Exhibit 99.1

Disrupting the Cell Cycle to Treat AML and MDS
BioCentury Newsmakers in the Biotech Industry Conference
September 2014

Disclaimer
This presentation contains forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 about financial results and estimates, business strategy, clinical trial plans and research and development programs of Cyclacel Pharmaceuticals, Inc. By their nature, forward-looking statements and forecasts involve risks and uncertainties because they relate to events and depend on circumstances that will occur in the future. For a further list and description of the risks and uncertainties the Company faces, please refer to our most recent Annual Report on Form 10-K and other periodic and current filings that have been filed with the Securities and Exchange Commission and are available at www.sec.gov. The information in this presentation is current as of this date. Cyclacel does not take any responsibility to update such information.
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 2

Cyclacel Highlights
Sapacitabine in front-line AML in the elderly: SEAMLESS Phase 3
– Oral agent for elderly AML patients; minimal options today
– Interim analysis for futility expected late 2014/early 2015
– Complete enrollment 2014/15; top-line data 2H15
Sapacitabine in high-risk MDS after HMA failure
– “Impressive” Phase 2 survival data in 2nd/3rd Line MDS
– Phase 2b RCT planned to start in 2015
Strong financial position & earlier-stage pipeline
– Sufficient capital beyond SEAMLESS Phase 3 data readout
– Sapacitabine in solid tumors; CDK and PLK inhibitors
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 20143

Sapacitabine for AML © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 4
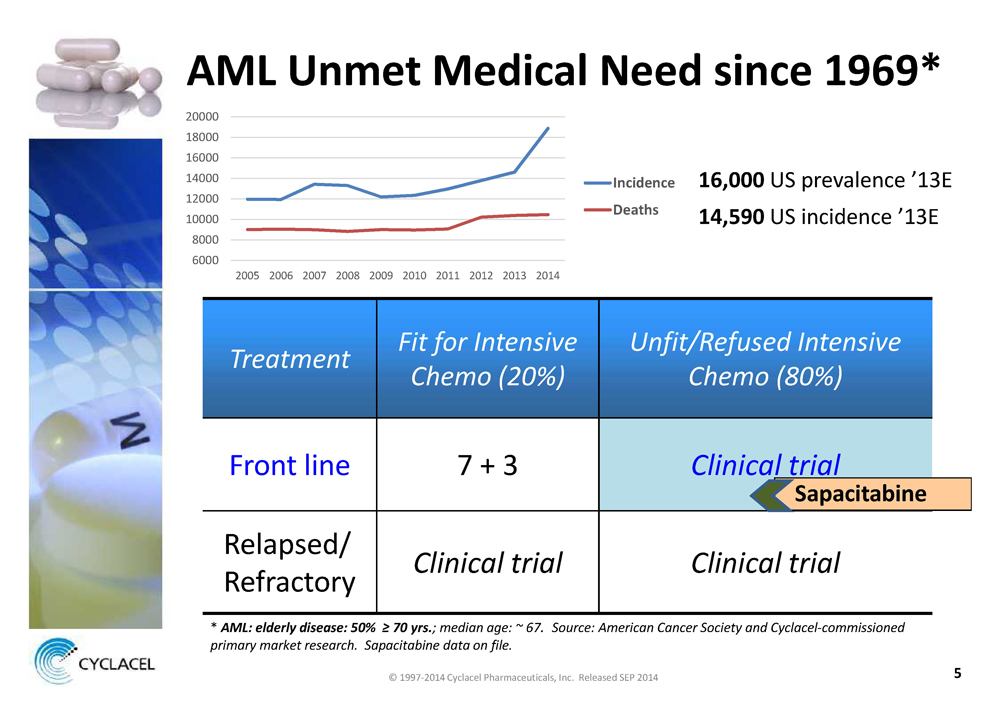
AML Unmet Medical Need since 1969*
8000 10000 12000 14000 16000 18000 20000 6000
2005 2006 2007 2008 2009 2010 2011 2012 2013 2014
Incidence Deaths
16,000US prevalence ’13E14,590US incidence ’13E
Treatment Fit for Intensive Chemo (20%) Unfit/Refused Intensive Chemo (80%)
Front line 7 + 3Clinical trial
Relapsed/ RefractoryClinical trial Clinical trial
*AML: elderly disease: 50% ≥ 70 yrs.; median age: ~ 67. Source: American Cancer Society and Cyclacel-commissioned primary market research. Sapacitabine data on file.
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 5
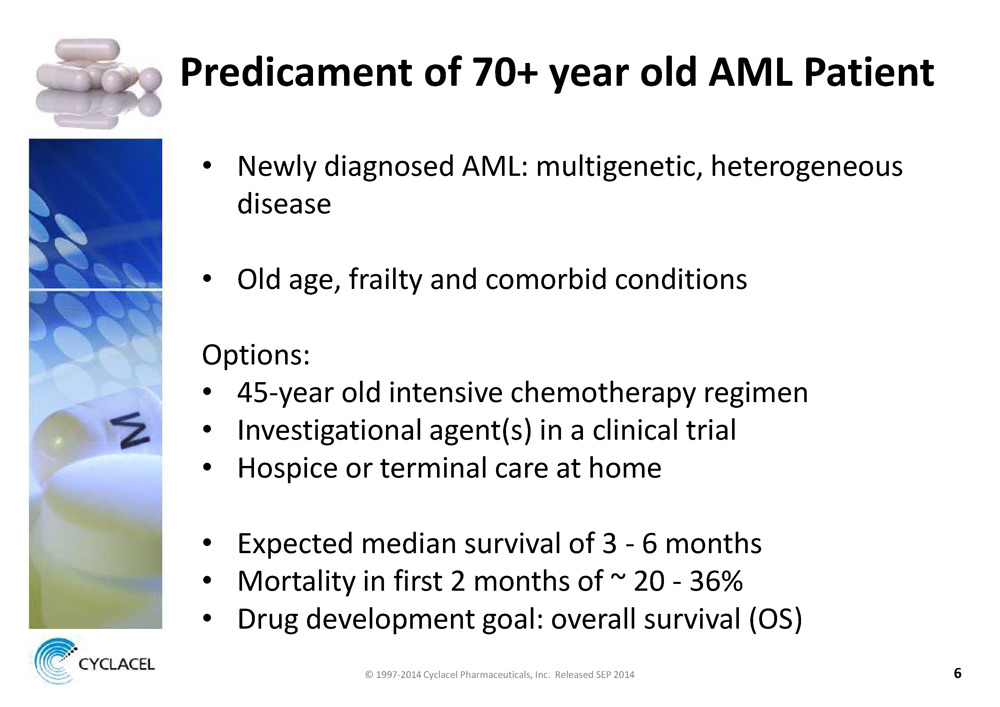
Predicament of 70+ year old AML Patient
• Newly diagnosed AML: multigenetic, heterogeneous disease
• Old age, frailty and comorbid conditions
Options:
• 45-year old intensive chemotherapy regimen
• Investigational agent(s) in a clinical trial
• Hospice or terminal care at home
• Expected median survival of 3 - 6 months
• Mortality in first 2 months of ~ 20 - 36%
• Drug development goal: overall survival (OS)
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 6
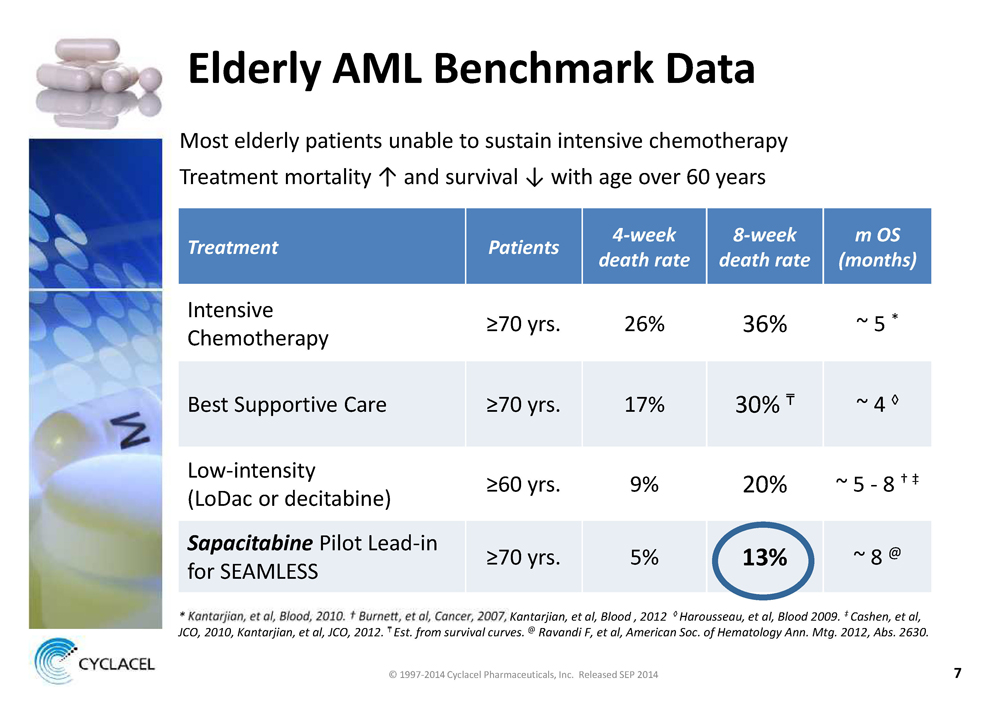
Elderly AML Benchmark Data
Most elderly patients unable to sustain intensive chemotherapy Treatment mortality ↑ and survival ↓ with age over 60 years
Treatment Patients 4-week death rate 8-week death rate m OS (months)
Intensive Chemotherapy ≥70 yrs. 26% 36% ~ 5 *
Best Supportive Care ≥70 yrs. 17% 30% ~ 4 ◊
Low-intensity (LoDac or decitabine) ≥60 yrs. 9% 20% ~ 5 - 8 † ‡
SapacitabinePilot Lead-in for SEAMLESS ≥70 yrs. 5%13%~ 8 @
* Kantarjian, et al, Blood , 2012 ◊ Harousseau, et al, Blood 2009. ‡ Cashen, et al,
JCO, 2010, Kantarjian, et al, JCO, 2012. Est. from survival curves.@Ravandi F, et al, American Soc. of Hematology Ann. Mtg. 2012, Abs. 2630.
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 7
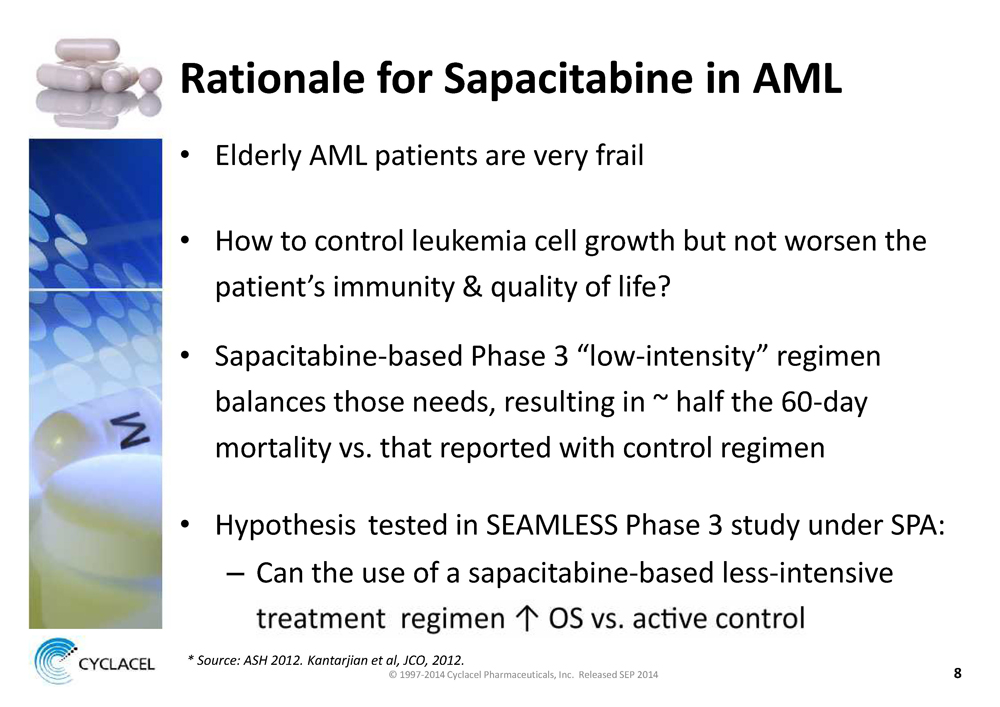
Rationale for Sapacitabine in AML
• Elderly AML patients are very frail
• How to control leukemia cell growth but not worsen the patient’s immunity & quality of life?
• Sapacitabine-based Phase 3 “low-intensity” regimen
balances those needs, resulting in ~ half the 60-day mortality vs. that reported with control regimen
• Hypothesis tested in SEAMLESS Phase 3 study under SPA:
– Can the use of a sapacitabine-based less-intensive
* Source: ASH 2012. Kantarjian et al, JCO, 2012.
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 8
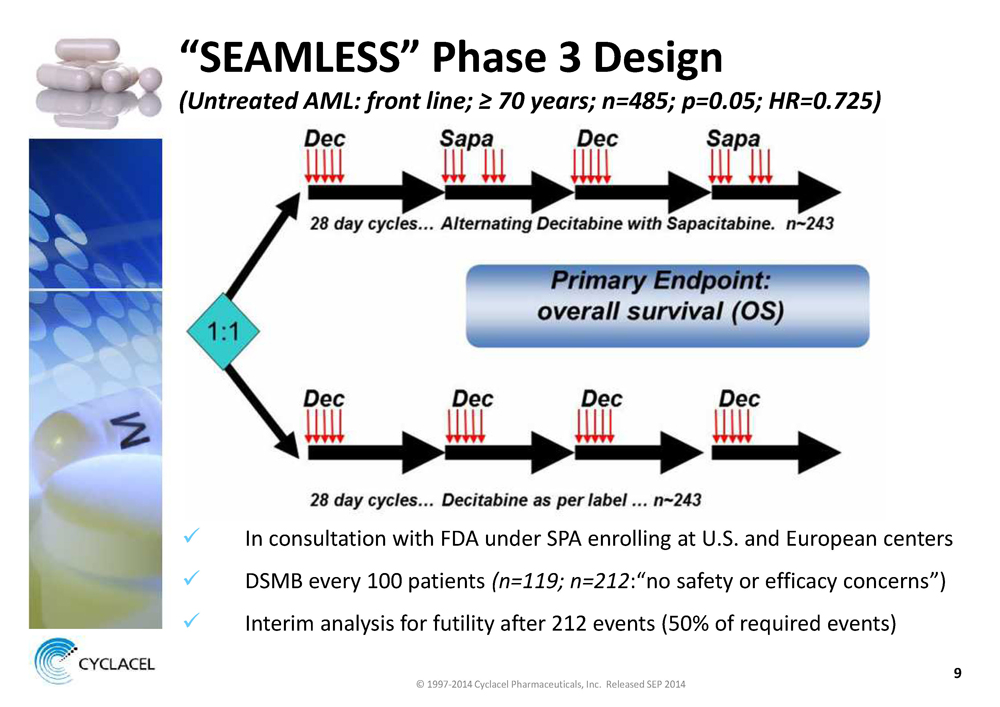
“SEAMLESS” Phase 3 Design
(Untreated AML: front line; ≥ 70 years; n=485; p=0.05; HR=0.725)
üIn consultation with FDA under SPA enrolling at U.S. and European centers
üDSMB every 100 patients(n=119; n=212:“no safety or efficacy concerns”)
üInterim analysis for futility after 212 events (50% of required events)
© 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 9

SEAMLESS Milestones • DSMB review at ~ 300 patients: 2H14 • Interim analysis for futility: Late 2014/Early 2015 • Enrollment > 75%; completion: Late ’14/Early ’15 • Top-line data: 2H15 © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 10
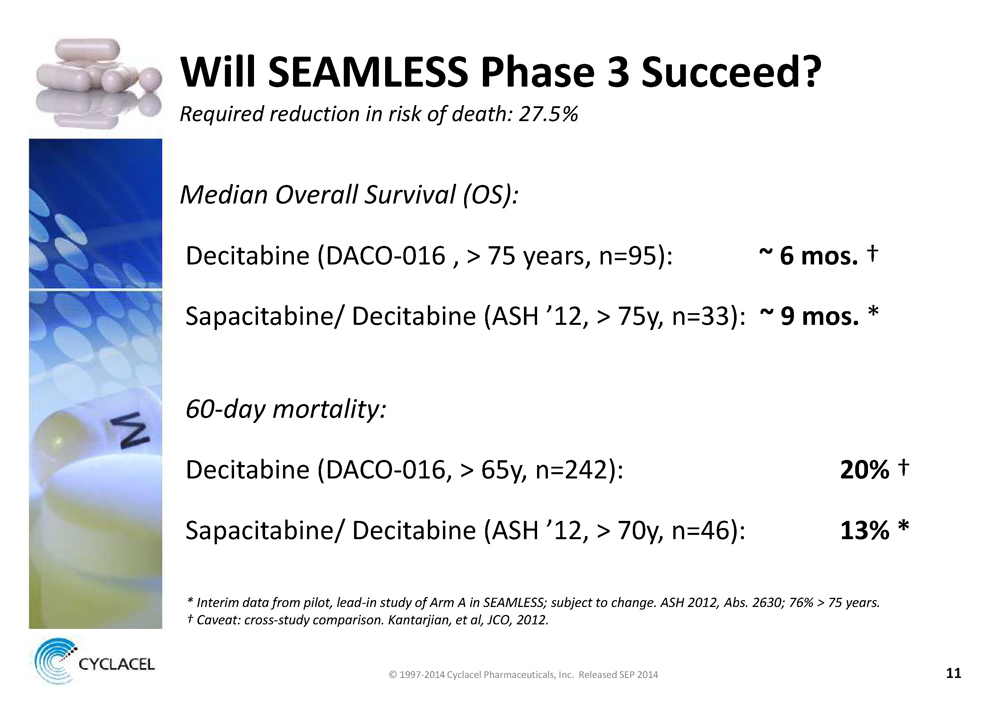
WILL SEAMLESS PHASE 3 SUCCEED? REQUIRED REDUCTION IN RISK OF DEATH: 27.5% MEDIAN OVERALL SURVIVAL (OS): Decitabine (DACO-016 , > 75 years, n=95): ~ 6 MOS. † Sapacitabine/ Decitabine (ASH ’12, > 75y, n=33): ~ 9 MOS. * 60-DAY MORTALITY: Decitabine (DACO-016, > 65y, n=242): 20% † Sapacitabine/ Decitabine (ASH ’12, > 70y, n=46): 13% * * INTERIM DATA FROM PILOT, LEAD-IN STUDY OF ARM A IN SEAMLESS; SUBJECT TO CHANGE. ASH 2012, ABS. 2630; 76% > 75 YEARS. † CAVEAT: CROSS-STUDY COMPARISON. KANTARJIAN, ET AL, JCO, 2012. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
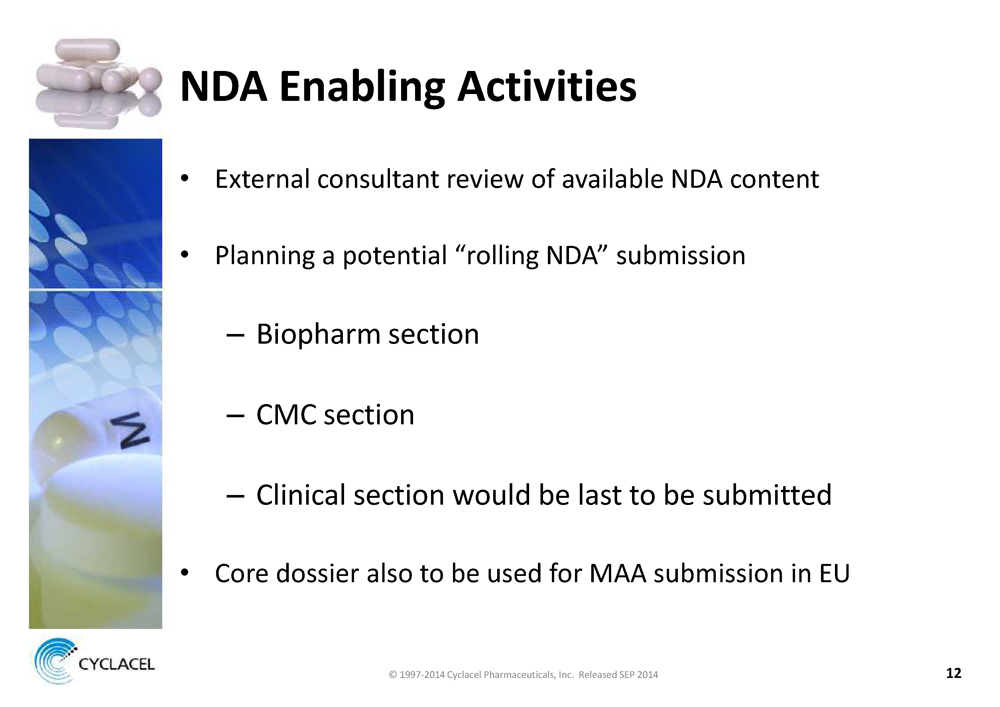
NDA ENABLING ACTIVITIES • External consultant review of available NDA content • Planning a potential “rolling NDA” submission – Biopharm section – CMC section – Clinical section would be last to be submitted • Core dossier also to be used for MAA submission in EU © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014

SAPACITABINE FOR MDS © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
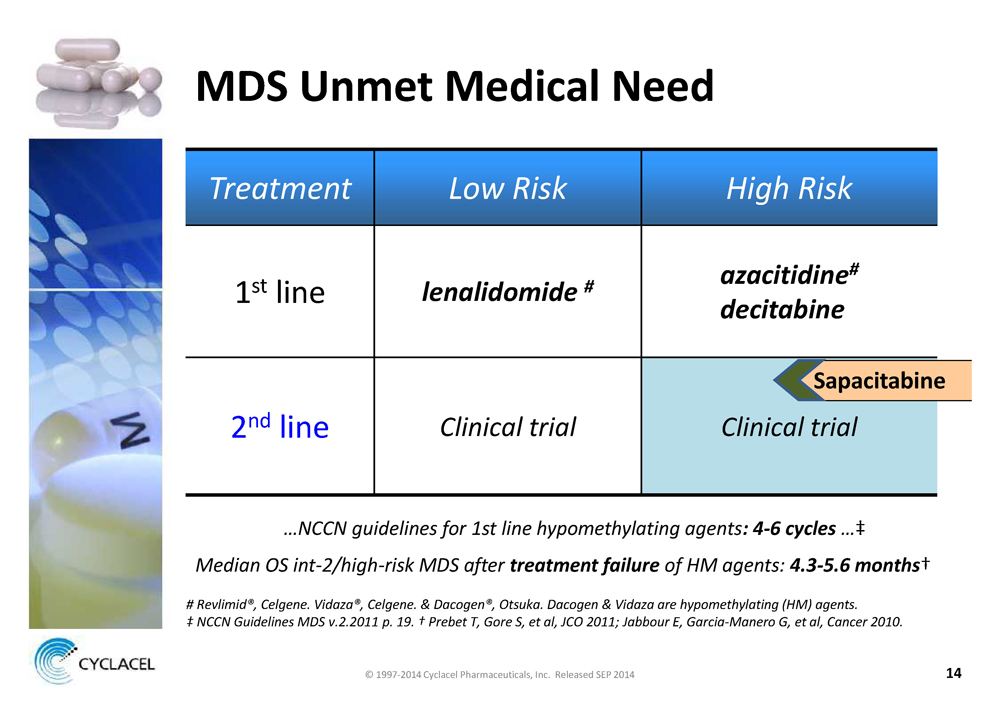
MDS UNMET MEDICAL NEED TREATMENT LOW RISK HIGH RISK 1st line LENALIDOMIDE # AZACITIDINE# DECITABINE 2nd line CLINICAL TRIAL CLINICAL TRIAL SAPACITABINE …NCCN GUIDELINES FOR 1ST LINE HYPOMETHYLATING AGENTS: 4-6 CYCLES …‡ MEDIAN OS INT-2/HIGH-RISK MDS AFTER TREATMENT FAILURE OF HM AGENTS: 4.3-5.6 MONTHS† # REVLIMID®, CELGENE. VIDAZA®, CELGENE. & DACOGEN®, OTSUKA. DACOGEN & VIDAZA ARE HYPOMETHYLATING (HM) AGENTS. ‡ NCCN GUIDELINES MDS V.2.2011 P. 19. † PREBET T, GORE S, ET AL, JCO 2011; JABBOUR E, GARCIA-MANERO G, ET AL, CANCER 2010. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
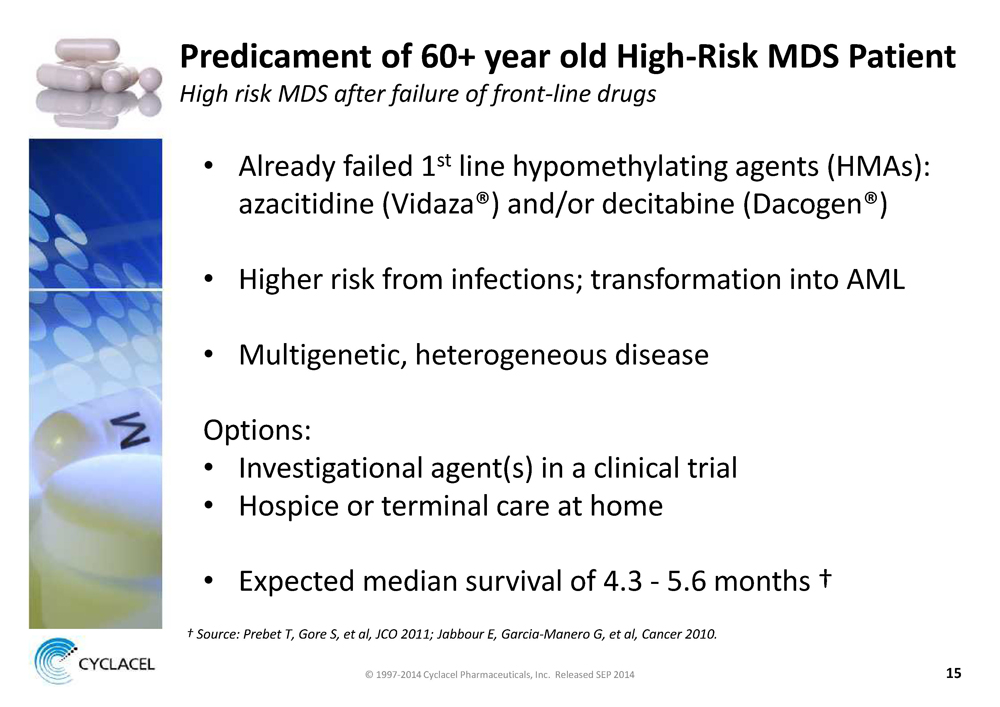
PREDICAMENT OF 60+ YEAR OLD HIGH-RISK MDS PATIENT HIGH RISK MDS AFTER FAILURE OF FRONT-LINE DRUGS • Already failed 1st line hypomethylating agents (HMAs): azacitidine (Vidaza®) and/or decitabine (Dacogen®) • Higher risk from infections; transformation into AML • Multigenetic, heterogeneous disease Options: • Investigational agent(s) in a clinical trial • Hospice or terminal care at home • Expected median survival of 4.3 - 5.6 months † † SOURCE: PREBET T, GORE S, ET AL, JCO 2011; JABBOUR E, GARCIA-MANERO G, ET AL, CANCER 2010. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
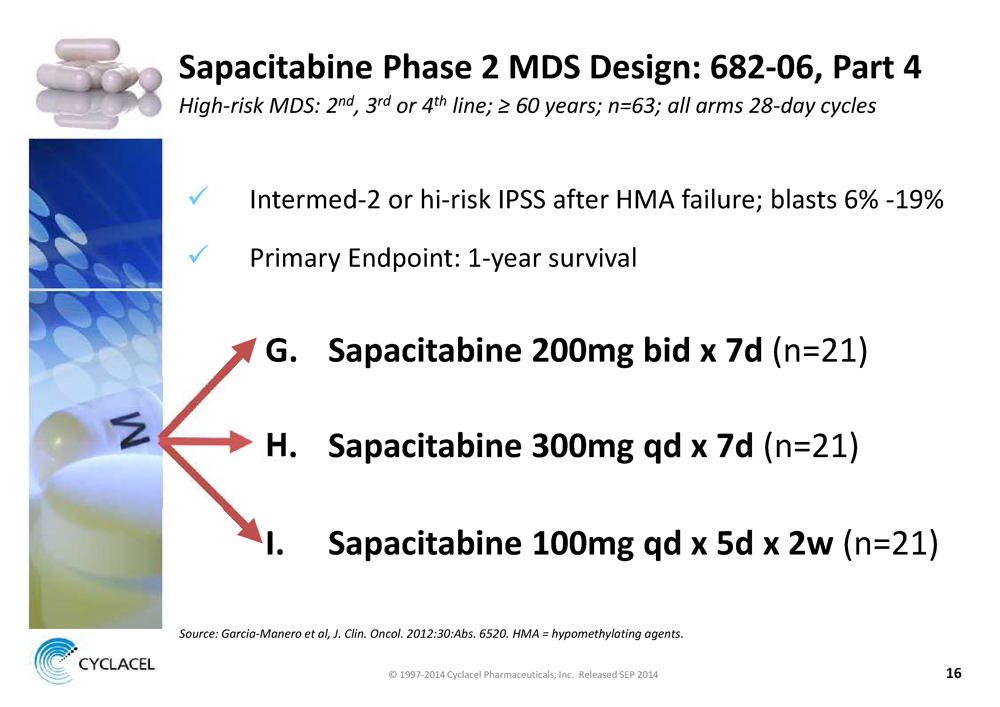
SAPACITABINE PHASE 2 MDS DESIGN: 682-06, PART 4 HIGH-RISK MDS: 2ND, 3RD OR 4TH LINE; ≥ 60 YEARS; N=63; ALL ARMS 28-DAY CYCLES x Intermed-2 or hi-risk IPSS after HMA failure; blasts 6% -19% x Primary Endpoint: 1-year survival G. SAPACITABINE 200MG BID X 7D (n=21) H. SAPACITABINE 300MG QD X 7D (n=21) I. SAPACITABINE 100MG QD X 5D X 2W (n=21) SOURCE: GARCIA-MANERO ET AL, J. CLIN. ONCOL. 2012:30:ABS. 6520. HMA = HYPOMETHYLATING AGENTS. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
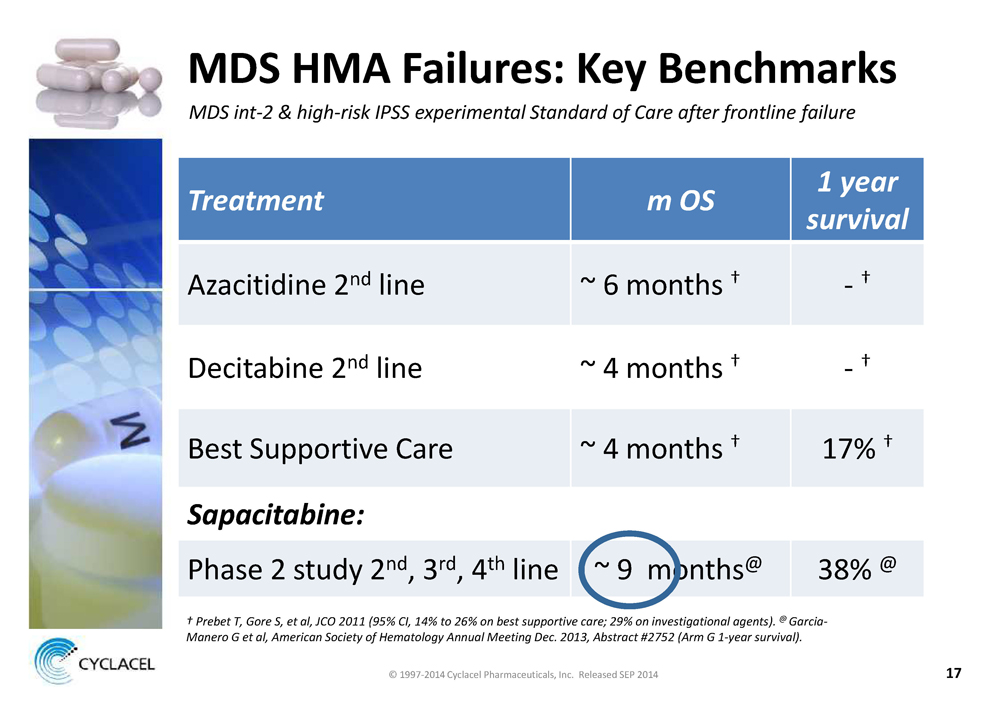
MDS HMA FAILURES: KEY BENCHMARKS MDS INT-2 & HIGH-RISK IPSS EXPERIMENTAL STANDARD OF CARE AFTER FRONTLINE FAILURE TREATMENT M OS 1 YEAR SURVIVAL Azacitidine 2nd line ~ 6 months † - † Decitabine 2nd line ~ 4 months † - † Best Supportive Care ~ 4 months † 17% † SAPACITABINE: Phase 2 study 2nd, 3rd, 4th line ~ 9 months@ 38% @ † PREBET T, GORE S, ET AL, JCO 2011 (95% CI, 14% TO 26% ON BEST SUPPORTIVE CARE; 29% ON INVESTIGATIONAL AGENTS). @ GARCIA- MANERO G ET AL, AMERICAN SOCIETY OF HEMATOLOGY ANNUAL MEETING DEC. 2013, ABSTRACT #2752 (ARM G 1-YEAR SURVIVAL). © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
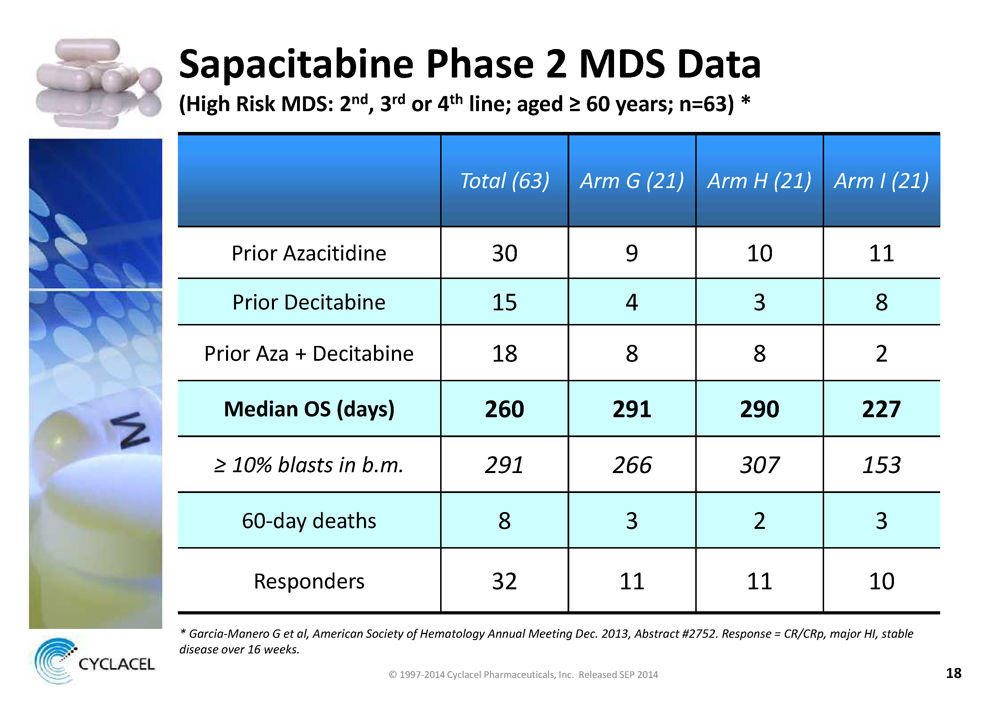
SAPACITABINE PHASE 2 MDS DATA (HIGH RISK MDS: 2ND, 3RD OR 4TH LINE; AGED ≥ 60 YEARS; N=63) * TOTAL (63) ARM G (21) ARM H (21) ARM I (21) Prior Azacitidine 30 9 10 11 Prior Decitabine 15 4 3 8 Prior Aza + Decitabine 18 8 8 2 MEDIAN OS (DAYS) 260 291 290 227 ≥ 10% BLASTS IN B.M. 291 266 307 153 60-day deaths 8 3 2 3 Responders 32 11 11 10 * GARCIA-MANERO G ET AL, AMERICAN SOCIETY OF HEMATOLOGY ANNUAL MEETING DEC. 2013, ABSTRACT #2752. RESPONSE = CR/CRP, MAJOR HI, STABLE DISEASE OVER 16 WEEKS. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
SAPACITABINE MDS PHASE 2B RCT STUDY OBJECTIVES » Prolong overall survival » Convenient outpatient treatment ACTIVE CONTROL OPTIONS 1. Low dose cytarabine (LoDAC) ‒ Differentiated mechanism ‒ Outpatient convenience ‒ Activity in 1st line setting * 2. OTHER HMA ‒ PATIENTS FAILED/PROGRESSED 1ST LINE HMA ‒ IV ADMINISTRATION ‒ HMA CROSS-TREATMENT DATA INCONCLUSIVE © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014 * ZWIERZINA H ET AL, LEUKEMIA, 2005
RATIONALE FOR RANDOMIZED PHASE 2B RCT • Limited knowledge • Genetic heterogeneity & treatment complexity • Sapacitabine Phase 2 clinical data encouraging • Cyclacel approach — Review recent MDS trials — Confer with MDS KOLs — Conduct feasibility assessment • Goal: determine path that may — Add to understanding of sapacitabine’s role in the indication — If RCT data exceptional, discuss with regulators © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
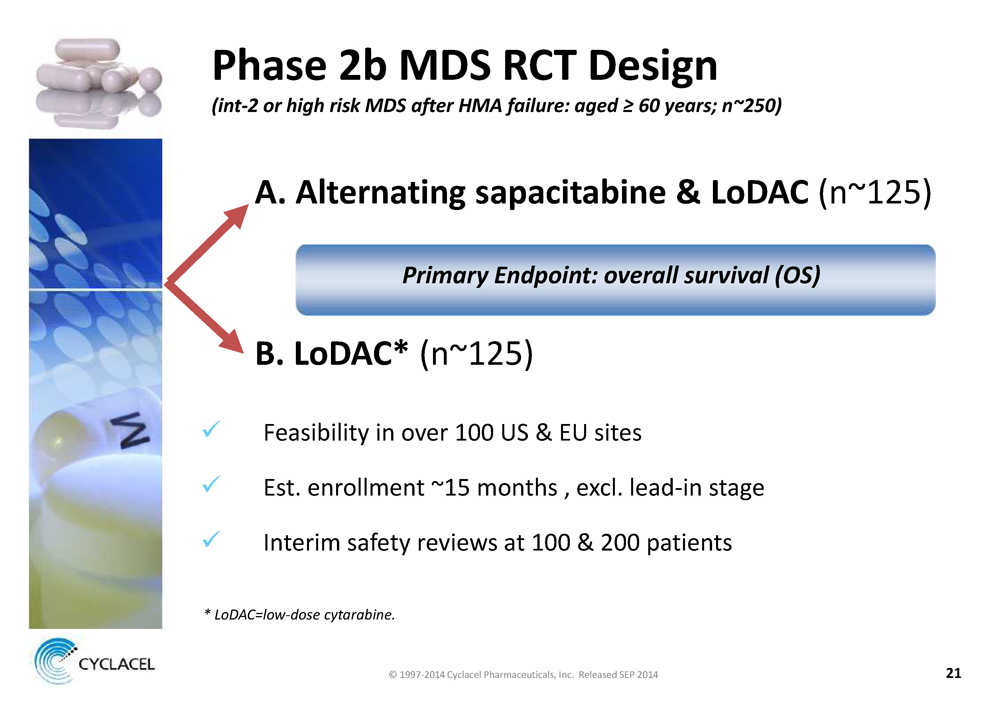
PHASE 2B MDS RCT DESIGN (INT-2 OR HIGH RISK MDS AFTER HMA FAILURE: AGED ≥ 60 YEARS; N~250) A. ALTERNATING SAPACITABINE & LODAC (n~125) PRIMARY ENDPOINT: OVERALL SURVIVAL (OS) B. LODAC* (n~125) x Feasibility in over 100 US & EU sites x Est. enrollment ~15 months , excl. lead-in stage x Interim safety reviews at 100 & 200 patients * LODAC=LOW-DOSE CYTARABINE © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
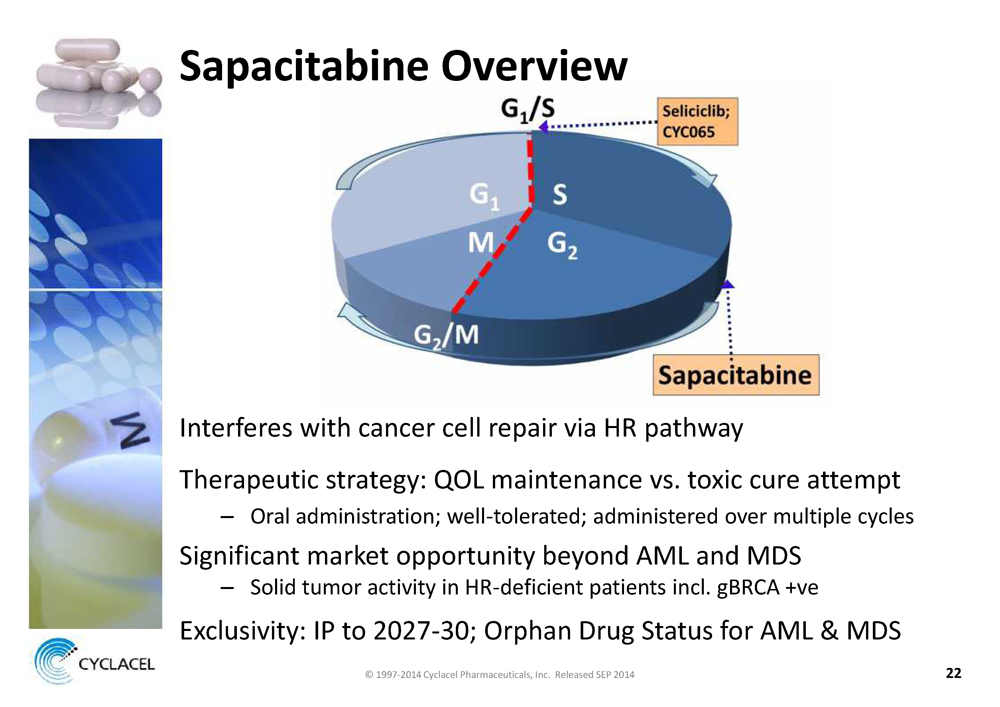
SAPACITABINE OVERVIEW Interferes with cancer cell repair via HR pathway Therapeutic strategy: QOL maintenance vs. toxic cure attempt – Oral administration; well-tolerated; administered over multiple cycles Significant market opportunity beyond AML and MDS – Solid tumor activity in HR-deficient patients incl. gBRCA +ve Exclusivity: IP to 2027-30; Orphan Drug Status for AML & MDS © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
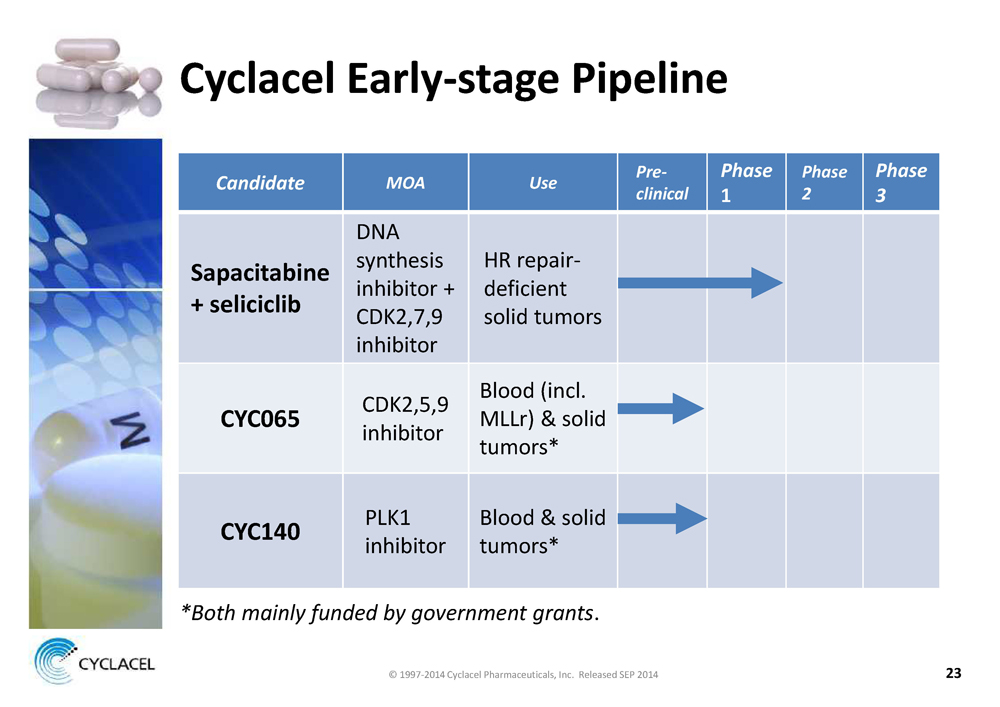
CYCLACEL EARLY-STAGE PIPELINE CANDIDATE MOA USE PRECLINICAL PHASE 1 PHASE 2 PHASE 3 SAPACITABINE + SELICICLIB DNA synthesis inhibitor + CDK2,7,9 inhibitor HR repairdeficient solid tumors CYC065 CDK2,5,9 inhibitor Blood (incl. MLLr) & solid tumors* CYC140 PLK1 inhibitor Blood & solid tumors* *BOTH MAINLY FUNDED BY GOVERNMENT GRANTS. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
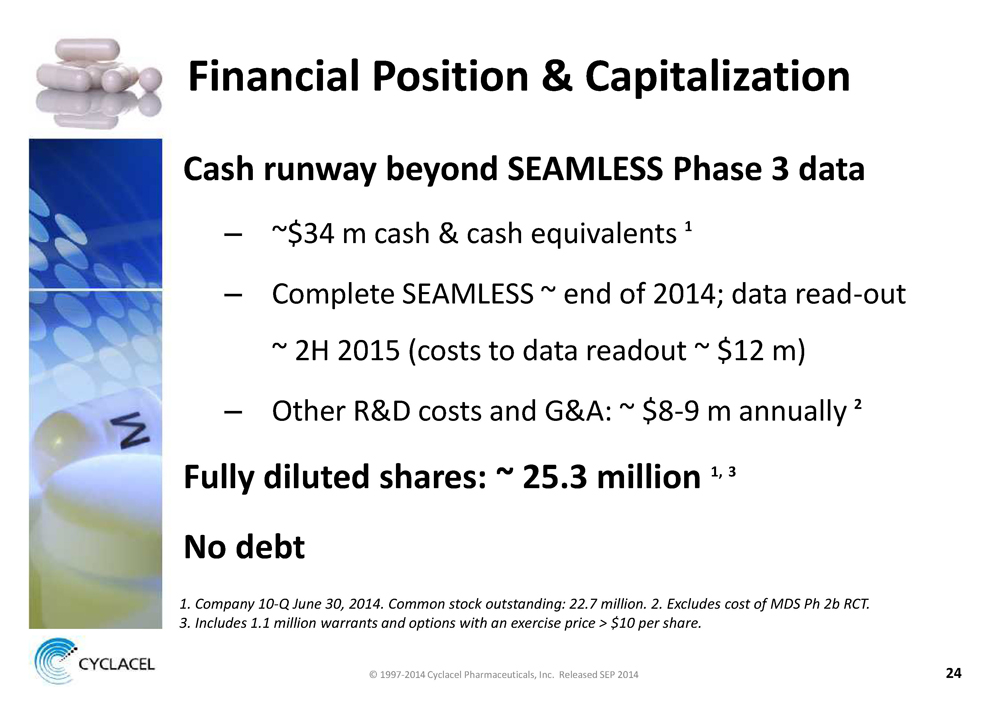
FINANCIAL POSITION & CAPITALIZATION CASH RUNWAY BEYOND SEAMLESS PHASE 3 DATA – ~$34 m cash & cash equivalents 1 – Complete SEAMLESS ~ end of 2014; data read-out ~ 2H 2015 (costs to data readout ~ $12 m) – Other R&D costs and G&A: ~ $8-9 m annually 2 FULLY DILUTED SHARES: ~ 25.3 MILLION 1, 3 NO DEBT 1. COMPANY 10-Q JUNE 30, 2014. COMMON STOCK OUTSTANDING: 22.7 MILLION. 2. EXCLUDES COST OF MDS PH 2B RCT. 3. INCLUDES 1.1 MILLION WARRANTS AND OPTIONS WITH AN EXERCISE PRICE > $10 PER SHARE. © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014

KEY MILESTONES SAPACITABINE – SEAMLESS: 300-patient DSMB review – SEAMLESS: interim analysis for futility – SEAMLESS: complete enrollment – MDS: open enrollment of Phase 2b after HMA failure – Sapacitabine & seliciclib in patients with solid tumors: update Phase 1 data OTHER – Advance early-stage pipeline © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
SUMMARY • SAPACITABINE OPPORTUNITY IN FRONT LINE AML: SEAMLESS APPROACHING COMPLETION • SAPACITABINE IN MDS: PHASE 2 DATA, HIGH-REWARD • STRONG FINANCIAL POSITION: SUFFICIENT CAPITAL BEYOND SEAMLESS DATA READ-OUT • EARLY-STAGE PIPELINE ADDRESSING HIGH-INTEREST TARGETS & MECHANISMS OF ACTION © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014
CYCLACEL PHARMACEUTICALS CELL CYCLE PIONEERS IMPROVING PATIENT LIVES WITH ORALLY-AVAILABLE INNOVATIVE MEDICINES © 1997-2014 Cyclacel Pharmaceuticals, Inc. Released SEP 2014


























