TCRs with efficacy in Phase 2 Pivotal • IND can start while Phase 2 continues for other indications/TCRs • Multiple TCRs for use in multiple cancer types Vision for Commercial Product • Early guidance from FDA supports our approach 14
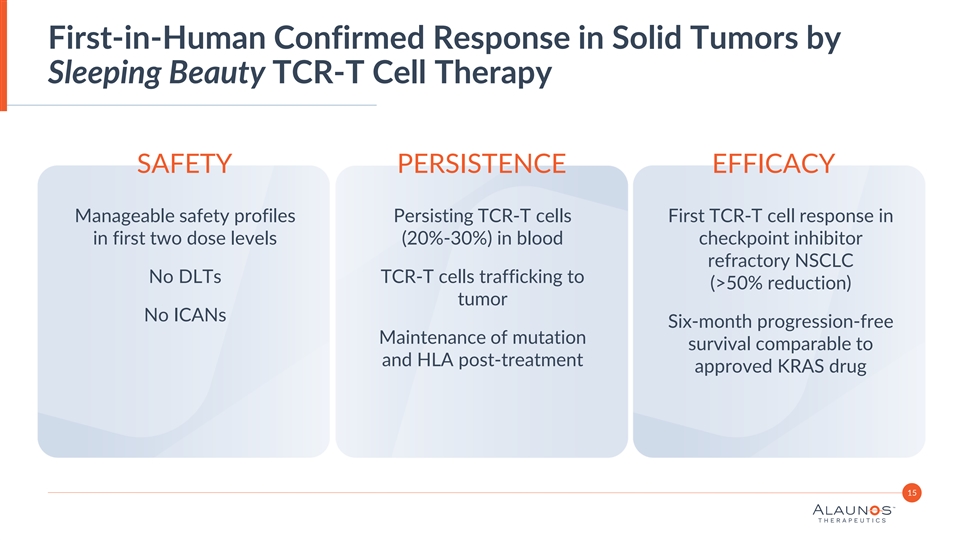
First-in-Human Confirmed Response in Solid Tumors by Sleeping Beauty TCR-T Cell Therapy SAFETY PERSISTENCE EFFICACY Manageable safety profiles Persisting TCR-T cells First TCR-T cell response in in first two dose levels (20%-30%) in blood checkpoint inhibitor refractory NSCLC No DLTs TCR-T cells trafficking to (>50% reduction) tumor No ICANs Six-month progression-free Maintenance of mutation survival comparable to and HLA post-treatment approved KRAS drug 15
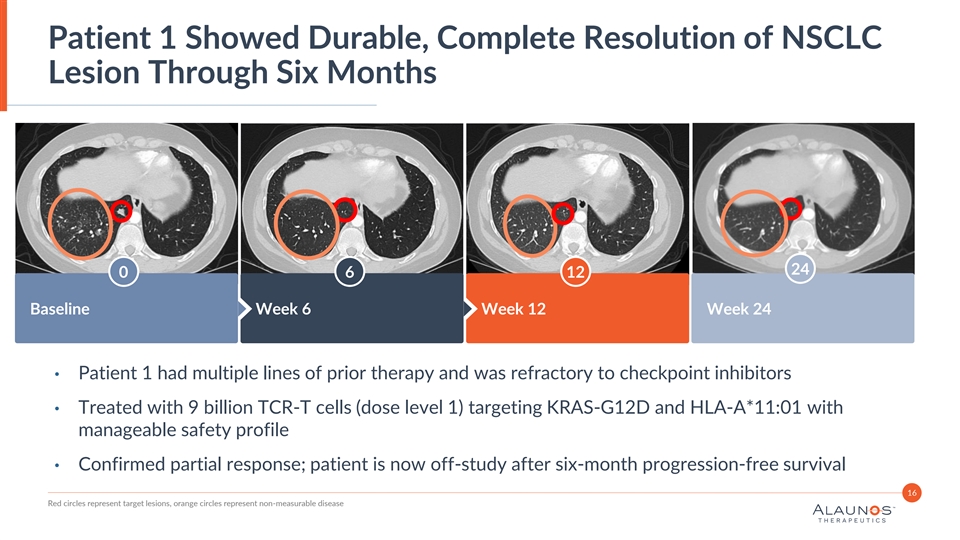
Patient 1 Showed Durable, Complete Resolution of NSCLC Lesion Through Six Months Insert Image Here Insert Image Here Insert Image Here 24 0 6 12 Baseline Week 6 Week 12 Week 24 • Patient 1 had multiple lines of prior therapy and was refractory to checkpoint inhibitors • Treated with 9 billion TCR-T cells (dose level 1) targeting KRAS-G12D and HLA-A*11:01 with manageable safety profile • Confirmed partial response; patient is now off-study after six-month progression-free survival 16 Red circles represent target lesions, orange circles represent non-measurable disease
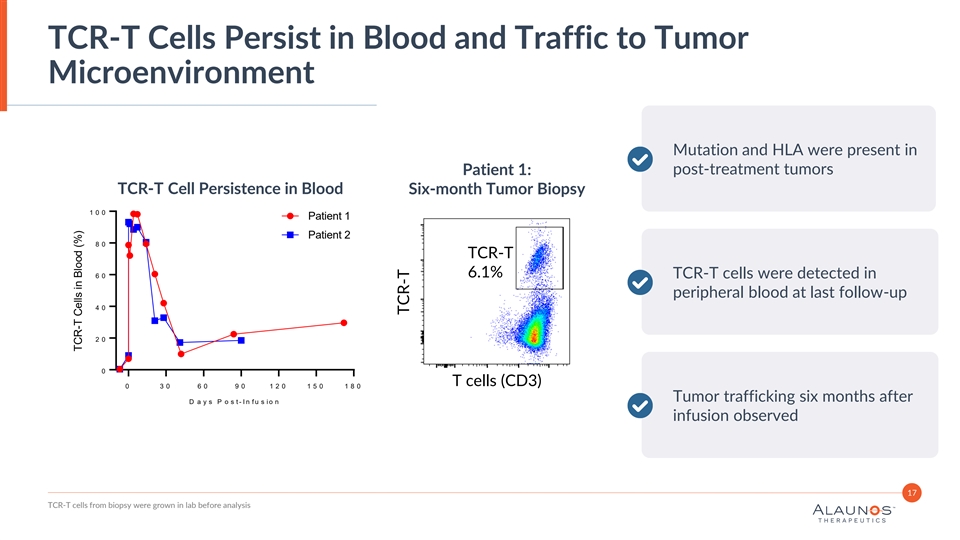
TCR-T Cells Persist in Blood and Traffic to Tumor Microenvironment Mutation and HLA were present in post-treatment tumors Patient 1: TCR-T Cell Persistence in Blood Six-month Tumor Biopsy 1 0 0 Patient 1 Patient 2 8 0 TCR-T 6.1% 6 0 TCR-T cells were detected in peripheral blood at last follow-up 4 0 2 0 0 T cells (CD3) 0 3 0 6 0 9 0 1 2 0 1 5 0 1 8 0 Tumor trafficking six months after D a y s P o s t - I n f u s i o n infusion observed 17 TCR-T cells from biopsy were grown in lab before analysis TCR-T Cells in Blood (%) TCR-T
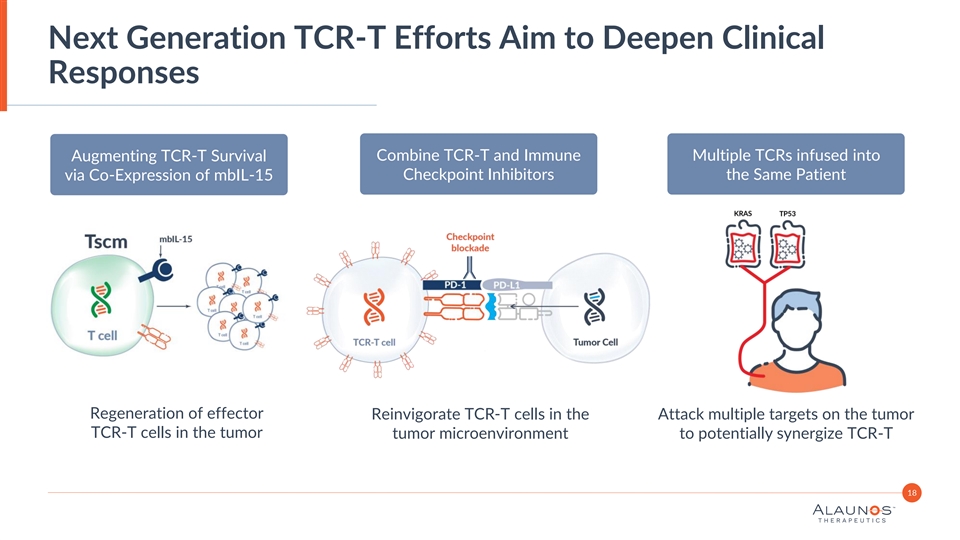
Next Generation TCR-T Efforts Aim to Deepen Clinical Responses Augmenting TCR-T Survival Combine TCR-T and Immune Multiple TCRs infused into Checkpoint Inhibitors the Same Patient via Co-Expression of mbIL-15 Regeneration of effector Reinvigorate TCR-T cells in the Attack multiple targets on the tumor TCR-T cells in the tumor tumor microenvironment to potentially synergize TCR-T 18

® hunTR Potentially Expands TCR Library, Increases Addressable Market and Enables Multiplexed TCR-T Cell Therapy Add new HLAs to existing mutations and add 2024+ more key mutations within EGFR, KRAS, TP53 40+ TCRs 2023 Matching data from clinical efforts inform which 15 HLA/mutation combinations to prioritize TCRs >200,000 2022 10 Sleeping Beauty expected to allow for cost- effective and efficient expansion of TCR library TCRs ~60,000 for clinic ~30,000 Expect out-licensing opportunities of selected proprietary TCRs Multiple TCRs Matching 19 Potential Patients per Year

Experienced Management Team Melinda Lackey Kevin S. Boyle, Sr. SVP Legal Chief Executive Officer Drew Deniger, PhD Abhishek Srivastava, PhD Mike Wong VP Research & Development VP Technical Operations VP Finance Melinda Lackey 20
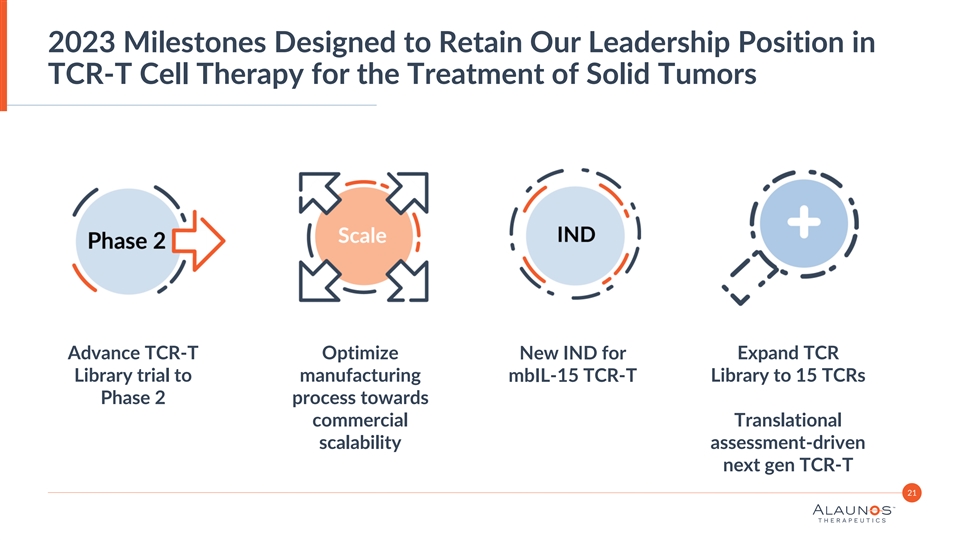
2023 Milestones Designed to Retain Our Leadership Position in TCR-T Cell Therapy for the Treatment of Solid Tumors Advance TCR-T Optimize New IND for Expand TCR Library trial to manufacturing Library to 15 TCRs mbIL-15 TCR-T Phase 2 process towards commercial Translational scalability assessment-driven next gen TCR-T 21




















