exclusive and further information concerning Akebia and Keryx and their respective businesses, including factors that potentially could materially affect their respective businesses, financial condition or operating results, may emerge from time to time. Readers are urged to consider these factors carefully in evaluating these forward-looking statements, and not to place undue reliance on any forward-looking statements. Readers should also carefully review the risk factors described in other documents that Akebia and Keryx file from time to time with the SEC. The forward-looking statements in these materials speak only as of the date of these materials. Except as required by law, Akebia and Keryx assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
Akebia Therapeutics Contact
John Garabo
Director, Corporate Communications
(617)844-6130
jgarabo@akebia.com
Footnotes:
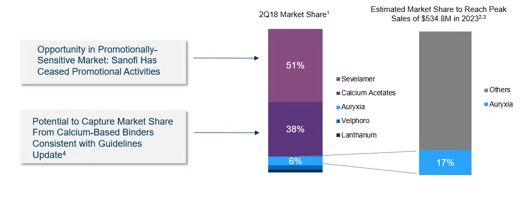
1. Keryx consolidated data based on data received from IMS and specialty pharmacies (Fresenius Rx, DaVita Rx)
2. Definitive Proxy Statement/Prospectus filed by Akebia Therapeutics, Inc. with the U.S. Securities and Exchange Commission on October 30, 2018 (see “The Merger—Certain Akebia Management Unaudited Prospective Financial Information – Akebia Management Keryx Projections”). This estimate of peak sales is unaudited and was based upon Akebia assumptions made in preparation for the June 28, 2018, merger announcement, including upon publicly filed financial information of Keryx, certain financial information provided to Akebia management by Keryx, and certain assumptions made by the Akebia management, including estimates of revenue growth for U.S. sales of Auryxia and associated operational costs, and has not been updated since that time. Furthermore, this estimate of peak sales was not adjusted for a number of critical risks, including the recent changes to reimbursement coverage for Auryxia that could have a material adverse effect on Auryxia sales and profitability. See the Forward-Looking Statements section herein for additional information regarding risks.
3. Akebia management internal estimates based on market research. This Auryxia market share estimate formed the basis of certain information included in the Definitive Proxy Statement/Prospectus filed by Akebia Therapeutics, Inc. with the U.S. Securities and Exchange Commission on October 30, 2018 (see “The Merger—Certain Akebia Management Unaudited Prospective Financial Information – Akebia Management Keryx Projections”) and was based upon Akebia assumptions made in preparation for the June 28, 2018, merger announcement, including upon publicly filed financial information of Keryx, certain financial information provided to Akebia management by Keryx, and certain assumptions made by the Akebia management, including estimates of revenue growth for U.S. sales of Auryxia and associated operational costs, and has not been updated since that time. Furthermore, this market share estimate was not adjusted for a number of critical risks, including the recent changes to reimbursement coverage for Auryxia that could have a material adverse effect on Auryxia sales and profitability. See the Forward-Looking Statements section herein for additional information regarding risks.
4.Prevention and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD_MBD); Vol. 7, Issue 1. July 2017
5. Reason Research Q3 Auryxia ATU
6. Spherix Global Anemia 1Q Pulse (2018); aided awareness data
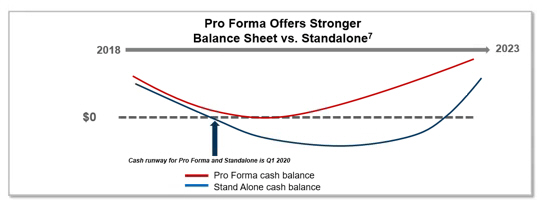
7. Definitive Proxy Statement/Prospectus filed by Akebia Therapeutics, Inc. with the U.S. Securities and Exchange Commission on October 30, 2018 (see “The Merger—Certain Akebia Management Unaudited Prospective Financial Information”). These cash balance estimates are unaudited and were based upon Akebia assumptions made in preparation for the June 28, 2018, merger announcement, including assumptions related to timing for clinical trial completion and commercial launch, estimated operational costs, including R&D, manufacturing and general and administrative costs, and estimates of revenue growth for U.S. sales of Auryxia, and have not been updated since that time. Furthermore, these cash balance estimates are not adjusted for a number of critical risks, including the risks and probability of success of vadadustat, delays of any clinical trials or commercial launch, the financial implications of Akebia’s collaborations and other relationships with third parties, and the recent changes to reimbursement coverage for Auryxia that could have a material adverse effect on Auryxia sales and profitability. See the Forward-Looking Statements section herein for additional information regarding risks.
###