Exhibit 99.1

CURIS TM
Curis, Inc. (NASDAQ: CRIS) Corporate Overview June 20, 2005
©2005 Curis Inc.

Forward Looking Statements
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements about Curis’ drug discovery and development programs. Such statements may contain the words “believes”, “expects”, “anticipates”, “plans”, “seeks”, “estimates” or similar expressions. These forward looking statements are not guarantees of future performance and involve risks, uncertainties, assumptions and other factors that may cause Curis’ actual results to be materially different from those indicated by such forward-looking statements. Actual results can be affected by a number of important factors including, among other things: adverse results in Curis’ and its strategic partners’ product development programs; and difficulties or delays in completing clinical trials and obtaining or maintaining required regulatory approvals for such development programs; Curis’ ability to obtain or maintain the patent and other proprietary intellectual property protection necessary for the development and commercialization of products based on its technologies; changes in or Curis’ inability to execute its realigned business strategy; the risk that Curis does not obtain the additional funding required to conduct research and development of its product candidates and execute on its business plan, including the capital required to fund its co-development option with Genentech; unplanned cash requirements and expenditures; risks relating to Curis’ ability to enter into and maintain important strategic partnerships, including its ability to maintain its current collaboration agreements with Ortho Biotech, Genentech, and Wyeth; the risk that competitors will discover and develop signaling pathway-based therapeutics or alternative competing therapeutics faster and more successfully than Curis and its collaborators are able to; and other risk factors identified in Curis’ most recent Annual Report on Form 10-K, Quarterly Report on 10-Q and any subsequent reports filed with the Securities and Exchange Commission. In addition, any forward-looking statements represent the Company’s views only as of today and should not be relied upon as representing its views as of any subsequent date. Curis disclaims any intention or obligation to update any of the forward-looking statements after the date of this presentation whether as a result of new information, future events or otherwise.
©2005 Curis Inc.
2

Presentation Outline
Business Model
Technology Overview
Collaborations and Product Candidates
Patents/Financials
©2005 Curis Inc.
3

Curis Business Strategy
Collaboration strategy improves probability of success
Portfolio approach results in product diversity (i.e., technology platform, compound type, and target indication)
Payments from collaborators lessen future dependence on capital markets
• Cash milestones under top four deals total $650 million
Diverse collaboration structures allow for calculated risk assumption and retained technology rights
• Curis is co-developing a skin cancer product with Genentech
Research capabilities ensures pipeline of new drug development candidates
4
©2005 Curis Inc.

Curis Business Strategy
Collaboration strategy improves probability of success
Portfolio approach results in product diversity (i.e., technology platform, compound type, and target indication)
Payments from collaborators lessen future dependence on capital markets
• Cash milestones under top four deals total $650 million
Diverse collaboration structures allow for calculated risk assumption
Curis is co-developing a skin cancer product with Genentech
Research capabilities ensures pipeline of new drug development candidates
5
©2005 Curis Inc.
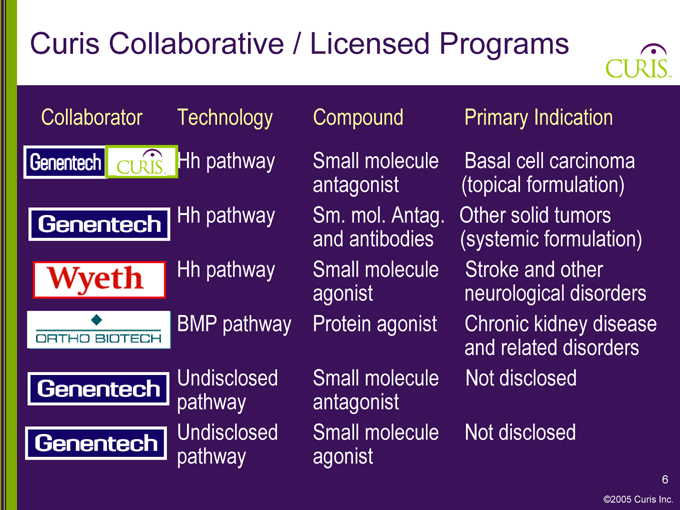
Curis Collaborative / Licensed Programs
Collaborator Technology Compound Primary Indication
Genentech CURIS Hh pathway Small molecule Basal cell carcinoma
antagonist (topical formulation)
Genentech Hh pathway Sm. mol. Antag. Other solid tumors
and antibodies (systemic formulation)
Wyeth Hh pathway Small molecule Stroke and other
agonist neurological disorders
ORTHO BIOTECH BMP pathway Protein agonist Chronic kidney disease
and related disorders
Genentech Undisclosed Small molecule Not disclosed
pathway antagonist
Genentech Undisclosed Small molecule Not disclosed
pathway agonist
6
©2005 Curis Inc.
Curis Business Strategy
CURIS TM
Collaboration strategy improves probability of success
Portfolio approach results in product diversity (i.e., technology platform, compound type, and target indication)
Payments from collaborators lessen future dependence on capital markets
• Cash milestones under top four deals total $650 million
Diverse collaboration structures allow for calculated risk assumption
• Curis is co-developing a skin cancer product with Genentech
Research capabilities ensures pipeline of new drug development candidates
7
©2005 Curis Inc.
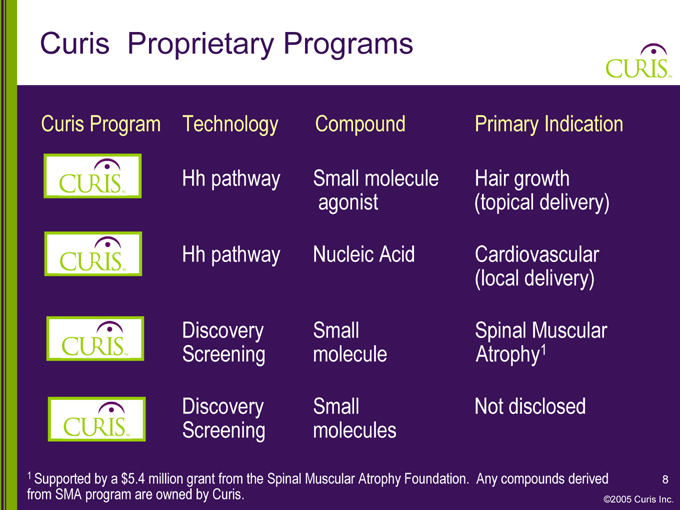
Curis Proprietary Programs
CURIS TM
Curis Program Technology Compound Primary Indication
CURIS Hh pathway Small molecule Hair growth
agonist (topical delivery)
CURIS Hh pathway Nucleic Acid Cardiovascular
(local delivery)
CURIS Discovery Small Spinal Muscular
Screening molecule Atrophy1
CURIS Discovery Small Not disclosed
Screening molecules
1 Supported by a $5.4 million grant from the Spinal Muscular Atrophy Foundation. Any compounds derived from SMA program are owned by Curis.
8
©2005 Curis Inc.

Presentation Outline
CURIS TM
Business Model
Technology Overview
Collaborations and Product Candidates
Patents/Financials/Summary
9
©2005 Curis Inc.
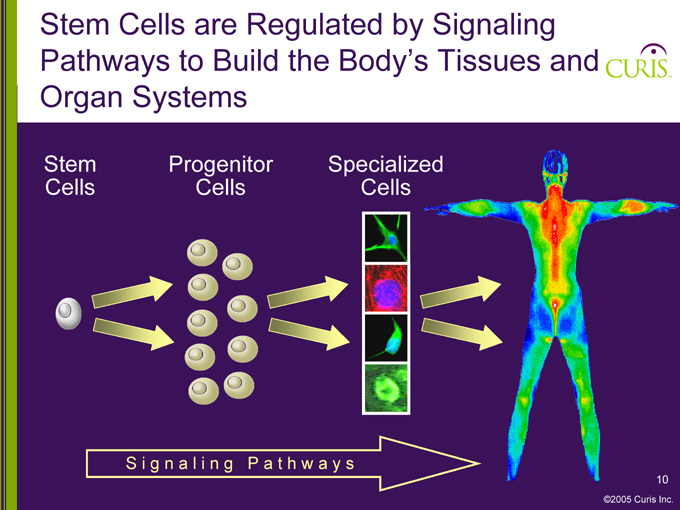
Stem Cells are Regulated by Signaling Pathways to Build the Body’s Tissues and Organ Systems
Stem Progenitor Specialized Cells Cells Cells
Signaling Pathways
10
©2005 Curis Inc.
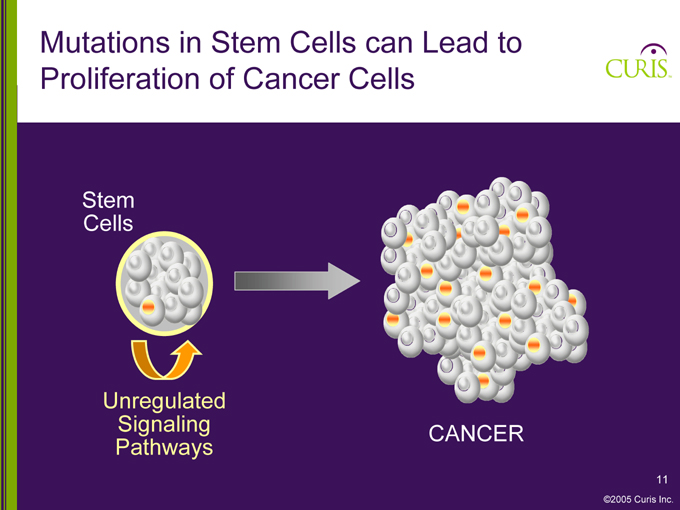
Mutations in Stem Cells can Lead to Proliferation of Cancer Cells
Stem Cells
Unregulated Signaling Pathways
CANCER
11
©2005 Curis Inc.
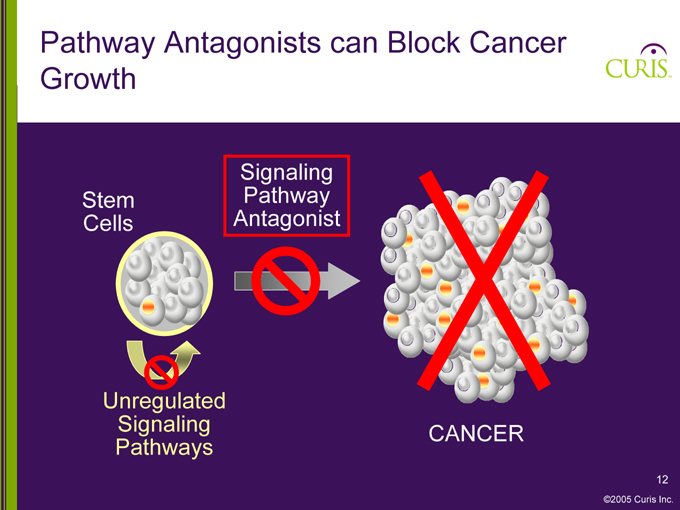
Pathway Antagonists can Block Cancer Growth
Stem Cells
Signaling Pathway Antagonist
Unregulated Signaling Pathways
CANCER
12
©2005 Curis Inc.
Presentation Outline
Business Model
Technology Overview
Collaborations and Product Candidates
Patents/Financials/Summary
13
©2005 Curis Inc.
Hedgehog Pathway Antagonist Program
Basal Cell Carcinoma and other Solid Tumor Cancers
Collaboration with Genentech
©2005 Curis Inc.

Genentech Hh Antagonist Collaboration
Established: June 2003
Deal structure: $8.5 million up-front payment, development milestones, product royalties, minimum two years R&D support, BCC co-development option in U.S.
Primary indications: Topical treatment for basal cell carcinoma (co-development program with Curis) and systemic treatment of other solid tumors
15
©2005 Curis Inc.

Genentech Hedgehog Antagonist Collaboration: Recent Progress
Genentech doubles the number of Curis personnel on Hedgehog antagonist program from 8 to 16
Curis personnel are supporting systemic solid tumor program
Genentech extends research funding under the Hedgehog antagonist program from June 2005 to at least December 2005
Curis exercises co-development option with Genentech for an equal share of U.S. costs and profits for a basal cell carcinoma product candidate
IND for basal cell carcinoma product candidate is filed and approved by FDA
First patients treated in Phase I clinical trial
16
©2005 Curis Inc.
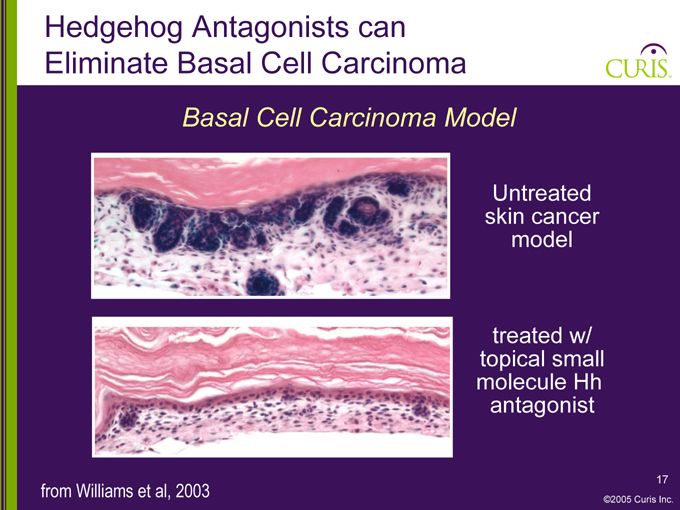
Hedgehog Antagonists can Eliminate Basal Cell Carcinoma
Basal Cell Carcinoma Model
Untreated skin cancer model
treated w/ topical small molecule Hh antagonist
from Williams et al, 2003
17
©2005 Curis Inc.
Basal Cell Carcinoma (BCC) Genentech Curis Co-Development
Co-development: Profit sharing structure commensurate with cost-sharing contributions; equal participation option exercised February 2005
BCC Phase I initiation: April 2005
Disease incidence: 800,000 to 1 million cases annually in the U.S.*
Marketing advantage: Non-surgical treatment for skin cancer that occurs primarily on face and hands
* American Cancer Society; Skin Cancer Foundation
18
©2005 Curis Inc.
BCC – Hh Antagonist Pilot Human Clinical Study
Study goal: To assess the potential of a Hedgehog (Hh) pathway antagonist as a treatment for basal cell carcinoma (BCC)
Hh antagonist: Cyclopamine
Study design: Four patients with established BCCs, who were scheduled for excision surgery, treated topically with cream containing cyclopamine or placebo (4X/d, 3-6 days)
Literature citation: Eur. J. Dermatol. 2004 14(2):96-102 from Ta and Avci, 2004
19
©2005 Curis Inc.
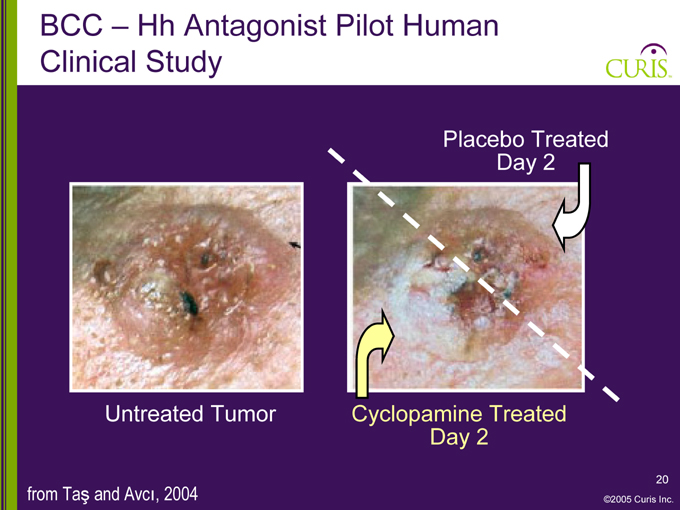
BCC – Hh Antagonist Pilot Human Clinical Study
Placebo Treated Day 2
Untreated Tumor Cyclopamine Treated Day 2 from Ta and Avci, 2004
20
©2005 Curis Inc.
BCC – Hh Antagonist Pilot Human Clinical Study
Study results: All cyclopamine-treated tumors regressed rapidly
Histological analysis showed inhibition of tumor cell proliferation and induction of tumor cell apoptosis
No adverse effects were noted on normal skin tissue
Results suggest that transient inhibition of Hedgehog pathway provides potential treatment modality for BCC
from Ta and Avci, 2004
21
©2005 Curis Inc.
Other Cancers Associated with Abnormal Expression of Hedgehog
Medulloblastoma1 Small Cell Lung Cancer 2 Pancreatic Carcinoma 3,4 Stomach Cancer 4 Esophageal Cancer 4 Colorectal Cancer 5 Prostate Cancer 6,7,8 Breast Cancer 9
1 Romer et al, 2004, 2 Watkins et al, 2003, 3 Thayer et al, 2003,
4 Berman et al, 2003, 5 Qualtrough et al 2004, 6 Fan et al, 2004,
7 Karhadkar et al, 2004, 8 Sanchez et al, 2004, 9Kubo et al, 2004
22
©2005 Curis Inc.
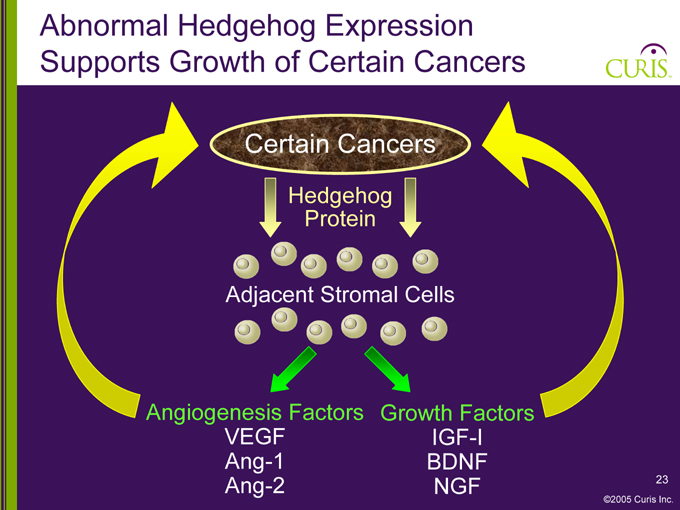
Abnormal Hedgehog Expression Supports Growth of Certain Cancers
Certain Cancers
Hedgehog Protein
Adjacent Stromal Cells
Angiogenesis Factors Growth Factors
VEGF Ang-1 Ang-2
IGF-I BDNF NGF
23
©2005 Curis Inc.
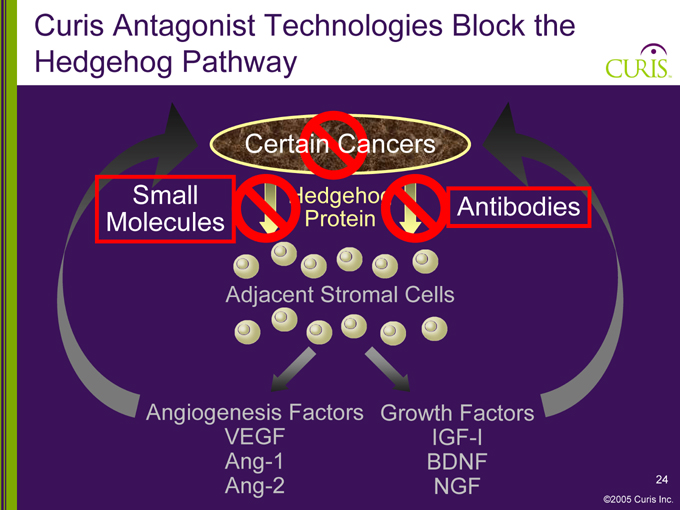
Curis Antagonist Technologies Block the Hedgehog Pathway
Certain Cancers
Small Molecules
Hedgehog Protein
Antibodies
Adjacent Stromal Cells
Angiogenesis Factors Growth Factors
VEGF Ang-1 Ang-2
IGF-I BDNF NGF
24
©2005 Curis Inc.
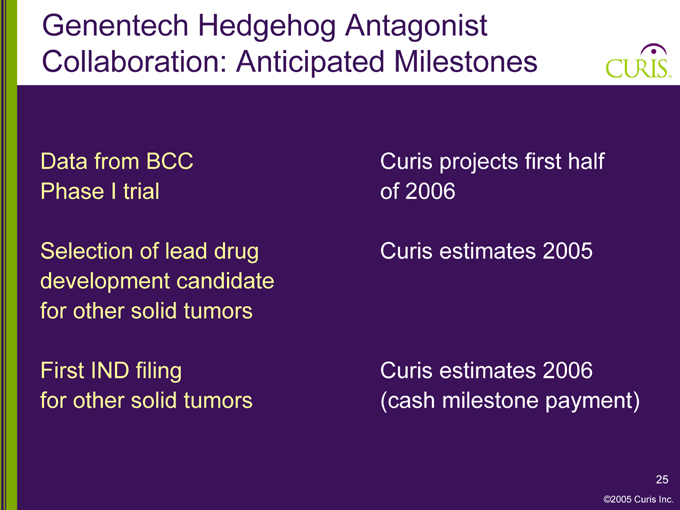
Genentech Hedgehog Antagonist Collaboration: Anticipated Milestones
Data from BCC Curis projects first half
Phase I trial of 2006
Selection of lead drug Curis estimates 2005
development candidate
for other solid tumors
First IND filing Curis estimates 2006
for other solid tumors (cash milestone payment)
©2005 Curis Inc.
25
Hedgehog Pathway Agonist Program
Neurological and other Disorders
Collaboration with
©2005 Curis Inc. ©2003

Wyeth Hedgehog Agonist Collaboration
Established: January 2004
Deal structure: $3 million up-front payment,
development milestones of
up to $170 million, product
royalties, minimum two
years R&D support (1)
Primary indications: Stroke and other
neurological disorders
(1) – Development milestone total is based on the successful development and regulatory approval of two products.
27
©2005 Curis Inc.
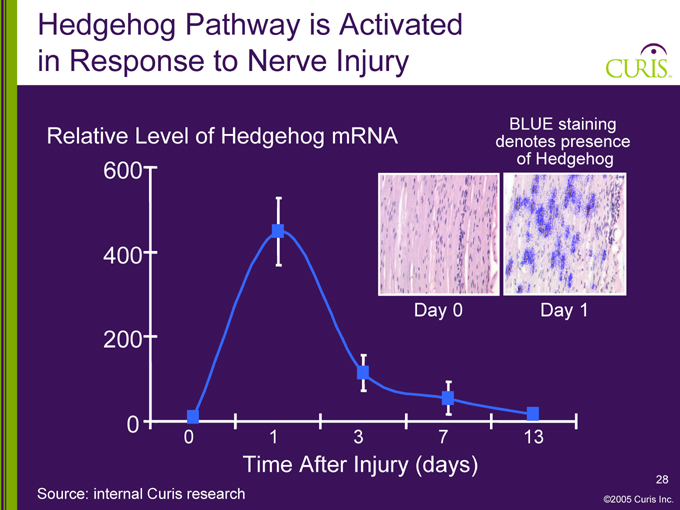
Hedgehog Pathway is Activated in Response to Nerve Injury
Relative Level of Hedgehog mRNA
BLUE staining denotes presence of Hedgehog
600 400 200 0
Day 0
Day 1
0 1 3 7 13
Time After Injury (days)
Source: internal Curis research
28
©2005 Curis Inc.
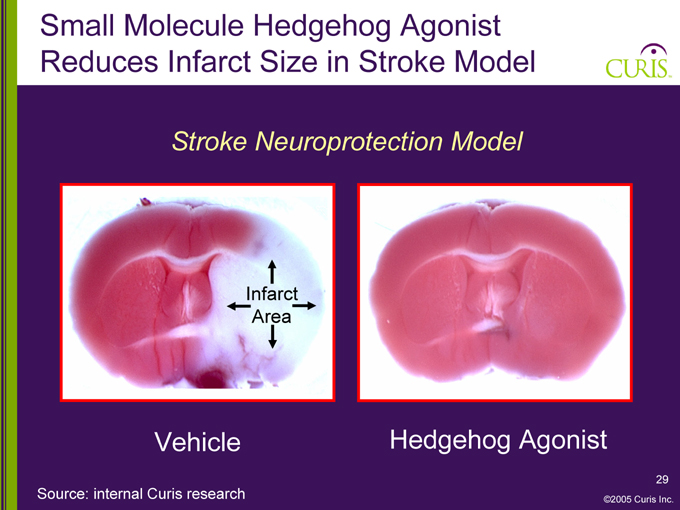
Small Molecule Hedgehog Agonist Reduces Infarct Size in Stroke Model
Stroke Neuroprotection Model
Infarct Area
Vehicle Hedgehog Agonist
Source: internal Curis research
29
©2005 Curis Inc.

Wyeth Hedgehog Agonist Collaboration: Anticipated Milestones—Market
Estimated IND filing: Curis estimates late 2006
(cash milestone payment)
Disease incidence: Approximately 700,000 patients suffer a stroke each year in U.S.1
Marketing advantage: Presently no effective therapies for treating stroke
1 American Heart Association, Heart Disease and Stroke Statistics – 2005 Update
30
©2005 Curis Inc.

BMP Pathway Program
Kidney Disease and Associated Disorders
Licensed to a subsidiary of
©2005 Curis Inc.
Ortho Biotech (J&J) BMP License
Established: November 2002
Transitioned to Centocor in 2005
Deal structure: $3.5 million up-front payment, development milestones including $30 million on NDA approval, product royalties
Primary indications: Chronic kidney disease and disorders associated with end stage kidney disease
32
©2005 Curis Inc.
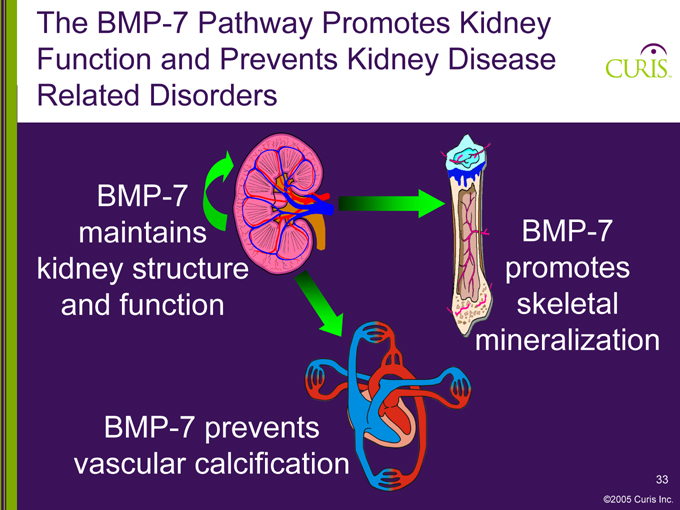
The BMP-7 Pathway Promotes Kidney Function and Prevents Kidney Disease Related Disorders
BMP-7 maintains kidney structure and function
BMP-7 promotes skeletal mineralization
BMP-7 prevents vascular calcification
33
©2005 Curis Inc.
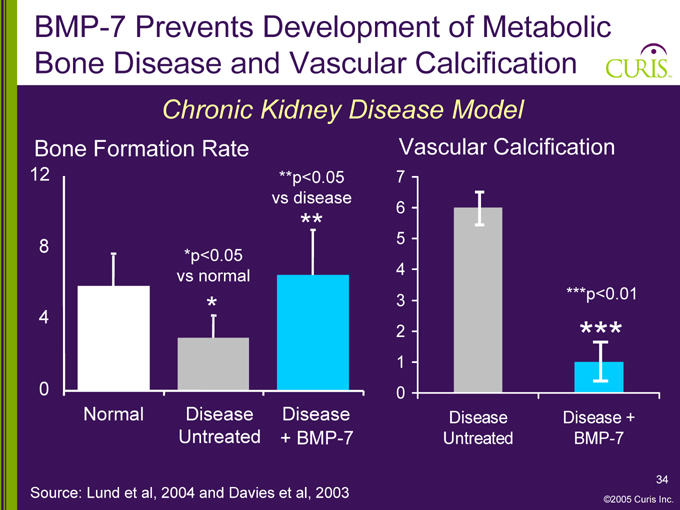
BMP-7 Prevents Development of Metabolic Bone Disease and Vascular Calcification
Chronic Kidney Disease Model
Bone Formation Rate
[GRAPHIC]
*p<0.05 vs normal
**p<0.05 vs disease
Normal Disease Disease Untreated + BMP-7
Vascular Calcification
7 6 5 4 3 2 1 0
***p<0.01
Disease Disease + Untreated BMP-7
Source: Lund et al, 2004 and Davies et al, 2003
34
©2005 Curis Inc.
Ortho Biotech (J&J) BMP License: Anticipated Milestone - Market
Estimated IND filing: 2007 - 2008 1
Disease incidence: Approximately 423,000 patients have end-stage renal disease in U.S.2
Marketing advantage: Presently no effective therapies for treating renal disease and associated complications
1 BMP development recently transferred to Centocor (a separate division of Johnson & Johnson) to address issues relating to protein manufacturing.
2 U.S. Renal Data System, 2004 Annual Report
35
©2005 Curis Inc.

Undisclosed Pathway Antagonist/Agonist Program
Collaboration with
©2005 Curis Inc.
Genentech Undisclosed Pathway Antagonist/Agonist Program
Established: April 2005
Deal structure: $3 million up-front payment, development milestones of up to $140 million, product royalties, minimum two years R&D support ($6M) (1)
Primary indications: Antagonist: not disclosed
Agonist: not disclosed
(1) – Development milestone total is based on the successful development and regulatory approval of two products in each of the agonist and antagonist 37
programs. ©2005 Curis Inc.
37
©2005 Curis Inc.
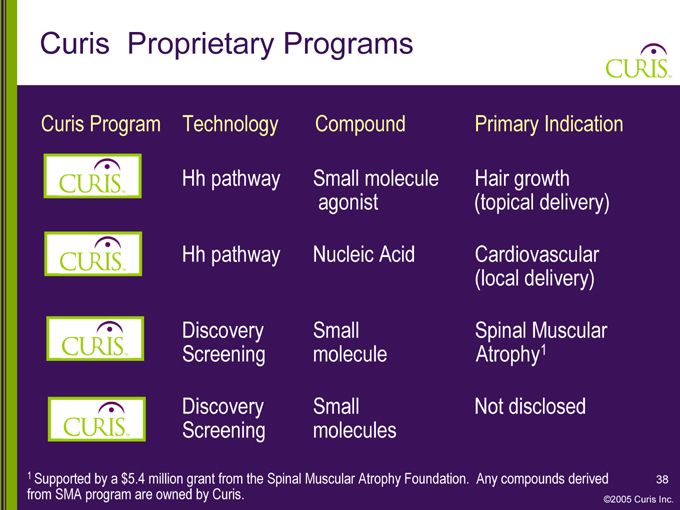
Curis Proprietary Programs
Curis Program Technology Compound Primary Indication
Hh pathway Small molecule Hair growth agonist (topical delivery)
Hh pathway Nucleic Acid Cardiovascular (local delivery)
Discovery Small Spinal Muscular Screening molecule Atrophy1
Discovery Small Not disclosed Screening molecules
1 Supported by a $5.4 million grant from the Spinal Muscular Atrophy Foundation. Any compounds derived 38 from SMA program are owned by Curis. ©2005 Curis Inc.
38
©2005 Curis Inc.
Hair Growth Regulation
(Hedgehog Agonist Technologies)
Development by
©2004 Curis Inc.
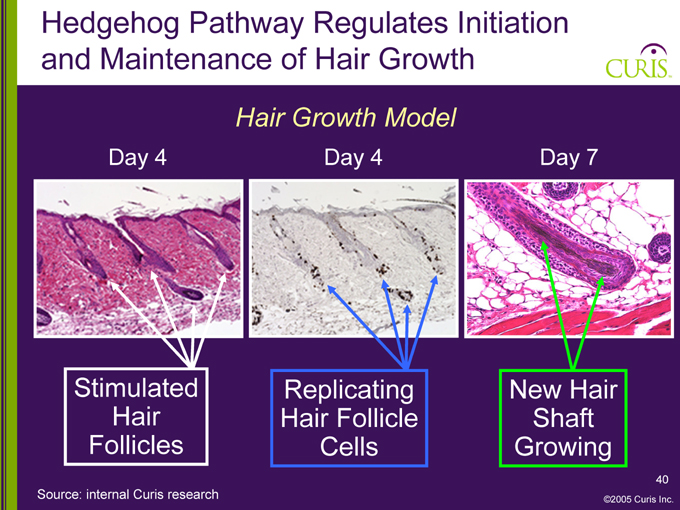
Hedgehog Pathway Regulates Initiation and Maintenance of Hair Growth
Hair Growth Model
Day 4 Day 4 Day 7
Stimulated Hair Follicles
Replicating Hair Follicle Cells
New Hair Shaft Growing
Source: internal Curis research
40
©2005 Curis Inc.
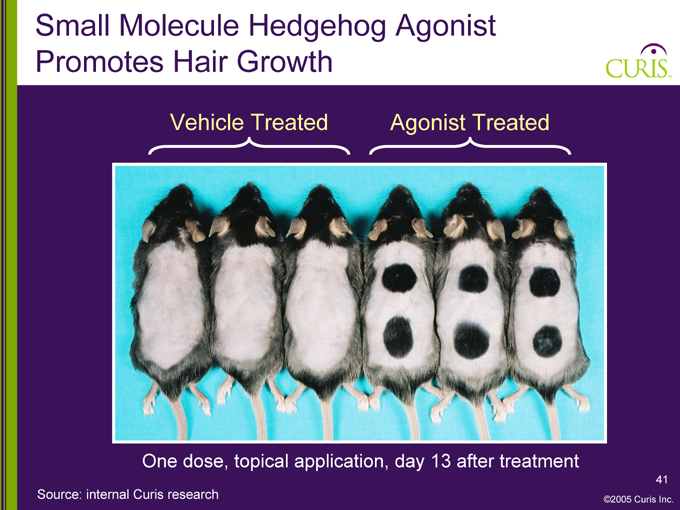
Small Molecule Hedgehog Agonist Promotes Hair Growth
Vehicle Treated Agonist Treated
One dose, topical application, day 13 after treatment
Source: internal Curis research
41
©2005 Curis Inc.

Presentation Outline
Business Model
Technology Overview
Collaborations and Product Candidates
Patents/Financials
42
©2005 Curis Inc.
Strong Intellectual Property Position
Approximately 200 US patents issued or allowed and over 100 pending in U.S. Approximately 300 foreign patents issued or allowed and 350 foreign patents pending Compositions of matter covering signaling proteins, receptors, antibodies, small molecule agonists and antagonists, and others Fields of medical use including renal disorders, metabolic bone diseases, neurology, oncology, cardiovascular disease, alopecia, and others Methods of screening for small molecules, manufacture, proliferation, purification, differentiation, and others
43
©2005 Curis Inc.
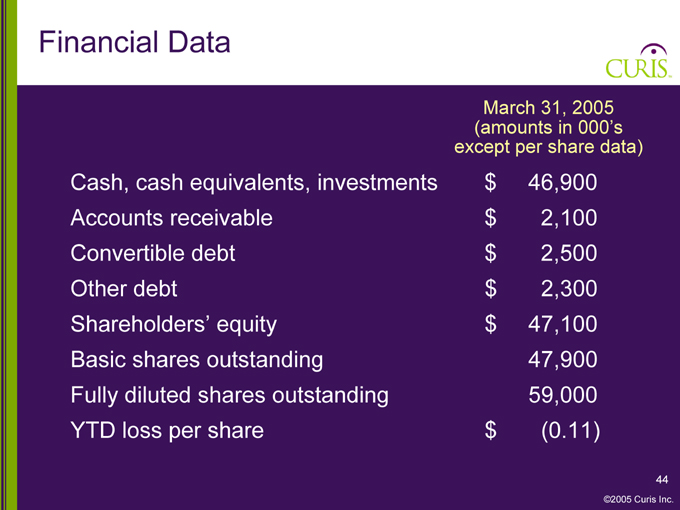
Financial Data
March 31, 2005
(amounts in 000’s
except per share data)
Cash, cash equivalents, investments $ 46,900
Accounts receivable $ 2,100
Convertible debt $ 2,500
Other debt $ 2,300
Shareholders’ equity $ 47,100
Basic shares outstanding 47,900
Fully diluted shares outstanding 59,000
YTD loss per share $ (0.11)
44
©2005 Curis Inc.

Financial Guidance
Cash life Mid-20071 BCC Co-development costs to Ph II completion (Mid 2007) $20 million
2005 Guidance:
Cash at December 31, 2005 $36 - $39 million Gross revenues $11 - $13 million BCC Co-development costs $8.5 - $9 million 2 Research and Development $13 - $15 million Administration $7.5 - $9 million
1 – Includes cash, marketable securities, investments and long-term investments. 2 – Amounts will be recorded as contra-revenue.
45
©2005 Curis Inc.

Curis, Inc. (NASDAQ: CRIS) Corporate Overview June 20, 2005
©2005 Curis Inc.













































