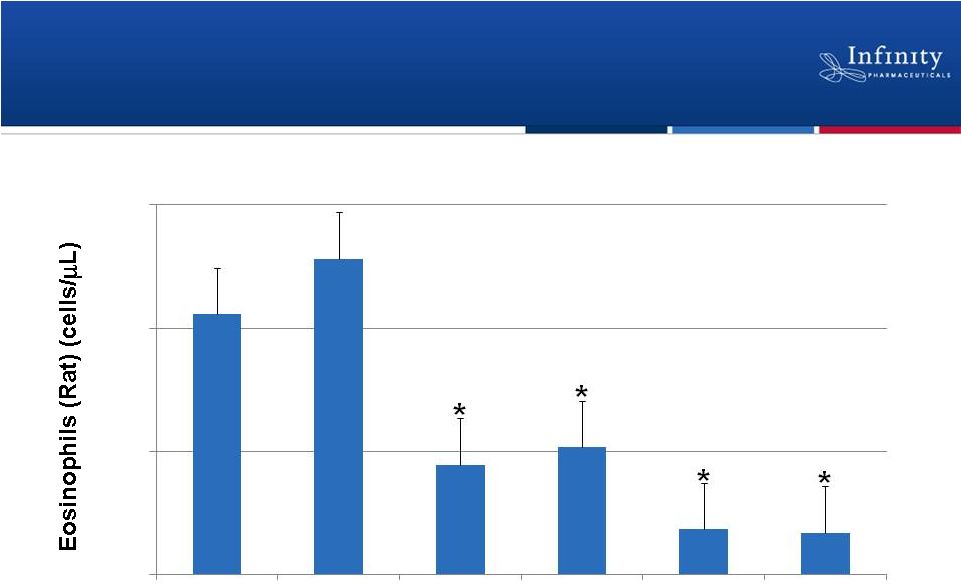
Forward Looking Statements 2 • This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. • These statements involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward-looking statements. • Such forward-looking statements include those regarding the expectation that Infinity will begin a Phase 2a study of IPI-145 in patients with rheumatoid arthritis during the fourth quarter of 2012, the expectation that Infinity’s Phase 1 trial of IPI-145 in patients with advanced hematologic malignancies will expand to additional cohorts (T-cell lymphomas, diffuse large B-cell lymphoma, acute lymphocytic leukemia and myeloproliferative neoplasms) once the maximum tolerated dose is determined, the expectation that Infinity will present data from its Phase 1 trial of IPI-145 in patients with advanced hematologic malignancies in the second half of 2012, the expectation that Infinity will present additional details from its Phase 1 study in healthy volunteers at a medical meeting in the second half of 2012, the expectation that enrollment of the trial evaluating retaspimycin HCl and docetaxel will complete this fall and that Infinity will report data from this trial in the first half of 2013, the expectation that Infinity will present top-line data from the dose escalation portion of its Phase 1b/2 trial evaluating retaspimycin HCl and everolimus, potential first-in-class and best-in-class opportunities, market size projections, the expectation that Infinity will receive $27.5 million from the planned equity investment by Purdue Pharma L.P. and the expectation that Infinity’s cash runway will extend into 2014. • For example, there can be no guarantee that Infinity will be able to complete the transactions contemplated in the securities purchase agreement with Purdue Pharma L.P. and certain associated entities, that any product candidate Infinity is developing will successfully complete necessary preclinical and clinical development phases or that development of any of Infinity’s product candidates will continue. Further, there can be no guarantee that any positive developments in Infinity’s product portfolio will result in stock price appreciation. Management’s expectations could also be affected by risks and uncertainties relating to: Infinity’s results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. FDA and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Infinity’s ability to enroll patients in its clinical trials; unplanned cash requirements and expenditures, including in connection with business development activities; development of agents by Infinity’s competitors for diseases in which Infinity is currently developing its product candidates; and Infinity’s ability to obtain, maintain and enforce patent and other intellectual property protection for any product candidates it is developing. • These and other risks which may impact management’s expectations are described in greater detail under the caption “Risk Factors” included in Infinity’s quarterly report on Form 10-Q for the quarter ended June 30, 2012, filed with the Securities and Exchange Commission on August 7, 2012. • Further, any forward-looking statements contained in this presentation speak only as of the date hereof, and Infinity expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. |