 Infinity Pharmaceuticals (NASDAQ: INFI) November 2007 Exhibit 99.1 |
 2 Forward-Looking Statements • This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. These statements involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward- looking statements. • Such forward-looking statements include statements regarding future preclinical and clinical trial activity for IPI- 504, IPI-493, and IPI-926; the timing of IND submissions and publication of data for IPI-504, IPI-493, and IPI-926; the collection of additional clinical information on IPI-504; the intended utilization and commercial potential of IPI-504; the ability to name clinical candidates in the company’s research programs; estimates of 2007 financial performance and year-end cash balance; and the expectation that Infinity will have cash to support its current operating plan into the fourth quarter of 2009. • Such statements are subject to numerous factors, risks and uncertainties that may cause actual events or results to differ materially from the company's current expectations. For example, there can be no guarantee that any product candidate Infinity is developing will successfully complete necessary preclinical and clinical development phases, be approved for sale in any market or that, if approved, revenue from sales of such product will reach any specific level. In particular, management's expectations could be affected by risks and uncertainties relating to: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities and investigational review boards at clinical trial sites; whether abstracts or other publications are accepted for presentation or publication; Infinity’s ability to enroll patients in its clinical trials; Infinity's dependence on its collaborations with MedImmune and Novartis; Infinity's ability to obtain additional funding required to conduct its research, development and commercialization activities; unplanned cash requirements and expenditures; and Infinity's ability to obtain, maintain and enforce patent and other intellectual property protection for any products it is developing. • These and other risks which may impact management's expectations are described in greater detail under the caption "Risk Factors" included Infinity's quarterly report on Form 10-Q for the quarter ended September 30, 2007, as filed with the Securities and Exchange Commission on November 7, 2007. • Further, any forward-looking statements contained in this presentation speak only as of the date hereof, and Infinity expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. • All trademarks used in this presentation are the property of their respective owners. |
 3 Profile of Infinity Pharmaceuticals (NASDAQ: INFI) Strategy Management Business Portfolio Emerging targets in oncology, areas of high patient need Build rapid registration paths, broaden market potential Led by proven drug developers and company-builders Committed corporate partners and strong balance sheet Lead clinical product: IPI-504, a novel Hsp90 inhibitor Hedgehog pathway inhibitor, IPI-926 Pipeline of internally-discovered cancer drug candidates * |
 1 Infinity Leadership Michael Curtis, Ph.D., Sr Dir Pharmaceutical Development TKT, Syntonix, Genzyme, Bristol-Myers Squibb David Grayzel, M.D., VP Clinical Development & Medical Affairs Dyax, Mass General Hospital Steven Kafka, Ph.D., VP Finance Millennium, Strategic Decisions Group John Keilty, Sr Dir Informatics Millennium, UMass Medical School Jeanette Kohlbrenner, Dir Human Resources Genetics Institute, Syntonix Vito Palombella, Ph.D., VP Drug Discovery Syntonix, Millennium, ProScript Gerald Quirk, Esq., VP & General Counsel Genzyme, Palmer & Dodge Jeffrey Tong, Ph.D., VP Corporate & Product Development McKinsey & Co, Harvard Center for Genomics Research Jim Wright, Ph.D., VP Pharmaceutical Development Millennium, Alkermes, Boehringer Ingelheim, Syntex, U. of Wisconsin Steven Holtzman, President & CEO Millennium, DNX Julian Adams, Ph.D., President R&D and CSO Millennium, ProScript, Boehringer Ingelheim, Merck Adelene Perkins, EVP & CBO Transform, Genetics Institute, Bain, GE |
 5 Lead clinical program IPI-504 – a novel Hsp90 inhibitor * * * * * |
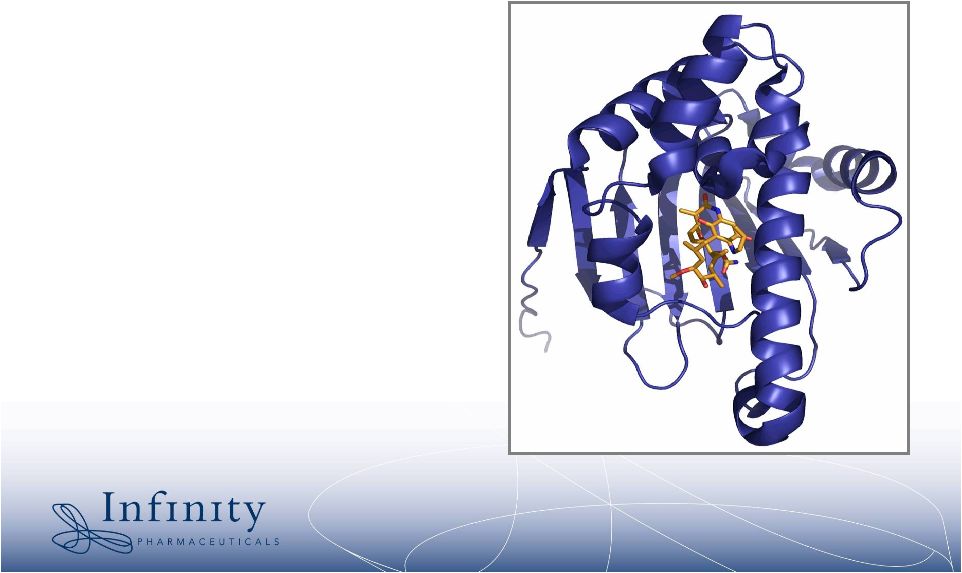 6 Heat shock protein 90 (Hsp90) is an emerging cancer target Function of Hsp90 • “Chaperone” protein responsible for proper folding and function of some proteins Function of Hsp90 in cancer cells • Many oncogenic proteins rely on Hsp90 to function • Inhibiting Hsp90 is another way to inhibit the oncogenic proteins * |
 7 Many critical oncogenic proteins depend on Hsp90… Indication GIST NSCLC Prostate (PTEN -/-) Breast (HER2+) Melanoma Renal cell CML AML c-Kit EGFR p-Akt HER2 b-Raf VEGFR / HIF-1a Bcr-Abl Flt3 Hsp90 client proteins Broad clinical potential for Hsp90 inhibition * |
 8 Gleevec ® /Sutent ® Tarceva ® /Erbitux ® Investigational Herceptin ® /Tykerb ® Investigational Nexavar ® /Sutent ® Gleevec ® /Sprycel ® Investigational Targeted therapy …with well-validated commercial potential Indication GIST NSCLC Prostate (PTEN -/-) Breast (HER2+) Melanoma Renal cell CML AML c-Kit EGFR p-Akt HER2 b-Raf VEGFR / HIF-1a Bcr-Abl Flt3 Broad clinical and commercial potential for Hsp90 inhibition Hsp90 client proteins * |
 9 Cancer cell death Tyrosine kinase inhibitor (e.g., Gleevec, Tarceva) Normal protein Oncogenic protein drives cancer cell survival & growth Resistance mutations evade TKI therapy IPI-504 IPI-504: an alternative to direct inhibition of oncogenic proteins IPI-504 Dependent on Hsp90 for function Still dependent on Hsp90 for function * |
 10 Hsp90 clinical development strategy Focus on most rapid path to registration with IPI-504 i.v. – Evaluate for single-agent activity in refractory setting GIST and NSCLC – Inform Phase 2 dose and schedule In parallel, initiate additional trials for broader indications – Additional tumors (solid and hematological) in refractory settings – Combination therapy with standards of care to expand market potential Rapid development of oral dosage form – Ease of use for earlier lines of therapy and chronic settings – Two ansamycin-based molecules in preclinical development * |
 11 Hsp90 clinical development strategy Focus on most rapid path to registration with IPI-504 i.v. – Evaluate for single-agent activity in refractory setting GIST and NSCLC – Inform Phase 2 dose and schedule In parallel, initiate additional trials for broader indications – Additional tumors (solid and hematological) in refractory settings – Combination therapy with standards of care to expand market potential Rapid development of oral dosage form – Ease of use for earlier lines of therapy and chronic settings – Two ansamycin-based molecules in preclinical development * |
 12 IPI-504 i.v. Phase 1 trial in refractory GIST/STS Principal Investigator: • Dr. George Demetri, Dana-Farber Cancer Institute Objectives: • Safety, PK, dose-ranging • Establish Phase 2 dose Marker of response: • CT scans (RECIST) • PET scans Schedule • 3-week cycle: 2x-weekly followed by 10-day-off period, or “drug holiday” • If clinical benefit, receive additional cycles Trial description: • Phase 1 trial in patients with Gleevec-refractory metastatic gastrointestinal stromal tumors (GIST) and advanced soft tissue sarcomas (STS) * |
 13 Status of Phase 1 trial of IPI-504 i.v. in GIST • Completed dose-escalation portion of Phase 1 • Established recommended Phase 2 dose and schedule – 400 mg/m² – 3-week cycle: 2x-weekly treatment followed by 10 days off • Initiated expansion phase – 20 patients (10 GIST, 10 STS) – Further characterize safety and response to inform Phase 2 * |
 14 Data reported from IPI-504 Phase 1 trial in GIST/STS* • 28 patients treated with IPI-504 – Average 2.6 prior therapies (primarily Gleevec ® and Sutent ® ) • IPI-504 well-tolerated up to 400 mg/m² • 16 of 21 (76%) Stable Disease by RECIST • 7 of 20 (35%) received 5 or more cycles (15 wks) of therapy • Correlative activity observed – EORTC quantitative PET criteria • 15 of 18 (83%) Stable Disease or better • 4 of 18 (22%) Partial Response – Histological and CT changes *Results published at ASCO, June 2007: George D. Demetri, et al,“ Inhibition of the Heat Shock Protein 90 (Hsp90) chaperone with the novel agent IPI-504 to overcome resistance to tyrosine kinase inhibitors (TKIs) in metastatic GIST: Updated results of a phase I trial," (Poster Number 8, Abstract No: 10023) and A.D. Van den Abbeele, et al, "Inhibition and flare patterns of metabolic response to the heat shock protein 90 (Hsp90) inhibitor IPI-504 visualized by FDG-PET in patients (pts) with advanced gastrointestinal stromal tumors (GIST) resistant to tyrosine kinase inhibitor (TKI) therapy," (Poster Number 1, Abstract No: 3530) * |
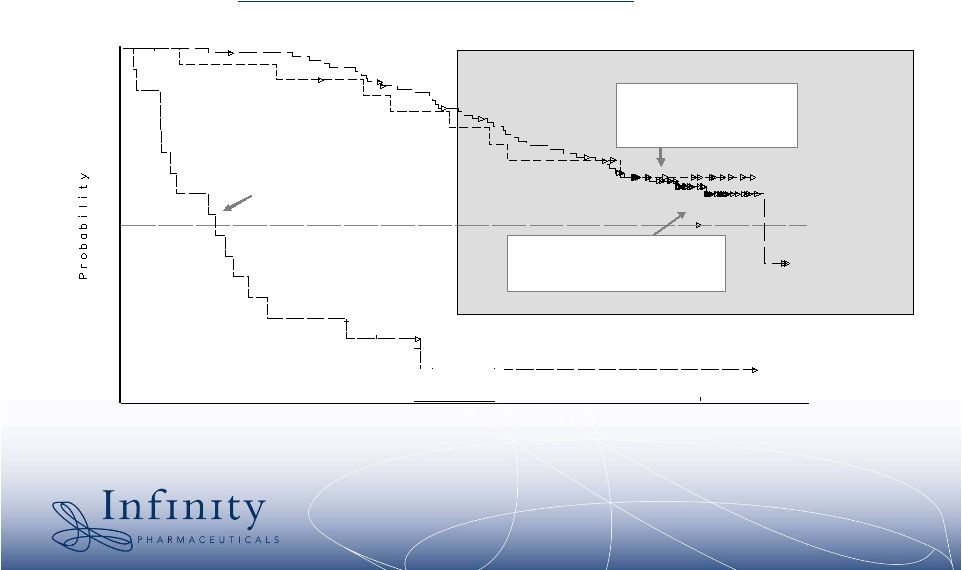 15 Number at Risk 0 2 40 2 80 2 Wks: Best Response CR Median Duration N/A 95% CI LL UL 172 N/A Number at Risk 0 98 40 97 80 92 Wks: Best Response PR Median Duration 248 Wks 95% CI LL UL 226 N/A Number at Risk 0 23 40 22 80 20 Wks: Best Response SD Median Duration N/A 95% CI LL UL 149 N/A Number at Risk 0 17 40 7 80 4 Wks: Best Response PD Median Duration 36 Wks 95% CI LL UL 15 56 Number at Risk 0 7 40 5 80 4 Wks: Best Resonse UNK Median Duration 144 Wks 95% CI LL UL 18 223 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 Weeks Post First Dose 0 12 24 36 48 60 72 84 96 108 120 132 144 156 168 180 192 204 216 228 240 252 264 PROGRESSION On Imatinib Median 36 wks Overall Survival by Best Response Achieved (Kaplan Meier Estimate) STABLE DISEASE PARTIAL RESPONSE 1 Blanke, CD, et al. J Clin Onc 2006 (ASCO Proceedings) Part I, Vol 24; No. 18S: 9528. Stable disease is important in GIST 1 Both SD and PR predict survival * |
 16 Stable disease for >6 weeks is meaningful in the refractory GIST setting 1 Results from the Phase 3 Sutent trial in GIST TTP on Placebo: 6.4 weeks TTP on Sutent: 27.3 weeks 1 Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JVS, Booth BP, Verbois SL, Morse DL, Liang CY, Chidambaram N, Jiang JX, Tang S, Mahjoob K, Justice R, Pazdur R. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res (2007) 13:1367-1373. * |
 17 IPI-504 PET response (at 400 mg/m²) Courtesy of Van den Abbeele & Demetri: Dana-Farber Cancer Institute Harvard Medical School • Baseline • Baseline • Cycle 1, Day 22 • 10 days off treatment • Cycle 1, Day 22 • 10 days off treatment • Cycle 1, Day 12 • Cycle 1, Day 12 • 24 hrs post 4th dose Patient discontinued for personal reasons * |
 18 Path forward for IPI-504 i.v. in refractory GIST • Expansion phase at recommended Phase 2 dose in progress – 20 patients (10 GIST, 10 STS) – Further characterize safety and response to inform Phase 2 trial • Phase 2 has potential for rapid approval path – Active consultation with FDA and advisors – Ongoing discussions of trial structure (e.g., control arm) • Issued U.S. patents • Granted U.S. and EU orphan drug designation * |
 19 0 200 400 600 800 1000 1200 1400 1600 1800 2000 15 21 24 28 32 37 Days Post Implant Vehicle IPI-504 at 25mg/kg IPI-504 at 50mg/kg IPI-504 at 100mg/kg IPI-504 oral is active in Tarceva- / Iressa-resistant NSCLC (T790M EGFR mutation) 25% TGI (total growth inhibition) 72% TGI 95% TGI Control • NCI-1975 xenograft • IPI-504 PO QOD * |
 20 Phase 1/2 trial in advanced NSCLC Principal Investigator: • Dr. Thomas Lynch, Massachusetts General Hospital Objectives: • Phase 1: Safety, PK, dose-ranging • Phase 2: Expand at MTD to further characterize response of patients with mutant or wt EGFR Markers of response: • CT scans (RECIST) • PET scans Trial description: • Phase 1/2 trial in Stage IIIb/IV NSCLC patients with previous TKI therapy Patient population: • Prior therapy (>12 weeks) on a TKI * |
 21 Data reported from IPI-504 i.v. Phase 1/2 trial in NSCLC* • 12 patients treated with IPI-504 through dose-escalation – Average 3.7 prior therapies • 7 of 9 (78%) Stable Disease by RECIST – 1 mEGFR experienced extended SD >6 months (28 weeks) • Correlative activity observed via PET – 4 of 4 qualitative PET responses – 2 of 4 Partial Response by EORTC quantitative PET criteria *Results published at AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, October 2007: Lecia V. Sequist, et al, “Phase 1/2 Trial of the Novel Hsp90 Inhibitor, IPI-504, in Patients with Relapsed and/r Refractory Stage IIIb or Stage IV Non-Small Cell Lunch Cancer (NSCLC) Stratified by EGFR Mutation Status," ( Abstract No: B79) * |
 22 IPI-504 PET response (at 150 mg/m²) Baseline Cycle 1, Day 8 Courtesy of: * |
 23 Path forward for IPI-504 i.v. in advanced NSCLC • Completed Phase 1 dose-escalation • Initiate Phase 2 portion – 400 mg/m², 3-week cycle: 2x-weekly treatment followed by 10 days off – Evaluate mutant EGFR and wild-type EGFR (10 pts. each) – Enroll 19 additional pts. per arm if expansion criteria met (PR or SD >12 wks.) • Evaluate combination therapy strategy – Phase 1 single agent activity drives interest in exploration of mechanistic combinations – Allows for use in earlier lines of treatment * * |
 24 Hsp90 clinical development strategy Focus on most rapid path to registration with IPI-504 i.v. – Evaluate for single-agent activity in refractory setting GIST and NSCLC – Inform Phase 2 dose and schedule In parallel, initiate additional trials for broader indications – Additional tumors (solid and hematological) in refractory settings – Combination therapy with standards of care to expand market potential Rapid development of oral dosage form – Ease of use for earlier lines of therapy and chronic settings – Two ansamycin-based molecules in preclinical development * |
 25 Days post implant IPI-504 + taxotere inhibits the growth of human PC-3 prostate xenografts * * |
 26 Phase 2 trial in HRPC Principal Investigator: • Dr. William Oh, Dana-Farber Cancer Institute Objectives: • Characterize response of patients with or without prior cytotoxic chemotherapy treatment Markers of response: • Bone scans • PSA levels • CT scans (RECIST) Trial description: • Phase 2 trial in hormone-refractory (castration- resistant) prostate cancer Patient population: • 2 groups: 1 pre- and 1 post-Taxotere ® treatment • Expand to additional 10 patients in each group if response observed in that group * |
 27 Hsp90 clinical development strategy Focus on most rapid path to registration with IPI-504 i.v. – Evaluate for single-agent activity in refractory setting GIST and NSCLC – Inform Phase 2 dose and schedule In parallel, initiate additional trials for broader indications – Additional tumors (solid and hematological) in refractory settings – Combination therapy with standards of care to expand market potential Rapid development of oral dosage form – Ease of use for earlier lines of therapy and chronic settings – Two ansamycin-based molecules in preclinical development * |
 28 Hsp90 Oral program progressing rapidly • Key strategic objective: flexibility and breadth • Two molecules in preclinical development, ansamycin class – Infinity expertise in geldanamycin natural product and analogs – IPI-504 PO – IPI-493, named as development candidate 2Q 2007 • Both molecules show potent and selective inhibition of Hsp90 – Good oral bioavailability • Preclinical studies and evaluation of comparative data ongoing – Determine which candidate to move into clinic – Anticipate IND filing early 2008 * |
 29 0% 20% 40% 60% 80% 100% 15 35 55 75 95 Days post BMT Collaboration: Shauguang Li, Jackson Labs Overcomes resistance to kinase inhibitors in preclinical models of refractory settings Oral IPI-504 prolongs survival in Bcr-Abl T315I mice (Gleevec-resistant) Placebo Gleevec IPI-504 (50 mg/kg, MWF) IPI-504 (100 mg/kg, MWF) * |
 30 Active against multiple resistant mutations 0 20 40 60 80 100 15 20 25 30 35 40 45 50 55 60 65 70 Days post BMT Placebo IPI-504 T315I 0 20 40 60 80 100 20 30 40 50 60 70 80 90 Days post BMT Placebo IPI-504 E225K 0 20 40 60 80 100 12 16 20 24 28 32 36 40 44 48 Days post BMT Placebo IPI-504 M351T 0 20 40 60 80 100 13 17 21 25 29 33 Days post BMT Placebo IPI-504 Y253F IPI-504 at 50 mg/kg, MWF Collaboration: Shauguang Li, Jackson Labs Oral IPI-504 in Bcr-Abl mice with various resistance mutations * |
 31 Hsp90 program milestones • Preliminary Phase 1/2 NSCLC data at EORTC • Complete enrollment in Phase 1 trial in GIST/STS • Initiate Phase 2 single-agent trial • Initiate combination trial • Updated Phase 1 GIST/STS data at ASCO • Oral Hsp90 candidate IND filing • Initiate Phase 2 trial in GIST 4Q07 1H08 * |
 32 Preclinical programs Hedgehog, Bcl-2 * * * * * * |
 33 Role of Hedgehog pathway in cancer • Embryonic pathway, typically quiescent in adults • Aberrantly active in a variety of cancers: Genetically-defined tumors Tumors with pathway up-regulation Examples: Childhood medulloblastoma Basal cell carcinoma Rhabdomyosarcoma Examples: Pancreatic Small cell lung Breast Ovarian * |
 34 Infinity’s Hedgehog program: IPI-926 • Selected as development candidate Q2 2007 – Clinical studies anticipated in 2008 • Derivative of natural product cyclopamine • Promising preclinical data – Highly potent, selective inhibition of Hedgehog pathway – Oral bioavailability, extended half life – Excellent therapeutic window – Activity in vivo • Issued composition of matter patent • Infinity owns 100% rights, royalty-free * |
 35 Hedgehog program milestones 2007 2008 2009 • Selected development candidate IPI-926 • IND-enabling studies • Preclinical data at AACR • File IND • Initiate Phase 1 • Expand clinical development * |
 36 Corporate Summary Strong validation, significant value retention * * * * * * |
 37 Traditional product-based license Bcl-2/Bcl-xL March 2006 Discovery • $30M upfront & committed • >$370M milestones • Significant WW royalty • Co-promotion right in US AstraZeneca acquisition • Hsp90 cited as key clinical stage program • Infinity regained Hh program rights, and has opt-in rights to ongoing AZ Hh research programs Worldwide collaboration; R&D and commercial jointly led • $70M upfront • $215M milestones • 50/50 R&D cost share • 50/50 WW profit split • Co-promotion right in US Hsp90 August 2006 Preclinical/Phase 1 Near-term financial strength and long-term value supported by product-based alliances * |
 38 Strong balance sheet and cash runway • September 2007 cash and equivalents (unaudited): $116M • Burn rate well-controlled via alliances – 50% cost-sharing of Hsp90 program with MedImmune – 100% of Bcl-2 program funded by Novartis • January 2008 cash and equivalents (projected) : >$100M • Cash runway into fourth quarter of 2009 – Based on current operating plan and assuming no financing or business development activity * |
 39 Upcoming milestones Hsp90 • Preliminary Phase 1/2 NSCLC data at EORTC(Q407) • Complete Phase 1 expansion enrollment in GIST/STS (Q407) • Initiate Phase 2 single-agent trial (Q407) • Initiate combination trial (Q407) • Oral Hsp90 preclinical comparisons, file IND (1H08) Hedgehog • Preclinical data at AACR • File IND, initiate clinical studies (2008) * |
 40 Product pipeline Current Programs Discovery Preclinical Phase 1 Phase 2 Hsp90 IPI-504 (IV) GIST / STS IPI-504 (IV) NSCLC IPI-504 (IV) Add’l tumors IPI-504 (IV) Combinations IPI-504 (PO) IPI-493 (PO) Hedgehog pathway IPI-926 (PO) Solid tumors Bcl-2 / Bcl-xL Early discovery Planned for next 12 months, pending data * |
 Infinity Pharmaceuticals (NASDAQ: INFI) November 2007 * |