UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
SCHEDULE 14A
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a)
of the Securities Exchange Act of 1934
Filed by the Registranto
Filed by a Party other than the Registrantþ
Check the appropriate box:
| o | | Preliminary Proxy Statement |
| |
| o | | Confidential, For Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) |
| |
| o | | Definitive Proxy Statement |
| |
| o | | Definitive Additional Materials |
| |
| þ | | Soliciting Material Under Rule 14a-12 |
IOMAI CORPORATION
(Name of Registrant as Specified in Its Charter)
INTERCELL AG
(Name of Person(s) Filing Proxy Statement, if Other Than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| þ | | No fee required. |
| |
| o | | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| | (1) | | Title of each class of securities to which transaction applies: |
| |
| | | | [ ] |
| |
| | (2) | | Aggregate number of securities to which transaction applies: |
| |
| | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): |
| |
| | | | $[ ] |
| |
| | (4) | | Proposed maximum aggregate value of transaction: |
| |
| | | | $[ ] |
| |
| | (5) | | Total fee paid: |
| |
| | | | $[ ] |
| o | | Fee paid previously with preliminary materials: |
| |
| o | | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the form or schedule and the date of its filing. |
| | (1) | | Amount previously paid: |
| |
| |
|
| |
| | (2) | | Form, Schedule or Registration Statement No.: |
| |
| |
|
| |
| | (3) | | Filing Party: |
| |
| |
|
| |
| | (4) | | Date Filed: |
| |
| |
|
Driving Vaccine Innovation ROADSHOW PRESENTATION
Intercell develops vaccines for the prevention and treatment of infectious diseases
For more information be invited to: www.intercell.com
Safe Harbour Statement
These materials do not constitute an offer to sell or the solicitation of an offer to sell or the solicitation of an offer to buy any securities.
During the course of this presentation, Intercell AG (the “Company”) may make projections or other forward-looking statements regarding, among other things, the benefits of the proposed acquisition of Iomai Corporation (“Iomai”) by the Company, including future financial and operating results, the Company’s plans, objectives, expectations and intentions, the expected timing of completion of the transaction, and other statements that are not historical facts. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include risks and uncertainties relating to the ability to obtain regulatory approvals of the transaction on the proposed terms and schedule; the risk that the businesses will not be integrated successfully; the risk that the cost savings and any other synergies from the transaction may not be fully realized or may take longer to realize than expected; market acceptance for the transaction and approved products; risks of regulatory review and clinical trials; disruption from the transaction making it more difficult to maintain relationships with customers, employees or suppliers; competition and its effect on pricing, spending, third-party relationships and revenues; the need to acquire and develop new products; reliance on intellectual property and having limited patents and proprietary rights; general worldwide economic conditions and related uncertainties; and the effect of changes in governmental regulations.
In addition, projections or other forward-looking statements may relate to, among other things, the progress, timing and completion of the Company’s and Iomai’s research, development and clinical trials for product candidates, the Company’s and Iomai’s ability to market, commercialize and achieve market acceptance for product candidates, their ability to protect their intellectual property and operate their respective businesses without infringing on the intellectual property rights of others, the Company’s and Iomai’s estimates for future performance and their estimates regarding anticipated operating losses, future revenues, capital requirements and their needs for additional financing. In addition, even if the Company’s and Iomai’s actual results or development are consistent with the forward-looking statements contained in this presentation, those results or developments may not be indicative of the Company’s and/or Iomai’s results or developments in the future. In some cases, you can identify forward-looking statements by words such as “could,” “may,” “expects,” “anticipates,” “believes,” “intends,” “estimates,” or similar words. These forward-looking statements are based largely on the Company’s current expectations as of the date of this presentation and are subject to a number of known and unknown risks and uncertainties and other factors that may cause actual results, performance or achievements to be materially different from any future results, performance or achievement expressed or implied by these forward-looking statements. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements made during this presentation will in fact be realized. Except as otherwise required by applicable securities laws, we disclaim any intention or obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Additional Information and Where to Find It:
In connection with the proposed acquisition of Iomai by Intercell, Iomai will be filing a proxy statement for its stockholders and other documents regarding the proposed acquisition with the Securities and Exchange Commission (“SEC”). BEFORE MAKING ANY VOTING OR INVESTMENT DECISION, IOMAI STOCKHOLDERS AND INVESTORS ARE URGED TO READ THE PROXY STATEMENT AND OTHER RELEVANT MATERIALS CAREFULLY IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THE DOCUMENTS WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED TRANSACTION AND IOMAI. Investors and stockholders may obtain copies of the proxy statement and other relevant documents filed with the SEC by Iomai (when they are available) free of charge at the SEC’s website at http://www.sec.gov. In addition, investors and stockholders may obtain copies of the proxy statement and other relevant documents filed with the SEC by Iomai (when they are available) by going to Iomai’s Investor Relations page on its corporate website at http://www.Iomai.com.
Iomai and its directors, executive officers and other members of management may be deemed to be participants in the solicitation of proxies from Iomai’s stockholders with respect to the proposed transaction. Information regarding Iomai’s executive officers and directors, and their beneficial ownership of Iomai’s common stock as of March 26, 2008, is available in Iomai’s proxy statement for its 2008 Annual Meeting of Stockholders, which was filed with the SEC on April 4, 2008. Other information regarding the interests of such potential participants in the proxy solicitation will be contained in the proxy statement and other relevant materials to be filed with the SEC when they become available.
PAGE 1 ROADSHOW PRESENTATION MAY 2008
Delivering on promises — dedication to innovation KEY MANAGEMENT
Gerd Zettlmeissl, CEO Former CEO of Chiron Behring, co-inventor of Enbrel
Werner Lanthaler, CFO Former Senior Consultant with McKinsey & Company, former Executive Director with the Federation of Austrian Industry
Alexander von Gabain, CSO Former Chair of Department of Microbiology and Genetics at the Campus Vienna Biocenter, Foreign Adjunct Professor at the Karolinska Institute
Thomas Lingelbach, COO Former Vice President Industrial Operations Chiron Vaccines, Managing Director for Novartis Vaccines Germany
PAGE 2 ROADSHOW PRESENTATION MAY 2008
Agenda
Overview Introduction to Iomai — The Target Company Deal Rationale & Product Development Update Q1 2008: Results & Strategic Implications Intercell Going Forward
PAGE 3 ROADSHOW PRESENTATION MAY 2008
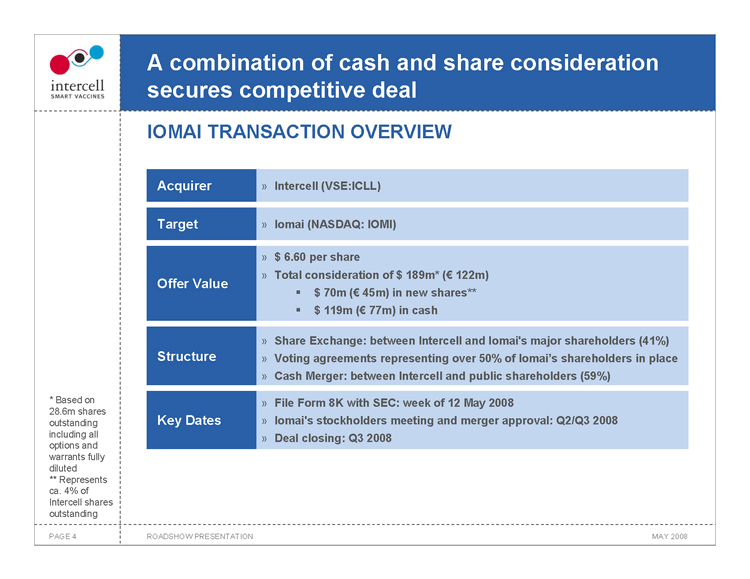
A combination of cash and share consideration secures competitive deal
IOMAI TRANSACTION OVERVIEW
Acquirer Intercell (VSE:ICLL)
Target Iomai (NASDAQ: IOMI)
$6.60 per share Total consideration of $189m* (€122m) Offer Value ??$70m (€45m) in new shares** ??$119m (€77m) in cash
Share Exchange: between Intercell and Iomai’s major shareholders (41%) Structure Voting agreements representing over 50% of Iomai’s shareholders in place Cash Merger: between Intercell and public shareholders (59%)
File Form 8K with SEC: week of 12 May 2008 Key Dates �� Iomai’s stockholders meeting and merger approval: Q2/Q3 2008 Deal closing: Q3 2008
• Based on 28.6m shares outstanding including all options and warrants fully diluted ** Represents ca. 4% of Intercell shares outstanding
PAGE 4 ROADSHOW PRESENTATION MAY 2008
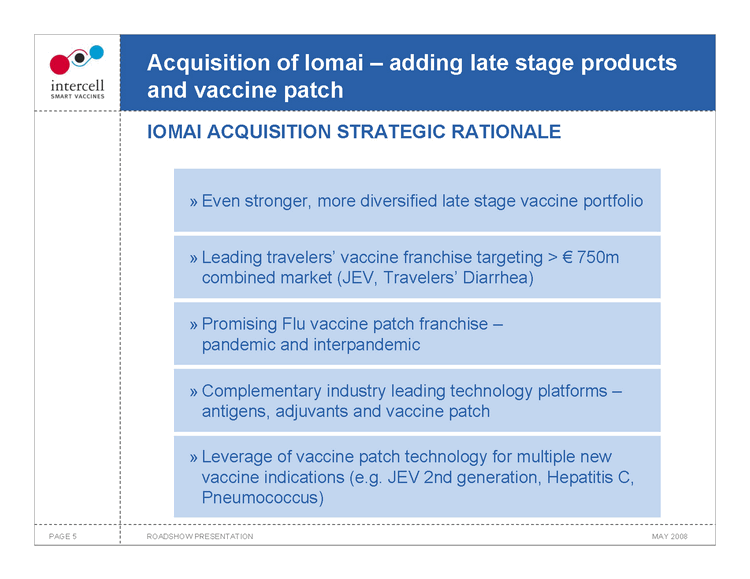
Acquisition of Iomai — adding late stage products and vaccine patch IOMAI ACQUISITION STRATEGIC RATIONALE
Even stronger, more diversified late stage vaccine portfolio
Leading travelers’ vaccine franchise targeting >€750m combined market (JEV, Travelers’ Diarrhea)
Promising Flu vaccine patch franchise —pandemic and interpandemic
Complementary industry leading technology platforms —antigens, adjuvants and vaccine patch
Leverage of vaccine patch technology for multiple new vaccine indications (e.g. JEV 2nd generation, Hepatitis C, Pneumococcus)
PAGE 5 ROADSHOW PRESENTATION MAY 2008
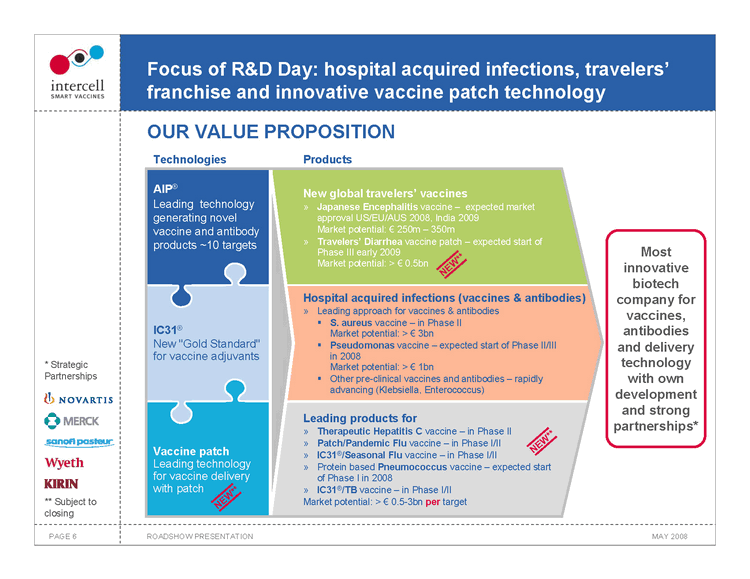
Focus of R&D Day: hospital acquired infections, travelers’ franchise and innovative vaccine patch technology
OUR VALUE PROPOSITION
Technologies Products
AIP® New global travelers’ vaccines Leading technology Japanese Encephalitis vaccine — expected market generating novel approval US/EU/AUS 2008, India 2009 vaccine and antibody Market potential:€250m — 350m products ~10 targets Travelers’ Diarrhea vaccine patch — expected start of Phase III early 2009 Market potential: >€0.5bn
NEW* *
Hospital acquired infections (vaccines & antibodies) Leading approach for vaccines & antibodies
??S. aureus vaccine — in Phase II
IC31® Market potential: >€3bn New “Gold Standard” ??Pseudomonas vaccine — expected start of Phase II/III for vaccine adjuvants in 2008 Market potential: >€1bn ? Other pre-clinical vaccines and antibodies — rapidly advancing (Klebsiella, Enterococcus)
Leading products for Therapeutic Hepatitis C vaccine — in Phase II *
Patch/Pandemic Flu vaccine — in Phase I/II NEW* Vaccine patch IC31®/Seasonal Flu vaccine — in Phase I/II Leading technology Protein based Pneumococcus vaccine — expected start of Phase I in 2008 for vaccine delivery with patch IC31®/TB vaccine — in Phase I/II NEW* * Market potential: >€0.5-3bn per target
Most innovative biotech company for vaccines, antibodies and delivery technology with own development and strong partnerships*
• Strategic Partnerships ** Subject to closing
PAGE 6 ROADSHOW PRESENTATION MAY 2008
Agenda
Overview Introduction to Iomai — The Target Company Deal Rationale & Product Development Update Q1 2008: Results & Strategic Implications Intercell Going Forward
PAGE 7 ROADSHOW PRESENTATION MAY 2008
Perfect match for our growth — advanced products and new delivery technology COMPANY SNAPSHOT
Late stage products targeting significant infectious disease opportunities
Travelers’ Diarrhea vaccine patch (>€0.5bn market opportunity) Vaccine enhancement patch for Pandemic Flu (€0.5-1.0bn market opportunity)
Leader in the application of vaccines using needle-free vaccine patch technology
Highly innovative vaccine delivery alternative to current injected vaccines Broad application opportunities for vaccine enhancement patch to reduce the dose/number of injections of existing vaccines
Clinical Validation: breakthrough results from needle-free vaccine patch
Excellent Phase II efficacy data for Travelers’ Diarrhea vaccine patch Promising Phase I/II efficacy data vaccine enhancement patch for Pandemic Flu
Headquartered in Gaithersburg, Maryland (USA), ~ 110 employees, incorporated in 1997
PAGE 8 ROADSHOW PRESENTATION MAY 2008
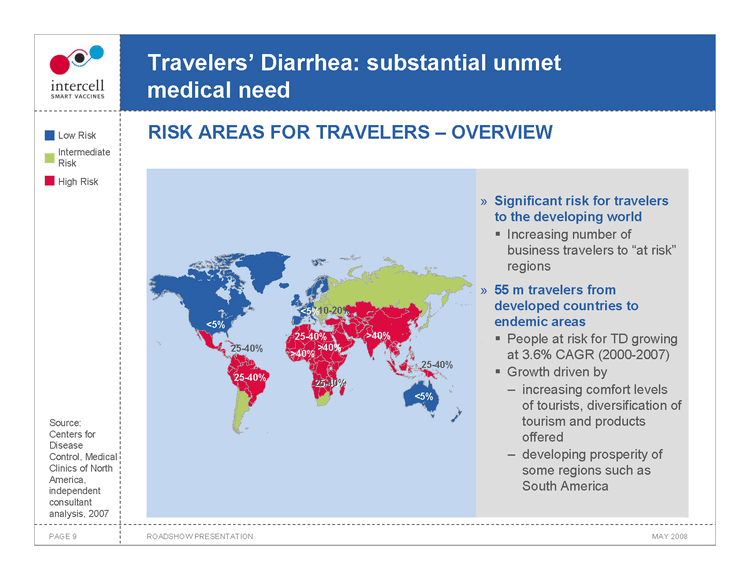
Travelers’ Diarrhea: substantial unmet medical need RISK AREAS FOR TRAVELERS — OVERVIEW
Significant risk for travelers to the developing world
Increasing number of business travelers to “at risk” regions
55 m travelers from developed countries to endemic areas
People at risk for TD growing at 3.6% CAGR (2000-2007) Growth driven by increasing comfort levels of tourists, diversification of tourism and products offered developing prosperity of some regions such as South America
Source: Centers for Disease Control, Medical Clinics of North America, independent consultant analysis, 2007
PAGE 9 ROADSHOW PRESENTATION MAY 2008
Low Risk Intermediate Risk High Risk
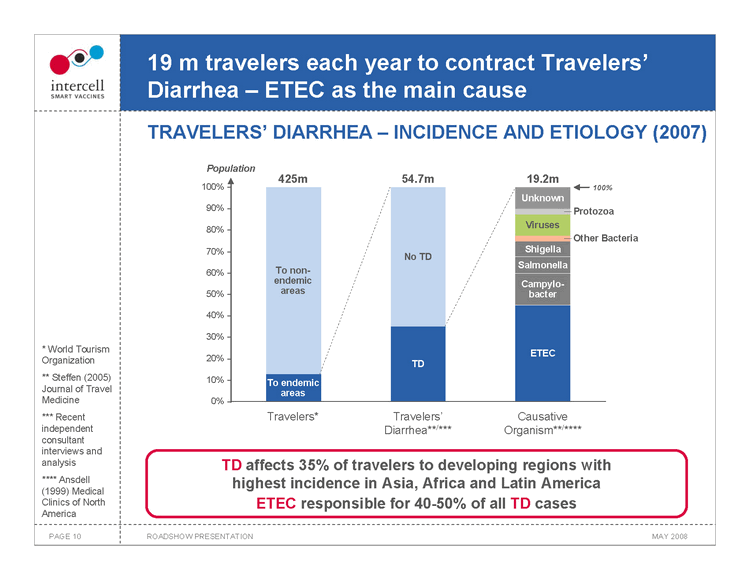
19 m travelers each year to contract Travelers’ Diarrhea — ETEC as the main cause
TRAVELERS’ DIARRHEA — INCIDENCE AND ETIOLOGY (2007)
Population 425m 54.7m 19.2m 100% 100% Unknown 90% Protozoa Viruses 80% Other Bacteria 70% Shigella No TD Salmonella 60% To non- endemic Campylo- 50% areas bacter 40%
30% ETEC 20% TD 10% To endemic areas 0% Travelers* Travelers’ Causative Diarrhea**/*** Organism**/ ****
TD affects 35% of travelers to developing regions with highest incidence in Asia, Africa and Latin America ETEC responsible for 40-50% of all TD cases
• World Tourism Organization ** Steffen (2005) Journal of Travel Medicine *** Recent independent consultant interviews and analysis **** Ansdell (1999) Medical Clinics of North America
PAGE 10 ROADSHOW PRESENTATION MAY 2008
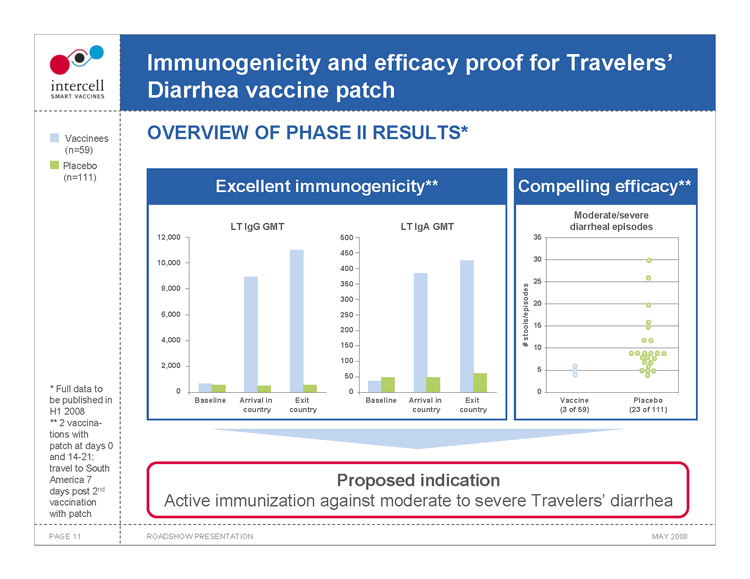
Immunogenicity and efficacy proof for Travelers’ Diarrhea vaccine patch OVERVIEW OF PHASE II RESULTS*
Vaccinees (n=59) Placebo (n=111)
Excellent immunogenicity**
LT IgG GMT LT IgA GMT 12,000 500 450 10,000 400 350 8,000 300 6,000 250 200 4,000 150 100 2,000 50 0 0 Baseline Arrival in Exit Baseline Arrival in Exit
country country country country
Compelling efficacy** Moderate/severe diarrheal episodes 35
30
25
20 # stools/episodes 15 10 5
0 Vaccine Placebo (3 of 59) (23 of 111)
• Full data to be published in H1 2008 ** 2 vaccinations with patch at days 0 and 14-21; travel to South America 7 days post 2nd vaccination with patch
Proposed indication Active immunization against moderate to severe Travelers’ diarrhea
PAGE 11 ROADSHOW PRESENTATION MAY 2008
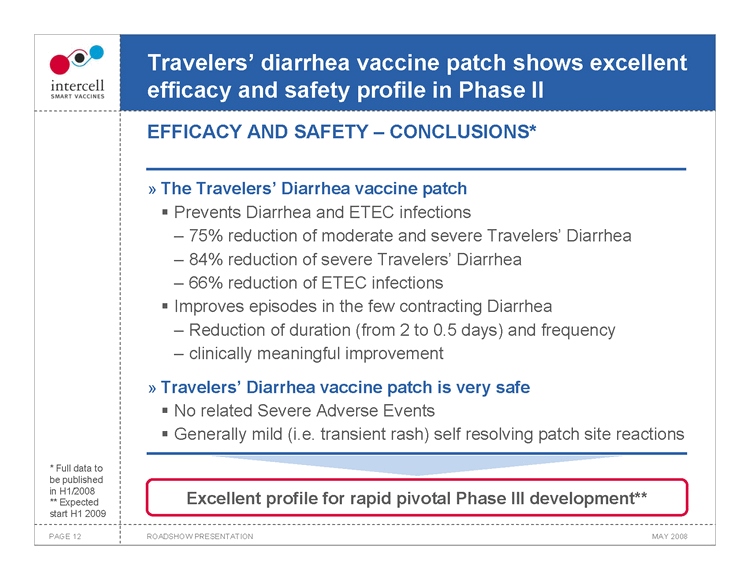
Travelers’ diarrhea vaccine patch shows excellent efficacy and safety profile in Phase II EFFICACY AND SAFETY — CONCLUSIONS*
The Travelers’ Diarrhea vaccine patch
Prevents Diarrhea and ETEC infections
75% reduction of moderate and severe Travelers’ Diarrhea 84% reduction of severe Travelers’ Diarrhea 66% reduction of ETEC infections
?Improves episodes in the few contracting Diarrhea
Reduction of duration (from 2 to 0.5 days) and frequency clinically meaningful improvement
Travelers’ Diarrhea vaccine patch is very safe
No related Severe Adverse Events Generally mild (i.e. transient rash) self resolving patch site reactions
• Full data to be published in H1/2008 ** Expected start H1 2009
Excellent profile for rapid pivotal Phase III development**
PAGE 12 ROADSHOW PRESENTATION MAY 2008
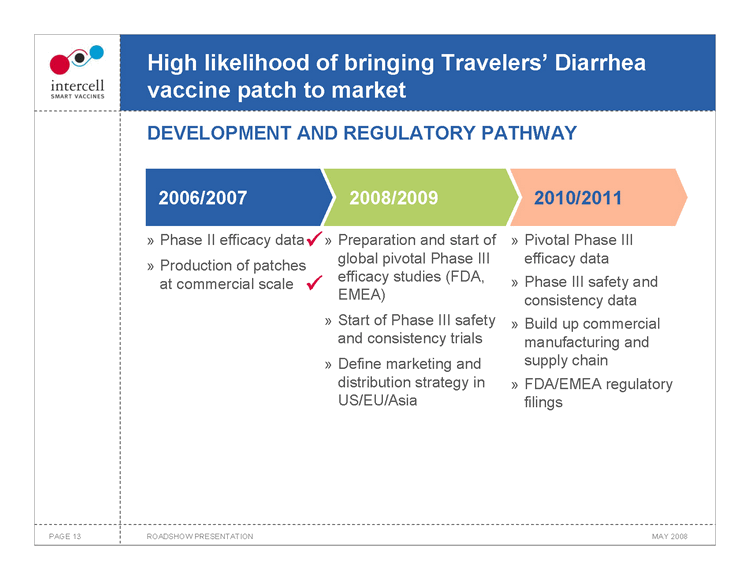
High likelihood of bringing Travelers’ Diarrhea vaccine patch to market DEVELOPMENT AND REGULATORY PATHWAY
2006/2007 2008/2009 2010/2011 Phase II efficacy data Preparation and start of global pivotal Phase III Pivotal Phase III efficacy data
Production of patches at commercial scale efficacy studies (FDA, EMEA) Phase III safety and consistency data
Start of Phase III safety and consistency trials Build up commercial manufacturing and supply chain
Define marketing and distribution strategy in US/EU/Asia FDA/EMEA regulatory filings
PAGE 13 ROADSHOW PRESENTATION MAY 2008
A “one dose” Pandemic Flu vaccine patch is urgently needed PROBLEM AND CURRENT STATUS
Permanent threat of a global pandemic outbreak Need to vaccinate total population with fast protection ?Lower antigen doses ?Protection with one dose vaccine Currently only US approved pandemic vaccine with suboptimal profile* ?High antigen dose (90 mcg/dose) ?Two doses (day 1 and day 28) No breakthrough yet with other injectable vaccines in development** US government $7.1bn initiative to prevent and prepare for an outbreak
• sanofi pasteur ** GSK, Novartis, sanofi pasteur
PAGE 14 ROADSHOW PRESENTATION MAY 2008
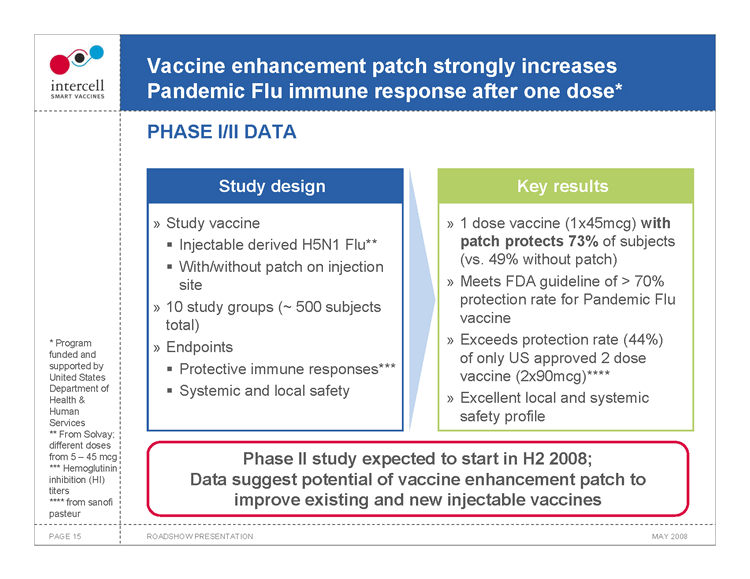
Vaccine enhancement patch strongly increases Pandemic Flu immune response after one dose* PHASE I/II DATA
Study design
Study vaccine ?Injectable derived H5N1 Flu** ?With/without patch on injection site 10 study groups (~ 500 subjects total) Endpoints ?Protective immune responses*** ?Systemic and local safety
Key results
1 dose vaccine (1x45mcg) with patch protects 73% of subjects (vs. 49% without patch) Meets FDA guideline of > 70% protection rate for Pandemic Flu vaccine Exceeds protection rate (44%) of only US approved 2 dose vaccine (2x90mcg)**** Excellent local and systemic safety profile
Phase II study expected to start in H2 2008; Data suggest potential of vaccine enhancement patch to improve existing and new injectable vaccines
• Program funded and supported by United States Department of Health & Human Services ** From Solvay; different doses from 5 — 45 mcg *** Hemoglutinin inhibition (HI) titers **** from sanofi pasteur
PAGE 15 ROADSHOW PRESENTATION MAY 2008
Agenda
Overview
Introduction to Iomai — The Target Company Deal Rationale & Product Development Update Q1 2008: Results & Strategic Implications Intercell Going Forward
PAGE 16 ROADSHOW PRESENTATION MAY 2008
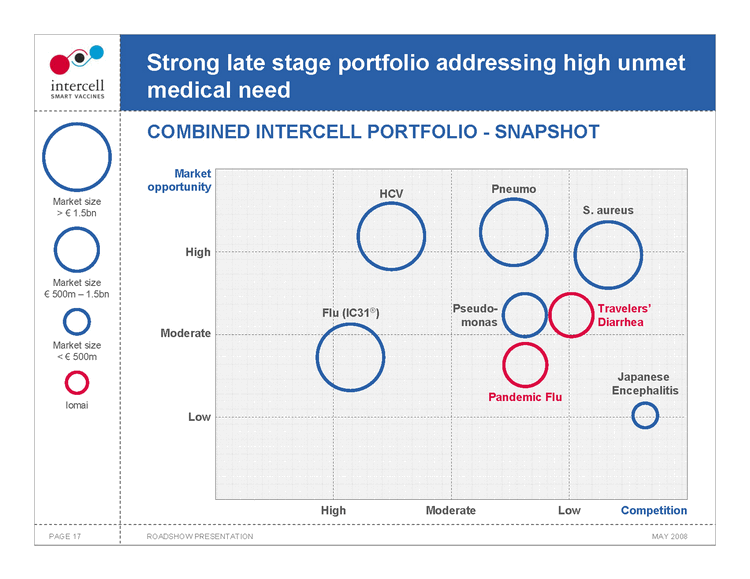
Strong late stage portfolio addressing high unmet medical need COMBINED INTERCELL PORTFOLIO — SNAPSHOT
Market size >€1.5bn Market size€ 500m — 1.5bn
Market size <€500m Iomai
Market opportunity Pneumo HCV
S. aureus
High
Flu (IC31®)Pseudo- Travelers’ monas Diarrhea Moderate
Japanese Encephalitis Pandemic Flu Low
High ModerateLowCompetition
PAGE 17 ROADSHOW PRESENTATION MAY 2008
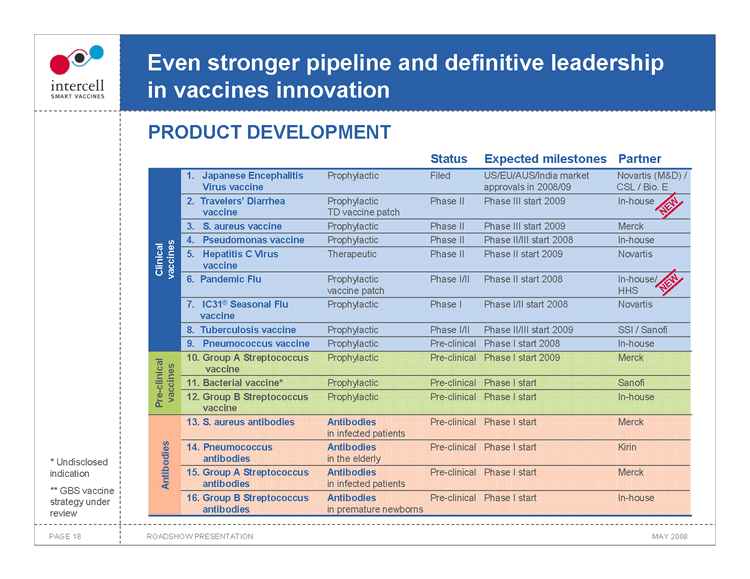
Even stronger pipeline and definitive leadership in vaccines innovation PRODUCT DEVELOPMENT
Clinical vaccines
Status Expected milestones Partner
1. Japanese Encephalitis Prophylactic Filed US/EU/AUS/India market Novartis (M&D) /
Virus vaccine approvals in 2008/09 CSL / Bio. E 2. Travelers’ Diarrhea Prophylactic Phase II Phase III start 2009 In-house vaccine TD vaccine patch NEW 3. S. aureus vaccine Prophylactic Phase II Phase III start 2009 Merck 4. Pseudomonas vaccine Prophylactic Phase II Phase II/III start 2008 In-house 5. Hepatitis C Virus Therapeutic Phase II Phase II start 2009 Novartis vaccine 6. Pandemic Flu Prophylactic Phase I/II Phase II start 2008 In-house/ NEW vaccine patch HHS 7. IC31® Seasonal Flu Prophylactic Phase I Phase I/II start 2008 Novartis vaccine 8. Tuberculosis vaccine Prophylactic Phase I/II Phase II/III start 2009 SSI / Sanofi 9. Pneumococcus vaccine Prophylactic Pre-clinical Phase I start 2008 In-house
10. Group A Streptococcus Prophylactic Pre-clinical Phase I start 2009 Merck
vaccine 11. Bacterial vaccine*ProphylacticPre-clinicalPhase I start Sanofi Pre-clinical vaccines 12. Group B Streptococcus ProphylacticPre-clinicalPhase I startIn-house vaccine 13. S. aureus antibodies AntibodiesPre-clinicalPhase I startMerck in infected patients 14. Pneumococcus AntibodiesPre-clinicalPhase I startKirin antibodies in the elderly 15. Group A Streptococcus AntibodiesPre-clinicalPhase I startMerck Antibodies antibodies in infected patients 16. Group B Streptococcus Antibodies Pre-clinical Phase I start In-house antibodies in premature newborns
• Undisclosed indication ** GBS vaccine strategy under review
PAGE 18 ROADSHOW PRESENTATION MAY 2008
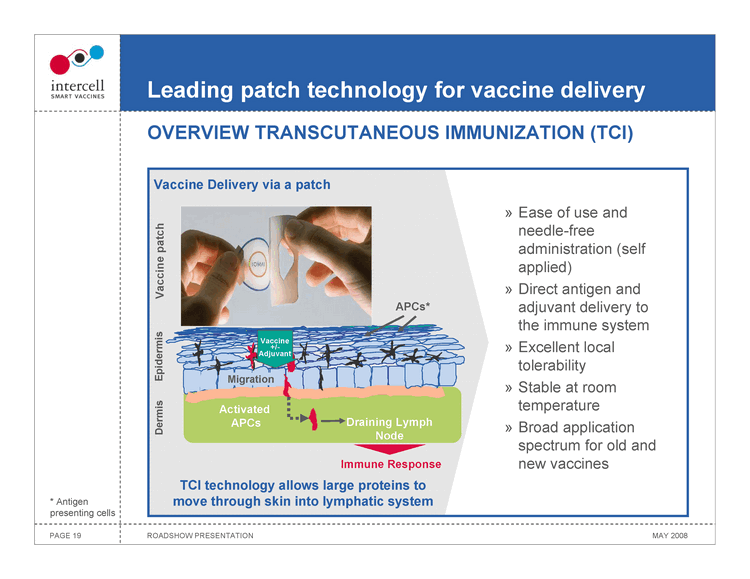
Leading patch technology for vaccine delivery
OVERVIEW TRANSCUTANEOUS IMMUNIZATION (TCI)
Vaccine Delivery via a patch
Vaccine patch
APCs*
Epidermis
Vaccine +/-Adjuvant Migration
Dermis
Activated APCs Draining Lymph Node
Ease of use and needle-free administration (self applied) Direct antigen and adjuvant delivery to the immune system Excellent local tolerability Stable at room temperature Broad application spectrum for old and new vaccines
Immune Response
TCI technology allows large proteins to move through skin into lymphatic system
• Antigen presenting cells
PAGE 19 ROADSHOW PRESENTATION MAY 2008
Numerous applications for vaccine patch (TCI) POTENTIAL STRATEGIC APPLICATIONS
Direct antigen/adjuvant delivery, e.g.
TD vaccine patch (Phase II) Pneumococcus vaccine patch Many others
Vaccine enhancement patch*, e.g. Pandemic Flu (Phase I/II) 2nd generation JEV (one dose) Reduction of injections for childhood vaccines Many others
PAGE 20 ROADSHOW PRESENTATION MAY 2008
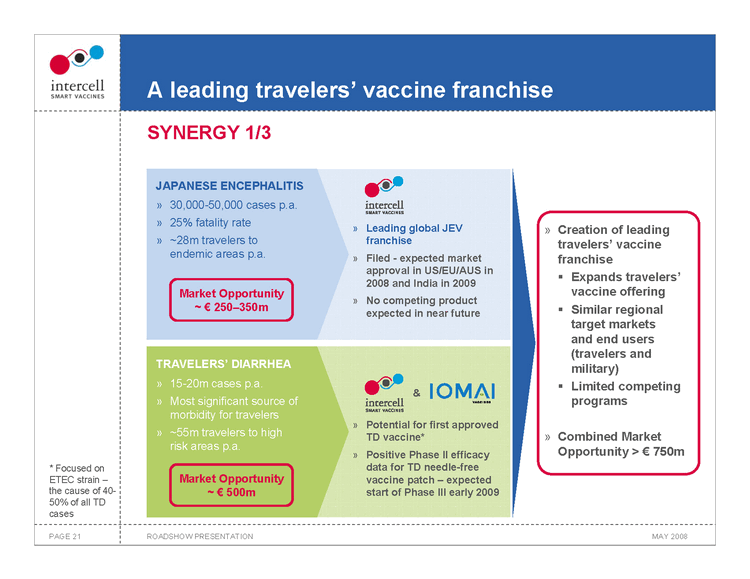
A leading travelers’ vaccine franchise SYNERGY 1/3
JAPANESE ENCEPHALITIS
30,000-50,000 cases p.a. 25% fatality rate ~28m travelers to endemic areas p.a. Market Opportunity ~€250—350m
Leading global JEV franchise Filed — expected market approval in US/EU/AUS in 2008 and India in 2009 No competing product expected in near future
TRAVELERS’ DIARRHEA
15-20m cases p.a. Most significant source of morbidity for travelers ~55m travelers to high risk areas p.a.
Market Opportunity ~€500m
Potential for first approved TD vaccine* Positive Phase II efficacy data for TD needle-free vaccine patch — expected start of Phase III early 2009
Creation of leading travelers’ vaccine franchise ?Expands travelers’ vaccine offering Similar regional target markets and end users (travelers and militry) ???Limited competing programs Combined Market Opportunity >€750m
• Focused on ETEC strain —the cause of 40-50% of all TD cases
PAGE 21 ROADSHOW PRESENTATION MAY 2008
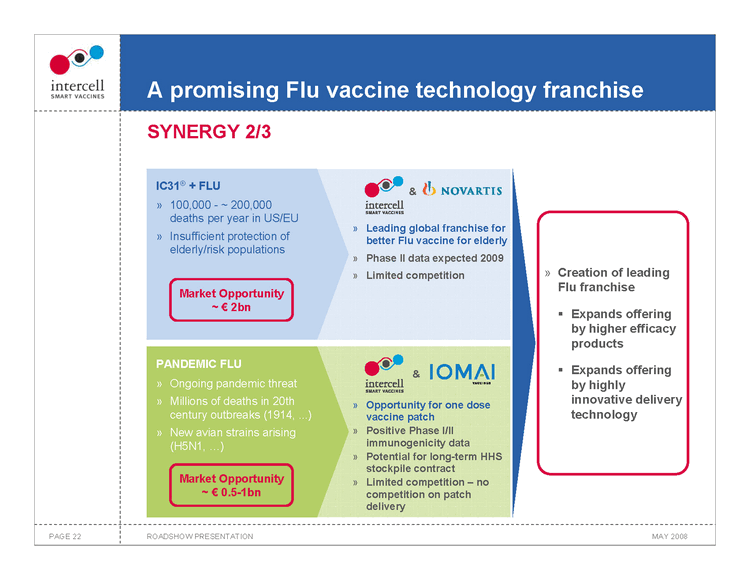
A promising Flu vaccine technology franchise SYNERGY 2/3
IC31® + FLU
100,000 ~ 200,000 deaths per year in US/EU Leading global franchise for Insufficient protection of better Flu vaccine for elderly elderly/risk populations Phase II data expected 2009 Limited competition Market Opportunity ~€2bn
PANDEMIC FLU
Ongoing pandemic threat Millions of deaths in 20th Opportunity for one dose century outbreaks (1914, ...) vaccine patch New avian strains arising Positive Phase I/II (H5N1, ...) immunogenicity data Potential for long-term HHS stockpile contract Market Opportunity Limited competition — no€0.5-1bn competition on patch delivery
Creation of leading Flu franchise ?Expands offering by higher efficacy products ?Expands offering by highly innovative delivery technology
PAGE 22 ROADSHOW PRESENTATION MAY 2008
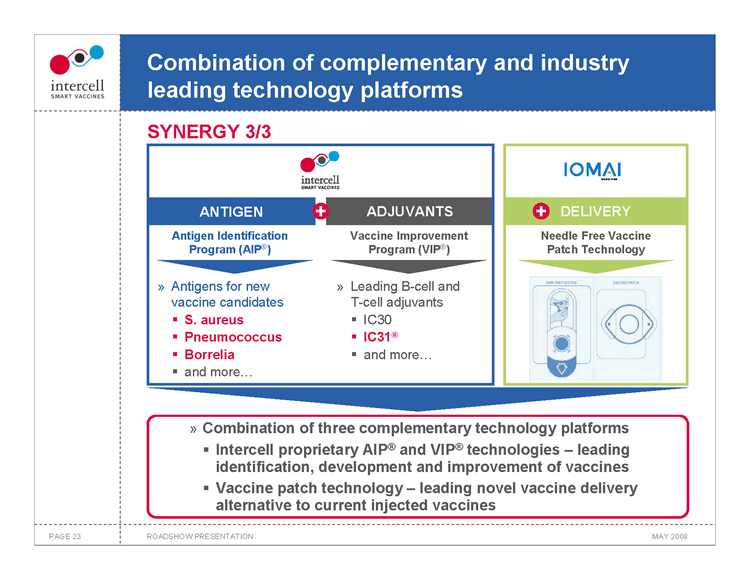
Combination of complementary and industry leading technology platforms SYNERGY 3/3
ANTIGEN ADJUVANTS Antigen Identification Vaccine Improvement Program (AIP®) Program (VIP®)
Antigens for new Leading B-cell and vaccine candidates T-cell adjuvants
?S. aureus ?IC30
?Pneumococcus ?IC31® ?Borrelia ?and more... ?and more...
DELIVERY Needle Free Vaccine Patch Technology
Combination of three complementary technology platforms ?Intercell proprietary AIP® and VIP® technologies — leading identification, development and improvement of vaccines ?Vaccine patch technology — leading novel vaccine delivery alternative to current injected vaccines
PAGE 23 ROADSHOW PRESENTATION MAY 2008
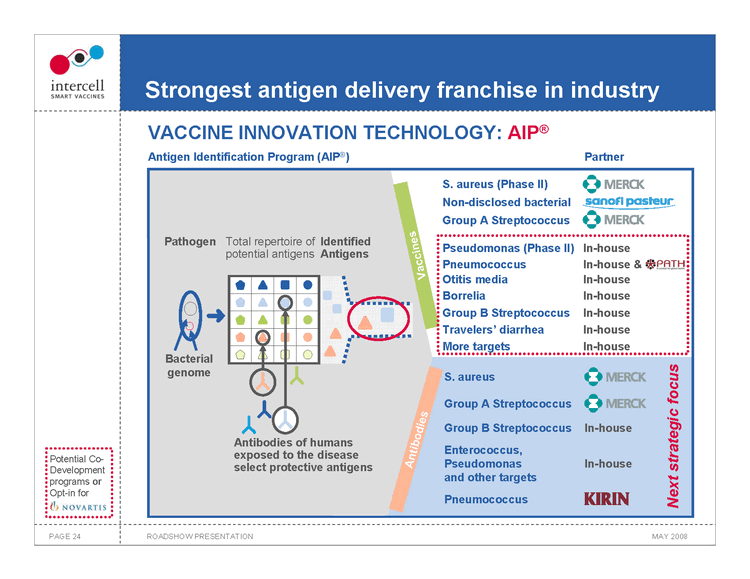
Strongest antigen delivery franchise in industry VACCINE INNOVATION TECHNOLOGY: AIP®
Antigen Identification Program (AIP®) Partner
Pathogen
Total repertoire of Identified potential antigens Antigens
Bacterial genome Antibodies Antibodies of humans exposed to the disease select protective antigens Vaccines
S. aureus (Phase II) Non-disclosed bacterial Group A Streptococcus
Pseudomonas (Phase II) In-house Pneumococcus In-house & Otitis media In-house Borrelia In-house Group B Streptococcus In-house Travelers’ diarrhea In-house More targets In-house
S. aureus
Group A Streptococcus Group B StreptococcusIn-house Enterococcus, Pseudomonas In-house and other targets Pneumococcus
Next strategic focus
PAGE 24 ROADSHOW PRESENTATION MAY 2008
Potential Co-Development programs or Opt-in for
Next Strategic Focus: Broadening the use of novel vaccine adjuvant VACCINE INNOVATION TECHNOLOGY IC31®
IC31® peptide + oligonucleotide
FormationAntigen uptake of Depotby APCs
Long lasting depot
Anti- bodies APC
CTLs
Humoral andMaturation/ cellular responseactivation of APCs
Prophylactic
Therapeutic
Target Partner Flu vaccine — exclusive (Phase I) Tuberculosis vaccine — exclusive (Phase I/II) Defined infectious disease vaccines — non-exclusive Malaria vaccine — non-exclusive Meningitis vaccine — non-exclusive Infectious Diseases, In-house / to Allergy, Cancer be partnered
HCV vaccine — co-development &
Infectious Diseases, In-house / to Allergy, Cancer be partnered
PAGE 25 ROADSHOW PRESENTATION MAY 2008
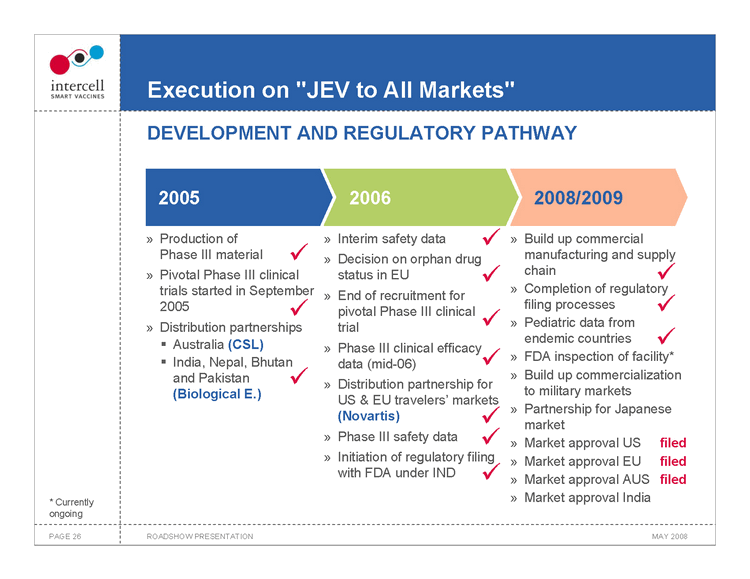
Execution on “JEV to All Markets” DEVELOPMENT AND REGULATORY PATHWAY
2005 2006 2008/2009
Production of Interim safety data ? Build up commercial Phase III material ? Decision on orphan drug manufacturing and supply Pivotal Phase III clinical status in EU ? chain ? trials started in September Completion of regulatory End of recruitment for 2005 filing processes ? ? pivotal Phase III clinical Distribution partnerships trial ? Pediatric data from endemic countries ? ?Australia (CSL) Phase III clinical efficacy ? India, Nepal, Bhutan FDA inspection of facility* data (mid-06) ? and Pakistan ? Build up commercialization Distribution partnership for (Biological E.) to military markets US & EU travelers’ markets Partnership for Japanese (Novartis) ? market Phase III safety data ? Market approval US filed Initiation of regulatory filing Market approval EU filed with FDA under IND ? Market approval AUS filed Market approval India
• Currently ongoing
PAGE 26 ROADSHOW PRESENTATION MAY 2008
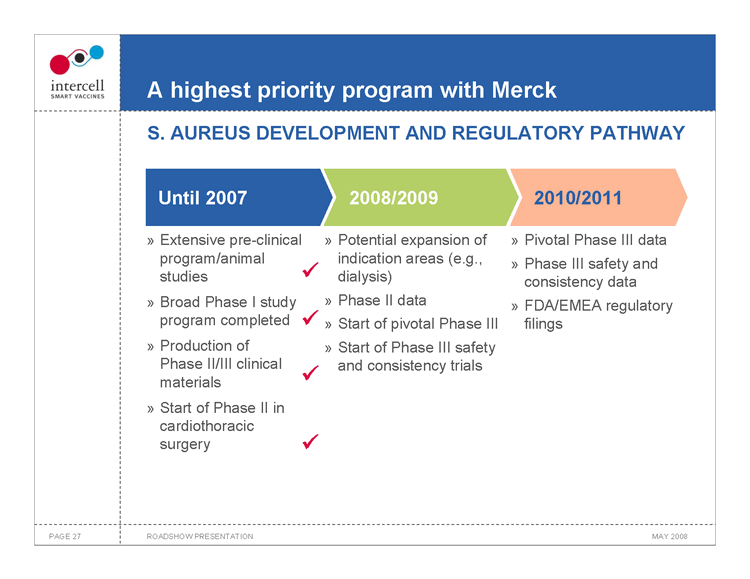
A highest priority program with Merck S. AUREUS DEVELOPMENT AND REGULATORY PATHWAY
Until 2007 2008/20092010/2011
Extensive pre-clinicalPotential expansion of» Pivotal Phase III data program/animal indication areas (e.g.,» Phase III safety and studies dialysis) consistency data Broad Phase I study Phase II data» FDA/EMEA regulatory program completed Start of pivotal Phase IIIfilings Production of Start of Phase III safety Phase II/III clinical and consistency trials materials Start of Phase II in cardiothoracic surgery
PAGE 27 ROADSHOW PRESENTATION MAY 2008
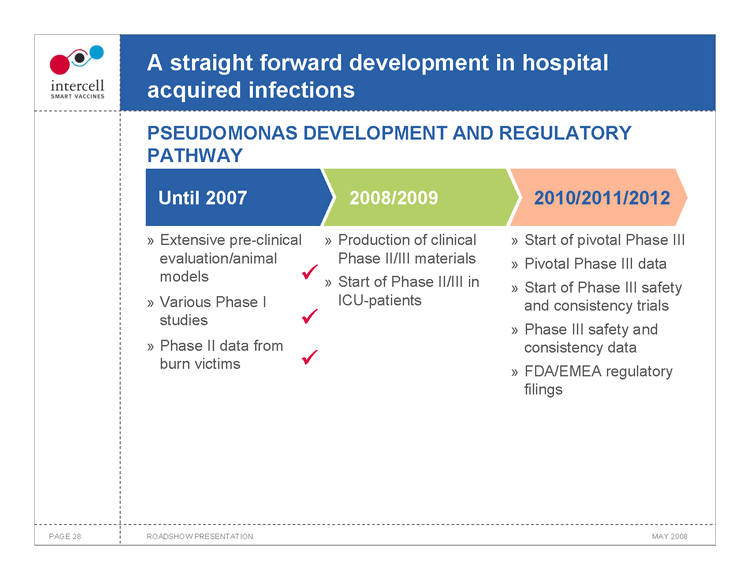
A straight forward development in hospital acquired infections PSEUDOMONAS DEVELOPMENT AND REGULATORY PATHWAY
Until 2007 2008/20092010/2011/2012
Extensive pre-clinical »Production of clinical » Start of pivotal Phase III evaluation/animal Phase II/III materials» Pivotal Phase III data models ?» Start of Phase II/III in » Start of Phase III safety Various Phase I ICU-patientsand consistency trials studies ? » Phase III safety and Phase II data from consistency data burn victims ? » FDA/EMEA regulatory filings
PAGE 28 ROADSHOW PRESENTATION MAY 2008
Agenda
Overview Introduction to Iomai — The Target Company Deal Rationale & Product Development Update Q1 2008: Results & Strategic Implications Intercell Going Forward
PAGE 29 ROADSHOW PRESENTATION MAY 2008
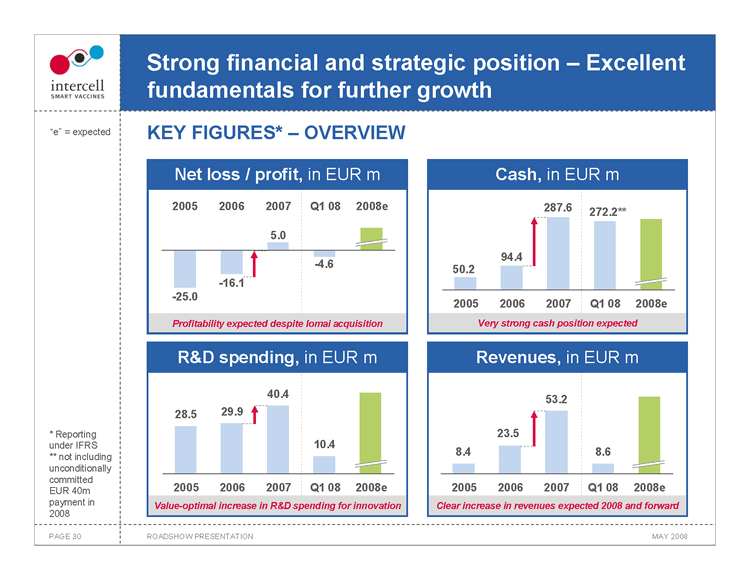
Strong financial and strategic position — Excellent fundamentals for further growth KEY FIGURES* — OVERVIEW
“e” = expected
Net loss / profit, in EUR m 2005 2006 2007 Q1 08 2008e
5.0
-4.6
-16.1
-25.0 Profitability expected despite Iomai acquisition Cash, in EUR m 287.6 272.2** 94.4
50.2 2005 2006 2007 Q1 08 2008e
Very strong cash position expected
R&D spending, in EUR m 40.4
28.5 29.9
10.4
2005 2006 2007 Q1 08 2008e
Value-optimal increase in R&D spending for innovation
Revenues, in EUR m 53.2
23.5
8.4 8.6
2005 2006 2007 Q1 08 2008e
Clear increase in revenues expected 2008 and forward
• Reporting under IFRS ** not including unconditionally committed EUR 40m payment in 2008
PAGE 30 ROADSHOW PRESENTATION MAY 2008
Agenda
Overview Introduction to Iomai — The Target Company Deal Rationale & Product Development Update Q1 2008: Results & Strategic Implications Intercell Going Forward
PAGE 31 ROADSHOW PRESENTATION MAY 2008
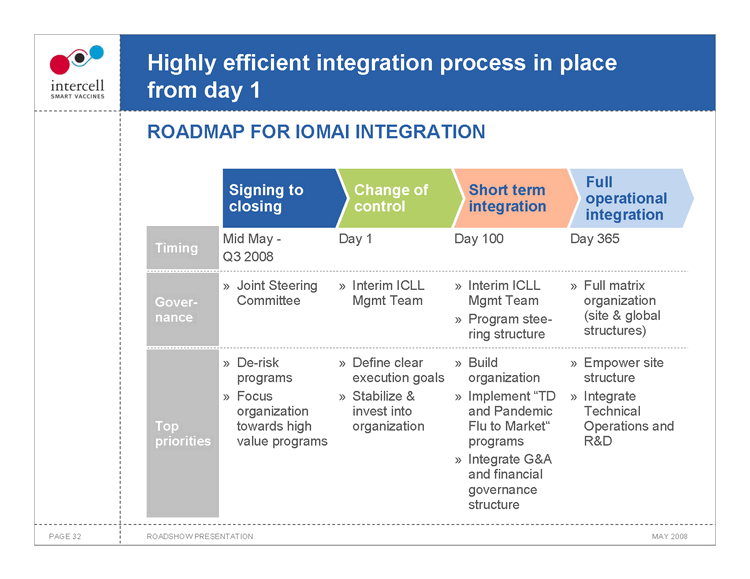
Highly efficient integration process in place from day 1 ROADMAP FOR IOMAI INTEGRATION
Signing to closingChange of controlShort term integration Full operational integration Mid May -Day 1Day 100 Day 365 Timing Q3 2008 Joint Steering» Interim ICLL» Interim ICLL » Full matrix Gover- CommitteeMgmt TeamMgmt Team organization nance » Program stee- (site & global ring structure structures) De-risk» Define clear» Build » Empower site programsexecution goalsorganization structure Focus» Stabilize &» Implement “TD » Integrate organizationinvest intoand Pandemic Technical Top towards highorganizationFlu to Market” Operations and priorities value programs programs R&D » Integrate G&A and financial governance structure
PAGE 32 ROADSHOW PRESENTATION MAY 2008
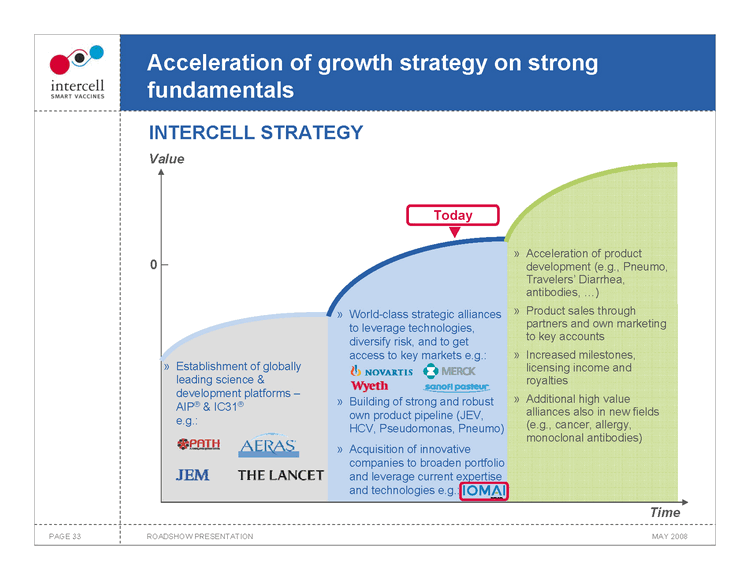
Acceleration of growth strategy on strong fundamentals INTERCELL STRATEGY
Value
Today
Acceleration of product development (e.g., Pneumo, Travelers’ Diarrhea, antibodies, ...)
World-class strategic alliances to leverage technologies, diversify risk, and to get access to key markets e.g.: Product sales through partners and own marketing to key accounts
Establishment of globally leading science & development platforms — AIP® & IC31® e.g.: Increased milestones,licensing income and royalties
Building of strong and robust own product pipeline (JEV, HCV, Pseudomonas, Pneumo) Additional high value alliances also in new fields(e.g., cancer, allergy, monoclonal antibodies)
Acquisition of innovative companies to broaden portfolio and leverage current expertise and technologies e.g.:
Time
PAGE 33 ROADSHOW PRESENTATION MAY 2008
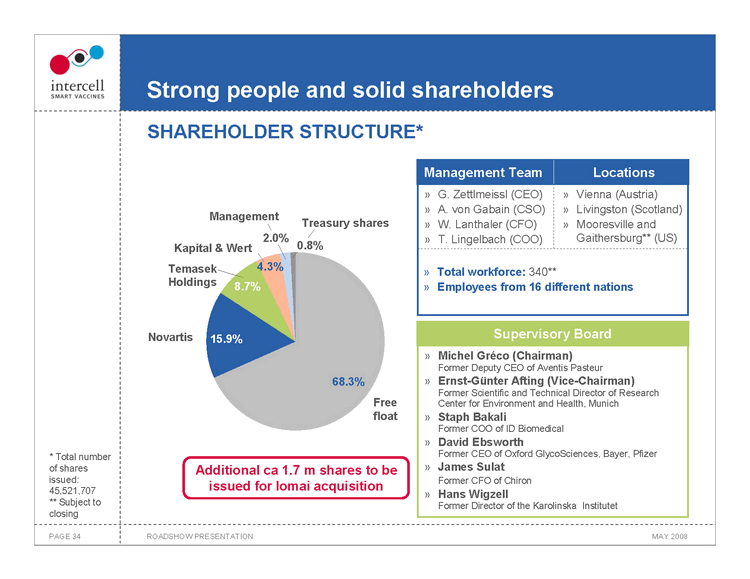
Strong people and solid shareholders SHAREHOLDER STRUCTURE*
Management Treasury shares Kapital & Wert 2.0% 0.8% Temasek 4.3% Holdings 8.7% Novartis 15.9% 68.3% Free float Management Team Locations
G. Zettlmeissl (CEO) Vienna (Austria) A. von Gabain (CSO) Livingston (Scotland) W. Lanthaler (CFO) Mooresville and T. Lingelbach (COO) Gaithersburg** (US)
Total workforce: 340** Employees from 16 different nations
Supervisory Board » Michel Gréco (Chairman)
Former Deputy CEO of Aventis Pasteur » Ernst-Günter Afting (Vice-Chairman)
Former Scientific and Technical Director of Research Center for Environment and Health, Munich » Staph Bakali
Former COO of ID Biomedical » David Ebsworth
Former CEO of Oxford GlycoSciences, Bayer, Pfizer » James Sulat
Former CFO of Chiron » Hans Wigzell
Former Director of the Karolinska Institutet
• Total number of shares issued: 45,521,707 ** Subject to closing
Additional ca 1.7 m shares to be issued for Iomai acquisition
PAGE 34 ROADSHOW PRESENTATION MAY 2008
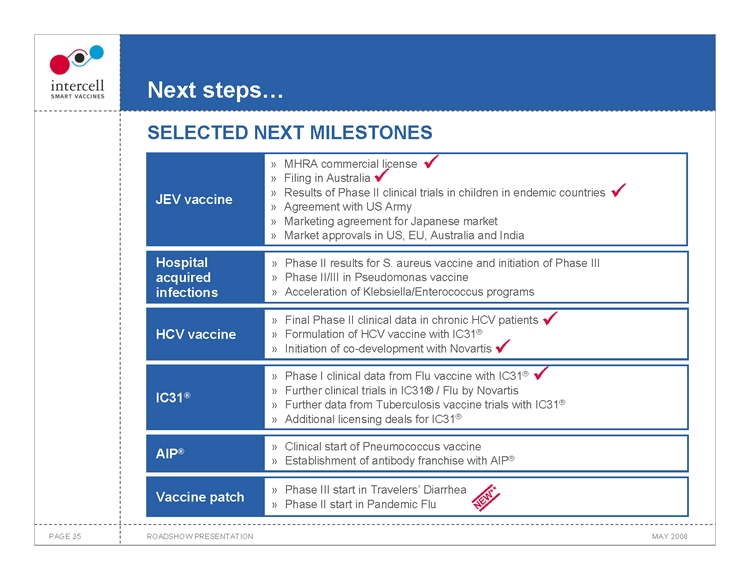
Next steps... SELECTED NEXT MILESTONES
MHRA commercial license ? Filing in Australia ? Results of Phase II clinical trials in children in endemic countries ?
JEV vaccine
Agreement with US Army Marketing agreement for Japanese market Market approvals in US, EU, Australia and India
Hospital Phase II results for S. aureus vaccine and initiation of Phase III acquired Phase II/III in Pseudomonas vaccine infections Acceleration of Klebsiella/Enterococcus programs Final Phase II clinical data in chronic HCV patients ? HCV vaccine Formulation of HCV vaccine with IC31® Initiation of co-development with Novartis ? Phase I clinical data from Flu vaccine with IC31® ? Further clinical trials in IC31® / Flu by Novartis IC31® Further data from Tuberculosis vaccine trials with IC31® Additional licensing deals for IC31®
Clinical start of Pneumococcus vaccine AIP® Establishment of antibody franchise with AIP®
Phase III start in Travelers’ Diarrhea Vaccine patch Phase II start in Pandemic Flu NEW**
PAGE 35 ROADSHOW PRESENTATION MAY 2008
QUESTIONS & ANSWERS
SMART VACCINES
For more information be invited to: www.intercell.com