 Introduction to Infinity Pharmaceuticals (NASDAQ: INFI) September 26, 2006 Exhibit 99.1 |
 2 Forward-Looking Statements • This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These statements involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by these forward- looking statements. • These forward-looking statements include, but may not be limited to: statements regarding the expected benefits of our merger with Discovery Partners; our ability to advance our pipeline of anti-cancer agents and create substantial value for patients and stockholders; the efficacy, safety, and intended utilization of our product candidates; the results of discovery efforts and clinical trials; plans regarding regulatory filings, future research and clinical trials; current and future collaborative activities; the strength of our patent position; and our expectation that we will have cash to support our current operating plan through at least December 31, 2009. • These forward-looking statements are subject to numerous factors, risks and uncertainties that may cause actual events or results to differ materially from our current expectations. For example, we cannot guarantee that any product candidate we are developing will successfully complete necessary preclinical and clinical development phases, be approved for sale in any market or that, if approved, revenues from sales of such product will reach any specific level. In particular, our expectations could be affected by risks and uncertainties relating to: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; our dependence on our collaborations with MedImmune and Novartis; our ability to obtain any additional funding necessary to conduct our research, development and commercialization activities; unplanned cash requirements and expenditures; and our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates. These and other risks that may impact our expectations are described in greater detail in the document entitled "Risk Factors" that we filed with the SEC on September 18, 2006 as Exhibit 99.2 to our current report on Form 8-K. • In addition, any forward-looking statements contained in this presentation speak only as of September 26, 2006, and we expressly disclaim any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. * |
 3 Mission To develop targeted therapies for the treatment of cancer and related conditions discovered through the use of our innovative small molecule drug technologies * |
 4 Strategy • Targets – “Well-credentialed, but not well-trodden” • Drugs – Internally discovered, novel small molecules • Products – Opportunity for first-in class or fast follower best-in-class * |
 5 • Lead clinical product in two disease-focused Phase I cancer studies – IPI-504, a novel Hsp90 inhibitor – Phase II expected in 2007 • Pipeline of internally-discovered cancer drug candidates – Hedgehog pathway inhibitors (systemically administered) – Bcl family inhibitors – Additional discovery programs • 5 Pharma/Biotech corporate alliances – MedImmune, Novartis (2), Amgen, and J & J • Significant cash position after reverse merger and MEDI alliance – ~$125M in cash as of 9/25/06, with another $35M to come MedImmune – Projected cash runway through end of 2009 – Achieve key value-driving events before additional financing required • Proven biotech leadership team Snapshot of Infinity Pharmaceuticals (NASDAQ: INFI) * |
 6 Lead clinical program IPI-504 – a novel Hsp90 inhibitor * * * * * * * * * |
 7 • Novel chemical entity • Two disease-focused Phase I trials – GIST, Multiple Myeloma • Preclinical evidence of broad therapeutic potential – Large therapeutic window consistent with targeted therapies – Activity in resistant settings • Patient-friendly formulations – Water-soluble IV – Oral under development • Strong intellectual property position • Ready for Phase II in 2007 Infinity’s lead clinical product: IPI-504 (Hsp90 inhibitor) IPI-504 OH N H N OH O OH Me O O O O NH 2 H H + Cl - * * |
 8 Heat Shock Protein 90 (Hsp90) is an emerging cancer target Hsp90 in cancer cells differs from Hsp90 in normal cells Function of Hsp90 in cancer cells • Stabilization and enablement of oncogenic proteins responsible for cancer cell proliferation and survival • Opportunity for preferential targeting of drug candidates to cancer cells * |
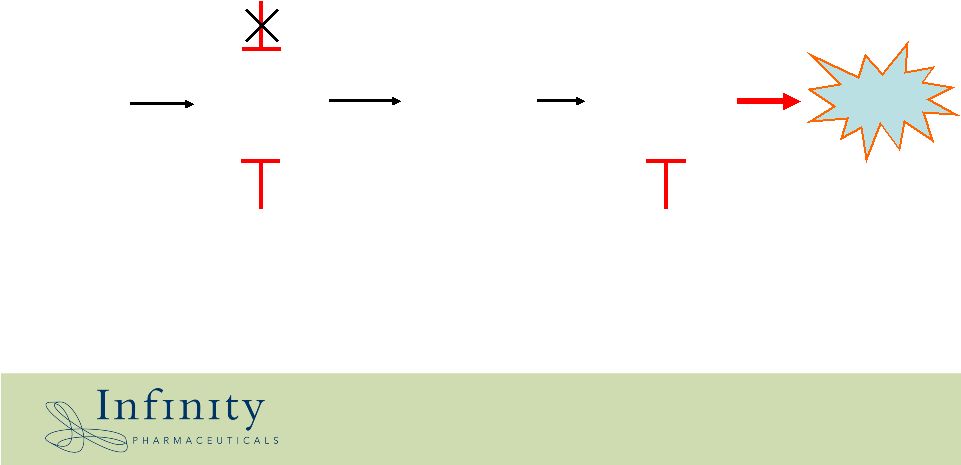 9 Dependence on Hsp90 Apoptosis Tyrosine kinase inhibitor (e.g Gleevec, Tarceva) Oncogene Cancer cell survival & proliferation Resistance mutations Targeting specific oncogenic Hsp90 client proteins Hsp90 inhibitor (single agent or combination) Hsp90 inhibitor (single agent) * |
 10 Velcade Gleevec / dasatinib Investigational Gleevec / Sutent Herceptin Tarceva / Erbitux Sorafenib / Sutent Sorafenib Investigational Targeted therapy The emerging world of targeted cancer therapies Indication Myeloma CML AML GIST Breast (HER2+) NSCLC Renal cell Melanoma Prostate (PTEN -/-) NF- B Bcr-Abl Flt3 c-Kit HER2 EGFR VEGFR / HIF-1a b-Raf p-Akt Molecular Target * |
 11 The emerging world of targeted cancer therapies NF- B Bcr-Abl Flt3 c-Kit HER2 EGFR VEGFR / HIF-1a b-Raf p-Akt Molecular Target • All are clients of Hsp90 • Inhibiting Hsp90 affects the stability of these targets • Attractive alternative to chasing tumor-specific resistance mutations * |
 12 IPI-504 advantages 17-AAG (multiple formulations) Highly soluble, allowing for patient- friendly formulations: • Water-soluble IV • Oral under development Novel chemical entity x Chemical entity is off-patent x Highly insoluble, requiring sub- optimal DMSO- and Cremophor- based formulations Equivalent potency and selectivity Potent & selective inhibitor of Hsp90 Well-tolerated to date in Phase I trials (dose-escalating at 400 mg/m 2 ) IPI-504 O N H H N O Me O OH Me Me O Me O O O NH Me Me IPI-504 OH N H N OH O OH Me O O O O NH 2 H H + Cl - * |
 13 GIST: Gleevec-resistant cells more sensitive to IPI-504 GIST 882* Gleevec-Sensitive (primary: exon 13, K642E) 10 100 1000 10 20 30 40 50 10000 60 70 Compounds concentrations (nM) 10 100 1000 10 20 30 40 50 10000 10000 60 70 Compounds concentrations (nM) IPI-504 : EC50 = 121 +/- 21 nM IM : EC50 = 147 +/- 42 nM Gleevec- Resistant (primary: exon 11, V560D + Gleevec resistance: exon 17, D820A) 10 100 1000 5 15 25 35 45 55 65 75 85 Compounds concentrations (nM) IPI-504 Imatinib GIST 48* IPI-504 : EC50 = 54 +/- 7 nM IM : 25% inhibition @ 10uM Collaboration: Fletcher, Demetri, DFCI * |
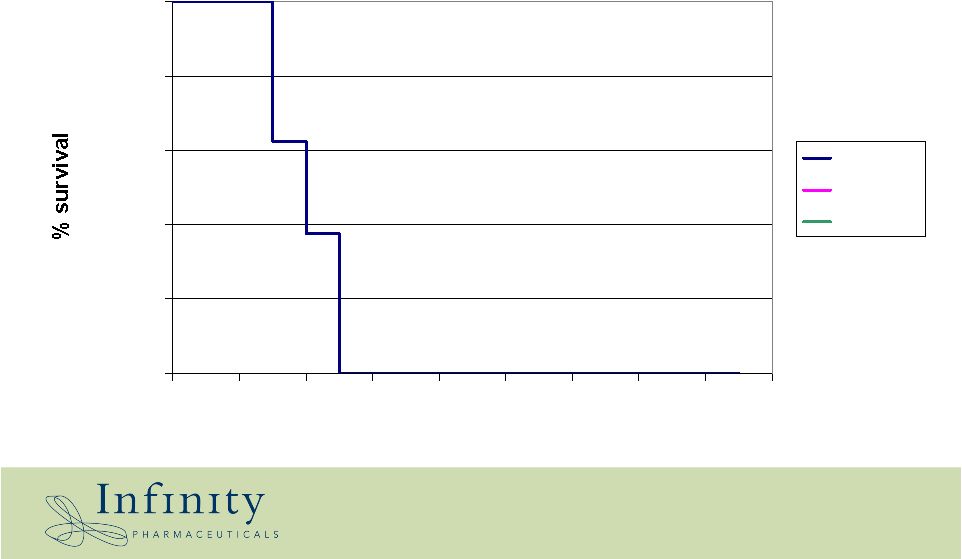 14 Placebo Gleevec IPI-504 Collaboration: Shauguang Li, Jackson Labs 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% 15 17 19 21 23 25 27 29 31 33 Days Oral IPI-504: survival benefit in Gleevec-resistant T315I CML transplantation model * |
 15 Placebo Gleevec IPI-504 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% 15 17 19 21 23 25 27 29 31 33 Days Oral IPI-504: survival benefit in Gleevec-resistant T315I CML transplantation model Collaboration: Shauguang Li, Jackson Labs * |
 16 Placebo Gleevec IPI-504 0.0% 20.0% 40.0% 60.0% 80.0% 100.0% 15 17 19 21 23 25 27 29 31 33 Days Oral IPI-504: survival benefit in Gleevec-resistant T315I CML transplantation model Collaboration: Shauguang Li, Jackson Labs * |
 17 • Development and registration of IPI-504 in hematologic malignancies and solid tumors – Preclinical support for broad role of Hsp90 • Early human proof-of-concept with most rapid path to registration – Strong scientific rationale – Trials targeted to homogenous patient population (disease-focused) – Surrogate markers – Rapid patient accrual – Single-agent activity in refractory setting (potential for expedited approval) • In parallel, initiate broader development for larger indications (additional diseases, combination therapy, front-line therapy) IPI-504 Clinical Development Strategy * |
 18 Principal Investigator: • Dr. George Demetri, DFCI Objectives: • Safety, PK, dose-ranging • Establish Phase II dose Surrogate marker of response: • PET scans Solid Tumor Gastrointestinal Stromal Tumors (Gleevec-resistant) Schedule / status: • Days 1, 4, 8, 11 of 21 day • Continuing dose escalation Current ongoing phase I clinical trials Principal Investigator: • Dr. Paul Richardson, DFCI • Dr. Sundar Jagannath, SVCCC • Dr. David Siegel, HUMED Objectives: • Safety, PK, dose-ranging • Establish Phase II dose Surrogate marker of response: • M protein levels Hematologic Multiple Myeloma (relapsed, refractory) Schedule / status: • Days 1, 4, 8, 11 of 21 day • Continuing dose escalation * |
 19 Phase I dose escalation for IPI-504 (GIST) • 1 cycle = 21 days • 4 doses (days 1, 4, 8, 11 followed by 10 days off) Phase I schedule 25% 500 6 33% 400 5 33% 300 4 50% 225 3 66% 150 2 100% 90 1 Escalation over previous dose Dose (mg/m2) Group * |
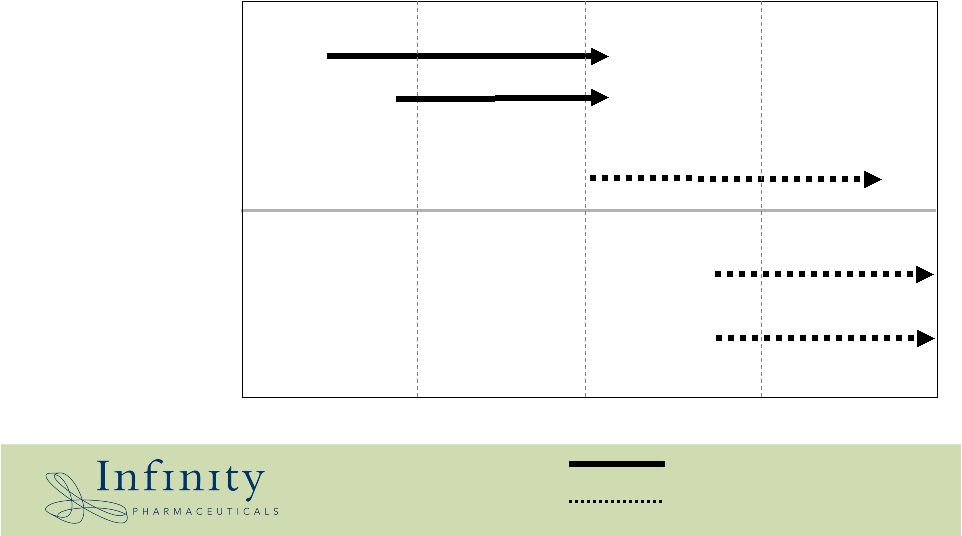 20 On-going trial Phase I/II – Solid 2005 2006 2007 2008 Phase I • Multiple myeloma • GIST • Solid tumor TBD Phase II • Solid tumor TBD • Hem. Tumor TBD • Others Phase II – Solid TBD based on data/results IPI-504: Clinical Plan Phase II – Hem. * |
 21 IPI-504 * |
 22 Lead preclinical program Hedgehog pathway inhibitors * * * * * * * * * |
 23 • Systemic inhibitors of the Hedgehog signaling pathway – Proprietary NCE’s – Systemic (sub-cu and oral) products • Broad anti-cancer potential – Strong preclinical data supporting pancreatic, metastatic prostate, SCLC, others – Single agent activity in preclinical studies – Potential for synergy with standards of care – Possible role in cancer progenitor cells • Multiple drug candidates – Lead molecule, IPI-609: IND-ready – Additional attractive analogs under evaluation – Select preferred clinical candidate over next 6-12 months Infinity’s Hedgehog inhibitor program * |
 24 Cancers have hijacked components of the Hedgehog pathway 1 Hahn et al., 1996, Cell 85: 841 2 Bale & Yu, 2001, Human Molec. Genetic. 10: 757 (review) 3 Berman et al., 2002 Science 297: 1559 4 Berman et al., 2003 Nature 425: 846 5 Kayed et al., 2004 Int. J. Cancer 110: 668 6 Thayer et al., 2003 Nature 425: 851 7 Karhadkar et al., 2004 Nature, 431: 707 8 Fan et al., 2004 Endocrinology 145: 3961 9 Watkins et al., 2003, Nature 422: 313 10 Sicklick 2005 ASCO; Mohini, 2005 AACR 11 Kubo et al., 2004 Cancer Res. 64 :6071 State Normal Basal cell carcinoma 1,2 Medulloblastoma 3 Pancreatic cancer 4,5,6 Prostate cancer 7,8 Small cell lung cancer 9 Hepatocellular cancer 10 Breast Cancer 11 Pathway activation OFF ON ON ON ON ON ON ON * * |
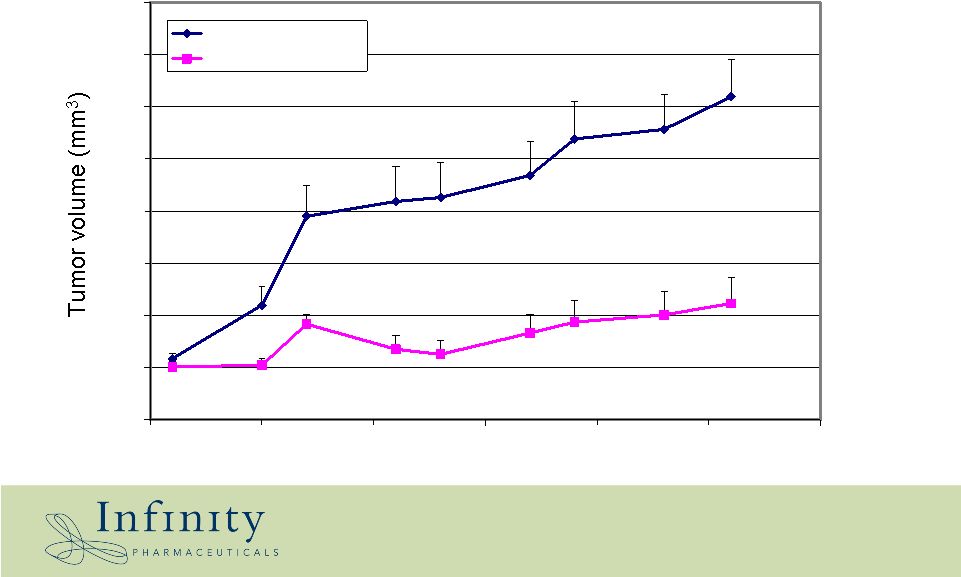 25 0 200 400 600 800 1000 1200 1400 1600 31 36 41 46 51 56 61 Days Vehicle IPI-609 10 mpk/day IPI-609 shows activity in PC-3 prostate xenograft * |
 26 IPI-609 slows tumor growth rates 0 200 400 600 800 1000 1200 30 35 40 45 50 55 60 Day Linear Fit Bivariate Fit of P 10 By Day 200 400 600 800 1000 1200 30 35 40 45 50 55 60 Day Linear Fit Bivariate Fit of VP 6 By Day Median vehicle-treated animals Median IPI-609 treated animals PC-3 prostate xenograft: * |
 27 Clinical development strategy of hedgehog pathway inhibitors • Strong scientific rationale supports targeting of cancers dependent on the Hedgehog pathway – Pancreatic – Small cell lung – Metastatic prostate – Metastatic breast – Ovarian – Others (medulloblastoma, glioma, basal cell carcinoma, etc.) • Identify a rapid path to registration – Potential for sole agent activity or – Combination with standard of care * |
 28 Corporate Alliances Strong validation, significant value retention * * * * * * * * * |
 29 • Diversity Oriented Synthesis (DOS) platform – Creates novel, “natural-product like” drug candidates – Potential to access previously “undruggable” targets • 2004 – 2006: > $60M upfront/committed cash • Additional milestone and royalty potential • No license of proprietary Infinity product rights • Amgen extension in 2006 Small Molecule Technology Access Alliances * |
 30 • Potential additional milestones Early product pipeline: Bcl family alliance with Novartis • Joint discovery of novel Bcl family (Bcl-2, Bcl-xL) targeted cancer drugs • Infinity participation in clinical development (at NVS expense) COLLABORATION • Infinity participation in US sales effort (at NVS expense) $30M • Upfront & committed funds FINANCIALS • Royalties on WW sales >$370M * |
 31 Cancer Drug Development and Worldwide Commercialization Agreement August 28, 2006 * * * |
 32 • Potential additional milestones Lead products: Hsp90 and Hedgehog alliance with MEDI • Infinity leads early translational development through proof of concept in humans • MedImmune leads later clinical development, registration, and sales and marketing, with Infinity participation COLLABORATION • Infinity has right to provide up to 35% of US promotional activity (cost shared by alliance) $70M • Upfront license fee FINANCIALS • 50/50 worldwide R&D cost share • 50/50 worldwide profit share (regardless of who sells) $430M * |
 33 Discovery Preclinical Start Clinical Trials Hsp90 (IPI-504) Bcl-2/Bcl-xL 2005 2007/2008 50% WW profit share with MEDI 50% WW profit share with MEDI Royalty from Novartis Non-exclusive – Amgen – Novartis – J&J Small molecule drug technologies Alliance and financing strategy: value retention Hedgehog Pathway inhibitors 2007/2008 * |
 34 Financing Creative access to capital * * * * * * |
 35 • Reverse merger employed to ensure certainty of valuation and amount of cash raised, with low market-based risk • Infinity received $78M in cash from DPI • Ownership of combined entity: – Issued and outstanding basis: 34% DPI 66% Infinity 19.4M shares – Fully-diluted basis: 31% DPI 69% Infinity 20.9M shares Infinity (NASDAQ: INFI) public via reverse merger (9/12/06) * |
 36 Team Proven track record * * * * * * * * * |
 37 Leadership Mr. Steven Holtzman, CEO Millennium, DNX Dr. Julian Adams, President & CSO Millennium, ProScript Boehringer Ingelheim, Merck Ms. Adelene Perkins, CBO Transform, Genetics Institute, Bain, GE Dr. David Grayzel, VP Clinical Development & Medical Affairs Dyax, Mass General Hospital Steven Kafka, Ph.D., VP Strategic Product Planning & Finance Millennium, Strategic Decisions Group Dr. Vito Palombella, VP Discovery Research Syntonix, Millennium, ProScript Gerald Quirk, Esq., VP & General Counsel Genzyme, Palmer & Dodge Dr. Jeffrey Tong, VP Corporate & Product Development McKinsey & Co, Harvard Center for Genomics Research Dr. Jim Wright, VP Pharmaceutical Development Millennium, Alkermes, Boehringer Ingelheim, Syntex, U. of Wisconsin * |
 38 Infinity Pharmaceuticals Summary * * * * * * * * * |
 39 • Product Pipeline – IPI-504 • Complete Phase I trials • Publish First Clinical Data • Successful execution of technology access alliances • At least one new corporate alliance • Financing event – Year-end cash runway: 12-24 months 2006 Goals and Achievements AMGN extension Expected at EORTC 11/7/06 NVS (Bcl) MEDI (Hsp90, HH) + Reverse merger with DPI (~$125M cash as of 9/25/06) + * |