Exhibit (a)(5)(H)
COMMONWEALTH OF MASSACHUSETTS
| | | | | | | | | | | | | |
| SUFFOLK, ss | | | | | | SUPERIOR COURT
| | | | |
| | | | | | | Civ. Action No. 09-0269-BLS
| | | | |
| | | | | | | | | | | | | |
MARTIN ALBRIGHT AND VITO CARUSO,
| | | ) | | | | | | | | | |
individually and on behalf of all others similarly
| | | ) | | | | | | | | | |
| situated, | | | ) | | | | | | | | | |
| | | | ) | | | | | | | | | |
| Plaintiffs, | | | ) | | | | | | | | | |
| | | | ) | | | AMENDED | | | | |
| v. | | | ) | | | CLASS ACTION | | | | |
| | | | ) | | | COMPLAINT | | | | |
MARK LEUCHTENBERGER, STÉPHANE
| | | ) | | | | | | | | | |
BANCEL, M.D., GAREN BOHLIN, JEFFREY
| | | ) | | | | | | | | | |
COURTNEY, ROSEMARY A. CRANE,
| | | ) | | | | | | | | | |
WILLIAM W. CROUSE, ERIC M. GORDON,
| | | ) | | | | | | | | | |
PH.D., DILIP MEHTA, M.D., PH.D,
| | | ) | | | | | | | | | |
TARGANTA THERAPEUTICS
| | | ) | | | | | | | | | |
CORPORATION, THE MEDICINES
| | | ) | | | | | | | | | |
COMPANY, AND BOXFORD
| | | ) | | | | | | | | | |
SUBSIDIARY CORPORATION,
| | | ) | | | | | | | | | |
| | | | ) | | | | | | | | | |
| Defendants. | | | ) | | | | | | | | | |
| | | | ) | | | | | | | | | |
| | | | | | | | | | | |
Plaintiffs, by their attorneys, allege upon information and belief, except for their own acts, which are alleged on knowledge, as follows:
1. Plaintiffs bring this action on behalf of the public shareholders of Targanta Therapeutics Corporation (“Targanta” or the “Company”) against Defendants Targanta and its Board of Directors seeking equitable relief for their breaches of fiduciary duty and other violations of state law arising out of a proposed transaction in which Defendants The Medicines Company Inc. (“Medicines”) and Boxford Subsidiary Corporation (“Boxford” or “Merger Sub”) plan to acquire all the outstanding shares of Targanta for $2.00 per share plus contractual rights to receive up to an additional $4.55 per share contingent upon certain regulatory and commercial milestones achieved in relation to Targanta’s products (the “Proposed Transaction”). The consideration being offered — $42 million, not including contingent payments — is grossly unfair
- 1 -
and significantly undervalues the Company. Moreover, in addition to accepting an unreasonable offer price, the Targanta Board has failed to provide shareholders with adequate disclosure concerning the Proposed Transaction, preventing them from making an informed decision as to whether or not to tender their shares into this transaction.
2. Accordingly, Plaintiffs seek injunctive and other equitable relief to prevent irreparable injury that the Company and its shareholders will continue to suffer absent judicial intervention.
PARTIES
3. Plaintiffs are, and have been at all relevant times, the owners of shares of common stock of Targanta.
4. Targanta is a corporation organized and existing under the laws of the State of Delaware. It maintains its principal corporate offices at 222 Third Street, Suite 2300, Cambridge, MA 02142-1122. Targanta is a biopharmaceutical company focused on developing and commercializing antibiotics to treat serious infections in the hospital and other institutional settings. The Company’s pipeline includes an intravenous version of oritavancin, a semi- synthetic lipoglycopeptide antibiotic currently awaiting EU regulatory approval, and a program to develop an oral version of oritavancin for the possible treatment of Clostidium difficile-related infection. The Company has operations in Cambridge, MA, Indianapolis, IN, and Montreal, Quebec, Canada.
5. Defendant Mark Leuchtenberger has been the President and Chief Executive Officer of the Company since September 2006.
6. Defendant Stéphane Bancel M.D. has been a Director of the Company since 2008.
7. Defendant Garen Bohlin has been a Director of the Company since May 2007.
- 2 -
8. Defendant Jeffrey Courtney has been a Director of the Company since December 2005.
9. Defendant Rosemary A. Crane has been a Director of the Company since 2008.
10. Defendant William W. Crouse has been a Director of the Company since December 2005.
11. Defendant Eric M. Gordon Ph.D. has been a Director of the Company since January 2007.
12. Defendant Dilip Mehta M.D., Ph.D. has been a Director of the Company since December 2005.
13. Defendants referenced in ¶¶ 4 through 11 are collectively referred to as the Individual Defendants and/or the Targanta Board. The Individual Defendants as officers and/or directors of Targanta, have a fiduciary relationship with Plaintiff and other public shareholders of Targanta and owe them the highest obligations of good faith, fair dealing, loyalty and due care.
14. Defendant Medicines is a Delaware Corporation with its headquarters located at 8 Campus Drive, Parsippany, NJ 07054. Medicines is focused on advancing the treatment of critical care patients through the delivery of innovative, cost-effective medicines to the worldwide hospital marketplace. The Company markets Angiomax® (bivalirudin) in the United States and other countries for use in patients undergoing coronary angioplasty, and Cleviprex® (clevidipine butyrate) injectable emulsion in the United States for the reduction of blood pressure when oral therapy is not feasible or not desirable. Medicines also has an investigational antiplatelet agent, cangrelor, in late-stage development and a serine protease inhibitor, CU-2010, in early-stage development.
- 3 -
15. Defendant Merger Sub is a Delaware Corporation wholly owned by Medicines that was created for the purpose of effectuating the Proposed Transaction.
INDIVIDUAL DEFENDANTS’ FIDUCIARY DUTIES
16. By reason of the Individual Defendants’ positions with the Company as officers and/or Directors, they are in a fiduciary relationship with Plaintiffs and the other public shareholders of Targanta and owe them, as well as the Company, a duty of the highest good faith, fair dealing, loyalty and full, candid and adequate disclosure, as well as a duty to maximize shareholder value.
17. Where the officers and/or Directors of a publicly traded corporation undertake a transaction that will result in either: (i) a change in corporate control; (ii) a break up of the corporation’s assets; or (iii) sale of the corporation, the Directors have an affirmative fiduciary obligation to obtain the highest value reasonably available for the corporation’s shareholders, and if such transaction will result in a change of corporate control, the shareholders are entitled to receive a significant premium. To diligently comply with their fiduciary duties, the Directors and/or officers may not take any action that:
(a) adversely affects the value provided to the corporation’s shareholders;
(b) favors themselves or will discourage or inhibit alternative offers to purchase control of the corporation or its assets;
(c) contractually prohibits them from complying with their fiduciary duties;
(d) will otherwise adversely affect their duty to search and secure the best value reasonably available under the circumstances for the corporation’s shareholders; and/or
(e) will provide the Directors and/or officers with preferential treatment at the expense of, or separate from, the public shareholders.
- 4 -
18. In accordance with their duties of loyalty and good faith, the Individual Defendants, as Directors and/or officers of Targanta, are obligated to refrain from:
(a) participating in any transaction where the Directors or officers’ loyalties are divided;
(b) participating in any transaction where the Directors or officers receive, or are entitled to receive, a personal financial benefit not equally shared by the public shareholders of the corporation; and/or
(c) unjustly enriching themselves at the expense or to the detriment of the public shareholders.
19. Plaintiffs allege herein that the Individual Defendants, separately and together, in connection with the Proposed Transaction are knowingly or recklessly violating their fiduciary duties, including their duties of loyalty, good faith and independence owed to Plaintiffs and other public shareholders of Targanta, or are aiding and abetting others in violating those duties.
20. Defendants also owe the Company’s shareholders a duty of truthfulness, which includes the disclosure of all material facts concerning the Proposed Transaction and, particularly, the fairness of the price offered for the shareholders’ equity interest. Defendants are knowingly or recklessly breaching their fiduciary duties of candor and good faith by failing to disclose all material information concerning the Proposed Transaction, and/or aiding and abetting other Defendants’ breaches.
CONSPIRACY, AIDING AND ABETTING AND CONCERTED ACTION
21. In committing the wrongful acts alleged herein, each of the Defendants has pursued, or joined in the pursuit of, a common course of conduct, and acted in concert with and conspired with one another, in furtherance of their common plan or design. In addition to the
- 5 -
wrongful conduct herein alleged as giving rise to primary liability, the Defendants further aided and abetted and/or assisted each other in breach of their respective duties as herein alleged.
22. During all relevant times hereto, the Defendants, and each of them, initiated a course of conduct which was designed to and did: (i) permit Medicines to attempt to eliminate the public shareholders’ equity interest in Targanta pursuant to a defective sales process, and (ii) permit Medicines to buy the Company for an unfair price and pursuant to unfair terms. In furtherance of this plan, conspiracy and course of conduct, Defendants, and each of them, took the actions as set forth herein.
23. Each of the Defendants herein aided and abetted and rendered substantial assistance in the wrongs complained of herein. In taking such actions, as particularized herein, to substantially assist the commission of the wrongdoing complained of, each Defendant acted with knowledge of the primary wrongdoing, substantially assisted the accomplishment of that wrongdoing, and was aware of his or her overall contribution to, and furtherance of, the wrongdoing. The Defendants’ acts of aiding and abetting included,inter alia, the acts each of them are alleged to have committed in furtherance of the conspiracy, common enterprise and common course of conduct complained of herein.
CLASS ACTION ALLEGATIONS
24. Plaintiffs bring this action on their own behalf and as a class action on behalf of all owners of Targanta common stock and their successors in interest, except Defendants and their affiliates (the “Class”).
25. This action is properly maintainable as a class action for the following reasons:
(a) the Class is so numerous that joinder of all members is impracticable. As of January 15, 2009, Targanta had approximately 21 million shares outstanding.
- 6 -
(b) questions of law and fact are common to the Class, including, inter alia, the following:
| | (i) | | Have the Individual Defendants breached their fiduciary duties owed by them to Plaintiffs and the others members of the Class; |
| |
| | (ii) | | Are the Individual Defendants, in connection with the Proposed Transaction of Targanta by Medicines and Merger Sub, pursuing a course of conduct that does not maximize Targanta’s value, in violation of their fiduciary duties; |
| |
| | (iii) | | Have the Individual Defendants misrepresented and omitted material facts in violation of their fiduciary duties owed by them to Plaintiffs and the other members of the Class; |
| |
| | (iv) | | Have Medicines and Merger Sub aided and abetted the Individual Defendants’ breaches of fiduciary duty; and |
| |
| | (v) | | Is the Class entitled to injunctive relief or damages as a result of Defendants’ wrongful conduct. |
(c) Plaintiffs are committed to prosecuting this action and has retained competent counsel experienced in litigation of this nature.
(d) Plaintiffs’ claims are typical of those of the other members of the Class.
(e) Plaintiffs have no interest that are adverse to the Class.
(f) The prosecution of separate actions by individual members of the Class would create the risk of inconsistent or varying adjudications for individual members of the Class and of establishing incompatible standards of conduct for Defendants.
- 7 -
26. Conflicting adjudications for individual members of the Class might as a practical matter be dispositive of the interests of the other members not parties to the adjudications or substantially impair or impede their ability to protect their interests.
SUBSTANTIVE ALLEGATIONS
THE PRICE IS UNFAIR
27. In a press release dated January 12, 2009, the Company announced that it had entered into an agreement to be acquired by Medicines through a merger agreement cash tender offer at $2.00 per share, stating:
PARSIPPANY, NJ—(MARKET WIRE)—Jan 12, 2009— The Medicines Company (NasdaqGS:MDCO — News) today announced that it has entered into a merger agreement with Targanta Therapeutics Corporation (NasdaqGM:TARG — News) under which The Medicines Company has agreed to commence a tender offer to acquire 100 percent of Targanta’s outstanding shares.
“The Medicines Company is pleased to announce our agreement to add the assets and capabilities of Targanta. The addition of Targanta’s oritavancin, a late stage product, will be another step toward execution of our strategic plan to become a global leader in critical care medicine,” said Clive Meanwell, M.D., Chairman and Chief Executive Officer of The Medicines Company. “Oritavancin has the potential to provide important patient outcome and economic advantages for hospitals. The growing hospital market for gram positive infections in the U.S. alone reached $1.1 billion in 2007. We believe that oritavancin can become an important anti-infective for serious infections involving difficult-to-treat bacteria in difficult-to-treat hospitalized patients. Many of those critically ill patients are the same patients treated with our existing products.”
Under the terms of the merger agreement, Targanta shareholders will receive $2.00 in cash up front for each common share tendered, or approximately $42 million. Targanta shareholders may also be entitled to receive additional contingent cash payments upon the achievement of specified regulatory and commercial milestones within agreed upon time periods:
— Upon Food and Drug Administration (FDA) approval of a new drug application (NDA) for oritavancin for cSSSI
- 8 -
(complicated skin and skin structure infections) using a single dose infusion, $1.20 per share. If FDA approval does not include single dose infusion labeling, this payment is reduced to $0.50 per share.
— Upon European Medicines Agency (EMEA) approval of a marketing authorisation application (MAA) for oritavancin for cSSSI during 2009, $1.00 per share. If EMEA approval occurs later, this payment is reduced to $0.75 per share if it occurs prior to June 30, 2010 or if later, $0.50 per share.
— On achievement of worldwide net sales adding up to a total of $400 million or more in the aggregate over four consecutive quarters, a one-time payment of $2.35 per share.
The transaction has been approved by the boards of directors of both companies, and Targanta’s Board of Directors has recommended that Targanta’s shareholders tender their shares into the tender offer, adopt the merger agreement and approve the merger.
“We believe that this transaction can create significant value for our shareholders and further expand our portfolio of critical care products. It adds a late stage product, with global rights, and the potential for near-term revenue, and could contribute significantly to our long-term growth. Oritavancin is a well characterized Phase 3 asset. We believe the deal terms reflect a balanced investment to expand our product portfolio and we agreed to pay for the transaction with cash to avoid share dilution. The addition of staged payments provides Targanta shareholders additional value if milestones are achieved and mitigates risk for The Medicines Company,” said Glenn Sblendorio, EVP & Chief Financial Officer.
Targanta’s lead product, oritavancin, is an innovative, investigational hospital-based antibiotic with potent bactericidal (killing) activity against a broad spectrum of gram-positive bacteria including staphylococcal strains with resistance to methicillin (MRSA) and vancomycin, Oritavancin has the potential to provide significant clinical advantages, including superior dosing options over current IV antibiotics that treat serious infections in the hospital setting. While conventional daily dosing with oritavancin would provide hospitals with a new treatment option, the potential for oritavancin to be a single dose product could deliver significant cost advantages and treatment benefits to the health-care system. Initial use of oritavancin is expected in critical care settings within the hospital including the ICU, surgical
- 9 -
suite and the emergency department, where The Medicines Company sales representatives promote our current products.
Oritavancin has been studied in two pivotal Phase 3 trials in the treatment of cSSSI (complicated skin and skin structure infections). Phase 2 trials have successfully studied the compound in gram positive bacteremia and have examined efficacy and safety of a single dose infusion in cSSSI (SIMPLIFI trial). Pre-clinical studies have shown unique anti-microbiological activity in Clostridium Difficile infection, a rapidly growing problem, which causes severe and occasionally life-threatening colitis in the hospital.
Invasive MRSA infection is a serious and growing public health care concern. In the U.S. over 94,000 patients suffered invasive infection in 2005, and, of these, almost 19,000 cases were associated with death (Klevens, RM et al, JAMA. 2007 Oct 17). As a result of this epidemic, the lack of efficacy provided by existing older drug treatments, and the availability of novel antibiotics similar to oritavancin delivered with conventional dosing, the US market for sales of gram-positive antibiotics grew to more than $1 billion in 2007, up 17% since 2006.
In December 2008, the U.S. Food and Drug Administration (FDA) issued a complete response letter to Targanta’s New Drug Application (NDA) indicating that it could not approve the NDA in its present form and that it would be necessary for Targanta to perform an additional adequate and well-controlled study to demonstrate the safety and efficacy of oritavancin in patients with cSSSI before the application may be approved. A Market Authorisation Application (MAA) for oritavancin is undergoing review by the European Medicines Agency (EMEA).
Following consummation of the transaction, The Medicines Company plans to consult with regulatory authorities with a view to initiating a confirmatory Phase 3 study, in the United States, of oritavancin given as a single dose infusion before the end of the year.
The tender offer will expire at midnight Eastern Time on the 20th business day following and including the commencement date, unless extended in accordance with the terms of the merger agreement and the applicable rules and regulations of the Securities and Exchange Commission. The tender offer, if successful, will be followed by a second-step merger in which any shares of Targanta not tendered into the offer will be converted into the right to receive the same per share consideration paid to
- 10 -
Targanta shareholders in the tender offer. The Medicines Company has entered into agreements with Targanta shareholders representing approximately 36% of the voting shares outstanding to tender their shares in the tender offer.
The consummation of the tender offer is subject to the satisfaction or waiver of certain conditions, including: (i) a majority of outstanding Targanta shares on a fully diluted basis having been tendered into the offer, (ii) the absence of litigation by any governmental agency relating to the transaction or any other litigation that would reasonably be expected to succeed and in which a judgment adverse to Targanta would reasonably be expected to result in a material adverse change with respect to Targanta, (iii) there not having been a material adverse change with respect to Targanta, and (iv) other customary conditions. The tender offer is not subject to a financing condition.
The Medicines Company plans to announce fourth quarter and full year 2008 financial results in February 2009. At that time, the Company will provide expected 2009 full-year net sales and net income estimates based upon completion of the valuation of the transaction and transition costs.
28. On that same day, the Company filed a Form 8-K with the United States Securities and Exchange Commission (“SEC”) wherein it disclosed the operating Agreement and Plan of Merger for the Proposed Transaction (the “Merger Agreement”). The announcement and filings reveal that the Proposed Transaction is the product of a flawed sales process and is being consummated at an unfair price.
29. The only guaranteed payment, however, is the $2.00 per shares and shareholders were given no assurances whatsoever that they would get the additional contingent payments. Specifically, in a January 13, 2008 investor conference call, Medicines Executive Vice President and Chief Financial Officer Glenn Sblendorio explained:
Under the terms of the merger agreement, Targanta’s shareholders will receive $2 in cash up front for each common share tendered, or approximately $42 million. Targanta’s shareholders may also be entitled to receive three additional contingent payments which we are calling contingent payment rights upon the achievement of
- 11 -
specified regulatory and commercial milestones within agreed upon time limits.
First, upon Food and Drug Administration, or FDA approval, of a new drug application for Oritavancin, specifically for cSSSI, which is complicated skin and skin structure infections, using a single dose infusion, shareholders would receive an additional $1.20 per share. If the FDA does not include single-dose infusion labeling, this payment is reduced to $0.50 a share.
Second, upon European Medicines Agency, or EMEA approval of a marketing authorization for Oritavancin, again for cSSSI, during 2009 shareholders would receive $1 per share. If the EMEA approval occurs later, this payment is reduced to $0.75 per share. If it occurs after June 30, 2010, shareholders would receive $0.50 a share.
And third, upon achievement of worldwide net sales adding up to $400 million over four consecutive quarters, a one-time payment of $2.35 per share. To provide you with a dollar figure for these three contingent payments, the total of the regulatory and commercial milestones, if achieved, could be approximately $70.3 million to $95.5 million, of which $49.3 million would be a one-time sales milestone.
30. Thus, the Company has not provided any assurances that the shareholders will receive the additional contingent payments. Moreover, it is unclear which party will bear the ultimate responsibility for making such future payments.
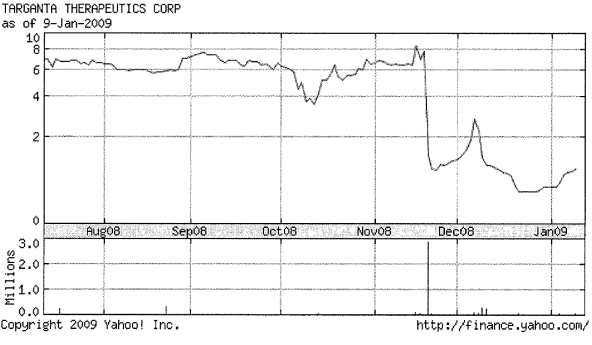
31. Furthermore, the offer price is unfair given that in the few months prior to the Proposed Transaction, Targanta stock was trading substantially in excess of the Proposed Transaction offer price of $2.00. In fact, during November and December 2008, Targanta stock traded around $7 to $8 per share. Moreover, the current market price of Targanta stock is approximately $2.50 per share - 25% above the $2.00 Proposed Transaction price.
- 12 -
32. In fact, Wall Street analysts value Targanta’s shares well in excess of the price in the Proposed Transaction. The average analyst valuation is $2.80 to $3 per share. Therefore, the offer price of $2.00 per share plus possible contingent payments, which are at best uncertain and depend on certain metrics that may or may not be achieved, represents a significant discount to the Company’s intrinsic value rather than the premium transaction boasted by the Company in the aforementioned press release.
33. Finally, section 2.7 of the Merger Agreement provides for a Top-Up option that permits Medicines to acquire sufficient shares to ensure that they can obtain 90% of the outstanding common shares of Targanta to successfully complete the Proposed Transaction.
SELF-DEALING
34. By reason of their positions with Targanta, the Individual Defendants are in possession of non-public information concerning the financial condition and prospects of Targanta, and especially the true value and expected increased future value of Targanta and its assets, which they have not disclosed to Targanta’s public shareholders. Moreover, despite their
- 13 -
duty to maximize shareholder value, the Defendants have clear and material conflicts of interest and are acting to better their own interests at the expense of Targanta’s public shareholders.
35. In particular, the Employment Agreement for officers and certain individual defendants provide for severance packages in the amount of $782,990.00, $386,230.00, $378,532.00, $365,605.00 and $128,625.00 for Mark W. Leuchtenberger, George A. Eldridge, Thomas R. Parr, Jr., Mona L. Haynes and Roger D. Miller, respectively. The aggregate of the severance packages represents approximately 4% of the upfront consideration in the Proposed Transaction.
36. Further, William W. Crouse is a Director for both the Company and Medicines.
37. The Proposed Transaction is wrongful, unfair and harmful to Targanta’s public shareholders, and represents an effort by defendants to aggrandize their own financial position and interests at the expense of and to the detriment of Class members. The Proposed Transaction is an attempt to deny Plaintiffs and the other members of the Class their rights while usurping the same for the benefit of defendants and Abbott on unfair terms
THE PRECLUSIVE DEAL PROTECTION DEVICES
38. As part of the Merger Agreement, Defendants agreed to certain onerous and preclusive deal protection devices that operate conjunctively to make the Proposed Transaction afait d’accompli and ensure that no competing offers will emerge for the Company.
39. First, section 7.1 of the Merger Agreement contains a strict “No Solicitation” provision prohibiting the members of the Targanta Board from taking any affirmative action to comply with their fiduciary duties to maximize shareholder value, including soliciting proposals relating to alternative tender offer or business combinations. The Merger Agreement also includes a strict “standstill” provision which prohibits, except under extremely limited circumstances, the Defendants from even engaging in discussions or negotiations relating to
- 14 -
proposals regarding alternative business combinations. In addition to the no-shop and standstill provisions, the Merger Agreement includes a $5,485,000 termination fee that in combination will all but ensure that no competing offer will be forthcoming.
40. Section 7.1(b) of the Merger Agreement severely constrains the Targanta Board from entering into any discussions or negotiations for a competing bid — they can do soonly“after consultation with outside counsel, in response to a bona fide, unsolicited written Acquisition Proposal made or received after the date of this Agreement that the Company Board reasonably determines in good faith after consultation with outside counsel and the Financial Advisor or another nationally recognized independent financial advisor is, or is reasonably likely to lead to, a Superior Proposal, in each case that did not result from a breach by the Company of this Section 7.1, and not earlier than three business days after providing the notice contemplated by Section 7.1(c), (x) furnish information with respect to the Company to the person making such Acquisition Proposal and its Representatives pursuant to a customary confidentiality agreement not less restrictive of the other party than the Confidentiality Agreement other than any standstill restrictions and (y) participate in discussions or negotiations (including solicitation of a revised Acquisition Proposal) with such person and its Representatives regarding any Acquisition Proposal.”
41. Further, Section 7.1(b)(A), permits the Board to recommend an alternative Acquisition Proposal only after “a majority of the Company Board determines (after consultation with outside counsel) that it is necessary to take such actions in order to comply with its fiduciary duties to the shareholders of the Company under applicable Law; provided, however, that no such Company Adverse Recommendation Change may be made until after the fourth calendar day following Parent’s receipt of written notice from the Company advising Parent that
- 15 -
the Company Board intends to take such action and specifying the reasons therefor, including the terms and conditions of any Superior Proposal that is the basis of the proposed action by the Company Board.” These provisions further discourage bidders from making a competing bid for the Company.
42. Thus, even if the Targanta Board receives an intervening bid that appeared to be “superior” to Medicines’ offer, they are precluded from even entering into discussions or negotiations unless they can first demonstrate that the alternative proposal is, in fact, “superior.” The provisions cited above prevent the Targanta Board from exercising its fiduciary duties to investigate competing proposals unless, as a prerequisite, the majority of the Targanta Board (without discussions from the potential competing bidder) first determines that the proposal is superior on its own—a virtually impossible task to complete without engaging the potential competing bidder.
43. In addition to the unreasonably high standard that must be met for the Board to even consider a competing bid, the Company must also notify Medicines promptly (and in any event within 24 hours) before recommending to accept that alternative bid, giving Medicines an opportunity to match the terms of any competing bid. This provision will undoubtedly be a huge obstacle to any competing offers emerging because no potential bidder will waste time and resources to make a competing bid that Medicines can simply match.
44. Further, the Merger Agreement, which locks up approximately 36% of the outstanding shares in favor of Medicines (as described below) is combined with a preclusive “Top-Up” option that permits Medicines to acquire additional shares of up to 90% of the outstanding common shares of Targanta so that Medicines could consummate a short-form merger transaction and acquire the entire Company regardless of the shareholders’ will. In other
- 16 -
words, the tender offer is nothing but a sham because the Merger Agreement in combination with the “Top-Up” option renders it virtually impossible for the Proposed Transaction not to be consummated.
45. The Proposed Transaction also includes a $5.485 million termination fee that together with the no shop, standstill, Merger Agreement, Top-Up and Voting Agreements (described below) ensure that no competing offer will ever be forthcoming.
VOTING AGREEMENTS
46. In addition to the preclusive deal protection devices described above, certain individual defendants or entities affiliated with them, as well certain shareholders, have entered into agreements with Medicines to tender in support of the Proposed Transaction. In particular, Radius Venture Partners II, LP, Radius Venture Partners III QP, LP, Radius Venture Partners III, LP, Radius Venture Partners III (OH), LP (each of which is affiliated with Dilip J. Mehta, M.D., Ph.D., a director of the Company); Skyline Venture Partners Qualified Purchaser Fund IV, L.P., Skyline Venture Partners Qualified Purchaser Fund III, L.P., Skyline Venture Partners III, L.P. (each of which is affiliated with Eric M. Gordon, Ph.D., a director of the Company); VenGrowth Advanced Life Sciences Fund Inc., and VenGrowth III Investment Fund Inc. (each of which is affiliated with Jeffrey Courtney, a director of the Company); Caduceus Private Investments III LP, OrbiMed Associates III, LP, Seaflower Health Ventures III, L.P., Seaflower Health Ventures III Companion Fund, L.P., and J&L Sherblom Family LLC, have agreed to tender in favor of the Proposed Transaction their combined common stock, restricted common stock and options and warrants to purchase common stock ownership that accounts for approximately 36% of the outstanding Company common stock (the “Voting Agreements”).
- 17 -
47. As a result of the Voting Agreements, the public shareholders of Targanta have little say as to whether the Proposed Transaction is consummated. In particular, given that 36% of the voting shares are already “locked-up” under the Voting Agreements and a Top-Up can be exercised by Medicines to get up to 90% to avoid shareholder vote if a majority of common stock are tendered in favor of Proposed Transaction, only an additional 14.1% of common stock is needed to approve the Proposed Transaction. Thus, the Proposed Transaction is afait accomplieven if the majority of public shareholders disapprove of the deal.
THE RECOMMENDATION STATEMENT IS
MATERIALLY MISLEADING AND/OR
INCOMPLETE
48. On January 27, 2009, the Company filed a Schedule 14D-9 Recommendation Statement (the “Recommendation Statement”) with the SEC in connection with the Proposed Transaction.
49. The Recommendation Statement fails to provide the Company’s shareholders with material information and/or provides them with materially misleading information, thereby rendering shareholders unable to make an informed decision on how to tender their shares in connection with Proposed Transaction.
50. For example, the Recommendation Statement completely fails to disclose the underlying methodologies, key inputs and ranges of ultimate values relied upon and observed by Leerink Swann, LLC (“Leerink Swann”) so that shareholders can properly assess the credibility of the various analyses performed by Leerink Swann and relied upon by the Board in recommending the Proposed Transaction. In particular, the Recommendation Statement is deficient and should provide,inter alia, as follows:
| | (i) | | Disclose the justifications for the Probability Projections to have identical figures for Pre-Tax Operating Income and Free Cash Flow for years 2009-14 and 2022-23. |
- 18 -
| | (ii) | | Disclose the assumptions and actual probability and timing for the Probability Projections provided to Leerink Swann to prepare its fairness opinion. |
| |
| | (iii) | | Disclose a complete set of Projections and Probability Projections for years 2009-2023 (i.e., EPS or Net Operating Income and outstanding shares). |
| |
| | (iv) | | Disclose the justifications for selecting a discounted annual rate of 15% in theHistorical Stock Trading Analysisperformed by Leerink Swann. |
| |
| | (v) | | Disclose the probability and timing for each scenario in theHistorical Stock Trading Analysisperformed by Leerink Swann. |
| |
| | (vi) | | Disclose the justifications for selecting companies in different markets and with different products and the relevance of the companies used in thePublicly Traded Analysisperformed by Leerink Swann. |
| |
| | (vii) | | Disclose the multiples applied or derived for each company (or at least the range: high/median/mean/low) in thePublicly Traded Analysis performed by Leerink Swann. |
| |
| | (viii) | | Disclose the multiples observed for each company resulting in a range of implied per share value (or at least the range: high/median/mean/low) in thePublicly Traded Analysisperformed by Leerink Swann. |
| |
| | (ix) | | Disclose the justification of selecting a discount rates of 12.5% to 17.5% and P/E multiples of 15.0x to 25.0x in 2014 in theDiscounted Stock Price Analysisperformed by Leerink Swann. |
| |
| | (x) | | Disclose the multiples applied or derived for each company (or at least the range: high/median/mean/low) in theDiscounted Stock Price Analysisperformed by Leerink Swann. |
| |
| | (xi) | | Disclose the range of premiums observed for each transaction in theRecent Small Capitalization Biopharma Transaction Premiums Analysis performed by Leerink Swann. |
| |
| | (xii) | | Disclose the range of premiums applied in the implied per share table in theRecent Small Capitalization Biopharma Transaction Premiums Analysisperformed by Leerink Swann, and how the particular premium correlates to the Proposed Transaction. |
| |
| | (xiii) | | Disclose the justification of selecting a discount rate of 12.5% to 17.5% in theDiscounted Cash Flow Analysisperformed by Leerink Swann. |
| |
| | (xiv) | | Disclose the terminal values selected (or if none were used the reason for it) for theDiscounted Cash Flow Analysisperformed by Leerink Swann. |
- 19 -
51. Further, the Recommendation Statement states that Leerink Swann was retained as the Company’s financial advisor in the Proposed Transaction, but fails to inform the shareholders which other investment advisors (if any) were considered, the specific experience in mergers and acquisitions or change in control transactions that Leerink Swann has worked on, as well as, whether Leerink Swann or any of its clients have any interest in the Company or future work that Leerink Swann has or will perform for Medicines. It is material for shareholders to be informed of previous or future relationships by a financial advisors as well any financial and economic interests by either Leerink Swann or its clients that could be perceived or create a conflict of interest.
52. The Recommendation Statement further neglects to provide shareholders with sufficient information to evaluate the potential growth prospects and risks associated with Targanta remaining as stand-alone Company, which is vital to shareholders when deciding whether or not to cash out their interest in the Company in this Proposed Transaction, or for that matter the probability for shareholders to actually receive the payments under the CPR Agreement if the Proposed Transaction goes forward.
53. Moreover, Leerink Swann approached 22 potential strategic partners to explore an acquisition opportunity. The Recommendation Statement, however, does not provide the criteria to select potential partners and whether they were a mix of strategic and financial partners and the specific breakdown. According to the Recommendation Statement, at least 3 unsolicited inquiries were received in January 2009 from parties that do not appear to have been included in the list of 22 potential strategic partners and with whom no negotiations took place, The Recommendation Statement needs to provide material disclosure related to the sales process for shareholders to be able to make an informed decision.
- 20 -
54. Lastly, according to the Recommendation Statement, on December 17, 2008 Leerink Swann advised the Board that exclusivity would be required to negotiate with Medicines based on prior experiences with Medicines. The Recommendation Statement omits to explain what prior experiences Leerink Swann has had and in what type of transactions. This information is germane to the sales process, especially in light of the unsolicited inquiries the Company received and was not able to explore due to the exclusivity entered with Medicines on December 21, 2009.
55. Accordingly, Plaintiffs seek injunctive and other equitable relief to prevent the irreparable injury that the Company and its shareholders will continue to suffer absent judicial intervention.
CLAIM FOR RELIEF
COUNT I
Breach of Fiduciary Duty — Failure to Maximize Shareholder Value
(Against All Individual Defendants)
56. Plaintiffs repeat all previous allegations as if set forth in full herein.
57. As Directors of Targanta, the Individual Defendants stand in a fiduciary relationship to Plaintiffs and the other public shareholders of the Company and owe them the highest fiduciary obligations of loyalty and care. The Individual Defendants’ recommendation of the Proposed Transaction will result in change of control of the Company which imposes heightened fiduciary responsibilities to maximize Targanta’s value for the benefit of the shareholders and requires enhanced scrutiny by the Court.
58. As discussed herein, the Individual Defendants have breached their fiduciary duties to Targanta shareholders by failing to engage in an honest and fair sale process.
59. As a result of the Individual Defendants’ breaches of their fiduciary duties, Plaintiffs and the Class will suffer irreparable injury in that they have not and will not receive
- 21 -
their fair portion of the value of Targanta’s assets and will be prevented from benefiting from a value-maximizing transaction.
60. Unless enjoined by this Court, the Individual Defendants will continue to breach their fiduciary duties owed to Plaintiffs and the Class, and may consummate the Proposed Transaction, to the irreparable harm of the Class.
61. Plaintiffs and the Class have no adequate remedy at law.
COUNT II
Breach of Fiduciary Duty — Disclosure
(Against Individual Defendants)
62. Plaintiffs repeat all previous allegations as if set forth in full herein.
63. The fiduciary duties of the Individual Defendants in the circumstances of the Proposed Transaction require them to disclose to Plaintiffs and the Class all information material to the decisions confronting Targanta’s shareholders.
64. As set forth above, the Individual Defendants have breached their fiduciary duty through materially inadequate disclosures and material disclosure omissions.
65. As a result, Plaintiffs and the Class members are being harmed irreparably.
66. Plaintiffs and the Class have no adequate remedy at law.
COUNT III
Aiding and Abetting
(Against Targanta, Medicines and Merger Sub)
67. Plaintiffs repeat all previous allegations as if set forth in full herein.
68. As alleged in more detail above, Targanta, Medicines and Merger Sub are well aware that the Individual Defendants have not sought to obtain the best available transaction for the Company’s public shareholders. Thus, Defendants Targanta, Medicines and Merger Sub aided and abetted the Individual Defendants’ breaches of fiduciary duties.
52. Plaintiffs and the Class have no adequate remedy at law.
- 22 -
PRAYER FOR RELIEF
WHEREFORE, Plaintiffs demand judgment against Defendants jointly and severally, as follows:
(A) declaring this action to be a class action and certifying Plaintiffs as the Class representatives and their counsel as Class counsel;
(B) enjoining, preliminarily and permanently, the Proposed Transaction;
(C) in the event that the transaction is consummated prior to the entry of this Court’s final judgment, rescinding it or awarding Plaintiffs and the Class rescissory damages;
(D) directing that Defendants account to Plaintiffs and the other members of the Class for all damages caused by them and account for all profits and any special benefits obtained as a result of their breaches of their fiduciary duties;
(E) awarding Plaintiffs the costs of this action, including a reasonable allowance for the fees and expenses of Plaintiff’s attorneys and experts; and
(F) granting Plaintiffs and the other members of the Class such further relief
(G) as the Court deems just and proper.
| | | | | |
| February 2, 2009 | | Respectfully submitted, | | |
| | | | | |
| | | BERMAN DEVALERIO | | |
| | | | | |
| | | /s/ Norman Berman | | |
| | | Norman Berman (BBO# 040460) | | |
| | | Jeffrey C. Block (BBO# 600747) | | |
| | | Kristin J. Moody (BBO# 661792) | | |
| | | One Liberty Square | | |
| | | Boston, MA 02109 | | |
| | | Telephone: (617) 542-8300 | | |
| | | Facsimile: (617) 542-1194 | | |
| | | | | |
| | | [proposed] Liaison Counsel | | |
- 23 -
| �� | | | | |
| | | LEVI & KORSINSKY, LLP | | |
| | | Eduard Korsinsky, Esq.(to be admittedpro hac vice) | | |
| | | Juan E. Monteverde, Esq. (to be admittedpro hac vice) | | |
| | | Jerald M. Stein, Esq. (to be admittedpro hac vice) | | |
| | | 39 Broadway, Suite 1601 | | |
| | | New York, New York 10006 | | |
| | | Telephone: (212) 363-7500 | | |
| | | Facsimile: (212) 363-7171 | | |
| | | | | |
| | | [proposed] Lead Counsel | | |
- 24 -