
BioCentury NewsMakers in the Biotech Industry 2013 New York City

Forward-Looking Disclaimer These slides accompany an oral presentation by NewLink Genetics Corporation, which contains forward-looking statements. The Company’s actual results may differ materially from those suggested here. Additional information concerning factors that could cause such a difference is contained in the Company’s Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2013 and other prior and subsequent regulatory filings. 2

NewLink Genetics Corporation Oncology-Focused Biopharmaceutical Company Founded in 1999; IPO in 2011, Currently 100+ employees Pipeline includes biologic and small molecule candidates Completed enrollment for IMPRESS phase 3 trial Strong IP position for technology platforms Added to NASDAQ Biotech Index May 2013 Advancing commercialization efforts for the U.S. market 3
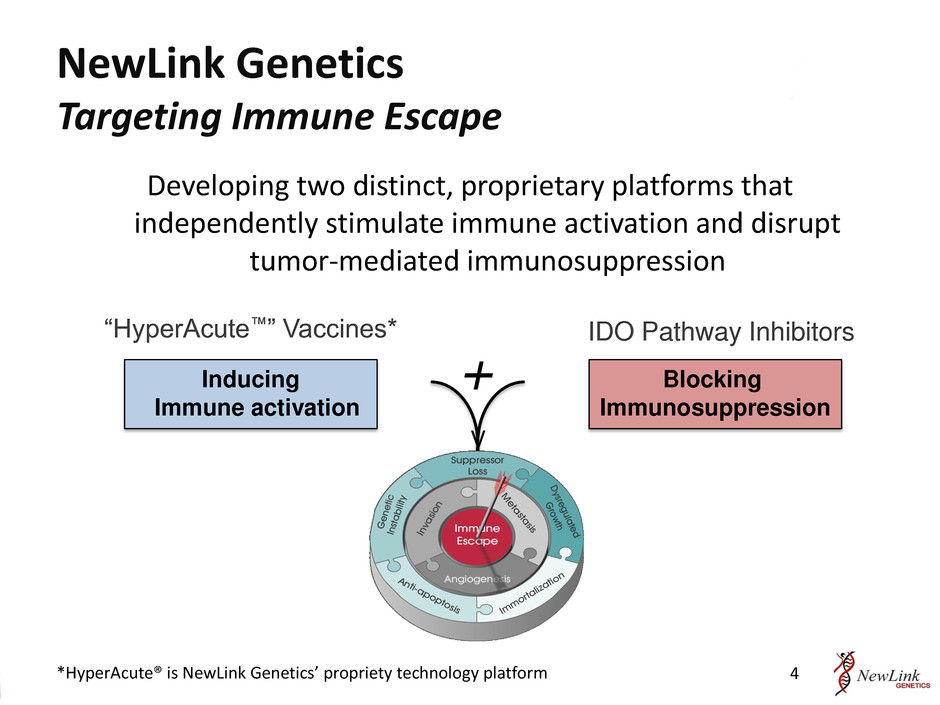
Blocking Immunosuppression “HyperAcute™” Vaccines* IDO Pathway Inhibitors Inducing Immune activation + V NewLink Genetics Targeting Immune Escape Developing two distinct, proprietary platforms that independently stimulate immune activation and disrupt tumor-mediated immunosuppression 4 *HyperAcute® is NewLink Genetics’ propriety technology platform

NewLink Clinical Development Pipeline Targeting Immune Activation & Immunosuppression Product/ Tumor Type Phase 1 Phase 2 Phase 3 HyperAcute™ Platform Algenpantucel-L (pancreas) Tergenpumatucel-L (lung) HyperAcute Melanoma HyperAcute Prostate HyperAcute Renal Expected to enter clinical trials in 2013 IDO Pathway Inhibitor Platform Indoximod – Solid Tumors Metastatic Prostate Metastatic Breast NLG 919 – Solid Tumors Expected to enter clinical trials in 2013 5

HyperAcute™ Immunotherapy “Stimulating the immune system to recognize and attack cancer cells.” 6

HyperAcute™ Platform Activation of Immune Response via Innate Immunity Novel biologics designed to express alpha-gal carbohydrate which stimulates the immune system to recognize and attack cancer cells Cellular immunotherapy that is tumor specific, but does not require tissue from the patient Anti-alpha-gal Ab’s in primates are responsible for “HyperAcute rejection” of xenotransplants Two Phase 3 studies underway for lead product candidate Technology derived from unexpected anti-tumor responses observed in unrelated gene therapy experiment 7 Post-Treatment Pre-Treatment

Mechanism of Action HyperAcute Response Tumor Specific Activity Dendritic Cells Macrophages Eosinophils NK Cells T-cells Complement Mediated Lysis Anti-α Gal Antibodies HyperAcute Immunotherapy Tumor Target Cell Tumor Specific Antibodies and T Cells CD8+ T Cells CD4+ T Cells Activated Dendritic Cells 8
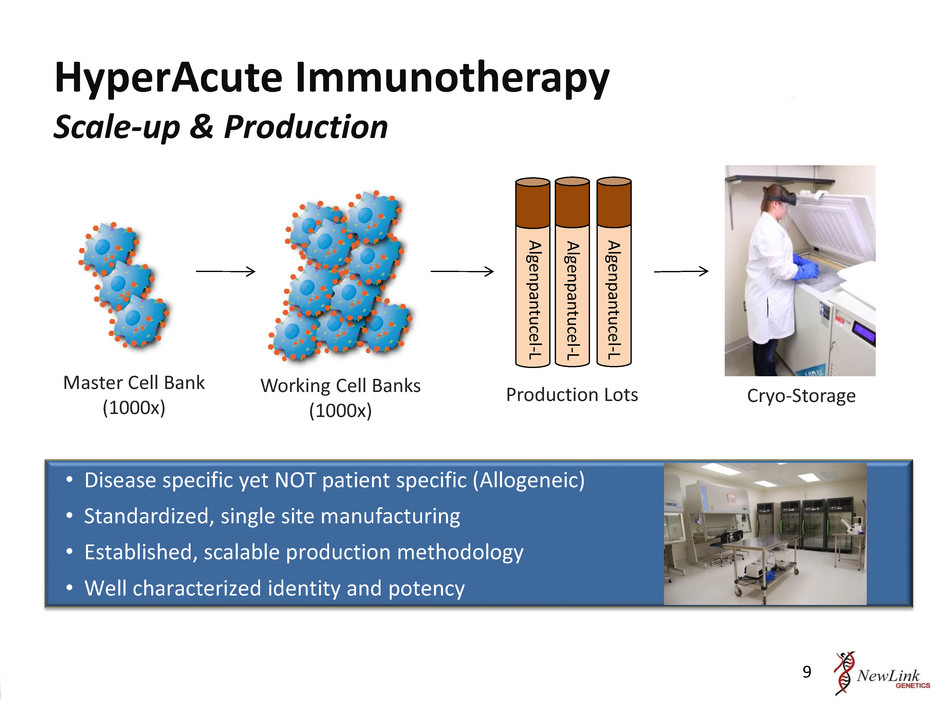
HyperAcute Immunotherapy Scale-up & Production • Disease specific yet NOT patient specific (Allogeneic) • Standardized, single site manufacturing • Established, scalable production methodology • Well characterized identity and potency Master Cell Bank (1000x) Production Lots Working Cell Banks (1000x) Cryo-Storage Al gen p an tu cel-L Al gen p an tu cel-L Al gen p an tu cel-L 9
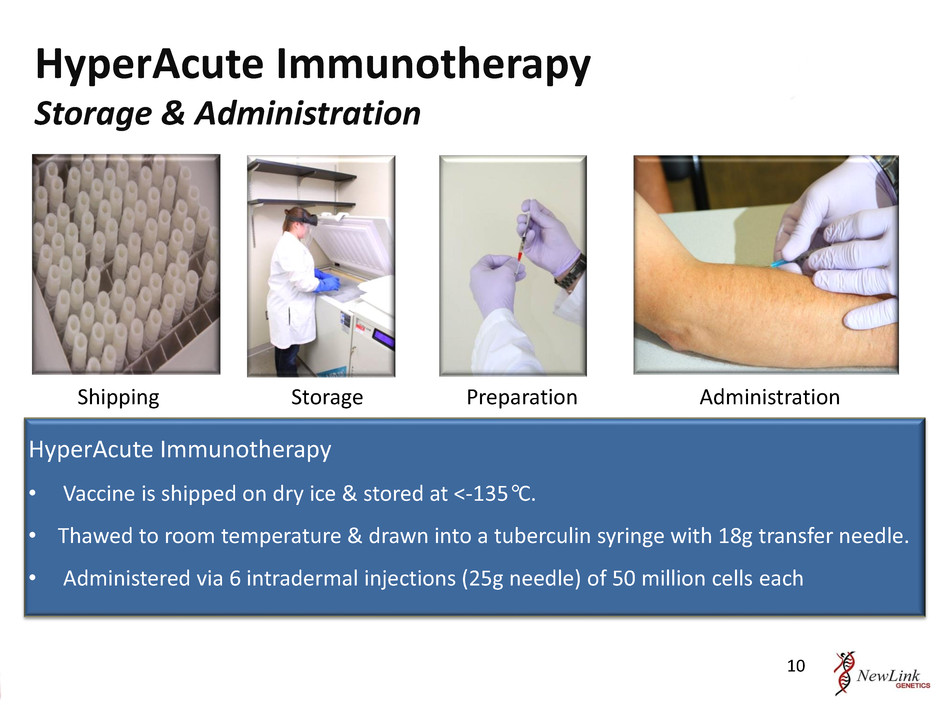
Shipping Storage Preparation Administration HyperAcute Immunotherapy • Vaccine is shipped on dry ice & stored at <-135°C. • Thawed to room temperature & drawn into a tuberculin syringe with 18g transfer needle. • Administered via 6 intradermal injections (25g needle) of 50 million cells each HyperAcute Immunotherapy Storage & Administration 10

Pancreatic Cancer Epidemiology & Pathophysiology 11 4th leading cause of cancer death in U.S.* All stages, 5 year survival** < 5% Stage IIB, resected, 5 year survival** <8% Resection rate 20-25% U.S.* Post resection standard of care Chemotherapy +/- Radiotherapy Gemcitabine +/- 5FU Concurrent Radiotherapy Annual Incidence in Major Markets Total U.S.* Europe Japan 117,000 43,000 45,000 29,000 *2013 Cancer Facts & Figures **Bilimoria et al., Cancer; August 15, 2007: Volume 110, Number 4: 738-744

Algenpantucel-L Phase 2 Results: Resected Pancreatic Cancer Eligibility & Treatment Post-resection patients with no evidence of residual disease SOC (gemcitabine+5FU+concurrent XRT) + algenpantucel-L Algenpantucel-L schedule: Q2weeks X 6 months High dose (300 million cells) & low dose (100 million cells) Phase 2 Trial NLG-0205 (n = 69) Multicenter(16), open label, 2 arm study Primary endpoint met: 1 year DFS 62% DFS: High dose (81%) superior to low dose (52%) p= 0.02 Secondary endpoints: Overall Survival(OS) OS: High dose (96 %) superior to low dose (79%) p= 0.049 Rocha Lima, C. et al, JCO, Vol 31,_suppl (May 15 supplement), ASCO 2013: Oral Abstract 3007 12

HyperAcute Immunotherapy Safety Minimal Grade 3 & No Grade 4 Events Most frequent AEs attributed to algenpantucel-L are grade 1/2 skin reactions at injection sites (51%) Grade 3 events possibly attributable to vaccine: lymphopenia (6%), skin reaction/pain (3%) & leukopenia/neutropenia (3%) No grade 4 drug related adverse events reported Hardacre, J. et al, JCO, Vol 30, No 15_suppl (May 20 supplement), ASCO 2012: Abstract 4049 13

Prognostic Variables Impacting Survival Nodal Status & Tumor Size have Greatest Impact 14 Brennan Nomogram2 Validated Prognostic Variables N=555 Prognostic Variable Hazard Ratio P-Value Nodal Status 1.53 (1.18-1.97) 0.001 Tumor Size 1.21 (0.95-1.53) 0.12 Surgical Margin Status 1.05 (0.80-1.37) 0.74 RTOG 97041 Prognostic Variables N=388 1 Regine et al, JAMA 2008; 299(9): 1019-1026 2 Brennan, M et al, Annals of Surgery 2004 , Volume 240(2): 293-298 Prognostic Variable P-Value Nodal Status 0.001 Tumor Size 0.002 Differentiation 0.002 Surgical Margin Status 0.74

15 Stage 20,000 Patient Death Registry1 NLG 02052 1 Year (%) 2 Year (%) 3 year (%) Overall Survival Patient Distribution IA 71.3 50.2 40.7 24.1 mo 1% IB 67.3 45.4 35.3 20.6 mo 3% IIA 60.7 34.9 23.8 15.4 mo 15% IIB 52.7 23.8 14.4 12.7 mo 81% 2 Hardacre, J. et al, JCO, Vol 30, No 15_suppl (May 20 supplement), ASCO 2012: Abstract 4049 Pancreatic Cancer: Survival by Disease Stage NLG 0205: 96% High Risk Patients 1 Hidalgo, M, The New England Journal of Medicine 2010, 362:1605

NLG0205: High Risk Patients w/ Poorer Prognosis Sponsor RTOG NewLink Study 97041 NLG 02052 Median Age 61 62 Gender (male) 53 % 53 % Whipple 85 % 86 % Lymph Node Positive 68 % 81 % Tumor ≥ 3 cm 61 % 66 % Poorly Differentiated 30 % 35 % CA 19-9 ≥180 9%** 18 % 16 2Hardacre, J. et al, JCO, Volume 30, No 15_suppl (May 20 supplement), ASCO 2012: Abstract 4049 NLG 0205 Additional High Risk Factors: 96% High Risk (Positive Nodes or Tumor ≥2cm) 90% Local Invasion Median Tumor Size = 3.2cm 1 Regine et al, JAMA 2008; 299(9): 1019-1026 **Berger, A et al, Int J Radiation Oncol Biol Phys, Vol 84, No 3 pp e291-297, 2012 (N=385)
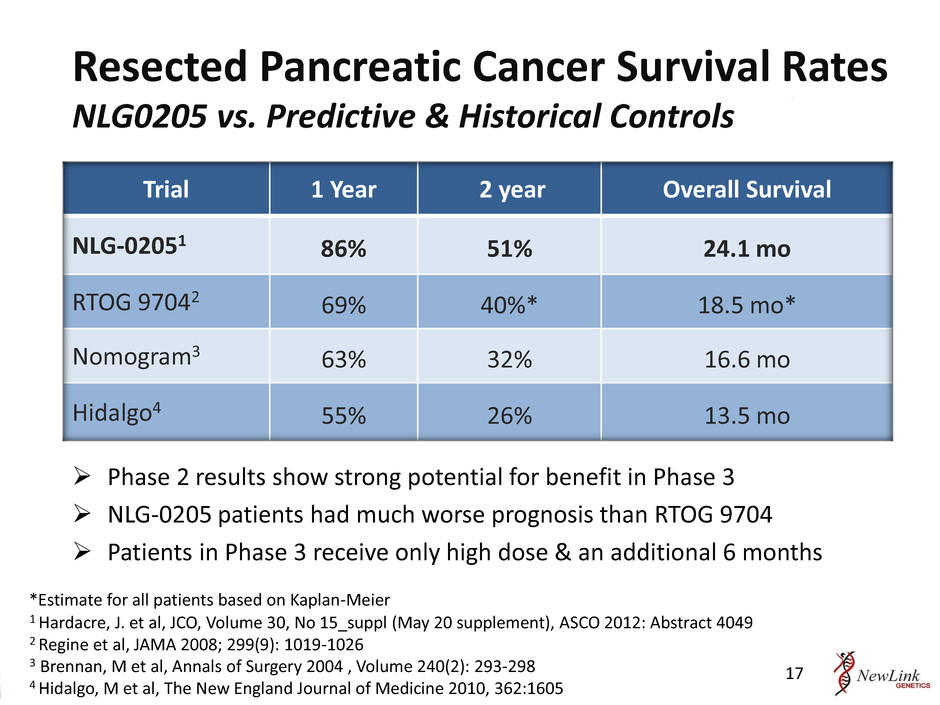
Resected Pancreatic Cancer Survival Rates NLG0205 vs. Predictive & Historical Controls 17 Trial 1 Year 2 year Overall Survival NLG-02051 86% 51% 24.1 mo RTOG 97042 69% 40%* 18.5 mo* Nomogram3 63% 32% 16.6 mo Hidalgo4 55% 26% 13.5 mo 1 Hardacre, J. et al, JCO, Volume 30, No 15_suppl (May 20 supplement), ASCO 2012: Abstract 4049 2 Regine et al, JAMA 2008; 299(9): 1019-1026 3 Brennan, M et al, Annals of Surgery 2004 , Volume 240(2): 293-298 4 Hidalgo, M et al, The New England Journal of Medicine 2010, 362:1605 Phase 2 results show strong potential for benefit in Phase 3 NLG-0205 patients had much worse prognosis than RTOG 9704 Patients in Phase 3 receive only high dose & an additional 6 months *Estimate for all patients based on Kaplan-Meier

Algenpantucel-L Phase 2 Results: Long Term Follow Up at 3 Years *All patients ≥ 3 year follow up ** Developed by Memorial Sloan Kettering Cancer Center and validated by Massachusetts General Hospital patient cohort **Brennan, M. et al, (2004) Annals of Surgery, Volume 240(2): p. 293-8 **Ferrone, CR et al, (2005) Journal of Clinical Oncology, Volume 23(30): p. 7529-35 Time 3 Years Overall Survival (OS) Disease Free Survival (DFS) NLG0205* 39% 26% Brennan ** Nomogram 19% NR Rocha Lima, C. et al, JCO, Vol 31,_suppl (May 15 supplement), ASCO 2013: Oral Abstract 3007 18

Algenpantucel-L Phase 2 Results: Overall Survival Hardacre, J. et al, JCO, Vol 30, No 15_suppl (May 20 supplement), ASCO 2012: Abstract 4049 NLG-0205 Nomogram Months Survi v a l Nomogram Median OS: 16.6 mo NLG0205 (All Patients) Observed Median OS: 24.1 mo 19 Relative survival benefit increases over time
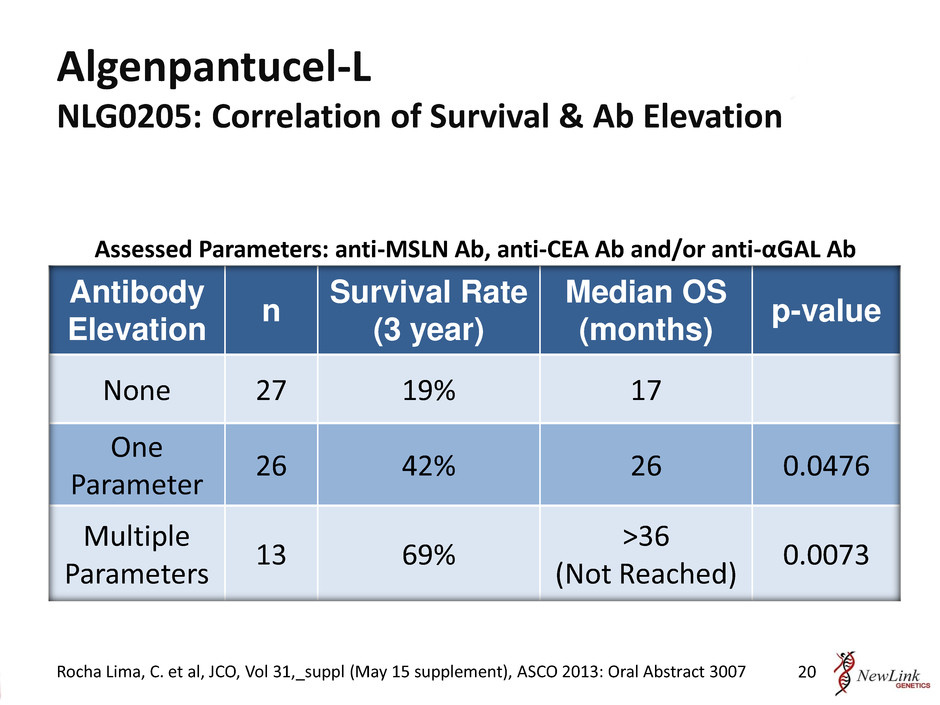
Algenpantucel-L NLG0205: Correlation of Survival & Ab Elevation Antibody Elevation n Survival Rate (3 year) Median OS (months) p-value None 27 19% 17 One Parameter 26 42% 26 0.0476 Multiple Parameters 13 69% >36 (Not Reached) 0.0073 Assessed Parameters: anti-MSLN Ab, anti-CEA Ab and/or anti-αGAL Ab Rocha Lima, C. et al, JCO, Vol 31,_suppl (May 15 supplement), ASCO 2013: Oral Abstract 3007 20

Algenpantucel-L Unexpected Clinical Observations Recall skin reactions can occur 1-2 year after last vaccination 70% of patients showed eosinophilia; 30% lasted ≥ 1 year CR to salvage chemotherapy (post algenpantucel-L therapy) in 3 patients with recurrent disease 0 2000 4000 6000 8000 10000 12000 14000 16000 18000 Jan-09 Feb-09 Aug-09 Nov-09 Dec-10 Jun-11 Aug-11 Jan-12 1 6 13 23 36 2884 16020 13 Normalized CA19-9 from 16,000 Regression of Liver Metastasis CA-19.9 Hardacre, J. et al, JCO, Vol 30, No 15_suppl (May 20 supplement), ASCO 2012: Abstract 4049 21

Phase 3 Registration Trial - IMPRESS Surgically Resected Pancreatic Cancer IMPRESS Trial* (n = 722) Initiated, May 2010 under SPA with the FDA FDA Fast Track and Orphan Drug Open label, 2 arm, randomized study Post surgical resection patients SOC +/- algenpantucel-L (SOC = gemcitabine +/- radiation) Algenpantucel-L: 300 million cells Q2wks X 6 mo Q1m X 6 mo** Overall Survival is the primary endpoint Stratified for Nodal Status, Radiotherapy & CA 19-9 Accrual Status and Endpoints Completed enrollment September 2013 (722 patients) First interim look when 222 events occur, final analysis at 444 events Designed to detect ≈20% difference in overall survival at final analysis *Clinicaltrials.gov NCT01072981 **Phase 2 trial administered 300 million Q2wks X 6 months 22

IMPRESS Patient Characteristics* Consistent with Previously Reported Large Studies Characteristics RTOG 97041 (n=221) IMPRESS (n≈700) Age (Median) 61 64 Gender (Male) 53 53 Disease Stage Ia N/R 4% Ib N/R 5% IIa N/R 21% IIb N/R 70% Nodal Status N0 32% 30% N1 68% 70% CA19-9 ≥180 9%** 9% 23 *Initial analysis includes approximately 700 patients with available data 1 Regine et al, JAMA 2008; 299(9): 1019-1026 **Berger, A et al, Int J Radiation Oncol Biol Phys, Vol 84, No 3 pp e291-297, 2012 (N=385)

Timing of IMPRESS Analysis Potential Factors Impacting Trigger Events Patient characteristics Age, gender, tumor size , stage, grade, Nodal status, CA19-9 etc… New salvage treatment regimens Gem+Abraxane, FOLFIRINOX, Tarceva Enrollment rate (IMPRESS is in line with study projections) Possible improvement in surgical or diagnostic techniques (PET?) Increased use of neoadjuvant therapy Delayed reporting of death events Treatment duration at 300M cell dose with Algenpantucel-L 24

Second Phase 3 Trial - PILLAR Locally Advanced Pancreatic Cancer Locally Advanced Trial* (n = 280) Launched, October 2012 (FPI, Q113) Open label, 2 arm, randomized study FOLFIRINOX +/- algenpantucel-L Algenpantucel-L: 300 million cells Q2 weeks up to 18 doses Overall Survival is the primary endpoint Includes borderline or locally advanced unresectable patients Open to Enrollment . 25 Positive results might more than double the treatable patient population of initial indication

Commercialization Strategy Algenpantucel-L for Patients with Pancreatic Cancer NewLink plans to execute an independent US commercial launch Leverage key pancreatic surgical centers as hubs Provide strong product support across entire multidisciplinary team Key launch components currently being assembled Utilizing state of the art cold chain distribution technology & services Conducting reimbursement analysis & establishing support services Corporate & product branding initiatives underway Partnered with ICC Lowe for corporate & product campaigns Planned launch of new website in Q413 Will Pursue Partnerships for Ex-U.S. commercialization Ongoing discussions with various global & regional companies Anticipate EMEA submission with IMPRESS results Evaluating global manufacturing & distribution options 26

Lung Cancer Epidemiology & Pathophysiology 27 Leading cause of cancer death in U.S.* All stages, 5 year survival 16% Advanced disease, 5 year survival <5% 40% of patients present with metastatic disease A significant unmet need exists for new treatment options for these patients U. S. Annual Incidence & Deaths (Lung Cancer) Incidence Deaths 228,190 159,480 *2013 ACS Cancer Facts & Figures

Tergenpumatucel-L Phase 2 Study – Previously Treated NSCLC Single agent survival and safety results for 28 NSCLC patients Median OS was 11.3 months with 46% of patients surviving one year Favorable safety profile, no grade 4 drug related adverse events Potential chemo-sensitization effect 16 patients received follow on chemotherapy post tergenpumatucel-L Partial response rate of 31% (5/16) in patients Stable disease achieved in an additional 25% (4/16) Correlative studies Significantly improved OS in patients with elevated IFN-ɣ secretion Acquired reactivity to CL4-H522 cell line, not part of vaccine, suggests antigen cross-priming to shared tumor antigens Morris, J. et al, JCO, Vol 31,_suppl (May 15 supplement), ASCO 2013: Abstract 8094 28

Single Agent in Previously Treated NSCLC Tergenpumatucel-L in NSCLC Phase 2: Survival Correlates with IFN-g Response8 Morris, J. et al, JCO, Vol 31,_suppl (May 15 supplement), ASCO 2013: Abstract 8094 29

30 Tergenpumatucel-L 300 M Cells Weekly or Biweekly Docetaxel 75 mg/m2 Q 3 weeks - 240 Patients - Previously Treated - Stage IIIb/IV NSCLC - PS 0-1 - >1,000 lymphocyte count Progression Standard Chemotherapy (gemicitabine, premetrexed or docetaxel) Standard Chemotherapy (gemicitabine or premetrexed) Tergenpumatucel-L NLG-0301 Randomized Phase 2b/3 NSCLC 1:1 Randomization Compare Response Primary Endpoint: Overall Survival Secondary Endpoints: Response to subsequent controlled chemotherapy, immunologic response and dosing schedule Progression http://clinicaltrials.gov/show/NCT01774578

IDO Pathway Inhibitors Indoleamine 2,3-dioxygenase (IDO) “Disrupting mechanisms by which tumors evade a patient’s immune system.” NewLink Genetics IDO Pathway Inhibitors Indoximod NLG-919 31

Cancer Immunosuppression Three Key Checkpoint Targets CTLA-4 PD-1 IDO B7-1 or B7-2 CTLA-4 T cell PDL-1 or PDL-2 IDO APC (Dendritic Cell or Macrophage PD-1 Manipulation of IDO pathway related targets such as CTLA-4 and PD-1 offer potential breakthrough approaches to cancer therapy, targeting mechanisms by which tumors evade immune mediated destruction 32

Regulates immune response to cancer like CTLA-4 and PD-1 Inhibits effector T-cells, activates suppressive Tregs Enables local tumor immune escape Overexpressed Within tumor cells or antigen presenting cells to directly suppress T- cells, must be targeted with a small molecule NewLink actively developing two distinct IDO pathway inhibitors Indoximod is being studied in various chemotherapy and immunotherapy combination clinical studies NLG919 is expected to enter clinical trials by the end of 2013 33 Indoleamine 2,3-dioxygenase (IDO) Supports Cancer Immunosuppression

Indoximod Phase 1: Key Results Summary Well tolerated, 200 mg QD to 2000 mg BID Most common adverse events: fatigue, anorexia & nausea Biologic effects observed in 3 patients previously sensitized to immunotherapy (low dose) & 2 non-sensitized patients (high dose) Five patients with SD for ≥6 months (2 melanoma, 2 sarcoma, 1 colon) Multiple mixed responses including regression of visceral metastases Hypophysitis Auto-immune event correlated with beneficial anti-tumor responses in ipilumimab trials Condition resolved with short course of steroids Soliman, H. et al, JCO, Vol 30, No 15,_suppl (May 20 supplement), ASCO 2012: Abstract 2501 34

Indoximod + Docetaxel Combination Phase 1: Efficacy Results 18% (4/22) partial response rate (2 breast, 1 lung, 1 thymic) 41% (9/22) rate of stable disease -100% -80% -60% -40% -20% 0% 20% 40% 60% Fold Change in Tumor Volume NSCLC Esophageal Laryngeal Breast Thymic Rectal Pancreatic Ovarian 35

36 Phase Design / Purpose/ Indication Status 1 Indoximod as single agent Dose escalation to determine MTD & PK All Solid malignancies Completed 1 Indoximod as single agent Dose escalation to determine MTD & PK All Solid malignancies Completed 1b/2 Indoximod + Docetaxel Dose escalation of both agents Breast, lung, ovarian, prostate Completed 1b/2 Indoximod + Dendritic cells loaded with Ad-p53 vaccine Dose escalation Breast, colon, lung Completed 2 Sipuleucel-T +/- Indoximod Refractory Metastatic Prostate Open 2 Docetaxel +/- Indoximod Metastatic Breast Open Indoximod Clinical Development
NLG-919 Preclinical Results & Next Steps Potent Inhibitor (nM) of IDO in vitro and in cell based assays Is orally bioavailable and has a favorable PK-Tox profile Blocked IDO-induced T cell suppression (EC50 ~ 100 nM) Enhanced antitumor activity in established tumors (B16F10) Shows synergistic T cell activation & antitumor activity w/ indoximod Expected to enter phase 1 by year end 2013 37 Mautino, M et al; AACR Annual Meeting Poster Presentation, Abstract 491, April 7th, 2013

NewLink Genetics Corporation Highlights & Future Direction Multiple near term value-generating milestones expected Accrual now complete for IMPRESS trial Conduct first interim analysis for IMPRESS trial Continue expansion of current clinical programs (PILLAR, lung, IDO, etc.) Initiate new clinical programs (HyperAcute Renal & NLG919) Strong financial position Sufficient cash to fund development to next data inflection points Poised to build a commercial presence Clear regulatory and commercial path for pancreatic market Building internal infrastructure for North America Seeking long term partnership(s) for ROW development Exploring potential partnerships for follow on products 38





































