
Developing Breakthrough Biologics, Life-changing Medicines® Post-ESMO Conference Call September 16, 2024

2 Legal Notices The information in this slide deck is current as of September 16, 2024, unless otherwise noted, and is qualified in its entirety by reference to MacroGenics’ Annual, Quarterly and Current Reports filed with the SEC. MacroGenics undertakes no obligation to update any of the information herein. Cautionary Note on Forward-Looking Statements Any statements in this presentation about future expectations, plans and prospects for MacroGenics (“Company”), including statements about the Company’s strategy, future operations, clinical development of and regulatory plans for the Company’s therapeutic candidates, expected timing of the release of final safety and efficacy data, including mature median rPFS and other statements containing the words “subject to”, "believe", “anticipate”, “plan”, “expect”, “intend”, “estimate”, “potential,” “project”, “may”, “will”, “should”, “would”, “could”, “can”, the negatives thereof, variations thereon and similar expressions, or by discussions of strategy constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: risks that TZIELD, vobramitamab duocarmazine, lorigerlimab, ZYNYZ, MARGENZA or any other product candidate’s revenue, expenses and costs may not be as expected, risks relating to TZIELD, vobramitamab duocarmazine, lorigerlimab, ZYNYZ, MARGENZA or any other product candidate’s market acceptance, competition, reimbursement and regulatory actions; future data updates, especially timing and results of mature median radiographic progression-free survival, other efficacy and safety data with respect to vobramitamab duocarmazine; our ability to provide manufacturing services to our customers; the uncertainties inherent in the initiation and enrollment of future clinical trials; the availability of financing to fund the internal development of our product candidates; expectations of expanding ongoing clinical trials; availability and timing of data from ongoing clinical trials; expectations for the timing and steps required in the regulatory review process; expectations for regulatory approvals; expectations of future milestone payments; the impact of competitive products; our ability to enter into agreements with strategic partners and other matters that could affect the availability or commercial potential of the Company's product candidates; business, economic or political disruptions due to catastrophes or other events, including natural disasters, terrorist attacks, civil unrest and actual or threatened armed conflict, or public health crises; costs of litigation and the failure to successfully defend lawsuits and other claims against us; and other risks described in the Company's filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views only as of the date hereof. The Company anticipates that subsequent events and developments will cause the Company's views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do so, except as may be required by law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date hereof. Trademarks DART, TRIDENT, MacroGenics, the MacroGenics logo and MARGENZA are trademarks or registered trademarks of MacroGenics, Inc. All third-party trademarks used herein are registered trademarks of their respective owners. Investigational Agents The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. September 16, 2024

3 Conference Call Agenda Introduction Scott Koenig, M.D., Ph.D. – President and CEO, MacroGenics Updated TAMARACK Study Results Stephen Eck, M.D. – SVP, Clinical Development and Chief Medical Officer Pipeline Update Scott Koenig, M.D., Ph.D. Q&A September 16, 2024

4 Conference Call Agenda Introduction Scott Koenig, M.D., Ph.D. – President and CEO, MacroGenics Updated TAMARACK Study Results Stephen Eck, M.D. – SVP, Clinical Development and Chief Medical Officer Pipeline Update Scott Koenig, M.D., Ph.D. Q&A September 16, 2024
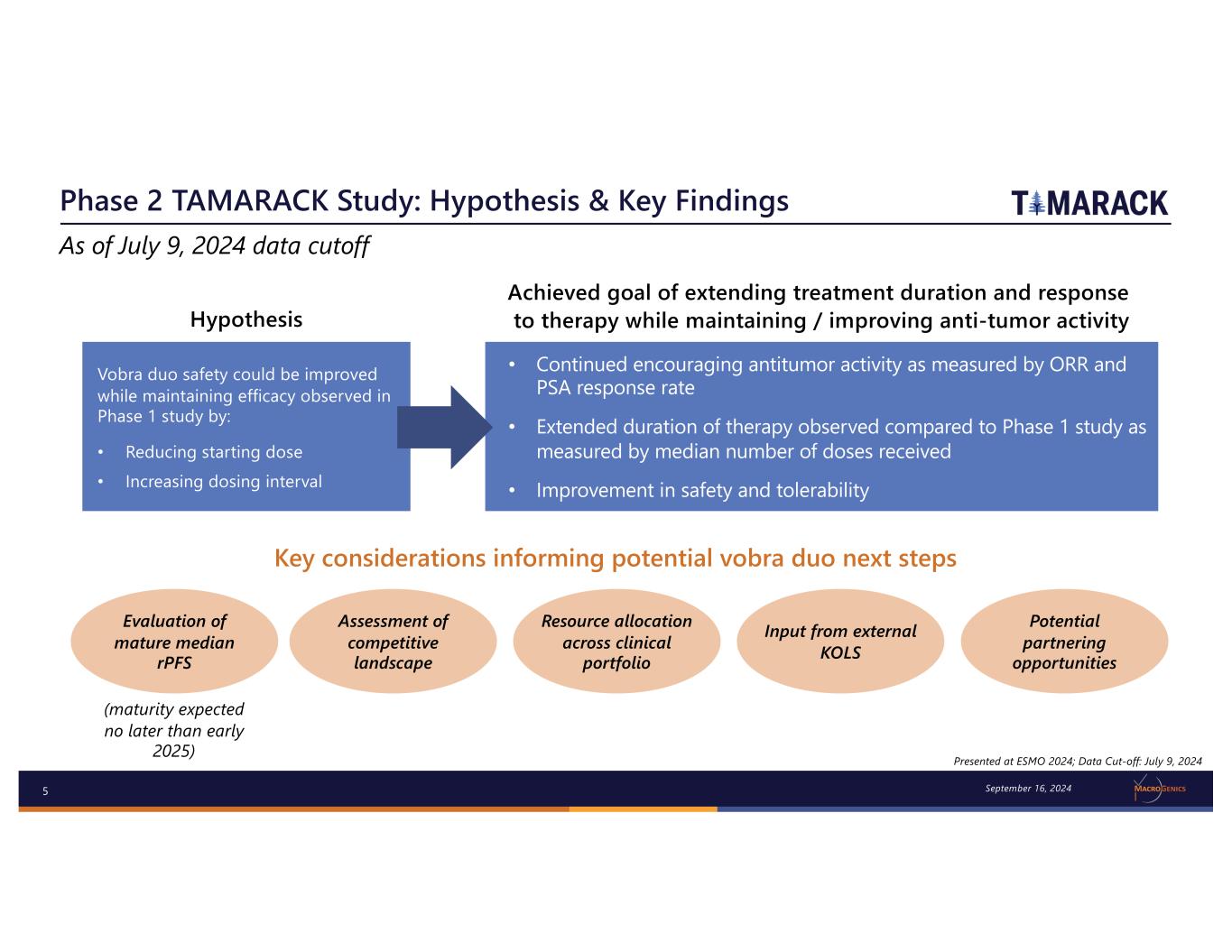
5 Phase 2 TAMARACK Study: Hypothesis & Key Findings Hypothesis • Continued encouraging antitumor activity as measured by ORR and PSA response rate • Extended duration of therapy observed compared to Phase 1 study as measured by median number of doses received • Improvement in safety and tolerability Achieved goal of extending treatment duration and response to therapy while maintaining / improving anti-tumor activity Vobra duo safety could be improved while maintaining efficacy observed in Phase 1 study by: • Reducing starting dose • Increasing dosing interval Key considerations informing potential vobra duo next steps Evaluation of mature median rPFS Assessment of competitive landscape Resource allocation across clinical portfolio (maturity expected no later than early 2025) Input from external KOLS Potential partnering opportunities Presented at ESMO 2024; Data Cut-off: July 9, 2024 As of July 9, 2024 data cutoff September 16, 2024

6 Conference Call Agenda Introduction Scott Koenig, M.D., Ph.D. – President and CEO, MacroGenics Updated TAMARACK Study Results Stephen Eck, M.D. – SVP, Clinical Development and Chief Medical Officer Pipeline Update Scott Koenig, M.D., Ph.D. Q&A September 16, 2024
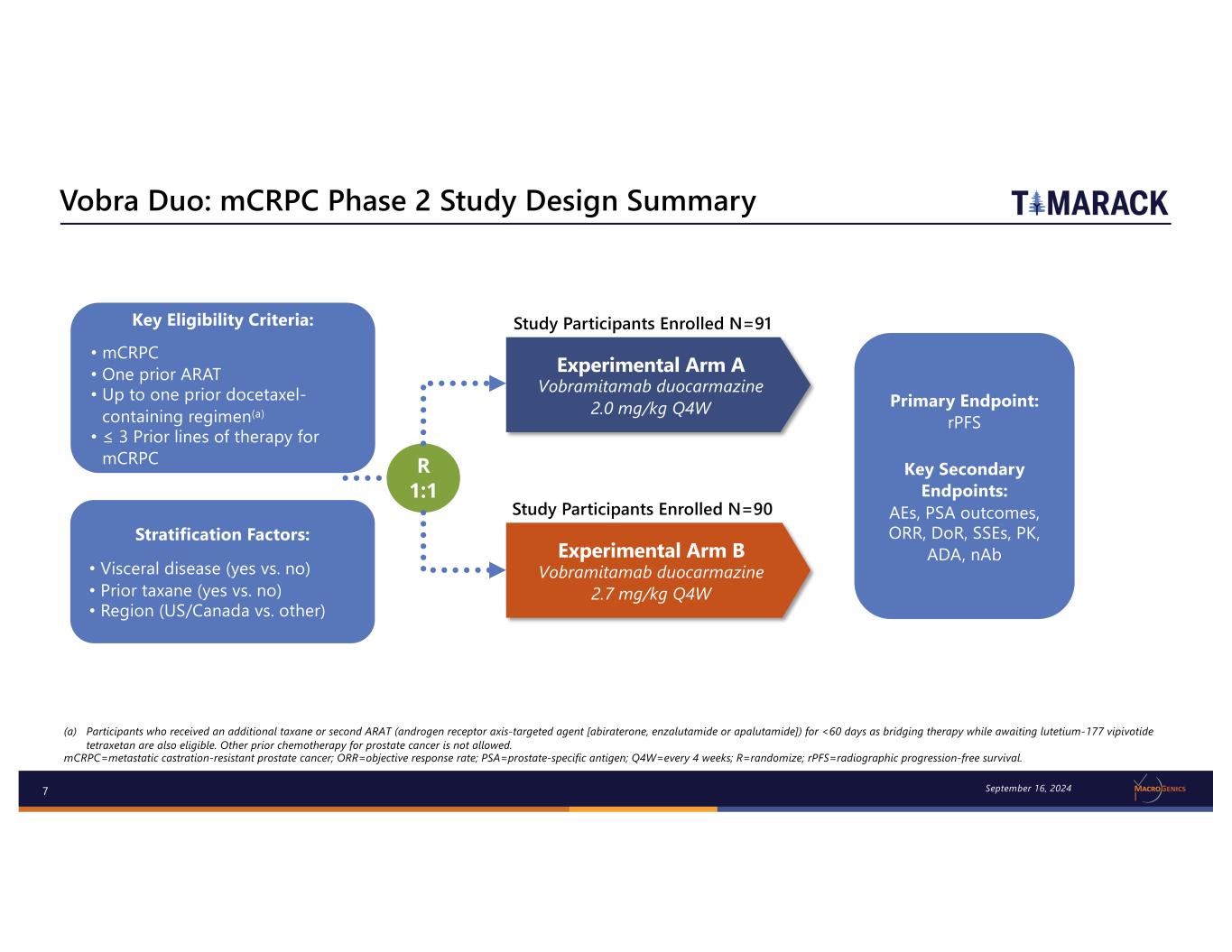
7 Experimental Arm A Vobramitamab duocarmazine 2.0 mg/kg Q4W Experimental Arm B Vobramitamab duocarmazine 2.7 mg/kg Q4W R 1:1 Study Participants Enrolled N=91 Primary Endpoint: rPFS Key Secondary Endpoints: AEs, PSA outcomes, ORR, DoR, SSEs, PK, ADA, nAb (a) Participants who received an additional taxane or second ARAT (androgen receptor axis-targeted agent [abiraterone, enzalutamide or apalutamide]) for <60 days as bridging therapy while awaiting lutetium-177 vipivotide tetraxetan are also eligible. Other prior chemotherapy for prostate cancer is not allowed. mCRPC=metastatic castration-resistant prostate cancer; ORR=objective response rate; PSA=prostate-specific antigen; Q4W=every 4 weeks; R=randomize; rPFS=radiographic progression-free survival. Vobra Duo: mCRPC Phase 2 Study Design Summary (TAMARACK) Stratification Factors: • Visceral disease (yes vs. no) • Prior taxane (yes vs. no) • Region (US/Canada vs. other) Key Eligibility Criteria: • mCRPC • One prior ARAT • Up to one prior docetaxel- containing regimen(a) • ≤ 3 Prior lines of therapy for mCRPC September 16, 2024 Study Participants Enrolled N=90
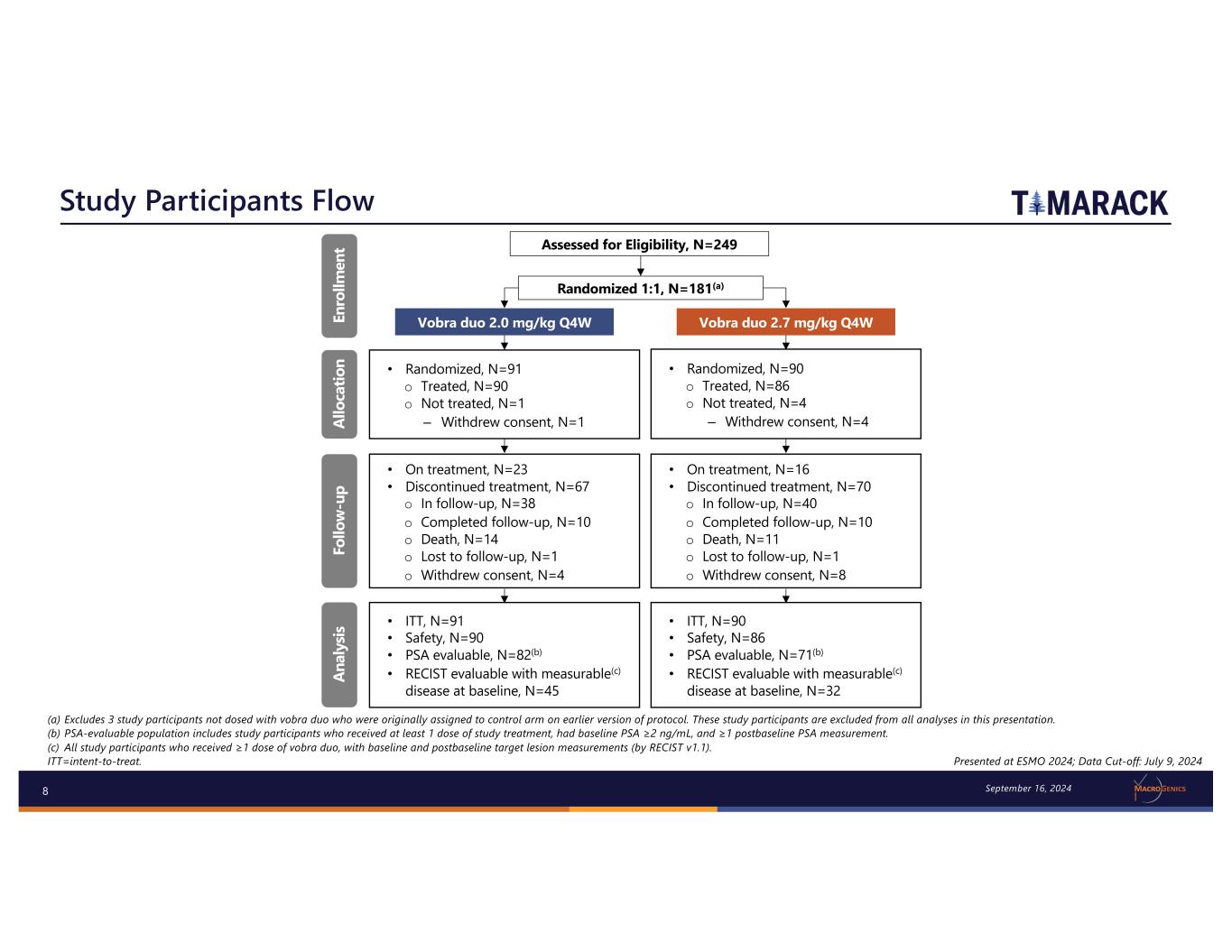
8 Study Participants Flow (a) Excludes 3 study participants not dosed with vobra duo who were originally assigned to control arm on earlier version of protocol. These study participants are excluded from all analyses in this presentation. (b) PSA-evaluable population includes study participants who received at least 1 dose of study treatment, had baseline PSA ≥2 ng/mL, and ≥1 postbaseline PSA measurement. (c) All study participants who received ≥1 dose of vobra duo, with baseline and postbaseline target lesion measurements (by RECIST v1.1). ITT=intent-to-treat. • Randomized, N=91 o Treated, N=90 o Not treated, N=1 – Withdrew consent, N=1 • Randomized, N=90 o Treated, N=86 o Not treated, N=4 – Withdrew consent, N=4 • ITT, N=91 • Safety, N=90 • PSA evaluable, N=82(b) • RECIST evaluable with measurable(c) disease at baseline, N=45 • ITT, N=90 • Safety, N=86 • PSA evaluable, N=71(b) • RECIST evaluable with measurable(c) disease at baseline, N=32 • On treatment, N=23 • Discontinued treatment, N=67 o In follow-up, N=38 o Completed follow-up, N=10 o Death, N=14 o Lost to follow-up, N=1 o Withdrew consent, N=4 • On treatment, N=16 • Discontinued treatment, N=70 o In follow-up, N=40 o Completed follow-up, N=10 o Death, N=11 o Lost to follow-up, N=1 o Withdrew consent, N=8 Randomized 1:1, N=181(a) Assessed for Eligibility, N=249 Vobra duo 2.0 mg/kg Q4W Vobra duo 2.7 mg/kg Q4WEn ro llm en t Al lo ca tio n Fo llo w -u p An al ys is Presented at ESMO 2024; Data Cut-off: July 9, 2024 September 16, 2024

9 Demographics and Baseline Characteristics (a) All randomized study participants, including the study participants not treated. PARP=poly (ADP-ribose) polymerase. Parameter Vobra duo 2.0 mg/kg Q4W N=91 Vobra duo 2.7 mg/kg Q4W N=90 Median (range) age, years 71 (46-89) 69 (35-86) ECOG PS, n (%) 0 42 (46.2) 51 (56.7) 1 48 (52.7) 37 (41.1) 2 1 (1.1) 2 (2.2) Disease status at first diagnosis, n (%) Local resectable 28 (30.8) 37 (41.1) Locally advanced unresectable 12 (13.2) 9 (10.0) Metastatic 51 (56.0) 44 (48.9) Type of disease progression at study entry, n (%) Radiographic progression of measurable disease 43 (47.3) 31 (34.4) Radiographic progression of bone disease (in >2 new bone lesions) 33 (36.3) 41 (45.6) PSA progression only 24 (26.4) 25 (27.8) PSA progression with any other type of progression 39 (42.9) 32 (35.6) Study participants with visceral disease, n (%) 15 (16.5) 15 (16.7) Study participants with prior taxane, n (%) 48 (52.7) 49 (54.4) Study participants with prior PARP, n (%) 6 (6.6) 8 (8.9) Number of prior ARAT, n (%) 1 82 (90.1) 84 (93.3) >1 9 (9.9) 6 (6.7) Baseline PSA n 89 85 Mean (standard deviation), ng/mL 180.5 (542.60) 182.6 (433.06) Median (range), ng/mL 26.4 (0.8-3447.0) 24.7 (0.2-2778.0) PSA ≥2 ng/mL, n (%) 83 (91.2) 74 (82.2) ITT population, N=181(a) Presented at ESMO 2024; Data Cut-off: July 9, 2024 September 16, 2024
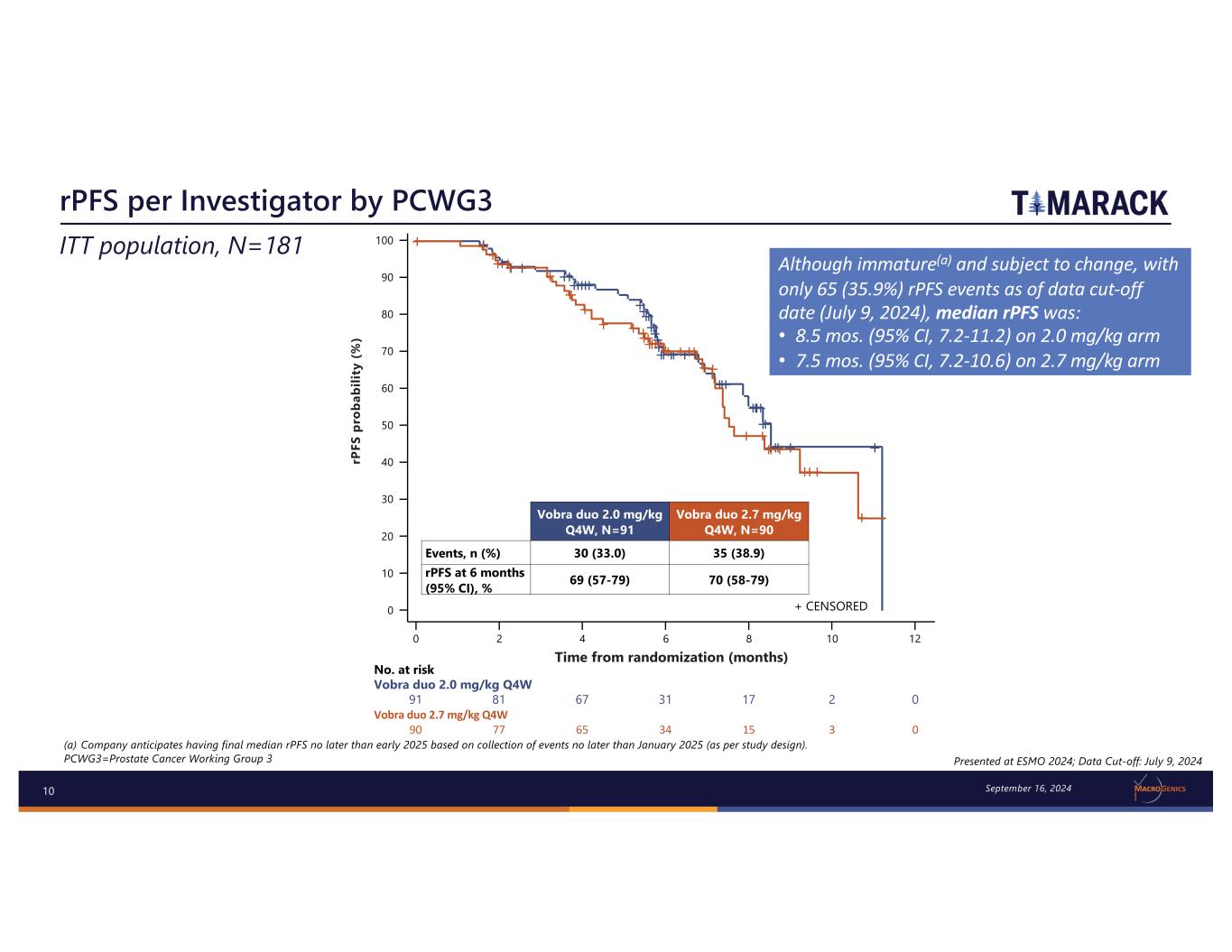
10 rPFS per Investigator by PCWG3 Presented at ESMO 2024; Data Cut-off: July 9, 2024 ITT population, N=181 100 90 80 70 60 50 40 30 20 10 0 0 2 4 6 8 10 12 No. at risk Vobra duo 2.0 mg/kg Q4W 91 81 67 31 17 2 0 Vobra duo 2.7 mg/kg Q4W 90 77 65 34 15 3 0 rP FS p ro ba bi lit y (% ) Time from randomization (months) Vobra duo 2.0 mg/kg Q4W, N=91 Vobra duo 2.7 mg/kg Q4W, N=90 Events, n (%) 30 (33.0) 35 (38.9) rPFS at 6 months (95% CI), % 69 (57-79) 70 (58-79) + CENSORED September 16, 2024 (a) Company anticipates having final median rPFS no later than early 2025 based on collection of events no later than January 2025 (as per study design). PCWG3=Prostate Cancer Working Group 3 Although immature(a) and subject to change, with only 65 (35.9%) rPFS events as of data cut-off date (July 9, 2024), median rPFS was: • 8.5 mos. (95% CI, 7.2-11.2) on 2.0 mg/kg arm • 7.5 mos. (95% CI, 7.2-10.6) on 2.7 mg/kg arm

11 Tumor and PSA Responses (a) All study participants who received ≥1 dose of vobra duo, with baseline and postbaseline target lesion measurements (by RECIST v1.1). (b) All study participants who received ≥1 dose of vobra duo, with a baseline PSA ≥2 ng/mL and ≥1 postbaseline PSA measurement. NE=not evaluable; SD=stable disease. Vobra duo 2.0 mg/kg Q4W Vobra duo 2.7 mg/kg Q4W RECIST response-evaluable population w/baseline measurable disease(a) N=45 N=32 Best overall response (confirmed), n (%) CR 0 1 (3.1) PR 9 (20.0) 12 (37.5) SD 30 (66.7) 15 (46.9) PD 5 (11.1) 2 (6.3) NE 1 (2.2) 2 (6.3) Confirmed ORR (CR + PR), n (%) 9 (20.0) 13 (40.6) Confirmed + unconfirmed ORR, n (%) 12 (26.7) 15 (46.9) Median (range) DOR of confirmed RECIST responders, months [n] 4.9 (1.94-6.47) [9] NE (1.54-9.46) [13] PSA response-evaluable population(b) N=82 N=71 PSA50 response (confirmed), n (%) 37 (45.1) 28 (39.4) PSA50 response (confirmed + unconfirmed), n (%) 41 (50.0) 37 (52.1) Median (range) DOR of confirmed PSA50 responders, months [n] NE (0.95-9.23) [37] NE (0.95-9.49) [28] Presented at ESMO 2024; Data Cut-off: July 9, 2024 September 16, 2024
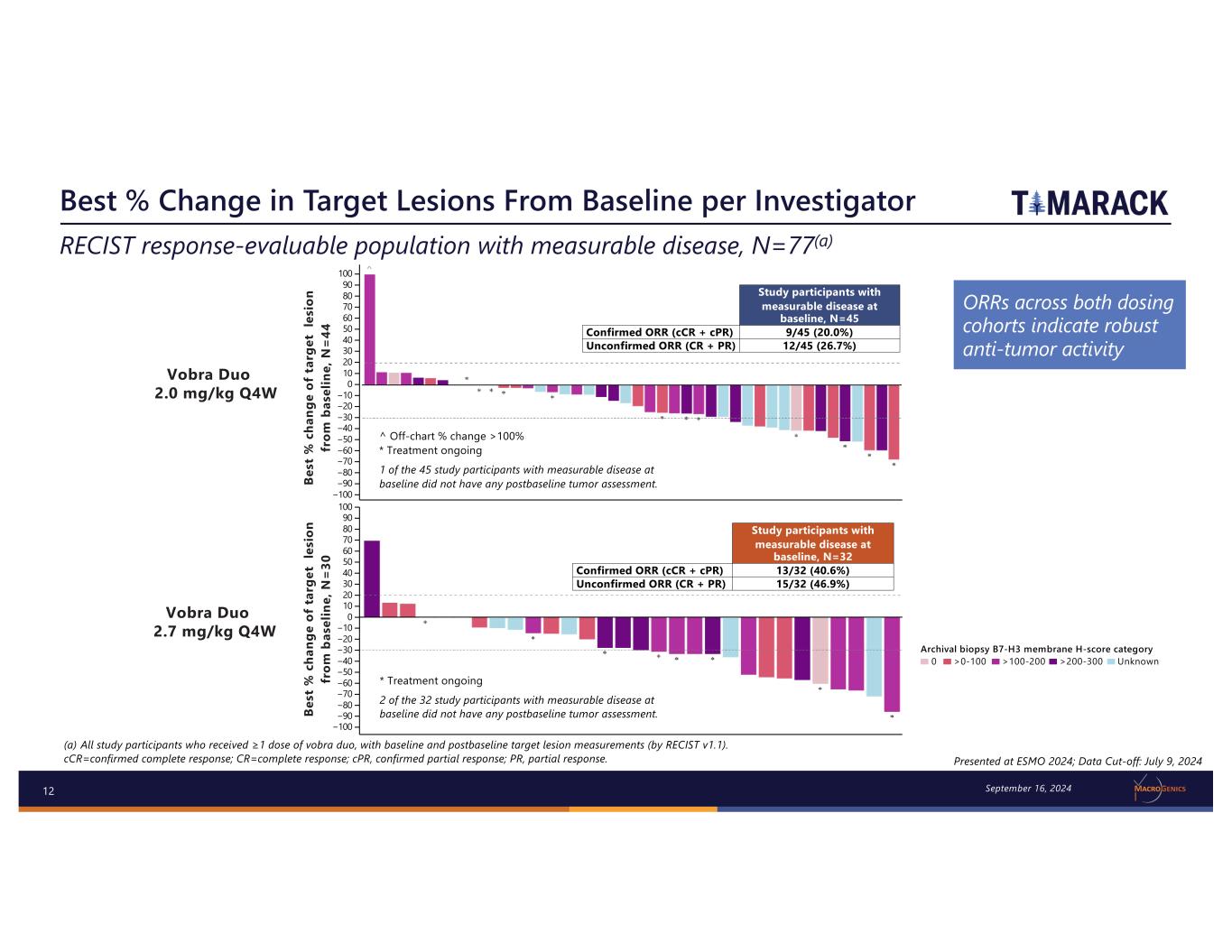
12 Best % Change in Target Lesions From Baseline per Investigator (a) All study participants who received ≥1 dose of vobra duo, with baseline and postbaseline target lesion measurements (by RECIST v1.1). cCR=confirmed complete response; CR=complete response; cPR, confirmed partial response; PR, partial response. Presented at ESMO 2024; Data Cut-off: July 9, 2024 RECIST response-evaluable population with measurable disease, N=77(a) Be st % c ha ng e of t ar ge t le si on fr om b as el in e, N = 44 Study participants with measurable disease at baseline, N=45 Confirmed ORR (cCR + cPR) 9/45 (20.0%) Unconfirmed ORR (CR + PR) 12/45 (26.7%) −100 100 90 80 70 60 50 40 30 20 10 0 −10 −20 −40 −60 −80 −30 −50 −70 −90 Archival biopsy B7-H3 membrane H-score category 0 >0-100 >100-200 >200-300 Unknown ^ Off-chart % change >100% * Treatment ongoing 1 of the 45 study participants with measurable disease at baseline did not have any postbaseline tumor assessment. Vobra Duo 2.0 mg/kg Q4W Be st % c ha ng e of t ar ge t le si on fr om b as el in e, N = 30 Vobra Duo 2.7 mg/kg Q4W Study participants with measurable disease at baseline, N=32 Confirmed ORR (cCR + cPR) 13/32 (40.6%) Unconfirmed ORR (CR + PR) 15/32 (46.9%) −100 100 90 80 70 60 50 40 30 20 10 0 −10 −20 −40 −60 −80 −30 −50 −70 −90 2 of the 32 study participants with measurable disease at baseline did not have any postbaseline tumor assessment. * Treatment ongoing September 16, 2024 ORRs across both dosing cohorts indicate robust anti-tumor activity
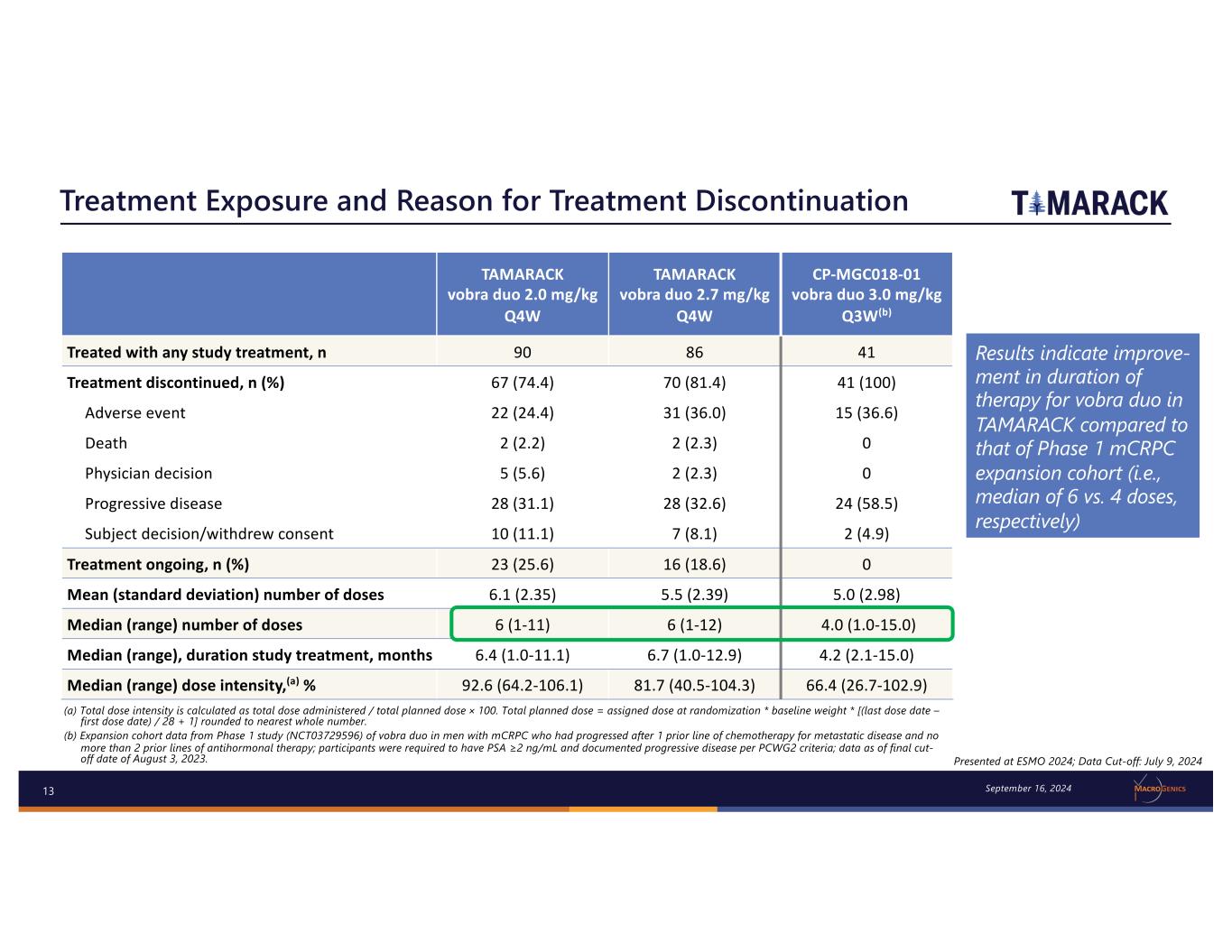
13 Treatment Exposure and Reason for Treatment Discontinuation (a) Total dose intensity is calculated as total dose administered / total planned dose × 100. Total planned dose = assigned dose at randomization * baseline weight * [(last dose date – first dose date) / 28 + 1] rounded to nearest whole number. (b) Expansion cohort data from Phase 1 study (NCT03729596) of vobra duo in men with mCRPC who had progressed after 1 prior line of chemotherapy for metastatic disease and no more than 2 prior lines of antihormonal therapy; participants were required to have PSA ≥2 ng/mL and documented progressive disease per PCWG2 criteria; data as of final cut- off date of August 3, 2023. TAMARACK vobra duo 2.0 mg/kg Q4W TAMARACK vobra duo 2.7 mg/kg Q4W CP-MGC018-01 vobra duo 3.0 mg/kg Q3W(b) Treated with any study treatment, n 90 86 41 Treatment discontinued, n (%) 67 (74.4) 70 (81.4) 41 (100) Adverse event 22 (24.4) 31 (36.0) 15 (36.6) Death 2 (2.2) 2 (2.3) 0 Physician decision 5 (5.6) 2 (2.3) 0 Progressive disease 28 (31.1) 28 (32.6) 24 (58.5) Subject decision/withdrew consent 10 (11.1) 7 (8.1) 2 (4.9) Treatment ongoing, n (%) 23 (25.6) 16 (18.6) 0 Mean (standard deviation) number of doses 6.1 (2.35) 5.5 (2.39) 5.0 (2.98) Median (range) number of doses 6 (1-11) 6 (1-12) 4.0 (1.0-15.0) Median (range), duration study treatment, months 6.4 (1.0-11.1) 6.7 (1.0-12.9) 4.2 (2.1-15.0) Median (range) dose intensity,(a) % 92.6 (64.2-106.1) 81.7 (40.5-104.3) 66.4 (26.7-102.9) Presented at ESMO 2024; Data Cut-off: July 9, 2024 September 16, 2024 Results indicate improve- ment in duration of therapy for vobra duo in TAMARACK compared to that of Phase 1 mCRPC expansion cohort (i.e., median of 6 vs. 4 doses, respectively)

14 Overall Summary of TEAEs on TAMARACK and CP-MGC018-01 (a) Includes events with causality assessments of “possible,” “probable,” or “definite,” per investigator. (b) Expansion cohort data from Phase 1 study (NCT03729596) of vobra duo in men with mCRPC who had progressed after 1 prior line of chemotherapy for metastatic disease and no more than 2 prior lines of antihormonal therapy; participants were required to have PSA ≥2 ng/mL and documented progressive disease per PCWG2 criteria; data as of final cut-off date of August 3, 2023. SAE=serious adverse event. AEs, n (%) Vobra duo 2.0 mg/kg Q4W N=90 Vobra duo 2.7 mg/kg Q4W N=86 CP-MGC018-01 3.0 mg/kg Q3W(b) N=41 Any TEAE 89 (98.9) 86 (100) 41 (100) Treatment-related AEs(a) 87 (96.7) 84 (97.7) 41 (100) Any grade ≥3 TEAE 59 (65.6) 54 (62.8) 33 (80.5) Grade ≥3 treatment-related AE(a) 42 (46.7) 45 (52.3) 32 (78.0) Any SAE 34 (37.8) 38 (44.2) 23 (56.1) Treatment-related SAEsa 23 (25.6) 24 (27.9) 19 (46.3) Fatal treatment-related AEs 5 (5.6) 3 (3.5) 2 (4.9) TEAEs resulting in vobra duo discontinuation 23 (25.6) 33 (38.4) 15 (36.6) TEAEs resulting in vobra duo dose reductions 45 (50.0) 47 (54.7) 28 (68.3) TEAEs resulting in vobra duo interruption 46 (51.1) 51 (59.3) 28 (68.3) Presented at ESMO 2024; Data Cut-off: July 9, 2024 September 16, 2024 Overall improvement in safety and tolerability in TAMARACK compared to that of Phase 1 mCRPC expansion cohort

15 (Safety population, N=176) (a) Includes one treatment-related pleural effusion event reported to be grade 3 but with a fatal outcome; site query to correct the discrepancy is pending. TEAEs Reported in ≥10% of Study Participants in Either Arm Presented at ESMO 2024; Data Cut-off: July 9, 2024 Asthenia – Pleural effusion(a) – Decreased appetite – Edema peripheral – Nausea – PPE syndrome – Stomatitis – Neutropenia – Fatigue – Diarrhea – Anemia – Constipation – Dyspnea – Conjunctivitis – Headache – Pericardial effusion – Pyrexia – Cough – Thrombocytopenia – Back pain – Insomnia – Dysgeusia – Abdominal pain – Dry eye – Lymphopenia – Infusion-related reaction – Platelet count decreased – Weight decreased – Dry skin – Atrial fibrillation – Rash – Arthralgia – Vomiting – Vobra Duo 2.0 mg/kg Q4W Vobra Duo 2.7 mg/kg Q4W % of study participants with TEAEs 51.1% 59.3% 28.9% 44.2% 35.6% 39.5% 36.7% 37.2% 35.6% 30.2% 18.9% 27.9% 13.3% 26.7% 18.9% 25.6% 26.7% 23.3% 27.8% 23.3% 23.3% 23.3% 24.4% 23.3% 10.0% 19.8% 12.2% 19.8% 13.3% 17.4% 13.3% 17.4% 13.3% 15.1% 7.8% 15.1% 6.7% 15.1% 10.0% 14.0% 3.3% 11.6% 11.1% 11.6% 4.4% 11.6% 12.2% 10.5% 4.4% 10.5% 4.4% 10.5% 8.9% 10.5% 11.1% 9.3% 18.9% 9.3% 10.0% 8.1% 10.0% 8.1% 13.3% 8.1% 15.6% 5.8% Grade 4 Grade 3 Grade 2 Grade 1 Grade 5 100 90 80 70 60 50 40 30 20 10 0 10 20 30 40 50 60 70 80 90 100 September 16, 2024
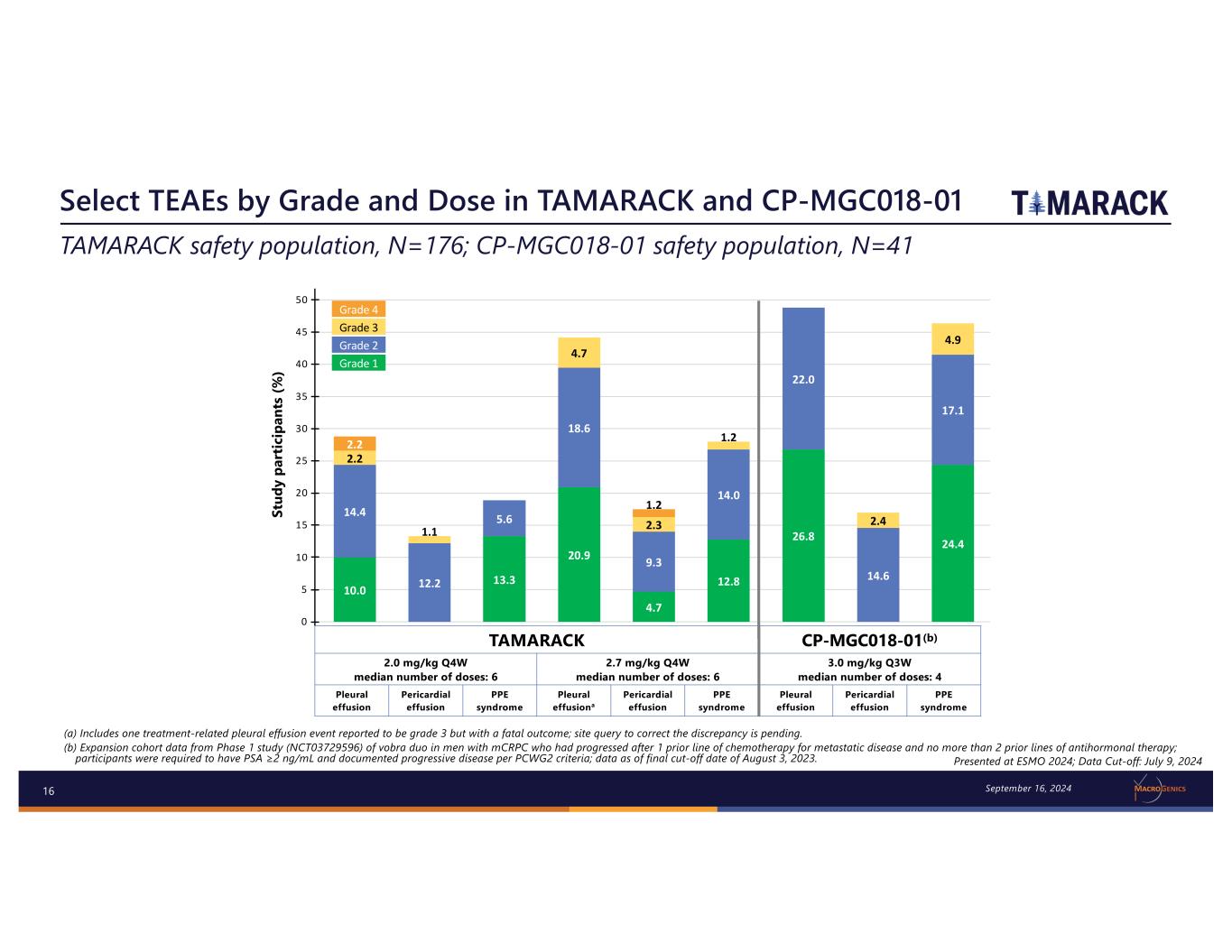
16 Select TEAEs by Grade and Dose in TAMARACK and CP-MGC018-01 (a) Includes one treatment-related pleural effusion event reported to be grade 3 but with a fatal outcome; site query to correct the discrepancy is pending. (b) Expansion cohort data from Phase 1 study (NCT03729596) of vobra duo in men with mCRPC who had progressed after 1 prior line of chemotherapy for metastatic disease and no more than 2 prior lines of antihormonal therapy; participants were required to have PSA ≥2 ng/mL and documented progressive disease per PCWG2 criteria; data as of final cut-off date of August 3, 2023. TAMARACK safety population, N=176; CP-MGC018-01 safety population, N=41 Presented at ESMO 2024; Data Cut-off: July 9, 2024 10.0 13.3 20.9 4.7 12.8 26.8 24.4 14.4 12.2 5.6 18.6 9.3 14.0 22.0 14.6 17.1 2.2 1.1 4.7 2.3 1.2 2.4 4.9 2.2 1.2 0 5 10 15 20 25 30 35 40 45 50 Grade 1 Grade 2 Grade 3 Grade 4 St ud y pa rt ic ip an ts (% ) Grade 4 Grade 3 Grade 2 Grade 1 TAMARACK CP-MGC018-01(b) 2.0 mg/kg Q4W median number of doses: 6 2.7 mg/kg Q4W median number of doses: 6 3.0 mg/kg Q3W median number of doses: 4 Pleural effusion Pericardial effusion PPE syndrome Pleural effusiona Pericardial effusion PPE syndrome Pleural effusion Pericardial effusion PPE syndrome September 16, 2024

17 Summary As of July 9, 2024 data cutoff • Data demonstrated vobra duo’s antitumor activity by ORR and PSA response rate • Anticipate having final TAMARACK median rPFS no later than early 2025 • Company believes it has better understanding of vobra duo’s overall safety and tolerability and is considering ways to further improve molecule’s safety as it awaits final median rPFS • Through dose reduction and increase in dosing interval, events of neutropenia, anemia, thrombocytopenia, pleural effusion and PPE syndrome improved compared to Phase 1 dose expansion in mCRPC; also, on average, TAMARACK study participants stayed on treatment longer than did those in Phase 1 • Company is considering exploring whether adverse events associated with prolonged exposure to vobra duo could be mitigated by strategies such as further increasing dosing intervals or utilizing loading doses Presented at ESMO 2024; Data Cut-off: July 9, 2024 September 16, 2024

18 Conference Call Agenda Introduction Scott Koenig, M.D., Ph.D. – President and CEO, MacroGenics Updated TAMARACK Study Results Stephen Eck, M.D. – SVP, Clinical Development and Chief Medical Officer Pipeline Update Scott Koenig, M.D., Ph.D. Q&A September 16, 2024
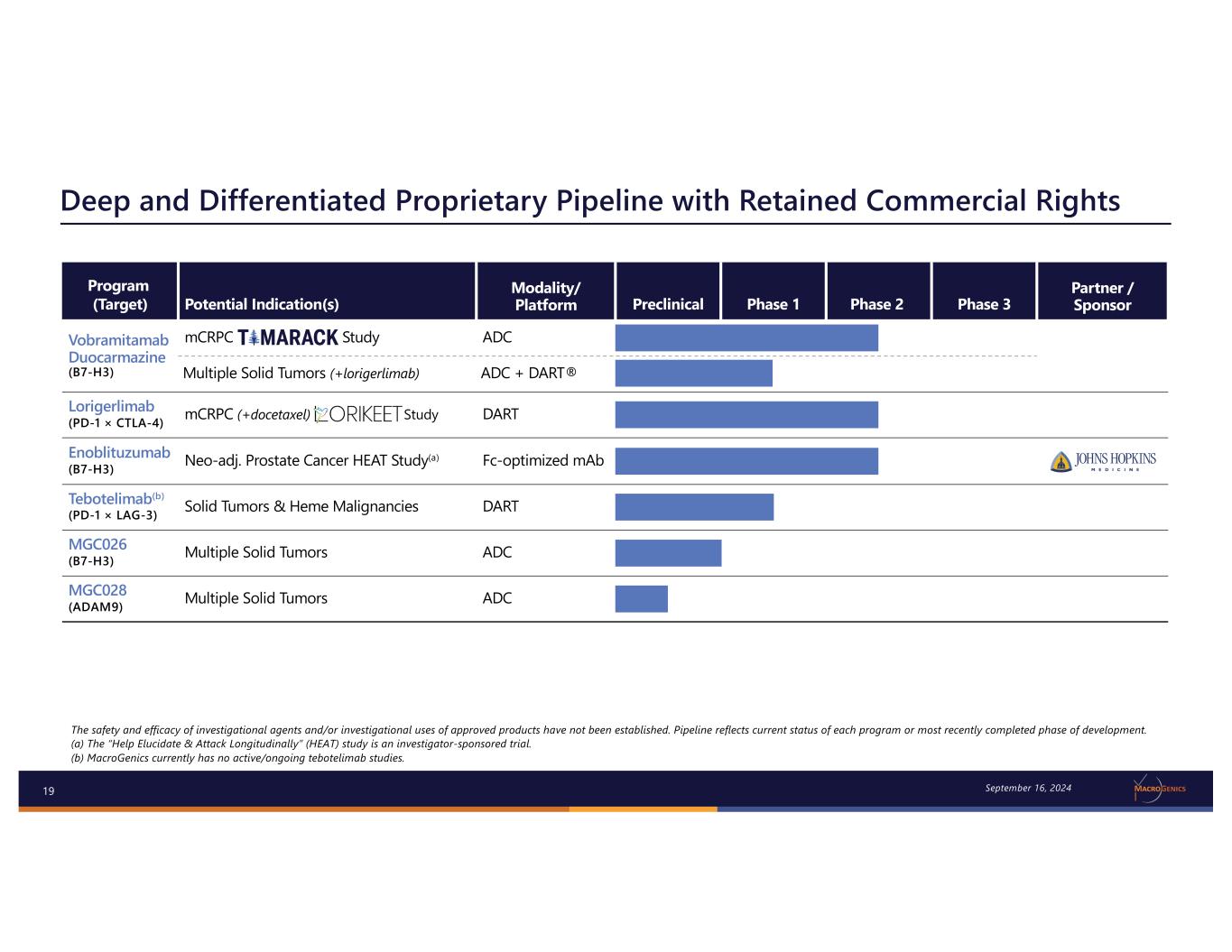
19 Deep and Differentiated Proprietary Pipeline with Retained Commercial Rights The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. Pipeline reflects current status of each program or most recently completed phase of development. (a) The “Help Elucidate & Attack Longitudinally” (HEAT) study is an investigator-sponsored trial. (b) MacroGenics currently has no active/ongoing tebotelimab studies. September 16, 2024 Program (Target) Potential Indication(s) Modality/ Platform Preclinical Phase 1 Phase 2 Phase 3 Partner / Sponsor Vobramitamab Duocarmazine (B7-H3) mCRPC Study ADC Multiple Solid Tumors (+lorigerlimab) ADC + DART® Lorigerlimab (PD-1 × CTLA-4) mCRPC (+docetaxel) Study DART Enoblituzumab (B7-H3) Neo-adj. Prostate Cancer HEAT Study(a) Fc-optimized mAb Tebotelimab(b) (PD-1 × LAG-3) Solid Tumors & Heme Malignancies DART MGC026 (B7-H3) Multiple Solid Tumors ADC MGC028 (ADAM9) Multiple Solid Tumors ADC
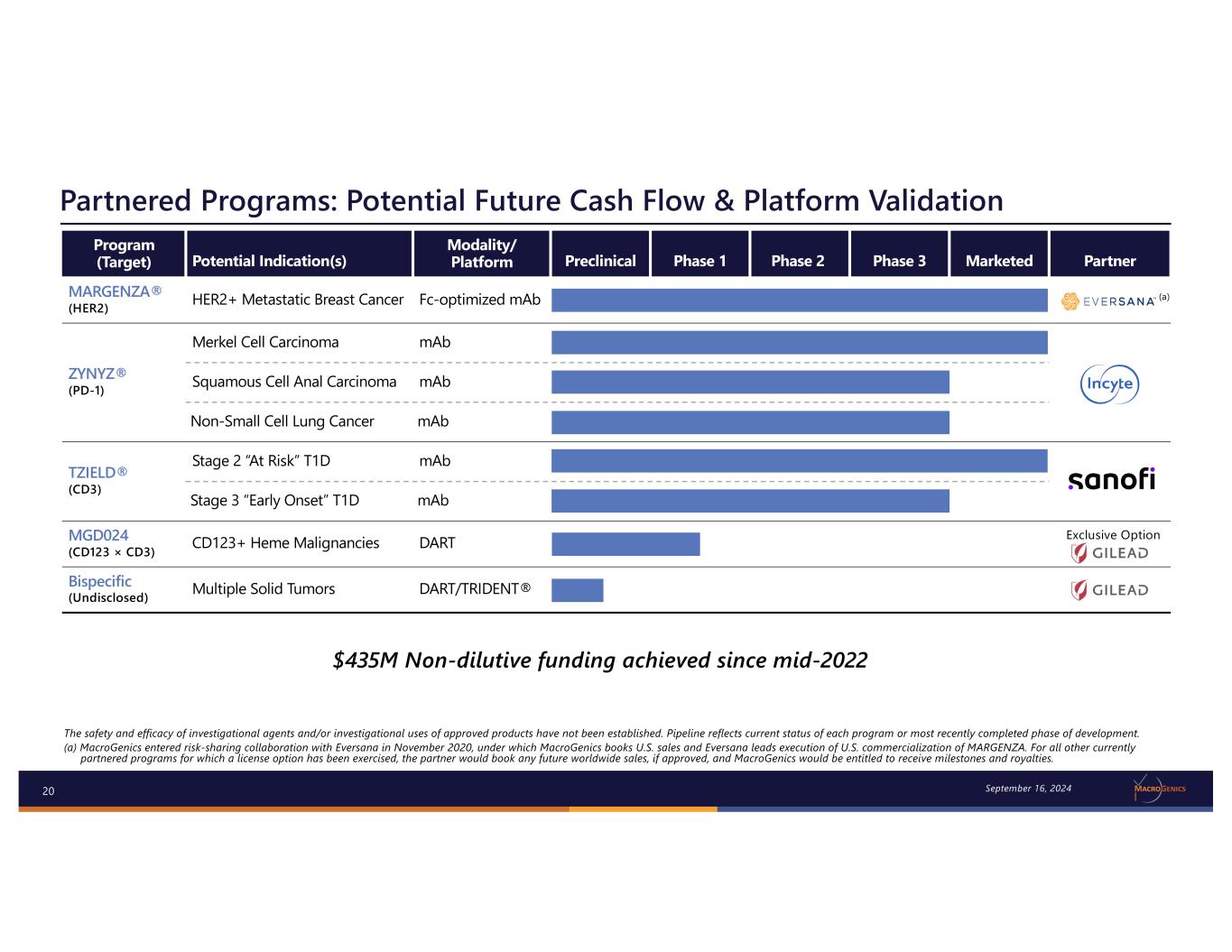
20 The safety and efficacy of investigational agents and/or investigational uses of approved products have not been established. Pipeline reflects current status of each program or most recently completed phase of development. (a) MacroGenics entered risk-sharing collaboration with Eversana in November 2020, under which MacroGenics books U.S. sales and Eversana leads execution of U.S. commercialization of MARGENZA. For all other currently partnered programs for which a license option has been exercised, the partner would book any future worldwide sales, if approved, and MacroGenics would be entitled to receive milestones and royalties. Partnered Programs: Potential Future Cash Flow & Platform Validation September 16, 2024 $435M Non-dilutive funding achieved since mid-2022 Program (Target) Potential Indication(s) Modality/ Platform Preclinical Phase 1 Phase 2 Phase 3 Marketed Partner MARGENZA® (HER2) HER2+ Metastatic Breast Cancer Fc-optimized mAb ZYNYZ® (PD-1) Merkel Cell Carcinoma mAb Squamous Cell Anal Carcinoma mAb Non-Small Cell Lung Cancer mAb TZIELD® (CD3) Stage 2 “At Risk” T1D mAb Stage 3 “Early Onset” T1D mAb MGD024 (CD123 × CD3) CD123+ Heme Malignancies DART Exclusive Option Bispecific (Undisclosed) Multiple Solid Tumors DART/TRIDENT® (a)

21 Multiple data catalysts Studies: Experience in combining novel targets with differentiated drug- linker technology Flexible platforms with clinical and/or partner validation Three approved products generated from our pipeline(a) fuel potential revenue $140M Cash as of 6/30/24, plus $100M in milestones subsequently received from Incyte, and other anticipated and potential future payments, should provide cash runway into 2026 Unique Capabilities to Develop Next Generation Antibodies for Treating Cancer (a) TZIELD® was sold to Provention Bio (Sanofi) and is marketed by Sanofi; ZYNYZ™ was licensed to, and is marketed by, Incyte. (b) The “Help Elucidate & Attack Longitudinally” (HEAT) neo-adjuvant prostate cancer study is an investigator-sponsored trial. September 16, 2024 Multiple Phase 2 Programs in Prostate Cancer Broad Capabilities for Drug Conjugates Proprietary Platforms for Multispecifics Proven R&D Track Record Well Funded to Deliver on Plan HEAT(b)

22 Conference Call Agenda Introduction Scott Koenig, M.D., Ph.D. – President and CEO, MacroGenics Updated TAMARACK Study Results Stephen Eck, M.D. – SVP, Clinical Development and Chief Medical Officer Pipeline Update Scott Koenig, M.D., Ph.D. Q&A September 16, 2024

23 Thank You! Jim Karrels – SVP, Chief Financial Officer 301-354-2681 | karrelsj@macrogenics.com Link to our latest presentations: http://ir.macrogenics.com/events.cfm www.macrogenics.com Investor Relations Inquiries: Business Development Inquiries: September 16, 2024 Eric Risser – Chief Operating Officer rissere@macrogenics.com Harish Krishnaswamy – Vice President, BD krishnaswamyh@macrogenics.com






















