Exhibit 99.1
Preliminary Safety Results and Antiviral Activity from the Open-label Pilot Portion of a Phase 3 Study to Evaluate Brincidofovir (BCV) for the Treatment of Adenovirus (AdV) Infection
 Session: Oral Abstract Session: Late Breaker Oral Abstracts
Session: Oral Abstract Session: Late Breaker Oral Abstracts
Saturday, October 11, 2014: 10:50 AM
Room: The Pennsylvania Convention Center: 105-AB
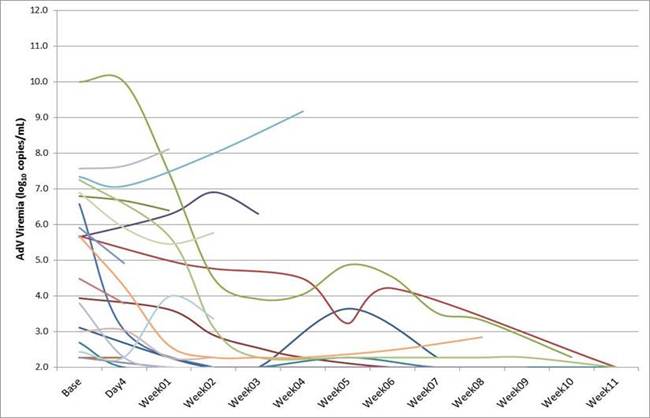
Background:AdV is a serious, often rapidly fatal infection in the immunocompromised, especially hematopoietic cell transplant (HCT) recipients. There is no approved treatment (tx) for AdV; intravenous cidofovir (CDV) has been reportedly used off-label despite the risk of renal injury. BCV (CMX001), an orally available lipid-conjugate of CDV in Phase 3 development for cytomegalovirus (CMV) prevention in HCT, has demonstrated antiviral activity in Phase 2 in AdV infection. The pilot portion of a Phase 3 study (NCT02087306) was initiated in March 2014; preliminary results for 25 subjects enrolled through 09 July 2014 are described.Methods:Adult and pediatric subjects with AdV infection receive open-label BCV 100 mg (adults ≥ 50 kg) or 2 mg/kg (≤ 12 y or< 50 kg) twice a week for 12 wks followed by a 24-wk follow-up period.Results:Mean (range) age: 9.7 (0-29) y, 68% ≤ 12 y; 64% male; 72% White; 80% HCT; median AdV viremia (VL) for 21 subjects at baseline (BL) = 5.6 (range: detected, < 2.3 to > 10.0) log10c/mL; other double-stranded DNA virus coinfections: 9 BKV viruria, 5 CMV viremia, 2 EBV viremia. Median (range) BCV duration at this reporting is 2 (1-12) wks. Fifteen subjects have > 1 wk of AdV PCR VL data (Figure 1). Of these 15, 3 subjects had AdV VL< 1000 c/mL at BL, with 2 undetectable by Wk 1. Twelve subjects had AdV VL ≥ 1000 c/mL at BL, 6 were AdV undetectable during BCV tx: by Wk 2 (n=3), Wk 6 (n=1) and Wk 11 (n=2). One subject's AdV declined from > 10 log10 c/mL to below the limit of quantitation (BLQ, 2.3 log10) by Wk 10. Two subjects had AdV VL increases on tx. Nine subjects have died, all but one within 4 wks of enrollment, attributed to AdV (5), bacterial sepsis (1), septic shock (1), multiorgan failure/pulmonary hemorrhage (1), or progression of underlying condition (1). One subject had two drug-related serious adverse events (AEs) of diarrhea and nausea. No subjects have discontinued due to BCV-related AEs.Conclusions:The majority of evaluable subjects to date had reduction or clearance of AdV viremia during BCV therapy. These preliminary results support the continued development of BCV for AdV infection and will be used to inform the final design of the Phase 3 study. Additional data from recently enrolled subjects will be included in the presentation.
Figure 1: Smooth-line Scatter Plots of AdV Viremia (log10 copies/mL) Over Time
Jo-Anne Young, MD1, Michael Grimley, MD2, David Jacobsohn, MD, ScM3, Gabriela Maron, MD4, Greg Chittick5, Thomas Brundage, MS5, Herve Mommeja-Marin, MD5 and Michelle Berrey, MD, MPH5, (1)University of Minnesota Medical Center, Minneapolis, MN, (2)Cincinnati Children’s Hospital, Cincinnati, OH, (3)George Washington University School of Medicine and Health Sciences, Washington, DC, (4)St. Jude Children's Research Hospital, Memphis, TN, (5)Chimerix, Inc., Durham, NC
 Session: Oral Abstract Session: Late Breaker Oral Abstracts
Session: Oral Abstract Session: Late Breaker Oral Abstracts