Exhibit 99.1

OraSure Technologies Analyst Day November 29, 2016 Nasdaq MarketSite

Agenda • Welcome and introductions • Infectious Disease – HCV Country-Wide Elimination Programs – HIV Self-Testing – R&D: Emerging Diseases -Ebola, Zika – Tuberculosis (TB) • Molecular – Personal Genomics – Microbiome – R&D: Sample Optimization • Financial review / Business Development • Summary

Forward-looking statements These slides and the associated presentation contain certain forward-looking statements, including statements with respect to revenues, earnings, technology, new products, product performance, markets, clinical development, regulatory filings and approvals, and business plans. Factors affecting these statements include, but are not limited to, the ability to develop new technology, technology changes, ability to fund research and development, required regulatory approvals, product performance and market acceptance of products. Please see the Company’s SEC filings, including its registration statements, and the Company’s most recent Form 10-K and Form 10-Q, for a more detailed description of specific factors that may cause actual results or events to differ materially from those described in the forward-looking statements. The Company undertakes no duty to update these statements.

Welcome and introductions Tony Zezzo Executive Vice President, Business Unit Lead, Infectious Disease Mike Reed, Ph.D. Senior Vice President, Research & Development and Chief Science Officer Cassandra Kelly-Cirino, Ph.D. Vice President, Infectious Disease TB and Emerging Diseases Program Lead Brian Smith Senior Vice President Business Unit Lead, Molecular Aaron Del Duca Vice President, Technology Microbiome Program Lead Rafal Iwasiow, Ph.D. Vice President, R&D, Molecular

Our company vision Empower healthcare providers and patients worldwide to improve global health through access to accurate, essential information. We will accomplish this through a deep understanding of our customers’ needs and a commitment to innovative infectious disease and molecular solutions.
Core strategic growth pillars Grow Molecular business Grow Infectious Disease business Driving unprecedented global access to our existing and new Infectious Disease diagnostics Leveraging the strength of our Molecular business into new growth markets, with existing and new technology
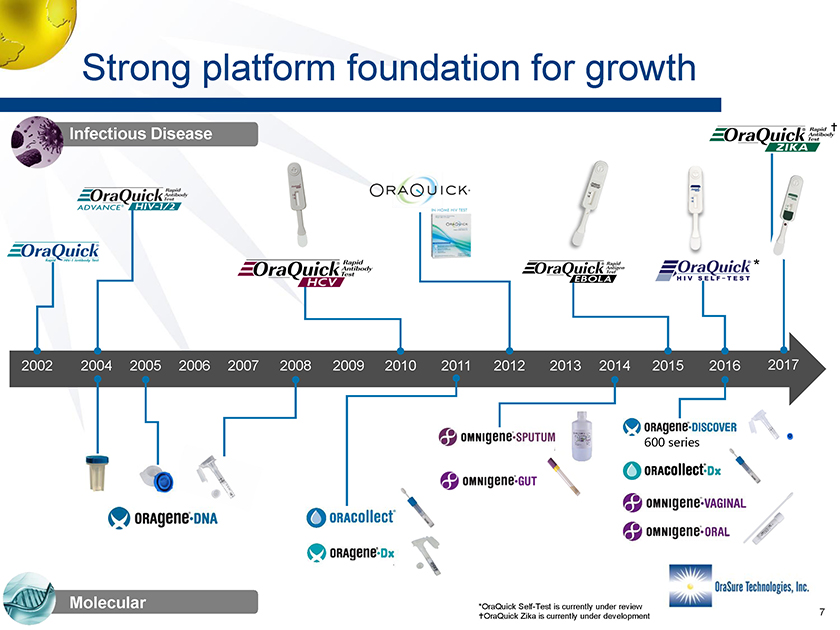
Strong platform foundation for growth 2004 2011 2005 2006 2007 20102002 2008 2009 2012 2013 2014 2015 2016 2017 Infectious Disease Molecular * *OraQuick Self-Test is currently under review †OraQuick Zika is currently under development 7 Strong platform foundation for growth 2004 2011 2005 2006 2007 20102002 2008 2009 2012 2013 2014 2015 2016 2017 Infectious Disease Molecular * *OraQuick Self-Test is currently under review OraQuick Zika is currently under development 7
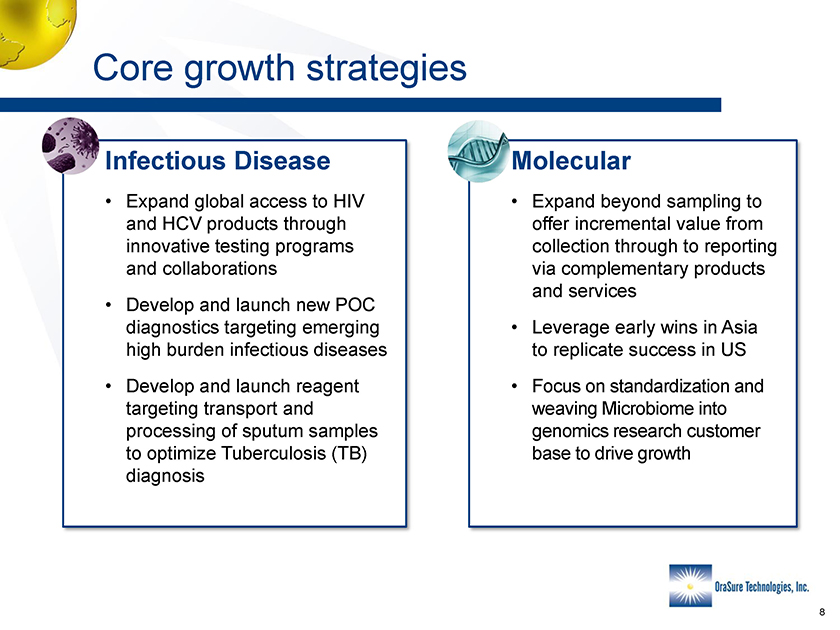
Core growth strategies Molecular • Expand beyond sampling to offer incremental value from collection through to reporting via complementary products and services • Leverage early wins in Asia to replicate success in US • Focus on standardization and weaving Microbiome into genomics research customer base to drive growth Infectious Disease • Expand global access to HIV and HCV products through innovative testing programs and collaborations • Develop and launch new POC diagnostics targeting emerging high burden infectious diseases • Develop and launch reagent targeting transport and processing of sputum samples to optimize Tuberculosis (TB) diagnosis

HCV Country-Wide Elimination Programs Tony Zezzo Executive Vice President Business Unit Lead, Infectious Disease OraSure Technologies, Inc.
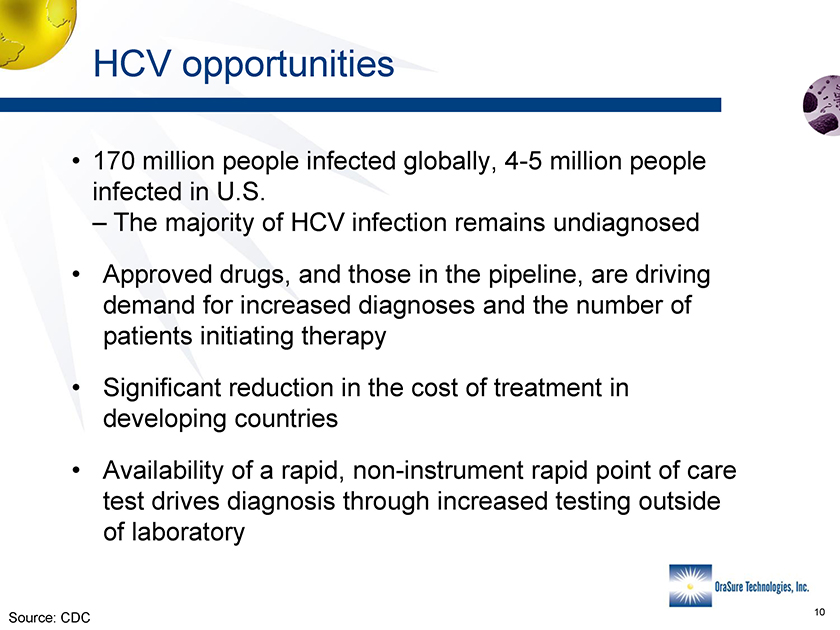
HCV opportunities • 170 million people infected globally, 4-5 million people infected in U.S. – The majority of HCV infection remains undiagnosed • Approved drugs, and those in the pipeline, are driving demand for increased diagnoses and the number of patients initiating therapy • Significant reduction in the cost of treatment in developing countries • Availability of a rapid, non-instrument rapid point of care test drives diagnosis through increased testing outside of laboratory Source: CDC
OraQuick® HCV Rapid Antibody Test Quality matters • Accurate – sensitivity and specificity comparable to lab-based immunoassays • Versatile -reach patients outside traditional laboratory testing channels • Simple – extremely easy to use three step process; use with oral fluid outside U.S. • 20 minute results – allows for immediate linkage of patient to care and treatment
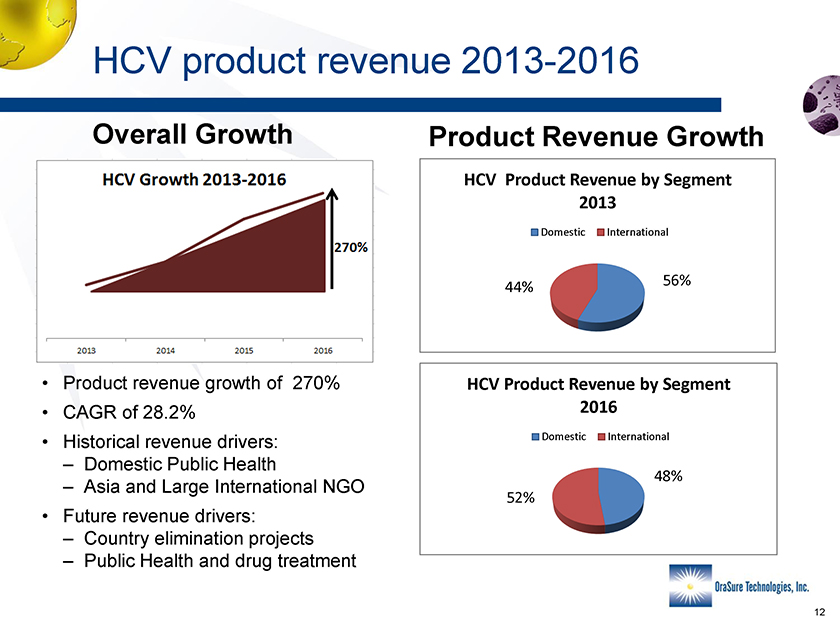
HCV product revenue 2013-2016 Overall Growth Product Revenue Growth HCV Product Revenue by Segment 2013 Domestic International 56%44% • Product revenue growth of 270% • CAGR of 28.2% • Historical revenue drivers: – Domestic Public Health – Asia and Large International NGO • Future revenue drivers: – Country elimination projects – Public Health and drug treatment HCV Product Revenue by Segment 2016 Domestic International 48% 52%
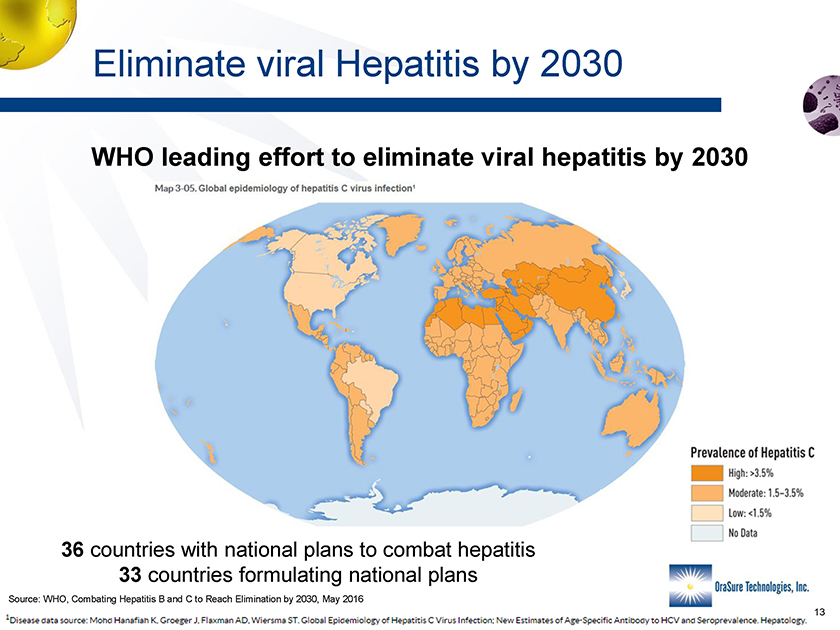
Eliminate viral Hepatitis by 2030 WHO leading effort to eliminate viral hepatitis by 2030 36 countries with national plans to combat hepatitis 33 countries formulating national plans Source: WHO, Combating Hepatitis B and C to Reach Elimination by 2030, May 2016 13
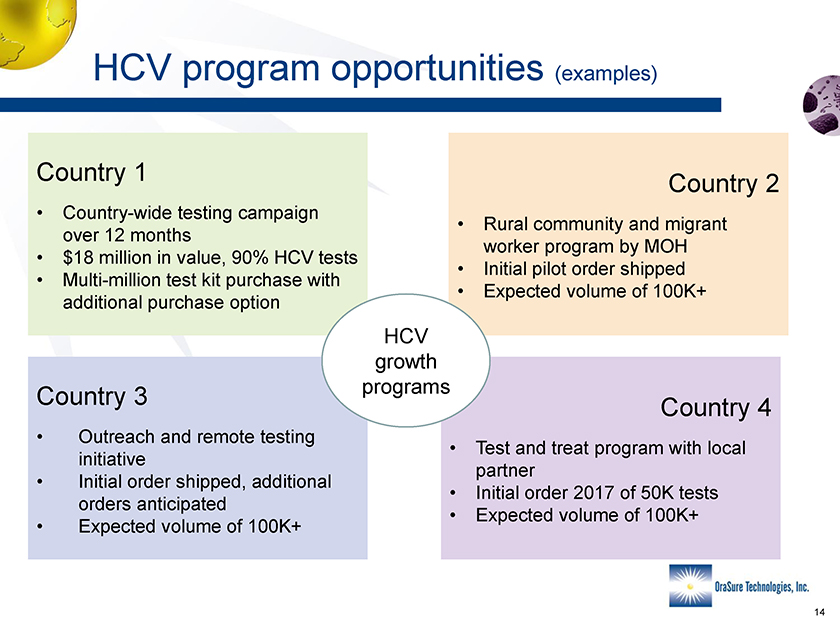
HCV program opportunities (examples) Country 1 • Country-wide testing campaign over 12 months • $18 million in value, 90% HCV tests • Multi-million test kit purchase with additional purchase option Country 3 • Outreach and remote testing initiative • Initial order shipped, additional orders anticipated • Expected volume of 100K+ Country 2 • Rural community and migrant worker program by MOH • Initial pilot order shipped • Expected volume of 100K+ Country 4 • Test and treat program with local partner • Initial order 2017 of 50K tests • Expected volume of 100K+ HCV growth programs

Excellent growth prospects • Recognized bodies (WHO) have prioritized Hepatitis and Hepatitis C for elimination • Price of HCV therapy is significantly reduced in developing countries • Large scale test and treat pilots and country elimination programs are being executed • Recognition that all test and treat programs will need a quality rapid test to optimize success • OraSure’s HCV test is seen as an ideal solution and is being incorporated into test and treat programs

HIV Self-Testing Tony Zezzo Executive Vice President Business Unit Lead, Infectious Disease OraSure Technologies, Inc.

The need and opportunity • ~100MM HIV rapid tests are deployed annually, funded largely by donor agencies • 36.7 million people globally were living with HIV (end 2015), only half of those individuals know their status • The majority of undiagnosed individuals have limited or no access to health services • Stigma associated with HIV remains an issue • The down stream costs of these issues has and will financially burden health systems • Implementation of UNAIDS 90:90:90 initiative to address situation Source: unaids
State of HIV self-testing • The first studies conducted by the London School of Hygiene and Tropical Medicine • Other studies followed by several notable organizations • Two important outcomes developed: – More people chose to test when offered a self test – OraQuick was used in all studies for its quality and ease of use • UNAIDS sited self testing as an innovation critical to achieving the 90:90:90 goals

The OraQuick® HIV self-test Self-test product contains: Standard OraQuick device and vial, single use stand and a package insert placed in an over-pouch for personal use/carry. Over-pouched for single use Standard device/vial Package insert + single use stand

OraQuick and self-testing Competitive advantage • OraQuick track record of success • Endorsement from leading KOLs • Ease of use and oral fluid matrix • Foundation and validation studies well underway
State of HIV self-testing WHO/UNITAID release landscape report on Rapid HIV self-testing • Market for HIV self testing tools is clearly growing – Drivers are replacement, frequency of testing and uptake of testing – Early estimates indicate self testing could easily reach 23M tests annually • Additional information on demand estimates is expected by the end of 2016 • 16 countries have adopted HIV self testing policies – others are currently developing them • Growing interest from international donors could make low-cost and quality self-testing tools available faster than ever before

Key progress to date • UNITAID/PSI STAR program phase 1 initiated in Zimbabwe, Malawi and Zambia • Shipped over 400K tests to pilot countries • Additional studies initiated in 6 countries with early stage interest • WHO submission received 10/7/2016 • WHO pre-qualification opens up funding through various organizations • Funding organizations highly interested (Global Fund, PEPFAR, UNITAID)

Next steps • Complete Phase 1 (750K units) of UNITAID/PSI initiative and prepare for Phase 2 (1.9M units) • Continue ongoing discussions with additional 6 countries to deploy once WHO prequalification is achieved • Initiate studies in additional countries

Summary • Major funding organizations are showing high interest in self-testing • Volume growth will be significant over the next few years • The OraQuick HIV Self-test is very well positioned as the easiest, high quality, oral fluid rapid test available • Self testing is a critical tool in reaching the undiagnosed in middle and low income countries and toward achieving the 90:90:90 millennium development goal

R&D: Emerging Diseases – Ebola & Zika Michael Reed, Ph.D Senior Vice President, Research & Development and Chief Science OraSure Technologies, Inc.

Emerging Diseases “An emerging disease is one that has appeared in a population for the first time, or that may have existed previously but is rapidly increasing in incidence or geographic range”… World Health Organization
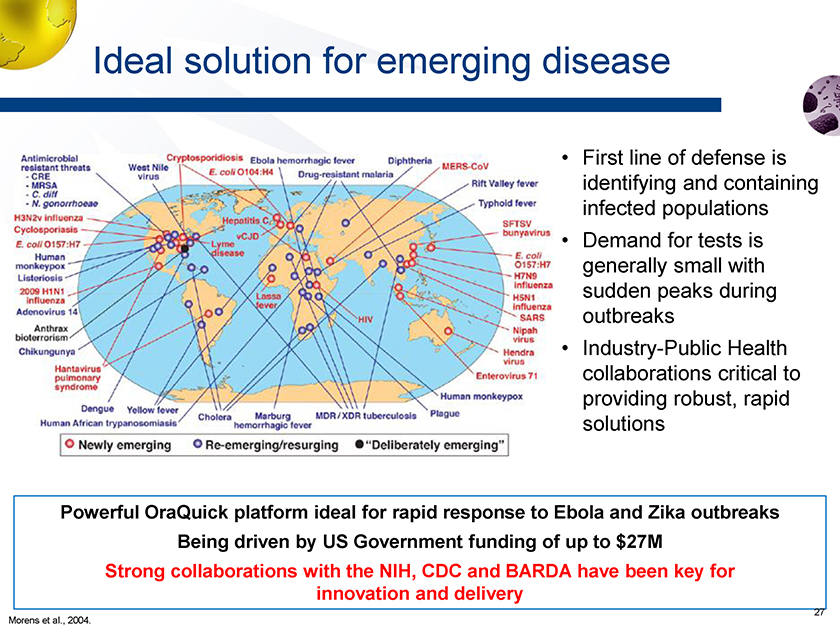
Ideal solution for emerging disease • First line of defense is identifying and containing infected populations • Demand for tests is generally small with sudden peaks during outbreaks • Industry-Public Health collaborations critical to providing robust, rapid solutions Powerful OraQuick platform ideal for rapid response to Ebola and Zika outbreaks Being driven by US Government funding of up to $27M Strong collaborations with the NIH, CDC and BARDA have been key for innovation and delivery Morens et al., 2004.
OraQuick EBOLA Rapid Antigen Test
28 28
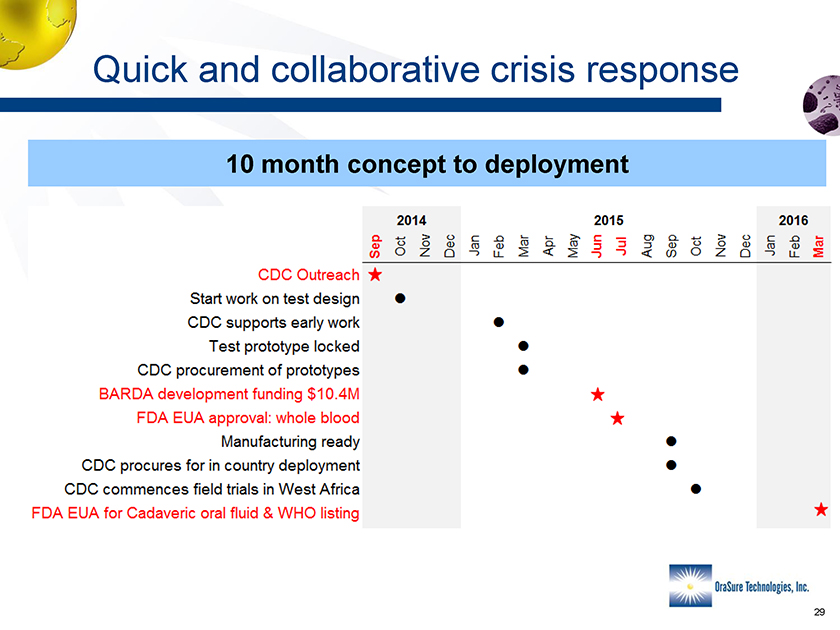
Quick and collaborative crisis response 10 month concept to deployment
��
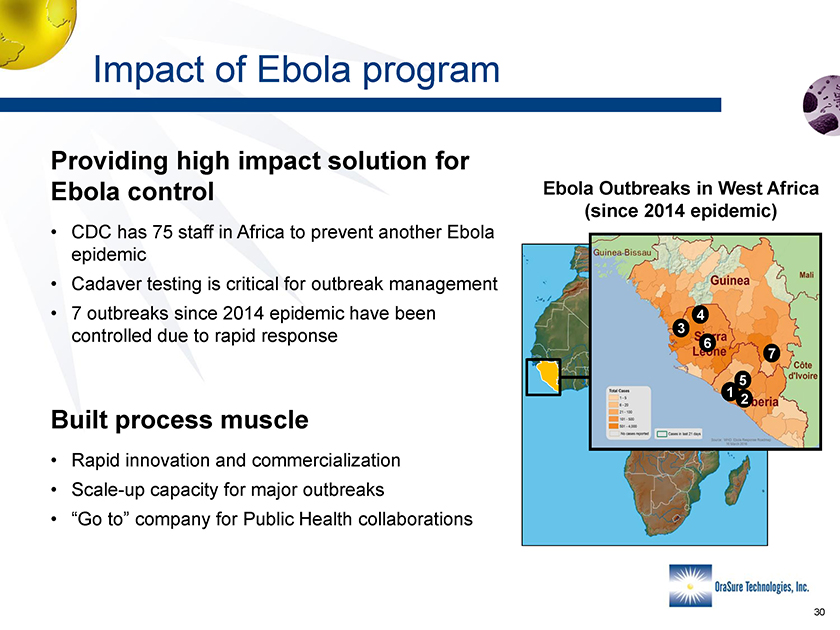
Impact of Ebola program Providing high impact solution for Ebola Outbreaks in West Africa Ebola control (since 2014 epidemic) • CDC has 75 staff in Africa to prevent another Ebola epidemic • Cadaver testing is critical for outbreak management • 7 outbreaks since 2014 epidemic have been controlled due to rapid response Built process muscle • Rapid innovation and commercialization • Scale-up capacity for major outbreaks • “Go to” company for Public Health collaborations 1 2 5 4 3 6 7
OraQuick ZIKA Rapid Antibody Test 31
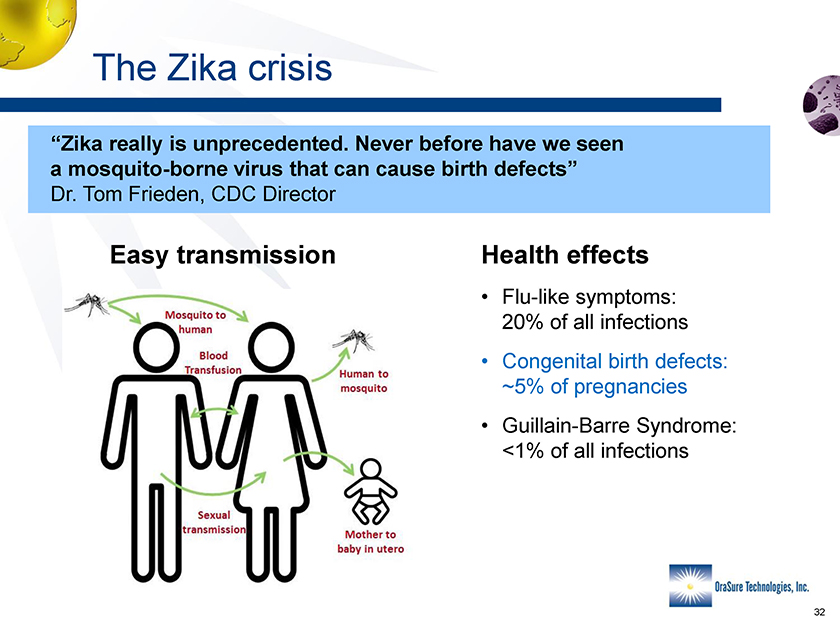
The Zika crisis “Zika really is unprecedented. Never before have we seen a mosquito-borne virus that can cause birth defects” Dr. Tom Frieden, CDC Director Easy transmission Health effects • Flu-like symptoms: 20% of all infections • Congenital birth defects: ~5% of pregnancies • Guillain-Barre Syndrome: <1% of all infections
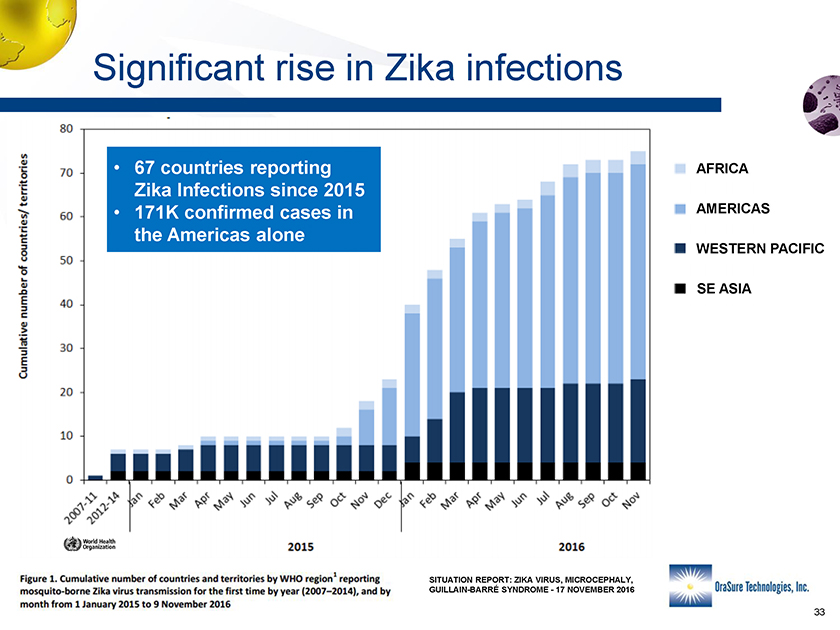
Significant rise in Zika infections • 67 countries reporting Zika Infections since 2015 • 171K confirmed cases in the Americas alone AFRICA SE ASIA WESTERN PACIFIC AMERICAS SITUATION REPORT: ZIKA VIRUS, MICROCEPHALY, GUILLAIN-BARRÉ SYNDROME -17 NOVEMBER 2016
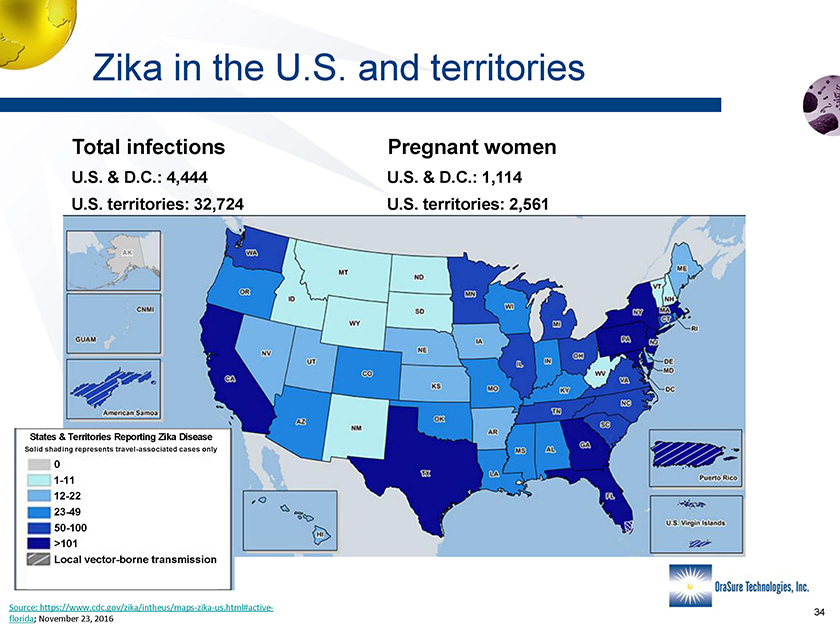
Zika in the U.S. and territories Total infections Pregnant women U.S. & D.C.: 4,444 U.S. & D.C.: 1,114 U.S. territories: 32,724 U.S. territories: 2,561 0 1-11 12-22 23-49 50-100 >101 Local vector-borne transmission States & Territories Reporting Zika Disease Solid shading represents travel-associated cases only Source: https://www.cdc.gov/zika/intheus/maps-zika-us.html#activeflorida; November 23, 2016

Zika health impact “All pregnant women in the U.S. and U.S. territories should be assessed for possible Zika virus exposure at each prenatal care visit.” ACOG Practice Advisory, October 18, 2016 “Regardless of how WHO defines Zika, it is unprecedented, and it’s an extraordinary risk for pregnant women.” Tom Frieden, CDC Director Deployed
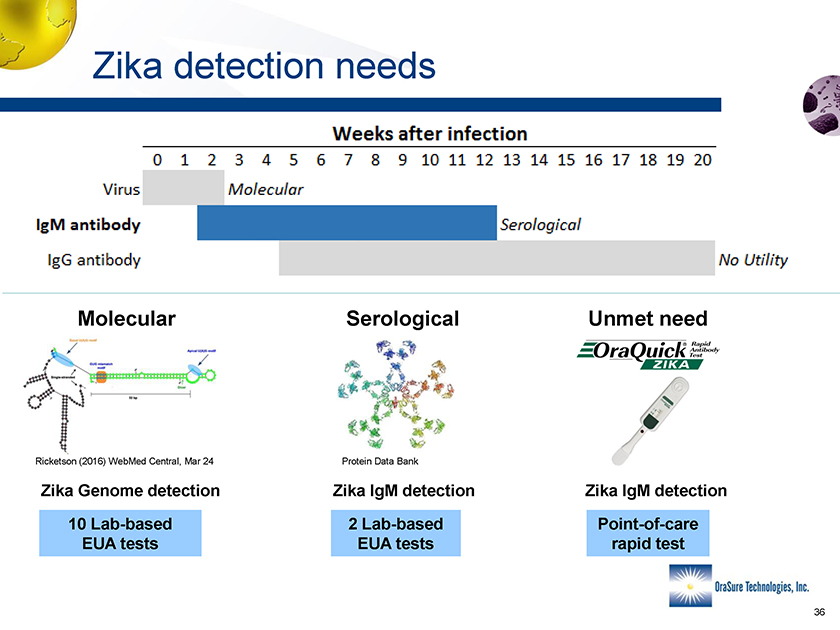
Zika detection needs Molecular Serological Unmet need Ricketson (2016) WebMed Central, Mar 24 Protein Data Bank Zika Genome detection Zika IgM detection Zika IgM detection 10 Lab-based EUA tests 2 Lab-based EUA tests Point-of-care rapid test

Awarded $16.6 M BARDA contract THE WALL STREET JOURNAL YOU ARE READING A PREVIEW OF A PAID ARTICLE SUBSCRIBE NOW TO GET CONTENT.
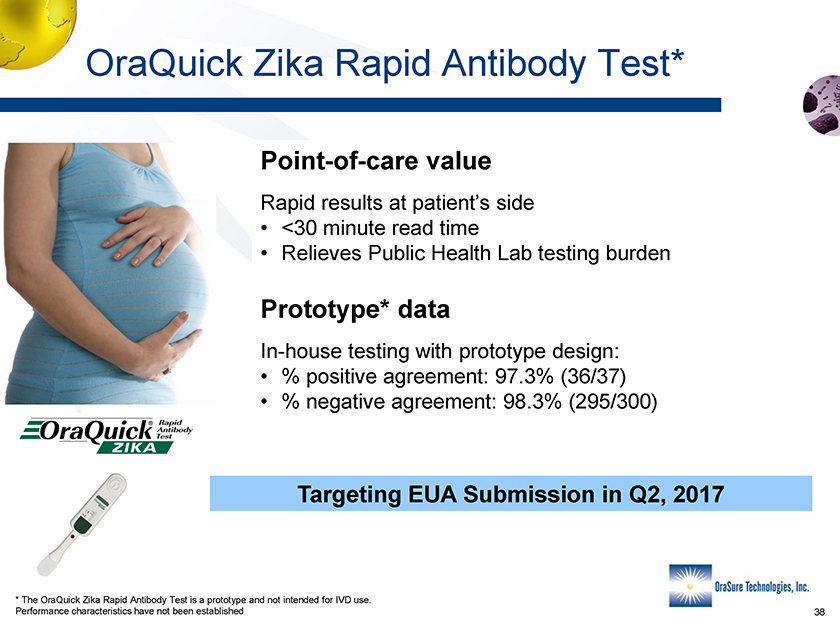
OraQuick Zika Rapid Antibody Test* Point-of-care value Rapid results at patient’s side • <30 minute read time • Relieves Public Health Lab testing burden Prototype* data In-house testing with prototype design: • % positive agreement: 97.3% (36/37) • % negative agreement: 98.3% (295/300) Targeting EUA Submission in Q2, 2017 * The OraQuick Zika Rapid Antibody Test is a prototype and not intended for IVD use. Performance characteristics have not been established
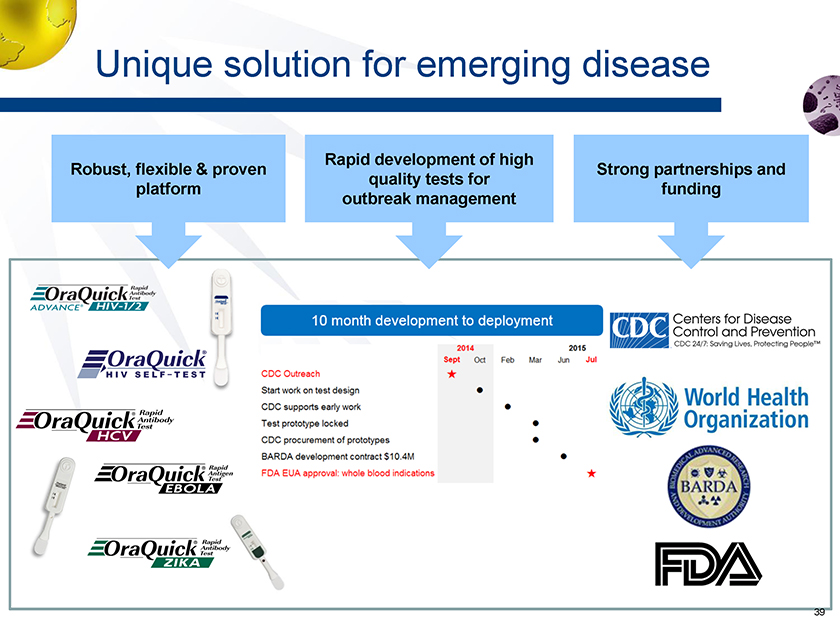
Unique solution for emerging disease Rapid development of high quality tests for outbreak management Robust, flexible & proven platform Strong partnerships and funding 39
Tuberculosis (TB) Cassandra Kelly-Cirino, Ph.D. Vice President, Infectious Disease TB and Emerging Diseases Program Lead DNA Genotek
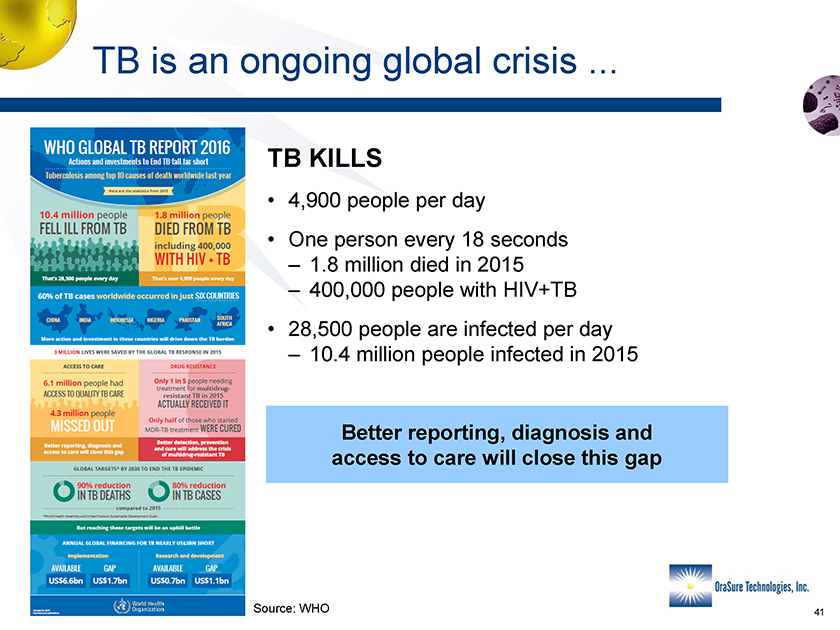
TB is an ongoing global crisis . TB KILLS • 4,900 people per day • One person every 18 seconds – 1.8 million died in 2015 – 400,000 people with HIV+TB • 28,500 people are infected per day – 10.4 million people infected in 2015 Better reporting, diagnosis and access to care will close this gap Source: WHO

WHO’s end TB strategy End the global TB epidemic • Reduce TB deaths by 90% • Cut new cases by 90% between 2015 and 2035 • Ensure that no family is burdened with catastrophic expenses due to TB

Why is TB eradication such a challenge? • Easy to catch – TB is highly contagious – spread through the air, most often by coughing • Difficult to reach patients – Poor patient access – rural settings with no infrastructure – Need to collect, transport and process viable sputum samples • Hard to diagnose – Inefficient laboratory processes – Diagnostic tests for TB are insensitive, slow and/or expensive • Hard to treat – Increasing antibiotic resistance
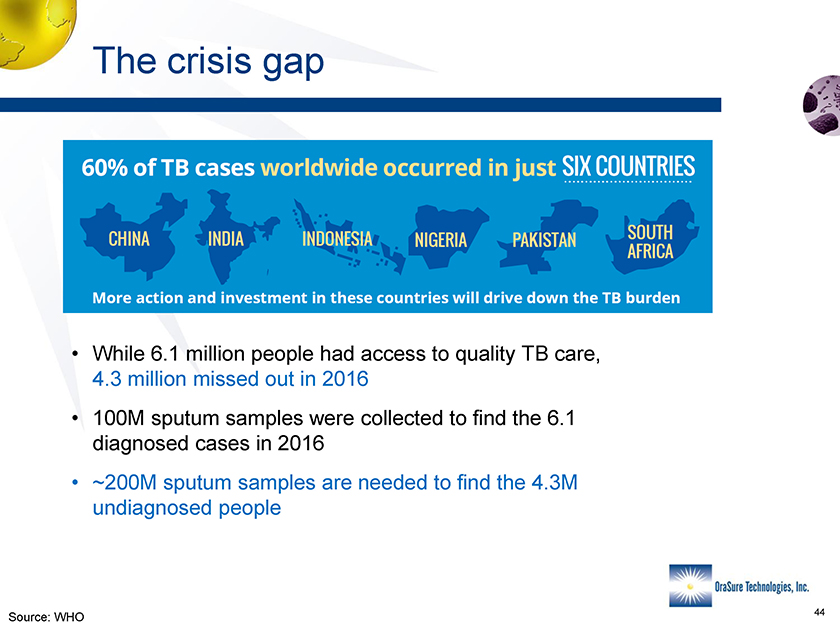
The crisis gap • While 6.1 million people had access to quality TB care, 4.3 million missed out in 2016 • 100M sputum samples were collected to find the 6.1 diagnosed cases in 2016 • ~200M sputum samples are needed to find the 4.3M undiagnosed people Source: WHO
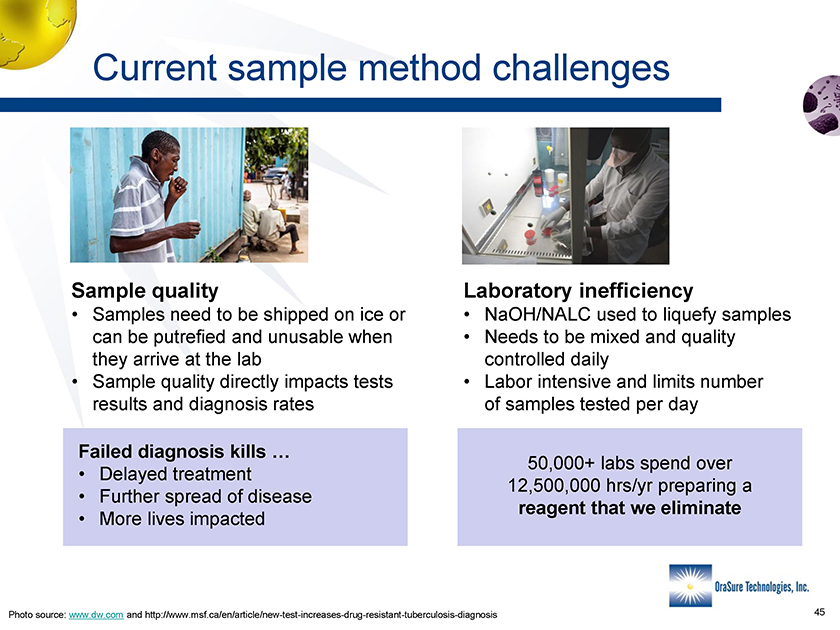
Current sample method challenges Current sample method challenges Sample quality • Samples need to be shipped on ice or can be putrefied and unusable when they arrive at the lab • Sample quality directly impacts tests results and diagnosis rates Laboratory inefficiency • NaOH/NALC used to liquefy samples • Needs to be mixed and quality controlled daily • Labor intensive and limits number of samples tested per day 50,000+ labs spend over 12,500,000 hrs/yr preparing a reagent that we eliminate Failed diagnosis kills … • Delayed treatment • Further spread of disease • More lives impacted Photo source: www.dw.com and http://www.msf.ca/en/article/new-test-increases-drug-resistant-tuberculosis-diagnosis

Our solution Leader in Biospecimen Optimization • A reagent designed to maintain MTb viability for 8 days between 39oF and 104oF while liquefying and decontaminating sputum samples. • IP protected Sample transport and • CE/IVD marked decontamination reagent
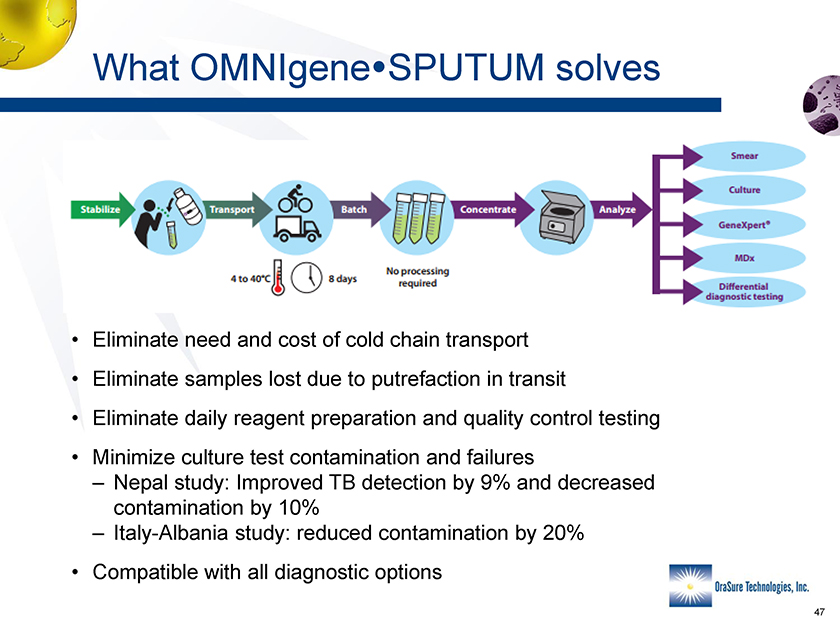
What OMNIgene SPUTUM solves • Eliminate need and cost of cold chain transport • Eliminate samples lost due to putrefaction in transit • Eliminate daily reagent preparation and quality control testing • Minimize culture test contamination and failures – Nepal study: Improved TB detection by 9% and decreased contamination by 10% – Italy-Albania study: reduced contamination by 20% • Compatible with all diagnostic options
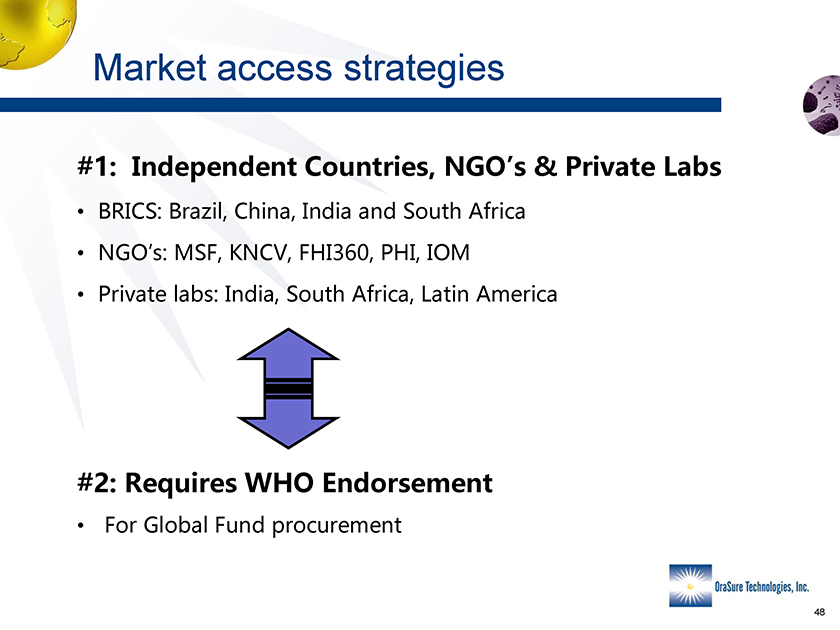
Market access strategies #1 Independent Countries, NGO’s & Private Labs • BRICS: BRAZIL, China, India and South Africa •NGO’s MSF, KNCV, FHI360, PHI IOM Private labs:India, South Africa, Latin America #2: Requires WHO Endorsement • For Global Fund Procurement
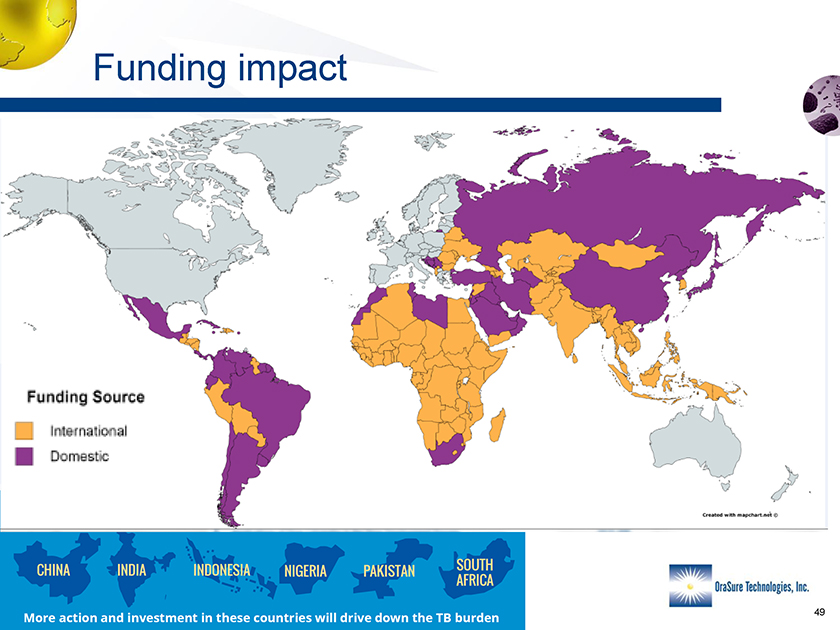
Funding impact

Market access plan In country evaluations to speed adoption upon WHO endorsement Peer reviewed publication data (open independent funding) ->ongoing 3 complete 1 more submitted 2014 2015 2016 2017 2018 CE/IVD registrations Unlock funding (USAID, Global Fund) > WHO endorsement – target Q2 2017 Establish global procurement routes -> GDF, Global Fund, MSF, UNICEF, USAID, PEPFAR and FIND Establish early adopter countries -> working with FIND and STOPTB to identify and ramp early adopter countries Scale use in early adopter countries Replicating model to other countries ~67 countries ongoing Future publications Endorsement dependent
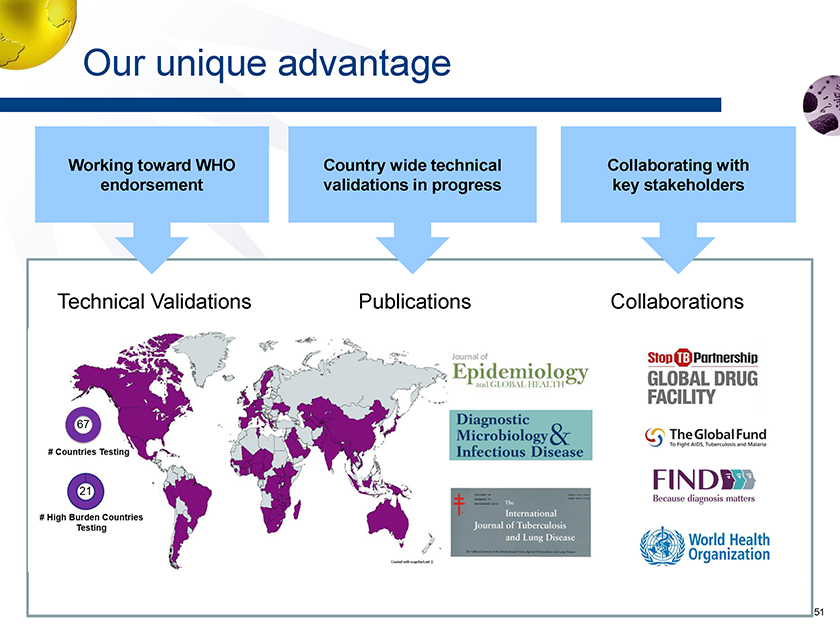
Our unique advantage Technical Validations Publications Collaborations Country wide technical validations in progress Working toward WHO endorsement Collaborating with key stakeholders

Q&A

Personal Genomics Brain Smith Senior Vice President and Business Unit Lead, Molecular DNA Genotek
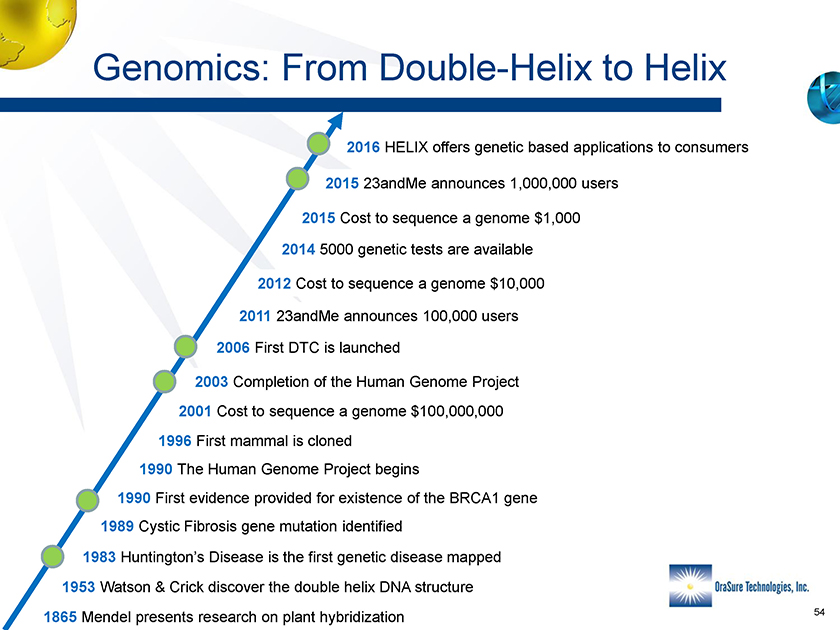
Genomics: From Double-Helix to Helix 1865 Mendel presents research on plant hybridization 1953 Watson & Crick discover the double helix DNA structure 1983 Huntington’s Disease is the first genetic disease mapped 1996 First mammal is cloned 2003 Completion of the Human Genome Project 2006 First DTC is launched 2011 23andMe announces 100,000 users 2015 23andMe announces 1,000,000 users 1989 Cystic Fibrosis gene mutation identified 1990 First evidence provided for existence of the BRCA1 gene 1990 The Human Genome Project begins 2014 5000 genetic tests are available 2016 HELIX offers genetic based applications to consumers 2001 Cost to sequence a genome $100,000,000 2012 Cost to sequence a genome $10,000 2015 Cost to sequence a genome $1,000 54 Genomics: From Double-Helix to Helix 1865 Mendel presents research on plant hybridization 1953 Watson & Crick discover the double helix DNA structure 1983 Huntington’s Disease is the first genetic disease mapped 1996 First mammal is cloned 2003 Completion of the Human Genome Project 2006 First DTC is launched 2011 23andMe announces 100,000 users 2015 23andMe announces 1,000,000 users 1989 Cystic Fibrosis gene mutation identified 1990 First evidence provided for existence of the BRCA1 gene 1990 The Human Genome Project begins 2014 5000 genetic tests are available 2016 HELIX offers genetic based applications to consumers 2001 Cost to sequence a genome $100,000,000 2012 Cost to sequence a genome $10,000 2015 Cost to sequence a genome $1,000 54
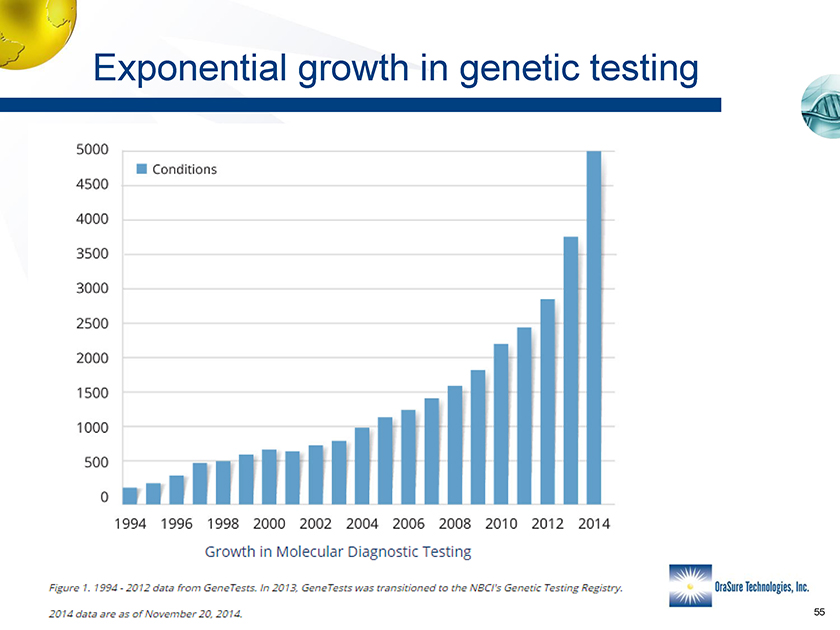
Exponential growth in genetic testing
Personal genomics segments

HELIX BUSINESS INSIDER TECH INSIDER A new $100 million company could transorm the way we interact with our own DNA One sequence Limitless learning Source: www.Helix.com

23andMe 23andMe Genotypes One Millionth Customer Direct-to-Consumer Genetic Testing Company Reaches Milestone; Increases Potential for Genetic Research 58 Source: www.23andMe.com

Growing worldwide market opportunity THE GLOBAL PLATFORM FOR GENOMIC BIG DATA WuXi NextCODE is a genomic information company using sequence data to improve health for people around the world.
Enabling personal genomics Access to patients/consumers No established collection infrastructure Competing with reference labs Reference labs • Established brick and mortar service clinics for sample collection Emerging Personal Genomics companies • In clinic or at home access to patients for sample collection • Personalized experience • Results back directly or via practitioner
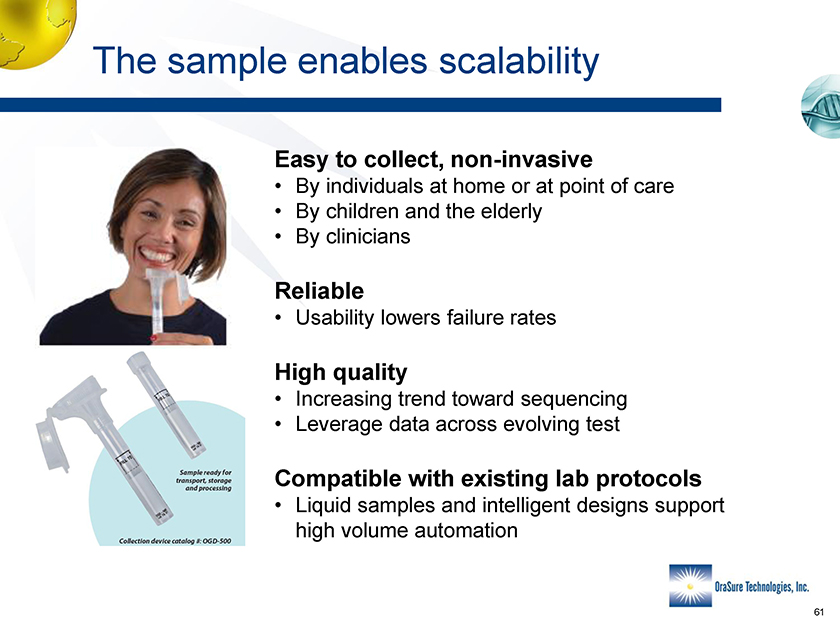
The sample enables scalability Easy to collect, non-invasive • By individuals at home or at point of care • By children and the elderly • By clinicians Reliable • Usability lowers failure rates High quality • Increasing trend toward sequencing • Leverage data across evolving test Compatible with existing lab protocols • Liquid samples and intelligent designs support high volume automation

Proven solutions First and only FDA 510(k) cleared devices Leader in Biospecimen optimization Oragene Dx and ORAcollect Dx are the only FDA 510(k) cleared devices• proven for collection, stabilization and ambient temperature transportation and storage of DNA from saliva. • FDA cleared for in vitro diagnostic use with the eSensor Warfarin Sensitivity Saliva Test.
Our unique advantage • Customizations • Donor recruitment • Logistical support • Single Order Fulfillment Products CustomersService and support Regulatory and quality focus Collaborating with leaders and innovators in the space End-to-end service and support 63

Microbiome Aaron Del Duca Vice President, Technology and Microbiome Program Lead DNA Genotek G J Q
What is the Microbiome? The human-associated microbiome comprises the trillions of micro organisms that live on us and in us.
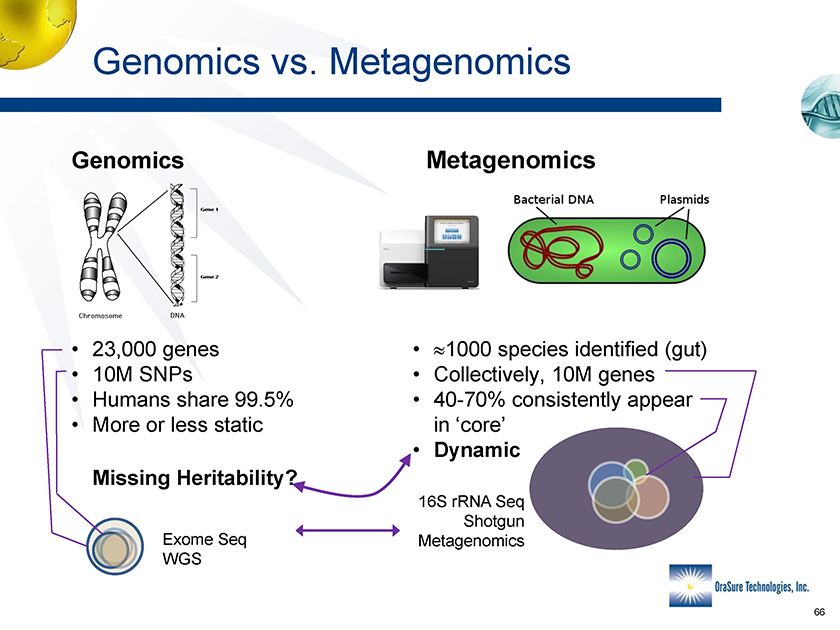
Genomics vs. Metagenomics Genomics Metagenomics • 23,000 genes • 1000 species identified (gut) • 10M SNPs • Collectively, 10M genes • Humans share 99.5% • More or less static in ‘core’• Dynamic Missing Heritability? 16S rRNA Seq Shotgun Exome Seq Metagenomics WGS • 40-70% consistently appear
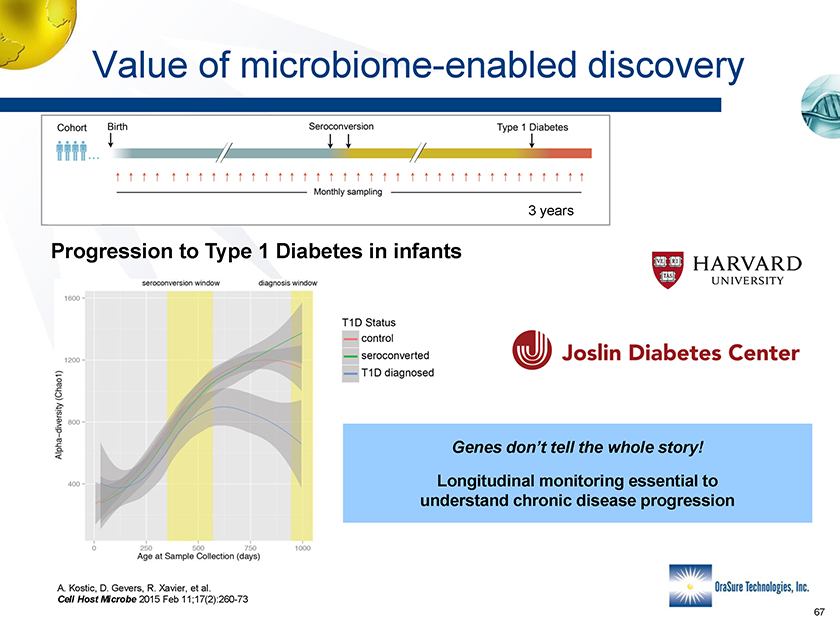
Value of microbiome-enabled discovery 3 years Progression to Type 1 Diabetes in infants Genes don’t tell the whole story! Longitudinal monitoring essential to understand chronic disease progression A. Kostic, D. Gevers, R. Xavier, et al. Cell Host Microbe 2015 Feb 11;17(2):260-73
Microbiome impact to healthcare • It influences extremely high burden/high value medical conditions – Gastrointestinal diseases, Type 1&2 Diabetes, Skin conditions, the urinary tract, women’s health and neonatal health • It provides a means of intercepting disease and personalizing treatments – Diagnostics, therapeutics, and even preventive medicine are all enabled with this new perspective on health • Microbiome science is quickly translating from research to clinical applications
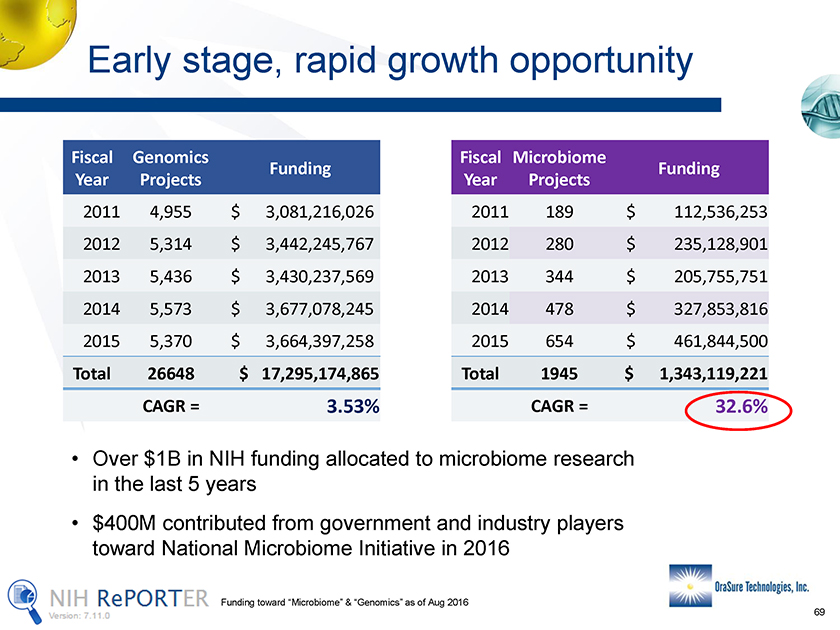
Early stage, rapid growth opportunity Fiscal Year Genomics Projects Funding Fiscal Year Microbiome Projects Funding 2011 4,955 $ 3,081,216,026 2012 5,314 $ 3,442,245,767 2013 5,436 $ 3,430,237,569 2013 344 $ 205,755,751 2014 5,573 $ 3,677,078,245 2015 5,370 $ 3,664,397,258 2014 478 $ 327,853,816 2015 654 $ 461,844,500 Total 26648 $ 17,295,174,865 CAGR = 3.53% • Over $1B in NIH funding allocated to microbiome research in the last 5 years • $400M contributed from government and industry players toward National Microbiome Initiative in 2016 2011 189 $ 112,536,253 2012 280 $ 235,128,901 Total 1945 $ 1,343,119,221 CAGR = 32.6% Funding toward “Microbiome” & “Genomics” as of Aug 2016
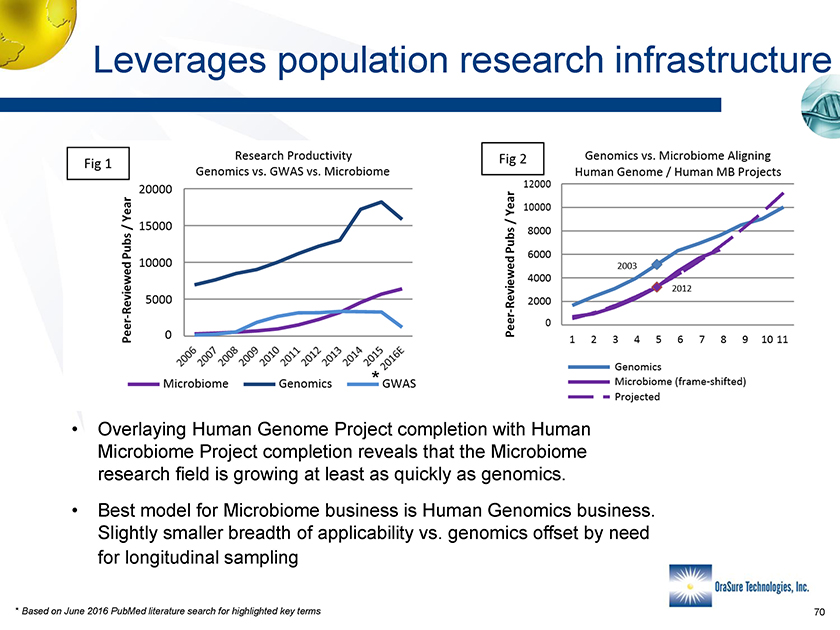
Leverages population research infrastructure * • Overlaying Human Genome Project completion with Human Microbiome Project completion reveals that the Microbiome research field is growing at least as quickly as genomics. • Best model for Microbiome business is Human Genomics business. Slightly smaller breadth of applicability vs. genomics offset by need for longitudinal sampling * Based on June 2016 PubMed literature search for highlighted key terms
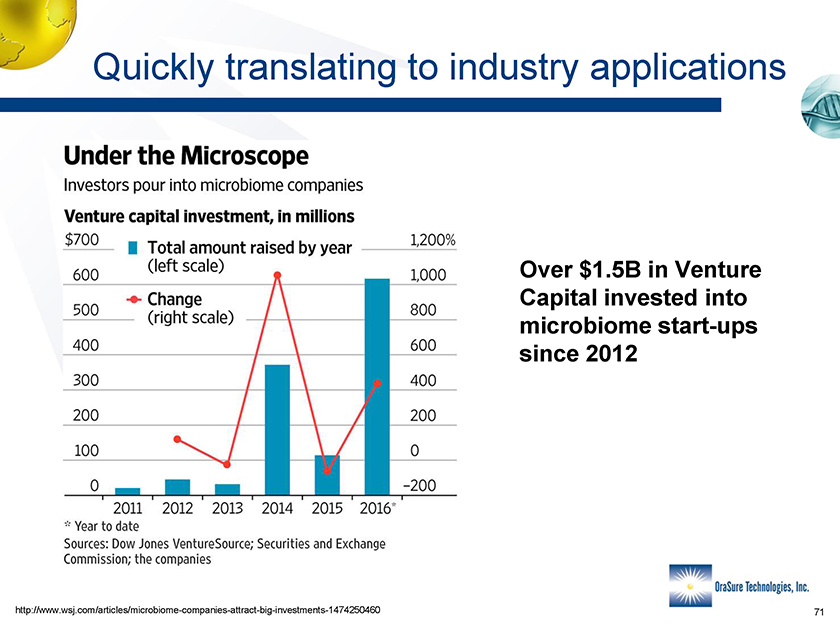
Quickly translating to industry applications Over $1.5B in Venture Capital invested into microbiome start-ups since 2012 http://www.wsj.com/articles/microbiome-companies-attract-big-investments-1474250460
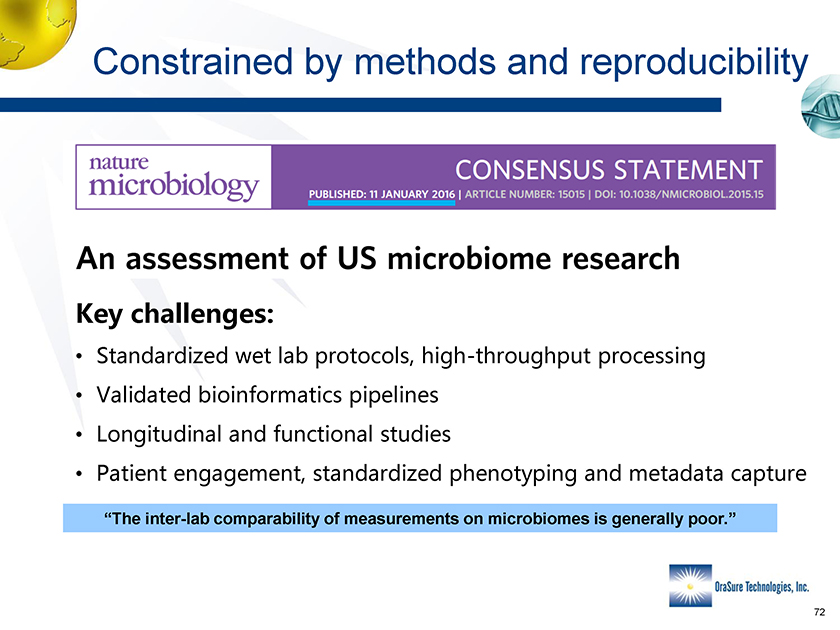
Constrained by methods and reproducibility • M • Y • Y • K “The inter-lab comparability of measurements on microbiomes is generally poor.”
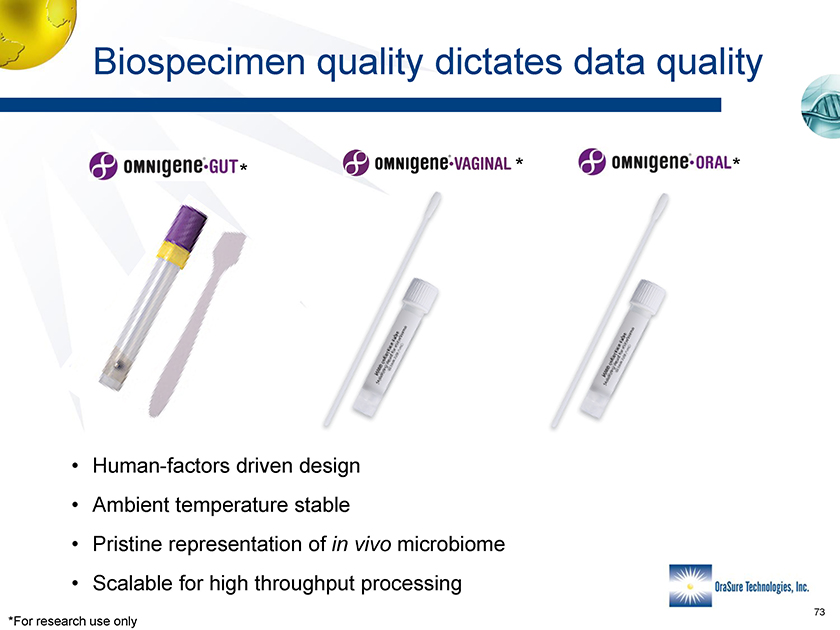
Biospecimen quality dictates data quality * * * • Human-factors driven design • Ambient temperature stable • Pristine representation of in vivo microbiome • Scalable for high throughput processing *For research use only
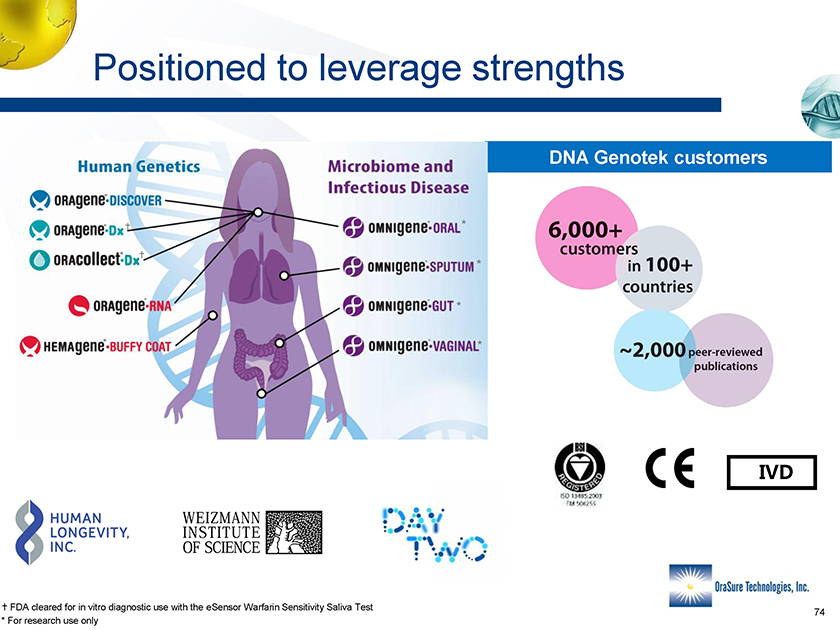
Positioned to leverage strengths DNA Genotek customers * * * * • FDA cleared for in vitro diagnostic use with the eSensor Warfarin Sensitivity Saliva Test * For research use only
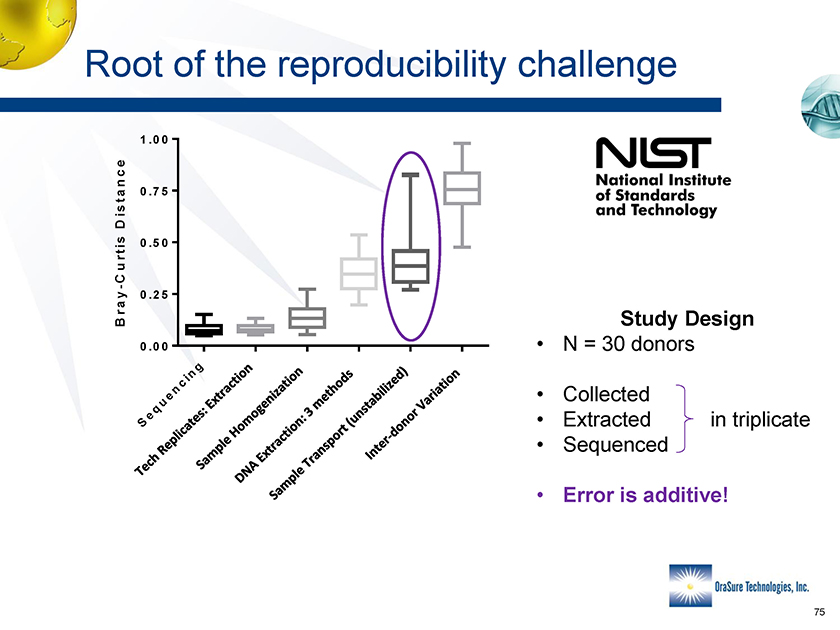
Root of the reproducibility challenge 1.00 0.75 0.50 0.25 Study Design 0.00 Sequencing Wth oge on B hodsiliz Donor onor • N = 30 donors • Collected • Extracted in triplicate • Sequenced • Error is additive! Bray-Curtis Distance
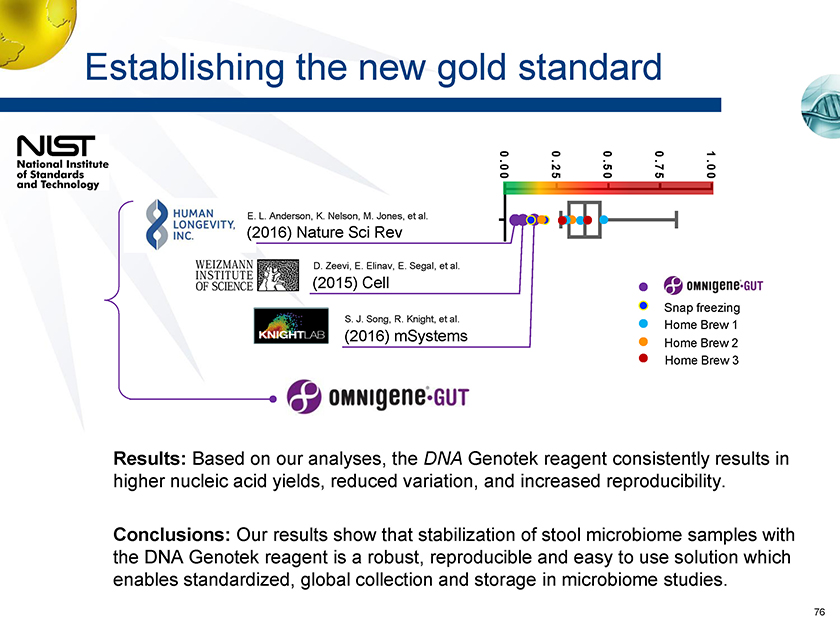
Establishing the new gold standard 1.00 0.75 0.50 0.25 0.00 E. L. Anderson, K. Nelson, M. Jones, et al. (2016) Nature Sci Rev D. Zeevi, E. Elinav, E. Segal, et al. (2015) Cell Snap freezing S. J. Song, R. Knight, et al. Home Brew 1 (2016) mSystems Home Brew 2 Home Brew 3 Results: Based on our analyses, the DNA Genotek reagent consistently results in higher nucleic acid yields, reduced variation, and increased reproducibility. Conclusions: Our results show that stabilization of stool microbiome samples with the DNA Genotek reagent is a robust, reproducible and easy to use solution which enables standardized, global collection and storage in microbiome studies.
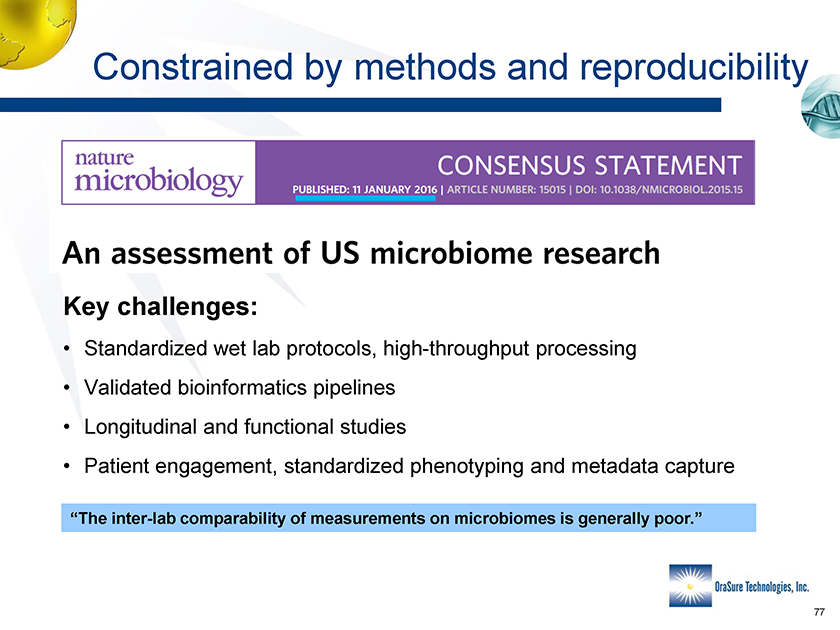
Constrained by methods and reproducibility Constrained by methods and reproducibility Key challenges: • Standardized wet lab protocols, high-throughput processing • Validated bioinformatics pipelines • Longitudinal and functional studies • Patient engagement, standardized phenotyping and metadata capture “The inter-lab comparability of measurements on microbiomes is generally poor.”
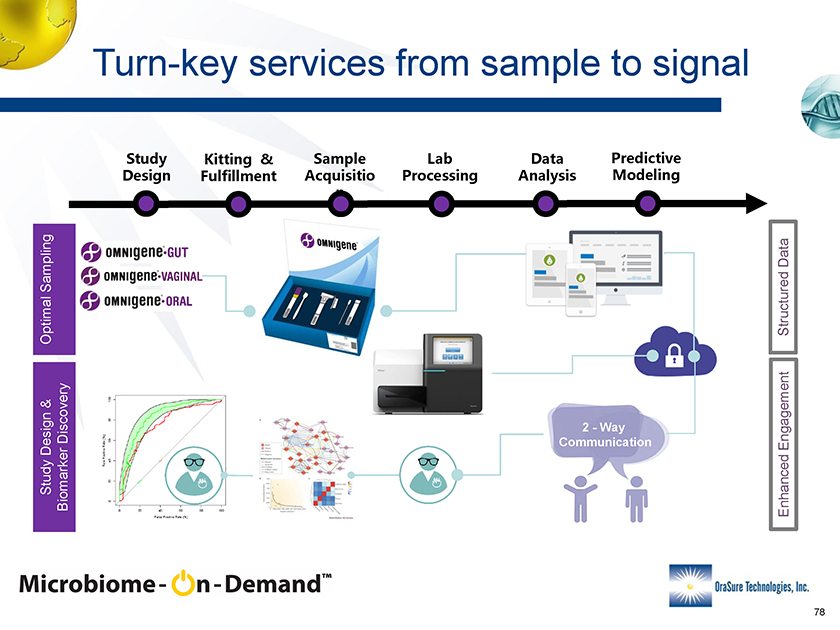
Turn-key services from sample to signal T Z U M Y M Optimal SamplingStudy Design & Biomarker Discovery Structured DataEnhanced Engagement 2 -Way Communication
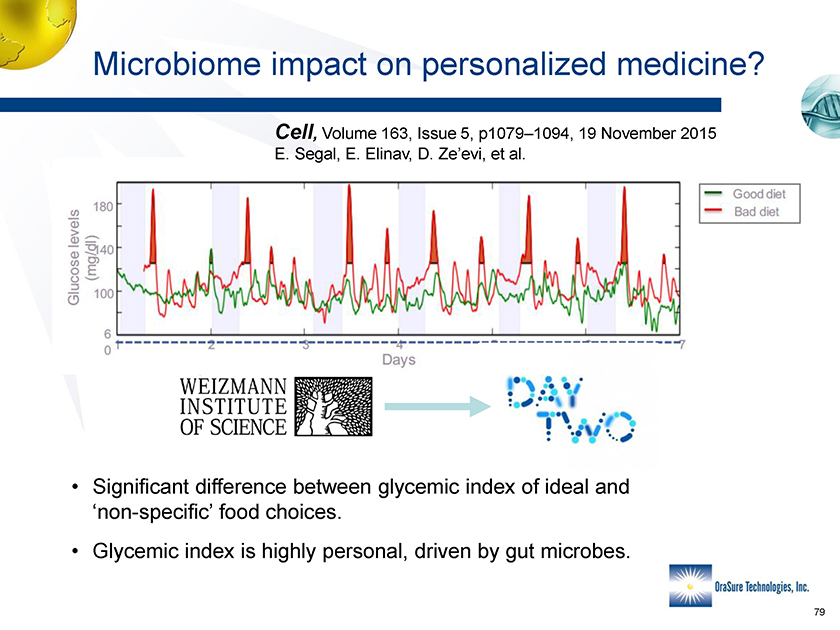
Microbiome impact on personalized medicine? Cell, Volume 163, Issu 5, p1079-19 November 2015 E. Segal, E. Elinav, D. Ze’evi, et al. E. Segal, E. Elinav, D. Ze’evi, et al. Microbiome impact on personalized medicine? Cell, Volume 163, Issue 5, p1079–1094, 19 November 2015 • Significant difference between glycemic index of ideal and ‘non-specific’ food choices. • Glycemic index is highly personal, driven by gut microbes.
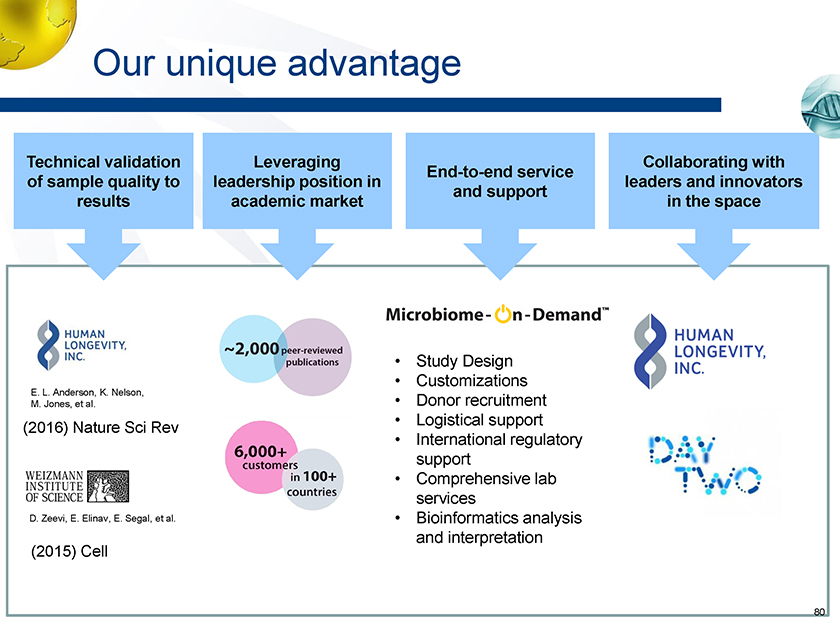
Our unique advantage End-to-end service and support Technical validation of sample quality to results Leveraging leadership position in academic market • Study Design • Customizations E. L. Anderson, K. Nelson, • Donor recruitment M. Jones, et al. • Logistical support (2016) Nature Sci Rev • International regulatory support • Comprehensive lab services D. Zeevi, E. Elinav, E. Segal, et al. • Bioinformatics analysis and interpretation (2015) Cell Collaborating with leaders and innovators in the space
R&D: Sample Optimization Rafal Iwasiow, Ph.D. Vice President R&D Molecular DNA GEnotek
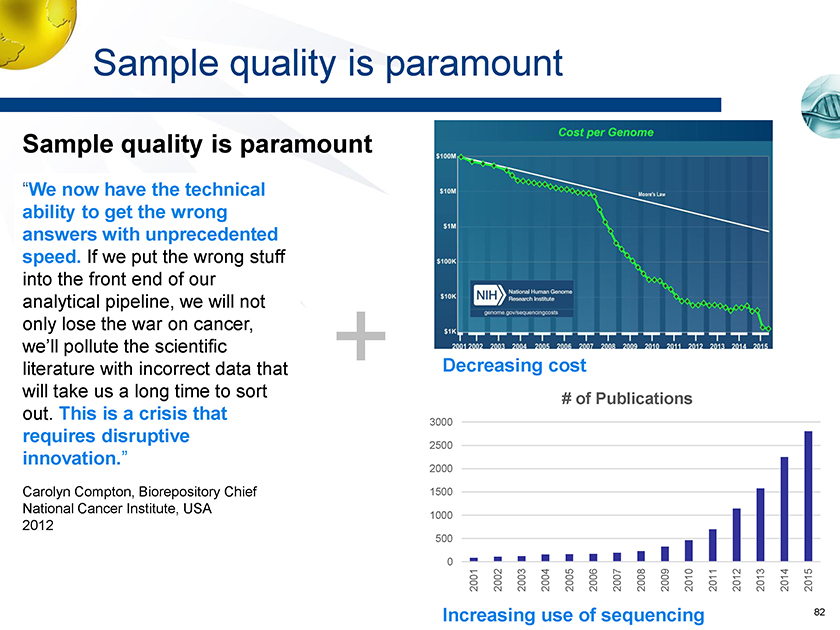
Sample quality is paramount Sample quality is paramount “We now have the technical ability to get the wrong answers with unprecedented speed. If we put the wrong stuff into the front end of our analytical pipeline, we will not only lose the war on cancer, we’ll pollute the scientific + literature with incorrect data that will take us a long time to sort # of Publications out. This is a crisis that 3000 requires disruptive 2500 innovation.” 2000 Carolyn Compton, Biorepository Chief 1500 National Cancer Institute, USA 1000 500 0 Decreasing cost 200120022003200420052006200720082009201020112012201320142015 Increasing use of sequencing
Our current solutions – optimized samples optimized samples Leader in Biospecimen Optimization • Easy and reliable collection • Stabilize and protect the in vivo state • Ship and store at ambient temperature • Integrate into lab protocols and automation • High quality DNA/RNA for all applications • Increase sensitivity and improve results of assays
Proven platforms accelerate growth Molecular Platforms Combined physical design and reagent to optimize products to address market needs 2004 2011 2005 2006 2007 20102002 2008 2009 2012 2013 2014 2015 2016 2017 • FDA cleared for in vitro diagnostic use with the eSensor Warfarin Sensitivity Saliva Test
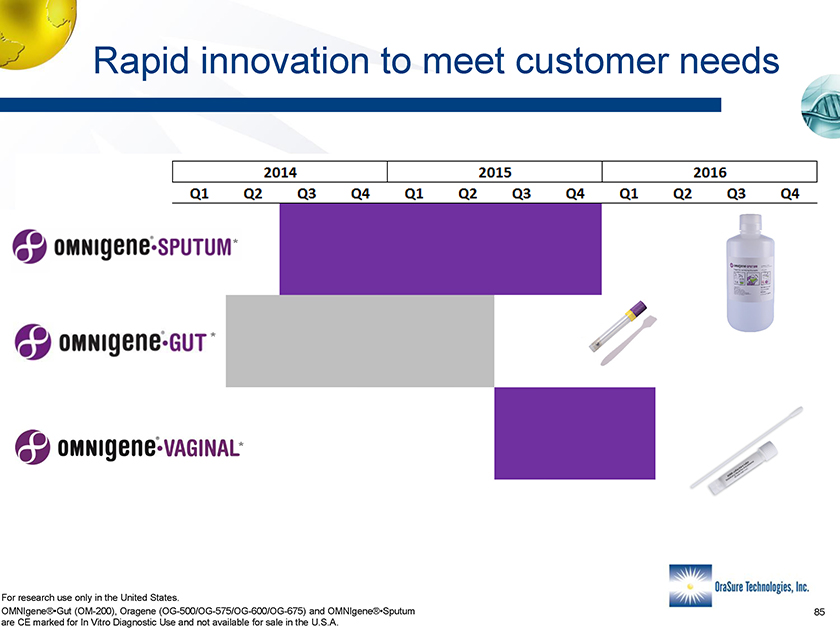
Rapid innovation to meet customer needs * * * For research use only in the United States. OMNIgene®•Gut (OM-200), Oragene (OG-500/OG-575/OG-600/OG-675) and OMNIgene®•Sputum are CE marked for In Vitro Diagnostic Use and not available for sale in the U.S.A.
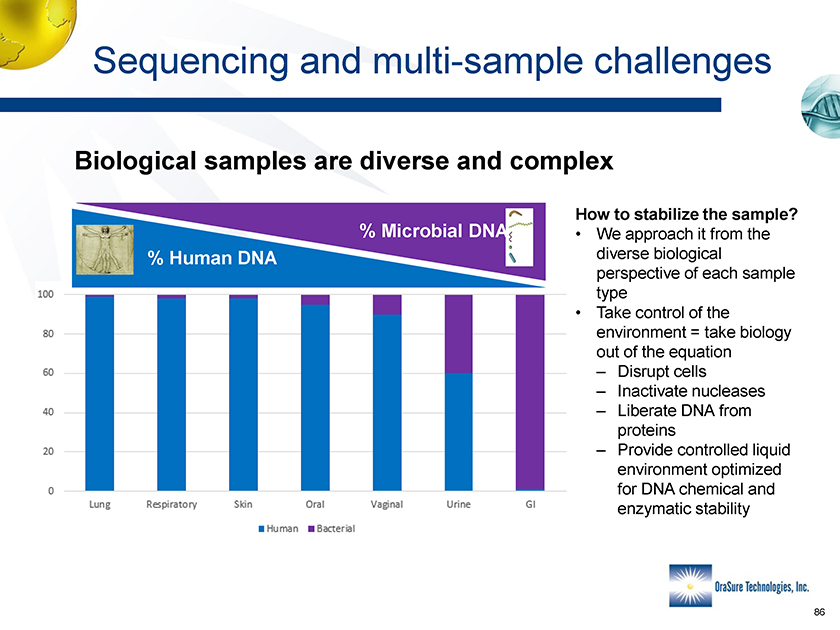
Sequencing and multi-sample challengs Biological samples are diverse and complex % Microbial DNA % Human DNA How to stabilize the sample? • We approach it from the diverse biological perspective of each sample type • Take control of the environment = take biology out of the equation – Disrupt cells – Inactivate nucleases – Liberate DNA from proteins – Provide controlled liquid environment optimized for DNA chemical and enzymatic stability
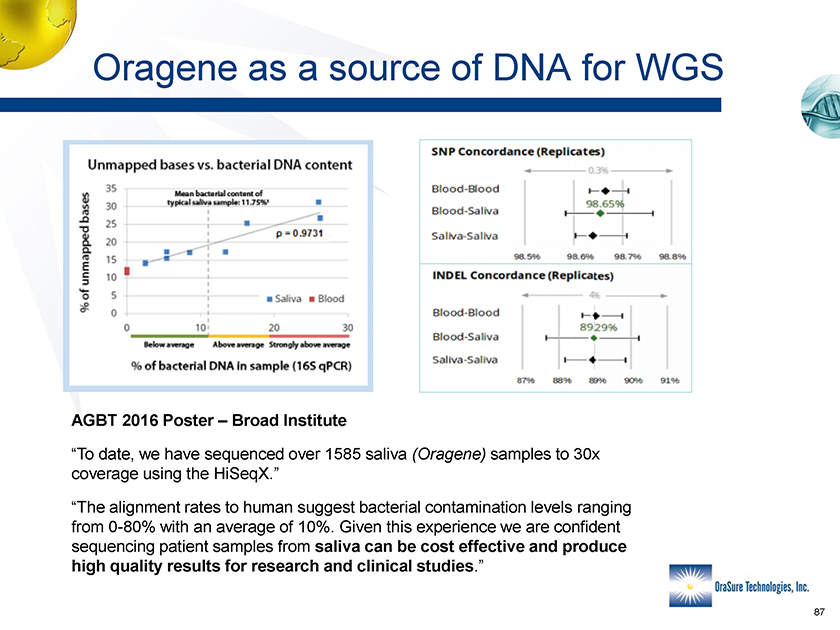
Oragene as a source of DNA for WGS AGBT 2016 Poster – Broad Institute “To date, we have sequenced over 1585 saliva (Oragene) samples to 30x coverage using the HiSeqX.” “The alignment rates to human suggest bacterial contamination levels ranging from 0-80% with an average of 10%. Given this experience we are confident sequencing patient samples from saliva can be cost effective and produce high quality results for research and clinical studies.”
New platform opportunity Y K K T New platform opportunity Targeted market solutions for optimized sequencing workflow Upsell for existing customers
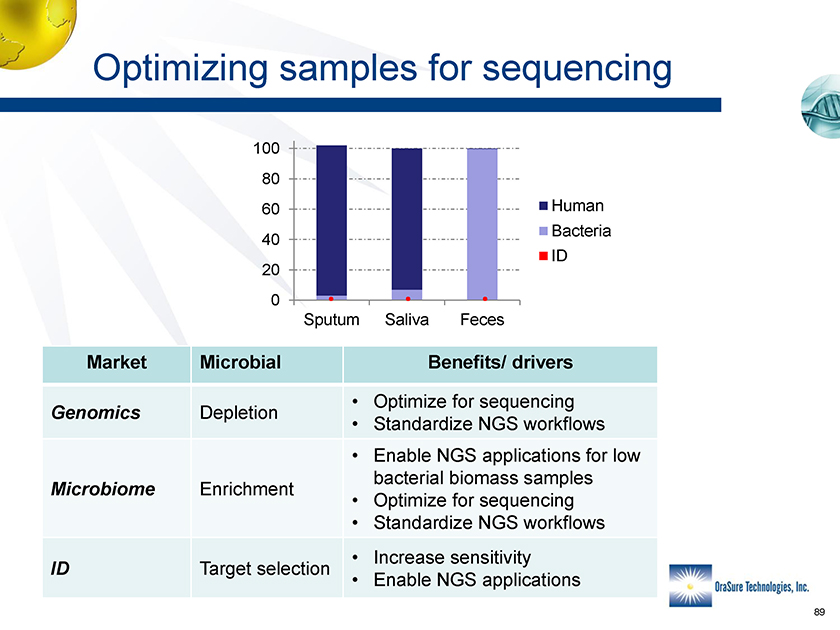
Optimizing samples for sequencing 100 80 60 40 20 0 Sputum Saliva Feces Human Bacteria ID Market Microbial Benefits/ drivers • Optimize for sequencing Genomics Depletion • Standardize NGS workflows • Enable NGS applications for low bacterial biomass samples Microbiome Enrichment • Optimize for sequencing • Standardize NGS workflows • Increase sensitivity ID Target selection • Enable NGS applications

Financial Review / Business Development Ron Spair Chief Operating Officer and Chief Financial Officer

Business development program Over $120 million in cash and marketable securities and no debt Our criteria: • Seeking opportunity to in-license, partner or acquire a product or company that complements or leverages our existing business • Focused on sample prep, enrichment, collection, microbiome and infectious disease • Preferably a late-stage or approved product(s) • Favorable reimbursement and regulatory profile • Disciplined buyers
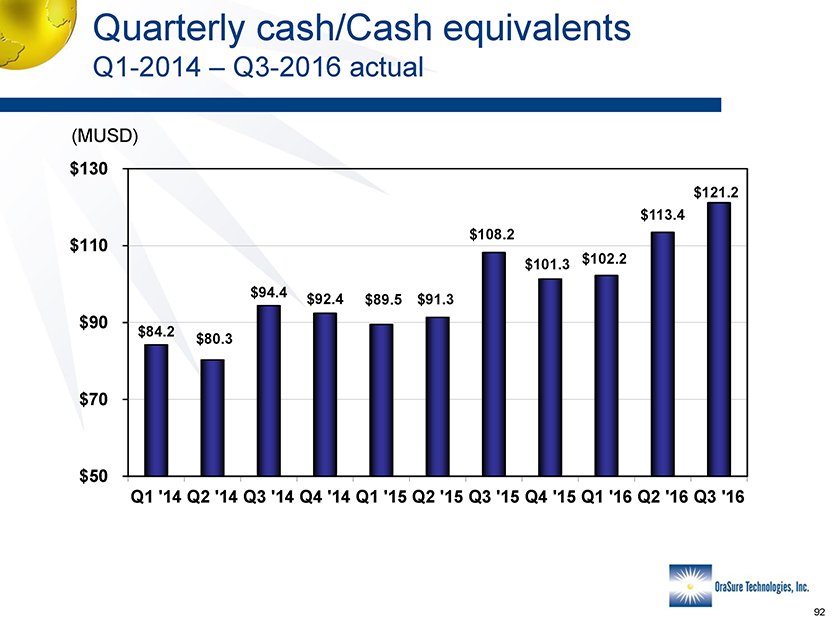
Quarterly cash/Cash equivalents Q1-2014 – Q3-2016 actual (MUSD) $130 $110 $90 $70 $50 Q1 ‘14 Q2 ‘14 Q3 ‘14 Q4 ‘14 Q1 ‘15 Q2 ‘15 Q3 ‘15 Q4 ‘15 Q1 ‘16 Q2 ‘16 Q3 ‘16 $84.2 $80.3 $94.4 $92.4 $89.5 $91.3 $108.2 $101.3 $102.2 $113.4 $121.2
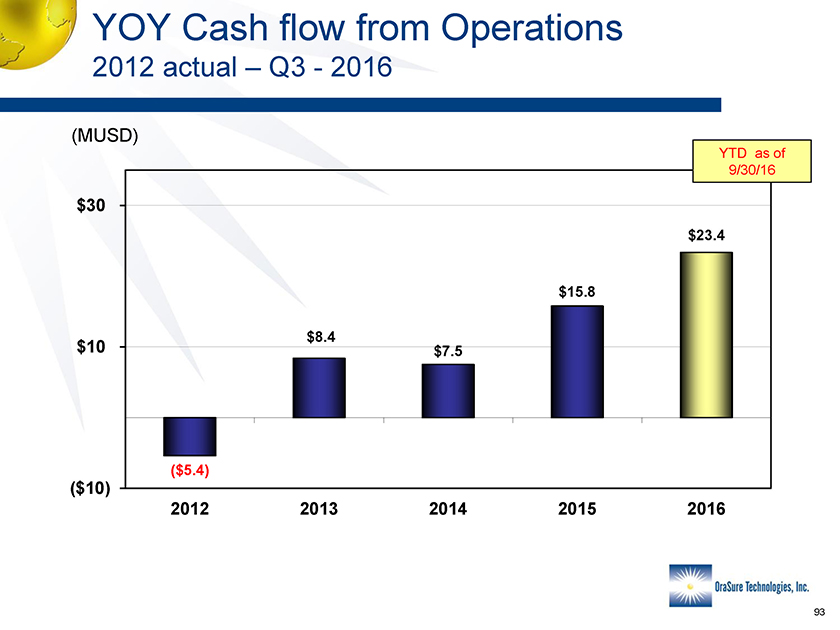
YOY Cash flow from Operations 2012 actual – Q3 -2016 (MUSD) $30 $10 ($10) ($5.4) $8.4 $7.5 $15.8 $23.4 2012 2013 2014 2015 2016 YTD as of 9/30/16

ID business development strategy Infectious Disease strategies drive specific business development opportunities to investigate: • Technology to enhance current lateral flow devices (e.g. digital reader, assay, technology licenses) • Technologies to expand point of care testing portfolio (lateral flow, POC MDx, other) • Access to low cost products or enabling technology that may lower manufacturing costs

Molecular business development strategy Molecular business strategies drive specific business development needs to investigate: • Collection, stabilization, enrichment and prep technologies that are disruptive and enable innovation in NGS market (small volume blood, liquid biopsy, sample to sequence) • Analytics that directly support microbiome expansion, and potentially lab services
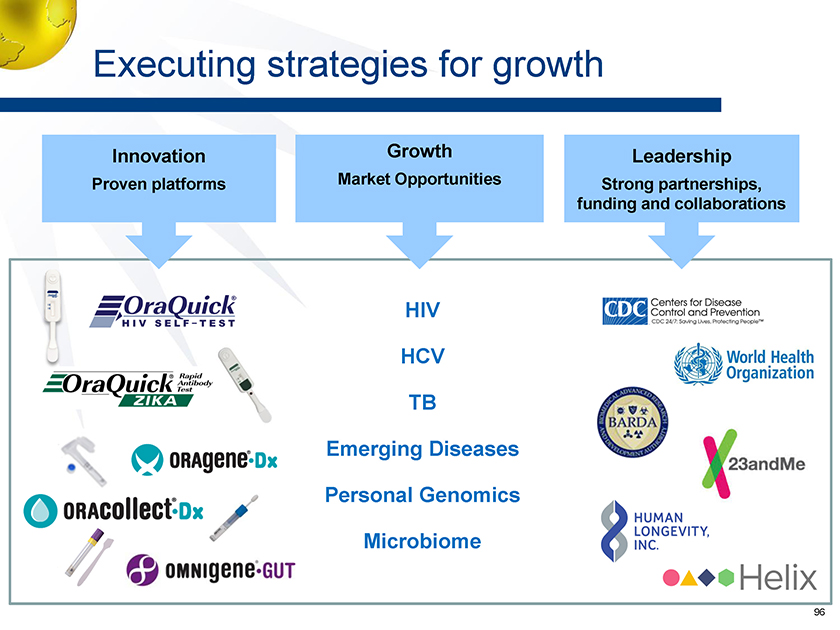
Executing strategies for growth Growth Market Opportunities Innovation Proven platforms Leadership Strong partnerships, funding and collaborations HIV HCV TB Emerging Diseases Personal Genomics Microbiome

Q&A

Additional information

Glossary BARDA: Biomedical Advanced Research and Development Authority FIND: Foundation for Innovative New Diagnostics GDF: Global Drug Facility IgM: Immunoglobulin M IOM: International Organization for Migration KNCV: Royal Netherlands Chemical Society MSF: Doctors without Boarders NGO: Non-Government Organization PEPFAR: President’s Emergency Plan for AIDS Relief PHI: Public Health Institute PSI: Population Services International UNAIDS: The Joint United Nations Programme on HIV/AIDS UNITAID: Innovative Financing Mechanism for Global Health USAID: United States Agency for International Development WHO: World Health Organization


































































































