Valcent Products Inc.
Management Discussion and Analysis
March 31, 2009
(as Amended October 15, 2009)
The attached March 31, 2009 Management Discussion and Analysis have been updated for a clerical error in that a prior period adjustment was not recorded in the March 31, 2009 financial statements, originally filed on September 30, 2009 in Canada.
The error is a result of incorrect closing balances from the March 31, 2008 financial statements being applied to the March 31, 2009 financial statements. The effect on the previously reported March 31, 2009 financial statements was to increase share capital by $350,452, commitment to issue shares by $329,106 and accumulated deficit during the development stage by $679,558. There was no effect on working capital, shareholders deficiency, net loss and comprehensive loss, loss per share or cash flow.
Certain of the comparative March 31, 2008 information which related to this error has been updated.
Also, as a result of this clerical error the Company has updated and corrected its March 31, 2009 financial statements, originally filed September 30, 2009.
The revised information outlined above had no effect on information filed on the Company's 20-F on October 15, 2009.
VALCENT PRODUCTS INC.
THE ATTACHED AMENDED CONSOLIDATED FINANCIAL STATEMENTS DATED MARCH 31, 2009 FORM AN INTEGRAL PART OF THIS MANAGEMENT DISCUSSION AND ANALYSIS
Management Discussion and Analysis as of September 21, 2009, as amended October 13, 2009
By certificate of amendment dated April 15, 2005, we changed our name from Nettron.com, Inc. to Valcent Products Inc. to reflect a newly adopted business plan.
On May 3, 2005 we delisted from the TSX Venture Exchange and effected a consolidation of our common shares on a one-for-three basis. We maintained our OTC Bulletin Board listing, however, our trading symbol was changed to “VCTPF”, which it remained through to July 15, 2009.
Due to economic circumstances and to make our Company more conducive to investment the shareholders of the Company approved a special resolution to reorganize the capital structure of the Company by a share consolidation of its common shares on the basis of one new share for each eighteen old shares. This share consolidation became effective July 16, 2009. Also effective July 16, 2009 our trading symbol changed to “VCTZF” and our CUSIP number changed to 91881 20 2. Our website is located at www.valcent.net
Unless otherwise noted, all references to the number of common shares are stated on a post-consolidation basis.
Our common share authorized share capital remains unlimited.
All amounts are stated in Canadian dollars unless otherwise noted.
We are at present a life sciences targeted, development stage company focused primarily on:
| (i) | the development and commercialization of our “High Density Vertical Growth System” (“VerticropTM” or “HDVG System”) designed to produce vegetables and other plant crops, |
| (ii) | the development of a commercial algae growing technology via Vertigro Algae Technologies LLC (“Vertigro Algae”) with Global Green Solutions, Inc. (“Global Green”), and |
| (iii) | the development and marketing of the Tomorrow GardenTM consumer retail product. |
From inception, we have generated minimal revenues and experienced negative cash flows from operating activities and our history of losses has resulted in our continued dependence on external financing. Any inability to achieve or sustain profitability or otherwise secure additional external financing, will negatively impact our financial condition and raises substantial doubts as to our ability to continue as a going concern.
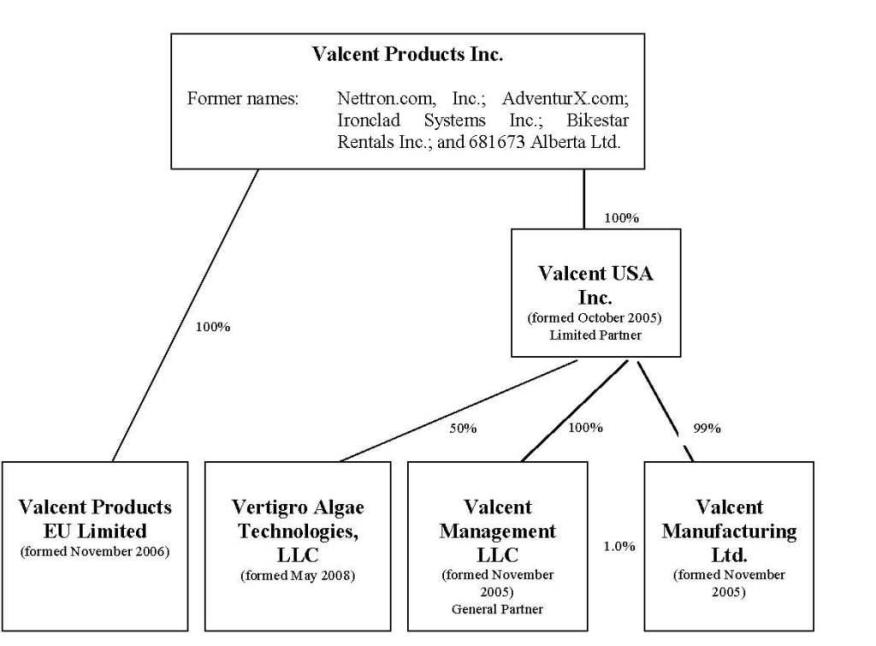
Organizational Structure
The following organizational chart sets forth our current corporate structure and reflects subsidiary interests relating to our various entities.
Corporate History
We were incorporated in accordance with the provisions of the Business Corporations Act (Alberta) on January 19, 1996, as 681673 Alberta Ltd., later changed to Ironclad Systems Inc. Beginning in 1996, following the completion of a public offering, our common shares began trading as a junior capital pool company on the Alberta Stock Exchange (later becoming part of the Canadian Venture Exchange, which was thereafter acquired and renamed the TSX Venture Exchange).
On May 8, 1999, while still operating our bicycle rental and eco-tour businesses through Bikestar Rentals Inc., we incorporated Nettron Media Group Inc., a wholly-owned subsidiary under the laws of the State of Texas, as a marketing enterprise focusing on products and services that could be effectively marketed through internet as well as more traditional business channels. Nettron Media Group Inc.’s primary focus was Cupid’s Web, an interactive online dating and marketing service. We also changed our name from Bikestar Rentals Inc. to AdventurX.com, Inc., and later to Nettron.com, Inc.
In 2000, and in connection with Cupid’s Web, we signed an agreement in principle to acquire all of the outstanding capital stock of a group of companies operating a worldwide dating service franchise, as well as a collection of dating magazines and websites.
On January 1, 2001, in order to fully focus on our interactive dating and marketing services, we disposed of all of the outstanding capital stock of Arizona Outback Adventures LLC and Bikestar Rentals Inc.
On February 18, 2002, due to general weakness in the equity markets, we terminated the agreement in principle to acquire the dating service franchise and related businesses originally entered into in 2000. On March 24, 2004, we disposed of our interest in Nettron Media Group Inc. and began exploring business opportunities that might allow us to restart commercial operations.
By certificate of amendment dated April 15, 2005, we changed our name from Nettron.com, Inc. to Valcent Products Inc. to reflect a newly adopted business plan. On May 3, 2005 we delisted our common stock from the TSX Venture Exchange, maintaining only our OTC Bulletin Board listing and changing our symbol to “VCTPF”. Effective May 3, 2005, and in order to render our capital structure more amenable to contemplated financing, we effected a consolidation of our common shares on a one-for-three-basis.
On August 5, 2005, we completed a licensing agreement with Pagic LLP, formerly MK Enterprises LLC, (“Pagic”) for the exclusive worldwide marketing rights to certain potential products and a right of first offer on future potential products.
In order to facilitate the business plan, the Company formed a wholly-owned Nevada corporation, Valcent USA, Inc. to conduct operations in the United States in October 2005. In turn, Valcent USA, Inc. organized Valcent Management, LLC, a wholly-owned limited liability corporation under the laws of Nevada, to serve as the general partner in Valcent Manufacturing Ltd., a limited partnership also formed by Valcent USA, Inc., under the laws of Texas, wherein Valcent USA, Inc. serves as its limited partner.
Also during the year ended March 31, 2007, Valcent Products EU Limited (“Valcent EU”) was incorporated by Valcent Products Inc. in the domicile of England to conduct operations in Europe.
On May 5, 2008, Valcent Vertigro Algae Technologies LLC, a Texas limited liability corporation, was formed as a 50% owned subsidiary to each of Valcent, USA Inc. and Global Green Solutions Inc. to develop algae related technologies.
The shareholders of the Company approved a special resolution on June 22, 2009 to reorganize the capital structure of the Company through a share consolidation of its common shares on the basis of one new share for each eighteen (1:18) old shares. This share consolidation became effective July 16, 2009. Also effective July 16, 2009 our trading symbol changed to “VCTZF” and our CUSIP number changed to 918881 20 2. Unless otherwise noted, all references to the number of common shares and or prices(s) per share are stated on a post-consolidation basis.
Evolution of License Agreements with Pagic
Original Master License Agreements - On July 29, 2005, we entered into five related definitive agreements (the “Pagic Agreements”) with Pagic LP (formerly MK Enterprises LLC), an entity controlled by Malcolm Glen Kertz, our former Chief Executive Officer, and President, including:
| | (i) | a master license agreement for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to three unrelated and proprietary potential consumer retail products that had previously been developed (the “Pagic Master License”), certain of which are patent pending by Pagic, including the Nova Skin Care System, the Dust WolfTM, and the Tomorrow GardenTM Kit (collectively, and together with any improvements thereon, the “Initial Products”); |
| (ii) | the Pagic Master License also included a license for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Initial Products (the “Initial Ancillaries”); |
| (iii) | a product development agreement pursuant to which we were granted a right for an initial period of five years to acquire a license for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to any new products developed by Pagic (any such products, collectively, the “Additional Products”, and, the agreement itself, the “Pagic Product Development Agreement”); |
| (iv) | the Pagic Product Development Agreement also included a license for a term continuing so long as royalty payments continue to be made as required for the exclusive worldwide marketing and distribution rights to any ancillary products developed and sold for use by consumers in connection with the Additional Products (the “Additional Ancillaries”); and |
| (v) | a related services agreement pursuant to which Pagic shall provide consulting support in connection with the Initial Products, the Initial Ancillaries, the Additional Products and the Additional Ancillaries (the “Pagic Consulting Agreement”), in exchange for the following: |
| 1) | 1,111,112 shares of our common stock which have been issued; |
| 2) | a one-time US$125,000 license fee (paid); |
| 3) | reimbursement for US$125,000 in development costs associated with each of the Initial Products since March 17, 2005 (paid); |
| 4) | consulting fees of US$156,000 per year, payable monthly in advance, which the Company has paid to date; and |
| 5) | the greater of the following, payable annually beginning in the second license year (beginning April 1, 2007): |
| (i) | US$400,000 inclusive of all consulting fees, royalty and other fees; or |
(ii) the aggregate of the following:
a minimum amount of US$37,500 per Initial Product during the second year of the Pagic Master License, and $50,000 US$ each year thereafter, continuing royalties payable quarterly at a rate of:
| Ø | US$10.00 US per Nova Skin Care System unit sold; |
| Ø | US$2.00 per Dust WolfTM unit sold; |
| Ø | 4.5% of annual net sales of the Tomorrow GardenTM Kit; and |
| Ø | 3% of annual net sales of Initial Ancillaries. |
| 6) | a one-time $50,000 US license fee for each Additional Product licensed (except for one pre-identified product); and |
| 7) | subject to a minimum amount of US$50,000 per year commencing with the second year of each corresponding license, continuing royalties of 4.5% of annual net sales and 3% on annual net sales of any Additional Ancillaries. |
As described below these agreements were terminated effective April 1, 2009.
Joint Venture of Algae Related Business Operations - Beginning on October 2, 2006, we granted certain rights to Global Green relating to our joint venture of our high density vertical bio-reactor technology named “Vertigro”, an algae biomass technology initiative. Refer to “PLAN OF OPERATIONS, “High Density Vertical Bio-Reactor and Global Green Joint Venture”, and “Technology License Agreement” between Pagic LP, West Peak Ventures of Canada Ltd. (“West Peak”), and Valcent Products, Inc.
Algae Technology License - On May 5, 2008, the joint venture arrangement pertaining to the development of the algae biomass technology initiative was terminated and Vertigro Algae executed a separate Technology License Agreement (“Technology License”) together with Pagic, and West Peak. The Technology License licenses certain algae biomass technology and intellectual property to Vertigro Algae for purposes of commercialization and exploitation for all industrial, commercial, and retail applications worldwide “Algae Biomass Technology”.
Discontinued Product Development Lines and Settlement of Licensed Technologies - Of the Initial Products under license as defined above, the Company ceased development of Dust Wolf and Nova Skin Care Systems during the year ended March 31, 2009 due to economic conditions, and increased corporate focus on life sciences plant growth technologies. The Company has continued with Tomorrow Garden product development and commercialization though the VerticropTM Technology Purchase Agreement.
VerticropTM Technology Purchase Agreement - Effective April 1, 2009, the Company executed a purchase agreement to acquire all ownership rights and intellectual property relating to its VerticropTM vertical plant growing technology and Tomorrow Garden kit technology (the “Technologies”) from Glen Kertz, Pagic, and West Peak and which provides the Company with all rights and know how to the Technologies (the “Purchase Agreement”). Pursuant to this agreement original master license agreements between the Company and Pagic were terminated and this agreement replaced all financial obligations the Company had with Pagic related to the original master license agreements, including annual payments, royalty burden, and all other associated licensing costs. As noted above, all agreement related to the Algae Biomass Technology are with Vertigro Algae and as such survive the termination of all other agreements.
Pursuant to the Purchase Agreement, the Company agreed to pay a total of US$2,000,000 plus issue 3% of its common stock on conclusion of the purchase agreement. The US$2,000,000 is payable on a cumulative basis as to US$65,000 on signing (paid) plus the greater of 3% of the gross monthly product sales less returns from exploitation of the technologies or US$12,000 per month until US$2,000,000 has been paid. The ownership of the Technologies will remain in escrow until fully paid or if the Company defaults in making payments. The issuance of the 3% of its common stock is payable upon release of the Technologies from escrow to the Company. The Company may at any time elect to pay out the remaining balance due. Should the Company default under this agreement the Technologies will revert back to Glen Kertz and Pagic and the Company’s obligations under the Purchase Agreement will cease. The Company expenses amounts paid under the Purchase Agreement.
From inception we have generated minimal revenues from our business operations and have traditionally met our ongoing obligations by raising capital through external sources of financing, such as convertible notes, promissory notes and director and shareholder advances.
At present, we do not believe that our current financial resources are sufficient to meet our working capital needs in the near term or over the next twelve months and, accordingly, we will need to secure additional external financing to continue our operations. We anticipate raising additional capital though further private equity or debt financings and shareholder loans. If we are unable to secure such additional external financing, we may not be able to meet our obligations as they come due or to fully implement our intended plan of operations, as set forth below, raising substantial doubts as to our ability to continue as a going concern.
In addition, during the year ended March 31, 2009 we reduced staffing at our El Paso offices and research facility, transferring our primary development directives previously located in the United States to our UK offices.
Our plan of operations over the course of the next twelve months, subject to adequate financing, is to focus primarily on the continued development and marketing of our VerticropTM vertical plant growing systems, development and distribution of our lines of potential consumer retail Tomorrow Garden products, and the development of our high density vertical algae growth technology.
Chris Bradford, our Managing Director, of Valcent EU, is responsible for UK business operations and the Company’s “Tomorrow GardenTM” retail plant sales initiative, as well as development of European based VerticropTM market development and sales rollout.
More specifically, our plan of operations with respect to each of our lines of potential retail and commercial products is provided as follows:
Vertigro Algae and the Algae High Density Vertical Bio-Reactor Technology
We are in the development stages of creating technology for a High Density Vertical Bio-Reactor (“Vertigro Project”). The objective of this technology is to produce algae related source products by utilizing the waste gas of carbon dioxide capable of growing micro-algae. Our High Density Vertical Bio-Reactor is configured in a manner intended to promote the rapid growth of various forms of micro-algae which is later processed to remove volatile oils suitable for the production of fuels, and/or other target products for commerce and industry. The design of our technology allows the reactors to be stacked on a smaller foot print of land than traditional growing methods require. We believe secondary potential markets for this technology include industrial, commercial and manufacturing businesses that produce carbon dioxide emissions. Technology development is ongoing and subject to adequate budgets for product development.
On May 5, 2008, the Company and Global Green concurrently organized Vertigro Algae and entered into an operating agreement (“Operating Agreement”). Pursuant to the Operating Agreement, Global Green and the Company each hold a 50% interest in Vertigro Algae and have committed to fund project development costs according to ownership allocation. Global Green will receive 70% of the net cash flow generated by Vertigro Algae until it has received US$3,000,000 in excess of its 50% interest in such cash flow and then thereafter it will be split equally.
Also on May 5, 2008, Vertigro Algae executed a Technology License Agreement (“Technology License”) together with Pagic and West Peak. The Technology License licenses certain algae biomass technology and intellectual property to Vertigro Algae for purposes of commercialization and exploitation for all industrial, commercial, and retail applications worldwide (“Algae Biomass Technology”). As consideration for the Technology License, the Company and Global Green agreed to issue 16,6667 common shares and 300,000 common shares respectively, to Pagic and also pay a one-time commercialization fee of US$50,000 upon the Algae Biomass Technology achieving commercial viability. The Technology License is subject to royalty of 4.5% of gross customer sales receipts for use of the Algae Biomass Technology and aggregate annual royalty minimum amounts of US$50,000 in 2009, US$100,000 in 2010 and US$250,000 in 2011 and each year thereafter in which the Technology License is effective. The Company issued the 16,667 common shares to Pagic on August 18, 2008 at a fair value of $190,746 which has been expensed to product development and has accrued and paid in full via equity debt settlement its share of the US$50,000 payment due on March 31, 2009.
As a result of economic conditions, the Company significantly reduced its staff at its El Paso research facility during the year ended March 31, 2009. The Company is currently negotiating for the continued development of the Company’s algae programs with certain universities.
VerticropTM Commercial Plant Growth Systems
Valcent Products Inc. has also introduced the “VerticropTM” HDVG System intended to grow a wide variety of crop products. The Company initially began experimenting with vegetable crops utilizing the growing system within its greenhouse production facilities in El Paso, Texas, however, during the year ended March 31, 2009 the Company’s development efforts shifted to Valcent EU where it has conducted detailed research and subsequently developed commercial scale growing systems.
HDVG System Technology – Concept and Advantages: The HDVG System technology provides a solution to rapidly increasing food costs caused by transportation/fuel due to the cost of oil. Together with higher cost comes a reduction in availability and nutritional values in the food people consume. The HDVG system is designed to grow vegetables and other plants much more efficiently and with greater food value than in agricultural field conditions.
As the world population increases, agricultural land and water resources rapidly diminish. Alternative and innovative solutions have to be found to feed people and reduce the consumption of water, land, energy, and food miles.
VerticropTM is an innovative and exciting vertical growing system which:
| · | Produces up to 20 times the normal production volume for field crops |
| · | Requires approximately 5% of the normal water requirements for field crops |
| · | Can be built on non arable lands and close to major city markets |
| · | Can work in a variety of environments: urban, suburban, countryside, etc. |
| · | Minimizes or eliminates the need for herbicides and insecticides |
| · | Will have very significant operating and capital cost savings over field agriculture |
| · | Will drastically reduce transportation costs to market, resulting in further savings, higher quality and fresher foods on delivery and less transportation pollution |
| · | Is modular and easily scalable from small to very large food production situations |
The HDVG System grows plants in closely spaced shelves vertically arranged on panels that are moving on an overhead conveyor system. The system is designed to provide maximum sunlight and precisely correct nutrients to each plant. Ultraviolet light and filter systems may exclude the need for herbicides and pesticides. Sophisticated control systems gain optimum growth performance through the correct distribution of nutrients, the accurate balancing of PH and the delivery of the correct amount of heat, light and water.
System Advantages
| | | reduced global transport costs and associated carbon emissions |
| | | food and fuel safety, security and sovereignty |
| | | local food is better for public health |
| | | building local economies |
| | | control of externalities and true costs |
In a rapidly urbanizing world where the majority of people now live in cities, localization requires that food and fuel be produced in an urban context. Urban agriculture presents a number of technological challenges. The main challenge is a lack of growing space.
Vertical growing is a new idea currently emerging in the sustainability discourse which offers great promise for increasing urban production. Vertical growing systems have been proposed as possible solutions for increasing urban food supplies while decreasing the ecological impact of farming. The primary advantage of vertical growing is the high density production it allows using a much reduced physical footprint and fewer resources relative to conventional agriculture. Vertical growing systems can be applied in combination with existing hydroponics, and greenhouse technologies which already address many aspects of the sustainable urban production challenge (i.e., soil-free, organic production, closed loop systems that maximize water and nutrient efficiencies, etc.). Vertical growing, hydroponics and greenhouse production have yet to be combined into an integrated commercial production system, but, such a system would have major potential for the realization of environmentally sustainable urban food and fuel production.
1/8th Acre HDVG System Commercial Production Model: The Company had been recently developing an HDVG System and specification of a 1/8th acre commercial scale plant capable of defining final operating and capital costs to maximize sales return. Completion of this facility located in El Paso is subject to budget allocations in the future. All current development and commercialization efforts relating to the Company’s vertical growing systems are continuing in the Company’s UK offices.
Commercial Deployment of First VerticropTM System – Paignton Zoo, Devon, UK: The Company via its UK subsidiary has in the summer months of 2009 deployed its first commercial test installation of its VerticropTM technology at the Paignton Zoo Environmental Park located in Devon, UK. The Paignton Zoo is one of the largest zoos in the UK. The Zoo is part of South West Environmental Parks Ltd which is owned by the Whitley Wildlife Conservation Trust. It is a combined zoo and botanic garden that welcomes over half a million visitors a year. The VerticropTM System installed at Paignton Zoo is meant to grow more plants in less room using less water and less energy. It will help to reduce food miles and bring down the Zoo’s annual costs for animal feed, which is currently in excess of £200,000 a year. The zoo will grow a whole range of herbs such as parsley and oregano, as well as leaf vegetables like lettuce and spinach, plus a range of fruits such as cherry tomato and strawberry. Reptiles, birds and most of the mammal collection - including primates and big cats -- will benefit from the production of year-round fresh food. The system which was a joint venture between Valcent Products EU Limited and the Paignton Zoo Environmental Park became operational on August 5, 2009, and will supply necessary data to the Company of semi-commercial crop yields, and other data for further commercialization of the VerticropTM System.
Verticrop Warehouse Systems: The Company is also developing a new VerticropTM product line for use in a warehouse environment. Using the latest horticultural lighting technologies, combined with state of the art irrigation and nutrient delivery systems, Valcent EU is in the final stages of developing a commercial application of its VerticropTM HDVG system suitable for installing in industrial type warehouses, as an alternative to polytunnels or glasshouses. A warehouse environment will provide a commercial grower with significant benefits, particularly in areas of climate extremes. Growing crops in a glasshouse or polytunnel can involve high energy costs to maintain stable temperatures suitable for healthy plant growth. Growing in a warehouse environment with a VerticropTM vertical farming system is designed to improve production and lower costs. Valcent EU’s research team has been working with two strategic partners who are acknowledged experts in the field of industrial lighting. This research has lead to the development of a commercially viable eco-friendly system, particularly well suited to application in a warehouse environment. The VerticropTM warehouse growing system will also use a hybrid lighting system, harnessing and channeling heat free natural daylight, complemented by the latest LED horticultural lighting technology.
Nova Skin Care System and Dust Wolf
The Company ceased development of Dust Wolf and Nova Skin Care Systems during the year ended March 31, 2009 due to economic conditions, and increased corporate focus on life sciences plant growth technologies. Any inventory related thereto amounting to raw materials of $314,012 and finished goods inventory of $330,451 were written off product development costs in the fiscal year ended March 31, 2009.
Agreement for Settlement and Reversion of Nova Skin Care System and Dust Wolf: On March 30, 2009, the Company entered into a letter of agreement with Pagic, Glen Kertz, and West Peak to settle royalties and other rights and obligations due under the Pagic Agreements (master license arrangements from July 2005) relating to Nova Skin Care Systems and Dust Wolf products that were discontinued by the Company. Under the settlement agreement, the Company relinquished all rights to the Nova Skin Care Systems and Dust Wolf products to Pagic and Kertz in exchange for a 3% royalty to the Company of future sales of the Nova Skin Care System or Dust Wolf on terms similar to those incurred by the Company under the Pagic Agreements, and the cessation of obligations of the Company under the Pagic Agreements relating to these technologies previously under license. West Peak agreed to forfeit any royalty rights it previously had relating to these technologies. There has been no further development of the Nova Skin Care System or Dust Wolf products that the Company is aware of.
Tomorrow GardenTM
Our Tomorrow GardenTM Kit is an indoor herb garden kit, designed to offer, direct to the consumer, an easy to use kit featuring herbs and plants not otherwise readily available in the marketplace. The Tomorrow GardenTM Kit offers an improved plant lifespan of up to three to six months, as opposed to the traditional shelf life of approximately seven to ten days for fresh herbs, and requires only ambient light, with no watering or other maintenance, to survive. Our Tomorrow GardenTM Plant Kit will be capable of supplying all of the standard herbs traditionally offered in grocery shops today, such as basil, mint, thyme, rosemary, parsley and cilantro, but may, in addition, supply more exotic herbs or pharmaceutical grade plants. Our Tomorrow GardenTM Kit is currently in the design, development, and test sales phase operating out of our offices located in Cornwall, England. First selected retail applications are aimed at children and education channels.
The Tomorrow Garden gift kits offer an easy to use growing system for a range of interesting and attractive plants.
Each Tomorrow Garden kit contains:
| · | a micropropagated plantlet |
| · | a membrane which enables the plants to grow and stay fresh for 3-6 months in normal light without the need for watering |
| · | a coir growing pot and compost |
The official launch of the Tomorrow Garden was premiered at the BBC Gardeners’ World Exhibition at the NEC in Birmingham on June 11 – 14, 2008, with a follow-up at the Royal Horticultural Society’s Hampton Court Flower Show on July 8 -13, 2008.
Currently our kits are aimed at the Junior/Educational market sector. As ferns have been around since pre-historic times, and are known to have provided a large proportion of the diet of the herbivore dinosaurs, the emphasis will be on inviting children in the 7yrs – 14 yrs age group to grow “dino food”. Growing kits have been designed in the appropriate packaging, reflecting the dinosaur theme.
Valcent EU is also culturing a number of more exotic plant species (such as orchids) so that it can both follow-up and compliment its initial products. It is also developing a range of culinary herbs and are reviewing a number of other options for special “Christmas” packs (e.g. the Christmas Rose). Finally, through “in house” expertise, Valcent EU is researching the development of a range of Chinese medicinal herbs, which can be sold in “growing kit” form through “alternate medicine” outlets or (ultimately) to pharmaceutical companies involved in this field of research.
Fluctuations in Results
During the period from March 24, 2004 through the year ended March 31, 2005, we had no meaningful operations and focused exclusively on identifying and adopting a suitable business plan and securing appropriate financing for its execution. As a result of the Company completing a licensing agreement with Pagic for the exclusive worldwide marketing rights to certain potential products and a right of first offer on future potential products during the fiscal year ended March 31, 2006 operating results have fluctuated significantly and past performance should not be used as an indication of future performance.
| Valcent Products Inc. |
| Selected Financial Data [Annual] |
| (Expressed in Canadian Dollars) |
| | 12 months ended |
| | | 2009 | 2008 | 2007 |
| Net Operating Revenues | $ | 0 | 0 | 0 |
| Loss from operations | $ | 15,337,285 | 12,028,222 | 8,171,090 |
| | | | | |
Other Loss (Income) | $ | 2,548,205 | 684,136 | (32,697) |
| Net loss per Canadian GAAP | $ | 17,885,490 | 12,712,358 | 8,138,393 |
| Loss per share | $ | 6.43 | 6.44 | 7.60 |
| | | | | |
| Share capital | $ | 21,957,516 | 16,691,282 | 8,196,982 |
| Common shares issued | | 3,008,977 | 2,459,796 | 1,703,670 |
| Weighted average shares outstanding | | 2,782,284 | 1,974,763 | 1,070,066 |
| Total Assets | $ | 2,351,963 | 4,605,914 | 4,142,485 |
| Net Liabilities | $ | 1,031,226 | 3,068,935 | 24,376 |
| | | | | |
Cash Dividends Declared per Common Shares | $ | 0 | 0 | 0 |
| | | | | |
Exchange Rates (CDN $ to 1 US $) period average | $ | 0.88839 | 0.97084 | 0.87896 |
Exchange Rates (CDN $ to 1 British Pound £) period average | $ | 0.52433 | 0.48368 | n/a |
Restructuring Initiatives
The debt settlements, lockup, and convertible debt restructuring (described below) are a part of the Company’s plan to substantially reduce its debt and restructure the Company’s capital structure as part of the Company’s efforts to further fund its business operations. As part of its restructuring, the Company held a special meeting of its shareholders on June 22, 2009 who approved a stock consolidation of one new share for each eighteen old shares. This share consolidation became effective on July 16, 2009. The Company continues to have obligations pursuant to the four convertible note holders in the aggregate of US$1,323,000, described below - July 2008 Convertible Note Amendments, and in conjunction with its re-organization and funding efforts incurred additional debt also described below – “2009 Debt and Conversion of Debt to Equity”.
Debt Settlement Agreements and Lockup Agreements
Concluding on May 11, 2009, but effective for accounting purposes as of March 31, 2009, Valcent Products, Inc. (the “Company”) entered into agreements with a significant number of the Company’s creditors to settle or restructure a significant portion of the Company’s indebtedness in consideration for shares of the Company’s common stock. The Company settled an aggregate of US$10,806,780 representing these balances was settled in exchange for 29,516,951 common shares issued on May 11, 2009. Included in these shares are 24,232,816 common shares which are subject to pooling restrictions with quarterly equal releases beginning on January 1, 2010, a further 2,634,135 common shares were subject to pooling restrictions until January 1, 2010. Also included in these shares are 1,316,424 shares were issued in settlements of debts involving current officers or directors of the Company, 391,298 were issued to a past director and officer of the Company, all of which are subject to the lockup agreements.
July 2008 Convertible Note Amendments
As part of the overall debt restructuring, the Company also entered into agreements with each of the Company’s secured creditors and amended the terms of the four secured convertible promissory notes issued in July 2008 in the aggregate principal amount of US$2,428,160 (collectively the “Notes”). One of the Notes is held by the Company’s chief financial officer and member of the board of directors (being a Note in the principal amount of US$188,160).
By their original terms the Notes were to be due on or before July 16, 2009, however all of the parties agreed to extend the maturity date of the Notes until December 31, 2009. On June 2, 2009, pursuant to the modified contractual arrangements, the Company paid US$400,000 to certain of the holders of the Notes (with the exception of the Company’s chief financial officer and member of the board of directors) to pay down the principal amount due and owing under the Notes. All holders of the Notes also agreed to provide the Company or its designee an option to purchase on or before December 31, 2009 the Notes and the remaining amounts due under them, being US$1,323,000 as of December 31, 2009. Further, through December 31, 2009 each Note holder has agreed not to effect any conversions of the Notes into shares of our common stock.
In consideration for the amendments and accommodations to the Notes, the Company agreed to pay each holder consideration that was comprised of the prepayment of interest that otherwise would have been due and owing on the Notes through December 31, 2009 and amounts that would have been due under the Notes pursuant to their terms, including the original issuance discount and prepayment premium. This consideration was paid to each Note holder in the form of Company common stock. In total the Company issued 2,892,036 shares of its common stock to the four Note holders. However, each of the holders entered into an agreement whereby each agreed to not sell these shares until January 1, 2010. The Company reserves the right to change lock up arrangements at its discretion.
Each Note holder also agreed to waive any adjustment to the exercise price of the warrants issued to the holders as part of the July 2008 financing that may have resulted from the issuances of shares of Company common stock as part of the Company’s overall debt restructuring and/or from certain other contemplated Company issuances. However, subject to certain exceptions, the Company agreed that if before March 31, 2010 the Company issues shares of its common stock at a price less than the valuation of the shares issued to each Note holder (US$0.40 per share), that the Company would issue each holder additional shares of Company common stock in a number equal to the difference between the number of shares each holder would have received had the consideration paid to the holder been paid in shares at the lower valuation. If the US$1,323,000 face amount required to retire these notes is not paid on or before December 31, 2009, the promissory notes revert to the terms and conditions original secured convertible note transaction documents including security agreement originally executed in July 2008 which remain valid and in effect.
2009 Debt and Conversion of Debt to Equity
On March 26, 2009, the Company entered into a subscription agreement for an investment of up to US$2,000,000 in convertible units with a single subscriber. The subscription funds bear interest at 10% and the issuance of the units are subject to the completion of the Company’s reverse consolidation which occurred on July 18, 2009. The subscription advances and accrued interest are convertible into units at the rate of US$0.125 per unit on the date upon which the Company effects a reverse share consolidation. Each unit will consist of one restricted common share and one-third share purchase warrant with each whole warrant exercisable into one common share at an exercise price of US$0.45 per share for a one year term from the date of issue. The warrants may be exercised on a cashless basis.
As at March 31, 2009, the Company had received US$500,000 ($630,650) which has been reflected as a promissory note payable in the financial statements. During the year ended March 31, 2009, the Company accrued US$685 interest on the principal balance of this advance. On May 22, 2009, the balance of US$1,500,000 was received.
The Company completed the share consolidation on June 22, 2009 and on July 17, 2009, the Company issued 16,303,562 common shares pursuant to the conversion of US$2,000,000 in convertible subscription advances and interest of US$37,945 and also issued 5,434,521 warrants to purchase 5,434,521 common shares at an exercise price of US$0.45 per share until July 17, 2010. The 5,434,521 warrants issued may be exercised on a cashless basis. Any shares issued to this subscriber upon the conversion of the notes would not be subject to lock up arrangements, but the subscriber is deemed an affiliate of the Company owing to shareholdings greater than 20% ownership and as such has certain selling restrictions
In addition, during the period April 1, 2009 through June 22, 2009, the Company received subscriptions to a 10% convertible promissory note in the aggregate of US$176,240. The note and accrued interest have been converted into units at the rate of US$0.40 per unit. Each unit of consists of one restricted common share and one share purchase warrant to purchase an additional restricted common share at an exercise price of US$0.60 per share for a two year term from the date of conversion. On July 17, 2009, the Company issued 458,139 common shares pursuant to the conversion of US$176,240 in convertible notes and interest of US$3,777 and also issued 458,139 warrants to purchase 458,139 common shares at an exercise price of US$0.60 per share until July 17, 2011
Selected Quarterly Financial Data
Valcent Products Inc. Selected Financial data [Unaudited] (Expressed in Canadian Dollars | | | | | | | |
| | | Quarter Ended 03/31/2009 | Quarter Ended 12/31/2008 | Quarter Ended 09/30/2008 | Quarter Ended 06/30/2008 | Quarter Ended 03/31/2008 | Quarter Ended 12/31/07 | Quarter Ended 09/30/07 | Quarter Ended 06/30/07 |
| | | | | | | | | | |
| Net Operating Revenues | $ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Loss from operations | $ | 3,704,702 | 4,547,838 | 4,077,677 | 3,007,068 | 5,140,674 | 3,482,692 | 2,121,037 | 1,283,819 |
| Net loss per Canadian GAAP | $ | 5,297,776 | 5,566,158 | 4,075,475 | 2,916,081 | 6,364,394 | 3,495,735 | 1,921,261 | 930,968 |
| Loss per share from continued operations | $ | 1.78 | 1.96 | 1.49 | 1.12 | 2.73 | 1.808 | 1.05 | 0.52 |
| Share Capital | $ | 21,957,516 | 20,543,236 | 19,482,091 | 18,620,606 | 16,691,282 | 13,322,958 | 9,333,316 | 12,604,100 |
| Common Shares issued | | 3,008,977 | 2,932,718 | 2,793,016 | 2,679,613 | 2,459,795 | 2,234,935 | 1,837,103 | 1,829,344 |
| Weighted average shares outstanding | | 2,982,909 | 2,832,856 | 2,725,336 | 2,590,304 | 2,328,672 | 1,857,651 | 1,835,443 | 1,785,232 |
| Total assets | $ | 2,351,963 | 4,001,862 | 4,230,595 | 4,330,428 | 4,605,914 | 4,434,893 | 3,585,751 | 4,520,482 |
| Net assets (Liabilities) | $ | (1,031,226) | (10,716,836) | (5,984,732) | (4,633,652) | (3,068,935) | (2,329,107) | (3,259,327) | (2,019,941) |
| | | | | | | | | | |
| Cash Dividends Declared Per common Shares | $ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| | | | | | | | | | |
| | | | | | | | | | |
YEAR ENDED MARCH 31, 2009 COMPARED WITH YEAR ENDED MARCH 31, 2008
Operating Results
For the year ended March 31, 2009, we focused on the following business initiatives:
| (i) | the development and commercialization of our VerticropTM HDVG System designed to produce vegetables and other plant crops, |
| (ii) | the development of a commercial algae growing technology via Vertigro Algae with Global Green Solutions, Inc., and |
| (iii) | the development and marketing of the Tomorrow GardenTM consumer retail product by Valcent EU. |
For the year ended March 31, 2008, the Company focused on:
| (i) | the development of a commercial algae growing technology via a joint venture with Global Green, |
| (ii) | the development of our VerticropTM HDVG System designed to more efficiently produce certain plant crops, |
| (iii) | the development and test marketing of the Tomorrow Garden TM consumer retail product by Valcent EU, |
| (iv) | the development of product inventories and direct test and sales initiatives and product introduction promotion relating to our Nova Skin Care System, and |
| (v) | ongoing research and development with tissue culture technologies, plant growth stimulation technologies, and other product and technology development initiatives. |
Our reported net loss increased by $5,173,132 to $17,885,490 ($6.43 basic loss per share) for the year ended March 31, 2009 as compared to $12,712,358 ($6.44 basic loss per share) for the same period ending March 31, 2008. The increase during the 2009 fiscal year is largely a result of increased research and development activity related to the discontinuance of the Nova Skin Care System, development of the Company’s Algae technology and the commercialization of the VerticropTM HDVG System. The Company also incurred non recurring costs relating to fixed asset impairment and a loss on settlement of debts related to the valuation of convertible debentures utilized to finance the Company. Finally, owing to changes in the value of the United States dollar, primarily, the Company also incurred a significant foreign exchange loss during the year ended March 31, 2009.
Revenues
For the years ended March 31, 2009 and March 31, 2008, the Company had no revenue.
However, as the Company is in the development stage, test sales are credited to product development costs. During the year ended March 31, 2009, the Company had test sales of $219,483 (2008 - $985,779) related to the Nova Skin Care System. As at March 31, 2009 the Company ceased the development of the Nova Skin Care System.
Operating Expenses
Product development expenses increased by $4,157,564 to $8,238,999 for the year ended March 31, 2009 as compared with the year ended March 31, 2008. The increase is due to write downs relating to, accounts receivable impairment, inventory impairment for the discontinuance of Nova Skin Care Products marketing and product testing, increased research and development costs relating to the commercialization of the VerticropTM System, and the advent of costs relating to the Tomorrow GardenTM product line. Product development expenses were $4,081,435 during the year ended March 2008.
In conjunction with convertible debenture financings the Company incurred $3,213,003 in interest, accretion, and financing on convertible notes in the twelve month period ended March 31, 2009. This represents a $296,045 increase from the $2,916,958 that had been incurred during the year ended March 31, 2008 stemming primarily from amortization and accretion of convertible debenture funding activity during the 2009 and 2008 fiscal years.
The Company incurred a loss on the settlement of debts in the amount of $885,292 during the year ended March 31, 2009 (2008 $0) with respect to the renegotiation and settlement of certain portions of convertible notes issued in July 2008.
Investor relations fees decreased $88,793 to $782,749 (2008 - $871,542) for the year ended March 31, 2009 as a result of the Company employing a decreasing number of third party consultants in advisory, business consulting services, and investor relations activities.
Professional fees increased by $92,946 to $524,216 for the year ended March 31, 2009 from $431,270 for the year ended March 31, 2008. The increase is primarily attributable to costs associated with increased finance activity and costs relating to complex financial accounting issues, as well as intellectual property legal services, and technology acquisition negotiations between the respective years.
Advertising and media development was $448,258 during the year ended March 31, 2009 (2008 $1,953,998) with such decrease reflecting the cessation of marketing development relating to the Nova Skin Care System.
Office and miscellaneous expenses increased $154,311 to $389,128 for the year ended March 31, 2009 from $234,817 for the year ended March 31, 2008. The increase is due to emphasis on project development initiatives in the Company’s UK subsidiary.
As a result of the issuance of options to directors, officers, employees and consultants the Company incurred stock option compensation expenses of $260,530 during the year ended March 31, 2009 (2008 - $990,305).
Travel expenses decreased by $55,024 to $236,441 (2008 - $291,465) for the year ended March 31, 2009 as a result of decreased activity in all of the Company’s operations, decreased number of active development projects, as well as cost streamlining due to global economic circumstances.
Amortization increased $134,155 to $181,618 (2008 - $47,463) for the year ended March 31, 2009 as a result of an increasing asset base and depreciation charges related thereto.
Rent expenses increased $53,120 to $131,038 for the year ended March 31, 2009 from $77,918 for the year ended March 31, 2008. The increase in rent costs incurred relates to our new offices and operations located in the United Kingdom.
Filing and transfer agent expenses decreased $22,350 to $22,150 for the year ended March 31, 2009, from $44,500 for the year ended March 31, 2008. The decrease is primarily attributable to costs associated with the proportionate decrease of financing and business activity during the 2009 fiscal year.
Insurance expense decreased to $19,640 for the year ended March 31, 2009, from $71,071 for the year ended March 31, 2008. The decrease is primarily attributable to the rebate of 2008 insurance costs received in the year ended March 31, 2009 and associated decreased insurance coverage required which was prorated over both 2008 and 2009 fiscal years.
Interest relating to long term debt decreased to $4,223 (2008 - $15,480) due to the payout of all long term debt in July, 2008.
Other Income and Expenses
Interest income for the years ended March 31, 2009 and March 31, 2008 were $19,491 and $10,805 respectively.
As a result of staffing cuts in our El Paso facility and due to the specialized nature of buildings and equipment, the Company impaired the carrying value of certain of its fixed assets relating to our El Paso, Texas operation; the Company’s fixed assets and land were valued at a net book value of $1,070,216 (2008 - $1,135,108) during the year ended March 31, 2009, and a one time write down of assets in the amount of $509,892 was incurred (2008 - $0).
Due primarily to fluctuations in the United States dollar the foreign exchange loss for the year ended March 31, 2009 was $2,027,804. For the year ended March 31, 2008 the Company had a foreign exchange gain of $611,133.
Liquidity and Capital Resources
Because we are organized in Canada, our March 31, 2009 financial statements have been prepared by our management in accordance with Canadian GAAP applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
Our accumulated losses during the development stage increased by $17,885,490 to $42,173,129 for the year ended March 31, 2009 from $24,317,639 for the year ended March 31, 2008.
Our working capital deficit decreased to $2,101,443 from $5,094,440 for the year ended March 31, 2009 representing a $2,992,997 decrease over the year ended March 31, 2008. Even though this decrease stemmed primarily from debt settlement and restructuring initiatives, as described in Note 1 to our March 31, 2009 financial statements, these conditions raise substantial doubt as to our ability to continue as a going concern.
The Company’s ability to continue as a going-concern is dependent upon the economic development of its products, the attainment of profitable operations and the Company’s ability to obtain further financing. The Company continues to explore additional funding to finance its operations and obligations. Management is considering all possible financing alternatives, including equity financing, debt financing, joint-venture, corporate collaboration and licensing arrangements. However, there can be no assurance that the Company will be successful in its financing efforts or in the success of its products.
During the year ended March 31, 2009, the Company engaged in a series of private offering transactions with institutional and other investors, pursuant to which we raised US$1,951,600 through private placements of equity comprised of common shares and warrants, US$191,664 from warrant exercises, US$2,168,000 from convertible debentures issuances, and approximately $1,662,578 in promissory notes, and short term advances.
During the year ended March 31, 2008, the Company engaged in a series of private offering transactions with institutional and other investors, pursuant to which we raised US$5,129,636 through private placements of equity comprised of common shares and warrants, US$367,671 from warrant and share option exercises, and US$1,291,000 from convertible debentures, promissory notes, and short term advances.
During the year ended March 31, 2009, we issued 16,667 common shares for technology valued at US$180,000, and issued 275,731 common shares for convertible note conversions received of US$1,422,206 in interest and principal.
Long term debt decreased from $158,612 at March 31, 2008 to $0 at March 31, 2009 as a consequence of freeing a first secured interest in the Company’s El Paso property in favour of holders of our July 2008 convertible note debt financing.
Our advances and amounts due from related parties decreased by $1,303,871 to $186,645 as at March 31, 2009 (2008 - $1,490,516) due to debt settlements made with debt holders on March 31, 2009 for common share equity as part of corporate restructuring initiatives.
Convertible notes decreased as at March 31, 2009 to $11,228 from $5,202,741 at March 31, 2008 due to debt settlements made with debt holders on March 31, 2009 for common share equity as part of corporate restructuring initiatives. Certain remaining principal portions of the notes were optioned to the Company until December 31, 2009 and were reclassified as secured promissory notes.
Promissory notes increased from $270,167 at March 31, 2008 to $2,643,514 at March 31, 2009 owing in part to reclassification of certain remaining principal portions of the July 2008 convertible notes to promissory notes due to renegotiation of these instruments, provisions made by negotiation for the repayment of US$400,000 of the July 2008 convertible notes in cash on June 2, 2009, and the receipt of US$500,000 as part of a private placement with a single investor which was later converted to common shares on July 17, 2009.
Accounts payable and accrued liabilities increased slightly to $541,802 at March 31, 2009 from $536,563 at March 31, 2009.
As a result of the cessation of Nova Skin Care System development certain of our inventories were impaired by $644,463 as at March 31, 2009. As at March 31, 2009, inventories related to the Company’s Tomorrow Garden project in the aggregate of $34,072 (2008 - $nil).
Prepaid expenses as at March 31, 2009 of $102,670 (2008 $2,119,546) include $16,606 (2008 - $2,015,837) related to the deferred portion of business consulting and investor relations services agreements with the balance of $86,064 (2008 - $103,709) consisting of in prepaid insurance and rental deposits.
As at March 31, 2009, accounts receivable of $920,235 consists of $812,048 due from Global Green Solutions, the Company’s joint venture partner in Vertigro Algae, with the balance primarily due from value added tax receivable by the Company’s subsidiary, Valcent Products EU Limited, and goods and services tax receivable by the Company, as well as receivables relating to Tomorrow GardenTM sales. As at March 31, 2008, accounts receivable of $462,156 consisted primarily of amounts due from the Company’s product fulfillment agent, GSI Commerce, Inc. (formerly Accretive Commerce Inc.) from test sales relating to the Company’s Nova Skin Care System, value added tax receivable by the Company’s subsidiary, Valcent Products EU Limited, and goods and services tax receivable by the Company.
Restricted cash as at March 31, 2008 consisted of certificates of deposit and interest earned of $108,471 that were pledged to secure long term debt relating to our purchased lands in El Paso. Restricted cash as at March 31, 2009 was $0 due to long term debt being paid in full in July, 2008 resulting in restricted cash being freed for corporate use.
During the year ended March 31, 2009, the Company determined that certain capital assets were not in use or impaired and accordingly wrote down the carrying value of those assets to their net recoverable amounts. The write-down amounting to $509,892 (2008 $0) was in addition to $181,618 (2008 $47,463) in amortization in the statement of operations for the year ended March 31, 2009.
As at March 31, 2009, we had $224,769 in cash (2008 - $163,437) and we currently have approximately $46,000 in cash as at September 15, 2009.
Due to uncertainty in determining future cash flows related to products under license, the Company, during the year ended March 31, 2008, wrote down the value of the product license by $1,306,074 to $1.
Details of the convertible notes are as follows:
Convertible note continuity:
| | US $ | CDN $ |
| | Balance | 2009 | 2009 | 2009 | 2009 | Balance | Balance |
| | March 31, | Issued | Equity | Interest/ | Conversions/ Conversions/ | March 31, | March 31, |
| Date of Issue | 2008 | Principal | Portion | Penalty | Repayments* | 2009 | 2009 |
| | | | | | | | |
| July/August 2005 | $ 259,825 | $ 0 | $ 0 | $ 15,647 | $ (275,472) | $ 0 | $ 0 |
| April 2006 | 534,442 | 0 | 0 | 29,287 | (563,729) | 0 | 0 |
| April 2006 | 85,542 | 0 | 0 | 4,673 | (90,215) | 0 | 0 |
| December 2006 | 1,659,782 | 0 | 0 | 150,544 | (1,810,326) | 0 | 0 |
| January 2007 | 1,569,183 | 0 | 0 | 468,170 | (2,037,353) | 0 | 0 |
| August 2007 | 678,567 | 0 | 0 | 102,260 | (780,827) | 0 | 0 |
| September 2007 | 297,433 | 0 | 0 | 154,295 | (442,826) | 8,902 | 11,228 |
| July 2008 | 0 | 2,168,000 | (1,315,003) | 1,262,529 | (2,115,526) | 0 | 0 |
| | $5,084,774 | $2,168,000 | $(1,315,003) | $2,187,405 | $(8,116,274) | $ 8,902 | $11,228 |
| | US $ | CDN $ |
| | Balance | 2008 | 2008 | 2008 | 2008 | Balance | Balance |
| | March 31, | Issued | Equity | Interest/ | | March 31, | March 31, |
| Date of Issue | 2007 | Principal | Portion | Penalty | Conversions | 2008 | 2008 |
| | | | | | | | |
| August 2005 | $ 316,957 | $ 0 | $ 0 | $ 22,389 | $ (79,521) | $ 259,825 | $ 265,583 |
| April 2006 | 495,607 | 0 | 0 | 38,835 | 0 | 534,442 | 546,841 |
| April 2006 | 79,115 | 0 | 0 | 6,427 | 0 | 85,542 | 87,527 |
| December 2006 | 670,486 | 0 | 0 | 989,296 | 0 | 1,659,782 | 1,698,289 |
| January 2007 | 813,084 | 0 | 0 | 967,767 | (211,668) | 1,569,183 | 1,605,558 |
| August 2007 | 0 | 650,000 | (230,007) | 258,574 | 0 | 678,567 | 694,310 |
| September 2007 | 0 | 391,000 | (213,249) | 119,682 | 0 | 297,433 | 304,633 |
| | $2,375,249 | $1,041,000 | $(443,256) | $2,402,970 | $ (291,189) | $5,084,774 | $5,202,741 |
* Includes commitments to convert the remaining amounts into common shares
US $1,277,200 July – August 2005 Convertible Note
To provide working capital for product development, during July and August 2005 the Company issued one-year, unsecured US$1,277,200 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 50,741 common shares of the Company at a price per share of US$9.00; and (ii) up to an additional 50,741 common shares of the Company at a price per share of US$18.00. Under their original terms, the holders of the convertible notes could elect to convert the notes into common shares of the Company at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; and (ii) US$9.90. Accrued and unpaid interest may be converted into common shares of the Company at US$9.00 per share. The Company may, subject to notice provisions and the common shares trading above US$27.00 per share for more than twenty consecutive trading days, elect to payout the notes and interest due by paying 130% of the amount due under the notes plus interest. The common stock purchase warrants carry a “net cashless” exercise feature (“Cashless Conversion Feature”) allowing the holder thereof, under certain limited circumstances, to exercise the warrants without payment of the stated exercise price, but rather solely in exchange for the cancellation of that number of common shares into which such warrants are exercisable. As a result of the issuance of the warrants in conjunction with the convertible notes, the Company recorded a non-cash financing expense of $1,328,337. These convertible notes were unsecured and due on demand.
In conjunction with this financing, the Company paid consultants an amount equal to 10% of the gross proceeds, which was included in investor relations during the year ended March 31, 2006 and issued 23,651 common shares at a deemed value of $285,242. There are 14,192 finders’ A warrants outstanding whereby the holders have the right to purchase 14,192 common shares at US$9.00 per share until August 5, 2008 and 23,652 finders’ B warrants whereby the holders shall have the right to purchase 23,652 common shares at US$13.50 per share until August 5, 2008. A total of US$82,200 in registration penalties incurred in the year ended March 31, 2007 were converted to a new convertible debenture in the same amount on April 6, 2007.
During the year ended March 31, 2009, principal of US$92,281 and interest of US$9,423 were converted to 14,845 common shares and interest of US$15,647 (2008 – US$22,389) was accrued on the unconverted principal balance of these convertible notes during the year.
Effective March 27, 2009, the Company agreed to settle the remaining principal, accrued interest and prepayment penalties comprising remaining unpaid elements of this convertible note aggregating US$173,768 for 595,006 common shares that will be restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 595,006 common shares were subsequently issued on May 11, 2009.
US $551,666 April 2006 Convertible Note
On April 6, 2006, the Company consummated a private offering transaction with and among a syndicated group of investors, pursuant to which the Company issued, in the aggregate, US$551,666 in 8% per annum convertible notes and three-year Class A and B warrants to acquire: (i) up to 40,864 shares of the Company’s common stock at a price per share of US$9.00; and (ii) up to an additional 40,864 shares of the Company’s common stock at a price per share of US$18.00. Subject to certain limitations under their original terms, the principal amount of the notes, together with any accrued interest could be converted into shares of the Company’s common stock at the lesser of:
| (i) | 70% of the average of the five lowest closing bid prices for the common stock for the ten trading days prior to conversion; or |
| (ii) | US$9.90. The convertible notes carry a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$27.00 per share for at least twenty consecutive trading days and there has otherwise been no default. The common stock purchase warrants carry a Cashless Conversion Feature. |
These convertible notes were unsecured and due on demand.
In conjunction with these private offering transactions, the Company paid consultants: (i) US$55,166 cash, representing 10% of the gross proceeds realized; (ii) 10,216 shares of common stock; (iii) three-year warrants to purchase up to 6,129 shares of common stock at a price per share of US$9.00; and (iv) three-year warrants to purchase up to 10,215 shares of common stock at a price per share of US$13.50.
During the year ended March 31, 2009, convertible notes and interest of US$460,679 were converted to 111,751 common shares and interest of US$21,312 (2008 – US$38,835) was accrued on the unconverted principal balance of these convertible notes.
Effective March 27, 2009, the Company agreed to settle the remaining principal, accrued interest and prepayment penalties comprising remaining unpaid elements of this convertible note aggregating US$103,050 for 473,101 common shares that will be restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 473,101 common shares were subsequently issued on May 11, 2009.
US $82,200 April 2006 penalty Convertible Note
On April 6, 2006, and in conjunction with certain private placements, the Company reached a verbal agreement with investors wherein the Company agreed to convert US$82,200 in accrued penalties associated with the July 25, 2005 through August 5, 2005 convertible notes into US$82,200 convertible penalty notes (Note 10(a)) carrying terms similar to the July 25, 2005 through August 5, 2005 convertible notes and an aggregate of 6,089 warrants. These warrants carry a Cashless Conversion Feature and each warrant entitles the holder to purchase additional common shares for three years at a price of US$13.50 per share. These convertible notes are unsecured and due on demand.
During the year ended March 31, 2009, convertible notes and interest of US$64,410 were converted to 17,994 common shares and interest of US$4,673 (2008 – US$6,427) was accrued on the unconverted principal balance of these convertible notes.
Effective March 27, 2009, the Company agreed to settle the remaining principal, accrued interest and prepayment penalties comprising remaining unpaid elements of this convertible note aggregating US$25,715 for 106,443 common shares that will be restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 106,443 common shares were subsequently issued on May 11, 2009.
Warrant Exercise Price Reduction and Registration Penalty Interest
Certain of the July and August 2005 and the April 6, 2006 convertible notes contained registration rights whereby the Company agreed to pay a penalty of 2% for every thirty day period after which the Company was obligated to file a registration statement and further reduce the warrant price of certain of the warrants issued by US$1.80. As a result of the Company not filing its registration statement until April 27, 2006, the Company incurred penalties, which have been included in interest expense. An aggregate of 189,299 previously issued share purchase warrants relating to certain of the July and August 2005 and the April 6, 2006 convertible notes have reduced exercise prices from US$9.00, US$13.50 and US$18.00 to US$7.20, US$11.70 and US$16.20, respectively. In 2007, the Company recognized $80,102 in interest expense with the corresponding amount to contributed surplus as a result of re-valuation of the warrants upon the change in the pricing.
US $1,500,000 December 2006 Convertible Note
On December 1, 2006, the Company accepted subscriptions of US$1,500,000 towards a private placement of 8% per annum, unsecured, convertible notes and three-year warrants to acquire: (i) up to an aggregate of 111,112 shares of the Company’s common stock at a price per share of US$9.00; and (ii) up to an additional 111,112 shares of the Company’s common stock at a price per share of US$18.00. Subject to certain limitations, the terms of the notes provided that the principal amount of the notes, together with any accrued interest may be converted into shares of the Company’s common stock, at the lesser of: (i) 70% of the average of the five lowest closing bid prices for the Company’s common stock for the ten trading days prior to conversion; or (ii) US$9.90. The convertible notes carried a redemption feature, which allows the Company to retire them, in whole or in part, for an amount equal to 130% of that portion of the face amount being redeemed, but only in the event that the common shares have a closing price of US$27.00 per share for at least twenty consecutive trading days and there has otherwise been no default. These convertible notes are unsecured and due on demand. The common stock purchase warrants may be exercised on a cashless basis. After December 31, 2007, this convertible note began accruing interest at the rate of 15% per annum.
The right of the note holders to convert into the Company’s common stock is subject to the contractual agreement between the parties that any conversion by the note holders may not lead at the date of such conversion to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities or any other financial instruments convertible into common equity.
During the year ended March 31, 2009, the Company accrued US$150,544 (2008 - US$989,296) in interest and accreted interest on the principal balance on the unconverted principal balance of these convertible notes; the principal portion of the notes was increased owing to the accretion of interest expense in the same amount relating to convertible debenture equity conversion component.
Effective March 27, 2009, the Company agreed to settle the remaining principal of US$1,500,000 and accrued interest of US$310,326 comprising remaining unpaid elements of this convertible note aggregating US$1,810,326 for 4,525,807 common shares that are restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 4,525,807 common shares were subsequently issued on May 11, 2009.
US $2,000,000 January 2007 Convertible Note
On January 29, 2007, the Company completed a private placement comprised of $2,000,000 convertible notes. The convertible notes matured on December 11, 2008, and carry interest at 6% per annum and are unsecured. The notes were convertible into “Units” at the note-holders’ discretion at a conversion price of US$9.00 per Unit. Each “Unit” consisted of one common share and one purchase warrant to purchase an additional common share at US$12.60 per share until December 11, 2008. The notes and any accrued interest were callable by the Company at any time after December 11, 2007 by providing thirty days’ written notice to the note-holders. Interest on the notes was compounded annually and cumulative until the earlier of either the date the Company achieves pre-tax earnings or the end of the term. At the discretion of the note-holders, interest on the notes was payable in either cash or units at US$9.00 per unit. In connection with this financing, the Company paid consultants US$108,000 in cash and issued 7,500 units exercisable at US$9.00 per unit, with each unit consisting of one common share and one share purchase warrant to purchase a further common share at US$12.60 per share until December 11, 2008. The Company was obligated to file a resale registration statement on the underlying securities within four months of closing, which it has failed to do.
As a result of the failure to file the registration statement, the Company recorded penalties of US$120,000 during the year ended March 31, 2007 and a further US$289,973 during the year ended March 31, 2008.
During the year ended, March 31, 2009, convertible note principal and accrued interest in the aggregate amount of US$709,995 was converted into 78,888 common shares and convertible note principal and accrued interest in the aggregate amount of US$28,238 was traded for new 10% interest convertible notes that convert automatically into units at a conversion price of US$0.40 per unit on the effective date of the reverse share consolidation where each unit is comprised of one common share and one 2-year share purchase warrant to purchase an additional common shares at $0.60 per share for a two year term from the date of issue of the units.
During the year ended March 31, 2009, the Company accrued US$468,170 (2008 - US$967,767) in interest and accreted interest on the principal balance on the unconverted principal balance of these convertible notes.
Effective March 27, 2009, the Company agreed to settle the remaining principal, and accrued interest comprising remaining unpaid elements of this convertible note aggregating US$1,299,120 for 3,247,797 common shares that will be restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 3,247,797 common shares were subsequently issued on May 11, 2009.
US $650,000 August 2007 Convertible Note
On August 10, 2007, the Company issued an unsecured convertible term promissory note in the amount of US$650,000 to a third party. The convertible note bears interest at 6% with both interest and principal convertible at the option of the lender into units at US$10.80 per unit, with each unit consisting of one common share and one-half share purchase warrant with each whole share purchase warrant exercisable at US$13.50 to purchase an additional common share. After November 25, 2008, this convertible note accrued interest at the rate of 15% per annum.
The Company was required to register for trading the securities underlying the conversion features of this convertible note on a best-efforts basis but failed to do so within terms agreed. A one-time financial penalty of US$28,567 for failure to register the securities underlying this convertible note within 180 days from the date of issuance was incurred in the year ended March 31, 2008.
The right of the note holder to convert into the Company’s common stock was subject to the contractual agreement between parties that any conversion by the note-holder may not lead, at the date of such conversion, to an aggregate equity interest in the common stock of the Company greater than 9.99% inclusive of any derivative securities including options, warrants, convertible debt, any other convertible debt securities or any other financial instruments convertible into common equity.
During the year ended March 31, 2009, the Company accrued US$102,260 (2008 - US$258,574) in interest and accreted interest on the principal balance on the unconverted principal balance of these convertible notes.
Effective March 27, 2009, the Company agreed to settle the remaining principal and accrued interest comprising remaining unpaid elements of this convertible note aggregating US$780,827 for 1,952,066 common shares that are restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 1,952,066 common shares were subsequently issued on May 11, 2009.
US $391,000 September 2007 Convertible Note
On September 27, 2007, the Company issued unsecured one-year term convertible notes bearing interest at 6% per annum in the amount of US$391,000 to third parties. Under the original terms, both interest and principal may be converted at the option of the lender at any time at US$10.80 per unit, with each unit consisting of one common share and one-half share purchase warrant, with each whole share purchase warrant exercisable at US$13.50 to purchase an additional common share for a two-year term from the date of conversion.
The Company was required to register for trading the securities underlying the conversion features of this convertible note on a best efforts basis but has failed to do so within terms agreed. A one-time financial penalty of US$23,460 for failure to register the securities underlying this convertible note within 90 days from the date of issuance has been incurred during the year ended March 31, 2008.
During the year ended March 31, 2009, the Company accrued US$154,295 (2008 - US$119,652) in interest and accreted interest on the principal balance on the unconverted principal balance of these convertible notes.
During the year ended March 31, 2009, the Company repaid US$7,500 of the principal on these convertible notes and, subsequent to year end, paid the balance of US$7,500 in principal and US$1,402 in interest.
Effective March 27, 2009, the Company agreed to settle the remaining principal and accrued interest comprising remaining unpaid elements of this convertible note aggregating US$435,326 for 1,024,667 common shares that will be restricted from trading and pooled with equal quarterly releases beginning in January 2010. The 1,024,667 common shares were subsequently issued on May 11, 2009.
US $2,168,000 July 2008 Convertible Note
On July 21, 2008, the Company closed a financing of zero coupon, 12% interest, senior secured convertible promissory notes in the amount of US$2,428,160 with an aggregate purchase price of US$2,168,000 with four investors, one of which was the Company’s Chief Financial Officer. The debt is convertible into shares of common stock at the lesser of US$9.18 per share (unless the conversion price has been adjusted pursuant to further contract covenants) and 70% of the average of the five lowest closing bid prices for the ten preceding trading days. The Company issued each purchaser in the private placement two warrants - one warrant being redeemable by the Company and the other being non-redeemable. The non-redeemable warrants are exercisable at US$9.90 and permit the holder to purchase shares of common stock equal to 100% of the number of shares issuable upon the conversion of the notes calculated on July 21, 2008. The redeemable warrants are exercisable at US$13.50 and permit the holder to purchase common stock equal to 50% of the number of shares issuable upon the conversion of the notes issued calculated on the closing date. In total, the Company issued redeemable warrants to purchase an aggregate of 264,506 shares of common stock and redeemable warrants to purchase an aggregate of 132,253 shares of common stock. Further, the Company issued 24,401 non-redeemable warrants and 12,201 redeemable warrants and US$160,000 in cash fees to close the transaction. Each non-redeemable warrant is exercisable at US$9.90, and each redeemable warrant is exercisable at US$13.50; warrants carry a term of five years from the date of closing of the financing. The redeemable warrants may be redeemed by the Company only if certain conditions have been satisfied including the Company’s common stock having closed at $27.00 per share for a period of 20 consecutive trading days and the warrant holder being able to resell the shares acquired upon exercise through a resale registration statement or under Rule 144 of the Securities Act.
During the year ended March 31, 2009, interest and accretion of the convertible debenture in the aggregate of US$1,326,959 was accrued on the principal balance of these convertible notes.
Effective March 27, 2009, the Company settled these notes by:
| · | Issuing a new promissory note with a face value of US$1,323,000 that accrues interest at 12% and is repayable by the Company anytime until maturity on December 31, 2009; |
| · | settled the non-cash portions of the convertible note including US$524,884 in prepayment penalty, US$260,160 in original issuer discounts and US$371,771 in interest which includes the interest payable on the new note through December 31, 2009 for an aggregate settlement of US$1,156,815 and agreed to issue 2,892,036 common shares that will be restricted from trading and pooled until January 1, 2010; |
| · | Repayment of US$400,000 (cash paid May 11, 2009); and |
| · | Conversion of US$250,000 face value of the original note into common shares of the Company. |
Each Note holder also agreed to waive any adjustment to the exercise price of the warrants issued to the holders as part of the July 2008 financing that may have resulted from the issuances of shares of Company common stock as part of the Company’s overall debt restructuring and/or from certain other contemplated Company issuances. However, subject to certain exceptions, the Company agreed that if before March 31, 2010 the Company issues shares of its common stock at a price less than the valuation of the shares issued to each Note holder, that the Company would issue each holder additional shares of Company common stock in a number equal to the difference between the number of shares each holder would have received had the consideration paid to the holder been paid in shares at the lower valuation.
If the US$1,323,000 face amount required to retire these notes is not paid on or before December 31, 2009, the promissory notes revert to the terms and conditions original secured convertible note transaction documents including security agreement originally executed in July 2008 which remain valid and in effect.
SUBSEQUENT EVENTS TO MARCH 31, 2009
Unless otherwise noted in this annual report, the following events occurred after March 31, 2009:
| (a) | On April 1, 2009 through June 22, 2009, the Company received subscriptions to a 10% convertible promissory note in the aggregate of US$176,240. The note and accrued interest have been converted into units at the rate of US$0.40 per unit. Each unit consists of one restricted common share and one share purchase warrant to purchase an additional restricted common share at an exercise price of US$0.60 per share for a two year term. On July 17, 2009, the Company issued 458,139 common shares pursuant to the conversion of US$179,478 in convertible notes and accrued interest of US$3,777, and also issued 458,139 warrants to purchase 458,139 common shares at an exercise price of US$0.60 per share until July 17, 2011. |
| (b) | On May 9, 2009, the Company completed a series of debt settlements that settled $10,806,780 in aggregate debt as at March 31, 2009 by issuing 29,516,951 post reverse consolidation shares. |
| (c) | On May 9, 2009, the Company issued 312,500 common shares at US$0.80 per share pursuant to secured convertible note principal conversions received in the amount of US$250,000. |
| (d) | On May 22, 2009, the Company received a subscription to a 10% convertible promissory note in the amount of US$1,500,000 from a single subscriber to complete a staged financing by a single subscriber. The note and accrued interest is convertible into units of equity at the rate of US$0.125 per post consolidated unit. Each unit is comprised of one restricted common share and one share purchase warrant to purchase an additional restricted common share at US$0.45 per post reverse consolidated share for a one year term from the date of conversion. The warrants may be exercised on a cashless basis. On July 17, 2009, the Company issued 16,303,562 common shares and 5,434,521 warrants to purchase an additional restricted common share at US$0.45 for a one year term from the date of issue pursuant to conversion of the US$1,500,000 and a further US$500,000 received during the year ended March 31, 2009 together with accrued interest of US$37,945. |
| (e) | On May 29, 2009, retroactive to April 1, 2009, the Company executed an agreement to acquire all ownership rights and intellectual property relating to its VertiCrop™ vertical plant growing technology and Tomorrow Garden™ plant kit technology, all previously obtained under master license arrangements with Pagic LP, a company controlled by the Company’s past President. The conditional sales agreement between the Company, Glen Kertz, Pagic LP, and West Peak Ventures of Canada Ltd., provides full ownership of all patents rights, know how, trademarks, research and development, improvements, test and development data, and all other intellectual property rights for VertiCrop™ and Tomorrow Garden™ technologies (the “Technologies”). The agreement terminates the previous master license agreements between Valcent and Pagic LP, and replaces all financial obligations the Company had under that agreement including annual payments, royalty burden, and all other associated licensing costs. The Company agreed to pay a total of US$2,000,000 plus issue 3% of its common stock on conclusion of the agreement. The US$2,000,000 is payable on a cumulative basis plus the greater of 3% of the gross monthly product sales less returns from exploitation of the Technologies or US$12,000 per month until US$2,000,000 has been paid. The Company has up to 10 years to make the total payment, with the ownership of the Technologies remaining in escrow until fully paid or Company defaults in making payments, with the Technologies reverting back to Glen Kertz and Pagic LP. The Company paid $153,295 since execution of the agreement. |
| (f) | On July 16, 2009, the Company effected a reverse share consolidation of its common shares on a one new share for eighteen old shares basis. The Company’s trading symbol changed to VCTZF on this date and its CUSIP number changed to 918881 20 2. |
| (g) | On July 31, 2009, the Company issued an aggregate of 75,000 common shares valued at US$43,500 pursuant to obligations under a public relations agreement and an internet based services agreement. |
YEAR ENDED MARCH 31, 2008 COMPARED WITH YEAR ENDED MARCH 31, 2007
Operating Results
For the year ended March 31, 2008, the Company focused on (i) the development of a commercial algae biomass technology via a joint venture with Global Green (the “Vertigro Project”), (ii) the development of our High Density Vertical Growth System designed to more efficiently produce vegetables and other plant crops, (iii) the development and test marketing of the Tomorrow GardenTM consumer retail product in our UK based subsidiary, (iv) the development of product inventories and direct test and sales initiatives and product introduction promotion relating to our Nova Skin Care System, (v) ongoing research and development with tissue culture technologies, plant growth stimulation technologies, and other product and technology development initiatives, and (vi) a series of private offering transactions with institutional and other investors, pursuant to which we raised US$5,129,636 through private placements of equity comprised of common shares and warrants, US$367,671 from warrant and share option exercises, and US$1,291,000 from convertible debentures, promissory notes, and short term advances.
For the year ended March 31, 2007, we focused (i) the development of our algae technology via a joint venture with Global Green, (ii) the development of product inventories and direct sales initiatives relating to our Nova Skin Care System, and (iii) the development and anticipated marketing of the Tomorrow GardenTM consumer retail product in our UK based subsidiary and, (iv) on a series of related private offering transactions with institutional and other investors, pursuant to which we raised $4,817,114 through the issuance of convertible debentures and $1,028,266 from the issuance of common shares. We incurred losses of $12,712,358 for the year ended March 31, 2008, as compared to $8,138,393 for the year ended March 31, 2007.
Revenues
For the years ended March 31, 2008 and March 31, 2007, revenue in connection with Nova Skin Care Systems product sales testing was netted against product development costs. The Company generated approximately US$598,775 (2007 $nil) in cash related to the Nova Skin Care system which has been netted against product development costs.
Interest income for the years ended March 31, 2008 and March 31, 2007 was $10,805 and $25,704 respectively.
The foreign exchange gain for the years ended March 31, 2008 and March 31, 2007 was $611,133 and $6,993 respectively.
Operating Expenses
Product development expenses increased by $2,519,014 to $4,081,435 for the year ended March 31, 2008 as compared with the year ended March 31, 2007. The increase was due to the larger scale and scope of a) Nova Skin Care Products marketing and product testing rollout with significant infomercial media advertising costs, b) increased Vertigro research and development costs, c) new development initiatives relating to tissue culture research, plant stimulator technologies, and HDVG System product development, and d) the advent of Valcent EU costs relating to the Tomorrow GardenTM and UK based HDVG System development initiatives, all pursuant to license arrangements acquired in the Pagic Agreements. Product development expenses were $1,562,421 during the year ended March 2007.
In conjunction with convertible debenture financings during the year ended 2008, the Company incurred $2,804,582 in interest, accretion and financing on convertible notes in the twelve month period ended March 31, 2008. This represents a $78,515 increase from the $2,726,067 that had been incurred during the year ended March 31, 2007 stemming primarily from amortization of convertible debenture funding activity during the 2007 and 2008 fiscal years.
Investor relations fees increased $583,708 to $871,542 (2007 - $287,834) for the year ended March 31, 2008 as a result of the Company employing an increasing number of third party consultants in advisory, business consulting services, and investor relations activities with the bulk of the increase represented by non-cash stock based compensation.
Professional fees decreased by $281,187 to $431,271for the year ended March 31, 2008 from $712,458 for the year ended March 31, 2007. The decrease is primarily attributable to costs associated with decreased business activity relating to intellectual property legal services, and executive search services between the respective years.
Advertising and media development was $1,953,998 during the year ended March 31, 2008 (2007 $1,092,917) that reflects the more advanced stages of marketing systems development hat include infomercial media purchases in connection with test sales initiatives of our Nova Skin Care System that commenced in December, 2006.
Office and miscellaneous expenses decreased $46,879 to $234,817 for the year ended March 31, 2008 from $281,696 for the year ended March 31, 2007. The decrease is due to cost streamlining and organizational emphasis on project development initiatives and research facility build out at our facility in El Paso, Texas.
As a result of the issuance of options to directors, officers, employees and consultants the Company incurred stock option compensation expenses of $990,305 during the year ended March 31, 2008 (2007 - $1,127,141).
Travel expenses increased by $134,967 to $291,465 (2007 - $156,498) for the year ended March 31, 2008 as a result of increased activity in all of the Company’s operations, increased number of active development projects, as well as new costs relating to an additional subsidiary interest located in the United Kingdom.
As a result of increasing operating capacity and existing and as new project build out at our El Paso, Texas operation, as well as the development of our new Cornwall, UK offices, the Company’s fixed assets and land were valued at a net book value of $1,135,108 (2007 - $348,487) during the year ended March 31, 2008, and incurred a depreciation and amortization charge of $47,463 (2007 - $25,288).
Rent expenses increased $12,225 to $77,918 for the year ended March 31, 2008 from $65,693 for the year ended March 31, 2007. The increase in rent costs incurred relates to our new offices and operations located in the United Kingdom.
Filing and transfer agent expenses increased $5,617 to $44,500 for the year ended March 31, 2008, from $38,883 for the year ended March 31, 2007. The increase is primarily attributable to costs associated with the proportionate increase of financing and business activity over the 2007 fiscal year.
Insurance expense decreased to $71,071 for the year ended March 31, 2008, from $78,784 for the year ended March 31, 2007. The decrease is primarily attributable to the rebate of 2007 insurance costs received in the year ended March 31, 2008 and associated decreased insurance coverage required which was prorated over both 2007 and 2008 fiscal years.
Interest relating to long term debt increased to $15,480 (2007 - $8,500) due to a complete year of debt repayments during fiscal 2008 as compared with a partial year of debt repayments during fiscal 2007 in connection with lands acquired for research relating to the Company’s development of its various projects and initiatives.
Due to fluctuations in the United States dollar in relation to the Canadian dollar, the Company incurred a foreign exchange gain of $611,133 (2007 - $6,993) during the year ended March 31, 2008.
Net Loss
Our reported loss increased by $4,573,965 to $12,712,358 ($6.44 basic loss per share) for the year ended March 31, 2008 as compared to $8,138,393 ($7.60 basic loss per share) for the same period ending March 31, 2007. The increase during the 2008 fiscal year is largely a result of a) the prior period correction in the amount of $3,346,835 pertaining primarily to accounting methods used to value and amortize convertible debenture instruments, and to a lesser degree an adjustment to prepaid expenses and insurance expense for amounts that relate to the 2008 fiscal year-end, b) $365,021 in aggregate expenses associated with increasing scale and scope of product development and marketing initiatives relating to all our projects under development, increasing Company consulting arrangements relating to increasing scale and scope of business operations, as well as expenses our new UK operating offices, and c) the write down of our product license by $1,306,074, all of which was offset by $611,133 of gain relating to foreign exchange accounts and $10,805 of interest income.
Liquidity and Capital Resources
Because we are organized in Canada, our March 31, 2008 financial statements have been prepared by our management in accordance with Canadian GAAP applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business.
Our accumulated losses during the development stage increased by $12,712,358 to $24,317,639 for the year ended March 31, 2008 from approximately $11,605,281 for the year ended March 31, 2007.
Our working capital deficit increased to $5,094,440 from $1,600,602 for the year ended March 31, 2008 representing a $3,493,838 increase over the year ended March 31, 2007.
We raised US$1,041,000 in net cash proceeds from the issuance of convertible debentures during the year ended March 31, 2008, as compared to US$4,133,866 for the year ended March 31, 2007.
For the year ended March 31, 2008, the Company received $5,081,103 from the issuance of common shares relating to private offering transactions with institutional and other investors. We raised a further $367,671 from the exercise of outstanding warrants and options.
We further raised $154,196 from interest bearing promissory notes during the year ended March 31, 2008 for an aggregate outstanding of $270,167 as at March 31, 2008 (2007 - $115,460). Our advances and amounts due from related parties increased by $560,341 to $1,490,516 as at March 31, 2008 (2007 - $930,175).
As a result of the Nova Skin Care System our inventories were $617,195 as at March 31, 2008 (2007 - $1,236,808).
As at March 31, 2008, accounts receivable of $462,156 consists of $374,502 (2007 $30,282) net of allowance for doubtful accounts of $90,263 (2007 $3,798) due from the Company’s product fulfillment agent, GSI Commerce, Inc. (formerly Accretive Commerce Inc.) from test sales relating to the Company’s Nova Skin Care System, ($7,806) (2007 $426,987) due from the Company to Global Green Solutions Inc., the Company’s joint venture partner in the development of the Company’s High Density Vertical Bio-Reactor technology, $51,472 in value added tax owed to the Company’s subsidiary, Valcent Products EU Limited, and goods and services tax receivable by the Company of $43,988.
Prepaid expenses as at March 31, 2008 consists of $2,015,837 (2007 - $285,690), which is the deferred portion of these agreements with the balance consisting of $103,709 (2007 - $71,071) in prepaid insurance and rental deposits.
Restricted cash as at March 31, 2008 consists of certificates of deposit and interest earned of $108,471 (2007 - $117,327) that are pledged to secure long term debt relating to our purchased lands in El Paso.
We purchased $834,084 (2007 - $310,448) in fixed assets during the year ended March 31, 2008. During the year ended March 31, 2007, we incurred long term debt in connection with a land purchase. As at March 31, 2008, we owed an aggregate of $174,862 relating to this debt (2007 - $209,114).
As at March 31, 2008, we had $163,437 in cash (2007 - $314,972) and we currently have approximately $755,000 in cash as at August 6, 2008.
Due to uncertainty in determining future cash flows related to products under license, the Company, during the year ended March 31, 2008, wrote down the value of the product license by $1,306,074 to $1.
RISKS
The business of the Company entails significant risks, and an investment in the securities of the Company should be considered highly speculative. An investment in the securities of the Company should only be undertaken by persons who have sufficient financial resources to enable them to assume such risks. The following is a general description of some of the material risks, which can adversely affect the business and in turn the financial results, ultimately affecting the value of an investment the Company.
We Have A History Of Operating Losses And We May Have Operating Losses And A Negative Cash Flow In the Future
We Need Additional Financing To Meet Our Current And Future Capital Needs And We May Not Be Able To Secure That Financing
We Have Only Limited Experience As A Public Reporting Company Which May Place Significant Demands On Our Operations
The Company’s Inability To Attract And Retain New Personnel Could Inhibit Our Ability To Grow Or Maintain Our Operations
There Is Only A Limited Market For Our Common Shares
The Price Of Our Common Shares May Be Volatile Which Could Result In Substantial Losses For Individual Shareholders
As Of The Date Of This Report The Company Is Not Carrying Any Insurance On Its EL Paso Facilities Which Could Result In A Significant Loss
OFF-BALANCE SHEET ARRANGEMENTS
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a material adverse affect on our financial condition or results of operations.
CONTRACTUAL OBLIGATIONS
As of March 31, 2009, we had the following contractual obligations not otherwise noted in this Management’s Discussion and Analysis:
On November 16, 2007, our wholly owned subsidiary, Valcent EU Limited leased office and development space in Launceston, Cornwall, UK, under a ten-year lease beginning November 15, 2007 and ending on November 15, 2017 at a quarterly cost of GB£12,550 ($24,020). There were 8 years and 7.5 months remaining on the lease as at March 31, 2009.
Fiscal yearly commitments are as follows:
| 2010 | $ 96,081 |
| 2011 | 96,081 |
| 2012 | 96,081 |
| 2013 | 96,081 |
| 2014 | 96,081 |
| Thereafter | 348,295 |
| | $ 828,700 |
RELATED PARTY TRANSACTIONS DURING THE YEAR ENDED MARCH 31, 2009
Amounts due to related parties are non-interest bearing and have no specific terms of repayment. Due to related parties includes the following amounts at March 31 in respect to certain of the transactions detailed below:
| | 2009 | 2008 |
(a) Pagic | $ 0 | $ 189,156 |
(b) CFO | 19,105 | 158,370 |
| (c) Consulting services and unsecured loan advances | 32,938 | 73,896 |
| (d) Consulting services and unsecured loan advances | 30,882 | 1,000,165 |
(e) Advertising | 0 | 52,145 |
(f) Operational management consulting | 103,720 | 16,784 |
| | | |
| | $ 186,645 | $ 1,490,516 |
Related party transactions are in the ordinary course of business and are measured at the exchange amount at the time the agreement is entered into or services are provided. Related party transactions not disclosed elsewhere in these financial statements are as follows:
| | 2009 | 2008 |
(a) Pagic, a company related by a past common officer and director for: (i) Net charges for product development expenses including royalties (ii) Issued 16,667 shares related to technology purchase (iii) Commitment to issue 391,298 common shares for debt settlement | $ 301,948 190,746 195,570 | $ 565,135 0 0 |
(b) The Company’s Chief Financial Officer (“CFO”) and director for: (i) Charges for professional fees (ii) Exchange of balances for 12% convertible note (Note 10(i)) (iii) Net short-term advance, at 8-10% interest per annum (iv) Original issuer discount, prepayment penalty and interest prepaid through December 31, 2009 (v) Commitment to issue 1,085,690 common shares for debt settlement | 36,000 188,992 52,605 128,889 370,087 | 35,370 0 150,000 0 0 |
(c) Charges from private companies with CFO and director in common for: (i)Office rent (ii)Consulting fee (iii)Unsecured loan advances (iv)Commitment to issue 114,826 common shares | 37,800 160,650 0 57,364 | 30,000 150,000 57,364 0 |
(d) West Peak and its principal shareholder, a beneficial owner of more than 5% of the Company’s common shares: | | |
(i) Unsecured loan advances, at 8% interest per annum (ii) Consulting fees (iv) Convertible notes purchased (v) Exchange of amounts owed at March 31, 2009 forfuture issuance of 4,722,204 common shares | 2,587,638 653,290 85,460 1,601,528 | 977,665 52,500 162,305 0 |
(e) Advertising services of a private company with a director in common | 0 | 244,861 |
(f) A director for: (i) Operational management consulting and investor relations services (ii) Commitment to issue 46,875 common shares | 202,421 23,428 | 151,825 0 |
(g) Bonus of 5,555 restricted common shares to a retiring director | 0 | 52,889 |
(h) A director for: (i) Loans received/repaid during the year with 10%interest (ii) Convertible notes purchased (iii) Commitment to issue 183,860 common shares | 99,390 91,893 $ 191,293 | 0 0 $ 0 |
Summary of Critical Accounting Policies
These financial statements have been prepared in accordance with Canadian GAAP and are presented in Canadian dollars. The following is a summary of certain of the significant accounting policies used in the March 31, 2009 consolidated financial statements. Readers are recommended to review Note 2 to the March 31, 2009 consolidated financial statements for a complete listing.
(a) Principles of consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries Valcent EU and Valcent USA and Valcent USA’s wholly-owned subsidiaries Valcent Management and Valcent Manufacturing, in which Valcent Management is the general partner and Valcent USA is the limited partner. All significant inter-company transactions and balances have been eliminated.
These consolidated financial statements also include the Company’s proportionate share of the assets, liabilities, income and expenses of the Vertigro JV.
(b) Research and product development costs
Research costs are expensed as incurred. Development costs, which meet Canadian GAAP criteria including reasonable assurance regarding recoverability, are capitalized and amortized over the expected economic useful life of the product. No development costs have been deferred to date. As the Company is in the development stage, any revenue derived from the test marketing and development of products is considered to be an expense recovery and is, therefore, netted against product development costs.
(c) Foreign currency transactions and translation
The Company presents its financial statements in Canadian dollars. Amounts recorded in foreign currencies are translated into Canadian dollars as follows:
(i) Monetary assets and liabilities, at the year-end exchange rates;
(ii) Non-monetary assets and liabilities, at historical exchange rates; and
(iii) Income and expense items, at the exchange rate in effect on the date of the transaction.
Gains and losses arising from the translation of foreign currency are included in operations for the year.
(d) Use of estimates
The preparation of financial statements in conformity with Canadian GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant areas requiring the use of estimates include the net realizable value of accounts receivable and inventories, impairment of property and equipment, rates of amortization, fair value of product license, amount of accrued liabilities, the variables used to calculate interest, accretion and financing costs on convertible notes, the variables used to calculate the fair value of stock-based compensation and the determination of the valuation allowance for future income tax assets. While management believes the estimates are reasonable, actual results could differ from those estimates and could impact future results of operations and cash flows.
(e) Stock-based compensation
The Company accounts for stock-based compensation expense using the fair value based method with respect to all stock-based awards to directors, employees and non-employees, including awards that are direct awards of stock and call for settlement in cash or other assets or stock appreciation rights that call for settlement by the issuance of equity instruments. Under this method, stock-based payments are recorded as an expense over the vesting period or when the awards or rights are granted, with a corresponding increase to contributed surplus. When stock options are exercised, the corresponding fair value is transferred from contributed surplus to share capital.
Revenue from the sales of products is recognized when the product has been delivered to the purchaser, the price is fixed or determinable and when collectability is reasonably assured. Sales during the development stage are netted against product development costs.
Interest income is recognized on an accrual basis as earned at the stated rate of interest of the investment over the term to maturity.
| (g) | Financial Instruments |
Financial instruments must be classified into one of these five categories: held-for-trading, held-to-maturity, loans and receivables, available-for-sale financial assets or other financial liabilities. All financial instruments are measured in the balance sheet at fair value except for loans and receivables, held-to-maturity investments and other financial liabilities, which are measured at amortized cost. Subsequent measurement and changes in fair value will depend on their initial classification as follows: held-for-trading financial assets are measured at fair value and changes in fair value are recognized in net income; available-for-sale financial instruments are measured at fair value with changes in fair value recorded in other comprehensive income until the investment is no longer recognized or impaired, at which time the amounts would be recorded in net income.
Transaction costs that are directly attributable to the acquisition or issue of financial instruments that are classified as other than held-for-trading, which are expensed as incurred, are included in the initial carrying value of such instruments and amortized using the effective interest method.
Changes in accounting policies
Effective April 1, 2008, the Company adopted the following standards of the Canadian Institute of Chartered Accountants (“CICA”):
(i) Capital Disclosures (Section 1535)
Section 1535 specifies the disclosure of: (i) an entity’s objectives, policies and procedures for managing capital; (ii) quantitative data about what the entity regards as capital; (iii) whether the entity has complied with any capital requirements; and (iv) if it has not complied, the consequences of such non-compliance.
As a result of the adoption of this standard, additional disclosure on the Company’s capital management strategy has been included in note 4 to the March 31, 2009 consolidated financial statements.
(ii) Financial Instruments - Disclosures and Presentation
Sections 3862 and 3863 replace Section 3861, “Financial Instruments – Disclosures and Presentation”, revising its disclosure requirements and carrying forward its presentation requirements. These new sections place increased emphasis on disclosures about the nature and extent of risks arising from financial instruments and how the entity manages those risks.
Section 3862 specifies disclosures that enable users to evaluate: (i) the significance of financial instruments for the entity’s financial position and performance; and (ii) the nature and extent of risks arising from financial instruments to which the entity is exposed and how the entity manages those risks.
As a result of the adoption of these standards, additional disclosures on the risks of certain financial instruments have been included in notes 3 and 4 to the March 31, 2009 consolidated financial statements.
(iii) Amendments to Section 1400 – Going Concern
CICA Section 1400, “General Standards of Financial Statement Presentation”, was amended to include requirements to assess and disclose an entity's ability to continue as a going concern. When financial statements are not prepared on a going concern basis, that fact shall be disclosed together with the basis on which the financial statements are prepared and the reason why the company is not considered a going concern. The Company’s accounting policies were already in accordance with the requirements of the amended section and there was no effect on the Company’s consolidated financial statements.
Future accounting changes
(i) International Financial Reporting Standards ("IFRS")
In 2006, the Canadian Accounting Standards Board ("AcSB") published a new strategic plan that will significantly affect financial reporting requirements for Canadian companies. The AcSB strategic plan outlines the convergence of Canadian GAAP with IFRS over an expected five year transitional period. In February 2008, the AcSB announced that the effective date for publicly-listed companies to use IFRS is for interim and annual financial statements relating to fiscal years beginning on or after January 1, 2011 which will require the restatement for comparative purposes of amounts reported by the Company for the year ended March 31, 2011. While the Company has begun assessing the adoption of IFRS for 2011, the financial reporting impact of the transition to IFRS cannot be reasonably estimated at this time.
(ii) Goodwill and intangible assets
In February 2008, the CICA issued Section 3064, “Goodwill and Intangible Assets”. Effective for fiscal years beginning on or after October 1, 2008, this section provides guidance on the recognition, measurement, presentation and disclosure for goodwill and intangible assets, other than the initial recognition of goodwill or intangible assets acquired in a business combination. Retroactive application to prior period financial statements will be required. The Company does not expect these changes to have an impact on its consolidated financial statements.
(iii) Business Combinations
In January 2009, the CICA issued Section 1582, “Business Combinations”, Section 1601, “Consolidations” and Section 1602, “Non-Controlling Interests”. These sections replace the former Section 1581, “Business Combinations” and Section 1600, “Consolidated Financial Statements” and establish a new section for accounting for a non-controlling interest in a subsidiary.
Sections 1582 and 1602 will require net assets, non-controlling interests and goodwill acquired in a business combination to be recorded at fair value and non-controlling interests will be reported as a component of equity. In addition, the definition of a business is expanded and is described as an integrated set of activities and assets that are capable of being managed to provide a return to investors or economic benefits to owners. Acquisition costs are not part of the consideration and are to be expensed when incurred. Section 1601 establishes standards for the preparation of consolidated financial statements.
These new sections apply to interim and annual consolidated financial statements relating to fiscal years beginning on or after January 1, 2011. Earlier adoption of these sections is permitted as of the beginning of a fiscal year. All three sections must be adopted concurrently.
Financial Instruments and Risks
The Company has designated its cash and cash equivalents and restricted cash as held-for-trading; accounts receivable as loans and receivables; and its accounts payable and accrued liabilities, promissory notes payable, amounts due to related parties, convertible notes and long-term debt as other financial liabilities.
The carrying values of cash and cash equivalents, accounts receivable, accounts payable and accrued liabilities, promissory note payable and amounts due to related parties approximated their fair values because of the short-term maturity of these financial instruments.
The carrying values of restricted cash and long-term debt approximated their fair values as these financial instruments bore interest at approximate market rates of interest.
Convertible debentures are shown at amortized cost.
Interest rate risk consists of two components:
| (b) | To the extent that payments made or received on the Company’s monetary assets and liabilities are affected by changes in prevailing market interest rates, the Company is exposed to interest rate cash flow risk. |
| (b) | To the extent that changes in prevailing market interest rates differ from the interest rates in the Company’s monetary assets and liabilities, the Company is exposed to interest rate price risk. |
The Company is not exposed to significant interest rate risk due to the short-term maturityof its monetary current assets and current liabilities.
The Company is exposed to interest rate cash flow risk on its long-term debt with variableinterest rates as the payments on the loan will fluctuate as interest rates fluctuate during theterm of the debt.
The Company is exposed to interest rate price risk on its promissory and convertible noteswith fixed interest rates as the market rate of interest differs from the interest rate of theconvertible notes.
The Company operates internationally and is therefore exposed to foreign exchange fluctuations in the value of foreign currencies in relation to the Canadian dollar. The Company’s foreign currency exposures comprise cash, accounts receivable, accounts payable and convertible notes denominated in British pounds and United States dollars as follows:
| Cash | £ | 17,200 | US$ | 146,800 |
| Accounts receivable | | 12,000 | | 637,000 |
| Accounts payable | | 7,000 | | 468,000 |
| Promissory notes payable | | 0 | | 2,223,000 |
| Convertible notes | | 0 | | 7,500 |
| | | | | |
The Company does not use derivatives or other techniques to manage foreign currency risk.
Based on the above net foreign currency exposure as at March 31, 2009 and assuming all other variables remain constant, a 10% weakening or strengthening of the Canadian dollar against the British pound and US dollar would result in the following increases or decreases in the Company’s loss :
| Increase/decrease in net loss – British pound | | $ 2,400 |
| Increase/decrease in net loss – US dollar | $ 241,500 |
Credit risk is the risk of financial loss to the Company if a customer or counterparty to a financial instrument fails to meet its contractual obligations. The Company is exposed to credit risk with respect to its cash and accounts receivable. The credit risk associated with cash is minimal as cash has been placed with major financial institutions. The Company is exposed to significant credit risk with respect to accounts receivable as the majority of the amount is due from a joint venture partner.
Concentration of credit risk exists with respect to the Company’s cash as all amounts are held at single major Canadian, American and British financial institutions. The Company’s concentration of credit risk and maximum exposure thereto is as follows:
| | 2009 | 2008 |
| | | |
| Cash | $ 224,769 | $ 163,437 |
| Accounts receivable | 920,235 | 462,156 |
| | | |
| | $ 1,145,004 | $ 625,593 |
Liquidity risk is the risk that the Company will encounter difficulty in obtaining funds to meet commitments. The Company manages its liquidity risk by forecasting cash flows from operations and anticipated investing and financing activities. At March 31, 2009, the cash balance of $224,769 (2008 - $163,437) is insufficient to meet the Company’s business requirements for the coming year. Therefore, the Company will be required to raise additional capital or sell one or more of its technologies in order to fund its operations for 2010.
At March 31, 2009, the Company had the following amounts coming due during the next year:
| | | Carrying amount | | Due date |
| Accounts payable | $ | 541,802 | | 90 days |
| Due to related parties | $ | 186,645 | | 90 days |
| US$400,000 note payable | $ | 504,520 | | 90 days |
| Convertible note | $ | 46,512 | | 90 days |
| US$500,000 subscription advance | $ | 630,650 | | Aug 31, 2009 |
| US$1,323,000 note payable | $ | 1,473,060 | | Dec 31, 2009 |
| (e) | Classification of financial instruments |
| | Loans and Receivables | Held for Trading | Other Liabilities |
| Cash | $ 0 | $ 224,769 | $ 0 |
| Accounts receivable | 920,235 | 0 | 0 |
| Accounts payable | 0 | 0 | 541,802 |
| Promissory notes | 0 | 2,643,514 | 0 |
| Due to related parties | 0 | 0 | 186,645 |
| Convertible notes | 0 | 11,228 | 0 |
| Total | $ 920,235 | $ 2,879,511 | $ 728,447 |
| | | | |
Capital Management
The Company’s objectives when managing capital are to safeguard the Company’s ability to continue as a going concern in order to pursue the development of its products. The Company relies mainly on equity and debt instrument issuances to raise new capital. In the management of capital, the Company includes the components of shareholders’ equity as well as cash. The Company estimates anticipated research and development expenditures to ensure that there is sufficient capital on hand to meet ongoing obligations. The Company’s investment policy is to invest its cash in highly liquid short‐term deposits with terms of one year or less and which can be liquidated after thirty days without interest penalty. The Company currently does not have sufficient capital to cover its administrative costs or fund research and development programs for the next twelve months. The Company will only be able to continue to develop its products with additional equity or debt instrument financing.
Legal Proceedings
Except as noted below; there are no legal actions either in process or pending and we are not aware of any contemplated, legal, governmental or arbitration proceedings, including those related to bankruptcy, receivership or those involving a third party which have, or may have, significant effects on our financial position or profitability.
· The Company is an OTC reporting issuer under British Columbia Instrument 51-509 and as suchhas certain reporting obligations in British Columbia, Canada. On August 21, 2009 the BritishColumbia Securities Commission issued a cease trade order pertaining to the trading of theCompany’s securities in British Columbia until it files its March 31, 2009 financial statements and management discussion and analysis (filed), its June 30, 2009 financial statements and management discussion (filed), annual information form for the year ended March 31, 2009 and the Executive Director of the British Columbia Securities Commission makes an order revoking the cease trade order.
· The Company also has certain reporting obligations in Alberta, Canada is currently listed as adefaulting issuer by the Alberta Securities Commission for failure to file its June 30, 2009financial statements and management discussion (contained herein). No further action has beentaken by the Alberta Securities Commission.
The Company believes that upon making its filings that it will be in compliance with its regulatory filings and that the British Columbia cease trade order will be lifted and the Company will be removed from the Alberta Securities Commissions list of defaulting issuers.
Advisory Regarding Forward-Looking Statements
This MD&A contains forward-looking information and statements including opinions, assumptions, estimates and expectations of future production, cash flows and exploration results. Forward-looking statements include information that does not relate strictly to historical or current facts. When used in this document, the words “anticipate”, “believe”, estimate”, “expect”, “forecast”, “intent”, “may”, “project”, “plan”, “potential”, “should” and similar expressions are intended to be among the statements that indentify forward-looking statements.
Forward-looking statements are not guarantees of future performance and are subject to a wide range of known and unknown risks and uncertainties, and although the Company believes that the expectations represented by such forward-looking statements are reasonable, there can be no assurance that such expectations will be realized. Any number of important factors could cause actual results, future actions, conditions or events to differ materially from those in the forward-looking statements, including, but not limited to, the ability to implement corporate strategies, the state of domestic capital markets, the ability to obtain financing, operating risks, changes in general economic conditions, and other factors. Undue reliance should not be placed on forward-looking statements as the Company can give no assurance that they will prove to be correct.
The forward-looking statements contained in this MD&A are made as of the date hereof. While the Company acknowledges that subsequent events and developments may cause the views expressed herein to change, the Company has no intention and undertakes no obligation to update, revise or correct such forward-looking information, whether as a result of new information, future events or otherwise, except as required by applicable securities law.
The Company believes that the expectations reflected in the forward-looking statements and information contained herein are reasonable, but no assurance can be given that these expectations, or the assumptions underlying these expectations, will prove to be correct and such forward-looking statements and information included in this document should not be unduly relied upon. The forward-looking information included herein represents the Company’s views as of the date of this document and such information should not be relied upon as representing the Company’s views as of any date subsequent to the date of this document. We have attempted to identify important factors that could cause actual results, performance or achievements to vary from those current expectations or estimates expressed or implied by the forward-looking information. However, these factors are not intended to represent a complete list of the factors that could affect us and there may be other factors that cause results, performance or achievements not to be as expected or estimated and that could cause actual results, performance or achievements to differ materially from current expectations. There can be no assurance that forward-looking information will prove to be accurate, as actual results and future events could differ materially from those expected or estimated in such statements. Accordingly, readers should not place undue reliance on forward-looking information.
The risks and uncertainties that could affect future events or the Company's future financial performance are more fully described in the Company's annual reports (on Form 20-F filed in the U.S. and Canada) and the other recent filings in the U.S. and Canada. These filings are available at www.sec.gov in the U.S. and www.sedar.com in Canada.
Company Information
Additional information related to Valcent Products, Inc. is available on the SEDAR’s website at www.sedar.com and EDGAR’s website at www.sec.gov.