
CA-4948 & CI-8993 Clinical Data Update January 2022 Exhibit 99.1

Cautionary Note Regarding Forward Looking Statements This presentation contains certain forward-looking statements about Curis, Inc. (“we,” “us,” or the “Company”) within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. Words such as “expect(s),” “believe(s),” “will,” “may,” “anticipate(s),” “focus(es),” “plans,” “mission,” “strategy,” “potential,” “estimate(s)”, "intend," "project," "seek," "should," "would" and similar expressions are intended to identify forward-looking statements. Forward-looking statements are statements that are not historical facts, reflect management’s expectations as of the date of this presentation, and involve important risks and uncertainties. Forward-looking statements herein include, but are not limited to, expectations of the potential for Company’s proprietary drug candidates CA-4948 and CI-8993, including with respect to the potency, anti-cancer activity, durability and tolerability of CA-4948 and CI-8993; future studies with respect to CA-4948 and CI-8993; the potential advantages and benefits of CA-4948, CI-8993 and checkpoint inhibitors over other therapies; and the Company’s plans to advance its development programs for CA-4948 and CI-8993, including with respect to anticipated results, clinical trials, regulatory and commercialization plans and timelines. These forward-looking statements are based on our current expectations and may differ materially from actual results due to a variety of important factors including, without limitation, risks relating to: whether any of our drug candidates will advance further in the clinical development process and whether and when, if at all, they will receive approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies; whether historical preclinical results will be predictive of future clinical trial results; whether historical clinical trial results will be predictive of future trial results; whether any of our drug candidate discovery and development efforts will be successful; whether any of our drug candidates will be successfully marketed if approved; our ability to achieve the benefits contemplated by our collaboration agreements; management’s ability to successfully achieve its goals; the sufficiency of our cash resources; our ability to raise additional capital to fund our operations on terms acceptable to us or the use of proceeds of any offering of securities or other financing; general economic conditions; competition; and the other risk factors contained in our periodic and interim reports filed with the Securities and Exchange Commission which are available on the SEC website at www.sec.gov. You are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events, except as required by law.

Today’s Participants James Dentzer President & Chief Executive Officer at Curis Robert Martell, MD, PhD Head of R&D at Curis Daniel DeAngelo, MD, PhDChief, Division of Leukemia at Dana-Farber Cancer Institute

Introductory Remarks Clinical update on CA-4948 (IRAK4) and CI-8993 (VISTA) CA-4948 addresses a novel target (IRAK4) and: (1) demonstrates clear anti-cancer activity as an oral single agent (2) is active in genetically-defined populations that can be identified and enrolled (3) has the added potential benefit of also hitting FLT3 CI-8993 addresses a novel target (VISTA) and: (1) has successfully cleared dose level where Janssen observed dose-limiting toxicity (DLT) (2) with pharmacodynamic effects suggesting multiple anti-cancer mechanisms are being activated

CA-4948: First-in-class IRAK4 Inhibitor Targeting Specific Genetic Populations in R/R AML and high-risk MDS (MDS)
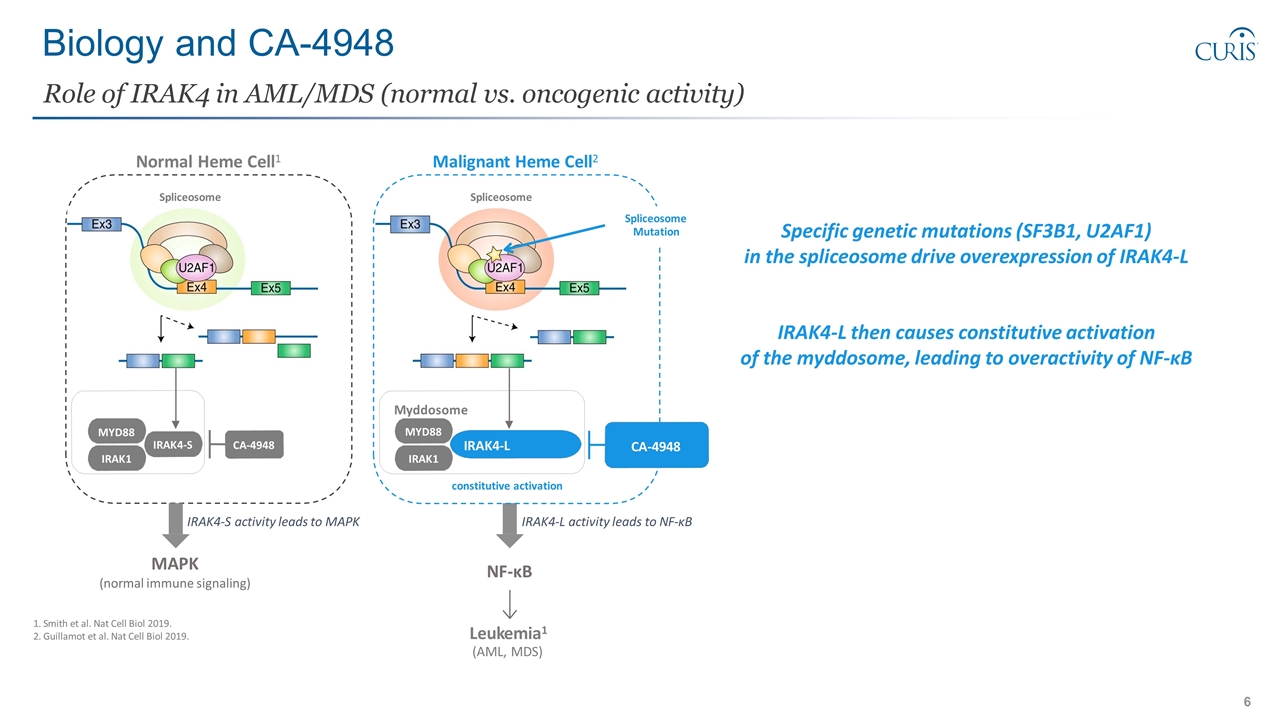
Biology and CA-4948 Role of IRAK4 in AML/MDS (normal vs. oncogenic activity) 1. Smith et al. Nat Cell Biol 2019. 2. Guillamot et al. Nat Cell Biol 2019. constitutive activation MYD88 IRAK1 IRAK4-S Myddosome IRAK1 IRAK4-L MYD88 Malignant Heme Cell2 Spliceosome Spliceosome Normal Heme Cell1 Spliceosome Mutation CA-4948 CA-4948 Leukemia1 (AML, MDS) NF-κB MAPK (normal immune signaling) IRAK4-L activity leads to NF-κB IRAK4-S activity leads to MAPK Specific genetic mutations (SF3B1, U2AF1) in the spliceosome drive overexpression of IRAK4-L IRAK4-L then causes constitutive activation of the myddosome, leading to overactivity of NF-κB
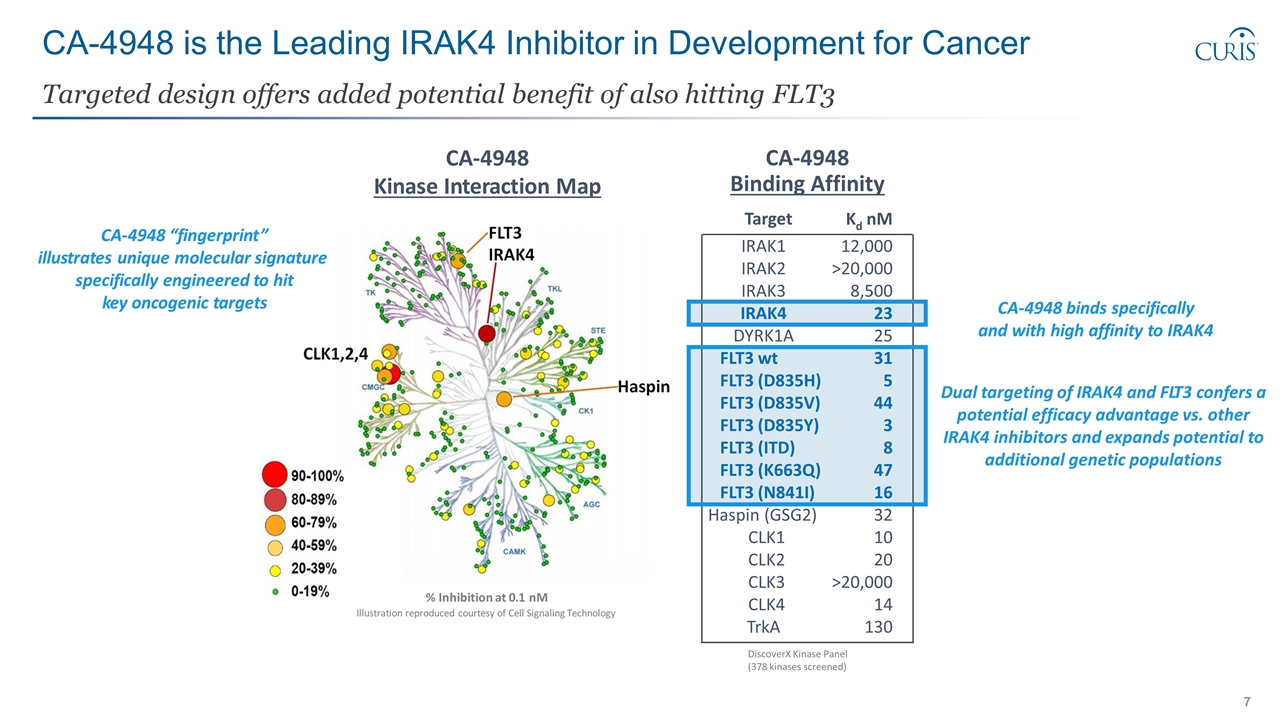
CA-4948 is the Leading IRAK4 Inhibitor in Development for Cancer Targeted design offers added potential benefit of also hitting FLT3 CA-4948 Kinase Interaction Map % Inhibition at 0.1 nM Illustration reproduced courtesy of Cell Signaling Technology CA-4948 binds specifically and with high affinity to IRAK4 CA-4948 “fingerprint” illustrates unique molecular signature specifically engineered to hit key oncogenic targets Target Kd nM IRAK1 12,000 IRAK2 >20,000 IRAK3 8,500 IRAK4 23 DYRK1A 25 FLT3 wt FLT3 (D835H) 31 5 FLT3 (D835V) 44 FLT3 (D835Y) 3 FLT3 (ITD) 8 FLT3 (K663Q) 47 FLT3 (N841I) 16 Haspin (GSG2) 32 CLK1 10 CLK2 20 CLK3 >20,000 CLK4 14 TrkA 130 CA-4948 Binding Affinity DiscoverX Kinase Panel (378 kinases screened) Dual targeting of IRAK4 and FLT3 confers a potential efficacy advantage vs. other IRAK4 inhibitors and expands potential to additional genetic populations

Clinical Study Overview Phase 1/2 Study in AML and MDS
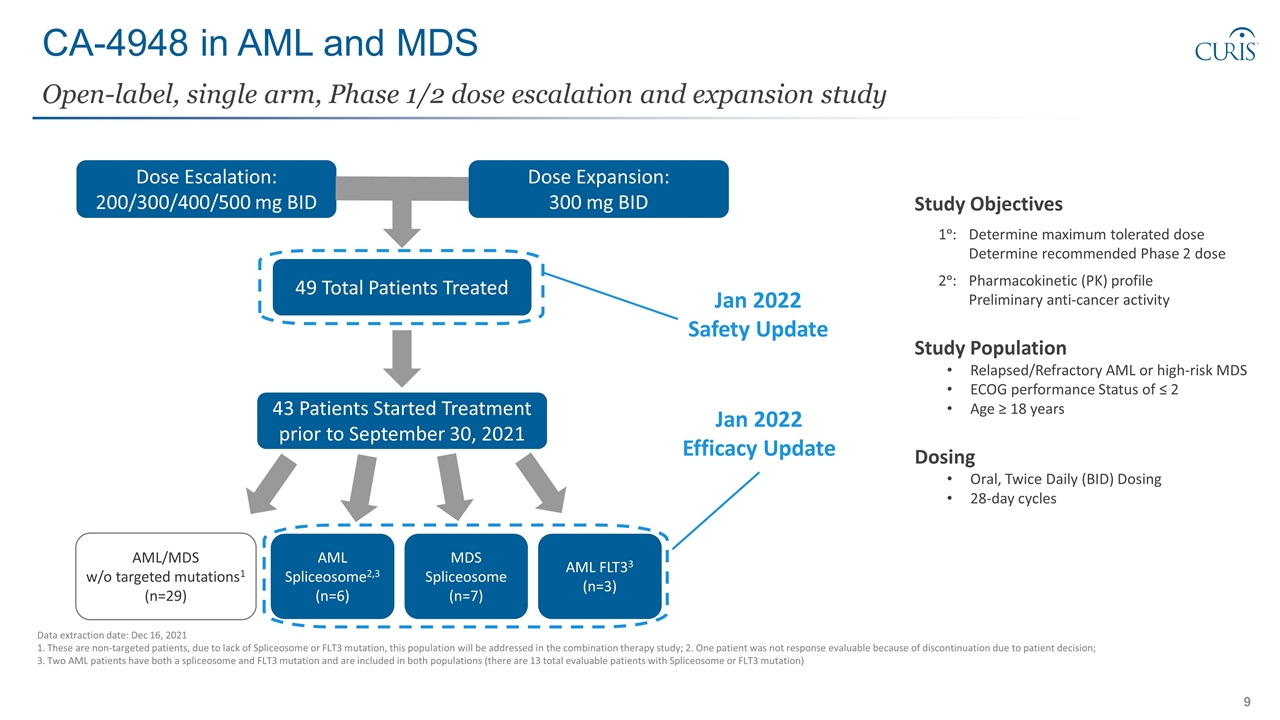
CA-4948 in AML and MDS Study Objectives 1ᵒ: Determine maximum tolerated dose Determine recommended Phase 2 dose 2ᵒ:Pharmacokinetic (PK) profile Preliminary anti-cancer activity Study Population Relapsed/Refractory AML or high-risk MDS ECOG performance Status of ≤ 2 Age ≥ 18 years Dosing Oral, Twice Daily (BID) Dosing 28-day cycles Open-label, single arm, Phase 1/2 dose escalation and expansion study Data extraction date: Dec 16, 2021 1. These are non-targeted patients, due to lack of Spliceosome or FLT3 mutation, this population will be addressed in the combination therapy study; 2. One patient was not response evaluable because of discontinuation due to patient decision; 3. Two AML patients have both a spliceosome and FLT3 mutation and are included in both populations (there are 13 total evaluable patients with Spliceosome or FLT3 mutation) Dose Escalation: 200/300/400/500 mg BID Dose Expansion: 300 mg BID 49 Total Patients Treated 43 Patients Started Treatment prior to September 30, 2021 AML Spliceosome2,3 (n=6) MDS Spliceosome (n=7) AML FLT33 (n=3) Jan 2022 Safety Update Jan 2022 Efficacy Update AML/MDS w/o targeted mutations1 (n=29)
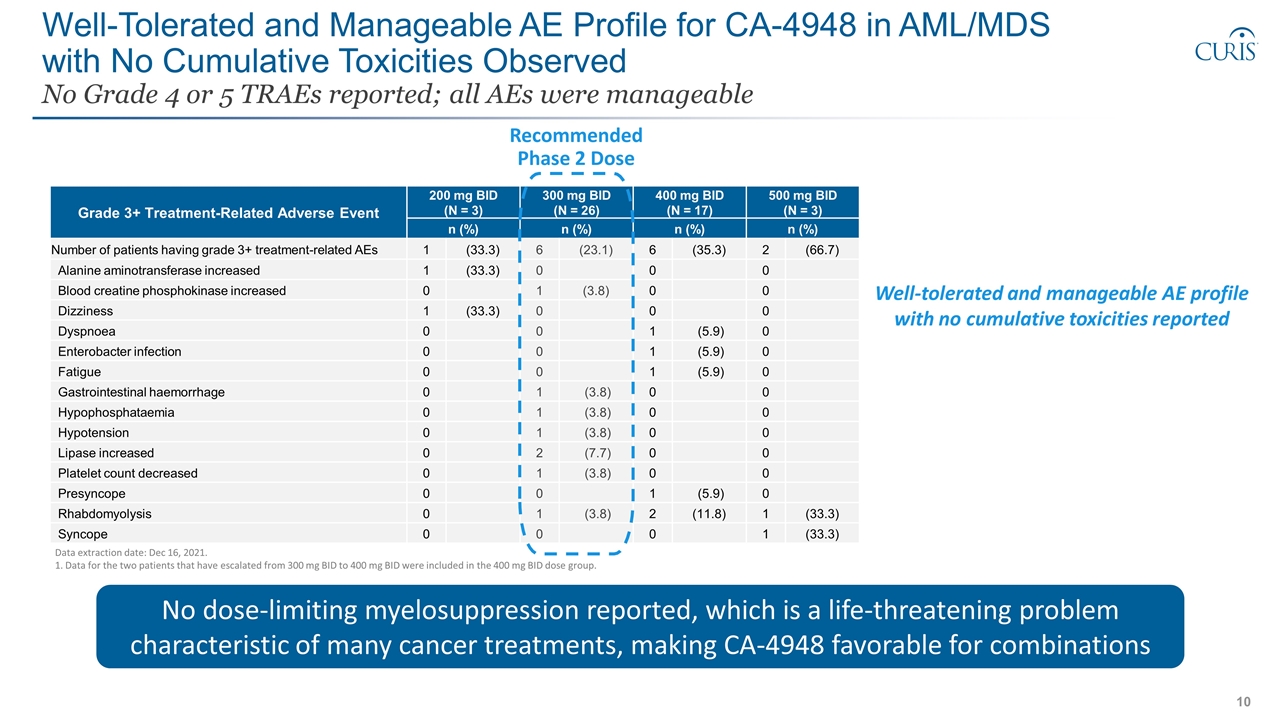
Well-Tolerated and Manageable AE Profile for CA-4948 in AML/MDS with No Cumulative Toxicities Observed No Grade 4 or 5 TRAEs reported; all AEs were manageable Data extraction date: Dec 16, 2021. 1. Data for the two patients that have escalated from 300 mg BID to 400 mg BID were included in the 400 mg BID dose group. Grade 3+ Treatment-Related Adverse Event 200 mg BID (N = 3) 300 mg BID (N = 26) 400 mg BID (N = 17) 500 mg BID (N = 3) Preferred Term n (%) n (%) n (%) n (%) Number of patients having grade 3+ treatment-related AEs 1 (33.3) 6 (23.1) 6 (35.3) 2 (66.7) Alanine aminotransferase increased 1 (33.3) 0 0 0 Blood creatine phosphokinase increased 0 1 (3.8) 0 0 Dizziness 1 (33.3) 0 0 0 Dyspnoea 0 0 1 (5.9) 0 Enterobacter infection 0 0 1 (5.9) 0 Fatigue 0 0 1 (5.9) 0 Gastrointestinal haemorrhage 0 1 (3.8) 0 0 Hypophosphataemia 0 1 (3.8) 0 0 Hypotension 0 1 (3.8) 0 0 Lipase increased 0 2 (7.7) 0 0 Platelet count decreased 0 1 (3.8) 0 0 Presyncope 0 0 1 (5.9) 0 Rhabdomyolysis 0 1 (3.8) 2 (11.8) 1 (33.3) Syncope 0 0 0 1 (33.3) No dose-limiting myelosuppression reported, which is a life-threatening problem characteristic of many cancer treatments, making CA-4948 favorable for combinations Well-tolerated and manageable AE profile with no cumulative toxicities reported Recommended Phase 2 Dose

Clinical Data Overview: Three Targeted Patient Populations (1) AML Spliceosome, (2) MDS Spliceosome, (3) AML FLT3
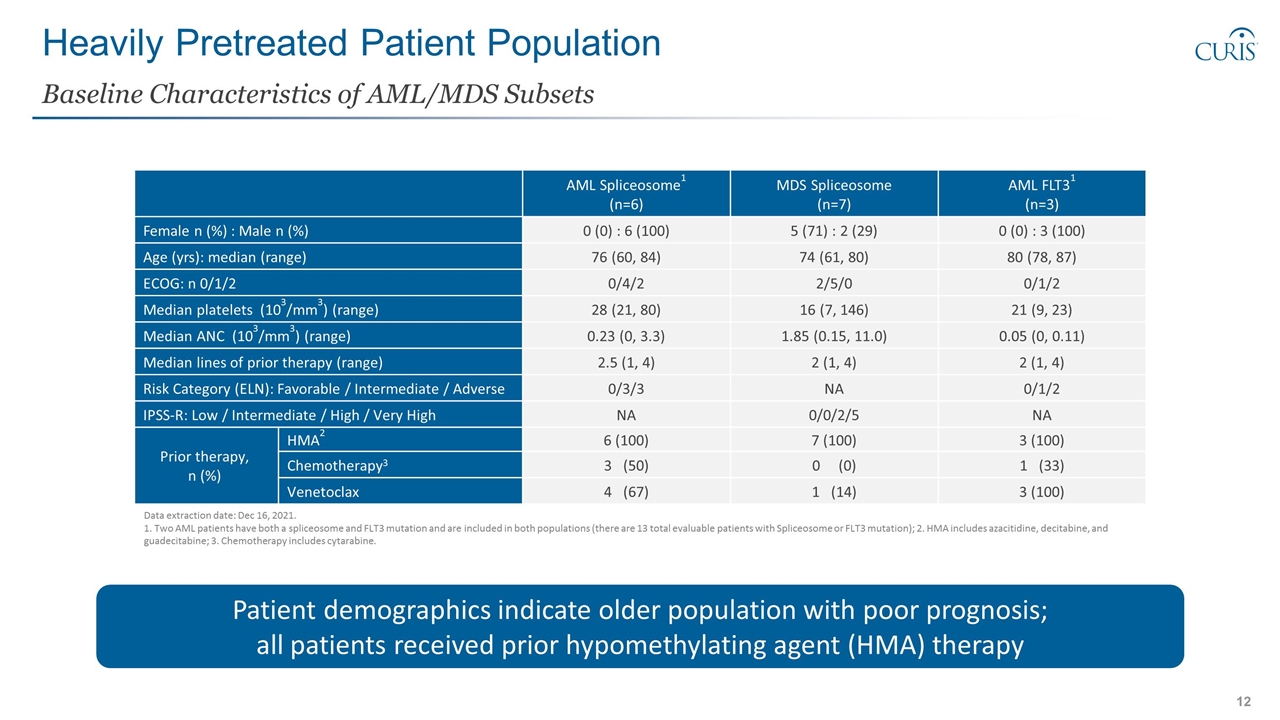
Heavily Pretreated Patient Population Baseline Characteristics of AML/MDS Subsets AML Spliceosome1 (n=6) MDS Spliceosome (n=7) AML FLT31 (n=3) Female n (%) : Male n (%) 0 (0) : 6 (100) 5 (71) : 2 (29) 0 (0) : 3 (100) Age (yrs): median (range) 76 (60, 84) 74 (61, 80) 80 (78, 87) ECOG: n 0/1/2 0/4/2 2/5/0 0/1/2 Median platelets (103/mm3) (range) 28 (21, 80) 16 (7, 146) 21 (9, 23) Median ANC (103/mm3) (range) 0.23 (0, 3.3) 1.85 (0.15, 11.0) 0.05 (0, 0.11) Median lines of prior therapy (range) 2.5 (1, 4) 2 (1, 4) 2 (1, 4) Risk Category (ELN): Favorable / Intermediate / Adverse 0/3/3 NA 0/1/2 IPSS-R: Low / Intermediate / High / Very High NA 0/0/2/5 NA Prior therapy, n (%) HMA2 6 (100) 7 (100) 3 (100) Chemotherapy3 3 (50) 0 (0) 1 (33) Venetoclax 4 (67) 1 (14) 3 (100) Data extraction date: Dec 16, 2021. 1. Two AML patients have both a spliceosome and FLT3 mutation and are included in both populations (there are 13 total evaluable patients with Spliceosome or FLT3 mutation); 2. HMA includes azacitidine, decitabine, and guadecitabine; 3. Chemotherapy includes cytarabine. Patient demographics indicate older population with poor prognosis; all patients received prior hypomethylating agent (HMA) therapy
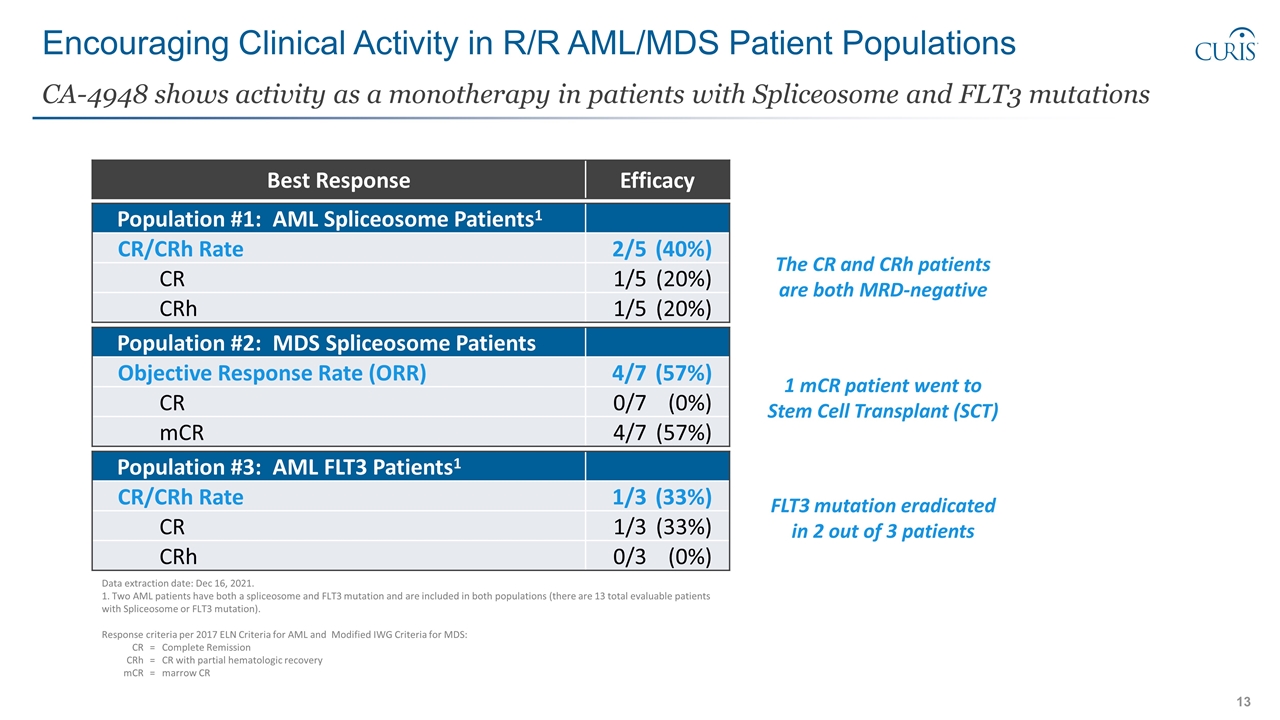
Encouraging Clinical Activity in R/R AML/MDS Patient Populations CA-4948 shows activity as a monotherapy in patients with Spliceosome and FLT3 mutations Population #1: AML Spliceosome Patients1 CR/CRh Rate 2/5(40%) CR 1/5(20%) CRh 1/5(20%) Population #2: MDS Spliceosome Patients Objective Response Rate (ORR) 4/7(57%) CR 0/7(0%) mCR 4/7(57%) Population #3: AML FLT3 Patients1 CR/CRh Rate 1/3(33%) CR 1/3(33%) CRh 0/3(0%) Data extraction date: Dec 16, 2021. 1. Two AML patients have both a spliceosome and FLT3 mutation and are included in both populations (there are 13 total evaluable patients with Spliceosome or FLT3 mutation). Response criteria per 2017 ELN Criteria for AML and Modified IWG Criteria for MDS: CR=Complete Remission CRh=CR with partial hematologic recovery mCR=marrow CR The CR and CRh patients are both MRD-negative 1 mCR patient went to Stem Cell Transplant (SCT) FLT3 mutation eradicated in 2 out of 3 patients Best Response Efficacy

Clinical Data: R/R AML Patients with Spliceosome Mutation Patient Population #1
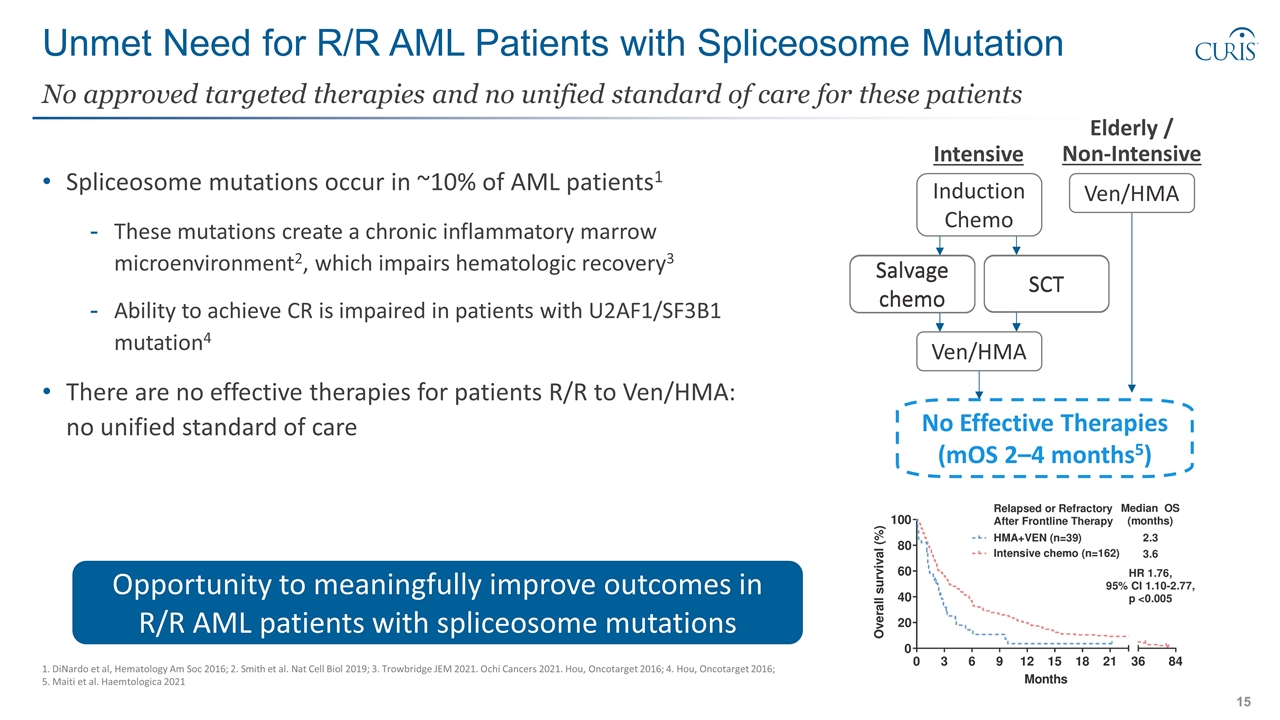
Unmet Need for R/R AML Patients with Spliceosome Mutation Spliceosome mutations occur in ~10% of AML patients1 These mutations create a chronic inflammatory marrow microenvironment2, which impairs hematologic recovery3 Ability to achieve CR is impaired in patients with U2AF1/SF3B1 mutation4 There are no effective therapies for patients R/R to Ven/HMA: no unified standard of care No approved targeted therapies and no unified standard of care for these patients 1. DiNardo et al, Hematology Am Soc 2016; 2. Smith et al. Nat Cell Biol 2019; 3. Trowbridge JEM 2021. Ochi Cancers 2021. Hou, Oncotarget 2016; 4. Hou, Oncotarget 2016; 5. Maiti et al. Haemtologica 2021 Opportunity to meaningfully improve outcomes in R/R AML patients with spliceosome mutations Intensive Elderly / Non-Intensive Induction Chemo SCT Ven/HMA Ven/HMA No Effective Therapies (mOS 2–4 months5) Salvage chemo SCT Salvage chemo
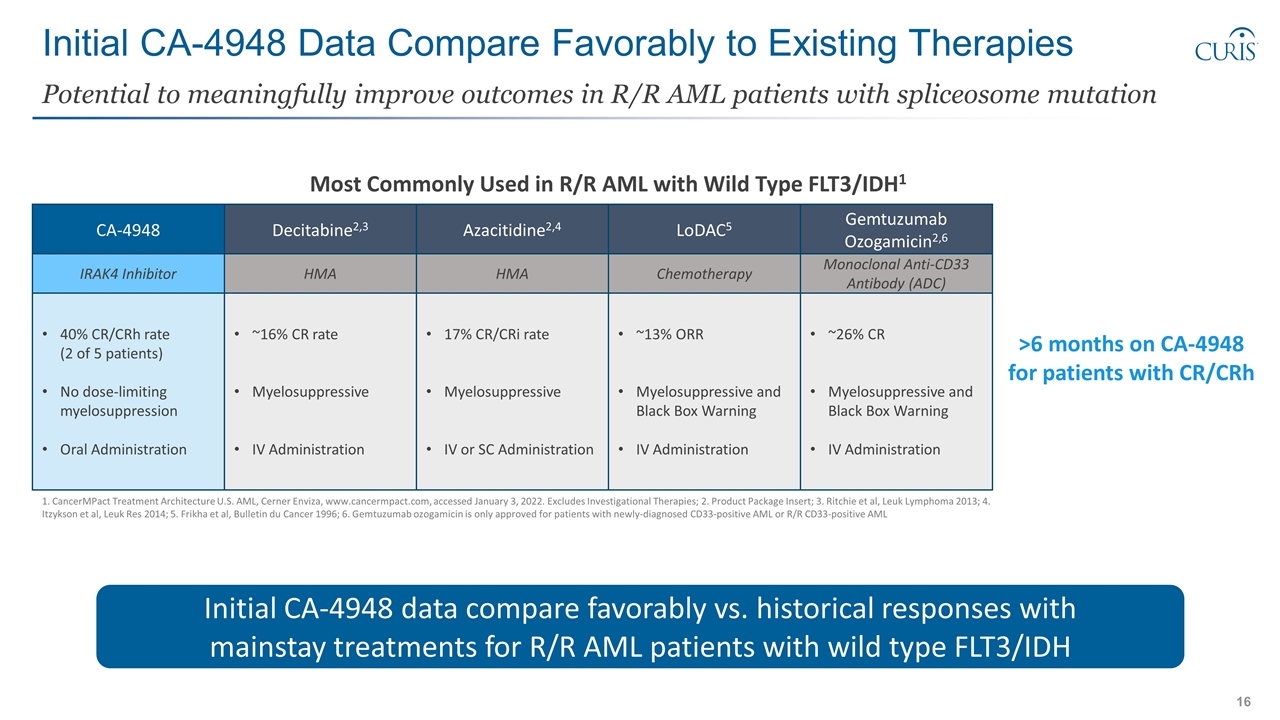
Initial CA-4948 Data Compare Favorably to Existing Therapies Potential to meaningfully improve outcomes in R/R AML patients with spliceosome mutation 1. CancerMPact Treatment Architecture U.S. AML, Cerner Enviza, www.cancermpact.com, accessed January 3, 2022. Excludes Investigational Therapies; 2. Product Package Insert; 3. Ritchie et al, Leuk Lymphoma 2013; 4. Itzykson et al, Leuk Res 2014; 5. Frikha et al, Bulletin du Cancer 1996; 6. Gemtuzumab ozogamicin is only approved for patients with newly-diagnosed CD33-positive AML or R/R CD33-positive AML Azacitidine2,4 LoDAC5 HMA Chemotherapy 17% CR/CRi rate Myelosuppressive IV or SC Administration ~13% ORR Myelosuppressive and Black Box Warning IV Administration Most Commonly Used in R/R AML with Wild Type FLT3/IDH1 Decitabine2,3 HMA ~16% CR rate Myelosuppressive IV Administration >6 months on CA-4948 for patients with CR/CRh CA-4948 40% CR/CRh rate (2 of 5 patients) No dose-limiting myelosuppression Oral Administration Gemtuzumab Ozogamicin2,6 Monoclonal Anti-CD33 Antibody (ADC) ~26% CR Myelosuppressive and Black Box Warning IV Administration IRAK4 Inhibitor Initial CA-4948 data compare favorably vs. historical responses with mainstay treatments for R/R AML patients with wild type FLT3/IDH
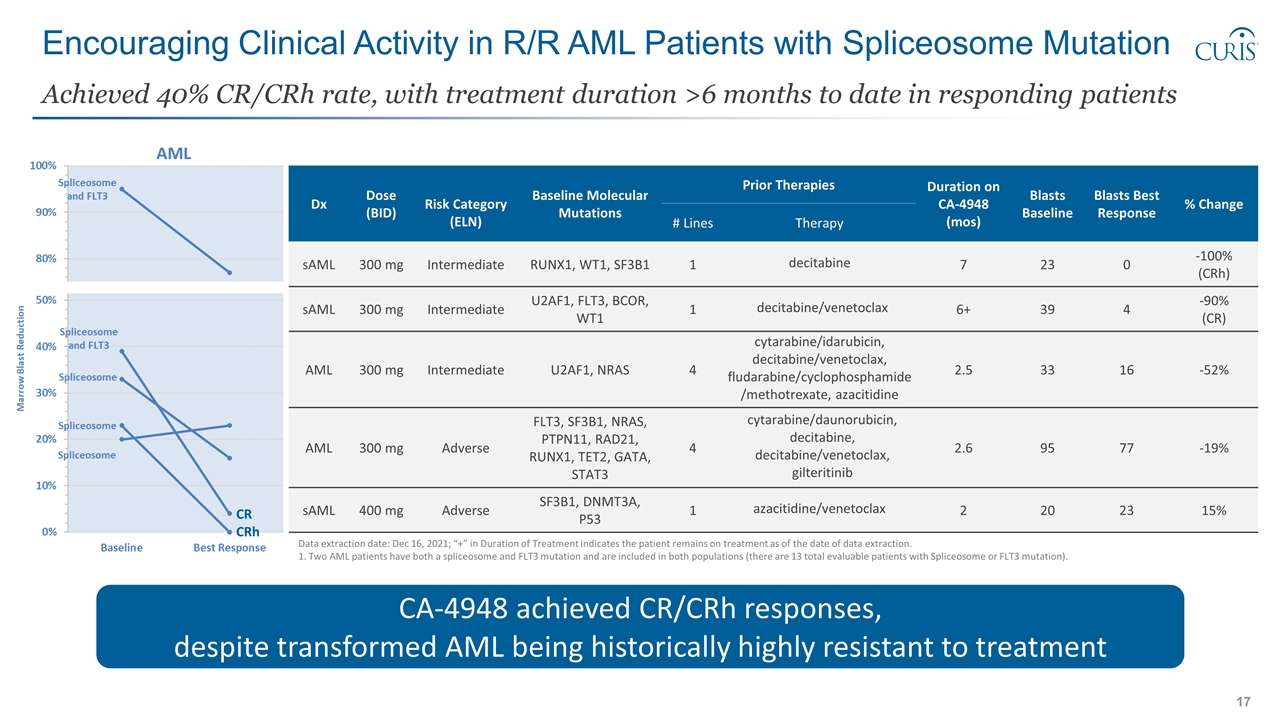
Encouraging Clinical Activity in R/R AML Patients with Spliceosome Mutation Achieved 40% CR/CRh rate, with treatment duration >6 months to date in responding patients CA-4948 achieved CR/CRh responses, despite transformed AML being historically highly resistant to treatment CR CRh Dx Dose (BID) Risk Category (ELN) Baseline Molecular Mutations Prior Therapies Duration on CA-4948 (mos) Blasts Baseline Blasts Best Response % Change # Lines Therapy sAML 300 mg Intermediate RUNX1, WT1, SF3B1 1 decitabine 7 23 0 -100% (CRh) sAML 300 mg Intermediate U2AF1, FLT3, BCOR, WT1 1 decitabine/venetoclax 6+ 39 4 -90% (CR) AML 300 mg Intermediate U2AF1, NRAS 4 cytarabine/idarubicin, decitabine/venetoclax, fludarabine/cyclophosphamide/methotrexate, azacitidine 2.5 33 16 -52% AML 300 mg Adverse FLT3, SF3B1, NRAS, PTPN11, RAD21, RUNX1, TET2, GATA, STAT3 4 cytarabine/daunorubicin, decitabine, decitabine/venetoclax, gilteritinib 2.6 95 77 -19% sAML 400 mg Adverse SF3B1, DNMT3A, P53 1 azacitidine/venetoclax 2 20 23 15% Data extraction date: Dec 16, 2021; “+” in Duration of Treatment indicates the patient remains on treatment as of the date of data extraction. 1. Two AML patients have both a spliceosome and FLT3 mutation and are included in both populations (there are 13 total evaluable patients with Spliceosome or FLT3 mutation).

Clinical Data: R/R MDS Patients with Spliceosome Mutation Patient Population #2
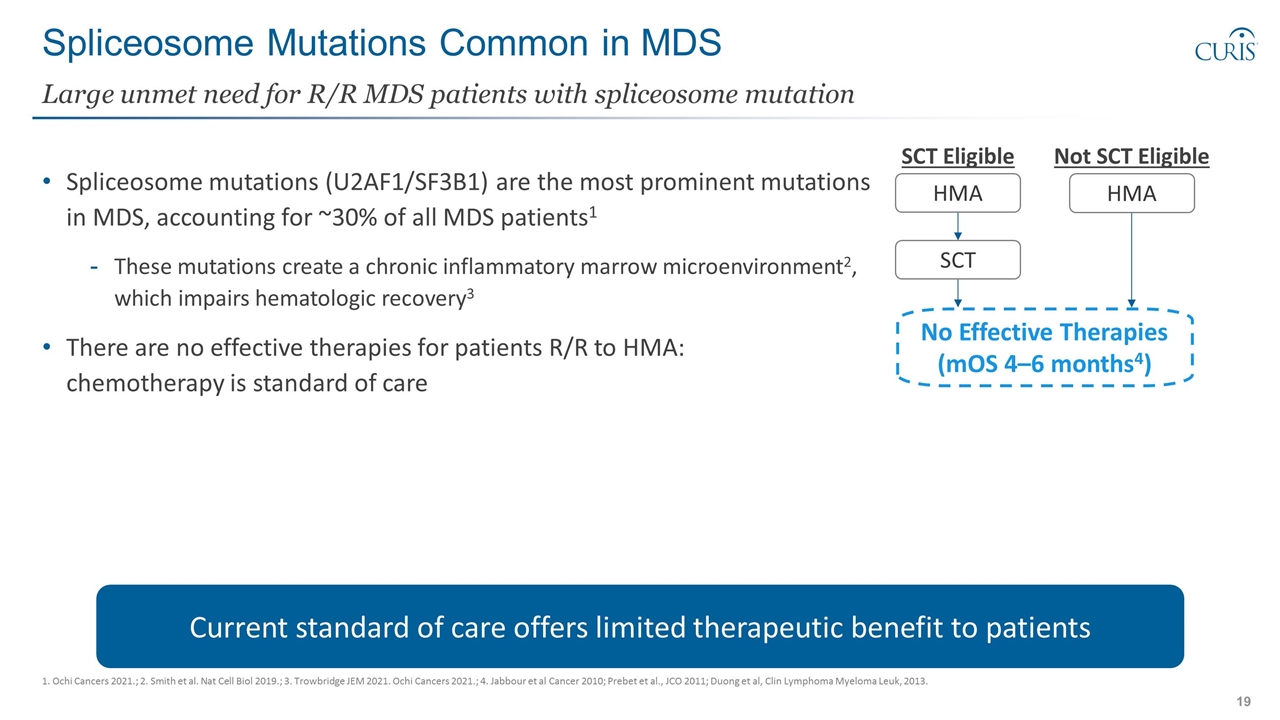
Spliceosome Mutations Common in MDS Spliceosome mutations (U2AF1/SF3B1) are the most prominent mutations in MDS, accounting for ~30% of all MDS patients1 These mutations create a chronic inflammatory marrow microenvironment2, which impairs hematologic recovery3 There are no effective therapies for patients R/R to HMA: chemotherapy is standard of care Large unmet need for R/R MDS patients with spliceosome mutation 1. Ochi Cancers 2021.; 2. Smith et al. Nat Cell Biol 2019.; 3. Trowbridge JEM 2021. Ochi Cancers 2021.; 4. Jabbour et al Cancer 2010; Prebet et al., JCO 2011; Duong et al, Clin Lymphoma Myeloma Leuk, 2013. Current standard of care offers limited therapeutic benefit to patients SCT Eligible Not SCT Eligible HMA SCT HMA No Effective Therapies (mOS 4–6 months4)
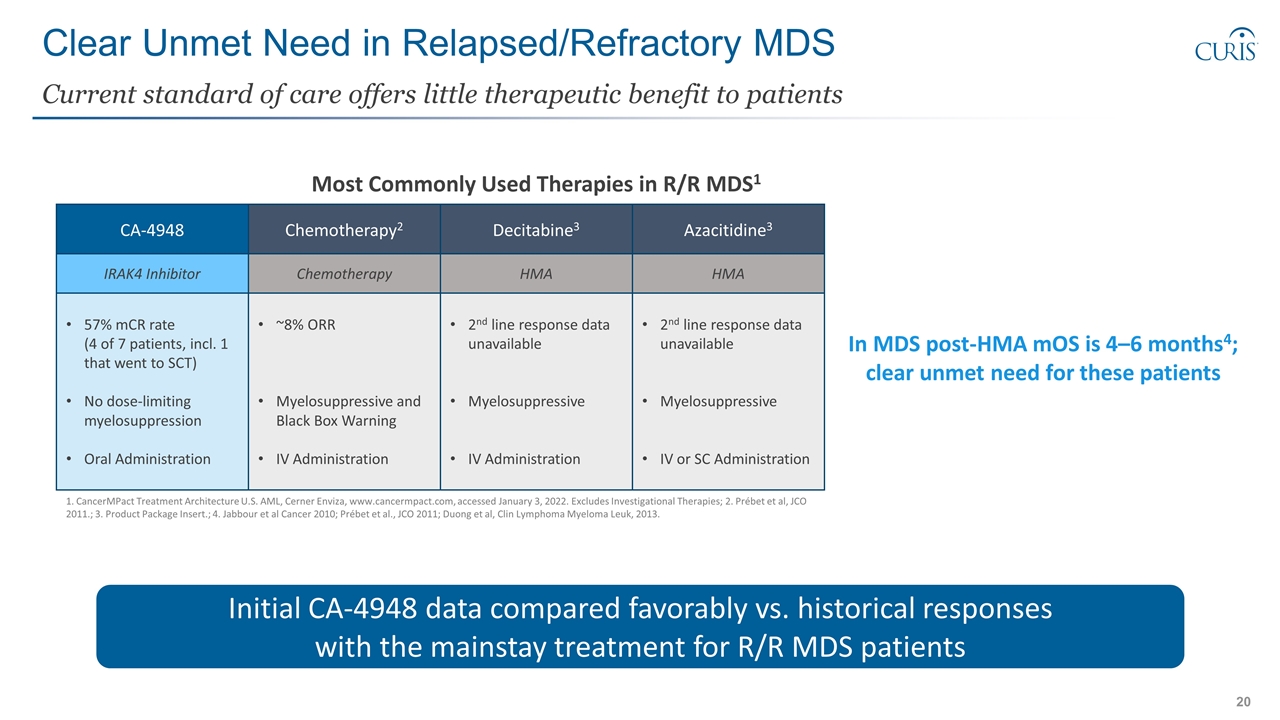
Clear Unmet Need in Relapsed/Refractory MDS Current standard of care offers little therapeutic benefit to patients 1. CancerMPact Treatment Architecture U.S. AML, Cerner Enviza, www.cancermpact.com, accessed January 3, 2022. Excludes Investigational Therapies; 2. Prébet et al, JCO 2011.; 3. Product Package Insert.; 4. Jabbour et al Cancer 2010; Prébet et al., JCO 2011; Duong et al, Clin Lymphoma Myeloma Leuk, 2013. Chemotherapy2 Decitabine3 Azacitidine3 CA-4948 Chemotherapy HMA HMA IRAK4 Inhibitor ~8% ORR Myelosuppressive and Black Box Warning IV Administration 2nd line response data unavailable Myelosuppressive IV Administration 2nd line response data unavailable Myelosuppressive IV or SC Administration 57% mCR rate (4 of 7 patients, incl. 1 that went to SCT) No dose-limiting myelosuppression Oral Administration Initial CA-4948 data compared favorably vs. historical responses with the mainstay treatment for R/R MDS patients In MDS post-HMA mOS is 4–6 months4; clear unmet need for these patients Most Commonly Used Therapies in R/R MDS1
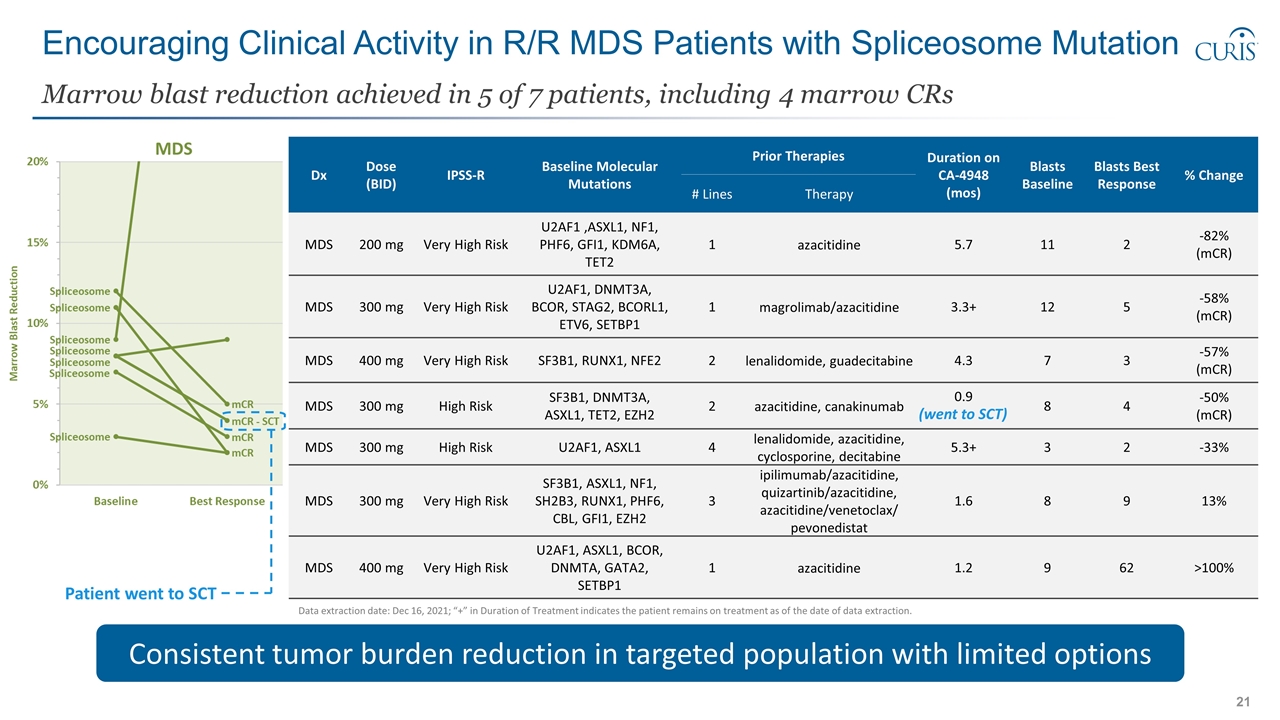
Dx Dose (BID) IPSS-R Baseline Molecular Mutations Prior Therapies Duration on CA-4948 (mos) Blasts Baseline Blasts Best Response % Change # Lines Therapy MDS 200 mg Very High Risk U2AF1 ,ASXL1, NF1, PHF6, GFI1, KDM6A, TET2 1 azacitidine 5.7 11 2 -82% (mCR) MDS 300 mg Very High Risk U2AF1, DNMT3A, BCOR, STAG2, BCORL1, ETV6, SETBP1 1 magrolimab/azacitidine 3.3+ 12 5 -58% (mCR) MDS 400 mg Very High Risk SF3B1, RUNX1, NFE2 2 lenalidomide, guadecitabine 4.3 7 3 -57% (mCR) MDS 300 mg High Risk SF3B1, DNMT3A, ASXL1, TET2, EZH2 2 azacitidine, canakinumab 0.9 8 4 -50% (mCR) MDS 300 mg High Risk U2AF1, ASXL1 4 lenalidomide, azacitidine, cyclosporine, decitabine 5.3+ 3 2 -33% MDS 300 mg Very High Risk SF3B1, ASXL1, NF1, SH2B3, RUNX1, PHF6, CBL, GFI1, EZH2 3 ipilimumab/azacitidine, quizartinib/azacitidine, azacitidine/venetoclax/ pevonedistat 1.6 8 9 13% MDS 400 mg Very High Risk U2AF1, ASXL1, BCOR, DNMTA, GATA2, SETBP1 1 azacitidine 1.2 9 62 >100% Encouraging Clinical Activity in R/R MDS Patients with Spliceosome Mutation Marrow blast reduction achieved in 5 of 7 patients, including 4 marrow CRs Consistent tumor burden reduction in targeted population with limited options (went to SCT) Data extraction date: Dec 16, 2021; “+” in Duration of Treatment indicates the patient remains on treatment as of the date of data extraction. Patient went to SCT

Clinical Data: R/R AML Patients with FLT3 Mutation Patient Population #3
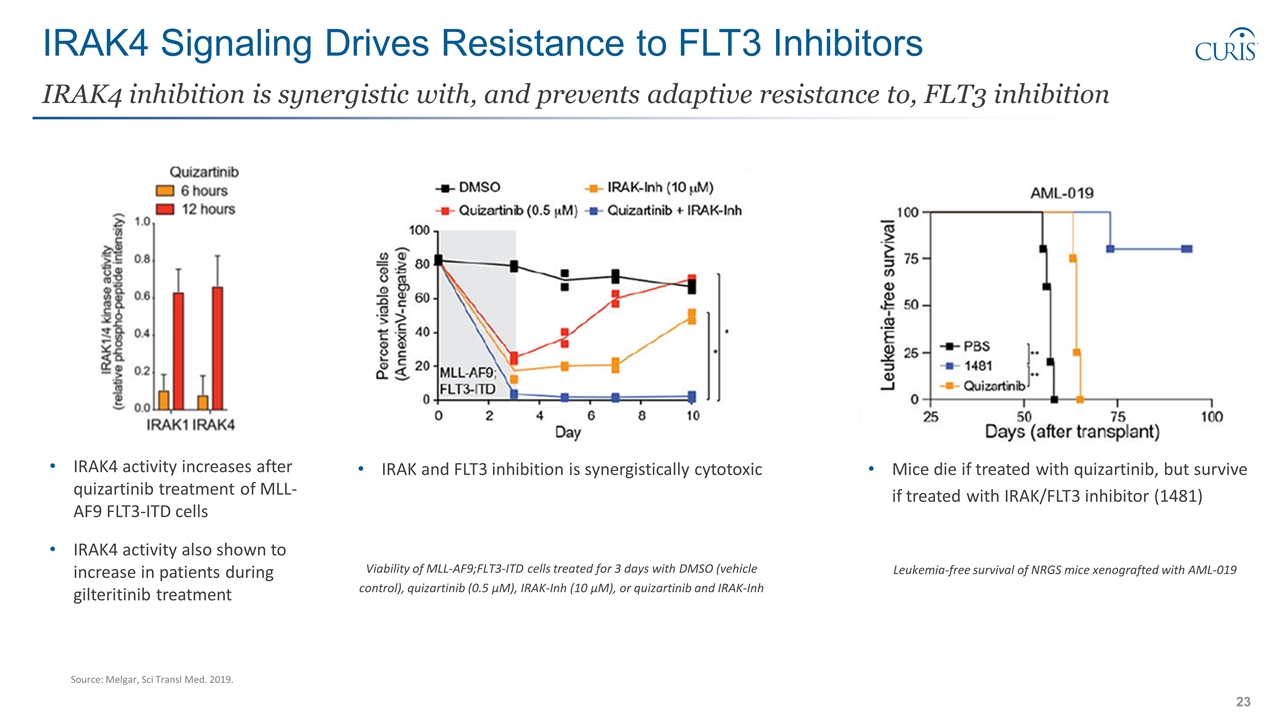
IRAK4 Signaling Drives Resistance to FLT3 Inhibitors IRAK4 inhibition is synergistic with, and prevents adaptive resistance to, FLT3 inhibition IRAK4 activity increases after quizartinib treatment of MLL-AF9 FLT3-ITD cells IRAK4 activity also shown to increase in patients during gilteritinib treatment IRAK and FLT3 inhibition is synergistically cytotoxic Viability of MLL-AF9;FLT3-ITD cells treated for 3 days with DMSO (vehicle control), quizartinib (0.5 μM), IRAK-Inh (10 μM), or quizartinib and IRAK-Inh Mice die if treated with quizartinib, but survive if treated with IRAK/FLT3 inhibitor (1481) Leukemia-free survival of NRGS mice xenografted with AML-019 Source: Melgar, Sci Transl Med. 2019.
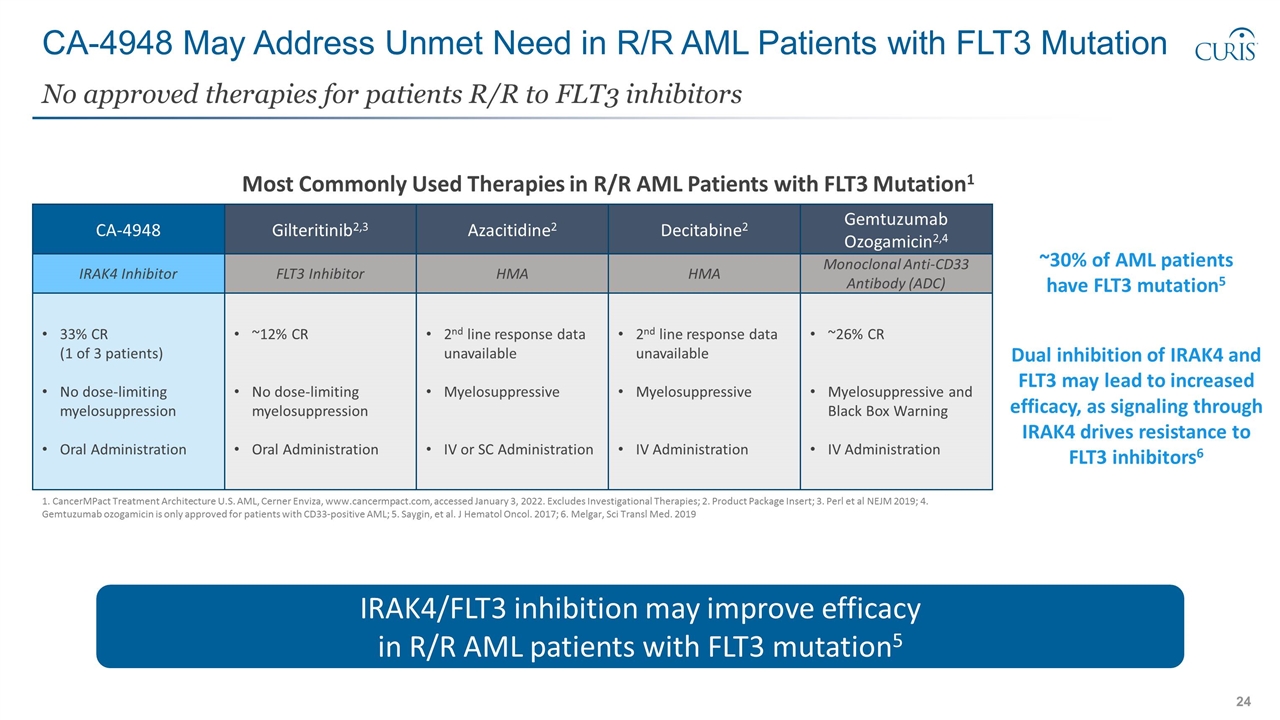
CA-4948 May Address Unmet Need in R/R AML Patients with FLT3 Mutation No approved therapies for patients R/R to FLT3 inhibitors 1. CancerMPact Treatment Architecture U.S. AML, Cerner Enviza, www.cancermpact.com, accessed January 3, 2022. Excludes Investigational Therapies; 2. Product Package Insert; 3. Perl et al NEJM 2019; 4. Gemtuzumab ozogamicin is only approved for patients with CD33-positive AML; 5. Saygin, et al. J Hematol Oncol. 2017; 6. Melgar, Sci Transl Med. 2019 Gilteritinib2,3 Azacitidine2 CA-4948 FLT3 Inhibitor HMA IRAK4 Inhibitor ~12% CR No dose-limiting myelosuppression Oral Administration 2nd line response data unavailable Myelosuppressive IV or SC Administration 33% CR (1 of 3 patients) No dose-limiting myelosuppression Oral Administration IRAK4/FLT3 inhibition may improve efficacy in R/R AML patients with FLT3 mutation5 Decitabine2 HMA 2nd line response data unavailable Myelosuppressive IV Administration Gemtuzumab Ozogamicin2,4 Monoclonal Anti-CD33 Antibody (ADC) ~26% CR Myelosuppressive and Black Box Warning IV Administration Most Commonly Used Therapies in R/R AML Patients with FLT3 Mutation1 Dual inhibition of IRAK4 and FLT3 may lead to increased efficacy, as signaling through IRAK4 drives resistance to FLT3 inhibitors6 ~30% of AML patients have FLT3 mutation5
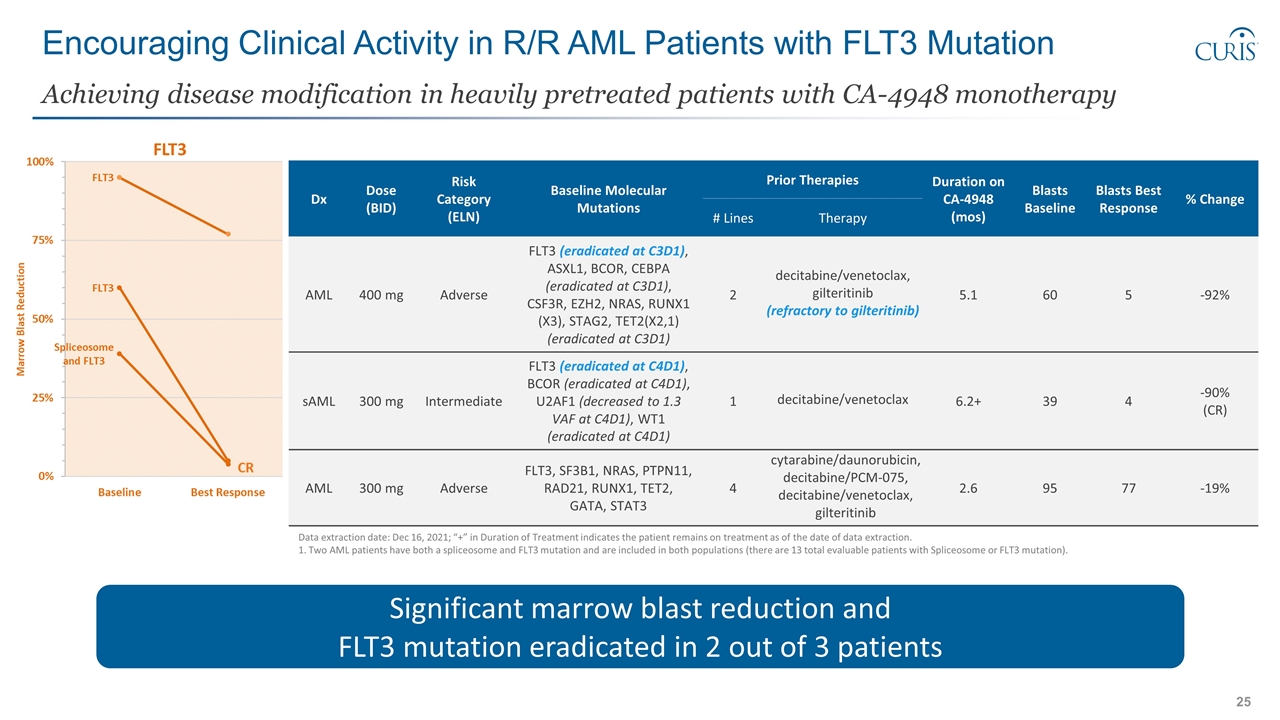
Encouraging Clinical Activity in R/R AML Patients with FLT3 Mutation Achieving disease modification in heavily pretreated patients with CA-4948 monotherapy Significant marrow blast reduction and FLT3 mutation eradicated in 2 out of 3 patients Data extraction date: Dec 16, 2021; “+” in Duration of Treatment indicates the patient remains on treatment as of the date of data extraction. 1. Two AML patients have both a spliceosome and FLT3 mutation and are included in both populations (there are 13 total evaluable patients with Spliceosome or FLT3 mutation). Dx Dose (BID) Risk Category (ELN) Baseline Molecular Mutations Prior Therapies Duration on CA-4948 (mos) Blasts Baseline Blasts Best Response % Change # Lines Therapy AML 400 mg Adverse FLT3 (eradicated at C3D1), ASXL1, BCOR, CEBPA (eradicated at C3D1), CSF3R, EZH2, NRAS, RUNX1 (X3), STAG2, TET2(X2,1) (eradicated at C3D1) 2 decitabine/venetoclax, gilteritinib (refractory to gilteritinib) 5.1 60 5 -92% sAML 300 mg Intermediate FLT3 (eradicated at C4D1), BCOR (eradicated at C4D1), U2AF1 (decreased to 1.3 VAF at C4D1), WT1 (eradicated at C4D1) 1 decitabine/venetoclax 6.2+ 39 4 -90% (CR) AML 300 mg Adverse FLT3, SF3B1, NRAS, PTPN11, RAD21, RUNX1, TET2, GATA, STAT3 4 cytarabine/daunorubicin, decitabine/PCM-075, decitabine/venetoclax, gilteritinib 2.6 95 77 -19%

Summary CA-4948 in Three Targeted Patient Populations

Comments from Clinical Investigator, Daniel DeAngelo, MD, PhD Encouraging clinical activity in patients with R/R disease, all of whom had prior HMA treatment 4 out of 5 patients with spliceosome-mutated AML achieved blast reduction 2 patients achieved CR/CRh with significant hematologic improvement, including 1 full CR 5 out of 7 patients with spliceosome-mutated MDS achieved blast reduction 4 patients achieved marrow CR, 1 of whom proceeded to Stem Cell Transplant All 3 patients with FLT3-mutated AML achieved blast reduction 2 patients achieved eradication of FLT3 mutation, including 1 full CR Manageable Toxicity/Safety Profile Oral drug with a well-tolerated and manageable safety profile Supports further development, both as a single agent and in combination therapy Chief, Division of Leukemia at Dana-Farber Cancer Institute
CA-4948 Has Potential to Address Clear Unmet Need in AML and MDS First-in-class IRAK4 inhibitor targets specific genetic populations in AML and MDS Next Steps in Expansion Monotherapy: Spliceosome mutation Monotherapy: FLT3 mutation Combination: CA-4948 + azacitidine Combination: CA-4948 + venetoclax Plan to discuss potential for a rapid registrational path with FDA in 1H 2022 CA-4948 addresses a novel target (IRAK4) and: (1) demonstrates clear anti-cancer activity as an oral single agent (2) is active in genetically-defined populations that can be identified and enrolled (3) has the added potential benefit of also hitting FLT3 Well-tolerated and manageable safety profile may provide advantage to existing standard of care therapies as a single agent, and also suggests CA-4948 may be a favorable candidate for addition to combination therapy regimens Dual targeting of IRAK4 and FLT3 may prevent adaptive resistance to current FLT3 inhibitors1 1. Melgar, Sci Transl Med. 2019

CI-8993: First-in-class VISTA Antagonist Novel Immune Checkpoint Inhibitor for Solid Tumors
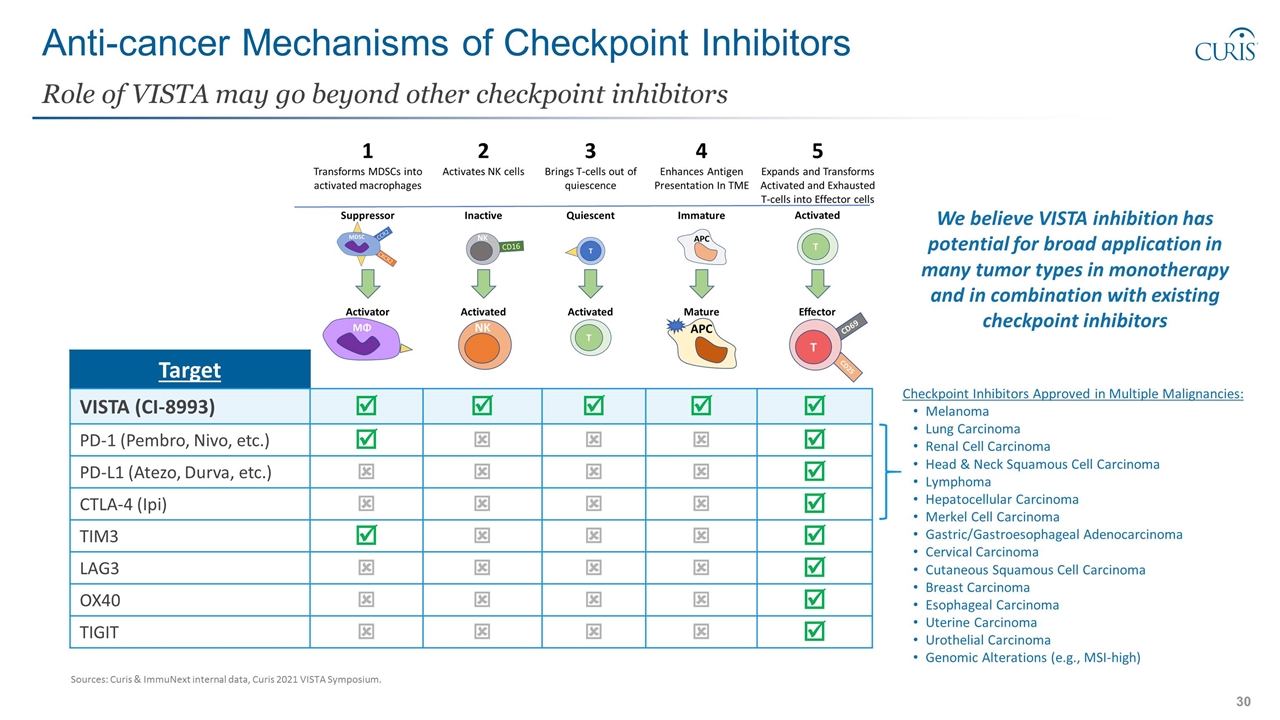
Anti-cancer Mechanisms of Checkpoint Inhibitors Role of VISTA may go beyond other checkpoint inhibitors Target VISTA (CI-8993) þ þ þ þ þ PD-1 (Pembro, Nivo, etc.) þ ý ý ý þ PD-L1 (Atezo, Durva, etc.) ý ý ý ý þ CTLA-4 (Ipi) ý ý ý ý þ TIM3 þ ý ý ý þ LAG3 ý ý ý ý þ OX40 ý ý ý ý þ TIGIT ý ý ý ý þ We believe VISTA inhibition has potential for broad application in many tumor types in monotherapy and in combination with existing checkpoint inhibitors Checkpoint Inhibitors Approved in Multiple Malignancies: Melanoma Lung Carcinoma Renal Cell Carcinoma Head & Neck Squamous Cell Carcinoma Lymphoma Hepatocellular Carcinoma Merkel Cell Carcinoma Gastric/Gastroesophageal Adenocarcinoma Cervical Carcinoma Cutaneous Squamous Cell Carcinoma Breast Carcinoma Esophageal Carcinoma Uterine Carcinoma Urothelial Carcinoma Genomic Alterations (e.g., MSI-high) Sources: Curis & ImmuNext internal data, Curis 2021 VISTA Symposium.

Incorporated Learnings from CI-8993 Prior Clinical Study CI-8993 is the first anti-VISTA monoclonal antibody (IgG1κ) to be studied in clinical trials Janssen initiated a Ph1 study in 2016 and enrolled 12 patients1 Transient Cytokine Release Syndrome (CRS) observed in several patients at 0.15mg/kg Transient grade 3 CRS-associated encephalopathy observed at 0.3mg/kg, after which Janssen halted the study Pharmacodynamic activity (cytokine release) observed in initial clinical study CI-8993 Protocol Designed to Manage Expected CRS CRS is likely an on-target effect; indicates drug is hitting the target and inducing immune response NCCN guidelines were issued in 2018; oncology community now familiar with managing CRS Desensitizing step-dose of CI-8993, and pre/post-infusion medication, administered to mitigate CRS 1. ClinicalTrials.gov, Trial #NCT02671955
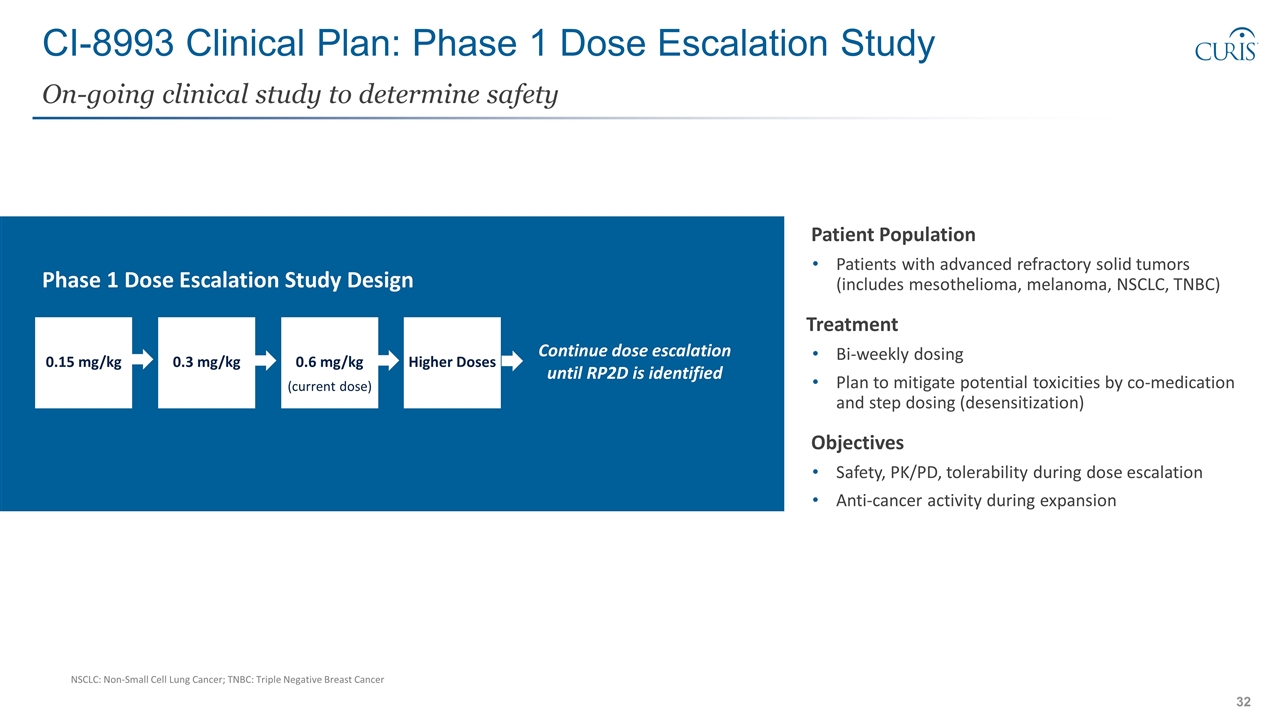
CI-8993 Clinical Plan: Phase 1 Dose Escalation Study On-going clinical study to determine safety Patient Population Patients with advanced refractory solid tumors (includes mesothelioma, melanoma, NSCLC, TNBC) Treatment Bi-weekly dosing Plan to mitigate potential toxicities by co-medication and step dosing (desensitization) Objectives Safety, PK/PD, tolerability during dose escalation Anti-cancer activity during expansion Continue dose escalation until RP2D is identified Higher Doses 0.6 mg/kg (current dose) 0.3 mg/kg 0.15 mg/kg Phase 1 Dose Escalation Study Design NSCLC: Non-Small Cell Lung Cancer; TNBC: Triple Negative Breast Cancer
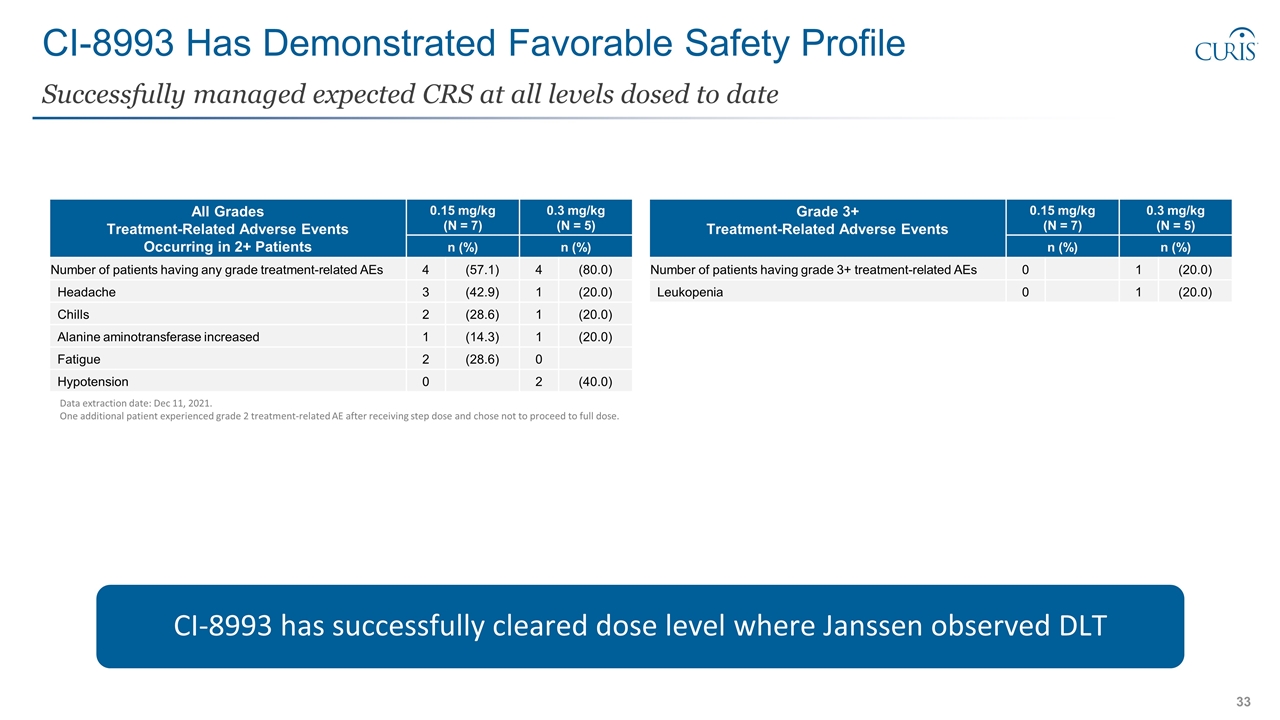
CI-8993 Has Demonstrated Favorable Safety Profile Successfully managed expected CRS at all levels dosed to date Data extraction date: Dec 11, 2021. One additional patient experienced grade 2 treatment-related AE after receiving step dose and chose not to proceed to full dose. CI-8993 has successfully cleared dose level where Janssen observed DLT All Grades Treatment-Related Adverse Events Occurring in 2+ Patients 0.15 mg/kg (N = 7) 0.3 mg/kg (N = 5) Preferred Term n (%) n (%) Number of patients having any grade treatment-related AEs 4 (57.1) 4 (80.0) Headache 3 (42.9) 1 (20.0) Chills 2 (28.6) 1 (20.0) Alanine aminotransferase increased 1 (14.3) 1 (20.0) Fatigue 2 (28.6) 0 Hypotension 0 2 (40.0) Grade 3+ Treatment-Related Adverse Events 0.15 mg/kg (N = 7) 0.3 mg/kg (N = 5) Preferred Term n (%) n (%) Number of patients having grade 3+ treatment-related AEs 0 1 (20.0) Leukopenia 0 1 (20.0)
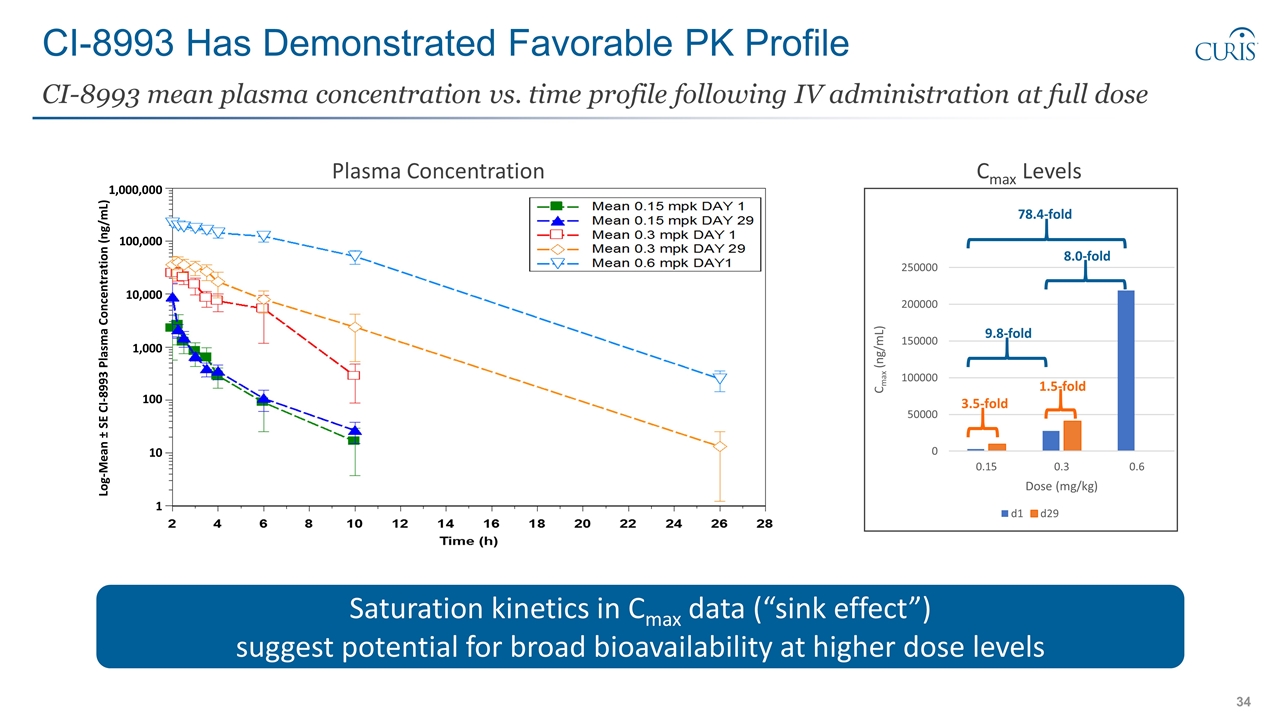
CI-8993 Has Demonstrated Favorable PK Profile CI-8993 mean plasma concentration vs. time profile following IV administration at full dose 3.5-fold 1.5-fold 9.8-fold 8.0-fold 78.4-fold Cmax Levels Log-Mean ± SE CI-8993 Plasma Concentration (ng/mL) 100,000 1,000,000 10,000 1,000 100 10 1 Saturation kinetics in Cmax data (“sink effect”) suggest potential for broad bioavailability at higher dose levels Plasma Concentration
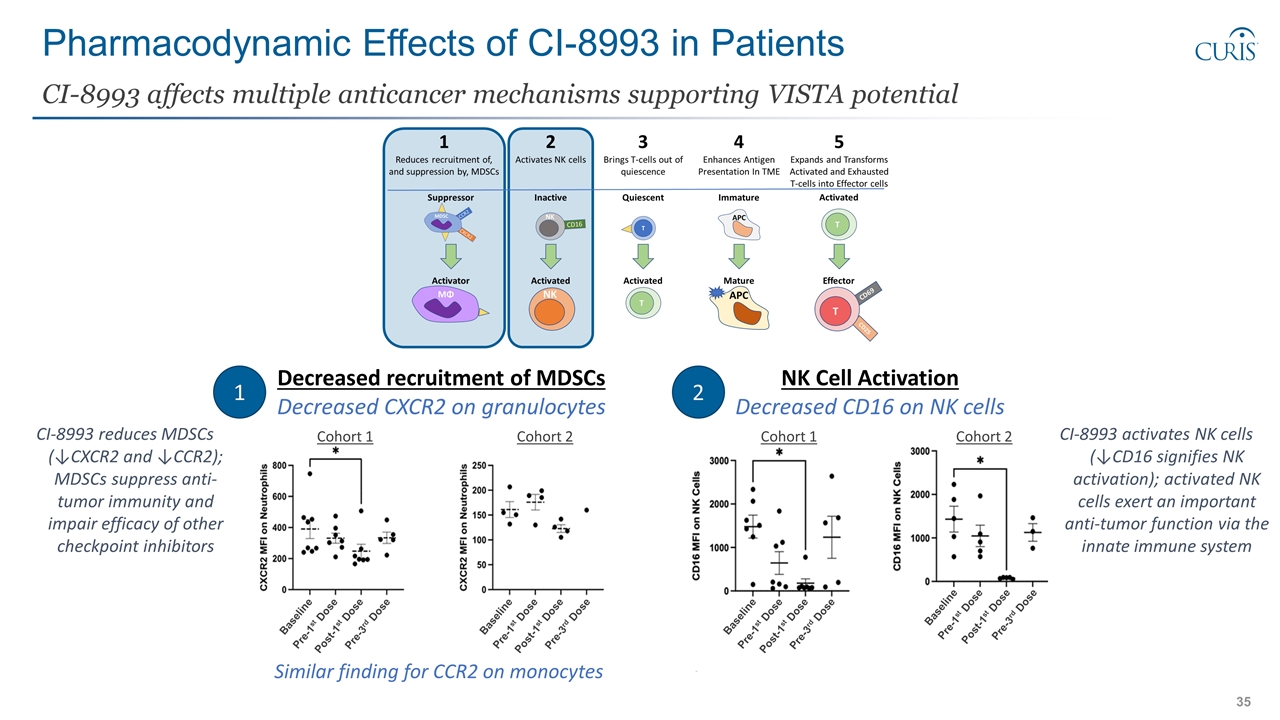
Pharmacodynamic Effects of CI-8993 in Patients CI-8993 affects multiple anticancer mechanisms supporting VISTA potential Decreased recruitment of MDSCs Decreased CXCR2 on granulocytes 1 2 NK Cell Activation Decreased CD16 on NK cells Baseline Pre-1st Dose Post-1st Dose Pre-3rd Dose Baseline Pre-1st Dose Post-1st Dose Pre-3rd Dose Baseline Pre-1st Dose Post-1st Dose Pre-3rd Dose Baseline Pre-1st Dose Post-1st Dose Pre-3rd Dose Cohort 1 Cohort 2 Cohort 1 Cohort 2 Similar finding for CCR2 on monocytes CI-8993 reduces MDSCs (↓CXCR2 and ↓CCR2); MDSCs suppress anti-tumor immunity and impair efficacy of other checkpoint inhibitors CI-8993 activates NK cells (↓CD16 signifies NK activation); activated NK cells exert an important anti-tumor function via the innate immune system
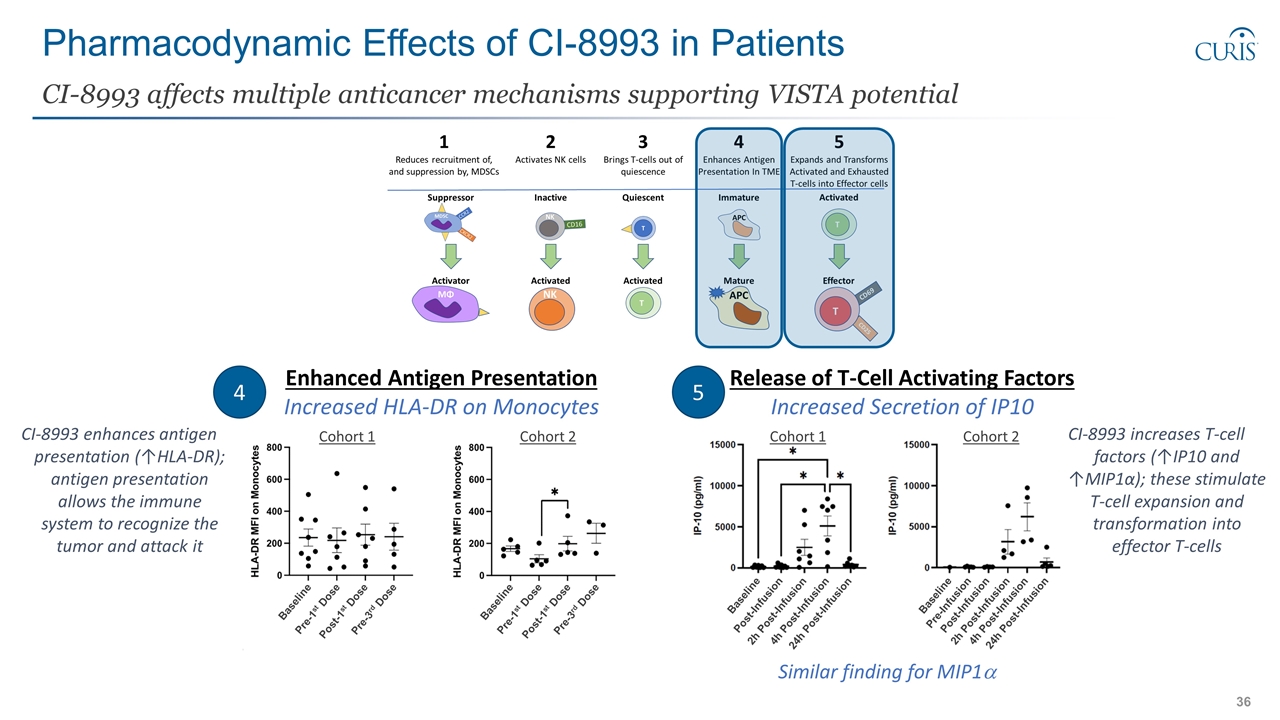
Pharmacodynamic Effects of CI-8993 in Patients CI-8993 affects multiple anticancer mechanisms supporting VISTA potential Enhanced Antigen Presentation Increased HLA-DR on Monocytes 4 5 Release of T-Cell Activating Factors Increased Secretion of IP10 Similar finding for MIP1a Baseline Pre-1st Dose Post-1st Dose Pre-3rd Dose Baseline Pre-1st Dose Post-1st Dose Pre-3rd Dose Baseline Post-Infusion 4h Post-Infusion 2h Post-Infusion 24h Post-Infusion Baseline Pre-Infusion 2h Post-Infusion Post-Infusion 4h Post-Infusion 24h Post-Infusion CI-8993 enhances antigen presentation (↑HLA-DR); antigen presentation allows the immune system to recognize the tumor and attack it CI-8993 increases T-cell factors (↑IP10 and ↑MIP1α); these stimulate T-cell expansion and transformation into effector T-cells Cohort 1 Cohort 2 Cohort 1 Cohort 2
CI-8993 Cleared Initial Safety Hurdle First-in-class CI-8993 has potential for broad applicability in immune checkpoint therapy Encouraging initial safety data appears to demonstrate effectiveness of procedures implemented to manage expected CRS Pharmacokinetic profile of CI-8993 exhibits saturation kinetics, suggesting potential to overcome “sink effect” Pharmacodynamic effects of CI-8993 in patients suggest multiple anti-cancer mechanisms may be activated Next Steps in Dose Escalation Continue dose-escalation for signs of anti-cancer activity and determination of RP2D

Closing Comments
Initial clinical data demonstrate clear anti-cancer activity with a single, oral agent in specific genetic populations of AML/MDS Initial safety data appear to demonstrate that expected immune effects (cytokine release) can be managed, and early PK/PD data show that anti-cancer mechanisms are being activated Closing Remarks & Next Steps Clinical update on CA-4948 (IRAK4) and CI-8993 (VISTA) Next Steps Plan to discuss potential for a rapid registrational path with FDA in 2022 Next Steps Continue dose-escalation for signs of anti-cancer activity and determination of RP2D CA-4948 CI-8993

Q&A







































