MANAGEMENT'S DISCUSSION & ANALYSIS
September 30, 2023
November 2, 2023
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This discussion and analysis should be read in conjunction with our unaudited condensed interim consolidated financial statements and notes thereto as at and for the three and nine months ended September 30, 2023, and should also be read in conjunction with the audited consolidated financial statements and Management's Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) contained in our annual report for the year ended December 31, 2022. The financial statements have been prepared in accordance with International Financial Reporting Standards ("IFRS") as issued by the International Accounting Standards Board ("IASB"). Unless otherwise indicated, all references to "$" and "dollars" in this discussion and analysis mean Canadian dollars.
All references in this MD&A to "the Company," "Oncolytics," "we," "us," or "our" and similar expressions refer to Oncolytics Biotech Inc. and the subsidiaries through which it conducts its business unless otherwise indicated.
Forward-Looking Statements
The following discussion contains forward-looking statements, within the meaning of Section 21E of the United States Securities Exchange Act of 1934, as amended and forward-looking information under applicable Canadian securities laws (such forward-looking statements and forward-looking information are collectively referred to herein as "forward-looking statements"). Forward-looking statements, including: our belief as to the potential, mechanism of action of pelareorep, an intravenously delivered immunotherapeutic agent, as a cancer therapeutic; our business strategy, goals, focus, and objectives for the development of pelareorep, including our immediate primary focus on advancing our programs in hormone receptor-positive / human epidermal growth factor 2-negative metastatic breast cancer and advanced/metastatic pancreatic ductal adenocarcinoma (PDAC) to phase 3 licensure-enabling studies; our belief that our approach will increase opportunities for expanding our clinical program and business development and partnering opportunities, has the most promise for generating clinically impactful data and offers the most expeditious path to regulatory approval; our exploration of opportunities for registrational programs in other gastrointestinal cancers; our expectation that we will incur substantial losses and will not generate significant revenues until and unless pelareorep becomes commercially viable; our belief that our combined cash resources are sufficient to fund our presently planned operations for at least the next 12 months from the balance sheet date; our belief that if the results of our clinical trial of pelareorep in combination with modified FOLFIRINOX are encouraging, the treatment combination may be advanced to late-stage clinical development through the Precision PromiseSM adaptive clinical trial platform; our belief that we currently have sufficient drug product to support our clinical development program; the impact of the COVID-19 pandemic, global political conflicts, and recent bank failures on our research and development activities, business operations, and financial condition; our primary objectives and focus for the remainder of 2023; our ongoing evaluation of all types of financing arrangements; our continued management of our research and development plan; the factors that affect our cash usage; our approach to credit rate, interest rate, foreign exchange, and liquidity risk mitigation; our expectations regarding the performance of counterparties in connection with our BRACELET-1 study; the effectiveness of our internal control systems; and other statements that are not historical facts or which are related to anticipated developments in our business and technologies. We may be impacted by business interruptions resulting from COVID-19 coronavirus and global political conflicts. It is unknown whether and how the Company may be affected if the COVID-19 pandemic and global political conflicts persist for an extended period of time. Recent bank failures could impair our ability to access our existing cash, cash equivalents, and marketable securities and to timely pay key vendors and others. We may incur expenses or delays relating to such events outside of our control, which could have a material adverse impact on our business, operating results, and financial condition. In any forward-looking statement in which we express an expectation or belief as to future results, such expectations or beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation, or belief will be achieved. Forward-looking statements involve known and unknown risks and uncertainties, which could cause our actual results to differ materially from those in the forward-looking statements.
Such risks and uncertainties include, among others, the need for and availability of funds and resources to pursue research and development projects, the efficacy of pelareorep as a cancer treatment, the success and timely completion of clinical studies and trials, our ability to successfully commercialize pelareorep, uncertainties related to the research, development, and manufacturing of pelareorep, uncertainties related to competition, changes in technology, the regulatory process, and general changes to the economic environment.
With respect to the forward-looking statements made within this MD&A, we have made numerous assumptions regarding, among other things: our ability to recruit and retain employees, our continued ability to obtain financing to fund our clinical development plan, our ability to receive regulatory approval to commence enrollment in the clinical studies which are part of our clinical development plan, our ability to maintain our supply of pelareorep, and future expense levels being within our current expectations.
Investors should consult our quarterly and annual filings with the Canadian and U.S. securities commissions for additional information on risks and uncertainties relating to the forward-looking statements. Forward-looking statements are based on assumptions, projections, estimates, and expectations of management at the time such forward-looking statements are made, and such assumptions, projections, estimates and/or expectations could change or prove to be incorrect or inaccurate. Investors are cautioned against placing undue reliance on forward-looking statements. We do not undertake any obligation to update these forward-looking statements except as required by applicable law.
Company Overview
We are a clinical-stage biopharmaceutical company developing pelareorep, a safe and well-tolerated intravenously delivered immunotherapeutic agent that activates the innate and adaptive immune systems and weakens tumor defense mechanisms. This improves the ability of the immune system to fight cancer, making tumors more susceptible to a broad range of oncology treatments.
Pelareorep is a proprietary isolate of a naturally occurring, non-pathogenic double-stranded RNA (dsRNA) virus commonly found in environmental waters, known as reovirus. Pelareorep has demonstrated the ability to create a more permissive tumor microenvironment (TME) and conditions the tumor for multiple treatment combinations, including chemotherapies, checkpoint inhibitors, and other immuno-oncology drugs, like CAR T therapies, bispecific antibodies, and CDK4/6 inhibitors. Pelareorep creates a new army of tumor-reactive T cells, helps these cells to infiltrate the tumor through an inflammatory process, and promotes the overexpression of PD-1/PD-L1. By priming the immune system with pelareorep, we believe we can increase the proportion of patients who respond to immunotherapies and other cancer treatments, especially in cancers where immunotherapies have failed or provided limited benefit.
As our clinical development program advances, we anticipate pelareorep's ability to enhance innate and adaptive immune responses within the TME will play an increasingly important role. This greatly increases opportunities for expanding our clinical program, business development, and partnering opportunities to address a broad range of cancers in combination with various other therapies. We believe this approach has the most promise for generating clinically impactful data and offers the most expeditious path to regulatory approval.
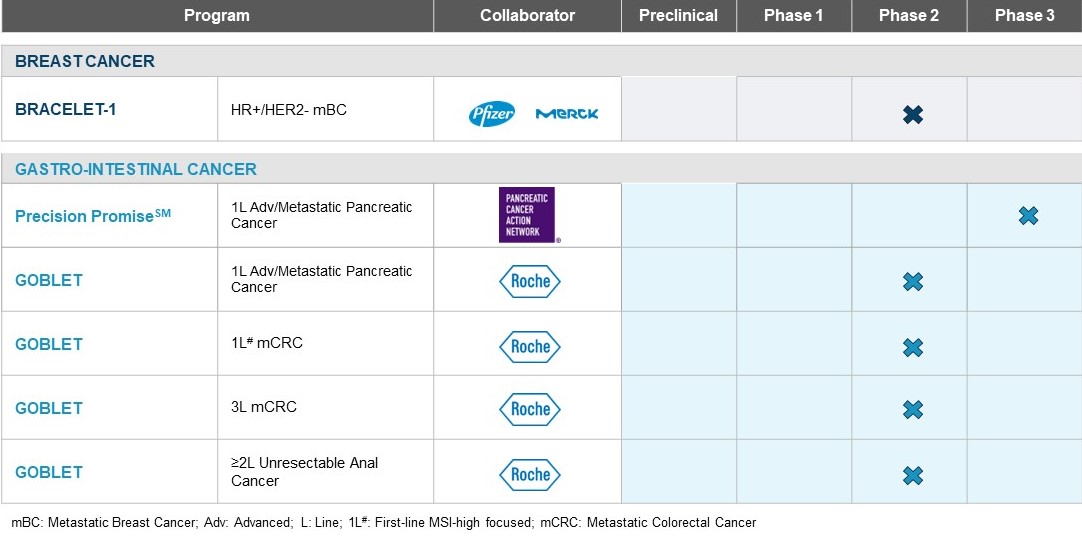
Our primary focus is to advance our programs in hormone receptor-positive / human epidermal growth factor 2-negative (HR+/HER2-) metastatic breast cancer (mBC) and advanced/metastatic pancreatic ductal adenocarcinoma (PDAC) to phase 3 licensure-enabling studies. In addition, we are exploring opportunities for registrational programs in other gastrointestinal cancers through our GOBLET platform study.
We have not been profitable since our inception and expect to continue to incur substantial losses as we continue research and development efforts. We do not expect to generate significant revenues until and unless pelareorep becomes commercially viable. As at September 30, 2023, we had cash and cash equivalents of $39,981. We believe we have sufficient existing cash resources to fund our presently planned operations for at least the next twelve months from the balance sheet date.
Third Quarter 2023 Pelareorep Development Update
Clinical Trial Program
Gastrointestinal cancer program
Recipient of PanCAN Therapeutic Accelerator Award
In the third quarter of 2023, we were selected by the Pancreatic Cancer Action Network (PanCAN) as the recipient of its US$5 million Therapeutic Accelerator Award. This grant will enable us to continue our research focused on a clinical trial with pelareorep in combination with modified FOLFIRINOX chemotherapy with or without an immune checkpoint inhibitor in pancreatic cancer patients. If results are encouraging, the treatment combination may be advanced to late-stage clinical development through the Precision PromiseSM adaptive clinical trial platform.
Inclusion in Precision PromiseSM: Phase 3 Platform Trial
In the second quarter of 2023, PanCAN selected pelareorep for inclusion as a new investigational treatment in the Precision PromiseSM study, an innovative adaptive Phase 3 clinical trial. In the third quarter, we continued working to finalize the definitive agreements for the Precision PromiseSM phase 3 platform trial and preparing the design of the investigational treatment arm to support regulatory approval for first-line advanced/metastatic PDAC.
Collaboration with Roche and AIO-Studien-gGmbH: GOBLET platform study
Our GOBLET platform study is a collaboration with Roche and AIO-Studien-gGmbH, a leading academic cooperative medical oncology group based in Germany. The study is investigating the use of pelareorep, in combination with Roche's anti-PD-L1 checkpoint inhibitor atezolizumab (Tecentriq®), in patients with first-line advanced/metastatic PDAC, first- and third-line metastatic colorectal, and advanced anal cancers. Approximately 55 patients are planned for enrollment across these four separate cohorts, and the study is being conducted at 12 centers in Germany. The study's co-primary endpoints are safety and objective response rate at week 16. Key secondary and exploratory endpoints include additional efficacy assessments and evaluation of potential blood-based biomarkers. In 2022, we received clearance from the Paul Ehrlich Institute (PEI; Germany's medical regulatory body) for full enrollment of the trial’s four cohorts. We also received FDA Fast Track designation for the treatment of advanced/metastatic PDAC using pelareorep in combination with atezolizumab, gemcitabine, and nab-paclitaxel. Fast Track designation is designed to facilitate the development and expedite the review of therapies to treat serious conditions and fill an unmet medical need. A clinical program that receives Fast Track designation may benefit from more frequent meetings and communications with the FDA to discuss development plans and ensure the collection of appropriate data needed to support approval.
In the third quarter of 2023, we continued enrolling and treating patients. The advanced/metastatic PDAC cohort/Stage 1 and third-line metastatic colorectal (CRC) cohort/Stage 1 have been fully enrolled, and we continue to monitor patients and patient
outcomes. In our first-line metastatic colorectal and advanced anal cancer cohorts, we continued enrolling patients and evaluating patient outcomes.
Subsequent to the quarter, in October 2023, positive updated results from GOBLET's advanced/metastatic PDAC cohort were presented at the European Society for Medical Oncology meeting. We also presented GOBLET's third-line metastatic CRC cohort achieved efficacy criteria for enrollment expansion. This is the second consecutive arm within the GOBLET platform study to meet its respective success criteria to move to full enrollment.
The updated PDAC arm data included:
Tumor Responses:
•Objective Response Rate of 62% (54% confirmed by two or more scans)
•A Disease Control Rate of 85%
Survival data:
•Median duration of response was 5.7 months
•Median PFS was 7.2 months
•Interim 12-month survival rate was 46%
•Interim median OS was 10.6 months
T-Cell populations analysis of the changes of T-cell clones and tumor-infiltrating lymphocytes (TILs) showed:
•Mean baseline TIL cell levels of 22%
•Expansion of pre-existing and new T cell clones, including the expansion of TIL-specific clones
•A correlation between the expansion in the blood of TIL-specific clones and tumor response
Safety:
•The treatment combination has been well tolerated with no safety concerns
•Most common grade 3 and 4 treatment-related adverse events were related to red and white blood cell counts (anemia, neutropenia and decreased neutrophil counts)
The interim results from GOBLET's third-line metastatic CRC cohort included:
•6 of 15 enrolled patients had stable disease as their best response, including 4 patients demonstrating stable disease at week 16
•These patients demonstrated a 40% disease control rate, a PFS of 2.8 months, a median overall survival of 8.0 months, and a 12-month survival rate of 33%
•Pelareorep uptake by tumor cells and stimulating T cell expansion even in heavily pre-treated colorectal cancer patients
Breast cancer program
Co-development Agreement with Pfizer Inc. and Merck KGaA, Darmstadt, Germany: BRACELET-1 study
In 2019, we entered into a co-development agreement with Merck KGaA, Darmstadt, Germany and Pfizer Inc. to co-develop pelareorep in combination with paclitaxel and avelumab (Bavencio®), a human anti-PD-L1 antibody, for the treatment of HR+/HER2- mBC. This phase 2 clinical trial is jointly funded by Oncolytics and Pfizer. The study, known as BRACELET-1, is a randomised open-label study that enrolled 48 patients into three cohorts: paclitaxel alone, paclitaxel in combination with pelareorep, and paclitaxel in combination with both pelareorep and avelumab. PrECOG LLC, a leading cancer research network, is managing the BRACELET-1 study. We completed patient enrollment in the second quarter of 2022.
The study is examining the expression of immune-related biomarkers to identify changes in the T cell population between pretreatment and on-treatment biopsies and seeks to confirm our previously identified biomarker. It is designed to assess efficacy in terms of overall response rate (ORR) at week 16 per RECIST 1.1. Key secondary and exploratory endpoints include the safety of the combination along with progression-free survival (PFS) and overall survival (OS). In the second quarter of 2023, we announced data that showed pelareorep driving robust increases in PFS and confirmed ORR. We featured these data at an oral presentation at the 2023 American Society of Clinical Oncology Annual Meeting (ASCO) and a subsequent key opinion leader webinar.
In the third quarter of 2023, we continued monitoring patients still on-study treatment and following patients through the survival follow-up timepoint, which we expect to occur in 2024. We also reviewed our BRACELET-1 data with key opinion leaders to investigate different trial designs as we move towards defining our breast cancer phase 3 licensure-enabling study.
Manufacturing and Process Development
While we currently have sufficient drug product supply to support our clinical development program, we continued our activities to expand our production capabilities as we focus on advancing our active drug substance and finished drug product towards registration readiness. During the third quarter of 2023, we executed a scaled-up engineering production run and the related batch testing, where we also implemented new procedures to match industry standards and evolving environmental regulations. As well, we completed release testing of a new master cell bank to support the use of the cell bank for potency assay validation. These activities ensure alignment with the clinical development timeline and anticipated phase 3 program. We also incurred storage and distribution costs to maintain our product supply. Ongoing bulk manufacturing and expanded filling capabilities are both part of the planned process validation. Continued process validation is required to ensure that the resulting product meets the specifications and quality standards and will form part of our submission to regulators, including the FDA, for product approval.
Intellectual Property
At the end of the third quarter of 2023, we had been issued over 153 patents, including 19 U.S. and 7 Canadian patents, as well as issuances in other jurisdictions. We have an extensive patent portfolio covering the oncolytic reovirus and formulations that we use in our clinical trial program. These patent rights extend to at least the end of 2031.
Financing Activity
Public offering
In the third quarter of 2023, pursuant to an underwritten public offering, we issued 7,667,050 units for gross proceeds of $23,262 (US$17,251) at a price of US$2.25 per unit. Each unit consisted of one common share and one common share purchase warrant ("warrant"), which were immediately separable and issued separately in this offering. Each warrant entitles the holder to purchase one common share at an exercise price of US$2.81 up to 60 months from the date of issuance, subject to certain acceleration provisions (see Notes 6 and 7 of our condensed interim consolidated financial statements). In consideration of the services rendered by the underwriter, we issued 536,693 compensation warrants. Each compensation warrant is exercisable into one common share at an exercise price of US$2.25 up to 60 months from the date of issuance. Net proceeds from the offering were $20,770.
Cash Resources
We ended the third quarter of 2023 with cash and cash equivalents of $39,981 (see "Liquidity and Capital Resources").
Subsequent Events
AWARE-1 study
In November 2023, we reported additional data from the AWARE-1 study at the 38th Annual Society for Immunotherapy of Cancer Annual Meeting. AWARE-1 evaluated early-stage breast cancer patients in two cohorts who received pelareorep and letrozole with and without atezolizumab. The study met its primary endpoint of CelTIL score (a measurement of cellularity and tumor-infiltrating lymphocytes), with the arm that included atezolizumab showing enhanced outcomes. Further analysis of the tumor microenvironment of patient tissue samples was performed using imaging mass cytometry. The data showed increased PD-L1 positive cells and an increase in cytotoxic T cells, amongst other benefits, which make the tumor microenvironment more amenable to treatment.
Global Business Conditions
General market conditions resulting from high inflation, high interest rates, global supply chain issues, global political conflicts, COVID-19, bank failures, general economic uncertainty and other macroeconomic factors, as well as market conditions affecting companies in the life sciences industry in general, may make it difficult for us to obtain financing from the capital markets on attractive terms, or at all.
We face various risks related to public health issues, including epidemics, pandemics, and other outbreaks, such as the lingering effects of the COVID-19 pandemic. The effects and potential effects of the COVID-19 pandemic, including, but not limited to, its impact on general economic conditions, trade and financing markets, changes in customer behavior and continuity in business operations, create significant uncertainty. In addition, the COVID-19 pandemic may cause an increase in costs resulting from our efforts to mitigate the effects. The extent to which the COVID-19 pandemic may continue to affect our business will depend on continued developments, including the duration of the pandemic and the extent of any further
resurgences in cases in geographic areas where we operate, the emergence of new variants, some of which have been, and may be in the future, more transmissible or virulent than the initial strain, the timing, availability and acceptance of effective medical treatments and vaccines, the impact on capital and financial markets and the related impact on consumer confidence and spending, all of which are uncertain and cannot be predicted. Even if the COVID-19 pandemic subsides, we may continue to suffer an adverse impact on our business due to the global economic effect of the pandemic, including any economic recession that has occurred or may occur in the future.
In recent years, there have been various global political conflicts. The extent and duration of the military action, sanctions, and resulting market disruptions could be significant and could potentially have a substantial negative impact on the global economy and/or our business for an unknown period of time. The ramifications of the hostilities and sanctions may not be limited to these geopolitical areas, and may spill over to and negatively impact other regional and global economic markets (including Europe and the United States), companies in other countries, and on various sectors, industries and markets for securities and commodities globally. Any such volatility and disruptions may also magnify the impact of other financial market risks and uncertainties described herein.
Recent bank failures could impair our ability to access our existing cash, cash equivalents, and marketable securities and to timely pay key vendors and others. For example, on March 10, 2023, Silicon Valley Bank (SVB) was placed into receivership with the Federal Deposit Insurance Corporation (FDIC), which resulted in all funds held at SVB being temporarily inaccessible by SVB’s customers. Although we did not have any funds in SVB or other institutions that have been closed, we cannot guarantee that the banks or other financial institutions that hold our funds will not experience similar issues. If other banks and financial institutions with whom we have banking relationships enter receivership or become insolvent in the future, we may be unable to access, and we may lose, some or all of our existing cash, cash equivalents, and marketable securities to the extent those funds are not insured or otherwise protected by the FDIC or Canadian Deposit Insurance Corporation (CDIC). In addition, in such circumstances we might not be able to timely pay key vendors and others. We regularly maintain cash balances that are not insured or are in excess of the FDIC/CDIC’s insurance limit. Any delay in our ability to access our cash, cash equivalents, and marketable securities (or the loss of some or all of such funds) or to timely pay key vendors and others could have a material adverse effect on our operations and cause us to need to seek additional capital sooner than planned.
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on terms favorable to us, or at all, and could have material adverse impacts on our liquidity, our business, financial condition or results of operations.
Pelareorep Development for the Remainder of 2023
Our primary clinical objectives for the remainder of 2023 will focus on delivering interim or updated data from our GOBLET clinical study and assessing our clinical data to help form the nature of our registration strategy, our path to approval, and other possible clinical development opportunities. We expect to finalize the definitive agreements associated with the Precision PromiseSM study and work towards obtaining the necessary regulatory approvals to allow us to commence enrollment.
Our 2023 manufacturing program is focused on progressing a process development program implementing single-use equipment for our drug substance production process, including a current Good Manufacturing Practices (cGMP) production run in the fourth quarter of 2023. A product fill and the associated analytical testing are also anticipated in the fourth quarter of 2023. Distribution of pelareorep to our various clinical sites is ongoing. Additionally, we will advance other product and analytical development activities toward registration readiness. Finally, our intellectual property program includes filings for additional patents and monitoring activities required to protect our patent portfolio.
Results of Operations
Comparison of the three months ended September 30, 2023, and 2022:
Unless otherwise indicated, all amounts below are presented in thousands of Canadian dollars, except for share amounts.
Net loss for the three months ended September 30, 2023, was $9,925 compared to $4,408 for the three months ended September 30, 2022.
Research and Development Expenses (“R&D”)
Our R&D expenses increased by $2,133 from $3,678 for the three months ended September 30, 2022, to $5,811 for the three months ended September 30, 2023. The following table summarizes our R&D expenses for the three months ended September 30, 2023, and 2022:
| | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | |
| 2023 | | 2022 | | Change |
| Clinical trial expenses | $ | 1,010 | | | $ | 1,364 | | | $ | (354) | |
| Manufacturing and related process development expenses | 2,979 | | | 651 | | | 2,328 | |
| Intellectual property expenses | 84 | | | 60 | | | 24 | |
| Translational science expenses | — | | | 110 | | | (110) | |
| Personnel-related expenses | 1,317 | | | 1,187 | | | 130 | |
| Share-based compensation expense | 399 | | | 274 | | | 125 | |
| Other expenses | 22 | | | 32 | | | (10) | |
| Research and development expenses | $ | 5,811 | | | $ | 3,678 | | | $ | 2,133 | |
The increase in our R&D expenses in the third quarter of 2023 was primarily due to higher manufacturing and related process development expenses associated with completing a scaled-up engineering production run and the associated batch testing. As part of the production run, we also implemented new procedures to match industry standards and evolving environmental regulations. The increase was partly offset by lower clinical trial expenses related to clinical and safety data management.
General and Administrative Expenses ("G&A")
Our G&A expenses increased by $2,855 from $2,382 for the three months ended September 30, 2022, to $5,237 for the three months ended September 30, 2023. The following table summarizes our G&A expenses for the three months ended September 30, 2023, and 2022:
| | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | |
| 2023 | | 2022 | | Change |
| Public company-related expenses | $ | 4,180 | | | $ | 1,371 | | | $ | 2,809 | |
| Office expenses | 754 | | | 688 | | | 66 | |
| Share-based compensation expense | 200 | | | 226 | | | (26) | |
| Depreciation - property and equipment | 20 | | | 23 | | | (3) | |
| Depreciation - right-of-use assets | 83 | | | 74 | | | 9 | |
| General and administrative expenses | $ | 5,237 | | | $ | 2,382 | | | $ | 2,855 | |
The increase in our G&A expenses in the third quarter of 2023 was primarily due to higher public-company related expenses associated with higher investor relations activities and the portion of the 2023 public offering transaction costs allocated to warrants (see Note 6 of our condensed interim consolidated financial statements).
Foreign Exchange
Our foreign exchange gain was $310 for the third quarter of 2023 compared to a gain of $1,525 for the third quarter of 2022. The foreign exchange impact mainly reflected the fluctuation of the U.S. dollar versus the Canadian dollar throughout the respective periods, primarily on our U.S. dollar-denominated cash and cash equivalents and marketable securities balances.
Comparison of the nine months ended September 30, 2023, and 2022:
Net loss for the nine months ended September 30, 2023, was $23,803 compared to $16,281 for the nine months ended September 30, 2022.
Research and Development Expenses (“R&D”)
Our R&D expenses increased by $2,461 from $10,590 for the nine months ended September 30, 2022, to $13,051 for the nine months ended September 30, 2023. The following table summarizes our R&D expenses for the nine months ended September 30, 2023, and 2022:
| | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, | | |
| 2023 | | 2022 | | Change |
| Clinical trial expenses | $ | 2,941 | | | $ | 3,675 | | | $ | (734) | |
| Manufacturing and related process development expenses | 4,856 | | | 1,671 | | | 3,185 | |
| Intellectual property expenses | 327 | | | 416 | | | (89) | |
| Translational science expenses | — | | | 250 | | | (250) | |
| Personnel-related expenses | 4,071 | | | 3,592 | | | 479 | |
| Share-based compensation expense | 735 | | | 911 | | | (176) | |
| Other expenses | 121 | | | 75 | | | 46 | |
| Research and development expenses | $ | 13,051 | | | $ | 10,590 | | | $ | 2,461 | |
The increase in our R&D expenses for the nine months ended September 30, 2023, was primarily due to the following:
•Increased manufacturing and related process development expenses associated with completing a process development production run, a scaled-up engineering production run, and the related batch testing. As part of the production run, we also implemented new procedures to match industry standards and evolving environmental regulations as we focus on advancing toward registration readiness; and
•Increased personnel-related expenses due to changes in salary levels and the strengthening of the U.S. dollar.
The above increases were partly offset by the following:
•Decreased clinical trial expenses due to lower BRACELET-1 and AWARE-1 study costs, as well as reduced clinical and safety data management. The BRACELET-1 trial was in the patient follow-up phase throughout the first nine months of 2023, whereas patients were enrolled and treated during the same period in the previous year. We incurred AWARE-1 data analysis costs during the first nine months of 2022 for various conference presentations;
•Decreased translational science expenses as we focus on biomarker activities related to our ongoing clinical trials. In the first nine months of 2022, we incurred expenses related to our bispecific antibodies and CAR T studies; and
•Decreased share-based compensation expense reflecting the impact of the vesting of options granted in prior periods and the third quarter of 2023.
General and Administrative Expenses ("G&A")
Our G&A expenses increased by $4,065 from $7,826 for the nine months ended September 30, 2022, to $11,891 for the nine months ended September 30, 2023. The following table summarizes our G&A expenses for the nine months ended September 30, 2023, and 2022:
| | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, | | |
| 2023 | | 2022 | | Change |
| Public company-related expenses | $ | 8,868 | | | $ | 4,787 | | | $ | 4,081 | |
| Office expenses | 2,304 | | | 2,028 | | | 276 | |
| Share-based compensation expense | 423 | | | 718 | | | (295) | |
| Depreciation - property and equipment | 62 | | | 71 | | | (9) | |
| Depreciation - right-of-use assets | 234 | | | 222 | | | 12 | |
| General and administrative expenses | $ | 11,891 | | | $ | 7,826 | | | $ | 4,065 | |
The increase in our G&A expenses for the nine months ended September 30, 2023, was primarily due to the following:
•Increased public-company related expenses associated with higher investor relations activities, the portion of the 2023 public offering transaction costs allocated to warrants (see Note 6 of our condensed interim consolidated financial statements), and higher annual general meeting of shareholders costs; and
•Increased office expenses caused by a change in salary level and an increase in headcount.
The above increases were partly offset by decreased share-based compensation expense reflecting the impact of the vesting of options granted in prior periods and the third quarter of 2023.
Foreign Exchange
Our foreign exchange loss for the nine months ended September 30, 2023, was $83 compared to a gain of $1,939 for the nine months ended September 30, 2022. The foreign exchange impact mainly reflected the fluctuation of the U.S. dollar versus the Canadian dollar throughout the respective periods, primarily on our U.S. dollar-denominated cash and cash equivalents and marketable securities balances.
Summary of Quarterly Results
Historical patterns of expenditures cannot be taken as an indication of future expenditures. Our current and future expenditures are subject to numerous uncertainties, including the duration, timing, and costs of R&D activities ongoing during each period and the availability of funding from investors and prospective partners. As a result, the amount and timing of expenditures and, therefore, liquidity and capital resources may vary substantially from period to period.
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| 2023 | 2022 | 2021 |
| Sept.(3) | June | March | Dec.(3) | Sept. | June | March | Dec.(3) |
| Revenue | — | | — | | — | | — | | — | | — | | — | | — | |
Net loss(1)(2) | (9,925) | | (7,441) | | (6,437) | | (8,554) | | (4,407) | | (5,095) | | (6,779) | | (7,751) | |
Basic and diluted loss per common share(1)(2) | $ | (0.14) | | $ | (0.12) | | $ | (0.10) | | $ | (0.14) | | $ | (0.08) | | $ | (0.09) | | $ | (0.12) | | $ | (0.14) | |
Total assets(4) | 46,089 | 31,966 | 35,328 | 37,334 | | 38,959 | | 40,239 | | 44,446 | | 45,880 | |
Total cash, cash equivalents, and marketable securities(4) | 39,981 | | 24,351 | 29,670 | 32,138 | | 32,362 | | 33,689 | | 39,483 | | 41,262 | |
| Total long-term debt | — | | — | | — | | — | | — | | — | | — | | — | |
Cash dividends declared(5) | Nil | Nil | Nil | Nil | Nil | Nil | Nil | Nil |
(1)Included in consolidated net loss and loss per common share are share-based compensation expenses of $599, $242, $317, $749, $500, $490, $639, and $1,129, respectively.
(2)Included in consolidated net loss and loss per common share are foreign exchange gain (loss) of $310, $(394), $1, $(274), $1,526, $888, $(474), and $(326), respectively.
(3)During the quarter ended September 30, 2023, we completed an engineering production run, resulting in higher manufacturing and related process development expenses. We also incurred higher public-company related expenses associated with higher investor relations activities and the portion of the 2023 public offering transaction costs allocated to warrants (see Note 6 of our condensed interim consolidated financial statements). During the quarters ended December 31, 2022, and 2021, we incurred expenses related to annual short-term incentive awards.
(4)We issued 12,070,933 common shares for net cash proceeds of $30.0 million in 2023 (2022 - 6,284,125 common shares for net cash proceeds of $12.6 million).
(5)We have not declared or paid any dividends since incorporation.
Liquidity and Capital Resources
As a clinical-stage biopharmaceutical company, we have not been profitable since our inception. We expect to continue to incur substantial losses as we continue our research and development efforts. We do not expect to generate significant revenues until and unless pelareorep becomes commercially viable. To date, we have funded our operations mainly through issuing additional capital via public offerings, equity distribution arrangements, and the exercise of warrants and stock options. For the nine months ended September 30, 2023, we were able to raise funds through our U.S. ATM and our 2023 public offering.
We have no assurances that we will be able to raise additional funds through the sale of our common shares. Consequently, we will continue to evaluate all types of financing arrangements. On June 16, 2022, we renewed our short form base shelf prospectus (the "Base Shelf") that qualifies for distribution of up to $150.0 million of common shares, subscription receipts, warrants, or units (the "Securities") in either Canada, the U.S. or both. Under a Base Shelf, we may sell Securities to or through underwriters, dealers, placement agents, or other intermediaries. We may also sell Securities directly to purchasers or through agents, subject to obtaining any applicable exemption from registration requirements. The distribution of Securities may be
affected from time to time in one or more transactions at a fixed price or prices, which may be subject to change, at market prices prevailing at the time of sale or at prices related to such prevailing market prices to be negotiated with purchasers and as set forth in an accompanying Prospectus Supplement.
Renewing our Base Shelf provides additional flexibility when managing our cash resources as, under certain circumstances, it shortens the time required to close a financing and is expected to increase the number of potential investors that may be prepared to invest in the Company. Funds received from using our Base Shelf would be used in line with our Board approved budget and multi-year plan. Our renewed Base Shelf will be effective until July 16, 2024.
Our Base Shelf allowed us to enter our ATM equity distribution agreements and our 2023 public offering (see Note 7 of our condensed interim consolidated financial statements). We use these equity arrangements to assist us in achieving our capital objective. These arrangements provide us with the opportunity to raise capital and better manage our cash resources.
As at September 30, 2023, and December 31, 2022, we had cash and cash equivalents and marketable securities as follows:
| | | | | | | | | | | |
| September 30,
2023 | | December 31,
2022 |
| Cash and cash equivalents | $ | 39,981 | | | $ | 11,666 | |
| Marketable securities | $ | — | | | $ | 20,472 | |
| | | |
We have no debt other than accounts payable and accrued liabilities and lease liabilities. We have commitments and contingent obligations relating to completing our research and development of pelareorep.
The following table summarizes our cash flows for the periods indicated:
| | | | | | | | | | | | | | | | | |
| Nine Months Ended September 30, | | |
| 2023 | | 2022 | | Change |
| Cash used in operating activities | $ | (22,324) | | | $ | (17,416) | | | $ | (4,908) | |
| Cash provided by (used in) investing activities | 20,225 | | | (56) | | | 20,281 | |
| Cash provided by financing activities | 30,252 | | | 6,335 | | | 23,917 | |
| Impact of foreign exchange on cash and cash equivalents | 162 | | | 2,237 | | | (2,075) | |
| Increase (decrease) in cash and cash equivalents | $ | 28,315 | | | $ | (8,900) | | | $ | 37,215 | |
Cash used in operating activities
The change reflected higher net operating activities and non-cash working capital changes.
Cash used in operating activities for the nine months ended September 30, 2023, consisted of a net loss of $23,803 less non-cash adjustments of $1,240 and non-cash working capital changes of $239. Non-cash items primarily included share-based compensation expense and change in fair value of warrant derivative. Non-cash working capital changes mainly reflected increased accounts payable and accrued liabilities and decreased prepaid expenses.
Cash used in operating activities for the nine months ended September 30, 2022, consisted of a net loss of $16,281 less non-cash adjustments of $314 and non-cash working capital changes of $1,449. Non-cash items primarily included share-based compensation expense and unrealized foreign exchange gain. Non-cash working capital changes mainly reflected increased accounts payable and accrued liabilities and decreased prepaid expenses.
Cash provided by (used in) investing activities
The change was primarily related to the maturities of marketable securities in the first nine months of 2023.
Cash provided by financing activities
The change was mainly due to our 2023 public offering, whereby we issued 7,667,050 units for gross proceeds of $23,262 (US$17,251) at a price of US$2.25 per unit. Each unit consisted of one common share and one warrant. Each warrant entitles the holder to purchase one common share at an exercise price of US$2.81 up to 60 months from the date of issuance.
We desire to maintain adequate cash reserves to support our planned activities, including our clinical trial program, product manufacturing, administrative costs, and intellectual property protection. To do so, we estimate our future cash requirements by preparing a budget and a multi-year plan annually for review and approval by our Board. The budget establishes the approved activities for the upcoming year and estimates the associated costs. The multi-year plan estimates future activity along with the potential cash requirements and is based on our assessment of our current clinical trial progress along with the expected results
from the coming year’s activity. Budget to actual variances are prepared and reviewed by management and are presented quarterly to the Board.
We continue to manage our research and development plan to ensure optimal use of our existing resources as we expect to fund our expenditure requirements and commitments with existing working capital. Additional activities continue to be subject to adequate resources, and we believe we will have sufficient existing cash resources to fund our presently planned operations for at least the next twelve months from the balance sheet date. Factors that will affect our anticipated cash usage for which additional funding might be required include, but are not limited to, expansion of our clinical trial program, the timing of patient enrollment in our approved clinical trials, the actual costs incurred to support each clinical trial, the number of treatments each patient will receive, the timing of R&D activity with our clinical trial research collaborations, the number, timing and costs of manufacturing runs required to conclude the validation process and supply product to our clinical trial program, and the level of collaborative activity undertaken.
We are not subject to externally imposed capital requirements, and there have been no changes in how we define or manage our capital in 2023.
Contractual Obligations and Commitments
The following table summarizes our significant contractual obligations as at September 30, 2023:
| | | | | | | | | | | | | | | | | |
|
Total | Less than 1 year |
2 -3 years |
4 - 5 years | More than
5 years |
| Accounts payable and accrued liabilities | $ | 4,536 | | $ | 4,536 | | $ | — | | $ | — | | $ | — | |
| Lease obligations | 624 | | 262 | | 345 | | 17 | | — | |
| Total contractual obligations | $ | 5,160 | | $ | 4,798 | | $ | 345 | | $ | 17 | | $ | — | |
In addition, we are committed to payments totaling approximately $12.3 million for activities mainly related to our clinical trial and manufacturing programs, which are expected to occur over the next three years. We are able to cancel most of these agreements with notice. The ultimate amount and timing of these payments are subject to changes in our research and development plan.
Off-Balance Sheet Arrangements
As at September 30, 2023, we had not entered into any off-balance sheet arrangements.
Transactions with Related Parties
During the three and nine months ended September 30, 2023, and 2022, we did not enter into any related party transactions other than compensation paid to key management personnel. Key management personnel are those persons having authority and responsibility for planning, directing, and controlling our activities as a whole. We have determined that key management personnel comprise the Board of Directors, Executive Officers, President, and Vice Presidents.
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended September 30, | | Nine Months Ended September 30, |
| 2023 | | 2022 | | 2023 | | 2022 |
| Compensation and short-term benefits | $ | 1,010 | | | $ | 812 | | | $ | 3,036 | | | $ | 2,405 | |
| | | | | | | |
| Share-based compensation expense | 541 | | | 359 | | | 941 | | | 1,128 | |
| $ | 1,551 | | | $ | 1,171 | | | $ | 3,977 | | | $ | 3,533 | |
Critical Accounting Policies and Estimates
In preparing our condensed interim consolidated financial statements, we use IFRS as issued by the IASB. IFRS requires us to make certain estimates, judgements, and assumptions that we believe are reasonable based on the information available in applying our accounting policies. These estimates and assumptions affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed interim consolidated financial statements, and the reported amounts of revenue and expenses during the reporting periods presented. Actual results could differ from those estimates, and such differences could be material.
Our critical accounting policies and estimates are described in our audited consolidated financial statements for the year ended December 31, 2022, and available on SEDAR+ at www.sedarplus.ca and contained in our annual report on Form 20-F filed on EDGAR at www.sec.gov/edgar.
There were no material changes to our critical accounting policies in the nine months ended September 30, 2023.
Adoption of New Accounting Standards
IAS 1 Presentation of Financial Statements
In February 2021, the IASB issued amendments to IAS 1 Presentation of Financial Statements and IFRS Practice Statement 2 Making Materiality Judgements, in which it provides guidance and example to help entities apply materiality judgements to accounting policy disclosures. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our condensed interim consolidated financial statements.
IAS 8 Accounting Policies, Changes in Accounting Estimates and Errors
In February 2021, the IASB issued amendments to IAS 8, in which it introduces a new definition of 'accounting estimates'. The amendments clarify the distinction between changes in accounting estimates and changes in accounting policies, and the correction of errors. Also, the amendments clarify how entities use measurement techniques and inputs to develop accounting estimates. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our condensed interim consolidated financial statements.
IAS 12 Income Taxes
In May 2021, the IASB issued amendments to IAS 12, which narrows the scope of the initial recognition exception under IAS 12, so that it no longer applies to transactions that give rise to equal taxable and deductible temporary differences. The amendments became effective on January 1, 2023. Adopting the amendments did not have a material impact on our condensed interim consolidated financial statements.
Accounting Standards and Interpretations Issued but Not Yet Effective
IAS 1 Classification of Liabilities as Current or Non-Current
In October 2022, the IASB issued amendments to clarify how conditions with which an entity must comply within 12 months after the reporting period affect the classification of a liability, in addition to the amendment from January 2020 where the IASB issued amendments to IAS 1 Presentation of Financial Statements, to provide a more general approach to the presentation of liabilities as current or non-current based on contractual arrangements in place at the reporting date. These amendments specify that the rights and conditions existing at the end of the reporting period are relevant in determining whether the Company has a right to defer settlement of a liability by at least 12 months, provided that management's expectations are not a relevant consideration as to whether the Company will exercise its rights to defer settlement of a liability and clarify when a liability is considered settled. The amendments are effective for annual periods beginning on or after January 1, 2024, and are to be applied retrospectively. The adoption of this standard is not expected to have a material impact on our consolidated financial
statements.
Financial Instruments and Other Instruments
Our financial instruments consist of cash and cash equivalents, marketable securities, other receivables, accounts payable and accrued liabilities, and warrant derivative. As at September 30, 2023, and December 31, 2022, the carrying amount of our cash and cash equivalents, marketable securities, other receivables, and accounts payable and accrued liabilities approximated their fair value due to their short-term maturity. The warrant derivative is a recurring Level 2 fair value measurement as these warrants have not been listed on an exchange and, therefore, do not trade on an active market. As at September 30, 2023, the fair value of our warrant derivative was $5,198 (December 31, 2022 - $79). The change was mainly due to warrants issued as part of our 2023 public offering. These warrants have an exercise price denominated in a currency that differs from our
functional currency and have been treated as a derivative measured at fair value with subsequent changes in fair value accounted for through profit and loss (see Note 6 of our condensed interim consolidated financial statements). We use the Black-Scholes valuation model to estimate fair value.
Credit risk
Credit risk is the risk of a financial loss if a counterparty to a financial instrument fails to meet its contractual obligations. We are exposed to credit risk on our cash and cash equivalents and other receivables from Pfizer in connection with the BRACELET-1 study (see Note 4 of our condensed interim consolidated financial statements) in the event of non-performance by counterparties, but we do not anticipate such non-performance. Our maximum exposure to credit risk at the end of the period is the carrying value of our cash and cash equivalents and other receivables from Pfizer.
We mitigate our exposure to credit risk connected to our cash and cash equivalents by maintaining our primary operating and investment bank accounts with Schedule I banks in Canada. For our foreign-domiciled bank accounts, we use referrals or recommendations from our Canadian banks to open foreign bank accounts. Our foreign-domiciled bank accounts are used solely for the purpose of settling accounts payable and accrued liabilities or payroll.
Interest rate risk
Interest rate risk is the risk that a financial instrument's fair value or future cash flows will fluctuate because of changes in market interest rates. We hold our cash and cash equivalents in bank accounts or high-interest investment accounts with variable interest rates. We mitigate interest rate risk through our investment policy that only allows the investment of excess cash resources in investment-grade vehicles while matching maturities with our operational requirements.
Fluctuations in market interest rates do not significantly impact our results of operations due to the short-term maturity of the investments held.
Foreign exchange risk
Foreign exchange risk arises from changes in foreign exchange rates that may affect the fair value or future cash flows of our financial assets or liabilities. We are primarily exposed to the risk of changes in the Canadian dollar relative to the U.S. dollar, as a portion of our financial assets and liabilities are denominated in such currency. The impact of a $0.01 increase in the value of the U.S. dollar against the Canadian dollar would have decreased our net comprehensive loss in 2023 by approximately $216.
We mitigate our foreign exchange risk by maintaining sufficient foreign currencies by purchasing foreign currencies or receiving foreign currencies from financing activities to settle our foreign accounts payable.
Significant balances in foreign currencies at September 30, 2023, were as follows:
| | | | | | | | |
|
U.S. dollars | | | |
| Cash and cash equivalents | $ | 27,834 | | | | |
| Accounts payable and accrued liabilities | (2,291) | | | | |
| Warrant derivative | (3,842) | | | | |
| $ | 21,701 | | | | |
Liquidity risk
Liquidity risk is the risk that we will encounter difficulty meeting obligations associated with financial liabilities. We manage liquidity risk by managing our capital structure as outlined in Note 11 of our condensed interim consolidated financial statements. Accounts payable and accrued liabilities are all due within the current operating period.
Use of Proceeds
2023 Public Offering and Use of Proceeds
The following table provides an update on the anticipated use of proceeds raised as part of the public offering of common shares and warrants which was completed in the third quarter of 2023 along with amounts actually expended. As at September 30, 2023, the following expenditures have been incurred (in thousands of U.S. dollars):
| | | | | | | | | | | | | | | | | | | | | | | |
| Item | Amount to Spend | | Spent to Date | | Adjustments | | Remaining to Spend |
| Pancreatic Cancer Program | $ | 10,500 | | | $ | — | | | $ | — | | | $ | 10,500 | |
| Breast Cancer Program | 500 | | | — | | | — | | | 500 | |
| General and Administrative Expenses | 2,650 | | | — | | | — | | | 2,650 | |
| Total | $ | 13,650 | | | $ | — | | | $ | — | | | $ | 13,650 | |
ATM Facility
On June 17, 2022, we entered into an ATM equity distribution agreement with Canaccord Genuity Inc. The ATM allows us to issue common shares, at prevailing market prices, with an aggregate offering value of up to US$65.0 million over a 25-month period through the facilities of the Nasdaq Capital Market in the United States. During the nine months ended September 30, 2023, we sold 4,205,240 common shares for gross proceeds of $9.1 million (US$6.8 million). Approximately $71.1 million (US$52.6 million) remains unused under the ATM equity distribution agreement.
Other MD&A Requirements
We have 73,398,847 common shares outstanding at November 2, 2023. If all of our options and restricted share awards (6,615,834) and common share purchase warrants (8,267,778) were exercised, we would have 88,282,459 common shares outstanding.
Our 2022 annual report on Form 20-F is available on SEDAR+ at www.sedarplus.ca and EDGAR at www.sec.gov/edgar.
Disclosure Controls and Procedures
Disclosure controls and procedures (“DC&P”) are designed to provide reasonable assurance that information required to be disclosed by the Company in its reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time periods specified in the securities legislation and include controls and procedures designed to ensure that information required to be disclosed by the Company in its reports filed or submitted under securities legislation is accumulated and communicated to the Company’s management, including its certifying officers, as appropriate to allow timely decisions regarding required disclosure. There were no changes in our DC&P during the three months ended September 30, 2023, that materially affected or are reasonably likely to materially affect, our DC&P.
Internal Controls over Financial Reporting
The Chief Executive Officer ("CEO") and Chief Financial Officer ("CFO") are responsible for designing internal controls over financial reporting (“ICFR”) or causing them to be designed under their supervision in order to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with IFRS. The CEO and CFO have designed, or caused to be designed under their supervision, ICFR to provide reasonable assurance that: (i) material information relating to the Company is made known to the Company's CEO and CFO by others; and (ii) information required to be disclosed by the Company in its reports filed or submitted by it under securities legislation is recorded, processed, summarized and reported within the time period specified in securities legislation. The Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) 2013 framework provides the basis for management’s design of internal controls over financial reporting. There were no changes in our ICFR during the three months ended September 30, 2023, that materially affected or are reasonably likely to materially affect, our ICFR.
Management, including the CEO and CFO, does not expect that our internal controls and procedures over financial reporting will prevent all errors and all fraud. A control system can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within the Company have been detected. These inherent
limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the control. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving our stated goals under all potential future conditions. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Risks and Uncertainties
We are a clinical-stage biopharmaceutical company. Prospects for biotechnology companies in the research and development stage should generally be regarded as speculative. It is not possible to predict, based on studies in animals, or early studies in humans, whether a new therapeutic will ultimately prove to be safe and effective in humans or whether necessary and sufficient data can be developed through the clinical trial process to support a successful product application and approval. If a product is approved for sale, product manufacturing at a commercial scale and significant sales to end users at a commercially reasonable price may not be successful. There can be no assurance that we will generate adequate funds to continue development or will ever achieve significant revenues or profitable operations. Many factors (e.g., competition, patent protection, appropriate regulatory approvals) can influence the revenue and product profitability potential. In developing a pharmaceutical product, we rely on our employees, contractors, consultants and collaborators, and other third-party relationships, including the ability to obtain appropriate product liability insurance. There can be no assurance that this reliance and these relationships will continue as required. In addition to developmental and operational considerations, market prices for securities of biotechnology companies generally are volatile, and may or may not move in a manner consistent with the progress we have made or are making.
Investment in our common shares involves a high degree of risk. An investor should carefully consider, among other matters, the risk factors in addition to the other information in our annual report on Form 20-F filed with the U.S. Securities and Exchange Commission (the "SEC"), as well as our other public filings with the Canadian securities regulatory authorities and the SEC, when evaluating our business because these risk factors may have a significant impact on our business, financial condition, operating results or cash flow. If any of the described material risks in our annual report or in subsequent reports we file with the regulatory authorities actually occur, they may materially harm our business, financial condition, operating results or cash flow. Additional risks and uncertainties that we have not yet identified or presently consider to be immaterial may also materially harm our business, financial condition, operating results, or cash flow. For information on risks and uncertainties, please refer to the "Risk Factors" section of our most recent annual report on Form 20-F and our other public filings available on www.sedarplus.ca and www.sec.gov/edgar.