UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
| | |
| þ | | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the fiscal year ended December 31, 2010 |
|
| or |
| |
| ¨ | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d)
OF THE SECURITIES EXCHANGE ACT OF 1934 |
| | For the transition period from to |
Commission File Number 000-51329
XenoPort, Inc.
(Exact name of registrant as specified in its charter)
| | |
| Delaware | | 94-3330837 |
(State or other jurisdiction of incorporation or organization) | | (IRS employer identification no.) |
3410 Central Expressway, Santa Clara, California | | 95051 (Zip code) |
| (Address of principal executive offices) | |
(408) 616-7200
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| | |
Title of Each Class | | Name of Each Exchange on Which Registered |
Common stock, par value $0.001 per share
Preferred share purchase rights | | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. þ
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| | | | | | |
| Large accelerated filer ¨ | | Accelerated filer þ | | Non-accelerated filer ¨ | | Smaller reporting company ¨ |
| | (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
As of June 30, 2010 (the last business day of the registrant’s most recently completed second quarter), the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $281.6 million based on the closing sale price as reported on The NASDAQ Global Select Market for such date. Excludes an aggregate of 1,850,436 shares of the registrant’s common stock held by officers, directors and stockholders that the registrant has concluded are affiliates of the registrant. Exclusion of such shares should not be construed to indicate that the holder of any such shares possesses the power, direct or indirect, to direct or cause the direction of the management or policies of the registrant or that such person is controlled by, or under common control with, the registrant.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date.
| | |
Class | | Outstanding at February 1, 2011 |
| Common stock, par value $0.001 per share | | 35,263,668 shares |
DOCUMENTS INCORPORATED BY REFERENCE
| | |
Document | | Parts Into Which Incorporated |
| Portions of the Definitive Proxy Statement for the Annual Meeting of Stockholders to be held on or about May 11, 2011 to be filed with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year covered by this Form 10-K are incorporated by reference. | | Part III, Items 10-14 |
XENOPORT, INC.
TABLE OF CONTENTS
XENOPORT, the XenoPort logo and Transported Prodrug are trademarks of XenoPort, Inc.
Horizant, Requip and Requip XL are trademarks of GlaxoSmithKline.
2
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, which are subject to the “safe harbor” created by those sections. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions. In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions intended to identify forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors, which may cause our actual results, performance, time frames or achievements to be materially different from any future results, performance, time frames or achievements expressed or implied by the forward-looking statements. We discuss many of these risks, uncertainties and other factors in this Annual Report on Form 10-K in greater detail under the heading “Risk Factors.” Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date of this filing. You should read this Annual Report on Form 10-K completely and with the understanding that our actual future results may be materially different from what we expect. We hereby qualify our forward-looking statements by these cautionary statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
PART I.
Item 1. Business.
Overview
We are a biopharmaceutical company focused on developing and commercializing a portfolio of internally discovered product candidates that utilize the body’s natural nutrient transport mechanisms to improve the therapeutic benefits of existing drugs. Our innovative product candidates, which we refer to as Transported Prodrugs, are created by modifying the chemical structure of currently marketed drugs, referred to as parent drugs, and are designed to correct limitations in the oral absorption, distribution and/or metabolism of the parent drug. We intend to focus our development and commercialization efforts on potential treatments of diseases with significant unmet medical needs, with an emphasis on central nervous system, or CNS, disorders. Each of our product candidates is an orally available, patented or patentable new chemical entity that addresses large potential markets.
Our lead product candidate, gabapentin enacarbil (previously known as XP13512), is licensed to Astellas Pharma Inc. in Japan and five Asian countries and to Glaxo Group Limited, or GSK, in the United States. Astellas has filed a new drug application, or NDA, with the Pharmaceuticals and Medical Device Agency, or PMDA, for approval of gabapentin enacarbil as a treatment for restless legs syndrome in Japan. Restless legs syndrome is a neurological condition that causes an irresistible urge to move the legs. In November 2010, the U.S. Food and Drug Administration, or FDA, accepted for review GSK’s NDA resubmission for approval to market gabapentin enacarbil, known in the United States by the trade nameHorizant (gabapentin enacarbil) Extended-Release Tablets, for the treatment of moderate-to-severe primary restless legs syndrome, or RLS. The FDA has designated theHorizant NDA resubmission as a Class 2 response and set a new Prescription Drug User Fee Act, or PDUFA, date of April 6, 2011.
GSK is also evaluatingHorizant for the potential treatment of post-herpetic neuralgia, or PHN, a chronic type of neuropathic pain that can follow the resolution of shingles, andHorizant has successfully completed several Phase 2 clinical trials for the management of PHN in the United States. In addition, GSK evaluatedHorizant for the potential treatment of diabetic peripheral neuropathy, or DPN, and as a potential prophylactic therapy for migraine headaches.Horizant did not show statistically significant separation from placebo in the primary endpoints of these trials. GSK remains responsible for the development of Horizant for RLS and PHN in the United States; any further potential development ofHorizant for other indications, including PHN to the extent that a product label would reflect a superiority claim, would be conducted by us.
3
We are evaluating our second product candidate, arbaclofen placarbil, or AP (and previously known as XP19986), as a potential treatment for patients with spasticity. We have successfully completed a Phase 2 clinical trial of AP as a potential treatment of spasticity in patients with spinal cord injury, and in September 2010, we announced our plans to move AP into Phase 3 development as a potential treatment of spasticity in multiple sclerosis, or MS, patients. Based on discussions with the FDA, we intend to conduct a multi-center, randomized, double-blind, placebo-controlled study designed to assess the efficacy and safety of AP as a treatment for spasticity in MS patients. Patients who complete this study would have the option to enter an extension study to evaluate the safety of AP in MS patients. Favorable results from these studies and preclinical and clinical pharmacology studies could lead to the filing of an NDA with the FDA under Section 505(b)(2) seeking approval of AP for the treatment of spasticity. The Section 505(b)(2) application would enable us to reference published literature and the FDA’s previous findings of safety and effectiveness for baclofen, a drug that has been approved by the FDA for the alleviation of signs and symptoms of spasticity resulting from MS. We intend to initiate this Phase 3 clinical program in the first half of 2011.
We are also evaluating AP for the potential adjunctive treatment of gastroesophageal reflux disease, or GERD, in patients who do not experience complete relief of GERD symptoms while being treated with proton pump inhibitors, or PPIs. We are conducting a multi-dose, randomized, placebo-controlled Phase 2b clinical trial to evaluate the efficacy and safety of AP in approximately 450 patients with GERD who are incomplete responders to PPIs. We have completed enrollment in this trial and anticipate reporting top-line results in the first quarter of 2011.
We are evaluating our third product candidate, XP21279, for the potential treatment of patients with Parkinson’s disease and are conducting a randomized, cross-over Phase 2 clinical trial of XP21279 in Parkinson’s disease patients with motor fluctuations that is designed to compare the efficacy, safety and pharmacokinetics of individual patient-optimized doses of a new bi-layer tablet of XP21279/carbidopa to patient-optimized doses of Sinemet (L-Dopa/carbidopa). We anticipate reporting top-line results in the second half of 2011.
We have entered into development and commercialization agreements with Astellas and GSK. In December 2005, we entered into a collaboration with Astellas for the development and commercialization of gabapentin enacarbil, also known as ASP8825, pursuant to which we licensed to Astellas exclusive rights to develop and commercialize gabapentin enacarbil in Japan, Korea, the Philippines, Indonesia, Thailand and Taiwan. In February 2007, we entered into an exclusive collaboration with GSK to develop and commercialize gabapentin enacarbil, also known as GSK1838262, worldwide, excluding the Astellas territory. In November 2010, we amended and restated our collaboration agreement with GSK, pursuant to which we reacquired all rights to gabapentin enacarbil outside of the United States previously granted to GSK (which excludes the Astellas territory) and obtained the right to pursue development ofHorizant for: (i) the potential treatment of DPN; (ii) the potential treatment of PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug; and (iii) any additional indications in the United States. GSK remains responsible for seeking approval of the NDA for RLS in the United States, further development and regulatory matters with respect toHorizant for the potential treatment of PHN and commercialization ofHorizant in the United States for all indications. We plan to enter into additional agreements with pharmaceutical companies: (1) when access to a primary care physician sales force is necessary to maximize the commercial potential of our product candidates in the United States; (2) for the development and commercialization of our product candidates outside the United States; or (3) to develop and commercialize product candidates that fall outside our therapeutic areas of interest.
Transported Prodrugs
Critical to the success of any drug is its ability to access the targeted tissues, achieve and maintain effective concentrations at the site of therapeutic action for an appropriate period of time and have minimal side effects. In addition, convenient administration is frequently necessary to ensure patient compliance. Many marketed drugs do not possess all of these attributes, leading to limitations in their therapeutic benefit and commercial potential.
The conventional approach to designing new oral drugs is to rely on the drug’s ability to passively diffuse through the intestinal wall to enter the bloodstream and reach the targeted tissue. However, this can be a difficult
4
task, since the chemical and physical properties that allow a drug to bind to its cellular target and cause the intended therapeutic effect frequently impair the drug’s ability to passively diffuse through the wall of the intestines. If the medical need is high, drugs with poor absorption from the gastrointestinal, or GI, tract are still developed and marketed, but often with suboptimal therapeutic benefit. In some cases, drugs that are poorly absorbed from the GI tract are marketed as injectable medicines, which is inconvenient for patients. Another problem frequently encountered by drug designers occurs when a drug is well-absorbed from the intestines but does not last in the bloodstream for a sufficient period of time to maintain a therapeutic benefit. In this situation, frequent oral dosing is required, which is inconvenient for patients and can lead to poor compliance. In addition, drugs requiring frequent dosing often exhibit unwanted side effects when the drug is present in high concentration and then ineffectiveness when the concentration of the drug is insufficient. Sustained-release formulations that deliver medicine slowly as a pill travels through the entire GI tract can sometimes improve the utility of drugs that exhibit suboptimal therapeutic properties. However, drugs absorbed only in the upper GI tract do not benefit from sustained-release formulations.
Since most nutrients contain chemical features that prevent effective passive diffusion through cellular barriers, the human body contains specific membrane proteins, known as transporters, which are responsible for carrying nutrients into cells and across cell barriers. There are hundreds of different transporters in the human body that vary in the types of molecules they recognize and their localization to certain cells and tissue barriers. Active transport refers to cellular transporter mechanisms that capture nutrients and carry them across membranes.
Our proprietary technology utilizes the body’s natural mechanisms for actively transporting nutrients through cellular barriers to permit certain parent drugs with suboptimal oral absorption to be effectively and efficiently delivered into the body after the oral administration of our product candidate.
We have identified specific, high-capacity nutrient transporter proteins in the intestines and chemically modified the structure of the parent drug to create a Transported Prodrug that utilizes these transporters to gain efficient absorption into the bloodstream through active transport. Our Transported Prodrugs are engineered to split apart, releasing the parent drug and natural substances that generally have well-studied, favorable safety characteristics. In some cases, our product candidates target transporter proteins that are present throughout the entire GI tract, including the colon, so they can be formulated using sustained-release technology and thereby maintain effective blood concentrations for an extended period after dosing. As a result of their improved oral absorption, our product candidates may have improved therapeutic benefits compared to the parent drugs, such as superior clinical efficacy, reduced side effects and less frequent dosing, which result in improved patient convenience and compliance.
5
Our Product Candidates
Our current portfolio of proprietary product candidates includes the following:
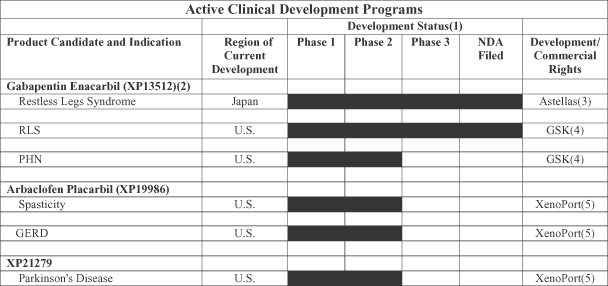
| (1) | Development Status indicates the most advanced stage of development that has been completed or is in process. |
| (2) | Known asHorizant in the United States. |
| (3) | Astellas holds rights in Japan and five Asian countries. |
| (4) | GSK holds commercialization and certain development rights in the United States. XenoPort holds co-promotion and certain development rights in the United States. |
| (5) | XenoPort holds rights worldwide. |
Gabapentin Enacarbil (Known as Horizant in the United States) — A Transported Prodrug of Gabapentin
Our most advanced product candidate is being developed in Japan for the potential treatment of restless legs syndrome and in the United States for the potential treatment of RLS and PHN. We hold composition-of-matter patents and methods-of-synthesis patents onHorizant in the United States and composition-of-matter patents on gabapentin enacarbil in Japan. We also hold patents or pending patent applications in the United States and outside the United States that are directed to the formulations and methods of synthesis and use of gabapentin enacarbil.
Parent Drug Background
Gabapentin enacarbil is metabolized by the body to release gabapentin, a drug that has been sold by Pfizer Inc as Neurontin since 1993 and is currently sold as a generic drug by a number of companies. Gabapentin is approved for marketing in the United States as adjunctive therapy in the treatment of partial seizures in patients with epilepsy and for the management of PHN. In addition, based on a variety of published medical studies, gabapentin is prescribed by physicians to treat a wide range of psychiatric, neurological and pain conditions. Gabapentin has a side effect profile that is considered favorable, with dizziness and somnolence, or drowsiness, as the most commonly reported side effects.
Despite its substantial commercial success, we believe that gabapentin therapy can be significantly improved. Gabapentin absorption is highly variable among patients, and there is a limit on the gabapentin exposure that can be achieved by direct oral administration of the parent drug. Published results from clinical trials of gabapentin in epilepsy patients indicated that, for the same dose level, some patients absorbed as little as
6
10% of the dose of gabapentin administered while others absorbed more than 70%. We have also conducted a clinical trial of gabapentin in neuropathic pain patients in which the high variability of gabapentin absorption was confirmed. In addition, the short duration of gabapentin in blood after oral dosing requires that it be administered three times a day, which may lead to poor compliance with the dosing regimen and, therefore, reduced efficacy in some patients.
We believe that these suboptimal characteristics of gabapentin result from the mechanism responsible for the absorption of gabapentin. Gabapentin is actively transported across the GI tract after administration. However, the specific transporter mechanism responsible for gabapentin absorption appears to have limited capacity, which seems to vary among individuals, and which is predominantly expressed in the upper GI tract. Due to gabapentin’s poor absorption in the lower GI tract, the use of traditional sustained-release formulations to correct the frequent dosing requirement has not been possible.
Our Transported Prodrug
Gabapentin enacarbil is designed to address the limitations of gabapentin by targeting high-capacity nutrient transporter mechanisms expressed throughout the length of the intestinal tract. We believe that this approach can address the variable and suboptimal exposure to gabapentin experienced by patients. By targeting transporters expressed throughout the length of the intestinal tract, we have been able to develop a sustained-release formulation of gabapentin enacarbil that we believe provides more consistent absorption of gabapentin and has overcome the need for frequent dosing of gabapentin.
Gabapentin enacarbil is designed to rapidly convert to gabapentin once absorbed from the GI tract, resulting in limited systemic exposure to the intact Transported Prodrug. In addition to producing gabapentin, gabapentin enacarbil is metabolized to release other components with well-studied, favorable safety characteristics. We believe that gabapentin enacarbil has demonstrated a favorable safety profile in clinical trials conducted in humans to date, which profile is comparable to that of gabapentin.
Phase 1 Clinical Trials
We have completed multiple safety, tolerability and pharmacokinetic Phase 1 clinical trials of gabapentin enacarbil. The results of these Phase 1 clinical trials indicated that all doses of gabapentin enacarbil were rapidly absorbed and converted to gabapentin, that doses up to 6000 mg produced dose-proportional gabapentin levels in the blood and that there was no evidence of saturation of drug absorption. Reported adverse events were consistent with those reported previously for gabapentin; somnolence and dizziness were the most frequently reported adverse events. Exposure to the intact Transported Prodrug was low and transient compared to the level of gabapentin produced at all dose levels.
Initial Target Indications
Restless Legs Syndrome
Background on Restless Legs Syndrome. Restless legs syndrome is a neurological condition that causes an irresistible urge to move the legs. This urge is usually accompanied by unpleasant sensations of burning, creeping, tugging or tingling inside the patients’ legs, ranging in severity from uncomfortable to painful. These restless legs syndrome-related symptoms typically begin or worsen during periods of rest or inactivity, particularly when lying down or sitting, and may be temporarily relieved by movement such as walking or massaging the legs. Symptoms often worsen at night, and disturbed sleep is a common result of restless legs syndrome. Left untreated, restless legs syndrome may cause exhaustion, daytime fatigue, inability to concentrate and impaired memory.
Potential Markets. In the United States, GSK is seeking FDA approval forHorizant as a potential treatment of RLS. Although the exact prevalence rate of RLS is uncertain, a study published inMovement Disorders in 2010 indicated that approximately 2% of people in the United States are afflicted with RLS.
7
According to Datamonitor’s 2008 Stakeholder Opinions: Restless Legs Syndrome report, there are approximately 8 million sufferers of RLS in the United States. We estimate that in 2010 there were approximately 4.6 million prescriptions written for drugs that are approved for the treatment of RLS in the United States.
In Japan, Astellas is seeking PMDA approval of gabapentin enacarbil as a potential treatment for restless legs syndrome. Although the exact prevalence is uncertain, Astellas estimates that there are approximately 3.9 million patients with restless legs syndrome in Japan.
Current Treatments. In the United States, the currently approved and most widely prescribed treatments for RLS belong to a class of drugs called dopamine agonists and include ropinirole (marketed as Requip by GSK), pramipexole (marketed as Mirapex by Boehringer Ingelheim GmbH) and generic comparables of these drugs. Physicians also prescribe opioids, benzodiazepines and anticonvulsants, such as gabapentin, to treat patients with restless legs syndrome. In Japan, pramipexole was approved in 2010 for the treatment of restless legs syndrome.
GSK’s U.S. Regulatory Filing. We evaluatedHorizant in a Phase 3 clinical program for the treatment of RLS, and in January 2009, GSK filed an NDA with the FDA forHorizantas a treatment for RLS. In February 2010, GSK received a Complete Response letter from the FDA regarding the NDA forHorizantfor RLS. In the Complete Response letter, the FDA concluded that the NDA provides substantial evidence of effectiveness forHorizant as a treatment for patients with RLS and that the FDA had not identified a clinical safety concern that would prevent approval of the 600 mg dose ofHorizant. However, a preclinical signal of pancreatic acinar cell tumors in rats was determined to be of sufficient concern to preclude approval of theHorizant NDA for RLS at that time. In the Complete Response letter, the FDA acknowledged that similar preclinical findings were known for gabapentin, the parent drug ofHorizant, at the time of the FDA’s approval of gabapentin for refractory epilepsy, but concluded that the seriousness and severity of refractory epilepsy and the benefit to patients provided by gabapentin justified the potential risk. In the Complete Response letter, the FDA also acknowledged that findings in laboratory animals are not necessarily translatable to risk in humans, and the FDA noted that gabapentin products have been available for over 15 years and they do not appear to be associated with a clinical signal for pancreatic cancer based on an analysis of spontaneous reports in the FDA’s Adverse Event Reporting System. However, the FDA concluded that the absence of a finding in analyses of post-marketing reports cannot be reliably interpreted as evidence of the absence of risk.
In October 2010, GSK submitted its response to questions raised by the FDA in the Complete Response letter. GSK’s response to the FDA included new data from non-clinical studies ofHorizant and two epidemiology studies, conducted by GSK, exploring gabapentin use and cancer based on the UK General Practice Research Database. The resubmission also included a final safety update that provided updated or new safety information on patients in clinical studies who have been treated withHorizant. In order for the FDA to be able to consider published gabapentin non-clinical data in their assessment ofHorizant, GSK amended the NDA forHorizant from a Section 505(b)(1) to a Section 505(b)(2) application.
In November 2010, the FDA accepted for review GSK’s response to the Complete Response letter forHorizantas a treatment of RLS. The FDA designated the resubmission as a Class 2 response and set a new PDUFA date of April 6, 2011.
XenoPort’s Clinical Program. The Phase 3 clinical program encompassed multiple U.S. trials, including one 12-week, randomized, double-blind, placebo-controlled trial, known as the PIVOT (Patient Improvement in Vital Outcomes following Treatment) RLS I clinical trial (previously known as XP052), designed to evaluate the safety and efficacy of 1200 mg ofHorizant versus placebo administered once a day at approximately 5:00 p.m., and a second 12-week, randomized, double-blind, placebo-controlled trial, known as the PIVOT RLS II clinical trial (previously known as XP053), designed to evaluate the safety and efficacy of 600 mg or 1200 mg ofHorizant versus placebo administered once a day at approximately 5:00 p.m. The co-primary outcome measures for these trials were defined to be the change from baseline in the International Restless Legs Syndrome, or IRLS, rating scale score and the Investigator Clinical Global Impression of Improvement, or CGI-I, scale at the end of treatment. Secondary endpoints for both trials included onset of efficacy and subjective sleep, pain, mood and quality of life assessments.
8
The PIVOT RLS I trial, which commenced in March 2006, enrolled 222 patients at 23 sites who were diagnosed with RLS. In April 2007, we reported top-line results demonstrating that treatment with 1200 mg ofHorizant was associated with a statistically significant improvement in the co-primary endpoints compared to placebo. Improvements in the IRLS rating scale score were significantly greater forHorizant than for placebo (-13.2 vs. -8.8; p=0.0002). At the end of treatment, significantly more patients treated withHorizant were reported as “much improved” or “very much improved” on the Investigator CGI-I scale compared to those treated with placebo (76% vs. 39%; p < 0.0001). During treatment over the 12-week period, the most commonly reported adverse events forHorizant versus placebo were somnolence (27%Horizant; 7% placebo) and dizziness (20%Horizant; 5% placebo). There were no reported serious adverse events inHorizant-treated patients.
The PIVOT RLS II trial, which commenced in August 2006, enrolled 325 patients who were diagnosed with RLS. In February 2008, we reported top-line results demonstrating that treatment with 1200 mg ofHorizant was associated with a statistically significant improvement in the co-primary endpoints compared to placebo. Improvements in the IRLS rating scale score were significantly greater for 1200 mg ofHorizant than for placebo (-13.0 vs. -9.8; p=0.0015). At the end of treatment, significantly more patients treated with 1200 mg ofHorizant were reported as “much improved” or “very much improved” on the Investigator CGI-I scale compared to those treated with placebo (78% vs. 45% for placebo; p<0.0001).
This trial also demonstrated that treatment with 600 mg ofHorizant was associated with a statistically significant improvement in the co-primary endpoints compared to placebo. Improvements in the IRLS rating scale score were significantly greater for 600 mg ofHorizant than for placebo (-13.8 vs. -9.8. p<0.0001). At the end of treatment, significantly more patients treated with 600 mg ofHorizant were reported as “much improved” or “very much improved” on the Investigator CGI-I scale compared to those treated with placebo (73% vs. 45%, p<0.0001).
During the 12-week treatment period, the most commonly reported adverse events forHorizant were dizziness (24% 1200 mgHorizant; 10% 600 mgHorizant; 5% placebo) and somnolence (18% 1200 mgHorizant; 22% 600 mgHorizant; 2% placebo). These adverse events were generally mild or moderate in intensity. Withdrawals due to adverse events were 7% in the 1200 mgHorizant group, 6% in the 600 mgHorizant group and 6% in the placebo group. There were three reported serious adverse events in the study (one in the placebo group, two in the 600 mgHorizant group), none of which were considered treatment-related.
In addition to these two 12-week trials, the Phase 3 program also included a clinical trial, known as the PIVOT RLS Maintenance clinical trial (previously known as XP060), to assess the long-term efficacy ofHorizant. The trial, which commenced in May 2006, was designed to evaluate the potential ofHorizant to maintain efficacy over the course of nine months in patients with RLS. The multi-center, double-blind, randomized, placebo-controlled, parallel-group clinical trial enrolled 327 patients diagnosed with RLS. All patients were administered 1200 mg ofHorizant, taken at approximately 5:00 p.m., for 24 weeks. Patients were assessed to determine treatment response at the end of this single-blind phase, and responders then entered the 12-week, randomized, double-blind phase of the clinical trial. Patients randomized to the placebo group received 600 mg ofHorizant for two weeks and then received placebo for an additional ten weeks. Patients randomized to theHorizant treatment group continued to receive 1200 mg ofHorizant for the entire 12-week, double-blind period. In January 2008, we reported top-line results that showed thatHorizant was generally well-tolerated during the treatment period and that there was a statistically significant difference between the percentage of patients treated withHorizant and placebo who met a pre-specified relapse criteria during the randomized phase of the study. Two hundred twenty one patients completed the 24-week, single-blind portion of the clinical trial, of which 194 (88%) met the responder criteria and were randomized to double-blind treatment. Analysis of the primary endpoint indicated that treatment withHorizant resulted in a statistically significant lower proportion of relapses compared to placebo during the double-blind treatment period (23% placebo compared to 9%Horizant; p= 0.0158).
The most commonly reported adverse events during the single-blind phase of this clinical trial were somnolence (30%) and dizziness (22%), which were generally mild or moderate in intensity and transient in nature. The incidence of somnolence and dizziness inHorizant-treated patients during the double-blind portion of the trial were 3% and 2%, respectively. During the trial, there was one death that was determined to be unrelated toHorizant treatment. There were five other serious adverse events, only one of which was judged as possibly related toHorizant treatment.
9
We have also conducted clinical trials and collected information that is typically required for submission of an NDA to the FDA, including an examination of the exposure/response relationship, pharmacokinetics in a special population, drug/drug interactions, cognition, driving performance and cardiovascular safety. In addition, we have completed an open-label safety extension study that included patients from the two 12-week clinical trials to enable assessment of the safety ofHorizant treatment extending up to 12 months. Data from this trial was also included in the NDA filing. The results of the Phase 3 clinical trials, combined with the results from otherHorizant clinical trials in RLS patients, are intended to meet the International Committee for Harmonization, or ICH, guidelines for safety assessment.
In addition, GSK conducted a polysomnography, or sleep laboratory measurement, study of Horizant in RLS patients to explore further the potential sleep benefits of Horizant. Results from this trial showed statistically significant benefits of Horizant versus placebo in several objective measurements of sleep.
Astellas’ Clinical Program and Regulatory Filing. In March 2009, Astellas reported results from a Phase 2 clinical trial of gabapentin enacarbil for the treatment of symptoms in restless legs syndrome patients in Japan. The trial was a 12-week, double-blind, placebo-controlled study that enrolled 474 patients who were diagnosed with restless legs syndrome. Patients were treated with 600, 900 or 1200 mg of gabapentin enacarbil or placebo, given once per day after the evening meal. The primary endpoint for the clinical trial was the change from baseline for the IRLS rating scale score at end of treatment.
Treatment with 1200 mg of gabapentin enacarbil was associated with a statistically significant improvement in the primary endpoint compared to placebo. Statistically significant improvements over placebo were also observed on some secondary endpoints, including the investigator-rated CGI-I scale, which achieved statistical significance for each of the 600 mg, 900 mg and 1200 mg dosing cohorts.
The most commonly reported adverse events for gabapentin enacarbil were somnolence and dizziness, which were generally transient and mild to moderate in severity. There were no treatment-emergent serious adverse events during the study period in gabapentin enacarbil-treated subjects.
In November 2009, Astellas filed an NDA with the PMDA for approval of gabapentin enacarbil as a potential treatment for restless legs syndrome in Japan. The evidence of efficacy for the NDA filing was based on data from Astellas’ successful Phase 2 trial in restless legs syndrome patients conducted in Japan and our clinical program conducted in the United States.
Neuropathic Pain
Background on Neuropathic Pain. Neuropathic pain is pain that results from damage to nerves. The damage may result from a variety of causes, including injury or illnesses such as diabetes, HIV and shingles. In addition, the toxic effects of therapy used to treat patients with cancer or HIV may also cause nerve damage leading to neuropathic pain.
One form of chronic neuropathic pain is PHN. PHN is a complication of shingles, a painful outbreak of rash or blisters on the skin caused by a reactivation of the same virus that causes chicken pox. PHN is often characterized as constant stabbing, burning or electric shock-like sensations in the area affected by shingles after the rash has cleared. Approximately 10% to 15% of all patients with shingles develop PHN, which can persist for many years. DPN is another form of neuropathic pain that is associated with a family of nerve disorders caused by diabetes. Over time, people with diabetes can experience damage to nerves leading to numbness and sometimes pain and weakness in the hands, feet and legs.
Potential Market. We estimate that the prevalence of PHN is less than 200,000 patients in the United States. In May 2006, Merck & Co. received FDA approval for Zostavax, a live attenuated vaccine, to help prevent shingles. In October 2006, the U.S. Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to recommend that adults 60 years of age and older be vaccinated with Zostavax for the prevention of shingles. While Zostavax is not a treatment for shingles or PHN, the availability of this vaccine could impact the future market for therapies for PHN.
Diabetes is the leading cause of neuropathy in the Western world, and neuropathy is the most common complication and greatest source of morbidity and mortality in diabetes patients. In 2007, the National Institute
10
of Diabetes and Digestive and Kidney Diseases estimated that 17.9 million people in the United States had been diagnosed with diabetes. Epidemiology studies indicate that about 20% of community-dwelling diabetes patients suffer from DPN, indicating that approximately 3.5 million people in the United States are afflicted with DPN.
Current Treatments. Current classes of drugs used to treat patients with neuropathic pain include anticonvulsants, antidepressants and tricyclic drugs, with anticonvulsants representing the largest share of the neuropathic pain market. Of the anticonvulsants, gabapentin is the market leader, and pregabalin (marketed as Lyrica by Pfizer) is also widely prescribed for the treatment of neuropathic pain. Duloxetine (marketed as Cymbalta by Eli Lilly and Company) is an antidepressant that is also prescribed for the treatment of DPN. Also, the FDA recently approved a once-daily formulation of gabapentin (known as Gralise from Depomed Inc. and its partner for pain indications, Abbott Laboratories) for PHN. Other treatments used in selected patients include a capsaicin patch (marketed as Qutenza by NeurogesX, Inc.) and local application of lidocaine.
Phase 2 Clinical Trial Results. We have completed a randomized, double-blind, parallel, placebo-controlled Phase 2a clinical trial ofHorizant for the management of PHN. The trial included 101 patients at 18 clinical sites in the United States. The objective of this trial was to assess the safety, tolerability, pharmacokinetics and efficacy of 1200 mg ofHorizant administered twice a day for 14 days and to compare the response toHorizant against the response to placebo. The trial included a Neurontin treatment phase to enable the evaluation of blood levels of Neurontin.
The trial met the primary endpoint of the study, demonstrating that treatment withHorizant was associated with a statistically significant reduction in pain as measured by an 11-point numerical pain scale compared to placebo (p=0.032). Additional analyses were conducted on data from those patients who received both Neurontin andHorizant. When administeredHorizant, patients experienced on average a 17% increase in the steady-state average blood concentration of gabapentin compared to a dose of Neurontin that contained roughly 50% more gabapentin (p=0.005), indicating higher bioavailability ofHorizant. Thirty-six percent of evaluated patients had an increased steady-state average blood concentration of greater than 30%. For all patients who receivedHorizant, the change in average pain score between the last seven days of theHorizant treatment from the final seven days of Neurontin treatment was determined. A statistically significant reduction in pain score at the end ofHorizant treatment was observed (p=0.045).Horizant was well-tolerated. The most common adverse event in theHorizant treatment group was dizziness, which was mild to moderate in severity.
GSK has evaluatedHorizant in two Phase 2 clinical trials for the potential treatment of PHN. In September 2009, GSK announced top-line results from a 14-week, double-blind, placebo-controlled Phase 2b clinical trial that enrolled 376 subjects with PHN who had been experiencing pain for at least three months following healing of the herpes zoster skin rash. Subjects were randomized to receive placebo, 1200, 2400 or 3600 mg/day ofHorizant divided into twice-daily doses. The primary endpoint of the trial was the change from baseline to the end of maintenance treatment in the 24-hour average pain intensity score. All doses ofHorizant demonstrated statistically significant improvements over placebo on the primary endpoint, with the adjusted mean change from baseline in the 24-hour average pain intensity score of -1.66 for placebo, -2.47 for 1200 mg/dayHorizant, -2.36 for 2400 mg/dayHorizant and -2.72 for 3600 mg/dayHorizant. The pre-specified statistical analysis included adjustment for comparisons of multipleHorizant doses to placebo. The adjusted p-values for comparison of 1200, 2400 and 3600 mg/day to placebo were 0.013, 0.029 and 0.002, respectively.Horizant was generally well tolerated at all doses in this trial. The most common adverse events were dizziness (17% 1200 mgHorizant, 26% 2400 mgHorizant, 30% 3600 mgHorizantand 15% placebo) and somnolence (10% 1200 mgHorizant, 11% 2400 mgHorizant, 14% 3600 mgHorizantand 8% placebo), and most of these adverse events were mild or moderate in intensity. Withdrawals due to adverse events were 6% in the 1200 mgHorizant group, 15% in the 2400 mgHorizant group, 18% in the 3600 mgHorizant group and 13% in the placebo group. There was one serious adverse event (gastritis) in the 3600 mg/day dose group that was judged by the investigator to be related to treatment.
In October 2009, GSK announced top-line results from a double-blind, two-period, cross-over Phase 2 clinical trial that enrolled 138 subjects diagnosed with PHN who had been experiencing pain for at least three months following healing of the herpes zoster skin rash. Subjects with a history of inadequate response to gabapentin entered a baseline period during which they received a dose of 1800 mg/day of gabapentin for two weeks. Subjects (N=96) who had a 24-hour average pain intensity score of at least four on the 11-point pain
11
intensity rating scale were then randomized to receive either 1200 mg/day ofHorizant for the first 28-day treatment period followed by 3600 mg/day for the second 28-day treatment period, or 3600 mg/day followed by 1200 mg/day. Subjects received 2400 mg/day ofHorizant for four days in between the two treatment periods. The primary endpoint in this trial was the change from baseline to the end of the treatment period in the 24-hour average pain intensity score. A greater reduction in the 24-hour average pain score was observed for the 3600 mg/day dose than for the 1200 mg/day dose, which reduction was statistically significant.Horizant was well tolerated at both doses in this study. The only treatment-emergent adverse event occurring in greater than or equal to 5% of subjects takingHorizant was nasopharyngitis.
GSK has also evaluatedHorizant for the potential treatment of DPN. In April 2009, GSK completed a 14-week, double-blind, placebo-controlled, Phase 2 clinical trial ofHorizant as a potential treatment for DPN patients. In the trial, 421 patients who were diagnosed with either Type 1 or Type 2 diabetes mellitus with signs and symptoms of DPN were randomized to receive either 1200 mg/day, 2400 mg/day or 3600 mg/day ofHorizant administered in divided doses taken twice daily, 300 mg/day of pregabalin as an active control, administered in divided doses three times daily, or placebo. NeitherHorizant nor pregabalin, the active control, demonstrated a statistically significant improvement on the primary endpoint when compared to placebo, based on the change from baseline to end of treatment on the 24-hour average pain intensity score, which may have been a consequence of the unexpectedly high placebo response rate observed in the study. The highest dose of 3600 mg/day ofHorizant showed consistent trends towards efficacy across multiple pain endpoints.Horizant was generally well tolerated; the two most frequently reported adverse events were dizziness and somnolence.
Clinical Development of Horizant in Neuropathic Pain. The development of Horizant as a potential treatment for PHN has been delayed based on the February 2010 Complete Response letter for RLS. GSK intends to have discussions with the FDA regarding the filing of an NDA, including the possibility of pursuing a Section 505(b)(2) NDA regulatory pathway, forHorizant as a potential treatment of PHN. Although we do not have active development programs underway, we continue to evaluate our resources and potential for pursuing development ofHorizant in the United States for the potential treatment of DPN, the potential treatment of PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug, and for any additional potential indications.
Migraine Prophylaxis
GSK has also evaluatedHorizant for the potential prophylactic treatment of migraine headaches. In July 2010, GSK announced top-line results from a Phase 2b dose-ranging clinical trial evaluating the efficacy, safety and tolerability ofHorizant in adults diagnosed with migraine headache (with or without aura) according to the International Headache Society criteria.Horizant did not demonstrate a statistically significant improvement compared to placebo as a prophylactic treatment for migraine headaches. The 30-week, double-blind, placebo-controlled study randomized 526 patients to receive 1200, 1800, 2400 or 3000 mg/day ofHorizant or placebo, administered twice daily. The primary efficacy endpoint was the change from baseline in the number of migraine headache days during the last four weeks of treatment prior to taper.Horizant did not demonstrate a statistically significant improvement over placebo on the primary endpoint, which may have been a consequence of the unexpectedly high placebo response rate observed in the study. The most common adverse event was dizziness, which was generally mild or moderate and did not lead to discontinuation in the majority of patients. Two patients who receivedHorizant died, one due to bronchopneumonia and the other due to an accidental overdose involving medications other thanHorizant. We do not intend to pursue further development ofHorizant as a prophylactic treatment of migraine headaches.
Horizant/Gabapentin Enacarbil Development, Commercialization and Partnering Strategy
Due to the large market potential forHorizant, the requirement of a primary care physician sales force to address these markets in the United States and our desire to focus our commercialization efforts in the United States, we have entered into agreements with pharmaceutical partners to maximize the potential commercial value ofHorizant. In December 2005, we entered into a license agreement with Astellas for exclusive rights to develop and commercialize gabapentin enacarbil in Japan and five Asian countries. Astellas made an up-front
12
payment to us of $25.0 million, has paid additional milestones of $23.0 million and may make additional milestone payments to us of up to $37.0 million. We will receive royalties on any net sales of gabapentin enacarbil in the Astellas territory. Under the terms of the agreement, Astellas is responsible for all future development costs and Astellas is solely responsible for the manufacturing of gabapentin enacarbil to support its development and commercialization within the Astellas territory. Astellas may terminate the collaboration at its discretion. In such event, all gabapentin enacarbil product rights would revert to us and we would be entitled to specified transition assistance from Astellas.
Additionally, in February 2007, we announced an exclusive collaboration with GSK to develop and commercializeHorizant/gabapentin enacarbil worldwide, excluding the Astellas territory. In November 2010, we amended and restated our collaboration agreement with GSK, pursuant to which we reacquired all rights to gabapentin enacarbil outside of the United States previously granted to GSK (which excludes the Astellas territory) and obtained the right to pursue development ofHorizant for: (i) the potential treatment of DPN; (ii) the potential treatment of PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug; and (iii) any additional indications in the United States. GSK remains responsible for seeking approval of the NDA for RLS in the United States, further development and regulatory matters with respect toHorizant for the potential treatment of PHN, possibly seeking NDA approval through a 505(b)(2) approval process, and commercialization ofHorizant in the United States for all indications.
Under the terms of the amended and restated collaboration agreement, the aggregate clinical and regulatory milestone payments that we are eligible to receive, have been increased by $37.5 million from a total of $275.0 million to $312.5 million, of which $85.0 million has been received to date. We remain eligible to receive up to an additional $290.0 million upon the achievement of specified sales levels; however, the associated sales levels that give rise to these payments were lowered from the terms of our original agreement.
We plan to enter into additional agreements with pharmaceutical companies for the development and commercialization of gabapentin enacarbil outside the United States and the Astellas territory to the extent that we are able to find partners and negotiate agreement terms that are suitable to us.
Arbaclofen Placarbil, or AP — A Transported Prodrug of R-baclofen
We are developing our product candidate, AP, a Transported Prodrug of R-baclofen, for the potential treatment of spasticity and for the potential adjunctive treatment of patients with GERD. We were previously evaluating AP as a potential treatment for acute back spasms, but have discontinued development following an unsuccessful Phase 2a clinical trial in this indication. We hold a composition-of-matter patent and methods-of-synthesis patents in the United States on AP, and hold patents or pending patent applications directed to AP formulations and methods of use in the United States and other jurisdictions.
Parent Drug Background
Baclofen is thought to act selectively on the target known as the GABA(B) receptor. Baclofen is racemic, which means it is a mixture of R and S isomers. Only the R isomer is active at GABA(B) receptors. Baclofen, which is now sold as a generic drug in the United States, has been used since 1977 for the alleviation of the signs and symptoms of spasticity in patients with MS and may also be of some value in patients with spinal cord injuries and other spinal cord diseases. Published studies indicate that baclofen may also be effective in treating GERD. Although baclofen has acceptable oral absorption, its short duration in blood of three to four hours necessitates oral dosing at least three times per day. This dosing regimen produces substantial peaks and troughs in drug exposure, which may be the cause of side effects such as significant drowsiness, weakness and dizziness during peak drug levels and diminished efficacy during trough drug levels. However, due to its poor absorption in the colon, a less frequently dosed sustained-release formulation of baclofen that produces a more constant level of baclofen in the blood has proven challenging to date. To address these limitations of oral baclofen, an implantable pump that delivers baclofen directly into the spinal cord fluid via a catheter has been developed. However, physicians typically reserve this invasive surgical procedure for those patients for whom oral baclofen is not effective.
13
Our Transported Prodrug
AP was designed to address the limitations of baclofen by targeting high-capacity nutrient transporter mechanisms expressed throughout the length of the entire GI tract, including the colon. By targeting these transporters, we believe that AP can be formulated in a sustained-release pill and thereby require less frequent dosing than baclofen. AP is a chiral molecule, which means that it exists as a single isomeric form, and produces only the R isomer of baclofen, known as R-baclofen.
AP was designed to rapidly convert to R-baclofen upon absorption, with limited systemic exposure to the intact Transported Prodrug. Once absorbed, AP converts to R-baclofen and natural substances that have well-studied, favorable safety characteristics. We believe that the inherently safe nature of the metabolic breakdown products of AP could provide AP with a safety profile that is comparable to, and potentially better than, that seen with racemic baclofen.
We have sustained-release formulations of AP that may be suitable for once- or twice-daily dosing for the potential treatment of spasticity and GERD.
Phase 1 Clinical Trials
We have completed multiple Phase 1 clinical trials of AP that included a total of over 250 healthy volunteers. The results of these Phase 1 clinical trials indicated that AP was well absorbed and rapidly converted to the R isomer of baclofen. Exposure to the intact Transported Prodrug was low compared to the level of R-baclofen produced at all dose levels. Comparison of these data with historical pharmacokinetic data for racemic baclofen suggests that AP taken once a day or twice a day should be associated with a decreased peak-to-trough ratio of R-baclofen blood levels over 24 hours compared to racemic baclofen dosed three or four times a day.
Initial Target Indications
Spasticity
Background on Spasticity. Spasticity is a debilitating condition that is associated with some common neurological disorders, such as MS, stroke and cerebral palsy, as well as spinal cord injury. The underlying cause of spasticity is unknown, but it is believed to result from an imbalance of inhibitory and excitatory functioning within the central nervous system. Patients with spasticity may experience abnormal increases in muscle tone that are associated with loss of range of motion, increased muscle stretch reflexes, weakness and problems with coordination. Common complications of spasticity include joint and muscle contracture, pain and difficulty performing activities of daily living.
Potential Market. According to “We Move”, a non-profit organization providing patient information and continuing medical education to professionals, two out of every 1,000 people in North America suffer from MS and roughly 200,000 people in the United States suffer from spinal cord injury. It is estimated that spasticity affects between 37% and 78% of MS patients and 40% of spinal cord injury patients.
Current Treatments. According to data from Wolters Kluwer Health, Pharmaceutical Audit Suite, there were approximately 8.7 million prescriptions written in the United States in 2010 for the two most widely prescribed drugs for the treatment of spasticity, baclofen and tizanidine. Besides baclofen and tizanidine, treatments for spasticity include diazepam and dantrolene sodium. Although these medications may provide symptom relief in some people, they are often only partially effective and generally require dosing three or more times a day. In addition, these medications are often associated with unwanted side effects such as sedation and weakness, as well as issues with bladder, bowel and sexual function. We believe that a Transported Prodrug of R-baclofen that can be taken twice each day to provide a steady exposure of R-baclofen may more adequately address the needs of spasticity patients than current therapies, including racemic baclofen.
Phase 2 Clinical Trial Results. In June 2009, we announced preliminary results from a multi-dose, randomized, placebo-controlled, crossover Phase 2 clinical trial of AP in spinal cord injury patients with
14
spasticity. This trial enrolled 37 subjects at ten sites in the United States and Canada. Patients received either AP (10, 20 or 30 mg given twice daily, or BID) or placebo in the first treatment segment of the two-segment crossover design. The primary endpoint in the study was the difference in Ashworth Scale score during the placebo and AP treatment segments for the muscle group with the highest Ashworth Scale score at baseline. Ashworth Scale scores were determined by the investigator prior to dosing, and again two, four and six hours after the morning dose. The primary analysis used a repeated-measures analysis of variance model and included data from the 35 subjects who completed both treatment segments.
Mean maximum baseline Ashworth Scale scores were 3.2 (n=10), 3.1 (n=12) and 3.1 (n=13) for the 10, 20 and 30 mg BID AP dose cohorts, respectively. For the primary endpoint, the overall adjusted mean differences between placebo and AP over the six-hour assessment period for these cohorts were -0.17 (not significant), -0.60 (p=0.0059) and -0.88 (p=0.0007), respectively. AP treatment was associated with statistically significant differences from placebo at all time points in the 20 and 30 mg BID AP dose cohorts, indicating a treatment effect over the 12-hour dosing interval. In a secondary analysis, 20 and 30 mg BID of AP also showed a statistically significant difference from placebo in the average Ashworth Scale score for all six muscle groups.
AP was well tolerated at all dose levels. There were no withdrawals due to adverse events during the trial. The most commonly reported adverse events while on any AP dose were urinary tract infection (11% AP; 9% placebo), pain in extremity (8% AP; 0% placebo), insomnia (8% AP; 0% placebo) and nasopharyngitis (8% AP; 3% placebo). Side effects were generally mild to moderate in intensity. There were no drug-related serious adverse events.
Planned Clinical Development of AP in Spasticity. Based on discussions with the FDA, we intend to conduct a single randomized, double-blind, placebo-controlled, multi-center Phase 3 efficacy clinical trial and an open-label, extension, safety study of AP in MS patients with spasticity. Favorable results from these studies could lead to the filing of an NDA with the FDA under Section 505(b)(2) seeking approval of AP for the treatment of spasticity. The 505(b)(2) application would enable us to reference published literature and the FDA’s previous findings of safety and effectiveness for baclofen, which has been approved by the FDA for the alleviation of signs and symptoms of spasticity resulting from MS.
Gastroesophageal Reflux Disease
Background on GERD. GERD is a chronic digestive system disorder caused primarily by transient relaxations of the lower esophageal sphincter, or LES, which is a combination of muscles that controls the junction between the esophagus and the stomach. This results in frequent, undesirable passage of stomach contents into the esophagus that can cause heartburn, regurgitation and potential damage to the lining of the esophagus. Current treatments for GERD reduce the acidity of stomach contents but do not treat the underlying transient relaxations of the LES, resulting in inadequate treatment of GERD in many patients.
Potential Market. According to a survey conducted by the American Gastroenterological Association in 2008, GERD affects an estimated 25% to 35% of the U.S. population. According to data from Wolters Kluwer Health, Pharmaceutical Audit Suite, there were approximately 110 million prescriptions written in the United States in 2010 for PPIs. While treatment with PPIs improves symptoms in the majority of GERD patients, it is estimated that nearly 40% of patients on daily PPI therapy continue to experience breakthrough symptoms.
Current Treatments. Conventional treatment for GERD encompasses medications that suppress stomach acid, including PPIs, such as Nexium, Prilosec and Prevacid, H2 receptor antagonists, such as Tagamet, Pepcid and Zantac, as well as over-the-counter antacids. However, these treatments are not effective in all patients, and there is a subset of patients who suffer from reflux of stomach contents that are not acidic, such as bile, who do not respond to these acid-suppression treatments.
Baclofen has been the subject of clinical trials indicating that it may also be effective in treating GERD. Unlike acid-suppressing agents, baclofen exerts its effects on the function of the LES that controls passage of material between the esophagus and the stomach. Baclofen reduces the frequency of transient LES relaxations and, therefore, passage of gastric contents into the esophagus. Such a mechanism potentially may be effective alone in treating GERD or in combination with acid suppressants to increase the effectiveness of GERD treatment. One study published in 2003 indicated that baclofen was effective when compared to placebo in
15
reducing the number of reflux episodes and the percentage of time that the esophagus was acidic. Another study published in 2003 indicated that baclofen, when combined with a PPI, was more effective in reducing the number of reflux episodes as compared to the PPI alone. In these studies, baclofen was taken three or four times a day.
While these studies suggest a potential role for baclofen in the treatment of GERD, it is currently not approved for this indication, and we believe that it is unlikely that an approval of baclofen for this indication will be pursued because of the requirement for frequent dosing. We believe that providing a steady exposure of the R isomer of baclofen to patients with a once- or twice-daily dosage of AP may result in reduced side effects compared to racemic baclofen and may demonstrate improved efficacy in the treatment of GERD.
Phase 2a Clinical Trial Results. AP was evaluated in a single-dose, randomized, double-blind, crossover, placebo-controlled, clinical trial that included 50 GERD patients at three sites in the United States. Patients received single doses of AP (10, 20, 40 or 60 mg) or placebo in separate 12-hour testing periods with four to seven days between testing periods. Reflux-provoking meals were consumed at two hours and six hours after dosing, and patients were required to lie on their right side for two hours after each meal to further provoke LES relaxations. Reflux was monitored using a pH/impedance probe placed in the esophagus.
AP showed a statistically significant difference from placebo in the primary endpoint, which was the median change in total reflux episodes after AP treatment compared to placebo (median change=-9.5; p=0.005). Analysis was performed by combining the AP responses and comparing them with the combined placebo responses. Acid and non-acid reflux were analyzed as secondary endpoints. AP treatment compared to placebo was associated with a statistically significant reduction in the median number of acid reflux episodes during the 12-hour monitoring period (median=-9.5; p=0.0027). AP was well tolerated at all dose levels with few reported adverse events. The incidence of adverse events during AP treatment was similar during placebo treatment.
Phase 2 Multi-Dose Clinical Trial Results. AP was evaluated in a randomized, parallel-group, double-blind, placebo-controlled Phase 2 clinical trial that evaluated the efficacy, safety and tolerability of a sustained-release formulation of AP in patients with symptomatic GERD. The trial enrolled 156 subjects at 16 sites in the United States. Enrolled subjects had reflux symptoms occurring at least three days a week and had either no history of taking PPIs, or PPI-Naïve, or a history of at least a partial symptom response to PPI therapy, or PPI-Experienced. Enrolled subjects discontinued prior therapy for GERD other than rescue antacids. During the second week of a two-week washout period, baseline data regarding frequency and severity of GERD symptoms were recorded in an electronic diary as they occurred. Each subject who met the entry criteria was randomized to one of five treatment arms: placebo; three dose levels of AP (20 mg, 40 mg or 60 mg) administered once a day in the morning; or AP (30 mg) administered twice daily. PPI history was used as a stratification criterion during randomization. The treatment period was four weeks, which included an up-titration period. At the end of four weeks, subjects were tapered off treatment.
The primary efficacy analysis involved the difference in the change in total number of weekly heartburn episodes between the AP dose groups and placebo through four weeks of treatment. The primary efficacy analysis compared pooled AP treatment groups (60 mg dosed once a day and 30 mg dosed twice a day; and 60 mg and 40 mg dosed once a day) with the placebo group and included both PPI-Experienced and PPI-Naïve subjects. This analysis did not reach statistical significance.
The primary analysis indicated that the status of a subject as either PPI-Naïve or PPI-Experienced had a significant impact on the outcome of the analysis. The prospective statistical analysis plan specified separate analyses of the PPI-Naïve and the PPI-Experienced populations. In the PPI-Experienced population, which represented 63% of all subjects, AP demonstrated a significantly greater reduction in heartburn episodes compared to placebo for the 30 mg twice-daily dosage group.
A number of pre-defined secondary analyses were conducted on subjects in the PPI-Experienced population. All AP dose groups showed a greater adjusted mean percent reduction from baseline at week four in weekly heartburn episodes that was statistically significant compared to placebo.
In addition, a dose-dependent effect on the complete relief of heartburn symptoms during the last seven days of the four-week treatment period was observed for subjects in the PPI-Experienced population. The comparison of the 30 mg twice-daily group with the placebo group was statistically significant.
16
AP was generally well tolerated at all dose levels. There were no treatment emergent serious adverse events. Among all subjects receiving study medication, the most common adverse events for placebo, 20 mg, 40 mg and 60 mg dosed once daily and 30 mg of AP dosed twice daily were somnolence, at rates of 3%, 3%, 12%, 16% and 13%, respectively, and dizziness, at rates of 10%, 10%, 6%, 13% and 20%, respectively. Most reported adverse events were mild or moderate in severity. Withdrawals due to adverse events were 6%, 0%, 3%, 9% and 10%, respectively.
Clinical Development of AP in GERD. We are conducting a multi-dose, randomized, placebo-controlled Phase 2b clinical trial to evaluate the efficacy and safety of AP as adjunctive treatment in approximately 450 patients with GERD who are incomplete responders to PPIs. The clinical trial is being conducted in multiple study centers in the United States and Canada. GERD patients with a history of incomplete response to a PPI undergo up to a four-week run-in period on PPI therapy. Subjects who remain symptomatic and meet the entrance criteria are then randomized to a six-week treatment period on PPI therapy plus either 20 mg or 40 mg of AP dosed once daily, 20 mg or 30 mg of AP dosed twice daily or placebo. The primary endpoint of the study examines heartburn events. Regurgitation will be assessed as a key secondary endpoint. We have completed enrollment in this trial and anticipate reporting top-line results in the first quarter of 2011.
AP Development, Commercialization and Partnering Strategy
We may seek to partner the development and commercialization of AP for the potential treatment of GERD and/or spasticity. Factors that we will consider in determining a strategy to partner AP include: the results of our clinical trials; whether a potential partner seeks development and commercialization rights in or outside of the United States; and whether we believe that access to a large primary care physician sales force is necessary to address our target markets.
XP21279 — A Transported Prodrug of L-Dopa
We are developing our product candidate, XP21279, a Transported Prodrug of L-Dopa, for the potential treatment of Parkinson’s disease. We hold a composition-of-matter patent and a formulation patent in the United States on XP21279, and hold patents or pending patent applications directed to the XP21279 methods of synthesis and use in the United States. We have also filed applications directed to the XP21279 composition of matter and methods of synthesis and use in other jurisdictions.
Parent Drug Background
Patients with Parkinson’s disease have a deficiency of the neurotransmitter dopamine resulting from neuronal degeneration within certain nerve cells in an area of the brain collectively known as the substantia nigra. L-Dopa is an immediate precursor of dopamine that, unlike dopamine, readily crosses the blood brain barrier. When administered in conjunction with carbidopa (and, in some cases, with benzerazide or carbidopa and entacapone), L-Dopa is protected from rapid degradation by enzymes that are outside of the brain and is able to be converted to dopamine at its desired site of action in the brain. L-Dopa is widely viewed as one of the most effective treatments of Parkinson’s disease, and virtually all patients with Parkinson’s disease ultimately require it. However, L-Dopa has many undesirable pharmacokinetic characteristics, including its rapid breakdown by gastric and other peripheral enzymes, a short duration in blood after oral dosing that leads to the fluctuation of drug plasma concentrations upon frequent dosing and a narrow absorption window within the GI tract. The poor colonic absorption of L-Dopa has precluded the development of a satisfactory sustained-release formulation of L-Dopa that would prolong absorption beyond the small intestine.
Our Transported Prodrug
We believe that XP21279 has the potential to improve upon the limitations of L-Dopa. XP21279 is designed to engage natural nutrient transport mechanisms located throughout the length of the GI tract and then be rapidly converted to L-Dopa by the body’s naturally occurring enzymes. In addition to L-Dopa, the metabolic breakdown products of XP21279 are substances with favorable safety characteristics. Because XP21279 is designed to be
17
well absorbed from the lower GI tract, we believe that it can be formulated for sustained release, thus reducing fluctuations of L-Dopa levels in the bloodstream. From December 2002 to December 2004, we were engaged in a collaboration with the ALZA division of Johnson & Johnson to jointly develop Transported Prodrugs of L-Dopa. In March 2005, ALZA relinquished all rights to such Transported Prodrugs, subject to a royalty upon net sales of certain product candidates if they are ultimately commercialized.
Phase 1 Clinical Trials
We have conducted three Phase 1 clinical trials that included a total of 82 healthy volunteers. The trials evaluated the pharmacokinetic profile of different formulations of XP21279 administered with carbidopa compared to a combination of L-Dopa/carbidopa. The results of these Phase 1 clinical trials indicated that XP21279/carbidopa was well absorbed and rapidly converted to L-Dopa. Exposure to the intact Transported Prodrug was negligible. Data from the trials indicated that compared to the pharmacokinetic data of L-Dopa/carbidopa, XP21279/carbidopa was associated with a decreased peak-to-trough ratio of L-Dopa blood levels over 24 hours compared to L-Dopa/carbidopa. XP21279 was generally well tolerated, with no serious adverse events reported in these trials.
Target Indication
Parkinson’s Disease
Background on Parkinson’s Disease. Parkinson’s disease is a motor system disorder that results from the loss of dopamine-producing nerve cells in the brain. Dopamine is a chemical that is naturally produced by the body. It is responsible for smooth, coordinated function of the body’s muscles and movement. When approximately 80% of dopamine-producing cells are damaged, the symptoms of Parkinson’s disease appear. The primary symptoms of Parkinson’s disease are tremor or shaking, slowness of movement, rigidity or stiffness and difficulty with balance.
Potential Market. It is estimated that as many as 1.5 million people in North America are living with Parkinson’s disease. According to the National Institute of Neurological Disorders and Stroke, the average age of onset is 60, though some people are diagnosed at age 40 or younger. In 2010, there were approximately 4.0 million prescriptions written for L-Dopa drugs indicated for the treatment of Parkinson’s disease in the United States, according to Wolters Kluwer Pharma Solutions, Pharmaceutical Audit.
Current Treatments. At present, there is no cure for Parkinson’s disease, but a variety of medications provide relief from the symptoms. L-Dopa acts to replenish dopamine in the brain. It is usually administered with benzerazide or carbidopa, or a combination of carbidopa and entacapone, which delays the premature conversion of L-Dopa to dopamine in peripheral tissues. According to the National Institute of Neurological Disorders and Stroke, treatment with L-Dopa helps patients in at least three-quarters of Parkinson’s disease cases.
Another class of drugs, called dopamine agonists, is also commonly used to treat Parkinson’s disease. Dopamine agonists, which include bromocriptine, pergolide, pramipexole and ropinirole, mimic the role of dopamine in the brain, which causes neurons to react as they would to dopamine. In spite of their wide use, both L-Dopa and dopamine agonists remain suboptimal in treating the symptoms of Parkinson’s disease. L-Dopa therapy has been associated with “wearing-off,” a condition where treatment effects diminish over time as the disease progresses, and “on-off” dyskinesias, or impairment of movement, due to changes in L-Dopa plasma concentrations. Dopamine agonists are generally considered the next most powerful drug class in treating the symptoms of Parkinson’s disease, but are more likely to cause hallucinations, confusion and psychosis, especially in the elderly.
Phase 1b Clinical Trial Result. In January 2010, we reported preliminary results from an open-label, crossover, Phase 1b clinical trial of XP21279 administered with carbidopa in ten Parkinson’s disease patients who were sequentially administered L-Dopa/carbidopa three or four times per day for 14 days followed by administration of XP21279/carbidopa three times per day for 14 days. Dosing for both L-Dopa/carbidopa and XP21279/carbidopa was optimized to minimize “off-time” (the period in which patients believe their medication is not working well or causing worsening of Parkinson’s symptoms), with no appreciable increase in duration of
18
dyskinesias (involuntary movements). The primary objective of the study was the comparison of pharmacokinetic profiles of XP21279/carbidopa compared to L-Dopa/carbidopa. XP21279 taken three times a day showed less variation in average L-Dopa concentrations over 16 hours compared to L-Dopa/carbidopa dosed three or four times a day, with a lower peak to trough ratio for XP21279. Efficacy assessments at the end of each treatment period showed improvements with XP21279 over L-Dopa. However, because the trial was not blinded, i.e., subjects knew what treatment was administered, the results of the efficacy analyses must be viewed cautiously. XP21279 was well tolerated.
Planned Clinical Development of XP21279 in Parkinson’s Disease
We have developed a new bi-layer tablet formulation of XP21279 with carbidopa and have initiated a randomized, double-blind, crossover Phase 2 clinical trial in patients with Parkinson’s disease that is designed to evaluate safety, efficacy and pharmacokinetics of our new bi-layer formulation of XP21279 versus Sinemet.
XP21279 Development, Commercialization and Partnering Strategy
We plan to continue development of XP21279 and retain rights to this product candidate in the United States, while seeking a partner for the development and commercialization of XP21279 as a treatment for Parkinson’s disease outside the United States.
Preclinical Development Candidates
Our portfolio of potential additional product candidates that are in preclinical development includes a novel prodrug of methylhydrogenfumarate, or MHF, known as XP23829, that we believe may provide higher blood levels of MHF and exhibit less gastric irritation than dimethylfumarate, or DMF, another prodrug of MHF. Prodrugs of MHF, a molecule that appears to have anti-inflammatory properties, may be useful in the potential treatments of patients with MS or psoriasis. We have also developed a novel prodrug of acamprosate that appears to be more readily absorbed after oral administration than the current formulation of acamprosate. In November 2010, we were awarded a grant through the Michael J. Fox Foundation for Parkinson’s Research to support a preclinical study of the efficacy and safety of our acamprosate prodrug in reducing L-Dopa-induced dyskinesias in a pre-clinical model of Parkinson’s disease. We plan to continue our development of XP23829, our acamprosate prodrug and other preclinical assets, such as XP21510, which was previously licensed to Xanodyne Pharmaceuticals, Inc., as resources permit.
Future Applications for Our Technology
While our resource constraints preclude development at this time, we believe that there are a number of other generic parent drugs that could be candidates for our Transported Prodrug technology. As resources permit, we intend to apply our proprietary technology to selected parent drugs that have low or regionally restricted absorption in the GI tract that results in suboptimal therapy, have a chemical structure that is amenable to prodrug manipulation and are economical to manufacture.
Additionally, we believe that our proprietary technology could have broad applicability beyond improving absorption from the GI tract, such as improving the penetration of drugs into the CNS. For example, the blood brain barrier is an important obstacle to the effectiveness of compounds acting on CNS targets. The highly restrictive endothelium of the brain capillary bed and the protective epithelial layer of a part of the brain known as the choroid plexus comprise a formidable barrier of cells through which drugs must pass from the blood to enter the brain. However, many natural compounds needed to feed the high metabolic activity of the brain are selectively absorbed into the CNS, particularly through the extensive capillary beds in the brain. In some cases, large amounts of these compounds are actively pumped from the blood to the brain by transporter proteins. From November 2003 to November 2005, we were engaged in a collaboration with Pfizer to jointly develop transporter technology to enhance the delivery of drugs to the brain. The program was exclusive during the term of the collaboration and provided Pfizer with non-exclusive rights to resulting technologies.
19
We also believe that our proprietary technology could be utilized to rehabilitate those product candidates of third parties that initially demonstrated potential therapeutic benefits but whose limitations in absorption, distribution and pharmacokinetics have prevented successful drug development or commercialization. Resources permitting, we would select other drug molecules for this approach based on our ability to license from third parties these product candidates, the medical need for an improved version of the third party’s drug, the size of the commercial opportunity and the amenability of our chemistry to the drug’s particular structure.
Our Strategic Alliances
Astellas Pharma Inc.
In December 2005, we entered into an agreement in which we licensed to Astellas exclusive rights to develop and commercialize gabapentin enacarbil in Japan, Korea, the Philippines, Indonesia, Thailand and Taiwan. Under the terms of this agreement, we received an initial license payment of $25.0 million and have subsequently received $23.0 million in milestone payments as of December 31, 2010. In addition, we are eligible to receive clinical and regulatory milestone payments totaling up to an additional $37.0 million. We will receive royalties on any net sales of gabapentin enacarbil in the Astellas territory at a royalty rate in the mid-teens on a percentage basis. Astellas is solely responsible for the manufacturing of gabapentin enacarbil to support its development and commercialization within the Astellas territory. Astellas may terminate the collaboration at its discretion; in such event, all gabapentin enacarbil product rights would revert to us and we would be entitled to specified transition assistance from Astellas.
Glaxo Group Limited
In February 2007, we entered into an exclusive collaboration with GSK to develop and commercializeHorizant/gabapentin enacarbil worldwide, excluding the Astellas territory. In November 2010, we amended and restated our collaboration agreement with GSK, pursuant to which we reacquired all rights to gabapentin enacarbil outside of the United States previously granted to GSK (which excludes the Astellas territory) and obtained the right to pursue development ofHorizant for: (i) the potential treatment of DPN; (ii) the potential treatment of PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug; and (iii) any additional indications in the United States. GSK remains responsible for seeking approval of the NDA for RLS in the United States, further development and regulatory matters with respect toHorizant for the potential treatment of PHN, possibly seeking NDA approval through a 505(b)(2) approval process, and commercialization ofHorizant in the United States for all indications.
Under the terms of the amended and restated collaboration agreement, the aggregate clinical and regulatory milestone payments that we are eligible to receive, have been increased by $37.5 million from a total of $275.0 million to $312.5 million, of which $85.0 million has been received as of December 31, 2010. We remain eligible to receive up to an additional $290.0 million upon the achievement of specified sales levels; however, the associated sales levels that give rise to these payments have been lowered from our original agreement.
We exercised our right to the co-promotion arrangement in April 2009, under which all allowable expenses and any potential future sales ofHorizant are accounted for using a joint profit and loss, or P&L, statement, in which we and GSK share in the resulting operating pre-tax profits and losses. Under the amended and restated collaboration agreement, our participation in the co-promotion and joint P&L arrangements remain unchanged, except that we can delay the deployment of our sales force for up to three years following the potential approval ofHorizant in the United States and our share of losses from the joint P&L will be forgiven up to a maximum of $10.0 million. Our payment of additional losses, if any, would be deferred and payable without interest over a period of time following the first quarter in which the joint P&L is profitable. In addition, we no longer have the right to detail Requip XL, GSK’s product for Parkinson’s disease, as this right would have terminated under the original agreement upon the earlier of the launch of a generic form of Requip XL or July 1, 2011. Pending FDA approval of Horizant, GSK is responsible for: establishing pricing and reimbursement; creating promotional and advertising materials; managed care contracting; receiving, accepting and filling orders; distributing; controlling invoicing, order processing and collecting accounts receivable; and recording sales of Horizant in the United
20
States. Expenses that can be charged to the joint P&L statement are the cost of goods and certain costs directly related to Horizant marketing and sales.
Upon approval, we would share any profits on sales ofHorizant in the United States at tiered rates that escalate as a function of annual net sales levels, from a low of 20% to a maximum of 50%. For example (and for illustrative purposes only), if the annual net sales ofHorizant reach $250.0 million, $500.0 million and $1.0 billion, we would be entitled to blended profit share rates of 25%, 34% and 42%, respectively. We may terminate our co-promotion right and participation in the profit share arrangement at any time upon notice to GSK with no penalty to us, resulting in a royalty-based compensation structure, whereby we would receive royalties on annual net sales in the United States at tiered rates that escalate as a function of net sales levels from a low of 15% to a maximum of 30%. For example (and for illustrative purposes only), if the annual net sales ofHorizant reach $250.0 million, $500.0 million and $1.0 billion, we would be entitled to blended royalty rates of 17%, 21% and 25%, respectively. GSK may terminate our co-promotion right for our not meeting a minimum sales requirement, for our uncured material breach in conducting co-promotional activities or upon our change of control in certain circumstances. GSK may terminate our collaboration agreement in its entirety for any reason and at any time. In such event, certainHorizant product rights would revert to us, and we would be entitled to specified transition assistance from GSK.
Patents and Proprietary Rights
We will be able to protect our technology from unauthorized use by third parties only to the extent that our technology is covered by valid and enforceable patents or effectively maintained as trade secrets and able to be utilized without infringing the proprietary rights of others. Our success in the future will depend in part on obtaining patent protection for our technologies and product candidates. Accordingly, patents and other proprietary rights are essential elements of our business. Our policy is to actively seek in the United States and selected foreign countries patent protection for novel technologies and compositions of matter that are commercially important to the development of our business.
Issued U.S. and foreign patents generally expire 20 years after filing. We hold a number of issued patents in the United States, including composition-of-matter patents onHorizant/gabapentin enacarbil, AP and XP21279. We have a number of pending patent applications in the United States. Of the U.S. patents that we hold, many patents are related to compounds, pharmaceutical compositions containing the compounds and therapeutic methods of using the compounds and compositions. We also have U.S. patents that are related to methods of synthesis, proteomics methodology and screening methodology. We also hold a number of issued foreign patents. We have pending Patent Cooperation Treaty, known as PCT, regional applications that permit us to pursue patents outside of the United States, pending European regional patent applications that permit us to pursue patents in various European countries and foreign national patent applications. The claims in these various patents and patent applications are directed to compositions of matter, including claims covering product candidates, lead compounds and key intermediates, pharmaceutical compositions, methods of use and processes for making our compounds, along with methods of design, synthesis, selection and use of Transported Prodrugs in general and to our research and development programs in particular.
The patent rights relating toHorizant, its synthesis, formulations and methods of use are owned by us and consist of issued U.S. patents that expire at the earliest in 2022 and a number of pending U.S. patent applications. For gabapentin enacarbil, we also own pending counterpart PCT regional patent applications, issued foreign patents and foreign national applications in a number of jurisdictions, including Asia and Europe. Rights under these patents and applications have been exclusively licensed to Astellas within its territory. The patent rights relating to AP and its synthesis, formulations and methods of use are owned by us and consist of issued U.S. patents that expire at the earliest in 2025, a number of pending U.S. patent applications, and issued foreign patents or foreign national applications. The patent rights relating to XP21279 and its synthesis, formulations and methods of use are owned by us and consist of an issued U.S. patent that expires in 2025, pending U.S. patent applications and counterpart PCT applications and a number of foreign national applications.
In September 2008, a law firm, on behalf of an undisclosed client, filed an opposition against the patent grant of one of our European patent applications covering gabapentin enacarbil. The European patent office, at an
21
opposition hearing in April 2010, undertook a full review of the grant of the European patent, and ruled that our European patent covering the composition of matter of gabapentin enacarbil is valid. While the law firm that filed the opposition initially appealed the ruling on behalf of the undisclosed client, that appeal was withdrawn in November 2010.
The composition-of-matter patent on gabapentin, the parent drug ofHorizant/gabapentin enacarbil, expired in 2000, but Pfizer sold gabapentin exclusively based on a formulation patent until September 2004. This formulation patent is the subject of ongoing litigation between Pfizer and several generic manufacturers, including Alpharma, Inc. and Teva Pharmaceutical Industries, Ltd. Pfizer currently markets generic gabapentin through its Greenstone Ltd. subsidiary. Alpharma and Teva, along with many others, currently market gabapentin as a generic drug. In July 2006, the United States District Court for the District of New Jersey ruled in favor of the generic gabapentin makers, including Teva, and Pfizer appealed that ruling. In September 2007, the Court of Appeals for the Federal Circuit overturned the July 2006 District Court ruling that was in favor of the generic gabapentin makers, including Teva, and the suit has been remanded to the District Court to continue with the trial. We are not a party to this litigation, and we believe that the manufacturing process for gabapentin enacarbil does not infringe the patent that is the subject of this litigation. However, in case of an adverse event in this litigation, such as a decision to enjoin or limit the sale of generic gabapentin, GSK and/or Astellas may be limited in their choices of potential suppliers. This could increase the cost of supply of generic gabapentin and potentially impair our and our collaborative partners’ ability to commercialize this product candidate.
Certain product candidates that we develop may be submitted to the FDA for approval under Section 505(b)(2) of the Food, Drug and Cosmetic Act, or FDCA, which was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by, or for, the applicant and for which the applicant has not obtained a right of reference. In accordance with the Hatch-Waxman Act, such NDAs may be required to include certifications, known as Paragraph IV certifications, that certify that any patents listed in the Patent and Exclusivity Information Addendum of the FDA’s publication, Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, with respect to any product referenced in the Section 505(b)(2) application, are invalid, unenforceable or will not be infringed by the manufacture, use or sale of the product that is the subject of the Section 505(b)(2) application. Under the Hatch-Waxman Act, the holder of patents that the Section 505(b)(2) application references may file a patent infringement lawsuit after receiving notice of the Paragraph IV certification. Filing of a patent infringement lawsuit within 45 days of the patent owner’s receipt of notice triggers a one-time, automatic, 30-month stay of the FDA’s ability to approve the 505(b)(2) application. Accordingly, we may invest a significant amount of time and expense in the development of one or more products only to be subject to significant delay and patent litigation before such products may be commercialized, if at all.
We also rely on trade secret protection and confidentiality agreements to protect our proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery process that involve proprietary know-how and technology that is not covered by patent applications, especially where patent protection is not believed to be appropriate or obtainable. We require all of our employees, consultants and advisors to enter into confidentiality agreements. Where it is necessary to share our proprietary information or data with outside parties, our policy is to make available only that information and data required to accomplish the desired purpose and only pursuant to a duty of confidentiality on the part of those parties.
Manufacturing
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of any of our product candidates. To date, we have relied on, and we expect to continue to rely on, a limited number of third-party compound manufacturers and active pharmaceutical ingredient, or API, formulators for the production of preclinical, clinical and commercial quantities of our product candidates. We do not have commercial supply agreements with any of these third parties, and our agreements with these parties are generally terminable at will by either party at any time.
22
Under the terms of our collaboration with GSK, GSK is solely responsible for the manufacture ofHorizant to support its development and commercialization within the United States. GSK is currently relying on a single source supplier for clinical supplies ofHorizant. If GSK fails to qualify alternative manufacturers ofHorizant, the current contract manufacturer terminates its agreement with GSK, and GSK is otherwise unable to manufacture or contract to manufacture sufficient quantities ofHorizant, the development and commercialization ofHorizant could be impaired or delayed. Under the terms of our collaboration with Astellas, Astellas is solely responsible for the manufacture of gabapentin enacarbil to support its development and commercialization within the Astellas territory. To our knowledge, Astellas is currently relying on a single source supplier for clinical supplies of gabapentin enacarbil. As a result, if Astellas fails to manufacture or contract to manufacture sufficient quantities of gabapentin enacarbil, development and commercialization of gabapentin enacarbil could be impaired or delayed in the Astellas territory. If we pursue development with respect to the rights we maintain on Horizant/gabapentin enacarbil, we will need to obtain clinical supplies from GSK or another supplier. As a result, if we are unable to obtain sufficient quantities of Horizant/gabapentin enacarbil from GSK at prices that are commercially attractive, and if we are unable to qualify an alternative supplier, it could delay the development of, and impair our ability to commercialize, this product candidate.
We rely on two suppliers of R-baclofen, the active agent used to make AP, under purchase orders issued from time to time. In the event that such suppliers determine to not sell R-baclofen to us at a price that is commercially attractive, and if we were unable to qualify an alternative supplier of R-baclofen, this could delay the development of, and impair our ability to commercialize, this product candidate.
We rely on a single source supplier of our current worldwide requirements of AP in API form under a manufacturing services and product supply agreement. Our current agreement with this supplier does not provide for the entire supply of the API necessary for all additional Phase 2 and Phase 3 clinical trials or for full-scale commercialization. In the event that the parties cannot agree to the terms and conditions for the manufacturer to provide some or all of our API clinical and commercial supply needs, we would not be able to manufacture API until a qualified alternative supplier is identified, which could also delay the development of, and impair our ability to commercialize, this product candidate. The API is manufactured using a four-step synthetic process that uses commercially available starting materials for each step. There are no complicated chemistries or unusual equipment required in the manufacturing process.
We rely on a single source supplier for AP formulated in sustained-release tablets for future clinical trials at specific transfer prices under quotations agreed upon by the parties as a part of a master services agreement. In the event that such supplier terminates the agreement under specified circumstances, we would not be able to manufacture AP sustained-release tablets until an alternative supplier is qualified. This could delay the development of, and impair our ability to commercialize, AP.
We rely on a single source supplier of L-Dopa, the active pharmaceutical ingredient used to make XP21279, under purchase orders issued from time to time. We are aware of several alternative suppliers of L-Dopa, and we believe at least one alternative supplier could potentially supply L-Dopa in the event that our current supplier determines to not sell L-Dopa to us at a price that is commercially attractive. If we were unable to qualify an alternative supplier of L-Dopa, this could delay the development of, and impair our ability to commercialize, XP21279.
We rely on a single source supplier of XP21279 in API form under a manufacturing services and product supply agreement. In the event that such supplier terminates the agreement under specified circumstances, we would not be able to manufacture API until a qualified alternative supplier is identified, which could also delay the development of, and impair our ability to commercialize, this product candidate. The API is manufactured by a four-step synthetic process that uses commercially available starting materials. There are no complicated chemistries or unusual equipment required in the manufacturing process.
We have purchased XP21279 formulated in sustained-release tablets from a single source supplier at specified transfer prices under quotations agreed upon by the parties as part of a master services agreement. We have recently qualified a supplier for the manufacture of XP21279 with carbidopa bi-layer tablets to be supplied under quotations agreed upon by the parties as part of a master services agreement. In the event that either supplier terminates their agreement under specified circumstances for manufacture of XP21279 sustained-release
23
tablets or carbidopa bi-layer tablets, we would not be able to manufacture XP21279 until an alternative supplier is qualified. This could delay the development of, and impair our ability to commercialize, XP21279.
Our contract manufacturers may own process technology related to the manufacture of our compounds. This would increase our reliance on this manufacturer. However, we have been successful in negotiating agreements with our contract manufacturers that include licenses, with the right to grant sublicenses, to any technology incorporated into the manufacture of our compounds or that is invented by employees of the contract manufacturers during the course of work conducted on our product candidates.
Research and Development
Since inception, we have devoted a significant amount of resources to develop our product candidates. For the years ended December 31, 2010, 2009 and 2008, we recorded $52.5 million, $70.7 million and $83.2 million, respectively, in research and development expenses. As part of a restructuring that we implemented in March 2010 as a response to the Complete Response letter GSK received from the FDA with respect toHorizant, we eliminated our discovery research department, which will prevent our ability to discover additional product candidates at this time.
Marketing and Sales
Under the terms of our agreement with GSK, we have co-promotion rights forHorizant in the United States. Pending FDA approval ofHorizant, GSK is responsible for: establishing pricing and reimbursement; creating promotional and advertising materials; managed care contracting; receiving, accepting and filling orders; distributing; controlling invoicing, order processing and collecting accounts receivable; and recording sales ofHorizant in the United States. Under the agreement, we can delay the deployment of our sales force for up to three years following the potential approval ofHorizant in the United States. We may terminate our co-promotion right at any time upon notice to GSK with no penalty to us, resulting in a compensation structure based on a royalty of GSK’s sales ofHorizant.
If gabapentin enacarbil is approved within the Astellas territory, Astellas will be responsible for all commercial activities related to the marketing and sale of gabapentin enacarbil.
We plan to establish additional development and commercialization partnerships with pharmaceutical and biotechnology companies to accelerate the completion of regulatory approval and product introduction and to maximize the breadth of the commercial opportunity of our other product candidates.
We also plan to license to third parties for development, marketing and sales other potential drug candidates that are discovered by us but do not fall within the CNS therapeutic area, our primary area of interest.
Competition
The pharmaceutical and biotechnology industries are intensely competitive. Any product candidate developed by us would compete with existing drugs and therapies. There are many pharmaceutical companies, biotechnology companies, public and private universities, government agencies and research organizations actively engaged in research and development of products targeting the same markets as our product candidates. Many of these organizations have substantially greater financial, technical, manufacturing and marketing resources than we have. Several of them have developed or are developing therapies that could be used for treatment of the same diseases that we are targeting. In addition, many of these competitors have significantly greater commercial infrastructures than we have. Our ability to compete successfully will depend largely on our ability to leverage our experience in drug development to:
| | • | | develop products that are superior to other products in the market; |
| | • | | attract and retain qualified scientific, product development and commercial personnel; |
| | • | | obtain patent and/or other proprietary protection for our products and technologies; |
24
| | • | | obtain required regulatory approvals; and |
| | • | | successfully collaborate with pharmaceutical companies in the development and commercialization of new products. |
We expect to compete on, among other things, product efficacy and safety, time to market, price, extent of adverse side effects experienced and convenience of treatment procedures. In order to compete successfully, we will need to develop and exploit these pharmaceutical products commercially before others are able to develop competitive products.
In addition, our ability to compete may be affected if insurers and other third-party payors seek to encourage the use of generic products, making branded products less attractive to buyers from a cost perspective.
We believe that our product development programs will be subject to significant competition from companies utilizing alternative technologies. In addition, as the principles of active transport become more widely known and appreciated based on patent and scientific publications and regulatory filings, we expect the field to become highly competitive. Pharmaceutical companies, biotechnology companies and academic and research institutions may succeed in developing products based upon the principles underlying our proprietary technologies earlier than us, obtaining approvals for such products from the FDA more rapidly than us or developing products that are safer, more effective and/or more cost effective than those under development or proposed to be developed by us.
Our objective is to discover, develop and commercialize new medicines with superior efficacy, convenience, tolerability and/or safety. To the extent that we are able to develop medicines, they are likely to compete with existing drugs that have long histories of effective and safe use and with new therapeutic agents. We expect that any medicines that we commercialize with our collaborative partners or on our own will compete with existing, market-leading medicines.
Horizant/Gabapentin Enacarbil. We anticipate that, if approved in the United States for RLS,Horizant will compete with currently approved treatments for RLS that all belong to a class of drugs called dopamine agonists, including the following: ropinirole (marketed as Requip by GSK); generic ropinirole (marketed by, among others, CorePharma, LLC, Mylan Pharmaceuticals Inc. and Wockhardt USA LLC); pramipexole (marketed as Mirapex by Boehringer Ingelheim); and generic pramipexole (marketed by, among others, Teva, Norvartis AG and Watson Pharmaceuticals, Inc.). In addition, we could also experience competition from the rotigotine transdermal system (a dopamine agonist patch marketed as Neupro by UCB). UCB filed its NDA for the treatment of RLS with the FDA in 2007. In April 2010, the FDA provided UCB a Complete Response letter that recommended reformulation of rotigotine before making it available in the U.S. market for the treatment of restless legs syndrome. We anticipate that, if gabapentin enacarbil is approved in Japan for the treatment of restless legs syndrome, it will compete with pramipexole, which was approved in 2010 for the treatment of restless legs syndrome.
We anticipate that, if approved for neuropathic pain in the United States,Horizant would compete with generic gabapentin (marketed by Alpharma, IVAX Corp., Pfizer and Teva, among others). Other drugs targeting neuropathic pain will represent substantial competition. These include pregabalin (marketed as Lyrica by Pfizer), duloxetine (marketed as Cymbalta by Eli Lilly) and a capsaicin patch (marketed as Qutenza by NeurogesX, Inc.). Transdermal patches containing the anesthetic known as lidocaine are sometimes used for the management of PHN. In addition, in January 2011, the FDA approved a once-daily formulation of gabapentin (known as Gralise from Depomed and its partner for pain indications, Abbott) for the treatment of PHN.
AP. We anticipate that, if approved, AP would experience competition from several generic drugs approved for the treatment of spasticity, including racemic baclofen, diazepam, dantrolene sodium and tizanidine. In addition, in March 2010, the FDA approved onabotulinumtoxin A (marketed as Botox by Allergan Inc.) to treat spasticity in the flexor muscles of the elbow, wrist and fingers in adults, which could also pose a competitive threat to AP. We know of at least one therapy that is in development for the treatment of spasticity, IPX056 (an extended-release formulation of baclofen being developed by Impax Laboratories, Inc). Products that could compete with AP in the GERD therapeutic area include: pantoprazole sodium (marketed as Protonix by Pfizer); lansoprazole (marketed as Prevacid by Takeda Pharmaceutical Company Limited); esomeprazole and
25
omeprazole (marketed as Nexium and Prilosec, respectively, by AstraZeneca Pharmaceuticals LP); rabeprazole (marketed as Aciphex by Eisai/Johnson & Johnson); and generic H2 receptor antagonists such as cimetidine, ranitidine, famotidine and nizatidine.
XP21279. We anticipate that, if approved, XP21279 would compete with generic L-Dopa/carbidopa drugs and other drugs for the treatment of Parkinson’s disease. These include a combination therapy of L-Dopa/carbidopa/entacapone (marketed as Stalevo in the United States by Novartis Group) and dopamine agonists, including pramipexole and ropinirole (marketed as Mirapex and Requip by Boehringer-Ingelheim and GSK, respectively) as well as generic dopamine agonists, including ropinirole (marketed by, among others, CorePharma, Mylan and Wockhardt) and pramipexole (marketed by, among others, Teva, Norvartis and Watson). In addition, we could also experience future competition from the rotigotine transdermal system (a dopamine agonist patch known as Neupro by UCB) that was previously approved in 2007 for the treatment of Parkinson’s disease, but was withdrawn from the U.S. market in 2008 due to a product stability issue. In April 2010, the FDA recommended reformulation of Neupro before making it available in the U.S. market for the treatment of Parkinson’s disease. Other therapies under development in the United States include L-Dopa/carbidopa formulations. For example, IPX066 from Impax, an extended-release formulation of L-Dopa/carbidopa, and Duodopa (a L-Dopa/carbidopa gel delivered by a portable pump directly into the duodenum being developed by Solvay) are among the product candidates in development that represent potential competition for XP21279.
There may be other compounds of which we are not aware that are at an earlier stage of development and may compete with our product candidates. If any of those compounds are successfully developed and approved, they could compete directly with our product candidates.
Government Regulation
Product Approval Process
The clinical testing, manufacturing, labeling, storage, record keeping, advertising, promotion, export and marketing, among other things, of our product and product candidates are subject to extensive regulation by governmental authorities in the United States and other countries. The FDA, under the FDCA, regulates pharmaceutical products in the United States. The steps required before a drug may be approved for marketing in the United States generally include:
| | • | | preclinical laboratory tests and animal tests; |
| | • | | the submission to the FDA of an investigational new drug application, or IND, for human clinical testing, which must become effective before human clinical trials commence; |
| | • | | adequate and well-controlled human clinical trials to establish the safety and efficacy of the product; |
| | • | | the submission to the FDA of an NDA; |
| | • | | FDA acceptance, review and approval of the NDA; and |
| | • | | satisfactory completion of an FDA inspection of the manufacturing facilities at which the product is made to assess compliance with current Good Manufacturing Practices, or cGMPs. |
The testing and approval process requires substantial time, effort and financial resources, and the receipt and timing of any approval is uncertain. The FDA may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
Preclinical studies include laboratory evaluations of the product candidate, as well as animal studies to assess the potential safety and efficacy of the product candidate. The results of the preclinical studies, together with manufacturing information and analytical data, are submitted to the FDA as part of the IND, which must become effective before clinical trials may be commenced. The IND will become effective automatically 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the trials as outlined in the IND prior to that time. In this case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can proceed.
26
Clinical trials involve the administration of the product candidates to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at each institution at which the clinical trial will be conducted. The IRB will consider, among other things, ethical factors, the safety of human subjects and the possible liability of the institution.
Clinical trials typically are conducted in three sequential phases prior to approval, but the phases may overlap. These phases generally include the following:
Phase 1. Phase 1 clinical trials represent the initial introduction of a product candidate into human subjects, frequently healthy volunteers. In Phase 1, the product candidate is usually tested for safety, including adverse effects, dosage tolerance, absorption, distribution, metabolism, excretion and pharmacodynamics.
Phase 2. Phase 2 clinical trials usually involve studies in a limited patient population to (1) evaluate the efficacy of the product candidate for specific indications, (2) determine dosage tolerance and optimal dosage and (3) identify possible adverse effects and safety risks. Although there are no statutory definitions for Phase 2a and Phase 2b, Phase 2a is commonly used to describe a Phase 2 clinical trial evaluating efficacy, adverse effects and safety risks, and Phase 2b is commonly used to describe a subsequent Phase 2 clinical trial that also evaluates dosage tolerance and optimal dosage.
Phase 3. If a product candidate is found to be potentially effective and to have an acceptable safety profile in Phase 2 studies, the clinical trial program will be expanded to Phase 3 clinical trials to further demonstrate clinical efficacy, optimal dosage and safety within an expanded patient population at geographically dispersed clinical study sites.
Phase 4 clinical trials are conducted after approval to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations, or when otherwise requested by the FDA in the form of post-market requirements or commitments. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirements. These clinical trials are often referred to as Phase 3/4 post-approval clinical trials. Failure to promptly conduct Phase 4 clinical trials could result in withdrawal of approval for products approved under accelerated approval regulations.
The results of preclinical studies and clinical trials, together with detailed information on the manufacture and composition of the product, are submitted to the FDA in the form of an NDA requesting approval to market the product. The FDA has substantial discretion in the approval process and may refuse to accept any application or decide that our or our collaborative partners’ data is insufficient for approval and require additional preclinical, clinical or other studies. For example, in November 2008, GSK and we agreed to withdraw the initial NDA submission forHorizant following discussions with the FDA in which the FDA requested that we reformat existing data for a submitted Phase 3 clinical trial. The NDA for this product candidate was then resubmitted in January 2009 with the appropriate data reformatted, and subsequently, in February 2010, GSK received a Complete Response letter from the FDA regarding the NDA forHorizant. The letter indicated that a preclinical finding of pancreatic acinar cell tumors in rats was of sufficient concern to preclude approval of theHorizant NDA for RLS in its form at that time. In November 2010, the FDA accepted for review GSK’s NDA resubmission forHorizant for the treatment of RLS, designated theHorizant NDA resubmission as a Class 2 response and set a new PDUFA date of April 6, 2011.
Generally, regulatory approval of a new drug by the FDA may follow one of three routes. The most traditional of these routes is the submission of a full NDA under Section 505(b)(1) of the FDCA. A second route, which is possible where an applicant chooses to rely in part on data generated or approvals obtained previously by other parties and/or on data described in published literature, is to submit a more limited NDA described in Section 505(b)(2) of the FDCA. The final route is the submission of an Abbreviated New Drug Application for products that are shown to be pharmaceutically and therapeutically equivalent to previously approved drug products as permitted under Section 505(j) of the FDCA.
27
Both Section 505(b)(1) and Section 505(b)(2) applications are required by the FDA to contain full reports of investigations of safety and effectiveness. However, in contrast to a traditional NDA submitted pursuant to Section 505(b)(1) in which the applicant submits all of the data demonstrating safety and effectiveness, we believe an application submitted pursuant to Section 505(b)(2) can rely upon previous findings by the FDA that the parent drug is safe and effective in that indication, and/or upon data described in published literature. As a consequence, the preclinical and clinical development programs leading to the submission of an NDA under Section 505(b)(2) may be less expensive to carry out and could be concluded in a shorter period of time than programs required for a Section 505(b)(1) application. In its review of any NDA submission, however, the FDA has broad discretion to require an applicant to generate additional data related to safety and efficacy, and it is impossible to predict the number or nature of the studies that may be required before the FDA will grant approval.
Pursuant to the terms of our collaboration with GSK, GSK is the sponsor of the NDA forHorizantfor the treatment of RLS, and GSK is responsible for leading the registration ofHorizant for any additional indications in the United States. For our other product candidates that are undergoing clinical trials, we intend to follow the development pathway permitted under the FDCA that will maximize the commercial opportunities for these Transported Prodrugs. We are evaluating both Section 505(b)(1) and Section 505(b)(2) NDA routes for our Transported Prodrugs. In the event that we decide to utilize Section 505(b)(2) of the FDCA to pursue an approval of our Transported Prodrugs in indications for which the relevant parent drug has previously been approved, we will engage in discussions with the FDA to determine which, if any, portions of our development program can be modified.
In addition, for NDAs submitted under Section 505(b)(2) of the FDCA, the patent certification and related provisions of the Hatch-Waxman Act apply. In accordance with the Hatch-Waxman Act, such NDAs may be required to include certifications, known as Paragraph IV certifications, that certify that any patents listed in the Patent and Exclusivity Information Addendum of the FDA’s publication, Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, with respect to any product referenced in the Section 505(b)(2) application, are invalid, unenforceable or will not be infringed by the manufacture, use or sale of the product that is the subject of the Section 505(b)(2) application. Under the Hatch-Waxman Act, the holder of patents that the Section 505(b)(2) application references may file a patent infringement lawsuit after receiving notice of the Paragraph IV certification. Filing of a patent infringement lawsuit within 45 days of the patent owner’s receipt of notice triggers a one-time, automatic, 30-month stay of the FDA’s ability to approve the 505(b)(2) application. A Section 505(b)(2) application may also not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. The FDA may also require us to perform one or more additional clinical studies or measurements to support the change from the approved product. The FDA may also reject our future Section 505(b)(2) submissions and require us to file such submissions under Section 505(b)(1) of the FDCA, which could cause delay and be considerably more expensive and time consuming.
Once the NDA submission has been accepted for filing, the FDA typically takes one year to review the application and respond to the applicant. The review process is often extended by FDA requests for additional information or clarification. Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured and will not approve the product unless the manufacturing facility complies with cGMPs. The FDA may delay approval of an NDA if applicable regulatory criteria are not satisfied, the FDA requires additional testing or information and/or the FDA requires post-marketing testing and surveillance to monitor safety or efficacy of a product. FDA approval of any NDA submitted by us or GSK will be at a time the FDA chooses. For example, although the FDA has accepted for review GSK’s NDA resubmission forHorizant for the treatment of RLS and set a new PDUFA date of April 6, 2011, the FDA has the discretion to exercise its option to extend the PDUFA timing goal an additional three months.
Post-Marketing Regulations
If the FDA grants regulatory approval of a product, such approval may entail limitations on the indicated uses for which such product may be marketed. The FDA may withdraw the product approval if compliance with
28
pre- and post-marketing regulatory standards and requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require additional clinical trials, including Phase 4 post-marketing studies, to provide additional data on safety, to monitor the effect of approved products or for other reasons. The failure of such trials can result in a range of regulatory actions, including limiting further marketing of the product or withdrawal of the product from the market.
If we or our collaborative partners obtain regulatory approval for a product, this clearance will be limited to those diseases and conditions for which the FDA agrees that the product is safe and effective, as demonstrated through clinical trials and as described in the FDA-approved product label. Thus, further studies will be required to gain approval for the use of a product as a treatment for clinical indications other than the indication for which the product was initially approved. Even if regulatory approval is obtained, a marketed product, its manufacturer and its manufacturing facilities are subject to continual review and periodic inspections by the FDA. Further, if there are any modifications to the drug, including any change in indication, manufacturing process, labeling or manufacturing facility, it is necessary to submit an application to the FDA seeking approval of such changes. Also, the FDA can place restrictions on approval and marketing utilizing its authority under applicable regulations. Discovery of previously unknown problems with a medicine, manufacturer or facility may result in restrictions on the marketing or manufacturing of an approved product, including costly recalls or withdrawal of the product from the market. The FDA has broad post-market regulatory and enforcement powers, including the ability to suspend or delay issuance of approvals, seize or recall products, withdraw approvals, enjoin violations and institute criminal prosecution. Additionally, the FDA regulates the labeling, storage, record-keeping, advertising and promotion of prescription pharmaceuticals. In the course of practicing medicine, physicians may legally prescribe FDA-approved drugs for an indication that has not been approved by the FDA and which, therefore, is not described in the product’s approved labeling. This prescribing practice is known as “off-label use.” The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA and other governmental agencies do, however, restrict communications on the subject of off-label use by a manufacturer or those acting on behalf of a manufacturer, and companies may not promote FDA-approved drugs for off-label uses. Marketed products are subject to continued regulatory oversight by the Office of Medical Policy, Division of Drug Marketing, Advertising, and Communications. Certain products approved by the FDA may only be marketed if the promotional materials advertising such products carry certain warnings. Failure to comply with applicable regulations could result in marketing restrictions, financial penalties and/or other sanctions.
The Controlled Substances Act imposes various registration, record-keeping and reporting requirements, procurement and manufacturing quotas, labeling and packaging requirements, security controls and a restriction on prescription refills on certain pharmaceutical products. A principal factor in determining the particular requirements, if any, applicable to a product is its actual or potential abuse profile. The U.S. Drug Enforcement Agency, or DEA, regulates chemical compounds as Schedule I, II, III, IV or V substances, with Schedule I substances considered to present the highest risk of substance abuse and Schedule V substances the lowest risk. Pregabalin is classified as a controlled substance (Schedule V), which could increase the possibility thatHorizantwould be classified as a controlled substance since they are believed to act on the same therapeutic target. If any of our product candidates contains a scheduled substance, it would be subject to DEA regulations relating to manufacturing, storage, distribution and physician prescription procedures, and the DEA would regulate the amount of the scheduled substance that would be available for clinical trials and commercial distribution.
We and our collaborative partners also will be subject to a variety of foreign regulations governing clinical trials and the marketing of our products. Outside the United States, our ability to market a product depends upon receiving a marketing authorization from the appropriate regulatory authorities. Pursuant to the terms of our collaboration with Astellas, Astellas has filed in Japan an NDA for gabapentin enacarbil for restless legs syndrome, and Astellas will lead the development and registration of gabapentin enacarbil for any other indications in the Astellas territory. The requirements governing the conduct of clinical trials, marketing authorization, pricing and reimbursement vary widely from country to country. In any country, however, we and our collaborative partners will only be permitted to commercialize our products if the appropriate regulatory authority is satisfied that we have presented adequate evidence of safety, quality and efficacy. Whether or not FDA approval has been obtained, approval of a product by the comparable regulatory authorities of foreign countries must be obtained prior to the commencement of marketing of the product in those countries. The time
29
needed to secure approval may be longer or shorter than that required for FDA approval. The regulatory approval and oversight process in other countries includes all of the risks associated with the FDA process described above.
Pharmaceutical Pricing and Reimbursement
Political, economic and regulatory influences are subjecting the healthcare industry in the United States to fundamental change. Initiatives to reduce the federal deficit and to reform healthcare delivery are increasing cost-containment efforts. We anticipate that Congress, state legislatures and the private sector will continue to review and assess alternative benefits, controls on healthcare spending through limitations on the growth of private health insurance premiums and Medicare and Medicaid spending, the creation of large insurance purchasing groups, price controls on pharmaceuticals and other fundamental changes to the healthcare delivery system. Any proposed or actual changes could limit or eliminate our spending on development projects and affect our ultimate profitability. In March 2010, the Patient Protection Affordable Care Act and the Health Care and Education Reconciliation Act of 2010 were signed into law. Such laws are anticipated to have a wide range of effects on the management of healthcare in the Untied States, including potentially on the pricing and availability of government reimbursement for pharmaceutical products. The full effect of the healthcare reform acts are still unknown, and we are evaluating the impact such laws could have on our business. Legislative debate is expected to continue in the future, and market forces are expected to drive reductions of healthcare costs.
In both domestic and foreign markets, sales of any products for which we or our collaborative partners receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. These third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare product candidates. We or our collaborative partners may need to conduct expensive pharmacoeconomic studies in order to demonstrate the cost-effectiveness of our products. Our product candidates may not be considered cost-effective.
Pursuant to the Medicare Prescription Drug Improvement and Modernization Act of 2003, Medicare beneficiaries are eligible to obtain subsidized prescription drug coverage from a choice of private sector plans. Approximately 90 percent of Medicare beneficiaries now have coverage for prescription medicines. The use of pharmaceuticals has increased slightly among some patients as the result of the expanded access to medicines afforded by coverage under Medicare. However, such expanded utilization has been largely offset by increased pricing pressure and competition due to the enhanced purchasing power of the private sector plans that negotiate on behalf of Medicare beneficiaries and by an increase in the use of generic medicines in this population.
Facilities
We lease approximately 162,000 square feet of office and laboratory space in two adjacent buildings in Santa Clara, California, where we conduct our operations in one of the buildings and the other is vacant. The leases expire concurrently in August 2013, although we have the option to extend both leases for two additional terms of five years each. The 2010 aggregate annual rental amount payable under the leases was approximately $5.5 million, subject to periodic increases. Although our facilities are adequate for our existing needs, we may require additional space as our business expands.
Employees
As of December 31, 2010, we had 108 full-time employees, 67 of whom were engaged in product development activities. Sixty-three employees hold post-graduate degrees, including three with M.D. degrees and 26 with Ph.D. degrees. Our employees are not represented by a collective bargaining agreement. We believe our relations with our employees are good.
30
Executive Officers of the Registrant
The following sets forth certain information regarding our executive officers as of February 1, 2011:
| | | | | | |
Name | | Age | | | Position |
Ronald W. Barrett, Ph.D. | | | 55 | | | Chief Executive Officer and Director |
| | |
Vincent J. Angotti | | | 43 | | | Senior Vice President, Chief Commercialization Officer |
| | |
Gianna M. Bosko. | | | 41 | | | Senior Vice President, Chief Administrative Officer, General Counsel and Secretary |
| | |
Kenneth C. Cundy, Ph.D. | | | 51 | | | Senior Vice President of Preclinical and Clinical Sciences |
| | |
William G. Harris | | | 52 | | | Senior Vice President of Finance and Chief Financial Officer |
| | |
David R. Savello, Ph.D. | | | 65 | | | Senior Vice President of Development Operations |
Ronald W. Barrett is one of our founders and has served as our chief executive officer since September 2001. He served as our chief scientific officer from 1999 to 2001. Dr. Barrett has been a director since August 1999. From 1989 to 1999, he held various positions at Affymax Research Institute, a company employing combinatorial chemistry and high-throughput target screening for drug discovery, the most recent of which was senior vice president of research. Glaxo Wellcome plc, a pharmaceutical company, acquired Affymax Research Institute in 1995. Glaxo Wellcome subsequently merged with SmithKline Beecham plc, a pharmaceutical company, in 2000 to form GlaxoSmithKline plc, a pharmaceutical company. Prior to Affymax Research Institute, Dr. Barrett was a molecular pharmacologist in the Neuroscience Group at Abbott Laboratories, a healthcare company, from 1986 to 1989. Dr. Barrett received a B.S. from Bucknell University and a Ph.D. in pharmacology from Rutgers University.
Vincent J. Angotti has been our senior vice president and chief commercialization officer since May 2008. From 2001 to 2008, he held several positions with Reliant Pharmaceuticals, Inc., a pharmaceutical company, the most recent of which was senior vice president of sales and marketing. GlaxoSmithKline acquired Reliant Pharmaceuticals in 2008. Prior to Reliant Pharmaceuticals, from 1991 to 2001, Mr. Angotti held several positions at Novartis Pharmaceuticals Corporation, a pharmaceutical company, most recently as executive director, field operations. Mr. Angotti received a B.S. from Cornell University and an M.B.A. from Columbia University.
Gianna M. Bosko has been our senior vice president, chief administrative officer, general counsel and secretary since August 2010. She was previously our vice president, general counsel and secretary from 2007 to 2010, and senior corporate counsel from 2005 to 2007. From 2004 to 2005, Ms. Bosko was a legal consultant, providing general corporate and in-house legal consulting services for private and public companies, including XenoPort. From 1996 to 2004, she was an associate at CooleyLLP, a law firm, practicing general corporate and securities law, with an emphasis on securities transactions and mergers and acquisitions. Ms. Bosko received an A.B. from Stanford University and a J.D. from the University of Chicago Law School.
Kenneth C. Cundyhas been our senior vice president of preclinical and clinical sciences since November 2010. He was previously our senior vice president of preclinical development from 2004 to November 2010. He was our vice president of biopharmaceutics from 2000 to 2004. From 1992 to 2000, he was senior director of biopharmaceutics at Gilead Sciences, Inc. Prior to Gilead Sciences, from 1988 to 1992, Dr. Cundy was principal research investigator at Sterling Drug, a pharmaceutical division of Eastman Kodak Company. He received a B.S. from the University of Manchester and a Ph.D. in pharmaceutical sciences from the University of Kentucky.
William G. Harris has been our senior vice president of finance and chief financial officer since November 2001. From 1996 to 2001, he held several positions with Coulter Pharmaceutical, Inc., a biotechnology company engaged in the development of novel therapies for the treatment of cancer and autoimmune diseases, the most recent of which was senior vice president and chief financial officer. Corixa Corp., a developer of immunotherapeutic products, acquired Coulter Pharmaceutical in 2000. Prior to Coulter Pharmaceutical, from 1990 to 1996, Mr. Harris held several positions at Gilead Sciences, the most recent of which was director of finance. Mr. Harris received a B.A. from the University of California, San Diego and an M.B.A. from Santa Clara University, Leavey School of Business and Administration.
31
David R. Savellohas been our senior vice president of development operations since November 2010. He was previously our senior vice president of development from 2007 to November 2010. He was responsible for our regulatory affairs, quality and project management from 2005 to 2007. From 1999 to 2005, Dr. Savello was executive vice president and chief scientific officer for the Pharmaceutical Technology and Services Sector of Cardinal Health, Inc. Prior to joining Cardinal Health, from 1997 to 1999, he was senior vice president for drug development at Guilford Pharmaceuticals Inc. From 1985 to 1997, Dr. Savello held several positions at Glaxo and Glaxo Wellcome including both vice president of drug development and vice president of regulatory affairs and compliance. Prior to that, he held R&D management and executive management positions at Boehringer Ingelheim GmbH, and 3M Company. Dr. Savello received his B.S. degree from the Massachusetts College of Pharmacy and both an M.S. and a Ph.D. in pharmaceutics from the University of Maryland School of Pharmacy.
About XenoPort
We were incorporated in Delaware in May 1999. Our principal offices are located at 3410 Central Expressway, Santa Clara, California 95051, and our telephone number is (408) 616-7200. Our Web site address is www.XenoPort.com. Information found on, or accessible through, our Web site is not a part of, and is not incorporated into, this Annual Report on Form 10-K. XENOPORT, the XenoPort logo and Transported Prodrug are our trademarks. Service marks, trademarks and trade names appearing in this Annual Report on Form 10-K are the property of their respective owners. Unless the context requires otherwise, references in this Annual Report on Form 10-K to “the company,” “we,” “us” and “our” refer to XenoPort, Inc.
Available Information
We file electronically with the U.S. Securities and Exchange Commission our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our Web site at www.XenoPort.com, free of charge, copies of these reports as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. Further, copies of these reports are located at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a Web site that contains reports, proxy and information statements, and other information regarding our filings, at www.sec.gov.
Item 1A. Risk Factors.
The following risks and uncertainties may have a material adverse effect on our business, financial condition or results of operations. Investors should carefully consider the risks described below before making an investment decision. The risks described below are not the only ones we face. Additional risks not presently known to us or that we believe are immaterial may also significantly impair our business operations. Our business could be harmed by any of these risks. The trading price of our common stock could decline due to any of these risks, and investors may lose all or part of their investment.
Risks Related to Our Business and Industry
We have incurred cumulative operating losses since inception, we expect to continue to incur losses for the foreseeable future and we may never sustain profitability.
We have a limited operating history and have incurred cumulative losses of $387.4 million since our inception in May 1999, including net loss of $82.5 million, $66.3 million and $62.5 million for the years ended December 31, 2010, 2009 and 2008, respectively. Subject to regulatory approval of any of our product candidates, we may incur significant expenses associated with the establishment of a North American specialty sales force. Annual losses have had, and will continue to have, an adverse effect on our stockholders’ equity.
Because of the numerous risks and uncertainties associated with drug development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be able to achieve or sustain profitability. We have no products approved for commercial sale and, to date, we have not generated any product revenues. We
32
have financed our operations primarily through the sale of equity securities, non-equity payments from collaborative partners and interest earned on investments. We have devoted substantially all of our efforts to research and development, including clinical trials. If we or our collaborative partners are unable to develop and commercialize our product candidates, if development is delayed or if sales revenue from a product candidate that receives marketing approval is insufficient, we may never become profitable. Even if we do become profitable, we may not be able to sustain or increase our profitability on a quarterly or annual basis.
Our success depends substantially on our most advanced product candidates, which are still under development. If we or our collaborative partners are unable to bring any or all of these product candidates to market, or experience significant delays in doing so, our ability to generate product revenue and our likelihood of success will be reduced.
Our ability to generate product revenue in the future will depend heavily on the successful development and commercialization of our product candidates. In February 2010, we and our partner, Glaxo Group Limited, or GSK, announced that GSK received a Complete Response letter from the U.S. Food and Drug Administration, or FDA, regarding the new drug application, or NDA, submitted by GSK seeking approval ofHorizant (gabapentin enacarbil) Extended-Release Tablets for the treatment of moderate-to- severe primary restless legs syndrome, or RLS. A Complete Response letter is issued by the FDA’s Center of Drug Evaluation and Research when the review of a file is completed and questions remain that preclude the approval of the NDA in its current form. In the Complete Response letter, the FDA indicated that a preclinical finding of pancreatic acinar cell tumors in rats was of sufficient concern to preclude approval of theHorizant NDA for RLS at that time. In October 2010, GSK responded to the Complete Response letter with an NDA resubmission, which included additional nonclinical data ofHorizant and epidemiological data related to gabapentin use and cancer. In order for the FDA to be able to consider published gabapentin nonclinical data in their assessment ofHorizant, GSK amended theHorizantNDA from a Section 505(b)(1) to a Section 505(b)(2) application. The Complete Response letter and NDA resubmission process has delayed, and may prevent, the approval ofHorizant for RLS. We believe the Complete Response letter has also delayed the development by GSK ofHorizant in neuropathic pain. In addition, in July 2010, GSK announced top-line results from a Phase 2b clinical trial in whichHorizant did not demonstrate a statistically significant improvement compared to placebo as a prophylactic treatment for migraine headaches.
In November 2009, our partner, Astellas Pharma Inc., submitted an NDA for gabapentin enacarbil (previously known as XP13512) for the treatment of restless legs syndrome in Japan. Our other product candidates are either in Phase 1 or Phase 2 clinical development or in various stages of preclinical development. Any of our product candidates could be unsuccessful if it:
| | • | | does not demonstrate acceptable safety and efficacy in preclinical studies or clinical trials or otherwise does not meet applicable regulatory standards for approval; |
| | • | | does not offer therapeutic or other improvements over existing or future drugs used to treat the same conditions; |
| | • | | is not capable of being produced in commercial quantities at acceptable costs; or |
| | • | | is not accepted in the medical community and by third-party payors. |
For example, in September 2009, we announced that we would no longer be pursuing further development of arbaclofen placarbil, or AP (and previously known as XP19986), for acute back spasms following the completion of a Phase 2 clinical trial of AP in patients with acute moderate to severe muscle spasms in the lumbar region that did not demonstrate AP efficacy over placebo. If we or our collaborative partners are unable to make additional product candidates commercially available, we may not be able to generate substantial product revenues, which would adversely affect our business and financial condition. The results of our clinical trials to date do not provide assurance that acceptable efficacy or safety will be shown upon completion of future clinical trials.
33
If we or our partners are not able to obtain required regulatory approvals, we or our partners will not be able to commercialize our product candidates, our ability to generate revenue will be materially impaired and our business will not be successful.
Our product candidates and the activities associated with their development and commercialization are subject to comprehensive regulation by the FDA and other agencies in the United States and by comparable authorities in other countries. The inability to obtain FDA approval or approval from comparable authorities in other countries would prevent us and our collaborative partners from commercializing our product candidates in the United States or other countries. We or our collaborative partners may never receive regulatory approval for the commercial sale of our product candidates. For example, in February 2010, GSK received a Complete Response letter from the FDA in which a preclinical finding of pancreatic acinar cell tumors in rats precluded approval of theHorizant NDA for the treatment of RLS at that time. In October 2010, GSK responded to questions raised by the FDA in the Complete Response letter with an NDA resubmission, which included new data from nonclinical studies ofHorizantand two epidemiology studies conducted by GSK exploring gabapentin use and cancer based on the UK General Practice Research Database, as well as a final safety update that provided updated or new safety information on patients in clinical studies who have been treated withHorizant. In order for the FDA to be able to consider published gabapentin nonclinical data in their assessment ofHorizant, GSK amended theHorizant NDA from a Section 505(b)(1) to a Section 505(b)(2) application. However, it is unknown when, or if, we and GSK will obtain FDA approval forHorizant for RLS or any indication. If we are unable to obtain regulatory approval ofHorizant, we may not achieve profitability and our business will be severely harmed. Moreover, if the FDA requires that any of our products or product candidates be scheduled by the U.S. Drug Enforcement Agency, or DEA, we or our collaborative partners will be unable to begin commercial sale of that product until the DEA completes scheduling proceedings. If any of our products or product candidates is classified as a controlled substance by the DEA, we or our collaborative partners would have to register annually with the DEA and those product candidates would be subject to additional regulation.
We have only limited experience in preparing and filing the applications necessary to gain regulatory approvals. The process of applying for regulatory approval is expensive, often takes many years and can vary substantially based upon the type, complexity and novelty of the product candidates involved. The application process begins with the submission of an NDA that the FDA initially reviews and either accepts or rejects for filing. NDA submissions are complex electronic filings, which include vast compilations of data sets, integrated documents and data calculations. The FDA has substantial discretion in the submission process and may refuse to accept an NDA submission if there are errors or omissions relating to the electronic transmittal process, data entry, data compilation or formatting. For example, in November 2008, GSK withdrew a previously submitted NDA forHorizant for the treatment of RLS in connection with the FDA’s request that the data from a single study be reformatted. The Section 505(b)(1) NDA forHorizant was resubmitted in January 2009 and the FDA accepted the Section 505(b)(1) NDA for review in March 2009.
Changes in the regulatory approval policy during the development period, changes in, or the enactment of additional, regulations or statutes or changes in regulatory review for each submitted product application may cause delays in the approval or rejection of an NDA. If the FDA were to miss a Prescription Drug User Fee Act, or PDUFA, timing goal for one of our product candidates, the development and commercialization of the product candidate could be delayed or impaired. For example, in November 2009, the FDA notified GSK that it was extending the PDUFA timing goal forHorizant for the treatment of RLS to February 2010. Similarly, in connection with theHorizant NDA resubmission, the FDA has set a new PDUFA timing goal of April 6, 2011. However, the FDA has the discretion to exercise its one-time option to extend the PDUFA timing goal an additional three months. In addition, the Food and Drug Administration Amendments Act of 2007, or FDAAA, mandates FDA advisory committee reviews of all new molecular entities as part of the NDA approval process, although the FDA maintains discretion under FDAAA to approve NDAs for new molecular entities without advisory committee reviews in certain instances. Although the FDA did not convene an advisory committee during its review of the initialHorizant NDA filing, the FDA may convene an advisory committee at any time during the review process, including following an NDA resubmission. The advisory committee review process can be a lengthy and uncertain process that could delay the FDA’s NDA approval and delay or impair the development and commercialization of our product candidates.
34
The FDA has substantial discretion in the approval process and may refuse to approve any application or decide that our or our collaborative partners’ data is insufficient for approval and require additional preclinical, clinical or other studies. Varying interpretations of the data obtained from preclinical and clinical testing or epidemiology studies could delay, limit or prevent regulatory approval of any of our product candidates. For example, in the epidemiology studies that GSK conducted in connection with theHorizant NDA resubmission, some significant associations between gabapentin use and cancer were seen in the analyses, but only when no time lag was used and/or cumulative dose was low, duration of exposure was low or number of prescriptions was low. When analyses were conducted that took into account two common potential sources of bias in epidemiology studies — protopathic bias and carcinogenic latency – the studies did not demonstrate significant associations between gabapentin use and cancer. In total, we believe that these observations were inconsistent with gabapentin being a carcinogen. Protopathic bias can occur when a pharmaceutical agent is prescribed for an early manifestation of a disease that has not yet been diagnostically detected or formally recorded in the medical record. In this case, gabapentin use could appear to be associated with cancer because it could be prescribed for pain (a common symptom of certain cancers) prior to the diagnosis of the underlying cancer. Carcinogenic latency refers to the time gap between the moment that a drug initiates a carcinogenic process and the time that a tumor is diagnosed and entered into the database. Epidemiologists often use a time lag between drug use and cancer diagnosis when selecting cases to be included in analyses in order to eliminate false positives that are biologically implausible. The FDA does not define standards for controlling protopathic bias or appropriate time lags for considering carcinogenic latency. The FDA may interpret the GSK epidemiology data differently, and there can be no assurances that the FDA will agree with our and GSK’s conclusion thatHorizant does not significantly raise the risk of pancreatic or other cancers in humans.
The FDA could also require additional studies or trials to satisfy particular safety concerns noted in our preclinical or clinical testing or epidemiological studies. In particular, to satisfy a preclinical safety concern expressed in the Complete Response letter with respect toHorizant, the FDA may require us to undertake additional preclinical studies or trials or epidemiological studies prior to approvingHorizant. Even if we were to undertake additional studies or trials, there are no assurances that it would be sufficient to obtain approval ofHorizant for RLS. In addition, given the FDA’s substantial discretion in the approval process, the FDA may raise new or unexpected issues at any time in the review process. For example, in October 2010, Amylin Pharmaceuticals, Inc. announced that they received a second Complete Response letter from the FDA regarding Amylin’s NDA seeking approval of BYDUREON (exenatide extended-release for injectable suspension). Amylin indicated that although the issue was not raised in the FDA’s initial Complete Response letter, the FDA requested a thorough QT study with exposures of exenatide higher than typical therapeutic levels of BYDUREON in a second Complete Response letter. If the FDA were to raise new issues in a subsequent Complete Response letter with respect toHorizant, its approval, if granted, would be delayed and our business would be harmed.
Even if the FDA or other regulatory agency approves a product candidate, the approval may impose significant restrictions on the indicated uses, conditions for use, labeling, advertising, promotion, marketing and/or production of such product and may impose ongoing commitments or requirements for post-approval studies, including additional research and development and clinical trials. The FDA and other agencies also may impose various civil or criminal sanctions for failure to comply with regulatory requirements, including withdrawal of product approval.
We and our collaborative partners will need to obtain regulatory approval from authorities in foreign countries to market our product candidates in those countries. Approval by one regulatory authority does not ensure approval by regulatory authorities in other jurisdictions. If we or our collaborative partners fail to obtain approvals from foreign jurisdictions, the geographic market for our product candidates would be limited.
We depend on collaborations to complete the development and commercialization of some of our product candidates. These collaborations may place the development of our product candidates outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us.
In December 2005, we entered into a collaboration with Astellas for the development and commercialization of gabapentin enacarbil, also known as ASP8825, in Japan, Korea, the Philippines, Indonesia, Thailand and
35
Taiwan. In February 2007, we entered into an exclusive collaboration with GSK to develop and commercialize gabapentin enacarbil, also known as GSK1838262 and by the trade nameHorizant (gabapentin enacarbil) Extended-Release Tablets in the United States, worldwide, excluding the Astellas territory. In November 2010, we amended and restated our collaboration agreement with GSK, pursuant to which we reacquired all rights to gabapentin enacarbil outside of the United States previously granted to GSK and obtained the right to pursue development ofHorizant for: (i) the potential treatment of diabetic peripheral neuropathy, or DPN; (ii) the potential treatment of post-herpetic neuralgia, or PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug; and (iii) any additional indications in the United States.
We may enter into additional collaborations with third parties to develop and commercialize some of our product candidates. Our dependence on Astellas and GSK for the development and commercialization of gabapentin enacarbil/Horizant subjects us to, and our dependence on future collaborators for development and commercialization of our product candidates will subject us to, a number of risks, including:
| | • | | we are not able to control the amount and timing of resources that GSK and Astellas devote to the development or commercialization of our product candidate or to its marketing and distribution; |
| | • | | we may not be able to control the amount and timing of resources that our potential future collaborators may devote to the development or commercialization of product candidates or to their marketing and distribution; |
| | • | | collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing; |
| | • | | disputes may arise between us and our collaborators that result in the delay or termination of the research, development or commercialization of our product candidates or that result in costly litigation or arbitration that diverts management’s attention and resources; |
| | • | | collaborators may experience financial difficulties; |
| | • | | collaborators may not be successful in their efforts to obtain regulatory approvals in a timely manner, or at all; |
| | • | | collaborators may receive regulatory sanctions relating to other aspects of their business that could adversely affect the approval of our product candidates; |
| | • | | collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our proprietary information or expose us to potential litigation; |
| | • | | business combinations or significant changes in a collaborator’s business strategy may also adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement; |
| | • | | a collaborator could independently move forward with a competing product candidate developed either independently or in collaboration with others, including our competitors; |
| | • | | where we co-promote a product with a collaborator, if we do not receive timely and accurate information from our collaborator regarding sales activities, expenses and resulting operating profits and losses, our estimates at a given point of time could be incorrect and we could be required to record adjustments in future periods or restate our financial results for prior periods; and |
| | • | | the collaborations may be terminated or allowed to expire, which would delay the development and may increase the cost of developing our product candidates. |
For example, in October 2007, we entered into a collaboration with Xanodyne Pharmaceuticals, Inc. for the development and commercialization of XP21510 in the United States. Effective July 2009, Xanodyne terminated the collaboration agreement.
As a further example, we cannot control the process for securing FDA approval ofHorizant for the potential treatment of RLS. GSK is responsible for all interactions with the FDA. If the FDA requires additional studies or
36
trials evaluating the safety or efficacy ofHorizantfor RLS, or imposes conditions on any approval ofHorizantfor the treatment of RLS, GSK would be responsible for performing such studies or trials and would control decisions with respect to the acceptance and implementation of any conditions to approval. We cannot control the amount and timing of resources that GSK or Astellas may devote to the development or commercialization of Horizant/gabapentin enacarbil or its marketing and distribution. In February 2010, GSK announced that it proposed to cease discovery research in certain neuroscience areas, including depression and pain. IfHorizantis not approved for RLS in the United States, GSK may not developHorizantfor PHN. GSK or Astellas may abandon further development or the pursuit of regulatory approval ofHorizantor gabapentin enacarbil, and may terminate their respective collaboration agreements with us at any time, which could delay or impair the development and commercialization of Horizant/gabapentin enacarbil and harm our business.
In addition, GSK recently announced new corporate policies, including that it would no longer use sales targets as a component of compensation for its sales force. Assuming FDA approval ofHorizant, if GSK’s change in its compensation practices does not effectively motivate its sales force, the commercial potential ofHorizant may be harmed or limited and our business could suffer.
If we do not establish collaborations for our product candidates, we will have to alter our development and commercialization plans.
Our strategy includes selectively collaborating with leading pharmaceutical and biotechnology companies to assist us in furthering development and potential commercialization of some of our product candidates. We intend to do so especially for indications that involve a large, primary care market that must be served by large sales and marketing organizations. We face significant competition in seeking appropriate collaborators, and these collaborations are complex and time consuming to negotiate and document. We may not be able to negotiate additional collaborations on acceptable terms, or at all. We are unable to predict when, if ever, we will enter into any additional collaborations because of the numerous risks and uncertainties associated with establishing additional collaborations. If we are unable to negotiate additional collaborations, we may have to curtail the development of a particular product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of our sales or marketing activities or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to bring our product candidates to market and generate product revenues.
We will continue to need additional funding and may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs or commercialization efforts.
We will continue to need to raise additional capital to fund our operations and complete the development of our product candidates. If any product candidate receives regulatory approval for commercial sale, we may need to raise additional capital to fund our commercialization efforts. Our future funding requirements will depend on many factors, including:
| | • | | the scope, rate of progress, results and cost of our preclinical testing, clinical trials and other research and development activities; |
| | • | | the receipt of FDA approval forHorizant and the timing and success of further studies and trials necessary to secure this approval, if any; |
| | • | | the extent of product development funding under our current collaborative arrangements; |
| | • | | the cost of manufacturing clinical, and establishing commercial, supplies of our product candidates and any products that we may develop; |
| | • | | the timing of any milestone payments under our collaborative arrangements; |
37
| | • | | the number and characteristics of product candidates that we pursue, including rights related toHorizant that we obtained pursuant to our amended and restated collaboration agreement with GSK; |
| | • | | the cost, timing and outcomes of regulatory approvals, if any; |
| | • | | the terms and timing of any collaborative, licensing and other arrangements that we may establish or modify; |
| | • | | the timing and amount of our share of operating losses from our GSK collaboration; |
| | • | | the timing, receipt and amount of sales, profit sharing or royalties, if any, from our potential products; |
| | • | | the cost and expenses associated with the pending and potential additional related purported securities class action lawsuits, as well as any unforeseen litigation; |
| | • | | the cost of preparing, filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| | • | | the extent to which we acquire or invest in businesses, products or technologies that complement our business, although we have no commitments or agreements relating to any of these types of transactions. |
Until we can generate a sufficient amount of product revenues, if ever, we expect to finance future cash needs through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements, as well as through interest income earned on cash balances. If we raise additional funds by issuing our common stock, or securities convertible into or exchangeable or exercisable for common stock, our stockholders will experience dilution. Any debt financing or additional equity that we raise may contain terms that are not favorable to our stockholders or us. To the extent that we raise additional capital through licensing arrangements or arrangements with collaborative partners, we may be required to relinquish, on terms that are not favorable to us, rights to some of our technologies or product candidates that we would otherwise seek to develop or commercialize ourselves.
We believe that our existing capital resources and anticipated milestone payments, together with interest thereon, will be sufficient to meet our projected operating requirements into the fourth quarter of 2013. We have based our cash sufficiency estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect. Further, our operating plan may change, and we may need additional funds to meet operational needs and capital requirements for product development and commercialization sooner than planned. We have no credit facility or committed sources of capital other than potential milestones receivable under our current collaborations.
Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not, or we anticipate that they may not be, available on a timely basis, we may:
| | • | | terminate or delay clinical trials for one or more of our product candidates; |
| | • | | curtail significant drug development programs that are designed to identify new product candidates; |
| | • | | delay our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our product candidates; or |
| | • | | exercise the right to revert to a net sales royalty-based compensation structure and forego the right to co-promoteHorizant in the United States. |
For example, in January 2009, we suspended preclinical development activities for XP20925, our Transported Prodrug of propofol, to focus our resources on development of later-stage product candidates. In addition, in March 2010, as a result of the Complete Response letter that precluded approval of theHorizant NDA for RLS in its form at that time, we implemented a restructuring plan to reduce expenses, focus our resources on advancement of our later-stage product candidates and eliminate our discovery research efforts. In connection with this restructuring, we postponed the commencement of additional clinical trials of AP as a potential treatment for spasticity until 2011 to focus our clinical development resources on the completion of the Phase 2b clinical trial of AP as a potential treatment for gastroesophageal reflux disease, or GERD.
38
If our preclinical studies do not produce successful results or our clinical trials do not demonstrate safety and efficacy in humans, we will not be able to commercialize our product candidates.
To obtain the requisite regulatory approvals to market and sell any of our product candidates, we must demonstrate, through extensive preclinical studies and clinical trials, that the product candidate is safe and effective in humans. Preclinical and clinical testing is expensive, can take many years and has an uncertain outcome. A failure of one or more of our clinical trials could occur at any stage of testing. For example, in July 2010, GSK announced top-line results from a 30-week, double-blind, placebo-controlled, Phase 2b clinical trial ofHorizantas a potential prophylactic treatment for migraine headaches in whichHorizantdid not demonstrate a statistically significant improvement on the primary endpoint when compared to placebo. Long-term safety concerns may also prevent the approval of any of our product candidates by a regulatory authority. For example, in February 2010, safety concerns related to a preclinical finding of pancreatic acinar cell tumors in rats precluded FDA approval of theHorizantNDA for RLS in its form at that time. In addition, success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. We may experience numerous unforeseen events during, or as a result of, preclinical testing and the clinical trial process, which could delay or prevent our or our collaborative partners’ ability to commercialize our product candidates, including:
| | • | | regulators or institutional review boards may not authorize us to commence a clinical trial at a prospective trial site; |
| | • | | our preclinical testing or clinical trials may produce negative or inconclusive results, which may require us to conduct additional preclinical or clinical testing or to abandon projects that we expect to be promising; |
| | • | | we may suspend or terminate our clinical trials if the participating patients are being exposed to unacceptable health risks; |
| | • | | risks associated with clinical trial design may result in a failure of the clinical trial to show statistically significant results even if the product candidate is effective; |
| | • | | regulators or institutional review boards may suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; and |
| | • | | the effects of our product candidates may not be the desired effects or may include undesirable side effects. |
As an example of an unforeseen event, after having been discharged from a Phase 1 clinical trial in which a single dose ofHorizant was administered almost two days earlier, a volunteer died of a self-inflicted gunshot wound following a domestic dispute. We do not believe that this incident was related toHorizant. However, any unforeseen event could cause us to experience significant delays in, or the termination of, clinical trials. Any such events would increase our costs and could delay or prevent our ability to commercialize our product candidates, which would adversely impact our financial results.
Any failure or delay in commencing or completing clinical trials for our product candidates could severely harm our business.
The commencement and completion of clinical trials for our product candidates may be delayed or terminated as a result of many factors, including:
| | • | | delays in patient enrollment, which we have experienced in the past and which we are experiencing in our current Phase 2 clinical trial of XP21279 for Parkinson’s disease, and variability in the number and types of patients available for clinical trials; |
| | • | | our inability or the inability of our collaborators or licensees to manufacture or obtain from third parties materials sufficient for use in preclinical studies and clinical trials; |
| | • | | difficulty in maintaining contact with patients after treatment, resulting in incomplete data; |
39
| | • | | poor effectiveness of product candidates during clinical trials; |
| | • | | unforeseen safety issues or side effects; and |
| | • | | governmental or regulatory delays and changes in regulatory requirements, policy and guidelines. |
For example, based on the results of a planned interim analysis of the clinical data, although no safety concerns were noted, Astellas terminated its Phase 2 clinical trial of gabapentin enacarbil as a potential treatment for DPN due to difficulty in demonstrating a statistically significant advantage of gabapentin enacarbil over placebo. As a result, Astellas does not intend to continue the development of gabapentin enacarbil in Japan as a potential treatment for DPN at this time. Any delay in commencing or completing clinical trials for our product candidates would delay commercialization of our product candidates and severely harm our business and financial condition. In addition, unforeseen safety issues or side effects could result from our collaborators’ current or future clinical trials, which could delay or negatively impact commercialization of our product candidates. It is also possible that none of our product candidates will complete clinical trials in any of the markets in which we or our collaborators intend to sell those product candidates. Accordingly, we or our collaborators would not receive the regulatory approvals needed to market our product candidates, which would severely harm our business and financial condition.
We rely on third parties to conduct our clinical trials. If these third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for, or commercialize, our product candidates.
We do not have the ability to independently conduct clinical trials for our product candidates, and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators, collaborative partners and contract laboratories, to conduct our clinical trials. We have, in the ordinary course of business, entered into agreements with these third parties. Nonetheless, we are responsible for confirming that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. Moreover, the FDA requires us to comply with regulations and standards, commonly referred to as good clinical practices, for conducting and recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. For example, we need to prepare, and ensure our compliance with, various procedures required under good clinical practices, even though third-party contract research organizations have prepared and are complying with their own, comparable procedures. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for, or successfully commercialize, our product candidates.
An NDA submitted under Section 505(b)(2) of the Food, Drug and Cosmetic Act subjects us to the risk that we or our partner may be subject to a patent infringement lawsuit that would delay or prevent the review and approval of our product candidate.
Certain product candidates that we develop may be submitted to the FDA for approval under Section 505(b)(2) of the Food, Drug and Cosmetic Act, or FDCA, which was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, also known as the Hatch-Waxman Act. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by, or for, the applicant and for which the applicant has not obtained a right of reference. In connection with the October 2010HorizantNDA resubmission, GSK amended theHorizantNDA from a Section 505(b)(1) to a Section 505(b)(2) application in order for the FDA to be able to consider published gabapentin nonclinical data in their assessment ofHorizant. In addition, if we receive positive results in planned Phase 3 clinical trials of AP as a potential treatment for spasticity in multiple sclerosis, or MS, patients, we intend to file an NDA with the FDA under Section 505(b)(2) seeking approval of AP in this
40
indication. The Section 505(b)(2) application would enable us to reference published literature and the FDA’s previous finding of safety and effectiveness for baclofen, a drug that has been approved by the FDA for the alleviation of signs and symptoms of spasticity resulting from MS.
For NDAs submitted under Section 505(b)(2) of the FDCA, the patent certification and related provisions of the Hatch-Waxman Act apply. In accordance with the Hatch-Waxman Act, such NDAs may be required to include certifications, known as Paragraph IV certifications, that certify that any patents listed in the Patent and Exclusivity Information Addendum of the FDA’s publication, Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book, with respect to any product referenced in the Section 505(b)(2) application, are invalid, unenforceable or will not be infringed by the manufacture, use or sale of the product that is the subject of the Section 505(b)(2) application. Under the Hatch-Waxman Act, the holder of patents that the Section 505(b)(2) application references may file a patent infringement lawsuit after receiving notice of the Paragraph IV certification. Filing of a patent infringement lawsuit within 45 days of the patent owner’s receipt of notice triggers a one-time, automatic, 30-month stay of the FDA’s ability to approve the 505(b)(2) application. Accordingly, we may invest a significant amount of time and expense in the development of one or more products only to be subject to significant delay and patent litigation before such products may be commercialized, if at all. A Section 505(b)(2) application may also not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the referenced product has expired. The FDA may also require us to perform one or more additional clinical studies or measurements to support the change from the approved product. The FDA may also reject our future Section 505(b)(2) submissions and require us to file such submissions under Section 505(b)(1) of the FDCA, which could cause delay and be considerably more expensive and time consuming. These factors, among others, may limit our ability to successfully commercialize our product candidates.
If some or all of our patents expire, are invalidated or are unenforceable, or if some or all of our patent applications do not yield issued patents or yield patents with narrow claims, competitors may develop competing products using our intellectual property and our business will suffer.
Our success will depend in part on our ability to obtain and maintain patent and trade secret protection for our technologies and product candidates both in the United States and other countries. We have a number of U.S. and foreign patents, patent applications and rights to patents related to our compounds, product candidates and technology, but we cannot guarantee that issued patents will be enforceable or that pending or future patent applications will result in issued patents. Alternatively, a third party may successfully circumvent our patents. Our rights under any issued patents may not provide us with sufficient protection against competitive products or otherwise cover commercially valuable products or processes.
The degree of future protection for our proprietary technologies and our product candidates is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. For example:
| | • | | we might not have been the first to make the inventions covered by each of our pending patent applications and issued patents; |
| | • | | we might not have been the first to file patent applications for these inventions; |
| | • | | others may independently develop similar or alternative technologies or duplicate any of our technologies; |
| | • | | it is possible that none of our pending patent applications will result in issued patents; |
| | • | | any patents issued to us or our collaborators may not provide a basis for commercially viable products or may be challenged by third parties; or |
| | • | | the patents of others may have an adverse effect on our ability to do business. |
Even if valid and enforceable patents cover our product candidates and technologies, the patents will provide protection only for a limited amount of time.
41
Our and our collaborators’ ability to obtain patents is highly uncertain because, to date, some legal principles remain unresolved, there has not been a consistent policy regarding the breadth or interpretation of claims allowed in patents in the United States and the specific content of patents and patent applications that are necessary to support and interpret patent claims is highly uncertain due to the complex nature of the relevant legal, scientific and factual issues. Furthermore, the policies governing biotechnology patents outside the United States are even more uncertain. Changes in either patent laws or interpretations of patent laws in the United States and other countries may diminish the value of our intellectual property or narrow the scope of our patent protection.
Even if patents are issued regarding our product candidates or methods of using them, those patents can be challenged by our competitors who can argue such patents are invalid and/or unenforceable. For example, in September 2008, a law firm on behalf of an undisclosed client filed an opposition against the patent grant of one of our European patent applications covering gabapentin enacarbil. The European patent office, at an opposition hearing in April 2010, undertook a full review of the grant of the European patent, and ruled that our European patent covering the composition of matter of gabapentin enacarbil is valid. While the law firm that filed the opposition initially appealed the ruling on behalf of the undisclosed client, that appeal was withdrawn in November 2010. Patents also may not protect our product candidates if competitors devise ways of making these or similar product candidates without legally infringing our patents. The FDCA and FDA regulations and policies provide incentives to manufacturers to challenge patent validity and these same types of incentives encourage manufacturers to submit NDAs that rely on literature and clinical data not prepared for or by the drug sponsor.
Subject to possible patent term extension, the entitlement for which and the term of which we cannot predict, patent protection in the United States coveringHorizantwill expire no earlier than 2022. We believe that in all countries in which we hold or have licensed rights to patents or patent applications related to Horizant/gabapentin enacarbil, the composition-of-matter patents relating to gabapentin have expired. For AP, U.S. composition-of-matter patents have issued that will expire no earlier than 2025. For XP21279, a U.S. composition-of-matter patent has issued that will expire no earlier than 2025. Although third parties may challenge our rights to, or the scope or validity of, our patents, to date, other than the European opposition described above, we have not received any communications from third parties challenging our patents or patent applications covering our product candidates.
We may obtain patents for certain product candidates many years before marketing approval is obtained for those products. Because patents have a limited life, which may begin to run prior to the commercial sale of the related product, the commercial value of the patent may be limited. However, we may be able to apply for patent term extensions.
As part of the approval process of our product candidates in the United States, the FDA may determine that the product candidates be granted an exclusivity period during which other manufacturers’ applications for approval of generic versions of our products will not be granted. Generic manufacturers often wait to challenge the patents protecting products that have been granted exclusivity until one year prior to the end of the exclusivity period. While the FDA has historically granted a five-year new chemical entity exclusivity to prodrugs such asHorizantand AP, a lawsuit was filed by a generic drug company against the FDA challenging the grant of the five-year exclusivity to another company’s prodrug. Following a review of applicable statutes and regulations and a period for public comment, the FDA reaffirmed its decision to grant the five-year exclusivity to the prodrug. In March 2010, the U.S. District Court for the District of Columbia granted summary judgment to the FDA confirming the grant of five-year exclusivity to the prodrug. The District Court’s decision was affirmed on appeal, although a further appeal to the U.S. Supreme Court is possible. If the decision is overturned on appeal, it could mean thatHorizantand AP receive shorter or no exclusivity periods. It is also possible that generic manufacturers are considering attempts to seek FDA approval for a similar or identical drug as our product candidate through an abbreviated NDA, which is the application form typically used by manufacturers seeking approval of a generic drug. If our patents are subject to challenges, we may need to spend significant resources to defend such challenges and we may not be able to defend our patents successfully.
We also rely on trade secrets to protect our technology, especially where we do not believe that patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. Our employees, consultants, contractors, outside scientific collaborators and other advisors may unintentionally or willfully
42
disclose our confidential information to competitors. Enforcing a claim that a third party illegally obtained and is using our trade secrets is expensive and time-consuming, and the outcome is unpredictable. Failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Our research and development collaborators may have rights to publish data and other information in which we have rights. In addition, we sometimes engage individuals or entities to conduct research that may be relevant to our business. The ability of these individuals or entities to publish or otherwise publicly disclose data and other information generated during the course of their research is subject to certain contractual limitations. In most cases, these individuals or entities are, at the least, precluded from publicly disclosing our confidential information and are only allowed to disclose other data or information generated during the course of the research after we have been afforded an opportunity to consider whether patent and/or other proprietary protection should be sought. If we do not apply for patent protection prior to such publication or if we cannot otherwise maintain the confidentiality of our technology and other confidential information, then our ability to receive patent protection or protect our proprietary information may be jeopardized.
Third-party claims of intellectual property infringement would require us to spend significant time and money and could prevent us from developing or commercializing our products.
Our commercial success depends in part on not infringing the patents and proprietary rights of other parties and not breaching any licenses that we have entered into with regard to our technologies and products. Because others may have filed, and in the future are likely to file, patent applications covering products or other technologies of interest to us that are similar or identical to ours, patent applications or issued patents of others may have priority over our patent applications or issued patents. For example, we are aware of a family of third-party patent applications relating to prodrugs of gabapentin. We believe the applications have been abandoned in the United States, the European Patent Office, Canada, Australia and the United Kingdom. Additionally, we are aware of third-party patents relating to the use of baclofen in the treatment of GERD. If the patents are determined to be valid and construed to cover AP, the development and commercialization of AP could be affected. With respect to the claims contained in these patent applications and patents, we believe that our activities do not infringe the patents at issue and/or that the third-party patent or patent applications are invalid. However, it is possible that a judge or jury will disagree with our conclusions regarding non-infringement and/or invalidity, and we could incur substantial costs in litigation if we are required to defend against patent suits brought by third parties or if we initiate these suits. Any legal action against our collaborators or us claiming damages and seeking to enjoin commercial activities relating to the affected products and processes could, in addition to subjecting us to potential liability for damages, require our collaborators or us to obtain a license to continue to manufacture or market the affected products and processes. Licenses required under any of these patents may not be available on commercially acceptable terms, if at all. Failure to obtain such licenses could materially and adversely affect our ability to develop, commercialize and sell our product candidates. We believe that there may continue to be significant litigation in the biotechnology and pharmaceutical industry regarding patent and other intellectual property rights. If we become involved in litigation, it could consume a substantial portion of our management and financial resources and we may not prevail in any such litigation.
Furthermore, our commercial success will depend, in part, on our ability to develop additional product candidates in current indications of interest or opportunities in other indications. Some of these activities may involve the use of genes, gene products, screening technologies and other research tools that are covered by third-party patents. Court decisions have indicated that the exemption from patent infringement afforded by 35 U.S.C. § 271(e)(1) does not encompass all research and development activities associated with product development. In some instances, we may be required to obtain licenses to such third-party patents to conduct our development activities, including activities that may have already occurred. It is not known whether any license required under any of these patents would be made available on commercially acceptable terms, if at all. Failure to obtain such licenses could materially and adversely affect our ability to maintain a pipeline of potential product candidates and to bring new products to market. If we are required to defend against patent suits brought by third parties relating to third-party patents that may be relevant to our development activities, or if we initiate such suits, we could incur substantial costs in litigation. Moreover, an adverse result from any legal action in which we are involved could subject us to damages and/or prevent us from conducting some of our development activities.
43
If third parties do not manufacture our product candidates in sufficient quantities or at an acceptable cost, clinical development and commercialization of our product candidates would be delayed.
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of any of our products or product candidates. To date, we have relied on, and we expect to continue to rely on, a limited number of third-party compound manufacturers and active pharmaceutical ingredient, or API, formulators for the production of preclinical, clinical and commercial quantities of our product candidates. We do not have commercial supply agreements with any of these third parties, and our agreements with these parties are generally terminable at will by either party at any time. If, for any reason, these third parties are unable or unwilling to perform under our agreements or enter into new agreements, we may not be able to locate alternative manufacturers or formulators or enter into favorable agreements with them. Any inability to acquire sufficient quantities of our product candidates in a timely manner from these third parties could delay clinical trials and prevent us or our partners from developing and commercializing our product candidates in a cost-effective manner or on a timely basis.
Under the terms of our collaboration with GSK, GSK is solely responsible for the manufacture ofHorizant to support its development and commercialization within the United States. GSK is currently relying on a single source supplier for clinical supplies ofHorizant. If GSK fails to qualify alternative manufacturers ofHorizant, the current contract manufacturer terminates their agreement with GSK and GSK is otherwise unable to manufacture or contract to manufacture sufficient quantities ofHorizant, the development and commercialization ofHorizantcould be impaired or delayed. Under the terms of our collaboration with Astellas, Astellas is solely responsible for the manufacture of gabapentin enacarbil to support its development and commercialization within the Astellas territory. To our knowledge, Astellas is currently relying on a single source supplier for clinical supplies of gabapentin enacarbil. As a result, if Astellas fails to manufacture or contract to manufacture sufficient quantities of gabapentin enacarbil, development and commercialization of gabapentin enacarbil could be impaired or delayed in the Astellas territory. If we pursue development with respect to the rights we maintain on Horizant/gabapentin enacarbil, we will need to obtain clinical supplies from GSK or another supplier. As a result, if we are unable to obtain sufficient quantities of Horizant/gabapentin enacarbil from GSK at prices that are commercially attractive, and if we are unable to qualify an alternative supplier, it could delay the development of, and impair our ability to commercialize, this product candidate.
We rely on two suppliers of R-baclofen, the active agent used to make AP, under purchase orders issued from time to time. In the event that both suppliers determine to not sell R-baclofen to us at a price that is commercially attractive, and if we were unable to qualify an alternative supplier of R-baclofen, this could delay the development of, and impair our ability to commercialize, this product candidate.
We rely on a single source supplier of our current worldwide requirements of AP in API form under a manufacturing services and product supply agreement. Our current agreement with this supplier does not provide for the entire supply of the API necessary for our Phase 2 and Phase 3 clinical trials or for full-scale commercialization. In the event that the parties cannot agree to the terms and conditions for this supplier to provide some or all of our API clinical and commercial supply needs, we would not be able to manufacture API until a qualified alternative supplier is identified, which could also delay the development of, and impair our ability to commercialize, this product candidate.
We rely on a single source supplier of AP formulated in sustained-release tablets for future clinical trials at specified transfer prices under quotations agreed upon by the parties as a part of a master services agreement. In the event that such supplier terminates the agreement under specified circumstances, we would not be able to commercialize AP sustained-release tablets until an alternative supplier is qualified. This could delay the development of, and impair our ability to commercialize, AP.
We rely on a single source supplier of L-Dopa, which is used to make XP21279, under purchase orders issued from time to time. We are aware of several alternative suppliers of L-Dopa, and we believe at least one alternative manufacturer could potentially supply L-Dopa in the event that our supplier determines to not sell L-Dopa to us at a price that is commercially attractive. If we were unable to qualify an alternative supplier of L-Dopa, this could delay the development of, and impair our ability to commercialize, XP21279.
44
We rely on a single source supplier of XP21279 in API form under a manufacturing services and product supply agreement. In the event that such supplier terminates the agreement under specified circumstances, we would not be able to manufacture API until a qualified alternative supplier is identified, which could also delay the development of, and impair our ability to commercialize, this product candidate.
We have purchased XP21279 formulated in sustained-release tablets from a single source supplier at specified transfer prices under quotations agreed upon by the parties as part of a master services agreement. We have recently qualified another supplier for the manufacture of XP21279 with carbidopa bi-layer tablets to be supplied under quotations agreed upon by the parties as part of a master services agreement. In the event that either supplier terminates its agreement under specified circumstances for the manufacture of XP21279 sustained-release tablets or carbidopa bi-layer tablets, we would not be able to manufacture XP21279 until an alternative supplier is qualified. This could delay the development of, and impair our ability to commercialize, XP21279.
If we are required to obtain alternate third-party manufacturers, it could delay or prevent the clinical development and commercialization of our product candidates.
We may not be able to maintain or renew our existing or any other third-party manufacturing arrangements on acceptable terms, if at all. If we are unable to continue relationships with GSK for supplies of Horizant/gabapentin enacarbil, or our other suppliers for AP and XP21279, or to continue relationships at an acceptable cost or if these suppliers fail to meet our requirements for these product candidates for any reason, we would be required to obtain alternative suppliers. Any inability to obtain qualified alternative suppliers, including an inability to obtain, or delay in obtaining, approval of an alternative supplier from the FDA, would delay or prevent the clinical development and commercialization of these product candidates.
Use of third-party manufacturers may increase the risk that we or our partners will not have adequate supplies of our product candidates.
Our current reliance, and our and our partners’ anticipated future reliance, on third-party manufacturers will expose us and our partners to risks that could result in higher costs or lost product revenues or delay or prevent:
| | • | | the initiation or completion of clinical trials by us or our partners; |
| | • | | the submission of applications for regulatory approvals; and |
| | • | | the approval of our products by the FDA or foreign regulatory authorities or the commercialization of our products. |
In particular, our or our partner’s contract manufacturers:
| | • | | could encounter difficulties in achieving volume production, quality control and quality assurance or suffer shortages of qualified personnel, which could result in their inability to manufacture sufficient quantities of drugs to meet clinical schedules or to commercialize our product candidates; |
| | • | | could terminate or choose not to renew manufacturing agreements, based on their own business priorities, at a time that is costly or inconvenient for us or our partners; |
| | • | | could fail to establish and follow FDA-mandated current good manufacturing practices, or cGMPs, which are required for FDA approval of our product candidates, or fail to document their adherence to cGMPs, either of which could lead to significant delays in the availability of material for clinical study, delay or prevent marketing approval for our product candidates or require costly recalls of products already having received approval; |
| | • | | could encounter financial difficulties that would interfere with their obligations to supply our product candidates; and |
| | • | | could breach, or fail to perform as agreed under, manufacturing agreements. |
45
If we or our partners are not able to obtain adequate supplies of our product candidates, it will be more difficult to develop our product candidates and compete effectively. Our product candidates and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities.
In addition, the manufacturing facilities of certain of our suppliers are located outside of the United States. This may give rise to difficulties in importing our product candidates or their components into the United States or other countries as a result of, among other things, regulatory agency import inspections, incomplete or inaccurate import documentation or defective packaging.
Safety issues with the parent drugs or other components of our product candidates, or with approved products of third parties that are similar to our product candidates, could give rise to delays in the regulatory approval process, restrictions on labeling or product withdrawal.
Discovery of previously unknown problems, or increased focus on a known problem, with an approved product may result in restrictions on its permissible uses, including withdrawal of the medicine from the market. Although gabapentin, baclofen (which includes the R-isomer of baclofen) and L-Dopa, the parent drugs of Horizant/gabapentin enacarbil, AP and XP21279, respectively, have been used successfully in patients for many years, newly observed toxicities or worsening of known toxicities, in preclinical studies of, or patients receiving, gabapentin, baclofen and L-Dopa, or reconsideration of known toxicities of gabapentin, baclofen or L-Dopa in the setting of new indications, could result in increased regulatory scrutiny of Horizant/gabapentin enacarbil, AP and XP21279, respectively. The FDA has substantial discretion in the NDA approval process and may refuse to approve any application if the FDA concludes that the risk/benefit analysis of a potential drug treatment for a specific indication does not warrant approval. For example, in February 2010, safety concerns related to a preclinical finding of pancreatic acinar cell tumors in rats precluded FDA approval of theHorizantNDA in RLS in its form at that time. Although there were similar findings of rat pancreatic acinar cell tumors following treatment with gabapentin, the parent drug ofHorizant, the FDA has, to date, not prevented the use of gabapentin. In the February 2010 Complete Response letter, the FDA noted that they had concluded that the seriousness and severity of refractory epilepsy and the benefit to patients provided by gabapentin justified the potential risk at that time. Thus, although the parent drug for one of our product candidates may be approved by the FDA in a particular indication, the FDA may conclude that our product candidate’s risk/benefit profile does not warrant approval in a different indication, and the FDA may refuse to approve our product candidate. Such conclusion and refusal would prevent us from developing and commercializing our product candidates and severely harm our business and financial condition.
As a further example, the label for baclofen, the R-isomer of which is the parent drug of AP, includes a warning that hallucinations and seizures have occurred on abrupt withdrawal of baclofen dosing without proper tapering in spasticity patients. Although the FDA has approved baclofen for the treatment of spasticity from MS, we do not know how the FDA would consider the risk/benefit analysis for AP as a potential adjunctive treatment of GERD. The FDA may conclude that AP’s risk/benefit profile does not warrant approval in adjunctive GERD treatment and refuse to approve AP for this indication.
Our product candidates are engineered to be broken down by the body’s natural metabolic processes and to release the parent drug and other substances. While these breakdown products are generally regarded as safe, it is possible that there could be unexpected toxicity associated with these breakdown products that will cause any or all of Horizant/gabapentin enacarbil, AP and XP21279 to be poorly tolerated by, or toxic to, humans. Any unexpected toxicity of, or suboptimal tolerance to, our Transported Prodrugs would delay or prevent commercialization of these product candidates.
Additionally, problems with approved products marketed by third parties that utilize the same therapeutic target or that belong to the same therapeutic class as the parent drug of our product candidates could adversely affect the development of our product candidates. For example, the product withdrawals of Vioxx from Merck & Co., Inc. and Bextra from Pfizer in 2005 due to safety issues have caused other drugs that have the same therapeutic target, such as Celebrex from Pfizer, to receive additional scrutiny from regulatory authorities. If either gabapentin or pregabalin, drugs from Pfizer that are marketed as Neurontin and Lyrica, respectively,
46
encounters unexpected toxicity problems in humans, the FDA may delay or prevent the regulatory approval ofHorizantsince it is believed to share the same therapeutic target as gabapentin and pregabalin. In 2005, the FDA requested that all makers of epilepsy drugs analyze their clinical trial data to determine whether these drugs increase the risk of suicide in patients. In December 2008, the FDA added warnings to 11 antiepileptic drugs, including gabapentin, regarding an increased risk of suicide or suicidal thoughts. In April 2009, the FDA approved safety label changes for all approved antiepileptic drugs, except those indicated only for short-term use, to include a warning about an increased risk of suicidal thoughts or actions.Horizant, as a compound that is believed to share the same therapeutic target as gabapentin and pregabalin, would, if approved by the FDA, require a similar warning in its label. In September 2010, the FDA released draft guidance recommending that prospective suicidality assessments be performed in clinical trials of any drug with central nervous system activity. We expect that the FDA will follow this guidance, and we will be required to perform suicidality assessments in all of our clinical trials, including Phase 1 trials, of any of our product candidates with central nervous system activity. Finally, if the FDA determines that a drug may present a risk of substance abuse, it can recommend to the DEA that the drug be scheduled under the Controlled Substances Act. While gabapentin is not a scheduled drug at the present time, pregabalin has been scheduled as a controlled substance. Since pregabalin is a scheduled drug, it is possible that the FDA may require additional testing ofHorizant, the results of which could lead the FDA to conclude thatHorizantshould be scheduled as well. Scheduled substances are subject to DEA regulations relating to manufacturing, storage, distribution and physician prescription procedures, and the DEA regulates the amount of a scheduled substance that is available for clinical trials and commercial distribution. Accordingly, any scheduling action that the FDA or DEA may take with respect toHorizantmay delay its clinical trial and approval process. Any failure or delay in commencing or completing clinical trials or obtaining regulatory approvals for our product candidates would delay commercialization of our product candidates, and severely harm our business and financial condition.
We may not be successful in our efforts to develop additional Transported Prodrug candidates.
An important element of our strategy is to develop and commercialize Transported Prodrugs that improve upon the absorption, distribution and/or metabolism of drugs that have already received regulatory approval. Programs to develop and commercialize new product candidates require substantial technical, financial and human resources. These programs may initially show promise with respect to potential product candidates, yet fail to yield product candidates for clinical development for a number of reasons, including:
| | • | | the methodology used may not be successful in identifying potential product candidates for development; or |
| | • | | potential product candidates may, on further study, be shown to have inadequate efficacy, harmful side effects, suboptimal pharmaceutical profile or other characteristics suggesting that they are unlikely to be effective products. |
As part of our restructuring in March 2010, we eliminated our discovery research department, which will prevent our ability to discover additional product candidates at this time. If we are unable to develop suitable product candidates from our current preclinical pipeline, we may pursue additional product candidates through in-licensing. Any growth through in-licensing would depend upon the availability of suitable product candidates at favorable prices and upon advantageous terms and conditions. To obtain additional product candidates, we may also reconstitute our discovery research department, which would require the expenditure of significant resources and the identification and hiring of a number of highly-skilled employees. Such efforts could divert the time and resources from the later-stage development or commercialization of our product candidates.
If we are unable to develop or obtain suitable product candidates, we will not be able to increase our revenues in future periods, which could result in significant harm to our financial position and adversely impact our stock price.
47
Our product candidates, even if they receive marketing approval, will remain subject to ongoing regulatory review. If we or our collaborative partners fail to comply with continuing regulations, these approvals could be rescinded and the sale of our products could be suspended.
Even if we or our collaborative partners receive regulatory approval to market another product candidate, the approval could be conditioned on conducting additional, costly, post-approval studies, implementing a risk evaluation and mitigation strategy or could limit the indicated uses included in the labeling. Moreover, the product may later cause adverse effects that limit or prevent its widespread use, force us or our collaborative partners to withdraw it from the market or impede or delay our or our collaborative partners’ ability to obtain regulatory approvals in additional countries or indications. In addition, the manufacturer of the product and its facilities will continue to be subject to FDA review and periodic inspections to ensure adherence to applicable regulations. After receiving marketing approval, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record keeping related to the product will remain subject to extensive regulatory requirements.
If we or our collaborative partners fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities or previously unknown problems with our products, manufacturers or manufacturing processes are discovered, we and our partners could be subject to administrative or judicially imposed sanctions, including:
| | • | | restrictions on the products, manufacturers or manufacturing processes; |
| | • | | civil or criminal penalties or fines; |
| | • | | product seizures, detentions or import bans; |
| | • | | voluntary or mandatory product recalls and publicity requirements; |
| | • | | suspension or withdrawal of regulatory approvals; |
| | • | | total or partial suspension of production; and |
| | • | | refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications. |
Because we have a number of product candidates and are considering a variety of target indications, we may expend our limited resources to pursue a particular candidate or indication and fail to capitalize on candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we must focus on product candidates for the specific indications that we believe are the most promising. As a result, we may forego or delay pursuit of opportunities with other product candidates or other indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. In addition, we may spend valuable time and managerial and financial resources on product candidates for specific indications that ultimately do not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in situations where it would have been more advantageous for us to retain sole rights to development and commercialization.
48
The commercial success of any products that we or our partners may develop will depend upon the degree of market acceptance among physicians, patients, healthcare payors and the medical community.
Any products that result from our product candidates may not gain market acceptance among physicians, patients, healthcare payors and the medical community. If these products do not achieve an adequate level of acceptance, we may not generate material product revenues and we may not become profitable. The degree of market acceptance of any products resulting from our product candidates will depend on a number of factors, including:
| | • | | demonstration of efficacy and safety in clinical trials; |
| | • | | the prevalence and severity of any side effects; |
| | • | | potential or perceived advantages over alternative treatments; |
| | • | | perceptions about the relationship or similarity between our product candidates and the parent drug upon which each Transported Prodrug candidate is based; |
| | • | | the timing of market entry relative to competitive treatments; |
| | • | | the ability to offer product candidates for sale at competitive prices; |
| | • | | relative convenience and ease of administration; |
| | • | | the strength of marketing and distribution support; |
| | • | | sufficient third-party coverage or reimbursement; and |
| | • | | the product labeling or product insert required by the FDA or regulatory authorities in other countries. |
If we are unable to establish sales and marketing capabilities or enter into additional agreements with third parties to market and sell our product candidates, we may be unable to generate product revenues.
We have a limited sales and marketing organization and have limited experience in the sales, marketing and distribution of pharmaceutical products. There are risks involved with establishing our own sales and marketing capabilities, as well as entering into arrangements with third parties to perform these services. Developing an internal sales force is expensive and time-consuming. On the other hand, if we enter into arrangements with third parties to perform sales, marketing and distribution services, as we have for Horizant/gabapentin enacarbil, our product revenues will be lower than if we market and sell any products that we develop ourselves.
In the United States, if we receive approval from the FDA of an NDA forHorizant, until such time, if any, as we exercise the right to revert to a net sales royalty, we will share marketing and commercialization costs that exceed $10.0 million and share operating profits from net sales ofHorizant, if any. IfHorizantis approved, we may establish our own specialty sales force to sell and market our products.
Factors that may inhibit our efforts to commercialize our products after approval include:
| | • | | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| | • | | the inability of sales personnel to obtain access to adequate numbers of physicians to provide information on the advantages and risks of prescribing our products; |
| | • | | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage compared to companies with more extensive product lines; and |
| | • | | unforeseen costs and expenses associated with creating an independent sales and marketing organization. |
Because of the numerous risks and uncertainties involved with establishing our own sales and marketing capabilities, we are unable to predict when, or if, we will establish our own sales and marketing capabilities. If we are not successful in recruiting sales and marketing personnel or in building a sales and marketing infrastructure, we will have difficulty commercializing our product candidates, which would adversely affect our business and financial condition.
49
Our ability to generate revenue from any products that we may develop will depend on reimbursement and drug pricing policies and regulations.
Many patients may be unable to pay for any products that we or our collaborative partners may develop. In the United States, many patients will rely on Medicare, Medicaid, private health insurers and other third-party payors to pay for their medical needs. Our and our partners’ ability to achieve acceptable levels of reimbursement for drug treatments by governmental authorities, private health insurers and other organizations will have an effect on our and our partners’ ability to successfully commercialize, and attract additional collaborators to invest in the development of, our product candidates. We cannot be sure that reimbursement in the United States, Europe or elsewhere will be available for any products that we or our partners may develop, and any reimbursement that may become available may be decreased or eliminated in the future. Third-party payors increasingly are challenging prices charged for medical products and services, and many third-party payors may refuse to provide reimbursement for particular drugs when an equivalent generic drug is available. Although we believe any products that may result from our product candidates represent an improvement over the parent drugs upon which they are based and should be considered unique and not subject to substitution by a generic parent drug, it is possible that a third-party payor may consider our product candidate and the generic parent drug as equivalents and only offer to reimburse patients for the generic drug. Even if we show improved efficacy or improved convenience of administration with our product candidate, pricing of the existing parent drug may limit the amount we will be able to charge for our product candidate. If reimbursement is not available or is available only at limited levels, we or our partners may not be able to successfully commercialize our product candidates, and may not be able to obtain a satisfactory financial return on such products.
The trend toward managed healthcare in the United States and the changes in health insurance programs, as well as the recently enacted healthcare reform bill, may result in lower prices for pharmaceutical products, including any products that may result from our product candidates. In addition, any future regulatory changes regarding the healthcare industry or third-party coverage and reimbursement may affect demand for any products that we may develop and could harm our sales and profitability.
Pursuant to the Medicare Prescription Drug Improvement and Modernization Act of 2003, or the 2003 Medicare Modernization Act, Medicare beneficiaries are eligible to obtain subsidized prescription drug coverage from a choice of private sector plans. Approximately 90 percent of Medicare beneficiaries now have coverage for prescription medicines. The use of pharmaceuticals has increased slightly among some patients as the result of the expanded access to medicines afforded by coverage under Medicare. However, such expanded utilization has been largely offset by increased pricing pressure and competition due to the enhanced purchasing power of the private sector plans that negotiate on behalf of Medicare beneficiaries and by an increase in the use of generic medicines in this population. In addition, legislative changes have been proposed to mandate government rebates in Medicare and to allow the federal government to directly negotiate prices with pharmaceutical manufacturers. If legislation were enacted to mandate rebates or provide for direct government negotiation in Medicare prescription drug benefits, access and reimbursement for our product candidates upon commercialization could be restricted.
Current healthcare laws and regulations and future legislative or regulatory reforms to the healthcare system may affect our ability to profitably sell any products that we may develop.
The United States and some foreign jurisdictions are considering, or have enacted, a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
50
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively, PPACA, became law in the United States. PPACA substantially changes the way healthcare is financed by both governmental and private insurers and significantly affects the pharmaceutical industry. Among the provisions of PPACA of greatest importance to the pharmaceutical industry are the following:
| | • | | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents, apportioned among these entities according to their market share in certain government healthcare programs, beginning in 2011; |
| | • | | an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program, retroactive to January 1, 2010, to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; |
| | • | | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during their overage gap period, as a condition for the manufacturer’s outpatient drugs to be covered under Medicare Part D, beginning in 2011; |
| | • | | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, effective March 23, 2010; |
| | • | | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals beginning in April 2010 and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the Federal Poverty Level beginning in 2014, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| | • | | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program, effective in January 2010; |
| | • | | new requirements to report certain financial arrangements with physicians, including reporting any “transfer of value” made or distributed to prescribers and other healthcare providers, effective March 30, 2013, and reporting any investment interests held by physicians and their immediate family members during the preceding calendar year; |
| | • | | a new requirement to annually report drug samples that manufacturers and distributors provide to physicians, effective April 1, 2012; |
| | • | | a licensure framework for follow-on biologic products; and |
| | • | | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
We anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for any approved product, and could seriously harm our business. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors.
We also cannot be certain that any products that may result from our product candidates will successfully be placed on the list of drugs covered by particular health plan formularies, nor can we predict the negotiated price for such products, which will be determined by market factors. Many states have also created preferred drug lists and include drugs on those lists only when the manufacturers agree to pay a supplemental rebate. If the products that may result from our product candidates are not included on these preferred drug lists, physicians may not be inclined to prescribe it to their Medicaid patients, thereby diminishing the potential market for such products.
If our competitors are able to develop and market products that are more effective, safer or less costly than any products that we may develop, our commercial opportunity will be reduced or eliminated.
We face competition from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies and private and public research institutions. Our commercial
51
opportunity will be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects or are less expensive than any products that we may develop. In addition, significant delays in the development of our product candidates could allow our competitors to bring products to market before us and impair our ability to effectively commercialize our product candidates.
Products that we believe could compete withHorizantin the United States include the following drugs approved for the treatment of RLS: Mirapex (pramipexole) from Boehringer Ingelheim GmbH; generic pramipexole that is marketed by, among others, Teva Pharmaceuticals Industries, Ltd., Norvartis AG and Watson Pharmaceuticals, Inc.; Requip (ropinirole) from GSK; and generic ropinirole that is marketed by, among others, CorePharma, LLC, Mylan Pharmaceuticals Inc. and Wockhardt USA LLC. In addition, we could experience competition from Neupro (the rotigotine transdermal system), a dopamine agonist patch from UCB, which filed its NDA for the treatment of RLS with the FDA in 2007, and in April 2010, the FDA provided UCB a complete response letter that recommended reformulation of Neupro before making it available in the U.S. market for the treatment of restless legs syndrome. We anticipate that, if gabapentin enacarbil is approved in Japan for the treatment of restless legs syndrome, it will compete with pramipexole, which was approved in 2010 for the treatment of restless legs syndrome. Products that we believe could compete withHorizantfor the treatment of neuropathic pain include drugs that act on the same target asHorizant, such as Lyrica (pregabalin) and Neurontin (gabapentin) from Pfizer, and generic gabapentin that is marketed by Alpharma Inc., IVAX Corp, Pfizer and Teva, among others. Competition forHorizantcould also include Cymbalta (duloxetine) from Eli Lilly and Company, which is approved for the management of DPN. In addition, in January 2011, the FDA approved a once-daily formulation of gabapentin (known as Gralise from Depomed, Inc. and its partner for pain indications, Abbott Laboratories) for the treatment of PHN. We believe that AP, our product candidate that is a Transported Prodrug of R-baclofen, could experience competition from several generic drugs approved for the treatment of spasticity, including racemic baclofen, diazepam, dantrolene sodium and tizanidine. In addition, the FDA has approved Botox (onabotulinumtoxin A) from Allergan Inc. to treat spasticity in the flexor muscles of the elbow, wrist and fingers in adults. A therapy in development for the treatment of spasticity is IPX056, an extended-release formulation of baclofen, from Impax Laboratories, Inc. Products that could compete with AP in the GERD therapeutic area include: Protonix (pantoprazole sodium) from Pfizer; Prevacid (lansoprazole) from Takeda Pharmaceutical Company Limited; Nexium (esomeprazole) and Prilosec (omeprazole) from AstraZeneca Pharmaceuticals LP; Aciphex (rabeprazole) from Eisai/Johnson & Johnson; and generic H2 receptor antagonists such as cimetidine, ranitidine, famotidine and nizatidine. Products that could compete with XP21279, our product candidate that is a Transported Prodrug of L-Dopa, include: generic L-Dopa/carbidopa drugs and other drugs approved for the treatment of Parkinson’s disease, including Stalevo, a combination therapy of L-Dopa/carbidopa/entacapone that is marketed in the United States by Novartis; dopamine agonists such as Mirapex (pramipexole) and Requip (ropinirole) which are marketed by Boehringer-Ingelheim and GSK respectively; as well as generic dopamine agonists, including pramipexole that is marketed by, among others, Teva, Norvartis and Watson and ropinirole that is marketed by, among others, CorePharma, Mylan and Wockhardt. In addition, we could also experience future competition from the rotigotine transdermal system (a dopamine agonist patch known as Neupro by UCB) that was previously approved for the treatment of Parkinson’s disease, but in April 2010, the FDA provided UCB a complete response letter that recommended reformulation of Neupro before making it available in the U.S. market for the treatment of Parkinson’s disease. Other therapies under development in the United States include L-Dopa/carbidopa formulations. For example, IPX066 from Impax, an extended-release formulation of L-Dopa/carbidopa, has completed a Phase 3 trial. There may be other compounds of which we are not aware that are at an earlier stage of development and may compete with our product candidates. If any of those compounds are successfully developed and approved, they could compete directly with our product candidates.
Many of our competitors have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Established pharmaceutical companies may invest heavily to quickly discover and develop novel compounds that could make our product candidates obsolete. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. In addition, these third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical
52
trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA approval or discovering, developing and commercializing medicines before we do. We are also aware of other companies that may currently be engaged in the discovery of medicines that will compete with the product candidates that we are developing. In addition, in the markets that we are targeting, we expect to compete against current market-leading medicines. If we are not able to compete effectively against our current and future competitors, our business will not grow and our financial condition will suffer.
Off-label sale or use of generic gabapentin products could decrease sales of Horizant/gabapentin enacarbil and could lead to pricing pressure if such products become available at competitive prices and in dosages that are appropriate for the indications for which we or our collaborative partners are developing Horizant/gabapentin enacarbil.
Physicians are permitted to prescribe legally available drugs for uses that are not described in the drug’s labeling and that differ from those uses tested and approved by the FDA. The occurrence of such off-label uses could significantly reduce our or our partners’ ability to market and sell any other products that we or our partners may develop.
We believe that in all countries in which we hold or have licensed rights to patents or patent applications related to Horizant/gabapentin enacarbil, the composition-of-matter patents relating to gabapentin have expired. Off-label prescriptions written for gabapentin for indications for which we or our partners are developing Horizant/gabapentin enacarbil could adversely affect our ability to generate revenue from the sale of Horizant/gabapentin enacarbil, if approved for commercial sale in such indications. This could result in reduced sales and increased pricing pressure on Horizant/gabapentin enacarbil, if approved in such indications, which in turn would reduce our ability to generate revenue and have a negative impact on our results of operations.
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop or commercialize our product candidates.
Our success depends on our continued ability to attract, retain and motivate highly qualified management, clinical and scientific personnel and on our ability to develop and maintain important relationships with leading clinicians. If we are not able to retain Drs. Ronald Barrett, Kenneth Cundy and David Savello, we may not be able to successfully develop or commercialize our product candidates. Competition for experienced scientists and development staff may limit our ability to hire and retain highly qualified personnel on acceptable terms. In addition, none of our employees have employment commitments for any fixed period of time and could leave our employment at will. We do not carry “key person” insurance covering members of senior management or key scientific personnel. If we fail to identify, attract and retain qualified personnel, we may be unable to continue our development and commercialization activities.
We will need to hire additional employees in order to commercialize our product candidates. Any inability to manage future growth could harm our ability to commercialize our product candidates, increase our costs and adversely impact our ability to compete effectively.
In order to commercialize our product candidates, we will need to expand the number of our managerial, operational, financial and other employees. Because the projected timeframe of hiring these additional employees depends on the development status of our product candidates and because of the numerous risks and uncertainties associated with drug development, we are unable to project when we will hire these additional employees. The competition for qualified personnel in the pharmaceutical and biotechnology field is intense, and we may experience difficulties in recruiting, hiring and retaining qualified individuals.
Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. Our future financial performance and our ability to commercialize our product candidates and compete effectively will depend, in part, on our ability to manage any future growth effectively.
53
If product liability lawsuits are brought against us, we will incur substantial liabilities and may be required to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop. If we cannot successfully defend ourselves against claims that our product candidates or products that we successfully develop caused injuries, we will incur substantial liabilities.
Regardless of merit or eventual outcome, liability claims may result in:
| | • | | decreased demand for any product candidates or products that we may develop; |
| | • | | injury to our reputation; |
| | • | | withdrawal of clinical trial participants; |
| | • | | costs to defend the related litigation; |
| | • | | substantial monetary awards to clinical trial participants or patients; |
| | • | | the inability to commercialize any products that we may develop. |
We have product liability insurance that covers our clinical trials up to a $10.0 million annual aggregate limit. We intend to expand our insurance coverage to include the sale of commercial products if marketing approval is obtained for any products that we may develop. Insurance coverage is increasingly expensive, and we may not be able to maintain insurance coverage at a reasonable cost and we may not be able to obtain insurance coverage that will be adequate to satisfy any liability that may arise.
If we use biological and hazardous materials in a manner that causes contamination or injury or violates laws, we may be liable for damages.
Our development activities involve the use of potentially harmful biological materials as well as hazardous materials, chemicals and various radioactive compounds. We cannot completely eliminate the risk of accidental contamination or injury from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for damages that result, and any liability could exceed our resources. We, the third parties that conduct clinical trials on our behalf and the third parties that manufacture our product candidates are subject to federal, state and local laws and regulations governing the use, storage, handling and disposal of these materials and waste products. The cost of compliance with these laws and regulations could be significant. The failure to comply with these laws and regulations could result in significant fines and work stoppages and may harm our business.
Our facility is located in California’s Silicon Valley, in an area with a long history of industrial activity and use of hazardous substances, including chlorinated solvents. Environmental studies conducted prior to our leasing of the site found levels of metals and volatile organic compounds in the soils and groundwater at our site. While these constituents of concern predated our occupancy, certain environmental laws, including the U.S. Comprehensive, Environmental Response, Compensation and Liability Act of 1980, impose strict, joint and several liability on current operators of real property for the cost of removal or remediation of hazardous substances. These laws often impose liability even if the owner or operator did not know of, or was not responsible for, the release of such hazardous substances. As a result, while we have not been, we cannot rule out the possibility that we could in the future be held liable for costs to address contamination at the property beneath our facility, which costs could be material.
Our facility is located near known earthquake fault zones, and the occurrence of an earthquake, extremist attack or other catastrophic disaster could cause damage to our facilities and equipment, which could require us to cease or curtail operations.
Our facility is located near known earthquake fault zones and, therefore, is vulnerable to damage from earthquakes. In October 1989, a major earthquake struck this area and caused significant property damage and a
54
number of fatalities. We are also vulnerable to damage from other types of disasters, including power loss, attacks from extremist organizations, fire, floods and similar events. If any disaster were to occur, our ability to operate our business could be seriously impaired. We may not have adequate insurance to cover our losses resulting from disasters or other similar significant business interruptions, and we do not plan to purchase additional insurance to cover such losses due to the cost of obtaining such coverage. Any significant losses that are not recoverable under our insurance policies could seriously impair our business and financial condition.
Risks Related to Ownership of our Common Stock
Our stock price is volatile, and purchasers of our common stock could incur substantial losses.
The market prices for securities of biopharmaceutical companies in general have been highly volatile. The market price of our common stock may be influenced by many factors, including:
| | • | | announcement of FDA approvability, approval or non-approval of our product candidates, and the timing of the FDA review process; |
| | • | | adverse results or delays in our or our collaborative partners’ clinical trials; |
| | • | | the timing of achievement of our clinical, regulatory, partnering and other milestones, such as the commencement of clinical development, the completion of a clinical trial, the filing for regulatory approval or the establishment of commercial partnerships for one or more of our product candidates; |
| | • | | actions taken by regulatory agencies with respect to our product candidates, our clinical trials or our sales and marketing activities; |
| | • | | actions taken by regulatory agencies with respect to products or drug classes related to our product candidates; |
| | • | | the commercial success of any of our products approved by the FDA or its foreign counterparts; |
| | • | | developments in our relationships with GSK or Astellas, including the termination or modification of our respective agreements; |
| | • | | changes in our collaborators’ business strategies; |
| | • | | regulatory developments in the United States and foreign countries; |
| | • | | changes in the structure of healthcare payment systems; |
| | • | | any intellectual property matter involving us, including infringement lawsuits; |
| | • | | actions taken by regulatory agencies with respect to our or our partners’ compliance with regulatory requirements; |
| | • | | announcements of technological innovations or new products by us or our competitors; |
| | • | | market conditions for equity investments in general, or the biotechnology or pharmaceutical industries in particular; |
| | • | | changes in financial estimates or recommendations by securities analysts; |
| | • | | sales of large blocks of our common stock; |
| | • | | sales of our common stock by our executive officers, directors and significant stockholders; |
| | • | | restatements of our financial results and/or material weaknesses in our internal controls; and |
| | • | | the loss of any of our key scientific or management personnel. |
The stock markets in general and the markets for biotechnology stocks in particular, have experienced extreme volatility that has often been unrelated to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock. In the past, purported class action lawsuits have often been instituted against companies, including our company, whose securities have
55
experienced periods of volatility in market price. Any such litigation brought against us, including the purported securities class action lawsuit brought against us in July 2010, could result in substantial costs, which would hurt our financial condition and results of operations, divert management’s attention and resources and possibly delay our clinical trials or commercialization efforts.
We have been named a defendant in a purported securities class action lawsuit. This, and potential similar or related litigation, could result in substantial damages and may divert management’s time and attention from our business.
In July 2010, a purported securities class action lawsuit was filed in the United States District Court for the Northern District of California, naming us and certain of our officers and directors as defendants. The lawsuit alleges violations of the Securities Exchange Act of 1934, as amended, in connection with allegedly false, misleading and incomplete statements issued by us related toHorizant as a potential treatment of RLS, which allegedly made it impossible for investors to meaningfully understand the drug’s potential for FDA approval. The plaintiff alleges that we failed to disclose information regarding previous results in lab rats treated with gabapentin showing an increased risk of pancreatic acinar cell tumors, which plaintiff alleges presented a risk that the FDA would not approveHorizant/gabapentin enacarbil for the treatment of RLS. The plaintiff seeks damages, an award of its costs and injunctive and/or equitable relief on behalf of a purported class of stockholders who purchased our common stock during the period between May 5, 2009 and February 17, 2010. Another lawsuit was filed in September 2010 in the United States District Court for the Northern District of California making substantially similar allegations, on behalf of a purported class of stockholders who purchased our common stock during the period between March 16, 2009 and May 5, 2010. A motion to consolidate the complaints and appoint a lead plaintiff was granted in November 2010, and the lead plaintiff filed a consolidated complaint in January 2011. In February 2011, we responded to the complaint with a motion to dismiss. The lead plaintiff’s opposition brief to our motion is due on April 4, 2011. A hearing on the motion to dismiss is currently scheduled for May 20, 2011. It is possible that additional suits will be filed, or allegations received from stockholders, with respect to these same matters and also naming us and/or our officers and directors as defendants.
We believe that we have meritorious defenses and intend to defend the lawsuit vigorously. The lawsuit and any other related lawsuits are subject to inherent uncertainties, and the actual cost of defending the lawsuit will depend upon many unknown factors. The outcome of the litigation is necessarily uncertain, we could be forced to expend significant resources in the defense of the lawsuit and we may not prevail. Monitoring and defending against legal actions is time-consuming for our management and detracts from our ability to fully focus our internal resources on our business activities. In addition, we may incur substantial legal fees and costs in connection with the litigation. We are not able to estimate the possible cost to us from these matters, as this lawsuit is currently at an early stage and we cannot be certain how long it may take to resolve these matters or the possible amount of any damages that we may be required to pay. We have not established any reserves for any potential liability relating to the lawsuit. It is possible that we could, in the future, incur judgments or enter into settlements of claims for monetary damages. A decision adverse to our interests on the action could result in the payment of substantial damages, or possibly fines, and could have a material adverse effect on our cash flow, results of operations and financial position. In addition, the uncertainty of the pending litigation could lead to more volatility in our stock price.
Failure to maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could have a material adverse effect on our stock price.
Section 404 of the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the SEC require annual management assessments of the effectiveness of our internal control over financial reporting and a report by our independent registered public accounting firm attesting to, and reporting on, the effectiveness of our internal control over financial reporting. If we fail to maintain the adequacy of our internal control over financial reporting, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 and the related rules and regulations of the
56
SEC. If we cannot favorably assess, or our independent registered public accounting firm is unable to provide an unqualified attestation report on, the effectiveness of our internal control over financial reporting, investor confidence in the reliability of our financial reports may be adversely affected, which could have a material adverse effect on our stock price.
Fluctuations in our operating results could cause our stock price to decline.
The following factors are likely to result in fluctuations of our operating results from quarter to quarter and year to year:
| | • | | announcement of FDA approvability, approval or non-approval of our product candidates and the timing of the FDA review process; |
| | • | | adverse results or delays in our or our collaborative partners’ clinical trials; |
| | • | | the timing and achievement of our clinical, regulatory, partnering and other milestones, such as the commencement of clinical development, the completion of a clinical trial, the filing for regulatory approval or the establishment of a commercial partnership for one or more of our product candidates; |
| | • | | actions taken by regulatory agencies with respect to our product candidates, our clinical trials or our sales and marketing activities; |
| | • | | actions taken by regulatory agencies with respect to products or drug classes related to our product candidates; |
| | • | | the commercial success of any of our products approved by the FDA or its foreign counterparts; |
| | • | | developments in our relationships with GSK or Astellas, including the termination or modification of our respective agreements; |
| | • | | changes in our collaborators’ business strategies; |
| | • | | actions taken by regulatory agencies with respect to our or our partners’ compliance with regulatory requirements; |
| | • | | regulatory developments in the United States and foreign countries; |
| | • | | changes in the structure of healthcare payment systems; |
| | • | | litigation, including the purported securities class action lawsuit commenced against us and certain of our officers and directors; |
| | • | | any intellectual property matter involving us, including patent infringement lawsuits; and |
| | • | | announcements of technological innovations or new products by us or our competitors. |
Due to these fluctuations in our operating results, a period-to-period comparison of our results of operations may not be a good predictor of our future performance. For example, due to the recognition of revenues from up-front and milestone payments from our collaborations with Astellas, GSK and Xanodyne, we were profitable in the three-month periods ended June 30, September 30 and December 31, 2007, and for the year ended December 31, 2007. However, while recognition of these revenues resulted in a profitable year for 2007, we incurred net losses in 2008, 2009, 2010, and we expect to incur net losses in 2011. In any particular financial period, the actual or anticipated fluctuations could be below the expectations of securities analysts or investors and our stock price could decline.
Because a small number of existing stockholders own a large percentage of our voting stock, they may be able to exercise significant influence over our affairs, acting in their best interests and not necessarily those of other stockholders.
As of February 1, 2011, our executive officers, directors and holders of 5% or more of our outstanding common stock, based upon information known to us and derived from Schedules 13G filed with the SEC,
57
beneficially owned approximately 44.1% of our common stock. The interests of this group of stockholders may not always coincide with our interests or the interests of other stockholders. This concentration of ownership could also have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquiror from attempting to obtain control of us, which in turn could reduce the price of our common stock.
Our stockholder rights plan and anti-takeover provisions in our charter documents and under Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our amended and restated certificate of incorporation and bylaws may delay or prevent an acquisition of us, a change in our management or other changes that stockholders may consider favorable. These provisions include:
| | • | | a classified board of directors; |
| | • | | a prohibition on actions by our stockholders by written consent; |
| | • | | the ability of our board of directors to issue preferred stock without stockholder approval, which could be used to make it difficult for a third party to acquire us; |
| | • | | notice requirements for nominations for election to the board of directors; and |
| | • | | limitations on the removal of directors. |
Moreover, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
We have adopted a rights agreement under which certain stockholders have the right to purchase shares of a new series of preferred stock, at an exercise price of $140.00 per one one-hundredth of a share, if a person acquires more than 15% of our common stock. The rights plan could make it more difficult for a person to acquire a majority of our outstanding voting stock. The rights plan could also reduce the price that investors might be willing to pay for shares of our common stock and result in the market price being lower than it would be without the rights plan. In addition, the existence of the rights plan itself may deter a potential acquiror from acquiring us. As a result, either by operation of the rights plan or by its potential deterrent effect, mergers and acquisitions of us that our stockholders may consider in their best interests may not occur.
If there are large sales of our common stock, the market price of our common stock could drop substantially.
If our existing stockholders sell a large number of shares of our common stock or the public market perceives that existing stockholders might sell shares of our common stock, the market price of our common stock could decline significantly. As of February 1, 2011, we had 35,263,668 outstanding shares of common stock. Of these shares, up to 14,906,948 shares of common stock are tradable under Rule 144 under the Securities Act of 1933, as amended, or the Securities Act, subject to the volume limitations and manner of sale requirements under Rule 144, and the remainder of the shares outstanding as of February 1, 2011, have been registered under the Securities Act and are freely tradable. In addition, 1,136,398 shares are held by our directors and executive officers and their affiliates and will be subject to volume limitations and manner of sale requirements under Rule 144 under the Securities Act after the lock-up agreements pertaining to our December 2010 public common stock offering expire in March 2011.
Item 1B. Unresolved Staff Comments.
Not applicable.
58
We lease approximately 162,000 square feet of office and laboratory space in two adjacent buildings in Santa Clara, California. We conduct our operations in one of the buildings and the other is vacant. The leases expire concurrently in August 2013, although we have the option to extend both leases for two additional terms of five years each. The 2010 aggregate annual rental amount payable under the leases was approximately $5.5 million, subject to periodic increases. Although our facilities are adequate for our existing needs, we may require additional space as our business expands.
Item 3. Legal Proceedings.
In July 2010, a purported securities class action lawsuit was filed in the United States District Court for the Northern District of California, naming us and certain of our officers and directors as defendants. The lawsuit alleges violations of the Securities Exchange Act of 1934, as amended, in connection with allegedly false, misleading and incomplete statements issued by the defendants related to our product candidate,Horizant (gabapentin enacarbil) Extended-Release Tablets, as a potential treatment of moderate-to-severe primary restless legs syndrome, which allegedly made it impossible for investors to meaningfully understand the drug’s potential for U.S. Food and Drug Administration approval. The plaintiff seeks damages, an award of its costs and injunctive and/or equitable relief on behalf of a purported class of stockholders who purchased our common stock during the period between May 5, 2009 and February 17, 2010. Another lawsuit was filed in September 2010 in the United States District Court for the Northern District of California making substantially similar allegations, on behalf of a purported class of stockholders who purchased our common stock during the period between March 16, 2009 and May 5, 2010. In November 2010, a motion to consolidate the complaints and appoint a lead plaintiff was granted. In January 2011, the lead plaintiff filed a consolidated complaint. In February 2011, we responded to the complaint with a motion to dismiss. The lead plaintiff’s opposition brief to our motion is due on April 4, 2011. A hearing on the motion to dismiss is currently scheduled for May 20, 2011.
We believe that we have meritorious defenses and intend to defend the lawsuit vigorously. The lawsuit and any other related lawsuits are subject to inherent uncertainties, and the actual cost of defending the lawsuit will depend upon many unknown factors. The outcome of the litigation is necessarily uncertain, we could be forced to expend significant resources in the defense of the lawsuits, and we may not prevail.
From time to time, we may be involved in additional litigation relating to claims arising out of our ordinary course of business.
Item 4. (Removed and Reserved.)
59
PART II.
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Our common stock is traded on The NASDAQ Global Select Market under the symbol “XNPT.” As of February 1, 2011, there were approximately 85 holders of record of our common stock. No cash dividends have been paid on our common stock to date, and we intend to utilize any earnings for development of our business. The following table sets forth, for the periods indicated, the range of high and low intraday sales prices of our common stock as quoted on The NASDAQ Global Select Market for the two most recent fiscal years.
| | | | | | | | |
| | | High | | | Low | |
2010 | | | | | | | | |
4th Quarter | | $ | 9.33 | | | $ | 6.93 | |
3rd Quarter | | | 9.80 | | | | 5.66 | |
2nd Quarter | | | 11.82 | | | | 8.70 | |
1st Quarter | | | 20.97 | | | | 6.39 | |
| | |
2009 | | | | | | | | |
4th Quarter | | $ | 22.03 | | | $ | 15.13 | |
3rd Quarter | | | 25.42 | | | | 17.25 | |
2nd Quarter | | | 23.90 | | | | 13.36 | |
1st Quarter | | | 29.52 | | | | 17.45 | |
The closing price for our common stock as reported by The NASDAQ Global Select Market on February 1, 2011 was $7.74 per share.
Issuer Purchases of Equity Securities
None.
60
Performance Measurement Comparison(1)
The following graph shows the total stockholder return of an investment of $100 in cash on December 31, 2005 for: (i) our common stock; (ii) the NASDAQ Composite Index; and (iii) the NASDAQ Biotechnology Index for the five-year period ended December 31, 2010. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends; however, no dividends have been declared on our common stock to date. The stockholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
Comparison of Cumulative Total Return on Investment
| | (1) | This section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any filing of XenoPort under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing. |
61
Item 6. Selected Financial Data.
You should read the following selected financial data together with our audited financial statements and related notes and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other financial information included in this Annual Report on Form 10-K.
| | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | (In thousands, except per share amounts) | |
Statement of Operations Data: | | | | | | | | | | | | | | | | | | | | |
Revenues: | | | | | | | | | | | | | | | | | | | | |
Net revenue from unconsolidated joint operating activities | | $ | 1,364 | | | $ | 24,758 | | | $ | 28,981 | | | $ | 104,898 | | | $ | — | |
Collaboration revenue | | | 1,515 | | | | 9,515 | | | | 13,015 | | | | 8,924 | | | | 10,606 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenues | | | 2,879 | | | | 34,273 | | | | 41,996 | | | | 113,822 | | | | 10,606 | |
| | | | | | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | | | | | |
Research and development | | | 52,546 | | | | 70,747 | | | | 83,172 | | | | 74,397 | | | | 65,434 | |
Selling, general and administrative | | | 28,323 | | | | 31,807 | | | | 26,391 | | | | 18,755 | | | | 14,921 | |
Restructuring charges | | | 5,275 | | | | — | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total operating expenses | | | 86,144 | | | | 102,554 | | | | 109,563 | | | | 93,152 | | | | 80,355 | |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) from operations | | | (83,265 | ) | | | (68,281 | ) | | | (67,567 | ) | | | 20,670 | | | | (69,749 | ) |
Interest and other income | | | 796 | | | | 1,229 | | | | 4,640 | | | | 8,198 | | | | 5,634 | |
Interest and other expense | | | — | | | | (4 | ) | | | (19 | ) | | | (53 | ) | | | (198 | ) |
| | | | | | | | | | | | | | | | | | | | |
Income (loss) before income taxes | | | (82,469 | ) | | | (67,056 | ) | | | (62,946 | ) | | | 28,815 | | | | (64,313 | ) |
Income tax provision (benefit) | | | — | | | | (722 | ) | | | (406 | ) | | | 622 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Net income (loss) | | $ | (82,469 | ) | | $ | (66,334 | ) | | $ | (62,540 | ) | | $ | 28,193 | | | $ | (64,313 | ) |
| | | | | | | | | | | | | | | | | | | | |
Basic net income (loss) per share | | $ | (2.68 | ) | | $ | (2.31 | ) | | $ | (2.48 | ) | | $ | 1.14 | | | $ | (2.91 | ) |
| | | | | | | | | | | | | | | | | | | | |
Diluted net income (loss) per share | | $ | (2.68 | ) | | $ | (2.31 | ) | | $ | (2.48 | ) | | $ | 1.08 | | | $ | (2.91 | ) |
| | | | | | | | | | | | | | | | | | | | |
Shares used to compute basic net income (loss) per share | | | 30,813 | | | | 28,766 | | | | 25,180 | | | | 24,773 | | | | 22,101 | |
| | | | | | | | | | | | | | | | | | | | |
Shares used to compute diluted net income (loss) per share | | | 30,813 | | | | 28,766 | | | | 25,180 | | | | 25,992 | | | | 22,101 | |
| | | | | | | | | | | | | | | | | | | | |
Balance Sheet Data: | | | | | | | | | | | | | | | | | | | | |
Cash, cash equivalents and short-term investments | | $ | 108,595 | | | $ | 143,668 | | | $ | 152,783 | | | $ | 160,141 | | | $ | 118,854 | |
Working capital | | | 99,314 | | | | 131,749 | | | | 128,835 | | | | 138,685 | | | | 101,527 | |
Restricted investments | | | 1,948 | | | | 1,933 | | | | 1,824 | | | | 1,771 | | | | 1,699 | |
Total assets | | | 121,229 | | | | 160,212 | | | | 169,097 | | | | 172,877 | | | | 128,665 | |
Current portion of equipment financing obligations | | | — | | | | — | | | | — | | | | 176 | | | | 500 | |
Noncurrent portion of equipment financing obligations | | | — | | | | — | | | | — | | | | 5 | | | | 181 | |
Accumulated deficit | | | 387,406 | | | | 304,937 | | | | 238,603 | | | | 176,063 | | | | 204,256 | |
Total stockholders’ equity | | | 93,959 | | | | 127,276 | | | | 121,974 | | | | 125,537 | | | | 83,285 | |
62
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Overview
We are a biopharmaceutical company focused on developing and commercializing a portfolio of internally discovered product candidates that utilize the body’s natural nutrient transport mechanisms to improve the therapeutic benefits of existing drugs. Our innovative product candidates, which we refer to as Transported Prodrugs, are created by modifying the chemical structure of currently marketed drugs, referred to as parent drugs, and are designed to correct limitations in the oral absorption, distribution and/or metabolism of the parent drug. We intend to focus our development and commercialization efforts on potential treatments of diseases with significant unmet medical needs, with an emphasis on central nervous system, or CNS, disorders. Each of our product candidates is an orally available, patented or patentable new chemical entity that addresses large potential markets.
Our lead product candidate, gabapentin enacarbil (previously known as XP13512), is licensed to Astellas Pharma Inc. in Japan and five Asian countries and to Glaxo Group Limited, or GSK, in the United States. Astellas has filed a new drug application, or NDA, with the Pharmaceuticals and Medical Device Agency, or PMDA, for approval of gabapentin enacarbil as a treatment for restless legs syndrome in Japan. Restless legs syndrome is a neurological condition that causes an irresistible urge to move the legs. In November 2010, the U.S. Food and Drug Administration, or FDA, accepted for review GSK’s NDA resubmission for approval to market gabapentin enacarbil, known in the United States by the trade nameHorizant (gabapentin enacarbil) Extended-Release Tablets, for the treatment of moderate-to-severe primary restless legs syndrome, or RLS. The FDA has designated theHorizant NDA resubmission as a Class 2 response and set a new Prescription Drug User Fee Act, or PDUFA, date of April 6, 2011.
GSK is also evaluatingHorizant for the potential treatment of post-herpetic neuralgia, or PHN, a chronic type of neuropathic pain that can follow the resolution of shingles, andHorizant has successfully completed several Phase 2 clinical trials for the management of PHN in the United States. In addition, GSK evaluatedHorizant for the potential treatment of diabetic peripheral neuropathy, or DPN, and as a potential prophylactic therapy for migraine headaches.Horizant did not show statistically significant separation from placebo in the primary endpoints of these trials. GSK remains responsible for the development of Horizant for RLS and PHN in the United States; any further potential development of Horizant for other indications, including PHN to the extent that a product label would reflect a superiority claim, would be conducted by us.
We are evaluating our second product candidate, arbaclofen placarbil, or AP (and previously known as XP19986), as a potential treatment for patients with spasticity. We have successfully completed a Phase 2 clinical trial of AP as a potential treatment of spasticity in patients with spinal cord injury, and in September 2010, we announced our plans to move AP into Phase 3 development as a potential treatment of spasticity in multiple sclerosis, or MS, patients. Based on discussions with the FDA, we intend to conduct a multi-center, randomized, double-blind, placebo-controlled study designed to assess the efficacy and safety of AP as a treatment for spasticity in MS patients. Patients who complete this study would have the option to enter an extension study to evaluate the safety of AP in MS patients. Favorable results from these studies and preclinical and clinical pharmacology studies could lead to the filing of an NDA with the FDA under Section 505(b)(2) seeking approval of AP for the treatment of spasticity. The Section 505(b)(2) application would enable us to reference published literature and the FDA’s previous findings of safety and effectiveness for baclofen, a drug that has been approved by the FDA for the alleviation of signs and symptoms of spasticity resulting from MS. We intend to initiate this Phase 3 clinical program in the first half of 2011.
We are also evaluating AP for the potential adjunctive treatment of gastroesophageal reflux disease, or GERD, in patients who do not experience complete relief of GERD symptoms while being treated with proton pump inhibitors, or PPIs. We are conducting a multi-dose, randomized, placebo-controlled Phase 2b clinical trial to evaluate the efficacy and safety of AP in approximately 450 patients with GERD who are incomplete responders to PPIs. We have completed enrollment in this trial and anticipate reporting top-line results in the first quarter of 2011.
We are evaluating our third product candidate, XP21279, for the potential treatment of patients with Parkinson’s disease and are conducting a randomized, cross-over Phase 2 clinical trial of XP21279 in
63
Parkinson’s disease patients with motor fluctuations that is designed to compare the efficacy, safety and pharmacokinetics of individual patient-optimized doses of a new bi-layer tablet of XP21279/carbidopa to patient-optimized doses of Sinemet (L-Dopa/carbidopa). We anticipate reporting top-line results in the second half of 2011.
We have entered into development and commercialization agreements with Astellas and GSK. In December 2005, we entered into a collaboration with Astellas for the development and commercialization of gabapentin enacarbil, also known as ASP8825, pursuant to which we licensed to Astellas exclusive rights to develop and commercialize gabapentin enacarbil in Japan, Korea, the Philippines, Indonesia, Thailand and Taiwan. In February 2007, we entered into an exclusive collaboration with GSK to develop and commercialize gabapentin enacarbil, also known as GSK1838262, worldwide, excluding the Astellas territory. In November 2010, we amended and restated our collaboration agreement with GSK, pursuant to which we reacquired all rights to gabapentin enacarbil outside of the United States previously granted to GSK (which excludes the Astellas territory) and obtained the right to pursue development ofHorizant for: (i) the potential treatment of DPN; (ii) the potential treatment of PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug; and (iii) any additional indications in the United States. GSK remains responsible for seeking approval of the NDA for RLS in the United States, further development and regulatory matters with respect toHorizant for the potential treatment of PHN and commercialization ofHorizant in the United States for all indications. We plan to enter into additional agreements with pharmaceutical companies: (1) when access to a primary care physician sales force is necessary to maximize the commercial potential of our product candidates in the United States; (2) for the development and commercialization of our product candidates outside the United States; or (3) to develop and commercialize product candidates that fall outside our therapeutic areas of interest.
We believe that our existing capital resources and anticipated milestone payments, together with interest thereon, will be sufficient to meet our projected operating requirements into the fourth quarter of 2013.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments related to each of our critical accounting areas. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Revenue Recognition
We have current collaboration agreements with Astellas and GSK and a terminated collaboration agreement with Xanodyne Pharmaceuticals, Inc., each of which contains multiple elements. We account for these agreements in accordance with the provisions of theRevenue Recognition — Multiple-Element Arrangements andCollaborative Arrangements topics of the Financial Accounting Standards Board, or FASB, Accounting Standards Codification, or the Codification. We considered a variety of factors in determining the appropriate method of revenue recognition under these arrangements, such as whether the various elements can be considered separate units of accounting, whether there is objective and reliable evidence of fair value for these elements and whether there is a separate earnings process associated with a particular element of an agreement.
Where there are multiple deliverables combined as a single unit of accounting, revenues are deferred and recognized over the period that we remain obligated to perform services. The specific methodology for the recognition of the revenue (e.g., straight-line or according to specific performance criteria) is determined on a case-by-case basis according to the facts and circumstances applicable to a given agreement.
64
We account for our current revenue activities as follows:
Up-front, licensing-type fees. To date, these types of fees have been classified within the collaboration agreements as license fees, access fees, rights fees and initial licensing fees, and each of them was non-refundable and payable in connection with the execution of the contract. Up-front, licensing-type payments are assessed to determine whether or not the licensee is able to obtain any stand-alone value from the license. Where this is not the case, we do not consider the license deliverable to be a separate unit of accounting, and we defer the revenue with revenue recognition for the license fee being assessed in conjunction with the other deliverables that constitute the combined unit of accounting.
Milestones. We assess milestones on an individual basis and recognize revenue from these milestones when earned, as evidenced by acknowledgment from our collaborator, provided that (i) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement, (ii) the milestone represents the culmination, or progress towards the culmination, of an earnings process and (iii) the milestone payment is non-refundable. Milestones that are received after all substantive deliverables have occurred are considered to be bonus payments and are recognized when earned, assuming all of the other revenue recognition criteria are met. Where separate milestones do not meet these criteria, we use a performance-based model, with revenue recognition following delivery of effort as compared to an estimate of total expected effort.
Profit and loss sharing. This represents our share of the profits and losses from the co-promotion ofHorizant with GSK. Amounts are recognized in the period in which the related activities occur, and their financial statement classification is based on our assessment that these activities constitute part of our ongoing central operations.
Our current collaboration agreements also include potential payments for product royalties and detail reimbursements. To date, we have not received revenues from these activities.
Accrued Expenses
As part of the process of preparing financial statements, we are required to estimate accrued expenses. This process involves communicating with our applicable personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of actual cost. The majority of our service providers invoice us monthly in arrears for services performed. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us. We periodically confirm the accuracy of our estimates with selected service providers and make adjustments, if necessary. To date, we have not adjusted our estimate at any particular balance sheet date by any material amount. Examples of estimated accrued expenses include:
| | • | | fees paid to contract research organizations in connection with preclinical and toxicology studies and clinical trials; |
| | • | | fees paid to investigative sites in connection with clinical trials; |
| | • | | fees paid to contract manufacturers in connection with the production of clinical trial materials; and |
| | • | | professional service fees. |
We base our expenses related to clinical trials on our estimates of the services received and efforts expended pursuant to contracts with multiple research institutions and clinical research organizations that conduct and manage clinical trials on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If we do not identify costs that we have begun to incur or if we underestimate or overestimate the level of services performed or the costs of these services, our actual expenses could differ from our estimates.
65
Fair Value Measurements
We account for the fair value of our financial instruments in accordance with the provisions of theFair Value Measurements and Disclosures topic of the Codification. The carrying amounts of certain of our financial instruments, including cash equivalents and short-term investments, continue to be valued at fair value on a recurring basis.
As defined in theFair Value Measurements and Disclosures topic of the Codification, fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the measurement date. We utilize market data or assumptions that we believe market participants would use in pricing assets or liabilities, including assumptions about risk and the risks inherent in the inputs to the valuation technique. These inputs can be readily observable, market corroborated or generally unobservable. We apply the market approach valuation technique for fair value measurements on a recurring basis and attempt to maximize the use of observable inputs and minimize the use of unobservable inputs. All of our cash equivalents and short-term investments are valued using quoted prices in active markets and are valued at Level 1 or Level 2 within the fair value hierarchy.
Stock-Based Compensation
The provisions of theCompensation — Stock Compensationtopic of the Codification establish accounting for stock-based awards exchanged for employee services. In accordance with the topic, for stock options, awards and stock purchase rights granted under the 2005 Employee Stock Purchase Plan, or the ESPP, stock-based compensation cost is measured on the grant date, based on the fair value of the award, and is recognized as expense over the requisite employee service period.
We estimate the fair value of stock options and stock purchase rights using a Black-Scholes valuation model. The fair value of each option grant is estimated on the date of grant using the Black-Scholes option valuation model, and the resulting charge is expensed using the straight-line attribution method over the vesting period. Restricted stock units are measured at the fair value of our common stock on the date of grant and expensed over the period of vesting using the straight-line attribution approach. TheCompensation — Stock Compensationtopic of the Codification requires the use of option-pricing models that were not developed for use in valuing employee stock options. The Black-Scholes option-pricing model was developed for use in estimating the fair value of short-lived, exchange-traded options that have no vesting restrictions and are fully transferable. In addition, option-pricing models require the input of highly subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. Both the expected stock price volatility and the weighted-average expected life assumptions were determined using data obtained from similar entities, taking into consideration factors such as industry, stage of life cycle, size and financial leverage.
We account for stock compensation arrangements to non-employees in accordance with theEquity-Based Payments to Non-Employees topic of the Codification, using a fair value approach. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
Research and Development Expenses
Research and development expenses consisted of costs associated with both partnered and unpartnered research activities, as well as costs associated with our drug discovery efforts, conducting preclinical studies and clinical trials, manufacturing development efforts and activities related to regulatory filings. Research and development expenses are comprised of: external research and development expenses incurred under agreements with third-party contract research organizations and investigative sites, where a substantial portion of our preclinical studies and all of our clinical trials are conducted, with third-party manufacturing organizations, where a substantial portion of our preclinical supplies and all of our clinical supplies are produced, and with consultants; employee-related expenses, which include salaries and benefits; and facilities, depreciation and amortization and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, depreciation of leasehold improvements and equipment and laboratory and other supplies. We use our employee and infrastructure resources across multiple research projects, including our drug development programs. We do not allocate our employee and infrastructure costs on a project-by-project basis.
66
Our current portfolio of proprietary product candidates includes the product candidates summarized in the table below. The table summarizes those product candidates’ development initiatives, including the related stages of development for each product candidate in development and the direct, third-party research and development expenses recognized in connection with each product candidate. The information in the column labeled “Estimated Completion of Current Phase” is our current estimate of the timing of completion. The actual timing of completion could differ materially from the estimates provided in the table. For a discussion of the risks and uncertainties associated with the timing of completing a product development phase, see the “Our success depends substantially on our most advanced product candidates, which are still under development. If we or our collaborative partners are unable to bring any or all of these product candidates to market, or experience significant delays in doing so, our ability to generate product revenue and our likelihood of success will be reduced;” “If we or our partners are not able to obtain required regulatory approvals, we or our partners will not be able to commercialize our product candidates, our ability to generate revenue will be materially impaired and our business will not be successful;” “We depend on collaborations to complete the development and commercialization of some of our product candidates. These collaborations may place the development of our product candidates outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us;” “If we do not establish collaborations for our product candidates, we will have to alter our development and commercialization plans;” “If our preclinical studies do not produce successful results or our clinical trials do not demonstrate safety and efficacy in humans, we will not be able to commercialize our product candidates;” “Any failure or delay in commencing or completing clinical trials for our product candidates could severely harm our business;” “We rely on third parties to conduct our clinical trials. If these third parties do not perform as contractually required or expected, we may not be able to obtain regulatory approval for, or commercialize, our product candidates;” “If third parties do not manufacture our product candidates in sufficient quantities or at an acceptable cost, clinical development and commercialization of our product candidates would be delayed;” “If we are required to obtain alternate third-party manufacturers, it could delay or prevent the clinical development and commercialization of our product candidates;” and “Use of third-party manufacturers may increase the risk that we or our partners will not have adequate supplies of our product candidates” sections of “Risk Factors.”
| | | | | | | | | | | | | | | | | | | | | | | | |
Product Candidate | | Description | | | Phase of Development | | | Estimated
Completion of
Current Phase | | | Related R&D Expenses
Year Ended December 31, | |
| | | | | 2010 | | | 2009 | | | 2008 | |
| | | | | | | | | | | | (In thousands) | |
Preclinical and clinical development | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Gabapentin enacarbil* | |
| RLS/Restless
legs syndrome |
| |
| NDA resubmission
filed in the United
States/NDA filed
in Japan |
| |
| PDUFA
date of
April 6,
2011/second
half of 2011 |
| | $ | 310 | | | $ | 2,988 | | | $ | 8,856 | |
| | | | | | |
AP** | | | Spasticity | | | | Phase 2 | | | | Completed | | | | 15,729 | | | | 15,602 | | | | 19,847 | |
| | | | | | |
| | | GERD | | | | Phase 2 | | | | 2011 | | | | | | | | | | | | | |
| | | | | | |
XP21279 | |
| Parkinson’s
disease |
| | | Phase 2 | | | | 2011 | | | | 2,580 | | | | 4,024 | | | | 2,361 | |
| | | | | | |
Other(1) | | | | | | | | | | | | | | | 24,384 | | | | 27,686 | | | | 33,377 | |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Total preclinical and clinical development | | | | | | | | | | | | | | | 43,003 | | | | 50,300 | | | | 64,441 | |
| | | | | | |
Research(2) | | | | | | | | | | | | | | | 9,543 | | | | 20,447 | | | | 18,731 | |
| | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total research and development | | | | | | | | | | | | | | $ | 52,546 | | | $ | 70,747 | | | $ | 83,172 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
67
| * | Known asHorizant in the United States and previously known as XP13512. |
| ** | Arbaclofen placarbil, previously known as XP19986. Related R&D expenses include costs for both GERD and spasticity indications. |
| (1) | “Other” constitutes preclinical and clinical development costs for our product candidates that are not directly allocated to gabapentin enacarbil, AP or XP21279. For the year ended December 31, 2010, “other” expenses consisted primarily of personnel costs of $17.9 million and office and facilities overhead costs of $5.1 million. |
| (2) | For the year ended December 31, 2010, “research” expenses consisted primarily of personnel costs of $4.5 million and office and facilities overhead costs of $2.9 million. |
The largest component of our total operating expenses is our ongoing investment in our research and development activities, including the clinical development of our product candidate pipeline. The process of conducting the clinical research necessary to obtain FDA approval is costly and time consuming. We consider the active management and development of our clinical pipeline to be critical to our long-term success. The actual probability of success for each product candidate and clinical program may be impacted by a variety of factors, including, among others, the quality of the product candidate, early clinical data, investment in the program, competition, manufacturing capability and commercial viability. Furthermore, our strategy includes entering into additional collaborations with third parties to participate in the development and commercialization of at least some of our product candidates. In situations in which third parties have control over the preclinical development or clinical trial process for a product candidate, the estimated completion date is largely under the control of that third party and not under our control. We cannot forecast with any degree of certainty which of our product candidates, if any, will be subject to future collaborations or how such arrangements would affect our development plans or capital requirements.
As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development projects or when and to what extent we will generate revenues from the commercialization and sale of any of our product candidates.
Results of Operations
Years Ended December 31, 2010, 2009 and 2008
Revenues
Our collaboration revenue consisted of the recognition of revenues from up-front and milestone payments from our collaborations with Astellas and Xanodyne. Our agreement with Xanodyne terminated in July 2009. Our net revenue from unconsolidated joint operating activities consisted of the recognition of revenues from up-front and milestone payments and the recognition of our share of pre-launch operating losses resulting from our election to co-promoteHorizant in the United States with GSK. In connection with the amendment and restatement of our collaboration agreement with GSK in November 2010, our share of pre-launch operating losses is forgiven up to a maximum of $10.0 million.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | 2009 to 2010
Change | | | 2008 to 2009
Change | |
| | | 2010 | | | 2009 | | | 2008 | | | $ | | | % | | | $ | | | % | |
| | | (In thousands, except percentages) | |
Net revenue from unconsolidated joint operating activities | | $ | 1,364 | | | $ | 24,758 | | | $ | 28,981 | | | $ | (23,394 | ) | | | (94 | )% | | $ | (4,223 | ) | | | (15 | )% |
Collaboration revenue | | | 1,515 | | | | 9,515 | | | | 13,015 | | | | (8,000 | ) | | | (84 | )% | | | (3,500 | ) | | | (27 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | $ | 2,879 | | | $ | 34,273 | | | $ | 41,996 | | | $ | (31,394 | ) | | | (92 | )% | | $ | (7,723 | ) | | | (18 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Revenues in 2010 and 2009 resulted from our collaborations with Astellas and GSK. Revenues in 2008 resulted from our collaborations with Astellas, GSK and Xanodyne.
68
The decrease in net revenue from unconsolidated joint operating activities in 2010 compared to 2009 was primarily due to the receipt and recognition of a significant portion of the $20.0 million milestone payments from GSK related to the FDA’s acceptance for review of theHorizant NDA in 2009. As a result of our amended and restated collaboration agreement with GSK in November 2010, our share of losses from the joint profit and loss is forgiven up to a maximum of $10.0 million leading to the reversal in 2010 of the $1.1 million loss recognized in 2009.
The decrease in net revenue from unconsolidated joint operating activities in 2009 compared to 2008 was the result of a $3.1 million decrease in revenues recognized from up-front license and milestone payments under our GSK agreement and the recognition of a $1.1 million charge representing our share of pre-launch operating losses ofHorizantin 2009 as a result of our election of the co-promotion option.
The decrease in collaboration revenue in 2010 compared to 2009 was the result of an $8.0 million decrease in revenues recognized under our Astellas agreement from milestone payments related to the FDA’s acceptance for review of the NDA forHorizant in the United States and the acceptance of filing of the NDA for gabapentin enacarbil with the PMDA in Japan.
The decrease in collaboration revenue in 2009 compared to 2008 was the result of an $11.5 million decrease in revenues recognized under our Xanodyne agreement, partially offset by an $8.0 million increase in revenues recognized under our Astellas agreement from milestone payments related to the FDA’s acceptance for review of the NDA forHorizant in the United States and the acceptance of filing of the NDA for gabapentin enacarbil with the PMDA in Japan.
We expect revenues to fluctuate in the future primarily depending upon the potential further development and commercialization ofHorizant/gabapentin enacarbil, the timing of milestone-related activities under our Astellas and GSK collaborations and the extent to which we enter into new, or modify existing, collaborative agreements.
Research and Development Expenses
Of the total research and development expenses for the years ended December 31, 2010, 2009 and 2008, the costs associated with research and preclinical and clinical development activities approximated the following:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | 2009 to 2010
Change | | | 2008 to 2009
Change | |
| | | 2010 | | | 2009 | | | 2008 | | | $ | | | % | | | $ | | | % | |
| | | (In thousands, except percentages) | |
Research | | $ | 9,543 | | | $ | 20,447 | | | $ | 18,731 | | | $ | (10,904 | ) | | | (53 | )% | | $ | 1,716 | | | | 9 | % |
Preclinical and clinical development | | | 43,003 | | | | 50,300 | | | | 64,441 | | | | (7,297 | ) | | | (15 | )% | | | (14,141 | ) | | | (22 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total research and development | | $ | 52,546 | | | $ | 70,747 | | | $ | 83,172 | | | $ | (18,201 | ) | | | (26 | )% | | $ | (12,425 | ) | | | (15 | )% |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
The decrease in research and development expenses for 2010 compared to 2009 was principally due to the following:
| | • | | decreased net costs forHorizant/gabapentin enacarbil of $2.7 million primarily due to decreased manufacturing and clinical costs; |
| | • | | decreased net costs for XP21279 of $1.4 million primarily due to decreased toxicology costs; and |
| | • | | decreased personnel costs of $12.6 million primarily due to decreased headcount, including decreased non-cash stock-based compensation of $2.2 million; partially offset by |
| | • | | a credit in 2009 of $2.0 million from Astellas for manufacturing costs under the supply arrangement. |
The decrease in research and development expenses for 2009 compared to 2008 was principally due to the following:
| | • | | decreased net costs forHorizant/gabapentin enacarbil of $7.8 million primarily due to decreased clinical costs; |
69
| | • | | decreased net costs for AP of $4.2 million primarily due to decreased clinical costs; and |
| | • | | decreased net costs for our other development programs of $5.6 million primarily due to decreased toxicology and manufacturing costs; partially offset by |
| | • | | increased net costs for XP21279 of $1.7 million primarily due to increased toxicology costs; and |
| | • | | increased personnel costs of $3.3 million primarily due to increased non-cash stock-based compensation of $1.9 million. |
We expect our research and development expenses to remain relatively constant with 2010 levels. The timing and amount of expenses incurred will primarily depend upon the extent of current or future clinical trials for AP and XP21279, as well as the related expenses associated with our development organization, regulatory requirements, advancement of our preclinical programs and product candidate manufacturing costs.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consisted principally of salaries and other related costs for personnel in executive, finance, accounting, business development, information technology, legal, sales, marketing and human resources functions. Other selling, general and administrative expenses included facility costs not otherwise included in research and development expenses, patent-related costs and professional fees for legal, consulting and accounting services.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | 2009 to 2010
Change | | | 2008 to 2009
Change | |
| | | 2010 | | | 2009 | | | 2008 | | | $ | | | % | | | $ | | | % | |
| | | (In thousands, except percentages) | |
Selling, general and administrative | | $ | 28,323 | | | $ | 31,807 | | | $ | 26,391 | | | $ | (3,484 | ) | | | 11 | % | | $ | 5,416 | | | | 21 | % |
The decrease in selling, general and administrative expenses in 2010 compared to 2009 was principally due to decreased personnel costs of $2.8 million primarily due to decreased headcount.
The increase in selling, general and administrative expenses in 2009 compared to 2008 was principally due to increased personnel costs of $4.8 million primarily due to increased headcount and increased non-cash stock-based compensation of $1.8 million.
We expect selling, general and administrative expenses to remain relatively constant with 2010 levels. The timing and amount of selling, general and administrative expenses incurred will primarily depend upon the NDA approval process for theHorizant NDA and, assuming such approval, the timing and costs associated with establishing our sales force in support of the potential commercialization ofHorizant for RLS, which we have up to three years to deploy following the potential approval ofHorizant in the United States under the amended and restated collaboration agreement with GSK.
Restructuring Charges
As a result of the implementation of our March 2010 restructuring plan that resulted in a reduction in force of 107 employees, or approximately 50% of our workforce, we recorded restructuring charges of $5.3 million in the year ended December 31, 2010. The restructuring charges consisted primarily of $3.9 million of leave of absence pay, severance and healthcare benefits, $0.9 million of non-cash stock-based compensation and $0.4 million of property and equipment write-offs. We do not expect to incur additional charges in relation to the March 2010 restructuring plan, and we have made all cash payments in association with this restructuring plan.
70
Interest and Other Income and Interest and Other Expense
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year Ended December 31, | | | 2009 to 2010
Change | | | 2008 to 2009
Change | |
| | | 2010 | | | 2009 | | | 2008 | | | $ | | | % | | | $ | | | % | |
| | | (In thousands, except percentages) | |
Interest and other income | | $ | 796 | | | $ | 1,229 | | | $ | 4,640 | | | $ | (433 | ) | | | (35 | )% | | $ | (3,411 | ) | | | (74 | )% |
Interest and other expense | | | — | | | | 4 | | | | 19 | | | | (4 | ) | | | (100 | )% | | | (15 | ) | | | (79 | )% |
Interest and other income for 2010 resulted primarily from the awards that totaled $0.5 million received and recognized through the Qualifying Therapeutic Discovery Project program under section 48D of the Internal Revenue Code, which was enacted as part of thePatient Protection and Affordable Care Act of 2010, and, to a lesser extent, earnings on cash equivalents and short-term investments. Interest and other income for 2009 and 2008 resulted primarily from earnings on cash equivalents and short-term investments. The decrease in interest income in 2010 compared to 2009 and 2009 compared to 2008 was primarily due to lower interest rates.
Income Taxes
We recorded $0, $0.7 million and $0.4 million of current income tax benefit for the years ended December 31, 2010, 2009 and 2008, respectively. In the year ended December 31, 2009, $0.4 million of current income tax benefit recognized was due to the adoption of a provision in theWorker, Homeownership, and Business Assistance Act of 2009 that allows businesses with NOLs in 2008 or 2009 to carry back those losses for up to five-years and $0.3 million of current income tax benefit recognized was due to the adoption of a provision in theAmerican Recovery and Reinvestment Tax Act of 2009 that allows corporations to convert carry-forward research and development and Alternative Minimum Tax, or AMT, credits into a refundable credit amount, which we claimed and received as a refund in cash in 2010. The income tax benefit recognized for the year ended December 31, 2008 was primarily due to the adoption of a provision in theHousing and Economic Recovery Act of 2008that allowed corporations to convert carry-forward research and development and AMT credits into a refundable credit amount, which we claimed and received as a refund in cash in 2009.
Liquidity and Capital Resources
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
Cash provided by (used in): | | | | | | | | | | | | |
Operating activities | | $ | (64,481 | ) | | $ | (57,680 | ) | | $ | (45,242 | ) |
Investing activities | | | 20,227 | | | | (25,631 | ) | | | 49,604 | |
Financing activities | | | 31,191 | | | | 53,516 | | | | 43,727 | |
Capital expenditures (included in investing activities above) | | | (646 | ) | | | (2,891 | ) | | | (7,441 | ) |
Due to our significant research and development expenditures and the lack of regulatory agency approvals to sell products, we have generated cumulative operating losses since we incorporated in 1999. As such, we have funded our research and development operations primarily through sales of our equity securities, non-equity payments from our collaborators and interest earned on investments. At December 31, 2010, we had available cash and cash equivalents and short-term investments of $108.6 million. Our cash and investment balances are held in a variety of interest-bearing instruments, including corporate debt securities, investments backed by U.S. government-sponsored agencies, U.S. treasury securities and money market accounts. Cash in excess of immediate requirements is invested with regard to liquidity and capital preservation, and we seek to minimize the potential effects of concentration and degrees of risk.
Net cash used in operating activities was $64.5 million, $57.7 million and $45.2 million in the years ended December 31, 2010, 2009 and 2008, respectively. The net cash used in operating activities in 2010 and 2008 primarily reflected our net loss, partially offset by non-cash stock-based compensation. The net cash used in operating activities in 2009 primarily reflected our net loss and, to a lesser extent, changes in operating assets and liabilities, partially offset by non-cash stock-based compensation.
71
Net cash provided by (used in) investing activities was $20.2 million, $(25.6) million and $49.6 million in the years ended December 31, 2010, 2009 and 2008, respectively. The net cash provided by investing activities in 2010 was primarily related to the proceeds from maturities of investments, partially offset by purchases of investments. The net cash used in investing activities in 2009 was primarily related to the purchases of investments, partially offset by proceeds from maturities of investments. The net cash provided by investing activities in 2008 was primarily related to the proceeds from sales and maturities of investments, partially offset by purchases of investments and, to a lesser extent, capital expenditures.
Net cash provided by financing activities was $31.2 million, $53.5 million and $43.7 million in the years ended December 31, 2010, 2009 and 2008, respectively. The net cash provided by financing activities in 2010, 2009 and 2008 primarily reflected the net proceeds from the issuance of common stock and warrants and the exercise of stock options.
We believe that our existing capital resources and anticipated milestone payments, together with interest thereon, will be sufficient to meet our projected operating requirements into the fourth quarter of 2013. We have based our estimate of cash sufficiency on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we expect. Further, our operating plan may change, and we may need additional funds to meet operational needs and capital requirements for product development and commercialization sooner than planned. We have no credit facility or committed sources of capital other than potential milestones receivable under our collaborations. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement and involves risks and uncertainties, and actual results could vary as a result of a number of factors, including the factors discussed in “Risk Factors.” Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, and the extent to which we enter into additional collaborations with third parties to participate in their development and commercialization, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials. Our future funding requirements will depend on many factors, including:
| | • | | the scope, rate of progress, results and cost of our preclinical testing, clinical trials and other research and development activities; |
| | • | | the receipt of FDA approval forHorizant and the timing and success of further studies and trials necessary to secure this approval, if any; |
| | • | | the extent of product development funding under our current collaborative arrangements; |
| | • | | the cost of manufacturing clinical, and establishing commercial, supplies of our product candidates and any products that we may develop; |
| | • | | the timing of any milestone payments under our collaborative arrangements; |
| | • | | the number and characteristics of product candidates that we pursue, including rights related toHorizant that we obtained pursuant to our amended and restated collaboration agreement with GSK; |
| | • | | the cost, timing and outcomes of regulatory approvals, if any; |
| | • | | the terms and timing of any collaborative, licensing and other arrangements that we may establish or modify; |
| | • | | the timing and amount of our share of operating losses from our GSK collaboration; |
| | • | | the timing, receipt and amount of sales, profit sharing or royalties, if any, from our potential products; |
| | • | | the cost and expenses associated with the pending and potential additional related purported securities class action lawsuits, as well as any unforeseen litigation; |
| | • | | the cost of preparing, filing, prosecuting, defending and enforcing any patent claims and other intellectual property rights; and |
| | • | | the extent to which we acquire or invest in businesses, products or technologies that complement our business, although we have no commitments or agreements relating to any of these types of transactions. |
72
If we need to raise additional money to fund our operations, funding may not be available to us on acceptable terms, or at all. If we are, or anticipate that we may be, unable to raise additional funds when needed, we may terminate or delay clinical trials for one or more of our product candidates, we may delay our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our product candidates or we may curtail significant drug development programs that are designed to identify new product candidates. In addition, at any time upon advance notice to GSK, we may exercise the right to revert to a net sales royalty-based compensation structure and forego the right to co-promoteHorizant in the United States. We may seek to raise any necessary additional funds through equity or debt financings, collaborative arrangements with corporate partners or other sources. To the extent that we raise additional capital through licensing arrangements or arrangements with collaborative partners, we may be required to relinquish, on terms that are not favorable to us, rights to some of our technologies or product candidates that we would otherwise seek to develop or commercialize ourselves. To the extent that we raise additional capital through equity financings, dilution to our stockholders would result. Any debt financing or additional equity that we raise may contain terms that are not favorable to our stockholders or us.
Off-Balance Sheet Arrangements
We have no material off-balance sheet arrangements as defined in Regulation S-K 303(a)(4)(ii).
Contractual Obligations
Our future contractual obligations at December 31, 2010 were as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | |
Contractual Obligations | | Total | | | Less Than
1 Year | | | 1-3
Years | | | 3-5
Years | | | Greater
Than 5
Years | |
Operating lease obligations | | $ | 11,910 | | | $ | 5,547 | | | $ | 6,363 | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
Operating lease obligations do not assume the exercise by us of any termination or extension options.
Recent Accounting Pronouncements
In September 2009, the FASB Emerging Issues Task Force, or EITF, reached a consensus on Accounting Standards Update, or ASU, 2009-13 (Topic 605),Multiple-Deliverable Revenue Arrangements,or ASU 2009-13. ASU 2009-13 applies to multiple-deliverable revenue arrangements that are currently within the scope of theRevenue Arrangements — Multiple-Element Arrangementstopic of the Codification. ASU 2009-13 provides principles and application guidance on whether multiple deliverables exist and how the arrangement should be separated and the consideration allocated. ASU 2009-13 requires an entity to allocate revenue in an arrangement using estimated selling prices of deliverables if a vendor does not have vendor-specific objective evidence or third-party evidence of selling price. ASU 2009-13 eliminates the use of the residual method and requires an entity to allocate revenue using the relative selling price method and also significantly expands the disclosure requirements for multiple-deliverable revenue arrangements. ASU 2009-13 will be effective prospectively for revenue arrangements entered into, or materially modified in, fiscal years beginning on or after June 15, 2010, with earlier application permitted. As a result, ASU 2009-13 will be applied by us on a prospective basis for revenue arrangements entered into, or materially modified, beginning in the first quarter of fiscal 2011.
In April 2010, the FASB EITF reached a consensus on ASU 2010-17 (Topic 605),Milestone Method of Revenue Recognition,or ASU 2010-17. ASU 2010-17 provides guidance on defining a milestone and determining whether the milestone method of revenue recognition is appropriate. ASU 2010-17 will be effective for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, with earlier adoption permitted. As a result, ASU 2010-17 will be applied by us on a prospective basis for milestones achieved starting in the first quarter of fiscal 2011, including the revised clinical and regulatory milestone payments that we are eligible to receive as a result of the amended and restated agreement with GSK. We are currently evaluating the potential impact of the adoption of ASU 2010-17 on our financial position and results of operations.
73
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
The primary objective of our investment activities is to preserve our capital to fund operations. We also seek to maximize income from our investments without assuming significant risk. To achieve our objectives, we maintain a portfolio of cash equivalents and investments in a variety of securities of high-credit quality. As of December 31, 2010, we had cash and cash equivalents and short-term investments of $108.6 million consisting of cash and highly liquid investments deposited in highly rated financial institutions in the United States. A portion of our investments may be subject to interest rate risk and could fall in value if market interest rates increase. However, because our investments are short-term in duration, we believe that our exposure to interest rate risk is not significant and a 1% movement in market interest rates would not have a significant impact on the total value of our portfolio. We actively monitor changes in interest rates.
We contract for the conduct of certain manufacturing activities with a contract manufacturer in Europe. We made payments in the aggregate amount of $1.1 million, $3.5 million and $2.8 million during the years ended December 31, 2010, 2009 and 2008, respectively, to this European contract manufacturer. We are subject to exposure to fluctuations in foreign exchange rates in connection with agreements with this European contract manufacturer. To date, the effect of the exposure to these fluctuations in foreign exchange rates has not been material, and we do not expect it to be material in the foreseeable future. We do not hedge our foreign currency exposures. We have not used derivative financial instruments for speculation or trading purposes.
Item 8. Financial Statements and Supplementary Data.
The information required by this item is incorporated herein by reference to the financial statements and schedule listed in Item 15 (1) and (2) of Part IV of this Annual Report on Form 10-K.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
Not applicable.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Based on their evaluation as required by paragraph (b) of Rules 13a-15 or 15d-15 of the Securities Exchange Act of 1934, as amended, as of December 31, 2010, our chief executive officer and chief financial officer concluded that our disclosure controls and procedures (as defined in Securities Exchange Act Rules 13a-15(e) or 15d-15(e)) were effective.
Limitations on the Effectiveness of Controls. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within an organization have been detected. Accordingly, our disclosure controls and procedures are designed to provide reasonable, not absolute, assurance that the objectives of our disclosure control system are met and, as set forth above, our chief executive officer and chief financial officer have concluded, based on their evaluation as of December 31, 2010, that our disclosure controls and procedures were effective to provide reasonable assurance that the objectives of our disclosure control system were met.
74
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Securities Exchange Act Rules 13a-15(f) and 15d-15(f). Our internal control system is designed to provide reasonable assurance regarding the preparation and fair presentation of financial statements for external purposes in accordance with generally accepted accounting principles. All internal control systems, no matter how well designed, have inherent limitations and can provide only reasonable assurance that the objectives of the internal control system are met.
Under the supervision and with the participation of our management, including our chief executive officer and chief financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) inInternal Control — Integrated Framework. Based on our evaluation, we concluded that our internal control over financial reporting was effective as of December 31, 2010.
Ernst & Young LLP, an independent registered public accounting firm, has audited our financial statements included herein and has issued an audit report on the effectiveness of our internal control over financial reporting, which report is included below.
75
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of XenoPort, Inc.
We have audited XenoPort, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). XenoPort, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, XenoPort, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2010, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of XenoPort, Inc. as of December 31, 2010 and 2009, and the related statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010 of XenoPort, Inc., and our report dated March 1, 2011 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 1, 2011
76
Changes in Internal Controls Over Financial Reporting
There were no significant changes in our internal controls over financial reporting during the fourth quarter of 2010 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
PART III.
Certain information required by Part III is omitted from this Annual Report on Form 10-K since we intend to file our definitive proxy statement for our 2011 annual meeting of stockholders, or the Proxy Statement, pursuant to Regulation 14A of the Securities Exchange Act, not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K, and certain information to be included in the Proxy Statement is incorporated herein by reference.
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item with respect to our executive officers may be found under the caption, “Executive Officers of the Registrant” in Item 1 of this Annual Report on Form 10-K. The information required by this item relating to our directors and nominees, including information with respect to our audit committee, audit committee financial experts and procedures by which stockholders may recommend nominees to our board of directors, may be found under the section entitled “Proposal 1 — Election of Directors” appearing in the Proxy Statement. Such information is incorporated herein by reference. Information regarding compliance with Section 16(a) of the Securities Exchange Act may be found under the section entitled “Section 16(a) Beneficial Ownership Reporting Compliance” appearing in our Proxy Statement. Such information is incorporated herein by reference.
In 2005, we adopted a code of ethics that applies to our employees, officers and directors and incorporates guidelines designed to deter wrongdoing and to promote honest and ethical conduct and compliance with applicable laws and regulations. In addition, the code of ethics incorporates our guidelines pertaining to topics such as conflicts of interest and workplace behavior. We have posted the text of our code of ethics on our Web site at www.XenoPort.com in connection with “Investor Relations/Corporate Governance” materials. In addition, we intend to promptly disclose (1) the nature of any amendment to our code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions and (2) the nature of any waiver, including an implicit waiver, from a provision of our code of ethics that is granted to one of these specified officers, the name of such person who is granted the waiver and the date of the waiver on our Web site in the future.
Item 11. Executive Compensation.
The information required by this item is included in our Proxy Statement under the sections entitled “Executive Compensation,” “Director Compensation,” “Compensation Committee Interlocks and Insider Participation” and “Compensation Committee Report” and is incorporated herein by reference.
77
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
Equity Compensation Plan Information
The following table provides certain information regarding our equity compensation plans in effect as of December 31, 2010:
| | | | | | | | | | | | |
Plan Category | | Number of Securities
to be Issued Upon
Exercise of
Outstanding Options,
Warrants and Rights
(a) | | | Weighted-Average
Exercise Price of
Outstanding
Options, Warrants
and Rights
(b) | | | Number of Securities
Remaining Available for
Future Issuance Under
Equity Compensation
Plans (Excluding
Securities Reflected
in Column
(a)) | |
Equity compensation plans approved by security holders: | | | | | | | | | | | | |
1999 Stock Plan(1) | | | 437,778 | | | $ | 4.34 | | | | — | |
2005 Equity Incentive Plan(2) | | | 4,044,059 | | | $ | 21.70 | | | | 802,596 | |
2005 Non-Employee Directors’ Stock Option Plan(3) | | | 391,666 | | | $ | 25.66 | | | | 122,917 | |
2005 Employee Stock Purchase Plan(4) | | | — | | | | — | | | | 477,211 | |
Equity compensation plans not approved by security holders: | | | | | | | | | | | | |
New Hire Option Agreement with Vincent J. Angotti(5) | | | 140,612 | | | $ | 42.59 | | | | — | |
New Hire Stock Unit Award Agreement with Vincent J. Angotti(6) | | | 5,000 | | | | — | | | | — | |
New Hire Option Agreement with David A. Stamler, M.D.(7) | | | 81,601 | | | $ | 39.55 | | | | — | |
2010 Inducement Award Plan(8) | | | 167,350 | | | $ | 7.67 | | | | 182,650 | |
| | | | | | | | | | | | |
Total | | | 5,268,066 | | | $ | 20.89 | | | | 1,585,374 | |
| | | | | | | | | | | | |
| (1) | In December 1999, we adopted the 1999 Stock Plan, or the 1999 Plan, which was terminated in June 2005 in connection with our initial public offering so that no further awards may be granted under the 1999 Plan. Although the 1999 Plan has terminated, all outstanding options will continue to be governed by their existing terms. |
| (2) | In January 2005, we adopted the 2005 Equity Incentive Plan, or the 2005 Incentive Plan, which became effective in June 2005 in connection with our initial public offering. A total of 2,000,000 shares of common stock were initially authorized for issuance under the 2005 Incentive Plan. Our board of directors may increase the share reserve of the 2005 Incentive Plan as of each January 1, from January 1, 2006 through January 1, 2015, by an amount determined by our board; provided, however that the increase for any year may not exceed the lesser of (1) 2.5% of the total number of shares of our common stock outstanding on the December 31st of the preceding calendar year or (2) 2,000,000 shares. During the year ended December 31, 2010, the annual increase to the 2005 Incentive Plan reserve was 760,076 shares. Restricted stock unit awards and a performance stock unit award have been granted under the 2005 Incentive Plan and are included in column (a). The outstanding performance stock unit award has a variable amount of securities that may be issued under it depending on certain performance measures. The maximum number of shares of common stock that may be issued under such award, 200,000, has been included in column (a). The weighted-average exercise price in column (b) does not take the performance stock unit award into account, but does include the effect of the restricted stock unit awards under the 2005 Incentive Plan, which awards do not carry an exercise price. At December 31, 2010, the weighted-average exercise price of outstanding options under the 2005 Incentive Plan was $27.67, excluding the restricted stock unit awards. |
| (3) | In January 2005, we adopted the 2005 Non-Employees Directors’ Stock Option Plan, or the Directors’ Plan, which became effective in June 2005 in connection with our initial public offering. The Directors’ Plan |
78
| | provides for the automatic grant of options to purchase shares of our common stock to non-employee directors. A total of 150,000 shares of our common stock were initially authorized for issuance under the Directors’ Plan. Our board of directors may increase the share reserve of the Directors’ Plan as of each January 1, from January 1, 2006 through January 1, 2015, by an amount determined by our board; provided, however that the increase for any year may not exceed the excess of (1) the number of shares of our common stock subject to options granted under the Directors’ Plan during the preceding calendar year over (2) the number of shares added back to the share reserve of the Directors’ Plan during the preceding calendar year from cancellations. During the year ended December 31, 2010, the annual increase to the Directors’ Plan reserve was 87,083 shares. |
| (4) | In January 2005, we adopted the 2005 Employee Stock Purchase Plan, or the ESPP, which became effective in June 2005 in connection with our initial public offering. The ESPP allows for qualified employees (as defined in the ESPP) to purchase shares of our common stock at a price equal to the lower of 85% of the closing price of our common stock at the beginning of the offering period or 85% of the closing price of our common stock on the date of purchase. A total of 250,000 shares of our common stock were initially authorized for issuance under the ESPP. Our board of directors may increase the share reserve of the ESPP as of each January 1, from January 1, 2006 through January 1, 2015, by an amount determined by our board; provided, however that the increase for any year may not exceed the lesser of (1) 1% of the total number of shares of our common stock outstanding on the December 31st of the preceding calendar year or (2) 250,000 shares. During the year ended December 31, 2010, the share reserve of the ESPP was sufficient and did not require an annual increase. |
| (5) | On May 1, 2008, Mr. Angotti was granted a new employee inducement stock award outside of our stockholder approved equity plans consisting of nonqualified stock options to purchase 140,612 shares of our common stock. The stock options have a per share exercise price of $42.59, the closing trading price of our common stock on the NASDAQ Global Market on May 1, 2008. The stock options have a ten-year term and vest over four years, with 25% cliff vesting on the first anniversary of the May 1, 2008 grant date, and 1/48th of the shares subject to the options vesting monthly thereafter. |
| (6) | On May 1, 2008, Mr. Angotti was granted a new employee inducement stock award outside of our stockholder approved equity plans consisting of restricted stock units for 10,000 shares of our common stock. The restricted stock units vest in four equal annual installments on each anniversary of the May 1, 2008 grant date. The restricted stock units have no exercise price. |
| (7) | On July 14, 2008, Dr. Stamler was granted a new employee inducement stock award outside of our stockholder approved equity plans consisting of nonqualified stock options to purchase 139,888 shares of the Company’s common stock. The stock options have a per share exercise price of $39.55, the closing trading price of our common stock on the NASDAQ Global Market on July 14, 2008. The stock options have a ten-year term and initially vested over four years, with 25% cliff vesting on the first anniversary of the July 14, 2008 grant date, and 1/48th of the shares subject to the options vesting monthly thereafter until his termination. The remaining unvested stock options were cancelled due to Dr. Stamler’s departure in 2010. Dr. Stamler has the right to exercise the vested stock options until February 19, 2011. |
| (8) | In May 2010, the 2010 Inducement Award Plan, or the 2010 Inducement Plan, was adopted by our board of directors and became effective. We intend to grant awards under the 2010 Inducement Plan to persons not previously employees or directors of ours (or followingbona fide periods of non-employment by us and our affiliates) as inducements material to such individuals entering into employment with us and to provide incentives for such persons to exert maximum efforts for our success. A total of 350,000 shares of common stock were initially authorized for issuance under the 2010 Inducement Plan and no additional shares were authorized for issuance in 2010. The 2010 Inducement Plan provides for the grant of stock options, stock appreciation rights, restricted stock awards, restricted stock unit awards and other stock awards. Restricted stock unit awards have been granted under the 2010 Inducement Plan and are included in column (a). The weighted-average exercise price in column (b) includes the effect of the restricted stock unit awards under the 2010 Inducement Plan, which awards do not carry an exercise price. At December 31, 2010, the weighted-average exercise price of outstanding options under the 2010 Inducement Plan was $8.34, excluding the restricted stock unit awards. |
79
Security Ownership of Certain Beneficial Owners and Management
The information required by this item relating to security ownership of certain beneficial owners and management is included in our Proxy Statement under the section entitled “Security Ownership of Certain Beneficial Owners and Management” and is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is included in our Proxy Statement under the sections entitled “Transactions with Related Persons” and “Proposal 1 — Election of Directors” and is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
The information required by this item is incorporated herein by reference to the information included in our Proxy Statement under the section entitled “Proposal 4 — Ratification of Selection of Independent Registered Public Accounting Firm.”
PART IV.
Item 15. Exhibits, Financial Statement Schedules.
1. Index to Financial Statements
The following Financial Statements are included herein:
2. Index to Financial Statement Schedules
None.
All other schedules are omitted because they are not applicable, or not required, or because the required information is included in the financial statements or notes thereto.
80
3. Exhibits — The following exhibits are included herein or incorporated herein by reference:
| | |
Exhibit Number | | Description of Document |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation(1) |
| |
| 3.2 | | Amended and Restated Bylaws(1) |
| |
| 3.3 | | Certificate of Designation of Series A Junior Participating Preferred Stock(2) |
| |
| 4.1 | | Specimen Common Stock Certificate(3) |
| |
| 4.2 | | Form of Right Certificate(4) |
| |
| 4.3 | | Form of Registered Direct Common Warrant(5) |
| |
| 10.1* | | Form of Indemnification Agreement between the Company and its officers and directors(6) |
| |
| 10.2* | | Form of Employee Proprietary Information Agreement between the Company and its executive officers(7) |
| |
| 10.3 | | Lease Agreement, dated September 24, 2001, by and between the Company and Sobrato Interests(7) |
| |
| 10.3.1 | | First Amendment to Lease Agreement, dated February 29, 2008, by and between the Company and Sobrato Interests(8) |
| |
| 10.4 | | Lease, dated February 29, 2008, by and between the Company and Sobrato Interests(9) |
| |
| 10.5* | | 1999 Stock Plan(7) |
| |
| 10.6* | | Form of Stock Option Agreement under the 1999 Stock Plan(7) |
| |
| 10.7* | | 2005 Equity Incentive Plan(6) |
| |
| 10.8* | | Form of Option Agreement under the 2005 Equity Incentive Plan(6) |
| |
| 10.9* | | Form of Stock Unit Award Agreement under the 2005 Equity Incentive Plan(10) |
| |
| 10.10* | | 2005 Non-Employee Directors’ Stock Option Plan(11) |
| |
| 10.11* | | Form of Stock Option Agreement under the 2005 Non-Employee Directors’ Stock Option Plan(12) |
| |
| 10.12* | | 2005 Employee Stock Purchase Plan(13) |
| |
| 10.13* | | Form of 2005 Employee Stock Purchase Plan Offering Document(14) |
| |
| 10.14* | | New Hire Option Agreement between Vincent J. Angotti and the Company(15) |
| |
| 10.15* | | New Hire Stock Unit Award Agreement between Vincent J. Angotti and the Company(16) |
| |
| 10.16* | | New Hire Option Agreement between David A. Stamler, M.D., and the Company(17) |
| |
| 10.17* | | 2010 Inducement Award Plan(18) |
| |
| 10.18* | | Form of Option Agreement under the 2010 Inducement Award Plan(19) |
| |
| 10.19* | | Form of Stock Unit Award Agreement under the 2010 Inducement Award Plan(20) |
| |
| 10.20* | | Performance Stock Unit Agreement, dated May 13, 2010, between the Company and Ronald W. Barrett, Ph.D.(21) |
| |
| 10.21* | | Performance Stock Unit Agreement, dated May 13, 2010, between the Company and William J. Rieflin(22) |
| |
| 10.22* | | Form of Change of Control Agreement between the Company and certain of its officers(23) |
| |
| 10.23* | | Change of Control Agreement between the Company and Ronald W. Barrett, dated November 7, 2007(24) |
81
| | |
Exhibit Number | | Description of Document |
| |
| 10.24* | | Change of Control Agreement between the Company and William G. Harris, dated November 7, 2007(25) |
| |
| 10.25* | | Change of Control Agreement between the Company and Kenneth C. Cundy, dated November 7, 2007(26) |
| |
| 10.26* | | Change of Control Agreement between the Company and David R. Savello, dated November 7, 2007(27) |
| |
| 10.27* | | Change of Control Agreement between the Company and Vincent J. Angotti, dated May 1, 2008(28) |
| |
| 10.28* | | Amended and Restated Change of Control Agreement between the Company and Gianna M. Bosko, dated February 25, 2011 |
| |
| 10.29* | | Consulting Letter Agreement, dated August 26, 2010, between the Company and William J. Rieflin(29) |
| |
| 10.30* | | Release and Severance Agreement, dated November 19, 2010, between the Company and David A. Stamler, MD |
| |
| 10.31* | | XenoPort, Inc. Corporate Bonus Plan(30) |
| |
| 10.32* | | Termsheet for Director Cash Compensation(31) |
| |
| 10.33* | | 2011 Executive Compensation(32) |
| |
| 10.34† | | Amended and Restated Distribution and License Agreement, dated as of October 31, 2009 between the Company and Astellas Pharma Inc.(33) |
| |
| 10.35†† | | Amended and Restated Development and Commercialization Agreement, dated November 7, 2010, by and between the Company and Glaxo Group Limited |
| |
| 10.36 | | Rights Agreement, dated as of December 15, 2005, by and between the Company and Mellon Investor Services LLC(34) |
| |
| 10.37 | | Form of Purchase Agreement between the Company and certain stockholders of the Company(35) |
| |
| 23.1 | | Consent of Independent Registered Public Accounting Firm |
| |
| 24.1 | | Power of Attorney (included in the signature page hereto) |
| |
| 31.1 | | Certification of the Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 31.2 | | Certification of the Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 32.1 | | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350)(36) |
| |
| 101.INS | | XBRL Instance Document(37) |
| |
| 101.SCH | | XBRL Taxonomy Extension Schema Document(37) |
| |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document(37) |
| |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document(37) |
| |
| 101.LAB | | XBRL Taxonomy Extension Labels Linkbase Document(37) |
| |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document(37) |
| * | Represents a management contract or compensation plan or arrangement. |
| † | Confidential treatment has been granted for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
82
| †† | Confidential treatment has been requested for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| (1) | Incorporated herein by reference to the same numbered exhibit of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2005, as filed with the SEC on August 11, 2005. |
| (2) | Incorporated herein by reference to Exhibit 3.1 of our current report on Form 8-K, filed with the SEC on December 16, 2005. |
| (3) | Incorporated herein by reference to the same numbered exhibit of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on April 13, 2005. |
| (4) | Incorporated herein by reference to Exhibit 4.1 of our current report on Form 8-K, filed with the SEC on December 16, 2005. |
| (5) | Incorporated herein by reference to Exhibit 4.1 of our current report on Form 8-K, filed with the SEC on December 30, 2008. |
| (6) | Incorporated herein by reference to the same numbered exhibit of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on March 2, 2005. |
| (7) | Incorporated herein by reference to the same numbered exhibit of our registration statement on Form S-1 (File No. 333-122156), as filed with the SEC on January 19, 2005. |
| (8) | Incorporated herein by reference to the same numbered exhibit of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended March 31, 2008, as filed with the SEC on May 8, 2008. |
| (9) | Incorporated herein by reference to Exhibit 10.32 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended March 31, 2008, as filed with the SEC on May 8, 2008. |
| (10) | Incorporated herein by reference to Exhibit 10.9 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2008, as filed with the SEC on August 7, 2008. |
| (11) | Incorporated herein by reference to Exhibit 10.9 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on April 13, 2005. |
| (12) | Incorporated herein by reference to Exhibit 10.10 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on April 13, 2005. |
| (13) | Incorporated herein by reference to Exhibit 10.11 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on March 2, 2005. |
| (14) | Incorporated herein by reference to Exhibit 10.12 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on March 2, 2005. |
| (15) | Incorporated herein by reference to Exhibit 99.4 of our registration statement on Form S-8 (File No. 333-150730), as filed with the SEC on May 8, 2008. |
| (16) | Incorporated herein by reference to Exhibit 99.5 of our registration statement on Form S-8 (File No. 333-150730), as filed with the SEC on May 8, 2008. |
| (17) | Incorporated herein by reference to Exhibit 10.39 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2008, as filed with the SEC on August 7, 2008. |
| (18) | Incorporated herein by reference to Exhibit 99.3.1 of our registration statement on Form S-8 (File No. 333-166760), as filed with the SEC on May 12, 2010. |
| (19) | Incorporated herein by reference to Exhibit 99.3.2 of our registration statement on Form S-8 (File No. 333-166760), as filed with the SEC on May 12, 2010. |
| (20) | Incorporated herein by reference to Exhibit 99.3.3 of our registration statement on Form S-8 (File No. 333-166760), as filed with the SEC on May 12, 2010. |
| (21) | Incorporated herein by reference to Exhibit 10.41 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2010, as filed with the SEC on August 6, 2010. |
| (22) | Incorporated herein by reference to Exhibit 10.42 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2010, as filed with the SEC on August 6, 2010. |
83
| (23) | Incorporated herein by reference to Exhibit 10.13 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (24) | Incorporated herein by reference to Exhibit 10.14 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (25) | Incorporated herein by reference to Exhibit 10.17 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (26) | Incorporated herein by reference to Exhibit 10.31 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (27) | Incorporated herein by reference to Exhibit 10.33 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (28) | Incorporated herein by reference to Exhibit 10.34 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended March 31, 2008, as filed with the SEC on May 8, 2008. |
| (29) | Incorporated herein by reference to Exhibit 10.43 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2010, as filed with the SEC on November 9, 2010. |
| (30) | Incorporated herein by reference to Exhibit 10.24 of our current report on Form 8-K, filed with the SEC on February 2, 2007. |
| (31) | Incorporated herein by reference to Exhibit 10.25 of our current report on Form 8-K, filed with the SEC on August 4, 2006. |
| (32) | Incorporated herein by reference to Exhibit 10.45 of our current report on Form 8-K, filed with the SEC on January 14, 2011. |
| (33) | Incorporated herein by reference to Exhibit 10.35 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2009, as filed with the SEC on November 4, 2009. |
| (34) | Incorporated herein by reference to Exhibit 4.2 of our current report on Form 8-K, filed with the SEC on December 16, 2005. |
| (35) | Incorporated herein by reference to Exhibit 10.1 of our current report on Form 8-K, filed with the SEC on December 30, 2008. |
| (36) | This certification accompanies the annual report on Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of the Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing. |
| (37) | Pursuant to applicable securities laws and regulations, we are deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and are not subject to liability under any anti-fraud provisions of the federal securities laws as long as we have made a good faith attempt to comply with the submission requirements and promptly amend the interactive data files after becoming aware that the interactive data files fail to comply with the submission requirements. In accordance with Rule 406T of Regulation S-T, the information in these exhibits is furnished and deemed not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of Section 18 of the Exchange Act of 1934, and otherwise is not subject to liability under these sections and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing. |
84
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | |
| | | XenoPort, Inc. |
| | (Registrant) |
| |
| March 1, 2011 | | /s/ Ronald W. Barrett |
| | Ronald W. Barrett Chief Executive Officer and Director |
| |
| March 1, 2011 | | /s/ William G. Harris |
| | William G. Harris Senior Vice President of Finance and Chief Financial Officer (Principal Financial and Accounting Officer) |
| |
| March 1, 2011 | | /s/ Martyn J. Webster |
| | Martyn J. Webster Vice President of Finance |
85
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Ronald W. Barrett and William G. Harris, and each of them, as his or her true and lawful attorneys-in-fact and agents, each with the full power of substitution for him or her, and in his or her name and in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorneys-in-fact and agents, and any of them or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| | | | |
Signature | | Title | | Date |
| | |
/s/ Ronald W. Barrett Ronald W. Barrett | | Chief Executive Officer and Director (Principal Executive Officer) | | March 1, 2011 |
| | |
/s/ William G. Harris William G. Harris | | Senior Vice President of Finance and Chief Financial Officer (Principal Financial and Accounting Officer) | | March 1, 2011 |
| | |
/s/ Paul L. Berns Paul L. Berns | | Director | | March 1, 2011 |
| | |
/s/ Dennis M. Fenton Dennis M. Fenton | | Director | | March 1, 2011 |
| | |
/s/ John G. Freund John G. Freund | | Director | | March 1, 2011 |
| | |
/s/ Catherine J. Friedman Catherine J. Friedman | | Director | | March 1, 2011 |
| | |
/s/ Jeryl L. Hilleman Jeryl L. Hilleman | | Director | | March 1, 2011 |
| | |
/s/ William J. Rieflin William J. Rieflin | | Director | | March 1, 2011 |
| | |
/s/ Wendell Wierenga Wendell Wierenga | | Director | | March 1, 2011 |
86
EXHIBIT INDEX
| | |
Exhibit Number | | Description of Document |
| |
| 3.1 | | Amended and Restated Certificate of Incorporation(1) |
| |
| 3.2 | | Amended and Restated Bylaws(1) |
| |
| 3.3 | | Certificate of Designation of Series A Junior Participating Preferred Stock(2) |
| |
| 4.1 | | Specimen Common Stock Certificate(3) |
| |
| 4.2 | | Form of Right Certificate(4) |
| |
| 4.3 | | Form of Registered Direct Common Warrant(5) |
| |
| 10.1* | | Form of Indemnification Agreement between the Company and its officers and directors(6) |
| |
| 10.2* | | Form of Employee Proprietary Information Agreement between the Company and its executive officers(7) |
| |
| 10.3 | | Lease Agreement, dated September 24, 2001, by and between the Company and Sobrato Interests(7) |
| |
| 10.3.1 | | First Amendment to Lease Agreement, dated February 29, 2008, by and between the Company and Sobrato Interests(8) |
| |
| 10.4 | | Lease, dated February 29, 2008, by and between the Company and Sobrato Interests(9) |
| |
| 10.5* | | 1999 Stock Plan(7) |
| |
| 10.6* | | Form of Stock Option Agreement under the 1999 Stock Plan(7) |
| |
| 10.7* | | 2005 Equity Incentive Plan(6) |
| |
| 10.8* | | Form of Option Agreement under the 2005 Equity Incentive Plan(6) |
| |
| 10.9* | | Form of Stock Unit Award Agreement under the 2005 Equity Incentive Plan(10) |
| |
| 10.10* | | 2005 Non-Employee Directors’ Stock Option Plan(11) |
| |
| 10.11* | | Form of Stock Option Agreement under the 2005 Non-Employee Directors’ Stock Option Plan(12) |
| |
| 10.12* | | 2005 Employee Stock Purchase Plan(13) |
| |
| 10.13* | | Form of 2005 Employee Stock Purchase Plan Offering Document(14) |
| |
| 10.14* | | New Hire Option Agreement between Vincent J. Angotti and the Company(15) |
| |
| 10.15* | | New Hire Stock Unit Award Agreement between Vincent J. Angotti and the Company(16) |
| |
| 10.16* | | New Hire Option Agreement between David A. Stamler, M.D., and the Company(17) |
| |
| 10.17* | | 2010 Inducement Award Plan(18) |
| |
| 10.18* | | Form of Option Agreement under the 2010 Inducement Award Plan(19) |
| |
| 10.19* | | Form of Stock Unit Award Agreement under the 2010 Inducement Award Plan(20) |
| |
| 10.20* | | Performance Stock Unit Agreement, dated May 13, 2010, between the Company and Ronald W. Barrett, Ph.D.(21) |
| |
| 10.21* | | Performance Stock Unit Agreement, dated May 13, 2010, between the Company and William J. Rieflin(22) |
| |
| 10.22* | | Form of Change of Control Agreement between the Company and certain of its officers(23) |
| |
| 10.23* | | Change of Control Agreement between the Company and Ronald W. Barrett, dated November 7, 2007(24) |
| |
| 10.24* | | Change of Control Agreement between the Company and William G. Harris, dated November 7, 2007(25) |
| |
| 10.25* | | Change of Control Agreement between the Company and Kenneth C. Cundy, dated November 7, 2007(26) |
87
| | |
Exhibit Number | | Description of Document |
| |
| 10.26* | | Change of Control Agreement between the Company and David R. Savello, dated November 7, 2007(27) |
| |
| 10.27* | | Change of Control Agreement between the Company and Vincent J. Angotti, dated May 1, 2008(28) |
| |
| 10.28* | | Amended and Restated Change of Control Agreement between the Company and Gianna M. Bosko, dated February 25, 2011 |
| |
| 10.29* | | Consulting Letter Agreement, dated August 26, 2010, between the Company and William J. Rieflin(29) |
| |
| 10.30* | | Release and Severance Agreement, dated November 19, 2010, between the Company and David A. Stamler, MD |
| |
| 10.31* | | XenoPort, Inc. Corporate Bonus Plan(30) |
| |
| 10.32* | | Termsheet for Director Cash Compensation(31) |
| |
| 10.33* | | 2011 Executive Compensation(32) |
| |
| 10.34† | | Amended and Restated Distribution and License Agreement, dated as of October 31, 2009 between the Company and Astellas Pharma Inc.(33) |
| |
| 10.35†† | | Amended and Restated Development and Commercialization Agreement, dated November 7, 2010, by and between the Company and Glaxo Group Limited |
| |
| 10.36 | | Rights Agreement, dated as of December 15, 2005, by and between the Company and Mellon Investor Services LLC(34) |
| |
| 10.37 | | Form of Purchase Agreement between the Company and certain stockholders of the Company(35) |
| |
| 23.1 | | Consent of Independent Registered Public Accounting Firm |
| |
| 24.1 | | Power of Attorney (included in the signature page hereto) |
| |
| 31.1 | | Certification of the Chief Executive Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 31.2 | | Certification of the Chief Financial Officer pursuant to Securities Exchange Act Rules 13a-14(a) and 15d-14(a) as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| |
| 32.1 | | Certification required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code (18 U.S.C. §1350)(36) |
| |
| 101.INS | | XBRL Instance Document(37) |
| |
| 101.SCH | | XBRL Taxonomy Extension Schema Document(37) |
| |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document(37) |
| |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document(37) |
| |
| 101.LAB | | XBRL Taxonomy Extension Labels Linkbase Document(37) |
| |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document(37) |
| * | Represents a management contract or compensation plan or arrangement. |
| † | Confidential treatment has been granted for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
| †† | Confidential treatment has been requested for portions of this exhibit. Omitted portions have been filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended. |
88
| (1) | Incorporated herein by reference to the same numbered exhibit of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2005, as filed with the SEC on August 11, 2005. |
| (2) | Incorporated herein by reference to Exhibit 3.1 of our current report on Form 8-K, filed with the SEC on December 16, 2005. |
| (3) | Incorporated herein by reference to the same numbered exhibit of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on April 13, 2005. |
| (4) | Incorporated herein by reference to Exhibit 4.1 of our current report on Form 8-K, filed with the SEC on December 16, 2005. |
| (5) | Incorporated herein by reference to Exhibit 4.1 of our current report on Form 8-K, filed with the SEC on December 30, 2008. |
| (6) | Incorporated herein by reference to the same numbered exhibit of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on March 2, 2005. |
| (7) | Incorporated herein by reference to the same numbered exhibit of our registration statement on Form S-1 (File No. 333-122156), as filed with the SEC on January 19, 2005. |
| (8) | Incorporated herein by reference to the same numbered exhibit of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended March 31, 2008, as filed with the SEC on May 8, 2008. |
| (9) | Incorporated herein by reference to Exhibit 10.32 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended March 31, 2008, as filed with the SEC on May 8, 2008. |
| (10) | Incorporated herein by reference to Exhibit 10.9 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2008, as filed with the SEC on August 7, 2008. |
| (11) | Incorporated herein by reference to Exhibit 10.9 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on April 13, 2005. |
| (12) | Incorporated herein by reference to Exhibit 10.10 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on April 13, 2005. |
| (13) | Incorporated herein by reference to Exhibit 10.11 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on March 2, 2005. |
| (14) | Incorporated herein by reference to Exhibit 10.12 of our registration statement on Form S-1, as amended (File No. 333-122156), as filed with the SEC on March 2, 2005. |
| (15) | Incorporated herein by reference to Exhibit 99.4 of our registration statement on Form S-8 (File No. 333-150730), as filed with the SEC on May 8, 2008. |
| (16) | Incorporated herein by reference to Exhibit 99.5 of our registration statement on Form S-8 (File No. 333-150730), as filed with the SEC on May 8, 2008. |
| (17) | Incorporated herein by reference to Exhibit 10.39 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2008, as filed with the SEC on August 7, 2008. |
| (18) | Incorporated herein by reference to Exhibit 99.3.1 of our registration statement on Form S-8 (File No. 333-166760), as filed with the SEC on May 12, 2010. |
| (19) | Incorporated herein by reference to Exhibit 99.3.2 of our registration statement on Form S-8 (File No. 333-166760), as filed with the SEC on May 12, 2010. |
| (20) | Incorporated herein by reference to Exhibit 99.3.3 of our registration statement on Form S-8 (File No. 333-166760), as filed with the SEC on May 12, 2010. |
| (21) | Incorporated herein by reference to Exhibit 10.41 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2010, as filed with the SEC on August 6, 2010. |
| (22) | Incorporated herein by reference to Exhibit 10.42 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended June 30, 2010, as filed with the SEC on August 6, 2010. |
| (23) | Incorporated herein by reference to Exhibit 10.13 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (24) | Incorporated herein by reference to Exhibit 10.14 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
89
| (25) | Incorporated herein by reference to Exhibit 10.17 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (26) | Incorporated herein by reference to Exhibit 10.31 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (27) | Incorporated herein by reference to Exhibit 10.33 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2007, as filed with the SEC on November 9, 2007. |
| (28) | Incorporated herein by reference to Exhibit 10.34 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended March 31, 2008, as filed with the SEC on May 8, 2008. |
| (29) | Incorporated herein by reference to Exhibit 10.43 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2010, as filed with the SEC on November 9, 2010. |
| (30) | Incorporated herein by reference to Exhibit 10.24 of our current report on Form 8-K, filed with the SEC on February 2, 2007. |
| (31) | Incorporated herein by reference to Exhibit 10.25 of our current report on Form 8-K, filed with the SEC on August 4, 2006. |
| (32) | Incorporated herein by reference to Exhibit 10.45 of our current report on Form 8-K, filed with the SEC on January 14, 2011. |
| (33) | Incorporated herein by reference to Exhibit 10.35 of our quarterly report on Form 10-Q (File No. 000-51329) for the period ended September 30, 2009, as filed with the SEC on November 4, 2009. |
| (34) | Incorporated herein by reference to Exhibit 4.2 of our current report on Form 8-K, filed with the SEC on December 16, 2005. |
| (35) | Incorporated herein by reference to Exhibit 10.1 of our current report on Form 8-K, filed with the SEC on December 30, 2008. |
| (36) | This certification accompanies the annual report on Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of the Registrant under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing. |
| (37) | Pursuant to applicable securities laws and regulations, we are deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and are not subject to liability under any anti-fraud provisions of the federal securities laws as long as we have made a good faith attempt to comply with the submission requirements and promptly amend the interactive data files after becoming aware that the interactive data files fail to comply with the submission requirements. In accordance with Rule 406T of Regulation S-T, the information in these exhibits is furnished and deemed not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, is deemed not filed for purposes of Section 18 of the Exchange Act of 1934, and otherwise is not subject to liability under these sections and shall not be incorporated by reference into any registration statement or other document filed under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing. |
90
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of XenoPort, Inc.
We have audited the accompanying balance sheets of XenoPort, Inc. as of December 31, 2010 and 2009, and the related statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of XenoPort, Inc. at December 31, 2010 and 2009, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2010, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), XenoPort, Inc.’s internal control over financial reporting as of December 31, 2010, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated March 1, 2011, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Palo Alto, California
March 1, 2011
91
XENOPORT, INC.
BALANCE SHEETS
| | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2009 | |
| | | (In thousands,
except per share amount) | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 23,192 | | | $ | 36,255 | |
Short-term investments | | | 85,403 | | | | 107,413 | |
Prepaids and other current assets | | | 2,206 | | | | 3,719 | |
| | | | | | | | |
Total current assets | | | 110,801 | | | | 147,387 | |
Property and equipment, net | | | 7,209 | | | | 10,726 | |
Restricted investments and other assets | | | 3,219 | | | | 2,099 | |
| | | | | | | | |
Total assets | | $ | 121,229 | | | $ | 160,212 | |
| | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 515 | | | $ | 2,031 | |
Accrued compensation | | | 2,493 | | | | 5,653 | |
Accrued preclinical and clinical costs | | | 4,884 | | | | 3,109 | |
Accrued unconsolidated joint operating activities | | | — | | | | 1,095 | |
Other accrued liabilities | | | 976 | | | | 911 | |
Deferred rent | | | 1,104 | | | | 1,055 | |
Deferred revenue | | | 1,515 | | | | 1,784 | |
| | | | | | | | |
Total current liabilities | | | 11,487 | | | | 15,638 | |
Deferred revenue | | | 15,783 | | | | 17,298 | |
Commitments and contingencies | | | | | | | | |
Stockholders’ equity: | | | | | | | | |
Common stock, $0.001 par value; 60,000 shares authorized; 35,227 and 30,403 shares issued and outstanding at December 31, 2010 and 2009, respectively | | | 35 | | | | 30 | |
Additional paid-in capital | | | 481,336 | | | | 432,157 | |
Accumulated other comprehensive income (loss) | | | (6 | ) | | | 26 | |
Accumulated deficit | | | (387,406 | ) | | | (304,937 | ) |
| | | | | | | | |
Total stockholders’ equity | | | 93,959 | | | | 127,276 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 121,229 | | | $ | 160,212 | |
| | | | | | | | |
The accompanying notes are an integral part of these financial statements.
92
XENOPORT, INC.
STATEMENTS OF OPERATIONS
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands, except per share amounts) | |
Revenues: | | | | | | | | | | | | |
Net revenue from unconsolidated joint operating activities | | $ | 1,364 | | | $ | 24,758 | | | $ | 28,981 | |
Collaboration revenue | | | 1,515 | | | | 9,515 | | | | 13,015 | |
| | | | | | | | | | | | |
Total revenues | | | 2,879 | | | | 34,273 | | | | 41,996 | |
| | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | |
Research and development | | | 52,546 | | | | 70,747 | | | | 83,172 | |
Selling, general and administrative | | | 28,323 | | | | 31,807 | | | | 26,391 | |
Restructuring charges | | | 5,275 | | | | — | | | | — | |
| | | | | | | | | | | | |
Total operating expenses | | | 86,144 | | | | 102,554 | | | | 109,563 | |
| | | | | | | | | | | | |
Loss from operations | | | (83,265 | ) | | | (68,281 | ) | | | (67,567 | ) |
Interest and other income | | | 796 | | | | 1,229 | | | | 4,640 | |
Interest and other expense | | | — | | | | (4 | ) | | | (19 | ) |
| | | | | | | | | | | | |
Loss before income taxes | | | (82,469 | ) | | | (67,056 | ) | | | (62,946 | ) |
Income tax benefit | | | — | | | | (722 | ) | | | (406 | ) |
| | | | | | | | | | | | |
Net loss | | $ | (82,469 | ) | | $ | (66,334 | ) | | $ | (62,540 | ) |
| | | | | | | | | | | | |
Basic and diluted net loss per share | | $ | (2.68 | ) | | $ | (2.31 | ) | | $ | (2.48 | ) |
| | | | | | | | | | | | |
Shares used to compute basic and diluted net loss per share | | | 30,813 | | | | 28,766 | | | | 25,180 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
93
XENOPORT, INC.
STATEMENTS OF STOCKHOLDERS’ EQUITY
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Common Stock | | | Additional
Paid-In
Capital | | | Accumulated
Other
Comprehensive
Income
(Loss) | | | Accumulated
Deficit | | | Total
Stockholders’
Equity | |
| | | Shares | | | Amount | | | | | |
| | | (In thousands, except share amounts) | |
Balance at December 31, 2007 | | | 24,988,683 | | | $ | 25 | | | $ | 301,084 | | | $ | 491 | | | $ | (176,063 | ) | | $ | 125,537 | |
Issuance of common stock upon exercise of options, vesting of restricted stock units and vesting of early exercised options | | | 319,586 | | | | — | | | | 2,731 | | | | — | | | | — | | | | 2,731 | |
Issuance of common stock in connection with Employee Stock Purchase Plan | | | 50,339 | | | | — | | | | 1,636 | | | | — | | | | — | | | | 1,636 | |
Repurchase of common stock | | | (987 | ) | | | — | | | | (6 | ) | | | — | | | | — | | | | (6 | ) |
Employees stock-based compensation expense | | | — | | | | — | | | | 14,867 | | | | — | | | | — | | | | 14,867 | |
Proceeds from common stock and warrants issued upon registered direct offering, net of offering costs | | | 1,889,467 | | | | 2 | | | | 39,699 | | | | — | | | | — | | | | 39,701 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized gains (losses) on investments | | | — | | | | — | | | | — | | | | 48 | | | | — | | | | 48 | |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (62,540 | ) | | | (62,540 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss: | | | — | | | | — | | | | — | | | | — | | | | — | | | | (62,492 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2008 | | | 27,247,088 | | | | 27 | | | | 360,011 | | | | 539 | | | | (238,603 | ) | | | 121,974 | |
Issuance of common stock upon exercise of options and vesting of restricted stock units | | | 160,930 | | | | — | | | | 587 | | | | — | | | | — | | | | 587 | |
Issuance of common stock in connection with Employee Stock Purchase Plan | | | 120,039 | | | | — | | | | 1,714 | | | | — | | | | — | | | | 1,714 | |
Employees stock-based compensation expense | | | — | | | | — | | | | 18,633 | | | | — | | | | — | | | | 18,633 | |
Proceeds from common stock issued upon public offering, net of offering costs | | | 2,875,000 | | | | 3 | | | | 51,212 | | | | — | | | | — | | | | 51,215 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized gains (losses) on investments | | | — | | | | — | | | | — | | | | (513 | ) | | | — | | | | (513 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (66,334 | ) | | | (66,334 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss: | | | — | | | | — | | | | — | | | | — | | | | — | | | | (66,847 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2009 | | | 30,403,057 | | | | 30 | | | | 432,157 | | | | 26 | | | | (304,937 | ) | | | 127,276 | |
Issuance of common stock upon exercise of options and vesting of restricted stock units | | | 119,605 | | | | — | | | | (358 | ) | | | — | | | | — | | | | (358 | ) |
Issuance of common stock in connection with Employee Stock Purchase Plan | | | 104,100 | | | | — | | | | 859 | | | | — | | | | — | | | | 859 | |
Employees stock-based compensation expense | | | — | | | | — | | | | 17,993 | | | | — | | | | — | | | | 17,993 | |
Proceeds from common stock issued upon public offering, net of offering costs | | | 4,600,000 | | | | 5 | | | | 30,685 | | | | — | | | | — | | | | 30,690 | |
Comprehensive loss: | | | | | | | | | | | | | | | | | | | | | | | | |
Change in unrealized gains (losses) on investments | | | — | | | | — | | | | — | | | | (32 | ) | | | — | | | | (32 | ) |
Net loss | | | — | | | | — | | | | — | | | | — | | | | (82,469 | ) | | | (82,469 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Comprehensive loss: | | | — | | | | — | | | | — | | | | — | | | | — | | | | (82,501 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2010 | | | 35,226,762 | | | $ | 35 | | | $ | 481,336 | | | $ | (6 | ) | | $ | (387,406 | ) | | $ | 93,959 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
94
XENOPORT, INC.
STATEMENTS OF CASH FLOWS
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands) | |
Operating activities | | | | | | | | | | | | |
Net loss | | $ | (82,469 | ) | | $ | (66,334 | ) | | $ | (62,540 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: | | | | | | | | | | | | |
Depreciation and amortization | | | 4,163 | | | | 3,635 | | | | 2,762 | |
Accretion of investment discounts and amortization of investment premiums, net | | | 1,090 | | | | 1,438 | | | | (1,603 | ) |
Stock-based compensation expense | | | 17,993 | | | | 18,633 | | | | 14,867 | |
Changes in assets and liabilities: | | | | | | | | | | | | |
Accounts receivable | | | — | | | | — | | | | 1,392 | |
Prepaids and other current and noncurrent assets | | | 1,513 | | | | (799 | ) | | | (238 | ) |
Accounts payable | | | (1,516 | ) | | | (230 | ) | | | 614 | |
Accrued compensation | | | (3,160 | ) | | | 821 | | | | 909 | |
Accrued preclinical and clinical costs | | | 1,775 | | | | (6,598 | ) | | | 981 | |
Accrued unconsolidated joint operating activities | | | (1,095 | ) | | | 1,095 | | | | — | |
Other accrued liabilities | | | 65 | | | | (1,090 | ) | | | (568 | ) |
Deferred revenue | | | (1,784 | ) | | | (7,368 | ) | | | (1,995 | ) |
Deferred rent | | | (1,056 | ) | | | (883 | ) | | | 177 | |
| | | | | | | | | | | | |
Net cash used in operating activities | | | (64,481 | ) | | | (57,680 | ) | | | (45,242 | ) |
| | | | | | | | | | | | |
Investing activities | | | | | | | | | | | | |
Purchases of investments | | | (144,085 | ) | | | (231,650 | ) | | | (205,569 | ) |
Proceeds from sales of investments | | | — | | | | — | | | | 79,719 | |
Proceeds from maturities of investments | | | 164,973 | | | | 209,019 | | | | 182,948 | |
Change in restricted investments | | | (15 | ) | | | (109 | ) | | | (53 | ) |
Purchases of property and equipment | | | (646 | ) | | | (2,891 | ) | | | (7,441 | ) |
| | | | | | | | | | | | |
Net cash provided by (used in) investing activities | | | 20,227 | | | | (25,631 | ) | | | 49,604 | |
| | | | | | | | | | | | |
Financing activities | | | | | | | | | | | | |
Net proceeds from issuance of common stock and warrants and exercise of stock options | | | 31,191 | | | | 53,516 | | | | 43,908 | |
Payments on equipment financing obligations | | | — | | | | — | | | | (181 | ) |
| | | | | | | | | | | | |
Net cash provided by financing activities | | | 31,191 | | | | 53,516 | | | | 43,727 | |
| | | | | | | | | | | | |
Net increase (decrease) in cash and cash equivalents | | | (13,063 | ) | | | (29,795 | ) | | | 48,089 | |
Cash and cash equivalents at beginning of period | | | 36,255 | | | | 66,050 | | | | 17,961 | |
| | | | | | | | | | | | |
Cash and cash equivalents at end of period | | $ | 23,192 | | | $ | 36,255 | | | $ | 66,050 | |
| | | | | | | | | | | | |
Supplemental schedule of non-cash investing and financing activities | | | | | | | | | | | | |
Vesting of common stock from early exercises of stock options | | $ | — | | | $ | — | | | $ | 154 | |
| | | | | | | | | | | | |
Supplemental disclosure of cash flow information | | | | | | | | | | | | |
Income taxes refunded | | $ | 722 | | | $ | 388 | | | $ | 191 | |
| | | | | | | | | | | | |
The accompanying notes are an integral part of these financial statements.
95
XENOPORT, INC.
NOTES TO FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Nature of Operations
XenoPort, Inc., or the Company, was incorporated in the state of Delaware on May 19, 1999. The Company is a biopharmaceutical company focused on developing and commercializing a portfolio of internally discovered product candidates that utilize the body’s natural nutrient transport mechanisms to improve the therapeutic benefits of existing drugs. The Company intends to focus its development and commercialization efforts on potential treatments of diseases with significant unmet medical needs, with an emphasis on central nervous system disorders. Its facilities are located in Santa Clara, California.
Basis of Preparation
In June 2009, the Financial Accounting Standards Board, or FASB, issued the FASB Accounting Standards Codification, or the Codification. Effective September 2009, the Codification became the single source for all authoritative U.S. generally accepted accounting principles, or GAAP, recognized by the FASB and was required to be applied to financial statements issued for interim and annual periods ending after September 15, 2009. The Codification does not change GAAP and did not impact the Company’s financial position or results of operations. The adoption of the Codification only affects the specific references to GAAP literature noted in the Company’s financial statements.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from these estimates.
Fair Value of Financial Instruments
The carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents and short-term investments, approximate fair value due to their short maturities. The Company accounts for the fair value of its financial instruments in accordance with the provisions of theFair Value Measurements and Disclosurestopic of the Codification.
Fair value is the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the measurement date. The Company utilizes market data or assumptions that the Company believes market participants would use in pricing assets or liabilities, including assumptions about risk and the risks inherent in the inputs to the valuation technique. These inputs can be readily observable, market corroborated or generally unobservable. The Company applies the market approach valuation technique for fair value measurements on a recurring basis and attempts to maximize the use of observable inputs and minimize the use of unobservable inputs. All of the Company’s cash equivalents and short-term investments are valued using quoted prices in active markets and are valued at Level 1 or Level 2 within the fair value hierarchy.
Cash Equivalents and Short-Term Investments
The Company considers all highly liquid investments with original maturities of 90 days or less at the time of purchase to be cash equivalents, which primarily consist of money market funds and U.S. government-sponsored agencies.
Management determines the appropriate classification of securities at the time of purchase. All investments have been designated as available-for-sale. The Company views its available-for-sale portfolio as available for use in current operations. Accordingly, the Company has classified all investments as short-term, even though the
96
stated maturity may be one year or more beyond the current balance sheet date. Available-for-sale securities are carried at estimated fair value with unrealized gains and losses reported as a component of accumulated other comprehensive income (loss) in stockholders’ equity.
The amortized cost of securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization and accretion is included in interest income. Realized gains and losses and declines in value judged to be other-than-temporary on available-for-sale securities, if any, are recorded in interest income and expense. The cost of securities sold is based on the specific-identification method. Interest and dividends are included in interest income.
Restricted Investments
Under a facilities operating lease agreement, the Company is required to secure a letter of credit with cash or securities. At December 31, 2010 and 2009, the Company recorded $1,699,000 and $1,685,000, respectively, of restricted investments related to the letter of credit (see Note 6).
In connection with the Company’s license to use radioactive materials in its research facilities, it must maintain a $225,000 letter of credit with the Radiological Health Branch of the State of California. This requirement has been fulfilled through certificates of deposit with a financial institution. The fair value of the secured amount of $249,000 and $248,000 was classified as restricted investments in the accompanying balance sheets at December 31, 2010 and 2009, respectively.
Concentrations of Risk
The Company invests cash that is not being used for operational purposes. This exposes the Company to credit risk in the event of default by the institutions holding the cash and cash equivalents and available-for-sale securities. The credit risk is mitigated by the Company’s investment policy, which allows for the purchase of low risk debt securities issued by the U.S. government, U.S. government-sponsored agencies and very highly rated banks and corporations, subject to certain concentration limits. The maturities of these securities are maintained at no longer than 18 months. The Company believes its established guidelines for investment of its excess cash maintains safety and liquidity through its policies on diversification and investment maturity.
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and available-for-sale investment securities in high-credit quality debt securities issued by the U.S. government, U.S. government-sponsored enterprises and very highly rated banks and corporations. The carrying amounts of cash equivalents and available-for-sale investment securities approximate fair value due to their short-term nature.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation and amortization is computed using the straight-line method over the estimated useful lives of the respective assets, which is generally five years for the Company’s laboratory equipment and furniture and fixtures and generally three years for the Company’s computer equipment and software. Leasehold improvements are amortized over their estimated useful lives or the remaining lease term, whichever is shorter.
Revenue Recognition
Revenue arrangements are accounted for in accordance with the provisions of theRevenue Recognition-Multiple-Element ArrangementandCollaborative Arrangementstopics of the Codification. A variety of factors are considered in determining the appropriate method of revenue recognition under these arrangements, such as whether the various elements can be considered separate units of accounting, whether there is objective and reliable evidence of fair value for these elements and whether there is a separate earnings process associated with a particular element of an agreement.
97
Where there are multiple deliverables combined as a single unit of accounting, revenues are deferred and recognized over the period during which the Company remains obligated to perform services. The specific methodology for the recognition of the revenue (e.g., straight-line or according to specific performance criteria) is determined on a case-by-case basis according to the facts and circumstances applicable to a given agreement. For contracts with specific performance criteria, the Company utilizes the performance-based expected revenue method of revenue recognition, which requires that the Company estimate the total amount of costs to be expended for a given unit of accounting and then recognize revenue equal to the portion of costs expended to date. The estimated total costs to be expended are subject to revision from time-to-time as the underlying facts and circumstances change.
Payments received in excess of revenues recognized are recorded as deferred revenue until such time as the revenue recognition criteria have been met.
Collaboration revenue includes revenues from the Company’s current collaboration agreement with Astellas Pharma Inc. and a collaboration agreement with Xanodyne Pharmaceuticals, Inc. that terminated in July 2009. Net revenue from unconsolidated joint operating activities includes all revenue that results solely from the Company’s current collaboration agreement with Glaxo Group Limited, or GSK. The Company accounts for the revenue activities of these collaboration agreements as follows:
| | • | | Up-front, licensing-type fees. Up-front, licensing-type payments are assessed to determine whether or not the licensee is able to obtain any stand-alone value from the license. Where this is not the case, the Company does not consider the license deliverable to be a separate unit of accounting, and the revenue is deferred with revenue recognition for the license fee being assessed in conjunction with the other deliverables that constitute the combined unit of accounting. |
| | • | | Milestones. Milestones are assessed on an individual basis, and revenue is recognized from these milestones when earned, as evidenced by acknowledgment from collaborators, provided that (i) the milestone event is substantive and its achievability was not reasonably assured at the inception of the agreement, (ii) the milestone represents the culmination, or progress towards the culmination, of an earnings process and (iii) the milestone payment is non-refundable. Milestones that are received after all substantive deliverables have occurred are considered to be bonus payments and are recognized when earned, assuming all of the other revenue recognition criteria are met. Where separate milestones do not meet these criteria, the Company uses a performance-based model, with revenue recognition following delivery of effort as compared to an estimate of total expected effort. |
| | • | | Profit and loss sharing. This represents the Company’s share of the profits and losses from the co-promotion ofHorizant with GSK. Amounts are recognized in the period in which the related activities occur, and their financial statement classification is based on the Company’s assessment that these activities constitute part of the Company’s ongoing central operations. |
The Company’s current collaboration agreements also include potential payments for product royalties and detail reimbursements. To date, the Company has not received any revenue from these activities.
Research and Development
All research and development costs, including those funded by third parties, are expensed as incurred. Research and development costs consist of salaries, employee benefits, laboratory supplies, costs associated with clinical trials, including amounts paid to clinical research organizations, other professional services and facility costs.
Clinical Trials
The Company accrues and expenses the costs for clinical trial activities performed by third parties based upon estimates of the percentage of work completed over the life of the individual study in accordance with agreements established with contract research organizations and clinical trial sites. The Company determines the estimates through discussions with internal clinical personnel and external service providers as to progress or stage of completion of trials or services and the agreed upon fee to be paid for such services. Costs of setting up clinical trial sites for participation in the trials are expensed immediately as research and development expenses. Clinical trial site costs related to patient visits are accrued as patients progress through the trial and are reduced
98
by any payments made to the clinical trial site. Non-refundable advance payments for research and development goods or services are recognized as expense as the related goods are delivered or the related services are provided in accordance with the provisions of theResearch and Development Arrangements topic of the Codification.
Stock-Based Compensation
TheCompensation — Stock Compensationtopic of the Codification establishes accounting for stock-based awards exchanged for employee services. In accordance with this topic, for stock options, awards and stock purchase rights granted under the 2005 Employee Stock Purchase Plan, or the ESPP, stock-based compensation cost is measured at grant date, based on the fair value of the award, and is recognized as expense over the requisite employee service period.
The effect of recording stock-based compensation under theCompensation — Stock Compensationtopic was as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands, except per share amounts) | |
Stock-based compensation by type of award: | | | | | | | | | | | | |
Employee stock options | | $ | 17,347 | | | $ | 17,835 | | | $ | 14,315 | |
ESPP | | | 646 | | | | 798 | | | | 552 | |
| | | | | | | | | | | | |
Total stock-based compensation | | $ | 17,993 | | | $ | 18,633 | | | $ | 14,867 | |
| | | | | | | | | | | | |
Effect on basic and diluted net loss per share | | $ | (0.58 | ) | | $ | (0.65 | ) | | $ | (0.59 | ) |
| | | | | | | | | | | | |
The Company’s employee non-cash stock-based compensation, excluding non-cash stock-based compensation resulting from the Company’s restructuring plan, was reported as follows:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | 2010 | | | 2009 | | | 2008 | |
| | (In thousands) | |
Research and development | | $ | 7,930 | | | $ | 10,101 | | | $ | 8,167 | |
Selling, general and administrative | | | 9,210 | | | | 8,532 | | | | 6,700 | |
| | | | | | | | | | | | |
| | $ | 17,140 | | | $ | 18,633 | | | $ | 14,867 | |
| | | | | | | | | | | | |
Valuation Assumptions
The Company estimates the fair value of all of its stock options and stock purchase rights on the date of grant using a Black-Scholes valuation model, and the Company expenses the resulting charge using the straight-line attribution method over the vesting period. Restricted stock units are measured at the fair value of the Company’s common stock on the date of grant and expensed over the period of vesting using the straight-line attribution approach. The calculation of the Black-Scholes valuations used the following weighted-average assumptions:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
Dividend yield | | | 0 | % | | | 0 | % | | | 0 | % |
Volatility for options | | | 0.74 | | | | 0.72 | | | | 0.61 | |
Volatility for ESPP | | | 1.18 | | | | 0.80 | | | | 0.43 | |
Weighted-average expected life of options (years) | | | 5.26 | | | | 5.36 | | | | 4.96 | |
Weighted-average expected life of ESPP rights (years) | | | 0.5 | | | | 0.5 | | | | 0.5 | |
Risk-free interest rate for options | | | 1.18-2.58 | % | | | 1.60-2.71 | % | | | 1.52-3.49 | % |
Risk-free interest rate for ESPP rights | | | 0.19-0.24 | % | | | 0.24-1.74 | % | | | 1.74-4.55 | % |
99
TheCompensation — Stock Compensationtopic of the Codification requires the use of option-pricing models that were not developed for use in valuing employee stock options. The Black-Scholes option-pricing model was developed for use in estimating the fair value of short-lived, exchange-traded options that have no vesting restrictions and are fully transferable. In addition, option-pricing models require the input of highly subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. The Company derives the expected stock price volatility and the weighted-average expected life assumptions using data obtained from similar entities, taking into consideration factors such as industry, stage of life cycle, size and financial leverage. The risk-free interest rate input is based on the U.S. Treasury yield curve in effect at the time of grant.
Income Taxes
Income taxes are accounted for in accordance with theIncome Taxestopic of the Codification using the liability method. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities as measured by the enacted tax rates that will be in effect when these differences reverse. The Company provides a valuation allowance against net deferred tax assets if, based upon the available evidence, it is more-likely-than-not that the deferred tax assets will not be realized.
The recognition, derecognition and measurement of a tax position is based on management’s best judgment given the facts, circumstances and information available at the reporting date.
As of December 31, 2010, the Company continued to have no unrecognized tax benefits and expected no significant changes in unrecognized tax benefits in the next 12 months.
The Company’s policy is to recognize interest and penalties related to the underpayment of income taxes as a component of income tax expense. To date, there have been no interest or penalties charged to the Company in relation to the underpayment of income taxes.
Comprehensive Loss
The Company displays comprehensive loss and its components as part of the statements of stockholders’ equity. Comprehensive loss is comprised of net loss and unrealized gains (losses) on available-for-sale securities.
100
Net Loss per Share
Basic net loss per share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period, less the weighted-average number of unvested common shares subject to repurchase, without consideration for potential common shares. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of common shares outstanding for the period, less the weighted-average number of unvested common shares subject to repurchase, plus any dilutive potential common shares for the period determined using the treasury-stock method. For purposes of this calculation, restricted stock units, options to purchase stock and warrants are considered to be potential common shares and are only included in the calculation of diluted net loss per share when their effect is dilutive.
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | (In thousands, except per share amounts) | |
Numerator: | | | | | | | | | | | | |
Net loss | | $ | (82,469 | ) | | $ | (66,334 | ) | | $ | (62,540 | ) |
| | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | |
Weighted-average common shares outstanding | | | 30,813 | | | | 28,766 | | | | 25,200 | |
Less: Weighted-average unvested common shares subject to repurchase | | | — | | | | — | | | | (20 | ) |
| | | | | | | | | | | | |
Denominator for basic and diluted net loss per share | | | 30,813 | | | | 28,766 | | | | 25,180 | |
| | | | | | | | | | | | |
Basic and diluted net loss per share | | $ | (2.68 | ) | | $ | (2.31 | ) | | $ | (2.48 | ) |
| | | | | | | | | | | | |
Outstanding securities not included in the computation of diluted net loss per share as they had an anti-dilutive effect: | | | | | | | | | | | | |
Restricted stock units and options to purchase common stock | | | 5,168 | | | | 4,206 | | | | 3,496 | |
Warrants outstanding | | | 305 | | | | 305 | | | | 305 | |
| | | | | | | | | | | | |
| | | 5,473 | | | | 4,511 | | | | 3,801 | |
| | | | | | | | | | | | |
Recent Accounting Pronouncements
In September 2009, the FASB Emerging Issues Task Force, or EITF, reached a consensus on Accounting Standards Update, or ASU, 2009-13 (Topic 605),Multiple-Deliverable Revenue Arrangements,or ASU 2009-13. ASU 2009-13 applies to multiple-deliverable revenue arrangements that are currently within the scope of theRevenue Recognition — Multiple-Element Arrangements topic of the Codification. ASU 2009-13 provides principles and application guidance on whether multiple deliverables exist and how the arrangement should be separated and the consideration allocated. ASU 2009-13 requires an entity to allocate revenue in an arrangement using estimated selling prices of deliverables if a vendor does not have vendor-specific objective evidence or third-party evidence of selling price. ASU 2009-13 eliminates the use of the residual method and requires an entity to allocate revenue using the relative selling price method and also significantly expands the disclosure requirements for multiple-deliverable revenue arrangements. ASU 2009-13 will be effective prospectively for revenue arrangements entered into, or materially modified, in fiscal years beginning on or after June 15, 2010, with earlier application permitted. As a result, ASU 2009-13 will be applied by the Company on a prospective basis for revenue arrangements entered into, or materially modified, beginning in the first quarter of fiscal 2011.
In April 2010, the FASB EITF reached a consensus on ASU 2010-17 (Topic 605),Milestone Method of Revenue Recognition,or ASU 2010-17. ASU 2010-17 provides guidance on defining a milestone and determining whether the milestone method of revenue recognition is appropriate. ASU 2010-17 will be effective for milestones achieved in fiscal years, and interim periods within those years, beginning on or after June 15, 2010, with earlier adoption permitted. As a result, ASU 2010-17 will be applied by the Company on a
101
prospective basis for milestones achieved starting in the first quarter of fiscal 2011, including the revised clinical and regulatory milestone payments that the Company is eligible to receive as a result of the amended and restated agreement with GSK. The Company is evaluating the potential impact of the adoption of ASU 2010-17 on the Company’s financial position and results of operations.
2. Collaboration Agreements
Astellas Pharma Inc.
In December 2005, the Company entered into an agreement in which it licensed to Astellas exclusive rights to develop and commercialize the Company’s most advanced product candidate, gabapentin enacarbil (previously known as XP13512), in Japan, Korea, the Philippines, Indonesia, Thailand and Taiwan. The Company received an initial license payment of $25,000,000 in December 2005, which has been deferred and is being recognized on a straight-line basis over a period that the Company expects to remain obligated to provide services. In addition, the Company is eligible to receive potential total payments of $60,000,000 upon the achievement of additional clinical and regulatory milestones, of which $23,000,000 has been received to date. The Company is also entitled to receive percentage-based royalties on any net sales of gabapentin enacarbil in the Astellas territory. In each of the years ended December 31, 2010, 2009 and 2008, the Company recognized revenue of $1,515,000 representing amortization of the up-front license payment under this agreement. In the year ended December 31, 2009, the Company recognized revenue of $8,000,000, representing the recognition of milestone payments under this agreement. As of December 31, 2010, the Company had recognized an aggregate of $30,702,000 of revenue pursuant to this agreement. At December 31, 2010, $17,298,000 of revenue was deferred under this agreement, of which $1,515,000 was classified within current liabilities and the remaining $15,783,000 was recorded as a noncurrent liability. In addition, the agreement allows Astellas to request that the Company conduct development activities and required Astellas to source all drug product and both clinical and commercial supplies of the active pharmaceutical ingredient, or API, form of gabapentin enacarbil from the Company under a specified supply agreement. In October 2009, all of the Company’s remaining manufacturing or supply obligations to Astellas for gabapentin enacarbil API or finished drug product ceased. The Company remains obligated to provide certain services as originally specified in the December 2005 arrangement. Under the supply arrangement and requested development activities, the Company recorded a net offset to research and development expenses of $168,000, $528,000 and $2,737,000 in the years ended December 31, 2010, 2009 and 2008, respectively. Included in the net offset to research and development expenses in the year ended December 31, 2008 is a non-recurring reimbursement of $2,145,000 related to the transfer of gabapentin enacarbil drug substance to Astellas.
Glaxo Group Limited
In February 2007, the Company entered into an exclusive collaboration with GSK to develop and commercialize gabapentin enacarbil, known in the United States by the trade nameHorizant(gabapentin enacarbil) Extended-Release Tablets, for the treatment of moderate-to-severe primary restless legs syndrome, or RLS, in all countries of the world excluding the Astellas territory. In November 2010, the Company amended and restated its collaboration agreement with GSK, pursuant to which the Company reacquired all rights to gabapentin enacarbil outside of the United States previously granted to GSK (which excludes the Astellas territory) and obtained the right, not the obligation, to pursue development ofHorizant for: (i) the potential treatment of diabetic peripheral neuropathy; (ii) the potential treatment of post-herpetic neuralgia, or PHN, to the extent that a product label would reflect a superiority claim over a currently approved drug; and (iii) any additional indications in the United States. GSK remains responsible for seeking approval of the new drug application, or NDA, for RLS in the United States, which currently has a new Prescription Drug User Fee Act, or PDUFA, goal date of April 6, 2011; further development and regulatory matters with respect toHorizant for the potential treatment of PHN, possibly seeking NDA approval through a 505(b)(2) approval process; and manufacturing and commercialization ofHorizant in the United States for all indications.
In March 2007, GSK made an up-front, non-refundable license payment of $75,000,000. Under the terms of the amended and restated collaboration, the aggregate clinical and regulatory milestone payments that the
102
Company is eligible to receive, have been increased by $37,500,000 from a total of $275,000,000 to $312,500,000, of which $85,000,000 has been received to date. The Company remains eligible to receive up to $290,000,000 upon the achievement of specified sales levels; however, the associated sales levels that give rise to these payments have been lowered from the original agreement. The Company concluded that the up-front license payment did not have value to GSK on a stand-alone basis without the benefit of the specified development activities that the Company performed in connection withHorizant and that $85,000,000 of milestones payable for clinical trial and pre-clinical activities were either not sufficiently substantive or not sufficiently at risk to be accounted for using the “when-earned” model. Accordingly, these milestones and the up-front payment were combined into one unit of accounting that was recognized over the best estimate of the development period to commercialization of the product, during which time delivery of substantially all of the efforts required for the completion of the Company’s contractual responsibilities under the GSK agreement has occurred, and the Company has determined that no additional performance obligations resulted from the amended agreement. As of December 31, 2010, the Company had recognized an aggregate of $160,000,000 of up-front license and milestone revenue pursuant to this agreement and no revenue was deferred under this agreement.
The Company exercised its right to the co-promotion arrangement in April 2009, under which all allowable expenses and any potential future sales ofHorizant are accounted for using a joint profit and loss, or P&L, statement, in which the Company and GSK share in the resulting operating pre-tax profits and losses. Under the amended and restated collaboration, the Company’s participation in the co-promotion and joint P&L arrangements remain unchanged, except that the Company can delay the deployment of its sales force for up to three years following the potential approval ofHorizant in the United States and its share of losses from the joint P&L will be forgiven up to a maximum of $10,000,000. The Company’s payment of additional losses, if any, would be deferred and payable without interest over a period of time following the first quarter in which the joint P&L is profitable. In addition, the Company no longer has the right to detail Requip XL, GSK’s product for Parkinson’s disease, as this right would have terminated upon the earlier of the launch of a generic form of Requip XL or July 1, 2011, under the original agreement. Pending FDA approval ofHorizant, GSK is responsible for: establishing pricing and reimbursement; creating promotional and advertising materials; managed care contracting; receiving, accepting and filling orders; distributing; controlling invoicing, order processing and collecting accounts receivable; and recording sales ofHorizant in the United States. Expenses that can be charged to the joint P&L statement are the cost of goods and certain costs directly related toHorizant marketing and sales. Sales and marketing expenses ofHorizantthat the Company incurs that are not charged to the joint P&L statement are classified as selling, general and administrative operating expenses within the Company’s statements of operations. The Company has concluded that under the original and amended agreement, the potential detail ofHorizant and the amount from the joint P&L statement together constitute one unit of accounting separate from the previously established milestone and up-front payment unit of accounting. The Company also has determined the commercialization of its portfolio of product candidates to be part of its core operations, and accordingly concluded that all revenue resulting from the Company’s GSK collaboration agreement is presented in the net revenue from unconsolidated joint operating activities line item in the revenues section of the statements of operations in the period the related activities occur. The Company began recording its share of pre-launch operating losses from the joint P&L statement ofHorizant in the second quarter of 2009, and the total pre-launch operating losses of $1,095,000 recorded as of December 31, 2009 were forgiven and therefore reversed in the fourth quarter of 2010 as a result of the amended and restated development and commercialization agreement in November 2010. No detailing activities occurred and no detail reimbursements were recognized in the years ended December 31, 2010 and 2009.
Upon approval, the Company would share any profits on sales ofHorizant in the United States at tiered rates that escalate as a function of annual net sales levels, from a low of 20% to a maximum of 50%. For example (and for illustrative purposes only), if the annual net sales ofHorizant reach $250,000,000, $500,000,000 and $1,000,000,000, the Company would be entitled to blended profit share rates of 25%, 34% and 42%, respectively. The Company may terminate its co-promotion right and participation in the profit share arrangement at any time upon notice to GSK with no penalty to the Company, resulting in a royalty-based compensation structure, whereby the Company would receive royalties on annual net sales in the United States at tiered rates that escalate as a function of net sales levels from a low of 15% to a maximum of 30%. For example (and for illustrative purposes only), if the annual net sales ofHorizant reach $250,000,000, $500,000,000 and
103
$1,000,000,000, the Company would be entitled to blended royalty rates of 17%, 21% and 25%, respectively. GSK may terminate the Company’s co-promotion right for the Company not meeting a minimum sales requirement, for the Company’s uncured material breach in conducting co-promotional activities or upon the Company’s change of control in certain circumstances. GSK may terminate the collaboration agreement in its entirety for any reason and at any time. In such event, certainHorizant product rights would revert to the Company, and the Company would be entitled to specified transition assistance from GSK.
The Company’s net revenue from unconsolidated joint operating activities from the GSK collaboration agreement was comprised of the following:
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | 2010 | | | 2009 | | | 2008 | |
| | (In thousands) | |
Up-front license and development milestone revenue | | $ | 269 | | | $ | 25,853 | | | $ | 28,981 | |
XenoPort’s share of pre-launch operating losses | | | 1,095 | | | | (1,095 | ) | | | — | |
| | | | | | | | | | | | |
Net revenue from unconsolidated joint operating activities | | $ | 1,364 | | | $ | 24,758 | | | $ | 28,981 | |
| | | | | | | | | | | | |
Xanodyne Pharmaceuticals, Inc.
In October 2007, the Company licensed to Xanodyne exclusive rights to develop and commercialize the Company’s product candidate XP21510 in the United States, including for the potential treatment of women diagnosed with menorrhagia. In exchange for these rights, the Company received and recognized non-refundable cash payments totaling $13,000,000, of which $6,000,000 was paid to the Company upon execution of the agreement, $6,000,000 was paid in October 2008 and $1,000,000 was paid in April 2008 as a milestone payment. In July 2009, the collaboration agreement with Xanodyne terminated and all XP21510 product rights reverted to the Company. In the years ended December 31, 2010, 2009 and 2008, the Company recognized revenue of $0, $0 and $11,500,000, respectively, under this agreement. At December 31, 2010, no revenue was deferred under this agreement.
The following table presents the Company’s total revenues that have been recognized pursuant to all of its collaborations (in thousands):
| | | | | | | | | | | | |
| | | Year Ended December 31, | |
| | 2010 | | | 2009 | | | 2008 | |
Astellas | | $ | 1,515 | | | $ | 9,515 | | | $ | 1,515 | |
GSK | | | 1,364 | | | | 24,758 | | | | 28,981 | |
Xanodyne | | | — | | | | — | | | | 11,500 | |
| | | | | | | | | | | | |
| | $ | 2,879 | | | $ | 34,273 | | | $ | 41,996 | |
| | | | | | | | | | | | |
104
3. Cash and Cash Equivalents, Short-Term Investments and Restricted Investments
The following are summaries of cash and cash equivalents, short-term investments and restricted investments (in thousands):
| | | | | | | | | | | | | | | | |
| | | Cost | | | Gross
Unrealized
Gains | | | Gross
Unrealized
Losses | | | Estimated
Fair Value | |
As of December 31, 2010: | | | | | | | | | | | | | | | | |
Cash | | $ | 915 | | | $ | — | | | $ | — | | | $ | 915 | |
Money market funds | | | 9,917 | | | | — | | | | — | | | | 9,917 | |
U.S. treasury securities | | | 5,515 | | | | 1 | | | | — | | | | 5,516 | |
U.S. government-sponsored agencies | | | 38,552 | | | | 2 | | | | (11 | ) | | | 38,543 | |
Corporate debt securities | | | 53,702 | | | | 10 | | | | (8 | ) | | | 53,704 | |
Certificates of deposit | | | 1,948 | | | | — | | | | — | | | | 1,948 | |
| | | | | | | | | | | | | | | | |
| | $ | 110,549 | | | $ | 13 | | | $ | (19 | ) | | $ | 110,543 | |
| | | | | | | | | | | | | | | | |
Reported as: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | | | | | $ | 23,192 | |
Short-term investments | | | | | | | | | | | | | | | 85,403 | |
Restricted investments | | | | | | | | | | | | | | | 1,948 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | $ | 110,543 | |
| | | | | | | | | | | | | | | | |
| | | Cost | | | Gross
Unrealized
Gains | | | Gross
Unrealized
Losses | | | Estimated
Fair Value | |
As of December 31, 2009: | | | | | | | | | | | | | | | | |
Cash | | $ | 4,115 | | | $ | — | | | $ | — | | | $ | 4,115 | |
Money market funds | | | 18,260 | | | | — | | | | — | | | | 18,260 | |
U.S. treasury securities | | | 22,024 | | | | — | | | | (7 | ) | | | 22,017 | |
U.S. government-sponsored agencies | | | 99,243 | | | | 40 | | | | (7 | ) | | | 99,276 | |
Certificates of deposit | | | 1,933 | | | | — | | | | — | | | | 1,933 | |
| | | | | | | | | | | | | | | | |
| | $ | 145,575 | | | $ | 40 | | | $ | (14 | ) | | $ | 145,601 | |
| | | | | | | | | | | | | | | | |
Reported as: | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | | | | | $ | 36,255 | |
Short-term investments | | | | | | | | | | | | | | | 107,413 | |
Restricted investments | | | | | | | | | | | | | | | 1,933 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | $ | 145,601 | |
| | | | | | | | | | | | | | | | |
At December 31, 2010 and 2009, the contractual maturities of all investments held were less than one year.
The Company recognized $445,000 in the year ended December 31, 2008 of gross realized gains on sales of short-term investments based on the specific identification method. No gross realized gains or losses were recognized in 2010 and 2009.
105
The Company’s available-for-sale investments, which include cash equivalents and short-term investments, are measured at fair value using the following inputs (in thousands):
| | | | | | | | | | | | | | | | |
| | | | | | Fair Value Measurements at Reporting Date Using | |
Description | | Total As of
December 31,
2010 | | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | | Significant
Other
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Money market funds | | $ | 9,917 | | | $ | 9,917 | | | $ | — | | | $ | — | |
U.S. treasury securities | | | 5,516 | | | | — | | | | 5,516 | | | | — | |
U.S. government-sponsored agencies | | | 38,543 | | | | — | | | | 38,543 | | | | — | |
Corporate debt securities | | | 53,704 | | | | — | | | | 53,704 | | | | — | |
| | | | | | | | | | | | | | | | |
Total | | $ | 107,680 | | | $ | 9,917 | | | $ | 97,763 | | | $ | — | |
| | | | | | | | | | | | | | | | |
| | |
Description | | Total As of
December 31,
2009 | | | Fair Value Measurements at Reporting Date Using | |
| | | Quoted Prices in
Active Markets
for Identical
Assets
(Level 1) | | | Significant
Other
Observable
Inputs
(Level 2) | | | Significant
Unobservable
Inputs
(Level 3) | |
Money market funds | | $ | 18,260 | | | $ | 18,260 | | | $ | — | | | $ | — | |
U.S. treasury securities | | | 22,017 | | | | — | | | | 22,017 | | | | — | |
U.S. government-sponsored agencies | | | 99,276 | | | | — | | | | 99,276 | | | | — | |
| | | | | | | | | | | | | | | | |
Total | | $ | 139,553 | | | $ | 18,260 | | | $ | 121,293 | | | $ | — | |
| | | | | | | | | | | | | | | | |
4. Property and Equipment
Property and equipment consisted of the following (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2009 | |
Laboratory equipment | | $ | 11,836 | | | $ | 11,916 | |
Furniture and fixtures | | | 1,287 | | | | 1,233 | |
Computer equipment and software | | | 5,345 | | | | 4,940 | |
Leasehold improvements | | | 4,596 | | | | 4,571 | |
Construction in-progress | | | 198 | | | | 205 | |
| | | | | | | | |
| | | 23,262 | | | | 22,865 | |
Less: Accumulated depreciation and amortization | | | (16,053 | ) | | | (12,139 | ) |
| | | | | | | | |
Property and equipment, net | | $ | 7,209 | | | $ | 10,726 | |
| | | | | | | | |
5. Restructuring
On March 5, 2010, as a result of the Complete Response letter from the FDA, the Company implemented a restructuring plan to reduce expenses, focus the Company’s resources on advancement of its later-stage product candidates and eliminate the Company’s discovery research efforts. The restructuring plan resulted in a reduction in force of 107 employees, or approximately 50% of the Company’s workforce. The Company provided affected employees with up to 60 days of leave of absence pay in accordance with theWorker Adjustment and Retraining Notification Act, and provided 60 days of employee benefits and continued vesting of stock options and awards. Qualified affected employees were also eligible to receive severance payments, transition pay, continuation of medical insurance under COBRA, a two-year extension of exercisability of stock options vested as of May 4, 2010 and outplacement services.
106
As a result of this restructuring, the Company recorded restructuring charges of $5,275,000 in the three months ended March 31, 2010, which were included on a separate line in the Company’s statements of operations, in accordance with theExit or Disposal Cost Obligations topic of the Codification. The Company does not expect to incur additional charges in relation to the March 2010 restructuring plan. The restructuring components are summarized in the following table (in thousands):
| | | | | | | | | | | | | | | | | | | | |
| | | Paid Leave,
Severance
and Other
Benefits | | | Non-Cash
Stock-Based
Compensation | | | Property and
Equipment | | | Legal and
Other | | | Total | |
Net restructuring charges | | $ | 3,910 | | | $ | 853 | | | $ | 437 | | | $ | 75 | | | $ | 5,275 | |
Cash payments | | | (966 | ) | | | — | | | | — | | | | (16 | ) | | | (982 | ) |
Non-cash charges | | | — | | | | (853 | ) | | | (437 | ) | | | (19 | ) | | | (1,309 | ) |
| | | | | | | | | | | | | | | | | | | | |
Ending liability balance at March 31, 2010 | | | 2,944 | | | | — | | | | — | | | | 40 | | | | 2,984 | |
Cash payments | | | (2,692 | ) | | | — | | | | — | | | | (40 | ) | | | (2,732 | ) |
| | | | | | | | | | | | | | | | | | | | |
Ending liability balance at June 30, 2010 | | | 252 | | | | — | | | | — | | | | — | | | | 252 | |
Cash payments | | | (252 | ) | | | — | | | | — | | | | — | | | | (252 | ) |
| | | | | | | | | | | | | | | | | | | | |
Ending liability balance at September 30 and December 31, 2010 | | $ | — | | | $ | — | | | $ | — | | | $ | — | | | $ | — | |
| | | | | | | | | | | | | | | | | | | | |
6. Commitments and Contingencies
Operating Leases
In February 2008, the Company entered into a lease for approximately 59,000 square feet of office space in a building at 3400 Central Expressway, Santa Clara, California, or the 3400 Lease. The term of the 3400 Lease runs for 60 months.
Also in February 2008, the Company amended its lease with respect to the Company’s current office space at 3410 Central Expressway, Santa Clara, California, or, as amended, the 3410 Lease, that commenced in December 2001. This amendment extended the term of the 3410 Lease for approximately two years from the original expiration date of December 10, 2011, so that the 3410 Lease will expire in 2013, on the same date as the 3400 Lease.
The Company has the option to extend both the 3410 Lease and 3400 Lease for two additional terms of five years each.
In connection with the 3410 Lease, the Company entered into a letter of credit agreement of $1,500,000 in December 2006. The fair value of the certificate of deposit is presented as restricted investments on the balance sheet at $1,699,000 and $1,685,000 at December 31, 2010 and 2009, respectively. This letter of credit is required until the termination of the lease.
The Company is recognizing rent expense on a straight-line basis over the applicable lease terms. The Company began recognizing rent expense on the 3400 Lease in May 2008. Rent expense was $4,443,000, $4,443,000 and $4,140,000 for the years ended December 31, 2010, 2009 and 2008, respectively. Net deferred rent of $(67,000) and $988,000 at December 31, 2010 and 2009, respectively, represented the difference between rent expense recognized and actual cash payments related to the Company’s operating leases. At December 31, 2010, net deferred rent was comprised of a current deferred rent liability of $1,104,000 and a noncurrent deferred rent asset of $1,171,000.
At December 31, 2010, future minimum payments under all non-cancelable operating leases were as follows (in thousands):
| | | | |
Year ending December 31: | | | | |
2011 | | $ | 5,547 | |
2012 | | | 3,723 | |
2013 | | | 2,640 | �� |
| | | | |
Total minimum lease payments | | $ | 11,910 | |
| | | | |
107
Guarantees and Indemnifications
The Company, as permitted under Delaware law and in accordance with its bylaws, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, while the officer or director is or was serving at the Company’s request in such capacity. The term of the indemnification period is for the officer’s or director’s lifetime. The Company may terminate the indemnification agreements with its officers and directors upon 90 days’ written notice, but termination will not affect claims for indemnification relating to events occurring prior to the effective date of termination. The maximum amount of potential future indemnification is unlimited; however, the Company has a director and officer insurance policy that limits its exposure and may enable it to recover a portion of any future amounts paid. The Company believes the fair value of these indemnification agreements is minimal. Accordingly, the Company had not recorded any liabilities for these agreements as of December 31, 2010.
Contingencies
In July 2010, a purported securities class action lawsuit was filed in the United States District Court for the Northern District of California, naming the Company and certain of its officers and directors as defendants. The lawsuit alleges violations of the Securities Exchange Act of 1934, as amended, in connection with allegedly false, misleading and incomplete statements issued by the defendants related toHorizant as a potential treatment of moderate-to-severe primary RLS, which allegedly made it impossible for investors to meaningfully understand the drug’s potential for FDA approval. The plaintiff seeks damages, an award of its costs and injunctive and/or equitable relief on behalf of a purported class of stockholders who purchased the Company’s common stock during the period between May 5, 2009 and February 17, 2010. Another lawsuit was filed in September 2010 in the United States District Court for the Northern District of California making substantially similar allegations, on behalf of a purported class of stockholders who purchased the Company’s common stock during the period between March 16, 2009 and May 5, 2010. In November 2010, a motion to consolidate the complaints and appoint a lead plaintiff was granted. In January 2011, the lead plaintiff filed a consolidated complaint. In February 2011, the Company responded to the complaint with a motion to dismiss. The lead plaintiff’s opposition brief to the Company’s motion is due on April 4, 2011. A hearing on the motion to dismiss is currently scheduled for May 20, 2011.
The Company believes that it has meritorious defenses and intends to defend this lawsuit vigorously. The Company is not able to estimate the possible cost to the Company from this matter, as this lawsuit is at an early stage and the Company cannot be certain how long it may take to resolve this matter or the possible amount of any damages that the Company may be required to pay. Therefore, the Company has not established any reserves for any potential liability relating to this lawsuit.
7. Stockholders’ Equity
Common Stock
At December 31, 2010 and 2009, the Company was authorized to issue 60,000,000 shares of common stock.
Stockholders’ Rights Plan
On December 16, 2005, the Company adopted a preferred stock rights plan pursuant to which each share of common stock outstanding on January 13, 2006, and each subsequently issued share, will receive a non-taxable dividend. The dividend will confer the purchase right, or a right, that confers the right to purchase one one-hundredth of a share of a new class of preferred stock and will be exercisable only if a person or group acquires 15% or more of the Company’s common stock or announces a tender offer for 15% or more of the Company’s common stock. If such a person acquires 15% or more of the Company’s common stock, all rights holders, except the 15% acquiror, will be entitled to acquire the Company’s common stock at a discount through the exercise of the preferred stock. The rights plan has been designed to discourage acquisitions of more than 15% of the Company’s common stock without negotiations with the board of directors. The rights expire on January 13, 2016. The rights will trade with the Company’s common stock, unless and until they are separated upon the occurrence of certain future events. The board of directors may terminate the rights plan at any time or redeem the rights prior to the time the rights are triggered.
108
Equity Incentive Plans
1999 Stock Plan
Under the terms of the 1999 Stock Plan, or the 1999 Plan, options or stock purchase rights were granted by the board of directors to employees, directors and consultants. Options granted were either incentive stock options or non-statutory stock options. Incentive stock options were granted to employees with exercise prices of no less than the fair value, and non-statutory options were granted to employees, directors or consultants at exercise prices of no less than 85% of the fair value, of the common stock on the grant date as determined by the board of directors. Options vest as determined by the board of directors, generally at the rate of 25% at the end of the first year, with the remaining balance vesting ratably over the next three years for initial employee grants and ratably over four years for subsequent grants. Options granted under the 1999 Plan expire no more than ten years after the date of grant. All options granted under the 1999 Plan have vested.
2005 Equity Incentive Plan
In January 2005, the Company’s board of directors adopted the 2005 Equity Incentive Plan, or the 2005 Plan. Under the terms of the 2005 Plan, options, stock purchase rights, stock bonus rights, stock appreciation rights and other stock awards and rights may be granted by the board of directors to employees, directors and consultants. Options granted may be either incentive stock options or non-statutory stock options. Incentive stock options may be granted to employees with exercise prices of no less than the fair value, and non-statutory options may be granted to employees, directors or consultants at exercise prices of no less than 85% of the fair value, of the common stock on the grant date. Options vest as determined by the board of directors, generally at the rate of 25% at the end of the first year, with the remaining balance vesting ratably over the next three years for initial employee grants and ratably over four years for subsequent grants. Options granted under the 2005 Plan expire no more than ten years after the date of grant.
In January 2007, the Company’s board of directors approved the use of grants of restricted stock units to employees under the 2005 Plan as part of the Company’s long-term incentive compensation program. Restricted stock units have no exercise price, are valued using the closing market price on the date of grant and vest as determined by the board of directors, typically in annual tranches over a four-year period at the rate of 25% at the end of each year. Employees can elect to have the Company withhold a portion of shares to pay for their payroll taxes in connection with the vesting of restricted stock units, where the Company would then make a cash payment for the associated payroll taxes on behalf of the employees, or employees can elect to make the cash payment for the associated payroll taxes.
In May 2010, the Company granted performance stock unit awards to two executive employees. Each performance stock unit award is scheduled to vest three years from the grant date, with the actual number of shares of common stock of the Company subject to issuance to be between 0% and 200% of the target amount, based on the performance of the Company’s total shareholder return as compared to the total shareholder returns of a group of pre-selected pharmaceutical companies over a performance period ending on the third anniversary of the grant date. The target amount of shares of common stock of the Company that were subject to issuance under the performance stock unit awards was 140,000, and the grant date fair value using a lattice valuation model of these performance stock unit awards was $2,675,000. In 2010, a performance stock unit award representing a target amount of 40,000 shares was cancelled due to the departure of one of the two executive employees. At December 31, 2010, a performance stock unit award representing a target amount of 100,000 shares was outstanding, and the associated expense recognized in the year ended December 31, 2010 was $405,000.
Stock purchase rights, stock bonus rights, stock appreciation rights and other stock awards and rights may be granted by the board of directors to employees, directors and consultants and may be subject to such terms and conditions as the board of directors deems appropriate, although such awards may not be granted with a purchase price below the par value of the stock. Under the terms of the 2005 Plan, the maximum number of shares that may be issued shall not exceed the total of 2,000,000, plus any shares issuable from options previously granted from the 1999 Plan at the date of the Company’s initial public offering, plus an annual increase equal to the lesser of (i) 2.5% of the total number of common shares outstanding at the end of the preceding calendar year and
109
(ii) 2,000,000 common shares. During the year ended December 31, 2010, the annual increase to the 2005 Plan reserve was 760,076 shares. At December 31, 2010 and 2009, there were 902,596 and 1,098,762 shares, respectively, remaining and available for future grant under the 2005 Plan.
New Employee Inducement Stock Awards
In May 2008, the Company’s Senior Vice President and Chief Commercialization Officer was granted a new employee inducement stock award outside of the Company’s stockholder-approved equity plans consisting of nonqualified stock options to purchase 140,612 shares of the Company’s common stock. The stock options have a per share exercise price of $42.59, the closing trading price of the Company’s common stock on the NASDAQ Global Market on the May 1, 2008 grant date. The stock options have a ten-year term and vest over four years, with 25% cliff vesting on the first anniversary of the May 1, 2008 grant date, and 1/48th of the shares subject to the options vesting monthly thereafter. The Company also granted to the Company’s Senior Vice President and Chief Commercialization Officer a new employee inducement stock award outside of the Company’s stockholder-approved equity plans consisting of restricted stock units for 10,000 shares of the Company’s common stock. The restricted stock units shall vest in four equal annual installments on each anniversary of the May 1, 2008 grant date.
In July 2008, the Company’s Senior Vice President and Chief Medical Officer was granted a new employee inducement stock award outside of the Company’s stockholder-approved equity plans consisting of nonqualified stock options to purchase 139,888 shares of the Company’s common stock. The stock options have a per share exercise price of $39.55, the closing trading price of the Company’s common stock on the NASDAQ Global Market on the July 14, 2008 grant date. The stock options have a ten-year term and vest over four years, with 25% cliff vesting on the first anniversary of the July 14, 2008 grant date, and 1/48th of the shares subject to the options vesting monthly thereafter. The Company also granted to the Company’s Senior Vice President and Chief Medical Officer a new employee inducement stock award outside of the Company’s stockholder-approved equity plans consisting of restricted stock units for 10,000 shares of the Company’s common stock. The restricted stock units shall vest in four equal annual installments on each anniversary of the August 1, 2008 grant date. As of December 31, 2010, no restricted stock units were outstanding due to the departure of the Company’s Senior Vice President and Chief Medical Officer and 81,601 nonqualified stock options remained outstanding.
2010 Inducement Award Plan
In May 2010, the Company’s board of directors adopted the 2010 Inducement Award Plan, or the 2010 Inducement Plan. Under the terms of the 2010 Inducement Plan, options, stock purchase awards, stock bonus awards, stock appreciation rights, stock unit awards and other stock awards may be granted by the board of directors or the independent compensation committee of the board of directors to persons entering into employment with the Company and not previously employees or directors of the Company (or followingbona fide periods of non-employment with the Company) as an inducement material to the new employees entering into employment with the Company in accordance with NASDAQ Market Place Rule 5635(c)(4). Options granted may be non-statutory stock options with exercise prices of no less than 100% of the fair value of the Company’s common stock on the grant date. Options vest as determined by the board of directors or the compensation committee of the board of directors, generally at the rate of 25% at the end of the first year, with the remaining balance vesting ratably over the next three years. Options granted under the 2010 Inducement Plan expire no more than ten years after the date of grant. Restricted stock units have no exercise price, are valued using the closing market price on the date of grant and vest as determined by the board of directors or the compensation committee of the board of directors, typically in annual tranches over a four-year period at the rate of 25% at the end of each year.
Under the terms of the 2010 Inducement Plan, the maximum number of shares that may be issued shall not exceed the total of 350,000. At December 31, 2010, there were 182,650 shares remaining and available for future grant under the 2010 Inducement Plan.
110
2005 Non-Employee Directors’ Stock Option Plan
In January 2005, the Company’s board of directors adopted the 2005 Non-Employee Directors’ Stock Option Plan, or the 2005 Directors’ Plan, under which non-statutory options are automatically granted to non-employee directors. Any individual who first becomes a non-employee director automatically receives an option to purchase 25,000 shares subject to vesting in four equal successive annual installments. Non-employee directors serving on the date of each annual meeting of stockholders receive an option to purchase 10,000 shares subject to vesting in 12 successive equal monthly installments measured from the grant date. Stock options may be granted at exercise prices no less than the fair value on the grant date and may expire no more than ten years after the date of grant. Under the terms of the 2005 Directors’ Plan, the maximum number of shares that may be issued shall not exceed the total of 150,000, plus an annual increase equal to the excess of (i) the number of shares subject to options granted in the preceding calendar year, over (ii) the number of shares added back to the share reserve from cancellations, provided that such increase shall not exceed 150,000 shares. During the year ended December 31, 2010, the annual increase to the 2005 Directors’ Plan reserve was 87,083 shares. At December 31, 2010 and 2009, there were 122,917 and 62,917 shares, respectively, remaining and available for future grant under the 2005 Directors’ Plan.
A summary of option activity as of December 31, 2010 is presented below:
| | | | | | | | | | | | | | | | |
| | | Shares | | | Weighted-
Average
Exercise
Price | | | Weighted-
Average
Remaining
Contractual
Term | | | Aggregate
Intrinsic
Value | |
| | | | | | | | | | | | (In thousands) | |
Outstanding at January 1, 2010 | | | 3,926,455 | | | $ | 27.47 | | | | | | | | | |
Options granted | | | 1,069,500 | | | $ | 16.67 | | | | | | | | | |
Options cancelled | | | (734,609 | ) | | $ | 26.82 | | | | | | | | | |
Options exercised | | | (41,304 | ) | | $ | 3.20 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Outstanding at December 31, 2010 | | | 4,220,042 | | | $ | 25.08 | | | | 6.12 | | | $ | (69,901 | ) |
| | | | | | | | | | | | | | | | |
Exercisable at December 31, 2010 | | | 3,006,912 | | | $ | 25.52 | | | | 5.15 | | | $ | (51,118 | ) |
| | | | | | | | | | | | | | | | |
A summary of restricted stock and performance stock unit activity as of December 31, 2010 is presented below:
| | | | | | | | |
| | | Shares | | | Weighted-
Average
Grant Date
Fair Value | |
Outstanding at January 1, 2010 | | | 279,765 | | | $ | 32.45 | |
Awards granted | | | 1,024,950 | | | $ | 11.81 | |
Awards cancelled | | | (237,634 | ) | | $ | 21.08 | |
Awards vested | | | (119,057 | ) | | $ | 32.13 | |
| | | | | | | | |
Outstanding at December 31, 2010 | | | 948,024 | | | $ | 13.02 | |
| | | | | | | | |
The aggregate intrinsic value of all options outstanding and exercisable at December 31, 2010 was based on a closing stock price of $8.52.
The weighted-average grant date fair values of options granted in the years ended December 31, 2010, 2009 and 2008 were $10.45, $14.09 and $24.98 per share, respectively. The weighted-average grant date fair values of restricted stock units and performance stock units granted in the years ended December 31, 2010, 2009 and 2008 were $11.81, $25.01 and $50.10 per share, respectively.
The total intrinsic value of options exercised in the years ended December 31, 2010, 2009 and 2008 was $403,000, $1,969,000 and $10,701,000, respectively. The total fair value of restricted stock units that vested in the year ended December 31, 2010 was $3,826,000.
111
As of December 31, 2010, the total compensation cost related to 1,213,120 unvested options and unvested awards covering 948,024 shares not yet recognized was $23,587,000. This amount will be recognized over an estimated weighted-average amortization period of 2.24 years.
Employee Stock Purchase Plan
As of December 31, 2010, the Company had reserved a total of 945,555 shares of common stock for issuance under the ESPP. In addition, the board of directors may increase the share reserve as of each January 1 through January 1, 2015, by an amount not to exceed the lesser of (i) 1% of the total number of shares of common stock outstanding on December 31 of the preceding calendar year or (ii) 250,000 shares. The ESPP permits eligible employees to purchase common stock at a discount through payroll deductions during defined offering periods. The price at which the stock is purchased is equal to the lower of 85% of the fair market value of the common stock at the beginning of an offering period or after a purchase period ends. During the years ended December 31, 2010 and 2009, 104,100 shares and 120,039 shares, respectively, were purchased under the ESPP. At December 31, 2010 and 2009, there were 477,211 and 581,311 shares, respectively, remaining and available for future grant under the ESPP.
Warrants
At December 31, 2010, 304,752 warrants were outstanding, of which 21,332 were exercisable for shares of common stock at $15.00 per share and 283,420 were exercisable for shares of common stock at $25.40 per share. The warrants expire at various dates from June 2012 to December 2013.
8. Preferred Stock
At December 31, 2010 and 2009, the Company was authorized to issue 5,000,000 shares of preferred stock.
9. Income Taxes
The Company recorded $0, $722,000 and $406,000 of current income tax benefit for the years ended December 31, 2010, 2009 and 2008, respectively. In the year ended December 31, 2009, $444,000 of current income tax benefit recognized was due to the adoption of a provision in theWorker, Homeownership, and Business Assistance Act of 2009 that allows businesses with net operating loss, or NOLs, in 2008 or 2009 to carry back those losses for up to five years, and $278,000 of current income tax benefit recognized was due to the adoption of a provision in theAmerican Recovery and Reinvestment Tax Act of 2009 that allows corporations to convert carry-forward research and development and Alternative Minimum Tax, or AMT, credits into a separate refundable amount, which the Company claimed and received as a refund in cash in 2010. The income tax benefit recognized for the year ended December 31, 2008 was primarily due to the adoption of a provision in theHousing and Economic Recovery Act of 2008that allowed corporations to convert carry-forward research and development and AMT credits into a separate refundable amount, which the Company claimed and received as a refund in cash in 2009.
112
Deferred income taxes reflect the net tax effects of NOL and tax credit carryovers and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company’s net deferred tax assets were as follows (in thousands):
| | | | | | | | |
| | | December 31, | |
| | | 2010 | | | 2009 | |
Net operating loss carryforwards | | $ | 108,753 | | | $ | 78,163 | |
Research credit carryforwards | | | 26,924 | | | | 23,933 | |
Capitalized research and development | | | 14,259 | | | | 16,916 | |
Deferred revenue | | | 7,048 | | | | 7,665 | |
Stock options | | | 14,648 | | | | 10,543 | |
Other | | | 928 | | | | 2,016 | |
| | | | | | | | |
Total net deferred tax assets | | | 172,560 | | | | 139,236 | |
Valuation allowance | | | (172,560 | ) | | | (139,236 | ) |
| | | | | | | | |
Net deferred tax assets | | $ | — | | | $ | — | |
| | | | | | | | |
Realization of net deferred tax assets is dependent upon the Company generating future taxable income, the timing and amount of which are uncertain. Accordingly, the net deferred tax assets have been fully offset by a valuation allowance. The valuation allowance increased by $33,324,000, $27,686,000 and $28,648,000 during 2010, 2009 and 2008, respectively.
As of December 31, 2010, the Company had NOL carryforwards for federal income tax purposes of $271,478,000, which expire in the years 2022 through 2030, and federal research and development tax credits of $19,557,000, which expire in the years 2021 through 2030.
As of December 31, 2010, the Company had NOL carryforwards for state income tax purposes of $269,499,000, which expire in the years 2013 through 2030, and state research and development tax credits of $11,494,000, which do not expire.
Approximately $529,000 of the valuation allowance for net deferred tax assets relates to benefits of stock option deductions that, when recognized, will be allocated directly to additional paid-in capital.
The Company files income tax returns in the U.S. federal jurisdiction and the California state jurisdiction. To date, the Company has not been audited by the Internal Revenue Service or any state income tax jurisdiction. Tax years 2002 to 2010 remain subject to examination by the U.S. federal jurisdiction and the California state jurisdiction.
Utilization of the Company’s NOL and credit carryforwards may be subject to substantial annual limitation due to the ownership change limitations provided by the Internal Revenue Code and similar state provisions. Such annual limitation could result in the expiration of the net operating loss and credit carryforwards before utilization. As of December 31, 2010, based on the analyses performed on annual limitation as a result of ownership changes that may have occurred from inception through December 2010, the Company expects to be able to use all of the NOL and tax credit carryforwards before their respective expiration periods.
113
10. Quarterly Financial Data (Unaudited)
The following table summarizes the unaudited quarterly financial data for the last two fiscal years (in thousands, except per share data):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Quarter Ended | |
| | | Dec. 31,
2010 | | | Sept. 30,
2010 | | | June 30,
2010 | | | March 31,
2010 | | | Dec. 31,
2009 | | | Sept. 30,
2009 | | | June 30,
2009 | | | March 31,
2009 | |
Selected Quarterly Data: | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Total revenues | | $ | 1,906 | | | $ | 389 | | | $ | 491 | | | $ | 93 | | | $ | 5,762 | | | $ | 403 | | | $ | 1,831 | | | $ | 26,277 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Net loss | | $ | (14,823 | ) | | $ | (19,899 | ) | | $ | (19,522 | ) | | $ | (28,225 | ) | | $ | (18,348 | ) | | $ | (24,374 | ) | | $ | (20,914 | ) | | $ | (2,698 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Basic and diluted net loss per share | | $ | (0.47 | ) | | $ | (0.65 | ) | | $ | (0.64 | ) | | $ | (0.93 | ) | | $ | (0.60 | ) | | $ | (0.81 | ) | | $ | (0.76 | ) | | $ | (0.10 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
114

