
Corporate Presentation © Curis, Inc. 2017 – All Rights Reserved Exhibit 99.1

This presentation contains certain forward-looking statements about Curis, Inc. (“we,” “us,” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as “expect(s),” “feel(s),” “believe(s),” “will,” “may,” “anticipate(s)” and similar expressions are intended to identify forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this presentation, and involve risks and uncertainties. Forward-looking statements herein include, but are not limited to, statements with respect to the timing and results of future clinical and pre-clinical milestones; the timing of future preclinical studies and clinical trials and results of these studies and trials; the clinical and therapeutic potential of our drug candidates; and management’s ability to successfully achieve its goals. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of important factors including, without limitation, risks relating to: whether any of our drug candidates will advance further in the clinical development process and whether and when, if at all, they will receive approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies; whether historical preclinical results will be predictive of future clinical trial results; whether historical clinical trial results will be predictive of future trial results; whether any of our drug candidate discovery and development efforts will be successful; whether any of our drug candidates will be successfully marketed if approved; our ability to achieve the benefits contemplated by our collaboration agreements; management’s ability to successfully achieve its goals; our ability to raise additional capital to fund our operations on terms acceptable to us; general economic conditions; competition; and the other risk factors contained in our periodic and interim reports filed with the Securities and Exchange Commission which are available on the SEC website at www.sec.gov. You are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events, except as required by law. Forward Looking Statements
Mission: Innovative Medicines to Treat Cancer Effectively Precision Oncology Oncology-Focused Biotech Pipeline of differentiated small molecule cancer drug candidates Developing and intend to commercialize oncology drugs Partnerships: Aurigene, Genentech/Roche Immuno-oncology
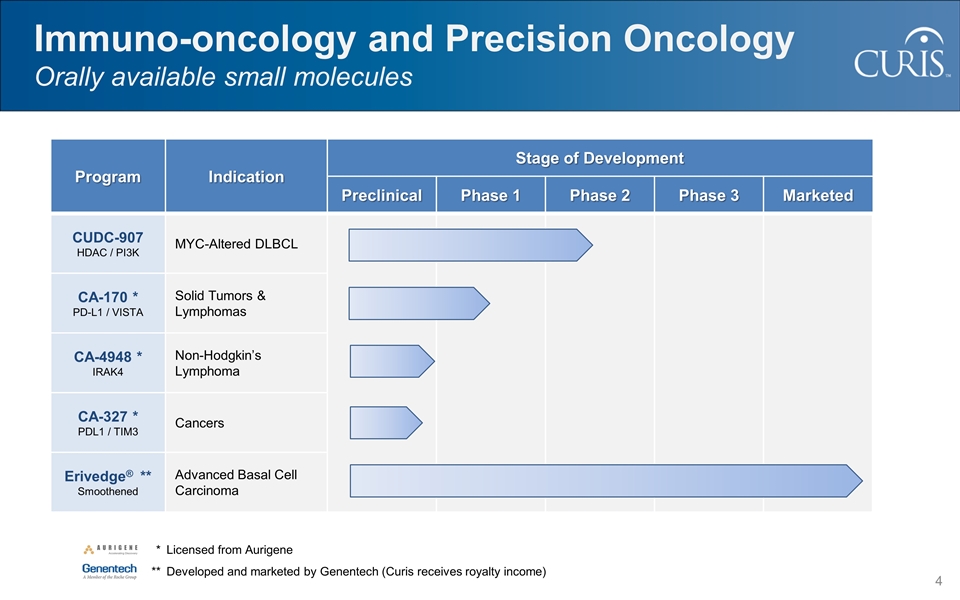
Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CUDC-907 HDAC / PI3K MYC-Altered DLBCL CA-170 * PD-L1 / VISTA Solid Tumors & Lymphomas CA-4948 * IRAK4 Non-Hodgkin’s Lymphoma CA-327 * PDL1 / TIM3 Cancers Erivedge® ** Smoothened Advanced Basal Cell Carcinoma Immuno-oncology and Precision Oncology Orally available small molecules * Licensed from Aurigene **Developed and marketed by Genentech (Curis receives royalty income)
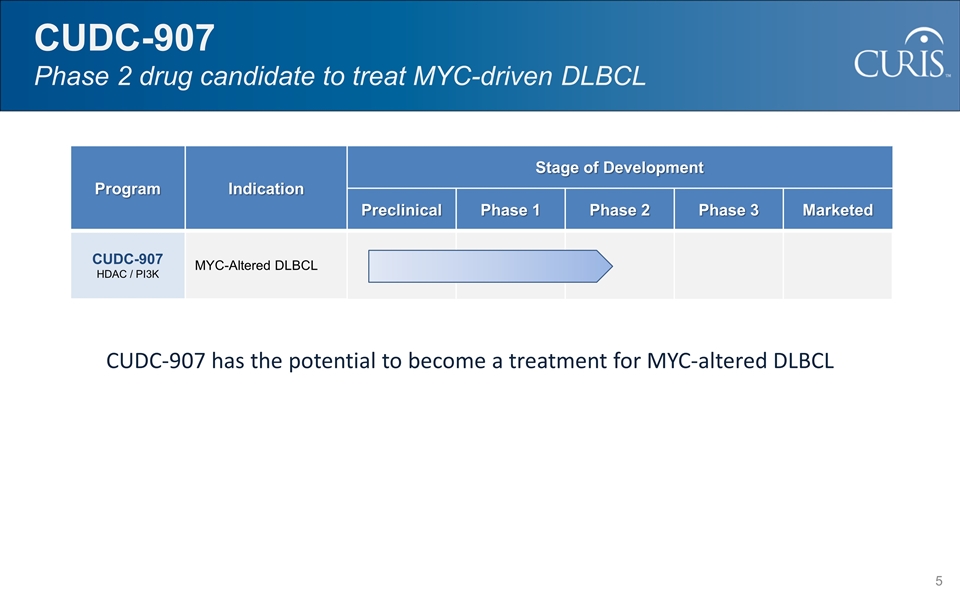
CUDC-907 has the potential to become a treatment for MYC-altered DLBCL CUDC-907 Phase 2 drug candidate to treat MYC-driven DLBCL Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CUDC-907 HDAC / PI3K MYC-Altered DLBCL
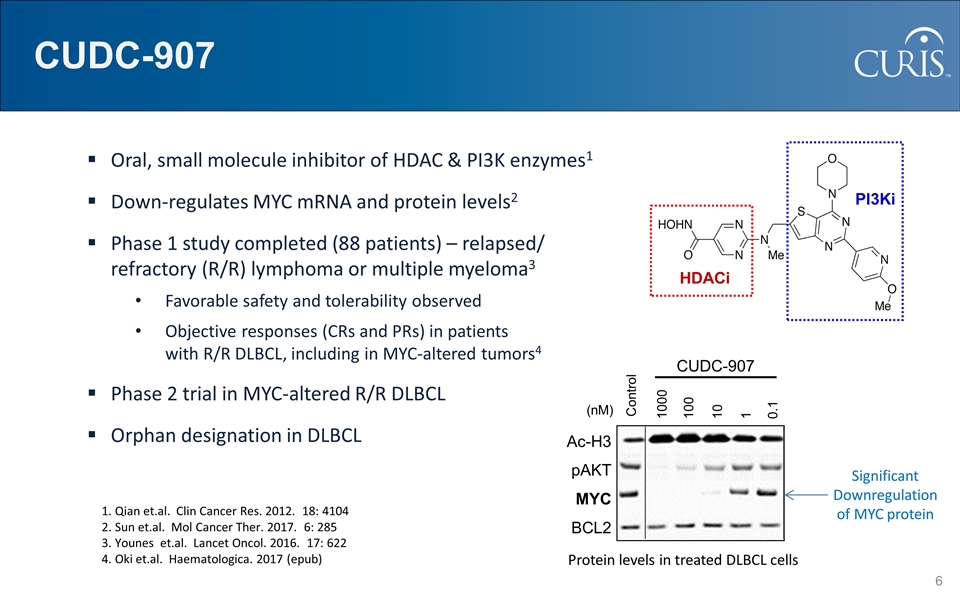
HDACi PI3Ki CUDC-907 Oral, small molecule inhibitor of HDAC & PI3K enzymes1 Down-regulates MYC mRNA and protein levels2 Phase 1 study completed (88 patients) – relapsed/ refractory (R/R) lymphoma or multiple myeloma3 Favorable safety and tolerability observed Objective responses (CRs and PRs) in patients with R/R DLBCL, including in MYC-altered tumors4 Phase 2 trial in MYC-altered R/R DLBCL Orphan designation in DLBCL CUDC-907 Control 1000 100 10 1 0.1 Ac-H3 pAKT MYC BCL2 (nM) 1. Qian et.al. Clin Cancer Res. 2012. 18: 4104 2. Sun et.al. Mol Cancer Ther. 2017. 6: 285 3. Younes et.al. Lancet Oncol. 2016. 17: 622 4. Oki et.al. Haematologica. 2017 (epub) Significant Downregulation of MYC protein Protein levels in treated DLBCL cells
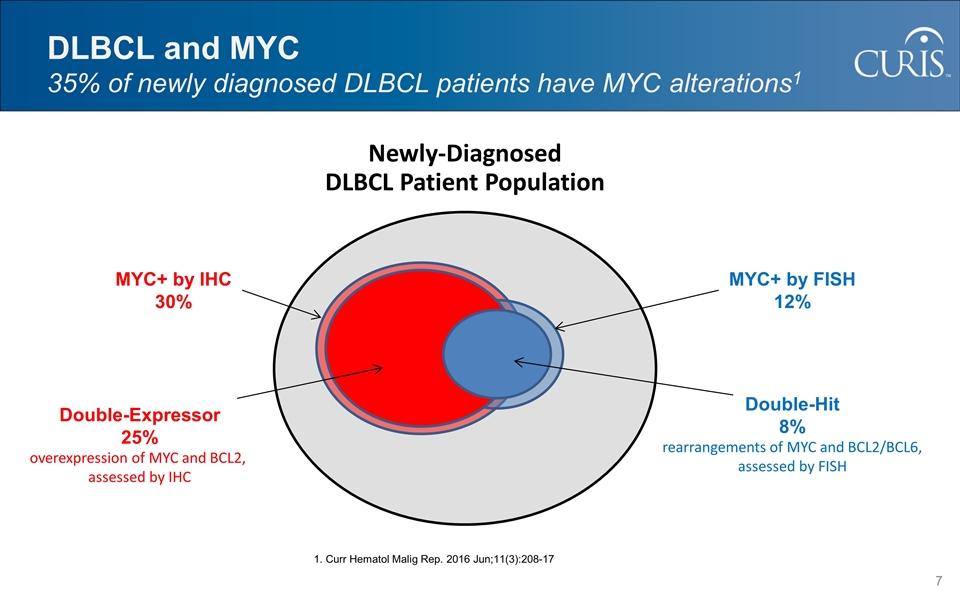
DLBCL and MYC 35% of newly diagnosed DLBCL patients have MYC alterations1 Newly-Diagnosed DLBCL Patient Population MYC+ by IHC 30% Double-Expressor 25% overexpression of MYC and BCL2, assessed by IHC MYC+ by FISH 12% Double-Hit 8% rearrangements of MYC and BCL2/BCL6, assessed by FISH 1. Curr Hematol Malig Rep. 2016 Jun;11(3):208-17

CUDC-907 Phase 1 and Phase 2 Trials in DLBCL Study population Relapsed or refractory DLBCL after ≥2 prior regimens Total of 105 patients enrolled in Phase 1 (37) and Phase 2 (68) Treatment: oral, once daily dosing – RP2D 5/2 (5 days on, 2 days off): 60mg continuous dosing Safety and tolerability No DLT at RP2D Phase 1 DLT of diarrhea (1 pt) and hyperglycemia (1 pt) Interim results for Phase 2 show that most common (> 10% of patients) Grade ≥ 3 TEAE observed to date: diarrhea (14%), neutropenia (14%), thrombocytopenia (19%)
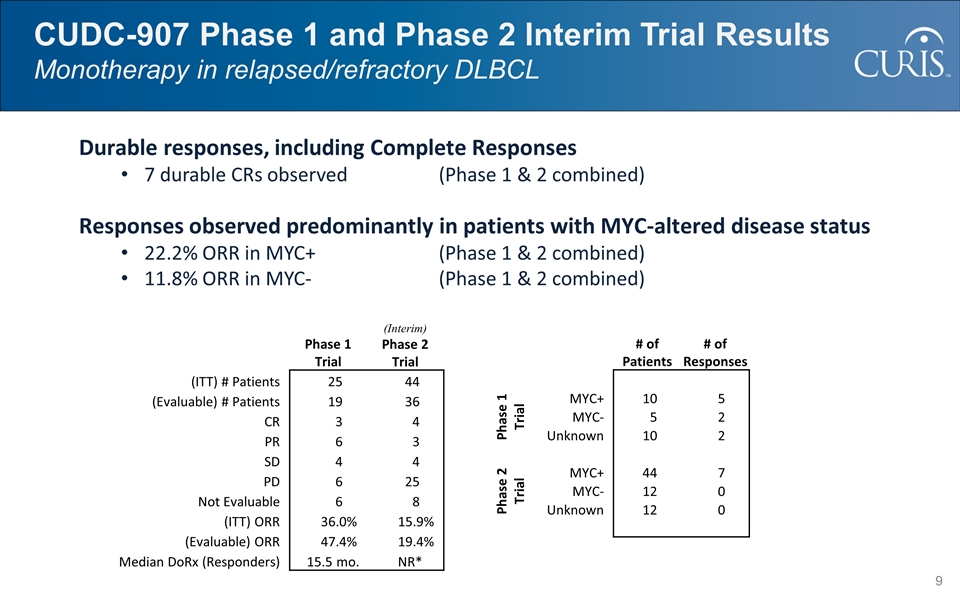
CUDC-907 Phase 1 and Phase 2 Interim Trial Results Monotherapy in relapsed/refractory DLBCL Durable responses, including Complete Responses 7 durable CRs observed (Phase 1 & 2 combined) Responses observed predominantly in patients with MYC-altered disease status 22.2% ORR in MYC+(Phase 1 & 2 combined) 11.8% ORR in MYC-(Phase 1 & 2 combined) Phase 1 Trial (Interim) Phase 2 Trial (ITT) # Patients 25 44 (Evaluable) # Patients 19 36 CR 3 4 PR 6 3 SD 4 4 PD 6 25 Not Evaluable 6 8 (ITT) ORR 36.0% 15.9% (Evaluable) ORR 47.4% 19.4% Median DoRx (Responders) 15.5 mo. NR* # of Patients # of Responses Phase 1 Trial MYC+ 10 5 MYC- 5 2 Unknown 10 2 Phase 2 Trial MYC+ 44 7 MYC- 12 0 Unknown 12 0
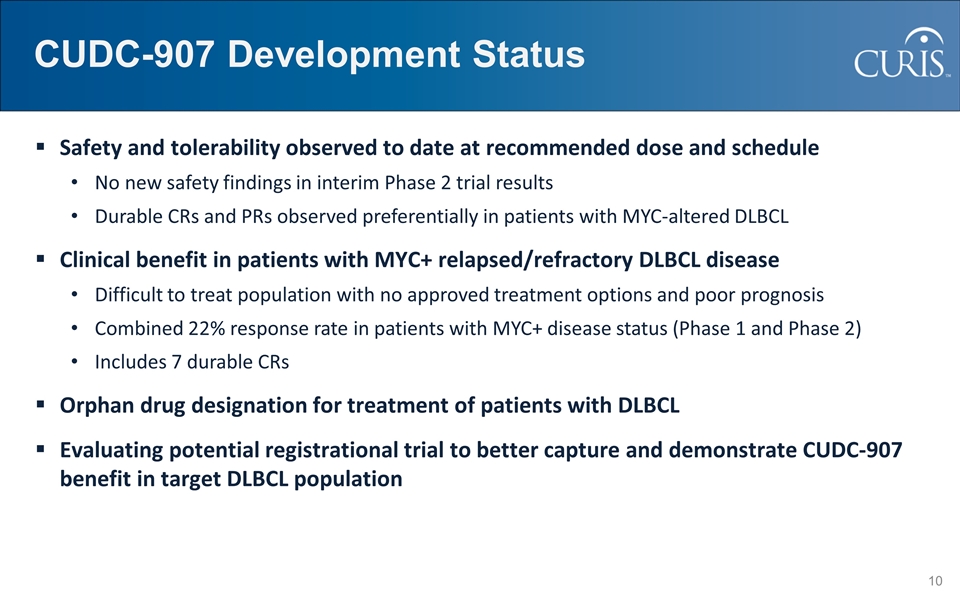
CUDC-907 Development Status Safety and tolerability observed to date at recommended dose and schedule No new safety findings in interim Phase 2 trial results Durable CRs and PRs observed preferentially in patients with MYC-altered DLBCL Clinical benefit in patients with MYC+ relapsed/refractory DLBCL disease Difficult to treat population with no approved treatment options and poor prognosis Combined 22% response rate in patients with MYC+ disease status (Phase 1 and Phase 2) Includes 7 durable CRs Orphan drug designation for treatment of patients with DLBCL Evaluating potential registrational trial to better capture and demonstrate CUDC-907 benefit in target DLBCL population
CA-170 is a first-in-class oral small molecule immune checkpoint inhibitor In Phase 1 clinical development CA-170 Oral, small molecule checkpoint inhibitor – PDL1 and VISTA Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CA-170 PDL1 / VISTA Solid Tumors & Lymphomas
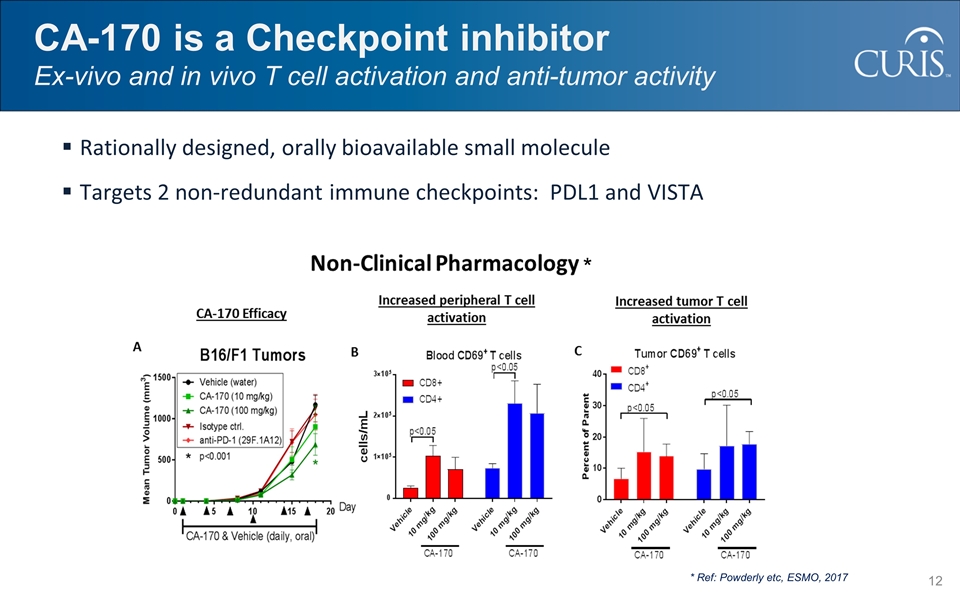
CA-170 is a Checkpoint inhibitor Ex-vivo and in vivo T cell activation and anti-tumor activity * Ref: Powderly etc, ESMO, 2017 * Rationally designed, orally bioavailable small molecule Targets 2 non-redundant immune checkpoints: PDL1 and VISTA
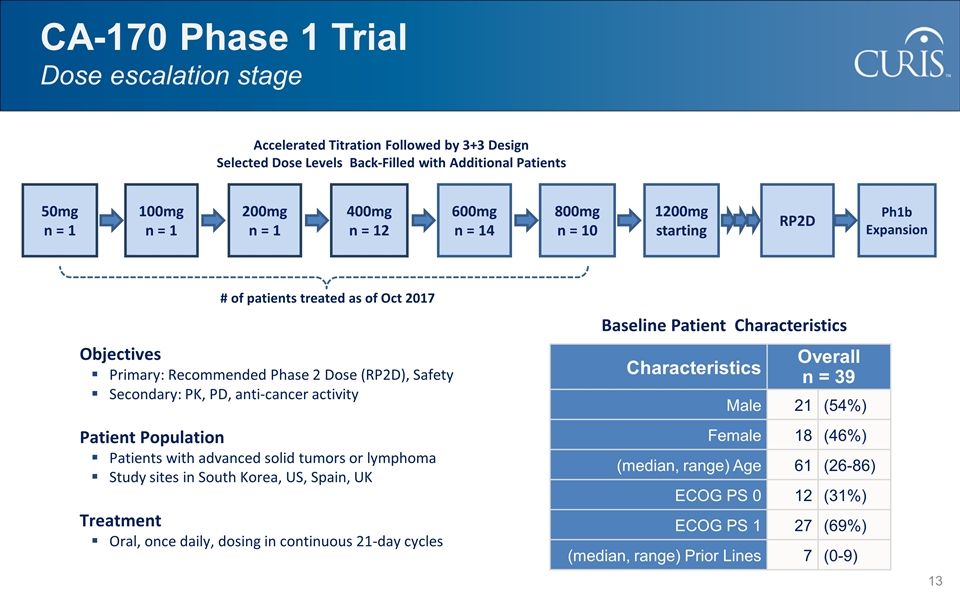
CA-170 Phase 1 Trial Dose escalation stage Objectives Primary: Recommended Phase 2 Dose (RP2D), Safety Secondary: PK, PD, anti-cancer activity Patient Population Patients with advanced solid tumors or lymphoma Study sites in South Korea, US, Spain, UK Treatment Oral, once daily, dosing in continuous 21-day cycles Characteristics Overall n = 39 Male 21 (54%) Female 18 (46%) (median, range) Age 61 (26-86) ECOG PS 0 12 (31%) ECOG PS 1 27 (69%) (median, range) Prior Lines 7 (0-9) Accelerated Titration Followed by 3+3 Design Selected Dose Levels Back-Filled with Additional Patients 50mg n = 1 100mg n = 1 400mg n = 12 600mg n = 14 800mg n = 10 1200mg starting # of patients treated as of Oct 2017 Baseline Patient Characteristics RP2D Ph1b Expansion 200mg n = 1
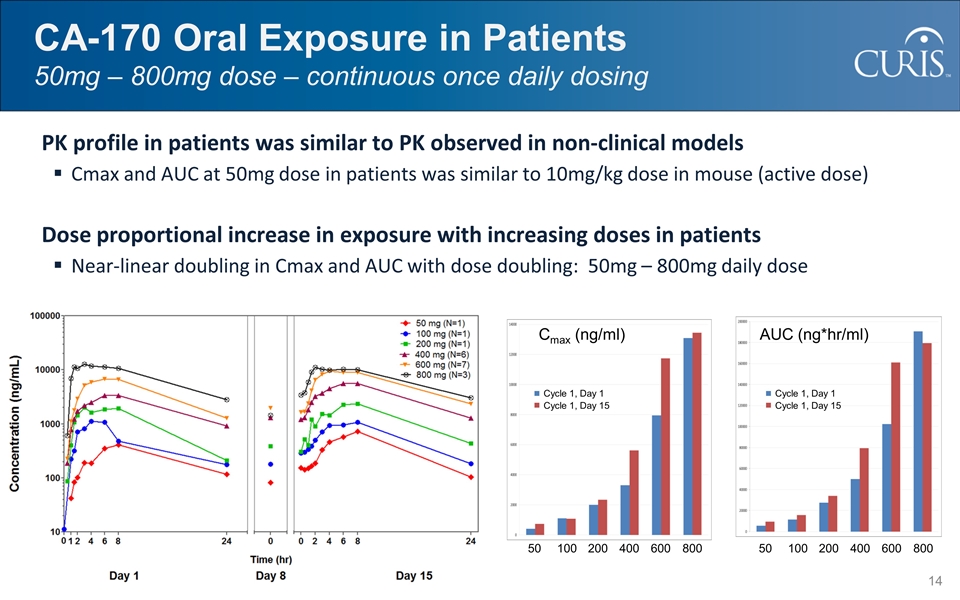
CA-170 Oral Exposure in Patients 50mg – 800mg dose – continuous once daily dosing PK profile in patients was similar to PK observed in non-clinical models Cmax and AUC at 50mg dose in patients was similar to 10mg/kg dose in mouse (active dose) Dose proportional increase in exposure with increasing doses in patients Near-linear doubling in Cmax and AUC with dose doubling: 50mg – 800mg daily dose Human PK Profile 50 100 200 400 600 800 50 100 200 400 600 800 Cycle 1, Day 1 Cycle 1, Day 15 Cmax (ng/ml) Cycle 1, Day 1 Cycle 1, Day 15 AUC (ng*hr/ml)
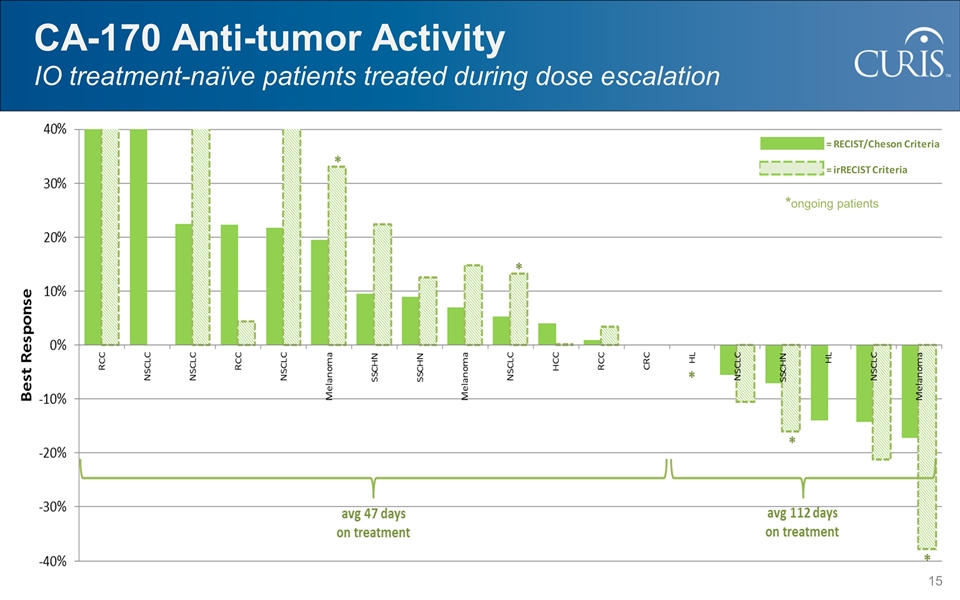
CA-170 Anti-tumor Activity IO treatment-naïve patients treated during dose escalation *ongoing patients
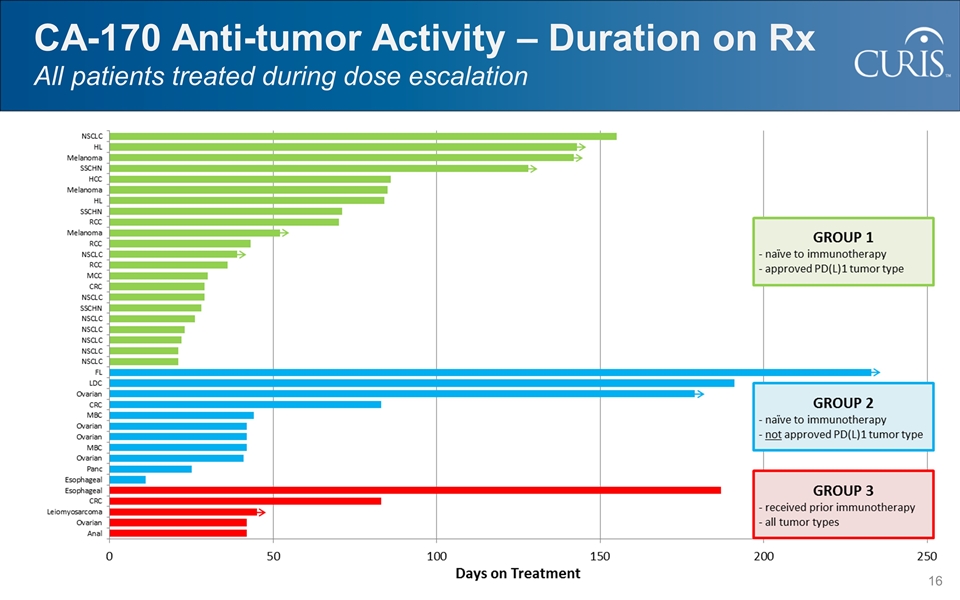
CA-170 Anti-tumor Activity – Duration on Rx All patients treated during dose escalation
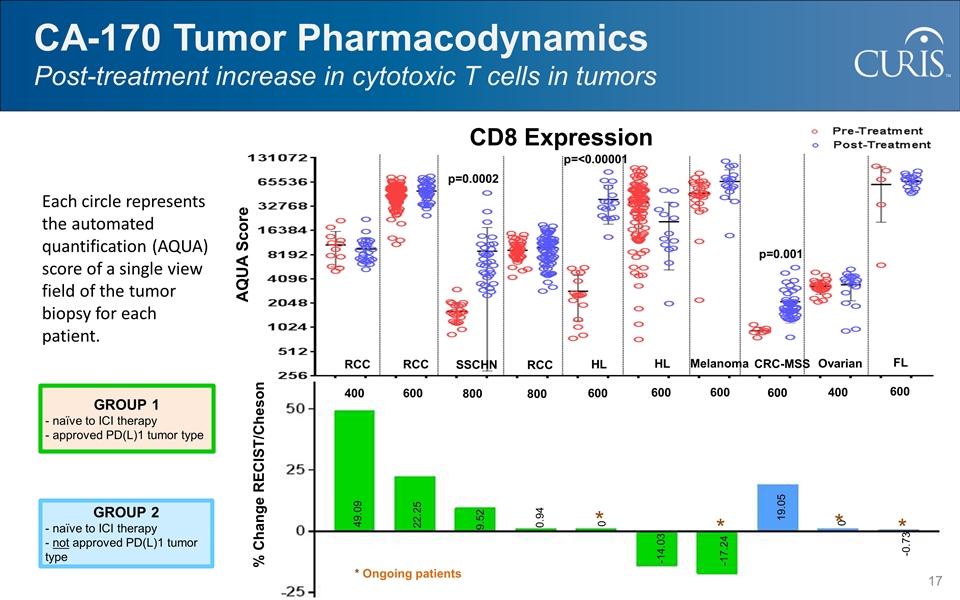
CA-170 Tumor Pharmacodynamics Post-treatment increase in cytotoxic T cells in tumors GROUP 1 - naïve to ICI therapy - approved PD(L)1 tumor type GROUP 2 - naïve to ICI therapy - not approved PD(L)1 tumor type -17.24 49.09 22.25 9.52 0.94 0 -14.03 19.05 0 -0.73 % Change RECIST/Cheson * * * * * Ongoing patients RCC 400 RCC 600 SSCHN 800 RCC 800 HL 600 CRC-MSS 600 Melanoma 600 HL 600 Ovarian 400 FL 600 AQUA Score CD8 Expression p=0.001 p=0.0002 p=<0.00001 Each circle represents the automated quantification (AQUA) score of a single view field of the tumor biopsy for each patient.
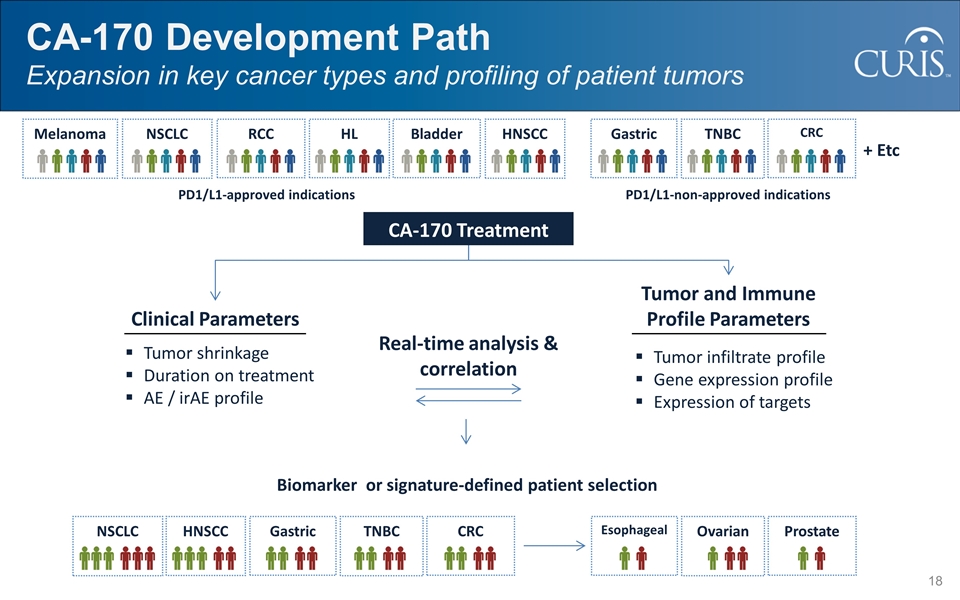
CA-170 Development Path Expansion in key cancer types and profiling of patient tumors Melanoma NSCLC RCC HL TNBC HNSCC CRC Bladder Gastric + Etc CA-170 Treatment Clinical Parameters Tumor and Immune Profile Parameters Tumor shrinkage Duration on treatment AE / irAE profile Tumor infiltrate profile Gene expression profile Expression of targets Real-time analysis & correlation PD1/L1-approved indications PD1/L1-non-approved indications Biomarker or signature-defined patient selection NSCLC TNBC HNSCC CRC Gastric Ovarian Prostate Esophageal
CA-327 is an oral, small molecule immune checkpoint inhibitor IND filing expected in 1H 2018 CA-327 Oral, small molecule checkpoint inhibitor – PDL1 and TIM3 Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CA-327 PD-L1 / TIM3 Cancers
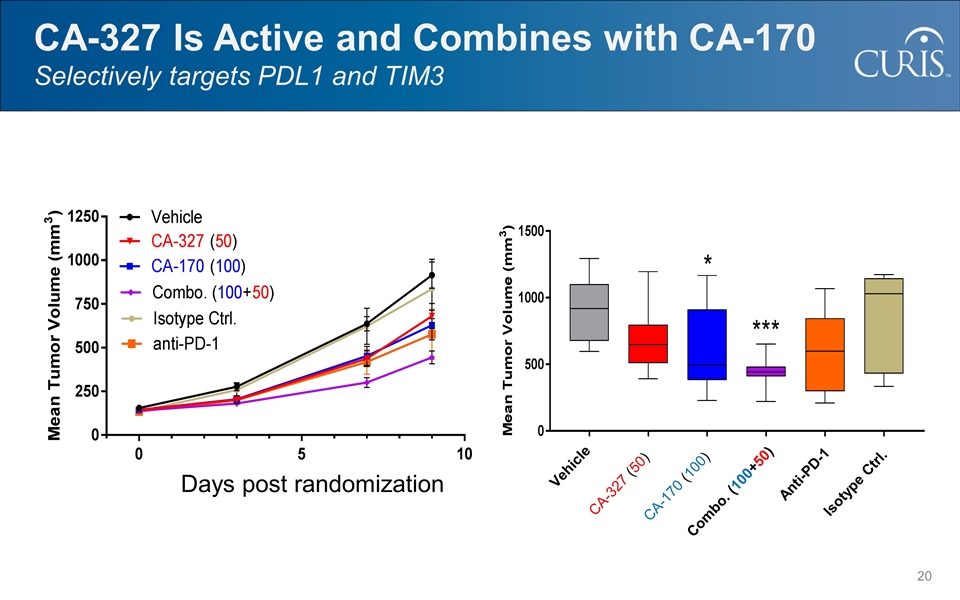
CA-327 Is Active and Combines with CA-170 Selectively targets PDL1 and TIM3 CA-327 (50) CA-170 (100) Combo. (100+50) Anti-PD-1 Isotype Ctrl. Vehicle Days post randomization
CA-4948 is an oral, small molecule inhibitor of IRAK4 Patient enrollment expected in 4Q 2017 (Phase 1 trial in Non-Hodgkin’s Lymphoma) CA-4948 Oral, small molecule inhibitor of IRAK4 Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CA-4948 IRAK4 Non-Hodgkin’s Lymphoma
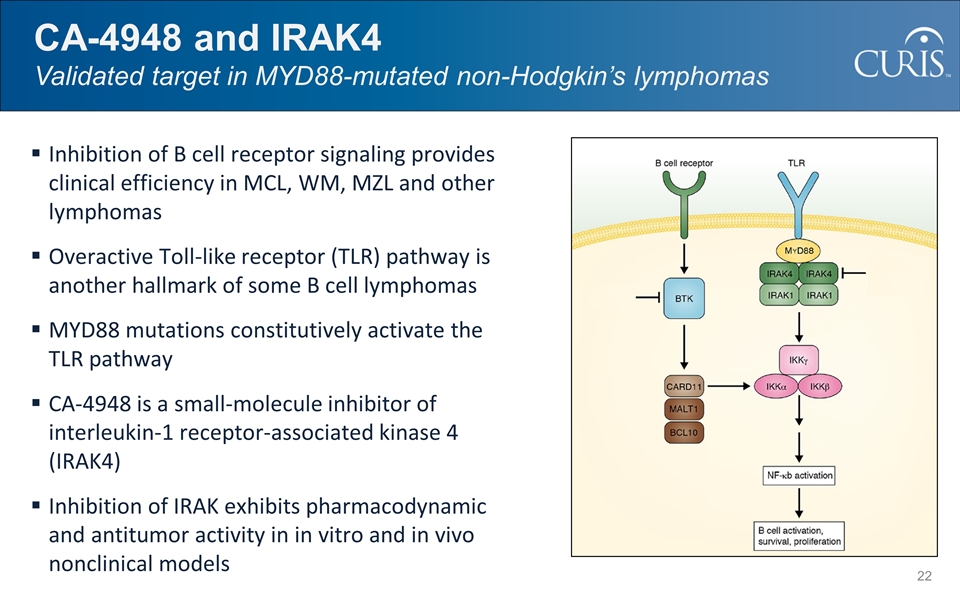
Inhibition of B cell receptor signaling provides clinical efficiency in MCL, WM, MZL and other lymphomas Overactive Toll-like receptor (TLR) pathway is another hallmark of some B cell lymphomas MYD88 mutations constitutively activate the TLR pathway CA-4948 is a small-molecule inhibitor of interleukin-1 receptor-associated kinase 4 (IRAK4) Inhibition of IRAK exhibits pharmacodynamic and antitumor activity in in vitro and in vivo nonclinical models CA-4948 and IRAK4 Validated target in MYD88-mutated non-Hodgkin’s lymphomas
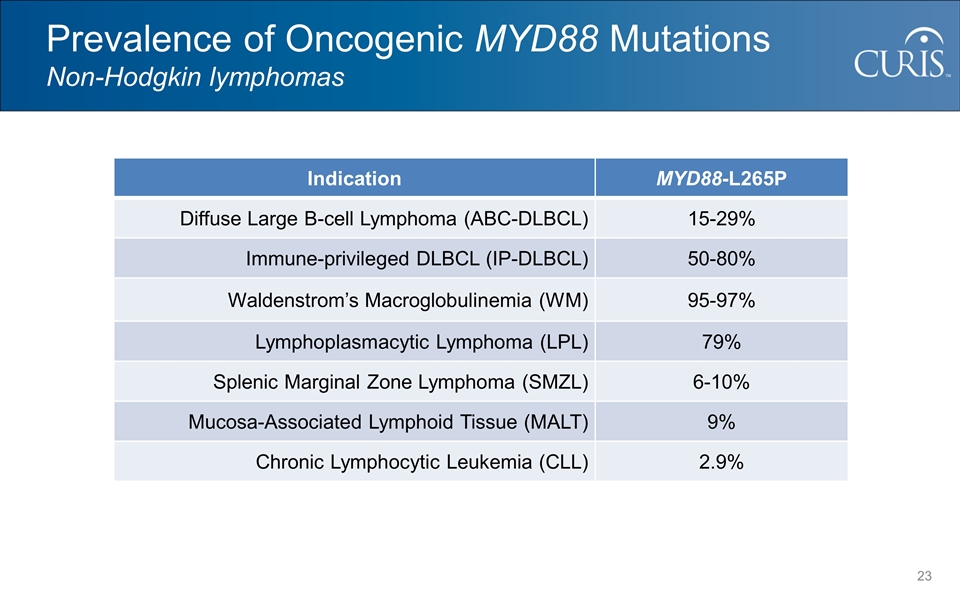
Prevalence of Oncogenic MYD88 Mutations Non-Hodgkin lymphomas Indication MYD88-L265P Diffuse Large B-cell Lymphoma (ABC-DLBCL) 15-29% Immune-privileged DLBCL (IP-DLBCL) 50-80% Waldenstrom’s Macroglobulinemia (WM) 95-97% Lymphoplasmacytic Lymphoma (LPL) 79% Splenic Marginal Zone Lymphoma (SMZL) 6-10% Mucosa-Associated Lymphoid Tissue (MALT) 9% Chronic Lymphocytic Leukemia (CLL) 2.9%
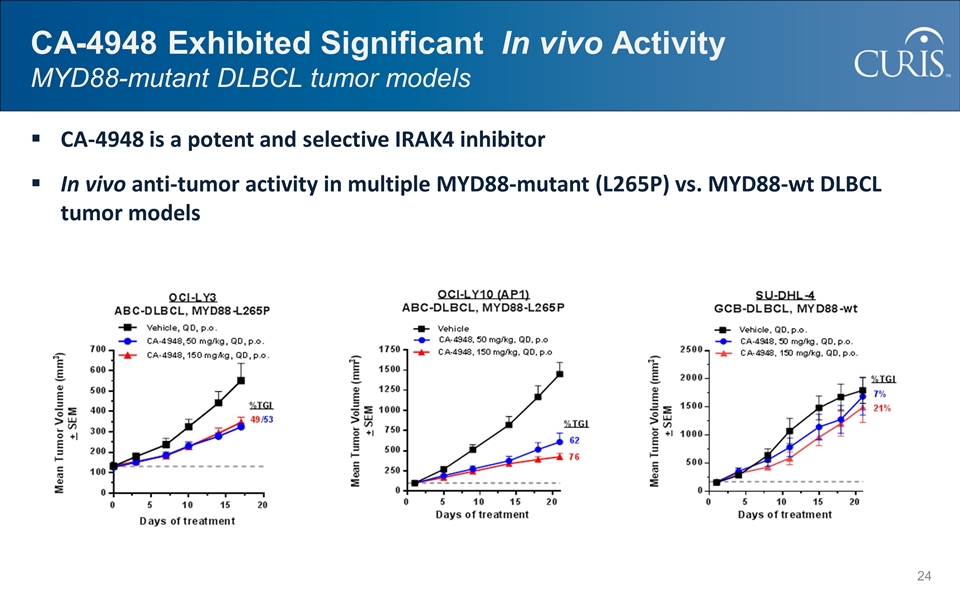
CA-4948 Exhibited Significant In vivo Activity MYD88-mutant DLBCL tumor models CA-4948 is a potent and selective IRAK4 inhibitor In vivo anti-tumor activity in multiple MYD88-mutant (L265P) vs. MYD88-wt DLBCL tumor models
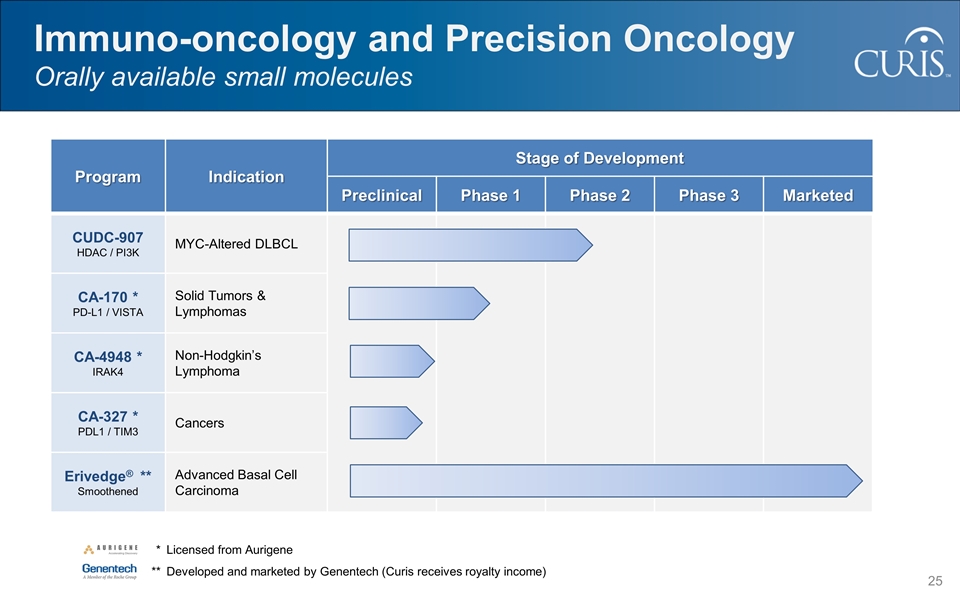
Program Indication Stage of Development Preclinical Phase 1 Phase 2 Phase 3 Marketed CUDC-907 HDAC / PI3K MYC-Altered DLBCL CA-170 * PD-L1 / VISTA Solid Tumors & Lymphomas CA-4948 * IRAK4 Non-Hodgkin’s Lymphoma CA-327 * PDL1 / TIM3 Cancers Erivedge® ** Smoothened Advanced Basal Cell Carcinoma Immuno-oncology and Precision Oncology Orally available small molecules * Licensed from Aurigene **Developed and marketed by Genentech (Curis receives royalty income)

CUDC-907 Updated regulatory path n Q1 2018 CA-170 Phase 1 dose escalationn Ongoing Phase 2 expansion trial n Q4 2017 – in India CA-4948 Phase 1 dose escalation n Q4 2017 CA-327 IND filing n 1H 2018 Projected Milestones
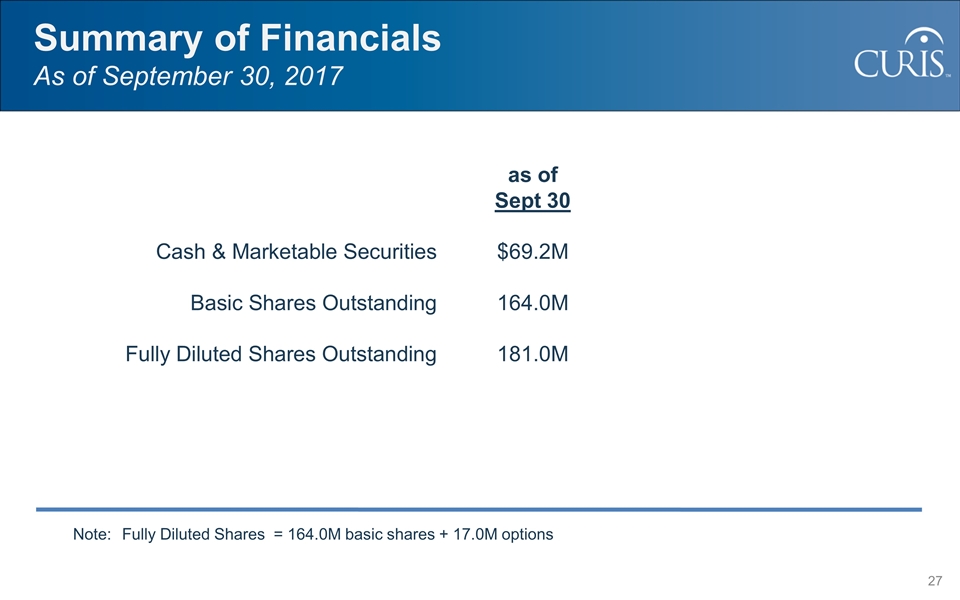
Summary of Financials As of September 30, 2017 as of Sept 30 Cash & Marketable Securities $69.2M Basic Shares Outstanding164.0M Fully Diluted Shares Outstanding 181.0M Note: Fully Diluted Shares = 164.0M basic shares + 17.0M options

END



























