Exhibit 99.1

Phase1/2 Trial of Subcutaneously Administered Factor IX Variant CB 2679d/ISU304: Pharmacokinetics and Activity Chur Woo You, MD PhD, Ho-Jin Shin, MD, Howard Levy MBBCh PhD, Martin Lee, PhD, Seung-Beom Hong, PhD, Jamie Ellen Siegel, MD and June Young Park, MD 87 Session 322. Disorders of Coagulation or Fibrinolysis: Novel Therapies and Clinical Trials in Bleeding Disorders ASH 9 December 2017
Disclosure Employee and Stockholder of Catalyst Biosciences
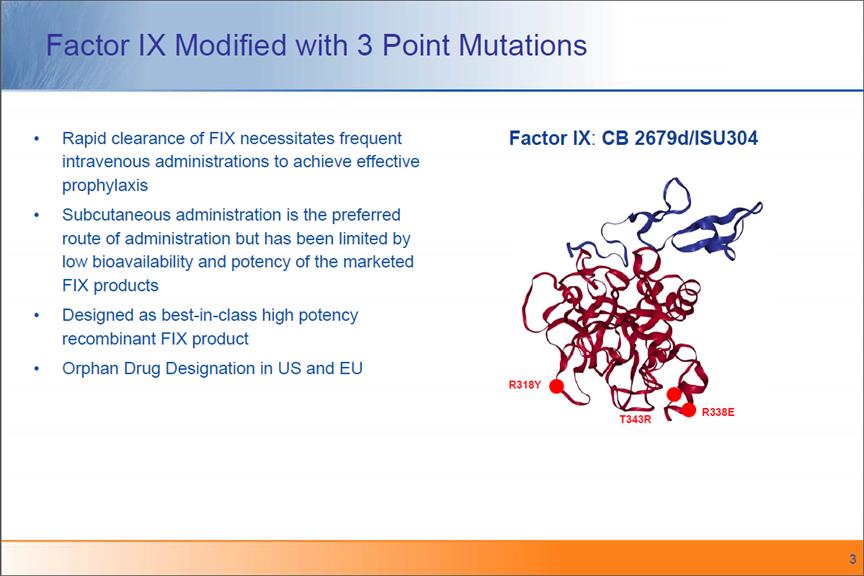
Factor IX Modified with 3 Point Mutations Rapid clearance of FIX necessitates frequent intravenous administrations to achieve effective prophylaxis Subcutaneous administration is the preferred route of administration but has been limited by low bioavailability and potency of the marketed FIX products Designed as best-in-class high potency recombinant FIX product Orphan Drug Designation in US and EU Factor IX: CB 2679d/ISU304
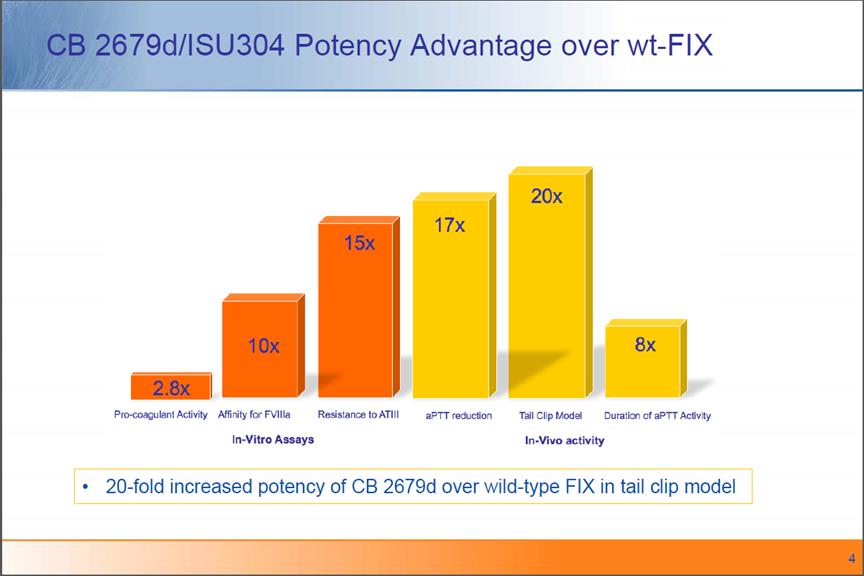
CB 2679d/ISU304 Potency Advantage over wt-FIX 20-fold increased potency of CB 2679d over wild-type FIX in tail clip model
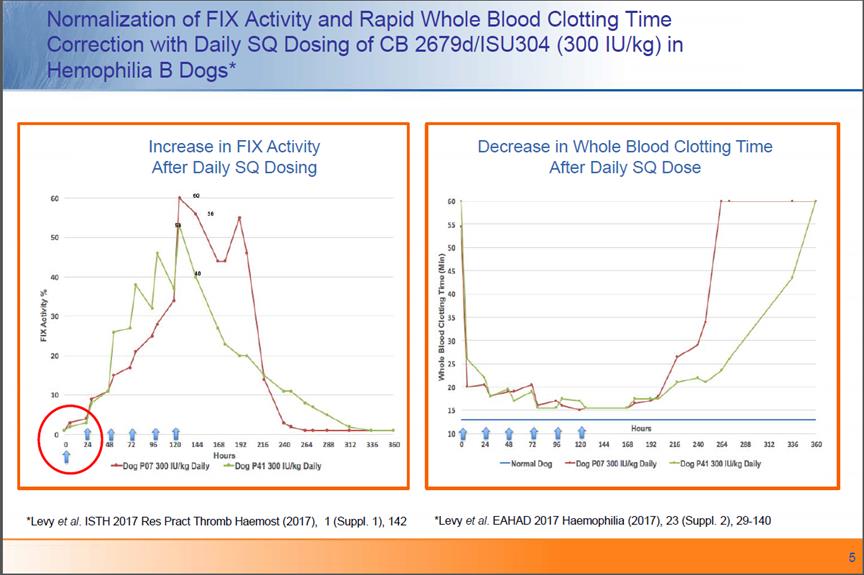
Normalization of FIX Activity and Rapid Whole Blood Clotting Time Correction with Daily SQ Dosing of Cb 2679d/ISU304 (300 IU/kg) in Hemophilia B Dogs*
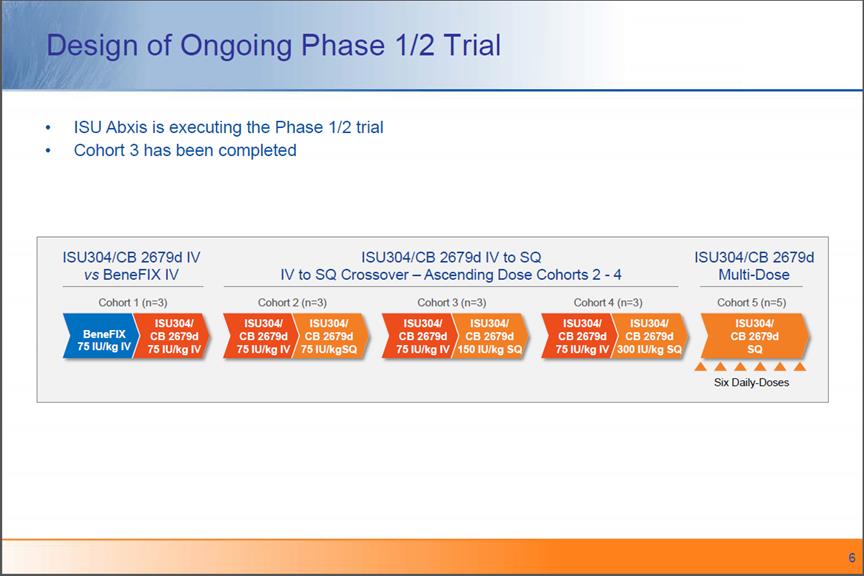
Design of Ongoing Phase 1/2 Trial ISU Abxis is executing the Phase 1/2 trial Cohort 3 has been completed
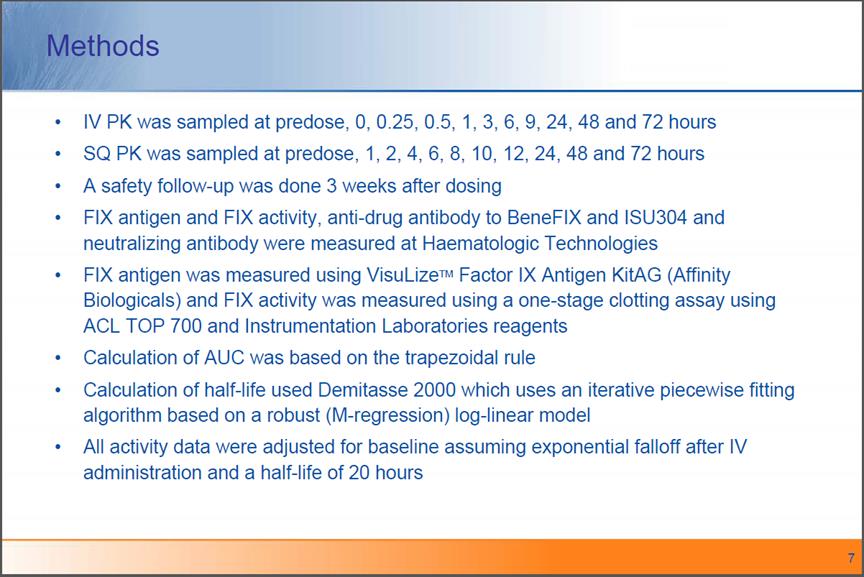
Methods IV PK was sampled at predose, 0, 0.25, 0.5, 1, 3, 6, 9, 24, 48 and 72 hours SQ PK was sampled at predose, 1, 2, 4, 6, 8, 10, 12, 24, 48 and 72 hours A safety follow-up was done 3 weeks after dosing FIX antigen and FIX activity, anti-drug antibody to BeneFIX and ISU304 and neutralizing antibody were measured at Haematologic Technologies FIX antigen was measured using VisuLize™ Factor IX Antigen KitAG (Affinity Biologicals) and FIX activity was measured using a one-stage clotting assay using ACL TOP 700 and Instrumentation Laboratories reagents Calculation of AUC was based on the trapezoidal rule Calculation of half-life used Demitasse 2000 which uses an iterative piecewise fitting algorithm based on a robust (M-regression) log-linear model All activity data were adjusted for baseline assuming exponential falloff after IV administration and a half-life of 20 hours
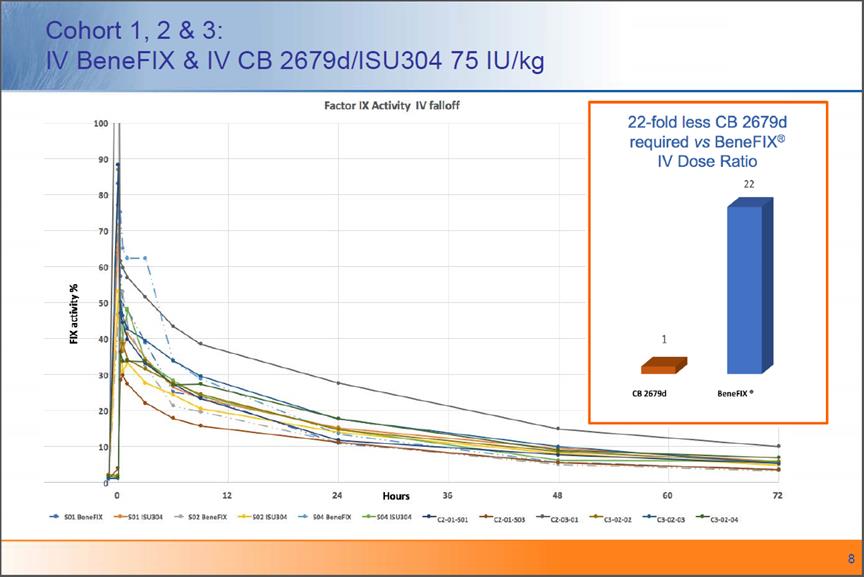
Cohort 1, 2 & 3: IV BeneFIX & IV CB 2679d/ISU304 75 IU/kg
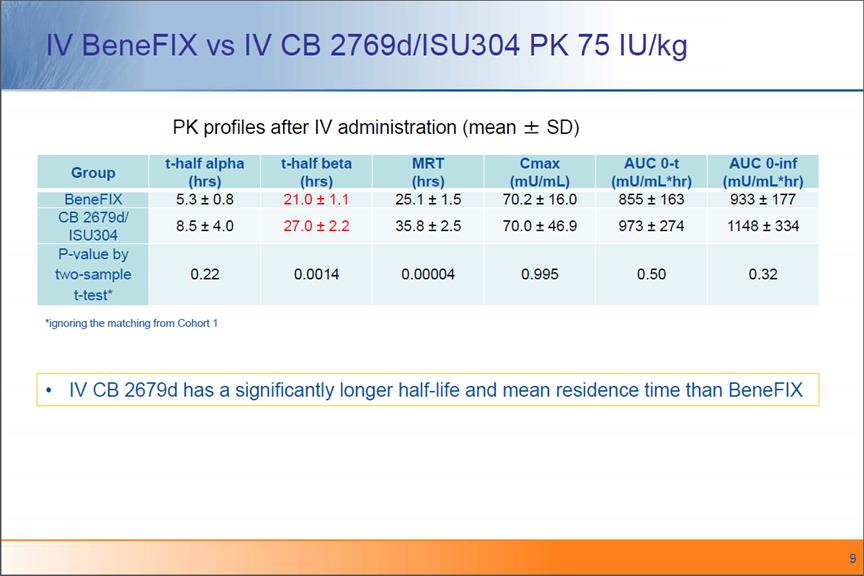
IV BeneFIX vs IV CB 2769d/ISU304 PK 75 IU/kg PK profiles after IV administration (mean ± SD) Groupt-half alpha (hrs) t-half beta (hrs) MRT (hrs)Cmax(mU/mL)AUC 0-t (mU/mL hr)AUC 0-inf (mU/mL*hr)BeneFIX5.3 ±0.8 21.0 ± 1.1 25.1 ± 1.570.2 ± 16.0 855±163933±177 CB 2679d/ ISU3048.5 ±4.027.0 ±2.235.8 ±2.570.0 ±46.9973 ± 2741148 ±334P-value by two-sample0.220.00140.000040.9950.500.32 *ignoring the matching from Cohort 1t-test*
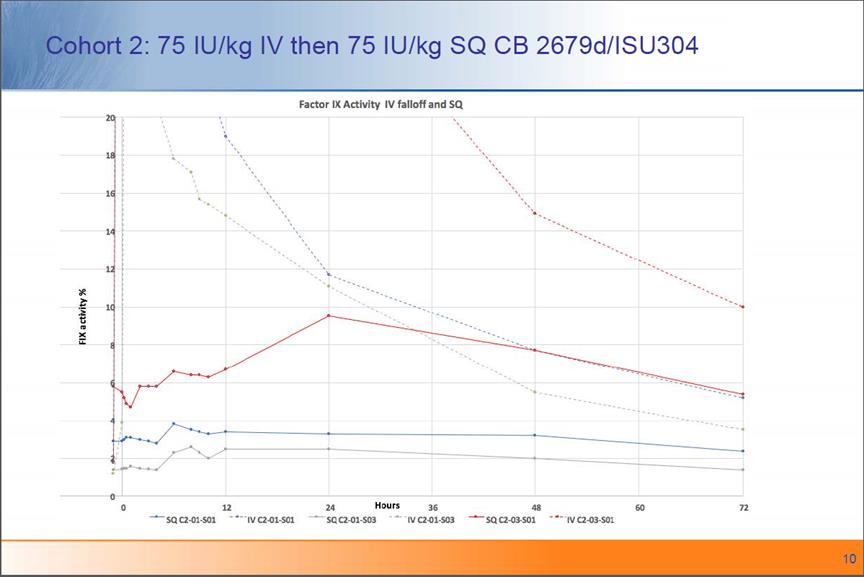
Cohort 2: 75 IU/kg IV then 75 IU/kg SQ CB 2679d/ISU304 10
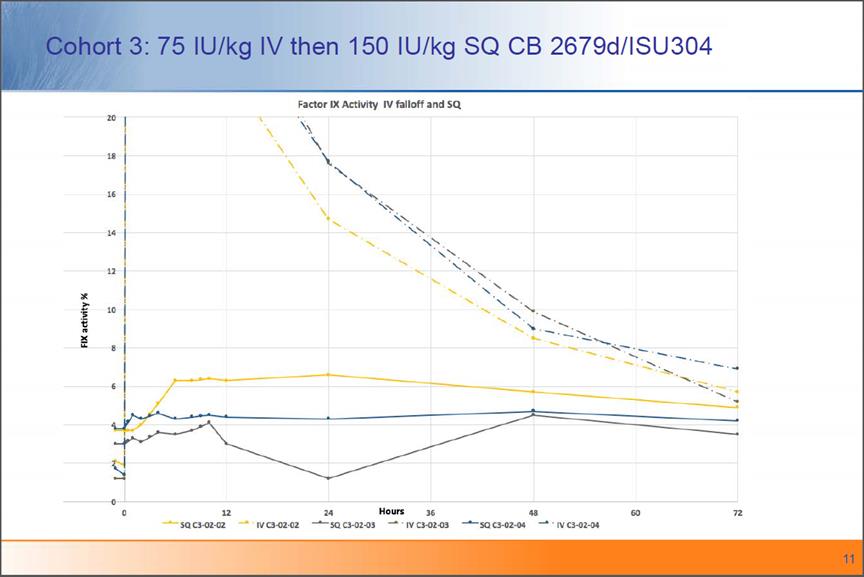
Cohort 3: 75 IU/kg IV then 150 IU/kg SQ CB 2679d/ISU304

CB 2769d - ISU304-001 PK:SQ vs IV has 3.6-fold Increase in Half-life Cohort 2 & 3: PK activity profiles after IV and SQ CB 2679d/ISU304 administration 98.7 hour SQ CB 2679d half-life is similar to IV agents dosed biweekly or weekly: Alprolix 86.52 hours Idelvion 104-118 hours Rebinyn/Refixia114.9 hours

ISU-304-001 Safety One subject had a mild general reaction within 1 hour of injection Fatigue/Boredom Headache Dizziness Transient mild AEs were reported in cohorts 2 and 3 and all resolved without sequelae: Itching Tenderness Erythema Solidification Injection site discomfort General ache [moderate severity]
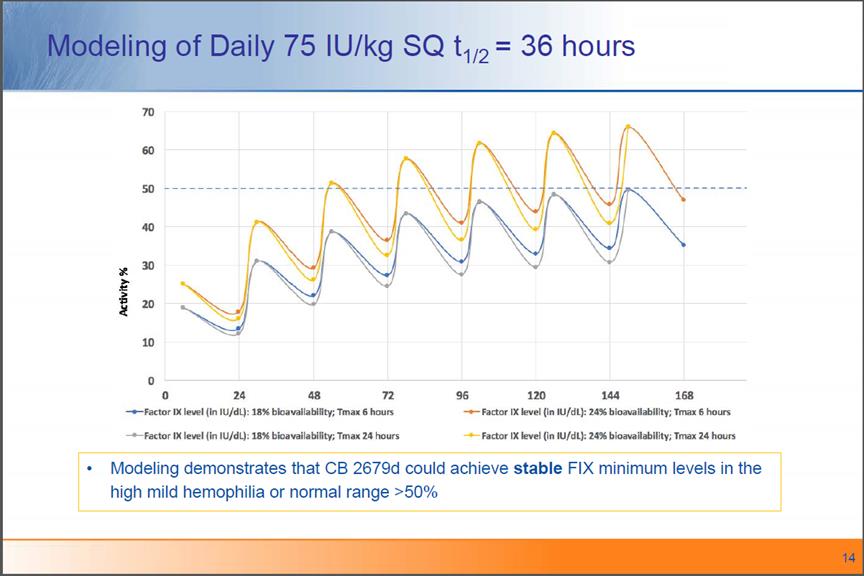
Modeling of Daily 75 IU/kg SQ t1/2= 36 hoursModeling demonstrates that CB 2679dcouldachieve stable FIX minimum levels in the high mild hemophilia or normal range >50%
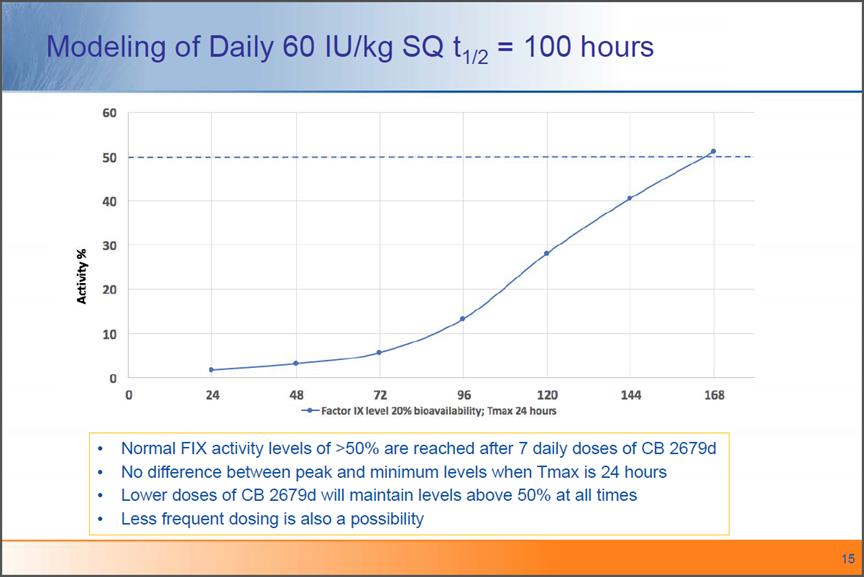
Modeling of Daily 60 IU/kg SQ t1/2 = 100 hours Normal FIX activity levels of >50% are reached after 7 daily doses ofCB 2679d No difference between peak and minimum levels when Tmax is 24 hours Lower doses of CB 2679d will maintain levels above 50% at all times Less frequent dosing is also a possibility

Modeling Predicts Subcutaneous Administration may be a Superior Prophylaxis Regimen Compared with IV Agents Time in Mild to Normal Levels Predicts Protection from Spontaneous Bleeds Illustrative Clotting Agent Activity Level Time after Dosing SQ Subcutaneous Drug Administration IV Drug Administration

CB 2679d/ISU304 Program Conclusions CB 2679d is designed as best-in-class high potency recombinant Factor IX product 22-fold potency advantage allows subcutaneous administration Normal trough factor IX blood levels achieved after 6 daily subcutaneous doses in hemophilia B dogs Phase 1/2 subcutaneous trial is ongoing Cohort 3 (150 IU/kg SQ) has been completed Multi-dose SQ data anticipated Q1 2018 IV CB 2679d has a longer half-life of 27 hours than 21 hours of wt-FIX SQ delivery significantly increases half-life 3.6-fold to 98.7 hours SQ dosing may provide superior prophylaxis to IV extended half-life agents Orphan drug designations have been granted in US and EU
















