
Third Quarter 2018 Financial Results NewLink Genetics Corporation Nasdaq: NLNK November 1, 2018
Introduction •Lisa Miller, Director of Investor Relations Clinical Priorities • Charles J. Link, Jr, MD, Chairman, CEO & CSO Clinical Updates and Guidance on Timing of Data •Eugene Kennedy, MD, Chief Medical Officer Third Quarter 2018 Financial Results • Carl Langren, Chief Financial Officer 2

Cautionary Note Regarding Forward-Looking Statements This presentation contains forward-looking statements of NewLink Genetics that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation are forward- looking statements, within the meaning of The Private Securities Litigation Reform Act of 1995. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "target," "potential," "will," "could," "should," "seek" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among others, statements about NewLink Genetics' financial guidance for 2018; results of its clinical trials for product candidates; its timing of release of data from ongoing clinical studies; its plans related to execution of clinical trials; plans related to moving additional indications into clinical development; NewLink Genetics' future financial performance, results of operations, cash position and sufficiency of capital resources to fund its operating requirements; the effects of its organizational realignment, and any other statements other than statements of historical fact. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that NewLink Genetics makes due to a number of important factors, including those risks discussed in "Risk Factors" and elsewhere in NewLink Genetics' Annual Report on Form 10-K for the year ended December 31, 2017 and other reports filed with the U.S. Securities and Exchange Commission (SEC). The forward-looking statements in this presentation represent NewLink Genetics' views as of the date of this presentation. NewLink Genetics anticipates that subsequent events and developments will cause its views to change. However, while it may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. You should, therefore, not rely on these forward-looking statements as representing NewLink Genetics' views as of any date subsequent to the date of this presentation. 3

Indoximod Clinical Programs Front-line diffuse intrinsic pontine glioma (DIPG) Indoximod plus radiotherapy for pediatric patients with DIPG Early data show all patients demonstrated initial symptomatic improvement on therapy Phase 1b trial ongoing with updated data anticipated 2019 Recurrent malignant pediatric brain tumors Indoximod plus radio-chemotherapy for pediatric patients with malignant brain tumors Phase 1b trial ongoing with updated data anticipated 1H 2019 Front-line acute myeloid leukemia (AML) Indoximod plus standard-of-care chemotherapy for patients with front-line AML Updated data July 2018 showed promising MRD-negativity with indoximod Phase 1b trial ongoing with updated data in oral presentation at ASH, December 2018 NLG802, prodrug of indoximod Preclinical data show significantly higher PK levels with NLG802 Phase 1 trial ongoing with updated data at SITC, November 2018 4

Indoximod plus Chemotherapy in Acute Myeloid Leukemia (AML) Phase 1b Exploring Minimal Residual Disease as a Surrogate Endpoint Combination with current Data to be presented by Emadi, et al at standard of care (7+3) ASH, December 2018 chemotherapy • Indoximod was well tolerated and does not appear to add significant toxicity • 21/25 (84%) ITT patients achieved morphologic Minimal complete response (CR) residual disease • 15/19 (79%) per protocol patients achieved CR (MRD) evaluated by • 10/12 (83%) patients MRD- at end of induction sensitive flow • 11/11 (100%) patients MRD- at end of cytometry assay consolidation Currently enrolling Phase 1b expansion cohort 5

Abstracts Accepted for Upcoming Medical Meetings Abstract accepted for oral presentation at the ASH Annual Meeting, December 1-4, 2018 • Indoximod combined with standard induction chemotherapy is well tolerated and induces a high rate of complete remission with MRD-negativity in patients with newly diagnosed AML: results from a Phase 1 trial, Emadi, A., et al. Abstracts accepted for presentation at the SITC Annual Meeting, November 7-11, 2018 • A phase 1a clinical trial of NLG802, a prodrug of indoximod with enhanced pharmacokinetic properties, Rixe, O., et al. • The immunogenomic impact of indoximod on the tumor microenvironment of melanoma patients, Yu, J., et al. • Effects of indoximod plus gemcitabine/nab-paclitaxel on tumor microenvironment of patients with metastatic pancreatic cancer, Yu, J., et al. 6
Other Opportunities Clinical Programs Under Evaluation . NLG207 (formerly CRLX101) – Nanoparticle formulation of the topoisomerase 1 inhibitor camptothecin – Acquired from Cerulean Pharma – Phase 2 trial to evaluate NLG207 plus weekly paclitaxel in recurrent ovarian cancer – Trial conducted in conjunction with GOG – Phase 2 data anticipated in 2019 . Pursuing additional opportunities to expand our pipeline Multiple clinical opportunities addressing areas of unmet need 7
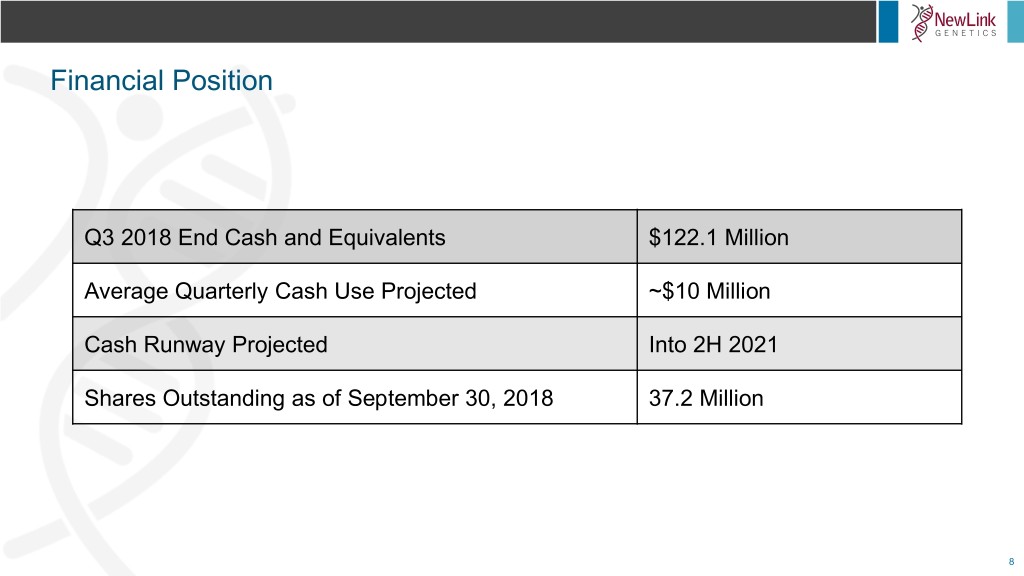
Financial Position Q3 2018 End Cash and Equivalents $122.1 Million Average Quarterly Cash Use Projected ~$10 Million Cash Runway Projected Into 2H 2021 Shares Outstanding as of September 30, 2018 37.2 Million 8
NewLink Genetics: Key Takeaways Clinical development plan targeting the most promising programs • Recurrent pediatric brain tumors, front-line DIPG, front-line AML, NLG802, NLG207 Strong cash position • Cash on hand at Q3 end $122.1 million • Estimated cash runway into the second half of 2021 excluding Ebola PRV monetization • Substantial financial interest in Priority Review Voucher (PRV) affiliated with approval of the Ebola vaccine licensed by NewLink Genetics Indoximod data to be presented at upcoming medical conferences in 2018 • December 2018: Updated Phase 1b AML data presented in oral presentation at ASH • November 2018: Initial Phase 1 NLG802 data & Phase 2 indoximod biomarker data from melanoma and pancreatic cancer patients to be presented at SITC 9

Q & A 10









