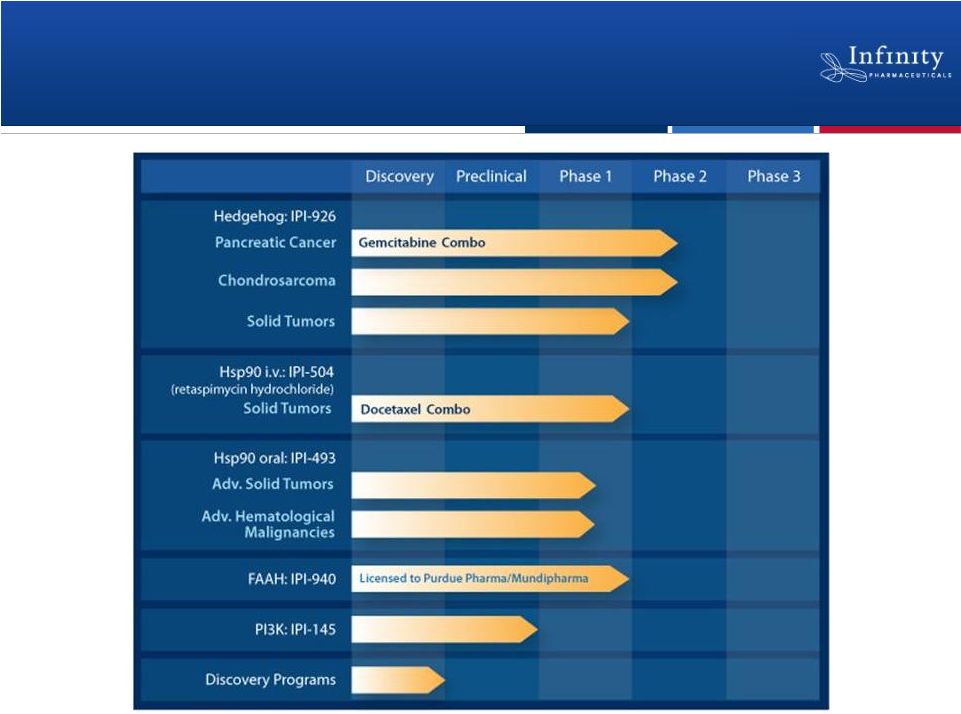
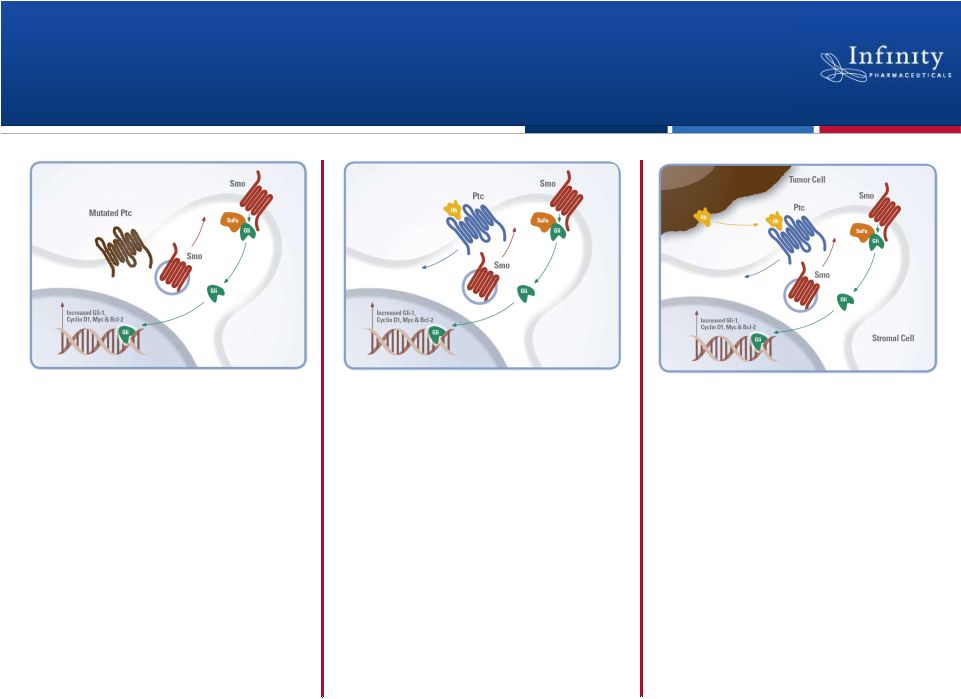
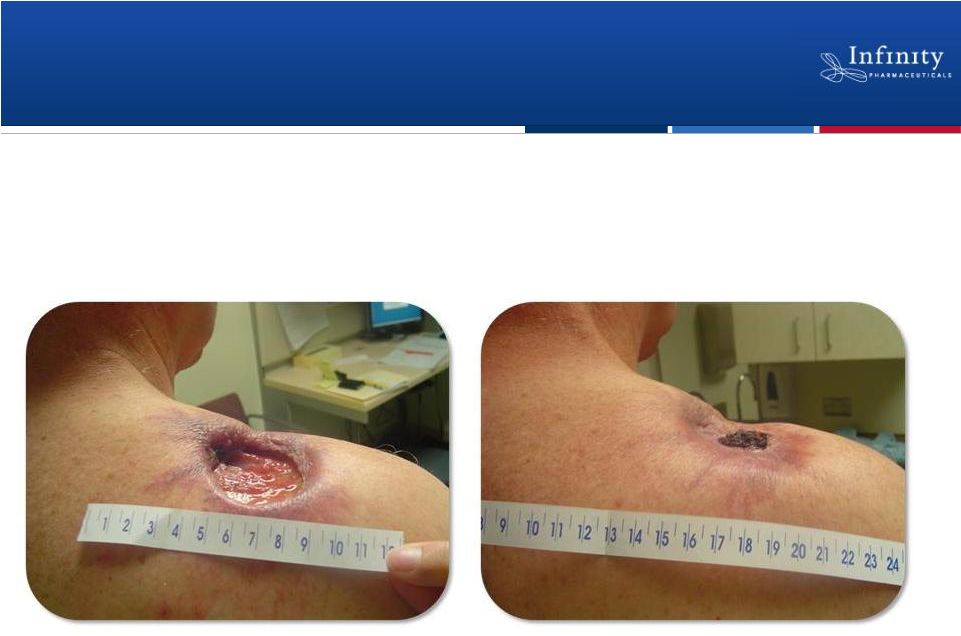
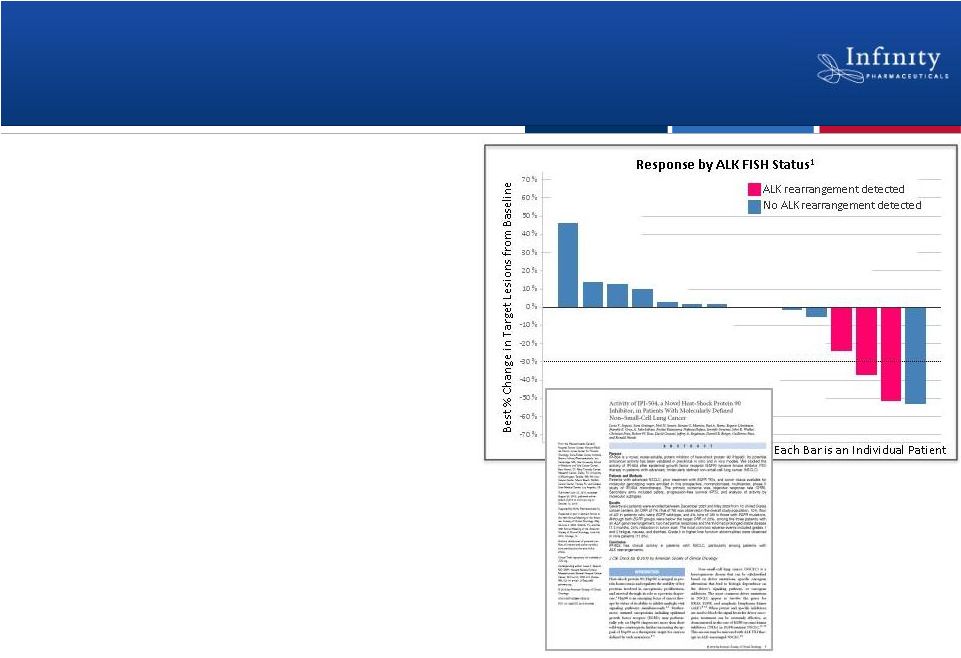
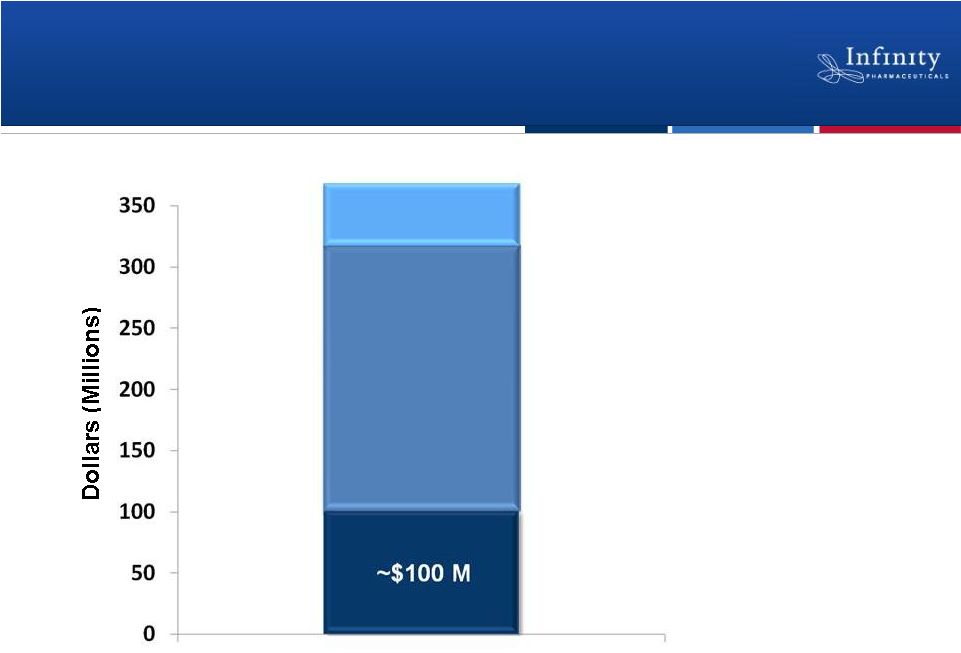
Forward Looking Statements • This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. • These statements involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. • Such forward-looking statements include statements regarding: the therapeutic potential of Infinity’s Hedgehog pathway, FAAH, PI3K and Hsp90 chaperone inhibitors; the potential of IPI-926 in addressing chondrosarcoma, pancreatic cancer and other cancers, presentation of rationale for development of IPI-926 in chondrosarcoma, clinical trial enrollment expectations, an investigator sponsored trial program with IPI-926, presentation of data from Infinity’s trials of IPI-926 pancreatic cancer and chondrosarcoma and of IPI-504 in combination with docetaxel, plans for additional Phase 2 trials of IPI-926, announcement of data and future plans for the company’s Hsp90 program, the commencement of Phase 1 development of IPI-145, Phase 2 development of IPI-940 by Purdue; completing transition activities to enable Phase 2 development by Purdue; the naming of a new drug development candidate, estimates of 2011 financial performance (including cash burn and year-end cash and investments balance), and the expectation that Infinity will have capital to support its current operating plan into 2014. • Such forward-looking statements are subject to numerous factors, risks and uncertainties that may cause actual events or results to differ materially from the company's current expectations. For example, there can be no guarantee that Infinity’s strategic alliance with Purdue/Mundipharma will continue for its expected term or that these entities will fund Infinity’s programs as agreed, or that any product candidate Infinity is developing will successfully complete necessary preclinical and clinical development phases. Further, there can be no guarantee that any positive developments in Infinity’s product portfolio will result in stock price appreciation. Infinity’s expectations could also be affected by risks and uncertainties relating to: results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities, investigational review boards at clinical trial sites, and publication review bodies; Infinity's ability to enroll patients in its clinical trials; unplanned cash requirements and expenditures, including in connection with business development activities; market acceptance of any products Infinity or its partners may successfully develop; and, Infinity's ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidate it is developing. • These and other risks which may impact management's expectations are described in greater detail under the caption "Risk Factors" included in Infinity's annual report on Form 10-K filed with the U.S. Securities and Exchange Commission on March 16, 2011. • Further, any forward-looking statements contained in this presentation speak only as of the date hereof, and Infinity expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. • All trademarks used in this presentation are the property of their respective owners. • Our Internet website is http://www.infi.com. We regularly use our website to post information regarding our business, product development programs and governance. We encourage investors to use www.infi.com, particularly the information in the section entitled “Investors/Media,” as a source of information about Infinity. References to www.infi.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.infi.com into this presentation by reference. 2 |