EXHIBIT 1.1

THE WESTAIM CORPORATION
ANNUAL INFORMATION FORM
APRIL 29, 2003
EXHIBIT 1.1
| 1. | | The Annual Information Form dated April 29, 2003 for the year ended December 31, 2002 is attached. |
| |
| 2. | | Contractual Obligations |
| |
| | | The Company’s obligations with respect to future commitments are disclosed in note 18 to the Company’s annual audited consolidated financial statements for the year ended December 31, 2002 which are attached as Exhibit 1.3 to this Form 40-F filing. |
THE WESTAIM CORPORATION
ANNUAL INFORMATION FORM
APRIL 29, 2003
TABLE OF CONTENTS
| | | | | | | |
| | | | | PAGE |
| | | | |
|
| CORPORATE STRUCTURE | | | 1 | |
| GENERAL DEVELOPMENT OF THE BUSINESS | | | 2 | |
| DESCRIPTION OF THE BUSINESS | | | 3 | |
| | Emerging Technologies | | | 3 | |
| | | iFire Technology Inc. | | | 3 | |
| | | Nucryst Pharmaceuticals Corp. | | | 5 | |
| | Industrial Technologies | | | 8 | |
| | | Ambeon | | | 8 | |
| | Discontinued Operations | | | 9 | |
| | Investments | | | 9 | |
| | Revenue by Segment | | | 10 | |
| | Supplies and Raw Materials | | | 10 | |
| | Environmental | | | 10 | |
| | Employees | | | 11 | |
| RISK FACTORS | | | 11 | |
| FORWARD-LOOKING STATEMENTS | | | 15 | |
| SELECTED CONSOLIDATED FINANCIAL INFORMATION | | | 15 | |
| | Quarterly Information | | | 16 | |
| MANAGEMENT’S DISCUSSION AND ANALYSIS | | | 16 | |
| MARKET FOR SECURITIES | | | 16 | |
| DIRECTORS AND OFFICERS | | | 16 | |
| ADDITIONAL INFORMATION | | | 18 | |
CORPORATE STRUCTURE
The Westaim Corporation (“Westaim” or the “Company”) was incorporated under theBusiness Corporations Act (Alberta)by Articles of Incorporation dated May 7, 1996, as a wholly owned subsidiary of Viridian (“Viridian” means Viridian Inc. and its predecessors). The Company’s Articles were amended effective June 26, 1996 to remove private company restrictions on the transfer of securities, to create preferred share classes designated as Class A Preferred Shares and Class B Preferred Shares and to increase the maximum number of directors to 15. The shareholders of Westaim approved a further amendment to the Articles on April 17, 2000 to permit meetings of shareholders to be held in certain specified cities outside of Alberta, or in any other city in Canada or the United States.
On June 26, 1996, the Company, through a reorganization, issued 75,000,000 common shares to Viridian in exchange for $180 million of cash along with its specialty materials businesses and related research and development activities, plus other industrial businesses. The transaction was effective June 1, 1996 and was accounted for as a reorganization, with the carrying value of the assets and liabilities transferred to the Company reflected in the Company’s balance sheet as at June 1, 1996 at Viridian’s carrying value.
On July 8, 1996 and September 16, 1996, Viridian distributed to holders of its common shares, as dividends-in-kind, an aggregate total of 70,923,248 common shares of Westaim. Viridian subsequently disposed of the remainder of its Westaim shares through market sales. Westaim’s principal and head office is located at 144 – 4th Avenue S.W., Calgary, Alberta, T2P 3N4.
The following are the material subsidiaries of the Company, including jurisdiction of incorporation or continuance and percentage share ownership by the Company and the operating divisions of the Company:
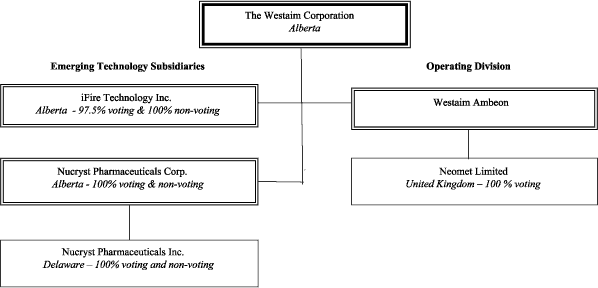
1
GENERAL DEVELOPMENT OF THE BUSINESS
In 1990, as a result of Viridian’s research and development capabilities in advanced industrial materials, Viridian entered into an agreement with the governments of Alberta and Canada to expand its specialty materials businesses. The undertaking, formed as a result of this agreement, is referred to as the “Westaim Initiative”. During the six-year period of the Westaim Initiative, $140 million was spent on research and development of advanced industrial materials. Of this $140 million, $40 million was funded by the Alberta Government, $30 million was funded by the Government of Canada, and $70 million was funded by Viridian and other industry research participants.
In 1997, the Company sold substantially all its assets related to the battery materials and cobalt powders business units for cash proceeds of $36 million. Also in 1997, the Company’s investment in its electronic ceramics and structural ceramics technologies, together with $15.4 million in cash, were rolled into a new corporate entity, ThermicEdge Corporation. In March of 1998, the Company distributed approximately 90.6% of the shares of ThermicEdge Corporation to the common shareholders of Westaim by way of a dividend-in-kind. The balance of 9.4% of the shares was transferred to the Province of Alberta as partial consideration for the release of Westaim by the Province of Alberta of any remaining obligations under the Westaim Initiative.
As noted in the chart above, the Company holds its interest in its two emerging technologies through wholly-owned, or majority-owned, subsidiaries. The related assets and intellectual property of these technologies were rolled into these subsidiaries effective January 1, 1998.
In February 2000, the Company’s subsidiary, iFire Technology Inc. (“iFire”), entered into a strategic business arrangement with Japan-based TDK Corporation for technology collaboration and production of iFire™ Displays. In addition, TDK Corporation purchased a 2.5% common equity investment in iFire for US$7.5 million.
In December 2000, the Company sold substantially all assets related to its Chemicals business segment for proceeds of $18 million.
In May 2001, the Company’s subsidiary, Nucryst Pharmaceuticals Corp., sold its North American burn dressing business and entered into an exclusive global licensing agreement for the sale and marketing of Acticoat™ Burn and Wound Care Dressings as discussed more in the Emerging Technologies section below.
On May 29, 2002, the Company announced its intention to sell non-core assets and to increase the focus of management and financial resources on its two high-potential emerging businesses, iFire and Nucryst Pharmaceuticals Corp. In July 2002, the Coinage business was shut down permanently and in February 2003 the ethylene coatings business (Surface Engineered Products Corporation) was shut down. See “Discontinued Operations”.
In January 2003, the Company’s Board of Directors retained Griffiths McBurney & Partners (“GMP”) as its financial advisor to undertake a review during 2003 of the operations of the Corporation. GMP has been tasked to either reaffirm the Company’s commitment to its high potential technologies and operating businesses or recommend a revised Corporate strategy.
2
DESCRIPTION OF THE BUSINESS
Westaim develops, commercializes and launches high potential technologies into the fast growing sectors of the economy. Westaim’s portfolio of business opportunities includes flat panel displays and anti-microbial medical devices and pharmaceuticals. These proprietary technologies resulted from the Westaim Initiative and from the Company’s advanced materials expertise. In addition, Westaim develops, manufactures and markets nickel composite powders and expects that this business will provide a source of cash flow to help fund the Company’s operations and research and development expenditures.
Westaim’s strategy is to develop the independent technical, operating and marketing and sales capabilities of each technology investment through the early years of product introduction and commercialization with the objective of taking these technologies public through initial public offerings. Management recognizes that, in circumstances where it lacks technical or marketing expertise or the necessary capital to complete development of a product, it may be in Westaim’s best interests to pursue commercialization through joint venture arrangements, strategic acquisitions, alliances, licensing or selling its technology.
EMERGING TECHNOLOGIES
iFire Technology Inc.
iFire Technology Inc. (“iFire”) has developed a proprietary flat panel display with solid state, thick-film dielectric electroluminescent (“TDEL”) technology. iFire is commercializing its proprietary technology for both large-screen consumer television applications and small graphic displays. The Company’s primary target is the mid-30-inch diagonal screen size television market. iSuppli/Stanford Resources, an independent research firm that follows the flat panel display industry, predicts that the market opportunity for mid-30-inch TVs will exceed US$23 billion by 2006 and the market for small graphic displays to exceed US$6 billion by 2005.
For mid-30-inch screens, the Company believes that in high-volume production, iFire™ displays will have a 30% to 40% cost advantage over other flat panel technologies due to TDEL’s simpler structure, less complex manufacturing methods and fewer processing steps compared with liquid crystal displays (“LCD”) and plasma display panels (“PDP”). Unlike other flat panel technologies, the iFire™ displays do not contain gases (as with PDP), liquids (as with LCD) or vacuum (as with the cathode ray tube), making it inherently rugged, less susceptible to shock, vibration and breakage. TDEL technology’s solid state structure and thick-film manufacturing process also make an iFire™ display less sensitive to cleanroom contamination that is associated with PDP and LCD, resulting in lower capital investment, higher manufacturing yield and reduced production cost. In addition, iFire™ displays feature full colour, rapid video response, unrestricted viewing angles and a wide operating temperature range. The technology is scalable from five inches to 50 inches.
iFire’s primary strategy is to become a significant supplier of high definition modules for large-screen flat panel televisions. The Company intends to initially target the mid-30-inch screen size market due to the lack of competitor presence. PDP technology is best suited for TVs greater than 40-inches; LCD technology is best suited for TVs less than 30-inches; and organic light emitting diodes (“OLED”) are best suited for small graphics applications with screen sizes less than 10-inches. iFire intends to establish partnerships with commercial consumer electronics companies to jointly manufacture iFire™ flat panel modules for consumer television sets. iFire believes that its technology can achieve significant market penetration in the flat panel television market segment and achieve a retail price advantage compared with PDP and LCD.
In 2002, iFire entered a non-exclusive technology collaboration agreement with Sanyo Electric Company, Ltd. (“Sanyo”) of Japan. The agreement focuses on the advancement of iFire’s TDEL technology for large-screen flat
3
panel televisions through a series of joint development projects. iFire retains exclusive use of any new joint developments in the inorganic electroluminescent display sector while Sanyo has exclusive rights to use any joint developments in the OLED field. Nine projects have been identified and initiated in the product categories of video electronics, manufacturing technologies for defect reduction and phosphor process development. The projects are on schedule with five completed. Two additional projects have been identified and are expected to begin in 2003.
In March 2003, iFire announced a non-exclusive joint development agreement with Dai Nippon Printing Co., Ltd. (“DNP”) of Japan for commercial production of mid-30-inch screen size flat panel television modules. To support the partnership with DNP, iFire and Sanyo have expanded their technology collaboration agreement to transfer technological and electronics advancements on 17-inch prototypes to the larger, mid-30-inch displays.
Under the terms of the agreement, DNP intends to utilize its primary flat panel production line in Kashiwa, Japan in 2003 for developing front-end manufacturing processes for iFire’s TDEL technology, including the substrate preparation and the fabrication of the row electrodes and thick-film dielectric layer. Back-end processes such as the deposition of phosphors, column electrodes and colour correction layers, as well as electronics assembly, are planned to be developed by iFire at its facility in Toronto, Canada. Three projects related to the development of low-cost manufacturing process for large size substrates have been identified. Two have been completed and one is underway. A fourth project has been proposed and is expected to begin in 2003.
For small graphic applications, iFire has pursued a licensing strategy. In February 2000, iFire entered into a non-exclusive licensing agreement with Japan-based TDK Corporation (“TDK”) for technology collaboration and production of iFire™ displays less than 12-inches. The agreement included a 2.5% equity investment in iFire, an up-front licensing fee and ongoing royalties on all sales. In 2001, TDK began demonstrating prototypes and in 2002, TDK announced that it plans to begin production at the earliest possible date.
In March 2001, iFire entered into an agreement with Technology Partnerships Canada (“TPC”), a technology investment fund established by the Government of Canada under which TPC will contribute up to $30 million over two years towards eligible research and development costs and related capital expenditures incurred by iFire. In consideration for this funding, TPC will receive a royalty of 1.065% on future sales of iFire products and has received warrants to purchase common shares of iFire for future consideration of $6.3 million. The warrants expire on April 30, 2007 and represented approximately 0.5% of the outstanding common shares of iFire as at March 31, 2001. In 2002, contribution claims totaled $9.1 million (2001 – $14.5 million). Of this amount, $8.1 million (2001 – $12.9 million) was credited to research and development expense and $1.0 million (2001 – $1.6 million) was credited to capital assets. The final contribution, amounting to $6.5 million, will be received in 2003.
In 2002, iFire demonstrated a 17-inch flat panel prototype with commercial-quality luminance, colour, contrast and pixel geometries equivalent to a 34-inch screen with a wide XGA (1280 x 768) pixel format. In addition, iFire prepared for mid-30-inch panel development by commissioning new column driver integrated circuits that are capable of supporting HDTV signals and are expected to be capable of driving iFire™ displays of up to 42 inches. iFire has begun securing the tools needed to produce a mid-30-inch television module. iFire also developed a new, lower cost, Efficient Resonant Integrated Supply (“ERIS”) power management system that should allow iFire™ wide- XGA products in the mid-30-inch screen size range to operate on less than 200 watts of power. Additionally, iFire substantially completed the basic material development to achieve the luminance required for a commercial product and identified a lower-cost substrate that will lead to manufacturing cost savings and better performance.
In April 2003, iFire announced a simplification of their manufacturing process which will result in capital and production cost reductions of approximately 15% compared to previous estimates. The development, called “Colour by Blue”, will replace the current triple patterned (red, green and blue) phosphor system with a single high performance blue phosphor system, simplifying the manufacturing process.
iFire will begin the technology transfer to scale up to larger modules in 2003 which the Company believes will lead to an initial HDTV product in the mid-30-inch screen size market priced lower than comparably sized LCD and PDP products. Other flat panel display technologies have not been able to competitively offer products in this screen
4
size, creating a major opportunity for iFire. In addition, this segment is currently one of the most lucrative parts of the television market because it is a size that is attractive to a broader range of consumers.
In conjunction with industry partners, production of mid-30-inch screen size products in both high definition and standard definition is expected to begin in the 2005 timeframe. This strategy includes iFire’s non-exclusive agreements with Sanyo and DNP.
While there are numerous current and emerging flat panel technologies, to date we know of no other competitor that has produced a full-colour, thick-film surface luminescent technology based on solid state electroluminescence. Westaim believes that the iFire™ display will have superior characteristics at a lower cost than competing technologies. However, successful commercialization is dependent upon meaningful progress in partner discussions which, in turn, are influenced by many factors including the state of the economy in the potential partner’s home market, particularly Japan, and the success or failure of competing technologies (previous and current) to profitably penetrate the flat panel display market with cost-effective products. As a result, the Company has found that investment in new technologies by large commercial electronics companies is taking longer and requiring lengthier technical due diligence than in the past.
The Company owns and operates a 36,000-square-foot research and trial manufacturing facility in Toronto, Ontario. The Toronto facility is capable of producing displays from 2-inches to 17-inches in diagonal screen size. In preparation for mid-30-inch display production, iFire has leased an adjacent 49,700 square-foot building. iFire employed 155 people at December 31, 2002.
With respect to iFire’s technology, the Company holds 7 patents related to TDEL technology in the United States, has 25 pending patent applications in the United States for advancements and improvements to the technology, and has numerous corresponding issued patents and patent applications in various other countries. Where appropriate, iFire also maintains certain proprietary technology as trade secrets.
Nucryst Pharmaceuticals Corp.
Nucryst Pharmaceuticals Corp. (“NUCRYST”) has developed a proprietary nanocrystalline form of noble metals such as silver, gold and platinum. While all three of these metals have medical uses, the Company’s first focus is nanocrystalline silver. Silver has antimicrobial properties and has been used in medicine for many years for infections. To be an effective antimicrobial agent, normally stable silver must be converted into a soluble form. NUCRYST’s proprietary technology can produce thin soluble films that release an effective concentration of silver over several days. As silver is consumed, additional silver is released to provide continued antimicrobial activity.
Medical Devices –NUCRYST’s technology can be applied to a wide range of medical devices, including wound dressings and various implants, to prevent infections. The Company chose the in-patient burn segment of the North American wound dressing market as its first target market because large burns present a life-threatening risk of infection.
In 1998, the Company launched its first product, Acticoat™ antimicrobial dressings for serious wounds. The product is currently being used in more than 100 of 120 major burn centres in North America and, in 2002, sales of Acticoat™ dressings were up more than 50% from 2001. NUCRYST’s dressings have been approved by the U.S. Food and Drug Administration (“FDA”) and by the Health Protection Branch in Canada and have also been given European certification (CE mark) which permits the marketing of the products in the United States, Canada and Europe.
5
As part of Westaim’s strategy to enter into partnerships and licensing agreements to accelerate its products’ market penetration, in May 2001, NUCRYST entered into a global partnership with Smith & Nephew plc (“Smith & Nephew”). Under a series of agreements, Smith & Nephew acquired NUCRYST’s North American burn dressing business and entered into an exclusive global licensing agreement for the sale and marketing of Acticoat™ wound care dressings developed by NUCRYST. NUCRYST receives reimbursement of manufacturing costs and royalties based on Smith & Nephew’s sales of these products, as well as milestone payments for the achievement of certain regulatory and sales accomplishments. In 2001, NUCRYST received a US$1.0 million milestone payment for the first Acticoat™ sale in Europe. Under a collaborative agreement, the two companies are working together to develop new wound care products and obtain additional regulatory approvals. In January 2003, NUCRYST received a US$3.0 million payment following the achievement of a regulatory milestone in Europe in which NUCRYST and Smith & Nephew collaborated to provide additional scientific evidence reinforcing the superior antimicrobial performance of Acticoat™ products. NUCRYST continues to manufacture all Acticoat™ products for Smith & Nephew at its Fort Saskatchewan, Alberta plant under a long-term manufacturing agreement.
Smith & Nephew is now selling Acticoat™ burn and chronic would products in 22 countries around the world. Chronic wounds affect more than 4 million patients in North America alone and the Company estimates that more than US$1 billion is spent annually on chronic wound products worldwide. Chronic wounds include: diabetic foot ulcers, a serious condition affecting 700,000 individuals in the United States alone (Medical Data International, 1996) that results in over 80,000 amputations each year (Center for Disease Control, Diabetes Surveillance, 1999); venous stasis ulcers, a condition caused by venous insufficiency which affects 1.2 million individuals in the United States every year (Medical Data International, 1996); and pressure ulcers, which commonly affect elderly, bed-ridden or chair-bound patients, of which, there are more than 2 million cases per year (Frost & Sullivan, 1998). Currently, there is no dominant treatment in the chronic wound dressing market.
NUCRYST believes that its products are superior to other existing products. The most commonly used competitive products use silver in various chemical forms in creams and solutions which must be applied to wounds several times a day. NUCRYST’s antimicrobial technology releases silver over several days, leaves the wound bed undisturbed while fighting infection and has been shown in the laboratory to be effective against more than 150 pathogens. Findings from in vitro studies published in peer-reviewed journals have demonstrated that NUCRYST’s coatings kill bacteria rapidly and have a longer release of silver than conventional treatments. Use of Acticoat™ dressings provides health care cost savings by preventing infections and reducing the required number of dressing changes, thereby saving nursing time and medical supply costs.
Already used commercially on Acticoat™ dressings, NUCRYST’s proprietary nanocrystalline silver coatings, trademarked Silcryst™, have proven safe for use by humans and have demonstrated their efficacy as a barrier to infection. NUCRYST will now focus on developing drugs based on customized formulations of its patented nanocrystalline technology. These drugs may be offered in a variety of delivery formats including creams, solutions or gels.
Pharmaceuticals– NUCRYST’sRx nanocrystalsappear to have two very important therapeutic effects: broad-spectrum antimicrobial activity and anti-inflammatory activity. This dual activity could be useful for treating a wide range of diseases. Pre-clinical work by NUCRYST has pointed to two broad areas: dermatological and respiratory diseases. In both these areas, the Company sees new product opportunities. Diseases of the skin affect millions of people and are not optimally treated with today’s drugs. Nosocomial (hospital acquired) lung infections are a leading cause of death among hospitalized patients. The total US market size is over US$2 billion for dermatology products and US$3 billion for respiratory products (IMS 2002 data).
The first indication that NUCRYST plans to target is eczema, a very common skin disease characterized by inflammation and itching. Infection often accompanies eczema as a result of excessive scratching, which damages or excoriates the skin, and because eczema patients may lack endogenous antimicrobial substances in their skin. The two leading anti-inflammatory therapies are topical steroids and topical immunomodulators (“TIMS”). Today,
6
there are no drugs available for eczema that are both anti-inflammatory and antimicrobial. Due to nanocrystalline silver’s dual action (providing both antimicrobial and anti-inflammatory activity), NUCRYST believes that its proprietary silver formulation will be more effective than the leading drug classes available today.
Topical steroids are typically the first line of treatment for many skin diseases, but are not always the most effective. Steroids are known to cause many side effects such as atrophy (thinning of the skin), telangiectasia (visible “spider-like” blood vessels) and striation (striping) of the skin. In addition, topical steroids are vulnerable to tachyphylaxis (loss of efficacy over time) and run the risk of systemic absorption.
TIMS produce their anti-inflammatory effect by inhibiting the immune system. Since they are potent immunosuppressants, they are generally indicated after other treatments (such as topical steroids) have proven to be ineffective or are deemed inadvisable. In addition to a burning sensation often experienced upon application, side effects may include the increased risk of superficial skin infections, such as shingles (herpes zoster virus infection) or eczema herpeticum, due to TIMS potent immunosuppressant activity.
NUCRYST’s nanocrystalline silver does not appear to significantly suppress the immune system or damage the skin. Nanocrystalline silver has the added potential benefit of combating secondary infections that are common to eczema sufferers, something that steroids and TIMS cannot do. NUCRYST’sin vivo laboratory results are very encouraging.
Despite the risks and limitations of both topical steroids and TIMS, they have a sizeable market, generating US$665 million and US$165 million in 2002 sales respectively (IMS 2002 data), in the United States alone. TIMS have only been on the market for two years, demonstrating that physicians and patients are seeking and willing to try new, more effective treatments. NUCRYST believes that a new dual action drug that addresses both inflammation and infection without the risks associated with steroids and TIMS will be well received by doctors and patients.
NUCRYST has filed an Investigational New Drug (“IND”) application with the FDA for a nanocrystalline silver cream. NUCRYST began Phase I clinical studies in April 2003 following clearance of the IND.
In general, the regulatory pathway to FDA product approval for pharmaceuticals includes:
| (i) | | Pre-clinical Animal Studies– These studies evaluate the safety and potential efficacy of a therapeutic drug or medical device and are necessary prior to initiation of human clinical studies. |
| |
| (ii) | | Phase I Clinical Studies– These studies test the product in a small number of volunteers to determine toxicity (safety), maximum dose tolerance and pharmacokinetic (the absorption, distribution and excretion of a drug) properties. |
| |
| (iii) | | Phase II Clinical Studies– These studies assess safety and efficacy in patients. |
| |
| (iv) | | Phase III Clinical Studies– These studies involve a controlled evaluation in an expanded patient population at multiple sites to determine longer-term clinical safety and efficacy. It is from the data of these studies that the benefit/risk relationship is established and the final drug labeling claims are defined. |
Results of these controlled clinical studies are detailed in the filing of a New Drug Application (“NDA”) for pharmaceuticals, together with detailed information related to the drug manufacturing process. The FDA full approval process is expected to take four to seven years.
7
The Company owns and operates a 43,000 sq. ft. production facility in Fort Saskatchewan, Alberta and leases 19,000 sq. ft. of office and laboratory space in Wakefield, Massachusetts for administration, marketing, and pharmaceutical research and development. Depending on product specifications, the Fort Saskatchewan facility is capable of producing up to $60 million of burn or wound care product per year.
NUCRYST employed 62 people in Canada and the U.S. as at December 31, 2002.
NUCRYST holds 14 issued patents in the United States, has 19 pending applications in the United States, and has numerous corresponding patents and patent applications in various other countries. Where appropriate, NUCRYST also maintains certain proprietary technology as trade secrets.
Acticoat™ is a trademark of Smith & Nephew and Silcryst™ is a trademark of NUCRYST.
INDUSTRIAL TECHNOLOGIES
Ambeon
The Ambeon division designs, manufactures and markets advanced materials and products under three major product lines: turbine engine materials, electronics materials and catalyst materials.
Turbine Engine Materials – Ambeon produces and markets composite powders and honeycomb for use in gas turbine engines (under the trademarks Durabrade™ and Neomet™, respectively). The principal market for these turbine engines is commercial and military airplanes. The Company’s products create an abradable seal between moving and stationary metal parts. These products are also used in land-based turbine power generators.
As a turbine engine heats up, metal parts expand; conversely, as the engine cools, metal parts contract. The Company’s composite powders and honeycomb are designed to minimize the clearance between the stationary and rotating parts of the engine resulting in improved efficiency and fuel economy. As an engine blade rotates, the outer edge of the blade cuts through the material allowing precise control of the gap between stationary and moving parts, effectively creating a seal.
The Company’s competitive advantage lies in its ability to identify opportunities to leverage its extensive experience in the manufacturing processes that it has developed to produce composite powders in commercial quantities. Additionally it has extensive experience in the qualification and certification of products to meet aerospace standards.
Ambeon has a long history and successful track record of design, qualification and large-scale production of composite powders technology. In addition, the Company’s honeycomb products are complementary to its composite powders with higher temperature capabilities that enable the Company to provide material for abradable seals in most sections of the turbine engine. Ambeon is actively pursuing key performance advances in coating materials that will help its customers define and implement the next generation of abradable seals to work in all temperature ranges of turbine engines. Many of these products are currently being tested for approval by key customers.
Principal customers are major jet engine manufacturers and distributors to the jet engine repair and overhaul market. The Company is an ISO 9002 approved supplier to all three major original equipment manufacturers, (Rolls-Royce, Pratt & Whitney, GE Aircraft Engines) and holds a National Aerospace and Defence Contractors Accreditation Program (“NADCAP”) certification. These approvals are difficult to obtain and represent a significant barrier to entry for new competitors.
8
Electronics Materials – The Company also produces materials for use in electromagnetic shielding applications. The Company’s powders are incorporated into tapes, gaskets, adhesives and other materials used in the manufacture of portable devices such as cell phones. The powders provide these materials with the conductive and shielding characteristics required for their high-tech applications, allowing electronic devices to be made smaller and lighter without losing functionality. In 2002, Ambeon introduced gold-coated composite powders for the conductive materials used in electronics packaging.
Catalyst Materials– During 2002, Ambeon used its proprietary technology and existing production capability to make nickel-based catalyst powder materials. The Company currently produces a unique catalyst that is used in the production of hydrogen from natural gas. Sales of catalyst materials in other industrial product areas are currently being developed. Catalyst material production capability is expected to lead to the Company’s participation in the growing hydrogen fuel cell market within the next 3 to 5 years.
The Company owns a 62,000 sq. ft. production facility for aerospace and electronics powders in Fort Saskatchewan, Alberta, with an annual capacity of approximately 300 tonnes of composite nickel powders for turbine engines and electronics materials. Westaim manufactures its nickel-based honeycomb products at a 13,000 sq. ft. leased facility in Marple, England, operated by its wholly-owned subsidiary, Neomet Limited. This facility has an annual capacity of approximately 15 tonnes of honeycomb.
The Company protects its coating and catalyst products’ intellectual property through patents, where appropriate, or through trade secrets. The Company has 14 issued patent applications for advancements and improvements to its technology worldwide related to its current product lines and 16 pending patent applications.
The Westaim Ambeon business segment employed 109 people as at December 31, 2002.
DISCONTINUED OPERATIONS
In May 2002, the Company announced its intention to close the Coinage division and to sell or close its ethylene coatings business. As a result, these businesses have been accounted for as discontinued operations.
The decision to discontinue the Coinage business followed several quarters of operating losses. The outlook for the business was unsustainable as a result of weak demand and global over-capacity in the coin blank industry following completion of the multi-year Euro coin project. The Company continues to own and maintain the 120,000 sq. ft. former Coinage facility in Fort Saskatchewan, Alberta, which is available for, and in partial use by, other Westaim businesses. Production assets were sold in late 2002. At December 31, 2002, the Company had no employees engaged in the Coinage division.
The ethylene coatings business, Surface Engineered Products (“SEP”), produced anti-coking coatings for furnaces used in the production of ethylene. SEP’s main target market, the ethylene industry, has been experiencing harsh economic conditions and the rate of adoption of the anti-coking technology has not met expectations. SEP operations were discontinued in February 2003 after the completion of contracted production. The Company plans to sell the operating assets and the 120,000 sq. ft. former ethylene coatings manufacturing facility in Edmonton, Alberta. SEP had 39 employees at December 31, 2002.
INVESTMENTS
Until early 2002, Westaim, and its Westaim Partners division, made early stage investments in emerging technology businesses. In May 2002, the Company made the decision to discontinue further early stage investments and began reducing its existing investment portfolio. The Company recorded equity losses and write downs related to these
9
investments of $3.4 million in 2002. The Company’s voting interest in each of the remaining investments is 12% or less and is accounted for on a cost basis, with a total book value of $0.5 million.
REVENUE BY SEGMENT
| | | | | | | | | | |
| | | | | | | | Year Ended |
| | | | Year Ended | | December 31, 2001 |
| ($000) | | December 31, 2002 | | (restated) (Note 2) |
| |
| |
|
| Emerging Technologies | | | | | | | | |
| | Nucryst Pharmaceuticals | | $ | 8,344 | | | $ | 9,835 | |
| | iFire Technology | | | 4,270 | | | | 4,272 | |
| | Ambeon (Note 1) | | | 36,449 | | | | 36,160 | |
| | | |
| | | |
| |
| | | $ | 49,063 | | | $ | 50,267 | |
| | Discontinued Operations (Note 2) | | | 10,283 | | | | 18,616 | |
| | | |
| | | |
| |
Consolidated Operations | | $ | 59,346 | | | $ | 68,883 | |
| | | |
| | | |
| |
| | | | | | | | | |
| | | |
| Note 1 | | In 2002, Ambeon recorded revenue from one customer which represented 20.3% (2001 – 21.7%) of revenue from continuing operations of the Company. |
| |
| Note 2 | | In 2002, the Coinage division and the ethylene coatings business, Surface Engineered Products, have been accounted for on a discontinued basis. 2001 information has been restated accordingly. |
SUPPLIES AND RAW MATERIALS
Owing to the diversity of its products, Westaim purchases its raw materials from a number of sources. Westaim suppliers are both domestic and international. Nickel, graphite, hydrogen, and ammonia are the major raw materials required by the Company, most of which can be purchased in the open market.
Westaim is provided certain utilities, services and materials from an unrelated company at the Fort Saskatchewan plant site under long term supply and service agreements. These agreements ensure the continued supply of these inputs which are required for Westaim’s operations and which Westaim is not presently equipped to supply itself. Westaim has the right under these agreements to construct new facilities to supply its needs or to obtain supplies from third parties in the open market.
ENVIRONMENTAL
Westaim’s businesses are subject to extensive federal, provincial and municipal environmental statutes and regulations, including those relating to air emissions, wastewater discharges, contaminated soil and groundwater and the handling and disposal of hazardous substances and wastes. Westaim believes that its operations are in substantial compliance with these statutes and regulations, and it has an extensive environmental program in place to comply with environmental regulations and to maintain its facilities in an environmentally safe condition.
The Company anticipates that it will continue to incur capital expenditures and operating costs to comply, on an ongoing basis, with environmental statutes and regulations. It does not believe that the costs of compliance will have a material adverse affect on its operations, its competitiveness or its financial position. However, Westaim’s businesses involve potential environmental risks, including the risk of harmful substances entering the environment, which could cause damage or injury.
10
Environmental liabilities under applicable statutes and regulations may arise in respect of events which occurred prior to completion of the “Viridian Reorganization”, when the businesses transferred to Westaim belonged to Viridian. The agreements under which the “Viridian Reorganization” was completed provide that Viridian, or its successors, will bear all costs associated with these environmental liabilities that existed prior to June 1996 for the Fort Saskatchewan site. With respect to Westaim’s operations in Ontario, the previous owner of iFire’s trial manufacturing plant has provided a limited environmental indemnity to Westaim in respect of any pre-existing soil or groundwater contamination.
Westaim’s operations in Alberta are conducted under an environmental operating approval issued under the Environmental Protection and Enhancement Act (Alberta) on March 31, 1998 and expiring on February 28, 2008. iFire operates under a Certificate of Approval granted by the Ministry of the Environment (Ontario) on February 16, 1999.
EMPLOYEES
As at December 31, 2002, Westaim and its subsidiaries employed a total of 399 employees which included 26 hourly employees represented by the Communications, Energy and Paperworkers Union. Union-management relations are positive and stable. The current collective agreement expires in March 2004. Westaim’s workforce includes a number of highly skilled professional, technical and operational personnel. Many employees have specialized knowledge and skills and are leaders in their fields.
RISK FACTORS
Westaim is primarily in the business of developing and commercializing new technologies. There are a number of risks inherent in this type of business model, including those set out below. The occurrence of events arising from these risks could have a material adverse effect on the Company’s business, financial condition, and results from operations.
Westaim may be unable to develop commercially viable products.
Many of the Company’s products, such as iFire’s flat panel television displays, are still in the developmental stage. Westaim will likely incur significant research and development costs before any of these products are commercially viable, and there is no assurance that any of its products will ever reach this stage or that the Company will achieve the level of market penetration that it expects. Some or all of the technological obstacles that will need to be overcome in order to make these products commercially viable may prove to be insurmountable. Conversely, a market for these products may never materialize, even if they prove to be technically sound. If new products have reliability or quality problems, then reduced orders, higher manufacturing costs, delays in acceptance of and payment for new products, and additional service and warranty expenses may result.
Some of the Company’s proposed products, such as medical devices and pharmaceutical products, will require regulatory approval before the Company is allowed to sell them. The Company expects the regulatory approval process to be lengthy and expensive and the Company will have the burden of proving that its products are safe and effective. Regulatory agencies may make their approval subject to conditions that the Company may be unable to satisfy or that, if satisfied, would cause its products to become prohibitively expensive. There is no assurance that the Company will ever obtain regulatory approval to sell any of its proposed products, or that the conditions imposed by regulators will be satisfactory to the Company. Regulatory requirements imposed on its products could limit the Company’s ability to test, manufacture and commercialize its products.
11
If Westaim fails to raise the capital necessary to fund its operations, it may be unable to advance the development and commercialization of its technologies.
A commitment of substantial resources by the Company and its collaborators to conduct research and development, construct pilot manufacturing facilities and conduct clinical studies will be required to successfully commercialize products under development. It has not been necessary for the Company to raise new capital since its inception and the Company believes that its current financial position is strong. Nevertheless, the Company believes that many of its businesses, particularly the flat panel television display, medical products and pharmaceuticals businesses, may grow extremely rapidly and the Company may not be able to raise additional capital at the time it is needed to complete product development and build manufacturing facilities. Additional capital may be required to fund operations, fund clinical studies, continue the research and development of product candidates, commercialize products and construct pilot and full scale manufacturing facilities.
If the Company is unable to raise additional funds when required, it may be necessary to delay, reduce or eliminate some or all of its development programs.
The commercial potential of the Company’s products depends upon certain issues regarding pricing, production costs and medical reimbursement.
The Company’s ability to commercialize its pharmaceutical products successfully will depend in part on the extent to which reimbursement for the cost of such products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Third party payers are increasingly challenging the price of medical products and services. Significant uncertainty exists as to the reimbursement status of newly approved healthcare products and there can be no assurance that adequate third party coverage will be available for the Company to realize an appropriate return on its investment in product development.
The ability to commercialize iFire’s flat-screen technology successfully will depend in part on its ability to price its products at a point that will generate consumer demand, while allowing for an adequate profit margin. The Company believes that its product can be produced at costs lower than other flat-screen technologies but there is no assurance that there will be consumer demand for the iFire product or that competing products will not be developed and priced below the prices required by iFire to be profitable.
Westaim will need to form partnerships to develop and sell its products.
The Company’s ability to successfully develop, manufacture and market its current and proposed products will depend, to a large extent, on its ability to form partnerships or joint ventures with established corporations or other collaborators. Except as described elsewhere in this document, the Company has not yet entered into any material partnerships or joint ventures for the development or marketing of these products, nor will it necessarily be able to do so in the future. The Company may be unable to find suitable partners or be unable to form a partnership or joint venture on terms that are beneficial. If the Company does enter into a partnership or joint venture, it may suffer loss if the partner becomes insolvent or otherwise fails to meet its obligations to the Company.
Westaim’s products may become technologically obsolete.
The Company competes, and intends to compete, in markets that are characterized by rapid adaptation to technological change. These markets include, but are not limited to the medical devices, pharmaceuticals and flat-screen television monitor markets. The Company’s current and future products may be quickly rendered obsolete and unmarketable. The Company will need to continually develop new products and enhance existing products to keep pace with evolving technologies, customer preferences and industry standards.
12
Westaim is developing products for highly competitive markets.
The Company faces, and will face, competition from a number of other companies including major domestic and international companies which have substantially greater financial, technical, marketing, sales, distribution and other resources. Many of these competitors may also have greater name or brand recognition. The Company’s competitors may produce more technologically-advanced products, at a lower cost, than the Company is capable of producing. Competition may cause the Company to lose market share and may reduce profit margins on any products that it is able to sell.
Westaim may be unable to protect its intellectual property.
In order to succeed, the Company will need to prevent its intellectual property from being misappropriated by third parties. To protect its intellectual property, the Company relies primarily on its confidentiality agreements, physical security at research and manufacturing facilities, as well as the copyright, trade secret, trademark and patent laws of Canada, the United States, and other countries in which the Company conducts, or will conduct, business. The laws of other countries may not protect intellectual property rights to the same extent as the laws of Canada and the United States and, in any event, the methods that the Company has chosen may fail to adequately prevent misappropriation of its intellectual property.
The Company currently holds over 210 patents worldwide and an additional 190 patent applications are pending. The Company cannot provide assurance that it will succeed in obtaining new patents, that it will be able to enforce existing patents against third parties, or that existing patents will not be successfully challenged by third parties.
Even if the Company is able to prevent the misappropriation of intellectual property, others may independently, and legally, develop technologies that are substantially equivalent or superior.
Westaim may become involved in expensive intellectual property or product liability litigation.
The Company may be required to commence litigation to enforce its intellectual property rights. Others may claim that it has infringed upon their intellectual property rights and commence litigation against the Company. The Company believes that it will be subject to an increasing number of infringement claims as it begins to produce more products in more industries.
Some of the Company’s existing and proposed products, such as its medical devices and pharmaceuticals, are part of a class of product that is particularly vulnerable to product liability litigation for a number of reasons:
| – | | These types of products are extremely complex and the Company may fail to discover product faults, despite its best efforts; |
| |
| – | | These types of products will interact with very complex biological and man-made systems and the Company’s products may interact with these systems in harmful ways that it was unable to anticipate, despite its best efforts; and |
| |
| – | | Because these products may be used by a large number of people, if the Company’s products do cause harm, it may be exposed to a large number of claims for damages. |
The Company has tried to protect itself against product liability litigation by including limitation of liability provisions in some of its sales agreements. There is no assurance, however, that existing or future limitation of liability provisions will be sufficient to protect the Company in all circumstances, nor can the Company provide assurance that any of these provisions will be held to be enforceable by the Courts.
The Company believes that it has obtained sufficient product liability insurance coverage to protect it against claims. However, the wording of its insurance policies may exclude some claims. Furthermore, the Company cannot provide assurance that its insurance limits will be sufficient, nor can it ensure that it will be able to acquire satisfactory insurance in the future.
13
Westaim may be unable to retain the required highly skilled people.
The Company’s technology businesses are dependent upon the talents and knowledge of certain key individuals in each of the businesses. The marketplace for people with these skills is highly competitive, and the Company may not be able to retain a sufficient number of people with the skills that it requires.
Some of Westaim’s businesses may inadvertently harm the environment.
The Company is subject to extensive federal, state, provincial and local laws in Canada, the United States, and elsewhere, regarding pollution, the protection of the environment and the use or cleanup of hazardous substances. Although Westaim believes that it is in compliance with all of these laws, some of its businesses may inadvertently harm the environment in violation of these laws. If this occurs, the Company may be required to pay significant fines, the cost of investigation and cleanup and civil damages. The Company may also be ordered to permanently stop some of its operations, or it may be ordered to cease its operations temporarily until it has adopted new and potentially expensive environmental controls. The Company may suffer a significant loss of reputation and risk lengthy and expensive litigation if it harms the environment in breach of environmental laws.
Westaim is subject to certain risks because of the international character of its business.
The Company estimates that sales to international customers accounted for approximately 98% of its net sales in the fiscal year ended December 31, 2002 and Westaim anticipates that international sales will continue to represent a material portion of net sales in the future. International sales are subject to inherent risks, including variations in local economies, fluctuating exchange rates, greater difficulty in the collection of accounts receivable, changes in tariffs and other trade barriers, adverse foreign tax consequences and burdens of complying with a variety of foreign laws. The Company may also encounter exchange rate risk in the event international sales are denominated in a currency other than Canadian dollars.
The Company’s financial results are reported in Canadian dollars. A significant portion of the Company’s revenue and expenses, as well as accounts payable, accounts receivable and other balance sheet items, are frequently denominated in currencies other than the Canadian dollar, primarily in United States dollars. Fluctuations in the exchange rate between these other currencies and the Canadian dollar could reduce the Company’s reported revenue, increase the Company’s costs or give rise to a charge related to foreign currency translation, all of which could adversely affect operating results.
Volatility of Share Price
Market prices for securities of companies developing new technologies are generally volatile. Factors such as announcements of technological innovations, new commercial products, patents, the development of proprietary rights, results of clinical trials, regulatory actions, publications, quarterly financial results, the Company’s financial position, public concern over the safety of biotechnology, future sales of shares by the Company or by our current shareholders, and other factors, could have a significant effect on the market price and volatility of the Company’s common shares.
14
FORWARD-LOOKING STATEMENTS
Forward-looking statements involve significant risks, uncertainties and assumptions, and Westaim’s actual results could differ materially from those anticipated by these forward-looking statements for various reasons generally beyond Westaim’s control.
Certain statements contained in this Annual Information Form, as well as other public statements by Westaim, include forward-looking statements within the meaning of Section 21E of the U.S. Securities Exchange Act of 1934. The words “may”, “could”, “should”, “would”, “will”, “suspect”, “outlook”, “believe”, “anticipate”, “estimate”, “expect”, “intend”, “plan”, and words and expressions of similar import, are intended to identify forward-looking statements concerning expected iFire and NUCRYST product introductions, performance, market penetration, technology development, production costs, price advantages and applications; potential size of the market for iFire and NUCRYST technology; the timing of clinical studies and expected date and cost of certain regulatory approvals, including from U.S. and Canadian authorities, for NUCRYST pharmaceutical products; the expected commencement of iFire product manufacturing and its introduction of new products into the market for consumer television products; and the expected global demand in 2006 for television products, including for flat panel televisions. Forward-looking statements are not guarantees of future performance. They involve significant risks, uncertainties and assumptions and the Company’s actual results could differ materially from those anticipated by these forward-looking statements for various reasons generally beyond our control, including: (i) hurdles in the completion and patenting of the iFire technology; (ii) complexities associated with developing the flat screen manufacturing process; (iii) market and competing technology developments which might affect the willingness of potential iFire partners to manufacture and market iFire products; (iv) cost estimates based upon assumptions which may prove not to be realistic; (v) delays or problems in receiving regulatory approvals for NUCRYST’s products; (vi) market or economic conditions which might affect the product’s development, clinical studies and demand for iFire’s or NUCRYST’s products; (vii) general economic and financing conditions which may affect the ability to raise new capital or affect potential partner ability to contribute financially; (viii) general industry and market conditions and growth rates; and (ix) the other risk factors set forth above.
SELECTED CONSOLIDATED FINANCIAL INFORMATION
The following table sets forth certain financial information for the Company for 2000 – 2002:
| | | | | | | | | | | | | |
| | | Year Ended | | Year Ended | | Year Ended |
| ($000, except per share data) | | Dec. 31, 2002 | | Dec. 31, 2001 | | Dec. 31, 2000 |
| |
| |
| |
|
| Revenue from Continuing Operations | | $ | 49,063 | | | $ | 50,267 | | | $ | 51,324 | |
| Loss from Continuing Operations | | | (30,095 | ) | | | (8,428 | ) | | | (18,541 | ) |
| Loss per Common Share from Continuing Operations | | | (0.39 | ) | | | (0.11 | ) | | | (0.24 | ) |
| Net Loss | | | (49,762 | ) | | | (67,459 | ) | | | (14,648 | ) |
| Net Loss per Common Share | | | (0.64 | ) | | | (0.87 | ) | | | (0.19 | ) |
| Total Assets | | | 185,748 | | | | 238,179 | | | | 309,482 | |
| Total Long Term Debt | | | – | | | | – | | | | – | |
| Dividends Declared | | | – | | | | – | | | | – | |
In 2002, the Company made the decision to close the Coinage division and to divest or shutdown the ethylene coatings business. Accordingly, the results of these operations and the cost of shutdown have been accounted for on a discontinued basis. Prior years’ information has been restated accordingly.
In 2000, the Company sold substantially all its assets related to the Chemicals business segment for proceeds of $18 million. Accordingly, the results of these operations and the costs of disposition have been accounted for on a discontinued basis.
15
QUARTERLY INFORMATION
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| ($000, except per share data) | | | | | | | | | | | | | | Restated (Note 1) |
| | | | | | | | | | | | | | | | | | |
|
| | | Q1 | | Q2 | | Q3 | | Q4 | | Q1 | | Q2 | | Q3 | | Q4 |
| | | 2002 | | 2002 | | 2002 | | 2002 | | 2001 | | 2001 | | 2001 | | 2001 |
| | |
| |
| |
| |
| |
| |
| |
| |
|
| Revenue from Continuing Operations | | $ | 12,018 | | | $ | 11,638 | | | $ | 11,698 | | | $ | 13,709 | | | $ | 13,639 | | | $ | 11,660 | | | $ | 12,510 | | | $ | 12,458 | |
| Loss from Continuing Operations | | | (5,997 | ) | | | (11,464 | ) | | | (6,338 | ) | | | (6,296 | ) | | | (3,274 | ) | | | 8,628 | | | | (4,846 | ) | | | (8,936 | ) |
| Loss per Common Share from Continuing Operations | | | (0.08 | ) | | | (0.15 | ) | | | (0.08 | ) | | | (0.08 | ) | | | (0.04 | ) | | | 0.11 | | | | (0.06 | ) | | | (0.11 | ) |
| Net Loss | | | (8,362 | ) | | | (25,617 | ) | | | (6,338 | ) | | | (9,445 | ) | | | (8,005 | ) | | | 2,018 | | | | (7,718 | ) | | | (53,754 | ) |
| Net Loss Per Common Share | | | (0.11 | ) | | | (0.33 | ) | | | (0.08 | ) | | | (0.12 | ) | | | (0.10 | ) | | | 0.03 | | | | (0.10 | ) | | | (0.69 | ) |
| | | |
| Note 1 | | In 2002, the Company made the decision to close the Coinage division and to divest or shutdown the ethylene coatings business. Accordingly, the results of these operations and the cost of shutdown have been accounted for on a discontinued basis. Prior years’ information has been restated accordingly. |
MANAGEMENT’S DISCUSSION AND ANALYSIS
The information set out under the heading “Management’s Discussion and Analysis”, on pages 18 through 23 of the Company’s 2002 Annual Report, is incorporated herein by reference.
MARKET FOR SECURITIES
The Common Shares of Westaim are listed on The Toronto Stock Exchange under the symbol WED and on NASDAQ under the symbol WEDX.
16
DIRECTORS AND OFFICERS
The following table sets forth the names, municipality of residence and principal occupation of the directors of the Company, and the period of service as a director of the Company.
| | | | | |
| Name and Municipality | | Principal Occupation | | Period of Service |
| Of Residence | | At Present5 | | As Director |
| |
| |
|
Neil Carragher1,3,4
Toronto, Ontario | | Chairman of The Corporate Partnership Ltd. | | May 1996 to date |
| | | | | |
Ian W. Delaney4
Toronto, Ontario | | Chairman of Sherritt International Corporation | | May 1996 to date |
| | | | | |
Barry M. Heck
Calgary, Alberta | | President and Chief Executive Officer of the Company | | January 2003 to date |
| | | | | |
Frank W. King2,4
Calgary, Alberta | | President of Metropolitan Investment Corporation | | May 1996 to date |
| | | | | |
Edward M. Lakusta1,2,4
Calgary, Alberta | | Private Business and Energy Consultant | | May 1996 to date |
| | | | | |
Daniel P. Owen2,3,4
Toronto, Ontario | | Chairman of Molin Holdings Limited | | May 1996 to date |
| | | | | |
Guy J. Turcotte1,4
Calgary, Alberta | | President and Chief Executive Officer of Western Oil Sands Inc. | | April 1998 to date |
| | | | | |
Bruce V. Walter3,4
Toronto, Ontario | | Vice-Chairman of Dynatec Corporation | | May 1997 to date |
Notes:
| 1. | | Messrs. Turcotte, Carragher and Lakusta are members of the Audit Committee. |
| |
| 2. | | Messrs. King, Lakusta and Owen are members of the Compensation Committee. |
| |
| 3. | | Messrs. Walter, Carragher and Owen are members of the Environmental, Health and Safety Committee. |
| |
| 4. | | Messrs. Delaney, Carragher, King, Lakusta, Owen, Turcotte and Walter are members of the Corporate Governance Committee. |
| |
| 5. | | Each of the Directors has been engaged for more than five years in his present principal occupation except the following: |
| |
| | | Barry M. Heck– Prior to becoming President and Chief Executive Officer of the Company on January 15, 2003, Mr. Heck was Senior Vice President of the Company from January 1997. |
| |
| | | Guy J. Turcotte– Mr. Turcotte became Chief Executive Officer of Western Oil Sands Inc. in July 1999 and President in January 2002. He has held the position of Chairman of Fort Chicago Energy Partners L.P. since December 1997 and was Chief Executive Officer of that company from December 1997 to December 2002. |
| |
| | | Bruce V. Walter– Prior to becoming Vice-Chairman of Dynatec Corporation in March 2002, Mr. Walter was Chief Executive Officer of Four Mile Investments Inc. from August 1993; Managing Director, BMO Nesbitt Burns Inc. from February 1999 to November, 2001; Chief Executive Officer of Plaintree Systems Inc. from April 1993 to June 1998 and Vice-Chairman of that company until October 1998. |
Each of the directors will hold office until the next meeting of shareholders or until a successor is duly elected or appointed. The Westaim Corporation does not have a standing executive committee.
17
The following table sets forth the names, municipality of residence and office of the executive officers of the Company.
| | | |
| Names and Municipality of Residence | | Office with Company |
| |
|
Ian W. Delaney
Toronto, Ontario | | Chairman of the Board (non-employee) |
| | | |
Barry M. Heck
Calgary, Alberta | | President and Chief Executive Officer |
| | | |
G.A. (Drew) Fitch
Calgary, Alberta | | Senior Vice President and Chief Financial Officer |
| | | |
Anthony B. Johnston
Calgary, Alberta | | Senior Vice President |
| | | |
Brian D. Heck
Sherwood Park, Alberta | | General Counsel and Corporate Secretary |
Barry Heck– Before becoming President and Chief Executive Officer of the Company on January 15, 2003, Mr. Heck was Senior Vice President of the Company from January 1997.
Brian Heck– Mr. Heck has been Counsel to the law firm of Henning Byrne Whitmore & McKall since June 2001. From November 1999 through October 2000, Mr. Heck practiced in the areas of corporate law and mergers and acquisitions with the law firm of Bennett Jones. From October 1997 to May 1999, Mr. Heck was Vice President, General Counsel and Corporate Secretary to ThermicEdge Corporation and MicroWear Corporation.
All other officers have been in their current positions and these have been their principal occupations for the past five years.
The percentage of the Company’s common shares beneficially owned, directly or indirectly, or over which control or direction is exercised by all directors and executive officers of the Company, as a group, as at April 30, 2003 was 1.95%.
ADDITIONAL INFORMATION
Additional information, including directors’ and officers’ remuneration and indebtedness, principal holders of the Company’s securities, options to purchase securities and interests of insiders in material transactions, where applicable, is contained in the Company’s information circular with respect to its most recent annual meeting of shareholders. Additional financial information is contained in the Company’s comparative financial statements for its most recently completed fiscal year.
The Company undertakes, upon request to the Corporate Secretary, The Westaim Corporation, to provide to any person, when the securities of the Company are in the course of a distribution pursuant to a short form prospectus or when a preliminary short form prospectus has been filed in respect of a distribution of its securities:
| (i) | | one copy of the Company’s AIF, together with one copy of any document, or the pertinent pages of any document, incorporated by reference in the AIF; |
18
| (ii) | | one copy of the Company’s comparative financial statements for its most recently completed financial year together with the accompanying report of the auditors and one copy of the most recent interim financial statements that have been filed for any period after the end of its most recently completed financial year; |
| |
| (iii) | | one copy of the Company’s information circular in respect of its most recent annual meeting of shareholders that involved the election of directors; and |
| |
| (iv) | | one copy of any other documents that are incorporated by reference into the preliminary short form prospectus or the short form prospectus and are not required to be provided under (i) to (iii) above; |
or, at any other time, subject to payment of a reasonable charge if the request is made by a person who is not a security holder of the Company, one copy of any document referred to in (i), (ii) and (iii) above.
19

