- INFIQ Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
425 Filing
Infinity Pharmaceuticals (INFIQ) 425Business combination disclosure
Filed: 11 Sep 06, 12:00am
Filed by Discovery Partners International, Inc. Pursuant to Rule 425
Under the Securities Act of 1933
and Deemed Filed Pursuant to Rule 14a-12
Under the Securities Exchange Act of 1934
Subject Company: Infinity Pharmaceuticals, Inc.
Commission File No. 333-134438
Additional Information about the DPI-Infinity Merger and Where to Find It
In connection with the proposed merger between Discovery Partners International, Inc. (DPI) and Infinity, on August 7, 2006, DPI filed an amended registration statement on Form S-4 that contains a proxy statement/prospectus, which registration statement has been declared effective by the SEC. Investors and security holders of DPI and Infinity are urged to read the proxy statement/prospectus (including any amendments or supplements to the proxy statement/prospectus) regarding the proposed merger because it contains important information about DPI, Infinity and the proposed merger. Security holders will be able to obtain a copy of the proxy statement/prospectus, as well as other filings containing information about DPI and Infinity, without charge, at the SEC’s Internet site (http://www.sec.gov). Copies of the proxy statement/prospectus can also be obtained, without charge, by directing a request to Discovery Partners International, Inc., 9640 Towne Centre Drive, San Diego, CA 92121, Attention: Investor Relations, Telephone: (858) 455-8600.
Participants in the solicitation
DPI and its directors and executive officers and Infinity and its directors and executive officers may be deemed to be participants in the solicitation of proxies from the stockholders of DPI in connection with the proposed merger of DPI with Infinity. Information regarding the special interests of these directors and executive officers in the merger transaction is included in the proxy statement/prospectus referred to above. Additional information regarding the directors and executive officers of DPI is also included in DPI’s proxy statement for its 2006 Annual Meeting of Stockholders, which was filed with the SEC on April 6, 2006. This document is available free of charge at the SEC’s web site (www.sec.gov) and from Investor Relations at DPI at the address described above.
Infinity gave the following presentation on September 8, 2006.
 Introduction to Infinity September 8, 2006 |
 Forward-Looking Statements • This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 that involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. You are urged to consider statements that include the words “may,” “will,” “would,” “could,” “should,” “believes,” “estimates,” “projects,” “potential,” “expects,” “plans,” “anticipates,” “intends,” “continues,” “forecast,” “designed,” “goal,” or the negative of those words or other comparable words to be uncertain and forward- looking. Such forward-looking statements include statements regarding the expected benefits of the merger of Infinity and DPI for stockholders of the combined company, the expectation that the merger will enable the combined company to be well positioned to drive forward its pipeline of anti-cancer agents and create substantial value for patients and stockholders, and the expectation that the combined company will have cash to support its current operating plan through at least December 31, 2009. Such statements are subject to numerous factors, risks and uncertainties that may cause actual events or results to differ materially from the combined company's current expectations. For example, there can be no guarantee that any product candidate the combined company is developing will successfully complete necessary preclinical and clinical development phases, be approved for sale in any market or that, if approved, revenues from sales of such product will reach any specific level. In particular, management's expectations could be affected by risks and uncertainties relating to: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the combined company's dependence on its collaborations with MedImmune and Novartis; the combined company's ability to obtain additional funding required to conduct its research, development and commercialization activities; unplanned cash requirements and expenditures; and the company's ability to obtain, maintain and enforce patent and other intellectual property protection for any products it is developing. These and other risks which may impact management's expectations are described in greater detail under the caption "Risk Factors" in DPI's registration statement on Form S-4, as amended, as filed with the Securities and Exchange Commission and DPI’s other SEC reports. • Any forward-looking statements contained in this presentation speak only as of the date hereof and Infinity expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. |
 Mission To develop targeted therapies for the treatment of cancer and related conditions discovered through the use of our innovative small molecule drug technologies |
 • Lead product candidate: IPI-504, a novel Hsp90 inhibitor – Two ongoing Phase I cancer studies in GIST and multiple myeloma – Phase II expected 2007 • Pipeline of preclinical cancer drug candidates – Internally discovered and developed, chemistry platform • 5 Pharma/Biotech corporate alliances – MedImmune, Novartis (2), Amgen, and J & J • Proven biotech leadership team • Significant cash position after MEDI alliance and DPI merger – Funds sufficient for projected operating expenses through end of 2009 Infinity Snapshot |
 Strategy • Drugs – Internally discovered, novel small molecules • Targets – “Well-credentialed, but not well-trodden” • Products – Opportunity for first-in class or fast follower best-in-class |
 Overview • Founded in late 2001 (~5 years old) • Team – Recognized biotechnology investor, business and R&D leaders – ~115 employees (~55 PhD / MDs) • Alliance and Financing Strategy – Hsp90 and Hedgehog pathway product alliance with MedImmune – Bcl family product alliance with Novartis – Small molecule technology access alliances with Amgen, J&J and Novartis – Public financing via Reverse Merger with Discovery Partners • IPI-504 – lead proprietary oncology drug candidate (Hsp90) – Phase I in GIST and multiple myeloma commenced 2005 – Phase II anticipated in 2007 • Hedgehog pathway – preclinical oncology candidate |
 Our Team: ~115 full-time employees Infinity headcount Biology/Clinical/Regulatory 36 Chemistry 50 Management & other 12 (~55 MD or PhDs) R&D Total 98 Total 115 G&A 17 • Well-balanced • Moderate near-term growth anticipated – Primarily in “downstream” disciplines (i.e. clinical, regulatory, CMC/ADME/tox) |
 Leadership Mr. Steven Holtzman, CEO Millennium, DNX Dr. Julian Adams, President & CSO Millennium, ProScript Boehringer Ingelheim, Merck Ms. Adelene Perkins, CBO Transform, Genetics Institute, Bain, GE Dr. David Grayzel, VP Clinical Development & Medical Affairs Dyax, Mass General Hospital Dr. Vito Palombella, VP Discovery Biology Syntonix, Millennium, ProScript Dr. Jeffrey Tong, VP Corp & Prod Dev McKinsey & Co, Harvard Center for Genomics Research Dr. Jim Wright, VP Pharm Dev Millennium, Alkermes, Boehringer Ingelheim, Syntex, U. of Wisconsin |
 SAB – Oncology & Chemistry • Co-chair: Stuart Schreiber, PhD - Co-Director Broad Institute, Prof. of Chemistry and Chemical Biology Harvard University • Co-chair: Rick Klausner, MD – Column Group, former Head of the NCI • Arnie Levine, PhD - Institute for Advanced Study • Eric Lander, PhD - Co-Director Broad Institute, Whitehead, MIT, Harvard • Todd Golub, MD - DFCI, Broad Institute, Harvard, MIT • David Livingston, MD – Professor of Medicine, Harvard Medical School, DFCI • Ken Anderson, MD - Robert Kraft Prof. of Medicine Harvard Medical School, DFCI • Matthew Shair, PhD – Professor of Chemistry, Harvard University • Vicki Sato, PhD – former President Vertex Pharmaceuticals • Phil Needleman, PhD - former Head of R&D Searle, Pharmacia |
 Investors Venture Capitalists • Prospect Venture Partners • Venrock Associates • Advent Venture Partners • HBM BioVentures • Vulcan Ventures • Novartis BioVentures • Wellcome Trust • POSCO BioVentures • Tallwood • Alexandria Equities • Lotus BioScience Pharmaceutical Companies • Amgen • Novartis • J&J |
 Overview • Founded in late 2001 (~5 years old) • Team – Recognized biotechnology investor, business and R&D leaders – ~115 employees (~55 PhD / MDs) • Alliance and Financing Strategy – Hsp90 and Hedgehog pathway product alliance with MedImmune – Bcl family product alliance with Novartis – Small molecule technology access alliances with Amgen, J&J and Novartis – Public financing via Reverse Merger with Discovery Partners • IPI-504 – lead proprietary oncology drug candidate (Hsp90) – Phase I in GIST and multiple myeloma commenced 2005 – Phase II anticipated in 2007 • Hedgehog pathway – preclinical oncology candidate |
 DOS Small Molecule Technology: Discovery and Alliance Engine • Innovative small molecule platform, diversity oriented synthesis (DOS), enables the creation of novel, “natural product-like” synthetic drug candidates • Potential to access previously “undruggable” drug targets • Unique asset for: – Internal drug discovery – Value-accretive technology access alliances |
 • Diversity Oriented Synthesis (DOS) • 2004 – 2006: > $60 million upfront/committed cash • Additional milestone and royalty potential • No license of proprietary Infinity product rights Small Molecule Technology Access Alliances |
 • Total payments >$400M Early product pipeline: Bcl family alliance with Novartis • Joint discovery of novel Bcl family (Bcl-2, Bcl-xL) targeted cancer drugs • Infinity participation in clinical development (at NVS expense) COLLABORATION • Infinity participation in US sales effort (at NVS expense) $30M • Upfront & committed funds FINANCIALS • Royalties on WW sales |
 • Clinical and commercial milestones Lead products: Hsp90 and Hedgehog alliance with MedImmune • Infinity leads early translational development through proof of concept • MedImmune leads later clinical development, worldwide registration and sales and marketing, with Infinity participation COLLABORATION • Infinity has right to provide up to 35% of US promotional activity (cost shared by alliance) $70M • Upfront funds FINANCIALS • 50% R&D cost sharing $430M • 50% worldwide profit split |
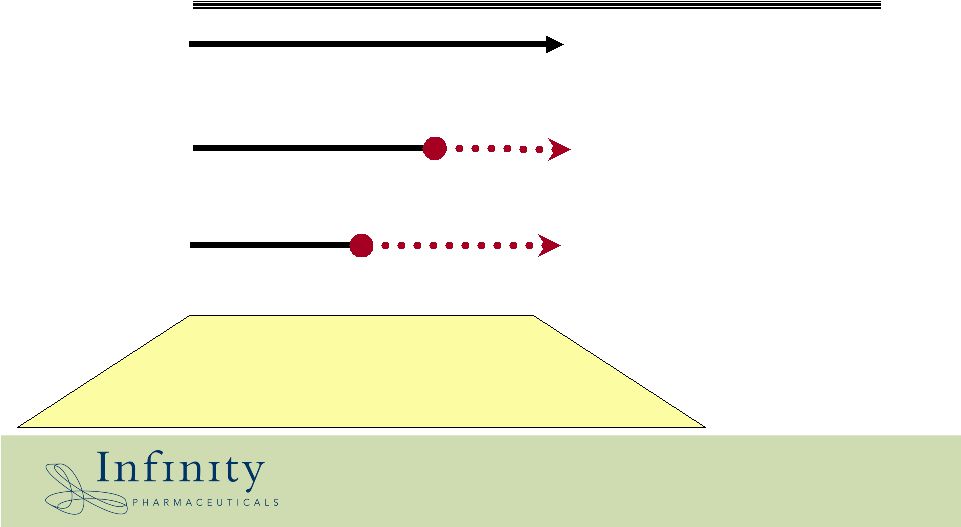 Discovery Preclinical Start Clinical Trials Hsp90 (IPI-504) Bcl-2/Bcl-xL 2005 2007/2008 50% WW profit share with MEDI 50% WW profit share with MEDI Royalty from Novartis Non-exclusive – Amgen – Novartis – J&J Small molecule drug technologies Alliance and financing strategy: value retention Hedgehog Pathway (IPI-609) 2007 |
 Reverse Merger with Discovery Partners International, Inc. (NASDAQ: DPII) * * * * * * |
 DPI reverse merger opportunity • Discovery Partners International – Publicly traded company on NASDAQ (DPII) – Cash position 1/1/06: > $83M – Board mandate (Q1, 2006): • Shut down existing business • Seek alternative, high-value biotech investment opportunity • DPI undertakes extensive evaluation of merger candidates • DPI selects Infinity as preferred partner |
 • A financing event only – NO programs, employees, partnerships, or obligations of DPI transferred to Infinity – DPI “invests” cash and divests operating units – 7/7/06: Sale of all DPI operating assets to Galapagos • If DPI cash between $70M and $75M, ownership: – DPII stockholders = 31% – Infinity stockholders = 69% • If cash above $75M or below $70M, adjustment applied • 4:1 reverse stock split approved by DPI board to lower share number and bring share price >$10 The reverse merger: a creative financing and access to public markets |
 • Lead clinical product in two ongoing Phase I cancer studies – Phase II expected 2007 • Pipeline of preclinical cancer drug candidates – Internally discovered and developed, chemistry platform • 5 Pharma/Biotech corporate alliances – MedImmune, Novartis (2), Amgen, and J & J • Proven biotech leadership team • Significant cash position – Projected cash runway through end of 2009 – Enough cash to reach key value-driving events before any additional alliances or financing Snapshot of Post-Merger Infinity (NASDAQ: INFI) |
 Status of Reverse Merger Announce merger File Initial S4 S-4 is Declared Effective S-4 mailed to DPI and IPI Stockholders Stockholder meeting/vote scheduled Deal Closes, INFI publicly traded April 12, 2006 July 11, 2006 August 7, 2006 August 9-10, 2006 September 12, 2006 Following successful vote |
 Overview • Founded in late 2001 (~5 years old) • Team – Recognized biotechnology investor, business and R&D leaders – ~115 employees (~55 PhD / MDs) • Alliance and Financing Strategy – Hsp90 and Hedgehog pathway product alliance with MedImmune – Bcl family product alliance with Novartis – Small molecule technology access alliances with Amgen, J&J and Novartis – Public financing via Reverse Merger with Discovery Partners • IPI-504 – lead proprietary oncology drug candidate (Hsp90) – Phase I in GIST and multiple myeloma commenced 2005 – Phase II anticipated in 2007 • Hedgehog pathway – preclinical oncology candidate |
 • Novel Hsp90 inhibitor • Currently in 2 phase I clinical trials: • GIST • Multiple myeloma • Ready for Phase II in 2007 • Both IV (water-soluble) and oral formulations Cl - Infinity’s lead clinical product: IPI-504 (Hsp90 inhibitor) IPI-504 OH N H N OH O OH Me O O O O NH 2 H H + |
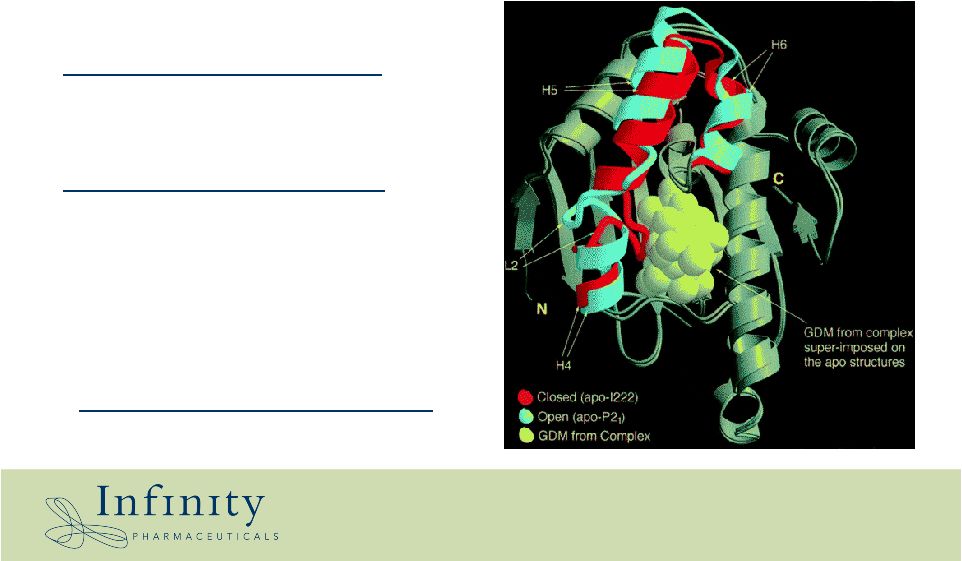 Heat Shock Protein 90 (Hsp90) is an emerging cancer target Hsp90 in cancer cells differs from Hsp90 in normal cells Function of Hsp90 in cancer cells • General chaperone function – essential for protein homeostasis • Specific chaperone function – stabilization of oncogenic proteins in key cell signaling pathways • Preferential targeting to cancer |
 Dependence on Hsp90 Apoptosis Tyrosine kinase inhibitor (e.g Gleevec, Tarceva) Oncogene Cancer cell survival & proliferation Resistance mutations Hsp90 inhibitor Targeting specific oncogenic Hsp90 client proteins Hsp90 inhibitor |
 Velcade Gleevec / dasatinib Investigational Gleevec / Sutent Herceptin Tarceva / Erbitux Sorafenib / Sutent Sorafenib Investigational Targeted therapy The emerging world of targeted cancer therapies Indication Myeloma CML AML GIST Breast (HER2+) NSCLC Renal cell Melanoma Prostate (PTEN -/-) NF- B Bcr-Abl Flt3 c-Kit HER2 EGFR VEGFR / HIF-1a b-Raf p-Akt Molecular Target |
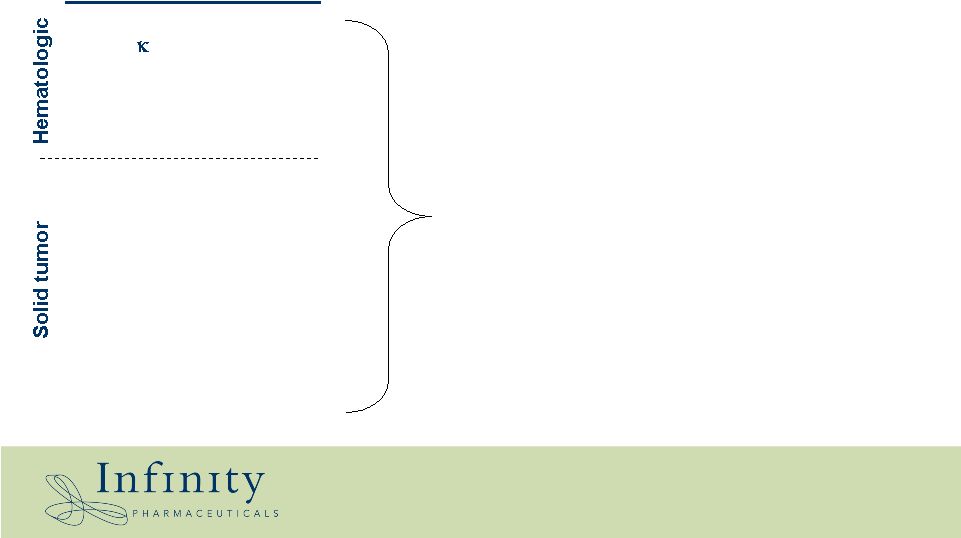 The emerging world of targeted cancer therapies NF- B Bcr-Abl Flt3 c-Kit HER2 EGFR VEGFR / HIF-1a b-Raf p-Akt Molecular Target • All are clients of Hsp90 • Inhibiting Hsp90 affects the stability of these targets • Attractive alternative to chasing tumor-specific resistance mutations |
 History of Geldanamycin analogs • 17-AAG is a semi-synthetic natural product, derived from Geldanamycin • 17-AAG activity: – Potent & selective inhibitor of Hsp90 – Well-tolerated in humans (>400 patients tested in multiple Phase I trials) – Removed chemical reactivity of geldanamycin • Problems: – Highly insoluble – Sub-optimal DMSO-and Cremophor based formulations – Off-patent O N H H N O Me O OH Me Me O Me O O O N H Me Me 17-AAG *Reference: Kamal et al, Nature, 2003, 425,.407-410 |
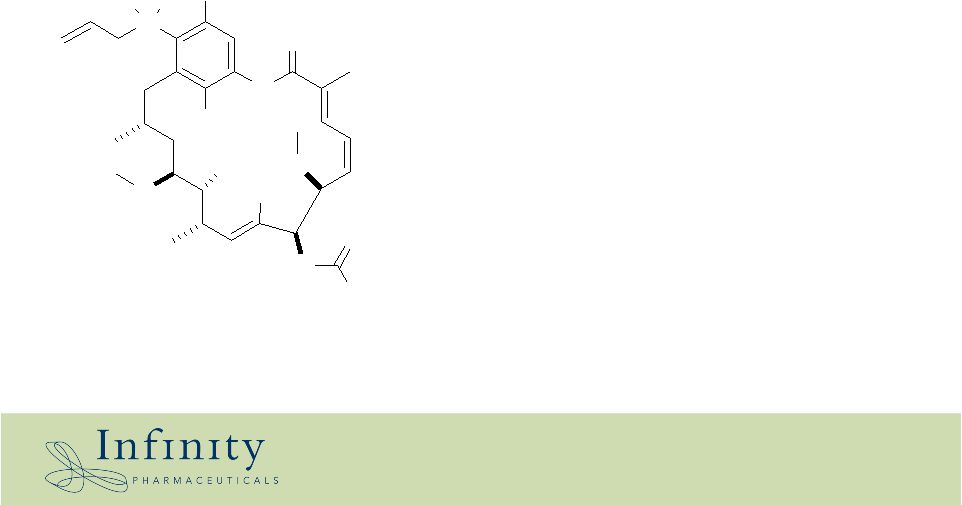 • Novel chemical entity • Patient-friendly formulations – IV in two Phase 1 trials – Oral under development • Broad therapeutic potential – Large therapeutic window consistent with targeted therapies – Activity in resistant settings • Strong intellectual property position • Ready for Phase 2 in 2007 Cl - Infinity’s lead clinical product: IPI-504 (HSP90 inhibitor) IPI-504 OH N H N OH O OH Me O O O O NH 2 H H + |
 IPI-504 |
 IPI-504 competitive landscape for IV formulation POTENCY NCE PATENT? DELIVERY CHEMICAL PROPERTIES MTD COMPOUND COMPANY 17-DMAG KOS-1022 ~25-50 nM Yes IV 60–120 min Chemically reactive alkylating agent <24 mg/m² Kosan 17-AAG KOS-953 ~25-50 nM No IV 60-120 min in Cremophor Special tubing Steroid pretreatment Emulsion changes distribution and PK Dose escalation ongoing; > 340 mg/m² Kosan Emulsion changes distribution and PK 17-AAG CNF-1010 ~25-50 nM No IV 60 min in lipid emulsion 175 mg/m² Biogen/ Conforma Emulsion changes distribution and PK 17-AAG ~25-50 nM No IV 60 min in DMSO/Egg 220 mg/m² Kosan IPI-504 ~25-50 nM IV 30 min Diffusion controlled distribution Dose escalation ongoing at 400 mg/m² Yes Infinity |
 IPI-504 competitive landscape for PO formulations IPI-504 (same molecule as IV) 17-DMAG CNF-2024 Small Molecule Small Molecule Small Molecule Compound Company Phase of Development Infinity Kosan Biogen Idec Serenex Novartis / Vernalis Synta Pre-clinical Phase I Phase I Preclinical Preclinical Preclinical No competitive oral product is significantly more advanced Novel small molecules not derived from geldanamycin |
 Intellectual property protection for IPI-504 • Composition of matter • Formulations (IV and PO) • Methods of making • Methods of using Infinity has broad patent applications pending for IPI-504 |
 IPI-504 Preclinical Data * * * * * * * * |
 Highly responsive to Hsp90 inhibition T315I T790M T670I Preclinical evidence of potential as salvage therapy BCR-ABL EGFR KIT Hsp90 Client Disease Drug CML NSCLC GIST Gleevec, Dasatinib Tarceva, Iressa Gleevec, Sutent Kinase Inhibitor Resistance Mutation |
 CML / Bcr-Abl Wild-type protein Bcr Abl Non-cancer related Protein status Entity Function Hsp90- dependent Gain-of-function mutant Bcr-Abl fusion Constitutively activated signaling Drug-resistant mutant Bcr-Abl (T315I) TKI-resistant kinase |
 Gleevec-refractory primary CML cells sensitive to IPI-504 0 10 20 30 40 50 60 70 Pt 1 Pt 2 (T315I) Pt 3 Control 0.5 uM IPI-504 2.0 uM IPI-504 Collaboration: Kapil Bhalla, Moffitt Cancer Center |
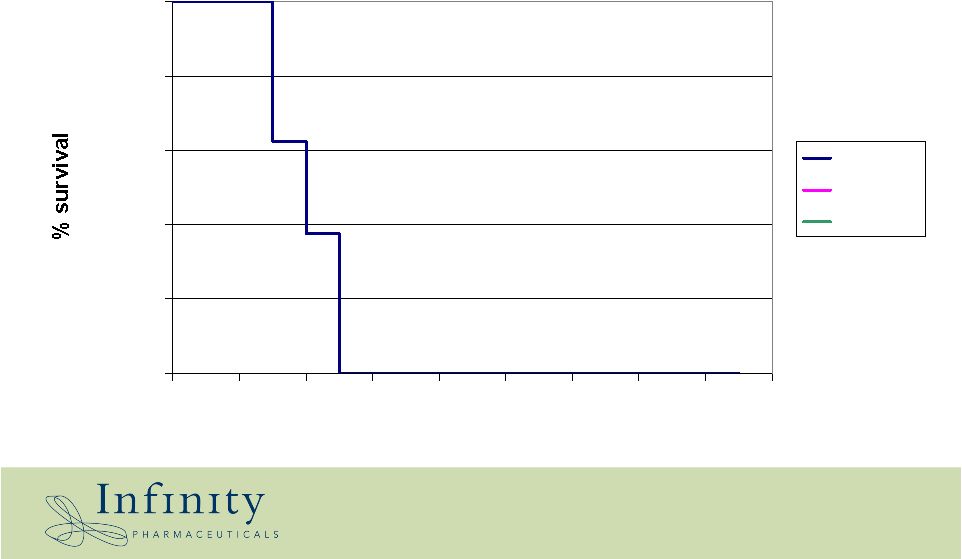 Placebo Gleevec IPI-504 Collaboration: Shauguang Li, Jackson Labs 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% 15 17 19 21 23 25 27 29 31 33 Days Oral IPI-504: survival benefit in Gleevec-resistant T315I CML transplantation model |
 Placebo Gleevec IPI-504 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% 15 17 19 21 23 25 27 29 31 33 Days Oral IPI-504: survival benefit in Gleevec-resistant T315I CML transplantation model Collaboration: Shauguang Li, Jackson Labs |
 Placebo Gleevec IPI-504 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% 15 17 19 21 23 25 27 29 31 33 Days Oral IPI-504: survival benefit in Gleevec-resistant T315I CML transplantation model Collaboration: Shauguang Li, Jackson Labs |
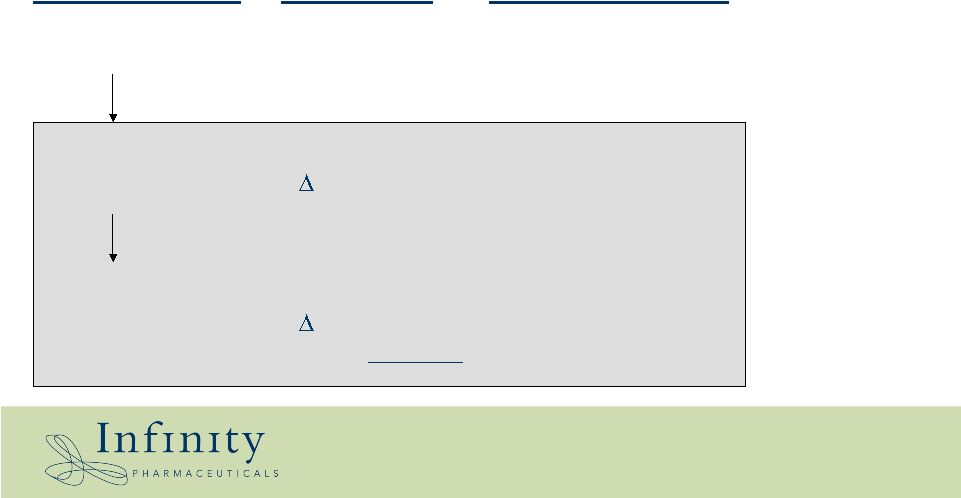 NSCLC / EGFR Wild-type protein EGFR Ligand-dependent RTK Protein status Entity Function Hsp90- dependent Gain-of-function mutant EGFR ( exon19 or L858R) Ligand- hypersensitive RTK Drug-resistant mutant EGFR ( exon19 or L858R + T790M) TKI-resistant, ligand hypersensitive RTK |
 0 500 1000 1500 2000 2500 3000 3500 4000 12 15 19 22 26 27 32 Days Post-Implant IPI-504 Vehicle, IP Gefitinib Vehicle, PO 100mpk Gefitinib, PO 100mpk IPI-504, IP • 100mpk IPI-504 2X weekly IP; 100mpk Gefitinib daily PO for 3 weeks • 21% difference in tumor volumes between vehicle and Gefitinib treated groups (p=0.54) • 69% difference in tumor volumes between vehicle and IPI-504 treated groups (p=0.009) 69% Non small cell lung cancer xenograft with T790M EGFR Tarceva/Iressa-resistance mutation |
 GIST / Kit Wild-type protein Kit Ligand-dependent RTK Protein status Entity Function Hsp90- dependent Gain-of-function mutant c-Kit Ligand-independent RTK Drug-resistant mutant c-Kit (T670I) TKI-resistant, ligand-independent RTK |
 GIST: Gleevec-resistant cells more sensitive to IPI-504 GIST 882* Gleevec-Sensitive (primary: exon 13, K642E) 10 100 1000 10 20 30 40 50 10000 60 70 Compounds concentrations (nM) 10 100 1000 10 20 30 40 50 10000 10000 60 70 Compounds concentrations (nM) IPI-504 : EC50 = 121 +/- 21 nM IM : EC50 = 147 +/- 42 nM Gleevec- Resistant (primary: exon 11, V560D + Gleevec resistance: exon 17, D820A) 10 100 1000 5 15 25 35 45 55 65 75 85 Compounds concentrations (nM) IPI-504 Imatinib GIST 48* IPI-504 : EC50 = 54 +/- 7 nM IM : 25% inhibition @ 10uM Collaboration: Fletcher, Demetri, DFCI |
 IPI-504 Clinical Development Strategy * * * * * * * * * |
 • Development and registration of IPI-504 in hematologic malignancies and solid tumors – Preclinical support for broad role of Hsp90 • Early human proof-of-concept with most rapid path to registration – Strong scientific rationale – Trials targeted to homogenous patient population (disease-focused) – Surrogate marker – Rapid patient accrual – Single-agent activity in refractory setting (potential for expedited approval) • In parallel, initiate broader development for larger indications (additional diseases, combination therapy, front-line therapy) IPI-504 Clinical Development Strategy |
 Principal Investigator: • Dr. George Demetri, DFCI Objectives: • Safety, PK, dose-ranging • Establish Phase II dose Surrogate marker of response: • PET scans Solid Tumor Gastrointestinal Stromal Tumors (Gleevec-resistant) Schedule / status: • Days 1, 4, 8, 11 of 21 day • Continuing dose escalation Current ongoing phase I clinical trials Principal Investigator: • Dr. Paul Richardson, DFCI • Dr. Sundar Jagannath, SVCCC • Dr. David Siegel, HUMED Objectives: • Safety, PK, dose-ranging • Establish Phase II dose Surrogate marker of response: • M protein levels Hematologic Multiple Myeloma (relapsed, refractory) Schedule / status: • Days 1, 4, 8, 11 of 21 day • Continuing dose escalation |
 Phase I dose escalation for IPI-504 (GIST) • 1 cycle = 21 days • 4 doses (days 1, 4, 8, 11 followed by 10 days off) Phase I schedule 25% 500 6 33% 400 5 33% 300 4 50% 225 3 66% 150 2 100% 90 1 Escalation over previous dose Dose (mg/m2) Group |
 Near-term sequence of additional clinical indications (2006/2007) Resistance Mutation Disease PI T. Lynch T. Kipp, CLL consortium Matsui, Smith / Bhalla NSCLC CLL CML Tarceva-R (T790M ) Zap-70 T315I • Focused trials would determine IPI-504 activity in patients with known resistance to targeted therapy • If positive, trials provide opportunity to rapidly advance to market • Additional indications to follow Site MGH UCSD JHU, Moffitt |
 Overview • Founded in late 2001 (~5 years old) • Team – Recognized biotechnology investor, business and R&D leaders – ~115 employees (~55 PhD / MDs) • Alliance and Financing Strategy – Hsp90 and Hedgehog pathway product alliance with MedImmune – Bcl family product alliance with Novartis – Small molecule technology access alliances with Amgen, J&J and Novartis – Public financing via Reverse Merger with Discovery Partners • IPI-504 – lead proprietary oncology drug candidate (Hsp90) – Phase I in GIST and multiple myeloma commenced 2005 – Phase II anticipated in 2007 • Hedgehog pathway – preclinical oncology candidate |
 • Potential for first-in-class systemic hedgehog inhibitor • Proprietary NCE’s • Systemic (sub-cu and oral) products • Lead molecule (IPI-609) in advanced preclinical development • First in man expected in 2007 • Broad anti-cancer potential • Strong data supporting pancreatic, metastatic prostate, SCLC, others • Single agent activity • Potential for synergy with standards of care Infinity’s Hedgehog program |
 History of cyclopamine – chemical discovery 1950’s • Lambs born in Idaho with cyclopic features (defect in development of left-right asymmetry) • USDA determines that pregnant ewes grazed on the plant Veratrum californicum • Cyclopamine identified as the teratogenic substance in V. californicum • Purified cyclopamine given to animals recapitulates cyclopic features and other birth defects V. californicum cyclopamine |
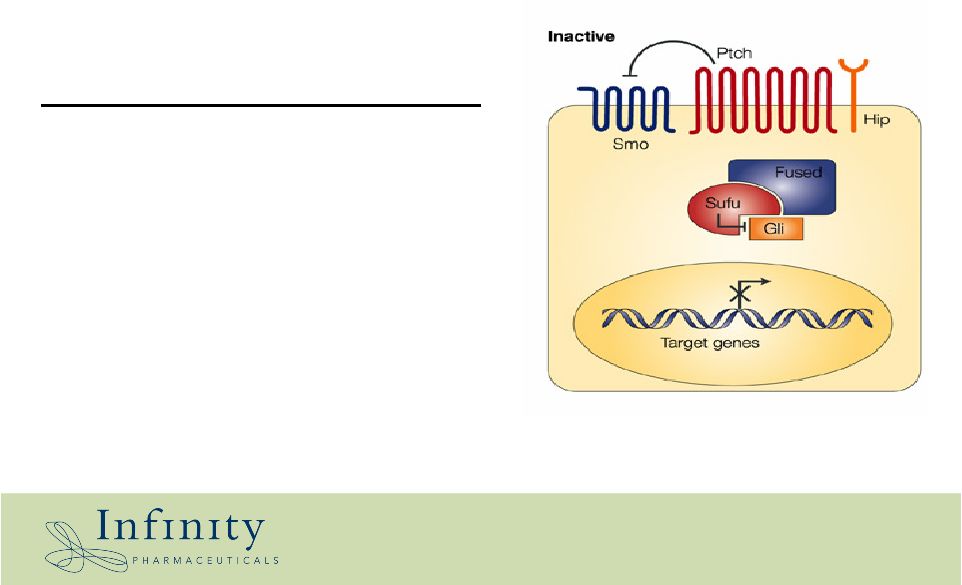 History of hedgehog – genetics 40 years later (1980’s to today) • Genes are discovered that control embryonic development and pattern formation • One such gene is called “hedgehog” • Hedgehog mutations in the Drosophila fruit fly result in cyclopia • Hedgehog function in humans related to development of the pancreas, gut, and other elements of GI tract |
 Cyclopamine chemistry meets hedgehog genetics Chemistry The chemical cyclopamine results in cyclopic animals Genetics Mutation of hedgehog pathway results in cyclopic animals Might the chemical cyclopamine interact with genes in the hedgehog pathway? YES |
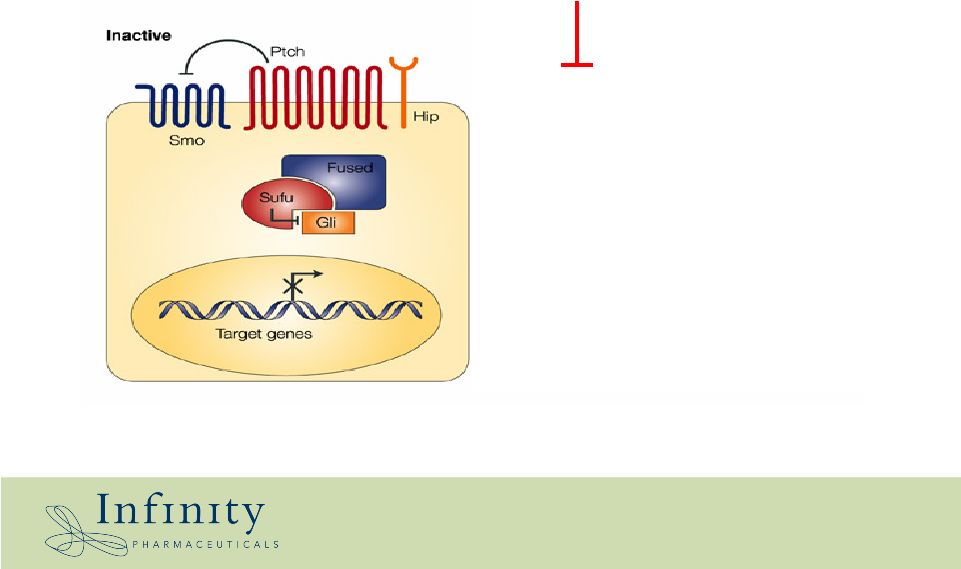 Cyclopamine is a smoothened antagonist *Chen et al., 2002 G&D 16:2743 Cyclopamine Normal Cancer |
 Cancers have hijacked components of the hedgehog pathway # ON = active repressor of Smo * Mutation in Patched 1 Hahn et al., 1996, Cell 85: 841 2 Bale & Yu, 2001, Human Molec. Genetic. 10: 757 (review) 3 Berman et al., 2002 Science 297: 1559 4 Berman et al., 2003 Nature 425: 846 5 Kayed et al., 2004 Int. J. Cancer 110: 668 6 Thayer et al., 2003 Nature 425: 851 7 Karhadkar et al., 2004 Nature, 431: 707 8 Fan et al., 2004 Endocrinology 145: 3961 9 Watkins et al., 2003, Nature 422: 313 10 Sicklick 2005 ASCO; Mohini, 2005 AACR 11 Kubo et al., 2004 Cancer Res. 64 :6071 State Normal Basal cell carcinoma* 1,2 Medulloblastoma*³ Pancreatic cancer 4,5,6 Prostate cancer 7,8 Small cell lung cancer 9 Hepatocellular cancer 10 Breast Cancer 11 Smoothened OFF ON ON ON ON ON ON ON Patched # ON Mutant - OFF Mutant - OFF OFF OFF OFF OFF OFF Hedgehog OFF OFF OFF Turned ON Turned ON Turned ON Turned ON Turned ON Frequency --- 95% 30-40% 100% 100% 50% n/a 100% |
 Cyclopamine validates Hedgehog as a cancer target • Cyclopamine is a plant natural product produced by Veratrum californicum Cyclopamine activity: • Potent inhibitor of Smoothened • Highly active in pancreatic, prostate, small cell lung cancer animal models Drawbacks: • Insoluble • Caustic formulations • Off-patent HO O HN H H H H H |
 Infinity’s lead Hedgehog pathway inhibitors • Novel candidates based on cyclopamine • On mechanism • Superior to cyclopamine: – More chemically stable – More potent – More soluble • Most advanced candidate (IPI-609) in late-preclinical development – First in man 2007 – i.v., s.c., or oral formulations – Better oral bioavailability – Better tumor PK |
 IPI-609 competitive landscape • CUR-61414 – Curis and Genentech Hedgehog antagonist – Highly insoluble: not suitable for systemic administration – Topical formulation failed in Phase 1 Basal Cell Carcinoma trial; failure attributed to formulation, not pathway • Curis and Genentech-have-expressed continued interest in the Hedgehog pathway for systemic agents |
 Intellectual property protection for IPI-609 • Novel scaffold for IPI-609 and analogs with patent applications pending • We believe there are no patents preventing us from marketing IPI- 609 or its analogs |
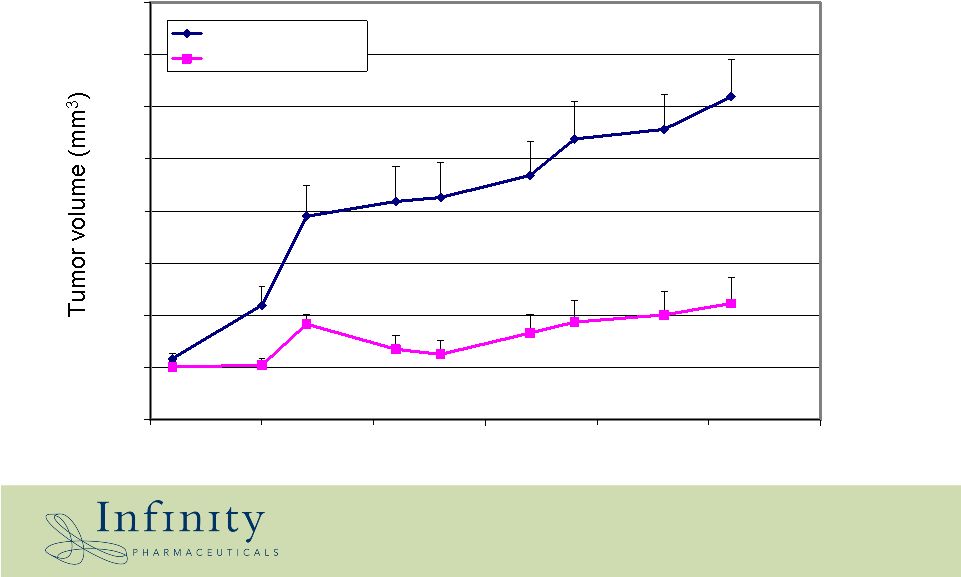 0 200 400 600 800 1000 1200 1400 1600 31 36 41 46 51 56 61 Days Vehicle IPI-609 10 mpk/day IPI-609 efficacious in PC-3 prostate xenograft |
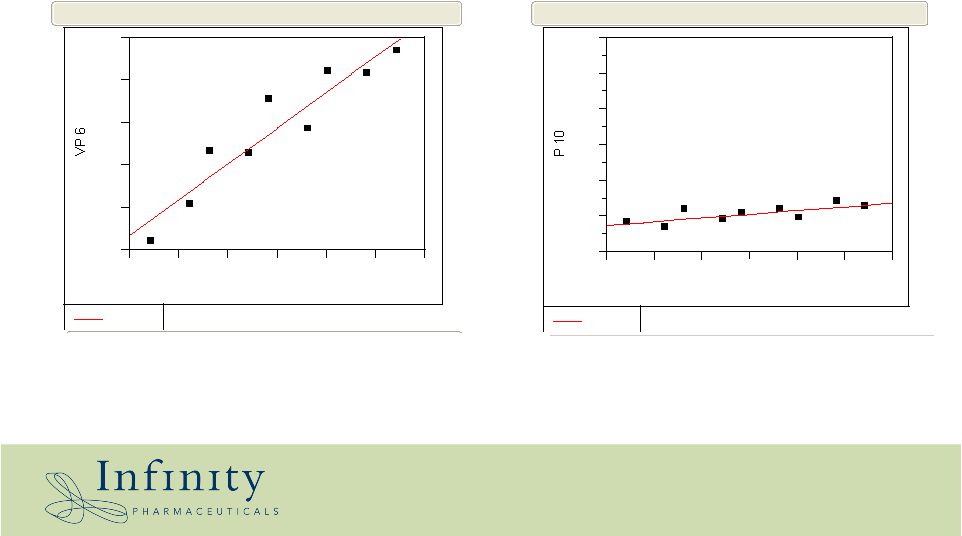 IPI-609 slows tumor growth rates 0 200 400 600 800 1000 1200 30 35 40 45 50 55 60 Day Linear Fit Bivariate Fit of P 10 By Day 200 400 600 800 1000 1200 30 35 40 45 50 55 60 Day Linear Fit Bivariate Fit of VP 6 By Day Median vehicle-treated animals Median IPI-609 treated animals |
 Clinical development strategy of hedgehog pathway inhibitors • Strong scientific rationale supports targeting of cancers dependent on the Hedgehog pathway – Pancreatic – Small cell lung – Metastatic prostate – Metastatic breast – Ovarian – Others (medulloblastoma, glioma, basal cell carcinoma, etc.) • Identify a rapid path to registration – Potential for sole agent activity or – Combination with a single Standard of Care |
 Key Principal Investigator relationships established • Pancreatic cancer Manuel Hidalgo, MD Johns Hopkins (PCRT – Dan Van Hoff, MD) • Small cell lung cancer Charles Rudin, MD Johns Hopkins • Prostate cancer Phil Kantoff, MD DFCI Howard Scher, MD MSKCC Chris Logothetis, MD MD Anderson Prostate Consortium • Breast Max Wicha, MD U of Michigan • Heme malignancies Doug Smith, MD Johns Hopkins Bill Matsui, MD Johns Hopkins Kapil Bhalla, MD Moffitt Cancer Ctr |
 Infinity Pharmaceuticals Summary * * * * * * |
 • Product Pipeline – IPI-504: Complete Phase I trials • Publish First Clinical Data – IPI-504: Expect to initiate Phase II in 2007 – Hedgehog Pathway: Expect to initiate Phase I in 2007 • Successful alliance execution • At least one new corporate alliance • Financing event – Year-end cash runway: 12-24 months 2006/Early 2007 Goals, Achievements and Anticipated News Flow Pending DPII merger AMGN extension Expected at EORTC 11/7/06 NVS (Bcl) MEDI (Hsp90, HH) |