
CHIMERIX EXCLUSIVE LICENSE OF CX - 01 TRANSACTION SUMMARY July 31, 2019 1 Exhibit 99.2

2 Forward - Looking Statements These slides contain forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward - looking statements include those relating to, among other things, the potential benefits to be derived from the License and Development Agreement with Cantex Pharmaceuticals, including any statements related to CX - 01; Chimerix’s ability to develop disease modifying and potentially curative treatments for diseases, including AML. Among the factors and risks that could cause actual results to differ materially from those indicated in the forward - looking statements are risks that the benefits of the agreement with Cantex may never be realized; risks that CX - 01 may not obtain regulatory approval from the FDA or such approval may be delayed or conditioned; risks that development activities related to CX - 01 may not be completed on time or at all; Chimerix’s reliance on a sole source third - party manufacturer for CX - 01; risks that ongoing or future clinical trials may not be successful or replicate previous clinical trial results, or may not be predictive of real - world results or of results in subsequent clinical trials; risks and uncertainties relating to competitive products and technological changes that may limit demand for CX - 01; risks that CX - 01 may be precluded from commercialization by the proprietary rights of third parties; and additional risks set forth in the Company's filings with the Securities and Exchange Commission. These forward - looking statements represent the Company's judgment as of the date of this release. The Company disclaims, however, any intent or obligation to update these forward - looking statements.
3 CX - 01 provides compelling opportunity for development in first - line (1L) AML (1) , as well as other hematologic indications ▪ High unmet need in AML (5 year survival < 30%, mOS (2) < 1 year in high risk pts) ▪ Strong / consistent clinical data ▪ Multi - modal mechanism positions well vs competition ▪ Attractive safety profile ▪ Rationale for combination with standard chemotherapy and targeted agents ▪ Platform technology in other hematologic indications ▪ Fast track designation and orphan drug designation in the U.S. in AML (1) acute myeloid leukemia (2) median overall survival

4 Transaction summary ▪ Exclusive WW license to CX - 01 from Cantex Pharmaceuticals, Inc for all uses – Chimerix took assignment of long - term exclusive manufacturing agreement with Scientific Protein Laboratories for production of CX - 01 ▪ Chimerix has full rights to develop and commercialize CX - 01 in all markets globally and will incur 100% of the development and commercial costs ▪ Financial Terms – $30 million upfront – No additional payments owed Cantex until first approval – Milestones for first approval in US, EU and Japan totaling $105 million (1) – Milestones for subsequent approvals in US, EU and Japan totaling $97.5 million (1) – Sales milestones for achievement of certain revenue amounts totaling $385 million (1) – Tiered royalties on net sales starting at 10% and ending in high - teens – Equity: 10,000,000 shares of Chimerix common stock (1) No milestone to be paid more than once
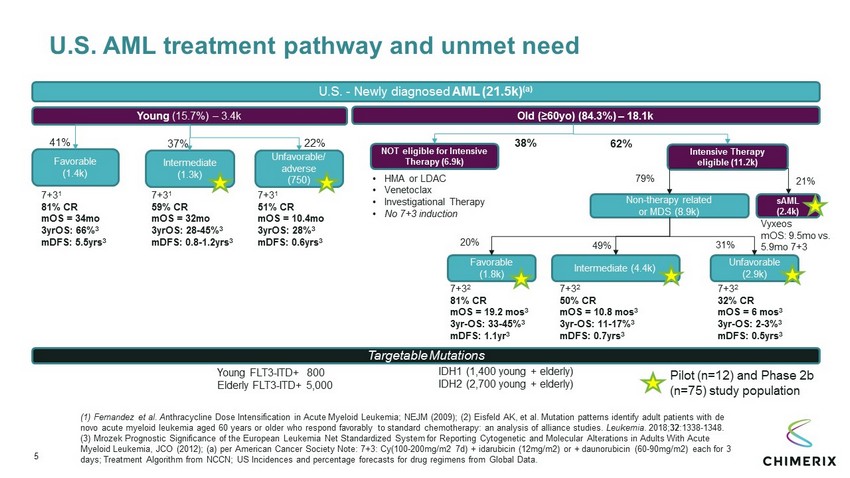
U.S. AML treatment pathway and unmet need U.S. - Newly diagnosed AML (21.5k) (a) Intensive Therapy eligible (11.2k) NOT eligible for Intensive Therapy (6.9k) 20% 62% 38% 49% 31% Young (15.7%) – 3.4k Old (≥60yo) (84.3%) – 18.1k Favorable (1.4k) Unfavorable/ adverse (750) Intermediate (1.3k) 7+3 1 81% CR mOS = 34mo 3yrOS: 66% 3 mDFS : 5.5yrs 3 7+3 1 59% CR mOS = 32mo 3yrOS: 28 - 45% 3 mDFS : 0.8 - 1.2yrs 3 Targetable Mutations Young FLT3 - ITD+ 800 Elderly FLT3 - ITD+ 5,000 41% 37% 22% • HMA or LDAC • Venetoclax • Investigational Therapy • No 7+3 induction sAML (2.4k) Non - therapy related or MDS (8.9k) 21% Vyxeos mOS : 9.5mo vs. 5.9mo 7+3 Favorable (1.8k) 79% Unfavorable (2.9k) Intermediate (4.4k) 7+3 2 32% CR mOS = 6 mos 3 3yr - OS: 2 - 3% 3 mDFS : 0.5yrs 3 7+3 1 51% CR mOS = 10.4mo 3yrOS: 28% 3 mDFS : 0.6yrs 3 IDH1 (1,400 young + elderly) IDH2 (2,700 young + elderly) 7+3 2 81% CR mOS = 19.2 mos 3 3yr - OS: 33 - 45% 3 mDFS : 1.1yr 3 7+3 2 50% CR mOS = 10.8 mos 3 3yr - OS: 11 - 17% 3 mDFS : 0.7yrs 3 Pilot (n=12) and Phase 2b (n=75) study population (1) Fernandez et al. A nthracycline Dose Intensification in Acute Myeloid Leukemia ; NEJM (2009); (2) Eisfeld AK, et al. Mutation patterns identify adult patients with de novo acute myeloid leukemia aged 60 years or older who respond favorably to standard chemotherapy: an analysis of alliance st udi es. Leukemia . 2018; 32 :1338 - 1348. (3) Mrozek Prognostic Significance of the European Leukemia Net Standardized System for Reporting Cytogenetic and Molecular Alterations in Adults With Acute Myeloid Leukemia, JCO (2012) ; (a) per American Cancer Society Note: 7+3: Cy(100 - 200mg/m2 7d) + idarubicin (12mg/m2) or + daunorubicin (60 - 90mg/m2) each for 3 days; Treatment Algorithm from NCCN; US Incidences and percentage forecasts for drug regimens from Global Data. 5
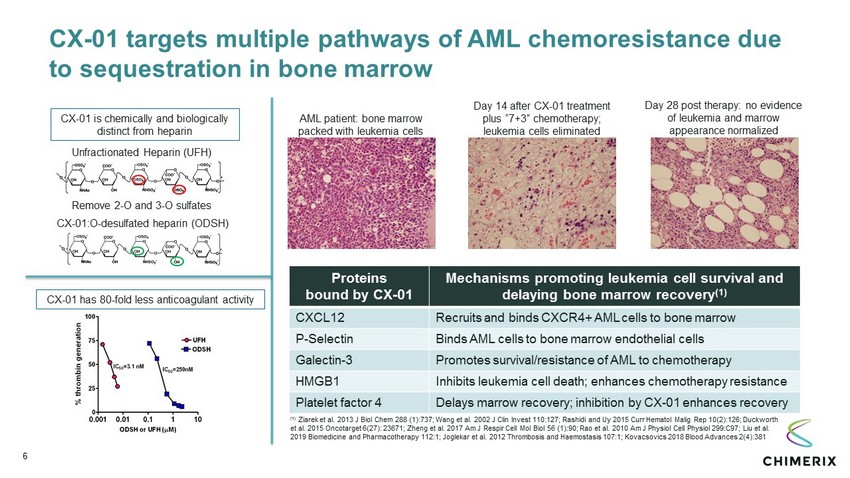
CX - 01 targets multiple pathways of AML chemoresistance due to sequestration in bone marrow 6 CX - 01 has 80 - fold less anticoagulant activity Unfractionated Heparin (UFH) Remove 2 - O and 3 - O sulfates CX - 01:O - desulfated heparin (ODSH) CX - 01 is chemically and biologically distinct from heparin AML patient: bone marrow packed with leukemia cells Day 14 after CX - 01 treatment plus “7+3” chemotherapy; leukemia cells eliminated Day 28 post therapy: no evidence of leukemia and marrow appearance normalized Proteins bound by CX - 01 Mechanism s promoting leukemia cell survival and delaying bone marrow recovery (1) CXCL12 Recruits and binds CXCR4 + AML cells to bone marrow P - Selectin Binds AML cells to bone marrow endothelial cells Galectin - 3 Promotes survival/resistance of AML to chemotherapy HMGB1 Inhibits leukemia cell death; enhances chemotherapy resistance Platelet factor 4 Delays marrow recovery; inhibition by CX - 01 enhances recovery (1) Ziarek et al. 2013 J Biol Chem 288 (1):737; Wang et al. 2002 J Clin Invest 110:127; Rashidi and Uy 2015 Curr Hematol Malig Rep 10(2):126; Duckworth et al. 2015 Oncotarget 6(27): 23671; Zheng et al. 2017 Am J Respir Cell Mol Biol 56 (1):90; Rao et al. 2010 Am J Physiol Cell Physiol 299:C97; Liu et al. 2019 Biomedicine and Pharmacotherapy 112:1; Joglekar et al. 2012 Thrombosis and Haemostasis 107:1; Kovacsovics 2018 Blood Advances 2(4):381
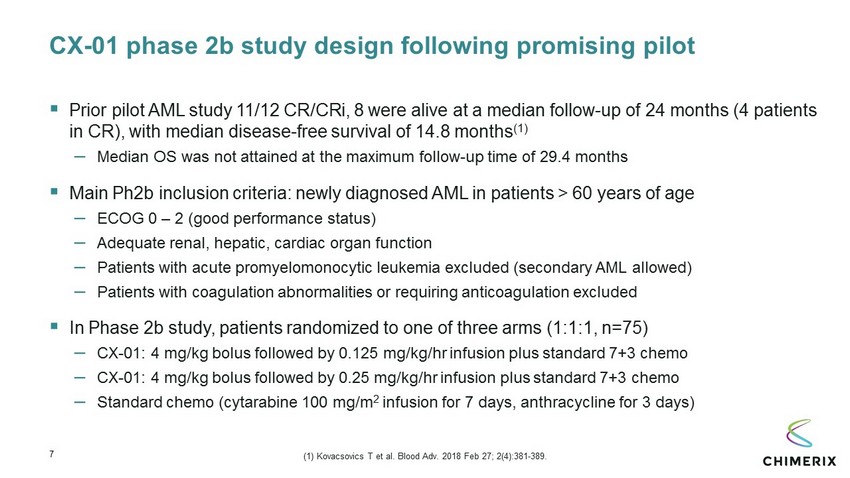
7 CX - 01 phase 2b study design following promising pilot ▪ Prior pilot AML study 11/12 CR/ CRi , 8 were alive at a median follow - up of 24 months (4 patients in CR), with median disease - free survival of 14.8 months (1) – Median OS was not attained at the maximum follow - up time of 29.4 months ▪ Main Ph2b inclusion criteria: newly diagnosed AML in patients > 60 years of age – ECOG 0 – 2 (good performance status) – Adequate renal, hepatic, cardiac organ function – Patients with acute promyelomonocytic leukemia excluded (secondary AML allowed) – Patients with coagulation abnormalities or requiring anticoagulation excluded ▪ In Phase 2b study, patients randomized to one of three arms (1:1:1, n=75) – CX - 01: 4 mg/kg bolus followed by 0.125 mg/kg/ hr infusion plus standard 7+3 chemo – CX - 01: 4 mg/kg bolus followed by 0.25 mg/kg/ hr infusion plus standard 7+3 chemo – Standard chemo (cytarabine 100 mg/m 2 infusion for 7 days, anthracycline for 3 days) (1) Kovacsovics T et al. Blood Adv. 2018 Feb 27; 2(4):381 - 389.
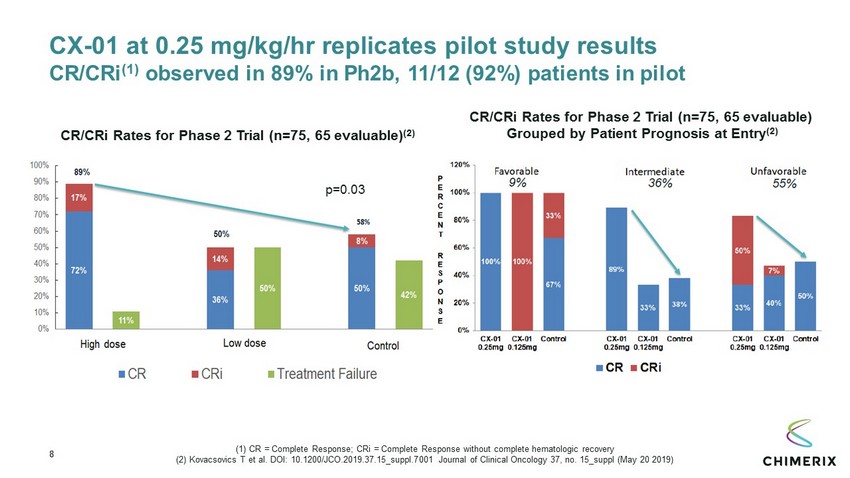
8 CX - 01 at 0.25 mg/kg/ hr replicates pilot study results CR/ CRi (1) observed in 89% in Ph2b, 11/12 (92%) patients in pilot CR/ CRi Rates for Phase 2 Trial (n=75, 65 evaluable) (2) CR/ CRi Rates for Phase 2 Trial (n=75, 65 evaluable) Grouped by Patient Prognosis at Entry (2) p=0.03 (1) CR = Complete Response; CRi = Complete Response without complete hematologic recovery (2) Kovacsovics T et al. DOI: 10.1200/JCO.2019.37.15_suppl.7001 Journal of Clinical Oncology 37, no. 15_suppl (May 20 2019)
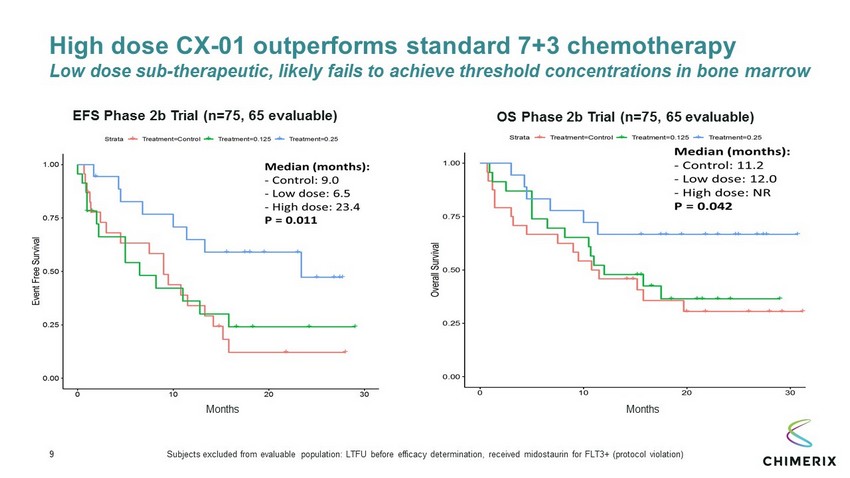
9 High dose CX - 01 outperforms standard 7+3 chemotherapy Low dose sub - therapeutic, likely fails to achieve threshold concentrations in bone marrow EFS Phase 2b Trial (n=75, 65 evaluable) OS Phase 2b Trial (n=75, 65 evaluable) Months Months Subjects excluded from evaluable population: LTFU before efficacy determination, received midostaurin for FLT3+ (protocol violation)
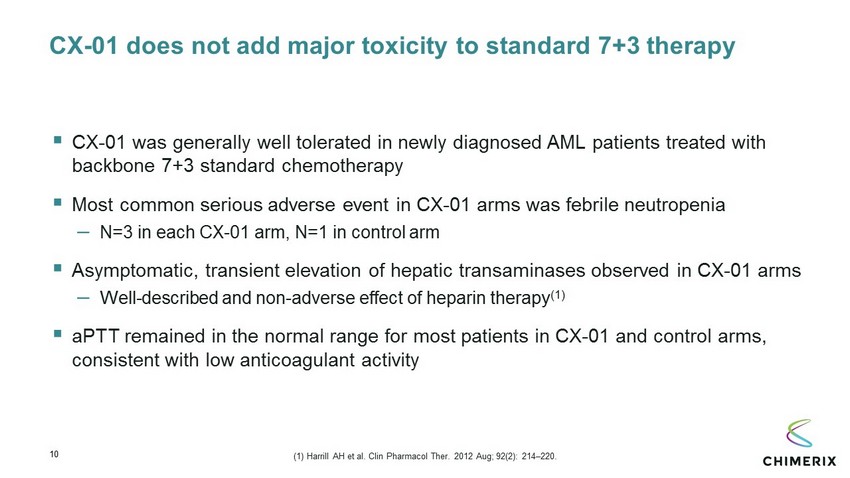
10 CX - 01 does not add major toxicity to standard 7+3 therapy ▪ CX - 01 was generally well tolerated in newly diagnosed AML patients treated with backbone 7+3 standard chemotherapy ▪ Most common serious adverse event in CX - 01 arms was febrile neutropenia – N=3 in each CX - 01 arm, N=1 in control arm ▪ Asymptomatic, transient elevation of hepatic transaminases observed in CX - 01 arms – Well - described and non - adverse effect of heparin therapy (1) ▪ aPTT remained in the normal range for most patients in CX - 01 and control arms, consistent with low anticoagulant activity (1) Harrill AH et al. Clin Pharmacol Ther . 2012 Aug; 92(2): 214 – 220.
Market opportunity Significant commercial opportunity in 1L AML with backbone chemotherapy ▪ US: 21,000+ AML patients diagnosed in 2019 (1) , growing 1.9% annually (2) , equating to 26,000+ newly diagnosed patients forecast by 2030 ▪ ~ 40 - 50% are eligible for intensive therapy and have an intermediate or unfavorable prognosis and thus likely candidates for CX - 01’s profile (10,000 to 13,000 U.S. patients eligible in 2030) ▪ Pricing decisions will be made following an evaluation of any benefit shown in a Phase 3 pivotal study 11 (1) American Cancer Society (2) SEER database

12 Strategic priorities ▪ Quickly advance CX - 01 in to a pivotal registration study – End of Phase 2 meeting with U.S. FDA – Phase 3 protocol finalization ▪ Evaluate new indications to expand and maximize CX - 01 opportunities ▪ Complete execution of work to advance brincidofovir to NDA as a medical countermeasure for smallpox – Non - dilutive funding to invest in CX - 01 and other potential programs











