Use these links to rapidly review the document
EXACT SCIENCES CORPORATION ANNUAL REPORT ON FORM 10-K YEAR ENDED DECEMBER 31, 2002 TABLE OF CONTENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended: December 31, 2002
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number: 000-32179
EXACT SCIENCES CORPORATION
(Exact Name of registrant as specified in its charter)
DELAWARE
(State or other jurisdiction of
incorporation or organization) | | 02-0478229
(IRS Employer Identification No.) |
63 Great Road, Maynard, Massachusetts
(Address of principal executive offices) |
|
01754
(zip code) |
Registrant's telephone number, including area code: (978) 897-2800
Securities registered pursuant to Section 12(b) of the Act:
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $.01 Par Value
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such report(s), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by checkmark whether or the registrant is an accelerated filer (as defined in the Exchange Act Rule 12B-2). Yes ý No o
The aggregate market value of the voting stock held by non-affiliates of the Registrant, as of the last business day of the Registrant's most recently completed second fiscal quarter was approximately $221,720,000 (based on the closing price of the Registrant's Common Stock on June 28, 2002 of $15.97 per share).
The number of shares outstanding of the Registrant's $.01 par value Common Stock as of March 14, 2003 was 19,031,882.
DOCUMENT INCORPORATED BY REFERENCE
The registrant intends to file a definitive proxy statement pursuant to Regulation 14A within 120 days of the end of the fiscal year ended December 31, 2002. Portions of such proxy statement are incorporated by reference into Part III of this Form 10-K.
EXACT SCIENCES CORPORATION
ANNUAL REPORT ON FORM 10-K
YEAR ENDED DECEMBER 31, 2002
TABLE OF CONTENTS
i
PART I
Item 1. Business
This Business section and other parts of this Form 10-K contain forward-looking statements that involve risk and uncertainties. Our actual results may differ significantly from the results discussed in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those set forth in "Management's Discussion and Analysis of Financial Condition and Results of Operations—Factors That May Affect Future Results" and elsewhere in this Form 10-K.
Overview
EXACT Sciences Corporation (Nasdaq: EXAS) has developed and continues to develop proprietary technologies in applied genomics (our "PreGen™ technologies") that we believe will revolutionize the early detection of colorectal cancer and other types of common cancers. We believe that medical practitioners will order tests based on our PreGen technologies as part of a regular screening program for the early detection of these cancers. We also believe that the widespread and periodic application of tests utilizing our PreGen technologies will reduce mortality, morbidity and the costs associated with these cancers.
We have selected colorectal cancer as the first application of our PreGen technologies because it is the most deadly cancer among non-smokers, curable if detected early and is well understood from a genomics point of view. There are an estimated 80 million Americans age 50 and over for whom the American Cancer Society recommends regular colorectal cancer screening. Current detection methods for colorectal cancer have proven to be inadequate as screening tools due to invasiveness, inadequate performance characteristics, or poor patient compliance.
Our first major commercial application derived from our PreGen technologies is PreGen-Plus™, a proprietary, non-invasive DNA-based test for the early detection of colorectal cancer in the average-risk population. In June 2002, we entered into an agreement to exclusively license Laboratory Corporation of America® Holdings ("LabCorp®") our PreGen technologies necessary to perform fecal-based colorectal cancer screening. Additionally, we have also agreed to provide LabCorp with access to additional PreGen technologies that we develop that may improve the efficacy or efficiency of PreGen-Plus through the five-year exclusivity period.
As part of our strategic alliance with LabCorp, LabCorp will conduct colorectal cancer screening tests using our PreGen technologies at their facilities and will be responsible for all billing and collection activities relating to tests LabCorp performs, and we will receive a per-test royalty fee on each test.
PreGen-Plus utilizes our proprietary PreGen technologies to isolate minute amounts of human DNA that are shed from the colon into stool. From that DNA, we then identify mutations in the DNA that are shed from abnormal cells and that are indicative of colorectal cancer and pre-cancerous lesions. We have conducted blinded clinical studies at leading medical institutions that we believe indicate that cancer screening using our PreGen technologies are able to detect colorectal cancer at an early stage more accurately in patients who have the disease than existing non-invasive screening methods currently available. Early detection results in less expensive and more effective treatment of patients. We believe that the benefits of early detection and the ease of use and accuracy of tests using our PreGen technologies will convince medical practitioners and patients to use these screening tests. We have conducted, and are currently conducting, clinical studies of our PreGen technologies to detect colorectal cancer in a variety of patients, including several studies focusing on asymptomatic patients over the age of 50. We also plan to develop, and work with others to develop, other commercial products and services based on our PreGen technologies.
1
We were incorporated in the State of Delaware on February 10, 1995 as Lapidus Medical Systems, Inc. We changed our corporate name to EXACT Laboratories, Inc. on December 11, 1996, to EXACT Corporation on September 12, 2000 and to EXACT Sciences Corporation on December 1, 2000. Our executive offices are currently located at 63 Great Road, Maynard, Massachusetts 01754. Our telephone number is (978) 897-2800. Our web address is www.exactsciences.com. We make available on our Internet website free of charge our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports as soon as practicable after we electronically file such material with the SEC. The information contained in our website is not incorporated by reference in this Annual Report on Form 10-K.
Colorectal Cancer
Colorectal cancer is the most deadly cancer in the U.S. among non-smokers and the second most deadly cancer overall. The only cancer that kills more people each year is lung cancer. The American Cancer Society estimates that in the U.S. there will be approximately 148,000 new cases of colorectal cancer and approximately 57,000 people will die from colorectal cancer in 2003. Unfortunately, almost 50% of the patients with a new diagnosis of colorectal cancer will die from the disease within five years of diagnosis.
Despite these risks, current non-invasive colorectal cancer screening methods, such as fecal occult blood testing ("FOBT'), find only indirect evidence of cancer. These tests suffer from poor sensitivity or specificity, require patients to touch or manipulate their stool, and modify their diet or medications, which results in poor patient compliance. Current alternative procedures-based detection technologies, such as flexible sigmiodoscopy, colonoscopy and virtual colonoscopy, while more effective than FOBT in the early detection of colorectal cancer, require patients to modify their diet or medications and undergo bowel preparation for the procedure which also results in poor patient compliance. Additionally, procedures such as flexible sigmiodoscopy and colonoscopy are considered invasive and suffer problems of scalability because of the short supply of clinicians, endoscopy suites and fees associated with these tests. As a result, mortality and the cost of treatment of colorectal cancer remains high.
We have designed our PreGen technologies to detect colorectal cancer using a single whole stool sample obtained non-invasively and in the privacy of one's own home. Unlike other screening tests, patients are not required to touch or manipulate their stool, modify their diet or medications or undergo any bowel preparation, which we believe will result in higher patient compliance. Higher compliance could dramatically decrease mortality from this curable disease.
Medical practitioners commonly classify colorectal cancer into four stages at the time of diagnosis as shown in the following table:
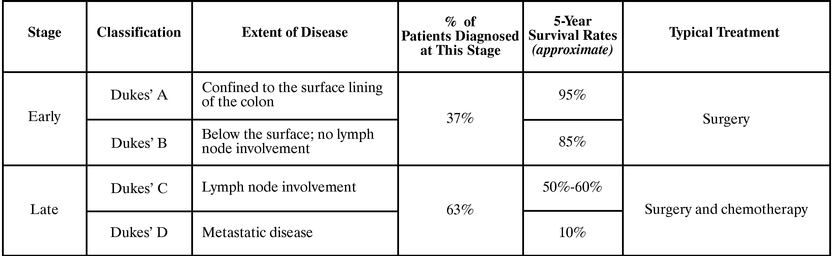
2
Detection of pre-cancerous adenomas and cancer in its earliest stages increases the likelihood of survival and reduces the cost of treatment and care. As a result, the American Cancer Society recommends that the 80 million Americans age 50 and above undergo regular colorectal cancer screening.
Genomics, a relatively new discipline, broadly defined, is the study of the genome. Initial efforts in human genomics centered on identifying, mapping, sequencing and analyzing the definitive sequence of every gene in the human genome. Scientists are now focusing on applying that knowledge to the development of novel technologies used for the detection and management of disease, as well as the development of improved therapeutics.
Cancer begins to develop when the DNA in a single normal cell mutates or changes in such a way that ultimately results in unregulated cell growth. In a ground-breaking paper published in theNew England Journal of Medicine in 1988, Dr. Bert Vogelstein, one of our scientific collaborators, and his colleagues described a multi-step model of colorectal cancer development. In 1990, Dr. Eric Fearon, a former member of our scientific advisory board, and Dr. Vogelstein published a diagram depicting the development of colorectal cancer. An updated version of this diagram showing many of the genomic events involved in the development of colorectal cancer is shown below:
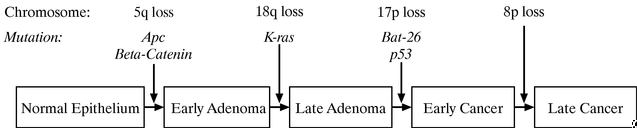
The diagram illustrates that cancer develops in steps that results from alterations in multiple genes in an individual cell, and occurs frequently with chromosome loss. The diagram shows that these alterations lead to pathologic changes in the colon from normal epithelium—the tissue that lines the surface of the colon—through early and late adenomas, which are a form of pre-cancerous growth, to early cancer and late cancer. These alterations, shown in the above diagram, usually accumulate over many years, and are typically due to:
- •
- mutations in individual genes, such as theApc,K-ras andp53 genes;
- •
- larger scale effects in which large parts of a chromosome or even entire chromosome and chromosome arms, such as 5q, 18q, 17p and 8p, are deleted; or
- •
- inactivation of the mismatch repair genes (the genes responsible for correcting misincorporated bases of DNA after DNA replication) that manifest themselves as deletions in polynucleotide DNA regions such as BAT-26.
The multi-step process provides an abundance of genomic targets that may be used for the early detection of cancer. By detecting genetic alterations associated with cancer, the disease can be treated at its most curable stage. While most other colorectal cancer screening tests seek to detect the pathologic changes (polyps, adenomas, lesions, etc.) thatresult from genetic alterations at the cellular level, our PreGen technologies seek to detect the genetic alterations directly. We believe this increases the likelihood of early detection.
3
Our Solution
Today, millions of Americans avoid current colorectal screening methods for a variety of reasons, including embarrassment, fear and discomfort due to invasiveness, bowel preparation, the potential for the need to touch or manipulate their stool and modification of their diet or medications. This creates a dilemma for today's medical practitioner who knows colorectal screening is essential but is faced with patients who simply choose not to get tested or who choose the least invasive, least sensitive method available, FOBT. We believe medical practitioners will view PreGen-Plus as a more effective alternative for the millions of Americans for whom the American Cancer Society recommends regular screening.
Through regular screening, we believe PreGen-Plus will improve patient screening compliance and thereby enable the detection of colorectal cancer and adenomas earlier so that patients can be treated more effectively. Using our PreGen technologies, a laboratory will isolate the human DNA shed from the surface of the colon into a stool sample. The laboratory will then use our PreGen technologies to identify mutations in the human DNA shed from abnormal cells associated with adenomas and colorectal cancer. If an individual tests positive using the PreGen-Plus test, the ordering physician should refer the patient for an appropriate diagnostic test such as colonoscopy.
We believe colorectal cancer screening tests using our PreGen technologies will become widely-accepted and used routinely for screening as a result of the following features and benefits:
- •
- Earlier Detection. Early detection saves lives. Based upon data from completed clinical studies, we believe colorectal cancer screening tests using our PreGen technologies will detect Dukes' A and B cancers, as well as some pre-cancerous lesions. We believe that this will represent a marked improvement over other non-invasive colorectal cancer screening methods being used today.
- •
- Higher Sensitivity. Since the fall of 1998, we have conducted a series of blinded clinical studies in collaboration with leading medical institutions using our colorectal cancer screening tests. In all of these clinical studies, the sensitivity of colorectal cancer screening tests utilizing our PreGen technologies substantially exceeded the sensitivity reported for FOBT and flexible sigmiodoscopy.
- •
- Higher Compliance. We designed our PreGen technologies to detect colorectal cancer using a single whole stool sample obtained non-invasively and in the privacy of one's own home. Unlike other screening tests, patients are not required to touch or manipulate their stool, modify their diet or medications or undergo any bowel preparation. Moreover, we believe that, based on the results of our clinical studies and trials, we will be able to educate physicians about the potential for improving detection of colorectal cancer with tests based on our PreGen technologies. We also believe that this will lead many primary care physicians to include regular colorectal cancer screening as a part of their periodic physical examinations of patients aged 50 and above.
- •
- Cost-effective Prevention and Treatment. We believe that colorectal cancer screening tests using our PreGen technologies will detect early stage lesions more effectively than currently available non-invasive screening methods. As a result of this early detection, medical practitioners will have the ability to treat early stage colorectal cancer and pre-cancerous lesions which is less expensive and more effective than treating late stage cancer.
- •
- Scalability. Screening 80 million Americans age 50 and above requires the ability to efficiently test a large population. Procedures such as flexible sigmiodoscopy and colonoscopy suffer problems of scalability because of the short supply of clinicians, endoscopy suites and reimbursement. We believe tests using our PreGen technologies will enable efficient mass screening on a regular basis.
4
Our Testing Process
Diagnostic tests typically require sample collection and preparation procedures as well as detection methods. We have developed a three-step sample collection and preparation process and five detection methods that apply genomics discoveries to the early detection of colorectal cancer. As of December 31, 2002, we have 26 issued U.S. patents and 30 pending U.S. patent applications relating to our testing process.

Specimen Collection and Transportation. Our PreGen technologies for colorectal cancer are based on collecting a single whole stool sample in an easy, non-invasive manner. Utilizing our specially designed sample container, samples can be either brought by the patient directly to the laboratory performing the colorectal cancer-screening test or sent directly from the patient's home using one of the many national couriers.
Representative Sampling. Before we developed our PreGen technologies, no one had been able to reproducibly extract human DNA and consistently find mutations in stool. We believe that this was due to the non-uniform distribution of abnormal DNA in stool. We have invented proprietary homogenization methods designed to ensure that the portion of stool sample that is processed at the laboratory will contain uniformly distributed DNA throughout the portion of the sample being tested, and that the stool sample is, therefore, representative of the entire stool and colon. Based upon our data to date, we believe these methods lead to increased sensitivity and reproducible results.
DNA Extraction, Purification and Amplification. The isolation and amplification of human DNA found in stool is technically challenging because over 99% of DNA in stool is not human DNA, but is actually DNA from bacteria normally found in the colon. In addition, there are substances in stool that make the isolation and amplification of human DNA a difficult task. Our proprietary PreGen technologies allow for the reproducible isolation and amplification of human DNA found in stool.
Cancer Detection Methods. We have designed proprietary methods for detecting and identifying genomic markers associated with colorectal cancer that can be performed on existing instruments commonly available in clinical laboratories conducting molecular testing.
Our Proprietary PreGen Technologies
Our PreGen technologies are comprised of a variety of proprietary tools and techniques that continue to evolve as we seek to optimize sensitivity, specificity, and the operational efficiency of our PreGen technologies. Our first major commercial application, PreGen-Plus, is derived from our suite of PreGen technologies and represents an assay that will likely evolve over time based on our on-going research and development activities. Our scientists regularly explore the efficacy, impact, and relationship among each of the PreGen technologies to ensure the combination of our PreGen technologies are best suited to optimize sensitivity, specificity and operational efficiency for an assay. They also evaluate our PreGen technologies for other potential standalone applications or products that may be developed in the future.
The information below sets forth those PreGen technologies that we believe will be important to PreGen-Plus, either at the time of commercial launch, or over the long-term. Each of the technologies below enable the early detection of cancer by identifying the presence of minute amounts of altered human DNA relative to the large amounts of normal DNA naturally present in stool. Each of these proprietary methods for detecting and identifying genomic markers associated with colorectal cancer can be performed on existing instruments commonly available in clinical laboratories conducting
5
molecular testing. Our proprietary testing process includes the cancer detection technologies described below as well as other proprietary methods important to the overall efficacy of PreGen-Plus.
Name
| | Role in Detection
| | Our Scientific Advance
|
|---|
| Multiple Mutation Detection (MuMu®) | | • Each element of MuMu detects a specific mutation within a cancer-related gene | | • Sensitive and specific detection of rare DNA mutations in a heterogeneous environment |
| Deletion Technology | | • Detects short deletions and insertions in BAT-26 | | • Sensitive and specific detection of rare DNA insertions and deletions mutations in a heterogeneous environment |
| DNA Integrity Assay (DIA®) | | • Detects abnormally long human DNA fragments associated with colorectal abnormality | | • Proprietary marker associated with cancer that does not require knowledge of which specific genes cause cancer |
| Enumerated Loss of Heterozygosity (e-LOH) | | • Enumerates ratio of paternal DNA to maternal DNA at a given genomic site to identify chromosomal loss that is characteristic of many cancers | | • Statistical method that applies a commonly used analytical technique to indicate a large portion of a chromosome is missing and does not require knowledge of which specific genes cause cancer |
| DNA isolation based on acquired Hybrigel™ technology | | • Cost effective, automated DNA isolation platform that increases the amount of DNA that can be isolated from fecal specimens | | • Technology platform that will increase the DNA purity and yield within samples collected from all bodily fluids |
| Oligonucleotide Tiling | | • Mutation scanning technology that can be used to screen for abnormalities in DNA segments such as Apc | | • Proprietary platform that can be used to scan genes or segments of DNA for mutations, alterations, transitions or transversions without prior knowledge of the site of mutation |
Multiple Mutation. Multiple Mutation, or MuMu, identifies DNA mutations at specific sites. We have currently selected 21 sites that are commonly mutated in the colorectal cancer-related genesApc,p53 andK-ras. We have designed our proprietary MuMu method to allow simultaneous probing of different DNA sequences and to allow analysis even though only a small amount of DNA in the sample is derived from abnormal cells while the vast majority is derived from normal human cells or bacteria.
Deletion Technology. Deletion Technology detects short deletions and insertions in segments of DNA that are indications of defects in cellular mechanisms for DNA repair. Approximately 15% of sporadic colorectal cancers, referred to as mismatch-repair cancers, result from inactivation of the proteins that normally repair errors in DNA after DNA replication. We have developed a proprietary method for identifying this condition by detecting the presence of short deletions and insertions in a DNA segment known as BAT-26 in samples that have less than 1% of mutant BAT-26 in an excess of normal Bat-26. This altered DNA segment appears in virtually all colorectal cancers with defects in the mismatch repair mechanism.
6
DNA Integrity Assay. DNA recovered from the stool of many cancer patients contains a small but detectable population of DNA that is longer than DNA recovered from individuals who do not have cancer and have never had cancer or adenomas. Use of this proprietary detection method does not require knowledge of which genes cause cancer. In addition to its utility for our colorectal cancer tests, we believe that this discovery may lead us to the development of markers for other types of cancers.
Enumerated Loss of Heterozygosity. In normal cells, the quantity of DNA inherited from each parent is generally equal. This is not true for cells from many different types of cancers, including virtually all non-mismatch repair colorectal cancers. This condition, which is an imbalance of maternal and paternal chromosomal fragments, is called loss of heterozygosity, or LOH. Prior to our development efforts, we believe that scientists were unable to detect LOH in stool samples. We have developed proprietary methods for detecting LOH in a highly heterogeneous DNA sample such as stool by enumerating the ratio of fragments of DNA that are inherited from each parent at defined locations in the genome. We call this detection method e-LOH. Use of this detection method does not require knowledge of which genes cause cancer. We believe that our novel e-LOH detection method may also be broadly applicable to early cancer detection using a variety of bodily fluids.
DNA Isolation Based On Hybrigel Technology. This technology involves the isolation and/or detection of DNA fragments using DNA probes affixed into an elecrophoretic medium. It is our intention to use this as a sample preparation platform for isolation of human DNA from stool specimens. To date, we have an active program validating this platform, developing laboratory consumables and exploring manufacturing options. We believe this platform will be capable of isolating and purifying DNA from numerous bodily fluids in a cost-effective and efficient manner.
Oligonucleotide Tiling. This is a technology that we have developed to allow us to scan large regions of DNA for mutations. For example 80% of the mutations that occur in theApc gene occur in an approximate 800 base pair segment. Currently, technologies like sequencing and protein truncation testing are costly and laborious to perform. Oligonucleotide tiling is efficient, sensitive and can be performed using most laboratory equipment. Additionally, this technology may be used for scanning mutations in many different genes for both sporadic and hereditary cancers.
Clinical Studies
In collaboration with the Mayo Clinic, we have completed three blinded clinical studies since the fall of 1998. These clinical studies included stool samples from 219 patients seen at the Mayo Clinic, 58 of whom were diagnosed with colorectal cancer by means of colonoscopy at the Mayo Clinic. The first two clinical studies were conducted using frozen, partial stool samples. The sensitivity for each of these two clinical studies was 91% and 67%, respectively. In the spring of 2000, we conducted a third clinical study with the Mayo Clinic in which we collected fresh, whole stool samples. The sensitivity in this clinical study was 78%. Specificity in these studies ranged from 93% to 100% across all three clinical studies.
During 2002, we presented results from three additional studies. The first of these was conducted in collaboration with the University of Nebraska and was presented at the Digestive Disease Week Conference ("DDW") in May 2002. This study included 17 patients with colorectal cancer who had their cancer diagnosed by means of a colonoscopy. The sensitivity of this clinical study was 65% as our PreGen technologies were able to detect 11 of the 17 cancers. Three separate stool samples from each patient were analyzed and the results demonstrated that analysis of a single stool sample appeared to be equivalent to analyzing three stools from the same patient.
The second study was in collaboration with the Kaiser Clinic in Sacramento, California and was also presented at DDW in 2002. In this study, stool samples were obtained from patients who were
7
diagnosed with colorectal cancer by means of a flexible sigmiodoscopy. The sensitivity of this clinical study was 67% as our PreGen technologies were able to detect 26 of 39 invasive cancers. In addition, the results from this study indicated that our PreGen technologies were at least equally effective in detecting early stage (Dukes' A and B) disease (20/26, 77%) as they were in detecting late stage (Dukes' C and D) disease (5/11, 45%). Most early stage cancers can be readily cured by surgery, and hence the importance of early detection.
The third study was conducted in the greater Boston area in collaboration with four leading academic medical institutions and was presented at the American College of Gastroenterology meeting held in October 2002. In this study, patients who had been diagnosed with colorectal cancer by means of a colonoscopy provided a stool sample prior to undergoing surgical resection of their cancer. The sensitivity of this clinical study was 64% as our PreGen technologies were able to detect 30 of 47 invasive cancers. In addition, stool samples were obtained one month after surgery in some of these patients, and the analysis of those stool samples demonstrated that mutations from the cancer were generally not present after surgery.
The results of all of these studies are summarized in the following table:
Study
| | Completed
| | Number of
Cancer Patients
| | Sensitivity
| |
|---|
| Mayo Clinic I Pilot Study | | 1999 | | 22 | | 91 | % |
| Mayo Clinic II Study | | 2000 | | 27 | | 67 | % |
| Mayo Clinic III Study | | 2000 | | 9 | | 78 | % |
| University of Nebraska Study | | 2002 | | 17 | | 65 | % |
| Kaiser Clinic Study | | 2002 | | 39 | | 67 | % |
| Greater Boston Area Study | | 2002 | | 47 | | 64 | % |
| | | | |
| | | |
| Total | | | | 161 | | | |
These sensitivity rates are superior to the 25%-30% sensitivity of the fecal occult blood test and the approximately 48% sensitivity of flexible sigmiodoscopy for colorectal cancers located throughout the colon.
In the third quarter of 2001, we initiated a blinded multi-center clinical trial that is expected to include an estimated 5,500 patients from over 80 academic and community-based practices who are asymptomatic, age 50 and older. The goal of this clinical trial will be to provide additional data supporting the superiority of tests utilizing our Pre-Gen technology versus the most widely used brand of FOBT, Hemoccult II®, in detecting colorectal cancer in this average-risk population. We are conducting this clinical trial in accordance with the applicable guidelines of the United States Food and Drug Administration, or FDA, so that the results may be used in any application that we may make to the FDA in the future. We expect patient enrollment in this clinical trial to conclude by the end of the first quarter of 2003 and expect that study data will be available in the fourth quarter of 2003.
In October 2001, we signed a Clinical Trial Agreement with the Mayo Clinic in which our PreGen technologies will be the subject of an independent study by the Mayo Clinic for which the Mayo Clinic received a $4.9 million grant from the National Cancer Institute of the National Institutes of Health. Dr. David Ahlquist, a director of the Colorectal Neoplasia Clinic at Mayo, is the principal investigator of this clinical trial and has assisted us in certain of our previous clinical trials and in the use of PreGen technologies in the detection of colorectal cancer. This three-year study will involve approximately 4,000 patients at average risk for developing colorectal cancer, and will compare the results of our non-invasive, genomics-based screening technology with those of FOBT, a common first-line colorectal cancer screening option. The Mayo Clinic has indicated that it expects enrollment in this clinical trial to be completed sometime in 2004.
8
While most adenomas do not progress to cancer in a patient's lifetime, those that do are more likely to have villous features characterized by an irregular surface and associated with more rapid growth. Our results for detecting adenomas using our PreGen technologies have varied in different studies. In the Mayo Clinic I study, there were 11 patients with advanced adenomas greater than one centimeter in size, of which 9, or 82%, were detected. In the Kaiser Clinic study, there were 19 patients with advanced adenomas, of which 13, or 68%, were detected. In the Boston study, there were 14 patients with advanced adenomas, of which five, or 36% were detected. We believe that by detecting adenomas more likely to progress to cancer during a patient's lifetime, through a non-invasive screening procedure, will provide additional medical value for our PreGen technologies. We intend to test our ability to detect advanced adenomas in our 5,500-patient clinical trial.
Research and Development
Our research and development efforts focus on developing multiple DNA-based methods for the early detection of cancer and pre-cancerous lesions. We believe that the evaluation of these methods in a clinical setting will determine the best approaches for commercialization. We also focus on developing methods to automate and simplify the collection, preparation and analysis of samples to produce cost-effective commercial tests. Our research and development expense, including stock-based compensation, for fiscal 2000, 2001 and 2002 was $6.1 million, $14.2 million and $20.5 million, respectively.
Assay Development. We continue to focus our research and development efforts on improving the sensitivity, throughput and cost of PreGen-Plus while maintaining our specificity level. We continue to evaluate new potential cancer gene markers, chemistries and technologies that we believe may be useful in increasing the clinical sensitivity and throughput, and decreasing the cost of PreGen-Plus.
Process Development. We have undertaken a multi-year effort to automate the testing process and reduce the cost of processing stool samples. Our objectives include eliminating many of the manual steps, reducing the use of expensive reagents and increasing screening throughput. This effort is important so that our strategic partners can offer products based upon our PreGen technologies at commercially reasonable prices.
Extensions to Other Cancers. Our proprietary DIA detection method uses a marker that we believe may be broadly applicable to the detection of other cancers in addition to colorectal cancer. In the course of our blinded clinical studies with the Mayo Clinic, we tested 50 stool samples from patients diagnosed with aero-digestive cancers, such as cancer in the lung, pancreas, esophagus, stomach and duodenum, gall bladder and bile ducts. Combined, these cancers kill more people than colorectal cancer. The results from these studies indicated sensitivity that varies between 20% and over 90%, depending on the original site of the cancer. These studies were conducted in patients with advanced cancer, and we will have to demonstrate that we can detect these cancers at an early stage in order for our PreGen technologies to be clinically and commercially useful. Additional studies are underway to refine the methodology and evaluate patients with earlier stage disease. If the results are promising, we intend to develop methods and technologies to detect these cancers.
Adenomas. While our research focus has been the detection of invasive colorectal cancer, we intend to conduct research on improved methods for adenoma detection, particularly those advanced adenomas that are most likely to progress to invasive colorectal cancer (size greater than 1cm, villous features or high grade dysplasia). As part of this effort, we have invented a new method for scanning regions of DNA at sites often associated with adenoma development. In addition, our Hybrigel technology allows us to retrieve more DNA from a stool sample which we believe should increase the likelihood of adenoma detection.
9
Strategic Alliance
We established our first licensing agreement with LabCorp in July 2001, and expanded the relationship in June 2002, when we entered into an exclusive, long-term strategic alliance to commercialize PreGen-Plus™, a proprietary, non-invasive DNA-based technology for the early detection of colorectal cancer in the average-risk population based upon our PreGen technologies. Pursuant to the license agreement, we licensed to LabCorp all U.S. and Canadian patents and patent applications owned by us relating to our PreGen technologies for the detection of colorectal cancer in the average-risk population. The license is exclusive for a five-year period, followed by a non-exclusive license for the life of the patents. As a result of this agreement, LabCorp will be responsible for the processing of all colorectal cancer screening tests in the United States and Canada using our PreGen technologies at their facilities, including the billing and collection of tests performed.
In return for the exclusive license, LabCorp has agreed to pay us certain upfront, milestone and performance-based payments, and a per-test royalty fee. LabCorp made an initial payment of $15 million to us upon the signing of the agreement, and a second payment of $15 million is to be made upon the commercial launch of PreGen-Plus. In addition, we may be eligible for milestone payments from LabCorp of up to $30 million based upon deliverables related to scientific acceptance, reimbursement approval and technology improvements, and up to an additional $15 million based upon the achievement of significant LabCorp revenue thresholds. In addition to these payments, we will receive a royalty fee for each PreGen-Plus test performed by LabCorp. In conjunction with this strategic alliance, we have issued to LabCorp a warrant to purchase 1,000,000 shares of our common stock, exercisable over a three-year period at an exercise price of $16.09 per share. We assigned a value to the warrant of $6.6 million under the Black-Scholes option-pricing model which has been recorded as a reduction in the initial upfront deferred license fee of $15 million.
We are actively engaged with LabCorp in preparing for the commercial availability of PreGen-Plus. The companies are currently focusing their efforts on: assay validation, technology transfer and licensing; contracting with manufacturers and suppliers; physician education and demand creation; broad-based reimbursement initiatives; advocacy development; and sales force training. While some of these efforts will be on-going after the launch of PreGen-Plus, others, such as assay validation, technology transfer and licensing and contracting with manufacturers and suppliers, must be completed prior to LabCorp making PreGen-Plus commercially available. Additionally, there are a number of significant initiatives that are primarily LabCorp's responsibility that must be accomplished prior to commercialization, if we are to be successful. Some of the major initiatives include assay validation at LabCorp's facility, the creation of an adequate distribution infrastructure for sample movement, the build-out of adequate laboratory space, the hiring and training of personnel, the establishment of supply agreements with vendors, and the licensing of certain third-party technologies for use in the PreGen-Plus assay as it is currently configured.
10
Sales and Marketing
We continue to build our sales and marketing organization to support the commercialization of PreGen-Plus. While our PreGen technologies may extend themselves to several other types of cancer or lend themselves to other product opportunities over time, the current primary focus of our sales and marketing organization is the commercialization of PreGen-Plus for colorectal cancer.
Our PreGen-Plus commercialization strategy, being executed vigorously with LabCorp, is designed to address the needs of four major constituencies: (i) primary care physicians (including family practice, generalists, internists, and obstetricians and gynecologists, together "PCPs"); (ii) gastroenterology thought leaders; (iii) consumers; and (iv) third-party payors.
- •
- PCPs drive most colorectal cancer screening activities; as such they are principal targets of our promotional activities.
- •
- Gastroenterologists are highly vocal in advocating colorectal cancer screening, and perform the vast majority of the reference standard screening procedure, colonoscopy. Because they are key to establishing new tests as standard of care and are highly influential with local primary care physicians, we are working closely with gastroenterology thought leaders.
- •
- Consumers can be very influential in the screening process as well; as such they are important promotional targets.
- •
- All promotional targets, PCPs, gastroenterologists and consumers, will bring important pressure on the fourth major constituency, third party payors, such as Medicare, major national and regional managed care organizations and insurance carriers, and self-insured employer groups. Activities addressing these groups have as their goal payment for PreGen-Plus, and, later, formal inclusion in plan reimbursement policies.
To address these four important constituencies, we have engaged in five broad sales and marketing activities: (i) direct sales to physicians; (ii) medical education programs; (iii) advocacy development; (iv) consumer marketing initiatives; and (v) managed care activities.
- •
- Sales initiatives to date have included direct detailing of medical professionals at numerous conventions and in their individual offices, resulting in widespread awareness of the product. We have also initiated a robust, six-month training program designed to educate and prepare all of LabCorp's sales representatives to support the upcoming product launch of PreGen-Plus. After launch, LabCorp's sales force will execute the majority of physician calls.
- •
- We are executing numerous educational initiatives directed at luminaries in the field, as well as local PCPs, to promote the potential value of PreGen-Plus in their practices. These include continuing medical education ("CME") and non-CME symposia, publications, and speaker's bureau programming. The goal of these efforts is to enhance of our credibility and, long-term, gain inclusion in formal clinical practice guidelines.
- •
- We are working with most influential advocacy groups to promote their awareness of PreGen-Plus, its performance characteristics, and its potential value in clinical practice toward the goal of reducing mortality from colorectal cancer. We intend to build on growing public awareness of colorectal cancer through our activities with these advocacy groups. Our efforts to date have led to inclusion of PreGen-Plus in various well-circulated brochures, and radio and television broadcasts.
- •
- Because PreGen-Plus promises to be a more consumer-friendly screening option, patients are more likely to ask their doctor for PreGen-Plus which, in turn, should help drive sales. Accordingly, we are evaluating various consumer-marketing initiatives that may enhance awareness of PreGen-Plus and increase consumer advocacy for the test among physicians.
11
- •
- Finally, we have been educating Medicare, major national and regional managed care organizations and insurance carriers, and self-insured employer groups about the need and clinical rationale for PreGen-Plus. Along with LabCorp, we are having discussions with key decision makers at most of the major payors, with the goal of shortening the review time and gaining approval for the inclusion of PreGen-Plus in formal practice guidelines within each payor's plan following commercial launch. In addition, we also continue to address reimbursement for PreGen-Plus from government payors, primarily the Centers for Medicare and Medicaid Services ("CMS", formerly known as the Health Care Financing Administration) by educating their senior staff about the need and clinical rationale for PreGen-Plus (See "Reimbursement").
Reimbursement
We are currently working to obtain national coverage and reimbursement approval for tests using our PreGen technologies from Medicare as well as major national and regional managed care organizations and insurance carriers, and self-insured employer groups. We currently do not have formal reimbursement approval from any organization or public agency, and do not anticipate such approval until some time after the commercialization of PreGen-Plus. Medicare and other third-party payors will independently evaluate our PreGen technologies by, among other things, reviewing the published literature with respect to the results obtained from our clinical studies. We intend to assist these organizations in evaluating our PreGen technologies by providing scientific and clinical data in support of our assertions regarding the superiority and appropriateness of our PreGen technologies. In addition, we intend to present analysis showing the benefits of early disease detection and the resulting cost-effectiveness of our PreGen technologies. Current molecular diagnostic procedural terminology ("CPT") codes are available which will allow our PreGen technologies to be billed following completion of a test prescribed (ordered) by a physician for a patient. We believe that the existence of current CPT codes with applicability to our screening test will help facilitate Medicare's reimbursement process.
The Federal Balanced Budget Act of 1997 required Medicare to reimburse for colorectal cancer screening for average-risk patients beginning on January 1, 1998 and mandated Medicare coverage for FOBT preformed by the guaiac method and flexible sigmiodoscopy. Congress amended the Budget Act of 1997 to include coverage for double contrast barium enema, a radiographic imaging test used to detect colorectal cancer in areas beyond the reach of flexible sigmiodoscopy. This was further expanded to include a screening colonoscopy every 10 years as an available option effective July 2001. We believe these actions provide evidence of the public interest in colorectal cancer screening methods and the federal government's willingness to fund these methods.
Most importantly, the Federal Balanced Budget Act of 1997 allows new technologies to be included as colorectal cancer screening tests by action of the Secretary of Health and Human Services without the need for additional Congressional action. In the spring of 1999, we met with senior staff members of CMS to apprise them of our progress and to determine the steps we would need to take prior to a reimbursement determination. Following that meeting, we successfully petitioned the CMS staff to cover all medical expenses of a patient participating in our clinical studies who tests positive for colorectal cancer, which we believe was a favorable departure from prior CMS policy of not reimbursing for these costs.
In October of 2002, we met with CMS to discuss the reimbursement process. Subsequent to that meeting, CMS published its approach to expanding the colorectal cancer screening benefit to include new technologies by use of a national coverage decision process, thereby avoiding the time-consuming notice and comment procedures otherwise applicable.
12
In addition, we continue to work on building support in Congress and have met with several members of Congressional staffs and national organizations with an interest in colorectal cancer. In October 1999, we testified before the Subcommittee on Health of the House Ways and Means Committee in support of the Eliminate Colorectal Cancer Act of 1999. The Eliminate Colorectal Cancer Act of 1999 requires private insurers to cover colorectal cancer screening tests deemed appropriate by physicians and patients to the same extent as the Federal Balanced Budget Act of 1997 covers for Medicare.
We believe that colorectal cancer screening tests based on our PreGen technologies will add a lifesaving and cost-effective alternative to currently available colorectal cancer screening methods. We believe that reimbursement for FOBT tests ranges from $5 to $30, but, as stated earlier, FOBT sensitivity only falls in the 25% to 30% range, and is most effective in detecting later stage cancers when survival rates are low and treatment costs are high. We believe that reimbursement for flexible sigmiodoscopy ranges from $80 to $500, but at best, can directly detect no more than half of all colorectal cancers and adenomas since it only reaches the first third of the colon, where approximately 50% of lesions develop. Medicare and some private insurers currently reimburse for colonoscopy for cancer screening once every 10 years in average risk individuals. We believe that the cost of this procedure ranges from $700 to $2,000, and while colonoscopy is sensitive, the use of colonoscopy as a screening test to date has been limited due to low patient compliance and capacity constraints which result in generally long scheduling lead times for the procedure.
Government Regulation
Certain of our activities are, or have the potential to be, subject to regulatory oversight by the FDA under provisions of the Federal Food, Drug and Cosmetic Act and regulations thereunder, including regulations governing the development, marketing, labeling, promotion, manufacturing and export of our products. Failure to comply with applicable requirements can lead to sanctions, including withdrawal of products from the market, recalls, refusal to authorize government contracts, product seizures, civil money penalties, injunctions and criminal prosecution.
Generally, certain categories of medical devices, a category that may be deemed to include products based upon our PreGen technologies, require FDA pre-market approval or clearance before they may be marketed and placed into commercial distribution. The FDA has not, however, actively regulated in-house laboratory tests that have been developed and validated by the laboratory providing the tests. Additionally, the FDA has demonstrated prior enforcement discretion and is currently undergoing internal review on its legal authority for regulating these products. Pre-market clearance or approval is not currently required for this category of products. The FDA does regulate the sale of certain reagents, including some of our reagents, used in laboratory tests. The FDA refers to the reagents used in these tests as analyte specific reagents. Analyte specific reagents react with a biological substance including those intended to identify a specific DNA sequence or protein. These reagents generally do not require FDA pre-market approval or clearance if they are (i) sold to clinical laboratories certified by the government to perform high complexity testing and (ii) labeled in accordance with FDA requirements, including a statement that their analytical and performance characteristics have not been established. A similar statement would also be required on all advertising and promotional materials relating to analyte specific reagents such as those used in our test. Laboratories also are subject to restrictions on the labeling and marketing of tests that have been developed using analyte specific reagents. The analyte specific reagent regulatory category is relatively new and its regulatory boundaries are not well defined. We believe that in-house testing based upon our PreGen technologies, and any analyte specific reagents that we intend to sell to leading clinical reference laboratories currently do not require FDA approval or clearance. We cannot be sure, however, that the FDA will not change its policy in a manner that would result in tests based upon our
13
PreGen technologies, or a combination of reagents, to require pre-market approval or clearance. In addition, we cannot be sure that the FDA will not change its position in ways that could negatively affect our operations either through regulation or new enforcement initiatives.
Regardless of whether a medical device requires FDA approval or clearance, a number of other FDA requirements apply to its manufacturer and to those who distribute it. Device manufacturers must be registered and their products listed with the FDA, and certain adverse events, correction and removals must be reported to the FDA. The FDA also regulates the product labeling, promotion, and in some cases, advertising, of medical devices. Manufacturers must comply with the FDA's Quality System Regulation which establishes extensive requirements for design, quality control, validation and manufacturing. Thus, manufacturers and distributors must continue to spend time, money and effort to maintain compliance, and failure to comply can lead to enforcement action. The FDA periodically inspects facilities to ascertain compliance with these and other requirements.
We are also subject to U.S. and state laws and regulations regarding the operation of clinical laboratories. The federal Clinical Laboratory Improvement Amendments of 1988 ("CLIA") and laws of certain other states impose certification requirements for clinical laboratories, and establish standards for quality assurance and quality control, among other things. Clinical laboratories are subject to inspection by regulators, and the possible sanctions for failing to comply with applicable requirements. Sanctions available under CLIA include prohibiting a laboratory from running tests, requiring a laboratory to implement a corrective plan, and imposing civil monetary penalties. If we fail to meet the requirements of CLIA or state law, it could cause us to incur significant expense.
Any diagnostic test kits that we, or our partners, may sell would require FDA clearance or approval before they could be placed into commercial distribution. There are two regulatory review procedures by which a product may receive such approval or clearance. Some products may qualify for clearance under a pre-market notification, or 510(k) process. Under such a process, the manufacturer provides to the FDA a pre-market notification that it intends to begin marketing the product, and demonstrates to the FDA's satisfaction, through appropriate studies, that the product is substantially equivalent to a comparative product that has been legally marketed and is currently in commercial distribution. Clearance of a 510(k) means that the product has the equivalent intended use, is as safe and effective as, and does not raise significant questions of safety and effectiveness than a legally marketed device. A 510(k) submission for anin vitro diagnostic device generally must include labeling information, performance data, and in some cases, it must include data from human clinical studies. Marketing may commence under a 510(k) submission when the FDA issues a clearance letter determining the product to be substantially equivalent to a comparative device.
If a medical device does not qualify for the 510(k) submission process by not being substantially equivalent or raising new issues of safety and effectiveness, the FDA may require submission of a pre-market approval application, or PMA, before marketing can begin. PMA applications must demonstrate, among other matters, that the medical device is safe and effective. A PMA application is a more comprehensive submission than a 510(k) submission, resulting in longer review and approval timeframes and usually includes the results of extensive pre-clinical and clinical studies and detailed information on the product, design and manufacturing system. Before the FDA will approve an original PMA, the manufacturer must undergo and pass a pre-approval inspection that assesses its compliance with the requirements of the FDA's Quality System Regulations.
We believe that if our products are sold in FDA approved diagnostic test kit form; they would likely require PMA approval. As compared to the 510(k) process, the PMA process is traditionally more lengthy and costly, and we cannot be sure that the FDA will approve PMAs for our products in a timely fashion, or at all. Additionally, FDA requests for additional studies during the review period are
14
not uncommon, and can significantly delay approvals. Even if we were able to gain approval of a product for one indication, changes to the product, its indication, or its labeling would likely require additional approvals in the form of a PMA Supplement.
Once a physician orders a test, the patient will need to receive a specimen container to collect the patient's stool. Although specimen transport and storage containers are also medical devices regulated by the FDA, such containers generally have been exempted by regulation from the FDA's pre-market clearance or approval requirement. We believe that our specimen container falls within an applicable exemption, but we cannot be sure that the FDA will not assert that our container is not exempt and seek to impose a pre-market clearance or approval requirement.
Intellectual Property
In order to protect our proprietary PreGen technologies, we rely on combinations of patent, trademark, and copyright protection, as well as confidentiality agreements with employees, consultants, and third parties.
We have pursued an aggressive patent strategy designed to maximize our patent position with respect to third parties. Generally, we have filed patents and patent applications that cover the methods we have designed to detect colorectal cancer as well as other cancers. We have also filed patent applications covering the preparation of stool samples and the extraction of DNA from heterogeneous stool samples. As part of our strategy, we seek patent coverage in the United States and in foreign countries on aspects of our PreGen technologies that we believe will be significant to our market strategy or that we believe provide barriers to entry for our competition.
As of December 31, 2002, we had 26 patents issued and 30 pending patent applications in the United States and, in foreign jurisdictions, 8 patents issued and 107 pending applications. Our success depends to a significant degree upon our ability to develop proprietary products and technologies and to obtain patent coverage for such products and technologies. We intend to continue to file patent applications covering newly-developed products or technologies.
Each of our patents generally has a term of 20 years from its respective priority filing dates. Consequently, our first patents are set to expire in 2016. We have filed terminal disclaimers in certain later-filed patents, which means that such later-filed patents will expire earlier than the twentieth anniversary of their priority filing dates.
A third-party institution has asserted co-inventorship rights with respect to one of our issued patents relating to use of our e-LOH detection method on pooled samples from groups of patients. Our current cancer screening detection methods do not include pooled samples. To date, no legal proceedings have been initiated by this third party. If any third party, including the third party discussed above, asserting co-inventorship rights with respect to any of our patents is successful in challenging our inventorship determination, such patent may become unenforceable or we may be required to add that third party inventor to the applicable patent, resulting in co-ownership of such patent with the third party. Co-ownership of a patent allows the co-owner to exercise all rights of ownership, including the right to use, transfer and license the rights protected by the applicable patent.
We and a third-party institution have filed a joint patent application under the Patent Cooperation Treaty that will be co-owned by us and the third-party institution relating to the use of various DNA markers, including the DNA Integrity Assay, to detect cancers of the lung, pancreas, esophagus, stomach, small intestine, bile duct, naso-pharyngeal, liver and gall bladder in stool. This patent application does not relate to the detection of colorectal cancer and designates the United States, Japan, Europe and Canada as the territories in which rights are sought.
15
We license on a non-exclusive basis certain polymerase chain reaction ("PCR") technology from Roche Molecular Systems, Inc. This license relates to a gene amplification process used in almost all genetic testing, and the patent that we utilize expires in mid-2004. In exchange for the license, we have agreed to pay Roche a royalty based on net revenues we receive from commercial tests that we perform at our facility. Our strategic relationship with LabCorp, however, contemplates commercial tests being performed in LabCorp's facilities, rather than in our facility, and therefore, this obligation becomes the responsibility of LabCorp. Roche may terminate this license upon notice if we fail to pay royalties, fail to submit reports or breach a material term of the license agreement.
We license on a non-exclusive basis technology from Genzyme Corporation, a licensee of patents owned by Johns Hopkins University and of which Dr. Vogelstein is an inventor. This license relates to the use of theApc andp53 genes and methodologies related thereto in connection with our products and services and lasts through 2013, the life of the patent term of the last-licensed Genzyme patent. In exchange for the license, we have agreed to pay Genzyme a royalty based on net revenues we receive from commercial tests we perform at our facility and the sale of analyte specific diagnostic test kits, as well as certain milestone payments and maintenance fees. In addition, we must use reasonable efforts to make products and services based on these patents available to the public. Genzyme may terminate this license upon notice if we fail to pay milestone payments and royalties, achieve a stated level of sales or submit reports. In addition, if we fail to request FDA clearance for a diagnostic test as required by the agreement, Genzyme may terminate the license. As noted previously, our strategic relationship with LabCorp contemplates commercial tests being performed in LabCorp's facilities, rather than our facility, and therefore, this obligation becomes the responsibility of LabCorp.
We license on an exclusive basis, in the field of stool-based colorectal cancer screening, from Matrix Technologies Corporation, d/b/a Apogent Discoveries, certain patents owned by Apogent relating to its Acrydite™ technologies. The license provides us and our sublicensees, with the ability to manufacture and use the Acrydite technology in the PreGen-Plus assay. The Acrydite technology is useful in connection with our proprietary electrophoretic DNA gel capture technology used in the isolation of nucleic acids and the diagnosis of disease that we purchased from MT Technologies.
We license on an exclusive basis from Johns Hopkins University certain patents owned by JHU that relate to digital amplification of DNA. We believe that this license will allow us and our partners to develop and commercialize novel detection technologies to enhance the performance of our current technologies. In exchange for the license, we have agreed to pay JHU certain royalties on revenues received by us relating to our or our sublicensees' sales of products and services that incorporate the JHU technology.
We and LabCorp are currently negotiating additional third-party technology license and supply agreements that are necessary to the commercial launch of the PreGen-Plus assay.
Competition
To our knowledge, none of the large genomics or diagnostics companies is developing tests to conduct stool-based DNA testing. However, these companies may be working on similar tests that have not yet been announced. In addition, other companies may succeed in developing novel or improving existing technologies and marketing products and services that are more effective or commercially attractive than ours. Some of these companies may be larger than we are and can commit significantly greater financial and other resources to all aspects of their business, including research and development, marketing, sales and distribution.
We also face competition from alternative procedures-based detection technologies such as flexible sigmiodoscopy, colonoscopy and virtual colonoscopy as well as traditional screening tests such as the Hemoccult II. Virtual colonoscopy involves a new approach that requires patients to undergo bowel preparation similar to a colonoscopy after which they are scanned by a spiral CT scanner. Three-
16
dimensional images are constructed to allow a radiologist to virtually travel through the colon and visualize the colon for polyps and cancers.
In addition, certain of our competitors are developing serum-based tests, an alternative cancer-screening approach that is based on detection of proteins or nucleic acids that are produced by colon cancers and may be found circulating in blood. We believe serum-based testing is not able to detect disease at the earliest stages of cancer at levels of sensitivity and specificity comparable to that of stool-based testing.
We believe the principal competitive factors in the cancer screening market include:
- •
- high sensitivity;
- •
- high specificity;
- •
- non-invasiveness;
- •
- acceptance by the medical community, especially primary care medical practitioners;
- •
- adequate reimbursement from Medicare and other third-party payors;
- •
- cost-effectiveness; and
- •
- patent protection.
Employees
As of December 31, 2002, we had seventy-five employees, eight of whom have Ph.D.s and two of whom have M.D.s. Forty-seven employees are engaged in research and development, ten employees in sales and marketing and eighteen employees in general and administration. None of our employees is represented by a labor union. We consider our relationship with our employees to be good. As of December 31, 2002, we also had twenty contractual employees who were primarily involved in performing tests associated with our on-going clinical trials.
Item 2. Properties
We currently lease approximately 20,000 square feet of space in our headquarters located in Maynard, Massachusetts under various leases that expire on June 30, 2003 and November 1, 2003. In January 2003, we signed a lease for approximately 56,000 square feet of space in Marlborough, Massachusetts for a seven-year term and expect to relocate substantially all of our operations into this new facility by October 2003. We believe that this new facility will be adequate to meet our space requirements for the foreseeable future.
Item 3. Legal Proceedings
From time to time we are a party to various legal proceedings arising in the ordinary course of our business. The outcome of litigation cannot be predicted with certainty and some lawsuits, claims or proceedings may be disposed of unfavorably to us. Intellectual property disputes often have a risk of injunctive relief which, if imposed against us, could materially and adversely affect our financial condition, or results of operations. From time to time, third parties have asserted and may in the future assert intellectual property rights to technologies that are important to our business and have demanded and may in the future demand that we license their technology. We are not currently a party to any material legal proceedings.
Item 4. Submission of Matters to a Vote of Security Holders
No matters were submitted to a vote of security holders during the fourth quarter of fiscal 2002.
17
PART II
Item 5. Market for Registrant's Common Equity and Related Stockholder Matters
Our common stock has been listed for trading on the Nasdaq National Market under the symbol "EXAS" since the effective date of our initial public offering on January 30, 2001. Prior to that time, there was no public market for our common stock. On March 14, 2003, the last reported price of our common stock on the Nasdaq National Market was $9.51 per share. Based upon information supplied to us by the registrar and transfer agent for our common stock, the number of common stockholders of record on March 14, 2003 was approximately 150, not including beneficial owners in nominee or street name. We believe that a significant number of shares of our common stock are held in nominee name for beneficial owners. The high and low common stock prices per share subsequent to our initial public offering in January 2001 were as follows:
| | High
| | Low
|
|---|
| Fiscal 2002 Quarter Ended: | | | | | | |
| March 31 | | $ | 12.16 | | $ | 7.27 |
| June 30 | | $ | 17.40 | | $ | 9.32 |
| September 30 | | $ | 15.90 | | $ | 9.75 |
| December 31 | | $ | 15.99 | | $ | 9.65 |
| Fiscal 2001 Quarter Ended: | | | | | | |
| March 31 | | $ | 15.38 | | $ | 7.50 |
| June 30 | | $ | 14.15 | | $ | 5.30 |
| September 30 | | $ | 15.57 | | $ | 7.75 |
| December 31 | | $ | 11.75 | | $ | 6.75 |
We have not paid any cash dividends on our common stock and we currently intend to retain any future earnings for use in our business. Accordingly, we do not anticipate that any cash dividends will be declared or paid on the common stock in the foreseeable future.
EQUITY COMPENSATION PLAN INFORMATION
The Company maintains the following three equity compensation plans under which our equity securities are authorized for issuance to our employees and/or directors: the 1995 Stock Option Plan, the 2000 Stock Option and Incentive Plan and the 2000 Employee Stock Purchase Plan. Each of the foregoing equity compensation plans was approved by our stockholders. The following table presents information about these plans as of December 31, 2002.
Plan Category
| | Number Of Securities To Be
Issued Upon Exercise Of
Outstanding Options,
Warrants, And Rights
| | Weighted Average Exercise
Price Of Outstanding
Options, Warrants And Rights
| | Number Of Securities Remaining
Available For Future Issuance
Under Equity Compensation Plans
(Excluding Securities Outstanding)
|
|---|
| Equity compensation plans approved by security holders | | 2,892,291 | | $ | 7.07 | | 702,162 |
| Equity compensation plans not approved by security holders | | None | | | None | | None |
| | |
| |
| |
|
| Total | | 2,892,291 | | $ | 7.07 | | 702,162 |
No further grants will be made under the 1995 Stock Option Plan.
18
Item 6. Selected Financial Data
The selected historical financial data set forth below as of December 31, 2001 and 2002 and for the years ended December 31, 2000, 2001 and 2002, are derived from our financial statements, which have been audited by Ernst & Young LLP, independent auditors, as of December 31, 2002 and for the year then ended; and by Arthur Andersen LLP, our former independent public accountants, as of December 31, 2001 and for the years ended December 31, 2000 and 2001, and which are included elsewhere in this Form 10-K. The selected historical financial data as of December 31, 1998, 1999 and 2000 and for the years ended December 31, 1998 and 1999 are derived from our audited financial statements, which have been audited by Arthur Andersen LLP, our former independent public accountants and which are not included elsewhere in this Form 10-K.
The selected historical financial data should be read in conjunction with, and are qualified by reference to "Management's Discussion and Analysis of Financial Condition and Results of Operations," our financial statements and notes thereto and the report of independent public auditors included elsewhere in this Form 10-K.
| | 1998
| | 1999
| | 2000
| | 2001
| | 2002
| |
|---|
| | (Dollars in thousands, except share and per share data)
| |
|---|
| Statement of Operations Data: | | | | | | | | | | | | | | | | |
| | Revenue | | $ | — | | $ | — | | $ | — | | $ | 51 | | $ | 897 | |
| | Cost of revenues | | | — | | | — | | | — | | | — | | | 9 | |
| | Research and development | | | 2,849 | | | 3,689 | | | 5,332 | | | 13,335 | | | 19,989 | |
| | Selling, general and administrative | | | 1,170 | | | 1,560 | | | 4,814 | | | 9,078 | | | 9,701 | |
| | Stock-based compensation (1) | | | 2 | | | 14 | | | 3,184 | | | 3,788 | | | 2,043 | |
| | |
| |
| |
| |
| |
| |
| | Loss from operations | | | (4,021 | ) | | (5,263 | ) | | (13,330 | ) | | (26,150 | ) | | (30,845 | ) |
| | Interest income | | | 443 | | | 299 | | | 1,447 | | | 2,665 | | | 962 | |
| | |
| |
| |
| |
| |
| |
| | | Net loss | | $ | (3,578 | ) | $ | (4,964 | ) | $ | (11,883 | ) | $ | (23,485 | ) | $ | (29,883 | ) |
| | |
| |
| |
| |
| |
| |
| Net loss per common share: | | | | | | | | | | | | | | | | |
| | Basic and diluted | | $ | (6.08 | ) | $ | (5.32 | ) | $ | (8.13 | ) | $ | (1.42 | ) | $ | (1.62 | ) |
| | |
| |
| |
| |
| |
| |
| Weighted average common shares outstanding: | | | | | | | | | | | | | | | | |
| | Basic and diluted | | | 588,143 | | | 932,593 | | | 1,461,726 | | | 16,487,499 | | | 18,433,400 | |
| | |
| |
| |
| |
| |
| |
| Balance Sheet Data: | | | | | | | | | | | | | | | | |
| | Cash and cash equivalents | | $ | 8,826 | | $ | 3,553 | | $ | 26,470 | | $ | 56,843 | | $ | 17,439 | |
| | Marketable securities | | | — | | | — | | | — | | | — | | | 26,407 | |
| | Total assets | | | 9,708 | | | 4,754 | | | 29,059 | | | 63,100 | | | 50,086 | |
| | Stockholders' equity | | | 9,298 | | | 4,410 | | | 27,700 | | | 58,967 | | | 38,349 | |
- (1)
- The following summarizes the departmental allocation of stock-based compensation:
| | 1998
| | 1999
| | 2000
| | 2001
| | 2002
|
|---|
| | Research and development | | $ | 2 | | $ | 9 | | $ | 810 | | $ | 898 | | $ | 478 |
| | Selling, general and administrative | | | — | | | 5 | | | 2,374 | | | 2,890 | | | 1,565 |
| | |
| |
| |
| |
| |
|
| | Total | | $ | 2 | | $ | 14 | | $ | 3,184 | | $ | 3,788 | | $ | 2,043 |
| | |
| |
| |
| |
| |
|
19
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
This report and other documents we have filed with the SEC contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended, and are subject to the "safe harbor" created by those sections. Some of the forward-looking statements can be identified by the use of forward-looking terms such as "believes," "expects," "may," "will," "should," "could," "seek," "intends," "plans," "estimates," "anticipates" or other comparable terms. Forward-looking statements involve inherent risks and uncertainties. A number of important factors could cause actual results to differ materially from those in the forward-looking statements. We urge you to consider the risks and uncertainties discussed below and elsewhere in this report and in the other documents filed with the SEC in evaluating our forward-looking statements. We have no plans to update our forward-looking statements to reflect events or circumstances after the date of this report. We caution readers not to place undue reliance upon any such forward-looking statements, which speak only as of the date made.
Overview
We apply proprietary genomic technologies to the early detection of common cancers. We have selected colorectal cancer screening as the first application of our PreGen technologies platform. Since our inception on February 10, 1995, our principal activities have included:
- •
- researching and developing our PreGen technologies for colorectal cancer screening;
- •
- conducting clinical studies to validate our colorectal cancer screening tests;
- •
- negotiating licenses for intellectual property of others incorporated into our technologies;
- •
- developing relationships with opinion leaders in the scientific and medical communities;
- •
- conducting market studies and analyzing potential approaches for commercializing our PreGen technologies;
- •
- hiring research and clinical personnel;
- •
- hiring management and other support personnel;
- •
- raising capital;
- •
- licensing our proprietary PreGen technologies to LabCorp; and
- •
- working with LabCorp on activities necessary to prepare for commercial launch of PreGen-Plus.
On June 26, 2002, we entered into a license agreement with LabCorp for an exclusive, long-term strategic alliance between the parties to commercialize PreGen-Plus, our proprietary, non-invasive technology for the early detection of colorectal cancer in the average-risk population. Pursuant to the license agreement, we agreed to license to LabCorp all U.S. and Canadian patents and patent applications owned by us relating to our PreGen technologies for the detection of colorectal cancer in an average-risk population. The license is exclusive for a five-year period, followed by a non-exclusive license for the life of the patents. In return for the license, LabCorp has agreed to pay us certain upfront, milestone and performance-based payments, and a per-test royalty fee. LabCorp made an initial payment of $15 million upon the signing of the agreement, and a second payment of $15 million is to be made upon the commercial launch of PreGen-Plus. In addition, we may be eligible for milestone payments from LabCorp totaling up to $30 million based upon Company deliverables related to scientific acceptance, reimbursement approval and technology improvements and up to $15 million upon the achievement of significant LabCorp revenue thresholds. In addition to these payments, the Company will receive a royalty fee for each PreGen-Plus test performed by LabCorp. In conjunction with the strategic alliance, the Company has issued to LabCorp a warrant to purchase 1,000,000 shares
20
of its common stock, exercisable over a three-year period at an exercise price of $16.09 per share. The Company assigned a value to the warrant of $6,550,000 under the Black-Scholes option-pricing model, which has been recorded as a reduction in the initial upfront deferred license fee of $15 million.
We have generated no material operating revenues since our inception, and do not expect any product licensing revenues until at least the second half of 2003. As of December 31, 2002, we had an accumulated deficit of approximately $76.5 million. Our losses have historically resulted from costs incurred in conjunction with our research and development initiatives, and more recently, costs associated with selling, general and administrative expenses as we hire additional personnel, initiate marketing programs and build our infrastructure to support the commercial launch of PreGen-Plus, our average-risk colorectal cancer test, and to support future growth.
Research and development expenses include costs related to scientific and laboratory personnel, clinical studies and reagents and supplies used in the development of our PreGen technologies. There are two on-going clinical trials that comprise the majority of our research and development expense during 2002.
In the third quarter of 2001, we initiated our blinded multi-center clinical trial, which is expected to include an estimated 5,500 asymptomatic, age 50 and older, patients from over 80 academic and community-based practices. The goal of this clinical trial will be to provide additional data supporting the superiority of tests utilizing our Pre-Gen technology versus the most widely used brand of FOBT, Hemoccult II, in detecting colorectal cancer in this average-risk population. We expect patient enrollment in this clinical trial to conclude by the end of the first quarter of 2003 and expect that study data will be available in the fourth quarter of 2003. The estimated cost to complete this clinical trial is approximately $3.0 million to $4.0 million depending on the ultimate number of patients recruited and the final number of stool samples processed.
In October 2001, we initiated a clinical trial in collaboration with the Mayo Clinic in which our PreGen technologies will be the subject of an independent study by the Mayo Clinic for which the Mayo Clinic received a $4.9 million grant from the National Cancer Institute of the National Institutes of Health. This three-year study will involve approximately 4,000 patients at average risk for developing colorectal cancer, and will compare the results of our non-invasive, genomics-based screening technology with those of FOBT, a common first-line colorectal cancer screening option. The Mayo Clinic has indicated that it expects enrollment in this clinical trial to be completed sometime in 2004. In addition to the costs already incurred, our estimated cost to complete this clinical trial is approximately $3.5 million to $4.5 million depending on the ultimate number of patients recruited and the final number of stool samples processed. We expect that the cost of our research and development activities will decrease in 2003 slightly from 2002 as we conclude our 5,500 patient blinded multi-center clinical trial.
Selling, general and administrative expenses consist primarily of non-research personnel salaries, office expenses and professional fees. We expect selling, general and administrative expenses to increase in 2003 as we hire additional sales and marketing personnel and implement marketing and sales initiatives in conjunction with LabCorp, to support the commercialization of PreGen-Plus.
Stock-based compensation expense, a non-cash expense, primarily represents the difference between the exercise price and fair value of common stock on the date of grant for certain options granted prior to our initial public offering. The stock compensation expense is being amortized over the vesting period of the applicable options, which is generally 60 months. Currently, we expect to recognize stock-based compensation expense related to employee, consultant and director options of approximately $1.2 million, $600,000 and $200,000 during the years ended December 31, 2003, 2004 and 2005, respectively.
21
Significant Accounting Policies
Financial Reporting Release No. 60, which was recently issued by the Securities and Exchange Commission ("SEC"), requires all registrants to discuss critical accounting policies or methods used in the preparation of the financial statements. The notes to the consolidated financial statements included in this annual report on Form 10-K includes a summary of the significant accounting policies and methods used in the preparation of our consolidated financial statements.
Further, we have made a number of estimates and assumptions that affect reported amounts of assets, liabilities, revenues and expenses, and actual results may differ from those estimates. The areas that require the greatest degree of management judgment are the assessment of the recoverability of long-lived assets, primarily intellectual property and the accrual of costs related to patient recruitment for our multi-center study.
Patent costs, which historically consisted of related legal fees, are capitalized as incurred and are amortized beginning when patents are issued in the United States over an estimated useful life of five years. In November 2001, however, the Company purchased certain intellectual property of MT Technologies (formerly known as Mosaic Technologies, Inc.) relating to its Hybrigel™ technology which consisted of four issued patents and 40 pending patent applications. The purchase price for the assets consisted of $1.3 million in cash and warrants to purchase 40,000 shares of common stock, immediately exercisable over a three-year period at an exercise price of $7.33 per share, which the Company valued at $188,261 in accordance with Emerging Issues Task Force 96-18, Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods or Services, using the Black-Scholes option pricing model. Capitalized patent costs are expensed upon disapproval or upon a decision by us to no longer pursue the patent.
The Company accrues the estimated cost of patient recruitment associated with its large multi-center clinical trial as patients are enrolled in the trial. These costs consist primarily of payments made to the clinical centers, investigators and patients for participating in the Company's clinical trial. As actual or expected costs become known, they may differ from estimated costs previously accrued for and this clinical trial accrual would be adjusted accordingly.
We believe that full consideration has been given to all relevant circumstances that we may be subject to, and the financial statements accurately reflect our best estimate of the results of operations, financial position and cash flows for the periods presented.
Results of Operations
Comparison of the years ended December 31, 2002 and 2001
Revenue. Revenue increased to $897,000 for the year ended December 31, 2002 from $51,000 for the year ended December 31, 2001. This revenue is primarily composed of amortization of up-front technology license fees associated with agreements signed in July 2001 and June 2002 with LabCorp that are being amortized on a straight-line basis over the respective license periods.
Cost of revenues. Cost of revenues for the year ended December 31, 2002 of $9,000 represents the estimated cost of performing colorectal screening tests at our facility.
Research and development expenses. Research and development expenses, excluding departmental allocations of stock-based compensation, increased to $20.0 million for the year ended December 31, 2002 from $13.3 million for the year ended December 31, 2001. This increase was primarily attributable with the initiation of our blinded multi-center clinical trial in October 2001 and included increases of $1.3 million in personnel-related expenses, $253,000 in professional fees and expenses, $1.6 million in laboratory expenses, $1.9 million in trials and studies expenses and $1.6 million related to the leasing of additional laboratory space.
22
Selling, general and administrative expenses. Selling, general and administrative expenses, excluding departmental allocations of stock-based compensation, increased to $9.7 million for the year ended December 31, 2002 from $9.1 million for the year ended December 31, 2001. This increase was attributable primarily to additional personnel hired to build our infrastructure and the initiation of other corporate and marketing programs to support future growth and included increases of $162,000 in personnel-related expenses, $722,000 in professional fees and expenses, $62,000 in travel-related expenses partially offset by lower costs of $322,000 related to office space and related office expenses.
Stock-based compensation. Stock-based compensation, a non-cash expense, decreased to $2.0 million for the year ended December 31, 2002, of which $478,000 related to research and development personnel and $1.6 million related to general and administrative personnel from $3.8 million for the year ended December 31, 2001. The decrease in stock-based compensation in 2002 from 2001 is due to the accelerated method of amortization being used to record this expense.
Interest income. Interest income decreased to $962,000 for the year ended December 31, 2002 from $2.7 million for the year ended December 31, 2001. This decrease was primarily due to lower interest rates on our investments and overall decreases in our average cash, cash equivalents and marketable securities balances.
Comparison of the years ended December 31, 2001 and 2000
Revenue. Revenue was $51,000 for the year ended December 31, 2001. This revenue is primarily composed of amortization of up-front technology license fees associated with an agreement signed in July 2001 with Laboratory Corporation of America Holdings, Inc. that is being amortized on a straight-line basis over the license period.
Research and development expenses. Research and development expenses, excluding departmental allocations of stock-based compensation, increased to $13.3 million for the year ended December 31, 2001 from $5.3 million for the year ended December 31, 2000. This increase was primarily attributable with the initiation of our blinded multi-center clinical trial and included increases of $1.1 million in personnel-related expenses, $808,000 in professional fees and expenses, $821,000 in laboratory expenses, $4.5 million in trials and studies expenses and $642,000 related to the leasing of additional laboratory space.
Selling, general and administrative expenses. Selling, general and administrative expenses, excluding departmental allocations of stock-based compensation, increased to $9.1 million for the year ended December 31, 2001 from $4.8 million for the year ended December 31, 2000. This increase was attributable primarily to additional personnel hired to build our infrastructure and the initiation of other corporate and marketing programs to support future growth and included increases of $1.8 million in personnel-related expenses, $1.9 million in professional fees and expenses, $81,000 in travel-related expenses and $503,000 related to the leasing of additional office space and related office expenses.
Stock-based compensation. Stock-based compensation, a non-cash expense, increased to $3.8 million for the year ended December 31, 2001, of which $898,000 related to research and development personnel and $2.9 million related to general and administrative personnel. Stock-based compensation was $3.2 million for the year ended December 31, 2000, of which $810,000 related to research and development personnel and $2.4 million related to general and administrative personnel.
Interest income. Interest income increased to $2.7 million for the year ended December 31, 2001 from $1.4 million for the year ended December 31, 2000. This increase was primarily due to an increase in our cash and cash equivalents balances resulting from the issuance of common stock in February 2001.
23
Liquidity and Capital Resources
We have financed our operations since inception primarily through private sales of preferred stock, as well as the completion of an initial public offering of our common stock in January 2001. As of December 31, 2002, we had approximately $43.8 million in cash, cash equivalents and marketable securities.
Net cash used in operating activities was $11.9 million for the year ended December 31, 2002, $15.8 million in 2001 and $8.0 million in 2000. Excluding the impact of the upfront deferred licensing fee of $15 million from LabCorp, net cash used in operating activities would have been $26.9 million for the year ended December 31, 2002. This increase was primarily due to our increase in operating losses resulting from higher spending in research and development expenses as the Company commenced its blinded multi-center clinical trial in October 2001. In addition, selling, general and administrative expenses increased as we initiated certain corporate, selling and marketing programs to support the commercialization of PreGen-Plus and build our infrastructure to support future growth.
Net cash used in investing activities was $28.1 million for the year ended December 31, 2002, $4.6 million in 2001 and $1.1 million in 2000. The majority of this increase was due to the purchase of $26.3 million in investments during 2002 as we began to invest in longer-term securities in order to increase the return on our excess cash position consistent with our investment policy guidelines. Cash used for purchasing property and equipment was $1.3 million for the year ended December 31, 2002, $2.6 million in 2001 and $762,000 in 2000. This investment for 2002 and 2001 is primarily the result of the expansion of our laboratory space to prepare for our multi-center clinical trial. Cash used for our intellectual property portfolio was $417,000 for the year ended December 31, 2002, $2.0 million in 2001 and $318,000 in 2000. The investment for 2001 includes the purchase of intellectual property from MT Technologies relating to its Hybrigel technology which consisted of 4 issued patents and 40 pending patent applications for $1.3 million in cash. Patent costs, which historically consisted of related legal fees, are capitalized as incurred.
Net cash provided by financing activities was $615,000 for the year ended December 31, 2002, $50.8 million in 2001 and $32.0 million in 2000. Cash provided by financing for the year ended December 31, 2002 resulted from issuance of $371,000 of common stock under our stock option and stock purchase plans along with the repayment of $248,000 of stock subscription receivables. Cash provided by financing for the years ended December 31, 2001 resulted primarily from the sale of our common stock from our initial public offering in 2001 and the sale of preferred stock in 2000.
We expect that cash, cash equivalents and short-term investments on hand at December 31, 2002, together with the additional milestone and royalty payments expected to be received from LabCorp, will be sufficient to fund our operations for the foreseeable future. Our future capital requirements include, but are not limited to, continuing our research and development programs, supporting our clinical study efforts, supporting our marketing efforts associated with the commercialization of PreGen-Plus and capital expenditures primarily associated with leasehold improvements and other moving costs of approximately $2.0 million for our new facility and purchases of additional laboratory
24
equipment. We do not have any significant fix obligations and commitments other than payments required under operating leases for leased facilities as follows:
Year Ending December 31,
| |
|
|---|
| 2003 | | $ | 765,000 |
| 2004 | | | 1,293,000 |
| 2005 | | | 1,289,000 |
| 2006 | | | 1,318,000 |
| 2007 | | | 1,347,000 |
| Thereafter | | | 3,370,000 |
| | |
|
| Total lease payments | | $ | 9,382,000 |
| | |
|
Our future capital requirements will depend on many factors, including the following:
- •
- the success of our clinical studies;
- •
- the scope of and progress made in our research and development activities; and
- •
- the successful commercialization of Pre-Gen Plus.
Net Operating Loss Carryforwards
As of December 31, 2002, we had net operating loss carryforwards of approximately $46.3 million and tax credit carryforwards of approximately $1.3 million. The net operating loss and tax credit carryforwards will expire at various dates through 2022, if not utilized. The Internal Revenue Code and applicable state laws impose substantial restrictions on a corporation's utilization of net operating loss and tax credit carryforwards if an ownership change is deemed to have occurred.
A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before we are able to realize their benefit, or that future deductibility is uncertain. In general, companies that have a history of operating losses are faced with a difficult burden of proof on their ability to generate sufficient future income within the next two years in order to realize the benefit of the deferred tax assets. We have recorded a valuation against our deferred tax assets based on our history of losses. The deferred tax assets are still available for us to use in the future to offset taxable income, which would result in the recognition of tax benefit and a reduction to our effective tax rate.
Factors That May Affect Future Results
We operate in a rapidly changing environment that involves a number of risks, some of which are beyond our control. This discussion highlights some of the risks which may affect future operating results.
We may never successfully commercialize any of our products or services or earn a profit.
We have incurred losses since we were formed. From our date of inception on February 10, 1995 through December 31, 2002, we have accumulated a total deficit of approximately $76.5 million. We do not expect to have any operating revenue from the licensing of our products and services until at least the second half of 2003. Even after we begin selling our products and services, we expect that our losses will continue and increase as a result of continuing research and development expenses, as well as increased sales and marketing expenses. We cannot assure you that the revenue from any of our products or services will be sufficient to make us profitable.
25
Dependence on our strategic partnership
We have a long-term strategic alliance agreement with LabCorp whereby we license PreGen technologies to LabCorp that are required for the commercialization of PreGen-Plus, our proprietary, non-invasive technology for the early detection of colorectal cancer in the average-risk population. The license to LabCorp is exclusive within North America for a five-year term followed by a non-exclusive license for the life of the underlying patents. LabCorp has the ability to terminate this agreement for, among other things, a material breach by us. If LabCorp were to terminate the agreement, or fail to meet its obligation under the agreement, we would incur significant delays and expense in the commercialization of PreGen-Plus and we cannot guarantee that we would be able to enter into a similar agreement to commercialize this technology. Further, if we do not achieve certain milestones, or LabCorp does not achieve certain revenue thresholds, within the time periods prescribed in the agreement, we may not fully realize the expected benefits of the agreement to us.
We are actively working together with LabCorp to create and execute an operational and business plan for commercializing PreGen-Plus. The companies are currently focusing their efforts on: assay validation, technology transfer and licensing; contracting with manufacturers and suppliers; physician education and demand; broad-based reimbursement initiatives; advocacy development; and sales force training. Additionally, there are a number of significant initiatives that are primarily LabCorp's responsibility that must be accomplished prior to commercialization, if we are to be successful. Some of the major initiatives include the creation of an adequate distribution infrastructure for sample movement, the build-out of adequate laboratory space, the hiring and training of personnel, the establishment of supply agreements with vendors, and the licensing of certain third-party technologies used with the PreGen-Plus assay as it is currently configured. Once completed, LabCorp will also need to process an adequate number of stool samples at their facilities to validate test results. Failure to adequately complete any of these initiatives, or to do so in a timely manner, could impact the timing and success of the commercialization of PreGen-Plus.
In addition, LabCorp may decide to delay the commercialization of PreGen-Plus despite the achievement of technology transfer, validation, etc. or may decide that the commercialization of PreGen-Plus is no longer appropriate to its business. Should that occur, there is no assurance that we would be able to license PreGen-Plus to another clinical reference laboratory or otherwise successfully commercialize the technology.
Our business would suffer if we are unable to license certain technologies or if certain of our licenses were terminated.
The current configuration of PreGen-Plus that we expect to commercialize with LabCorp requires access to certain technologies and supply of raw materials for which we or LabCorp will need to enter into licensing and supply agreements prior to commercialization. While we believe that such agreements can be entered into with necessary third parties on favorable terms and conditions, no assurances can be given that we or LabCorp will be able to accomplish this objective on a timely basis, if at all. If we or LabCorp are unable to obtain these technologies on acceptable terms, the PreGen-Plus assay will need to be reconfigured which could significantly delay its commercialization.
We license certain technologies from Roche Molecular Systems, Inc. and Genzyme Corporation that are key to our PreGen technologies. The Roche license for the polymerase chain reaction ("PCR) technology, which relates to a gene amplification process used in almost all genetic testing, is a non-exclusive license through 2004, the date on which the patent that we utilize expires. Roche may terminate the license upon notice if we fail to pay royalties, submit certain reports or breach any other material term of the license agreement. The Genzyme license is a non-exclusive license to use theApc and p53 genes, methods for the detection of target nucleic acid by analysis of stool and other methodologies relating to the genes in connection with our products and services through 2013, the
26
date on which the term of the patent that we utilize expires. Genzyme may terminate the license upon notice if we fail to pay milestone payments and royalties, achieve a certain level of sales, or submit certain reports. In addition, if we fail to use reasonable efforts to make products and services based on these patents available to the public or fail to request FDA clearance for a diagnostic test kit as required by the agreement, Genzyme may terminate the license. If either Roche or Genzyme were to terminate the licenses, and if we were, at the time of license termination, generating material revenue from tests performed at our Company's facility, we would incur significant delays and expense to change a portion of our testing methods and we cannot guarantee that we would be able to change our testing methods without affecting the performance characteristics of our tests.
Additionally, a third-party has asserted claims of patent infringement against certain entities that are anticipated suppliers of materials necessary to the PreGen-Plus assay as it is currently configured. Although to date, no legal proceedings have been initiated against us, if any third party, including the third party discussed above, is successful in challenging the supply of materials needed for the PreGen-Plus assay as it is currently configured, commercialization of our PreGen technologies may be significantly delayed, sales of the PreGen-Plus assay may become interrupted, and our revenue may become impacted.
Dependence on collaborative relationships.
In addition to our dependence on LabCorp for the commercialization of PreGen-Plus, if any other party with which we, or LabCorp, have established, or will establish, licensing, supply, or collaborative agreements were unable to satisfy its contractual obligations to us or LabCorp, there can be no assurance that substantially similar agreements could be negotiated and executed with other third parties on acceptable terms, if at all, or that any such new agreements would be successful. While we believe third parties will meet their contractual responsibilities under current and future agreements, there can be no assurance that this will be the case or that such future agreements will in fact be negotiated and entered into. There can be no assurance that any of our current contractual arrangements between us and third parties, us and LabCorp, or between our strategic partners and other third parties, will be continued, entered into, or not breached or terminated early, or that we or our strategic partners will be able to enter into any future relationships necessary to the commercial launch of PreGen-Plus or necessary to our realization of material revenues. The failure to do so could have a material adverse effect on our business, results of operations and financial condition. Further, LabCorp or any other strategic partner may commercialize technologies or products for colorectal cancer outside of their relationship with us that compete directly with our PreGen technologies, and such technologies or products may prove to be more effective and cost-efficient than our PreGen technologies. Because our revenue will be dependent upon our and LabCorp's successful commercial introduction of PreGen-Plus, failure to successfully maintain our relationship with LabCorp or any other strategic partner would have a material adverse effect on our operations and ability to generate operating revenue.
If our clinical studies do not prove the superiority of our PreGen technologies, we and our partners may never sell our products and services.
In the third quarter of 2001, we initiated a large multi-center clinical study that will include approximately 5,500 patients with average-risk profiles. In October 2001, we also signed a Clinical Trial Agreement with the Mayo Clinic in which our PreGen technologies will be the subject of an independent study by Mayo Clinic for which Mayo Clinic received a $4.9 million grant from the National Cancer Institute of the National Institutes of Health. This three-year study will involve approximately 4,000 patients at average-risk for developing colorectal cancer and, similar to our 5,500 patient study, will compare the results of our PreGen technologies with those of the Hemoccult II, a fecal occult blood test, a common first-line colorectal cancer screening option. The results of these
27
clinical studies may not be favorable or show that tests using our PreGen technologies are sufficiently superior to existing non-invasive screening methods. In that event, we may have to devote significant financial and other resources to further research and development. In addition, we may experience reluctance or refusal on the part of third-party payors to pay for tests using our PreGen technologies which could slow the demand and/or commercialization of these tests, or commercialization may never occur. Our earlier clinical studies were small and included samples from high-risk or symptomatic patients. The results from these earlier studies may not be representative of the results we obtain from any future studies, including our multi-center study, which will include substantially more samples from average-risk patients.
Scarcity of Raw Materials
We rely on contract manufacturers and suppliers for certain components for our PreGen technologies. We believe that there are relatively few manufacturers that are currently capable of supplying commercial quantities of the raw materials necessary for the current configuration of the PreGen-Plus assay that is intended for commercialization. Although we have identified suppliers that we believe are capable of supplying these raw materials in sufficient quantity today, there can be no assurance that we, or LabCorp, will be able to enter into agreements with such suppliers on a timely basis on acceptable terms, if at all. Furthermore, PreGen-Plus has never been offered on a commercial scale, and there can be no assurance that the raw materials necessary to meet demand will be available in sufficient quantities or on acceptable terms, if at all. If we or LabCorp should encounter delays or difficulties in securing the necessary raw materials for PreGen-Plus, we may need to reconfigure our assay which would result in delays in commercialization or an interruption in sales which could materially adversely impact our revenues.
We may not be able to sustain commercialization of our PreGen technologies if we are not able to lower costs through automating and simplifying key operational processes.
Currently, colorectal cancer screening tests using our PreGen technologies are expensive because they are labor-intensive and use highly complex processes and expensive reagents. In order to generate significant profits and make our PreGen technologies more commercially attractive, we will need to reduce substantially the costs of tests using our PreGen technologies through significant automation of key operational processes and other cost savings procedures. If we fail to create and improve PreGen technologies that sufficiently reduce costs, tests using our PreGen technologies either may not be commercially viable or may generate little, if any, profitability for our partners, or for us.
If Medicare and other third-party payors, including managed care organizations, do not provide adequate reimbursement for our products and services, it is unlikely that most clinical reference laboratories will use our products or license our PreGen technologies to perform cancer screening tests.
Most clinical reference laboratories will not perform colorectal cancer screening tests using our PreGen technologies unless they are adequately reimbursed by third-party payors such as Medicare and managed care organizations. There is significant uncertainty concerning third-party reimbursement for the use of any test incorporating new technology. Reimbursement by a third-party payor may depend on a number of factors, including a payor's determination that tests using our PreGen technologies are sensitive for colorectal cancer, not experimental or investigational, medically necessary, appropriate for the specific patient and cost-effective. While we have had some success in obtaining reimbursement from a limited number of third-party payors for tests performed in-house, to date, we have not secured any broad-based reimbursement approval for tests using our PreGen technologies from any third-party payor, nor do we expect any such approvals in the immediate future.
Reimbursement by Medicare will require a review that may be lengthy and which may be performed under the provisions of a National Coverage Decision process. The Federal Balanced
28
Budget Act of 1997 provides for adding new technologies to the colorectal cancer screening benefit, such as ours, with such frequency and payment limits as the Secretary of Health and Human Services ("HHS") determines appropriate. We cannot guarantee that the Secretary of HHS will act to approve tests based on our PreGen technologies on a timely basis, or at all. While the current procedural terminology codes facilitate Medicare reimbursement, the level of any eventual Medicare reimbursement is unknown at this time.
Since policy level reimbursement approval is required from each payor individually, seeking such approvals is a time-consuming and costly process. If we are unable to obtain adequate reimbursement approval from Medicare and private payors, our ability to generate revenue from our PreGen technologies will be limited.
If we are unable to convince medical practitioners to order tests using our PreGen technologies, our revenue and profitability may be limited.
If we fail to convince medical practitioners to order tests using our PreGen technologies, we will not be able to create sufficient demand for products using our PreGen technologies in sufficient volume for us to become profitable. We will need to make thought-leading gastroenterologists and primary care physicians aware of the benefits of tests using our PreGen technologies through published papers, presentations at scientific conferences and favorable results from our clinical studies. Our failure to be successful in these efforts would make it difficult for us to convince medical practitioners to order colorectal cancer screening tests using our PreGen technologies for their patients.
We may experience limits on our revenue and profitability if only a small number of people decide to be screened for colorectal cancer using our PreGen technologies.
Even if our PreGen technologies are superior to alternative colorectal cancer screening technologies, adequate third-party reimbursement is obtained and medical practitioners order tests using our PreGen technologies, an immaterial number of people may decide to be screened for colorectal cancer. Despite the availability of current colorectal cancer screening methods as well as the recommendations of the American Cancer Society that all Americans age 50 and above be screened for colorectal cancer, most of these individuals decide not to complete a colorectal cancer screening test. If only a small portion of the population decides to utilize colorectal cancer screening tests using our PreGen technologies, we will, despite our efforts, experience limits on our revenue and profitability.
If we or our partners fail to comply with FDA requirements, we may not be able to market our products and services and may be subject to stringent penalties.
The FDA does not actively regulate laboratory tests that have been developed and used by the laboratory to conduct in-house testing. The FDA does regulate specific reagents, some of which are used with our PreGen technologies that react with a biological substance including those designed to identify a specific DNA sequence or protein. The FDA's regulations provide that most such reagents, which the FDA refers to as analyte specific reagents, are exempt from the FDA's pre-market review requirements. We believe that analyte specific reagents that we may provide fall within these exemptions. However, if the FDA were to decide to more actively regulate in-house developed laboratory tests, the commercialization of our products and services could be impacted by being delayed, halted or prevented. If the FDA were to view any of our actions as non-compliant, it could initiate enforcement action such as a regulatory warning letter and possible imposition of penalties. Finally, analyte specific reagents that we may provide will be subject to a number of FDA requirements, including compliance with the FDA's Quality System Regulation, which establishes extensive regulations for quality assurance and control as well as manufacturing procedures. Failure to comply with these regulations could result in enforcement action for us, our partners, or our contract
29
manufacturers. Adverse FDA action in any of these areas could significantly increase our expenses and limit our revenue and profitability.
We may be subject to substantial costs and liability or be prevented from selling our screening tests for cancer as a result of litigation or other proceedings relating to patent rights.
Third parties may assert infringement or other intellectual property claims against our licensors, our suppliers, our licensees, our strategic partners, or us. We pursue an aggressive patent strategy that we believe provides us with a competitive advantage in the early detection of colorectal cancer and other common cancers. As of December 31, 2002, we have 26 issued U.S. patents and 30 pending patent applications in the United States. Because the U.S. Patent & Trademark Office maintains patent applications in secrecy until a patent application publishes or the patent is issued, others may have filed patent applications for technology covered by our pending applications. There may be third-party patents, patent applications and other intellectual property relevant to our potential products that may block or compete with our products or processes. Even if third-party claims are without merit, defending a lawsuit may result in substantial expense to us and may divert the attention of management and key personnel. In addition, we cannot provide assurance that we would prevail in any of these suits or that the damages or other remedies if any, awarded against us would not be substantial. Claims of intellectual property infringement may require that we, or our strategic partners, enter into royalty or license agreements with third parties that may not be available on acceptable terms, if at all. These claims may also result in injunctions against the further development and use of our PreGen technologies, which would have a material adverse effect on our business, financial condition and results of operations.
Additionally, a third-party has asserted claims of patent infringement against certain entities that are anticipated suppliers of materials necessary to the PreGen-Plus assay as it is currently configured. Although to date, no legal proceedings have been initiated against us, if any third party, including the third party discussed above, is successful in challenging the supply of materials needed for the PreGen-Plus assay as it is currently configured, commercialization of our PreGen technologies may be significantly delayed, sales of the PreGen-Plus assay may become interrupted, and our revenue may become impacted.
Also, patents and applications owned by us may become the subject of interference proceedings in the United States Patent and Trademark Office to determine priority of invention, which could result in substantial cost to us, as well as a possible adverse decision as to the priority of invention of the patent or patent application involved. An adverse decision in an interference proceeding may result in the loss of rights under a patent or patent application subject to such a proceeding.
If we fail to gain relief from the U.S. Department of Transportation ("DOT") regulation for the transport of diagnostic specimens, it could increase the cost of transporting stool specimens and limit revenue growth.
On August 14, 2002, the DOT issued revised Hazardous Materials Regulations for the packaging and transport of infectious materials, including diagnostic specimens. In anticipation of the application of these regulations to our current specimen container and transport system, we submitted an exemption request to the DOT to minimize the changes that would be necessary for our specimen collection system, while still providing an equivalent level of safety. On February 13, 2003, the DOT issued a formal determination that stool samples intended for clinical research or diagnostic purpose would not be deemed an infectious substance subject to the Hazardous Materials Regulations. While this decision is favorable to us and our partners, we cannot be certain that the DOT will not more actively regulate or restrict the transportation of stool samples, such as those used in our diagnostic tests.
30
Other companies may develop and market novel or improved methods for detecting colorectal cancer, which may make our PreGen technologies less competitive, or even obsolete.
The market for colorectal cancer screening is large, approximating 80 million Americans age 50 and above, and has attracted competitors, some of which have significantly greater resources than we have. Currently, we face competition from alternative procedures-based detection technologies such as flexible sigmiodoscopy, colonoscopy, virtual colonoscopy, a new procedure being performed in which a radiologist views the inside of the colon through a scanner, as well as existing and improved traditional screening tests such as FOBT. In addition, some competitors are developing serum-based tests, or screening tests based on the detection of proteins or nucleic acids produced by colon cancer. These and other companies may also be working on additional methods of detecting colon cancer that have not yet been announced. We may be unable to compete effectively against these competitors either because their test is superior or because they may have more expertise, experience, financial resources and stronger business relationships.
The loss of key members of our senior management team could adversely affect our business.
Our success depends largely on the skills, experience and performance of key members of our senior management team, including Don M. Hardison, our President and Chief Executive Officer, John A. McCarthy, Jr., our Executive Vice President, Chief Operating Officer, Chief Financial Officer and Treasurer, and Anthony P. Shuber, our Executive Vice President and Chief Technology Officer. Anthony P. Shuber, together with Stanley N. Lapidus, our Chairman, has been critical to the development of our technologies and business. Although Messrs. Hardison, McCarthy and Shuber have each signed a non-disclosure and assignment of intellectual property agreement and a non-compete agreement, they have no employment agreements currently in place. We also have a severance agreement with each of Messrs. Hardison, McCarthy and Shuber that provides for twelve months severance under certain circumstances. The efforts of each of these persons will be critical to us as we continue to develop our technologies and our testing process and as we transition to a company with commercialized products and services. If we were to lose one or more of these key employees, we may experience difficulties in competing effectively, developing our technologies and implementing our business strategies.
If we are unable to protect our intellectual property effectively, we may be unable to prevent third parties from using our PreGen technologies, which would impair our competitive advantage.
We rely on patent protection as well as a combination of trademark, copyright and trade secret protection, and other contractual restrictions to protect our proprietary PreGen technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. If we fail to protect our intellectual property, we will be unable to prevent third parties from using our PreGen technologies and they will be able to compete more effectively against us.
As of December 31, 2002, we have 26 issued patents and 30 pending patent applications in the United States. We also have 8 issued foreign patents and 107 pending foreign patent applications. We cannot assure you that any of our currently pending or future patent applications will result in issued patents, and we cannot predict how long it will take for such patents to be issued. Further, we cannot assure you that other parties will not challenge any patents issued to us, or that courts or regulatory agencies will hold our patents to be valid or enforceable.
31
A third-party institution has asserted co-inventorship rights with respect to one of our issued patents relating to pooling patient samples in connection with our loss of heterozygosity detection method. We cannot guarantee you that we will be successful in defending this or other challenges made in connection with our patents and patent applications. Any successful third-party challenge to our patents could result in co-ownership of such patents with a third party or the unenforceability or invalidity of such patents. In addition, we and a third-party institution have filed a joint patent application that is co-owned by us and that third-party institution relating to the use of various DNA markers, including one of our detection methods, to detect cancers of the lung, pancreas, esophagus, stomach, small intestine, bile duct, naso-pharyngeal, liver and gall bladder in stool under the Patent Cooperation Treaty. This patent application designates the United States, Japan, Europe and Canada. Co-ownership of a patent allows the co-owner to exercise all rights of ownership, including the right to use, transfer and license the rights protected by the applicable patent.
In addition to our patents, we rely on contractual restrictions to protect our proprietary technology. We require our employees and third parties to sign confidentiality agreements and employees to sign agreements assigning to us all intellectual property arising from their work for us. Nevertheless, we cannot guarantee that these measures will be effective in protecting our intellectual property rights.
We cannot guarantee that the patents issued to us will be broad enough to provide any meaningful protection nor can we assure you that one of our competitors may not develop more effective technologies, designs or methods to test for colorectal cancer or any other common cancer without infringing our intellectual property rights or that one of our competitors might not design around our proprietary PreGen technologies.
We may incur substantial costs to protect and enforce our patents.
We have pursued an aggressive patent strategy designed to maximize our patent protection against third parties in the U.S. and in foreign countries. We have filed patent applications that cover the methods we have designed to detect colorectal cancer and other cancers, as well as patent applications that cover our testing process. In order to protect or enforce our patent rights, we may initiate actions against third parties. Any actions regarding patents could be costly and time-consuming, and divert our management and key personnel from our business. Additionally, such actions could result in challenges to the validity or applicability of our patents.
If we lose the support of our key scientific collaborators, it may be difficult to establish tests using our PreGen technologies as a standard of care for colorectal cancer screening, which may limit our revenue growth and profitability.
We have established relationships with leading scientists, including members of our scientific advisory board, and research and academic institutions, such as the Mayo Clinic and John Hopkins University that we believe are key to establishing tests using our PreGen technologies as a standard of care for colorectal cancer screening. If our collaborators determine that colorectal cancer screening tests using our PreGen technologies are not superior to available colorectal cancer screening tests or that alternative technologies would be more effective in the early detection of colorectal cancer, we would encounter significant difficulty establishing tests using our PreGen technologies as a standard of care for colorectal cancer screening, which would limit our revenue growth and profitability.
Our inability to apply our proprietary PreGen technologies successfully to detect other common cancers may limit our revenue growth and profitability.
While to date we have focused substantially all of our research and development efforts on colorectal cancer, we have used our PreGen technologies to detect cancers of the lung, pancreas,
32
esophagus, stomach and gall bladder. As a result, we intend to devote personnel and financial resources in the future to extending our PreGen technology platform to the development of screening tests for these common cancers. To do so, we may need to overcome technological challenges to develop reliable screening tests for these cancers. There can be no assurance that our PreGen technologies will be capable of reliably detecting cancers, beyond colorectal cancer, with the sensitivity and specificity necessary to be clinically and commercially useful for such other cancers, or that we can develop such technologies. We may never realize any benefits from our research and development activities.
Changes in healthcare policy could subject us to additional regulatory requirements that may delay the commercialization of our tests and increase our costs.
Healthcare policy has been a subject of discussion in the executive and legislative branches of the federal and many state governments. We developed our commercialization strategy for PreGen-Plus based on existing healthcare policies. Changes in healthcare policy, if implemented, could substantially delay the use of PreGen-Plus, increase costs, and divert management's attention. We cannot predict what changes, if any, will be proposed or adopted or the effect that such proposals or adoption may have on our business, financial condition and results of operations.
Our inability to raise additional capital on acceptable terms in the future may limit our growth.
Although we believe that the Company will not need to raise additional funds from the capital markets for the foreseeable future, if our capital resources become insufficient to meet future requirements, we will have to raise additional funds to continue the development and commercialization of our PreGen technologies. Our inability to raise capital would seriously harm our business and development efforts. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operations. These funds may not be available on favorable terms, or at all. If adequate funds are not available on attractive terms, we may have to restrict our operations significantly or obtain funds by entering into agreements on unattractive terms. Further, to the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in dilution to our stockholders.
Conviction of Arthur Andersen LLP.
Prior to July 17, 2002, Arthur Andersen LLP ("Arthur Andersen") served as the Company's independent auditors. On March 14, 2002, Arthur Andersen was indicted on federal obstruction of justice charges arising from the government's investigation of Enron Corporation and on June 15, 2002, Arthur Andersen was found guilty. Arthur Andersen informed the SEC that it would cease practicing before the SEC by August 31, 2002, unless the SEC determined that another date was appropriate. On May 7, 2002, the Company dismissed Arthur Andersen and retained Ernst & Young LLP as its independent auditors for its current fiscal year ended December 31, 2002. SEC rules require the Company to present historical audited financial statements in various SEC filings, such as registration statements, along with Arthur Andersen's consent to the Company's inclusion of Arthur Andersen's audit report in those filings. Since the Company's former engagement partner and audit manager have left Arthur Andersen and in light of the announced cessation of Arthur Andersen's SEC practice, the Company will not be able to obtain the consent of Arthur Andersen to the inclusion of Arthur Andersen's audit report in the Company's relevant current and future filings. The SEC recently has provided regulatory relief designed to allow companies that file reports with the SEC to dispense with the requirement to file a consent of Arthur Andersen in certain circumstances, but purchasers of securities sold under the Company's registration statements, which were not filed with the consent of Arthur Andersen to the inclusion of Arthur Andersen's audit report will not be able to sue Arthur Andersen pursuant to Section 11(a)(4) of the Securities Act of 1933 and therefore the purchasers' right
33
of recovery under that section may be limited as a result of the lack of the Company's ability to obtain Arthur Andersen's consent.
Our executive officers, directors and principal stockholders own a significant percentage of our Company and could exert significant influence over matters requiring stockholder approval.
As of December 31, 2002, our executive officers, directors and principal stockholders and their affiliates together control approximately 20.2% of our outstanding common stock, without giving effect to the exercise of outstanding options under our stock plans. As a result, these stockholders, if they act together, will have significant influence over matters requiring stockholder approval, such as the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying, preventing or deterring a change in control, could deprive other stockholders the opportunity to receive a premium for their common stock as part of a sale and could adversely affect the market price of our common stock.
Certain provisions of our charter, by-laws and Delaware law may make it difficult for you to change our management and may also make a takeover difficult.
Our corporate documents and Delaware law contain provisions that limit the ability of stockholders to change our management and may also enable our management to resist a takeover. These provisions include a staggered board of directors, limitations on persons authorized to call a special meeting of stockholders and advance notice procedures required for stockholders to make nominations of candidates for election as directors or to bring matters before an annual meeting of stockholders. These provisions might discourage, delay or prevent a change of control or in our management. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors and cause us to take other corporate actions. In addition, the existence of these provisions, together with Delaware law, might hinder or delay an attempted takeover other than through negotiations with our board of directors.
Our stock price may be volatile.
The market price of our stock is likely to be highly volatile and could fluctuate widely in price in response to various factors, many of which are beyond our control, including:
- •
- technological innovations or new products and services by us or our competitors;
- •
- clinical trial results relating to our tests or those of our competitors;
- •
- reimbursement decisions by Medicare and other managed care organizations;
- •
- FDA regulation of our products and services;
- •
- the establishment of collaborative partnerships;
- •
- health care legislation;
- •
- intellectual property disputes and other litigation;
- •
- additions or departures of key personnel;
- •
- a delay, or anticipated delay, in commercialization of our PreGen technologies; and
- •
- sales of our common stock.
Because we are a company with no operating revenue expected until at least the second half of 2003, you may consider one of these factors to be material. Our stock price may fluctuate widely as a result of any of the above.
34
In addition, the Nasdaq National Market and the market for biotechnology companies in particular, has experienced significant price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies.
Future sales by our existing stockholders could depress the market price of our common stock.
If our existing stockholders sell a large number of shares of our common stock, the market price of our common stock could decline significantly. Moreover, the perception in the public market that our existing stockholders might sell shares of common stock could adversely affect the market price of our common stock.
Item 7a. Quantitative and Qualitative Disclosures about Market Risk
The Company's exposure to market risk is principally confined to its cash, cash equivalents and marketable securities. We invest our cash, cash equivalents and marketable securities in securities of the U.S. governments and its agencies and in investment-grade, highly liquid investments consisting of commercial paper, bank certificates of deposit and corporate bonds, all of which are currently invested in the U.S and are classified as available-for-sale. We place our cash equivalents and marketable securities with high-quality financial institutions, limit the amount of credit exposure to any one institution and have established investment guidelines relative to diversification and maturities designed to maintain safety and liquidity.
Based on a hypothetical ten percent adverse movement in interest rates, the potential losses in future earnings, fair value of risk-sensitive financial instruments, and cash flows are immaterial, although the actual effects may differ materially from the hypothetical analysis.
35
Item 8. Financial Statements and Supplementary Data
EXACT SCIENCES CORPORATION
Index to Financial Statements
| | Page
|
|---|
| Report of Independent Auditors | | 37 |
| Report of Independent Public Accountants | | 38 |
| Consolidated Balance Sheets as of December 31, 2001 and 2002 | | 39 |
| Consolidated Statements of Operations for the Years Ended December 31, 2000, 2001 and 2002 | | 40 |
| Consolidated Statements of Stockholders' Equity the Years Ended December 31, 2000, 2001 and 2002 | | 41 |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2000, 2001 and 2002 | | 42 |
| Notes to Consolidated Financial Statements | | 43 |
36
Report of Independent Auditors
The Board of Directors and Stockholders
EXACT Sciences Corporation
We have audited the accompanying consolidated balance sheet of EXACT Sciences Corporation as of December 31, 2002, and the related consolidated statements of operations, stockholders' equity, and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. The financial statements of EXACT Sciences Corporation for the years ended December 31, 2001 and 2000 were audited by other auditors who have ceased operations and whose report dated January 28, 2002, expressed an unqualified opinion on those statements.
We conducted our audit in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the 2002 consolidated financial statements referred to above present fairly, in all material respects the consolidated financial position of EXACT Sciences Corporation at December 31, 2002, and the consolidated results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States.
/s/ Ernst & Young LLP
Boston, Massachusetts
January 17, 2003
37
THE FOLLOWING REPORT IS A COPY OF THE ACCOUNTANT'S REPORT PREVIOUSLY ISSUED BY ARTHUR ANDERSEN LLP. THIS REPORT HAS NOT BEEN REISSUED BY
ARTHUR ANDERSEN LLP.
Report of Independent Public Accountants
To EXACT Sciences Corporation:
We have audited the accompanying consolidated balance sheets of EXACT Sciences Corporation (a Delaware corporation in the development stage) and subsidiary as of December 31, 2000 and 2001, and the related consolidated statements of operations, stockholders' equity and cash flows for the three years in the period ended December 31, 2001 and the period from inception (February 10, 1995) to December 31, 2001. These financial statements are the responsibility of management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with auditing standards generally accepted in the United States. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of EXACT Sciences Corporation and subsidiary as of December 31, 2000 and 2001, and the results of their operations and their cash flows for the three years in the period ended December 31, 2001 and the period from inception (February 10, 1995) to December 31, 2001, in conformity with accounting principles generally accepted in the United States.
/s/ARTHUR ANDERSEN LLP
Boston, Massachusetts
January 28, 2002
38
EXACT SCIENCES CORPORATION
Consolidated Balance Sheets
(Amounts in thousands, except share and per share data)
| | December 31,
| |
|---|
| | 2001
| | 2002
| |
|---|
| ASSETS | | | | | | | |
| Current Assets: | | | | | | | |
| | Cash and cash equivalents | | $ | 56,843 | | $ | 17,439 | |
| | Marketable securities | | | — | | | 26,407 | |
| | Prepaid expenses | | | 721 | | | 1,110 | |
| | |
| |
| |
| | | Total current assets | | | 57,564 | | | 44,956 | |
| Property and Equipment, at cost: | | | | | | | |
| | Laboratory equipment | | | 2,497 | | | 3,427 | |
| | Office and computer equipment | | | 1,178 | | | 1,278 | |
| | Leasehold improvements | | | 581 | | | 823 | |
| | Furniture and fixtures | | | 212 | | | 272 | |
| | |
| |
| |
| | | | 4,468 | | | 5,800 | |
| Less—Accumulated depreciation and amortization | | | (1,884 | ) | | (3,544 | ) |
| | |
| |
| |
| | | | 2,584 | | | 2,256 | |
| Patent Costs and Other Assets, net of accumulated amortization of approximately $397 and $902 at December 31, 2001 and 2002, respectively | | | 2,952 | | | 2,874 | |
| | |
| |
| |
| | | $ | 63,100 | | $ | 50,086 | |
| | |
| |
| |
| LIABILITIES AND STOCKHOLDERS' EQUITY | | | | | | | |
| Current Liabilities: | | | | | | | |
| | Accounts payable | | $ | 1,176 | | $ | 1,157 | |
| | Accrued expenses | | | 2,407 | | | 2,466 | |
| | Deferred licensing fees, current portion | | | 550 | | | 1,621 | |
| | |
| |
| |
| | | Total current liabilities | | | 4,133 | | | 5,244 | |
| Deferred Licensing Fees, less current portion | | | — | | | 6,493 | |
| Commitments and Contingencies | | | | | | | |
| Stockholders' Equity: | | | | | | | |
| | Common stock, $0.01 par value | | | | | | | |
| | | Authorized—100,000,000 shares | | | | | | | |
| | | Issued and outstanding—18,790,807 and 19,070,767 shares at December 31, 2001 and 2002, respectively | | | 188 | | | 191 | |
| | Additional paid-in capital | | | 110,497 | | | 117,256 | |
| | Treasury stock, at cost, 50,646 and 60,959 shares at December 31, 2001 and 2002, respectively | | | (8 | ) | | (12 | ) |
| | Notes receivable | | | (947 | ) | | (699 | ) |
| | Deferred compensation | | | (4,179 | ) | | (1,977 | ) |
| | Unrealized gain on marketable securities | | | — | | | 57 | |
| | Accumulated deficit | | | (46,584 | ) | | (76,467 | ) |
| | |
| |
| |
| | | Total stockholders' equity | | | 58,967 | | | 38,349 | |
| | |
| |
| |
| | | $ | 63,100 | | $ | 50,086 | |
| | |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
39
EXACT SCIENCES CORPORATION
Consolidated Statements of Operations
(Amounts in thousands, except per share data)
| | Year Ended December 31,
| |
|---|
| | 2000
| | 2001
| | 2002
| |
|---|
| Revenue | | $ | — | | $ | 51 | | $ | 897 | |
| Cost of revenues | | | — | | | — | | | 9 | |
| | |
| |
| |
| |
| | | Gross margin | | | — | | | 51 | | | 888 | |
| Operating Expenses: | | | | | | | | | | |
| | Research and development | | | 5,332 | | | 13,335 | | | 19,989 | |
| | Selling, general and administrative | | | 4,814 | | | 9,078 | | | 9,701 | |
| | Stock-based compensation (1) | | | 3,184 | | | 3,788 | | | 2,043 | |
| | |
| |
| |
| |
| | | | 13,330 | | | 26,201 | | | 31,733 | |
| | |
| |
| |
| |
| | | Loss from operations | | | (13,330 | ) | | (26,150 | ) | | (30,845 | ) |
| Interest income | | | 1,447 | | | 2,665 | | | 962 | |
| | |
| |
| |
| |
| | | Net loss | | $ | (11,883 | ) | $ | (23,485 | ) | $ | (29,883 | ) |
| | |
| |
| |
| |
| Net loss per share—basic and diluted: | | $ | (8.13 | ) | $ | (1.42 | ) | $ | (1.62 | ) |
| | |
| |
| |
| |
| Weighted average common shares outstanding—basic and diluted | | | 1,462 | | | 16,487 | | | 18,433 | |
| | |
| |
| |
| |
- (1)
- The following summarizes the departmental allocation of
stock-based compensation:
| | Research and development | | $ | 810 | | $ | 898 | | $ | 478 |
| | Selling, general and administrative | | | 2,374 | | | 2,890 | | | 1,565 |
| | |
| |
| |
|
| | | Total | | $ | 3,184 | | $ | 3,788 | | $ | 2,043 |
| | |
| |
| |
|
The accompanying notes are an integral part of these consolidated financial statements.
40
EXACT SCIENCES CORPORATION
Consolidated Statements of Stockholders' Equity
(Amounts in thousands, except share data)
| | Series A
Convertible
Preferred Stock
| | Series B
Convertible
Preferred Stock
| | Series C
Convertible
Preferred Stock
| | Series D
Convertible
Preferred Stock
| | Common Stock
| |
| | Treasury Stock
| |
| |
| |
| |
| |
| |
|---|
| | Number of
Shares
| | $0.01
Par Value
| | Number of
Shares
| | $0.01
Par Value
| | Number of
Shares
| | $0.01
Par Value
| | Number of
Shares
| | $0.01
Par Value
| | Number of
Shares
| | $0.01
Par Value
| | Additional
Paid-In
Capital
| | Number of
Shares
| | Value
| | Notes
Receivable
| | Deferred
Compensation
| | Deficit
Accumulated
| | Total
Stockholders'
Equity
| | Other
Comprehensive
Income
| |
|---|
| Balance, December 31, 1999 | | 902,414 | | $ | 9 | | 996,196 | | $ | 10 | | 1,007,186 | | $ | 10 | | — | | $ | — | | 1,582,848 | | $ | 16 | | $ | 15,675 | | — | | $ | — | | $ | (40 | ) | $ | (54 | ) | $ | (11,216 | ) | $ | 4,410 | | $ | — | |
| Sale of Series D convertible preferred stock, net of issuance costs of $172 | | — | | | — | | — | | | — | | — | | | — | | 1,417,534 | | | 14 | | — | | | — | | | 31,708 | | — | | | — | | | — | | | — | | | — | | | 31,722 | | | — | |
| Sale of common stock | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 48,125 | | | — | | | 18 | | — | | | — | | | — | | | — | | | — | | | 18 | | | — | |
| Repurchase of common stock | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | 27,844 | | | (5 | ) | | 5 | | | — | | | — | | | — | | | — | |
| Retirement of treasury stock | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | (27,841 | ) | | — | | | (5 | ) | (27,844 | ) | | 5 | | | — | | | — | | | — | | | — | | | — | |
| Exercise of common stock options | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 1,186,449 | | | 12 | | | 1,177 | | — | | | — | | | (1,023 | ) | | — | | | — | | | 166 | | | — | |
| Repayment of subscription receivable | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | 82 | | | — | | | — | | | 82 | | | — | |
| Compensation expense related to issuance of stock options | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 11,359 | | — | | | — | | | — | | | (8,524 | ) | | — | | | 2,835 | | | — | |
| Non-cash cost related to issuance of warrant | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 349 | | — | | | — | | | — | | | — | | | — | | | 349 | | | — | |
| Net loss | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | — | | | — | | | (11,883 | ) | | (11,883 | ) | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, December 31, 2000 | | 902,414 | | | 9 | | 996,196 | | | 10 | | 1,007,186 | | | 10 | | 1,417,534 | | | 14 | | 2,789,581 | | | 28 | | | 60,281 | | — | | | — | | | (976 | ) | | (8,578 | ) | | (23,099 | ) | | 27,699 | | | — | |
| Repurchase of common stock | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | 50,646 | | | (8 | ) | | — | | | — | | | — | | | (8 | ) | | — | |
| Issuance shares under stock purchase plan | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 4,737 | | | — | | | 32 | | — | | | — | | | — | | | — | | | — | | | 32 | | | — | |
| Exercise of common stock options | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 107,354 | | | 1 | | | 164 | | — | | | — | | | (50 | ) | | — | | | — | | | 115 | | | — | |
| Sale of common stock at initial public offering, net of issuance costs of $5,442 | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 4,000,000 | | | 40 | | | 50,518 | | — | | | — | | | — | | | — | | | — | | | 50,558 | | | — | |
| Repayment of subscription receivable | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | 79 | | | — | | | — | | | 79 | | | — | |
| Conversion of convertible preferred stock into common stock at initial public offering | | (902,414 | ) | | (9 | ) | (996,196 | ) | | (10 | ) | (1,007,186 | ) | | (10 | ) | (1,417,534 | ) | | (14 | ) | 11,889,135 | | | 119 | | | (76 | ) | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| Compensation expense related to issuance of stock options | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | (610 | ) | — | | | — | | | — | | | 4,399 | | | — | | | 3,789 | | | — | |
| Non-cash cost related to issuance of warrant | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 188 | | — | | | — | | | — | | | — | | | — | | | 188 | | | — | |
| Net loss | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | — | | | — | | | (23,485 | ) | | (23,485 | ) | | — | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Balance, December 31, 2001 | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 18,790,807 | | | 188 | | | 110,497 | | 50,646 | | | (8 | ) | | (947 | ) | | (4,179 | ) | | (46,584 | ) | | 58,967 | | | — | |
| Repurchase of common stock | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | 10,313 | | | (4 | ) | | — | | | — | | | — | | | (4 | ) | | — | |
| Issuance shares under stock purchase plan | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 17,840 | | | — | | | 137 | | — | | | — | | | — | | | — | | | — | | | 137 | | | — | |
| Exercise of common stock options | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | 262,120 | | | 3 | | | 231 | | — | | | — | | | — | | | — | | | — | | | 234 | | | — | |
| Repayment of subscription receivable | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | 248 | | | — | | | — | | | 248 | | | — | |
| Compensation expense related to issuance (forfeitures) of stock options | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | (159 | ) | — | | | — | | | — | | | 2,202 | | | — | | | 2,043 | | | — | |
| Non-cash cost related to issuance of warrant | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | 6,550 | | — | | | — | | | — | | | — | | | — | | | 6,550 | | | — | |
| Net loss | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | — | | | — | | | (29,883 | ) | | (29,883 | ) | | (29,883 | ) |
| Other comprehensive income | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | — | | | — | | | — | | — | | | — | | | — | | | — | | | — | | | — | | | 57 | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Comprehensive loss | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | $ | (29,826 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| |
| Balance, December 31, 2002 | | — | | $ | — | | — | | $ | — | | — | | $ | — | | — | | $ | — | | 19,070,767 | | $ | 191 | | $ | 117,256 | | 60,959 | | $ | (12 | ) | $ | (699 | ) | $ | (1,977 | ) | $ | (76,467 | ) | $ | 38,292 | | | | |
| | |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| |
| | | | |
The accompanying notes are an integral part of these consolidated financial statements.
41
EXACT SCIENCES CORPORATION
Consolidated Statements of Cash Flows
(Amounts in thousands)
| | Year Ended December 31,
| |
|---|
| | 2000
| | 2001
| | 2002
| |
|---|
| Cash Flows from Operating Activities: | | | | | | | | | | |
| | Net loss | | $ | (11,883 | ) | $ | (23,485 | ) | $ | (29,883 | ) |
| | | Adjustments to reconcile net loss to net cash used in operating activities— | | | | | | | | | | |
| | | | Depreciation | | | 326 | | | 895 | | | 1,663 | |
| | | | Amortization | | | 78 | | | 173 | | | 495 | |
| | | | Non-cash stock-based compensation expense | | | 2,834 | | | 3,788 | | | 2,043 | |
| | | | Amortization of deferred licensing fees | | | — | | | (50 | ) | | (886 | ) |
| | | | Non-cash expense associated with the issuance of warrants | | | 350 | | | — | | | — | |
| | | Changes in assets and liabilities: | | | | | | | | | | |
| | | | Prepaid expenses | | | (712 | ) | | 17 | | | (389 | ) |
| | | | Accounts payable | | | 386 | | | 594 | | | (19 | ) |
| | | | Deferred license fees | | | — | | | 600 | | | 15,000 | |
| | | | Accrued expenses | | | 630 | | | 1,631 | | | 59 | |
| | |
| |
| |
| |
| | | | | Net cash used in operating activities | | | (7,991 | ) | | (15,837 | ) | | (11,917 | ) |
| Cash Flows from Investing Activities: | | | | | | | | | | |
| | Purchase of marketable securities | | | — | | | — | | | (26,350 | ) |
| | Purchases of property and equipment | | | (763 | ) | | (2,615 | ) | | (1,335 | ) |
| | Increase in patent costs and other assets | | | (318 | ) | | (1,951 | ) | | (417 | ) |
| | |
| |
| |
| |
| | | | | Net cash used in investing activities | | | (1,081 | ) | | (4,566 | ) | | (28,102 | ) |
| Cash Flows from Financing Activities: | | | | | | | | | | |
| | Proceeds from exercise of common stock
options and stock purchase plan | | | 166 | | | 147 | | | 371 | |
| | Repayment of notes receivable | | | 82 | | | 79 | | | 248 | |
| | Purchase of treasury shares | | | — | | | (8 | ) | | (4 | ) |
| | Net proceeds from sale of common stock | | | 18 | | | 50,558 | | | — | |
| | Net proceeds from sale of convertible preferred stock | | | 31,723 | | | — | | | — | |
| | |
| |
| |
| |
| | | | | Net cash provided by financing activities | | | 31,989 | | | 50,776 | | | 615 | |
| | |
| |
| |
| |
| Net Increase (Decrease) in Cash and Cash Equivalents | | | 22,917 | | | 30,373 | | | (39,404 | ) |
| Cash and Cash Equivalents, beginning of period | | | 3,553 | | | 26,470 | | | 56,843 | |
| | |
| |
| |
| |
| Cash and Cash Equivalents, end of period | | $ | 26,470 | | $ | 56,843 | | $ | 17,439 | |
| | |
| |
| |
| |
Supplemental Disclosure of Non-Cash Investing and
Financing Activities: | | | | | | | | | | |
| | Sale of restricted stock through issuance of notes receivable | | $ | 1,023 | | $ | 50 | | $ | — | |
| | |
| |
| �� |
| |
| | Issuance of warrants | | $ | — | | $ | 188 | | $ | 6,550 | |
| | |
| |
| |
| |
The accompanying notes are an integral part of these consolidated financial statements.
42
EXACT SCIENCES CORPORATION
Notes to Consolidated Financial Statements December 31, 2002
(Amounts in thousands, except share and per share data)
(1) ORGANIZATION
EXACT Sciences Corporation (the "Company") was incorporated on February 10, 1995. The Company applies proprietary genomics technologies to the early detection of several types of common cancers. The Company has selected colorectal cancer as the first application of its technology platform. The Company is currently devoting a majority of its efforts toward research and development primarily related to the completion of several large in-process multi-centered clinical trials and has begun plans to market its proprietary, non-invasive technology for the early detection of colorectal cancer in the average-risk population, PreGen-Plus™. Prior to this year, the Company considered itself a development stage company. With the Company signing its first major licensing agreement as discussed below, the Company no longer considers itself a development stage company, and as such, has reflected this presentation for its financial statements for all years presented.
On February 5, 2001, the Company completed an initial public offering of 4,000,000 shares of its common stock at $14.00 per share. The Company received net proceeds of approximately $50,558 after deducting the underwriters' commission and issuance costs. Upon consummation of the initial public offering, all previously issued shares of preferred stock outstanding automatically converted into 11,889,135 shares of common stock.
(2) SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of the Company's wholly owned subsidiary, EXACT Sciences Securities Corporation, a Massachusetts securities corporation. All significant intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of 90 days or less at the time of acquisition to be cash equivalents. Cash equivalents primarily consist of money market funds at December 31, 2001 and 2002.
Marketable Securities
The Company accounts for its investments in marketable securities in accordance with Statement of Financial Accounting Standards ("SFAS") No. 115,Accounting for Certain Investments in Debt and Equity Securities. Management determines the appropriate classification of debt securities at the time of purchase and re-evaluates such designation as of each balance sheet date. Debt securities are classified as held-to-maturity when the Company has the positive intent and ability to hold the securities to maturity. Marketable equity securities and debt securities not classified as held-to-maturity are classified
43
as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses, net of tax, reported in other comprehensive income. The amortized cost of debt securities in this category is adjusted for amortization of premiums and accretion of discounts to maturity computed under the effective interest method. Such amortization is included in investment income. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities are included in investment income. The cost of securities sold is based on the specific identification method. Interest and dividends on securities classified as available-for-sale are included in investment income.
All of the Company's investments are comprised of fixed income investments and all are deemed available-for-sale. The objectives of this portfolio are to provide liquidity and safety of principal while striving to achieve the highest rate of return, consistent with these two objectives. The Company's investment policy limits investments to certain types of instruments issued by institutions with investment grade credit ratings and places restrictions on maturities and concentration by type and issuer. Available-for-sale securities consist of corporate debt securities as of December 31, 2002 of which $22,229 and $4,178 mature in 2003 and 2004, respectively.
For the year ended December 31, 2002, the gross unrealized gains on available-for-sale securities totaled approximately $57 while there were no realized gains or losses on the sales of available-for sale securities.
Depreciation and Amortization
Depreciation and amortization of fixed assets is computed using the straight-line method based on the estimated useful lives of the related assets, as follows:
Asset Classification
| | Estimated
Useful Life
|
|---|
| Laboratory equipment | | 3 years |
| Office and computer equipment | | 3 years |
| Leasehold improvements | | Lesser of the remaining
lease life or useful life |
| Furniture and fixtures | | 3 years |
Patent Costs
Patent costs, which historically consisted of related legal fees, are capitalized as incurred and are amortized beginning when patents are approved over an estimated useful life of five years. In November 2001, however, the Company purchased intellectual property of MT Technologies (formerly known as Mosaic Technologies, Inc.) relating to its Hybrigel technology which consisted of 4 issued patents and 40 pending patent applications. The purchase price for the assets included $1,250 in cash and warrants to purchase 40,000 shares of common stock immediately, exercisable over a three-year period, at an exercise price of $7.33 per share which the Company valued at $188 in accordance with Emerging Issues Task Force 96-18, Accounting for Equity Instruments that are Issued to Other than Employees for Acquiring, or in Conjunction with Selling Goods or Services, using the Black-Scholes option pricing model. During the second quarter of 2002, these two warrants were exercised utilizing the net
44
settlement (cashless) election per the warrant agreements, which resulted in the Company issuing 19,881 shares of common stock. Capitalized patent costs are expensed upon disapproval or upon a decision by the Company to no longer pursue the patent. Other assets principally consist of license fees and deposits. The amortization of those patents that we have currently capitalized and are amortizing at as of December 31, 2002 will result in amortization as follows:
Year
| | Amount
|
|---|
| 2003 | | $ | 495 |
| 2004 | | | 419 |
| 2005 | | | 396 |
| 2006 | | | 343 |
| 2007 | | | 13 |
| | |
|
| Total | | $ | 1,666 |
| | |
|
The Company has approximately $1.1 million of additional intangible assets as of December 31, 2002 that have not yet commenced amortization due to uncertainty as to the timing of issuance, and are therefore, not included in the table above.
The Company applies SFAS No. 144,Accounting for the Impairment or Disposal of Long-Lived Assets and for Long-Lived Asset, which requires the Company to continually evaluate whether events or circumstances have occurred that indicate that the estimated remaining useful life of long-lived assets and certain identifiable intangibles and goodwill may warrant revision or that the carrying value of these assets may be impaired.
Net Loss Per Share
Basic and diluted net loss per share is presented in conformity with SFAS No. 128,Earnings per Share, for all periods presented. In accordance with SFAS No. 128, basic net loss per common share was determined by dividing net loss applicable to common stockholders by the weighted average common shares outstanding during the period, less shares subject to repurchase. Basic and diluted net loss per share are the same because all outstanding common stock equivalents have been excluded as they are anti-dilutive. All shares issuable upon conversion of outstanding preferred stock and exercise of stock options to purchase 1,771,621; 2,228,077; and 2,892,291 common shares, unvested restricted common shares of 996,806; 649,963; and 342,391, and outstanding warrants to purchase common shares of 48,125; 88,125; and 1,000,000, have therefore been excluded from the computations of diluted weighted average shares outstanding for the years ended December 31, 2000, 2001 and 2002, respectively.
In accordance with the Securities Exchange Commission ("SEC") Staff Accounting Bulletin ("SAB") No. 98,Earnings Per Share in an Initial Public Offering, the Company has determined that there were no nominal issuances of the Company's common stock prior to the Company's initial public offering.
45
Pro Forma Net Loss
The Company's historical capital structure is not indicative of its capital structure subsequent to its initial public offering due to the automatic conversion of all shares of preferred stock into 11,889,135 shares of common stock concurrent with the closing of the Company's initial public offering on February 5, 2001. Accordingly, pro forma net loss per share is presented below for the years ended December 31, 2000 and 2001, assuming the conversion of all outstanding shares of preferred stock into common stock upon the closing of the Company's initial public offering using the if-converted method from the respective dates of issuance.
| | Year Ended December 31,
| |
|---|
| | 2000
| | 2001
| |
|---|
| Net loss | | $ | (11,883 | ) | $ | (23,485 | ) |
| Weighted average common shares outstanding | | | 1,462 | | | 16,487 | |
| Weighted conversion of preferred stock to common stock | | | 10,849 | | | 1,024 | |
| | |
| |
| |
| | Pro forma weighted average common shares outstanding | | | 12,311 | | | 17,511 | |
| | |
| |
| |
| Pro forma basic and diluted net loss per share | | $ | (0.96 | ) | $ | (1.34 | ) |
| | |
| |
| |
Accounting for Stock-Based Compensation
The Company accounts for its stock-based compensation plan under Accounting Principal Bulletin Opinion ("APB") No. 25,Accounting for Stock Issued to Employees. SFAS No. 123,Accounting for Stock-Based Compensation, establishes the fair-value-based method of accounting for stock-based compensation plans. The Company has adopted the disclosure-only alternative for options granted to employees and directors under SFAS No. 123, which requires disclosure of the pro forma effects on earnings as if SFAS No. 123 had been adopted, as well as certain other information. Options granted to scientific advisory board members and other non-employees are recorded at fair value based on the fair value measurement criteria of SFAS No. 123. Compensation expense, computed using the Black-Scholes option pricing model, of $529, $167 and $68 was recorded in the accompanying consolidated statements of operations for the years ended December 31, 2000, 2001 and 2002, respectively
In connection with certain 1999 and 2000 stock option grants to employees and directors, the Company recorded deferred compensation of $52 and $11,359 during the years ended December 31, 1999 and 2000, respectively. The deferred compensation represents the aggregate difference between the option exercise price and the estimated fair value of the common stock on the date of grant and is being charged to operations over the related vesting period using the accelerated method prescribed under FASB Interpretation 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans—An Interpretation of APB Opinion Nos. 15 and 25.
The Company has computed the pro forma disclosures required under SFAS No. 123 for all stock options granted to employees and directors of the Company as of December 31, 2000, 2001 and 2002, using the Black-Scholes option pricing model prescribed by SFAS No. 123.
46
The assumptions used for the years ended December 31, 2000, 2001 and 2002 are as follows:
| | December 31,
|
|---|
| | 2000
| | 2001
| | 2002
|
|---|
| Risk-free interest rates | | 4.98%–6.16% | | 2.86%–4.98% | | 1.71%–3.71% |
| Expected lives | | 7 years | | 7 years | | 7 years |
| Expected volatility | | 100% | | 100% | | 100% |
| Dividend yield | | 0% | | 0% | | 0% |
| Weighted average fair value of grants | | $2.91 | | $3.07 | | $3.61 |
The effect of applying SFAS No. 123 would be as follows:
| | December 31,
| |
|---|
| | 2000
| | 2001
| | 2002
| |
|---|
| Net loss as reported | | $(11,883 | ) | $(23,485 | ) | $(29,883 | ) |
| Add: Stock-based compensation included in reported net loss | | 3,184 | | 3,788 | | 2,043 | |
| Deduct: Total stock based employee compensation determined under SFAS 123 for all awards | | (3,256 | ) | (6,918 | ) | (5,524 | ) |
| | |
| |
| |
| |
| Pro forma net loss—SFAS 123 | | $(11,955 | ) | $(26,615 | ) | $(33,364 | ) |
| | |
| |
| |
| |
| Basic and diluted net loss per share: | | | | | | | |
| | As reported | | $(8.13 | ) | $(1.42 | ) | $(1.62 | ) |
| | |
| |
| |
| |
| | Pro forma—SFAS 123 | | $(8.18 | ) | $(1.61 | ) | $(1.81 | ) |
| | |
| |
| |
| |
Revenue Recognition
The Company's revenue for the years ended December 31, 2001 and 2002 is primarily composed of amortization of up-front technology license fees associated with the Company's two strategic alliance agreements with Laboratory Corporation of America® Holdings ("LabCorp®") which are being amortized on a straight-line basis over the license periods (Note 3). Fees for the licensing of product rights on initiation of strategic agreements are recorded as deferred revenue upon receipt and recognized as income on a straight-line basis over the license period. As such, the Company will amortize the first two payments of $15,000 from LabCorp, net of the $6,550 value of the warrant, as license fee revenue over the remaining exclusive license period. Revenue from milestone and other performance-based payments, as well as per-test royalty fees on each test performed will be recognized as revenue when earned and collection of the receivable is probable.
The Company recognizes revenue from performing tests in its facility upon delivery of the results to the prescribing physician provided there is persuasive evidence of an agreement, the fee is fixed or determinable and collection of the receivable is probable.
47
Clinical Trial Accrual
The Company accrues the estimated cost of patient recruitment associated with its large multi-center clinical trial as patients are enrolled in the trial. These costs consist primarily of payments made to the clinical centers, investigators and patients for participating in the Company's clinical trial. As actual or expected costs become known, they may differ from estimated costs previously accrued for and this clinical trial accrual would be adjusted accordingly.
Advertising Costs
The Company expenses the costs of media advertising at the time the advertising take place.
Comprehensive Income
SFAS No. 130,Reporting Comprehensive Income, establishes presentation and disclosure requirements for comprehensive income (loss). For the Company, comprehensive loss consists of net loss and the change in unrealized gains and losses on marketable securities. Prior to December 31, 2002, the Company's net loss equaled its comprehensive loss.
Segment Information
SFAS No. 131,Disclosures about Segments of an Enterprise and Related Information, requires companies to report selected information about operating segments, as well as enterprise-wide disclosures about products, services, geographic areas and major customers. Operating segments are determined based on the way management organizes its business for making operating decisions and assessing performance. The Company's chief decision-maker, as defined under SFAS No. 131, is a combination of the President and Chief Executive Officer and Chief Financial Officer. The Company has determined that it conducts its operations in one business segment. The Company conducts its business in the United States. As a result, the financial information disclosed herein represents all of the material financial information related to the Company's principal operating segment.
Fair Value of Financial Instruments
SFAS No. 107,Disclosures about Fair Value of Financial Instruments, requires disclosures about fair value of financial instruments. Financial instruments consist of cash, cash equivalents, marketable securities, accounts payable and capital lease obligations. Marketable securities are carried at fair value. The estimated fair value of all other financial instruments approximates their carrying values due to their short-term maturity.
Concentration of Credit Risk
SFAS No. 105,Disclosure of Information about Financial Instruments with Off-Balance-Sheet Risk and Financial Instruments with Concentrations of Credit Risk, requires disclosure of any significant off-balance-sheet risk and credit risk concentration. The Company has no significant off-balance-sheet risk, such as foreign exchange contracts or other hedging arrangements. Financial instruments that subject the Company to credit risk consist of cash, cash equivalents and marketable securities. The Company maintains its cash equivalents with financial institutions with high credit ratings.
48
(3) STRATEGIC ALLIANCE AGREEMENT
On June 26, 2002, the Company entered into a license agreement with LabCorp for an exclusive, long-term strategic alliance between the parties to commercialize PreGen-Plus, the Company's proprietary, non-invasive DNA-based technology for the early detection of colorectal cancer in the average-risk population. Pursuant to the agreement, the Company licensed to LabCorp all U.S. and Canadian patents and patent applications owned by the Company relating to its PreGen™ technology. The license is exclusive for a five-year period, followed by a non-exclusive license for the life of the patents. In return for the license, LabCorp has agreed to pay the Company certain upfront, milestone and performance-based payments, and a per-test royalty fee. LabCorp made an initial payment of $15,000 upon the signing of the agreement, and a second payment of $15,000 is to be made upon the commercial launch of PreGen-Plus. In addition, the Company may be eligible for milestone payments from LabCorp up to $30,000 based upon Company deliverables related to scientific acceptance, reimbursement approval and technology improvements and up to $15,000 based upon the achievement of significant LabCorp revenue thresholds. In addition to these payments, the Company will receive a royalty fee for each PreGen-Plus test performed by LabCorp. In conjunction with the strategic alliance, the Company has issued to LabCorp a warrant to purchase 1,000,000 shares of its common stock, exercisable over a three-year period at an exercise price of $16.09 per share. The Company assigned a value to the warrant of $6,550 under the Black-Scholes option-pricing model which has been recorded as a reduction in the initial upfront deferred license fee of $15,000.
The Company will amortize the first two payments of $15,000, net of the $6,550 value of the warrant, as license fee revenue over the remaining exclusive license period. The milestone and performance-based payments, as well as the royalty fee on each test performed by LabCorp, will be recorded as revenue when earned and collection is probable.
(4) INCOME TAXES
The Company accounts for income taxes in accordance with SFAS No. 109,Accounting for Income Taxes. Under SFAS No. 109, deferred tax assets or liabilities are computed based on the differences between the financial statement and income tax bases of assets and liabilities using the enacted tax rates. Deferred income tax expense or benefit represents the change in the deferred tax assets or liabilities from period to period. At December 31, 2002, the Company had net operating loss and research tax credit carryforwards of approximately $46,274 and $1,326, respectively, for financial reporting purposes, which may be used to offset future taxable income. The carryforwards expire through 2022 and are subject to review and possible adjustment by the Internal Revenue Service. The Internal Revenue Code contains provisions that may limit the net operating loss and research tax credit carryforwards in the event of certain changes in the ownership interests of significant stockholders.
49
The components of the net deferred tax asset with the approximate income tax effect of each type of carryforward, credit and temporary differences are as follows:
| | December 31,
| |
|---|
| | 2001
| | 2002
| |
|---|
| Deferred tax assets: | | | | | | | |
| | Operating loss carryforwards | | $ | 14,811 | | $ | 18,329 | |
| | Tax credit carryforwards | | | 943 | | | 1,326 | |
| | Deferred revenue | | | 218 | | | 3,214 | |
| | Other temporary differences | | | 3,597 | | | 3,522 | |
| | |
| |
| |
| Tax assets before valuation allowance | | | 19,569 | | | 26,391 | |
| Less—valuation allowance | | | (19,569 | ) | | (26,391 | ) |
| | |
| |
| |
| Net deferred tax asset | | $ | — | | $ | — | |
| | |
| |
| |
The Company has recorded a full valuation allowance against its net deferred tax asset because, based on the weight of available evidence, the Company believes it is more likely than not that the deferred tax assets will not be realized in the future.
(5) NOTES RECEIVABLE
Prior to the initial public offering in February 2001, the Company had issued more than 2.2 million restricted common shares to employees, primarily as a result of early exercise of common stock options. The shares were sold at the then fair market value or the exercise price of the common stock options and vested over the remaining option vesting period or, generally, three to five years. At December 31, 2002, 342,391 common shares were still restricted.
The Company issued full recourse notes receivable to various employees and executives for the purchase of the restricted stock. The notes originally had interest rates ranging from 8.5% to 9.5% with principal and interest payments due over a five to ten year period. In December 2001, the Company elected to reduce the prospective interest rate on all notes receivable to executives and employees to 5% to reflect the current interest rate environment and individual borrowing rates. All other provisions of the notes remained in effect.
(6) RELATED PARTY TRANSACTIONS
In February 1998, the Company entered into a letter agreement with one of its stockholders, the Mayo Foundation, for a clinical study. The Company paid approximately $229 during the year ended December 31, 2000 related to this study and recorded this as research and development expense. In December 2000, the Company issued a warrant to the same stockholder to purchase 48,125 shares of common stock at an exercise price of $10.91 per share. The Company valued the warrant using the Black-Scholes model and recorded as research and development stock-based compensation of $349 in 2000. During the second quarter of 2002, this warrant was exercised utilizing the net settlement (cashless) election per the warrant agreement, which resulted in the Company issuing 11,334 shares of common stock.
50
In October 2001, the Company signed a Clinical Trial Agreement with the Mayo Foundation and Mayo Clinic pursuant to which the Company's genomics-based colorectal cancer technology (PreGen™) will be the subject of an independent study by Mayo Clinic. This three-year study will involve approximately 4,000 patients at average risk for developing colorectal cancer and compare the results of Company's non-invasive, genomics-based screening technology with those of the fecal occult blood test, a common first-line colorectal cancer screening option. Using its proprietary PreGen technologies, the Company agreed to process all the stool samples at its CLIA-approved laboratory and to pay total fees of $654 over approximately three years. The Company paid approximately $109 and $218 to the Mayo Clinic for the years ended December 31, 2001 and 2002, respectively, related to this study and recorded these as research and development expense as incurred.
In March 2001, the Company entered into a consulting agreement with a member of its Board of Directors. The Company paid approximately $37 and $55 for services provided under the agreement for the years ended December 31, 2001 and 2002, respectively.
(8) EMPLOYEE BENEFIT PLAN
The Company maintains a qualified 401(k) retirement savings plan (the "401(k) Plan") covering all employees. Under the 401(k) Plan, the participants may elect to defer a portion of their compensation, subject to certain limitations. Company matching contributions may be made at the discretion of the Board of Directors. There have been no discretionary contributions made by the Company to the 401(k) Plan through December 31, 2002.
51
(9) EMPLOYEE STOCK PURCHASE PLAN
The 2000 Employee Stock Purchase Plan (the "2000 Purchase Plan") provides for the issuance of up to an aggregate of 300,000 shares of common stock to participating employees. The 2000 Purchase Plan provides that the number of shares authorized for issuance will automatically increase on each February 1, by the greater of 0.75% of the outstanding number of shares of common stock on the immediately preceding December 31, or that number of shares issued during the one-year period prior to such February 1, or such lesser number as may be approved by the Board of Directors.
The compensation committee of the Board of Directors administers the 2000 Purchase Plan. Generally, all employees who have completed three months of employment and whose customary employment is more than 20 hours per week and for more than five months in any calendar year are eligible to participate in the 2000 Purchase Plan. The right to purchase common stock under the 2000 Purchase Plan will be made available through a series of offerings. Participating employees will be required to authorize an amount, between 1% and 10% of the employee's compensation, to be deducted from the employee's pay during the offering period. On the last day of the offering period, the employee will be deemed to have exercised the option, at the option exercise price, to the extent of accumulated payroll deductions. Under the terms of the 2000 Purchase Plan, the option exercise price is an amount equal to 85% of the fair market value, as defined under the plan, of one share of common stock on either the first or last day of the offering period, whichever is lower. No employee may be granted an option that would permit the employee's rights to purchase common stock to accrue in excess of $25,000 in any calendar year. The first offering period under the 2000 Purchase Plan commenced on the date at which shares were issued in connection with the Company's initial public offering of its common stock (January 30, 2001) and continued through July 31, 2001. Thereafter, the offering periods will begin on each February 1 and August 1. Options granted under the 2000 Purchase Plan terminate upon an employee's voluntary withdrawal from the plan at any time or upon termination of employment. The Company issued the following shares of common stock under the 2000 Purchase Plan.
Offering Period Ended
| | Number
of Shares
| | Price
Per Share
|
|---|
| July 31, 2001 | | 4,737 | | $ | 6.85 |
| January 31, 2002 | | 7,388 | | $ | 6.86 |
| July 31, 2002 | | 10,422 | | $ | 8.32 |
(10) STOCK OPTION PLANS
1995 Stock Option Plan
Under the 1995 stock option plan (the "1995 Option Plan"), the Board of Directors could grant incentive and non-qualified stock options to purchase an aggregate of 3,987,500 shares of common stock to employees and consultants of the Company. Non-qualified stock options may be granted to any employee or consultant of the Company. The exercise price of each option is determined by the Board of Directors. Incentive stock options may not be less than the fair market value of the stock on the date of grant, as defined by the Board of Directors. Options granted under the 1995 Option Plan vest over a three-to-five-year period and expire 10 years from the grant date.
The 1995 Option Plan was terminated on January 31, 2001, the effective date of the Company's registration statement in connection with its initial public offering. Options granted prior to the date of
52
termination will remain outstanding and may be exercised in accordance with their terms, unless sooner terminated by vote of the Board of Directors. At December 31, 2002, 1,358,496 shares were outstanding under the 1995 Option Plan.
2000 Stock Option Plan
The Company adopted the 2000 Stock Option and Incentive Plan (the "2000 Option Plan") on October 17, 2000. At December 31, 2002, a total of 1,935,957 shares of common stock have been authorized and reserved for issuance under the 2000 Option Plan. The 2000 Option Plan provides that the number of shares authorized for issuance will automatically increase on each January 1, by the greater of 5% of the outstanding number of shares of common stock on the preceding December 31, or that number of shares underlying option awards issued during the one-year period prior to such January 1, or such lesser number as may be approved by the Board of Directors. Under the terms of the 2000 Option Plan, the Company is authorized to grant incentive stock options as defined under the Internal Revenue Code, non-qualified options, stock awards or opportunities to make direct purchases of common stock to employees, officers, directors, consultants and advisors.
The 2000 Option Plan is administered by the compensation committee of the Board of Directors, which selects the individuals to whom equity-based awards will be granted and determines the option exercise price and other terms of each award, subject to the provisions of the 2000 Option Plan. The 2000 Option Plan provides that upon an acquisition, all options to purchase common stock will accelerate by a period of one year. In addition, upon the termination of an employee without cause or for good reason prior to the first anniversary of the completion of the acquisition, all options then outstanding under the 2000 Option Plan held by that employee will immediately become exercisable. At December 31, 2002, options to purchase 1,533,795 were outstanding under the 2000 Option Plan and 402,162 shares were available for future grant under the 2000 Option Plan.
53
Information with respect to activity under the 1995 and 2000 Option Plans is as follows:
| | Number
of Shares
| | Weighted
Exercise Price
|
|---|
| Outstanding, December 31,1999 | | 1,097,830 | | $ | 0.30 |
| Granted | | 1,901,492 | | | 3.41 |
| Exercised | | (1,186,449 | ) | | 1.00 |
| Canceled | | (41,252 | ) | | 0.38 |
| | |
| |
|
| Outstanding, December 31, 2000 | | 1,771,621 | | | 3.16 |
| Granted | | 690,500 | | | 11.21 |
| Exercised | | (107,354 | ) | | 1.54 |
| Canceled | | (126,690 | ) | | 5.84 |
| | |
| |
|
| Outstanding, December 31, 2001 | | 2,228,077 | | | 5.58 |
| Granted | | 1,013,150 | | | 9.09 |
| Exercised | | (239,204 | ) | | 1.00 |
| Canceled | | (109,732 | ) | | 8.74 |
| | |
| |
|
| Outstanding, December 31, 2002 | | 2,892, 291 | | $ | 7.07 |
| | |
| |
|
| Exercisable, December 31, 2000 | | 360,722 | | $ | 0.14 |
| | |
| |
|
| Exercisable, December 31, 2001 | | 819,174 | | $ | 3.90 |
| | |
| |
|
| Exercisable, December 31, 2002 | | 1,071,983 | | $ | 5.65 |
| | |
| |
|
The following table summarizes information relating to currently outstanding and exercisable stock options as of December 31, 2002:
| | Outstanding
| | Exercisable
|
|---|
Exercise
Price
| | Number of
Shares
| | Weighted
Average
Remaining
Contractual
Life (Years)
| | Exercisable
Weighted
Average
Exercise
Price
| | Number of
Shares
| | Weighted
Average
Exercise
Price
|
|---|
| $0.04–$0.38 | | 442,088 | | 6.46 | | $ | 0.34 | | 347,133 | | $ | 0.33 |
| $2.05–$6.95 | | 433,408 | | 7.45 | | | 2.05 | | 194,035 | | | 2.05 |
| $7.27–$8.00 | | 1,013,025 | | 8.75 | | | 7.75 | | 146,461 | | | 7.28 |
| $8.07–$9.90 | | 218,000 | | 8.70 | | | 8.87 | | 57,531 | | | 8.87 |
| $10.10–$11.70 | | 359,020 | | 8.33 | | | 10.98 | | 169,500 | | | 10.86 |
| $12.10–$13.61 | | 286,750 | | 8.92 | | | 12.92 | | 67,323 | | | 12.77 |
| $14.00–$14.33 | | 140,000 | | 8.73 | | | 14.12 | | 90,000 | | | 14.00 |
| | |
| |
| |
| |
| |
|
| | | 2,892,291 | | 8.17 | | $ | 7.07 | | 1,071,983 | | $ | 5.65 |
| | |
| |
| |
| |
| |
|
Shares reserved for issuance
The Company has reserved the following shares of its authorized common shares to be issued upon exercise or issuance of shares related to its employee stock purchase, stock options plans,
54
including all outstanding stock option grants noted above, and outstanding warrants at December 31, 2002:
Plan
| | Shares
Reserved
|
|---|
| 2000 Purchase Plan | | 300,000 |
| 1995 Option Plan | | 1,358,496 |
| 2000 Option Plan | | 1,935,957 |
| Outstanding warrants | | 1,000,000 |
| | |
|
| Total | | 4,594,453 |
| | |
|
(10) COMMITMENTS
The Company leases certain equipment and conducts its operations in leased facilities under noncancelable operating leases expiring through July 2010, including the leasing of approximately 56,000 square feet of space over a seven-year term signed in January 2003. Future minimum rental payments under operating leases as of December 31, 2002 are approximately as follows:
| Year Ending December 31, | | | |
| | 2003 | | $ | 765 |
| | 2004 | | | 1,293 |
| | 2005 | | | 1,289 |
| | 2006 | | | 1,318 |
| | 2007 | | | 1,347 |
| | Thereafter | | | 3,370 |
| | |
|
| | | Total lease payments | | $ | 9,382 |
| | |
|
Rent expense included in the accompanying consolidated statements of operations was approximately $216, $301 and $348 for the years ended December 31, 2000, 2001 and 2002, respectively.
Royalty Agreements
The Company licenses, on a non-exclusive basis, certain technologies that are, or may be, incorporated into its technology under several license agreements. Generally, these licenses require the Company to pay royalties based on net revenues received using the technologies, may require minimum royalty amounts or maintenance fees, and in one instance, required the Company to pay an upfront license fee of $150 that is being amortized over the life of the contract. The Company has recorded research and development expense associated with these agreements of $50 for each of the years ended December 31, 2000, 2001 and 2002, respectively.
55
(11) ACCRUED EXPENSES
Accrued expenses at December 31, 2001 and 2002 consisted of the following:
| | December 31,
|
|---|
| | 2001
| | 2002
|
|---|
| Research and trial-related expenses | | $ | 1,095 | | $ | 940 |
| Payroll and payroll-related | | | 620 | | | 864 |
| Occupancy costs | | | 224 | | | 246 |
| Professional fees | | | 206 | | | 179 |
| Consulting | | | 164 | | | 98 |
| Other | | | 98 | | | 139 |
| | |
| |
|
| | | $ | 2,407 | | $ | 2,466 |
| | |
| |
|
(12) QUARTERLY RESULTS OF OPERATIONS (UNAUDITED)
The following table sets forth unaudited quarterly statement of operations data for each the eight quarters ended December 31, 2002. In the opinion of management, this information has been prepared on the same basis as the audited financial statements appearing elsewhere in this Form 10-K, and all necessary adjustments, consisting only of normal recurring adjustments, have been included in the amounts stated below to present fairly the unaudited quarterly results of operations. The quarterly data
56
should be read in conjunction with our audited financial statements and the notes to the financial statements appearing elsewhere in this Form 10-K.
| | Quarter ended
| |
|---|
| | March 31,
| | June 30,
| | September 30,
| | December 31,
| |
|---|
| | (Amounts in thousands, except per share data)
| |
|---|
| 2002 | | | | | | | | | | | | | |
| Revenue | | $ | 39 | | $ | 40 | | $ | 410 | | $ | 408 | |
| Cost of revenues | | | — | | | 2 | | | 4 | | | 3 | |
| Research and development | | | 5,043 | | | 5,066 | | | 5,131 | | | 4,749 | |
| Selling, general and administrative | | | 2,289 | | | 2,534 | | | 2,396 | | | 2,482 | |
| Stock-based compensation | | | 568 | | | 544 | | | 544 | | | 387 | |
| | |
| |
| |
| |
| |
| Loss from operations | | | (7,861 | ) | | (8,106 | ) | | (7,665 | ) | | (7,213 | ) |
| Interest income | | | 275 | | | 231 | | | 251 | | | 205 | |
| | |
| |
| |
| |
| |
| Net loss | | $ | (7,586 | ) | $ | (7,875 | ) | $ | (7,414 | ) | $ | (7,008 | ) |
| | |
| |
| |
| |
| |
| Net loss per common share—basic and diluted | | $ | (0.42 | ) | $ | (0.43 | ) | $ | (0.40 | ) | $ | (0.38 | ) |
| | |
| |
| |
| |
| |
| Weighted average common shares outstanding— | | | | | | | | | | | | | |
| | Basic and diluted | | | 18,165 | | | 18,376 | | | 18,551 | | | 18,642 | |
| | |
| |
| |
| |
| |
| 2001 | | | | | | | | | | | | | |
| Revenue | | $ | — | | $ | — | | $ | 13 | | $ | 38 | |
| Research and development | | | 2,543 | | | 2,849 | | | 3,535 | | | 4,408 | |
| Selling, general and administrative | | | 2,583 | | | 2,607 | | | 1,965 | | | 1,923 | |
| Stock-based compensation | | | 1,052 | | | 1,055 | | | 865 | | | 816 | |
| | |
| |
| |
| |
| |
| Loss from operations | | | (6,178 | ) | | (6,511 | ) | | (6,352 | ) | | (7,109 | ) |
| Interest income | | | 778 | | | 800 | | | 623 | | | 464 | |
| | |
| |
| |
| |
| |
| Net loss | | $ | (5,400 | ) | $ | (5,711 | ) | $ | (5,729 | ) | $ | (6,645 | ) |
| | |
| |
| |
| |
| |
| Net loss per common share—basic and diluted | | $ | (0.44 | ) | $ | (0.32 | ) | $ | (0.32 | ) | $ | (0.37 | ) |
| | |
| |
| |
| |
| |
| Weighted average common shares outstanding— | | | | | | | | | | | | | |
| | Basic and diluted | | | 12,191 | | | 17,818 | | | 17,887 | | | 18,055 | |
| | |
| |
| |
| |
| |
57
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
On May 14, 2002, we announced the dismissal of our independent public accountants, Arthur Andersen LLP and the engagement of Ernst & Young LLP as our independent auditors. There have been no disagreements with accountants on accounting or financial disclosure matters during our two most recent fiscal years.
PART III
Item 10. Directors and Executive Officers of the Registrant
The information under the Sections "Election of Directors," "Occupations of Directors, The Nominee for Director and Officers" and "Section 16(a) Beneficial Ownership Reporting Compliance" from the registrant's definitive proxy statement for the annual meeting of stockholders to be held on June 12, 2003, which is to be filed with the Securities and Exchange Commission not later than 120 days after the close of the registrant's fiscal year ended December 31, 2002, is hereby incorporated by reference.
Our policy governing transactions in our securities by directors, officers and employees permits our officers, directors and certain other persons to enter into trading plans complying with Rule 10b5-1 under the Securities Exchange Act of 1934, as amended. We have been advised that Anthony P. Shuber, our Executive Vice President and Chief Technology Officer, has entered into trading plans in accordance with Rule 10b5-1 and our policy governing transactions in our securities. We anticipate that, as permitted by Rule 10b5-1 and our policy governing transactions in our securities, some or all of our officers, directors and employees may establish trading plans in the future. We intend to disclose the names of officers and directors who establish a trading plan in compliance with Rule 10b5-1 and the requirements of our policy governing transactions in our securities in our future quarterly and annual reports on Form 10-Q and 10-K filed with the Securities and Exchange Commission. However, we undertake no obligation to update or revise the information provided herein, including for revision or termination of an established trading plan, other than in such quarterly and annual reports.
Item 11. Executive Compensation and Other Information
The information under the Section "Compensation and Other Information Concerning Directors and Officers" from the registrant's definitive proxy statement for the annual meeting of stockholders to be held on June 12, 2003, which is to be filed with the Securities and Exchange Commission not later than 120 days after the close of the registrant's fiscal year ended December 31, 2002, is hereby incorporated by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management
The information under the Section "Securities Ownership of Certain Beneficial Owners and Management" from the registrant's definitive proxy statement for the annual meeting of stockholders to be held on June 12, 2003, which is to be filed with the Securities and Exchange Commission not later than 120 days after the close of the registrant's fiscal year ended December 31, 2002, is hereby incorporated by reference.
Item 13. Certain Relationships and Related Transactions
The information under the Sections "Compensation and Other Information Concerning Directors and Officers" and "Compensation Committee Interlocks, Insider Participation and Other Related Transactions" from the registrant's definitive proxy statement for the annual meeting of stockholders to be held on June 12, 2003, which is to be filed with the Securities and Exchange Commission not later
58
than 120 days after the close of the registrant's fiscal year ended December 31, 2002, is hereby incorporated by reference.
Item 14. Controls and Procedures
Within the 90 days prior to the date of this report, the Company carried out an evaluation, under the supervision and with the participation of the Company's management, including the Company's President and Chief Executive Officer and Executive Vice President, Chief Operating Officer, Chief Financial Officer and Treasurer, of the effectiveness of the design and operation of the Company's disclosure controls and procedures, as defined in Exchange Act Rule 15d-14(c). Based upon that evaluation, the Company's President and Chief Executive Officer and Executive Vice President, Chief Operating Officer, Chief Financial Officer and Treasurer concluded that the Company's disclosure controls and procedures are effective in enabling the Company to record, process, summarize and report information required to be included in the Company's periodic SEC filings within the required time period. There have been no significant changes in the Company's internal controls or in other factors that could significantly affect internal controls subsequent to the date the Company carried out its evaluation.
PART IV
Item 15. Exhibits, Financial Statement Schedule and Reports on Form 8-K
- (a)
- The following documents are filed as part of this Form 10-K:
- (1)
- Financial Statements (see "Financial Statements and Supplementary Data" at Item 8 and incorporated herein by reference).
- (2)
- Financial Statement Schedules (Schedules to the Financial Statements have been omitted because the information required to be set forth therein is not applicable or is shown in the accompanying Financial Statements or notes thereto).
- (3)
- Exhibits
The following exhibits are filed as part of and incorporated by reference into this Form 10-K:
Exhibit
Number
| | Description
|
|---|
| 3.1 | | Sixth Amended and Restated Certificate of Incorporation of the Registrant (previously filed as Exhibit 3.3 to our Registration Statement on Form S-1 File No. 333-48812), which is incorporated herein by reference) |
| 3.2 | | Amended and Restated By-Laws of the Registrant (previously filed as Exhibit 3.4 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 4.1 | | Specimen certificate representing the Registrant's Common Stock (previously filed as Exhibit 4.1 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 4.5 | | Warrant between the Registrant and Laboratory Corporation of America Holdings, Inc. dated June 26, 20022002 (previously filed as Exhibit 4.1 to our Quarterly Report on Form 10-Q for the quarterly period (File No. 000-32179) which is incorporated herein by reference) |
| 10.1* | | 1995 Stock Option Plan (previously filed as Exhibit 10.1 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
59
| 10.2* | | 2000 Stock Option and Incentive Plan (previously filed as Exhibit 10.2 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.3* | | 2000 Employee Stock Purchase Plan (previously filed as Exhibit 10.3 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.4* | | Sixth Amended and Restated Registration Rights Agreement between the Registrant and the parties named therein dated as of April 7, 2000 (previously filed as Exhibit 10.4 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.5* | | Restricted Stock Purchase Agreement between the Registrant and Stanley N. Lapidus dated February 11, 1998 (previously filed as Exhibit 10.5 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.6* | | Restricted Stock Purchase Agreement between the Registrant and Stanley N. Lapidus dated as of March 31, 2000 (previously filed as Exhibit 10.6 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.7* | | Restricted Stock Purchase Agreement between the Registrant and Don M. Hardison dated as of June 23, 2000, as amended (previously filed as Exhibit 10.7 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.10* | | Secured Promissory Note between the Registrant and Don M. Hardison dated as of June 23, 2000 (previously filed as Exhibit 10.10 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.11 | | Lease Agreement, dated December 10, 1996, between C.B. Realty Limited Partnership and the Registrant, as amended (previously filed as Exhibit 10.11 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.12 | | Fourth Amendment to Lease Agreement, dated February 7, 2001, between C.B. Realty Limited Partnership and the Registrant |
| 10.13 | | License Agreement between the Registrant and Genzyme Corporation dated as of March 25, 1999 (previously filed as Exhibit 10.12 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) (certain portions of this agreement have been accorded confidential treatment until March 25, 2003) |
| 10.14 | | PCR Diagnostic Services Agreement between the Registrant and Roche Molecular Systems, Inc. (previously filed as Exhibit 10.13 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) (certain portions of this agreement have been accorded confidential treatment until July 2004) |
| 10.15 | | Mayo Foundation for Medical Education and Research (the "Foundation") Technology License Contract between the Registrant and the Foundation dated as of July 7, 1998, as amended (previously filed as Exhibit 10.14 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.16 | | Letter Agreement by and between The Mayo Foundation for Medical Education and Research and the Registrant dated February 4, 1998 (previously filed as Exhibit 10.15 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.17 | | Form of Consulting Agreement by and between the Registrant and certain members of the scientific advisory board (previously filed as Exhibit 10.16 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
60
| 10.18* | | Restricted Stock Purchase Agreement between the Registrant and John A. McCarthy, Jr. dated as of November 28, 2000 (previously filed as Exhibit 10.17 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.19* | | Full Recourse Promissory Note between the Registrant and John A. McCarthy, Jr. dated as of November 28, 2000 (previously filed as Exhibit 10.18 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.20* | | Pledge Agreement between the Registrant and John A. McCarthy, Jr. dated as of November 30, 2000 (previously filed as Exhibit 10.19 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.21* | | Severance Agreement between the Registrant and Stanley N. Lapidus dated January 4, 2001 (previously filed as Exhibit 10.20 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.22* | | Severance Agreement between the Registrant and Don M. Hardison dated January 4, 2001 (previously filed as Exhibit 10.21 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.23* | | Severance Agreement between the Registrant and John A. McCarthy, Jr. dated January 4, 2001 (previously filed as Exhibit 10.22 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.24* | | Severance Agreement between the Registrant and Anthony P. Shuber dated January 4, 2001 (previously filed as Exhibit 10.23 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.25 | | Warrant Agreement between the Registrant and The Mayo Foundation for Medical Research dated December 28, 2000 (previously filed as Exhibit 10.26 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.26* | | Amendment No 1. to Full Recourse Promissory Note between the Registrant and Stanley N. Lapidus dated as of November 30, 2001 (previously filed as Exhibit 10.26 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.27* | | Amendment No 1. to Full Recourse Promissory Note between the Registrant Don M. Hardison dated as of November 30, 2001 (previously filed as Exhibit 10.27 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.28* | | Amendment No 1. to Full Recourse Promissory Note between the Registrant and John A. McCarthy, Jr. dated as of November 30, 2001 (previously filed as Exhibit 10.28 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.29* | | Executive Cash Incentive Plan dated October 15, 2001 (previously filed as Exhibit 10.29 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.30** | | Agreement between the Registrant and Laboratory Corporation of America Holdings, Inc. dated June 26, 2002 (previously filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q (File No. 000-32179) which is incorporated herein by reference) |
| 10.31+ | | Lease Agreement, dated January 23, 2003, between Marlborough Campus Limited Partnership and the Registrant |
| 10.32**+ | | Exclusive License Agreement between Matrix Technologies Corporation, d/b/a Apogent Discoveries, and the Registrant dated as of November 26, 2002 |
61
| 21.1 | | Subsidiaries of the Registrant (previously filed as Exhibit 21.1 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 23.1+ | | Consent of Ernst & Young LLP |
| 23.1(b)+ | | Information regarding Arthur Andersen LLP |
| 24.1 | | Power of Attorney (included on signature page) |
| 99.1+ | | Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 99.2+ | | Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
- *
- Indicates a management contract or any compensatory plan, contract or arrangement.
- **
- Confidential Treatment requested for certain portions of this Agreement.
- +
- Filed herewith.
- (b)
- Reports on Form 8-K.
During the quarter ended December 31, 2002, we filed one report on Form 8-K, dated October 18, 2002, which announced a conference call to discuss our third quarter of 2002 financial results. We filed no other reports on Form 8-K during the quarter ended December 31, 2002.
62
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | EXACT SCIENCES CORPORATION |
Date: March 26, 2003 |
|
By: |
/s/ DON M. HARDISON
Don M. Hardison
President, Chief Executive Officer and Director (Principal Executive Officer) |
Date: March 26, 2003 |
|
By: |
/s/ JOHN A. MCCARTHY, JR.
John A. McCarthy, Jr.
Executive Vice President, Chief Operating Officer, Chief Financial Officer and Treasurer
(Duly Authorized Officer and Principal
Financial Officer) |
63
POWER OF ATTORNEY AND SIGNATURES
We, the undersigned officers and directors of EXACT Sciences Corporation, hereby severally constitute and appoint Don M. Hardison and John A. McCarthy, Jr., and each of them singly, our true and lawful attorneys, with full power to them and each of them singly, to sign for us and in our names in the capacities indicated below, any amendments to this Annual Report on Form 10-K, and generally to do all things in our names and on our behalf in such capacities to enable EXACT Sciences Corporation to comply with the provisions of the Securities Exchange Act of 1934, as amended, and all the requirements of the Securities Exchange Commission.
Pursuant to the requirements of the Securities and Exchange Act of 1934, as amended, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
SIGNATURE
| | TITLE
| | DATE
|
|---|
|
|
|
|
|
/s/ STANLEY N. LAPIDUS
Stanley N. Lapidus | | Chairman of the Board and Director | | March 26, 2003 |
/s/ DON M. HARDISON
Don M. Hardison |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 26, 2003 |
/s/ JOHN A. MCCARTHY, JR.
John A. McCarthy, Jr. |
|
Executive Vice President, Chief Operating Officer, Chief Financial Officer and Treasurer (Duly Authorized Officer and Principal Financial Officer) |
|
March 26, 2003 |
/s/ RICHARD W. BARKER
Richard W. Barker |
|
Director |
|
March 26, 2003 |
/s/ SALLY W. CRAWFORD
Sally W. Crawford |
|
Director |
|
March 26, 2003 |
/s/ WILLIAM W. HELMAN
William W. Helman |
|
Director |
|
March 26, 2003 |
/s/ EDWIN M. KANIA, JR.
Edwin M. Kania, Jr. |
|
Director |
|
March 26, 2003 |
/s/ CONNIE MACK, III
Connie Mack, III |
|
Director |
|
March 26, 2003 |
/s/ LANCE WILLSEY
Lance Willsey |
|
Director |
|
March 26, 2003 |
/s/ PATRICK J. ZENNER
Patrick J. Zenner |
|
Director |
|
March 26, 2003 |
64
Certifications
I, Don M. Hardison, certify that:
- 1.
- I have reviewed this annual report on Form 10-K of EXACT Sciences Corporation;
- 2.
- Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
- 3.
- Based on my knowledge, the financial statements, and other financial information included in this annual report on Form 10-K, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report;
- 4.
- The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and we have:
- a)
- designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared;
- b)
- evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the "Evaluation Date"); and
- c)
- presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
- 5.
- The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent function):
- a)
- all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
- b)
- any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
- 6.
- The registrant's other certifying officers and I have indicated in this annual report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
| Date: March 26, 2003 | | By: /s/ DON M. HARDISON
Don M. Hardison
President, Chief Executive Officer and Director
(Principal Executive Officer) |
65
Certifications
I, John A. McCarthy, Jr., certify that:
- 1)
- I have reviewed this annual report on Form 10-K of EXACT Sciences Corporation;
- 2)
- Based on my knowledge, this annual report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this annual report;
- 3)
- Based on my knowledge, the financial statements, and other financial information included in this annual report on Form 10-K, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this annual report;
- 4)
- The registrant's other certifying officers and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-14 and 15d-14) for the registrant and we have:
- a)
- designed such disclosure controls and procedures to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this annual report is being prepared;
- b)
- evaluated the effectiveness of the registrant's disclosure controls and procedures as of a date within 90 days prior to the filing date of this annual report (the "Evaluation Date"); and
- c)
- presented in this annual report our conclusions about the effectiveness of the disclosure controls and procedures based on our evaluation as of the Evaluation Date;
- 5)
- The registrant's other certifying officers and I have disclosed, based on our most recent evaluation, to the registrant's auditors and the audit committee of registrant's board of directors (or persons performing the equivalent function):
- a)
- all significant deficiencies in the design or operation of internal controls which could adversely affect the registrant's ability to record, process, summarize and report financial data and have identified for the registrant's auditors any material weaknesses in internal controls; and
- b)
- any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant's internal controls; and
- 6)
- The registrant's other certifying officers and I have indicated in this annual report whether or not there were significant changes in internal controls or in other factors that could significantly affect internal controls subsequent to the date of our most recent evaluation, including any corrective actions with regard to significant deficiencies and material weaknesses.
| Date: March 26, 2003 | | By: /s/ JOHN A. MCCARTHY, JR.
John A. McCarthy, Jr.
Executive Vice President, Chief Operating Officer,
Chief Financial Officer and Treasurer
(Duly Authorized Officer and Principal
Financial Officer) |
66
Exhibit Index to Annual Report on Form 10-K
for Fiscal Year Ended December 31, 2002
Exhibit
Number
| | Description
|
|---|
| 3.1 | | Sixth Amended and Restated Certificate of Incorporation of the Registrant (previously filed as Exhibit 3.3 to our Registration Statement on Form S-1 File No. 333-48812), which is incorporated herein by reference) |
| 3.2 | | Amended and Restated By-Laws of the Registrant (previously filed as Exhibit 3.4 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 4.1 | | Specimen certificate representing the Registrant's Common Stock (previously filed as Exhibit 4.1 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 4.5 | | Warrant between the Registrant and Laboratory Corporation of America Holdings, Inc. dated June 26, 20022002 (previously filed as Exhibit 4.1 to our Quarterly Report on Form 10-Q for the quarterly period (File No. 000-32179) which is incorporated herein by reference) |
| 10.1* | | 1995 Stock Option Plan (previously filed as Exhibit 10.1 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.2* | | 2000 Stock Option and Incentive Plan (previously filed as Exhibit 10.2 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.3* | | 2000 Employee Stock Purchase Plan (previously filed as Exhibit 10.3 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.4* | | Sixth Amended and Restated Registration Rights Agreement between the Registrant and the parties named therein dated as of April 7, 2000 (previously filed as Exhibit 10.4 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.5* | | Restricted Stock Purchase Agreement between the Registrant and Stanley N. Lapidus dated February 11, 1998 (previously filed as Exhibit 10.5 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.6* | | Restricted Stock Purchase Agreement between the Registrant and Stanley N. Lapidus dated as of March 31, 2000 (previously filed as Exhibit 10.6 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.7* | | Restricted Stock Purchase Agreement between the Registrant and Don M. Hardison dated as of June 23, 2000, as amended (previously filed as Exhibit 10.7 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.10* | | Secured Promissory Note between the Registrant and Don M. Hardison dated as of June 23, 2000 (previously filed as Exhibit 10.10 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.11 | | Lease Agreement, dated December 10, 1996, between C.B. Realty Limited Partnership and the Registrant, as amended (previously filed as Exhibit 10.11 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.12 | | Fourth Amendment to Lease Agreement, dated February 7, 2001, between C.B. Realty Limited Partnership and the Registrant |
67
| 10.13 | | License Agreement between the Registrant and Genzyme Corporation dated as of March 25, 1999 (previously filed as Exhibit 10.12 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) (certain portions of this agreement have been accorded confidential treatment until March 25, 2003) |
| 10.14 | | PCR Diagnostic Services Agreement between the Registrant and Roche Molecular Systems, Inc. (previously filed as Exhibit 10.13 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) (certain portions of this agreement have been accorded confidential treatment until July 2004) |
| 10.15 | | Mayo Foundation for Medical Education and Research (the "Foundation") Technology License Contract between the Registrant and the Foundation dated as of July 7, 1998, as amended (previously filed as Exhibit 10.14 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.16 | | Letter Agreement by and between The Mayo Foundation for Medical Education and Research and the Registrant dated February 4, 1998 (previously filed as Exhibit 10.15 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.17 | | Form of Consulting Agreement by and between the Registrant and certain members of the scientific advisory board (previously filed as Exhibit 10.16 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.18* | | Restricted Stock Purchase Agreement between the Registrant and John A. McCarthy, Jr. dated as of November 28, 2000 (previously filed as Exhibit 10.17 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.19* | | Full Recourse Promissory Note between the Registrant and John A. McCarthy, Jr. dated as of November 28, 2000 (previously filed as Exhibit 10.18 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.20* | | Pledge Agreement between the Registrant and John A. McCarthy, Jr. dated as of November 30, 2000 (previously filed as Exhibit 10.19 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.21* | | Severance Agreement between the Registrant and Stanley N. Lapidus dated January 4, 2001 (previously filed as Exhibit 10.20 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.22* | | Severance Agreement between the Registrant and Don M. Hardison dated January 4, 2001 (previously filed as Exhibit 10.21 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.23* | | Severance Agreement between the Registrant and John A. McCarthy, Jr. dated January 4, 2001 (previously filed as Exhibit 10.22 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.24* | | Severance Agreement between the Registrant and Anthony P. Shuber dated January 4, 2001 (previously filed as Exhibit 10.23 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 10.25 | | Warrant Agreement between the Registrant and The Mayo Foundation for Medical Research dated December 28, 2000 (previously filed as Exhibit 10.26 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
68
| 10.26* | | Amendment No 1. to Full Recourse Promissory Note between the Registrant and Stanley N. Lapidus dated as of November 30, 2001 (previously filed as Exhibit 10.26 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.27* | | Amendment No 1. to Full Recourse Promissory Note between the Registrant Don M. Hardison dated as of November 30, 2001 (previously filed as Exhibit 10.27 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.28* | | Amendment No 1. to Full Recourse Promissory Note between the Registrant and John A. McCarthy, Jr. dated as of November 30, 2001 (previously filed as Exhibit 10.28 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.29* | | Executive Cash Incentive Plan dated October 15, 2001 (previously filed as Exhibit 10.29 to our Annual Report on Form 10-K for the period ended December 31, 2002 (File No. 000-32179) which is incorporated herein by reference) |
| 10.30** | | Agreement between the Registrant and Laboratory Corporation of America Holdings, Inc. dated June 26, 2002 (previously filed as Exhibit 10.1 to our Quarterly Report on Form 10-Q (File No. 000-32179) which is incorporated herein by reference) |
| 10.31+ | | Lease Agreement, dated January 23, 2003, between Marlborough Campus Limited Partnership and the Registrant |
| 10.32**+ | | Exclusive License Agreement between Matrix Technologies Corporation, d/b/a Apogent Discoveries, and the Registrant dated as of November 26, 2002 |
| 21.1 | | Subsidiaries of the Registrant (previously filed as Exhibit 21.1 to our Registration Statement on Form S-1 (File No. 333-48812), which is incorporated herein by reference) |
| 23.1+ | | Consent of Ernst & Young LLP |
| 23.1(A)+ | | Information regarding Arthur Andersen LLP |
| 24.1 | | Power of Attorney (included on signature page) |
| 99.1+ | | Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 99.2+ | | Certification Pursuant to 18 U.S.C Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
- *
- Indicates a management contract or any compensatory plan, contract or arrangement.
- **
- Confidential Treatment requested for certain portions of this Agreement.
- +
- Filed herewith.
69


