
1 First Quarter 2022 Financial Results & Clinical Update May 10, 2022

2 Forward Looking Statements This presentation contains forward-looking statements of Lumos Pharma, Inc. that involve substantial risks and uncertainties. All such statements contained in this presentation are forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995, a law that gives us the opportunity to share our outlook for the future without fear of litigation if it turns out our predictions were not correct. We are passionate about our business, including LUM-201 and the potential it may have to help patients in the clinic. This passion feeds our optimism that our efforts will be successful and bring about meaningful change for patients. Please keep in mind that actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make. We have attempted to identify forward-looking statements by using words such as “projected,” "upcoming," "will," “would,” "plan," “intend,” "anticipate," "approximate," "expect," “potential,” “imminent,” and similar references to future periods or the negative of these terms. Not all forward-looking statements contain these identifying words. Examples of forward-looking statements include, among others, statements we make regarding progress in our clinical efforts including comments concerning screening and enrollment for our trials, momentum building in our LUM-201 program for PGHD, anticipated timing of interim analyses of trials, our belief that the interim data should provide an early indication of efficacy and safety of oral LUM-201 versus standard of care daily rhGH injections in PGHD, LUM-201’s therapeutic potential when administered to pediatric subjects with idiopathic growth hormone deficiency, that the interim sample size should be adequate to provide an initial indication of LUM 201’s impact, expecting the primary outcome data readout for our trials, the potential to expand our LUM-201 platform into other indications, future financial performance, results of operations, cash position, cash use rate and sufficiency of our cash resources to fund our operating requirements through the primary outcome data readout from the OraGrowtH210 and OraGrowtH212 Trials, and any other statements other than statements of historical fact. We wish we were able to predict the future with 100% accuracy, but that just is not possible. In addition to other considerations referenced in this paragraph, the recent conflict between Ukraine and Russia has increased the uncertainty in that region and may impact our business in the future. Our forward-looking statements are neither historical facts nor assurances of future performance. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements that we make due to a number of important factors, including the effects of pandemics, other widespread health problems or the Ukraine-Russia conflict, the outcome of our future interactions with regulatory authorities, our ability to project future cash utilization and reserves needed for contingent future liabilities and business operations, the ability to obtain the necessary patient enrollment for our product candidate in a timely manner, the ability to successfully develop our product candidate, the timing and ability of Lumos to raise additional equity capital as needed and other risks that could cause actual results to differ materially from those matters expressed in or implied by such forward-looking statements. You should not rely on any of these forward-looking statements and, to help you make your own risk determinations, we have provided an extensive discussion of risks that could cause actual results to differ materially from our forward-looking statements in the "Risk Factors" section and elsewhere in Lumos Pharma’s Annual Report on Form 10-K for the year ended December 31, 2021, as well as other reports filed with the SEC including our Quarterly Reports on Form 10-Q. All of these documents are available on our website. Before making any decisions concerning our stock, you should read and understand those documents. We anticipate that subsequent events and developments will cause our views to change. We may choose to update these forward-looking statements at some point in the future, however, we disclaim any obligation to do so. As a result, you should not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. 5.10.2022

3 Agenda • Lisa Miller, Senior Director of Investor Relations • Rick Hawkins, Chief Executive Officer & Chairman • David B. Karpf, MD, Chief Medical Officer • Lori Lawley, Chief Financial Officer • John McKew, PhD, President & Chief Scientific Officer
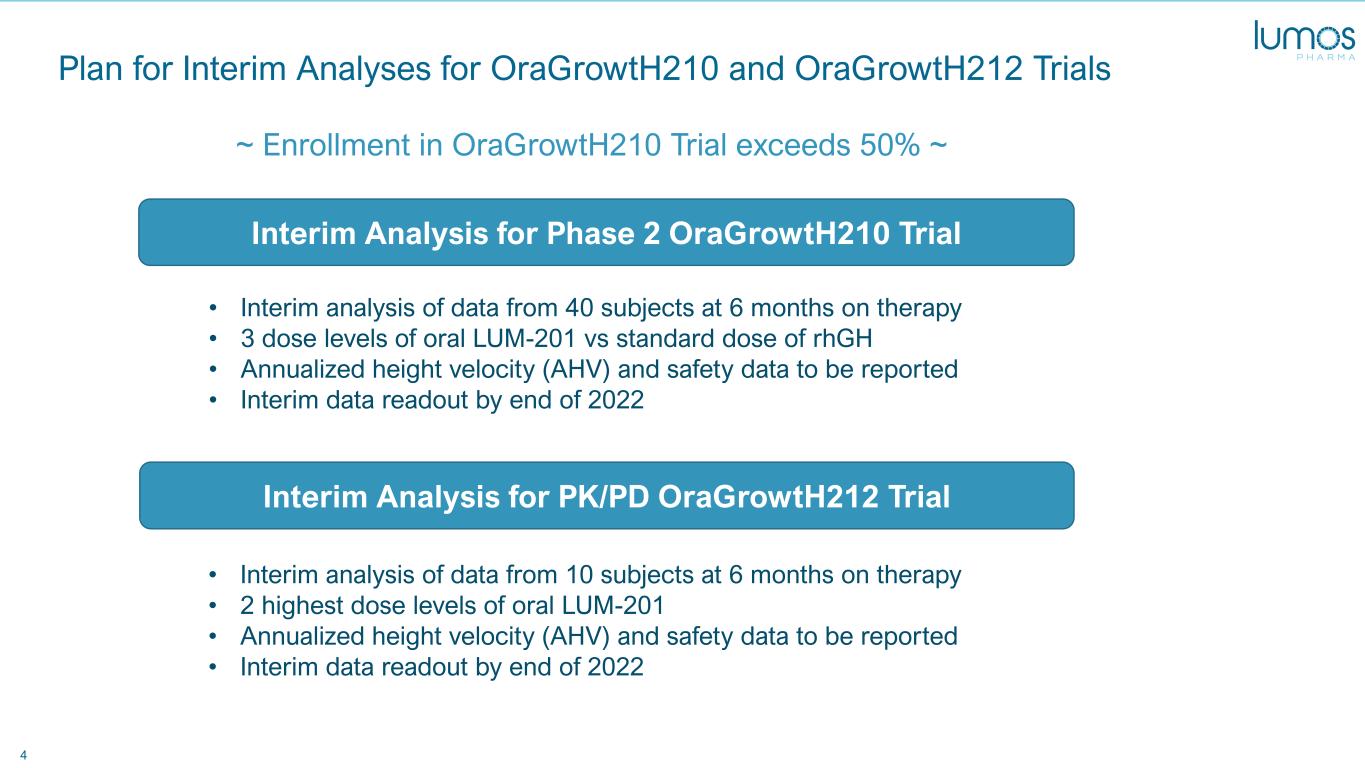
4 Plan for Interim Analyses for OraGrowtH210 and OraGrowtH212 Trials ~ Enrollment in OraGrowtH210 Trial exceeds 50% ~ Interim Analysis for Phase 2 OraGrowtH210 Trial Interim Analysis for PK/PD OraGrowtH212 Trial • Interim analysis of data from 40 subjects at 6 months on therapy • 3 dose levels of oral LUM-201 vs standard dose of rhGH • Annualized height velocity (AHV) and safety data to be reported • Interim data readout by end of 2022 • Interim analysis of data from 10 subjects at 6 months on therapy • 2 highest dose levels of oral LUM-201 • Annualized height velocity (AHV) and safety data to be reported • Interim data readout by end of 2022
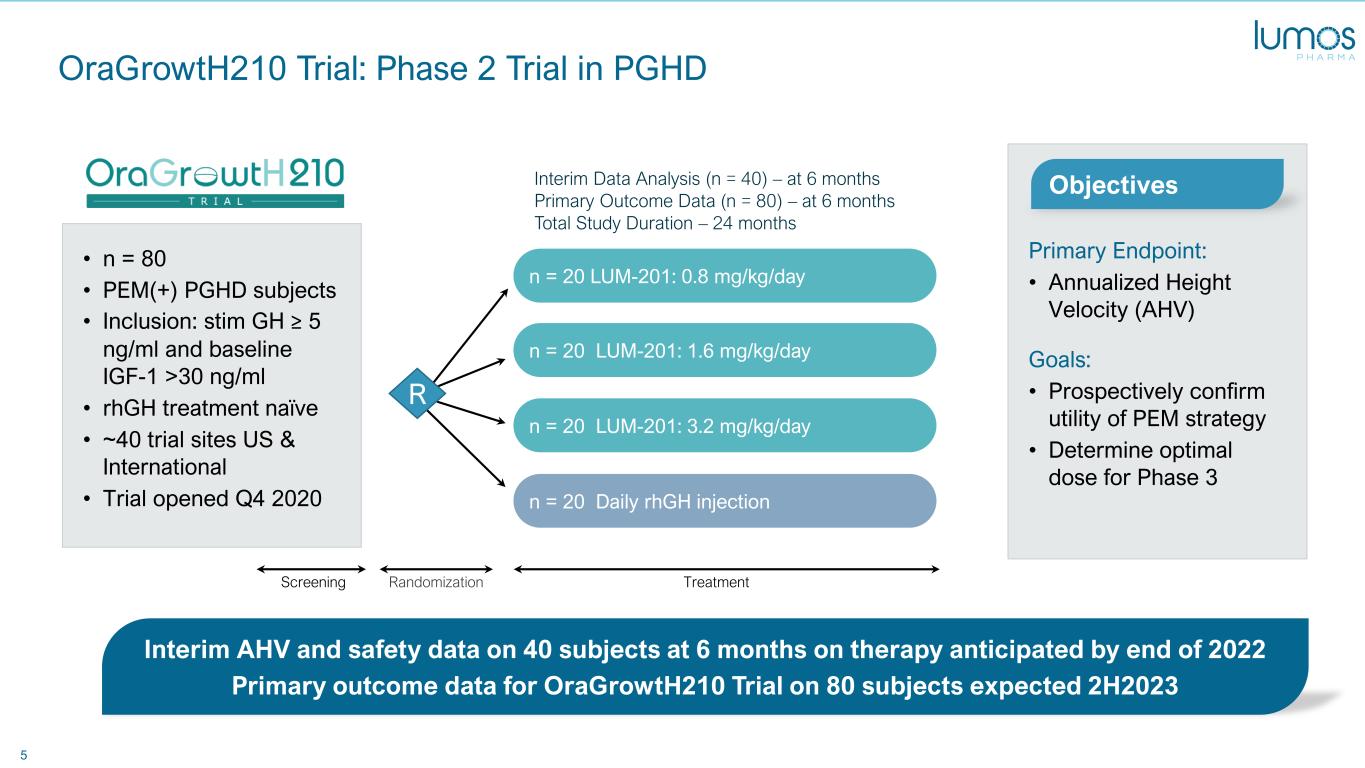
5 OraGrowtH210 Trial: Phase 2 Trial in PGHD n = 20 Daily rhGH injection n = 20 LUM-201: 3.2 mg/kg/day n = 20 LUM-201: 1.6 mg/kg/day n = 20 LUM-201: 0.8 mg/kg/day • n = 80 • PEM(+) PGHD subjects • Inclusion: stim GH ≥ 5 ng/ml and baseline IGF-1 >30 ng/ml • rhGH treatment naïve • ~40 trial sites US & International • Trial opened Q4 2020 Primary Endpoint: • Annualized Height Velocity (AHV) Goals: • Prospectively confirm utility of PEM strategy • Determine optimal dose for Phase 3 Interim AHV and safety data on 40 subjects at 6 months on therapy anticipated by end of 2022 Primary outcome data for OraGrowtH210 Trial on 80 subjects expected 2H2023 Interim Data Analysis (n = 40) – at 6 months Primary Outcome Data (n = 80) – at 6 months Total Study Duration – 24 months Objectives TreatmentRandomizationScreening R

6 • n = up to 24 • Open-label study • PGHD patients • rhGH-treatment naïve • 12-month dosing • Single, specialized clinical site • Q10 minute GH sampling for 12 hours OraGrowtH212 Trial: Pharmacokinetic / Pharmacodynamic Trial in PGHD Primary Endpoints: • Assess LUM-201 effect on endogenous GH pulsatility and Annualized Height Velocity (AHV) • Evaluate PK/PD in children Goals: • Confirm prior PK/PD data in adults & subset of Merck 020 trial • Support future regulatory filings & commercialization Interim AHV and safety data on 10 subjects anticipated by end of 2022 Objectives n = up to 12 - LUM-201: 3.2 mg/kg/day n = up to 12 - LUM-201: 1.6 mg/kg/day R TreatmentRandomizationScreening Interim Data Analysis (n =10) – at 6 months Primary Outcome Data (n = up to 24) – at 6 months Total Study Duration – 12 months* * Proposed protocol amendment to extend total study duration beyond 12 months

7 Other Updates FDA permits LUM-201 treatment beyond 12 months • After review of unblinded LUM-201 data from OraGrowtH Trials, FDA lifts partial clinical hold • OraGrowtH210 and OraGrowtH212 Trials extended beyond 12 months • Primary outcome data for OraGrowtH210 & OraGrowtH212 Trials at 6-months on treatment Peer-reviewed analysis of prior LUM-201 data published in journal • Peer-reviewed article, LUM-201 Elicits Greater GH Response than Standard GH Secretagogues in Pediatric Growth Hormone Deficiency, published online in journal, Hormone Research in Paediatrics, March 2022

8 • n = 10 • Adult NAFLD subjects with relative GH/IGF-1 deficiency • Open-label • Single-site pilot study • 6-month dosing Investigator Initiated Phase 2 Pilot Trial in Non-Alcoholic Fatty Liver Disease (NAFLD) Primary Objective: • Determine changes in intra- hepatic lipid content, inflammation, and potentially fibrosis resulting from LUM-201 induced GH augmentation compared to historical placebo-treated controls Massachusetts General Hospital (MGH) initiated pilot study of oral LUM-201 in NAFLD approved by FDA Objectives n = 10 – LUM-201 at dose level of 25 mg/day Study Duration – 6 months MGH Initiated Phase 2 Pilot Trial

9 Lumos Pharma Expands Leadership Team Pisit Pitukcheewanont, MD – renowned pediatric endocrinologist • Vice President of Global Clinical Development and Medical Affairs for Lumos Pharma • Professor of Clinical Pediatrics at the Children’s Hospital of Los Angeles, Keck School of Medicine of the University of Southern California; on staff since 1998 • Former president of the Human Growth Foundation • Authored over 70 publications • Previously, Vice President of Medical Affairs and Vice President, Global Medical Ambassador and Medical Education for Ascendis Pharma
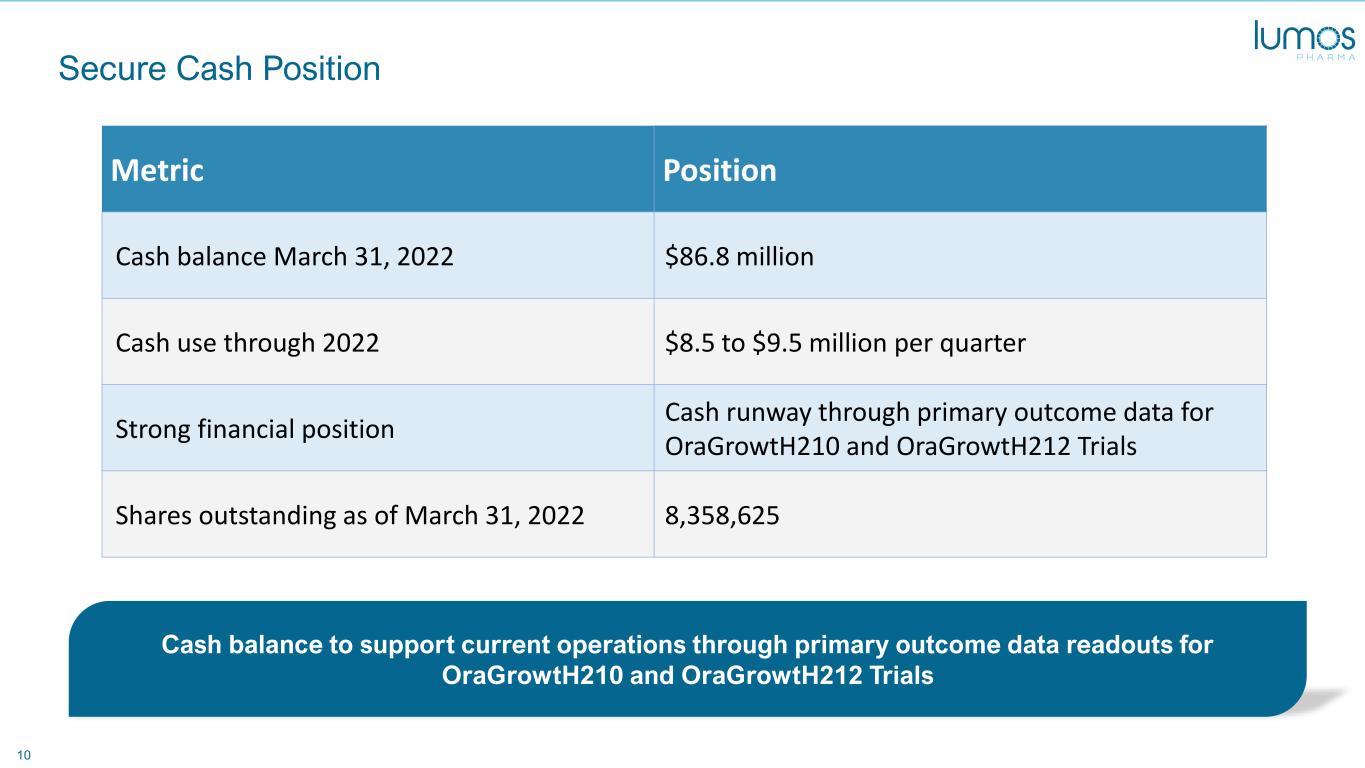
10 Secure Cash Position Cash balance to support current operations through primary outcome data readouts for OraGrowtH210 and OraGrowtH212 Trials Metric Position Cash balance March 31, 2022 $86.8 million Cash use through 2022 $8.5 to $9.5 million per quarter Strong financial position Cash runway through primary outcome data for OraGrowtH210 and OraGrowtH212 Trials Shares outstanding as of March 31, 2022 8,358,625
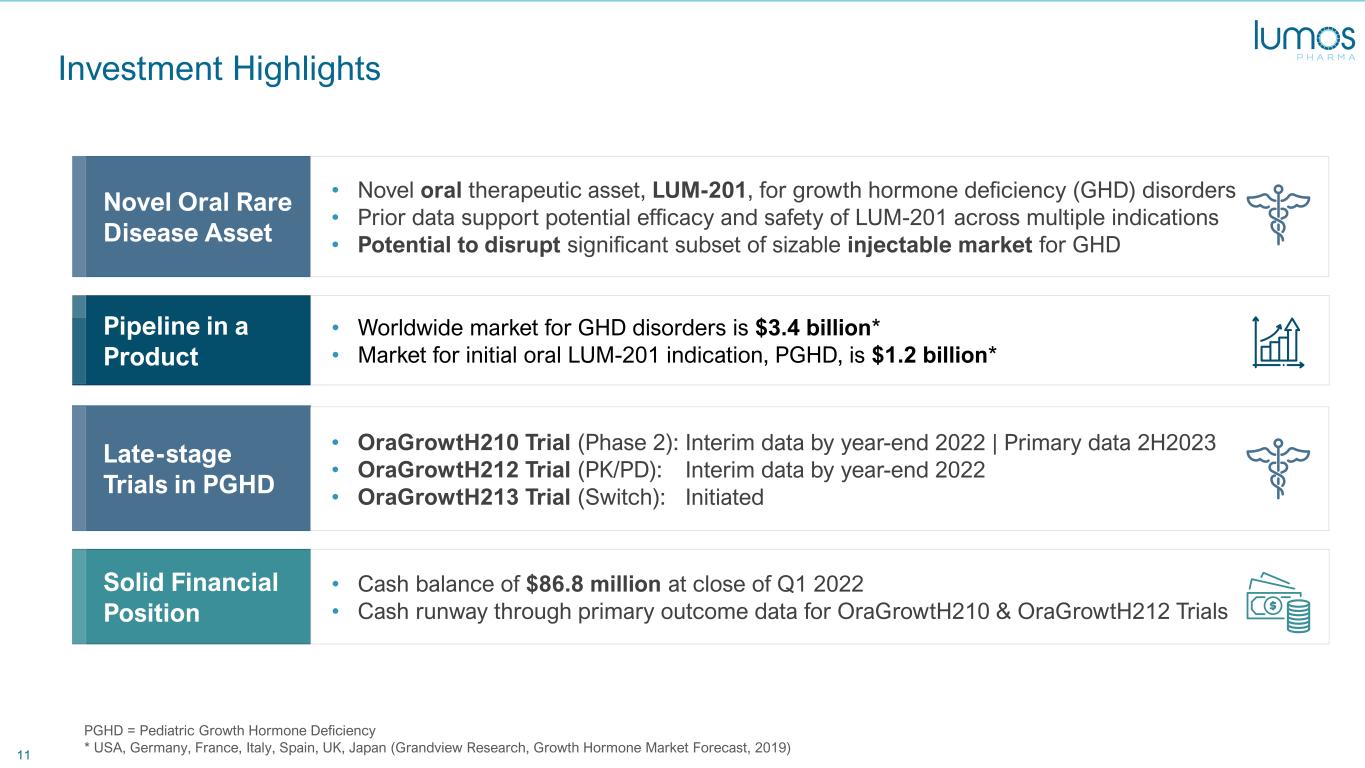
11 Investment Highlights PGHD = Pediatric Growth Hormone Deficiency * USA, Germany, France, Italy, Spain, UK, Japan (Grandview Research, Growth Hormone Market Forecast, 2019) Novel Oral Rare Disease Asset Pipeline in a Product Solid Financial Position • Novel oral therapeutic asset, LUM-201, for growth hormone deficiency (GHD) disorders • Prior data support potential efficacy and safety of LUM-201 across multiple indications • Potential to disrupt significant subset of sizable injectable market for GHD • Worldwide market for GHD disorders is $3.4 billion* • Market for initial oral LUM-201 indication, PGHD, is $1.2 billion* • Cash balance of $86.8 million at close of Q1 2022 • Cash runway through primary outcome data for OraGrowtH210 & OraGrowtH212 Trials Late-stage Trials in PGHD • OraGrowtH210 Trial (Phase 2): • OraGrowtH212 Trial (PK/PD): • OraGrowtH213 Trial (Switch): Interim data by year-end 2022 | Primary data 2H2023 Interim data by year-end 2022 Initiated










