UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark
One)
ý | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | |
For the fiscal year ended December 31, 2005 |
|
Or |
|
o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 000-31141
Discovery Partners International, Inc.
(Exact name of registrant as specified in its charter)
Delaware State or other jurisdiction of
incorporation or organization | | 33-0655706 (I.R.S. Employer Identification No.) |
| | |
9640 Towne Centre Drive, San Diego, California (Address of principal executive offices) | | 92121 (Zip Code) |
| | | | |
Registrant’s telephone number, including area code: (858) 455-8600
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $.001 par value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405of the Securities Act.
o Yes ý No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
o Yes ý No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer o | | Accelerated filer ý | | Non-accelerated filer o |
Indicate by check mark where the registrant is a shell company (as defined in Rule 12b-2 of the Act). o Yes ý No
The aggregate market value of the Common Stock of the registrant (the “Common Stock”) held by non-affiliates of the Registrant, based on the last sale price of the Common Stock on June 30, 2005 (the last business day of the registrants most recently completed second fiscal quarter) of $2.86 per share as reported by the Nasdaq National Market, was approximately $75,300,000. Shares of Common Stock held by each officer, director and holder of 10% or more of the outstanding Common Stock, if any, have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for any other purposes. As of March 1, 2006 there were 26,436,931 shares of Common Stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive Proxy Statement to be filed with the Securities and Exchange Commission by May 1, 2006 are incorporated by reference into Part III of this Annual Report on Form 10-K.
DISCOVERY PARTNERS INTERNATIONAL, INC.
FORM 10-K
For the Year Ended December 31, 2005
TABLE OF CONTENTS
2
PART I
Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements that involve a high degree of risk and uncertainty. Such statements include, but are not limited to, statements containing the words “believes,” “anticipates,” “expects,” “estimates” and words of similar import. Our actual results could differ materially from any forward-looking statements, which reflect management’s opinions only as of the date of this report, as a result of risks and uncertainties that exist in our operations, development efforts and business environment. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements You should carefully review “Risk Factors” described elsewhere in this Annual Report on Form 10-K and the risk factors described in other documents that we file from time to time with the Securities and Exchange Commission, or SEC, including our Quarterly Reports on Form 10-Q.
We were originally incorporated in California on March 22, 1995 as IRORI. In October 1998, we changed our name to Discovery Partners International and in July 2000 we reincorporated in the state of Delaware. In October 2005, we sold the assets related to our instrumentation product lines. Our consolidated financial statements and selected financial data contained herein have been recast to reflect the financial position, results of operations and cash flows of the instrumentation product lines as a discontinued operation.
We own the following trademark among others: Xenometrix®. The following trademarks, among others, are currently pending registration: µARCS™ and ChemCard™. All other brand names or trademarks appearing in this report are the property of their respective holders. Use or display by us of other parties’ trademarks, trade dress, or products in this report is not intended to, and does not, imply a relationship with, or endorsements or sponsorship of, us by the trademark or trade dress owners.
Item 1. Business.
Overview
We collaborate with pharmaceutical and biopharmaceutical companies to advance their drug discovery process through our integrated and highly efficient collection of drug discovery technologies, products and services focused from the point immediately following identification of a drug target through when a drug candidate is ready for pre-clinical studies. Despite numerous technological advances in chemistry, high throughput screening, genomics and proteomics, the process of drug discovery remains slow, expensive and often unsuccessful. In order to make the drug discovery process faster, more efficient and more likely to generate a drug candidate, we offer an integrated platform of drug discovery technologies, including assay development, high throughput screening, design and synthesis of proprietary libraries of compounds for screening and primary hit-to-lead expansion, lead compound optimization, drug discovery informatics and in vitro toxicology profiling. These products and services can be provided individually or as an integrated solution, depending on our customers’ requirements. We believe our depth of knowledge and experience, and our range of product offerings, across these areas of drug discovery differentiates us from our competitors. During 2005, we increased the focus on offering integrated drug discovery services as part of long-term collaborations, while we continued to work with companies worldwide in all aspects of drug discovery research. In late 2004, we inaugurated
3
our compound management facility sponsored under our contract with the National Institute of Mental Health of the National Institutes of Health, or NIH, as part of the new NIH chemo-genomic Roadmap Initiative. Our core compound management operation has the ability to select, manage and curate a compound collection of up to one million compounds and has begun to provide samples to the nine national screening centers that have been selected by the NIH to participate in its Roadmap Initiative. In 2005, we generated revenue from 46 customers worldwide, including Pfizer, The National Institute of Mental Health, Actelion, Allergan and Renovis.
However, even with this steady progress, it has become evident during 2005 that the basic business sector in drug discovery contract research and services was undergoing a major and quite unfavorable market shift. Worldwide improvements in communications and shipping, coupled with entrepreneurial efforts in rapidly developing locations such as India, China and Eastern Europe, enabled the highly skilled scientists in those areas to build companies providing a similar range of products and services to us and our peer group, but at significantly lower prices. New guarantees of protection of intellectual property in these locations has offered the necessary assurances to the biotech and pharmaceutical industry that the decision to outsource basic drug discovery offshore has become driven by low price. This shift has essentially resulted in the loss of our ability to consummate synthetic chemistry library contracts, the principal basis of our business in preceding years.
In the fourth quarter of 2005, discussions with Pfizer to renew our contract were ended. With the absence of a new contract with Pfizer, we began the process of reducing our combinatorial chemistry and library synthesis operational capacity through a restructuring of our South San Francisco facility and consolidation of our chemistry platform into our San Diego facility. The NIH Roadmap compound management facility remains fully staffed and operational in our South San Francisco location. In the fourth quarter of 2005, we sold our instrumentation product line, as it was not consistent with our collaborations strategy. We also believe that offshore pricing pressure on biology services, similar to those that already noted in chemistry services, has and will continue to force us to reduce our reliance on fee-for-service work as the primary basis of our business.
We enter 2006 cognizant of these changes in our business under reorganized management and with an imperative from our Board of Directors to make the best use of our current financial and scientific assets to accelerate our entry into more substantial value-creating activities. We have engaged consultants to assist us in evaluating a range of options to best deploy our resources in order to improve stockholder value, including divestiture of assets and merger or acquisition opportunities that are specifically identified to create or enhance a drug development-based product portfolio with defined risk and timelines to clinical milestones with generally acknowledged market value, and which may involve a change in control of our company. As we transition our strategic initiatives and reorganize our operational capacity, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results based on our past operational contract services model are not indicative of future results. We cannot assure that we will be successful in accomplishing any of these strategic initiatives or that any strategic transaction that may occur will be accomplished on favorable terms.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of our shareholders’ equity. Lastly, in the event that we are unsuccessful
4
with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
Industry Background
The Genomics/Proteomics Revolution
The drug discovery process continues to undergo fundamental changes as a result of advances in genomics and proteomics, the studies of genes and the proteins they encode, and how those genes and proteins cause or prevent disease. Prior to these advances, pharmaceutical and biopharmaceutical companies addressed fewer than 500 identified drug targets in the development of drugs. Industry experts now agree that the application of genomics and proteomics has led to the identification of thousands of potential new drug targets, whose roles in disease pathology will not be fully clarified for decades. Drug targets are a subset of the numerous biological molecules, such as enzymes, receptors, other proteins and nucleic acids, which may play a role in the onset, maintenance and progression of a disease. The Pharmaceutical Research and Manufacturers of America, or PhRMA, reported that its members alone spent an estimated $38.8 billion worldwide on research and development in 2004, with approximately 25% of this total amount being spent on the stages of drug discovery in which we focus.
Genomics and proteomics have been the subject of intense scientific and commercial focus. Genomics has led to the identification of large numbers of genes encoding potential drug targets, increasing the demand for drug discovery products and services. Once a company has identified a potential drug target, it must still devote significant time and resources to validating the target’s role in the disease process and screening libraries of compounds against the target to discover potential drug candidates, which must be optimized further before commencement of human testing.
The Drug Discovery Process
Despite numerous advances and technological breakthroughs in genomics, proteomics, high throughput screening and chemistry, the process of discovering drug candidates from drug targets, as illustrated in the following figure and described below, remains slow, expensive and often unsuccessful.
5
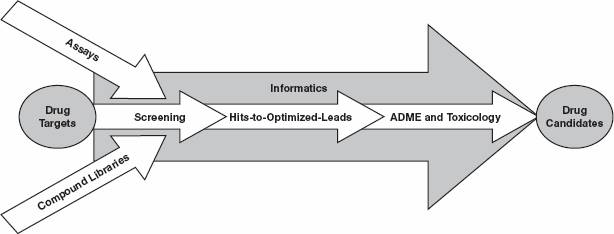
Drug targets. According to the official website of the Human Genome Project a program under the stewardship of the National Institutes of Health, Department of Health and Human Services, of the United States government the genomics revolution has identified between 20,000 and 25,000 human genes that encode the information for cells to produce the proteins that determine human physiology and disease. Drug discovery organizations often advance these new drug targets into discovery with varying degrees of understanding about their role in disease processes and their susceptibility to modulation by chemical compounds. Modulation is defined as the process of selectively increasing or decreasing the biological activity of a particular drug target.
Assays. Once a drug target has been identified and has been validated as having a role in a disease process, a corresponding set of biological assays, or tests, that relate to the activity of the drug target in the disease process must be developed. These assays are designed to show the effect of chemical compounds on the drug target and/or the disease process. Additionally, assays indicate the relative potency and specificity of interaction between the target and the compounds. The more potent and specific the interaction between the target and the compound, the more likely the compound is to become a drug.
Compound libraries. Typically, biologists or biochemists conduct assays in which they screen compound libraries - collections consisting of thousands of compounds each - to find those few compounds that are active in modulating the behavior of the drug targets. Up through the late 1980’s, chemists generated these compounds for testing mostly by synthesizing them one at a time, or painstakingly isolating them from natural sources. During the last decade, the pharmaceutical industry has developed more sophisticated synthetic chemistry approaches, including modular building block techniques, known as combinatorial chemistry, to generate many more diverse compounds far more quickly.
Screening. Screening is the process of testing compounds in assays to determine their potential therapeutic value. A typical screening campaign at a pharmaceutical company will entail screening hundreds of thousands of compounds from multiple compound libraries. Today’s automated high throughput screening, or HTS, systems can test hundreds of thousands of compounds per day and require only very small amounts of each compound and target material. To address the impact of chemicals on complex systems, the drug discovery industry introduced the capability of High Content Screening, or HCS. HCS enables the analysis of multiple independent or interacting targets in intact cells, thereby providing a deeper understanding of drug action and target validity.
Hit-to-lead chemistry. A successful screening process will identify a number of compounds, or hits, that show activity against the drug target. One or more of the hits are then selected for optimization based on their potency and specificity against the drug target. The hits selected for the optimization process are generally referred to as leads.
6
Optimizing a lead compound involves repeatedly producing several slight chemical variants of the lead compound and screening them in assays to discover the relationship between the changes in the molecular structure of compounds and the positive or negative effect on biological activity of the target in the assay. These relationships are called structure-activity relationships, or SARs, and are used to identify the compounds that have the optimal effect on the biological activity of the target in the assay. Traditionally, defining SARs was painstakingly slow. Within the last several years, combinatorial chemistry methods have helped to speed up this process by creating focused libraries that are comprised of dozens to hundreds of compounds, computationally designed to explore the SARs of leads.
ADME and toxicology. Once a lead compound with a well understood SAR is selected for further development, researchers undertake the process of establishing its absorption, distribution, metabolism and excretion, or ADME, and toxicology characteristics. Leads are studied in biochemical assays and pre-clinical animal studies to determine, among other things, whether they are likely to be safe in humans and stay in the body long enough to perform their intended function. Traditionally, these ADME and toxicology studies are performed at the end of the drug discovery process. There is a significant push in the industry, however, to attempt to provide ADME and toxicology information earlier in the process in order to avoid large expenditures on compounds that could ultimately fail due to their poor ADME and toxicology characteristics.
Drug candidates. If the results of the ADME and toxicology studies performed on a lead are favorable in pre-clinical studies, an investigational new drug application, or IND, may be filed with the Food and Drug Administration requesting permission to begin clinical trials of the drug candidate in humans.
Limitations of the Current Industry
To treat diseases and to meet growth expectations, pharmaceutical companies are under intense pressure to introduce new drugs, and they have increased research and development expenses more than 300% from $8 billion in 1990 to $38.8 billion in 2004 according to PhRMA. Despite major scientific and technological advances in areas such as genomics, HTS, HCS and combinatorial chemistry, the drug discovery process remains lengthy, expensive and often unsuccessful. We believe this is due to the following significant limitations to the current process of drug discovery:
Insufficient validation of targets. Drug discovery organizations are advancing many potential new drug targets into discovery without significantly understanding their role in disease processes and their susceptibility to modulation by compounds. Resources spent on pursuing these potential drug targets could be saved if there were better biological or chemical methods to eliminate, early in the process, those drug targets exhibiting undesirable characteristics in these areas.
Inadequate informatics and computational tools. Success of many drug discovery programs is predicated on screening large numbers of compounds, followed by the synthesis and testing of compounds for optimization and for their ADME and toxicology characteristics. This sequential approach is time-consuming and costly. Although many of the recent advances in drug discovery have been targeted at streamlining this process and have allowed large numbers of compounds to be generated and tested in higher throughput, these advances have been in small increments. In addition, the identification of thousands of new drug targets through the application of genomics and proteomics technologies has resulted in large amounts of data being generated. Pharmaceutical companies can save large expenditures of time and money by using informatics and computational tools to manage the data and develop increased and earlier knowledge about which targets are likely to be receptive to chemical modulation, the likely interaction of chemicals and biological targets and which compounds are likely to have unacceptable ADME and toxicological characteristics.
7
Lack of an integrated, noncompeting drug discovery solution. Many of the companies that provide drug discovery services to the pharmaceutical and biopharmaceutical industries provide only selected services. As a result, they are unable to provide the knowledge and efficiencies that can be gained by broad experience in all facets of drug discovery. Further, customers seeking a totally outsourced solution must use valuable resources to manage multiple vendors and integrate inconsistent or incompatible products. Many drug discovery service providers also compete with their potential customers by conducting internal, proprietary drug discovery activities.
Limited predictive value of model systems. Drug candidates are normally tested in animal models or in selected in vitro and ex vivo models to evaluate their efficacy. Many of these models only partially reflect the drug candidates’ effects in humans. Proof of efficacy can often only be obtained in clinical studies. Methods and systems which allow a compound to continue through the pre-clinical phase in a cost effective manner and add to the understanding of the mechanisms of action of drug candidates in complex systems might significantly improve the discovery success rate.
Our Solution
We collaborate with pharmaceutical and biopharmaceutical companies to advance their drug discovery process through our integrated and highly efficient collection of drug discovery technologies focused from the point immediately following identification of a drug target through when a drug candidate is ready for pre-clinical studies. Our customers include many major pharmaceutical companies and numerous biopharmaceutical companies. We do not discover or develop drugs for our own account and we do not compete with our customers. We believe the broad range of products and services we offer or intend to offer, either as part of long-term collaborations, or as fee-for-service contracts, will provide the following benefits:
Target validation. We have developed, through outright purchase, license and proprietary methodologies, a large number of libraries of highly diverse synthetic and natural product compounds that are expected, and in some cases, specifically designed, to modulate many drug targets. We believe the use of these compound libraries, which are not sold on a stand-alone basis but rather offered as part of an integrated drug discovery solution, may provide early information about whether a drug target is susceptible to chemical modulation and, if so, whether modulation of its activity has an important effect on the disease process or outcome. If these libraries are successful in providing this information early in the drug discovery process, our partners can save significant amounts of time and resources by abandoning the pursuit of targets that do not exhibit favorable chemical and biological characteristics.
High quality synthetic compound libraries. Our synthetic chemistries are easily replicated and our compounds rapidly replenishable because we produce detailed synthesis protocols for each chemical compound library. We are able to rapidly create focused libraries containing slight variations of hits from our original discovery or targeted libraries to study SARs. Working with our customers, we design libraries for maximum diversity using commercial and proprietary computer algorithms. Finally, after synthesis of a compound, we use multiple analytical methods to ensure a high degree of compound purity. As a consequence, our libraries contain highly diverse, drug-like compounds of high purity.
Purified natural product libraries. Our acquisition of the assets of Biofrontera Discovery GmbH, in Heidelberg, Germany, was completed in April 2005. Among the assets acquired by us at that time are rights to a large and diverse collection of bacterial and fungal microbial strains, and a library of natural products, which are chemical compounds produced by those microbes under specifically defined laboratory conditions. Our natural product libraries are differentiated from other natural product sources by their unique microbial origins, and by the methodology that is used to purify and characterize the resulting compounds. We do not screen fermentation broths or crude extracts. Rather, the libraries we utilize in screening are both fully purified
8
(approximately 100,000 samples each containing a single natural product compound), and pre-purified (approximately 145,000 samples each containing between 8 and12 natural product compounds), using a proprietary serial high performance liquid chromatography, or HPLC, process. These natural product samples can be applied to any HTS project in the same way that synthetic chemical libraries are used. Following the identification of hits, libraries are then generated by scale-up fermentation and purification, followed by semi-synthetic transformations to produce focused analog libraries for SAR determination and subsequent preclinical evaluation.
Broad range of products and services for assay development, chemistry and screening. We currently offer a broad range of drug discovery products and services to pharmaceutical and biopharmaceutical companies targeted at assay development, chemistry and screening, either as part of multi-target collaborations, or under more limited fee-for-service contracts. We have performed more than 190 discovery projects for our customers and provide access to more than 650,000 discrete synthetic chemical compounds. Our approach for efficiently finding screening hits or drug leads combines proprietary computational methods for compound selection and data mining with our high throughput screening platform. In addition, our team of chemists and biologists has worked on several hit and lead optimization projects for our customers. When applicable, we employ our µARCS technology to improve the ease of access to screening and cost effectiveness of the screening process. In addition, we possess the capability to quantitatively study the effect of drugs on a sub-cellular level.
Development of an informatics and computational tools knowledge base. We apply sophisticated computational software tools to generate predictive information in the early stages of drug discovery. We use our tools to correlate information available on families of drug targets and compounds with screening data to predict which drug targets are likely to be receptive to chemical modulation, and which chemical structures are likely to react favorably with large families of drug targets or produce unacceptable ADME or toxicological results. We have also developed computer algorithms that reduce the number of compounds that must be screened to identify hits. We believe our computational tools complement the drug discovery process and reduce the time and resources involved.
Integrated drug discovery products and services. As part of long-term collaborations, we offer an integrated drug discovery platform that provides unique value to our customers. We believe our focus on our customers’ needs, rather than our own drug development efforts, makes our product and service offerings more attractive. Additionally, we believe we have the ability to collaborate with multiple customers effectively and to efficiently maintain confidential proprietary information while successfully providing products and services to our customers.
Our Strategy
Our strategy has been to be a leading fee-for-service provider of a complete, integrated and highly efficient drug discovery technology platform designed to overcome many of the limitations associated with the slow and expensive traditional drug discovery process. However, it has become evident during 2005 that the market for drug discovery contract research and services has undergone a major and negative shift. Worldwide improvements in communications and shipping, coupled with entrepreneurial efforts in rapidly developing locations such as India, China and Eastern Europe, have enabled the highly skilled scientists in those areas to build companies providing a similar range of products and services to us and our peer group, but at significantly lower prices. New guarantees of protection of intellectual property in these locations has offered the necessary assurances to the biotech and pharmaceutical industry that the decision to outsource basic drug discovery offshore has become driven by low price. This shift has resulted in the loss of our ability to consummate synthetic chemistry library contracts, the principal basis of our business in preceding years.
In the fourth quarter of 2005, discussions with Pfizer to renew our contract were ended. With the absence of a new contract with Pfizer, we have reduced our combinatorial chemistry and
9
library synthesis operational capacity through a restructuring of our South San Francisco facility, consolidating our chemistry platform into our San Diego facility. The NIH Roadmap compound management facility remains fully staffed and operational in our South San Francisco location. We sold our instrumentation product line, as it was not consistent with our collaborations strategy. We also believe that offshore pricing pressure on biology services, similar to that already noted in chemistry services, has and will continue to force us to reduce our reliance on fee-for-service work as the primary basis of our business.
We have determined though both our own marketing efforts and the investigations of independent consultants we retained that our past strategy of providing contract research is no longer viable in the context of a public company due to the severe pricing pressures that have developed in the last year from low-cost offshore competitors. Remaining in this sector as our sole business strategy would require significant expenditures of capital over many years as a co-investment with customers. We would incur significant losses and introduce significant risk for our business as a whole.
We enter 2006 cognizant of these changes in our business under reorganized management and with an imperative from our Board of Directors to make best use of our current financial and scientific assets to accelerate our entry into more substantial value-creating activities. We are currently exploring a range of options to best deploy our resources in order to improve stockholder value, including divestiture of assets and merger or acquisition opportunities that are specifically identified to create or enhance a drug development-based product portfolio with defined risk and timelines to clinical milestones with generally acknowledged market value, and which may involve a change in control of our company. As we transition our strategic initiatives and reorganize our operational capacity, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results based on our past operational contract services model are not indicative of future results. We cannot assure that we will be successful in accomplishing any of these strategic initiatives or that any strategic transaction that may occur will be accomplished on favorable terms.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
Our Technology Platform
Our technology platform has been designed to make the drug discovery process faster, more efficient and more likely to generate a high quality drug candidate. We currently have capabilities in many functional disciplines of the drug discovery process that can be purchased individually or as integrated solutions, depending on our customers’ requirements. We have continued to add to
10
our functional offerings in order to provide a comprehensive and integrated suite of drug discovery products and services to our pharmaceutical and biopharmaceutical customers.
Assays
We provide assay development services through our team of scientists who are experienced in working with major disease target classes such as protein kinases, G-protein-coupled receptors, nuclear receptors, phosphatases, and proteases. Biological systems about which we have expertise include enzymes, receptor-ligand interaction, protein-protein interaction, ion channel assays, reporter-gene assays in prokaryotic and eukaryotic cells, cellular proliferation, differentiation and physiologic response, and microbial growth. Most recently we established HCS technology in-house. This allows us to profile compounds for their effect on multiple intracellular events in one assay. We have the ability to provide assay development services through our subsidiary, Discovery Partners International AG.
We have established, through both internal development and sublicense, a high-throughput panel of in vitro assays that provide data on multiple parameters relevant to the pharmaceutical, pharmacokinetic and metabolic properties of individual compounds or whole libraries. This panel is comprised of approximately 20 assays, including those for solubility, permeability, metabolic liability, and potential for interaction with key enzymes involved in metabolism of drugs, and a critical receptor indicative of potential cardiac side effects. The resulting PK-ADMET profile, reflecting pharmacokinetic-absorption-distribution-metabolism-excretion-toxicity data, profile is a valuable adjunct data set to the potency and selectivity SAR data used to select compounds for further evaluation in subsequent more expensive and time-consuming activities.
We offer unique cell-based assays with multiple gene response indicators, which give specific information on the potential beneficial and harmful biological activities of a given pharmaceutical compound. Genetically engineered living cells allow us to determine the on and off state of gene promoters in the presence of compounds. Our portfolio of reporter cell lines may provide important efficacy and safety information to help optimize the selection of drug candidates before moving to the more costly stages of pre-clinical and clinical testing.
Proprietary Libraries of Compounds
We offer the following broad range of highly purified compound libraries for assay screening and rapid hit-to-lead activities:
Discovery libraries. We generate and sell discovery libraries, which are collections of diverse, drug-like compounds that are designed using computer programs to systematically explore specified areas of chemical space or types of chemistry. They are used in the initial stages of screening in which very little information is known about which compounds will alter the activity of the drug target in the assay.
Targeted libraries. We design and sell targeted libraries selected for a specified type of drug target. These libraries are a group of highly related compounds used much like discovery libraries, but they provide a more insightful medicinal chemistry starting point.
Focused libraries. We are able to rapidly generate focused libraries based on hits from our discovery or targeted libraries because we have previously invested significant resources to produce detailed synthesis protocols in the development of each library of compounds. Focused libraries explore subtle changes in the compound structure to quickly elicit SARs and evolve lead compounds. In addition, we develop focused libraries from hits generated by our customers.
11
Chemistry protocols. In conjunction with the provision of proprietary compounds, we generally provide detailed protocols for generating our libraries to customers that purchase those libraries. This enables our customers to replenish compounds and to create additional compounds. We use a proprietary combinatorial chemistry technology platform to generate compound libraries that employs parallel synthesis and our directed sorting technology. Our approach provides the following advantages:
• Purity: Maximum purity is important to minimize false positives during screening. We can deliver compounds that are greater than the current industry standard of 90% pure depending on customer specifications. Our quality control measures include high performance liquid chromatography, mass spectroscopy, nuclear magnetic resonance, evaporative light scattering detection and weight percent analysis. We achieve the required purity using several purification technologies including our proprietary ARW high throughput purification process;
• Diversity: Each discovery library of approximately 1,000 to 5,000 drug-like compounds is designed to contain a set of highly diverse compounds using our chemical mapping and differentiation software;
• Ease of optimization: The individual chemistries for each library are highly validated and characterized. This allows rapid generation of focused libraries around hits and rapid follow-up and modification by medicinal chemistry programs; and
• Re-supply and reproducibility: Our synthesis approaches produce large quantities that allow rapid and cost effective restocking of customers’ supplies. Our highly validated chemistries allow us or our customers to re-synthesize larger quantities on demand.
Screening
We offer high throughput screening services through an experienced staff of scientists located at our facility near Basel, Switzerland. We also offer our customers access to compounds from many of the world’s leading compound suppliers as well as a significant collection of internally developed compounds. This allows our partners access to a large and diverse collection of compounds without the need to store and manage the compound collections in their own facilities.
Our HTS modules are equipped to quickly and efficiently process the particular assay being carried out. A module consists of the appropriate plate and liquid handling equipment, coupled with the best read out technology for the assay being run. We deliver a list of validated hits to our screening customers. We also provide hit follow-up and verification services and, when desired, actual physical samples of the hit compounds.
Hit-to-Lead Chemistry
We have developed products and services to advance early stage screening hits to optimized drug leads. These products and services include the following:
Custom focused libraries. In addition to our collection of proprietary libraries, we design and produce custom, focused libraries based upon hits identified from screening. These hits may be from our compound libraries, the customer’s internal compound collection or even from another compound library supplier. Focused libraries consist of compounds that represent systematic variations of hits. Medicinal chemists use these focused libraries to begin refining hits to optimize the properties that have an effect on the drug target in the assay. Because we invest significant resources in the development of each of our compound libraries, we are able to
12
generate focused libraries based on hits from our discovery libraries or targeted libraries more rapidly than when we begin from an isolated hit resulting from a customer’s compound collection.
Medicinal chemistry. We provide a wide range of medicinal chemistry and other lead optimization services. This includes the synthesis of compounds that modify the original hit or lead for improved potency, selectivity and other pharmaceutical characteristics. We have an experienced group of synthetic organic chemists and medicinal chemists with expertise in both solid phase chemistry and solution phase chemistry. In some cases we provide medicinal chemistry services in conjunction with our computational drug discovery efforts to design and construct small libraries of compounds to act on specific targets of known structure.
Biological profiling in the hit-to-lead phase. We also provide a broad range of biological profiling including the primary screening test, specificity assays, cellular assays, ADME and in vitro toxicology tests. Our multi-parameter analysis tools allow efficient data analysis and selection of compounds that fit the product profile.
Drug Discovery Informatics; ADME and Toxicology
We employ computational tools that we believe will allow us to continue to increase our knowledge of the characteristics of targets, leads, and ligand-target interactions and which we believe can be applied throughout the drug discovery process to significantly reduce the time and cost of developing a drug. We currently have computer algorithms that allow us to design libraries of compounds with high diversity, thereby increasing the likelihood of finding hits during screening. When screened against large numbers of potential drug targets, we believe these large and highly diverse libraries will provide significant information about which drug targets are amenable to modulation by chemical means. We have developed novel algorithms to aid in the understanding and utilization of the data resulting from high throughput screening experiments. We have also developed a proprietary analysis tool, which we believe will allow us to use screening data to correlate drug target families with the types of compounds that will likely bind to them. Using this tool, we will seek to design highly effective targeted libraries for whole drug target families. In addition, we will seek to use this tool to efficiently design potent compounds for a particular drug target and to efficiently search databases of compounds available from other vendors for likely leads.
We expect to further use our computational tools and screening data to help predict ADME and toxicological reactions to classes of compounds. This will allow our customers to avoid spending money and time on hits and leads that will ultimately fail due to their ADME and toxicological characteristics.
Integrated Drug Discovery Programs
When some or all of the above discreet technology capabilities need to be accessed by a single partner on a single or multiple biological targets, we offer an integrated collaborative drug discovery program that provides access to the full range of our capabilities needed to find and advance leads to pre-clinical candidates. In these collaborations we seek to provide and manage integrated access to our computational design and analysis, chemistry, and biology capabilities for the purpose of developing a pre-clinical lead for the partner’s target of choice. As a result, these collaborations will provide our partners with the knowledge and efficiencies that we have gained from our broad experience in a number of areas of drug discovery. In addition, partners seeking a totally outsourced solution are not required to use valuable resources to manage multiple collaborators and integrate inconsistent or incompatible products. Each integrated drug discovery program is customized to increase the likelihood of success. Milestone payments, which are due upon lead compounds demonstrating specified potency and selectivity requirements, may be included in addition to service fees. In some future collaborative efforts, these contracts may include an element of risk-sharing with us, such that some of our fixed costs will only be offset by
13
milestone payments and, in some cases, royalties on future product sales resulting from the collaboration. In 2005, milestone payments represented an immaterial portion of our total revenue and are not anticipated to be material to total revenue in the foreseeable future.
Component Supply
The raw materials used in the research and development of our products and the offering of our services are available from more than one supplier.
Customers
During 2005, we generated revenue from 46 customers worldwide. The most significant by dollar volume and which we have previously disclosed are as follows:
Actelion Ltd. | | Merck & Co., Inc. |
Allergan Inc. | | National Institute of Mental Health |
Chroma Therapeutics, Ltd. | | Ono Pharmaceutical Co., Ltd. |
Renovis | | Pfizer Inc |
In 2005, 2004 and 2003, 54%, 62% and 69%, respectively, of our revenue from continuing operations came from our chemistry contracts with Pfizer Inc. Our contract with Pfizer ended by its terms on January 6, 2006, and, in the fourth quarter of 2005, discussions with Pfizer to renew our contract ended. The NIH represented 14% of our revenue in 2005 and there were no other customers that represented over 10% of our revenue in 2004 or 2003.
Sales and Marketing
We have a skilled team of business development professionals targeting pharmaceutical and biopharmaceutical customers worldwide. Additionally, our senior executives coordinate global management of our key customers and manage our general sales and marketing efforts for our drug discovery offerings to major pharmaceutical customers and prospective partners worldwide. In 2005, to supplement the efforts of our business development and marketing groups, our Chief Scientific Officer (currently acting Chief Executive Officer) initiated a new round of outreach visits to approximately 40 major pharmaceutical and biotech companies to gauge both the basic interest in, and the market potential for, our expanded integrated offerings. In addition to these direct selling efforts we also use industry trade shows and industry journal advertising for sales and marketing.
Research and Development
Our research and development expenses totaled approximately $3.9 million in 2005, $1.5 million in 2004, and $407,000 in 2003. None of these expenses were funded by outside parties. Research and development expenses increased in 2005 primarily due to the acquisition of the natural compound based discovery business from Biofrontera Discovery GmbH in April 2005, which represented $2.1 million of expenses, and from the redeployment of development scientists and engineers from direct revenue generating activities of customer funded R&D programs and collaborations to internal programs focused on in silico tools, screening assays and drug discovery process development. During 2005, we conducted internal research and development programs to enhance our technology platforms in drug discovery informatics, assay development and high-throughput screening, and natural products.
14
Changes in Comparability
In October 2005, we sold the assets related to the IRORI chemical synthesis, Crystal Farm automated protein crystallization, and Universal Store™ compound storage systems product lines to preserve our cash resources in eliminating the investment expenses required for the engineering innovation required to develop new product lines. The income and cash flows related to the instrumentation product lines and the direct transaction costs of the sale of those product lines have been presented as discontinued operations in the Condensed Consolidated Statements of Operations and Consolidated Statements of Cash Flows in the Consolidated Financial Statements to this Annual Report on Form 10-K. The assets and liabilities related to the sale of those product lines have been reclassified within the 2004 Balance Sheet in the Consolidated Financial Statements to this Annual Report on Form 10-K. The presentation of geographical data and selected financial data for 2004 and all earlier years has been adjusted to conform to the presentation in 2005.
There were no individual foreign countries where the revenue represented 10% or more of total revenues, from continuing operations, for the periods presented below. The following table presents the geographic breakdown of our revenue, from continuing operations, for our last three fiscal years.
| | Years Ended December
31, | |
| | 2005 | | 2004 | | 2003 | |
United States | | 84 | % | 82 | % | 80 | % |
Foreign Countries | | 16 | % | 18 | % | 20 | % |
The following table presents the geographic breakdown of our long-lived assets, from continuing operations, for our last two fiscal years.
| | As of December 31, | |
| | 2005 | | 2004 | |
United States | | 63 | % | 79 | % |
Foreign Countries | | 37 | % | 21 | % |
Our total backlog as of February 15, 2006 was approximately $26 million, which compares to approximately $48 million on February 27, 2005. We expect to realize approximately 62% of our total current backlog by December 31, 2006.
Backlog measures are not defined by generally accepted accounting principles and our measurement of backlog may vary from that used by others. While we believe that long-term backlog trends serve as a useful metric for assessing the growth prospects for our business, backlog is not a guarantee of future revenues and provides no information about the timing on which future revenue may be recorded.
Agreement with Pfizer
In February 2004, we entered into a broadened collaboration agreement with Pfizer that replaced our prior collaboration with Pfizer that we entered into in December 2001. Under this agreement, we collaborated with Pfizer to design and develop compounds that are owned by and exclusive to Pfizer. We manufactured and purified the compounds to high purity standards using, among other methods, our proprietary ARW purification technology. The agreement expired by its natural terms on January 6, 2006. In the fourth quarter of 2005, discussions with Pfizer to renew our contract were ended.
15
Agreement with The National Institutes of Health
Effective August 20, 2004, we entered into a multi-year contract with The NIH to establish and maintain a Small Molecule Repository to manage and provide up to one million chemical compounds to multiple NIH Screening Centers as part of the NIH Roadmap Initiative. The estimated funding available to us under this contract for the base period (August 2004 through December 2008) is approximately $24 million, assuming the contract continues for its full term. The agreement expires by its natural terms on November 30, 2008, but may be renewed on an annual basis by the NIH up to November 30, 2013. It is uncertain at this time whether the NIH will renew this agreement or whether we will be successful in entering into new agreements with this customer. This contract is funded in its entirety by the NIH and Department of Health and Human Services. Payment to us for performance under this contract is subject to audit by the Defense Contract Audit Agency and is subject to government funding. Costs incurred that are billable to the NIH under this contract include indirect costs that are based on provisional rates estimated by management at the time we submitted our proposal. Actual indirect costs may be greater or lower than our provisional rates. Negotiation for recovery of higher costs with the government may not be successful and any costs billed to the government in excess of actual costs may be required to be reimbursed to the NIH.
Intellectual Property
We pursue patents, copyrights and trademarks and otherwise endeavor to protect our technology, inventions and improvements that are commercially important to the development of our business. We also rely upon trade secrets and proprietary know-how that may be important to the development of our business.
Our success will depend in large part on our ability to:
• obtain and maintain patent and other proprietary protection for the technology, inventions and improvements we consider important to our business;
• defend our patents;
• preserve the confidentiality of our trade secrets; and
• operate without infringing the patents and proprietary rights of third parties.
We have implemented a patent strategy designed to maximize our intellectual property rights. We are pursuing patent coverage in the United States and those foreign countries that are home to the majority of our anticipated customer base. We currently own 4 issued patents in the United States.
Our United States patents generally have a term of 20 years from the application filing date or earlier claimed priority. Our foreign patents generally have a term of 20 years from the date of filing of the patent application. Because the time from filing to issuance of patent applications is often several years, this process may result in a shortened period of patent protection, which may adversely affect our ability to exclude competitors from our markets. Our issued United States patents have expiration dates ranging from 2014 through 2017. Our success will depend to a significant degree upon our ability to develop proprietary products and technologies and to obtain patents having claims that cover such products and technologies. We intend to continue to file patent applications covering any newly developed products and technologies.
Patents provide some degree of protection for our proprietary technology. However, the pursuit and assertion of patent rights, particularly in areas like pharmaceuticals and
16
biopharmaceuticals, involve complex determinations and, therefore, are characterized by some uncertainty. In addition, the laws governing patentability and the scope of patent coverage continue to evolve, particularly in the area of biopharmaceuticals, and due to the time between the filing and granting of a patent application, we may be infringing upon the patent rights of a third party without any knowledge of the patent. As a result, patents might not issue from any of our patent applications or from applications licensed to us. The scope of any of our issued patents may not be sufficiently broad to offer meaningful protection. In addition, our issued patents or patents licensed to us may be successfully challenged, invalidated, circumvented or rendered unenforceable so that our patent rights might not create an effective competitive barrier. Moreover, the laws of some foreign countries may not protect our proprietary rights to the same extent as do the laws of the United States. Any patents issued to us or our collaborators may not provide a legal basis for establishing an exclusive market for our products or services or provide us with any competitive advantages. In addition, patents issued to us or our collaborators may not ensure that the patents of others will not have an adverse effect on our ability to do business or to continue to use our technologies freely. In view of these factors, our intellectual property positions bear some degree of uncertainty.
We also rely in part on trade secret protection for certain of our technologies and proprietary know-how. The source code for our proprietary software is protected both as a trade secret and as copyrighted works. We attempt to protect our trade secrets by entering into confidentiality agreements with third parties, employees and consultants. Our employees also sign agreements requiring that they assign to us their interests in inventions and original expressions and any corresponding patents and copyrights arising from their work for us. However, it is possible that these agreements may be breached, invalidated or rendered unenforceable, and if so, there may not be an adequate corrective remedy available. Despite the measures we have taken to protect our intellectual property, parties to our agreements may breach the confidentiality provisions in our contracts or infringe or misappropriate our patents, copyrights, trademarks, trade secrets and other proprietary rights. In addition, third parties may independently discover or invent competing technologies or reverse engineer our trade secrets or other technology.
Third parties may file claims asserting that our technologies or products infringe upon their intellectual property. We cannot predict whether third parties will assert such claims against us or our licensees or against the licensors of technology licensed to us, or whether those claims will harm our business. If we are forced to defend against such claims, whether they are with or without merit, and whether they are resolved in our favor or against us, our licensees or our licensors, we will incur significant expenses and experience diversion of management’s attention and resources. As a result of any disputes over intellectual property, we may have to develop at a substantial cost non-infringing technology or enter into costly licensing agreements. These agreements, if necessary, may be unavailable on terms acceptable to us, or at all, which could seriously harm our business or financial condition.
Competition
We compete with companies in the United States and abroad that engage in the provision of drug discovery technology and services to the pharmaceutical and biotechnology industry. These competitors include companies engaged in the following areas of drug discovery:
• Assay development and screening;
• Synthetic compound libraries and lead optimization;
• Natural products libraries and chemistry;
• Informatics; and
• Gene expression profiling.
17
We face competition based on a number of factors, including size, relative expertise and sophistication, speed and costs of identifying and optimizing potential lead compounds and of developing and optimizing chemical processes. We compete with the research departments of pharmaceutical companies, biopharmaceutical companies, combinatorial chemistry companies, contract research companies, contract drug manufacturing companies and research and academic institutions. Many of these competitors have greater financial and other resources and more experience in research and development than we do. Smaller companies may also prove to be significant competitors, particularly through arrangements with large corporate collaborators.
Historically, pharmaceutical companies have maintained close control over their research and development activities, including the synthesis, screening and optimization of chemical compounds and the development of chemical processes. Many of these companies, which represent a significant potential market for our products and services, are developing or already possess in-house technologies and services offered by us. Academic institutions, governmental agencies and other research organizations are also conducting research in areas in which we provide services either on their own or through collaborative efforts.
We have faced and anticipate that we will continue to face increased competition as new companies enter the market and advanced technologies become available. Our services and expertise may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors. The existing approaches of our competitors or new approaches or technologies developed by our competitors may be more effective than those developed by us. We cannot assure that our competitors will not develop more effective or more affordable technologies or services, thus rendering our technologies and/or services obsolete, uncompetitive or uneconomical. For example, advances in informatics and virtual screening may render some of our technologies, such as our large compound libraries, obsolete.
In addition, due to improvements in global communications, combined with the supply of lower cost PhD level scientific talent, we have recognized the significant effects of the real and direct price-based competition for our chemistry, computational chemistry, and high-throughput screening services from low-cost offshore locations such as China, India and Eastern Europe. New guarantees of protection of intellectual property in these locations has offered the necessary assurances to the biotech and pharmaceutical industry so that the decision to outsource basic drug discovery offshore has become driven by low price. This shift has resulted in the loss of our ability to consummate synthetic chemistry library contracts, the principal basis of our business in preceding years. We also believe that offshore pricing pressure on biology services, similar to that already noted in chemistry services, have and will continue to force us to reduce our reliance on fee-for-service work as the primary basis of our business.
Government Regulation
We are subject to various federal, state and local laws and regulations relating to the protection of the environment. In the course of our business, we handle, store and dispose of chemicals. The laws and regulations applicable to our operations include provisions that regulate the discharge of materials into the environment. Usually these environmental laws and regulations impose strict liability, rendering a person liable without regard to negligence or fault on the part of such person. Such environmental laws and regulations may expose us to liability for the conduct of, or conditions caused by, others. We have not been required to expend material amounts in connection with our efforts to comply with environmental requirements, and we do not believe compliance with such requirements will have a material adverse effect upon our capital expenditures, results of operations or competitive position. Because the requirements imposed by these laws and regulations frequently change, we are unable to predict the cost of compliance
18
with these requirements in the future, or the effect of these laws on our capital expenditures, results of operations or competitive position.
Employees
As of February 28, 2006, we had 133 full-time employees worldwide. None of our employees are covered by a collective bargaining agreement. We believe our relationship with our employees is generally satisfactory.
Web Site Access to SEC Filings
We maintain an Internet website at www.discoverypartners.com. We make available free of charge on our Internet website our Annual Report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC.
Item 1A. Risk Factors.
In addition to the other information contained herein, you should carefully consider the following risk factors in evaluating our company.
We may engage in strategic transactions, which could adversely affect our business.
We are currently exploring a range of options to best deploy our resources and improve stockholder value in light of the changes in our business and an imperative from our Board of Directors to make best use of our current financial and scientific assets to accelerate our entry into more substantial value-creating activities. The options we are considering include divestiture of assets and merger or acquisition opportunities that are specifically identified to create or enhance a product portfolio with defined risk and timelines to milestones, which may involve a change in control of our company. As we transition our strategic initiatives and reorganize our operational capacity, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results are not indicative of future results.
We cannot assure that we will be successful in accomplishing any of these strategic initiatives or that any strategic transaction that may occur will be accomplished on favorable terms. Our consideration and completion of any strategic transaction is subject to a variety of risks that could materially and adversely affect our business and financial results, including risks that we will forego business opportunities while any transaction is being considered or is pending; that our business, including our ability to retain key employees, may suffer due to uncertainty; risks inherent in negotiating and completing any transaction; and challenges in integrating businesses and technologies in the event any transaction is completed.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly
19
less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
Our strategy of placing a high degree of emphasis on integrated drug discovery collaborations is untested, involves higher risk and complexity, requires significant upfront funding, and will likely result in continued operating losses for the foreseeable future.
We have a limited history of offering our integrated drug discovery platform in the form of a collaborative model to the pharmaceutical and biopharmaceutical industries. Our marketing efforts in this area during 2005 included discussions with over 40 current and prospective customers. Based on these discussions and other market-based analyses of competitors’ cost and pricing structures, it is uncertain whether our current service-based customers will migrate to this new business offering or whether new collaborators will enter into collaborations with us. In order to be successful, our drug discovery technology platform must meet the requirements of the pharmaceutical and biopharmaceutical industries, and we must convince potential customers to collaborate with us instead of either performing these services internally or utilizing other companies with competing drug discovery technology platforms. Because of these and other factors, some of which are beyond our control, our integrated drug discovery collaboration offering may not gain sufficient market acceptance.
In addition, our strategy of focusing on more significant value added integrated drug discovery collaborations with biotechnology companies relies upon a relatively complex form of customer engagement to generate revenue. As a result of the inherent complexity of such collaborations, we have an increased risk of being unable to reach agreement or delays in reaching agreement with the prospective customer for such collaborations or of structuring sub-optimal arrangements that fail to adequately compensate us for the risks inherent in such collaborations. If we are unable to enter into these collaborations or experience delays in entering into these collaborations, we will not be able to generate revenues when expected or at all, which would adversely affect our business. In 2005, we focused on offering long-term collaborations and experienced longer lead times for entering into these collaborations and a lower percentage of completed agreements than previously experienced in fee-for-service arrangements.
As it is unlikely that we will be able to enter into integrated drug discovery collaborations using our previous fee-for-service pricing structure, we will need to invest, at our own cost, in the feasibility phase of various projects in exchange for higher downstream rewards in the form of access fees and milestone payments that could be received during or at the conclusion of such projects. As such, our risk profile is increased as we will likely generate losses into the foreseeable future and our future success will depend on our ability to enter into collaborations where the cumulative payments received from our partners exceed our initial investment. Later-stage milestone payments and success fees dependent on confirmation of biological or pharmacological activity by compounds generated as part of such collaborations are particularly at risk for being achieved, as compounds may either lack the desired effects in preclinical studies or in clinical trials, or possess additional activities manifested as side effects that limit or preclude the advancement of such compounds in preclinical and clinical development. Additionally, even if such compounds advance through clinical development, there is no guarantee that compounds eligible for royalty payments will either be approved by the Food and Drug Administration, or similar agencies worldwide, for marketing.
20
We have incurred significant operating and net losses since our inception. As of December 31, 2005, we had an accumulated deficit of $113.9 million. Although we generated net income during 2004 and 2003 of $3.9 million and $1.1 million, respectively, we had net losses of $14.2 million and $62.1 million for the years ended December 31, 2005 and 2002, respectively. We also expect in the future to incur operating and net losses and negative cash flow from operations. We did not achieve operating profitability until the third quarter of 2003 and we may not be able to achieve or maintain profitability in any quarter in the future. We experienced an operating loss in 2005 and currently expect an operating loss in 2006. Based on the major changes in our business sector described elsewhere, we do not expect to achieve profitability in the future based on our need for continued investment in the collaborative value-based activities.
Our collaborations and services involve significant scientific risk of fulfillment.
Our ability to achieve future success-based revenues from integrated drug discovery collaborations will rely upon our scientific success. Our drug discovery collaborations may fail to meet our or our collaborators’ drug discovery objectives on a timely basis or at all. In this event, we would not achieve the success-based milestone payments necessary to recover our upfront investment in a given project.
In addition, a large portion of our revenues relies upon our customers’ scientific success. Our services and technologies may fail to assist our customers in achieving their drug discovery objectives, on a timely basis or at all. For example, when our customers deliver proteins to us for assay development or chemistry library design ideas for chemical compound development and production, we rely on our customers for timely delivery of those deliverables, and our customers rely on us for timely and effective assay design or compound library development and production that fulfills our scientific obligations to them. To the extent that either we experience delays or failures in receiving specific deliverables required for us to complete our objectives or we encounter delays in our ability to meet, or are unable to meet, our scientific obligations, we may be unable to receive and recognize revenues in accordance with our expectations.
We derive a significant percentage of our revenues from a single customer. If this customer relationship terminated, we would incur a larger net loss from operations.
A significant portion of our actual 2005 and anticipated annual 2006 revenues were and will be, as the case may be, derived from the compound procurement and management contract that we entered into with the NIH in September, 2004. The agreement expires by its natural terms on November 30, 2008, but may be renewed on an annual basis by the NIH up to November 30, 2013; however the agreement is subject to continued government funding. During the year ended December 31, 2005, revenue from the NIH represented 14% of our total revenue. Revenues under the NIH contract are earned as costs are incurred to procure, inspect and ship compounds to NIH designated screening centers. In addition, revenues are earned as compounds are purchased on behalf of the NIH at such time that the compounds pass certain quality standards as specified by the NIH and payment is made to the compound vendors. Timing of revenues earned is partially dependent on the timing of the NIH selection of compounds, the timing of procurement and processing of acquired compounds and the volume of screening activity at the NIH designated screening centers. In the event the NIH is delayed in the selection process of acquiring compounds, as it was in 2005, or such acquired compounds fail to meet the NIH specified standards, or if there are delays in the ramp up in the demand of the NIH designated screening centers, revenues recognized under this contract may be deferred to future periods. It is uncertain at this time whether the NIH will renew this agreement or whether we will be successful in entering into new agreements with this customer. Our agreement with the NIH is also subject to the risk that the U.S. government will not continue to provide the funding to support the activities pursuant to this agreement.
21
A significant portion of our actual revenues over the past three years resulted from the agreement we entered into with Pfizer in February 2004. In 2005, 2004 and 2003, 54%, 62% and 69%, respectively, of our revenue from continuing operations came from our chemistry contracts with Pfizer. Our contract with Pfizer ended by its terms on January 6, 2006, and, in the fourth quarter of 2005, discussions with Pfizer to renew our contract were ended. The loss of this customer relationship will result in a larger net loss from operations for 2006 than in previous years.
If our revenues decline, we will not be able to correspondingly reduce our operating expenses.
A large portion of our expenses, including expenses for facilities, equipment and personnel, is relatively fixed. A significant percentage of our fixed costs is directly related to the cost of operating as a public company in maintaining compliance with the regulatory requirements. Accordingly, if revenues continue to decline, we expect we will not be able to correspondingly reduce our operating expenses. Failure to achieve anticipated levels of revenues could therefore significantly harm our operating results for a particular fiscal period.
The drug discovery industry is highly competitive and subject to technological changes, and we may not have the resources necessary to compete successfully.
We compete with companies in the United States and abroad that engage in the provision of drug discovery technology to the pharmaceutical and biotechnology industry. These competitors include companies engaged in the following areas of drug discovery:
• Assay development and screening;
• Synthetic compound libraries and lead optimization;
• Natural products libraries and chemistry;
• Informatics; and
• Gene expression profiling.
Academic institutions, governmental agencies and other research organizations also conduct research in areas in which we provide services, either on their own or through collaborative efforts. Also, substantially all of our pharmaceutical and biopharmaceutical company customers have internal departments that provide some or all of the products and services we sell, so these customers may have limited needs for our products and services. Many of our competitors have more experience and have access to greater financial, technical, research, marketing, sales, distribution, service and other resources than we do. We may not yet be large enough to achieve satisfactory market recognition or operating efficiencies, particularly in comparison to some competitors.
Moreover, the pharmaceutical and biopharmaceutical industries are characterized by continuous technological innovation. We have faced and will continue to face increased competition as new companies enter the market and our competitors make advanced technologies available. Technological advances or entirely different approaches that we or one or more of our competitors develop may render our products, services and expertise obsolete or uneconomical. For example, advances in informatics and virtual screening may render some of our technologies, such as our large compound libraries, obsolete. Additionally, the existing approaches of our competitors or new approaches or technologies that our competitors develop may be more effective than those we develop. We may not be able to compete successfully with existing or future competitors.
22
Our financial performance will depend on the prospects of the pharmaceutical and biopharmaceutical industries and the extent to which these industries engage outside parties to perform one or more aspects of their drug discovery process.
Our revenues depend almost exclusively, with the exception of the NIH contract, on research and development expenditures by the pharmaceutical and biopharmaceutical industries and companies in these industries outsourcing research and development projects. These expenditures are based on a wide variety of factors, including the resources available for purchasing research equipment, the spending priorities among various types of research and policies regarding expenditures during recessionary periods. In recent years, pharmaceutical companies have been attempting to contain spending on drug discovery and many biotechnology companies have found it difficult to raise capital to fund drug discovery activities. Geopolitical uncertainty or general economic downturns in our customers’ industries or any decrease in research and development expenditures could harm our operations, as could increased acceptance of management theories that counsel against outsourcing of critical business functions. Any decrease in drug discovery spending by pharmaceutical and biopharmaceutical companies would cause our revenues to decline and require us to further increase our net cash burn if we continued to pursue our current strategy.
In addition, due to improvements in global communications, combined with the supply of significantly lower cost PhD level scientific talent, we face the existing and growing threat of real and direct price-based competition for our chemistry, computational chemistry, and high-throughput screening services from low-cost offshore locations such as China, India and Eastern Europe. New guarantees of protection of intellectual property in these locations offered the necessary assurances to the biotech and pharmaceutical industry that the decision to outsource basic drug discovery offshore has become driven by low price. This shift has essentially resulted in the loss of our ability to consummate synthetic chemistry library contracts, the principal basis of our business in preceding years. We also believe that offshore pricing pressure on biology services, similar to those already noted in chemistry services, has and will continue to force us to reduce our reliance on fee-for-service work as the primary basis of our business.
The concentration of the pharmaceutical industry and the current trend toward increasing consolidation could hurt our business prospects.
The pharmaceutical customer segment of the market for our services is highly concentrated, with approximately 50 large pharmaceutical companies conducting drug discovery research. We have lost customers due to consolidation of pharmaceutical companies and the continuation of this trend may reduce the number of our current and potential customers even further. As a result, a small number of customers could account for a substantial portion of our revenues.
Additional risks associated with a concentrated customer base include:
• larger companies may develop and utilize in-house technology and expertise rather than using our products and services; and
• larger customers may negotiate price discounts or other terms for our products and services that are unfavorable to us.
We may fail to expand customer relationships through the integration of services.
We may not be able to use existing relationships with customers in individual areas of our business to sell services in multiple areas of drug discovery. We may not be successful in selling
23
our offerings in combination across the range of drug discovery disciplines we serve because integrated combinations of our services may not achieve time and cost efficiencies for our customers, especially our large pharmaceutical company customers. Biotechnology companies may desire our integrated offerings but are often not sufficiently capitalized to pay for these services. In addition, we may not succeed in further integrating our offerings. If we do not achieve integration of our services, we may not be able to take advantage of potential revenue opportunities and differentiate ourselves from competitors. Moreover, such integrated offerings may require us to expend at-risk capital as part of the co-investment in potential future value of the compounds resulting from such work.
Our services and technologies may never help discover drugs that receive Food and Drug Administration approval, which may make it difficult for us to gain new business.
To date, we are not aware of any of our customers having used any of our drug discovery products, services or technologies to develop a drug that ultimately has been approved by the Food and Drug Administration, and our customers may never do so. Whether our customers use our drug discovery services and technologies to develop any drugs that ultimately receive Food and Drug Administration approval will depend heavily on our scientific success and our customers’ scientific success, as well as on our customers’ ability to meet applicable Food and Drug Administration regulatory requirements. Our products, services and technologies may fail to assist our customers in achieving their drug discovery objectives, either on a timely basis or at all. For example, when our customers deliver proteins to us for assay development or chemistry library design ideas for chemical compound development and production, we may design assays or develop chemical compound libraries that fail to fully characterize the applicable protein’s or compound’s therapeutic potential, which could cause its further development to be delayed or abandoned. Additionally, our customers may not deliver to us proteins for assay development or chemistry library design ideas for chemical compound development and production that yield promising lead compounds for further development. Our customers may also lack the resources or experience or be otherwise unable to comply with the Food and Drug Administration’s clinical trial requirements. Certain of our competitors are able to claim that their drug discovery services have been used in developing drugs that received Food and Drug Administration approval. To the extent that potential customers consider demonstrated therapeutic success an important factor in selecting between us and our competitors, we may be competitively disadvantaged, which would negatively impact our ability to generate new business.
Our financial performance will depend on improved market conditions in the segments of the drug discovery and development process in which we participate.
The drug discovery and development process can be broadly separated into the following stages: Target identification; target validation; lead discovery; lead optimization; pre-clinical development; IND filing; clinical trials, phases I-III; new drug application, or NDA; and post market surveillance. We currently participate in the areas of lead discovery and lead optimization. Based on current industry averages, the cost of acquiring a validated target plus the costs of lead discovery and lead optimization are greater than the expected proceeds of out-licensing a potential drug candidate during the pre-clinical phase of drug development. This is primarily due to the negative imbalance between the relatively high cost of obtaining pre-clinical drug candidates, the high failure rate of such pre-clinical candidates, and the relatively low demand for such pre-clinical candidates that exists at present. It is estimated that a positive expected return on investment is not obtained until a drug candidate has passed through phase II clinical trials, which requires a significant commitment of resources to attain. Therefore, many drug companies may be deterred from engaging in drug discovery unless they have the substantial financial resources necessary to fund the drug discovery process all the way through phase II clinical trials. Unless advances are made to either reduce the cost or improve the success rate of pre-clinical
24
drug candidates, or unless the market demand for such pre-clinical drug candidates improves, we may continue to face difficult market conditions for our services which may inhibit our growth.
Many of our collaboration and services offerings have lengthy sales cycles, which could cause our operating results to fluctuate significantly from quarter to quarter.
Business development activities related to the marketing of our collaboration and service offerings typically involve significant technical evaluation and commitment of expense or capital by our customers. Accordingly, the sales cycles, or the time from finding a prospective customer through closing the collaboration or sale, associated with these collaborations or sale typically range from six to eighteen months. Sales of these services and the formation of these collaborations are subject to a number of significant risks, including customers’ budgetary constraints and internal acceptance reviews that are beyond our control. Due to these lengthy and unpredictable sales cycles, our operating results could fluctuate significantly from quarter to quarter. We expect to continue to experience significant fluctuations in quarterly operating results due to a variety of factors, such as general and industry specific economic conditions, that may affect the research and development expenditures of pharmaceutical and biopharmaceutical companies.
The intellectual property rights on which we rely to protect the technology underlying our techniques may not be adequate, which could enable third parties to use our technology or very similar technology and could reduce our ability to compete in the market.
Our success will depend, in part, on our ability to obtain, protect and enforce patents on our technology and to protect our trade secrets. We also depend, in part, on patent rights that third parties license to us. Any patents we own or license may not afford meaningful protection for our technology and products. Others may challenge our patents or the patents of our licensors and, as a result, these patents could be narrowed, invalidated or rendered unenforceable. In addition, current and future patent applications on which we depend may not result in the issuance of patents in the United States or foreign countries. Competitors may develop products similar to ours that are not covered by our patents. Further, since there is a substantial backlog of patent applications at the U.S. Patent and Trademark Office, the approval or rejection of our or our competitors’ patent applications may take several years.
Our European eukaryotic gene profiling patent was opposed by various companies. Oral proceedings were held before the Opposition Division of the European Patent Office in January 2003. At the conclusion of the hearing, the Opposition Division maintained our patent in amended form. The period during which an appeal of the Opposition Division decision could be made has expired. As amended, the patent claims kits and methods for identifying and characterizing the potential toxicity of a compound using expression profiles of four categories of stress.
In addition to patent protection, we also rely on copyright protection, trade secrets, know-how, continuing technological innovation and licensing opportunities. In an effort to maintain the confidentiality and ownership of our trade secrets and proprietary information, we require our employees, consultants and advisors to execute confidentiality and proprietary information agreements. However, these agreements may not provide us with adequate protection against improper use or disclosure of confidential information, and there may not be adequate remedies in the event of unauthorized use or disclosure. Furthermore, like many technology companies, we may from time to time hire scientific personnel formerly employed by other companies involved in one or more areas similar to the activities conducted by us. In some situations, our confidentiality and proprietary information agreements may conflict with, or be subject to, the rights of third
25
parties with whom our employees, consultants or advisors have prior employment or consulting relationships.
Although we require our employees and consultants to maintain the confidentiality of all confidential information of previous employers, their prior affiliations may subject us or these individuals to allegations of trade secret misappropriation or other similar claims. Finally, others may independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets. Our failure to protect our proprietary information and techniques may inhibit or limit our ability to exclude certain competitors from the market.
The drug discovery industry has a history of intellectual property litigation and we may be involved in intellectual property lawsuits, which may be lengthy and expensive.
In order to protect or enforce our patent rights, we may have to initiate legal or administrative proceedings against third parties. In addition, others may sue us or initiate interference proceedings against us for infringing their intellectual property rights, or we may find it necessary to initiate a lawsuit seeking a declaration from a court that we are not infringing the proprietary rights of others. The patent positions of pharmaceutical, biopharmaceutical and drug discovery companies are generally uncertain. A number of pharmaceutical companies, biopharmaceutical companies, independent researchers, universities and research institutions may have filed patent applications or may have been granted patents that cover technologies similar to the technologies owned by, or licensed to, us or our collaborators. A number of patents may have been issued or may be issued in the future that could cover certain aspects of our technology and that could prevent us from using technology that we use or expect to use. In addition, we are unable to determine all of the patents or patent applications that may materially affect our ability to make use or sell any potential products. Legal or administrative proceedings relating to intellectual property would be expensive, take significant time and divert management’s attention from other business concerns, no matter whether we win or lose. The cost of such litigation, interference or administrative proceedings could hurt our profitability.
Further, an unfavorable judgment in an administrative proceeding, interference or infringement lawsuit brought against us, in addition to any damages we might have to pay, could prevent us from obtaining intellectual property protection for our technology, require us to stop the infringing activity or obtain a license. Any required license may not be available to us on acceptable terms, or at all. In addition, some licenses may be nonexclusive, and therefore, our competitors may have access to the same technology that is licensed to us. If we fail to obtain a required license or are unable to design around a patent, we may be unable to sell some of our products or services.
Our stock price will likely be volatile.
The trading price of our common stock has been and will likely continue to be volatile and could be subject to fluctuations in price in response to various factors, many of which are beyond our control, including:
• actual or anticipated variations in quarterly operating results;
• announcements of technological innovations by us or our competitors;
• new products or services introduced or announced by us or our competitors;
• changes in financial estimates by (or the beginning or cessation of research coverage by) securities analysts;
26
• the announcements by us or our competitors of financial results that do not meet or exceed the results anticipated by the public markets;
• conditions or trends in the pharmaceutical and biopharmaceutical industries or in the drug discovery services industry;
• announcements by us or our competitors of significant acquisitions, divestitures or other strategic transactions, collaborations, joint ventures or capital commitments, or terminations of collaborations or joint ventures;
• the implementation or wind-down of stock buyback programs;
• additions or departures of key personnel;
• economic and political factors; and
• sales of our common stock, including sales by any of our stockholders who beneficially own more than 5% of our common stock and who could potentially sell large amounts of our common stock at any one time.
In addition, price and volume fluctuations in the stock market in general, and the Nasdaq National Market and the market for technology companies in particular, have often been unrelated or disproportionate to the operating performance of those companies. Further, the market prices of securities of life sciences companies have been particularly volatile. Conditions or trends in the pharmaceutical and biopharmaceutical industries generally may cause further volatility in the trading price of our common stock, because the market may incorrectly perceive us as a pharmaceutical or biopharmaceutical company and our customers are pharmaceutical and biopharmaceutical companies. These broad market and industry factors may harm the market price of our common stock, regardless of our operating performance. In the past, plaintiffs have often instituted securities class action litigation following instances of volatility in the market price of a company’s securities. A securities class action suit against us could result in potential liabilities, substantial costs and the diversion of management’s attention and resources, regardless of whether we win or lose.
Our customers may restrict our use of scientific information, which could prevent us from using this information for additional revenue.
We plan to generate and use information that is not proprietary to our customers and which we derive from performing drug discovery services for our customers. However, our customers may not allow us to use information such as the general interaction between types of chemistries and types of drug targets that we generate when performing drug discovery services for them. Our current contracts typically restrict our use of certain scientific information we generate for our customers, such as the biological activity of chemical compounds with respect to drug targets, and future contracts also may restrict our use of additional scientific information. To the extent that our use of information is restricted, we may not be able to collect and aggregate scientific data and take advantage of potential revenue opportunities.
Our ability to maintain the current infrastructure will depend on our attracting and retaining key executives, experienced scientists and business development personnel.
Our future success will depend to a significant extent on our ability to attract, retain and motivate highly skilled scientists and business development personnel. In addition, our business would be significantly harmed if we lost the services of Michael C. Venuti, Ph.D., our Acting Chief Executive Officer. Additionally, it is difficult for us to find qualified business development personnel that are experienced in our business model. We do not maintain life insurance on any of our
27
officers. Our ability to maintain, expand or renew existing collaborations with our customers, enter into new collaborations and provide additional services to our existing customers depends, in large part, on our ability to hire and retain scientists with the skills necessary to keep pace with continuing changes in drug discovery technologies personnel who are highly motivated. Our U.S. employees are at will employees, which means that they may resign at any time, and we may dismiss them at any time (subject, in some cases, to severance payment obligations). We believe that there is a shortage of, and significant competition for, scientists with the skills and experience in the sciences necessary to perform the services we offer. We compete with pharmaceutical companies, biopharmaceutical companies, combinatorial chemistry companies, contract research companies and academic institutions for new personnel. If we do not attract new scientists or retain or motivate our existing personnel, we may not be able to maintain the current infrastructure.
We have acquired several businesses and face risks associated with integrating these businesses and potential future drug discovery technology-driven acquisitions.
To enhance and expand our technology platforms, we have acquired several businesses and review potential acquisition and other strategic opportunities in the ordinary course of our business. Acquisitions of technology-based assets intended to improve or supplement our then-current offerings involve numerous risks, including, among others, difficulties and expenses incurred in the consummation of acquisitions and assimilation of the operations, personnel and services or products of the acquired companies, difficulties of operating new businesses, the diversion of management’s attention from other business concerns and the potential loss of key employees of the acquired company. In addition, acquired businesses may have management structures incompatible with our own and may experience difficulties in maintaining their existing levels of business after joining us. If we do not successfully integrate and grow the businesses we have acquired or any businesses we may acquire in the future, our business will suffer. Additionally, acquisition candidates may not be available in the future or may not be available on terms and conditions acceptable to us. Acquisitions of foreign companies also may involve additional risks of assimilating different business practices, overcoming language and cultural barriers and foreign currency translation. We currently have no agreements or commitments with respect to any acquisition, and we may never successfully complete any additional acquisitions.
We may incur write-downs or write-offs in connection with potential future acquisitions, and exit costs, losses and liabilities in connection with potential future business divestitures or shutdowns.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
We incurred $4.7 million in impairment charges in 2005 related to long-lived assets. Approximately $3.7 million of the impairment charges related to our prepaid royalty to Abbott Laboratories related to the µARCS screening technology. In connection with the restructuring of our chemistry operations we decided to discontinue the commercialization of the µARCS screening technology. We incurred an additional $1.0 million of impairment charges related to our gene profiling technology.
We incurred a $50.9 million goodwill impairment charge during the fourth quarter of 2002, which represented the write-off of goodwill that we had accumulated in connection with several acquisitions. In the event that we make future acquisitions, we may take additional write-downs or write-offs associated with acquired assets, which could have a material adverse effect on our results of operations and financial condition. Any future acquisitions we make may also not improve our business as much as we expect, or be accretive to our earnings, which could cause the trading price of our common stock to decline. In addition, if any future acquisitions we make do not improve our business as much as we expect, we may choose to discontinue the businesses associated with those acquisitions by divestiture or by shutting those businesses down. We may
28
also choose to divest or shut down existing businesses or product or service lines for strategic reasons. We may incur substantial exit costs, losses and liabilities in connection with any such divestiture or shut-down.
Our operations could be interrupted by damage to our facilities.
Our results of operations are dependent upon the continued use of our highly specialized laboratories and equipment. Our operations are primarily concentrated in facilities in San Diego, California, South San Francisco, California, Heidelberg, Germany and Allschwil, Switzerland. Natural disasters, such as earthquakes or fires, or terrorist acts could damage our laboratories or equipment and these events may materially interrupt our business. We maintain business interruption insurance to cover lost revenues caused by such occurrences. However, this insurance would not compensate us for the loss of opportunity and potential adverse impact on relations with existing customers created by an inability to meet our customers’ needs in a timely manner, and may not compensate us for the physical damage to our facilities.
We are subject to foreign currency risk related to conducting business in multiple currencies.
Currency fluctuations between the U.S. dollar and the currencies in which we do business, including the Japanese yen, the Swiss franc, and the Euro, will cause foreign currency translation gains and losses. We cannot predict the effects of exchange rate fluctuations on our future operating results because of the number of currencies involved, changes in the percentage of our revenue that will be invoiced in foreign currencies, the variability of currency exposure and the potential volatility of currency exchange rates. Because we conduct business in multiple currencies we are subjected to economic and earnings risk. We do not currently engage in foreign exchange hedging transactions to manage our foreign currency exposure; however, we may begin to hedge certain transactions between the Swiss franc and other currencies that are invoiced from our Swiss affiliate in order to minimize foreign exchange transaction gains and losses.
We may be subject to liability regarding hazardous materials.
Our products and services as well as our research and development processes involve the controlled use of hazardous materials. For example, we often use dangerous acids, bases, oxidants, radio isotopic and flammable materials. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of such materials and certain waste products. We cannot completely eliminate the risk of accidental contamination or injury from these materials. In the event of such an accident, we could be held liable for any damages that result, and any such liability could exceed our resources and disrupt our business. In addition, we may have to incur significant costs to comply with environmental laws and regulations related to the handling or disposal of such materials or waste products in the future, which would require us to spend substantial amounts of money.
Because it is unlikely that we will pay dividends, our stockholders will only be able to benefit from holding our stock if the stock price appreciates.
We have never paid cash dividends on our capital stock and do not anticipate paying any cash dividends in the foreseeable future.
29
Anti-takeover provisions in our stockholder rights plan and in our charter and bylaws could make a third-party acquisition of us difficult.
In 2003 we adopted a stockholder rights plan (a so-called “poison pill”). Also, our certificate of incorporation and bylaws contain provisions that could make it more difficult for a third party to acquire us, even if doing so would be beneficial to our stockholders. These provisions could limit the price that investors might be willing to pay in the future for shares of our common stock.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
We believe that our currently leased and occupied facilities are generally well maintained, in good operating condition and are sufficient to meet our needs for the near term.
Leased Properties
Locations | | Square
Feet | | Use | | Lease
Expiration Dates | |
San Diego, California | | 34,500 | | Corporate headquarters Marketing and Laboratory | | August 31, 2008 | |
South San Francisco, California | | 52,200 | | Laboratory and Office | | November 30, 2008 | |
Heidelberg, Germany | | 22,800 | | Laboratory and Office | | June 12, 2013 | |
Basel, Switzerland | | 20,000 | | Laboratory and Office | | January 31, 2013 | |
Tokyo, Japan | | 140 | | Office | | November 9, 2006 | |
In March 2006, we vacated approximately 70% of our South San Francisco facility in connection with our restructuring efforts announced in November 2005. Although we continue to lease all of the property in South San Francisco, we are currently seeking to sublease the unoccupied space.
Item 3. Legal Proceedings.
From time to time, we may be involved in litigation that arises through the normal course of business. As of the date of this Report, we are not a party to any material legal proceedings or disputes and obligations to any government related to tax compliance matters.
Item 4. Submission of Matters to a Vote of Security Holders.
There were no matters submitted to a vote of security holders during the quarter ended December 31, 2005.
30
PART II
Item 5. Market for the Company’s Common Equity, Related Stockholder Matters and Issuer Repurchases of Equity Securities.
Market Information
Our common stock is traded on the Nasdaq National Market, under the symbol DPII. The following table sets forth the range of high and low sales prices on the Nasdaq National Market of our common stock for the quarterly periods indicated, as reported by Nasdaq. Such quotations represent inter-dealer prices without retail mark up, mark down or commission and may not necessarily represent actual transactions.
| | High | | Low | |
Year Ended December 31, 2005: | | | | | |
First Quarter | | $ | 4.76 | | $ | 3.10 | |
Second Quarter | | 3.54 | | 2.79 | |
Third Quarter | | 3.50 | | 2.80 | |
Fourth Quarter | | 3.46 | | 2.24 | |
Year Ended December 31, 2004: | | | | | |
First Quarter | | $ | 6.50 | | $ | 5.48 | |
Second Quarter | | 6.40 | | 4.54 | |
Third Quarter | | 5.91 | | 4.08 | |
Fourth Quarter | | 5.47 | | 4.11 | |
Holders
As of March 1, 2006, there were 106 holders of record of our common stock.
Dividends
We have never paid cash dividends on our common stock, and we do not expect to pay any cash dividends in the foreseeable future.
Equity Compensation Plan Information
The following table provides certain information with respect to all of our equity compensation plans in effect as of December 31, 2005:
Plan Category | | Number of securities
to be issued upon
exercise of
outstanding options,
warrants and rights
(a) | | Weighted average
exercise price of
outstanding options,
warrants and rights
(b) | | Number of securities
remaining available
for
future issuance under
equity compensation
plans (excluding
securities reflected in
column) (a) (c)(1)(2) | |
Equity compensation plans approved by
security holders | | 3,221,602 | | $ | 4.67 | | 2,185,346 | |
Equity compensation plans not approved
by security holders | | — | | — | | — | |
Total | | 3,221,602 | | $ | 4.67 | | 2,185,346 | |
31
(1) The number of securities available for future issuance under our stock incentive plan automatically increases on the
first trading day in January each calendar year by an amount equal to 2% of the total number of shares of common stock outstanding on the last trading day in December of the preceding calendar year, but in no event shall any such annual increase exceed 2,000,000 shares. The number of securities available for future issuance under our employee stock purchase plan automatically increases on the first trading day in January each calendar year by an amount equal to 1.5% of the total number of shares of common stock outstanding on the last trading day in December of the preceding calendar year, but in no event shall any such annual increase exceed 500,000 shares.
(2) 1,908,761 of these securities are attributable to our Employee Stock Purchase Plan.
Changes in Securities and Use of Proceeds
The registration statement (File No. 333-36638) for our initial public offering was declared effective by the SEC on July 27, 2000. We received net proceeds from the Offering of approximately $94.7 million. Through December 31, 2005, we had used approximately $18.5 million of the net proceeds for acquisitions of companies, $6.0 million for prepaid µARCS royalties, $15.4 million for capital expenditures and $2.6 million for costs associated with restructuring.
The registration statement (File No. 333-113488) for our secondary public offering was declared effective by the SEC on May 4, 2004. A total of 8,305,300 shares of common stock at a price of $5.00 per share were made available to the public. Axys Pharmaceuticals, Inc., then a stockholder of Discovery Partners International, Inc., registered 7,222,000 shares for resale, with the remaining 1,083,300 shares registered for sale by the Company to the underwriters to cover over-allotments. We received proceeds from the offering, of the shares registered for sale by the Company, of $5.1 million net of underwriters discounts.
There have been no sales or new issues of unregistered securities in the last three fiscal years other than as previously disclosed in this section.
Issuer Purchases of Equity Securities
The following table discloses the purchases of our equity securities during the fourth fiscal quarter of 2005.
Period | | Total
Number of
Shares
Purchased
(1) | | Average
Price Paid
per Share | | Total Number
of Shares
Purchased as
Part of Publicly
Announced
Plans or
Programs (2) | | Maximum Number
(or Approximate
Dollar Value) of
Shares that May
Yet Be Purchased
under the Plans or
Programs (2) | |
October 1, 2005 through October 31, 2005 | | — | | $ | — | | — | | 1,850,000 | |
November 1, 2005 through November 30, 2005 | | 21,523 | | 2.72 | | — | | 1,850,000 | |
December 1, 2005 through December 31, 2005 | | — | | — | | — | | 1,850,000 | |
Total | | 21,523 | | $ | 2.72 | | — | | | |
(1) In November 2005, we accepted 21,523 shares of our common stock in lieu of cash from former employees in payment of obligations to the Company totaling $58,000.
32
(2) On October 4, 2001, our Board of Directors authorized a Stock Repurchase Plan, whereby we were authorized to repurchase up to 2,000,000 shares of our common stock at no more than $3.50 per share. In October 2001, we purchased 35,000 shares of our common stock for a total of $119,250 pursuant to our Stock Repurchase Plan. In February 2003, an additional 115,000 shares were purchased for a total of $289,000.
33
Item 6. Selected Financial Data.
The financial data below has been recast to reflect the results of operations and financial positions of our instrumentation product lines as a discontinued operation. The amounts included in the results for discontinued operations consist of the revenues, cost of sales and operating expenses associated with the operations of the instrumentation product lines excluding any allocations for facilities and other corporate support. The selected consolidated financial data should be read in conjunction with our financial statements and related notes thereto and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Annual Report on Form 10-K. The historical results are not necessarily indicative of the results that may be expected for any future period.
Selected Consolidated Financial Information
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | | 2002 | | 2001 | |
| | (In thousands, except per share data) | |
Consolidated Statement of Operations Data: | | | | | | | | | | | |
Revenues | | $ | 34,837 | | $ | 44,268 | | $ | 45,209 | | $ | 36,407 | | $ | 35,221 | |
Cost of revenues: | | | | | | | | | | | |
Cost of revenues before additional charges | | 25,108 | | 25,145 | | 27,615 | | 25,837 | | 17,514 | |
Additional charges: | | | | | | | | | | | |
Provision for discontinued products and obsolete inventory | | — | | — | | — | | 5,781 | | 4,397 | |
Anticipated contract loss | | — | | — | | — | | 1,485 | | — | |
Total cost of revenues | | 25,108 | | 25,145 | | 27,615 | | 33,103 | | 21,911 | |
Gross margin | | 9,729 | | 19,123 | | 17,594 | | 3,304 | | 13,310 | |
Operating expenses: | | | | | | | | | | | |
Research and development | | 3,919 | | 1,546 | | 407 | | 3,393 | | 10,020 | |
Selling, general and administrative | | 15,068 | | 14,432 | | 13,342 | | 12,140 | | 10,909 | |
Amortization of stock-based compensation and other non-cash compensation charges | | 1,017 | | 947 | | 502 | | 623 | | 1,074 | |
Restructuring | | 1,040 | | — | | 1,873 | | — | | — | |
Impairment of goodwill and other intangible assets | | 4,721 | | — | | — | | 51,091 | | — | |
Amortization of goodwill | | — | | — | | — | | — | | 5,849 | |
Total operating expenses | | 25,765 | | 16,925 | | 16,124 | | 67,247 | | 27,852 | |
Income (loss) from continuing operations | | (16,036 | ) | 2,198 | | 1,470 | | (63,943 | ) | (14,542 | ) |
Interest income, net | | 2,018 | | 1,419 | | 1,758 | | 2,037 | | 3,252 | |
Foreign currency transaction gains (losses) and other income (expense), net | | 310 | | (176 | ) | 60 | | 160 | | 246 | |
Income (loss) from continuing operations before provision for income taxes | | (13,708 | ) | 3,441 | | 3,288 | | (61,746 | ) | (11,044 | ) |
Provision for income taxes | | 13 | | 56 | | 10 | | 9 | | 8 | |
Net income (loss) from continuing operations | | (13,721 | ) | 3,385 | | 3,278 | | (61,755 | ) | (11,052 | ) |
Discontinued operations: | | | | | | | | | | | |
Gain on sale of discontinued operations | | 394 | | — | | — | | — | | — | |
Gain (loss) from discontinued operations | | (838 | ) | 518 | | (2,219 | ) | (358 | ) | (96 | ) |
Net income (loss) | | $ | (14,165 | ) | $ | 3,903 | | $ | 1,059 | | $ | (62,113 | ) | $ | (11,148 | ) |
| | | | | | | | | | | |
Net income (loss) per share for continuing operations, basic and diluted | | $ | (0.53 | ) | $ | 0.13 | | $ | 0.13 | | $ | (2.54 | ) | $ | (0.46 | ) |
Net income (loss) per share for discontinued operations, basic and diluted | | $ | (0.02 | ) | $ | 0.02 | | $ | (0.09 | ) | $ | (0.01 | ) | $ | (0.00 | ) |
Net income (loss) per share, basic and diluted | | $ | (0.55 | ) | $ | 0.15 | | $ | 0.04 | | $ | (2.55 | ) | $ | (0.46 | ) |
Shares used in calculating net income (loss) per share, basic | | 25,919 | | 25,319 | | 24,344 | | 24,315 | | 24,016 | |
Shares used in calculating net income (loss) per share, diluted | | 25,919 | | 26,272 | | 25,077 | | 24,315 | | 24,016 | |
Other Data: | | | | | | | | | | | |
Net cash provided by (used in) operating activities | | $ | 5,547 | | $ | 3,659 | | $ | 7,845 | | $ | (3,314 | ) | $ | (2,187 | ) |
Net cash provided by operating activities from discontinued operations | | 510 | | 1,091 | | 674 | | 1,179 | | 658 | |
Net cash provided by (used in) investing activities | | 4,804 | | (5,243 | ) | (8,410 | ) | (39,284 | ) | (44,917 | ) |
Net cash used in investing activities from discontinued operations | | (52 | ) | (123 | ) | (81 | ) | (362 | ) | (533 | ) |
Net cash provided by (used in) financing activities | | 129 | | 5,598 | | (634 | ) | (610 | ) | 477 | |
34
| | As of December 31, | |
| | 2005 | | 2004 | | 2003 | | 2002 | | 2001 | |
| | | | | (In thousands) | | | | |
Selected Consolidated Balance Sheet Data: | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 83,486 | | $ | 80,019 | | $ | 72,574 | | $ | 69,636 | | $ | 77,265 | |
Working capital | | 85,757 | | 93,368 | | 77,540 | | 75,788 | | 85,659 | |
Total assets | | 102,280 | | 115,643 | | 105,194 | | 101,609 | | 162,223 | |
Long-term obligations, less current portion | | 528 | | 155 | | 98 | | 411 | | 1,177 | |
Total stockholders’ equity | | 95,074 | | 108,407 | | 98,247 | | 96,532 | | 157,042 | |
| | | | | | | | | | | | | | | | |
35
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion of our financial condition contains certain statements that are not strictly historical and are “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995 and involve a high degree of risk and uncertainty. Our actual results may differ materially from those projected in the forward-looking statements due to risks and uncertainties that exist in our operations, development efforts and business environment, including those set forth under the Section entitled “Risks and Uncertainties” in Item 1, and other documents we file with the Securities and Exchange Commission. All forward-looking statements included in this report are based on information available to us as of the date hereof, and we assume no obligation to update any such forward-looking statement.
Overview
We collaborate with pharmaceutical and biopharmaceutical companies to advance their drug discovery process through our integrated and highly efficient collection of drug discovery technologies, products and services focused from the point immediately following identification of a drug target through when a drug candidate is ready for pre-clinical studies. We offer an integrated platform of drug discovery technologies, including assay development, high throughput screening, design and synthesis of proprietary libraries of compounds for screening and primary hit-to-lead expansion, lead compound optimization, drug discovery informatics and in vitro toxicology profiling, as described in more detail below. These products and services can be provided individually or as an integrated solution, depending on our customers’ requirements. During 2005, we focused on offering integrated drug discovery services as part of long-term collaborations, as we continued to work with companies worldwide in all aspects of drug discovery research.
However, even with this steady progress, it has become evident during 2005 that the basic business sector in drug discovery contract research and services was undergoing a major and quite unfavorable market shift. Worldwide improvements in communications and shipping, coupled with entrepreneurial efforts in rapidly developing locations such as India, China and Eastern Europe, enabled the highly skilled scientists in those areas to build companies providing a similar range of products and services to us and our peer group, but at significantly lower prices. New guarantees of protection of intellectual property in these locations has offered the necessary assurances to the biotech and pharmaceutical industry that the decision to outsource basic drug discovery offshore has become driven by low price. This shift has resulted in the loss of our ability to consummate synthetic chemistry library contracts, the principal basis of our business in preceding years.
In the fourth quarter of 2005, discussions with Pfizer to renew our contract were ended. With the absence of a new contract with Pfizer, we have reduced our combinatorial chemistry and library synthesis operational capacity through a restructuring of our South San Francisco facility and consolidation of our chemistry platform into our San Diego facility. The NIH Roadmap compound management facility remains fully staffed and operational in our South San Francisco location. We sold our instrumentation product line, as it was not consistent with our collaborations strategy. We also believe that offshore pricing pressure on biology services, similar to that already noted in chemistry services, have and will continue to force us to reduce our reliance on fee-for-service work as the primary basis of our business.
We enter 2006 cognizant of these changes in our business under reorganized management and with an imperative from our Board of Directors to make best use of our current financial and scientific assets to accelerate our entry into more substantial value-creating activities. We are
36
currently exploring a range of options to best deploy our resources in order to improve stockholder value, including divestiture of assets and merger or acquisition opportunities that are specifically identified to create or enhance a drug development-based product portfolio with defined risk and timelines to clinical milestones with generally acknowledged market value, and which may involve a change in control of our company. As we transition our strategic initiatives and reorganize our operational capacity, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results based on our past operational contract services model are not indicative of future results. We cannot assure that we will be successful in accomplishing any of these strategic initiatives or that any strategic transaction that may occur will be accomplished on favorable terms.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of the our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
Our current major services are as follows:
Chemistry Services
Compounds. We develop and synthesize a broad range of highly purified compound libraries that can be screened using biological assays. After compounds are screened, promising compounds, or hits, are then improved, or optimized, to generate drug candidates, or leads. As a result of the acquisition of substantially all of the assets of Biofrontera Discovery GmbH, in April 2005, we are able to offer collections of unique purified mixtures and purified natural compounds that can be screened using assays and that can then further be characterized or/and modified to generate drug candidates.
Medicinal Chemistry. We provide a wide range of medicinal chemistry and other lead optimization services. This includes the synthesis of compounds that modify the original hit for improved potency, selectivity and other pharmaceutical characteristics. In some cases we provide medicinal chemistry services in conjunction with our computational drug discovery efforts to design and synthesize small libraries of compounds to act on specific targets that have known structures.
Drug Discovery Informatics; ADME and Toxicology. We have developed computational tools that we believe allow us to substantially increase our knowledge of the characteristics of targets and leads, and their interaction with certain molecules. We believe these tools could potentially be applied throughout the drug discovery process to significantly reduce the time and cost of developing a drug. We currently have computer algorithms that allow us to design libraries of compounds with high diversity, thereby increasing the likelihood of finding hits during screening.
37
We have developed novel algorithms to aid in the understanding and utilization of the data resulting from high throughput screening experiments. We expect to use our computational tools to help predict absorption, distribution, metabolism, and excretion, or ADME, and toxicological reactions to classes of compounds. This could allow our customers to avoid spending money and time on hits and leads that will ultimately fail due to their unfavorable ADME and toxicological characteristics.
Compound Repository. We also provide services to establish, maintain and manage compound repositories for third parties such as the NIH.
Screening Services
Screening. We offer high throughput and high content screening services at our facility in Allschwil, Switzerland. We also offer our customers access to a proprietary collection of chemical compounds comprised of compounds from many commercial suppliers as well as those that have been internally developed.
Assays. We provide assay development services to help our customers better select drug candidates before moving to the more costly stages of pre-clinical and clinical testing. Our team of scientists are experienced in working with major target classes in a number of significant therapeutic areas, such as cardiovascular, neurology, oncology and ophthalmology.
Other licenses and services
Royalties. We license our proprietary gene profiling system, under the Xenometrix patent licensing agreements, that characterizes a cell’s response upon exposure to compounds and other agents by the pattern of gene expression.
Customer concentration
The following table illustrates customers, from continuing operations, that provided more than 10% of our revenues:
| | For the Years Ended | |
| | 2005 | | 2004 | | 2003 | |
| | | | | | | |
Pfizer | | 54 | % | 62 | % | 69 | % |
National Institutes of Health (NIH) | | 14 | % | 3 | % | 0 | % |
Others | | 32 | % | 35 | % | 31 | % |
| | | | | | | |
In February 2004, we entered into a broadened collaboration agreement with Pfizer that replaced our prior collaboration with Pfizer that we entered into in December 2001. Under this agreement, we collaborated with Pfizer to design and develop compounds that are owned by and exclusive to Pfizer. The agreement expired by its natural terms on January 6, 2006.
In August 2004 we entered into a multi-year contract with the NIH to establish and maintain a Small Molecule Repository to acquire, manage and provide up to one million chemical compounds to multiple NIH screening centers as part of the NIH Roadmap Initiative. The estimated funding available to us under this contract for the period from August 2004 through November 2008 is approximately $24 million, assuming the contract continues for its full term. The agreement expires by its natural terms on November 30, 2008, but may be renewed on an annual basis by the NIH up to November 30, 2013. Based on experienced cost under-runs, it is also possible that the estimated funding available to us under this contract could be extended
38
beyond the specified contractual time period. It is uncertain at this time whether the NIH will renew this agreement or whether we will be successful in entering into new agreements with this customer. This contract is funded in its entirety by NIH, Department of Health and Human Services. Payments to us for performance under this contract are subject to audit by the Defense Contract Audit Agency and are subject to government funding.
Strategic Initiatives
The pharmaceutical and biopharmaceutical industries provide substantially all of our revenues. This industry has experienced economic difficulties in recent periods and is under intense pressure to bring new products to market as quickly as possible in order to both offset the impact of existing drug patent expirations and to grow revenues and profits. As a result, investment priorities for the industry have shifted more to development activities from discovery activities in order to address these near term pressures and an increasing emphasis is being placed on the achievement of cost savings in discovery research activities. In 2005, we experienced significant competitive pressure from lower cost offshore providers in the chemistry services we offer to our customers. As a result we made a strategic decision to reorganize and refocus our resources to build value through more highly integrated drug discovery services as part of long-term collaborations. In adapting to this shift in our business strategy we executed on the following transactions:
• In April 2005, we acquired, for $1.5 million, the assets of Biofrontera Discovery GmbH in the field of natural products, which secured for us a large library of purified natural products highly complementary to our synthetic compound screening libraries.
• In October 2005, we sold, for $1.7 million, the assets related to the IRORI® chemical synthesis, Crystal Farm® automated protein crystallization, and Universal Store™ compound storage systems product lines to preserve our cash resources by eliminating the investment expenses required for the engineering innovation required to develop new product lines.
• In November 2005, we decided to cease commercialization of the mARCS screening technology and to streamline our chemistry offerings to those that add value to a long-term collaboration.
In November 2005, discussions with Pfizer regarding a new collaboration to extend our services in the design and development of compounds exclusively for Pfizer were ended. In the absence of a new contract with Pfizer, we reduced our combinatorial chemistry and library synthesis operational capacity in a restructuring of our South San Francisco facility. In the fourth quarter of 2005, we recorded a $928,000 charge for restructuring activities resulting from this decision, which consisted of accrued one-time termination benefits. In addition, we recorded $3.9 million in non-cash write-downs of our prepaid royalty to Abbott Laboratories for the mARCS screening technology, recorded as impairment of long-lived assets, and inventories that are non-essential to our current focus, recorded in cost of revenues.
We have a limited history of offering our integrated drug discovery platform in the form of a collaborative model to the pharmaceutical and biopharmaceutical industries. It is uncertain whether our current “service-based” customers will migrate to this new business offering or whether new collaborators will enter into collaborations with us. In addition, our existing fee-for-service screening services and chemistry services continue to operate under increasing price pressures and will continue to force us to reduce our reliance on such fee-for-service work as the primary basis of our business. If we are unsuccessful in our new strategic efforts, we may be required to undertake additional restructuring activities.
We are currently exploring a range of options to best deploy our resources and improve stockholder value in light of the changes in our business and an imperative from our Board of Directors to make best use of our current financial and scientific assets to accelerate our entry
39
into more substantial value-creating activities. The options we are considering include divestiture of assets and merger or acquisition opportunities that are specifically identified to create or enhance a product portfolio with defined risk and timelines to milestones, which may involve a change in control of our company. As we transition our strategic initiatives and reorganize our operational capacity, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results are not indicative of future results. We cannot assure that we will be successful in accomplishing any of these strategic initiatives or that any strategic transaction that may occur will be accomplished on favorable terms.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
Discontinued Operations
In October 2005, we sold the assets related to our instrumentation product lines to former members of our management team for a total of $1.9 million, of which $1.7 million has been paid in cash with a reserved receivable of $200,000 at December 31, 2005, which will be recognized when collected. Our consolidated financial statements and related notes contained herein have been recast to reflect the financial position, results of operations and cash flows of the instrumentation product lines as a discontinued operation.
We did not account for our instrumentation product lines as a separate legal entity. Therefore, the following selected financial data for our discontinued operations is presented for informational purposes only and does not necessarily reflect what the net sales or earnings would have been had the businesses operated as a stand-alone entity. The financial information for our discontinued operations excludes allocations of facilities and other corporate expenses related to those operations that were not transferred in the sale of those assets. These amounts are considered by management to reflect most fairly or reasonably the incremental financial results related to those operations. See Note 3, “Discontinued Operations,” in the notes to the consolidated financial statements listed under Item 15(a) of Part IV of this report.
40
Selected Financial Data for Discontinued Operations
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
| | | | (In thousands) | | | |
| | | | | | | |
Revenues | | $ | 2,026 | | $ | 7,296 | | $ | 4,617 | |
Cost of revenues | | 842 | | 3,953 | | 3,784 | |
Gross margin | | 1,184 | | 3,343 | | 833 | |
Research and development | | 1,462 | | 2,252 | | 1,979 | |
Selling, general and administrative | | 507 | | 518 | | 1,059 | |
Amortization of stock-based compensation | | 53 | | 55 | | 14 | |
Total operating expenses | | 2,022 | | 2,825 | | 3,052 | |
Gain (loss) from discontinued operations | | $ | (838 | ) | $ | 518 | | $ | (2,219 | ) |
Net cash flows provided by operating activities | | $ | 510 | | $ | 1,091 | | $ | 674 | |
Net cash flows used in investing activities | | $ | (52 | ) | $ | (123 | ) | $ | (81 | ) |
Amounts included in fiscal 2005 represent the results of operations to the instrumentation product lines for the period January 1, 2005 through October 7, 2005, the effective date of the sale of the assets.
41
Critical Accounting Policies
This discussion and analysis of our financial condition and results of operations is based upon our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses. On an ongoing basis, we evaluate our estimates. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates, and the estimates themselves might be different if we used different assumptions.
We believe the following critical accounting policies involve significant judgments and estimates that are used in the preparation of our consolidated financial statements.
Revenue recognition. Revenue is recognized as follows:
Chemistry services. Revenue from the sale of chemical compounds delivered under our chemistry collaborations is recorded as the compounds are shipped. Revenue under chemistry service agreements that are compensated on a full-time equivalent, or FTE, basis is recognized on a monthly basis and is based upon the number of FTE employees that actually worked on each project and the agreed-upon rate per FTE per month. Beginning in April 2004, in accordance with our agreement with Pfizer, we were compensated based on predetermined limits to reserve sufficient resources to complete specific compound related activities, at the customer’s request, whether or not utilized. Revenue for reserving these resources was recognized based on the predetermined limits stipulated in the contract.
Compound repository services. In August 2004, we entered into a multi-year contract with the NIH to establish and maintain a Small Molecule Repository to manage and provide up to one million chemical compounds to multiple NIH Screening Centers as part of the NIH Roadmap Initiative. Revenue under this contract is recorded as costs are incurred, which include indirect costs that are based on provisional rates estimated by management at the time we submitted our proposal. We have calculated our actual indirect costs to be greater than our provisional rates and management fully intends to negotiate recovery of these higher costs with the government. Since this is our first government contract we have no historical experience negotiating final indirect cost rates with the government and therefore all cost overruns have been expensed and any potential recovery will be recognized as revenue upon receipt of monies. This contract is funded, in its entirety, by the NIH and the Department of Health and Human Services. Payment to us for performance under this contract is subject to audit by the Defense Contract Audit Agency and is subject to government funding. We provide a reserve against our receivables for estimated losses that may result from rate negotiations, audit adjustments and/or lack of government funding availability. As of December 31, 2005, no reserve was considered necessary. To the extent that we incur adjustments due to rate negotiations or lack of government funding availability, our revenue may be impacted.
Screening services. High throughput screening service revenues are recognized on the proportional performance method. Advances received under these high throughput screening service agreements are initially recorded as deferred revenue, which is then recognized proportionately as costs are incurred over the term of the contract. Certain of these contracts may allow the customer the right to reject the work performed; however, we have no history of material rejections and, as a result, historically we have recognized revenue without providing for such contingency.
42
Other licenses. Other licenses revenue includes royalty revenue due to us under the Xenometrix patent licensing agreements. Royalty revenue is recognized upon receipt of monies, provided we have no future obligation with respect to such payments.
Integrated drug discovery collaborations may provide chemistry services revenue, screening services revenue, milestone payments and other revenues. Revenue for each of these elements of such collaborations is recognized as described above. Revenue from milestone payments would be recognized upon receipt of monies.
Valuation of long-lived assets. In accounting for long-lived assets, we make estimates about the expected useful lives and the potential for impairment. Changes in the marketplace, technology or our operations could result in changes to these estimates. If a change to the estimate of the expected useful life is identified, the impact of accelerated depreciation is recognized in the period of the change. In connection with the restructuring of our South San Francisco facility in 2005, we identified long-lived assets that would cease to be used beyond the first quarter of fiscal 2006 (the period when the restructuring would be complete). The change to the estimate of the useful lives of these assets resulted in $202,934 of accelerated depreciation charges recognized in the fourth quarter of fiscal 2005. We expect approximately $200,000 of additional charges related to this change in estimated useful lives to be recognized in the first quarter of 2006. Our long-lived assets are evaluated for impairment when events and circumstances indicate that the assets may be impaired. If impairment is indicated, we reduce the carrying value of the asset to fair value. During the year ended December 31, 2005, we recorded $4.7 million in impairment charges on long-lived assets.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
43
Valuation of investments in marketable securities. In accounting for investments in marketable securities, we classify our investments as Available-for-Sale and record such assets at estimated fair value in the consolidated balance sheets, with unrealized gains and losses, if any, reported in stockholders’ equity (other comprehensive income). We invest our excess cash balances in marketable debt securities, primarily government securities, corporate bonds and notes and asset-backed securities, with strong credit ratings. We limit the amount of investment exposure to institutions, maturity and investment type. The realized gains and losses of securities sold is determined based on the specific identification method.
We will record an impairment charge if the securities continue to be impaired beyond twelve months or other factors indicate there is permanent impairment. We regularly monitor and evaluate the realizable value of our marketable securities. When assessing marketable securities for other-than-temporary declines in value, we consider such factors as, among other things, how significant the decline in value is as a percentage of the original cost, how long the market value of the investment has been less than its original cost and the market in general.
Restructuring charges. In accounting for restructuring charges we consider the primary elements to our restructuring plans: one-time termination benefits and the consolidation of excess facilities. We recognize the fair value of one-time termination benefits when we have taken actions or have the appropriate approval for taking action, and when a liability is incurred (when the plan has been communicated to employees). If employees are required to render service beyond a 60 day minimum retention period, the fair value of the obligation is determined on the date of the communication to the employee and recognized over the service period. In determining our costs to consolidate excess facilities, we estimate the fair value of the obligation at the cease-use date based on the remaining lease rentals, reduced by estimated sublease rentals that could be reasonably obtained for the property, even if we are unsuccessful in entering into a sublease. We recognize charges for consolidation of excess facilities when we have vacated the premises. We recognize the cumulative effect of any changes to the plan subsequent to the communication date and cease-use date in the period of the change.
Results of Operations
Revenues. Total revenue in 2005 decreased 21% to $34.8 million from $44.3 million in 2004 and $45.2 million in 2003. The decrease from 2004 to 2005 resulted primarily from decreases in chemistry services revenue and screening services revenue partially offset by increases in compound repository service revenue. The decrease in chemistry services revenue of approximately $7.2 million was due primarily to lower revenues generated from Pfizer and decreases in all other chemistry services. In the fourth quarter of 2004, we exercised our right to deliver additional compounds in 2004 under our contract with Pfizer, not to exceed the number of compounds scheduled for delivery in the first quarter of 2005 as stipulated in the contract. These additional shipments in 2004 equaled our allotment for the first quarter of 2005 and resulted in additional revenue of $4.2 million in the fourth quarter of 2004 that was not recognized in the first
44
quarter of 2005. The decrease in screening services revenue of approximately $2.3 million resulted primarily from decreases in screening service activity. These decreases were partially offset by an increase of $3.8 million of compound repository service revenue generated by our contract with the NIH. The increase from 2003 to 2004 resulted primarily from increases in screening services revenue due primarily to new contracts as well as additional services provided on existing contracts in 2004.
In 2005, 2004 and 2003, 54%, 62% and 69%, respectively, of our revenue came from our chemistry contract with Pfizer. The agreement expired by its natural terms on January 6, 2006.
In 2005 and 2004, 14% and 3%, respectively, of our revenues came from the NIH funded contract. Revenues under the NIH contract are earned as costs are incurred to procure, inspect and ship compounds to NIH designated screening centers. In addition, the component of revenue earned, as compounds are purchased on behalf of the NIH, is recognized at the point the compounds pass certain quality standards, as specified by the NIH, and payment is made to the compound vendors. The timing of revenues earned is partially dependent on the timing of the NIH selection of compounds, the timing of procurement and processing of acquired compounds and the volume of screening activity at the NIH designated screening centers. In the event the NIH is delayed in the selection process of acquiring compounds, or such acquired compounds fail to meet the NIH specified standards, or if there are delays in the ramp up in the demand of the NIH designated screening centers, revenues recognized under this contract may be deferred to future periods. We anticipate this contract will generate revenues of approximately $8.3 million in fiscal 2006. The NIH contract is subject to continued government funding.
Gross margins. Gross margin decreased to $9.7 million in 2005 from $19.1 million in 2004 and $17.6 million in 2003. The decrease in gross margin in 2005 from 2004 is primarily due to the decrease in chemistry and screening service revenue volume as costs of sales remained constant. In the fourth quarter of 2004, we exercised our right to deliver additional compounds to Pfizer in 2004, not to exceed the number of compounds scheduled for delivery in the first quarter of 2005 as stipulated in the contract. These additional shipments in 2004 equaled our allotment for the first quarter of 2005 and resulted in additional gross margin of $3.1 million in the fourth quarter of 2004 that was not recognized in the first quarter of 2005. The increase in gross margin for 2004 over 2003 primarily relates to improved gross margins derived from higher volumes on chemistry and screening services.
Gross margin as a percentage of revenues decreased to 28% in 2005 from 43% in 2004 and 39% in 2003. The decrease in gross margin as a percentage of revenue for 2005 is primarily due to decreased volume in higher margin chemistry revenues and screening services. The decrease in chemistry gross margin as a percentage of revenue was partially offset by increased volume of lower margin NIH business in 2005 as this contract began in August of 2004. Gross margin as a percentage of chemistry services revenue decreased due to decreased revenues under our Pfizer agreement, decreases in medicinal chemistry services, and charges related to the restructuring of our South San Francisco facility in the fourth quarter of 2005, which included the impact of accelerated depreciation and reserves on raw material inventory totaling $380,000. The improvement in gross margin as a percentage of revenue for 2004 over 2003 was primarily related to improvements on chemistry and screening services as a result of improved margins as a percentage of revenue on the Pfizer contract and lower levels of excess capacity. We anticipate that gross margin as a percentage of revenue in 2006 will continue to decline due to the expiration of the Pfizer contract and change in revenue mix.
Research and development expenses. Research and development expenses consist primarily of salaries and benefits, supplies and expensed development materials, and facilities costs including equipment depreciation. Research and development expenses increased 153% in 2005 to $3.9 million compared to $1.5 million in 2004. Research and development expenses increased 279% in 2004 to $1.5 million compared to $407,000 in 2003. Research and development expenses increased primarily due to the increased operating costs in the later half of 2005 as a result of the acquisition of the natural compound based discovery business from Biofrontera Discovery GmbH in April 2005 which represented $2.1 million of operating expenses, and
45
from the redeployment of development scientists and engineers from direct revenue generating activities of customer funded R&D programs and collaborations to internal programs focused on in silico tools, screening assays and drug discovery process development. The increase in research and development expense in 2004 from 2003 is primarily due to an increase in internal research programs focused on in silico tools, targeted libraries, screening assays and drug discovery process development. Research and development expenses as a percentage of revenues were 11% in 2005, 3% in 2004 and 1% in 2003. We anticipate increased spending in research and development in 2006 as we redirect our resources to co-funded research and development collaborations.
Selling, general and administrative expenses. Selling, general and administrative expenses consist primarily of salaries and benefits for sales, marketing and administrative personnel, advertising and promotional expenses, professional services, and facilities costs. Selling, general and administrative expenses increased 4% to $15.1 million in 2005 compared to $14.4 million in 2004. Selling, general and administrative expenses increased 8% to $14.4 million in 2004 compared to $13.3 million in 2003. The increase in 2005 is primarily due to $1.3 million in termination benefits paid to our former Chief Executive Officer, Chief Operating Officer and other members of senior management in 2005 and $600,000 in increased consulting fees related to the evaluation of corporate strategic initiatives. These increases were partially offset by $1.1 million decrease in business development activities and decreases in various administrative costs. The increase from 2003 to 2004 was due primarily to an increase in business development activities and professional services fees primarily related to Sarbanes-Oxley compliance. These increases were partially offset by decreased incentive compensation costs due to underperformance against corporate goals. Selling, general and administrative expenses as a percentage of revenues were 43% in 2005, 33% in 2004 and 30% in 2003. We anticipate decreased spending in selling, general and administrative expenses in 2006 as a result of the restructuring and lower termination benefit charges.
Stock-based compensation. We awarded 142,500 shares of restricted stock and rights to acquire 700,000 shares of restricted stock in August 2003, July 2004 and April 2005, collectively. As a result, we have recorded deferred stock-based compensation to be amortized on an accelerated basis over the period that these restricted stock grants and rights to acquire restricted stock vest. The deferred stock-based compensation expense totaled $1.0 million in 2005, $947,000 in 2004 and $501,000 in 2003.
Impairment of long-lived assets. We have stated within this Annual Report on Form 10-K that the Board of Directors is contemplating various strategic options that include the divestiture of various operating assets, a merger or acquisition and, if necessary, a liquidation of all of our assets. These events and circumstances have been evaluated in determining if the carrying amount of our assets at December 31, 2005 are impaired. We considered all available evidence and determined there is insufficient information to establish whether or not the carrying amount of the assets may be fully recovered should one of these events occur. As such, no related impairment charges were recorded at December 31, 2005.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of the our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
We recorded $4.7 million in impairment charges on long-lived assets in 2005. Approximately $3.7 million of the impairment charges related to our prepaid royalty to Abbott Laboratories related to the µARCS screening technology. In connection with the restructuring of our chemistry operations we decided to discontinue the commercialization of the µARCS screening technology. We considered all available evidence and determined that no further benefit would be realized by use of this asset in current revenue generating or operating activities nor would any future cash flows be generated by use of this asset. $1.0 million of the impairment charges relate to patent rights to a proprietary gene profiling system that is licensed and which enables us to offer toxicology research products and services. The loss of a customer required the reevaluation of the recoverability of the gross carrying value of the asset. We considered all available evidence and developed estimates based on historical rates of attrition of the customer base, future cash generating capacity and future expenditures necessary to maintain the asset. We utilized an expected present value technique, in which a series of cash flow scenarios that reflect the range of possible outcomes are discounted, to estimate the fair value of the asset. If we are not successful in generating sufficient revenues in the future from this asset, we may be required to record additional impairment charges up to $718,000. The restructuring of our chemistry operations in South San Francisco and anticipated expiration of our chemistry collaboration with Pfizer required the reevaluation of the recoverability of the gross carrying value of the long-lived assets used in this facility. We determined the carrying value (after consideration of the change in estimated useful lives discussed above) was recoverable through the first quarter of 2006 and no additional impairment charges were required at December 31, 2005. We have
46
begun the transition to integrated service offerings. In addition, we anticipate continued price pressures on our screening service offerings. If we are unsuccessful in penetrating the market under our new business model and if we continue to incur losses, we may be required to re-estimate the useful lives of our remaining long-lived assets. As of December 31, 2005, property, plant and equipment, net totaled approximately $8.0 million.
Restructuring expenses. In the fourth quarter of 2005, discussions with Pfizer regarding a new collaboration to extend our services in the design and development of compounds exclusively for Pfizer were ended. In the absence of a new contract with Pfizer, we began the process of reducing our combinatorial chemistry and library synthesis operational capacity in a restructuring of our South San Francisco facility. In the fourth quarter of 2005 we recorded a $928,000 charge for restructuring activities resulting from this decision, which consisted of accrued one-time termination benefits. Restructuring expenses related to the closure of our Tucson facility were $112,000 in 2005 and $1.9 million in 2003 consisting of moving, relocation and other costs. We expect to incur approximately $1.6 million in additional restructuring charges in 2006, subject to changes in actual sublease income on the excess facility and actual termination benefits paid. We do not expect to incur any additional restructuring charges related to the Tucson closure.
Interest income, net of interest expense. We realized $2.0 million in net interest income in 2005, compared to net interest income of approximately $1.4 million in 2004 and $1.8 million in 2003. The increase in net interest income in 2005 compared to 2004 is due primarily to higher yields on short-term investments in 2005. The decrease in 2004 compared to 2003 is primarily due to lower yields and losses realized in 2004.
Foreign currency transaction gains and losses. We realized approximately $39,000 in foreign currency transaction gains in 2005, compared to losses of $265,000 in 2004 and $13,000 in 2003. The prior year loss is primarily a result of the completion of two significant contracts performed by our Swiss-based subsidiary which were denominated in U.S. dollars where no such significant settlements occurred in 2005 and 2003.
Discontinued operations. In October 2005, we sold the assets related to the IRORI® chemical synthesis, Crystal Farm® automated protein crystallization, and Universal Store™
47
compound storage system product lines for $1.9 million, resulting in a net gain on sale of $394,000. The results of discontinued operations presented in 2004 and 2003 relate to the assets sold in 2005.
Income taxes. At December 31, 2005, we had federal and California income tax net operating loss carryforwards of approximately $28.9 million and $14.7 million, respectively. In addition, we had foreign tax net operating loss carryforwards of approximately $9.93 million which will begin to expire in 2008. The difference between the federal and California net tax operating loss carryforwards is primarily attributable to the capitalization of research and development expenses and the percentage limitation on the carryover of net operating losses for California income tax purposes. The federal tax loss carryforwards will begin to expire in 2010 unless previously utilized. The California tax loss carryforwards will continue to expire in 2006. We also have federal and California research tax credit carryforwards of approximately $2.7 million and $1.4 million, respectively. The federal research tax credit carryforwards will begin to expire in 2011 unless previously utilized. The California research tax credits will carry forward indefinitely. Pursuant to Internal Revenue Code Sections 382 and 383, use of our net operating loss and credit carryforwards may be limited because of a cumulative change in ownership of more than 50%, which may have occurred for tax purposes. As of December 31, 2005, we had approximately $30.2 million in tax-deductible goodwill and other intangibles related to the purchase of Axys Advanced Technologies in April 2000. The majority of this amount is amortized over a 15-year period for tax purposes. We have provided a 100% valuation allowance against the related deferred tax assets as realization of such tax benefits is uncertain.
Inflation. We do not believe that inflation has had a material impact on our business or operating results during the periods presented.
Recent events. As we transition our strategic initiatives and reorganize our operational capacity, it is uncertain at this time whether we will be successful in entering into any collaborative arrangements in sufficient amounts to absorb the current operating capacity levels. In addition, the majority of our operating costs are fixed in nature. Accordingly, if revenues continue to decline as anticipated, we may not be able to correspondingly reduce our operating expenses, which would negatively impact our future operating results for a particular fiscal period. We expect to continue to incur losses in 2006.
Given the change in our business strategy, we believe our historical operating results are not indicative of future results. Results may fluctuate significantly from quarter to quarter due to the possibility of fluctuations in revenues as well as other factors, many of which are outside of our control, such as, customers’ budgetary constraints. Consequently, we believe that quarter-to-quarter comparisons of our operating results are not a good indication of our future performance. In addition, as we consider strategic opportunities, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results based on our past operational contract services model are not indicative of future results.
Liquidity and Capital Resources
Since our inception, we have funded our operations with $39.0 million of private equity financings and $94.7 million of net proceeds from our initial public offering in July 2000.
48
In May 2004 our secondary public offering was declared effective by the SEC. A total of 8,305,300 shares of common stock at a price of $5.00 per share were made available to the public. Axys Pharmaceuticals, Inc., then a stockholder of Discovery Partners International, Inc., registered 7,222,000 shares for resale, with the remaining 1,083,300 shares registered for sale by the Company to the underwriters to cover over-allotments. We received proceeds from the offering, of the shares registered for sale by the Company, of $5.1 million net of underwriters discounts.
At December 31, 2005, cash and cash equivalents and short-term investments totaled approximately $83.5 million, compared to $80.0 million at December 31, 2004 and $72.6 million at December 31, 2003.
We are currently exploring a range of options to best deploy our resources and improve stockholder value in light of the changes in our business and an imperative from our Board of Directors to make best use of our current financial and scientific assets to accelerate our entry into more substantial value-creating activities. The options we are considering include divestiture of assets and merger or acquisition opportunities that are specifically identified to create or enhance a product portfolio with defined risk and timelines to milestones, which may involve a change in control of our company. As we transition our strategic initiatives and reorganize our operational capacity, the trends and risks that apply to our business will change from those that are described in this Annual Report on Form 10-K based on our business to date, and we believe our historical operating results are not indicative of future results. We cannot assure that we will be successful in accomplishing any of these strategic initiatives or that any strategic transaction that may occur will be accomplished on favorable terms.
In the event that we divest the various operating assets of the company, it is possible that we may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that are reflected on our consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of our long-lived assets. In the event that we engage in a merger or acquisition transaction, it is possible that the value realized by our shareholders in such a transaction might be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005, due to the fact that our market capitalization is significantly below the book value of the our shareholders’ equity. Lastly, in the event that we are unsuccessful with the divestiture of our assets or are unable to successfully conclude any merger or acquisition activity, it is possible that our Board of Directors could decide to liquidate all of our assets, in which event the value realized by our shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on our consolidated financial statements as of December 31, 2005.
Operating Activities. We rely on cash on hand and cash flows from operations to provide working capital for current and future operations. We believe we have sufficient cash resources to fund existing operations through 2006. Cash flows provided by operating activities for continuing operations totaled $5.5 million in 2005 compared to $3.7 million in 2004 and $7.8 million in 2003. The increase in operating cash flows in 2005 compared to 2004 was primarily due to the impact of a reduction in working capital requirements, caused by lower revenue volumes, and the impact of non-cash adjustments related to asset impairment and restructuring charges which more than offset the impact of negative operating results for the year. The decrease in operating cash flows from 2004 compared to 2003 is primarily due to a significant decrease in prepayments received from our customers and a significant increase in 2003 incentives paid to key employees in the first quarter of 2004 offset partially by improved operating results, a decrease in inventory and a decrease in payments made against the restructuring accrual. We currently expect an operating loss in 2006, which would negatively impact our cash flows from operations in the future.
49
Additionally, on November 29, 2005 we announced the restructuring of our South San Francisco facility. The restructuring plan consisted of a reduction in workforce and the consolidation of excess facilities. As of December 31, 2005, we have incurred $928,000 of charges relating to the benefits payable to terminated employees. We anticipate incurring an additional $455,000 of termination benefits and $1.1 million in lease obligations in the first quarter of 2006. Amounts are subject to changes based on actual terminations and actual sublease income.
Investing Activities. Cash provided by investing activities from continuing operations totaled $4.8 million in 2005 compared to cash used of $5.2 million in 2004 and $8.4 million in 2003. The increase in cash available from investing activities is primarily a result of a decrease in the investment of short-term marketable securities as we redirected more cash resources to highly liquid investments, reflected as cash equivalents. The decrease in cash used in investing activities in 2004 compared to 2003 is due primarily to a $2.0 million royalty prepayment made in the first quarter of 2003 as required under our exclusive µARCS license agreement with Abbott Laboratories, which was fully impaired at December 2005. No additional prepayments are required under this agreement. The primary objective for our investment portfolio is to preserve principal while maintaining adequate liquidity to meet projected cash requirements. A secondary objective is to achieve a yield on investments commensurate with the risk levels associated with the primary objective.
We currently anticipate utilizing approximately $3.0 million and $4.0 million to pursue strategic initiatives that contemplate merger or acquisition opportunities that consist of consulting fees, key employee retention benefits and legal and accounting support. We currently anticipate investing approximately $1.0 million to $1.4 million in 2006 for leasehold improvements and capital equipment necessary to support the NIH contract and approximately $1.0 million to $1.5 million in 2006 to support all other continuing operations. Our actual future capital requirements will depend on a number of factors, including our ability to enter into long-term collaboration arrangements, expenses associated with unforeseen litigation, regulatory changes, competition and technological developments, and potential future merger and acquisition activity as well as research and development spending.
Financing Activities. Cash provided by financing activities totaled $129,000 in 2005 compared to $5.6 million in 2004 and cash used in financing activities totaling $634,000 in 2003. This change is primarily due to the sale of approximately 1.1 million shares of our common stock generating $5.1 million in net proceeds during the second quarter of 2004. Historically, we had debt obligations under lease and line of credit agreements. Net payments made under these agreements totaled $245,000 in 2005 and $1.1 million in 2003. As of December 31, 2005, we have no debt obligations. Absent any significant merger and acquisition activity, we do not expect to incur debt in 2006.
On October 4, 2001, our board of directors approved a Stock Repurchase Plan, authorizing us to repurchase up to 2,000,000 shares of common stock at no more than $3.50 per share. In 2003, we purchased 115,000 shares under this Plan for $289,000. We did not purchase any shares in 2005 or 2004 pursuant to this Plan. We continue to have the authority to purchase additional shares in the future.
50
Contractual Obligations
We have entered into various agreements that obligate us to make future payments. The table below sets forth the contractual cash obligations that exist as of December 31, 2005:
| | Payments Due by Period | |
Contractual Obligations | | Total | | Less than 1Year | | 1-3 Years | | 4-5 Years | | More than
5 Years | |
Minimum license fees (A) | | $ | 90,000 | | $ | 15,000 | | $ | 25,000 | | $ | 20,000 | | $ | 30,000 | |
Firm purchase orders | | 2,012,913 | | 2,008,812 | | 4,101 | | — | | — | |
Operating leases | | 14,378,234 | | 3,433,276 | | 6,567,258 | | 2,260,415 | | 2,117,285 | |
Other contractual commitments | | 280,000 | | 280,000 | | — | | — | | — | |
Total contractual cash obligations | | $ | 16,761,147 | | $ | 5,737,088 | | $ | 6,596,359 | | $ | 2,280,415 | | $ | 2,147,285 | |
(A) The terms of the license agreements generally range from the remaining life of the patent up to 25 years.
We do not have any off-balance sheet arrangements.
We have entered into employment agreements with key executives that provide for the continuation of salary if terminated for reasons other than cause, as defined in those agreements. The future employment contract commitments for such key executives totaled approximately $500,000 for the fiscal year ended December 31, 2005 and none for years thereafter.
Recent Accounting Pronouncements
In November 2005, the FASB issued SFAS 115-1 and SFAS 124-1 relating to the determination and measurement of other-than-temporary losses for investments. The guidance in these statements shall be applied to reporting periods beginning after December 15, 2005 and earlier application is permitted. We do not believe adopting this statement will have a material impact on our financial condition or results of operation. There are no investments held at December 31, 2005, which are considered to be temporarily or other-than-temporarily impaired beyond 12 months. We will record an impairment charge if securities held continue to be impaired beyond twelve months or if other factors indicate there is permanent impairment. We regularly monitor and evaluate the realizable value of our marketable securities. When assessing marketable securities for other-than-temporary declines in value, we consider such factors as, among other things, how significant the decline in value is as a percentage of the original cost, how long the market value of the investment has been less than its original cost and the market in general. We believe that the decline in the value of our marketable securities is temporary and related to the change in market interest rates since purchase. The decline is not related to any company or industry specific event, and all portfolio investments are investment grade quality. We anticipate full recovery of amortized cost with respect to these securities at maturity or sooner in the event of a change in the market interest rate environment.
In March 2005, the SEC released Staff Accounting Bulletin (SAB) No. 107, Share-Based Payment (SAB 107). SAB 107 provides the SEC staff position regarding the application of SFAS No. 123R, Share-Based Payment. SAB 107 contains interpretive guidance related to the interaction between SFAS No. 123R and certain SEC rules and regulations, as well as provides the Staff’s views regarding the valuation of share-based payment arrangements for public companies. SAB 107 also highlights the importance of disclosures made related to the accounting for share-based payment transactions. We are currently reviewing the effect of SAB 107 on our condensed consolidated financial statements as we prepare to adopt SFAS No. 123R.
In December 2004, the Financial Accounting Standards Board (FASB) issued SFAS No. 123R (revised 2004), Shared-Based Payment, which is a revision of SFAS No. 123, Accounting for Stock-Based Compensation. SFAS No. 123R supersedes APB Opinion No. 25 (Opinion No.25), Accounting for Stock Issued to Employees, and amends SFAS No. 95, Statement of Cash Flows. Generally, the approach to accounting for share-based payments in SFAS No. 123R is similar to the approach described in SFAS No. 123. However, SFAS No. 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statement based on their fair values. In April 2005, the SEC delayed implementation of SFAS No.123R for publicly traded companies such that they must apply this Standard as of the beginning of the next fiscal year that begins after December 16, 2005. SFAS No. 123R permits public companies to adopt its requirements using one of two methods:
1. A “modified prospective” method in which compensation cost is recognized beginning with the effective date (a) based on the requirements of SFAS No. 123R for all share-based payments granted after the effective date and (b) based on the requirements of SFAS No. 123 for all awards granted to employees prior to the effective date of SFAS 123R that remain unvested on the effective date.
51
2. A “modified retrospective” method which includes the requirements of the modified prospective method described above, but also permits entities to restate based on the amounts previously recognized under SFAS No. 123 for purposes of pro forma disclosures either (a) all prior periods presented or (b) prior interim periods of the year of adoption.
We plan to adopt SFAS No. 123R using the modified-prospective method, which will impact all periods beginning after December 31, 2005. As permitted by SFAS No. 123, we currently account for share-based payments to employees using Opinion No. 25’s intrinsic value method and, as such, generally do not recognize compensation cost for employee stock options. Accordingly, the adoption of SFAS No. 123R’s fair value method will have a significant impact on our future results from operations, although it will have no impact on our overall financial position. As a result of the anticipated adoption of SFAS No. 123R, the Compensation Committee of the Board of Directors approved the acceleration of vesting on stock options with exercise prices of $5.75 or more effective February 21, 2005, which did not result in an accounting charge under the Opinion 25 intrinsic value method. We have not determined the impact of adoption of SFAS No. 123R since it will depend on levels of share-based payments granted in the future. SFAS No. 123R also requires the benefits of tax deductions in excess of recognized compensation cost to be reported as a financing cash flow, rather than as an operating cash flow as required under current literature. This requirement will reduce net operating cash flows and increase net financing cash flows in periods after adoption. While we cannot estimate what those amounts will be in the future (because they depend on, among other things, when employees exercise stock options), we have not recognized excess tax deductions historically due to our accumulated loss position.
52
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
Short-term investments. Our interest income is sensitive to changes in the general level of U.S. interest rates, particularly since a significant portion of our investments are and will be in short-term marketable securities, U.S. government securities, asset-backed securities and corporate bonds. Due to the nature and maturity of our short-term investments, we have concluded that there is no material market risk exposure to our principal. The average maturity of our investment portfolio is six months. A 1% change in interest rates would have an effect of approximately $432,000 on the value of our portfolio.
Foreign currency rate fluctuations. The functional currency for our Discovery Partners International AG (DPI AG) group is the Swiss franc. The financial statements of Discovery Partners International GmbH (DPI GmbH), which is wholly-owned by DPI AG, are re-measured using the Swiss Franc as the functional currency, after transacting in its local currency, the Euro. The financial statements of DPI GmbH are consolidated with the DPI AG financials. DPI AG accounts are translated from their local currency to the U.S. dollar using the current exchange rate in effect at the balance sheet date for the balance sheet accounts, and using the average exchange rate during the period for revenues and expense accounts. The effects of translation for our consolidated DPI AG group are recorded as a separate component of stockholders’ equity (accumulated other comprehensive income (loss)). DPI AG conducts its business with customers in local currencies. Exchange gains and losses arising from these transactions are recorded using the actual exchange differences on the date the transaction is settled.
The financial statements of DPI LLC are remeasured from the local currency, the Japanese Yen, to its functional currecy, the U.S dollar. Exchange gains and losses arising from transactions are recorded using the actual exchange differences on the date the transaction is settled.
We have not in the past taken any action to reduce our exposure to changes in foreign currency exchange rates, such as options or futures contracts, with respect to transactions with DPI AG, DPI GmbH and DPI LLC or transactions with our worldwide customers, but anticipate that we could begin to hedge against foreign exchange transaction gains and losses resulting from non-Swiss franc invoices issued to customers by DPI AG in the future. A 10% change in the value of the Swiss franc, Euro and Japanese Yen, collectively, relative to the U.S. dollar throughout 2005 would have resulted in a 2% change in revenue for the year ended December 31, 2005.
Item 8. Financial Statements and Supplementary Data.
Our financial statements appear in a separate section of this Annual Report on Form 10-K beginning on page F-1.
53
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
During our two most recent fiscal years and since then through today, we have not had a change in our independent auditors nor have there been any reportable disagreements between us and our independent auditors.
Item 9A. Controls and Procedures.
Changes in Internal Control Over Financial Reporting
There have been no significant changes in our internal control over financial reporting during the fourth quarter ended December 31, 2005 that have materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of our disclosure controls and procedures, as such term is defined under Rule 13a-15(e) promulgated under the Securities Exchange Act of 1934, as amended (the Exchange Act). Based on this evaluation, our principal executive officer and our principal financial officer concluded that our disclosure controls and procedures were designed and operating effectively as of the end of the period covered by this Annual Report on Form 10-K.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2005 and no material weaknesses have been identified.
Our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2005 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their attestation report which follows this report herein.
54
Report of Independent Registered Public Accounting Firm
To the Shareholders and the
Board of Directors of Discovery Partners International, Inc.
We have audited management’s assessment, included in the accompanying Management’s Report on Internal Control over Financial Reporting, that Discovery Partners International, Inc. (the “Company”) maintained effective internal control over financial reporting as of December 31, 2005, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the “COSO criteria”). The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment about the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that the Company maintained effective internal control over financial reporting as of December 31, 2005, is fairly stated, in all material respects, based on the COSO criteria. Also, in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2005, based on the COSO criteria.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of the Company as of December 31, 2005 and 2004 and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2005, and our report dated March 10, 2006 expressed an unqualified opinion thereon.
San Diego, California
March 10, 2006
Item 9B. Other Information.
None.
55
PART III
Item 10. Directors and Executive Officers of the Registrant.
The sections titled “Directors and Nominees”, “Board Meetings and Committees,” “Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance” appearing in the definitive Proxy Statement which we will file related to the Annual Meeting of Stockholders to be held May 11, 2006 are incorporated herein by reference.
Code of Ethics
We have adopted a Code of Business Conduct that applies to all officers, directors and employees. The Code of Business Conduct is filed herewith as Exhibit 14. If we make any substantive amendments to the Code of Business Conduct or grant any waiver from a provision of the code to any executive officer or director, we will promptly disclose the nature of the amendment or waiver on our website at www.discoverypartners.com.
Item 11. Executive Compensation.
The section titled “Executive Compensation and Other Information” appearing in the definitive Proxy Statement which we will file related to the Annual Meeting of Stockholders to be held May 11, 2006 is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The section titled “Principal Stockholders and Security Ownership of Directors and Management” appearing in the definitive Proxy Statement which we will file related to the Annual Meeting of Stockholders to be held May 11, 2006 is incorporated herein by reference.
See Item 5 of Part II of this Form 10-K for the “Equity Compensation Plan Information”.
Item 13. Certain Relationships and Related Transactions.
The section titled “Certain Transactions” appearing in the definitive Proxy Statement which we will file for the Annual Meeting of Stockholders to be held May 11, 2006 is incorporated herein by reference.
Item 14. Principal Accounting Fees and Services.
The section titled “Ratification of Independent Registered Public Accounting Firm” appearing in the definitive Proxy Statement which we will file for the Annual Meeting of the Stockholders to be held May 11, 2006 is incorporated herein by reference.
56
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a) Documents filed as part of this report:
(1) Financial Statements:
The following financial statements of Discovery Partners International, Inc. are included in a separate section of this Annual Report on Form 10-K commencing on the pages referenced below:
| Page |
Consolidated Financial Statements of Discovery Partners International, Inc. | |
Report of Independent Registered Public Accounting Firm | F-2 |
Consolidated Balance Sheets as of December 31, 2005 and 2004 | F-3 |
Consolidated Statements of Operations for the Years Ended December 31, 2005, 2004 and 2003 | F-4 |
Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2005, 2004 and 2003 | F-5 |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2005, 2004 and 2003 | F-6 |
Notes to the Consolidated Financial Statements | F-7 |
(2) Financial Statement Schedules:
All schedules have been omitted, since they are not applicable or not required, or the relevant information is included in the consolidated financial statements or the notes thereto.
(3) Exhibits:
Exhibit
Number | | Title | | Method of Filing |
2.1 | | Business Transfer Agreement, dated April 22, 2005, between Biofrontera Discovery GmbH and Discovery Partners International GmbH | | Incorporated by Reference to Exhibit 2.1 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 9, 2005 |
| | | | |
3.1 | | Certificate of Incorporation of the Company | | Incorporated by Reference to Exhibit 3.2 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on June 23, 2000 |
| | | | |
3.2 | | Bylaws of the Company | | Incorporated by Reference to Exhibit 3.4 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on June 23, 2000 |
57
4.2 | | Rights Agreement between the Company and American Stock Transfer & Trust Company, which includes the form of Certificate of Designation for the Series A junior participating preferred stock as Exhibit A, the form of Rights Certificate as Exhibit B and the Summary of Rights to Purchase Series A Preferred Stock as Exhibit C, dated as of February 13, 2003. | | Incorporated by Reference to Exhibit 4.2 to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission on February 24, 2003 |
| | | | |
10.1 | | Second Amended and Restated Investors’ Rights Agreement among the Company and the investors listed on Schedule A thereto, dated April 28, 2000, as amended. | | Incorporated by Reference to Exhibit 10.2 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on July 26, 2000. |
| | | | |
10.2 | | Patent License Agreement between the Company and Abbott Labs, Incorporated, dated January 2, 2001. | | Incorporated by Reference to Exhibit 10.22 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 27, 2001 |
| | | | |
10.3 | | Leasehold Contract between Swiss Accident Insurance Agency (formerly Basler Kantonalbank) and Discovery Technologies, Ltd., dated June 18, 1997. | | Incorporated by Reference to Exhibit 10.47 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on June 23, 2000 |
| | | | |
10.4* | | Key Employment Agreement between the Company and Riccardo Pigliucci, dated April 17, 1998. | | Incorporated by Reference to Exhibit 10.51 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on May 9, 2000 |
| | | | |
10.5* | | 2000 Stock Incentive Plan. | | Incorporated by Reference to Exhibit 10.59 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on July 21, 2000 |
| | | | |
10.6* | | 2000 Stock Incentive Plan, Form of Notice of Grant. | | Incorporated by Reference to Exhibit 10.44 to the Company’s |
58
| | | | Form 10-K filed with the Securities and Exchange Commission on March 27, 2001 |
| | | | |
10.7* | | 2000 Stock Incentive Plan, Form of Stock Option Agreement. | | Incorporated by Reference to Exhibit 10.45 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 27, 2001 |
| | | | |
10.8* | | 2000 Stock Incentive Plan, Form of Stock Issuance Agreement. | | Incorporated by Reference to Exhibit 10.46 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 27, 2001 |
| | | | |
10.9* | | 2000 Employee Stock Purchase Plan. | | Incorporated by Reference to Exhibit 10.60 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on July 21, 2000 |
| | | | |
10.10* | | 2000 Employee Stock Purchase Plan, Form of Stock Purchase Agreement | | Incorporated by Reference to Exhibit 10.48 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 27, 2001 |
| | | | |
10.11* | | Form of Indemnification Agreement between the Company and each of its directors and officers. | | Incorporated by Reference to Exhibit 10.61 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on June 23, 2000 |
| | | | |
10.12 | | Leasehold Contract between Swiss Accident Insurance Agency (formerly Basler Kantonalbank) and Discovery Partners Technologies, Ltd., dated January 31, 2000. | | Incorporated by Reference to Exhibit 10.63 to the Company’s Registration Statement No. 333-36638 on Form S-1 filed with the Securities and Exchange Commission on June 23, 2000 |
| | | | |
10.13* | | Offer letter between the Company and Craig Kussman, dated October 29, 2001 | | Incorporated by Reference to Exhibit 10.55 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 29, 2002 |
| | | | |
10.14† | | Protocol Development and Compound Production Agreement between the Company and Pfizer Inc., dated December 19, 2001. | | Incorporated by Reference to Exhibit 10.56 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 29, 2002 |
| | | | |
10.15* | | Offer letter between the Company and Taylor J. Crouch, dated June 18, 2002. | | Incorporated by Reference to Exhibit 10.57 to the Company’s Quarterly Report on Form 10-Q filed |
59
| | | | with the Securities and Exchange Commission on August 9, 2002 |
| | | | |
10.16* | | Promissory Note issued by Taylor J. Crouch, dated July 29, 2002. | | Incorporated by Reference to Exhibit 10.58 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 9, 2002 |
| | | | |
10.17† | | Amendment No. 1 to the 2001 Agreement between the Company and Pfizer Inc. effective May 15, 2002. | | Incorporated by Reference to Exhibit 10.59 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2002 |
| | | | |
10.18† | | Amendment No. 2 to the 2001 Agreement between the Company and Pfizer Inc. amended August 13, 2002. | | Incorporated by Reference to Exhibit 10.60 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2002 |
| | | | |
10.19* | | Offer letter between the Company and Douglas A. Livingston, dated November 13, 2002 | | Incorporated by Reference to Exhibit 10.61 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 21, 2003 |
| | | | |
10.20† | | Amendment No. 3 to the 2001 Agreement between the Company and Pfizer Inc. amended December 12, 2002. | | Incorporated by Reference to Exhibit 10.62 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 21, 2003 |
| | | | |
10.21* | | Amendment No. 1 to Notice of Grant of Stock Option between the Company and Craig Kussman, dated January 24, 2003 | | Incorporated by Reference to Exhibit 10.63 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 21, 2003 |
| | | | |
10.22* | | Employment Agreement between Discovery Technologies Ltd (since renamed Discovery Partners International AG) and Urs Regenass dated January 20, 2001 | | Incorporated by Reference to Exhibit 10.70 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on April 30, 2003 |
| | | | |
10.23* | | Change in Control Agreement between the Company and Riccardo Pigliucci, dated August 8, 2003 | | Incorporated by Reference to Exhibit 10.71 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.24* | | Change in Control Agreement between the Company and Craig Kussman, dated August 8, 2003 | | Incorporated by Reference to Exhibit 10.72 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.25* | | Change in Control Agreement between the Company and Taylor | | Incorporated by Reference to Exhibit 10.73 to the Company’s |
60
| | Crouch, dated August 8, 2003 | | Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.26* | | Change in Control Agreement between the Company and John Lillig, dated August 8, 2003 | | Incorporated by Reference to Exhibit 10.74 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.27* | | Change in Control Agreement between the Company and Urs Regenass, dated August 8, 2003 | | Incorporated by Reference to Exhibit 10.75 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.28* | | Change in Control Agreement between the Company and Richard Neale, dated August 8, 2003 | | Incorporated by Reference to Exhibit 10.76 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.29* | | Change in Control Agreement between the Company and Douglas Livingston, dated August 8, 2003 | | Incorporated by Reference to Exhibit 10.77 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 13, 2003 |
| | | | |
10.30† | | Worldwide Distribution and Strategic Alliance Agreement between the Company and Bruker AXS Inc., dated July 24, 2003 | | Incorporated by Reference to Exhibit 10.78 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 14, 2003 |
| | | | |
10.31 | | Industrial Lease between ChemRx Advanced Technologies, Inc. and Shelton International Holdings, Inc. | | Incorporated by Reference to Exhibit 10.79 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 10, 2004 |
| | | | |
10.32† | | Chemistry Products and Services Agreement between the Company and Pfizer Inc., dated February 13, 2004. | | Incorporated by Reference to Exhibit 10.80 to the Company’s Form 10-K filed with the Securities and Exchange Commission on March 10, 2004 |
| | | | |
10.33† | | Agreement between the Company and National Institutes of Mental Health, dated August 20, 2004 | | Incorporated by Reference to Exhibit 10.81 to the Company’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on November 9, 2004 |
| | | | |
10.34* | | Separation Agreement between the Company and Taylor Crouch, dated January 18, 2005 | | Incorporated by reference to Exhibit 10.82 to the Company’s Annual Report of Form 10-K filed with the Securities and Exchange Commission on March 10, 2005 |
| | | | |
10.35* | | Employment Agreement between | | Incorporated by Reference to the |
61
| | Discovery Partners International, Inc. and Michael C. Venuti, Ph.D., dated March 31, 2005 | | Company’s Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2005 |
| | | | |
10.36* | | Form of Stock Issuance Grant Notice | | Incorporated by Reference to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2005 |
| | | | |
10.37* | | Second Form of Stock Issuance Agreement | | Incorporated by Reference to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2005 |
| | | | |
10.38* | | Change in Control Agreement between the Company and Michael C. Venuti, Ph.D., dated March 31, 2005 | | Incorporated by Reference to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission on April 6, 2005 |
| | | | |
10.39* | | Separation Agreement between the Company and Riccardo Pigliucci, dated November 11, 2005 | | Incorporated by Reference to the Company’s Report on Form 8-K filed with the Securities and Exchange Commission on November 14, 2005 |
| | | | |
10.40 | | Asset Purchase Agreement between the Company and Irori Discovery, Inc., dated October 7, 2005 | | Filed Herewith |
| | | | |
14 | | Code of Business Conduct | | Incorporated by reference to Exhibit 14 to the Company’s Annual Report of Form 10-K filed with the Securities and Exchange Commission on March 10, 2005 |
| | | | |
21.1 | | Subsidiaries of the Registrant | | Filed Herewith |
| | | | |
23.1 | | Consent of Independent Registered Public Accounting Firm | | Filed Herewith |
| | | | |
31.1 | | Certification of the Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | Filed Herewith |
| | | | |
31.2 | | Certification of the Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) of the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 | | Filed Herewith |
| | | | |
32.1 | | Certification of the Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | Filed Herewith |
| | | | |
32.2 | | Certification of the Chief Financial | | Filed Herewith |
62
| | Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | | |
† Certain confidential portions of this Exhibit were omitted by means of redacting a portion of the text (the “Mark”). This Exhibit has been filed separately with the Secretary of the Commission without the Mark pursuant to the Company’s Application Requesting Confidential Treatment under Rule 24b-2 under the Securities Exchange Act of 1934.
* Indicates management contract or compensatory plan or arrangement.
63
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| DISCOVERY PARTNERS INTERNATIONAL, INC. |
| | |
| | |
| By: | /s/ MICHAEL C. VENUTI | |
| | Michael C. Venuti
Acting Chief Executive Officer |
Date: March 15, 2006
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | | Title | | Date |
| | | | |
/s/ MICHAEL C. VENUTI | | Acting Chief Executive Officer and Director | | March 15, 2006 |
Michael C. Venuti | | (Principal Executive Officer) | | |
| | | | |
/s/ CRAIG KUSSMAN | | Chief Financial Officer | | March 15, 2006 |
Craig Kussman | | (Principal Financial Officer and Principal
Accounting Officer) | | |
| | | | |
/s/ HARRY F. HIXSON, JR. | | Chairman and Director | | March 15, 2006 |
Harry F. Hixson, Jr. | | | | |
| | | | |
/s/ ALAN LEWIS | | Director | | March 15, 2006 |
Alan Lewis | | | | |
| | | | |
/s/ COLIN T. DOLLERY | | Director | | March 15, 2006 |
Colin T. Dollery | | | | |
| | | | |
/s/ HERM ROSENMAN | | Director | | March 15, 2006 |
Herm Rosenman | | | | |
64
DISCOVERY PARTNERS INTERNATIONAL, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page |
Report of Independent Registered Public Accounting Firm | F-2 |
Consolidated Balance Sheets as of December 31, 2005 and 2004 | F-3 |
Consolidated Statements of Operations for the Years Ended December 31, 2005, 2004 and 2003 | F-4 |
Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2005, 2004 and 2003 | F-5 |
Consolidated Statements of Cash Flows for the Years Ended December 31, 2005, 2004 and 2003 | F-6 |
Notes to Consolidated Financial Statements | F-7 |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
Discovery Partners International, Inc.
We have audited the accompanying consolidated balance sheets of Discovery Partners International, Inc. as of December 31, 2005 and 2004, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2005. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Discovery Partners International, Inc. at December 31, 2005 and 2004, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2005, in conformity with accounting principles generally accepted in the United States.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of Discovery Partners International, Inc.’s internal control over financial reporting as of December 31, 2005, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 10, 2006 expressed an unqualified opinion thereon.
San Diego, California
March 10, 2006
F-2
DISCOVERY PARTNERS INTERNATIONAL, INC.
CONSOLIDATED BALANCE SHEETS
| | December 31, | |
| | 2005 | | 2004 | |
ASSETS | | | | | |
Current assets: | | | | | |
Cash and cash equivalents | | $ | 24,231,257 | | $ | 13,148,242 | |
Short-term investments | | 59,254,873 | | 66,870,268 | |
Accounts receivable, net | | 5,673,509 | | 12,786,101 | |
Inventories, net | | 578,842 | | 2,390,608 | |
Prepaid expenses | | 1,734,030 | | 1,748,423 | |
Other current assets | | 961,715 | | 995,792 | |
Assets of discontinued operations | | — | | 2,508,920 | |
Total current assets | | 92,434,226 | | 100,448,354 | |
Restricted cash | | 1,060,753 | | 1,120,050 | |
Property and equipment, net | | 7,950,765 | | 7,095,978 | |
Prepaid royalty, net | | — | | 4,827,715 | |
Patent and license rights, net | | 717,707 | | 1,890,924 | |
Other assets, net | | 116,230 | | 259,720 | |
Total assets | | $ | 102,279,681 | | $ | 115,642,741 | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | |
Current liabilities: | | | | | |
Accounts payable and accrued expenses | | $ | 2,093,095 | | $ | 2,639,798 | |
Restructuring accrual | | 927,890 | | 293,929 | |
Accrued compensation | | 1,298,425 | | 2,296,206 | |
Deferred revenue | | 2,357,915 | | 884,734 | |
Liabilities of discontinued operations | | — | | 965,832 | |
Total current liabilities | | 6,677,325 | | 7,080,499 | |
Deferred rent | | 420,067 | | 155,159 | |
Other long-term liabilities | | 108,000 | | — | |
Total liabilities | | 7,205,392 | | 7,235,658 | |
Stockholders’ equity: | | | | | |
Preferred stock, $.001 par value, 1,000,000 shares authorized, no shares issued and outstanding at December 31, 2005 and 2004 | | — | | — | |
Common stock, $.001 par value, 100,000,000 shares authorized, 26,441,902 and 26,117,509 issued and outstanding at December 31, 2005 and 2004, respectively | | 26,442 | | 26,118 | |
Common stock issuable | | 1,596,500 | | 2,656,600 | |
Treasury stock, at cost, 306,933 and 228,702 shares at December 31, 2005 and 2004, respectively | | (1,037,190 | ) | (793,813 | ) |
Additional paid-in capital | | 209,237,267 | | 207,804,460 | |
Deferred compensation | | (919,217 | ) | (2,187,229 | ) |
Accumulated other comprehensive income | | 63,779 | | 629,502 | |
Accumulated deficit | | (113,893,292 | ) | (99,728,555 | ) |
Total stockholders’ equity | | 95,074,289 | | 108,407,083 | |
Total liabilities and stockholders’ equity | | $ | 102,279,681 | | $ | 115,642,741 | |
See accompanying notes
F-3
DISCOVERY PARTNERS INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
Revenues: | | | | | | | |
Services | | $ | 34,836,977 | | $ | 44,267,657 | | $ | 45,209,251 | |
Cost of revenues: | | | | | | | |
Services | | 25,107,968 | | 25,144,923 | | 27,615,269 | |
Gross margin | | 9,729,009 | | 19,122,734 | | 17,593,982 | |
Operating expenses: | | | | | | | |
Research and development | | 3,919,065 | | 1,546,091 | | 407,472 | |
Selling, general and administrative | | 15,066,812 | | 14,432,672 | | 13,341,645 | |
Amortization of stock-based compensation | | 1,017,370 | | 946,504 | | 501,499 | |
Impairment of long-lived assets | | 4,721,367 | | — | | — | |
Restructuring | | 1,040,258 | | — | | 1,872,986 | |
Total operating expenses | | 25,764,872 | | 16,925,267 | | 16,123,602 | |
Income (loss) from continuing operations | | (16,035,863 | ) | 2,197,467 | | 1,470,380 | |
Interest income | | 2,023,836 | | 1,424,860 | | 1,797,980 | |
Interest expense | | (4,808 | ) | (6,136 | ) | (40,745 | ) |
Foreign currency transaction gains (losses), net | | 38,978 | | (264,646 | ) | (12,803 | ) |
Other income, net | | 270,565 | | 89,219 | | 73,044 | |
Income (loss) from continuing operations before provision for income taxes | | (13,707,292 | ) | 3,440,764 | | 3,287,856 | |
Provision for income taxes | | 13,243 | | 55,584 | | 10,075 | |
Net income (loss) from continuing operations | | (13,720,535 | ) | 3,385,180 | | 3,277,781 | |
Discontinued operations: | | | | | | | |
Gain on sale from discontinued operations | | 393,899 | | — | | — | |
Gain (loss) from discontinued operations | | (838,101 | ) | 517,592 | | (2,218,868 | ) |
Net income (loss) | | $ | (14,164,737 | ) | $ | 3,902,772 | | $ | 1,058,913 | |
Basic and diluted: | | | | | | | |
Continuing operations | | $ | (0.53 | ) | $ | 0.13 | | $ | 0.13 | |
Discontinued operations | | (0.02 | ) | 0.02 | | (0.09 | ) |
Net income (loss) per share | | $ | (0.55 | ) | $ | 0.15 | | $ | 0.04 | |
Weighted average shares outstanding: | | | | | | | |
Basic | | 25,919,393 | | 25,318,937 | | 24,343,721 | |
Diluted | | 25,919,393 | | 26,271,625 | | 25,076,805 | |
See accompanying notes.
F-4
DISCOVERY PARTNERS INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| | CommonStock | | Common Stock | | Treasury Stock | | Additional | | Deferred | | Accumulated
Other
Comprehensive | | Accumulated | | Total
Stockholders’ | |
| | Shares | | Amount | | Issuable | | Shares | | Amount | | Paid in Capital | | Compensation | | Income (loss) | | Deficit | | Equity | |
Balance at December 31, 2002 | | 24,371,131 | | $ | 24,371 | | $ | — | | (35,000 | ) | $ | (119,250 | ) | $ | 200,691,363 | | $ | (260,226 | ) | $ | 885,485 | | $ | (104,690,240 | ) | $ | 96,531,503 | |
Exercise of options to purchase common stock | | 267,430 | | 267 | | — | | — | | — | | 597,713 | | — | | — | | — | | 597,980 | |
Amortization of stock-based compensation | | — | | — | | — | | — | | — | | — | | 514,929 | | — | | — | | 514,929 | |
Repurchase of company stock | | — | | — | | — | | (181,886 | ) | (656,201 | ) | — | | — | | — | | — | | (656,201 | ) |
Issuance of common stock | | 53,770 | | 54 | | — | | — | | — | | 113,270 | | — | | — | | — | | 113,324 | |
Issuance of restricted stock and rights to common stock | | 52,500 | | 53 | | 1,026,000 | | — | | — | | 283,447 | | (1,309,500 | ) | — | | — | | — | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | — | | — | | — | | — | | — | | — | | — | | 781,393 | | — | | 781,393 | |
Unrealized gain (loss) on investments | | — | | — | | — | | — | | — | | — | | — | | (694,908 | ) | — | | (694,908 | ) |
Net income | | — | | — | | — | | — | | — | | — | | — | | — | | 1,058,913 | | 1,058,913 | |
Comprehensive loss | | — | | — | | — | | — | | — | | — | | — | | — | | — | | 1,145,398 | |
Balance at December 31, 2003 | | 24,744,831 | | $ | 24,745 | | $ | 1,026,000 | | (216,886 | ) | $ | (775,451 | ) | $ | 201,685,793 | | $ | (1,054,797 | ) | $ | 971,970 | | $ | (103,631,327 | ) | $ | 98,246,933 | |
Exercise of options to purchase common stock | | 135,591 | | 136 | | — | | — | | — | | 375,830 | | — | | — | | — | | 375,966 | |
Amortization of stock-based compensation | | — | | — | | — | | — | | — | | 73,121 | | 942,915 | | — | | — | | 1,016,036 | |
Repurchase of company stock | | — | | — | | — | | (11,816 | ) | (18,362 | ) | (43,192 | ) | 28,653 | | — | | — | | (32,901 | ) |
Issuance of common stock | | 1,147,087 | | 1,147 | | — | | — | | — | | 5,239,598 | | — | | — | | — | | 5,240,745 | |
Issuance of restricted stock and rights to common stock | | 90,000 | | 90 | | 1,630,600 | | — | | — | | 473,310 | | (2,104,000 | ) | — | | — | | — | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | — | | — | | — | | — | | — | | — | | — | | 18,695 | | — | | 18,695 | |
Unrealized gain (loss) on investments | | — | | — | | — | | — | | — | | — | | — | | (361,163 | ) | — | | (361,163 | ) |
Net income | | — | | — | | — | | — | | — | | — | | — | | — | | 3,902,772 | | 3,902,772 | |
Comprehensive income | | — | | — | | — | | — | | — | | — | | — | | — | | — | | 3,560,304 | |
Balance at December 31, 2004 | | 26,117,509 | | $ | 26,118 | | $ | 2,656,600 | | (228,702 | ) | $ | (793,813 | ) | $ | 207,804,460 | | $ | (2,187,229 | ) | $ | 629,502 | | $ | (99,728,555 | ) | $ | 108,407,083 | |
Exercise of options to purchase common stock | | 200,310 | | 200 | | — | | — | | — | | 300,239 | | — | | — | | — | | 300,439 | |
Amortization of stock-based compensation | | — | | — | | — | | — | | — | | 26,582 | | 1,044,039 | | — | | — | | 1,070,621 | |
Repurchase of company stock | | — | | — | | — | | (33,231 | ) | (104,527 | ) | — | | — | | — | | — | | (104,527 | ) |
Issuance of common stock | | 52,583 | | 52 | | — | | — | | — | | 131,081 | | — | | — | | — | | 131,133 | |
Issuance of restricted stock and rights to common stock | | 71,500 | | 72 | | 257,900 | | — | | — | | 386,028 | | (644,000 | ) | — | | — | | — | |
Forfeiture of rights to common stock | | — | | — | | (1,318,000 | ) | (45,000 | ) | (138,850 | ) | 588,877 | | 867,973 | | — | | — | | — | |
Comprehensive income: | | | | | | | | | | | | | | | | | | | | | |
Foreign currency translation adjustment | | — | | — | | — | | — | | — | | — | | — | | (980,129 | ) | — | | (980,129 | ) |
Unrealized gain (loss) on investments | | — | | — | | — | | — | | — | | — | | — | | 414,406 | | — | | 414,406 | |
Net loss | | — | | — | | — | | — | | — | | — | | — | | — | | (14,164,737 | ) | (14,164,737 | ) |
Comprehensive income | | — | | — | | — | | — | | — | | — | | — | | — | | — | | (14,730,460 | ) |
Balance at December 31, 2005 | | 26,441,902 | | $ | 26,442 | | $ | 1,596,500 | | (306,933 | ) | $ | (1,037,190 | ) | $ | 209,237,267 | | $ | (919,217 | ) | $ | 63,779 | | $ | (113,893,292 | ) | $ | 95,074,289 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes.
F-5
DISCOVERY PARTNERS INTERNATIONAL, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
Operating activities | | | | | | | |
Net income (loss) | | $ | (14,164,737 | ) | $ | 3,902,772 | | $ | 1,058,913 | |
Adjustments to reconcile net income (loss) to cash provided by operating activities: | | | | | | | |
Depreciation and amortization | | 5,212,888 | | 5,439,567 | | 4,791,870 | |
Amortization of stock based compensation | | 1,017,370 | | 946,504 | | 501,499 | |
Impairment of long-lived assets | | 4,721,367 | | — | | — | |
Restructuring expense | | 1,040,258 | | — | | 1,872,986 | |
Gain on sale of discontinued operations | | (393,899 | ) | — | | — | |
Loss on disposal of fixed assets | | 112,943 | | 161,157 | | — | |
Realized loss on investments | | 133,810 | | 180,125 | | 21,177 | |
Change in operating assets and liabilities: | | | | | | | |
Accounts receivable | | 6,566,563 | | (2,905,908 | ) | (1,505,639) | |
Inventories | | 1,782,596 | | (115,887 | ) | 827,144 | |
Other current assets | | 7,115 | | (542,734 | ) | (224,384 | ) |
Accounts payable and accrued expenses | | (2,005,618 | ) | (1,213,513 | ) | 2,380,077 | |
Contract loss accrual | | — | | — | | (837,522 | ) |
Restructuring accrual | | (406,297 | ) | (450,212 | ) | (1,128,845 | ) |
Deferred revenue | | 1,599,811 | | (1,876,882 | ) | 343,371 | |
Deferred rent | | 265,792 | | 57,195 | | (6,976 | ) |
Restricted cash | | 56,415 | | 76,622 | | (248,382 | ) |
Net cash provided by operating activities | | 5,546,379 | | 3,658,806 | | 7,845,289 | |
Net cash provided by operating activities from discontinued operations | | 510,196 | | 1,091,168 | | 674,457 | |
Investing activities | | | | | | | |
Purchases of property and equipment | | (2,846,344 | ) | (2,070,629 | ) | (2,091,102 | ) |
Net cash paid for certain tangible assets of Biofrontera Discovery GmbH | | (1,477,487 | ) | — | | — | |
Proceeds from sale of discontinued operations | | 1,736,610 | | — | | — | |
Other assets | | 5,324 | | 43,936 | | 380,976 | |
Purchase of patents, license rights and prepaid royalties | | (2,274 | ) | (94,864 | ) | (2,210,617 | ) |
Purchases of short term investments | | (45,953,012 | ) | (57,982,297 | ) | (63,541,103 | ) |
Proceeds from sales and maturity of short term investments | | 53,340,885 | | 54,860,404 | | 59,052,271 | |
Net cash provided by (used in) investing activities | | 4,803,702 | | (5,243,450 | ) | (8,409,575 | ) |
Net cash used in investing activities from discontinued operations | | (52,241 | ) | (122,847 | ) | (80,794 | ) |
Financing activities | | | | | | | |
Principal payments on capital leases and equipment notes payable | | (244,656 | ) | — | | (1,056,270 | ) |
Net proceeds from issuance of common stock | | 454,151 | | 5,616,711 | | 711,304 | |
Purchase of treasury stock | | (80,915 | ) | (18,354 | ) | (289,000 | ) |
Net cash provided by (used in) financing activities | | 128,580 | | 5,598,357 | | (633,966 | ) |
Effect of exchange rate changes | | 146,399 | | 320,182 | | 141,346 | |
Net increase (decrease) in cash and cash equivalents | | 11,083,015 | | 5,302,216 | | (463,243 | ) |
Cash and cash equivalents at beginning of year | | 13,148,242 | | 7,846,026 | | 8,309,269 | |
Cash and cash equivalents at end of year | | $ | 24,231,257 | | $ | 13,148,242 | | $ | 7,846,026 | |
Supplemental disclosure of cash flow information | | | | | | | |
Interest paid | | $ | 4,908 | | $ | — | | $ | 38,601 | |
Income taxes paid | | $ | 5,580 | | $ | 42,007 | | $ | 8,339 | |
Supplemental schedule of non cash investing and financing activities | | | | | | | |
Unrealized gain (loss) on investments | | $ | 147,932 | | $ | (361,163 | ) | $ | (694,908 | ) |
Common stock received in payment of notes receivable | | $ | 23,612 | | $ | — | | $ | 367,201 | |
Deferred compensation related to the issuance of restricted stock | | $ | 644,000 | | $ | 2,104,000 | | $ | 1,309,500 | |
Purchases of property and equipment also included in accounts payable at year end | | $ | (483,000 | ) | $ | — | | $ | — | |
| | | | | | | | | | | |
See accompanying notes.
F-6
DISCOVERY PARTNERS INTERNATIONAL, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Amounts in dollars, except where noted)
1. Organization and Basis of Presentation
Organization and Business
Discovery Partners International, Inc. (the Company) was incorporated in California on March 22, 1995, under the name IRORI. The Company develops and offers libraries of drug-like compounds, drug discovery services, computational tools to generate compound libraries, and testing and screening services to optimize potential drugs. Additionally, the Company licenses proprietary gene profiling systems. In 1998, the Company changed its name to Discovery Partners International, Inc. In July 2000, the Company reincorporated in Delaware.
Basis of Presentation
The consolidated financial statements include all of the accounts of the Company and its wholly owned subsidiaries: Discovery Partners International AG (DPI AG), which wholly owns Discovery Partners International GmbH (DPI GmbH); ChemRx Advanced Technologies, Inc.; Xenometrix, Inc.; Discovery Partners International L.L.C. (DPI LLC); Structural Proteomics, Inc. (substantially inactive); Systems Integration Drug Discovery Company, Inc. (substantially inactive) and Irori Europe, Ltd. (substantially inactive). All intercompany accounts and transactions have been eliminated.
The consolidated financial statements have been recast to reflect the results of operations, financial positions and cash flows of our former instrumentation product lines as discontinued operations. The amounts included in the results for discontinued operations consist of revenues, cost of sales and operating expenses associated with the former operations of the instrumentation product lines excluding any allocations for facilities and other corporate support. All footnotes included herein exclude the amounts related to the assets and liabilities sold as part of the instrumentation product lines.
2. Summary of Significant Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, such as inventory, prepaid royalty, patents, license rights, property, plant, and equipment and restructuring accruals, at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash Equivalents
The Company invests its excess cash in marketable securities, principally asset-backed securities, corporate notes and government securities. The Company has established guidelines that maintain safety and liquidity of its cash equivalents. These guidelines are periodically reviewed and modified if necessary.
F-7
The Company considers all highly liquid investments with a remaining maturity of less than three months when purchased to be cash equivalents. At December 31, 2005 and 2004, the cost of cash equivalents was the same as the market value. Accordingly, there were no unrealized gains and losses. The Company evaluates the financial strength of institutions at which significant investments are made and believes the related credit risk is limited to an acceptable level.
Investments
The Company applies Statement of Financial Accounting Standards (SFAS) No. 115, Accounting for Certain Investments in Debt and Equity Securities, to its investments. Under SFAS No. 115, the Company classifies its investments as Available-for-Sale and records such assets at estimated fair value in the balance sheet, with unrealized gains and losses, if any, reported in stockholders’ equity (other comprehensive income). The Company invests its excess cash balances in marketable debt securities, consisting primarily of, government securities, corporate bonds and notes and asset-backed securities, with strong credit ratings. The Company limits the amount of investment exposure as to institutions, maturity and investment type. The realized gains and losses of securities sold is determined based on the specific identification method.
Short-term investments consist of the following:
| | Maturity | | Amortized | | Unrealized | | Market | |
December 31, 2005 | | in Years | | Cost | | Gains | | Losses | | Value | |
Asset Backed Securities | | 1 or less | | $ | 12,826,353 | | $ | 10 | | $ | (11,546 | ) | $ | 12,814,817 | |
Corporate Securities | | 1 or less | | 7,499,879 | | 1,391 | | — | | 7,501,270 | |
Total short-term investments | | | | 20,326,232 | | 1,401 | | (11,546 | ) | 20,316,087 | |
US Government Securities | | 1 to 2 | | 8,748,292 | | — | | (54,575 | ) | 8,693,717 | |
Asset Backed Securities | | 1 to 2 | | 21,341,979 | | 2,923 | | (158,360 | ) | 21,186,542 | |
Corporate Securities | | 1 to 2 | | 8,110,503 | | 501 | | (52,477 | ) | 8,058,527 | |
Certificate of Deposits | | 1 to 2 | | 1,000,000 | | — | | — | | 1,000,000 | |
Total long-term investments | | | | 39,200,774 | | 3,424 | | (265,412 | ) | 38,938,786 | |
| | | | $ | 59,527,006 | | $ | 4,825 | | $ | (276,958 | ) | $ | 59,254,873 | |
| | Maturity | | Amortized | | Unrealized | | Market | |
December 31, 2004 | | in Years | | Cost | | Gains | | Losses | | Value | |
US Government Securities | | 1 or less | | $ | 23,393,889 | | $ | — | | $ | (44,290 | ) | 23,349,599 | |
Asset Backed | | 1 or less | | 2,000,000 | | 204 | | — | | 2,000,204 | |
Corporate Securities | | 1 or less | | 6,624,211 | | 223 | | (29,753 | ) | 6,594,681 | |
Equities | | 1 or less | | 5,000,000 | | — | | — | | 5,000,000 | |
Total short-term investments | | | | $ | 37,018,100 | | $ | 427 | | $ | (74,043 | ) | $ | 36,944,484 | |
US Government Securities | | 1 to 2 | | 6,281,935 | | $ | — | | (78,657 | ) | 6,203,278 | |
Asset Backed | | 1 to 2 | | 20,200,016 | | 1,015 | | (550,457 | ) | 19,650,574 | |
Corporate Securities | | 1 to 2 | | 4,082,763 | | — | | (10,831 | ) | 4,071,932 | |
Total long-term investments | | | | $ | 30,564,714 | | $ | 1,015 | | $ | (639,945 | ) | $ | 29,925,784 | |
| | | | $ | 67,582,814 | | $ | 1,442 | | $ | (713,988 | ) | $ | 66,870,268 | |
F-8
The Company had realized losses on the sale of investments totaling $133,810, $180,125 and $21,177 in 2005, 2004 and 2003, respectively. All realized gains and losses are reclassified out of other comprehensive income (loss) in the period recognized based on specific identification of each security disposed. Proceeds from the sale of short-term investments totaled $7,328,537, $7,885,040 and $13,832,408 in the years ended December 31, 2005, 2004 and 2003, respectively. Interest receivable on investment securities at December 31, 2005 and 2004 totaled $339,225 and $321,517, respectively, and is included in other current assets in the consolidated balance sheets.
Investments considered to be temporarily impaired at December 31, 2005 are as follows:
| | | | Less than 12 months of
temporary impairment | |
| | Number of | | | | Unrealized | |
| | Investments | | Fair Value | | Losses | |
Asset Backed Securities | | 24 | | | $ | 27,570,601 | | $ | (169,906 | ) |
US Government Securities | | 8 | | | 8,693,718 | | (54,575 | ) |
Corporate Securities | | 4 | | | 7,490,041 | | (52,477 | ) |
Total temporarily impaired securities | | 36 | | | $ | 43,754,360 | | $ | (276,958 | ) |
There are no investments held at December 31, 2005, which are considered to be temporarily impaired beyond 12 months. The Company will record an impairment charge if the securities continue to be impaired beyond twelve months or other factors indicate there is permanent impairment. The Company regularly monitors and evaluates the realizable value of its marketable securities. When assessing marketable securities for other-than-temporary declines in value, the Company considers such factors as, among other things, how significant the decline in value is as a percentage of the original cost, how long the market value of the investment has been less than its original cost and the market in general.
The Company believes that the decline in value of its marketable securities is temporary and related to the change in market interest rates since purchase. The decline is not related to any company or industry specific event, and all portfolio investments are investment grade quality. The Company anticipates full recovery of amortized cost with respect to these securities at maturity or sooner in the event of a change in the market interest rate environment.
Accounts Receivable, net
Accounts receivable, net consists of the following:
| | December 31, | |
| | 2005 | | 2004 | |
Receivables | | $ | 5,250,288 | | $ | 12,308,406 | |
Unbilled receivables | | 496,474 | | 527,695 | |
Allowance for doubtful accounts | | (73,253 | ) | (50,000 | ) |
| | $ | 5,673,509 | | $ | 12,786,101 | |
F-9
Unbilled receivables are a difference in contractually based billing terms versus amounts recognized in accordance with our revenue recognition policies. We establish an allowance for doubtful accounts using the specific identification method.
Long-Lived Assets
The Company assesses potential impairments to its long-lived and intangible assets when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered, in accordance with SFAS No. 144, Accounting for Impairment or Disposal of Long-lived Assets. An impairment loss is recognized when the carrying amount of the long-lived and intangible asset is not recoverable and exceeds its fair value. The carrying amount of a long-lived and intangible asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Any required impairment loss is measured as the amount by which the carrying amount of a long-lived and intangible asset exceeds its fair value and is recorded as a reduction in the carrying value of the related asset and a charge to operating expense.
The Company has stated within this Annual Report on Form 10-K that it is contemplating various strategic options that include the divestiture of various operating assets, a merger or acquisition and, if necessary, a liquidation of all of the assets of the Company. These events and circumstances have been evaluated in determining if the carrying amount of the Company’s assets at December 31, 2005 are impaired. The Company considered all available evidence and determined there is insufficient information to establish whether or not the carrying amount of the assets may be fully recovered should one of these events occur. As such, no additional related impairment charges were recorded at December 31, 2005.
In the event the Company divests the various operating assets of the Company, it is possible that it may not successfully recover the $8.8 million of total long-lived assets (excluding restricted cash) that is reflected on the consolidated balance sheet at December 31, 2005, which may result in future impairment charges up to this amount. There are one or more viable alternatives that would not lead to a loss on the recoverability of the Company’s long-lived assets. In the event the Company engages in a merger or acquisition transaction, it is possible that the value realized by its shareholders in such a transaction may be significantly less than the $95.1 million of shareholders’ equity recorded on its consolidated financial statements as of December 31, 2005, due to the fact that the Company’s market capitalization is significantly below the book value of shareholders’ equity. Lastly, in the event that the Company is unsuccessful with a divestiture of its assets or is unable to successfully conclude any merger or acquisition activity, it is possible that the Company’s Board of Directors could decide to liquidate all of the Company’s assets, in which event the value realized by its shareholders would be significantly less than the $95.1 million of shareholders’ equity recorded on the consolidated financial statements as of December 31, 2005.
Fair Value of Financial Instruments
Financial instruments, including cash and cash equivalents, accounts payable and accrued liabilities, are carried at cost, which management believes approximates fair value because of the short-term maturity of these instruments.
Inventories
Inventories consist of the following:
| | December 31, | |
| | 2005 | | 2004 | |
Raw materials | | $ | 457,752 | | $ | 439,583 | |
Work-in process | | 400,546 | | 1,967,118 | |
Finished goods | | 17,359,676 | | 18,063,966 | |
| | 18,217,974 | | 20,470,667 | |
Less reserves | | (17,639,132 | ) | (18,080,059 | ) |
| | $ | 578,842 | | $ | 2,390,608 | |
Inventories are recorded at the lower of cost or market. The cost of inventory includes the cost of raw materials, labor and related overhead. The Company records write-downs of inventory for estimated obsolescence or non-marketability if there is an excess of cost of inventory over the
F-10
estimated market value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than we have projected, additional inventory write-downs may be required. As of December 31, 2005, 98% of the inventory reserve is associated with chemical compound finished goods inventory. A portion of the net inventory balance represents work-in-process related to ongoing chemistry projects with three customers. Estimated losses on any deliverables are recorded when they become apparent. As of December 31, 2005, we have reserved approximately $73,653 against the work-in-process representing the anticipated losses on the sale of certain chemical compound libraries. The anticipated losses are based on estimated revenue that will be recognized upon shipment of the compound libraries as well as the total historical and estimated future costs associated with these revenues. The actual losses on the sale of these libraries could differ from management’s estimates.
As of December 31, 2005, $159,304 of the inventory reserve is associated with raw materials inventory deemed obsolete as a result of the restructuring of the facilities in South San Francisco and the termination of the Pfizer contract. Estimated losses on any deliverables are recorded when they become apparent.
Property and Equipment
Property and equipment consists of the following:
| | December 31, | |
| | 2005 | | 2004 | |
Furniture and equipment | | $ | 23,710,779 | | $ | 20,796,435 | |
Software | | 1,902,692 | | 4,751,112 | |
Leasehold improvements | | 5,719,965 | | 6,524,780 | |
| | 31,333,436 | | 32,072,327 | |
Less accumulated depreciation and amortization | | (23,382,671 | ) | (24,976,349 | ) |
| | $ | 7,950,765 | | $ | 7,095,978 | |
Property and equipment, including equipment under capital leases, are stated at cost and depreciated over the estimated useful lives of the assets (three to seven years) or the term of the related lease, whichever is shorter, using the straight-line method. Maintenance and repairs are charged to operations as incurred. Amortization of assets acquired under capital leases is included in depreciation expense. Depreciation and amortization expense of property and equipment totaled $3,496,863, $3,309,336 and $4,520,686 for the years ended December 31, 2005, 2004 and 2003, respectively. Any costs related to satisfying contractual obligations upon the retirement or abandonment of assets is measured at fair value at the time of purchase of the asset and recorded as a long-term liability and an additional cost of the asset. Such amounts are recognized in line with depreciation expense.
During 2005, the Company announced the restructuring of its chemistry operations in South San Francisco in connection with the non-renewal of its chemistry collaboration with Pfizer (representing 54% of its revenues in 2005). This event required the reevaluation of the recoverability of the gross carrying value of the long-lived assets used in this facility. The
F-11
Company identified property and equipment that would cease to be used beyond the first quarter of fiscal 2006 (the period when the restructuring would be complete). The change to the estimate of the useful lives of these assets resulted in $202,934 of accelerated depreciation charges recognized in the fourth quarter of fiscal 2005. The Company determined the carrying value (after consideration of the change in estimated useful lives discussed above) was recoverable through the first quarter of fiscal 2006 and no additional impairment charges were required.
Prepaid Royalty, Patents and License Rights
Prepaid royalty, patents and license rights consist of the following:
| | December 31, 2005 | | December 31, 2004 | |
| | Gross | | | | | | Gross | | | | | |
| | Carrying | | Accumulated | | | | Carrying | | Accumulated | | | |
| | Value | | Amortization | | Net | | Value | | Amortization | | Net | |
Prepaid royalty | | $ | — | | $ | — | | $ | — | | $ | 6,034,643 | | $ | (1,206,928 | ) | $ | 4,827,715 | |
Patents | | 1,864,293 | | (1,146,586 | ) | 717,707 | | 2,862,020 | | (971,096 | ) | 1,890,924 | |
License rights | | 6,667 | | (6,667 | ) | — | | 256,666 | | (256,666 | ) | — | |
Total intangible assets | | $ | 1,870,960 | | $ | (1,153,253 | ) | $ | 717,707 | | $ | 9,153,329 | | $ | (2,434,690 | ) | $ | 6,718,639 | |
Amortization expense related to amortizable intangible assets was $1,382,416, $1,536,487, and $776,252 for the years ended December 31, 2005, 2004 and 2003, respectively. During 2005 the Company sold patents in connection with sale of its instrumentation product lines that resulted in a reduction of the gross carrying value of patents of $1.0 million.
During 2005, the Company recorded $4.7 million in impairment charges on intangible long-lived assets. Approximately $3.7 million of the impairment charges related to the prepaid royalty to Abbott Laboratories related to the µARCS screening technology. In connection with the restructuring of the chemistry operations the Company decided to discontinue the commercialization of the µARCS screening technology. All available evidence was considered and determined that no further benefit would be realized by use of this asset in current revenue generating or operating activities nor would any future cash flows be generated by use of this asset. In addition, $1.0 million of impairment charges related to patent rights to a proprietary gene profiling system that is licensed and enables the Company to offer toxicology research products and services. The loss of a customer required the reevaluation of the recoverability of the gross carrying value of the asset. The Company considered all available evidence and developed estimates based on historical rates of attrition of the customer base, future cash generating capacity and future expenditures necessary to maintain the asset. An expected present value technique, in which a series of cash flow scenarios that reflect the range of possible outcomes were discounted to estimate the fair value of the asset.
F-12
The estimated annual amortization expense of all intangible assets for the years ended December 31 after 2005 is as shown in the following table. Actual amortization expense to be reported in future periods could differ from these estimates as a result of acquisitions, divestitures, asset impairments and other factors.
2006 | | $ | 134,580 | |
2007 | | 134,580 | |
2008 | | 134,580 | |
2009 | | 134,580 | |
2010 | | 134,580 | |
Thereafter | | 44,807 | |
| | $ | 717,707 | |
Other Assets
Other assets consist of chemical compounds purchased by DPI AG for its screening services. The compounds are stated at cost and depreciated over the estimated useful lives of the assets (four years) using the straight-line method. The net carrying value of these assets approximates or is less than their net realizable value.
Revenue Recognition
Chemistry services. Revenue from the sale of chemical compounds delivered under chemistry collaborations is recorded as the compounds are shipped. Revenue under chemistry service agreements that are compensated on a full-time equivalent, or FTE, basis is recognized on a monthly basis and is based upon the number of FTE employees that actually worked on each project and the agreed-upon rate per FTE per month.
Compound repository services. In August 2004, the Company entered into a multi-year contract with the NIH to establish and maintain a Small Molecule Repository to manage and provide up to one million chemical compounds to multiple NIH Screening Centers as part of the NIH Roadmap Initiative. Revenue under this contract is recorded as costs are incurred, which include indirect costs that are based on provisional rates estimated by management at the time a proposal is submitted. The Company has determined that actual indirect costs are greater than the provisional rates and management fully intends to negotiate recovery of these higher costs with the government. Since this is the first government contract and the Company has no historical experience negotiating final indirect cost rates with the government, all cost overruns have been expensed and any potential recovery will be recognized as revenue upon receipt of monies. This contract is funded, in its entirety, by the NIH, Department of Health and Human Services. Payments to the Company for performance under this contract are subject to audit by the Defense Contract Audit Agency (DCAA) and is subject to government funding. The Company records a reserve against receivables for estimated losses that may result from rate negotiations and/or lack of government funding availability. As of December 31, 2005, no such reserve was considered necessary.
Screening services. High throughput screening service revenues are recognized on the proportional performance method. Advances received under these high throughput screening service agreements are initially recorded as deferred revenue, which is then recognized proportionately as costs
F-13
are incurred over the term of the contract. Certain of these contracts may allow the customer the right to reject the work performed; however, there is no history of material rejections and historically the Company has been able to recognize revenue without realizing any losses from any rejections.
Other licenses. Other licenses revenue includes royalty revenue due to the Company under the Xenometrix patent licensing agreements. Royalty revenue is recognized upon receipt of monies, provided there is no future obligation with respect to such payments.
Integrated drug discovery collaborations may provide chemistry services revenue, screening services revenue, milestone payments and other revenues. Revenue for each of these elements of such collaborations is recognized as described above. Revenue from milestone payments would be recognized upon receipt of monies.
From time to time the Company may receive requests from customers to bill and hold goods for them. In these cases, as long as the specific revenue recognition criteria under accounting principles generally accepted in the United States at the time of the bill and hold are met, including the customer accepting the risk of loss and the transfer of ownership of such goods occurring prior to shipment, the revenue is recognized.
Shipping and Handling Costs
Costs incurred for shipping and handling of products are included in cost of revenues. Amounts billed to customers for such costs are reported as revenue.
Restructuring Costs
The Company accounts for restructuring costs in accordance with SFAS No. 146, Accounting for Costs Associated with Exit or Disposal Activities. The Company recognizes the fair value of one-time termination benefits when both the appropriate approval for taking action has been obtained, and when a liability is incurred (i.e., when the plan has been communicated to employees). If employees are required to render service beyond a 60 day minimum retention period, the fair value of the obligation is determined on the date of the communication to the employee and recognized over the service period. In determining costs to consolidate excess facilities, the Company estimates the fair value of the obligation at the cease-use date based on the remaining lease rentals, reduced by estimated sublease rentals that could be reasonably obtained for the property. As a result, in the event we are unsuccessful in entering into a sublease, the Company could incur future restructuring charges. Liabilities related to the consolidation of excess facilities are recorded when the premises have been vacated. The cumulative effect of any changes to the plan subsequent to the communication date and cease-use date are recognized in the period of the change.
F-14
Research and Development Costs
Costs incurred in connection with research and development are charged to operations as incurred.
Stock-Based Compensation
As permitted by SFAS No. 123, Accounting for Stock-Based Compensation, the Company accounts for common stock options granted to employees and directors using the intrinsic value method and, thus, recognizes no compensation expense for such stock-based awards where the exercise prices are equal to or greater than the fair value of the Company’s common stock on the date of the grant. The Company has recorded deferred stock compensation related to certain stock options which were granted with exercise prices below estimated fair value, restricted stock and rights to acquire restricted stock (see Note 6). In accordance with APB Opinion No. 25, deferred compensation is included as a reduction of stockholders’ equity and is being amortized to expense on an accelerated basis in accordance with Financial Accounting Standards Board Interpretation No. 28 over the vesting period of the options, restricted stock and rights to acquire restricted stock.
Pro forma information regarding net income or loss is required by SFAS No. 123, and has been determined as if the Company had accounted for its employee stock options and shares issued pursuant to the Company’s 2000 Employee Stock Purchase Plan under the fair value method of that Statement. The fair value of these options was estimated at the date of grant using the Black-Scholes option pricing model with the following weighted average assumptions used for option grants:
| | Years Ended
December 31, | |
| | 2005 | | 2004 | | 2003 | |
Risk-free interest rate | | 4.0 | % | 3.5 | % | 3.5 | % |
Dividend yield | | 0 | % | 0 | % | 0 | % |
Volatility factor | | 78 | % | 79 | % | 87 | % |
Weighted average life in years | | 5.3 | | 5.8 | | 6.7 | |
For purposes of adjusted pro forma disclosures, the estimated fair value of the options is amortized to expense, on an accelerated basis, over the vesting period. The Company’s adjusted pro forma information is as follows:
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
Net income (loss), as reported | | $ | (14,164,737 | ) | $ | 3,902,772 | | $ | 1,058,913 | |
Deduct: Total stock-based compensation expense determined under fair value based method | | (2,292,441 | ) | (2,900,964 | ) | (2,979,216 | ) |
Pro forma net income (loss) | | $ | (16,457,178 | ) | $ | 1,001,808 | | $ | (1,920,303 | ) |
Income (loss) per share: | | | | | | | |
Basic and diluted—as reported | | $ | (0.55 | ) | $ | 0.15 | | $ | 0.04 | |
Basic and diluted—pro forma | | $ | (0.63 | ) | $ | 0.04 | | $ | (0.08 | ) |
F-15
Comprehensive Income (Loss)
SFAS No. 130, Reporting Comprehensive Income, requires the Company to report in the consolidated financial statements, in addition to net income, comprehensive income (loss) and its components including foreign currency items and unrealized gains and losses on certain investments in debt and equity securities. For the three years in the period ended December 31, 2005, the Company has disclosed comprehensive income (loss) in its consolidated statements of stockholders’ equity. The accumulated balances for each item included in accumulated other comprehensive income (loss) is as follows:
| | December 31, | |
| | 2005 | | 2004 | | 2003 | |
Foreign currency translation adjustment | | $ | 361,919 | | $ | 1,342,048 | | $ | 1,323,353 | |
Unrealized loss on investments | | (298,140 | ) | (712,546 | ) | (351,383 | ) |
Accumulated other comprehensive income | | $ | 63,779 | | $ | 629,502 | | $ | 971,970 | |
F-16
Net Income (Loss) Per Share
Basic and diluted net income (loss) per share is presented in conformity with SFAS No. 128, Earnings per Share. In accordance with SFAS No. 128, basic net income (loss) per share has been computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding (including vested deferred stock units) during the period, less shares subject to repurchase. Diluted net income (loss) per share has been computed by dividing net income (loss) by the weighted-average number of common and common stock equivalent shares outstanding during the period calculated using the treasury stock method, less shares subject to repurchase. Common equivalent shares, composed of outstanding stock options, restricted stock and contingently issuable stock are included in diluted net income (loss) per share to the extent these shares are dilutive. The computations for basic and diluted earnings per share are as follows:
| | Income | | Shares | | Earnings | |
| | (Numerator) | | (Denominator) | | Per Share | |
Year Ended December 31, 2005 | | | | | | | |
Basic earnings per share, as reported: | | | | | | | | | |
Net loss | | $ | (14,164,737 | ) | 25,919,393 | | $ | (0.55 | ) |
Diluted earnings per share: | | | | | | | |
Dilutive stock options | | — | | — | | — | |
Common stock issuable | | — | | — | | — | |
Net loss plus assumed conversions | | $ | (14,164,737 | ) | 25,919,393 | | $ | (0.55 | ) |
Year Ended December 31, 2004 | | | | | | | |
Basic earnings per share, as reported: | | | | | | | | | |
Net income | | $ | 3,902,772 | | 25,318,937 | | $ | 0.15 | |
Diluted earnings per share: | | | | | | | |
Dilutive stock options | | — | | 587,742 | | — | |
Common stock issuable | | — | | 364,946 | | — | |
Net income plus assumed conversions | | $ | 3,902,772 | | 26,271,625 | | $ | 0.15 | |
The total number of shares issuable upon exercise of stock options excluded from the calculation of diluted earnings per share since they are anti-dilutive were 2,278,043, 1,940,315 and 1,845,012 in 2005, 2004 and 2003, respectively.
Segment Reporting
The Company considers its operations to be a single reportable segment.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents and short-term investments. The Company believes it
F-17
has reduced its exposure to credit loss to an acceptably low level by placing its cash, cash equivalents and investments with financial institutions and corporations that are believed to be of high credit quality and by limiting its exposure to any single investment.
Recently Issued Accounting Standards
In December 2004, the FASB issued SFAS No. 123R (revised 2004), Shared-Based Payment, which is a revision of SFAS No. 123, Accounting for Stock-Based Compensation. SFAS No. 123R supersedes APB Opinion No. 25, Accounting for Stock Issued to Employees (Opinion No. 25), and amends SFAS No. 95, Statement of Cash Flows. Generally, the approach to accounting for share-based payments in SFAS No. 123R is similar to the approach described in SFAS No. 123. However, SFAS No. 123R requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statement based on their fair values. SFAS No. 123R is effective at the beginning of the first interim or annual period beginning after December 16, 2005. SFAS No. 123R permits public companies to adopt its requirements using one of two methods:
1. A “modified prospective” method in which compensation cost is recognized beginning with the effective date (a) based on the requirements of SFAS No. 123R for all share-based payments granted after the effective date and (b) based on the requirements of SFAS No. 123 for all awards granted to employees prior to the effective date of SFAS No. 123R that remain unvested on the effective date.
2. A “modified retrospective” method which includes the requirements of the modified prospective method described above, but also permits entities to restate based on the amounts previously recognized under SFAS No. 123 for purposes of pro forma disclosures either (a) all prior periods presented or (b) prior interim periods of the year of adoption.
The Company plans to adopt SFAS No. 123R using the modified-prospective method, which will impact all periods beginning after December 16, 2005. As permitted by SFAS No. 123, the Company currently accounts for share-based payments to employees using the intrinsic value method provided by Opinion No. 25 and, as such, generally recognizes no compensation cost for employee stock options. Accordingly, the adoption of SFAS No. 123R’s fair value method will have a significant impact on our future results from operations, although it will have no impact on our overall financial position. As a result of the anticipated adoption of SFAS No. 123R, the Compensation Committee of the Board of Directors approved the acceleration of vesting on stock options with exercise prices of $5.75 or more effective February 21, 2005, which did not result in an accounting charge under the Opinion No. 25 intrinsic value method. The Company has not determined the impact of adoption of SFAS No. 123R since it will depend on levels of share-based payments granted in the future. SFAS No. 123R also requires the benefits of tax deductions in excess of recognized compensation cost to be reported as a financing cash flow, rather than as an operating cash flow as required under current literature. This requirement will reduce net operating cash flows and increase net financing cash flows in periods after adoption. While the Company cannot estimate what those amounts will be in the future (because they depend on, among other things, when employees exercise stock options), we have not recognized excess tax deductions historically due to our accumulated loss position.
F-18
Foreign Currency Translation
The financial statements of DPI AG are measured using the local currency, the Swiss Franc, as the functional currency. The financial statements of DPI GmbH are re-measured using the Swiss Franc as the functional currency, after transacting in its local currency, the Euro. The financial statements of DPI GmbH are consolidated with the DPI AG financials. DPI AG accounts are translated from their local currency to the U.S. dollar using the current exchange rate in effect at the balance sheet date for the balance sheet accounts, and using the average exchange rate during the period for revenues and expense accounts. The effects of translation for consolidated DPI AG are recorded as a separate component of stockholders’ equity (accumulated other comprehensive income (loss)). DPI AG conducts its business with customers in local currencies. Exchange gains and losses arising from these transactions are recorded using the actual exchange differences on the date the transaction is settled. The carrying value of consolidated net assets of DPI AG at December 31, 2005 totaled $4,795,736 (excluding intercompany balances).
The financial statements of DPI LLC are remeasured from the local currency, the Japanese Yen, to its functional currency, the U.S. dollar. Exchange gains and losses arising from transactions are recorded using the actual exchange differences on the date the transaction is settled. The carrying value of net liabilities of DPI LLC at December 31, 2005 totaled $15,474 (excluding intercompany balances).
3. Discontinued Operations
On October 7, 2005, the Company entered into an Asset Purchase Agreement with IRORI Discovery, Inc., now known as Nexus Biosystems (Nexus), pursuant to which the Company
F-19
agreed to sell certain assets and liabilities to Nexus, including the IRORI® chemical synthesis products, the Crystal Farm® automated protein crystallization products, and the Universal Store™ compound storage systems products for a purchase price of $1,941,576, inclusive of a purchase price adjustment. Nexus is a California company whose Chief Executive Officer, John Lillig, was previously the Chief Technology Officer, Vice President and General Manager, Discovery Systems for the Company. The sale of net assets pursuant to the Asset Purchase Agreement was completed on October 12, 2005. The sale price for the assets was effectively based on the total book value of the net assets and the cash cost of operations for the assets sold through the closing date. As of December 31, 2005, the Company had received $1,736,610 in proceeds from Nexus for payment of the net assets. The remaining amount due of $204,966 is included in other current assets within the balance sheet at December 31, 2005 and is fully reserved. The Company recognized a gain on sale of the net assets totaling $393,899 for the year ended December 31, 2005. The Company incurred $177,616 for the year ended December 31, 2005 in costs related to the sale of these net assets that are included in the gain on sale.
The Company’s consolidated financial statements and related notes contained herein have been recast to reflect the financial position, results of operations and cash flows of the instrumentation product lines as a discontinued operation. The Company did not account for its instrumentation product lines as a separate legal entity. Therefore, the following selected financial data for the Company’s discontinued operations is presented for informational purposes only and does not necessarily reflect what the net sales or earnings would have been had the businesses operated as a stand-alone entity. The financial information for the Company’s discontinued operations excludes allocations of certain DPI assets, liabilities and expenses to those operations, such as facility charges. These amounts have been excluded from discontinued operations on the basis that these assets, liabilities and expenses were not transferred in the sale of these product lines and are considered by management to reflect most fairly or reasonably the incremental results of operations that were sold.
F-20
The following tables set forth, for the periods indicated, selected financial data of the Company’s discontinued operations:
Selected Financial Data for Discontinued Operations
Statement of Operations and Cash Flows
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
| | | | | | | |
Revenues | | $ | 2,026,280 | | $ | 7,296,391 | | $ | 4,617,341 | |
Cost of revenues | | 841,858 | | 3,953,467 | | 3,783,939 | |
Gross margin | | 1,184,422 | | 3,342,924 | | 833,402 | |
Research and development | | 1,462,407 | | 2,252,453 | | 1,978,512 | |
Selling, general and administrative | | 506,865 | | 517,894 | | 1,060,028 | |
Amortization of stock-based compensation | | 53,251 | | 54,985 | | 13,730 | |
Total operating expenses | | 2,022,523 | | 2,825,332 | | 3,052,270 | |
Gain (loss) from discontinued operations | | $ | (838,101 | ) | $ | 517,592 | | $ | (2,218,868 | ) |
Balance Sheet
(Assets and Liabilities Held for Sale)
| | December 31,
2004 | |
| | | |
Accounts receivable, net | | $ | 1,547,676 | |
Inventories, net | | 451,079 | |
Prepaids and other current assets | | 4,150 | |
Property and equipment, net | | 110,182 | |
Intangible assets, net | | 395,833 | |
Total Assets | | $ | 2,508,920 | |
| | | |
Accounts payable and accrued expenses | | $ | 778,329 | |
Deferred revenue | | 187,503 | |
Total Liabilities | | $ | 965,832 | |
F-21
4. Restructuring Accrual
In November 2005, the Company announced the termination of discussions with Pfizer around a new collaboration for services in the design and development of compounds exclusively for Pfizer. With the absence of a new contract with Pfizer, the Company initiated the process of reducing its chemistry operational capacity in a restructuring of its South San Francisco facility. Under the restructuring plan, 50 employees were involuntary terminated, including scientific, operational and administrative staff. In the fourth quarter of 2005, the Company recorded $928,000 of restructuring expenses, which consisted of accrued one-time termination benefits. The Company required all employees to render service for a minimum of 60 days to receive termination benefits. As a result, the fair value of the obligation was determined on the date of communication to the employee and will be recognized over the service period. In determining costs to consolidate excess facilities, the Company estimates the fair value of the obligation at the cease-use date based on the remaining lease rentals, reduced by estimated future sublease rentals that could be reasonably obtained for the property, even if the Company is unsuccessful in entering into a sublease. Liabilities related to the consolidation of excess facilities are recorded when the premises have been vacated. As of December 31, 2005, no such accrual for lease obligations was recognized as the facility will be utilized through the first quarter of 2006. Moving, relocation and other costs related to consolidation of facilities are expensed as incurred and are included in operating expenses. The Company expects to incur a total of approximately $2.5 million related to this restructuring event, inclusive of termination benefits and lease obligations (net of sublease income). The Company expects to utilize future accruals related to this event by December 2008.
In April 2003, the Company announced that it would consolidate its domestic chemistry facilities into two locations: in South San Francisco for primary screening library design and synthesis programs and in San Diego for lead optimization and medicinal chemistry projects. Restructuring charges totaled $1,872,986 for the year ended December 31, 2003 and $112,368 for the year ended December 31, 2005. There were no additional restructuring costs incurred 2004. During the year ended December 31, 2005 all final payments were made under the restructuring plan and the estimated charges of $17,304 previously accrued for were reversed to reflect actual payments made. Restructuring charges were comprised of the following in the aggregate:
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
Severance and Retention Bonuses for Involuntary Employee
Terminations | | $ | 927,890 | | $ | — | | $ | 375,599 | |
Costs to Exit Certain Contractual and Lease Obligations | | 112,368 | | — | | 919,171 | |
Moving, Relocation and Other Costs Related to Consolidation
of Facilities | | — | | — | | 578,216 | |
| | | | | | | |
Total Restructuring Expense | | $ | 1,040,258 | | $ | — | | $ | 1,872,986 | |
F-22
The following table summarizes the activity and balances of the restructuring reserve:
| | Severance | | | | | |
| | and Retention | | Costs to | | | |
| | Bonuses | | Exit Certain | | | |
| | for Involuntary | | Contractual | | | |
| | Employee | | and Lease | | | |
| | Terminations | | Obligations | | Total | |
Balance at December 31, 2003 | | $ | — | | $ | 744,141 | | $ | 744,141 | |
Reserve Established | | — | | — | | — | |
Utilization of reserve: | | | | | | | |
Payments | | — | | (450,212 | ) | (450,212 | ) |
Balance at December 31, 2004 | | — | | 293,929 | | 293,929 | |
Reserve Established | | 927,890 | | 112,368 | | 1,040,258 | |
Utilization of reserve: | | | | | | | |
Payments | | — | | (406,297 | ) | (406,297 | ) |
Balance at December 31, 2005 | | $ | 927,890 | | $ | — | | $ | 927,890 | |
The Company expects to incur additional restructuring charges associated with lease obligations in 2006, which will be utilized through fiscal 2008. As terminated employees complete their service arrangements, the Company will incur additional restructuring charges associated termination benefits through fiscal 2006. . The Company expects to complete the utilization of the obligation recorded at December 31, 2005 by January 2006.
5. Commitments and Contingencies
Leases
The Company leases certain buildings under operating leases, which expire at varying dates through June 2013. Rent expense was $3,244,134, $2,727,901 and $2,495,943 for the years ended December 31, 2005, 2004, and 2003, respectively.
F-23
Annual future minimum lease obligations under the Company’s operating leases as of December 31, 2005 are as follows:
| | Operating | |
| | Leases | |
2006 | | $ | 3,433,276 | |
2007 | | 3,283,629 | |
2008 | | 3,283,629 | |
2009 | | 1,237,025 | |
2010 | | 1,023,390 | |
Thereafter | | 2,117,285 | |
Total minimum lease payments | | $ | 14,378,234 | |
At December 31, 2005 and 2004, there were no capital lease obligations.
Licensing and Purchase Commitments
The Company sells certain products under licensing and purchasing agreements. The licensing agreements require payments based upon various percentages of sales from products. Terms of the licensing agreements generally range from the remaining life of the patent up to 25 years. Total license costs incurred under these agreements were $44,075, $104,502 and $34,403 for the years ended December 31, 2005, 2004 and 2003, respectively.
To maintain exclusivity, certain of the licensing agreements require guaranteed minimum annual license payments. Future minimum guaranteed payments at December 31, 2005 are as follows:
| | Minimum payments | |
2006 | | $ | 15,000 | |
2007 | | 15,000 | |
2008 | | 10,000 | |
2009 | | 10,000 | |
2010 | | 10,000 | |
Thereafter | | 30,000 | |
Total minimum license payments | | $ | 90,000 | |
The Company also has purchase commitments from time to time for the purchase of capital expenditures and raw materials. Obligations under these commitments totaled $2,012,913 at December 31, 2005. Purchase commitments for equipment expire in 2006.
Executive Employment Agreements
The Company has entered into employment agreements with key executives that provide for the continuation of salary if terminated for reasons other than cause, as defined in those agreements. At December 31, 2005, the future employment contract commitments for such key
F-24
executives totaled approximately $500,000 for the fiscal year ending December 31, 2005 and none for years thereafter.
The Company has entered into change in control agreements with the following current officers of the Company: Michael Venuti, Craig Kussman, Urs Regenass, Douglas Livingston, Richard Neale and Daniel Harvey. In the event of both a change in control and termination of an officer’s employment (either by the Company without cause or by the officer for good reason) either before, and in connection with, the change in control or within 365 days after the change in control, the officer will be entitled to a severance payment equal to the officer’s average bonus for the three prior full calendar years of employment with the Company multiplied by the number of days in the calendar year through the date of termination divided by 365 and the greater of 100% of the officer’s annual base salary in effect immediately prior to the change in control of the Company or the officer’s annual base salary in effect at the time notice of termination is given. Additionally, for purposes of determining the vesting of the officers’ awards made under the 2000 Stock Incentive Plan, as well as any unvested shares of Company stock acquired pursuant to that Plan, the officer will be treated as if he had completed an additional 12 months of service immediately before the date on which his employment is terminated.
The initial term of these agreements expires December 31, 2004 and automatically renews thereafter on an annual basis unless either party gives notice by September 30th of the preceding year and no change of control of the Company has occurred during the 18 months before that notice.
Restricted Cash
The Company has restricted cash of $1,060,753 and $1,120,050 as of December 31, 2005 and 2004, respectively, collateralizing obligations under operating lease and line of credit agreements.
6. Stockholders’ Equity
Common Stock
On July 27, 2000, the Company sold 5,000,000 shares of common stock at $18.00 per share through an Initial Public Offering. On August 27, 2000, the underwriters exercised their option to acquire an additional 750,000 shares, also at $18.00 per share.
On May 4, 2004, our secondary public offering was declared effective by the SEC. A total of 8,305,300 shares of common stock at a price of $5.00 per share were made available to the public. Axys Pharmaceuticals, Inc., then a stockholder of the Company, registered 7,222,000 shares for resale, with the remaining 1,083,300 shares registered for sale by the Company to the underwriters to cover over-allotments. We received proceeds from the offering, of the shares registered for sale by the Company, of $5.1 million net of discounts.
F-25
On October 4, 2001, the Company’s Board of Directors authorized a Stock Repurchase Plan, whereby the Company was authorized to repurchase up to 2,000,000 shares of the Company’s common stock at no more than $3.50 per share. In October 2001, the Company purchased 35,000 shares of its common stock for a total of $119,250 pursuant to its Stock Repurchase Plan. In February 2003, an additional 115,000 shares were purchased for a total of $289,000.
For the year ended December 31, 2005, the Company accepted 33,231 shares of the Company’s common stock in lieu of cash from former employees in payment of obligations to the Company totaling $104,527.
Stock Options
In November 1995, the Company adopted the 1995 Stock Option/Stock Issuance Plan, under which 2,350,000 shares of common stock were reserved for issuance of stock and stock options granted by the Company. In July 2000, the Company adopted the 2000 Stock Incentive Plan (the “Plan”) as the successor plan to the 1995 Stock Option/Stock Issuance Plan. 3,300,000 shares of common stock were reserved under the Plan, including shares rolled over from its 1995 Plan. The Plan provides for the grant of incentive and nonstatutory options. The exercise price of options must equal at least the fair value on the date of grant. The options generally vest over a four-year period. Options granted prior to January 1, 2003 are exercisable immediately, subject to the Company’s right of repurchase. Options granted after January 1, 2003 are exercisable as the options vest. All options expire no later than ten years after the date of grant.
A summary of the Company’s stock option activity and related information is as follows:
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
| | Options | | Weighted-
Average
Exercise
Price | | Options | | Weighted-
Average
Exercise
Price | | Options | | Weighted-
Average
Exercise
Price | |
Outstanding at beginning of period | | 3,362,362 | | $ | 5.40 | | 3,396,560 | | $ | 5.34 | | 3,566,852 | | $ | 5.44 | |
Granted | | 163,700 | | 3.66 | | 370,100 | | 5.75 | | 475,000 | | 3.74 | |
Exercised | | (200,310 | ) | 1.50 | | (135,591 | ) | 2.78 | | (263,873 | ) | 2.23 | |
Forfeited | | (506,169 | ) | 6.21 | | (268,707 | ) | 6.36 | | (382,119 | ) | 6.50 | |
Outstanding at end of period | | 2,819,583 | | $ | 5.32 | | 3,362,362 | | $ | 5.40 | | 3,395,860 | | $ | 5.34 | |
Exercisable | | 2,614,939 | | $ | 5.46 | | 2,851,667 | | $ | 5.48 | | 2,992,117 | | $ | 5.52 | |
F-26
Following is a further breakdown of the options outstanding as of December 31, 2005:
Range of Exercise Prices | | Options
Outstanding | | Weighted
Average
Remaining Life
in Years | | Weighted
Average
Exercise Price | | Options
Exercisable | | Weighted
Average
Exercise Price
of Options
Exercisable | |
$0.20 – 2.50 | | 62,583 | | 2.8 | | $ | 1.82 | | 62,583 | | $ | 1.82 | |
$2.51 – 5.00 | | 1,220,142 | | 4.5 | | $ | 3.63 | | 1,030,583 | | $ | 3.67 | |
$5.01 – 12.00 | | 1,473,183 | | 3.9 | | $ | 6.29 | | 1,458,098 | | $ | 6.29 | |
$12.01 – 24.00 | | 63,675 | | 3.9 | | $ | 18.93 | | 63,675 | | $ | 18.93 | |
| | 2,819,583 | | | | | | 2,614,939 | | | |
Exercise prices for options outstanding as of December 31, 2005 ranged from $0.20 to $24.00 per share. The weighted-average remaining contractual life of those options is approximately 4.1 years. The weighted-average fair value of the options granted in 2005, 2004 and 2003 are $2.45, $4.39 and $2.97 per share, respectively.
At December 31, 2005, options for 3,475,667 shares were available for future grant under the Plan.
Employee Stock Purchase Plan
In June 2000, the Board of Directors and stockholders adopted the Employee Stock Purchase Plan (the “Purchase Plan”). The Purchase Plan permits eligible employees to purchase common stock at a discount, but only through payroll deductions, during defined offering periods. The price at which stock is purchased under the Purchase Plan is equal to 85% of the fair market value of the common stock on the first or last day of the offering period, whichever is lower. In addition, the Purchase Plan provides for annual increases of shares available for issuance under the Purchase Plan beginning with fiscal 2001. Employee participation in the Purchase Plan commenced August 1, 2002. As of December 31, 2005 a total of 1,931,280 shares of the Company’s common stock were reserved for future issuance under the Purchase Plan of which 958,380 remain unregistered at December 31, 2005. Pursuant to the Purchase Plan, the participating employees purchased 52,583 shares of the Company’s common stock during 2005.
Deferred Stock Compensation
The Company awarded 142,500 shares of restricted stock and rights to acquire 500,000 shares of restricted stock in August 2003 and July 2004, collectively, pursuant to the Company’s 2000 Stock Incentive Plan to certain of the Company’s key employees resulting in an increase in deferred compensation of $3.4 million. The restricted stock and rights to acquire restricted stock vest in annual installments over a four-year period. In April 2005, Michael C. Venuti, PhD, upon employment as the Company’s Chief Scientific Officer, was awarded a restricted stock grant for 200,000 shares of the Company’s common stock pursuant to the Company’s 2000 Stock Incentive Plan resulting in an increase in deferred compensation of $644,000. The restricted stock will vest
F-27
at the end of five years from the grant date except that vesting can be accelerated if certain conditions are met.
In accordance with Opinion No. 25, deferred compensation is included as a reduction of stockholders’ equity and is being amortized to expense on an accelerated basis in accordance with Financial Accounting Standards Board Interpretation No. 28 over the vesting period of the options, restricted stock and rights to acquire restricted stock. During 2005 and 2004, the Company recorded stock-based compensation expense, for continuing operations, of $1,017,370 and $946,504, respectively. Common stock issuable represents the fair value at the time of grant of the shares issuable in the future.
Stockholder Rights Agreement
On February 13, 2003, the Company’s Board of Directors adopted a Rights Agreement (the Agreement). The Agreement provides for a dividend distribution of one preferred share purchase right for each outstanding share of the Company’s common stock held of record at the close of business on February 24, 2003. The rights are not currently exercisable. Under certain conditions involving an acquisition or proposed acquisition by any person or group holding 15 percent or more of the Company’s outstanding common stock, the rights permit the holders to purchase from the Company one unit consisting of one-thousandth of a share of the Company’s Series A junior participating preferred stock at a price of $19.00 per unit, subject to adjustment. Under certain conditions, the rights may be redeemed by the Company’s Board of Directors in whole, but not in part, at a price of $0.01 per right.
Common Shares Reserved For Future Issuance
At December 31, 2005 common shares reserved for future issuance consist of the following:
Stock and stock options | | 3,475,667 | |
Employee stock purchase plan | | 1,931,280 | |
| | 5,406,947 | |
7. Income Taxes
At December 31, 2005, the Company had federal and California income tax net operating loss carryforwards of approximately $28.9 million and $14.7 million, respectively. At December 31, 2005, the Company also had foreign income tax net operating loss carryforwards of approximately $9.93 million which will begin to expire in 2008. The difference between the federal and California tax net operating loss carryforwards is primarily attributable to the capitalization of research and development expenses and the percentage limitation on the carryover of net operating losses for California income tax purposes.
F-28
The federal tax loss carryforwards will begin to expire in 2010 unless previously utilized. The California tax loss carryforwards will continue to expire in 2006. The Company also has federal and California research tax credit carryforwards of approximately $2.7 million and $1.4 million, respectively. The federal research tax credit carryforwards will begin to expire in 2011 unless previously utilized. The California research tax credits will carry forward indefinitely. Pursuant to Internal Revenue Code Sections 382 and 383, use of the net operating loss and credit carryforwards may be limited because of a cumulative change in ownership of more than 50%, which may have occurred for tax purposes. As of December 31, 2005, the Company had approximately $30.2 million in tax-deductible goodwill and other intangibles related to the purchase of Axys Advanced Technologies in May 2000. The majority of this amount is amortized over a 15-year period for tax purposes.
Significant components of the Company’s deferred tax assets are shown below. A valuation allowance of $40.0 million has been recognized to offset the deferred tax assets as realization of such assets is uncertain.
| | December 31, | |
| | 2005 | | 2004 | |
Deferred tax assets: | | | | | |
Net operating loss carryforwards | | $ | 12,685,617 | | $ | 7,268,542 | |
Research and development credits | | 3,889,479 | | 3,889,479 | |
Capitalized research and development expenses | | 189,773 | | 236,593 | |
Intangible assets | | 14,625,512 | | 13,797,211 | |
Inventory reserves | | 7,187,240 | | 7,682,888 | |
Other, net | | 1,587,391 | | 1,150,848 | |
Total deferred tax assets | | 40,165,012 | | 34,025,561 | |
Valuation allowance for deferred tax assets | | (39,985,857 | ) | (33,846,406 | ) |
Net deferred tax assets | | 179,155 | | 179,155 | |
Deferred tax liabilities: | | | | | |
Acquisitions | | (179,755 | ) | (179,155 | ) |
Net deferred tax assets | | $ | — | | $ | — | |
8. Retirement Plan
In 1996, the Company established a 401(k) plan covering substantially all domestic employees. The Company pays all administrative fees of the plan. The plan contains provisions allowing for the Board of Directors to declare a discretionary match. In 2003, the Board of Directors authorized a matching contribution equal to 50% of the first 6% deferred by the employee to be awarded annually unless rescinded by a future decision by the Board of Directors. Accordingly, $229,729 and $350,822 were accrued at December 31, 2005 and 2004, respectively which were subsequently paid in January of the following fiscal year. Plan administration costs
F-29
totaled $22,296, $22,752 and $20,978 for the years ended December 31, 2005, 2004 and 2003, respectively.
9. Significant Customers, Suppliers and Foreign Operations
Most of the Company’s operations and long-lived assets are based in the United States. DPI AG, located near Basel, Switzerland, had long-lived assets totaling $1,657,450 and $2,839,038 (net of amortization) at December 31, 2005 and 2004, respectively. DPI GmbH, located in Heidelberg, Germany, had long-lived assets totaling $1,456,706 (net of amortization) at December 31, 2005. Net loss for consolidated DPI AG totaled $3,687,428 for the year ended December 31, 2005. Net income for DPI AG totaled $1,063,557 and 94,988 for the years ended December 31, 2004 and 2003, respectively.
The following table presents the geographic breakdown of our revenue, from continuing operations, for our last three fiscal years.
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
United States | | 84 | % | 82 | % | 80 | % |
Foreign Countries | | 16 | % | 18 | % | 20 | % |
The following table presents the geographic breakdown of our long-lived assets, from continuing operations, for our last two fiscal years.
| | As of December 31, | |
| | 2005 | | 2004 | |
United States | | 63 | % | 79 | % |
Switzerland (DPI AG) | | 32 | % | 21 | % |
Germany (DPI GmbH) | | 5 | % | 0 | % |
Major customers are defined as those responsible for 10% or more of revenues and have historically included collaborative partners, pharmaceutical and biopharmaceutical companies. The following table illustrates our major customers for the last three fiscal years from continuing operations:
F-30
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
| | | | | | | |
Pfizer | | 54 | % | 62 | % | 69 | % |
National Institutes of Health (NIH) | | 14 | % | 3 | % | 0 | % |
Others | | 32 | % | 35 | % | 31 | % |
10. Related Party Transactions
In June 2002, the Company hired Taylor J. Crouch and in July 2002 he was appointed its President and Chief Operating Officer. In connection with Mr. Crouch’s employment offer, the Company agreed to assist him in his relocation from Massachusetts to California. On July 29, 2002, the Company loaned Mr. Crouch $300,000 against his full recourse non-interest bearing promissory note. On January 18, 2005, the Company entered into a separation agreement with Taylor Crouch whereby Mr. Crouch’s employment with the Company ended effective January 18, 2005 (Separation Agreement). Pursuant to the terms of the Separation Agreement, Mr. Crouch received a lump sum payment of $378,538 on January 28, 2005. Additionally, the balance owed totaling $300,000 by Mr. Crouch pursuant to the promissory note made by Mr. Crouch to the Company, was reduced by the amount equivalent to the amount that Mr. Crouch could have earned from participation in the Company’s incentive compensation plan for fiscal year 2004, of approximately $106,000, plus an amount equivalent to the sum of the fair market value, on January 18, 2005, of 21,250 shares of the Company Stock under a stock grant as if such stock grant had vested as to an additional 25% plus an amount equivalent to the fair market value, as of January 18, 2005, of 8,750 vested shares of the Company’s Common Stock, less any applicable withholding taxes. The remaining balance was paid in full by Mr. Crouch.
On November 14, 2005, the Company announced that Riccardo Pigliucci had resigned as the Company’s Chief Executive Officer and as the Chairman of the Company’s Board of Directors, effective as of November 14, 2005. In connection with Mr. Pigliucci’s resignation, on November 14, 2005, the Company and Mr. Pigliucci entered into a separation agreement. Pursuant to the separation agreement and in satisfaction of the Company’s obligations under its employment agreement with Mr. Pigliucci, Mr. Pigliucci received a payment of $475,000, which equals Mr. Pigliucci’s 2005 annual base salary, along with a bonus of $75,000 for Mr. Pigliucci’s service to the Company during 2005. The Company also agreed to pay the monthly premiums for continued health insurance coverage under COBRA for Mr. Pigliucci for a period of twelve months from the effective date of his resignation, and the Company paid Mr. Pigliucci’s legal expenses in connection with the separation agreement totaling $10,000 in 2005. In addition, the Company agreed to partially accelerate the vesting of one of his restricted stock grants, such that the grant was deemed vested as to 56,250 shares at the time of his resignation. Mr. Pigliucci is also entitled to receive an additional lump sum payment of $475,000 in the event that the Company is acquired pursuant to a change of control transaction on or before June 30, 2006.
F-31
11. Revenues by Product Category
The Company operates in one industry segment: the development and marketing of services to make the drug discovery process more efficient, less expensive and more likely to generate a drug target. Such services include libraries of drug-like compounds, drug discovery services, compound management services, computational tools to generate compound libraries, and testing and screening services to optimize potential drugs. Additionally, the Company licenses proprietary gene profiling systems. The Company’s services are complementary, and share the same customers and marketing strategies. In addition, in making operating and strategic decisions, the Company’s management evaluates revenues based on the worldwide revenues of each major type of service, and profitability on an enterprise-wide basis. Revenue by service category is as follows:
| | Years Ended December 31, | |
| | 2005 | | 2004 | | 2003 | |
Chemistry services | | $ | 26,502,598 | | $ | 34,720,549 | | $ | 37,350,781 | |
Screening services | | 7,049,796 | | 9,326,265 | | 7,049,248 | |
Other licenses and services | | 284,583 | | 220,843 | | 809,222 | |
Total revenues | | $ | 34,836,977 | | $ | 44,267,657 | | $ | 45,209,251 | |
12. Acquisition of Tangible Assets
On April 22, 2005, the Company, through its subsidiary Discovery Partners International AG (parent to Discovery Partners International GmbH), acquired substantially all of the assets (primarily drug discovery equipment) and assumed certain liabilities of Biofrontera Discovery GmbH, a subsidiary of Biofrontera AG, located in Heidelberg, Germany. Discovery Partners International GmbH provides natural products-based drug discovery capabilities, which will enhance our current drug discovery offerings in lead optimization and candidate selection. Biofrontera Discovery GmbH was a development stage company with no customer contracts or revenues and was not considered a business as defined by EITF Issue No. 98-3, Determining Whether a Nonmonetary Transaction Involves Receipt of Productive Assets or of a Business.
Total acquisition cost was as follows:
Cash paid for assets | | $ | 1,369,266 | |
Acquisition-related expenses | | 108,221 | |
Total purchase price | | $ | 1,477,487 | |
Allocated to assets and liabilities as follows:
Fair value of tangible assets acquired | | $ | 1,824,321 | |
Fair value of liabilities assumed | | (346,834 | ) |
| | $ | 1,477,487 | |
F-32
12. Quarterly Financial Data (Unaudited)
The financial data below has been recast to reflect the results of operations and financial positions of our instrumentation product lines as a discontinued operation. The amounts included in the results for discontinued operations consist of the revenues, cost of sales and operating expenses associated with the operations of the instrumentation product lines excluding any allocations for facilities and other corporate support. The following financial information reflects all normal recurring adjustments which are, in the opinion of management, necessary for a fair presentation of the results of the interim periods. Summarized quarterly data for fiscal 2005 and 2004 are as follows (in thousands, except per share data):
| | 2005 Quarter Ended | |
| | Previously
reported | | Restated | | Previously
Reported | | Restated | | Previously
Reported | | Restated | | | |
| | Mar 31 | | Mar 31 | | Jun 30 | | Jun 30 | | Sep 30 | | Sep 30 | | Dec 31 | |
Revenues | | $ | 7,068 | | $ | 6,734 | | $ | 11,362 | | $ | 11,004 | | $ | 11,080 | | $ | 9,746 | | $ | 7,354 | |
Cost of revenues | | 5,413 | | 5,179 | | 7,662 | | 7,321 | | 6,496 | | 6,012 | | 6,597 | |
Gross margin | | $ | 1,655 | | $ | 1,555 | | $ | 3,700 | | $ | 3,683 | | $ | 4,584 | | $ | 3,734 | | $ | 757 | |
Loss from continuing operations | | $ | (5,093 | ) | $ | (4,506 | ) | $ | (1,817 | ) | $ | (1,337 | ) | $ | (938 | ) | $ | (1,214 | ) | $ | (8,990 | ) |
Net loss from continuing operations | | $ | (4,548 | ) | $ | (3,961 | ) | $ | (1,368 | ) | $ | (888 | ) | $ | (341 | ) | $ | (617 | ) | $ | (8,255 | ) |
Gain (loss) from discontinued operations (1) | | $ | — | | (587 | ) | — | | (480 | ) | — | | 276 | | 347 | |
Net loss | | $ | (4,548 | ) | $ | (4,548 | ) | $ | (1,368 | ) | $ | (1,368 | ) | $ | (341 | ) | $ | (341 | ) | $ | (7,907 | ) |
Net loss per share, basic and diluted (2) | | $ | (0.18 | ) | $ | (0.18 | ) | $ | (0.05 | ) | $ | (0.05 | ) | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.30 | ) |
| | 2004 Quarter Ended | |
| | Previously
reported | | Restated | | Previously
Reported | | Restated | | Previously
Reported | | Restated | | Previously
Reported | | Restated | |
| | Mar 31 | | Mar 31 | | Jun 30 | | Jun 30 | | Sep 30 | | Sep 30 | | Dec 31 | | Dec 31 | |
Revenues | | $ | 11,808 | | $ | 9,520 | | $ | 12,999 | | $ | 11,530 | | $ | 12,575 | | $ | 10,886 | | $ | 14,182 | | $ | 12,331 | |
Cost of revenues | | 6,551 | | 5,294 | | 7,807 | | 6,895 | | 6,820 | | 5,770 | | 8,261 | | 7,186 | |
Gross margin | | $ | 5,257 | | $ | 4,226 | | $ | 5,192 | | $ | 4,635 | | $ | 5,755 | | $ | 5,116 | | $ | 5,921 | | $ | 5,145 | |
Income from continuing operations | | $ | 632 | | $ | 192 | | $ | 561 | | $ | 631 | | $ | 401 | | $ | 447 | | $ | 1,121 | | $ | 928 | |
Net income from continuing operations | | $ | 1,046 | | $ | 606 | | $ | 740 | | $ | 810 | | $ | 750 | | $ | 796 | | $ | 1,367 | | $ | 1,173 | |
Gain (loss) from discontinued operations (1) | | $ | — | | $ | 440 | | $ | — | | $ | (70 | ) | $ | — | | $ | (46 | ) | $ | — | | $ | 194 | |
Net income | | $ | 1,046 | | $ | 1,046 | | $ | 740 | | $ | 740 | | $ | 750 | | $ | 750 | | $ | 1,367 | | $ | 1,367 | |
Net income per share, basic and diluted (2) | | $ | 0.04 | | $ | 0.04 | | $ | 0.03 | | $ | 0.03 | | $ | 0.03 | | $ | 0.03 | | $ | 0.05 | | $ | 0.05 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-33
(1) The amounts reflect the results of the discontinued operations associated with the instrumentation product line.
(2) Net income (loss) per share is calculated independently for each of the quarters presented. Therefore, the sum of the quarterly net income (loss) per share will not necessarily equal the total for the year.
F-34
