Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT
TO SECTIONS 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2006
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 001-16537
ORASURE TECHNOLOGIES, INC.
(Exact Name of Registrant as Specified in Its Charter)
| Delaware | 36-4370966 | |
(State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification No.) | |
220 East First Street Bethlehem, Pennsylvania | 18015 | |
| (Address of Principal Executive Offices) | (Zip Code) | |
(610) 882-1820
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class | Name of Each Exchange on Which Registered | |
| Common Stock $0.000001 par value per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ Nox
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes¨ Nox
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yesx No¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K.x
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act.
Large accelerated filer¨ | Accelerated filerx | Non-accelerated filer¨ |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ Nox
State the aggregate market value of the voting and non-voting common equity held by nonaffiliates, computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the Registrant’s most recently completed second fiscal quarter (June 30, 2006): $434,917,582
Indicate the number of shares outstanding of each of the Registrant’s classes of common stock, as of March 12, 2007: 46,127,212 shares.
Documents Incorporated by Reference:
Portions of the Registrant’s Definitive Proxy Statement for the 2007 Annual Meeting of Stockholders are incorporated by reference into Part III of this Report.
Table of Contents
| Page | ||||
| PART I | ||||
| ITEM 1. | 1 | |||
| ITEM 1A. | 19 | |||
| ITEM 1B. | 33 | |||
| ITEM 2. | 33 | |||
| ITEM 3. | 33 | |||
| ITEM 4. | 34 | |||
| PART II | ||||
| ITEM 5. | 35 | |||
| ITEM 6. | 37 | |||
| ITEM 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 38 | ||
| ITEM 7A. | 55 | |||
| ITEM 8. | 55 | |||
| ITEM 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 56 | ||
| ITEM 9A. | 56 | |||
| ITEM 9B. | 57 | |||
| PART III | ||||
| ITEM 10. | 58 | |||
| ITEM 11. | 58 | |||
| ITEM 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 58 | ||
| ITEM 13. | Certain Relationships and Related Transactions, and Director Independence | 58 | ||
| ITEM 14. | 58 | |||
| PART IV | ||||
| ITEM 15. | 59 | |||
Table of Contents
This Report contains certain “forward-looking statements,” within the meaning of the Federal securities laws. These may include statements about our expected revenues, earnings, expenses or other financial performance, future product performance or development, expected regulatory filings and approvals, planned business transactions, views of future industry, competitive or market conditions, and other factors that could affect our future operations, results of operations or financial position. These statements often include words, such as “believes,” “expects,” “anticipates,” “intends,” “plans,” “estimates,” “may,” “will,” “should,” “could,” or similar expressions.
Forward-looking statements are not guarantees of future performance or results. Known and unknown factors could cause actual performance or results to be materially different from those expressed or implied in these statements. Factors that could affect our results are discussed more fully under Item 1A., entitled “Risk Factors,” and elsewhere in this Annual Report. Although forward-looking statements help to provide complete information about us, readers should keep in mind that forward-looking statements may not be reliable. Readers are cautioned not to place undue reliance on the forward-looking statements. The forward-looking statements are made as of the date of this Report and we undertake no duty to update these statements.
Our business principally involves the development, manufacture, marketing and sale of oral fluid specimen collection devices using our proprietary oral fluid technologies, as well as other diagnostic products including immunoassays and otherin vitro diagnostic tests that are used on other specimen types, and other medical devices. Our diagnostic products include tests which are processed in a laboratory and tests which are performed on a rapid basis at the point of care. These products are sold in the United States and internationally to various clinical laboratories, hospitals, clinics, community-based organizations and other public health organizations, distributors, government agencies, physicians’ offices, and commercial and industrial entities. One of our products is also sold in the over-the-counter (“OTC”) or consumer retail market in the United States, Canada, Europe, Australia and certain other foreign countries.
In vitro diagnostic testing is the process of analyzing oral fluid, blood, urine and other bodily fluids or tissue for the presence of specific substances or markers for infectious diseases, drugs of abuse or other conditions.In vitro diagnostic tests are performed outside the body, in contrast toin vivo tests, which are performed directly on or within the body. The substance or marker that a diagnostic test is intended to detect is generally referred to as an analyte.
Immunodiagnostic testing is the leading method ofin vitro testing for antigens and antibodies. When an infectious disease is caused by pathogens, such as bacteria, viruses and fungi, or other substances are present, the body responds by producing an antibody. Substances that stimulate production of antibodies are generally referred to as antigens. An antibody binds specifically with an antigen in a lock-and-key fashion that initiates a biochemical reaction to attempt to neutralize and, ultimately, eliminate the antigen. The ability of an antibody to bind with a specific antigen provides the basis for immunodiagnostic testing.
Our Company was formed in May 2000 under Delaware law solely for the purposes of combining two companies, STC Technologies, Inc. (“STC” or “STC Technologies”) and Epitope, Inc. (“Epitope”), and changing the state of incorporation of Epitope from Oregon to Delaware. STC Technologies and Epitope were merged into our Company on September 29, 2000. Our principal offices are located at 220 East First Street, Bethlehem, Pennsylvania 18015, and our telephone number is (610) 882-1820.
Additional information about us can be found on our website. Our website address is www.orasure.com. We make available free of charge through a link provided at such website our Annual Reports on Form 10-K, our
1
Table of Contents
Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K, as well as any amendments to those Reports. These Reports are made available as soon as reasonably practicable after they are filed or furnished to the Securities and Exchange Commission. Our Internet website and the information contained in or connected to that website are not intended to be incorporated by reference into this Annual Report.
Products
The following is a summary of our principal products and their regulatory and commercial status:
Product | Description | Regulatory Status | Commercial Status | |||
OraQuick ADVANCE® HIV-1/2 | A rapid, point-of-care test for antibodies to the Human Immunodeficiency Virus Type 1 (“HIV-1”) and Type 2 (“HIV-2” and together with HIV-1, “HIV-1/2”) that can be visually read at the point of care in approximately 20 minutes. | Premarket approval (“PMA”) approved by the U.S. Food and Drug Administration (“FDA”) (March 2004—June 2004) for use with oral fluid, finger-stick and venous whole blood, and plasma. CLIA (Clinical Laboratory Improvement Amendments of 1988) waived for use with oral fluid, finger-stick and venous whole blood (June 2004). | Marketed | |||
| CE mark application filed. | Pending | |||||
| Registered in Mexico. | Marketed | |||||
OraQuickADVANCE® OTC | In development. | |||||
OraQuick® HCV | A rapid, point-of-care test for antibodies to the hepatitis C virus (“HCV”) | In development. | ||||
OraSure® | Oral fluid collection device for the detection of antibodies to HIV-1 in an oral fluid sample in a laboratory setting. | PMA approved by FDA in December 1994. | Marketed | |||
| Also FDA 510(k) cleared for use in detecting cocaine and cotinine (an indicator of nicotine) in oral fluid. | Marketed | |||||
| CE marked and registered in the United Kingdom. Also registered in Mexico, Canada, Columbia, South Africa, Afghanistan, Argentina, Brazil and Trinidad. | Marketed | |||||
Intercept® | Oral fluid collection device, along with nine related immunoassays, for oral fluid drugs of abuse (“DOA”) testing in a laboratory setting. | Collection device—FDA 510(k) cleared in 2000. | Marketed | |||
MICRO-PLATE DOA Assays | Used to detect the following drugs in an oral fluid sample: marijuana, cocaine, opiates, amphetamines, methamphetamines, PCP, benzodiazepines, barbiturates and methadone. | Nine drug assays—FDA 510(k) cleared during 2000-2001. | Marketed | |||
| Intercept® device CE marked and registered in the United Kingdom. Various assays are CE marked and registered in the United Kingdom, Austria, Belgium, Denmark, Finland, France, Germany, Ireland, Italy, Luxembourg, Mexico, Netherlands, Portugal, Spain, Sweden, Korea, Canada, Afghanistan and Brazil. | Marketed | |||||
2
Table of Contents
Product | Description | Regulatory Status | Commercial Status | |||
Homogeneous DOA Assays | Homogeneous fully-automated oral fluid DOA assays. | In development. | ||||
Cryosurgical Systems—Professional | Cryosurgical system for the removal of warts and other benign skin lesions, marketed under the Histofreezer® tradename primarily to the physicians’ office market. | Nine indications—FDA 510(k) cleared during 1991—1999. | Marketed | |||
| CE marked and registered in Europe, Venezuela, Thailand, New Zealand, Hong Kong, Brazil, Mexico, Canada and Afghanistan. | Marketed | |||||
Cryosurgical Systems—OTC | Cryosurgical (freezing) system for the removal of common and plantar warts, sold in the OTC markets in the United States and Canada under the Compound W Freeze Off® tradename by Prestige Brands Holdings, Inc., in Europe, Australia and New Zealand under the Scholl and Dr. Scholl Freeze Spray tradenames by SSL International plc. and in Mexico under the POINTTS tradename by Genomma Labs. | FDA 510(k) cleared for two Freeze Off® indications in February 2003.
Freeze Off® registered in Canada.
Scholl Freeze Spray CE marked and registered in several European countries.
POINTTS registered in Mexico. | Marketed
Marketed
Marketed
Marketed | |||
Cryosurgical Systems—OTC Product Line Extensions | Cryosurgical system for an indication other than common warts or plantar warts. | In development. | ||||
| Cryosurgical system combined with salicylic acid. | In development. |
In addition to the above products, we also sell certain immunoassay tests and reagents for insurance risk assessment, substance abuse testing and forensic toxicology applications; an oral fluid Western blot HIV-1 confirmatory test approved by the FDA for confirming positive HIV-1 test results obtained from the use of our OraSure® collection device; and the FDA 510(k) cleared Q.E.D.® point-of-care saliva alcohol test.
OraQuick® Rapid Test Platform
OraQuick® is our rapid test platform designed to test oral fluid, whole blood (i.e., both finger-stick and venous) and plasma samples for the presence of various antibodies or analytes. The device uses a porous flat pad to collect an oral fluid specimen. After collection, the pad is inserted into a vial containing a pre-measured amount of developer solution and allowed to develop. When whole blood or plasma is to be tested, a loop collection device is used to collect a drop of blood or plasma and mix it in the developer solution, after which the collection pad is inserted into the solution and allowed to develop. In all cases, the specimen and developer solution then flow through the testing device where test results are observable in approximately 20 minutes. The OraQuick® device is a screening test and requires a confirmation test where an initial positive result is obtained.
We have commercialized this technology in the form of our OraQuickADVANCE® rapid HIV-1/2 antibody test. This is a rapid, point-of-care test which has received FDA approval for the detection of antibodies to both HIV-1 and HIV-2 in oral fluid, finger-stick and venous whole blood and plasma. This test is available for use by the nearly 40,000 locations in the United States certified under the Clinical Laboratory Improvements Amendment of 1988 (“CLIA”) to perform moderately complex tests. We have also received a CLIA waiver for use of the OraQuickADVANCE® test with oral fluid and finger-stick and venous whole blood. As a result, the test can be used by approximately 140,000 additional sites in the United States not certified under CLIA to perform moderately complex tests, such as outreach clinics, community-based organizations and physicians’ offices.
3
Table of Contents
On the international front, we are in the process of obtaining a CE mark for our OraQuickADVANCE® test so that we will be able to sell this product in Europe. We have a distributor in place for the United Kingdom and are pursuing distribution arrangements in several additional European countries. We are selling OraQuick® in Mexico and Africa and are completing registrations of our OraQuick® test in several countries in Latin America, Asia, the Middle East and Russia. We are aggressively seeking to expand our distribution network for this product throughout the world.
We believe that the OraQuickADVANCE® device, because it is approved for detecting antibodies to both HIV-1 and HIV-2 in finger-stick and venous whole blood, oral fluid and plasma samples, provides a significant competitive advantage in the market for rapid HIV testing in the United States and elsewhere around the world. Demand for OraQuickADVANCE® has quickly grown since the launch of that product in late 2004.
OraSure®/Intercept® Collection Devices
Our OraSure® oral fluid collection device is used in conjunction with screening and confirmatory tests for HIV-1 antibodies and other analytes. This device consists of a small, treated cotton-fiber pad on a handle that is placed in a person’s mouth for two to five minutes. The device collects oral mucosal transudate (“OMT”), a serum-derived fluid that contains higher concentrations of certain antibodies and analytes than saliva. As a result, OMT testing is a highly accurate method for detecting HIV-1 infection and other analytes.
We believe that oral fluid testing has several significant advantages over blood or urine-based systems for infectious disease testing, for both health care professionals and the individuals being tested. These advantages include eliminating the risk of needle-stick accidents, providing a non-invasive collection technique, requiring minimal training to administer, providing rapid and efficient collection in almost any setting, and reducing the cost of administration by a trained health care professional.
We have received premarket approval from the FDA to sell the OraSure® collection device for use with a laboratory-based enzyme immunoassay (“EIA”) screening test for HIV-1 antibody detection. This EIA screening test has been approved by the FDA for use with our OraSure® device and is manufactured and sold by bioMerieux, Inc. (“BMX”).
HIV-1 antibody detection using the OraSure® collection device involves three steps:
| • | Collection of an oral fluid specimen using the OraSure® device; |
| • | Screening of the specimen for HIV-1 antibodies at a laboratory with an EIA screening test approved by the FDA for use with the OraSure® device; and |
| • | Laboratory confirmation of any positive screening test results with our oral fluid Western blot HIV-1 confirmatory test (described below). |
A trained health care professional then conveys test results and provides appropriate counseling to the individual who was tested. We have also received FDA 510(k) clearance for use of the OraSure® collection device with EIAs to test for cocaine and cotinine (a metabolite of nicotine) in oral fluid specimens primarily for insurance risk assessment purposes.
In late 2006, BMX announced that it will discontinue manufacturing the HIV-1 EIA screening test during 2007 and that, due to quality problems, it may have difficulty supplying this screening test prior to the time it ceases manufacturing. As a result, we are working with BMX, the FDA and Centers for Disease Control and Prevention (“CDC”), our major laboratory customers and other potential suppliers to find or develop an alternative HIV-1 EIA screening test that can be used with oral fluid samples collected with our OraSure® device.
A collection device that is substantially similar to the OraSure® device is sold under the name Intercept®, and is used to collect OMT for oral fluid drug testing. We have received FDA 510(k) clearance to use the
4
Table of Contents
Intercept® collection device with laboratory-based EIAs to test for drugs of abuse commonly identified by the National Institute for Drug Abuse (“NIDA”) as the NIDA-5 (i.e., cannabinoids (marijuana), cocaine, opiates, amphetamines/methamphetamines and phencyclidine (“PCP”)), and for barbiturates, methadone and benzodiazepines. Each of these EIAs is also FDA 510(k) cleared for use with the Intercept® device.
We have received a CE mark for the Intercept® and OraSure® devices and both are distributed in Canada, the United Kingdom and Mexico. The OraSure® device and our oral fluid drugs of abuse assays are also sold in several other foreign countries.
We believe that the Intercept® device has several advantages over competing urine and other drugs-of-abuse testing products, including its lower total testing cost, its non-invasive nature, mobility and accuracy, the ease of maintaining a chain-of-custody, the treatment of test subjects with greater dignity, no requirement for specially-prepared collection facilities and difficulty of sample adulteration. The availability of an oral fluid test is intended to allow our customers to test for drug impairment, eliminate scheduling costs and inconvenience, and thereby streamline the testing process.
Cryosurgical Systems (Skin Lesion Removal Products)
The Histofreezer® cryosurgical removal system is a low-cost alternative to liquid nitrogen and other methods for removal of warts and other benign skin lesions by physicians. The Histofreezer® product mixes two environmentally friendly cryogenic gases in a small aerosol canister. When released, these gases are delivered to a specially designed foam bud, cooling the bud to a maximum of –50°C to –55°C. The frozen bud is then applied to the wart or lesion for 15 to 40 seconds (depending on the type of lesion) creating localized destruction of the target area by freezing. We have received 510(k) clearance for use of the Histofreezer® product to remove common warts and eight other types of benign skin lesions, and this product has been CE marked and registered for distribution throughout Europe.
We have also received FDA 510(k) clearance to market and sell a cryosurgical product similar to the Histofreezer® product in the OTC or retail market for the removal of common and plantar warts only. This product is being distributed in the United States and Canadian OTC markets under the name Freeze Off® by Prestige Brands Holdings, Inc. (“Prestige”), the owner of the Compound W® line of wart removal products. Prestige is the owner of both the Freeze Off® and Compound W® tradenames. In September 2006, Prestige announced that it had acquired the Wartner® cryosurgical wart removal product line, which competes with the Freeze Off® product in the U.S. and Canadian OTC markets. Because we believe that the Wartner acquisition constitutes a breach of our agreement with Prestige, we have initiated an arbitration proceeding against Prestige. As a result, it is uncertain whether Prestige will continue to distribute the Freeze Off® product after 2007. For a more detailed description of our dispute with Prestige, see Item 3, “Legal Proceedings,” in this Annual Report.
Internationally, we distribute a similar CE marked cryosurgical wart removal product into the OTC footcare market in Europe, Australia and New Zealand through our distributor, SSL International plc (“SSL”), under the Scholl and Dr. Scholl trademarks. SSL is the owner of the Scholl and Dr. Scholl trademarks in countries outside North and South America. We have also launched an OTC cryosurgical product in Mexico through our distributor Genomma Labs, under the POINTTS tradename.
Immunoassay Tests and Reagents
We develop and sell immunoassay tests in two formats, known as MICRO-PLATE and AUTO-LYTE®, to meet the specific needs of our customers.
In a MICRO-PLATE kit, the sample to be tested is placed into a small plastic receptacle, called a microwell, along with the reagents. The result of the test is determined by the color of the microwell upon completion of the reaction. Controlling the reaction involves the use of a variety of reagents by laboratory personnel. Test results
5
Table of Contents
are analyzed by any of a variety of commercially available laboratory instruments, which we may also provide to our laboratory customers. MICRO-PLATE tests can be performed on commonly used instruments and can detect drugs in urine, serum and sweat specimens. MICRO-PLATE tests are also used as part of the Intercept® product line to detect drugs of abuse in oral fluid specimens.
AUTO-LYTE® tests are sold in the form of bottles of liquid reagents. These reagents are run on commercially available laboratory-based automated analytical instruments, which are manufactured by a variety of third parties. AUTO-LYTE® is typically used in high volume, automated, commercial reference insurance laboratories to detect certain drugs or chemicals in urine. Test results are produced quickly, allowing for high throughput. In recent years, sales of our AUTO-LYTE® tests have been substantially reduced largely because of competition from cheaper “home-brew” tests used by our laboratory customers. As a result, we eventually expect to stop selling our AUTO-LYTE® tests.
Western blot HIV-1 Confirmatory Test
We sell an oral fluid Western blot HIV-1 confirmatory test that received premarket approval from the FDA in 1996. This test uses the original specimen collected with the OraSure® oral fluid collection device to confirm positive results of initial oral fluid HIV-1 EIA screening tests. The oral fluid Western blot HIV-1 confirmatory test is currently marketed under an exclusive arrangement with BMX.
In March 2007, BMX notified us that it will not renew the agreement under which it supplies the HIV-1 antigen used to manufacture our oral fluid Western blot HIV-1 confirmatory test or the agreement under which it distributes that product on an exclusive, world-wide basis. As a result, these agreements will terminate on December 31, 2007. Pursuant to the terms of the antigen supply agreement, we have the right to purchase an additional two-year supply of the antigen following termination so that we can continue to manufacture and sell our oral fluid Western blot test. When this additional two-year supply is combined with our existing inventory of the HIV-1 antigen, we believe we will have a sufficient supply of HIV-1 antigen to meet the demand for our Western blot test for three to four years after the agreement terminates. We also intend to pursue a long-term supply agreement directly with the vendor (a former affiliate of BMX) used by BMX to manufacture the HIV-1 antigen. During 2006, sales of our oral fluid Western blot HIV-1 confirmatory test totaled approximately $330,000.
Q.E.D.® Saliva Alcohol Test
Our Q.E.D.® saliva alcohol test is a point-of-care test device that is a cost-effective alternative to breath or blood alcohol testing. The test is a quantitative, saliva-based method for the detection of ethanol, has been cleared for sale by the FDA and has received a CLIA waiver. The U.S. Department of Transportation (“DOT”) has also approved the test for purchase.
Each Q.E.D.® test kit contains a collection stick that is used to collect a sample of saliva and a disposable detection device that displays results in a format similar to a thermometer. The Q.E.D.® device is easy to operate and instrumentation is not required to read the result. The product has a testing range of 0 to 0.145% blood alcohol and produces results in approximately two minutes.
Products Under Development
OraQuick® Platform
We believe that OraQuick® has significant potential as a point-of-care testing platform for clinics and other public health entities, hospitals, physicians’ offices and other markets. Because the OraQuick® platform is simple to use and can operate in a non-invasive manner with oral fluid, we believe it will be suitable for use by consumers without the assistance of a doctor or other medical professional. We also believe that OraQuick® provides a platform technology that can be modified for detection of a variety of infectious diseases in addition to HIV, such as viral hepatitis and certain sexually transmitted diseases.
6
Table of Contents
We are currently devoting significant resources to obtaining FDA approval to sell our OraQuickADVANCE® HIV-1/2 test in the United States OTC market. We have completed several laboratory-based operational studies and have initiated and will continue to perform additional clinical studies, including label comprehension studies, in support of our application for FDA approval. We are also developing a counseling and referral system and product packaging and labeling suitable for the OTC market, all of which will be key components of our clinical studies. We expect this clinical work to continue during 2007 and early 2008, after which we intend to submit an application for FDA approval.
During 2005, we obtained a license from Ortho-Clinical Diagnostics and Chiron Corporation to patents relating to the Hepatitis C virus, or HCV, and we have made substantial progress in developing a rapid HCV test using the OraQuick® platform. In addition, in late 2006 we entered into an agreement with Schering-Plough Corporation (“Schering-Plough”) to collaborate on the development and promotion of our OraQuick® HCV test for use with oral fluid. Under the terms of our agreement, we will be reimbursed by Schering-Plough for a portion of our costs to develop the test, and Schering-Plough will provide detailing and other promotional support for the test in the U.S. physicians’ office market, once the test is approved by the FDA. We are also in negotiations to obtain rights to a rapid HCV test manufactured by a third party that we intend to distribute into international markets.
OraSure®/Intercept® Applications
Oral mucosal transudate, or OMT, contains many constituents found in blood and serum, although in lower concentrations. We believe the OraSure® and Intercept® devices are a platform technology with a wide variety of potential applications, where laboratory testing is available. For example, the OraSure® device may be useful for the collection of a variety of antibodies or markers for infectious diseases or conditions in addition to HIV-1, such as antibodies to viral hepatitis.
In 2004, the Substance Abuse and Mental Health Services Administration (“SAMHSA”) issued proposed regulations for oral fluid drug testing for federal workers. When issued in final form, these regulations may require certain modifications to our Intercept® product in order to permit its use by federal workers. As a result, we are developing modifications to the Intercept® collection device that we anticipate will be required by these regulations or are otherwise likely to be desired by our customers.
We are also currently developing additional drugs of abuse assays for use with our Intercept® collection device. In October 2006, we signed a letter of intent with Roche Diagnostics to negotiate a joint development and commercialization agreement for homogeneous fully-automated oral fluid drugs of abuse assays that can be run on random access chemistry analyzers. The oral fluid assays will be developed for use with our Intercept® collection device and Roche’s KIMS (kinetic information of microparticles in solution) technology. The assays will be designed to run on various automated analyzers and to allow oral fluid samples to be processed with the same efficiency currently achieved with urine-based drug tests. The parties are in the process of negotiating a definitive joint development and commercialization agreement.
In light of BMX’s announced decision to cease production of the HIV-1 EIA screening assay used with our OraSure® device, we are working with BMX, the FDA and CDC, our major laboratory customers and other potential suppliers to find or develop an alternative HIV-1 EIA screening test that can be used with our OraSure® device.
OTC Cryosurgical Systems Products
We currently sell our Histofreezer® cryosurgical systems product in the physicians’ office or professional market. This product has been approved by the FDA for the treatment of a total of nine different types of benign skin lesions. Our OTC cryosurgical product has been approved by the FDA for two types of skin lesions—common warts and plantar warts.
7
Table of Contents
We believe that one or more of the seven remaining Histofreezer® indications may be attractive to the OTC market. We are developing an OTC cryosurgical product for one of these indications, and we intend to seek FDA 510(k) clearance of that product during 2007. In addition, we are developing an extension of our existing OTC cryosurgical wart removal product in order to sell that product in combination with salicylic acid for the treatment of common and plantar warts. We also intend to seek FDA clearance of this product extension in 2007.
Business Strategy
We have adopted a multi-part growth strategy, pursuant to which we intend to leverage our extensive diagnostic experience in order to maximize the available opportunities from our existing products and technologies, and supplement our existing product pipeline by accessing other technologies and products. We intend to follow a disciplined approach to maximize the value of our business for the benefit of our stockholders.
Our overall vision is to become a recognized global leader focused on providing innovative diagnostic solutions that add substantial value to existing and emerging healthcare needs. In order to achieve this vision, our business strategy includes the following key elements:
| • | Extension of Base Businesses. We intend to maximize the sales potential of our existing product lines and technologies in the markets where they are currently sold, with a focus on expanding, where possible, the number of our oral fluid product offerings. Under this part of the strategy, we intend to fully capitalize on the potential market reach of our OraQuick®, OraSure®, Intercept®, Histofreezer® and Freeze Off® products by investing in our sales and marketing efforts where appropriate, making product improvements and enhancements, and optimizing our distribution channels. We also intend to expand the reach of our existing products and technology platforms into new markets and will focus specifically on expanding into international markets. |
| • | Infectious Disease Testing. We will pursue new products and technology platforms in the infectious disease, point-of-care testing business to supplement our existing product pipeline. This may include either the development of new infectious disease products or the acquisition of new technologies or products. One new product we are pursuing is the development of a rapid HCV test on our OraQuick® platform. |
| • | OTC Opportunities. We intend to identify or develop products that can be sold in the OTC or retail marketplace. A significant opportunity that we are pursuing under this part of our strategy is to seek FDA approval to sell our OraQuickADVANCE® rapid HIV-1/2 antibody test in the United States OTC market. We are also working to expand the distribution of our OTC cryosurgical product internationally beyond Europe, Australia, New Zealand and Mexico where the product is currently distributed. |
| • | Operational Improvements. We intend to remain focused on the continuous improvement of our operations. These improvements will include, but not be limited to, expanding the use of automated manufacturing for our product lines as demand increases, expanding the global sourcing of components and assemblies to achieve efficiencies and cost improvements, making infrastructure and information technology investments as needed to improve effectiveness and productivity, and modifying our processes in order to continuously improve quality and the effectiveness of our operations. |
Research and Development
In 2006, our research and development activities focused primarily on the development of a rapid HCV test using our OraQuick® technology platform, clinical and regulatory activities related to obtaining a CE mark for the OraQuickADVANCE® test, preliminary work to obtain FDA approval for use of OraQuickADVANCE® in the United States OTC market, and development of certain improvements to existing products in both the Intercept® and cryosurgical wart removal product lines.
From time to time, we supplement our own research and development activities by funding external research at universities and certain other entities. We may continue to fund external research.
8
Table of Contents
Research and development expenses totaled $8.6 million in 2006, $5.3 million in 2005 and $6.1 million in 2004. These expenses include the costs associated with research and development, regulatory affairs, clinical trials and product support.
Sales and Marketing
We attempt to reach our major target markets through a combination of direct sales, strategic partnerships, and independent distributors. Our marketing strategy is to raise awareness through a full array of marketing activities, which include trade shows, print advertising, special programs and distributor promotions, to support sales in each target market.
We market our products in the United States and internationally. Revenues attributable to customers in the United States were $56.8 million, $59.9 million and $47.8 million in 2006, 2005 and 2004, respectively. Revenues attributable to international customers amounted to $11.4 million, $9.5 million and $6.2 million, or 17%, 14% and 11% of our total revenues, in 2006, 2005 and 2004, respectively.
Infectious Disease Testing
We market the OraQuickADVANCE® rapid HIV-1/2 antibody test directly to customers in the public health market for HIV testing. This market consists of a broad range of clinics and laboratories and includes states, counties, the CDC, SAMHSA and other governmental agencies, family planning clinics, colleges and universities, correctional facilities and the military. There are also a number of organizations in the public health market, such as AIDS service organizations and various community-based organizations set up primarily for the purpose of encouraging and enabling HIV testing.
Abbott Laboratories (“Abbott”) was appointed as our exclusive distributor in the U.S. hospital market and as a non-exclusive distributor in the U.S. physicians’ office marketplace. As our exclusive distributor to hospitals, Abbott sells OraQuickADVANCE® to federal hospitals under the terms of our Federal Supply Schedule on file with the General Services Administration. Under our agreement with Abbott, we have retained exclusive rights for all other markets, including sales to the public health and criminal justice markets, the military, the CDC, SAMHSA and other governmental agencies. We have a small sales force that supports Abbott in order to maximize the penetration of OraQuickADVANCE® in the hospital market.
Abbott recently announced that it will sell part of its diagnostics business, including its rights to distribute OraQuickADVANCE®, to General Electric (“GE”). This transaction is expected to close during the first half of 2007. We intend to meet with executives from GE to discuss their plans for the OraQuickADVANCE® product.
We currently distribute our OraQuick® test in several foreign countries. We expect the number of countries to increase as we find new distributors, complete registrations in additional countries and obtain a CE mark for this product.
We also market the OraSure® oral fluid collection device for HIV-1 testing, separately and as a kit in combination with laboratory testing services. To better serve our public health customers, we have entered into agreements with two commercial laboratories to provide prepackaged OraSure® test kits, with prepaid laboratory testing and specimen shipping costs included. We also sell the OraSure® device in the international public health market.
Substance Abuse Testing
Our substance abuse testing products are marketed to laboratories serving the workplace testing, forensic toxicology, criminal justice and drug rehabilitation markets.
We have entered into agreements for the distribution of Intercept® collection devices and associated MICRO-PLATE assays for drugs-of-abuse testing in the workplace testing market in the United States and
9
Table of Contents
Canada through several laboratory distributors, including Quest Diagnostics (“Quest”) and Clinical Reference Laboratory, and internationally for workplace, criminal justice and forensic toxicology testing through Bio-Rad Laboratories, Concateno (which recently acquired Altrix HealthCare, plc) and other distributors. In some cases, we assist our laboratory customers in customizing their testing services by selling them equipment required to test oral fluid specimens collected with the Intercept® device.
The forensic toxicology market in the United States for our substance abuse testing products consists of 250—300 laboratories including federal, state and county crime laboratories, medical examiner laboratories and reference laboratories. The criminal justice market consists of a wide variety of entities in the criminal justice system that require drug screening, such as pre-trial services, parole and probation officials, police forces, drug courts, prisons, drug treatment programs and community/family service programs.
We also distribute our Q.E.D.® saliva alcohol test primarily through various distributors in the United States and internationally. The markets for alcohol testing are relatively small and fragmented with a broad range of legal and procedural barriers to entry. Markets range from law enforcement testing to workplace testing of employees in safety sensitive occupations. Typical usage situations include pre-employment, random, post-accident, reasonable-cause and return-to-duty testing.
Cryosurgical Systems
Most of our Histofreezer® sales occur in the United States to distributors that, in turn, resell the product to primary care physicians and podiatrists in the United States. Major U.S. distributors include Cardinal Healthcare, McKesson HBOC, Physicians Sales & Service, AmerisourceBergen Corporation, and Henry Schein. Internationally, we established a sales office in Reeuwijk, The Netherlands, and we are selling the Histofreezer® product through a network of distributors in more than 20 countries worldwide.
We sell Freeze Off®, a product similar to Histofreezer®, in the OTC market in the U.S. and Canada pursuant to a distribution agreement with Prestige Brands, the owner of the Compound W® line of wart removal products. Additionally, we distribute cryosurgical wart removal products in the OTC footcare market in Europe, Australia and New Zealand through our distributor, SSL, under its Scholl and Dr. Scholl tradenames, and in the OTC market in Mexico under the POINTTS tradename through our distributor, Genomma Labs. For a description of our pending dispute with Prestige Brands, see Item 3, “Legal Proceedings,” in this Annual Report.
Insurance Risk Assessment
We currently market the OraSure® oral fluid collection device for use in screening life insurance applicants in the United States and internationally to test for three of the most important underwriting risk factors: HIV-1, cocaine and cotinine (a metabolite of nicotine). Devices are sold to insurance testing laboratories, including Quest Diagnostics, Heritage Labs and Clinical Reference Laboratory. These laboratories in turn provide the devices to insurance companies, usually in combination with testing services.
We also maintain a direct sales force that promotes use of the OraSure® device directly to insurance companies for life insurance risk assessment. Insurance companies then make their own decision regarding which laboratory to use to supply their collection devices and testing services. Our OraSure® Western blot confirmatory test is distributed through BMX to laboratories and is used to confirm oral fluid specimens collected with our OraSure® device that initially test positive for HIV-1. For a description of BMX’s recent election not to renew the Western blot agreements after December 31, 2007, see the Section entitled, “Western blot HIV-1 Confirmatory Test,” in this Annual Report.
Because insurance companies are in various stages of their adoption of the OraSure® device, there exists a wide range of policy limits where the product is being applied. Some insurance companies have chosen to extend their testing to lower policy limits where they did not test at all before, while others have used OraSure® to
10
Table of Contents
replace some of their blood and urine-based testing. In general, most of our insurance company customers use the OraSure® device in connection with life insurance policies having face amounts of up to $250,000, with some customers using the device for policies of up to $500,000 in amount.
In recent years, we have experienced a decline in sales of OraSure® and related assays for insurance testing, primarily due to a reduction in the number of applications for life insurance policies and changes in underwriting requirements, as well as some consolidation in the industry leading to a reevaluation of testing methods. However, our sales force continues to encourage additional insurance companies to use OraSure® and to extend the use of the product by existing customers. We believe there are several factors which could help expand the use of our device, including increasing acceptance of the reliability of oral fluid testing, the high quality of test results, the low cost of oral fluid testing relative to blood tests and the ease of use of the OraSure® device.
We also sell our AUTO-LYTE® assays and reagents in the insurance testing market directly to laboratories, including Heritage Labs and Clinical Reference Laboratory.
International Markets
We sell most of our products into international markets primarily through distributors with knowledge of their local markets. Principal markets include physicians’ offices, insurance risk assessment, substance abuse, public health and laboratory testing.
We assist our international distributors in registering the products and obtaining required regulatory approvals in each country, and we provide training and support materials. Our international marketing program includes direct assistance to distributors in arranging for laboratory services, cooperation from screening test manufacturers and performance of Western blot confirmatory tests when necessary.
Significant Products and Customers
Several different products have contributed significantly to our financial performance, accounting for 10% or more of total revenues during the past three years. The OraSure® and Intercept® oral fluid collection devices, cryosurgical systems products, and OraQuick® rapid HIV test accounted for total revenues of $15.1 million, $17.3 million and $25.6 million in 2006, $15.9 million, $22.7 million and $21.6 million in 2005, and $14.6 million, $20.2 million and $10.2 million in 2004, respectively. As new products are developed and commercialized, we expect to receive a greater portion of our revenues from these new products.
We currently have two customers, Quest and Abbott, which accounted for 14% and 10% of our total revenues, respectively, during 2006. The loss of Quest or Abbott, or a significant decrease in the volume of products purchased by either customer, could have a material adverse effect on our results.
Supply and Manufacturing
We manufacture our OraQuickADVANCE® test in our Bethlehem, Pennsylvania facility. In addition, we have entered into a supply agreement for the assembly of the OraQuick® device in Thailand, in order to supply certain international markets. This supply agreement had an initial term of one year, and automatically renews for additional annual periods unless either party provides a timely notice of termination prior to the end of an annual period. We believe that other firms would be able to manufacture the OraQuick® test on terms no less favorable than those set forth in the agreement if the Thailand contractor would be unable or unwilling to continue manufacturing this product.
We can purchase the HIV antigen and the nitrocellulose required for the OraQuick® test only from a limited number of sources. The antigen is currently purchased from a single contract supplier under a long-term agreement with an initial term ending in January 2010 and one-year automatic renewal terms thereafter. The
11
Table of Contents
nitrocellulose used in the test is also provided by a single contract supplier, under a supply agreement with a five-year term ending in 2009. If for any reason these suppliers are unwilling or no longer able to supply our antigen or nitrocellulose needs, we believe that alternative supplies could be obtained at a competitive cost. However, a change in the antigen or nitrocellulose would require FDA approval and some additional development work. This in turn would require significant time to complete and could disrupt our ability to manufacture and sell the OraQuick® device.
We manufacture both the OraSure® and Intercept® collection devices in our Bethlehem, Pennsylvania facility, and we expect to continue to do so for the foreseeable future.
The oral fluid Western blot HIV-1 confirmatory test is currently manufactured in our Bethlehem, Pennsylvania facility. The HIV antigen needed to manufacture the Western blot test is currently available from only a limited number of sources. For many years, we have purchased the antigen for this product from BMX on an exclusive basis. BMX is also the exclusive distributor of the Western blot test kits. Our agreements with BMX provide for the supply by BMX of the HIV-1 antigen and distribution of the oral fluid Western blot product by BMX on an exclusive worldwide basis. In March 2007, BMX notified us that it will not renew the agreements under which it supplies the HIV-1 antigen and distributes that product on an exclusive, world-wide basis, beyond December 31, 2007. For a further description of BMX’s election not to renew the Western blot agreements, see the Section entitled, “Western blot HIV-1 Confirmatory Test,” in this Annual Report.
Histofreezer® is assembled in The Netherlands by Koninklijke, Utermöhlen, N.V. (“Utermöhlen”), the company from which we acquired the product in 1998. We purchase the product pursuant to an exclusive production agreement. Utermöhlen also supplies Freeze Off®, the OTC cryosurgical product for the U.S. and Canadian markets. Assuming minimum purchase requirements are met, our agreement with Utermöhlen will terminate at the end of 2008 with respect to the Histofreezer® product. The Utermöhlen agreement was scheduled to terminate at the end of 2006 with respect to the Freeze Off® product, subject to a minimum purchase requirement. However, we did not meet that requirement by the end of 2006 due to unexpectedly low purchases of the product by our distributor, Prestige. As a result, we expect to continue to purchase product for the U.S. OTC market from Utermöhlen for the foreseeable future. The cryosurgical wart removal products distributed in international OTC markets are supplied by vendors located in the United States.
We believe that additional suppliers of all of our cryosurgical products are available on terms no less favorable than the terms of our existing supply agreements in the event that our current suppliers would be unable or unwilling to continue manufacturing these products.
Our AUTO-LYTE® and MICRO-PLATE assays are manufactured in our Bethlehem, Pennsylvania facility. These tests require the production of highly specific and sensitive antibodies corresponding to the antigen of interest. Substantially all our antibody requirements are provided by contract suppliers. We believe that we have adequate reserves of antibody supplies and that we have access to sufficient raw materials for these products.
The Q.E.D.® saliva alcohol test is manufactured and packaged for shipment in our Bethlehem, Pennsylvania facility.
Employees
As of December 31, 2006, we had 250 full-time employees, including 66 in sales, marketing and client services; 26 in research and development; 105 in operations, manufacturing, quality control, information systems, purchasing and shipping; 19 in regulatory affairs and quality assurance, and 34 in administration and finance. This compares to 233 employees as of December 31, 2005. Our employees are not currently represented by a collective bargaining agreement.
12
Table of Contents
Competition
The diagnostic industry is a multi-billion dollar international industry and is intensely competitive. Many of our competitors are substantially larger than we are, and they have greater financial, research, manufacturing and marketing resources.
Important competitive factors for our products include product quality, price, ease of use, customer service and reputation. Industry competition is based on the following:
| • | Scientific and technological capability; |
| • | Proprietary know-how; |
| • | The ability to develop and market products and processes; |
| • | The ability to obtain FDA or other regulatory approvals; |
| • | The ability to manufacture products that meet applicable FDA requirements (i.e., good manufacturing practices); |
| • | Access to adequate capital; |
| • | The ability to attract and retain qualified personnel; and |
| • | The availability of patent protection. |
A few large corporations produce a wide variety of diagnostic tests and other medical devices and equipment. A larger number of mid-size companies generally compete only in the diagnostic industry and a significant number of small companies produce only a few diagnostic products. As a result, the diagnostic test industry is highly fragmented and segmented.
The future market for diagnostic tests is expected to be characterized by consolidation, greater cost consciousness and tighter reimbursement policies. The purchasers of diagnostic products are expected to place increased emphasis on lowering costs, reducing inventory levels, automation, service and volume discounts. The increased complexity of the market is expected to force many competitors to enter into joint ventures or license certain products or technologies.
We expect competition to intensify as technological advances are made and become more widely known, and as new products reach the market. Furthermore, new testing methodologies could be developed in the future that render our products impractical, uneconomical or obsolete. There can be no assurance that our competitors will not succeed in developing or marketing technologies and products that are more effective than those we develop or that would render our technologies and products obsolete or otherwise commercially unattractive. In addition, there can be no assurance that our competitors will not succeed in obtaining regulatory approval for these products, or introduce or commercialize them before we can do so. These developments could have a material adverse effect on our business, financial condition and results of operations.
Several companies market or have announced plans to market oral specimen collection devices and tests both within and outside the United States. We expect the number of devices competing with our Intercept®, OraQuick® and OraSure® devices to increase as the benefits of oral specimen-based testing become more widely accepted.
Competition in the market for HIV testing is intense and is expected to increase. We believe that the principal competition will come from existing laboratory-based blood tests, point-of-care rapid blood tests, laboratory-based urine assays, or other oral fluid-based tests that may be developed. Our competitors include specialized biotechnology firms, as well as pharmaceutical companies with biotechnology divisions and medical diagnostic companies.
13
Table of Contents
Significant competitors for our OraQuickADVANCE® rapid test, such as the Ortho Diagnostics division of Johnson & Johnson, Bio-Rad Laboratories, Abbott and BMX, sell laboratory-based HIV-1/2 EIAs, and Maxim Biomedical (formerly Calypte, Inc.) sells an HIV-1 screening test for urine, in the United States. MedMira and Trinity Biotech each sell competing rapid HIV-1 blood tests, and Bio-Rad Laboratories and Chembio sell competing rapid HIV-1/2 blood tests in the United States. These tests compete with our OraQuickADVANCE® test in hospitals or other laboratory settings. In addition, Trinity Biotech and Chembio have received CLIA waivers for their rapid HIV tests, and these tests compete with our OraQuickADVANCE® test in the markets outside of the traditional hospital and laboratory settings. These companies, or others, may continue to expand the bodily fluids with which a rapid HIV test may be performed, or develop and commercialize new rapid HIV tests, which would provide further competition for our OraQuickADVANCE® test. We believe other companies may also seek FDA approval to sell competing rapid HIV tests in the future.
Internationally, our OraQuickADVANCE® test competes against rapid HIV tests sold by a number of other entities, and often these competing tests are sold at prices substantially below the prices we charge for our OraQuickADVANCE® test. Calypte has developed a rapid oral fluid HIV test which is now being sold in certain foreign countries.
The Intercept® drug testing system competes with laboratory-based drug testing products and services using testing matrices such as urine, hair, sweat and oral fluid. Major competitors include Ansys Technologies, Inc., Dade Behring, Psychemedics and Immunalysis.
Our MICRO-PLATE oral fluid drug assays, which are sold for use with the Intercept® and OraSure® collection devices, are expected to come under increasing competitive pressure from “home-brew” assays developed internally by our laboratory customers. Our oral fluid MICRO-PLATE assays also compete with urine-based homogeneous assays that are run on fully-automated, random access analyzers. These tests provide strong competitive pressure because they provide the benefits of automation, including lower costs and short turn-around times. In addition, we believe our competitors are developing oral fluid tests suitable for use on these fully automated homogeneous assay systems and these assays, if and when they are developed and commercialized, will represent a significant competitive threat to our oral fluid MICRO-PLATE business. In order to meet this competition, we executed a letter of intent with Roche Diagnostics in October 2006 to enter into an agreement for the joint development and commercialization of fully-automated homogeneous oral fluid drugs of abuse assays for use with our Intercept® device. We expect final agreements with Roche to be executed during the first half of 2007.
Our MICRO-PLATE drugs-of-abuse reagents sold in the forensic toxicology market are targeted to forensic testing laboratories where sensitivity, automation and “system solutions” are important. In the past, these laboratories have typically had to rely on radioimmunoassay test methods to provide an adequate level of sensitivity. Radioimmunoassays require radioactive materials, which have a short shelf-life and disposal problems. Our MICRO-PLATE tests meet the laboratories’ sensitivity needs, run on automated equipment, are not radioimmunoassays, and are offered to the laboratory as a complete system solution of reagents, instrumentation and software to meet the specific needs of each customer. We compete with both homogeneous and heterogeneous tests manufactured by many companies. Significant competitors in the market for these assays include Microgenics, Inc., Roche Diagnostics and Immunalysis.
Sales of our AUTO-LYTE® urine assays have declined substantially during the past several years, primarily due to competition from assays developed internally by our laboratory customers (i.e., “home brews”), which can be produced at a cost lower than the price typically paid for our products. Many of our customers no longer purchase our AUTO-LYTE® assays, and we eventually expect to stop selling this product line.
The Histofreezer® product’s delivery system and operating temperature, which is warmer than liquid nitrogen, provide us with the opportunity to target sales to primary care physicians, such as family practitioners, pediatricians and podiatrists. We do not generally target sales to dermatologists because they have the volume of
14
Table of Contents
patients required to support the capital costs associated with a liquid nitrogen delivery system, which is also used to remove warts and other benign skin lesions. There is limited competition for convenient cryosurgical products for wart removal in the primary care physician market. Major competitors for the Histofreezer® product include Cryosurgery, Inc. in the United States and Wartner in Europe. Wartner may also eventually compete with Histofreezer® in the physician market in the United States.
The Freeze Off® product, sold by Prestige under its Compound W® tradename, competes with other OTC wart removal products in the United States. Schering-Plough sells a competing cryosurgical wart removal product under its Dr. Scholl’s brand and another competing cryosurgical wart removal product is sold in the OTC market under the Wartner® name. Wartner also sells a product that competes with our cryosurgical product in the European OTC footcare market. During 2006, Prestige acquired the Wartner® product sold in the United States and Canada, and we believe this action constitutes a material breach of our agreement with Prestige. For a further discussion of our dispute with Prestige regarding this matter, see Item 3, “Legal Proceedings,” in this Annual Report.
Q.E.D.® has two direct competitors, Ansys Technologies, Inc. and Chematics. These companies offer semi-quantitative saliva-based alcohol tests and have received DOT approval. Indirect competitors who offer breath testing equipment include Intoximeters, Dräger and CMI. Although there are lower priced tests on the market that use oral fluid or breath as a test medium, these tests are qualitative tests that are believed to be substantially lower in quality and provide fewer benefits than our Q.E.D.® test.
Patents and Proprietary Information
We seek patent and other intellectual property rights to protect and preserve our proprietary technology and our right to capitalize on the results of our research and development activities. We also rely on trade secrets, know-how, continuing technological innovations and licensing opportunities to provide competitive advantages for our products in our markets and to accelerate new product introductions. We regularly search for third-party patents in fields related to our business to shape our own patent and product commercialization strategies as effectively as possible and to identify licensing opportunities. United States patents generally have a maximum term of 20 years from the date an application is filed.
We have 16 United States patents and numerous foreign patents for the OraSure® and Intercept® collection devices and technology relating to oral fluid collection, containers for oral fluids, methods to test oral fluid, formulations for the manufacture of synthetic oral fluid, and methods to control the volume of oral fluid collected and dispersed. We have also applied for additional patents, in both the United States and certain foreign countries, on such products and technology.
We have one issued patent for our OraQuickADVANCE® rapid HIV-1/2 antibody test in the United States, and we received notice that the claims of a related patent have been allowed. We also have several related patent applications pending for this product in the United States and internationally. We have obtained licenses to certain lateral flow patents and to certain HIV-1 and HIV-2 patents held by other parties. We also have obtained a license to certain HCV patents which we intend to use to manufacture and sell a rapid HCV test on the OraQuick® or other technology platform. We obtained these licenses through the payment of certain upfront fees and an agreement to pay ongoing royalties. We believe these fees and royalties are comparable to those generally paid by other companies under similar arrangements.
We may need to obtain licenses or other rights under, or enter into distribution or other business arrangements in connection with, certain other intellectual property patents in order to manufacture and sell the OraQuickADVANCE® test or other tests that use the same or similar technology platform. See Section 1A, entitled “Risk Factors,” for a further discussion of these issues.
We have five United States patents and numerous foreign patents issued for apparatuses and methods for the topical removal of skin lesions relating to our cryosurgical wart removal products, and we have a pending patent
15
Table of Contents
application related to these products. We have also licensed another patent relating to apparatuses and methods for the topical removal of skin lesions relating to our cryosurgical wart removal products.
We have four United States patents and numerous foreign patents and patent applications for the technology used in the Q.E.D.® test. These patents are related to the analog-to-digital technology color control systems and methods, systems and devices for the test, and detection of biochemical molecules.
We have one United States patent relating to the method for detecting blood in urine specimens using our AUTO-LYTE® products.
We require our employees, consultants, outside collaborators and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed by or made known to the individual during the course of the individual’s relationship with us, is to be kept confidential and not disclosed to third parties except in specific circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual during his or her tenure with us will be our exclusive property.
We own rights to trademarks and service marks that we believe are necessary to conduct our business as currently operated. In the United States, we own the OraSure®, Intercept®, OraQuick®, OraQuickADVANCE®, Histofreezer®, Q.E.D.® and AUTO-LYTE® trademarks. We also own many of these marks and others in several foreign countries. The Compound W® and Freeze Off® trademarks are owned by Prestige, or its affiliates, in the United States and Canada, the Scholl and Dr. Scholl tradenames are owned by SSL in Europe, Australia, New Zealand and other countries outside North and South America, and the POINTTS tradename is owned by Genomma Labs in Mexico.
Although important, the issuance of a patent or existence of trademark or trade secret protection does not in itself ensure the success of our business. Competitors may be able to produce products competing with our patented products without infringing our patent rights. Issuance of a patent in one country generally does not prevent manufacture or sale of the patented product in other countries. The issuance of a patent is not conclusive as to validity or as to the enforceable scope of the patent. The validity or enforceability of a patent can be challenged by litigation after its issuance. If the outcome of such litigation is adverse to the owner of the patent, the owner’s rights could be diminished or withdrawn. Trade secret protection does not prevent independent discovery and exploitation of the secret product or technique.
Government Regulation
General
Most of our products are regulated by the FDA, certain state and local agencies and comparable regulatory bodies in other countries. This regulated environment governs almost all aspects of development, production, and marketing, including product testing, authorizations to market, labeling, promotion, manufacturing and recordkeeping.
All of our FDA-regulated products require some form of action by the FDA before they can be marketed in the United States. After approval or clearance by the FDA, we must continue to comply with other FDA requirements applicable to marketed products. Both before and after approval or clearance, failure to comply with the FDA’s requirements can lead to significant penalties or could disrupt our ability to sell these products. In addition, the FDA could refuse permission to obtain certificates needed to export our products if the agency determines that we are not in compliance.
Domestic Regulation
Most of our products are regulated in the United States as medical devices.
16
Table of Contents
There are two mechanisms by which regulated medical devices can be placed on the market in the United States. Some products may qualify for clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act. To obtain this clearance from the FDA, the manufacturer must provide a premarket notification that it intends to begin marketing the product, and show that the product is substantially equivalent to another legally marketed product (i.e., that it has the same intended use and is as safe and effective as a legally marketed device and does not raise different questions of safety and effectiveness). In some cases, the submission must include data from human clinical studies. Marketing may commence when the FDA issues a clearance letter finding substantial equivalence. An applicant must submit a 510(k) application at least 90 days before marketing of the affected product commences. Although FDA clearance may be granted within that 90-day period, in some cases as much as a year or more may be required before clearance is obtained, if at all.
If the medical device does not qualify for the 510(k) procedure (either because it is not substantially equivalent to a legally marketed device or because it is required by statute and the FDA’s regulations to have an approved premarket application), the FDA must approve a premarket application, or PMA, before marketing can begin. PMAs must demonstrate, among other matters, that the medical device provides a reasonable assurance of safety and effectiveness. A PMA is typically a complex submission, including the results of preclinical and clinical studies. Preparing a PMA is a detailed and time-consuming process. Once a PMA has been submitted, the FDA is required to review the submission within 180 days. However, the FDA’s review may, and often is, much longer, often requiring one year or more, and may include requests for additional data and facility inspections before approval is granted, if at all.
Some of our products are used for non-medical purposes and many of our drugs-of-abuse products sold to state crime labs are for forensic use. The FDA does not currently regulate products used for these purposes.
Every company that manufactures medical devices distributed in the United States must comply with the FDA’s Quality System Regulations (“QSRs”). These regulations govern the manufacturing process, including design, manufacture, testing, release, packaging, distribution, documentation and purchasing. In complying with QSRs, manufacturers must continue to expend time, money and effort in the area of production and quality assurance to ensure full technical compliance. Companies are also subject to other post-market and general requirements, including restrictions imposed on marketed products, promotional standards and requirements for recordkeeping and reporting of certain adverse reactions. If there are any modifications made to our marketed devices, a premarket notification or PMA may be required to be submitted to, and cleared or approved by, the FDA, before the modified device may be marketed. The FDA regularly inspects companies to determine compliance with QSRs and other post-market requirements. Failure to comply with statutory requirements and the FDA’s regulations can result in warning letters, monetary penalties, suspension or withdrawal of regulatory approvals, operating restrictions, total or partial suspension of production, injunctions, product recalls, seizure of products and criminal prosecution.
The Clinical Laboratory Improvements Amendments of 1988, or CLIA, prohibit laboratories from performing tests for the purpose of providing information for the diagnosis, prevention or treatment of any disease or impairment of, or the assessment of, the health of human beings, unless there is in effect for such laboratories a certificate issued by the U.S. Department of Health and Human Services applicable to the category of examination or procedure performed. We consider the applicability of the requirements of CLIA in the design and development of our products. We have obtained a waiver of the CLIA requirements for both our OraQuickADVANCE® rapid HIV-1/2 antibody test and our Q.E.D.® alcohol saliva test and may seek similar waivers for certain other products. A CLIA waiver allows certain customers to use the waived products that may not have been able to use them without complying with certain quality control and other requirements.
Certain of our products may also be affected by state regulations in the United States. For example, there are several states that restrict or do not currently permit oral fluid drug testing in the workplace or other markets. In addition, several states prohibit or limit the use of rapid, point-of-care HIV testing. We are presently working with legislators or regulators in certain of these states in an effort to modify or remove any restrictions affecting our ability to sell products.
17
Table of Contents
International
We are also subject to regulations in foreign countries governing products, human clinical trials and marketing, and may need to obtain approval from international public health agencies, such as the World Health Organization, in order to sell products in certain countries. Approval processes vary from country to country, and the length of time required for approval or to obtain other clearances may in some cases be longer than that required for U.S. governmental approvals. We generally pursue approval only in those countries that we believe have a significant market opportunity.
The International Organization for Standardization (“ISO”) is a worldwide federation of national standards bodies from some 130 countries, established in 1947. The mission of the ISO is to promote the development of standardization and related activities in the world with a view to facilitating the international exchange of goods and services. ISO certification is a pre-requisite to use of the CE mark and indicates that our quality system complies with standards applicable to activities ranging from initial product design and development through production and distribution. The CE mark is a European Union (“EU”) requirement to sell products that fall under the scope of the Medical Devices Directive (“MDD”) and the In Vitro Diagnostic Directive (“IVDD”). The CE mark is evidence that the manufacturer and the product meet the requirements of all applicable directives, including the MDD and IVDD.
We received authorization to use the CE mark for the OraSure® and Intercept® collection devices and our Histofreezer® product line, and SSL International has obtained authorization to use the CE mark for our cryosurgical wart removal product in the OTC European footcare market. In addition, we are currently in the process of obtaining authorization to affix a CE mark to our OraQuickADVANCE® HIV-1/2 test.
We must also comply with certain registration requirements as dictated by Health Canada, prior to commencing sales in Canada. We have completed this process for several of our current products and may do so with respect to other products in the future. In addition, Canadian law requires manufacturers of medical devices to have a quality management system that meets various ISO requirements in order to obtain a license to sell their devices in Canada.
Anti-Kickback Laws
The Federal Anti-Kickback Statute prohibits the knowing and willful offer, payment, solicitation, or receipt of any form of remuneration in return for, or to induce:
| • | The referral of a person; |
| • | The furnishing or arranging for the furnishing of items or services reimbursable under Medicare, Medicaid or other governmental programs; or |
| • | The purchase, lease, or order of, or the arrangement or recommendation of the purchasing, leasing, or ordering of any item or service reimbursable under Medicare, Medicaid, or other governmental programs. |
Our products are or may be purchased by customers that will seek or receive reimbursement under Medicare, Medicaid or other governmental programs. Noncompliance with the federal anti-kickback legislation can result in exclusion from Medicare, Medicaid, or other governmental programs, restrictions on our ability to operate in certain jurisdictions, as well as civil and criminal penalties, any of which could have an adverse effect on our business and results of operations.
The Federal Civil Monetary Penalties Law prohibits the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services. Noncompliance can result in civil money penalties of up to $10,000 for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the Federal healthcare programs.
18
Table of Contents
Many states have also adopted some form of anti-kickback laws. A determination of liability under such laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
We believe that we are operating in compliance with these laws.
Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act (“FCPA”) prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity. Our present and future business has and will continue to be subject to the FCPA and various other laws, rules and/or regulations applicable to us as a result of our international sales.
Environmental Regulation
Because of the nature of our current and proposed research, development, and manufacturing processes, we are subject to stringent federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge and handling and disposal of materials and wastes. We believe that we have complied with these laws and regulations in all material respects.
The foregoing discussion of our business should be read in conjunction with the Financial Statements and accompanying notes included in Item 15 of this Annual Report.
The following is a discussion of certain significant risk factors that could potentially negatively impact our financial condition, performance and prospects.
Regulatory Risks
The Need to Obtain Regulatory Approvals and Respond to Changes in Regulatory Requirements Could Adversely Affect Our Business.
Many of our proposed and existing products are subject to regulation by the FDA and other governmental or public health agencies. In particular, we are subject to strict governmental controls on the development, manufacture, labeling, distribution and marketing of our products. In addition, we are often required to obtain approval or registration with foreign governments or regulatory bodies before we can import and sell our products in foreign countries.
The process of obtaining required approvals or clearances from governmental or public health agencies can involve lengthy and detailed laboratory testing, human clinical trials, sampling activities and other costly, time- consuming procedures. These approvals can require the submission of a large amount of clinical data which may require significant time to obtain. It is also possible that a product will not perform at a level needed to generate the clinical data required to obtain an approval or clearance. The submission of an application to the FDA or other regulatory authority does not guarantee that an approval or clearance to market the product will be received. Each authority may impose its own requirements and delay or refuse to grant approval or clearance, even though a product has been approved in another country or by another agency.
Moreover, the approval or clearance process for a new product can be complex and lengthy. This time span increases our costs to develop new products as well as the risk that we will not succeed in introducing or selling them in the United States or other countries.
19
Table of Contents
We are in the process of conducting clinical studies to support an application for FDA approval of our OraQuickADVANCE® HIV-1/2 test for sale in the United States OTC market. We also expect to conduct clinical trials in support of our application for FDA approval of our OraQuick® HCV test for professional use. There can be no assurance that these clinical trials will generate sufficient data to support FDA approval of either product or that FDA approval will be obtained. Failure to obtain or any delay in obtaining FDA approval for either product could significantly reduce future revenues and could adversely affect our financial performance.
Newly promulgated or changed regulations could also require us to undergo additional trials or procedures, or could make it impractical or impossible for us to market our products for certain uses, in certain markets, or at all. For example, during 2004 SAMHSA, which is part of the U.S. Department of Health and Human Services, issued proposed regulations for the use of oral fluid drug testing for federal workers. Although the SAMHSA regulations have been withdrawn, if and when they are issued in final form, they could permit us to market and sell our oral fluid drug tests for use with federal workers only if certain modifications are made to our products. If we are unable to make these modifications, or if the modifications require significant time to develop, our ability to sell our oral fluid drug testing products in that market could be limited. In addition, the extent to which the final SAMHSA regulations permit the sale of our oral fluid drug tests for use with federal workers may influence whether customers in the workplace, criminal justice or other unregulated markets use our products.
The regulations in some states may restrict our ability to sell products in those states. For example, certain states restrict or do not allow the testing of oral fluid for drugs of abuse or the rapid, point-of-care testing for HIV. While we intend to work with state legislators and regulators to remove or modify any applicable restrictions, there is no guarantee we will be successful in these efforts.
In addition, allin vitro diagnostic products that are to be sold in the EU must bear the CE mark indicating conformance with the essential requirements of the IVDD. We are not permitted to sell our products in the EU without a CE mark, which could lead to the termination of strategic alliances and agreements for sales of those products in the EU. We have obtained the CE mark for several of our existing products and we intend to CE mark our OraQuickADVANCE® test and certain of our future products and are not aware of any material reason why we will be unable to do so. However, there can be no assurance that compliance with all provisions of the IVDD will be demonstrated and the CE mark will be obtained for all products that we desire to sell in the EU.
The Inability to Extend the Shelf Life of Our OraQuick ADVANCE® Test Could Adversely Affect Our Business.
The shelf life of a product is the period of time from the date of manufacture during which the product is expected to perform in accordance with its specifications and labeling. In order to successfully sell our products, they need to have a shelf life that is long enough to cover the time required to distribute the product to a customer and provide the customer with a reasonable period to use that product.
Where a product has a short shelf life, our ability to sell that product may be adversely affected. In order to extend the shelf life, we may be required to submit real time stability data supporting such an extension to the FDA for approval.
Our OraQuickADVANCE® HIV-1/2 test has a shelf life of six months. While this shelf life has not prevented us from selling into the public health and hospital markets in the United States, it has limited our ability to sell that test internationally. We also believe a shelf life of at least 12 months will be required to sell the OraQuickADVANCE® test successfully into the United States OTC market.
We are working to extend the shelf life of our OraQuickADVANCE® test. However, there can be no assurance that we will be successful in obtaining such an extension or its approval by the FDA. If we are unsuccessful in obtaining a shelf life extension, our ability to sell the OraQuick® test may be adversely affected and we may not be able to sell it successfully into the United States OTC market.
20
Table of Contents
Failure to Comply With FDA or Other Regulatory Requirements May Require Us to Suspend Production of Our Products or Institute a Recall Which Could Result in Higher Costs and a Loss of Revenues.
We can manufacture and sell many of our products, both in the United States and internationally, only if we comply with regulations of government agencies such as the FDA. We have implemented quality assurance and other systems that are intended to comply with applicable regulations.
Although we believe that we have adequate processes in place to ensure compliance with these requirements, the FDA or other regulatory bodies could force us to stop manufacturing or selling our products if it concludes that we are out of compliance with applicable regulations. The FDA and other regulatory bodies could also require us to recall products if we fail to comply with applicable regulations, which could force us to stop manufacturing such products. Such actions by the FDA could adversely affect our revenues. See the Section entitled “Government Regulation” in Item 1 of this Annual Report for a further discussion of applicable regulatory requirements.
Risks Relating to Our Industry, Business and Strategy
Our Ability to Sell Products Could be Affected by Competition From New and Existing Diagnostic Products and by Treatments or Other Non-Diagnostic Products Which May be Developed.
The diagnostic industry is focused on the testing of biological specimens in a laboratory or at the point of care and is highly competitive and rapidly changing. Many of our principal competitors have considerably greater financial, technical and marketing resources. As new products enter the market, our products may become obsolete or a competitor’s products may be more effective or more effectively marketed and sold than ours. If we fail to maintain and enhance our competitive position, our customers may decide to use products developed by competitors which could result in a loss of revenues.
We also face competition from products which may be sold at a lower price. To the extent this competition arises, customers may choose to buy lower cost products from third parties or we may be forced to sell our products at a lower price, both of which could result in a loss of revenues or a lower gross margin contribution from the sale of our products.
In addition, the development and commercialization of products outside of the diagnostics industry could adversely affect sales of our product. For example, the development of a safe and effective vaccine to prevent HIV or preventative treatments for other diseases or conditions that our products are designed to detect, could reduce, or eventually eliminate, the demand for our HIV or other diagnostic products and thereby result in a loss of revenues.
Our Research, Development and Commercialization Efforts May Not Succeed and Our Competitors May Develop and Commercialize More Effective or Successful Diagnostic Products.
In order to remain competitive, we must regularly commit substantial resources to research and development and the commercialization of new products.
The research and development process generally takes a significant amount of time from inception to commercial product launch. This process is conducted in various stages. During each stage there is a substantial risk that we will not achieve our goals on a timely basis, or at all, and we may have to abandon a product in which we have invested substantial amounts.
During 2006, 2005 and 2004, we incurred $8.6 million, $5.3 million and $6.1 million, respectively, in research and development expenses. We expect to continue to incur significant costs from our research and development activities.
21
Table of Contents
Successful products require significant development and investment, including testing, to demonstrate their cost-effectiveness or other benefits prior to commercialization. In addition, regulatory approval must be obtained before most products may be sold. Additional development efforts on these products will be required before any regulatory authority will review them. Regulatory authorities may not approve these products for commercial sale. In addition, even if a product is developed and all applicable regulatory approvals are obtained, there may be little or no market for the product. Accordingly, if we fail to develop commercially successful products, or if competitors develop more effective products or a greater number of successful new products, customers may decide to use products developed by our competitors. This would result in a loss of revenues and adversely affect our results of operations, cash flows and business.
If We Lose Our Key Personnel or Are Unable to Attract and Retain Qualified Personnel as Necessary, Our Business Could be Harmed.
Our success will depend to a large extent upon the contributions of our executive officers, management and sales, marketing, operations and scientific staff. We may not be able to attract or retain qualified employees in the future due to the intense competition for qualified personnel among medical products businesses.
If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that will adversely affect our ability to effectively manufacture, sell and market our products, to meet the demands of our strategic partners in a timely fashion, or to support internal research and development programs. Although we believe we will be successful in attracting and retaining qualified personnel, competition for experienced scientists and other personnel from numerous companies and academic and other research institutions may limit our ability to do so on acceptable terms.
Future Acquisitions or Investments Could Disrupt Our Ongoing Business, Distract Our Management, Increase Our Expenses and Adversely Affect Our Business.
We may consider strategic acquisitions or investments as a way to expand our business in the future. These activities, and their impact on our business, are subject to the following risk factors:
| • | Suitable acquisitions or investments may not be found or consummated on terms that are satisfactory to us; |
| • | We may be unable to successfully integrate an acquired company’s personnel, assets, management systems and technology into our business; |
| • | Acquisitions may require substantial expense and management time and could disrupt our business; |
| • | An acquisition and subsequent integration activities may require greater capital resources than originally anticipated at the time of acquisition; |
| • | An acquisition may result in the incurrence of unexpected expenses, the dilution of our earnings or our existing stockholders’ percentage ownership, or potential losses from undiscovered liabilities not covered by an indemnification from the seller(s) of the acquired business; |
| • | An acquisition may result in the loss of existing key personnel or customers or the loss of the acquired company’s key personnel or customers; |
| • | The benefits expected to be derived from an acquisition may not materialize and could be affected by numerous factors, such as regulatory developments, general economic conditions and increased competition; and |
| • | An acquisition of a foreign business may involve additional risks, including, but not limited to, foreign currency exposure, liability under foreign laws or regulations and not being able to successfully assimilate differences in foreign business practices or overcome language or cultural barriers. |
The occurrence of one or more of the above or other factors may prevent us from achieving all or a significant part of the benefits expected from an acquisition or investment. This may adversely affect our
22
Table of Contents
financial condition, results of operations and ability to grow our business or otherwise achieve our financial or strategic objectives.
Our Revenues Could be Affected by Third-Party Reimbursement Policies and Potential Cost Constraints.
The end-users of our products are expected to increasingly include hospitals, physicians and other healthcare providers. Use of our products could be adversely impacted if these end-users do not receive reimbursement for the cost of our products by their patients’ healthcare insurers or payors. Our net sales could also be adversely affected by changes in reimbursement policies of governmental or private healthcare payors, including in particular the level of reimbursement for our products. In the United States, healthcare providers such as hospitals and physicians who purchase diagnostic products generally rely on third-party payors, principally private health insurance plans, Medicare and Medicaid, to reimburse all or part of the cost of the product and procedure. We believe that the overall escalating cost of medical products and services has led to, and will continue to lead to, increased pressures on the healthcare industry, both foreign and domestic, to reduce the cost of products and services. Given the efforts to control and reduce healthcare costs in the United States in recent years, currently available levels of reimbursement may not continue to be available in the future for our existing products or products under development. Third-party reimbursement and coverage may not be available or adequate in either the United States or foreign markets, current reimbursement amounts may be decreased in the future and future legislation, and regulation or reimbursement policies of third-party payors may reduce the demand for our products or our ability to sell our products on a profitable basis.
Increases in Demand for Our Products Could Require Us to Expend Considerable Resources to Meet the Demand or Harm Our Customer Relationships if We are Unable to Meet Demand.
If we experience significant or unexpected increases in the demand for our products, we and our suppliers may not be able to meet that demand without expending additional capital resources. These capital resources could involve the cost of new machinery or even the cost of new manufacturing facilities. This would increase our capital costs, which could adversely affect our earnings. Our suppliers may be unable or unwilling to expend the necessary capital resources or otherwise expand their capacity. In addition, new manufacturing equipment or facilities may require FDA approval before they can be used to manufacture our products. To the extent we are unable to obtain or are delayed in obtaining such approvals, our ability to meet the demand for our products could be adversely affected.
If we or our suppliers are unable to develop necessary manufacturing capabilities in a timely manner, our net sales could be adversely affected. Failure to cost-effectively increase production volumes, if required, or lower than anticipated yields or production problems encountered as a result of changes that we or our suppliers make in our manufacturing processes to meet increased demand, could result in shipment delays or interruptions and increased manufacturing costs, which could also have a material adverse effect on our revenues and profitability.
Our inability to meet customer demand for our products could also harm our customer relationships and impair our reputation within the industry. This, in turn, could have a material adverse effect on our business and prospects.
Risks Relating to Collaborators
Our Failure to Maintain Existing Distribution Channels, or Develop New Distribution Channels, May Result in Lower Revenues.
We have marketed many of our products by collaborating with laboratories, diagnostic companies and distributors. Our sales depend to a substantial degree on our ability to sell products to these customers and on the marketing abilities of the companies with which we collaborate.
23
Table of Contents
We currently sell our OraQuickADVANCE® rapid HIV-1/2 antibody test to the diagnostics division of Abbott Laboratories for distribution into the United States hospital market on an exclusive basis. Abbott recently announced that it will sell a portion of its diagnostics business to General Electric (“GE”), including its rights to the OraQuickADVANCE® product. This sale has not yet closed, and it is unclear what, if any, effect it may have on sales of our OraQuickADVANCE® test in the hospital market.
Some of our distributors or other customers may not fulfill their contractual obligations to us. Although we will try to maintain and expand our business with our distributors and customers and require that they fulfill their contractual obligations, there can be no assurance that such companies will continue to purchase or distribute our products, maintain historic order volumes or otherwise meet their purchase or other obligations, or that new distribution channels will be available on satisfactory terms. The failure of these distributors or other customers to purchase our products could adversely affect our revenues.
In September 2006, Prestige Brands, Inc., the domestic distributor of the Freeze Off® cryosurgical wart removal product, announced that it had acquired the Wartner® cryosurgical product, which directly competes with the Freeze Off® product in the United States and Canadian OTC markets. We believe this acquisition constitutes a material breach of our agreement with Prestige, and we are pursuing arbitration to resolve this matter. We believe we have already suffered significant damages from this breach and Prestige’s actions may continue to impact future sales of the Freeze Off® product. If we are unable to successfully resolve this matter with Prestige or find an alternative arrangement for distributing our cryosurgical product into the United States and Canada OTC markets, our revenues could be negatively affected.
Some of our distributors have also consolidated in recent years and such consolidation has had, and may continue to have, an adverse impact on the level of orders for our products. In addition, some distributors have experienced, and may continue to experience, pressure from their customers to reduce the price of their products and testing services. For example, several of our insurance testing laboratories are facing this pressure and are using lower cost “home brew” insurance testing assays that they have developed internally or purchased from our competitors. This has reduced our assay sales and is expected to lower sales of these products in 2007 and beyond.
The Use of Sole Supply Sources or Third Party Suppliers For Critical Components of Our Products Could Adversely Affect Our Business.
We currently purchase certain critical components of our products from sole supply sources or other third party suppliers. For example, all of the HIV antigen and nitrocellulose required to make our OraQuickADVANCE® rapid HIV-1/2 antibody test is purchased from sole source suppliers. In addition, the conjugates used in our MICROPLATE oral fluid drugs of abuse assays are obtained from third party suppliers.
If these suppliers are unable or unwilling to supply the required component or if they make changes in the component or do not supply materials meeting our specifications, we may need to find another source and perform additional development work. We may also need to obtain FDA or other regulatory approvals for the use of the alternative component for our products. Completing that development and obtaining such approvals could require significant time to complete and may not occur at all. The availability of critical components from sole supply sources or other third parties could also reduce our control over pricing, quality and timely delivery. These events could either disrupt our ability to manufacture and sell certain of our products, or completely prevent us from doing so or increase our costs. Any such event could have a material adverse effect on our results of operations, cash flows and business.
The Unavailability of Certain Products Distributed by a Third Party Could Adversely Affect Sales of Our OraSure® Oral Fluid Collection Device.
In testing an oral fluid sample collected with an OraSure® device for HIV-1 in the United States, our customers must use an HIV-1 EIA screening test approved by the FDA for use with our OraSure® device. Where
24
Table of Contents
an oral fluid sample screens positive for HIV-1, our customers must then use our oral fluid Western blot HIV-1 confirmatory test, which has also been approved by the FDA for use with our OraSure® device, to confirm that positive indication.
BMX currently manufactures and sells the only oral fluid HIV-1 EIA screening test that has received FDA approval for use in detecting HIV-1 in an oral fluid specimen collected with our OraSure® collection device. BMX also supplies the HIV-1 antigen used to manufacture our oral fluid Western blot HIV-1 confirmatory test and is the exclusive world-wide distributor of that product.
In late 2006, BMX announced that it will discontinue manufacturing the HIV-1 EIA screening test during 2007 and that, due to quality problems, it may have difficulty supplying this screening test prior to the time it ceases manufacturing. As a result, we are working with BMX, the FDA and CDC, our major laboratory customers and other potential suppliers to find or develop an alternative HIV-1 EIA screening test that can be used with oral fluid samples collected with our OraSure® device.
In March 2007, BMX notified us that it will not renew the agreement under which it supplies the HIV-1 antigen used to manufacture our oral fluid Western blot HIV-1 confirmatory test or the agreement under which it distributes that product on an exclusive, world-wide basis. As a result, these agreements will terminate on December 31, 2007. Pursuant to the terms of the antigen supply agreement, we have the right to purchase an additional two-year supply of the antigen following termination so that we can continue to manufacture and sell our oral fluid Western blot test. When this additional two-year supply is combined with our existing inventory of the HIV-1 antigen, we believe we will have a suffucient supply of HIV-1 antigen to meet the demand for our Western blot test for three to four years after the agreement terminates. We also intend to pursue a long-term supply agreement directly with the vendor (a former affiliate of BMX) used by BMX to manufacture the HIV-1 antigen. During 2006, sales of our oral fluid Western blot HIV-1 confirmatory test totaled approximately $330,000.
If at some point in the future our customers cannot obtain either an HIV-1 EIA screening test or a Western blot or other HIV-1 confirmatory test that has been approved by the FDA for use with our OraSure® collection device, sales of our OraSure® device could be negatively affected.
We May Need Strategic Partners to Assist in Developing and Commercializing Some of Our Diagnostic Products.
Although we intend to pursue some product opportunities independently, opportunities that require a significant level of investment for development and commercialization or a distribution network beyond our existing sales force may necessitate involving one or more strategic partners. Our strategy for development and commercialization of products may entail entering into arrangements with distributors or other corporate partners, universities, research laboratories, licensees and others. We may be required to transfer material rights to such strategic partners, licensees and others. While we expect that our current and future partners, licensees and others have and will have an economic motivation to succeed in performing their contractual responsibilities, there is no assurance that they will do so and the amount and timing of resources to be devoted to these activities will be controlled by others. Consequently, there can be no assurance that any revenues or profits will be derived from such arrangements.
We may need to collaborate with one or more third parties or find new product distribution channels in order to commercialize our OraQuickADVANCE® HIV-1/2 test should we receive approval from the FDA for use in the United States OTC market. In order to successfully commercialize our OraQuickADVANCE® test in the OTC market, we may need to invest significantly in advertising and promotion and obtain distribution channels to the OTC market. If we are unable to collaborate with a third party having sufficient resources to assist in these efforts or find alternative distribution channels to access the OTC market, we may need to incur significant costs for advertising and promotion, and our ability to maximize our future revenues for this opportunity could be adversely affected.
25
Table of Contents
Risks Relating to Our Financial Results, Structure and Need for Financing
We Have a History of Losses Prior to 2005.
We achieved our first full years of profitability in 2005 and 2006. However, as of December 31, 2006, the Company had an accumulated deficit of $98.4 million.
Our ability to achieve continued profitability in the future will be dependent upon a number of factors including, without limitation, the following:
• | Creating market acceptance for and selling increasing volumes of our OraQuickADVANCE® rapid HIV-1/2 antibody test, Intercept® drug testing product and OraSure® collection device; |
| • | The degree to which certain of our new products may replace sales of our existing products and the financial impact of that change, including the degree to which our OraQuickADVANCE® test will replace our OraSure® collection device for HIV-1 testing or sales of our cryosurgical wart removal products in the OTC market will replace sales of our Histofreezer® product to physicians’ offices or other professional markets; |
| • | The degree to which our major distributors comply with their contractual obligations, including minimum purchase commitments; |
• | Whether we are able to extend the shelf life of our OraQuickADVANCE® HIV-1/2 test; |
| • | Our ability to successfully resolve claims or litigation, including patent infringement litigation; |
• | The level of expenditures we are required to make in order to develop and obtain regulatory approvals for our new products, including our OraQuickADVANCE® HIV-1/2 test for use in the OTC market and an OraQuick® HCV test for professional use; |
| • | Achieving growth in sales of our wart removal products in the OTC market and selling other products, such as our OraQuickADVANCE® HIV-1/2 test, in the OTC market; |
| • | Whether we are able to find a replacement for the BMX HIV-1 EIA screening test for use in connection with oral fluid samples collected with our OraSure® device; |
| • | Achieving growth in international markets with our OraQuickADVANCE® HIV-1/2 test, cryosurgical wart removal products and other products; |
| • | Changes in the level of competition, such as would occur if larger and financially stronger competitors introduced new or lower priced products to compete with our products; |
| • | Changes in economic conditions in domestic or international markets, such as economic downturns, reduced demand, inflation and currency fluctuations; |
| • | Failure to achieve our targets for growth in revenues; |
| • | Changes in distributor buying patterns or a buildup of significant quantities in our distributors’ inventories or distribution channels; and |
| • | Commercially developing, and obtaining regulatory approvals and creating market acceptance for new products in a time frame consistent with our objectives. |
Even though we achieved profitability for 2005 and 2006, there can be no assurance that we will be able to sustain such profitability in the future.
Utilization of Our Deferred Tax Assets May Be Limited and is Dependent on Future Taxable Income.
As of December 31, 2006, we had federal net operating loss (“NOL”) carryforwards of $53.0 million for federal income tax purposes. The Tax Reform Act of 1986 contains provisions under Section 382 of the Internal
26
Table of Contents
Revenue Code that limit the NOLs that may be used in any given year in the event of specified occurrences, including significant ownership changes. If these specified events occur, we may lose some or all of the tax benefits of these carryforwards.
During 2005, we determined, based on our assessment of both positive and negative evidence, which takes into consideration our forecasted taxable income, that it was more likely than not that we will benefit from the use of a significant portion of our deferred tax assets, and therefore we reduced our valuation allowance on our deferred tax assets related to these NOLs. If in the future we determine, based on our assessment of both positive and negative evidence, that it is more likely than not that we will not realize all or a portion of the deferred tax assets, we will record a valuation allowance on the deferred tax assets which would result in recognition of income tax expense.
We May Require Future Additional Capital.
Our future liquidity and ability to meet our future capital requirements will depend on numerous factors, including, but not limited to, the following:
| • | The costs and timing of the expansion of our manufacturing capacity; |
| • | The success of our research and product development efforts; |
| • | The magnitude of capital expenditures; |
| • | Changes in existing and potential relationships with distributors and other business partners; |
| • | The time and cost of obtaining regulatory approvals; |
| • | The costs involved in obtaining and enforcing patents, proprietary rights and necessary licenses; |
| • | The costs and liability associated with patent infringement or other types of litigation; |
| • | The costs and timing of expansion of sales and marketing activities; |
| • | The timing of the commercial launch of new products; |
| • | The extent to which existing and new products gain market acceptance; |
| • | The scope and results of clinical testing; |
| • | Competing technological and market developments; and |
| • | The scope and timing of strategic acquisitions. |
If additional financing is needed, we may seek to raise funds through the sale of equity or other securities or through bank borrowings. There can be no assurance that financing through the sale of securities, bank borrowings or otherwise, will be available to us on satisfactory terms, if at all.
An Economic Downturn, Terrorist Attacks or National Disasters May Adversely Affect Our Business.
Changes in economic conditions could adversely affect our business. For example, in a difficult economic environment, customers may be unwilling or unable to invest in new diagnostic products, may elect to reduce the amount of their purchases or may perform less drug testing because of declining employment levels or the issuance of fewer life insurance policies. A weakening business climate could also cause longer sales cycles and slower growth, and could expose us to increased business or credit risk in dealing with customers or suppliers adversely affected by economic conditions.
Terrorist attacks such as those occurring on September 11, 2001, or national disasters such as Hurricane Katrina, and subsequent governmental responses to these events could cause economic instability. These actions
27
Table of Contents
could adversely affect economic conditions both within and outside the United States and reduce demand for our products. Terrorist attacks and natural disasters could cause governments and regulatory agencies, such as the FDA or agencies that perform similar functions outside the United States, to focus their resources on other matters, such as relief efforts or vaccines or other products intended to address the threat of biological or chemical warfare. This diversion of resources could eliminate or delay the bulk procurement of our products by government agencies or delay our ability to obtain regulatory approvals required to manufacture, market or sell our products in the United States and other countries. These events could also disrupt the operations of our customers and suppliers and eliminate, reduce or delay our customers’ ability to purchase and use our products and our suppliers’ ability to provide raw materials and finished products.
Our Stock Price Could Continue to be Volatile.
Our stock price has been volatile, has fluctuated substantially in the past and may be volatile in the future and could experience substantial declines. The following factors, among others, could have a significant impact on the market for our Common Stock:
| • | Future announcements concerning us or our products; |
| • | Future announcements concerning our competitors or industry; |
| • | Developments in patent or other proprietary rights; |
| • | Litigation or threatened litigation; |
| • | Public concern as to the performance or safety of products that we or others have developed or sold; |
| • | Failure to achieve, or changes in, financial estimates by securities analysts and comments or opinions about us by securities analysts or major stockholders; |
| • | Governmental regulation; |
| • | Clinical results with respect to our products in development or those of our competitors; |
| • | Changes in the level of competition; |
| • | Loss of or declines in sales to major distributors or customers; |
| • | The relatively low trading volume for our Common Stock; |
| • | Period to period fluctuations in our operating results; |
| • | General market and economic conditions; and |
| • | Terrorist attacks, civil unrest, war and national disasters. |
Future Sales of Our Common Stock by Existing Stockholders, Executive Officers or Directors Could Depress the Market Price of Our Common Stock and Make It More Difficult For Us to Sell Stock in the Future.
Sales of our Common Stock in the public market, or the perception that such sales could occur, could negatively impact the market price of our Common Stock. We are unable to estimate the number of shares of our Common Stock that may actually be resold in the public market since this will depend on the market price for our Common Stock, the individual circumstances of the sellers and other factors.
We have a number of institutional stockholders that own significant blocks of our Common Stock. If one or more of these stockholders sell large portions of their holdings in a relatively short time, for liquidity or other reasons, the prevailing market price of our Common Stock could be negatively affected. In addition, it is possible that one or more of our executive officers or non-employee members of our Board of Directors could sell shares of our Common Stock during an open trading window under our Insider Trading Policy. These transactions and
28
Table of Contents
the perceived reasons for these transactions could have a negative effect on the prevailing market price of our Common Stock.
Our Reported Financial Results May be Adversely Affected by Changes in Accounting Principles Generally Accepted in the United States.
We prepare our financial statements in conformity with accounting principles generally accepted in the United States. These accounting principles are subject to creation or interpretation by the Financial Accounting Standards Board, the SEC and various bodies formed to interpret and create appropriate accounting principles. A change in these principles or interpretations could have a significant effect on our reported financial results, and could affect the reporting of transactions completed before the announcement of a change.
Risks Relating to Intellectual Property
Our Success Depends on Our Ability to Protect Our Proprietary Technology.
The diagnostics industry places considerable importance on obtaining patent, trademark and trade secret protection, as well as other intellectual property rights, for new technologies, products and processes. Our success depends, in part, on our ability to develop and maintain a strong intellectual property portfolio or obtain licenses to patents for products and technologies both in the United States and in other countries.
As appropriate, we intend to file patent applications and obtain patent protection for our proprietary technology. These patent applications and patents will cover, as applicable, compositions of matter for our products, methods of making those products, methods of using those products and apparatus relating to the use or manufacture of those products. We will also rely on trade secrets, know-how and continuing technological advancements to protect our proprietary technology.
We have entered, and will continue to enter, into confidentiality agreements with our employees, consultants, advisors and collaborators. However, these parties may not honor these agreements and we may not be able to successfully protect our rights to unpatented trade secrets and know-how. Others may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets and know-how.
Many of our employees, including scientific and management personnel, were previously employed by competing companies. Although we encourage and expect all of our employees to abide by any confidentiality agreement with a prior employer, competing companies may allege trade secret violations and similar claims against us.
We may collaborate with universities and governmental research organizations which, as a result, may acquire part of the rights to any inventions or technical information derived from collaboration with them.
To facilitate development and commercialization of a proprietary technology base, we may need to obtain licenses to patents or other proprietary rights from other parties. Obtaining and maintaining such licenses may require the payment of substantial amounts. In addition, if we are unable to obtain these types of licenses, our product development and commercialization efforts may be delayed or precluded.
We may incur substantial costs and be required to expend substantial resources in asserting or protecting our intellectual property rights, or in defending suits against us related to intellectual property rights. Disputes regarding intellectual property rights could substantially delay product development or commercialization activities. Disputes regarding intellectual property rights might include state, federal or foreign court litigation, as well as patent interference, patent reexamination, patent reissue, or trademark opposition proceedings in the
29
Table of Contents
United States Patent and Trademark Office. Opposition or revocation proceedings could be instituted in a foreign patent office. An adverse decision in any proceeding regarding intellectual property rights could result in the loss or limitation of our rights to a patent, an invention or trademark.
We are Involved in Pending, and May Become Involved in Future, Intellectual Property Infringement Disputes, Which are Costly and Could Limit or Eliminate Our Ability to Sell Our Products or Use Certain of Our Technologies in the Future.
From time to time, we may seek to enforce our patents or other intellectual property rights through litigation. In addition, there are a large number of patents and patent applications in our product areas, and additional patents may be issued to third parties relating to our product areas. We may be sued for infringement of patents or misappropriation of other intellectual property rights with respect to one or more of our products. Litigation in our industry regarding patent and other intellectual property rights is prevalent and is expected to continue.
Our involvement in litigation with respect to patents or other intellectual property or to determine rights in proprietary technology, either as a plaintiff or defendant, could adversely affect our revenues, market share, results of operations and business because:
| • | As is common with major litigation, it could consume a substantial portion of managerial and financial resources; |
| • | Its outcome would be uncertain and a court may find that our patents are invalid or unenforceable in response to claims by another party or that the third-party patent claims are valid and infringed by our products; |
| • | An adverse outcome could subject us to the loss of the protection of our patents or to liability in the form of past royalty payments, penalties, special and punitive damages, or future royalty payments significantly affecting our future earnings; |
| • | Failure to obtain a necessary license upon an adverse outcome could prevent us from selling our current products or other products we may develop or acquire; and |
| • | A court could award a preliminary and/or permanent injunction which would prevent us from selling our current or future products. |
We have sued Schering-Plough for infringement of several of our patents related to the technology for the cryosurgical removal of warts and other benign skin lesions. This litigation has been costly to prosecute, and there is no assurance that we will be successful in our obtaining either injunctive relief or an award of damages in this matter. It is also possible, based on the defenses asserted by Schering-Plough, that one or more of our patents could be found to be invalid or unenforceable. For a further description of the Schering-Plough litigation, see Item 3, “Legal Proceedings,” in this Annual Report.
The Sales Potential for OraQuick ADVANCE® Could be Affected by Our Ability to Obtain Certain Licenses.
Our OraQuickADVANCE® test is a lateral flow assay device that tests for specific antibodies or other substances. The term “lateral flow” generally refers to a test strip through which a sample flows and which provides a test result on a portion of the strip downstream from where the sample is applied. There are numerous patents in the United States and other countries which claim lateral flow assay methods and devices. Some of these patents may broadly cover the technology used in the OraQuickADVANCE® test and are in force in the United States and other countries. We may not be able to make or sell the OraQuickADVANCE® test in the United States or other countries where these patents are in force.
We have obtained licenses under several lateral flow patents, which we believe should be sufficient to permit the manufacturing and sale of the OraQuickADVANCE® device as currently contemplated. However,
30
Table of Contents
licenses under additional patents may be required and it is possible that a third party could seek to enforce one or more lateral flow patents against us. In the event that we are unable to successfully defend against such litigation or it is determined that a license is required and it is not possible to negotiate or otherwise obtain a license agreement on reasonable terms under a necessary patent, our ability to manufacture and sell the OraQuickADVANCE® device could be limited. In such case, we may be able to modify the OraQuickADVANCE® test such that a license would not be necessary. However, this alternative could delay or limit our ability to sell the OraQuickADVANCE® test in the United States and other markets, which would adversely affect our results of operations, cash flows and business.
Risks Relating to Products, Marketing and Sales
A Market for Our Products May Not Develop.
Our future success will depend, in part, on the market acceptance, and the timing of such acceptance, of our products such as the OraQuickADVANCE® rapid HIV-1/2 antibody test, the Intercept® drug test and other new products or technologies that may be developed or acquired. To achieve market acceptance, we must make substantial marketing efforts and spend significant funds to inform potential customers and the public of the perceived benefits of these products. There may be limited evidence on which to evaluate the market reaction to products that may be developed, and there can be no assurance that any products will obtain market acceptance and fill the market need that is perceived to exist.
If Acceptance and Adoption of Our Oral Fluid Testing Does Not Continue, Our Future Results May Suffer.
We have made significant progress in gaining acceptance of oral fluid testing for HIV in the insurance, public health and other markets. We have also made significant progress in gaining acceptance of oral fluid testing for drugs of abuse in the workplace and criminal justice testing markets. However, the ultimate degree of acceptance in these markets is uncertain, and other markets may resist the adoption of oral fluid testing as a replacement for other testing methods in use today. In addition, certain state laws prohibit or restrict the use of oral fluid testing for drugs of abuse in certain markets or the rapid, point-of-care testing for HIV. As a result, there can be no assurance that we will be able to expand the use of our oral fluid testing products in these or other markets.
We May be Sued for Product Liabilities for Injuries Resulting From the Use of Our Products.
We may be held liable if any of our products, or any product which is made with the use or incorporation of any of our technologies, causes injury of any type or is found otherwise unsuitable during product testing, manufacturing, marketing, sale or usage. Although we have obtained product liability insurance, this insurance may not fully cover potential liabilities. As we bring new products to market, we may need to increase our product liability coverage.
We are selling cryosurgical wart removal products in the consumer or OTC market in the United States, Canada, and Europe. We expect to expand the OTC sales of these products to other countries and to eventually distribute other types of products in the domestic and international OTC markets, such as our OraQuickADVANCE® HIV-1/2 test. We believe the sale of products in the OTC market increases the risk of potential product liability exposure and possibly the required level of insurance coverage that we will need to maintain. Inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could affect our decision to commercialize new products and our results of operations.
Performance of Our Products May Affect Our Revenues and Stock Price.
Our products are generally sold with labeling that contains performance claims approved or cleared by the FDA or other regulators. If our products fail to perform in accordance with the applicable label claims or
31
Table of Contents
otherwise in accordance with the expectations or needs of our customers, customers may switch to a competing product or otherwise stop using our products, and our revenues could be adversely affected. In addition, poor performance by one or more of our products and publicity surrounding such performance could have an adverse effect on our reputation, our continuing ability to sell products and the prevailing market price of our Common Stock.
Our Increasing International Presence May be Affected by Regulatory, Cultural or Other Restraints.
We intend to increase revenue derived from international sales of our products. Our international sales accounted for $11.4 million or 17% of total revenues for 2006, $9.5 million or 14% of total revenues for 2005, and $6.2 million or 11% of total revenues for 2004.
A number of factors can slow or prevent international sales, or substantially increase the cost of international sales, including those set forth below:
| • | Regulatory requirements (including compliance with applicable customs regulations) may slow, limit, or prevent the offering of products in foreign countries; |
| • | The unavailability of licenses to certain patents in force in a foreign country which cover our products may restrict our ability to sell into that country; |
| • | The inability to maintain ISO certification for our or our suppliers’ manufacturing facilities could preclude, interrupt or delay our ability to manufacture products for sale in Europe or other international territories; |
| • | Our inability to obtain the CE mark on our products in a timely manner may preclude or delay our ability to sell products in the European Union; |
| • | Our inability to identify international distributors and negotiate acceptable terms for distribution agreements may delay or reduce our sales; |
| • | Cultural and political differences may make it difficult to effectively market, sell and gain acceptance of products in foreign countries; |
| • | Inexperience in international markets may slow or limit our ability to sell products in foreign countries; |
| • | Exchange rates, currency fluctuations, tariffs and other barriers, extended payment terms and dependence on and difficulties in managing international distributors or representatives may affect our revenues even when product sales occur; |
| • | The creditworthiness of foreign distributors may be less certain and foreign accounts receivable collection may be more difficult; |
| • | Economic conditions, the absence of available funding sources, terrorism, civil unrest, war and natural disasters may slow or limit our ability to sell our products in foreign countries; |
| • | Our exposure to liability under the Foreign Corrupt Practices Act and various other laws, rules and/or regulations applicable to us as a result of our international sales may affect our ability to sell into international markets; |
| • | International markets often have long sales cycles, especially for sales to foreign governments, quasi-governmental agencies and international public health agencies, thereby delaying or limiting our ability to sell our products; and |
| • | We may be at a disadvantage if competitors in foreign countries sell competing products at prices at or below such competitors’ or our cost. |
In addition, we have entered into a contract for the manufacture and supply of our OraQuick® HIV-1/2 test in Thailand, and the Histofreezer® and Freeze Off® cryosurgical products are currently manufactured in The
32
Table of Contents
Netherlands. We may enter into agreements to manufacture other products in foreign countries as well. However, factors such as economic and political conditions and foreign regulatory requirements may slow or prevent the manufacture of our products in countries other than the United States. Interruption of the supply of our products could reduce revenues or cause us to incur significant additional expenses in finding an alternative source of supply. In addition, foreign currency fluctuations and economic conditions in foreign countries could increase the costs of manufacturing our products in foreign countries.
ITEM 1B. Unresolved Staff Comments.
Not Applicable.
We own a 48,000 square foot facility, which is our primary corporate office and manufacturing facility, and a second 31,700 square foot facility that houses our sales and marketing and research and development offices. Both of these facilities are located in Bethlehem, Pennsylvania. These facilities were previously leased until we exercised our option to purchase them on June 30, 2006.
We also own an additional 33,500 square foot building in Bethlehem, Pennsylvania, which is used for manufacturing activities.
We rent additional warehouse space on an as-needed basis. We also lease space for a small sales office in Reeuwijk, The Netherlands.
We believe that the facilities described above are adequate for our current requirements.
Schering-Plough Patent Infringement Litigation
On July 23, 2004, we filed a lawsuit against Schering-Plough for infringement of several of our patents relating to technology for the cryosurgical removal (i.e., freezing) of warts and other benign skin lesions. The suit was commenced in the United States District Court for the Eastern District of Pennsylvania, and alleges that Schering-Plough’s manufacture and sale of its Dr. Scholls® Freeze Away® cryosurgical wart removal product in the United States OTC market infringes the following United States patents: Nos. 5,738,682; 6,092,527 and 4,865,028. We are requesting permanent injunctive relief and the payment of damages. Schering-Plough has asserted various defenses in this matter, including that its Dr. Scholls® Freeze Away® product does not infringe our patents and that one or more of our patents are invalid and unenforceable.
On November 2, 2005, an initial pretrial conference was held on this matter, at which the Court heard oral argument on motions for summary judgment and certain evidentiary and other motions filed by the parties. Those motions remain pending. We expect the Court to set a final trial schedule once a decision on the motions has been rendered.
Prestige Brands Dispute
Prestige, through an affiliate, is the exclusive distributor of our cryosurgical wart removal product in the OTC market in the United States and Canada. Prestige distributes this product under its Compound W Freeze Off® trade name. In September 2006, Prestige announced that it had acquired the Wartner® cryosurgical wart removal product line, which directly competes with the Freeze Off® product in the OTC market.
Our distribution agreement with Prestige contains a covenant not to compete which precludes Prestige from acquiring, manufacturing, distributing or selling a cryosurgical product that directly competes with the Freeze
33
Table of Contents
Off® product. We notified Prestige that its acquisition of the Wartner product constitutes a material breach of the distribution agreement and that certain of its other actions constitute additional breaches under the agreement.
On September 27, 2006, we filed a special petition and application with the Supreme Court of the State of New York, New York County, for a preliminary injunction against Prestige pursuant to New York Civil Practice Law and Rules § 7502(c) in support of the arbitration to be commenced between the parties. On November 8, 2006, the Court issued a decision dated October 30, 2006 finding that Prestige had breached the covenant not to compete, but denied our application. By decision dated December 20, 2006, the Court denied our motion for reargument and reconsideration of its October 30 decision. We have since filed an appeal of the Court’s denial of our request for a preliminary injunction with the Appellate Division—First Department of the New York Supreme Court and that appeal remains pending.
We have also initiated the alternative dispute resolution procedures required under our agreement with Prestige, which include mediation and binding arbitration. The parties’ efforts to resolve this matter through mediation were not successful, and an arbitration pursuant to the rules of the American Arbitration Association has been commenced, pursuant to the terms of the agreement.
Unless we are able to resolve this matter with Prestige, we intend to vigorously enforce our rights and remedies under the agreement, including seeking specific performance of the covenant not to compete.
ITEM 4. Submission of Matters to a Vote of Security Holders.
No matters were submitted to a vote of security holders during the fourth quarter of the year ended December 31, 2006.
34
Table of Contents
| ITEM 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Market Information
Our Common Stock is listed for trading on the Global Market tier of The Nasdaq Stock Market LLC (“NASDAQ”) under the symbol OSUR. High and low sales prices reported by NASDAQ during the periods indicated are shown below.
| Year ended December 31, | ||||||||||||
| 2006 | 2005 | |||||||||||
| High | Low | High | Low | |||||||||
First Quarter | $ | 11.67 | $ | 8.80 | $ | 7.45 | $ | 5.35 | ||||
Second Quarter | 10.89 | 7.89 | 10.23 | 6.91 | ||||||||
Third Quarter | 10.16 | 6.15 | 11.83 | 8.42 | ||||||||
Fourth Quarter | 8.70 | 7.27 | 14.14 | 7.74 | ||||||||
On March 1, 2007, there were 578 holders of record and approximately 16,000 holders in street name of the Common Stock, and the closing price of the Common Stock was $7.76 per share.
Dividends
We have never paid any cash dividends and our Board of Directors does not anticipate paying cash dividends in the foreseeable future. We are generally not permitted to pay dividends or make other distributions to our stockholders under the terms of our credit facilities with Comerica Bank, without first obtaining Comerica’s consent. We intend to retain any future earnings to provide funds for the operation and expansion of our business.
35
Table of Contents
Performance Graph
The following graph compares the cumulative total returns to investors in the Company’s Common Stock, the NASDAQ Composite Index and the NASDAQ Biotechnology Index for the period from December 31, 2001 through December 31, 2006. The graph assumes that $100 was invested on December 31, 2001 in the Company’s Common Stock and in each of the above-mentioned indices, and that all dividends were reinvested.
The NASDAQ Composite Index was chosen because it is a broad index of companies whose equity securities are traded on the NASDAQ Stock Market. The NASDAQ Biotechnology Index was chosen because it includes a number of our competitors. Stockholders are cautioned that the graph shows the returns to investors only as of the dates noted and may not be representative of the returns for any other past or future period.
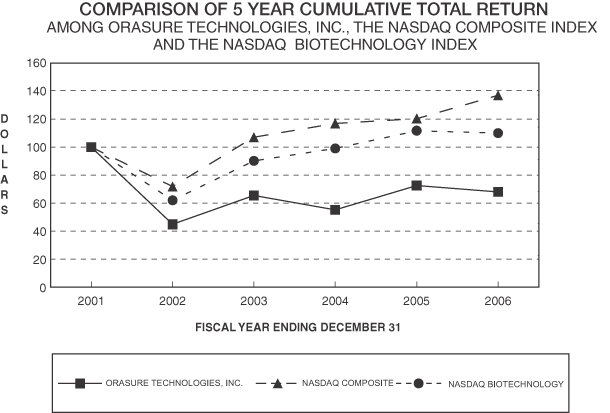
| 12/2001 | 12/2002 | 12/2003 | 12/2004 | 12/2005 | 12/2006 | |||||||
OraSure Technologies, Inc. | 100.00 | 44.86 | 65.51 | 55.31 | 72.59 | 67.98 | ||||||
NASDAQ Composite Index | 100.00 | 71.97 | 107.18 | 117.07 | 120.50 | 137.02 | ||||||
NASDAQ Biotechnology Index | 100.00 | 62.08 | 90.27 | 99.08 | 111.81 | 110.06 |
The performance graph set forth above shall not be deemed “soliciting material” or “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to liability under that Section. This graph will not be deemed “incorporated by reference” into any filing under the Securities Act of 1933, as amended, or the Exchange Act, whether such filing occurs before or after the date hereof, regardless of any general incorporation language in such filing.
36
Table of Contents
ITEM 6. Selected Financial Data.
The following table sets forth selected financial data of the Company. This information should be read in conjunction with the Financial Statements and notes thereto included in Item 15 and the information set forth in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Selected Financial Data
(In thousands, except per share data)
| Year ended December 31, | ||||||||||||||||||||
| 2006 | 2005 | 2004 | 2003 | 2002 | ||||||||||||||||
Operating Results: | ||||||||||||||||||||
Revenues | $ | 68,155 | $ | 69,366 | $ | 54,008 | $ | 40,451 | $ | 32,010 | ||||||||||
Costs and expenses | 62,692 | 61,793 | 55,365 | 41,737 | 35,550 | |||||||||||||||
Operating income (loss) | 5,463 | 7,573 | (1,357 | ) | (1,286 | ) | (3,540 | ) | ||||||||||||
Other income (expense), net | 3,599 | 2,146 | 797 | 177 | 198 | |||||||||||||||
Income tax provision (benefit) | 3,794 | (17,729 | )1 | — | 27 | — | ||||||||||||||
Net income (loss) | 5,268 | 27,448 | 1 | (560 | ) | (1,136 | ) | (3,342 | ) | |||||||||||
Earnings (loss) per share | ||||||||||||||||||||
Basic | $ | 0.11 | $ | 0.61 | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.09 | ) | |||||||
Diluted | $ | 0.11 | $ | 0.59 | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.09 | ) | |||||||
Shares used in computing earnings (loss) per share | ||||||||||||||||||||
Basic | 45,910 | 45,110 | 44,464 | 39,794 | 37,583 | |||||||||||||||
Diluted | 46,580 | 46,147 | 44,464 | 39,794 | 37,583 | |||||||||||||||
Cash Flow: | ||||||||||||||||||||
Cash flow provided by (used in) operating activities | $ | 16,886 | $ | 10,392 | $ | 3,438 | $ | 2,702 | $ | (518 | ) | |||||||||
| December 31, | ||||||||||||||||||||
| 2006 | 2005 | 2004 | 2003 | 2002 | ||||||||||||||||
Financial Position: | ||||||||||||||||||||
Cash, cash equivalents, and short-term investments | $ | 91,001 | $ | 77,620 | $ | 66,723 | $ | 64,024 | $ | 14,908 | ||||||||||
Working capital | 95,979 | 90,670 | 68,910 | 67,171 | 18,931 | |||||||||||||||
Deferred tax asset | 23,522 | 26,708 | — | — | — | |||||||||||||||
Total assets | 156,565 | 130,747 | 88,064 | 86,151 | 35,737 | |||||||||||||||
Long-term debt, excluding current portion | 10,031 | 884 | 1,334 | 2,456 | 3,409 | |||||||||||||||
Accumulated deficit | (98,414 | ) | (103,682 | ) | (131,130 | ) | (130,570 | ) | (129,435 | ) | ||||||||||
Stockholders’ equity | 129,504 | 118,919 | 75,577 | 73,509 | 26,019 | |||||||||||||||
1 | Includes an income tax benefit of $18,165 resulting from the elimination of a significant portion of the valuation allowance on our deferred tax assets (see Note 9 to the Notes to Financial Statements). |
37
Table of Contents
ITEM 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Statements below regarding future events or performance are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Our actual results could be quite different from those expressed or implied by the forward-looking statements. Factors that could affect results are discussed more fully under the Item 1A, entitled “Risk Factors,” and elsewhere in this Annual Report. Although forward-looking statements help to provide complete information about us, readers should keep in mind that forward-looking statements may not be reliable. Readers are cautioned not to place undue reliance on the forward-looking statements.
The following discussion should be read in conjunction with the financial statements contained herein and the notes thereto, along with the Section entitled “Critical Accounting Policies and Estimates,” set forth below.
Overview
We operate primarily in the worldwide $22 billionin vitro diagnostics business. We develop, manufacture and market oral fluid specimen collection devices using proprietary oral fluid technologies, diagnostic products including immunoassays, and otherin vitro diagnostic tests. We also manufacture and sell medical devices for the removal of warts and other benign skin lesions by cryosurgery, or freezing.
Our diagnostic product offerings primarily target the infectious disease and substance abuse testing segments of the largerin vitro diagnostic market, and are used in both laboratories as well as the emerging, and rapidly growing, point-of-care marketplace. Our OraSure® and Intercept® oral fluid collection devices, and their related assays, are processed in a laboratory, while the OraQuickADVANCE® rapid HIV-1/2 antibody test is designed for use at the point-of-care. Our cryosurgical products are also used at the point-of-care.
In vitro diagnostics have traditionally used blood or urine as the bodily fluids upon which tests are conducted. However, we have targeted the use of oral fluid in our products as a differentiating factor and believe that it provides a significant competitive advantage over blood and urine. Our oral fluid tests have sensitivity and specificity comparable to blood and/or urine tests. When combined with their ease of use, non-invasive and dignified nature, and cost effectiveness, our oral fluid tests represent a very competitive alternative to the more traditional testing methods in the diagnostic space.
During the year ended December 31, 2006, our total revenues were $68.2 million, which represents a 2% decrease from 2005. Our net income for 2006 was $5.3 million and includes a pre-tax charge of $3.4 million for stock options which are now required to be expensed under the new accounting rules. Our liquidity continued to improve, as we reported $16.9 million in cash flow from operating activities in 2006 and a balance of $91.0 million in cash, cash equivalents and short-term investments as of December 31, 2006.
Sales into the infectious disease testing market segment increased significantly in 2006 due to the continued market acceptance of our OraQuickADVANCE® HIV-1/2 test. This increase resulted largely from sales directly to various public health organizations and sales through Abbott into the hospital market. These increases were offset by the absence of government bulk purchases by SAMHSA and the CDC.
In February 2005, we entered into an agreement for the distribution of OraQuickADVANCE® with Abbott. Under this agreement, Abbott was appointed as our exclusive distributor in the U.S. hospital market and as a non-exclusive distributor in the U.S. physicians’ office marketplace. As our exclusive distributor to hospitals, Abbott sells OraQuickADVANCE® to federal hospitals under the terms and conditions of our Federal Supply Schedule that is on file with the U.S. General Services Administration. We have retained exclusive rights to all other markets, including the public health and criminal justice markets, the military, the CDC, SAMHSA and other government agencies. We utilize a small internal sales force to support Abbott and work together with them to maximize the penetration of OraQuickADVANCE® in the hospital market.
38
Table of Contents
Competition in the market for HIV testing is intense and is expected to increase. We believe that the principal competition will come from existing laboratory-based blood tests, point-of-care rapid blood tests, laboratory-based urine assays or other oral fluid-based tests that may be developed. Our competitors include specialized biotechnology firms, as well as pharmaceutical companies with biotechnology divisions and medical diagnostic companies.
Significant competitors for our OraQuickADVANCE® rapid test, such as the Ortho Diagnostics division of Johnson & Johnson, Bio-Rad Laboratories, Abbott and BMX, sell laboratory-based HIV-1/2 EIAs, and Maxim Biomedical (formerly Calypte, Inc.) sells an HIV-1 screening test for urine, in the United States. MedMira and Trinity Biotech each sell competing rapid HIV-1 blood tests, and Bio-Rad Laboratories and Chembio sell competing rapid HIV-1/2 blood tests in the United States. These tests compete with our OraQuickADVANCE® test in hospitals or other laboratory settings. In addition, Trinity Biotech and Chembio have received CLIA waivers for their rapid HIV tests and these tests compete with our OraQuickADVANCE® test in the markets outside of the traditional hospital and laboratory settings. These companies, or others, may continue to expand the bodily fluids with which a rapid HIV test may be performed, or develop and commercialize new rapid HIV tests, which would provide further competition for our OraQuickADVANCE® test. We believe other companies may also seek FDA approval to sell competing rapid HIV tests in the future.
Sales to the substance abuse testing market also increased during 2006, reflecting the growing acceptance of our Intercept® collection device and related oral fluid drug assays, as both domestic and international corporate customers continued to adopt oral fluid based drug testing and shift away from traditional urine-based drug testing. We expect continued growth in the utilization of our Intercept® product line, primarily in the United States.
In April 2004, SAMHSA published proposed guidelines that would, if adopted, include oral fluid testing as an accepted drug testing method for federal employees. During the summer of 2006, these proposed guidelines were withdrawn with no action taken and it is unclear when further action, if any, will be taken to permit the use of oral fluid as an accepted drug testing method in this market.
Sales to the cryosurgical systems market declined during 2006. The cryosurgical systems market represents sales of Histofreezer® into both the domestic and international physicians’ office markets and sales of the OTC formulation of this product to our domestic distributor, Prestige, and our international distributor, SSL. Prestige distributes our cryosurgical wart removal product under its Compound W Freeze Off® tradenames in the OTC market in the United States and Canada. SSL distributes a similar product under its Scholl’s and Dr. Scholl trademarks in the OTC footcare market in several European countries. Sales in the U.S. OTC cryosurgery marketplace declined primarily as a result of the disappointing performance by Prestige. Sales to Prestige for 2006 were $5.2 million, or more than 50% less than the $11.6 million in sales recorded for 2005.
In September 2006, Prestige announced that it had acquired the Wartner® cryosurgical wart removal product line, which directly competes with the Freeze Off® product in the OTC market. Our distribution agreement with Prestige contains a covenant not to compete which precludes Prestige from acquiring, manufacturing, distributing or selling a cryosurgical product that directly competes with the Freeze Off® product. We notified Prestige that its acquisition of the Wartner product constitutes a material breach of the distribution agreement and that certain of its other actions constitute additional breaches under the agreement. We initiated the alternative dispute resolution procedures required under the agreement, which include mediation and binding arbitration. The parties’ efforts to resolve this matter through mediation were not successful and an arbitration pursuant to the rules of the American Arbitration Association has been commenced, pursuant to the terms of the agreement. We are evaluating alternative arrangements for distributing this product in the event a resolution with Prestige cannot be reached. As a result of this ongoing dispute, it is not possible to predict at this time the potential impact this matter may have on sales of Freeze Off® in 2007 or beyond.
In July 2004, we filed a lawsuit against Schering-Plough for infringement of several of our patents relating to the technology for the cryosurgical removal (i.e., freezing) of warts and other benign skin lesions. The suit was
39
Table of Contents
commenced in the United States District Court for the Eastern District of Pennsylvania and alleges that Schering- Plough’s manufacture and sale of its Dr. Scholl’s® Freeze Away® cryosurgical wart removal product in the OTC market infringes three of our patents. We are seeking injunctive relief and the payment of damages and Schering-Plough has raised several defenses, including that their Freeze Away® device does not infringe our patents and that one or more of our patents are either invalid or unenforceable. On November 2, 2005, a pretrial conference was held in this matter, at which the Court heard oral argument on motions for summary judgment filed by the parties. These motions remain pending. We expect a new trial schedule to be set after the Court rules on these motions.
Sales to the insurance risk assessment market continued to decline in 2006, primarily because of a reduction in the number of applications for life insurance and changes in underwriting requirements. For higher face-value policies, it appears insurance companies are more likely to use a blood test for multiple risk factors, rather than an oral-fluid test. We currently expect that our 2007 revenues in this market will decline, or at best, remain at approximately the levels attained in 2006.
Because of the regulatory approvals needed for most of our products, we often are required to rely on sole source providers for critical components and materials and on related products supplied by third parties. This is particularly true for our OraQuickADVANCE® test, our OraSure® oral fluid collection device and our oral fluid Western blot HIV-1 confirmatory product. If we are unable to obtain necessary components or materials from these sole sources, the time required to develop replacements and obtain the required FDA approvals could disrupt our ability to sell the affected products.
BMX currently manufactures and sells the only oral fluid HIV-1 screening test that has received FDA approval for use in detecting HIV-1 in an oral fluid specimen collected with our OraSure® collection device. BMX also supplies the HIV-1 antigen used to manufacture our oral fluid Western blot HIV-1 confirmatory test and is the exclusive world-wide distributor of that product. BMX recently notified us that they intend to discontinue manufacturing their HIV-1 EIA screening test during 2007. BMX also notified us that it has elected not to renew the Western blot agreements beyond December 31, 2007. We are working closely with BMX, the FDA and CDC, our main laboratory customers and other potential suppliers to find or develop an alternative HIV-1 EIA screening test and confirmatory test for use with our OraSure® collection device. If our customers cannot obtain an HIV-1 EIA screening test or a confirmatory test that has been approved by the FDA for use in connection with our OraSure® collection device, these customers would likely stop purchasing our OraSure® device and our revenues would be adversely affected.
We also rely heavily on distributors to purchase and resell many of our products. For example, Prestige has exclusive distribution rights to the Freeze Off® product in the OTC markets in United States and Canada and SSL has exclusive rights to a similar product in the OTC footcare market in Europe, Australia and New Zealand. Similarly, Abbott has exclusive rights to distribute our OraQuickADVANCE® test to hospitals in the U.S. We expect to enter into additional distribution agreements for new and future products, for distribution in the U.S. and internationally. If our distributors are unable or unwilling to meet the minimum purchase commitments set forth in their agreements or otherwise substantially reduce the volume of their purchases, our revenues and results of operations could be adversely affected.
We generated 83% of our 2006 revenues in the U.S. marketplace. Consequently, we are evaluating strategies to increase our sales penetration in markets outside the U.S. As our business in foreign countries increases, we could be exposed to other economic, political, exchange rate, regulatory and cultural risks.
Results of Operations
Year Ended December 31, 2006 Compared to December 31, 2005
Total revenues decreased by 2% to $68.2 million in 2006 from $69.4 million in 2005, primarily as a result of declines in domestic OTC cryosurgical product revenues and insurance risk assessment revenues, partially
40
Table of Contents
offset by increased sales of our OraQuickADVANCE® rapid HIV-1/2 antibody test, our Intercept® oral fluid collection device and related drug assays, and our international cryosurgical products. Revenues derived from products sold in countries outside the U.S. were $11.4 million and $9.5 million, or 17% and 14% of total revenues, for the years ended December 31, 2006 and 2005, respectively.
The table below shows the amount of our total revenues (in thousands, except %) generated in each of our principal markets and by licensing and product development activities.
| Year ended December 31, | |||||||||||||||
| Dollars | % Change | Percentage of Total Revenues | |||||||||||||
Market | 2006 | 2005 | 2006 | 2005 | |||||||||||
Infectious disease testing | $ | 29,180 | $ | 25,988 | 12 | % | 43 | % | 37 | % | |||||
Substance abuse testing | 15,752 | 13,519 | 17 | 23 | 19 | ||||||||||
Cryosurgical systems | 17,333 | 22,744 | (24 | ) | 25 | 33 | |||||||||
Insurance risk assessment | 5,565 | 6,815 | (18 | ) | 8 | 10 | |||||||||
Product revenues | 67,830 | 69,066 | (2 | ) | 99 | 99 | |||||||||
Licensing and product development | 325 | 300 | 8 | 1 | 1 | ||||||||||
Total revenues | $ | 68,155 | $ | 69,366 | (2 | )% | 100 | % | 100 | % | |||||
Sales to the infectious disease testing market increased 12% to $29.2 million in 2006, primarily as a result of higher sales of our OraQuick® HIV-1/2 test in both the public health and hospital markets through our distributor, Abbott. OraQuick® and OraSure® sales during 2006 totaled $25.6 million and $3.6 million, as compared to $21.6 million and $4.4 million in 2005, respectively.
The table below shows a breakdown of our total OraQuick® revenues (in thousands, except %) during 2006 and 2005.
| Years ended December 31, | % Change | ||||||||
Customers | 2006 | 2005 | |||||||
Direct to U.S. Public Health | $ | 15,268 | $ | 8,292 | 84 | % | |||
Abbott | 6,897 | 4,929 | 40 | ||||||
SAMHSA | 406 | 3,741 | (89 | ) | |||||
CDC | 1,291 | 2,322 | (44 | ) | |||||
International | 1,694 | 1,530 | 11 | ||||||
Direct to Hospitals | — | 740 | — | ||||||
Total OraQuick® revenues | $25,556 | $21,554 | 19% | ||||||
During 2006, OraQuick® revenue derived from direct sales to the U.S. public health market increased by 84% as compared to 2005. This increase is the result of continued growth in usage of the OraQuickADVANCE® rapid HIV-1/2 antibody test in public health settings, including city-wide testing programs.
For the year ended December 31, 2006, sales to our hospital distributor, Abbott, grew 40% to $6.9 million, as compared to $4.9 million in 2005. This increase is a result of continued penetration of the hospital market by Abbott. Abbott recently announced that it will sell part of its diagnostics business, including its rights to distribute OraQuickADVANCE®, to General Electric (“GE”). This transaction is expected to close during the first half of 2007. We intend to meet with executives from GE to discuss their plans for the OraQuickADVANCE® product.
During 2006, we shipped a total of $1.7 million in OraQuickADVANCE® HIV-1/2 tests to the CDC and SAMHSA, as compared to $6.1 million in 2005. We believe that federal, state and other governmental agencies
41
Table of Contents
may make future bulk purchases of OraQuickADVANCE® for further distribution to the public health and other markets throughout the United States. There is no assurance, however, that comparable-size bulk purchase orders from these governmental entities or others will be received in the future. Likewise, any delay in receiving or deploying such bulk orders could adversely affect our financial performance.
We believe that our OraQuickADVANCE® device, which is FDA-approved for detecting antibodies to both HIV-1 and HIV-2 in oral fluid, finger-stick and venous whole blood, and plasma samples, and is CLIA-waived for use with all sample types except plasma, provides a significant competitive advantage, thereby enabling us to fully implement a strategy for selling OraQuick® internationally. We recently received notification that our OraQuickADVANCE® product will be recommended to receive a CE mark, pending successful completion of a facility inspection. CE-marking will allow us to sell our product in Europe. Our goal is to complete the facility inspection in the immediate future, secure a CE mark for OraQuickADVANCE®, and then obtain several country-specific registrations in order to permit the launch of this product in Europe as soon as possible.
We are beginning to see evidence that sales of OraQuickADVANCE® are negatively impacting sales of our OraSure® oral fluid collection device in the infectious disease testing market. Some customers who have purchased our OraSure® device for laboratory HIV-1 testing in the past are now electing instead to purchase our OraQuickADVANCE® test. It is not possible at this time to estimate the extent of such change in purchasing patterns or the financial impact of replacing OraSure® sales with sales of our OraQuickADVANCE® test.
Sales to the substance abuse testing market increased 17% to $15.8 million in 2006, as a result of increased sales of both our Intercept® oral fluid drug testing service and our Q.E.D.® rapid oral fluid alcohol test. Intercept® sales increased as a result of continued growth for this product, especially in the workplace and international markets. Increased sales of our Q.E.D.® test are attributed to a change in government regulations, which increased demand for this product.
The table below shows a breakdown of our total Intercept® revenues (in thousands, except %) generated in each market during 2006 and 2005.
| Years ended December 31, | % Change | ||||||||
Market | 2006 | 2005 | |||||||
Workplace testing | $ | 6,616 | $ | 5,661 | 17 | % | |||
Criminal Justice | 2,398 | 2,269 | 6 | ||||||
International | 2,314 | 1,956 | 18 | ||||||
Direct | 728 | 563 | 29 | ||||||
Total Intercept® revenues | $ | 12,056 | $ | 10,449 | 15 | % | |||
We expect continued growth in Intercept® sales in 2007 as customers continue to shift from urine-based to oral-fluid based testing methods. However, our microplate oral fluid drug assays, which are sold for use with the Intercept® collection device, are expected to come under increasing competitive pressure in the future from “home-brew” assays developed internally by our laboratory customers. In addition, our oral fluid microplate assays compete with urine-based homogeneous assays that are run on fully-automated, random access analyzers. We believe our competitors are developing oral fluid tests suitable for use on these fully automated homogeneous assay systems and these assays, if and when they are developed and commercialized, could represent a significant competitive threat to our oral fluid microplate business. In order to meet this competition, we recently signed a letter of intent to negotiate an agreement with Roche Diagnostics to jointly develop and commercialize fully-automated homogeneous oral fluid drugs of abuse assays for use with our Intercept® device.
Sales of our products in the cryosurgical systems market (which includes both the physicians’ office and OTC markets) decreased 24% to $17.3 million in 2006. This decrease was primarily the result of lower sales of our domestic OTC and professional cryosurgical products when compared to 2005.
42
Table of Contents
The table below shows a breakdown of our total cryosurgery revenues (in thousands, except %) generated in each market during 2006 and 2005.
| Years ended December 31, | % | ||||||||
Market | 2006 | 2005 | Change | ||||||
Professional domestic | $ | 5,360 | $ | 5,888 | (9 | )% | |||
Professional international | 2,284 | 2,018 | 13 | ||||||
OTC domestic | 5,174 | 10,560 | (51 | ) | |||||
OTC international | 4,515 | 4,278 | 6 | ||||||
Total cryosurgery revenues | $ | 17,333 | $ | 22,744 | (24 | )% | |||
Our domestic OTC cryosurgical product, called Freeze Off® , is distributed in the United States and Canada by Prestige, owner of the Compound W® line of wart removal products. In September 2006, Prestige announced that it had acquired the Wartner® cryosurgical wart removal product line, which directly competes with the Freeze Off® product in the OTC market. Our distribution agreement with Prestige contains a covenant not to compete which precludes Prestige from acquiring, manufacturing, distributing or selling a cryosurgical product that directly competes with the Freeze Off® product. We notified Prestige that its acquisition of the Wartner product constitutes a material breach of the distribution agreement and that certain of its other actions constitute additional breaches under the agreement. Efforts to resolve this matter through mediation were not successful and an arbitration pursuant to the rules of the American Arbitration Association has been commenced, pursuant to the terms of the agreement. Sales to Prestige were $5.2 million and $11.6 million, in 2006 and 2005, respectively. We are evaluating alternative arrangements for distributing this product in the event a resolution with Prestige cannot be reached. As a result of this ongoing dispute, it is not possible to predict at this time the potential impact this matter may have on sales of Freeze Off® in 2007 or beyond.
In June 2005, we entered into an agreement with SSL under which we manufacture and supply, and SSL distributes on an exclusive basis, the Company’s cryosurgical wart removal product in the OTC footcare market in Europe, Australia and New Zealand. The product is manufactured and sold under SSL’s Scholl and Dr. Scholl trademarks and initially was made available for retail purchase in pharmacies and other outlets in several European countries during the fourth quarter of 2005. Sales to SSL under the distribution agreement were $4.5 million and $3.2 million in 2006 and 2005, respectively. SSL continues to build distribution networks in pharmacies and mass merchandisers throughout Europe and expects to launch the OTC product in additional countries during 2007.We expect revenues from SSL to increase significantly in 2007. During 2007, we have agreed to co-invest in SSL’s marketing activities and will reimburse SSL for certain out-of-pocket costs of advertising and promoting this product in the international OTC market.
We have also launched our OTC cryosurgical wart removal product in Mexico and intend to establish distributor relationships for similar products in OTC markets in other Latin American countries.
Sales of our Histofreezer® product to physicians’ offices in the United States decreased 9% to $5.4 million in 2006, as compared to $5.9 million in 2005. Sales of Histofreezer® in the international market increased 13% to $2.3 million in 2006, as compared to $2.0 million in 2005. We believe these changes were due to fluctuations in distributor ordering patterns and inventory levels during 2006 and, in the case of domestic Histofreezer® sales, increased competition.
We are beginning to see some evidence that sales of our OTC cryosurgical products may reduce the number of individuals that will seek to obtain treatment of their warts by a physician, which in turn could negatively affect sales of our Histofreezer® product in the professional market. However, it is not possible at this time to estimate the magnitude of the financial impact of this change.
43
Table of Contents
Sales to the insurance risk assessment market declined by 18% to $5.6 million in 2006 from $6.8 million in 2005, primarily because of a reduction in the number of applications for life insurance and changes in underwriting requirements. For higher face-value policies, it appears insurance companies are more likely to use a blood test for multiple risk factors, rather than an oral fluid test. We currently expect that our 2007 revenues in this market will decline, or at best, remain at approximately the levels attained in 2006.
Licensing and product development revenues increased 8% to $325,000 in 2006, from $300,000 in 2005. Licensing and product development revenues in both years were primarily related to our collaborative oral fluid research project with the University of Pennsylvania and New York University, under a grant awarded by the National Institutes of Health. This grant expired in June 2006. In January 2007, the Company entered into a collaboration agreement with Schering-Plough Corporation, for the development and promotion of a rapid oral fluid test for the detection of antibodies to the hepatitis C virus (HCV). As a result of this agreement, licensing and product development revenues are expected to increase to approximately $2.0 million during 2007.
Quest and Abbott accounted for 14% and 10% of total revenues for 2006, respectively.
The Company’s gross margin was 64% in 2006, compared to 60% in 2005. Our 2006 gross margin was positively impacted by the absence of a $1.5 million charge related to the Company’s UPlink® assets, which was recorded in 2005, and increased efficiencies in manufacturing operations.
Research and development expenses increased 64% to $8.6 million in 2006, from $5.3 million in 2005, primarily as a result of costs associated with the development of an OraQuick® HCV test, preparation for OTC clinical trials for our OraQuickADVANCE® HIV-1/2 test, a $1.0 million charge for acquired in-process research and development related to future HIV-related products, costs related to additional research personnel hired during 2006 and charges for stock-based compensation expense. Research and development costs are expected to increase by approximately $8.0 million in 2007. The vast majority of this expected increase is related to the clinical trial work associated with the development of our OraQuickADVANCE® HIV-1/2 OTC test and our OraQuick® HCV test. This amount also includes costs expected to be incurred for FDA approval of new cryosurgical offerings, product registrations in foreign countries and development of fully-automated homogeneous oral fluid drugs of abuse assays.
Sales and marketing expenses decreased 1% to $15.9 million in 2006 from $16.1 million in 2005. This decrease was primarily the result of lower market research, commission, consulting and advertising expenses, partially offset by higher staffing costs and charges for stock-based compensation expense. Included in advertising expense was $540,000 and $1.8 million for 2006 and 2005, respectively, paid to Prestige as reimbursement for marketing expenses incurred for the Compound W® Freeze Off® product. We expect that sales and marketing expenses will increase in 2007 as we add sales personnel for the infectious disease market in order to drive OraQuickADVANCE® sales in the public health, hospital and international markets. During 2007, we also expect to co-invest in SSL’s marketing activities and reimburse SSL for certain out-of-pocket costs of advertising and promoting our cryosurgical product in the international OTC market.
General and administrative expenses increased 7% to $13.4 million in 2006 from $12.5 million in 2005. This increase was primarily attributable to charges for stock-based compensation expense, higher staffing costs and certain consulting costs related to implementation of a new enterprise resource planning system. This increase was partially offset by a reduction in legal fees associated with delays in the Schering-Plough litigation and a reduction in rent expense due to the purchase of two of our Bethlehem, PA facilities in June 2006. General and administrative expenses are expected to increase in 2007, as a result of increased legal expense related to both the Prestige dispute and the Schering-Plough patent infringement matter, as well as increased costs related to full-year staffing levels.
Interest expense increased to $405,000 in 2006 from $97,000 in 2005, as a result of higher outstanding debt balances during the second half of the year, resulting from the financing for our purchase of two previously
44
Table of Contents
leased facilities in Bethlehem, PA in June 2006. Interest expense will increase in 2007, as our borrowings for the building purchase will be outstanding for a full year. Interest income increased to $4.1 million in 2006 from $2.2 million in 2005, as a result of higher yields on our investment portfolio and larger balances available for investment.
We purchase some of our cryosurgical products from, or utilize the services of, vendors located in The Netherlands. As a result of the decline in the exchange rate between the United States dollar and the Euro, we recorded a $94,000 loss on foreign currency transactions for the year ended December 31, 2006, versus a $57,000 gain on foreign currency transactions recorded for the year ended December 31, 2005.
During 2006, we provided for income taxes at a rate equivalent to our estimated combined federal and state effective rates. As such, we recorded a $3.8 million provision for income taxes in 2006, which represents a 41.9% effective tax rate. During 2005, a net income tax benefit of $17.7 million was recorded, which reflected the release of a significant portion of the previous valuation allowance on our deferred tax asset. At December 31, 2005, the Company had federal net operating loss (“NOL”) carryforwards of $66.6 million. Prior to December 31, 2005, a valuation allowance had been established for the full amount of the deferred tax asset created by these carryforwards and other items. Based on taxable income in 2005 and future forecasted taxable earnings, a significant portion of the valuation allowance was released in the fourth quarter of 2005. This resulted in the recognition of $26.7 million of the deferred tax asset, of which $18.2 million was recorded as an income tax benefit in the statement of operations and $8.5 million was recorded directly as an increase in stockholders’ equity. Partially offsetting this benefit was an income tax provision of $436,000 that was recorded for the year ended December 31, 2005.
Year Ended December 31, 2005 Compared to December 31, 2004
Total revenues increased 28% to $69.4 million in 2005 from $54.0 million in 2004, primarily as a result of increased sales of our OraQuickADVANCE® rapid HIV-1/2 antibody test, our Intercept® oral fluid collection device and related drug assays, and our international OTC cryosurgical product, partially offset by declines in domestic OTC cryosurgical product revenues and revenues in the insurance risk assessment market. Revenues derived from products sold in countries outside the U.S. were $9.5 million and $6.2 million, or 14% and 11% of total revenues for the years ended December 31, 2005 and 2004, respectively.
The table below shows the amount of our total revenues (in thousands, except %) generated in each of our principal markets and by licensing and product development activities.
| Years ended December 31, | |||||||||||||||
| Dollars | % Change | Percentage of Total Revenues | |||||||||||||
Market | 2005 | 2004 | 2005 | 2004 | |||||||||||
Infectious disease testing | $ | 25,988 | $ | 15,526 | 67 | % | 37 | % | 29 | % | |||||
Substance abuse testing | 13,519 | 10,108 | 34 | 19 | 19 | ||||||||||
Cryosurgical systems | 22,744 | 20,193 | 13 | 33 | 37 | ||||||||||
Insurance risk assessment | 6,815 | 7,777 | (12 | ) | 10 | 14 | |||||||||
Product revenues | 69,066 | 53,604 | 29 | 99 | 99 | ||||||||||
Licensing and product development | 300 | 404 | (26 | ) | 1 | 1 | |||||||||
Total revenues | $ | 69,366 | $ | 54,008 | 28 | % | 100 | % | 100 | % | |||||
Sales to the infectious disease testing market increased 67% to $26.0 million in 2005, primarily as a result of higher sales of our OraQuick® rapid HIV-1/2 antibody test. OraQuick® and OraSure® sales during 2005 totaled $21.6 million and $4.4 million, respectively, as compared to $10.2 million and $5.3 million, respectively, for 2004.
45
Table of Contents
The table below shows a breakdown of our total OraQuick® revenues (in thousands, except %) during 2005 and 2004.
| Years ended December 31, | % Change | ||||||||
Customers | 2005 | 2004 | |||||||
Direct to U.S. Public Health | $ | 8,292 | $ | 4,093 | 103 | % | |||
Abbott | 4,929 | 1,983 | 149 | ||||||
SAMHSA | 3,741 | — | N/A | ||||||
CDC | 2,322 | 2,327 | — | ||||||
International | 1,530 | 1,178 | 30 | ||||||
Direct to Hospitals | 740 | 649 | 14 | ||||||
Total OraQuick® revenues | $ | 21,554 | $ | 10,230 | 111 | % | |||
During 2004, we received a total of $6.3 million in purchase orders from the CDC and SAMHSA for OraQuickADVANCE® rapid HIV-1/2 antibody tests, of which $6.1 million and $72,000 were shipped in 2005 and 2004, respectively.
Sales to the substance abuse testing market increased 34% to $13.5 million in 2005, as a result of higher sales of our Intercept® oral fluid collection device and related drug assays in all marketplaces.
The table below shows a breakdown of our total Intercept® revenues (in thousands, except %) generated in each market during 2005 and 2004.
| Years ended December 31, | % Change | ||||||||
Market | 2005 | 2004 | |||||||
Workplace testing | $ | 5,661 | $ | 3,030 | 87 | % | |||
Criminal Justice | 2,269 | 1,566 | 45 | ||||||
International | 1,956 | 1,684 | 16 | ||||||
Direct | 563 | 378 | 49 | ||||||
Total Intercept® revenues | $ | 10,449 | $ | 6,658 | 57 | % | |||
Revenues from our UPlink® rapid point-of-care oral fluid drug detection system were $286,000 and $564,000 in 2005 and 2004, respectively. As part of a strategic business review we completed in late 2004, we concluded that the roadside drugs-of-abuse testing market for UPlink® may not be as attractive as a number of other opportunities we are pursuing. During the first half of 2005, we explored our options with respect to the UPlink® product, including transitioning the manufacturing of the product to our distribution partner, Dräger Safety. Throughout this period, we were not able to reach an agreement with Dräger Safety or determine an alternative outlet for this product. In addition, we were advised that Dräger will no longer promote the sale of the UPlink® product. As a result, we recorded a $1.5 million charge in June 2005 to reflect a provision on inventory and fixed assets related to our UPlink® product. We subsequently terminated our existing research, development and distribution agreements with Dräger for the UPlink® product.
Sales of our products in the cryosurgical systems market (which includes both the physicians’ office and OTC markets) increased 13% to $22.7 million in 2005.
46
Table of Contents
The table below shows a breakdown of our total cryosurgery revenues (in thousands, except %) generated in each market during 2005 and 2004.
| Years ended December 31, | % Change | ||||||||
Market | 2005 | 2004 | |||||||
Professional domestic | $ | 5,888 | $ | 5,225 | 13 | % | |||
Professional international | 2,018 | 1,634 | 24 | ||||||
OTC domestic | 10,560 | 13,334 | (21 | ) | |||||
OTC international | 4,278 | — | N/A | ||||||
Total cryosurgery revenues | $ | 22,744 | $ | 20,193 | 13 | % | |||
The increase in total cryosurgery revenues was primarily due to the international launch of our OTC cryosurgical product pursuant to our agreement with SSL, the launch of the Freeze Off® product by Prestige in Canada, and an increase in sales of Histofreezer® to both United States and international physicians’ offices. This increase was partially offset by a reduction in sales of the Freeze Off® product to Prestige for distribution in the United States, to $10.6 million in 2005, compared to $13.3 million during 2004.
The Freeze Off® product is being sold under Prestige’s Compound W® trademark. The distribution agreement with Prestige, which was initiated in 2003, requires minimum purchases of at least $2.0 million each year over the life of the contract in order for Prestige to maintain its exclusive distribution rights to the OTC market in the United States. During the second half of 2005, Prestige also launched our OTC cryosurgical product in Canada. Sales of our cryosurgical product to Prestige for distribution in Canada were $1.0 million in 2005.
In June 2005, we entered into an agreement with SSL under which we manufacture and supply, and SSL distributes on an exclusive basis, the Company’s cryosurgical wart removal product in the OTC footcare market in Europe, Australia and New Zealand. The product is manufactured and sold under SSL’s Scholl and Dr. Scholl trademarks, and was made initially available for retail purchase in pharmacies and retail outlets in several European countries during the fourth quarter of 2005. Sales to SSL under the distribution agreement were $3.2 million in 2005.
Sales of our Histofreezer® product to physicians’ offices in the U.S. and international markets increased 13% and 24% to $5.9 million and $2.0 million, respectively, in 2005, when compared to 2004, primarily as a result of higher distributor purchases.
Sales to the insurance risk assessment market declined by 12% to $6.8 million in 2005 from $7.8 million in 2004, primarily as a result of decreased OraSure® device purchases by our insurance lab testing partners. We believe this decrease was a result of an overall reduction in life insurance application activity in the United States and changes in underwriting requirements.
Licensing and product development revenues decreased 26% to $300,000 in 2005, from $404,000 in the comparable period in 2004. Licensing and product development revenues in both years were primarily related to our collaborative oral fluid research project with the University of Pennsylvania and New York University, under a grant awarded by the National Institutes of Health.
We had two customers, Prestige and Quest, which accounted for 17% and 13% of total revenues for 2005, respectively.
The Company’s gross margin was 60% in 2005, compared to 59% in 2004. Our 2005 gross margin was positively impacted by more efficient utilization of the Company’s manufacturing capacity and renegotiated terms for the assembly and supply of the U.S. OTC cryosurgical product, offset by a less favorable product sales mix and the $1.5 million charge related to the Company’s UPlink® assets.
47
Table of Contents
Research and development expenses decreased 13% to $5.3 million in 2005, from $6.1 million in 2004, primarily as a result of lower overall staffing costs and lower expenses for clinical trials, partially offset by fees paid and restricted stock granted to recruit and relocate the Company’s new Chief Science Officer and Senior Vice President, Regulatory Affairs/Quality Assurance.
Sales and marketing expenses increased 6% to $16.1 million in 2005 from $15.2 million in 2004. This increase was primarily the result of increased levels of staffing, market research, travel and commission expenses, partially offset by lower advertising expenses. Included in advertising expenses was $1.8 million and $2.9 million for 2005 and 2004, respectively, paid to Prestige as reimbursement for marketing expenses incurred for the Compound W® Freeze Off® product.
General and administrative expenses increased 4% to $12.5 million in 2005 from $12.0 million in 2004. This increase was primarily attributable to legal fees associated with the Schering-Plough litigation, increased amortization of restricted stock grants to management, and increased staffing related expenses. This increase was partially offset by a reduction in consulting expenses, a reduction in transition expenses related to the Company’s former Chief Executive Officer, and a reduction in rent expense due to the expiration of the lease for our Oregon facilities in January 2005. Legal fees associated with the Schering-Plough patent infringement litigation were $2.4 million and $1.2 million in 2005 and 2004, respectively.
Interest expense decreased to $97,000 in 2005 from $134,000 in 2004, as a result of lower outstanding debt balances. Interest income increased to $2.2 million in 2005 from $984,000 in 2004, as a result of higher yields on our investment portfolio and larger balances available for investment.
A gain on foreign currency transactions of $57,000 was recorded for the year ended December 31, 2005, versus a loss on foreign currency transactions of $53,000 recorded for the year ended December 31, 2004.
During the year ended December 31, 2005, a net income tax benefit of $17.7 million was recorded, while no provision or benefit was recorded in 2004. The tax benefit recorded during 2005 reflects the release of a significant portion of the valuation allowance on our deferred tax asset. At December 31, 2005, the Company had federal net operating loss (“NOL”) carryforwards of $66.6 million. Our ability to use the NOLs and tax credit carryforwards to offset future income tax obligations could be limited by changes in the ownership of the Company’s capital stock. Internal Revenue Code Section 382 (“Section 382”) contains provisions that limit the amount of NOLs and tax credit carryforwards that can be used in any given year, in the event of a significant ownership change. In the fourth quarter of 2005, the Company engaged in an analysis, with the assistance of independent tax specialists, to determine if any Section 382 ownership changes have occurred that would limit the amount of NOLs that could be utilized to offset future taxable income. As a result of this analysis, the Company concluded that prior period ownership changes may impose a limitation on the amount of NOLs that can be utilized in a given year. The Company does not believe, however, that this limitation will impair our future ability to utilize NOLs to offset our forecasted taxable income or to realize the related deferred tax asset.
Prior to December 31, 2005, a valuation allowance had been established for the full amount of the deferred tax asset created by these carryforwards and other items. Based on the 2005 and forecasted taxable earnings, a significant portion of the valuation allowance was released in the fourth quarter of 2005, resulting in the recognition of $26.7 million of the deferred tax asset of which $18.2 million was recorded as an income tax benefit in the statement of operations and $8.5 million was recorded directly as an increase in stockholders’ equity. Partially offsetting this benefit was an income tax provision of $436,000 that was recorded for the year ended December 31, 2005 related to certain state income taxes and federal alternative minimum tax.
48
Table of Contents
Liquidity and Capital Resources
| December 31, 2006 | December 31, 2005 | |||||
| (In thousands) | ||||||
Cash and cash equivalents | $ | 19,950 | $ | 32,827 | ||
Short-term investments | 71,051 | 44,793 | ||||
Working capital | 95,979 | 90,670 | ||||
Our cash, cash equivalents and short-term investments increased $13.4 million during 2006 to $91.0 million at December 31, 2006, primarily as a result of $16.9 million in positive cash flow from operating activities, $10.0 million in borrowings of long-term debt, and $457,000 in proceeds from the exercise of stock options, partially offset by $701,000 of debt repayments, $12.6 million of property and equipment purchases, and $632,000 associated with the retirement of common stock to pay minimum tax withholding obligations on the vesting of restricted shares. At December 31, 2006, our working capital was $96.0 million.
Net cash provided by operating activities was $16.9 million in 2006, primarily generated by our net income of $5.3 million for the year, non-cash charges for depreciation and amortization of $1.9 million, stock-based compensation expense of $5.6 million, and a deferred income tax provision of $3.1 million resulting from utilization of our NOL carryforwards. Other factors which contributed to the $16.9 million in net cash provided by operating activities at December 31, 2006, also included a non-cash provision for excess and obsolete inventories of $750,000, $1.0 million in acquired in-process technology, an increase of $600,000 in accounts payable and accrued expenses primarily related to increased accruals for royalties, legal fees and customer prepayments, and a decrease in accounts receivable of $1.3 million resulting from increased collection efforts and lower outstanding balances due from Prestige and SSL. Offsetting these sources of operating cash were a $2.2 million increase in inventories primarily related to higher levels of raw material purchases associated with our OTC cryosurgical product for SSL and our OraQuickADVANCE® test, and a $453,000 increase in prepaid expenses resulting from the timing of prepayments for insurance and real estate taxes.
Net cash used in investing activities during 2006 was $38.9 million, of which $26.1 million was for the purchase of short-term investments. We invested $12.6 million in additions to property and equipment, of which $9.1 million related to the purchase of our two previously leased facilities in Bethlehem, PA. We also paid $200,000 for certain acquired in-process technology.
During the year ending December 31, 2007, we expect to invest $7.5 million in capital expenditures, primarily to purchase additional equipment, upgrade certain older equipment and make improvements to our facilities.
Net cash provided by financing activities was $9.1 million for the year ended December 31 2006, reflecting the $10.0 million in new borrowings from Comerica Bank to finance the purchase of our two previously leased facilities and $457,000 received from the exercise of stock options. Partially offsetting these sources of cash were $701,000 of loan principal repayments and $632,000 used for the purchase and retirement of common stock.
During 2006, we had in place an $11.9 million credit facility (the “Credit Facility”) with Comerica Bank, which is comprised of an $887,000 mortgage loan, a $3.0 million term loan, a $4.0 million non-revolving line of credit for the purchase of both capital equipment and software, and a $4.0 million revolving working capital line of credit. Interest on outstanding borrowings under the non-revolving line of credit accrues at a rate, selected at our option, equal to the bank’s prime rate, 180-day or 360-day LIBOR plus 2.625%, or the 4-year Treasury Note rate plus 2.30%, determined at the time of initial borrowing. Interest on outstanding borrowings under the revolving working capital line of credit accrues at a rate, selected at our option, equal to the bank’s prime rate less 0.25%, or 30-day LIBOR plus 2.55%, determined at the time of initial borrowing.
On June 27, 2006, we executed an amendment to our Credit Facility, pursuant to which we are permitted to borrow up to an additional $15.0 million in advances in order to fund the purchase and future expansion of two
49
Table of Contents
previously leased facilities in Bethlehem, Pennsylvania. On June 29, 2006, we borrowed $10.0 million under the terms of this Credit Facility, as amended, and purchased our two Bethlehem facilities. We can borrow the remaining $5.0 million at any time before June 30, 2007. At our option, interest on outstanding borrowings is payable monthly at either a fixed rate equal to the five-year U.S. Treasury Note rate plus 1.03% to 1.73%, or a variable rate equal to the 30, 180, or 360-day LIBOR rate plus 0.55% to 1.25%. In each case, the interest rate is determined at the date of the advance and is based upon the amount of cash and cash equivalents we invest and retain at Comerica Securities, Inc. We can also choose the fixed rate option, without penalty, at the expiration of a previously elected LIBOR period. Principal is repayable in periodic installments, based upon the rate option that we elect, with the remaining balance of unpaid principal due on June 27, 2011. This amendment also extended the maturity date of our $4.0 million revolving working capital line of credit to June 29, 2007. All other terms of the Credit Facility, as previously amended, remain in effect, except for our financial covenant related to liquidity, which was modified to require a minimum liquidity, as defined by Comerica, of not less than $25.0 million, of which at least $15.0 million must be held by Comerica or its affiliates.
At December 31, 2006, interest on the new $10.0 million borrowing was payable monthly, at the 360-day LIBOR rate plus 0.9%, or 6.1894%. Principal is repayable in installments, due at the end of each LIBOR rate period, based upon a twenty-year amortization schedule and the number of months in the expiring LIBOR rate period. Accordingly, on December 27, 2007, we will be required to make a $500,000 principal repayment and the interest rate on this loan will reset. The outstanding balance of the loan at December 31, 2006 was $9.75 million.
As of December 31, 2006, we had no outstanding borrowings under the $3.0 million term loan, the $4.0 million non-revolving line of credit, or the $4.0 million revolving working capital line of credit.
All borrowings under the Credit Facility are collateralized by a first priority security interest in all of our assets, including present and future accounts receivable, chattel paper, contracts and contract rights, equipment and accessories, general intangibles, investments, instruments, inventories, and a mortgage on our three facilities in Bethlehem, Pennsylvania. Borrowings under the equipment and software non-revolving line and the revolving working capital line are limited to commercially standard percentages of equipment and software purchases and accounts receivable, respectively. The Credit Facility contains certain covenants that set forth minimum requirements for our quick ratio, liquidity and tangible net worth. We were in full compliance with all covenants at December 31, 2006 and expect to remain in compliance with all covenants during 2007. The Credit Facility also restricts our ability to pay dividends, to make certain investments, to incur additional indebtedness, to sell or otherwise dispose of a substantial portion of assets and to merge or consolidate operations with an unaffiliated entity, without the consent of Comerica.
At December 31, 2006, we had NOL carryforwards of $53.0 million for federal income tax purposes. During the fourth quarter of 2005, the Company retained independent tax specialists to perform an ownership change study and analysis to determine the annual limitation amount applicable to utilization of the NOL carryforwards due to past ownership changes, as defined in Section 382 of the Internal Revenue Code. As a result of this study, we do not believe that the ownership change limitations would impair our ability to use our NOLs against our current forecasted taxable income.
The combination of our current cash position, cash flow from operations and available borrowings under our Credit Facility is expected to be sufficient to fund our operating and capital needs for at least the next twelve months. However, our cash requirements may vary materially from those now planned due to many factors, including, but not limited to, the scope and timing of strategic acquisitions, the cost and timing of the expansion of our manufacturing capacity, the progress of our research and development programs, the scope and results of clinical testing, the magnitude of capital expenditures, changes in existing and potential relationships with business partners, the time and cost of obtaining regulatory approvals, the costs involved in obtaining and enforcing patents, proprietary rights and any necessary licenses, the cost and timing of expansion of sales and marketing activities, the timing of market launch of new products, market acceptance of new products, competing technological and market developments and other factors.
50
Table of Contents
Recent Accounting Pronouncements
In July 2006, the Financial Accounting Standards Board (“FASB”) issued FASB Interpretation (“FIN”) No. 48, “Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109,” which clarifies what criteria must be met prior to recognition of the financial statement benefit of a position taken in a tax return. FIN No. 48 will require companies to include additional qualitative and quantitative disclosures within their financial statements. The disclosures will include potential tax benefits from positions recognized for tax return purposes but not recognized for financial reporting purposes, as well as a tabular presentation of significant changes in such benefits during each period. The disclosures will also include a discussion of the nature of uncertainties, factors that could cause a change, and an estimated range of reasonably possible changes in tax uncertainties. FIN No. 48 will require a company to recognize a financial statement benefit for a position taken for tax return purposes when it will be more-likely-than-not that the position will be sustained. FIN No. 48 is effective for fiscal years beginning after December 15, 2006. We are currently assessing the impact FIN No. 48 will have on our financial statements.
In September 2006, the United States Securities and Exchange Commission (“SEC”) issued Staff Accounting Bulletin (“SAB”) No. 108, “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements.” SAB No. 108 requires companies to evaluate the materiality of identified unadjusted errors using both the income statement approach and the balance sheet approach. In the initial year of adoption, if a company determines that an adjustment to prior year financial statements is required under either approach, SAB No. 108 allows for a one-time cumulative-effect adjustment to beginning retained earnings. SAB No. 108 is effective for interim periods of the first fiscal year ending after November 15, 2006. The adoption of SAB No. 108 did not have any impact on our financial statements.
In September 2006, the FASB issued Statement of Financial Accounting Standards (“SFAS”) No. 157,“Fair Value Measurements.” This Statement defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are currently assessing the impact, if any, that SFAS No. 157 will have on our financial statements.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities—Including an amendment of FASB Statement No. 115.” SFAS No. 159 permits entities to elect to measure many financial instruments and certain other items at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be recognized in earnings at each subsequent reporting date. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007. We are currently assessing the impact SFAS No. 159 will have on our financial statements.
Contractual Obligations and Commercial Commitments. The following sets forth our approximate aggregate obligations at December 31, 2006 for future payments under contracts and other contingent commitments, for the years 2007 and beyond:
Contractual Obligations | Total | Payments due by December 31, | |||||||||||||||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | Thereafter | ||||||||||||||||
Long-term debt1 | $ | 10,639,136 | $ | 608,595 | $ | 593,087 | $ | 597,346 | $ | 552,670 | $ | 7,796,730 | $ | 490,708 | |||||||
Operating leases2 | 161,759 | 81,866 | 68,480 | 11,413 | — | — | |||||||||||||||
Employment contracts3 | 2,529,425 | 1,804,625 | 724,800 | — | — | — | — | ||||||||||||||
Purchase obligations4 | 5,349,799 | 5,349,799 | — | — | — | — | — | ||||||||||||||
Minimum commitments under contracts5 | 5,791,667 | 500,000 | 500,000 | 500,000 | 500,000 | 500,000 | 3,291,667 | ||||||||||||||
Total contractual obligations | $ | 24,471,786 | $ | 8,344,885 | $ | 1,886,367 | $ | 1,108,759 | $ | 1,052,670 | $ | 8,296,730 | $ | 3,782,375 | |||||||
51
Table of Contents
| 1 | Represents principal repayments required under notes payable to our lenders. See Note 8 to the financial statements included herein. |
| 2 | Represents payments required under our operating leases. |
| 3 | Represents salary payments payable under the terms of employment agreements executed by us with certain executives. See Note 11 to the financial statements included herein. |
| 4 | Represents payments required by non-cancellable purchase orders related to inventory, capital expenditures and other goods or services. See Note 11 to the financial statements included herein. |
| 5 | Represents payments required pursuant to certain, licensing agreements executed by the Company. These agreements are cancellable within a specified number of days after communication by the Company of its intent to terminate. See Note 11 to the financial statements included herein. Additional payments of up to $5,500,000 may be required pursuant to one of these licensing agreements for the achievement of specific development and/or commercial milestones. |
Off-Balance Sheet Arrangements. We do not have any off-balance sheet arrangements, as defined in Item 303(a) (4) (ii) of Regulation S-K under the Securities Exchange Act of 1934, as amended.
Critical Accounting Policies and Estimates
This Management’s Discussion and Analysis of Financial Condition and Results of Operations discusses our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires that we make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. On an on-going basis, we evaluate our judgments and estimates, including those related to bad debts, inventories, investments, intangible assets, income taxes, revenue recognition, restructuring costs, contingencies and litigation. We base our judgments and estimates on historical experience and on various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Our significant accounting policies are described in Note 2 to the financial statements included in Item 15 of this Annual Report. We consider the following accounting estimates, which have been discussed with our Audit Committee, to be most critical in understanding the more complex judgments that are involved in preparing our financial statements and the uncertainties that could impact our results of operations, financial condition and cash flows.
Revenue Recognition. We follow SAB No. 104, “Revenue Recognition.” This bulletin draws on existing accounting rules and provides specific guidance on revenue recognition for up-front non-refundable licensing and development fees. We license certain products or technology to outside third parties, in return for which we receive up-front licensing fees. Some of these fees can be significant. In accordance with SAB No. 104, we recognize this revenue ratably over the related license period.
We also enter into research and development contracts with corporate, government and/or private entities. These contracts generally provide for payments to us upon achievement of certain research or development milestones. Product development revenues from these contracts are recognized only if the specified milestone is achieved and accepted by the customer and payment from the customer is probable. Any amounts received prior to the performance of product development efforts are recorded as deferred revenues. Recognition of revenue under these contracts can be sporadic, as it is the result of achieving specific research and development milestones. Furthermore, revenue from future milestone payments will not be recognized if the underlying research and development milestone is not achieved.
52
Table of Contents
We recognize product revenues when there is persuasive evidence that an arrangement exists, the price is fixed or determinable, title has passed and collection is reasonably assured. Product revenues are net of allowances for any discounts or rebates. We do not grant price protection or product return rights to our customers, except for warranty returns. Where a product fails to comply with its limited warranty, we can either replace the product or provide the customer with a refund of the purchase price or credit against future purchases. Historically, returns arising from warranty issues have been infrequent and immaterial. Accordingly, we expense warranty returns as incurred. While such returns have been immaterial in the past, we cannot guarantee that we will continue to experience the same rate of warranty claims as we have in the past. Any significant increase in product warranty claims could have a material adverse impact on our operating results for the period in which the claims occur.
Allowance for Uncollectible Accounts Receivable. Accounts receivable are reduced by an estimated allowance for amounts that may become uncollectible in the future. On an ongoing basis, we perform credit evaluations of our customers and adjust credit limits based upon the customer’s payment history and creditworthiness, as determined by a review of their current credit information. We also continuously monitor collections and payments from our customers.
Based upon historical experience and any specific customer collection issues that are identified, we use our judgment to establish and evaluate the adequacy of our allowance for estimated credit losses, which was $200,094 at December 31, 2006. While credit losses have been within our expectations and the allowance provided, these losses can vary from period to period ($16,022, ($4,771), and $3,541 in 2006, 2005 and 2004, respectively). Furthermore, there is no assurance that we will experience credit losses at the same rates as we have in the past. Also, at December 31, 2006, $4.5 million, or 44% of our accounts receivable, was due from four major customers. Any significant changes in the liquidity or financial position of these customers, or others, could have a material adverse impact on the collectibility of our accounts receivable and future operating results.
Inventories. Our inventories are valued at the lower of cost or market, determined on a first-in, first-out basis, and include the cost of raw materials, labor and overhead. The majority of our inventories are subject to expiration dating. We continually evaluate the carrying value of our inventories and when, in the opinion of management, factors indicate that impairment has occurred, either a reserve is established against the inventories’ carrying value or the inventories are completely written off. We base these decisions on the level of inventories on hand in relation to our estimated forecast of product demand, production requirements over the next twelve months and the expiration dates of raw materials and finished goods. During 2006, 2005 and 2004, we wrote-off inventory which had a cost of $751,000, $2.1 million and $839,000, respectively, as a result of scrap and product expiration issues and a $1.3 million provision for loss on our UPlink® product in 2005. Although we make every effort to ensure the accuracy of our forecasts of future product demand, any significant unanticipated changes in demand could have a significant impact on the carrying value of our inventories and reported operating results.
Stock-based Compensation. Commencing January 1, 2006, we adopted SFAS No. 123 (revised 2004), “Share-Based Payment,” which requires us to recognize the fair value of equity-based awards as compensation expense in our statement of operations. The fair value of our stock option awards was estimated using a Black-Scholes option valuation model. This valuation model incorporates highly subjective assumptions, such as the expected stock price volatility and the estimated life of each award, in the model’s computations. The fair value of awards, after considering the effect of expected forfeitures, is then amortized, generally on a straight-line basis, over the related vesting period of the award.
Long-lived and Intangible Assets. Our long-lived assets are comprised of property and equipment and an investment in a nonaffiliated entity, and our intangible assets primarily consist of patents and product rights. Together, these assets have a net book value of $24.0 million, or 15.4% of our total assets, at December 31, 2006.
53
Table of Contents
Our investment in a privately-held nonaffiliated company is recorded under the cost method of accounting because we do not have a controlling interest in this company, nor do we have the ability to exert significant influence over the operating and financial policies of this investee company. Property and equipment, patents and product rights are depreciated or amortized on a straight-line basis over their useful lives, which we determine based upon our estimate of the period of time over which each asset will generate revenues. In August 2005, we recorded a $1.5 million intangible asset related to a payment under a license agreement to certain patents related to the Hepatitis C Virus. We recorded an additional $3.0 million related to this license in 2006. Management’s intent in executing this license is to provide for various alternatives for use, including uses in the international market that would not require additional research and development efforts or regulatory approvals. This $4.5 million asset was capitalized based on management’s estimate of the cash flows to be received from future product sales in these international markets. A similar analysis of estimated future cash flows will be prepared upon payment of additional license fees under this agreement, or upon changes in circumstances, to determine the appropriate accounting treatment for payments under this license agreement. An impairment of long-lived or intangible assets could occur whenever events or changes in circumstances indicate that the net book value of these assets may not be recoverable. Events which could trigger an asset impairment include significant underperformance relative to expected historical or projected future operating results, significant changes in the manner of our use of an asset or in our overall business strategy, significant negative industry or economic trends, shortening of product life-cycles or changes in technology, and negative financial performance of the nonaffiliated investee company. If we believe impairment of an asset has occurred, we measure the amount of such impairment by comparing the net book value of the affected assets to the fair value of these assets, which is generally determined based upon the present value of the expected cash flows associated with the use of these assets. If the net book value exceeds the fair value of the impaired assets, we would incur an impairment expense equal to this difference. In June 2005, we recorded a $196,000 provision for loss on our UPlink® fixed assets as a result of our inability to reach an agreement to transfer these assets to our distribution partner or determine an alternative outlet for these assets. We currently believe the future cash flows to be received from all other long-lived and intangible assets will exceed their book value and, as such, we have not recognized any additional impairment losses through December 31, 2006. Any unanticipated significant impairment in the future, however, could have a material adverse impact to our balance sheet and future operating results.
Deferred Tax Assets. At December 31, 2006, we had federal NOL carryforwards of $53.0 million. The net deferred tax asset associated with these NOLs and other temporary differences was $23.5 million at December 31, 2006. In assessing the realizability of deferred tax assets, we consider whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the period in which those temporary differences become deductible or the NOLs and credit carryforwards can be utilized. We consider the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment.
Our ability to use our NOL carryforwards to offset future federal income tax obligations, could be limited by changes in the ownership of our stock. Internal Revenue Code (“IRC”) Section 382 contains provisions that limit the amount of federal NOL carryforwards that can be used in any given year in the event of specified occurrences, including significant ownership changes. In the fourth quarter of 2005, the Company completed an analysis, with the assistance of independent tax specialists, to determine if any IRC Section 382 ownership changes have occurred that would limit the amount of NOLs that could be utilized to offset future taxable income. As a result of this analysis, the Company concluded that prior period ownership changes may impose a limitation on the amount of NOLs that can be utilized in a given year. The Company does not believe, however, that this limitation will impair our future ability to utilize NOLs to offset our forecasted taxable income or to realize the related deferred tax asset.
Prior to December 31, 2005, a valuation allowance had been established for the full amount of the deferred tax asset created by these carryforwards and other items. Based on our 2005 results and our projections for future taxable income over the periods in which the deferred tax assets are deductible or the NOLs and credit carryforwards can be utilized, we believe a significant portion of the deferred tax asset was realizable at
54
Table of Contents
December 31, 2005. As such, we recorded the estimated net realizable value of the deferred tax asset at December 31, 2005 and have begun providing for income taxes at a rate equal to our combined federal and state effective rates. Subsequent revisions to the estimated net realizable value of the deferred tax asset could cause our provision for income taxes to vary significantly from period to period.
Contingencies. In the ordinary course of business, we have entered into various contractual relationships with strategic corporate partners, customers, distributors, research laboratories and universities, licensors, licensees, suppliers, vendors and other parties. As such, we could be subject to litigation, claims or assessments arising from any or all of these relationships. We account for contingencies such as these in accordance with SFAS No. 5, “Accounting for Contingencies.” SFAS No. 5 requires us to record an estimated loss contingency when information available prior to issuance of our financial statements indicates that it is probable that an asset has been impaired or a liability has been incurred at the date of the financial statements and the amount of the loss can be reasonably estimated. Accounting for contingencies arising from contractual or legal proceedings requires that we use our best judgment when estimating an accrual related to such contingencies. As additional information becomes known, our accrual for a loss contingency could fluctuate, thereby creating variability in our results of operations from period to period. Likewise, an actual loss arising from a loss contingency which significantly exceeds the amount accrued for in our financial statements could have a material adverse impact on our operating results for the period in which such actual loss becomes known.
ITEM 7A. Quantitative and Qualitative Disclosures About Market Risk.
We do not hold any amounts of derivative financial instruments or derivative commodity instruments and, accordingly, we have no material derivative risk to report under this Item.
A significant portion of our assets is comprised of certificates of deposit, commercial paper, U.S. government and agency obligations, and U.S. corporate bonds. All such instruments are classified as available-for-sale securities. The primary objective of our investment activities is to preserve principal while maximizing the related income without significantly increasing risk. Even so, some of the securities in which we invest may be subject to market risk. Market risk is the risk that a change in prevailing interest rates may cause the fair value of an investment to fluctuate. As interest rates increase, the fair value of a debt instrument would be expected to decrease. Correspondingly, if interest rates decrease the fair value of a debt instrument would be expected to increase. To minimize market risk, we have the ability to hold such debt instruments to maturity, at which time the debt instrument would be redeemed at its stated or face value. We also typically invest in the shorter end of the maturity spectrum. As such, we do not believe that we have a material exposure to market risk.
At December 31, 2006, approximately $10.5 million of the Company’s long-term debt bears interest at a variable or floating rate tied to either the United States prime rate or the London Interbank Offered Rate. A one percentage point increase in these interest rates would cost the Company approximately $105,000 in additional interest expense.
As of December 31, 2006, we did not have any foreign currency exchange contracts or purchase currency options to hedge local currency cash flows. We have operations in The Netherlands, which are subject to foreign currency fluctuations. As currency rates change, translation of revenues and expenses for these operations from euros to U.S. dollars affects year-to-year comparability of operating results. Sales denominated in a foreign currency represented approximately $135,000 of our total revenues for the year ended December 31, 2006. We do not expect the risk of foreign currency fluctuations to be material in the near future.
ITEM 8. Financial Statements and Supplementary Data.
Information with respect to this Item is contained in our Financial Statements included in Item 15 of this Annual Report on Form 10-K.
55
Table of Contents
ITEM 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
Not applicable.
ITEM 9A. Controls and Procedures.
(a) Evaluation of Disclosure Controls and Procedures.
The Company’s management, with the participation of the Company’s Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the Company’s disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934) as of December 31, 2006. Based on that evaluation, the Company’s management, including such officers, concluded that the Company’s disclosure controls and procedures are adequate and effective to ensure that information required to be disclosed by the Company in the reports that we file or submit under the Securities Exchange Act of 1934 is accumulated and communicated to the Company’s management, including the Chief Executive Officer and Chief Financial Officer, to allow timely decisions regarding required disclosure and is recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission.
(b) Management’s Report on Internal Control Over Financial Reporting.
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a(f) and 15d-15(f) under the Securities Exchange Act of 1934. Under the supervision and with the participation of the Company’s management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework inInternal Control—Integrated Frameworkissued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework, our management concluded that our internal control over financial reporting was effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles as of December 31, 2006.
Our management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2006 has been audited by KPMG LLP, an independent registered public accounting firm, as stated in their report, which is included below.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
(c) Changes in Internal Control Over Financial Reporting.
There was no change in the Company’s internal control over financial reporting identified in connection with the evaluation referred to in paragraph (a) above that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
(d) Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
OraSure Technologies, Inc.:
We have audited management’s assessment, included in the accompanying Management’s Report on Internal Control over Financial Reporting, that OraSure Technologies, Inc. maintained effective internal control over financial reporting as of December 31, 2006, based on criteria established inInternal Control—Integrated
56
Table of Contents
Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). OraSure Technologies, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting. Our responsibility is to express an opinion on management’s assessment and an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, evaluating management’s assessment, testing and evaluating the design and operating effectiveness of internal control, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, management’s assessment that OraSure Technologies, Inc. maintained effective internal control over financial reporting as of December 31, 2006, is fairly stated, in all material respects, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Also, in our opinion, OraSure Technologies, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2006, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of OraSure Technologies, Inc. as of December 31, 2006 and 2005, and the related statements of operations, stockholders’ equity and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2006, and our report dated March 16, 2007 expressed an unqualified opinion on those financial statements.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 16, 2007
Not applicable.
57
Table of Contents
We have omitted from Part III the information that will appear in our Definitive Proxy Statement for our 2007 Annual Meeting of Stockholders (the “Proxy Statement”), which will be filed within 120 days after the end of our fiscal year pursuant to Regulation 14A.
ITEM 10. Directors, Executive Officers and Corporate Governance.
Certain information required by this Item is incorporated by reference to the information under the captions, “Corporate Governance—Committees of the Board—Audit Committee,” “Election of Directors,” “Executive Officers,” and “Section 16(a) Beneficial Ownership Reporting Compliance,” in the Proxy Statement.
Our Board of Directors has adopted a Code of Business Conduct and Ethics that applies to our principal executive officer, principal financial officer and principal accounting officer, as well as to the members of our Board of Directors and our other officers and employees. This Code of Business Conduct and Ethics is available on our website at www.orasure.com. We intend to satisfy the amendment and waiver disclosure requirements under applicable securities regulations by posting any amendments of, or waivers to, the Code of Business Conduct and Ethics on our website.
ITEM 11. Executive Compensation.
The information required by this Item is incorporated by reference to the information under the caption, “Executive Compensation,” in the Proxy Statement.
| ITEM 12. Security | Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The information required by this Item with respect to the securities ownership of certain beneficial owners and management, and equity compensation plan information, is incorporated by reference to the information under the captions, “Principal Stockholders” and “Equity Compensation Plan Information,” respectively, in the Proxy Statement.
ITEM 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this Item is incorporated by reference to the information under the captions, “Transactions with Related Persons” and “Corporate Governance—Director Independence,” in the Proxy Statement.
ITEM 14. Principal Accountant Fees and Services.
The information required by this Item is incorporated by reference to the information under the caption, “Audit Fees; Audit-Related Fees; Tax Fees; All Other Fees,” in the Proxy Statement.
58
Table of Contents
ITEM 15. Exhibits and Financial Statement Schedules.
(a)(1) and (a)(2).Financial Statements and Schedules. For a list of the Financial Statements filed herewith, see the Index to Financial Statements following the signature page to this Annual Report. No schedules are included with the Financial Statements because the required information is inapplicable or is presented in the Financial Statements or related notes thereto.
(a)(3).Exhibits. See Index to Exhibits following the Financial Statements in this Annual Report.
59
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 16, 2007.
| ORASURE TECHNOLOGIES, INC. | ||
| By: | /s/ DOUGLAS A. MICHELS | |
| Douglas A. Michels | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed on March 16, 2007, by the following persons on behalf of the Registrant and in the capacities indicated.
SIGNATURE | TITLE | |
/s/ DOUGLAS A. MICHELS Douglas A. Michels | President, Chief Executive Officer and Director (Principal Executive Officer) | |
/s/ RONALD H. SPAIR Ronald H. Spair | Chief Operating Officer, Chief Financial Officer and Director (Principal Financial Officer) | |
/s/ MARK L. KUNA Mark L. Kuna | Senior Vice President, Finance and Controller (Principal Accounting Officer) | |
*MICHAEL CELANO Michael Celano | Director | |
*JACK GOLDSTEIN, PH.D. Jack Goldstein, Ph.D. | Director | |
Frank G. Hausmann | Director | |
*RONNY B. LANCASTER Ronny B. Lancaster | Director | |
*CHARLES W. PATRICK Charles W. Patrick | Director | |
*ROGER L. PRINGLE Roger L. Pringle | Director | |
*DOUGLAS G. WATSON Douglas G. Watson | Director | |
*By: | /s/ JACK E. JERRETT | |
Jack E. Jerrett (Attorney-in-Fact) | ||
60
Table of Contents
| Page | ||
| F-2 | ||
| F-3 | ||
| F-4 | ||
Statements of Stockholders’ Equity and Comprehensive Income (Loss) | F-5 | |
| F-6 | ||
| F-7 | ||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders
OraSure Technologies, Inc.:
We have audited the accompanying balance sheets of OraSure Technologies, Inc. as of December 31, 2006 and 2005, and the related statements of operations, stockholders’ equity and comprehensive income (loss), and cash flows for each of the years in the three-year period ended December 31, 2006. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of OraSure Technologies, Inc. as of December 31, 2006 and 2005, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2006, in conformity with U.S. generally accepted accounting principles.
As discussed in Note 2 to the financial statements, effective January 1, 2006, the Company adopted the fair value method of accounting for stock-based compensation as required by Statement of Financial Accounting Standards No. 123R,Share-Based Payment.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the effectiveness of OraSure Technologies, Inc.’s internal control over financial reporting as of December 31, 2006, based on criteria established inInternal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated March 16, 2007 expressed an unqualified opinion on management’s assessment of, and the effective operation of, internal control over financial reporting.
/s/ KPMG LLP
Philadelphia, Pennsylvania
March 16, 2007
F-2
Table of Contents
BALANCE SHEETS
| December 31, | ||||||||
| 2006 | 2005 | |||||||
| ASSETS | ||||||||
CURRENT ASSETS: | ||||||||
Cash and cash equivalents | $ | 19,949,821 | $ | 32,826,740 | ||||
Short-term investments | 71,051,482 | 44,793,046 | ||||||
Accounts receivable, net of allowance for doubtful accounts of $200,094 | 10,357,287 | 11,602,127 | ||||||
Inventories | 5,534,567 | 4,128,029 | ||||||
Deferred income taxes | 3,675,785 | 6,503,946 | ||||||
Prepaid expenses and other | 1,989,882 | 1,553,545 | ||||||
Total current assets | 112,558,824 | 101,407,433 | ||||||
PROPERTY AND EQUIPMENT, net | 17,374,718 | 5,815,233 | ||||||
PATENTS AND PRODUCT RIGHTS, net | 6,328,344 | 2,879,958 | ||||||
DEFERRED INCOME TAXES | 19,845,789 | 20,204,352 | ||||||
OTHER ASSETS | 457,788 | 440,227 | ||||||
| $ | 156,565,463 | $ | 130,747,203 | |||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
CURRENT LIABILITIES: | ||||||||
Current portion of long-term debt | $ | 608,595 | $ | 456,541 | ||||
Accounts payable | 3,311,968 | 2,546,621 | ||||||
Accrued expenses and other | 12,659,149 | 7,733,941 | ||||||
Total current liabilities | 16,579,712 | 10,737,103 | ||||||
LONG-TERM DEBT | 10,030,541 | 884,021 | ||||||
OTHER LIABILITIES | 451,235 | 207,037 | ||||||
COMMITMENTS AND CONTINGENCIES (Note 11) | ||||||||
STOCKHOLDERS’ EQUITY: | ||||||||
Preferred stock, par value $0.000001; 25,000,000 shares authorized, | — | — | ||||||
Common stock, par value $0.000001; 120,000,000 shares authorized, 45,994,752 and 45,775,625 shares issued and outstanding | 46 | 46 | ||||||
Additional paid-in capital | 228,069,433 | 226,218,469 | ||||||
Deferred compensation | — | (3,334,792 | ) | |||||
Accumulated other comprehensive loss | (151,197 | ) | (282,825 | ) | ||||
Accumulated deficit | (98,414,307 | ) | (103,681,856 | ) | ||||
Total stockholders’ equity | 129,503,975 | 118,919,042 | ||||||
| $ | 156,565,463 | $ | 130,747,203 | |||||
The accompanying notes are an integral part of these statements.
F-3
Table of Contents
STATEMENTS OF OPERATIONS
| For the year ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
REVENUES: | ||||||||||||
Product | $ | 67,830,561 | $ | 69,066,152 | $ | 53,604,124 | ||||||
Licensing and product development | 324,359 | 300,040 | 404,140 | |||||||||
| 68,154,920 | 69,366,192 | 54,008,264 | ||||||||||
COST OF PRODUCTS SOLD | 24,756,195 | 27,973,907 | 22,143,190 | |||||||||
Gross profit | 43,398,725 | 41,392,285 | 31,865,074 | |||||||||
OPERATING EXPENSES: | ||||||||||||
Research and development | 8,647,484 | 5,269,083 | 6,062,275 | |||||||||
Sales and marketing | 15,921,467 | 16,060,413 | 15,154,174 | |||||||||
General and administrative | 13,367,111 | 12,490,074 | 12,005,309 | |||||||||
| 37,936,062 | 33,819,570 | 33,221,758 | ||||||||||
Operating income (loss) | 5,462,663 | 7,572,715 | (1,356,684 | ) | ||||||||
INTEREST EXPENSE | (404,680 | ) | (96,632 | ) | (133,652 | ) | ||||||
INTEREST INCOME | 4,097,860 | 2,185,486 | 983,841 | |||||||||
FOREIGN CURRENCY GAIN (LOSS) | (94,390 | ) | 57,329 | (53,147 | ) | |||||||
Income (loss) before income taxes | 9,061,453 | 9,718,898 | (559,642 | ) | ||||||||
INCOME TAX PROVISION (BENEFIT) | 3,793,904 | (17,729,189 | ) | — | ||||||||
NET INCOME (LOSS) | $ | 5,267,549 | $ | 27,448,087 | $ | (559,642 | ) | |||||
EARNINGS (LOSS) PER SHARE | ||||||||||||
BASIC | $ | 0.11 | $ | 0.61 | $ | (0.01 | ) | |||||
DILUTED | $ | 0.11 | $ | 0.59 | $ | (0.01 | ) | |||||
SHARES USED IN COMPUTING EARNINGS (LOSS) PER SHARE | ||||||||||||
BASIC | 45,909,990 | 45,109,580 | 44,463,861 | |||||||||
DILUTED | 46,580,266 | 46,146,612 | 44,463,861 | |||||||||
The accompanying notes are an integral part of these statements.
F-4
Table of Contents
STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE INCOME (LOSS)
For the years ended December 31, 2006, 2005 and 2004
| Common Stock | Additional Paid-in Capital | Deferred Compensation | Accumulated Other Comprehensive Loss | Accumulated Deficit | Total | |||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||
Balance at January 1, 2004 | 44,260,931 | $ | 44 | $ | 204,867,765 | $ | (614,515 | ) | $ | (173,704 | ) | $ | (130,570,301 | ) | $ | 73,509,289 | ||||||||||
Common stock issued upon exercise | 370,800 | 1 | 1,904,160 | — | — | — | 1,904,161 | |||||||||||||||||||
Restricted stock grants to employees | — | — | 3,176,150 | (3,176,150 | ) | — | — | — | ||||||||||||||||||
Amortization of deferred compensation expense | — | — | — | 874,162 | — | — | 874,162 | |||||||||||||||||||
Comprehensive loss: | ||||||||||||||||||||||||||
Net loss | — | — | — | — | — | (559,642 | ) | (559,642 | ) | |||||||||||||||||
Currency translation adjustment | — | — | — | — | (12,983 | ) | — | (12,983 | ) | |||||||||||||||||
Net unrealized loss on marketable securities | — | — | — | — | (137,982 | ) | — | (137,982 | ) | |||||||||||||||||
Total comprehensive loss | (710,607 | ) | ||||||||||||||||||||||||
Balance at December 31, 2004 | 44,631,731 | 45 | 209,948,075 | (2,916,503 | ) | (324,669 | ) | (131,129,943 | ) | 75,577,005 | ||||||||||||||||
Common stock issued upon exercise | 1,009,794 | 1 | 6,067,868 | — | — | — | 6,067,869 | |||||||||||||||||||
Compensation expense for stock option grants | — | — | 123,916 | — | — | — | 123,916 | |||||||||||||||||||
Vesting of restricted stock | 197,180 | — | — | — | — | — | — | |||||||||||||||||||
Restricted stock grants to employees | — | — | 1,918,343 | (1,918,343 | ) | — | — | — | ||||||||||||||||||
Purchase and retirement of treasury shares | (63,080 | ) | — | (601,347 | ) | — | — | — | (601,347 | ) | ||||||||||||||||
Amortization of deferred compensation expense | — | — | — | 1,500,054 | — | — | 1,500,054 | |||||||||||||||||||
Deferred taxes related to stock options | — | — | 8,761,614 | — | — | — | 8,761,614 | |||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||
Net income | — | — | — | — | — | 27,448,087 | 27,448,087 | |||||||||||||||||||
Currency translation adjustment | — | — | — | — | (39,095 | ) | — | (39,095 | ) | |||||||||||||||||
Unrealized gain on marketable securities, net of tax provision of $26,268 | — | — | — | — | 80,939 | — | 80,939 | |||||||||||||||||||
Total comprehensive income | 27,489,931 | |||||||||||||||||||||||||
Balance at December 31, 2005 | 45,775,625 | 46 | 226,218,469 | (3,334,792 | ) | (282,825 | ) | (103,681,856 | ) | 118,919,042 | ||||||||||||||||
Reclassification of deferred compensation | — | — | (3,334,792 | ) | 3,334,792 | — | — | — | ||||||||||||||||||
Reclassification of liability-classified awards | — | — | (230,659 | ) | — | — | — | (230,659 | ) | |||||||||||||||||
Common stock issued upon exercise | 85,013 | — | 457,334 | — | — | — | 457,334 | |||||||||||||||||||
Vesting of restricted stock | 199,633 | — | — | — | — | — | — | |||||||||||||||||||
Purchase and retirement of treasury shares | (65,519 | ) | — | (631,509 | ) | — | — | — | (631,509 | ) | ||||||||||||||||
Compensation cost for restricted stock | — | — | 1,969,178 | — | — | 1,969,178 | ||||||||||||||||||||
Compensation cost for stock option grants | — | — | 3,621,412 | — | — | — | 3,621,412 | |||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||
Net income | — | — | — | — | — | 5,267,549 | 5,267,549 | |||||||||||||||||||
Currency translation adjustment | — | — | — | — | 6,787 | — | 6,787 | |||||||||||||||||||
Unrealized gain on marketable securities, net of tax provisions of $72,979 | — | — | — | — | 124,841 | — | 124,841 | |||||||||||||||||||
Total comprehensive income | 5,399,177 | |||||||||||||||||||||||||
Balance at December 31, 2006 | 45,994,752 | $ | 46 | $ | 228,069,433 | $ | — | $ | (151,197 | ) | $ | (98,414,307 | ) | $ | 129,503,975 | |||||||||||
The accompanying notes are an integral part of these statements
F-5
Table of Contents
STATEMENTS OF CASH FLOWS
| For the year ended December 31, | ||||||||||||
| 2006 | 2005 | 2004 | ||||||||||
OPERATING ACTIVITIES: | ||||||||||||
Net income (loss) | $ | 5,267,549 | $ | 27,448,087 | $ | (559,642 | ) | |||||
Adjustments to reconcile net income (loss) to net cash provided by operating activities: | ||||||||||||
Deferred income taxes | 3,113,745 | (18,165,068 | ) | — | ||||||||
Stock-based compensation | 5,590,590 | 1,623,970 | 874,162 | |||||||||
Depreciation and amortization | 1,923,275 | 2,346,861 | 2,487,121 | |||||||||
Acquired in-process technology | 1,000,000 | — | — | |||||||||
Provision for loss on property and equipment, net | — | 196,011 | 4,339 | |||||||||
Provision for excess and obsolete inventories | 750,542 | 2,062,855 | 839,130 | |||||||||
Changes in assets and liabilities: | ||||||||||||
Accounts receivable | 1,250,552 | (4,553,469 | ) | 1,159,881 | ||||||||
Inventories | (2,156,302 | ) | (1,240,049 | ) | (1,787,590 | ) | ||||||
Prepaid expenses and other | (452,502 | ) | (360,945 | ) | (272,265 | ) | ||||||
Accounts payable | 465,344 | 457,543 | (1,066,064 | ) | ||||||||
Accrued expenses and other liabilities | 133,011 | 576,540 | 1,759,144 | |||||||||
Net cash provided by operating activities | 16,885,804 | 10,392,336 | 3,438,216 | |||||||||
INVESTING ACTIVITIES: | ||||||||||||
Purchases of property and equipment | (12,643,266 | ) | (2,048,167 | ) | (912,144 | ) | ||||||
Proceeds from the sale of property and equipment | — | — | 66,427 | |||||||||
Purchase of patents, product rights, or acquired in-process technology | (200,000 | ) | (1,800,000 | ) | (600,000 | ) | ||||||
Purchases of short-term investments | (91,494,215 | ) | (54,071,801 | ) | (65,638,600 | ) | ||||||
Proceeds from maturities and redemptions of short-term investments | 65,433,600 | 65,935,674 | 42,226,980 | |||||||||
(Increase) decrease in other assets | — | (24,801 | ) | 80,160 | ||||||||
Net cash (used in) provided by investing activities | (38,903,881 | ) | 7,990,905 | (24,777,177 | ) | |||||||
FINANCING ACTIVITIES: | ||||||||||||
Borrowings of long-term debt | 10,000,000 | — | — | |||||||||
Repayments of long-term debt | (701,426 | ) | (1,116,129 | ) | (1,126,186 | ) | ||||||
Proceeds from issuance of common stock | 457,334 | 6,067,869 | 1,904,161 | |||||||||
Purchase and retirement of common stock | (631,509 | ) | (601,347 | ) | — | |||||||
Net cash provided by financing activities | 9,124,399 | 4,350,393 | 777,975 | |||||||||
EFFECT OF FOREIGN EXCHANGE RATE CHANGES ON CASH | 16,759 | (28,102 | ) | (12,983 | ) | |||||||
NET (DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS | (12,876,919 | ) | 22,705,532 | (20,573,969 | ) | |||||||
CASH AND CASH EQUIVALENTS, BEGINNING OF YEAR | 32,826,740 | 10,121,208 | 30,695,177 | |||||||||
CASH AND CASH EQUIVALENTS, END OF YEAR | $ | 19,949,821 | $ | 32,826,740 | $ | 10,121,208 | ||||||
The accompanying notes are an integral part of these statements.
F-6
Table of Contents
NOTES TO THE FINANCIAL STATEMENTS
| 1. | THE COMPANY: |
We develop, manufacture and market oral specimen collection devices using our proprietary oral fluid technologies, diagnostic products includingin vitro diagnostic tests, and other medical devices. These products are sold in the United States and internationally to various clinical laboratories, hospitals, clinics, community-based organizations and other public health organizations, distributors, government agencies, physicians’ offices, and commercial and industrial entities. One of our products is also sold in the over-the-counter or consumer retail markets in the United States, Canada, Europe and Mexico.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES: |
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
We consider all highly liquid investments with a purchased maturity of ninety days or less to be cash equivalents. As of December 31, 2006 and 2005, cash equivalents consisted of commercial paper, U.S. government agency obligations, state and local government agency obligations, corporate bonds, and certificates of deposit.
Short-term Investments
We consider all short-term investments to be available-for-sale securities, in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 115, “Accounting for Certain Investments in Debt and Equity Securities.” These securities are comprised of certificates of deposits, commercial paper, U.S. government and agency obligations, state and local government agency obligations, and corporate bonds, all with purchased maturities greater than ninety days. Available-for-sale securities are carried at fair value, based upon quoted market prices, with unrealized gains and losses reported in stockholders’ equity as a component of accumulated other comprehensive loss. There were no securities held as of December 31, 2006 in a continuous unrealized loss position for twelve or more months.
F-7
Table of Contents
The following is a summary of our available-for-sale securities at December 31, 2006 and 2005:
| Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | ||||||||||
December 31, 2006 | |||||||||||||
Certificates of deposit | $ | 800,000 | $ | — | $ | — | $ | 800,000 | |||||
Commercial paper | 28,079,352 | 165,064 | — | 28,244,416 | |||||||||
Government and agency bonds | 3,331,455 | — | (4,618 | ) | 3,326,837 | ||||||||
Corporate bonds | 38,713,921 | 2,264 | (35,956 | ) | 38,680,229 | ||||||||
Total available-for-sale securities | $ | 70,924,728 | $ | 167,328 | $ | (40,574 | ) | $ | 71,051,482 | ||||
December 31, 2005 | |||||||||||||
Certificates of deposit | $ | 10,385,000 | $ | — | $ | (464 | ) | $ | 10,384,536 | ||||
Commercial paper | 1,984,999 | — | (1,059 | ) | 1,983,940 | ||||||||
Government and agency bonds | 18,544,871 | — | (43,851 | ) | 18,501,020 | ||||||||
State and local government agency obligations | 75,000 | — | — | 75,000 | |||||||||
Corporate bonds | 13,874,242 | 1,261 | (26,953 | ) | 13,848,550 | ||||||||
Total available-for-sale securities | $ | 44,864,112 | $ | 1,261 | $ | (72,327 | ) | $ | 44,793,046 | ||||
At December 31, 2006, maturities of our available-for-sale securities were as follows: | |||||||||||||
Less than one year | $ | 67,851,766 | $ | 165,064 | $ | (40,574 | ) | $ | 67,976,256 | ||||
One to two years | 3,072,962 | 2,264 | �� | — | 3,075,226 | ||||||||
Total available-for-sale securities | $ | 70,924,728 | $ | 167,328 | $ | (40,574 | ) | $ | 71,051,482 | ||||
Supplemental Cash Flow Information
In 2006, 2005, and 2004, we paid interest of $350,535, $99,728, and $137,112, respectively.
In 2006 and 2005, we paid federal and state income taxes of $695,765 and $170,000, respectively. No income tax payments were made in 2004.
For 2006, 2005, and 2004, we recorded through the statement of operations a reduction in our allowance for doubtful accounts of $61,950, $71,962, and $10,360, respectively. We had write-offs/(recoveries) of $16,022, $(4,771), and $3,541 against the allowance for doubtful accounts in 2006, 2005, and 2004, respectively.
For 2006, 2005, and 2004, we recorded accruals for purchases of property and equipment of $346,258, $57,923, and $72,394, respectively.
In 2006, we recorded a $4,000,000 accrual related to two licensing agreements. In 2004, we recorded a $300,000 accrual related to a new license agreement.
Accounts Receivable
Accounts receivable have been reduced by an allowance for amounts that may become uncollectible in the future. This estimated allowance is based primarily on management’s evaluation of specific balances as the balances become past due, the financial condition of our customers and our historical experience related to write-offs. If not reserved through these specific examination procedures, our policy is to reserve for uncollectible accounts by applying fixed percentages to the aging categories of accounts receivable.
F-8
Table of Contents
Inventories
Inventories are stated at the lower of cost or market determined on a first-in, first-out basis, and include the cost of raw materials, labor and overhead. The majority of our inventories are subject to expiration dating. We continually evaluate quantities on hand and the carrying value of our inventories to determine the need for reserves for excess and obsolete inventories, based primarily on the estimated forecast of product sales. When factors indicate that impairment has occurred, either a reserve is established against the inventories’ carrying value or the inventories are completely written off, as in the case of lapsing expiration dates. In addition to reserving for these items identified through specific identification procedures, we also reserve for unidentified scrap or spoilage under a fixed-formula methodology. We currently buy a portion of our cryosurgical product line from a foreign vendor, with such purchases payable in Euros. Changes in the exchange rate of the Euro will impact our product cost.
Property and Equipment
Property and equipment are stated at cost. Additions or improvements are capitalized, while repairs and maintenance are charged to expense. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets. Buildings are depreciated over 20-40 years, while computer equipment, machinery and equipment, and furniture and fixtures are depreciated over three to ten years. Building improvements are amortized over their estimated useful lives. When assets are sold or otherwise disposed of, the related property amounts are relieved from the accounts, and any gain or loss is recorded in the statement of operations.
Patents and Product Rights
Patents and product rights consist of costs associated with the acquisition of patents, licenses and product distribution rights. Patents and product rights are amortized using the straight-line method over their estimated useful lives of three to ten years.
Other Assets
Included in other assets is a $337,253 investment, representing a 7.7% ownership interest in a privately-held nonaffiliated company. We do not have a controlling interest in this company, nor do we have an ownership or voting interest which allows us to exert significant influence over the operating and financial policies of this investee company. Accordingly, we have accounted for this investment using the cost method of accounting. In January, 2007, this privately-held nonaffiliated company was sold, and we received approximately $1,750,000 for our ownership interest.
Impairment of Long-Lived Assets
In accordance with SFAS No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets,” if indicators of impairment exist, we assess the recoverability of the affected long-lived assets, which include property and equipment and patents and product rights, by determining whether the carrying value of such assets can be recovered through the sum of the undiscounted future cash flows from the use and eventual disposition of the asset. If impairment is indicated, we measure the amount of such impairment by comparing the carrying value of the assets to the fair value of these assets, which is generally determined based on the present value of the expected future cash flows associated with the use of the asset. During 2005, we recorded a $196,011 impairment on certain equipment because its carrying value exceeded the future cash flows to be received from those assets. We believe the future cash flows to be received from our long-lived assets will exceed the assets’ carrying value, and accordingly we have not recognized any additional impairment losses through December 31, 2006.
F-9
Table of Contents
Revenue Recognition
We recognize product revenues when there is persuasive evidence that an arrangement exists, the price is fixed or determinable, title has passed and collection is reasonably assured. Product revenues are recorded net of allowances for any discounts or rebates. We do not grant price protection or product return rights to our customers, except for warranty returns. Historically, returns arising from warranty issues have been infrequent and immaterial. Accordingly, we expense warranty returns as incurred.
Up-front licensing fees are deferred and recognized ratably over the related license period. Product development revenues are recognized over the period in which the related product development efforts are performed. Amounts received prior to the performance of product development efforts are recorded as deferred revenues. Grant revenue is recognized as the related work is performed and costs are incurred. We record shipping and handling charges billed to our customers as product revenue and the related expense as cost of products sold.
Significant Customer Concentration
During 2006, Quest Diagnostics, including its wholly-owned subsidiary, LabOne, Inc. (“Quest”), and Abbott Laboratories (“Abbott”) accounted for 14 percent and 10 percent of our total revenues, respectively.
Additionally, four customers each accounted for more than 10 percent of our accounts receivable at December 31, 2006. Prestige Brands Holdings, Inc. (“Prestige”), Quest, SSL International plc, and Abbott accounted for 12 percent, 11 percent, 10 percent and 11 percent, respectively, of our accounts receivable at December 31, 2006.
During 2005 and 2004, two of our customers accounted for more than 10 percent of our total revenues. Prestige accounted for 17% and 25% of total revenues for 2005 and 2004, respectively. Quest accounted for 13% and 14% of our total revenues for 2005 and 2004, respectively.
At December 31, 2005, Prestige and SSL International plc accounted for 15% and 20% of our accounts receivable, respectively.
Research and Development
Research and development costs are charged to expense as incurred.
Advertising Expenses
Advertising costs are charged to expense as incurred. During 2006, 2005, and 2004, we incurred $805,936, $2,232,945, and $3,512,037, respectively, in advertising expenses. Included in advertising expenses for 2006, 2005 and 2004 were $539,856, $1,820,022 and $2,883,145, respectively, in reimbursement for marketing expenses incurred for the Compound W® Freeze Off® product.
Stock-Based Compensation
Prior to 2006, we accounted for stock-based compensation to employees and directors using the intrinsic value method in accordance with Accounting Principles Board (“APB”) Opinion No. 25, “Accounting for Stock Issued to Employees,” and related interpretations. Under APB Opinion No. 25, no stock-based compensation expense was recognized for stock options granted to employees or directors, as the exercise price was equal to the market price of our common stock on the date of grant. We account for stock-based compensation to nonemployees using the fair value method in accordance with SFAS No. 123 (revised 2004), (“SFAS No. 123R”), “Share-Based Payment,” and Emerging Issues Task Force (“EITF”) Issue No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services.”
F-10
Table of Contents
Effective January 1, 2006, we adopted SFAS No. 123R, which eliminated the ability to account for stock-based compensation under APB Opinion No. 25 and requires us to recognize compensation expense based on the fair value of our stock-based awards. We elected the modified prospective transition method as permitted by SFAS No. 123R. Accordingly, results from prior periods have not been restated. Under this transition method, stock-based compensation expense for the year ended December 31, 2006 includes:
| (a) | compensation expense for all stock-based awards granted prior to January 1, 2006, but not yet vested, based on the grant date fair value previously estimated in accordance with the original provisions of SFAS No. 123, “Accounting for Stock-Based Compensation,” and |
| (b) | compensation expense for all stock-based awards granted, modified or settled subsequent to January 1, 2006, based on the grant-date fair value estimated in accordance with the provisions of SFAS No. 123R. |
Upon the adoption of SFAS No. 123R, our deferred compensation balance of $3,334,792 was reclassified against additional paid-in capital. Consistent with our past practice under the disclosure requirements of SFAS No. 123, we have elected to recognize compensation expense for stock option awards issued to employees and directors on a straight-line basis over the requisite service period of the award. To satisfy the exercise of options or to issue new restricted stock, we normally issue new shares rather than purchase shares on the open market.
Pursuant to the disclosure requirements of SFAS No. 123, the table below illustrates the effect on net income (loss) and earnings (loss) per share had compensation expense for our stock-based awards been determined based upon the fair value of the awards at the date of grant for the years ended December 31, 2005 and 2004:
| Year ended December 31, | ||||||||
| 2005 | 2004 | |||||||
Net income (loss): | ||||||||
As reported | $ | 27,448,087 | $ | (559,642 | ) | |||
Add: stock-based employee compensation expense included in net income (loss), net of tax | 945,634 | 874,162 | ||||||
Deduct: total stock-based employee compensation expense determined under the fair value-based method for all awards, net of tax | (3,004,813 | ) | (5,921,957 | ) | ||||
Pro forma | $ | 25,388,908 | $ | (5,607,437 | ) | |||
Earnings (loss) per share: | ||||||||
Basic | ||||||||
As reported | $ | 0.61 | $ | (0.01 | ) | |||
Pro forma | $ | 0.56 | $ | (0.13 | ) | |||
Diluted | ||||||||
As reported | $ | 0.59 | $ | (0.01 | ) | |||
Pro forma | $ | 0.55 | $ | (0.13 | ) | |||
Income Taxes
We follow the asset and liability method for accounting for income taxes. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and the respective tax basis of assets and liabilities, and operating loss and credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates that are expected to apply to taxable income in the years in which those temporary differences and operating loss and credit
F-11
Table of Contents
carryforwards are expected to be recovered, settled or utilized. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
Foreign Currency Translation
Pursuant to SFAS No. 52, “Foreign Currency Translation,” the assets and liabilities of our foreign operations are translated from Euros into U.S. dollars at current exchange rates as of the balance sheet date, and revenues and expenses are translated at average exchange rates for the period. Resulting translation adjustments are reflected in accumulated other comprehensive loss, which is a separate component of stockholders’ equity.
Earnings (Loss) Per Share
We have presented basic and diluted earnings (loss) per share pursuant to SFAS No. 128, “Earnings per Share.” In accordance with SFAS No. 128, basic earnings (loss) per share is computed by dividing net income (loss) by the weighted-average number of shares of common stock outstanding during the period. Diluted earnings per share is computed in a manner similar to basic earnings per share except that the weighted average number of shares outstanding is increased to include incremental shares from the assumed vesting or exercise of dilutive securities, such as common stock options, warrants and unvested restricted stock. The number of incremental shares is calculated by assuming that outstanding stock options and warrants were exercised and unvested restricted shares were vested, and the proceeds from such exercises or vesting were used to acquire shares of common stock at the average market prices during the reporting period.
The computations of basic and diluted earnings (loss) per share are as follows:
| Year ended December 31, | ||||||||||
| 2006 | 2005 | 2004 | ||||||||
Net income (loss) | $ | 5,267,549 | $ | 27,448,087 | $ | (559,642 | ) | |||
Weighted average shares of common stock outstanding: | ||||||||||
Basic | 45,909,990 | 45,109,580 | 44,463,861 | |||||||
Dilutive effect of stock options, warrants and restricted shares | 670,276 | 1,037,032 | — | |||||||
Diluted | 46,580,266 | 46,146,612 | 44,463,861 | |||||||
Earnings (loss) per share: | ||||||||||
Basic | $ | 0.11 | $ | 0.61 | $ | (0.01 | ) | |||
Diluted | $ | 0.11 | $ | 0.59 | $ | (0.01 | ) | |||
For the years ended December 31, 2006, 2005, and 2004, outstanding common stock options, warrants and unvested restricted stock representing 1,308,809, 1,069,181, and 5,479,504 shares, respectively, were excluded from the computation of diluted earnings (loss) per share as their inclusion would have been anti-dilutive.
Other Comprehensive Income (Loss)
We follow SFAS No. 130, “Reporting Comprehensive Income.” This statement requires the classification of items of other comprehensive income (loss) by their nature and disclosure of the accumulated balance of other comprehensive income (loss), separately from accumulated deficit and additional paid-in capital, in the stockholders’ equity section of our balance sheet.
Fair Value of Financial Instruments
As of December 31, 2006, the carrying values of cash and cash equivalents, short-term investments, accounts receivable, accounts payable, and accrued expenses approximate their respective fair values based on
F-12
Table of Contents
their short-term nature. In addition, we believe the carrying value of our debt instruments, which do not have readily ascertainable market values, approximate their fair values, given that the interest rates on outstanding borrowings approximate market rates.
Recent Accounting Pronouncements
In July 2006, the Financial Accounting Standards Board (“FASB”) issued FASB Interpretation (“FIN”) No. 48, “Accounting for Uncertainty in Income Taxes—an Interpretation of FASB Statement No. 109,” which clarifies what criteria must be met prior to recognition of the financial statement benefit of a position taken in a tax return. FIN No. 48 will require companies to include additional qualitative and quantitative disclosures within their financial statements. The disclosures will include potential tax benefits from positions recognized for tax return purposes but not recognized for financial reporting purposes, as well as a tabular presentation of significant changes in such benefits during each period. The disclosures will also include a discussion of the nature of uncertainties, factors that could cause a change, and an estimated range of reasonably possible changes in tax uncertainties. FIN No. 48 will require a company to recognize a financial statement benefit for a position taken for tax return purposes when it will be more-likely-than-not that the position will be sustained. FIN No. 48 is effective for fiscal years beginning after December 15, 2006. We are currently assessing the impact FIN No. 48 will have on our financial statements.
In September 2006, the United States Securities and Exchange Commission issued Staff Accounting Bulletin No. 108 (“SAB No. 108”) “Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements.” SAB No. 108 requires companies to evaluate the materiality of identified unadjusted errors using both the income statement approach and the balance sheet approach. In the initial year of adoption, if a company determines that an adjustment to prior year financial statements is required under either approach, SAB No. 108 allows for a one-time cumulative-effect adjustment to beginning retained earnings. SAB No. 108 is effective for interim periods of the first fiscal year ending after November 15, 2006. The adoption of SAB No. 108 did not have any impact on our financial statements.
In September 2006, the FASB issued SFAS No. 157,“Fair Value Measurements.” This Statement defines fair value, establishes a framework for measuring fair value in generally accepted accounting principles, and expands disclosures about fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are currently assessing the impact, if any, that SFAS No. 157 will have on our financial statements.
In February 2007, the FASB issued SFAS No. 159, “The Fair Value Option for Financial Assets and Financial Liabilities—Including an amendment of FASB Statement No. 115.” SFAS No. 159 permits entities to elect to measure many financial instruments and certain other items at fair value. Unrealized gains and losses on items for which the fair value option has been elected will be recognized in earnings at each subsequent reporting date. SFAS No. 159 is effective for fiscal years beginning after November 15, 2007. We are currently assessing the impact SFAS No. 159 will have on our financial statements.
| 3. | INVENTORIES: |
| December 31, | ||||||
| 2006 | 2005 | |||||
Raw materials | $ | 3,868,301 | $ | 2,625,889 | ||
Work in process | 533,470 | 718,804 | ||||
Finished goods | 1,132,796 | 783,336 | ||||
| $ | 5,534,567 | $ | 4,128,029 | |||
In 2005, we recorded a $1,288,655 provision on our UPlink® inventory as a result of the inability to determine an alternative use for this product and the termination of an agreement with our distribution partner.
F-13
Table of Contents
| 4. | PROPERTY AND EQUIPMENT: |
| December 31, | ||||||||
| 2006 | 2005 | |||||||
Land | $ | 1,117,788 | $ | — | ||||
Buildings and improvements | 13,454,065 | 4,895,253 | ||||||
Machinery and equipment | 8,623,415 | 7,855,947 | ||||||
Computer equipment | 1,577,073 | 2,052,741 | ||||||
Furniture and fixtures | 781,103 | 847,512 | ||||||
Construction in progress | 3,672,005 | 1,966,125 | ||||||
| 29,225,449 | 17,617,578 | |||||||
Less—Accumulated depreciation | (11,850,731 | ) | (11,802,345 | ) | ||||
| $ | 17,374,718 | $ | 5,815,233 | |||||
On June 30, 2006, we exercised purchase options contained in the commercial leases for two of our facilities located in Bethlehem, Pennsylvania and acquired both facilities for an aggregate purchase price of approximately $9,100,000, including settlement costs. As a result of these purchases, the leases for these facilities, which required minimum annual rental payments of $1,051,000, were terminated.
Depreciation expense was $1,371,400, $1,573,852, and $1,739,733 for 2006, 2005, and 2004, respectively. In addition, we retired or disposed of $1,323,014 of fully depreciated assets during 2006. No gain or loss was recognized on these disposals.
| 5. | PATENTS, PRODUCT RIGHTS AND ACQUIRED IN-PROCESS TECHNOLOGY: |
Patents product rights and licenses were as follows:
| December 31, | ||||||||
| 2006 | 2005 | |||||||
HIV-related | $ | 1,900,000 | $ | 1,900,000 | ||||
HCV-related | 4,500,000 | 1,500,000 | ||||||
Lateral flow-related | 1,500,000 | 500,000 | ||||||
Cryosurgery-related | 2,548,620 | 2,548,620 | ||||||
| 10,488,620 | 6,448,620 | |||||||
Less accumulated amortization | (4,120,276 | ) | (3,568,662 | ) | ||||
| $ | 6,328,344 | $ | 2,879,958 | |||||
Amortization expense for 2006, 2005, and 2004 was $551,614, $700,377, and $705,808, respectively. Amortization expense for each of the five succeeding fiscal years is estimated at $1,066,565 for 2007, $896,619 for 2008, $789,515 for 2009, $789,515 for 2010, and $789,515 for 2011.
In June 1998, we acquired the patents and exclusive worldwide distribution rights to our cryosurgical product line. The purchase price of $2,548,620, including transaction costs, has been recorded as patents and product rights and is being amortized using the straight-line method over an estimated useful life of ten years. In connection with this acquisition, we also entered into a product purchase agreement with the manufacturer of the cryosurgical product line, with a current term extending through 2008.
In June 2004, we entered into a sublicense agreement with a third party, pursuant to which we have been granted a limited, worldwide, non-exclusive sublicense to certain HIV-2 patents held by such party. The agreement required us to pay the third party a one-time non-refundable license fee of $900,000, which was
F-14
Table of Contents
recorded as patent and product rights on our balance sheet and is being amortized through June 30, 2014. This agreement also contained an option to expand the application of this sublicense to other immunoassay platforms, in addition to our OraQuick® platform. In June 2006, we exercised this option, which requires us to pay the third party a non-refundable license fee of $600,000, of which $200,000 was paid in July 2006. Two remaining contractually obligated payments of $200,000 each are due in July 2007 and 2008. As of December 31, 2006, $200,000 of this obligation is included in accrued expenses, with the remaining $200,000 included in other liabilities. We have recognized this $600,000 license fee as acquired in-process technology, which is included in research and development expense in our statement of operations, because other immunoassay platforms for the detection of HIV-2 will require additional research and development efforts and subsequent regulatory approvals.
In August 2005, we entered into a license agreement with third parties, pursuant to which we have been granted a limited, personal, non-transferable, non-exclusive license related to certain Hepatitis C Virus (“HCV”) patents held by such parties. The agreement required us to pay the third parties a one-time non-refundable license fee of $1,500,000, which was paid in August 2005. In December 2006, an additional milestone payment of $3,000,000 was due and is included in accrued expenses as of December 31, 2006. We may also be required to pay additional license fees of up to $5,500,000, upon the achievement of specific development and/or commercial milestones.
Management’s intent in executing the HCV license agreement is to provide for various alternatives for use of the licensed patents. Some of these uses require additional research and development efforts and regulatory approvals, while others, specifically in the international market, do not require additional research and development efforts or regulatory approvals. Based on management’s estimate of the cash flows to be received from future product sales in international markets, we capitalized both the $1,500,000 and $3,000,000 license fees in the accompanying balance sheet. We are amortizing these amounts to cost of products sold on a straight-line basis over ten years, which represents management’s estimate of the remaining useful life of the licensed patents.
Under the terms of the HCV license agreement, we are also obligated to pay royalties based on our net sales of certain products, which incorporate the technology covered by the licensed patents. Royalties under the license agreement vary based upon the geographical territory where the product is sold. No sales have been made under the terms of this license agreement through December 31, 2006.
In December 2006, we amended a license agreement with third parties, pursuant to which we have been granted a limited, non-exclusive license to certain lateral flow technology patents held by such parties. The amendment provides for the renewal of our license to certain lateral flow patents held by these parties, the expansion of these patents to future product applications to be developed by our Company, and the settlement of prior royalty obligations arising prior to the amendment date. It requires us to pay the third parties a one-time non-refundable fee of $1,750,000. We allocated the $1,750,000 fee based upon the relative fair values of the items contained in the agreement. Accordingly, at December 31, 2006, we capitalized $1,000,000 as patent and product rights and will amortize this amount through December 2011. Of the remaining $750,000, we recognized $400,000 as acquired in-process technology as such amount was allocated to royalties for future product applications that will require additional research and development efforts and regulatory approvals. The remaining $350,000 was recorded as a reduction of the accrued prior royalty obligations, which resulted in the reversal of $738,983 of royalty expense during 2006. The $1,750,000 fee is included in accrued expenses at December 31, 2006.
F-15
Table of Contents
| 6. | ACCRUED EXPENSES AND OTHER: |
| December 31, | ||||||
| 2006 | 2005 | |||||
Payroll and related benefits | $ | 2,117,630 | $ | 2,510,240 | ||
Deferred revenue | 1,877,546 | 1,302,791 | ||||
Professional fees | 681,850 | 487,712 | ||||
Royalties | 2,813,102 | 1,925,679 | ||||
Advertising | 201,509 | 757,906 | ||||
License fees | 4,200,000 | — | ||||
Laboratory testing fees | 155,996 | 210,604 | ||||
Other | 611,516 | 539,009 | ||||
| $ | 12,659,149 | $ | 7,733,941 | |||
At December 31, 2006, accrued payroll and related benefits decreased primarily as a result of a decrease in annual bonuses. Deferred revenue includes customer prepayments of $1,727,546 and $1,012,891 at December 31, 2006 and 2005, respectively. Professional fees at December 31, 2006 increased primarily as a result of legal fees related to current litigation. Accrued royalties and advertising expenses at December 31, 2006 and 2005 are primarily related to our OraQuick® and Freeze Off® products, respectively. License fees at December 31, 2006 are related to the sublicense agreements, which we entered into in December 2006, as discussed in Note 5.
| 7. | CREDIT FACILITIES: |
On June 27, 2006, we executed an amendment to our existing $11,900,000 credit facility (the “Credit Facility”) with Comerica Bank (“Comerica”). This amendment permitted us to borrow up to an additional $15,000,000 in advances in order to fund the purchase and future expansion of two leased facilities in Bethlehem, Pennsylvania. The original Credit Facility was comprised of an $887,000 mortgage loan, a $3,000,000 term loan, a $4,000,000 non-revolving line of credit for the purchase of both capital equipment and software, and a $4,000,000 revolving working capital line of credit. On June 29, 2006, we borrowed $10,000,000 under the terms of this Credit Facility, as amended, and purchased our two Bethlehem facilities. We can borrow the remaining $5,000,000 at any time before June 30, 2007. At our option, interest on outstanding borrowings is payable monthly at either a fixed rate equal to the five-year U.S. Treasury Note rate plus 1.03% to 1.73%, or a variable rate equal to the 30, 180, or 360-day LIBOR rate plus 0.55% to 1.25%. In each case, the interest rate is determined at the date of the advance and is based upon the amount of cash and cash equivalents we invest and retain at Comerica Securities, Inc. We also can choose the fixed rate option, without penalty, at the expiration of a previously elected LIBOR period. Principal is repayable in periodic installments, based upon the rate option that we elect, with the remaining balance of unpaid principal due on June 27, 2011. This amendment also extended the maturity date of our $4,000,000 revolving working capital line of credit to June 29, 2007. All other terms of the Credit Facility, as previously amended, remain in effect, except for our financial covenant related to liquidity, which was modified to require a minimum liquidity, as defined by Comerica, of not less than $25,000,000, of which at least $15,000,000 must be held by Comerica or its affiliates.
As of December 31, 2006, we had no outstanding borrowings under the $3,000,000 term loan, the $4,000,000 non-revolving line of credit, or the $4,000,000 revolving working capital line of credit.
At December 31, 2006, interest on the new $10,000,000 borrowing is payable monthly, at the 360-day LIBOR rate plus 0.9%, or 6.1894%. Principal is repayable in installments, due at the end of each LIBOR rate period, based upon a twenty-year amortization schedule and the number of months in the expiring LIBOR rate period. Accordingly, on December 27, 2007, we will be required to make a $500,000 principal repayment and the interest rate on this loan will reset.
F-16
Table of Contents
All borrowings under the Credit Facility are collateralized by a first priority security interest in all of our assets, including present and future accounts receivable, chattel paper, contracts and contract rights, equipment and accessories, general intangibles, investments, instruments, inventories, and a mortgage on our three facilities in Bethlehem, Pennsylvania. Borrowings under the revolving working capital line of credit are limited to commercially standard percentages of accounts receivable. The Credit Facility contains certain covenants that set forth minimum requirements for our quick ratio, liquidity, and tangible net worth. We were in full compliance with all covenants at December 31, 2006. The Credit Facility also restricts our ability to pay dividends, to make certain investments, to incur additional indebtedness, to sell or otherwise dispose of a substantial portion of assets, and to merge or consolidate operations with an unaffiliated entity, without the consent of Comerica.
| 8. | LONG-TERM DEBT: |
| December 31, | ||||||||
| 2006 | 2005 | |||||||
Note payable to bank, interest payable monthly at the 360-day LIBOR rate plus 0.9%, (6.1894% at December 31, 2006), principal payments due at the end of each LIBOR rate period through June 2011, at which time the remaining unpaid principal balance is payable, secured by a first priority security interest in all of our assets. | $ | 9,750,000 | $ | — | ||||
Mortgage loan payable to bank, interest at an annual floating rate equal to the bank’s prime rate (8.25% at December 31, 2006), fixed monthly installments of principal and interest of $7,426 through September 2007, at which time the interest rate and fixed monthly repayment amount is reset for the remaining sixty monthly installments, secured by our building. | 689,442 | 722,233 | ||||||
Notes payable to bank, matured in 2006. | — | 311,210 | ||||||
Note payable to bank, interest at an annual floating rate equal to the bank’s prime rate (8.25% at December 31, 2006), monthly principal installments of $2,144, plus interest, through June 2007, secured by certain equipment | 12,863 | 38,590 | ||||||
Note payable to bank, interest at an annual floating rate equal to the bank’s prime rate (8.25% at December 31, 2006), monthly principal installments of $2,264, plus interest, through March 2007, secured by certain equipment. | 6,793 | 33,963 | ||||||
Note payable to Pennsylvania Industrial Development Authority, interest at 2%, monthly installments of principal and interest of $4,893 through March 2010, secured by a second lien on our building. | 180,038 | 234,566 | ||||||
| 10,639,136 | 1,340,562 | |||||||
Less—Current portion | (608,595 | ) | (456,541 | ) | ||||
| $ | 10,030,541 | $ | 884,021 | |||||
Long-term debt maturities as of December 31, 2006 are as follows:
2007 | $ | 608,595 | |
2008 | 593,087 | ||
2009 | 597,346 | ||
2010 | 552,670 | ||
2011 | 7,796,730 | ||
Thereafter | 490,708 | ||
| $ | 10,639,136 | ||
Certain of these notes payable require, among other items, the maintenance of certain financial covenants. We were in compliance with these covenants as of December 31, 2006.
F-17
Table of Contents
| 9. | INCOME TAXES: |
The components of the provision (benefit) for income taxes for the years ended December 31, 2006, 2005 and 2004 are as follows:
| 2006 | 2005 | 2004 | |||||||||
Current | |||||||||||
Federal | $ | 251,074 | $ | 224,153 | $ | — | |||||
State | 429,085 | 211,726 | — | ||||||||
| 680,159 | 435,879 | — | |||||||||
Deferred | |||||||||||
Federal | 2,976,992 | 3,285,740 | (123,009 | ) | |||||||
State | 136,753 | 495,298 | (18,823 | ) | |||||||
| 3,113,745 | 3,781,038 | (141,832 | ) | ||||||||
Change in valuation allowance | — | (21,946,106 | ) | 141,832 | |||||||
| 3,113,745 | (18,165,068 | ) | — | ||||||||
Total provision (benefit) | $ | 3,793,904 | $ | (17,729,189 | ) | $ | — | ||||
A reconciliation of the statutory United States federal tax rate to our effective tax rate for the year ended December 31, is as follows:
| 2006 | 2005 | 2004 | |||||||
Statutory U.S. federal tax rate—expense (benefit) | 34.0 | % | 34.0 | % | (34.0 | )% | |||
State income taxes, net of federal benefit | 4.1 | 4.8 | (2.2 | ) | |||||
Nondeductible expenses and other | 3.8 | 4.6 | 10.9 | ||||||
Net change in valuation allowance, federal and state | — | (225.8 | ) | 25.3 | |||||
Effective tax rate | 41.9 | % | (182.4 | )% | — | % | |||
Deferred income taxes reflect the tax effects of temporary differences between the basis of assets and liabilities recognized for financial reporting purposes and tax purposes, and net operating loss and tax credit carryforwards. Significant components of our total deferred tax asset as of December 31, 2006 and 2005 are as follows:
| 2006 | 2005 | |||||
Net operating loss carryforwards | $ | 18,019,056 | $ | 22,827,988 | ||
Inventory | 1,315,262 | 1,165,890 | ||||
Capitalized research and development costs | 552,740 | 665,353 | ||||
Accruals and reserves currently not deductible | 573,819 | 579,584 | ||||
Patent costs | 764,311 | 579,347 | ||||
Depreciation and amortization | 472,586 | 391,677 | ||||
Stock-based compensation | 1,418,655 | 359,573 | ||||
Alternative minimum tax credit carryforwards | 405,145 | 138,886 | ||||
Total deferred tax asset | $ | 23,521,574 | $ | 26,708,298 | ||
F-18
Table of Contents
In assessing the realizability of our deferred tax asset, we follow the guidance contained within SFAS No. 109, “Accounting for Income Taxes,” which requires that deferred income tax assets be reduced by a valuation allowance, if after considering all relevant positive and negative evidence, it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The realization of the gross deferred tax assets is dependent on several factors, including the generation of sufficient taxable income prior to the expiration of the net operating loss (“NOL”) carry forwards. We conclude that it is more likely than not that we will generate sufficient taxable income to recognize the deferred tax assets. Our federal NOL carryforwards expire as follows:
Year of Expiration | NOLs | ||
2008 | $ | 596,830 | |
2009 | 7,053,000 | ||
2010 | 795,394 | ||
2011 | 7,731,587 | ||
2017-2021 | 30,428,371 | ||
2022-2025 | 6,383,686 | ||
| $ | 52,988,868 | ||
The Tax Reform Act of 1986 contains provisions that limit the annual amount of NOLs available to be used in any given year in the event of a significant change in ownership. On September 29, 2000, two separate companies, STC Technologies, Inc. and Epitope, Inc. merged to form our Company. A significant change in ownership, as defined by Section 382 of the Internal Revenue Code, occurred in connection with this merger. As such, the utilization of NOLs generated prior to September 29, 2000 is limited to approximately $13,700,000 per year. We do not believe that this limitation will have a material adverse impact on the utilization of our NOL carryforwards.
At the end of 2005, we concluded that it was more likely than not that the majority of our deferred tax asset would be recovered. Accordingly, we reduced our valuation allowance by approximately $26,700,000. Included in the 2005 reduction of the valuation allowance was approximately $8,500,000 related to prior years’ tax benefits associated with the exercise of employee stock options. This amount was recognized as an increase to additional paid-in capital in 2005. A tax benefit of approximately $245,000 associated with employee exercises of non-qualified stock options and disqualifying dispositions of stock acquired with incentive stock options during the year ended December 31, 2005 was also recorded as additional paid-in capital during 2005.
| 10. | STOCKHOLDERS’ EQUITY: |
Stock-based Awards
We grant stock-based awards under the OraSure Technologies, Inc. 2000 Stock Award Plan, as amended and restated (the “2000 Plan”). The 2000 Plan permits stock-based awards to employees, outside directors and consultants or other third-party advisors. Awards which may be granted under the 2000 Plan include qualified incentive stock options, nonqualified stock options, stock appreciation rights, restricted awards, performance awards and other stock-based awards.
Under the terms of the 2000 Plan, qualified incentive stock options for shares of our common stock may be granted to eligible employees, including our officers. To date, options generally have been granted with ten-year exercise periods and an exercise price not less than the fair market value on the date of grant. Options generally vest over four years, with one quarter of the options vesting one year after grant and the remainder vesting on a monthly basis over the next three years. The 2000 Plan also provides that nonqualified options may be granted at a price not less than 75 percent of the fair market value of a share of common stock on the date of grant. The option term and vesting schedule of such awards may either be unlimited or have a specified period in which to vest and be exercised.
F-19
Table of Contents
As of December 31, 2006, 2,749,391 shares were available for future grants under the 2000 Plan.
The fair value of each stock option was estimated on the date of the grant using the Black-Scholes option-pricing model using the following weighted-average assumptions:
| Year Ended December 31, | |||||||||
Black-Scholes Option Valuation Assumptions | 2006 | 2005 | 2004 | ||||||
Risk-free interest rate(1) | 4.53 | % | 3.72 | % | 3.21 | % | |||
Expected dividend yield | — | — | — | ||||||
Expected stock price volatility(2) | 56 | % | 58 | % | 65 | % | |||
Expected life of stock options (in years)(3) | 5 | 4 | 5 | ||||||
| (1) | Based on the Treasury Securities constant maturity interest rate whose term is consistent with the expected life of our stock options. |
| (2) | Expected stock price volatility is based upon historical experience. |
| (3) | Expected life of stock options is based upon historical experience. |
The weighted-average grant date fair value of stock options granted during the years ended December 31, 2006, 2005 and 2004 was $4.85, $3.17 and $4.60, respectively.
Amounts recognized in the financial statements related to stock options were as follows:
| Year Ended December 31, 2006 | ||||
Total compensation cost during the year | $ | 3,621,412 | ||
Amounts capitalized into inventory and property and equipment during the year | (468,754 | ) | ||
Amounts recognized in cost of products sold for amounts previously capitalized | 264,038 | |||
Amounts charged against income, before income tax benefit | $ | 3,416,696 | ||
Amount of related income tax benefit | $ | 842,239 | ||
As a result of adopting SFAS No. 123R, the Company’s income before income taxes and net income for the year ended December 31, 2006 were $3,416,696 and $2,574,457 lower, respectively, than if it had continued to account for share-based compensation under APB Opinion No. 25. Basic and diluted earnings per share for the year ended December 31, 2006 would have been $0.06 per share higher if the Company had not adopted SFAS No. 123R.
As a result of modifying the vesting provisions of two grants made to former members of the Company’s Board of Directors, the Company recognized $123,916 in compensation cost related to these modifications in the year ended December 31, 2005.
The aggregate intrinsic value of options (the amount by which the market price of the stock on the date of exercise exceeded the exercise price) exercised during the years ended December 31, 2006, 2005 and 2004 was $332,261, $4,338,143, and $1,326,796, respectively.
F-20
Table of Contents
The following table summarizes the stock option activity under the 2000 Plan:
| Options | Weighted-Average Exercise Price Per Share | Weighted-Average Contractual Term | Aggregate Intrinsic Value | ||||||||
Outstanding on January 1, 2004 | 3,935,463 | $ | 6.42 | ||||||||
Granted | 1,629,891 | 8.07 | |||||||||
Exercised | (370,800 | ) | 5.14 | ||||||||
Forfeited | (320,050 | ) | 7.89 | ||||||||
Outstanding on December 31, 2004 | 4,874,504 | 6.98 | |||||||||
Granted | 778,572 | 6.60 | |||||||||
Exercised | (1,009,794 | ) | 6.01 | ||||||||
Forfeited | (426,497 | ) | 7.55 | ||||||||
Outstanding on December 31, 2005 | 4,216,785 | 7.08 | |||||||||
Granted | 801,900 | 9.22 | |||||||||
Exercised | (85,013 | ) | 5.38 | ||||||||
Forfeited | (145,254 | ) | 7.99 | ||||||||
Balance, December 31, 2006 | 4,788,418 | $ | 7.44 | 7.57 | $ | 4,409,463 | |||||
Vested or expected to vest as of December 31, 2006 | 4,638,197 | $ | 7.44 | 7.57 | $ | 4,271,130 | |||||
Exercisable on December 31, 2006 | 3,536,575 | $ | 7.12 | 7.23 | $ | 4,366,963 | |||||
As of December 31, 2006, there was $4,637,882 of unrecognized compensation expense related to unvested option awards that is expected to be recognized over a weighted-average period of 1.7 years.
Net cash proceeds from the exercise of stock options were $457,334, $6,067,869 and $1,904,161 for the years ended December 31, 2006, 2005 and 2004, respectively. As a result of the Company’s net operating loss carryforward position, no actual income tax benefit was realized from stock option exercises for these periods.
The following table summarizes information about stock options outstanding at December 31, 2006:
Options outstanding | Options exercisable | |||||||||||
Range of exercise prices | Number outstanding | Weighted- average remaining life, in years | Weighted- average exercise price | Number exercisable | Weighted- average exercise price | |||||||
$ 0.80–$5.60 | 858,895 | 11.91 | $ | 4.75 | 679,406 | $ | 4.53 | |||||
$ 5.76–$6.84 | 486,953 | 5.23 | 5.96 | 480,160 | 5.96 | |||||||
$6.87 | 63,333 | 5.39 | 6.87 | 63,333 | 6.87 | |||||||
$6.96 | 509,452 | 6.08 | 6.96 | 501,251 | 6.96 | |||||||
$ 6.98–$7.77 | 863,943 | 5.91 | 7.49 | 669,359 | 7.42 | |||||||
$ 7.90–$8.18 | 106,000 | 8.23 | 8.05 | 32,603 | 8.03 | |||||||
$8.20 | 711,448 | 7.04 | 8.20 | 563,156 | 8.20 | |||||||
$ 8.36–$9.56 | 818,184 | 8.55 | 9.26 | 230,643 | 9.18 | |||||||
$ 9.78–$12.23 | 365,210 | 5.54 | 10.57 | 311,664 | 10.63 | |||||||
$12.69 | 5,000 | 4.68 | 12.69 | 5,000 | 12.69 | |||||||
| 4,788,418 | 7.57 | $ | 7.44 | 3,536,575 | $ | 7.12 | ||||||
F-21
Table of Contents
The 2000 Plan also permits us to grant restricted shares of our common stock to eligible employees, including officers. Generally, these shares are nontransferable and are subject to three-year vesting requirements or forfeiture, as determined by the Compensation Committee of our Board of Directors. The market value of these shares at the date of grant is recognized on a straight-line basis over the period during which the restrictions lapse. Compensation cost of $1,969,178, $1,500,054 and $874,162 related to restricted shares was recognized during the years ended December 31, 2006, 2005 and 2004, respectively.
The following table summarizes restricted stock award activity under the 2000 Plan:
| Shares | Weighted-Average Fair Value | |||||
Issued and unvested, January 1, 2004 | 75,000 | $ | 8.68 | |||
Granted | 410,000 | 7.75 | ||||
Vested | — | — | ||||
Forfeited | — | — | ||||
Issued and unvested, December 31, 2004 | 485,000 | 7.89 | ||||
Granted | 332,188 | 6.00 | ||||
Vested | (197,180 | ) | 9.26 | |||
Forfeited | (13,563 | ) | 5.60 | |||
Issued and unvested, December 31, 2005 | 606,445 | 6.90 | ||||
Granted | 465,313 | 9.16 | ||||
Vested | (199,633 | ) | 6.99 | |||
Forfeited | (65,071 | ) | 7.56 | |||
Issued and unvested, December 31, 2006 | 807,054 | $ | 8.13 | |||
Issued and expected to vest, December 31, 2006 | 802,242 | $ | 8.08 | |||
As of December 31, 2006, there was $5,136,029 of unrecognized compensation expense related to unvested restricted stock awards that is expected to be recognized over a weighted average period of 3.7 years.
In connection with the vesting of restricted shares during the years ended December 31, 2006 and 2005, we purchased and immediately retired 65,519 and 63,080 shares with aggregate values of $631,509 and $601,347, respectively, in satisfaction of minimum tax withholding obligations. No shares were purchased and retired during the year ended December 31, 2004.
SFAS No. 123R addresses the accounting for awards issued as part of share-based payment arrangements in exchange for employee services. Certain of the Company’s share-based payment arrangements are outside the scope of SFAS No. 123R and are subject to EITF Issue No. 00-19, “Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock,” which requires that vested stock options held by certain nonemployee consultants be accounted for as liability-classified awards. The fair value of these vested and unexercised awards was estimated using the Black-Scholes option pricing model and $109,080 was reclassified from equity to other liabilities as of January 1, 2006. An additional $121,579 was reclassified for options that vested during 2006. The fair value of these awards are remeasured at each financial reporting date until the awards are settled or expire. As of December 31, 2006, $228,475 was included in other liabilities for stock options to acquire 63,000 shares of common stock which remained unexercised.
Common Stock Warrants
As of December 31, 2006, warrants to purchase 120,000 shares of common stock at $6.13 per share were outstanding. These warrants were issued on September 30, 1998 and expire on September 30, 2008.
F-22
Table of Contents
| 11. | COMMITMENTS AND CONTINGENCIES: |
Sublicense Agreement
In June 2004, we entered into a sublicense agreement with a third party, pursuant to which we have been granted a limited, worldwide, non-exclusive sublicense to certain HIV-2 patents held by such party. Under the terms of this sublicense agreement, we are obligated to pay royalties based on a percentage of our net sales of certain products, which incorporate the technology covered by the licensed patents. Future minimum payments under this agreement are as follows:
2007 | $ | 500,000 | |
2008 | 500,000 | ||
2009 | 500,000 | ||
2010 | 500,000 | ||
2011 | 500,000 | ||
Thereafter | 3,291,667 | ||
| $ | 5,791,667 | ||
Royalties from our commercial sale of products covered by the sublicense can be credited against these minimum royalty obligations.
License Agreement
In August 2005, we entered into a license agreement with third parties, pursuant to which we have been granted a limited, personal, non-transferable, non-exclusive license related to certain HCV patents held by such parties. Under the terms of the HCV license agreement, we are also obligated to pay royalties based on our net sales of certain products which incorporate the technology covered by the licensed patents. Royalties under the license agreement vary based upon the geographical territory where the product is sold. No minimum payments are required under this agreement in 2007 or thereafter. We may, however, be required to pay additional license fees of up to $5,500,000, upon the achievement of specific development and/or commercial milestones.
Leases
We lease office and warehouse facilities under operating lease agreements. Future payments required under these non-cancelable leases are as follows:
2007 | $ | 81,866 | |
2008 | 68,480 | ||
2009 | 11,413 | ||
| $ | 161,759 | ||
Rent expense for 2006, 2005 and 2004 was $624,479, $1,146,697, and $1,690,858, respectively.
Purchase Commitments
As of December 31, 2006, we had outstanding non-cancelable purchase commitments in the amount of $5,349,799, of which $3,749,692, $198,340 and $1,401,767 are related to inventory, capital expenditures, and other goods or services, respectively.
F-23
Table of Contents
Employment Agreements
Under terms of employment agreements with certain executive officers, extending through 2008, we are required to pay each individual a base salary for continuing employment with our Company. The agreements require payments of $1,804,625 and $724,800 in 2007 and 2008, respectively.
Litigation
From time-to-time, we are involved in certain legal actions arising in the ordinary course of business. In management’s opinion, based upon the advice of counsel, the outcome of such actions are not expected to have a material adverse effect on our future financial position or results of operations.
| 12. | RETIREMENT PLANS: |
Substantially all employees of the Company are eligible to participate in the OraSure Technologies, Inc. 401(k) Plan (the “401(k) Plan”). The 401(k) Plan permits voluntary employee contributions to be excluded from an employee’s current taxable income under provisions of Internal Revenue Code Section 401(k) and the regulations thereunder. The 401(k) Plan also provides for us to match employee contributions up to $4,000 per year. Contributions to the 401(k) Plan, net of forfeitures, were $397,970, $361,629, and $330,552 in 2006, 2005, and 2004, respectively.
| 13. | GEOGRAPHIC INFORMATION: |
Based on guidance in SFAS No. 131, “Disclosures about Segments of an Enterprise and Related Information,” we believe we operate within one reportable segment. Our products are sold principally in the United States and Europe. Segmentation of operating income and identifiable assets is not applicable since our revenues outside the United States are export sales, and we do not have significant operating assets outside the United States.
The following table represents total revenues by geographic area, based on the location of the customer (amounts in thousands):
| 2006 | 2005 | 2004 | |||||||
United States | $ | 56,780 | $ | 59,859 | $ | 47,843 | |||
Europe | 9,521 | 7,868 | 4,318 | ||||||
Other regions | 1,854 | 1,639 | 1,847 | ||||||
| $ | 68,155 | $ | 69,366 | $ | 54,008 | ||||
F-24
Table of Contents
| 14. | QUARTERLY DATA (Unaudited): |
The following tables summarize the quarterly results of operations for each of the quarters in 2006 and 2005. These quarterly results are unaudited, but in the opinion of management, have been prepared on the same basis as our audited financial information and include all adjustments (consisting only of normal recurring adjustments) necessary for a fair presentation of the information set forth herein (all amounts in thousands, except per share amounts).
| 2006 Results | |||||||||||||||
| Three months ended | Year ended December 31, 2006 | ||||||||||||||
| March 31, 2006 | June 30, 2006 | September 30, 2006 | December 31, 2006 | ||||||||||||
Revenues | $ | 15,217 | $ | 17,564 | $ | 17,639 | $ | 17,735 | $ | 68,155 | |||||
Costs and expenses | 14,331 | 16,261 | 15,160 | 16,940 | 62,692 | ||||||||||
Operating income | 886 | 1,303 | 2,479 | 795 | 5,463 | ||||||||||
Other income (expense), net | 791 | 896 | 920 | 992 | 3,599 | ||||||||||
Income before income taxes | 1,677 | 2,199 | 3,399 | 1,787 | 9,062 | ||||||||||
Income tax provision | 777 | 991 | 1,265 | 761 | 3,794 | ||||||||||
Net income | $ | 900 | $ | 1,208 | $ | 2,134 | $ | 1,026 | $ | 5,268 | |||||
Earnings per share(1) | |||||||||||||||
Basic | $ | 0.02 | $ | 0.03 | $ | 0.05 | $ | 0.02 | $ | 0.11 | |||||
Diluted | $ | 0.02 | $ | 0.03 | $ | 0.05 | $ | 0.02 | $ | 0.11 | |||||
| 2005 Results | |||||||||||||||||
| Three months ended | Year ended December 31, 2005 | ||||||||||||||||
| March 31, 2005 | June 30, 2005 | September 30, 2005 | December 31, 2005 | ||||||||||||||
Revenues | $ | 15,828 | $ | 17,430 | $ | 18,077 | $ | 18,031 | $ | 69,366 | |||||||
Costs and expenses | 14,613 | 16,468 | 14,863 | 15,849 | 61,793 | ||||||||||||
Operating Income | 1,215 | 962 | 3,214 | 2,182 | 7,573 | ||||||||||||
Other income (expense), net | 346 | 481 | 594 | 725 | 2,146 | ||||||||||||
Income before income taxes | 1,561 | 1,443 | 3,808 | 2,907 | 9,719 | ||||||||||||
Income taxes (benefit) | — | — | — | (17,729 | ) | (17,729 | ) | ||||||||||
Net income | $ | 1,561 | $ | 1,443 | $ | 3,808 | $ | 20,636 | $ | 27,448 | |||||||
Earnings per share(1) | |||||||||||||||||
Basic | $ | 0.03 | $ | 0.03 | $ | 0.08 | $ | 0.45 | $ | 0.61 | |||||||
Diluted | $ | 0.03 | $ | 0.03 | $ | 0.08 | $ | 0.44 | $ | 0.59 | |||||||
| (1) | The summation of the quarterly amounts may not equal the year-end amounts due to the use of weighted-average shares for each period. |
F-25
Table of Contents
INDEX TO EXHIBITS
Exhibit Number | Exhibit | |
| 3.1.1 | Certificate of Incorporation of OraSure Technologies, Inc. is incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form S-4 (No. 333-39210), filed June 14, 2000. | |
| 3.1.2 | Certificate of Amendment to Certificate of Incorporation dated May 23, 2000 is incorporated by reference to Exhibit 3.1.1 to the Company’s Registration Statement on Form S-4 (No. 333-39210), filed June 14, 2000. | |
| 3.1.3 | Certificate of Designation of Series A Preferred Stock of OraSure Technologies (filed as Exhibit A to the Rights Agreement referred to in Exhibit 4.1). | |
| 3.2 | Amended and Restated Bylaws of OraSure Technologies, effective as of February 4, 2003, are incorporated by reference to Exhibit 3.2 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002. | |
| 4.1 | Rights Agreement, dated as of May 6, 2000, between OraSure Technologies, Inc. and ChaseMellon Shareholder Service, L.L.C. (now called Mellon Investor Services LLC), as Rights Agent, is incorporated by reference to Exhibit 4.2 to Amendment No. 1 to the Company’s Registration Statement on Form S-4 (No. 333-39210), filed August 8, 2000. | |
| 10.1 | Form of Indemnification Agreement (and list of parties to such agreement) is incorporated by reference to Exhibit 10.1 to Amendment No. 3 to the Company’s Registration Statement on Form S-4 (No. 333-39210), filed August 30, 2000.* | |
| 10.2 | Employment Agreement, dated as of June 22, 2004, between OraSure Technologies, Inc. and Douglas A. Michels, is incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.* | |
| 10.3 | Employment Agreement, dated as of July 1, 2004, between OraSure Technologies, Inc. and Ronald H. Spair, is incorporated by reference to Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.* | |
| 10.4 | Employment Agreement, dated September 23, 2005, between OraSure Technologies, Inc. and Stephen R. Lee, Ph.D., is incorporated herein by reference to Exhibit 99 to the Company’s Current Report on Form 8-K filed September 28, 2005.* | |
| 10.5 | Employment Agreement, dated as of July 1, 2004, between OraSure Technologies, Inc. and P. Michael Formica, is incorporated by reference to Exhibit 10.5 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.* | |
| 10.6 | Employment Agreement, dated as of July 1, 2004, between OraSure Technologies, Inc. and Joseph E. Zack, is incorporated by reference to Exhibit 10.6 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.* | |
| 10.7 | Employment Agreement, dated as of July 1, 2004, between OraSure Technologies, Inc. and Jack E. Jerrett, is incorporated by reference to Exhibit 10.7 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.* | |
| 10.8 | Employment Agreement, dated as of October 2, 2006, between Mark L. Kuna and OraSure Technologies, Inc., is incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K filed October 5, 2006.* | |
| 10.9 | Description of Nonemployee Director Compensation Policy, as amended as of November 15, 2005, is incorporated by reference to Exhibit 10.8 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005.* | |
| 10.10 | Amended and Restated Epitope, Inc. 1991 Stock Award Plan is incorporated by reference to Exhibit 10.9 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002.* | |
Table of Contents
Exhibit Number | Exhibit | |
| 10.11 | OraSure Technologies, Inc. Employee Incentive and Non-Qualified Stock Option Plan, as amended and restated effective September 29, 2000, is incorporated by reference to Exhibit 10.12 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2000.* | |
| 10.12 | OraSure Technologies, Inc. 2000 Stock Award Plan, as amended and restated effective as of May 16, 2006, is incorporated by reference to Exhibit 10 to the Company’s Current Report on Form 8-K filed May 18, 2006.* | |
| 10.13 | Form of Restricted Share Grant Agreement is incorporated by reference to Exhibit 10.2.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004.* | |
| 10.14 | Incentive Stock Option General Terms and Conditions (Officers and Employees) is incorporated by reference to Exhibit 10.12 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004.* | |
| 10.15 | Nonqualified Stock Option Award General Terms and Conditions (Officers and Employees) is incorporated by reference to Exhibit 10.13 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004.* | |
| 10.16 | Nonqualified Stock Option Award General Terms and Conditions (Non-Employee Directors) is incorporated by reference to Exhibit 10.14 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004.* | |
| 10.17 | Description of the OraSure Technologies, Inc. 2007 Management Incentive Plan is incorporated by reference to Item 5.02 to the Company’s Current Report on Form 8-K filed February 8, 2007.* | |
| 10.18 | Description of the OraSure Technologies, Inc. 2006 Self-Funding Management Incentive Plan is incorporated by reference to Exhibit 10.17 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005.* | |
| 10.19 | Description of the OraSure Technologies, Inc. Management Stock Award Guidelines dated January 26, 2005 is incorporated by reference to Exhibit 10.16 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2004.* | |
| 10.20 | Description of the OraSure Technologies, Inc. 2007 Stock Guidelines is incorporated by reference to Item 5.02 to the Company’s Current Report on Form 8-K filed February 8, 2007.* | |
| 10.21 | Distribution Agreement, dated as of April 24, 2003, between OraSure Technologies, Inc. and Medtech Holdings, Inc., is incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2004. | |
| 10.22 | Amendment No. 1 to Distribution Agreement, dated as of February 10, 2006, among OraSure Technologies, Inc., Medtech Holdings, Inc., Medtech Products, Inc. (as assignee of Medtech Holdings, Inc.) and Prestige Brands Holdings, Inc., as guarantor, is incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2006. | |
| 10.23 | Distribution Agreement, dated as of June 1, 2005, between OraSure Technologies, Inc. and SSL International plc, is incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005. | |
| 10.24 | Loan and Security Agreement, dated as of September 10, 2002, between Comerica Bank—California and OraSure Technologies, Inc., is incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2002. | |
| 10.25 | First Amendment to Loan and Security Agreement, dated as of May 23, 2003, between OraSure Technologies, Inc. and Comerica Bank – California, is incorporated by reference to Exhibit 10.24 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2003. | |
Table of Contents
Exhibit Number | Exhibit | |
| 10.26 | Second Amendment to Loan and Security Agreement, dated as of September 12, 2003, between OraSure Technologies, Inc. and Comerica Bank, is incorporated by reference to Exhibit 99.1 to the Company’s Current Report on Form 8-K, dated September 17, 2003. | |
| 10.27 | Third Amendment to Loan and Security Agreement, dated as of April 21, 2005, between OraSure Technologies, Inc. and Comerica Bank, is incorporated by reference to Exhibit 10 to the Company’s Current Report on Form 8-K filed April 27, 2005. | |
| 10.28 | Fourth Amendment to Loan and Security Agreement, dated as of June 27, 2006, between OraSure Technologies, Inc. and Comerica Bank, is incorporated by reference to Exhibit 10 to the Company’s Current Report on Form 8-K filed June 30, 2006. | |
| 10.29 | Supply and Distribution Agreement, dated as of February 11, 2005, between Abbott Laboratories and OraSure Technologies, Inc.** | |
| 10.30 | Amendment No. 1 to Supply and Distribution Agreement, dated as of July 21, 2005, between Abbott Laboratories and OraSure Technologies, Inc.** | |
| 23 | Consent of KPMG LLP, Independent Registered Public Accounting Firm. | |
| 24 | Powers of Attorney. | |
| 31.1 | Certification of Douglas A. Michels required by Rule 13a-14(a) or Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended. | |
| 31.2 | Certification of Ronald H. Spair required by Rule 13a-14(a) or Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended. | |
| 32.1 | Certification of Douglas A. Michels required by Rule 13a-14(b) or Rule 15a-14(b) under the Securities Exchange Act of 1934, as amended, and 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2 | Certification of Ronald H. Spair required by Rule 13a-14(b) or Rule 15a-14(b) under the Securities Exchange Act of 1934, as amended, and 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| * | Management contract or compensatory plan or arrangement. |
| ** | Portions of this exhibit were omitted pursuant to an application for confidential treatment and filed separately with the Securities and Exchange Commission. |