|
Exhibit 99.1 
|
At Keryx, the patient comes first. Our goal is to bring innovative therapies to market that provide unique and meaningful advantages to patients with renal disease and their healthcare providers – because we know that when patient care improves, everybody succeeds. NEW YORK BOSTON Keryx Biopharmaceuticals, Inc. Keryx Biopharmaceuticals, Inc. Corporate Presentation 750 Lexington Ave., 20th Floor One Marina Drive, Tenth Floor New York, NY 10022 Boston, MA 02210 1.212.531.5965 tel 1. 617.466.3500 tel NASDAQ: KERX | December 2014 1.212.531.5961 fax 1. 617.466.3501 fax www.keryx.com Copyright © 2014 by Keryx Biopharmaceuticals, Inc. |
|

|
Safe Harbor Statement Various remarks that we make about our future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Important factors may cause our actual results to differ materially, including: whether AuryxiaTM (ferric citrate), will be successfully launched and marketed in the U.S.; whether Riona® will be successfully marketed by our Japanese partner, Japan Tobacco, Inc. and Torii Pharmaceutical Co., Ltd; the risk that the EMA could ultimately deny approval of the MAA; the risk that the EMA may not concur with our interpretation of our Phase 3 study results, supportive data, conduct of the studies, or any other part of our MAA submission and could ultimately deny approval of the MAA; the risk that we may not be successful in the development of ferric citrate for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients; and other risk factors identified from time to time in our reports filed with the Securities and Exchange Commission. These and other important factors that may affect our results are discussed under the heading ``Risk Factors’’ in public filings including our 2013 Annual Report on Form 10-K, 1Q 2014 Form 10-Q, as well as other filings we periodically make with the SEC. In addition, any forward-looking statements made during this presentation speak only as of the date of this presentation. While we may update these forward-looking statements to reflect events or circumstances that occur after this date, we specifically disclaim any obligation to do so, even if our estimates and expectations change. Copyright © 2014 by Keryx Biopharmaceuticals, Inc. | 1
|
|
FDA Approved: AuryxiaTM (ferric citrate) Approved on September 5, 2014, by the U.S. Food and Drug Administration (FDA) AuryxiaTM (ferric citrate) is an absorbed, iron-based phosphate binder INDICATION: For the control of serum phosphorus levels in patients with chronic kidney disease (CKD) on dialysis U.S. launch of AuryxiaTM at YE Ferric Citrate is currently being marketed in Japan, under the trade name Riona, by the Company?s Japanese partner, Japan Tobacco Inc. and Torii Pharmaceutical Co. Ltd. Additional potential growth areas for Ferric Citrate Label expansion: Iron deficiency anemia in non-dialysis dependent chronic kidney disease Geographical expansion: EU and emerging markets Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 2
|
|
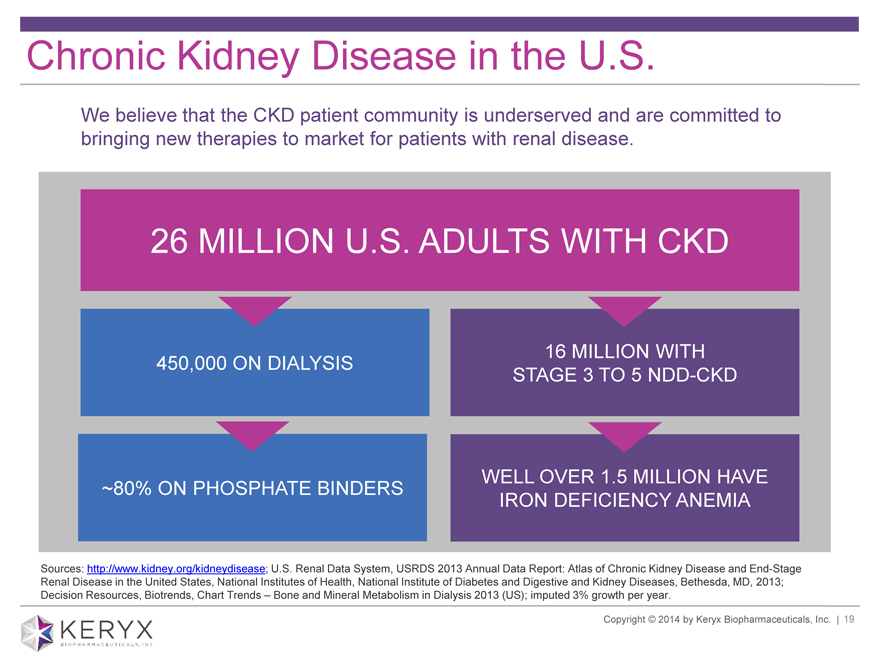
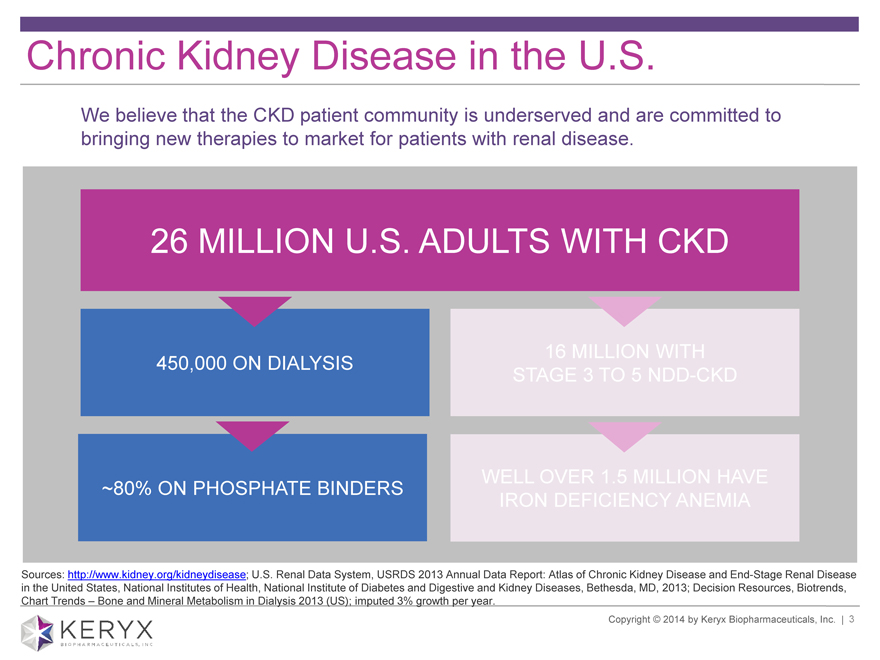
Chronic Kidney Disease in the U.S. We believe that the CKD patient community is underserved and are committed to bringing new therapies to market for patients with renal disease. 26 MILLION U.S. ADULTS WITH CKD 16 MILLION WITH 450,000 ON DIALYSIS STAGE 3 TO 5 NDD-CKD WELL OVER 1.5 MILLION HAVE ~80% ON PHOSPHATE BINDERS IRON DEFICIENCY ANEMIA Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; Decision Resources, Biotrends, Chart Trends ? Bone and Mineral Metabolism in Dialysis 2013 (US); imputed 3% growth per year. Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 3
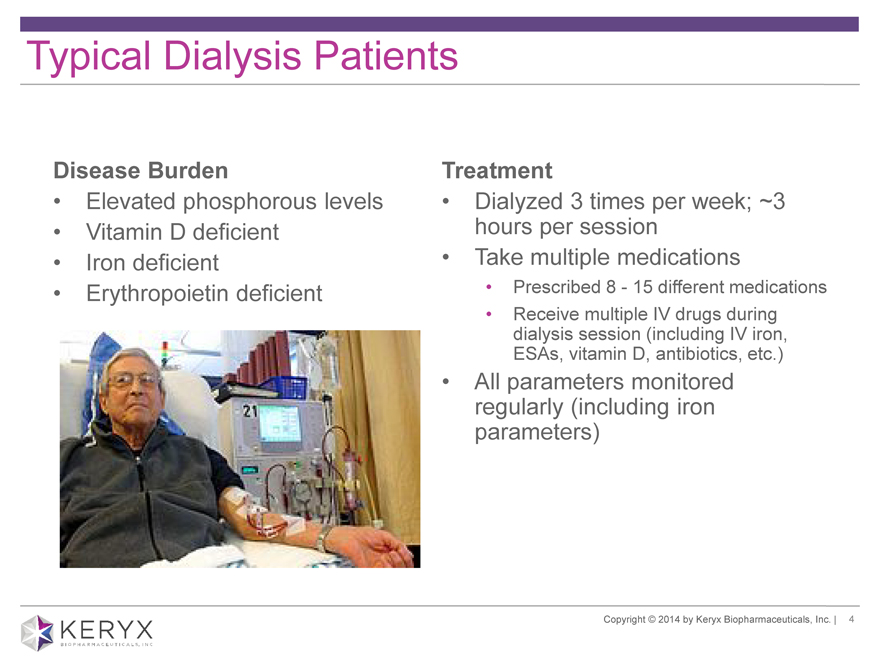
Typical Dialysis Patients
Disease Burden Treatment
Elevated phosphorous levels Dialyzed 3 times per week; ~3
Vitamin D deficient hours per session
Iron deficient Take multiple medications
Erythropoietin deficient Prescribed 8—15 different medications
Receive multiple IV drugs during
dialysis session (including IV iron,
ESAs, vitamin D, antibiotics, etc.)
All parameters monitored
regularly (including iron
parameters)
Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 4
Dialysis Providers U.S. Top 15 U.S. providers oversee care of ~90% of dialysis patients Davita, Fresenius, DCI, Satellite, U.S. Renal, etc. Own dialysis centers, employ nurses, renal dietitians, social workers Responsible for clinical care and operations Bundled payment system Dialysis providers receive payment from Medicare per dialysis session Dialysis session and IV drugs included in bundle; IV iron, ESAs, vitamin D, etc. Oral drugs (e.g. phosphate binders) are not included in the bundle until 2024 Injectable drugs represent a cost center for the providers Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 5
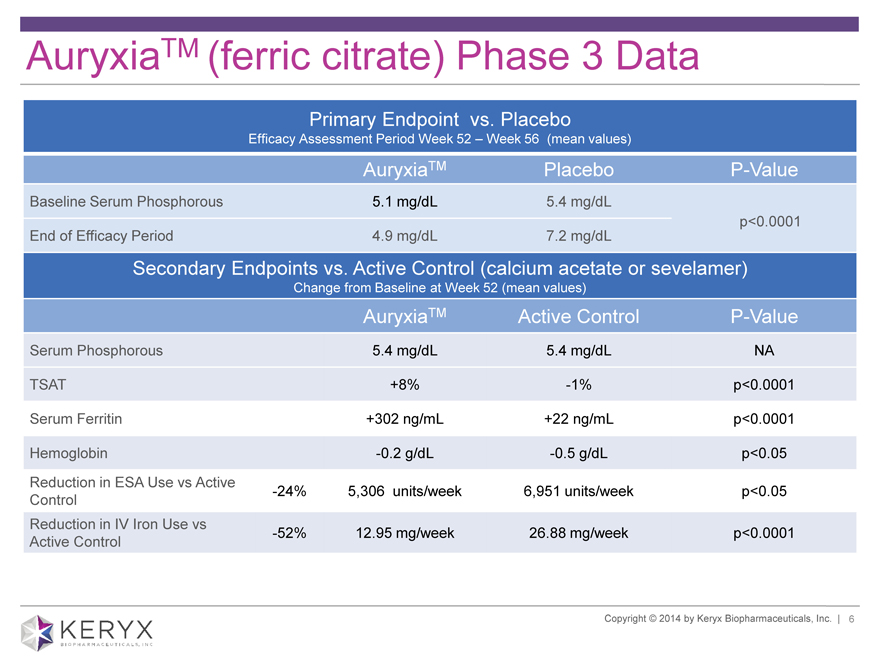
AuryxiaTM (ferric citrate) Phase 3 Data
Primary Endpoint vs. Placebo
Efficacy Assessment Period Week 52 – Week 56 (mean values)
AuryxiaTM Placebo P-Value
Baseline Serum Phosphorous 5.1 mg/dL 5.4 mg/dL
p<0.0001
End of Efficacy Period 4.9 mg/dL 7.2 mg/dL
Secondary Endpoints vs. Active Control (calcium acetate or sevelamer)
Change from Baseline at Week 52 (mean values)
AuryxiaTM Active Control P-Value
Serum Phosphorous 5.4 mg/dL 5.4 mg/dL NA
TSAT +8% -1% p<0.0001
Serum Ferritin +302 ng/mL +22 ng/mL p<0.0001
Hemoglobin -0.2 g/dL -0.5 g/dL p<0.05
Reduction in ESA Use vs Active -24% 5,306 units/week 6,951 units/week p<0.05
Control
Reduction in IV Iron Use vs
Active Control -52% 12.95 mg/week 26.88 mg/week p<0.0001
Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 6
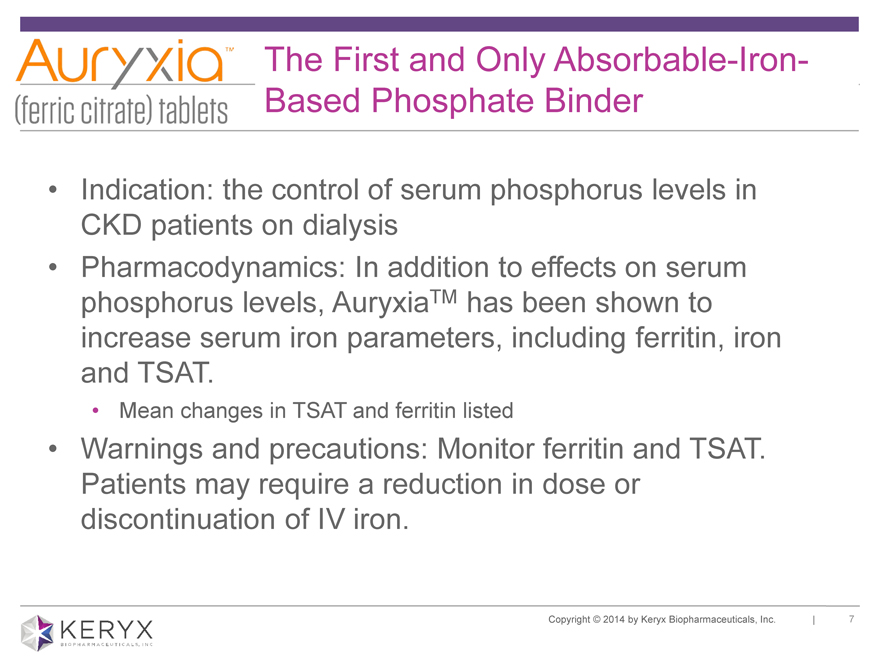
The First and Only Absorbable-Iron-Based Phosphate Binder Indication: the control of serum phosphorus levels in CKD patients on dialysis Pharmacodynamics: In addition to effects on serum phosphorus levels, AuryxiaTM has been shown to increase serum iron parameters, including ferritin, iron and TSAT. Mean changes in TSAT and ferritin listed Warnings and precautions: Monitor ferritin and TSAT. Patients may require a reduction in dose or discontinuation of IV iron. Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 7
|
|
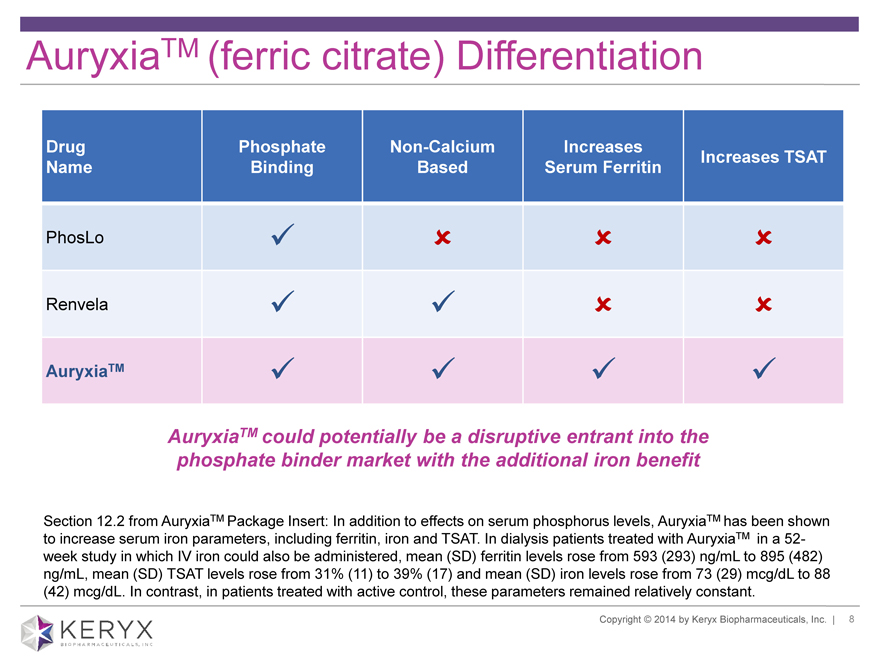
AuryxiaTM (ferric citrate) Differentiation Drug Phosphate Non-Calcium Increases Increases TSAT Name Binding Based Serum Ferritin PhosLo Renvela AuryxiaTM AuryxiaTM could potentially be a disruptive entrant into the phosphate binder market with the additional iron benefit Section 12.2 from AuryxiaTM Package Insert: In addition to effects on serum phosphorus levels, AuryxiaTM has been shown to increase serum iron parameters, including ferritin, iron and TSAT. In dialysis patients treated with AuryxiaTM in a 52-week study in which IV iron could also be administered, mean (SD) ferritin levels rose from 593 (293) ng/mL to 895 (482) ng/mL, mean (SD) TSAT levels rose from 31% (11) to 39% (17) and mean (SD) iron levels rose from 73 (29) mcg/dL to 88 (42) mcg/dL. In contrast, in patients treated with active control, these parameters remained relatively constant. Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 8
|

|
Preparing for Launch NEW YORK | BOSTON | WWW.KERYX.COM Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 9
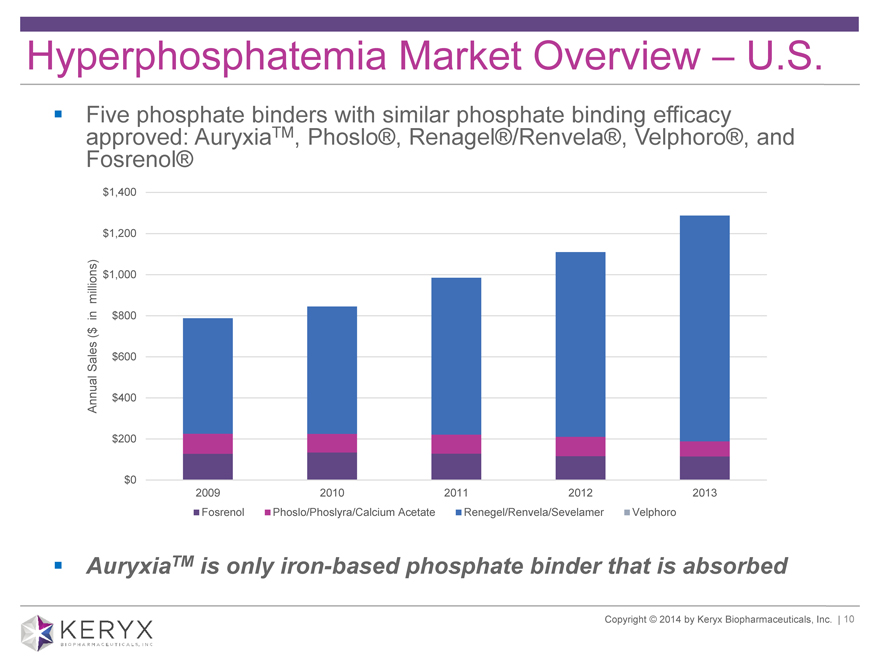
Hyperphosphatemia Market Overview – U.S. ? Five phosphate binders TM with similar phosphate binding efficacy approved: Auryxia , Phoslo®, Renagel®/Renvela®, Velphoro®, and Fosrenol®
$1,400
$1,200
millions) $1,000
in $800
$
(
Sales $600
Annual $400
$200
$0
2009 2010 2011 2012 2013
Fosrenol Phoslo/Phoslyra/Calcium Acetate Renegel/Renvela/Sevelamer Velphoro
? AuryxiaTM is only iron-based phosphate binder that is absorbed Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 10
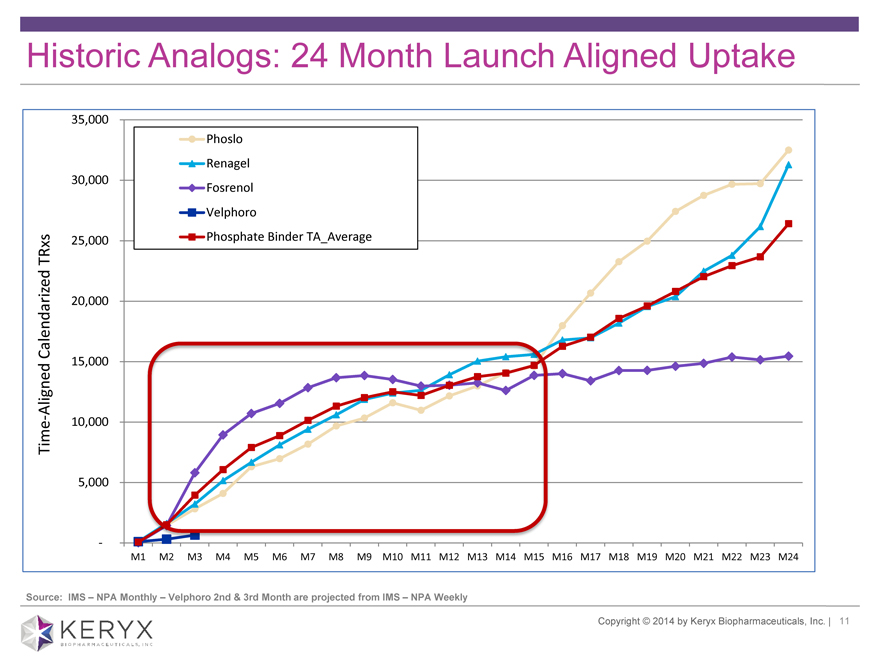
Historic Analogs: 24 Month Launch Aligned Uptake
35,000
Phoslo
Renagel
30,000 Fosrenol
Velphoro
TRxs 25,000 Phosphate Binder TA_Average
Calendarized 20,000
Aligned 15,000
—10,000
Time
5,000
-
M1 M2 M3 M4 M5 M6 M7 M8 M9 M10 M11 M12 M13 M14 M15 M16 M17 M18 M19 M20 M21 M22 M23 M24
Source: IMS NPA Monthly Velphoro 2nd & 3rd Month are projected from IMS ? NPA Weekly Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 11
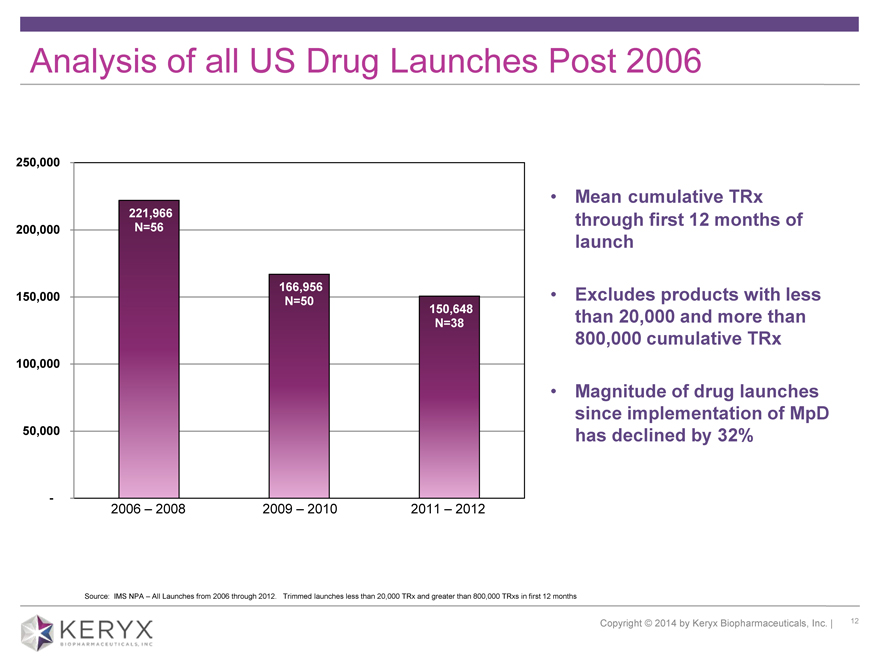
Analysis of all US Drug Launches Post 2006
250,000
•
221,966
200,000 N=56
150,000 166,956 N=50 •
150,648
N=38
100,000
•
50,000
-
2006 – 2008 2009 – 2010 2011 – 2012
Mean cumulative TRx through first 12 months of launch Excludes products with less than 20,000 and more than 800,000 cumulative TRx Magnitude of drug launches since implementation of MpD has declined by 32% Source: IMS NPA All Launches from 2006 through 2012. Trimmed launches less than 20,000 TRx and greater than 800,000 TRxs in first 12 months Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 12
|
|
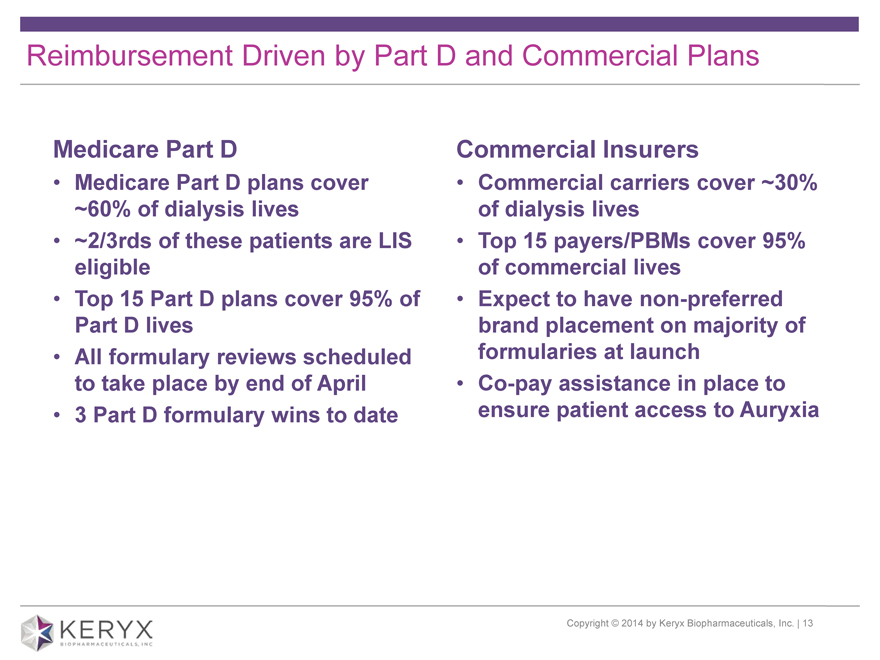
Reimbursement Driven by Part D and Commercial Plans Medicare Part D Commercial Insurers Medicare Part D plans cover Commercial carriers cover ~30% ~60% of dialysis lives of dialysis lives ~2/3rds of these patients are LIS Top 15 payers/PBMs cover 95% eligible of commercial lives Top 15 Part D plans cover 95% of Expect to have non-preferred Part D lives brand placement on majority of All formulary reviews scheduled formularies at launch to take place by end of April Co-pay assistance in place to 3 Part D formulary wins to date ensure patient access to Auryxia Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 13
|
|
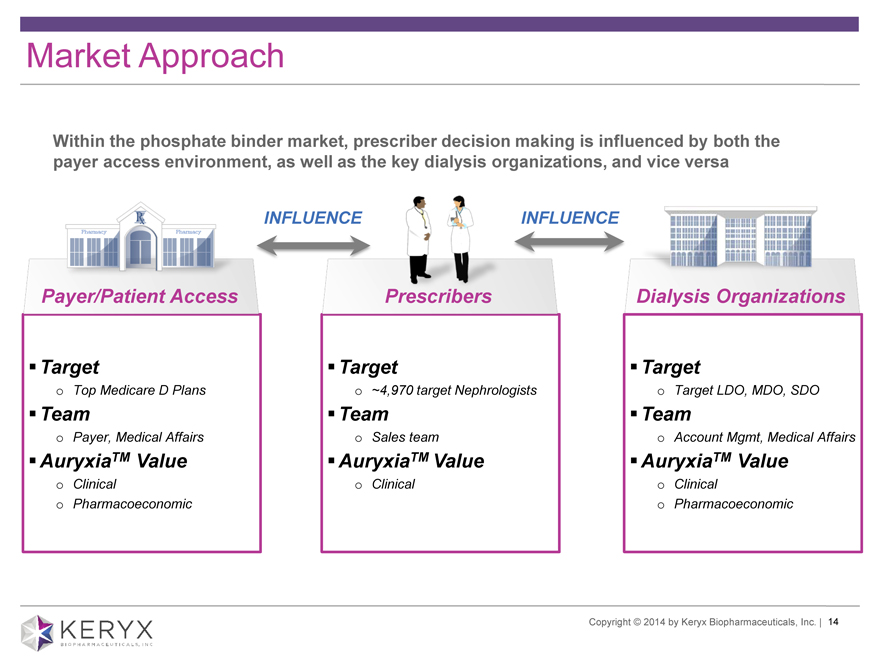
Market Approach Within the phosphate binder market, prescriber decision making is influenced by both the payer access environment, as well as the key dialysis organizations, and vice versa INFLUENCE INFLUENCE Payer/Patient Access Prescribers Dialysis Organizations ?Target?Target?Target o Top Medicare D Plans o ~4,970 target Nephrologists o Target LDO, MDO, SDO ?Team?Team?Team o Payer, Medical Affairs o Sales team o Account Mgmt, Medical Affairs ?AuryxiaTM Value?AuryxiaTM Value?AuryxiaTM Value o Clinical o Clinical o Clinical o Pharmacoeconomic o Pharmacoeconomic Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 14
|
|
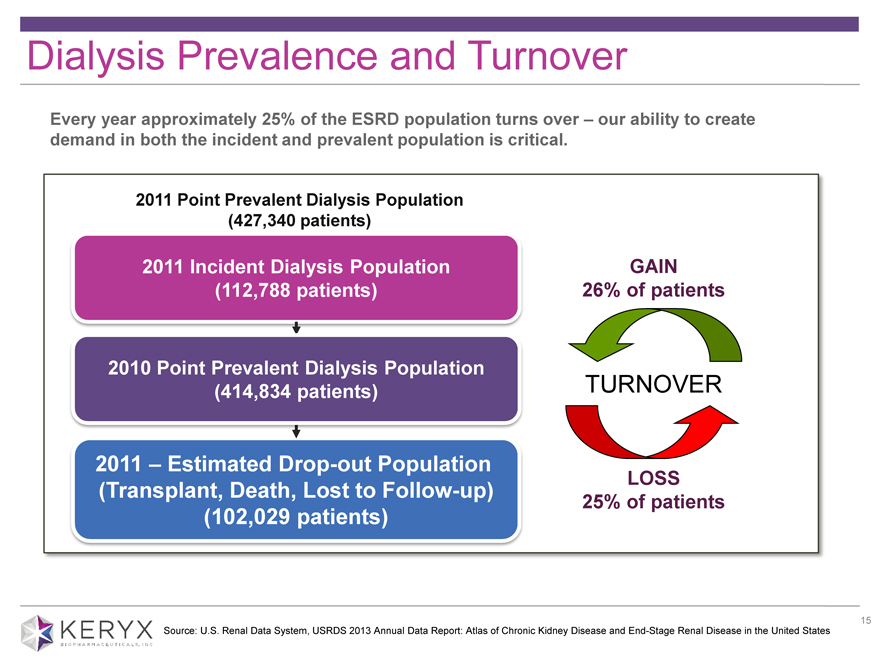
Dialysis Prevalence and Turnover Every year approximately 25% of the ESRD population turns over ? our ability to create demand in both the incident and prevalent population is critical. 2011 Point Prevalent Dialysis Population (427,340 patients) 2011 Incident Dialysis Population (112,788 patients) 2010 Point Prevalent Dialysis Population (414,834 patients) 2011 ? Estimated Drop-out Population (Transplant, Death, Lost to Follow-up) (102,029 patients) GAIN 26% of patients TURNOVER LOSS 25% of patients Source: U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States 15
|
|
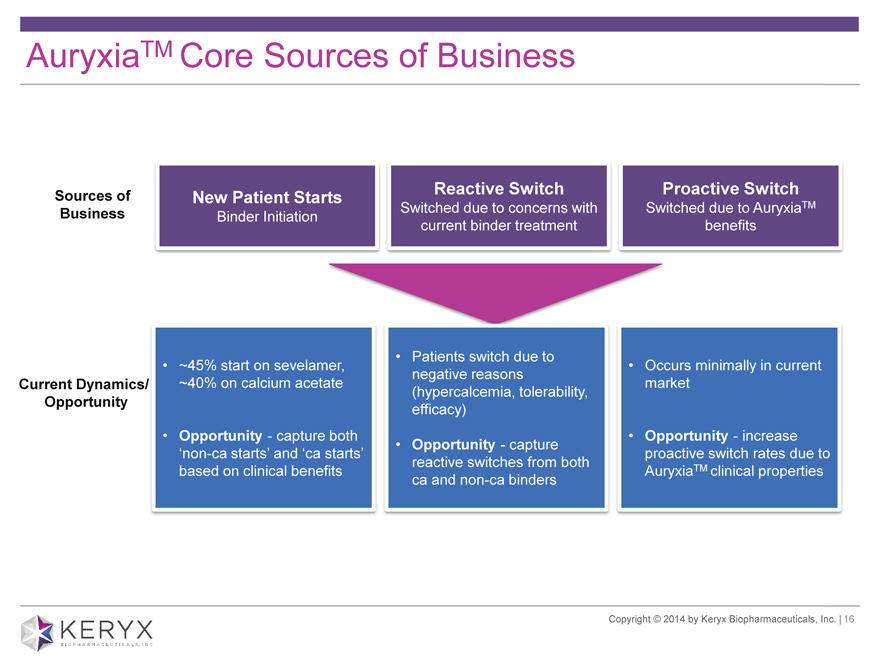
AuryxiaTM Core Sources of Business Sources of Business New Patient Starts Binder Initiation Reactive Switch Switched due to concerns with current binder treatment Proactive Switch Switched due to AuryxiaTM benefits Current Dynamics/ Opportunity ~45% start on sevelamer, ~40% on calcium acetate Opportunity—capture both ?non-ca starts? and ?ca starts? based on clinical benefits Patients switch due to negative reasons (hypercalcemia, tolerability, efficacy) Opportunity—capture reactive switches from both ca and non-ca binders Occurs minimally in current market Opportunity—increase proactive switch rates due to AuryxiaTM clinical properties Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 16
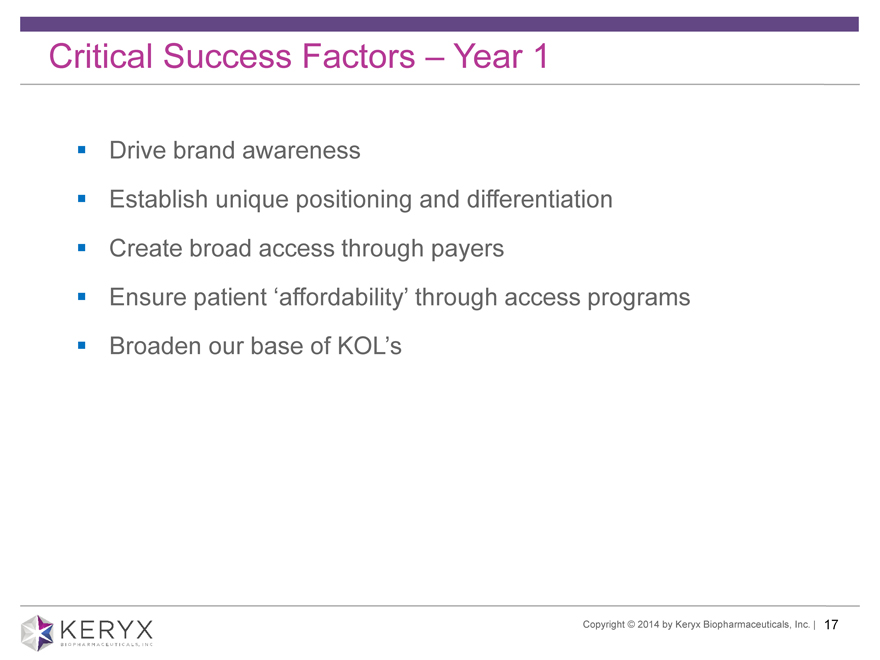
Critical Success Factors Year 1 Drive brand awareness Establish unique positioning and differentiation Create broad access through payers Ensure patient affordability through access programs Broaden our base of KOL’s Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 17
Growth Opportunities NEW YORK | BOSTON | WWW.KERYX.COM Copyright 2014 by Keryx Biopharmaceuticals, Inc. |
Chronic Kidney Disease in the U.S. We believe that the CKD patient community is underserved and are committed to bringing new therapies to market for patients with renal disease. 26 MILLION U.S. ADULTS WITH CKD 450,000 ON DIALYSIS ~80% ON PHOSPHATE BINDERS 16 MILLION WITH STAGE 3 TO 5 NDD-CKD WELL OVER 1.5 MILLION HAVE IRON DEFICIENCY ANEMIA Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; Decision Resources, Biotrends, Chart Trends Bone and Mineral Metabolism in Dialysis 2013 (US); imputed 3% growth per year. Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 19
|
|
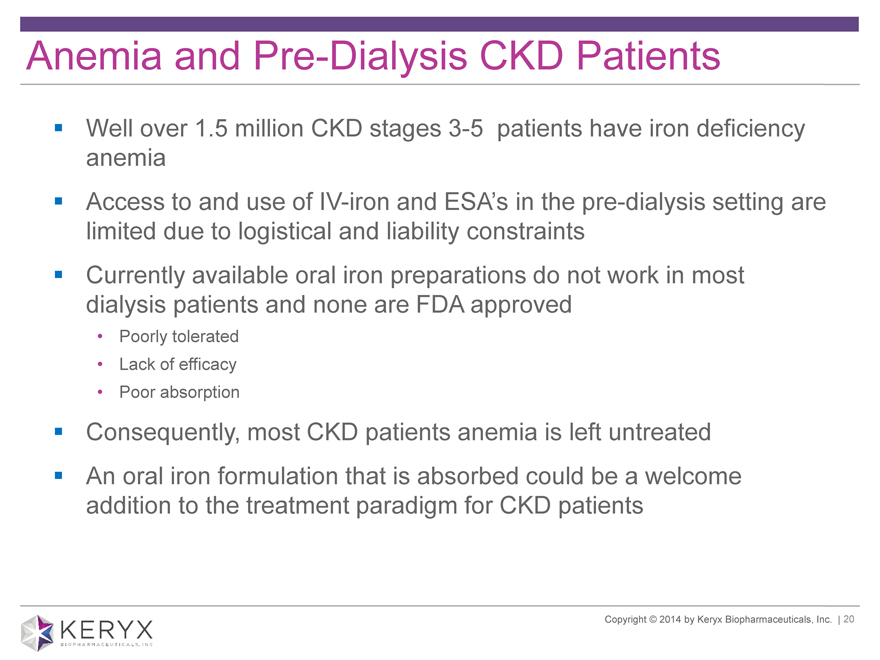
Anemia and Pre-Dialysis CKD Patients Well over 1.5 million CKD stages 3-5 patients have iron deficiency anemia Access to and use of IV-iron and ESA’s in the pre-dialysis setting are limited due to logistical and liability constraints Currently available oral iron preparations do not work in most dialysis patients and none are FDA approved Poorly tolerated Lack of efficacy Poor absorption Consequently, most CKD patients anemia is left untreated An oral iron formulation that is absorbed could be a welcome addition to the treatment paradigm for CKD patients Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 20
|
|
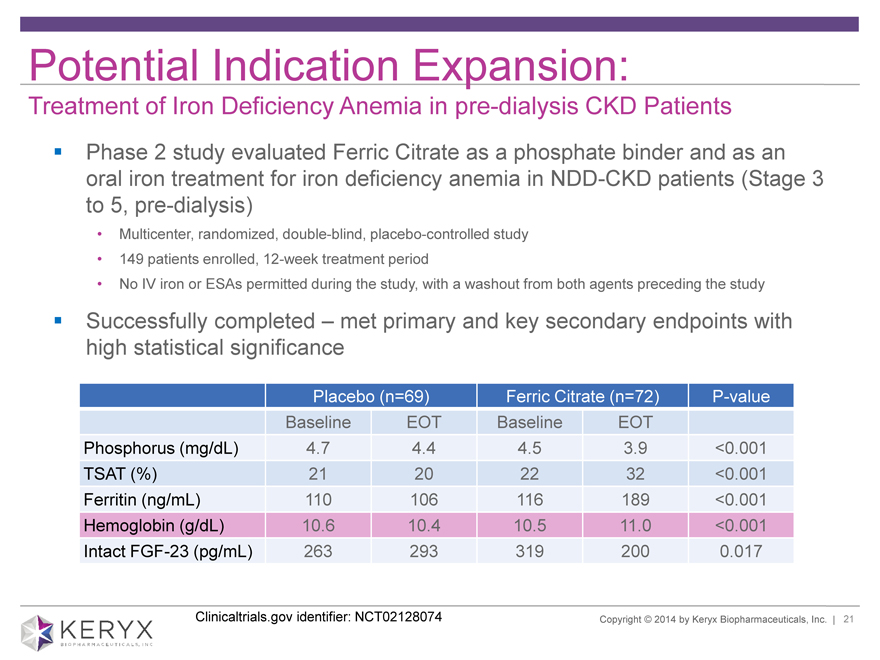
Potential Indication Expansion: Treatment of Iron Deficiency Anemia in pre-dialysis CKD Patients Phase 2 study evaluated Ferric Citrate as a phosphate binder and as an oral iron treatment for iron deficiency anemia in NDD-CKD patients (Stage 3 to 5, pre-dialysis) • Multicenter, randomized, double-blind, placebo-controlled study • 149 patients enrolled, 12-week treatment period • No IV iron or ESAs permitted during the study, with a washout from both agents preceding the study Successfully completed – met primary and key secondary endpoints with high statistical significance
Placebo (n=69) Ferric Citrate (n=72) P-value
Baseline EOT Baseline EOT
Phosphorus (mg/dL) 4.7 4.4 4.5 3.9 <0.001
TSAT (%) 21 20 22 32 <0.001
Ferritin (ng/mL) 110 106 116 189 <0.001
Hemoglobin (g/dL) 10.6 10.4 10.5 11.0 <0.001
Intact FGF-23 (pg/mL) 263 293 319 200 0.017
Clinicaltrials.gov identifier: NCT02128074 Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 21
Iron Deficiency Anemia Phase 3 Trial Study Initiated in September 2014 ~230 stage 3 5 CKD patients with iron deficiency anemia Primary endpoint: % of patient that achieve a 1 g/dL or greater increase in hemoglobin at any point during the 16-week randomization period 2-month safety follow up period, for a total of 24 weeks IND filed with Hematology/Oncology Division of FDA for this trial Clinicaltrials.gov identifier: NCT02268994 Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 22
International Expansion On January 17, 2014, our Japanese partner, JT/Torii, received marketing approval in Japan for Ferric Citrate (Riona) in CKD (dialysis and pre-dialysis) Japanese ESRD phosphate binder market is currently ~$350M and growing EU marketing authorization application is under review; decision expected mid-2015 Determining best path forward in other geographies, including Europe Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 23
FDA Approved: AuryxiaTM (ferric citrate) Approved on September 5, 2014, by the U.S. Food and Drug Administration (FDA) INDICATION: For the control of serum phosphorus levels in patients with chronic kidney disease (CKD) on dialysis First and only absorbed iron-based phosphate binder U.S. launch at YE Additional potential growth areas for Ferric Citrate Label expansion: Iron deficiency anemia in non-dialysis dependent chronic kidney disease Geographical expansion: EU and emerging markets Copyright 2014 by Keryx Biopharmaceuticals, Inc. | 24
|

|
At Keryx, the patient comes first. Our goal is to bring innovative therapies to market that provide unique and meaningful advantages to patients with renal disease and their healthcare providers – because we know that when patient care improves, everybody succeeds. Corporate Presentation NASDAQ: KERX | December 2014 NEW YORK Keryx Biopharmaceuticals, Inc. 750 Lexington Ave., 20th Floor New York, NY 10022 1.212.531.5965 tel 1.212.531.5961 fax www.keryx.com BOSTON Keryx Biopharmaceuticals, Inc. One Marina Drive, Tenth Floor Boston, MA 02210 1. 617.466.3500 tel 1. 617.466.3501 fax Copyright © 2014 by Keryx Biopharmaceuticals, Inc.